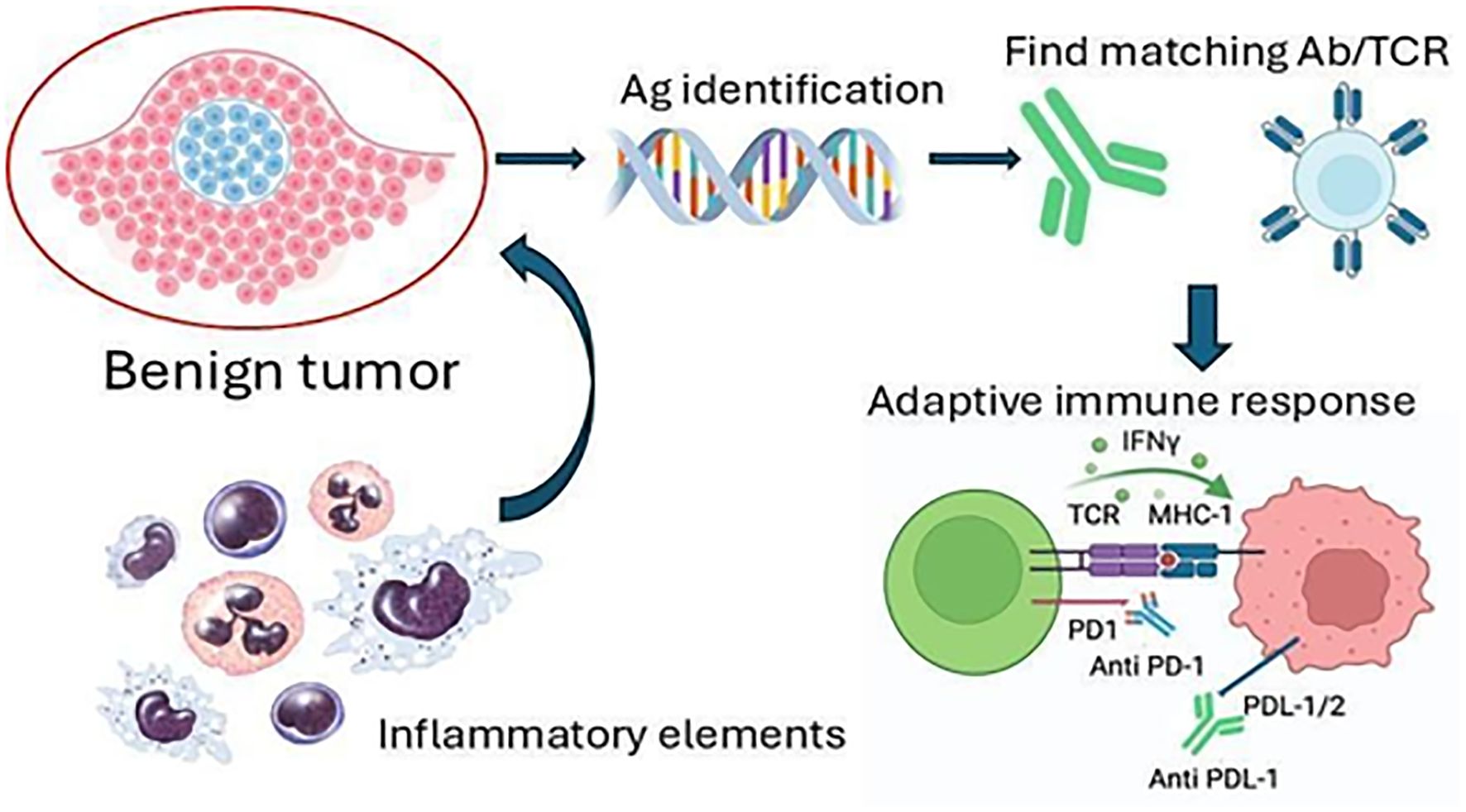
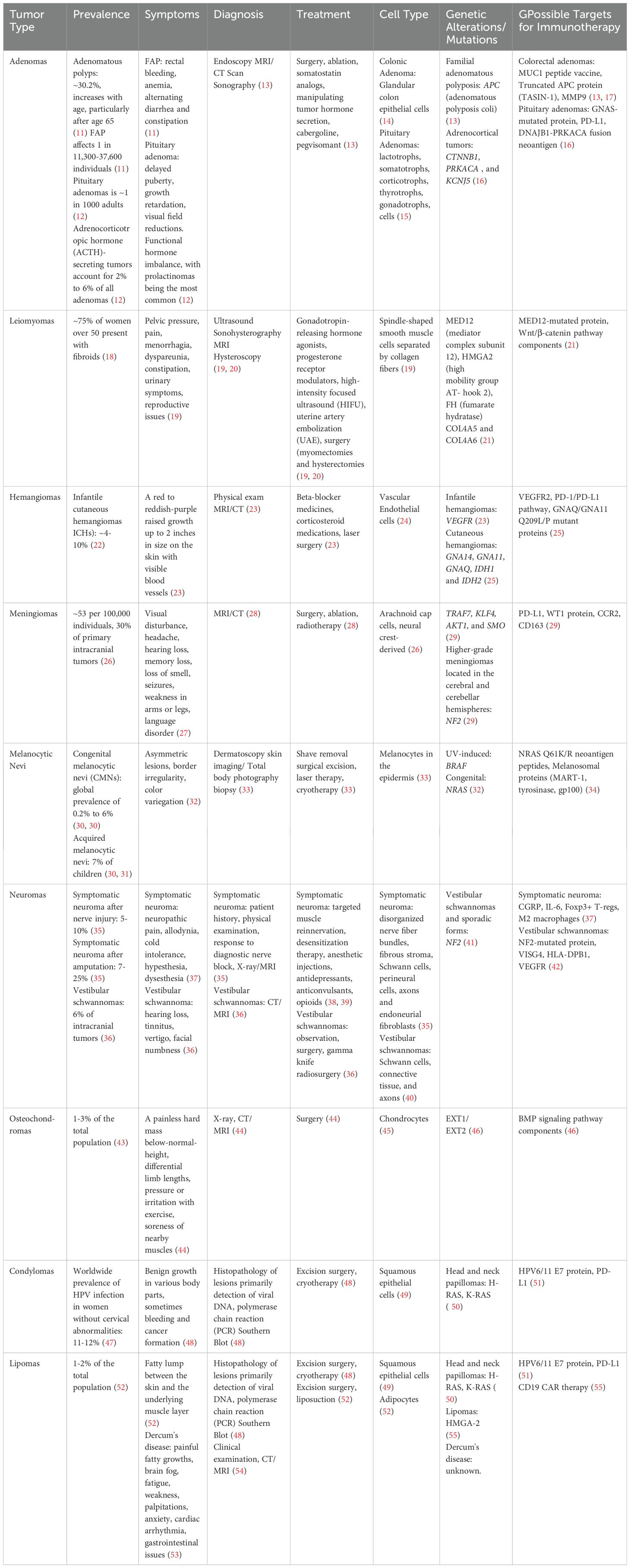
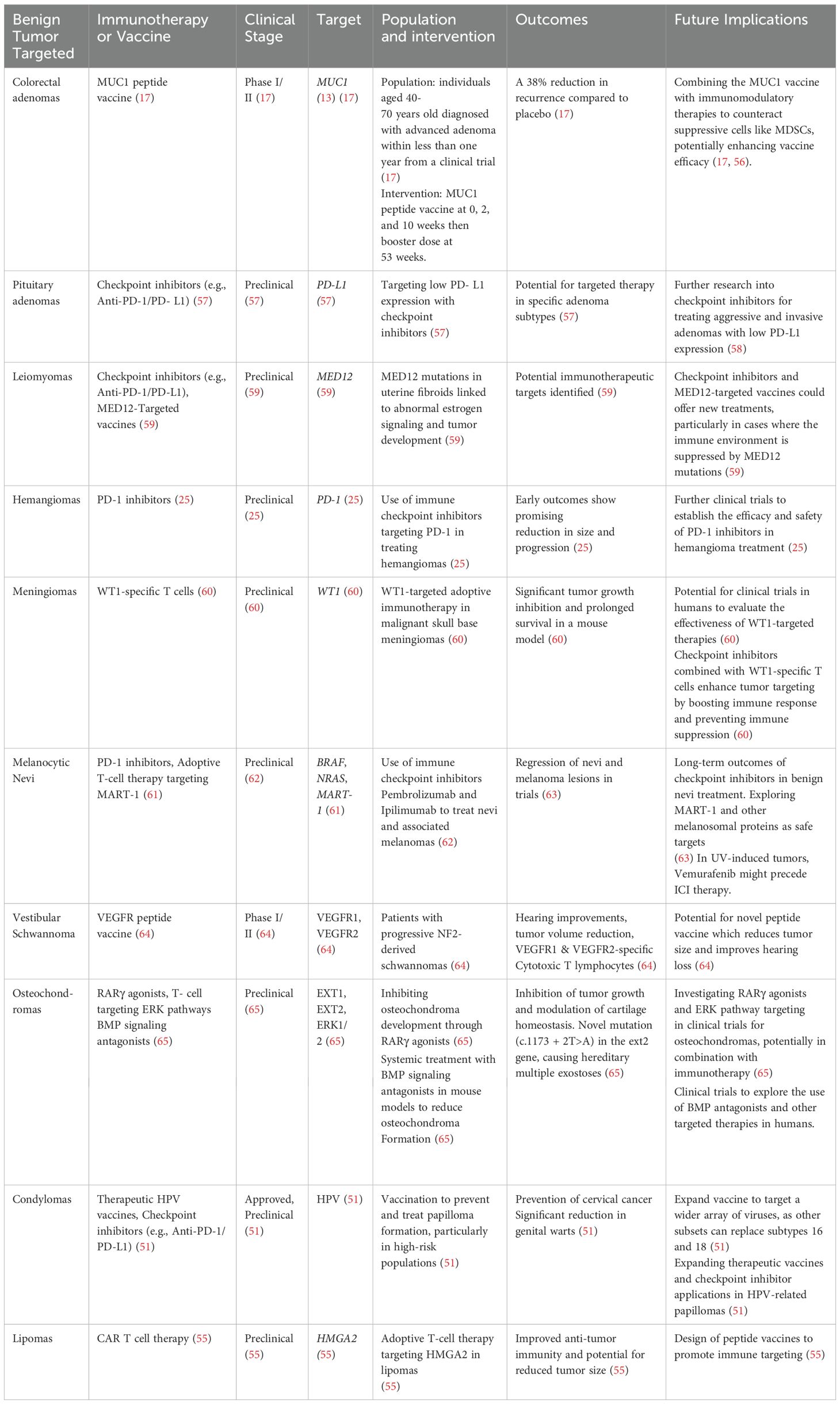
- 1Department of Internal Medicine, University of Texas Medical Branch, Galveston, TX, United States
- 2Department of Pathology, The University of Texas Medical Branch, Galveston, TX, United States
- 3Department of Medical Oncology, Ohio State University, Columbus, OH, United States
- 4Departments of Dermatology, Microbiology and Immunology, Robert H. Lurie Comprehensive Cancer Center, North Western University, Chicago, IL, United States
- 5Department of Pathology, Loyola University Medical Center, Maywood, IL, United States
Immunotherapy has shown significant potential for treating malignancies. Not yet widely considered is the opportunity to employ immunotherapy for the treatment of benign tumors. By focusing on targetable antigens expressed following specific genetic changes associated with individual benign tumors, immunotherapy may provide an effective approach to benign tumor treatment, circumventing the need for more conventional surgery. Immunotherapies can specifically recognize and target tumor cells, which could be especially beneficial for benign tumors given the extended timeframe available for treatment. Thus, benign tumors, offering a greater window of opportunity for treatment and a relatively stable phenotype associated with a limited mutation burden, can derive great benefit from immunotherapeutic approaches targeting antigens uniquely associated with each condition.
