- 1Department of Rheumatology, Children’s Hospital of Fudan University, National Pediatric Medical Center of China, Shanghai, China
- 2Department of Pathology, Children’s Hospital of Fudan University, National Pediatric Medical Center of China, Shanghai, China
- 3Department of Pediatric, The First People’s Hospital of Zunyi, The Third Affiliated Hospital of Zunyi Medical University, Zunyi, China
- 4Department of Nephrology, Children’s Hospital of Fudan University, National Pediatric Medical Center of China, Shanghai, China
Background: Primary hypertension (PHTN) and IgA vasculitis (IgAV) are two prevalent medical conditions that affect the kidneys. Despite their distinct pathophysiological mechanisms, clinical manifestations, and treatment approaches, both conditions significantly impact patients’ quality of life and overall health. Unfortunately, a boy developed hypertensive nephritis (HN) at the age of 4, followed by IgAV nephritis at the age of 10. The exact pathophysiological mechanisms underlying these conditions remain elusive. This study aims to report this unusual case and investigate the biological mechanisms associated with the differentially expressed genes (DEGs) related to PHTN and IgAV through the application of bioinformatics tools.
Methods: We present the case of a boy diagnosed with PHTN and IgAV. Additionally, we explore the molecular mechanisms underlying both conditions. DEGs were analyzed, and gene functional enrichment was performed using the DAVID database. A protein-protein interaction (PPI) network was constructed using the STRING database and visualized with Cytoscape software. The hub genes were identified using the MCODE plugin. CIBERSORT was used to assess the expression changes in immune cells and obtain the proportion of various types of immune cells. Furthermore, the Connectivity Map L1000 platform was utilized to identify potential therapeutic agents.
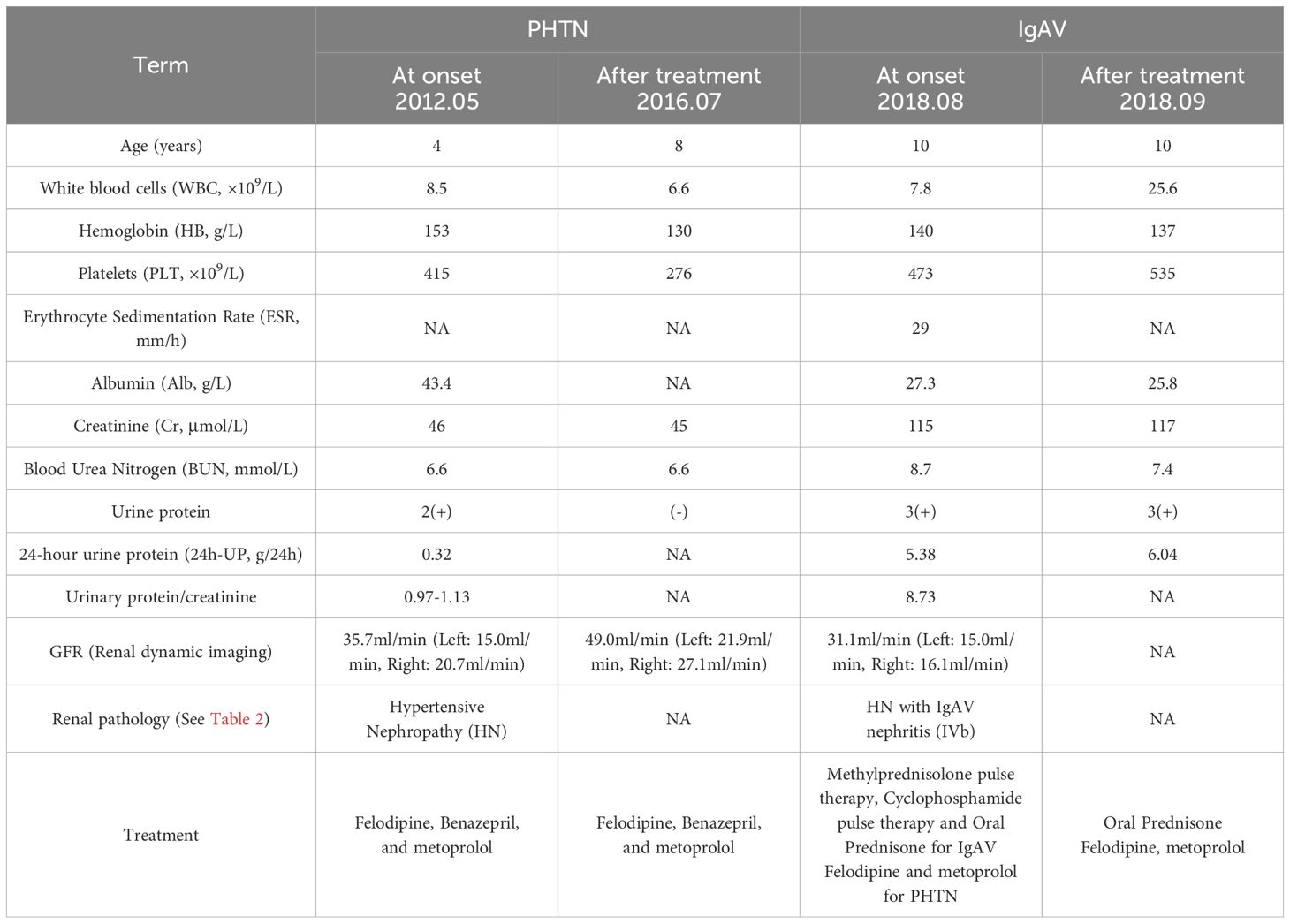
Results: The boy initially presented with malignant hypertension (MHT), and renal biopsy pathology indicated HN. Following regular use of antihypertensive medications, there was a significant improvement in blood pressure (BP), renal function, and the left ventricular hypertrophy index. However, he developed IgAV six years after the diagnosis of PHTN. A subsequent renal biopsy revealed IgAV nephritis, and the pathology associated with HN showed marked improvement. A total of 7,027 DEGs associated with PHTN and 90 DEGs linked to IgAV were identified, with 25 genes overlapping between the two sets. KEGG pathway analysis revealed that the DEGs were primarily associated with extracellular matrix (ECM) receptor interaction. Among EMC key components, fibronectin expression was markedly elevated in hypertensive nephritis and IgA nephropathy. Gene Ontology (GO) biological process analysis indicated that the 25 overlapping DEGs were significantly related to processes such as proteolysis, amyloid fibril formation, cAMP-mediated signaling, synaptic vesicle endocytosis, and receptor internalization. The significantly enriched terms related to changes in the cellular component of DEGs included platelet alpha granule membrane, nucleoplasm, and endocytic vesicle membrane. Changes in molecular function were primarily associated with protein binding. Furthermore, six hub genes implicated in both diseases were linked to cell adhesion molecules. We also found that neutrophils accounted for the majority of all infiltrating cells. And B cell naïve were downregulated in both diseases.Using the Connectivity Map (CMap) database, the top 10 potential therapeutic agents were identified.
Conclusion: We found that aggressive BP-lowering agents were necessary for managing PHTN. This study also reveals the common pathogenesis underlying both PHTN and IgAV. Moving forward, these shared hub genes could serve as novel targets for more in-depth mechanistic investigations and the development of new therapeutic interventions for individuals affected by PHTN and IgAV.
Highlights
● Aggressive blood pressure-lowering agents are necessary for managing PHTN.
● The mechanisms underlying the DEGs associated with PHTN and IgAV were explored for the first time.
● Extracellular matrix (ECM) receptor interaction participated in the pathogenesis of PHTN and IgAV.
● Fibronectin expression was markedly elevated in hypertensive nephritis and IgA nephropathy.
● We identified several compounds as potential treatment options for PHTN and IgAV.
1 Introduction
Primary hypertension (PHTN) and IgA vasculitis (IgAV) are two prevalent medical conditions that affect the kidneys. PHTN is a chronic condition characterized by consistently elevated blood pressure without an identifiable secondary cause (1). Hypertensive nephropathy (HN) refers to kidney damage resulting from prolonged high blood pressure (BP) (2). Genetic predisposition and environmental factors play significant roles in the development of PHTN (3). Several physiological mechanisms contribute to the onset of PHTN, including renal mechanisms, sympathetic nervous system activation, and endothelial dysfunction (4). HN develops as a consequence of sustained high blood pressure, leading to structural and functional changes in the kidneys. The cumulative effect of these changes results in decreased glomerular filtration rate (GFR), proteinuria, and ultimately chronic kidney disease (CKD) (5). In contrast, IgA vasculitis (IgAV), also known as Henoch-Schönlein purpura (HSP), is a small-vessel vasculitis characterized by a tetrad of symptoms: palpable purpura, often on the buttocks and legs, arthralgia or arthritis, abdominal pain, and renal involvement (6). IgAV is thought to be related to an abnormal immune response (7), with key features including IgA deposition and vascular inflammation (8).
