- 1Department of Clinical Pharmacy, Xiangtan Central Hospital (The Affiliated Hospital of Hunan University), Xiangtan, China
- 2Department of Infection Control, Xiangtan Central Hospital (The Affiliated Hospital of Hunan University), Xiangtan, China
Over the past decade, inflammasome and cGAS-STING have received significant attention as immune components that coordinate host immune homeostasis. Although the inflammasome and cGAS-STING are relatively independent immune signaling pathways, some studies have pointed out that there is an important association between cGAS-STING and the inflammasome. As the downstream of cGAS-STING, inflammasome plays an important role in many diseases. In this mini-review, we review the association between CGAS-STING and NLRP3 inflammasome, the progression of the cGAS/STING/NLRP3 signaling pathway in the pathogenesis of disease, the therapeutic strategies that targeted cGAS/STING/NLRP3 signaling pathway. We believe that targeting the cGAS/STING/NLRP3 signaling pathway in the future has broad prospects and significance for the treatment of disease.
1 Introduction
In 2008, American scientist Ishikawa H and his team found a transmembrane protein “Stimulator of Interferon Genes (STING)” (1). STING, also known as TMEM173, MITA, ERIS, and MPYS, is composed of 379 amino acids and has a molecular weight of 42 kU (2). STING is widely distributed in the endoplasmic reticulum of mammalian immune cells. Meanwhile, STING plays an important role in regulating the innate immune response. Activation of STING confers host immunity and is key to clearing a wide range of pathogens (viruses and bacteria), while its absence causes cells to fail to respond to cytoplasmic DNA or certain bacterial components (3). Subsequently, in 2013, Sun L and his team discovered that cytoplasmic DNA catalyzes the production of cGAMP by activating cyclic GMP-AMP synthase (cGAS) (4). cGAS, also known as C6ORF150 or MB21D1, is composed of 522 amino acids and has a molecular weight of 60 kU (5). As an innate immune sensor for cytoplasmic double-stranded DNA (dsDNA), the C-terminal of cGAS contains a nucleotide transferase domain for catalysis and multiple DNA binding sites (6). The inflammasome is a multiprotein complex in the cytoplasm. Meanwhile, it is also a relatively important part of the immune system. Over the past decade, inflammasome and cGAS-STING have received significant attention as immune components that coordinate host immune homeostasis. Although the inflammasome and cGAS-STING are relatively independent immune signaling pathways, some studies have pointed out that there is an important association between cGAS-STING and the inflammasome. As the downstream of cGAS-STING, inflammasome plays an important role in many diseases. In this mini-review, we review the association between cGAS-STING and NLRP3 inflammasome, the progression of the cGAS/STING/NLRP3 signaling pathway in the pathogenesis of disease, the therapeutic strategies that targeted cGAS/STING/NLRP3 signaling pathway. We believe that targeting the cGAS/STING/NLRP3 signaling pathway in the future has broad prospects and significance for the treatment of disease.
2 cGAS/STING signaling pathway
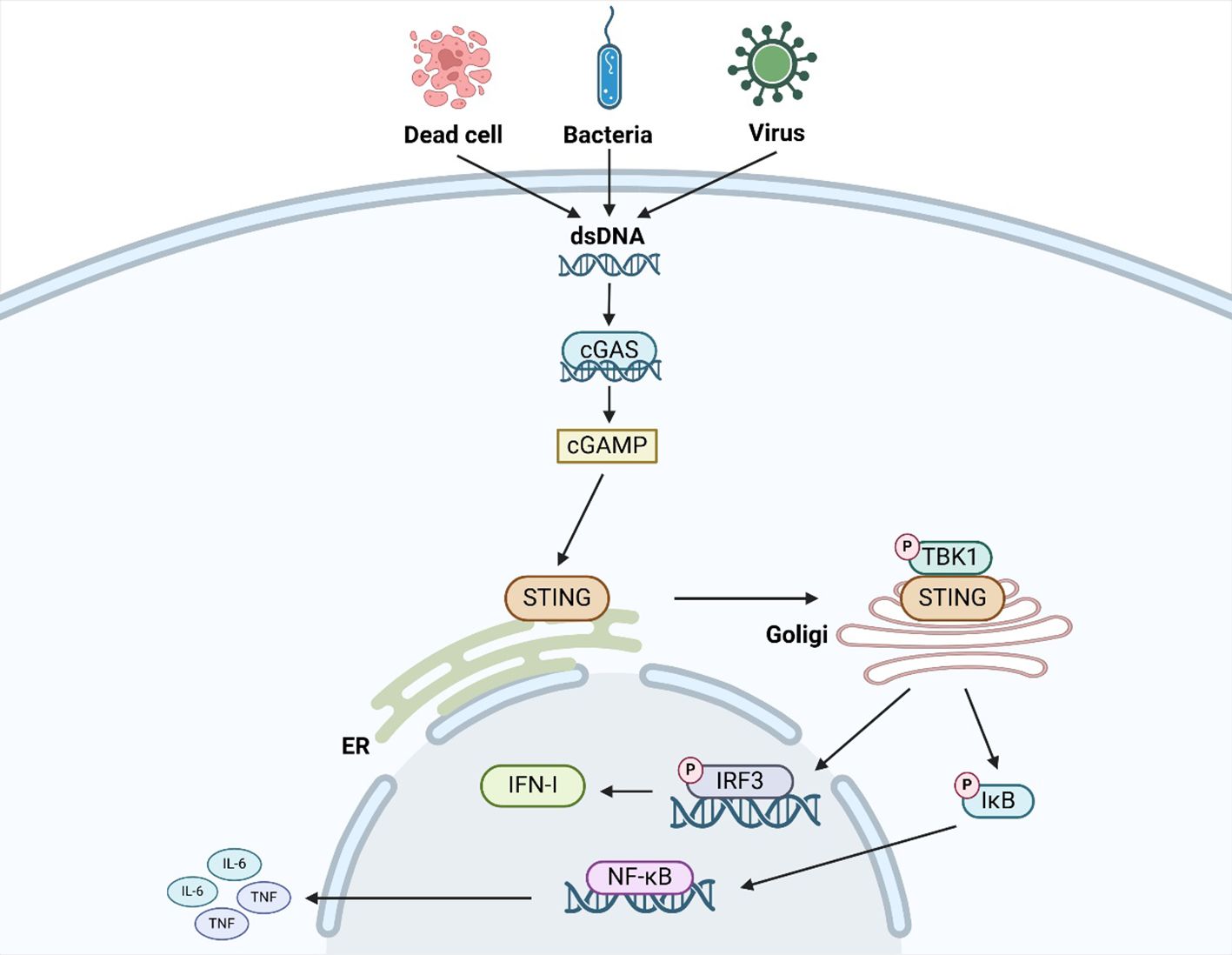
More and more studies have found that the cGAS/STING signaling pathway plays an important role in infectious diseases, neurodegenerative diseases, autoimmune diseases, inflammatory diseases, liver diseases, cardiovascular diseases, and tumors (7, 8). Therefore, it is very important for us to understand the mechanism of the cGAS/STING signaling pathway (Figure 1). cGAS is a key component in the cGAS/STING signaling pathway. By recognizing and binding the dsDNA, cGAS converts adenosine triphosphate(ATP) and guanosine triphosphate(GTP) into 2 ‘-3’ ring GMP-AMP(cGAMP) (9). As a second messenger, cGAMP can directly bind to STING. After binding to cGAMP, STING dimer undergoes a transition from an inactive state to an active state and triggers its translocation from the endoplasmic reticulum to the Golgi apparatus (9). STING is phosphorylated by TBK1, leading to recruitment of IRF3. After being phosphorylated by TBK1, IRF3 enters the nucleus to drive