- 1Division of Cardiovascular Medicine, Department of Medicine, University of Florida, Gainesville, FL, United States
- 2Biomedical Sciences Program, University of Florida, College of Medicine, Gainesville, FL, United States
- 3Center for Integrative Cardiovascular and Metabolic Disease, University of Florida, Gainesville, FL, United States
- 4Department of Physiology and Aging, University of Florida, Gainesville, FL, United States
- 5Department of Pharmacology and Therapeutics, University of Florida, Gainesville, FL, United States
- 6Congenital Heart Center, College of Medicine, University of Florida, Gainesville, FL, United States
- 7Department of Pediatrics, University of Florida, Gainesville, FL, United States
Myocarditis is an inflammatory heart disease that is more prevalent in men. The etiology of myocarditis is often multifactorial with viral infections being a predominant cause of myocarditis. Other etiologies such as autoimmune mediated or secondary to certain medical therapies such as immune checkpoint inhibitors are also seen however less commonly. The wide spectrum of clinical symptoms with which these patients present and the lack of reliable patterns or biomarkers of progression make it difficult to both diagnose and risk-stratify patients. Importantly, this disease is widely prevalent in pediatric populations and is a leading cause of sudden cardiac death in young patients. However, much of the knowledge of pathogenesis and treatment of this disease is extrapolated from adult studies. Current research in myocarditis has increasingly identified the role of hormones and the apparent sex differences seen predominantly in adult patients; however, such data is not well established in pediatric patients. Thus, there is an increased need to evaluate the age and sex-based differences in pediatric patients with myocarditis. Therefore, this review aims to present an overview of our current understanding of pathogenesis, diagnosis, and treatment strategies for myocarditis, with an emphasis on outlining both adult and pediatric studies to emphasize the continued need for research into this disease.
1 Introduction
Myocarditis is caused by acute or chronic inflammation of the cardiomyocytes, leading to myocardial edema, injury, and necrosis (1). There have been ongoing efforts to define myocarditis better, and the most recent AHA guidelines suggest four strata: biopsy-proven, cardiac magnetic resonance imaging (CMR)-confirmed clinically suspected, clinically suspected, and possible myocarditis (2). The pathophysiology of myocarditis is multifactorial; however, it occurs primarily due to viral infections, though other pathogens and hypersensitivity reactions are also other known triggers (3). Serology can be utilized to identify viruses that could be causative for myocarditis but it is well accepted that a definitive diagnosis of viral caused myocarditis can only be made via positive PCR on the endomyocardial biopsy. Clinically, patients have heterogeneous presentations ranging from mild symptoms such as non-specific chest pain to heart failure, life-threatening arrhythmias, and sudden cardiac death (4, 5). The natural history after the episode of acute inflammation includes the possibility of complete resolution of clinical symptoms, improvement of myocardial dysfunction, development of dilated cardiomyopathy, and need for heart transplantation or death (4–8).
The true prevalence of myocarditis is difficult to estimate due to inconsistency in definition, heterogeneous presentation, and often sub-clinical self-limiting illness. The Global Burden of Disease Study estimated the number of myocarditis cases using discharge records to be roughly 22 for every 100,000 patients (9). More recent estimates range from 10.2 to 105.6 per 100,000 worldwide, with an annual occurrence of around 1.8 million cases (10). It has been well documented that men are at two-to-four-fold increased risk of developing myocarditis compared to females (11–21). Additionally, it has been shown that males who develop myocarditis are also at an increased risk of progressing to dilated cardiomyopathy (22). Overall, the sex differences in the incidence and natural history of myocarditis have led to significant research evaluating the impact of sex hormones on the inflammatory response in myocarditis. Most studies discussing these sex differences have been conducted in adult patients. However, there is data in younger patients that points to male predominance and even an age-related increase in predominance of males with myocarditis (23, 24). Conversely, other studies have shown no significant difference in the proportion of males versus females with myocarditis (25). A recent study of over 15,000 patients with myocarditis showed that this sex ratio was consistent in their adult cohort but was not present among pediatric patients (26).
Although several studies have documented the incidence and outcomes of viral myocarditis in children, there is a paucity of research comparing the unique differences in pediatric versus adult patients with myocarditis. Viral myocarditis is more often seen in children than in adults, and children are more likely to present with acute myocarditis (sudden onset) rather than chronic myocarditis, which is typically seen in adults. Also, the incidence of myocarditis in children varies with age, having a bimodal distribution, being higher in infants and rising again in young adults. While there are fundamental clinical differences between these two patient populations, there is a need for foundational basic, translational, and clinical research specifically focusing on myocarditis in the pediatric population to further our understanding. Therefore, this paper aims to review the pathogenesis, diagnosis, and treatment of viral myocarditis, highlighting our current understanding of the differences between pediatric and adult populations.
2 Pathogenesis
While numerous viruses have been associated with myocarditis, including parvovirus B19, human herpesvirus 6, and adenovirus, one of the most common viral agents known to cause myocarditis is coxsackievirus B (CVB) (27, 28). CVB is a positive single-stranded RNA belonging to the enterovirus group within the Picornaviridae family (29). Like other enteroviruses, CVB is transmitted via a fecal-oral route, and initial infection is through the GI tract, though viral kinetic experiments have shown that replication occurs in the heart, spleen, thymus, and pancreas (30). The pathogenesis of myocarditis is still being investigated, and clinical samples and varied mouse models are being utilized to better understand disease development and progression. Some of the known immune cell involvement in viral myocarditis are illustrated in Figure 1.
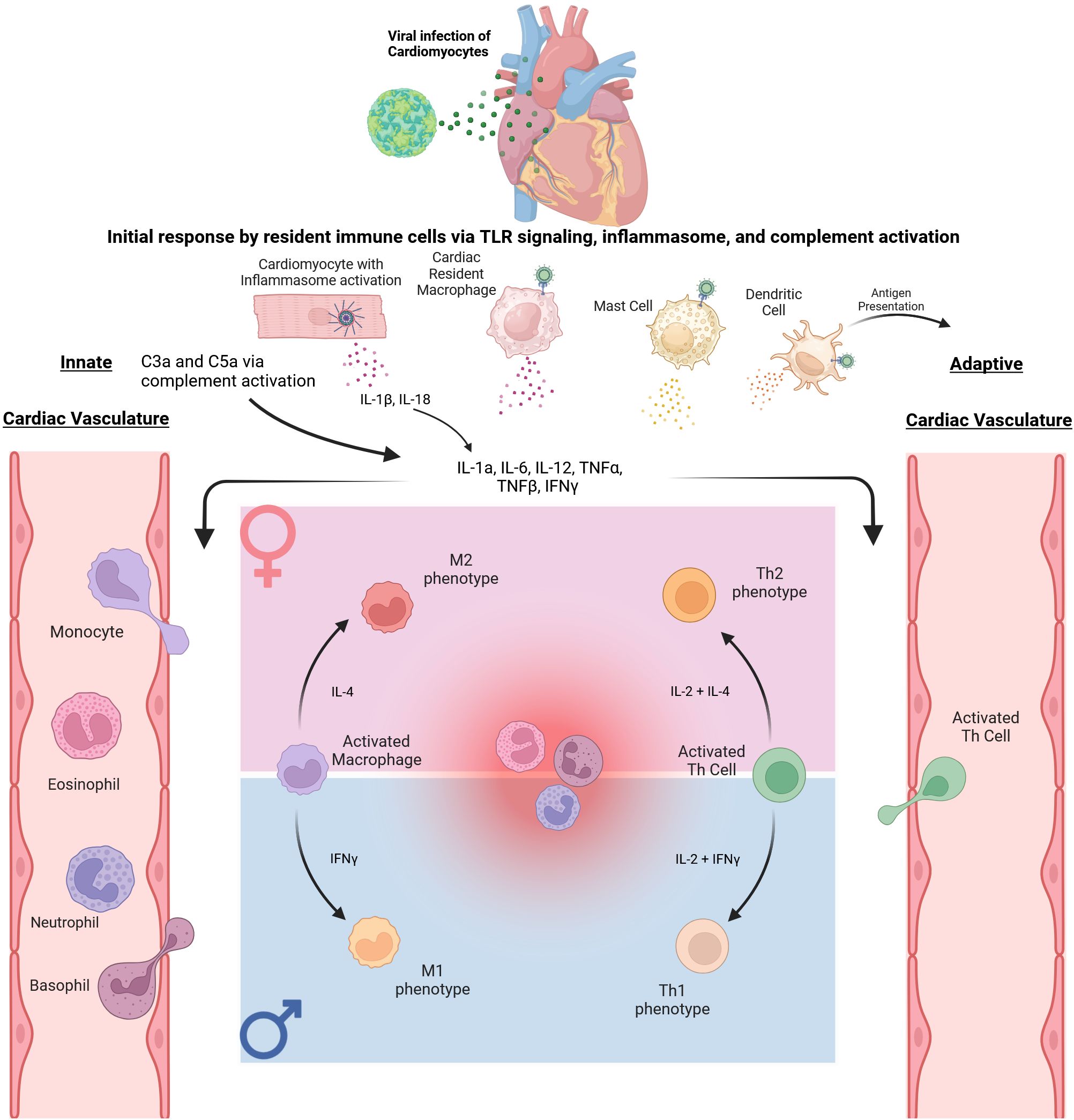
Figure 1. Immune response to Coxsackievirus B3 in Cardiac Tissue. The initial immune response begins with CVB3 trafficking to the heart. The initial infection of cardiomyocytes and resident immune cells triggers an innate inflammatory response via signaling through TLR 2, 4, and 7, as well as complement and inflammasome activation, ultimately leading to secretion of proinflammatory cytokines IL-1β, IL-18, IL-1α, IL-6, TNFα, TNFβ, and IFNγ. These cytokines promote extravasation of innate immune cells from the vasculature to the site of inflammation. Specific sex differences within this process are displayed via the pink and blue panels. In particular, as monocytes exit the vasculature and differentiate into macrophages, it has been shown that an M2 polarization may be favored in female mice. In contrast, an M1 polarization may be more prominent in male mice. As the immune response progresses, dendritic cells can migrate and present antigens to Naïve T cells. These subsequently activated T cells then migrate to the site of inflammation. Subsequent differentiation of activated CD4+ Th cells also displays sex differences as immune cells display androgen and estrogen receptors. An example is shown in the figure with CD4+ Th cells differentiating to the Th2 subtype in females while differentiating to the Th1 subtype in males.
Other etiologies for myocarditis include various parasites and bacteria (i.e. Trypanosoma Cruzi, Staphylococcus) (31, 32). Furthermore, myocarditis has also been reported as a side effect of many medications and vaccines, most recently the COVID-19 vaccine (33). Medication classes including antipsychotics (clozapine) and salicylates (mesalazine) have been associated with myocarditis (34). Additionally, though rare, myocarditis secondary to the use of immune checkpoint inhibitors has also been reported (35, 36). Immune checkpoint inhibitor medications, primarily those targeting programmed death (PD)-1, programmed death ligand (PDL)-1, and cytotoxic T-lymphocyte antigen (CTLA)-4, are widely used in the treatment of certain cancers. Though their mechanism of action is to primarily block these targets from suppressing the immune system, they can lead to cardiac toxicity through infiltration of multiple types of immune cells including CD8+ T cells, and CCR2+ macrophages (37–39).
Though viral etiologies are often identified as the most common cause of myocarditis in North America (40, 41), many autoimmune diseases also have cardiac manifestations, including myocarditis. A recent systematic review found that myocarditis is prevalent within a wide spectrum of autoimmune diseases such as cardiac sarcoidosis, systemic lupus erythematosus, systemic sclerosis, eosinophilic granulomatosis with polyangiitis, demato/polymyositis, and Takayasu’s arteritis. Interestingly, the presence of biopsy confirmed myocarditis in these diseases has been shown to be associated with a worse prognosis (42). Finally, although an area of ongoing research, genetic evidence gathered from familial cases of recurrent myocarditis and pediatric myocarditis support the idea of an underlying genetic susceptibility (43, 44). A recent study of 336 patient with myocarditis found that, compared to healthy controls, there was an increased enrichment of truncating variants of cardiomyopathy associated genes. In particular, truncated variants in the desmoplakin gene (DSP) were found in myocarditis patients with normal LV function and ventricular arrhythmia while truncated variants in the Titin gene (TTN) were found in myocarditis patients with reduced ejection fraction (45). Though more research is needed into the genetic aspects of myocarditis, it is an etiology that should be considered clinically.
Despite the varied etiologies, viral infections continue to be the predominant cause leading to myocarditis with differential impacts on various ages and varied clinical presentations.
2.1 Viral entry
Mechanisms of entry utilized by these viruses are multi-faceted and often strain-dependent. Usually, the virus gains entry to cardiomyocytes, endothelial cells, and stromal cells using virus-specific receptors. The coxsackie-adenovirus receptor (CAR) is a highly conserved, widely distributed transmembrane protein that CVB utilizes to aid in viral attachment as well as infection (46, 47). However, CAR has been shown to localize to tight junctions in polarized epithelial cells. Therefore, the mechanism of viral access to this receptor has been an ongoing topic of interest (48). Decay Accelerating Factor (DAF or CD55) is a member of the complement control family. It is an additional receptor for many of the enteroviruses, though, compared to CAR, the mode of binding is less conserved (49–51). However, DAF plays a critical role in viral infection via CAR. Viral binding to DAF on the apical cell surface has been shown to trigger a signaling cascade, ultimately leading to Rac-dependent actin rearrangements (52). These rearrangements allow for the delivery of the virus to the tight junctions where interactions with CAR can occur.
