- 1Chemistry Department, Biochemistry Division, Faculty of Science, Helwan University, Cairo, Egypt
- 2Department of Biology, College of Science, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia
- 3Zoology and Entomology Department, Faculty of Science, Helwan University, Cairo, Egypt
- 4Al-Ayen Scientific Research Center, Al-Ayen Iraqi University, AUIQ, An Nasiriyah, ThiQar, Iraq
- 5Molecular Biology and Tissue Culture, Medical Faculty of Medicine Ain Shams Research Institute (MASRI), Ain Shams University, Cairo, Egypt
Introduction: Sepsis-induced liver injury is a serious issue in critical care. Since antibiotics are insufficiently effective to combat the disease and avoid upcoming organ failure, treatment with mesenchymal stem cells (MSCs) is an alternate strategy for treating liver damage. Thus, our work aimed to boost the therapeutic potential of MSCs by pretreating them with selenium in the form of sodium selenite (Na₂SeO₃) and selenium nanoparticles (SeNPs) in the cecal ligation and puncture (CLP) rat model of sepsis.
Methods: Rats were split into groups that received MSCs alone, MSCs enhanced with Na₂SeO₃ (E1-MSCs), MSCs enhanced with SeNPs (E2-MSCs), antibiotics (Ab), and no therapy (CLP), in addition to the control and sham groups. Within 48 hours of the operation, liver tissues and blood samples were taken.
Results: MSC treatment, significantly augmented with selenium compounds, markedly reduced markers of liver injury and signs of oxidative stress (MDA, MPO, NO) while elevating levels of GSH and antioxidant enzymes (GPx, GR, SOD, CAT). Furthermore, the therapies attenuated pro-inflammatory cytokines (TNF-α, IL-1β, IL-8) and inflammatory pathways (iNOS, MAPK9, NF-κB). Additionally, MSCs and enhanced MSCs improved hepatic tissue by alleviating the immunomodulatory indicators (COX-2, PGE2) and regulating apoptosis by raising (Bcl-2) and minimizing (Cas-3 and Bax). Histopathological analysis showed that MSC therapies, particularly when enhanced, restored the natural architecture of the liver.
Discussion: This study concludes that MSCs enhanced with selenium compounds provide a promising therapeutic approach for liver dysfunction caused by sepsis, possibly through regulating antioxidants, anti-inflammatory processes, immunology, and hepatic tissue regeneration.
1 Introduction
Sepsis is a potentially lethal disorder that features organ failure resulting from an uncontrolled immunological defense to infection (1). It is a serious hazard to global health, accounting for 48.9 million new cases and 11 million deaths from sepsis each year, or 20% of all deaths worldwide (2). The sepsis pathophysiology includes alterations in the functions of several organs and uncontrolled infiltration of the body. This is caused by the in vivo release of significant amounts of inflammatory factors and neutrophil overactivation, leading to an inflammatory storm that ultimately results in edema formation, tissue damage, and organ dysfunction (3, 4). The liver, an essential organ that regulates immunity, metabolism, and detoxification, is especially susceptible to sepsis. Liver insufficiency, abnormalities in biochemical testing, and even liver failure can result from sepsis-related liver injury (SRLI) (5). Considering the severity of sepsis and its consequences on the liver, efficient treatments are urgently required.
Mesenchymal stem cells (MSCs) are currently an effective therapy for sepsis. According to Xiang et al. (6), these cells can differentiate and self-renew. They can also modulate inflammatory responses in liver tissue to produce an immunologically tolerant environment (7). Nonetheless, oxidative stress (OS) during cell culture and after transplantation restricts the curative effect of MSCs by reducing cell survival and functioning (8, 9). Increasing MSC survival and function with antioxidant supplementation has been the focus of recent research to solve this issue (10).
The antioxidative properties of selenium, a vital redox-active micronutrient, and its function in cellular defense processes attracted attention (11, 12). Selenium nanoparticles (SeNPs) are noteworthy because they have benefits over elemental Se, such as being more bioactive, soluble, and less poisonous (11). Additional benefits of nanosized selenium include its better antioxidant activity (13), involvement in enhanced glutathione peroxidase and thioredoxin reductase activity, and superior catalytic efficiency (14). Furthermore, Zhang et al. (15) research indicates that selenium deficiency is related to mortality risk in COVID-19 infection. Even though there may be advantages to combining MSCs and SeNPs, no study has explored how both work together to treat sepsis. This shortage in research offers an opportunity to explore a new therapeutic strategy that could enhance the effectiveness of MSC-based sepsis treatment. This work explores the possible therapeutic advantages of MSCs with SeNPs in a rat model of sepsis caused by cecal ligation and puncture (CLP). We hypothesize that MSCs combined with SeNPs will exhibit hepatoprotective and anti-inflammatory properties better than traditional MSC therapy.
2 Materials and methods
2.1 Ethical statement and experimental animals
Male adult albino rats (n = 41) from the experimental animal farm in El-Giza, Egypt, were used in the current study. The animals were kept in conventional circumstances in the Zoology and Entomology Department of Helwan University’s Faculty of Science. The study included 35 rats (n = 35, 200–220 g) divided randomly into 7 groups, five rats per cage, for endpoint experiments. We used an additional cage containing 6-week-old male rats for MSC isolation (n=6). The conditions included a 12-hour light/dark cycle, room temperature, unlimited access to water, and standard rat chow. A week before the trial, the rats were acclimated to the laboratory environment. Helwan University’s Institutional Animal Ethics Committee (permission No. HU-IACUC/Z/AEA0920-1) authorized all animal procedures, which adhered to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, eighth edition.
2.2 Cecal ligation and puncture model
According to Fujimura et al. (16), sepsis was created utilizing the CLP model. In brief, rats received intraperitoneal thiopental sodium (25 mg/kg) anesthesia after a 12-hour fast. A 3-cm midline laparotomy was carried out, and a 4.0 silk thread was used to ligate the cecum beneath the ileocecal valve. An 18-gauge needle was used twice to puncture the ventral side of the cecum to enable the dispersal of fecal matter. After the cecum was repositioned into the intraperitoneal space, the abdomen was closed using 3.0 silk sutures.
2.3 Preparation and characterization of selenium nanoparticles
According to Khaled et al. (11), SeNPs were produced by an ordinary chemical reduction process, including sodium selenite (Na2SeO3) and ascorbic acid. A mixture of a 0.125 M ascorbic acid solution (10 mL) and an 8.6% bovine serum albumin (w/v, 5 mL) solution was combined, then a 20 mM sodium selenite solution (12.5 mL) was added dropwise. After homogenizing on a magnetic stirrer for 30 minutes at 1000 rpm, protecting from light, and storing in a refrigerator between 4 and 8°C, the color changed from translucent to brick red, confirming the ascorbic acid-mediated reduction of Se4+ to Se0. SeNPs were characterized by X-ray diffraction (XRD) (Bruker D2-Phaser) to examine the crystalline structure, phase distribution, and purity of the produced selenium nanoparticles; also, for particle size, size distribution, and zeta potential, dynamic light scattering (DLS) (Zetasizer Nano ZN, Malvern Panalytical Ltd., United Kingdom). DLS data are shown as the intensity-weighted Z-average diameter and polydispersity index (PDI), and the samples undergo triplicate analysis. Moreover, transmission electron micrographs were also captured using a high-resolution transmission electron microscope (TEM; JEOL Ltd., Japan) equipped with an electron diffraction pattern.
2.4 Bone marrow-derived mesenchymal stem cell isolation and culture
Tibiae and femurs were used to isolate BM-MSCs from male rats (n=6). Dulbecco’s Modified Eagle Medium (DMEM; GIBCO/BRL, Waltham, MA) supplemented with 13% fetal bovine serum (GIBCO/BRL) was used to flush the bone marrow. Using a density gradient method (Ficoll/Paque [Pharmacia]) to isolate nucleated cells, the cells were then grown as a primary culture in standard media (500 mL DMEM, 13% FBS, 1.5% penicillin/streptomycin, and 0.01% fungizone) for 12 or 14 days (GIBCO/BRL) at 37°C with 5% CO2. Every two to three days, the media were changed.
