- 1Department of Biochemistry, Wonkwang University School of Medicine, Iksan, Republic of Korea
- 2Sarcopenia Total Solution Center, Wonkwang University School of Medicine, Iksan, Republic of Korea
- 3Department of Oriental Pharmacy, College of Pharmacy, Wonkwang-Oriental Medicines Research Institute, Wonkwang University, Iksan, Republic of Korea
- 4Department of Microbiology, Wonkwang University School of Medicine, Iksan, Republic of Korea
- 5Institute of Wonkwang Medical Science, Wonkwang University School of Medicine, Iksan, Republic of Korea
Extracellular vesicles (EVs), including exosomes and microvesicles, play crucial roles in cancer progression by mediating the communication between cancer cells and their microenvironment. Cancer cell-derived EVs promote tumor growth, metastasis, and immune evasion by carrying bioactive materials, such as proteins, RNAs, DNA fragments, and lipids but, immunotherapy aims to enhance the immune response against cancer; however, resistance remains a major challenge. Cancer cell-derived EVs contribute to this resistance by delivering immunosuppressive molecules that impair T cell activation, promote the expansion of regulatory T cells (Tregs), and reduce natural killer (NK) cell cytotoxicity, thereby allowing cancer cells to evade immune surveillance. Additionally, cancer cell-derived EVs can carry immune checkpoint proteins, such as Programmed Death-Ligand 1 (PD-L1), which bind to the Programmed Death-1 (PD-1) receptor on T cells, leading to T cell exhaustion and reduced anti-tumor activity. This mechanism reflects how cancer cells directly evade immune detection and contributes to the overall resistance to immune checkpoint blockade therapies, such as anti-PD-1 or anti-PD-L1 antibodies. By delivering these immunomodulatory molecules, EVs not only contribute to local immune suppression but also create a systemic environment that is less favorable for effective anticancer immunity. Therefore, understanding the role of EVs in the immunotherapy resistance is crucial for developing targeted strategies to counteract their effects and ultimately improve therapeutic outcomes. Here we encourage researchers to pay more attention to the role of cancer cell-derived EVs in overcoming immunotherapeutic resistance, because such efforts may be one of the most promising approaches to address immunotherapy resistance in the future.
1 Introduction
Extracellular vesicles (EVs) are nanosized, membrane-enclosed vesicles released by nearly all cell types, including cancer cells. These vesicles, mainly classified as exosomes (30–150 nm) and microvesicles (100–1,000 nm), function as intercellular messengers that transport bioactive molecules such as proteins, lipids, RNAs, and DNA fragments. By facilitating the exchange of these components, EVs influence a wide range of physiological and pathological processes, including immune modulation, tissue repair, and disease progression. In cancer, EVs play a pivotal role in shaping the tumor microenvironment (TME) and driving tumor growth, metastasis, and immune evasion through cell-to-cell communication (1). Cancer cell-derived EVs perform a range of functions and their influence on immune modulation has gained increasing attention. To evade immune detection, cancer cells utilize multiple strategies, and EVs play a crucial role in suppressing immune responses and facilitating immune evasion. EVs promote cancer progression while limiting the efficacy of immune-based therapies by transporting immunosuppressive factors, modulating immune cell activity, and reshaping the TME into an immune-resistant niche. A major concern regarding this phenomenon is the involvement of EVs in inducing resistance to immunotherapy, particularly to immune checkpoint inhibitors (ICIs), such as anti-Programmed Death-1 (PD-1) and anti- Programmed Death-Ligand 1 (PD-L1) antibodies. These therapies aim to restore anti-tumor immunity by reactivating exhausted T cells. However, growing evidence indicates that cancer cell-derived EVs can negatively regulate these responses by carrying immune checkpoint proteins, such as PD-L1, thus contributing to systemic immune suppression (2).
Although immunotherapy has the potential to transform cancer treatment, its overall success is hindered by primary or acquired resistance, which affects a substantial proportion of patients. A deeper understanding of the mechanisms underlying immunotherapy resistance is essential for developing novel therapeutic strategies to improve treatment outcomes. This review explores the role of EVs in cancer biology, emphasizing their involvement in immune evasion and resistance to immunotherapy. Additionally, we discuss potential strategies for counteracting EV-mediated immunosuppression and highlight future directions for enhancing cancer immunotherapy.
2 EVs in cancer biology
EVs are increasingly recognized as key mediators of intercellular communication in cancer, influencing tumor progression, immune modulation, metastasis, and therapeutic resistance. These vesicles, secreted by tumor cells, carry a diverse range of bioactive molecules, including proteins, lipids, nucleic acids, and metabolites, which contribute to shaping the TME. EVs contribute to fundamental cancer hallmarks by transferring oncogenic signals between cells, including uncontrolled proliferation, angiogenesis, immune evasion, and metastatic dissemination (1). Elucidation of these molecular mechanisms may enable the development of EV-targeted therapeutics and biomarker-driven strategies for precision oncology.
Cancer cells exploit EVs to enhance their survival and proliferation by transmitting oncogenic factors that support tumor growth and metabolic adaptation. These vesicles carry miRNAs, including miR-21 and miR-155, which regulate pathways involved in cancer cell proliferation, apoptosis, and chemoresistance (3, 4). Additionally, EVs contain metabolic regulators including hexokinase 2 and lactate dehydrogenase, which promote the Warburg effect, a metabolic reprogramming process that enhances glucose uptake and lactate production to sustain rapid tumor growth. This metabolic shift provides cancer cells with a continuous energy supply, while modifying the surrounding environment to favor tumor expansion (5).
As tumors grow, they require an adequate blood supply, and EVs contribute to this process by promoting angiogenesis. Cancer cell-derived EVs carry key angiogenic factors, such as vascular endothelial growth factor, fibroblast growth factor, and hypoxia-inducible factor-1α (HIF-1α), which enhance endothelial cell proliferation and migration, leading to the formation of new blood vessels (6). Additionally, EVs containing miR-210 suppress antiangiogenic regulators, thereby promoting the angiogenic switch (7). These molecular interactions not only ensure sufficient oxygen and nutrient supply to tumor cells, but also facilitate their infiltration into surrounding tissues (8).
Beyond local tumor progression, EVs play a pivotal role in metastatic dissemination by priming the colonization of distant organs. A key mechanism involves EV-associated integrins α6β4 and α6β1, which guide metastatic cancer cells to specific organs, such as the lungs, liver, and brain (9). In addition, EVs facilitate extracellular matrix remodeling by delivering matrix metalloproteinases that degrade structural barriers, allowing tumor cells to invade and establish secondary tumors. These interactions create a conducive environment for circulating tumor cells to initiate metastasis, ultimately inducing cancer progression to an advanced stage (10).
In addition to inducing tumor growth and metastasis, EVs contribute to evasion of the immune system, allowing cancer cells to escape immune surveillance. This is achieved by impairing T cell function through the induction of exhaustion and reduced cytotoxic activity, ultimately leading to diminished responsiveness to ICIs. Furthermore, EVs promote the expansion of regulatory T-cells (Tregs), contributing to the impairment of anti-tumor immunity by dampening cytotoxic T cell function. Another key immunosuppressive mechanism is the induction of myeloid-derived suppressor cells (MDSCs), which are expanded and activated through EV-mediated signaling, thereby suppressing T cell proliferation and anti-tumor immune responses (11–13). Additionally, EVs impair natural killer (NK) cell activity by downregulating activating receptors required for NK cell-mediated tumor elimination (14). By modulating immune regulatory pathways, EVs facilitate cancer immune evasion and ultimately reduce the efficacy of immune-based therapies.