2.2 Immune response
Focusing on CVB infection and immune response in the heart, during the initial phase of acute viremia, there is a near immediate response from resident cells in the myocardium, including increased expression of IL-1α, IL-1β, IL-6, IL-18, TNFα, TNFβ, and IFNγ (53, 54). This initial response is predominantly due to the presence and activation of Toll-like Receptors (TLRs) on the cell surface and cytosol. While the role of TLRs in CVB immune response still needs better understanding, three classes of TLRs are integral to the initial immune response: TLR 2, 4, and 7 (55, 56). Activating these receptors provides chemotactic signaling for other innate immune cells and creates a cytokine profile that impacts eventual adaptive immune response.
As viral replication and early inflammatory response progress, cells of the innate immune system, particularly Natural Killer (NK) cells, and macrophages, are recruited to the site of damage (57). While both cell types are key to the innate immune response and controlling viral replication, macrophages also present a unique response to infection in their ability to polarize (58, 59). In response to specific cytokine profiles, macrophages can either take on a proinflammatory M1 or anti-inflammatory M2 polarization (60, 61). Interestingly, sex differences in polarization exist in CVB myocarditis, with males having a predominant M1 profile compared to females, who tend to develop an M2 profile (62, 63). Though sex hormones have been shown to impact this polarization process (64), this effect has been analyzed primarily in adult mice, and the role of age has not been investigated.
With innate immune cell infiltration, there is an accompanying increase in pro-inflammatory cytokine levels and eventual activation of the adaptive immune system via antigen presentation on Major Histocompatibility Complexes (MHCs) to naïve CD4+ Th cells (65). This antigen presentation, combined with specific cytokine profiles, leads to the differentiation of activated CD4+ T-cells into four possible subtypes of helper T cells: Th1, Th2, Th17, or regulatory T cells (Treg). Th1 is involved with responses to intracellular pathogens by expressing IFNγ and TNFα, while Th2 and Th17 cells are primarily involved in extracellular pathogen responses expressing IL-6, IL-4 and IL-10 and IL-17, IL-22, and IL-21, respectively (66, 67). In contrast, Treg cells primarily aim to control the immune response and maintain homeostasis (68). While this process of differentiation is well reviewed in response to CVB (69), there are notable sex differences in the ratios of these subtypes, as estrogen and androgen receptors are present in many immune cells, including T cells (70). Specifically, it has been shown that estrogen favors a Th2 response and promotes IL-4 and IL-10 expression while also inhibiting the Th1 response (71–75). Conversely, testosterone leads to a predominant Th1 response, though there is evidence that inflammation in males is attributed more to innate rather than adaptive cells (76, 77).
2.3 Role of aging
The immune system constantly evolves throughout our lives, beginning in infancy, peaking near puberty, and subsequently declining with age (78). Given this evolution, the impact of sex hormones on the immune system has been a significant research topic and has been well-reviewed (79). Moreover, the interplay between sex hormones and the immune system in the context of CVB myocarditis is well documented and does support the sex differences seen in adult patients.
However, while puberty may be a convenient dividing line, sexual dimorphisms in the immune system may be present before the onset of puberty. Rosen et al. analyzed age and sex differences in mice both pre- and post-puberty. Interestingly, they found that in 3-week-old mice, mitogenic response to ionomycin and phorbol ester was more significant in female splenocytes than male splenocytes. Yet, in mice 4–6 weeks of age, this was reversed, with males displaying a more substantial response (80). Additionally, Castro demonstrated that prepubertal orchiectomy in mice resulted in relative thymic hyperplasia compared to controls (81). Overall, future work should include an investigation into the prepubertal immune response, particularly with respect to CVB myocarditis.
3 Diagnosis
Diagnosis of myocarditis presents a significant difficulty as patients may present with a wide array of symptoms ranging from mild viral illness to new onset heart failure, and even with evidence of ST-elevation myocardial infarction (STEMI) (82–85). Currently, the gold standard diagnostic test for myocarditis is endomyocardial biopsy. While recent large-scale prospective studies have shown that EMB is safe and prognostically valuable in both adults and children, the United States tends to trail behind Europe (86–88). In 2021, a joint position statement from the American and Japanese Heart Failure Societies outlined potential clinical indications for EMB, including suspected acute myocarditis with acute heart failure or suspected myocarditis in a hemodynamically stable patient (89). In 2024, the American College of Cardiology (ACC) released their consensus decision pathway on the diagnosis and management of myocarditis and recommended that EMB be performed in “clinical scenarios where the prognostic and diagnostic value of the information gained outweigh the procedural risks” (90). Thus, while no “one-size-fits-all” test can provide a definitive diagnosis, currently available testing methods can help the medical team differentiate myocarditis from other cardiac pathologies.
3.1 Clinical presentation
Clinical presentation of acute myocarditis is heterogeneous, ranging from non-specific chest pain to cardiogenic shock. In Table 1 we have summarized various presenting features described in the literature as the presenting symptoms in pediatric and adult patients. These symptoms are not an exhaustive list, and many patients have overlapping clinical features. Additionally, patients may present with any combination of these symptoms (i.e., adult patients often do not present with fever or constitutional symptoms). In adult patients with myocarditis, chronic presentations may also be seen with cardiac symptoms such as chest pain, often with preserved systolic function and histologic evidence of persistent myocardial inflammation. The pediatric population usually will have a history of a viral prodrome in about two-thirds of patients, arrhythmias in about 45%, syncope in 10%, and occasionally may present as sudden cardiac death. The clinical symptoms may overlap in adults and pediatric patients, with fatigue, shortness of breath, fever, chest pain, dyspnea, and cough being common presenting signs. The patient usually may demonstrate subtle signs of heart failure to cardiogenic shock depending on the severity of the presentation. Especially in pediatric patients the symptoms differ among age groups. For example, infants with myocarditis may present with persistent tachypnea, poor feeding, getting tired with feeding, recurrent respiratory infections, diaphoresis and/or other overt signs of heart failure. However, an adolescent may have a presentation similar to a young adult with fatigue, shortness of breath, tiredness, poor appetite or abdominal pain especially with severely reduced ventricular function. Other presentations include acute coronary syndrome (more common in adults) and new or worsening heart failure symptoms. Subacute or chronic myocarditis, seen more commonly in adults, usually presents with an indistinct onset of illness (generally more than three months), which has been described as either chronic active or chronic persistent myocarditis. On the other hand, pediatric patients may be more likely to present with fulminant disease (2). Regardless, in patients with clinical suspicion, further evaluation is warranted using laboratory studies and other biomarkers to diagnose myocarditis further.
3.2 Laboratory evaluation
According to the 2024 ACC and AHA expert consensus documents on the diagnosis and management of myocarditis, initial laboratory evaluation should include CBC with differential, biomarkers of cardiac necrosis such as high-sensitivity troponin and creatinine kinase-MB and nonspecific inflammatory biomarkers such as C-reactive protein and erythrocyte sedimentation rates should be obtained (90, 91). Viral swabs and antibodies are also obtained to ascertain the etiology of the myocarditis. There continues to be a lack of universal diagnostic criteria for myocarditis and heterogeneity of clinical presentation makes it even more challenging to compare various studies. Generally, these initial evaluations are mirrored in the recommendations for pediatric populations though disease specific guidelines are not directly published (92). For both populations, there is support for genetic evaluation. However, genetic evaluation is more common in children and often screens for genes in which there is definitive evidence of mutations causing cardiac disease while in adults there may be more focus on genetic variants that are overrepresented in acute myocarditis populations (93, 94).
While there are no definitive laboratory markers for the diagnosis of myocarditis, ongoing developments in the area of acute phase reactants and markers of inflammation or damage have shown promise. A study of 44 patients with confirmed myocarditis showed that myoglobin levels in peripheral blood positively correlated with disease severity. Upon further testing, it was shown that, with a cutoff level of ≥ 87 μg/L, myoglobin levels were able to identify mice with myocarditis with a sensitivity and specificity of 92% and 80%, respectively (95). Recently, Villatore et al. showed that pentraxin 3, an acute phase protein, was detected in the plasma of 86.7% (26/30) myocarditis patients compared to 0% (0/10) healthy controls. Additionally, pentraxin 3 detection was performed in cardiac samples from all patients, but they noted that higher signal intensity was associated with viral myocarditis compared to autoimmune myocarditis (96). MicroRNA has also been shown to be helpful in the diagnosis of myocarditis. Specifically, a microRNA (mmu-miR-721), which has a human homolog (hsa-miR-Chr8:96), is synthesized by Th17 cells. Using this microRNA, patients with myocarditis could be distinguished from those with myocardial infarction with an AUROC of 0.927 (0.879-0.975) (97). Serum Anti-heart and Antinuclear antibodies have also been shown to be predictive of immunosuppressive treatment response in biopsy proven myocarditis. However, this study only considered autoimmune etiologies (98). Despite this, there is evidence that autoantibodies may be a valuable marker in viral myocarditis. In particular, it has been shown that autoantibodies are produced in response to cardiac injury during coxsackievirus infection (99). Furthermore, anti-myosin antibodies identified in myocarditis patients were found to bind to β-adrenergic receptors on the heart thus impacting heart rate and contractility (100). Nevertheless, the role of autoantibodies in the pathogenesis and progression of myocarditis remains unclear and should be a focus of research moving forward (101).
Finally, serum sST2 (also known as interleukin-1 receptor-like 1) may be a valuable biomarker in differentiating myocarditis. The Fairweather group has shown that, within a subset of patients with clinically suspected or biopsy-confirmed myocarditis, elevated sST2 levels were associated with increased risk of heart failure based on New York Heart Association (NYHA) class in men under 50 years of age (13). Interestingly, though sera sST2 has been shown to be elevated in Cardiovascular Disease (CVD) compared to healthy controls, sex differences are present for subsets with myocarditis and cardiomyopathy. Notably, in patients under 50 years of age with myocarditis, males have a significantly higher sera sST2 level compared to females. However, this sex difference is not present in myocarditis patients greater than 50 years of age (102). Two studies have assessed the value of sST2 as a biomarker for myocarditis in children, although the focus was on fulminant myocarditis. One retrospective study of 203 patients under 11 years of age found that elevated levels of sST2 correlated with fulminant myocarditis, while another study of 144 patients under 14 years of age showed that, with a cut-off value of 63.8 ng/mL, sST2 had a sensitivity and specificity of 84.13% and 88.9%, respectively (103, 104). Ultimately, further research is needed into potential biomarkers for myocarditis with specific attention to the possible impacts of sex as well as age.
An electrocardiogram (EKG) is a valuable diagnostic tool; however, in myocarditis, it has been shown to lack specificity (105). Chen et al. reported that among adult patients admitted with a diagnosis of acute myocarditis, a wide QRS-T angle (>100°) was present in 13% of patients and was a predictor of increased morbidity and mortality (106). Like acute myocardial infarction, myocarditis may be associated with regional ST elevations and Q waves. Some studies have shown that the presence of Q waves or left bundle branch block was associated with higher rates of death or transplantation (107) while other studies have not been able to confirm these findings (108, 109).
Although not specific to CVB myocarditis, utilizing lab values and EKG findings to monitor and predict disease progression has been a clinical research focus, particularly during the COVID-19 pandemic. Patel et al. found that among 99 patients with a median age of 3 years old (0–22) who were hospitalized for symptomatic heart failure, lymphocytopenia, hyponatremia, and elevated serum creatinine were independent predictors for risk of death or need for a heart transplant (110). Regarding electrocardiographic findings, children with DCM who have increased P maximum, P distribution, QT dispersion, QTc maximum, QTc dispersion, Tp-emax, Tp-e dispersion, or QRS duration had an increased risk of death (111). However, specific studies designed for the comparison of EKG findings between pediatric and adult populations with myocarditis remain to be conducted.
3.3 Echocardiography
Echocardiography is an integral tool in cardiac evaluation for myocarditis within both pediatric and adult populations (90–92). Echocardiographic findings may vary from mild to severe ventricular dysfunction with or without evidence of ventricular dilation and/or wall motion abnormalities. However, conventional 2D echocardiography lacks the ability to detect potential deformations or changes in cardiac function in all but severe cases of myocarditis (112, 113). Though its utility in diagnosing acute myocarditis is variable, echocardiography has a role in monitoring patients, particularly children, who have a history of myocarditis and dilated cardiomyopathy (DCM). Decreased ejection fraction, myocardial performance index, and increased left ventricular end-systolic and end-diastolic dimensions (LVESD and LVEDD, respectively) have been shown to be associated with an increased risk of death or transplant in pediatric patients (111). The transplant/mortality likelihood increased by 41.6% and 19.8% for each unit increase in LVEDD and LVESD z scores, respectively (80). Ryo et al. also showed that increased left ventricular ejection fraction (LVEF), mitral E-deceleration time, right ventricular (RV) fractional area change, and tricuspid annular plane systolic excursion were associated with reduced mortality and transplant risk (114).