Despite their divergent pathophysiological origins, we encountered a rare case of concurrent PHTN and IgAV in a pediatric patient. This clinical overlap raises intriguing questions about potential shared molecular mechanisms. Understanding these common pathways and mechanisms is crucial for advancing treatment strategies. This study aims to present this remarkable case and delve into the biological mechanisms that govern the differentially expressed genes (DEGs) associated with PHTN and IgAV. By employing advanced bioinformatics tools, we seek to uncover the intricate relationships and underlying pathways that contribute to these conditions.
2 Methods
2.1 Case report
This study was approved by the Institutional Review Board of Children’s Hospital of Fudan University (No. 2021-169), and informed consent was obtained from the participant. We collected demographic, clinical, laboratory characteristics, and follow-up information for the patients included in this study. We report the clinical manifestations, diagnostic processes, and prognosis of the patient.
2.2 Microarray data
NCBI-GEO (http://www.ncbi.nlm.nih.gov/geo) serves as a centralized repository for high-throughput functional genomics data, including microarray and RNA sequencing datasets (9). We conducted a search using the keywords “primary hypertension” OR “essential hypertension,” which yielded 51 results in the GEO Database. After filtering for Homo sapiens, 27 results remained. We carefully examined these 27 entries. In this study, we aim to explore the differentially expressed genes (DEGs) between patients with PHTN and normal controls, ultimately identifying only GSE24752 for further analysis (10). Additionally, we searched using the keywords “IgA vasculitis,” which returned 14 results in the GEO Database. Our goal is to investigate the DEGs between IgAV patients and normal controls, leading us to focus on GSE102114 (11).
2.3 Identification of DEGs
The GEO2R online tool was utilized for the identification of DEGs (9). A log fold change (FC) greater than 1 and a P-value less than 0.05 were considered statistically significant. The raw data were analyzed using Venn software to identify common DEGs. DEGs with a log FC less than 0 were classified as downregulated, while those with a log FC greater than 0 were classified as upregulated.
2.4 Functional enrichment and PPI analysis
The Database for Annotation, Visualization, and Integrated Discovery (DAVID; http://david.ncifcrf.gov) is an online biological information resource (12). The Kyoto Encyclopedia of Genes and Genomes (KEGG) serves as a database for understanding high-level functions and biological systems derived from large-scale molecular datasets generated by high-throughput experimental technologies (13). Gene Ontology (GO) is a key bioinformatics tool used for annotating genes and analyzing their biological processes (14). In this study, DAVID 6.8 Bioinformatics Resources was employed for pathway annotations, with statistical significance set at P < 0.05. A protein-protein interaction (PPI) network was constructed using the Search Tool for the Retrieval of Interacting Genes (STRING) (version 10.0) available at https://string-db.org/, with interactions exhibiting a combined score greater than 0.4 considered statistically significant. The PPI network was visualized using Cytoscape (version 3.7.2) (https://cytoscape.org/).
2.5 Profiling infiltrating immune cells with CIBERSORT
To systematically evaluate compositional alterations in immune cell populations and quantify subtype-specific lymphocyte proportions, we employed the web-based CIBERSORT analytical tool (https://cibersort.stanford.edu/). This machine learning approach, based on deconvolution principles, enables precise characterization of immune cell fractions through linear support vector regression analysis of gene expression data. Comparative analyses were conducted using a standardized leukocyte gene signature matrix (LM22) that distinguishes 22 functionally distinct immune cell subtypes. The analytical workflow incorporated 1000 permutations with p-value thresholding (p<0.05) to ensure statistically robust cell fraction estimations.
2.6 CMap analysis
The Connectivity Map (CMap) was utilized to identify potential agents for PHTN and IgAV. The 25 DEGs were queried using the Connectivity Map online tool (L1000 platform; https://clue.io/l1000-query) (15). Upon submitting a list of 20 upregulated genes, connectivity scores ranging from -1 to 1 were generated to reflect the similarity between expression profiles. A positive score indicates a promoted effect, while a negative score signifies an inhibited effect.
2.7 Pathological findings of renal biopsies
The renal biopsy specimens were embedded in OCT compound (Sakura, Hayward, CA, USA) and paraffin wax, respectively, to prepare frozen sections and paraffin sections. Routine pathological staining methods, including hematoxylin-eosin (HE) staining and immunofluorescence staining, were performed on the sections. Furthermore, Masson’s trichrome staining was conducted on the renal tissue sections, and immunofluorescence staining for Fibronectin was employed to observe its expression pattern in renal tissues.
3 Result
3.1 Case presentation
In April 2012, a 4-year-old boy presented to a local hospital with recurrent dizziness for 3 weeks. Initial evaluation revealed severe hypertension. BP was 210/150 mmHg. After treatment with sodium nitroprusside, nifedipine, phentolamine, and benazepril, his BP was maintained at 130-150/72-90 mmHg. However, subsequent nonadherence to the prescribed regimen led to uncontrolled hypertension, prompting referral to the Children’s Hospital of Fudan University on October 13, 2012, for specialized evaluation and management.
Physical examination demonstrated marked hypertension (BP: 140/100 mmHg (>99th percentile for age/height: 118/77 mmHg). Anthropometric measurements revealed normal growth parameters: height 107 cm (50th percentile), weight 16.6 kg (25th-50th percentile), and BMI was 14.4 kg/m². Neurological assessment showed an alert, interactive child with no signs of encephalopathy (e.g., seizures, altered consciousness) or volume overload (e.g., edema, oliguria). Nutritional status and developmental milestones were age-appropriate, with preserved appetite. No significant pre-existing medical conditions were documented. However, familial medical history remained unavailable due to the patient’s adoptive status.
Laboratory investigations ruled out secondary hypertension etiologies: autoantibodies including ANA, ANCA, anti-dsDNA returned negative, and endocrine profiles including thyroid function, cortisol, renin-angiotensin-aldosterone system were within normal ranges. Renal involvement was evidenced by proteinuria (++), microscopic hematuria (3–4 RBCs/high-power field), and elevated urinary protein excretion (urine protein/creatinine ratio: 0.97–1.13; 24-hour urine protein: 0.32 g). Blood biochemistry demonstrated preserved renal function (serum creatinine: 46.0 µmol/L) and normoalbuminemia (43.4 g/L). Histopathological analysis of renal biopsy specimens confirmed malignant nephrosclerosis, characterized by arteriolar fibrinoid necrosis and ischemic glomerular collapse. Advanced imaging studies excluded structural anomalies: Normal brain and pituitary gland on MRI (plain + contrast-enhanced sequences). Vascular imaging: No renal artery stenosis (CTA), urinary tract malformations (MCU), or vascular lesions (DSA). Cardiac evaluation revealed left ventricular hypertrophy with preserved systolic function (left ventricular ejection fraction: 77%; cardiac output: 3.56 L/min). Electrocardiographic findings included left axis deviation and left ventricular high voltage, consistent with pressure overload. Ophthalmologic assessment ruled out hypertensive retinopathy, with fundoscopic examination showing no vascular abnormalities.
Secondary hypertension etiologies—including connective tissue disorders, endocrine pathologies (hyperthyroidism, Cushing’s syndrome, pheochromocytoma), Takayasu arteritis, and CNS tumors—were systematically excluded based on comprehensive laboratory and imaging findings. The patient was thus diagnosed with PHTN complicated by HN. Antihypertensive therapy with felodipine, benazepril, and metoprolol was initiated, achieving blood pressure stabilization (100–110/60–70 mmHg) by discharge on November 6, 2012. From 2013 to 2017, strict adherence to the regimen maintained normotension. Serial monitoring demonstrated preserved renal function (normal urinalysis, serum creatinine) and resolved cardiac remodeling (normalized ECG and echocardiographic parameters).
In October 2017, when the boy was 9 years old, he developed purpura. prompting a diagnosis of IgA vasculitis (IgAV) at a local hospital, with symptom resolution following undocumented treatment. Post-discharge renal monitoring (urinalysis, creatinine) was neglected. By August 2018, at the age of 10, he re-presented with systemic symptoms (vomiting, diarrhea, headache, dizziness) and nephritic-nephrotic syndrome features: gross hematuria, foamy urine, nephrotic-range proteinuria (urine protein+++, UPCR 8.73, 24-hour protein 5.38–6.04 g), hypoalbuminemia (27.3 g/L), and elevated creatinine (115 µmol/L). Renal biopsy confirmed IgAV nephritis (ISKDC grade IVb), prompting pulse methylprednisolone (1 g/day ×3) and cyclophosphamide induction, followed by oral prednisone, alongside antihypertensive optimization (felodipine/metoprolol continued; benazepril halted due to renal impairment). Post-therapy urinalysis showed persistent proteinuria (+++) and microscopic hematuria (36 RBCs/hpf), with stabilized BP (100–110/60–70 mmHg) prior to discharge on September 20, 2018.
