- Department of otorhinolaryngology, Renmin Hospital of Wuhan University, Wuhan, Hubei, China
Objective: This study aimed to investigate the relationship between prognostic nutritional index (PNI) and prognosis in patients with head and neck squamous cell carcinoma (HNSCC).
Methods: A systematic review was conducted across three major databases—Embase, PubMed, and the Cochrane Library—to identify studies examining the association between PNI and outcomes in HNSCC patients. The search included all records from database inception through January 20, 2025. Outcomes assessed included hazard ratios (HRs) for overall survival (OS), cancer-specific survival (CSS), disease-free survival (DFS), and progression-free survival (PFS), as well as odds ratios (ORs) for objective response rate (ORR) and disease control rate (DCR).
Results: A total of 27 articles involving 4,400 patients were included. Patients with low PNI had significantly shorter OS (HR: 2.42, 95% CI: 2.15–2.73, p < 0.001), CSS (HR: 2.05, 95% CI: 1.09–3.84, p = 0.026), DFS (HR: 1.89, 95% CI: 1.58–2.27, p < 0.001), and PFS (HR: 2.23, 95% CI: 1.90–2.62, p < 0.001) compared to those with high PNI. Additionally, low PNI was associated with lower ORR (OR: 0.40, 95% CI: 0.22–0.73, p = 0.002) and DCR (OR: 0.30, 95% CI: 0.17–0.53, p < 0.001). Subgroup analyses confirmed consistent associations between PNI and OS, DFS, and PFS across different Cox models, cancer types, treatment modalities (immune checkpoint inhibitors and surgery), countries, and PNI cut-off values.
Clinical trial registration: This study underscores the prognostic significance of PNI in predicting survival outcomes and treatment responses in HNSCC patients. The findings highlight the importance of incorporating PNI into routine prognostic assessments to improve clinical decision-making and patient management in HNSCC.
1 Introduction
Head and neck squamous cell carcinoma (HNSCC) encompasses a diverse group of tumors originating from various anatomical sites, including the oral cavity, oropharynx, hypopharynx, and larynx (1, 2). Therapeutic approaches for HNSCC include surgical resection, radiotherapy, chemotherapy, targeted molecular therapies, and immune checkpoint inhibitors (ICIs) (3). Over the past decade, advances in treatment strategies have significantly improved therapeutic outcomes. However, predicting prognosis remains a major challenge for head and neck surgeons. To optimize treatment strategies, there is an urgent need to identify reliable biomarkers that can more accurately predict both prognosis and treatment response (4, 5).
The prognostic nutritional index (PNI) is calculated based on serum albumin concentration and lymphocyte count. Serum albumin is a recognized biomarker of nutritional status and has been linked to comorbidities and cancer prognosis (6, 7). Lymphocytes, as key mediators of cell-mediated immunity, play a critical role in suppressing cancer cell proliferation and invasion (8). As a result, PNI provides an integrated measure of both the nutritional and immunological health of a patient. Initially introduced as a predictor of postoperative complications in gastrointestinal cancer patients (9), recent research has established its relevance in predicting clinical outcomes across various cancer types (10–13).
The predictive value of PNI in HNSCC patients, however, remains controversial. For instance, studies by Abe et al., Matsumura et al., and Miyamoto et al. reported that HNSCC patients with high PNI levels had longer overall survival (OS) (14–16). In contrast, studies by Ikeguchi et al., Song et al., and Tada et al. suggested that PNI levels were not significantly correlated with prognosis (17–19).
This study aims to resolve the controversy by systematically synthesizing all available evidence, thereby enhancing our understanding of the clinical significance of PNI in predicting prognosis for HNSCC patients. To the best of our knowledge, this is the first pooled analysis to comprehensively evaluate the role of PNI in predicting both prognosis and treatment response in patients with HNSCC.
2 Methods
2.1 Search strategy
An electronic search was initiated on January 20, 2025, across major bibliographic databases, including EMBASE, PubMed, and the Cochrane Library. The search used predefined terms such as “Squamous Cell Carcinoma of Head and Neck” [Mesh], “Oral Tongue Squamous Cell Carcinoma,” “Hypopharyngeal Squamous Cell Carcinoma,” “Oropharyngeal Squamous Cell Carcinoma,” “Laryngeal Squamous Cell Carcinoma,” and “prognostic nutritional index,” covering all relevant domains. The search was limited to human studies published in English. A detailed description of the search strategy is provided in Supplementary Material 1. Additionally, grey literature was sourced from Google Scholar, and reference lists of relevant studies were manually reviewed. Following Cochrane collaboration guidelines, findings from both manual and electronic searches were compiled in Covidence software for data management.
2.2 Inclusion and exclusion criteria
We established the following inclusion criteria for article selection: (i) studies involving patients diagnosed with HNSCC; (ii) studies assessing the prognostic significance of baseline PNI; and (iii) studies reporting at least one of the following clinical outcomes: OS, progression-free survival (PFS), disease-free survival (DFS), cancer-specific survival (CSS), objective response rate (ORR), or disease control rate (DCR). The exclusion criteria were: (i) studies based on animal models, literature reviews, case reports, or conference abstracts; (ii) studies lacking hazard ratios (HRs) or odds ratios (ORs) for outcome evaluation, either from the main text or published data. In cases where multiple studies included overlapping patient cohorts, preference was given to those with more comprehensive data and robust methodological quality.
2.3 Data extraction and quality assessment
During data extraction, we systematically collected key details, including authorship, publication year, study period, geographic location, cancer types, treatment modalities, sample size, demographic information (age and gender), and PNI cut-off values. The primary data sources for HRs, ORs, and their respective 95% confidence intervals (CIs) were multivariate analyses. When these were unavailable, data were either derived from univariate analyses or extracted from survival plots using Engauge Digitizer software. The quality of the included observational studies was assessed using the Newcastle-Ottawa Scale (NOS), with studies scoring six or above considered of high quality. The nine-point NOS criteria evaluate areas such as patient selection, study comparability, and outcome measurement. All stages of the process, from literature retrieval and screening to data extraction and quality evaluation, were independently performed by two researchers, with discrepancies resolved through consultation with the senior author.
2.4 Statistical methods
Statistical analyses were conducted using Stata 18.0, with results visualized through forest plots. Heterogeneity was assessed using Cochran’s Q test and I² statistics, with significant heterogeneity defined as a p-value < 0.1 and I² > 50%. In cases of substantial heterogeneity, the DerSimonian-Laird random-effects model was employed, while the Inverse Variance fixed-effects model was used otherwise (20). To evaluate the potential for publication bias, we used funnel plots when the number of included studies for a specific outcome was ≥12, in accordance with PRISMA and MOOSE guidelines. For outcomes with fewer than 12 studies, the statistical power of funnel plot asymmetry tests is limited. Therefore, we applied Begg’s tests to assess publication bias in these cases (21). The robustness of the findings was tested through sensitivity analyses by systematically excluding individual studies (22). Additionally, subgroup analyses were performed, focusing on different Cox models, cancer types, treatment modalities, countries, and PNI cut-off values. A two-tailed p-value < 0.05 was considered statistically significant.
3 Results
3.1 Search results and included studies
The initial search strategy, combined with manual review, identified 397 potentially relevant articles. After removing 70 duplicates, 272 articles were excluded for failing to meet the inclusion criteria based on their titles and abstracts. A thorough evaluation of the remaining 55 full-text articles led to the exclusion of 28, as they did not fulfill the established criteria. As a result, 27 articles with 29 studies were ultimately deemed eligible for inclusion (Figure 1) (1, 4, 14–19, 23–41).
3.2 Study characteristics
Table 1 summarizes the key characteristics of the studies included in this analysis. The cohort comprised 4,400 patients, of whom 73.65% were male. Sample sizes ranged from 42 to 661 individuals. Among the studies, 14 were conducted in Japan, seven in China, and two in the United States. Additionally, one study was conducted in Canada, one in Hungary, one in Italy, one in Korea, one in Tottori, and one in the USA. Treatment modalities varied: 14 studies involved surgical treatment, 6 studies used ICIs, 3 studies utilized chemoradiotherapy, and 3 studies applied comprehensive therapy. All studies were retrospective, with NOS scores ranging from 6 to 8, indicating a low risk of bias (Table 1).