More recently, there has been increased interest in the changes in myocardial strain with myocarditis. Strain, or deformation, imaging allows for quantifying regional myocardial function, yet its adoption has been slow in clinical echocardiography, which primarily utilizes visual assessment of wall motion (115–118). Fairweather et al. showed in their CVB murine model that there was a significant reduction in global longitudinal strain in infected mice compared to healthy controls as well as sex differences, with males having a more severe reduction compared to females (113). In humans, global longitudinal peak systolic strain, as well as global circumferential strain, were lower in patients with acute myocarditis, and a multiparametric model including epicardial left ventricular global longitudinal strain was able to identify patients who were subsequently diagnosed with myocarditis correctly (119, 120).
Use of strain calculations for acute viral myocarditis in pediatric patients is limited, though one study from Gursu et al. showed strain values were significantly reduced in patients (mean age 12.3 years) compared to healthy age-matched controls (112). Other studies of strain in pediatric patients have focused on multisystem inflammatory syndrome in response to COVID-19 but show similar effects (121, 122).
3.4 Cardiac magnetic resonance
While endomyocardial biopsy remains the gold standard for diagnosing myocarditis, Cardiac magnetic resonance (CMR) is increasingly being used for non-invasive diagnosis. Due to its non-invasive nature, it is recommended over endomyocardial biopsy except in certain specific scenarios (90). CMR imaging enables the detection of various features of myocarditis, including evidence of inflammatory hyperemia and edema, late gadolinium enhancement (LGE) suggestive of myocyte necrosis and scar, changes in ventricular size and geometry, regional and global wall motion abnormalities, and identification of accompanying pericardial effusion. Myocardial inflammation is diagnosed on CMR via the Lake Louise Criteria based on the following criteria: myocardial edema on T2 mapping (demonstrated by regional or global increase of either native T2 times or T2 signal intensity) or non-ischemic myocardial injury (demonstrated by regional or global increase of either native T1 time, extracellular volume, or a regional increase in late gadolinium enhancement (LGE)) (123). Overall, CMR data has been highly effective in diagnosing myocarditis and providing valuable prognostic indicators and risk-stratifying patients (124–127).
However, there are potential limitations in terms of sensitivity. Mainly, CMR has been shown to have high diagnostic sensitivity for myocarditis in patients with an “infarct-like” (fever, chest pain, troponinemia, and ST changes) pattern compared those with “cardiomyopathic” (LV dysfunction with no EKG changes) or “arrhythmic” (acute onset of life-threatening arrhythmia) patterns (128). Moreover, this difference in sensitivity has also been shown to persist even after use of parametric tissue mapping as recommended by the updated Lake Louise Criteria (129).
Finally, it is important to consider that there is evidence of reduced diagnostic sensitivity beyond 8 weeks from symptom onset (130) and the baseline sex-related differences in the patient population, especially adolescents (131). Moreover, performing CMR in younger patients can be difficult as they may have difficulty following breathing instructions or may potentially be too sick to undergo anesthesia safely (132).
3.5 Other diagnostic tools
Cardiac catheterization with coronary angiography may be used in select patients presenting with acute coronary syndrome or in those with high-risk features for ischemic heart disease on noninvasive testing. While not widely used clinically, there has been additional research into other diagnostic tools for diagnosing myocarditis. Lekakis et al. in 1995 showed that Indium-111 monoclonal anti-myosin antibody uptake ratios between the heart and lung were significantly higher than controls (133). Additionally, using 18F-FDG PET may be beneficial in diagnosing myocarditis, particularly if arrhythmias are present or if the time since symptom onset is more prolonged. However, prospective trials are still necessary (134, 135). In mice, it has been shown that translocator protein 18kDa (TSPO) is upregulated in male mice on CD11-b(+) immune cells, and this upregulation translates to significantly higher uptake of [ (125)I]-IodoDPA-713 on microSPECT in CVB3 infected males compared to uninfected controls (136). The use of TSPO in diagnosing myocarditis patients has not been translated to human patients at this time.
An additional imaging modality that has the potential to aid clinicians in diagnosing myocarditis is magnetocardiography (MCG). While MCG has been present since the 1960s, it has primarily been researched in the setting of ischemic heart disease. Still, its use has more recently extended into myocarditis and inflammatory cardiomyopathies. MCG relies on detecting the magnetic field generated by the physiological current that passes through the heart with each cycle. Using this technology, it has been shown that MCG was able to not only differentiate those patients with cardiomyopathy from those without but also showed improving values 7 days after treatment initiation compared to 30 days when echo was used (137).
Finally, genetic evaluation may have a role. A recent study published the results of forty-two pediatric patients with biopsy-proven myocarditis who underwent genetic screening. Among those patients who progressed to DCM at a faster rate, potential pathogenic variants in BAG3, DSP, LMNA, MYH7, TNNI3, TNNT2, and TTN were seen (138). Therefore, there may still be value in genetic testing patients with newly diagnosed myocarditis to predict which patients may be more likely to progress to DCM. Overall, while there has been considerable research into the diagnosis of myocarditis, research into the differences between pediatric and adult populations remains sparse. Thus, future work to better understand these differences may be beneficial for accurately diagnosing myocarditis within each of these populations.
4 Treatment
Treatment for myocarditis remains a significant therapeutic challenge due to the lack of specific targeted therapies. Current treatment guidelines primarily focus on symptomatic relief and treatment of any associated heart failure symptoms in line with heart failure guidelines. There may also be a role for anti-inflammatory medications use which function to decrease the body’s inflammatory response as well as immunosuppressive therapies which suppress the body’s immune response. However, while there is agreement on the use of immunosuppressive medications in specific noninfectious presentations, there remains disagreement on the utility of immunosuppression in lymphocytic myocarditis in which viral PCR of the EMB sample is negative. However, intravenous immunoglobulin (IVIG) may be considered in inflammatory conditions (90). Pocapavir is an investigational enteroviral capsid inhibitor that has shown promise in a limited number of cases of severe neonatal enterovirus myocarditis. At this time, there are no disease-specific approved antiviral therapies for patients with myocarditis (139, 140). However, the JACC 2024 consensus statement on myocarditis notes that these medications may be an acceptable alternative though recommends consultation with an infectious disease expert (90). Overall, this highlights the need for robust prospective clinical trials and further research into treatment strategies specific to myocarditis.
4.1 Immunosuppressive and anti-inflammatory therapies
Corticosteroids have been extensively studied for their role in managing viral myocarditis. A landmark trial by Mason et al. failed to show a significant improvement in LVEF with the use of corticosteroids with either cyclosporine or azathioprine (141). A 2013 Cochrane meta-analysis of eight randomized controlled trials (RCTs) involving 719 participants revealed mixed outcomes (142). Of these eight trials, two focused specifically on pediatric populations. The analysis showed no significant reduction in mortality or rates of death/heart transplant and no improvement in the NYHA class. However, short-term improvements in LVEF at 1–3 months were observed with corticosteroids. This trend continued when the pediatric studies were analyzed separately. Long-term improvements in LVEF and decreased levels of biomarkers such as CK-MB and α-HBDH were also noted, though these results were only reported in adult studies. Notably, a 2010 study involving 68 children (mean age 3.7 ± 2.9 years) demonstrated that prednisolone significantly improved LVEF, with more children achieving over 60% LVEF compared to the control group (143). Another study in pediatric patients demonstrated that high-dose steroids in conjunction with IVIG to treat acute myocarditis can be safe without significant infections or long-term side effects. However, there still is a need for a prospective comparison of a combination of high-dose steroids with IVIG versus other therapies (144). A Taiwanese study showed that high-dose steroid or IVIG therapy had no significant effects on major in-hospital complications, late heart failure hospitalization, and long-term mortality (145). These conflicting results highlight the need for RCTs to answer these questions and the ongoing need for an enhanced understanding of the pathogenesis to develop targeted therapies.
A 2021 study investigating colchicine’s efficacy in myocarditis, as determined by cardiac MRI (CMR) criteria, enrolled 146 participants, with eighty-six receiving colchicine. The results were promising, as 64% of patients treated with colchicine achieved complete resolution of myocarditis, compared to only 19% in the control group (146). Biopsy samples of patients with myocarditis demonstrate an amplified expression of NLRP3 inflammasome and related cytokines including interleukin (IL)-1β and IL-18, reflecting greater myocardial injury. Colchicine targets these pathways by reducing superoxide production and inhibition of inflammasomes and IL-1β production. Additionally, colchicine has shown to have antifibrotic and endothelial-protective features. Overall, colchicine may be useful in patients with myocarditis by targeting the underlying inflammatory processes however further studies with larger cohorts are warranted.
Anakinra, an interleukin-1 receptor antagonist, was investigated in the ARAMIS trial (Anakinra Versus Placebo for the Treatment of Acute Myocarditis; NCT03018834), which is the largest randomized trial in myocarditis to date. This double-blind RCT enrolled 120 symptomatic patients to evaluate the efficacy of Anakinra in mitigating the inflammatory cascade associated with acute myocarditis. They found that patients receiving a short course of Anakinra did not have a significantly increased number of days free of myocarditis complications (147). There was also a trial of this agent in pericarditis during the COVID-19 pandemic, but it was terminated early due to drastic benefits seen within the first 24 hours of administration (148). However, the FDA has not yet approved Anakinra for use in either indication.
A 2020 study evaluating immunosuppressive therapy in acute inflammatory cardiomyopathy showed that immunosuppressive therapy did not significantly improve outcomes compared to those who did not receive it. However, patients who did not receive the therapy had higher rates of poor lymphocytic infiltrates at endomyocardial biopsy, suggesting potential differences in disease pathology (149).
Finally, a recent study was published that evaluated the use of individualized prednisone and azathioprine dosing strategies, compared to optimal medical therapy for heart failure, in biopsy proven myocarditis (150). This study was an extension of the TIMIC trial which showed potential efficacy of these medications in virus-negative inflammatory cardiomyopathy and at prespecified doses and durations (151). Among 91 patients receiving tailored immunosuppressive therapy, compared to the 267 receiving the optimal therapy, it was shown that, at baseline, patients in the immunosuppressive groups tended to have lower cardiac ejection fraction at baseline. At 5-year follow-up, there was no significant difference in overall survival between the two groups. However, it should be noted that this study included all etiologies of myocarditis and only enrolled adult patients. Moreover, in the cases where viral etiologies may be suspected, an ESC position statement recommends that viral PCR of endomyocardial biopsy be negative before initiation of any immunosuppressive therapy to mitigate risk of exacerbating potential underlying infections (152).
4.2 Adjunctive and emerging therapies
The role of IVIG in myocarditis treatment has been explored in both pediatric and adult populations. IVIG has anti-inflammatory, antiviral, and immunomodulatory effects and is relatively safe, with minimal side effects prompting its use, especially when patients present in severe cardiogenic shock with suspected myocarditis. A 2020 Cochrane meta-analysis yielded insights showing no significant improvement in event-free survival or overall survival among adults. There were no changes in LVEF, peak oxygen consumption, or complete recovery rates. Similar findings were reported from the analysis of the single pediatric study (153). However, a separate 2014 study involving 41 patients (aged 19-80) reported improved survival in the IVIG group, though most parameters except lymphocyte and monocyte fractions remained unchanged (154). Another study in pediatric patients with acute encephalitis complicated with myocarditis showed that the use of IVIG was associated with improved LVEF and survival (155). These conflicting results underscore the need for further investigation. There has also been interest in leveraging aspects of fatty acid oxidation. While full mechanisms of action are yet to be understood, trimetazidine is an inhibitor of the long-chain 3-ketoacyl-CoA thiolase, a key enzyme in fatty acid breakdown. Inhibition of this enzyme shifts the primary energy source of the heart from fatty acids to glucose, which leads to lower oxygen consumption and improved myocardial perfusion (156). Combination therapy with Coenzyme Q10 (CoQ10) and Trimetazidine, an inhibitor of fatty acid oxidation, has shown additive benefits in acute viral myocarditis. A 2016 study involving 124 participants demonstrated that CoQ10 and Trimetazidine independently reduced inflammatory markers and improved LVEF. The combination therapy resulted in significantly greater improvements than either treatment alone (157). Additionally, pocapavir, an enteroviral capsid inhibitor, has been shown to be beneficial in treating myocarditis secondary to enterovirus in isolated case reports. However, more extensive studies are still needed (158, 159).
4.3 Pediatric-specific treatment studies
Pediatric-specific research highlights unique therapeutic considerations. However, most large-scale randomized control trials for drug approval are conducted predominantly in adult populations, with results being extrapolated to the pediatric population. A 2024 study with 120 children (aged <10 years) compared different doses of creatine phosphate sodium combined with immunoglobulin. The findings revealed that groups receiving higher doses of creatine (1.25g and 1.5g) showed superior therapeutic effects compared to the lowest dose (1g). Significant reductions in biomarkers such as cTnI, CK-MB, LDH, and IL-6, alongside improved LVEF and immune parameters (CD4+/CD8+ ratios), were noted (160).