According to our hospital’s post-treatment evaluation records, only four weeks since the initial treatment. And the patient exhibited no clinically significant improvement (Table 1). Consequently, the child was transferred to a local medical institution for continued care management. Unfortunately, the patient was lost to follow-up after discharge. Due to the sensitive nature of the patient’s adoptive status, the guardians declined to provide additional demographic or clinical details beyond mandatory reporting requirements. And his current condition remains unknown.
3.2 Renal pathology at different stage
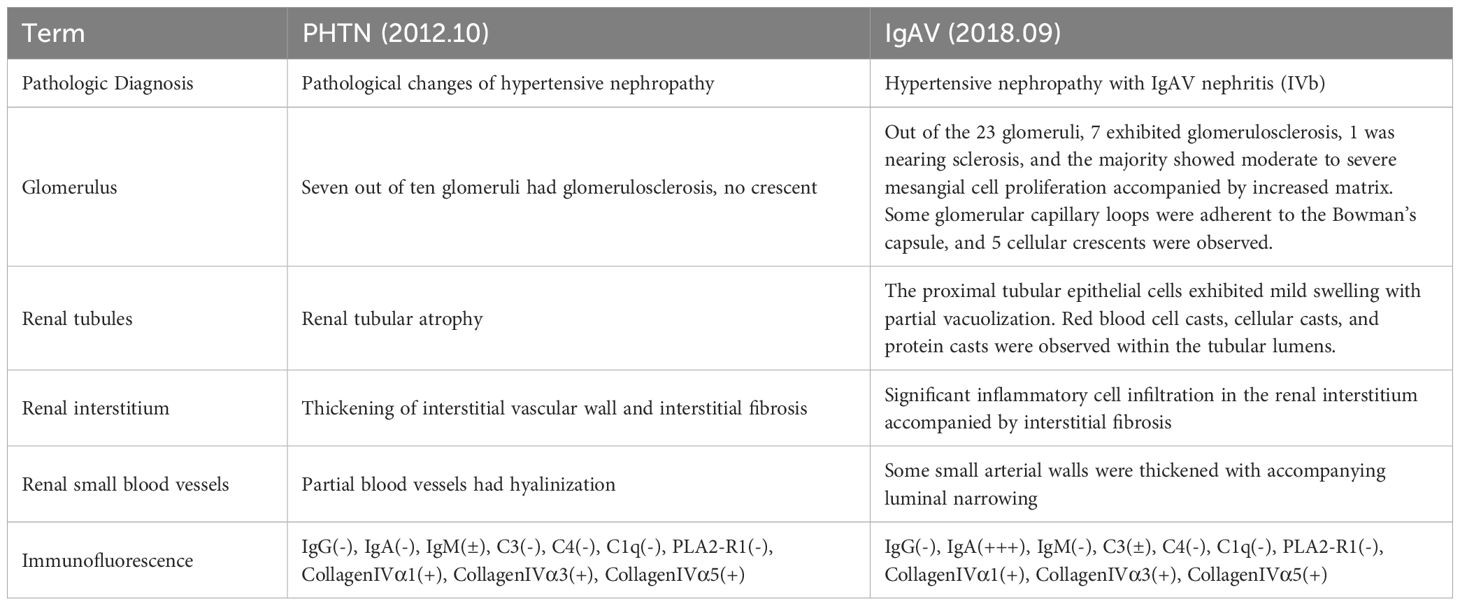
In october 2012, the pathologic diagnosis indicated HN. HE staining displayed that seven out of ten glomeruli had glomerulosclerosis, no crescent were seen. Renal tubular had atrophy. Thickening of interstitial vascular wall and interstitial fibrosis were seen and partial blood vessels had hyalinization (Figure 1A). Immunofluorescence displayed that IgG(-), IgA(-) (Figure 1B), IgM(±), C3(-), C4(-), C1q(-), PLA2-R1(-), CollagenIVα1(+), CollagenIVα3(+), CollagenIVα5(+) (Table 2).
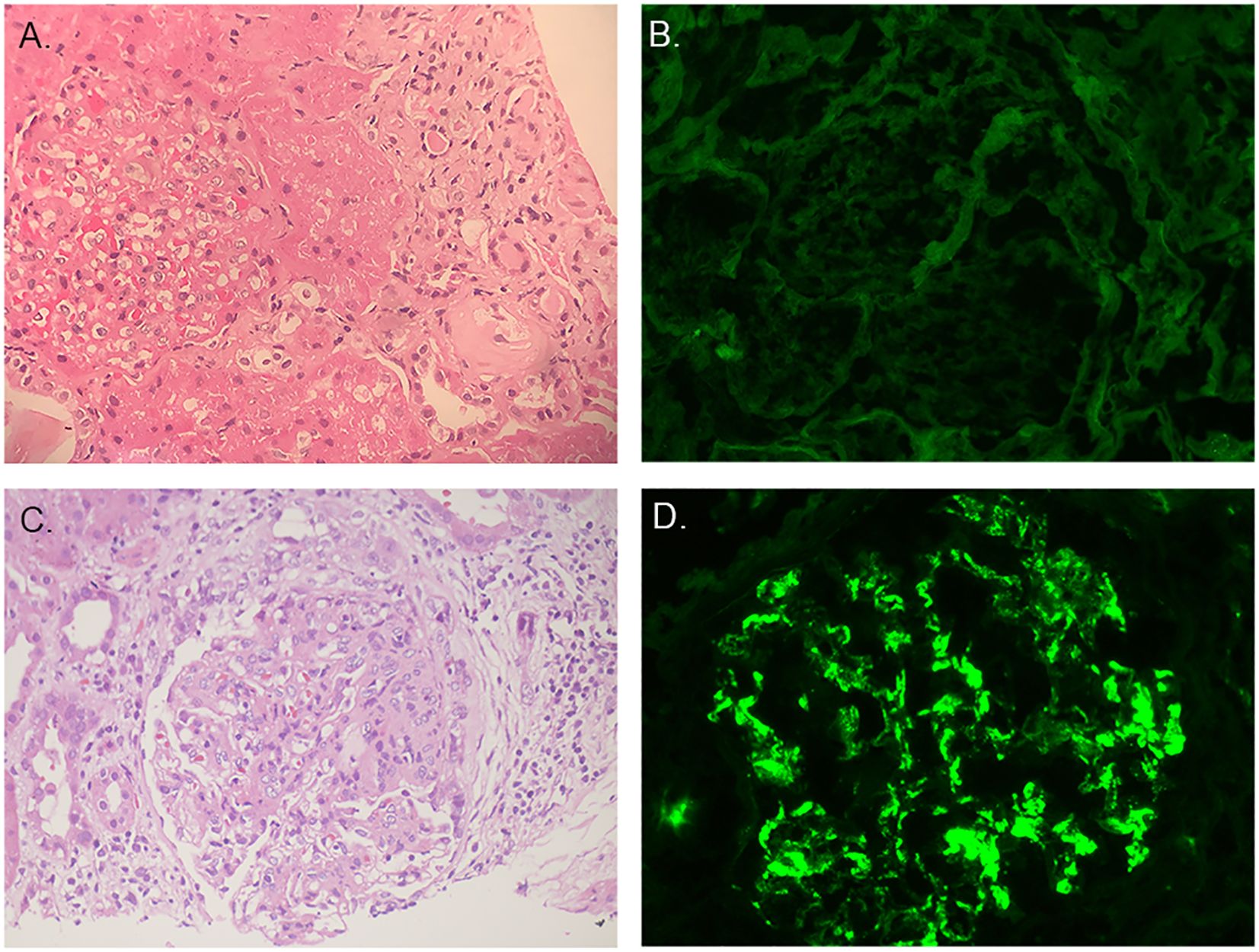
Figure 1. Pathological findings of renal biopsies. (A) The first renal biopsy: H&E staining. (B) The first renal biopsy: immunofluorescence staining showed IgA was negative. (C) The second renal biopsy: H&E staining. (D) The second renal biopsy: immunofluorescence staining showed IgA was positive.
In August 2018, the boy was diagnosed with IgAV and developed hematuria and proteinuria, so a second renal biopsy was performed. The pathologic diagnosis was HN with IgAV nephritis (IVb).
Out of the 23 glomeruli, 7 exhibited glomerulosclerosis, 1 was nearing sclerosis, and the majority showed moderate to severe mesangial cell proliferation accompanied by increased matrix. Some glomerular capillary loops were adherent to the Bowman’s capsule, and 5 cellular crescents were observed. The proximal tubular epithelial cells exhibited mild swelling with partial vacuolization. Red blood cell casts, cellular casts, and protein casts were observed within the tubular lumens. Significant inflammatory cell infiltration in the renal interstitium accompanied by interstitial fibrosis. Some small arterial walls were thickened with accompanying luminal narrowing (Figure 1C). Immunofluorescence displayed that IgG(-), IgA(+++) (Figure 1), IgM(-), C3(±), C4(-), C1q(-), PLA2-R1(-),CollagenIVα1(+), CollagenIVα3(+), CollagenIVα5(+) (Table 2).