For enhancing MSCs, adherent cells at 60-70% confluency (second passage) were treated with either 100 nM sodium selenite (17) or 50 ng/mL SeNPs (10) until massive colonies developed, achieving a confluence of 80% to 90%. Cells were trypsinized by incubating with a mixture of trypsin (0.25%) and one mM ethylenediaminetetraacetic acid (EDTA; GIBCO/BRL) for five minutes at 37°C after two rounds of washing with phosphate-buffered saline. Cells were then immersed in media enriched with serum and kept in 50 cm² culture flasks for incubation (18). The adherent colonies were counted on day 14 (Figure 1). BM-MSCs were characterized by morphology and surface marker expression by flow cytometry using CD44-FITC (IM7, Cat#11-0441-82), CD45-PerCP-Cy5.5 (HI30, Cat #45-0459-42), and CD19-APC-Cy7 (SJ25-C1, Cat #A15429). Cells were analyzed using logarithmic SSC. At Mean Fluorescence Intensity (MFI) ≥4x baseline (Region ALL: X-Mean=1.03), positivity thresholds were established.
Figure 1. Bone marrow-derived mesenchymal stem cells at days of culturing: (A) Untreated MSCs, (B) mesenchymal stem cells enhanced with Na2SeO3 (E1-MSCs), and (C) mesenchymal stem cells enhanced with SeNPs (E2-MSCs). All photos were taken at 80% of confluency.
2.5 Animal groups and treatment protocol
The seven groups (n = 5 per group) of rats utilized in this investigation were randomly assigned. According to the study of Luo et al. (19), treatments were administered 3 hours post-CLP via intraperitoneal injection and then studied 48 hours later, as shown below:
Control group: Rats were given a single saline solution injection.
Sham-operated group: Rats were only given anesthesia to carry out the laparotomy and cecum manipulation operations; they were not exposed to CLP.
Untreated CLP-induced septic (CLP) group: Rats were subjected to CLP, left untreated for 48 hours to develop sepsis, and then euthanized without treatment.
CLP-induced sepsis treated with bone marrow-derived mesenchymal stem cells (CLP-MSCs) group: Rats were exposed to CLP to develop sepsis and then treated with 1 × 106 BM-MSCs.
CLP-induced sepsis treated with bone marrow-derived mesenchymal stem cells enhanced with Na2SeO3 (CLP+Na2SeO3+MSCs; CLP-E1-MSCs) group: Rats were exposed to CLP to develop sepsis, then treated with 1 × 106 Na2SeO3-enhanced BM-MSCs.
CLP-induced sepsis treated with bone marrow-derived mesenchymal stem cells-enhanced with SeNPs (CLP+SeNPs+MSCs; CLP-E2-MSCs) group: Rats were exposed to CLP to develop sepsis and then injected with 1 × 106 SeNPs-enhanced BM-MSCs.
CLP-induced sepsis treated with antibiotic (CLP-Ab) group: Rats were exposed to CLP to develop sepsis and then treated with one dosage of ceftriaxone (100 mg/kg) (20).
2.6 Collecting and processing samples
Forty-eight hours after treatment, rats were euthanized by cervical dislocation and pentobarbital anesthesia (300 mg/kg, i.p.) in each group. For analysis, liver tissues and blood samples were collected. Three pieces of liver tissue were divided as follows:
The initial homogenate, 10% (w/v), was centrifuged for 10 minutes at 4°C at 3000 x g after being mixed with 50 mM Tris-HCl (pH 7.4) in an ice-cold solution. Biochemical analysis was then performed on the obtained supernatants. The second part was kept at -80°C for gene expression investigation. The remainder was stored in 10% neutral formalin for histological H&E and immunohistochemical studies.
2.7 Biochemical analysis
2.7.1 Liver function parameters
The serum levels of alkaline phosphatase (ALP), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were measured using standard diagnostic kits.
2.7.2 Markers of oxidative stress
The peroxidation of lipids was detected by measuring the level of malondialdehyde (MDA) in the liver (21). The O-dianisidine approach was used to measure MPO activity, a marker of leukocyte migration and aggregation (22). At 460 nm, spectroscopy was used to calculate the alteration in absorbance. Using the Ellman (23) approach, 5,5′-dithiobis (2-nitrobenzoic acid) was converted to a yellowish 5-thionitrobenzoic acid to quantify glutathione (GSH) spectrophotometrically at 405 nm.
2.7.3 Estimation of antioxidant enzymes
Superoxide dismutase activity was assessed by determining how well it prevented phenazine methosulfate from reducing nitroblue tetrazolium dyeing (24). By detecting the rate of H2O2 breakdown at 240 nm, catalase activity was quantified (25). Ursini et al. (26) method was used to test glutathione peroxidase (GPx) activity indirectly through a coupled reaction with glutathione reductase (GR). In contrast, the activity of GR was calculated by measuring the reduction absorbance at 340 nm, which was caused by GR-catalyzed GSSG oxidation to NADP− in the presence of NADPH (27).
2.7.4 Inflammatory biomarkers
Green et al. (28) used a colorimetric approach to estimate nitrite/nitrate (nitric oxide; NO) levels. TNF-α, IL-1β, and IL-8 levels in the liver tissue supernatant were measured using commercial ELISA kits as directed by the manufacturer. The sandwich ELISA technique measures NF-κB (Cat. no.: MBS287521).
2.7.5 Assessment of immunomodulatory markers
The levels of PGE2 and COX-2 in the liver homogenates were evaluated according to the manufacturer’s instructions using commercially available ELISA kits (R&D Systems, Minneapolis, MN, USA).
2.7.6 Measurement of apoptosis-related biomarkers
The ELISA kit’s instructions were followed, lysing both treated and untreated samples. The Bax and Bcl-2 proteins obtained from the cell lysate were coupled to the primary antibody, while HRP-conjugated secondary antibodies were used to detect them. The protein levels of Bax and Bcl-2 were then determined by measuring the absorbance at 450 nm using a microplate reader. To determine the signal transduction route causing apoptosis following the destruction of the liver, we investigated the activity of caspase-3 and how it inhibits apoptosis. The caspase-3 family, an essential player downstream of apoptotic death signaling, was measured using an ELISA kit per the guidelines set forth by the manufacturer.
2.8 Histological examination
Histological investigations were conducted to evaluate liver injury. Liver tissues were immersed in paraffin after being fixed in 10% buffered formaldehyde. These immersed tissues were sectioned (5 μm) and stained using eosin and hematoxylin to assess the general histological characteristics. A semi-quantitative approach was followed to score them. When evaluating liver tissues, we ranked the degradation of the hepatocytes and inflammation of the portals and lobules from 0 to 3. The tissue specimen’s score revealed the mean score across ten different fields. With a 100x magnification light microscope (Eclipse E200-LED; Nikon, Kawasaki, Japan). The severity of all pathological changes was graded semi-quantitatively, as previously recorded by Ahmed et al. (29). The pathological alterations investigated are inflammatory cell infiltration, disorganized hepatic strands, damaged hepatocytes, and pyknotic and apoptotic hepatocytes. H&E was evaluated on a scale of 0 to 3 by two blind investigators.
2.9 Immunohistochemistry examination
The immunological response of NF-κB and TNF-α was evaluated in hepatic tissue slices for all regimens. After deparaffinization, tissue blocks were hydrated again in ethyl alcohol and graded sequentially. The antigen was extracted using an EDTA (pH 8) solution, and endogenous peroxidases were inactivated with a five-minute incubation in a hydrogen peroxide solution. Next, the microscope slide was incubated with the primary monoclonal antibody against NF-κB p65 (2A12A7, Cat #33-9900) or TNF-α (28401, Cat #MA5-23720) for 60 minutes to avoid nonspecific binding, followed by washing with PBS. Next, the slide was incubated with anti-rat IgG, a second antibody, for 10 minutes (1:1000 dilution). Brown staining was visualized using the substrate solution containing diaminobenzidine (DAB), and it was counterstained for ten minutes using Mayer’s hematoxylin. The measurement of the positive immunoreactions was taken in ten non-overlapping high-power fields (40×) of paraffin sections of the liver for each group, utilizing ImageJ software (version 1.46, NIH, USA) and subsequently analyzed statistically.
2.10 Gene expression analyses
The expression level of inflammatory mediators (MAPK 9, iNOS, caspase-3, and Bax) was measured by quantitative real-time polymerase chain reaction (qRT-PCR). In brief, total RNA was isolated from the liver tissue using RNeasy Plus Mini kits. cDNA was synthesized using a Scripting cDNA synthesis kit (Bio-Rad, CA, USA) from 100 ng of total RNA, following the manufacturer’s instructions. qRT-PCR was performed using Power SYBR Green in Applied Biosystems 7500 (Applied Biosystems, USA), with duplicates for each reaction/sample. Gene primer sequences that were analyzed are included in Table 1.
