- 1Center for Food Animal Health, Department of Animal Sciences, College of Food, Agricultural, and Environmental Sciences, The Ohio State University, Wooster, OH, United States
- 2Department of Population Medicine and Diagnostic Sciences, Animal Health Diagnostic Center, College of Veterinary Medicine, Cornell University, Ithaca, NY, United States
- 3Department of Veterinary Biosciences, College of Veterinary Medicine, The Ohio State University, Columbus, OH, United States
In addition to neutralizing activity, antibodies can contribute to protection against viral infections through antibody-dependent cellular cytotoxicity (ADCC) and antibody-mediated complement-dependent cell cytotoxicity (CDC) mediated via Fcy receptors. Swine is a suitable large-animal biomedical model for influenza research, because it is a natural host for influenza like humans exhibiting comparable clinical and immunological responses. Unfortunately, there are currently limited insights into ADCC and CDC functions to swine influenza A virus (SwIAV) in pigs due to lack of adequate immunological tools. Therefore, the present study was aimed at optimizing the ADCC and CDC assays to evaluate the cytotoxicity mediated by virus-specific antibodies in response to vaccination of pigs with chitosan nanoparticle-based inactivated monovalent and commercial multivalent SwIAV vaccines administered through intranasal and intramuscular route, respectively. Using these assays, we quantified and compared the antibody-mediated cytotoxicity induced in pigs by intranasal chitosan nanoparticle-based inactivated monovalent whole SwIAV vaccine and intramuscular administered commercial multivalent SwIAV vaccine. Our results revealed that maternal antibody-positive pigs following vaccination with whole inactivated virus failed to elicit specific ADCC-mediating antibodies, but production of CDC antibodies was not affected. However, after exposure of vaccinated animals to challenge infection, high levels of ADCC antibodies were elicited. Further, it was observed that the function of virus-specific neutralizing and non-neutralizing antibodies are influenced by route of vaccination (intranasal versus intramuscular), vaccine type (monovalent versus multivalent) and adjuvant formulation. Overall, we observed a positive trend among the magnitude of ADCC, CDC, antibody avidity, Nabs, and HA inhibition (HAI) antibody responses in vaccinated and influenza virus-infected pigs. In conclusion, measuring ADCC- and CDC-mediating antibodies in pigs is important for evaluating the protective immunity against influenza by vaccines. Monitoring the function of both virus-neutralizing and non-neutralizing antibodies in vaccinated animals aid in the development of innovative cross-protective vaccine formulations to fight against constantly evolving influenza viruses.
Introduction
Swine influenza A virus (SwIAV) causes an acute respiratory illness in pigs, with periodical zoonotic infections in humans from few SwIAV strains (1), like the 2009 pandemic influenza (2). Similar to humans, the most common circulating subtypes of IAV in swine population are H1N1, H1N2, and H3N2 (3). An effective vaccination is recommended to reduce the disease burden in the swine population caused by SwIAV and its transmission to humans (4). The development of a universal influenza vaccine capable of eliciting cross-protective antibodies remains one of the top priorities globally. While neutralizing antibodies (nAbs) can block the infection and reduce the influenza virus replication, desired levels of its titer against variant strains of virus have not yet been achieved by existing vaccines. Despite the fact that non-nAbs (nNAbs) can bind viruses, they are unable to prevent infection in the cell culture system, and such antibodies are documented in influenza, rotavirus, HIV, cytomegalovirus, and SARS-CoV-2 infections (5). Interestingly, the role of nNAbs in vivo in protection against viruses has not been widely appreciated by the scientific community due to the lack of optimized immunological tools, especially in pigs and other large animal species, unlike in rodent models and humans. However, increasing evidence suggests that the induction of virus-specific nNAbs is an important correlation of protection (4). While nNAbs cannot prevent the entry of viruses into their host cell, they facilitate the recruitment of effector proteins or immune cells that can destroy the immune complex containing virus (4). Several nNAbs also mediate their effector function intracellularly either by direct blocking of the virus replication or by recruiting the Fc receptor expressing phagocytic cells (5).
Hemagglutinin inhibition (HAI) and virus neutralization (VN) assays are commonly employed for the detection and quantification of influenza-specific antibodies following infection or vaccination. Generally, HAI antibody titers are accepted as proxy for VN antibodies and are widely used to assess the efficacy of influenza vaccines (6). However, limited application of HAI assay has been reported, pointing the relevance of some non-HAI-mediated process as additional correlates of protection (7). The HA glycoprotein of influenza virus is composed of two domains: variable globular head as immunodominant receptor binding domain (RBD) and conserved stalk domain. The IAV-specific NAbs are typically directed to epitopes located in and around the viral RBD preventing virus binding to the target cells (8). Hence, mismatch between circulating strains of influenza results in compromised neutralization; however, it is likely that nNAbs and non-HAI antibodies might play a crucial role in protection in vaccine-mediated immune response (9, 10). Effector functions of the HA-stalk-specific nNAbs includes antibody-dependent phagocytosis (ADP) (11, 12), antibody-dependent cellular cytotoxicity (ADCC) (13), and antibody-mediated complement-dependent cell cytotoxicity (CDC), which may be critical in broader cross-protection against influenza (14–16). All these effector functions of nNAbs and non-HAI antibodies are mediated by Fcy receptors, contributing to protective immunity against influenza and other viral infections (17–19). Mostly, the conserved regions of influenza virus surface proteins are target for nNAbs, hence partially overcoming the emergence of NAbs escape mutants mediated by antigenic drift and antigenic shift in IAV (20).
NK cells are an important component of innate immunity during influenza virus infection because of their direct involvement in viral clearance (21, 22). NK cells mediate ADCC effector function by binding the antigen-bound IgG via FcyRIII (CD16). This results in the activation of NK cells and, consequently, the degranulation process to release lytic granules (perforin/granzymes) as well as secretion of antiviral cytokines such as tumor necrosis factor alpha (TNFα) and interferon gamma (IFNy). The role of the complement system (both classical and alternative pathways) is also critical in influenza virus infection or vaccination. Virion opsonization and its efficient lysis mediated by nNAbs through the complement system induced in response to infection or vaccination is another important humoral response against influenza (16).
Demonstration of the presence of influenza-specific ADCC and CDC antibodies was started in 1978 (23, 24). In mouse and human systems for measuring ADCC and CDC functions, we have optimized commercial kits. The antibody IgG1 isotype is involved in Fc‐effector functions of ADCC via FcγRIIIa expressed on macrophages and NK cells (25). The IgG1 isotype was shown to be superior to other isotypes, inducing ADCC as well as CDC functions (26). However, in the pig system, we do not have satisfactory tools to precisely delineate the IgG isotypes. Strategies to assess the role of cell-mediated immunity in response to SwIAV vaccines in pigs have already been demonstrated (27, 28); however, there have been limited studies and tools for estimating the SwIAV-specific ADCC and CDC antibodies in pigs. Therefore, developing and validating the ADCC and CDC assays in pig system is important.
Studies have shown that maternally derived antibody (MDA)-positive piglets have interference in induction of immunity following intramuscular SwIAV vaccination (29–32). In this study, we optimized the lactate dehydrogenase (LDH) release assay for the detection of pig HA-specific antibodies having ADCC and CDC activity. We validated the assays using serum samples archived from our previous two intranasal administered monovalent SwIAV nanoparticle-based vaccine trials, including a commercial multivalent SwIAV vaccine as control, conducted in both specific pathogen (influenza)-free and MDA-positive pigs (33, 34). Furthermore, when the ADCC and CDC data with VN, HAI, and antibody avidity data were compared, we did find an overall positive trend among all the different functional antibodies in pigs following vaccination and influenza infection. However, preexisting influenza-specific MDA strongly interfered with the generation of ADCC, but not CDC antibodies to inactivated whole SwIAV vaccines in pigs. In conclusion, we optimized the ADCC and CDC antibody functions specific to SwIAV in pigs, validated the assays, and revealed the trends among different antibody functions to influenza vaccination and infection in pigs.
Material and methods
Generation of MDCK cells stably expressing HA
To generate stable MDCK cells expressing HA from the SwIAV A/H1N1/OH/2007 strain, the full-length HA sequence was modified by replacing its transmembrane domain with that of PDGFRβ and adding a Myc-tag epitope to the 3' end. The PCR amplification of the HA region from the parental SwIAV A/H1N1/OH/2007 virus was performed using the following primers: forward primer 5'-ACTGCGGATCCATGAAGGCCATCCTGGTGGTGCTGCTGTACACCTTCACCA and reverse primer 3'-ACTGCCTCGAGCGGCCGCCTAACGTGGCTTCTTCT (1,808 bp). The amplified HA construct was then cloned into the lentiviral vector, pScalps (Addgene plasmid 99636, Watertown, Massachusetts, USA) (35) via enzymatic digestion with BamHI and XhoI (New England Biolabs, Ipswich, MA, USA), which corresponded to restriction sites included in the forward and reverse primers, respectively. Successful integration of the HA gene was confirmed by plasmid digestion followed by 1% agarose gel electrophoresis and detection of the Myc-tag epitope in transfected HEK 293T cells by immunofluorescence assay (IFA) (data not shown) and Western Blot (Figure 1) using an anti-Myc-tag antibody (9B11, Cell Signaling Technology, Danvers, MA, USA).
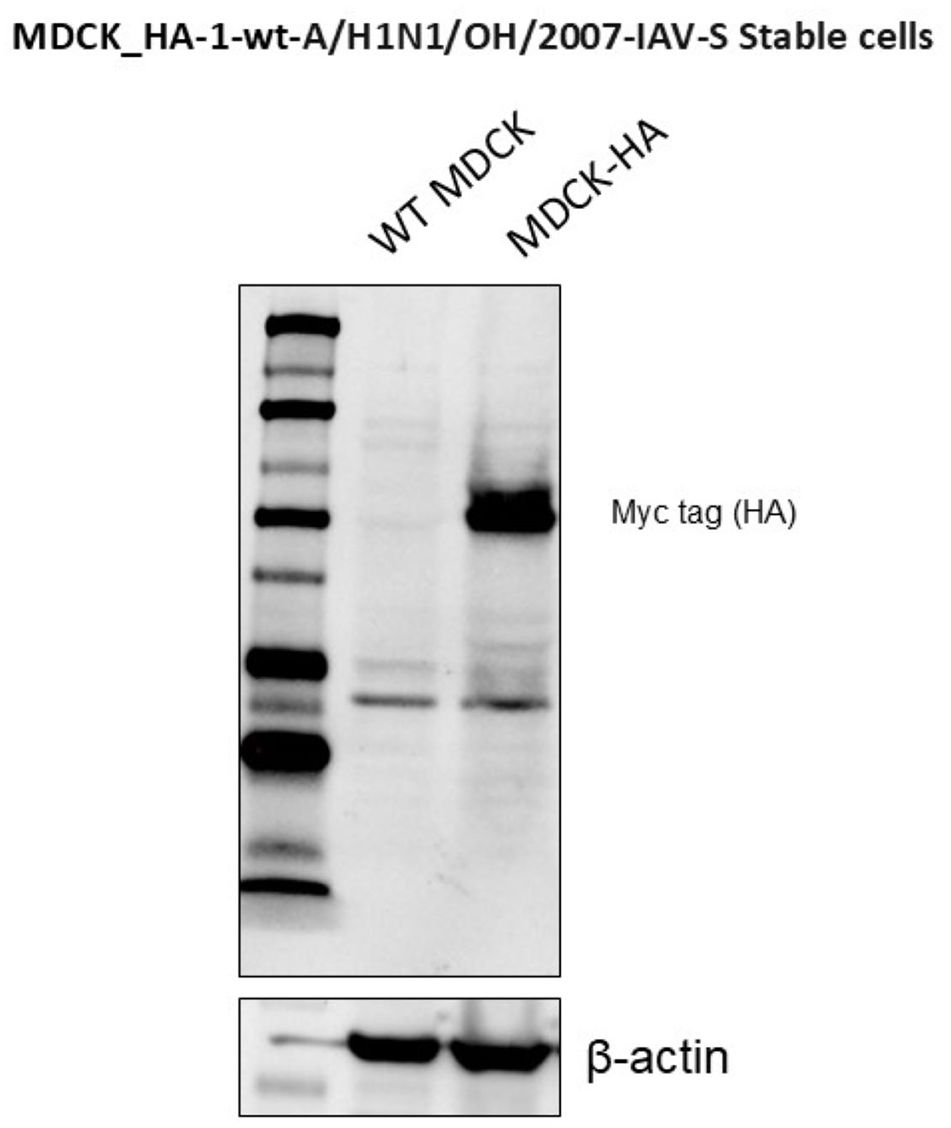
Figure 1. Stable expression of HA protein in transfected MDCK cells was detected throughout at different passages by Western blot.
