- 1Department of Thoracic Surgery, Fujian Medical University Union Hospital, Fuzhou, China
- 2Key Laboratory of Cardio-Thoracic Surgery (Fujian Medical University), Fujian Province University, Fuzhou, China
- 3Key Laboratory of Ministry of Education for Gastrointestinal Cancer, Fujian Medical University, Fuzhou, China
- 4Fujian Key Laboratory of Tumor Microbiology, Fujian Medical University, Fuzhou, China
- 5Department of Cardiothoracic Surgery, The Affiliated Hospital of Putian University, Putian, China
- 6Department of Thoracic Surgery, Quanzhou First Hospital, Quanzhou, China
- 7Department of Thoracic Surgery, Gaozhou People’s Hospital, Gaozhou, Guangdong, China
- 8Department of Thoracic Surgery, Zhongshan Hospital of Xiamen University, School of Medicine, Xiamen University, Xiamen, China
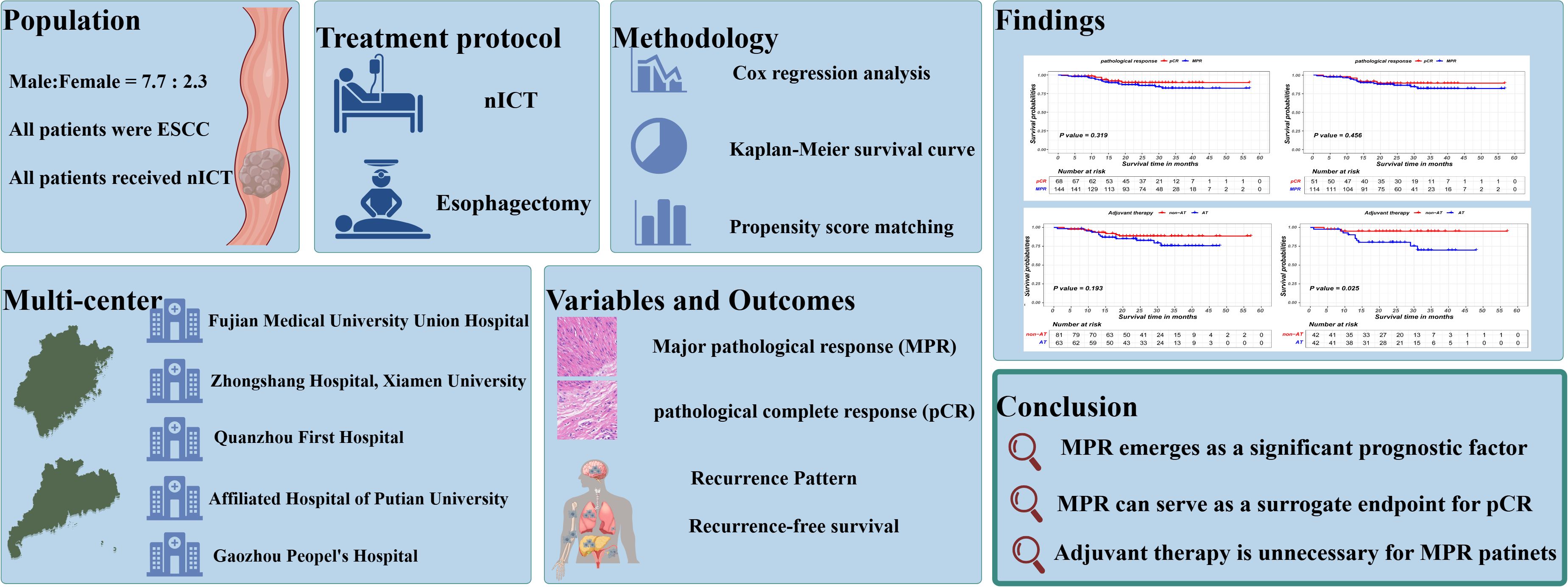
Background: For esophageal squamous cell carcinoma(ESCC), neoadjuvant chemoimmunotherapy (nICT) constitutes an innovative therapeutic strategy. However, The relationship between its short-term efficacy and long-term prognosis requires further clarification. Therefore, this study aims to evaluate the prognostic significance of major pathological response (MPR) in ESCC patients receiving nICT.
Method: This is a retrospective multi-center study enrolling 306 ESCC patients undergoing nICT. The primary endpoints were recurrence-free survival (RFS) and recurrence patterns. Propensity score matching (PSM) was applied to address heterogeneity between groups. Kaplan-Meier curves and Cox regression analysis were utilized to analyze survival difference.
Results: 144 achieved a MPR, while 68 achieved a pathological complete response (pCR). Cox regression analysis identified MPR as an independent prognostic factor [HR = 0.48, 95%CI= (0.28 - 0.82), P = 0.007]. Survival analysis demonstrated that MPR patients experienced significantly improved RFS compared to non-MPR patients, before (P<0.001) and after PSM (P = 0.016). Importantly, the RFS of MPR patients was comparable to that of pCR patients (P = 0.319 in the unmatched cohort; P = 0.456 in the matched cohort). Furthermore, adjuvant therapy did not provide additional recurrence-free benefits for MPR patients. Compared to pCR patients, MPR patients exhibited a similar recurrence rate, with similar recurrence sites.
Conclusion: MPR represents a significant prognostic indicator in ESCC patients undergoing nICT, demonstrating prognostic outcomes comparable to those of pCR. These findings indicated that MPR could function as a surrogate endpoint for pCR, potentially influencing treatment strategies by refining follow-up protocol and the implementation of adjuvant therapy.
1 Introduction
Esophageal cancer (EC) is the seventh most prevalent malignant neoplasm worldwide and is the sixth leading cause of cancer-related mortality (1). A significant proportion of patients with esophageal squamous cell carcinoma (ESCC) are initially diagnosed at a locally advanced stage. To improve prognosis, the standard treatment approach for these patients entails the administration of neoadjuvant therapy followed by surgical intervention.
Extensive clinical evidence supported the recommendation of neoadjuvant chemotherapy (nCT) and neoadjuvant chemoradiotherapy (nCRT) as standard treatments for locally advanced esophageal squamous cell carcinoma (LA-ESCC) patients (2, 3). With recent advancements in immunotherapy, its potential as a therapeutic strategy for LA-ESCC has garnered significant attention. The safety profile of neoadjuvant chemoimmunotherapy(nICT) is supported by evidence from multiple clinical trials (4–6), which demonstrate no significant increase in postoperative complications, such as anastomotic leakage. Furthermore, its efficacy is reinforced by elevated rates of major pathological response (MPR) and pathological complete response (pCR), both of which are critical indicators of improved treatment outcomes. In 2012, the U.S. Food and Drug Administration (FDA) recognized pCR as a surrogate endpoint for survival in studies of neoadjuvant therapy, based on the premise that pCR may serve as a predictor of clinical benefit (7). However, apart from pCR, MPR can also serve as an indicator of the sensitivity and efficacy of treatment modalities. Our prior study has demonstrated that MPR can function as a survival surrogate, providing a clinical reference and an evidence-based foundation for decision-making in ESCC patients undergoing nCT/nICT (8).
Previous research has demonstrated that EC patients treated with nCRT who exhibited microscopic residual disease faced an elevated risk of recurrence, however their overall survival paralleled that of patients achieving a pCR (9). Nevertheless, different treatment modalities have been observed to exhibit differing short-term efficacy and long-term prognoses in LA-ESCC patients (10, 11). To the best of our knowledge, there is a paucity of studies directly comparing the prognostic difference of MPR with pCR in ESCC patients undergoing nICT, underscoring the necessity for further research in this area. Therefore, this study aims to examine the prognostic value and clinical significance of MPR in ESCC patients undergoing nICT.
2 Method
2.1 Study population
This study recruited patients diagnosed with EC from five medical centers between January 1, 2019, and August 31, 2024. The inclusion criteria were (1): histologically confirmed ESCC and diagnosed as cT3-4aNanyM0 or cT1-2N+M0 (2); receipt of at least one cycle of neoadjuvant chemoimmunotherapy (3); receipt of esophagectomy following neoadjuvant chemoimmunotherapy; and (4) availability of complete clinical data. Exclusion criteria included (1): the presence of other primary malignant tumors (2); unsuitability for surgical resection; and (3) undergoing salvage or palliative surgery.
2.2 Treatment protocol
Among the five medical centers, there are slight difference in preoperative examinations and nICT protocols; however, a high degree of consistency is maintained across all centers. Pre-treatment diagnosis and clinical staging were conducted through gastroscopy, contrast-enhanced computed tomography, and neck color Doppler ultrasound. Positron emission tomography was performed when required.
