- 1Centre for Tumour Immunology and Microenvironment, Department of Zoology, Mar Ivanios College, Thiruvananthapuram, Kerala, India
- 2Department of Biotechnology, CEPCI Laboratory and Research Institute, Kollam, India
- 3Department of Botany and Biotechnology, St. Xaviers College, Thiruvananthapuram, Kerala, India
Chemokines are tiny chemotactic cytokines which play a crucial role in pathophysiology by maintaining homeostasis and inflammation. Their role in the tumour microenvironment is very much puzzling because of both pro- and anti-tumourigenic effects. Chemokines have gained much attention today, since it has been recognized that they are game changers in the TME via controlling immune cell recruitment, angiogenesis, metastasis, tumour growth and drug resistance. In this review, we are exploring the role of several chemokines and their receptors in the TME with special focus on immune cell recruitment, immune surveillance, regulation of immune checkpoints and epithelial mesenchymal transition. We are also reviewing the possibility of targeting chemokines along with immunotherapy for better outcome and disease-free survival. A better understanding on the dual role of chemokine in the TME might help to implement novel therapeutic interventions and adopt precision in targeted therapy.
1 Introduction
Cancer, a comprehensive disease with a progressive lurch of immunologic, metabolic, neuroendocrinal and microbial features, remains a significant economic and social burden for both developed and developing countries (1). The disease is not merely a cluster of cancer cells; rather, a collection of infiltrating as well as host immune cells along with stromal cells, fibroblast cells, extracellular matrix and blood-lymphatic vascular networks, collectively called the tumour micro environment (TME) (2). In fact, hypoxic, acidic, and immune/inflammatory TMEs are enriched with non-cellular components such as cytokines, chemokines, growth factors and metabolites (3). The interaction of tumour mass with this surrounding milieu will mediate the tumour development, immune surveillance and life-threatening distant metastasis (4). According to global cancer statistics by international agency for research on cancer - GLOBOCAN 2022, lung, colorectal, liver, breast and stomach cancers are the leading causes of cancer death worldwide. Female breast cancer (BC) has the highest global cancer incidence with approximately 2.3 million new cases followed closely by lung cancer, which ranks second with 2.2 million new cancer cases universally (5).
Surgery, chemotherapy and radiation therapies remain to be the foundational modalities of conventional cancer treatment strategies. Even though radiation therapy is a non-surgical way to control cancer cells, the treatment option has its own limitations including DNA fragmentation in adjacent healthier cells and serious side effects depending on the dosage (6). Conversely, surgery cannot be suggested as a complete treatment for the disease, rather impactful only when combined with other therapies (7). Among these therapies, chemotherapy is considered to be a successful choice for metastatic tumours. Adverse effects caused by chemotherapy such as myelosuppression, nausea, vomiting, diarrhoea and alopecia, affect the quality of life very much (8). Moreover, treatment-induced inflammatory microenvironment, drug resistance and activated cancer stemness will hinder the anti-tumoural activity of chemotherapy. Tumour cells with all their distinct pathologic features, subvert the normal physiological conditions including non-cellular components such as chemokines. Emphasising these components offers a more rational and potentially effective approach for cancer treatments (9). In fact, Hanahan and Weinberg state that the chemokines and their receptors have been involved in the majority hallmark processes of cancers (10). Upon binding, chemokine – chemokine receptor interaction activates intracellular signalling pathways leading to cell-cell interaction, migration (chemotaxis), coordination and regulation of the immune system by several modulations in gene transcription. Diverse and dynamically regulated expression of chemokine ligands and their receptors by TME facilitates immune cell recruitment and activation. Here the review focuses on the complex and diverse functions of chemokines within the tumour microenvironment (TME), highlighting their roles in tumour progression, immune evasion and disease prognosis as well as their anti-tumoural effects. The review also attempts to give an account on chemokines as therapeutic targets aiding in immune surveillance and tumour suppression.
2 Chemokines and their receptors
Chemokines are a large family of small (8–12 kDa), soluble and secretory chemotactic cytokines that regulates the migratory patterns and positioning of immune cells, specifically at the time of development, homeostasis and pathological conditions (11). The family consists of 50 chemokine ligands and 20 GPCR signalling receptors, classified into four groups - CC, CXC, CX3C and C - based on the number and location of highly conserved cysteine residues at the N terminus of the ligand, with their corresponding nomenclature designated as CCL, CXCL, CX3CL and CL for the ligands and CCR, CXCR, CX3CR and CR for the receptors (12). Chemokines and their receptors are highly compatible with each other and at the same time, both are ambiguous and are susceptible to degeneration as well I.e. Many chemokines have the binding capacity to multiple receptors and some receptors binds to many ligands. (Figure 1) (13). CXC chemokines are again subdivided into the ELR+ (CXCL1 - CXCL3, CXCL5, CXCL6, CXCL7 and CXCL8) and ELR- (CXCL9 - 11) chemokine ligands based on the presence or absence of ELR (Glutamic acid - Leucine - Arginine) motif regions (14). These two subgroups execute entirely different functional roles, as follows, ELR+ chemokines promote angiogenesis, whereas ELR- chemokines mediate anti-angiogenesis except CXCL12, which does not contain an ELR motif but exhibits angiogenic property (15).
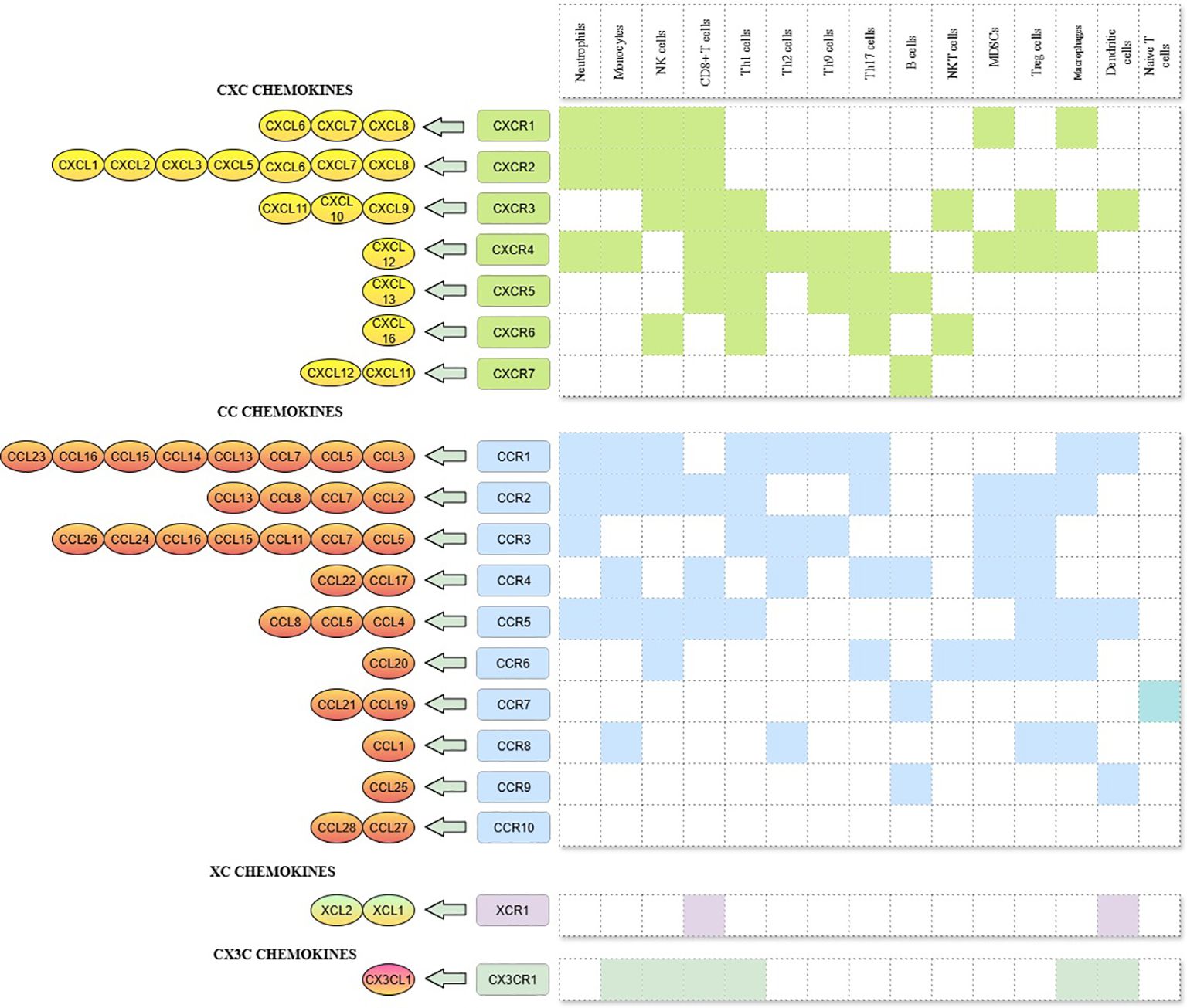
Figure 1. CXC, CC, XC, and CX3C chemokines, its corresponding receptors and immune cells involved in the chemokine-receptor signalling.
Based on the functional role, chemokines can also be categorised into two – those which are generated and secreted constitutively to maintain homeostasis and those which are produced during pro-inflammatory stimulus to raise an immune response (16). Chemokines involved in homeostasis (e.g., CXCL12) constitutively express and mediate the migration of immune cells to the lymphoid organs, blood and peripheral tissues (17). On the other hand, inflammatory chemokines, also known as inducible chemokines, expressed only at the time of pathogenic conditions, enable the trafficking of inflammatory leukocytes to damaged tissues (18). Given the fact that chemokines recruit both pro- and anti-tumourigenic immune cells, inhibiting chemokines that mediate the recruitment of pro-tumoural immune cells and promoting the expression of chemokines which recruit anti-tumoural immune cells could be a better option in therapeutic strategies (19).
3 Role of chemokines in tumour microenvironment
The complex, ever-changing environment that surrounds and interacts with the tumour is called the tumour microenvironment. The cellular and non-cellular components of TME including circulatory system mediates cell survival, local invasion and metastatic dispersion (20). As compared to healthy tissues, the tumour microenvironment exhibits substantially different expression of chemokines and their receptors, facilitating the recruitment of pro- as well as anti- tumoural immune cells (21). A string of them, including, regulatory T cells (Tregs), tumour associated macrophages (TAMs), tumour associated neutrophils (TANs) and myeloid-derived suppressor cells (MDSCs) create an immunosuppressive microenvironment that allows cancer cells to evade immune surveillance and simultaneously thrive on their own (22). Alternatively, CD8+ T cells, CD4+ Th1 cells, NK cells and B cells help in the detection and elimination of tumour cells through immune surveillance and contribute to tumour elimination (23). In this segment, we are attempting to describe the pro-tumoural and anti-tumoural roles of chemokines in the TME.
3.1 Pro-tumoural effect
Chemokines play a crucial role in recruiting immune cells into the TME and shaping it into a pro-tumourigenic state. An aberrant chemokine profile in TME facilitates the infiltration of immune suppressive pro-tumourigenic cells into tumours such as Tregs, MDSCs, TANs and TAMs. Chemokine-mediated immune modulatory pathways are also often altered upon oncogenic transformation. Some of these cells and signalling proteins basically show both pro- and anti-tumourigenic responses. However, when they interact with the tumour progressive-immunosuppressive cells, they will transform into a pro-tumourigenic response (24). Besides immune evasion, chemokines also play a significant role in angiogenesis, metastasis, epithelial-mesenchymal transition, cancer stemness and drug resistance to keep the TME in a pro-tumoural state. Taking one example, CXCL8 and CXCL12 upregulate VEGF leads to the process angiogenesis. Furthermore, some chemokines can also accelerate angiogenesis by recruiting angiogenic factors producing leukocytes such as macrophages to the TME (25). This section elaborates on the key roles of chemokines in shaping a pro-tumoural microenvironment.
3.1.1 Immune evasion and infiltration of immune suppressive cells
The complexity of immune escape mechanisms is the major hindrance to the effectiveness of cancer therapies. The initiation of anti-apoptotic pathways, activation of immune checkpoint pathways and dysfunction and exhaustion of immune systems enhance the process of immune evasion (26). Immune suppressive factors namely, chemokines, cytokines and inflammatory factors expressed by immune cells, promote the tumour immune invasion and metastasis. For instance, Tregs, which abundantly express CCR4, CCR8 and CCR10, were directed to hepatocellular carcinoma (HCC) sites, where their corresponding ligands, CCL22 and CCL28, were present, affecting the treatment outcome by evading the immune system (27). Similarly, the increased recruitment of MDSCs by the expression of CXCL2 inhibits the action of CD8+ T cells and promotes tumour growth in ovarian cancer (28). Tumour associated macrophages is another kind of non-tumour stromal cells, that support tumour growth and immune evasion by secreting factors such as TGF-β, VEGF, FGF-2, and various matrix metalloproteinases (MMPs). Interactions like CCL2-CCR2 axis contribute to the recruitment of macrophages to the tumour milieu. The role of chemokine – chemokine receptor interactions in the recruitment of key immune cells involved in the immune evasion will be reviewed in the following sections.
3.1.1.1 Regulatory T cells
Tregs, a specific subset of CD4+ CD25+ FoxP3+ T lymphocytes, suppress the anti-tumour immune responses and stimulate tumour growth (29). The suppressive mechanisms mediated by Tregs includes, secretion of immunosuppressive cytokines such as IL10, TGFβ, and IL35 (30) and inhibition of dendritic cells’ functions like ability to costimulate naïve T cells by CTLA4 expression (31). The chemotaxis of Tregs to the TME driven by the CCL17/CCL22-CCR4, CCL1-CCR8, CCL28-CCR10, and CXCL9/10/11-CXCR3 axis has also been a widely studied concept among cancer biologists (32). The hypoxic tumour microenvironment upregulates the expression of CCL28 in many tumours and thereby promotes the infiltration of CCR10 expressing Treg cells, which secrete vascular endothelial growth factor A (VEGF-A) and promote angiogenesis and metastasis (33). To explicate the role of CCL28 in the hypoxia-induced Treg recruitment, Ren, Li, et al. blocked the CCL28 interaction by knockdown and anti-CCL28 antibody treatment, resulting in the reversion of hypoxia-induced Treg recruitment (34). CCL17/CCL22-CCR4 axis is also a well-documented pathway which leads to the accumulation of Tregs in tumours in squamous cell carcinoma (35). Blockade of CCL1-CCR8 axis is being explored as a therapeutic option to control the accumulation of CCR8+ Treg cells (36). CXC chemokines also play a vital role in the Treg infiltration. In particular, CXCR3, a key chemokine receptor, takes part in the recruitment of activated Treg cells to human colorectal cancer (CRC) tissues overexpressing their cognate ligand, CXCL11 (21, 37). Interferon γ produced by CD8 + helps to secrete CXCL9 and CXCL10 from the stromal cells and leads to the recruitment of CXCR3+ Treg cells to the TME (38). Additionally, in bone metastasis of prostate cancer, the elevated level of Treg cells with a memory phenotype is observed, along with the presence of functional CXCR4 receptors. The abundance of Tregs in metastasised bone marrow tumours create an immune suppressive tumour microenvironment (39). Thus, the chemokines, as mediators in the influx of regulatory T cells act as a therapeutic target with their pro-tumoural, immune suppressive characteristics.
3.1.1.2 Tumour associated macrophages
Macrophages that infiltrate into tumour tissues or reside within the microenvironment of solid tumours are referred to as tumour associated macrophages (40). Their ability to show different and opposing phenotypes in response to environmental cues is embedded within known “plasticity,” which leads to the polarisation of undifferentiated macrophages (M0) to classically activated macrophages (M1) and alternatively activated macrophages (M2). Even though M1 and M2 populations exhibit anti-tumoural and pro-tumoural behaviour, respectively, TAMs primarily show a pro-tumourigenic M2 phenotype (41, 42). Considering the role of chemokines in TAM recruitment, the inactivation of retinoblastoma (RB), a tumour suppressor gene, enhances the secretion of CCL2, promoting tumour microenvironment by the recruitment of TAMs (43). Similarly, the blockade of CCR2 restored anti-tumour immunity in the preclinical pancreatic ductal adenocarcinoma by decreasing the number of tumour associated macrophages (44). CCL5 neutralisation in the BC cells significantly reduces the cell migration mediated by lactate activated TAMs, further strengthening the role of chemokines in developing a pro-tumoural microenvironment (45). CCL20 attracts more immature myeloid cells and differentiate into TAMs (46). Similarly, CXCL12, secreted by cancer associated fibroblasts, also recruits macrophages to the TME and promote more M2 polarisation (47).