Given their extensive role in shaping the TME, EVs have emerged as promising therapeutic targets. Efforts to block EV production, neutralize their cargo, or harness them for drug delivery are being actively investigated to counteract their tumor-promoting effects. Moreover, the presence of cancer cell-derived EVs in bodily fluids highlights their potential as biomarkers for cancer diagnosis and treatment response monitoring. Despite advances in our understanding of EV functions, further research is essential to develop effective strategies that can selectively target EV-mediated signaling pathways without disrupting essential physiological processes.
3 Mechanisms of EV-mediated immune evasion and immunotherapy resistance
EVs mediate immune suppression, enabling cancer cells to evade immune destruction and resist immunotherapy. By delivering immunomodulatory molecules, EVs impair T-cell function, promote Treg expansion, inhibit NK cell cytotoxicity, and foster an immunosuppressive TME. Moreover, cancer cell-derived EVs carry immune checkpoint proteins that contribute to ICI resistance, thereby diminishing the efficacy of immunotherapy (15). However, one of the most critical mechanisms of EV-mediated immune suppression is the direct inhibition of T cell function. EVs suppress T cell function by delivering PD-L1, FasL, TGF-β, and IL-10, leading to T cell exhaustion and reduced anti-tumor immunity. Although this mechanism resembles PD-L1 expression in tumor cells, PD-L1-containing EVs have a broader impact by systemically circulating and suppressing immune responses at distant sites. Furthermore, EVs deliver FasL, which triggers apoptosis in activated T cells and further reduces anti-tumor immunity (16). By modulating these pathways, EVs establish an immunosuppressive environment that supports tumor survival and progression.
In addition, cancer-derived EVs enhance glycolysis in tumor-associated macrophages and myeloid-derived suppressor cells, further acidifying the TME and impairing effector T cell infiltration (17–19). Hypoxic EVs enriched in HIF-1α promote immune suppression by recruiting immunosuppressive myeloid cells and inhibiting cytotoxic T cell function (20–22). Notably, EVs also promote Treg expansion via TGF-β and IL-10, intensifying immune suppression within the TME. Consequently, EV-mediated Treg expansion contributes to immunotherapy resistance, because excessive Treg activity diminishes the efficacy of immune checkpoint blockade therapy (23). Similarly, cancer cell-derived EVs impair NK cell cytotoxicity via TGF-β, CD73, and FasL, downregulating NKG2D and facilitating immune evasion (24). Moreover, EVs contribute to MDSC expansion via HSP72- and miR-21-induced STAT3 activation, further suppressing T-cell responses (25, 26). Additionally, EVs facilitate metabolic reprogramming within the TME by transferring enzymes and metabolites that promote local hypoxia and acidity, creating an environment that is hostile to immune cells, but conducive to tumor progression (27).
A major concern in EV-mediated immune evasion is its contribution to resistance against ICIs, such as anti-PD-1 and anti-PD-L1 antibodies. Although ICIs are designed to restore T-cell function by blocking inhibitory signals, cancer cell-derived EVs act as decoys by carrying PD-L1, thereby neutralizing the therapeutic efficacy of these drugs. As a result, circulating PD-L1+ EVs not only suppress T cell activation, but also sequester checkpoint inhibitors, reducing their ability to block tumor-associated PD-L1 (28). Importantly, the role of EVs in immune evasion and therapy resistance may differ depending on tumor type. Notably, their immunosuppressive functions have been more extensively studied in solid tumors, such as melanoma, triple-negative breast cancer (TNBC), and hepatocellular carcinoma, where EVs carry immune checkpoint ligands or immunomodulatory RNAs that suppress anti-tumor immunity (29, 30). In contrast, EVs in hematological malignancies often participate more prominently in altering the bone marrow microenvironment, promoting niche remodeling and facilitating chemoresistance (31, 32). Such tumor-specific variations necessitate the development of more sophisticated and individualized EV-based therapeutic approaches tailored to the distinct biological contexts of each cancer type. Thus, targeting EVs production or their immunosuppressive cargo may enhance the efficacy of immunotherapy.
Emerging approaches include pharmacological inhibition of EVs release, with agents such as GW4869, a neutral sphingomyelinase inhibitor that effectively reduces exosome secretion and tumor-promoting signals (33). In addition, neutralization of immunosuppressive EVs-associated cargo, such as PD-L1, has been shown to restore T-cell activity and enhance responses to ICIs (34). The development of engineered EVs capable of delivering therapeutic agents to specific immune or tumor targets represents another innovative approach (35). Moreover, the identification of EV-derived biomarkers predictive of immunotherapy response holds significant promise for advancing precision oncology, although clinical validation remains an ongoing challenge (36, 37). Collectively, these strategies highlight the therapeutic potential of EV-targeting interventions, but systematic preclinical and clinical validation is needed to translate these findings into clinical practice.
4 Cancer cell-derived EVs and immunotherapy resistance
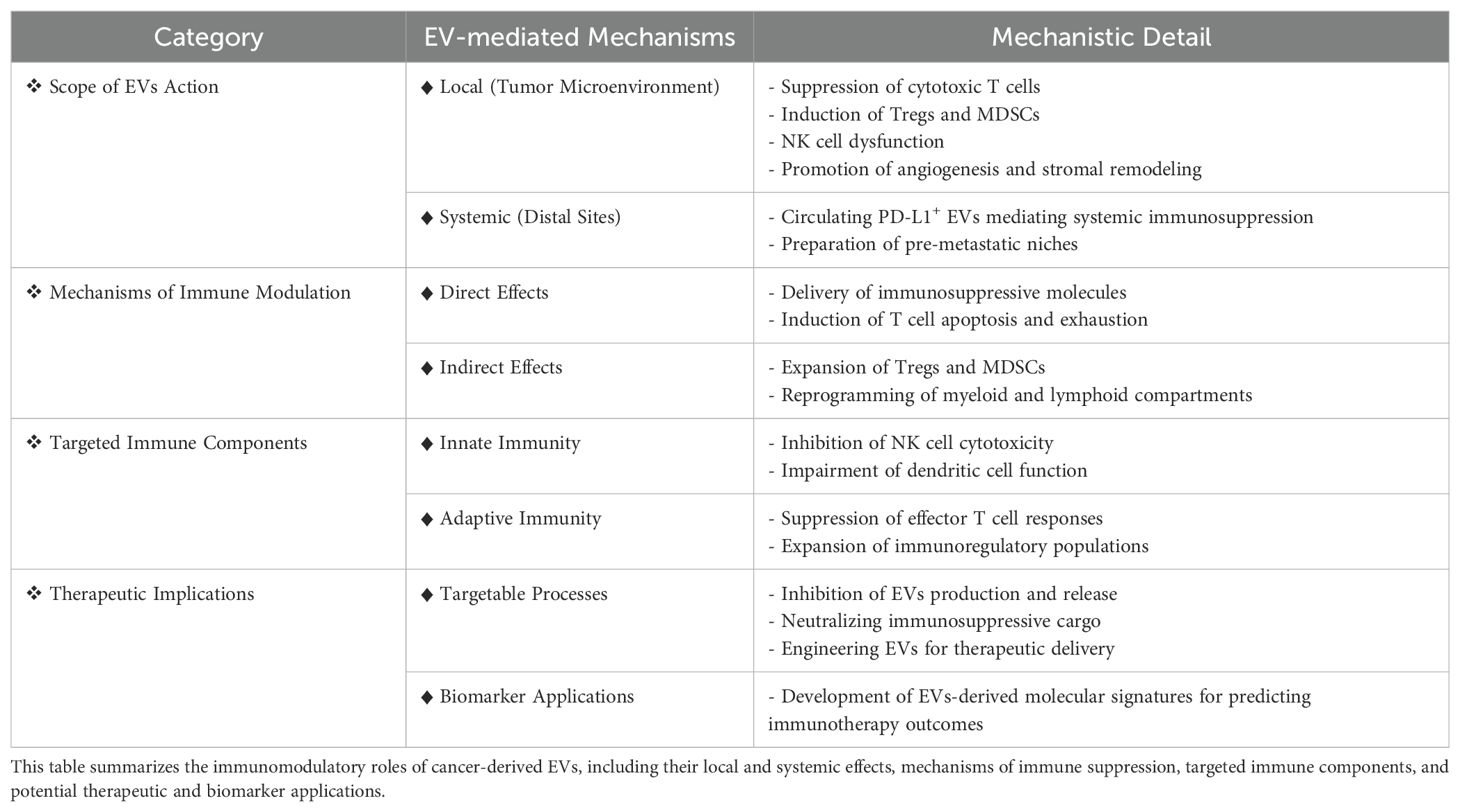
Immunotherapy has emerged as an innovative approach that uses the immune system to treat cancer. However, resistance to immunotherapy remains a significant challenge, and increasing evidence suggests that cancer cell-derived EVs play a pivotal role in this process. In this section, we describe the role of cancer cell-derived EVs in immune suppression and resistance to cancer therapy based on previous studies. These data are summarized in Table 1.