Commonly, the chemotherapy regimen consisted primarily of platinum combined with paclitaxel or docetaxel, administered once every 3 weeks. On the basis of nCT, the immune drug was applied in nICT group, using one of the following five types: camrelizumab, pembrolizumab, sintilimab, tislelizumab, and toripalimab. The treatment regimen and its dosage will be established through multidisciplinary consultations and adjusted based on the patient’s physical condition, particularly in response to adverse reactions. The efficacy of the treatment should be assessed 3–4 weeks after the completion of the final neoadjuvant therapy cycle. If surgical intervention is deemed appropriate, patients will undergo a minimally invasive esophagectomy. In instances where preoperative evaluation indicates the possibility of cervical lymph node metastasis, a three-field lymphadenectomy will be conducted.
2.3 Relevant definition
The primary endpoint of this study is recurrence-free survival (RFS), which is defined as the interval from the date of surgical resection to the occurrence of locoregional recurrence or/and distant metastasis. Pathological complete response (pCR) is characterized by the absence of residual tumor cells in both the primary tumor tissue and the lymph nodes. Major pathological response (MPR) refers to the presence of 10% or fewer viable tumor cells in resected tumor (12, 13). Various scoring systems were employed to evaluate the pathological response in patients with LA-ESCC undergoing neoadjuvant therapy (14). In this study, the tumor regression grade (TRG) was utilized to assess the pathological response. In accordance with the guidelines established by the College of American Pathologists (CAP) and the National Comprehensive Cancer Network (NCCN), MPR was equated to TRG 0-1, while it corresponds to TRG 1–2 under the Mandard scoring system (15–17).
2.4 Follow-up and adjuvant therapy
The postoperative follow-up schedule for ESCC patients was typically consistent in our study. During the first two years, ESCC patients underwent routine follow-up evaluations every 3 to 6 months. From the third to the fifth year, follow-ups were performed every 6 months, and subsequently on an annual basis. Furthermore, follow-up was carried out via outpatient visits and telephone consultations in our study.
In our study, adjuvant therapy is advised for ESCC patients diagnosed with ypT0-4aN+M0. Conversely, for patients with ypT0-4aN0M0, especially those achieving a pCR, both surveillance and adjuvant therapy are deemed suitable options. Additionally, a multidisciplinary team conducted consultations to evaluate the need for adjuvant therapy, taking into account the postoperative pathological outcomes, the overall health status of the patients, and their personal treatment preferences. The adjuvant therapy regimens comprised chemotherapy (aCT), immunotherapy (aIT), or a combination of both therapies (aICT). The detail information of drugs used in nICT and AT was shown in Supplementary Table 1.
2.5 Statistical analysis
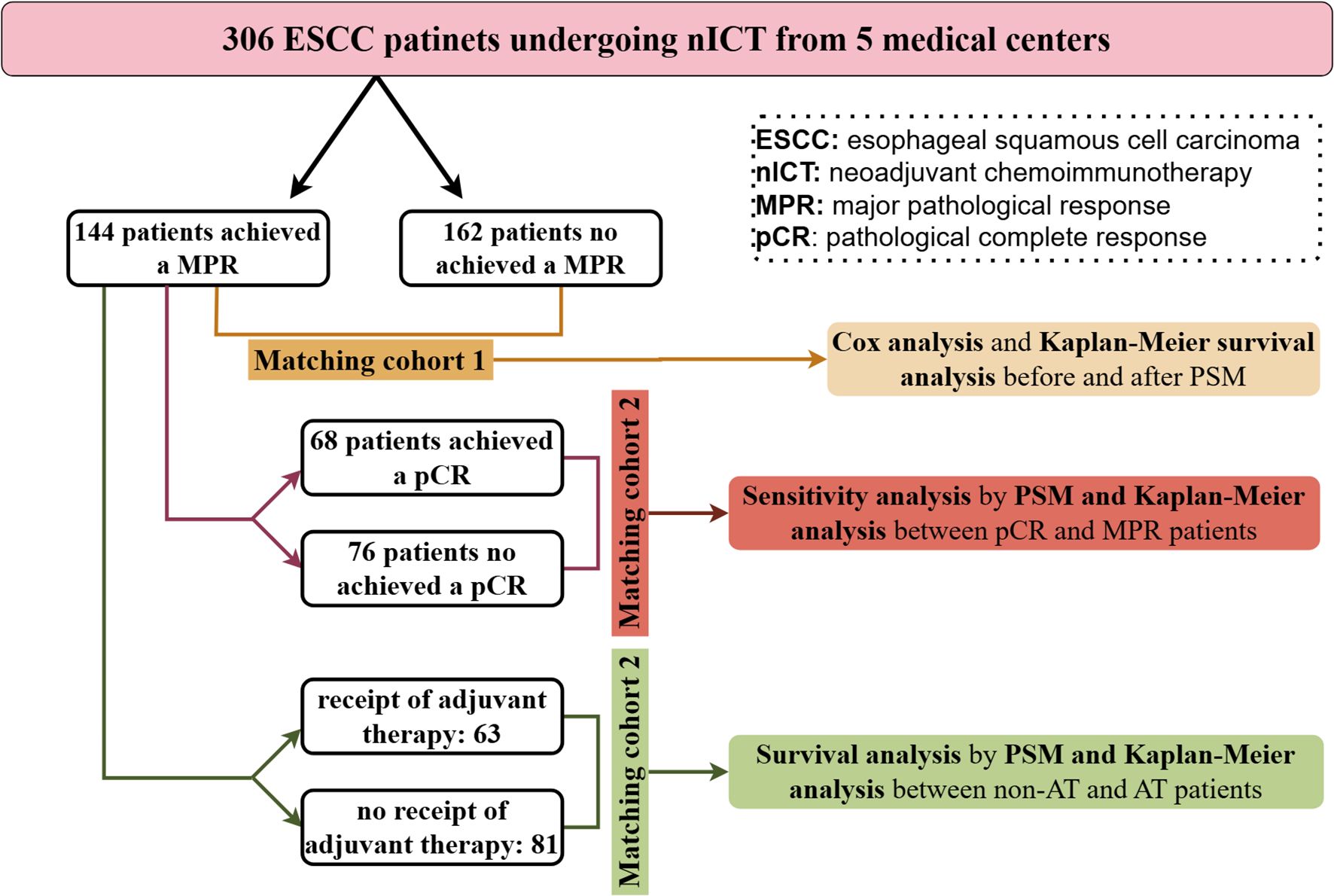
Categorical data were presented as counts and percentages, compared using Chi-square or Fisher's exact tests. Propensity score matching (PSM) was employed to mitigate bias arising from confounding variables, utilizing logistic regression to generate scores and implementing nearest neighbor matching without replacement, with a caliper of 0.05 and a ratio of 1:1. Matching parameters included sex, age, smoking history, drinking history, BMI, tumor location, clinical stage. Survival difference was evaluated using Kaplan-Meier survival curves. To investigate risk factors, Cox regression analysis was conducted. Variables exhibiting statistical significance in the univariate Cox regression analysis were incorporated as predictors in the multivariate analysis. Additionally, a backward stepwise regression approach was employed in the multivariate analysis. The data were processed with SPSS version 27 and R version 4.3.1. Statistical significance was set at P < 0.05. The flow chart of statistical analysis process of this study was as shown in Figure 1.