HEK 293T cells were cultured in complete media comprising Minimum Essential Medium (MEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mM L-glutamine, and antibiotics (penicillin, streptomycin, and gentamicin) at a density of 4 × 105 cells per well in a six-well plate. After 24 h, the media was replaced, and cells were transfected with 1,000 ng of the generated lentiviral transfer plasmid, 750 ng of the human immunodeficiency virus (HIV) lentiviral packaging plasmid psPAX, and 1,000 ng of the VSV-G-encoding plasmid pMD2.G, using the Lipofectamine 3000 transfection reagent (Life Technologies, Carlsbad, CA, USA). Cell supernatants were harvested at 72 h post-transfection, centrifuged, and used for cell transduction.
MDCK cells were cultured in complete media at a similar density in a six-well plate. After 24 h, the media was removed, and cells were transduced with 500 µL of the lentiviral preparation per well. Control non-transduced cells received 500 µL of MEM cultured either with or without puromycin selection. During the 2-h adsorption period at 37°C, plates were gently tilted every 15–20 min. Subsequently, 1.5 mL of fresh complete media was added to each well, and cells were incubated at 37°C for 48 h. A puromycin kill curve was performed to determine the optimal concentration for selection, with concentrations ranging from 0 to 10 µg/mL. The higher concentration following the one causing 100% cell death was selected for generating stable cells. After 48 h of incubation, puromycin selection was initiated and continued until stable MDCK-HA cells were established.
Antibody-dependent cellular cytotoxicity
Stably transfected MDCK-HA cells were used as target cells for the ADCC assay. The assay was performed as described previously with several modifications (36). Briefly, 8,000 MDCK-HA cells per well were seeded in round bottom 96-well plates overnight at 37°C with 5% CO2. Cells were washed thrice with 1× phosphate buffered saline (PBS) and incubated with heat-inactivated serum samples (1:25 dilution) for 30 min at 37°C. Following three washings with 1× PBS, freshly isolated pig peripheral blood mononuclear cells (PBMCs) (source of effector cells) from healthy slaughtered adult pigs were added in pre-titrated 80:1 effector-to-target ratio (E:T) to the round bottom 96-well plates and incubated for 4 h at 37°C with 5% CO2. Both the target and effector cells were cultured in serum-free AIM-V medium containing 5% Immune Cell Serum Replacement (Life Technologies, UK) along with 100 IU/mL penicillin and 100 µg/mL streptomycin. Following incubation, 50 μL of cell-free supernatant was collected and transferred into a 96-well flat bottom plate and the LDH release is measured using the Cytotox 96 non-radioactive cytotoxicity assay kit (Promega, Leiden, Netherlands) according to the manufacturer’s instructions. The absorbance was measured at an optical density (OD) of 490 nm in a plate reader. The ADCC activity of serum samples was assessed using the following formula: [corrected experimental OD490 (experimental sample with target cells and PBMC) − corrected effector spontaneous OD490 (PBMC spontaneous release without target cells) − corrected target spontaneous OD490 (target spontaneous release without effector cells)] divided by [corrected target maximum OD490 (maximum release of target cells in the presence of 0.8% Triton® X-100) − corrected target spontaneous OD490 (target spontaneous release without effector cells)] × 100. We optimized the dilution of serum 1:25 to treat target cells, which provided the least background with high specific ADCC activity.
Complement-dependent cell cytotoxicity
The CDC was determined as described previously with slight modifications (37). Briefly, 30,000 MDCK-HA cells per well were seeded in round bottom 96-well plates overnight at 37°C with 5% CO2. Subsequently, cells were washed thrice with 1× PBS and incubated with heat-inactivated serum samples at pre-titrated 1:25 dilution in serum-free AIM-V medium (Life Technologies, UK) containing 100 IU/mL penicillin and 100 µg/mL streptomycin for 30 min at 37°C. Following three washes with 1× PBS, 1:10 diluted Low-Tox rabbit complement (Cedarlane, Burlington, Ontario, Canada) in serum-free AIM-V medium was added, and the plate was incubated for 2 h at 37°C with 5% CO2. Subsequently, 50 μL of cell-free supernatant was transferred into a 96-well flat bottom plate and complement-dependent lysis of target cells was determined by measuring the LDH release using the Cytotox 96 nonradioactive cytotoxicity assay kit (Promega, Leiden, Netherlands) according to the manufacturer’s recommendations. The percentage specific lysis mediated by complement with serum samples was assessed using the following formula: (% lysis of target cells by antibody and complement − % lysis of target cells by complement alone)/(% maximum lysis of target cells in the presence of 0.8% Triton® X-100 − % lysis of target cells by complement alone) × 100.
Pig samples used to validate the assays
To validate our developed and optimized ADCC and CDC assays, we used sera samples from our earlier two vaccine trials in pigs with intranasal chitosan nanoparticle-based SwIAV vaccines or a commercial intramuscular administered multivalent SwIAV (FluSure XP®, Zoetis) vaccine (33, 34). The vaccines and related information are summarized in Table 1 and Supplementary Table 1.
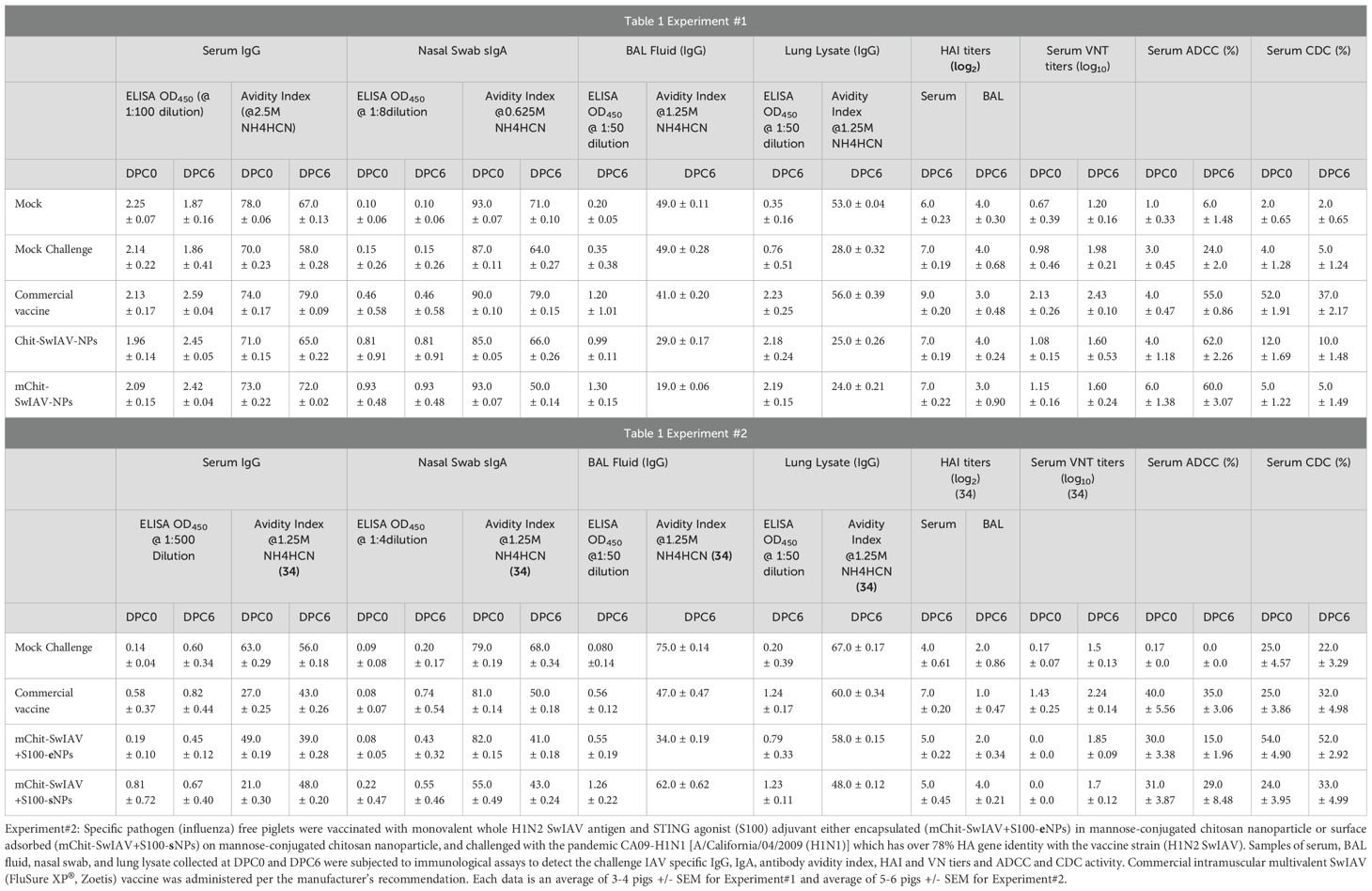
Table 1. Experiment#1: Conventional maternal antibody positive weaned piglets were vaccinated with mannose-conjugated or -unconjugated chitosan nanoparticle-based inactivated monovalent whole H1N2 SwIAV nanovaccines (Chit/mChit-SwIAV-NPs) and were challenged with the SwIAV H1N1-OH7 (A/Swine/OH/24366/2007) which has over 77% HA gene identity with the vaccine strain (H1N2 SwIAV).
Virus neutralization assay
Virus nAb titers were determined as described previously with slight modifications (38). Pig sera collected at both DPC 0 and 6 were subjected to heat inactivation for 30 min at 56°C. Briefly, a twofold serial dilution (starting from 1:5) of respective serum samples was mixed with an equal volume of SwIAV H1N1/OH/2007 (100 TCID50/well) in a 96-well round-bottom plate and incubated at 37°C in 5% CO2 for 1.5 h. One hundred microliters of sample and virus mix was transferred onto pre-adhered MDCK cells monolayer of over 90% confluency and incubated at 37°C in 5% CO2 for 1.5 h before adding additional 100 µL/well 1× DMEM containing TPCK-treated trypsin (2.0 µg/mL), and incubated for 44 h. The neutralizing activity of each serum sample was recorded as the reciprocal of the highest dilution showing complete inhibition of virus infection in MDCK cells assessed by fluorescent antibody test. Finally, the virus nAb titers were transformed to log10 values for comparison between different experimental vaccine groups.