An additional study in 2023 involving eighty-three children (aged 2–12 years) reported that creatine phosphate sodium reduced heart rate and improved cardiac output, stroke volume, and LVEF within 14 days. The therapy also lowered inflammatory and myocardial injury markers while enhancing immune responses (161).
4.4 Clinical trials
Multiple ongoing clinical trials focus on treating myocarditis, which is presented in Table 2. The MYTHS trial (Myocarditis Therapy with Steroids; NCT05150704) is focused on treating acute myocarditis with pulsed IV methylprednisolone. In their single-blind randomized trial, pulsed IV steroids will be compared to current standard therapy (162). The ARCHER trial (Impact of CardiolRx on Myocardial Recovery in Patients with Acute Myocarditis; NCT05180240) is a phase II trial focusing on the impact of CardiolRxTM, a cannabidiol preparation, on myocardial recovery in acute myocarditis (163). Interestingly, cannabidiol has been shown to have immunosuppressive effects in both the innate and adaptive systems (164, 165). Additionally, a study in Egypt is recruiting and analyzing the potential benefits of heart rate-lowering therapy in patients with acute myocarditis (NCT06312891). Finally, the ARGO trial (Colchicine Versus Placebo in Acute Myocarditis Patients; NCT05855746) out of France is an ongoing Phase III trial evaluating the impact of colchicine on acute myocarditis. Though multiple trials are ongoing, none have focused their efforts on potential therapeutics considering sex and age as biological variates.
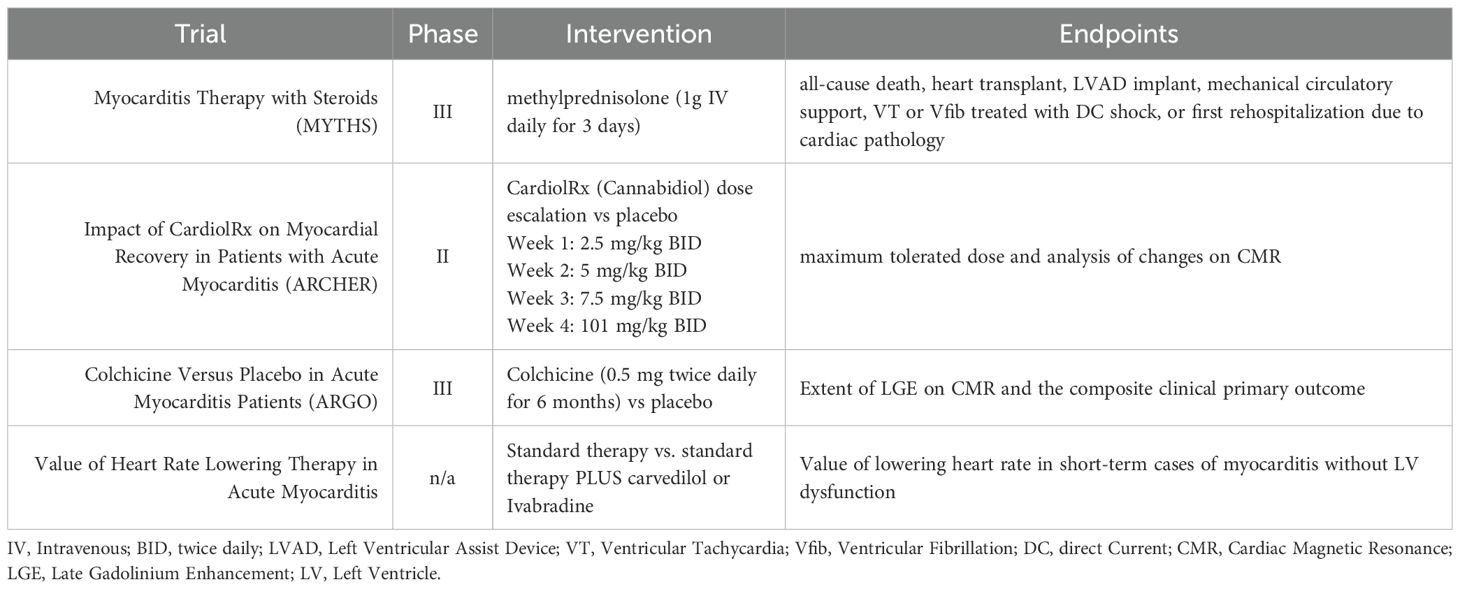
Table 2. Current ongoing therapeutic trials in myocarditis. All trials listed are currently being conducted with adult patients.
4.5 Mechanical circulatory support
The use of mechanical circulatory support in the setting of acute myocarditis is relatively low, and guidelines generally reserve these strategies for patients with electrical or hemodynamic instability (90, 166). Short-term mechanical circulatory support is primarily provided using venoarterial membrane oxygenation (VA-ECMO), Impella (usually in >30kg), or implantable devices such as PediMag/Centrimag. Younger patients who require ECMO have a higher mortality risk. However, IVIG administration has been shown to have a protective effect in this population (167–169). In studies specifically looking at children, elevated serum Troponin, female sex, vomiting, seizures, and arrhythmia at presentation may predict the need for ECMO (170, 171). Once initiated, rates of survival and transition off ECMO across studies range from 50% to 70%, which is similar rates seen in studies of adults (172–176).
According to a statement by the AHA, patients who do not recover despite mechanical support transition to a durable ventricular assist device should be considered (177). While rare, recovery of myocardial function with ventricular assist device (VAD) placement can occur, and patients do undergo an explanation of the device in this case (90, 178). However, VAD placement is often used as an effective bridge to eventual cardiac transplant both in pediatrics and adults (179–181). For those patients who do undergo transplant, outcomes for both pediatric and adult patients with myocarditis are similar to those with other cardiomyopathies (182, 183). Conversely, children with clinically or biopsy-diagnosed myocarditis at presentation had significantly higher post-transplantation mortality compared with children without myocarditis. The impact of a history of myocarditis on long-term allograft survival in children may be related to the inflammatory milieu in the individual patients (184).
5 Conclusions
Myocarditis is prevalent in both pediatric and adult populations, and the broad spectrum of causes, as well as clinical symptoms, presents a diagnostic difficulty for providers. While there have been great strides in understanding the pathogenesis of this disease, there are still many gaps in understanding. Though future research should continue to investigate the causes of increased risk in adult males, there should also be a stronger focus on research on the pathogenesis of this disease in the pediatric population and, specifically, the differences that may be present between these two populations. As basic and clinical research in this area increases, it may reveal specific mechanisms or biomarkers that may allow for more effective identification and risk stratification in these patients.
Despite advancements in myocarditis treatment, significant gaps remain. Immunosuppressive and anti-inflammatory therapies show variable efficacy, while adjunctive treatments like CoQ10 and Trimetazidine offer promising results. Pediatric-specific studies highlight the importance of tailoring therapies to age-specific needs.
Future research should focus on large-scale, age-stratified RCTs to validate current findings, personalized treatment strategies leveraging biomarkers and advanced imaging techniques, and long-term follow-up studies to assess the durability of therapeutic effects. The evolving understanding of myocarditis pathophysiology offers hope for more effective, targeted therapies in the future.
Author contributions
JR: Methodology, Supervision, Investigation, Conceptualization, Writing – review & editing, Visualization, Project administration, Writing – original draft. NF: Writing – review & editing, Investigation, Writing – original draft, Methodology. CD: Data curation, Writing – review & editing, Visualization. KR: Visualization, Data curation, Writing – review & editing. LP: Writing – review & editing, Visualization, Data curation. PT: Visualization, Data curation, Writing – review & editing. AD: Writing – review & editing, Visualization. KG: Resources, Project administration, Data curation, Writing – review & editing. DG: Visualization, Writing – review & editing, Conceptualization, Writing – original draft, Supervision. KB: Methodology, Writing – original draft, Resources, Visualization, Supervision, Project administration, Conceptualization, Funding acquisition, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Funding for this work is generously provided by the University of Florida Gatorade Funds, National Institutes of Health (NIH) awards (R21 AI163302, R21 AI180863), the American Heart Association (23SCEFIA1153413), Department of Defense (CDMRP PR210385), and the For Elysa Foundation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ammirati E, Frigerio M, Adler DE, Basso C, Birnie HD, Brambatti M, et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy. Circulation: Heart Failure. (2020) 13. doi: 10.1161/CIRCHEARTFAILURE.120.007405
2. Law YM, Lal AK, Chen S, Cihakova D, Cooper LT Jr., Deshpande S, et al. Diagnosis and management of myocarditis in children: A scientific statement from the american heart association. Circulation. (2021) 144:e123–e35. doi: 10.1161/CIR.0000000000001001
4. Ammirati E, Veronese G, Bottiroli M, Wang WD, Cipriani M, Garascia A, et al. Update on acute myocarditis. Trends Cardiovasc Med. (2021) 31:370–9. doi: 10.1016/j.tcm.2020.05.008
5. Tschöpe C, Ammirati E, Bozkurt B, Caforio PLA, Cooper TL, Felix BS, et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. (2021) 18:169–93. doi: 10.1038/s41569-020-00435-x
6. Amdani S and Gupta D. Improving outcomes for critically ill children with myocarditis. J Cardiac Failure. (2024) 30:359–61. doi: 10.1016/j.cardfail.2023.06.013
7. Law MY, Lal KA, Chen S, Čiháková D, Cooper TL, Deshpande S, et al. Diagnosis and management of myocarditis in children. Circulation. (2021) 144:e132–5. doi: 10.1161/CIR.0000000000001001
8. Sanna G, Serrau G, Bassareo PP, Neroni P, Fanos V, and Marcialis AM. Children’s heart and COVID-19: Up-to-date evidence in the form of a systematic review. Eur J Pediatrics. (2020) 179:1079–87. doi: 10.1007/s00431-020-03699-0
9. Collaborators GBoDS. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. (2015) 386:721–2. doi: 10.1016/S0140-6736(15)60692-4
10. Golpour A, Patriki D, Hanson JP, Mcmanus B, and Heidecker B. Epidemiological impact of myocarditis. J Clin Med. (2021) 10:603. doi: 10.3390/jcm10040603
11. Laufer-Perl M, Havakuk O, Shacham Y, Steinvil A, Letourneau-Shesaf S, Chorin E, et al. Sex-based differences in prevalence and clinical presentation among pericarditis and myopericarditis patients. Am J Emerg Med. (2017) 35:201–5. doi: 10.1016/j.ajem.2016.10.039
12. Kyto V, Sipila J, and Rautava P. The effects of gender and age on occurrence of clinically suspected myocarditis in adulthood. Heart. (2013) 99:1681–4. doi: 10.1136/heartjnl-2013-304449
13. Coronado MJ, Bruno KA, Blauwet LA, Tschope C, Cunningham MW, Pankuweit S, et al. Elevated sera sST2 is associated with heart failure in men </=50 years old with myocarditis. J Am Heart Assoc. (2019) 8:e008968. doi: 10.1161/JAHA.118.008968
14. Lynge TH, Nielsen TS, Gregers Winkel B, Tfelt-Hansen J, and Banner J. Sudden cardiac death caused by myocarditis in persons aged 1–49 years: a nationwide study of 14–294 deaths in Denmark. Forensic Sci Res. (2019) 4:247–56. doi: 10.1080/20961790.2019.1595352
15. Fu M, Kontogeorgos S, Thunstrom E, Zverkova Sandstrom T, Kroon C, Bollano E, et al. Trends in myocarditis incidence, complications and mortality in Sweden from 2000 to 2014. Sci Rep. (2022) 12:1810. doi: 10.1038/s41598-022-05951-z
16. Wong BTW and Christiansen JP. Clinical characteristics and prognostic factors of myocarditis in New Zealand patients. Heart Lung Circ. (2020) 29:1139–45. doi: 10.1016/j.hlc.2020.01.007
17. Ozieranski K, Tyminska A, Kruk M, Kon B, Skwarek A, Opolski G, et al. Occurrence, trends, management and outcomes of patients hospitalized with clinically suspected myocarditis-ten-year perspectives from the MYO-PL nationwide database. J Clin Med. (2021) 10. doi: 10.3390/jcm10204672
18. Baritussio A, Schiavo A, Basso C, Giordani AS, Cheng CY, Pontara E, et al. Predictors of relapse, death or heart transplantation in myocarditis before the introduction of immunosuppression: negative prognostic impact of female gender, fulminant onset, lower ejection fraction and serum autoantibodies. Eur J Heart Fail. (2022) 24:1033–44. doi: 10.1002/ejhf.2496
19. Patriki D, Kottwitz J, Berg J, Landmesser U, Luscher TF, and Heidecker B. Clinical presentation and laboratory findings in men versus women with myocarditis. J Womens Health (Larchmt). (2020) 29:193–9. doi: 10.1089/jwh.2018.7618
20. Shah Z, Mohammed M, Vuddanda V, Ansari MW, Masoomi R, and Gupta K. National trends, gender, management, and outcomes of patients hospitalized for myocarditis. Am J Cardiol. (2019) 124:131–6. doi: 10.1016/j.amjcard.2019.03.036
21. Hassan K, Kyriakakis C, Doubell A, Van Zyl G, Claassen M, Zaharie D, et al. Prevalence of cardiotropic viruses in adults with clinically suspected myocarditis in South Africa. Open Heart. (2022) 9. doi: 10.1136/openhrt-2021-001942
22. Jain A, Norton N, Bruno KA, Cooper LT Jr., Atwal PS, and Fairweather D. Sex differences, genetic and environmental influences on dilated cardiomyopathy. J Clin Med. (2021) 10. doi: 10.3390/jcm10112289
23. Vasudeva R, Bhatt P, Lilje C, Desai P, Amponsah J, Umscheid J, et al. Trends in acute myocarditis related pediatric hospitalizations in the United States, 2007-2016. Am J Cardiol. (2021) 149:95–102. doi: 10.1016/j.amjcard.2021.03.019
24. Arola A, Pikkarainen E, Sipila JO, Pykari J, Rautava P, and Kyto V. Occurrence and features of childhood myocarditis: A nationwide study in Finland. J Am Heart Assoc. (2017) 6. doi: 10.1161/JAHA.116.005306
25. Klugman D, Berger JT, Sable CA, He J, Khandelwal SG, and Slonim AD. Pediatric patients hospitalized with myocarditis: a multi-institutional analysis. Pediatr Cardiol. (2010) 31:222–8. doi: 10.1007/s00246-009-9589-9
26. Thevathasan T, Kenny MA, Gaul AL, Paul J, Krause FJ, Lech S, et al. Sex and age characteristics in acute or chronic myocarditis A descriptive, multicenter cohort study. JACC Adv. (2024) 3. doi: 10.1016/j.jacadv.2024.100857
27. Andreoletti L, Leveque N, Boulagnon C, Brasselet C, and Fornes P. Viral causes of human myocarditis. Arch Cardiovasc Dis. (2009) 102:559–68. doi: 10.1016/j.acvd.2009.04.010
28. Leone O, Pieroni M, Rapezzi C, and Olivotto I. The spectrum of myocarditis: from pathology to the clinics. Virchows Arch. (2019) 475:279–301. doi: 10.1007/s00428-019-02615-8
29. Heckenberg E, Steppe JT, and Coyne CB. Enteroviruses: The role of receptors in viral pathogenesis. Adv Virus Res. (2022) 113:89–110. doi: 10.1016/bs.aivir.2022.09.002
30. Bopegamage S, Borsanyiova M, Vargova A, Petrovicova A, Benkovicova M, and Gomolcak P. Coxsackievirus infection of mice. I. Viral kinetics and histopathological changes in mice experimentally infected with coxsackieviruses B3 and B4 by oral route. Acta Virol. (2003) 47:245–51.