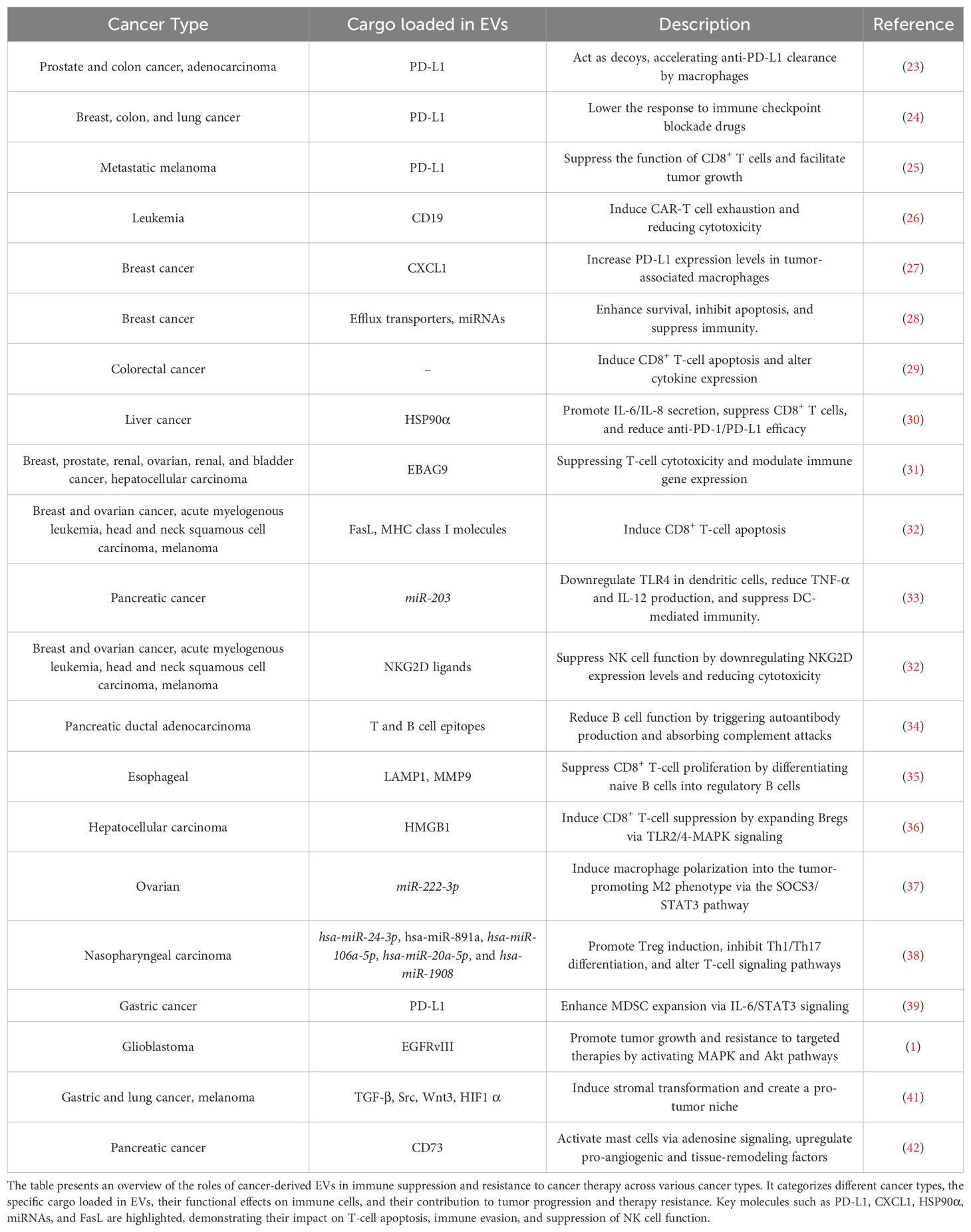
Table 1. Role of cancer-derived extracellular vesicles in immune suppression and resistance to cancer therapy.
Cancer cell-derived EVs directly contribute to immunotherapy resistance by interfering with ICIs and chimeric antigen receptor (CAR)-T cells. ICIs, such as anti-PD-1/PD-L1 antibodies, are powerful treatments that enhance the ability of the immune system to recognize and attack cancer cells by blocking inhibitory signals that suppress T cell activity. PD-L1 present in cancer cell-derived EVs can contribute to immunotherapy resistance by acting as a decoy that binds to anti-PD-L1 antibodies (38). Tumor-cell-derived EVs carrying PD-L1 also interact with PD-1 on T cells, leading to immune suppression and reduced efficacy of immune checkpoint blockade therapies (39). PD-L1-containing EVs have been shown to correlate with the patient response to anti-PD-1 therapy in metastatic melanoma (37). In addition, cancer cell-derived EVs impair the efficacy of CAR-T cell therapy. EVs containing CD19 interact with CAR-T cells, inducing proinflammatory cytokine secretion and promoting T cell exhaustion. This interaction weakens the therapeutic efficacy and reduces CAR-T cell cytotoxicity (40).
Chemotherapy is widely used for cancer treatment; however, several studies have suggested that cancer cell-derived EVs formed after chemotherapy reduce the efficacy of immunotherapy. Chemotherapy-induced cancer cell-derived EVs increase PD-L1 expression levels in tumor-associated macrophages (TAMs) (41). Similarly, EVs from drug-resistant breast cancer cells deliver efflux transporters and miRNAs that enhance cell survival, inhibit apoptosis, and inhibit immune responses (42). In addition, after chemotherapy, cancer cell-derived EVs induce CD8+ T-cell apoptosis and alter cytokine-related gene expression, leading to an immunosuppressive TME that hinders effective anti-tumor immunity (43).
Cancer cell-derived EVs contain various molecules that significantly contribute to immunotherapy resistance by affecting both immune and immunosuppressive cells. For example, cancer cell-derived EVs carrying HSP90-alpha promote the secretion of IL-6 and IL-8 by monocytes and neutrophils, resulting in CD8+ T cell suppression and reduced efficacy of anti-PD-1/PD-L1 treatment (44). Cancer cell-derived EVs transfer estrogen receptor-binding fragment-associated antigen 9 to T cells, suppressing cytotoxicity and modulating immune-related gene expression (45). Cancer cell-derived EVs can also induce CD8+ T-cell apoptosis via the membrane-associated form of the FasL and MHC class I molecules (46).
