- Department of Interventional Radiology, Guangxi Medical University Cancer Hospital, Nanning, Guangxi, China
A Commentary on
Lung enteric-type adenocarcinoma with gastric metastasis: a rare case report and literature review
by Li X, Ma K, Ma X, Zhao X, Fan M and Xu Y (2024). Front. Immunol. 15:1486214. doi: 10.3389/fimmu.2024.1486214
We read with great interest the case report by Li et al. entitled “Lung enteric-type adenocarcinoma with gastric metastasis: a rare case report and literature review” (1). The authors present the first documented case of pulmonary enteric-type adenocarcinoma (ETAC) demonstrating gastric antral metastasis, providing valuable insights into the biological behavior of this rare malignancy. While the comprehensive documentation of diagnostic procedures (including imaging, histopathological analysis, and molecular profiling) and therapeutic strategies establishes an important clinical reference, we would like to address several methodological considerations regarding the imaging surveillance protocol.
The authors used only non-contrast-enhanced CT (NCECT) for postoperative surveillance from July 2021 to March 2024. Of particular concern is that, for malignant lesions demonstrating “heterogeneous enhancement” on contrast-enhanced CT (CECT) as documented in this ETAC case, the inherent limitations of NCECT in soft tissue characterization may substantially reduce diagnostic specificity. Take a case of ETAC in our center as an example (see Figures 1A-D). The vascular heterogeneity and necrotic components characteristic of malignant tumors produce distinctive enhancement patterns on CECT, whereas both postoperative changes (e.g., fibrotic remodeling, atelectasis) and inflammatory processes manifest as isodense shadows on NCECT. This makes it challenging to differentiate tumor recurrence from benign structural alterations. Current evidence from multiple studies demonstrates the superior diagnostic performance of CECT in detecting locoregional recurrences (including hilar, mediastinal, and pleural recurrences) during postoperative surveillance of pulmonary malignancies (2–4). In the present case, exclusive reliance on NCECT for assessing disease progression (August 2023) and monitoring response to PD-1 inhibitor therapy (October 2023 and March 2024) may preclude accurate characterization of soft tissue density biology (viable tumor versus scar tissue versus inflammatory changes), potentially confounding the objective interpretation of therapeutic outcomes.
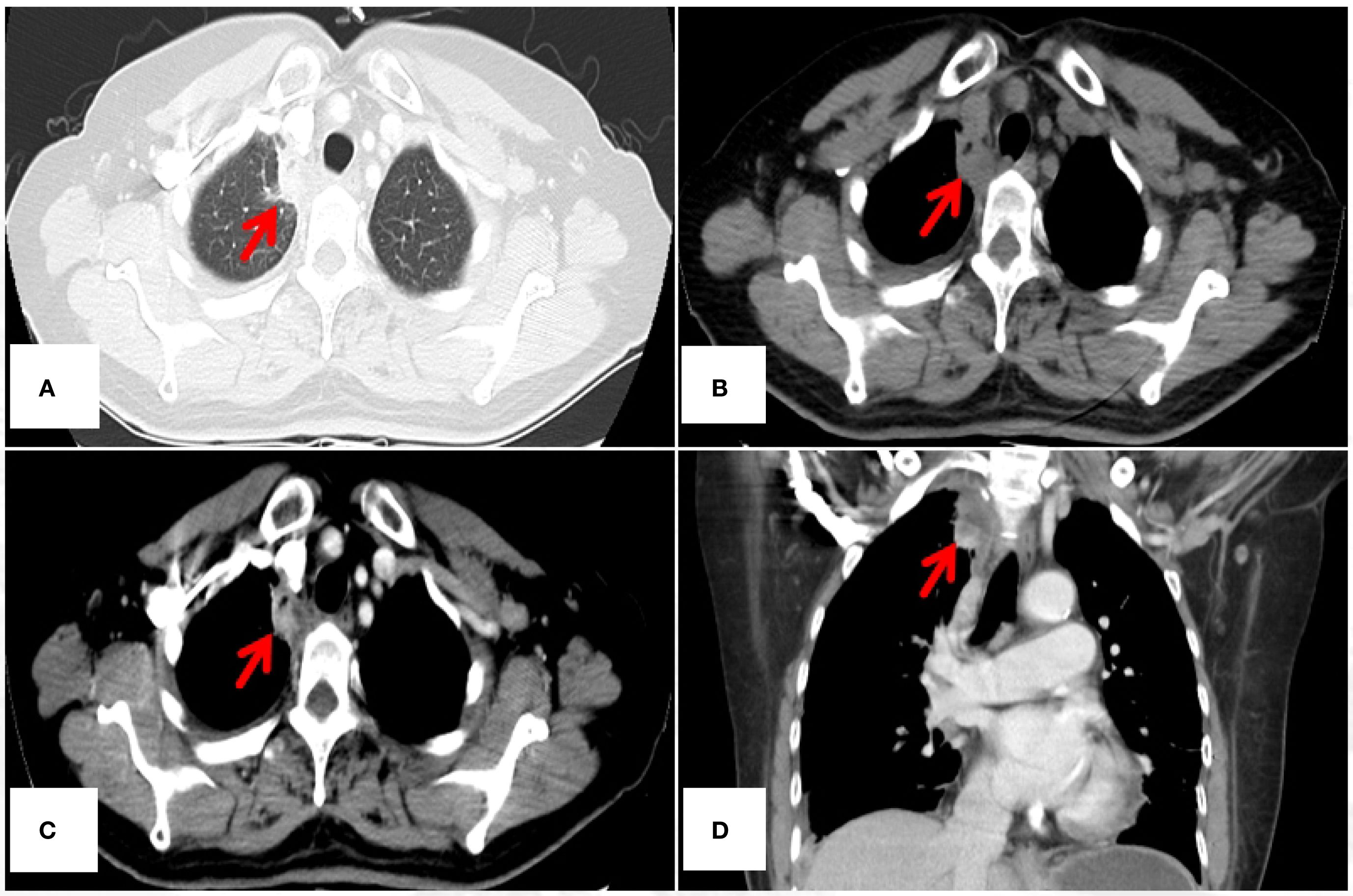
Figure 1. 45-year-old female diagnosed with ETAC. Immunocytochemistry shows: Ber - EP4(+), CK7(+), TTF - 1(SPT24)(-), Napsin A(-), Villin(+), CDX - 2(+), GATA3(-), PAX8(-), CR(-). (A) Lesion under lung window (arrow); (B) Lesion under mediastinal window non – contrast (arrow), (C, D) Lesion under mediastinal window contrast - enhanced, showing heterogeneous enhancement(arrow). CT, computed tomography.
