- 1Department of Hematology, First Affiliated Hospital of Nanjing Medical University, Nanjing, China
- 2Department of Oncology, First Affiliated Hospital of Nanjing Medical University, Nanjing, China
- 3Department of Oncology, The Affiliated Suqian First People’s Hospital of Nanjing Medical University, Suqian, China
Editorial on the Research Topic
Vital role of innate immunity in cancer immunotherapy
The complement system, a cornerstone of innate immunity, acts as a ‘double-edged sword’ in cancer immunotherapy (1). As highlighted by Yang et al., it can both combat malignancy by enhancing the efficacy of therapeutic antibodies and promote tumor progression by shaping the tumor microenvironment (TME). This duality depends on factors such as cell type-specific complement distribution, signaling balance within the TME, and tumor proliferation dynamics (Figure 1). For example, in lung cancer, cytotoxic T cells efficiently eliminate tumor cells expressing strong antigens. In contrast, in pancreatic cancer, the same antigens may accelerate disease progression due to a lack of dendritic cells needed for T-cell activation (2). Therefore, the complement system may contribute, directly or indirectly, to the development of drug resistance and reduce the effectiveness of cancer therapies.
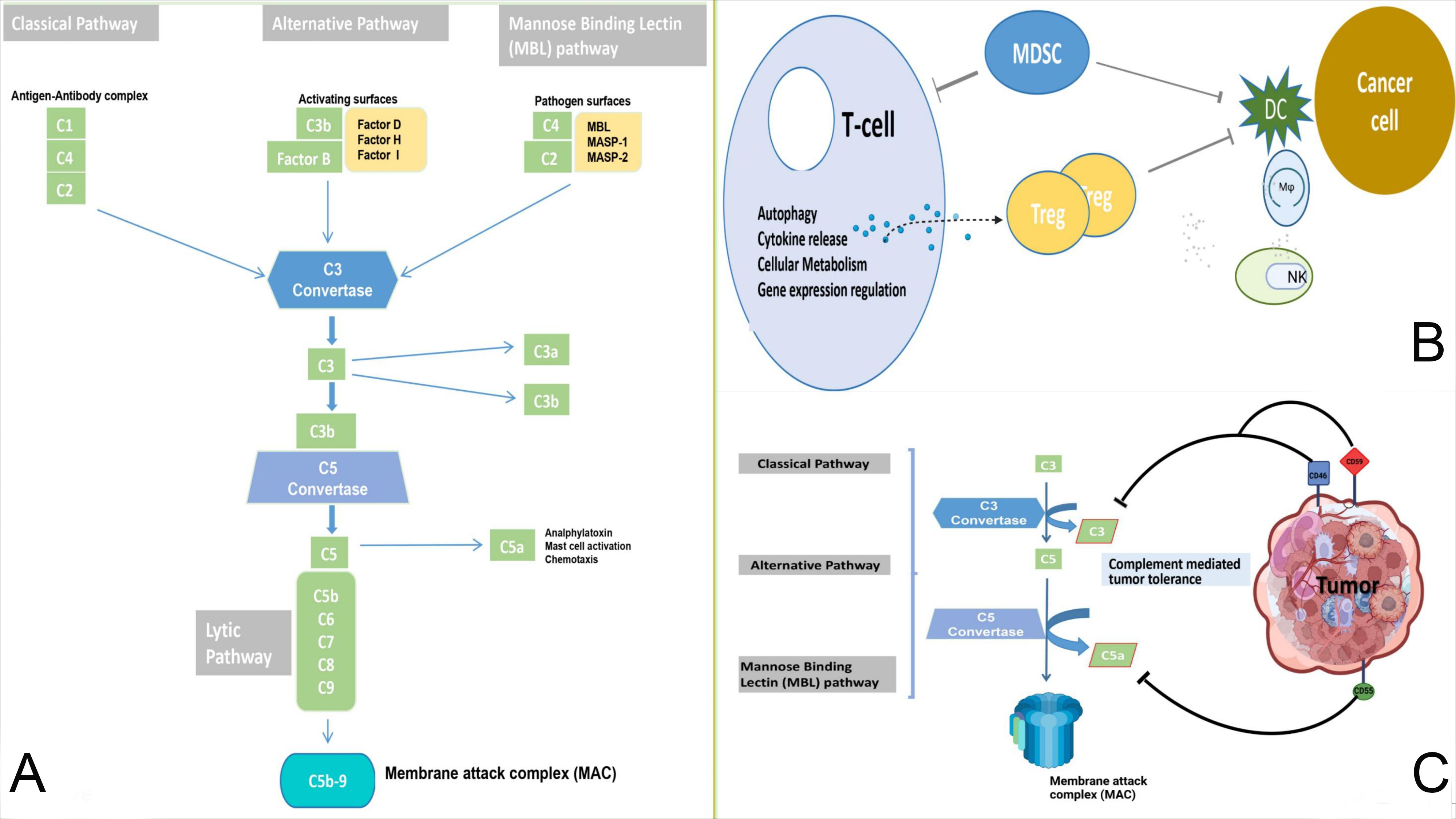
Figure 1. (A): Normal anti-tumor immunity and complement pathways. The classical, lectin, and alternative pathways are used to form C3 convertase and C5 convertase, which results in the cleavage of C5 into C5a and C5b. This process facilitates the formation of MAC and cytotoxicity against cancer cells. (B, C): The complement system mediates tumor immune tolerance. Complement components and inhibitory receptors are produced by cancer cells to facilitate their growth and suppress the immune response. These components contribute to the establishment of an immunosuppressive environment, promote Treg differentiation, and influence T cells. Additionally, they impede the function of antigen-presenting cells, recruit MDSCs, and influence macrophage polarization and NK cell function. Mφ, macrophage; MDSCs, myeloid-derived suppressor cells; DC, dendritic cells; NK, natural killer cell.