Introduction
Neoplastic somatic cells form benign or malignant tumors in diverse organs. Benign tumors are typically non- invasive and well-circumscribed compared to more aggressive malignant tumors. While benign tumors generally do not present an acute risk to life, they considerably affect an individual’s quality of life by manifesting various symptoms and complications. For example, they may provoke an overproduction of hormones, causing systemic side effects such as hypertension. Other benign tumors are more directly life-threatening due to their location. Intracranial benign tumors, for example, can disrupt vital functions such as breathing by their growth in the brainstem and other critical areas (1). When left unaddressed, the progression of benign tumors can cause compression of nearby structures or potentially fatal obstruction of blood and lymph flow (2).
We present benign tumors as potential targets for immunotherapy, expanding upon the concept of our prior studies with transgenic T cells for the treatment of lymphangioleiomyomatosis (LAM) and tuberous sclerosis complex (TSC) (3). While immunotherapy has achieved remarkable successes in treating malignancies including melanoma and non-small cell lung carcinoma, its application to benign tumors remains underexplored. Historically, benign tumors have been considered less impactful for patient quality of life, prompting limited therapeutic innovation. However, some benign tumor patients face unmet medical needs, making them strong candidates for immunotherapy. This review examines the risk-benefit profile of these approaches and identifies benign tumor subtypes with the greatest potential for therapeutic intervention. As potential therapeutic approaches, we considered currently approved therapies such as immune checkpoint inhibitors (ICI), transgenic T cells and next generation modalities including personalized mRNA vaccines (4).
We recognize the importance of considering combinatorial approaches to establish lasting responses (5). Ongoing innovation continues to bring new platforms, engineered safety mechanisms, and increasingly effective strategies to be tailored for benign tumor conditions. This progress is reflected in strategies such as suicide genes inserted in the constructs used for T cell transduction in order to eliminate the therapeutic cells as needed, including cytokine genes to sustain transgenic T cell activity, combining anti-PD1 with transgenic T cells to prevent their premature exhaustion, preceding checkpoint inhibition by anti-tumor vaccination and other combined measures (6).
Treating benign tumors by immunotherapy presents compelling advantages. Unlike malignancies, benign tumors are relatively slow-growing with limited metastatic activity, offering a broader window of opportunity and more localized areas for treatment. The extended time frame allows for careful adaptation of immunotherapy strategies, maximizing the potential for sustained responses. Benign tumors are frequently associated with chronic inflammation, suggesting recognition by the immune system (7). However, ineffective development of full adaptive immunity ultimately leads to disease progression. Like malignancies, benign tumors result from specific genetic alterations. Cancer-driving mutations provide malignant tumors the ability to invade and metastasize, and mutations in similar genes exist in benign tumors (8). Mutations linked to benign tumor conditions are more consistent and the corresponding disrupted biology can be used to identify tumor- specific antigens. While generic immune approaches including immune checkpoint inhibitors are used, it must be recognized that mutations are relatively sparse in benign tumors, resulting in limited specificity and efficacy. The tumor specific antigens associated with individual benign tumor types meanwhile offer steady targets for directed approaches. A critical factor in the efficacy of immune-based interventions is the immune-tumor interaction. The malignant tumor microenvironment becomes increasingly immunosuppressive, which can render immunotherapies ineffective (9). Benign tumors are more likely to be responsive to immunotherapeutics, especially when antigens of premalignancy are identified to guide adaptive responses and local inflammation can be overcome (10). Conventional approaches to benign tumor treatment include surgery, radiation and targeted chemotherapy. Some limitations of these approaches include disfigurement and infection risk, the possibility of introducing mutations that facilitate malignant transformation and halting progression rather than mediating tumor elimination. These current benign tumor treatments can bring extreme physical and emotional stress.
Immunotherapy has become a transformative development in oncology. Meanwhile, current benign tumor treatments, including surgery and hormone treatments, can bring extreme physical and emotional stress, and the possibility of a recurrence after treatment is significant. Early introduction of immunotherapy could facilitate limiting the reliance on invasive procedures to safeguard the quality of life. These factors provide a rationale for considering immunotherapy as a treatment option for benign tumors.
Methods
An extensive literature review was conducted to explore therapeutic approaches for benign tumors, encompassing scientific studies, clinical trials, and reviews focused on promoting adaptive immune responses to benign tumors. Data sources included peer-reviewed journals, clinical trial registries, and medical databases. Research involved identifying and analyzing mutated genes associated with benign tumors; using this genetic information, strategies were derived from methodologies and treatments used for other conditions. Understanding the molecular background of benign tumors is important to predict the resulting physiologic changes in specific tumor types. Existing therapies were reviewed, but the primary focus was on developing novel approaches tailored to the identified genetic alterations to expand the current therapeutic landscape. Summarizing our findings, Tables 1, 2 provide common benign tumor types, their symptoms, current treatment options, mutations that drive these tumors, and the current treatment options.
Adenomas
Familial adenomatous polyposis
Adenomas commonly develop from glandular tissues and their clinical presentations vary based on organ location and on specific glandular functions (14). Nearly all FAP patients progress to colorectal cancer if left untreated (11, 14). Pathogenic variants of benign adenomas provide insight into tumor pathogenesis. FAP diagnosis involves endoscopic examination and genetic screening for mutated Adenomatous Polyposis Coli (APC), a multifunctional tumor suppressor gene, and MUTYH, a base-excision repair gene protecting against DNA damage from oxidative stress; germline mutations in APC are present in 80% of FAP patients (13). Tumor recurrence is common within the first year of follow-up, involving new lesions, de novo mutations, or remnants from incomplete resection (13).
Immune prevention strategies have been explored for adenomas. MUC1 glycoprotein was identified as a tumor associated antigen for gastric cancers (66) In a phase II study, most patients with advanced colorectal adenomas treated with a MUC1 vaccine plus adjuvant poly-ICLC (polyinosinic-polycytidylic acid, carboxymethylcellulose, and poly-L-lysine) exhibited significant anti-MUC1 IgG antibody titers, sustained responses and a 38% reduced adenoma recurrence (17) Alternatively, three bispecific antibodies (bsAbs) for bile duct carcinoma (BDC) immunotherapy: anti-MUC1 x anti-CD3 (M x 3), anti-MUC1 x anti-CD28 (M x 28), and anti-MUC1 x anti-CD2 (M x 2) were developed (67). Combining all three bsAbs together with T lymphokine-activated killer cells (T-LAK) cells rendered the greatest tumor- killing efficacy in vitro in BDC-grafted mice, indicating a potential treatment strategy. This could mark a significant shift in the management of FAP and offer a preventive approach to complement or replace surgery.
Pituitary adenomas
Pituitary adenomas (Figure 1A) arise from the anterior pituitary gland (12) from either gonadotropin-producing cells, CD15+ cells, or tumor stem-like cells (15). These cellular origins exhibit unique stem cell gene expression profiles, growth behavior and hormone secretory activity. Some variants display a more aggressive clinical behavior with higher recurrence rates and resistance to standard therapies. These include sparsely granulated somatotroph adenomas, silent corticotroph adenomas, Crooke’s cell adenomas, and immature PIT1-lineage adenomas (15).