31. Hidron A, Vogenthaler N, Santos-Preciado JI, Rodriguez-Morales AJ, Franco-Paredes C, and Rassi A Jr. Cardiac involvement with parasitic infections. Clin Microbiol Rev. (2010) 23:324–49. doi: 10.1128/CMR.00054-09
32. Haddad F, Berry G, Doyle RL, Martineau P, Leung TK, and Racine N. Active bacterial myocarditis: a case report and review of the literature. J Heart Lung Transplant. (2007) 26:745–9. doi: 10.1016/j.healun.2007.04.010
33. Ferone E, Segev A, Tempo E, Gentile P, Elsanhoury A, Baggio C, et al. Current treatment and immunomodulation strategies in acute myocarditis. J Cardiovasc Pharmacol. (2024) 83:364–76. doi: 10.1097/FJC.0000000000001542
34. Nguyen LS, Cooper LT, Kerneis M, Funck-Brentano C, Silvain J, Brechot N, et al. Systematic analysis of drug-associated myocarditis reported in the World Health Organization pharmacovigilance database. Nat Commun. (2022) 13:25. doi: 10.1038/s41467-021-27631-8
35. Milutinovic S, Jancic P, Jokic V, Petrovic M, Dumic I, Rodriguez AM, et al. Pembrolizumab-associated cardiotoxicity: A retrospective analysis of the FDA adverse events reporting system. Pharm (Basel). (2024) 17:1372. doi: 10.3390/ph17101372
36. Chen C, Chen T, Liang J, Guo X, Xu J, Zheng Y, et al. Cardiotoxicity induced by immune checkpoint inhibitors: A pharmacovigilance study from 2014 to 2019 based on FAERS. Front Pharmacol. (2021) 12:616505. doi: 10.3389/fphar.2021.616505
37. Becker RC. Immune checkpoint inhibitors and cardiovascular toxicity: immunology, pathophysiology, diagnosis, and management. J Thromb Thrombolysis. (2025). doi: 10.1007/s11239-025-03146-7
38. Zhu H, Galdos FX, Lee D, Waliany S, Huang YV, Ryan J, et al. Identification of pathogenic immune cell subsets associated with checkpoint inhibitor-induced myocarditis. Circulation. (2022) 146:316–35. doi: 10.1161/CIRCULATIONAHA.121.056730
39. Ma P, Liu J, Qin J, Lai L, Heo GS, Luehmann H, et al. Expansion of pathogenic cardiac macrophages in immune checkpoint inhibitor myocarditis. Circulation. (2024) 149:48–66. doi: 10.1161/CIRCULATIONAHA.122.062551
40. Elamm C, Fairweather D, and Cooper LT. Pathogenesis and diagnosis of myocarditis. Heart. (2012) 98:835–40. doi: 10.1136/heartjnl-2012-301686
41. Kuhl U, Pauschinger M, Seeberg B, Lassner D, Noutsias M, Poller W, et al. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation. (2005) 112:1965–70. doi: 10.1161/CIRCULATIONAHA.105.548156
42. Cheng CY, Baritussio A, Giordani AS, Iliceto S, Marcolongo R, and Caforio ALP. Myocarditis in systemic immune-mediated diseases: Prevalence, characteristics and prognosis. A systematic review. Autoimmun Rev. (2022) 21:103037. doi: 10.1016/j.autrev.2022.103037
43. Portig I, Wilke A, Freyland M, Wolf MJ, Richter A, Ruppert V, et al. Familial inflammatory dilated cardiomyopathy. Eur J Heart Fail. (2006) 8:816–25. doi: 10.1016/j.ejheart.2006.02.010
44. Kontorovich AR, Tang Y, Patel N, Georgievskaya Z, Shadrina M, Williams N, et al. Burden of cardiomyopathic genetic variation in lethal pediatric myocarditis. Circ Genom Precis Med. (2021) 14:e003426. doi: 10.1161/CIRCGEN.121.003426
45. Lota AS, Hazebroek MR, Theotokis P, Wassall R, Salmi S, Halliday BP, et al. Genetic architecture of acute myocarditis and the overlap with inherited cardiomyopathy. Circulation. (2022) 146:1123–34. doi: 10.1161/CIRCULATIONAHA.121.058457
46. Martino TA, Petric M, Weingartl H, Bergelson JM, Opavsky MA, Richardson CD, et al. The coxsackie-adenovirus receptor (CAR) is used by reference strains and clinical isolates representing all six serotypes of coxsackievirus group B and by swine vesicular disease virus. Virology. (2000) 271:99–108. doi: 10.1006/viro.2000.0324
47. He Y, Chipman PR, Howitt J, Bator CM, Whitt MA, Baker TS, et al. Interaction of coxsackievirus B3 with the full length coxsackievirus-adenovirus receptor. Nat Struct Biol. (2001) 8:874–8. doi: 10.1038/nsb1001-874
48. Cohen CJ, Shieh JT, Pickles RJ, Okegawa T, Hsieh JT, and Bergelson JM. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc Natl Acad Sci U S A. (2001) 98:15191–6. doi: 10.1073/pnas.261452898
49. Tuthill TJ, Groppelli E, Hogle JM, and Rowlands DJ. Picornaviruses. Curr Top Microbiol Immunol. (2010) 343:43–89. doi: 10.1007/82_2010_37
50. Lea S. Interactions of CD55 with non-complement ligands. Biochem Soc Trans. (2002) 30:1014–9. doi: 10.1042/bst0301014
51. Powell RM, Ward T, Goodfellow I, Almond JW, and Evans DJ. Mapping the binding domains on decay accelerating factor (DAF) for haemagglutinating enteroviruses: implications for the evolution of a DAF-binding phenotype. J Gen Virol. (1999) 80:3145–52. doi: 10.1099/0022-1317-80-12-3145
52. Coyne CB and Bergelson JM. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell. (2006) 124:119–31. doi: 10.1016/j.cell.2005.10.035
53. Fuse K, Chan G, Liu Y, Gudgeon P, Husain M, Chen M, et al. Myeloid differentiation factor-88 plays a crucial role in the pathogenesis of Coxsackievirus B3-induced myocarditis and influences type I interferon production. Circulation. (2005) 112:2276–85. doi: 10.1161/CIRCULATIONAHA.105.536433
54. Seko Y, Takahashi N, Yagita H, Okumura K, and Yazaki Y. Expression of cytokine mRNAs in murine hearts with acute myocarditis caused by coxsackievirus b3. J Pathol. (1997) 183:105–8. doi: 10.1002/(SICI)1096-9896(199709)183:1<105::AID-PATH1094>3.0.CO;2-E
55. Roberts BJ, Dragon JA, Moussawi M, and Huber SA. Sex-specific signaling through Toll-Like Receptors 2 and 4 contributes to survival outcome of Coxsackievirus B3 infection in C57Bl/6 mice. Biol Sex Differ. (2012) 3:25. doi: 10.1186/2042-6410-3-25
56. de Marcken M, Dhaliwal K, Danielsen AC, Gautron AS, and Dominguez-Villar M. TLR7 and TLR8 activate distinct pathways in monocytes during RNA virus infection. Sci Signal. (2019) 12. doi: 10.1126/scisignal.aaw1347
57. Esfandiarei M and McManus BM. Molecular biology and pathogenesis of viral myocarditis. Annu Rev Pathol. (2008) 3:127–55. doi: 10.1146/annurev.pathmechdis.3.121806.151534
58. Godeny EK and Gauntt CJ. Involvement of natural killer cells in coxsackievirus B3-induced murine myocarditis. J Immunol. (1986) 137:1695–702. doi: 10.4049/jimmunol.137.5.1695
59. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, and Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. (2004) 25:677–86. doi: 10.1016/j.it.2004.09.015
60. Porta C, Riboldi E, Ippolito A, and Sica A. Molecular and epigenetic basis of macrophage polarized activation. Semin Immunol. (2015) 27:237–48. doi: 10.1016/j.smim.2015.10.003
61. Wang N, Liang H, and Zen K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front Immunol. (2014) 5:614. doi: 10.3389/fimmu.2014.00614
62. Di Florio DN, Sin J, Coronado MJ, Atwal PS, and Fairweather D. Sex differences in inflammation, redox biology, mitochondria and autoimmunity. Redox Biol. (2020) 31:101482. doi: 10.1016/j.redox.2020.101482
63. Frisancho-Kiss S, Coronado MJ, Frisancho JA, Lau VM, Rose NR, Klein SL, et al. Gonadectomy of male BALB/c mice increases Tim-3(+) alternatively activated M2 macrophages, Tim-3(+) T cells, Th2 cells and Treg in the heart during acute coxsackievirus-induced myocarditis. Brain Behav Immun. (2009) 23:649–57. doi: 10.1016/j.bbi.2008.12.002
64. Enright S and Werstuck GH. Investigating the effects of sex hormones on macrophage polarization. Int J Mol Sci. (2024) 25:951. doi: 10.3390/ijms25020951
65. Park S, Krshnan L, Call MJ, Call ME, and Im W. Structural conservation and effects of alterations in T cell receptor transmembrane interfaces. Biophys J. (2018) 114:1030–5. doi: 10.1016/j.bpj.2018.01.004
66. Gagliani N and Huber S. Basic aspects of T helper cell differentiation. Methods Mol Biol. (2017) 1514:19–30. doi: 10.1007/978-1-4939-6548-9_2
67. Zhu X and Zhu J. CD4 T helper cell subsets and related human immunological disorders. Int J Mol Sci. (2020) 21:8011. doi: 10.3390/ijms21218011
68. Lee GR. The balance of th17 versus treg cells in autoimmunity. Int J Mol Sci. (2018) 19:730. doi: 10.3390/ijms19030730
69. Zheng SY and Dong JZ. Role of toll-like receptors and th responses in viral myocarditis. Front Immunol. (2022) 13:843891. doi: 10.3389/fimmu.2022.843891
70. Huber SA and Pfaeffle B. Differential Th1 and Th2 cell responses in male and female BALB/c mice infected with coxsackievirus group B type 3. J Virol. (1994) 68:5126–32. doi: 10.1128/jvi.68.8.5126-5132.1994
71. Frisancho-Kiss S, Davis SE, Nyland JF, Frisancho JA, Cihakova D, Barrett MA, et al. Cutting edge: cross-regulation by TLR4 and T cell Ig mucin-3 determines sex differences in inflammatory heart disease. J Immunol. (2007) 178:6710–4. doi: 10.4049/jimmunol.178.11.6710
72. Frisancho-Kiss S, Nyland JF, Davis SE, Frisancho JA, Barrett MA, Rose NR, et al. Sex differences in coxsackievirus B3-induced myocarditis: IL-12Rbeta1 signaling and IFN-gamma increase inflammation in males independent from STAT4. Brain Res. (2006) 1126:139–47. doi: 10.1016/j.brainres.2006.08.003
73. Huber SA. Coxsackievirus B3-induced myocarditis: infection of females during the estrus phase of the ovarian cycle leads to activation of T regulatory cells. Virology. (2008) 378:292–8. doi: 10.1016/j.virol.2008.05.015
74. Zhou N, Yue Y, and Xiong S. Sex hormone contributes to sexually dimorphic susceptibility in CVB3-induced viral myocarditis via modulating IFN-gamma(+) NK cell production. Can J Cardiol. (2018) 34:492–501. doi: 10.1016/j.cjca.2018.01.002
75. Nakaya M, Yamasaki M, Miyazaki Y, Tachibana H, and Yamada K. Estrogenic compounds suppressed interferon-gamma production in mouse splenocytes through direct cell-cell interaction. In Vitro Cell Dev Biol Anim. (2003) 39:383–7. doi: 10.1290/1543-706X(2003)039<0383:ECSIPI>2.0.CO;2
76. Coronado MJ, Brandt JE, Kim E, Bucek A, Bedja D, Abston ED, et al. Testosterone and interleukin-1beta increase cardiac remodeling during coxsackievirus B3 myocarditis via serpin A 3n. Am J Physiol Heart Circ Physiol. (2012) 302:H1726–36. doi: 10.1152/ajpheart.00783.2011
77. Barcena ML, Niehues MH, Christiansen C, Estepa M, Haritonow N, Sadighi AH, et al. Male macrophages and fibroblasts from C57/BL6J mice are more susceptible to inflammatory stimuli. Front Immunol. (2021) 12:758767. doi: 10.3389/fimmu.2021.758767
78. Hirokawa K, Utsuyama M, Kasai M, Kurashima C, Ishijima S, and Zeng YX. Understanding the mechanism of the age-change of thymic function to promote T cell differentiation. Immunol Lett. (1994) 40:269–77. doi: 10.1016/0165-2478(94)00065-4