3 Results
3.1 Patients characteristic
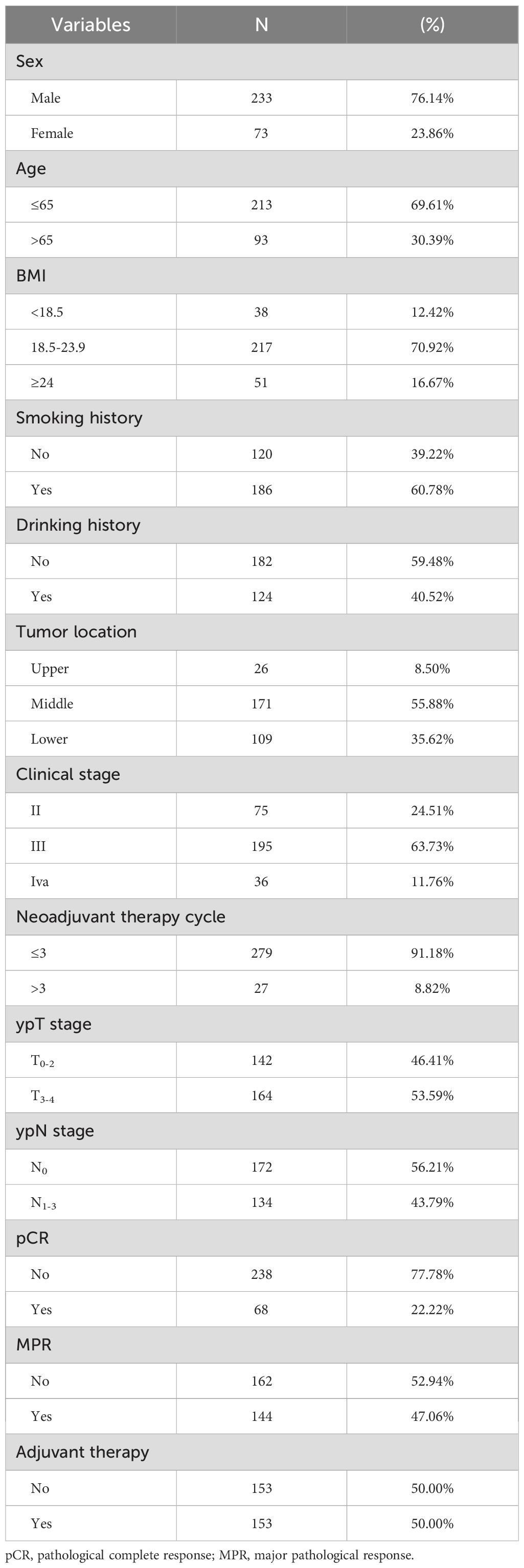
In this study, a cohort of 306 patients diagnosed with ESCC and treated with nICT from five medical centers was analyzed. Among these patients, 233 cases (76.14%) were male, and 213 (69.61%) were aged 65 years or younger. Postoperative pathological outcomes showed that 144 patients achieved a MPR, while 68 attained a pCR. Following nICT and surgical intervention, 153 patients received adjuvant therapy. Detailed baseline characteristics of this patient population are presented in Table 1.
3.2 The Cox regression analysis for ESCC patients undergoing nICT
Cox regression analysis was employed to examine the independent risk factors in ESCC patients following nICT. The univariate Cox analysis identified ypT stage, ypN stage, MPR, and pCR as prognostic factors influencing this patient cohort. In the subsequent multivariate Cox analysis, ypN stage [HR= 1.88, 95%CI=(1.13 - 3.10), P = 0.014] and MPR [HR = 0.48, 95%CI= (0.28 - 0.82), P = 0.007] emerged as independent risk factors, as detailed in Table 2.
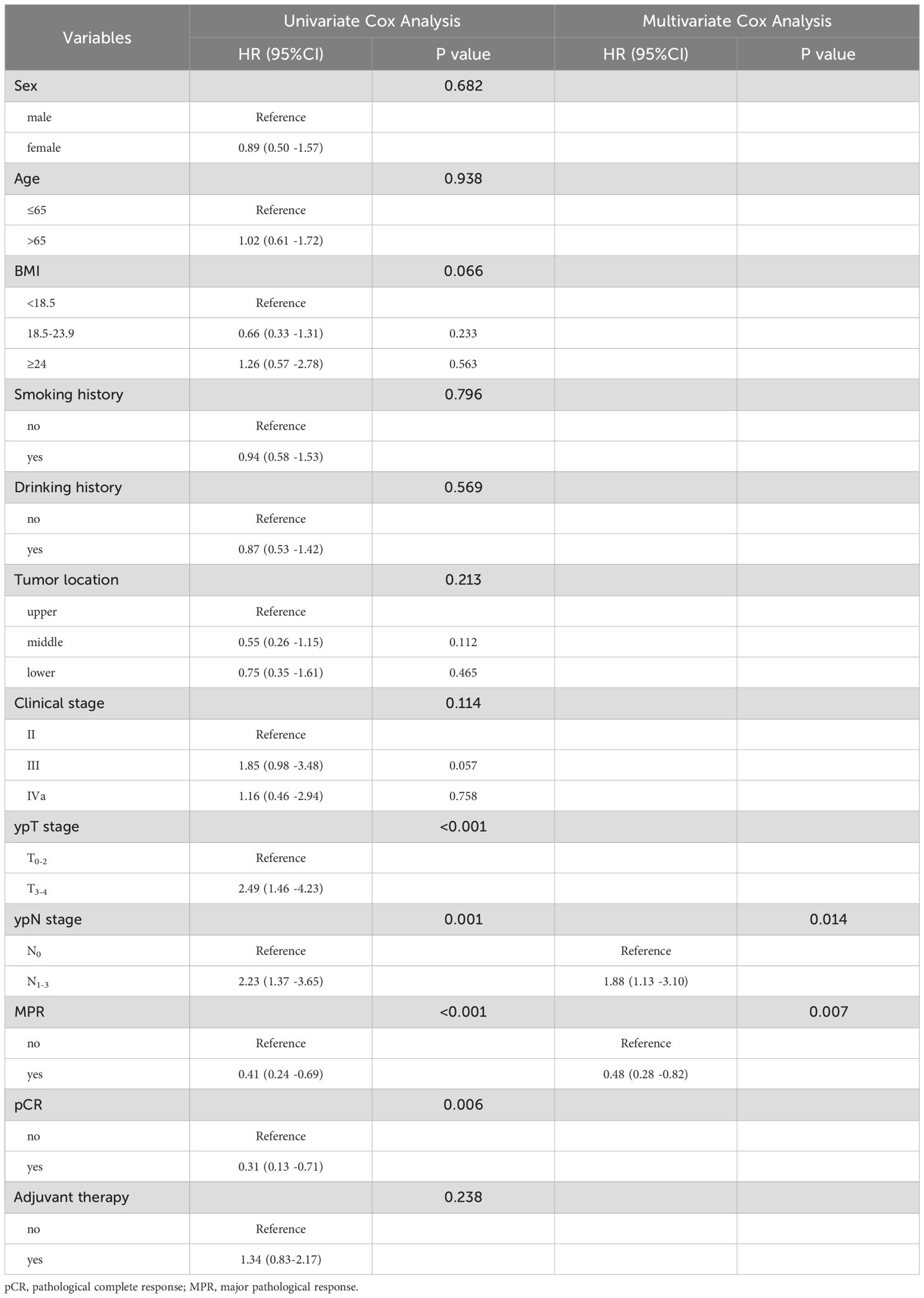
Table 2. Univariate and Multivariate Cox analysis for recurrence-free survival in ESCC patients after nICT.
3.3 The survival comparison of MPR patients with non-MPR patients and pCR patients
The propensity score matching method was employed to mitigate baseline differences between the MPR patient group and the non-MPR patient group, as illustrated in Table 3. The survival curve by Kaplan-Meier analysis indicated that the recurrence-free survival of MPR patients was significantly superior than that of non-MPR patients, both before (P<0.001) and after PSM (P = 0.016), as is shown in Figures 2A, B.