type I interferon(IFN-I)expression. Meanwhile, STING also recruits IκB kinase (IKK), which phosphorylates iκBα (an inhibitor of NF-κB), resulting in the translocation of NF-κB to the nucleus and expression of pro-inflammatory cytokines (such as IL-6, TNF) (10). As mentioned above, The cGAS/STING/TBK1/IRF3 signaling pathway is the canonical cGAS/STING signaling pathway. This signaling pathway is characterized by the activation of IFN-I and inflammatory factors. Therefore, targeting this signaling pathway plays an important role in improving viral infections, tumors, autoimmune diseases, and inflammatory diseases. In addition to the canonical cGAS/STING signaling pathway, many noncanonical cGAS/STING signaling pathways have also been revealed in recent years. For example, Zhang D and his colleagues identified a noncanonical signaling pathway mediated by STING protein on the endoplasmic reticulum: the cGAS/STING/PERK/eIF2α signaling pathway (7). STING at the ER binds and directly activates the ER-located kinase PERK via their intracellular domains, which precedes TBK1–IRF3 activation and is irrelevant to the unfolded protein response. The activated PERK phosphorylates eIF2α, forming an inflammatory program. Inhibiting this signaling pathway can significantly improve cellular senescence, the pathological process of pulmonary fibrosis, and the pathological process of liver fibrosis. Liu D and his colleagues identified a noncanonical signaling pathway mediated by STING protein on endoplasmic reticulum Golgi intermediates (ERGIC): cGAS/STING/LC3-mediated autophagy (8). As a potential autophagy receptor, STING promotes the esterification reaction of LC3 and induces the occurrence of autophagy. Inhibiting this signaling pathway can significantly improve viral infections and inflammatory diseases. In short, the cGAS/STING signaling pathway is one of the most popular signaling pathways in recent years. It is of great significance to explore the mechanism and downstream pathway of this signaling pathway in these diseases.
3 NLRP3 inflammasome
The inflammasome is a multiprotein complex in the cytoplasm. Meanwhile, it is also a relatively important part of the immune system. The inflammasome includes NLRP1, NLRP2, NLRP3, NLRP6, NLRP12, NLRC4, AIM2. Among them, the NLRP3 inflammasome is the most studied and popular inflammasome (11). The NLRP3 inflammasome is composed of NLRP3, ASC, and pro-Caspase-1 (12). As a sensor for danger signals, NLRP3 inflammasome can be activated by pathogen-associated molecular patterns(PAMPs) and damage-associated molecular patterns(DAMPs) and then induce the maturation of pro-IL-1β and pro-IL-18 (12). In the physiological state, intracellular NLRP3 and downstream(pro-IL-1β and pro-IL-18) are at extremely low levels to maintain a low inflammatory state (13). When PAMPs or DAMPs are recognized by the corresponding PRRs, NF-κB nuclear translocation is triggered, and then the transcriptional expression of NLRP3, IL-1β, and IL-18 genes is activated (13). Oligomerization of the NLRP3 structural protein causes it to bind to the PYD of the splice protein ASC, and then the CARD of ASC binds to the CARD of pro-Caspase-1 to form an active NLRP3 inflammasome. The NLRP3 inflammasome promotes the self-cleavage of pro-Caspase-1 to produce active Caspase-1. Caspase-1 has the function of cutting GSDMD. On the one hand, Caspase-1 directly cleaved GSDMD to initiate pyroptosis (14). On the other hand, Caspase-1 can also induce the conversion of pro-IL-1β and pro-IL-18 to IL-1β and IL-18, which are secreted outside the cell to induce inflammation. This process is induced by a number of endogenous and exogenous agonists(ATP, cholesterol, silica, alum, amyloid). These agonists may activate the NLRP3 inflammasome through a number of common signaling pathways. At present, the activation pathways of NLRP3 inflammasome mainly include endoplasmic reticulum stress, mitochondrial damage, and lysosome damage (14). As an important substance in immune regulation, NLRP3 plays an important role in various diseases. Therefore, it is very interesting and meaningful for us to explore the relationship between the cGAS/STING signaling pathway and NLRP3.
