- 1Department of Laboratory Medicine, Laboratory of Medical Immunology, Radboud University Medical Center, Nijmegen, Netherlands
- 2Department of Transplantation Immunology, Maastricht University Medical Center+, Maastricht, Netherlands
- 3GROW – Research Institute for Oncology and Reproduction, Maastricht University, Maastricht, Netherlands
- 4Department of Obstetrics and Gynecology, Maastricht University Medical Center+, Maastricht, Netherlands
- 5Department of Obstetrics and Gynecology, Radboud University Medical Center, Nijmegen, Netherlands
Introduction: Recurrent pregnancy loss (RPL) is associated with altered immune phenotypes and functions. It has been proposed that physical exercise might impact the immune system. Therefore, we evaluated the effect of a personalized 3-month moderate-intensity aerobic exercise intervention on the immune system of women with unexplained RPL (uRPL). Given the suggested supportive role of Natural Killer (NK) cells during early pregnancy, we focused on numerical, phenotypic, and functional changes in peripheral NK (pNK) and uterine NK (uNK) cells.
Methods: Mononuclear cells were isolated from peripheral blood (PB) (n=23) and menstrual blood (MB) (n=22) of women with uRPL. NK cell phenotypes were assessed with comprehensive flow cytometry panels. NK cell function was assessed with degranulation assays and intracellular staining of interferon-γ (IFN-γ), perforin and granzyme-B in a subgroup of women due to lower availability of samples (n=12).
Results: Attendance to the exercise intervention was overall 95%, which resulted in effects on the phenotype and function of pNK cells. We found a significant reduction in the median fluorescent intensity of CD161 (464 vs 410, p=0.011), NKp30 (432 vs 376, p=0.018), and NKG2A (886 vs 732, p=0.039) in pNK cells after exercise, while no differences were observed in uNK cells. We also observed decreased percentages of IFN-γ+ pNK cells (49% vs 25.2%, p=0.027) after exercise.
Discussion: Our study shows promising results, suggesting that exercise can impact pNK cell phenotype and function in women with uRPL. Following the changes in pNK phenotype and function suggest a lower pro-inflammatory state post-exercise. Whether these exercise-induced phenotypic and functional changes of pNK cells impact subsequent pregnancies remains to be studied. The study details are available through HYPERLINK “https://clinicaltrials.gov/”Home | ClinicalTrials.gov, trial ID: HMOVE
1 Introduction
Recurrent pregnancy loss (RPL) is a pregnancy disorder affecting 1-2% of women attempting to conceive and is defined by the failure of two or more clinically recognized pregnancies before 24 weeks of gestation (1). The etiologies for RPL include advanced maternal age, abnormal parental karyotyping (if indicated), endocrine disorders, uterine abnormalities, antiphospholipid syndrome, lifestyle factors, and embryonic factors (1). In addition, women with RPL have a higher incidence of metabolic disorders (2–4), and an elevated risk of developing cardiovascular disease (5–7). However, in approximately 50% of cases, the underlying cause remains unknown, referred to as “unexplained” RPL (uRPL) (8).
It is well-recognized that the maternal immune system plays a critical role in the establishment and maintenance of pregnancy (9, 10). During early pregnancy, 70% of all infiltrating leukocytes are uterine Natural Killer (uNK) cells (11). They are primarily CD56brightCD16- and exhibit functional and phenotypic differences compared to peripheral NK (pNK) cells, which are predominantly CD56dimCD16+ (12). It is known that uNK cells are involved in regulating trophoblast invasion and spiral artery remodeling (13–16). Numerous studies have investigated the role of NK cells in the etiology of RPL by examining uterine tissue and peripheral blood (PB) (15, 17–19). Some of these studies reported altered numbers and percentages of NK cells in the endometrium and decidua (20, 21) of women with RPL compared to controls. Additionally, several studies have demonstrated altered NK cell receptor expression (20, 22–25) and increased NK cell cytotoxicity in women with RPL (26–28). However, there are also studies indicating no changes in NK cells of women with RPL (29, 30).
Multiple studies have shown that physical exercise can modulate the immune system and influence the distribution, phenotype, and activity of different immune cell subsets, as summarized in various reviews (31–33). NK cells seem to be particularly responsive to exercise, acutely mobilizing into the circulation immediately after exercise (34–36). Notably, this mobilization primarily involves more mature and differentiated NKG2A-/Killer Immunoglobulin-like (KIR)+ NK cells compared to the less mature NKG2A+/KIR- NK cells in PB (37). It is hypothesized that after rapid mobilization to the bloodstream NK cells redistribute to peripheral tissues, as a drop in circulating NK cells to baseline levels was observed 2 hours after acute exercise in men (38). Moreover, a recent meta-analysis showed that the cytolytic activity of NK cells increases after acute exercise (39). However, the effects of exercise on tissue-resident immune cells, such as those in the uterine environment, remain understudied.
Considering altered numbers, phenotypes and function of NK cells have been reported in uRPL, we aimed to investigate the potential effects of a moderate-intensity aerobe exercise intervention on NK cell percentages, phenotype and function. Moreover, we have also investigated the impact of exercise on different T- and B-cell population frequencies. In this feasibility study, we focused on the impact of a three-month moderate-intensity aerobic exercise intervention on both peripheral immune cells and uterine immune cells by sampling menstrual blood (MB) as source of uterine-derived immune cells (40).
2 Materials and methods
2.1 Study population
In this multi-center intervention study, 49 women with uRPL were included (Supplementary Figure 1). uRPL was defined as having two or more pregnancy losses before 24 weeks of gestation without an identifiable cause. Women were referred to the department of Obstetrics and Gynecology at either the Radboud University Medical Centre (RUMC) or the Maastricht University Medical Centre+ (MUMC+) and were included when no cause for their miscarriages could be identified according to the guidelines of the European Society of Human Reproduction and Embryology (ESHRE). The clinical work-up included a standardized medical history of the couple, screening for uterine abnormalities, thyroid abnormalities, anti-phospholipid syndrome, paternal screening for sperm DNA fragmentation and parental karyotyping (if indicated) (41). Participants were excluded from the study if they met one or more of the exclusion criteria presented in Supplementary Table 1.
This study was conducted in accordance with the Declaration of Helsinki, the Medical Research Involving Human Subjects Act, and the guidelines for Good Clinical Practice (GCP). After obtaining informed consent, according to the Medical Ethical Committee of the Radboud University Medical Centre and Maastricht University Medical Centre+ NL77307.091.21, women received a questionnaire for assessing baseline characteristics. PB and MB were collected for immunophenotyping.
The intervention consisted of a 12-week, personalized, moderate-intensity, aerobe exercise program for a stationary bike. The exercise program was personalized to individual heart rate reserves (HRR). The first six weeks the participants were instructed to train two times a week for 50 minutes at 50-60% of their HRR, the subsequent six weeks for three times a week for one 50 minutes at 50-60% HRR. Their heart rate was monitored during exercise using a Polar H10 heartrate monitor chest strap (Polar Electro, Inc, Kempele Finland). Women needed to attend a minimum of 80% of 30 prescribed trainings after completion of the intervention in order to be eligible for data analysis. All women were asked not to change their pre-existing training schedules (if applicable) and not to change their diet during the intervention. Before and after the 12-week exercise intervention, identical immune analyses were conducted.
Of the 49 women who were included in the study, 24 women completed the intervention (data is shown only of these women) (Table 1). Both PB and MB samples were collected. However, due to collection issues, PB was available from 23 participants, and MB was available from 22 participants. Different figures might show variable numbers of women included in analyses if unreliable individual markers were excluded due to technical staining issues. Sufficient remaining cells (n=12) were frozen for later functional analyses.
2.2 Isolation of menstrual blood mononuclear cells and peripheral blood mononuclear cells
To study uterine lymphocytes, MB was collected using a menstrual cup (40) at 3 time points of 12 hours right after the start of menses, for a total of 36 hours. After every 12 hours, the cup was emptied in a new collection tube, containing RPMI1640 medium (ThermoFisher Scientific, Waltham, MA, USA) supplemented with 10% human pooled serum (HPS) (Zen-Bio, Durham, NC, USA), 1 mM pyruvate (ThermoFisher Scientific), 2 mM GlutaMAX™ (ThermoFisher Scientific), 100 U/mL penicilin/100 μg/mL streptomycin (ThermoFisher Scientific), and 0.3% sodium citrate (Merck, Darmstadt, Germany). PB (10 mL) was collected in ethylene diamine tetra acetic acid (EDTA) tubes.