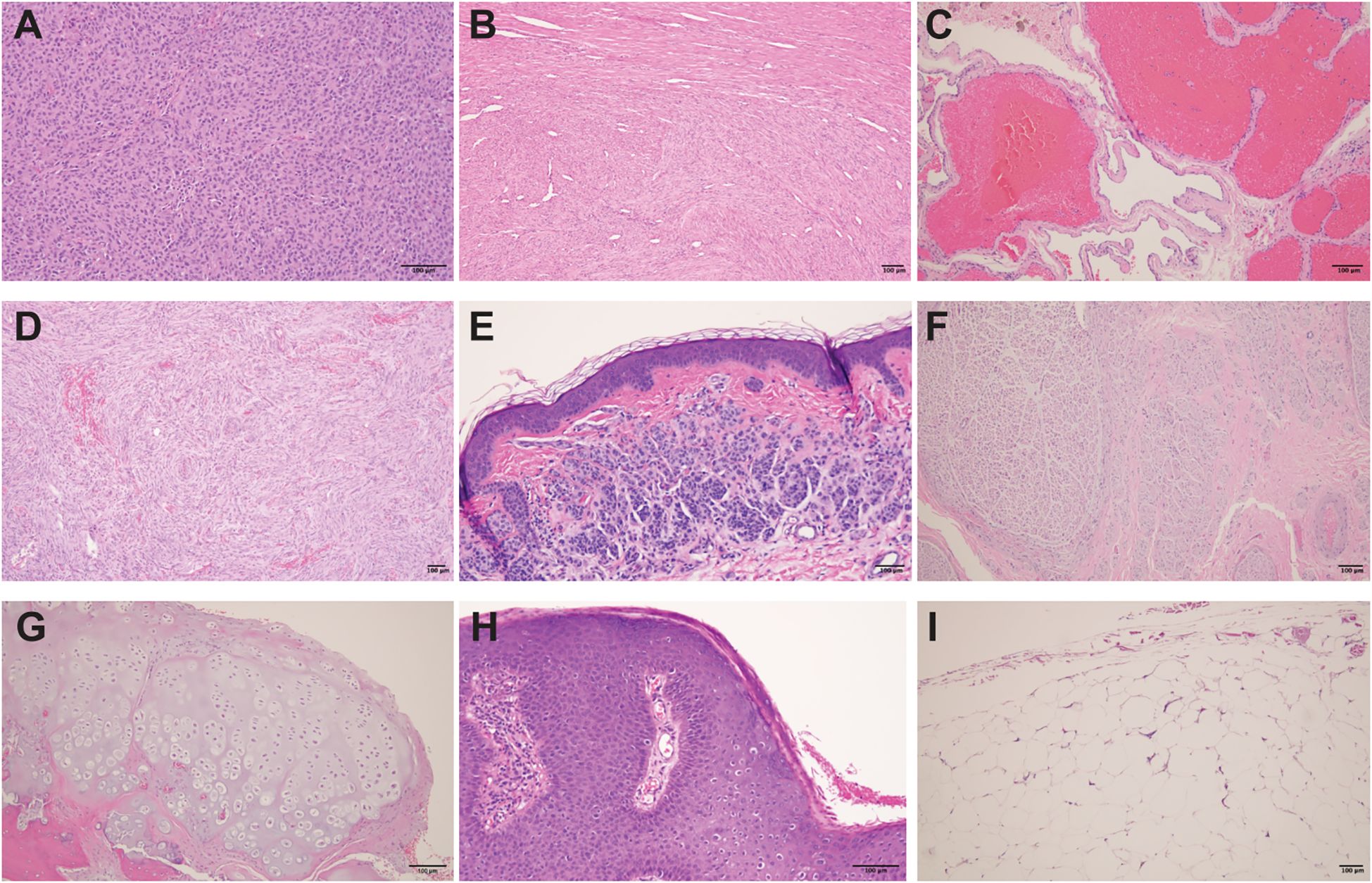
Figure 1. Histological features of various benign tumors and lesions. (A) Pituitary neuroendocrine tumor (pituitary adenoma): Solid sheets to nests of epithelioid cells with abundant acidophilic cytoplasm within a fibrovascular stroma. (B) Uterine leiomyoma (fibroid): Benign tumor of smooth muscle origin, composed of intersecting fascicles of spindle-shaped cells with indistinct borders, eosinophilic cytoplasm, and cigar-shaped nuclei. (C) Section of liver with cavernous hemangioma: Benign vascular tumor composed of variably sized, dilated and thin- walled vessels lined by a single layer of flat endothelial cells. (D) Meningioma: Benign neoplasm of cerebral meninges composed of spindle cells with meningothelial whorls and psammoma bodies. (E) Acquired intradermal melanocytic nevus: Nested proliferation of melanocytes in the dermis. The cells have scant cytoplasm, regular nuclei and are separated by a collagenous stroma. (F) Neuroma: Disorganized spindle cell proliferation of nerve components. (G) Osteochondroma: Mature hyaline cartilage with overlying fibrous perichondrium. (H) Condyloma acuminatum: Hyperplastic papillomatous squamous proliferation with a fibrovascular core and focal koilocytosis. (I) Lipoma: Benign soft tissue tumors characterized by uniform proliferation of mature adipose tissue. All images represent H&E (hematoxylin and eosin) stained tissue sections of paraffin-embedded tissues, showing the physiologic variety to indicate that benign tumors might be selectively targeted.
Pituitary adenomas are triggered by somatic mutations that disrupt intra-pituitary signaling and promote benign cell proliferation (12) Mutations in genes such as ACAsCTNNB1, PRKACA, and KCNJ5 underlie adrenocortical tumors (16). GNAS (guanine nucleotide- binding protein alpha subunit) mutations, particularly at R201 and Q227, are linked to uncontrolled cAMP accumulation and cellular proliferation in approximately 35% of functioning pituitary adenomas (58). Constitutive activation of the Gsα protein results in continuous stimulation of adenylate cyclase, causing elevated cyclic AMP (cAMP) and cellular proliferation. Vaccines have been developed to target the mutated Gsα protein, eliciting an immune response (68). Early studies show promise, particularly when combined with immune checkpoint inhibitors (57). Therapeutics impacting downstream signaling molecules within the cAMP/PKA/CREB pathway could further improve treatment outcomes (58). High invasiveness of pituitary adenoma subtypes are correlated with low expression of MSH6/2 and PD-L1, and early intervention is key.
CTAG2 and TSPYL6 nonfunctional pituitary adenomas show promise for immunotherapy, with studies suggesting benefits including CD8+ T cell infiltration (69). Counter-intuitively, the presence of tumor-infiltrating lymphocytes (TILs) in pituitary adenomas is linked to immunosuppression and recurrence, with significantly worse post-surgery outcomes; 61% of patients with TILs experience tumor persistence or recurrence involving increased immune suppression by regulatory T cells (Tregs) and derivative cytokines (70). Thus, targeting immunosuppressive components among TILs might provide a therapeutic avenue for pituitary adenoma patients. Moreover, tumor-associated macrophages (TAMs) in pituitary adenomas are correlated with increased tumor growth (71). Targeting MMP9, overexpressed in CTNNB1- mutant hepatocellular carcinoma, restored CD8+ T cell function and enhanced anti-PD-1 efficacy (72). We therefore suggest a strategy targeting MMP9 in adrenocortical adenomas with CTNNB1 mutations.
Leiomyoma (fibroma)
Leiomyomas (Figure 1B) primarily consist of smooth muscle growth and seventy % of leiomyomas are driven by mutations in MED12 (Table 2). MED12 helps the multiprotein Mediator complex regulate transcription (18). Pathogenic MED12 mutations disrupt interactions with the cyclin C-CDK8/CDK19 complex, leading to upregulated estrogen signaling and leiomyoma development (21). MED12 mutations activate the Wnt pathway, potentially cooperating with estrogen in leiomyoma development. MED12 mutations and HMGA2 gene rearrangements represent two mutually exclusive pathways in leiomyoma development, with MED12-mutated tumors typically being smaller but more numerous (21, 73). Beyond initiating fibroid development, MED12 mutations are also associated with immune suppression within fibromas, rendering them potential targets for immunotherapy to enhance immune cell infiltration and activation. This alternative to surgery potentially enables long-term management (59).
Hemangiomas
Hemangiomas are common neoplasms of proliferating vascular endothelial cells (24) and are classified as capillary, cavernous (Figure 1C), tufted, or mixed based on their histology. With an incidence of 10%, hemangiomas are divided into congenital hemangiomas or infantile hemangiomas (IH) (22) Though most infantile hemangiomas self-resolve by age 9, up to 8% may cause cosmetic issues and require treatment (22). While most hemangiomas occur sporadically, IH was linked to chromosome 5q31-33, containing candidate genes FGFR4, PDGFR-β, and FLT4 (23). These genes are involved in blood vessel growth, angiogenesis, VEGF signaling and MAPK regulation pathways.
The immune environment of infantile hemangiomas is marked by gross overrepresentation of CD11b+ dendritic cells that contribute to tumor development and immunity (74). Activated dendritic cells interact with CD4+ T cells, including regulatory T cells, releasing proangiogenic VEGF, IL-6, TNF-α, and IL-8. These support endothelial cell survival and promote hemangioma stem cell (HemSC) proliferation (75). CD163- expressing M2-polarized macrophages that express DC-SIGN further stimulate angiogenesis, inflammatory responses, and IH growth (76).
Checkpoint inhibitors, adoptive cell transfer, and vaccine-based therapies offer promise for the treatment of hemangiomas (Table 3). Prominent PD-1 expression by TILs is a sign of T cell activation in IH (80). VEGFR2 and endoglin (CD105) are highly expressed in proliferating IH, providing distinct opportunities for immunotherapy (75). Overexpressed on hemangioma cells, they could be targets for vaccines that ‘educate’ the immune system. A phase 1 glioblastoma study revealed that combining a VEGFR-2 DNA vaccine and anti-PD1 generated safe and detectable immune responses to VEGFR-2 (25). T cells might specifically eliminate hemangioma cells displaying these antigens. These immunotherapeutic strategies could provide new treatments for hemangiomas, especially those resistant to conventional therapies.