Hemagglutination inhibition assay
The HAI titer specific to SwIAV H1N1/OH/2007 and 2009 pandemic CA09-H1N1 IAV in sera and bronchoalveolar lavage (BAL) fluid (Table 1) was determined as described previously (39). Briefly, heat-inactivated serially 2-fold diluted BAL fluid samples were mixed with 8 HAU of SwIAV (H1N1/OH/2007) in 50 µL and incubated for 1 h at 37°C. The HAI titers were determined as the reciprocal of the highest dilution of the samples that completely prevented the hemagglutination of 1% turkey red blood cells. The HAI titers were transformed into log2 values for comparison between different experimental groups. The HAI titers in serum and BAL fluid samples were reported earlier (33, 34) and are mentioned in Table 1 for comparison purposes.
Avidity of virus-specific antibodies
Determination of influenza virus-specific antibody avidity present in serum, BAL fluid, lung lysate, and nasal specimens collected from experimental animals was performed by an avidity enzyme-linked immunosorbent assay (ELISA) as described earlier (40). The dilutions of samples:serum (1:100), BAL fluid (1:50), lung lysate (1:50), and nasal swab specimens (1:8) were used in the assay. Briefly, the 96-well ELISA plates were adsorbed with pre-titrated inactivated SwIAV antigen (H1N1/OH/2007) and the procedure followed the same steps of ELISA as described earlier with an additional step of incubating the plate with a chaotropic agent, ammonium thiocyanate (NH4SCN), at different indicated molar concentrations (0 to 5 M) for 15 min at room temperature following treatment with test samples. The antibody avidity index was calculated as mean OD450 (NH4SCN-treated samples)/mean OD450 (PBST-treated samples) and multiplied by 100. The antibody avidity data in serum, BAL fluid, lung lysate, and nasal swab specimens in Experiment #2 (and not Experiment #1) were already reported (34) and are mentioned in Table 1 for comparison purposes.
Statistical analysis
Statistical differences between multi-group comparisons were performed using one-way ANOVA or two-way ANOVA followed by Tukey’s multiple comparison test in Prism 10 (GraphPad Software, Inc., CA, USA). The group data are presented as the mean ± SEM of three to four pigs (Experiment #1) and six to seven pigs (Experiment # 2) with a statistical significance of p < 0.05. A box-and-whisker plot shows interquartile ranges, and horizontal lines show group median.
Results
Stable expression of SwIAV-HA in MDCK cells
Expression of SwIAV H1N1 HA protein in stably transfected MDCK cells was confirmed by the indirect IFA on both permeabilized and non-permeabilized cells (data not shown), and by WB analysis detected in transfected MDCK cells at different passages using the anti-Myc-tag antibody (9B11, Cell Signaling Technology, Danvers, MA, USA) (Figure 1). Non-transduced MDCK cells served as negative control, while β-actin served as a loading control in the WB analysis.
Optimization of SwIAV-specific ADCC activity
We optimized the conditions such as temperature and duration of incubation of pig serum-treated target cells with effectors to measure the ADCC activity in pigs. In an earlier study, influenza-specific ADCC antibodies measured in pigs had few limitations. In that study, H1N1-specific pig immune serum was diluted 1:10 and detected a maximum 10% ADCC activity over the high background activity of greater than 15% in negative sera-treated MDCK-HA target cells, used at an E:T ratio of 20:1, with healthy pig PBMC as effectors (36). We made use of serum samples collected from unvaccinated sows (negative) and sows vaccinated with a commercial SwIAV multivalent (FluSure XP®) vaccine to optimize the ADCC assay. We specifically determined the ratio of effector pig PBMC to target MDCK-HA cells that allowed the detection of ADCC activity with high specificity and the least background. The target MDCK-HA cells were pre-incubated with sera at 1:25 dilution for 4 h at 37°C and then co-cultured with effector cells. Under these experimental conditions, sera from unvaccinated sows showed some background ADCC activity at an E:T of 20:1 and 40:1 (Figure 2), while the sera of vaccinated sows exhibited ADCC activity of 50% and 80% at a target ratio of 40:1 and 20:1, respectively, which was significantly higher than the background ADCC signals of 15% and 25% induced by sera from unvaccinated sows (Figure 2). Therefore, the E:T of 80:1 was used in subsequent studies.
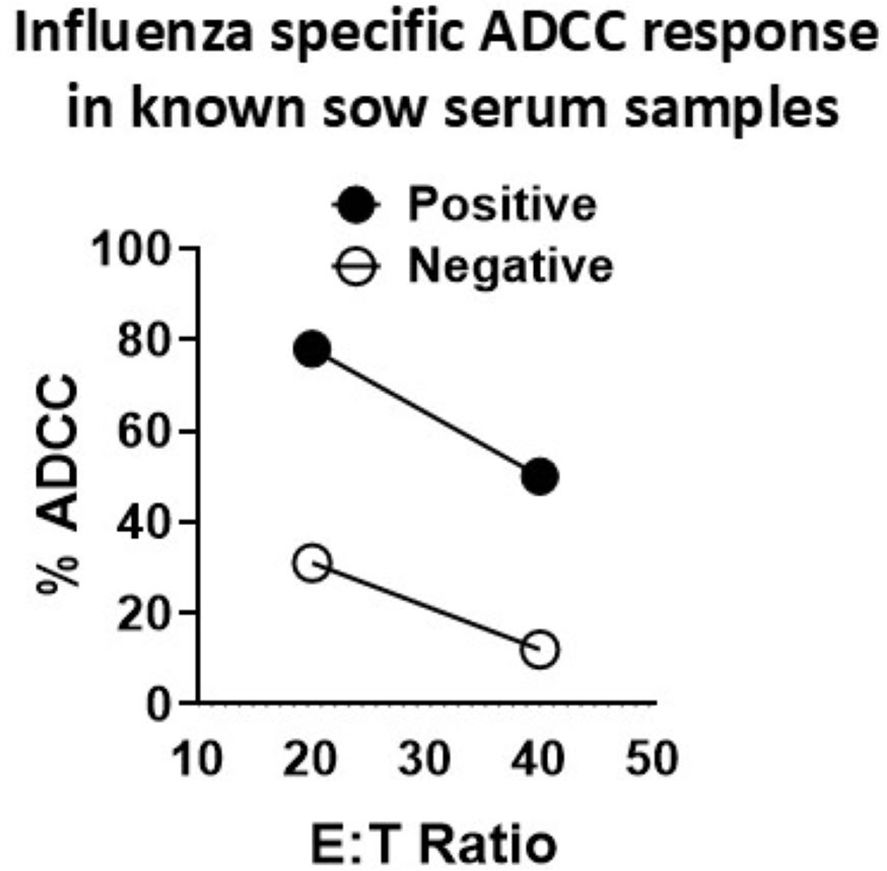
Figure 2. Optimization of influenza A virus-specific ADCC activity in the serum of adult vaccinated pigs. Serum samples collected from sows vaccinated (positive) or unvaccinated (negative) twice with a commercial swine influenza multivalent vaccine were used to optimize the ADCC activity measured by LDH release assay. Freshly isolated pig peripheral blood mononuclear cells (PBMCs) from healthy slaughtered pigs served as effector cells and the MDCK-HA cells served as targets, which were pretreated with SwIAV-specific positive or -negative sera at 1:25 dilution for 4 h at 37°C, and the effector-to-target ratios (E:T) used in the assay were 20:1 and 40:1. Each marking on a line graph represents the % lysis of target cells treated with a known influenza antibody-positive or -negative serum to determine the virus-specific ADCC activity.
Optimization of SwIAV-specific CDC activity
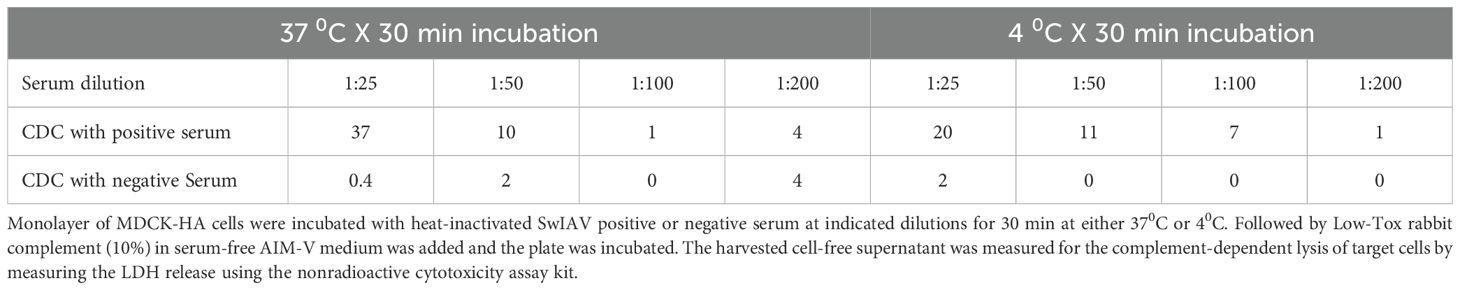
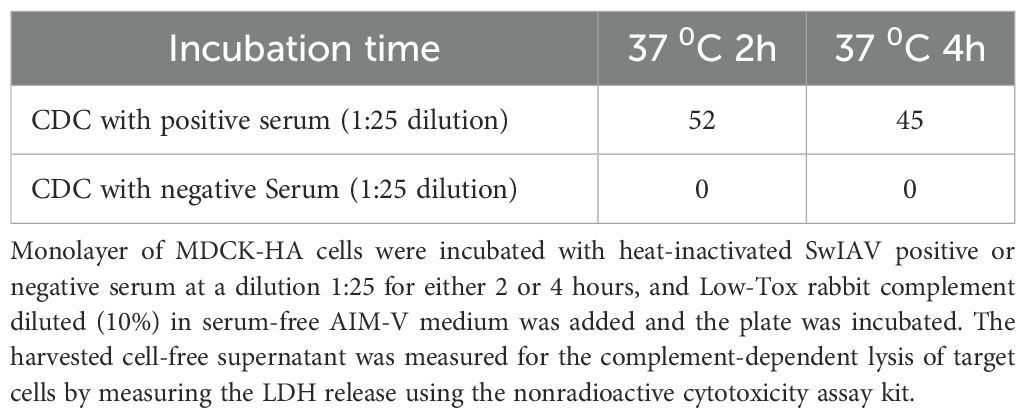
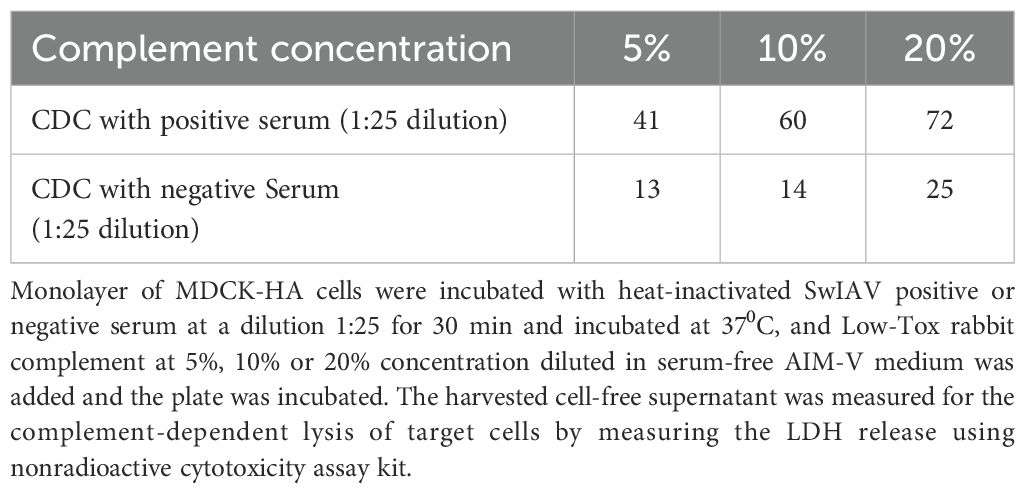
Serum samples collected from unvaccinated sows (negative) or sows vaccinated with a commercial SwIAV multivalent (FluSure XP®) vaccine were used to optimize the CDC assay. Similar to ADCC, serum dilution was optimized at 1:25 in serum-free AIM-V medium, which resulted in high signal and low background values. The 10% Low Tox rabbit complement provided the least background with high specific CDC activity when incubated at 37°C for 2 h in the assay (Tables 2–4).
