- 1Department of Orthopaedics, The 960th Hospital of People’s Liberation Army of China (PLA), Jinan, China
- 2Department of Reproductive Medicine, The 960th Hospital of People's Liberation Army of China (PLA), Jinan, China
Objective: This study aims to investigate the mechanism by which Negative Pressure Wound Therapy (NPWT) regulates local immune responses in spinal infection through Piezo1-mediated mechanical stress, and elucidate its potential role in the treatment of spinal infections.
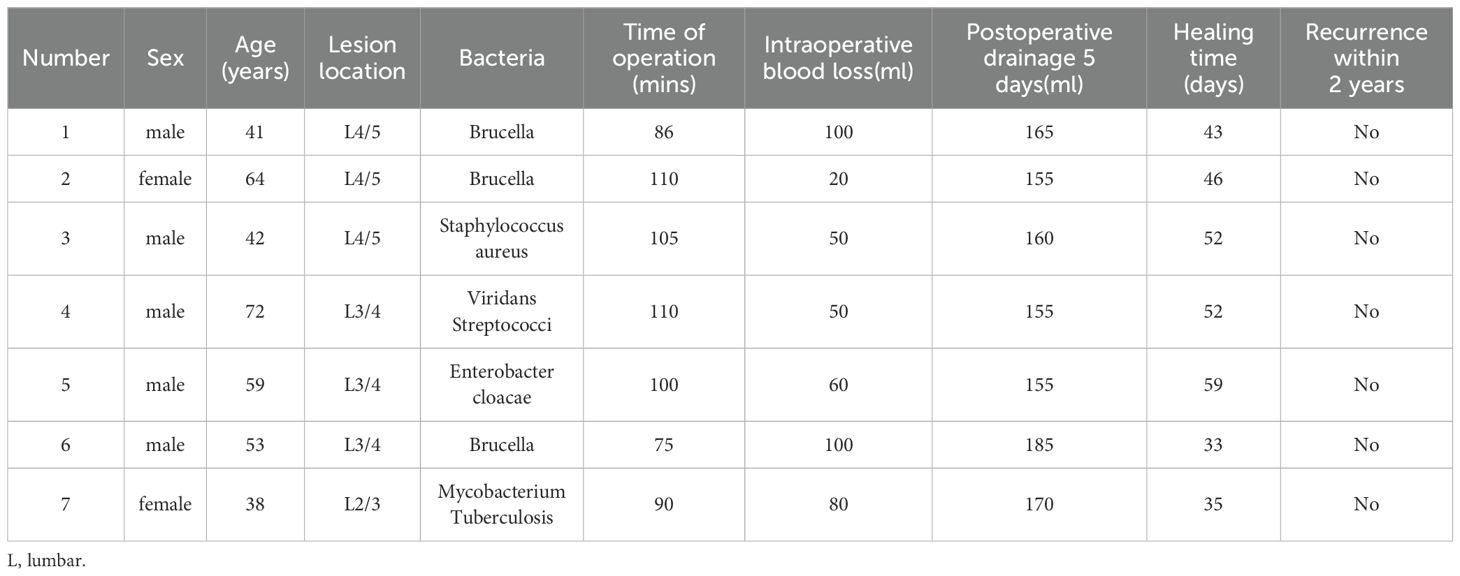
Methods: From July 2021 to April 2022, a total of 7 patients with spinal infection treated with NPWT at our department were included in the study. The study analyzed clinical outcomes of spinal infection surgeries, including operative duration, intraoperative blood loss, postoperative drainage, improvements in pain levels as measured by the Visual Analogue Scale (VAS), and inflammatory markers such as C-reactive protein (CRP) and Erythrocyte Sedimentation Rate (ESR) measured one week before and after the procedure. Additionally, healing times and recurrence rates within two years post-surgery were assessed. In addition, lesion specimens were retained during surgery and changes in Piezo1, Interleukin-1β (IL-1β), IL-6, IL-8, and Tumor Necrosis Factor-α (TNF-α) in lesion tissues were observed before and after immunohistochemical analysis.
Results: All 7 patients with spinal infections successfully underwent NPWT treatment and were ultimately cured. The average healing time was 45.71 ± 9.49 days, and there were no cases of recurrence or death during the two-year follow-up period. Surgical data showed a surgery duration of 96.57 ± 13.31 minutes, intraoperative blood loss of 65.71 ± 29.36 milliliters, and postoperative drainage of 163.57 ± 11.07 milliliters. Postoperatively, CRP, ESR, and VAS all significantly improved compared to preoperative levels (all p<0.05), which was superior to traditional treatment methods. Following NPWT intervention, the expression of Piezo1 protein at the lesion site significantly increased (0.03 ± 0.11 vs. 0.27 ± 0.22; p<0.05), while the expression levels of IL-1β, IL-6, IL-8, and TNF-α in the local immune microenvironment of the infected lesion significantly decreased (0.26 ± 0.11 vs. 0.16 ± 0.09, 0.27 ± 0.12 vs. 0.15 ± 0.67, 0.26 ± 0.18 vs. 0.10 ± 0.12, 0.35 ± 0.21 vs. 0.15 ± 0.11; p<0.05).