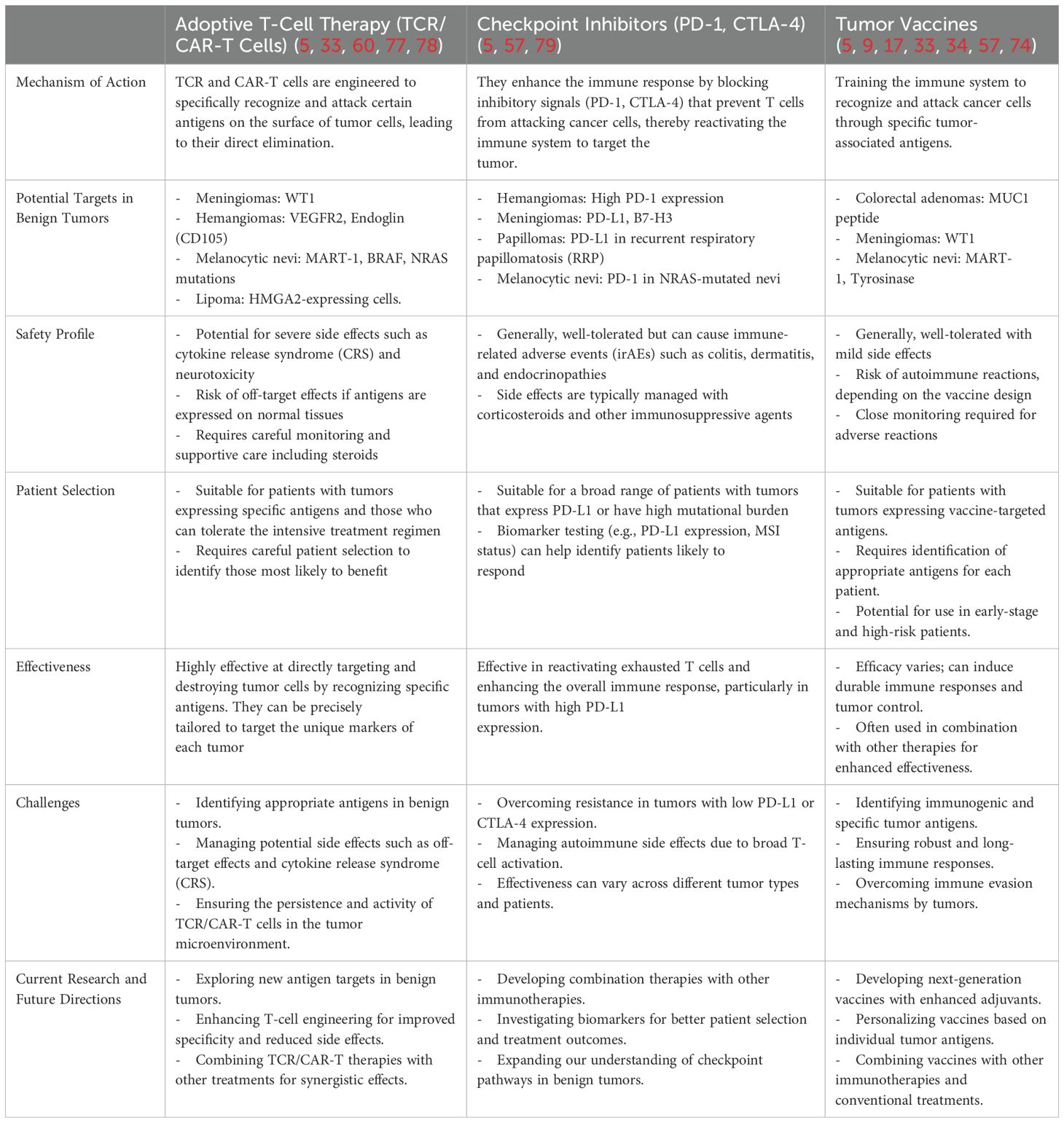
Table 3. Potential target antigens for TCR and CAR transgenic T cells compared with other immunotherapeutic agents.
Meningiomas
Meningiomas (Figure 1D) form from the arachnoid cap and meningothelial cells originating from neural crest tissue (Table 1) (26). They are classified as Grade I (benign), Grade II (atypical), or Grade III (anaplastic), wherein approximately 90% are benign (81). NF2, regulating contact-inhibited cell growth, is mutated in approximately 49% of all meningiomas. Other affected genes can include TRAF7, AKT1, KLF4, POLR2A, chromatin genes KDM6A, CHD2, and SMARCB1 and tumor suppressor PTEN. Meningiomas segregate into four groups according to integrated molecular data (29) providing superior prediction of recurrence-free survival over WHO classification.
Chromosomal instabilities and genomic abnormalities can lead to increased metabolic aggressiveness (82). The complex immune environment of meningiomas involves mature memory/effector T and B cells, regulatory T cells, and cells expressing immune checkpoint molecules (83), indicating an opportunity for immune-based therapies (84). Adoptive T cell transfer targeting WT1 (Table 2) holds potential for treating skull base malignant meningiomas, revealing efficacy in mice and providing a promising approach for such challenging cases (77). Overexpressed WT-1 could be targeted vaccines to treat meningiomas (60). A major prognostic factor in low-grade meningiomas involves immune infiltration by dendritic cells and M2 macrophages, and CSF-1R inhibitors to decrease M2 macrophage populations or targeting MDSCs with IDO- or arginase inhibitors may further improve meningioma immune responses (78).
Nevi
Congenital melanocytic nevi (CMN)(moles), consist of benign proliferating melanocytes protruding into the deep dermis. Nevi are classified by size, shape, and color. Large and giant CMN are associated with an increased risk of developing melanoma and neurocutaneous melanosis (NCM) (33) NCM is caused by rare proliferation of melanocytes within the CNS. Immunotherapies can well serve to treat recurrent, premalignant nevi, large neurocutaneous melanosis, and large congenital melanocytic nevi to avert the risk of progression (32).
Acquired nevi (Figure 1E) often harbor BRAF mutations linked to aging and UV exposure, whereas CMN typically exhibit NRAS mutations (Table 1), indicating a distinct molecular basis. While BRAF and NRAS mutations are mutually exclusive, both activate the MAPK pathway to drive melanocytic neoplasia (32). Somatic mutations in codon 61 of NRAS are prevalent among CMN and NCM (33). Immunotherapy can induce regression, often by mechanisms shared with melanoma treatment. Herein immune checkpoint inhibitors (Table 3) including anti-CTLA-4 enhance T cell activation, mediating regression of atypical nevi and a vitiliginous reaction (62). NRAS-mutated melanomas exhibit improved clinical responses and progression-free survival over BRAF-mutated melanomas after IL-2 or checkpoint inhibitor therapy (79). These findings pave the way for immunotherapy targeting nevi.
Melanocytic nevi express melanosomal proteins including MART-1, tyrosinase, and gp100 as potential targets for adoptive cell transfer or vaccine-based treatments (34). MART-1-specific CTLs (Table 2) can regress both benign nevi and melanoma lesions in an antigen-specific manner (61). Nevus-resident type 1 CD4+ T cells also rejected benign nevi in an antigen-specific manner, highlighting the use for melanosomal antigens as effective immunotherapy targets (63). The target molecules in nevi are shared exclusively with melanocytes, and are predominantly melanosomal in nature. This selectivity would be advantageous for immunotherapies (62).
Neuromas
Symptomatic neuromas
Neuromas (Figure 1F) in the PNS arise from failed tissue repair and abnormal cell growth after nerve damage, occurring in 5-10% of nerve injury patients and more in individuals undergoing amputations (35). By exception, most neuromas are not associated with mutations, yet the predictable pathophysiology of symptomatic neuromas can provide opportunities for immune targeting. Neuromas present as disorganized nerve fiber tangles encapsulated in fibrous tissue. Nerve myelination is greatly reduced with evidence of chronic inflammation caused by infiltrating mast cells, proliferating fibroblasts and glycosaminoglycan rich stromatic tissue. SMA+ myofibroblasts are overabundant. Secretion of neuropeptides like CGRP and pro-inflammatory cytokines is upregulated (37). M1 Macrophages are recruited, exacerbating the pain response (85). Treatment with anti-inflammatories might reduce pain and neuroma sizes (38, 39). Modulating the immune environment can remove proinflammatory molecules from the blood, and have shown potential in attenuating disease progression.
Vestibular schwannomas (acoustic neuromas)
Vestibular schwannomas (VS) are benign growth of Schwann cells on the vestibular portion of the eighth cranial nerve (86). While 95% of VS are sporadic, 5% are associated with mutations in NF2 encoding merlin, a tumor suppressor protein related to the ERM (ezrin-radixin-moesin) family (87). Neurofibromatosis covers multiple tumor types, including acoustic neuromas. Alternate types include mutations in NF1 which are more susceptible to malignant transformation. The epidermal growth factor receptor HER1 was found to be overexpressed in the latter tumor type, prompting the development of immunosuppression-resistant CAR T-cells that displayed anti-tumor efficacy in vitro and in tumor spheroids (88). Its loss from Schwann cells dysregulates signaling pathways such as PI3k-Akt, Wnt, Hippo-YAP/TAZ mTORC1 and EGFR and stimulates Schwann cell growth and migration (89). Upon de-differentiation, Schwannoma cells promote nerve repair and immune cell recruitment (90). marked by elevated cytokines that drive tumor progression, immunosuppression, and stroma formation (40). Recruited immune cells include macrophages, T-lymphocytes including regulatory T cells and NK cells (91).
The tumor microenvironment contributes to VS pathogenesis, and offers potential therapeutic targets (92). VSIG4 is expressed on M2 macrophages, inhibiting CD8+ T cell cytotoxicity after binding to ligands (92). Treatment with VISG4 antibodies reduced disease severity in a mouse model of experimental autoimmune encephalomyelitis, and might serve schwannoma treatment as well (42). Angiogenesis plays a key role in VS lesion progression (41). Treatment with an anti- vascular endothelial growth factor (VEGF) antibody reduced tumor burden and improved the hearing of NF2 patients (93). VS tumor cells expressed high levels of VEGFR1 and VEGFR2, and VEGF receptor peptide vaccination in NF2 vestibular schwannomas also demonstrated signs of intratumoral apoptosis (64).
Osteochondromas
Osteochondromas (OC) (Figure 1G) develop as bony outgrowths with a cartilage cap projecting from the bone surface, typically located in the metaphysis of long bones (45). Most are solitary lesions, while 15% of cases are associated with hereditary multiple osteochondroma (HMO), an autosomal dominant disorder (94). Malignant transformation occurs in 1% of solitary and 10% of HMO cases. Recurrence is observed in approximately 5% of sporadic and 20% of HMO osteochondroma patients, dropping to less than 2% if complete resection is achieved (44). Pathogenic variants in EXT1 or EXT2 were detected in 85% of HMO cases and 80% of solitary cases (46). The genes encode enzymes critical for heparan sulfate biosynthesis, important for cell differentiation and tissue morphogenesis. Heparan sulfate binds growth factors and extracellular matrix proteins, and supports leukocyte migration and recruitment (95). High EXT1 expression is associated with an abundance of CD8+ T cells, while EXT2 expression is associated with reduced CD4+ T cells, macrophages, neutrophils, and dendritic cells in head and neck squamous cell carcinoma (HNSC) (96).
ERK signaling suppresses chondrocyte proliferation, lineage commitment and differentiation, while its loss in CD4+ cells leads to osteochondroma-like structures lacking immune cell infiltration in mice (97). T cell loss accelerated tumor growth. A patient with biallelic germline mutations in Nuclear Factor of Activated T Cells-2 (NFATC2) presented clinically with recurrent B cell lymphoma and osteochondromas (65). NFATC2 encodes the NFAT1 transcription factor which responds to calcium signaling and controls gene expression in immune cells and chondrocytes. NFAT1-deficient CD4+ T cells expressed elevated PD-1 and reduced TNF-α and IFN-γ, underscoring a role for the immune system (65). There would be great benefit to developing osteochondroma immunotherapies such as checkpoint inhibitors, which restore T-cell activation and enhance anti-tumor immunity by inhibiting immune checkpoint signaling.