3.3 Baseline prognostic nutritional index and overall survival and cancer-specific survival
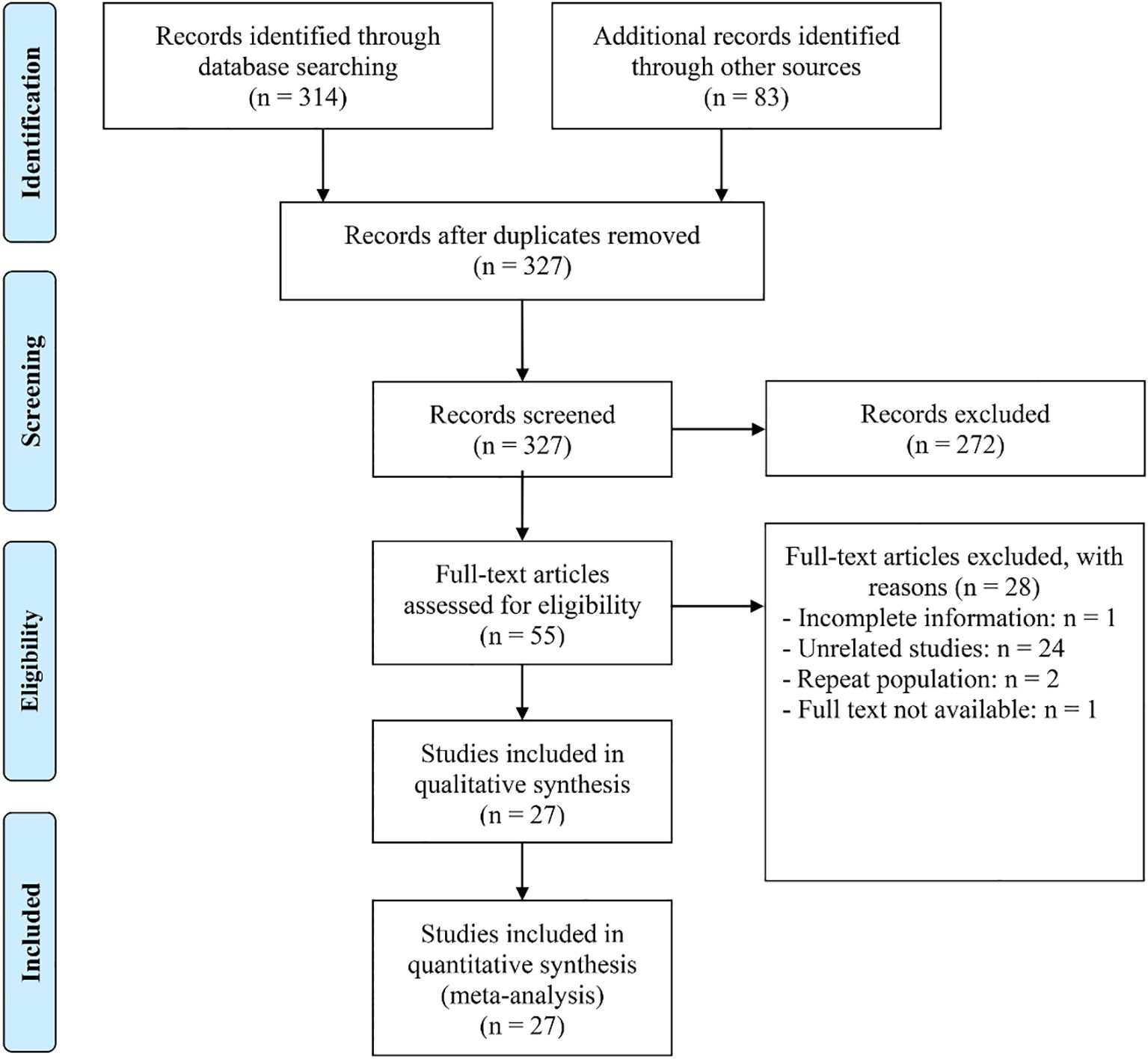
In this study, we included 28 studies comprising a total of 3,739 cancer patients to examine the impact of high and low PNI on OS in patients with HNSCC. The analysis revealed that patients with low PNI had significantly shorter OS (HR: 2.42, 95% CI: 2.15–2.73, p < 0.001, Figure 2A) compared to those with high PNI. No significant heterogeneity across studies was found, as indicated by Cochran’s Q test and I² statistics (I² = 26.3%, p = 0.102). Therefore, a fixed-effects model was applied. In addition, three studies treated PNI as a continuous variable and found that higher PNI was associated with longer OS in patients (I² = 1.3%, p = 0.363; HR: 0.94, 95% CI: 0.93–0.96, p < 0.001, Figure 2B).
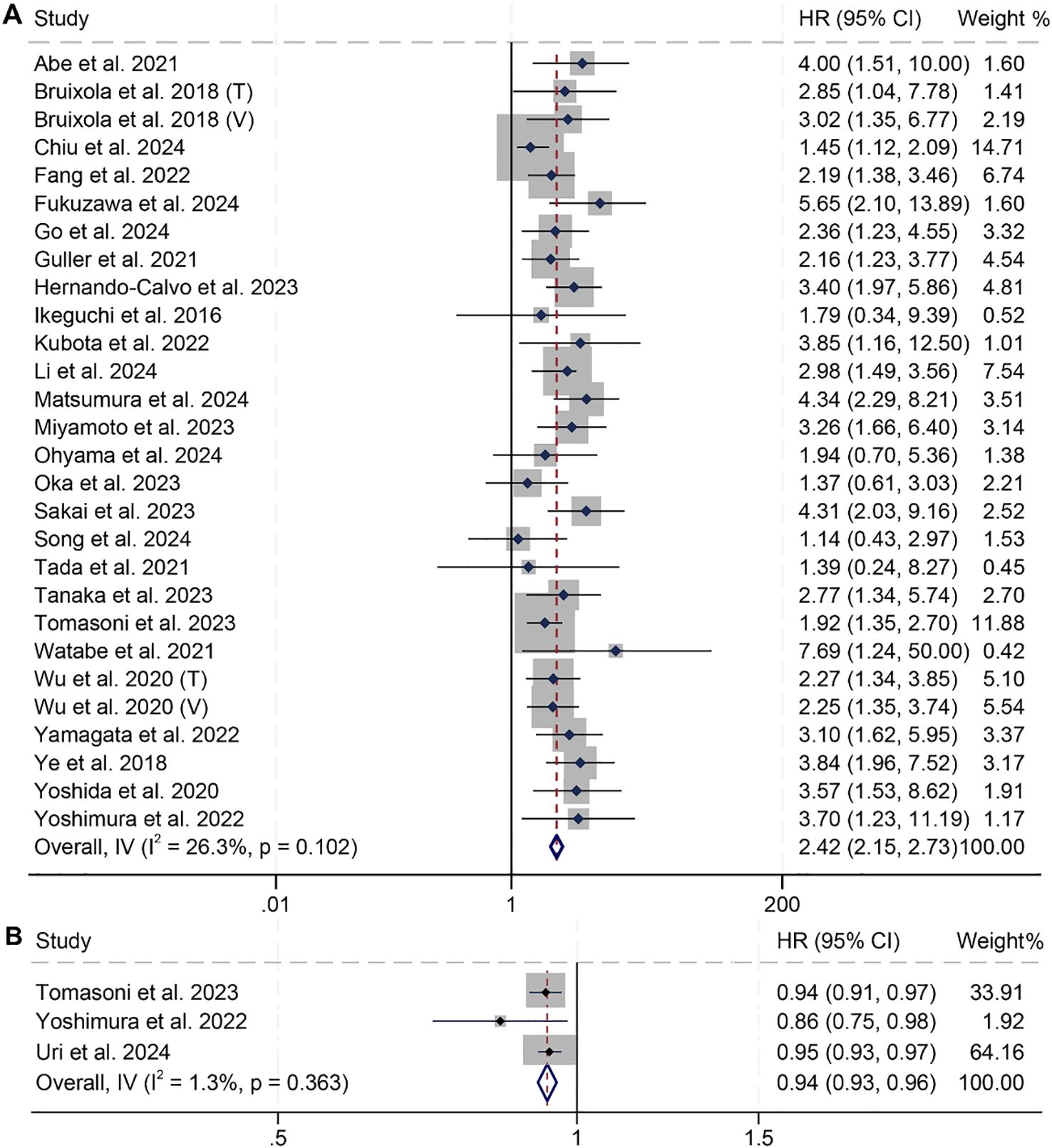
Figure 2. Forest plots showing the association between prognostic nutritional index (PNI) and overall survival (OS). (A) PNI analyzed as a binary variable (high vs. low); (B) PNI analyzed as a continuous variable (per unit increase). HR, hazard ratio; CI, confidence interval.