3.3 DEG identification
From the GSE24752 dataset, a total of 90 DEGs were successfully identified, comprising 79 upregulated and 11 downregulated genes. In the GSE102114 dataset, we observed 7,027 DEGs, which included 2,600 upregulated and 4,427 downregulated genes. Among all the DEGs identified, 25 were common to both datasets, as illustrated in the Venn diagram (Figure 2). These 25 DEGs included 5 downregulated genes and 20 upregulated genes. The DEGs are depicted in Figure 2, where red dots represent upregulated genes and green dots represent downregulated genes.
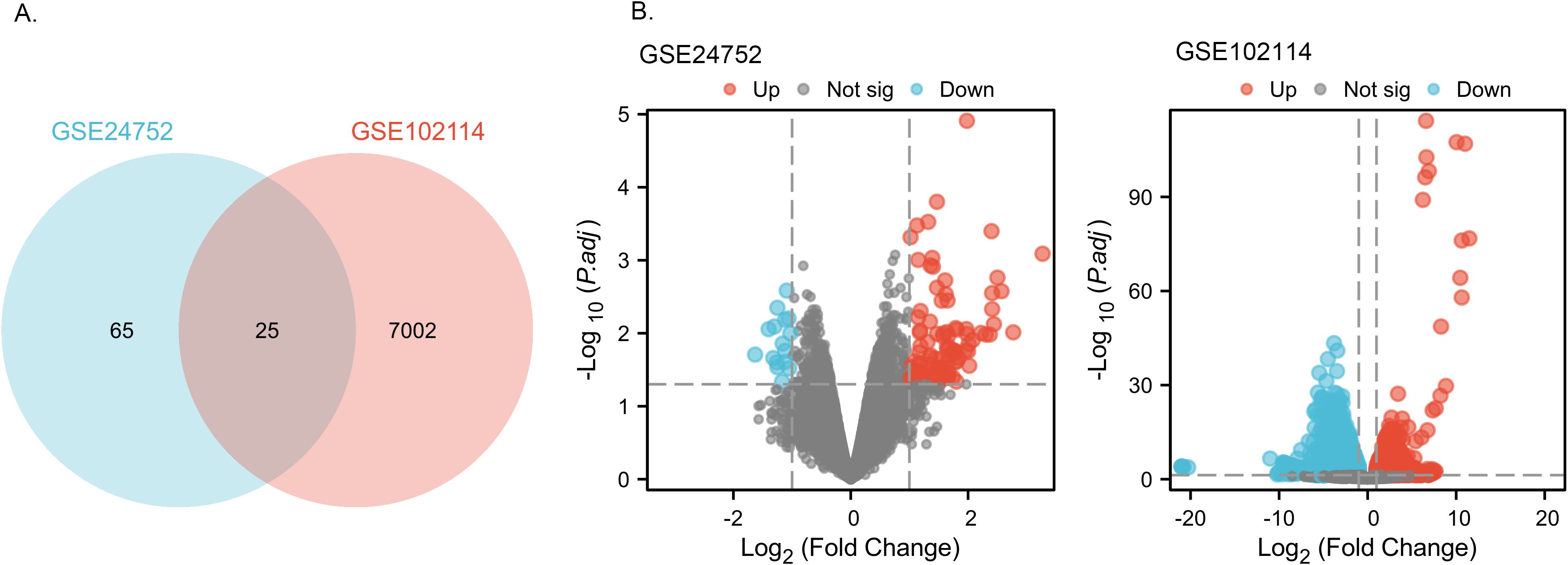
Figure 2. Identification of common DEGs from GSE24752, GSE102114 datasets. (A) Venn diagram of DEGs based on the two GEO datasets. (B) Volcano plot of the DEGs. Red, upregulation; green, downregulation.
3.4 GO annotation and KEGG pathway enrichment analyses
To gain deeper insight into the biological roles of these 25 DEGs, we conducted functional and pathway enrichment analyses using DAVID. The enriched GO terms and KEGG pathways are presented in Figure 3. KEGG pathway analysis revealed that the DEGs were primarily associated with extracellular matrix (ECM) receptor interaction. GO biological process analysis indicated that the 25 DEGs were significantly associated with proteolysis, amyloid fibril formation, cAMP-mediated signaling, synaptic vesicle endocytosis and receptor internalization. The significantly enriched terms regarding changes in cell component of DEGs were platelet alpha granule membrane, nucleoplasm and endocytic vesicle membrane. Changes in molecular function are primarily associated with protein binding.
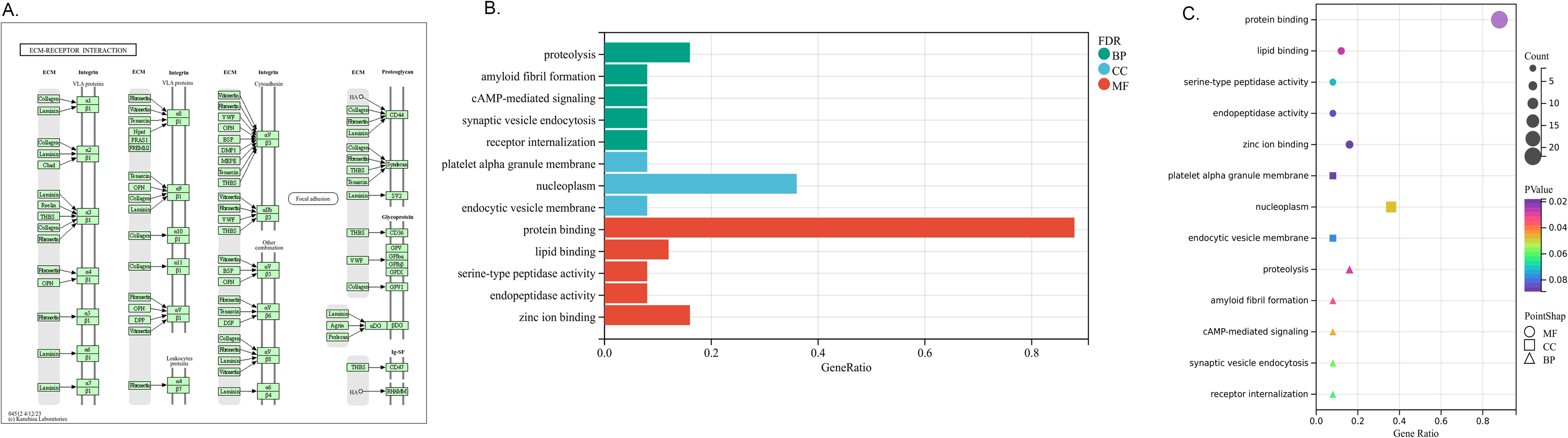
Figure 3. Distribution of integrated DEGs for different enriched functions. (A) KEGG pathway- EMC receptor interaction. (B) The DEGs enrichment of BP, MF, CC in bar chart. (C) The DEGs enrichment of BP, MF, CC in bubble chart.
3.5 PPI analysis and infiltrating immune cells
A total of 25 DEGs were imported into the PPI network complex, comprising 34 nodes and 49 edges (Figures 4A, B). We then applied Cytotype MCODE for further analysis, and the results are shown in Figure 4. Six hub genes were identified in both diseases, and they were linked to cell adhesion molecules.