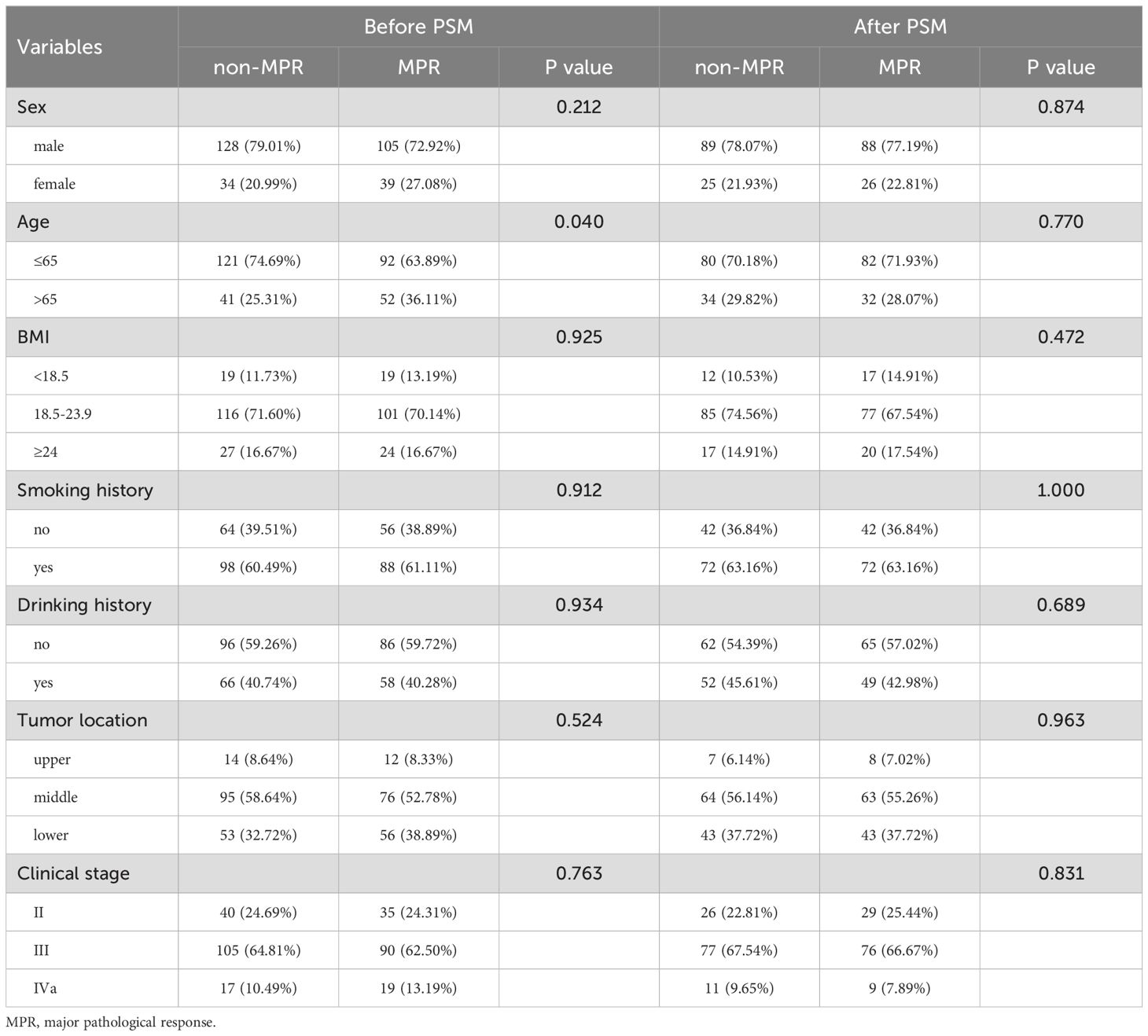
Table 3. Characteristics comparison of MPR and non-MPR patients in nICT group before and after matching.
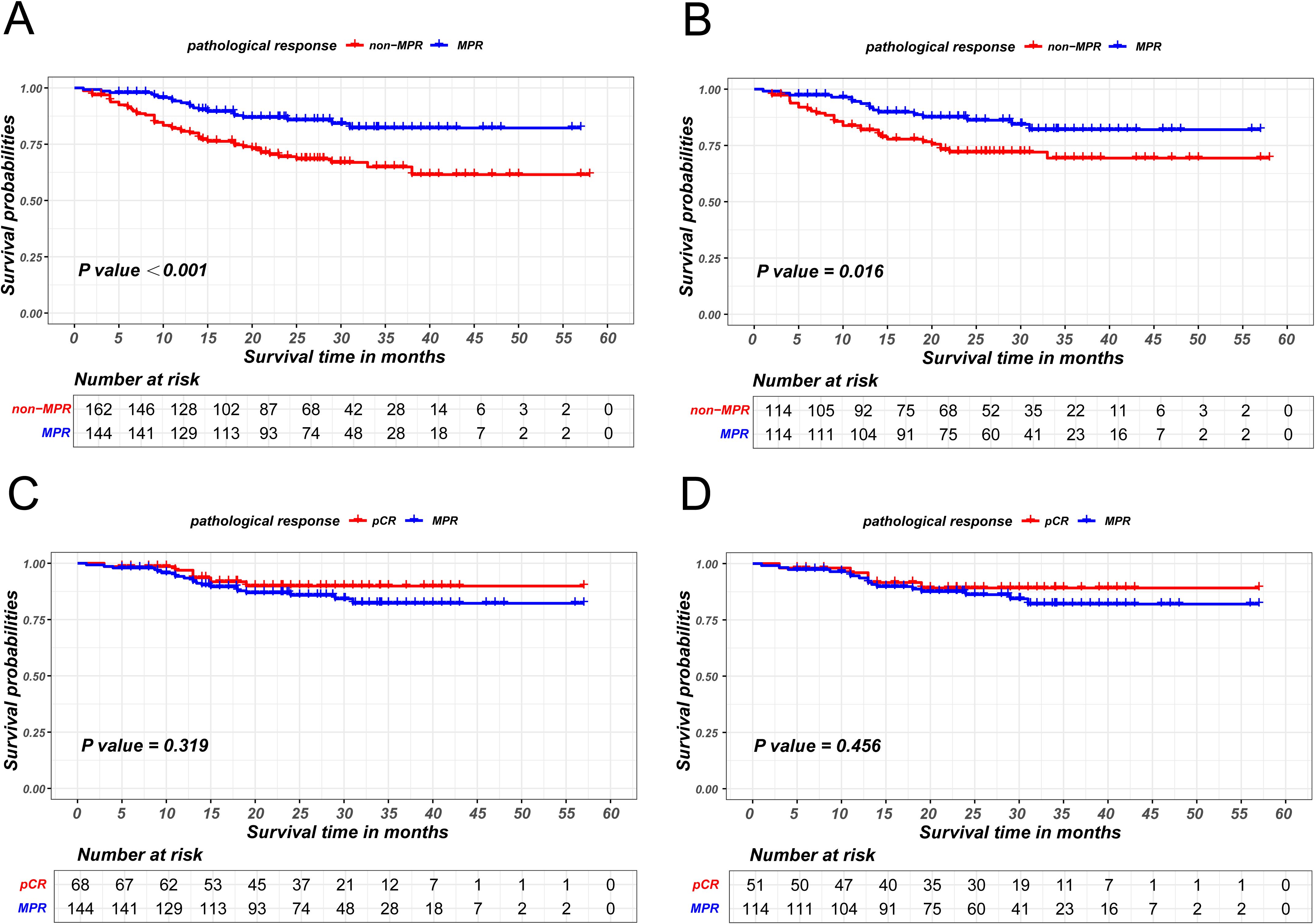
Figure 2. Kaplan-Meier survival curves of recurrence-free survival between non-MPR and MPR patients before PSM (A) and after PSM (B). Kaplan-Meier survival curves of recurrence-free survival between pCR and MPR patients before PSM (C) and after PSM (D).
Survival analysis also revealed that the recurrence-free survival of patients achieving pCR were comparable to those of patients achieving MPR (P = 0.319 in the unmatched cohort; P = 0.456 in the matched cohort), as illustrated in Figures 2C, D.
3.4 The sensitivity analysis between non-pCR and pCR patients in MPR patients cohort
Given that patients achieving pCR are encompassed within the MPR cohort, a sensitivity analysis was utilized to explore survival difference between non-pCR and pCR patients within the MPR group. MPR patients were stratified in two groups based on pCR status and PSM was used to eliminate intergroup bias, as is shown in Supplementary Table S2. In the unmatched group, the Kaplan-Meier survival analysis indicated the absence of a statistically significant difference in disease-free survival between non-pCR and pCR patients within MPR cohort (P = 0.119). Similarly, the survival difference was not observed between two groups in the matched group (P = 0.193), as is shown in Figure 3.
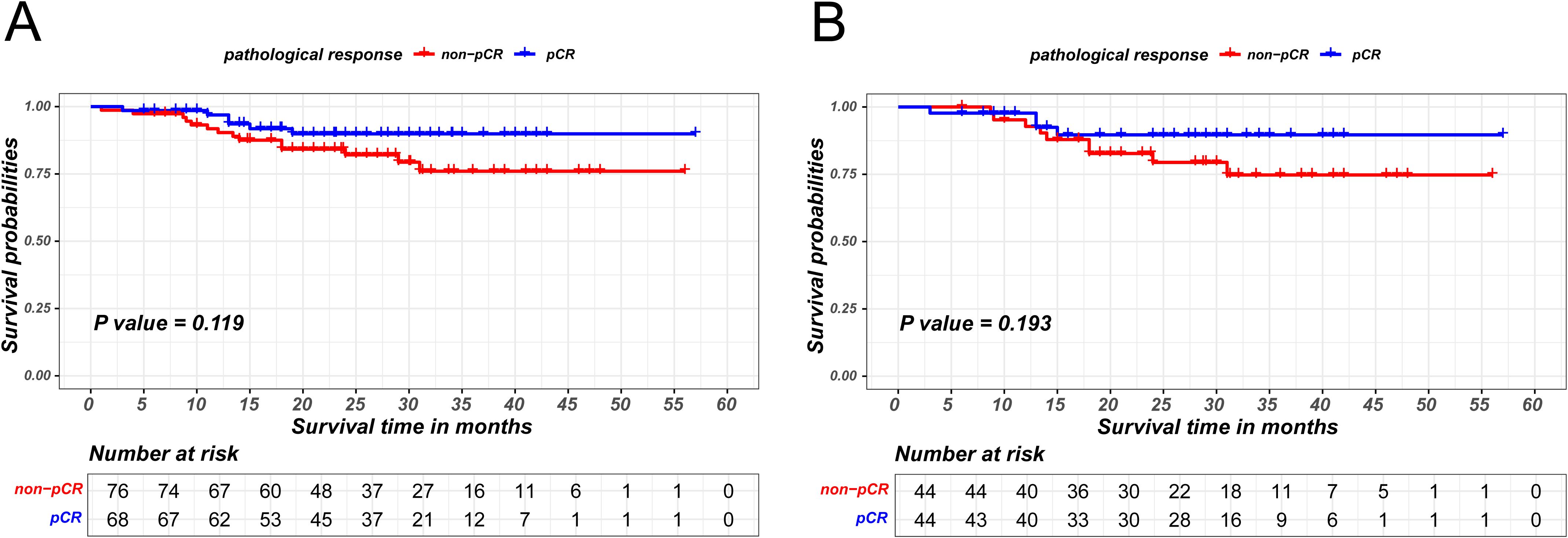
Figure 3. Kaplan-Meier survival curves of recurrence-free survival between pCR and non-pCR patients in MPR group before PSM (A) and after PSM (B).