Subgroup analyses confirmed that the association between PNI and OS was consistent across subgroups with different Cox models, treatment modalities, countries, and PNI cut-off values (Table 2).
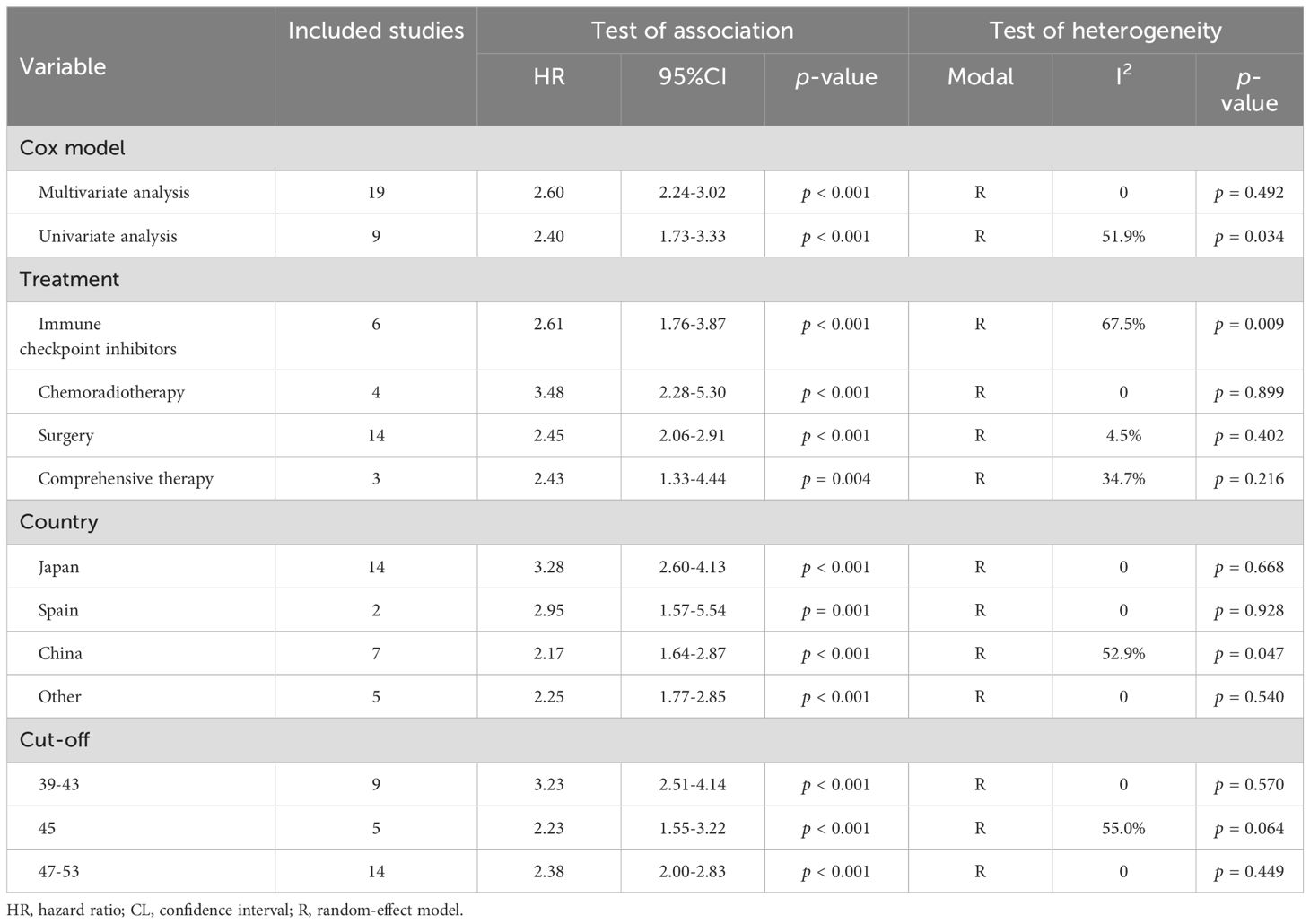
Table 2. Subgroup analysis of the association between prognostic nutritional index and overall survival in patients with head and neck squamous cell carcinoma.
Sensitivity analysis, which systematically excluded each study, demonstrated that the pooled HRs for OS remained stable and robust (Figure 3A). Assessments of publication bias using funnel plots and Begg’s test showed no significant bias (Begg’s test: p = 0.441, Figure 3B).
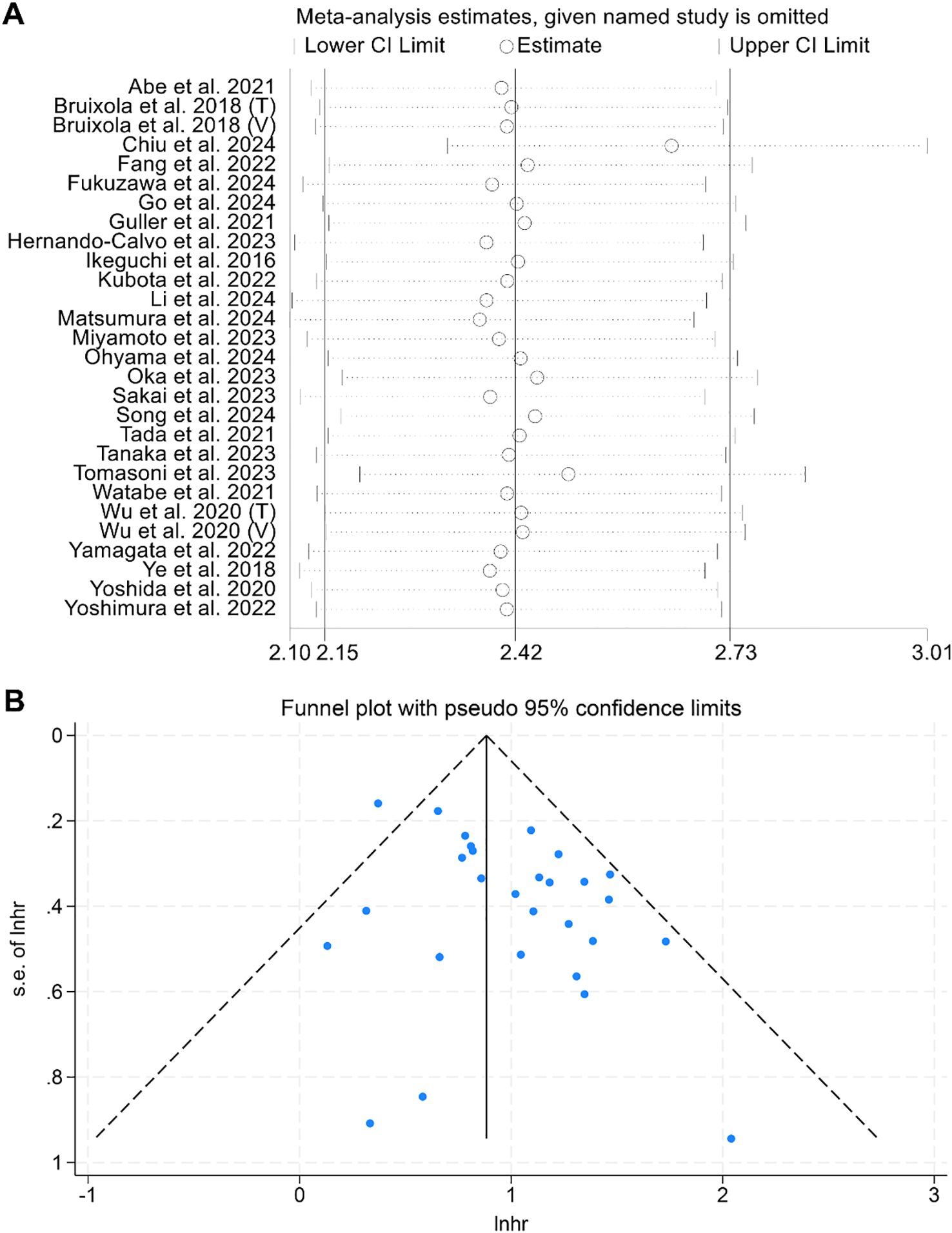
Figure 3. (A) Sensitivity analysis of the association between prognostic nutritional index (PNI) and overall survival (OS), based on sequential exclusion of each included study. (B) Funnel plot assessing publication bias in the analysis of PNI and OS. HR, hazard ratio; CI, confidence interval.