PB was diluted 1:1 with phosphate buffered saline (PBS) (Fresenius Kabi, GmbH, Graz, Austria). The collected MB from 3 different timepoints was first pooled, after which it was washed with PBS. After centrifugation, the pellet of the MB was filtered through a 70 μm cell strainer (PluriSelect Life Science, Leipzig, Germany) by creating a vacuum using a connector ring (PluriSelect) and a 30 mL syringe (Terumo Corporation, Tokyo, Japan). The filtered MB was then incubated for 20 minutes at room temperature with RosetteSep™ Human Granulocyte Depletion Cocktail (STEMCELL Technologies, Vancouver, Canada) according to manufacturer’s instructions, after which the sample was diluted with an equal volume of PBS+2%HPS.
Subsequently, MB mononuclear cells (MBMCs) and PB mononuclear cells (PBMCs) were isolated using Lymphoprep (STEMCELL Technologies) according to manufacturer’s instructions. The samples were centrifuged for 15 min at 800g, no brake at 20°C. Isolated MBMCs and PBMCs were washed twice with PBS+2%HPS, and resuspended in RPMI1640 + 10% HPS.
2.3 Antibodies and flow cytometry analyses
Immunophenotyping of MBMCs and PBMCs was done using flow cytometry. For the phenotyping, 500.000 MBMCs and 250.000 PBMCs were used and stained in 50 μL and 25 μL respectively. First, the cells were stained for 30 minutes at 4°C with eBioscience™ Fixable Viability Dye eFluor 780 (ThermoFisher Scientific) to stain for dead cells. Subsequently, the cells were stained for 30 minutes at 4°C for specific surface markers stated in Supplementary Table 2. Because of the use of multiple BD Horizon™ Brilliant Violet Dyes (BD, Franklin Lakes, NJ, USA), BD Horizon™ Brilliant Staining Buffer (BD) was used in combination with regular FACS buffer (PBS+1%BSA+0.02% Sodium Azide (Merck, Rahway, NJ, USA)) according to manufacturer’s recommendations. After surface staining, all cells were fixated and permeabilized using the eBioscience™ Foxp3/Transcription Factor Staining Buffer Set according to the manufacturer’s instructions (ThermoFisher Scientific). After fixation/permeabilization, part of the cells were stained for the transcription factor FoxP3 (ThermoFisher Scientific). All samples were measured with standardized settings on the BD FACSLyric™ (BD). Data was analyzed using the FlowJo™ v10.8 software (Flowjo™, Ashland, OR, USA) as either a percentage or as Median Fluorescent Intensity (MFI). MFIs of immune markers were normalized against MFIs of Fluorescence Minus One (FMO) controls, critical to ensure that the signals observed are truly due to the marker of interest and not artefacts from overlapping fluorescent markers. Certain markers for specific signals, timepoints or participants could not be analyzed due to various reasons such as sample or reagent availability. PBMCs and MBMCs that were not directly used in the flow cytometry staining were frozen in Recovery™ Cell Culture Freezing Medium (ThermoFisher Scientific).
2.4 Degranulation assay and staining of intracellular molecules
To assess the functional capacity of NK cells in MB and PB, we performed a CD107a degranulation assay and an intracellular staining on frozen PBMCs and MBMCs. Following thawing of a subgroup of available samples (n=12), 200,000 cells were plated in a round-bottom 96-wells plate. Cells that were used to investigate CD107a and interferon-γ (IFN-γ) expression were stimulated with 100 U/mL IL-2 and 10 ng/mL IL-15. An unstimulated condition was taken as a control. The cells that were used to investigate granzyme B and perforin expression were left unstimulated. The cells were incubated at 37°C and 5% CO2. After 20 hours of incubation, 25 μL of a 1:50 dilution of CD107a-PE (BD Biosciences) was added to the respective wells. One hour later, 20,000 K562 target cells and 5 μg/mL of Brefeldin A and 5 μg/mL of Monensin were added to inhibit the internalization of CD107a molecules and enhance the detection of intracellular proteins. The cells in the wells for investigation of granzyme B and perforin expression were not stimulated with K562. The cells were then incubated for an additional 3 hours at 37°C in 5% CO2. Following the incubation period, cell viability was assessed, and surface markers were stained with the following antibodies: anti-CD16 Alexa Fluor 700 (BioLegend), anti-CD45 Krome Orange (Beckman Coulter), anti-CD3 BV605 (BD Biosciences), and anti-CD56 BV711 (BD Biosciences). Subsequently, intracellular staining was performed using anti-granzyme B PerCP-Cy5.5 (BioLegend), anti-IFNγ PE-Cy7 (ThermoFisher Scientific), and anti-perforin Pacific Blue (BioLegend) (Supplementary Table 2). All samples were analyzed on a BD FACSLyric™ flow cytometer (BD Biosciences).
2.5 Statistics
Percentages of immune cells or percentages/MFIs of immune markers before and after exercise were analyzed with a Wilcoxon signed-rank test. All analyses were performed using IBM SPSS statistics, version 29.0.0.0 (SPSS Inc, Chicago, IL, USA) and P-values <0.05 were considered statistically significant. The boxplots were prepared in RStudio using ggplot2 package (42, 43).
3 Results
3.1 Moderate-intensity aerobic exercise does not alter immune cell frequencies in MB and PB of women with uRPL
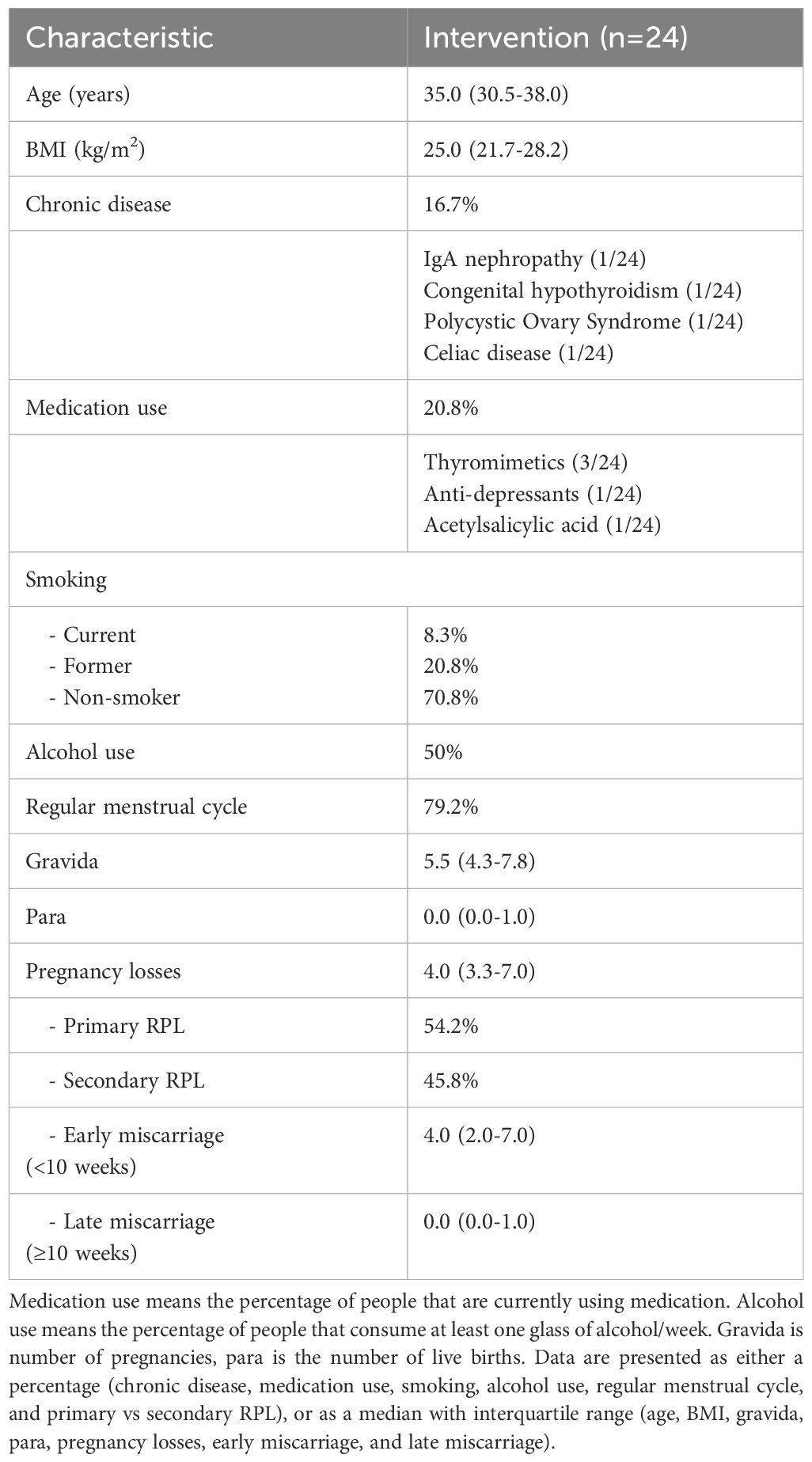
To determine the effect of exercise on specific immune cell subsets, we analyzed frequencies of T, B, and NK cells using high-dimensional flow cytometry in both PB and MB from women with uRPL (Figure 1A). Baseline characteristics, of the included 24 women who completed the intervention with an overall attendance of 95%, are shown in Table 1.