4 cGAS/STING signaling pathway and NLRP3
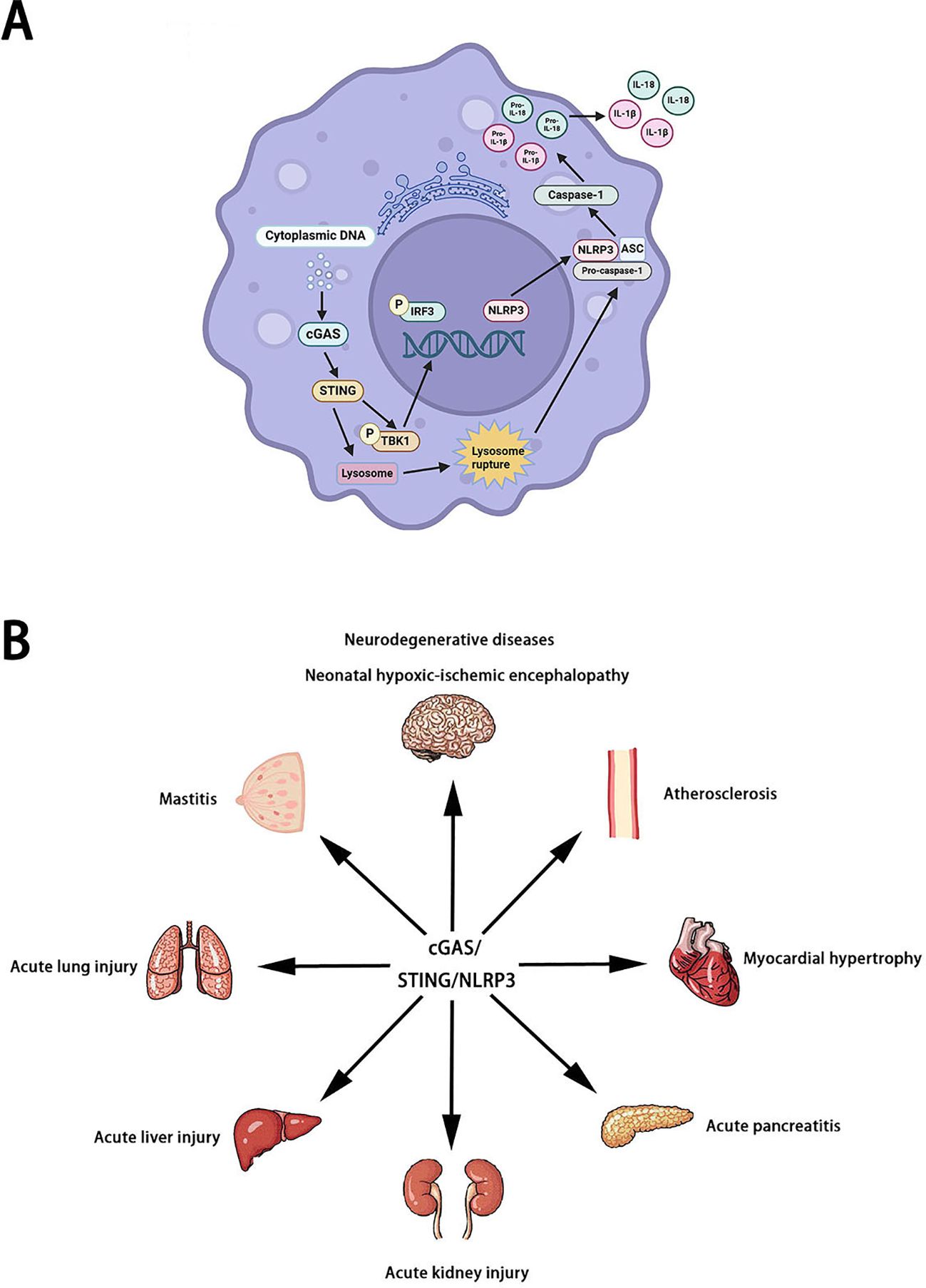
At present, more and more studies have proved that NLRP3 is the downstream of the cGAS/STING signaling pathway. NLRP3 is important for the cGAS/STING signaling pathway to perform various roles (15). NLRP3 deficiency increased the production of IFN-I (16). In neutrophils of sepsis mice, cGAS/STING signaling pathway can induce the activation of NLRP3 inflammasome to promote neutrophil pyroptosis (17). In myelodysplastic syndromes, cGAS/STING signaling pathway can activate the NLRP3 inflammasome by inducing ISG (18). STING/NLRP3 signaling pathway can also participate in lipopolysaccharide-induced inflammation and pyroptosis by activating NLRP3. In wild-type mice and cardiomyocytes, lipopolysaccharide stimulated the binding of STING to IRF3 and phosphorylate IRF3. Phosphorylated IRF3 is subsequently translocated into the nucleus and increases the expression of NLRP3 (19). Therefore, it is very interesting and meaningful to explore how the cGAS/STING signaling pathway acts on NLRP3. Available studies indicate that STING can promote the activation of NLRP3 inflammasome in a variety of ways under the stimulation of cytoplasmic DNA. On the one hand, STING activates NLRP3 inflammasome by recruiting NLRP3 and deubiquitinating NLRP3 (20). On the other hand, STING is activated and transported to lysosomes, triggering membrane penetration and leading to lysosomal cell death. After the death of lysozyme cells, the lysed cathepsin leaks into the cytoplasm, changes the permeability of the plasma membrane, activates K+ efflux(upstream of NLRP3), and finally induces pyroptosis (21, 22). In conclusion, NLRP3 is a potential downstream target for the cGAS/STING signaling pathway (Figure 2).