We also found that HNSCC patients with low PNI had shorter CSS compared to those with high PNI (Binary variables: HR: 2.05, 95% CI: 1.09–3.84, p = 0.026; Continuous variables: HR: 0.94, 95% CI: 0.92–0.97, p < 0.001) (Supplementary Figures S1A, B).
3.4 Pretreatment prognostic nutritional index and disease-free survival and progression-free survival
A total of 10 studies involving 1,551 patients and 11 studies with 1,379 patients examined the predictive value of PNI on DFS and PFS in HNSCC patients, respectively. The findings revealed that cancer patients with low PNI had significantly poorer DFS (I² = 6.2%, p = 0.384; HR: 1.89, 95% CI: 1.58–2.27, p < 0.001, Figure 4A) and PFS (I² = 8.1%, p = 0.367; HR: 2.23, 95% CI: 1.90–2.62, p < 0.001, Figure 4B). Subgroup analyses further demonstrated that low PNI was significantly associated with worse DFS and PFS across various subgroups, with detailed results presented in Tables 3, 4.
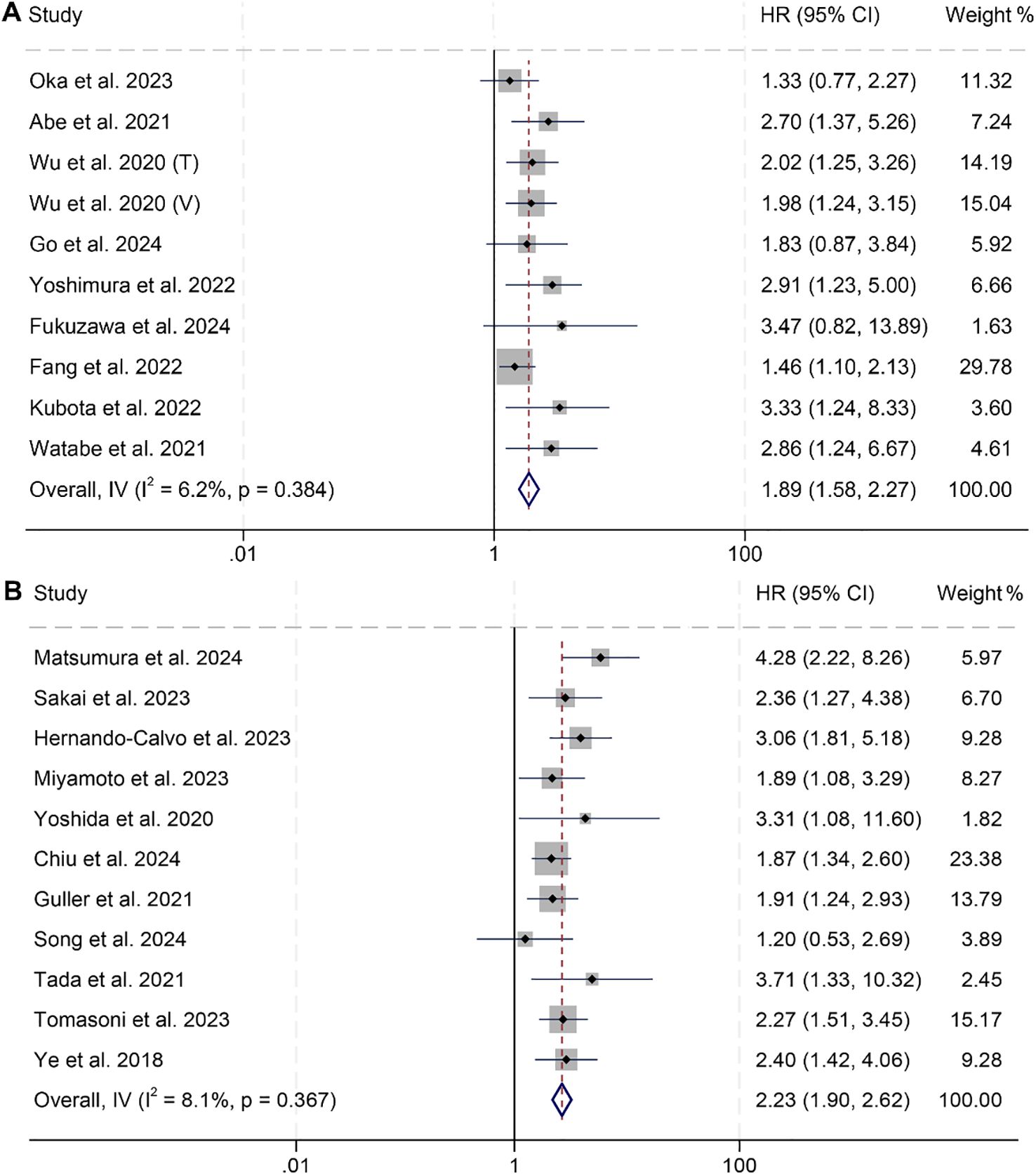
Figure 4. Forest plots showing the association between prognostic nutritional index (PNI) and survival outcomes: (A) Disease-free survival (DFS); (B) Progression-free survival (PFS). Both panels represent analyses using PNI as a binary variable (high vs. low). HR, hazard ratio; CI, confidence interval.
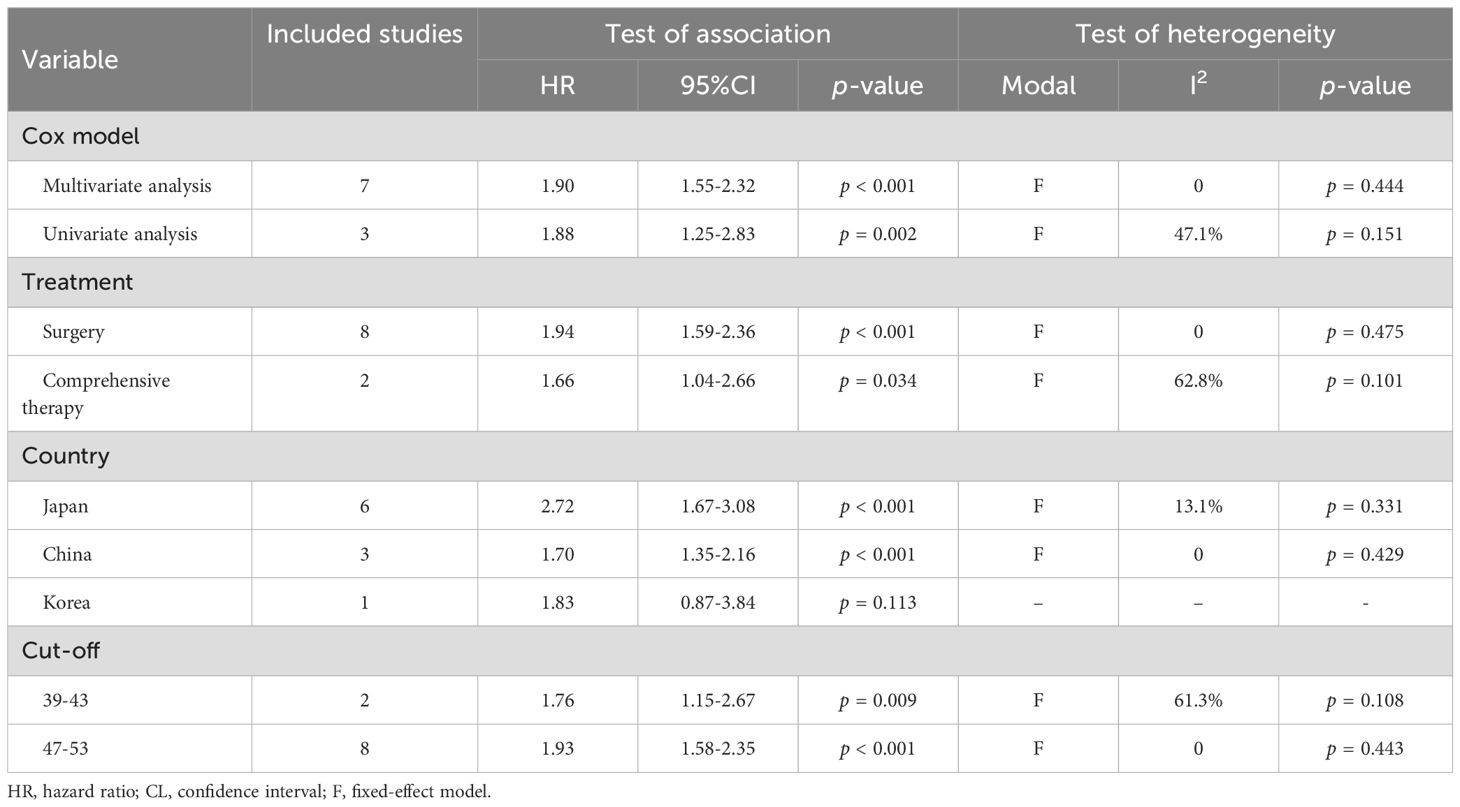
Table 3. Subgroup analysis of the association between prognostic nutritional index and disease-free survival in patients with head and neck squamous cell carcinoma.