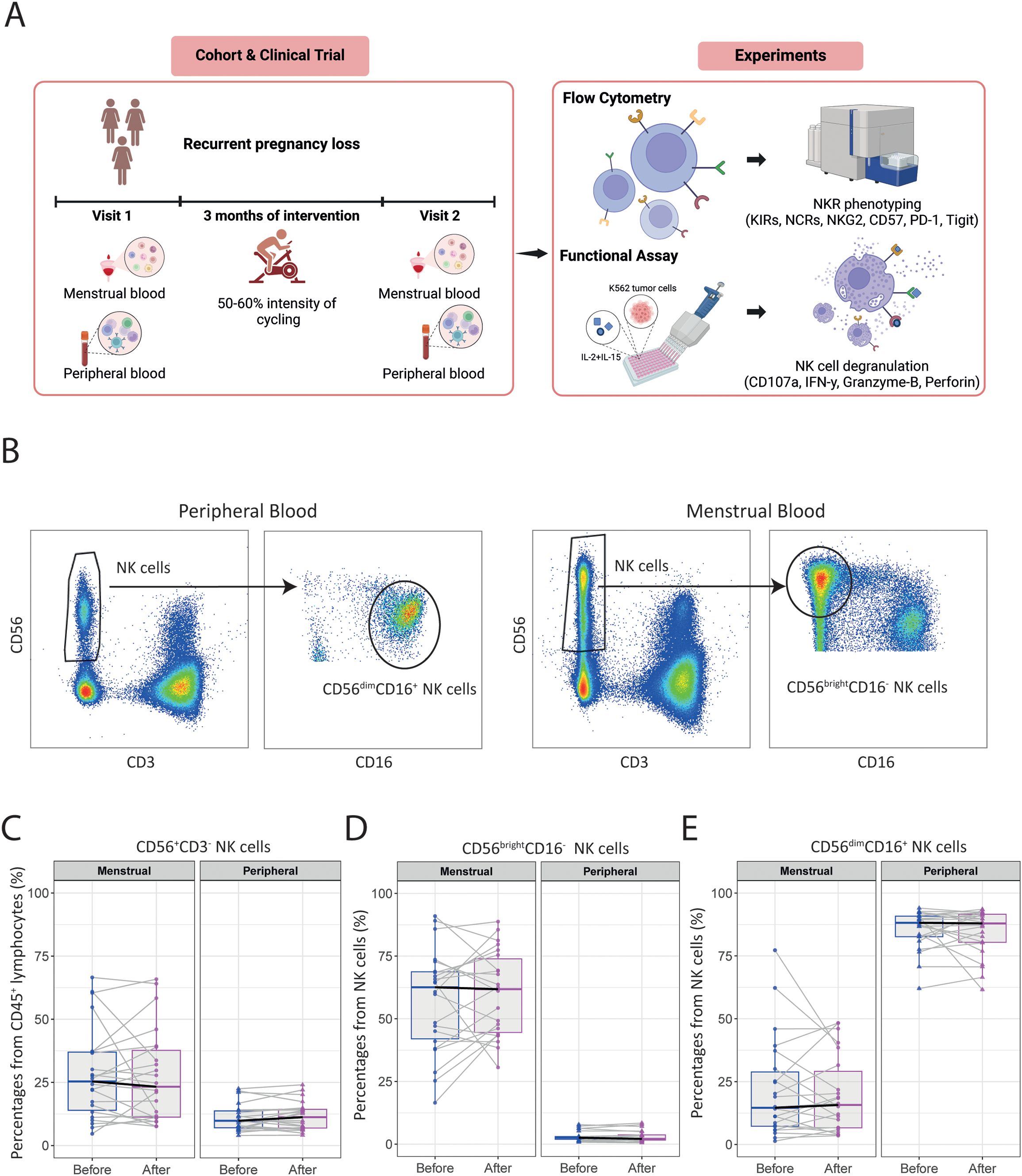
Figure 1. The moderate-intensity aerobic exercise intervention does not alter immune cell frequencies in MB or PB of women with uRPL. (A) Overview of the study and the experiments performed on the isolated immune cells. (B) Representative dot plots showing the gating strategies for NK cell subsets in PB and MB. (C) The percentage of total CD56+CD3- NK cells from CD45+ lymphocytes in MB and PB before and after the moderate-intensity aerobic exercise intervention. (D) The percentage of CD56brightCD16- NK cells of total NK cells. (E) The percentage of CD56dimCD16+ NK cells of total NK cells. PB n=23, MB n=22. The dots represent the uterine NK cells, the triangles the peripheral NK cells (blue=before intervention, magenta=after intervention). Boxplots visualize the median (bold black line) and the interquartile ranges. A Paired Wilcoxon signed-rank test was used to determine statistical significance.
The role of NK cells in PB and MB are different. While the majority of pNK cells consist of a subset of CD56dimCD16+ NK cells with high cytotoxic capabilities, uNK cells in MB mainly have a CD56brightCD16- phenotype and a immunoregulatory functions (12). Results of these two subsets (Figure 1B) revealed no significant differences in the frequency of total CD56+CD3-, CD56brightCD16- and CD56dimCD16+ NK cells after intervention in either PB or MB (Figures 1C–E). Interestingly, we observed significant heterogeneity in the baseline frequencies of uNK cells among women with uRPL, as well as in their response to the intervention. While some women exhibited increased frequencies of uNK cells, others showed a decrease post-intervention.
Likewise, the frequencies of total CD3+ T cells and their subsets (including conventional effector CD4+, cytotoxic CD8+ T, and CD127lowCD25+FoxP3+ regulatory T cells) remained similar in both PB and MB when comparing pre- and post-intervention measurements (Supplementary Figures 2A–C). Although there was a trend towards an increased frequency of total CD19+CD20+ B cells in PB as well as MB, the frequencies of B cell subsets did not change significantly after intervention (Supplementary Figures 2D–F).
3.2 CD161 (KLRB1) and NKp30 expression by pNK cells is decreased after a moderate-intensity aerobic exercise intervention in women with uRPL
To study the effect of the intervention on the phenotype of pNK and uNK cells, we performed comprehensive immunophenotyping by flow cytometry to evaluate changes in activating and inhibitory NK cell receptors (NKRs), frequently described to be involved in the regulation of NK cell activity.
We found a significant decrease in median fluorescent intensity (MFI) of CD161 (KLRB1) in total pNK cells (464 [364-623] to 410 [277-483]; p=0.011), in the CD56brightCD16- pNK cell subset (353 [247-478] to 296 [193-367]; p=0.033), and in the CD56dimCD16+ pNK cell subset (493 [393-648] to 424 [296-559]; p=0.035), while no significant differences were observed in uNK cells post-intervention (Figures 2A–D).