This context-dependent plasticity of innate immune cells underscores the need to unravel their dual mechanisms. Such insights could drive innovations in biomarkers and therapies, i.e., unlocking novel T cell-based immunotherapies that interconnect innate and adaptive immunity. These cells produce pro-inflammatory cytokines (e.g., IFN-γ, IL-12, IL-18) and exhibit innate-like cytotoxicity while retaining adaptive features via T-cell receptors (TCRs) (3). The development of immunotherapy has transformed the clinical management of cancer, positioning it as a key strategy that activates innate immunity and promotes durable, protective T-cell responses. Immunotherapies, including cell-based treatments, immune checkpoint inhibitors (ICIs), and tumor vaccines, have demonstrated substantial anticancer activity (4). Despite these advances, long-term cancer control remains challenging. Further research is needed to overcome current limitations and to identify new therapeutic targets that can expand the effectiveness and applicability of immunotherapy.
Decroos et al. investigated CD8+ T cells in leukemia patients who achieved treatment-free remission (TFR) despite the persistence of residual cancer cells. Their study revealed a strong correlation between elevated natural killer (NK) cell ativity—key anti-tumor effectors—and TFR. They also identified a subset of innate CD8+ T cells co-expressing Eomesodermin and inhibitory markers (KIR/NKG2A) that exhibited enhanced cytotoxicity through perforin upregulation. In addition, reduced PD-1 expression on effector T cells was found to serve as a predictive biomarker for TFR in chronic myeloid leukemia (CML). Although these findings are promising, their applicability to other hematological malignancies beyond CML remains limited, particularly when targeting ICIs such as anti-PD-1. A more established role of innate CD8+ T cells has been observed in solid tumors, including ovarian cancer (5). Integrating past and current insights from leading journals such as Frontiers in Immunology may stimulate new ideas and collaborative initiatives to advance translational research and further elucidate the critical role of innate immunity in cancer immunotherapy.
In clinical practice, cancer patients often develop resistance to therapy, highlighting the need for improved immunotherapeutic strategies. Although immune checkpoint therapies activate immune cells by modulating cytokines and chemokines, they can also promote tumorigenesis through changes in the TME and can cause systemic toxicity. An ideal cancer immunotherapy should stimulate both systemic innate and adaptive immune responses while minimizing systemic damage. Therefore, targeting both innate and adaptive mechanisms is critical to establishing a durable and effective anti-tumor immune response (6).
The immune system eliminates disease by recognizing common pathogen components and activating innate and adaptive immune pathways, which are also essential for cancer immunosurveillance, often mediated by cytokines and chemokines. Xiao et al. conducted a comprehensive review of publications from 2006 to 2024, identifying emerging strategies involving neutrophil extracellular traps (NETs) and bacterial therapies. NETs, once thought to be solely protective against infection, are now implicated in cancer progression. This review highlights the role of NETs in promoting an immunosuppressive TME, positioning NET-targeted therapies as a promising frontier in cancer immunotherapy. Historically, systemic bacterial administration has faced major toxicity challenges. For example, Coley demonstrated that intravenous (i.v.) administration was effective but often caused uncontrollable toxicity. Encouragingly, modern approaches have shown greater promise, as illustrated by the FDA-approved Bacillus Calmette-Guérin (BCG) vaccine, derived from attenuated Mycobacterium bovis, which has demonstrated clinical efficacy in the treatment of bladder cancer (7–9).
