- School of Basic Medical Sciences, Chengdu University of Traditional Chinese Medicine, Chengdu, China
Obesity is a common metabolic syndrome in which an imbalance between energy intake and consumption is the main cause of excessive accumulation of body fat. The increasing prevalence of obesity and its associated complications poses significant challenges to public health. Activation of the transient receptor potential vanilloid subtype 1 (TRPV1) cascade plays a key role in lipid metabolism and energy intake. TRPV1 is expressed across the central nervous system and peripheral organs is involved in the regulation of hormone secretion, appetite and mitochondrial function, and is recognized as one of the key targets for preventing obesity. The current treatments for obesity exhibit limited efficacy and are associated with numerous side effects. Targeting TRPV1 represents a potentially effective approach for managing obesity. In this work, by combining the recent mechanism of the role of TRPV1 in neuroendocrine regulation, we hope to provide novel approaches to block or even reverse the development of obesity.
1 Introduction
The increasing prevalence of obesity is expected to affect 4 billion people by 2035 (1–3). As a common metabolic syndrome, an imbalance in energy storage and expenditure is the main cause of excessive accumulation of body fat (4–7). Recent studies have shown that neuroendocrine modulation plays an important role in regulating adipose tissue thermogenesis and lipid metabolism (8). Body weight homeostasis is regulated by the coordinated interactions of nutrients, circulating neuroendocrine hormones, the central nervous system, and peripheral nerves, and even the release of hormonal signals from endocrine tissues is largely regulated by the peripheral nervous system (PNS) (9–13). Sympathetic nervous system (SNS) innervation of adipose tissue has been demonstrated in recent studies (14–16), and activation of the SNS prevents obesity by promoting brown fat thermogenesis and energy expenditure via the hypothalamic neuropeptide Y and norepinephrine. The SNS is an integral part of metabolism-related organs, but our understanding of the mechanisms by which the nervous system regulates the endocrine system to affect obesity is still lacking. understanding.
Activation of transient receptor potential vanilloid subtype 1 (TRPV1) sustains centrally regulated thermogenesis in peripheral tissues. TRPV1 is a nonselective cation channel (17). TRPV1 has a tetrameric structure consisting of three parts, the N-terminus and C-terminus located intracellularly, and six transmembrane regions (S1–S6) (18–20), with the N-terminal end playing a role in the activation of the channel. Early studies of TRPV1 focused on thermal and inflammatory pain transmission, and recent studies have revealed that TRPV1 also plays an important role in the regulation of tissue energy metabolism. A study of TRPV1 involvement in white adipose tissue (WAT) browning revealed (21) that the gene levels of TRPV1, silent message regulator 1 (Sirt1), and uncoupling protein-1 (UCP1) were suppressed in high-fat diet-fed mice, whereas capsaicin-fed mice presented a reversal of the expression levels of all these genes. Increasing evidence suggests that TRPV1 plays a key role in the regulation of body weight and lipid metabolism and is therefore considered a potential target for the treatment of obesity (22–26).
Given the increasing incidence of obesity annually, the resulting complications place heavy psychological and economic pressure on patients. Therefore, revealing the neuroendocrine regulatory mechanism of TRPV1 in obesity is particularly important. This paper reveals the core mechanism of TRPV1 in the endocrine system and the central nervous system of obese patients by reviewing previous studies on obesity and TRPV1 to provide a theoretical basis for stopping or even reversing obesity.
2 The potential role of TRPV1 in obesity
2.1 Neuromodulatory mechanisms of TRPV1
2.1.1 Regulation of feeding behavior and energy metabolism by TRPV1 activation in the central nervous system
The TRPV1 protein in the central nervous system (CNS) plays an important role in the regulation of feeding behavior. TRPV1-positive neurons are widely distributed in the CNS (27–30), especially in the hypothalamus and nucleus tractus solitarius (NTS), which are closely related to food intake and energy expenditure (31, 32). TRPV1 expression in the hypothalamus of high-fat diet (HFD)-fed mice was significantly downregulated (33), whereas capsaicin restored its expression level, and activation of TRPV1 was able to increase energy expenditure and reduce body weight.
The function of TRPV1 is related to its distribution. In the hypothalamus, TRPV1-positive neurons coexpress a variety of neuropeptides [including calcitonin gene-related peptide (CGRP), NPY, and substance P (SP)] to regulate peripheral thermogenesis and dietary intake (33). TRPV1 activation induces Ca2+ influx, which may be crucial for the release and function of CGRP (34, 35), and CGRP may inhibit food intake by increasing cyclic adenosine monophosphate (cAMP) and cholecystokinin (CCK) expression in the hypothalamus, downregulating the expression of appetite-inducing neuropeptides (NPY and MCH), and increasing skin temperature and brown adipose tissue (BAT) thermogenesis (34, 36). In POMC neurons, which regulate appetite and satiety, TRPV1 activation releases α-melanocyte-stimulating hormone (α-MSH) to act on satiety centers, leading to a reduction in appetite (37), and this process is TRPV1 dependent. In terms of hypothalamic gene expression profiles in HFD-fed mice, TRPV1 activation upregulates the expression of satiety-related neuropeptide genes (e.g., UCN, PYY, RAMP3, GRP, BDNF, and CARTPT) and downregulates the expression of appetite-stimulating genes (e.g., CNR1, GALR1, GHRL, ADRA2B, and GHSR), reducing food intake and body weight (33, 38–41). (Figure 1).
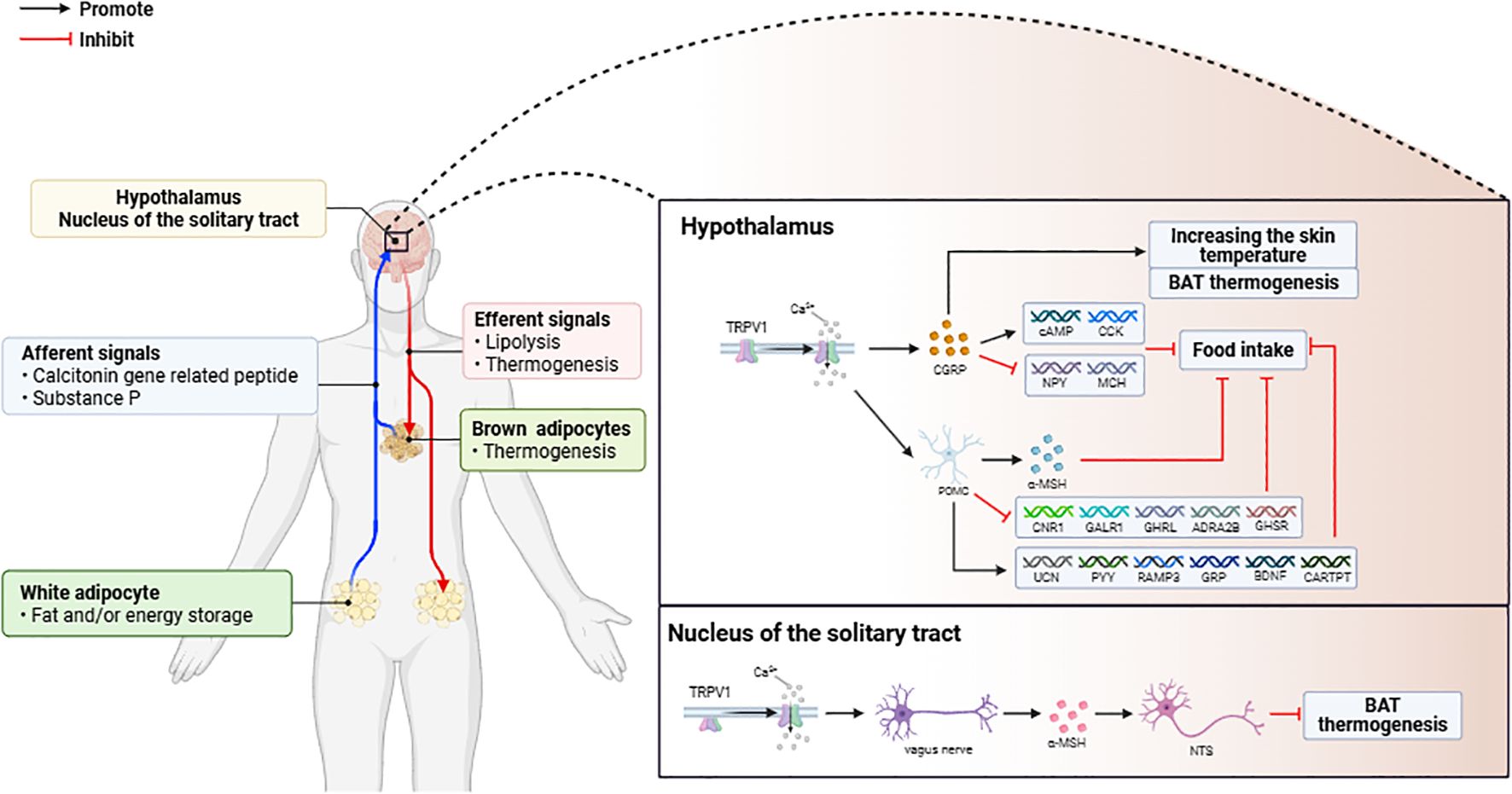
Figure 1. (1) Activation of TRPV1 in adipose tissue triggers the release of CGRP and SP, which mediate signal transduction in the central nervous system to regulate fat metabolism. (2) Activation of TRPV1 in the central nervous system (CNS) modulates the expression of appetite and adipose thermogenesis-related genes, leading to weight loss through increased energy expenditure.
