- 1The NHC Key Laboratory of Male Reproduction and Genetics, Family Planning Research Institute of Guangdong Province, Guangzhou, China
- 2Department of Central Laboratory, Guangdong Provincial Reproductive Science Institute (Guangdong Provincial Fertility Hospital), Guangzhou, China
- 3College of Animal Science and Technology, Northwest A&F University, Xianyang, Shaanxi, China
Male infertility is influenced by genetic abnormalities, hormonal imbalances, lifestyle factors, and environmental exposures. Recently, Damage-Associated Molecular Patterns (DAMPs) have emerged as key players in male reproductive health, particularly in regulating inflammatory responses and tissue damage. This review highlights the role of critical DAMPs, such as HMGB1, HSPs, ATP, eCIRP, histones, and cfDNA, in processes like spermatogenesis, sperm maturation, and fertilization. Released through mechanisms like necrosis, apoptosis, pyroptosis, and exosomes, DAMPs significantly influence immune regulation, thereby affecting male fertility. Understanding these roles offers new therapeutic avenues targeting DAMPs to improve male reproductive health and treat infertility.
1 Introduction
Male infertility is a condition characterized by the inability to achieve pregnancy after a year of unprotected intercourse (1). It is a significant cause of reproductive challenges, affecting a man’s ability to father children naturally (2). The condition can result from various factors, including genetic abnormalities (3), hormonal imbalances (4), lifestyle choices (5), and environmental exposures (6). The impact of male infertility is profound, often leading to prolonged periods of attempting conception and requiring medical interventions such as assisted reproductive technologies.
Damage-Associated Molecular Patterns (DAMPs) are endogenous molecules released by stressed or damaged cells (7). They play a crucial role in the body’s inflammatory response by activating the innate immune system (8). When released, DAMPs bind to pattern recognition receptors (PRRs) on immune cells, triggering a cascade of inflammatory processes (9). This response, while protective in the context of acute injury, can become detrimental if chronic, leading to tissue damage and impaired function (10). This review aims to explore the role of DAMPs in the male reproductive process, from spermatogenesis to fertilization, and discuss potential therapeutic strategies targeting these molecular patterns. Understanding the connection between DAMPs and the male reproductive process could provide novel insights into the mechanisms underlying spermatogenesis and fertilization, and pave the way for innovative treatments.
This narrative review employed systematic literature retrieval from PubMed and Web of Science databases using keywords: DAMPs, male infertility, spermatogenesis, sperm maturation, fertilization, and associated terms. Inclusion criteria prioritized: (1) original research elucidating DAMPs’ mechanistic roles in male reproduction; (2) clinical/animal studies linking DAMPs to sperm parameters; (3) peer-reviewed publications in English. Final analysis integrated 145 studies emphasizing the role of DAMPs in male reproduction.
2 Types and release mechanisms of DAMPs
2.1 Major types of DAMPs
The immune system’s ability to distinguish ‘self’ from ‘non-self’ is fundamental in initiating immune responses against pathogens (11). While innate immune cells use PRRs like Toll-like receptors (TLRs) to detect pathogen-associated molecular patterns (PAMPs), they also recognize DAMPs released from stressed or damaged cells (12). DAMPs activate innate immune cells, including neutrophils (13), macrophages (14), and dendritic cells (15), leading to the release of cytokines and chemokines that trigger adaptive immune responses. DAMPs also stimulate non-immune cells such as epithelial (16), endothelial (17), and fibroblast cells (18), causing them to release inflammatory mediators. Major DAMPs include but are not limited to HMGB1, HSPs, ATP, extracellular cold-inducible RNA-binding protein (eCIRP), histones, extracellular RNAs (exRNAs), cell-free DNA (cfDNA), and uric acid (19). These molecules are detected by PRRs such as TLRs, NOD-like receptors (NLRs), and RIG-I-like receptors (20). Upon DAMP recognition, TLRs activate downstream signaling pathways involving myeloid differentiation primary response 88 (MyD88) (21) and TIR-domain-containing adapter-inducing interferon-β (TRIF) (22), which in turn activate transcription factors like activator protein-1 (AP-1) (23) and nuclear factor kappa B (NF-κB) (24). Additionally, DAMPs can signal through receptors like RAGE and P2X7 (25), further propagating inflammatory responses. This complex network of signaling pathways underscores the pivotal role of DAMPs in modulating immune and inflammatory responses.
2.2 Release mechanisms of DAMPs
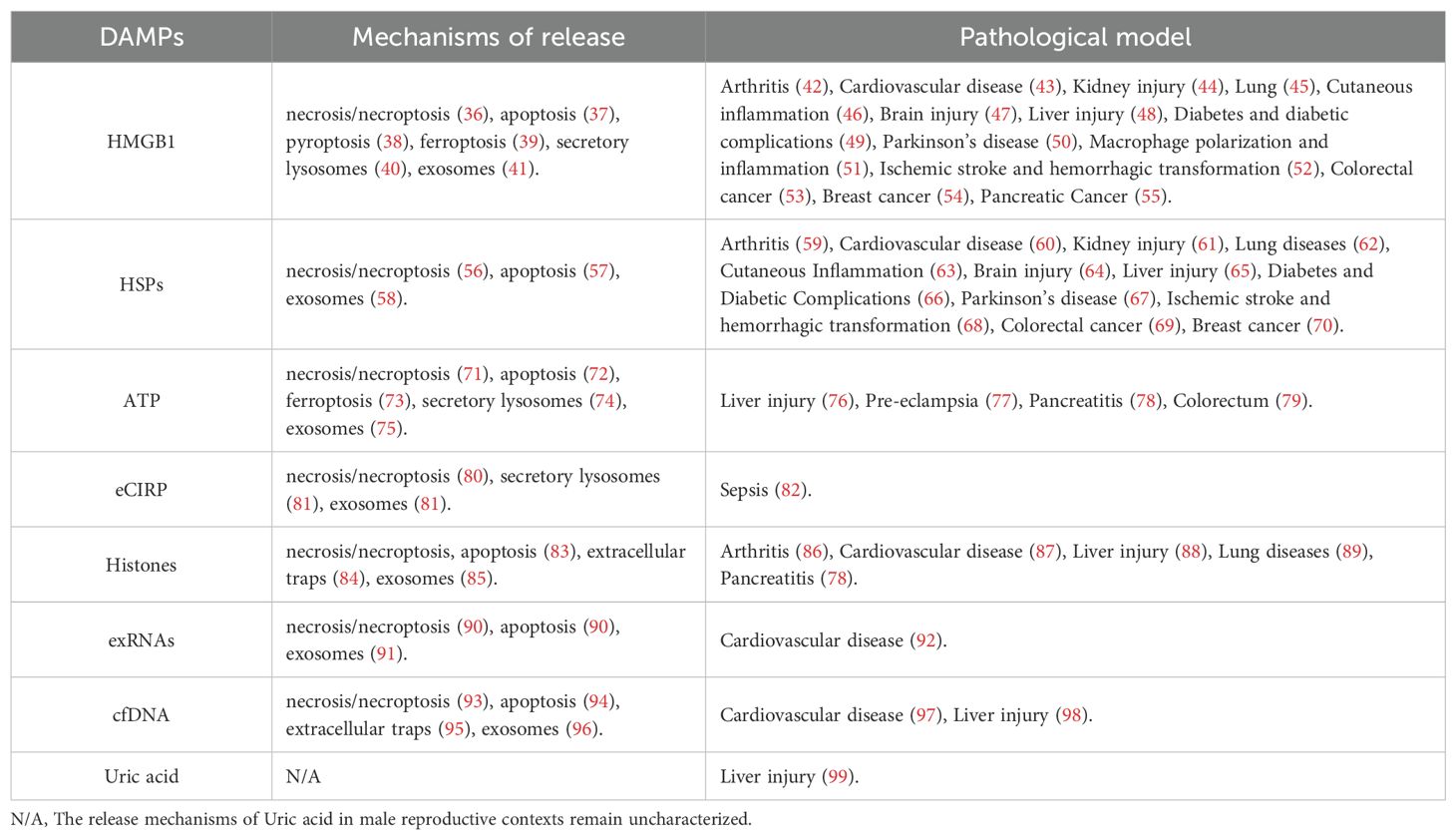
Building on the descriptions of the various types of DAMPs, their release mechanisms are diverse and complex, encompassing necrosis/necroptosis (26), apoptosis (27), pyroptosis (28), ferroptosis (29), extracellular traps (30), secretory lysosomes (7), and exosomes (31). Each of these mechanisms contributes to the release of DAMPs under different pathological and physiological conditions. For instance, necrosis and necroptosis typically result in the uncontrolled release of intracellular contents, including DAMPs, due to cell membrane rupture (32). In contrast, apoptosis generally involves a more controlled release of apoptotic bodies containing DAMPs (33). Pyroptosis and ferroptosis also contribute to DAMPs release through distinct forms of regulated cell death characterized by inflammatory responses and lipid peroxidation, respectively (34). Additionally, DAMPs can be actively secreted through extracellular traps formed by immune cells, as well as through secretory lysosomes and exosomes (35), which are specialized vesicles that facilitate intercellular communication (Figure 1). The specific release mechanisms and the tissue types involved in these processes are detailed in Table 1. These DAMP release pathways, though general in cellular biology, are also active in male reproductive tissues such as the testes and epididymis. The physiological consequences of these mechanisms—including their effects on sperm development, motility, and function—are discussed in detail in Section 4.