Cancer cell-derived EVs suppress the function of other immune cells, including dendritic, NK, and B cells, contributing to immune evasion and tumor progression. Pancreatic-cancer cell-derived EVs transfer miR-203 to dendritic cells (DCs), leading to TLR4 downregulation and reduced TNF-α and IL-12 production, thereby suppressing DC-mediated immune responses (47). Cancer cell-derived EVs suppress NK cell function by down-regulating NKG2D receptor expression and decreasing cytotoxicity (46). They present tumor antigens to B cells to trigger autoantibody production, while simultaneously acting as decoys that absorb complement attacks, thereby reducing complement-mediated cytotoxicity against cancer cells (48). Cancer cell-derived EVs not only impair B cell function, but also induce their differentiation into regulatory B cells (Bregs), a subset of B cells with immunosuppressive functions that inhibit excessive immune responses and promote immune tolerance. Esophageal cancer cell–derived EVs induce naive B cells to differentiate into Bregs, which suppress CD8+ T cell proliferation (49). In hepatocellular carcinoma, Cancer cell-derived exosomes induce the expansion of Bregs via HMGB1-TLR2/4-MAPK signaling, enhancing their ability to suppress CD8+ T cell activity (50).
Cancer cell-derived EVs promote the functions of other immunosuppressive cells. Epithelial ovarian cancer cell–derived EVs contain miR-222-3p, which regulates the SOCS3/STAT3 pathway and induces macrophage polarization to the tumor-promoting M2 phenotype (51). Cancer cell-derived EVs from nasopharyngeal carcinoma promote Treg induction by inhibiting Th1 and Th17 differentiation and altering T-cell signaling pathways, contributing to immune suppression (52). MDSC expansion can be promoted by cancer cell-derived EVs carrying PD-L1 via IL-6/STAT3 signaling, enhancing immune suppression and tumor progression in gastric cancer (53).
Cancer cell-derived EVs can also act as messengers that facilitate communication between cancer cells, potentially contributing to resistance to immunotherapy. Cancer cell-derived EVs transfer EGFRvIII between tumor cells, activating the MAPK and Akt signaling pathways, which promote tumor growth and metastasis, leading to enhanced resistance to targeted therapies (1). Additionally, EVs facilitate intercellular communication and tumor progression by transferring nucleic acids and proteins between tumor cells and modulating the TME to form a pre-metastatic niche (54).
The TME, which regulates various signals and immunosuppressive cells, significantly influences the efficacy of immunotherapy. For instance, cancer cell-derived EVs containing TGF-β, Src, Wnt3, and HIF1α are taken up by TAMs, which subsequently release membrane blebs, transferring these components to stromal cells (55). Blebs secreted by TAMs carrying cancer cell-derived components induce myofibroblastic changes in the recipient stromal cells, creating a favorable niche for cancer cells (55). Cancer cell-derived EVs activate mast cells through CD73-mediated adenosine signaling, leading to the upregulation of pro-angiogenic and tissue remodeling factors, which contribute to an immunosuppressive TME (56).
Despite significant advances in elucidating the immunosuppressive roles of tumor-derived EVs, emerging evidence points to their dual and context-dependent functions. As demonstrated in preclinical vaccination models, some EVs can stimulate antitumor immunity by delivering tumor-associated antigens and MHC molecules to dendritic cells (57). These contrasting findings highlight the complexity of EVs-mediated immune modulation and indicate the need to identify the molecular and contextual factors determining whether EVs suppress or activate immunity. Moreover, EVs heterogeneity within the tumor microenvironment remains a critical yet underexplored factor. Addressing these challenges through standardization efforts, such as the MISEV guidelines (58), and conducting research towards greater standardization in future studies. While certain EVs-derived signatures show promise as predictive immunotherapy response biomarkers, clinical validation remains incomplete. Addressing these challenges will be crucial for fully harnessing the translational potential of EVs in cancer immunotherapy.
5 Conclusions
Cancer cell-derived EVs play a crucial role in immunotherapy resistance by modulating the immune system and promoting an immunosuppressive TME (Table 2). These vesicles carry various immunosuppressive molecules, including PD-L1, TGF-β, and FasL, which impair the function of immune cells such as T cells, NK cells, and DCs. By influencing immune regulation and fostering immune evasion, EVs can contribute to the failure of ICIs and CAR-T cell therapies. Furthermore, EVs facilitate communication between tumor cells, thereby enhancing metastasis and resistance to targeted therapies. Understanding the mechanisms by which EVs contribute to immunotherapy resistance is essential for developing novel therapeutic strategies that can counteract their effects and improve the efficacy of cancer immunotherapies. Targeting EVs is a promising approach to overcome resistance and enhance treatment outcomes in cancer therapy. In this review, we highlighted the critical role of cancer cell-derived EVs in promoting immunotherapy resistance, underscoring the need for targeted strategies to counteract EV-mediated immune evasion and improve therapeutic outcomes (Figure 1).
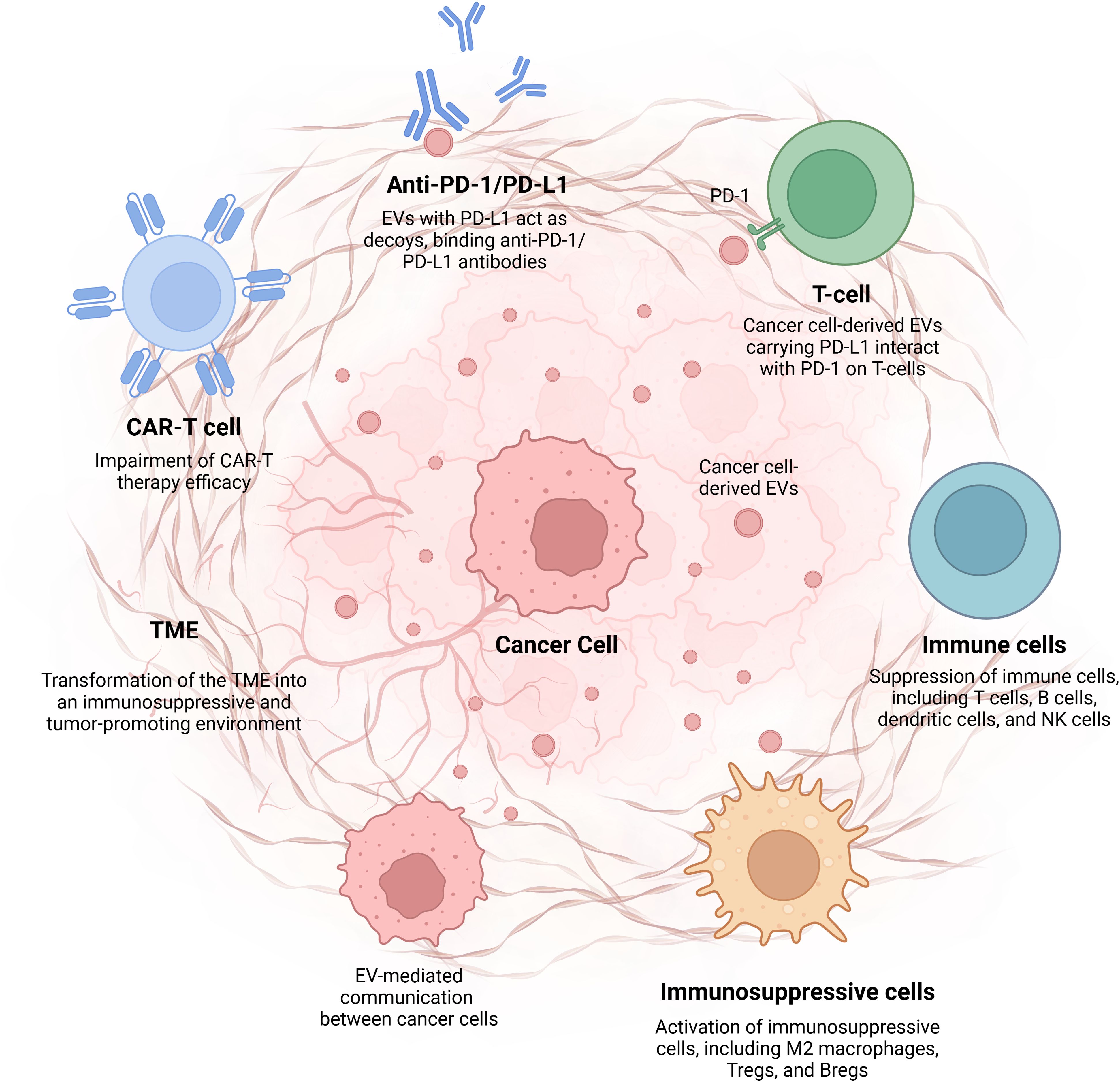
Figure 1. Cancer cell-derived EVs and their role in immunotherapy resistance. This figure illustrates the mechanisms by which cancer cell-derived extracellular vehicles (EVs) contribute to immunotherapy resistance by modulating the tumor microenvironment and suppressing the immune response. EVs, Extracellular Vesicles; PD-LI, Programmed Death-Ligand 1; PD-1, Programmed Death-1; NK, Natural Killer; Tregs, Regulatory T cells; Bregs, Regulatory B cells; TME, Tumor Microenvironment; CAR-T cell, Chimeric Antigen Receptor-T cell. Image was created with BioRender (https://biorender.com/)
Although EV-targeted therapies show promise, several limitations must be considered. Systemic inhibition of EVs may lead to unintended toxicity by interfering with physiological intercellular communication. Moreover, off-target effects remain a concern due to the challenge of distinguishing tumor-derived EVs from those released by normal cells (58). Strategies for selective targeting, such as ligand-directed delivery or nanoparticle engineering, are under development but require further optimization (59, 60).