Activation of TRPV1 in the solitary tract nucleus (NTS) inhibits BAT activation in HFD-fed rats. The levels of linoleic acid metabolites (LAs) are elevated in the NTS of HFD-fed rats, and these metabolites can act as endogenous TRPV1 activators (42). The activation of TRPV1 at the afferent terminals of the vagus nerve induced the release of glutamate to increase the activity of the neurons in the NTS, which in turn inhibited the sympathetic excitatory neurons of the BAT, forming a pathway to inhibit brown adipose tissue (BAT) thermogenesis and energy expenditure. pathway. Therefore, the energy metabolism regulatory function of TRPV1 is spatially inhibitory (Figure 1).
2.1.2 Central regulation of tissue energy metabolism is dependent on activation of TRPV1 in peripheral sensory nerves
Peripheral sensory nerves are involved in regulating adipose tissue thermogenesis and WAT browning processes. Studies have shown that BAT-specific denervation in rats is associated with increased body weight; decreased resting metabolic rates; decreased BAT mass; decreased adipocyte and mitochondrial numbers; downregulated UCP1 protein expression; and decreased core body temperature (43–45). In contrast, both unilateral and bilateral ablation of subcutaneous WAT in mice upregulated the expression of thermogenic genes and was accompanied by beige adipocyte formation (8). These findings suggest that neuromodulation is necessary to maintain the homeostasis of fat energy metabolism.
The regulation of energy metabolism in adipose tissue is dependent on neuropeptide secretion following TRPV1 activation. Mammalian adipose tissue function is regulated by the peripheral nervous system (8), and the activation of TRPV1 in BAT and WAT sensory neurons results in the expression of the neuropeptides CGRP and SP (46, 47), which transmit information from adipose tissue to the central nervous system (hypothalamus, solitary tract nucleus, etc.) through synaptic links between neurons, and the removal of sensory nerves of the adipose tissue results in compensatory hyperplasia, further demonstrating the involvement of sensory signaling in systemic adipose homeostasis (48, 49). Tracing of sympathetic nerves and sensory nerves innervating the BAT revealed colocalization in the central nervous system (hypothalamus, solitary tract nucleus, and other brain regions) (49, 50), suggesting that there is a direct interaction between sympathetic and sensory signals at the center. Thus, sympathetic and sensory nerves synergistically regulate fat metabolism through a bidirectional loop, with sympathetic nerves dominating lipolysis and thermogenesis and sensory nerves feeding back on fat status to modulate sympathetic output, a mechanism that is functionally specific in WAT and BAT but shares some central nodes.
In addition, the neuropeptides CGRP and SP released upon sensory nerve activation exert regulatory effects on adipose tissue metabolism. Previous studies have shown that CGRP has hormonal effects as a neuropeptide (51). Lipid metabolism regulation by CGRP may occur through changes in plasma catecholamine, cortisol, glucagon, insulin, lactate, and adipokine levels, as well as in the blood supply of adipose tissue (36, 52–60). SP upregulates neurokinin 1 receptor (NK1R) mRNA and protein expression levels in human preadipocytes (61). SP also promotes lipolysis in 3T3L1 adipocytes, blocks insulin-mediated fatty acid uptake, and inhibits the accumulation of lipid droplets during differentiation (62). In contrast, high-fat diet-induced weight gain was inhibited in NK1R-/- mice, circulating levels of insulin and leptin were reduced, and insulin-dependent glucose uptake was improved (63). Thus, neuropeptides secreted upon the activation of sensory nerve TRPV1 not only play a role in central regulation but also play a role in regulating local adipose tissue (Figure 1).
2.2 Endocrine regulatory mechanisms of TRPV1
2.2.1 Adipose tissue TRPV1 activation promotes mitochondrial oxidation
Adipose tissue is an important part of the human body. Owing to its structure and function, it can be divided into WAT, which is responsible for storing fat, maintaining body temperature and regulating metabolism throughout the body, and BAT, which generates a large amount of heat energy through the catabolism and oxidation of lipids and helps to maintain body temperature.
Adipose tissue TRPV1 activation to promote mitochondrial energy metabolism is required for WAT browning. WAT browning has been used as a novel strategy to improve metabolic health (6), and WAT browning is able to inhibit energy intake-induced weight loss by triggering thermogenesis to promote energy expenditure (40, 64, 65). Upon the activation of WAT-expressed TRPV1 (66, 67), the intracellular Ca2+ concentration increases, and the activation of calmodulin kinase II (CaMKII) causes the phosphorylation of AMP protein kinase (AMPK), leading to the activation of sirtuin 1 (SIRT-1), which serves as a sensor of cellular metabolism and energy utilization (68, 69), the activation of which leads to the deacetylation of PPARγ and PRDM-16, both of which promote WAT browning (70, 71). With the activation of TRPV1, the expression of UCP-1 and bone morphogenetic protein 8B (BMP8B) is upregulated. UCP-1 is localized on the inner mitochondrial membrane, and when activated, it short-circuits the mitochondrial proton gradient, thus promoting thermogenesis (66, 72). By increasing p38 MAPK/CREB signaling and adiponectin activity, BMP8B enhances the sensitivity of BAT to NE to promote energy expenditure (73). Upon activation of TRPV1, the mitochondrial deacetylase SIRT-3 is activated, leading to a decrease in ROS production (74) and an increase in energy metabolism due to increased mitochondrial activity. SIRT-3 was also able to downregulate the expression of H3K27ac on the mitochondrial calcium unidirectional transporter (MCU) promoter via an AMPK-dependent pathway, which inhibited mitochondrial calcium ion overload to prevent BAT whitening. The expression of the adipogenic regulators Pparγ2 and PPARγ coactivator 1α (Pgc-1α) in BAT is also upregulated upon activation of TRPV1 (75). Pparγ2 promotes transcriptional cascades involved in adipocyte function (76, 77), whereas Pgc-1α stimulates mitochondrial biogenesis as well as BAT cell function, including transcriptional activation of Ucp1 (78). Mitochondrial homeostasis in BAT is critical for maintaining BAT thermogenesis, and mitochondrial Ca2+ regulates the activity of essential metabolic enzymes and transporter proteins (79). TRPV1 maintains mitochondrial Ca2+ homeostasis in BAT by repressing the expression of the ion channel protein LETM1 (80). When genes regulating TRPV1 expression are knocked down, the expression of UCP1 and LETM1 tends to increase, leading to disturbances in mitochondrial Ca2+ homeostasis in BAT and aggravating obesity (Figure 2).
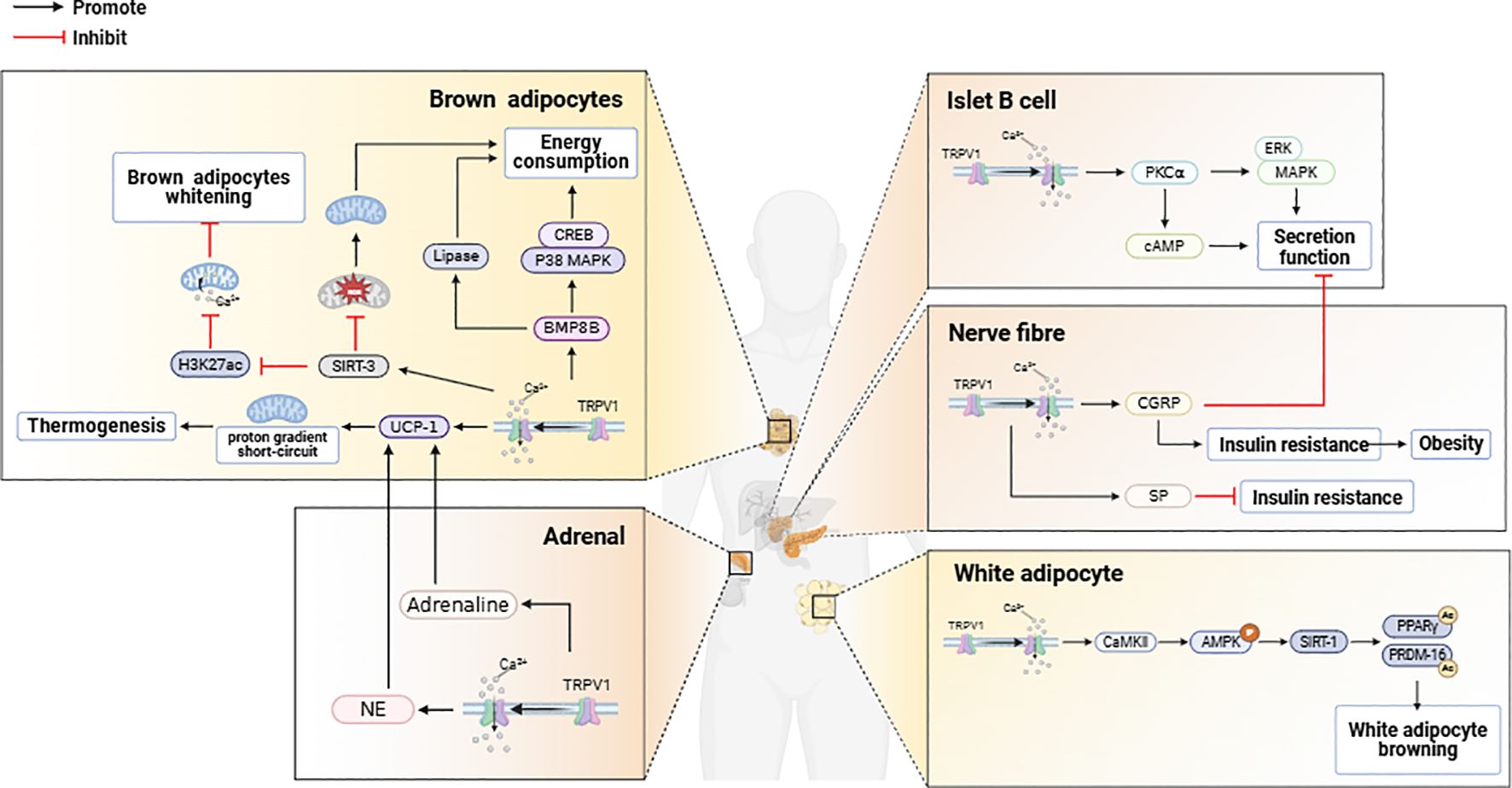
Figure 2. TRPV1 activation in brown adipose tissue, white adipose tissue, pancreas, and adrenal glands exerts regulatory effects on energy metabolism.
