- Department of Pathophysiology, School of Basic Medicine, Key Laboratory of Neurological Diseases of Hubei Province and National Education Ministry, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Meningeal lymphatic vessels (MLVs) form an important bridging structure between the brain and periphery, which drains cerebral metabolites in cerebrospinal fluid (CSF) and brain antigens to deep cervical lymph nodes (dCLNs), to maintain brain homeostasis. Increasing evidence reveals the importance of MLVs in brain ageing and various central nervous system (CNS) diseases, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), traumatic brain injury (TBI), and multiple sclerosis (MS). Advances in research techniques have provided detailed insights into the structure and functions of MLVs, highlighting the therapeutic potential of targeting MLVs for related diseases. Here, we perform a systematic review of the features and functional regulation of MLVs, their associations with brain disorders, as well as some methodological advances in imaging of MLVs and the drainage pathway.
Background
The blood-brain barrier (BBB), a highly selective structure, isolates the CNS from peripheral systems while hindering substance exchange between the periphery and CNS (1). Accordingly, the homeostasis of the brain is traditionally thought to depend mainly on stations of neurovascular units (NVUs), which involve neurons, astrocytes, capillary-associated microglia, pericytes, endothelial cells and perivascular macrophages, and the signal communications among them (1, 2). A growing number of studies shedding light on the interactions between the brain and periphery, such as peripheral organs, peripheral metabolic system, peripheral immune system and gut microbiota, have indicated that the homeostasis of brain is closely related to the overall state of the body, underscoring the importance of communication pathways between the brain and periphery. Recently, MLVs have been identified as important bridging structures between the periphery and CNS, which drains CSF, metabolic waste, and immune cells that carry antigens from brain towards dCLNs (3, 4), revolutionizing our understanding of brain homeostasis.
MLVs were first named by Antoine Louveau and colleagues. They demonstrated the presence of a lymphatic vascular system around the dural sinuses of mouse meninges, marked by the expression of the lymphatic endothelial hyaluronic acid receptor-1 (LYVE-1), a lymphatic endothelial cell (LEC) marker (3). In fact, as early as the late 18th century, an Italian anatomist named Paolo Mascagni first mentioned the possible existence of meningeal lymphatic tissue in the dura, but it remained unrecognized by the scientific mainstream (5). In the following two centuries, owing to the limitations of experimental techniques, scientists did not make substantial progress in exploring the meningeal lymphatic system. In 2015, Antoine Louveau and Aleksanteri Aspelund et al. confirmed the existence of MLVs and discovered their role in transporting macromolecules in the brain to dCLNs (3, 6). Subsequently in 2017, the localization of MLVs in zebrafish (7), rats (8), humans and nonhuman primates (9) was respectively reported.
MLVs can participate in maintaining brain homeostasis from two aspects: substances drainage and immunity. On the one hand, MLVs can remove metabolic wastes from the brain to the periphery and reduce the deposition of harmful substances in CNS (3, 10). On the other hand, MLVs can transport brain antigens (4), and there are many immune cells (including the antigen presenting cells and T cells) on the dura mater for immune surveillance of the CNS (11). In addition, impaired MLVs can also reshape the morphology and function of microglia (12, 13). In 2016, Antoine Louveau et al. proposed that strategies for promoting meningeal lymphatic drainage may be beneficial for some neurological disorders, which opened the prelude to the study of MLVs in CNS diseases (14). In this review, we aim to summarize the relevant characteristics of MLVs, the pathway for CSF clearance and immune cell trafficking, the functional regulation of MLVs and their connection with some neurological diseases, as well as several related imaging methods.
Location of MLVs
In studies of the peripheral lymphatic system, markers of LECs, such as LYVE-1 and prospero homeobox protein-1 (Prox-1), are often used to show the location and morphological structure of lymphatic vessels (15–17). Additionally, these markers are found in MLVs specifically in the brain. Immunofluorescence staining of these markers combined with high-resolution imaging technologies helped to identify the presence of MLVs (3, 6). Based on the location, MLVs can be mainly categorized into dorsal MLVs and basal MLVs. The dorsal MLVs are situated near the superior sagittal sinus (SSS) and transverse sinus (TS), wrapped in the dura and far from the subarachnoid space (Figure 1A) (3, 6, 18). The basal MLVs are located near the petrosquamosal sinus (PSS) and sigmoid sinus (SS), along the middle meningeal artery (MMA), pterygopalatine artery (PPA), and vein of Galen, and close to the subarachnoid space (Figure 1B) (3, 6, 18). Although the positioning is different, the basal MLVs and the dorsal MLVs are connected and eventually leave the CNS from the base of the skull (Figures 1B, 2B). In addition, there are some MLVs distributing near the cribriform plate, cavernous sinus and pituitary gland (6, 18). In the eyes and vertebrae, there are also some lymphatic vessels similar to MLVs (19, 20). The anterior and posterior chambers of the eyes have distinct lymphatic drainage systems, while the latter can drain to the dCLNs through lymphatics in the optic nerve sheath (19). Vertebral lymphatic vessels (VLVs) are mainly distributed in epidural space and arranged in segments along the vertebrae, forming a loop between each vertebra and connecting to peripheral lymphatics through the ligamentum flavum, dorsal facet joint and intervertebral foramen (20).
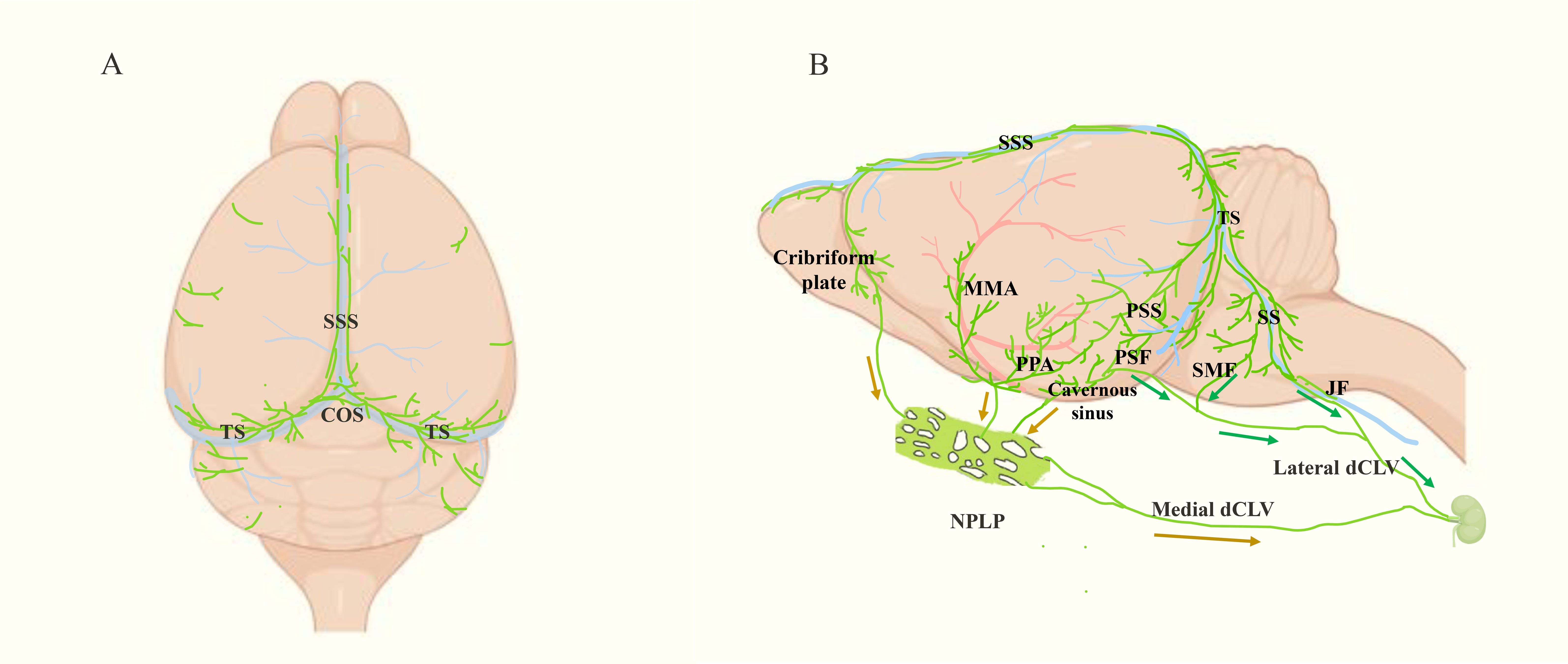
Figure 1. Localization and drainage pathway of mouse MLVs (green). (A) Top view of MLVs, which mainly shows the dorsal MLVs located along SSS and TS. SSS: superior sagittal sinus, TS, transverse sinus. (B) Side view of the meningeal lymphatic system, including the basal MLVs located near the PSS, SS, MMA and PPA. Besides, the drainage pathways from CNS to dCLN are shown following the arrows. Among them, the green arrows point to the direction of basal MLVs leaving the intracranial cavity. They get out of the skull at PSF, JF and SMF, then reach the dCLN via the lateral deep cervical lymph vessels (dCLV). The brown arrows indicate the lymphatic drainage pathway of the three upstream regions of nasopharyngeal lymphatic plexus (NPLP), which finally arrive at dCLN via medial dCLV. PSS, petrosquamosal sinus; SS, sigmoid sinus; MMA, middle meningeal artery; PPA, pterygopalatine artery; PSF, petrosquamous fissure; JF, jugular foramen; SMF, stylomastoid foramina.
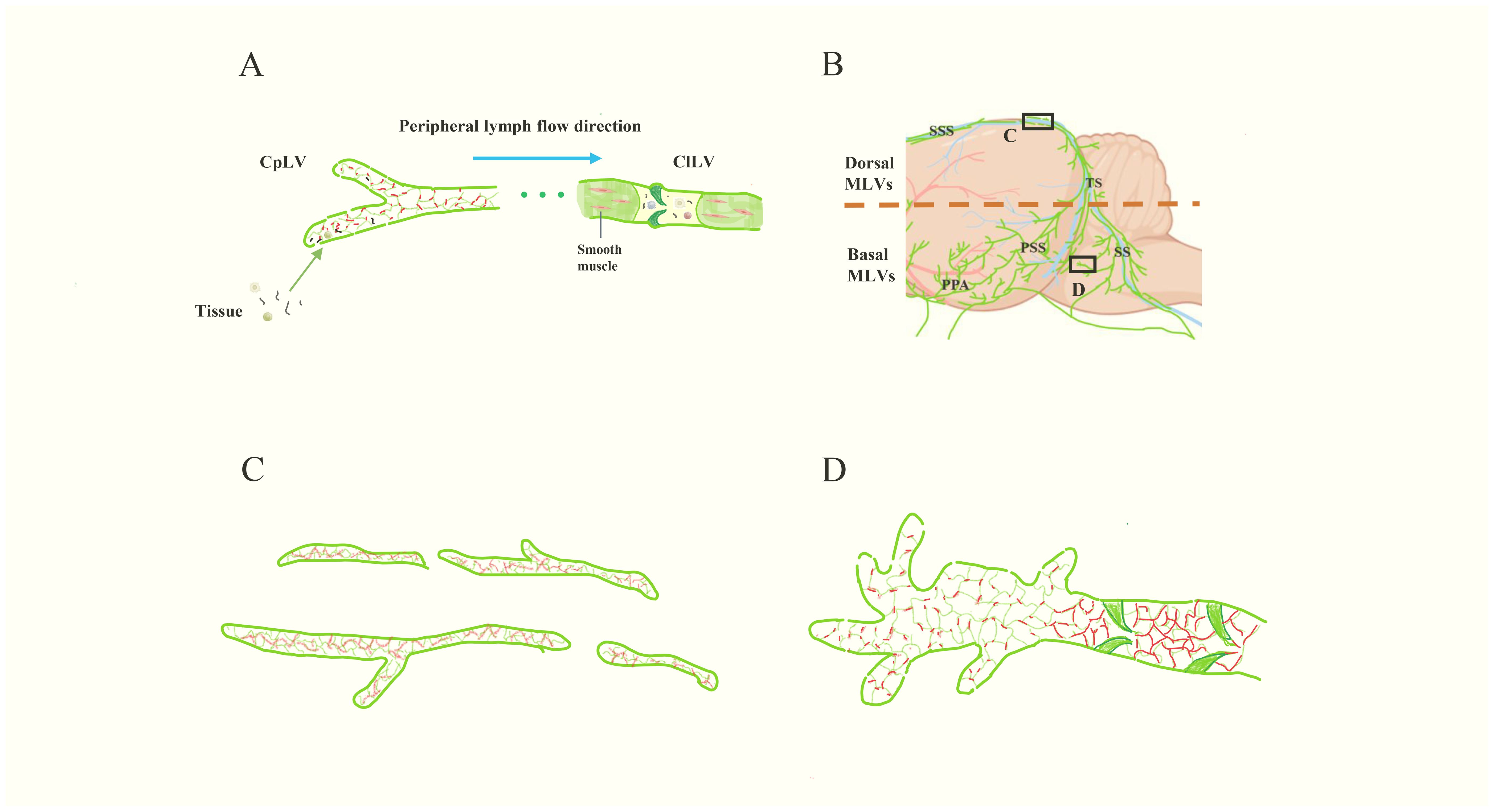
Figure 2. The structure of peripheral lymphatic vessels and MLVs. (A) The schematic diagram of peripheral lymphatic vessels. The left are CpLVs, which have loosely arranged endothelium like buttons (red) and drain ISF and large molecules. Then the lymph inside flows into ClLVs, which have tightly arranged endothelium, smooth muscle and lymphatic valves. (B) The schematic image of MLVs (green). The lymphatic vessels in upper part are dorsal MLVs and the lymphatic vessels in the lower part are basal MLVs. (C, D) are the magnifications of the images in the black boxes in (B). (C) represents the dorsal MLVs, which have few branches, tightly arranged endothelium like zipper (red), discontinuous structure and small diameter. (D) represents the basal MLVs with large lumens, lymphatic valves, abundant branches, and loosely arranged endothelium, which is conducive to CSF drainage. Besides, there are lymphatic valves at the proximal part and the endothelium is closely arranged like the ClLVs.
Before birth, the initial MLVs in mice are located near the foramen magnum and PPA. After birth, MLVs begin to develop along the MMA and PPA, grow along the dorsolateral side of SS, and reach the TS at the postnatal day 8 (P8). Following that, MLVs congregate at the confluence of the sinuses (COS) along the bilateral TS at P16, then extend along the SSS, and reach the rostral end of the SSS at P28, completing the basic development process (Figure 3) (21, 22).