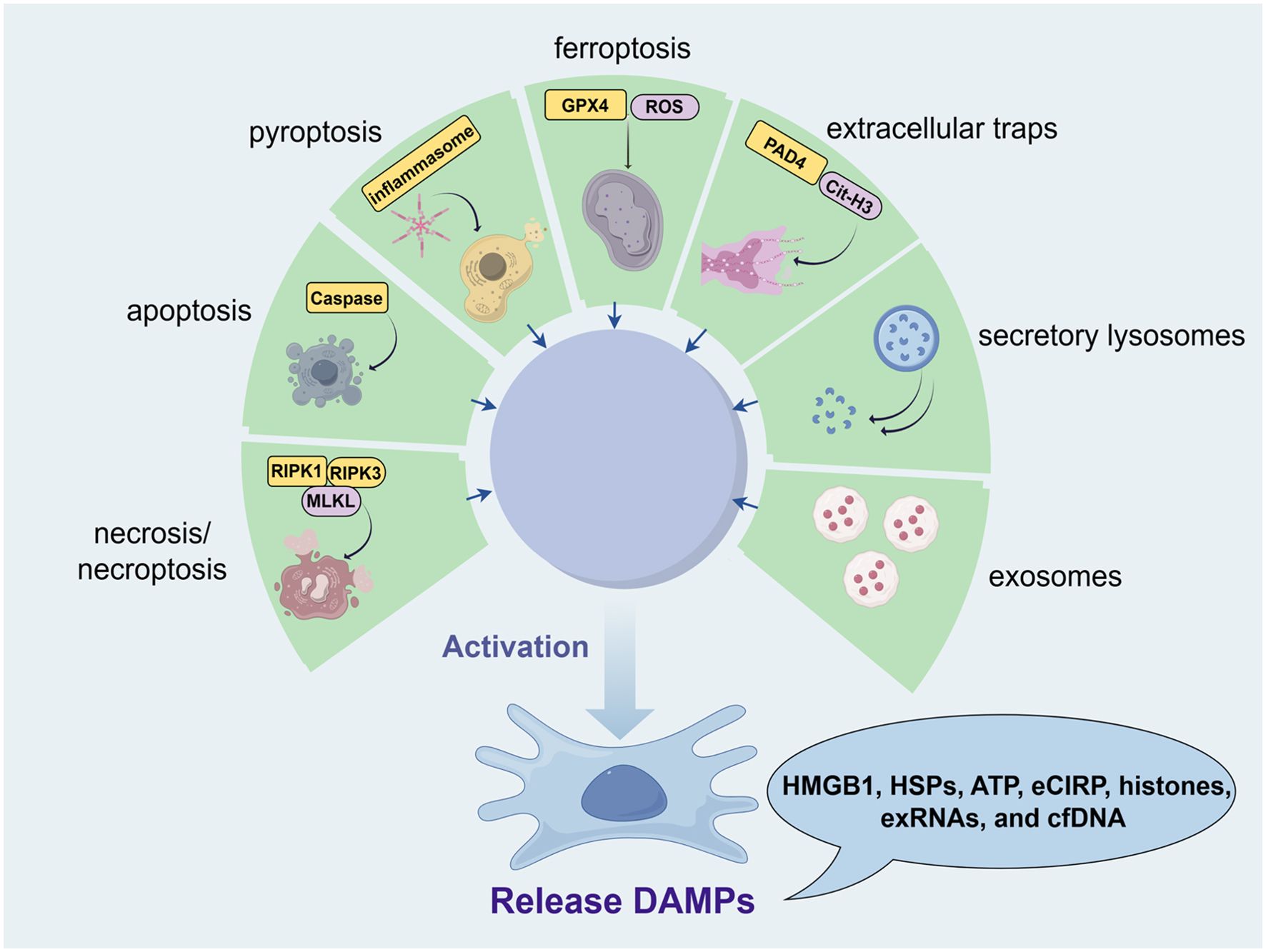
Figure 1. Release mechanisms of DAMPs in male reproductive health (from Figdraw, www.figdraw.com). Various forms of cellular stress, including necrosis/necroptosis, apoptosis, pyroptosis, ferroptosis, and immune responses, contribute to the release of DAMPs such as HMGB1, HSPs, ATP, eCIRP, histones, exRNAs, and cfDNA. Necrosis and necroptosis result in the uncontrolled release of intracellular contents due to cell membrane rupture, involving RIPK1, RIPK3, and MLKL. Apoptosis allows for a controlled release of DAMPs through apoptotic bodies mediated by caspase activity. Pyroptosis, driven by inflammasome activation, leads to DAMP release via gasdermin (GSDMD) pore formation. Ferroptosis, characterized by lipid peroxidation involving GPX4 and ROS, also releases DAMPs. Additionally, immune cells can release DAMPs through extracellular traps (e.g., Cit-H3) or via secretory lysosomes and exosomes, facilitating intercellular communication.
3 Roles of DAMPs in spermatogenesis, sperm maturation and fertilization
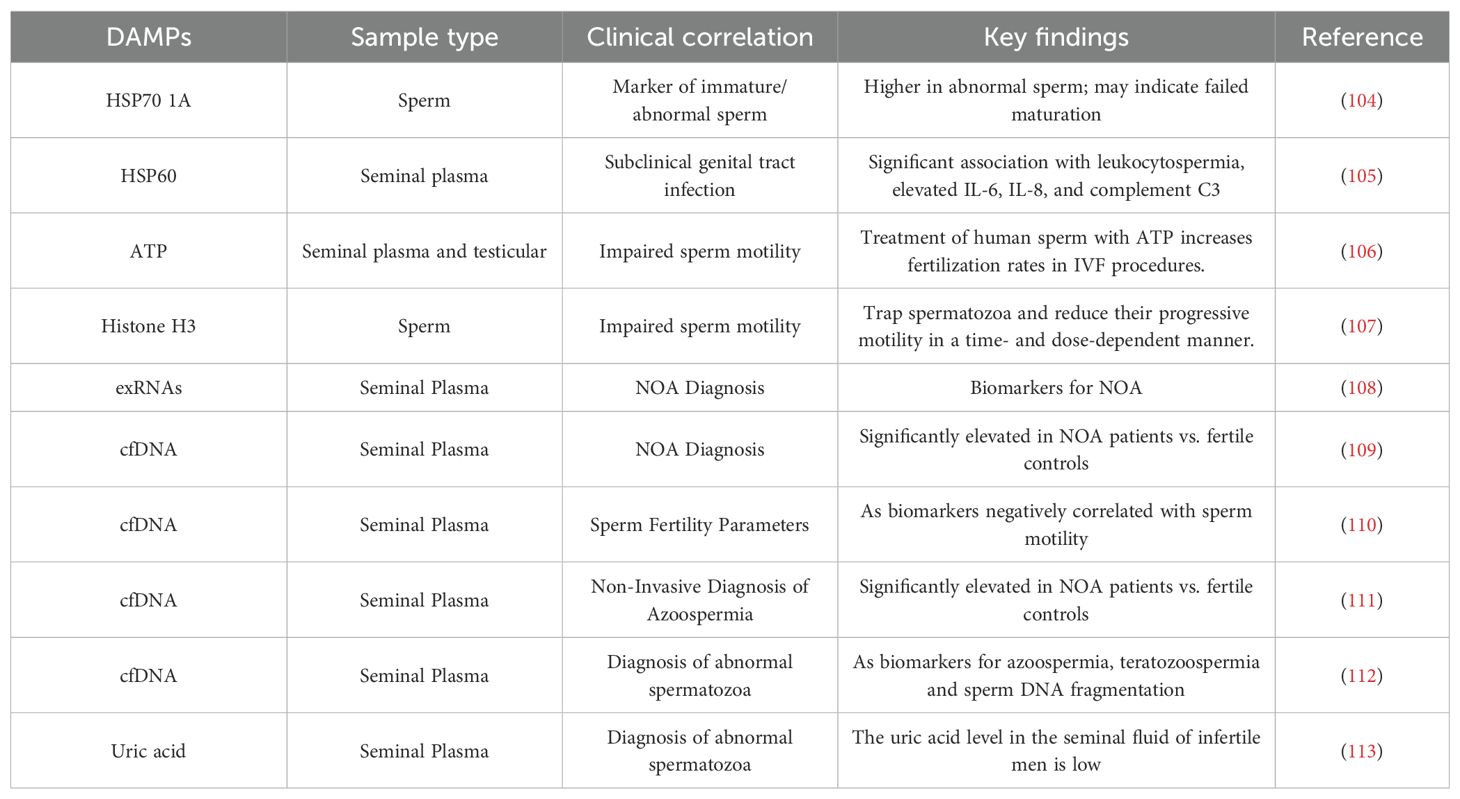
Inflammation, along with various forms of cellular stress and damage, can significantly impact male reproductive processes, including spermatogenesis (100), sperm maturation (101), and fertilization (102). The presence of these stressors in the male reproductive tract often leads to the release of DAMPs. DAMPs play crucial roles in spermatogenesis, sperm maturation, and fertilization. Research has demonstrated the involvement of various DAMPs in different regions of the male reproductive tract, impacting these critical processes (103). Clinically, the levels of specific DAMPs have been established as valuable biomarkers, reflecting distinct infertility phenotypes (Table 2).
3.1 Spermatogenesis
DAMPs play a critical role in regulating spermatogenesis and have been implicated in various mechanisms leading to male infertility. Notably, DAMPs regulate spermatogenesis by activating the inflammasome pathway (IP), particularly the NLRP3 inflammasome (114). In patients with varicocele (VCL)-associated infertility, DAMPs released by testicular cells activate the NLRP3 inflammasome, leading to the release of pro-inflammatory cytokines such as IL-1α, IL-1β, and TNF-α, which are closely associated with abnormal sperm production (115). Studies have shown that the NLRP3 inflammasome is activated in the testes of VCL patients, resulting in significantly increased levels of Caspase-1 and IL-1β, further highlighting the crucial role of the inflammasome in male infertility (116). Within the testicular microenvironment, DAMPs activate immune responses primarily through testicular macrophages, triggering robust TLR/NF-κB signaling and resulting in the secretion of pro-inflammatory cytokines and ROS, which directly contribute to spermatogenic damage (117). Moreover, HMGB1, a prototypical DAMP, also plays a significant role in spermatogenesis. In an in vivo rat model, linagliptin protects against cadmium-induced testicular injury by inhibiting the HMGB1/TLR4 pathway, reducing testicular inflammation, and improving sperm quantity and motility (118). Linagliptin further mitigates testicular damage by suppressing the HMGB1/TLR4/NLRP3 inflammasome axis, leading to decreased caspase-1 activity and reduced release of pro-inflammatory cytokines IL-1β and IL-18. This inhibition is associated with attenuated testicular cell apoptosis and enhanced autophagy flux (118). Studies have shown that mice lacking Hmgb2 (which belongs to the same HMGB family as Hmgb1) exhibit reduced fertility and impaired spermatogenesis (119), further emphasizing the importance of HMGB family proteins in spermatogenesis. Although Hmgb1 is not directly mentioned in the text, its homology suggests it may have similar effects. In experimental autoimmune orchitis (EAO), HMGB1 translocates from testicular cells, and its action can be blocked by ethyl pyruvate (EP), which reduces disease progression and spermatogenic damage (120). Excessive expression of HMGB1 in testicular cells is associated with inflammation and impaired spermatogenic function. Specifically, HMGB1 activates TLR4 on testicular macrophages, driving p38 MAPK/NF-κB-dependent production of TNF-α and IL-6, and further promoting ROS release, thereby amplifying immune-mediated spermatogenic disruption (120). In an in vivo rat model, high-fat diet increased testicular HMGB1 and NLRP3 levels, impairing spermatogenesis, while zinc supplementation reduced HMGB1 expression and improved sperm quantity and motility (121). Additionally, eugenol can alleviate torsion/reperfusion injury (IRI)-induced testicular damage by inhibiting the HMGB1/NF-κB axis and endoplasmic reticulum stress (122). HSPs are essential for proper spermatogenesis. For example, the conditional deletion of Hspa5 leads to spermatogenesis failure and infertility in mice (123). Specific roles of HSP isoforms in protecting sperm cells from stress and apoptosis have been detailed in various studies (124). Hyperthermia-induced stress and the expression of HSP27 are linked to disruptions in spermatogenesis and male fertility (125). Extracellular ATP plays multiple roles in sperm function, impacting both spermatogenesis and fertilization processes (126). ATP signaling in peritubular cells drives testicular sperm transport, showcasing its crucial role (127). eCIRP plays a crucial role in spermatogenesis, particularly under heat stress conditions, where its expression is downregulated, leading to impaired germ cell function (128). Studies have shown that CIRP, as a molecular chaperone, can protect germ cells from oxidative stress and apoptosis, which is especially important during testicular torsion/detorsion (129). Reduced CIRP expression is associated with varicocele and heat-induced infertility (130), suggesting that upregulating CIRP expression may be a new approach to treating male infertility. Lastly, cfDNA holds potential as a biomarker for reproductive health (131), further emphasizing the broad significance of DAMPs in spermatogenesis and male fertility (Figure 2). While rodent models consistently demonstrate HMGB1/NLRP3-driven spermatogenic impairment in cadmium/HFD exposure contexts, human clinical evidence remains predominantly correlative, constrained by two critical limitations: most human studies measure DAMP concentrations in semen without establishing causal mechanisms, and translational gaps persist as murine-targeted NLRP3 inhibitors lack validation in human male infertility trials.