Studies have shown that TRPV1 activation in murine and human adipose precursor cells upregulates the cytoplasmic receptor responsible for calcium cycling (α1-AR), the calcium-sensing enzyme (CaMKII), and mitochondrial calcium transporters (VDAC and MCU), leading to increased intracellular Ca²+ concentrations, which suppress adipogenesis in adipose precursor cells and promote UCP1-dependent thermogenesis (81, 82). Following the activation of TRPV1, the mRNA levels of hormone-sensitive lipase (HSL), carnitine palmitoyltransferase Ia (CPT-Ia), which is a rate-limiting enzyme in mitochondrial fatty acid oxidation, and uncoupling protein 2 (UCP2) are increased (83–86). This results in increased lipolysis in adipocytes and a reduction in the intracellular lipid content (Figure 2).
2.2.2 Pancreatic β-cell TRPV1 activation regulates pancreatic function
The pancreas is an important visceral organ in the regulation of glucose metabolism, and insulin, an anabolic hormone, is synthesized and secreted by pancreatic beta cells (87). In peripheral tissues, insulin promotes glucose uptake in adipose tissue and inhibits lipolysis, promoting fat storage in adipocytes (88, 89), and in the central nervous system, insulin acts as an appetite suppressant to decrease food intake and body weight (90).
TRPV1 activation in pancreatic β-cells increases insulin secretion. Previous studies have shown that TRPV1 affects pancreatic function and insulin secretion in both humans and animals (91), and the activation of TRPV1 expressed on pancreatic β-cells by calcium influx increases insulin secretion, a process that involves the regulation of protein kinase C alpha (PKC alpha) and cyclic adenosine monophosphate cAMP (92, 93). In addition, TRPV1 is coexpressed with CGRP in pancreatic nerve fibers (94), and inhibition of TRPV1 signaling decreases CGRP secretion, thereby increasing insulin secretion. Insulin sensitizes TRPV1 in sensory nerve endings (95, 96), and TRPV1-activated neurons regulate pancreatic β-cell function through the release of neuropeptides such as SP and CGRP (97–100), where an increase in CGRP secretion decreases insulin release from pancreatic β-cells (98). Sustained high levels of circulating CGRP can lead to insulin resistance and obesity, whereas increased SP secretion can alleviate insulin resistance (97). Furthermore, TRPV1 is coexpressed with CCK-sensitive vagal afferent neurons. Studies have revealed that TRPV1 activation-induced calcium influx enhances the responsiveness of vagal afferent neurons to CCK, leading to increased vagal signaling, which regulates pancreatic secretory function to maintain metabolic homeostasis. This mechanism may involve low-affinity CCK binding to CCK1 receptors (CCK1Rs), triggering downstream signaling (e.g., Gq proteins or β-arrestin) (101). Recent studies have indicated that TRPV1 activation may contribute to β-cell dysfunction and acute pancreatitis, whereas TRPV1 antagonists restore SP/CGRP expression levels, increase the islet area, reduce pancreatic β-cell vacuolization, decrease proinflammatory cytokine (TNF-α, IL-1β) release, and increase anti-inflammatory IL-10 secretion (102). This process may involve the modulation of the JAK2-STAT3 signaling pathway (103). (Figure 2).
2.2.3 Regulation of energy metabolism by adrenal TRPV1 activation
The adrenal gland is composed of the cortex and medulla and is involved in the regulation of energy metabolism as an endocrine gland (104). The adrenal cortex synthesizes and secretes steroids, whereas the medulla produces catecholamines and neuropeptides (105–107). Epinephrine and norepinephrine maintain energy production through lipolysis and ketogenesis during hypoglycemia and malnutrition (108–110). Catecholamine binding stimulates β3-adrenergic receptors, leading to increased intracellular cAMP concentrations and the activation of cyclic AMP-dependent protein kinase A (PKA), which leads to the phosphorylation and activation of hormone-sensitive lipase (HSL) to increase adipocyte lipolysis (111). In addition, catecholamine stimulation of α2-adrenergic receptors inhibits lipolysis (112). These adrenergic responses depend on the density of these two receptor families, their relative affinities, and the location and amount of adipose tissue (113). Obesity may alter the sensitivity of alpha- and beta-adrenergic receptors in adipose tissue, thereby altering the effects of catecholamines on lipolytic processes and increasing fat storage (114, 115). Glucocorticoids (GCS) plays an important role in the regulation of metabolic homeostasis (116). Chronical elevation of GCs can alter body fat distribution, increasing visceral obesity and metabolic abnormalities (117, 118). (Figure 2).
TRPV1 plays an important role in metabolic pathways related to energy homeostasis and insulin signaling. In the rat adrenal gland, there are TRPV1-positive nerve fibers; in particular, TRPV1-positive fibers are observed in the adrenal tegument, cortex and medulla (119), where 35% of the medullary cells and 20% of the cortical cells express this cation channel (120). The colocalization of TRPV1 in the adrenal medulla with CGRP, which is stored in sensory nerve endings, is associated with pain perception, inflammatory responses and increased catecholamine secretion in the adrenal medulla (121, 122). Catecholamines serve as an important class of neurotransmitters and hormones, including epinephrine, norepinephrine, and dopamine, which play key roles in the regulation of the nervous system, cardiovascular system, and energy metabolism (123). Sensory nerves expressing TRPV1 promote energy expenditure by activating sympathetic nerves and promoting noradrenaline secretion (124).
TRPV1 channels may be activated by acidic contents released by adrenal medullary cells (125), and synergistically with the activation of P2X3 receptors, they lead to the secretion of catecholamine hormones by the adrenal medulla. After the activation of TRPV1, the secretion of norepinephrine is stimulated via β2 adrenergic receptors and β3 adrenergic receptors, which increase the expression of UCP1 in BAT. This leads to a reduction in visceral fat content in obese rats induced by a high-fat diet (78, 126, 127). BAT plays a major role in diet-induced thermogenesis, and UCP1 is thought to be a key thermogenic regulator of BAT (78). Previous studies have demonstrated that the activation of TPPV1 enhances the secretion of epinephrine (128, 129), which increases energy expenditure and thermogenesis through the activation of adrenergic receptors (130, 131), whereas in adrenal-depleted rats, thermogenesis resulting from TPPV1 activation is markedly attenuated (132). In addition, adrenergic receptor activation upregulated UCP1 expression in BAT, increasing WAT browning and BAT thermogenesis (78, 133–140). In addition, TRPV1 activation in adrenocortical cells leads to an increase in intracellular calcium ion levels, which in turn inhibits GC secretion, reducing the occurrence of visceral obesity (141). (Figure 2).
3 TRPV1-targeted therapy
At this stage, the treatment strategy for obesity still emphasizes lifestyle changes, such as avoiding a sedentary lifestyle, proper exercise and a balanced diet. However, the incidence of obesity remains high. Previous studies on TRPV1-targeted therapy have focused mostly on relieving discomfort, such as pain and itching caused by the disease (142, 143), and with increasing research, TRPV1 has been recognized as a potential target with preventive effects against obesity (25). TRPV1 plays an important role in the regulation of pain sensation, body heat production and energy metabolism. In previous clinical trials, the use of TRPV1 antagonists increased the thermal threshold and increased the risk of burns, which makes it difficult for related drugs to enter clinical phase III trials (144). Most TRPV1 antagonists used in clinical studies involve varying degrees of body temperature elevation in experimental subjects. Most TRPV1 antagonists have shown different degrees of temperature elevation in subjects in clinical studies (144, 145), and their molecular mechanisms are still poorly understood. Moreover, TRPV1 agonists such as capsaicin have been shown to induce a decrease in body temperature in animal studies (146). In a related study, resveratrol significantly improved the discomfort induced by TRPV1 agonists (147), suggesting that these side effects could be avoided or even eliminated. A new generation of TRPV1 antagonists has been reported to have a weaker effect on body temperature in clinical trials (148). The clinically safe TRPV1 antagonist XEN-D0501 is currently under development as an oral drug for overactive bladder (148); undoubtedly, these findings provide valuable clinical data for the development of TRPV1-targeted drugs to treat obesity.