Conclusion: Clinical results demonstrate that NPWT treatment for spinal infections exhibits remarkable efficacy, accompanied by a notable augmentation in local Piezo1 protein consistency. It is hypothesized that the mechanical force employed in NPWT treatment stimulates the Piezo1 protein, thereby modulating local immune cells and factors, ultimately bolstering local immunity. This study not only provides a molecular biology basis for a deeper understanding of the therapeutic effects of NPWT, but also offers new insights for optimizing treatment strategies for spinal infections.
1 Introduction
With the arrival of an aging population, the incidence of bone and soft tissue infections is increasing, with spinal infections being common (1–3). Kehrer found that the incidence of spinal infections has increased dramatically to 2.2-5.8/100000 since 2000 (4). If spinal infections are not taken seriously or if treatment is ineffective, it can easily lead to the spread of infection and the formation of abscesses in the spine, causing serious complications such as nerve root or spinal cord compression and neurological dysfunction. Current surgical approaches often advocate for one-stage posterior lesion clearance combined with bone graft fusion and internal fixation, but they have drawbacks such as high trauma, low cure rates, and high mortality rates (5). Studies have shown that if the infection is not completely cleared from the body, it can lead to the continuous production of pro-inflammatory cytokines such as IL-1β, IL-6, IL-8, and TNF-α, which can limit the formation of granulation tissue and microvessels (6).
In recent years, multiple randomized controlled trials have highlighted the significant advantages of NPWT in treating infected wounds. NPWT is a sealed dressing system that applies mechanical stress to the wound, promoting healing through various biological mechanisms (7, 8). Piezo1, a mechanosensitive, non-selective cation channel, plays a pivotal role in transmitting mechanical-chemical signals that regulate cellular responses to external mechanical stress (9). Previous clinical evidence has demonstrated the benefits of NPWT in treating spinal infections, including promoting neutrophil chemotaxis to control infection spread and improving systemic inflammatory markers (10, 11). The primary mechanisms by which NPWT aids in treating infected wounds involve enhancing microvascular formation, stimulating granulation tissue generation, reducing bacterial load, and alleviating inflammation and edema (12–14). However, research on the impact of NPWT on the local immune microenvironment in spinal infections remains limited. Therefore, this study aims to further investigate the mechanisms underlying NPWT’s regulation of the local immune response in spinal infections, focusing on Piezo1-mediated mechanical stress and its effects on local inflammatory factors, with the goal of providing a more comprehensive theoretical foundation for the clinical application of this therapy.
2 Materials and methods
2.1 Standards for inclusion and exclusion, follow-up time and follow-up loss rate
This study was conducted from July 2021 to April 2022, with a total of 7 patients included. Inclusion criteria were: (1) Patients aged ≥18 years diagnosed with spinal infection through medical history, imaging, and pathology; (2) Patients receiving NPWT during the study period and able to provide sufficient and complete pathological specimens before and after treatment. Exclusion criteria were: (1) Patients with postoperative wound infections due to spinal surgery; (2) Patients receiving immunosuppressive therapy (such as biologics, immunosuppressive drugs, etc.), corticosteroids, or chemotherapy; (3) Patients with severe heart, kidney, liver, or other organ dysfunction, or other acute critical illnesses; (4) Patients refusing NPWT treatment for personal reasons or other factors. All patients will be followed up for at least 24 months after treatment. Patients who fail to attend follow-up appointments as scheduled and cannot be contacted by phone or other means will be considered lost to follow-up. From July 2021 to April 2022, our team treated a total of 21 patients with spinal infections, with 9 cases not receiving NPWT treatment and 12 cases receiving NPWT treatment. Out of these, 7 cases with pre- and post-operative pathological specimens were included in the study.
2.2 Data collection
Collect information on patient gender, age, diagnosis, pathogenic microorganisms, as well as surgical time, intraoperative blood loss, postoperative drainage volume, pre- and post-operative improvement in VAS, CRP, ESR, healing time, and 2-year recurrence rate. Additionally, obtain tissue samples from the infected lesion before and after NPWT treatment for spinal infection, and analyze the expression changes of Piezo1, IL-1β, IL-6, IL-8, and TNF-α in the lesion tissue using hematoxylin-eosin staining and immunohistochemical analysis.
2.3 Surgical procedure
All 7 patients with spinal infections underwent NPWT treatment during surgery. Sterile polyvinyl alcohol foam dressings were used intraoperatively, tailored to fit the size of the intervertebral infection wound. The dressings extended to the skin surface while another dressing covered the surgical incision on the skin surface, followed by a polyurethane medical dressing to create a sealed microenvironment. The foam dressings included a suction tube extending to the outside of the body, connected to an extension tube leading to a negative pressure collection bottle, which was connected to an adjustable negative pressure source (-125mmHg) (15) (as shown in Figure 1). Patients underwent adequate pathological tissue sampling from the infection site intraoperatively and 7 days postoperatively.