Figure 2. CD161 (KLRB1) and NKp30 expression by peripheral NK cells is decreased after the moderate-intensity aerobic exercise intervention in women with uRPL. (A) Representative histograms of CD161 expression by total NK cells (red), CD56dimCD16+ NK cells (blue), CD56brightCD16- NK cells (green), and the fluorescence minus one (FMO) control (gray) of MB and PB. (B) Median fluorescent intensity (MFI) of CD161 by total uterine NK (uNK) and peripheral NK (pNK) cells before and after the moderate-intensity aerobic exercise intervention. (C) MFI of CD161 by CD56brightCD16- NK cells. (D) MFI of CD161 by CD56dimCD16+ NK cells. (E) Representative histograms of NKp30 expression by total NK cells (red), CD56dimCD16+ NK cells (blue), CD56brightCD16- NK cells (green), and the FMO control (gray) of MB and PB. (F) MFI of NKp30 by NK cells. (G) MFI of NKp30 by CD56brightCD16- NK cells. (H) MFI of NKp30 by CD56dimCD16+ NK cells. PB n=21, MB n=22. All MFIs were normalized to the MFI of the FMO control. The dots represent the uNK cells, the triangles the pNK cells (blue=before intervention, magenta=after intervention). Boxplots visualize the median (bold black line) and the interquartile ranges. A Paired Wilcoxon signed-rank test was performed to determine statistical significance (*p<0.05, **p<0.005).
Among the natural cytotoxicity receptors (NCRs) analyzed, the MFI of NKp30 significantly decreased in total pNK cells (432 [317-494] to 376 [252-481]; p=0.018) and in the CD56dimCD16+ pNK subset (468 [290-550] to 369 [228-520]; p=0.004) post-intervention, whereas this was not observed in uNK cells (Figures 2E–H). No significant changes were found in the expression intensities of other NCRs (NKp44 and NKp46) after intervention (Supplementary Figures 3A, B).
3.3 Moderate-intensity aerobic exercise impacts NKG2A and NKG2C expression by pNK cells in women with uRPL
To study the effect of exercise on NK cell markers involved in maturation and memory, we analyzed the frequency and expression intensity of NKG2A and NKG2C before and after intervention. While the frequencies of NKG2A+ NK cells did not significantly change after intervention in either MB or PB (Supplementary Figures 4A–C), a marked decrease in the MFI of NKG2A was observed in total pNK cells (886 [781-1218] to 732 [555-962]; p=0.039) and CD56brightCD16+ pNK cells (2472 [1583-3116] to 1497 [1113-1790]; p=0.034) (Figures 3A–C). In contrast, uNK cells did not show any significant changes in NKG2A expression (Figures 3A–D). Furthermore, no significant changes were observed in both frequency and expression intensity of NKG2C in both MB and PB, except for a slight increase the percentage of in NKG2C+ CD56dimCD16+ pNK cells (5.4 [3.3-13.7] to 5.9 [2-8.9]; p=0.021) (Supplementary Figure 4F).
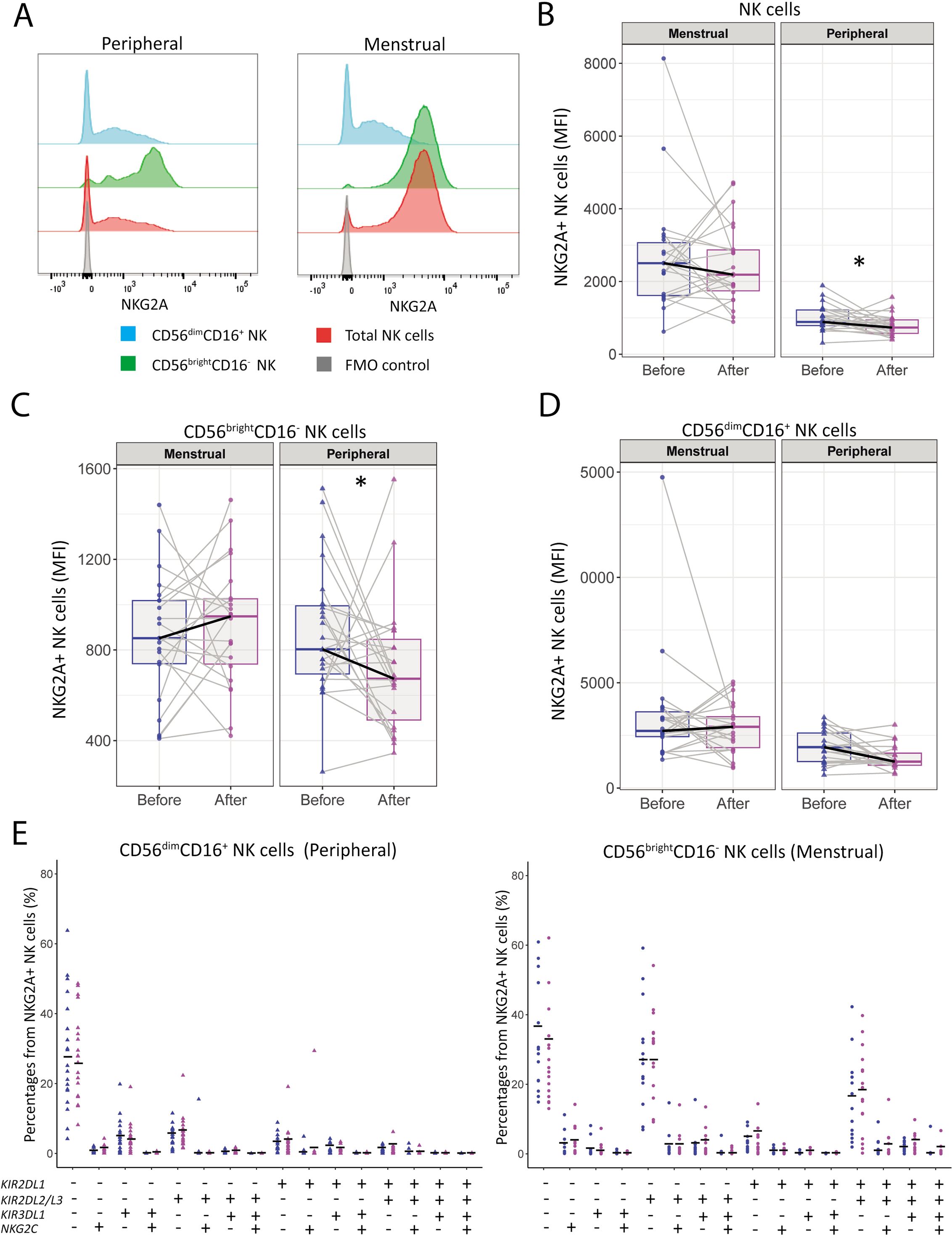
Figure 3. NKG2A expression by peripheral NK is decreased after the moderate-intensity aerobic exercise intervention in women with uRPL. (A) Representative histograms of NKG2A expression by total NK cells (red), CD56DimCD16+ NK cells (blue), CD56brightCD16- NK cells (green), and the fluorescence minus one (FMO) control (gray) of MB and PB. (B) Median fluorescent intensity (MFI) of NKG2A by total NK cells in MB and PB before and after the moderate-intensity aerobic exercise intervention. (C) MFI of NKG2A by CD56brightCD16- NK cells. (D) MFI of NKG2A by CD56dimCD16+ NK cells. PB n=23, MB n=22. (E) Tree plots showing percentages of NK cell clusters positive for NKRs (KIR2DL1, KIR2DL2/L3, KIR3DL1, NKG2C) in NKG2A+ CD56dimCD16+ peripheral NK (pNK) cells and CD56brightCD16- uterine NK (uNK) cells (PB n=18, MB n=16). MFIs were normalized to the MFI of the FMO control. The dots represent the uNK cells, the triangles the pNK cells (blue=before intervention, magenta=after intervention). Boxplots visualize the median (bold black line) and the interquartile ranges. A Paired Wilcoxon signed-rank test was performed to determine statistical significance (*p<0.05).
Next we investigated co-expression of NKRs by analyzing the expression of NKG2A, NKG2C, and KIRs, on CD56dimCD16+ pNK cells and CD56brightCD16- uNK cells. No significant changes were found in the CD56dimCD16+ pNK cells or CD56brightCD16- uNK cells populations (Figure 3E). Additionally, the frequencies of CD57 and KIRs remained unchanged (Supplementary Figures 3C–F).