79. Olsen NJ and Kovacs WJ. Gonadal steroids and immunity. Endocr Rev. (1996) 17:369–84. doi: 10.1210/edrv-17-4-369
80. Rosen JL, Tran HT, Lackey A, and Viselli SM. Sex-related immune changes in young mice. Immunol Invest. (1999) 28:247–56. doi: 10.3109/08820139909060859
81. Castro JE. Orchidectomy and the immune response. I. Effect of orchidectomy on lymphoid tissues of mice. Proc R Soc Lond B Biol Sci. (1974) 185:425–36. doi: 10.1098/rspb.1974.0027
82. Anzini M, Merlo M, Sabbadini G, Barbati G, Finocchiaro G, Pinamonti B, et al. Long-term evolution and prognostic stratification of biopsy-proven active myocarditis. Circulation. (2013) 128:2384–94. doi: 10.1161/CIRCULATIONAHA.113.003092
84. Biesbroek PS, Beek AM, Germans T, Niessen HW, and van Rossum AC. Diagnosis of myocarditis: Current state and future perspectives. Int J Cardiol. (2015) 191:211–9. doi: 10.1016/j.ijcard.2015.05.008
85. Wu S, Yang YM, Zhu J, Wan HB, Wang J, Zhang H, et al. Clinical characteristics and outcomes of patients with myocarditis mimicking ST-segment elevation myocardial infarction: Analysis of a case series. Med (Baltimore). (2017) 96:e6863. doi: 10.1097/MD.0000000000006863
86. Caforio ALP, Kaski JP, Gimeno JR, Elliott PM, Laroche C, Tavazzi L, et al. Endomyocardial biopsy: safety and prognostic utility in paediatric and adult myocarditis in the European Society of Cardiology EURObservational Research Programme Cardiomyopathy and Myocarditis Long-Term Registry. Eur Heart J. (2024) 45:2548–69. doi: 10.1093/eurheartj/ehae169
87. Shah Z, Vuddanda V, Rali AS, Pamulapati H, Masoomi R, and Gupta K. National trends and procedural complications from endomyocardial biopsy: results from the national inpatient sample, 2007-2014. Cardiology. (2018) 141:125–31. doi: 10.1159/000493786
88. Keller K, Gobel S, Gori T, Munzel T, Wenzel P, and Hobohm L. A nationwide trend analysis on the usage of endomyocardial biopsy. Clin Cardiol. (2024) 47:e24198. doi: 10.1002/clc.24198
89. Seferovic PM, Tsutsui H, McNamara DM, Ristic AD, Basso C, Bozkurt B, et al. Heart Failure Society of America and Japanese Heart Failure Society Position statement on endomyocardial biopsy. Eur J Heart Fail. (2021) 23:854–71. doi: 10.1002/ejhf.2190
90. Writing C, Drazner MH, Bozkurt B, Cooper LT, Aggarwal NR, Basso C, et al. 2024 ACC expert consensus decision pathway on strategies and criteria for the diagnosis and management of myocarditis: A report of the american college of cardiology solution set oversight committee. J Am Coll Cardiol. (2025) 85:391–431. doi: 10.1016/j.jacc.2024.10.080
91. Ammirati E, Frigerio M, Adler ED, Basso C, Birnie DH, Brambatti M, et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy: an expert consensus document. Circ Heart Fail. (2020) 13:e007405. doi: 10.1161/CIRCHEARTFAILURE.120.007405
92. Tunuguntla H, Jeewa A, and Denfield SW. Acute myocarditis and pericarditis in children. Pediatr Rev. (2019) 40:14–25. doi: 10.1542/pir.2018-0044
93. Landstrom AP, Kim JJ, Gelb BD, Helm BM, Kannankeril PJ, Semsarian C, et al. Genetic testing for herit able cardiovascular diseases in pediatric patients: A scientific statement from the american heart association. Circ Genom Precis Med. (2021) 14:e000086. doi: 10.1161/HCG.0000000000000086
94. McNally EM and Selgrade DF. Genetic testing for myocarditis. JACC Heart Fail. (2022) 10:728–30. doi: 10.1016/j.jchf.2022.07.003
95. Kottwitz J, Bruno KA, Berg J, Salomon GR, Fairweather D, Elhassan M, et al. Myoglobin for detection of high-risk patients with acute myocarditis. J Cardiovasc Transl Res. (2020) 13:853–63. doi: 10.1007/s12265-020-09957-8
96. Villatore A, Monno A, Sciorati C, Rovere-Querini P, Sala S, Carino D, et al. Pentraxin 3 in myocarditis: proof-of-principle assessment as a diagnostic and prognostic biomarker. J Cardiovasc Transl Res. (2024) 17:1048–58. doi: 10.1007/s12265-024-10506-w
97. Blanco-Dominguez R, Sanchez-Diaz R, de la Fuente H, Jimenez-Borreguero LJ, Matesanz-Marin A, Relano M, et al. A novel circulating microRNA for the detection of acute myocarditis. N Engl J Med. (2021) 384:2014–27. doi: 10.1056/NEJMoa2003608
98. Giordani AS, Baritussio A, Vicenzetto C, Brigiari G, Pilichou K, Gregori D, et al. Serum anti-heart and antinuclear autoantibodies are independent predictors of response to immunosuppressive therapy in autoimmune biopsy-proven inflammatory cardiomyopathy. JACC Basic Transl Sci. (2025) 10:101310. doi: 10.1016/j.jacbts.2025.05.005
99. Wolfgram LJ, Beisel KW, and Rose NR. Heart-specific autoantibodies following murine coxsackievirus B3 myocarditis. J Exp Med. (1985) 161:1112–21. doi: 10.1084/jem.161.5.1112
100. Limas CJ, Goldenberg IF, and Limas C. Autoantibodies against beta-adrenoceptors in human idiopathic dilated cardiomyopathy. Circ Res. (1989) 64:97–103. doi: 10.1161/01.RES.64.1.97
101. Fairweather D, Petri MA, Coronado MJ, and Cooper LT. Autoimmune heart disease: role of sex hormones and autoantibodies in disease pathogenesis. Expert Rev Clin Immunol. (2012) 8:269–84. doi: 10.1586/eci.12.10
102. Beetler DJ, Bruno KA, Di Florio DN, Douglass EJ, Shrestha S, Tschope C, et al. Sex and age differences in sST2 in cardiovascular disease. Front Cardiovasc Med. (2022) 9:1073814. doi: 10.3389/fcvm.2022.1073814
103. Hou W, Shi T, Li Y, Li W, Xu M, and Peng F. Soluble suppression of tumorigenicity 2 associated with fulminant myocarditis in children: A retrospective observational study. Med (Baltimore). (2023) 102:e34784. doi: 10.1097/MD.0000000000034784
104. Huang Y, Lin Y, Fu M, and Zhang W. Diagnostic efficacy of soluble ST2 in pediatric fulminant myocarditis. Front Pediatr. (2025) 13:1417341. doi: 10.3389/fped.2025.1417341
105. Morgera T, Di Lenarda A, Dreas L, Pinamonti B, Humar F, Bussani R, et al. Electrocardiography of myocarditis revisited: clinical and prognostic significance of electrocardiographic changes. Am Heart J. (1992) 124:455–67. doi: 10.1016/0002-8703(92)90613-Z
106. Chen S, Hoss S, Zeniou V, Shauer A, Admon D, Zwas DR, et al. Electrocardiographic predictors of morbidity and mortality in patients with acute myocarditis: the importance of QRS-T angle. J Card Fail. (2018) 24:3–8. doi: 10.1016/j.cardfail.2017.11.001
107. Nakashima H, Katayama T, Ishizaki M, Takeno M, Honda Y, and Yano K. Q wave and non-Q wave myocarditis with special reference to clinical significance. Jpn Heart J. (1998) 39:763–74. doi: 10.1536/ihj.39.763
108. Caforio AL, Calabrese F, Angelini A, Tona F, Vinci A, Bottaro S, et al. A prospective study of biopsy-proven myocarditis: prognostic relevance of clinical and aetiopathogenetic features at diagnosis. Eur Heart J. (2007) 28:1326–33. doi: 10.1093/eurheartj/ehm076
109. Ukena C, Mahfoud F, Kindermann I, Kandolf R, Kindermann M, and Bohm M. Prognostic electrocardiographic parameters in patients with suspected myocarditis. Eur J Heart Fail. (2011) 13:398–405. doi: 10.1093/eurjhf/hfq229
110. Patel MS, Berg AM, Vincent RN, and Mahle WT. Serum parameters and echocardiographic predictors of death or need for transplant in newborns, children, and young adults with heart failure. Am J Cardiol. (2010) 105:1798–801. doi: 10.1016/j.amjcard.2010.01.357
111. Ture M, Balik H, Akin A, Bilici M, and Nergiz A. The relationship between electrocardiographic data and mortality in children diagnosed with dilated cardiomyopathy. Eur J Pediatr. (2020) 179:813–9. doi: 10.1007/s00431-020-03569-9
112. Gursu HA, Cetin II, Azak E, Kibar AE, Surucu M, Orgun A, et al. The assessment of treatment outcomes in patients with acute viral myocarditis by speckle tracking and tissue Doppler methods. Echocardiography. (2019) 36:1666–74. doi: 10.1111/echo.14449
113. Di Florio DN, Macomb LP, Giresi PG, Beetler DJ, Bonvie-Hill NE, Shapiro KA, et al. Sex differences in left-ventricular strain in a murine model of coxsackievirus B3 myocarditis. iScience. (2023) 26:108493. doi: 10.1016/j.isci.2023.108493
114. Ishii R, Steve Fan CP, Mertens L, Manlhiot C, and Friedberg MK. Longitudinal prediction of transplant-free survival by echocardiography in pediatric dilated cardiomyopathy. Can J Cardiol. (2021) 37:867–76. doi: 10.1016/j.cjca.2020.12.010
115. Heimdal A, Stoylen A, Torp H, and Skjaerpe T. Real-time strain rate imaging of the left ventricle by ultrasound. J Am Soc Echocardiogr. (1998) 11:1013–9. doi: 10.1016/S0894-7317(98)70151-8
116. D’Hooge J, Heimdal A, Jamal F, Kukulski T, Bijnens B, Rademakers F, et al. Regional strain and strain rate measurements by cardiac ultrasound: principles, implementation and limitations. Eur J Echocardiogr. (2000) 1:154–70. doi: 10.1053/euje.2000.0031
117. Urheim S, Edvardsen T, Torp H, Angelsen B, and Smiseth OA. Myocardial strain by Doppler echocardiography. Validation of a new method to quantify regional myocardial function. Circulation. (2000) 102:1158–64. doi: 10.1161/01.cir.102.10.1158
118. Gorcsan J 3rd and Tanaka H. Echocardiographic assessment of myocardial strain. J Am Coll Cardiol. (2011) 58:1401–13. doi: 10.1016/j.jacc.2011.06.038
119. Poyraz E and Dinc Asarcikli L. Dynamic change of left ventricular mechanics in patients with acute myocarditis with preserved left ventricular systolic function: A 2-year follow-up study. Turk Kardiyol Dern Ars. (2022) 50:485–91. doi: 10.5543/tkda.2022.22358
120. Degiovanni A, Pastore MC, Spinoni EG, Focardi M, Cameli M, and Patti G. Usefulness of a multiparametric evaluation including global longitudinal strain for an early diagnosis of acute myocarditis. Int J Cardiovasc Imaging. (2021) 37:3203–11. doi: 10.1007/s10554-021-02299-9
121. McAree D, Hauck A, Arzu J, Carr M, Acevedo J, Patel AB, et al. Clinical predictors of subacute myocardial dysfunction in multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19. Pediatr Cardiol. (2024) 45:876–87. doi: 10.1007/s00246-022-03021-9
122. Sirico D, Basso A, Reffo E, Cavaliere A, Castaldi B, Sabatino J, et al. Early echocardiographic and cardiac MRI findings in multisystem inflammatory syndrome in children. J Clin Med. (2021) 10:3360. doi: 10.3390/jcm10153360
123. Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. (2018) 72:3158–76. doi: 10.1016/j.jacc.2018.09.072
124. Mewton N, Dernis A, Bresson D, Zouaghi O, Croisille P, Flocard E, et al. Myocardial biomarkers and delayed enhanced cardiac magnetic resonance relationship in clinically suspected myocarditis and insight on clinical outcome. J Cardiovasc Med (Hagerstown). (2015) 16:696–703. doi: 10.2459/JCM.0000000000000024