Condylomas
Condylomas (Figure 1H) are benign epithelial growths often caused by human papillomavirus (HPV) types 6 and 11 (49). HPV is a significant global health issue, with a prevalence of 11–12% or more for oncogenic types 16 and 18 in females regardless of cervical abnormalities (47). Preventive vaccines are highly effective against the main HPV types responsible for benign genital warts and cervical malignancies (48).
The HPV6 E5 protein decreases MHC class 1 expression, rendering infected cells less detectable to cytotoxic T cells (98). Clinical HPV vaccine trials, particularly those targeting precancerous papillomas, have shown great promise (99). Trials with therapeutic mRNA-based and nanoparticle vaccines targeting the HPV E7 protein likewise revealed promising outcomes (51). Immune checkpoint proteins, growth factors, and immunoregulatory proteins are expressed within the papilloma microenvironment, highlighting a complex immunoregulatory landscape (100). Overall, the development of HPV vaccines offers promise for the treatment for premalignant HPV lesions.
Lipomas
Lipomas (Figure 1I) are soft tissue tumors characterized by adipocyteproliferation and they often arise in the subcutaneous tissue (52). In 5–15% of cases, patients present with multiple lipomas, which may occur sporadically (e.g. Dercum’s disease) or result from inherited genetic abnormalities such as PTEN hamartoma syndromes (e.g. Cowden syndrome) and neurofibromatosis type 1 (52). Dercum’s disease, a rare inflammatory condition characterized by painful subcutaneous fat masses and alterations in lymphatic vessels, represents a promising indication for immunotherapy due to its underlying immune dysregulation and inflammatory nature (53).
Deoxycholic Acid injections triggered adipose degradation by macrophages, tumor shrinkage and pain mitigation (101). Emerging research suggests potential immunotherapeutic targets within the lipoma microenvironment. Macrophage polarization, particularly the predominance of M2 macrophages, may contribute to lipoma persistence, suggesting that CSF1R inhibitors could shift the immune response toward M1 activation to promote adipocyte apoptosis (102). Additionally, immune checkpoint inhibitors such as PD-1/PD-L1 blockade (pembrolizumab, nivolumab) may enhance T-cell activity if lipomas exhibit immune evasion mechanisms. Lipomas are associated with gene rearrangements involving, for example, lipoma preferred protein (LPP) or HMGA2. Tumor-associated overexpression of the resulting proteins provides a rationale for the design of peptide vaccines to promote immune targeting. The potential efficacy of immune-based approaches for lipomas is evidenced by the remarkable tumor shrinkage observed in a patient treated by CD19 CAR therapy (55).
Discussion
Benign tumors, perceived as less severe or life-threatening than malignancies, have historically been overlooked for therapeutic innovation. However, these tumors can cause debilitating symptoms that profoundly impact patient quality of life and lead to life-threatening complications. Through our analysis of the latest research, clinical outcomes, and existing therapies, we identified promising opportunities to explore immunotherapies as transformative treatments for benign tumors, some of which are already being put to the test.
Sporadic examples of immunotherapeutics used for the treatment of benign tumors in vivo include Garzon-Muvdi et al. (103) who reviewed ICI as an option for meningiomas, combined or not with NK cells in preclinical models. Azab et al., 2023 (104), also made a case for meningiomas, describing MDSCs as tumor drivers and showing that infiltration by relevant T cells and PD1 and PD-L1 expression are reliable prognostic predictors, while discussing mutations that could provide neoantigens for infiltrating T cells. IFNα has been among the earlier proposed immunotherapeutics tested for meningiomas, with some efficacy (105). Pituitary adenomas have also been the subject of preclinical studies, where animal models of Cushing’s disease and clinical studies did reveal survival benefit and treatment efficacy (106) from checkpoint inhibitor therapy. In a patient with laryngeal papillomas, the IL-5 receptor on eosinophils proved a target for antibody therapy (107). Halkola et al. (108) favored anti-angiogenic treatment for benign tumors, following suggestions of the same by Hannan et al. (109), though the treatment not only limits oxygen access but also access to immune cells. In patients with HNSCC, benign tumors provided a greater opportunity to propagate cytotoxic NKT cells than malignant ones (110). Warts were successfully injected with skin test antigens to induce T cell infiltration (111). Candida antigen has been used in this respect (56). Immune stimulation with imiquimod +/- green tea derivatives has shown some benefit (112). Hyperthermia was an early treatment to evoke immune responses, supportive of tumor regression in cows (113).
In solid organ transplant patients, BCG treatment did not temper the risk for developing bladder tumor (114), this outcome is potentially related to immunosuppressive treatment provided to this population. Patients might benefit from ICI and other less specific treatments, yet we promote the concept that knowledge of benign tumor physiology offers an opportunity to direct immune responses to the tumors, for greater specificity and efficacy. Our own studies showed marked efficacy of GD3 CAR T cells against benign tumors modeling TSC (3). Likewise, gp100/PMEL proved a credible target for T cells in lymphangioleiomyomatosis (LAM) (115). We subsequently showed efficacy for adoptive transfer of gp100/Pmel-reactive T cells for the condition in preclinical studies (116).
Moreover, Liu et al. (117) have shown that in preclinical mouse studies, CTLA-4 blockade combined with anti-PD1 provided greater tumor clearance of TSC-associated tumors than anti-PD1 treatment alone. The paper further describes a supportive mechanism for this observation by demonstrating increased T cell infiltration accompanied by elevated IFNγ and TNFα production, potentially overcoming B7-H3-mediated immune evasion reported in LAM (118). This work supports the very real potential of checkpoint inhibition for the treatment of benign tumors, which might be further enhanced when including antigen specificity to the treatment regimen.
When considering immunotherapeutic strategies, it will be important to understand if these tumors form as part of a hereditary syndrome or as a consequence of de novo mutations only, to understand the likelihood of the mutated gene product or its downstream effectors to be recognized by the patient’s immune system. In this respect, patients with hereditary tumor syndromes might draw specific benefit from immunotherapeutic approaches, if recurring tumors can be eliminated by T cell recall responses. The observed persistence of adoptively transferred CAR T-cells holds specific significance for these patients (19). Another consideration is the potential for side effects. ICIs for example can elicit adverse events ranging from skin rash to gastric or cardiac events; including an antigenic vaccine can provide direction to the immune response and limit such side effects, but such vaccines, in turn, deserve some scrutiny to understand if the epitopes included might be shared with homologous tissue antigens or with expression outside the tumor itself and could incite on target, of tumor responses that would do harm. These risks have formed the greatest impediment to applying immunotherapy for the treatment of benign tumors until the present day. This is not different from any other therapeutic application used to treat the condition, and with caution and consideration for potential consequences, the benefit of immunotherapy can well outweigh the risks.
Methods traditionally successful in treating malignant tumors like adoptive T-cell transfer, checkpoint inhibitors, or tumor vaccines (Table 3) could be beneficial. Immunotherapy strategies for benign tumors focus on preventing tumor progression, recurrence, or malignant transformation by leveraging immune modulation. Vaccination can be used if the benign tumor expresses new antigens, training the immune system to recognize and eliminate abnormal cells early. Varying the mode of application (e.g., peptide vaccines, mRNA, dendritic cells) enhances immune activation and prevents tolerance. Anti-CTLA-4 therapy (e.g., ipilimumab) promotes antigen-specific T cell responses, while anti-PD-1 inhibitors (e.g., pembrolizumab) sustain immune surveillance by preventing T cell exhaustion; other targets can finetune the outcomes. In cases requiring more targeted intervention, adoptive immunotherapy with transgenic T cells (e.g., CAR-T or TCR-T cells) can selectively eliminate tumor cells while preserving healthy tissue. This immunotherapeutic approach aims to control benign tumors, reduce recurrence, and prevent malignant progression. While immunotherapeutics have faced challenges in the clinic, the less aggressive nature and more predictable biology of benign tumors may create more favorable conditions for success. Furthermore, identifying tumor specific antigens has become more feasible as deeper genomic, phenotypic, and molecular data of these benign tumors become available. In conclusion, immunotherapeutics hold great promise for treating benign tumors. In future clinical trials, these concepts can be validated to establish new standards of care, ultimately enhancing the management of benign tumors.