6 Discussion
Concrete bioengineering strategies involve genetic modification of donor cells to enrich EVs with therapeutic cargos (e.g., siRNAs, ICIs), electroporation-based loading methods, and surface functionalization with antibodies or targeting ligands (e.g., anti-EGFR antibodies) (60–63). Preclinical models utilizing engineered EVs have shown promising results in overcoming immune resistance and enhancing the delivery of checkpoint inhibitors (64).
Recent advances in detection technologies such as next-generation sequencing (NGS), nanoplasmonic platforms, and microfluidic enrichment have improved EV analysis by enhancing the sensitivity and specificity of EV-based diagnosis (65–68). For instance, plasma-derived EV RNA signatures have been employed to predict therapy response in non-small cell lung cancer (NSCLC) (69). Several clinical studies have demonstrated the prognostic and predictive value of EV biomarkers, such as PD-L1+ EVs, in lung cancer, melanoma, and colorectal cancer (70–73). Ongoing clinical trials further support these findings, evaluating EV-based biomarkers for treatment response monitoring, minimal residual disease detection, and early cancer diagnosis across various tumor types such as lung cancer, breast cancer, pancreatic cancer, and colorectal cancer (74).
Despite the growing interest in EVs as key players in cancer progression, several controversies and unresolved challenges remain. A major issue is their heterogeneity and the classification of EVs to distinguish them from exosomes, microvesicles, and other subtypes remains unclear, because of overlapping size ranges and the absence of universally accepted markers (75). This complicates the standardization of EV isolation and characterization, leading to inconsistencies across studies (76). Additionally, although cancer cell-derived EVs carry oncogenic cargo, distinguishing them from normal-cell-derived EVs remains difficult, limiting their diagnostic potential (77–79).
Beyond tumor progression, cancer cell-derived EVs critically contribute to immune evasion and resistance to immunotherapy. ICI therapies, such as anti-PD-1/PD-L1 therapies, can induce the release of PD-L1+ EVs, which act as decoys to neutralize therapeutic antibodies and suppress T-cell responses (80). Similarly, tumors treated with CAR-T cells secrete EVs carrying inhibitory molecules that induce T cell exhaustion (81). Furthermore, chemotherapy-induced EVs can enhance PD-L1 expression levels in TAMs, creating an immunosuppressive TME (82). These EVs also carry immunosuppressive molecules, including PD-L1, TGF-β, and IL-10, which inhibit T cell activation or promote components of immune suppressive TME, such as Tregs and MDSCs, further dampening anti-tumor immunity (83, 84). The ability of EVs to establish an immunosuppressive microenvironment directly contributes to tumor immune escape and reduces the efficacy of ICIs.
To fully exploit EVs in cancer diagnosis and therapy, future research should focus on standardizing high-throughput EV isolation techniques (85) and utilizing advanced analytical tools, such as super-resolution microscopy and microfluidics-based platforms, to better understand EV heterogeneity (86). Additionally, engineered EVs are being explored as therapeutic vesicles using approaches such as selectively loading EVs with ICIs or small interfering RNAs to counteract tumor-induced immune suppression (87).
Bridging the gap between EV research and clinical applications requires collaborations among researchers, clinicians, and engineers. Large-scale clinical trials are needed to validate EV-based biomarkers and therapies for regulatory approval and widespread adoption (88). PD-L1-expressing EVs are emerging predictive biomarkers of ICI efficacy, enabling patient stratification for personalized treatment (89). Engineered EVs carrying immunomodulatory agents may offer novel strategies to overcome immune resistance (90). These emerging applications emphasize the need for further research on EVs as mediators and therapeutic targets in immunotherapeutic resistance.