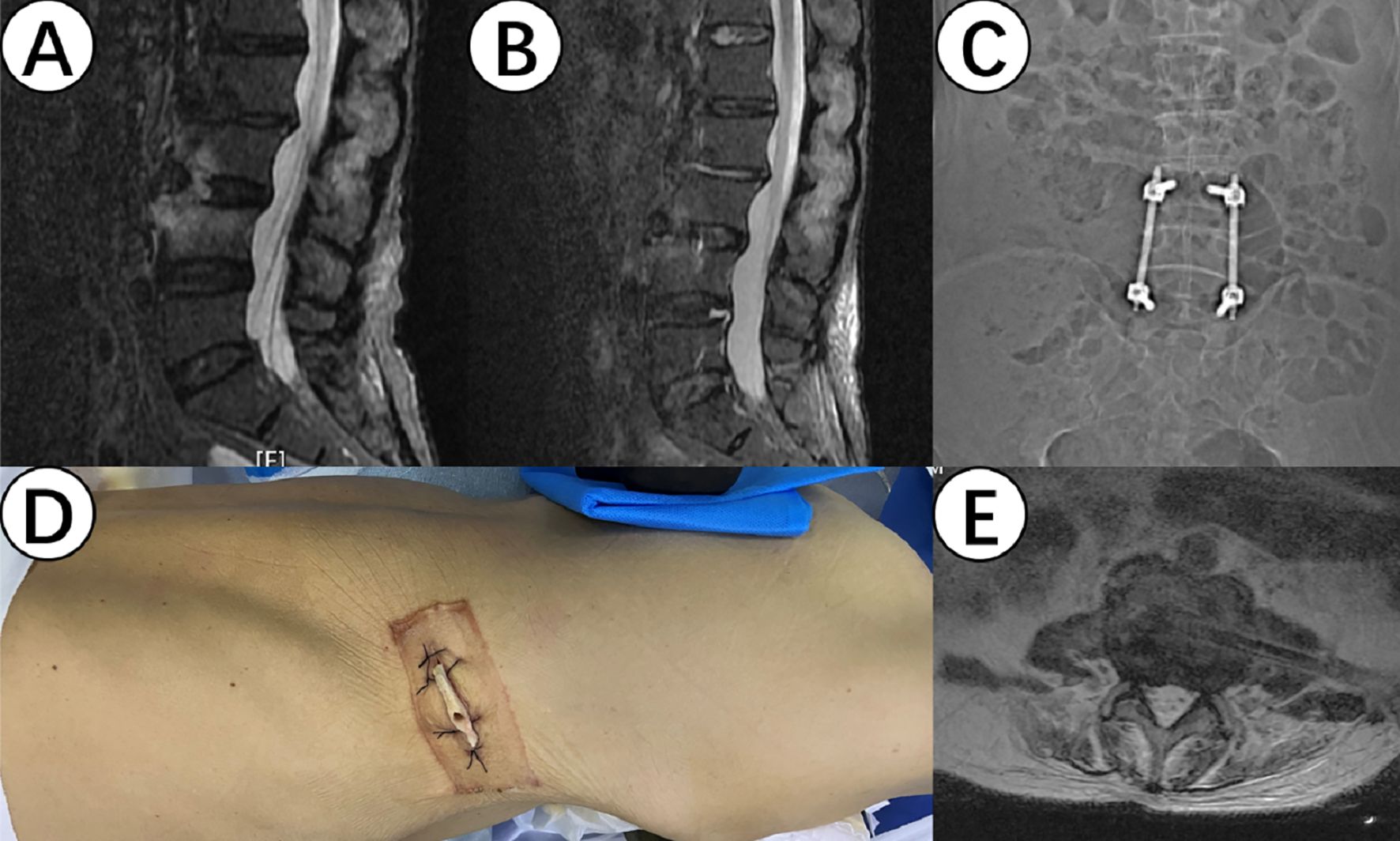
Figure 1. (A, B) show the magnetic resonance imaging (MRI) results of the lesion site before and after surgery, demonstrating a significant improvement in inflammatory signals. (C) shows internal fixation for intersegmental fixation to prevent lumbar instability and collapse. (D) shows the implantation of a drainage sponge through an extremely lateral approach. (E) shows the position of the drainage sponge in the intervertebral gap on the MRI.
2.4 Immunohistochemical analysis
Immunohistochemical analysis method was used to detect the expression of Piezo1, IL-1β, IL-6, IL-8, and TNF-α. Tissue samples were fixed in 10% buffered formalin, embedded in paraffin, and cut into standard 3μm sections using a pathological slicer (Shanghai Leica Instrument Co., RM2016). The sections were deparaffinized, underwent antigen retrieval, blocked endogenous peroxidase, and then blocked with Bovine Serum Albumin (BSA). Primary antibodies were incubated overnight at 4°C, followed by secondary antibodies incubated at 37°C for 50 minutes. Finally, the sections were counterstained with hematoxylin to stain the cell nuclei, dehydrated, and coverslipped (Figure 2). The immunohistochemistry slides were analyzed using Aipathwell digital pathology image analysis software (Servicebio®), and the positive staining areas were recorded.