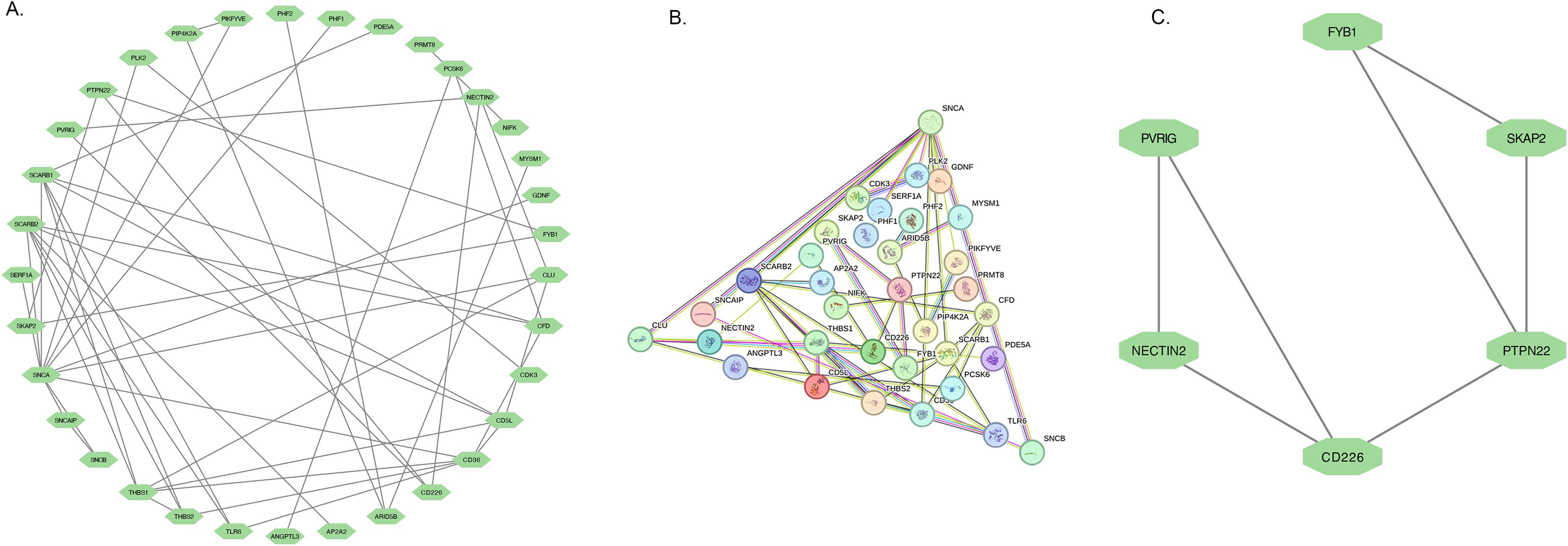
Figure 4. PPI network and the significant module of DEGs. (A) The significant module was obtained from PPI network constructed using Cytoscape with 34 nodes and 49 edges. (B) The PPI network of DEGs. (C) The 6 hub genes were analysed by Cytoscape MCODE.
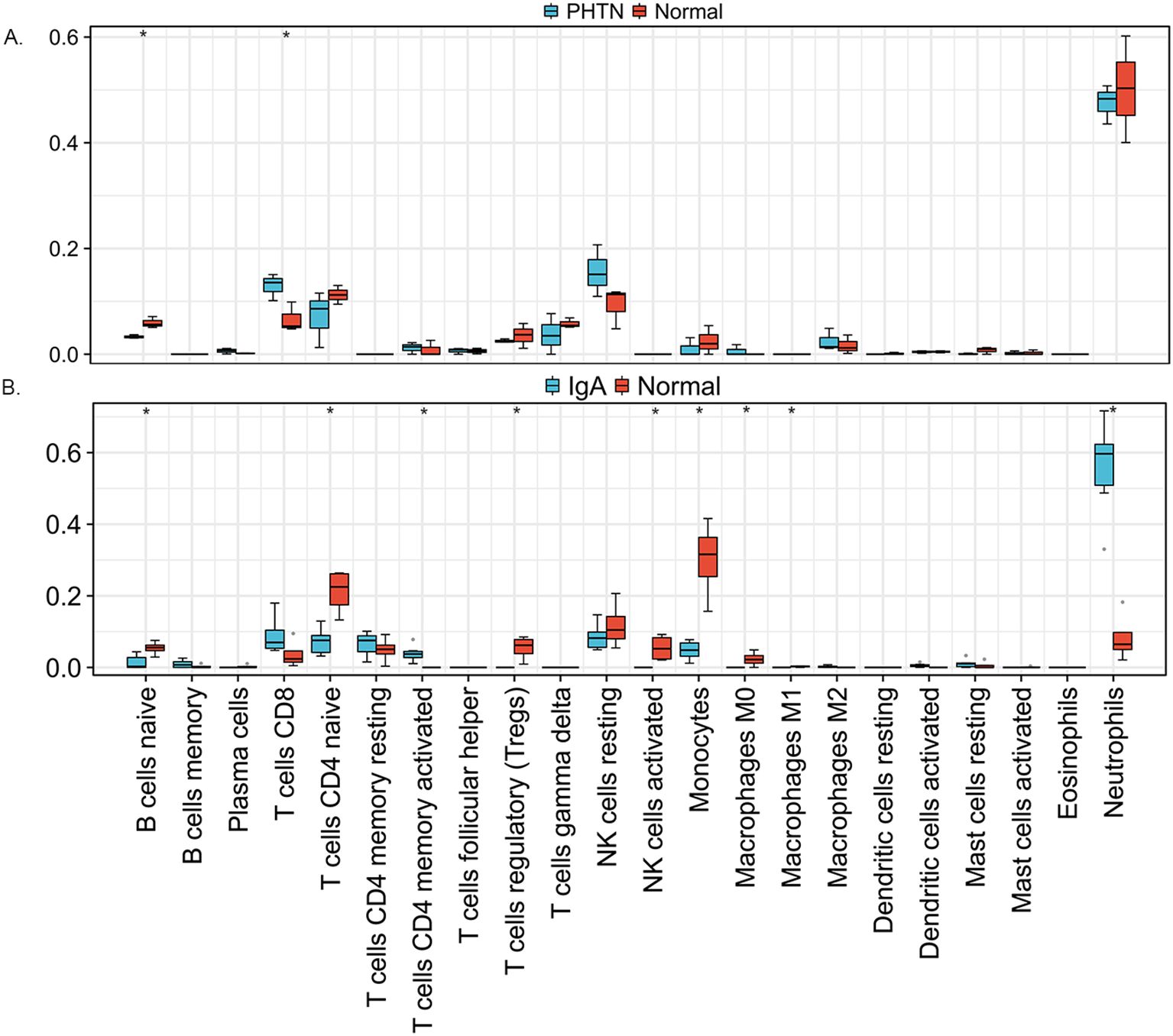
We used the online CIBERSORT algorithm to assess the expression changes in immune cells. We found that neutrophils accounted for the majority of all infiltrating cells (Figure 5). The differential expression proportion of immune-infiltrating cells in the PHTN and normal groups are B cell naïve (downregulated) and T cell CD4 (upregulated). We found that B cell naïve, T cell CD4 naïve, regulatory T cells (Tregs), activated NK cells, monocytes, macrophages M0 and M1 were downregulated. T cell CD4 memory activated and neutrophils were upregulated (Figure 6).
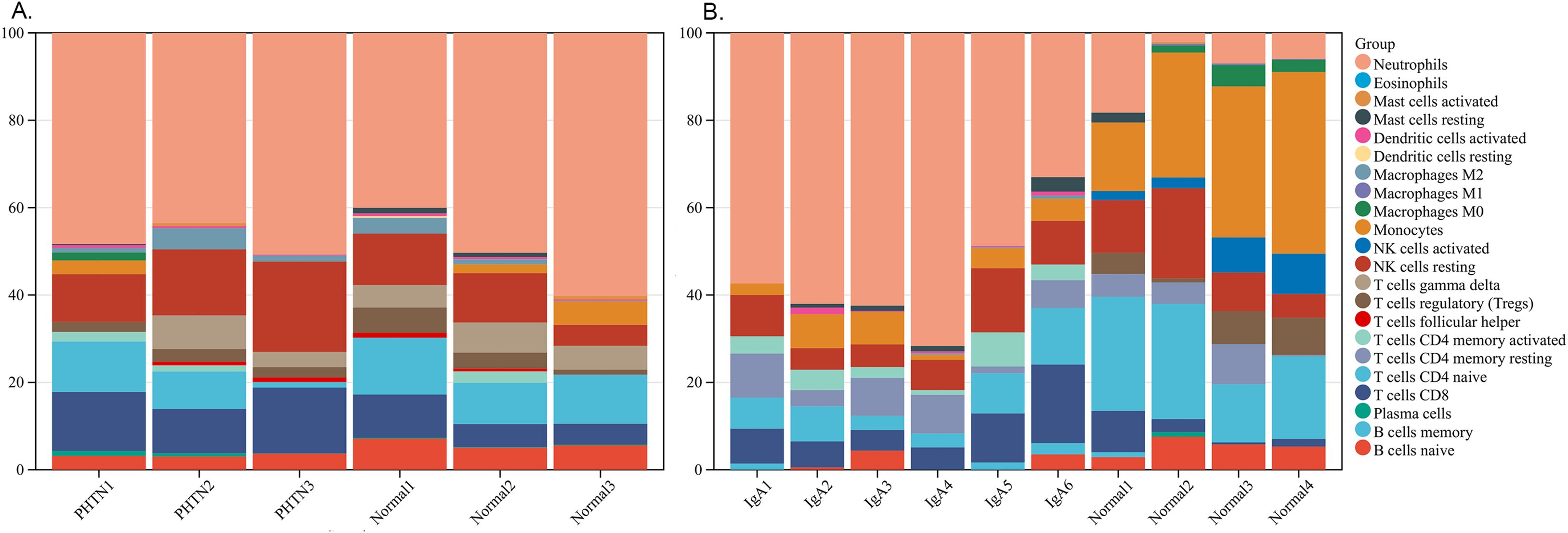
Figure 5. Stacked bar charts of 22 immune cell proportions. (A) PHTN, (B) IgA. Neutrophils accounted for the majority.