Author contributions
MY: Writing – original draft, Methodology, Project administration, Software, Writing – review & editing. HA: Project administration, Software, Methodology, Writing – review & editing, Writing – original draft. BM: Software, Writing – review & editing, Supervision. MP: Writing – review & editing, Supervision, Resources, Visualization. IL: Supervision, Writing – review & editing, Conceptualization, Funding acquisition, Visualization, Project administration, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Support for the preparation of this paper was provided in part by NIH R01HL165841 to IL.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Grebenc ML, Rosado de Christenson ML, Burke AP, Green CE, and Galvin JR. Primary cardiac and pericardial neoplasms: radiologic-pathologic correlation. Radiographics. (2000) 20:1073–103; quiz 1110. doi: 10.1148/radiographics.20.4.g00jl081073
2. Jain RK, Martin JD, and Stylianopoulos T. The role of mechanical forces in tumor growth and therapy. Annu Rev BioMed Eng. (2014) 16:321–46. doi: 10.1146/annurev-bioeng-071813-105259
3. Thomas A, Sumughan S, Dellacecca ER, Shivde RS, Lancki N, Mukhatayev Z, et al. Benign tumors in TSC are amenable to treatment by GD3 CAR T cells in mice. JCI Insight. (2021) 6(22):e152014. doi: 10.1172/jci.insight.152014
4. Guasp P, Reiche C, Sethna Z, and Balachandran VP. RNA vaccines for cancer: Principles to practice. Cancer Cell. (2024) 42:1163–84. doi: 10.1016/j.ccell.2024.05.005
5. Butterfield LH and Najjar YG. Immunotherapy combination approaches: mechanisms, biomarkers and clinical observations. Nat Rev Immunol. (2024) 24:399–416. doi: 10.1038/s41577-023-00973-8
6. Abodunrin F, Olson DJ, Emehinola O, and Bestvina CM. Adopting tomorrow’s therapies today: a perspective review of adoptive cell therapy in lung cancer. Ther Adv Med Oncol. (2025) 17:17588359251320280. doi: 10.1177/17588359251320280
7. Zannotti A, Greco S, Pellegrino P, Giantomassi F, Delli Carpini G, Goteri G, et al. Macrophages and immune responses in uterine fibroids. Cells. (2021) 10(5):982. doi: 10.3390/cells10050982
8. Boutry J, Tissot S, Ujvari B, Capp J-P, Giraudeau M, Nedelcu AM, et al. The evolution and ecology of benign tumors. Biochim Biophys Acta Rev Cancer. (2022) 1877:188643. doi: 10.1016/j.bbcan.2021.188643
9. Baker DJ, Arany Z, Baur JA, Epstein JA, and June CH. CAR T therapy beyond cancer: the evolution of a living drug. Nature. (2023) 619:707–15. doi: 10.1038/s41586-023-06243-w
10. Emens LA, Romero PJ, Anderson AC, Bruno TC, Capitini CM, Collyar D, et al. Challenges and opportunities in cancer immunotherapy: a Society for Immunotherapy of Cancer (SITC) strategic vision. J Immunother Cancer. (2024) 12(6):e009063. doi: 10.1136/jitc-2024-009063
11. Half E, Bercovich D, and Rozen P. Familial adenomatous polyposis. Orphanet J Rare Dis. (2009) 4:22. doi: 10.1186/1750-1172-4-22
12. Molitch ME. Diagnosis and treatment of pituitary adenomas: A review. JAMA. (2017) 317:516–24. doi: 10.1001/jama.2016.19699
13. Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. (1991) 66:589–600. doi: 10.1016/0092-8674(81)90021-0
14. Banskota S and Adamson DC. Pituitary adenomas: from diagnosis to therapeutics. Biomedicines. (2021) 9(5):494. doi: 10.3390/biomedicines9050494
15. Melmed S, Kaiser UB, Lopes MB, Bertherat J, Syro LV, Raverot G, et al. Clinical biology of the pituitary adenoma. Endocr Rev. (2022) 43:1003–37. doi: 10.1210/endrev/bnac010
16. Zheng G-Y, Zhang X-B, Li H-Z, Zhang Y-S, Deng J-H, and Wu X-C. Sum of high-risk gene mutation (SHGM): A novel attempt to assist differential diagnosis for adrenocortical carcinoma with benign adenoma, based on detection of mutations of nine target genes. Biochem Genet. (2021) 59:902–18. doi: 10.1007/s10528-021-10039-w
17. Finn OJ, Boardman L, Cruz-Correa M, Bansal A, Kastenberg D, Hurr C, et al. Abstract CT236: Randomized, double-blind, placebo-controlled trial of preventative MUC1 vaccine in patients with newly diagnosed advanced adenomas: Results from one-year booster. Cancer Res. (2019) 79:CT236–6. doi: 10.1158/1538-7445.AM2019-CT236
18. Philibert RA, Winfield SL, Damschroder-Williams P, Tengstrom C, Martin BM, and Ginns EI. The genomic structure and developmental expression patterns of the human OPA-containing gene (HOPA). Hum Genet. (1999) 105:174–8. doi: 10.1007/s004399900084
19. Myers RM, Li Y, Barz Leahy A, Barrett DM, Teachey DT, Callahan C, et al. Humanized CD19-targeted chimeric antigen receptor (CAR) T cells in CAR-naive and CAR-exposed children and young adults with relapsed or refractory acute lymphoblastic leukemia. J Clin Oncol. (2021) 39:3044–55. doi: 10.1200/JCO.20.03458
20. Ciarmela P, Delli Carpini G, Greco S, Zannotti A, Montik N, Giannella L, et al. Uterine fibroid vascularization: from morphological evidence to clinical implications. Reprod BioMed Online. (2022) 44:281–94. doi: 10.1016/j.rbmo.2021.09.005
21. Markowski DN, Bartnitzke S, Löning T, Drieschner N, Helmke BM, and Bullerdiek J. MED12 mutations in uterine fibroids–their relationship to cytogenetic subgroups. Int J Cancer. (2012) 131:1528–36. doi: 10.1002/ijc.v131.7
22. George A, Mani V, and Noufal A. Update on the classification of hemangioma. J Oral Maxillofac Pathol. (2014) 18:S117–20. doi: 10.4103/0973-029X.141321
23. Boscolo E and Bischoff J. Vasculogenesis in infantile hemangioma. Angiogenesis. (2009) 12:197–207. doi: 10.1007/s10456-009-9148-2
24. Drolet BA, Frommelt PC, Chamlin SL, Haggstrom A, Bauman NM, Chiu YE, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics. (2013) 131:128–40. doi: 10.1542/peds.2012-1691
25. Wick W, Wick A, Chinot O, Sahm F, von Deimling A, Jungk C, et al. KS05.6.A Oral DNA vaccination targeting VEGFR2 combined with the anti-PD-L1 antibody avelumab in patients with progressive glioblastoma - final results. NCT03750071. Neuro Oncol. (2022) 24:ii6–6.
26. Saito R, Chambers JK, and Uchida K. Immunohistochemical study of autophagy associated molecules and cell adhesion molecules in canine intracranial granular cell tumors. J Vet Med Sci. (2022) 84:1474–9. doi: 10.1292/jvms.22-0359
27. Chang WI, Kim I-H, Choi SH, Kim TM, Lee S-T, Won JK, et al. Risk stratification to define the role of radiotherapy for benign and atypical meningioma: A recursive partitioning analysis. Neurosurgery. (2022) 90:619–26. doi: 10.1227/neu.0000000000001904
28. Minja EJ, Tan M, Gibbs MJ, Kazimi MM, Hundley JC, and Pollinger HS. Massive leiomyomatous uterine proliferation following kidney transplantation: A case report and literature review. Case Rep Transpl. (2018) 2018:3874937. doi: 10.1155/2018/3874937
29. Nassiri F, Liu J, Patil V, Mamatjan Y, Wang JZ, Hugh-White R, et al. A clinically applicable integrative molecular classification of meningiomas. Nature. (2021) 597:119–25. doi: 10.1038/s41586-021-03850-3
30. Amirjamshidi A, Mehrazin M, and Abbassioun K. Meningiomas of the central nervous system occurring below the age of 17: report of 24 cases not associated with neurofibromatosis and review of literature. Childs Nerv Syst. (2000) 16:406–16. doi: 10.1007/s003819900205
31. Ingordo V, Gentile C, Iannazzone SS, Cusano F, and Naldi L. Congenital melanocytic nevus: an epidemiologic study in Italy. Dermatol (Basel). (2007) 214:227–30. doi: 10.1159/000099587
32. Colebatch AJ, Ferguson P, Newell F, Kazakoff SH, Witkowski T, Dobrovic A, et al. Molecular genomic profiling of melanocytic nevi. J Invest Dermatol. (2019) 139:1762–8. doi: 10.1016/j.jid.2018.12.033
33. Mologousis MA, Tsai SY-C, Tissera KA, Levin YS, and Hawryluk EB. Updates in the management of congenital melanocytic nevi. Children (Basel). (2024) 11(1):62. doi: 10.3390/children11010062
34. Seiter S, Monsurro V, Nielsen M-B, Wang E, Provenzano M, Wunderlich JR, et al. Frequency of MART-1/MelanA and gp100/PMel17-specific T cells in tumor metastases and cultured tumor-infiltrating lymphocytes. J Immunother. (2002) 25:252–63. doi: 10.1097/00002371-200205000-00008
35. Arnold DMJ, Wilkens SC, Coert JH, Chen NC, Ducic I, and Eberlin KR. Diagnostic criteria for symptomatic neuroma. Ann Plast Surg. (2019) 82:420–7. doi: 10.1097/SAP.0000000000001796
36. Valiukeviciene S, Miseviciene I, and Gollnick H. The prevalence of common acquired melanocytic nevi and the relationship with skin type characteristics and sun exposure among children in Lithuania. Arch Dermatol. (2005) 141:579–86. doi: 10.1001/archderm.141.5.579
37. Hwang CD, Hoftiezer YAJ, Raasveld FV, Gomez-Eslava B, van der Heijden EPA, Jayakar S, et al. Biology and pathophysiology of symptomatic neuromas. Pain. (2024) 165:550–64. doi: 10.1097/j.pain.0000000000003055
38. Zimmermann M. Pathobiology of neuropathic pain. Eur J Pharmacol. (2001) 429:23–37. doi: 10.1016/S0014-2999(01)01303-6
39. Minarelli J, Davis EL, Dickerson A, Moore WC, Mejia JA, Gugala Z, et al. Characterization of neuromas in peripheral nerves and their effects on heterotopic bone formation. Mol Pain. (2019) 15:1744806919838191. doi: 10.1177/1744806919838191
40. Guo S, Zheng X, Chen W, Raza U, Zeng A, Akter F, et al. From bench to bedside: Advancing towards therapeutic treatment of vestibular schwannomas. Neurooncol Adv. (2024) 6:vdae107. doi: 10.1093/noajnl/vdae107
41. Cazzador D, Astolfi L, Daloiso A, Tealdo G, Simoni E, Mazzoni A, et al. Tumor microenvironment in sporadic vestibular schwannoma: A systematic, narrative review. Int J Mol Sci. (2023) 24(7):6522. doi: 10.3390/ijms24076522
42. Huang X, Feng Z, Jiang Y, Li J, Xiang Q, Guo S, et al. VSIG4 mediates transcriptional inhibition of Nlrp3 and Il-1β in macrophages. Sci Adv. (2019) 5:eaau7426.