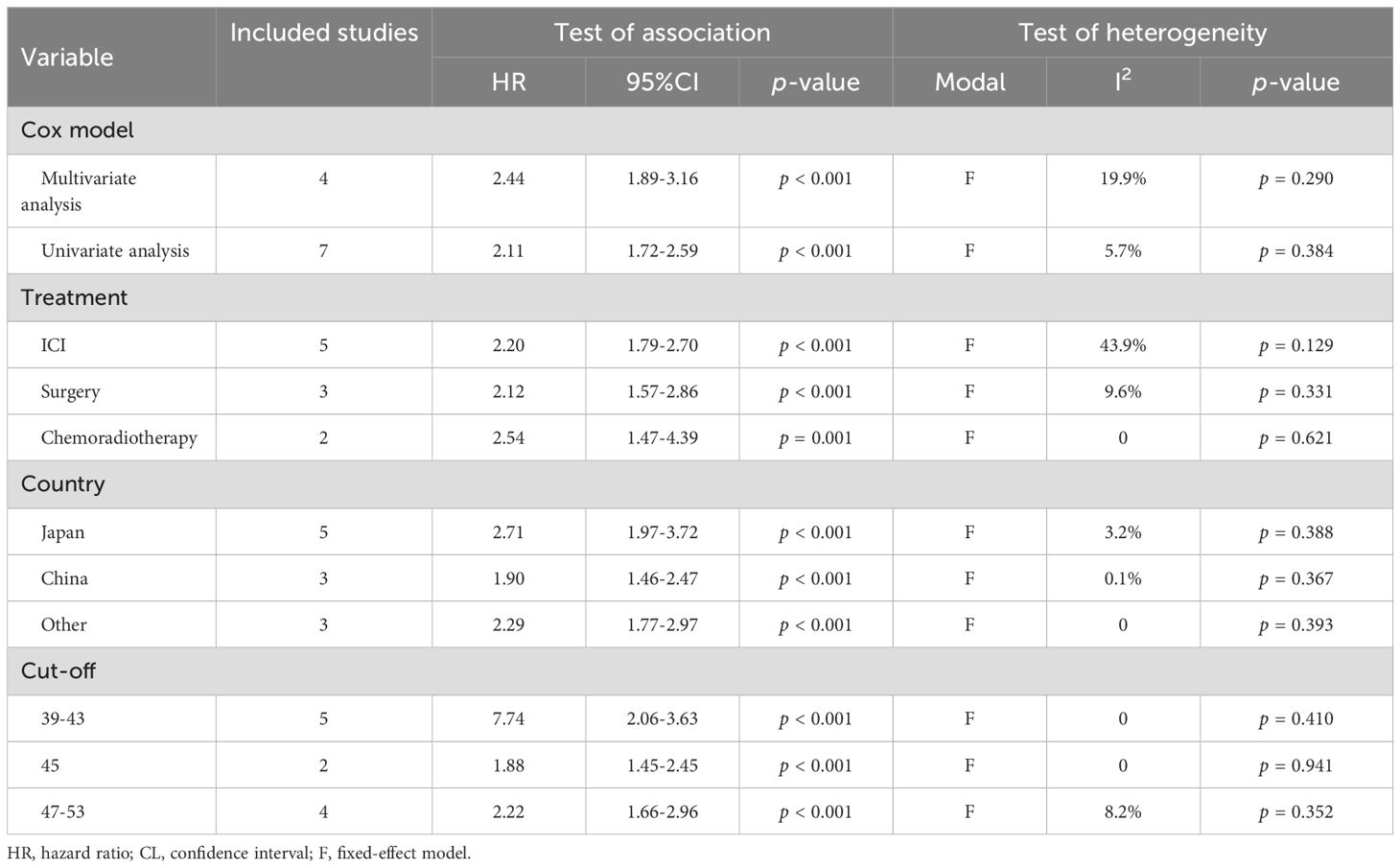
Table 4. Subgroup analysis of the association between prognostic nutritional index and progression-free survival in patients with head and neck squamous cell carcinoma.
Sensitivity analysis, in which each study was systematically removed, demonstrated that the pooled HRs for both DFS and PFS remained stable and robust (Supplementary Figures S2A, S3A). The Begg’s test indicated no significant publication bias for DFS (p = 0.107) or PFS (p = 0.213). However, the funnel plot for DFS pooled results was not symmetrically distributed (Supplementary Figure S2B, S3B). To address the possibility of missing studies, the trim-and-fill method was applied. The results showed that the pooled HR did not change significantly, even after accounting for potential missing studies.
3.5 Baseline prognostic nutritional index and objective response rate and disease control rate
We further investigated the relationship between PNI and ORR and DCR in HNSCC patients, based on three studies involving 301 individuals. Notably, no significant heterogeneity was observed across the studies (ORR, I² = 0, p = 0.443; DCR, I² = 0, p = 0.606), justifying the use of a fixed-effects model. The findings clearly indicated that patients with low PNI had a lower ORR (OR: 0.40, 95% CI: 0.22–0.73, p = 0.002, Figure 5A) and DCR (OR: 0.30, 95% CI: 0.17–0.53, p < 0.001, Figure 5B) compared to those with high PNI.
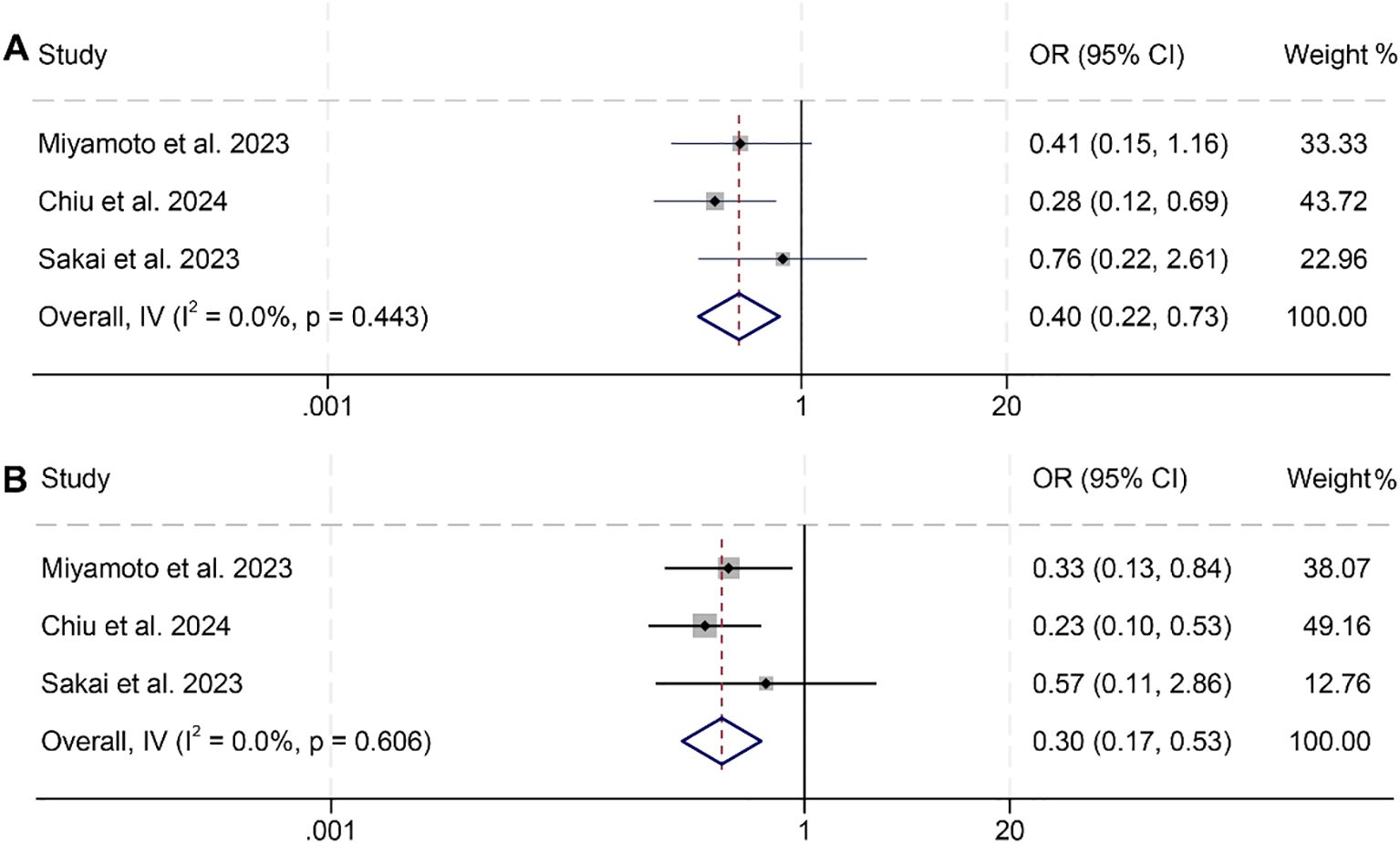
Figure 5. Forest plots showing the association between prognostic nutritional index (PNI) and treatment response: (A) Objective response rate (ORR); (B) Disease control rate (DCR). Both analyses were conducted using PNI as a binary variable (high vs. low).OR, odds ratio; CI, confidence interval.