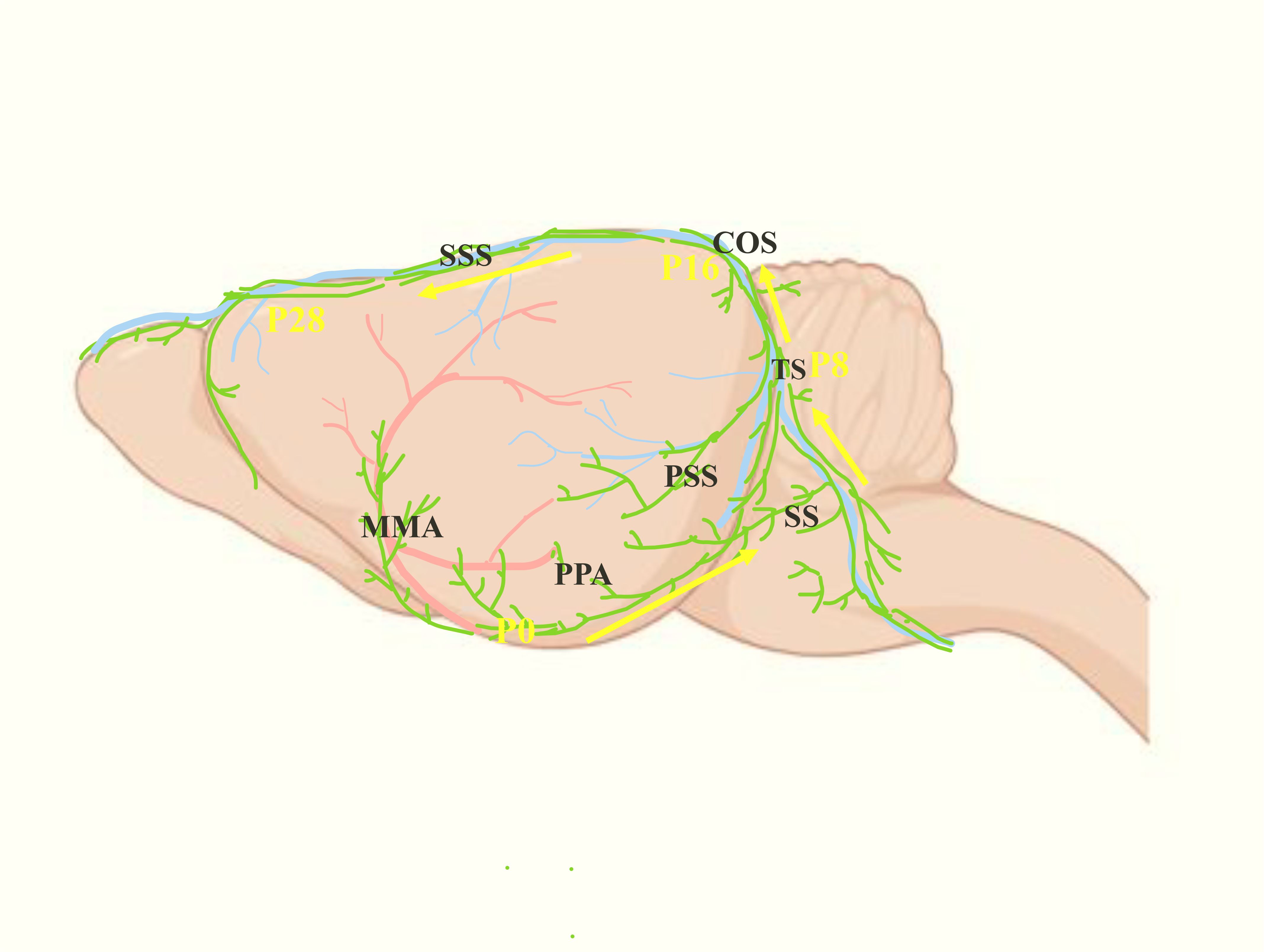
Figure 3. Postnatal development progress of the MLVs (green). The yellow arrows represent the development direction of MLVs after birth: from MMA and PPA to SS, then to TS, and finally to SSS after intersecting at COS.
Structures of MLVs
In the periphery, lymphatic vessels are divided into capillary lymphatic vessels (CpLVs) and collecting lymphatic vessels (ClLVs), with distinct structural features (Figure 2A) (17, 23). CpLVs have loosely arranged endothelium resembling buttons, which is conducive to draining interstitial fluid (ISF) and absorbing large molecules such as proteins. Anchoring filaments, another feature of initial lymphatic vessels, connect the membrane of LECs to surrounding elastic fibers, allowing CpLVs to remain open and absorb ISF (23, 24). CpLVs lack valves and smooth muscle, so lymphatic fluid can flow freely in all directions within the initial lymphatic plexus, whereas ClLVs transport lymphatic fluid from CpLVs to the lymph nodes and lymphatic trunks(Figure 2A) (17, 23, 25). The compact wall structure of ClLVs is divided into three layers: endothelial, smooth muscle and collagen tissue. ClLVs, like veins, have valves that permit fluid to flow in only one direction (proximal) (26). Through the functioning of smooth muscle, ClLVs undergo autonomous contractions to periodically propel lymph flow (26, 27).
MLVs present some characteristics consistent with initial lymphatic vessels, including the absence of smooth muscle and autonomous contractions (3, 6), leaving the driving force behind lymph flow in MLVs an enigma. The dorsal MLVs and basal MLVs are connected, but exhibit different structural characteristics(Figures 2B-D). The dorsal MLVs are small in diameter, discontinuous in structure, with few branches, and do not express integrin α-9, which is a marker of the lymphatic valve (Figure 2C) (28). Different from the directionless nature of CpLVs, the lymph in dorsal MLVs flows towards the cribriform plate in human (29). When Antoine Louveau et al. discovered MLVs in 2015, they proposed that Claudin-5 and vascular endothelial-cadherin (VE-Cadherin) have point-like distributions on dorsal MLVs (3). However, in 2019, Ji Hoon Ahn et al. reported that VE-Cadherin shows a continuous and closed distribution in dorsal MLVs, and the endothelial cells are closely connected like zippers, which are unsuitable for CSF drainage (Figure 2C) (18). In contrast, basal MLVs have large lumens, abundant branches, and the endothelial cells are leaf-like, loosely arranged like buttons, combining the characteristics of CpLVs and ClLVs (Figure 2D). However, at the proximal part, the endothelium is closely arranged like ClLVs and the lymphatic valves lead to the main flow direction of MLVs being intracranial to dCLNs (Figure 2D) (18). These structures make basal MLVs the main bearers of meningeal lymphatic drainage. However, the existence of anchoring filaments and layered structure in MLVs has not been reported so far, and how MLVs remain open and absorb CSF is still a question to be explored.
Drainage pathway through MLVs
At the beginning of the discovery of MLVs, researchers also revealed their function of draining CSF to dCLNs (3, 6). Initially, they were thought to get out of the skull following the cranial nerves, which were called perineural routes, including: along the olfactory nerve (near the cribriform plate), the trigeminal nerve and optic nerve (near the orbit), the facial nerve (through the stylomastoid foramina, SMF), as well as the glossopharyngeal, vagus, and accessory nerves (through the jugular foramen, JF) (6, 30). However, in 2019, Ji Hoon Ahn and colleagues discovered that MLVs remained intact after cranial nerve removal, suggesting that MLVs are not part of the perineuronal lymphatic system and may be simply concomitant with nerves (18). They observed basal MLVs draining CSF tracers from the petrosquamous fissure (PSF), JF, and SMF to periphery, connecting with extracranial lymphatics (lateral deep cervical lymph vessels, lateral dCLVs) and finally arriving to the dCLNs (Figure 1B) (18). In 2024, Jin-Hui Yoon et al. found that the nasopharyngeal lymphatic plexus (NPLP) is also a crucial hub for meningeal lymphatic drainage. The NPLP shares structural characteristics with basal MLVs, featuring both capillary and collecting lymphatic vessel traits: lacking smooth muscle coverage but having valves and a mixture of button-like and zipper-like junctions (31). There are three lymphatic drainage regions upstream of NPLP. Region 1 includes the lymphatics near the pituitary gland and cavernous sinus. Region 2 encompasses the lymphatics in the anterior region of the basolateral dura near the MMA and PSS, which flow along the PPA. Region 3 includes the lymphatics near the cribriform plate. The downstream of NPLP is connected to the dCLNs by the medial dCLVs (Figure 1B) (31).
However, at the starting point of meningeal lymphatic drainage pathways, how does CSF from the subarachnoid space access dural lymphatic vessels? This longstanding question was resolved in 2024 with the discovery of the arachnoid cuff exit (ACE) by Leon C. D. Smyth and colleagues, which provides a new perspective for CSF discharge other than arachnoid granules (32). In mouse models, they discovered that bridging veins traversing from the subarachnoid space into dural sinus are surrounded by cuff-like structures which are encased by arachnoid barrier cells and dural border cells, as well as a variety of immune cells. They termed the structure ACE points, where the phenotype of endothelial cells changes from that of the BBB (with specialized tight junctions) to that of peripheral blood vessels(with looser connection), providing an interface for molecular exchange (32, 33). Macromolecules from CSF were observed to migrate through ACE points into the dura mater, subsequently accessing meningeal lymphatic vessels. In human, magnetic resonance imaging (MRI) tracers transit along bridging veins in a similar manner (32). For immune cells aggregated near the dural sinus (11), the ACE point may also allow them to directly enter the subarachnoid space, realizing barrier-free trafficking of immune cells under neuroinflammatory conditions (32).
Traditionally, the meningeal lymphatic pathway has been usually described as an unidirectional channel from CNS to dCLNs. However, a study in 2024 revealed the bidirectional nature of lymphatic flow between CNS and dCLNs. Héctor M Ramos-Zaldívar et al. injected some nanoparticles, including superparamagnetic iron oxide, exosomes, gold nanorods, and Chinese ink nanoparticles, into dCLNs. Using MRI and histological analysis, they detected these nanoparticles in the brain, proving the reverse transport function of meningeal lymphatic pathway (34). This finding indicates that MLVs could serve as a novel route for delivering drugs to the brain, bypassing the BBB. In particular, they confirmed that exosomes can be transported backwards into CNS (34), which is a promising drug vehicle with perfect biocompatibility (35). Indeed, emerging evidence indicates that a similar lipid carrier named α-phospholipid nanoprobe (α-PLNPs) can carry drugs to prevent intracranial tumors via meningeal lymphatic reverse pathway (36).
The glymphatic system, another lymphatic system in CNS, is inextricably linked to MLVs, especially in terms of function. Serving as the bridge between CSF and brain ISF (33, 37), the glymphatic system can be considered as the upstream of meningeal lymphatic draining cerebral metabolites. Glymphatic system exchanges the solute between CSF and ISF, driven by cerebral arterial pulsation (38). Some cerebral metabolites and pathological molecule, like amyloid β (Aβ) and microtubule-associated protein (tau), can be cleared into CSF in order to be drained to periphery by MLVs, which function depends on the water channel aquaporin-4 (AQP4) located in the end feet of astrocytes (37–40). Enhancing the glymphatic system facilitates the discharge of molecules in CNS via meningeal lymphatic and may serve as a target for CNS waste removal (41, 42). Exploring the detailed connection between these two lymphatic systems in CNS is significant for clarifying the pathways of waste excretion in the brain and providing new targets for some CNS diseases.
Moreover, the characterization of human meningeal lymphatic flow has undergone transformative progress through advanced neuroimaging technologies. In 2017, Martina Absinta et al. first performed MRI on the meningeal lymphatic system in human and non-human primate by using a contrast agent (gadobutrol) to specifically differentiate lymphatic vessels and blood vessels, visualizing the MLVs located near the dural sinus, MMA and cribriform plate, some of which are completely enclosed within the dura (Figure 4) (9). In 2019, Phillip H. Kuo et al. found that the direction of lymph flow in the MLVs near the SSS is toward the cribriform plate and opposite to the direction of blood flow in the SSS (29). Jun-Hee Kim et al. proposed a noninvasive MLVs imaging technique based on an inter-slice blood perfusion MRI, called alternate ascending/descending directional navigation (ALADDIN), by which they verified the flow velocity of the dorsal MLVs in humans ranges between 2.2 and 2.7 mm/s (43). In 2020, Geir Ringstad et al. demonstrated that CSF tracers flow into the parasagittal dura in humans and Ying Zhou et al. discovered the old have the impairment of meningeal lymphatic drainage and thickening of lymphatic channels in the both dorsal and ventral regions (44, 45). Therewith in 2022, Laurent Jacob et al. demonstrated the extended anterior MLVs network around the cavernous sinus in human, with exit routes through the foramina of emissary veins, via real-time vessel-wall MRI (VW-MRI) (Figure 4) (46). Furthermore, Mehmet Sait Albayram et al. imaged not only intracranial MLVs but also the pathway of drainage from CNS to dCLNs. They classified the human meningeal lymphatic system into dorsal and ventral systems(Figure 4). The former refers to the MLVs near the dural sinus, which exits the skull through the JF and foramen magnum. The ventral system includes the MLVs located near the anterior cranial fossa, optic groove, Meckel’s caves and internal auditory canals, leaving the skull along the cranial nerves. The two eventually converge to dCLNs, and together participate in removing waste from the brain (47).
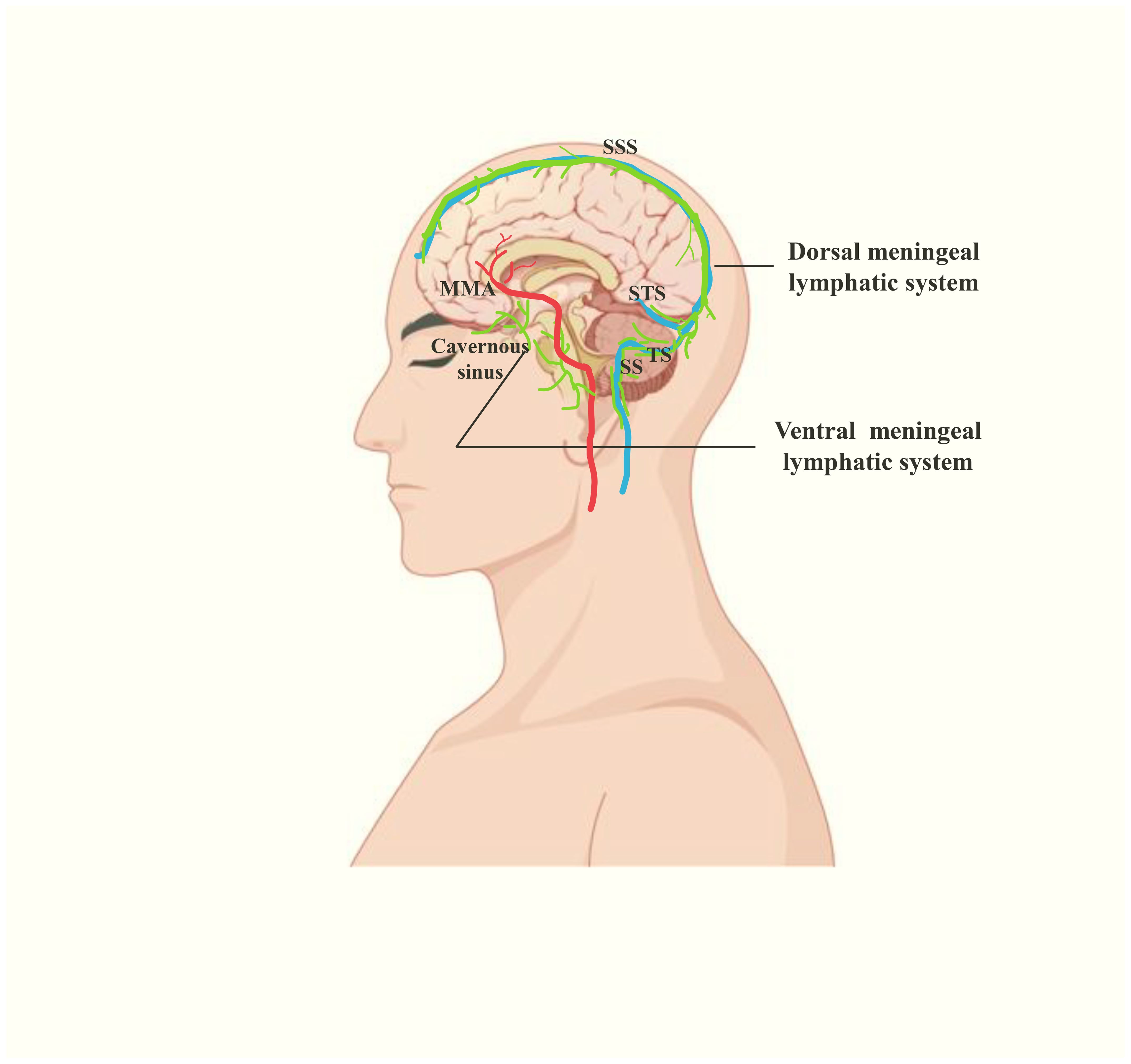
Figure 4. The human meningeal lymphatic system(green), including ventral and dorsal meningeal lymphatic systems. The former is located near the cavernous sinus, neural foraminas and MMA. The latter is located near the dural venous sinuses. STS, straight sinus.