3.1.1.3 Myeloid-derived suppressor cells
Myeloid-derived suppressor cells, a population of immature myeloid cells with immune suppressive functions, imply adverse clinical consequences and poor survival rates in many tumours (48). According to markers and morphology, MDSCs are sub-grouped into neutrophil like polymorphonuclear-MDSCs (PMN-MDSCs) and monocyte like monocytic-MDSCs (M-MDSCs) (49). Chemokines are involved in the MDSCs migration to the tumour microenvironment (50). The overexpression of CCL2 chemokine in tumours like glioblastoma, bladder, prostate, breast, ovarian, gastric, melanoma and renal cell carcinoma facilitates the recruitment of CCR2+ LY6C+ M-MDSCs, causing a higher tumour load and reduced overall survival (49, 51). Similarly, CCL15 secreted from the xenografted orthotopic CRC model recruits CCR1+ MDSCs and leads to aggressive tumour growth (52). The transcription factor SNAIL upregulates the expression of CXCL2, eventually increasing the recruitment of MDSCs into the TME, highlighting the role of CXC chemokines in the MDSCs recruitment (53). Aligned with this, Liu et al. demonstrated that CRIP1 facilitates MDSC trafficking in pancreatic ductal adenocarcinoma by promoting NF-κB/p65 translocation, further establishing the role of molecular drivers in the developing an immunosuppressive tumour microenvironment (54). Post androgen deprivation therapy in prostate cancer shows significant upregulation of certain chemokines such as CXCL8 and CXCL15, which facilitates the recruitment of PMN-MDSC to the tumour microenvironment (55).
3.1.1.4 Tumour associated neutrophils
Neutrophils, a plentiful constituent of leukocytes, traffic their way to the tumour tissue by local expression of chemo attractants main chemokines (56). The plasticity of tumour associated neutrophils helps to polarise neutrophils to anti-tumoural N1 and pro-tumoural N2 populations (57). Despite having differing nomenclature, N 2 TANs and Poly Morpho Nuclear -MDSCs (PMN-MDSCs) are both tumour-supporting, highly immunosuppressive neutrophil subsets that may represent the same or overlapping populations under various investigative frame works (58). The prominent neutrophil receptor, CXCR2, is responsible for the infiltration of neutrophils into tumour milieu, which regulates the involvement of neutrophils in tumourigenesis, angiogenesis and immune suppression (59). Likewise, targeted deletion of CXCR 2 in myeloid cells significantly increased anti-tumour immunity by altering the tumour microenvironment (60). The elevated expression of CXCL5 is also associated with extensive intra-tumoural neutrophil infiltration (61). In the case of many solid tumours, the potential regulatory role of TANs was mediated by the expression of elevated levels of CC chemokine receptors CCR1, CCR2 and CCR5, showcasing the contribution of CC chemokines to the TAN recruitment (12, 57, 61).
3.1.2 Angiogenesis
Angiogenesis is the physiological process that mediates the development of new capillaries from the pre-existing ones through the proliferation and differentiation of endothelial cells (62, 63). Cancer cells induce neovascularisation by chemo-attractants like chemokines, leading to the formation of immature, leaky and tortuous blood vessels (64). The tumour progression with these aberrant blood vessels develops hypoxia within the core and leads to the process “angiogenic switch” by classical regulators such as vascular endothelial growth factor receptors (VEGF-R), platelet derived growth factor receptor (PDGFR), fibroblast growth factor-2 (FGF-2), interleukins and angiopoietin (63). In addition to the nutrient supply, angiogenesis is fundamental in the transition of tumours from a benign to a malignant state and eventually metastasis. Chemokines influence angiogenesis by stimulating VEGF, which in turn activate endothelial cells, which may lead to the production of PDGF, resulting in the secretion of more VEGF, leading to the advancement of this process (65). Among chemokines, the ELR+ motif expressed CXC chemokines such as CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL7 and CXCL8, are correlated with angiogenic effect, whereas ELR- chemokines like CXCL4, CXCL9, CXCL10 and CXCL11 exhibit angiostatic behaviour. In contrast to ELR+ chemokines, CXCL12 (ELR-) exert a strong angiogenic effect on the TME (12). Elevated expression of CXCL8 has been correlated with VEGF expression in BC cell lines, suggesting their crucial role in promoting angiogenesis (66). In detail, CXCL8 significantly supports the survival and proliferation of endothelial cells, facilitating the release of VEGF, subsequently promoting further proliferation and sprouting. Additionally, CXCL8 operates in an autocrine fashion by promoting the expression of MMP2 and MMP9, leading to the extracellular matrix remodelling for the process of neovascularisation in BC (67). Moreover, CXCL8 generated by endothelial cells interacts with its primary receptor CXCR2 and transactivates VEGFR2 via Src kinase-mediated receptor phosphorylation and contributes to endothelial cell permeability (68). CXCL1 plays a significant role in tumour progression and is responsible for angiogenesis by directly acting on endothelial cells in breast cancer (69). CXCL6 is primarily secreted by fibroblasts and endothelial cells and acts as a growth factor that modulates the proliferation of both endothelial cells and tumour cells, resulting in angiogenesis. Along with CXCL6, CXCL2 also exhibits the same trend in the intratumoral space, where it recruits granulocytes, thereby enhancing cancer cell survival and angiogenesis by releasing additional chemokines (2). CXCL5 has been reported to enhance angiogenesis in human non-small cell lung cancer (NSCLC) by a cyclooxygenase (COX-2)- dependent mechanism (67). Interestingly, it has been noted that IL-8 plays a more significant role than VEGF-A in endothelial cell migration. Targeted blockade of IL-8 resulted in reduced HUVEC migration even in the presence of VEGF-A, which shows the VEGF-independent angiogenic activity of IL-8 (70).
Other than CXC chemokines, several CC chemokines are also involved in the stimulation of pathological neo vascularisation, including CCL1, CCL2, CCL5, CCL11, CCL15 and CCL16. The promotion of angiogenesis by CC chemokines is mediated indirectly by employing macrophages at the site, which recruits additional pro-angiogenic chemokines and growth factors (71). CCL18, the most abundant and specific chemokine released from TAMs, plays a crucial role in the recruitment of immune cells towards tumour sites, which is essential for tumour angiogenesis and endothelial cell survival. The elevated levels are linked to a poor prognosis in BC, making CCL18 targeted therapy, a promising approach for patients resistant to anti-VEGF therapies (72). Studies suggest that when tumour cells express CCL21, an anti-tumourigenic effect occurs through the inhibition of angiogenesis and increased recruitment of CD8+ T cells and dendritic cells. Nevertheless, the immune response may be suboptimal due to the insufficient dendritic cell maturation (73).
3.1.3 Epithelial-mesenchymal transition
A transient, reversible cellular reprogramming from an epithelial to a mesenchymal state helps in enhancing invasiveness and promoting metastasis, which is known as epithelial-mesenchymal transition (EMT) (74). Generally, the process in which the EMTs are involved is the normal embryonic development, tissue repair and wound healing. However, it is also correlated with tumourigenesis, metastasis, stemness and drug resistance (75). Cohesively arranged single layer of epithelial cells with apical-basal polarity switches to morphologically irregular, elongated, spindle-like cells with front-to-back polarity lacking cellular adhesion molecules in the process of EMT (76). Since the pathological reactivation of EMT is fundamental for tumour prognosis and metastasis, it must be tightly regulated with certain EMT-transcription factors (EMT-TFs) such as SNAIL, TWIST and ZEB (77).
Extensive research has demonstrated the oncogenic potential of chemokines to induce EMT and promote metastasis. For example, the CXCL12-CXCR4 axis mediates intracellular actin polymerisation and pseudopodia formation, thus playing a key role in EMT and cancer progression (78). Flavokawain A, a chalcone compound isolated from Chloranthus henryi, functionally inactivates PI3K/Akt/HIF-1α signalling pathways mediated by CXCL12 and thereby reducing the expression of a well-known EMT transcription factor, TWIST, in HCC (79). When human gastric carcinoma cells were simulated with CXCL12, their morphology changes from elongated to spindle-like fibroblast, along with decreased expression of E-cadherin and increased expression of vimentin, clearly demonstrating the relevance of the CXCL12-CXCR4 signalling axis in the process of EMT (80). CC chemokines such as CCL1, CCL2, CCL3, CCL21 and CCL25 are also associated with invasion and migration in many tumours. Upregulation of CCL8 in oesophageal squamous cell carcinoma activates the oncogenic pathway NF-κB to induce EMT (81). Also, stimulation of CCL21 in oral squamous carcinoma cells shows a reduction in E-cadherin and increase in vimentin, N-cadherin designating EMT transition (82).
3.1.4 Metastasis
Metastasis is responsible for approximately 90% of cancer-related deaths, making it the leading cause of cancer mortality. It is a process by which the primary tumour smartly moves from its primary site to the surroundings and distant organs by intravasation, extravasation and localisation (83). Tumour cells alone are feeble for the development of metastasis; rather, it is executed by multiple components of the tumour niche as well as complicated cross talks between stromal and tumour cells, which further release certain chemokines and cytokines (40). For instance, mesenchymal stem cells, lymphatic endothelial cells, cancer associated fibroblasts (CAFs), MDSCs, T cells and TAMs, along with tumour cells, aid the process of cancer metastasis (84). Induction of hypoxia activates various downstream signal cascades to promote hallmarks of tumours, including metastasis (85). CAFs recruit granulocytes to the TME by producing colony stimulating factor-1 (CSF-1) that promotes tumour growth and metastasis (86, 87). Previous investigations have demonstrated that the CXC chemokine family plays a vital role in promoting tumour growth and survival, and exhibits marked metastatic potential (67). CXC chemokines are well known for their pro-tumoural activities. Among them, CXCL12-CXCR4 is one of the most researched chemokine-receptor axes considering metastatic processes (67). Giving an example, the expression level of CXCL12 in the CAFs isolated from secondary brain tumours of BC is significantly higher than in the healthier fibroblasts and CAFs from primary breast tumours (88). CXCL8-CXCR1/2 is another CXC chemokine axis in mice, activates the gene matrix metalloproteinase and positively corelates with earlier distant metastasis in BC to the bone (89). Besides, the blockade of CXCR2, a high affinity receptor for CXCL8, reduced the chance of lung metastasis by 40% in a mammalian BC model (90). CXCR12 guides premetastatic tumour cells expressing CXCR4 to intravasate into circulation through the tumour microenvironment of metastasis (TMEM). TMEM is a CXCL12-rich, immunosuppressive environment where the CD8+ T cell population is exhausted, despite being present in high numbers, resulting in the homing of metastatic cells around TMEM gateways (91). CXCR4/CXCL12 signalling is involved in lung carcinoma metastasis, where CXCR4 is a chemokine that is universally upregulated in tumour cells which migrate to the bone (8, 9). Nevertheless, interleukin-8, also known as CXCL8, binds to the receptors CXCR1 and CXCR2 has been shown to mediate tumour progression, epithelial-mesenchymal transition, invasion, and angiogenesis. Neutralizing IL-8 signalling with antibodies resulted in reduced bone metastasis in mice bearing MDA-MET breast cancer cells (a bone homing derivative of MDA-MB-231) (92). Apart from the CXC chemokine family, the CC family also contributes to tumour progression and metastasis. Chemokines such as CCR7, CCR9, and CCR10, have been identified as having the potential to support the metastatic seeding of cancer cells (93). Overexpression of the CC chemokine CCL2 resulted in elevated bone metastasis in MDA-MB-231 via stimulating osteoclastogenesis. Blockade of the CCL2 chemokine using a neutralizing antibody reduced bone metastasis in the MDA-MB-231 cell line (94).
3.1.5 Hypoxia
In primary tumours, structurally and functionally abnormal blood vessels fail to deliver adequate oxygen, resulting in hypoxia (95). As stated by clinical studies, the measured oxygen levels in BC, cervical cancer and head and neck cancer are very less than that of normal tissues. Immunohistochemical staining of tumour biopsies manifests the increased HIF-1α protein expression, which positively mediates the expression of VEGF, Stromal cell-derived factor-1 (SDF-1) and stem cell factor and thereby induces the invasion and metastasis (96). In addition to the previously discussed roles of chemokines in cancer cell migration, invasion, and metastasis, this section examines the correlation between chemokines and hypoxia within the tumour microenvironment (TME). The chemokine axis CXCL8-CXCR1 involved in early inflammatory responses plays an important role in the migratory activity of different human breast cancer cells under hypoxic conditions. For example, IL1-β treatment under hypoxia results in the upregulation of CXCR1 by the transcriptional regulation of HIF-1α and enhances the migratory effects on BC (97). Hypoxia triggers CXCL12 expression in many tumours by the HIF-1 dependent expression of CXCL12 synthesis (39, 98). Another chemokine, CCL28 (mucosae associated epithelial chemokine) is also upregulated by hypoxia and HIF-1α in human ovarian cancer cell lines (99). Hypoxia induces the activation of CCL3 in multiple melanoma patient’s serum. These patients are also associated with worse prognosis in large B cell lymphoma (52).
3.1.6 Cancer stemness
Cancer stem cells (CSCs) are considered to be one of the primary contributors of tumourigenesis, metastasis, drug resistance to conventional therapy and tumour recurrence. CSCs are undifferentiated populations such as tumour initiating cells (TICs) or tumour propagating cells (TPCs), characterised by self- renewing, multi potent and tumour initiating properties. They are tightly maintained by TME (100, 101). Al-Hajj et al. had first reported about the importance of tumour initiating CSCs in BC. This is also known as the first identification of CSC population in solid tumours (102). Identification of cell surface markers helped the scientists in controlling the wild behaviour of CSCs when chemotherapeutic strategies failed in eradicating them (103). In addition to surface marker targeted therapies, manipulation of TME to destroy CSCs is regarded as a powerful treatment method. There are a number of cytokines and chemokines which play a prime role in the modulation of CSCs. Hence, upon targeting CXCL12-CXCR4 axis by a CXCR4 antagonist – Plerixafor, disrupt homing of leukaemia stem cell (LSC) to the bone marrow niche (104). The researchers found that evasion of primary tumours and establishment of distant metastasis in Pancreatic ductal adenocarcinoma were caused by pancreatic CSCs, co-expressed with CXCR4 chemokine receptors. These CXCR4+ CSCs chemoattracted to the highly metastatic sites express high levels of their ligand, CXCL12, which stimulate the small GTPase called, RhoA, vital for the extra cellular matrix remodelling and leading to metastasis (105). Additionally, in the glioma stem cells (GSCs), the stem cell properties are maintained by the induction of CXCL1 and CCL2 chemokines (106).
3.1.7 Drug resistance
Pharmacotherapy is recognised as one of the predominant clinical treatment strategies; however, the development of drug resistance which is an intricate process significantly undermines its effectiveness and contributes to disease progression (107). Tamoxifen, fulvestrants and aromatase inhibitors are some of the approved hormonal therapeutic agents used for the treatment of ER+ BCs; correspondingly, trastuzumab, lapatinib and pertuzumab are potential HER2 targeted agents (108). Several chemokines were significantly upregulated in drug resistant cells or patient samples than drug sensitive group when done in vitro, in vivo and clinical studies. The major chemokines involving in generating chemoresistance are CXCL8, CCL2, CCL5 and CCL20. They execute chemoresistance by the recruitment of immune cells, activation of the survival/proliferation pathways and promotion of invasion (109). In particular, TAM mediated CCL2 secretion promotes drug resistance and reduces apoptosis in BC cells through the activation of PI3K/Akt/mTOR pathway (110). By keeping a close watch on macrophages grown in the conditioned medium retrieved from tamoxifen-sensitive and resistant BC cells, Li, Dongbo, et al. observed that M2 polarisation due to TAM mediated CCL2 secretion was more apparent (110). Furthermore, CCL2-CCR2 axis recruit monocytes to TME and stimulate the release of pro-inflammatory cytokines such as IL1, IL6 and Tumour Necrosis Factor-α (TNF-α). Taken together, the transition of monocytes to TAM and secretion of pro-inflammatory cytokines activate PI3K/Akt/mTOR signalling pathways and mediate endocrine resistance in BC (41). CCL2 also exerts resistance to anti PD-L1 immunotherapy by the activation of PI3K/AKT and NF-κB signalling (111). In contrast, AKT signalling also promotes the differentiation of naïve CD4 T cells into T helper 1 (Th1), Th2, and Th17 cells and inhibits the transcription of Foxp3, a crucial transcription factor required for the development of T regulatory cells (Tregs). These scenarios highlight the dual role of AKT to modulate T cells in different ways in cancer immunotherapy (112). Another chemokine signalling axis, CCL15 -CCR1 also activates NF-κB pathway by recruiting monocytes, eosinophils and neutrophils to the tumour site ultimately inducing drug resistance (52).