4 Conclusion
Obesity is a metabolic syndrome characterized by excessive accumulation of fat in the body due to an imbalance between energy intake and expenditure. In previous studies, conventional treatments for obesity included lifestyle interventions (such as dietary restrictions and physical exercise), bariatric surgery, and drug therapy (149). At present, the drugs commonly used in clinical practice for treating obesity all have certain efficacy, but most of them cause various side effects (150). In this review, by summarizing the mechanism of the role of TRPV1 in the endocrine system and the central nervous system under conditions of obesity, we found that TRPV1 plays an important role in the occurrence and development of obesity and participates in the processes of energy intake and consumption. Strict control of energy homeostasis is crucial for maintaining a healthy weight or for helping with weight loss by expending more energy than is consumed. TRPV1 is involved in energy homeostasis, regulating both food intake and energy expenditure. TRPV1 may affect appetite by controlling the levels of appetite hormones, and it can also increase energy expenditure by generating heat.
TRPV1, a nonselective cation channel, is also an important receptor. The acute activation of TRPV1 leads to conformational changes in TRPV1, causing the opening of the TRPV1 channel, resulting in a large influx of Ca²+ and Na+, triggering cell depolarization. The influx of Ca²+ prompts sensory nerve endings to release CGRP and SP, mediating neurogenic inflammation (vasodilation, plasma extravasation). Chronic activation of TRPV1 (such as long-term exposure to capsaicin or inflammatory stimuli) causes changes in the phosphorylation state of TRPV1, leading to channel desensitization. Research has shown that normal rats typically lose weight after long-term capsaicin desensitization, and this process is associated with a reduction in fat accumulation (81, 151). Long-term activation of TRPV1 can also increase energy expenditure by enhancing the thermogenic capacity of brown adipose tissue and promoting the browning of white adipose tissue (67, 152). After capsaicin activates TRPV1, it increases the abundance of beneficial bacteria in the intestine, promoting the production of bile acids (BAs) and short-chain fatty acids (SCFAs) and increasing the secretion of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY), thereby increasing satiety, reducing food intake, and influencing energy metabolism and inflammatory responses (153, 154). Furthermore, obesity, a form of chronic inflammation, increases the circulating levels of fat and inflammatory cytokines (155, 156). The activation of TRPV1 is regulated by various inflammatory mediators, including nerve growth factor (NGF), prostaglandins (PGs), bradykinin (BK), leukotrienes (LTB4), etc. These mediators increase the sensitivity of TRPV1 through different signaling pathways (such as PKA, PKC, and MAPK), thereby activating TRPV1 (157, 158). When TRPV1 is activated, it regulates downstream pathways and participates in the regulation of obesity. The acute activation of TRPV1 serves as a warning system for the body to avoid harm (such as burns), whereas chronic activation induces adaptive protection (such as maintaining vascular homeostasis, anti-inflammatory desensitization, and metabolic regulation). Therefore, chronic activation of TRPV1 has broader application potential for metabolic diseases.
Although a large body of evidence points to a relationship between the development of obesity and TRPV1, the relationship between the two is still controversial in some studies. For example, TRPV1 knockout mice have been reported to lose body weight (22), but other studies have shown that TRPV1 knockout mice are not obese at young ages. However, the weight of TRPV1 knockout mice increases significantly during aging (159). These findings suggest that the regulatory effect of TRPV1 on obesity may be age dependent, and a similar relationship was also shown in healthy subjects (160). Therefore, age should be considered a potential influencing factor in the study of TRPV1 and obesity. Nevertheless, aberrant TRPV1 activation and expression may contribute to the onset and development of obesity. Therefore, TRPV1 may be a target for the treatment of weight loss disorders, and finding a drug or stimulation method (mechanical stimulation or temperature stimulation) that can treat obesity by acting on TRPV1 will be our next research direction.
Author contributions
JW: Writing – original draft, Writing – review & editing, Investigation, Conceptualization. ML: Writing – review & editing. LW: Writing – review & editing. PX: Writing – review & editing. JC: Writing – review & editing. XX: Supervision, Conceptualization, Writing – review & editing. WD: Writing – review & editing, Methodology, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work is supported by the National Natural Science Foundation of China (No. 82374176) and the Sichuan Science and Technology Program (No. 2023ZYD0048).
Acknowledgments
The image production in the article was created with Biorender.com.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Organization WH. One in eight people are now living with obesity(2024). Available online at: https://www.who.int/news/item/01-03-2024-one-in-eight-people-are-now-living-with-obesity (Accessed April 20, 2024).
2. Kelly T, Yang W, Chen CS, Reynolds K, and He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond). (2008) 32:1431–7. doi: 10.1038/ijo.2008.102
3. Saeed S, Bonnefond A, and Froguel P. Obesity: exploring its connection to brain function through genetic and genomic perspectives. Mol Psychiatry. (2024) 30:651–8. doi: 10.1038/s41380-024-02737-9
4. Zhou H, Gizlenci M, Xiao Y, Martin F, Nakamori K, Zicari EM, et al. Obesity-associated inflammation and alloimmunity. Transplantation. (2024) 109:588–96. doi: 10.1097/TP.0000000000005183
5. Kwok KH, Lam KS, and Xu A. Heterogeneity of white adipose tissue: molecular basis and clinical implications. Exp Mol Med. (2016) 48:e215. doi: 10.1038/emm.2016.5
6. Bartelt A and Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol. (2014) 10:24–36. doi: 10.1038/nrendo.2013.204
7. Cohen P and Kajimura S. The cellular and functional complexity of thermogenic fat. Nat Rev Mol Cell Biol. (2021) 22:393–409. doi: 10.1038/s41580-021-00350-0
8. Wang Y, Leung VH, Zhang Y, Nudell VS, Loud M, Servin-Vences MR, et al. The role of somatosensory innervation of adipose tissues. Nature. (2022) 609:569–74. doi: 10.1038/s41586-022-05137-7
9. Townsend KL. One nervous system: critical links between central and peripheral nervous system health and implications for obesity and diabetes. Diabetes. (2024) 73:1967–75. doi: 10.2337/dbi24-0004
10. Ren W, Chen J, Wang W, Li Q, Yin X, Zhuang G, et al. Sympathetic nerve-enteroendocrine L cell communication modulates GLP-1 release, brain glucose utilization, and cognitive function. Neuron. (2024) 112:972–90.e8. doi: 10.1016/j.neuron.2023.12.012
11. Alcantara IC, Tapia APM, Aponte Y, and Krashes MJ. Acts of appetite: neural circuits governing the appetitive, consummatory, and terminating phases of feeding. Nat Metab. (2022) 4:836–47. doi: 10.1038/s42255-022-00611-y
12. Gautron L, Elmquist JK, and Williams KW. Neural control of energy balance: translating circuits to therapies. Cell. (2015) 161:133–45. doi: 10.1016/j.cell.2015.02.023
13. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. (2019) 15:288–98. doi: 10.1038/s41574-019-0176-8
14. Bartness TJ, Liu Y, Shrestha YB, and Ryu V. Neural innervation of white adipose tissue and the control of lipolysis. Front Neuroendocrinol. (2014) 35:473–93. doi: 10.1016/j.yfrne.2014.04.001
15. Freire-Agulleiro Ó and López M. Sympathetic NPY ignites adipose tissue. Neuron. (2024) 112:3816–8. doi: 10.1016/j.neuron.2024.11.004
16. Cui X, Jing J, Wu R, Cao Q, Li F, Li K, et al. Adipose tissue-derived neurotrophic factor 3 regulates sympathetic innervation and thermogenesis in adipose tissue. Nat Commun. (2021) 12:5362. doi: 10.1038/s41467-021-25766-2
17. Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, and Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. (1997) 389:816–24. doi: 10.1038/39807
18. Zhao R and Tsang SY. Versatile roles of intracellularly located TRPV1 channel. J Cell Physiol. (2017) 232:1957–65. doi: 10.1002/jcp.25704
19. Cao E, Liao M, Cheng Y, and Julius D. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature. (2013) 504:113–8. doi: 10.1038/nature12823
20. Liao M, Cao E, Julius D, and Cheng Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature. (2013) 504:107–12. doi: 10.1038/nature12822