2.11 Statistical analysis
The data met ANOVA assumptions (equal variances by the Brown-Forsythe test, p > 0.05; normality validated by the Shapiro-Wilk tests, p > 0.05). A one-way ANOVA was used with Tukey’s post-hoc test to analyze the data. The statistical significance level is p < 0.05; the results are presented as mean ± SD. GraphPad Prism 8 and SPSS v.20.0 were used for the analyses.
3 Results
3.1 Characterization of SeNPs
A DLS analysis (Figure 2A) assessed the synthesized nanoparticles’ stability, size, and shape and showed a Z-average diameter of 262.5 ± 4.21 nm with a narrow polydispersity (PDI = 0.068 ± 0.021), confirming uniform SeNP synthesis. Zeta potential analysis indicated that the nano selenium particles had a zeta potential of 19.5 ± 0.11 mV, suggesting good stability (Figure 2B).
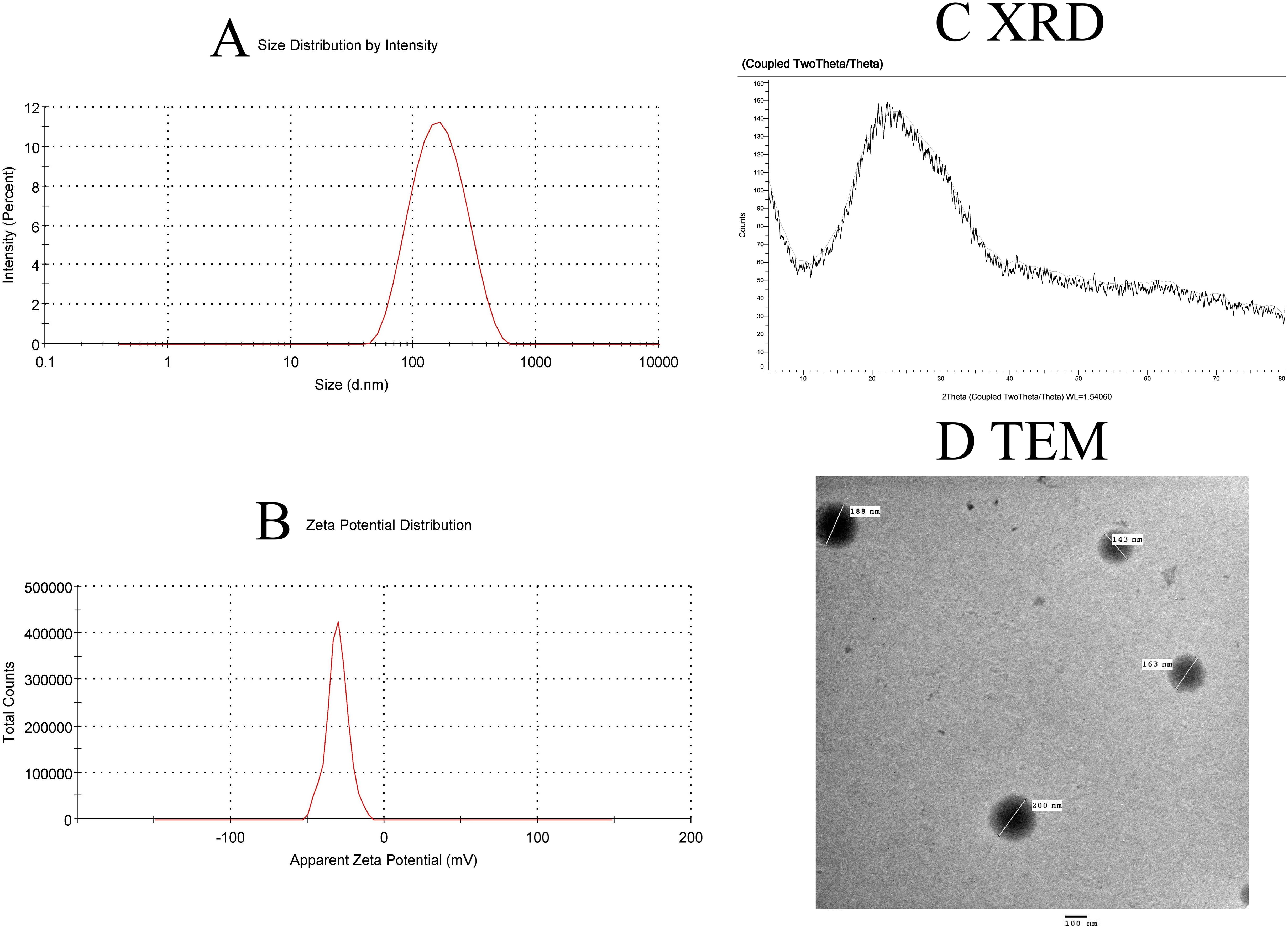
Figure 2. Characterization of SeNPs. (A) Intensity-weighted size distribution by dynamic light scattering (DLS), reporting Z-average diameter (262.5 nm) and polydispersity index (PDI = 0.068 ± 0.021, n=3). The logarithmic x-axis reflects the hydrodynamic size range. (B) Zeta potential distribution. (C) XRD pattern confirming crystalline structure. (D) TEM image confirming the revealed spherical particles with a diameter of less than 200 nm for the formed SeNPs.
The crystalline structure of the produced Se nanoparticle was confirmed by XRD analysis. The crystalline structure of the produced Se nanoparticles is well-defined (Figure 2C). At 2̟ Ɵ values of 24.03, 30.3, 44.2, 52.2, 54.5, and 56.6, the sharp-edged peaks were visible. These results are consistent with Anu Mary Ealia and Saravanakumar (30), who used JCPDS No. 04–0783 as a reference number to show that the Se nanoparticles are crystalline, cubic, and free of these contaminants. The EM image revealed spherical particles with a diameter of less than 200 nm for the formed SeNPs. These particles were uniformly dispersed and showed no signs of agglomeration (Figure 2D).
3.2 Identification of BM-derived MSCs from rat
During cell culture, BM-MSCs adhered to the plate. The cells’ morphology was spindle-shaped. The cells were screened before release and showed no signs of bacterial or fungal contamination. Before cell injection, the mean cell viability was 92.2% ± 2.5. Flow cytometry was used to analyze the immunophenotype of BM-MSCs. As illustrated in Figure 3, BM-MSCs exhibited substantial positive results for mesenchymal stem cell-specific markers, such as CD44 (82.46%, MFI=28.7), but negative results for CD45 (5.73%, MFI=10.9) and CD19 (2.96%, MFI=4.04). Specificity was confirmed by CD44+ cells, which displayed an MFI that was 28 times greater than baseline (28.7 vs. 1.03).
