- Department of Radiation Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen, China
Background: The average movement of esophagus is about 0.15 to 0.4 cm radially and 0.3 to 0.9 cm in the superior-inferior during radiotherapy. Little study has reported giant shift of esophagus during neoadjuvant chemotherapy and immunotherapy.
Case presentation: Here we presented a case of a 72-year-old male patient diagnosed with advanced lower thoracic esophageal squamous cell cancer, clinical T2N2M1, stage IVB (pulmonary metastasis). The esophagus moved about 4.2 cm radially from the right to the left of the aorta after 2 cycles of chemotherapy and immunotherapy.
Conclusion: All in all, we treated a patient with advanced lower thoracic esophageal squamous cell cancer that occurred a dramatic radial escape of about 4.2 cm after 2 cycles of chemotherapy and immunotherapy.
Introduction
Esophageal cancer, one of the most common malignancies, is ranked 7th in incidence and 6th in mortality globally in 2020 (1). According to its pathological characteristics, esophageal cancer can be divided into two types, esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC), which have substantial differences in behavioral characteristics, treatment and prognosis. Chemotherapy combined with immunotherapy is the standard first-line treatment for distant metastatic esophageal cancer (2, 3). However, the efficacy has reached a plateau. Recent studies have indicated that systemic therapy plus local radiotherapy can not only improve the swallowing status and improve the quality of life of patients, but also stimulate the immune response, which ultimately results in clinical benefits for patients (4–6). For resectable or potentially resectable locally advanced ESCC, the standard therapy strategy is neoadjuvant chemoradiotherapy followed by surgery, as evidenced by the 43-49% pathologic complete response rate and 60% 5-year overall survival rate in the CROSS study and NEOCRTEC5010 study (7–10). For unresectable locally advanced ESCC, definitive chemoradiotherapy is the standard treatment. Herein, we reported a patient with advanced lower thoracic esophageal squamous cell cancer whose esophagus moved about 4.2 cm radially from the right to the left of the aorta after 2 cycles of chemotherapy and immunotherapy.
Case presentation
A 72-year-old Chinese male patient was diagnosed with lower thoracic esophageal squamous cell cancer (cT2N2M1, stage IVB with pulmonary metastasis, AJCC 8th Edition) at National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. The esophagogastroduodenoscopy revealed a ring of 1/2 week ulcer mass extending from 34 to 39 cm from the incisors. The positron emission tomography computed tomography (PET/CT) showed a fludeoxyglucose avid primary mass with pulmonary metastasis (Figure 1). Therefore, the patient first received 4 cycles of chemotherapy and immunotherapy, followed by local palliative radiotherapy and immunotherapy. The chemotherapy and immunotherapy regimen were paclitaxel, cisplatin and Pembrolizumab. Interestingly, the CT revealed that malignant lesion of esophagus shifted about 4.2 cm radially from the right to the left of the aorta after 2 cycles of chemotherapy and immunotherapy. (Figures 2, 3) Moreover, there was a decrease in crosssectional area of malignant lesion. (Figure 2). The patient continued to follow the planned treatment regimen and the esophagus never shifted back to the location seen in the staging PET/CT scan before treatment.