Application of optimized ADCC and CDC assays to characterize antibody functions in intranasal vaccinated pigs
In vaccinated and virus-challenged pig serum samples (diluted 1:25), the optimum E:T ratio of 80:1 was found ideal, because we could achieve over 60% target ADCC lysis with less than 15% background activity in the assay (Figure 3). To validate the ADCC and CDC assays, we measured SwIAV-specific ADCC activity in the serum of pigs previously immunized with four different nanovaccine formulations and a commercial swine flu vaccine, while in influenza antibody-free pigs vaccinated with two types of nanovaccines containing a STING adjuvant, we observed relatively low levels of ADCC activity than the commercial SwIAV vaccine (Figure 3(i) A, B).
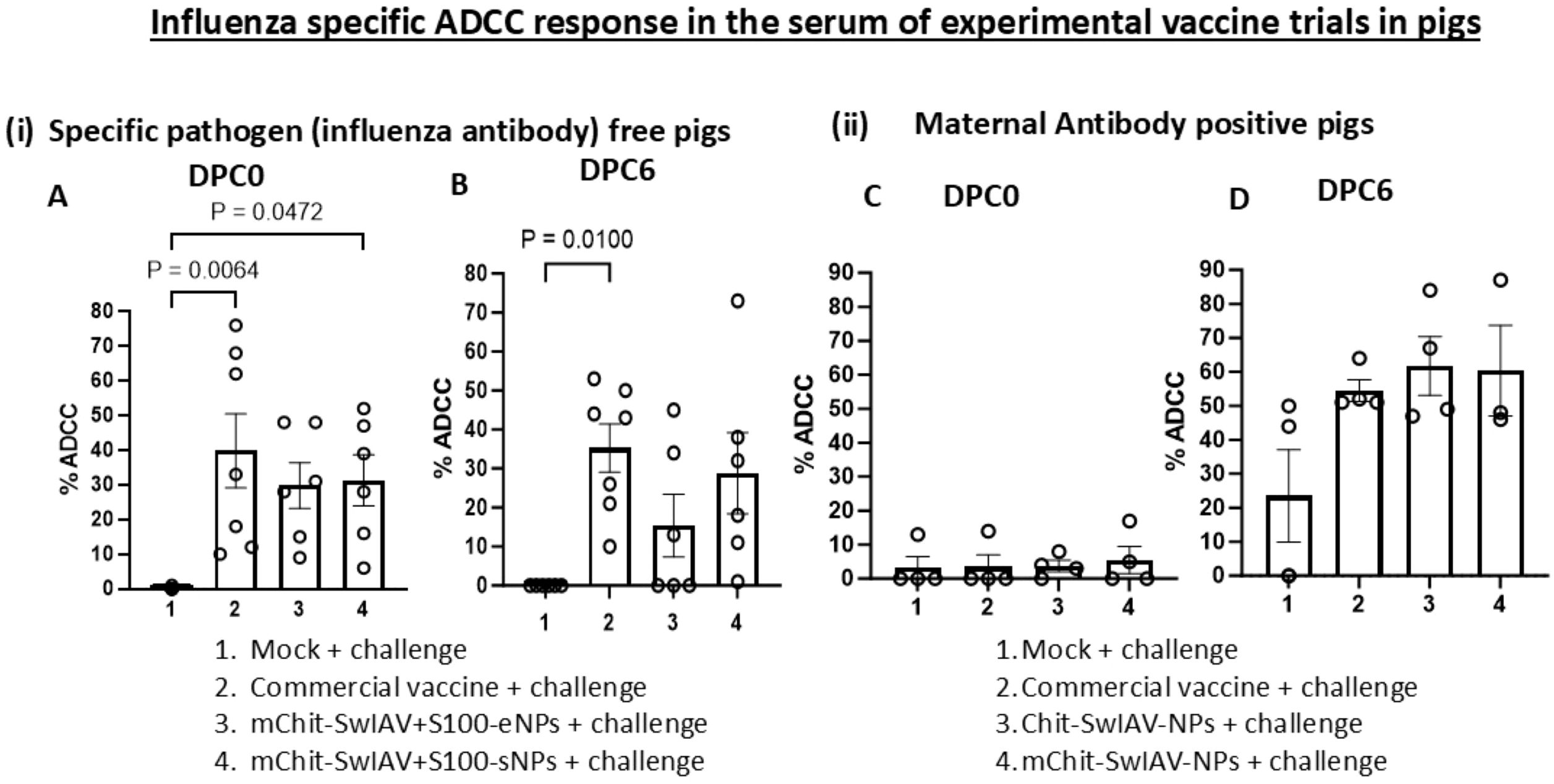
Figure 3. Influenza A virus-specific ADCC activity in the serum of experimental SwIAV vaccinated pigs. (i) Specific pathogen-free (6–7 per group) pigs and (ii) maternal antibody-positive (3–4 per group) pigs at 4–5 weeks of age were prime-boost vaccinated at 3-week intervals intranasally with Chit-SwIAV-NPs, mChit-SwIAV-NPs, mChit-SwIAV+S100-eNPs, and mChit-SwIAV+S100-sNPs, or intramuscularly with the commercial FluSure XP® vaccine and challenged with a heterologous (A, B) 2009 pandemic H1N1 IAV and (C, D) H1N1/OH/2007 SwIAV. Sera collected at (A, C) DPC0 and (B, D) DPC6 from different vaccinated pigs were used to pretreat the target cells at 1:25 dilution as mentioned in the Figure 2 legend. Freshly isolated PBMCs from healthy slaughtered pigs served as effectors used at an E:T ratio of 80:1 in the ADCC assay. Each marking on a bar represents the % lysis of target cells by individual pig serum and each bar is the mean ± SEM of each vaccine group. One-way ANOVA with Tukey’s multiple comparisons post-hoc test was employed to determine the p-values between virus-challenged vaccinated versus mock groups (p < 0.05).
In weaned MDA-positive piglets, interference in the induction of immunity to intramuscular administered inactivated SwIAV vaccines was observed, leading to poor induction of specific antibody responses, which is associated with evidence of vaccine-associated enhanced respiratory disease (29–32). Therefore, in this study, we assayed for SwIAV-specific ADCC and CDC activity in MDA-positive pigs that received inactivated SwIAV vaccine and challenged. Our data indicated that in MDA-positive pigs administered with two types of nanovaccines and a commercial swine flu vaccine, the ADCC activity was not detectable post prime-boost vaccination before challenge at DPC0, while after a challenge infection at DPC6, we observed enhanced induction of ADCC function (Figures 3(ii) C, D). The levels of ADCC activity with both nanovaccines and commercial swine flu vaccinates were comparable (Figures 3(ii) C, D).
In influenza antibody-free pigs vaccinated with two other types of nanovaccines containing a STING adjuvant (34), one of the nanovaccines containing whole inactivated SwIAV with STING adjuvant entrapped in NPs induced enhanced specific CDC activity compared to the cohort groups that received NP surface adsorbed vaccine cargo with adjuvant formulation and the commercial swine flu vaccine (Figures 4(i) A, B). The MDA positive pigs from commercial vaccine group displayed a significantly (p < 0.01) higher CDC activity as compared to both the nanovaccines group and mock challenge group (Figures 4(ii) C, D). Interestingly, the CDC activity did not increase further after the challenge infection at DPC6 compared to that at DPC0 in both vaccine trials (Figure 4i, ii).
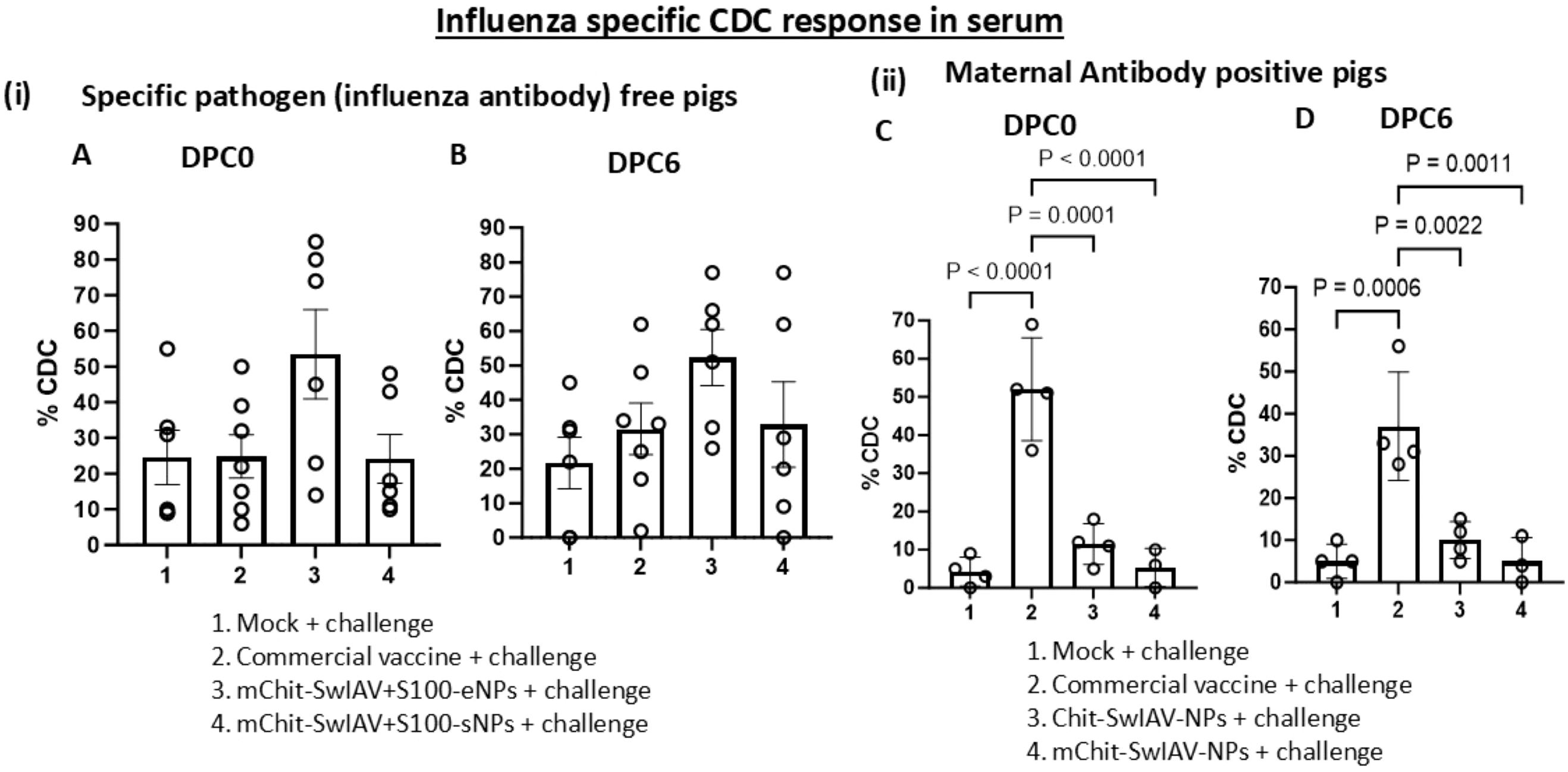
Figure 4. Influenza A virus-specific CDC activity in the serum of experimental SwIAV vaccinated pigs. (i) Specific pathogen-free (6–7 per group) and (ii) maternal antibody-positive (3–4 per group) pigs were prime-boost vaccinated and challenged with a heterologous SwIAV as described in the Figure 3 legend. Sera collected at (A, C) DPC0 and (B, D) DPC6 from different vaccinated groups were used to pretreat the MDCK-HA target cells at 1:25 dilution as mentioned in the Figure 2 legend in the presence of rabbit complement. The CDC-mediated killing (% lysis of target cells) was measured in duplicates by LDH release assay. Each marking on a bar represents the % lysis of target cells by individual pig serum, and each bar is the mean ± SEM. One-way ANOVA with Tukey’s multiple comparisons post-hoc test was employed to determine the p-values between virus-challenged vaccinated versus mock groups (p < 0.05).