5 cGAS/STING/NLRP3 signaling pathway and diseases
Neurodegenerative diseases are a class of diseases characterized by the gradual death of neurons. According to the different pathogenesis, neurodegenerative diseases can be divided into Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and Amyotrophic lateral sclerosis. More and more studies have shown that the cGAS/STING/NLRP3 signaling pathway plays an important role in Neurodegenerative diseases. Harmful inflammatory environments contribute to the progression of neurodegenerative disorders. Serum/glucocorticoid related kinase 1 (SGK1), a Serine/Threonine protein kinase, is widely expressed in the heart, brain, liver, kidney, and other tissues. Previous studies have shown that SGK1 is involved in the pathogenesis of various neurodegenerative diseases. Kwon OC et al. found that SGK1 inhibition corrects the pro-inflammatory properties by regulating the cGAS/STING/NLRP3 signaling pathway (23). In sevoflurane-induced cognitive dysfunction mice, the cGAS/STING/NLRP3 signaling pathway was activated to induce neuroinflammation (24). SENP7 (SUMO specific peptidase 7) is a multifunctional enzyme. Liu L found that SENP7 induced neuronal apoptosis by regulating the cGAS/STING/NLRP3 signaling pathway in sevoflurane-induced cognitive dysfunction mice (25). Heavy metal pollution is a growing global problem that poses significant risks to the environment and human health. Copper exposure-induced neurotoxicity and manganese exposure-induced neurotoxicity may be associated with excessive activation of the cGAS/STING/NLRP3 signaling pathway (26, 27). Notably, cadmium exposure-induced pulmonary toxicity may be associated with excessive activation of the cGAS/STING/NLRP3 signaling pathway (28). Some other environmental pollutants, such as hexafluoropropylene oxide trimer acid and polystyrene micronanomaterials, can also induce liver fibrosis and spermatogenesis dysfunction in mice by regulating the cGAS/STING/NLRP3 signaling pathway (29, 30). In addition to neurodegenerative diseases, inhibition of the cGAS/STING/NLRP3 signaling pathway can also improve neonatal hypoxic-ischemic encephalopathy and cerebral venous sinus thrombosis (31, 32). Cardiovascular disease (CVD) is a disease involving the heart or blood vessels, including atherosclerosis, heart failure, myocardial infarction, cardiomyopathy, arrhythmia, and coronary heart disease. In diabetic cardiomyopathy mice, free fatty acids promoted myocardial hypertrophy by activating cGAS/STING/NLRP3 signaling pathway (33). In myocardial infarction patients, high levels of dsDNA directly potentiate platelet activation via the cGAS/STING/NLRP3 signaling pathway (34). IQ motif-containing GTPase-activating protein1 (IQGAP1) is an important scaffolding protein. An C et al. found that IQGAP1 induces endothelial cell pyroptosis leading to atherosclerosis via the cGAS/STING/NLRP3 signaling pathway (35). In addition to neurodegenerative diseases and cardiovascular diseases, the cGAS/STING/NLRP3 signaling pathway is involved in the pathological process of acute pancreatitis (36), mastitis (37), acute kidney injury (38), acute liver injury (39), acute lung injury (40), systemic lupus erythematosus (41), and allergic rhinitis (42). Unfortunately, only a few studies have explored the relationship between the cGAS/STING/NLRP3 signaling pathway and disease. Therefore, it is very promising to explore the mechanism and relationship of the cGAS/STING/NLRP3 signaling pathway in various diseases in the future.
6 cGAS/STING/NLRP3 signaling pathway and therapeutic strategies
Because the cGAS/STING/NLRP3 signaling pathway plays an important role in the pathogenesis of various diseases, more and more studies are beginning to explore therapeutic strategies that target the cGAS/STING/NLRP3 signaling pathway. As we all know, natural products have the advantages of good efficacy, low toxicity, low cost, wide source, and high yield in the treatment of diseases. Gao D et al. found that tetrahydroxy stilbene glucoside inhibited neuroinflammation through the cGAS/STING/NLRP3 signaling pathway (43). Lai Y et al. found that Dihydrocapsaicin promoted the perforator flap survival by suppressing the cGAS/STING/NLRP3 signaling pathway (44). Tian Y et al. found that epigallocatechin gallate exerts anti-inflammatory activity by inhibiting the cGAS/STING/NLRP3 signaling pathway (45). Saikosaponin D is an active compound derived from the Radix bupleuri. In cerulein-induced pancreatic acinar cells (PACs) injury mode, Saikosaponin d protects PACs against pyroptosis by inhibiting the cGAS/STING/NLRP3 signaling pathway (46). Emodin is an anthraquinone derivative that is present in numerous herbal medicines. In acetaminophen-induced C57BL/6 mice, emodin protects hepatocytes from liver injury by inhibiting cGAS/STING/NLRP3 signaling pathway (47). Chlorogenic acid(CA), also known as 5-O-caffeoylquinic acid, is a phenolic compound. In varicocele rats, CA can improve spermatogenic dysfunction by inhibiting the cGAS/STING/NLRP3 signaling pathway (48). In addition to natural products, some chemical agents such as melatonin, Pulmozyme, and C-176 can also improve disease by regulating the cGAS/STING/NLRP3 signaling pathway (49–51). Interestingly, some non-drug treatment strategies have also been reported. For example, electroacupuncture alleviates depression in mice by regulating the cGAS/STING/NLRP3 signaling pathway (52). Transplanting fecal microbiota mitigated neurotoxicity by regulating the cGAS/STING/NLRP3 signaling pathway (53). Hydrogen sulfide improves cardiac dysfunction by inhibiting the cGAS/STING/NLRP3 signaling pathway (54). Together, these therapeutic strategies have important implications for improving and safeguarding human health. More treatment strategies need to be explored in the future.
