- 1Institute of Pathology, Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia
- 2Department of Nephrology, University Medical Centre Ljubljana, Ljubljana, Slovenia
- 3Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia
Introduction: Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is characterized by necrotizing small vessel vasculitis that frequently manifests as glomerulonephritis (AAV-GN). An accurate noninvasive biomarker reflecting active AAV-GN remains elusive. The knowledge of microRNAs (miRNAs), which have been considered as disease-specific biomarkers, is scarce and lacks validated data in AAV.
Methods: This study validated a renal tissue expression profile of candidate miRNAs specific to AAV-GN selected through prior screening using independent cohorts. The analysis was extended to serum samples to explore the potential of a circulating miRNA panel as a noninvasive biomarker for active AAV-GN. To substantiate the findings, we correlated the molecular data with clinical and histologic markers of AAV-GN activity.
Results: We identified miR-21-3p, miR-181a-5p, and miR-181d-5p as potential biomarkers distinguishing AAV-GN from non-AAV renal diseases and healthy controls. In addition, miR-21-3p and miR-181d-5p correlated with the presence of active AAV-GN, while miR-181a-5p differentiated AAV-GN subtypes based on ANCA antigen specificity. ROC curve analysis demonstrated that the combined serum expression of miR-21-3p and miR-181a-5p reliably distinguished AAV-GN from other renal pathologies, including ANCA-positive cases without histologic evidence of AAV-GN.
Conclusion: Our findings highlight the potential of circulating miRNA expression signature as a noninvasive biomarker for ongoing AAV-GN in the appropriate setting. Larger confirmatory studies are essential to support the clinical application of miRNA-based biomarkers in AAV-GN diagnostics and disease monitoring.
1 Introduction
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) encompasses a heterogeneous disease group characterized by autoimmune necrotizing small vessel vasculitis. A severe type of renal involvement is common in AAV, known as relapsing pauci-immune crescentic and necrotizing glomerulonephritis (AAV-GN). AAV-GN is an important determinant of disease-specific morbidity and mortality. While detectable ANCAs provide some correlation with ongoing renal involvement, the ‘ANCA-centric’ approach has limitations, including the presence of ANCAs in non-AAV diseases and the fact that rising titers do not always relate to AAV-GN activity/flare (1). An accurate noninvasive biomarker reflecting active AAV-GN thus remains elusive. Consequently, a renal biopsy, an invasive procedure with inherent risks, remains the diagnostic and prognostic gold standard in cases clinically suspected of AAV-GN.
Due to observed phenotypic and genotypic differences, AAV is best classified by the antigen specificity of ANCAs. The two primary antigens of pathogenic ANCAs are myeloperoxidase (MPO) and proteinase-3 (PR3). Notably, MPO-AAV and PR3-AAV are increasingly considered as entities with distinct pathogeneses, clinical features and management strategies (2, 3).
Altered macrophage polarization (4) and differentiation of CD4+ T-cells towards Th17 subsets (5) are two recognized biological processes implicated in the pathogenesis of AAV; however, the precise mechanisms driving their activation remain poorly understood. Epigenetic regulation through microRNAs (miRNAs) has been implicated in both macrophage polarization (6) and Th17 differentiation (7), but not in the specific context of AAV-GN. While miRNAs have been proposed as candidate biomarkers in various autoimmune diseases (8), validated data in AAV-GN are lacking. This study aimed to validate a renal miRNA expression profile identified as specific to AAV-GN (9) and investigate its potential as a circulating biomarker for active AAV-GN. Additionally, it aimed to identify miRNA expression differences between MPO- and PR3-positive AAG-GN cases and correlate the molecular data with clinical and histologic markers of disease activity to improve diagnostic accuracy.
2 Materials and methods
2.1 Study design
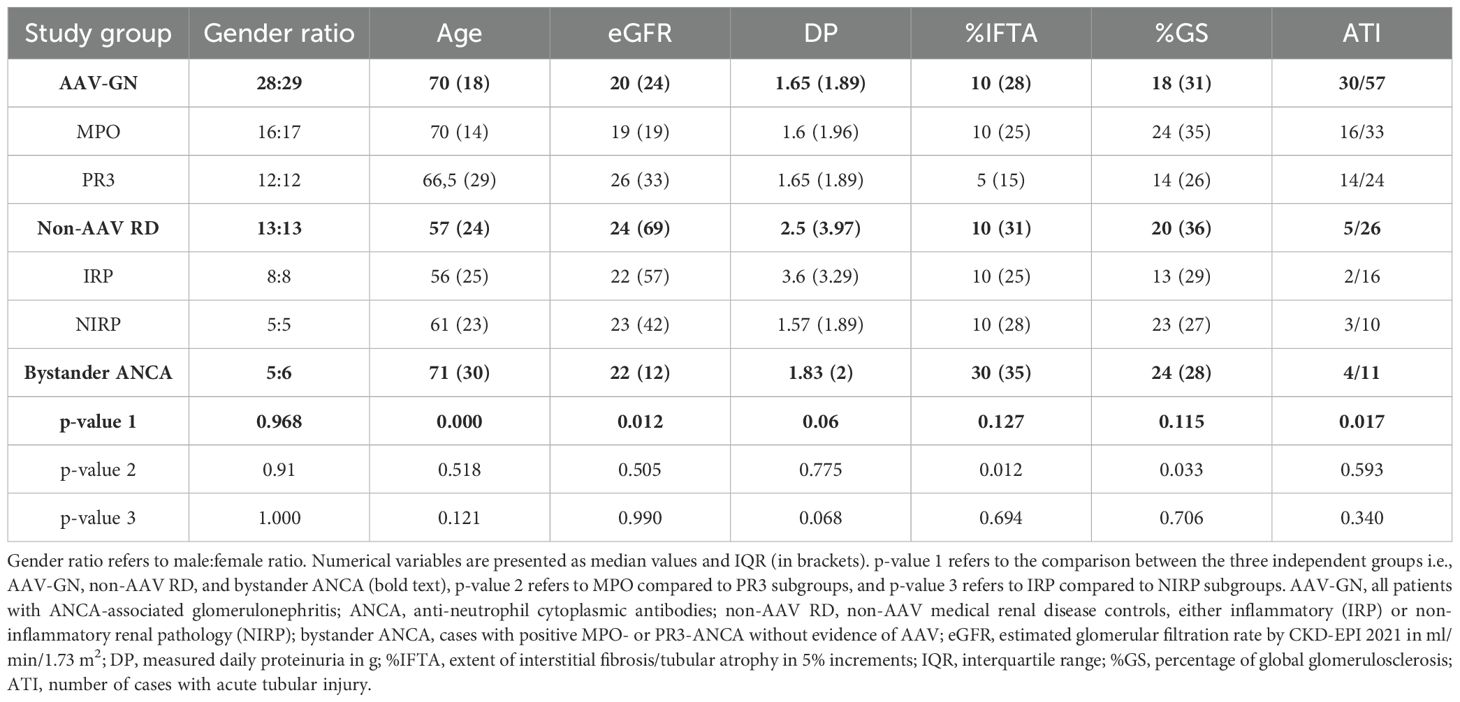
Matched renal tissue and serum samples were collected from 60 consecutive patients of AAV with biopsy-proven renal involvement in the form of AAV-GN, and from 28 individuals with non-AAV medical renal disease (non-AAV RD) between 2018 and 2023. Samples obtained during this period were included in analysis only if considered technically adequate for further processing after sample quality control (see Supplementary Materials & Methods). All AAV-GN cases demonstrated positive serological assays for ANCA (either MPO- or PR3-ANCA) on ELISA, which further stratified this group into MPO- and PR3-positive AAV-GN. The non-AAV RD group comprised a variety of inflammatory and non-inflammatory renal pathologies without detectable ANCA. A further 11 cases with positive MPO- or PR3-ANCA without clinical or histologic evidence of AAV-GN were included in the third group, referred to as the bystander ANCA group. The full list of diagnostic entities comprising the groups and the list of recorded clinical and histologic variables are available in Supplementary Materials & Methods.
All renal biopsies were performed at disease presentation and contained at least seven glomeruli and one interlobular-type artery to eliminate the potential confounding effect of suboptimal specimens. Matched serum samples were collected at the time of renal biopsy for ANCA serological assays by ELISA. All serum samples were snap-frozen and stored at –80°C within 72 hours of collection.