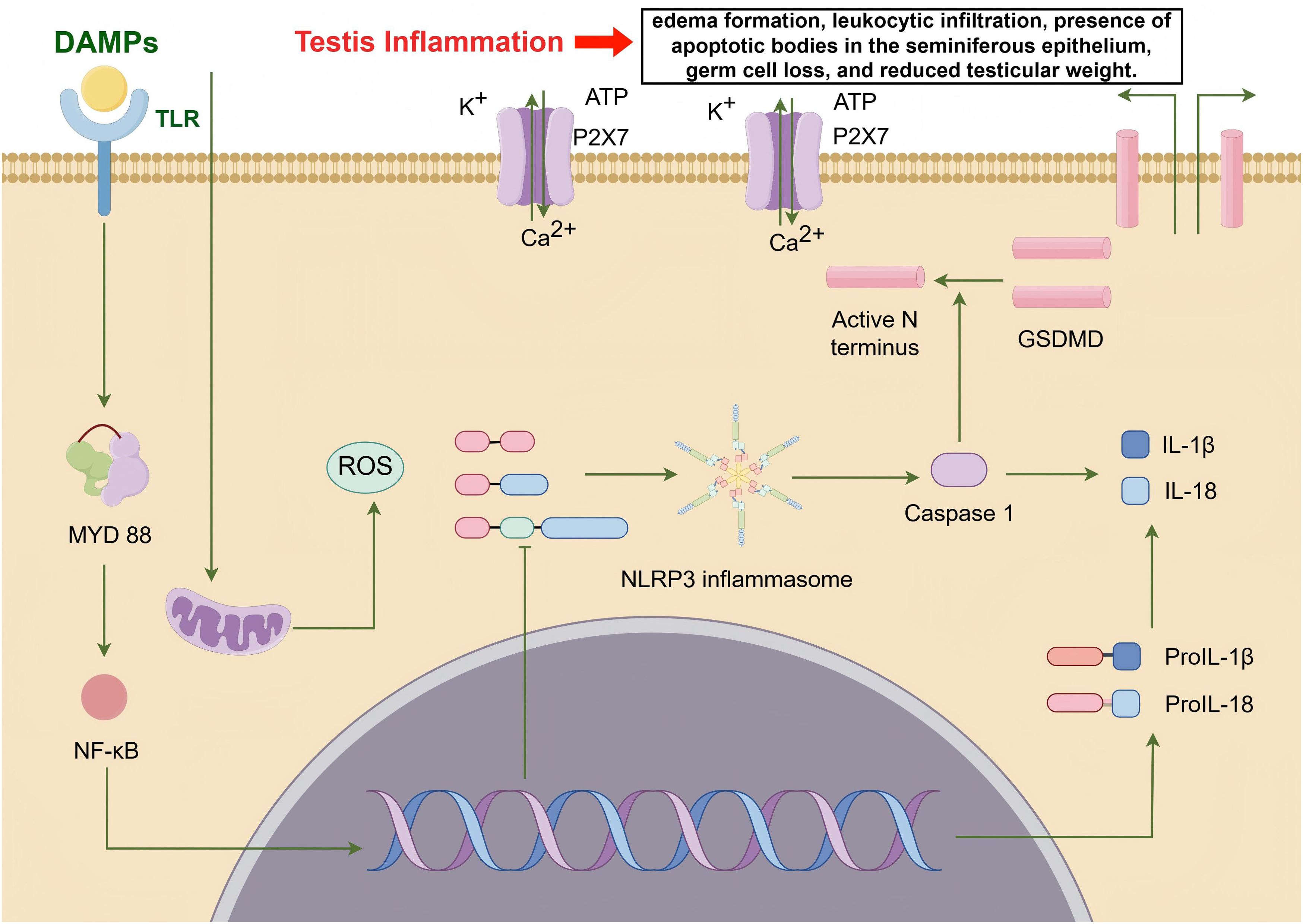
Figure 2. DAMPs promote testicular inflammation by regulating spermatogenesis through the activation of the inflammasome pathway (IP), particularly the NLRP3 inflammasome (from Figdraw, www.figdraw.com). DAMPs released by testicular cells activate the NLRP3 inflammasome via the TLR/MYD88/NF-κB signaling pathway, leading to the release of pro-inflammatory cytokines such as IL-1α, IL-1β, and TNF-α. Additionally, the NLRP3 inflammasome enhances the expression of Caspase-1 and IL-1β. Furthermore, DAMPs induce mitochondrial damage within cells, resulting in ROS accumulation and ATP efflux, thereby exacerbating testicular inflammation.
3.2 Sperm maturation
Studies suggest that the role of DAMPs in sperm maturation is still relatively underexplored, but existing research has highlighted their critical functions in the epididymis. HSPs exhibit differential expression in the epididymis and play a pivotal role in sperm maturation. For instance, it has been observed that HSPs are differentially expressed in the testis, epididymis, and vas deferens of domestic cats (Felis catus), indicating that HSPs may have specific regulatory functions during sperm maturation (103). Furthermore, the seasonal variations in HSP concentrations in the epididymides of roe deer (Capreolus capreolus) further support the regulatory role of HSPs in the process of sperm maturation (132). In addition, ATP also plays a significant role in sperm maturation. Research indicates that extracellular ATP significantly affects mammalian sperm physiology (133). The purinergic signaling pathways are crucial for the recruitment of V-ATPase to the apical membrane of acidifying epididymal clear cells, which is essential for sperm maturation (134). Moreover, studies have shown that extracellular ATP and dibutyryl cAMP can significantly enhance the freezability of rat epididymal sperm (135). Overall, although research on DAMPs in sperm maturation is limited, existing evidence suggests they play crucial roles in this process. Despite the established regulatory role of DAMPs in epididymal sperm maturation, contradictory evidence persists for ATP’s function: rodent models demonstrate enhanced maturation via P2X7 receptor activation, yet human sperm exhibit reduced motility at elevated ATP concentrations (>1mM) owing to Ca²+-mediated toxicity. This species-specific divergence underscores an urgent need for direct human epididymal tissue investigations.
3.3 Fertilization
DAMPs play a crucial role in the fertilization process, influencing sperm function and the interaction between sperm and oocyte. Studies have shown that HMGB1 is a key DAMP, and its levels in follicular fluid are associated with outcomes in in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) cycles. Higher levels of HMGB1 in follicular fluid are correlated with better fertilization outcomes, indicating that HMGB1 plays an important role in the fertilization process by modulating the inflammatory response (136). HSPs, particularly HSP70 and HSP90, are also critical during fertilization. HSP70 helps maintain sperm quality and function by stabilizing the sperm plasma membrane during cryopreservation (137). Additionally, the presence of HSPs in the female reproductive tract provides a protective environment for sperm, aiding in improving fertilization rates (138). Extracellular ATP significantly affects sperm function through various mechanisms. ATP activates purinergic receptors on sperm, increasing intracellular calcium levels and thereby enhancing sperm motility, which is crucial for successful fertilization (126). Treating sperm with extracellular ATP can improve fertilization rates in IVF, especially in cases of male factor infertility (139). ATP also promotes the acrosomal reaction in bovine sperm through P2 receptors, enhancing fertilization capability (140). Moreover, extracellular ATP shows a synergistic effect on the post-thaw quality and fertilization potential of Lohi ram sperm (141). In porcine sperm, surface ATP is essential for fertilization, linked to sperm proteasomal function (142). In human sperm, ATP significantly enhances sperm motility and fertilization potential (143). Histones also have a significant impact on fertilization. Studies have found that components of neutrophil extracellular traps (NETs) adversely affect bovine sperm function, indicating the importance of histones in sperm defense mechanisms (144). Inhibiting SOCE can reduce the formation of neutrophil extracellular traps induced by human sperm, thereby improving sperm motility (145). In porcine sperm, NETs entangle sperm and embryos, hindering the fertilization process (146). Additionally, leukocytes coincubated with human sperm trigger classic neutrophil extracellular trap formation, reducing sperm motility (107). cfDNA has also gained attention for its role in fertilization. Studies indicate that cfDNA levels can serve as biomarkers for embryo quality and are associated with IVF success rates (147). High-quality embryos usually exhibit lower cfDNA levels in follicular fluid and embryo culture media (148). Furthermore, cfDNA can influence maternal immune response, potentially affecting embryo implantation and development. Elevated levels of cfDNA are associated with lower pregnancy rates (147). Uric acid’s role in fertilization has also been studied. Elevated serum uric acid levels in women with polycystic ovary syndrome (PCOS) undergoing IVF or ICSI cycles are associated with adverse reproductive outcomes, suggesting that uric acid levels may impact follicular fluid metabolic characteristics, thereby affecting fertilization and embryo development (149). A significant clinical-translational disconnect persists: while HMGB1 levels in follicular fluid correlate with improved IVF outcomes, no therapeutics currently exist to modulate oviductal DAMPs due to major barriers such as ethical constraints in manipulating human reproductive tracts and unreplicated animal findings—exemplified by porcine NETs severely impairing fertilization versus negligible effects in bovine models.