7 Discussion
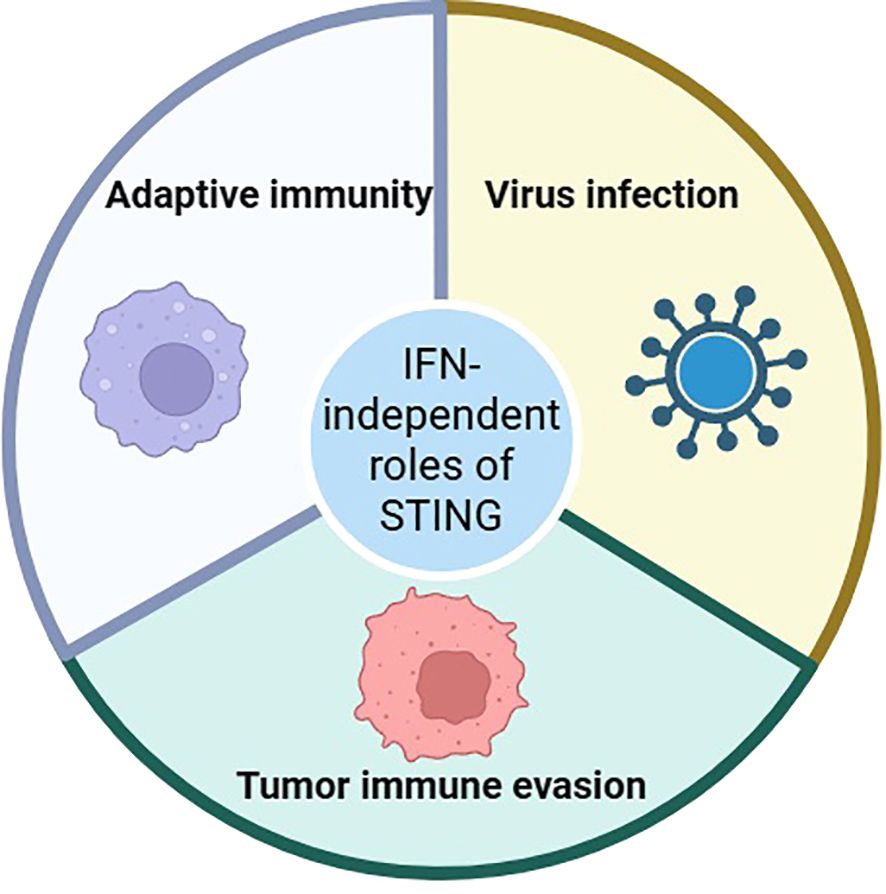
In recent years, as a hot signaling pathway, the cGAS/STING signaling pathway play an important role in bacterial infection, viral infection, immune diseases, and inflammatory diseases. On the one hand, the cGAS/STING signaling pathway induces the production of IFN-I by activating the TBK1 and IRF3. On the other hand, the cGAS/STING signaling pathway allows NF-κB to enter the nucleus and induce inflammatory responses by recruiting the IKK. At present, many noncanonical cGAS/STING signaling pathways have also been revealed. Studying IFN-independent roles of STING is challenging due to the overwhelming effects of IFN signaling when STING is activated by agonists, and IFN may mask other activities of STING. Interestingly, many IFN-independent roles of cGAS or STING have been reported to date. For example, Wu J et al. found that STING controls herpes simplex virus-1 (HSV-1) infection through IFN-independent activities (55). Wang K et al. found that STING controls hantaviral (HTNV) infection through IFN-independent way (56). Another important finding is that STING activates many signaling pathways in T cells and the majority of them are IFN-independent (57). These data demonstrate that mammalian STING possesses widespread IFN-independent activities that are important for restricting virus infection, tumor immune evasion, and adaptive immunity (Figure 3). It is very important to explore the connection between STING and NLRP3. The recent evidence indicates that stimulating STING prompts the activation of the NLRP3 inflammasome. STING comprises five putative transmembrane (TM) regions. TM5 (151-160 aa, human) is involved in the interaction with NLRP3, while TM2 (41-81 aa, human) participates in the assembly and activation of the NLRP3 inflammasome (58). STING binds NLRP3, and then it can promote the activation of the inflammasome via NLRP3 localization and the removal of NLRP3 polyubiquitination (59). Therefore, we believe that NLRP3 is a better downstream target for STING. As a downstream of the cGAS/STING signaling pathway, NLRP3 has been demonstrated in the pathogenesis of an increasing number of diseases. Many natural products, chemical agents, and non-drug treatment strategies can regulate this signaling pathway to improve disease. It is worth noting that some studies have also pointed out that the NLRP3 inflammasome and its downstream Caspase-1, GSDMD can also regulate the cGAS/STING signaling pathway. For example, Wang Y and his team found that Caspase-1 cleaved cGAS and inhibited STING-mediated IFN production during inflammasome activation (60). Banerjee I and his team found that GSDMD negatively regulates IFN in a manner independent of pyroptosis and IL-1β (61). These studies indicate the presence of crosstalk networks in the cGAS/STING signaling pathway and NLRP3. Therefore, more studies are needed in the future to explore the association between the cGAS/STING signaling pathway and NLRP3.