Author contributions
MA: Investigation, Writing – original draft. J-GM: Investigation, Writing – original draft. YH: Conceptualization, Supervision, Investigation, Writing – review & editing, Writing – original draft. JS: Writing – review & editing, Investigation, Conceptualization, Writing – original draft, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Wonkwang University in 2023.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, and Lyden D. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell. (2016) 30:836–48. doi: 10.1016/j.ccell.2016.10.009
2. Zhou K, Guo S, Li F, Sun Q, and Liang G. Exosomal pd-L1: new insights into tumor immune escape mechanisms and therapeutic strategies. Front Cell Dev Biol. (2020) 8:569219. doi: 10.3389/fcell.2020.569219
3. Sun LH, Tian D, Yang ZC, and Li JL. Exosomal mir-21 promotes proliferation, invasion and therapy resistance of colon adenocarcinoma cells through its target pdcd4. Sci Rep. (2020) 10:8271. doi: 10.1038/s41598-020-65207-6
4. Takahashi RU, Prieto-Vila M, Hironaka A, and Ochiya T. The role of extracellular vesicle micrornas in cancer biology. Clin Chem Lab Med. (2017) 55:648–56. doi: 10.1515/cclm-2016-0708
5. Pavlyukov MS, Yu H, Bastola S, Minata M, Shender VO, Lee Y, et al. Apoptotic cell-derived extracellular vesicles promote Malignancy of glioblastoma via intercellular transfer of splicing factors. Cancer Cell. (2018) 34:119–35 e10. doi: 10.1016/j.ccell.2018.05.012
6. Olejarz W, Kubiak-Tomaszewska G, Chrzanowska A, and Lorenc T. Exosomes in angiogenesis and anti-angiogenic therapy in cancers. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21165840
7. Zhang S, Yang J, and Shen L. Extracellular vesicle-mediated regulation of tumor angiogenesis- implications for anti-angiogenesis therapy. J Cell Mol Med. (2021) 25:2776–85. doi: 10.1111/jcmm.16359
8. Patel NJ, Ashraf A, and Chung EJ. Extracellular vesicles as regulators of the extracellular matrix. Bioeng (Basel). (2023) 10. doi: 10.3390/bioengineering10020136
9. Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature. (2015) 527:329–35. doi: 10.1038/nature15756
10. Schmidtmann M and D’Souza-Schorey C. Extracellular vesicles: biological packages that modulate tumor cell invasion. Cancers (Basel). (2023) 15. doi: 10.3390/cancers15235617
11. Czernek L and Duchler M. Functions of cancer-derived extracellular vesicles in immunosuppression. Arch Immunol Ther Exp (Warsz). (2017) 65:311–23. doi: 10.1007/s00005-016-0453-3
12. Li X, Zhong J, Deng X, Guo X, Lu Y, Lin J, et al. Targeting myeloid-derived suppressor cells to enhance the antitumor efficacy of immune checkpoint blockade therapy. Front Immunol. (2021) 12:754196. doi: 10.3389/fimmu.2021.754196
13. Lyu C, Sun H, Sun Z, Liu Y, and Wang Q. Roles of exosomes in immunotherapy for solid cancers. Cell Death Dis. (2024) 15:106. doi: 10.1038/s41419-024-06494-z
14. Knox MC, Ni J, Bece A, Bucci J, Chin Y, Graham PH, et al. A clinician’s guide to cancer-derived exosomes: immune interactions and therapeutic implications. Front Immunol. (2020) 11:1612. doi: 10.3389/fimmu.2020.01612
15. Marar C, Starich B, and Wirtz D. Extracellular vesicles in immunomodulation and tumor progression. Nat Immunol. (2021) 22:560–70. doi: 10.1038/s41590-021-00899-0
16. Ma F, Vayalil J, Lee G, Wang Y, and Peng G. Emerging role of tumor-derived extracellular vesicles in T cell suppression and dysfunction in the tumor microenvironment. J Immunother Cancer. (2021) 9. doi: 10.1136/jitc-2021-003217
17. Lee YJ, Seo CW, Chae S, Lee CY, Kim SS, Shin YH, et al. Metabolic reprogramming into a glycolysis phenotype induced by extracellular vesicles derived from prostate cancer cells. Mol Cell Proteomics. (2025) 24:100944. doi: 10.1016/j.mcpro.2025.100944
18. Beaumont JEJ, Barbeau LMO, Ju J, Savelkouls KG, Bouwman FG, Zonneveld MI, et al. Cancer ev stimulate endothelial glycolysis to fuel protein synthesis via mtor and ampkalpha activation. J Extracell Vesicles. (2024) 13:e12449. doi: 10.1002/jev2.12449
19. Fridman ES, Ginini L, and Gil Z. The role of extracellular vesicles in metabolic reprogramming of the tumor microenvironment. Cells. (2022) 11. doi: 10.3390/cells11091433
20. Wang X, Wu R, Zhai P, Liu Z, Xia R, Zhang Z, et al. Hypoxia promotes ev secretion by impairing lysosomal homeostasis in hnscc through negative regulation of atp6v1a by hif-1alpha. J Extracell Vesicles. (2023) 12:e12310. doi: 10.1002/jev2.12310
21. Muniz-Garcia A, Romero M, Falcomicronn-Perez JM, Murray P, Zorzano A, and Mora S. Hypoxia-induced hif1alpha activation regulates small extracellular vesicle release in human embryonic kidney cells. Sci Rep. (2022) 12:1443. doi: 10.1038/s41598-022-05161-7
22. Branco H, Xavier CPR, Riganti C, and Vasconcelos MH. Hypoxia as a critical player in extracellular vesicles-mediated intercellular communication between tumor cells and their surrounding microenvironment. Biochim Biophys Acta Rev Cancer. (2025) 1880:189244. doi: 10.1016/j.bbcan.2024.189244
23. Szajnik M, Czystowska M, Szczepanski MJ, Mandapathil M, and Whiteside TL. Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg). PloS One. (2010) 5:e11469. doi: 10.1371/journal.pone.0011469
24. Hou PP and Chen HZ. Extracellular vesicles in the tumor immune microenvironment. Cancer Lett. (2021) 516:48–56. doi: 10.1016/j.canlet.2021.05.032
25. Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, et al. Membrane-associated hsp72 from tumor-derived exosomes mediates stat3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. (2010) 120:457–71. doi: 10.1172/JCI40483
26. Liang L, Xu X, Li J, and Yang C. Interaction between micrornas and myeloid-derived suppressor cells in tumor microenvironment. Front Immunol. (2022) 13:883683. doi: 10.3389/fimmu.2022.883683
27. Yang E, Wang X, Gong Z, Yu M, Wu H, and Zhang D. Exosome-mediated metabolic reprogramming: the emerging role in tumor microenvironment remodeling and its influence on cancer progression. Signal Transduct Target Ther. (2020) 5:242. doi: 10.1038/s41392-020-00359-5
28. Zhang W, Ou M, Yang P, and Ning M. The role of extracellular vesicle immune checkpoints in cancer. Clin Exp Immunol. (2024) 216:230–9. doi: 10.1093/cei/uxae026
29. Ahmadi M, Abbasi R, and Rezaie J. Tumor immune escape: extracellular vesicles roles and therapeutics application. Cell Commun Signal. (2024) 22:9. doi: 10.1186/s12964-023-01370-3
30. Kuang L, Wu L, and Li Y. Extracellular vesicles in tumor immunity: mechanisms and novel insights. Mol Cancer. (2025) 24:45. doi: 10.1186/s12943-025-02233-w
31. Hayashi Y, Nishimura K, Tanaka A, and Inoue D. Extracellular vesicle-mediated remodeling of the bone marrow microenvironment in myeloid Malignancies. Int J Hematol. (2023) 117:821–9. doi: 10.1007/s12185-023-03587-x
32. Mendes M, Monteiro AC, Neto E, Barrias CC, Sobrinho-Simoes MA, Duarte D, et al. Transforming the niche: the emerging role of extracellular vesicles in acute myeloid leukaemia progression. Int J Mol Sci. (2024) 25. doi: 10.3390/ijms25084430