Regulation of meningeal lymphatic drainage
As a special lymphatic vessel, MLV drainage can be regulated by various molecules or stimulations, via modulating the structure or contractile function (Tables 1–3).
Molecular regulators of meningeal lymphatic function
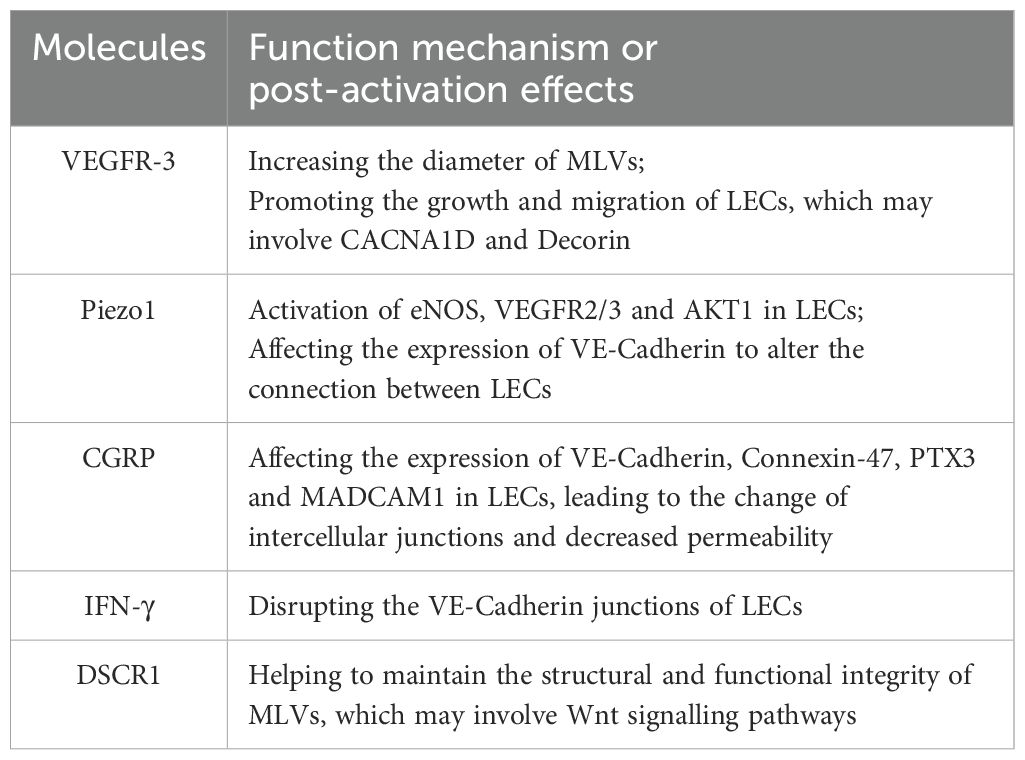
VEGFR-3
The vascular endothelial growth factor-C/D (VEGF-C/D) signaling pathway conducted by vascular endothelial growth factor receptor-3 (VEGFR-3) plays a significant role in lymphangiogenesis and development (48–51). K14-VEGFR-3-Ig transgenic mouse, which has an impaired VEGF-C/D–VEGFR3 pathway, exhibits disabled MLVs and significantly weakened function in draining macromolecules from the brain to dCLNs (6, 52). Expressing the extracellular domains 1 to 3 of murine VEGFR-3 by adeno-associated virus (AAV), which bind and sequester VEGF-C/D (VEGF-C/D trap), can induce meningeal lymphatic degeneration and drainage dysfunction (53). Administering a monoclonal antibody against VEGFR-3 or specifically knocking out VEGFR-3 in adult mouse LECs yields similar results (54). Conversely, intracranial overexpression of VEGF-C in aged mice through AAV, osmotic drug delivery from the skull through hydrogel, direct injection into cisterna magna, or delivery of VEGF-C mRNA all can increase the diameter of MLVs and enhance the drainage function (10, 55–58). The mechanism involves promoting the growth of LECs and changes in transcriptomics, including the increased calcium voltage-gated channel subunit α1 D (Cacna1D) expression and decreased Decorin expression, which are related to the proliferate and migrate of LECs (58). Moreover, skull progenitor cells secrete VEGF-C, which promotes the growth and migration of LECs and facilitates the growth and function of MLVs, playing an important role in craniosynostosis (59).
Piezo1
Piezo1 is a mechanically gated cation channel that senses the mechanical forces applied to the cell membrane and cytoskeleton (60, 61), playing a crucial part in the development and function of lymphatic vessels (62–64). Yoda1, a small molecule chemical agonist of Piezo1, lowers the mechanical force threshold for activation (65). Administering Yoda1 to mice stimulates MLVs to drain CSF. The mechanism may involve the activation of endothelial nitric oxide synthase (eNOS), VEGFR-2/3 and protein kinase B1 (AKT1) in LECs. Moreover, Yoda1 affects the expression of VE-Cadherin to alter the connection between LECs (66, 67). Specifically knocking out or overexpressing Piezo1 in the lymphatic vessels of mice also results in decreased or enhanced meningeal lymphatic drainage. Notably, knocking out or overexpressing Piezo1 for a short period does not cause changes in the structure of the MLVs, but the drainage function still appears weakened or enhanced (67).
CGRP
Calcitonin gene related peptide (CGRP) is related to sympathetic nerve excitation transmission and has strong vasodilator activity (68, 69). However, LECs even have higher expression levels of the CGRP receptor (a dimer formed by calcitonin receptor-like receptor, CLR and receptor activity modifying protein 1, RAMP1) than vascular endothelial cells (70, 71), and the abnormal expression of both will affect the development and function of lymphatic vessels (72, 73). At the cellular level, CGRP affects the VEGFR-3 signaling pathway and also alters the expression of some intercellular connection-related proteins such as VE-Cadherin (74, 75). In MLVs, CGRP stimulation reduces their permeability and inhibits their drainage function. The mechanism involves affecting the expression of VE-Cadherin, Connexin-47, Pentraxin3 (PTX3) and mucosal vascular addressin cell adhesion molecule 1 (MADCAM1) in meningeal LECs, leading to the changes of the connections between endothelial cells (76).
Interferon-γ
Interferon-γ (IFN-γ), a cytokine with roles in antiviral and anti-tumor responses, can be secreted by T cells, which can accumulate in the dura mater especially during the ageing (11, 77). A study conducted by Justin Rustenhoven discovered that the level of IFN-γ in meningeal LECs is increased in aged mice and the overexpression of IFN-γ disrupted VE-Cadherin junctions of LECs, impairing the meningeal lymphatic drainage (78). Manipulating the level of IFN-γ by intraperitoneally injecting anti-IFN-γ neutralizing antibodies can improve the flow of MLVs in aged mice, which may function by reducing the damage to VE-Cadherin caused by IFN-γ (78). Noteworthily, they administered peripherally but the lymphatic flow in CNS got significant improvement, highlighting its potential for clinical translation.
DSCR1
Down syndrome critical region 1 (DSCR1, also known as regulator of calcineurin 1, RCAN1) is upregulated in the tissues of patients with Down syndrome (79), especially in the brain, mechanistically linking to learning and memory impairments (80). However, in LECs, VEGF mediates the induction of DSCR1 (81), suggesting its potential role as a downstream effector in VEGF-driven lymphangiogenesis. Chiyeol Choi et al. found overexpressing DSCR1 potentiated dorsal MLVs and augmented intracranial lymphatic drainage capacity (82). In 5×FAD mice, increasing DSCR1 rescued the structural and functional integrity of MLVs and assisted to the discharge of Aβ, ultimately ameliorating cognitive deficits (82). Transcriptomic profiling of meningeal LECs implicated Wnt signaling pathways functioning in this reparative process, although the precise molecular mediators responsible for meningeal lymphangiogenesis remain to be elucidated.
Non-invasive modulation of meningeal lymphatic drainage
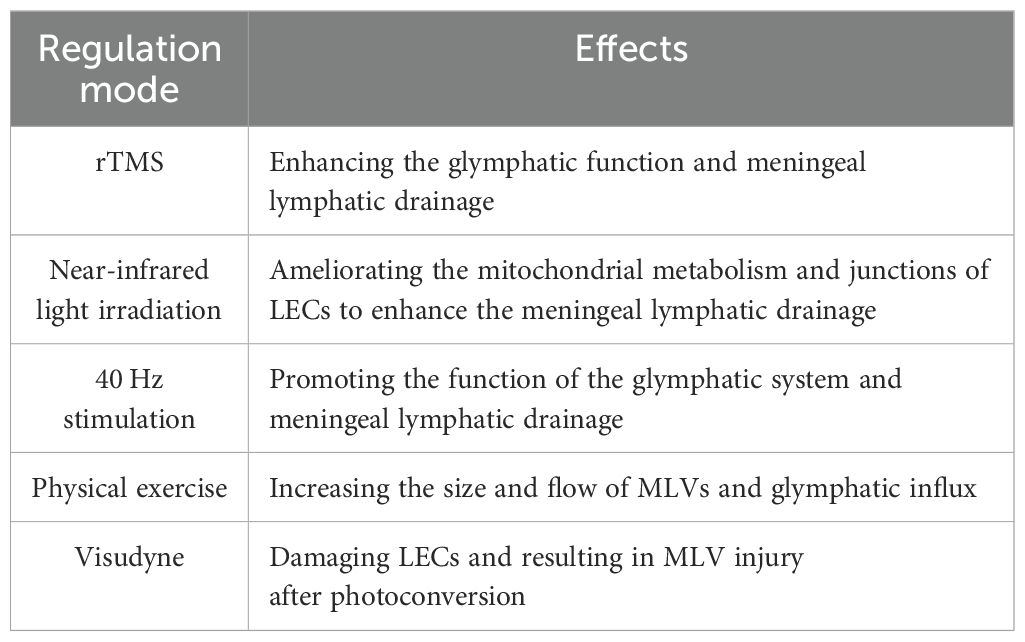
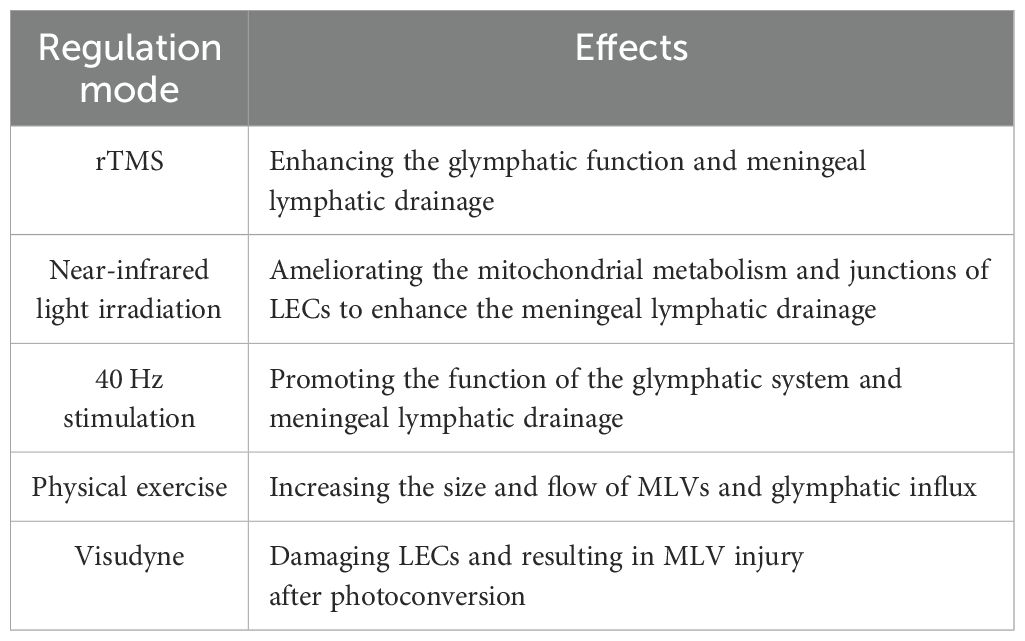
For its convenience, non-invasive stimulation has been extensively studied as a promising therapeutic target for various diseases, including some neurodegenerative diseases, such as AD and PD (83–85). Additionally, research has explored its effects on MLVs. Common stimulations include light, sound and magnetic stimulation. Yangyang Lin et al. found that repetitive transcranial magnetic stimulation (rTMS) promotes meningeal lymphatic drainage and glymphatic function in 5×FAD mice, reducing the Aβ deposition in the brain (86). Miao Wang and Dongyu Li et al. used near-infrared light irradiation and found enhanced drainage function in MLVs (87, 88). By transmission electron microscopy imaging and RNA sequencing, they found that light modulation ameliorated the mitochondrial metabolism and junctions of meningeal LECs, and subsequently enhanced the capability of MLVs in old and AD mice. In addition, Mitchell H. Murdock et al. and Wen Wu et al. respectively reported that multisensory 40 Hz stimulation (combining light and sound) or 40 Hz blue light exposure enhances the efflux of Aβ to dCLNs in AD mice, although this process is indirectly achieved by promoting the function of the glymphatic system, rather than directly acting on MLVs (41, 89).
Besides, regular voluntary exercise has been shown to increase the waste discharge through glymphatic system in mice brain (90). In healthy volunteers, Roh-Eul Yoo et al. investigated the impact of physical exercise on brain waste clearance via MRI (91). The results showed the size and flow of MLVs increased significantly after long-term exercise, which was associated with the downregulation of S100 calcium-binding protein A8 (S100A8), S100A9, proteasome subunit alpha type-3 (PSMA3), and defensin alpha 1 and alpha 3 (DEFA1A3) and the up-regulation of J chain in plasma (91).
In addition, to experimentally damage MLVs, researchers use a photodynamic drug named visudyne to ablate MLVs, which preferentially damages LECs after photoconversion (92). They inject visudyne into CSF and proceed photoconversion, resulting in MLV injury and loss of drainage function (4, 10, 55). Notably, repeating the above operations can prolong the effect of ablation (10), providing a model of long-term MLVs damage.
Modulation of extracranial lymphatics and clinical translation
As a component of the pathway from MLVs to dCLNs, dCLVs also play an important part in CSF drainage. Due to the existence of smooth muscle in dCLVs, some drugs that dilate or constrict blood vessels can also be applied in dCLVs. Phenylephrine can activate α1-adrenergic receptors in lymphatic smooth muscle cells and contract dCLVs, reducing the CSF drainage (31). By releasing nitric oxide (NO) and increasing the intracellular level of cyclic guanosine monophosphate (cGMP), sodium nitroprusside can act on smooth muscle cells and dilate dCLVs, enhancing the CSF outflow (31, 93). It is worth noting that a low concentration of phenylephrine can also dilate dCLVs and promote CSF drainage, probably through β2-adrenergic receptors (31). However, a study conducted by Ting Du et al. reported that the reduction of contraction frequency and flow velocity occurred in aged CLVs and slowed the CSF outflow (94). Topical application of prostaglandin F2α, a prostanoid that increases smooth muscle contractility, could restore lymphatic function and CNS clearance (94).