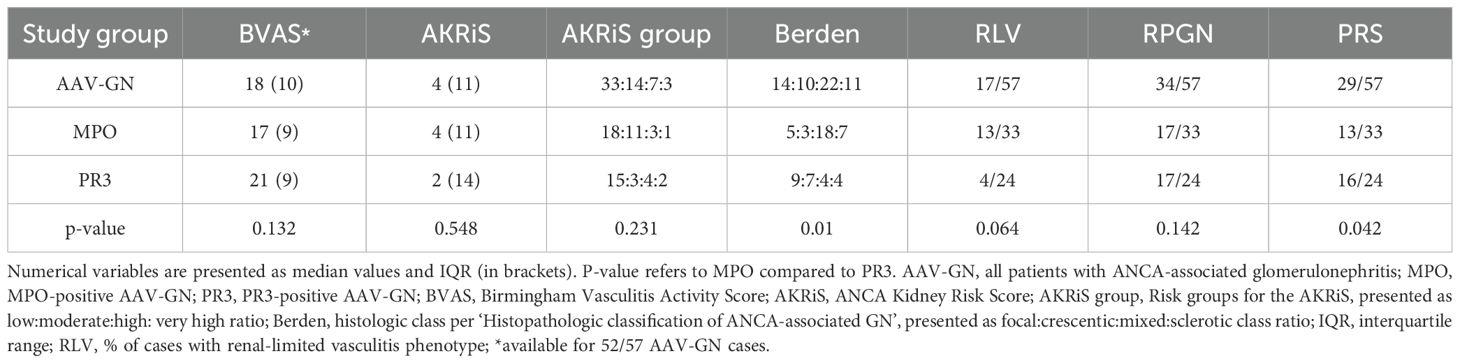
Clinical variables recorded for each case included age, gender, estimated glomerular filtration rate (eGFR in ml/min/1.73 m2, calculated using the CKD-EPI 2021 equation), ANCA serology, and measured daily proteinuria (24h urine total protein excretion in grams). For AAV-GN cases specifically, we documented the Birmingham Vasculitis Activity Score (BVAS) (10), the disease phenotype (renal limited or systemic), the presence of rapidly progressive glomerulonephritis (RPGN), the presence of pulmonary-renal syndrome, the extent of organ involvement (as total number of organs affected), and the application of pre-emptive immunosuppressive therapy and plasmapheresis at or prior to renal biopsy.
For each case, the following histologic variables were recorded: percentage of global glomerulosclerosis, extent of interstitial fibrosis/tubular atrophy in 5% increments and the presence of acute tubular injury. AAV-GN cases were classified according to two established scoring systems in AAV-GN i.e., histologic class per ‘Histopathologic classification of ANCA-associated glomerulonephritis (11)’ (Berden class) and ‘ANCA Kidney Risk Score (12)’ (AKRiS) were assigned. Additional histologic variables recorded in AAV-GN cases included the percentages of normal glomeruli (%NG) and cellular or fibrocellular glomerular crescents (%C), and the presence of medullary angiitis and arteritis in interlobular/arcuate-type arteries.
To enhance the specificity of our study at the serologic level, we included unmatched serum-only samples from 20 individuals without clinical or laboratory evidence of kidney disease and with negative ANCA serology, designated as healthy controls group (HC).
2.2 Total RNA isolation, reverse transcription and qPCR
Four 10-µm thick sections were cut from the formalin-fixed paraffin-embedded (FFPE) renal biopsy tissue blocks for RNA isolation into 2.0-ml microcentrifuge sterile tubes. Total RNA isolation was performed using a semi-automatic procedure with the Maxwell RSC (Promega, Madison, WI 53711-5399, USA), in combination with the Maxwell® RSC RNA FFPE kit (Promega, Madison, WI 53711-5399, USA; code AS1440) and Maxwell® RSC miRNA tissue kit (Promega, Madison, WI 53711-5399, USA; code AS1460).
For total RNA isolation from serum samples, 200 µl of stored serum was processed with miRNeasy Serum/Plasma Advanced Kit (Qiagen, Hilden, Germany).
See Supplementary Materials & Methods for detailed protocols of isolation, reverse transcription, reference genes (RGs) selection, and quantification by quantitative polymerase chain reaction. (qPCR).
2.3 Selection of candidate miRNAs for validation in renal tissue and serum samples
We identified 30 candidate miRNAs by (preceding) screenings using miRCURY LNA miRNA miRNOME panel (Qiagen, Hilden, Germany): 17 miRNAs that potentially distinguish AAV-GN from non-AAV GN controls and living donors (9), and 13 additional miRNAs that potentially distinguish MPO- and PR3-positive AAV-GN (the latter are presented in Results).
Candidate miRNAs from the two screening phases were selected for validation based on following criteria: (1) Cq values ≤ 30; (ii) candidate miRNAs belonging to the same miRNA family were included regardless of their Cq values; (iii) miRNAs from the three represented miRNA families that had not been statistically selected were also included for validation. The 21 miRNAs selected for validation in renal tissue samples and their specific inclusion criteria are presented in Supplementary Table S1. The selection process of candidate miRNAs for validation is described in more detail in Supplementary Materials & Methods.
Serum-based analysis and all correlations included candidate miRNAs with significant expression differences in the renal tissue between AAV-GN and non-AAV RD, MPO- and PR3-positive AAV-GN, and/or AAV-GN and bystander ANCA samples.
2.4 Technical yield and selection of samples included in the validation
The technical procedure (isolation and subsequent RT/qPCR) integrity was assessed by expression analysis of selected RG miRNAs in the renal tissue samples and of control spike-in RNAs in the serum samples as suggested by the manufacturer. In the renal tissue samples, comparable expression levels of RG miRNAs were identified in 57/60 AAV-GN, 26/28 non-AAV RD and 11/11 bystander ANCA samples. In the matched serum samples, the isolation and quantification of spike-in RNAs was successful in 38 of 57 AAV-GN, in 23 of 26 non-AAV RD and 6 of 11 bystander ANCA samples. In the unmatched serum samples of HC group, isolation and quantification of spike-in RNAs was successful in all 20 included samples.
2.5 Statistical analysis of miRNA expression and clinicopathologic characteristics
ΔCq was calculated to quantify the expression of individual miRNA gene relative to the geometric expression mean of RGs (13). The Kruskal-Wallis, Mann-Whitney, Chi-square, and Fischer’s exact tests were employed to analyze differences in ΔCq and in clinical and histologic characteristics. The Spearman’s rank correlation coefficient (Spearman’s rho) was used for all correlations, including calculation of statistical power and estimation of needed sample size. The receiver operating characteristic (ROC) curve analysis included the most promising validated serum-expressed miRNAs. All statistical analyses were performed using SPSS analytical software (IBM SPSS statistics, version 27.0, Armonk, NY, USA) with p-values < 0.05 considered statistically significant. Note that due to the nature of ΔCq value calculation, negative correlations in ΔCq values correspond to positive correlations in actual expression levels, and vice versa. See Supplementary Materials & Methods for detailed description of ΔCq calculation and its correlations and ROC curve analyses.
3 Results
A schematic workflow of this research is depicted in Figure 1. Briefly, we identified 17 candidate miRNAs that potentially distinguish AAV-GN as a group from non-AAV GN controls, as well as from individuals without renal disease (living donors) in the previous study (9). In this study, we performed a similar analysis of screening data and identified 13 candidate miRNAs that could distinguish MPO- from PR3-positive AAV-GN. The 21 out of 30 candidate miRNAs selected for validation in renal tissue samples and their specific inclusion criteria are presented in Supplementary Table S1.
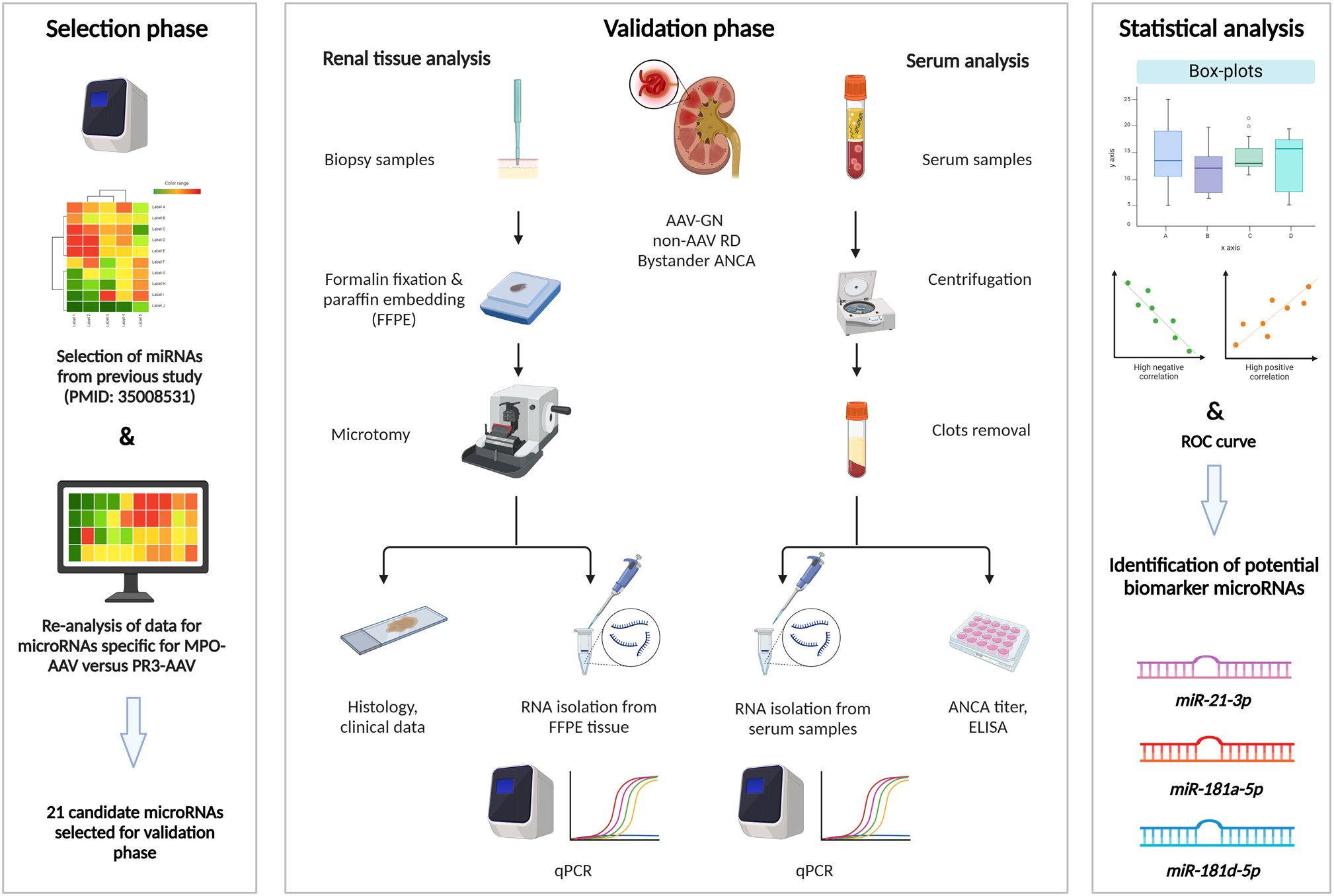
Figure 1. Research workflow scheme. Left: screening was based on qPCR in pooled tissue samples of AAV-GN compared to non-AAV RD and individuals without renal disease. Additional screening was performed within the AAV-GN group to identify candidate miRNAs that could distinguish MPO- from PR3-positive AAV-GN. After the statistical analysis of screening data, 21 candidate miRNAs were selected for validation. Middle: parallel analysis of expression of selected miRNAs was performed in matched renal biopsy and serum samples collected at the time of biopsy. We included 60 AAV-GN, 28 non-AAV RD and 11 bystander ANCA samples within the 2018–2023 timeframe. Fifty-seven tissue and 38 serum samples of AAV-GN, 26 tissue and 23 serum samples of non-AAV, and 11 tissue and 6 serum samples of bystander ANCA were analytically accepted after sample quality control. Validation was performed using qPCR. Right: The three most promising miRNAs were determined through statistical analysis of differential tissue and serum expression and through correlations to scoring systems for renal involvement and clinical variables of disease activity in AAV-GN. AAV-GN, patients with ANCA-associated glomerulonephritis; ANCA, anti-neutrophil cytoplasmic antibody; ELISA, enzyme-linked immunosorbent assay; non-AAV RD, non-AAV medical renal disease controls; qPCR, quantitative polymerase chain reaction.