33. Wan X, Fang Y, Du J, Cai S, and Dong H. Gw4869 can inhibit epithelial-mesenchymal transition and extracellular hsp90alpha in gefitinib-sensitive nsclc cells. Onco Targets Ther. (2023) 16:913–22. doi: 10.2147/OTT.S428707
34. Poggio M, Hu T, Pai CC, Chu B, Belair CD, Chang A, et al. Suppression of exosomal pd-L1 induces systemic anti-tumor immunity and memory. Cell. (2019) 177:414–27 e13. doi: 10.1016/j.cell.2019.02.016
35. Johnson V, Vasu S, Kumar US, and Kumar M. Surface-engineered extracellular vesicles in cancer immunotherapy. Cancers (Basel). (2023) 15. doi: 10.3390/cancers15102838
36. Theodoraki MN, Yerneni SS, Hoffmann TK, Gooding WE, and Whiteside TL. Clinical significance of pd-L1(+) exosomes in plasma of head and neck cancer patients. Clin Cancer Res. (2018) 24:896–905. doi: 10.1158/1078-0432.CCR-17-2664
37. Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, et al. Exosomal pd-L1 contributes to immunosuppression and is associated with anti-pd-1 response. Nature. (2018) 560:382–6. doi: 10.1038/s41586-018-0392-8
38. Chen J, Yang J, Wang W, Guo D, Zhang C, Wang S, et al. Tumor extracellular vesicles mediate anti-pd-L1 therapy resistance by decoying anti-pd-L1. Cell Mol Immunol. (2022) 19:1290–301. doi: 10.1038/s41423-022-00926-6
39. Lee CH, Bae JH, Choe EJ, Park JM, Park SS, Cho HJ, et al. Macitentan improves antitumor immune responses by inhibiting the secretion of tumor-derived extracellular vesicle pd-L1. Theranostics. (2022) 12:1971–87. doi: 10.7150/thno.68864
40. Wieckowski EU, Visus C, Szajnik M, Szczepanski MJ, Storkus WJ, and Whiteside TL. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated cd8+ T lymphocytes. J Immunol. (2009) 183:3720–30. doi: 10.4049/jimmunol.0900970
41. Wang S, Li J, Hong S, Wang N, Xu S, Yang B, et al. Chemotherapy-elicited extracellular vesicle cxcl1 from dying cells promotes triple-negative breast cancer metastasis by activating tam/pd-L1 signaling. J Exp Clin Cancer Res. (2024) 43:121. doi: 10.1186/s13046-024-03050-7
42. Santos P, Rezende CP, Piraine R, Oliveira B, Ferreira FB, Carvalho VS, et al. Extracellular vesicles from human breast cancer-resistant cells promote acquired drug resistance and pro-inflammatory macrophage response. Front Immunol. (2024) 15:1468229. doi: 10.3389/fimmu.2024.1468229
43. Ab Razak NS and Abu N. Chemotherapy-associated extracellular vesicles modulate T cells activity and cytokine release. J Cancer Res Updates. (2024) 13:75–8. doi: 10.30683/1929-2279.2024.13.10
44. Chen YQ, Man ZS, Zheng L, Zhang Y, Zhao CW, Ma YT, et al. Tumor cell-derived lc3b(+)Extracellular vesicles mediate the crosstalk between tumor microenvironment and immunotherapy efficacy in hepatocellular carcinoma via the hsp90alpha-il-6/il-8 signaling axis. Clin Immunol. (2024) 261:109925. doi: 10.1016/j.clim.2024.109925
45. Miyazaki T, Ikeda K, Sato W, Horie-Inoue K, and Inoue S. Extracellular vesicle-mediated ebag9 transfer from cancer cells to tumor microenvironment promotes immune escape and tumor progression. Oncogenesis. (2018) 7:7. doi: 10.1038/s41389-017-0022-6
46. Whiteside TL. Immune modulation of T-cell and nk (Natural killer) cell activities by texs (Tumour-derived exosomes). Biochem Soc Trans. (2013) 41:245–51. doi: 10.1042/BST20120265
47. Zhou M, Chen J, Zhou L, Chen W, Ding G, and Cao L. Pancreatic cancer derived exosomes regulate the expression of tlr4 in dendritic cells via mir-203. Cell Immunol. (2014) 292:65–9. doi: 10.1016/j.cellimm.2014.09.004
48. Capello M, Vykoukal JV, Katayama H, Bantis LE, Wang H, Kundnani DL, et al. Exosomes harbor B cell targets in pancreatic adenocarcinoma and exert decoy function against complement-mediated cytotoxicity. Nat Commun. (2019) 10:254. doi: 10.1038/s41467-018-08109-6
49. Li Y, An J, Huang S, He J, and Zhang J. Esophageal cancer-derived microvesicles induce regulatory B cells. Cell Biochem Funct. (2015) 33:308–13. doi: 10.1002/cbf.3115
50. Ye L, Zhang Q, Cheng Y, Chen X, Wang G, Shi M, et al. Tumor-derived exosomal hmgb1 fosters hepatocellular carcinoma immune evasion by promoting tim-1(+) regulatory B cell expansion. J Immunother Cancer. (2018) 6:145. doi: 10.1186/s40425-018-0451-6
51. Ying X, Wu Q, Wu X, Zhu Q, Wang X, Jiang L, et al. Epithelial ovarian cancer-secreted exosomal mir-222-3p induces polarization of tumor-associated macrophages. Oncotarget. (2016) 7:43076–87. doi: 10.18632/oncotarget.9246
52. Ye SB, Li ZL, Luo DH, Huang BJ, Chen YS, Zhang XS, et al. Tumor-derived exosomes promote tumor progression and T-cell dysfunction through the regulation of enriched exosomal micrornas in human nasopharyngeal carcinoma. Oncotarget. (2014) 5:5439–52. doi: 10.18632/oncotarget.2118
53. Li H, Chen X, Zheng S, Han B, Zhang X, Zheng X, et al. The expansion of mdscs induced by exosomal pd-L1 promotes the progression of gastric cancer. J Transl Med. (2024) 22:821. doi: 10.1186/s12967-024-05611-y
54. Qiao F, Pan P, Yan J, Sun J, Zong Y, Wu Z, et al. Role of tumor−Derived extracellular vesicles in cancer progression and their clinical applications (Review). Int J Oncol. (2019) 54:1525–33. doi: 10.3892/ijo.2019.4745
55. Umakoshi M, Takahashi S, Itoh G, Kuriyama S, Sasaki Y, Yanagihara K, et al. Macrophage-mediated transfer of cancer-derived components to stromal cells contributes to establishment of a pro-tumor microenvironment. Oncogene. (2019) 38:2162–76. doi: 10.1038/s41388-018-0564-x
56. Gorzalczany Y, Merimsky O, and Sagi-Eisenberg R. Mast cells are directly activated by cancer cell-derived extracellular vesicles by a cd73- and adenosine-dependent mechanism. Transl Oncol. (2019) 12:1549–56. doi: 10.1016/j.tranon.2019.08.005
57. Whiteside TL. Tumor-derived exosomes and their role in cancer progression. Adv Clin Chem. (2016) 74:103–41. doi: 10.1016/bs.acc.2015.12.005
58. Cheng W, Xu C, Su Y, Shen Y, Yang Q, Zhao Y, et al. Engineered extracellular vesicles: A potential treatment for regeneration. iScience. (2023) 26:108282. doi: 10.1016/j.isci.2023.108282
59. Liu Q, Li D, Pan X, and Liang Y. Targeted therapy using engineered extracellular vesicles: principles and strategies for membrane modification. J Nanobiotechnol. (2023) 21:334. doi: 10.1186/s12951-023-02081-0
60. Wiklander OPB, Mamand DR, Mohammad DK, Zheng W, Jawad Wiklander R, Sych T, et al. Antibody-displaying extracellular vesicles for targeted cancer therapy. Nat BioMed Eng. (2024) 8:1453–68. doi: 10.1038/s41551-024-01214-6
61. Rene CA and Parks RJ. Bioengineering extracellular vesicle cargo for optimal therapeutic efficiency. Mol Ther Methods Clin Dev. (2024) 32:101259. doi: 10.1016/j.omtm.2024.101259
62. Zhang F, Guo J, Zhang Z, Duan M, Wang G, Qian Y, et al. Application of engineered extracellular vesicles for targeted tumor therapy. J BioMed Sci. (2022) 29:14. doi: 10.1186/s12929-022-00798-y
63. Frawley T and Piskareva O. Extracellular vesicle dissemination of epidermal growth factor receptor and ligands and its role in cancer progression. Cancers (Basel). (2020) 12. doi: 10.3390/cancers12113200