Apart from pharmacological methods, surgical ligation of the afferent or efferent lymphatic vessels of dCLNs obstructs CSF drainage by MLVs (6, 10, 55, 95, 96). Some researchers even directly bridged the subdural space above the hippocampus with the submandibular lymph node to guide the lymphagiogenesis of MLVs (97). Targeting downstream components of the meningeal lymphatic axis represents a translational strategy to augment cerebral waste clearance without direct CNS intervention.
Recently, an operation named deep cervical lymphatic-venous anastomosis (dcLVA) has been performed on AD patients and reported to improve cognition (98). Surgeons connect dCLVs marked by ovalbumin with indocyanine green (ICG) with jugular veins, to enhance intracranial lymphatic drainage and waste removal in CSF, then evaluate curative effect by MRI and cognitive restoration (98). Besides, some similar surgical methods like lymph node-venous anastomosis (LNVA), lymphoid tissue-venous anastomosis have been also used for the treatment of AD (99). However, the feasibility still needs more clinical data to confirm, and long-term patency rates of surgical anastomoses remain undetermined (long-term follow-up data lacking). Moreover, with the same theory, we can even propose a bold hypothesis: does CSF shunt surgery like ventriculo-peritoneal shunt has a similar curative effect? Overall, dCLVs have their own advantages. Locating outside the CNS, dCLVs can be manipulated more easily to affect CSF drainage compared with MLVs, presenting a promising target for brain waste removal.
MLVs in brain ageing and CNS diseases
Brain ageing
Brain ageing is intricately linked to the deterioration of physical and mental health (100). Ageing triggers the changes in brain structure, such as reductions in regional cortical thickness, subcortical volume, rarefied white matter, and enlargement of the ventricles, and the worsening of this process contributes to the pathogenesis of neurodegenerative disorders, such as AD and PD. In aged mice, both the structural integrity and function of MLVs are impaired (10, 18, 30), while the deterioration of glymphatic function also occurs and is associated with the deficit of C-C chemokine receptor type 7 (CCR7) on meningeal T cells (101). These changes jointly lead to the weakening of meningeal lymphatic drainage, participating in causing some behavioral changes like suppression of exploratory activity and cognition (13, 101). As the main carrier of CSF drainage, basal MLVs show increasing in size, high branching and hyperplastic phenotypes but have fewer valves and impaired LEC junctions, ultimately leading to an attenuated drainage function (18). Noteworthily, giving VEGF-C or non-invasive modulations like near-infrared light treatment can delay the ageing of MLVs and restore their function (10, 87). Similar changes occur in aged humans. MRI results show that elderly individuals have worse meningeal lymphatic drainage and thickened MLVs (45, 47), causing metabolic waste retention in the brain, which may aggravate ageing in turn (Figure 5).
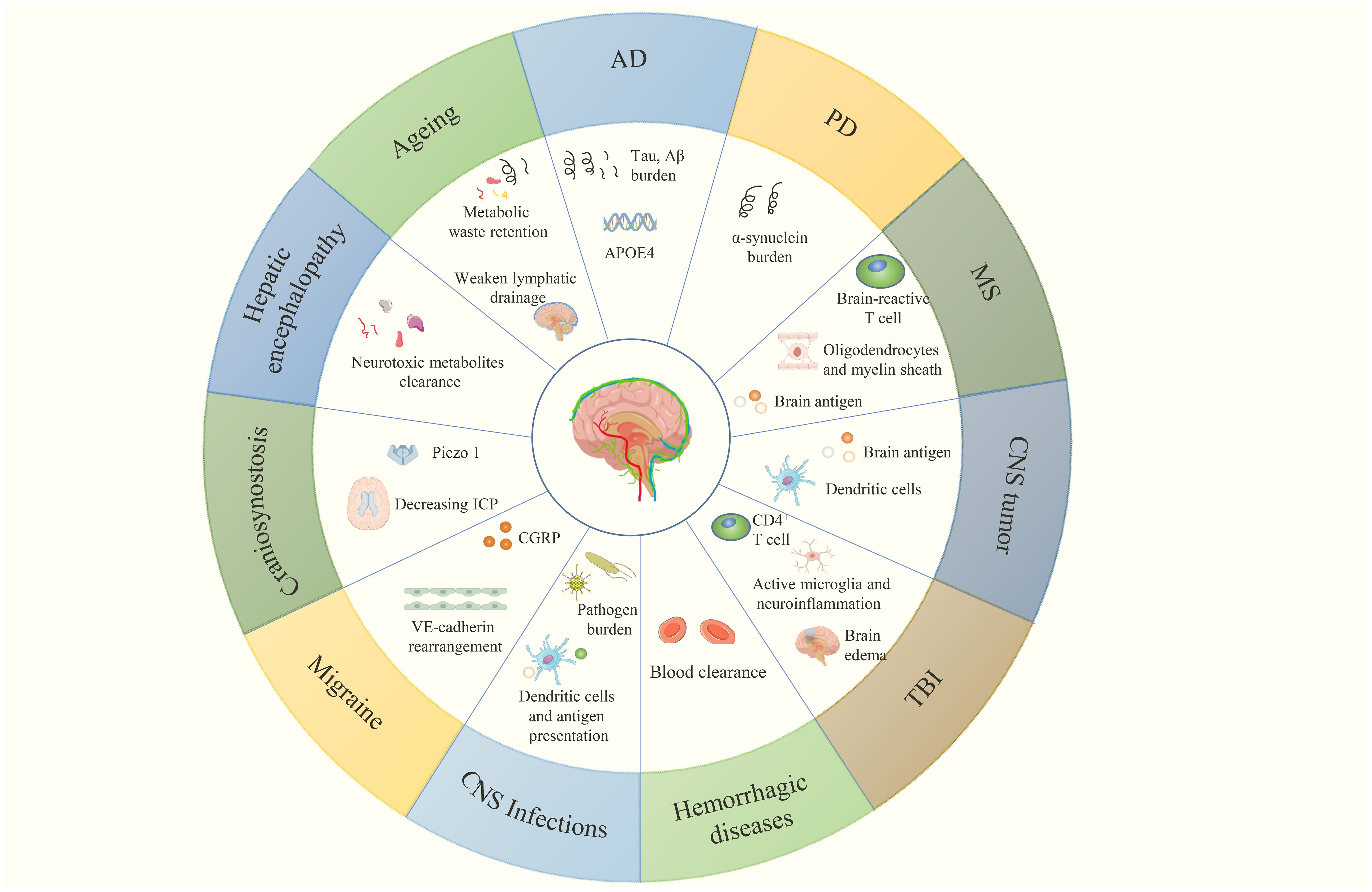
Figure 5. CNS diseases related to MLVs and their multiple aspects regulated by meningeal lymphatic (dys)function, which are discussed in this review.
AD
The pathogenesis of AD is closely related to the abnormal deposition of Aβ and neurofibrillary tangles formed by hyperphosphorylated tau in the brain (102). MLVs play a significant part in clearing these harmful molecules.
Interestingly, Aβ was initially isolated from the meningeal tissue of AD patients (103). Indeed, MLVs can transport Aβ from the brain to dCLNs. Disruption of meningeal lymphatic drainage(e.g., photodynamic ablating the MLVs, deficit in CCR7 or ligating the dCLVs) leads to Aβ deposition (Figure 5), exacerbated brain pathology, and decline in cognize and memory in AD transgenic mice including J20, 5×FAD and APP/PS1 (10, 96, 101, 104). Conversely, improving meningeal lymphatic drainage alleviates Aβ deposition. Giving VEGF-C to (old) AD transgenic mice in different ways improves MLVs function and promotes Aβ efflux to dCLNs (10, 56, 104). Non-invasive stimulations like near-infrared light irradiation and rTMS yield similar results in 5×FAD and APP/PS1 mice (86–88). Noteworthily, according to a study published in January 2025, cranial bone maneuver (CBM) promotes the lymphangiogenesis and drainage of MLVs, alleviating the Aβ deposition and memory deficits in 5×FAD mice for a long time (105). Besides, some traditional Chinese medicines (TCMs) have also shown promise in promoting meningeal lymphatic flow in AD. A study in 2023 revealed that borneol decreases the NO levels to constrict MLVs and increases VEGF-C and LYVE-1 to stimulate the lymphangiogenesis and valve plasticity, thereby contributing to Aβ efflux (106). A formula named Jiawei Xionggui decoction has also been proven to improve the Aβ efflux via MLVs and neuroinflammation by inhibiting the arachidonic acid pathway (107). However, in these studies about TMCs, the sample size limits the clarification of the problem. Only 3 to 5 samples were included in each group when studying the structure and drainage function of MLVs. Besides, all the above-mentioned treatment methods have been proved effective in the AD model mice, but whether they have same efficacy and safety in human remains to be verified.
Equally, tau can also be exported to the periphery by MLVs (108). Injecting labelled tau into the brains of K14-VEGFR-3-Ig transgenic mice results in more signal retention in the brain compared with mice having normal MLVs function, suggesting that MLVs regulate the clearance of extracellular tau from the CNS (52). Of course, without the action of glymphatic system, tau cannot get out from the brain parenchyma to CSF and enhancing glymphatic system facilitates the meningeal lymphatic clearance of tau in parenchyma (40). Interestingly, Mingqi Liu and Shiying Dong et al. found Interleukin 33(IL-33) and cannabidiol can enhance glymphatic function and intracranial lymphatic clearance, reducing tau accumulation and ameliorating memory and cognitive functions in the model of TBI (109, 110).
Moreover, apolipoprotein E4 (APOE4), the leading genetic risk factor for AD, may be associated with MLVs (Figure 5) (102, 111, 112). Although not yet proven, the connection between APOE4 and peripheral lymphatic (113) are always driving scientists to research its role in MLVs (111). By reanalyzing the previously published RNA-Seq data, Alexios-Fotios A. Mentis et al. proposed that induced pluripotent stem cells (iPSCs) carrying the APOE4 allele show lower expression of genes related to lymphatic markers and valve formation. Consequently, they put forward that APOE4 may induce abnormalities in the structure and function of MLVs (lymphosclerosis and lymphedema), then impair the clearance of waste like Aβ and exacerbate the manifestations of AD (111), although the authenticity of this conjecture has not yet been verified.
PD
PD is a neurodegenerative disease characterized by the loss of dopaminergic neurons in the substantia nigra and the formation of Lewy bodies, in which α-synuclein plays an essential role (114, 115). MLVs can drain oligomeric α-synuclein and reduce macrophage activation (116). Ligating the dCLVs of A53T mice that express mutated human α-synuclein aggravates α-synuclein accumulation (Figure 5), inflammation and dopaminergic neuronal loss (95). After injecting α-synuclein preformed fibrils into the brains of mice more than 6 months, Xue-Bing Ding et al. observed that the delayed clearance of CSF and loss of the tight connections between meningeal LECs, suggesting that α-synuclein may injure MLVs in PD (117). Besides, they also found that patients with idiopathic PD exhibit significantly reduced flow in the MLVs along the SSS and SS, as well as a delay in dCLNs perfusion, first demonstrating the relationship between PD and MLVs in human (117).
MS
MS is an autoimmune disease of CNS, characterized by mistaken immune system attack and demyelination of CNS (118). As components related to the immune system, Antoine Louveau et al. proposed that MLVs might participate in MS at the outset of discovery (14). In 2019, they found MLVs transport the brain antigens to T cells in dCLNs, causing T cells to recognize brain antigens and attack the CNS components (Figure 5) (4), indicating that MLVs do not always have a positive effect on CNS diseases. Ablating the MLVs or ligating the afferent lymphatic vessels of dCLNs relieves pathology (4). However, meningeal lymphatics also regulate oligodendrocyte function and brain myelination, and meningeal lymphatic dysfunction hinders brain myelination (53). Furthermore, MLVs did not have morphological changes (4), whereas the NPLP near the cribriform plate proliferated and drained both CSF and cells previously in the CNS parenchyma in experimental autoimmune encephalomyelitis (EAE) mice(a MS model) (119, 120), suggesting that NPLP may also play a part in neuroinflammation. At present, the main treatment method for MS in clinical practice is disease-modifying therapies (DMTs). Most DMTs have anti-inflammatory effects and function by inhibiting peripheral immunity and the entry of peripheral lymphocytes into CNS, which may lead to adverse consequences such as an increased risk of infection (121). Therefore, blocking the transport of brain antigens to the periphery is promising in the treatment of MS. How to block the brain antigens transport by MLVs without affecting oligodendrocytes may be the next research hotspot.
In 2025, a research conducted by Min Woo Kim et al. put forward that there are endogenous self-peptides existing in the brain’s borders and its lymphatic drainage pathway, which guide the immune response towards suppression, whereas neuroinflammation can impair the presentation of these peptides and worsen the immune response (122). Replenishment of these peptides in CSF ameliorates EAE, providing a new direction for the treatment of CNS autoimmune disease (122).
Tumors
As mentioned above, MLVs can export cells and antigens in the brain to dCLNs and present them to T cells (4, 123), which also function in CNS tumors. Glioma, a common CNS tumor, has always been a research hotspot. Qiaoli Ma et al. reported that mice with gliomas showed decrease of CSF outflow and lower signals in dCLNs after injecting CSF tracer (124). The glioma patients and mice both have low expression of VEGF-C, and administering VEGF-C to these mice prolongs their survival while ligating the dCLVs cancels this effect (57). In 2020, Xueting Hu et al. found that dorsal MLVs remodeling happened in mice with intracranial glioma or metastatic melanoma. And mice with defective or enhanced dorsal MLVs transported fewer or more dendritic cells (DCs, carrying the antigens in the brain) from CNS tumors to dCLNs (Figure 5) (125). Besides, combining checkpoint blockade therapies with VEGF-C delivery improves the therapeutic effect on glioma (57, 125). In 2022, Changping Zhou et al. reported that delivering VEGF-C mRNA significantly enhanced radiotherapy efficacy by MLVs in mice with brain tumors (126). Notably, VEGF-C shows low expression in patients with glioma and the level is strongly correlated with the anti-tumor immune response (57, 126). It is of great significance to conduct VEGF-C-related clinical trials on patients with glioma. In 2023, Minghuan Wang et al. found that patients with CNS malignant tumors presented elevated wash-in function and decreased wash-out function of MLVs, which represent the ability of MLVs to absorb waste from brain and expel it (127). These phenomena are associated with degree of tumors malignancy, isocitrate dehydrogenase (IDH) genotype and disease progression (127), but how these factors affect MLVs and whether MLVs are associated with CNS malignant tumor prognosis still need to be explored.
TBI
Studies in recent years have shown correlations between MLVs and the progression and recovery of TBI (109, 110, 128–133). In terms of gene expression, meningeal LECs of TBI mice show lower LYVE-1 expression but higher expression of FMS-like tyrosine kinase 4 and neuropilin 2 (markers of lymphangiogenesis), suggesting MLVs may function in the recovery of TBI (132). Indeed, TBI causes severe deficits in meningeal lymphatic drainage and increases intracranial pressure (ICP), while a high ICP injures MLVs, leading to a vicious cycle (130). In addition, K14-VEGFR-3-Ig mice show a significant reduction of infiltrating CD4+ T cells in the brain after TBI, suggesting that MLVs may participate in the neuro-immune response (Figure 5) (133). The mechanism of meningeal lymphatic drainage impairing after TBI mainly involves the “adrenaline storm” theory (129). Rashad Hussain et al. proved that the excessive systemic release of noradrenaline after TBI would cause a suppression of glymphatic fluid flow and reduced contractility of dCLVs, leading to the damage of meningeal lymphatic drainage. Pan-adrenergic receptor inhibition partly restored intracranial lymphatic flow and promoted the export of cellular debris, improving the brain edema and prognosis (129). Besides, pre-injured MLVs worsen the inflammation caused by TBI, which may be related to the upregulated expression of complement-related genes and the activation of microglia (128, 130). These findings suggest that the old with weaker MLV function may get more severe consequences after TBI. Some drugs that can improve the meningeal lymphatic drainage have been proven to have therapeutic effects in TBI mice. VEGF-C, ketoprofen and 9-cis retinoic acid improve the neuroinflammation and brain edema caused by TBI through stimulating MLVs to express LYVE-1, VEGFR-3, PROX-1 and forkhead box C2 (FOXC2) and enhancing drainage (130, 131). IL-33 and cannabidiol are also beneficial for the recovery of TBI by facilitating the lymphatic drainage of toxic waste (109, 110). They improve the exchange of CSF and ISF by reversing the dysregulation and depolarization of AQP-4. In other words, they can enhance the glymphatic system. Notably, these interventions can also inhibit the activation of glial cells and improve neuroinflammation, providing new methods for the reconstruction of neurological deficits in TBI patients (109, 110, 130, 131). Although the efficacy and safety (or side effects) in human still need to be verified through clinical trials.