3.1 Screening-based discovery of candidate renal tissue miRNAs differentially expressed in MPO- versus PR3-positive AAV-GN
The characteristics of the screening cohorts are described in detail in our previous research (9). We identified a candidate miRNA signature comprising 13 miRNAs that differentiated MPO- from PR3–positive AAV-GN. Among these, miR-181a-5p was already identified as capable of also distinguishing AAV-GN as a group from non-AAV GN, as well as from living donors without renal disease (9). Two other members of the miR-181 family were identified in this screening, namely miR-181a-2-3p and miR-181d-5p.
The results of the screening-based discovery of differentially expressed renal tissue miRNAs in MPO- compared to PR3-positive AAV-GN are illustrated in Supplementary Figure S1.
3.2 Validation of miRNA expression profiles in the renal tissue
Core clinicopathologic characteristics of the validation cohorts are presented in Table 1, while selected clinical and histologic variables specific to the AAV-GN group are presented in Table 2. See Supplementary Results for full clinicopathologic characteristics of the AAV-GN group.
3.2.1 AAV-GN versus non-AAV RD group
Significant expression differences were established for 8 out of 21 candidate miRNAs, namely miR-21-3p (p < 0.001), miR-30b-5p (p < 0.001), miR-142-5p (p = 0.001), miR-150-5p (p = 0.001), miR-181a-5p (p < 0.001), miR-181a-2-3p (p < 0.001), miR-181b-5p (p = 0.042), and miR-181d-5p (p < 0.001) in comparisons between AAV-GN and non-AAV RD samples. Figure 2 summarizes the results of renal tissue-based validation between AAV-GN and non-AAV RD samples.
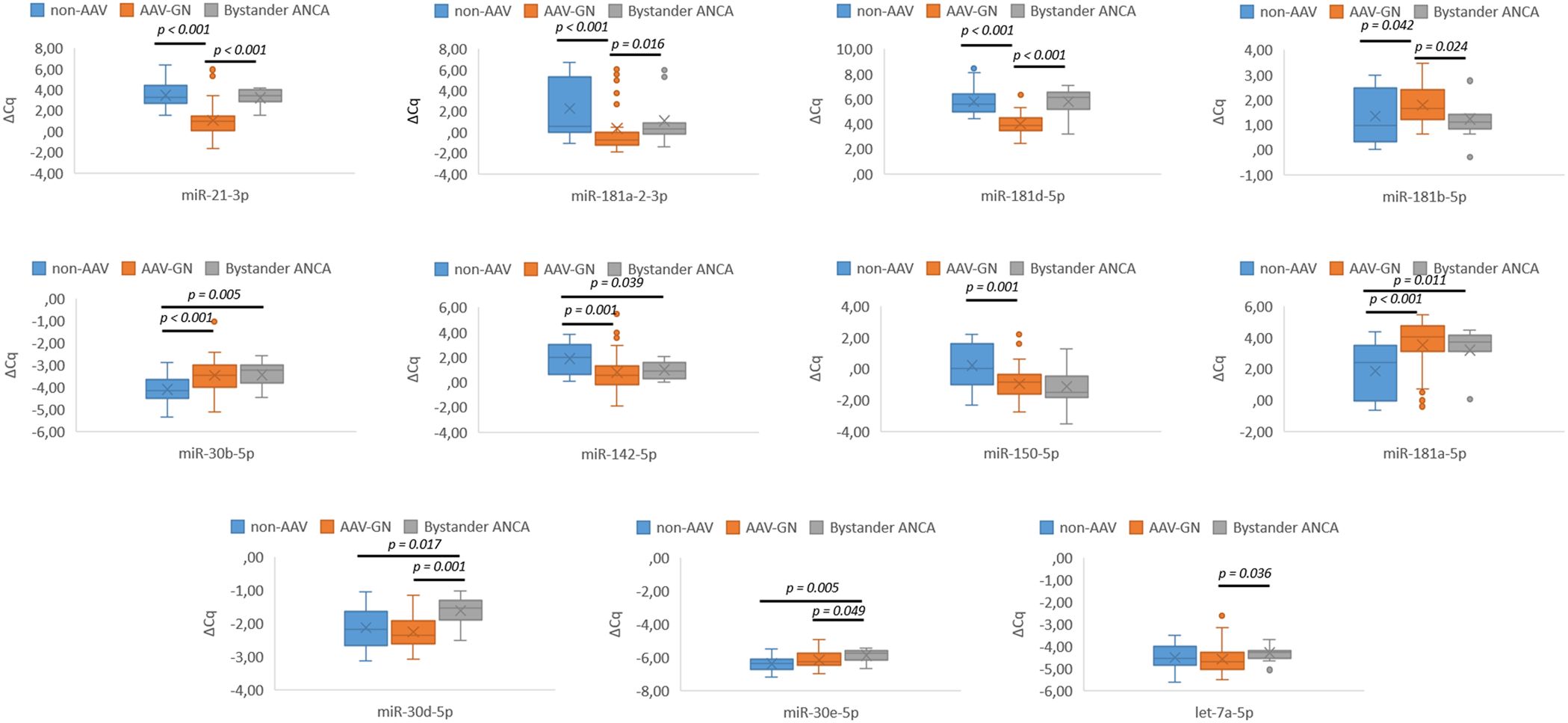
Figure 2. Differential expression of validated miRNAs in AAV-GN compared to non-AAV and bystander ANCA in renal tissue samples. AAV-GN, patients with ANCA-associated glomerulonephritis (n = 57); ANCA, anti-neutrophil cytoplasmic antibodies; non-AAV, non-AAV medical renal disease controls (n = 26); bystander ANCA, cases with positive MPO- or PR3-ANCA without evidence of AAV-GN (n = 11); ΔCq, delta quantitation cycle. All values were within 95% confidence interval.
3.2.2 MPO- versus PR3-positive AAV-GN
A significant expression difference was confirmed for miR-181a-5p (p = 0.04). The differential expression of any other candidate miRNA was not established. Figure 3A summarizes the validation results between MPO- and PR3-positive AAV-GN subgroups.