4 The impact of DAMPs-mediated inflammatory responses on sperm function
Studies have shown that DAMPs-mediated inflammatory responses, including necrosis, necroptosis, apoptosis, pyroptosis, and ferroptosis, have significant impacts on sperm function. During necrosis and necroptosis, the release of DAMPs such as HMGB1 triggers inflammatory responses that negatively affect sperm viability. In particular, necroptosis involves the release of DAMPs like HMGB1, which exacerbates inflammation through the TLR4 and RAGE pathways, compromising the integrity and function of sperm cells (118). On the other hand, studies have shown that reducing ATP levels can induce apoptosis or necrosis of spermatogonia (150). Apoptotic or necrotic cells can release cfDNA fragments, which negatively regulate the quality of embryos after ICSI (151). A study on normozoospermic and non-normozoospermic human samples indicated that HSP-70 expression is lower under normal conditions compared to abnormal conditions, suggesting that HSP-70 may respond to any stressor in non-normozoospermic patients. It can be inferred that HSP has anti-apoptotic effects, inhibiting the clearance of abnormal sperm cells and impairing sperm parameters (152). Additionally, in an in vivo rat model, Hany H. Arab et al. demonstrated that linagliptin inhibits the testicular HMGB1/TLR4/NLRP3 pro-inflammatory axis and apoptosis, thereby attenuating cadmium-induced testicular damage (118). Pyroptosis, an inflammatory form of cell death, is also a major pathway for DAMPs production. A study on semen samples from infertile patients with bilateral varicocele revealed that ROS exposure affects pathways related to pyroptosis and ferroptosis in human sperm, leading to decreased semen quality. Elevated levels of HSP90 in semen suggest a possible association with DAMPs release (153). Ferroptosis-induced ROS accumulation is related to sperm DNA damage. Increased cfDNA resulting from sperm DNA damage significantly reduces sperm fertilization ability (154). Finally, a study on a rat testicular torsion/detorsion (T/D) model found that T/D caused significant weight gain, distortion of the overall anatomical and cellular structure of the testes, poor sperm quality, redox imbalance, and inflammation in both ipsilateral and contralateral testes. This was accompanied by upregulation of xanthine oxidase/uric acid signaling and increased DNA fragmentation in the testes (155), which could be due to inflammation induced by urea and DNA release, ultimately leading to male infertility. In summary, these DAMPs-induced inflammatory responses can disrupt the environment of the testes and epididymis, negatively affecting sperm development and function. Collectively, the ‘DAMP-inflammation-sperm damage’ paradigm is predominantly established through toxin-induced rodent models. However, critical knowledge gaps persist regarding physiological DAMP functions and human disease heterogeneity. Unresolved questions include whether physiological DAMP concentrations contribute to sperm homeostasis maintenance, and why NLRP3 activation exhibits inconsistency among human varicocele patients. Resolution of these issues necessitates single-cell transcriptomic profiling of human testicular immune cells to delineate species-specific inflammatory cascades.
5 Conclusion
In this review, we have comprehensively discussed the role of damage-associated molecular patterns (DAMPs) in male infertility, highlighting their critical involvement in the pathogenesis of this condition. Our analysis reveals that DAMPs, through their diverse interactions with cellular and molecular pathways, significantly impact spermatogenesis and sperm function. Specifically, we have elucidated how DAMPs contribute to the disruption of normal sperm development and functionality, thereby exacerbating male infertility (Figure 3).
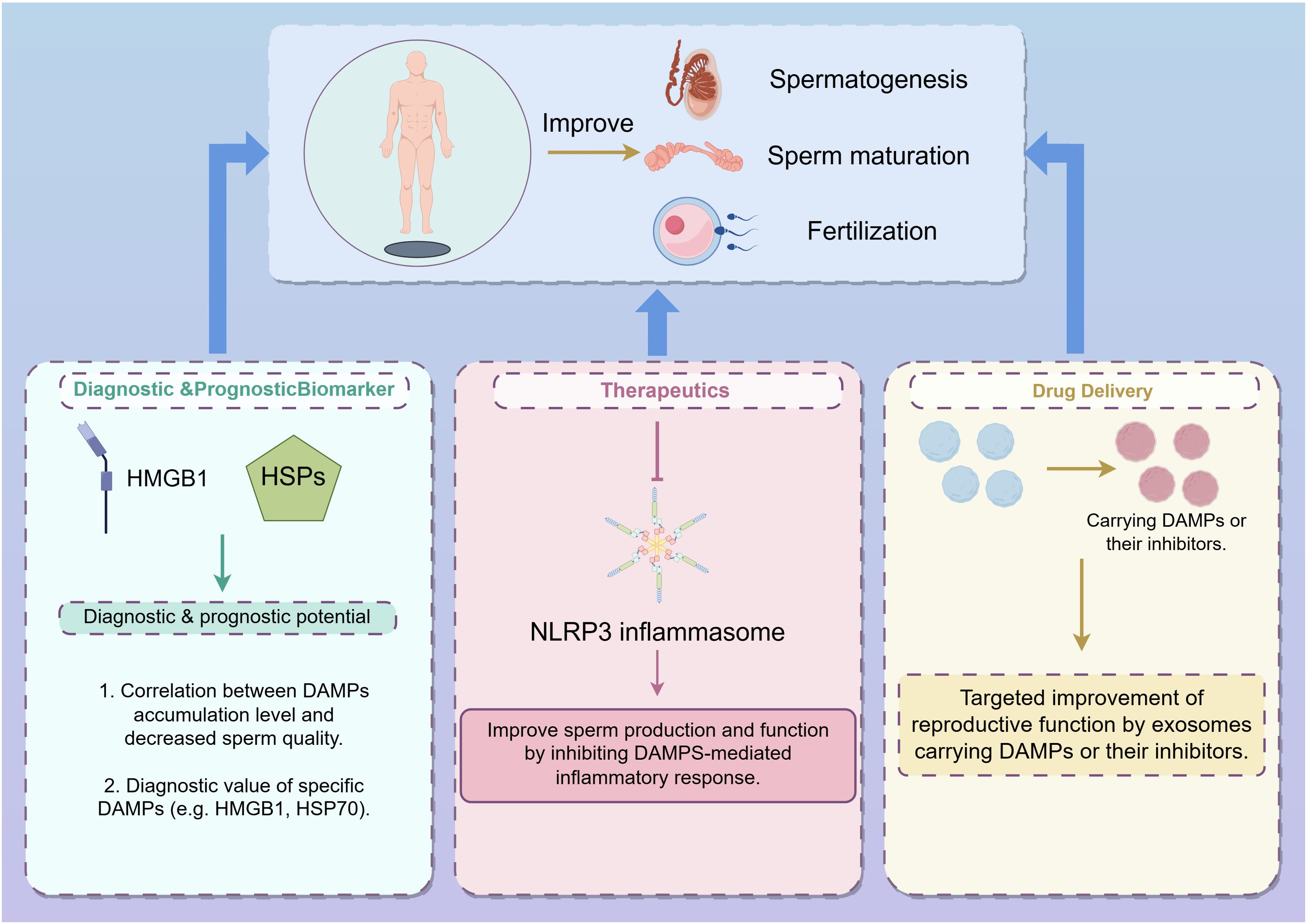
Figure 3. Strategies for improving spermatogenesis, sperm maturation, and fertilization through DAMPs modulation (from Figdraw, www.figdraw.com). This figure illustrates three main approaches to enhance male reproductive processes by targeting DAMPs: Diagnostic & Prognostic Biomarkers: DAMPs, such as HMGB1 and HSPs, are explored as potential diagnostic and prognostic markers for male infertility. The correlation between the accumulation of specific DAMPs and decreased sperm quality highlights their diagnostic value, particularly for DAMPs like HMGB1 and HSP70. Therapeutics: Targeting the NLRP3 inflammasome represents a therapeutic approach to improve sperm production and function. By inhibiting DAMPs-mediated inflammatory responses, it is possible to mitigate the negative effects of inflammation on male fertility. Drug Delivery: Exosomes carrying DAMPs or their inhibitors are depicted as a novel drug delivery system aimed at enhancing reproductive function. This targeted approach leverages the natural intercellular communication properties of exosomes to deliver therapeutic agents directly to the site of action, improving the efficacy of treatments for male infertility.
Our discussion illustrates the critical role of DAMPs in male infertility, emphasizing their potential as both biomarkers and therapeutic targets. The accumulation of DAMPs in the male reproductive tract and their effects on sperm quality and function present substantial implications for understanding the pathophysiology of male infertility. Targeting specific DAMPs or their signaling pathways could provide novel therapeutic avenues for managing and potentially reversing infertility conditions. Future research should focus on elucidating the precise molecular interactions between DAMPs and reproductive cells, as well as identifying specific DAMPs that could serve as reliable diagnostic markers or therapeutic targets. Investigations into how these molecules contribute to immune responses and cellular stress in the context of male infertility are crucial. Additionally, the development of targeted therapies aimed at modulating DAMPs could offer new strategies for improving treatment outcomes. In summary, the role of DAMPs in male infertility represents a promising field of research with significant clinical potential. Advancing our understanding of these mechanisms and their implications for male reproductive health will be instrumental in developing effective diagnostic and therapeutic strategies.
Author contributions
HC: Investigation, Writing – original draft. SL: Writing – review & editing, Data curation, Supervision. SC: Software, Writing – review & editing. HN: Writing – review & editing, Investigation. XL: Data curation, Writing – review & editing. WQ: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China grants (No.8210050633), the Natural Science Foundation of Guangdong Province (No.2020A1515110735), the Medical Research Foundation of Guangdong Province (A2023049, A2023057), the Science and Technology Planning Foundation of Guangzhou City (No.202102080102), the Guangdong Provincial Reproductive Science Institute Innovation Team grants (No.C-03).