Hemorrhagic diseases
Hemorrhagic diseases, including subarachnoid hemorrhage (SAH), intracerebral hemorrhage (ICH) and subdural hematoma (SDH), pose a serious threat to CNS.
The subarachnoid space is filled with CSF and MLVs can drain the CSF to periphery, so it is easy to consider MLVs when researching SAH. Indeed, in 2019, Tinglin Pu et al. found that the drainage function of MLVs decreased within one week after SAH in mice (134). Similarly, in larger animals like dogs, SAH can also disrupt MLVs (135). In 2020, Jinman Chen et al. demonstrated that MLVs drain the blood in subarachnoid space to dCLNs in SAH mice (Figure 5) (136). Additionally, they found that ablating MLVs or inhibiting VEGFR-3 reduces the clearance of the blood in subarachnoid space and aggravates the pathology of SAH (136). Subsequently, by single-cell RNA sequencing and spatial transcriptomics, Xiaoyu Wang et al. revealed that SAH induces MLV injury and the outcome of SAH is associated with thrombospondin 1 (THBS1) and S100A6. Furthermore, the THBS1-CD47 ligand-receptor pair functions in LECs apoptosis via signal transducer and activator of transcription 3/B-cell lymphoma 2 (STAT3/Bcl-2) signaling (137). Also, enhancing Th17 cell drainage through MLVs could alleviate neuroinflammation after SAH and influence prognosis (138).
ICH can also be relieved by MLVs (139, 140). After ICH, MLVs expand and become more functional, simultaneously drain the blood to dCLNs (140). During the late phase of ICH, MLVs proliferate (139). Ablation of MLVs or ligation of dCLVs inhibits the clearance, whereas administering VEGF-C promotes. Moreover, enhanced MLVs reduce the neuron loss, glial cell activation and hematoma volume (139). A non-invasive stimulation, rTMS, can enhance the clearance of intracranial lymphatic drainage after ICH (141). Also, near infrared photobiomodulation provides fast recovery after intraventricular hemorrhage (142). A drug named Panax Notoginseng Saponins (PNS) also promotes the drainage function of MLVs by stimulating the lymphangiogenesis in ICH (143). Additionally, in ischemic stroke, VEGF-C pretreatment enhanced MLVs, leading microglia-mediated inflammation mitigating and neuroprotective factors increasing, and helping to reduce stroke injury (58). Notably, cranial bone transport (CBT) has been found to contribute to the remission of neurological deficits in ischemic stroke patients (144), in which MLVs may also provide assistance (145). Interestingly, middle cerebral artery occlusion (MCAO) animals (an ischemic stroke model) have worsen meningeal lymphatic drainage and the animals with ablation MLVs or mutational VEGFR-3 get more grievous stroke injury after MCAO, however, the stroke mice induced by photothrombosis show meningeal lymphangiogenesis (145, 146). Why different molding methods have different effects on MLVs still remains unknown. Perhaps the laser used in photothrombosis has some stimulatory effect on meningeal LECs? This could be an interesting question to explore.
Equally, the SDH shares similar characteristics. Xuanhui Liu et al. found the red cells in dCLNs of SDH model rats, and ligating dCLVs aggravated the SDH (147). After SDH, the expression of lymphangiogenesis-related proteins in meninges, including LYVE1, FOXC2 and VEGF-C, is downregulated (147). In this process, MLVs are damaged, leading to progressive functional loss. The injury of MLVs, especially the basal MLVs, may be related to the dephosphorylation of extracellular signal-regulated kinase 1/2 (ERK1/2) within the LECs and the resulting change in connections between LECs (148). Besides, the vitamin D treatment significantly reduces SDH volume and improves the drainage of SDH to cervical lymph nodes (149).
CNS infections
The meningeal lymphatic system demonstrates functional involvement across major categories of CNS infections, encompassing bacterial, viral, and parasitic pathogens. In Listeria monocytogenes (LM) infection, bacterial invasion induces paradoxical responses in MLVs: lymphangiogenesis initially occurs, accompanied by the downregulation of critical lymphatic developmental regulators (FOXC2 and GATA binding protein 2, GATA2), ultimately culminating in structural degeneration and impaired drainage capacity (150). Notably, experimental ablation of MLVs exacerbates neuroinflammation and increases the bacterial burden in LM-infected mice (Figure 5) (150). Moreover, modulation of meningeal lymphatic function (including enhancement and inhibition) correspondingly alters outcomes in lipopolysaccharide-induced sepsis-associated encephalopathy (SAE) (151). This finding positions MLVs as a potential therapeutic target— capable of mitigating inflammation driven by infection.
Neuroinvasive viruses including Japanese encephalitis virus (JEV), rabies virus (RABV), and herpes simplex virus 1 (HSV-1), induce lymphangiectasia while impairing the physiological drainage of MLVs (55). MLVs mediate viral egress from CNS to dCLNs, and damaging or enhancing the MLVs gets similar results as the bacterial infection (55). Furthermore, as an important structure in CNS infection, a provocative hypothesis suggests MLVs may facilitate Human Immunodeficiency Virus (HIV) neuroinvasion, though the truth remains pending (152).
In parasitic infections such as Toxoplasma gondii invasion, MLVs orchestrate adaptive immune priming— DCs transport parasite antigens via MLVs to dCLNs, enabling peripheral T cells activation against CNS-resident pathogens (Figure 5) (153). VEGF-C treatment induces meningeal lymphangiogenesis and improves CSF outflow in chronically Toxoplasma gondii infected mice, although fails to alleviate infection-associated cerebral edema (154), highlighting the uncoupling of lymphatic flow and symptom improvement.
Other CNS diseases
In addition, several other CNS diseases are also associated with MLVs. In migraine pathophysiology, elevated meningeal levels of pro-inflammatory cytokine IL-12-p70 and CGRP correlate with lymphatic impairment (155). Neuroimaging studies have revealed meningeal lymphatic flow abnormalities in chronic migraine patients (156). Mechanistically, CGRP signaling induces VE-Cadherin rearrangement in MLVs (Figure 5), decreasing endothelial permeability and impairing drainage capacity— a pathway corroborated by migraine symptom alleviation following CGRP receptor blockade (76).
Therapeutic modulation of meningeal lymphatic function demonstrates translational potential in structural brain disorders. In craniosynostosis models, pharmacological activation of mechanosensitive Piezo1 via Yoda1 restores CSF drainage and normalizes the ICP (Figure 5) (66). Transplantation of VEGF-C-secreting skull progenitor cells induces meningeal lymphangiogenesis, effectively ameliorating craniosynostosis-associated neurological deficits (59).
Furthermore, in rats subjected to bile duct ligation, elevating VEGF-C in CSF enhances meningeal lymphatic clearance of neurotoxic metabolites and mitigates neuroinflammation and cognitive impairment in hepatic encephalopathy models (Figure 5) (157), implicating that MLVs take part in mediating the liver-brain axis interactions.
Imaging advances in MLVs and drainage path
Numerous innovative imaging methods have been employed in MLV research. Here, we make a summary of these methods.
Optical imaging
In addition to immunofluorescence (IF) of lymphatic markers, including LYVE-1, Prox-1, VEGFR-3 and podoplanin, some researchers label MLVs by injecting fluorescein into the cisterna magna or transgenic method (such as QD705 or Prox1-GFP mice), then expose the skull to the fluorescence stereo zoom microscope or multiphoton/two-photon microscope and obtain 3D or living images of MLVs (4, 18, 158). Moreover, another optical imaging method, optical coherence tomography (OCT), has been applied for visualizing MLVs in vivo (158). However, owing to the small size of MLVs, they believe that OCT can only image enlarged MLVs after BBB opening (158).
The dCLNs are the end points of intracranial lymphatic drainage. After injecting CSF fluorescent tracer, researchers observe the fluorescent signals of dCLNs and the pathway via histological sections or fluorescence stereo zoom microscopes (10, 18, 30, 55, 67), with which they quantify the drainage function and visualize the meningeal lymphatic flow. Pioneering work by Qiaoli Ma et al. established differential kinetics— near infrared (NIR) tracers appear in mouse dCLNs about ten minutes earlier than in saphenous veins, conclusively validating the existence of meningeal lymphatic pathway (30). Optical imaging has the advantage of high resolution in imaging vitro tissues. However, when imaging MLVs deep in the tissues in vivo, the penetrability of the optical signal limits its resolution.
MRI
To achieve higher penetration depth beyond the limitations of optical imaging, researchers have turned their attention to MRI. Owing to its superior imaging depth and noninvasive nature, MRI is widely applied in exploring the structure and function of MLVs, although its resolution is much lower than that of fluorescence microscopy imaging. Martina Absinta et al. intravenously injected gadobutrol, a gadolinium-based contrast agent with a high propensity to seep out of capillaries and into lymph vessels to enhance MLVs, and then collected T2-fluid attenuated inversion recovery (FLAIR) and T1-weighted black-blood images to visualize the meningeal lymphatic system of human and nonhuman primate (9). Since then, MRI has been extensively used in many other studies of MLVs. Although there are a variety of MRI sequences can be collected, many researchers choose the T2-FLAIR for its high sensitivity to gadolinium-based contrast agents (18, 44, 47, 117, 159, 160). For instance, Xue-Bing Ding et al. used this to compare the meningeal lymphatic drainage function between the normal controls and patients with PD (117). Noteworthily, Mehmet Sait Albayram et al. used 3D-T2-FLAIR to observe MLVs through the internal signals of protein-rich lymphatic fluid rather than contrast media (47). Furthermore, real-time VW-MRI has been applied in exploring the structure of MLVs (46). In addition, time-of-flight (TOF) angiography can distinguish the direction of flow in vessels without exogenous contrast agent, by which Phillip H. Kuo et al. explored the drainage direction of MLVs near the SSS (29), while Jun-Hee Kim et al. quantified the velocity of these MLVs via ALADDIN (43).
For its excellent temporal resolution, MRI has emerged as a powerful tool for real-time tracking of CSF dynamics, enabling researchers to map extracranial drainage pathways in vivo. Ji Hoon Ahn et al. collected T2-weighted 2D FLAIR and T1-weighted 3D fast low angle shot (FLASH) sequences with intrathecal gadolinium and delineated the pathway from basal MLVs to dCLNs (18). Mehmet Sait Albayram et al. used 3D-T2-FLAIR method without contrast media to capture the human CSF drainage with 0.9 mm resolution (47). With minimal invasiveness and high imaging depth, MRI is suitable for exploring human meningeal lymphatic systems and holds promise for detecting relevant pathological changes. However, visualizing submillimeter lymphatic structures without contrast agents remains technically challenging (47).
Photoacoustic imaging
To conduct high-resolution imaging of objects deep within tissues in vivo, researchers have developed new imaging techniques, such as photoacoustic imaging, which combines the advantages of optical resolution and acoustic penetration depth. Fei Yang et al. used the dual-contrast functional photoacoustic microscope (DCF-PAM) to image mouse MLVs and cerebral vessels (CVs) (161). Leveraging the properties of draining large molecules in CSF, they labelled MLVs with ICG and distinguished the CVs by hemoglobin, which have different photoacoustic characteristics. With this method, they obtained overall high-resolution 3D images of MLVs, as well as real-time monitored and quantified the CSF drainage in vivo (161). However, photoacoustic imaging also cannot be operated without the tracers, and the tracers need to have specific photoacoustic properties. Furthermore, when photoacoustic imaging is applied on human, the safety of the tracer also needs to be taken into consideration.
Synthesis and future perspectives
Since being rediscovered in 2015, MLVs have emerged as pivotal regulators of CNS homeostasis through two mechanistic axes: waste clearance and antigen presentation, protecting brain in terms of metabolism and immunity. Here we make a summary about previous related research on MLVs, at the same time, we enumerate some open questions in the way of explaining MLVs, which may be the next research hotspot.
The most compelling one is how MLVs drain CSF. Are the substances in the CSF actively absorbed by MLVs or freely diffused? Is there process selective in MLVs drain? What factors can restrict substances from entering MLVs? Besides, in peripheral CpLVs, anchoring filaments modulate the button junctions between LECs to control the inflow of ISF (24). However, whether anchoring filaments exist in MLVs has not been reported so far. Furthermore, several hydrodynamic parameters, such as flow, direction and velocity, have been fully discussed in peripheral lymphatics (25). However, these parameters and their mechanisms have not been fully elucidated in MLVs.
Moreover, the current research on the regulation of MLVs is still insufficient. Aside from known factors such as age and VEGF-C, do other factors, such as gender or certain hormones affect MLVs? After all, estrogen has been reported to promote the growth of peripheral lymphatic vessels by estrogen receptor-α expressed in LECs (162, 163). Besides, how to apply the treatments for MLVs in clinical practice is worth exploring. Although its feasibility and efficacy have not yet been fully demonstrated, dcLVA is a significant step in applying the theory of meningeal lymphatic drainage to clinical diseases (98, 99). In addition, the function of the drainage ISF of lymphatic vessels contribute to absorbing drugs. Coupled with the bidirectionality of meningeal lymphatic flow and the absence of BBB blocking effect, CNS administration by MLVs may be a research focus (34).
As a bridge of CNS and periphery, MLV plays a significant role in maintaining the brain homeostasis by modulating substance exchange and immune response. However, the mechanisms governing meningeal lymphatic drainage and how to apply these mechanisms on clinical patients still require further investigation. Systematic resolution of these issues will unlock the therapeutic potential of MLVs for many CNS disorders. In sum, with respect to MLVs, we are still in the way.
Author contributions
MZ: Investigation, Writing – original draft, Data curation, Software, Visualization, Writing – review & editing, Methodology, Conceptualization, Formal analysis. CY: Formal analysis, Methodology, Writing – review & editing, Investigation, Supervision. Y-CL: Supervision, Investigation, Writing – review & editing, Formal analysis. XW: Formal analysis, Writing – review & editing, Methodology, Supervision, Investigation. JF: Supervision, Writing – review & editing, Methodology. XL: Writing – review & editing, Supervision, Methodology, Investigation. RZ: Writing – review & editing, Supervision, Investigation, Methodology, Formal analysis. Y-ZL: Writing – review & editing, Supervision, Methodology. QT: Writing – review & editing, Supervision, Investigation, Funding acquisition, Formal analysis.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by a grant from the Natural Science Foundation of China (NSFC, 82071478).