4 Discussion
The PNI, an inexpensive alternative to tumor markers, can be easily measured using routine preoperative blood sampling techniques and serves as a valuable prognostic tool. In this study, we found that HNSCC patients with high PNI had significantly longer survival and demonstrated a higher therapeutic response. Subgroup analyses confirmed that the association between PNI and prognosis was consistent across subgroups with different Cox models, cancer types, treatment modalities, countries, and PNI cut-off values.
There is a well-documented association between malnutrition and HNSCC (42, 43). Nutritional disorders can result from both the tumor itself and its treatments (44). Dysphagia, characterized by difficulty swallowing, may arise due to direct tumor obstruction, nerve damage, or xerostomia (45). Odynophagia, or painful swallowing, along with frequent aspiration, can lead to food aversion and recurrent pneumonia (44, 46). Additionally, reduced appetite combined with tumor-induced metabolic changes often results in catabolic energy mobilization and cachexia (47).
The PNI reflects both the nutritional and immune statuses of cancer patients. A reduced PNI indicates diminished levels of albumin and/or lymphocytes. Serum albumin serves as a marker of the body’s nutritional condition and immune functionality. Additionally, albumin supports cellular proliferation, stabilizes DNA, and acts as a biochemical buffer in metabolic reactions. It also regulates sex hormones, which may counteract cancer progression. Low serum albumin has been consistently associated with unfavorable prognoses and reduced survival rates in cancer patients (8, 22, 48).
Lymphocytes, as key components of the immune system, play a critical role in initiating antitumor responses (49). They are essential for eliminating residual tumor cells and preventing micrometastases (50, 51). Prolonged T-cell activation in cancer patients promotes tumor cell apoptosis, while tumor-infiltrating lymphocytes (TILs) present tumor-associated antigens to lymphocytes, enhancing cancer cell eradication during chemoradiotherapy. As such, lymphocytes are vital for optimizing adjuvant therapies and reducing the likelihood of tumor recurrence (52). In summary, malnutrition and lymphocytopenia may signal a persistently compromised immune system, which contributes to poorer outcomes in cancer patients.
Recent studies suggest that the prognostic value of PNI may be attributed not only to general immune competence, but also to its reflection of tumor-immune microenvironment status (40, 53, 54). Lymphocytes, particularly cytotoxic CD8χ T cells and helper CD4χ subsets, are key effectors in antitumor immunity. Low peripheral lymphocyte counts, as captured by a reduced PNI, may reflect systemic immunosuppression or immune exhaustion—both of which are associated with poor infiltration of tumor-infiltrating lymphocytes (TILs), reduced effector cytokine production, and increased expression of inhibitory receptors such as PD-1 and CTLA-4 (40, 53, 55). In this regard, PNI could indirectly reflect the immunological fitness of the host, and its capacity to mount an effective antitumor response.
Of note, a subset of the included studies focused on HNSCC patients receiving ICIs, providing a unique opportunity to explore the relevance of PNI in the context of immunotherapy (56–58). Since successful ICI response depends heavily on pre-existing immune activation and adequate T cell function, a higher PNI—indicating preserved lymphocyte-mediated immunity—may be predictive of improved responsiveness to ICIs. Conversely, low PNI levels may suggest a state of immune exhaustion or systemic inflammation, which has been associated with primary resistance to immunotherapy (56, 57). Although formal subgroup analyses on ICI-treated patients were limited due to the number of available studies, this aspect highlights the potential utility of PNI as a baseline immune fitness biomarker that could guide ICI decision-making in HNSCC.
One notable limitation of this meta-analysis is the heterogeneity in PNI cutoff values used across the included studies, which ranged from 39 to 53. This variability reflects the lack of a universally accepted threshold for defining “low” versus “high” PNI in head and neck squamous cell carcinoma (HNSCC) and complicates direct comparisons across studies. Such inconsistencies also hinder the immediate translation of findings into clinical practice, as clinicians may be uncertain which threshold to apply for risk stratification. Due to the nature of our meta-analysis, which relied on aggregate data, we were unable to perform receiver operating characteristic (ROC) curve analyses to identify an optimal cutoff. Future prospective studies using individual patient-level data are needed to determine standardized, cancer-specific PNI thresholds—ideally derived from ROC-based methods and validated across diverse populations—to enhance the clinical applicability and consistency of this biomarker.
Certain limitations of this pooled analysis should be acknowledged. First, it is worth noting that all studies included in this analysis were retrospective cohort studies, which may limit the statistical robustness of the findings. Additionally, the majority of the studies were conducted in Asia, potentially limiting the generalizability of the results to other regions. Therefore, future studies should aim to validate these findings in more diverse, multinational cohorts to ensure broader applicability of PNI as a prognostic tool in global clinical practice.
5 Conclusion
This study underscores the prognostic significance of the prognostic nutritional index (PNI) in predicting survival outcomes and treatment responses in patients with head and neck squamous cell carcinoma (HNSCC). Given its simplicity, cost-effectiveness, and availability from routine laboratory data, PNI may serve as a valuable adjunct in clinical decision-making. Incorporating PNI into standard prognostic assessments could aid in identifying high-risk patients, tailoring treatment intensity, optimizing nutritional support, and improving overall patient management strategies in HNSCC.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Author contributions
YoW: Software, Investigation, Visualization, Funding acquisition, Formal analysis, Conceptualization, Methodology, Writing – original draft, Data curation. JW: Formal analysis, Writing – original draft, Data curation, Methodology, Investigation, Resources, Validation, Funding acquisition, Conceptualization, Supervision. BX: Validation, Methodology, Investigation, Writing – original draft. YuW: Resources, Validation, Supervision, Writing – review & editing. FH: Formal analysis, Writing – review & editing, Methodology, Investigation, Resources. YJ: Conceptualization, Resources, Validation, Visualization, Supervision, Writing – review & editing, Data curation, Project administration. TL: Supervision, Conceptualization, Project administration, Visualization, Resources, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by grants from the National Natural Science Foundation of China (Grant No: 82301294) and the Natural Science Foundation of Hubei Province (Grant No: 2023AFB264).