In conclusion, chemokines play a pivotal role in shaping the tumour microenvironment by critically regulating a wide range of processes such as immune evasion, modulation of immune cell infiltration, induction of epithelial-mesenchymal transition (EMT) and hypoxia. These functions significantly contribute to tumour progression, metastasis, angiogenesis, stemcellness and the development of drug resistance. The dual role of chemokines makes them more complex and is considered a potential strategic therapeutic target in cancer biology. Moreover, understanding the nuanced behaviour of pro-tumoural chemokines could encourage the possibility of effective therapies aimed at overcoming resistance to current treatment modalities (Figure 2).
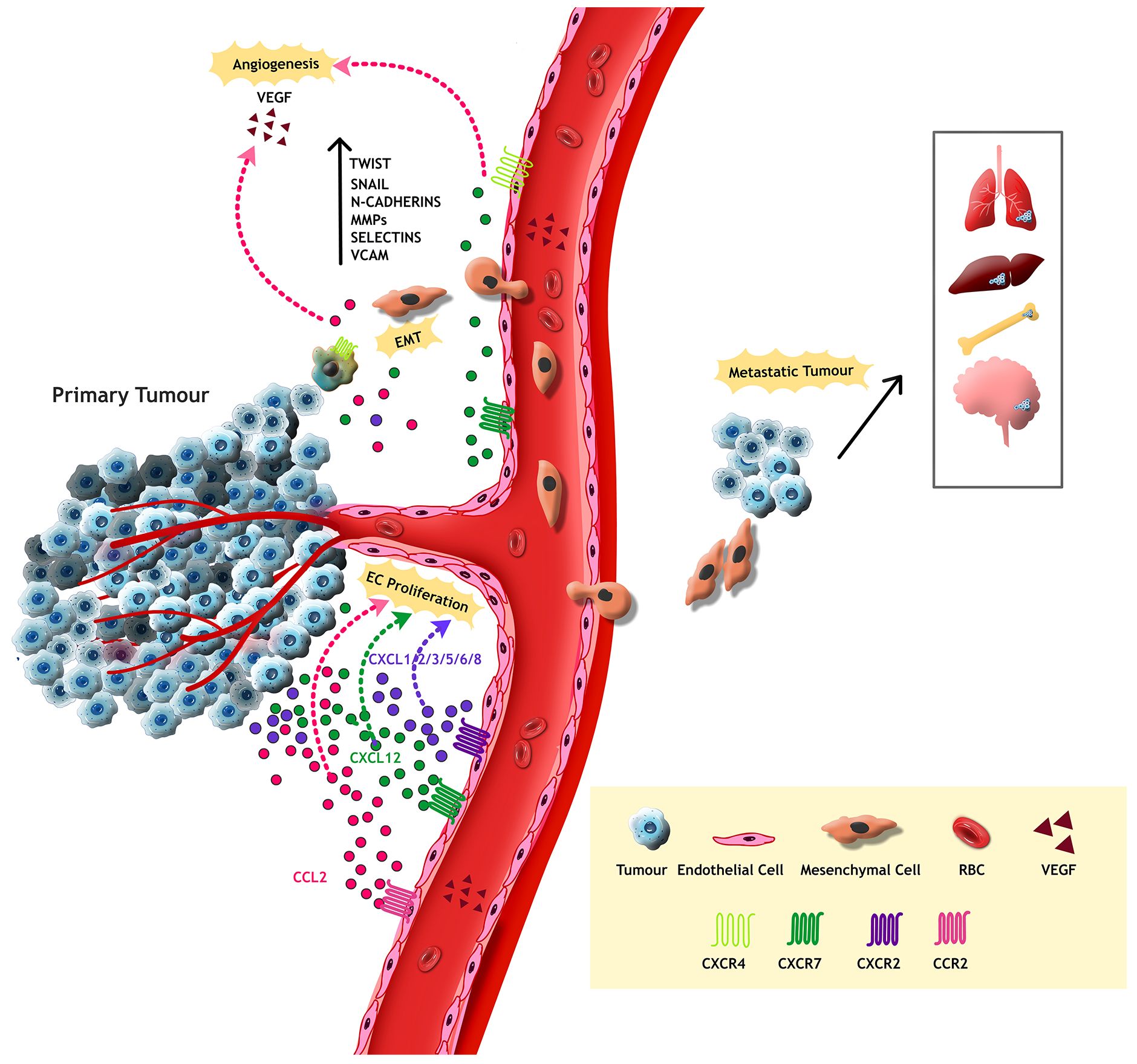
Figure 2. Tumour cells from the primary tumour attains mesenchymal properties through Epithelial Mesenchymal Transition, resulting in metastasis to adjacent or distant organs. Transcription factors such as Twist, Snail, MMPs and cell adhesion proteins like N-Cadherins, Selectins and VCAM assist this process. Moreover, the tumour supporting chemokines activate VEGF, thereby mediate endothelial cell proliferation and abnormal metastasis in the TME.
3.2 Anti-tumoural effects
The infiltration of anti-tumoural immune cells into the TME mediate the suppression of tumour growth and proliferation. CD4+ T cells, CD8+ T cells, B cells and NK cells are the fundamental cells that activate anti-tumoural immunity (113). Apart from immune cell infiltration, the modulation of cancerous niche by polarisation of neutrophils and macrophages to their anti-tumoural phenotype also facilitate the reduction of tumour growth and proliferation (114). When chemokines bind to their cognate receptors on targeted cells, they trigger cellular signalling pathways to recruit and organise pro- as well as anti-tumour immune cells (115). The ability of certain chemokines in mediating anti-tumoural activity would be appreciable in developing novel, targeted therapeutic strategies (20). The anti-neoplastic nature of chemokines will be emphasised in the following segment.
3.2.1 Chemokines in the modulation of anti-tumour microenvironment
Understanding the mechanism involved in the localisation and effects of anti-tumourigenic chemokines within the TME would be a great contribution to the field of immunotherapy (19). Cancer cells functionally sculpt their microenvironment by recruiting immune cells through the expression of various cytokines and chemokines (103). Tumours with the elevated CD8+ T cell infiltration and high tumour clearance capacity coupled with escalated pro-inflammatory cytokines can suppress the growth of tumours are referred to as immunologically “hot tumours” (116). Interestingly, chemokines such as CXCL9, CXCL10 and CXCL13 are involved in fostering the hot tumour milieu (117). Notably, CXCL9-CXCR3 axis stimulates the induction of apoptosis via the recruitment of CD8+ T cells, which produce granzyme B and perforin (118). Here, an attempt has been made to describe how the infiltration and polarisation of various immune cells modify TME.
3.2.1.1 CD8+ cytotoxic T lymphocytes
Activated CD8+ T cells are the mainstay of anti-tumour immunity, resulting in effective tumour immunotherapy. Cytotoxic T Lymphocytes (CTLs), a short-lived effector cell differentiated from the naïve CD8+ T cells, aim at killing potentially harmful intruders in the body like viruses, foreign antigens as well as tumour cells by apoptosis through the secretion of perforins and granzymes (119). In this section, we are exploring the significance of chemokines in the recruitment of CTLs in creating an anti-tumoural microenvironment. Intratumoural expression of anti-tumoural chemokines such as CXCL9, CXCL10 and CCL5 were reported in patients undergoing chemotherapy for multiple cancer types such as melanoma, non-small cell lung cancer (NSCLC) and BC (20). Higher levels of CXCL10 in NSCLC is associated with better prognosis by the recruitment of CXCR3+ CD8+ T cells to the tumour sites (120). Another study illustrated the involvement of CXCL9 in the recruitment of CD8+ T cells to the TME of melanoma mouse model providing effective anti-tumoural response (121). CXCR6 expressed on cytotoxic T cells helps in the positioning of CD8+ T cells crucial for the effective anti-tumour responses. CXCL16- CXCR6 deficiency impairs the efficacy of cancer vaccines by reducing CD8+ T cell resident memory cells (122). CC chemokines such as CCL3 and CCL4 secreted by CD8+ T cells helps to make a positive feedback loops to enhance recruitment and activation of T cells (123). Another chemokine- chemokine receptor axis involved in the anti-tumoral activity of CD8+ T cells is CCL9/CCL21-CCR7. These chemokine axis signalling helps to organise and maintain CD8+ T cell pool in the TME (124).
3.2.1.2 CD4+ T cells
CD4+ T cells play an indispensable role in anti-tumour immune responses, making them particularly significant in tumour immunology and immunotherapy. Several cytokines, specifically chemokines streamline the differentiation of CD4+ T cells to phenotypically distinct anti-tumoural subsets like Th1, Th17 and T follicular helper(Tfh)cells (125, 126). CXCR3, a common receptor for CXCL9 and CXCL10 recruits Th1 cells to the TME, suppressing tumour growth. Equally, upregulated expression of the receptor facilitates differentiation of naïve CD4+ T cells to anti-tumoural Th1 cells (127). Another fascinating study on the role of chemokines in recruiting antigen presenting cells revealed that CCL9/CCL21-CCR7 axis enables the entry of naïve CD4+ T cells into the tumour-draining lymph node (TDLNs), while CCL5-CCR5 facilitates their interactions with DCs (20). Another CC chemokines such as CCL19 and CCL21 facilitates CCR7+ CD4+ T cell recruitment and facilitates T cell priming in the tertiary lymphoid structure (128).
3.2.1.3 Macrophages
Among the total leukocyte tumour infiltrate, tumour associated macrophages are the most abundant population. The M1 subpopulation of TAMs promotes pro-inflammatory and anti-tumour immune responses, supported by the expression of TNF-α, IFN-γ, and iNOS (129). M2 population promotes tumour growth, metastasis and poor prognosis. Growing evidence suggests that, rather than focussing on the depletion of TAMs in TME, the ideal therapeutic strategy is to promote the repolarisation of M2 phenotype to M1, which is anti-tumoural phenotype (130). In an independent study by Ruytinx, Pieter, et al., it is demonstrated that the blockade of CCR5 induces the repolarisation of M2 phenotype to anti-tumoural M1 via the increased level of STAT-3 transcription factor (131). Similarly, another chemokine CCL5 also promotes M1 polarisation through the activation of oncogenic signalling pathways such as MAPK and NF-κB. The study was confirmed by downregulating CCR1/CCR5 receptors, reversing the action, underlining the role of CCL5 in anti-tumoural M1 macrophage polarisation (132).
3.2.1.4 Neutrophils
Polymorphonuclear leukocytes/neutrophils are the first immune cells reaching at the sites of inflammation. As discussed earlier, neutrophils exist in anti-tumoural N1 and pro-tumoural N2 phenotypes according to the presence of surface markers and the role in regulation of tumour and inflammation. Here in this section, we are scrutinising intricate processes of TAN polarisation influenced by chemokines signalling pathways (133). When evaluating the response to immunotherapy in HCC patient samples, it was identified that the upregulation of CCL21 and associated TAN-N1 polarisation (increased expression of N1 neutrophil markers such as FAS, NOS2 and TNF-α) are positively correlated with better prognosis (134). Suppression of pro-tumourigenic effects by inhibiting the recruitment of neutrophils is another possible strategy to modulate TME. In addition, overexpression of CXCR2, a key chemokine receptor in many tumour is linked to poor prognosis. However, the inhibition of CXCR2 signals promote polarisation of N1 anti-tumoural phenotype thereby providing better prognosis and overall survival (135). Inhibition of CXCR1/2 using SX-682 decreased infiltration of MDSCs and subsequently enhanced NK Cell-based immunotherapy in head and neck tumour models (136). In line with this, Kwong et al. found that targeting the IL-8/CXCR2 axis in myeloid cells helped to overcome the resistance in hepatocellular carcinoma towards immunotherapy by reprogramming the tumour microenvironment (137). Numerous studies revealed that chemokines such as CXCL1, CXCL2, IL-8 (CXCL8), CXCL5, CXCL12, CCL2, and MIP-1α (CCL3) promote the infiltration of neutrophil to the tumoural microenvironment (61).
3.2.2 Enhancement of immune check point inhibitor efficacy
Immune checkpoint pathways typically activate to prevent autoimmunity by inducing T cell anergy and exhaustion. Tumour cells hijack these checkpoint pathways to evade immune destruction by inhibiting the activity of activated T cells within the tumour microenvironment (113). Nevertheless, immune checkpoint inhibitors (ICI) reinvigorate the anti-tumour immune responses by blocking checkpoint inhibitory proteins and there by activating T cells in the TME. The advancement of therapies with ICIs are revolutionary milestones in the field of oncologic care (138). Carefully targeting the complex intercellular signalling of chemokine system combined with ICI monotherapy promises a better progression free survival and clinical improvements (139). The anti-tumour immune interventions are the result of combinatorial treatment of chemokines along with ICIs, which are achieved by escalated infiltration of T cells and inhibitions on their check points. For example, blockade of CXCL12-CXCR4 signalling along with anti-PD1 monotherapy significantly augments the anti-tumour effects. This is achieved by increasing the intratumoural CD8+ T cell infiltration which simultaneously inhibits the infiltration of Tregs and MDSCs (140). Recent studies on anti-PD1 monotherapy have demonstrated that CXCL9 and CXCL10 were crucial, since its receptor CXCR3 was found to be the arbitrator for the PD-1+ CD8+ T cells infiltration in B16 murine melanoma tumours (141). Similarly, another group studied on multimodal immunotherapy approach combining CXCR1/CXCR2 inhibition, neutralisation of TGF-β and blockade of PD-L1 signalling in Triple Negative Breast Cancer (TNBC). They observed a significantly higher distribution of CD8+ and CD4+ Tumour-Infiltrating Lymphocytes (TILs), which exhibit a small fraction of infiltrating CD4+ Foxp3+ Tregs indicating clinical effectiveness (142). Another ICI protein, anti-Cytotoxic T Lymphocyte associated Antigen-4 (CTLA-4) induces a stronger memory response than anti-PD-1 in tumour models (143). PD-1 and CTLA-4 inhibitory proteins are highly expressed in immune suppressive Tregs and MDSCs. The CXCL12-CXCR4 chemokine axis recruits these cells, contributing to immune suppression and resistance to immune checkpoint blockade (ICB) therapies in colon cancer. The chemokine axis activates downstream signalling pathways such as PI3K/AKT and MAPK/ERK, directing the migration of colon cancer cells to distant organs like liver and lungs. Furthermore, the blockade of CXCL12-CXCR4 axis along with anti-PD-1 and anti-CTLA-4 ICB therapies promises anti-tumour responses in colon cancer, emphasising the importance of combination therapies (144). The exclusion of T cells from tumours and deactivation of pre-existing T cells, characteristic of “immunologically cold” tumours is a limiting factor for effective ICI therapies in many malignancies. These immunologically cold tumours, with complete or hardly detectable presence of lymphocytes act as a major hindrance to anti-PD-L1 monoclonal therapies (145). A study in intrahepatic cholangiocarcinoma (ICC), found that accumulation of Tregs in the TME sequestered the activated CD8+ T cells and T helper cells at the tumour margin. These poorly perfused and hypoxic microenvironments make ICC tumours more immunologically cold. Nevertheless, chemotherapeutic agents like gemcitabine and cisplatin, combined with ICB treatment elevate the recruitment and activation of intratumoural CXCR3+ CD8+ T cells (146).
Collectively, chemokine mediated modulation of TME with the surplus of infiltered anti-tumoural immune cells give rise to a better immune recognition and immune response in many tumours by various cytotoxic activities thereby enhancing immune therapeutic efficacy (Figure 3).