Acknowledgments
The figures are drawn by Figdraw, bioicons and PowerPoint.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
MLVs: Meningeal lymphatic vessels
CSF: Cerebrospinal fluid
dCLNs: Deep cervical lymph nodes
CNS: Central nervous system
AD: Alzheimer’s disease
PD: Parkinson’s disease
TBI: Traumatic brain injury
MS: Multiple sclerosis
BBB: Blood-brain barrier
NVUs: Neurovascular units
LYVE-1: Lymphatic endothelial hyaluronic acid receptor 1
LEC: Lymphatic endothelial cell
Prox-1: Prospero homeobox protein 1
SSS: Superior sagittal sinus
TS: Transverse sinus
PSS: Petrosquamosal sinus
SS: Sigmoid sinus
MMA: Middle meningeal artery
PPA: Pterygopalatine artery
COS: Confluence of the sinuses
CpLVs: Capillary lymphatic vessels
ClLVs: Collecting lymphatic vessels
ISF: Interstitial fluid
VE-Cadherin: Vascular endothelial-cadherin
SMF: Stylomastoid foramina
JF: Jugular foramen
PSF: Petrosquamous fissure
dCLVs: Deep cervical lymph vessels
NPLP: Nasopharyngeal lymphatic plexus
MRI: Magnetic resonance imaging
α-PLNPs: α-phospholipid nanoprobe
Aβ: Amyloid β
AQP-4: Aquaporin-4
ALADDIN: Alternate ascending/descending directional navigation
VW-MRI: Vessel-wall MRI
VEGF-C/D: Vascular endothelial growth factor-C/D
VEGFR-3: Vascular endothelial growth factor receptor-3
AAV: Adeno-associated virus
eNOS: Endothelial nitric oxide synthase
Cacna1D: calcium voltage-gated channel subunit α1 D
AKT1: Protein kinase B1
CGRP: Calcitonin gene related peptide
CLR: Calcitonin receptor-like receptor
RAMP1: Receptor activity modifying protein 1
PTX3: Pentraxin3
MADCAM1: Mucosal vascular addressin cell adhesion molecule 1
IFN-γ: Interferon-γ
DSCR1: Down syndrome critical region 1
RCAN1: Regulator of calcineurin 1
rTMS: Repetitive transcranial magnetic stimulation
PSMA3: Proteasome subunit alpha type-3
DEFA1A3: Defensin alpha 1 and alpha 3
NO: Nitric oxide
cGMP: Cyclic guanosine monophosphate
dcLVA: Deep cervical lymphatic-venous anastomosis
LNVA: Lymph node-venous anastomosis
CBM: Cranial bone maneuver
IL-33: Interleukin 33
APOE4: Apolipoprotein E4
iPSCs: Induced pluripotent stem cells
EAE: Experimental autoimmune encephalomyelitis
DCs: Dendritic cells
IDH: Isocitrate dehydrogenase
ICP: Intracranial pressure
FOXC2: Forkhead box C2
SAH: Subarachnoid hemorrhage
ICH: Intracerebral hemorrhage
SDH: Subdural hematoma
THBS1: Thrombospondin 1
S100A8: S100 calcium-binding protein A8
STAT3: Signal transducer and activator of transcription 3
Bcl-2: B-cell lymphoma 2
PNS: Panax Notoginseng Saponins
CBT: Cranial bone transport
MCAO: Middle cerebral artery occlusion
ERK1/2: Extracellular signal-regulated kinase1/2
LM: Listeria monocytogenes
GATA2: GATA binding protein 2
SAE: Sepsis-associated encephalopathy
JEA: Japanese encephalitis virus
RABV: Rabies virus
HSV-1: Herpes simplex virus 1
HIV: Human Immunodeficiency Virus
OCT: Optical coherence tomography
NIR: Near infrared
FLAIR: Fluid attenuated inversion recovery
TOF: Time-of-flight
DCF-PAM: Dual-contrast functional photoacoustic microscope
CVs: Cerebral vessels
ICG: Indocyanine green
References
1. Segarra M, Aburto MR, and Acker-Palmer A. Blood-brain barrier dynamics to maintain brain homeostasis. Trends Neurosci. (2021) 44:393–405. doi: 10.1016/j.tins.2020.12.002
2. Segarra M, Aburto MR, Hefendehl J, and Acker-Palmer A. Neurovascular interactions in the nervous system. Annu Rev Cell Dev Biol. (2019) 35:615–35. doi: 10.1146/annurev-cellbio-100818-125142
3. Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. (2015) 523:337–41. doi: 10.1038/nature14432
4. Louveau A, Herz J, Alme MN, Salvador AF, Dong MQ, Viar KE, et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat Neurosci. (2018) 21:1380–91. doi: 10.1038/s41593-018-0227-9
5. Lukić IK, Glunčić V, Ivkić G, Hubenstorf M, and Marušić A. Virtual dissection: a lesson from the 18th century. Lancet. (2003) 362:2110–3. doi: 10.1016/S0140-6736(03)15114-8
6. Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. (2015) 212:991–9. doi: 10.1084/jem.20142290
7. Bower NI, Koltowska K, Pichol-Thievend C, Virshup I, Paterson S, Lagendijk AK, et al. Mural lymphatic endothelial cells regulate meningeal angiogenesis in the zebrafish. Nat Neurosci. (2017) 20:774. doi: 10.1038/nn.4558
8. Jung E, Gardner D, Choi D, Park E, Seong YJ, Yang S, et al. Development and characterization of a novel Prox1-EGFP lymphatic and schlemm’s canal reporter rat. Sci Rep. (2017) 7:5577. doi: 10.1038/s41598-017-06031-3
9. Absinta M, Ha SK, Nair G, Sati P, Luciano NJ, Palisoc M, et al. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife. (2017) 6:e29738. doi: 10.7554/eLife.29738
10. Da MS, Louveau A, Vaccari A, Smirnov I, Cornelison RC, Kingsmore KM, et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature. (2018) 560:185–91. doi: 10.1038/s41586-018-0368-8
11. Rustenhoven J, Drieu A, Mamuladze T, de Lima KA, Dykstra T, Wall M, et al. Functional characterization of the dural sinuses as a neuroimmune interface. Cell. (2021) 184:1000–16. doi: 10.1016/j.cell.2020.12.040
12. Kim K, Abramishvili D, Du S, Papadopoulos Z, Cao J, Herz J, et al. Meningeal lymphatics-microglia axis regulates synaptic physiology. Cell. (2025) 188:2705–19. doi: 10.1016/j.cell.2025.02.022
13. Goldman DH, Dykstra T, Smirnov I, Blackburn SM, Da Mesquita S, Kipnis J, et al. Age-associated suppression of exploratory activity during sickness is linked to meningeal lymphatic dysfunction and microglia activation. Nat Aging. (2022) 2:704. doi: 10.1038/s43587-022-00268-y
14. Louveau A, Da Mesquita S, and Kipnis J. Lymphatics in neurological disorders: a neuro-lympho-vascular component of multiple sclerosis and Alzheimer’s disease? Neuron. (2016) 91:957–73. doi: 10.1016/j.neuron.2016.08.027
15. Hong YK, Harvey N, Noh YH, Schacht V, Hirakawa S, Detmar M, et al. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn. (2002) 225:351–7. doi: 10.1002/dvdy.10163
16. Oliver G and Srinivasan RS. Endothelial cell plasticity: how to become and remain a lymphatic endothelial cell. Development. (2010) 137:363–72. doi: 10.1242/dev.035360
17. Alitalo K, Tammela T, and Petrova TV. Lymphangiogenesis in development and human disease. Nature. (2005) 438:946–53. doi: 10.1038/nature04480
18. Ahn JH, Cho H, Kim J, Kim SH, Ham J, Park I, et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature. (2019) 572:62–6. doi: 10.1038/s41586-019-1419-5
19. Yin XY, Zhang S, Lee JH, Dong HP, Mourgkos G, Terwilliger G, et al. Compartmentalized ocular lymphatic system mediates eye-brain immunity. Nature. (2024) 628:204. doi: 10.1038/s41586-024-07130-8
20. Jacob L, Boisserand L, Geraldo L, Neto JD, Mathivet T, Antila S, et al. Anatomy and function of the vertebral column lymphatic network in mice. Nat Commun. (2019) 10:4594. doi: 10.1038/s41467-019-12568-w
21. Antila S, Karaman S, Nurmi H, Airavaara M, Voutilainen MH, Mathivet T, et al. Development and plasticity of meningeal lymphatic vessels. J Exp Med. (2017) 214:3645–67. doi: 10.1084/jem.20170391
22. Izen RM, Yamazaki T, Nishinaka-Arai Y, Hong YK, and Mukouyama YS. Postnatal development of lymphatic vasculature in the brain meninges. Dev Dyn. (2018) 247:741–53. doi: 10.1002/dvdy.24624
23. Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. (2007) 204:2349–62. doi: 10.1084/jem.20062596
24. Leak LV and Burke JF. Ultrastructural studies on the lymphatic anchoring filaments. J Cell Biol. (1968) 36:129–49. doi: 10.1083/jcb.36.1.129
25. Ikomi F, Kawai Y, and Ohhashi T. Recent advance in lymph dynamic analysis in lymphatics and lymph nodes. Ann Vasc Dis. (2012) 5:258–68. doi: 10.3400/avd.ra.12.00046
26. von der Weid PY and Zawieja DC. Lymphatic smooth muscle: the motor unit of lymph drainage. Int J Biochem Cell Biol. (2004) 36:1147–53. doi: 10.1016/j.biocel.2003.12.008
27. Davis MJ and Zawieja SD. Pacemaking in the lymphatic system. J Physiol. (2024). doi: 10.1113/JP284752
28. Bazigou E, Xie S, Chen C, Weston A, Miura N, Sorokin L, et al. Integrin-α9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev Cell. (2009) 17:175–86. doi: 10.1016/j.devcel.2009.06.017
29. Kuo PH, Stuehm C, Squire S, and Johnson K. Meningeal lymphatic vessel flow runs countercurrent to venous flow in the superior sagittal sinus of the human brain. Tomography. (2018) 4:99–104. doi: 10.18383/j.tom.2018.00013
30. Ma Q, Ineichen BV, Detmar M, and Proulx ST. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat Commun. (2017) 8:1413–34. doi: 10.1038/s41467-017-01484-6
31. Yoon JH, Jin H, Kim HJ, Hong SP, Yang MJ, Ahn JH, et al. Nasopharyngeal lymphatic plexus is a hub for cerebrospinal fluid drainage. Nature. (2024) 625:768–77. doi: 10.1038/s41586-023-06899-4
32. Smyth L, Xu D, Okar SV, Dykstra T, Rustenhoven J, Papadopoulos Z, et al. Identification of direct connections between the dura and the brain. Nature. (2024) 627:165. doi: 10.1038/s41586-023-06993-7
34. Ramos-Zaldivar HM, Polakovicova I, Salas-Huenuleo E, Yefi CP, Silva-Ancahuail D, Jara-Guajardo P, et al. The cervical and meningeal lymphatic network as a pathway for retrograde nanoparticle transport to the brain. Int J Nanomedicine. (2024) 19:10725–43. doi: 10.2147/IJN.S477159
35. Liang YJ, Iqbal Z, Lu JP, Wang JH, Zhang H, Chen X, et al. Cell-derived nanovesicle-mediated drug delivery to the brain: principles and strategies for vesicle engineering. Mol Ther. (2023) 31:1207–24. doi: 10.1016/j.ymthe.2022.10.008
36. Dai YF, Yu X, Zhao YF, Wei JS, Lin D, Wang JL, et al. Targeted modulation of the meningeal lymphatic reverse pathway for immunotherapy of breast cancer brain metastases. ACS Nano. (2025) 19:4830–44. doi: 10.1021/acsnano.4c15860
37. Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. (2012) 4:111r–47r. doi: 10.1126/scitranslmed.3003748
38. Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A, Plog BA, Liao Y, et al. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci. (2013) 33:18190–9. doi: 10.1523/JNEUROSCI.1592-13.2013
39. Ishida K, Yamada K, Nishiyama R, Hashimoto T, Nishida I, Abe Y, et al. Glymphatic system clears extracellular tau and protects from tau aggregation and neurodegeneration. J Exp Med. (2022) 219:e20211275. doi: 10.1084/jem.20211275
40. Harrison IF, Ismail O, Machhada A, Colgan N, Ohene Y, Nahavandi P, et al. Impaired glymphatic function and clearance of tau in an Alzheimer’s disease model. Brain. (2020) 143:2576–93. doi: 10.1093/brain/awaa179
41. Murdock MH, Yang C, Sun N, Pao P, Blanco-Duque C, Kahn MC, et al. Multisensory gamma stimulation promotes glymphatic clearance of amyloid. Nature. (2024) 627:149–56. doi: 10.1038/s41586-024-07132-6
42. Wu CH, Liao WH, Chu YC, Hsiao MY, Kung Y, Wang JL, et al. Very low-intensity ultrasound facilitates glymphatic influx and clearance via modulation of the trpv4-aqp4 pathway. Adv Sci (Weinh). (2024) 11:e2401039. doi: 10.1002/advs.202401039
43. Kim JH, Yoo RE, Choi SH, and Park SH. Non-invasive flow mapping of parasagittal meningeal lymphatics using 2D interslice flow saturation MRI. Fluids Barriers CNS. (2023) 20:37. doi: 10.1186/s12987-023-00446-z
44. Ringstad G and Eide PK. Cerebrospinal fluid tracer efflux to parasagittal dura in humans. Nat Commun. (2020) 11:354. doi: 10.1038/s41467-019-14195-x
45. Zhou Y, Cai J, Zhang W, Gong X, Yan S, Zhang K, et al. Impairment of the glymphatic pathway and putative meningeal lymphatic vessels in the aging human. Ann Neurol. (2020) 87:357–69. doi: 10.1002/ana.25670
46. Jacob L, de Brito Neto J, Lenck S, Corcy C, Benbelkacem F, Geraldo LH, et al. Conserved meningeal lymphatic drainage circuits in mice and humans. J Exp Med. (2022) 219:e20220035. doi: 10.1084/jem.20220035
47. Albayram MS, Smith G, Tufan F, Tuna IS, Bostanciklioglu M, Zile M, et al. Non-invasive mr imaging of human brain lymphatic networks with connections to cervical lymph nodes. Nat Commun. (2022) 13:203. doi: 10.1038/s41467-021-27887-0
48. Aspelund A, Tammela T, Antila S, Nurmi H, Leppänen V, Zarkada G, et al. The Schlemm’s canal is a vegf-c/vegfr-3-responsive lymphatic-like vessel. J Clin Invest. (2014) 124:3975–86. doi: 10.1172/jci75395
49. Secker GA and Harvey NL. Vegfr signaling during lymphatic vascular development: From progenitor cells to functional vessels. Dev Dyn. (2015) 244:323–31. doi: 10.1002/dvdy.24227
50. Kukk E, Lymboussaki A, Taira S, Kaipainen A, Jeltsch M, Joukov V, et al. Vegf-c receptor binding and pattern of expression with vegfr-3 suggests a role in lymphatic vascular development. Development. (1996) 122:3829–37. doi: 10.1242/dev.122.12.3829
51. Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, et al. Vascular endothelial growth factor c is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. (2004) 5:74–80. doi: 10.1038/ni1013
52. Patel TK, Habimana-Griffin L, Gao X, Xu B, AChilefu S, Alitalo K, et al. Dural lymphatics regulate clearance of extracellular tau from the CNS. Mol Neurodegener. (2019) 14:11. doi: 10.1186/s13024-019-0312-x