Acknowledgments
The authors thank all the medical staff who contributed to the maintenance of the medical record database.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1597965/full#supplementary-material
References
1. Bruixola G, Caballero J, Papaccio F, Petrillo A, Iranzo A, Civera M, et al. Prognostic Nutritional Index as an independent prognostic factor in locoregionally advanced squamous cell head and neck cancer. ESMO Open. (2018) 3:e000425. doi: 10.1136/esmoopen-2018-000425
2. Feng C, Mao W, Yuan C, Dong P, and Liu Y. Nicotine-induced CHRNA5 activation modulates CES1 expression, impacting head and neck squamous cell carcinoma recurrence and metastasis via MEK/ERK pathway. Cell Death Dis. (2024) 15:785. doi: 10.1038/s41419-024-07178-4
3. Gore SM, Crombie AK, Batstone MD, and Clark JR. Concurrent chemoradiotherapy compared with surgery and adjuvant radiotherapy for oral cavity squamous cell carcinoma. Head Neck. (2015) 37:518–23. doi: 10.1002/hed.23626
4. Kubota K, Ito R, Narita N, Tanaka Y, Furudate K, Akiyama N, et al. Utility of prognostic nutritional index and systemic immune-inflammation index in oral cancer treatment. BMC Cancer. (2022) 22:368. doi: 10.1186/s12885-022-09439-x
5. Feng C, Jin X, Han Y, Guo R, Zou J, Li Y, et al. Expression and prognostic analyses of ITGA3, ITGA5, and ITGA6 in head and neck squamous cell carcinoma. Med Sci Monit. (2020) 26:e926800. doi: 10.12659/MSM.926800
7. Xue Y, Zhou X, Xue L, Zhou R, and Luo J. The role of pretreatment prognostic nutritional index in esophageal cancer: A meta-analysis. J Cell Physiol. (2019) 234:19655–62. doi: 10.1002/jcp.28565
8. Gupta D and Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. (2010) 9:69. doi: 10.1186/1475-2891-9-69
9. Onodera T, Goseki N, and Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. (1984) 85:1001–5.
10. Migita K, Takayama T, Saeki K, Matsumoto S, Wakatsuki K, Enomoto K, et al. The prognostic nutritional index predicts long-term outcomes of gastric cancer patients independent of tumor stage. Ann Surg Oncol. (2013) 20:2647–54. doi: 10.1245/s10434-013-2926-5
11. Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M, et al. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. (2013) 37:2688–92. doi: 10.1007/s00268-013-2156-9
12. Zhang L, Ma W, Qiu Z, Kuang T, Wang K, Hu B, et al. Prognostic nutritional index as a prognostic biomarker for gastrointestinal cancer patients treated with immune checkpoint inhibitors. Front Immunol. (2023) 14:1219929. doi: 10.3389/fimmu.2023.1219929
13. Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A, et al. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. (2011) 98:268–74. doi: 10.1002/bjs.7305
14. Abe A, Hayashi H, Ishihama T, and Furuta H. Prognostic impact of the prognostic nutritional index in cases of resected oral squamous cell carcinoma: a retrospective study. BMC Oral Health. (2021) 21:40. doi: 10.1186/s12903-021-01394-6
15. Matsumura S, Kaira K, Kuba K, Inoue H, Hamada M, Yamazaki T, et al. Potential of nutritional markers as predictors after immunotherapy in advanced head and neck squamous cell carcinoma. Anticancer Res. (2024) 44:4049–56. doi: 10.21873/anticanres.17234
16. Miyamoto N, Takenaka Y, Sudo T, Eguchi H, Tanaka H, Fukusumi T, et al. Prognostic significance of nutritional indices in patients with head and neck squamous cell carcinoma treated with immune checkpoint inhibitors. Acta Otolaryngol. (2023) 143:925–30. doi: 10.1080/00016489.2023.2288910
17. Ikeguchi M. Glasgow prognostic score and neutrophil-lymphocyte ratio are good prognostic indicators after radical neck dissection for advanced squamous cell carcinoma in the hypopharynx. Langenbecks Arch Surg. (2016) 401:861–6. doi: 10.1007/s00423-016-1453-9
18. Song Y, Tian Y, Lu X, Chen G, and Lv X. Prognostic value of 18F-FDG PET radiomics and sarcopenia in patients with oral squamous cell carcinoma. Med Phys. (2024) 51:4907–21. doi: 10.1002/mp.16949
19. Tada H, Nagata Y, Takahashi H, Matsuyama T, Ida S, Mito I, et al. Systemic immune responses are associated with molecular characteristics of circulating tumor cells in head and neck squamous cell carcinoma. Mol Clin Oncol. (2021) 15(1):147. doi: 10.3892/mco.2021.2309
20. Lilong Z, Kuang T, Li M, Li X, Hu P, Deng W, et al. Sarcopenia affects the clinical efficacy of immune checkpoint inhibitors in patients with gastrointestinal cancers. Clin Nutr. (2024) 43:31–41. doi: 10.1016/j.clnu.2023.11.009
21. Begg CB and Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
22. Zhang L, Wang K, Liu R, Kuang T, Chen C, Yao F, et al. Body composition as a prognostic factor in cholangiocarcinoma: a meta-analysis. Nutr J. (2024) 23:145. doi: 10.1186/s12937-024-01037-w
23. Chiu TJ, Huang TL, Chien CY, Huang WT, and Li SH. Hypoalbuminemia and hypercalcemia are independently associated with poor treatment outcomes of anti-PD-1 immune checkpoint inhibitors in patients with recurrent or metastatic head and neck squamous cell carcinoma. World J Surg Oncol. (2024) 22:242. doi: 10.1186/s12957-024-03522-2
24. Fang KH, Chang SW, Lee YC, Huang EI, Lai CH, Chang GH, et al. Preoperative prognostic nutritional index predicts prognosis of patients with oral cavity cancer. Oral Dis. (2022) 28:1816–30. doi: 10.1111/odi.13840
25. Fukuzawa S, Yamagata K, Takasaki R, Uchida F, Ishibashi-Kanno N, Bukawa H, et al. The effectiveness of onodera`s prognostic nutritional in predicting the prognosis of tongue squamous cell carcinoma. J Stomatol Oral Maxillofac Surg. (2024) 126(3S):102201. doi: 10.1016/j.jormas.2024.102201
26. Go JY, Lee YS, Choi YJ, Kim MJ, Kwon MS, Jung YH, et al. Discrete prognostic implication of sarcopenia according to nutritional status in surgically treated patients with hypopharyngeal cancer. World J Surg. (2024) 48:1892–901. doi: 10.1002/wjs.12246
27. Guller M, Herberg M, Amin N, Alkhatib H, Maroun C, Wu E, et al. Nutritional status as a predictive biomarker for immunotherapy outcomes in advanced head and neck cancer. Cancers (Basel). (2021) 13. doi: 10.3390/cancers13225772
28. Hernando-Calvo A, Mirallas O, Marmolejo D, Saavedra O, Vieito M, Assaf Pastrana JD, et al. Nutritional status associates with immunotherapy clinical outcomes in recurrent or metastatic head and neck squamous cell carcinoma patients. Oral Oncol. (2023) 140:106364. doi: 10.1016/j.oraloncology.2023.106364
29. Li Z, Ge S, Song C, Li Y, Xie X, Xu L, et al. Systemic immune-inflammation and prognostic immune nutritional index in oral squamous cell carcinoma patients. Biomarkers Med. (2024) 18:759–70. doi: 10.1080/17520363.2024.2394390
30. Ohyama Y, Inaba Y, Kubota M, Kanemaru T, and Hasegawa K. CT-assessed sarcopenia and prognostic nutritional index are associated with poor prognosis in oral squamous cell carcinoma. Oral Maxillofac Surg. (2024) 28:659–66. doi: 10.1007/s10006-023-01191-1
31. Oka T, Sato F, Ono T, Kawaguchi T, Murotani K, Sueyoshi S, et al. Prognostic values of systemic inflammation and nutrition-based prognostic indices in oropharyngeal carcinoma. Laryngoscope Investig Otolaryngol. (2023) 8:675–85. doi: 10.1002/lio2.1070
32. Sakai A, Ebisumoto K, Iijima H, Yamauchi M, Teramura T, Yamazaki A, et al. Chemotherapy following immune checkpoint inhibitors in recurrent or metastatic head and neck squamous cell carcinoma: clinical effectiveness and influence of inflammatory and nutritional factors. Discover Oncol. (2023) 14(1):158. doi: 10.1007/s12672-023-00774-4