Acknowledgments
We thank Figdraw (www.figdraw.com) for expert assistance in the pattern drawing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sharma A, Minhas S, Dhillo WS, and Jayasena CN. Male infertility due to testicular disorders. J Clin Endocrinol Metab. (2021) 106:e442–e59. doi: 10.1210/clinem/dgaa781
2. Gül M, Russo GI, Kandil H, Boitrelle F, Saleh R, Chung E, et al. Male infertility: new developments, current challenges, and future directions. World J Mens Health. (2024) 42:502–17. doi: 10.5534/wjmh.230232
3. Norris AC, Yazlovitskaya EM, Yang TS, Mansueto A, Stafford JM, and Graham TR. ATP10A deficiency results in male-specific infertility in mice. Front Cell Dev Biol. (2024) 12:1310593. doi: 10.3389/fcell.2024.1310593
4. Ubba V, Joseph S, Awe O, Jones D, Dsilva MK, Feng M, et al. Reproductive profile of neuronal androgen receptor knockout female mice with a low dose of DHT. Endocrinology. (2024) 165:bqad199. doi: 10.1210/endocr/bqad199
5. Biggs SN, Kennedy J, Lewis SL, Hearps S, O'Bryan MK, McLachlan R, et al. Lifestyle and environmental risk factors for unexplained male infertility: study protocol for Australian Male Infertility Exposure (AMIE), a case-control study. Reprod Health. (2023) 20:32. doi: 10.1186/s12978-023-01578-z
6. Bellingham M and Evans N. Impact Of Real-Life Environmental Exposures On Reproduction: Biosolids and male reproduction. Reproduction. (2024) 168:e240119. doi: 10.1530/REP-24-0119
7. Murao A, Aziz M, Wang H, Brenner M, and Wang P. Release mechanisms of major DAMPs. Apoptosis. (2021) 26:152–62. doi: 10.1007/s10495-021-01663-3
8. Ma M, Jiang W, and Zhou R. DAMPs and DAMP-sensing receptors in inflammation and diseases. Immunity. (2024) 57:752–71. doi: 10.1016/j.immuni.2024.03.002
9. Li X, Ye Y, Peng K, Zeng Z, Chen L, and Zeng Y. Histones: The critical players in innate immunity. Front Immunol. (2022) 13:1030610. doi: 10.3389/fimmu.2022.1030610
10. Chakraborty D, Zenker S, Rossaint J, Hölscher A, Pohlen M, Zarbock A, et al. Alarmin S100A8 activates alveolar epithelial cells in the context of acute lung injury in a TLR4-dependent manner. Front Immunol. (2017) 8:1493. doi: 10.3389/fimmu.2017.01493
11. Childs CE, Calder PC, and Miles EA. Diet and immune function. Nutrients. (2019) 11:1933. doi: 10.3390/nu11081933
12. Zhang Y, Wu J, Dong E, Wang Z, and Xiao H. Toll-like receptors in cardiac hypertrophy. Front Cardiovasc Med. (2023) 10:1143583. doi: 10.3389/fcvm.2023.1143583
13. Segal BH, Giridharan T, Suzuki S, Khan ANH, Zsiros E, Emmons TR, et al. Neutrophil interactions with T cells, platelets, endothelial cells, and of course tumor cells. Immunol Rev. (2023) 314:13–35. doi: 10.1111/imr.v314.1
14. Koncz G, Jenei V, Tóth M, Váradi E, Kardos B, Bácsi A, et al. Damage-mediated macrophage polarization in sterile inflammation. Front Immunol. (2023) 14:1169560. doi: 10.3389/fimmu.2023.1169560
15. Qian W, Ye J, and Xia S. DNA sensing of dendritic cells in cancer immunotherapy. Front Mol Biosci. (2024) 11:1391046. doi: 10.3389/fmolb.2024.1391046
16. DeWolf SE, Kasimsetty SG, Hawkes AA, Stocks LM, Kurian SM, and McKay DB. DAMPs released from injured renal tubular epithelial cells activate innate immune signals in healthy renal tubular epithelial cells. Transplantation. (2022) 106:1589–99. doi: 10.1097/TP.0000000000004038
17. DeWolf SE, Hawkes AA, Kurian SM, Gorial DE, Hepokoski ML, Almeida SS, et al. Human pulmonary microvascular endothelial cells respond to DAMPs from injured renal tubular cells. Pulm Circ. (2024) 14:e12379. doi: 10.1002/pul2.12379
18. Turner NA. Inflammatory and fibrotic responses of cardiac fibroblasts to myocardial damage associated molecular patterns (DAMPs). J Mol Cell Cardiol. (2016) 94:189–200. doi: 10.1016/j.yjmcc.2015.11.002
19. Patel S. Danger-associated molecular patterns (DAMPs): the derivatives and triggers of inflammation. Curr Allergy Asthma Rep. (2018) 18:63. doi: 10.1007/s11882-018-0817-3
20. Wicherska-Pawłowska K, Wróbel T, and Rybka J. Toll-like receptors (TLRs), NOD-like receptors (NLRs), and RIG-I-like receptors (RLRs) in innate immunity. TLRs, NLRs, and RLRs ligands as immunotherapeutic agents for hematopoietic diseases. Int J Mol Sci. (2021) 22:13397. doi: 10.3390/ijms222413397
21. Dinice L, Esposito G, Cacciamani A, Balzamino BO, Cosimi P, Cafiero C, et al. TLR2 and TLR4 are expressed in epiretinal membranes: possible links with vitreous levels of complement fragments and DAMP-related proteins. Int J Mol Sci. (2024) 25:7732. doi: 10.3390/ijms25147732
22. Díaz-Dinamarca DA, Salazar ML, Escobar DF, Castillo BN, Valdebenito B, Díaz P, et al. Surface immunogenic protein from Streptococcus agalactiae and Fissurella latimarginata hemocyanin are TLR4 ligands and activate MyD88- and TRIF dependent signaling pathways. Front Immunol. (2023) 14:1186188. doi: 10.3389/fimmu.2023.1186188
23. Lv X, Wang H, Su A, Xu S, and Chu Y. Herpes simplex virus type 2 infection triggers AP-1 transcription activity through TLR4 signaling in genital epithelial cells. Virol J. (2018) 15:173. doi: 10.1186/s12985-018-1087-3
24. Zou PF, Shen JJ, Li Y, Zhang ZP, and Wang YL. TRAF3 enhances TRIF-mediated signaling via NF-κB and IRF3 activation in large yellow croaker Larimichthys crocea. Fish Shellfish Immunol. (2020) 97:114–24. doi: 10.1016/j.fsi.2019.12.024
25. Genetzakis E, Gilchrist J, Kassiou M, and Figtree GA. Development and clinical translation of P2X7 receptor antagonists: A potential therapeutic target in coronary artery disease? Pharmacol Ther. (2022) 237:108228. doi: 10.1016/j.pharmthera.2022.108228
26. Lin SY, Hsieh SY, Fan YT, Wei WC, Hsiao PW, Tsai DH, et al. Necroptosis promotes autophagy-dependent upregulation of DAMP and results in immunosurveillance. Autophagy. (2018) 14:778–95. doi: 10.1080/15548627.2017.1386359
27. Li QF, Li YX, Yang YY, Dong PP, Mei CJ, Lu JL, et al. The egg ribonuclease SjCP1412 accelerates liver fibrosis caused by Schistosoma japonicum infection involving damage-associated molecular patterns (DAMPs). Parasitology. (2024) 151:260–70. doi: 10.1017/S0031182023001361
28. Wang YY, Li SL, Zhang XY, Jiang FL, Guo QL, Jiang P, et al. Multi-in-one" Yolk-shell structured nanoplatform inducing pyroptosis and antitumor immune response through cascade reactions. Small. (2024) 20:e2400254. doi: 10.1002/smll.202400254
29. Tang D, Kroemer G, and Kang R. Ferroptosis in immunostimulation and immunosuppression. Immunol Rev. (2024) 321:199–210. doi: 10.1111/imr.v321.1
30. Gando S, Levi M, and Toh CH. Trauma-induced innate immune activation and disseminated intravascular coagulation. J Thromb Haemost. (2024) 22:337–51. doi: 10.1016/j.jtha.2023.09.028
31. Fleshner M and Crane CR. Exosomes, DAMPs and miRNA: features of stress physiology and immune homeostasis. Trends Immunol. (2017) 38:768–76. doi: 10.1016/j.it.2017.08.002
32. Nakano H, Murai S, and Moriwaki K. Regulation of the release of damage-associated molecular patterns from necroptotic cells. Biochem J. (2022) 479:677–85. doi: 10.1042/BCJ20210604
33. Wickman GR, Julian L, Mardilovich K, Schumacher S, Munro J, Rath N, et al. Blebs produced by actin-myosin contraction during apoptosis release damage-associated molecular pattern proteins before secondary necrosis occurs. Cell Death Differ. (2013) 20:1293–305. doi: 10.1038/cdd.2013.69
34. Li JY, Yao YM, and Tian YP. Ferroptosis: A trigger of proinflammatory state progression to immunogenicity in necroinflammatory disease. Front Immunol. (2021) 12:701163. doi: 10.3389/fimmu.2021.701163
35. Murao A, Brenner M, Aziz M, and Wang P. Exosomes in sepsis. Front Immunol. (2020) 11:2140. doi: 10.3389/fimmu.2020.02140
36. Simpson J, Spann KM, and Phipps S. MLKL regulates rapid cell death-independent HMGB1 release in RSV infected airway epithelial cells. Front Cell Dev Biol. (2022) 10:890389. doi: 10.3389/fcell.2022.890389
37. Deng M, Tang Y, Li W, Wang X, Zhang R, Zhang X, et al. The endotoxin delivery protein HMGB1 mediates caspase-11-dependent lethality in sepsis. Immunity. (2018) 49:740–53.e7. doi: 10.1016/j.immuni.2018.08.016
38. Foley AE, Sulstarova S, and Goldenberg NM. Assessment of HMGB1 release during experimentally induced pyroptosis. Methods Mol Biol. (2023) 2641:189–202. doi: 10.1007/978-1-0716-3040-2_16
39. Li G, Liao C, Chen J, Wang Z, Zhu S, Lai J, et al. Targeting the MCP-GPX4/HMGB1 axis for effectively triggering immunogenic ferroptosis in pancreatic ductal adenocarcinoma. Adv Sci (Weinh). (2024) 11:e2308208. doi: 10.1002/advs.202308208
40. Cao S, Li S, Li H, Xiong L, Zhou Y, Fan J, et al. The potential role of HMGB1 release in peritoneal dialysis-related peritonitis. PloS One. (2013) 8:e54647. doi: 10.1371/journal.pone.0054647
41. Yang K, Fan M, Wang X, Xu J, Wang Y, Tu F, et al. Lactate promotes macrophage HMGB1 lactylation, acetylation, and exosomal release in polymicrobial sepsis. Cell Death Differ. (2022) 29:133–46. doi: 10.1038/s41418-021-00841-9
42. Andersson U and Tracey KJ. HMGB1 as a mediator of necrosis-induced inflammation and a therapeutic target in arthritis. Rheum Dis Clin North Am. (2004) 30:627–37, xi. doi: 10.1016/j.rdc.2004.04.007
43. Loukili N, Rosenblatt-Velin N, Li J, Clerc S, Pacher P, Feihl F, et al. Peroxynitrite induces HMGB1 release by cardiac cells in vitro and HMGB1 upregulation in the infarcted myocardium in vivo. Cardiovasc Res. (2011) 89:586–94. doi: 10.1093/cvr/cvq373
44. Wang Y, Zhang H, Chen Q, Jiao F, Shi C, Pei M, et al. TNF-α/HMGB1 inflammation signalling pathway regulates pyroptosis during liver failure and acute kidney injury. Cell Prolif. (2020) 53:e12829. doi: 10.1111/cpr.12829