3.5 The survival analysis of adjuvant therapy in MPR patients after nICT
The prognostic significance of AT in ESCC patients achieving MPR was also investigated. Similarly, the MPR patients cohort was stratified into AT and non-AT group based on the administration of AT and employed PSM to minimize bias between the two groups, as detailed in Supplementary Table S3. The matching parameters of this propensity score matching included sex, age, smoking history, drinking history, BMI, tumor location, ypT stage and ypN stage. The Kaplan-Meier survival analysis revealed that AT did not confer an additional recurrence-free survival benefit for patients attaining MPR (P=0.193 in the unmatched cohort;P=0.025 in the matched cohort), as is shown in Figure 4.
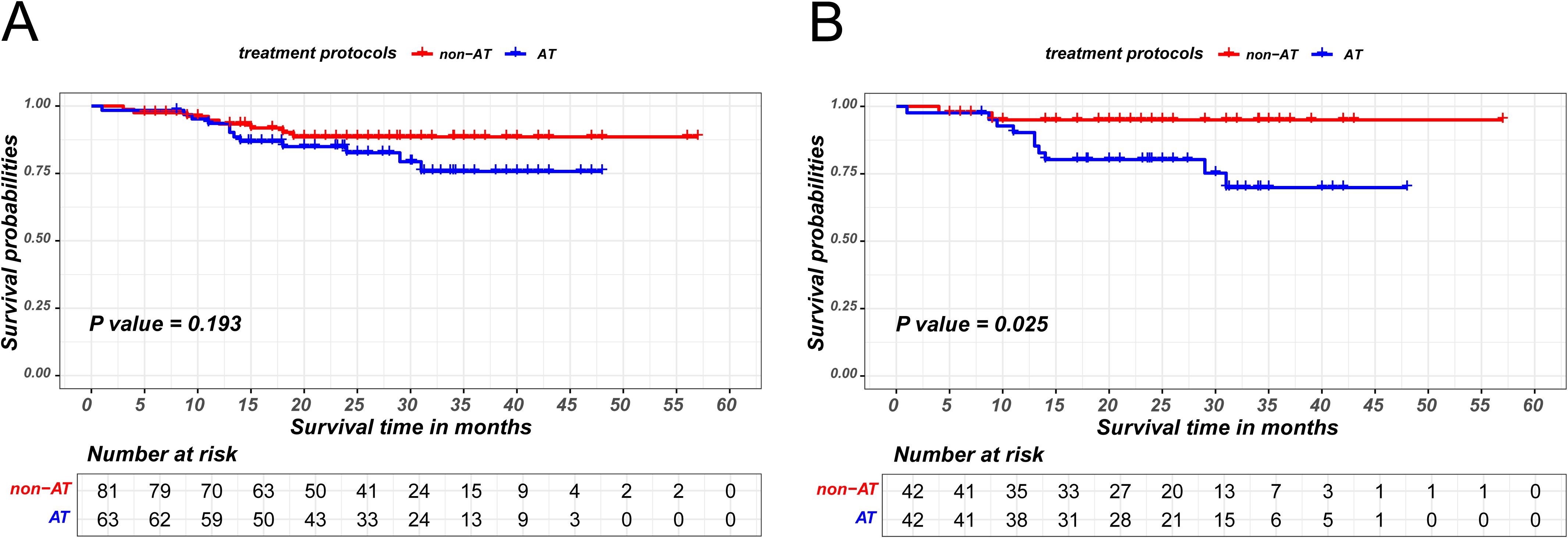
Figure 4. Kaplan-Meier survival curves of recurrence-free survival between non-AT and AT patients in MPR group before PSM (A) and after PSM (B).
3.6 Recurrence patterns comparison between MPR patients and pCR patients
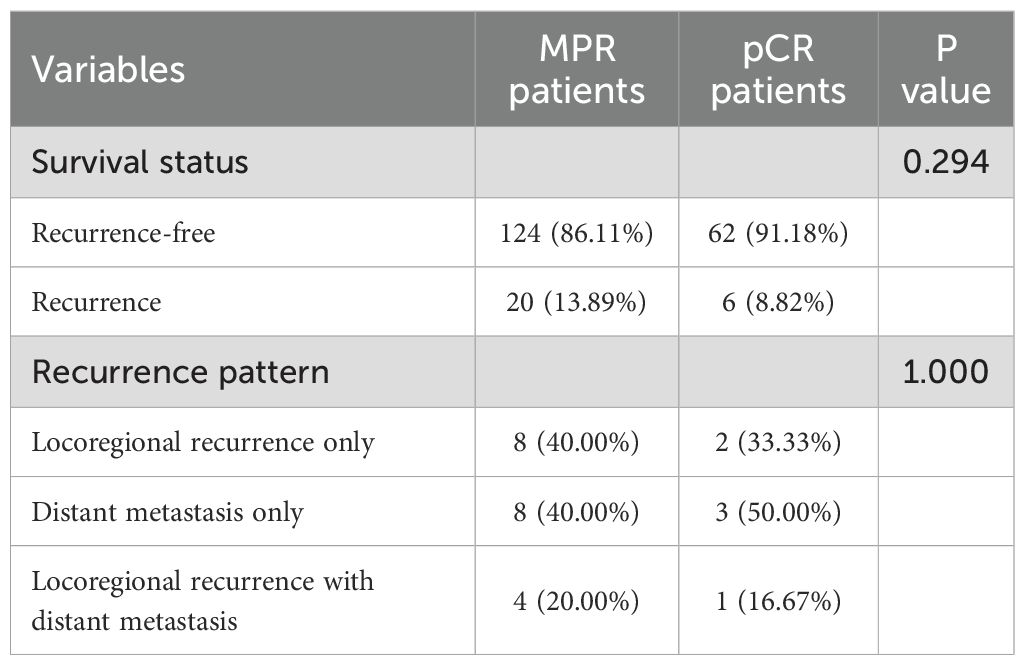
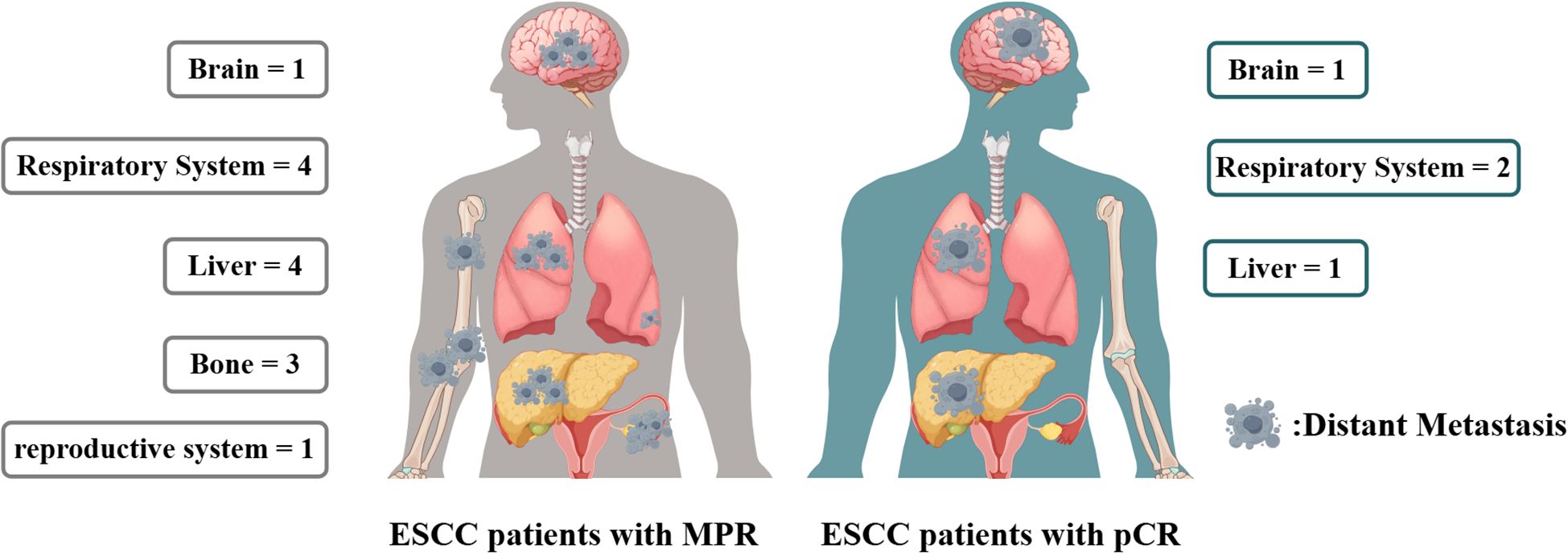
In the cohort of patients with MPR, 20 individuals experienced recurrence, with 8 presenting solely with locoregional recurrence and another 8 exhibiting only distant metastasis. In contrast, among the pCR patients, 6 individuals experienced recurrence, of whom 2 had only locoregional recurrence and 3 had only distant metastasis. A correlation analysis indicated that the recurrence patterns between the two groups were comparable, as detailed in Table 4 and Figure 5.