43. Fortnum H, O’Neill C, Taylor R, Lenthall R, Nikolopoulos T, Lightfoot G, et al. The role of magnetic resonance imaging in the identification of suspected acoustic neuroma: a systematic review of clinical and cost effectiveness and natural history. Health Technol Assess. (2009) 13:iii–iv, ix. doi: 10.3310/hta13180
44. Tepelenis K, Papathanakos G, Kitsouli A, Troupis T, Barbouti A, Vlachos K, et al. Osteochondromas: an updated review of epidemiology, pathogenesis, clinical presentation, radiological features and treatment options. In Vivo. (2021) 35:681–91. doi: 10.21873/invivo.12308
45. Li X, Su Y, Songfeng L, Changyoing S, and Pengfei H. Diagnostic features of lumbar canal osteochondroma and review of literature. FMSR. (2022) 4(1). doi: 10.25236/FMSR.2022.040106
46. Zuntini M, Pedrini E, Parra A, Sgariglia F, Gentile FV, Pandolfi M, et al. Genetic models of osteochondroma onset and neoplastic progression: evidence for mechanisms alternative to EXT genes inactivation. Oncogene. (2010) 29:3827–34. doi: 10.1038/onc.2010.135
47. Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, et al. Global burden of human papillomavirus and related diseases. Vaccine. (2012) 30 Suppl 5:F12–23. doi: 10.1016/j.vaccine.2012.07.055
48. Charde SH and Warbhe RA. Human papillomavirus prevention by vaccination: A review article. Cureus. (2022) 14:e30037. doi: 10.7759/cureus.30037
49. Lu B, Viscidi RP, Lee J-H, Wu Y, Villa LL, Lazcano-Ponce E, et al. Human papillomavirus (HPV) 6, 11, 16, and 18 seroprevalence is associated with sexual practice and age: results from the multinational HPV Infection in Men Study (HIM Study). Cancer Epidemiol Biomarkers Prev. (2011) 20:990–1002. doi: 10.1158/1055-9965.EPI-10-1160
50. Rusan M, Li YY, and Hammerman PS. Genomic landscape of human papillomavirus-associated cancers. Clin Cancer Res. (2015) 21:2009–19. doi: 10.1158/1078-0432.CCR-14-1101
51. Zhao X, Zhang Y, Trejo-Cerro O, Kaplan E, Li Z, Albertsboer F, et al. A safe and potentiated multi-type HPV L2-E7 nanoparticle vaccine with combined prophylactic and therapeutic activity. NPJ Vaccines. (2024) 9:119. doi: 10.1038/s41541-024-00914-z
52. Yee EJ, Stewart CL, Clay MR, and McCarter MM. Lipoma and its doppelganger: the atypical lipomatous tumor/well-differentiated liposarcoma. Surg Clin North Am. (2022) 102:637–56. doi: 10.1016/j.suc.2022.04.006
53. Beltran K, Wadeea R, and Herbst KL. Infections preceding the development of Dercum disease. IDCases. (2020) 19:e00682. doi: 10.1016/j.idcr.2019.e00682
54. Rydholm A and Berg NO. Size, site and clinical incidence of lipoma. Factors in the differential diagnosis of lipoma and sarcoma. Acta Orthop Scand. (1983) 54:929–34. doi: 10.3109/17453678308992936
55. Paradkar K, Krispinsky A, and Dulmage B. Cystic degeneration of a lipoma in a patient treated with CAR-T therapy. Int J Dermatol. (2023) 62:e182–4. doi: 10.1111/ijd.16097
56. Khozeimeh F, Alizadehsani R, Roshanzamir M, Khosravi A, Layegh P, and Nahavandi S. An expert system for selecting wart treatment method. Comput Biol Med. (2017) 81:167–75. doi: 10.1016/j.compbiomed.2017.01.001
57. Flatmark K, Torgunrud A, Fleten KG, Davidson B, Juul HV, Mensali N, et al. Peptide vaccine targeting mutated GNAS: a potential novel treatment for pseudomyxoma peritonei. J Immunother Cancer. (2021) 9. doi: 10.1136/jitc-2021-003109
58. Tirosh A, Jin DX, De Marco L, Laitman Y, and Friedman E. Activating genomic alterations in the Gs alpha gene (GNAS) in 274 694 tumors. Genes Chromosomes Cancer. (2020) 59:503–16. doi: 10.1002/gcc.22854
59. Tang Y, Tang S, Yang W, Zhang Z, Wang T, Wu Y, et al. MED12 loss activates endogenous retroelements to sensitise immunotherapy in pancreatic cancer. Gut. (2024) 73:1999–2011. doi: 10.1136/gutjnl-2024-332350
60. Jiang Y, Lv X, Ge X, Qu H, Zhang Q, Lu K, et al. Wilms tumor gent 1 (WT1)-specific adoptive immunotherapy in hematologic diseases. Int Immunopharmacol. (2021) 94:107504. doi: 10.1016/j.intimp.2021.107504
61. Yee C, Thompson JA, Roche P, Byrd DR, Lee PP, Piepkorn M, et al. Melanocyte destruction after antigen-specific immunotherapy of melanoma: direct evidence of t cell-mediated vitiligo. J Exp Med. (2000) 192:1637–44. doi: 10.1084/jem.192.11.1637
62. Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. (2002) 99:16168–73. doi: 10.1073/pnas.242600099
63. Schiferle EB, Cheon SY, Ham S, Son HG, Messerschmidt JL, Lawrence DP, et al. Rejection of benign melanocytic nevi by nevus-resident CD4+ T cells. Sci Adv. (2021) 7(26):eabg4498. doi: 10.1126/sciadv.abg4498
64. Tamura R, Fujioka M, Morimoto Y, Ohara K, Kosugi K, Oishi Y, et al. A VEGF receptor vaccine demonstrates preliminary efficacy in neurofibromatosis type 2. Nat Commun. (2019) 10:5758. doi: 10.1038/s41467-019-13640-1
65. Sharma M, Fu MP, Lu HY, Sharma AA, Modi BP, Michalski C, et al. Human complete NFAT1 deficiency causes a triad of joint contractures, osteochondromas, and B-cell Malignancy. Blood. (2022) 140:1858–74. doi: 10.1182/blood.2022015674
66. Vlad AM, Kettel JC, Alajez NM, Carlos CA, and Finn OJ. MUC1 immunobiology: from discovery to clinical applications. Adv Immunol. (2004) 82:249–93. doi: 10.1016/S0065-2776(04)82006-6
67. Kodama H, Suzuki M, Katayose Y, Shinoda M, Sakurai N, Takemura S, et al. Specific and effective targeting cancer immunotherapy with a combination of three bispecific antibodies. Immunol Lett. (2002) 81:99–106. doi: 10.1016/S0165-2478(01)00343-1
68. Jung H, Kim K, Kim D, Moon JH, Kim EH, Kim SH, et al. Associations of GNAS mutations with surgical outcomes in patients with growth hormone-secreting pituitary adenoma. Endocrinol Metab (Seoul). (2021) 36:342–50. doi: 10.3803/EnM.2020.875
69. Zhou W, Zhang C, Zhang D, Peng J, Ma S, Wang X, et al. Comprehensive analysis of the immunological landscape of pituitary adenomas: implications of immunotherapy for pituitary adenomas. J Neurooncol. (2020) 149:473–87. doi: 10.1007/s11060-020-03636-z
70. Lupi I, Manetti L, Caturegli P, Menicagli M, Cosottini M, Iannelli A, et al. Tumor infiltrating lymphocytes but not serum pituitary antibodies are associated with poor clinical outcome after surgery in patients with pituitary adenoma. J Clin Endocrinol Metab. (2010) 95:289–96. doi: 10.1210/jc.2009-1583
71. Ilie M-D, Vasiljevic A, Bertolino P, and Raverot G. Biological and therapeutic implications of the tumor microenvironment in pituitary adenomas. Endocr Rev. (2023) 44:297–311. doi: 10.1210/endrev/bnac024
72. Cai N, Cheng K, Ma Y, Liu S, Tao R, Li Y, et al. Targeting MMP9 in CTNNB1 mutant hepatocellular carcinoma restores CD8+ T cell-mediated antitumour immunity and improves anti-PD-1 efficacy. Gut. (2024) 73:985–99. doi: 10.1136/gutjnl-2023-331342
73. Piscuoglio S, Murray M, Fusco N, Marchiò C, Loo FL, Martelotto LG, et al. MED12 somatic mutations in fibroadenomas and phyllodes tumours of the breast. Histopathology. (2015) 67:719–29. doi: 10.1111/his.2015.67.issue-5
74. Gao X, Chen L, Zuo H, and Li Q. A novel hsa_circ_0006903 promotes tumor development and dendritic cells activated expression in infantile hemangioma. Heliyon. (2024) 10(14):e34186. doi: 10.21203/rs.3.rs-2275814/v1
75. Boscolo E, Mulliken JB, and Bischoff J. Pericytes from infantile hemangioma display proangiogenic properties and dysregulated angiopoietin-1. Arterioscler Thromb Vasc Biol. (2013) 33:501–9. doi: 10.1161/ATVBAHA.112.300929
76. Wang F-Q, Chen G, Zhu J-Y, Zhang W, Ren J-G, Liu H, et al. M2-polarised macrophages in infantile haemangiomas: correlation with promoted angiogenesis. J Clin Pathol. (2013) 66:1058–64. doi: 10.1136/jclinpath-2012-201286
77. Iwami K, Natsume A, Ohno M, Ikeda H, Mineno J, Nukaya I, et al. Adoptive transfer of genetically modified Wilms’ tumor 1-specific T cells in a novel Malignant skull base meningioma model. Neuro Oncol. (2013) 15:747–58. doi: 10.1093/neuonc/not007
78. Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. (2013) 19:1264–72. doi: 10.1038/nm.3337
79. Randic T, Kozar I, Margue C, Utikal J, and Kreis S. NRAS mutant melanoma: Towards better therapies. Cancer Treat Rev. (2021) 99:102238. doi: 10.1016/j.ctrv.2021.102238
80. Amaya CN, Wians FH, Bryan BA, and Torabi A. Enhanced expression of Programmed cell death 1 (PD-1) protein in benign vascular anomalies. Pathology. (2017) 49:292–6. doi: 10.1016/j.pathol.2016.10.015
81. Chamberlain MC. Meningiomas. Curr Treat Options Neurol. (2001) 3:67–76. doi: 10.1007/s11940-001-0025-6