Newman proposed the development of a systemically administered, attenuated, and killed bacteria-based multi-immune receptor agonist for anti-tumor immunotherapy. The study identified Toll-like receptors (TLRs) and their related agonists, with particular emphasis on the potent TLR4 agonist, lipopolysaccharide (LPS), which constitutes approximately 75% of the outer membrane of Gram-negative bacteria (G-NB). LPS was shown to serve as both a critical active component and a major contributor to the dose-limiting intravenous toxicity associated with G-NB. The study further demonstrated that attenuated, stabilized, intact bacterial products derived from non-pathogenic G-NB retained approximately 96% of their endotoxin activity. Notably, Decoy10, a formulation with agonist activity for TLR2, TLR4, TLR8, TLR9, NOD2, and STING, showed reduced intravenous toxicity in both mice and rabbits while preserving the ability to stimulate cytokine and chemokine secretion by human immune cells in vitro, comparable to unprocessed parental bacterial cells.
Using bacteria as delivery systems for cancer therapy offers a promising approach to achieve long-term protection through the induction of immunological memory, thereby supporting sustained cancer control. Nevertheless, further research and development are required to fully realize the potential of this strategy in immunotherapy.
These studies underscore the critical role of innate immunity in shaping the tumor microenvironment and advancing immunotherapy. They have identified additional targets that may overcome therapeutic resistance and improve treatment efficacy. The discovery of novel targets involving CD8+ T cells, NETs, and attenuated bacterial therapies may drive future research in this field. Ongoing investigations into the complexity of the innate immune system in cancer biology suggest that new therapeutic strategies may further enhance immunotherapy approaches. Moving forward, it will be essential to conduct research that builds on these findings to translate our expanding understanding of innate immunity and cancer immunotherapy into effective clinical applications.
Author contributions
SL: Writing – original draft, Writing – review & editing. JW: Writing – original draft, Writing – review & editing. XZ: Writing – review & editing, Writing – original draft.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lu P, Ma Y, Wei S, and Liang X. The dual role of complement in cancers, from destroying tumors to promoting tumor development. Cytokine. (2021) 143:155522. doi: 10.1016/j.cyto.2021.155522
2. Maiorino L, Dassler-Plenker J, Sun L, and Egeblad M. Innate immunity and cancer pathophysiology. Annu Rev Pathol. (2022) 17:425–57. doi: 10.1146/annurev-pathmechdis-032221-115501
3. Gutierrez-Arcelus M, Teslovich N, Mola AR, Polidoro RB, Nathan A, Kim H, et al. Lymphocyte innateness defined by transcriptional states reflects a balance between proliferation and effector functions. Nat Commun. (2019) 10:687. doi: 10.1038/s41467-019-08604-4
4. Sareen G, Mohan M, Mannan A, Dua K, and Singh TG. A new era of cancer immunotherapy: vaccines and miRNAs. Cancer Immunol Immunother. (2025) 74:163. doi: 10.1007/s00262-025-04011-5
5. Chap BS, Rayroux N, Grimm AJ, Ghisoni E, and Dangaj Laniti D. Crosstalk of T cells within the ovarian cancer microenvironment. Trends Cancer. (2024) 10:1116–30. doi: 10.1016/j.trecan.2024.09.001
6. Zhang Y, Xue W, Xu C, Nan Y, Mei S, Ju D, et al. Innate immunity in cancer biology and therapy. Int J Mol Sci. (2023) 24:11233. doi: 10.3390/ijms241411233
7. Morales A, Eidinger D, and Bruce AW. Intracavitary bacillus calmette-guerin in the treatment of superficial bladder tumors. J Urol. (2017) 197:S142–5. doi: 10.1016/j.juro.2016.10.101
8. Sieow BF, Wun KS, Yong WP, Hwang I Y, and Chang MW. Tweak to treat: reprograming bacteria for cancer treatment. Trends Cancer. (2021) 7:447–64. doi: 10.1016/j.trecan.2020.11.004
Keywords: innate immunity, complement, biomarkers, cancer, neutrophil extracellular traps, tumor microenvironment, immunotherapy, toll-like receptor
Citation: Luthuli S, Wu J and Zhou X (2025) Editorial: Vital role of innate immunity in cancer immunotherapy. Front. Immunol. 16:1594009. doi: 10.3389/fimmu.2025.1594009
Received: 15 March 2025; Accepted: 05 May 2025;
Published: 21 May 2025.
Edited and Reviewed by:
Peter Brossart, University of Bonn, GermanyCopyright © 2025 Luthuli, Wu and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiazhu Wu, d3VqaWF6aHUwOUBzaW5hLmNvbQ==; Xin Zhou, emhvdXhpbjU1MjNAanNwaC5vcmcuY24=
 Sibusiso Luthuli1
Sibusiso Luthuli1 Jiazhu Wu
Jiazhu Wu Xin Zhou
Xin Zhou