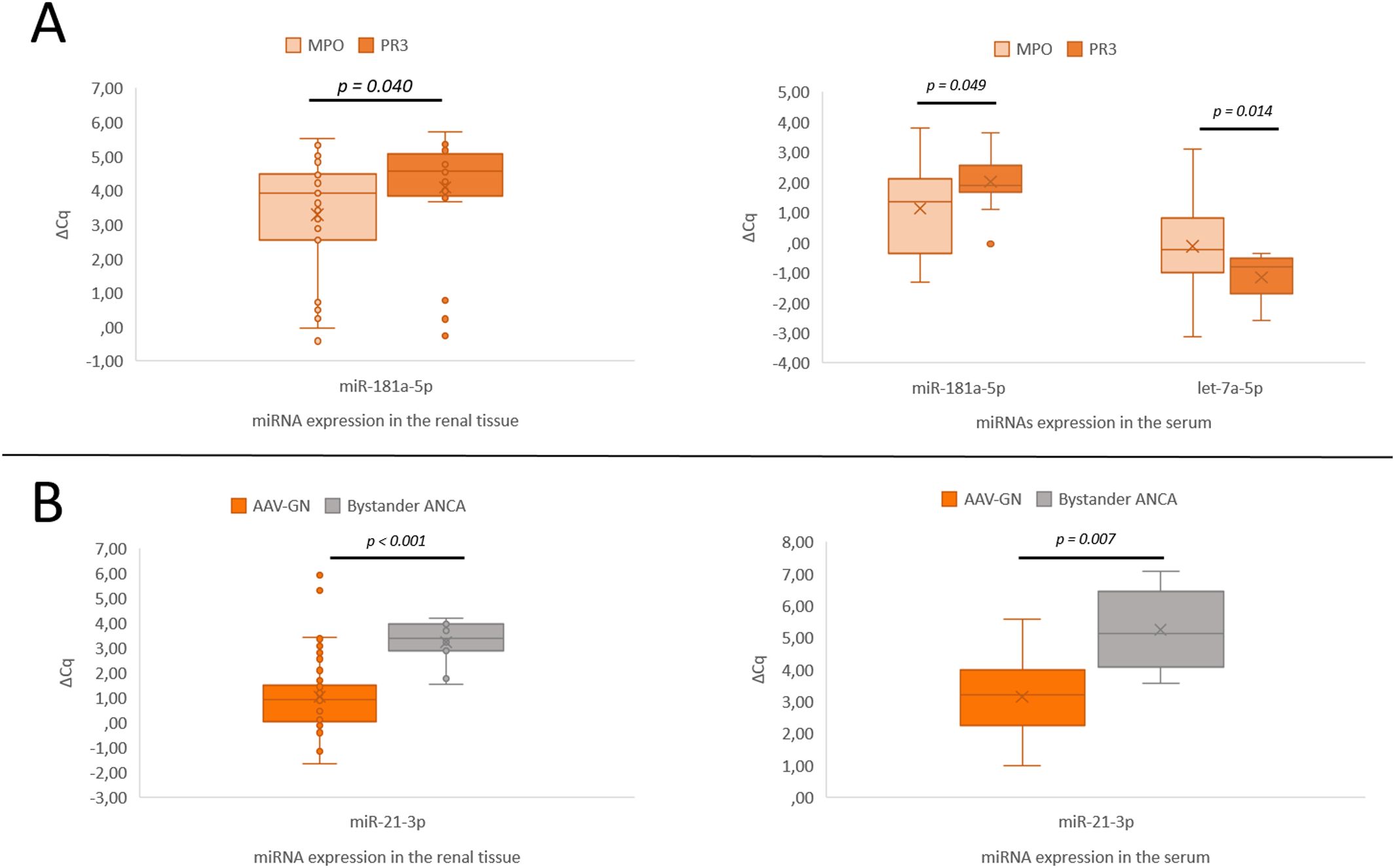
Figure 3. Significant expression differences of miRNAs in both renal tissue and serum samples. (A) Differential expression of miR-181a-5p between MPO- and PR3-positive AAV-GN. (B) Differential expression of miR-21-3p in AAV-GN compared to bystander ANCA in renal tissue and serum samples. AAV-GN, patients with ANCA-associated glomerulonephritis (n = 57 for tissue, n = 38 for serum); ANCA, anti-neutrophil cytoplasmic antibodies; MPO, MPO-positive AAV-GN (n = 33 for tissue, n = 23 for serum); PR3, PR3-positive AAV-GN (n = 24 for tissue, n = 15 for serum); bystander ANCA, cases with positive MPO- or PR3-ANCA without evidence of AAV-GN (n = 11 for tissue, n = 6 for serum); ΔCq, delta quantitation cycle. All values were within 95% confidence interval.
3.2.3 Bystander ANCA versus AAV-GN and non-AAV RD groups
Seven candidate miRNAs, namely miR-21-3p (p < 0.001), miR-30d-5p (p = 0.001), miR-30e-5p (p = 0.049), miR-181a-2-3p (p = 0.016), miR-181b-5p (p = 0.024), miR-181d-5p (p < 0.001), and let-7a-5p (p = 0.036) differentiated bystander ANCA from AAV-GN and five miRNAs, namely miR-30b-5p (p = 0.005), miR-30d-5p (p = 0.017), miR-30e-5p (p = 0.005), miR-142-5p (p = 0.039), and miR-181a-5p (p = 0.011) differentiated bystander ANCA from non-AAV RD samples. The expression profile of validated miRNAs in bystander ANCA samples is summarized in Figure 2 (for miRNAs without subsequent validation in serum samples) and Figure 3B (for miR-21-3p, which translated to serum samples).
3.3 Expression analysis of validated miRNAs in the serum samples
Serum-based analysis included 11 candidate miRNAs with significant expression differences in the renal tissue between AAV-GN and non-AAV RD, MPO- and PR3-positive AAV-GN, and/or AAV-GN and bystander ANCA samples (validated miRNAs). The expression of validated miRNAs (miR-21-3p, miR-30b-5p, miR-30d-5p, miR-30e-5p, miR-142-5p, miR-150-5p, miR-181a-5p, miR-181a-2-3p, miR-181b-5p, miR-181d-5p and let-7a-5p) was comparatively analyzed in the serum samples of AAV, non-AAV RD, bystander ANCA and HC groups.
3.3.1 AAV-GN versus non-AAV RD, and HC group
Five validated miRNAs, namely miR-21-3p (p < 0.001), miR-142-5p (p = 0.05), miR-150-5p (p = 0.012), miR-181a-5p (p = 0.002), and miR-181d-5p (p = 0.003) differentiated AAV-GN from non-AAV RD on serum samples. When AAV-GN was compared to HC group, the differential expressions of six validated miRNAs, namely miR-21-3p (p < 0.001), miR-30d-5p (p = 0.004), mir-181a-5p (p < 0.001), mir-181a-2-3p (p = 0.030), miR-181d-5p (p = 0.026), and let-7a-5p (p = 0.012) were significant. Conversely, when non-AAV RD group was compared to HC, the expression profile of validated miRNAs was broadly similar, except for miR-30b-5p (p < 0.001), miR-142-5p (p < 0.001), miR-181b-5p (p = 0.039) and let-7a-5p (p = 0.015).
Figure 4 summarizes the results of serum-based expression of validated miRNAs in AAV-GN compared to non-AAV RD and HC groups in box plot form.
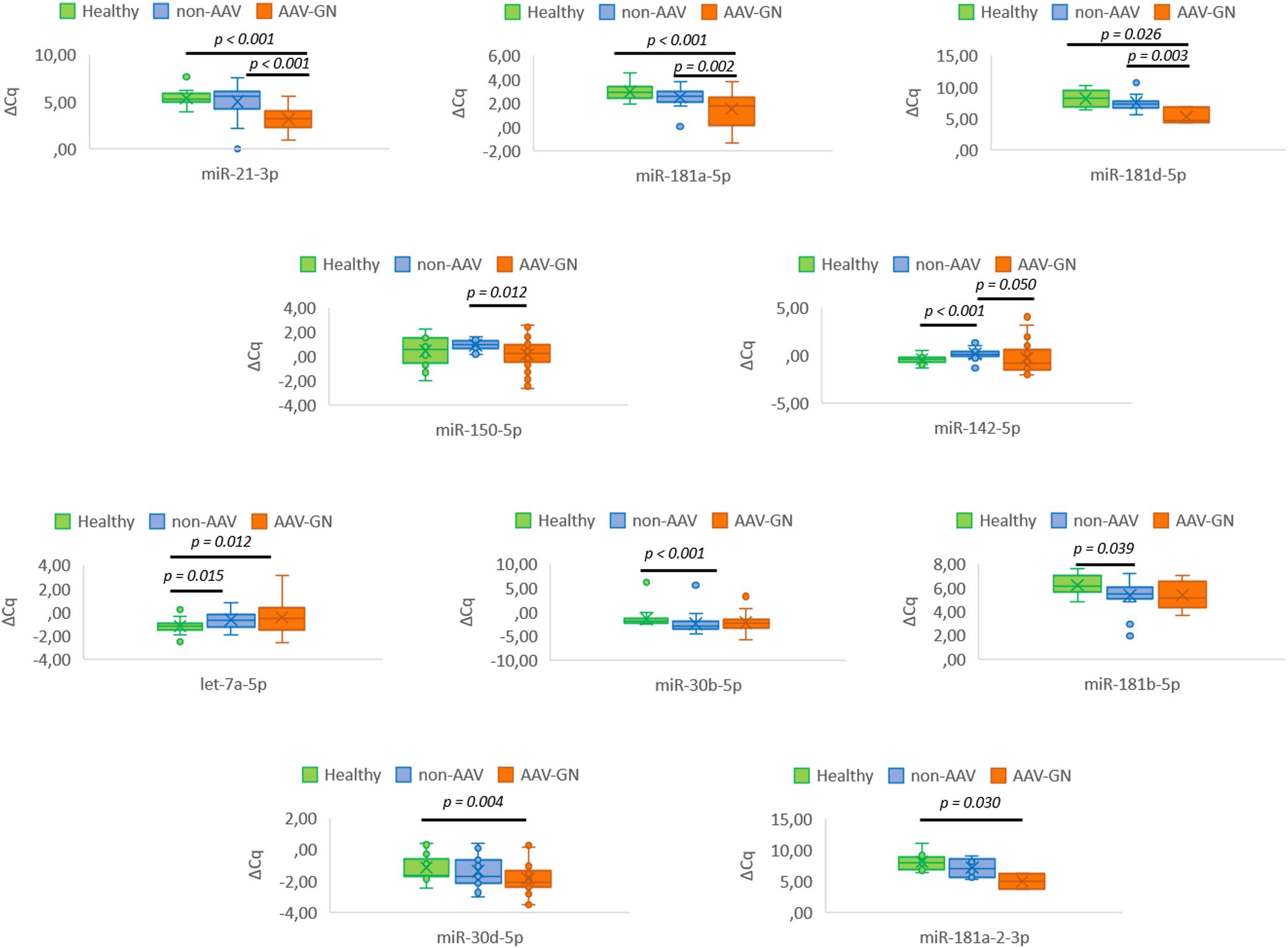
Figure 4. Serum-based differential expression of tissue-validated miRNAs in AAV-GN compared to non-AAV and HC groups. AAV-GN, patients with ANCA-associated glomerulonephritis (n = 38); ANCA, anti-neutrophil cytoplasmic antibodies; non-AAV, non-AAV medical renal disease controls (n = 23); Healthy, healthy serologic controls (n = 20); ΔCq, delta quantitation cycle. All values were within 95% confidence interval.