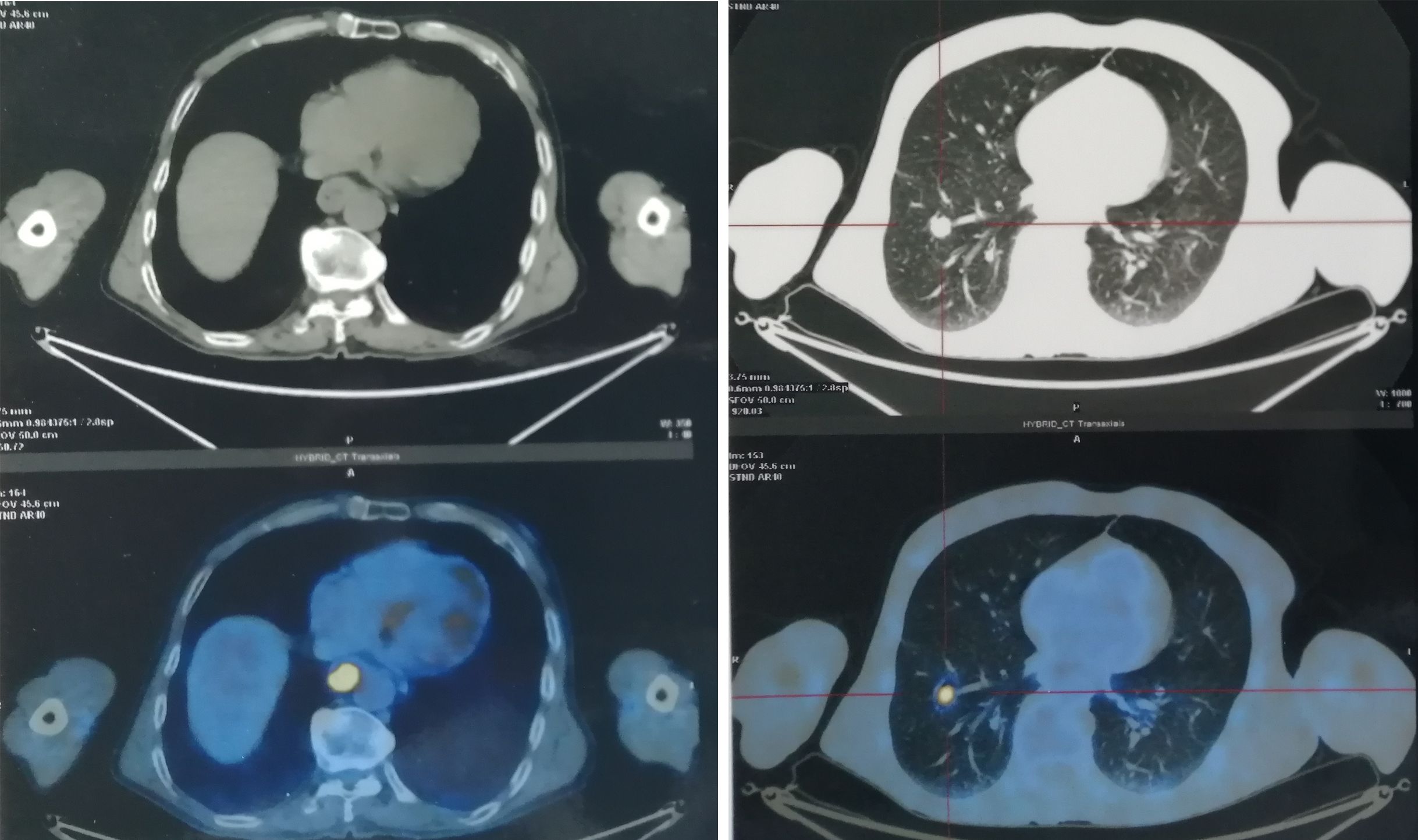
Figure 1. The PET/CT images showed a fludeoxyglucose avid primary mass of esophagus with pulmonary metastasis before treatment.
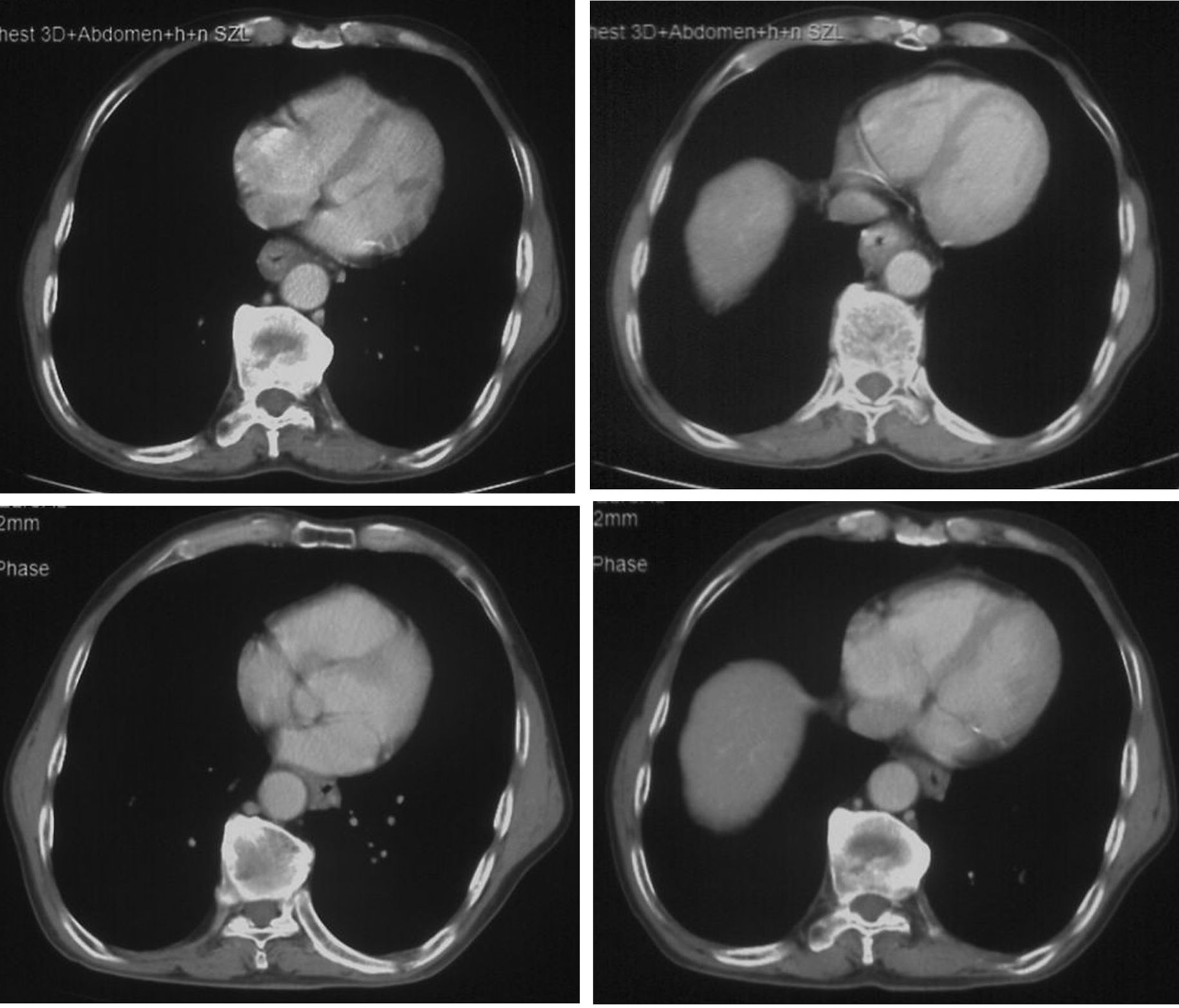
Figure 2. The CT images showed that esophagus had a dramatic radial escape after 2 cycles of chemotherapy and immunotherapy (lower) compared with the images before treatment (upper).
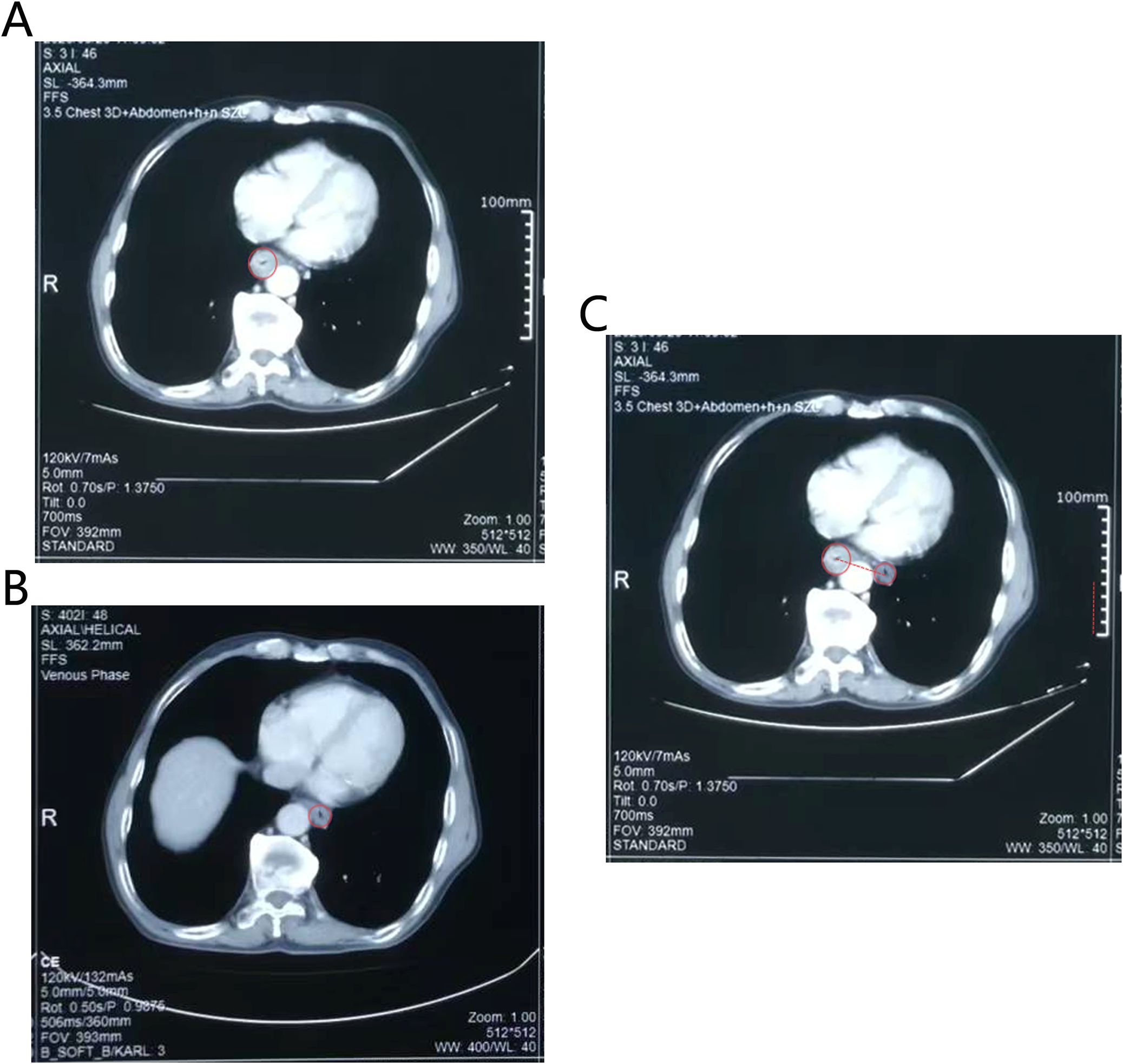
Figure 3. The CT images showed that esophagus had a dramatic radial escape after treatment. (A) The position of the esophagus before treatment. (B) The position of the esophagus after treatment. (C) The changes in the position of the esophagus before and after treatment. The red dotted line represented the distance that the esophagus moved. Technical details was in the CT images (e.g., slice thickness: 5mm).
Discussion