3.4 Exercise reduces the IFN-γ production by pNK cells in uRPL women
Lastly, we investigated the effect of the intervention on NK effector function by determining the levels of intracellular granzyme B and perforin. In addition, we analyzed NK cell degranulation (CD107a) and IFN-γ production upon stimulation with K562 target cells or cytokines (IL-2 and IL-15) before and after intervention. Our analysis revealed no significant changes in CD107a expression intensity or frequency in either pNK or uNK cells after intervention (Figures 4B, C, Supplementary Figure 5A). Notably, stimulation with K562 cells did not enhance CD107a expression in uNK cells compared to the unstimulated conditions (Figure 4C). IFN-γ production was significantly reduced by pNK cells (35.8 [3.5-50.4] to 23.1 [3.2-33.9]; p=0.028) after intervention, with no substantial change observed in uNK cells (Figures 4D, E). Overall, uNK cells demonstrated lower IFN-γ production compared to their peripheral counterparts upon stimulation and co-culture. Granzyme B and perforin production remained unchanged post-intervention in unstimulated pNK cells (Supplementary Figures 4B, C).
Figure 4. The moderate-intensity aerobic exercise intervention reduces the IFN-γ production by peripheral NK cells in women with uRPL. (A) Representative dot plots showing the gating strategy for determining IFN-γ+ and CD107a+ peripheral NK (pNK) cells after 24h of stimulation with IL-2 and IL-15 and a 4h co-culture with K562 tumor cells. (B) Percentages of CD107a+ pNK cells stimulated with IL-2 and IL-15, and co-cultured with K562 (IL-2/IL-15+K562, left panel), only co-cultured with K562 (No stim+K562, central panel), and unstimulated (No stimulation, right panel) conditions. (C) Percentages of CD107a+ uterine NK (uNK) cells. (D) Percentages of IFN-γ+ pNK cells. (E) Percentages of IFN-γ+ uNK cells. PB n=12, MB n=12. The dots represent the uNK cells, the triangles the pNK cells (blue=before intervention, magenta=after intervention). Boxplots visualize the median (bold black line) and the interquartile ranges. A Paired Wilcoxon signed-rank test was used for statistical significance (*p<0.05).
4 Discussion
This study investigated the effects of a moderate-intensity aerobic exercise intervention on the immune profiles of women with uRPL, focusing on immune cell frequencies, NK cell receptor repertoire, and NK cell functional responses. To our knowledge, this is the first study investigating the impact of an exercise intervention strategy on systemic (PB) and uterine (MB) immune cells, particularly in women with uRPL. The use of MB, a less commonly studied but highly relevant derivate of uterine tissue in reproductive research, can aid in providing better understanding whether exercise could impact the uterine immune environment. By conducting comprehensive analyses of NK cell receptor repertoires and functional degranulation assays in both PB and MB, we aim to provide new insights into characterization of potential immunomodulatory effects of exercise in women with uRPL.
The moderate-intensity aerobic exercise intervention was shown to induce phenotypic and functional changes in pNK cells. Firstly, we found a significant decrease in the expression of NKp30 by pNK cells after intervention, while no significant changes were detected in uNK cells. NKp30 is a critical activating receptor expressed on both mature resting and activated NK cells (44). Depending on the isoform, NKp30 can either enhance NK cell activation or promote the secretion of immunosuppressive cytokines, such as IL-10 (45). In the context of uRPL, some studies have reported a higher expression of NKp30 compared to healthy controls (46, 47), however, others have reported no significant differences (24, 25). In addition to a decreased expression of NKp30, we also found a reduced expression of CD161 (KLRB1) by pNK cells after exercise. Previously, Ntrivalas et al. investigated CD161 expression in uRPL patients and healthy controls but did not find any differences (48). It has been shown that high CD161 expression on NK cells correlates with pro-inflammatory NK cells indicating high cytokine responsiveness (49, 50). Crosslinking of CD161 with its ligand lectin-like transcript 1 (LLT1) can increase IFN-γ expression (49, 51). Indeed, our functional assay revealed decreased IFN-γ production by pNK cells following exercise suggesting potential association with decreased expression of CD161. In RPL patients, higher levels of IFN-γ have been found compared to controls, and this increase has been positively correlated with NK cell levels (52). However, it is important to note that only a small proportion of samples could be used in the functional assays. This reduces the ability to detect more subtle effects. In addition, we found significant decrease in expression intensity of NKG2A in pNK cells following intervention. Exercise has been shown to preferentially mobilize more mature and differentiated NKG2A-/KIR+ NK cells in PB (37). While we did not observe changes in the frequencies of KIRs after exercise, the decrease in NKG2A expression suggests a shift towards a more systemic mature NK cell phenotype, consistent with previous findings (34). Interestingly, NKG2A expression and frequency was not altered in uNK cells, however, a trend was visible towards an increase of NKG2A expression by CD56brightCD16- NK cells.
In this study, we collected paired PB and MB samples from the same menstrual cycle for each participant to compare the impact of exercise in systemic and uterine immune environment. Interestingly, the changes in the function and receptor expression of NK cells were confined to pNK cells and were not detected in uNK cells. It is well-established that pNK cells and uNK cells are distinct cell subsets (12), albeit the origin of uNK cells remains uncertain— some studies suggest that uNK cells originate from recruitment of CD34-CD117+CD94- NK precursor cells from the PB to the uterus (53–55), while others propose that these cells directly derive from CD34+ precursor cells present in the uterus (56, 57). This distinction could raise relevant questions about the diverse responsiveness of systemic versus tissue resident cells to exercise. Some studies have shown that pNK cells are functionally responsive to exercise (58–60). To the best of our knowledge, this is the first study to report effects of exercise on uterine NK cells. Therefore, in the future, it would be interesting to further investigate the differential responses of pNK and uNK cells to exercise.
The effect of exercise on immune cell numbers and function can vary significantly depending on the type, intensity, and duration of the exercise (60–63). Previous studies have reported increased leukocyte mobilization, particularly of NK cells, immediately after acute or high-intensity exercise (61, 62, 64, 65). However, we did not observe an increase in NK cells frequencies which might be attributed to a different duration and the choice for a moderate intensity exercise regimen. Additionally, it was shown that immune cell numbers typically peak shortly after an exercise session and later return to baseline levels (38, 65). Since we did not collect blood immediately after the final training session, the timing of our sample collection may explain the differences in our findings. Interestingly, we observed that the frequencies of immune cells varied between women with uRPL. Some exhibited increased NK cell percentages, while others showed reduced percentages, suggesting that the effects of exercise may differ between individuals. This variability could possibly be attributed to the lifestyle of women prior to the intervention. Previously, it has been shown that people with a sedentary lifestyle exhibited higher C-reactive protein and IL-6 levels, which could indicate low-grade inflammation (66, 67). Moreover, it has been shown that age can also contribute to differential immune responses to exercise. A study from de Almeida-Neto et al. showed that men of older chronological age (34.6 ± 8.3) versus males of a younger age (21.8 ± 1.8) responded differently to exercise in their T cell compartment (68). This highlights, that even within a narrow age-range, immune responses to exercise can differ. In our study, we included women with different lifestyles and ages (between 28 and 40 years old). Therefore, it would be relevant to study possible associations between, among others, prior lifestyle and chronological age in relation to the response of immune cells to an exercise intervention in more detail in the future.