3.3.2 MPO- versus PR3-positive AAV-GN
On serum samples, significant expression difference was maintained for miR-181a-5p (p = 0.049), while significant difference in let-7a-5p (p = 0.014) expression was additionally observed in contrast to renal-tissue based analysis between MPO- and PR3-positive AAV-GN (refer to Figure 3A for summarized results).
3.3.3 Bystander ANCA versus AAV-GN and non-AAV RD groups
Significant expression difference between bystander ANCA and AAV-GN samples was reiterated in the serum samples for miR-21-3p (p = 0.007, Figure 3B). None of the significant miRNA expression differences observed in the renal tissue translated to serum samples in comparisons between bystander ANCA and non-AAV RD samples.
3.4 Correlation of renal tissue and serum expression of validated miRNAs
Significant correlations were detected in miR-21-3p (ρ = 0.491, p < 0.001), miR-181a-5p (ρ = -0.292, p = 0.020), and miR-181d-5p (ρ = 0.540, p = 0.008) expressions in corresponding renal tissue and serum samples (see Figure 5 for summarized results). The differential expressions of some miRNAs separating AAV-GN from non-AAV RD and HC (miR-21-3p, miR-181a-5p, and miR-181d-5p) or distinguishing AAV-GN from bystander ANCA samples (miR-21-3p) in the serum were thus deducible from the expression differences established in the renal tissue and vice versa.
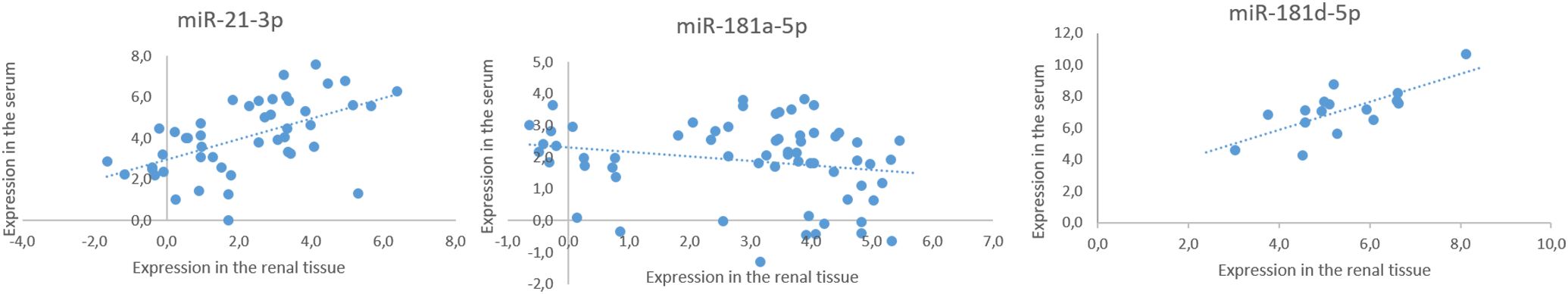
Figure 5. Expression correlation of validated miRNAs in matched renal tissue and serum samples. All values were within 95% confidence interval.
3.5 The ROC curve analysis
We conducted ROC curve analyses using miR-21-3p and miR-181a-5p as the most promising validated serum-expressed miRNAs. Although promising, miR-181d-5p was excluded from the model due to reliability concerns, specifically due to an insufficient rate of detectable serum expression in the analyzed samples. The combination of miR-21-3p and miR-181a-5p still effectively distinguished AAV-GN from the other independent groups. The combined ROC model using both miR-21-3p and miR-181a-5p is illustrated in Figure 6. ROC models using individual miRNAs and AUC values are presented in Supplementary Figure S2 and Supplementary Results, respectively.
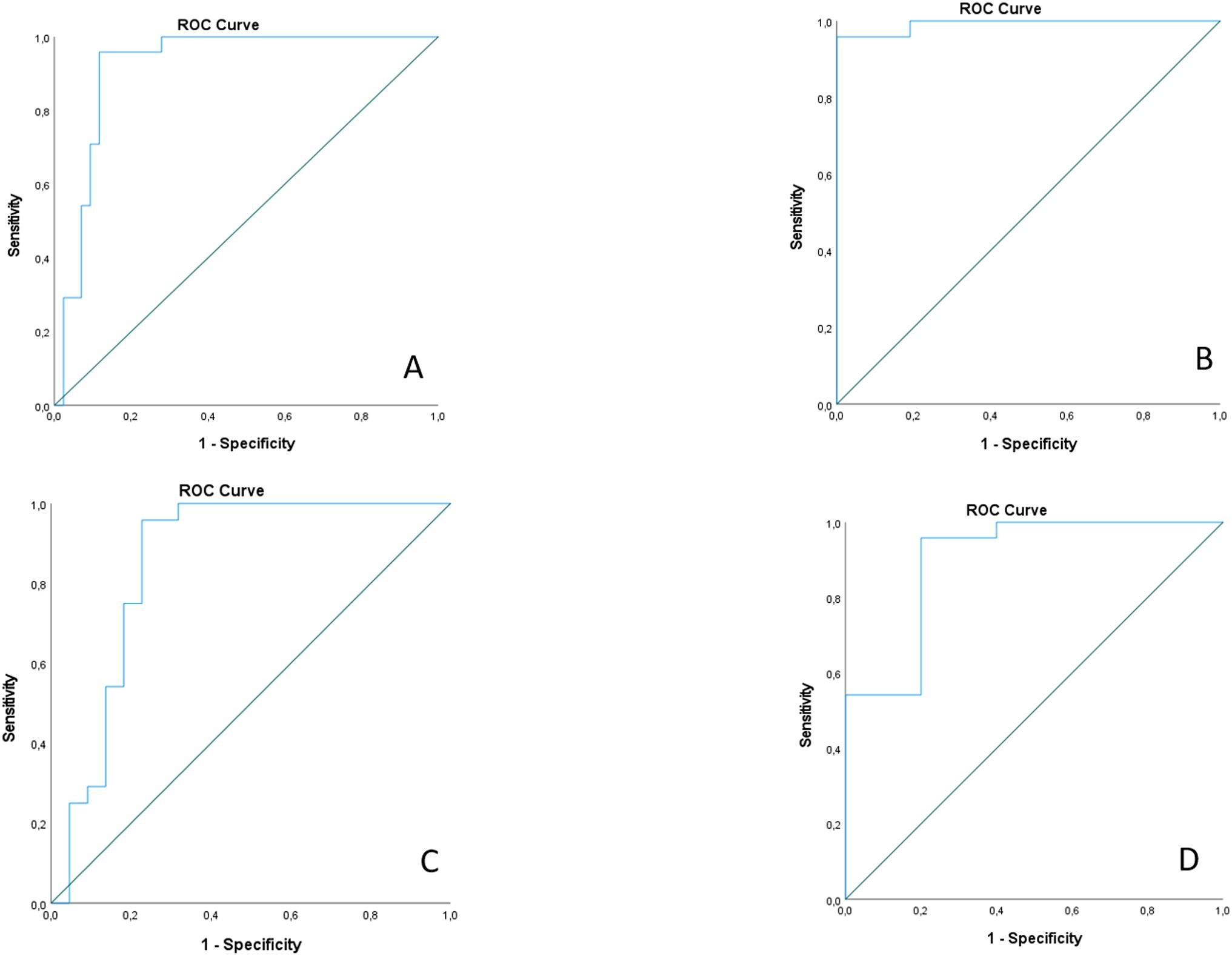
Figure 6. ROC curve analysis for combined miR-21-3p and miR-181a-5p serum expressions; (A) ROC curve for differentiation between AAV-GN and all other samples; (B) ROC curve for differentiation between AAV-GN and healthy controls; (C) ROC curve for differentiation between AAV-GN and non-AAV RD; (D) ROC curve for differentiation between AAV-GN and bystander ANCA. AAV-GN, patients with ANCA-associated glomerulonephritis (n = 38); ANCA, anti-neutrophil cytoplasmic antibodies; non-AAV, non-AAV medical renal disease controls (n = 23). All values were within 95% confidence interval.