45. Lu YN, Shen XY, Lu JM, Jin GN, Lan HW, Xu X, et al. Resveratrol inhibits Toxoplasma gondii-induced lung injury, inflammatory cascade and evidences of its mechanism of action. Phytomedicine. (2023) 108:154522. doi: 10.1016/j.phymed.2022.154522
46. Satoh TK. The role of HMGB1 in inflammatory skin diseases. J Dermatol Sci. (2022) 107:58–64. doi: 10.1016/j.jdermsci.2022.07.005
47. Chen S, Pan J, Gong Z, Wu M, Zhang X, Chen H, et al. Hypochlorous acid derived from microglial myeloperoxidase could mediate high-mobility group box 1 release from neurons to amplify brain damage in cerebral ischemia-reperfusion injury. J Neuroinflammation. (2024) 21:70. doi: 10.1186/s12974-023-02991-8
48. Qiao H, Morioka Y, Wang D, Liu K, Gao S, Wake H, et al. Protective effects of an anti-4-HNE monoclonal antibody against liver injury and lethality of endotoxemia in mice. Eur J Pharmacol. (2023) 950:175702. doi: 10.1016/j.ejphar.2023.175702
49. Yang K, Cao F, Wang W, Tian Z, and Yang L. The relationship between HMGB1 and autophagy in the pathogenesis of diabetes and its complications. Front Endocrinol (Lausanne). (2023) 14:1141516. doi: 10.3389/fendo.2023.1141516
50. Tian Y, Chen R, and Su Z. HMGB1 is a potential and challenging therapeutic target for parkinson's disease. Cell Mol Neurobiol. (2023) 43:47–58. doi: 10.1007/s10571-021-01170-8
51. Qu H, Heinbäck R, Salo H, Ewing E, Espinosa A, Aulin C, et al. Transcriptomic profiling reveals that HMGB1 induces macrophage polarization different from classical M1. Biomolecules. (2022) 12:779. doi: 10.3390/biom12060779
52. Li J, Wang Z, Li J, Zhao H, and Ma Q. HMGB1: A new target for ischemic stroke and hemorrhagic transformation. Transl Stroke Res. (2024) 16:990–1015. doi: 10.1007/s12975-024-01258-5
53. Cheng KJ, Alshawsh MA, Mejia Mohamed EH, Thavagnanam S, Sinniah A, and Ibrahim ZA. HMGB1: an overview of its versatile roles in the pathogenesis of colorectal cancer. Cell Oncol (Dordr). (2020) 43:177–93. doi: 10.1007/s13402-019-00477-5
54. Dong H, Zhang L, and Liu S. Targeting HMGB1: an available therapeutic strategy for breast cancer therapy. Int J Biol Sci. (2022) 18:3421–34. doi: 10.7150/ijbs.73504
55. Cebrián MJ, Bauden M, Andersson R, Holdenrieder S, and Ansari D. Paradoxical role of HMGB1 in pancreatic cancer: tumor suppressor or tumor promoter? Anticancer Res. (2016) 36:4381–9. doi: 10.21873/anticanres.10981
56. De Maio A. Extracellular Hsp70: export and function. Curr Protein Pept Sci. (2014) 15:225–31. doi: 10.2174/1389203715666140331113057
57. Fredly H, Ersvær E, Gjertsen BT, and Bruserud O. Immunogenic apoptosis in human acute myeloid leukemia (AML): primary human AML cells expose calreticulin and release heat shock protein (HSP) 70 and HSP90 during apoptosis. Oncol Rep. (2011) 25:1549–56. doi: 10.3892/or.2011.1229
58. Taha EA, Ono K, and Eguchi T. Roles of extracellular HSPs as biomarkers in immune surveillance and immune evasion. Int J Mol Sci. (2019) 20:4588. doi: 10.3390/ijms20184588
59. Wang Q, Kong X, Guo W, Liu L, Tian Y, Tao X, et al. HSP90 exacerbates bone destruction in rheumatoid arthritis by activating TRAF6/NFATc1 signaling. Inflammation. (2024) 47:363–75. doi: 10.1007/s10753-023-01914-2
60. Patnaik S, Nathan S, Kar B, Gregoric ID, and Li YP. The role of extracellular heat shock proteins in cardiovascular diseases. Biomedicines. (2023) 11:1557. doi: 10.3390/biomedicines11061557
61. Chebotareva N, Bobkova I, and Shilov E. Heat shock proteins and kidney disease: perspectives of HSP therapy. Cell Stress Chaperones. (2017) 22:319–43. doi: 10.1007/s12192-017-0790-0
62. Chen Z, Li P, Shen L, and Jiang X. Heat shock protein B7 (HSPB7) inhibits lung adenocarcinoma progression by inhibiting glycolysis. Oncol Rep. (2023) 50:196. doi: 10.3892/or.2023.8633
63. Ben Abdallah H, Bregnhøj A, Ghatnekar G, Iversen L, and Johansen C. Heat shock protein 90 inhibition attenuates inflammation in models of atopic dermatitis: a novel mechanism of action. Front Immunol. (2023) 14:1289788. doi: 10.3389/fimmu.2023.1289788
64. Li J, Shen S, and Shen H. Heat-shock protein A12A attenuates oxygen-glucose deprivation/reoxygenation-induced human brain microvascular endothelial cell dysfunction via PGC-1α/SIRT3 pathway. Drug Dev Res. (2024) 85:e22130. doi: 10.1002/ddr.22130
65. Lu JM, Xu X, Aosai F, Zhang MY, Zhou LL, and Piao LX. Protective effect of arctiin against Toxoplasma gondii HSP70-induced allergic acute liver injury by disrupting the TLR4-mediated activation of cytosolic phospholipase A(2) and platelet-activating factor. Int Immunopharmacol. (2024) 126:111254. doi: 10.1016/j.intimp.2023.111254
66. Esmaeilzadeh A, Mohammadi V, Elahi R, and Rezakhani N. The role of heat shock proteins (HSPs) in type 2 diabetes mellitus pathophysiology. J Diabetes Complications. (2023) 37:108564. doi: 10.1016/j.jdiacomp.2023.108564
67. Aghazadeh N, Beilankouhi EAV, Fakhri F, Gargari MK, Bahari P, Moghadami A, et al. Involvement of heat shock proteins and parkin/α-synuclein axis in Parkinson's disease. Mol Biol Rep. (2022) 49:11061–70. doi: 10.1007/s11033-022-07900-5
68. Banecka-Majkutewicz Z, Sawuła W, Kadziński L, Węgrzyn A, and Banecki B. Homocysteine, heat shock proteins, genistein and vitamins in ischemic stroke–pathogenic and therapeutic implications. Acta Biochim Pol. (2012) 59:495–9. doi: 10.18388/abp.2012_2083
69. Javid H, Hashemian P, Yazdani S, Sharbaf Mashhad A, and Karimi-Shahri M. The role of heat shock proteins in metastatic colorectal cancer: A review. J Cell Biochem. (2022) 123:1704–35. doi: 10.1002/jcb.v123.11
70. Yildiz MT, Tutar L, Giritlioğlu NI, Bayram B, and Tutar Y. MicroRNAs and heat shock proteins in breast cancer biology. Methods Mol Biol. (2022) 2257:293–310. doi: 10.1007/978-1-0716-1170-8_15
71. Luo R, Onyshchenko K, Wang L, Gaedicke S, Grosu AL, Firat E, et al. Necroptosis-dependent immunogenicity of cisplatin: implications for enhancing the radiation-induced abscopal effect. Clin Cancer Res. (2023) 29:667–83. doi: 10.1158/1078-0432.CCR-22-1591
72. Zhang H, Wang S, Zhang Z, Hou M, Du C, Zhao Z, et al. Cryo-EM structure of human heptameric pannexin 2 channel. Nat Commun. (2023) 14:1118. doi: 10.1038/s41467-023-36861-x
73. Li W, Fu H, Fang L, Chai H, Gao T, Chen Z, et al. Shikonin induces ferroptosis in multiple myeloma via GOT1-mediated ferritinophagy. Front Oncol. (2022) 12:1025067. doi: 10.3389/fonc.2022.1025067
74. Jung J, Shin YH, Konishi H, Lee SJ, and Kiyama H. Possible ATP release through lysosomal exocytosis from primary sensory neurons. Biochem Biophys Res Commun. (2013) 430:488–93. doi: 10.1016/j.bbrc.2012.12.009
75. Thakur A, Johnson A, Jacobs E, Zhang K, Chen J, Wei Z, et al. Energy sources for exosome communication in a cancer microenvironment. Cancers (Basel). (2022) 14:1698. doi: 10.3390/cancers14071698
76. Kaltenmeier C, Wang R, Popp B, Geller D, Tohme S, and Yazdani HO. Role of immuno-inflammatory signals in liver ischemia-reperfusion injury. Cells. (2022) 11:2222. doi: 10.3390/cells11142222
77. Fodor P, White B, and Khan R. Inflammation-The role of ATP in pre-eclampsia. Microcirculation. (2020) 27:e12585. doi: 10.1111/micc.12585
78. Kang R, Lotze MT, Zeh HJ, Billiar TR, and Tang D. Cell death and DAMPs in acute pancreatitis. Mol Med. (2014) 20:466–77. doi: 10.2119/molmed.2014.00117
79. Caballero-Herrero MJ, Jumilla E, Buitrago-Ruiz M, Valero-Navarro G, and Cuevas S. Role of damage-associated molecular patterns (DAMPS) in the postoperative period after colorectal surgery. Int J Mol Sci. (2023) 24:3862. doi: 10.3390/ijms24043862
80. Reilly B, Tan C, Murao A, Nofi C, Jha A, Aziz M, et al. Necroptosis-mediated eCIRP release in sepsis. J Inflammation Res. (2022) 15:4047–59. doi: 10.2147/JIR.S370615
81. Han J, Zhang Y, Ge P, Dakal TC, Wen H, Tang S, et al. Exosome-derived CIRP: An amplifier of inflammatory diseases. Front Immunol. (2023) 14:1066721. doi: 10.3389/fimmu.2023.1066721
82. Zhou M, Aziz M, and Wang P. Damage-associated molecular patterns as double-edged swords in sepsis. Antioxid Redox Signal. (2021) 35:1308–23. doi: 10.1089/ars.2021.0008
83. Wu D, Ingram A, Lahti JH, Mazza B, Grenet J, Kapoor A, et al. Apoptotic release of histones from nucleosomes. J Biol Chem. (2002) 277:12001–8. doi: 10.1074/jbc.M109219200
84. Nakazawa D, Marschner JA, Platen L, and Anders HJ. Extracellular traps in kidney disease. Kidney Int. (2018) 94:1087–98. doi: 10.1016/j.kint.2018.08.035
85. Ferrer-Diaz AI, Sinha G, Petryna A, Gonzalez-Bermejo R, Kenfack Y, Adetayo O, et al. Revealing role of epigenetic modifiers and DNA oxidation in cell-autonomous regulation of Cancer stem cells. Cell Commun Signal. (2024) 22:119. doi: 10.1186/s12964-024-01512-1
86. Jung J, Lee LE, Kim H, Kim JE, Jang SH, Roh JS, et al. Extracellular histones aggravate autoimmune arthritis by lytic cell death. Front Immunol. (2022) 13:961197. doi: 10.3389/fimmu.2022.961197
87. Shah M, He Z, Rauf A, Beikoghli Kalkhoran S, Heiestad CM, Stensløkken KO, et al. Extracellular histones are a target in myocardial ischaemia-reperfusion injury. Cardiovasc Res. (2022) 118:1115–25. doi: 10.1093/cvr/cvab139
88. Yang R and Tonnesseen TI. DAMPs and sterile inflammation in drug hepatotoxicity. Hepatol Int. (2019) 13:42–50. doi: 10.1007/s12072-018-9911-9
89. Lei B, Wang C, Snow K, Graton ME, Tighe RM, Fager AM, et al. Inhalation of an RNA aptamer that selectively binds extracellular histones protects from acute lung injury. Mol Ther Nucleic Acids. (2023) 31:662–73. doi: 10.1016/j.omtn.2023.02.021