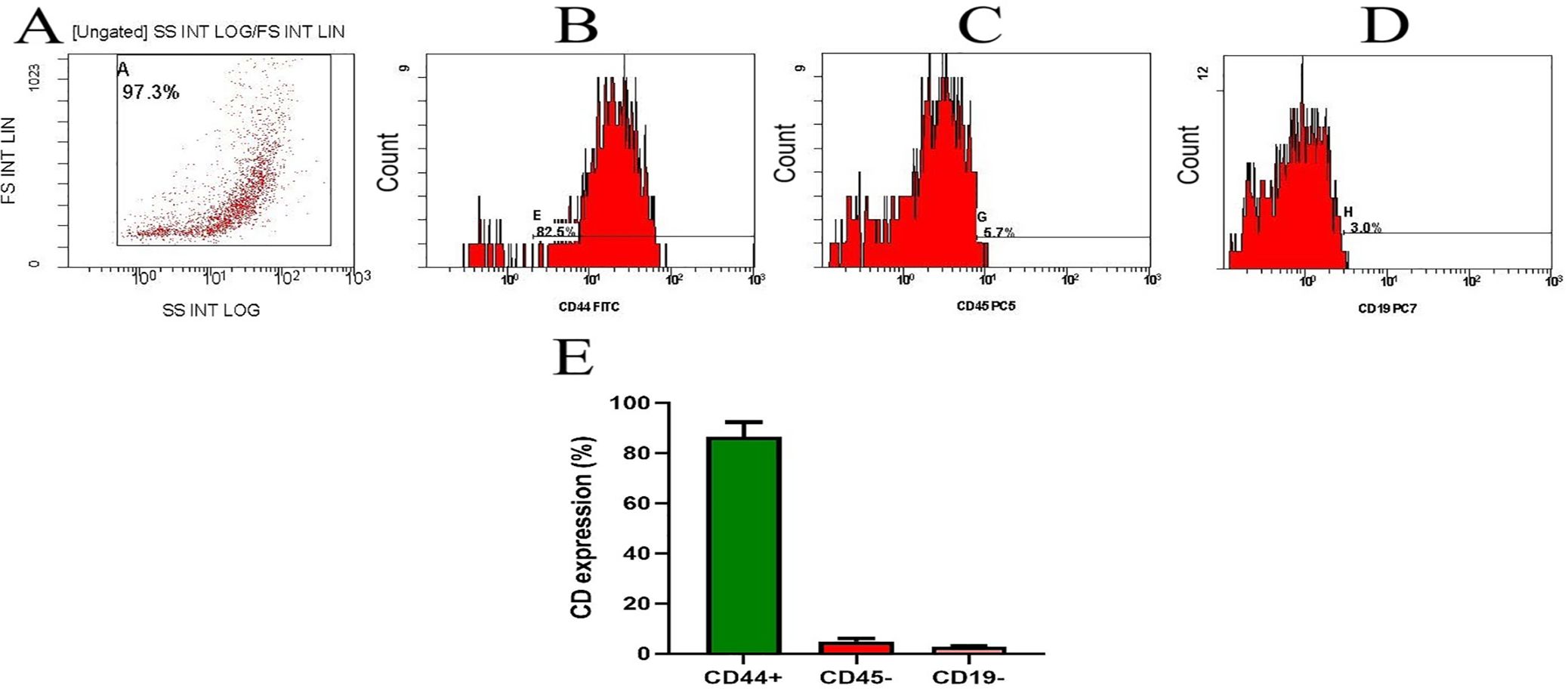
Figure 3. Flow cytometric characterization of bone marrow-derived MSCs. (A) Flow gate (logarithmic SSC vs. linear FSC). Note. Analysis of MSC cell surface marker expression. Cells were uniformly positive for (B) CD44 and negative for (C) CD45 and (D) CD19. (E) represents the CD percentage of two separate experiments and is presented as mean ± SD. Baseline MFI (Region ALL): 1.03.
3.3 The potential effect of enhanced MSCs on CLP-induced liver injury in rats
Serum levels of ALT, AST, and ALP were used to assess the extent of liver damage brought on by CLP. A significant elevation in blood levels of ALT (P < 0.0001, F (6, 28) = 49.74), AST (P < 0.0001, F (6, 28) = 21.69), and ALP (P < 0.0001, F (6, 28) = 14.01) was observed 48 hours after CLP surgery compared to the control group. There is a significant reduction in the blood levels of liver function enzymes in the treatment groups (MSCs, E1-MSCs, E2-MSCs, and Ab) as compared to the CLP group (P < 0.05, Figure 4). While there is no significant difference between the blood levels of alkaline phosphatase in the CLP group and the rats treated with antibiotics, the other treated groups exhibit modest significance.
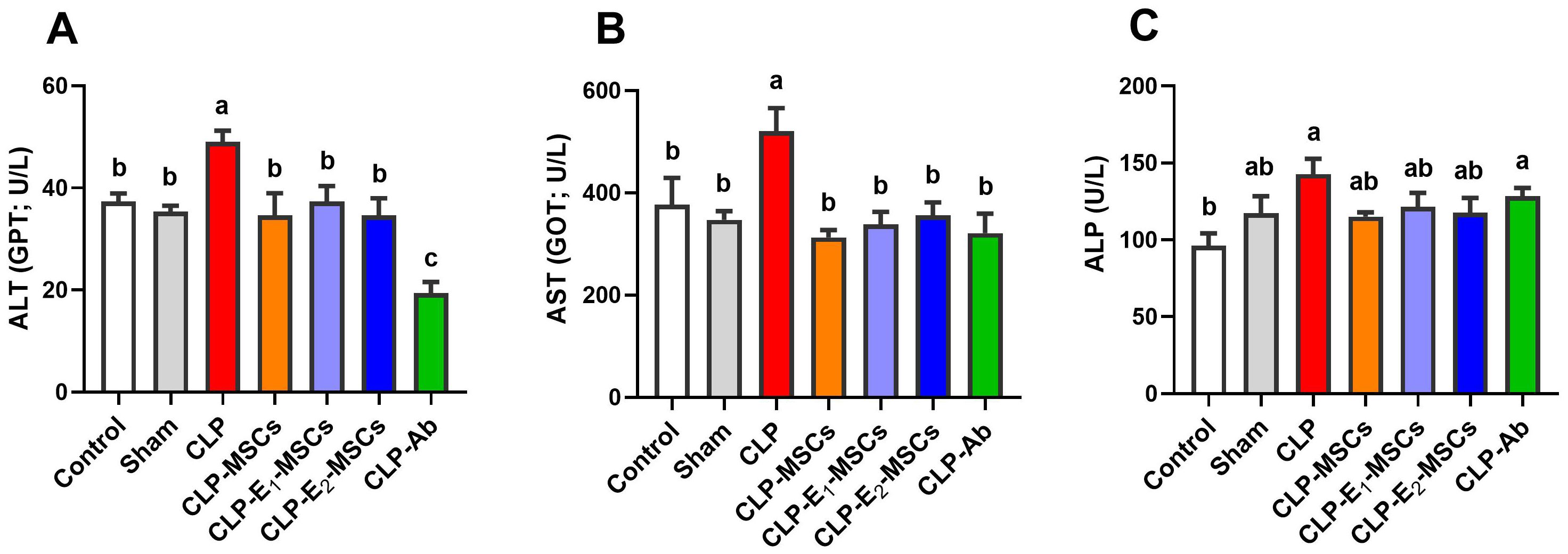
Figure 4. Effects of mesenchymal stem cells (MSCs), mesenchymal stem cells enhanced with Na2SeO3 (E1-MSCs), mesenchymal stem cells enhanced with SeNPs (E2-MSCs), or standard treatment antibiotics (Ab) on liver function tests in the serum of septic rats. (A) alanine aminotransferase (ALT), (B) aspartate aminotransferase (AST), and (C) alkaline phosphatase (ALP). The mean ± SD data displays the biochemical results (n = 5). Different letters show statistically significant differences between groups by one-way ANOVA, with Tukey’s test at P < 0.05. Groups with the same letter have no significant differences.
3.4 Synergistic effects of MSCs only or with enhancement on redox status of damaged liver in septic rats
The effects of sepsis induction and the ameliorating effects of MSCs, with or without augmentation, on the redox status within the liver’s tissue are illustrated in Figures 5 and 6. According to the results, the model group’s liver tissue had significantly higher levels of hepatic lipoperoxidation in terms of MDA [P < 0.0001, F (6, 28) = 39.43], MPO [P < 0.0001, F (6, 28) = 76.48], and NO [P < 0.0001, F (6, 28) = 80.96] than the control group. Alongside this, the CLP group’s GSH [P < 0.0001, F (6, 28) = 21.68] content and the activity of antioxidant enzymes [SOD (P < 0.0001, F (6, 28) = 27.26), CAT (P < 0.0001, F (6, 28) = 45.47), GPx (P < 0.0001, F (6, 28) = 18.83), and GR (P < 0.0001, F (6, 28) = 17.57)] significantly decreased in comparison to the control group. In contrast to the untreated CLP group, MSCs, E1-MSCs, E2-MSCs, or Ab treatments markedly (P < 0.05) increased these hepatic tissue-resident antioxidant enzymes. However, antibiotic treatment exhibits the least significance compared to other treated groups. MSCs inhibited oxidative damage significantly (P < 0.05) by lowering liver MDA, MPO, and NO levels alone or in combination with enhancers. In contrast, compared to the control rats, antibiotic therapy was unable to reduce these CLP-induced alterations in oxidative damage, particularly with regard to the NO level. This outcome suggests that the advantages of antibiotics are limited, even though MSCs protect the liver from oxidative stress, and MSC therapy might better treat oxidative damage in hepatic tissue after CLP-induced injury.