3.6 Correlations and associations of validated miRNAs with established scoring systems for renal involvement in AAV-GN and clinical variables of disease activity
The increasing AKRiS correlated with the increasing renal tissue expression of miR-142-5p, miR-150-5p and miR-181d-5p. When stratified into AKRiS risk groups, similar correlations were observed with the addition of correlation with renal tissue expression of miR-21-3p. Comparably, increasing Berden class was associated with increasing renal tissue expressions of miR-150-5p and miR-181d-5p. The renal tissue expression of miR-21-3p, miR-142-5p and miR-150-5p correlated to %NG, and miR-181d-5p correlated to %C. No correlations between any validated miRNA expressions and individual histologic variables or established scoring systems were identified in the serum samples except between increasing expression of miR-30b-5p and decreasing %C.
BVAS correlated with the expressions of miR-30b-5p and miR-181b-5p on renal tissue and serum samples, respectively. No validated miRNA in the renal tissue or serum samples correlated to ANCA titers. Additionally, the ANCA titers did not correlate with the BVAS (p = 0.496) or AKRiS (p = 0.262) and did not associate with the Berden class (p = 0.371).
Renal tissue expression of miR-21-3p (p = 0.035) and miR-142-5p (p = 0.022) differentiated cases presenting as RPGN. No significant miRNA expression differences in the renal tissue or serum were identified comparing samples of AAV-GN with renal-limited phenotype (RLV) to those with multiorgan involvement. Conversely, increasing expression of miR-30b-5p in the renal tissue associated with the increasing number of affected organs and the expression of miR-30d-5p in the serum (p = 0.003) differentiated cases with pulmonary-renal syndrome. Pre-emptive immunosuppression did not have any effect on the characteristic miRNA signature within the AAV-GN group.
Correlations of validated miRNAs with scoring systems and clinical variables of disease activity in renal tissue and serum samples are presented in Table 3. Associations and correlations of the validated miRNAs to established scoring systems for renal involvement in AAV-GN are further depicted in Figure 7. All correlations with biomarkers of renal function and histologic variables of chronicity are described in Supplementary Results and Supplementary Table S2 including calculation of statistical power and estimation of needed sample size.
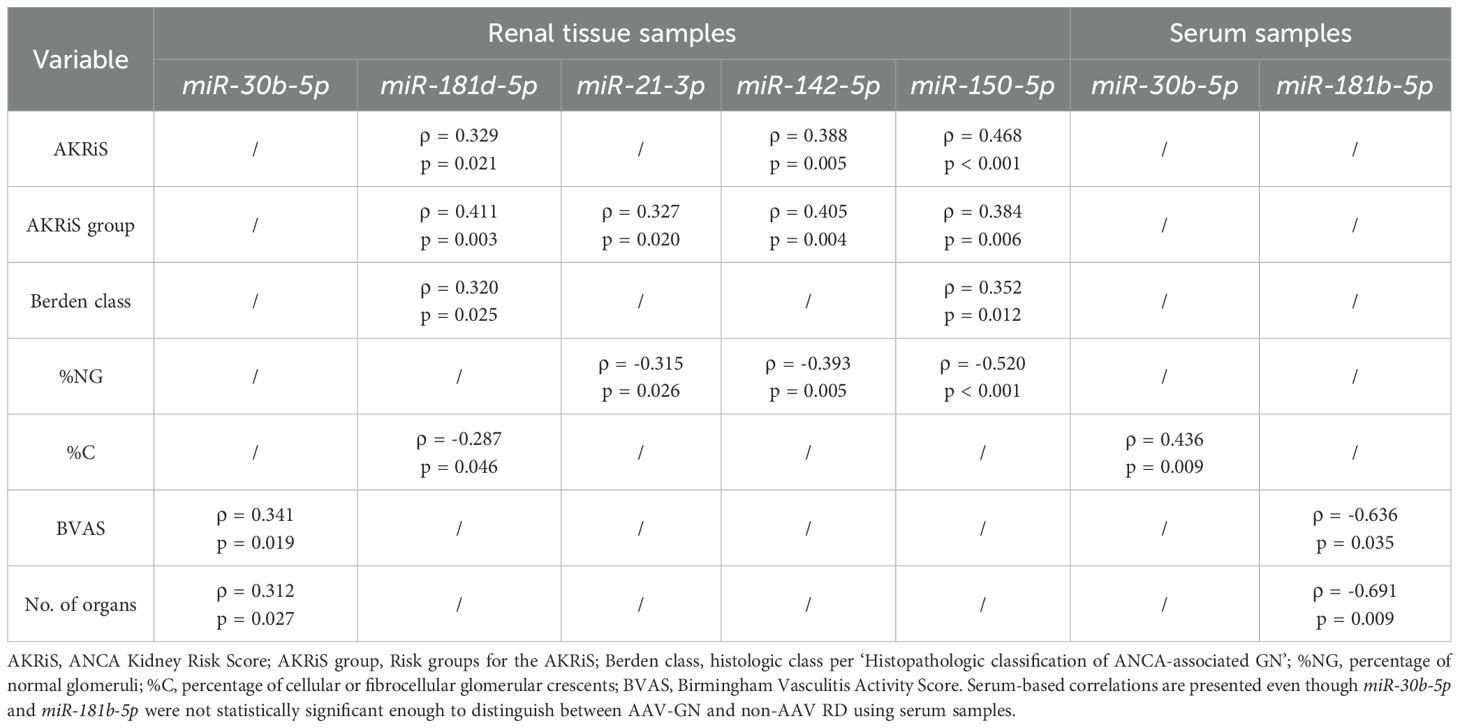
Table 3. Correlations and associations between the expression of validated miRNAs and scoring systems for renal involvement and clinical variables of disease activity in AAV-GN.
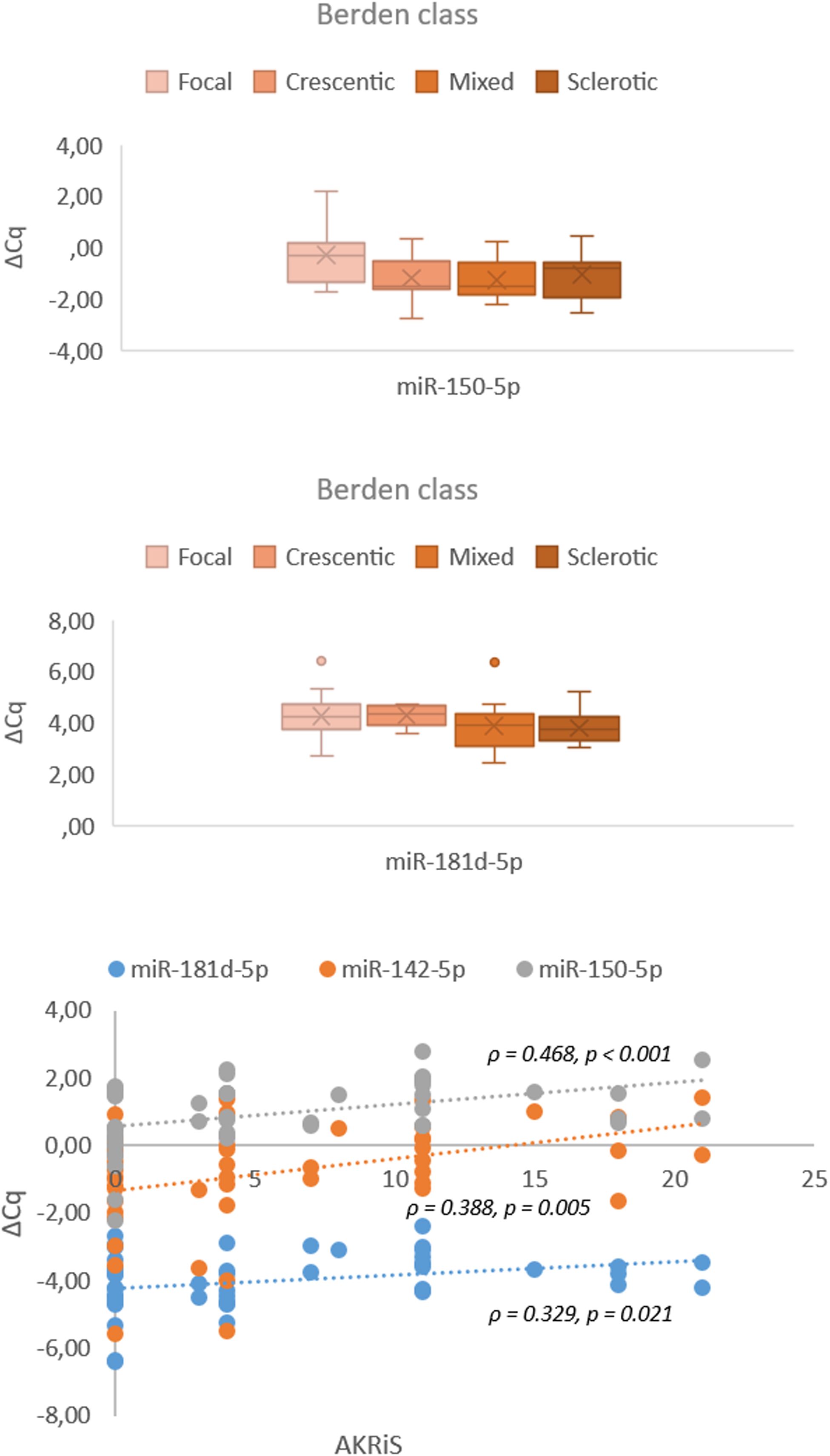
Figure 7. Associations and correlations of validated miRNAs in renal tissue samples to Berden class and AKRiS (n = 57). Berden class, histologic class per ‘Histopathologic classification of AAV-related GN’; AKRiS, ANCA Kidney Risk Score; ΔCq, delta quantitation cycle. All values were within 95% confidence interval.