21. Baskaran P, Covington K, Bennis J, Mohandass A, Lehmann T, and Thyagarajan B. Binding efficacy and thermogenic efficiency of pungent and nonpungent analogs of capsaicin. Molecules. (2018) 23:3198. doi: 10.3390/molecules23123198
22. Motter AL and Ahern GP. TRPV1-null mice are protected from diet-induced obesity. FEBS Lett. (2008) 582:2257–62. doi: 10.1016/j.febslet.2008.05.021
23. Chen J, Li L, Li Y, Liang X, Sun Q, Yu H, et al. Activation of TRPV1 channel by dietary capsaicin improves visceral fat remodeling through connexin43-mediated Ca2+ influx. Cardiovasc Diabetol. (2015) 14:22. doi: 10.1186/s12933-015-0183-6
24. Sun F, Xiong S, and Zhu Z. Dietary capsaicin protects cardiometabolic organs from dysfunction. Nutrients. (2016) 8:174. doi: 10.3390/nu8050174
25. Ludy MJ and Mattes RD. Comparison of sensory, physiological, personality, and cultural attributes in regular spicy food users and non-users. Appetite. (2012) 58:19–27. doi: 10.1016/j.appet.2011.09.018
26. Ma L, Zhong J, Zhao Z, Luo Z, Ma S, Sun J, et al. Activation of TRPV1 reduces vascular lipid accumulation and attenuates atherosclerosis. Cardiovasc Res. (2011) 92:504–13. doi: 10.1093/cvr/cvr245
27. Mezey E, Tóth ZE, Cortright DN, Arzubi MK, Krause JE, Elde R, et al. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci U S A. (2000) 97:3655–60. doi: 10.1073/pnas.97.7.3655
28. Sudbury JR and Bourque CW. Dynamic and permissive roles of TRPV1 and TRPV4 channels for thermosensation in mouse supraoptic magnocellular neurosecretory neurons. J Neurosci. (2013) 33:17160–5. doi: 10.1523/JNEUROSCI.1048-13.2013
29. Roberts JC, Davis JB, and Benham CD. 3H]Resiniferatoxin autoradiography in the CNS of wild-type and TRPV1 null mice defines TRPV1 (VR-1) protein distribution. Brain Res. (2004) 995:176–83. doi: 10.1016/j.brainres.2003.10.001
30. Inprasit C, Huang YC, and Lin YW. Evidence for acupoint catgut embedding treatment and TRPV1 gene deletion increasing weight control in murine model. Int J Mol Med. (2020) 45:779–92. doi: 10.3892/ijmm.2020.4462
31. Coveleskie K, Kilpatrick LA, Gupta A, Stains J, Connolly L, Labus JS, et al. The effect of the GLP-1 analogue Exenatide on functional connectivity within an NTS-based network in women with and without obesity. Obes Sci Pract. (2017) 3:434–45. doi: 10.1002/osp4.124
32. Lockie SH. Glucagon-like peptide-1 receptor in the brain: role in neuroendocrine control of energy metabolism and treatment target for obesity. J Neuroendocrinol. (2013) 25:597–604. doi: 10.1111/jne.12039
33. Baboota RK, Murtaza N, Jagtap S, Singh DP, Karmase A, Kaur J, et al. Capsaicin-induced transcriptional changes in hypothalamus and alterations in gut microbial count in high fat diet fed mice. J Nutr Biochem. (2014) 25:893–902. doi: 10.1016/j.jnutbio.2014.04.004
34. Lima WG, Marques-Oliveira GH, da Silva TM, and Chaves VE. Role of calcitonin gene-related peptide in energy metabolism. Endocrine. (2017) 58:3–13. doi: 10.1007/s12020-017-1404-4
35. Minowa S, Tsuchiya S, Someya A, Horie S, and Murayama T. Role of neuropeptide receptor systems in vanilloid VR1 receptor-mediated gastric acid secretion in rat brain. Eur J Pharmacol. (2004) 486:317–24. doi: 10.1016/j.ejphar.2004.01.006
36. Sun JY, Jing MY, Wang JF, and Weng XY. The approach to the mechanism of calcitonin gene-related peptide-inducing inhibition of food intake. J Anim Physiol Anim Nutr (Berl). (2010) 94:552–60. doi: 10.1111/j.1439-0396.2009.00937.x
37. Vicent MA, Mook CL, and Carter ME. POMC neurons in heat: A link between warm temperatures and appetite suppression. PloS Biol. (2018) 16:e2006188. doi: 10.1371/journal.pbio.2006188
38. Rios M. BDNF and the central control of feeding: accidental bystander or essential player? Trends Neurosci. (2013) 36:83–90. doi: 10.1016/j.tins.2012.12.009
39. Liao GY, An JJ, Gharami K, Waterhouse EG, Vanevski F, Jones KR, et al. Dendritically targeted Bdnf mRNA is essential for energy balance and response to leptin. Nat Med. (2012) 18:564–71. doi: 10.1038/nm.2687
40. Cao L, Choi EY, Liu X, Martin A, Wang C, Xu X, et al. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab. (2011) 14:324–38. doi: 10.1016/j.cmet.2011.06.020
41. Kaur J, Kumar V, Kumar V, Shafi S, Khare P, Mahajan N, et al. Combination of TRP channel dietary agonists induces energy expending and glucose utilizing phenotype in HFD-fed mice. Int J Obes (Lond). (2022) 46:153–61. doi: 10.1038/s41366-021-00967-3
42. Conceição EPS, Reynolds CA, Morrison SF, and Madden CJ. Activation of transient receptor potential vanilloid 1 channels in the nucleus of the solitary tract and activation of dynorphin input to the median preoptic nucleus contribute to impaired BAT thermogenesis in diet-induced obesity. eNeuro. (2021) 8:ENEURO.0048-21.2021. doi: 10.1523/ENEURO.0048-21.2021
43. Himms-Hagen J, Cui J, and Lynn Sigurdson S. Sympathetic and sensory nerves in control of growth of brown adipose tissue: Effects of denervation and of capsaicin. Neurochem Int. (1990) 17:271–9. doi: 10.1016/0197-0186(90)90149-N
44. Vaughan CH and Bartness TJ. Anterograde transneuronal viral tract tracing reveals central sensory circuits from brown fat and sensory denervation alters its thermogenic responses. Am J Physiol Regul Integr Comp Physiol. (2012) 302:R1049–58. doi: 10.1152/ajpregu.00640.2011
45. Cui J and Himms-Hagen J. Long-term decrease in body fat and in brown adipose tissue in capsaicin-desensitized rats. Am J Physiol. (1992) 262:R568–73. doi: 10.1152/ajpregu.1992.262.4.R568
46. Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, et al. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. (2005) 493:596–606. doi: 10.1002/cne.20794
47. Julius D. TRP channels and pain. Annu Rev Cell Dev Biol. (2013) 29:355–84. doi: 10.1146/annurev-cellbio-101011-155833
48. Harris RBS. Denervation as a tool for testing sympathetic control of white adipose tissue. Physiol Behav. (2018) 190:3–10. doi: 10.1016/j.physbeh.2017.07.008
49. Ryu V, Garretson JT, Liu Y, Vaughan CH, and Bartness TJ. Brown adipose tissue has sympathetic-sensory feedback circuits. J Neurosci. (2015) 35:2181–90. doi: 10.1523/JNEUROSCI.3306-14.2015
50. Garretson JT, Szymanski LA, Schwartz GJ, Xue B, Ryu V, and Bartness TJ. Lipolysis sensation by white fat afferent nerves triggers brown fat thermogenesis. Mol Metab. (2016) 5:626–34. doi: 10.1016/j.molmet.2016.06.013
51. Kruger L, Mantyh PW, Sternini C, Brecha NC, and Mantyh CR. Calcitonin gene-related peptide (CGRP) in the rat central nervous system: patterns of immunoreactivity and receptor binding sites. Brain Res. (1988) 463:223–44. doi: 10.1016/0006-8993(88)90395-2
52. Matsui S, Yamane T, Kobayashi-Hattori K, and Oishi Y. Ultraviolet B irradiation reduces the expression of adiponectin in ovarial adipose tissues through endocrine actions of calcitonin gene-related peptide-induced serum amyloid A. PloS One. (2014) 9:e98040. doi: 10.1371/journal.pone.0098040
53. Brain SD, Williams TJ, Tippins JR, Morris HR, and MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. (1985) 313:54–6. doi: 10.1038/313054a0
54. Uddman R, Edvinsson L, Ekblad E, Håkanson R, and Sundler F. Calcitonin gene-related peptide (CGRP): perivascular distribution and vasodilatory effects. Regul Pept. (1986) 15:1–23. doi: 10.1016/0167-0115(86)90071-6
55. Brain SD and Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev. (2004) 84:903–34. doi: 10.1152/physrev.00037.2003
56. Liu T, Kamiyoshi A, Sakurai T, Ichikawa-Shindo Y, Kawate H, Yang L, et al. Endogenous calcitonin gene-related peptide regulates lipid metabolism and energy homeostasis in male mice. Endocrinology. (2017) 158:1194–206. doi: 10.1210/en.2016-1510
57. Walker CS, Li X, Whiting L, Glyn-Jones S, Zhang S, Hickey AJ, et al. Mice lacking the neuropeptide alpha-calcitonin gene-related peptide are protected against diet-induced obesity. Endocrinology. (2010) 151:4257–69. doi: 10.1210/en.2010-0284
58. Moore MC, Lin DW, Colburn CA, Goldstein RE, Neal DW, and Cherrington AD. Insulin- and glucagon-independent effects of calcitonin gene-related peptide in the conscious dog. Metabolism. (1999) 48:603–10. doi: 10.1016/S0026-0495(99)90058-6
59. Danaher RN, Loomes KM, Leonard BL, Whiting L, Hay DL, Xu LY, et al. Evidence that alpha-calcitonin gene-related peptide is a neurohormone that controls systemic lipid availability and utilization. Endocrinology. (2008) 149:154–60. doi: 10.1210/en.2007-0583
60. Kuo T, Ouchi Y, Kim S, Toba K, and Orimo H. The role of activation of the sympathetic nervous system in the central pressor action of calcitonin gene-related peptide in conscious rats. Naunyn Schmiedebergs Arch Pharmacol. (1994) 349:394–400. doi: 10.1007/BF00170886
61. Sideri A, Bakirtzi K, Shih DQ, Koon HW, Fleshner P, Arsenescu R, et al. Substance P mediates pro-inflammatory cytokine release form mesenteric adipocytes in Inflammatory Bowel Disease patients. Cell Mol Gastroenterol Hepatol. (2015) 1:420–32. doi: 10.1016/j.jcmgh.2015.03.003