Virus neutralizing and hemagglutination inhibition antibody titers in vaccinated pigs
The virus neutralizing (VN) and HAI antibody titers in the serum samples of MDA-positive pigs that were vaccinated and challenged using the SwIAV H1N1-OH7 were determined. The lower detection limit of VN and HAI antibody titers were 1:5 and 1:2, respectively. At DPC0, only in the serum of the commercial vaccine group low levels of VN titers were detected, and the data were significantly (p < 0.05) higher compared to mock challenge and both the nanovaccine groups (Figure 5A). At DPC6, relatively higher VN titers were detected in the commercial vaccine compared to both the nanovaccines received pig groups (Figure 5B). Furthermore, no marked differences were observed in HAI titers in the BAL fluid of all the vaccinated and mock challenge pig groups (Figure 5C). It is evident that the commercial SwIAV vaccine is superior to nanovaccine candidates in eliciting relatively higher VN antibody titers in serum, but the same was not true for HAI titers in both the serum and BAL fluid (Figures 5A, C) (Table 1).
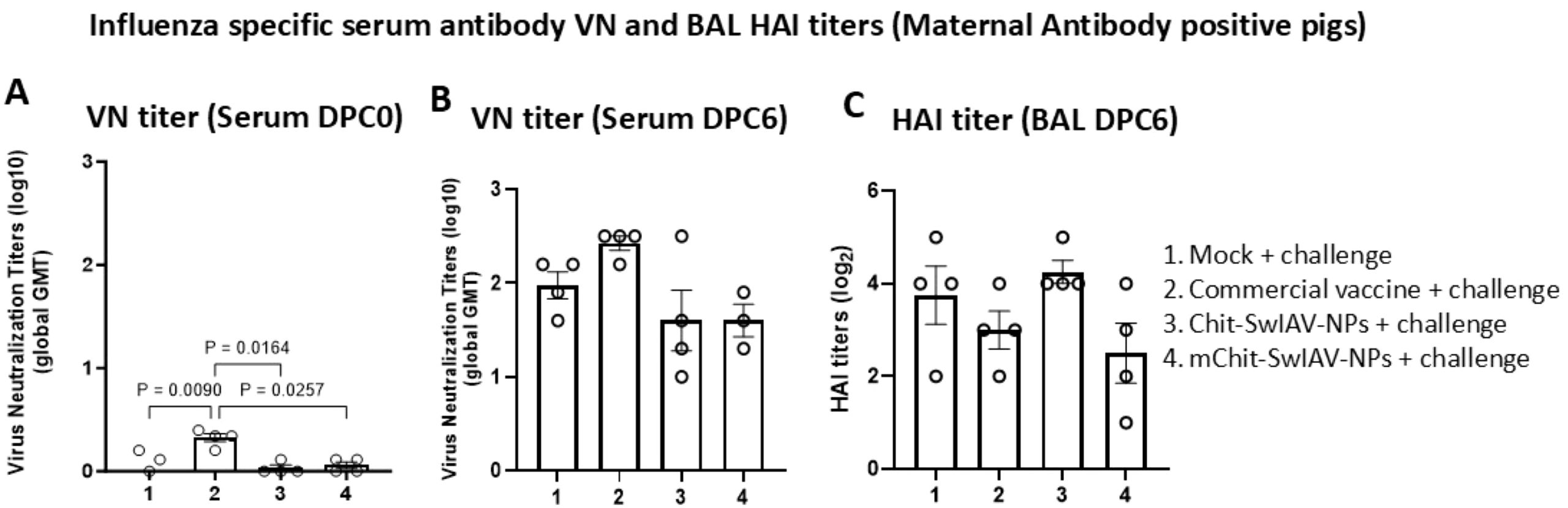
Figure 5. Influenza A virus-specific virus neutralization (VN) and hemagglutination inhibition (HAI) antibody titers in the serum and BAL fluid of experimental pigs, respectively. Maternal antibody-positive weaned 4-week-old pigs were prime-boost vaccinated at 3-week intervals intranasally with Chit-SwIAV-NPs and mChit-SwIAV-NPs vaccine or a commercial FluSure XP® vaccine intramuscularly and challenged with a heterologous H1N1/OH/2007 SwIAV. (A) VN titers in serum at DPC0 and (B) DPC6 and (C) BAL fluid HAI endpoint titers at DPC6 were assessed against the challenge virus (H1N1/OH/2007). The VN titer is the reciprocal endpoint dilution transformed to a log10 value and plotted as a geometric mean titer (GMT) with SEM from triplicate wells. The HAI titers is the reciprocal endpoint dilution transformed to log2 values. Each marking on a bar represents the titer of an individual pig, and each bar is the average of three to four pigs (mean ± SEM). One-way ANOVA with Tukey’s multiple comparisons post-hoc test was employed to determine the p-values between groups (p < 0.05).
SwIAV-specific antibodies (IgG and IgA) avidity in vaccinated pigs
Avidity refers to strength of binding of antigen and antibody, and it is considered as an important correlate of protective immunity induced by vaccines. As expected, the OD450 values declined in all the vaccine groups with increasing concentrations of NH4SCN ranging from 0 to 5.0 M. To determine the ideal concentration of the chaotropic agent to induce dissociation of immune complexes, a nonlinear regression model was plotted using the OD450 values obtained at twofold dilution of NH4SCN (5.0, 2.5, 1.25, 0.625, and 0.31 M) against control (0 M). At 2.5 M of NH4SCN, SwIAV-specific serum IgGs were still bound to the antigen, while at 1.25 and 0.625 M of NH4SCN solution, the lung lysate antibodies and the nasal swab IgA antibodies, respectively, were still bound to the SwIAV antigen (Figures 6A, C). Avidity of specific IgG in serum was comparable in both the nanovaccines and commercial SwIAV vaccinates (Figure 6A). In the nanovaccine-administered maternal antibody-positive pig groups, lung lysate and nasal swab antibodies had a significantly higher specific antibody avidity in some of the NH4SCN concentrations than in the commercial vaccine and mock challenge groups (Figures 6B, C). The avidity data of influenza antibody free pigs are presented in Table 1.
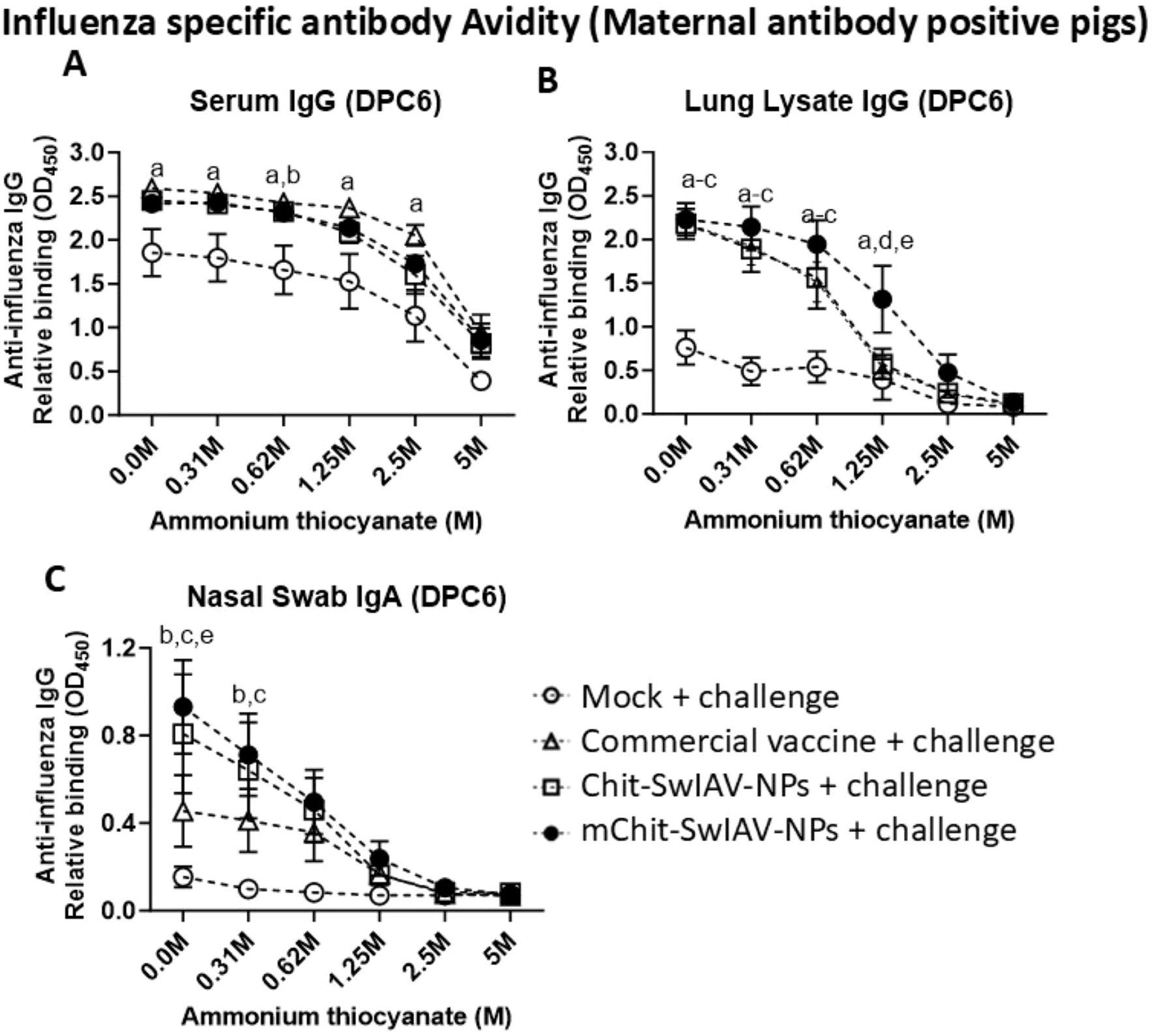
Figure 6. Influenza A virus-specific IgG and IgA antibody avidity in the serum, lung lysate, and nasal swab samples of experimental vaccinated pigs at different concentrations of ammonium thiocyanate (NH4SCN). Samples collected at DPC6 from the maternal antibody-positive weaned pigs were vaccinated and challenged as described in the Figure 5 legend. Relative binding avidity of SwIAV-specific IgG in (A) serum (1:100 dilution), (B) lung lysate (1:50 dilution), and (C) IgA in nasal swab (1:8 dilution), performed both in the absence and in the presence of NH4SCN at various molar concentrations. Each marking represents the average OD value of three to four pigs (mean ± SEM). The statistical difference between experimental groups is considered as significant and denoted with the following lowercase letters: a, mock + challenge vs. commercial vaccine; b, mock + challenge vs. Chit-SwIAV-NPs; c, mock + challenge vs. mChit-SwIAV-NPs; d, commercial vaccine vs. Chit-SwIAV-NPs; and e, commercial vaccine vs. mChit-SwIAV-NPs. Two-way ANOVA with Tukey’s multiple comparisons post-hoc test was employed to determine the p-values between the groups (p < 0.05).