We have added this part to the manuscript and made corresponding revisions to the methods, results, and discussion sections.
3.6 Identification of bioactive compounds by CMap analysis
Given the role of 25 DEGs in PHTN and IgAV, we probed for potential therapeutic compounds that might best be suited to target these genes in order to achieve a beneficial therapeutic outcome. The top 10 compounds are shown in Table 3. These compounds sequentially ordered by score are: dicoumarol, tramiprosate, delivert, levetiracetam, troglitazone, salirasib, finasteride, febuxostat, metformin and dapagliflozin. Target genes corresponding to each compound were also listed. Together, these compounds and target genes provide a promising list for researchers or companies interested in conducting pre-clinical research into the mechanisms of and treatments for PHTN and IgAV.
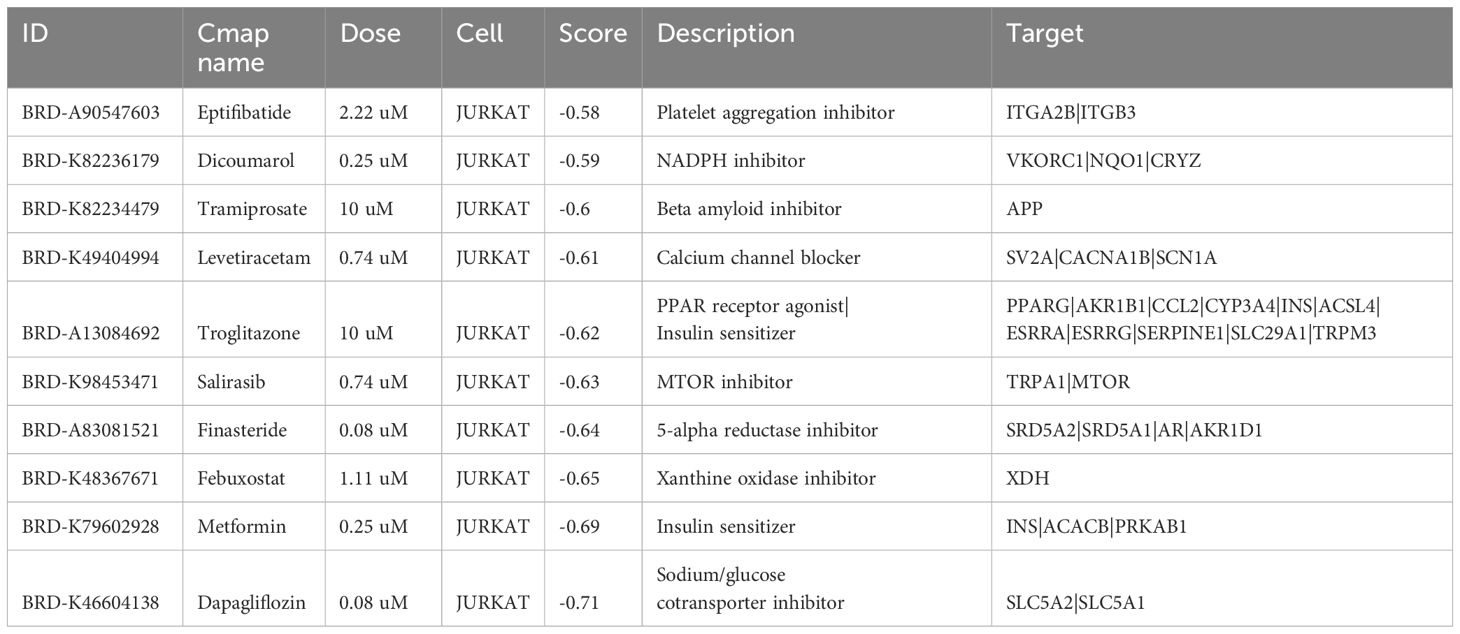
Table 3. Top 10 compounds predicted to have activity against PHTN and IgAV as predicted via connectivity map.
3.7 Expression of fibronectin in kidney tissue
Using immunofluorescence microscopy, we found that Fibronectin expression was markedly enhanced (Figure 7). Consistent with bioinformatics analysis results, Fibronectin expression was markedly elevated in hypertensive nephritis and IgA nephropathy.
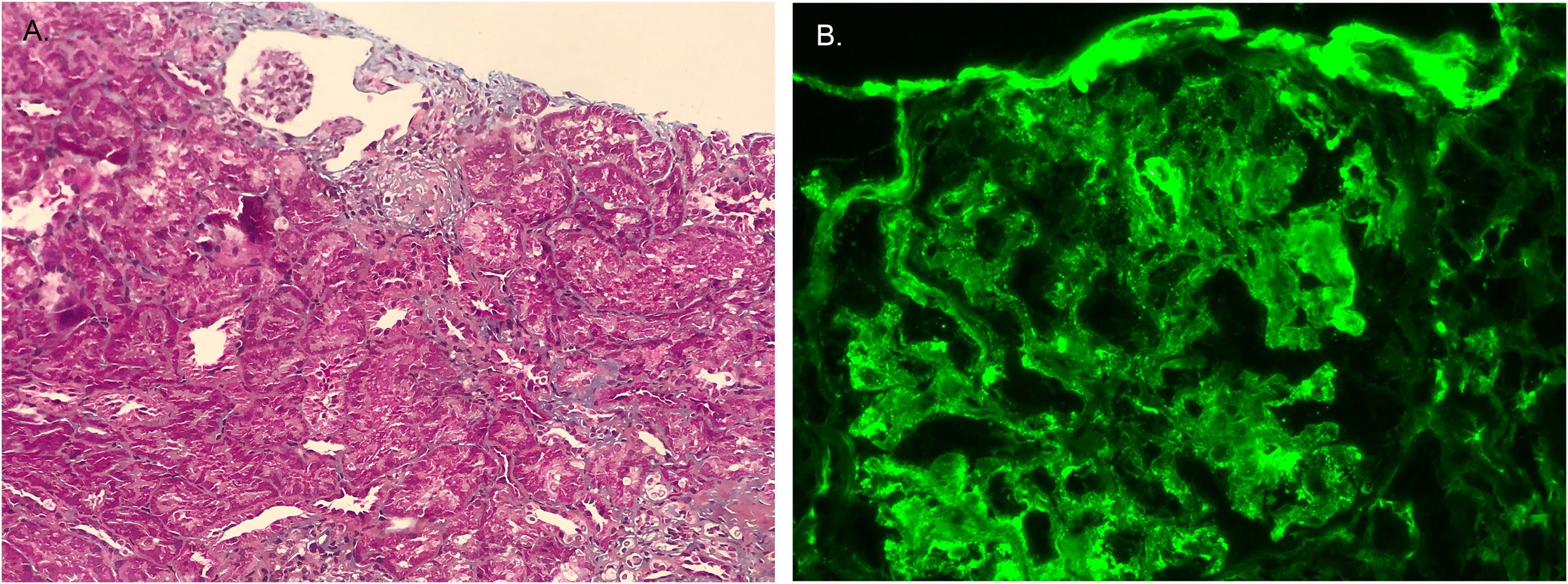
Figure 7. The expression of Fibronectin. (A) Fibronectin exhibits blue staining in Masson’s trichrome-stained renal tissue. (B) Immunofluorescence staining showed Fibronectin was positive.
4 Discussion
PHTN has become a major public health challenge, with prevalence rates rising to 3.5–5% in recent decades (16). Untreated PHTN predisposes children to long-term cardiovascular and renal complications, notably hypertensive nephropathy—a leading cause of progressive renal dysfunction and ESRD (17–19). IgAV/Henoch-Schönlein purpura, the most prevalent childhood small-vessel vasculitis (20), classically presents with palpable purpura, abdominal pain, arthralgia/arthritis, and renal involvement (IgAV nephritis, IgAVN) (21). Renal manifestations affect 20–54% of pediatric IgAV cases (22), ranging from self-limited hematuria to severe nephritis requiring intervention (23).
While PHTN and IgAV represent distinct entities, our case implies potential mechanistic overlaps. PHTN pathogenesis involves multifactorial interactions—genetic susceptibility, endothelial dysfunction (reduced nitric oxide bioavailability, elevated endothelin-1) (24), RAAS activation (25), sympathetic overactivity (26), sodium retention (27, 28), and chronic inflammation-driven vascular remodeling (29). In contrast, IgAV arises from IgA-dominant immune complex deposition triggered by infections or immune dysregulation (6, 30, 31), involving aberrant T-cell responses and elevated circulating IgA levels (32). These complexes deposit in small vessels, inciting inflammation across target organs (skin, gut, joints, kidneys) (33).