Author contributions
K-qC: Writing – original draft. W-rT: Writing – review & editing. XL: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ishikawa H and Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling [published correction appears in Nature. Nature. (2008) 455:674–8. doi: 10.1038/nature07317
2. Shang G, Zhang C, Chen ZJ, Bai XC, and Zhang X. Cryo-EM structures of STING reveal its mechanism of activation by cyclic GMP-AMP. Nature. (2019) 567:389–93. doi: 10.1038/s41586-019-0998-5
3. Ahn J and Barber GN. STING signaling and host defense against microbial infection. Exp Mol Med. (2019) 51:1–10. doi: 10.1038/s12276-019-0333-0
4. Sun L, Wu J, Du F, Chen X, and Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. (2013) 339:786–91. doi: 10.1126/science.1232458
5. Yu L and Liu P. Cytosolic DNA sensing by cGAS: regulation, function, and human diseases. Signal Transduct Target Ther. (2021) 6:170. doi: 10.1038/s41392-021-00554-y
6. Cohen D, Melamed S, Millman A, Shulman G, Oppenheimer-Shaanan Y, Kacen A, et al. Cyclic GMP-AMP signalling protects bacteria against viral infection. Nature. (2019) 574:691–5. doi: 10.1038/s41586-019-1605-5
7. Zhou J, Zhuang Z, Li J, and Feng Z. Significance of the cGAS-STING pathway in health and disease. Int J Mol Sci. (2023) 24:13316. doi: 10.3390/ijms241713316
8. Zhang Z, Zhou H, Ouyang X, Dong Y, Sarapultsev A, Luo S, et al. Multifaceted functions of STING in human health and disease: from molecular mechanism to targeted strategy. Signal Transduct Target Ther. (2022) 7:394. doi: 10.1038/s41392-022-01252-z
9. Peng Y, Zhuang J, Ying G, Zeng H, Zhou H, Cao Y, et al. Stimulator of IFN genes mediates neuroinflammatory injury by suppressing AMPK signal in experimental subarachnoid hemorrhage. J Neuroinflammation. (2020) 17:165. doi: 10.1186/s12974-020-01830-4
10. Chen Q, Sun L, and Chen ZJ. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol. (2016) 17:1142–9. doi: 10.1038/ni.3558
11. Chen KQ, Ke BY, Cheng L, Yu XQ, Wang ZB, and Wang SZ. Research and progress of inflammasomes in nonalcoholic fatty liver disease. Int Immunopharmacol. (2023) 118:110013. doi: 10.1016/j.intimp.2023.110013
12. Mridha AR, Wree A, Robertson AAB, Yeh MM, Johnson CD, Van Rooyen DM, et al. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J Hepatol. (2017) 66:1037–46. doi: 10.1016/j.jhep.2017.01.022
13. Xu J and Núñez G. The NLRP3 inflammasome: activation and regulation. Trends Biochem Sci. (2023) 48:331–44. doi: 10.1016/j.tibs.2022.10.002
14. Paik S, Kim JK, Silwal P, Sasakawa C, and Jo EK. An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell Mol Immunol. (2021) 18:1141–60. doi: 10.1038/s41423-021-00670-3
15. Wu T, Gao J, Liu W, Cui J, Yang M, Guo W, et al. NLRP3 protects mice from radiation-induced colon and skin damage via attenuating cGAS-STING signaling. Toxicol Appl Pharmacol. (2021) 418:115495. doi: 10.1016/j.taap.2021.115495
16. Zheng Y, Liu Q, Wu Y, Ma L, Zhang Z, Liu T, et al. Zika virus elicits inflammation to evade antiviral response by cleaving cGAS via NS1-caspase-1 axis. EMBO J. (2018) 37:e99347. doi: 10.15252/embj.201899347
17. Zhang H, Chen Z, Zhou J, Gu J, Wu H, Jiang Y, et al. NAT10 regulates neutrophil pyroptosis in sepsis via acetylating ULK1 RNA and activating STING pathway [published correction appears in Commun Biol. Commun Biol. (2022) 5:916. doi: 10.1038/s42003-022-03868-x
18. McLemore AF, Hou HA, Meyer BS, Lam NB, Ward GA, Aldrich AL, et al. Somatic gene mutations expose cytoplasmic DNA to co-opt the cGAS/STING/NLRP3 axis in myelodysplastic syndromes. JCI Insight. (2022) 7:e159430. doi: 10.1172/jci.insight.159430
19. Li N, Zhou H, Wu H, Wu Q, Duan M, Deng W, et al. STING-IRF3 contributes to lipopolysaccharide-induced cardiac dysfunction, inflammation, apoptosis and pyroptosis by activating NLRP3. Redox Biol. (2019) 24:101215. doi: 10.1016/j.redox.2019.101215
20. Wang W, Hu D, Wu C, Feng Y, Li A, Liu W, et al. STING promotes NLRP3 localization in ER and facilitates NLRP3 deubiquitination to activate the inflammasome upon HSV-1 infection. PLoS Pathog. (2020) 16:e1008335. doi: 10.1371/journal.ppat.1008335
21. Xiao Y, Zhao C, Tai Y, Li B, Lan T, Lai E, et al. STING mediates hepatocyte pyroptosis in liver fibrosis by Epigenetically activating the NLRP3 inflammasome. Redox Biol. (2023) 62:102691. doi: 10.1016/j.redox.2023.102691
22. Gaidt MM, Ebert TS, Chauhan D, Ramshorn K, Pinci F, Zuber S, et al. The DNA inflammasome in human myeloid cells is initiated by a STING-cell death program upstream of NLRP3. Cell. (2017) 171:1110–1124.e18. doi: 10.1016/j.cell.2017.09.039
23. Kwon OC, Song JJ, Yang Y, Kim SH, Kim JY, Seok MJ, et al. SGK1 inhibition in glia ameliorates pathologies and symptoms in Parkinson disease animal models. EMBO Mol Med. (2021) 13:e13076. doi: 10.15252/emmm.202013076