53. Das NS, Delivanoglou N, Ren Y, Cucuzza CS, Makuch M, Almeida F, et al. Meningeal lymphatic function promotes oligodendrocyte survival and brain myelination. Immunity. (2024) 57:2328–43. doi: 10.1016/j.immuni.2024.08.004
54. Li Z, Antila S, Nurmi H, Chilov D, Korhonen EA, Fang S, et al. Blockade of vegfr3 signaling leads to functional impairment of dural lymphatic vessels without affecting autoimmune neuroinflammation. Sci Immunol. (2023) 8:q375. doi: 10.1126/sciimmunol.abq0375
55. Li XJ, Qi LL, Yang D, Hao SJ, Zhang F, Zhu XG, et al. Meningeal lymphatic vessels mediate neurotropic viral drainage from the central nervous system. Nat Neurosci. (2022) 25:577. doi: 10.1038/s41593-022-01063-z
56. Wen YR, Yang JH, Wang X, and Yao ZB. Induced dural lymphangiogenesis facilities soluble amyloid-beta clearance from brain in a transgenic mouse model of Alzheimer’s disease. Neural Regener Res. (2018) 13:709–16. doi: 10.4103/1673-5374.230299
57. Song E, Mao T, Dong H, Boisserand L, Antila S, Bosenberg M, et al. Vegf-c-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature. (2020) 577:689–94. doi: 10.1038/s41586-019-1912-x
58. Boisserand L, Geraldo LH, Bouchart J, El Kamouh MR, Lee S, Sanganahalli BG, et al. Vegf-c prophylaxis favors lymphatic drainage and modulates neuroinflammation in a stroke model. J Exp Med. (2024) 221:e20221983. doi: 10.1084/jem.20221983
59. Ma L, Chang Q, Pei F, Liu M, Zhang W, Hong YK, et al. Skull progenitor cell-driven meningeal lymphatic restoration improves neurocognitive functions in craniosynostosis. Cell Stem Cell. (2023) 30:1472–85. doi: 10.1016/j.stem.2023.09.012
60. Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. (2010) 330:55–60. doi: 10.1126/science.1193270
61. Li J, Hou B, Tumova S, Muraki K, Bruns A, Ludlow MJ, et al. Piezol integration of vascular architecture with physiological force. Nature. (2014) 515:279–308. doi: 10.1038/nature13701
62. Lukacs V, Mathur J, Mao R, Bayrak-Toydemir P, Procter M, Cahalan SM, et al. Impaired Piezo1 function in patients with a novel autosomal recessive congenital lymphatic dysplasia. Nat Commun. (2015) 6:8329. doi: 10.1038/ncomms9329
63. Choi D, Park E, Jung E, Cha B, Lee S, Yu J, et al. Piezo1 incorporates mechanical force signals into the genetic program that governs lymphatic valve development and maintenance. JCI Insight. (2019) 4:e125068. doi: 10.1172/jci.insight.125068
64. Choi D, Park E, Yu RP, Cooper MN, Cho IT, Choi J, et al. Piezo1-regulated mechanotransduction controls flow-activated lymphatic expansion. Circ Res. (2022) 131:e2–e21. doi: 10.1161/CIRCRESAHA.121.320565
65. Syeda R, Xu J, Dubin AE, Coste B, Mathur J, Huynh T, et al. Chemical activation of the mechanotransduction channel Piezo1. Elife. (2015) 4:e07369. doi: 10.7554/eLife.07369
66. Matrongolo MJ, Ang PS, Wu J, Jain A, Thackray JK, Reddy A, et al. Piezo1 agonist restores meningeal lymphatic vessels, drainage, and brain-CSF perfusion in craniosynostosis and aged mice. J Clin Invest. (2023) 134:e171468. doi: 10.1172/JCI171468
67. Choi D, Park E, Choi J, Lu R, Yu JS, Kim C, et al. Piezo1 regulates meningeal lymphatic vessel drainage and alleviates excessive CSF accumulation. Nat Neurosci. (2024) 27:913–26. doi: 10.1038/s41593-024-01604-8
68. Brain SD, Williams TJ, Tippins JR, Morris HR, and MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. (1985) 313:54–6. doi: 10.1038/313054a0
69. Fisher LA, Kikkawa DO, Rivier JE, Amara SG, Evans RM, Rosenfeld MG, et al. Stimulation of noradrenergic sympathetic outflow by calcitonin gene-related peptide. Nature. (1983) 305:534–6. doi: 10.1038/305534a0
70. Petrova TV, Mäkinen T, Mäkelä TP, Saarela J, Virtanen I, Ferrell RE, et al. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. (2002) 21:4593–9. doi: 10.1093/emboj/cdf470
71. Hirakawa S, Hong YK, Harvey N, Schacht V, Matsuda K, Libermann T, et al. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am J Pathol. (2003) 162:575–86. doi: 10.1016/S0002-9440(10)63851-5
72. Mackie DI, Al Mutairi F, Davis RB, Kechele DO, Nielsen NR, Snyder JC, et al. Hcalcrl mutation causes autosomal recessive nonimmune hydrops fetalis with lymphatic dysplasia. J Exp Med. (2018) 215:2339–53. doi: 10.1084/jem.20180528
73. Mishima T, Ito Y, Nishizawa N, Amano H, Tsujikawa K, Miyaji K, et al. Ramp1 signaling improves lymphedema and promotes lymphangiogenesis in mice. J Surg Res. (2017) 219:50–60. doi: 10.1016/j.jss.2017.05.124
74. Xu WJ, Wittchen ES, Hoopes SL, Stefanini L, Burridge K, and Caron KM. Small GTPase Rap1A/B is required for lymphatic development and adrenomedullin-induced stabilization of lymphatic endothelial junctions. Arterioscler Thromb Vasc Biol. (2018) 38:2410–22. doi: 10.1161/ATVBAHA.118.311645
75. Harris NR, Nielsen NR, Pawlak JB, Aghajanian A, Rangarajan K, Serafin DS, et al. VE-Cadherin is required for cardiac lymphatic maintenance and signaling. Circ Res. (2022) 130:5–23. doi: 10.1161/CIRCRESAHA.121.318852
76. Nelson-Maney NP, Bálint L, Beeson A, Serafin DS, Kistner BM, Douglas ES, et al. Meningeal lymphatic CGRP signaling governs pain via cerebrospinal fluid efflux and neuroinflammation in migraine models. J Clin Invest. (2024) 134:e175616. doi: 10.1172/JCI175616
77. Zaidi MR and Merlino G. The two faces of interferon-γ in cancer. Clin Cancer Res. (2011) 17:6118–24. doi: 10.1158/1078-0432.CCR-11-0482
78. Rustenhoven J, Pavlou G, Storck SE, Dykstra T, Du S, Wan Z, et al. Age-related alterations in meningeal immunity drive impaired CNS lymphatic drainage. J Exp Med. (2023) 220:e20221929. doi: 10.1084/jem.20221929
79. Fuentes JJ, Genescà L, Kingsbury TJ, Cunningham KW, Pérez-Riba M, Estivill X, et al. DSCR1, overexpressed in Down syndrome, is an inhibitor of calcineurin-mediated signaling pathways. Hum Mol Genet. (2000) 9:1681–90. doi: 10.1093/hmg/9.11.1681
80. Martin KR, Corlett A, Dubach D, Mustafa T, Coleman HA, Parkington HC, et al. Over-expression of rcan1 causes Down syndrome-like hippocampal deficits that alter learning and memory. Hum Mol Genet. (2012) 21:3025–41. doi: 10.1093/hmg/dds134
81. Dieterich LC, Ducoli L, Shin JW, and Detmar M. Distinct transcriptional responses of lymphatic endothelial cells to vegfr-3 and vegfr-2 stimulation. Sci Data. (2017) 4:170106. doi: 10.1038/sdata.2017.106
82. Choi C, Park J, Kim H, Chang KT, Park J, and Min KT. DSCR1 upregulation enhances dural meningeal lymphatic drainage to attenuate amyloid pathology of Alzheimer’s disease. J Pathol. (2021) 255:296–310. doi: 10.1002/path.5767
83. Blanco-Duque C, Chan D, Kahn MC, Murdock MH, and Tsai LH. Audiovisual gamma stimulation for the treatment of neurodegeneration. J Intern Med. (2024) 295:146–70. doi: 10.1111/joim.13755
84. Iaccarino HF, Singer AC, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature. (2016) 540:230–5. doi: 10.1038/s41586-018-0351-4
85. Tanaka T, Takano Y, Tanaka S, Hironaka N, Kobayashi K, Hanakawa T, et al. Transcranial direct-current stimulation increases extracellular dopamine levels in the rat striatum. Front Syst Neurosci. (2013) 7:6. doi: 10.3389/fnsys.2013.00006
86. Lin YY, Jin J, Lv RK, Luo Y, Dai WP, Li WC, et al. Repetitive transcranial magnetic stimulation increases the brain’s drainage efficiency in a mouse model of Alzheimer’s disease. Acta Neuropathol Commun. (2021) 9:102. doi: 10.1186/s40478-021-01198-3
87. Wang M, Yan C, Li X, Yang T, Wu S, Liu Q, et al. Non-invasive modulation of meningeal lymphatics ameliorates ageing and Alzheimer’s disease-associated pathology and cognition in mice. Nat Commun. (2024) 15:1453. doi: 10.1038/s41467-024-45656-7
88. Li DY, Lin H, Sun SL, Liu SJ, Liu Z, and He YN. Photostimulation of lymphatic clearance of beta-amyloid from mouse brain: a new strategy for the therapy of Alzheimer’s disease. Front Optoelectron. (2023) 16:45. doi: 10.1007/s12200-023-00099-8
89. Wu W, Zhao YB, Cheng X, Xie XR, Zeng YX, Tao Q, et al. Modulation of glymphatic system by visual circuit activation alleviates memory impairment and apathy in a mouse model of Alzheimer’s disease. Nat Commun. (2025) 16:63. doi: 10.1038/s41467-024-55678-w
90. von Holstein-Rathlou S, Petersen NC, and Nedergaard M. Voluntary running enhances glymphatic influx in awake behaving, young mice. Neurosci Lett. (2018) 662:253–8. doi: 10.1016/j.neulet.2017.10.035
91. Yoo RE, Kim JH, Moon HY, Park JY, Cheon S, Shin HS, et al. Long-term physical exercise facilitates putative glymphatic and meningeal lymphatic vessel flow in humans. Nat Commun. (2025) 16:3360. doi: 10.1038/s41467-025-58726-1
92. Tammela T, Saaristo A, Holopainen T, Ylä-Herttuala S, Andersson LC, Virolainen S, et al. Photodynamic ablation of lymphatic vessels and intralymphatic cancer cells prevents metastasis. Sci Transl Med. (2011) 3:69ra11. doi: 10.1126/scitranslmed.3001699
93. Schultz K, Schultz K, and Schultz G. Sodium nitroprusside and other smooth muscle-relaxants increase cyclic gmp levels in rat ductus deferens. Nature. (1977) 265:750–1. doi: 10.1038/265750a0
94. Du T, Raghunandan A, Mestre H, Plá V, Liu GJ, Ladrón-de-Guevara A, et al. Restoration of cervical lymphatic vessel function in aging rescues cerebrospinal fluid drainage. Nat Aging. (2024) 4:1418–31. doi: 10.1038/s43587-024-00691-3
95. Zou WY, Pu TL, Feng WX, Lu M, Zheng Y, Du RH, et al. Blocking meningeal lymphatic drainage aggravates Parkinson’s disease-like pathology in mice overexpressing mutated α-synuclein. Transl Neurodegener. (2019) 8:7. doi: 10.1186/s40035-019-0147-y
96. Wang LM, Zhang YL, Zhao Y, Marshall C, Wu T, and Xiao M. Deep cervical lymph node ligation aggravates ad-like pathology of app/ps1 mice. Brain Pathol. (2019) 29:176–92. doi: 10.1111/bpa.12656
97. Topalis A, Dionyssiou D, Bekiari C, and Demiri E. Lymphatic brain decongestion in Alzheimer’s disease: experimental pilot study. J Plast Reconstr Aesthet Surg. (2024) 99:21. doi: 10.1016/j.bjps.2024.08.039
98. Lu H, Tan Y, and Xie Q. Preliminary observation of deep cervical lymphatic-venous anastomosis under off-eyepiece 3d microscope to treat an elderly patient with cognitive impairment. Chin J Microsurg. (2022) 45:570–4. doi: 10.3760/cma.j.cn441206-20210809-00194
99. Xie QP, Wang YL, Pan WR, Yang XD, Guo H, Xiao M, et al. Expert consensus on lymphatic surgical treatment for Alzheimer’s disease (2025 edition). Chin J Clin Anat. (2025) 43:121–7. doi: 10.13418/j.issn.1001-165x.2025.2.01
100. Hayat M, Syed RA, Qaiser H, Uzair M, Al-Regaiey K, Khallaf R, et al. Decoding molecular mechanisms: brain aging and Alzheimer’s disease. Neural Regener Res. (2025) 20:2279–99. doi: 10.4103/NRR.NRR-D-23-01403
101. Da Mesquita S, Herz J, Wall M, Dykstra T, de Lima KA, Norris GT, et al. Aging-associated deficit in ccr7 is linked to worsened glymphatic function, cognition, neuroinflammation, and β-amyloid pathology. Sci Adv. (2021) 7:eabe4601. doi: 10.1126/sciadv.abe4601
102. Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, et al. Alzheimer’s disease. Lancet. (2021) 397:1577–90. doi: 10.1016/S0140-6736(20)32205-4
103. Joachim CL, Duffy LK, Morris JH, and Selkoe DJ. Protein chemical and immunocytochemical studies of meningovascular beta-amyloid protein in Alzheimer’s disease and normal aging. Brain Res. (1988) 474:100–11. doi: 10.1016/0006-8993(88)90673-7
104. Da Mesquita S, Papadopoulos Z, Dykstra T, Brase L, Farias FG, Wall M, et al. Meningeal lymphatics affect microglia responses and anti-Aβ immunotherapy. Nature. (2021) 593:255–60. doi: 10.1038/s41586-021-03489-0
105. Lu X, Bai S, Feng L, Yan X, Lin Y, Huang J, et al. Cranial bone maneuver ameliorates alzheimer’s disease pathology via enhancing meningeal lymphatic drainage function. Alzheimers Dement. (2025) 21:e14518. doi: 10.1002/alz.14518
106. Wu Y, Zhang T, Li X, Wei Y, Li X, Wang S, et al. Borneol-driven meningeal lymphatic drainage clears amyloid-beta peptide to attenuate Alzheimer-like phenotype in mice. Theranostics. (2023) 13:106–24. doi: 10.7150/thno.76133
107. Liu X, Zhang H, Xiang J, Luo W, Zhang H, Wang P, et al. Jiawei Xionggui Decoction promotes meningeal lymphatic vessels clearance of β-amyloid by inhibiting arachidonic acid pathway. Phytomedicine. (2024) 135:156041. doi: 10.1016/j.phymed.2024.156041
108. Xin SH, Tan L, Cao X, Yu JT, and Tan L. Clearance of amyloid beta and tau in Alzheimer’s disease: from mechanisms to therapy. Neurotox Res. (2018) 34:733–48. doi: 10.1007/s12640-018-9895-1
109. Dong SY, Zhao HW, Nie M, Sha Z, Feng JC, Liu MQ, et al. Cannabidiol alleviates neurological deficits after traumatic brain injury by improving intracranial lymphatic drainage. J Neurotraum. (2024) 41:e2009–25. doi: 10.1089/neu.2023.0539
110. Liu MQ, Huang JH, Liu T, Yuan JY, Lv CX, Sha Z, et al. Exogenous interleukin 33 enhances the brain’s lymphatic drainage and toxic protein clearance in acute traumatic brain injury mice. Acta Neuropathol Commun. (2023) 11:61. doi: 10.1186/s40478-023-01555-4