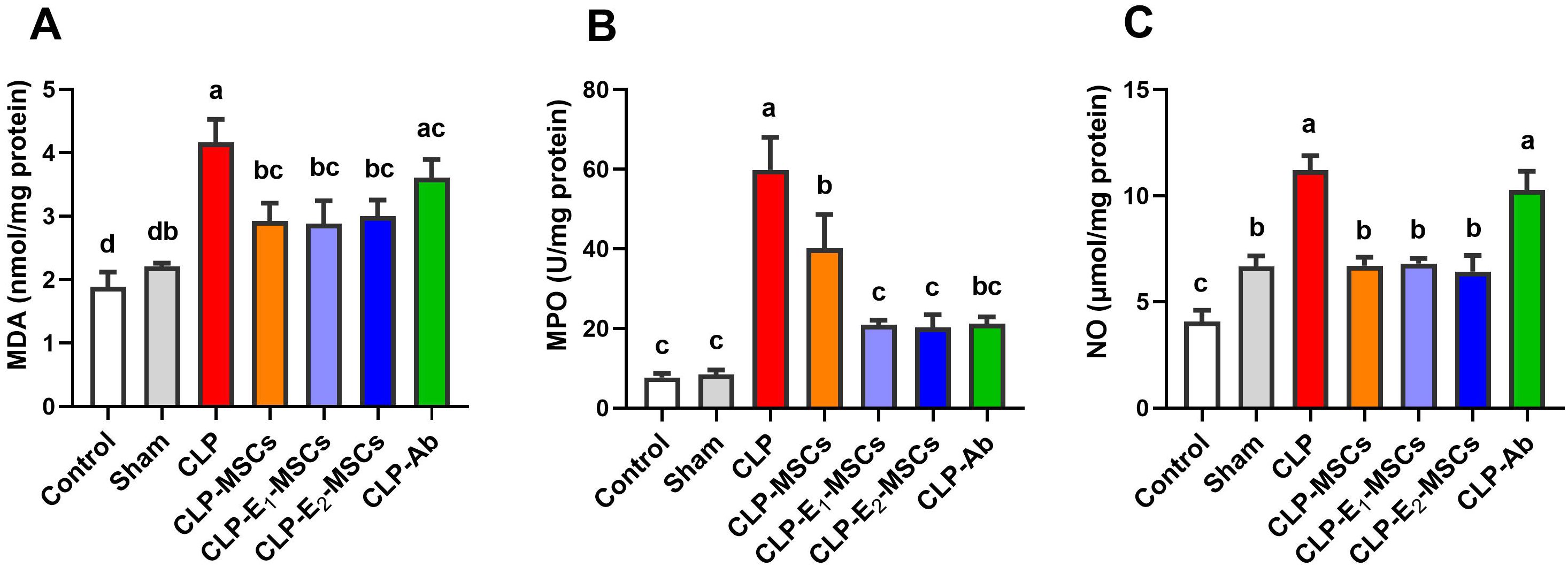
Figure 5. Effects of mesenchymal stem cells (MSCs), mesenchymal stem cells enhanced with Na2SeO3 (E1-MSCs), mesenchymal stem cells enhanced with SeNPs (E2-MSCs), or standard treatment antibiotics (Ab) on oxidative stress markers in damaged liver tissue of septic rats. (A) malondialdehyde (MDA), (B) myeloperoxidase (MPO), and (C) nitric oxide (NO). The mean ± SD data displays the biochemical results (n = 5). Different letters show statistically significant differences between groups by one-way ANOVA, with Tukey’s test at P < 0.05. Groups with the same letter have no significant differences.
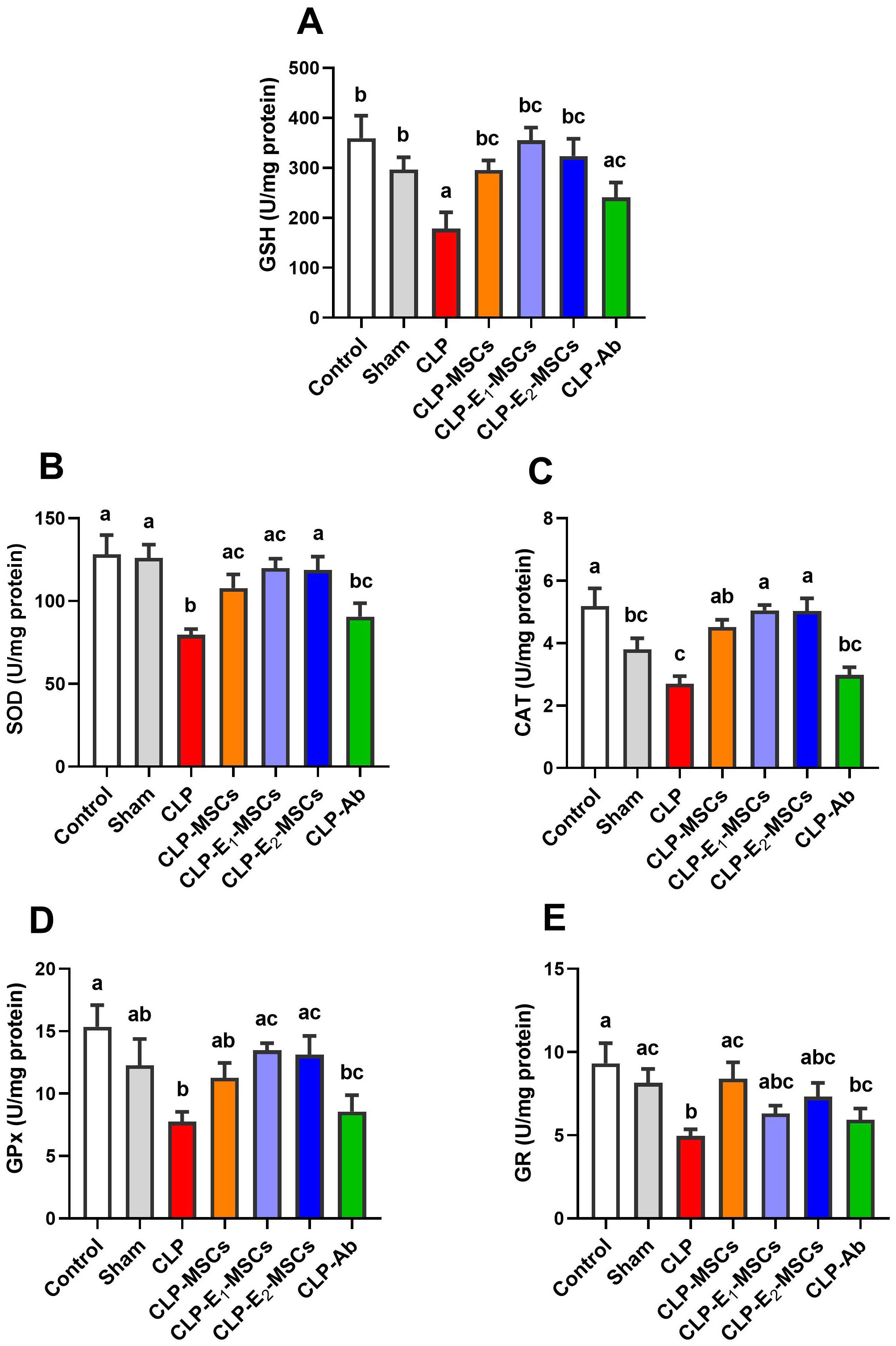
Figure 6. Effects of mesenchymal stem cells (MSCs), mesenchymal stem cells enhanced with Na2SeO3 (E1-MSCs), mesenchymal stem cells enhanced with SeNPs (E2-MSCs), or standard treatment antibiotics (Ab) on the antioxidant enzymes in damaged liver tissue of septic rats. (A) reduced glutathione (GSH), (B) superoxide dismutase (SOD), (C) catalase (CAT), (D) glutathione peroxidase (GPx), and (E) glutathione reductase (GR). The mean ± SD data displays the biochemical results (n = 5). Different letters show statistically significant differences between groups by one-way ANOVA, with Tukey’s test at P < 0.05. Groups with the same letter have no significant differences.