90. Tielking K, Fischer S, Preissner KT, Vajkoczy P, and Xu R. Extracellular RNA in central nervous system pathologies. Front Mol Neurosci. (2019) 12:254. doi: 10.3389/fnmol.2019.00254
91. Bryniarski K, Ptak W, Martin E, Nazimek K, Szczepanik M, Sanak M, et al. Free extracellular miRNA functionally targets cells by transfecting exosomes from their companion cells. PloS One. (2015) 10:e0122991. doi: 10.1371/journal.pone.0122991
92. Feng Y, Chen H, Cai J, Zou L, Yan D, Xu G, et al. Cardiac RNA induces inflammatory responses in cardiomyocytes and immune cells via Toll-like receptor 7 signaling. J Biol Chem. (2015) 290:26688–98. doi: 10.1074/jbc.M115.661835
93. Hu Z, Chen H, Long Y, Li P, and Gu Y. The main sources of circulating cell-free DNA: Apoptosis, necrosis and active secretion. Crit Rev Oncol Hematol. (2021) 157:103166. doi: 10.1016/j.critrevonc.2020.103166
94. Rostami A, Lambie M, Yu CW, Stambolic V, Waldron JN, and Bratman SV. Senescence, necrosis, and apoptosis govern circulating cell-free DNA release kinetics. Cell Rep. (2020) 31:107830. doi: 10.1016/j.celrep.2020.107830
95. Gould TJ, Vu TT, Swystun LL, Dwivedi DJ, Mai SH, Weitz JI, et al. Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arterioscler Thromb Vasc Biol. (2014) 34:1977–84. doi: 10.1161/ATVBAHA.114.304114
96. Wang W, Kong P, Ma G, Li L, Zhu J, Xia T, et al. Characterization of the release and biological significance of cell-free DNA from breast cancer cell lines. Oncotarget. (2017) 8:43180–91. doi: 10.18632/oncotarget.17858
97. Shah M, Yellon DM, and Davidson SM. The role of extracellular DNA and histones in ischaemia-reperfusion injury of the myocardium. Cardiovasc Drugs Ther. (2020) 34:123–31. doi: 10.1007/s10557-020-06946-6
98. Krenzien F, Katou S, Papa A, Sinn B, Benzing C, Feldbrügge L, et al. Increased cell-free DNA plasma concentration following liver transplantation is linked to portal hepatitis and inferior survival. J Clin Med. (2020) 9:1543. doi: 10.3390/jcm9051543
99. Petrasek J, Iracheta-Vellve A, Saha B, Satishchandran A, Kodys K, Fitzgerald KA, et al. Metabolic danger signals, uric acid and ATP, mediate inflammatory cross-talk between hepatocytes and immune cells in alcoholic liver disease. J Leukoc Biol. (2015) 98:249–56. doi: 10.1189/jlb.3AB1214-590R
100. Xu GL, Ye XL, Vashisth MK, and Zhao WZ. Correlation between PRDX2 and spermatogenesis under oxidative stress. Biochem Biophys Res Commun. (2023) 656:139–45. doi: 10.1016/j.bbrc.2023.03.050
101. Ko EY, Sabanegh ES Jr., and Agarwal A. Male infertility testing: reactive oxygen species and antioxidant capacity. Fertil Steril. (2014) 102:1518–27. doi: 10.1016/j.fertnstert.2014.10.020
102. Artini PG, Scarfò G, Marzi I, Fusi J, Obino ME, Franzoni F, et al. Oxidative stress-related signaling pathways predict oocytes' Fertilization In Vitro and Embryo Quality. Int J Mol Sci. (2022) 23:13442. doi: 10.3390/ijms232113442
103. Liman N. Heat shock proteins are differentially expressed in the domestic cat (Felis catus) testis, epididymis, and vas deferens. Microsc Microanal. (2023) 29:713–38. doi: 10.1093/micmic/ozac054
104. Cui Z, Sharma R, and Agarwal A. Proteomic analysis of mature and immature ejaculated spermatozoa from fertile men. Asian J Androl. (2016) 18:735–46. doi: 10.4103/1008-682X.164924
105. Eggert-Kruse W, Neuer A, Clussmann C, Boit R, Geissler W, Rohr G, et al. Seminal antibodies to human 60kd heat shock protein (HSP 60) in male partners of subfertile couples. Hum Reprod. (2002) 17:726–35. doi: 10.1093/humrep/17.3.726
106. Gur S, Kadowitz PJ, and Hellstrom WJ. Purinergic (P2) receptor control of lower genitourinary tract function and new avenues for drug action: an overview. Curr Pharm Des. (2007) 13:3236–44. doi: 10.2174/138161207782341277
107. Zambrano F, Carrau T, Gärtner U, Seipp A, Taubert A, Felmer R, et al. Leukocytes coincubated with human sperm trigger classic neutrophil extracellular traps formation, reducing sperm motility. Fertil Steril. (2016) 106:1053–60.e1. doi: 10.1016/j.fertnstert.2016.06.005
108. Barceló M, Mata A, Bassas L, and Larriba S. Exosomal microRNAs in seminal plasma are markers of the origin of azoospermia and can predict the presence of sperm in testicular tissue. Hum Reprod. (2018) 33:1087–98. doi: 10.1093/humrep/dey072
109. Fontana L, Sirchia SM, Pesenti C, Colpi GM, and Miozzo MR. Non-invasive biomarkers for sperm retrieval in non-obstructive patients: a comprehensive review. Front Endocrinol (Lausanne). (2024) 15:1349000. doi: 10.3389/fendo.2024.1349000
110. Costa F, Barbisan F, Assmann CE, Araújo NKF, de Oliveira AR, Signori JP, et al. Seminal cell-free DNA levels measured by PicoGreen fluorochrome are associated with sperm fertility criteria. Zygote. (2017) 25:111–9. doi: 10.1017/S0967199416000307
111. Li HM, Wan XY, Zhao JY, Liang XM, Dai Y, and Li HG. Promising novel biomarkers and therapy targets: The application of cell-free seminal nucleotides in male reproduction research. Transl Res. (2023) 256:73–86. doi: 10.1016/j.trsl.2022.12.006
112. Di Pizio P, Celton N, Menoud PA, Belloc S, Cohen Bacrie M, Belhadri-Mansouri N, et al. Seminal cell-free DNA and sperm characteristic's: An added biomarker for male infertility investigation. Andrologia. (2021) 53:e13822. doi: 10.1111/and.13822
113. Xu K, Shang X, Chen Y, Zhao F, Zhu P, and Huang Y. Measurement of uric acid of seminal plasma in fertile and infertile males. Zhonghua Nan Ke Xue. (2004) 10:900–1, 6.
114. Tavalaee M, Rahmani M, Drevet JR, and Nasr-Esfahani MH. The NLRP3 inflammasome: molecular activation and regulation in spermatogenesis and male infertility; a systematic review. Basic Clin Androl. (2022) 32:8. doi: 10.1186/s12610-022-00157-9
115. Sahin Z, Celik-Ozenci C, Akkoyunlu G, Korgun ET, Acar N, Erdogru T, et al. Increased expression of interleukin-1alpha and interleukin-1beta is associated with experimental varicocele. Fertil Steril. (2006) 85 Suppl 1:1265–75. doi: 10.1016/j.fertnstert.2005.10.025
116. Baazm M, Ghafarizadeh AA, Noshad Kamran AR, Beyer C, and Zendedel A. Presence of the NLRP3 inflammasome components in semen of varicocele patients. Int J Fertil Steril. (2020) 14:46–50. doi: 10.22074/ijfs.2020.5734
117. Fomichova O, Oliveira PF, and Bernardino RL. Exploring the interplay between inflammation and male fertility. FEBS J. (2025) 292:3321–49. doi: 10.1111/febs.v292.13
118. Arab HH, Elhemiely AA, El-Sheikh AAK, Khabbaz HJA, Arafa EA, Ashour AM, et al. Repositioning linagliptin for the mitigation of cadmium-induced testicular dysfunction in rats: targeting HMGB1/TLR4/NLRP3 axis and autophagy. Pharm (Basel). (2022) 15:852. doi: 10.3390/ph15070852
119. Ronfani L, Ferraguti M, Croci L, Ovitt CE, Schöler HR, Consalez GG, et al. Reduced fertility and spermatogenesis defects in mice lacking chromosomal protein Hmgb2. Development. (2001) 128:1265–73. doi: 10.1242/dev.128.8.1265
120. Aslani F, Schuppe HC, Guazzone VA, Bhushan S, Wahle E, Lochnit G, et al. Targeting high mobility group box protein 1 ameliorates testicular inflammation in experimental autoimmune orchitis. Hum Reprod. (2015) 30:417–31. doi: 10.1093/humrep/deu320
121. Elmorsy EH, Aly RG, Badae NM, Aboghazala MM, and Omar SS. Zinc alleviates high fat diet-induced spermatogenic dysfunction in Wistar rats: role of oxidative stress, HMGB1 and inflammasome. Rev Int Androl. (2024) 22:44–52. doi: 10.22514/j.androl.2024.007
122. Demir EA, Demir S, Kazaz IO, Kucuk H, Alemdar NT, Gecici OF, et al. Syringic acid ameliorates ischemia/reperfusion-induced testicular injury in rats via suppressing of HMGB1/NF-κB axis and endoplasmic reticulum stress. Eur J Trauma Emerg Surg. (2023) 49:1595–602. doi: 10.1007/s00068-023-02227-7
123. Wen Z, Zhu H, Wang J, Wu B, Zhang A, Zhao H, et al. Conditional deletion of Hspa5 leads to spermatogenesis failure and male infertility in mice. Life Sci. (2023) 314:121319. doi: 10.1016/j.lfs.2022.121319
124. Purandhar K, Jena PK, Prajapati B, Rajput P, and Seshadri S. Understanding the role of heat shock protein isoforms in male fertility, aging and apoptosis. World J Mens Health. (2014) 32:123–32. doi: 10.5534/wjmh.2014.32.3.123
125. Rockett JC, Mapp FL, Garges JB, Luft JC, Mori C, and Dix DJ. Effects of hyperthermia on spermatogenesis, apoptosis, gene expression, and fertility in adult male mice. Biol Reprod. (2001) 65:229–39. doi: 10.1095/biolreprod65.1.229
126. Cao H, Li L, Liu S, Wang Y, Liu X, Yang F, et al. The multifaceted role of extracellular ATP in sperm function: From spermatogenesis to fertilization. Theriogenology. (2024) 214:98–106. doi: 10.1016/j.theriogenology.2023.10.019
127. Fleck D, Kenzler L, Mundt N, Strauch M, Uesaka N, Moosmann R, et al. ATP activation of peritubular cells drives testicular sperm transport. Elife. (2021) 10:e62885. doi: 10.7554/eLife.62885
128. Liu J, Wei Q, Jin Y, Jin Y, and Jiang Y. Cold-induced RNA-binding protein and RNA-binding motif protein 3: two RNA molecular chaperones closely related to reproductive development and reproductive system diseases. Protein Pept Lett. (2023) 30:2–12. doi: 10.2174/0929866530666221124122507
129. Xia Z, Jiang K, Liu T, Zheng H, Liu X, and Zheng X. The protective effect of Cold-inducible RNA-binding protein (CIRP) on testicular torsion/detorsion: an experimental study in mice. J Pediatr Surg. (2013) 48:2140–7. doi: 10.1016/j.jpedsurg.2013.02.065
130. Nishiyama H, Danno S, Kaneko Y, Itoh K, Yokoi H, Fukumoto M, et al. Decreased expression of cold-inducible RNA-binding protein (CIRP) in male germ cells at elevated temperature. Am J Pathol. (1998) 152:289–96.