It is important to highlight several limitations that may have influenced the study’s conclusions. The first limitation is that we do not know if the changes induced by exercise are specific for uRPL patients. It may be that these changes also occur in women with uncomplicated pregnancies or, for pNK cells, also in age-matched men. Inclusion of a control group with non-uRPL women or males would be needed to further elucidate this. Second, there are no reference values for the markers that were affected by exercise. Furthermore, we were not able to evaluate multiple consecutive samples to determine the sample variation over time in individual patients by taking samples over multiple menstrual cycles or by sampling an intervention control group with uRPL that did not undergo the exercise intervention. For those markers, it would be interesting to include a larger cohort that could help confirm whether the changes we observe are purely exercise-mediated or whether additional factors may be involved as well. Some studies have already investigated the effects of the menstrual cycle on the expression of different uterine and peripheral NK cell receptors, including KIRs and NKG2A. Both studies showed that the expression was stable over different menstrual cycles (69, 70). Another point of attention is that there could have been a selection bias. It has been suggested that the endometrium of women with uRPL may be less selective for embryo quality, allowing low-quality embryos to implant (71, 72). Since these embryos may fail to develop properly, miscarriage is more likely to occur. In this study, participants were allowed to conceive during the intervention, leading to a high drop-out rate primarily due to pregnancy. As a result, we not only ended up with a relatively small sample size, but we also lost a subgroup of women who may have a higher propensity to conceive easily. As a follow up it would be interesting to perform more in-depth analysis of NK cell phenotypes or function in subgroups of women for example based on number of miscarriages, age, lifestyle factors. In the current study, our groups were underpowered to do this type of analysis. Finally, we were not able yet to correlate immune changes observed in response to the intervention with pregnancy outcomes due to the short follow-up of our study, currently limiting our ability to directly link these findings to clinical benefit. Despite these limitations, our intervention strategy may offer broader health benefits for women with uRPL, as regular physical activity is known to reduce risk factors commonly associated with RPL, such as metabolic syndrome and cardiovascular disease.
In conclusion, our study showed systemic effects of moderate-intensity aerobic exercise on immune phenotype and function in women with uRPL. Although it is difficult to translate these results into clinical significance, this research has offered new insights into the differential impact of exercise on both systemic pNK and tissue-resident uNK cells. Future research should focus on increasing the sample size, including a control cohort, and correlating the pregnancy outcomes to the exercise-dependent changes to identify the clinical relevance of these immune changes in pregnancy success or failure in women with uRPL.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the RUMC and the MUMC+. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AG: Conceptualization, Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. AL: Conceptualization, Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. DH: Conceptualization, Resources, Validation, Writing – review & editing. SA: Conceptualization, Writing – review & editing. MS: Conceptualization, Resources, Writing – review & editing. MdJ: Writing – review & editing. TM: Conceptualization, Funding acquisition, Resources, Writing – review & editing. LW: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing. RvdM: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Academic Incentive Radboud UMC/Maastricht UMC+ 2022.
Acknowledgments
We would like to thank all the women who participated in the study. We would also like to express our gratitude to the Radboud University Sports Department for providing free training sessions to participants enrolled at the Radboud UMC. Additionally, we would like thank all the people who helped with inclusion of the participants and processing of the samples: Anne van Uden, Imke van Wandeloo, Battice Westendorp, Lotte Jongen, Sanne Mol, Anouck van der Vlist, Claire Wenzler, Dieke Merks, Femke van Dalfsen, and Vera Valckx.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1602939/full#supplementary-material
References
1. RPL EGGo, Bender Atik R, Christiansen OB, Elson J, Kolte AM, Lewis S, et al. ESHRE guideline: recurrent pregnancy loss: an update in 2022. Hum Reprod Open. (2023) 2023:hoad002. doi: 10.1093/hropen/hoad002
2. Coumans ABC, Huijgens PC, Jakobs C, Schats R, de Vries JIP, van Pampus MG, et al. Haemostatic and metabolic abnormalities in women with unexplained recurrent abortion. Hum Reprod. (1999) 14:211–4. doi: 10.1093/humrep/14.1.211
3. Hantoushzadeh S, Kohandel Gargari O, Shafiee A, Seighali N, and Ghaemi M. Glucose metabolism tests and recurrent pregnancy loss: evidence from a systematic review and meta-analysis. Diabetol Metab Syndr. (2023) 15:3. doi: 10.1186/s13098-022-00973-z
4. Liu YJ, Du MY, Gan YX, Bao SH, Feng LP, and Zhang J. Triglyceride induced metabolic inflammation: potential connection of insulin resistance and recurrent pregnancy loss. Front Endocrinol. (2021) 12. doi: 10.3389/fendo.2021.621845
5. Westergaard D, Nielsen AP, Mortensen LH, Nielsen HS, and Brunak S. Phenome-wide analysis of short- and long-run disease incidence following recurrent pregnancy loss using data from a 39-year period. J Am Heart Assoc. (2020) 9:e015069. doi: 10.1161/JAHA.119.015069
6. Parikh NI, Jeppson RP, Berger JS, Eaton CB, Kroenke CH, LeBlanc ES, et al. Reproductive risk factors and coronary heart disease in the women’s health initiative observational study. Circulation. (2016) 133:2149–58. doi: 10.1161/CIRCULATIONAHA.115.017854
7. Wang YX, Minguez-Alarcon L, Gaskins AJ, Wang L, Ding M, Missmer SA, et al. Pregnancy loss and risk of cardiovascular disease: the Nurses’ Health Study II. Eur Heart J. (2022) 43:190–9. doi: 10.1093/eurheartj/ehab737
8. Christiansen OB. Special issue recurrent pregnancy loss: etiology, diagnosis, and therapy. J Clin Med. (2021) 10. doi: 10.3390/jcm10215040
9. Mor G and Cardenas I. The immune system in pregnancy: A unique complexity. Am J Reprod Immunol. (2010) 63:425–33. doi: 10.1111/j.1600-0897.2010.00836.x
10. Mor G, Cardenas I, Abrahams V, and Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann Ny Acad Sci. (2011) 1221:80–7. doi: 10.1111/j.1749-6632.2010.05938.x
11. Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. (2002) 2:656–63. doi: 10.1038/nri886
12. Gaynor LM and Colucci F. Uterine natural killer cells: functional distinctions and influence on pregnancy in humans and mice. Front Immunol. (2017) 8. doi: 10.3389/fimmu.2017.00467
13. Fraser R, Whitley GS, Johnstone AP, Host AJ, Sebire NJ, Thilaganathan B, et al. Impaired decidual natural killer cell regulation of vascular remodelling in early human pregnancies with high uterine artery resistance. J Pathol. (2012) 228:322–32. doi: 10.1002/path.2012.228.issue-3
14. Faas MM and de Vos P. Uterine NK cells and macrophages in pregnancy. Placenta. (2017) 56:44–52. doi: 10.1016/j.placenta.2017.03.001
15. Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. (2006) 12:1065–74. doi: 10.1038/nm1452
16. Robson A, Harris LK, Innes BA, Lash GE, Aljunaidy MM, Aplin JD, et al. Uterine natural killer cells initiate spiral artery remodeling in human pregnancy. FASEB J. (2012) 26:4876–85. doi: 10.1096/fj.12-210310
17. Seshadri S and Sunkara SK. Natural killer cells in female infertility and recurrent miscarriage: a systematic review and meta-analysis. Hum Reprod Update. (2014) 20:429–38. doi: 10.1093/humupd/dmt056
18. Tang AW, Alfirevic Z, and Quenby S. Natural killer cells and pregnancy outcomes in women with recurrent miscarriage and infertility: a systematic review. Hum Reprod. (2011) 26:1971–80. doi: 10.1093/humrep/der164
19. Tuckerman E, Mariee N, Prakash A, Li TC, and Laird S. Uterine natural killer cells in peri-implantation endometrium from women with repeated implantation failure after IVF. J Reprod Immunol. (2010) 87:60–6. doi: 10.1016/j.jri.2010.07.001
20. Wang S, Li YP, Ding B, Zhao YR, Chen ZJ, Xu CY, et al. Recurrent miscarriage is associated with a decline of decidual natural killer cells expressing killer cell immunoglobulin-like receptors specific for human leukocyte antigen C. J Obstet Gynaecol Re. (2014) 40:1288–95. doi: 10.1111/jog.2014.40.issue-5
21. Eskicioglu F, Özdemir AT, Özdemir RB, Turan GA, Akan Z, and Hasdemir SP. The association of HLA-G and immune markers in recurrent miscarriages. J Matern-Fetal Neo M. (2016) 29:3056–60. doi: 10.3109/14767058.2015.1114085
22. Giuliani E, Parkin KL, Lessey BA, Young SL, and Fazleabas AT. Characterization of uterine NK cells in women with infertility or recurrent pregnancy loss and associated endometriosis. Am J Reprod Immunol. (2014) 72:262–9. doi: 10.1111/aji.2014.72.issue-3
23. Ghafourian M, Karami N, Khodadadi A, and Nikbakhat R. Increase of CD69, CD161 and CD94 on NK cells in women with recurrent spontaneous abortion and in vitro fertilization failure. Iran J Immunol. (2014) 11:84–96.