62. Miegueu P, St-Pierre DH, Lapointe M, Poursharifi P, Lu H, Gupta A, et al. Substance P decreases fat storage and increases adipocytokine production in 3T3-L1 adipocytes. Am J Physiol Gastrointest Liver Physiol. (2013) 304:G420–7. doi: 10.1152/ajpgi.00162.2012
63. Karagiannides I, Stavrakis D, Bakirtzi K, Kokkotou E, Pirtskhalava T, Nayeb-Hashemi H, et al. (SP)-neurokinin-1 receptor (NK-1R) alters adipose tissue responses to high-fat diet and insulin action. Endocrinology. (2011) 152:2197–205. doi: 10.1210/en.2010-1345
64. Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessì-Fulgheri P, Zhang C, et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. (2012) 122:1022–36. doi: 10.1172/JCI59701
65. Bi P, Shan T, Liu W, Yue F, Yang X, Liang XR, et al. Inhibition of Notch signaling promotes browning of white adipose tissue and ameliorates obesity. Nat Med. (2014) 20:911–8. doi: 10.1038/nm.3615
66. Baskaran P, Krishnan V, Ren J, and Thyagarajan B. Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br J Pharmacol. (2016) 173:2369–89. doi: 10.1111/bph.13514
67. Baskaran P, Gustafson N, and Chavez N. TRPV1 Activation Antagonizes High-Fat Diet-Induced Obesity at Thermoneutrality and Enhances UCP-1 Transcription via PRDM-16. Pharm (Basel). (2024) 17:1098. doi: 10.3390/ph17081098
68. Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y, et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Pparγ. Cell. (2012) 150:620–32. doi: 10.1016/j.cell.2012.06.027
69. Krishnan V, Baskaran P, and Thyagarajan B. Troglitazone activates TRPV1 and causes deacetylation of PPARγ in 3T3-L1 cells. Biochim Biophys Acta Mol Basis Dis. (2019) 1865:445–53. doi: 10.1016/j.bbadis.2018.11.004
70. Villanueva CJ, Vergnes L, Wang J, Drew BG, Hong C, Tu Y, et al. Adipose subtype-selective recruitment of TLE3 or Prdm16 by PPARγ specifies lipid storage versus thermogenic gene programs. Cell Metab. (2013) 17:423–35. doi: 10.1016/j.cmet.2013.01.016
71. Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. (2008) 454:961–7. doi: 10.1038/nature07182
72. Hesselink MK, Mensink M, and Schrauwen P. Human uncoupling protein-3 and obesity: an update. Obes Res. (2003) 11:1429–43. doi: 10.1038/oby.2003.192
73. Whittle AJ, Carobbio S, Martins L, Slawik M, Hondares E, Vázquez MJ, et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. (2012) 149:871–85. doi: 10.1016/j.cell.2012.02.066
74. Gao P, Jiang Y, Wu H, Sun F, Li Y, He H, et al. Inhibition of mitochondrial calcium overload by SIRT3 prevents obesity- or age-related whitening of brown adipose tissue. Diabetes. (2020) 69:165–80. doi: 10.2337/db19-0526
75. Kida R, Yoshida H, Murakami M, Shirai M, Hashimoto O, Kawada T, et al. Direct action of capsaicin in brown adipogenesis and activation of brown adipocytes. Cell Biochem Funct. (2016) 34:34–41. doi: 10.1002/cbf.3162
76. Tang QQ and Lane MD. Adipogenesis: from stem cell to adipocyte. Annu Rev Biochem. (2012) 81:715–36. doi: 10.1146/annurev-biochem-052110-115718
77. Kajimura S, Seale P, and Spiegelman BM. Transcriptional control of brown fat development. Cell Metab. (2010) 11:257–62. doi: 10.1016/j.cmet.2010.03.005
78. Cannon B and Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. (2004) 84:277–359. doi: 10.1152/physrev.00015.2003
79. Kostic M, Katoshevski T, and Sekler I. Allosteric regulation of NCLX by mitochondrial membrane potential links the metabolic state and ca(2+) signaling in mitochondria. Cell Rep. (2018) 25:3465–75.e4. doi: 10.1016/j.celrep.2018.11.084
80. Li L, Ma L, Luo Z, Wei X, Zhao Y, Zhou C, et al. Lack of TRPV1 aggravates obesity-associated hypertension through the disturbance of mitochondrial Ca2+ homeostasis in brown adipose tissue. Hypertens Res. (2022) 45:789–801. doi: 10.1038/s41440-021-00842-8
81. Zhang LL, Yan Liu D, Ma LQ, Luo ZD, Cao TB, Zhong J, et al. Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ Res. (2007) 100:1063–70. doi: 10.1161/01.RES.0000262653.84850.8b
82. Abdillah AM and Yun JW. Capsaicin induces ATP-dependent thermogenesis via the activation of TRPV1/β3-AR/α1-AR in 3T3-L1 adipocytes and mouse model. Arch Biochem Biophys. (2024) 755:109975. doi: 10.1016/j.abb.2024.109975
83. Lee MS, Kim CT, Kim IH, and Kim Y. Effects of capsaicin on lipid catabolism in 3T3-L1 adipocytes. Phytother Res. (2011) 25:935–9. doi: 10.1002/ptr.3339
84. Morimoto C, Kameda K, Tsujita T, and Okuda H. Relationships between lipolysis induced by various lipolytic agents and hormone-sensitive lipase in rat fat cells. J Lipid Res. (2001) 42:120–7. doi: 10.1016/S0022-2275(20)32343-9
85. McGarry JD and Brown NF. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur J Biochem. (1997) 244:1–14. doi: 10.1111/j.1432-1033.1997.00001.x
86. Rousset S, Alves-Guerra MC, Mozo J, Miroux B, Cassard-Doulcier AM, Bouillaud F, et al. The biology of mitochondrial uncoupling proteins. Diabetes. (2004) 53 Suppl 1:S130–5. doi: 10.2337/diabetes.53.2007.S130
87. Sharma MD, Garber AJ, and Farmer JA. Role of insulin signaling in maintaining energy homeostasis. Endocr Pract. (2008) 14:373–80. doi: 10.4158/EP.ep.14.3.373
88. Cushman SW and Wardzala LJ. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. (1980) 255:4758–62. doi: 10.1016/S0021-9258(19)85561-8
89. Kolb H. Obese visceral fat tissue inflammation: from protective to detrimental? BMC Med. (2022) 20:494. doi: 10.1186/s12916-022-02672-y
90. Woods SC, Lotter EC, McKay LD, and Porte D Jr. Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. (1979) 282:503–5. doi: 10.1038/282503a0
91. Derbenev AV and Zsombok A. Potential therapeutic value of TRPV1 and TRPA1 in diabetes mellitus and obesity. Semin Immunopathol. (2016) 38:397–406. doi: 10.1007/s00281-015-0529-x
92. Olah Z, Karai L, and Iadarola MJ. Protein kinase C(alpha) is required for vanilloid receptor 1 activation. Evidence for multiple signaling pathways. J Biol Chem. (2002) 277:35752–9. doi: 10.1074/jbc.M201551200
93. Shibasaki T, Sunaga Y, Fujimoto K, Kashima Y, and Seino S. Interaction of ATP sensor, cAMP sensor, Ca2+ sensor, and voltage-dependent Ca2+ channel in insulin granule exocytosis. J Biol Chem. (2004) 279:7956–61. doi: 10.1074/jbc.M309068200
94. Tanaka H, Shimaya A, Kiso T, Kuramochi T, Shimokawa T, and Shibasaki M. Enhanced insulin secretion and sensitization in diabetic mice on chronic treatment with a transient receptor potential vanilloid 1 antagonist. Life Sci. (2011) 88:559–63. doi: 10.1016/j.lfs.2011.01.016
95. Van Buren JJ, Bhat S, Rotello R, Pauza ME, and Premkumar LS. Sensitization and translocation of TRPV1 by insulin and IGF-I. Mol Pain. (2005) 1:17. doi: 10.1186/1744-8069-1-17
96. Suri A and Szallasi A. The emerging role of TRPV1 in diabetes and obesity. Trends Pharmacol Sci. (2008) 29:29–36. doi: 10.1016/j.tips.2007.10.016
97. Tsui H, Razavi R, Chan Y, Yantha J, and Dosch HM. ‘Sensing’ autoimmunity in type 1 diabetes. Trends Mol Med. (2007) 13:405–13. doi: 10.1016/j.molmed.2007.07.006
98. Pettersson M, Ahrén B, Böttcher G, and Sundler F. Calcitonin gene-related peptide: occurrence in pancreatic islets in the mouse and the rat and inhibition of insulin secretion in the mouse. Endocrinology. (1986) 119:865–9. doi: 10.1210/endo-119-2-865
99. Razavi R, Chan Y, Afifiyan FN, Liu XJ, Wan X, Yantha J, et al. TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell. (2006) 127:1123–35. doi: 10.1016/j.cell.2006.10.038
100. Noble MD, Romac J, Wang Y, Hsu J, Humphrey JE, and Liddle RA. Local disruption of the celiac ganglion inhibits substance P release and ameliorates caerulein-induced pancreatitis in rats. Am J Physiol Gastrointest Liver Physiol. (2006) 291:G128–34. doi: 10.1152/ajpgi.00442.2005
101. Arnold RA, Fowler DK, and Peters JH. TRPV1 enhances cholecystokinin signaling in primary vagal afferent neurons and mediates the central effects on spontaneous glutamate release in the NTS. Am J Physiol Cell Physiol. (2024) 326:C112–c24. doi: 10.1152/ajpcell.00409.2023
102. Xu T, Yu Z, Liu Y, Lu M, Gong M, Li Q, et al. Hypoglycemic effect of electroacupuncture at ST25 through neural regulation of the pancreatic intrinsic nervous system. Mol Neurobiol. (2022) 59:703–16. doi: 10.1007/s12035-021-02609-1
103. Yu M, Tian H, Lu R, Quan N, and Qian L. TRPV1 promotes periodontitis tissue inflammation and oxidative damage by regulating STAT3 signaling pathway. J Periodontal Res. (2024). doi: 10.1111/jre.13368
104. Van Slycke S, Van Den Heede K, and Vandenwyngaerden E-A. Adrenal glands: anatomy, physiology, and pathophysiology. In: Shifrin AL, Raffaelli M, Randolph GW, and Gimm O, editors. Endocrine Surgery Comprehensive Board Exam Guide. Springer International Publishing, Cham (2021). p. 437–55.