4 Discussion
pCR and MPR are utilized as the principal endpoints in assessing the short-term efficacy of neoadjuvant trials. Previous researches showed that nICT could lead to higher rates of MPR and pCR compared to nCT alone (18, 19). Furthermore, in comparison to achieving a pCR, attaining a MPR is more readily achievable in ESCC patients undergoing neoadjuvant therapy (20). However, the question of whether ESCC patients achieving MPR have prognostic outcomes equivalent to those attaining pCR remains unclear. Our study found that patients who achieved MPR following nICT exhibited a more favorable prognosis compared to those who did not achieve MPR. Furthermore, their prognostic outcomes were comparable to those of patients who attained pCR.
This study demonstrated that patients exhibiting a MPR in the nICT group had a significantly improved prognosis compared to those without MPR, both prior to and following propensity score matching. The Cox regression analysis also identified MPR as an independent risk factor. From the perspective of tumor load, the status of MPR indicated that neoadjuvant therapy was particularly effective in reducing preoperative tumor burden and substantially decreasing the risk of tumor recurrence through the more efficient eradication of micro-metastases (21, 22). Furthermore, a distinctive characteristic of immunotherapy is its “tail effect” (23, 24). The tail effect is characterized by the sustained maintenance of therapeutic effects following treatment discontinuation, thereby providing long-term immune responses and better survival benefits for patients with advanced tumors. The development of the tail effect is linked to the “memory function” of immune cells, notably T cells. While, this effect requires a strong interaction between the patient’s immune system and the immunotherapeutic agent. Patients who achieve a MPR are more likely to exhibit an enhanced tail effect in the immune system during neoadjuvant therapy, which sustains anti-tumor activity postoperatively and results in improved clinical outcomes.
In our study, it was observed that the RFS of patients achieving a MPR was comparable to that of patients exhibiting a pCR. Sensitivity analysis indicated that, within the MPR cohort, the RFS of patients attaining pCR did not significantly differ from those who did not, corroborating previous findings (25). These findings implied that nICT may have yielded equivalent therapeutic outcomes to MPR patients and pCR patients. This observation also suggested that the MPR may serve as a surrogate endpoint for pCR in assessing the efficacy of nICT. Given the comparable RFS in MPR and pCR patients, MPR has the potential to merge as a more accessible and cost-effective prognostic indicator in clinical practice. The CROSS study demonstrated that nCRT significantly improves overall survival in EC patients compared to surgery alone; however, it does not confer enhanced survival benefits with respect to distant metastasis (26). Compared to nCRT, nICT offers a systemic therapeutic approach with a distinct advantage in the management of distant metastases, particularly in the control of microscopic metastasis (27).The integration of nICT and surgical intervention constitutes a comprehensive treatment modality that effectively manages locoregional recurrence and distant metastasis of tumors: nICT significantly reduces the quantity of tumor cells within the primary tumor and lymph nodes, especially in patients exhibiting drug sensitivity. During subsequent radical esophagectomy, the residual tumor cells can be effectively excised. The primary aims of neoadjuvant therapy are twofold: first, to achieve tumor downstaging, thereby enhancing the feasibility of curative surgical procedures (28); and second, to mitigate distant metastasis and micrometastasis of tumor cells through systemic drug administration, particularly when the tumor burden is substantial and the likelihood of metastasis is elevated. Numerous studies have documented that patients undergoing neoadjuvant therapy demonstrate superior recurrence-free survival rates in comparison to those receiving adjuvant therapy post-surgery, which implied that chemotherapy or immunotherapy might be more efficacious in managing distant metastasis when the tumor burden is elevated, as is the case prior to surgical intervention (29, 30). Both MPR and pCR reflect a significant sensitivity to the treatment protocol, showing the effective eradication of a considerable proportion of cancer cells. As a result, the prognosis for patients with MPR is notably enhanced due to the effective local tumor control attained through following standardized esophagectomy. This improvement may account for the similar RFS observed between ESCC patients with MPR and those with pCR following surgery.
In our study, pCR was found to be a prognostic factor but not an independent predictor of survival outcome, consistent with findings reported in other studies (31–33). This indicated that pCR can be a useful short-term indicator for evaluating treatment efficacy, but it may not be a reliable surrogate for long-term outcomes, especially in ESCC patients after nICT. The CheckMate 577 trial has shown that adjuvant immunotherapy can enhance survival outcomes for EC patients who have residual tumor cells after nCRT (34). Nonetheless, there are currently no established guidelines delineating the indications for adjuvant therapy in patients undergoing nICT. In our prior investigations, we found that the prognosis for ESCC patients who attained a pCR following nICT was unaffected by adjuvant therapy (35). In this study, it was observed that adjuvant therapy did not provide additional advantages in terms of disease-free survival for patients achieving a MPR, aligning with the outcomes observed in patients with pCR. Prior study has indicated that compared to those who did not undergo AT, the receipt of AT showed shorter disease-free survival following neoadjuvant chemoradiotherapy and surgery (36). Similarly, Yan’s study showed that adjuvant therapy failed to improve survival outcomes in patients receiving neoadjuvant chemotherapy and instead indicated a trend toward worse prognosis (37). It is well recognized that AT does not benefit all ESCC patients. There are three possible reasons for negative impact of AT. Firstly, postoperative impairments such as reduced food intake and swallowing difficulties and postoperative complications can significantly weaken the immune system of ESCC patients (38–40). In such circumstances, adjuvant therapy may further weaken the immune system’s ability to target cancer cells, potentially leading to disease recurrence (41, 42). Secondly, damage to the integrity of Lymph node, particularly as a result of systematic lymphadenectomy, may impair the activation of anti-tumor T cells, consequently diminishing the effectiveness of subsequent immunotherapeutic interventions (43, 44). Thirdly, the reduction of tumor burden after surgery may weaken the efficacy of adjuvant therapy. These findings suggested that clinicians might consider implementing a surveillance approach for patients achieving MPR after surgery, akin to the strategy employed for those with pCR. Besides, clinicians should take both MPR and pCR into account when assessing the prognosis of LA-ESCC patients, as this may facilitate the development of more tailored treatment strategies. However, we still believe this finding still requires further validation from through prospective, nationwide, multi-center clinical trials.
In the nICT cohort of patients with ESCC, the recurrence patterns observed in patients achieving MPR were analogous to those in patients achieving pCR, encompassing both distant metastasis and locoregional recurrence. Furthermore, in instances of distant metastasis, the predominant sites for both MPR and pCR patients were the brain, lungs, and liver. The analogous recurrence patterns and distribution of metastasis sites indicated the necessity for equal consideration to two patients group during follow-up. The recurrence pattern similarities between MPR and pCR patients also implied that these groups could be perceived as having comparable risk levels when devising follow-up strategies. Nonetheless, it is imperative to acknowledge that despite attaining MPR or pCR, patients remain at risk for recurrence. Ongoing surveillance is still essential to enhance prognostic outcomes.