125. Grani C, Eichhorn C, Biere L, Murthy VL, Agarwal V, Kaneko K, et al. Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J Am Coll Cardiol. (2017) 70:1964–76. doi: 10.1016/j.jacc.2017.08.050
126. Chopra H, Arangalage D, Bouleti C, Zarka S, Fayard F, Chillon S, et al. Prognostic value of the infarct- and non-infarct like patterns and cardiovascular magnetic resonance parameters on long-term outcome of patients after acute myocarditis. Int J Cardiol. (2016) 212:63–9. doi: 10.1016/j.ijcard.2016.03.004
127. Toussaint M, Gilles RJ, Azzabou N, Marty B, Vignaud A, Greiser A, et al. Characterization of benign myocarditis using quantitative delayed-enhancement imaging based on molli T1 mapping. Med (Baltimore). (2015) 94:e1868. doi: 10.1097/MD.0000000000001868
128. Francone M, Chimenti C, Galea N, Scopelliti F, Verardo R, Galea R, et al. CMR sensitivity varies with clinical presentation and extent of cell necrosis in biopsy-proven acute myocarditis. JACC Cardiovasc Imaging. (2014) 7:254–63. doi: 10.1016/j.jcmg.2013.10.011
129. Hassan K, Doubell A, Kyriakakis C, Joubert L, Robbertse PP, Van Zyl G, et al. Comparing the findings and diagnostic sensitivity of cardiovascular magnetic resonance in biopsy confirmed acute myocarditis with infarct-like vs. heart failure presentation. J Cardiovasc Magn Reson. (2022) 24:69. doi: 10.1186/s12968-022-00903-y
130. Gannon MP, Schaub E, Grines CL, and Saba SG. State of the art: Evaluation and prognostication of myocarditis using cardiac MRI. J Magn Reson Imaging. (2019) 49:e122–e31. doi: 10.1002/jmri.26611
131. Real C, Parraga R, Pizarro G, Garcia-Lunar I, Gonzalez-Calvo E, Martinez-Gomez J, et al. Magnetic resonance imaging reference values for cardiac morphology, function and tissue composition in adolescents. EClinicalMedicine. (2023) 57:101885. doi: 10.1016/j.eclinm.2023.101885
132. Ciet P, Tiddens HA, Wielopolski PA, Wild JM, Lee EY, Morana G, et al. Magnetic resonance imaging in children: common problems and possible solutions for lung and airways imaging. Pediatr Radiol. (2015) 45:1901–15. doi: 10.1007/s00247-015-3420-y
133. Lekakis J, Nanas J, Prassopoulos V, Kostamis P, and Moulopoulos S. Natural evolution of antimyosin scan and cardiac function in patients with acute myocarditis. Int J Cardiol. (1995) 52:53–8. doi: 10.1016/0167-5273(95)02440-8
134. Peretto G, Busnardo E, Ferro P, Palmisano A, Vignale D, Esposito A, et al. Clinical applications of FDG-PET scan in arrhythmic myocarditis. JACC Cardiovasc Imaging. (2022) 15:1771–80. doi: 10.1016/j.jcmg.2022.02.029
135. Chen W and Jeudy J. Assessment of myocarditis: cardiac MR, PET/CT, or PET/MR? Curr Cardiol Rep. (2019) 21:76. doi: 10.1007/s11886-019-1158-0
136. Fairweather D, Coronado MJ, Garton AE, Dziedzic JL, Bucek A, Cooper LT Jr., et al. Sex differences in translocator protein 18 kDa (TSPO) in the heart: implications for imaging myocardial inflammation. J Cardiovasc Transl Res. (2014) 7:192–202. doi: 10.1007/s12265-013-9538-0
137. Brala D, Thevathasan T, Grahl S, Barrow S, Violano M, Bergs H, et al. Application of magnetocardiography to screen for inflammatory cardiomyopathy and monitor treatment response. J Am Heart Assoc. (2023) 12:e027619. doi: 10.1161/JAHA.122.027619
138. Seidel F, Holtgrewe M, Al-Wakeel-Marquard N, Opgen-Rhein B, Dartsch J, Herbst C, et al. Pathogenic variants associated with dilated cardiomyopathy predict outcome in pediatric myocarditis. Circ Genom Precis Med. (2021) 14:e003250. doi: 10.1161/CIRCGEN.120.003250
139. Pollack A, Kontorovich AR, Fuster V, and Dec GW. Viral myocarditis–diagnosis, treatment options, and current controversies. Nat Rev Cardiol. (2015) 12:670–80. doi: 10.1038/nrcardio.2015.108
140. Krueger GR and Ablashi DV. Human herpesvirus-6: a short review of its biological behavior. Intervirology. (2003) 46:257–69. doi: 10.1159/000073205
141. Mason JW, O’Connell JB, Herskowitz A, Rose NR, McManus BM, Billingham ME, et al. A clinical trial of immunosuppressive therapy for myocarditis. The Myocarditis Treatment Trial Investigators. N Engl J Med. (1995) 333:269–75. doi: 10.1056/NEJM199508033330501
142. Chen HS, Wang W, Wu SN, and Liu JP. Corticosteroids for viral myocarditis. Cochrane Database Syst Rev. (2013) 2013:CD004471. doi: 10.1002/14651858.CD004471.pub3
143. Aziz KU, Patel N, Sadullah T, Tasneem H, Thawerani H, and Talpur S. Acute viral myocarditis: role of immunosuppression: a prospective randomised study. Cardiol Young. (2010) 20:509–15. doi: 10.1017/S1047951110000594
144. Schauer J, Newland D, Hong B, Albers E, Friedland-Little J, Kemna M, et al. Treating pediatric myocarditis with high dose steroids and immunoglobulin. Pediatr Cardiol. (2023) 44:441–50. doi: 10.1007/s00246-022-03004-w
145. Lin MS, Tseng YH, Chen MY, Chung CM, Tsai MH, Wang PC, et al. In-hospital and post-discharge outcomes of pediatric acute myocarditis underwent after high-dose steroid or intravenous immunoglobulin therapy. BMC Cardiovasc Disord. (2019) 19:10. doi: 10.1186/s12872-018-0981-3
146. Morgenstern D, Lisko J, Boniface NC, Mikolich BM, and Mikolich JR. Myocarditis and colchicine: a new perspective from cardiac MRI. J Cardiovasc Magnetic Resonance. (2016) 18:O100. doi: 10.1186/1532-429X-18-S1-O100
147. Morrow DA and Verbrugge FH. In-perspective: the ARAMIS double-blind randomized placebo-controlled trial of anakinra for the treatment of acute myocarditis. Eur Heart J Acute Cardiovasc Care. (2023) 12:627–8. doi: 10.1093/ehjacc/zuad102
148. Wohlford GF, Buckley LF, Vecchie A, Kadariya D, Markley R, Trankle CR, et al. Acute effects of interleukin-1 blockade using anakinra in patients with acute pericarditis. J Cardiovasc Pharmacol. (2020) 76:50–2. doi: 10.1097/FJC.0000000000000847
149. Merlo M, Gentile P, Ballaben A, Artico J, Castrichini M, Porcari A, et al. Acute inflammatory cardiomyopathy: apparent neutral prognostic impact of immunosuppressive therapy. Eur J Heart Fail. (2020) 22:1280–2. doi: 10.1002/ejhf.1821
150. Capasso R, Imperato MC, Serra N, Rodriguez R, Rivellini M, De Filippo M, et al. Infarct-like versus non-infarct-like clinical presentation of acute myocarditis: comparison of cardiac magnetic resonance (CMR) findings. Diagnostics (Basel). (2023) 13:2948. doi: 10.3390/diagnostics13152498
151. Frustaci A, Russo MA, and Chimenti C. Randomized study on the efficacy of immunosuppressive therapy in patients with virus-negative inflammatory cardiomyopathy: the TIMIC study. Eur Heart J. (2009) 30:1995–2002. doi: 10.1093/eurheartj/ehp249
152. Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. (2013) 34:2636–48, 48a-48d. doi: 10.1093/eurheartj/eht210
153. Robinson J, Hartling L, Vandermeer B, Sebastianski M, and Klassen TP. Intravenous immunoglobulin for presumed viral myocarditis in children and adults. Cochrane Database Syst Rev. (2020) 8:CD004370. doi: 10.1002/14651858.CD004370.pub4
154. Kishimoto C, Shioji K, Hashimoto T, Nonogi H, Lee JD, Kato S, et al. Therapy with immunoglobulin in patients with acute myocarditis and cardiomyopathy: analysis of leukocyte balance. Heart Vessels. (2014) 29:336–42. doi: 10.1007/s00380-013-0368-4
155. Bhatt GC, Sankar J, and Kushwaha KP. Use of intravenous immunoglobulin compared with standard therapy is associated with improved clinical outcomes in children with acute encephalitis syndrome complicated by myocarditis. Pediatr Cardiol. (2012) 33:1370–6. doi: 10.1007/s00246-012-0350-4
156. Chrusciel P, Rysz J, and Banach M. Defining the role of trimetazidine in the treatment of cardiovascular disorders: some insights on its role in heart failure and peripheral artery disease. Drugs. (2014) 74:971–80. doi: 10.1007/s40265-014-0233-5
157. Shao L, Ma A, Figtree G, and Zhang P. Combination therapy with coenzyme Q10 and trimetazidine in patients with acute viral myocarditis. J Cardiovasc Pharmacol. (2016) 68:150–4. doi: 10.1097/FJC.0000000000000396
158. Amdani SM, Kim HS, Orvedahl A, John AO, Said A, and Simpson K. Successful treatment of fulminant neonatal enteroviral myocarditis in monochorionic diamniotic twins with cardiopulmonary support, intravenous immunoglobulin and pocapavir. BMJ Case Rep. (2018) 2018. doi: 10.1136/bcr-2017-224133
159. Wittekind SG, Allen CC, Jefferies JL, Rattan MS, Madueme PC, Taylor BN, et al. Neonatal enterovirus myocarditis with severe dystrophic calcification: novel treatment with pocapavir. J Investig Med High Impact Case Rep. (2017) 5:2324709617729393. doi: 10.1177/2324709617729393
160. Wang M, Deng J, Xing S, and Li L. Clinical effect analysis of different doses of creatine phosphate sodium combined with immunoglobulin in the treatment of pediatric viral myocarditis. Pediatr Cardiol. (2024) 45:1048–54. doi: 10.1007/s00246-024-03450-8
161. Wang L, Chen SF, Huang XW, Wu ZY, and Zhang QM. Efficacy and safety of creatine phosphate sodium in the treatment of viral myocarditis: A systematic review and meta-analysis. PloS One. (2025) 20:e0317498. doi: 10.1371/journal.pone.0317498
162. Ammirati E, Conti N, Frea S, Uribarri A, Grosu A, Loffredo F, et al. Rationale and study design of the international randomized control trial MYocarditis THerapy with Steroids: the MYTHS trial. Eur Heart J. (2024) 45. doi: 10.1093/eurheartj/ehae666.1239
163. McNamara DM, Cooper LT, Arbel Y, Bhimaraj A, Bocchi E, Friedrich MG, et al. Impact of cannabidiol on myocardial recovery in patients with acute myocarditis: Rationale & design of the ARCHER trial. ESC Heart Fail. (2024) 11:3416–24. doi: 10.1002/ehf2.14889
164. Martinez Naya N, Kelly J, Corna G, Golino M, Abbate A, and Toldo S. Molecular and cellular mechanisms of action of cannabidiol. Molecules. (2023) 28:5980. doi: 10.3390/molecules28165980
165. Lee WS, Erdelyi K, Matyas C, Mukhopadhyay P, Varga ZV, Liaudet L, et al. Cannabidiol limits T cell-mediated chronic autoimmune myocarditis: implications to autoimmune disorders and organ transplantation. Mol Med. (2016) 22:136–46. doi: 10.2119/molmed.2016.00007
166. Pahuja M, Adegbala O, Mishra T, Akintoye E, Chehab O, Mony S, et al. Trends in the incidence of in-hospital mortality, cardiogenic shock, and utilization of mechanical circulatory support devices in myocarditis (Analysis of national inpatient sample data, 2005-2014). J Card Fail. (2019) 25:457–67. doi: 10.1016/j.cardfail.2019.04.012
167. Vishram-Nielsen JKK, Foroutan F, Rizwan S, Peck SS, Bodack J, Orchanian-Cheff A, et al. Patients with fulminant myocarditis supported with veno-arterial extracorporeal membrane oxygenation: a systematic review and meta-analysis of short-term mortality and impact of risk factors. Heart Fail Rev. (2023) 28:347–57. doi: 10.1007/s10741-022-10277-z