Discussion
The effectiveness of influenza vaccines is widely assessed by evaluating the humoral immune responses. In the process of development and validation of new broad-spectrum influenza vaccines, a mechanistic study to evaluate various types of functional antibodies—both virus neutralizing (NAbs) and non-neutralizing (nNAbs) through reliable methods for quantification in vaccinated animals—is important. This will help understand the correlates of protection, especially the cross-protection ability of vaccines. Therefore, measurement of different functional activities of antibodies including nNAbs should be prioritized in all the influenza vaccine preclinical and clinical trials, because both contribute significantly towards protection, leading to the development of novel influenza vaccination strategies directed towards boosting the quality of nNAbs. The effector functions of NAbs and nNAbs specific to influenza viruses are mediated through engagement of a constant region of antibodies (Fc), and it is not necessarily exclusive to nNAbs. Interestingly, it has been reported that NAbs specific to influenza and HIV elicit optimal function following their engagement with Fcy receptors (41, 42).
Both HA and neuraminidase (NA) proteins of influenza virus are considered as antigenic determinants for ADCC antibody (43). An anamnestic ADCC response has been observed following the IAV heterosubtypic challenge infection. Functional HA-specific antibodies capable of inducing ADCC appear before HAI antibodies and exhibit relatively higher breadth of binding to cognate antigen in comparison to NAbs (44). The ADCC-inducing HA-specific antibodies have been shown to reduce the severity of illness with increased virus clearance (45). In addition, increased ADCC activity positively correlates with robust HAI responses following vaccination (44). Even though HAI antibodies compete with ADCC antibodies to access the common antigenic epitopes (46), the role of ADCC in protection against influenza infection or vaccination remains elusive. Different animal models such as mouse, ferret, and non-human primates have been successfully used to assess the protective efficacy of ADCC-based vaccines (47, 48).
Despite numerous reports on NAbs and HAI antibodies induced by SwIAV vaccines, limited literature is available on vaccine-induced ADCC and CDC responses in pigs. The HA-targeting antibodies have been known to protect against influenza infections (49). Therefore, we used stably transfected MDCK cells engineered to express the full-length open reading frame of HA as target cells in both the ADCC and CDC assays and using the LDH release as a readout. The LDH release assay has been the most biologically relevant assay to determine the role of antibodies contributing to ADCC and CDC, as it directly measures the antibody-mediated killing of influenza-infected cells (50, 51). In human samples, the functional role of ADCC antibodies have been successfully demonstrated using target cells stably expressing HA protein (50). In pigs, this is the first report of detecting the ADCC and CDC antibodies induced in response to whole inactivated SwIAV vaccines (50). We propose that a blocking assay using monoclonal antibodies targeting specific HA domains could determine whether ADCC responses are primarily directed to the conserved HA stalk or more variable head regions.
Furthermore, validating the assays using samples of two relevant vaccine trials has provided a unique opportunity to quantify the ADCC and CDC antibodies. The generation of cross-reactive influenza-specific HAI-, VN-, ADCC-, and CDC-mediating antibodies is crucial following vaccination or infection. Our previous reports suggest that the SwIAV nanovaccines elicit cross-reactive NAbs and HAI antibodies against divergent viral strains, but we do not know the other functions of specific antibodies (33, 34). Therefore, our goal was to optimize the pig-specific ADCC and CDC assays and validate them using the archived samples of the two previous SwIAV nanovaccine trials (33, 34), determine the cross-reactive ADCC and CDC antibodies and correlate the data with specific VN and HAI titers and antibody avidity.
Our data indicated that there was a cross-reactive ADCC and CDC antibody activity, because the whole inactivated SwIAV H1N2 used in the nanovaccines was heterologous to both the challenge viruses (33, 34). The heterogeneity among the vaccine and challenge viruses was compared for HA gene identify by retrieving data of isolates from the GenBank public database (52) and using the BLAST server (53–56) (Supplementary Table 2). The commercial SwIAV multivalent vaccine has four SwIAV strains that belong to two H1N1, one H1N2, and one H3N2 subtype, and when compared among each other with both the challenge viruses, one of the H1N1 viruses in the vaccine has over 93% HA gene identity. Thus, the commercial swine flu vaccine and challenge viruses are considered homologous to each other.
Despite optimizing and validating the sensitivity of pig-specific ADCC and CDC assay using archived vaccinated piglets and sow serum samples, there are still some limitations such as high variability in the percentage cytotoxicity mediated by positive and negative serum samples within the treatment groups. Replacing the routine RPMI growth media with serum-free AIM-V medium containing 5% Immune Cell Serum Replacement for diluting serum samples and culturing of target and effector cells are critical for the assay, which is consistent with the previous report (57). Furthermore, ADCC and CDC assays were performed on sera only and not on BAL, nasal swabs, and lung lysate samples. Predominance of antibodies (IgG class) exhibiting ADCC and CDC activities in serum compared to other samples and the availability of limited samples (BAL, nasal swabs, and lung lysate) in our source directed us to perform these functional antibody assays using only serum.
We detected the cross-reactive HA-specific ADCC antibodies in pigs in response to nanovaccine candidates delivered intranasally. In the serum collected from influenza MDA-positive pigs at DPC0, the ADCC antibody was not induced, but upon challenge infection at DPC6, high levels of specific ADCC antibody were detected. In contrast, in influenza antibody-free pigs both at DPC0 and DPC6, high levels of virus-specific ADCC-mediating antibody production were detected, suggesting that in pigs having preexisting antibodies, there was a suppression in the induction of ADCC antibodies to an inactivated SwIAV vaccine, but the live virus exposure in vaccinates rescued that response. This pattern of induction of ADCC antibody is consistent with previous reports (58, 59). Interestingly, both the commercial swine flu and nanovaccines elicited high levels of specific ADCC antibody response, which may be associated with the identical HA subtype of the virus (H1) used in challenge and vaccine viruses, associated with the use of target MDCK cells expressing H1N1 HA. Our data revealed that MDA-positive pigs vaccinated intranasally with monovalent nanovaccines induced comparable levels of ADCC responses to that of a commercial swine flu vaccine, despite over 77% HA gene identify with the challenge virus. Furthermore, there are 13 amino acid differences in HA between vaccine and challenge viruses, which could have affected the functionality of ADCC antibodies (60). Hence, our findings support the previous observation that cross-reactive ADCC antibodies appear to confer a broad spectrum of protection and may contribute to the design of universal influenza vaccines in the future (59). In our study, maternal antibodies appear to suppress ADCC responses but not CDC responses, and we do not know the mechanistic explanation, which needs further investigation both in the pig and other animal models. It is possible that maternal antibodies neutralize the vaccine antigen prior to induction of ADCC-mediating antibodies, or if they happen to inhibit B-cell activation necessary for producing ADCC antibodies, the ADCC response can be consequently suppressed. Furthermore, the activation of pre-existing B cells may lead to class switching from IgG to non-ADCC isotypes (61).
The magnitude of ADCC based on serum IgG is, at least in part, influenced by the ratio of ADCC-inducing (nNAbs) and -inhibiting (NAbs) antibodies (62). Adjuvants may influence the pattern of antibody glycosylation generated by vaccines, thereby affecting the functionality of ADCC antibodies (63). Overall, the heightened specific ADCC antibody responses induced by intranasal delivered nanovaccines helped in the better clearance of the heterologous challenge virus in comparison to the commercial vaccine (33, 34), consistent with previous reports pointing to the cross-reactive and protective nature of ADCC-mediating antibodies in influenza infection/vaccination (45, 59, 64). The pre-existing IAV-specific neutralizing and HAI antibodies were reported to affect the potency of ADCC and the ADCC-based vaccine (46, 47). Therefore, the efficacy of the ADCC-based vaccine may be affected by pre-existing immunity (47). This is consistent with our data of not detecting any ADCC activity post-SwIAV vaccination.
The complement system can serve as a link between innate and adaptive immunity, and understanding its role in vaccine development is crucial. Under in vitro settings, the role of complement in the neutralization of the influenza virus using specific antibodies has been established (65, 66). Inclusion of the STING adjuvant in SwIAV nanovaccine formulation had a substantial effect on induction of specific CDC antibody reflected at both DPC0 and DPC6. The role of CDC has been investigated in other similar conditions such as HIV, hepatitis B, and herpes virus infections (13, 67). The major mechanism of CDC antibodies in virus clearance is through lysis of infected cells, thereby preventing maximal replication and release of infectious virions. Free virions released from infected cells can also be neutralized by specific CDC antibodies and subsequently removed by phagocytosis (68). The induction of specific CDC antibodies in MDA-positive pigs was not high at both DPC0 and DPC6 in nano-vaccinates compared to commercial vaccine, which elicited a robust CDC antibody response, suggesting the influence of route of vaccination (intramuscular versus intranasal), HA gene identity, and monovalent versus multivalent nature of the vaccines.
Antibody avidity indicates the priming of immunological memory, as vaccination results in antibody maturation and, hence, generation of antibodies with increased avidity. Antibody avidity is considered as a qualitative response index and can directly correlate with protection (69). Conversely, inadequate levels of avidity have been linked to antibody-mediated disease enhancement following pandemic influenza vaccinations (70). Virus-specific IgG avidity in serum was comparable in both commercial swine flu and nano-vaccinates, while the nasal passage known to be rich in sIgA showed significantly higher avidity compared to the commercial vaccine. In the lung lysate, which is known to possess both IgG and sIgA subclass antibodies, relatively higher antibody avidity in nano-vaccinates was observed compared to the commercial vaccine. This is important because both mucosal and systemic antibodies have been previously shown to be involved in protection against influenza infection (71). However, the immune response after vaccination may be influenced by several factors such as vaccine type and age of recipients. Our data in pigs vaccinated with a commercial multivalent vaccine and monovalent nanovaccines revealed a strong positive association among the NAbs, HAI, antibody avidity, ADCC, and CDC antibody activity (Table 1). This further highlights the importance of augmenting influenza specific both mucosal and systemic antibody responses using potent nasal vaccine delivery system like mucoadhesive chitosan nanoparticle platform for achieving the cross-protective immunity.