Thus, the following recommendations are put forward: firstly, if CECT is contraindicated (e.g., due to renal insufficiency or uncontrolled hyperthyroidism) or omitted per the institutional protocol, this should be clearly stated in the discussion section to enhance methodological transparency. This is because we found that at the time of the first CT examination in the articles, the patients were eligible for CECT, which also yielded good imaging. Secondly, for lesions with distinct CECT enhancement features, the monitoring protocol should prioritize CECT within the first two critical postoperative years to help differentiate recurrence from confounding factors like elevated diaphragm or pleural thickening. This aligns with the method used by Guo et al. (5), who dynamically assessed lesion morphology and enhancement patterns during treatment monitoring using consecutive enhanced CT scans. Thirdly, subsequent follow-up examinations using NCECT should include clear technical limitations in the graphic legends and discuss their potential impact on clinical interpretation.
This landmark study provides the first documentation of ETAC’s gastric metastatic potential, carrying significant implications for clinical practice. Our suggestions aim to emphasize the critical importance of precision imaging in monitoring rare malignancies while encouraging methodological optimization in future investigations.
Author contributions
GH: Conceptualization, Validation, Methodology, Writing – original draft. CZ: Supervision, Conceptualization, Validation, Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Li X, Ma K, Ma X, Zhao X, Fan M, Xu Y, et al. Lung enteric-type adenocarcinoma with gastric metastasis: a rare case report and literature review. Front Immunol. (2024) 15:1486214. doi: 10.3389/fimmu.2024.1486214
2. Jeon YJ, Kang D, Lee J, Park SY, Cho JH, Choi YS, et al. Efficacy of contrast versus non-contrast CT surveillance among patients surviving two years without recurrence after surgery for stage I lung cancer. Sci Rep. (2025) 15:6142. doi: 10.1038/s41598-025-90124-x
3. Lou F, Sima CS, Rusch VW, Jones DR, and Huang J. Differences in patterns of recurrence in early-stage versus locally advanced non-small cell lung cancer. Ann Thorac Surg. (2014) 98:1755–61. doi: 10.1016/j.athoracsur.2014.05.070
4. MacMahon H, Naidich DP, Goo JM, Lee KS, Leung ANC, Mayo JR, et al. Guidelines for management of incidental pulmonary nodules detected on CT images: from the fleischner society 2017. Radiology. (2017) 284:228–43. doi: 10.1148/radiol.2017161659
Keywords: lung enteric-type adenocarcinoma, gastric metastasis, NRAS gene exon 3 mutation, chemotherapy and immunotherapy, non-small cell lung cancer
Citation: Huang G and Zhao C (2025) Commentary: Lung enteric-type adenocarcinoma with gastric metastasis: a rare case report and literature review. Front. Immunol. 16:1598522. doi: 10.3389/fimmu.2025.1598522
Received: 23 March 2025; Accepted: 11 September 2025;
Published: 25 September 2025.
Edited by:
Mohd Farhan, Georgetown University Medical Center, United StatesReviewed by:
Santhosh Shanthi Bhupathi, West Virginia University, United StatesVertica Agnihotri, City of Hope Duarte Main Campus, United States
Copyright © 2025 Huang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chang Zhao, NzEwNTE5MTM3QHFxLmNvbQ==
 Ganfa Huang
Ganfa Huang Chang Zhao*
Chang Zhao*