These 25 DEGs are PIP4K2A, MIER3, MYSM1, PDE5A, LMTK3, ZNF652, ERAP1, CFD, SESN3, AP2A2, CD36, ATF1, PCSK6, CHURC1, ZDHHC14, GRAPL, HSPC102, SNCA, PVRIG, NIFK, TBC1D10A, MBNL3, VWA5A, PHF2 and SKAP2. Through systematic literature review, we identified that among the 25 DEGs analyzed, CFD was the only gene with documented research relevance in IgAV. Current evidence highlights CFD as a critical mediator of alternative complement pathway activation in IgAVN, with study reporting significantly elevated urinary CFD levels in pediatric IgAVN patients compared to non-nephritis cases and healthy controls (34). This aligns with its proposed role in amplifying glomerular inflammation and proteinuria. In contrast, the remaining 24 DEGs lack direct experimental or clinical validation in IgAV and PHTN, underscoring a significant knowledge gap in their mechanistic or diagnostic relevance to these diseases. Further investigation is warranted to explore potential roles of these uncharacterized DEGs in IgAV and PHTN pathogenesis or progression.
Currently, there is a lack of research linking ECM receptor interactions to PHTN and IgAV. The ECM is a dynamic, three-dimensional network of macromolecules that provides structural and biochemical support to surrounding cells. Among its key components, fibronectin play a pivotal role in mediating interactions between cells and the ECM. Immunofluorescence staining revealed a significant increase in fibronectin, suggesting that ECM receptor interactions play an important role.
Immune infiltration analysis revealed that neutrophils exhibited the highest proportion in both PHTN and IgAV, while naive B cells were consistently downregulated. IgAV is characterized by IgA immune complex deposition and vascular inflammation, where neutrophil infiltration may serve as a hallmark of its acute phase. Hypertension-induced oxidative stress may activate intrarenal neutrophils, and intraglomerular hypertension directly damages endothelial cells, triggering neutrophil chemotaxis. In IgAV, antigens likely drive the differentiation of naive B cells into plasma cells, leading to a reduced proportion of naive B cells in peripheral blood. In PHTN, chronic inflammation may promote B cell differentiation, thereby depleting the naive B cell population. However, no direct supporting literature has been reported for these mechanisms, and further validation is required.
Effective PHTN management is critical to halting hypertensive nephropathy progression. Aggressive blood pressure control preserves renal function and mitigates cardiovascular risks in chronic kidney disease (CKD) (35). Untreated hypertensive nephropathy progresses to end-stage renal disease (ESRD); notably, proteinuria resolution in our case post-antihypertensive therapy highlights the urgency of early intervention to reduce glomerular hypertension and nephron loss (36). We identified compounds with potential dual efficacy against PHTN and IgAV: Eptifibatide: GPIIb/IIIa inhibitor (antiplatelet) (37). Unclear role in PHTN/IgAV. Dicoumarol: Anticoagulant with anti-inflammatory/renal perfusion benefits (38). Tramiprosate: Anti-inflammatory agent for IgAV and hypertension (39). Levetiracetam: Anticonvulsant with anti-inflammatory properties (40). Troglitazone: PPARγ agonist improving insulin sensitivity and renal protection (41). Salirasib: Ras inhibitor targeting inflammation/fibrosis pathways (42). Finasteride: Reduces proteinuria and preserves renal function (43). Febuxostat: Urate-lowering agent with antioxidative/anti-inflammatory effects (44). Metformin: AMPK activator attenuating kidney injury via metabolic modulation (45). Dapagliflozin: SGLT2 inhibitor lowering intraglomerular pressure and offering renoprotection (46).
While this case report provides valuable insights into potential shared molecular mechanisms between PHTN and IgAV, the inherent limitations of single-case study must be acknowledged. The conclusions drawn from a single patient may lack generalizability due to the unique genetic, environmental, and clinical factors influencing disease progression in individuals. Nevertheless, we hope this case report will offer clinicians a valuable perspective when encountering similar clinical presentations in their practice.
5 Conclusions
After BP-lowering agent treatment, the BP was controlled within the normal range, and urine protein turned negative. Renal pathology also showed significant improvement. It reminds us that aggressive BP-lowering agent was needed in PHTN. We used bioinformatics to determine the common DEGs between PHTN and IgAV. Six hub genes implicated in both diseases were linked to cell adhesion molecules. Moving forward, these shared hub genes could serve as novel targets for more in-depth mechanistic investigations and the development of fresh therapeutic interventions for individuals affected by PHTN and IgAV. Further research into these agents’ mechanisms of action will be crucial for optimizing treatment regimens and improving patient outcomes across these interconnected diseases.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, http://www.ncbi.nlm.nih.gov/geo.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Children’s Hospital of Fudan University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
QW: Investigation, Funding acquisition, Formal analysis, Methodology, Writing – original draft, Data curation. JF: Writing – review & editing, Investigation, Software, Methodology. YM: Writing – original draft, Data curation, Conceptualization, Formal analysis. QS: Data curation, Writing – review & editing, Investigation. HX: Writing – review & editing, Investigation, Resources. LS: Visualization, Validation, Investigation, Writing – review & editing, Supervision. HL: Software, Visualization, Supervision, Project administration, Writing – review & editing, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by a grant from Shanghai Pujiang Young Rheumatologists Training Program (SPROG2403).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Falkner B, Gidding SS, Baker-Smith CM, Brady TM, Flynn JT, Malle LM, et al. Pediatric primary hypertension: an underrecognized condition: A scientific statement from the american heart association. Hypertension. (2023) 80:e101–11. doi: 10.1161/HYP.0000000000000228
2. Halbach SM and Flynn JT. Childhood primary hypertension: not uncommon, not benign. Hypertension. (2023) 80:1197–8. doi: 10.1161/HYPERTENSIONAHA.123.21041
3. Tran DC, Do MD, Le LHG, Thai TT, Hoang SV, and Truong BQ. Relationship between the AGT M235T genetic variant and the characteristics and prognosis of coronary atherosclerosis in patients with acute myocardial infarction. Mol Biol Rep. (2024) 51:1072. doi: 10.1007/s11033-024-09986-5
4. Saxena T, Ali AO, and Saxena M. Pathophysiology of essential hypertension: an update. Expert Rev Cardiovasc Ther. (2018) 16:879–87. doi: 10.1080/14779072.2018.1540301
5. VanSickle JS and Warady BA. Chronic kidney disease in children. Pediatr Clin North Am. (2022) 69:1239–54. doi: 10.1016/j.pcl.2022.07.010
6. Song Y, Huang X, Yu G, Qiao J, Cheng J, Wu J, et al. Pathogenesis of igA vasculitis: an up-to-date review. Front Immunol. (2021) 12:771619. doi: 10.3389/fimmu.2021.771619
7. Ait-Tigrine S, Liaudet L, Bogiatzi S, Yerly P, Hullin R, and Kirsch M. IgA vasculitis with henoch-schönlein purpura as an immune complication associated with left ventricle assist device insertion. ASAIO J. (2022) 68:e69–72. doi: 10.1097/MAT.0000000000001477
8. Neufeld M, Molyneux K, Pappelbaum KI, Mayer-Hain S, von Hodenberg C, Ehrchen J, et al. Galactose-deficient IgA1 in skin and serum from patients with skin-limited and systemic IgA vasculitis. J Am Acad Dermatol. (2019) 81:1078–85. doi: 10.1016/j.jaad.2019.03.029
9. Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, et al. NCBI GEO: archive for functional genomics data sets–update. Nucleic Acids Res. (2013) 41:D991–5. doi: 10.1093/nar/gks1193
10. Korkor MT, Meng FB, Xing SY, Zhang MC, Guo JR, Zhu XX, et al. Microarray analysis of differential gene expression profile in peripheral blood cells of patients with human essential hypertension. Int J Med Sci. (2011) 8:168–79. doi: 10.7150/ijms.8.168
11. Pang S, Lv J, Wang S, Yang G, Ding X, and Zhang J. Differential expression of long non-coding RNA and mRNA in children with Henoch-Schönlein purpura nephritis. Exp Ther Med. (2019) 17:621–32. doi: 10.3892/etm.2018.7038
12. Dennis G Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, et al. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. (2003) 4:P3. doi: 10.1186/gb-2003-4-5-p3
13. Kanehisa M and Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. (2000) 28:27–30. doi: 10.1093/nar/28.1.27
14. Gene Ontology Consortium. Gene Ontology Consortium: going forward. Nucleic Acids Res. (2015) 43:D1049–56. doi: 10.1093/nar/gku1179
15. Subramanian A, Narayan R, Corsello SM, Peck DD, Natoli TE, Lu X, et al. A next generation connectivity map: L1000 platform and the first 1,000,000 profiles. Cell. (2017) 171:1437–1452.e17. doi: 10.1016/j.cell.2017.10.049