64. Chen Y, Wang L, Zheng M, Zhu C, Wang G, Xia Y, et al. Engineered extracellular vesicles for concurrent anti-pdl1 immunotherapy and chemotherapy. Bioact Mater. (2022) 9:251–65. doi: 10.1016/j.bioactmat.2021.07.012
65. Testa A, Venturelli E, and Brizzi MF. Extracellular vesicles as a novel liquid biopsy-based diagnosis for the central nervous system, head and neck, lung, and gastrointestinal cancers: current and future perspectives. Cancers (Basel). (2021) 13. doi: 10.3390/cancers13112792
66. Pang B, Zhu Y, Ni J, Thompson J, Malouf D, Bucci J, et al. Extracellular vesicles: the next generation of biomarkers for liquid biopsy-based prostate cancer diagnosis. Theranostics. (2020) 10:2309–26. doi: 10.7150/thno.39486
67. Fairey A, Paproski RJ, Pink D, Sosnowski DL, Vasquez C, Donnelly B, et al. Clinical analysis of ev-fingerprint to predict grade group 3 and above prostate cancer and avoid prostate biopsy. Cancer Med. (2023) 12:15797–808. doi: 10.1002/cam4.6216
68. Rosa P, De Falco E, Pacini L, Piazza A, Ciraci P, Ricciardi L, et al. Next-generation sequencing comparative analysis of DNA mutations between blood-derived extracellular vesicles and matched cancer tissue in patients with grade 4 glioblastoma. Biomedicines. (2022) 10. doi: 10.3390/biomedicines10102590
69. Guo W, Zhou B, Zhao L, Huai Q, Tan F, Xue Q, et al. Plasma extracellular vesicle long rnas predict response to neoadjuvant immunotherapy and survival in patients with non-small cell lung cancer. Pharmacol Res. (2023) 196:106921. doi: 10.1016/j.phrs.2023.106921
70. Mathew M, Zade M, Mezghani N, Patel R, Wang Y, and Momen-Heravi F. Extracellular vesicles as biomarkers in cancer immunotherapy. Cancers (Basel). (2020) 12. doi: 10.3390/cancers12102825
71. Bollard SM, Howard J, Casalou C, Kelly BS, O’Donnell K, Fenn G, et al. Proteomic and metabolomic profiles of plasma-derived extracellular vesicles differentiate melanoma patients from healthy controls. Transl Oncol. (2024) 50:102152. doi: 10.1016/j.tranon.2024.102152
72. Kluszczynska K and Czyz M. Extracellular vesicles-based cell-cell communication in melanoma: new perspectives in diagnostics and therapy. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms24020965
73. Zhang Z, Liu X, Yang X, Jiang Y, Li A, Cong J, et al. Identification of faecal extracellular vesicles as novel biomarkers for the non-invasive diagnosis and prognosis of colorectal cancer. J Extracell Vesicles. (2023) 12:e12300. doi: 10.1002/jev2.12300
74. Makler A and Asghar W. Exosomal biomarkers for cancer diagnosis and patient monitoring. Expert Rev Mol Diagn. (2020) 20:387–400. doi: 10.1080/14737159.2020.1731308
75. De Sousa KP, Rossi I, Abdullahi M, Ramirez MI, Stratton D, and Inal JM. Isolation and characterization of extracellular vesicles and future directions in diagnosis and therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. (2023) 15:e1835. doi: 10.1002/wnan.1835
76. Witwer KW, Buzas EI, Bemis LT, Bora A, Lasser C, Lotvall J, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. (2013) 2. doi: 10.3402/jev.v2i0.20360
77. Kalluri R and McAndrews KM. The role of extracellular vesicles in cancer. Cell. (2023) 186:1610–26. doi: 10.1016/j.cell.2023.03.010
78. Ghosh S, Rajendran RL, Mahajan AA, Chowdhury A, Bera A, Guha S, et al. Harnessing exosomes as cancer biomarkers in clinical oncology. Cancer Cell Int. (2024) 24:278. doi: 10.1186/s12935-024-03464-5
79. Zhang W, Yang J, Cao D, You Y, Shen K, and Peng P. Regulation of exosomes released from normal ovarian epithelial cells and ovarian cancer cells. Tumour Biol. (2016) 37:15763–71. doi: 10.1007/s13277-016-5394-2
80. Yin Z, Yu M, Ma T, Zhang C, Huang S, Karimzadeh MR, et al. Mechanisms underlying low-clinical responses to pd-1/pd-L1 blocking antibodies in immunotherapy of cancer: A key role of exosomal pd-L1. J Immunother Cancer. (2021) 9. doi: 10.1136/jitc-2020-001698
81. Zhu X, Hu H, Xiao Y, Li Q, Zhong Z, Yang J, et al. Tumor-derived extracellular vesicles induce invalid cytokine release and exhaustion of cd19 car-T cells. Cancer Lett. (2022) 536:215668. doi: 10.1016/j.canlet.2022.215668
82. Yati S, Silathapanasakul A, Thakaeng C, Chanasakulniyom M, Songtawee N, Porntadavity S, et al. Extracellular vesicle-mediated il-1 signaling in response to doxorubicin activates pd-L1 expression in osteosarcoma models. Cells. (2022) 11. doi: 10.3390/cells11061042
83. Ye Z, Li G, and Lei J. Influencing immunity: role of extracellular vesicles in tumor immune checkpoint dynamics. Exp Mol Med. (2024) 56:2365–81. doi: 10.1038/s12276-024-01340-w
84. Reale A, Khong T, and Spencer A. Extracellular vesicles and their roles in the tumor immune microenvironment. J Clin Med. (2022) 11. doi: 10.3390/jcm11236892
85. Kapoor KS, Harris K, Arian KA, Ma L, Schueng Zancanela B, Church KA, et al. High throughput and rapid isolation of extracellular vesicles and exosomes with purity using size exclusion liquid chromatography. Bioact Mater. (2024) 40:683–95. doi: 10.1016/j.bioactmat.2024.08.002
86. Zhu J, Wu F, Li C, Mao J, Wang Y, Zhou X, et al. Application of single extracellular vesicle analysis techniques. Int J Nanomed. (2023) 18:5365–76. doi: 10.2147/IJN.S421342
87. Han Y, Jones TW, Dutta S, Zhu Y, Wang X, Narayanan SP, et al. Overview and update on methods for cargo loading into extracellular vesicles. Process (Basel). (2021) 9. doi: 10.3390/pr9020356
88. Asleh K, Dery V, Taylor C, Davey M, Djeungoue-Petga MA, and Ouellette RJ. Extracellular vesicle-based liquid biopsy biomarkers and their application in precision immuno-oncology. biomark Res. (2023) 11:99. doi: 10.1186/s40364-023-00540-2
89. Dou X, Hua Y, Chen Z, Chao F, and Li M. Extracellular vesicles containing pd-L1 contribute to cd8+ T-cell immune suppression and predict poor outcomes in small cell lung cancer. Clin Exp Immunol. (2022) 207:307–17. doi: 10.1093/cei/uxac006
Keywords: extracellular vesicles, tumor microenvironment, cancer therapy, immunotherapeutic resistance, immune checkpoint inhibitors, immune cells
Citation: Ahn M, Mun J-G, Han Y and Seo JH (2025) Cancer cell-derived extracellular vesicles: a potential target for overcoming tumor immunotherapy resistance and immune evasion strategies. Front. Immunol. 16:1601266. doi: 10.3389/fimmu.2025.1601266
Received: 27 March 2025; Accepted: 23 May 2025;
Published: 12 June 2025.
Edited by:
Sheila Spada, Hospital Physiotherapy Institutes (IRCCS), ItalyReviewed by:
Katia Cortese, University of Genoa, ItalyNoemi Aloi, CNR Area della Ricerca di Palermo, Italy
Anna Piro, University of Studies G. d’Annunzio Chieti and Pescara, Italy
Copyright © 2025 Ahn, Mun, Han and Seo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yohan Han, ZHlna3MxODc2QHdrdS5hYy5rcg==; Jae Ho Seo, YmlvbmlhbjlAd2t1LmFjLmty
†These authors have contributed equally to this work
 Minseo Ahn
Minseo Ahn Jeong-Geon Mun3†
Jeong-Geon Mun3† Jae Ho Seo
Jae Ho Seo