The primary first-line treatment for advanced esophageal cancer is chemotherapy plus immunotherapy (11, 12). However, as for locally advanced or node positive esophageal cancer, chemoradiotherapy is the main initial treatment strategy, with almost double the survival rate in the preoperative setting compared with surgery alone (9). Interestingly, a studies has shown that chemoradiotherapy combined with immunotherapy can improve the efficacy of patients with inoperable esophageal cancer and the toxicity is acceptable (13). Phase III studies of concurrent chemoradiotherapy combined with immunotherapy for inoperable esophageal cancer (e.g. Keynote975, Rationale311, KUNLUN et al.) are ongoing (14–16).
In light of the position near the lungs and diaphragm, the movement of esophagus is closely related to breathing, cardiac movement, and peristalsis (17–19). In addition, the esophagus shrinks in volume and tends to move from right to left during the treatment (20, 21). Studies have revealed that the average movement of the esophagus is about 0.15 to 0.4 cm radially and 0.3 to 0.9 cm in the superior-inferior during radiation therapy (20–22). However, we found an interesting phenomenon that there was a large escape of the esophagus after chemotherapy combined with immunotherapy, with a movement of about 4.2cm radially from the right to the left of the aorta in our patient. This dramatic escape was intricate and the underlying cause was still unclear. Until now, there were no specific biomarkers to explain this mechanism. Moreover, there was a pathological diagnosis before treatment, and taking pathological samples again after treatment was unacceptable to the patients and their families. Therefore, it was unknown whether there was treatment-induced fibrosis or immunotherapy-related inflammatory responses resulted in the huge movement of the esophagus. The case gives us an attention that radiation oncologists should focus on cone beam CT (CBCT) scans (once or twice a week) in order to timely discover poor target coverage of esophageal cancer when using chemoradiotherapy combined with immunotherapy.