105. Delarue C, Contesse V, Lenglet S, Sicard F, Perraudin V, Lefebvre H, et al. Role of neurotransmitters and neuropeptides in the regulation of the adrenal cortex. Rev Endocr Metab Disord. (2001) 2:253–67. doi: 10.1023/A:1011512415497
106. Ehrhart-Bornstein M, Hinson JP, Bornstein SR, Scherbaum WA, and Vinson GP. Intraadrenal interactions in the regulation of adrenocortical steroidogenesis. Endocr Rev. (1998) 19:101–43. doi: 10.1210/edrv.19.2.0326
107. Zouhal H, Jacob C, Delamarche P, and Gratas-Delamarche A. Catecholamines and the effects of exercise, training and gender. Sports Med. (2008) 38:401–23. doi: 10.2165/00007256-200838050-00004
108. Bahnsen M, Burrin JM, Johnston DG, Pernet A, Walker M, and Alberti KG. Mechanisms of catecholamine effects on ketogenesis. Am J Physiol. (1984) 247:E173–80. doi: 10.1152/ajpendo.1984.247.2.E173
109. Steiner KE, Stevenson RW, Adkins-Marshall BA, and Cherrington AD. The effects of epinephrine on ketogenesis in the dog after a prolonged fast. Metabolism. (1991) 40:1057–62. doi: 10.1016/0026-0495(91)90130-O
110. Nielsen TS, Jessen N, Jørgensen JO, Møller N, and Lund S. Dissecting adipose tissue lipolysis: molecular regulation and implications for metabolic disease. J Mol Endocrinol. (2014) 52:R199–222. doi: 10.1530/JME-13-0277
111. Dongdem JT, Etornam AE, Beletaa S, Alidu I, Kotey H, and Wezena CA. The β(3)-adrenergic receptor: structure, physiopathology of disease, and emerging therapeutic potential. Adv Pharmacol Pharm Sci. (2024) 2024:2005589. doi: 10.1155/2024/2005589
112. Lafontan M. Kidney, adipose tissue, adipocytes–what’s new]? Nephrol Ther. (2011) 7:69–79. doi: 10.1016/j.nephro.2010.11.004
113. Lafontan M and Berlan M. Do regional differences in adipocyte biology provide new pathophysiological insights? Trends Pharmacol Sci. (2003) 24:276–83. doi: 10.1016/S0165-6147(03)00132-9
114. Ravussin E, Lillioja S, Knowler WC, Christin L, Freymond D, Abbott WG, et al. Reduced rate of energy expenditure as a risk factor for body-weight gain. N Engl J Med. (1988) 318:467–72. doi: 10.1056/NEJM198802253180802
115. Ravussin E. Energy metabolism in obesity. Studies in the Pima Indians. Diabetes Care. (1993) 16:232–8. doi: 10.2337/diacare.16.1.232
116. Schoneveld OJ, Gaemers IC, and Lamers WH. Mechanisms of glucocorticoid signalling. Biochim Biophys Acta. (2004) 1680:114–28. doi: 10.1016/j.bbaexp.2004.09.004
117. Björntorp P. Do stress reactions cause abdominal obesity and comorbidities? Obes Rev. (2001) 2:73–86. doi: 10.1046/j.1467-789x.2001.00027.x
118. Björntorp P and Rosmond R. Obesity and cortisol. Nutrition. (2000) 16:924–36. doi: 10.1016/S0899-9007(00)00422-6
119. Ulrich-Lai YM, Harding-Rose CA, Guo A, Bowles WR, and Engeland WC. ACTH inhibits the capsaicin-evoked release of CGRP from rat adrenal afferent nerves. Am J Physiol Regul Integr Comp Physiol. (2001) 280:R137–42. doi: 10.1152/ajpregu.2001.280.1.R137
120. Rosenfeld D, Senko AW, Moon J, Yick I, Varnavides G, Gregureć D, et al. Transgene-free remote magnetothermal regulation of adrenal hormones. Sci Adv. (2020) 6:eaaz3734. doi: 10.1126/sciadv.aaz3734
121. Kuramoto H, Kondo H, and Fujita T. Calcitonin gene-related peptide (CGRP)-like immunoreactivity in scattered chromaffin cells and nerve fibers in the adrenal gland of rats. Cell Tissue Res. (1987) 247:309–15. doi: 10.1007/BF00218312
122. Kobata K, Iwasawa T, Iwasaki Y, Morita A, Suzuki Y, Kikuzaki H, et al. Capsaicinol: synthesis by allylic oxidation and its effect on TRPV1-expressing cells and adrenaline secretion in rats. Biosci Biotechnol Biochem. (2006) 70:1904–12. doi: 10.1271/bbb.60064
123. Song W, Luo Q, Zhang Y, Zhou L, Liu Y, Ma Z, et al. Organic cation transporter 3 (Oct3) is a distinct catecholamines clearance route in adipocytes mediating the beiging of white adipose tissue. PloS Biol. (2019) 17:e2006571. doi: 10.1371/journal.pbio.2006571
124. Uchida K, Dezaki K, Yoneshiro T, Watanabe T, Yamazaki J, Saito M, et al. Involvement of thermosensitive TRP channels in energy metabolism. J Physiol Sci. (2017) 67:549–60. doi: 10.1007/s12576-017-0552-x
125. Arribas-Blázquez M, Olivos-Oré LA, Barahona MV, Sánchez de la Muela M, Solar V, Jiménez E, et al. Overexpression of P2X3 and P2X7 receptors and TRPV1 channels in adrenomedullary chromaffin cells in a rat model of neuropathic pain. Int J Mol Sci. (2019) 20:155. doi: 10.3390/ijms20010155
126. Oi-Kano Y, Iwasaki Y, Nakamura T, Watanabe T, Goto T, Kawada T, et al. Oleuropein aglycone enhances UCP1 expression in brown adipose tissue in high-fat-diet-induced obese rats by activating β-adrenergic signaling. J Nutr Biochem. (2017) 40:209–18. doi: 10.1016/j.jnutbio.2016.11.009
127. Ootsuka Y, Kulasekara K, de Menezes RC, and Blessing WW. SR59230A, a beta-3 adrenoceptor antagonist, inhibits ultradian brown adipose tissue thermogenesis and interrupts associated episodic brain and body heating. Am J Physiol Regul Integr Comp Physiol. (2011) 301:R987–94. doi: 10.1152/ajpregu.00085.2011
128. Watanabe T, Sakurada N, and Kobata K. Capsaicin-, resiniferatoxin-, and olvanil-induced adrenaline secretions in rats via the vanilloid receptor. Biosci Biotechnol Biochem. (2001) 65:2443–7. doi: 10.1271/bbb.65.2443
129. Iwasaki Y, Tanabe M, Kobata K, and Watanabe T. TRPA1 agonists–allyl isothiocyanate and cinnamaldehyde–induce adrenaline secretion. Biosci Biotechnol Biochem. (2008) 72:2608–14. doi: 10.1271/bbb.80289
130. Blaak EE, Van Baak MA, Kemerink GJ, Pakbiers MT, Heidendal GA, and Saris WH. Beta-adrenergic stimulation of energy expenditure and forearm skeletal muscle metabolism in lean and obese men. Am J Physiol. (1994) 267:E306–15. doi: 10.1152/ajpendo.1994.267.2.E306
131. Landsberg L, Saville ME, and Young JB. Sympathoadrenal system and regulation of thermogenesis. Am J Physiol. (1984) 247:E181–9. doi: 10.1152/ajpendo.1984.247.2.E181
132. Kobayashi A, Osaka T, Namba Y, Inoue S, Lee TH, and Kimura S. Capsaicin activates heat loss and heat production simultaneously and independently in rats. Am J Physiol. (1998) 275:R92–8. doi: 10.1152/ajpregu.1998.275.1.R92
133. Himms-Hagen J, Cui J, Danforth E Jr., Taatjes DJ, Lang SS, Waters BL, et al. Effect of CL-316,243, a thermogenic beta 3-agonist, on energy balance and brown and white adipose tissues in rats. Am J Physiol. (1994) 266:R1371–82. doi: 10.1152/ajpregu.1994.266.4.R1371
134. Nagase I, Yoshida T, Kumamoto K, Umekawa T, Sakane N, Nikami H, et al. Expression of uncoupling protein in skeletal muscle and white fat of obese mice treated with thermogenic beta 3-adrenergic agonist. J Clin Invest. (1996) 97:2898–904. doi: 10.1172/JCI118748