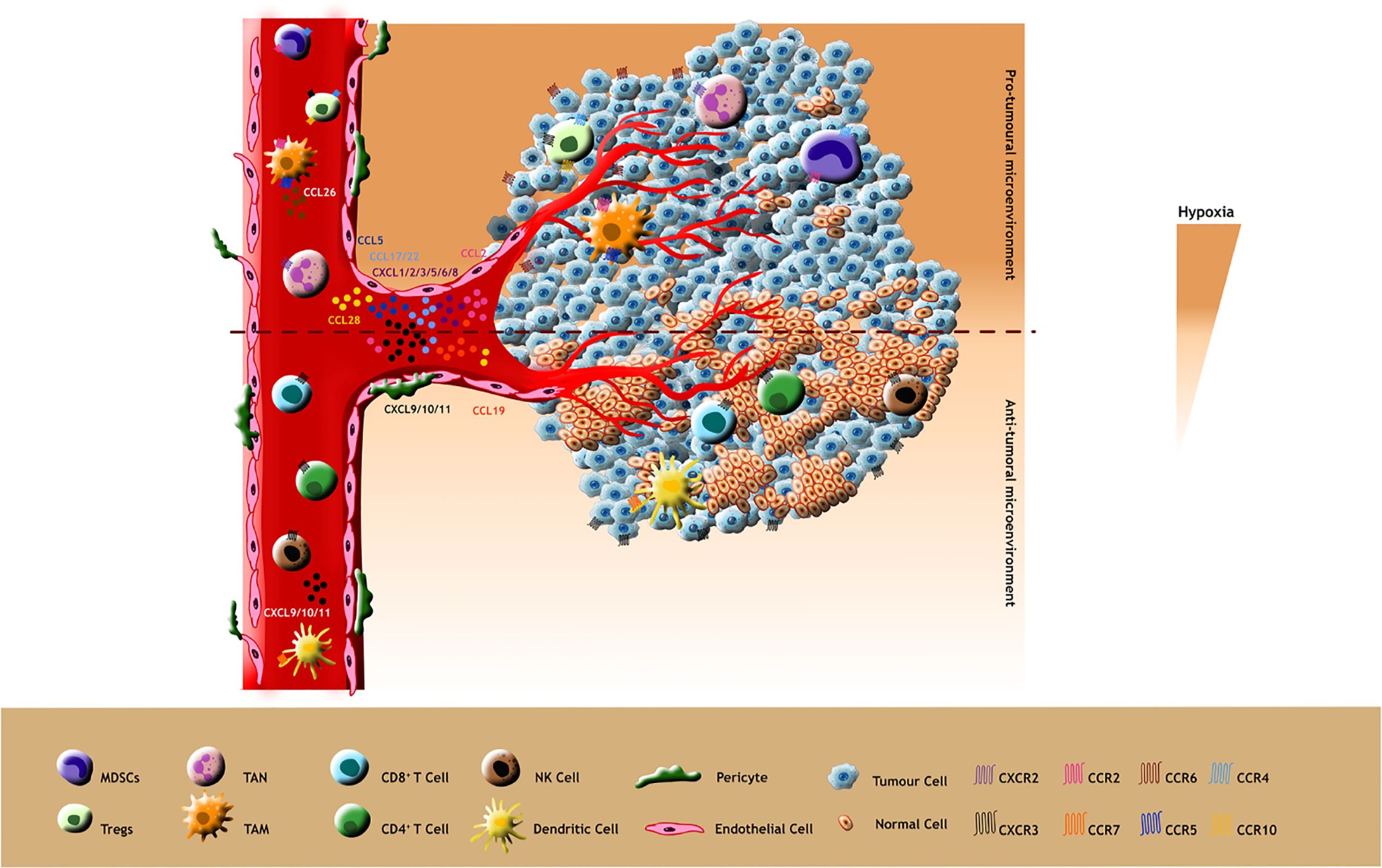
Figure 3. Pro-tumour effects: Pro-tumoural chemokines such as CCL5, CCL2, CCL28, CCL17/22 and CXCL1/2/3/5/6/8 released by tumour cells attract tumour promoting immune cells expressing their cognate receptors CCR5, CCR2, CCR10, CCR4 and CXCR2 respectively (Ligands and their receptors depicted as identical colours in the figure). Additionally, these immunosuppressive cells such as TAMs release pro-tumoral ligands like CCL26 and bind to their receptors in tumour cells which in turn stimulate the hypoxic TME for tumour progression. Anti-tumour effects: The less hypoxic TME promotes the anti-tumoral behaviour of chemokines making them attract immunostimulatory cells such as CD8+, CD4+, DC and NK cells. These immune supportive cells also release anti-tumoral chemokines aiding their infiltration into the TME.
4 Regulation of chemokine expression in tumour microenvironment
Chemokines play a pivotal role in the elicitation of immune responses by precisely arranging leukocytes in lymphoid organs and to the sites of inflammation. These tiny yet mighty bioactive molecules influence tumour growth via multiple mechanisms either by promoting or suppressing tumour growth (147). Chemokines often recruit pro-tumourigenic immune cells such as MDSCs, TAMs, TANs and regulatory T cells etc. to stimulate the growth and proliferation of tumour cells, while recruitment of CD4+ T cells, CD8+ T cells and NK cells inhibit tumour growth, invasion and metastasis. Due to the dual hatted characteristics of chemokine molecules, their expression in the TME must be tightly regulated to prevent excessive tissue inflammation, immune cell exhaustion and damage during immune responses (148). These regulation processes include modulation of chemokine mRNA stability, sequestration of chemokines by Atypical Chemokine Receptors (ACKRs) and post-translational modifications (149). The timely downregulation of chemokine mRNA is governed by a specific mRNA instability element, adenine - and uridine rich elements (AREs) located in the 3’ UTR. Upon recognising these AREs by several RNA - binding proteins (RBPs), mRNA will undergo degradation through various mechanisms like de-adenylation, decapping and exonucleolytic decay. For example, RPL22 - an RNA binding protein, execute the degradation of CCL2 chemokine, making its expression in a controlled manner (150). Similarly, the degradation of CXCL1 mRNA is overruled by the pro-inflammatory cytokine, IL-17 signalling (151).
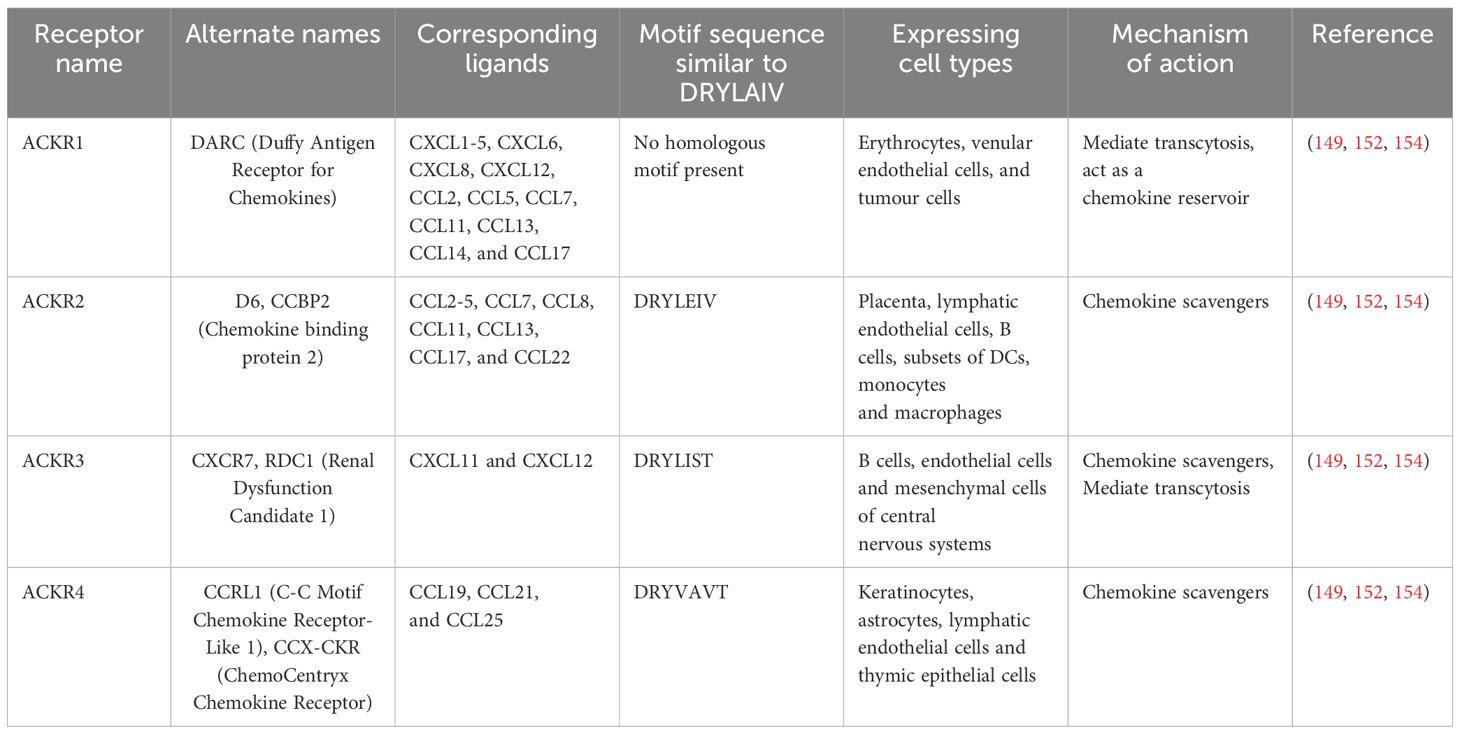
Atypical chemokine receptors (ACKRs), a unique subclass, functions to fine tune inflammation, immune cell migration and tissue homeostasis. The sub family includes ACKR1, ACKR2, ACKR3 and ACKR4, which are mainly expressed on non-lymphocytes but a small amount is detected in lymphocytes (152). Classic chemokine receptors switch on the downstream signalling pathways through DRYLAIF motif present in the GPCR. In contrast, ACKRs are able to regulate bioavailability of chemokines by its altered specific motifs (DRYLEIV, DRYLIST and DRYVAVT) through β-arrestin mediated non-canonical signalling pathways (153). The inability to promote cell migration along with their ability to scavenge and transport chemokines, makes them a true check point for chemokine expression (154). For instance, in NSCLCs, the tumour cells over expressing ACKR1 internalises and immobilise the bound ligands CXCL5 and CXCL8 thereby restricting the downstream signalling for angiogenesis and metastasis (155). ACKRs internalise and transport chemokines to degradative compartment, thereby controlling their concentration and bioavailability. Regulation by ACKR2, as a chemokine decoy or scavenger receptor, boosts the clearance of inflammatory CC chemokines. Inactivation of these receptors restore the secretion of inflammatory chemokines such as CCL2–5 and CCL12 in oral squamous cell carcinoma (156). In B6/129 ACKR2-deficient mice, treatment with tetradecanoyl phorbol acetate/dimethylbenz(a) anthracene (TPA, a tumour promoting agent for cutaneous malignancy/DMBA) caused elevated levels of CCL3, leading to inflammation and recruitment of polymorphonuclear cells (PMNs) and CD3+ T cells into papillomas. This increased inflammatory response promoted papilloma formation and made ACKR2-deficient mice more susceptible to keratinocytes proliferation. However, transgenic expression of ACKR2 in keratinocytes reduced inflammation and lowered the risk of skin tumours. These finding suggests that ACKR2 helps suppress the skin cancer by reducing chronic inflammation, likely through scavenging of CCL3 (156). The different subclasses of ACKRs, their ligands, cells expressing the receptors, motifs and roles in chemokine regulation are depicted in the Table 1.
Another mechanism in the regulation of chemokines include post-translational modifications like citrullination, nitration/nitrosylation, cleavage by the dipeptidyl peptidase- CD26 and other proteases (157). Citrullination of CXCL10 by Peptidyl Arginine Deiminase (PAD) reduces the chemotactic activity of T lymphocytes helping tumours evade immune surveillance (158). The CXCR3 receptor and its ligands were truncated by CD26 (a serine protease) in some cancers resulting in malignant progression. On the other hand, the inhibition of CD26 enhances anti-tumoural lymphocyte response and effectiveness of immunotherapy (159).
The altered metabolism of tumour cells also has a role in the regulation of chemokines. The acidic pH generated by the Warburg effect enhances the expression of CXCL8 in human ovarian cancer cells (160). Lactic acid produced during aerobic glycolysis activates NF-κB signalling, which in turn induces CXCL8 expression in breast and colon cancers (39). The hormonal regulation of chemokine is another interesting process. For instance, a study in BC showed a positive regulation of CXCL12 when treated with oestrogen, while the effects were reverted when re-treated with anti-oestrogen antibodies (161). Similarly, oestrogen-induced CXCR4 protein levels in HER2- and ER- positive breast cancer lead to the enhanced cell migration (162). In prostate cancer, androgen hormone induces the activity of transcriptional regulator, ERG, which increases the expression of CXCR4 thereby enhancing the pro-tumoural effects of CXCL12-CXCR4 signalling pathway (153). Understanding mechanisms behind the regulation of chemokine expression might be a great tool in immune surveillance and controlling tumour growth.
5 Chemokines and immunotherapy
US Food and Drug Administration (FDA) approved the first targeted therapeutic drug, trastuzumab, an anti-HER2 monoclonal antibody for the treatment of HER2 positive BC in 1998. This was followed by the approval of tyrosine kinase inhibitor, imatinib for Philadelphia chromosome positive chronic myelogenous leukaemia in 2001 (163). To date, a variety of molecularly targeted therapeutic agents have been clinically utilised for cancer treatment (164). TME contain many factors that are known to induce tumour growth. Targeting factors like VEGF, VEFGR, Transforming growth factor-β and various immune cells have proven their clinical efficacy in the field of cancer biology (165). In 2018, the Nobel prize in physiology and medicine was awarded for groundbreaking advancement in cancer therapy, specifically the revolutionary approach of inhibiting negative immune regulation. This recognition highlighted the significance of immunotherapy in leveraging the body’s immune system to combat cancer. James P. Allison and Tasuku Honjo were instrumental in chiselling out a transformative era in this field by emphasising its potential in immunotherapy which can act as a powerful tool in the fight against various forms of cancer (166). Analysis of current data demonstrates that chemokines and their receptors can have anti- or pro-immunogenic role depending upon the immune context on TME. Neutralisation or manipulation of chemokine gradient and its expression in tumour niche provide a promising approach for the treatment of diverse types of cancers (115). This section will delve into how the regulation of chemokine expression plays a crucial role in shaping the effectiveness of cancer immunotherapy.