The landmark CROSS trial had demonstrated that esophageal tumor contours were expanded with 1.5 cm radial and a 3- to 4-cm craniocaudal margin (9). Recent studies indicated that the large majority of tumor targets were covered by about 2 to 7.5 mm radially and 5 to 15 mm craniocaudally, with distal tumors requiring more distance (23). Despite the rapid development of radiation technology and machines, poor target coverage and toxicity of adjacent normal tissues is still a problem that cannot be ignored during radiotherapy. Image guidance, such as daily KV imaging or daily CBCT (once or twice a week), is an essential solution to this dilemma. Part of patients require resimulation and adaptive planning. a study suggested that radiotherapy treatment in esophageal cancer was adapted if the volume receiving 95% of the prescribed dose (V95%) coverage of CTV decreased > 1% or planning target volume (PTV) decreased by > 3% (24). However, this criteria of adaptive planning is definitely not applicable to this case, because of the huge displacement of the esophagus. Take this case for example. If the patient was diagnosed with locally advanced esophageal cancer and treated with combination of chemoradiotherapy and immunotherapy, he would benefit from twice or more a week CBCT and resimulation.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Cancer Hospital & Shenzhen Hospital Chinese Academy of Medical Sciences. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
RL: Writing – original draft, Writing – review & editing. JJ: Data curation, Methodology, Writing – original draft. JZ: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. JL: Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Shenzhen Science and Technology Program (grant no. KCXFZ20211020172542002), the Shenzhen Science and Technology Program (grant no. JCYJ20220530153801004), the Sanming Project of Medicine in Shenzhen (grant nos. SZSM202211030), Shenzhen Key Medical Discipline Construction Fund (No.SZXK013), Shenzhen Clinical Research Center for Cancer (No.[2021]287), and the Shenzhen High-level Hospital Construction Fund (No. grant number).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yang H, Wang F, Hallemeier CL, Lerut T, and Fu J. Oesophageal cancer. Lancet. (2024) 404:1991–2005. doi: 10.1016/S0140-6736(24)02226-8
2. Patel MA, Kratz JD, Lubner SJ, Loconte NK, and Uboha NV. Esophagogastric cancers: integrating immunotherapy therapy into current practice. J Clin Oncol. (2022) 40:2751–62. doi: 10.1200/JCO.21.02500
3. National Comprehensive Cancer Network. Esophageal and esophagogastric junction cancers(2022). Available online at: https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf/ (Accessed December 5, 2022).
4. Kroese TE, Buijs GS, Burger MDL, Ruurda JP, Mook S, Brosens LAA, et al. Metastasectomy or stereotactic body radiation therapy with or without systemic therapy for oligometastatic esophagogastric cancer. Ann Surg Oncol. (2022) 29:4848–57. doi: 10.1245/s10434-022-11541-0
5. Seyedin SN, Parekh KR, Ginader T, and Caster JM. The role of definitive radiation and surgery in metastatic esophageal cancer: an NCDB investigation. Ann Thorac Surg. (2021) 112:459–66. doi: 10.1016/j.athoracsur.2020.08.034
6. Li J, Wen YX, Xiang ZZ, Du H, Geng L, Yang X, et al. Radical radiotherapy for metachronous oligometastasis after initial treatment of esophageal cancer. Radiother Oncol. (2021) 154:201–6. doi: 10.1016/j.radonc.2020.09.042
7. Yang H, Liu H, Chen Y, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): A phase III multicenter, randomized, open-label clinical trial. J Clin Oncol. (2018) 36:2796–803. doi: 10.1200/JCO.2018.79.1483
8. Yang H, Liu H, Chen YP, Zhu C, Fang W, Yu Z, et al. Long-term efficacy of neoadjuvant chemoradiotherapy plus surgery for the treatment of locally advanced esophageal squamous cell carcinoma: the NEOCRTEC5010 randomized clinical trial. JAMA Surg. (2021) 156:721–9. doi: 10.1001/jamasurg.2021.2373
9. van Hagen P, Hulshof MCCM, van Lanschot JJB, Steyerberg EW, van Berge HMI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. (2012) 366:2074–84. doi: 10.1056/NEJMoa1112088
10. Eyck BM, van Lanschot JJB, Hulshof MCCM, van der Wilk BJ, Shapiro J, van Hagen P, et al. Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: the randomized controlled CROSS trial. J Clin Oncol. (2021) 39:1995–2004. doi: 10.1200/JCO.20.03614
11. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. (2021) 398:27–40. doi: 10.1016/S0140-6736(21)00797-2
12. Wang ZX, Cui CX, Yao J, Zhang Y, Li M, Feng J, et al. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cell. (2022) 40(3):277–88.e3. doi: 10.1016/j.ccell.2022.02.007
13. Zhang WC, Yan CH, Gao X, Li X, Cao F, Zhao G, et al. Safety and feasibility of radiotherapy plus camrelizumab for locally advanced esophageal squamous cell carcinoma. Oncologist. (2021) 26:e1110–24. doi: 10.1002/onco.13797
14. Shah MA, Bennouna J, Doi T, Shen L, Kato K, Adenis A, et al. KEYNOTE-975 study design: a Phase III study of definitive chemoradiotherapy plus pembrolizumab in patients with esophageal carcinoma. Future Oncol. (2021) 17:1143–53. doi: 10.2217/fon-2020-0969
15. Wang WF, Li JC, and Li t. A phaseIIl trial in progress comparing tislelizumabplus concurrent chemoradiotherapy (cCRT) with placebo plus cCRT in patients with localized esophageal squamous cell carcinoma (ESCC). ASCO-GI. (2020) abstract TPS475.