33. Tanaka K, Hirakawa H, Suzuki M, Higa T, Agena S, Hasegawa N, et al. Biomarkers for predicting anti-programmed cell death-1 antibody treatment effects in head and neck cancer. Curr Oncol. (2023) 30:5409–24. doi: 10.3390/curroncol30060410
34. Tomasoni M, Piazza C, Deganello A, Bossi P, Tirelli G, Nicolai P, et al. The prognostic-nutritional index in HPV-negative head and neck squamous cell carcinoma treated with upfront surgery: a multi-institutional series. Acta Otorhinolaryngol Ital. (2023) 43:170–82. doi: 10.14639/0392-100X-N2358
35. Uri I, Horváth A, Tamás L, Polony G, and Dános K. Prognostic nutritional index (PNI) correlates with survival in head and neck cancer patients more precisely than other nutritional markers - real world data. Eur Arch Otorhinolaryngol. (2024) 281:6599–611. doi: 10.1007/s00405-024-08865-w
36. Watabe Y, Aoki K, Ichikawa H, Matsuzaki H, Ito A, Tanaka JI, et al. A preoperative prognostic nutritional index is a prognostic indicator in oral squamous cell carcinoma patients undergoing radical surgery. Int J Oral Maxillofac Surg. (2021) 50:1413–21. doi: 10.1016/j.ijom.2021.01.009
37. Wu X, Jiang Y, Ge H, Diao P, Wang D, Wang Y, et al. Predictive value of prognostic nutritional index in patients with oral squamous cell carcinoma. Oral Dis. (2020) 26:903–11. doi: 10.1111/odi.13318
38. Yamagata K, Fukuzawa S, Uchida F, Terada K, Ishibashi-Kanno N, Bukawa H, et al. Does the geriatric nutrition risk index predict the prognosis of patients with oral squamous cell carcinoma? Br J Oral Maxillofac Surg. (2022) 60:475–81. doi: 10.1016/j.bjoms.2021.09.008
39. Ye LL, Oei RW, Kong FF, Du CR, Zhai RP, Ji QH, et al. The prognostic value of preoperative prognostic nutritional index in patients with hypopharyngeal squamous cell carcinoma: a retrospective study. J Transl Med. (2018) 16:12. doi: 10.1186/s12967-018-1391-0
40. Yoshida R, Gohara S, Sakata J, Matsuoka Y, Hirosue A, Kawahara K, et al. Onodera's prognostic nutritional index correlates with tumor immune environment and survival in patients with oral squamous cell carcinoma undergoing chemoradiotherapy. Transl Oncol. (2020) 13:100850. doi: 10.1016/j.tranon.2020.100850
41. Yoshimura T, Suzuki H, Takayama H, Higashi S, Hirano Y, Tezuka M, et al. Prognostic value of inflammatory biomarkers in aged patients with oral squamous cell carcinoma. Front Pharmacol. (2022) 13:996757. doi: 10.3389/fphar.2022.996757
42. Cederholm T, Jensen GL, Correia M, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition - A consensus report from the global clinical nutrition community. Clin Nutr. (2019) 38:1–9. doi: 10.1016/j.clnu.2018.08.002
43. Steer B, Loeliger J, Edbrooke L, Deftereos I, Laing E, Kiss N, et al. Malnutrition prevalence according to the GLIM criteria in head and neck cancer patients undergoing cancer treatment. Nutrients. (2020) 12(11):3493. doi: 10.3390/nu12113493
44. Raber-Durlacher JE, Brennan MT, Verdonck-de Leeuw IM, Gibson RJ, Eilers JG, Waltimo T, et al. Swallowing dysfunction in cancer patients. Support Care Cancer. (2012) 20:433–43. doi: 10.1007/s00520-011-1342-2
45. Manikantan K, Khode S, Sayed SI, Roe J, Nutting CM, Rhys-Evans P, et al. Dysphagia in head and neck cancer. Cancer Treat Rev. (2009) 35:724–32. doi: 10.1016/j.ctrv.2009.08.008
46. Eisbruch A, Schwartz M, Rasch C, Vineberg K, Damen E, Van As CJ, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. (2004) 60:1425–39. doi: 10.1016/j.ijrobp.2004.05.050
47. Muscaritoli M, Corsaro E, and Molfino A. Awareness of cancer-related malnutrition and its management: analysis of the results from a survey conducted among medical oncologists. Front Oncol. (2021) 11:682999. doi: 10.3389/fonc.2021.682999
48. Zhang L, Li X, Wang K, Wu M, Liu W, Wang W, et al. Prognostic impact of body composition in hepatocellular carcinoma patients with immunotherapy. Ann Med. (2024) 56:2395062. doi: 10.1080/07853890.2024.2395062
49. Rabinowich H, Cohen R, Bruderman I, Steiner Z, and Klajman A. Functional analysis of mononuclear cells infiltrating into tumors: lysis of autologous human tumor cells by cultured infiltrating lymphocytes. Cancer Res. (1987) 47:173–7.
50. Kuang T, Qiu Z, Wang K, Zhang L, Dong K, Wang W, et al. Pan-immune inflammation value as a prognostic biomarker for cancer patients treated with immune checkpoint inhibitors. Front Immunol. (2024) 15:1326083. doi: 10.3389/fimmu.2024.1326083
51. Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E, et al. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg. (2009) 137:425–8. doi: 10.1016/j.jtcvs.2008.05.046
52. Apetoh L, Tesniere A, Ghiringhelli F, Kroemer G, and Zitvogel L. Molecular interactions between dying tumor cells and the innate immune system determine the efficacy of conventional anticancer therapies. Cancer Res. (2008) 68:4026–30. doi: 10.1158/0008-5472.CAN-08-0427
53. Okadome K, Baba Y, Yagi T, Kiyozumi Y, Ishimoto T, Iwatsuki M, et al. Prognostic nutritional index, tumor-infiltrating lymphocytes, and prognosis in patients with esophageal cancer. Ann Surg. (2020) 271:693–700. doi: 10.1097/SLA.0000000000002985
54. Feng C, Zhao Y, Yuan C, Lu D, Wang S, Sun Z, et al. S. aureus upregulation of MUC13 modulates mucosal remodeling in chronic rhinosinusitis via MEK1/2 and WNT2B. J Allergy Clin Immunol. (2025) 30:S0091-6749(25)00701-8. doi: 10.1016/j.jaci.2025.06.023
55. Ooyama T, Hirayama M, Seki Y, Iwamoto A, Yoshida R, Nakayama H, et al. Pretreatment nutritional indices are associated with survival and T-cell exhaustion in recurrent or metastatic oral squamous cell carcinoma patients treated with immune checkpoint inhibitors: a retrospective cohort study. Int J Oral Maxillofac Surg. (2025) 54:685–94. doi: 10.1016/j.ijom.2025.01.010
56. Hou S, Song D, Hao R, Li L, Zhang Y, Zhu J, et al. Prognostic relevance of prognostic nutritional indices in gastric or gastro-esophageal junction cancer patients receiving immune checkpoint inhibitors: a systematic review and meta-analysis. Front Immunol. (2024) 15:1382417. doi: 10.3389/fimmu.2024.1382417
57. Yan X, Wang J, Mao J, Wang Y, Wang X, Yang M, et al. Identification of prognostic nutritional index as a reliable prognostic indicator for advanced lung cancer patients receiving immune checkpoint inhibitors. Front Nutr. (2023) 10:1213255. doi: 10.3389/fnut.2023.1213255
Keywords: prognostic nutritional index, head and neck squamous cell carcinoma, prognosis, overall survival (OS), disease-free survival (DFS)
Citation: Wang Y, Wang J, Xiao B, Wang Y, Huang F, Jiang Y and Liu T (2025) Prognostic significance of prognostic nutritional index in patients with head and neck squamous cell carcinoma. Front. Immunol. 16:1597965. doi: 10.3389/fimmu.2025.1597965
Received: 22 March 2025; Accepted: 24 July 2025;
Published: 27 August 2025.
Edited by:
Priyanka Bhateja, The Ohio State University, United StatesReviewed by:
Michihisa Kono, Dana–Farber Cancer Institute, United StatesChen Feng, Shandong University, China
Copyright © 2025 Wang, Wang, Xiao, Wang, Huang, Jiang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Jiang, anlkZDE5NzdAMTYzLmNvbQ==; Tianyi Liu, cm0wMDM4NjBAd2h1LmVkdS5jbg==
†These authors have contributed equally to this work
 Yongping Wang†
Yongping Wang† Jie Wang
Jie Wang Tianyi Liu
Tianyi Liu