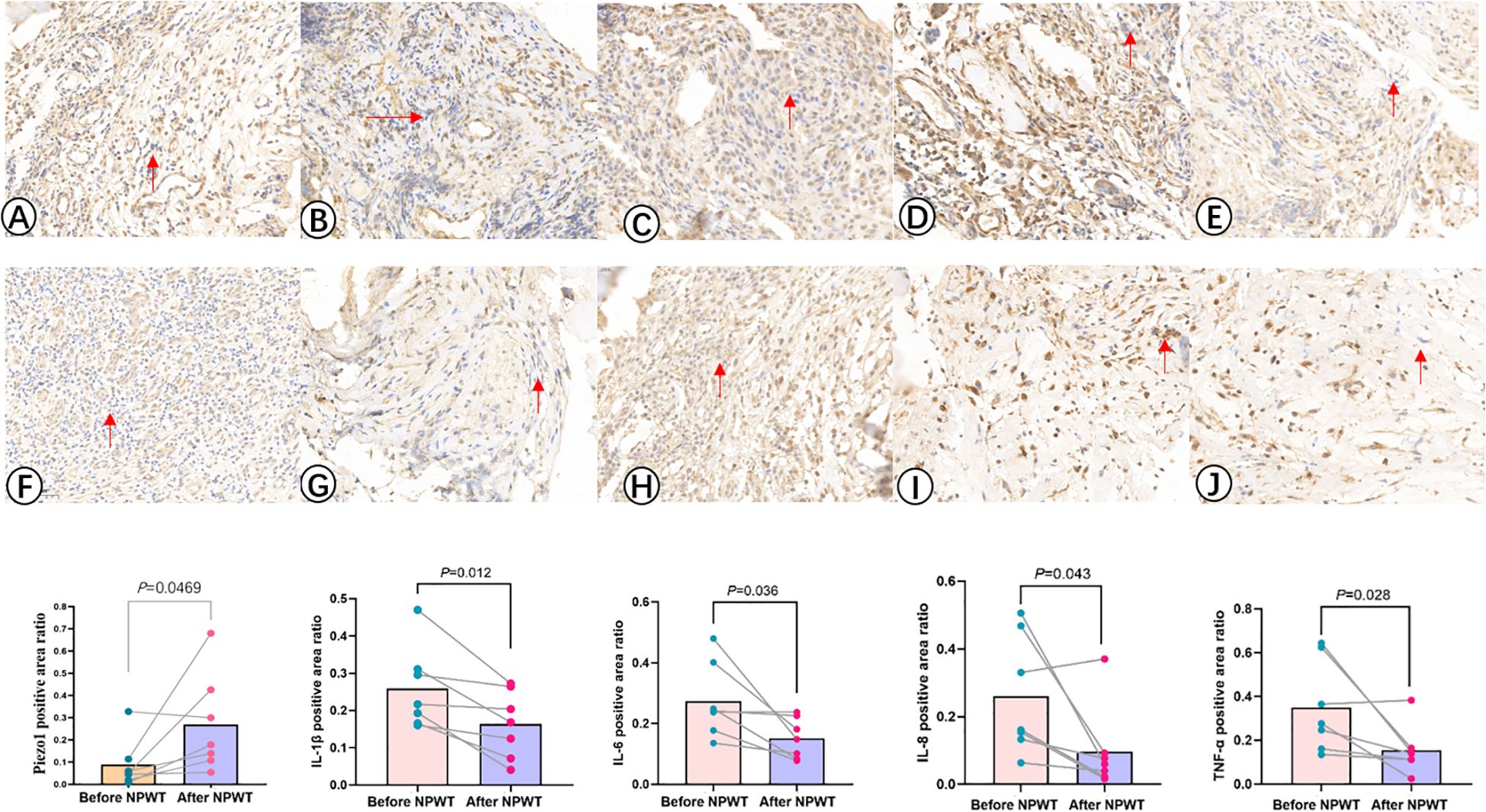
Figure 2. Immunohistochemistry images illustrate the expression levels of Piezo1, IL-1β, IL-6, IL-8, and TNF -α before (A–E) and after (F–J) NPWT treatment. The statistical result reveals a notable increase in the expression of Piezo1 after the treatment, along with a significant decrease in the expression of IL-1β, IL-6, IL-8, and TNF-α following NPWT intervention.
2.5 Statistical analysis
Statistical analysis was conducted using SPSS 21.0 software. Descriptive statistics for continuous data were presented as mean ± standard deviation, and normality and homogeneity of variance tests were performed. For normally distributed data, paired sample t-tests were used for comparison between the two groups; for non-normally distributed data, the Wilcoxon signed-rank test was used. A significance level of P < 0.05 was considered statistically significant.
3 Results
Among 7 patients diagnosed with spinal infections, 5 were male and 2 were female, with an average age of 52.7 ± 13.0 years (ranging from 38 to 72 years). The affected spinal levels were L2/3 in 1 case, L3/4 in 3 cases, and L4/5 in 3 cases. Following NPWT treatment, there was an improvement in systemic inflammatory markers such as CRP and ESR. Moreover, there was a significant short-term improvement in VAS (7.00 ± 0.82 Vs. 2.29 ± 0.76, P<0.05). The average time for complete healing was 45.71 ± 9.49 days, and there were no instances of recurrence or mortality during the 2-year postoperative follow-up. Surgical data revealed an average operation duration of 96.57 ± 13.31 mins, intraoperative blood loss of 65.71 ± 29.36ml, and postoperative drainage of 163.57 ± 11.07ml (Table 1).
The immunohistochemical analysis of Piezo1 and inflammatory factors in the infection lesions before and after treatment is shown in Table 2, Figure 2. The results of immunohistochemical examination suggest a significant decrease in inflammatory factors in the infected tissues after NPWT treatment, with a statistically significant difference.