This study has several limitations. First, despite being a multi-center study and employing rigorous selection criteria to mitigate selection bias, its retrospective design introduces inherent limitations. Secondly, this study was limited to patients with esophageal squamous cell carcinoma, and the extent to which our findings can be generalized to patients with adenocarcinoma remains uncertain. In future studies, we plan to conduct a separate analysis of esophageal adenocarcinoma. This approach will be pursued in subsequent research, although it will require additional time to collaborate with more centers due to the relatively low incidence of esophageal adenocarcinoma in China. The visual abstract of this study is shown in Figure 6.
5 Conclusion
In conclusion, MPR emerges as a significant prognostic factor in ESCC patients undergoing nICT, with outcomes similar to those achieving pCR. These findings warrant consideration of MPR as a potential surrogate endpoint for pCR and highlight the need to refine treatment strategies based on pathological responses. Further studies are necessary to confirm these results and to explore the integration of MPR into clinical practice, potentially leading to more personalized and effective treatment approaches for ESCC patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Fujian Medical University Union Hospital (Ethics number: 2025KY012). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Written informed consent was waived because of the retrospective design.
Author contributions
SX: Investigation, Conceptualization, Formal analysis, Writing – original draft, Data curation. SH: Writing – review & editing, Data curation. ZT: Data curation, Writing – review & editing. HZ: Writing – review & editing, Data curation. JXu: Data curation, Writing – review & editing. SK: Data curation, Writing – review & editing, Supervision. JXie: Data curation, Supervision, Writing – review & editing. RX: Writing – review & editing, Data curation, Supervision. YC: Supervision, Data curation, Writing – review & editing. ZH: Writing – review & editing, Supervision, Data curation. MK: Writing – review & editing, Conceptualization, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We appreciate the support by FigDraw.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1599526/full#supplementary-material.
Abbreviations
nCT, neoadjuvant chemotherapy; nCRT, neoadjuvant chemoradiotherapy; nICT, neoadjuvant chemoimmunotherapy; aIT, adjuvant immunotherapy; aICT, adjuvant chemoimmunotherapy; aCT, adjuvant chemotherapy; AT, adjuvant therapy; LA-ESCC, locally advanced ESCC; PSM, propensity score matching; RFS, recurrence-free survival; ESCC, esophageal squamous cell cancer.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Huang R, Qiu Z, Zheng C, Zeng R, Chen W, Wang S, et al. Neoadjuvant therapy for locally advanced esophageal cancers. Front Oncol. (2022) 12:734581–95. doi: 10.3389/fonc.2022.734581
3. Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. (2012) 19:68–74. doi: 10.1245/s10434-011-2049-9
4. Shen L, Kato K, Kim SB, Ajani JA, Zhao K, He Z, et al. Tislelizumab versus chemotherapy as second-line treatment for advanced or metastatic esophageal squamous cell carcinoma (RATIONALE-302): A randomized phase III study. J Clin Oncol. (2024) 42:486. doi: 10.1200/JCO.23.02629
5. Shitara K, Rha SY, Wyrwicz LS, Oshima T, Karaseva N, Osipov M, et al. Neoadjuvant and adjuvant pembrolizumab plus chemotherapy in locally advanced gastric or gastro-esophageal cancer (KEYNOTE-585): an interim analysis of the multicenter, double-blind, randomized phase 3 study. Lancet Oncol. (2024) 25:212–24. doi: 10.1016/S1470-2045(23)00541-7
6. Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced esophageal cancer (KEYNOTE-590): a randomized, placebo-controlled, phase 3 study. Lancet. (2021) 398:759–71. doi: 10.1016/S0140-6736(21)01234-4
7. Rose BS, Winer EP, and Mamon HJ. Perils of the pathologic complete response. J Clin Oncol. (2016) 34:3959–62. doi: 10.1200/JCO.2016.68.1718
8. Hong Z, Xie S, Xu H, Ke S, Liu W, Huang S, et al. Major pathologic response as a prognostic surrogate in esophageal squamous cell carcinoma patients receiving neoadjuvant chemotherapy/chemoimmunotherapy: A multi-center cohort study. Eur J Surg Oncol. (2025) 51(2):109500. doi: 10.1016/j.ejso.2024.109500
9. Kim MK, Cho KJ, Park SI, Kim YH, Kim JH, Song HY, et al. Initial stage affects survival even after complete pathologic remission is achieved in locally advanced esophageal cancer: analysis of 70 patients with pathologic major response after preoperative chemoradiotherapy. Int J Radiat Oncol Biol Phys. (2009) 75:115–21. doi: 10.1016/j.ijrobp.2008.10.074
10. von Döbeln GA, Klevebro F, Jacobsen AB, Johannessen HO, Nielsen NH, Johnsen G, et al. Neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the esophagus or gastroesophageal junction: long-term results of a randomized clinical trial. Dis Esophagus. (2019) 32. doi: 10.1093/dote/doy078
11. Tang H, Wang H, Fang Y, Zhu JY, Yin J, Shen YX, et al. Neoadjuvant chemoradiotherapy versus neoadjuvant chemotherapy followed by minimally invasive esophagectomy for locally advanced esophageal squamous cell carcinoma: a prospective multicenter randomized clinical trial. Ann Oncol. (2023) 34:163–72. doi: 10.1016/j.annonc.2022.10.508
12. Duan H, Shao C, Pan M, Liu H, Dong X, Zhang Y, et al. Neoadjuvant pembrolizumab and chemotherapy in resectable esophageal cancer: an open-label, single-arm study (PEN-ICE). Front Immunol. (2022) 13:849984. doi: 10.3389/fimmu.2022.849984
13. Wang Z, Shao C, Wang Y, Duan H, Pan M, Zhao J, et al. Efficacy and safety of neoadjuvant immunotherapy in surgically resectable esophageal cancer: A systematic review and meta-analysis. Int J Surg. (2022) 104:106767. doi: 10.1016/j.ijsu.2022.106767
14. Yun JK, Kim Y, Lee GD, Choi S, Kim YH, Kim DK, et al. Tumor regression grade combined with lymph node status in esophageal squamous cell carcinoma after neoadjuvant chemoradiotherapy. Cancer Med. (2022) 11:3623–32. doi: 10.1002/cam4.4748
15. Xu L, Wei XF, Li CJ, Yang ZY, Yu YK, Li HM, et al. Pathologic responses and surgical outcomes after neoadjuvant immunochemotherapy versus neoadjuvant chemoradiotherapy in patients with locally advanced esophageal squamous cell carcinoma. Front Immunol. (2022) 13:1052542. doi: 10.3389/fimmu.2022.1052542
16. Wang S, Di S, Lu J, Xie S, Yu Z, Liang Y, et al. 18 F-FDG PET/CT predicts the role of neoadjuvant immunochemotherapy in the pathological response of esophageal squamous cell carcinoma. Thorac Cancer. (2023) 14:2338–49. doi: 10.1111/1759-7714.15024
17. Liu J, Zhu L, Tang M, Huang X, Gu C, He C, et al. Efficacy of neoadjuvant immunochemotherapy and survival surrogate analysis of neoadjuvant treatment in IB-IIIB lung squamous cell carcinoma. Sci Rep. (2024) 14:5523. doi: 10.1038/s41598-024-54371-8
18. Wang P, Chen Y, Lei M, He H, Zhang D, Lin J, et al. Comparison of neoadjuvant chemoimmunotherapy and neoadjuvant chemotherapy for resectable esophageal squamous cell carcinoma: a retrospective study with 3-year survival analysis. J Cancer Res Clin Oncol. (2024) 150:477. doi: 10.1007/s00432-024-06004-w
19. Wang M, Dong W, Liu A, Lai T, Zhang B, and Sun Q. Efficacy and safety of neoadjuvant immunotherapy combined with chemotherapy for resectable esophageal cancer: a systematic review and meta-analysis. Transl Cancer Res. (2024) 13:2735–50. doi: 10.21037/tcr-24-198