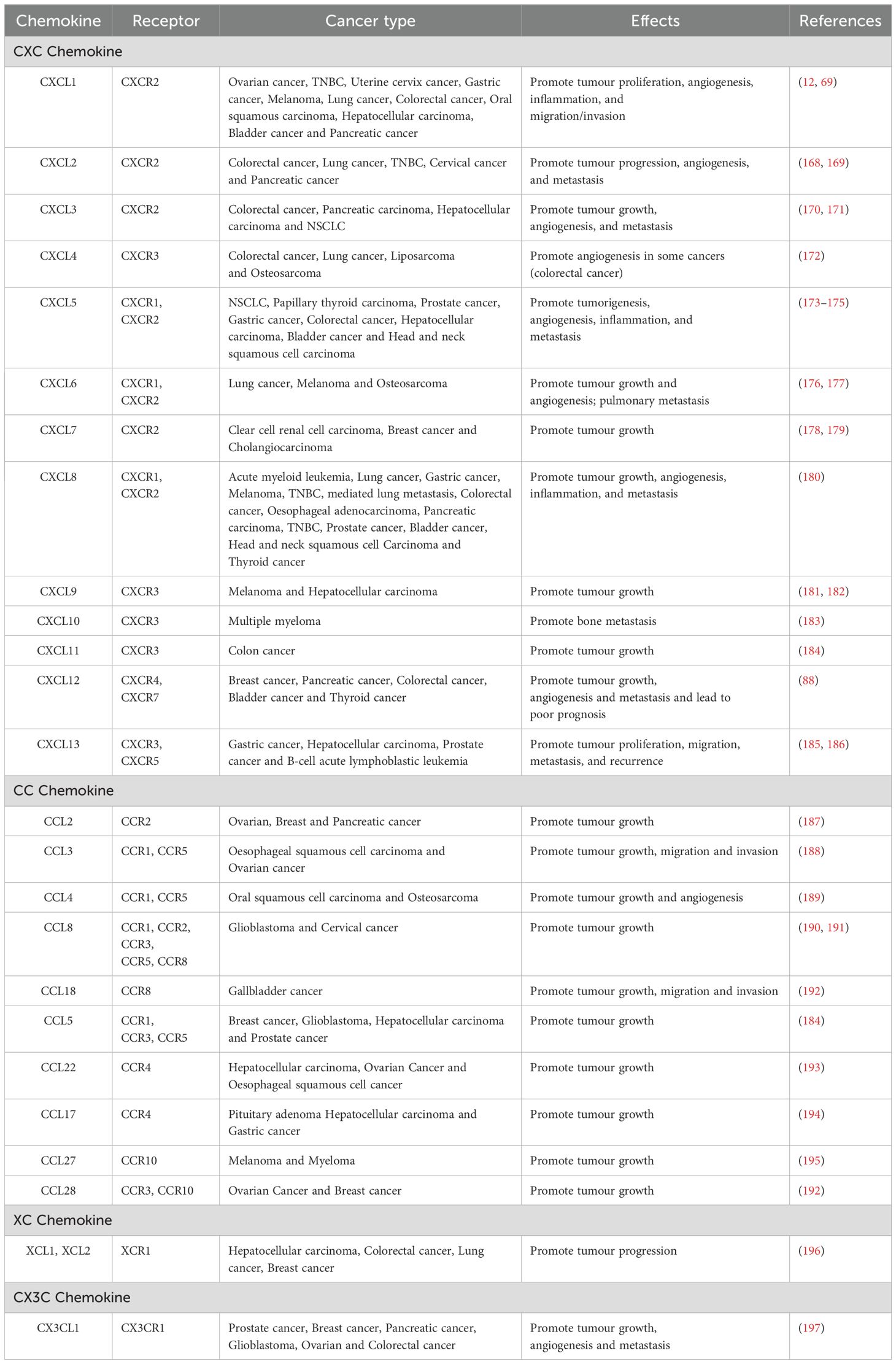
We know that migration and positioning of both pro- and anti-tumour immune cells directly modulate tumour growth by chemokine and its receptors making them the most crucial biomarker for tumour immunotherapy. Blocking chemokine - receptor signal axis that supports tumour growth and also promoting chemokine- receptor interactions that limit tumour growth are the two plausible ways to utilise the chemokine system as a therapeutic agent in immunotherapy (167). The chemokines exhibiting tumour promoting functions are shown in the Table 2. CCL3-CCR1 signal axis mediate the formation of osteolytic lesion and leads to tumour growth. CCXR721, a selective antagonist for CCR1, blocks the formation of mature osteoclast and experience a profound decrease in the tumour burden (198). The phase III clinical trial of mogamulizumab, an anti-CCR4 monoclonal antibody in the patients with cutaneious T-cell lymphoma showed a longer progression free survival and better quality of life (199). On the contrary, the enhancement of certain chemokines helps to reduce tumour growth. For example, anthracycline treatment enhances the recruitment of functional, antigen presenting, CD11b+ CD11c+ Ly6G- MHCII+ DC cells, stimulate the cytotoxic activity of effector T cells in fibrosarcoma model (200). The major obstacle in ICI therapies is the anergy and reluctance of effector T cell infiltration to the tumour sites. Targeting some prime chemokine - receptor axes simultaneously with ICI therapies can overcome this hurdle, contributing to tumour reduction (201). A combination of anti-PD-1 and anti-CXCR4 immunotherapy modulates tumour infiltration of CD8+ T cells can hence promote an anti-tumour immune response and improve survival rates (202). In brief, chemokines exhibiting elevated immune cell infiltration and inhibited pro-tumoural behaviour demonstrate better therapeutic strategies.
6 Future perspectives
Cancer treatments are continuously evolving progressing from traditional standard care to advanced immunotherapy shaping the future of Oncology. Over the years, a wide range of treatments including surgery, radiation therapy, chemotherapy, hormonal therapy, targeted therapy, and immunotherapy have been employed in the management of cancer. Tailoring treatments like adoptive cell therapies (ACTs) are the prime clinical methods currently in progress. Chimeric antigen receptor (CAR)-T cell therapy and engineered T cell receptor (TCR) therapy are the superior treatment methods in ACTs (203). CAR-T cell therapy uses a genetically altered CAR molecule, composed of an extra cellular antigen binding domain from an antibody and T cell receptor expressing intracellular signalling domain from patients own T cell. It has shown remarkable clinical responses (204). CAR-T cells exploit the anti-tumoural behaviour of chemokines in some cancers. For example, conditionally induced expression of chemokines such as CCL9 expressed on CAR-T cells increase the anti-tumour ability by enhancing their infiltration capacity in pancreatic ductal adenocarcinoma (205). The outcome of CAR-T cell mediated therapy against solid tumours are constrained by various reasons such as inadequate infiltration of effector cells, limited proliferation and declining effect of functional CAR-T cells etc. (206). CCL4 and CCL5 are the most robust and consistent chemokines present in the infiltration of CD8+ T cells. Hence, the CAR molecule engineered with CCR5, the receptor for CCL4 and CCL5, reinforce their migratory ability without affecting the cytotoxicity of CAR-T cells (206). Another ongoing therapeutic option is cancer vaccines. They efficiently eliminate malignant cells by amplifying and evoking the host immune system, making them an inevitable treatment method in the field of oncology. A phase I clinical trial of dendritic cell vaccines modified with the expression of CCL21 can potentially enhance the anti-tumour responses in NSCLC by increased immune cell infiltration (207). Several studies in cell-based immunotherapy like CAR-T cell therapies and ICI therapies (anti-PD-1, anti-PDL-1 and anti-CTLA-4) have been already transferred from preclinical to phase I and II clinical trials. Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) based technologies are also under trials in many cancer treatments. Clinical trials have shown significant results in patients with refractory cancer employing CRISPR-Cas9 in combination with T cell therapy, where CAR-T cells have been programmed using CRISPR-Cas9 (208). In short, precision medicine in the area of treatment and innovative insights mediated techniques in early diagnostics could reduce side effects and increase better prognosis.
7 Conclusion
Emerging therapeutic strategies are increasingly shifting focus from merely tumour cells to the broader tumour microenvironment (TME), paving the way for advanced approaches such as immunotherapy. Growing scientific evidence suggest that targeting TME derived factors including chemokines hold significant potential to enhance clinical outcomes. The immune sensitive milieu manipulates the chemokines and their receptors in favouring tumour growth. On the other hand, some of them contribute to anti-tumoural properties as well. The pro-tumoural behaviour of chemokines have been well researched. The immune suppressive cells such as Tregs, MDSCs, TAMs and TANs express chemokines which chemotactically binds to their cognate receptors on tumour, stromal cells and vice versa, advocating immune evasion and malignancy. Several chemokines - receptor axes mediate the oncogenic processes such as abnormal angiogenesis, EMT, metastasis and the other hallmarks of tumour like hypoxia, cancer stemness and drug resistance. It was noted that the gut microbiome as well as intratumoural microbiota in some cancers contribute to tumour promotion through chemokines. The anti-tumoural face of chemokines are rather seen when concentrating on CD4+ T cells, effector CD8+ T cells, B cells and NK cells along with M1 and N1 phenotypes of TAMs and TANs respectively. The ability of certain chemokines in mediating anti-tumoural activity would be a breakthrough in developing targeted and cutting-edge therapeutic strategies. Given the fact that immune checkpoint inhibitor therapy is greatly employed clinically and have successful outcomes, combination therapies targeting ICIs and chemokines would be a promising strategy in immunotherapy. Despite their role in both tumour enhancement and suppression, their levels are regulated in TME by several modifications and feedback mechanisms. The new and more feasible treatment applications in the field of oncology include tailored, precision medicines, adoptive cells therapies, CART-therapies and CRISPR based options. These are all plausible methods in this area. The paradoxical behaviour of chemokines can be strategically exploited to develop innovative and effective therapeutic approaches suppressing immune evasion and tumour growth while enhancing immune surveillance and improving prognosis.
Author contributions
HX: Writing – review & editing, Software, Writing – original draft, Validation. AG: Writing – review & editing, Writing – original draft, Validation. JT: Validation, Writing – review & editing. PS: Validation, Writing – review & editing. AS: Validation, Writing – review & editing. EB: Writing – review & editing, Validation. AB: Validation, Writing – review & editing. RD: Writing – review & editing, Validation. SB: Supervision, Writing – review & editing, Conceptualization, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Teachers Associateship for Research Excellence, SERB, Govt. of. India.(TAR/2021/000147) and Kairali Research Award (Biological Science), KSHEC, Govt. of Kerala (KSHEC-A3/352/Kairali Research Award/109/2021).
Acknowledgments
We thank Dr. Mary Susan, Retired Professor, Dept. of English, St. Xaviers College, Thumba, Thiruvananthapuram, Kerala, India, and Dr. Rani Alex, Assistant Professor, PG and Research Department of English, Mar Ivanios College, Nalanchira, Thiruvananthapuram, for proofreading and grammar corrections.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
TME: Tumour microenvironment
Tregs: Regulatory T cells
TAMs: Tumour associated macrophages
TANs: Tumour associated neutrophils
MDSC: Myeloid-derived suppressor cells
NK cells: Natural killer cells
VEGF-A: Vascular endothelial growth factor-A
RB: Retinoblastoma
PMN-MDSCs: Polymorphonuclear- myeloid-derived suppressor cells
M-MDSCs-: Monocyte-like monocytic- myeloid-derived suppressor cells
VEGF-R: Vascular endothelial growth factor receptors
PDGFR: Platelet derived growth factor receptor
FGF-2: Fibroblast growth factor -2
PDGF: Platelet derived growth factor
MMP2: Matrix Metalloproteinase-2
MMP-9: Matrix Metalloproteinase-9
VEGFR2: Vascular endothelial growth factor receptor-2
EMT: Epithelial mesenchymal transition
EMT-TFs: EMT-transcription factors
CAFs: Cancer associated fibroblasts
BC: Breast cancer
HIF-1 α: Hypoxia inducing factor 1 α
SDF-1: Stromal cell-derived factor-1
HCC: Hepatocellular carcinoma
NKT Cells: Natural killer T cells
CRC: Colorectal cancer
CSCs: Cancer stem cells
TICs: Tumour initiating cells
TPCs: Tumour propagating cells
LSC: Leukaemia stem cell
ER+: Estrogen receptor +ve
HER2: Human epidermal growth factor receptor 2
Tumor necrosis factor- αTLs
PD-L1: Programmed death ligand 1
CTLs: Cytotoxic T lymphocytes
NSCLC: non-small cell lung cancer
TFH: T Follicular helper cells
DCs: Dendritic cells
TNF- α: Tumour necrosis factor-alpha
IFN-γ: Interferon-gamma
iNOS: Inducible nitric oxide synthase
STAT 3: Signal transducer and activator of transcription 3
MAPK: Mitogen-activated protein kinase
NF-κB: Nuclear factor-kappa B
NOS2: Nitric oxide synthase-2
ICI: Immune checkpoint inhibitors
TGF-β: Transforming growth factor-beta
PD-1: Programmed cell death protein-1
TNBC: Triple negative breast cancer
CTLA-4: Cytotoxic T-lymphocyte -associated protein 4
ICB: Immune checkpoint blockade
ICC: Intrahepatic cholangiocarcinoma
ACKRs: Atypical chemokine receptors
AREs: Adenine- and uridine rich elements
RBPs: RNA- binding proteins
GPCR: G-Protein coupled receptor
PAD: Peptidyl arginine deiminase
FDA: Food and drug administration
DEGs: Differentially expressed genes
CAR-T: Chymeric antigen receptor- T-cell therapy
TCR: T- cell receptor
References
1. Kroemer G, Chan TA, Eggermont AMM, and Galluzzi L. Immunosurveillance in clinical cancer management. CA Cancer J Clin. (2024) 74:187–202. doi: 10.3322/caac.21818
2. Anderson NM and Simon MC. The tumor microenvironment. Curr Biol. (2020) 30:R921–5. doi: 10.1016/j.cub.2020.06.081
3. Zhong S, Jeong JH, Chen Z, Chen Z, and Luo JL. Targeting tumor microenvironment by small-molecule inhibitors. Transl Oncol. (2020) 13:57–69. doi: 10.1016/j.tranon.2019.10.001
4. Goubran HA, Kotb RR, Stakiw J, Emara ME, and Burnouf T. Regulation of tumor growth and metastasis: the role of tumor microenvironment. Cancer Growth Metastasis. (2014) 7:CGM.S11285. doi: 10.4137/CGM.S11285
5. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
6. Sachet NFM, Sajet HFM, Al-Owaidi MMN, Aljulihawi SRM, Al Idreis MKJ, Al-Gharibawi WNA, et al. Radiotherapy and Its Associated Side Effects in Breast, Head and neck, Liver, Thyroid, Non-melanoma skin Cancer and Recent Treatment Strategies. Curr Clin Med Educ. (2024) 2:106–15.
7. Liu M, Yang J, Xu B, and Zhang X. Tumor metastasis: Mechanistic insights and therapeutic interventions. MedComm. (2021) 2:587–617. doi: 10.1002/mco2.100
8. Chen G, Lu J, Li B, Zhao M, Liu D, Yang Z, et al. Efficacy and safety of Shenqi Fuzheng injection combined with chemotherapy for cancer: an overview of systematic reviews. Phytomedicine. (2024) 125:155293. doi: 10.1016/j.phymed.2023.155293
9. Behranvand N, Nasri F, Zolfaghari Emameh R, Khani P, Hosseini A, Garssen J, et al. Chemotherapy: a double-edged sword in cancer treatment. Cancer Immunol Immunother. (2022) 71:507–26. doi: 10.1007/s00262-021-03013-3
10. Hanahan D and Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
11. Griffith JW, Sokol CL, and Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. (2014) 32:659–702. doi: 10.1146/annurev-immunol-032713-120145
12. Do HTT, Lee CH, and Cho J. Chemokines and their receptors: multifaceted roles in cancer progression and potential value as cancer prognostic markers. Cancers. (2020) 12:287. doi: 10.3390/cancers12020287
13. Zajkowska M and Mroczko B. Chemokines in primary liver cancer. Int J Mol Sci. (2022) 23:8846. doi: 10.3390/ijms23168846
14. Gangele K, Jamsandekar M, Mishra A, and Poluri KM. Unraveling the evolutionary origin of ELR motif using fish CXC chemokine CXCL8. Fish Shellfish Immunol. (2019) 93:17–27. doi: 10.1016/j.fsi.2019.07.034
15. Wu T, Yang W, Sun A, Wei Z, and Lin Q. The role of CXC chemokines in cancer progression. Cancers. (2022) 15:167. doi: 10.3390/cancers15010167
16. Williams JL, Holman DW, and Klein RS. Chemokines in the balance: maintenance of homeostasis and protection at CNS barriers. Front Cell Neurosci. (2014) 8:154. doi: 10.3389/fncel.2014.00154
17. Cambier S, Gouwy M, and Proost P. The chemokines CXCL8 and CXCL12: molecular and functional properties, role in disease and efforts towards pharmacological intervention. Cell Mol Immunol. (2023) 20:217–51. doi: 10.1038/s41423-023-00974-6
18. Chen K, Bao Z, Tang P, Gong W, Yoshimura T, and Wang JM. Chemokines in homeostasis and diseases. Cell Mol Immunol. (2018) 15:324–34. doi: 10.1038/cmi.2017.134
19. Ozga AJ, Chow MT, and Luster AD. Chemokines and the immune response to cancer. Immunity. (2021) 54:859–74. doi: 10.1016/j.immuni.2021.01.012
20. Abdul-Rahman T, Ghosh S, Badar SM, Nazir A, Bamigbade GB, Aji N, et al. The paradoxical role of cytokines and chemokines at the tumor microenvironment: a comprehensive review. Eur J Med Res. (2024) 29:124. doi: 10.1186/s40001-024-01711-z
21. Susek KH, Karvouni M, Alici E, and Lundqvist A. The role of CXC chemokine receptors 1–4 on immune cells in the tumor microenvironment. Front Immunol. (2018) 9:2159. doi: 10.3389/fimmu.2018.02159
22. Fernandes Q and Folorunsho OG. Unveiling the nexus: The tumor microenvironment as a strategic frontier in viral cancers. Cytokine. (2025) 185:156827. doi: 10.1016/j.cyto.2024.156827
23. Edechi CA, Ikeogu N, Uzonna JE, and Myal Y. Regulation of immunity in breast cancer. Cancers. (2019) 11:1080. doi: 10.3390/cancers11081080
24. Bule P, Aguiar SI, Aires-Da-Silva F, and Dias JNR. Chemokine-directed tumor microenvironment modulation in cancer immunotherapy. Int J Mol Sci. (2021) 22:9804. doi: 10.3390/ijms22189804
25. Chow MT and Luster AD. Chemokines in cancer. Cancer Immunol Res. (2014) 2:1125–31. doi: 10.1158/2326-6066.CIR-14-0160
26. Li YR, Halladay T, and Yang L. Immune evasion in cell-based immunotherapy: unraveling challenges and novel strategies. J BioMed Sci. (2024) 31:5. doi: 10.1186/s12929-024-00998-8
27. Gao Y, You M, Fu J, Tian M, Zhong X, Du C, et al. Intratumoral stem-like CCR4+ regulatory T cells orchestrate the immunosuppressive microenvironment in HCC associated with hepatitis B. J Hepatol. (2022) 76:148–59. doi: 10.1016/j.jhep.2021.08.029
28. Du G, Dou C, Sun P, Wang S, Liu J, and Ma L. Regulatory T cells and immune escape in HCC: understanding the tumor microenvironment and advancing CAR-T cell therapy. Front Immunol. (2024) 15:1431211. doi: 10.3389/fimmu.2024.1431211
29. Ohue Y and Nishikawa H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci. (2019) 110:2080–9. doi: 10.1111/cas.14069
30. Li MO and Flavell RA. TGF-β: a master of all T cell trades. Cell. (2008) 134:392–404. doi: 10.1016/j.cell.2008.07.025
31. Os Q. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. (2011) 332:600–3. doi: 10.1126/science.1202947
32. Mailloux A and Young MRI. Regulatory T-cell trafficking: from thymic development to tumor-induced immune suppression. Crit Rev Immunol. (2010) 30(5). Available online at: https://www.dl.begellhouse.com/journals/2ff21abf44b19838,76236f9f086f9f9f,3738eae4718cee18.html.