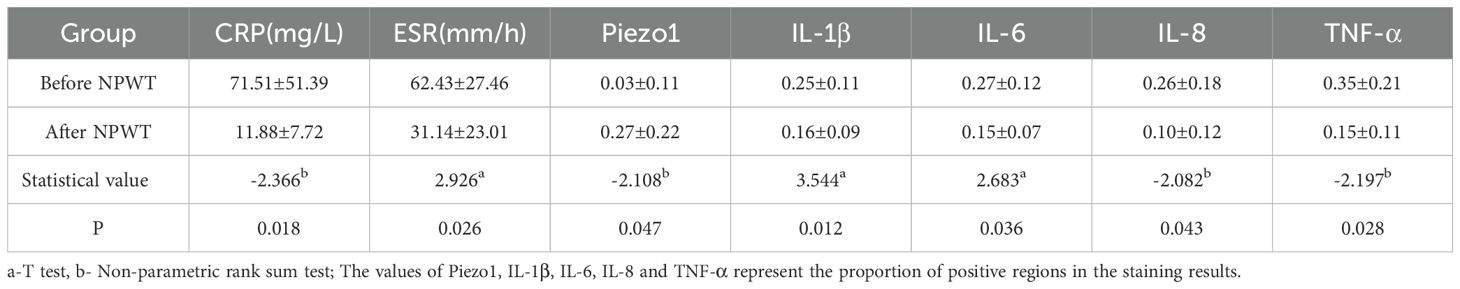
Table 2. Comparison of related detection and treatment before and after NPWT for spinal intervertebral space infection (7 cases).
4 Discussion
Spinal infection, as a special type of bone infection, has a higher recurrence rate and mortality rate compared to other bone infections, with reported recurrence rates of 10% and mortality rates reaching 20% (5). The main drawback of traditional treatment approaches is the inadequate blood supply to tissues, which significantly reduces the effectiveness of antibiotics. Additionally, the specific location of the wound also makes complete debridement challenging. NPWT has proven effective in promoting the healing of various complex wounds, such as extensive burns, postoperative incision infections, necrotizing fasciitis, and skin graft sites (16–19). Several randomized controlled trials have demonstrated that NPWT offers significant benefits in treating infected wounds by reducing local inflammation and edema, stimulating granulation tissue and microvascular formation, and lowering bacterial load (7, 8, 12–14). Our team has achieved favorable clinical outcomes using NPWT to treat spinal infections (10, 11). Pappalardo et al. (20) clearly highlighted the safety and effectiveness of NPWT in managing post-spinal surgery infections through a systematic review. In this study, all seven patients who received NPWT treatment experienced positive clinical outcomes, with an average healing time of 45.71 ± 9.49 days and no recurrence or mortality during the two-year follow-up period. Furthermore, all patients showed significant improvements in postoperative VAS scores, CRP, and ESR within a short period. Our clinical findings indicate that NPWT treatment is significantly more effective than traditional methods, consistent with reports in the literature. This suggests that NPWT not only facilitates drainage but also offers therapeutic benefits.
In addition to draining, NPWT also exerts mechanical force through negative pressure suction. Nonetheless, mechanical physical signals, in contrast to soluble chemical signals like cytokines, are less investigated as regulators of immune cell function. Atcha et al. (21) found that physical mechanical stress and soluble signals synergistically regulate macrophage morphology and function, proposing that the crosstalk between CD11b and Piezo1 plays a crucial role in mechanical transduction in macrophages. Orsini et al. (22) discussed the role of mechanical sensitive channels such as Piezo1 in immune cells under various physiological and pathological conditions. They believe that mechanical signals can activate various signaling pathways within cells through the activation of mechanosensitive ion channels and receptors, including MAPKs, YAP/TAZ, EDN1, NF-kB, and HIF-1a, thereby altering the physiological state of the cell. Mulhall et al. (23) used nanoscale fluorescence imaging technology to directly observe the conformational changes of Piezo1 within the cell membrane, demonstrating its direct response to mechanical stress. Following mechanical stimulation, Piezo1 enhances integrin β1 (ITGB1) activity, forming the Piezo1/ITGB1 signaling axis, which triggers cell cytoskeletal reorganization through the RhoA/ROCK pathway, thereby enhancing Piezo1’s mechanosensitivity and establishing a positive feedback loop (24, 25). Chen et al. (9) found that high blood flow shear stress activates Piezo1, leading to the activation of various inflammatory signaling pathways such as NF-κB in a rat model of pulmonary arterial hypertension induced by left pulmonary artery ligation. This results in upregulation of Piezo1 expression in pulmonary vascular endothelial cells. Similarly, by comparing infected tissue samples before and after NPWT treatment, we found a significant increase in Piezo1 expression levels after NPWT treatment, suggesting that the mechanical stress provided by NPWT and high blood flow shear stress may both upregulate Piezo1 expression in cells.
IL-1β, IL-6, IL-8, and TNF-α are inflammatory factors that play important roles in the immune microenvironment during the entire immune response process. IL-1β is an inductive cytokine mainly produced by activating M1 macrophages (26). Tao et al. (27) found that NPWT treatment for diabetic foot infection significantly reduced IL-1β expression after 7 days of use. IL-6 has a wide range of effects in immune regulation, acute phase response, regeneration, and bone homeostasis (28). Wang et al. (29) found that NPWT can significantly reduce the expression levels of IL-6 in infected wounds of diabetic foot patients, inhibit chronic inflammation, and promote wound healing. IL-8 is a leukocyte-specific chemotactic cytokine that plays an important role in recruiting polymorphonuclear neutrophils in the early inflammatory stage of wound healing (30). TNF-α is a classical pro-inflammatory cytokine mainly produced by activated macrophages, playing a crucial role in immune responses in the immune microenvironment, including regulating inflammation, cell apoptosis, and immune cell proliferation. Norbury et al. found that the levels of TNF-α in wound drainage fluid decreased after NPWT treatment in experimental animals (31). This study focuses on the changes in the expression levels of inflammatory factors IL-1β, IL-6, IL-8, and TNF-α in infected local tissues. Continuous application of NPWT mechanical stress was found to decrease the expression of IL-1β, IL-6, IL-8, and TNF-α.