4 Discussion
Our study validated significant renal tissue expression differences in 11 out of 21 candidate miRNAs selected through screening. This suggests that miRNA expression profiling in renal tissue can effectively distinguish AAV-GN from various non-AAV RD, including cases with detectable ANCA without clinical and histologic evidence of AAV-GN. Although tissue-based analysis provides valuable insights, it requires a renal biopsy to obtain sample material, which limits its applicability. Therefore, we also analyzed the expression of validated miRNAs in serum samples. Despite the inherent limitations of biofluid-based miRNA analysis (14), five validated miRNAs distinguished AAV-GN from non-AAV RD using matched serum samples. Notably, three of these five miRNAs also differentiated AAV-GN from both non-AAV RD and HC, consistent with a circulating miRNA expression profile specific to AAV. Additionally, miR-181a-5p also stratified AAV-GN into MPO- and PR3-positive.
Noteworthy observations apply to ‘bystander ANCA’ cases, ie. patients who underwent kidney biopsy, typically due to asymptomatic urinary abnormalities, and positive MPO- or PR3-ANCA but no histologic evidence of AAV-GN. Clinicians often struggle to distinguish between harmless (bystander) and pathogenic, disease-driving ANCAs in ANCA-positive patients. A reliable biomarker would be invaluable for guiding clinical decision-making in such cases. Comparative miRNA expression profiling in the renal tissue and serum revealed that bystander ANCA cases clustered separately from AAV-GN and non-AAV RD. While overall more aligned with non-AAV RD, bystander ANCA samples exhibited miR-142-5p, miR-150-5p, and miR-181a-5p expression patterns indistinguishable from AAV, suggesting a partial AAV-like epigenetic profile. This finding bears potential clinical implications as it raises questions about the ‘bystander’ nature of ANCAs in cases lacking clinical or histologic evidence of AAV-GN. Notably, miR-21-3p expression effectively differentiated AAV-GN from bystander ANCA cases in renal tissue or serum samples, highlighting its potential as a key biomarker in resolving this diagnostic uncertainty.
To refine the validated miRNA signature, the molecular data was correlated with established scoring systems for renal involvement in AAV-GN In the renal tissue, miR-142-5p, miR-150-5p and miR-181d-5p correlated with the AKRiS, while miR-150-5p and miR-181d-5p associated with the Berden classes. Accordingly, renal tissue expressions of miR-142-5p and miR-150-5p also correlated with %NG, supporting the importance of %NG in estimating risk in AAV-GN and corroborates the need for early diagnosis of kidney involvement in AAV (15–17).
Furthermore, the miRNA signature was analyzed in the context of clinical variables of disease activity. Notably, renal tissue expression of miR-21-3p and miR-142-5p differentiated AAV-GN cases presenting as RPGN, while the expression of miR-30b-5p in the renal tissue and the expression of miR-181b-5p in the serum associated with the increasing number of affected organs. Accordingly, BVAS correlated with the expressions of miR-30b-5p and miR-181b-5p on renal tissue and serum samples, respectively. Additionally, the expression of miR-30d-5p in the serum differentiated cases presenting with pulmonary-renal syndrome. Interestingly, no significant miRNA expression differences in the renal tissue or serum were identified comparing samples of AAV-GN labelled as RLV to those with multiorgan involvement.
Importantly, we could not directly translate any individual finding observed between variables of disease activity and validated miRNAs in the renal tissue to serum samples. This may be due to inadequate size of technically adequate serum samples, a lack of expression correlation between matched renal tissue and serum samples, or to expression levels of certain miRNAs in the serum falling below the detection threshold. Furthermore, some miRNAs may preferentially be expressed and thus detectable only in renal tissue. Conversely, aggregated miRNA expression in serum may also reflect influences from related or unrelated conditions in other organs or homeostatic mechanisms.
We would like to highlight miR-21-3p, miR-181a-5p and miR-181d-5p as the three most promising serum-expressed miRNAs. These miRNAs effectively differentiated AAV-GN from both non-AAV RD and HC cases. Moreover, the expression of miR-21-3p and miR-181d-5p differentiated the presence of RPGN and correlated with AKRiS in renal tissue samples, respectively, while miR-181a-5p differentiated MPO- from PR3-positive AAV-GN in renal tissue and serum samples. Furthermore, miR-21-3p distinguished AAV-GN from bystander ANCA cases in renal tissue and serum samples.
Importantly, miR-21-3p, miR-181a-5p and miR-181d-5p were the only validated miRNAs showing significant expression correlation between matched renal tissue and serum samples, underscoring their potential as robust noninvasive biomarkers for active AAV-GN. Additionally, ROC curve analyses demonstrated that combining serum levels of miR-21-3p and miR-181a-5p yielded a strong ability to differentiate AAV-GN from other groups. Beyond statistical analyses, functional annotations of miR-21-3p, miR-181a-5p and miR-181d-5p to biological processes implicated in AAV pathogenesis support their relevance. Namely, miR-21-3p and miR-181a-5p are implicated in altered macrophage polarization (6, 18). Indeed, alternatively activated (i.e., M2-polarized) macrophages are considered the dominant macrophage phenotype in AAV and are associated with a higher end-stage renal disease risk in AAV (4, 19).
miR-181a-5p also regulates the processes of B- and T-cell differentiation, namely the differentiation towards pathogenic autoantigen specific Th17 subsets in CD4+ T-cells and is involved in T-cell tolerance and selection stringency (7, 20). These processes are implicated in several autoimmune diseases, including AAV (5, 21, 22). Similarly, miR-21-3p overexpression promotes Th17 differentiation and activates the IL-23/IL-17 axis through MAP3K14, exacerbating inflammation in a psoriasis model (23). Elevated IL-23 and IL-17 levels were also observed in AAV (5). Interestingly, increased IL-17 levels in the context of Th17-predominant inflammation skew macrophage polarization toward the M2 phenotype (24). These insights not only align with established aspects of AAV pathogenesis but also suggest potential therapeutic implications, either by miRNA ago-/antagomirs and sponges, or by targeting implicated biologic processes with existent modalities, such as anti-IL23/IL-17 agents or abatacept (CTLA4-Ig).
It would be valuable to explore the exact function and target genes of miR-21-3p, miR-181a-5p and miR-181d-5p as the three most promising miRNAs. This can be achieved by experimentally validating the target genes of these miRNAs in appropriate cell line models. A commonly employed method involves cloning the 3’-untranslated region (3’-UTR) of the predicted miRNA target gene into a luciferase reporter construct and analyzing the change in luciferase expression as evidence of binding and regulatory activity of a particular miRNA. One such approach involves transfecting a cell line that expresses the miRNA of interest with a reporter containing either the wild-type UTR of a target gene or the UTR in which the miRNA binding site is mutated. If the construct containing the wild-type UTR shows a reduction in luciferase expression and this decrease is absent in the mutated version, this likely indicates that the endogenously expressed miRNA is capable of regulating that UTR. In this same setup, a miRNA inhibitor to block miRNA function could be used, which should lead to an increase in luciferase. Another approach is to transfect cells with both the luciferase reporter construct and increase amounts of the miRNA of interest. The advantage of introducing a miRNA is that if the miRNA really targets the binding site in the UTR, a dose-dependent effect on luciferase readout can be determined, with this response being absent with the mutated construct (25).
Several important study limitations should be acknowledged. The inherent variability of biofluid-based miRNA analysis, influenced by low isolation yields, expression variability, and the lack of standardized normalization methods, remains a challenge. This issue could be addressed by adjusting the sample volume or protocol (i.e., employing a more sensitive detection method, such as digital PCR). Additionally, the heterogeneity of renal tissue may lead to the omission or misidentification of compartment- or cell-specific miRNAs, which could be mitigated by spatial transcriptomics. Furthermore, the proposed miRNA signature relates specifically to AAV-GN and our findings may not apply to all AAV patients without renal involvement or ANCA-negative patients. Our study was also limited by small size of technically adequate samples (especially for bystander ANCA group), resulting in limited statistical power, incomplete correlation between renal tissue and serum miRNA expression for some miRNAs, and the exclusion of miR-181d-5p from the ROC curve analysis. Therefore, an independent validation in larger multicenter cohorts, using an alternative platform such as next-generation sequencing to reduce platform-dependent bias, is essential to confirm the proposed AAV-specific miRNA signature.