33. Sarkar T, Dhar S, and Sa G. Tumor-infiltrating T-regulatory cells adapt to altered metabolism to promote tumor-immune escape. Curr Res Immunol. (2021) 2:132–41. doi: 10.1016/j.crimmu.2021.08.002
34. Ren L, Yu Y, Wang L, Zhu Z, Lu R, and Yao Z. Hypoxia-induced CCL28 promotes recruitment of regulatory T cells and tumor growth in liver cancer. Oncotarget. (2016) 7:75763. doi: 10.18632/oncotarget.12409
35. Maruyama T, Kono K, Izawa S, Mizukami Y, Kawaguchi Y, Mimura K, et al. CCL17 and CCL22 chemokines within tumor microenvironment are related to infiltration of regulatory T cells in esophageal squamous cell carcinoma. Dis Esophagus. (2010) 23:422–9. doi: 10.1111/j.1442-2050.2009.01029.x
36. Plitas G, Konopacki C, Wu K, Bos PD, Morrow M, Putintseva EV, et al. Regulatory T cells exhibit distinct features in human breast cancer. Immunity. (2016) 45:1122–34. doi: 10.1016/j.immuni.2016.10.032
37. Yang S, Wang B, Guan C, Wu B, Cai C, Wang M, et al. Foxp3+IL-17+ T cells promote development of cancer-initiating cells in colorectal cancer. J Leukoc Biol. (2011) 89:85–91. doi: 10.1189/jlb.0910506
38. Tokunaga R, Zhang WU, Naseem M, Puccini A, Berger MD, Soni S, et al. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation–a target for novel cancer therapy. Cancer Treat Rev. (2018) 63:40–7. doi: 10.1016/j.ctrv.2017.11.007
39. Nagarsheth N, Wicha MS, and Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. (2017) 17:559–72. doi: 10.1038/nri.2017.49
40. Lin Y, Xu J, and Lan H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol OncolJ Hematol Oncol. (2019) 12:76. doi: 10.1186/s13045-019-0760-3
41. Gao J, Liang Y, and Wang L. Shaping polarization of tumor-associated macrophages in cancer immunotherapy. Front Immunol. (2022) 13:888713. doi: 10.3389/fimmu.2022.888713
42. Incio J, Suboj P, Chin SM, Vardam-Kaur T, Liu H, Hato T, et al. Metformin reduces desmoplasia in pancreatic cancer by reprogramming stellate cells and tumor-associated macrophages. PloS One. (2015) 10:e0141392. doi: 10.1371/journal.pone.0141392
43. Li F, Kitajima S, Kohno S, Yoshida A, Tange S, Sasaki S, et al. Retinoblastoma inactivation induces a protumoral microenvironment via enhanced CCL2 secretion. Cancer Res. (2019) 79:3903–15. doi: 10.1158/0008-5472.CAN-18-3604
44. Nywening TM, Wang-Gillam A, Sanford DE, Belt BA, Panni RZ, Cusworth BM, et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol. (2016) 17:651–62. doi: 10.1016/S1470-2045(16)00078-4
45. Lin S, Sun L, Lyu X, Ai X, Du D, Su N, et al. Lactate-activated macrophages induced aerobic glycolysis and epithelial-mesenchymal transition in breast cancer by regulation of CCL5-CCR5 axis: a positive metabolic feedback loop. Oncotarget. (2017) 8:110426. doi: 10.18632/oncotarget.22786
46. O’Hayre M, Salanga CL, Handel TM, and Allen SJ. Chemokines and cancer: migration, intracellular signalling and intercellular communication in the microenvironment. Biochem J. (2008) 409:635–49. doi: 10.1042/BJ20071493
47. Hojo S, Koizumi K, Tsuneyama K, Arita Y, Cui Z, Shinohara K, et al. High-level expression of chemokine CXCL16 by tumor cells correlates with a good prognosis and increased tumor-infiltrating lymphocytes in colorectal cancer. Cancer Res. (2007) 67:4725–31. doi: 10.1158/0008-5472.CAN-06-3424
48. Kurt FGO, Lasser S, Arkhypov I, Utikal J, and Umansky V. Enhancing immunotherapy response in melanoma: myeloid-derived suppressor cells as a therapeutic target. J Clin Invest. (2023) 133(13). Available online at: https://www.jci.org/articles/view/170762.
49. Li BH, Garstka MA, and Li ZF. Chemokines and their receptors promoting the recruitment of myeloid-derived suppressor cells into the tumor. Mol Immunol. (2020) 117:201–15. doi: 10.1016/j.molimm.2019.11.014
50. Umansky V, Blattner C, Gebhardt C, and Utikal J. The role of myeloid-derived suppressor cells (MDSC) in cancer progression. Vaccines. (2016) 4:36. doi: 10.3390/vaccines4040036
51. Eckstein M, Epple E, Jung R, Weigelt K, Lieb V, Sikic D, et al. CCL2 expression in tumor cells and tumor-infiltrating immune cells shows divergent prognostic potential for bladder cancer patients depending on lymph node stage. Cancers. (2020) 12:1253. doi: 10.3390/cancers12051253
52. Korbecki J, Kojder K, Simińska D, Bohatyrewicz R, Gutowska I, Chlubek D, et al. CC chemokines in a tumor: a review of pro-cancer and anti-cancer properties of the ligands of receptors CCR1, CCR2, CCR3, and CCR4. Int J Mol Sci. (2020) 21:8412. doi: 10.3390/ijms21218412
53. Jiang Y and Zhan H. Communication between EMT and PD-L1 signaling: New insights into tumor immune evasion. Cancer Lett. (2020) 468:72–81. doi: 10.1016/j.canlet.2019.10.013
54. Liu X, Tang R, Xu J, Tan Z, Liang C, Meng Q, et al. CRIP1 fosters MDSC trafficking and resets tumour microenvironment via facilitating NF-κB/p65 nuclear translocation in pancreatic ductal adenocarcinoma. Gut. (2023) 72:2329–43. doi: 10.1136/gutjnl-2022-329349
55. Lopez-Bujanda ZA, Haffner MC, Chaimowitz MG, Chowdhury N, Venturini NJ, Patel RA, et al. Castration-mediated IL-8 promotes myeloid infiltration and prostate cancer progression. Nat Cancer. (2021) 2:803–18. doi: 10.1038/s43018-021-00227-3
56. Disteldorf EM, Krebs CF, Paust HJ, Turner JE, Nouailles G, Tittel A, et al. CXCL5 drives neutrophil recruitment in TH17-mediated GN. J Am Soc Nephrol. (2015) 26:55–66. doi: 10.1681/ASN.2013101061
57. Bonavita O, Massara M, and Bonecchi R. Chemokine regulation of neutrophil function in tumors. Cytokine Growth Factor Rev. (2016) 30:81–6. doi: 10.1016/j.cytogfr.2016.03.012
58. Zhou J, Nefedova Y, Lei A, and Gabrilovich D. Neutrophils and PMN-MDSC: Their biological role and interaction with stromal cells. In: Seminars in immunology. Elsevier (2018). 35(19–28). Available online at: https://www.sciencedirect.com/science/article/pii/S1044532317300076.
59. Gong YT, Zhang LJ, Liu YC, Tang M, Lin JY, Chen XY, et al. Neutrophils as potential therapeutic targets for breast cancer. Pharmacol Res. (2023) 198:106996. doi: 10.1016/j.phrs.2023.106996
60. Yang J, Yan C, Vilgelm AE, Chen SC, Ayers GD, Johnson CA, et al. Targeted deletion of CXCR2 in myeloid cells alters the tumor immune environment to improve antitumor immunity. Cancer Immunol Res. (2021) 9:200–13. doi: 10.1158/2326-6066.CIR-20-0312
61. SenGupta S, Subramanian BC, and Parent CA. Getting TANned: How the tumor microenvironment drives neutrophil recruitment. J Leukoc Biol. (2019) 105:449–62. doi: 10.1002/JLB.3RI0718-282R
62. Oguntade AS, Al-Amodi F, Alrumayh A, Alobaida M, and Bwalya M. Anti-angiogenesis in cancer therapeutics: the magic bullet. J Egypt Natl Cancer Inst. (2021) 33:15. doi: 10.1186/s43046-021-00072-6
63. Liu ZL, Chen HH, Zheng LL, Sun LP, and Shi L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct Target Ther. (2023) 8:198. doi: 10.1038/s41392-023-01460-1
64. Rajabi M and Mousa SA. The role of angiogenesis in cancer treatment. Biomedicines. (2017) 5:34. doi: 10.3390/biomedicines5020034
65. Al-Ostoot FH, Salah S, Khamees HA, and Khanum SA. Tumor angiogenesis: Current challenges and therapeutic opportunities. Cancer Treat Res Commun. (2021) 28:100422. doi: 10.1016/j.ctarc.2021.100422
66. Madu CO, Wang S, Madu CO, and Lu Y. Angiogenesis in breast cancer progression, diagnosis, and treatment. J Cancer. (2020) 11:4474. doi: 10.7150/jca.44313
67. Marcuzzi E, Angioni R, Molon B, and Calì B. Chemokines and chemokine receptors: orchestrating tumor metastasization. Int J Mol Sci. (2018) 20:96. doi: 10.3390/ijms20010096
68. Guo F, Long L, Wang J, Wang Y, Liu Y, Wang L, et al. Insights on CXC chemokine receptor 2 in breast cancer: An emerging target for oncotherapy (Review). Oncol Lett. (2019) 18(6):5699–708. doi: 10.3892/ol.2019.10957
69. Korbecki J, Bosiacki M, Barczak K, Łagocka R, Brodowska A, Chlubek D, et al. Involvement in tumorigenesis and clinical significance of CXCL1 in reproductive cancers: breast cancer, cervical cancer, endometrial cancer, ovarian cancer and prostate cancer. Int J Mol Sci. (2023) 24:7262. doi: 10.3390/ijms24087262
70. Tan PHS, Chia SS, Toh SL, Goh JCH, and Nathan SS. The dominant role of IL-8 as an angiogenic driver in a three-dimensional physiological tumor construct for drug testing. Tissue Eng Part A. (2014) 20:1758–66. doi: 10.1089/ten.tea.2013.0245
71. Ridiandries A, Tan JT, and Bursill CA. The role of CC-chemokines in the regulation of angiogenesis. Int J Mol Sci. (2016) 17:1856. doi: 10.3390/ijms17111856
72. Harry JA and Ormiston ML. Novel pathways for targeting tumor angiogenesis in metastatic breast cancer. Front Oncol. (2021) 11:772305. doi: 10.3389/fonc.2021.772305
73. Rizeq B and Malki MI. The role of CCL21/CCR7 chemokine axis in breast cancer progression. Cancers. (2020) 12:1036. doi: 10.3390/cancers12041036
74. Dongre A and Weinberg RA. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. (2019) 20:69–84. doi: 10.1038/s41580-018-0080-4
75. Glaviano A, Lau HSH, Carter LM, Lee EHC, Lam HY, Okina E, et al. Harnessing the tumor microenvironment: targeted cancer therapies through modulation of epithelial-mesenchymal transition. J Hematol OncolJ Hematol Oncol. (2025) 18:6. doi: 10.1186/s13045-024-01634-6
76. Singh M, Yelle N, Venugopal C, and Singh SK. EMT: Mechanisms and therapeutic implications. Pharmacol Ther. (2018) 182:80–94. doi: 10.1016/j.pharmthera.2017.08.009
77. Goossens S, Vandamme N, Van Vlierberghe P, and Berx G. EMT transcription factors in cancer development re-evaluated: Beyond EMT and MET. Biochim Biophys Acta BBA-Rev Cancer. (2017) 1868:584–91. doi: 10.1016/j.bbcan.2017.06.006
78. Tian X, Wang J, Jiang L, Jiang Y, Xu J, and Feng X. Chemokine/GPCR signaling-mediated EMT in cancer metastasis. J Oncol. (2022) 2022:1–15. doi: 10.1155/2022/2208176
79. Xiao T, Bao J, Tian J, Lin R, Zhang Z, Zhu Y, et al. Flavokawain A suppresses the vasculogenic mimicry of HCC by inhibiting CXCL12 mediated EMT. Phytomedicine. (2023) 112:154687. doi: 10.1016/j.phymed.2023.154687
80. Cheng Y, Song Y, Qu J, Che X, Song N, Fan Y, et al. The chemokine receptor CXCR4 and c-MET cooperatively promote epithelial-mesenchymal transition in gastric cancer cells. Transl Oncol. (2018) 11:487–97. doi: 10.1016/j.tranon.2018.02.002
81. Huang CG, Liu Q, Zheng ST, Liu T, Tan YY, Peng TY, et al. Chemokines and their receptors: predictors of therapeutic potential in tumor microenvironment on esophageal cancer. Dig Dis Sci. (2024) 69:1562–70. doi: 10.1007/s10620-024-08392-y
82. Tangsiri M, Hheidari A, Liaghat M, Razlansari M, Ebrahimi N, Akbari A, et al. Promising applications of nanotechnology in inhibiting chemo-resistance in solid tumors by targeting epithelial-mesenchymal transition (EMT). BioMed Pharmacother. (2024) 170:115973. doi: 10.1016/j.biopha.2023.115973
83. Fu LQ, Du WL, Cai MH, Yao JY, Zhao YY, and Mou XZ. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell Immunol. (2020) 353:104119. doi: 10.1016/j.cellimm.2020.104119
84. Riaz F, Zhang J, and Pan F. Forces at play: exploring factors affecting the cancer metastasis. Front Immunol. (2024) 15:1274474. doi: 10.3389/fimmu.2024.1274474
85. Neophytou CM, Panagi M, Stylianopoulos T, and Papageorgis P. The role of tumor microenvironment in cancer metastasis: Molecular mechanisms and therapeutic opportunities. Cancers. (2021) 13:2053. doi: 10.3390/cancers13092053
86. Malla RR and Kiran P. Tumor microenvironment pathways: cross regulation in breast cancer metastasis. Genes Dis. (2022) 9:310–24. doi: 10.1016/j.gendis.2020.11.015
87. Kumar V, Donthireddy L, Marvel D, Condamine T, Wang F, Lavilla-Alonso S, et al. Cancer-associated fibroblasts neutralize the anti-tumor effect of CSF1 receptor blockade by inducing PMN-MDSC infiltration of tumors. Cancer Cell. (2017) 32:654–68. doi: 10.1016/j.ccell.2017.10.005
88. Shi Y, Riese DJ, and Shen J. The role of the CXCL12/CXCR4/CXCR7 chemokine axis in cancer. Front Pharmacol. (2020) 11:574667. doi: 10.3389/fphar.2020.574667
89. Liu Q, Li A, Tian Y, Wu JD, Liu Y, Li T, et al. The CXCL8-CXCR1/2 pathways in cancer. Cytokine Growth Factor Rev. (2016) 31:61–71. doi: 10.1016/j.cytogfr.2016.08.002
90. Mishra A, Suman KH, Nair N, Majeed J, and Tripathi V. An updated review on the role of the CXCL8-CXCR1/2 axis in the progression and metastasis of breast cancer. Mol Biol Rep. (2021) 48:6551–61. doi: 10.1007/s11033-021-06648-8
91. Anastasiadou DP, Couturier N, Goel S, Argyris DG, Vodopyanov S, Rivera-Sanchez L, et al. Intratumoral CXCL12 gradients contextualize tumor cell invasion, migration and immune suppression in breast cancer. bioRxiv. (2024). doi: 10.1101/2024.10.15.618571
92. Kamalakar A, Bendre MS, Washam CL, Fowler TW, Carver A, Dilley JD, et al. Circulating interleukin-8 levels explain breast cancer osteolysis in mice and humans. Bone. (2014) 61:176–85. doi: 10.1016/j.bone.2014.01.015