Research has shown a close association between Piezo1 and most inflammatory factors (32, 33). Piezo1, as a mechanosensitive ion channel protein, mediates calcium influx into immune cells such as macrophages, dendritic cells, and neutrophils under NPWT mechanical stress intervention, activating various downstream signaling pathways to elicit a series of immune response reactions. For example, Yang et al. found that Piezo1 activation enhances NF-κB pathway activity, promoting the production of inflammatory factors such as IL-1β, IL-6, IL-8, and TNF-α (34). However, in our clinical practice, we found that the expression levels of inflammatory factors in local tissues decreased after 7 days of NPWT mechanical stress intervention. This may be due to two reasons: (1) Piezo1 promotes wound healing by regulating the proliferation, migration, and collagen synthesis of fibroblasts, reducing continuous tissue damage and indirectly slowing down the duration and severity of the inflammatory response (35); (2) Piezo1 has a certain pro-angiogenic effect, which helps improve local blood circulation, reduce wound edema, and promote early wound healing (36).
During the early stages of normal healing, a temporary increase in inflammatory factors helps promote immune cells (such as neutrophils, macrophages, etc.) to accelerate the clearance of bacteria and tissue necrosis. Activation of Piezo1 can enhance local immune responses. However, in the late stages of healing, prolonged excessive presence of inflammatory cells and factors can lead to continued inflammation, hindering tissue repair and healing. Therefore, it is necessary to regulate inflammation appropriately to avoid excessive inflammation affecting the healing process. This study found that after 7 days of NPWT on spinal infection lesions, the expression of inflammatory factors such as IL-1β, IL-6, IL-8, and TNF-α in the local immune microenvironment significantly decreased, indicating that the spinal infection had gradually been controlled and entered the tissue repair stage. Therefore, it is speculated that NPWT can regulate local tissue immune changes, allowing various inflammatory factors to be produced, elevated, and decreased in an orderly manner, thereby promoting the clearance of local bacteria and tissue repair.
Although there is limited research on the mechanism of NPWT in treating spinal infections, we speculate that NPWT promotes healing by regulating the local immune microenvironment based on clinical and immunohistochemical analysis. Research has shown that inflammatory factors such as IL-1β, IL-6, IL-8, and TNF-α can induce and recruit various types of white blood cells to participate in immune responses, which may lead to tissue damage and affect the healing of infections if present long-term (26, 37, 38). Therefore, changes in the expression of inflammatory factors in the local immune microenvironment are closely related to the progression of infections. Piezo1, as a key protein that allows immune cells to receive mechanical signals and convert them into chemical signals, can sense the mechanical stress of NPWT and regulate immune response reactions in the local immune microenvironment of infections.
NPWT has the potential to enhance the local immune response in spinal infections through Piezo1 mediation. Clinical studies have shown that NPWT can improve outcomes, reduce healing times, and manage infection rates in patients with spinal infections. Immunohistochemical analysis has demonstrated an increase in Piezo1 protein expression levels and a significant decrease in inflammatory factors (IL-1β, IL-6, IL-8, TNF-α) in the immune microenvironment following NPWT treatment. This indicates that NPWT activates Piezo1 protein to regulate the immune response at the site of spinal infection, leading to inflammation control and tissue repair. However, further research involving cellular and animal experiments is necessary to fully understand the physiological functions of the Piezo1 signaling pathway. It is important to consider individual differences among patients that may impact treatment outcomes, and future studies should aim to include a larger sample size for more reliable conclusions.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Committee of Ethics at the 960th Hospital of the PLA. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. The manuscript presents research on animals that do not require ethical approval for their study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HX: Data curation, Formal Analysis, Investigation, Writing – original draft. JP: Formal Analysis, Methodology, Project administration, Writing – original draft. HL: Data curation, Formal Analysis, Investigation, Writing – original draft. YW: Data curation, Formal Analysis, Writing – original draft. ZC: Conceptualization, Data curation, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by Natural Science Foundation of Shandong Province (Award Number: ZR2023MH331; Grant Recipient: Zhengqi Chang).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Leowattana W, Leowattana P, and Leowattana T. Tuberculosis of the spine. World J Orthop. (2023) 14:275–93. doi: 10.5312/wjo.v14.i5.275