In conclusion, this study establishes miR-21-3p, miR-181a-5p, and miR-181d-5p as constituents of a circulating miRNA signature related to AAV-GN, providing a potential noninvasive biomarker for active AAV-GN in the appropriate clinical setting. By integrating renal tissue and serum miRNA expression profiles, we demonstrate that these miRNAs might effectively differentiate AAV-GN from non-AAV RD and correlate with disease activity markers. Future research, ideally as a multi-center study to increase sample sizes, should focus on refining presented findings to establish the role of identified miRNAs in the pathogenesis of AAV-GN and support the clinical application of miRNA-based biomarkers in AAV-GN diagnostics and disease monitoring.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by National Medical Ethics Committee (Republic of Slovenia, Ministry of Health), approval number 0120-102/2020/6. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MB: Conceptualization, Writing – original draft, Investigation. EB: Software, Formal analysis, Data curation, Writing – original draft, Writing – review & editing, Methodology, Visualization, Investigation, Supervision. ŽV: Data curation, Supervision, Investigation, Writing – review & editing, Conceptualization. JT: Data curation, Investigation, Formal Analysis, Writing – review & editing. ŽP: Writing – original draft, Methodology, Formal analysis, Investigation. NK: Supervision, Funding acquisition, Conceptualization, Writing – review & editing, Project administration, Resources, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Slovenian Research and Innovation Agency Program P3-0054 (Pathology and Molecular Genetics) funded this research. The funding agency had no role in the study design, data analysis, reporting and/or decision to submit the manuscript for publication.
Acknowledgments
We would like to thank the consultants from the Departments of Nephrology and Rheumatology, University Medical Centre Ljubljana and from the Department of Nephrology, University Medical Centre Maribor for their devotion to optimal patient management and care. We would like to extend our gratitude to our colleague, Maja Frelih, MD, for her contributions in providing renal biopsy services, as well as to the technicians at the Institute of Pathology, Faculty of Medicine, University of Ljubljana, for their meticulous pre-analytical laboratory work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1599043/full#supplementary-material
References
1. Moiseev S, Cohen Tervaert JW, Arimura Y, Bogdanos DP, Csernok E, Damoiseaux J, et al. 2020 international consensus on ANCA testing beyond systemic vasculitis. Autoimmun Rev. (2020) 19:102618. doi: 10.1016/j.autrev.2020.102618
2. Hilhorst M, van Paassen P, and Tervaert JWC. Limburg Renal Registry. Proteinase 3-ANCA vasculitis versus myeloperoxidase-ANCA vasculitis. J Am Soc Nephrol. (2015) 26:2314–27. doi: 10.1681/ASN.2014090903
3. Cohen Tervaert JW. Should proteinase-3 and myeloperoxidase anti-neutrophil cytoplasmic antibody vasculitis be treated differently: part 2. Nephrol Dial Transplant. (2019) 34:384–7. doi: 10.1093/ndt/gfy406
4. Vegting Y, Vogt L, Anders H-J, de Winther MPJ, Bemelman FJ, and Hilhorst ML. Monocytes and macrophages in ANCA-associated vasculitis. Autoimmun Rev. (2021) 20:102911. doi: 10.1016/j.autrev.2021.102911
5. Nogueira E, Hamour S, Sawant D, Henderson S, Mansfield N, Chavele K-M, et al. Serum IL-17 and IL-23 levels and autoantigen-specific Th17 cells are elevated in patients with ANCA-associated vasculitis. Nephrol Dial Transplant. (2010) 25:2209–17. doi: 10.1093/ndt/gfp783
6. Huang Y, Huang Y, Cai Z, Ferrari MW, Li C, Zhang T, et al. MiR-21-3p inhibitor exerts myocardial protective effects by altering macrophage polarization state and reducing excessive mitophagy. Commun Biol. (2024) 7:1371. doi: 10.1038/s42003-024-07050-3
7. Schaffert SA, Loh C, Wang S, Arnold CP, Axtell RC, Newell EW, et al. Mir-181a-1/b-1 modulates tolerance through opposing activities in selection and peripheral T cell function. J Immunol. (2015) 195:1470–9. doi: 10.4049/jimmunol.1401587
8. Pauley KM, Cha S, and Chan EKL. MicroRNA in autoimmunity and autoimmune diseases. J Autoimmun. (2009) 32:189–94. doi: 10.1016/j.jaut.2009.02.012
9. Bošnjak M, Večerić-Haler Ž, Boštjančič E, and Kojc N. Renal tissue miRNA expression profiles in ANCA-associated vasculitis-A comparative analysis. Int J Mol Sci. (2021) 23:105. doi: 10.3390/ijms23010105
10. Mukhtyar C, Lee R, Brown D, Carruthers D, Dasgupta B, Dubey S, et al. Modification and validation of the birmingham vasculitis activity score (version 3). Ann Rheum Dis. (2009) 68:1827–32. doi: 10.1136/ard.2008.101279
11. Berden AE, Ferrario F, Hagen EC, Jayne DR, Jennette JC, Joh K, et al. Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol. (2010) 21:1628–36. doi: 10.1681/ASN.2010050477
12. Bate S, McGovern D, Costigliolo F, Tan PG, Kratky V, Scott J, et al. The improved kidney risk score in ANCA-associated vasculitis for clinical practice and trials. J Am Soc Nephrol. (2024) 35:335–46. doi: 10.1681/ASN.0000000000000274
13. Latham GJ. Normalization of microRNA quantitative RT-PCR data in reduced scale experimental designs. Methods Mol Biol. (2010) 667:19–31. doi: 10.1007/978-1-60761-811-9_2
14. Connor KL and Denby L. MicroRNAs as non-invasive biomarkers of renal disease. Nephrol Dial Transplant. (2021) 36:428–9. doi: 10.1093/ndt/gfz183
15. Brix SR, Noriega M, Tennstedt P, Vettorazzi E, Busch M, Nitschke M, et al. Development and validation of a renal risk score in ANCA-associated glomerulonephritis. Kidney Int. (2018) 94:1177–88. doi: 10.1016/j.kint.2018.07.020
16. Sachez-Alamo B, Moi L, Bajema I, Berden A, Flossmann O, Hruskova Z, et al. Long-term outcome of kidney function in patients with ANCA-associated vasculitis. Nephrol Dial Transplant. (2024) 39:1483–93. doi: 10.1093/ndt/gfae018
17. Villacorta J, Diaz-Crespo F, Guerrero C, Acevedo M, Cavero T, and Fernandez-Juarez G. Long-term validation of the renal risk score for vasculitis in a Southern European population. Clin Kidney J. (2021) 14:220–5. doi: 10.1093/ckj/sfaa073
18. Jiang M, Dai J, Yin M, Jiang C, Ren M, and Tian L. LncRNA MEG8 sponging miR-181a-5p contributes to M1 macrophage polarization by regulating SHP2 expression in Henoch-Schonlein purpura rats. Ann Med. (2021) 53:1576–88. doi: 10.1080/07853890.2021.1969033
19. Bitton L, Vandenbussche C, Wayolle N, Gibier J-B, Cordonnier C, Verine J, et al. Tubulointerstitial damage and interstitial immune cell phenotypes are useful predictors for renal survival and relapse in antineutrophil cytoplasmic antibody-associated vasculitis. J Nephrol. (2020) 33:771–81. doi: 10.1007/s40620-019-00695-y
20. Sun X, Sit A, and Feinberg MW. Role of miR-181 family in regulating vascular inflammation and immunity. Trends Cardiovasc Med. (2014) 24:105–12. doi: 10.1016/j.tcm.2013.09.002
21. Pfeifle R, Rothe T, Ipseiz N, Scherer HU, Culemann S, Harre U, et al. Regulation of autoantibody activity by the IL-23-TH17 axis determines the onset of autoimmune disease. Nat Immunol. (2017) 18:104–13. doi: 10.1038/ni.3579
22. Krebs CF and Panzer U. Plasticity and heterogeneity of Th17 in immune-mediated kidney diseases. J Autoimmun. (2018) 87:61–8. doi: 10.1016/j.jaut.2017.12.005
23. Abdallah F, Henriet E, Suet A, Arar A, Clemençon R, Malinge J-M, et al. MiR-21-3p/IL-22 axes are major drivers of psoriasis pathogenesis by modulating keratinocytes proliferation-survival balance and inflammatory response. Cells. (2021) 10:2547. doi: 10.3390/cells10102547
24. Zizzo G and Cohen PL. IL-17 stimulates differentiation of human anti-inflammatory macrophages and phagocytosis of apoptotic neutrophils in response to IL-10 and glucocorticoids. J Immunol. (2013) 190:5237–46. doi: 10.4049/jimmunol.1203017
Keywords: ANCA, biomarker, glomerulonephritis, microRNA, vasculitis, epigenetics
Citation: Bošnjak M, Večerić-Haler Ž, Pipan Tkalec Ž, Tomšič J, Boštjančič E and Kojc N (2025) Circulating miRNAs as potential non-invasive biomarkers for ANCA-associated glomerulonephritis. Front. Immunol. 16:1599043. doi: 10.3389/fimmu.2025.1599043
Received: 24 March 2025; Accepted: 26 June 2025;
Published: 17 July 2025.
Edited by:
Chris Wincup, King’s College Hospital NHS Foundation Trust, United KingdomReviewed by:
Chaoqun Feng, Hospital of Chengdu University of Traditional Chinese Medicine, ChinaMarlon J. Sandino-Bermúdez, Mayo Clinic, United States
Copyright © 2025 Bošnjak, Večerić-Haler, Pipan Tkalec, Tomšič, Boštjančič and Kojc. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nika Kojc, bmlrYS5rb2pjQG1mLnVuaS1sai5zaQ==
 Matic Bošnjak
Matic Bošnjak Željka Večerić-Haler
Željka Večerić-Haler Živa Pipan Tkalec1
Živa Pipan Tkalec1 Emanuela Boštjančič
Emanuela Boštjančič Nika Kojc
Nika Kojc