16. Wang LH, Chen M, and Kato K. A phase 3 randomized, double-blind, placebo-controlled, multicenter, global study of durvalumab with and after chemoradiotherapy in patients with locally advanced, unresectable esophageal squamous cell carcinoma: KUNLUN. ASCO-GI. (2022) abstract TPS373:74. doi: 10.1200/JCO.2022.40.4_suppl.TPS373
17. Lorchel F, Dumas JL, Noël A, Wolf D, Bosset JF, and Aletti P. Esophageal cancer: determination of internal target volume for conformal radiotherapy. Radiother Oncol. (2006) 80:327–32. doi: 10.1016/j.radonc.2006.08.003
18. Zhao KL, Liao ZX, Bucci MK, Komaki R, Cox JD, H Yu ZQ, et al. Evaluation of respiratory-induced target motion for esophageal tumors at the gastroesophageal junction. Radiother Oncol. (2007) 84:283–9. doi: 10.1016/j.radonc.2007.07.008
19. Yamashita H, Kida S, Sakumi A, Haga A, Ito S, Onoe T, et al. Four-dimensional measurement of the displacement of internal fiducial markers during 320-multislice computed tomography scanning of thoracic esophageal cancer. Int J Radiat Oncol Biol Phys. (2011) 79:588–95. doi: 10.1016/j.ijrobp.2010.03.045
20. Wang JY, Lin SH, Dong L, Balter P, Mohan R, Komaki R, et al. Quantifying the interfractional displacement of the gastroesophageal junction during radiation therapy for esophageal cancer. Int J Radiat Oncol Biol Phys. (2012) 83:e273–80. doi: 10.1016/j.ijrobp.2011.12.048
21. Cohen RJ, Paskalev K, Litwin S, Price RA Jr, Feigenberg SJ, and Konski AA. Esophageal motion during radiotherapy: quantification and margin implications. Dis Esophagus. (2010) 23:473–9. doi: 10.1111/j.1442-2050.2009.01037.x
22. Fukada J, Hanada T, Kawaguchi O, Ohashi T, Takeuchi H, and Kitagawa Y. Detection of esophageal fiducial marker displacement during radiation therapy with a 2-dimensional on-board imager: analysis of internal margin for esophageal cancer. Int J Radiat Oncol Biol Phys. (2013) 85:991–8. doi: 10.1016/j.ijrobp.2012.07.2358
23. Ajani JA, D’Amico TA, and Bentrem DJ. Esophageal and esophagogastric junction cancers, version 2.2023, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2023) 21:393–422. doi: 10.6004/jnccn.2023.0019
Keywords: esophageal cancer, chemotherapy, immunotherapy, motion, radiotherapy
Citation: Liang R, Jin J, Zhang J and Liang J (2025) Case Report: The huge esophageal displacement: a case of dramatic esophageal interfraction motion during neoadjuvant chemotherapy and immunotherapy. Front. Immunol. 16:1602186. doi: 10.3389/fimmu.2025.1602186
Received: 29 March 2025; Accepted: 26 June 2025;
Published: 24 July 2025.
Edited by:
Xiaomeng Dai, Zhejiang University, ChinaReviewed by:
Jiaying Deng, Fudan University, ChinaYuan Tian, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, China
Copyright © 2025 Liang, Jin, Zhang and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Jin, amluamluZ0Bjc2NvLm9yZy5jbg==; Jianghu Zhang, emhhbmdqaWFuZ2h1Z2RAMTI2LmNvbQ==; Jun Liang, anVubGlhbmdzemNoQDE2My5jb20=
 Renba Liang
Renba Liang Jing Jin*
Jing Jin* Jianghu Zhang
Jianghu Zhang