3.5 The combined therapy of MSCs and SeNPs boosted the damaged hepatic tissue’s anti-inflammatory and immunomodulatory response
This research examined the impact of MSCs alone or enhanced with Na2SeO3 or SeNPs on inflammatory and immunomodulatory mediators in CLP rats (Figure 7). A significant increase in the levels of pro-inflammatory cytokines [TNF-α (P < 0.0001, F (6, 28) = 157.50), IL-1β (P < 0.0005, F (6, 28) = 8.45), and IL-8 (P < 0.0001, F (6, 28) = 69.56)], along with cytokine-induced upregulation of iNOS (P < 0.0001, F (6, 14) = 36.48), was observed in hepatic tissues of the experimental CLP group compared to the control group. However, treatment with MSCs, either alone or combined with Na2SeO3 or SeNPs, significantly subsided (P < 0.05) these mediators and tissue inflammatory reactions in the CLP model group. The anti-inflammatory mechanism of MSCs on CLP-induced liver damage was confirmed by inhibiting the MAPK9 signaling pathway. In addition, NF-κB is another key marker for clarifying the efficacy of MSCs in regulating inflammation in sepsis. The study revealed that MAPK9’s mRNA expression was significantly upregulated (P < 0.0001, F (6, 14) = 110.0), with a further notable rise in the NF-κB (P < 0.0001, F (6, 28) = 97.50) levels in inflamed hepatic tissue of the CLP group compared to control. On the contrary, the CLP groups that received different treatments, either MSCs, E1-MSCs, or E2-MSCs, displayed marked downregulation of MAPK9 expression and decreased (P < 0.05) values of NF-κB. Meanwhile, the liver tissue’s immunomodulatory circumstances in reaction to MSCs alone and SeNPs in septic model rats were verified by measuring the levels of PGE2 and COX-2. Results revealed that the CLP group has higher levels of PGE2 (P < 0.0001, F (6, 28) = 104.21) and COX-2 (P < 0.0001, F (6, 28) = 114.20) than the control group, but in comparison to the untreated CLP group, treated CLP animals with various therapies showed a significant (P < 0.05) decrease in these markers. Therefore, MSCs, particularly when incorporated with SeNPs, may be vital in suppressing liver inflammation and improving overall outcomes. Therefore, combined therapy of MSCs and SeNPs could be an intriguing strategy for curing inflammatory and immunomodulatory hepatic diseases.
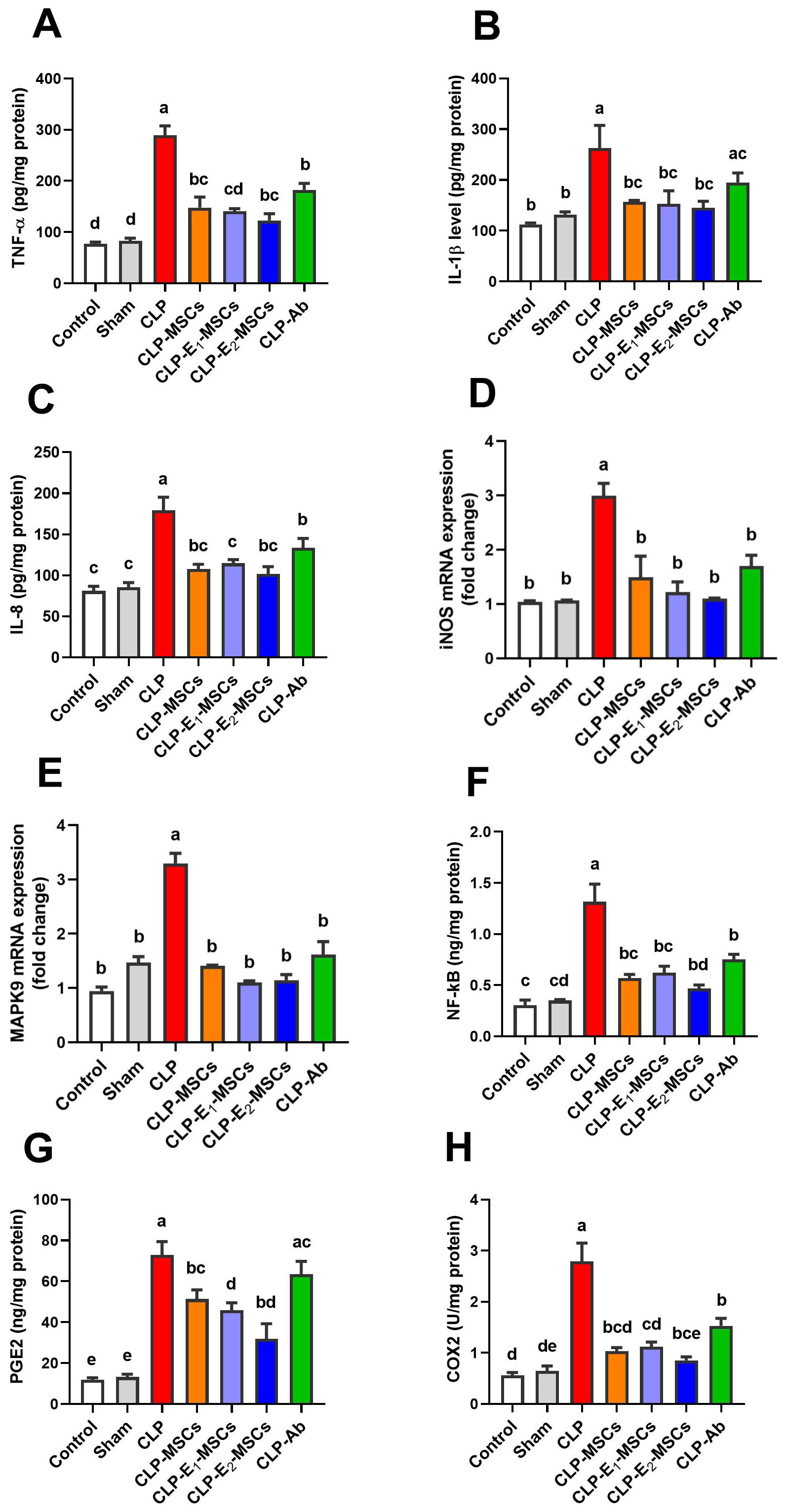
Figure 7. Effects of mesenchymal stem cells (MSCs), mesenchymal stem cells enhanced with Na2SeO3 (E1-MSCs), mesenchymal stem cells enhanced with SeNPs (E2-MSCs), or standard treatment antibiotics (Ab) on the inflammatory and immunomodulatory biomarkers in damaged liver tissue of septic rats. (A) tumor necrosis factor-alpha (TNF-α), (B) interleukin-1β (IL-1β), (C) interleukin-8 (IL-8), (D) inducible nitric oxide synthase (iNOS), (E) mitogen-activated protein kinases 9 (MAPK9), (F) nuclear factor kappa B (NF-κB), (G) prostaglandin E2 (PGE2), and (H) cyclooxygenase-2 (COX-2). The mean ± SD data displays the biochemical results (n = 5). Different letters show statistically significant differences between groups by one-way ANOVA, with Tukey’s test at P < 0.05. Groups with the same letter have no significant differences.
NF-κB and TNF-α immunostaining were performed to predict whether treatments with MSCs alone or with enhancement can spare hepatic cells from inflammation alongside necrosis induced by CLP. As shown in Figures 8–10, our results revealed that the inflamed hepatic cells had intense dark brown positivity staining for both NF-κB (Figure 8) and TNF-α (Figure 9) in the septic group with respect to the control group, indicating significant inflammation. Nuclear localization (Figure 10) was still discernible, despite the nucleus being less visible due to the dark stain. However, the control group lacked nuclear NF-κB and only showed light brown cytoplasmic staining. On the contrary, hepatic cells of the treated groups, either with MSCs alone or with enhancement, significantly decreased staining intensity, with NF-κB predominantly localized in the cytoplasm and distinctly visible blue nuclei, indicating reduced inflammation. Hence, administration of MSCs inhibits the translocation, activity, and binding of NF-κB to DNA molecules, consequently lowering the inflammatory response in the liver following CLP.