24. Yang NS, Zhong WJ, Sha HX, Zhang CY, Jin L, Duan JX, et al. mtDNA-cGAS-STING axis-dependent NLRP3 inflammasome activation contributes to postoperative cognitive dysfunction induced by sevoflurane in mice. Int J Biol Sci. (2024) 20:1927–46. doi: 10.7150/ijbs.91543
25. Liu L, Xiao F, Yang J, Yao H, and Hua K. Microglial pyroptosis induced by SENP7 via the cGAS/STING/IRF3 pathway contributes to neuronal apoptosis. Cytokine. (2025) 189:156893. doi: 10.1016/j.cyto.2025.156893
26. Liu J, Zhang X, and Wang H. The cGAS-STING-mediated NLRP3 inflammasome is involved in the neurotoxicity induced by manganese exposure. BioMed Pharmacother. (2022) 154:113680. doi: 10.1016/j.biopha.2022.113680
27. Shi W, Zhou Q, Lu L, Zhang Y, Zhang H, Pu Y, et al. Copper induced cytosolic escape of mitochondrial DNA and activation of cGAS-STING-NLRP3 pathway-dependent pyroptosis in C8-D1A cells. Ecotoxicol Environ Saf. (2024) 285:117085. doi: 10.1016/j.ecoenv.2024.117085
28. Zhang CY, Ou AJ, Jin L, Yang NS, Deng P, Guan CX, et al. Cadmium exposure triggers alveolar epithelial cell pyroptosis by inducing mitochondrial oxidative stress and activating the cGAS-STING pathway. Cell Commun Signal. (2024) 22:566. doi: 10.1186/s12964-024-01946-7
29. Yang H, Sun P, Zhou S, Tang Y, Li S, Li W, et al. Chlamydia psittaci infection induces IFN-I and IL-1β through the cGAS-STING-IRF3/NLRP3 pathway via mitochondrial oxidative stress in human macrophages. Vet Microbiol. (2024) 299:110292. doi: 10.1016/j.vetmic.2024.110292
30. Zhao M, Xie J, Zhang J, Zhao B, Zhang Y, Xue J, et al. Disturbance of mitochondrial dynamics led to spermatogenesis disorder in mice exposed to polystyrene micro- and nanoplastics. Environ Pollut. (2024) 362:124935. doi: 10.1016/j.envpol.2024.124935
31. Ding R, Li H, Liu Y, Ou W, Zhang X, Chai H, et al. Activating cGAS-STING axis contributes to neuroinflammation in CVST mouse model and induces inflammasome activation and microglia pyroptosis. J Neuroinflammation. (2022) 19:137. doi: 10.1186/s12974-022-02511-0
32. Shen H, Lu H, Mao L, and Song L. Inhibition of cGAS attenuates neonatal hypoxic-ischemic encephalopathy via regulating microglia polarization and pyroptosis. Transl Pediatr. (2024) 13:1378–94. doi: 10.21037/tp-24-148
33. Yan M, Li Y, Luo Q, Zeng W, Shao X, Li L, et al. Mitochondrial damage and activation of the cytosolic DNA sensor cGAS-STING pathway lead to cardiac pyroptosis and hypertrophy in diabetic cardiomyopathy mice. Cell Death Discov. (2022) 8:258. doi: 10.1038/s41420-022-01046-w
34. Zhang W, Zhang Y, Han L, Bo T, Qi Z, Zhong H, et al. Double-stranded DNA enhances platelet activation, thrombosis, and myocardial injury via cyclic GMP-AMP synthase. Cardiovasc Res. (2025) 121:353–66. doi: 10.1093/cvr/cvae218
35. An C, Sun F, Liu C, Huang S, Xu T, Zhang C, et al. IQGAP1 promotes mitochondrial damage and activation of the mtDNA sensor cGAS-STING pathway to induce endothelial cell pyroptosis leading to atherosclerosis. Int Immunopharmacol. (2023) 123:110795. doi: 10.1016/j.intimp.2023.110795
36. Peng Y, Yang Y, Li Y, Shi T, Xu N, Liu R, et al. Mitochondrial (mt)DNA-cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) signaling promotes pyroptosis of macrophages via interferon regulatory factor (IRF)7/IRF3 activation to aggravate lung injury during severe acute pancreatitis. Cell Mol Biol Lett. (2024) 29:61. doi: 10.1186/s11658-024-00575-9
37. Zhao Y, Li W, Xu J, Bao L, Wu K, Shan R, et al. Endogenous retroviruses modulate the susceptibility of mice to Staphylococcus aureus-induced mastitis by activating cGAS-STING signaling. Int Immunopharmacol. (2024) 142:113171. doi: 10.1016/j.intimp.2024.113171
38. Luo X, Zhao Y, Luo Y, Lai J, Ji J, Huang J, et al. Cytosolic mtDNA-cGAS-STING axis contributes to sepsis-induced acute kidney injury via activating the NLRP3 inflammasome. Clin Exp Nephrol. (2024) 28:375–90. doi: 10.1007/s10157-023-02448-5
39. Liu Z, Wang M, Wang X, Bu Q, Wang Q, Su W, et al. XBP1 deficiency promotes hepatocyte pyroptosis by impairing mitophagy to activate mtDNA-cGAS-STING signaling in macrophages during acute liver injury. Redox Biol. (2022) 52:102305. doi: 10.1016/j.redox.2022.102305
40. Ning L, Wei W, Wenyang J, Rui X, and Qing G. Cytosolic DNA-STING-NLRP3 axis is involved in murine acute lung injury induced by lipopolysaccharide. Clin Transl Med. (2020) 10:e228. doi: 10.1002/ctm2.228
41. Inokuchi S, Mitoma H, Kawano S, Ayano M, Kimoto Y, Akahoshi M, et al. Activation of caspase-1 is mediated by stimulation of interferon genes and NLR family pyrin domain containing 3 in monocytes of active systemic lupus erythematosus. Clin Exp Rheumatol. (2022) 40:522–31. doi: 10.55563/clinexprheumatol/eakvlv
42. Zheng Y, Xie Y, Li J, Cao Y, Li M, Cao Q, et al. CMPK2 promotes NLRP3 inflammasome activation via mtDNA-STING pathway in house dust mite-induced allergic rhinitis. Clin Transl Med. (2025) 15:e70180. doi: 10.1002/ctm2.70180