135. Lee YH, Mottillo EP, and Granneman JG. Adipose tissue plasticity from WAT to BAT and in between. Biochim Biophys Acta. (2014) 1842:358–69. doi: 10.1016/j.bbadis.2013.05.011
136. Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. (2012) 150:366–76. doi: 10.1016/j.cell.2012.05.016
137. Kozak LP. The genetics of brown adipocyte induction in white fat depots. Front Endocrinol (Lausanne). (2011) 2:64. doi: 10.3389/fendo.2011.00064
138. Collins S, Yehuda-Shnaidman E, and Wang H. Positive and negative control of Ucp1 gene transcription and the role of β-adrenergic signaling networks. Int J Obes (Lond). (2010) 34 Suppl 1:S28–33. doi: 10.1038/ijo.2010.180
139. Cao W, Daniel KW, Robidoux J, Puigserver P, Medvedev AV, Bai X, et al. p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol Cell Biol. (2004) 24:3057–67. doi: 10.1128/MCB.24.7.3057-3067.2004
140. Rim JS and Kozak LP. Regulatory motifs for CREB-binding protein and Nfe2l2 transcription factors in the upstream enhancer of the mitochondrial uncoupling protein 1 gene. J Biol Chem. (2002) 277:34589–600. doi: 10.1074/jbc.M108866200
141. Ferreira LGB, Prevatto JP, Freitas HR, Reis RAM, Silva PMR, Martins MA, et al. Capsaicin inhibits lipopolysaccharide-induced adrenal steroidogenesis by raising intracellular calcium levels. Endocrine. (2019) 64:169–75. doi: 10.1007/s12020-019-01849-5
142. Koivisto AP, Voets T, Iadarola MJ, and Szallasi A. Targeting TRP channels for pain relief: A review of current evidence from bench to bedside. Curr Opin Pharmacol. (2024) 75:102447. doi: 10.1016/j.coph.2024.102447
143. Qiao Z, Liu S, Zhai W, Jiang L, Ma Y, Zhang Z, et al. Novel dual-target FAAH and TRPV1 ligands as potential pharmacotherapeutics for pain management. Eur J Med Chem. (2024) 267:116208. doi: 10.1016/j.ejmech.2024.116208
144. Moran MM and Szallasi A. Targeting nociceptive transient receptor potential channels to treat chronic pain: current state of the field. Br J Pharmacol. (2018) 175:2185–203. doi: 10.1111/bph.14044
145. Szallasi A, Cortright DN, Blum CA, and Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov. (2007) 6:357–72. doi: 10.1038/nrd2280
146. Szallasi A and Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. (1999) 51:159–212. doi: 10.1016/S0031-6997(24)01403-0
147. Bölcskei K, Tékus V, Dézsi L, Szolcsányi J, and Petho G. Antinociceptive desensitizing actions of TRPV1 receptor agonists capsaicin, resiniferatoxin and N-oleoyldopamine as measured by determination of the noxious heat and cold thresholds in the rat. Eur J Pain. (2010) 14:480–6. doi: 10.1016/j.ejpain.2009.08.005
148. Round P, Priestley A, and Robinson J. An investigation of the safety and pharmacokinetics of the novel TRPV1 antagonist XEN-D0501 in healthy subjects. Br J Clin Pharmacol. (2011) 72:921–31. doi: 10.1111/j.1365-2125.2011.04040.x
149. Heymsfield SB and Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. (2017) 376:254–66. doi: 10.1056/NEJMra1514009
150. Kalarchian MA, Marcus MD, Courcoulas AP, Cheng Y, and Levine MD. Self-report of gastrointestinal side effects after bariatric surgery. Surg Obes Relat Dis. (2014) 10:1202–7. doi: 10.1016/j.soard.2014.08.007
151. Gram DX, Hansen AJ, Wilken M, Elm T, Svendsen O, Carr RD, et al. Plasma calcitonin gene-related peptide is increased prior to obesity, and sensory nerve desensitization by capsaicin improves oral glucose tolerance in obese Zucker rats. Eur J Endocrinol. (2005) 153:963–9. doi: 10.1530/eje.1.02046
152. Baskaran P, Markert L, Bennis J, Zimmerman L, Fox J, and Thyagarajan B. Assessment of pharmacology, safety, and metabolic activity of capsaicin feeding in mice. Sci Rep. (2019) 9:8588. doi: 10.1038/s41598-019-45050-0
153. Paternoster S and Falasca M. Dissecting the physiology and pathophysiology of glucagon-like peptide-1. Front Endocrinol (Lausanne). (2018) 9:584. doi: 10.3389/fendo.2018.00584
154. Kumar V, Mahajan N, Khare P, Kondepudi KK, and Bishnoi M. Role of TRPV1 in colonic mucin production and gut microbiota profile. Eur J Pharmacol. (2020) 888:173567. doi: 10.1016/j.ejphar.2020.173567
155. Song JX, Ren H, Gao YF, Lee CY, Li SF, Zhang F, et al. Dietary capsaicin improves glucose homeostasis and alters the gut microbiota in obese diabetic ob/ob mice. Front Physiol. (2017) 8:602. doi: 10.3389/fphys.2017.00602
156. Choi SE, Kim TH, Yi SA, Hwang YC, Hwang WS, Choe SJ, et al. Capsaicin attenuates palmitate-induced expression of macrophage inflammatory protein 1 and interleukin 8 by increasing palmitate oxidation and reducing c-Jun activation in THP-1 (human acute monocytic leukemia cell) cells. Nutr Res. (2011) 31:468–78. doi: 10.1016/j.nutres.2011.05.007
157. Devesa I, Planells-Cases R, Fernández-Ballester G, González-Ros JM, Ferrer-Montiel A, and Fernández-Carvajal A. Role of the transient receptor potential vanilloid 1 in inflammation and sepsis. J Inflammation Res. (2011) 4:67–81. doi: 10.2147/JIR.S12978
158. Huang J, Zhang X, and McNaughton PA. Inflammatory pain: the cellular basis of heat hyperalgesia. Curr Neuropharmacol. (2006) 4:197–206. doi: 10.2174/157015906778019554
159. Wanner SP, Garami A, and Romanovsky AA. Hyperactive when young, hypoactive and overweight when aged: connecting the dots in the story about locomotor activity, body mass, and aging in Trpv1 knockout mice. Aging (Albany NY). (2011) 3:450–4. doi: 10.18632/aging.100306
Keywords: transient receptor potential vanilloid subtype 1 (TRPV1), obesity, neuromodulatory mechanisms, endocrine mechanisms, energy metabolism
Citation: Wang J, Liu M, Wen L, Xing P, Chen J, Xia X and Ding W (2025) Role of TRPV1 in neuroendocrine regulation: a potential target against obesity? Front. Immunol. 16:1598804. doi: 10.3389/fimmu.2025.1598804
Received: 24 March 2025; Accepted: 19 June 2025;
Published: 03 July 2025.
Edited by:
Robson Xavier Faria, Oswaldo Cruz Foundation (Fiocruz), BrazilReviewed by:
Marilia Zaluar P. Guimaraes, Federal University of Rio de Janeiro, BrazilBin Xu, Nanjing University of Chinese Medicine, China
Copyright © 2025 Wang, Liu, Wen, Xing, Chen, Xia and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: WeiJun Ding, RGluZ3dlaWp1bkBjZHV0Y20uZWR1LmNu; Xiuwen Xia, eHhpb3V3ZW5AMTYzLmNvbQ==
 Jiexin Wang
Jiexin Wang Maohui Liu
Maohui Liu Lingmiao Wen
Lingmiao Wen Pengfei Xing
Pengfei Xing Jiawei Chen
Jiawei Chen Xiuwen Xia
Xiuwen Xia WeiJun Ding
WeiJun Ding