In conclusion, induction of ADCC response has been one of the important criteria for potential vaccine candidates by the World Health Organization. Therefore, understanding this response elicited by candidate vaccines irrespective of species may provide valuable insights into crucial immune correlates of protection. Together, our data provide evidence that both cross-reactive NAbs and nNAbs induced by vaccination could play an important role in broad-spectrum immunity against heterologous challenge influenza virus infection. Another notable finding was the positive trend between the magnitude of ADCC, antibody avidity, NAbs, and HAI responses observed at both pre- and post-challenge infection in vaccinates (Table 1). Mannose-conjugated inactivated whole SwIAV monovalent nanovaccine administered with or without a STING adjuvant generated high levels of cross-reactive neutralizing, HAI-, ADCC-, and CDC-mediating antibodies. Our data indicated that the level of HA gene identity between vaccine and challenge SwIAV potentially affects the breadth of antibody responses, and SwIAV vaccination using a nanovaccine candidate can induce both mucosal and systemic NAbs and nNAbs capable of clearing the heterologous SwIAVs from the respiratory tract.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Institutional Animal Care and Use Committee, The Ohio State University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
GR: Conceptualization, Data curation, Formal analysis, Funding acquisition, Supervision, Validation, Writing – review & editing. MS: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. GM: Methodology, Writing – original draft. SR: Methodology, Writing – review & editing. DB: Methodology, Writing – review & editing. OS: Methodology, Writing – review & editing. RS: Methodology, Writing – review & editing. JS: Methodology, Writing – review & editing. SD: Methodology, Writing – review & editing. DD: Methodology, Writing – review & editing. PB: Investigation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The work was supported in part by the USDA National Institute of Food and Agriculture (award 2019-67015–29814 and 2022-67015-36349). Salaries and research support were provided by state and federal funds appropriated to the CFAES, The Ohio State University. Mithilesh Singh received one year fellowship by the Indian Council of Medical Research (ICMR), Government of India.
Acknowledgments
We would like to acknowledge Dr. Juliette Hanson, Sara Tallmadge, Ronna Wood, and Megan Strother for their help in animal studies.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1600761/full#supplementary-material
References
1. Schultz-Cherry S, Olsen CW, and Easterday BC. History of swine influenza. In: Richt JA and Webby RJ, editors. Swine influenza. Springer Berlin Heidelberg, Berlin, Heidelberg (2013). p. 21–7.
2. Van Kerkhove MD, Vandemaele KA, Shinde V, Jaramillo-Gutierrez G, Koukounari A, Donnelly CA, et al. Infection, Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. . PloS Med. (2011) 8:e1001053. doi: 10.1371/journal.pmed.1001053
3. Vincent AL, Ma W, Lager KM, Janke BH, and Richt JA. Swine influenza viruses a North American perspective. Adv Virus Res. (2008) 72:127–54. doi: 10.1016/S0065-3527(08)00403-X
4. Powdrill TF, Nipp TL, and Rinderknecht JL. One health approach to influenza: assessment of critical issues and options. Emerg Infect Dis. (2010) 16:e1. doi: 10.3201/eid1608.100673
5. Chandler TL, Yang A, Otero CE, Permar SR, and Caddy SL. Protective mechanisms of nonneutralizing antiviral antibodies. PloS Pathog. (2023) 19:e1011670. doi: 10.1371/journal.ppat.1011670
6. Hobson D, Curry RL, Beare AS, and Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond). (1972) 70:767–77. doi: 10.1017/S0022172400022610
7. CDC. Past seasons vaccine effectiveness estimates. In: Seasonal influenza (Flu). Centers for Disease Control and Prevention: National Center for Immunization and Respiratory Diseases (2020). Available at: https://www.cdc.gov/flu/vaccines-work/past-seasons-estimates.html (Accessed June 13, 2024).
8. Knossow M, Gaudier M, Douglas A, Barrere B, Bizebard T, Barbey C, et al. Mechanism of neutralization of influenza virus infectivity by antibodies. Virology. (2002) 302:294–8. doi: 10.1006/viro.2002.1625
9. Xie H, Wan XF, Ye Z, Plant EP, Zhao Y, Xu Y, et al. H3N2 mismatch of 2014–15 northern hemisphere influenza vaccines and head-to-head comparison between human and ferret antisera derived antigenic maps. Sci Rep. (2015) 5:15279. doi: 10.1038/srep15279
10. de Jong JC, Beyer WE, Palache AM, Rimmelzwaan GF, and Osterhaus AD. Mismatch between the 1997/1998 influenza vaccine and the major epidemic A(H3N2) virus strain as the cause of an inadequate vaccine-induced antibody response to this strain in the elderly. J Med Virol. (2000) 61:94–9. doi: 10.1002/(SICI)1096-9071(200005)61:1<94::AID-JMV15>3.0.CO;2-C
11. Mullarkey CE, Bailey MJ, Golubeva DA, Tan GS, Nachbagauer R, He W, et al. Broadly neutralizing hemagglutinin stalk-specific antibodies induce potent phagocytosis of immune complexes by neutrophils in an fc-dependent manner. mBio. (2016) 7(5):e01624-16. doi: 10.1128/mBio.01624-16
12. Jegaskanda S. The potential role of fc-receptor functions in the development of a universal influenza vaccine. Vaccines (Basel). (2018) 6(2):27. doi: 10.3390/vaccines6020027
13. Huber M and Trkola A. Humoral immunity to HIV-1: neutralization and beyond. J Intern Med. (2007) 262:5–25. doi: 10.1111/j.1365-2796.2007.01819.x
14. Kopf M, Abel B, Gallimore A, Carroll M, and Bachmann MF. Complement component C3 promotes T-cell priming and lung migration to control acute influenza virus infection. Nat Med. (2002) 8:373–8. doi: 10.1038/nm0402-373
15. O’Brien KB, Morrison TE, Dundore DY, Heise MT, and Schultz-Cherry S. A protective role for complement C3 protein during pandemic 2009 H1N1 and H5N1 influenza A virus infection. PloS One. (2011) 6:e17377. doi: 10.1371/journal.pone.0017377
16. Rattan A, Pawar SD, Nawadkar R, Kulkarni N, Lal G, Mullick J, et al. Synergy between the classical and alternative pathways of complement is essential for conferring effective protection against the pandemic influenza A(H1N1) 2009 virus infection. PloS Pathog. (2017) 13:e1006248. doi: 10.1371/journal.ppat.1006248
17. Nachbagauer R, Feser J, Naficy A, Bernstein DI, Guptill J, Walter EB, et al. A chimeric hemagglutinin-based universal influenza virus vaccine approach induces broad and long-lasting immunity in a randomized, placebo-controlled phase I trial. Nat Med. (2021) 27:106–14. doi: 10.1038/s41591-020-1118-7
18. Bartsch YC, Cizmeci D, Kang J, Zohar T, Periasamy S, Mehta N, et al. Antibody effector functions are associated with protection from respiratory syncytial virus. Cell. (2022) 185:4873–4886 e10. doi: 10.1016/j.cell.2022.11.012
19. Geers D, Sablerolles RSG, van Baarle D, Kootstra NA, Rietdijk WJR, Schmitz KS, et al. Ad26.COV2.S priming provided a solid immunological base for mRNA-based COVID-19 booster vaccination. iScience. (2023) 26:105753. doi: 10.1016/j.isci.2022.105753
20. Fang Y, Banner D, Kelvin AA, Huang SS, Paige CJ, Corfe SA, et al. Seasonal H1N1 influenza virus infection induces cross-protective pandemic H1N1 virus immunity through a CD8-independent, B cell-dependent mechanism. J Virol. (2012) 86:2229–38. doi: 10.1128/JVI.05540-11
21. Fox A, Le NM, Horby P, van Doorn HR, Nguyen VT, Nguyen HH, et al. Severe pandemic H1N1–2009 infection is associated with transient NK and T deficiency and aberrant CD8 responses. PloS One. (2012) 7:e31535. doi: 10.1371/journal.pone.0031535
22. Beli E, Clinthorne JF, Duriancik DM, Hwang I, Kim S, and Gardner EM. Natural killer cell function is altered during the primary response of aged mice to influenza infection. Mech Ageing Dev. (2011) 132:503–10. doi: 10.1016/j.mad.2011.08.005
23. Greenberg SB, Criswell BS, Six HR, and Couch RB. Lymphocyte cytotoxicity to influenza virus-infected cells: response to vaccination and virus infection. Infect Immun. (1978) 20:640–5. doi: 10.1128/iai.20.3.640-645.1978
24. Hicks JT, Ennis FA, Kim E, and Verbonitz M. The importance of an intact complement pathway in recovery from a primary viral infection: influenza in decomplemented and in C5-deficient mice. J Immunol. (1978) 121:1437–45. doi: 10.4049/jimmunol.121.4.1437
25. Saunders KO. Conceptual approaches to modulating antibody effector functions and circulation half-life. Front Immunol. (2019) 10:1296. doi: 10.3389/fimmu.2019.01296
26. Bruggemann M, Williams GT, Bindon CI, Clark MR, Walker MR, Jefferis R, et al. Comparison of the effector functions of human immunoglobulins using a matched set of chimeric antibodies. J Exp Med. (1987) 166:1351–61. doi: 10.1084/jem.166.5.1351
27. Dhakal S, Renu S, Ghimire S, Shaan Lakshmanappa Y, Hogshead BT, Feliciano-Ruiz N, et al. Mucosal immunity and protective efficacy of intranasal inactivated influenza vaccine is improved by chitosan nanoparticle delivery in pigs. Front Immunol. (2018) 9:934. doi: 10.3389/fimmu.2018.00934
28. Dhakal S and Renukaradhya GJ. Nanoparticle-based vaccine development and evaluation against viral infections in pigs. Vet Res. (2019) 50:90. doi: 10.1186/s13567-019-0712-5
29. Sunwoo S-Y, Schotsaert M, Morozov I, Davis AS, Li Y, Lee J, et al. A universal influenza virus vaccine candidate tested in a pig vaccination-infection model in the presence of maternal antibodies. Vaccines. (2018) 6:64. doi: 10.3390/vaccines6030064
30. Sandbulte MR, Platt R, Roth JA, Henningson JN, Gibson KA, Rajão DS, et al. Divergent immune responses and disease outcomes in piglets immunized with inactivated and attenuated H3N2 swine influenza vaccines in the presence of maternally-derived antibodies. Virology. (2014) 464-465:45–54. doi: 10.1016/j.virol.2014.06.027
31. Markowska-Daniel I, Pomorska-Mól M, and Pejsak Z. The influence of age and maternal antibodies on the postvaccinal response against swine influenza viruses in pigs. Veterinary Immunol Immunopathology. (2011) 142:81–6. doi: 10.1016/j.vetimm.2011.03.019