2. Collaborators GUHD. Life expectancy by county, race, and ethnicity in the USA, 2000-19: a systematic analysis of health disparities. Lancet. (2022) 400:25–38. doi: 10.1016/S0140-6736(22)00876-5
3. Bai R, Liu Y, Zhang L, Dong W, Bai Z, and Zhou M. Projections of future life expectancy in China up to 2035: a modelling study. Lancet Public Health. (2023) 8:e915–22. doi: 10.1016/S2468-2667(22)00338-3
4. Kehrer M, Pedersen C, Jensen TG, and Lassen AT. Increasing incidence of pyogenic spondylodiscitis: a 14-year population-based study. J Infect. (2014) 68:313–20. doi: 10.1016/j.jinf.2013.11.011
5. Rutges JP, Kempen DH, van Dijk M, and Oner FC. Outcome of conservative and surgical treatment of pyogenic spondylodiscitis: a systematic literature review. Eur Spine J. (2016) 25:983–99. doi: 10.1007/s00586-015-4318-y
6. Zhang H, Zhou M, Wang Y, Zhang D, Qi B, and Yu A. Role of autologous fat transplantation combined with negative-pressure wound therapy in treating rat diabetic wounds. Plast Reconstr Surg. (2023) 152:561–70. doi: 10.1097/PRS.0000000000010226
7. Campitiello F, Mancone M, Corte AD, Guerniero R, and Canonico S. Expanded negative pressure wound therapy in healing diabetic foot ulcers: a prospective randomised study. J Wound Care. (2021) 30:121–9. doi: 10.12968/jowc.2021.30.2.121
8. Sahin E, Rizalar S, and Ozker E. Effectiveness of negative-pressure wound therapy compared to wet-dry dressing in pressure injuries. J Tissue Viability. (2022) 31:164–72. doi: 10.1016/j.jtv.2021.12.007
9. Chen J, Miao J, Zhou D, Liao J, Wang Z, Lin Z, et al. Upregulation of mechanosensitive channel Piezo1 involved in high shear stress-induced pulmonary hypertension. Thromb Res. (2022) 218:52–63. doi: 10.1016/j.thromres.2022.08.006
10. Xing W, Yang Y, Bai Y, Yu X, and Chang Z. A comparison of negative pressure and conventional therapy in spine infections: A single-center retrospective study. J Pers Med. (2023) 13(2):162. doi: 10.3390/jpm13020162
11. Li J and Chang Z. Case Report: A spinal infection with bilateral psoas abscesses was treated with NPWT to enhance the local infection by increasing the infiltration of neutrophil cells and draining the pus. Front Cell Infect Microbiol. (2023) 13:1228376. doi: 10.3389/fcimb.2023.1228376
12. Almeida IR, Coltro PS, Goncalves HOC, Westin AT, Almeida JB, Lima R, et al. The role of negative pressure wound therapy (NPWT) on the treatment of pyoderma gangrenosum: A systematic review and personal experience. Wound Repair Regener. (2021) 29:486–94. doi: 10.1111/wrr.12910
13. Huang C, Leavitt T, Bayer LR, and Orgill DP. Effect of negative pressure wound therapy on wound healing. Curr Probl Surg. (2014) 51:301–31. doi: 10.1067/j.cpsurg.2014.04.001
14. Pennington E, Bell S, and Hill JE. Should video laryngoscopy or direct laryngoscopy be used for adults undergoing endotracheal intubation in the pre-hospital setting? A critical appraisal of a systematic review. J Paramed Pract. (2023) 15:255–9. doi: 10.1002/14651858
15. Banwell PE and Teot L. Topical negative pressure (TNP): the evolution of a novel wound therapy. J Wound Care. (2003) 12:22–8. doi: 10.12968/jowc.2003.12.1.26451
16. Song H, Xu Y, Chang W, Zhuang J, and Wu X. Negative pressure wound therapy promotes wound healing by suppressing macrophage inflammation in diabetic ulcers. Regener Med. (2020) 15:2341–9. doi: 10.2217/rme-2020-0050
17. Peinemann F and Labeit A. Negative pressure wound therapy: A systematic review of randomized controlled trials from 2000 to 2017. J Evid Based Med. (2019) 12:125–32. doi: 10.1111/jebm.2019.12.issue-2
18. Agarwal A. Management of closed incisions using negative-pressure wound therapy in orthopedic surgery. Plast Reconstr Surg. (2019) 143:21S–6S. doi: 10.1097/PRS.0000000000005308
19. Wang Y, Beekman J, Hew J, Jackson S, Issler-Fisher AC, Parungao R, et al. Burn injury: Challenges and advances in burn wound healing, infection, pain and scarring. Adv Drug Delivery Rev. (2018) 123:3–17. doi: 10.1016/j.addr.2017.09.018
20. Pappalardo G, Schneider S, Kotsias A, Jeyaraman M, Schäfer L, and Migliorini F. Negative pressure wound therapy in the management of postoperative spinal wound infections: a systematic review. Eur J Orthop Surg Traumatol. (2024) 34:2303–13. doi: 10.1007/s00590-024-03983-x
21. Atcha H, Meli VS, Davis CT, Brumm KT, Anis S, Chin J, et al. Crosstalk between CD11b and piezo1 mediates macrophage responses to mechanical cues. Front Immunol. (2021) 12:689397. doi: 10.3389/fimmu.2021.689397