20. Liu C, Yu Y, Shao Y, He L, Wu T, Zheng J, et al. Real-world retrospective study of anti-PD-1 antibody in combination with chemotherapy as a neoadjuvant treatment strategy for locally advanced resectable esophageal squamous cell carcinoma. J Thorac Dis. (2024) 16:4106–19. doi: 10.21037/jtd-24-169
21. Hagi T, Makino T, Yamasaki M, Yamashita K, Tanaka K, Saito T, et al. Pathological regression of lymph nodes better predicts long-term survival in esophageal cancer patients undergoing neoadjuvant chemotherapy followed by surgery. Ann Surg. (2022) 275:1121–9. doi: 10.1097/SLA.0000000000004238
22. Agarwal A, Chang GJ, Hu CY, Taggart M, Rashid A, Park IJ, et al. Quantified pathologic response assessed as residual tumor burden is a predictor of recurrence-free survival in patients with rectal cancer who undergo resection after neoadjuvant chemoradiotherapy. Cancer. (2013) 119:4231–41. doi: 10.1002/cncr.28331
23. Zhu J, Lian J, Xu B, Pang X, Ji S, Zhao Y, et al. Neoadjuvant immunotherapy for colorectal cancer: Right regimens, right patients, right directions? Front Immunol. (2023) 14:1120684. doi: 10.3389/fimmu.2023.1120684
24. Fu C, Du H, Wang Q, Zhu W, Bian G, Zhong Z, et al. Case report: A golden tail of immunotherapy: significant tail effect in a chemotherapy-resistant advanced pulmonary sarcomatoid carcinoma patient treated by Sintilimab combined with Anlotinib. Front Immunol. (2024) 15:1452195. doi: 10.3389/fimmu.2024.1452195
25. Takeda FR, Tustumi F, de Almeida Obregon C, Yogolare GG, Navarro YP, Segatelli V, et al. Prognostic value of tumor regression grade based on ryan score in squamous cell carcinoma and adenocarcinoma of esophagus. Ann Surg Oncol. (2020) 27:1241–7. doi: 10.1245/s10434-019-07967-8
26. Eyck BM, van Lanschot JJB, Hulshof MCCM, van der Wilk BJ, Shapiro J, van Hagen P, et al. Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: the randomized controlled CROSS trial. J Clin Oncol. (2021) 39:1995–2004. doi: 10.1200/JCO.20.03614
27. Wu YY, Dai L, Yang YB, Yan WP, Cheng H, Fan MY, et al. Long-term survival and recurrence patterns in locally advanced esophageal squamous cell carcinoma patients with pathologic complete response after neoadjuvant chemotherapy followed by surgery. Ann Surg Oncol. (2024) 31:5047–54. doi: 10.1245/s10434-023-14809-1
28. Campbell NP and Villaflor VM. Neoadjuvant treatment of esophageal cancer. World J Gastroenterol. (2010) 16:3793–803. doi: 10.3748/wjg.v16.i30.3793
29. Pasquali S, Yim G, Vohra RS, Mocellin S, Nyanhongo D, Marriott P, et al. Survival after neoadjuvant and adjuvant treatments compared to surgery alone for resectable esophageal carcinoma: A network meta-analysis. Ann Surg. (2017) 265:481–91. doi: 10.1097/SLA.0000000000001905
30. Xiao X, Hong HG, Zeng X, Yang YS, Luan SY, Li Y, et al. , et al. The efficacy of neoadjuvant versus adjuvant therapy for resectable esophageal cancer patients: A systematic review and meta-analysis. World J Surg. (2020) 44:4161–74. doi: 10.1007/s00268-020-05721-w
31. Yu YK, Meng FY, Wei XF, Chen XK, Li HM, Liu Q, et al. Neoadjuvant chemotherapy combined with immunotherapy versus neoadjuvant chemoradiotherapy in patients with locally advanced esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg. (2024) 168:417–428.e3. doi: 10.1016/j.jtcvs.2023.12.030
32. Yang Y, Liu J, Liu Z, Zhu L, Chen H, Yu B, et al. Two-year outcomes of clinical N2–3 esophageal squamous cell carcinoma after neoadjuvant chemotherapy and immunotherapy from the phase 2 NICE study. J Thorac Cardiovasc Surg. (2024) 167:838–847.e1. doi: 10.1016/j.jtcvs.2023.08.056
33. Su F, Yang X, Yin J, Shen Y, and Tan L. Validity of using pathological response as a surrogate for overall survival in neoadjuvant studies for esophageal cancer: A systematic review and meta-analysis. Ann Surg Oncol. (2023) 30:7461–71. doi: 10.1245/s10434-023-13778-9
34. Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med. (2021) 384:1191–203. doi: 10.1056/NEJMoa2032125
35. Xie SH, Yang LT, Zhang H, Tang ZL, Lin ZW, Chen Y, et al. Adjuvant therapy provides no additional recurrence-free benefit for esophageal squamous cell carcinoma patients after neoadjuvant chemoimmunotherapy and surgery: a multi-center propensity score match study. Front Immunol. (2024) 15:1332492. doi: 10.3389/fimmu.2024.1332492
36. Li X, Luan S, Yang Y, Zhou J, Shang Q, Fang P, et al. Trimodal therapy in esophageal squamous cell carcinoma: role of adjuvant therapy following neoadjuvant chemoradiation and surgery. Cancers (Basel). (2022) 14:3721. doi: 10.3390/cancers14153721
37. Yan W, Zhao P, Fu H, Lin Y, Li Z, Dai L, et al. Survival after induction chemotherapy and esophagectomy is not improved by adjuvant chemotherapy. Ann Thorac Surg. (2019) 108:1505–13. doi: 10.1016/j.athoracsur.2019.04.106
38. Shiozaki A, Fujiwara H, Okamura H, Murayama Y, Komatsu S, Kuriu Y, et al. Risk factors for postoperative respiratory complications following esophageal cancer resection. Oncol Lett. (2012) 3:907–12. doi: 10.3892/ol.2012.589
39. van Sandick JW, Gisbertz SS, ten Berge IJ, Boermeester MA, van der Pouw Kraan TC, Out TA, et al. Immune responses and prediction of major infection in patients undergoing transhiatal or transthoracic esophagectomy for cancer. Ann Surg. (2003) 237:35–43. doi: 10.1097/00000658-200301000-00006
40. Kubo Y, Miyata H, Sugimura K, Shinno N, Asukai K, Hasegawa S, et al. Prognostic implication of postoperative weight loss after esophagectomy for esophageal squamous cell cancer. Ann Surg Oncol. (2021) 28:184–93. doi: 10.1245/s10434-020-08762-6
41. Ji Y, Du X, Zhu W, Yang Y, Ma J, Zhang L, et al. Efficacy of concurrent chemoradiotherapy with S-1 vs radiotherapy alone for older patients with esophageal cancer: A multicenter randomized phase 3 clinical trial. JAMA Oncol. (2021) 7:1459–66. doi: 10.1001/jamaoncol.2021.2705
42. Cheraghi A, Barahman M, Hariri R, Nikoofar A, and Fadavi P. Comparison of the pathological response and adverse effects of oxaliplatin and capecitabine versus paclitaxel and carboplatin in the neoadjuvant chemoradiotherapy treatment approach for esophageal and gastroesophageal junction cancer: A randomized control trial study. Med J Islam Repub Iran. (2021) 35:140. doi: 10.47176/mjiri.35.140
43. Xu ZY, Li ZZ, Cao LM, Zhong NN, Liu XH, Wang GR, et al. Seizing the fate of lymph nodes in immunotherapy: To preserve or not? Cancer Lett. (2024) 588:216740. doi: 10.1016/j.canlet.2024.216740
Keywords: neoadjuvant chemoimmunotherapy, major pathological response, adjuvant therapy, pathological complete response, recurrence pattern
Citation: Xie S, Huang S, Tang Z, Zhang H, Xu J, Ke S, Xie J, Xu R, Chen Y, Hong Z and Kang M (2025) The prognostic power of major pathological response in esophageal squamous cell carcinoma patients undergoing neoadjuvant chemoimmunotherapy: a multi-center cohort study. Front. Immunol. 16:1599526. doi: 10.3389/fimmu.2025.1599526
Received: 25 March 2025; Accepted: 13 June 2025;
Published: 07 July 2025.
Edited by:
Vera Rebmann, University of Duisburg-Essen, GermanyReviewed by:
Martin Leu, University Medical Center Göttingen, GermanyJiaying Deng, Fudan University, China
Copyright © 2025 Xie, Huang, Tang, Zhang, Xu, Ke, Xie, Xu, Chen, Hong and Kang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingqiang Kang, OTE5OTExNTA0NUBmam11LmVkdS5jbg==; Zhinuan Hong, aG9uZ3poaW51YW5AMTYzLmNvbQ==; Ying Chen, Y3lpbmd6eEAxNjMuY29t; Rongyu Xu, eHJ5NjQxMTI3QHNpbmEuY29t; Sunkui Ke, eG16c2tzazIwMjFAMTYzLmNvbQ==; Jinbiao Xie, amluYmlhb3hpZTEyM0AxNjMuY29t
†These authors have contributed equally to this work
 Shuhan Xie
Shuhan Xie Shijie Huang5†
Shijie Huang5† Hai Zhang
Hai Zhang Jinxin Xu
Jinxin Xu Zhinuan Hong
Zhinuan Hong Mingqiang Kang
Mingqiang Kang