93. Sarvaiya PJ, Guo D, Ulasov I, Gabikian P, and Lesniak MS. Chemokines in tumor progression and metastasis. Oncotarget. (2013) 4:2171. doi: 10.18632/oncotarget.1426
94. Coniglio SJ. Role of tumor-derived chemokines in osteolytic bone metastasis. Front Endocrinol. (2018) 9:313. doi: 10.3389/fendo.2018.00313
95. Korbecki J, Kojder K, Kapczuk P, Kupnicka P, Gawrońska-Szklarz B, Gutowska I, et al. The effect of hypoxia on the expression of CXC chemokines and CXC chemokine receptors—a review of literature. Int J Mol Sci. (2021) 22:843. doi: 10.3390/ijms22020843
96. Semenza GL. The hypoxic tumor microenvironment: A driving force for breast cancer progression. Biochim Biophys Acta BBA-Mol Cell Res. (2016) 1863:382–91. doi: 10.1016/j.bbamcr.2015.05.036
97. Filippi I, Carraro F, and Naldini A. Interleukin-1 β Affects MDAMB231 breast cancer cell migration under hypoxia: role of HIF-1 α and NF κ B transcription factors. Mediators Inflamm. (2015) 2015:789414. doi: 10.1155/2015/789414
98. Thomas JA, Gireesh Moly AG, Xavier H, Suboj P, Ladha A, Gupta G, et al. Enhancement of immune surveillance in breast cancer by targeting hypoxic tumor endothelium: Can it be an immunological switch point? Front Oncol. (2023) 13:1063051. doi: 10.3389/fonc.2023.1063051
99. Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and Treg cells. Nature. (2011) 475:226–30. doi: 10.1038/nature10169
100. Papaccio F, Paino F, Regad T, Papaccio G, Desiderio V, and Tirino V. Concise review: cancer cells, cancer stem cells, and mesenchymal stem cells: influence in cancer development. Stem Cells Transl Med. (2017) 6:2115–25. doi: 10.1002/sctm.17-0138
101. Zhang X, Powell K, and Li L. Breast cancer stem cells: biomarkers, identification and isolation methods, regulating mechanisms, cellular origin, and beyond. Cancers. (2020) 12:3765. doi: 10.3390/cancers12123765
102. Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, and Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci. (2003) 100:3983–8. doi: 10.1073/pnas.0530291100
103. Walcher L, Kistenmacher AK, Suo H, Kitte R, Dluczek S, Strauß A, et al. Cancer stem cells—origins and biomarkers: perspectives for targeted personalized therapies. Front Immunol. (2020) 11:1280. doi: 10.3389/fimmu.2020.01280
104. Desai A, Yan Y, and Gerson SL. Concise reviews: cancer stem cell targeted therapies: toward clinical success. Stem Cells Transl Med. (2019) 8:75–81. doi: 10.1002/sctm.18-0123
105. Ullah TR. The role of CXCR4 in multiple myeloma: Cells’ journey from bone marrow to beyond. J Bone Oncol. (2019) 17:100253. doi: 10.1016/j.jbo.2019.100253
106. Zhang S, Yang X, Wang L, and Zhang C. Interplay between inflammatory tumor microenvironment and cancer stem cells (Review). Oncol Lett. (2018) 16(1):679–6. doi: 10.3892/ol.2018.8716
107. Wang X, Zhang H, and Chen X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. (2019) 2:141. doi: 10.20517/cdr.2019.10
108. Mohamed A, Krajewski K, Cakar B, and Ma CX. Targeted therapy for breast cancer. Am J Pathol. (2013) 183:1096–112. doi: 10.1016/j.ajpath.2013.07.005
109. Shi Z, Tu J, Ying Y, Diao Y, Zhang P, Liao S, et al. CC chemokine ligand-2: a promising target for overcoming anticancer drug resistance. Cancers. (2022) 14:4251. doi: 10.3390/cancers14174251
110. Li D, Ji H, Niu X, Yin L, Wang Y, Gu Y, et al. Tumor-associated macrophages secrete CC-chemokine ligand 2 and induce tamoxifen resistance by activating PI3K/Akt/mTOR in breast cancer. Cancer Sci. (2020) 111:47–58. doi: 10.1111/cas.14230
111. Choi J, Lee HJ, Yoon S, Ryu HM, Lee E, Jo Y, et al. Blockade of CCL2 expression overcomes intrinsic PD-1/PD-L1 inhibitor-resistance in transglutaminase 2-induced PD-L1 positive triple negative breast cancer. Amer J Canc Res. (2020) 10(9):2878.
112. Abdullah L, Hills LB, Winter EB, and Huang YH. Diverse roles of Akt in T cells. Immunometabolism. (2021) 3:e210007. doi: 10.20900/immunometab20210007
113. Liu H, Yang Z, Lu W, Chen Z, Chen L, Han S, et al. Chemokines and chemokine receptors: A new strategy for breast cancer therapy. Cancer Med. (2020) 9:3786–99. doi: 10.1002/cam4.3014
114. Matsuo K, Yoshie O, and Nakayama T. Multifaceted roles of chemokines and chemokine receptors in tumor immunity. Cancers. (2021) 13:6132. doi: 10.3390/cancers13236132
115. Yi M, Li T, Niu M, Zhang H, Wu Y, Wu K, et al. Targeting cytokine and chemokine signaling pathways for cancer therapy. Signal Transduct Target Ther. (2024) 9:176. doi: 10.1038/s41392-024-01868-3
116. Khosravi G, Mostafavi S, Bastan S, Ebrahimi N, Gharibvand RS, and Eskandari N. Immunologic tumor microenvironment modulators for turning cold tumors hot. Cancer Commun. (2024) 44:521–53. doi: 10.1002/cac2.12539
117. Giacobbi NS, Mullapudi S, Nabors H, and Pyeon D. The chemokine CXCL14 as a potential immunotherapeutic agent for cancer therapy. Viruses. (2024) 16:302. doi: 10.3390/v16020302
118. Karin N. Chemokines and cancer: new immune checkpoints for cancer therapy. Curr Opin Immunol. (2018) 51:140–5. doi: 10.1016/j.coi.2018.03.004
119. Ahmed H, Mahmud AR, Siddiquee M, Shahriar A, Biswas P, Shimul M, et al. Role of T cells in cancer immunotherapy: Opportunities and challenges. Cancer Pathog Ther. (2023) 1:116–26. doi: 10.1016/j.cpt.2022.12.002
120. Nie S, Song Y, Hu K, Zu W, Zhang F, Chen L, et al. CXCL10 and IL15 co-expressing chimeric antigen receptor T cells enhance anti-tumor effects in gastric cancer by increasing cytotoxic effector cell accumulation and survival. OncoImmunology. (2024) 13:2358590. doi: 10.1080/2162402X.2024.2358590
121. Reschke R, Enk AH, and Hassel JC. Chemokines and cytokines in immunotherapy of melanoma and other tumors: from biomarkers to therapeutic targets. Int J Mol Sci. (2024) 25:6532. doi: 10.3390/ijms25126532
122. Karaki S, Blanc C, Tran T, Galy-Fauroux I, Mougel A, Dransart E, et al. CXCR6 deficiency impairs cancer vaccine efficacy and CD8+ resident memory T-cell recruitment in head and neck and lung tumors. J Immunother Cancer. (2021) 9:e001948. doi: 10.1136/jitc-2020-001948
123. Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, and Germain RN. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell–dendritic cell interaction. Nature. (2006) 440:890–5. doi: 10.1038/nature04651
124. Harada Y, Harada Y, Elly C, Ying G, Paik JH, DePinho RA, et al. Transcription factors Foxo3a and Foxo1 couple the E3 ligase Cbl-b to the induction of Foxp3 expression in induced regulatory T cells. J Exp Med. (2010) 207:1381–91. doi: 10.1084/jem.20100004
125. Shang Q, Yu X, Sun Q, Li H, Sun C, and Liu L. Polysaccharides regulate Th1/Th2 balance: a new strategy for tumor immunotherapy. BioMed Pharmacother. (2024) 170:115976. doi: 10.1016/j.biopha.2023.115976
126. Ivanova EA and Orekhov AN. T helper lymphocyte subsets and plasticity in autoimmunity and cancer: an overview. BioMed Res Int. (2015) 2015:1–9. doi: 10.1155/2015/327470
127. Wu B, Zhang B, Li B, Wu H, and Jiang M. Cold and hot tumors: from molecular mechanisms to targeted therapy. Signal Transduct Target Ther. (2024) 9:274. doi: 10.1038/s41392-024-01979-x
128. Shields JD, Kourtis IC, Tomei AA, Roberts JM, and Swartz MA. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science. (2010) 328:749–52. doi: 10.1126/science.1185837
129. Li L, Yu R, Cai T, Chen Z, Lan M, Zou T, et al. Effects of immune cells and cytokines on inflammation and immunosuppression in the tumor microenvironment. Int Immunopharmacol. (2020) 88:106939. doi: 10.1016/j.intimp.2020.106939
130. Yunna C, Mengru H, Lei W, and Weidong C. Macrophage M1/M2 polarization. Eur J Pharmacol. (2020) 877:173090. doi: 10.1016/j.ejphar.2020.173090
131. Ruytinx P, Proost P, Van Damme J, and Struyf S. Chemokine-induced macrophage polarization in inflammatory conditions. Front Immunol. (2018) 9:1930. doi: 10.3389/fimmu.2018.01930
132. Li M, Sun X, Zhao J, Xia L, Li J, Xu M, et al. CCL5 deficiency promotes liver repair by improving inflammation resolution and liver regeneration through M2 macrophage polarization. Cell Mol Immunol. (2020) 17:753–64. doi: 10.1038/s41423-019-0279-0
133. Wu Q, Mao H, Jiang Z, and Tang D. Tumour-associated neutrophils: Potential therapeutic targets in pancreatic cancer immunotherapy. Immunology. (2024) 172:343–61. doi: 10.1111/imm.13765
134. Xu W, Weng J, Xu M, Zhou Q, Liu S, Hu Z, et al. Chemokine CCL21 determines immunotherapy response in hepatocellular carcinoma by affecting neutrophil polarization. Cancer Immunol Immunother. (2024) 73:56. doi: 10.1007/s00262-024-03650-4
135. Sounbuli K, Mironova N, and Alekseeva L. Diverse neutrophil functions in cancer and promising neutrophil-based cancer therapies. Int J Mol Sci. (2022) 23:15827. doi: 10.3390/ijms232415827
136. Greene S, Robbins Y, Mydlarz WK, Huynh AP, Schmitt NC, Friedman J, et al. Inhibition of MDSC trafficking with SX-682, a CXCR1/2 inhibitor, enhances NK-cell immunotherapy in head and neck cancer models. Clin Cancer Res. (2020) 26:1420–31. doi: 10.1158/1078-0432.CCR-19-2625
137. Kwong TT, Xiong Z, Zhang Y, Wu H, Cao J, Wong PPC, et al. Overcoming immunotherapy resistance in hepatocellular carcinoma by targeting myeloid IL-8/CXCR2 signaling. Mol Ther. (2025). 33(4):1659–73.
138. Darvin P, Toor SM, Sasidharan Nair V, and Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. (2018) 50:1–11. doi: 10.1038/s12276-018-0191-1
139. Hu Y, Li S, Xiao H, Xiong Y, Lu X, Yang X, et al. Distinct circulating cytokine/chemokine profiles correlate with clinical benefit of immune checkpoint inhibitor monotherapy and combination therapy in advanced non-small cell lung cancer. Cancer Med. (2023) 12:12234–52. doi: 10.1002/cam4.5918
140. Zeng Y, Li B, Liang Y, Reeves PM, Qu X, Ran C, et al. Dual blockade of CXCL12-CXCR4 and PD-1–PD-L1 pathways prolongs survival of ovarian tumor–bearing mice by prevention of immunosuppression in the tumor microenvironment. FASEB J. (2019) 33:6596. doi: 10.1096/fj.201802067RR
141. Strazza M and Mor A. The complexity of targeting chemokines to promote a tumor immune response. Inflammation. (2020) 43:1201–8. doi: 10.1007/s10753-020-01235-8
142. Horn LA, Riskin J, Hempel HA, Fousek K, Lind H, Hamilton DH, et al. Simultaneous inhibition of CXCR1/2, TGF-β, and PD-L1 remodels the tumor and its microenvironment to drive antitumor immunity. J Immunother Cancer. (2020) 8(1): e000326. Available online at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7078948/.
143. Mok S, Liu H, Ağaç Çobanoğlu D, Anang NAAS, Mancuso JJ, Wherry EJ, et al. Anti-CTLA-4 generates greater memory response than anti-PD-1 via TCF-1. Proc Natl Acad Sci. (2025) 122:e2418985122. doi: 10.1073/pnas.2418985122
144. Abdul-Huseen SD and Alabassi HM. Estimate the relationship between CXCR4-SDF-1 axis and inhibitory molecules (CTLA4 and PD-1) in patients with colon cancer. Narra J. (2024) 4:e992. doi: 10.52225/narra.v4i3.992
145. Henriksen NL, Jensen PØ, and Jensen LK. Immune checkpoint blockade in experimental bacterial infections. J Infect. (2025) 90:106391. doi: 10.1016/j.jinf.2024.106391
146. Chen J, Amoozgar Z, Liu X, Aoki S, Liu Z, Shin SM, et al. Reprogramming the intrahepatic cholangiocarcinoma immune microenvironment by chemotherapy and CTLA-4 blockade enhances anti–PD-1 therapy. Cancer Immunol Res. (2024) 12:400–12. doi: 10.1158/2326-6066.CIR-23-0486
147. Harvanová G, Duranková S, and Bernasovská J. The role of cytokines and chemokines in the inflammatory response. Alergol Pol-Pol J Allergol. (2023) 10:210–9. doi: 10.5114/pja.2023.131708
148. Qin R, Ren W, Ya G, Wang B, He J, Ren S, et al. Role of chemokines in the crosstalk between tumor and tumor-associated macrophages. Clin Exp Med. (2022) 23:1359–73. doi: 10.1007/s10238-022-00888-z
149. Mempel TR, Lill JK, and Altenburger LM. How chemokines organize the tumour microenvironment. Nat Rev Cancer. (2024) 24:28–50. doi: 10.1038/s41568-023-00635-w
150. Das AS, Basu A, Kumar R, Borah PK, Bakshi S, Sharma M, et al. Post-transcriptional regulation of C-C motif chemokine ligand 2 expression by ribosomal protein L22 during LPS-mediated inflammation. FEBS J. (2020) 287:3794–813. doi: 10.1111/febs.15362
151. Sun D, Novotny M, Bulek K, Liu C, Li X, and Hamilton T. Treatment with IL-17 prolongs the half-life of chemokine CXCL1 mRNA via the adaptor TRAF5 and the splicing-regulatory factor SF2 (ASF). Nat Immunol. (2011) 12:853–60. doi: 10.1038/ni.2081
152. Bonecchi R and Graham GJ. Atypical chemokine receptors and their roles in the resolution of the inflammatory response. Front Immunol. (2016) 7:224. doi: 10.3389/fimmu.2016.00224
153. Gorbachev AV and Fairchild RL. Regulation of chemokine expression in the tumor microenvironment. Crit Rev Immunol. (2014) 34(2). Available online at: https://www.dl.begellhouse.com/journals/2ff21abf44b19838,6fd3c75b68d49e79,251e824f5fb393e6.html.