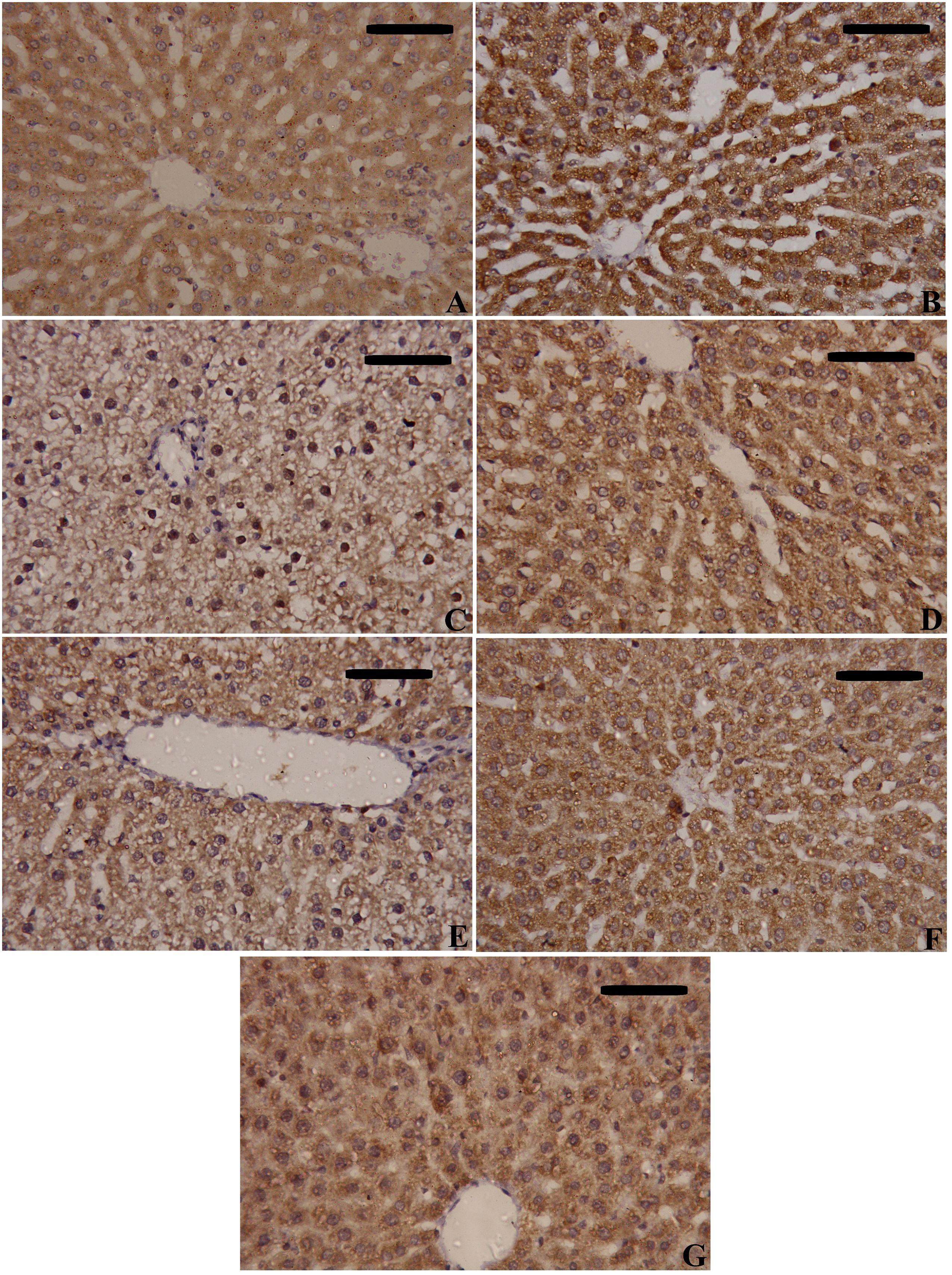
Figure 8. Effects of mesenchymal stem cells (MSCs), mesenchymal stem cells enhanced with Na2SeO3 (E1-MSCs), mesenchymal stem cells enhanced with SeNPs (E2-MSCs), or standard treatment antibiotics (Ab) on the inflammatory biomarkers’ immunohistochemistry analysis of nuclear factor kappa B (NF-κB). (A) Control; (B) Sham; (C) CLP; (D) CLP-MSCs; (E) CLP-E1-MSCs; (F) CLP-E2-MSCs; (G) CLP-Ab. Scale bar = 100 µm.
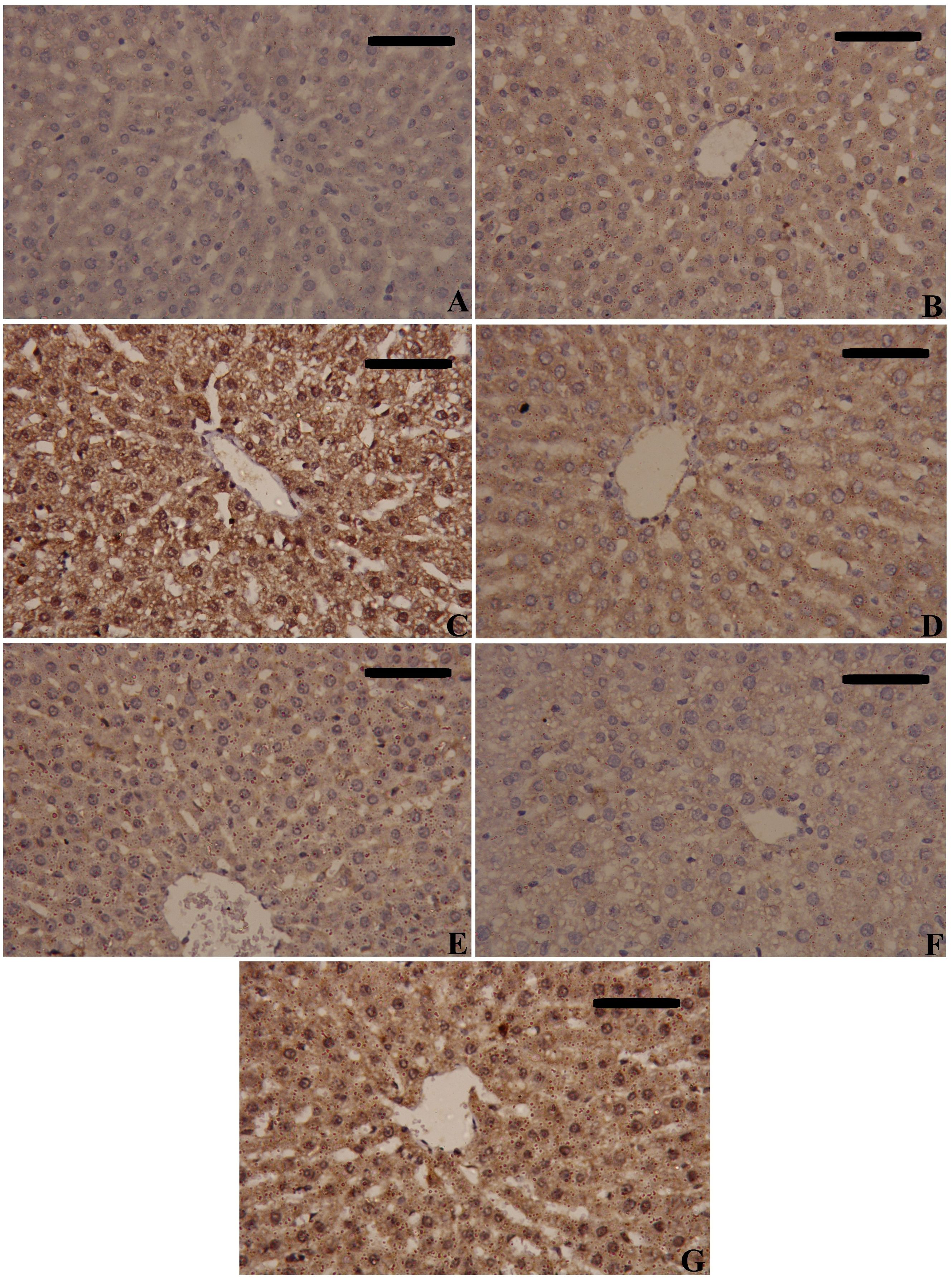
Figure 9. Effects of mesenchymal stem cells (MSCs), mesenchymal stem cells enhanced with Na2SeO3 (E1-MSCs), mesenchymal stem cells enhanced with SeNPs (E2-MSCs), or standard treatment antibiotics (Ab) on the inflammatory biomarkers’ immunohistochemistry analysis of tumor necrosis factor-alpha (TNF-α). (A) Control; (B) Sham; (C) CLP; (D) CLP-MSCs; (E) CLP-E1-MSCs; (F) CLP-E2-MSCs; (G) CLP-Ab. Scale bar = 100 µm.