32. Vincent AL, Ma W, Lager KM, Richt JA, Janke BH, Sandbulte MR, et al. Live attenuated influenza vaccine provides superior protection from heterologous infection in pigs with maternal antibodies without inducing vaccine-associated enhanced respiratory disease. J Virol. (2012) 86:10597–605. doi: 10.1128/JVI.01439-12
33. Renu S, Feliciano-Ruiz N, Patil V, Schrock J, Han Y, Ramesh A, et al. Immunity and protective efficacy of mannose conjugated chitosan-based influenza nanovaccine in maternal antibody positive pigs. Front Immunol. (2021) 12:584299. doi: 10.3389/fimmu.2021.584299
34. Bugybayeva D, Dumkliang E, Patil V, Yadagiri G, Suresh R, Singh M, et al. Evaluation of efficacy of surface coated versus encapsulated influenza antigens in mannose-chitosan nanoparticle-based intranasal vaccine in swine. Vaccines (Basel). (2024) 12(6):647. doi: 10.3390/vaccines12060647
35. Montagner S, Leoni C, Emming S, Della Chiara G, Balestrieri C, Barozzi I, et al. TET2 regulates mast cell differentiation and proliferation through catalytic and non-catalytic activities. Cell Rep. (2016) 15:1566–79. doi: 10.1016/j.celrep.2016.04.044
36. Morgan SB, Holzer B, Hemmink JD, Salguero FJ, Schwartz JC, Agatic G, et al. Therapeutic administration of broadly neutralizing FI6 antibody reveals lack of interaction between human igG1 and pig fc receptors. Front Immunol. (2018) 9:865. doi: 10.3389/fimmu.2018.00865
37. Bodewes R, Geelhoed-Mieras MM, Wrammert J, Ahmed R, Wilson PC, Fouchier RAM, et al. Assessment of the immunological significance of a human monoclonal antibody directed to the influenza A virus nucleoprotein. Clin Vaccine Immunol. (2013) 20:1333–7. doi: 10.1128/CVI.00339-13
38. Gauger PC and Vincent AL. Serum virus neutralization assay for detection and quantitation of serum neutralizing antibodies to influenza A virus in swine. Methods Mol Biol. (2020) 2123:321–33. doi: 10.1007/978-1-0716-0346-8_23
39. Spackman E and Sitaras I. Hemagglutination inhibition assay. Methods Mol Biol. (2020) 2123:11–28. doi: 10.1007/978-1-0716-0346-8
40. Hernandez-Franco JF, Yadagiri G, Patil V, Bugybayeva D, Dolatyabi S, Dumkliang E, et al. Intradermal vaccination against influenza with a STING-targeted nanoparticle combination adjuvant induces superior cross-protective humoral immunity in swine compared with intranasal and intramuscular immunization. Vaccines (Basel) 11. (2023) 11(11):1699. doi: 10.3390/vaccines11111699
41. Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. (2007) 449:101–4. doi: 10.1038/nature06106
42. DiLillo DJ, Palese P, Wilson PC, and Ravetch JV. Broadly neutralizing anti-influenza antibodies require Fc receptor engagement for in vivo protection. J Clin Invest. (2016) 126:605–10. doi: 10.1172/JCI84428
43. Hashimoto G, Wright PF, and Karzon DT. Antibody-dependent cell-mediated cytotoxicity against influenza virus-infected cells. J Infect Dis. (1983) 148:785–94. doi: 10.1093/infdis/148.5.785
44. Vanderven HA, Jegaskanda S, Wines BD, Hogarth PM, Carmuglia S, Rockman S, et al. Antibody-dependent cellular cytotoxicity responses to seasonal influenza vaccination in older adults. J Infect Dis. (2017) 217:12–23. doi: 10.1093/infdis/jix554
45. Jegaskanda S, Luke C, Hickman HD, Sangster MY, Wieland-Alter WF, McBride JM, et al. Generation and protective ability of influenza virus-specific antibody-dependent cellular cytotoxicity in humans elicited by vaccination, natural infection, and experimental challenge. J Infect Dis. (2016) 214:945–52. doi: 10.1093/infdis/jiw262
46. He W, Tan GS, Mullarkey CE, Lee AJ, Lam MM, Krammer F, et al. Epitope specificity plays a critical role in regulating antibody-dependent cell-mediated cytotoxicity against influenza A virus. Proc Natl Acad Sci U.S.A. (2016) 113:11931–6. doi: 10.1073/pnas.1609316113
47. Gao R, Sheng Z, Sreenivasan CC, Wang D, and Li F. Influenza A virus antibodies with antibody-dependent cellular cytotoxicity function. Viruses. (2020) 12:276. doi: 10.3390/v12030276
48. Florek KR, Weinfurter JT, Jegaskanda S, Brewoo JN, Powell TD, Young GR, et al. Modified vaccinia virus Ankara encoding influenza virus hemagglutinin induces heterosubtypic immunity in macaques. J Virol. (2014) 88:13418–28. doi: 10.1128/JVI.01219-14
49. Krammer F and Palese P. Influenza virus hemagglutinin stalk-based antibodies and vaccines. Curr Opin Virol. (2013) 3:521–30. doi: 10.1016/j.coviro.2013.07.007
50. Terajima M, Co MD, Cruz J, and Ennis FA. High antibody-dependent cellular cytotoxicity antibody titers to H5N1 and H7N9 avian influenza A viruses in healthy US adults and older children. J Infect Dis. (2015) 212:1052–60. doi: 10.1093/infdis/jiv181
51. Greenberg SB, Six HR, Drake S, and Couch RB. Cell cytotoxicity due to specific influenza antibody production in vitro after recent influenza antigen stimulation. Proc Natl Acad Sci U.S.A. (1979) 76:4622–6. doi: 10.1073/pnas.76.9.4622
53. Altschul SF, Gish W, Miller W, Myers EW, and Lipman DJ. Basic local alignment search tool. J Mol Biol. (1990) 215(3):403–10. doi: 10.1016/S0022-2836(05)80360-2
54. Ali A, Khatri M, Wang L, Saif YM, and Lee CW. Identification of swine H1N2/pandemic H1N1 reassortant influenza virus in pigs, United States. Vet Microbiol. (2012) 158:60–8. doi: 10.1016/j.vetmic.2012.02.014
55. Yassine HM, Khatri M, Zhang YJ, Lee CW, Byrum BA, O’Quin J, et al. Characterization of triple reassortant H1N1 influenza A viruses from swine in Ohio. Vet Microbiol. (2009) 139:132–9. doi: 10.1016/j.vetmic.2009.04.028
56. Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. (2009) 325:197–201. doi: 10.1126/science.1176225
57. Koh SK, Park J, Kim SE, Lim Y, Phan MT, Kim J, et al. Natural killer cell expansion and cytotoxicity differ depending on the culture medium used. Ann Lab Med. (2022) 42:638–49. doi: 10.3343/alm.2022.42.6.638
58. Tam YH, Valkenburg SA, Perera R, Wong JHF, Fang VJ, Ng TWY, et al. Immune responses to twice-annual influenza vaccination in older adults in hong kong. Clin Infect Dis. (2018) 66:904–12. doi: 10.1093/cid/cix900
59. Jegaskanda S, Weinfurter JT, Friedrich TC, and Kent SJ. Antibody-dependent cellular cytotoxicity is associated with control of pandemic H1N1 influenza virus infection of macaques. J Virol. (2013) 87:5512–22. doi: 10.1128/JVI.03030-12
60. Chen X, Sun HY, Lee CY, Rostad CA, Trost J, Abreu RB, et al. Functional antibody-dependent cell mediated cytotoxicity (ADCC) responses to vaccine and circulating influenza strains following vaccination. Virology. (2022) 569:44–55. doi: 10.1016/j.virol.2022.02.004
61. Horns F, Vollmers C, Croote D, Mackey SF, Swan GE, Dekker CL, et al. Lineage tracing of human B cells reveals the in vivo landscape of human antibody class switching. eLife 5. (2016) 5:e16578. doi: 10.7554/eLife.23066
62. Li APY, Cohen CA, Leung NHL, Fang VJ, Gangappa S, Sambhara S, et al. Immunogenicity of standard, high-dose, MF59-adjuvanted, and recombinant-HA seasonal influenza vaccination in older adults. NPJ Vaccines. (2021) 6:25. doi: 10.1038/s41541-021-00289-5
63. Alter G, Ottenhoff THM, and Joosten SA. Antibody glycosylation in inflammation, disease and vaccination. Semin Immunol. (2018) 39:102–10. doi: 10.1016/j.smim.2018.05.003
64. Valkenburg SA, Fang VJ, Leung NH, Chu DK, Ip DK, Perera RA, et al. Cross-reactive antibody-dependent cellular cytotoxicity antibodies are increased by recent infection in a household study of influenza transmission. Clin Transl Immunol. (2019) 8:e1092. doi: 10.1002/cti2.v8.11
65. Finco O and Rappuoli R. Rediscovering complement, the first barrier of innate immunity. Vaccine. (2008) 26 Suppl 8:I1–2. doi: 10.1016/j.vaccine.2008.11.048
66. Paudyal B, Mwangi W, Rijal P, Schwartz JC, Noble A, Shaw A, et al. Fc-mediated functions of porcine igG subclasses. Front Immunol. (2022) 13:903755. doi: 10.3389/fimmu.2022.903755
67. Qu Z, Liang X, Liu Y, Du J, Liu S, and Sun W. Hepatitis B virus sensitizes hepatocytes to complement-dependent cytotoxicity through downregulating CD59. Mol Immunol. (2009) 47:283–9. doi: 10.1016/j.molimm.2009.09.022
68. Terajima M, Cruz J, Co MD, Lee JH, Kaur K, Wrammert J, et al. Complement-dependent lysis of influenza a virus-infected cells by broadly cross-reactive human monoclonal antibodies. J Virol. (2011) 85:13463–7. doi: 10.1128/JVI.05193-11
69. Budroni S, Buricchi F, Cavallone A, Bourguignon P, Caubet M, Dewar V, et al. Antibody avidity, persistence, and response to antigen recall: comparison of vaccine adjuvants. NPJ Vaccines. (2021) 6:78. doi: 10.1038/s41541-021-00337-0
70. Smatti MK, Al Thani AA, and Yassine HM. Viral-induced enhanced disease illness. Front Microbiol. (2018) 9:2991. doi: 10.3389/fmicb.2018.02991
71. Clements ML, O’Donnell S, Levine MM, Chanock RM, and Murphy BR. Dose response of A/Alaska/6/77 (H3N2) cold-adapted reassortant vaccine virus in adult volunteers: role of local antibody in resistance to infection with vaccine virus. Infect Immun. (1983) 40:1044–51. doi: 10.1128/iai.40.3.1044-1051.1983
Keywords: swine influenza a virus, intranasal vaccine, chitosan nanoparticle, antibody functions, ADCC and CDC
Citation: Singh M, do Nascimento GM, Renu S, Bugybayeva D, Shekoni OC, Suresh R, Schrock J, Dolatyabi S, Diel DG, Boyaka PN and Renukaradhya GJ (2025) Influenza-specific antibody-mediated and complement-dependent cellular cytotoxicity-inducing antibodies in vaccinated and infected pigs. Front. Immunol. 16:1600761. doi: 10.3389/fimmu.2025.1600761
Received: 26 March 2025; Accepted: 29 May 2025;
Published: 30 June 2025.
Edited by:
Anke Huckriede, University Medical Center Groningen, NetherlandsReviewed by:
Victor C. Huber, University of South Dakota, United StatesRishi Kumar Jaiswal, University of Arkansas for Medical Sciences, United States
Basudev Paudyal, The Pirbright Institute, United Kingdom
Copyright © 2025 Singh, do Nascimento, Renu, Bugybayeva, Shekoni, Suresh, Schrock, Dolatyabi, Diel, Boyaka and Renukaradhya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gourapura J. Renukaradhya, Z291cmFwdXJhLjFAb3N1LmVkdQ==
 Mithilesh Singh
Mithilesh Singh Gabriela Mansano do Nascimento
Gabriela Mansano do Nascimento Sankar Renu
Sankar Renu Dina Bugybayeva
Dina Bugybayeva Olaitan C. Shekoni1
Olaitan C. Shekoni1 Jennifer Schrock
Jennifer Schrock Sara Dolatyabi
Sara Dolatyabi Diego G. Diel
Diego G. Diel Prosper N. Boyaka
Prosper N. Boyaka Gourapura J. Renukaradhya
Gourapura J. Renukaradhya