111. Mentis AA, Dardiotis E, and Chrousos GP. Apolipoprotein E4 and meningeal lymphatics in Alzheimer disease: a conceptual framework. Mol Psychiatry. (2021) 26:1075–97. doi: 10.1038/s41380-020-0731-7
112. Guo XD, Zhang GX, Peng QY, Huang LQ, Zhang ZH, and Zhang ZT. Emerging roles of meningeal lymphatic vessels in Alzheimer’s disease. J Alzheimers Dis. (2023) 94:S355–66. doi: 10.3233/JAD-221016
113. Lim HY, Rutkowski JM, Helft J, Reddy ST, Swartz MA, Randolph GJ, et al. Hypercholesterolemic mice exhibit lymphatic vessel dysfunction and degeneration. Am J Pathol. (2009) 175:1328–37. doi: 10.2353/ajpath.2009.080963
114. Spillantini MG, Schmidt ML, Lee V, Trojanowski JQ, Jakes R, and Goedert M. Alpha-synuclein in lewy bodies. Nature. (1997) 388:839–40. doi: 10.1038/42166
115. Kalia LV and Lang AE. Parkinson’s disease. Lancet. (2015) 386:896–912. doi: 10.1016/S0140-6736(14)61393-3
116. Liu ZR, Huang Y, Wang XJ, Li JY, Zhang C, Yang Y, et al. The cervical lymph node contributes to peripheral inflammation related to Parkinson’s disease. J Neuroinflamm. (2023) 20:93. doi: 10.1186/s12974-023-02770-5
117. Ding XB, Wang XX, Xia DH, Liu H, Tian HY, Fu Y, et al. Impaired meningeal lymphatic drainage in patients with idiopathic Parkinson’s disease. Nat Med. (2021) 27:411–8. doi: 10.1038/s41591-020-01198-1
118. Hemmer B, Kerschensteiner M, and Korn T. Role of the innate and adaptive immune responses in the course of multiple sclerosis. Lancet Neurol. (2015) 14:406–19. doi: 10.1016/S1474-4422(14)70305-9
119. Hsu M, Rayasam A, Kijak JA, Choi YH, Harding JS, Marcus SA, et al. Neuroinflammation-induced lymphangiogenesis near the cribriform plate contributes to drainage of CNS-derived antigens and immune cells. Nat Commun. (2019) 10:229. doi: 10.1038/s41467-018-08163-0
120. Hsu M, Laaker C, Madrid A, Herbath M, Choi YH, Sandor M, et al. Neuroinflammation creates an immune regulatory niche at the meningeal lymphatic vasculature near the cribriform plate. Nat Immunol. (2022) 23:581–93. doi: 10.1038/s41590-022-01158-6
121. Jakimovski D, Bittner S, Zivadinov R, Morrow SA, Benedict RH, Zipp F, et al. Multiple sclerosis. Lancet. (2024) 403:183–202. doi: 10.1016/S0140-6736(23)01473-3
122. Kim MW, Gao W, Lichti CF, Gu X, Dykstra T, Cao J, et al. Endogenous self-peptides guard immune privilege of the central nervous system. Nature. (2025) 637:176–83. doi: 10.1038/s41586-024-08279-y
123. Graham MS and Mellinghoff IK. Meningeal lymphatics prime tumor immunity in glioblastoma. Cancer Cell. (2021) 39:304–6. doi: 10.1016/j.ccell.2021.02.012
124. Ma Q, Schlegel F, Bachmann SB, Schneider H, Decker Y, Rudin M, et al. Lymphatic outflow of cerebrospinal fluid is reduced in glioma. Sci Rep. (2019) 9:14815. doi: 10.1038/s41598-019-51373-9
125. Hu X, Deng Q, Ma L, Li Q, Chen Y, Liao Y, et al. Meningeal lymphatic vessels regulate brain tumor drainage and immunity. Cell Res. (2020) 30:229–43. doi: 10.1038/s41422-020-0287-8
126. Zhou C, Ma L, Xu H, Huo Y, and Luo J. Meningeal lymphatics regulate radiotherapy efficacy through modulating anti-tumor immunity. Cell Res. (2022) 32:543–54. doi: 10.1038/s41422-022-00639-5
127. Wang M, Ran L, Liu B, Wei W, Zhu J, Long F, et al. Disturbed meningeal lymphatic function associated with Malignancy and progression in patients with intracranial Malignant tumors. Med. (2023) 4:898–912. doi: 10.1016/j.medj.2023.10.001
128. Virenque A, Koivisto H, Antila S, Zub E, Rooney EJ, Miszczuk D, et al. Significance of developmental meningeal lymphatic dysfunction in experimental post-traumatic injury. Brain Behav Immun Health. (2022) 23:100466. doi: 10.1016/j.bbih.2022.100466
129. Hussain R, Tithof J, Wang W, Cheetham-West A, Song W, Peng WG, et al. Potentiating glymphatic drainage minimizes post-traumatic cerebral oedema. Nature. (2023) 623:992. doi: 10.1038/s41586-023-06737-7
130. Bolte AC, Dutta AB, Hurt ME, Smirnov I, Kovacs MA, McKee CA, et al. Meningeal lymphatic dysfunction exacerbates traumatic brain injury pathogenesis. Nat Commun. (2020) 11:4524. doi: 10.1038/s41467-020-18113-4
131. Liao JW, Zhang MC, Shi ZC, Lu H, Wang LC, Fan WJ, et al. Improving the function of meningeal lymphatic vessels to promote brain edema absorption after traumatic brain injury. J Neurotraum. (2023) 40:383–94. doi: 10.1089/neu.2022.0150
132. Shimada R, Tatara Y, and Kibayashi K. Gene expression in meningeal lymphatic endothelial cells following traumatic brain injury in mice. PloS One. (2022) 17:e0273892. doi: 10.1371/journal.pone.0273892
133. Wojciechowski S, Virenque A, Vihma M, Galbardi B, Rooney EJ, Keuters MH, et al. Developmental dysfunction of the central nervous system lymphatics modulates the adaptive neuro-immune response in the perilesional cortex in a mouse model of traumatic brain injury. Front Immunol. (2021) 11:559810. doi: 10.3389/fimmu.2020.559810
134. Pu T, Zou W, Feng W, Zhang Y, Wang L, Wang H, et al. Persistent malfunction of glymphatic and meningeal lymphatic drainage in a mouse model of subarachnoid hemorrhage. Exp Neurobiol. (2019) 28:104–18. doi: 10.5607/en.2019.28.1.104
135. Wang JQ, Lv T, Jia F, Li Y, Ma WW, Xiao ZP, et al. Subarachnoid hemorrhage distinctively disrupts the glymphatic and meningeal lymphatic system in beagles. Theranostics. (2024) 14:6053–70. doi: 10.7150/thno.100982
136. Chen J, Wang L, Xu H, Xing L, Zhuang Z, Zheng Y, et al. Meningeal lymphatics clear erythrocytes that arise from subarachnoid hemorrhage. Nat Commun. (2020) 11:3159. doi: 10.1038/s41467-020-16851-z
137. Wang X, Zhang A, Yu Q, Wang Z, Wang J, Xu P, et al. Single-cell rna sequencing and spatial transcriptomics reveal pathogenesis of meningeal lymphatic dysfunction after experimental subarachnoid hemorrhage. Adv Sci (Weinh). (2023) 10:e2301428. doi: 10.1002/advs.202301428
138. Gao D, Zou B, Zhu K, Bi S, Zhang W, Yang X, et al. Enhancing th17 cells drainage through meningeal lymphatic vessels alleviate neuroinflammation after subarachnoid hemorrhage. J Neuroinflamm. (2024) 21:217–69. doi: 10.1186/s12974-024-03252-y
139. Tsai HH, Hsieh YC, Lin JS, Kuo ZT, Ho CY, Chen CH, et al. Functional investigation of meningeal lymphatic system in experimental intracerebral hemorrhage. Stroke. (2022) 53:987–98. doi: 10.1161/STROKEAHA.121.037834
140. Semyachkina-Glushkovskaya O, Navolokin N, Shirokov A, Terskov A, Khorovodov A, Mamedova A, et al. Meningeal lymphatic pathway of brain clearing from the blood after haemorrhagic injuries. Adv Exp Med Biol. (2020) 1232:63–8. doi: 10.1007/978-3-030-34461-0_9
141. Liu YH, Liu XH, Sun PJ, Li J, Nie M, Gong JJ, et al. rTMs treatment for abrogating intracerebral hemorrhage-induced brain parenchymal metabolite clearance dysfunction in male mice by regulating intracranial lymphatic drainage. Brain Behav. (2023) 13:e3062. doi: 10.1002/brb3.3062
142. Li DY, Liu SJ, Yu TT, Liu Z, Sun SL, Bragin D, et al. Photostimulation of brain lymphatics in male newborn and adult rodents for therapy of intraventricular hemorrhage. Nat Commun. (2023) 14:6104. doi: 10.1038/s41467-023-41710-y
143. Yu Z, Yang XY, Cai YQ, Hu E, Li T, Zhu WX, et al. Panax Notoginseng Saponins promotes the meningeal lymphatic system-mediated hematoma absorption in intracerebral hemorrhage. Phytomedicine. (2024) 135:156149. doi: 10.1016/j.phymed.2024.156149
144. Djachkov AN, Khudiaev AT, and Prudnikova OG. Application of external fixation in skull surgery. In: Solomin L, editor. The Basic Principles of External Skeletal Fixation Using the Ilizarov and Other Devices. Springer, Milan (2012). p. 1425–36.
145. Bai S, Lu X, Pan Q, Wang B, Pong UK, Yang Y, et al. Cranial bone transport promotes angiogenesis, neurogenesis, and modulates meningeal lymphatic function in middle cerebral artery occlusion rats. Stroke. (2022) 53:1373–85. doi: 10.1161/STROKEAHA.121.037912
146. Yanev P, Poinsatte K, Hominick D, Khurana N, Zuurbier KR, Berndt M, et al. Impaired meningeal lymphatic vessel development worsens stroke outcome. J Cereb Blood Flow Metab. (2020) 40:263–75. doi: 10.1177/0271678X18822921
147. Liu X, Gao C, Yuan J, Xiang T, Gong Z, Luo H, et al. Subdural haematomas drain into the extracranial lymphatic system through the meningeal lymphatic vessels. Acta Neuropathol Commun. (2020) 8:16. doi: 10.1186/s40478-020-0888-y
148. Yuan JY, Liu XH, Nie M, Chen YP, Liu MQ, Huang JH, et al. Inactivation of ERK1/2 signaling mediates dysfunction of basal meningeal lymphatic vessels in experimental subdural hematoma. Theranostics. (2024) 14:304–23. doi: 10.7150/thno.87633
149. Chen YP, Liu XH, Yuan JY, Dong SY, Nie M, Jiang WW, et al. Vitamin d accelerates the subdural hematoma clearance through improving the meningeal lymphatic vessel function. Mol Cell Biochem. (2024) 479:3129–40. doi: 10.1007/s11010-023-04918-6
150. Feng J, Ren YZ, Wang XL, Li XJ, Zhu XG, Zhang BK, et al. Impaired meningeal lymphatic drainage in Listeria monocytogenes infection. Front Immunol. (2024) 15:1382971. doi: 10.3389/fimmu.2024.1382971
151. Dong H, Dai X, Zhou Y, Shi C, Bhuiyan P, Sun Z, et al. Enhanced meningeal lymphatic drainage ameliorates lipopolysaccharide-induced brain injury in aged mice. J Neuroinflamm. (2024) 21:36. doi: 10.1186/s12974-024-03028-4
152. Lamers SL, Rose R, Ndhlovu LC, Nolan DJ, Salemi M, Maidji E, et al. The meningeal lymphatic system: a route for HIV brain migration? J Neurovirol. (2016) 22:275–81. doi: 10.1007/s13365-015-0399-y
153. Kovacs MA, Cowan MN, Babcock IW, Sibley LA, Still K, Batista SJ, et al. Meningeal lymphatic drainage promotes T cell responses against Toxoplasma gondii but is dispensable for parasite control in the brain. Elife. (2022) 11:e80775. doi: 10.7554/eLife.80775
154. Kovacs MA, Babcock IW, Marco AR, Sibley LA, Kelly AG, and Harris TH. Vascular Endothelial Growth Factor-C treatment enhances cerebrospinal fluid outflow during Toxoplasma gondii brain infection but does not improve cerebral edema. Am J Pathol. (2024) 194:225–37. doi: 10.1016/j.ajpath.2023.11.008
155. Mikhailov N, Virenque A, Koroleva K, Eme-Scolan E, Teleman M, Abdollahzadeh A, et al. The role of the meningeal lymphatic system in local meningeal inflammation and trigeminal nociception. Sci Rep. (2022) 12:8804. doi: 10.1038/s41598-022-12540-7
156. Wu CH, Chang FC, Wang YF, Lirng JF, Wu HM, Pan L, et al. Impaired glymphatic and meningeal lymphatic functions in patients with chronic migraine. Ann Neurol. (2024) 95:583–95. doi: 10.1002/ana.26842
157. Hsu SJ, Zhang CH, Jeong J, Lee SI, McConnell M, Utsumi T, et al. Enhanced meningeal lymphatic drainage ameliorates neuroinflammation and hepatic encephalopathy in cirrhotic rats. Gastroenterology. (2021) 160:1315–29. doi: 10.1053/j.gastro.2020.11.036
158. Semyachkina-Glushkovskaya O, Abdurashitov A, Dubrovsky A, Bragin D, Bragina O, Shushunova N, et al. Application of optical coherence tomography for in vivo monitoring of the meningeal lymphatic vessels during opening of blood-brain barrier: mechanisms of brain clearing. J BioMed Opt. (2017) 22:1–9. doi: 10.1117/1.JBO.22.12.121719
159. Fang Y, Sun Y, Lai T, Song X, Hu T, Zhao Y, et al. Comparative study of 3D-T2WI vs. 3D-T2-FLAIR MRI in displaying human meningeal lymphatics vessels. Clin Radiol. (2024) 81:106700. doi: 10.1016/j.crad.2024.09.006
160. Wu CH, Lirng JF, Ling YH, Wang YF, Wu HM, Fuh JL, et al. Noninvasive characterization of human glymphatics and meningeal lymphatics in an in vivo model of blood-brain barrier leakage. Ann Neurol. (2021) 89:111–24. doi: 10.1002/ana.25928
161. Yang F, Wang Z, Shi W, Wang M, Ma R, Zhang W, et al. Advancing insights into in vivo meningeal lymphatic vessels with stereoscopic wide-field photoacoustic microscopy. Light Sci Appl. (2024) 13:96. doi: 10.1038/s41377-024-01450-0
162. Nakamura R, Sugano M, Sone Y, Sakabe K, Itoh T, and Kiyono M. Establishment and characterization of a human lymphatic endothelial cell line. Biol Pharm Bull. (2014) 37:683–7. doi: 10.1248/bpb.b13-00733
Keywords: meningeal lymphatic vessels, brain homeostasis, cerebrospinal fluid drainage, neuroinflammation, neurological disorders, imaging technique
Citation: Zhao M-Y, Ye C-Y, Liu Y-C, Wang X-M, Fu J-C, Liu X-Y, Zhu R, Li Y-Z and Tian Q (2025) Role of meningeal lymphatic vessels in brain homeostasis. Front. Immunol. 16:1593630. doi: 10.3389/fimmu.2025.1593630
Received: 14 March 2025; Accepted: 04 June 2025;
Published: 19 June 2025.
Edited by:
Arianna Di Stadio, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Sandro Dá Mesquita, Mayo Clinic Florida, United StatesNikolaos Grigoriadis, Aristotle University of Thessaloniki, Greece
Che-Feng Chang, National Taiwan University, Taiwan
Copyright © 2025 Zhao, Ye, Liu, Wang, Fu, Liu, Zhu, Li and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qing Tian, dGlhbnFAaHVzdC5lZHUuY24=
 Meng-Ying Zhao
Meng-Ying Zhao Chao-Yuan Ye
Chao-Yuan Ye Yuan-Cheng Liu
Yuan-Cheng Liu Xiao-Ming Wang
Xiao-Ming Wang Qing Tian
Qing Tian