82. Monleón D, Morales JM, Gonzalez-Segura A, Gonzalez-Darder JM, Gil-Benso R, Cerdá-Nicolás M, et al. Metabolic aggressiveness in benign meningiomas with chromosomal instabilities. Cancer Res. (2010) 70:8426–34. doi: 10.1158/0008-5472.CAN-10-1498
83. Fang L, Lowther DE, Meizlish ML, Anderson RCE, Bruce JN, Devine L, et al. The immune cell infiltrate populating meningiomas is composed of mature, antigen-experienced T and B cells. Neuro Oncol. (2013) 15:1479–90. doi: 10.1093/neuonc/not110
84. Han SJ, Reis G, Kohanbash G, Shrivastav S, Magill ST, Molinaro AM, et al. Expression and prognostic impact of immune modulatory molecule PD-L1 in meningioma. J Neurooncol. (2016) 130:543–52. doi: 10.1007/s11060-016-2256-0
85. Chen H, Jiang L, Zhang D, Chen J, Luo X, Xie Y, et al. Exploring the correlation between the regulation of macrophages by regulatory T cells and peripheral neuropathic pain. Front Neurosci. (2022) 16:813751. doi: 10.3389/fnins.2022.813751
86. Ho SY and Kveton JF. Acoustic neuroma. Assessment and management. Otolaryngol Clin North Am. (2002) 35:393–404, viii. doi: 10.1016/S0030-6665(02)00004-X
87. Bachir S, Shah S, Shapiro S, Koehler A, Mahammedi A, Samy RN, et al. Neurofibromatosis type 2 (NF2) and the implications for vestibular schwannoma and meningioma pathogenesis. Int J Mol Sci. (2021) 22(2):690. doi: 10.3390/ijms22020690
88. Tang N, Cheng L, Hao J, Xu B, Pan X, Wei X, et al. Development of CAR-T cell therapy for NF1/SWN-related nerve sheath tumor treatment. Acta Neuropathol Commun. (2025) 13:45. doi: 10.1186/s40478-025-01965-6
89. Mohamed T, Melfi V, Colciago A, and Magnaghi V. Hearing loss and vestibular schwannoma: new insights into Schwann cells implication. Cell Death Dis. (2023) 14:629. doi: 10.1038/s41419-023-06141-z
90. Barrett TF, Patel B, Khan SM, Mullins RDZ, Yim AKY, Pugazenthi S, et al. Single-cell multi-omic analysis of the vestibular schwannoma ecosystem uncovers a nerve injury-like state. Nat Commun. (2024) 15:478. doi: 10.1038/s41467-023-42762-w
91. Nickl V, Ziebolz D, Rumpel C, Klein D, Nickl R, Rampeltshammer E, et al. Analysis of tumor microenvironment composition in vestibular schwannomas: insights into NF2-associated and sporadic variations and their clinical correlations. Front Oncol. (2024) 14:1340184. doi: 10.3389/fonc.2024.1340184
92. Yidian C, Chen L, Hongxia D, Yanguo L, and Zhisen S. Single-cell sequencing reveals the cell map and transcriptional network of sporadic vestibular schwannoma. Front Mol Neurosci. (2022) 15:984529. doi: 10.3389/fnmol.2022.984529
93. Plotkin SR, Stemmer-Rachamimov AO, Barker FG, Halpin C, Padera TP, Tyrrell A, et al. Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. N Engl J Med. (2009) 361:358–67. doi: 10.1056/NEJMoa0902579
94. Bovée JVMG. Multiple osteochondromas. Orphanet J Rare Dis. (2008) 3:3. doi: 10.1186/1750-1172-3-3
95. Sarrazin S, Lamanna WC, and Esko JD. Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol. (2011) 3(7):a004952. doi: 10.1101/cshperspect.a004952
96. Zak BM, Crawford BE, and Esko JD. Hereditary multiple exostoses and heparan sulfate polymerization. Biochim Biophys Acta. (2002) 1573:346–55. doi: 10.1016/S0304-4165(02)00402-6
97. Wehenkel M, Corr M, Edwards B, Calabrese C, Pagès G, Pouyssegur J, et al. Dysregulation of ERK signaling in CD4-expressing cells induces osteochondromas (IRC8P.441). J Immunol. (2015) 194:129.5–5. doi: 10.4049/jimmunol.194.Supp.129.5
98. Skalsky RL and Cullen BR. EBV noncoding RNAs. Curr Top Microbiol Immunol. (2015) 391:181–217. doi: 10.1007/978-3-319-22834-1_6
99. Movahed F, Darzi S, Mahdavi P, Salih Mahdi M, Qutaiba B Allela O, Naji Sameer H, et al. The potential use of therapeutics and prophylactic mRNA vaccines in human papillomavirus (HPV). Virol J. (2024) 21:124. doi: 10.1186/s12985-024-02397-9
100. Lam B, Miller J, Kung YJ, Wu TC, Hung C-F, Roden RBS, et al. Profiling of VEGF receptors and immune checkpoints in recurrent respiratory papillomatosis. Laryngoscope. (2024) 134:2819–25. doi: 10.1002/lary.v134.6
101. Silence C, Rice SM, Liteplo A, McFadden K, Al Jalbout N, Al Saud AA, et al. Deoxycholic acid for dercum disease: repurposing a cosmetic agent to treat a rare disease. Cutis. (2023) 111:E4–8. doi: 10.12788/cutis
102. Rodriguez-Perdigon M, Jimaja S, Haeni L, Bruns N, Rothen-Rutishauser B, and Rüegg C. Polymersomes-mediated delivery of CSF1R inhibitor to tumor associated macrophages promotes M2 to M1-like macrophage repolarization. Macromol Biosci. (2022) 22:e2200168. doi: 10.1002/mabi.202200168
103. Garzon-Muvdi T, Bailey DD, Pernik MN, and Pan E. Basis for immunotherapy for treatment of meningiomas. Front Neurol. (2020) 11:945. doi: 10.3389/fneur.2020.00945
104. Azab MA, Cole K, Earl E, Cutler C, Mendez J, and Karsy M. Medical management of meningiomas. Neurosurg Clin N Am. (2023) 34:319–33. doi: 10.1016/j.nec.2023.02.002
105. Sioka C and Kyritsis AP. Chemotherapy, hormonal therapy, and immunotherapy for recurrent meningiomas. J Neurooncol. (2009) 92:1–6. doi: 10.1007/s11060-008-9734-y
106. Dai C, Liang S, Sun B, and Kang J. The progress of immunotherapy in refractory pituitary adenomas and pituitary carcinomas. Front Endocrinol (Lausanne). (2020) 11:608422. doi: 10.3389/fendo.2020.608422
107. Krause KJ, Goldrich D, and Gniady J. Benralizumab as an adjuvant therapy for recurrent laryngeal papillomatosis. Laryngoscope. (2023) 133:863–5. doi: 10.1002/lary.v133.4
108. Halkola AS, Aittokallio T, and Parvinen K. Tumor microenvironment as a metapopulation model: The effects of angiogenesis, emigration and treatment modalities. J Theor Biol. (2022) 545:111147. doi: 10.1016/j.jtbi.2022.111147
109. Hannan CJ, Lewis D, O’Leary C, Donofrio CA, Evans DG, Stapleton E, et al. Beyond antoni: A surgeon’s guide to the vestibular schwannoma microenvironment. J Neurol Surg B Skull Base. (2022) 83:1–10. doi: 10.1055/s-0040-1716688
110. Ihara F, Sakurai D, Takami M, Kamata T, Kunii N, Yamasaki K, et al. Regulatory T cells induce CD4- NKT cell anergy and suppress NKT cell cytotoxic function. Cancer Immunol Immunother. (2019) 68:1935–47. doi: 10.1007/s00262-019-02417-6
111. Ghiasi MM and Zendehboudi S. Decision tree-based methodology to select a proper approach for wart treatment. Comput Biol Med. (2019) 108:400–9. doi: 10.1016/j.compbiomed.2019.04.001
112. Gross G. Genitoanal human papillomavirus infection and associated neoplasias. Curr Probl Dermatol. (2014) 45:98–122. doi: 10.1159/000358423
113. Kainer RA. Current concepts in the treatment of bovine ocular squamous cell tumors. Vet Clin North Am Large Anim Pract. (1984) 6:609–22. doi: 10.1016/S0196-9846(17)30013-7
114. Ederer IA, Lucca I, Hofbauer SL, Haidinger M, Haitel A, Susani M, et al. Histopathology and prognosis of de novo bladder tumors following solid organ transplantation. World J Urol. (2015) 33:2087–93. doi: 10.1007/s00345-015-1554-z
115. Klarquist J, Barfuss A, Kandala S, Reust MJ, Braun RK, Hu J, et al. Melanoma-associated antigen expression in lymphangioleiomyomatosisrenders tumor cells susceptible to cytotoxic T cells. Am J Pathol. (2009) 175(6):2463-72. doi: 10.2553/ajpath.2009.090525
116. Han F, Dellacecca ER, Barse LW, Cosgrove C, Henning SW, Ankney CM, et al. Adoptive T-cell transfer to treat lymphangioleiomyomatosis. Am J Respir Cell Mol Biol. (2020) 62:793–804. doi: 10.1165/rcmb.2019-0117OC
117. Liu H-J, Lizotte PH, Du H, Speranza MC, Lam HC, Vaughan S, et al. TSC2-deficient tumors have evidence of T cell exhaustion and respond to anti–PD-1/anti–CTLA-4 immunotherapy. JCI Insight. (2018) 3(8):e98674. doi: 10.1172/jci.insight.98674
Keywords: benign tumors, mutations, antigens, immunotherapy, checkpoint inhibitors, tumor vaccines, adoptive T cell therapy
Citation: Youssef MA, Al-Sharif H, McGrath BT, Picken MM and Le Poole IC (2025) Benign tumors broaden the field of application for immunotherapy. Front. Immunol. 16:1593960. doi: 10.3389/fimmu.2025.1593960
Received: 15 March 2025; Accepted: 03 June 2025;
Published: 24 June 2025.
Edited by:
Marina Yiasemidou, University of York, United KingdomReviewed by:
Elizabeth P. Henske, Brigham and Women’s Hospital and Harvard Medical School, United StatesAndrei Chitul, Barts Health NHS Trust, United Kingdom
Copyright © 2025 Youssef, Al-Sharif, McGrath, Picken and Le Poole. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: I. Caroline Le Poole, Y2Fyb2xpbmUubGVwb29sZUBub3J0aHdlc3Rlcm4uZWR1
†These authors have contributed equally to this work and share first authorship
 Mohamed A. Youssef
Mohamed A. Youssef Hisham Al-Sharif
Hisham Al-Sharif Brian T. McGrath
Brian T. McGrath Maria M. Picken
Maria M. Picken I. Caroline Le Poole
I. Caroline Le Poole