24. Habets DHJ, Schlutter A, van Kuijk SMJ, Spaanderman MEA, Al-Nasiry S, and Wieten L. Natural killer cell profiles in recurrent pregnancy loss: Increased expression and positive associations with TACTILE and LILRB1. Am J Reprod Immunol. (2022) 88:e13612. doi: 10.1111/aji.13612
25. Fukui A, Funamizu A, Fukuhara R, and Shibahara H. Expression of natural cytotoxicity receptors and cytokine production on endometrial natural killer cells in women with recurrent pregnancy loss or implantation failure, and the expression of natural cytotoxicity receptors on peripheral blood natural killer cells in pregnant women with a history of recurrent pregnancy loss. J Obstet Gynaecol Res. (2017) 43:1678–86. doi: 10.1111/jog.2017.43.issue-11
26. Shakhar K, Ben-Eliyahu S, Loewenthal R, Rosenne E, and Carp H. Differences in number and activity of peripheral natural killer cells in primary versus secondary recurrent miscarriage. Fertil Steril. (2003) 80:368–75. doi: 10.1016/S0015-0282(03)00611-3
27. Karami N, Boroujerdnia MG, Nikbakht R, and Khodadadi A. Enhancement of peripheral blood CD56(dim) cell and NK cell cytotoxicity in women with recurrent spontaneous abortion or in vitro fertilization failure. J Reprod Immunol. (2012) 95:87–92. doi: 10.1016/j.jri.2012.06.005
28. Yoo JH, Kwak-Kim J, Han AR, Ahn H, Cha SH, Koong MK, et al. Peripheral blood NK cell cytotoxicities are negatively correlated with CD8(+) T cells in fertile women but not in women with a history of recurrent pregnancy loss. Am J Reprod Immunol. (2012) 68:38–46. doi: 10.1111/j.1600-0897.2012.01133.x
29. Hosseini S, Zarnani AH, Asgarian-Omran H, Vahedian-Dargahi Z, Eshraghian MR, Akbarzadeh-Pasha Z, et al. Comparative analysis of NK cell subsets in menstrual and peripheral blood of patients with unexplained recurrent spontaneous abortion and fertile subjects. J Reprod Immunol. (2014) 103:9–17. doi: 10.1016/j.jri.2014.03.002
30. Benner M, Feyaerts D, Lopez-Rincon A, van der Heijden OWH, van der Hoorn ML, Joosten I, et al. A combination of immune cell types identified through ensemble machine learning strategy detects altered profile in recurrent pregnancy loss: a pilot study. F S Sci. (2022) 3:166–73. doi: 10.1016/j.xfss.2022.02.002
31. Chastin SFM, Abaraogu U, Bourgois JG, Dall PM, Darnborough J, Duncan E, et al. Effects of regular physical activity on the immune system, vaccination and risk of community-acquired infectious disease in the general population: systematic review and meta-analysis. Sports Med. (2021) 51:1673–86. doi: 10.1007/s40279-021-01466-1
32. Gustafson MP, Wheatley-Guy CM, Rosenthal AC, Gastineau DA, Katsanis E, Johnson BD, et al. Exercise and the immune system: taking steps to improve responses to cancer immunotherapy. J Immunother Cancer. (2021) 9. doi: 10.1136/jitc-2020-001872
33. Scheffer DDL and Latini A. Exercise-induced immune system response: Anti-inflammatory status on peripheral and central organs. Biochim Biophys Acta Mol Basis Dis. (2020) 1866:165823. doi: 10.1016/j.bbadis.2020.165823
34. Idorn M and Hojman P. Exercise-dependent regulation of NK cells in cancer protection. Trends Mol Med. (2016) 22:565–77. doi: 10.1016/j.molmed.2016.05.007
35. Quintana-Mendias E, Rodriguez-Villalobos JM, Gastelum-Arellanez A, Cervantes N, Carrasco-Legleu CE, and Espino-Solis GP. The effect of acute physical exercise on natural killer cells populations and cytokine levels in healthy women. Sports (Basel). (2023) 11. doi: 10.3390/sports11100189
36. Millard AL, Valli PV, Stussi G, Mueller NJ, Yung GP, and Seebach JD. Brief Exercise Increases Peripheral Blood NK Cell Counts without Immediate Functional Changes, but Impairs their Responses to ex vivo Stimulation. Front Immunol. (2013) 4:125. doi: 10.3389/fimmu.2013.00125
37. Bigley AB, Rezvani K, Chew C, Sekine T, Pistillo M, Crucian B, et al. Acute exercise preferentially redeploys NK-cells with a highly-differentiated phenotype and augments cytotoxicity against lymphoma and multiple myeloma target cells. Brain Behav Immun. (2014) 39:160–71. doi: 10.1016/j.bbi.2013.10.030
38. Hanson ED, Sakkal S, Que S, Cho E, Spielmann G, Kadife E, et al. Natural killer cell mobilization and egress following acute exercise in men with prostate cancer. Exp Physiol. (2020) 105:1524–39. doi: 10.1113/eph.v105.9
39. Rumpf C, Proschinger S, Schenk A, Bloch W, Lampit A, Javelle F, et al. The effect of acute physical exercise on NK-cell cytolytic activity: A systematic review and meta-analysis. Sports Med. (2021) 51:519–30. doi: 10.1007/s40279-020-01402-9
40. van der Molen RG, Schutten JH, van Cranenbroek B, ter Meer M, Donckers J, Scholten RR, et al. Menstrual blood closely resembles the uterine immune micro-environment and is clearly distinct from peripheral blood. Hum Reprod. (2014) 29:303–14. doi: 10.1093/humrep/det398
41. RPL EGGo, Bender Atik R, Christiansen OB, Elson J, Kolte AM, Lewis S, et al. ESHRE guideline: recurrent pregnancy loss. Hum Reprod Open. (2018) 2018:hoy004. doi: 10.1093/hropen/hoy004
42. Villanueva RAM and Chen ZJ. ggplot2: elegant graphics for data analysis, 2nd edition. Meas-Interdiscip Res. (2019) 17:160–7. doi: 10.1080/15366367.2019.1565254
43. Wickham H and Sievert C. ggplot2: Elegant Graphics for Data Analysis. (Vol. 10, pp. 978–0) New York: Springer (2009).