154. Borroni EM, Savino B, Bonecchi R, and Locati M. Chemokines sound the alarmin: The role of atypical chemokine in inflammation and cancer. In: Seminars in immunology. Elsevier (2018) 38:63–71. Available online at: https://www.sciencedirect.com/science/article/pii/S104453231830071X.
155. Crawford KS and Volkman BF. Prospects for targeting ACKR1 in cancer and other diseases. Front Immunol. (2023) 14:1111960. doi: 10.3389/fimmu.2023.1111960
156. Gowhari Shabgah A, Jadidi-Niaragh F, Mohammadi H, Ebrahimzadeh F, Oveisee M, Jahanara A, et al. The role of atypical chemokine receptor D6 (ACKR2) in physiological and pathological conditions; friend, foe, or both? Front Immunol. (2022) 13:861931. doi: 10.3389/fimmu.2022.861931
157. Hughes CE and Nibbs RJB. A guide to chemokines and their receptors. FEBS J. (2018) 285:2944. doi: 10.1111/febs.14466
158. Loos T, Mortier A, Gouwy M, Ronsse I, Put W, Lenaerts JP, et al. Citrullination of CXCL10 and CXCL11 by peptidylarginine deiminase: a naturally occurring posttranslational modification of chemokines and new dimension of immunoregulation. Blood J Am Soc Hematol. (2008) 112:2648–56. doi: 10.1182/blood-2008-04-149039
159. De Zutter A, Van Damme J, and Struyf S. The role of post-translational modifications of chemokines by CD26 in cancer. Cancers. (2021) 13:4247. doi: 10.3390/cancers13174247
160. Xu L and Fidler IJ. Acidic pH-induced elevation in interleukin 8 expression by human ovarian carcinoma cells. Cancer Res. (2000) 60(16):4610–6.
161. Boudot A, Kerdivel G, Habauzit D, Eeckhoute J, Le Dily F, Flouriot G, et al. Differential estrogen-regulation of CXCL12 chemokine receptors, CXCR4 and CXCR7, contributes to the growth effect of estrogens in breast cancer cells. PloS One. (2011) 6:e20898. doi: 10.1371/journal.pone.0020898
162. Sengupta S, Schiff R, and Katzenellenbogen BS. Post-transcriptional regulation of chemokine receptor CXCR4 by estrogen in HER2 overexpressing, estrogen receptor-positive breast cancer cells. Breast Cancer Res Treat. (2009) 117:243–51. doi: 10.1007/s10549-008-0186-z
163. Zhong L, Li Y, Xiong L, Wang W, Wu M, Yuan T, et al. Small molecules in targeted cancer therapy: advances, challenges, and future perspectives. Signal Transduct Target Ther. (2021) 6:1–48. doi: 10.1038/s41392-021-00572-w
164. Min HY and Lee HY. Molecular targeted therapy for anticancer treatment. Exp Mol Med. (2022) 54:1670–94. doi: 10.1038/s12276-022-00864-3
165. Zahavi D and Weiner L. Monoclonal antibodies in cancer therapy. Antibodies. (2020) 9:34. doi: 10.3390/antib9030034
166. Smyth MJ and Teng MW. 2018 Nobel Prize in physiology or medicine. Clin Transl Immunol. (2018) 7(10):e1041. Available online at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6200707/.
167. Karin N. Chemokines in the landscape of cancer immunotherapy: how they and their receptors can be used to turn cold tumors into hot ones? Cancers. (2021) 13:6317. doi: 10.3390/cancers13246317
168. Lv Y, Chen C, Han M, Tian C, Song F, Feng S, et al. CXCL2: a key player in the tumor microenvironment and inflammatory diseases. Cancer Cell Int. (2025) 25:133. doi: 10.1186/s12935-025-03765-3
169. Fernandez-Avila L, Castro-Amaya AM, Molina-Pineda A, Hernández-Gutiérrez R, Jave-Suarez LF, and Aguilar-Lemarroy A. The Value of CXCL1, CXCL2, CXCL3, and CXCL8 as potential prognosis markers in cervical cancer: Evidence of E6/E7 from HPV16 and 18 in chemokines regulation. Biomedicines. (2023) 11:2655. doi: 10.3390/biomedicines11102655
170. Sun X, He X, Zhang Y, Hosaka K, Andersson P, Wu J, et al. Inflammatory cell-derived CXCL3 promotes pancreatic cancer metastasis through a novel myofibroblast-hijacked cancer escape mechanism. Gut. (2022) 71:129–47. doi: 10.1136/gutjnl-2020-322744
171. Zhang L, Zhang L, Li H, Ge C, Zhao F, Tian H, et al. CXCL3 contributes to CD133+ CSCs maintenance and forms a positive feedback regulation loop with CD133 in HCC via Erk1/2 phosphorylation. Sci Rep. (2016) 6:27426. doi: 10.1038/srep27426
172. Steele CW, Karim SA, Leach JD, Bailey P, Upstill-Goddard R, Rishi L, et al. CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. Cancer Cell. (2016) 29:832–45. doi: 10.1016/j.ccell.2016.04.014
173. Wu K, Yu S, Liu Q, Bai X, Zheng X, and Wu K. The clinical significance of CXCL5 in non-small cell lung cancer. OncoTargets Ther. (2017) 10:5561–73. doi: 10.2147/OTT.S148772
174. Roca H, Jones JD, Purica MC, Weidner S, Koh AJ, Kuo R, et al. Apoptosis-induced CXCL5 accelerates inflammation and growth of prostate tumor metastases in bone. J Clin Invest. (2018) 128:248–66. doi: 10.1172/JCI92466
175. Mao Z, Zhang J, Shi Y, Li W, Shi H, Ji R, et al. CXCL5 promotes gastric cancer metastasis by inducing epithelial-mesenchymal transition and activating neutrophils. Oncogenesis. (2020) 9:63. doi: 10.1038/s41389-020-00249-z
176. Li J, Tang Z, Wang H, Wu W, Zhou F, Ke H, et al. CXCL6 promotes non-small cell lung cancer cell survival and metastasis via down-regulation of miR-515-5p. BioMed Pharmacother. (2018) 97:1182–8. doi: 10.1016/j.biopha.2017.11.004
177. Liu G, An L, Zhang H, Du P, and Sheng Y. Activation of CXCL6/CXCR1/2 axis promotes the growth and metastasis of osteosarcoma cells in vitro and in vivo. Front Pharmacol. (2019) 10:307. doi: 10.3389/fphar.2019.00307
178. Dufies M, Giuliano S, Viotti J, Borchiellini D, Cooley LS, Ambrosetti D, et al. CXCL7 is a predictive marker of sunitinib efficacy in clear cell renal cell carcinomas. Br J Cancer. (2017) 117:947–53. doi: 10.1038/bjc.2017.276
179. Wu Q, Tu H, and Li J. Multifaceted roles of chemokine CXC motif ligand 7 in inflammatory diseases and cancer. Front Pharmacol. (2022) 13:914730. doi: 10.3389/fphar.2022.914730
180. Li Y, Cheng J, Li Y, Jiang Y, Ma J, Li Q, et al. CXCL8 is associated with the recurrence of patients with acute myeloid leukemia and cell proliferation in leukemia cell lines. Biochem Biophys Res Commun. (2018) 499:524–30. doi: 10.1016/j.bbrc.2018.03.181
181. Ding Q, Lu P, Xia Y, Ding S, Fan Y, Li X, et al. CXCL9: evidence and contradictions for its role in tumor progression. Cancer Med. (2016) 5:3246–59. doi: 10.1002/cam4.934
182. Lan X, Xiao F, Ding Q, Liu J, Liu J, Li J, et al. The effect of CXCL9 on the invasion ability of hepatocellular carcinoma through up-regulation of PREX2. J Mol Histol. (2014) 45:689–96. doi: 10.1007/s10735-014-9593-0
183. Wang J, Peng Z, Guo J, Wang Y, Wang S, Jiang H, et al. CXCL10 recruitment of Γδ T cells into the hypoxic bone marrow environment leads to IL17 expression and multiple myeloma progression. Cancer Immunol Res. (2023) 11:1384–99. doi: 10.1158/2326-6066.CIR-23-0088
184. Wang J, Ouyang X, Zhu W, Yi Q, and Zhong J. The Role of CXCL11 and its Receptors in Cancer: Prospective but Challenging Clinical Targets. Cancer Control. (2024) 31:10732748241241162. doi: 10.1177/10732748241241162
185. Gao SH, Liu SZ, Wang GZ, and Zhou GB. CXCL13 in cancer and other diseases: biological functions, clinical significance, and therapeutic opportunities. Life. (2021) 11:1282. doi: 10.3390/life11121282
186. Kazanietz MG, Durando M, and Cooke M. CXCL13 and its receptor CXCR5 in cancer: inflammation, immune response, and beyond. Front Endocrinol. (2019) 10:471. doi: 10.3389/fendo.2019.00471
187. Lubowicka E, Przylipiak A, Zajkowska M, Piskór BM, Malinowski P, Fiedorowicz W, et al. Plasma chemokine CCL2 and its receptor CCR2 concentrations as diagnostic biomarkers for breast cancer patients. BioMed Res Int. (2018) 2018:1–9. doi: 10.1155/2018/2124390
188. Kodama T, Koma Yi, Arai N, Kido A, Urakawa N, Nishio M, et al. CCL3–CCR5 axis contributes to progression of esophageal squamous cell carcinoma by promoting cell migration and invasion via Akt and ERK pathways. Lab Invest. (2020) 100:1140–57. doi: 10.1038/s41374-020-0441-4
189. Lu CC, Tsai HC, Yang DY, Wang SW, Tsai MH, Hua CH, et al. The chemokine CCL4 stimulates angiopoietin-2 expression and angiogenesis via the MEK/ERK/STAT3 pathway in oral squamous cell carcinoma. Biomedicines. (2022) 10:1612. doi: 10.3390/biomedicines10071612
190. Garrido F, Wild CM, Mittelberger J, Dobler F, Schneider M, Ansorge N, et al. The role of chemokines in cervical cancers. Medicina (Mex). (2021) 57:1141. doi: 10.3390/medicina57111141
191. Aldinucci D, Borghese C, and Casagrande N. The CCL5/CCR5 axis in cancer progression. Cancers. (2020) 12:1765. doi: 10.3390/cancers12071765
192. Wertel I, Surówka J, Polak G, Barczyński B, Bednarek W, Jakubowicz-Gil J, et al. Macrophage-derived chemokine CCL22 and regulatory T cells in ovarian cancer patients. Tumor Biol. (2015) 36:4811–7. doi: 10.1007/s13277-015-3133-8
193. Gan W, Sun BY, Yang ZF, Ye C, Wang ZT, Zhou C, et al. Enhancing hepatocellular carcinoma management: prognostic value of integrated CCL17, CCR4, CD73, and HHLA2 expression analysis. J Cancer Res Clin Oncol. (2024) 150:325. doi: 10.1007/s00432-024-05832-0
194. Zhang A, Xu Y, Xu H, Ren J, Meng T, Ni Y, et al. Lactate-induced M2 polarization of tumor-associated macrophages promotes the invasion of pituitary adenoma by secreting CCL17. Theranostics. (2021) 11:3839–52. doi: 10.7150/thno.53749
195. Potential role of CCL27 and CCR10 expression in melanoma progression and immune escape - PubMed. Available online at.
196. Syed M, Dishman AF, Volkman BF, and Walker TL. The multifaceted role of XCL1 in health and disease. Protein Sci. (2025) 34:e70032. doi: 10.1002/pro.70032
197. Liu W, Jiang L, Bian C, Liang Y, Xing R, Yishakea M, et al. Role of CX3CL1 in diseases. Arch Immunol Ther Exp (Warsz). (2016) 64:371–83. doi: 10.1007/s00005-016-0395-9
198. Dairaghi DJ, Oyajobi BO, Gupta A, McCluskey B, Miao S, Powers JP, et al. CCR1 blockade reduces tumor burden and osteolysis in vivo in a mouse model of myeloma bone disease. Blood J Am Soc Hematol. (2012) 120:1449–57. doi: 10.1182/blood-2011-10-384784
199. Moore DC, Elmes JB, Shibu PA, Larck C, and Park SI. Mogamulizumab: an anti-CC chemokine receptor 4 antibody for T-cell lymphomas. Ann Pharmacother. (2020) 54:371–9. doi: 10.1177/1060028019884863
200. Ma Y, Mattarollo SR, Adjemian S, Yang H, Aymeric L, Hannani D, et al. CCL2/CCR2-dependent recruitment of functional antigen-presenting cells into tumors upon chemotherapy. Cancer Res. (2014) 74:436–45. doi: 10.1158/0008-5472.CAN-13-1265
201. Letourneur D, Danlos FX, and Marabelle A. Chemokine biology on immune checkpoint–targeted therapies. Eur J Cancer. (2020) 137:260–71. doi: 10.1016/j.ejca.2020.06.009
202. Wu A, Maxwell R, Xia Y, Cardarelli P, Oyasu M, Belcaid Z, et al. Combination anti-CXCR4 and anti-PD-1 immunotherapy provides survival benefit in glioblastoma through immune cell modulation of tumor microenvironment. J Neurooncol. (2019) 143:241–9. doi: 10.1007/s11060-019-03172-5
203. Zhang P, Zhang G, and Wan X. Challenges and new technologies in adoptive cell therapy. J Hematol OncolJ Hematol Oncol. (2023) 16:97. doi: 10.1186/s13045-023-01492-8
204. Pérez-Amill L, Bataller À, Delgado J, Esteve J, Juan M, and Klein-González N. Advancing CART therapy for acute myeloid leukemia: recent breakthroughs and strategies for future development. Front Immunol. (2023) 14:1260470. doi: 10.3389/fimmu.2023.1260470
205. Cheng Z, Cui X, Li S, Liang Y, Yang W, Ouyang J, et al. Harnessing cytokines to optimize chimeric antigen receptor-T cell therapy for gastric cancer: Current advances and innovative strategies. BioMed Pharmacother. (2024) 178:117229. doi: 10.1016/j.biopha.2024.117229
206. Tian Y, Zhang L, Ping Y, Zhang Z, Yao C, Shen C, et al. CCR5 and IL-12 co-expression in CAR T cells improves antitumor efficacy by reprogramming tumor microenvironment in solid tumors. Cancer Immunol Immunother. (2025) 74:55. doi: 10.1007/s00262-024-03909-w
207. Oh MS, Dumitras C, Salehi-Rad R, Tran LM, Krysan K, Lim RJ, et al. Characteristics of a CCL21 gene–modified dendritic cell vaccine utilized for a clinical trial in non–small cell lung cancer. Mol Cancer Ther. (2025) 24:286–98. doi: 10.1158/1535-7163.MCT-24-0435
Keywords: tumour microenvironment, chemokines, immune surveillance, immunotherapy, angiogenesis
Citation: Xavier H, Gireesh AGM, Thomas JA, Suboj P, Suresh A, Biju E, Baby A, Dominic RT and Babykutty S (2025) Chemokines: humble yet mighty players in the tumour microenvironment. Front. Immunol. 16:1601756. doi: 10.3389/fimmu.2025.1601756
Received: 28 March 2025; Accepted: 15 July 2025;
Published: 07 August 2025.
Edited by:
Petra Korać, University of Zagreb, CroatiaReviewed by:
Rodney Macedo Gonzales, Germans Trias i Pujol Health Science Research Institute, SpainAnn Richmond, Vanderbilt University, United States
Copyright © 2025 Xavier, Gireesh, Thomas, Suboj, Suresh, Biju, Baby, Dominic and Babykutty. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suboj Babykutty, c3Vib2ouYmFieWt1dHR5QG1pYy5hYy5pbg==
†These authors have contributed equally to this work
 Hima Xavier
Hima Xavier Athira Gireesh Gireesh Moly
Athira Gireesh Gireesh Moly Juvin Ann Thomas
Juvin Ann Thomas Priya Suboj
Priya Suboj Arya Suresh
Arya Suresh Emmanuel Biju
Emmanuel Biju Arya Baby
Arya Baby Roshin Thomas Dominic
Roshin Thomas Dominic Suboj Babykutty
Suboj Babykutty