131. Scalici E, Mullet T, Ferrières Hoa A, Gala A, Loup V, Anahory T, et al. Circulating nucleic acids and infertility. Gynecol Obstet Fertil. (2015) 43:593–8. doi: 10.1016/j.gyobfe.2015.07.016
132. Majewska AM, Kordan W, Koziorowska-Gilun M, and Wysocki P. Identification and changes in the seasonal concentrations of heat shock proteins in roe deer (Capreolus capreolus) epididymides. Reprod Domest Anim. (2017) 52:107–14. doi: 10.1111/rda.2017.52.issue-1
133. López-González I, Oseguera-López I, Castillo R, and Darszon A. Influence of extracellular ATP on mammalian sperm physiology. Reprod Fertil Dev. (2024) 36:RD23227. doi: 10.1071/RD23227
134. Belleannée C, Da Silva N, Shum WW, Brown D, and Breton S. Role of purinergic signaling pathways in V-ATPase recruitment to apical membrane of acidifying epididymal clear cells. Am J Physiol Cell Physiol. (2010) 298:C817–30. doi: 10.1152/ajpcell.00460.2009
135. Yamashiro H, Toyomizu M, Toyama N, Aono N, Sakurai M, Hiradate Y, et al. Extracellular ATP and dibutyryl cAMP enhance the freezability of rat epididymal sperm. J Am Assoc Lab Anim Sci. (2010) 49:167–72.
136. Li S, Yin T, Li W, Yang J, Xu W, and Zhou D. Association between follicular fluid levels of HMGB1 protein and outcomes in patients undergoing in vitro fertilization/intracytoplasmic sperm injection cycles. Exp Ther Med. (2015) 9:1611–6. doi: 10.3892/etm.2015.2369
137. Hristova I. Role of heat shock proteins (Hsp) in human and mammalian fertilization and pregnancy. Part II. Akush Ginekol (Sofiia). (2012) 51:37–40.
138. Pardede BP, Kusumawati A, Pangestu M, and Purwantara B. Bovine sperm HSP-70 molecules: a potential cryo-tolerance marker associated with semen quality and fertility rate. Front Vet Sci. (2023) 10:1167594. doi: 10.3389/fvets.2023.1167594
139. Rossato M, La Sala GB, Balasini M, Taricco F, Galeazzi C, Ferlin A, et al. Sperm treatment with extracellular ATP increases fertilization rates in in-vitro fertilization for male factor infertility. Hum Reprod. (1999) 14:694–7. doi: 10.1093/humrep/14.3.694
140. Luria A, Rubinstein S, Lax Y, and Breitbart H. Extracellular adenosine triphosphate stimulates acrosomal exocytosis in bovine spermatozoa via P2 purinoceptor. Biol Reprod. (2002) 66:429–37. doi: 10.1095/biolreprod66.2.429
141. Khan MT, Khan MI, Ahmad E, Yousaf MR, and Oneeb M. Synergistic effect of extracellular adenosine triphosphate and quercetin on post-thaw quality and fertilization potential of Lohi ram sperm. Cryobiology. (2023) 113:104593. doi: 10.1016/j.cryobiol.2023.104593
142. Yi YJ, Park CS, Kim ES, Song ES, Jeong JH, and Sutovsky P. Sperm-surface ATP in boar spermatozoa is required for fertilization: relevance to sperm proteasomal function. Syst Biol Reprod Med. (2009) 55:85–96. doi: 10.1080/19396360802699074
143. Edwards SE, Buffone MG, Knee GR, Rossato M, Bonanni G, Masiero S, et al. Effects of extracellular adenosine 5'-triphosphate on human sperm motility. Reprod Sci. (2007) 14:655–66. doi: 10.1177/1933719107306227
144. Moya C, Rivera-Concha R, Pezo F, Uribe P, Schulz M, Sánchez R, et al. Adverse effects of single neutrophil extracellular trap-derived components on bovine sperm function. Anim (Basel). (2022) 12:1308. doi: 10.3390/ani12101308
145. Zambrano F, Silva L, Uribe P, Gärtner U, Taubert A, Schulz M, et al. SOCE-inhibitor reduced human sperm-induced formation of neutrophil extracellular traps. Reproduction. (2021) 161:21–9. doi: 10.1530/REP-20-0185
146. Wei Z, Yu T, Wang J, Wang C, Liu X, Han Z, et al. Swine sperm induces neutrophil extracellular traps that entangle sperm and embryos. Reproduction. (2020) 160:217–25. doi: 10.1530/REP-19-0327
147. Czamanski-Cohen J, Sarid O, Cwikel J, Lunenfeld E, Douvdevani A, Levitas E, et al. Increased plasma cell-free DNA is associated with low pregnancy rates among women undergoing IVF-embryo transfer. Reprod BioMed Online. (2013) 26:36–41. doi: 10.1016/j.rbmo.2012.09.018
148. Alizadegan A, Akbarzadeh M, Soltani-Zangbar MS, Sambrani R, Hamdi K, Ghasemzadeh A, et al. Isolation of cfDNA from spent culture media and its association with implantation rate and maternal immunomodulation. BMC Res Notes. (2022) 15:259. doi: 10.1186/s13104-022-06151-8
149. Yang H, Wang G, Liu C, Ding L, Li Y, Chen Y, et al. Elevated serum uric acid level is associated with adverse reproductive outcomes in women with polycystic ovary syndrome undergoing in vitro fertilization or intracytoplasmic sperm injection embryo transfer cycles: a retrospective cohort study. Am J Obstet Gynecol. (2023) 228:324.e1–.e10. doi: 10.1016/j.ajog.2022.11.1287
150. Shinoda K, Mitsumori K, Uneyama C, and Uehara M. Induction and inhibition of testicular germ cell apoptosis by fluoroacetate in rats. Arch Toxicol. (2000) 74:33–9. doi: 10.1007/s002040050649
151. Debbarh H, Jamil M, Jelloul H, Zakaria A, Louanjli N, and Cadi R. Assessment of cell-free DNA and apoptosis in an oocyte microenvironment: promising biomarkers to predict intracytoplasmic sperm injection outcomes. Zygote. (2023) 31:296–302. doi: 10.1017/S0967199423000126
152. Karabulut S, Gürsoy Gürgen D, Kutlu P, and Keskin İ. The role of TNF-α and its target HSP-70 in triggering apoptosis in normozoospermic and non-normozoospermic samples. Biopreserv Biobank. (2022) 20:485–92. doi: 10.1089/bio.2021.0056
153. Sun TC, Li DM, Yu H, Song LL, Jia YJ, Lin L, et al. Bilateral varicocele leads to ferroptosis, pyroptosis and necroptosis of human spermatozoa and affects semen quality in infertile men. Front Cell Dev Biol. (2023) 11:1091438. doi: 10.3389/fcell.2023.1091438
154. Liu Y, Cao X, He C, Guo X, Cai H, Aierken A, et al. Effects of ferroptosis on male reproduction. Int J Mol Sci. (2022) 23:7139. doi: 10.3390/ijms23137139
155. Akhigbe RE, Hamed MA, Odetayo AF, Akhigbe TM, Ajayi AF, and Ajibogun FAH. Omega-3 fatty acid rescues ischaemia/perfusion-induced testicular and sperm damage via modulation of lactate transport and xanthine oxidase/uric acid signaling. BioMed Pharmacother. (2021) 142:111975. doi: 10.1016/j.biopha.2021.111975
Keywords: male infertility, DAMPs, spermatogenesis, sperm maturation, fertilization, inflammatory response
Citation: Cao H, Liu S, Cui S, Nie H, Liu X and Qin W (2025) From stress signals to fertility challenges: the role of damage-associated molecular patterns in male reproduction. Front. Immunol. 16:1598451. doi: 10.3389/fimmu.2025.1598451
Received: 24 March 2025; Accepted: 11 September 2025;
Published: 01 October 2025.
Edited by:
Konrad Andrzej Szychowski, University of Information Technology and Management in Rzeszow, PolandReviewed by:
Wei Zhang, Marine Bioproducts Cooperative Research Centre, AustraliaMaria Celeste Ruete, National Scientific and Technical Research Council (CONICET), Argentina
Copyright © 2025 Cao, Liu, Cui, Nie, Liu and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weibing Qin, cWlud2JAZ2RzemprLm9yZy5jbg==
 Heran Cao1,2
Heran Cao1,2 Shichao Cui
Shichao Cui Hua Nie
Hua Nie Xiaohua Liu
Xiaohua Liu Weibing Qin
Weibing Qin