168. Kondo T, Okumura T, Shibata N, Imaizumi T, Dohi K, Izawa H, et al. Differences in prognosis and cardiac function according to required percutaneous mechanical circulatory support and histological findings in patients with fulminant myocarditis: insights from the CHANGE PUMP 2 study. J Am Heart Assoc. (2022) 11:e023719. doi: 10.1161/JAHA.121.023719
169. Chou HW, Wang CH, Lin LY, Chi NH, Chou NK, Yu HY, et al. Prognostic factors for heart recovery in adult patients with acute fulminant myocarditis and cardiogenic shock supported with extracorporeal membrane oxygenation. J Crit Care. (2020) 57:214–9. doi: 10.1016/j.jcrc.2020.03.007
170. Wu HP, Lin MJ, Yang WC, Wu KH, and Chen CY. Predictors of extracorporeal membrane oxygenation support for children with acute myocarditis. BioMed Res Int. (2017) 2017:2510695. doi: 10.1155/2017/2510695
171. Butto A, Rossano JW, Nandi D, Ravishankar C, Lin KY, O’Connor MJ, et al. Elevated troponin in the first 72 h of hospitalization for pediatric viral myocarditis is associated with ECMO: an analysis of the PHIS+ Database. Pediatr Cardiol. (2018) 39:1139–43. doi: 10.1007/s00246-018-1871-2
172. Duncan BW, Bohn DJ, Atz AM, French JW, Laussen PC, and Wessel DL. Mechanical circulatory support for the treatment of children with acute fulminant myocarditis. J Thorac Cardiovasc Surg. (2001) 122:440–8. doi: 10.1067/mtc.2001.115243
173. Allan CK, Laussen PC, Newburger JW, Gauvreau K, and Thiagarajan RR. Management and outcomes in pediatric patients presenting with acute fulminant myocarditis. J Pediatr. (2011) 158:638–43.e1. doi: 10.1016/j.jpeds.2010.10.015
174. Alsoufi B, Al-Radi OO, Nazer RI, Gruenwald C, Foreman C, Williams WG, et al. Survival outcomes after rescue extracorporeal cardiopulmonary resuscitation in pediatric patients with refractory cardiac arrest. J Thorac Cardiovasc Surg. (2007) 134:952–9.e2. doi: 10.1016/j.jtcvs.2007.05.054
175. Hsu KH, Chi NH, Yu HY, Wang CH, Huang SC, Wang SS, et al. Extracorporeal membranous oxygenation support for acute fulminant myocarditis: analysis of a single center’s experience. Eur J Cardiothorac Surg. (2011) 40:682–8. doi: 10.1016/j.ejcts.2010.12.050
176. Asaumi Y, Yasuda S, Morii I, Kakuchi H, Otsuka Y, Kawamura A, et al. Favourable clinical outcome in patients with cardiogenic shock due to fulminant myocarditis supported by percutaneous extracorporeal membrane oxygenation. Eur Heart J. (2005) 26:2185–92. doi: 10.1093/eurheartj/ehi411
177. Kociol RD, Cooper LT, Fang JC, Moslehi JJ, Pang PS, Sabe MA, et al. Recognition and initial management of fulminant myocarditis: A scientific statement from the american heart association. Circulation. (2020) 141:e69–92. doi: 10.1161/CIR.0000000000000745
178. Guglin M and Miller L. Myocardial recovery with left ventricular assist devices. Curr Treat Options Cardiovasc Med. (2012) 14:370–83. doi: 10.1007/s11936-012-0190-9
179. Wilmot I, Morales DL, Price JF, Rossano JW, Kim JJ, Decker JA, et al. Effectiveness of mechanical circulatory support in children with acute fulminant and persistent myocarditis. J Card Fail. (2011) 17:487–94. doi: 10.1016/j.cardfail.2011.02.008
180. Sharma MS, Webber SA, Morell VO, Gandhi SK, Wearden PD, Buchanan JR, et al. Ventricular assist device support in children and adolescents as a bridge to heart transplantation. Ann Thorac Surg. (2006) 82:926–32. doi: 10.1016/j.athoracsur.2006.02.087
181. Topkara VK, Dang NC, Barili F, Martens TP, George I, Cheema FH, et al. Ventricular assist device use for the treatment of acute viral myocarditis. J Thorac Cardiovasc Surg. (2006) 131:1190–1. doi: 10.1016/j.jtcvs.2005.08.073
182. ElAmm CA, Al-Kindi SG, and Oliveira GH. Characteristics and outcomes of patients with myocarditis listed for heart transplantation. Circ Heart Fail. (2016) 9. doi: 10.1161/CIRCHEARTFAILURE.116.003259
183. Burstein DS, Li Y, Getz KD, Huang YV, Rossano JW, O’Connor MJ, et al. Mortality, resource utilization, and inpatient costs vary among pediatric heart transplant indications: A merged data set analysis from the united network for organ sharing and pediatric health information systems databases. J Card Fail. (2019) 25:27–35. doi: 10.1016/j.cardfail.2018.11.014
184. Pietra BA, Kantor PF, Bartlett HL, Chin C, Canter CE, Larsen RL, et al. Early predictors of survival to and after heart transplantation in children with dilated cardiomyopathy. Circulation. (2012) 126:1079–86. doi: 10.1161/CIRCULATIONAHA.110.011999
185. Martin ME, Moya-Mur JL, Casanova M, Crespo-Diez A, Asin-Cardiel E, Castro-Beiras JM, et al. Role of noninvasive antimyosin imaging in infants and children with clinically suspected myocarditis. J Nucl Med. (2004) 45:429–37.
186. Wu H-P, Lin M-J, Yang W-C, Wu K-H, and Chen C-Y. Predictors of extracorporeal membrane oxygenation support for children with acute myocarditis. BioMed Res Int. (2017) 2017:1–8. doi: 10.1155/2017/2510695
187. Patel RY, Louis WD, Atalay M, Agarwal S, and Shah RN. Cardiovascular magnetic resonance findings in young adult patients with acute myocarditis following mRNA COVID-19 vaccination: a case series. J Cardiovasc Magnetic Resonance. (2021) 23:101. doi: 10.1186/s12968-021-00795-4
188. Dionne A, Sperotto F, Chamberlain S, Baker AL, Powell AJ, Prakash A, et al. Association of myocarditis with BNT162b2 messenger RNA COVID-19 vaccine in a case series of children. JAMA Cardiol. (2021) 6:1446. doi: 10.1001/jamacardio.2021.3471
189. Hadley SM, Prakash A, Baker AL, De Ferranti SD, Newburger JW, Friedman KG, et al. Follow-up cardiac magnetic resonance in children with vaccine-associated myocarditis. Eur J Pediatrics. (2022) 181:2879–83. doi: 10.1007/s00431-022-04482-z
190. Das BB, Kohli U, Ramachandran P, Nguyen HH, Greil G, Hussain T, et al. Myopericarditis after messenger RNA Coronavirus Disease 2019 Vaccination in Adolescents 12 to 18 Years of Age. J Pediatrics. (2021) 238:26–32.e1. doi: 10.1016/j.jpeds.2021.07.044
191. Nahum E, Dagan O, Lev A, Shukrun G, Amir G, Frenkel G, et al. Favorable outcome of pediatric fulminant myocarditis supported by extracorporeal membranous oxygenation. Pediatr Cardiol. (2010) 31:1059–63. doi: 10.1007/s00246-010-9765-y
192. Durani Y, Egan M, Baffa J, Selbst MS, and Nager LA. Pediatric myocarditis: presenting clinical characteristics. Am J Emergency Med. (2009) 27:942–7. doi: 10.1016/j.ajem.2008.07.032
193. Saji T, Matsuura H, Hasegawa K, Nishikawa T, Yamamoto E, Ohki H, et al. Comparison of the clinical presentation, treatment, and outcome of fulminant and acute myocarditis in children. Circ J. (2012) 76:1222–8. doi: 10.1253/circj.CJ-11-1032
194. Barbandi M, Cordero-Reyes A, Orrego CM, Torre-Amione G, Seethamraju H, and Estep J. A case series of reversible acute cardiomyopathy associated with H1N1 Influenza infection. Methodist DeBakey Cardiovasc J. (2012) 8:42. doi: 10.14797/mdcj-8-1-42
195. Hao H, Ji M, Zhou K, Zhang Y, Zhang G, and Ruan L. Effect of Yangxin Huoxue Jiedu recipe on inflammatory factors and oxidative stress on viral myocarditis in children. Cardiol Young. (2024) 34:1521–8. doi: 10.1017/S1047951124000180
196. Ramachandra G, Shields L, Brown K, and Ramnarayan P. The challenges of prompt identification and resuscitation in children with acute fulminant myocarditis: case series and review of the literature. J Paediatrics Child Health. (2010) 46:579–82. doi: 10.1111/j.1440-1754.2010.01799.x
197. Fronza M, Thavendiranathan P, Chan V, Karur GR, Udell JA, Wald RM, et al. Myocardial injury pattern at MRI in COVID-19 vaccine–associated myocarditis. Radiology. (2022) 304:553–62. doi: 10.1148/radiol.212559
198. Chou H-W, Wang C-H, Lin L-Y, Chi N-H, Chou N-K, Yu H-Y, et al. Prognostic factors for heart recovery in adult patients with acute fulminant myocarditis and cardiogenic shock supported with extracorporeal membrane oxygenation. J Crit Care. (2020) 57:214–9. doi: 10.1016/j.jcrc.2020.03.007
199. Rady IH and Zekri H. Prevalence of myocarditis in pediatric intensive care unit cases presenting with other system involvement. Jornal Pediatria. (2015) 91:93–7. doi: 10.1016/j.jped.2014.05.011
200. Caforio ALP, Kaski JP, Gimeno JR, Elliott PM, Laroche C, Tavazzi L, et al. Endomyocardial biopsy: safety and prognostic utility in paediatric and adult myocarditis in the European Society of Cardiology EURObservational Research Programme Cardiomyopathy and Myocarditis Long-Term Registry. Eur Heart J. (2024) 45:2548–69. doi: 10.1093/eurheartj/ehae169
201. Krasic S, Prijic S, Ninic S, Nesic D, Bjelakovic B, Petrovic G, et al. Could the unfortunate outcome of pediatric acute myocarditis be predicted? Factors contributing to a poor outcome in myocarditis. Rev Portuguesa Cardiologia (English Edition). (2021) 40:631–8. doi: 10.1016/j.repce.2020.10.020
202. Simpson KE, Storch GA, Lee CK, Ward KE, Danon S, Simon CM, et al. High frequency of detection by PCR of viral nucleic acid in the blood of infants presenting with clinical myocarditis. Pediatr Cardiol. (2016) 37:399–404. doi: 10.1007/s00246-015-1290-6
203. Zhuang S-X, Shi P, Gao H, Zhuang Q-N, and Huang G-Y. Clinical characteristics and mortality risk prediction model in children with acute myocarditis. World J Pediatrics. (2023) 19:180–8. doi: 10.1007/s12519-022-00637-y
Keywords: myocarditis, dilated cardiomyopathy, pediatrics, coxsackievirus, age differences
Citation: Ricci JC, Farahani NA, Davis CJ, Ritter KG, Parrow LM, Tomerlin PI, Darakjian AA, Gegoutchadze K, Gupta D and Bruno KA (2025) A comparative review of myocarditis in pediatrics versus adults: pathogenesis, diagnosis, and management. Front. Immunol. 16:1601307. doi: 10.3389/fimmu.2025.1601307
Received: 27 March 2025; Accepted: 03 September 2025;
Published: 26 September 2025.
Edited by:
Alida L.P. Caforio, University of Padua, ItalyReviewed by:
Sally A. Huber, University of Vermont, United StatesAndrea Silvio Giordani, University of Padua, Italy
Copyright © 2025 Ricci, Farahani, Davis, Ritter, Parrow, Tomerlin, Darakjian, Gegoutchadze, Gupta and Bruno. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katelyn A. Bruno, a2F0ZWx5bi5icnVub0BtZWRpY2luZS51ZmwuZWR1
†These authors share first authorship
‡These authors share senior authorship
 Jacob C. Ricci
Jacob C. Ricci Nick A. Farahani1†
Nick A. Farahani1† Ashley A. Darakjian
Ashley A. Darakjian Katherine Gegoutchadze
Katherine Gegoutchadze Dipankar Gupta
Dipankar Gupta Katelyn A. Bruno
Katelyn A. Bruno