22. Orsini EM, Perelas A, Southern BD, Grove LM, Olman MA, and Scheraga RG. Stretching the function of innate immune cells. Front Immunol. (2021) 12:767319. doi: 10.3389/fimmu.2021.767319
23. Mulhall EM, Gharpure A, Lee RM, Dubin AE, Aaron JS, Marshall KL, et al. Direct observation of the conformational states of PIEZO1. Nature. (2023) 620:1117–25. doi: 10.1038/s41586-023-06427-4
24. Ma M, Li X, Jing M, Zhang P, Zhang M, Wang L, et al. Enhanced Tumor-Targeted Delivery of Arginine-Rich Peptides via a Positive Feedback Loop Orchestrated by Piezo1/integrin beta1 Signaling Axis. Adv Sci (Weinh). (2024) 11:e2409081. doi: 10.1002/advs.202409081
25. Emig R, Knodt W, Krussig MJ, Zgierski-Johnston CM, Gorka O, Gross O, et al. Piezo1 channels contribute to the regulation of human atrial fibroblast mechanical properties and matrix stiffness sensing. Cells. (2021) 10(3):663. doi: 10.3390/cells10030663
26. Facchin BM, Dos Reis GO, Vieira GN, Mohr ETB, da Rosa JS, Kretzer IF, et al. Inflammatory biomarkers on an LPS-induced RAW 264.7 cell model: a systematic review and meta-analysis. Inflammation Res. (2022) 71:741–58. doi: 10.1007/s00011-022-01584-0
27. Wang T, Fan L, Liu J, Tao Y, Li X, Wang X, et al. Negative pressure wound therapy promotes wound healing by inhibiting inflammation in diabetic foot wounds: A role for NOD1 receptor. Int J Low Extrem Wounds. (2022). doi: 10.1177/15347346221131844
28. Kang S, Narazaki M, Metwally H, and Kishimoto T. Correction: Historical overview of the interleukin-6 family cytokine. J Exp Med. (2020) 217(5):jem.2019034704212020c. doi: 10.1084/jem.2019034704212020c
29. Wang T, Li X, Fan L, Chen B, Liu J, Tao Y, et al. Negative pressure wound therapy promoted wound healing by suppressing inflammation via down-regulating MAPK-JNK signaling pathway in diabetic foot patients. Diabetes Res Clin Pract. (2019) 150:81–9. doi: 10.1016/j.diabres.2019.02.024
30. Jafri M, Li L, Liang B, and Luo M. The effect of heparin and other exogenous glycosaminoglycans (GAGs) in reducing IL-1beta-induced pro-inflammatory cytokine IL-8 and IL-6 mRNA expression and the potential role for reducing inflammation. Pharmaceuticals (Basel). (2024) 17(3):371. doi: 10.3390/ph17030371
31. Norbury K and Kieswetter K. Vacuum-assisted closure therapy attenuates the inflammatory response in a porcine acute wound healing model. Wounds. (2007) 19:97–106.
32. Shi S, Kang XJ, Zhou Z, He ZM, Zheng S, and He SS. Excessive mechanical stress-induced intervertebral disc degeneration is related to Piezo1 overexpression triggering the imbalance of autophagy/apoptosis in human nucleus pulpous. Arthritis Res Ther. (2022) 24:119. doi: 10.1186/s13075-022-02804-y
33. Fish A and Kulkarni A. Flow-induced shear stress primes NLRP3 inflammasome activation in macrophages via piezo1. ACS Appl Mater Interfaces. (2024) 16:4505–18. doi: 10.1021/acsami.3c18645
34. Yang Z, Zhao Y, Zhang X, Huang L, Wang K, Sun J, et al. Nano-mechanical immunoengineering: nanoparticle elasticity reprograms tumor-associated macrophages via piezo1. ACS Nano. (2024) 18:21221–35. doi: 10.1021/acsnano.4c04614
35. Liu S, Chen Y, Zhou G, Sun C, Ma M, Huang R, et al. Uniform and controllable surface nano-structure on polyetheretherketone implants can regulate mechanical property to enhance soft tissue integration through Piezo1/TGF-beta1 signaling axis. Mater Today Bio. (2025) 31:101645. doi: 10.1016/j.mtbio.2025.101645
36. Li J, Hou B, Tumova S, Muraki K, Bruns A, Ludlow MJ, et al. Piezo1 integration of vascular architecture with physiological force. Nature. (2014) 515:279–82. doi: 10.1038/nature13701
37. Zhu Y, Han Q, Wang L, Wang B, Chen J, Cai B, et al. Jinhua Qinggan granules attenuates acute lung injury by promotion of neutrophil apoptosis and inhibition of TLR4/MyD88/NF-kappaB pathway. J Ethnopharmacol. (2023) 301:115763. doi: 10.1016/j.jep.2022.115763
Keywords: spinal infection, negative pressure wound therapy, Piezo1, mechanical force, immune response
Citation: Xing H, Pan J, Liu H, Wang Y and Chang Z (2025) Preliminary research indicates that mechanical force through Pioze1 enhances local immunity during NPWT treatment for spinal infections. Front. Immunol. 16:1600194. doi: 10.3389/fimmu.2025.1600194
Received: 26 March 2025; Accepted: 14 May 2025;
Published: 03 June 2025.
Edited by:
Taotao Liang, Sports Medicine Center of the First Affiliated Hospital of Army Medical University, ChinaReviewed by:
Mingjie Kuang, Shandong Provincial Hospital, ChinaTing Wang, The Affiliated Hospital of Qingdao University, China
Huifeng Yang, General Hospital of Northern Theater Command, China
Copyright © 2025 Xing, Pan, Liu, Wang and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhengqi Chang, MjY3NjY3NzFAcXEuY29t
†These authors have contributed equally to this work
 Hao Xing
Hao Xing Junlin Pan2†
Junlin Pan2† Zhengqi Chang
Zhengqi Chang