43. Gao D, Hao JP, Li BY, Zheng CC, Miao BB, Zhang L, et al. Tetrahydroxy stilbene glycoside ameliorates neuroinflammation for Alzheimer’s disease via cGAS-STING. Eur J Pharmacol. (2023) 953:175809. doi: 10.1016/j.ejphar.2023.175809
44. Lai Y, Yang N, Chen X, Ma X, Chen Z, Dong C, et al. Dihydrocapsaicin suppresses the STING-mediated accumulation of ROS and NLRP3 inflammasome and alleviates apoptosis after ischemia-reperfusion injury of perforator skin flap. Phytother Res. (2024) 38:2539–59. doi: 10.1002/ptr.8167
45. Tian Y, Bao Z, Ji Y, Mei X, and Yang H. Epigallocatechin-3-gallate protects H2O2-induced nucleus pulposus cell apoptosis and inflammation by inhibiting cGAS/sting/NLRP3 activation. Drug Des Devel Ther. (2020) 14:2113–22. doi: 10.2147/DDDT.S251623
46. Chen H, Lu X, Xu B, Cheng G, Li Y, and Xie D. Saikosaponin d protects pancreatic acinar cells against cerulein-induced pyroptosis through alleviating mitochondrial damage and inhibiting cGAS-STING pathway. J Appl Toxicol. (2024) 44:1005–13. doi: 10.1002/jat.4594
47. Shen P, Han L, Chen G, Cheng Z, and Liu Q. Emodin Attenuates Acetaminophen-Induced Hepatotoxicity via the cGAS-STING Pathway. Inflammation. (2022) 45:74–87. doi: 10.1007/s10753-021-01529-5
48. Jia ZC, Liu SJ, Chen TF, Shi ZZ, Li XL, Gao ZW, et al. Chlorogenic acid can improve spermatogenic dysfunction in rats with varicocele by regulating mitochondrial homeostasis and inhibiting the activation of NLRP3 inflammasomes by oxidative mitochondrial DNA and cGAS/STING pathway. Bioorg Chem. (2024) 150:107571. doi: 10.1016/j.bioorg.2024.107571
49. Liu L, Li MZ, Yao MH, Yang TN, Tang YX, and Li JL. Melatonin inhibits atrazine-induced mitochondrial impairment in cerebellum of mice: Modulation of cGAS-STING-NLRP3 axis-dependent cell pyroptosis. Sci Total Environ. (2024) 912:168924. doi: 10.1016/j.scitotenv.2023.168924
50. Zhang Y, Li Z, Hong W, Hsu S, Wang B, Zeng Z, et al. STING-dependent sensing of self-DNA driving pyroptosis contributes to radiation-induced lung injury. Int J Radiat Oncol Biol Phys. (2023) 117:928–41. doi: 10.1016/j.ijrobp.2023.05.029
51. Wang B, Wang Y, Qiu J, Gao S, Yu S, Sun D, et al. The STING inhibitor C-176 attenuates MPTP-induced neuroinflammation and neurodegeneration in mouse parkinsonian models. Int Immunopharmacol. (2023) 124:110827. doi: 10.1016/j.intimp.2023.110827
52. Chen S, Li J, Yan L, Zhang X, Huang J, and Zhou P. Electroacupuncture alleviates the symptom of depression in mice by regulating the cGAS-STING-NLRP3 signaling. Aging (Albany NY). (2024) 16:6731–44. doi: 10.18632/aging.205596
53. Liu J, Zhang Z, Zhong S, Zhang X, Yang J, Zhou Q, et al. Fecal microbiome transplantation alleviates manganese-induced neurotoxicity by altering the composition and function of the gut microbiota via the cGAS-STING/NLRP3 pathway. Sci Total Environ. (2024) 951:175681. doi: 10.1016/j.scitotenv.2024.175681
54. Bai L, Dai J, Xia Y, He K, Xue H, Guo Q, et al. Hydrogen sulfide ameliorated high choline-induced cardiac dysfunction by inhibiting cGAS-STING-NLRP3 inflammasome pathway. Oxid Med Cell Longev. (2022) 2022:1392896. doi: 10.1155/2022/1392896
55. Wu J, Dobbs N, Yang K, and Yan N. Interferon-independent activities of mammalian STING mediate antiviral response and tumor immune evasion. Immunity. (2020) 53:115–126.e5. doi: 10.1016/j.immuni.2020.06.009
56. Wang K, Zhang J, Yang Y, Si Y, Zhou Z, Zhu X, et al. STING strengthens host anti-hantaviral immunity through an interferon-independent pathway. Virol Sin. (2023) 38:568–84. doi: 10.1016/j.virs.2023.06.006
57. Gui X, Yang H, Li T, Tan X, Shi P, Li M, et al. Autophagy induction via STING trafficking is a primordial function of the cGAS pathway. Nature. (2019) 567:262–6. doi: 10.1038/s41586-019-1006-9
58. Zheng W, Liu A, Xia N, Chen N, Meurens F, and Zhu J. How the innate immune DNA sensing cGAS-STING pathway is involved in apoptosis. Int J Mol Sci. (2023) 24:3029. doi: 10.3390/ijms24033029
59. Lu X, Li X, Li L, Han C, and Li S. Advances in the prerequisite and consequence of STING downstream signalosomes. Med Rev (2021). (2024) 4:435–51. doi: 10.1515/mr-2024-0016
60. Wang Y, Ning X, Gao P, Wu S, Sha M, Lv M, et al. Inflammasome activation triggers caspase-1-mediated cleavage of cGAS to regulate responses to DNA virus infection. Immunity. (2017) 46:393–404. doi: 10.1016/j.immuni.2017.02.011
Keywords: cGAS, STING, inflammasome, NLRP3, IFN
Citation: Chen K-q, Tang W-r and Liu X (2025) Research and progress of cGAS/STING/NLRP3 signaling pathway: a mini review. Front. Immunol. 16:1594133. doi: 10.3389/fimmu.2025.1594133
Received: 15 March 2025; Accepted: 01 May 2025;
Published: 20 May 2025.
Edited by:
Clett Erridge, Anglia Ruskin University, United KingdomReviewed by:
Monisankar Ghosh, Stony Brook University, United StatesCopyright © 2025 Chen, Tang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen-rui Tang, dHR3ZW5ydWlAeWVhaC5uZXQ=; Xiang Liu, TFgxOTg5MEAxNjMuY29t
 Ke-qian Chen
Ke-qian Chen Wen-rui Tang2*
Wen-rui Tang2*