44. Barrow AD, Martin CJ, and Colonna M. The natural cytotoxicity receptors in health and disease. Front Immunol. (2019) 10:909. doi: 10.3389/fimmu.2019.00909
45. Delahaye NF, Rusakiewicz S, Martins I, Ménard C, Roux S, Lyonnet L, et al. Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumors. Nat Med. (2011) 17:700–+. doi: 10.1038/nm.2366
46. Comins-Boo A, Cristobal I, Fernandez-Arquero M, Rodriguez de Frias E, Calvo Urrutia M, Pilar Suarez L, et al. Functional NK surrogate biomarkers for inflammatory recurrent pregnancy loss and recurrent implantation failure. Am J Reprod Immunol. (2021) 86:e13426. doi: 10.1111/aji.13426
47. Zhang Y, Huang C, Lian R, Xu J, Fu Y, Zeng Y, et al. The low cytotoxic activity of peripheral blood NK cells may relate to unexplained recurrent miscarriage. Am J Reprod Immunol. (2021) 85:e13388. doi: 10.1111/aji.13388
48. Ntrivalas EI, Bowser CR, Kwak-Kim J, Beaman KD, and Gilman-Sachs A. Expression of killer immunoglobulin-like receptors on peripheral blood NK cell subsets of women with recurrent spontaneous abortions or implantation failures. Am J Reprod Immunol. (2005) 53:215–21. doi: 10.1111/j.1600-0897.2005.00268.x
49. Kurioka A, Cosgrove C, Simoni Y, van Wilgenburg B, Geremia A, Björkander S, et al. CD161 defines a functionally distinct subset of pro-inflammatory natural killer cells. Front Immunol. (2018) 9. doi: 10.3389/fimmu.2018.00486
50. Konjevic G, Mirjacic Martinovic K, Vuletic A, Jurisic V, and Spuzic I. Distribution of several activating and inhibitory receptors on CD3-CD16+ NK cells and their correlation with NK cell function in healthy individuals. J Membr Biol. (2009) 230:113–23. doi: 10.1007/s00232-009-9191-3
51. Mathew PA, Chuang SS, Vaidya SV, Kumaresan PR, Boles KS, and Pham HTK. The LLT1 receptor induces IFN-γ production by human natural killer cells. Mol Immunol. (2004) 40:1157–63. doi: 10.1016/j.molimm.2003.11.024
52. Zargar M, Ghafourian M, Behrahi F, Nikbakht R, and Salehi AM. Association of recurrent implantation failure and recurrent pregnancy loss with peripheral blood natural killer cells and interferon-gamma level. Obstet Gynecol Sci. (2024) 67:112–9. doi: 10.5468/ogs.23120
53. Male V, Hughes T, McClory S, Colucci F, Caligiuri MA, and Moffett A. Immature NK cells, capable of producing IL-22, are present in human uterine mucosa. J Immunol. (2010) 185:3913–8. doi: 10.4049/jimmunol.1001637
54. Cerdeira AS, Rajakumar A, Royle CM, Lo A, Husain Z, Thadhani RI, et al. Conversion of peripheral blood NK cells to a decidual NK-like phenotype by a cocktail of defined factors. J Immunol. (2013) 190:3939–48. doi: 10.4049/jimmunol.1202582
55. Keskin DB, Allan DSJ, Rybalov B, Andzelm MM, Stern JNH, Kopcow HD, et al. TGFβ promotes conversion of CD16+ peripheral blood NK cells into CD16- NK cells with similarities to decidual NK cells. PNAS. (2007) 104:3378–83. doi: 10.1073/pnas.0611098104
56. McMenamin M, Lysakova-Devine T, Wingfield M, O’Herlihy C, and O’Farrelly C. Endometrial aspiration biopsy: a non-invasive method of obtaining functional lymphoid progenitor cells and mature natural killer cells. Reprod BioMed Online. (2012) 25:322–8. doi: 10.1016/j.rbmo.2012.05.001
57. Vacca P, Vitale C, Montaldo E, Conte R, Cantoni C, Fulcheri E, et al. CD34+ hematopoietic precursors are present in human decidua and differentiate into natural killer cells upon interaction with stromal cells. Proc Natl Acad Sci U.S.A. (2011) 108:2402–7. doi: 10.1073/pnas.1016257108
58. Jan P, Asp S, Daugaard JR, Richter EA, Klokker M, and Pedersen BK. Effect of eccentric exercise on natural killer cell activity. J Appl Physiol. (1995) 78:1442–6. doi: 10.1152/jappl.1995.78.4.1442
59. Nieman DC, Henson DA, Sampson CS, Herring JL, Suttles J, Conley M, et al. The acute immune response to exhaustive resistance exercise. Int J Sports Med. (1995) 16:322–8. doi: 10.1055/s-2007-973013
60. McFarlin BK, Flynn MG, Phillips MD, Stewart LK, and Timmerman KL. Chronic resistance exercise training improves natural killer cell activity in older women. J Gerontol a-Biol. (2005) 60:1315–8. doi: 10.1093/gerona/60.10.1315
61. Lancaster GI, Halson SL, Khan Q, Drysdale P, Wallace F, Jeukendrup AE, et al. Effects of acute exhaustive exercise and chronic exercise training on type 1 and type 2 T lymphocytes. Exerc Immunol Rev. (2004) 10:91–106.
62. Graff RM, Kunz HE, Agha NH, Baker FL, Laughlin M, Bigley AB, et al. beta(2)-Adrenergic receptor signaling mediates the preferential mobilization of differentiated subsets of CD8+ T-cells, NK-cells and non-classical monocytes in response to acute exercise in humans. Brain Behav Immun. (2018) 74:143–53. doi: 10.1016/j.bbi.2018.08.017
63. Rowbottom DG and Green KJ. Acute exercise effects on the immune system. Med Sci Sport Exer. (2000) 32:S396–405. doi: 10.1097/00005768-200007001-00004
64. Neves PRD, Tenorio TRD, Lins TA, Muniz MTC, Pithon-Curi TC, Botero JP, et al. Acute effects of high- and low-intensity exercise bouts on leukocyte counts. J Exerc Sci Fit. (2015) 13:24–8. doi: 10.1016/j.jesf.2014.11.003
65. Natale VM, Brenner IK, Moldoveanu AI, Vasiliou P, Shek P, and Shephard RJ. Effects of three different types of exercise on blood leukocyte count during and following exercise. Sao Paulo Med J. (2003) 121:9–14. doi: 10.1590/S1516-31802003000100003
66. Colbert LH, Visser M, Simonsick EM, Tracy RP, Newman AB, Kritchevsky SB, et al. Physical activity, exercise, and inflammatory markers in older adults: findings from the health, aging and body composition study. J Am Geriatr Soc. (1995) 52:1098–104. doi: 10.1111/j.1532-5415.2004.52307.x
67. Henson J, Yates T, Edwardson CL, Khunti K, Talbot D, Gray LJ, et al. Sedentary time and markers of chronic low-grade inflammation in a high risk population. PLoS One. (2013) 8:e78350. doi: 10.1371/journal.pone.0078350
68. de Almeida-Neto PF, Goncalves CAM, Wilde P, Jaggers JR, Junior GBC, de Farias Sales VS, et al. Influence of age and fitness level on immune responses of T and NK cells in healthy physically active subjects after strenuous aerobic exercise: a cross-sectional study. Front Immunol. (2023) 14:1252506. doi: 10.3389/fimmu.2023.1252506
69. Ivarsson MA, Stiglund N, Marquardt N, Westgren M, Gidlof S, and Bjorkstrom NK. Composition and dynamics of the uterine NK cell KIR repertoire in menstrual blood. Mucosal Immunol. (2017) 10:322–31. doi: 10.1038/mi.2016.50
70. Feyaerts D, Kuret T, van Cranenbroek B, van der Zeeuw-Hingrez S, van der Heijden OWH, van der Meer A, et al. Endometrial natural killer (NK) cells reveal a tissue-specific receptor repertoire. Hum Reprod. (2018) 33:441–51. doi: 10.1093/humrep/dey001
71. Salker M, Teklenburg G, Molokhia M, Lavery S, Trew G, Aojanepong T, et al. Natural selection of human embryos: impaired decidualization of endometrium disables embryo-maternal interactions and causes recurrent pregnancy loss. PLoS One. (2010) 5:e10287. doi: 10.1371/journal.pone.0010287
Keywords: NK cells, exercise intervention, recurrent pregnancy loss (RPL), menstrual blood, immunophenotyping
Citation: Gurbanova A, Lombardi AEM, Habets DHJ, Al-Nasiry S, Spaanderman MEA, de Jonge MI, Meuleman T, Wieten L and van der Molen RG (2025) Impact of a moderate-intensity aerobic exercise intervention on systemic and uterine natural killer cells in women with unexplained recurrent pregnancy loss. Front. Immunol. 16:1602939. doi: 10.3389/fimmu.2025.1602939
Received: 30 March 2025; Accepted: 20 May 2025;
Published: 04 June 2025.
Edited by:
Hadida Yasmin, Cooch Behar Panchanan Barma University, IndiaReviewed by:
Fernando Souza-Fonseca-Guimaraes, The University of Queensland, Faculty of Medicine, AustraliaAndrea Balduit, Institute for Maternal and Child Health Burlo Garofolo (IRCCS), Italy
Copyright © 2025 Gurbanova, Lombardi, Habets, Al-Nasiry, Spaanderman, de Jonge, Meuleman, Wieten and van der Molen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Renate G. van der Molen, cmVuYXRlLnZhbmRlcm1vbGVuQHJhZGJvdWR1bWMubmw=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
 Aysel Gurbanova
Aysel Gurbanova Amber E. M. Lombardi
Amber E. M. Lombardi Denise H. J. Habets
Denise H. J. Habets Salwan Al-Nasiry
Salwan Al-Nasiry Marc E. A. Spaanderman3,4,5
Marc E. A. Spaanderman3,4,5 Marien I. de Jonge
Marien I. de Jonge Tess Meuleman
Tess Meuleman Lotte Wieten
Lotte Wieten Renate G. van der Molen
Renate G. van der Molen