16. Hamdani G and Dagan A. PRIMARY HYPERTENSION IN CHILDREN AND ADOLESCENTS. Harefuah. (2021) 160:260–5.
17. Litwin M and Kułaga Z. Obesity, metabolic syndrome, and primary hypertension. Pediatr Nephrol. (2021) 36:825–37. doi: 10.1007/s00467-020-04579-3
18. Zhang Y, Arzaghi H, Ma Z, Roye Y, and Musah S. Epigenetics of hypertensive nephropathy. Biomedicines. (2024) 12:2622. doi: 10.3390/biomedicines12112622
19. Dong Z, Dai H, Feng Z, Liu W, Gao Y, Liu F, et al. Mechanism of herbal medicine on hypertensive nephropathy (Review). Mol Med Rep. (2021) 23:234. doi: 10.3892/mmr.2021.11873
20. González-Gay MA, López-Mejías R, Pina T, Blanco R, and Castañeda S. IgA vasculitis: genetics and clinical and therapeutic management. Curr Rheumatol Rep. (2018) 20:24. doi: 10.1007/s11926-018-0735-3
21. Neumann T. Update Immunglobulin-A-Vaskulitis [Update on immunoglobulin A vasculitis. Z Rheumatol. (2022) 81:305–12. doi: 10.1007/s00393-022-01162-z
22. Vivarelli M, Samuel S, Coppo R, Barratt J, Bonilla-Felix M, Haffner D, et al. IPNA clinical practice recommendations for the diagnosis and management of children with IgA nephropathy and IgA vasculitis nephritis. Pediatr Nephrol. (2025) 40:533–69. doi: 10.1007/s00467-024-06502-6
23. Delbet JD, Parmentier C, Herbez Rea C, Mouche A, and Ulinski T. Management of igA vasculitis with nephritis. Paediatr Drugs. (2021) 23:425–35. doi: 10.1007/s40272-021-00464-0
24. Konukoglu D and Uzun H. Endothelial dysfunction and hypertension. Adv Exp Med Biol. (2017) 956:511–40. doi: 10.1007/5584_2016_90
25. Jasiczek J, Trocha M, Derkacz A, Szahidewicz-Krupska E, and Doroszko A. Effect of the renin-angiotensin-aldosterone system reactivity on endothelial function and modulative role of valsartan in male subjects with essential hypertension. J Clin Med. (2021) 10:5816. doi: 10.3390/jcm10245816
26. Malheiros-Lima MR, Antunes VR, Takakura AC, and Moreira TS. Hypertension and sympathetic nervous system overactivity rely on the vascular tone of pial vessels of the rostral ventrolateral medulla in spontaneously hypertensive rats. Exp Physiol. (2020) 105:65–74. doi: 10.1113/EP088169
27. Nishimoto M, Griffin KA, Wynne BM, and Fujita T. Salt-sensitive hypertension and the kidney. Hypertension. (2024) 81:1206–17. doi: 10.1161/HYPERTENSIONAHA.123.21369
28. Nava E and Lüscher TF. Endothelium-derived vasoactive factors in hypertension: nitric oxide and endothelin. J Hypertens Suppl. (1995) 13:S39–48. doi: 10.1097/00004872-199508001-00007
29. Hertiš Petek T and Marčun Varda N. Childhood cardiovascular health, obesity, and some related disorders: insights into chronic inflammation and oxidative stress. Int J Mol Sci. (2024) 25:9706. doi: 10.3390/ijms25179706
30. Boudarbala H, Elouali A, Sara A, Rkain M, and Babakhouya A. Rheumatoid purpura in children: A retrospective study. Cureus. (2024) 16:e73443. doi: 10.7759/cureus.73443
31. Sugino H, Sawada Y, and Nakamura M. IgA vasculitis: etiology, treatment, biomarkers and epigenetic changes. Int J Mol Sci. (2021) 22:7538. doi: 10.3390/ijms22147538
32. Linke A, Tiegs G, and Neumann K. Pathogenic T-cell responses in immune-mediated glomerulonephritis. Cells. (2022) 11:1625. doi: 10.3390/cells11101625
33. Parums DV. A review of igA vasculitis (Henoch-schönlein purpura) past, present, and future. Med Sci Monit. (2024) 30:e943912. doi: 10.12659/MSM.943912
34. Marro J, Chetwynd AJ, Hawkes J, Northey SJ, and Oni L. Urinary markers of the alternative and lectin complement pathway are increased in IgA vasculitis nephritis. Clin Kidney J. (2023) 16:2703–11. doi: 10.1093/ckj/sfad236
35. Vallée A, Safar ME, and Blacher J. Hypertension artérielle permanente essentielle: définitions et revue hémodynamique, clinique et thérapeutique [Essential hypertension: Definitions, hemodynamic, clinical and therapeutic review. Presse Med. (2019) 48:19–28. doi: 10.1016/j.lpm.2018.11.017
36. Hughes D, Judge C, Murphy R, Loughlin E, Costello M, Whiteley W, et al. Association of blood pressure lowering with incident dementia or cognitive impairment: A systematic review and meta-analysis. JAMA. (2020) 323:1934–44. doi: 10.1001/jama.2020.4249
37. Tonin G and Klen J. Eptifibatide, an older therapeutic peptide with new indications: from clinical pharmacology to everyday clinical practice. Int J Mol Sci. (2023) 24:5446. doi: 10.3390/ijms24065446
38. Ge W, Zhang X, Wang Q, Mao J, Jia P, and Cai J. Dicoumarol attenuates NLRP3 inflammasome activation to inhibit inflammation and fibrosis in knee osteoarthritis. Mol Med Rep. (2024) 29:100. doi: 10.3892/mmr.2024.13224
39. Zielińska-Nowak E, Cichon N, Saluk-Bijak J, Bijak M, and Miller E. Nutritional supplements and neuroprotective diets and their potential clinical significance in post-stroke rehabilitation. Nutrients. (2021) 13:2704. doi: 10.3390/nu13082704
40. Thöne J, Ellrichmann G, Faustmann PM, Gold R, and Haghikia A. Anti-inflammatory effects of levetiracetam in experimental autoimmune encephalomyelitis. Int Immunopharmacol. (2012) 14:9–12. doi: 10.1016/j.intimp.2012.05.021
41. Plosker GL and Faulds D. Troglitazone: a review of its use in the management of type 2 diabetes mellitus. Drugs. (1999) 57:409–38. doi: 10.2165/00003495-199957030-00014
42. Wolfson E, Schmukler E, Schokoroy ST, Kloog Y, Pinkas-Kramarski R, and Enhancing FTS. (Salirasib) efficiency via combinatorial treatment. Biol Cell. (2015) 107:130–43. doi: 10.1111/boc.201400087
43. Plosker GL and Goa KL. Terazosin. A pharmacoeconomic evaluation of its use in benign prostatic hyperplasia. Pharmacoeconomics. (1997) 11:184–97. doi: 10.2165/00019053-199711020-00008
44. Yang XH, Zhang BL, Cheng Y, Fu SK, and Jin HM. Febuxostat provides renoprotection in patients with hyperuricemia or gout: a systematic review and meta-analysis of randomized controlled trials. Ann Med. (2024) 56:2332956. doi: 10.1080/07853890.2024.2332956
45. Bharath LP and Nikolajczyk BS. The intersection of metformin and inflammation. Am J Physiol Cell Physiol. (2021) 320:C873–9. doi: 10.1152/ajpcell.00604.2020
Keywords: primary hypertension, IgA vasculitis, blood pressure-lowering agents, bioinformatics analysis, differentially expressed genes
Citation: Wei Q, Feng J, Mu Y, Shen Q, Xu H, Sun L and Liu H (2025) Exploring the shared molecular mechanisms of primary hypertension and IgA vasculitis through a case report and combining bioinformatics analysis. Front. Immunol. 16:1596174. doi: 10.3389/fimmu.2025.1596174
Received: 19 March 2025; Accepted: 19 May 2025;
Published: 06 June 2025.
Edited by:
Nicola Susca, University of Bari Aldo Moro, ItalyReviewed by:
Jie Wang, First Affiliated Hospital of Xinjiang Medical University, ChinaBiao Huang, Second Affiliated Hospital of Chengdu Medical College, China
Copyright © 2025 Wei, Feng, Mu, Shen, Xu, Sun and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haimei Liu, bGl1X2htQDE2My5jb20=; Li Sun, bGlsbHlzdW5AMjYzLm5ldA==
†These authors have contributed equally to this work
 Qijiao Wei
Qijiao Wei Jiayan Feng2†
Jiayan Feng2†