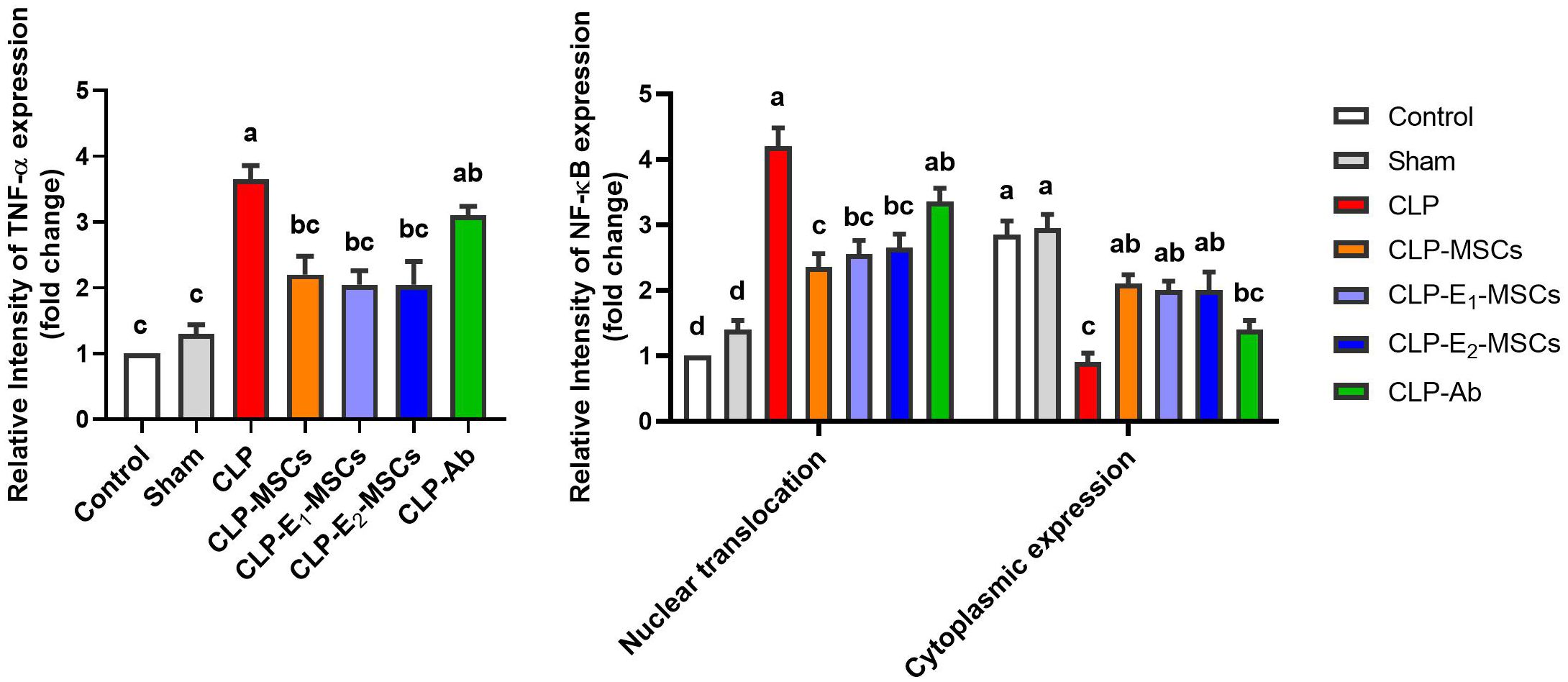
Figure 10. Quantitative analysis of tumor necrosis factor-alpha (TNF-α) and nuclear factor kappa B (NF-κB) either cytoplasmic or nuclear localization immunoreactions in the hepatic tissues of the different studied groups. The mean ± SD data displays the biochemical results. Different letters show statistically significant differences between groups by one-way ANOVA, with Tukey’s test at P < 0.05. Groups with the same letter have no significant differences.
3.6 MSCs, along with enhancement, restored the normal histology in hepatic tissues
Rats with CPL-induced sepsis showed remarkable pathologic changes, including hepatocyte necrosis, architectural destruction, inflammatory cell infiltration, liver steatosis, and hepatic fibroplasia in the portal system when compared to the typical liver architecture of control rats using H&E-stained sections (Figure 11, Supplementary Data, Supplementary Figure S1). On the other hand, different treatments using MSCs alone or with Na2SeO3/SeNPs helped improve these changes in the liver’s histopathology, and the typical structure of the tissue was slowly restored. Both parts of the combination therapy restored liver histology and improved liver function by lowering serum transaminase levels. Our findings further highlight the significance of combined therapy as a novel therapeutic approach in regenerative medicine for treating sepsis-induced liver damage.
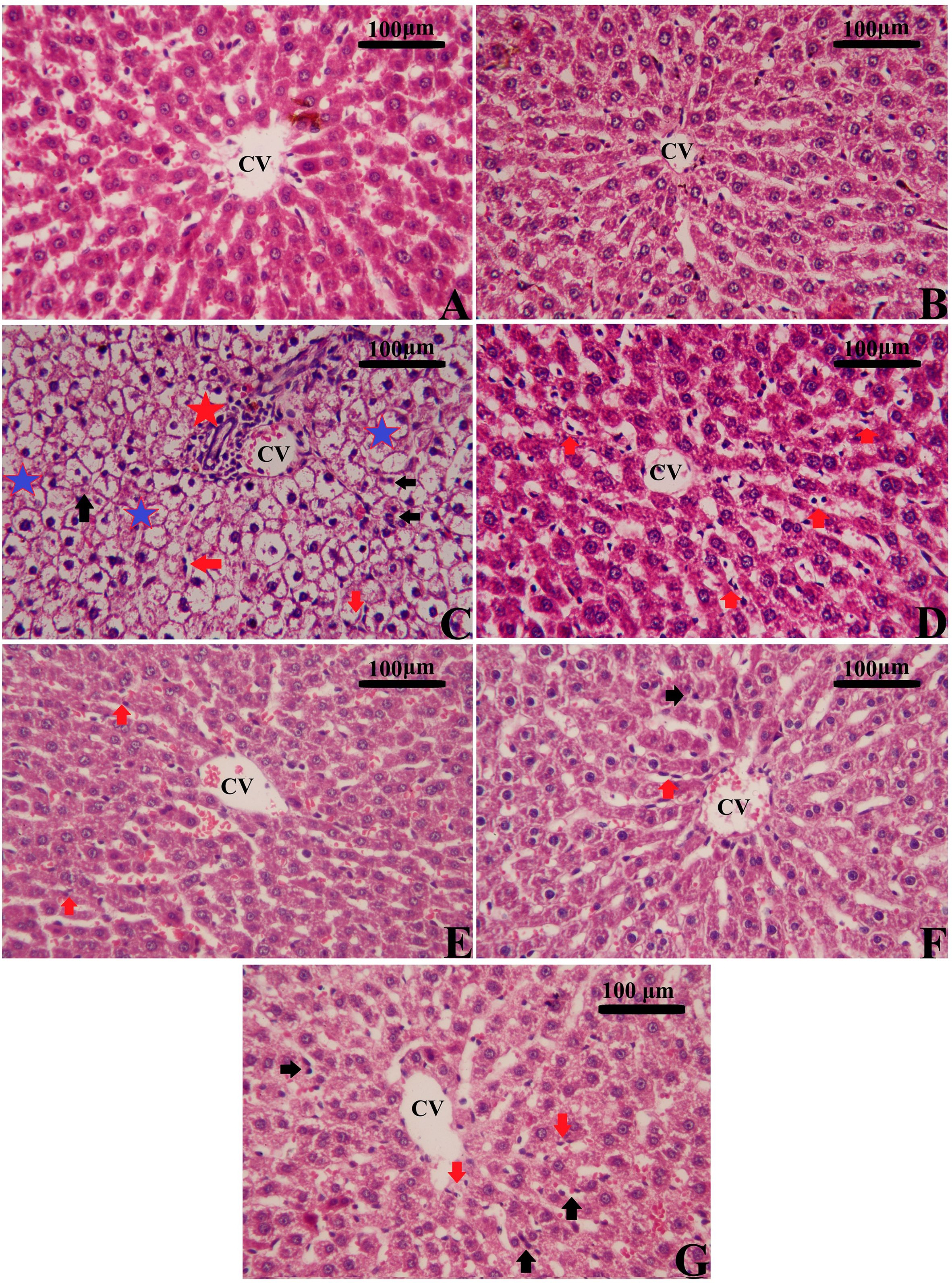
Figure 11. Effects of mesenchymal stem cells (MSCs), mesenchymal stem cells enhanced with Na2SeO3 (E1-MSCs), mesenchymal stem cells enhanced with SeNPs (E2-MSCs), and standard treatment antibiotics (Ab) on liver histopathology in CLP rats (H&E staining). Various magnifications were selected to illustrate typical tissue architecture and detailed histopathologic changes across broader areas. (A) Control; (B) Sham; (C) CLP; (D) CLP-MSCs; (E) CLP-E1-MSCs; (F) CLP-E2-MSCs; (G) CLP-Ab. CVs: central vein; black arrows: apoptotic nucleus; red arrows: inflammatory cells; red stars: focal inflammation; and blue stars: degenerative and vacuolated hepatocytes. Scale bar = 100 µm. Semi-quantitative analysis of histopathological changes is available in Supplementary Data, Supplementary Figure S1.
3.7 SeNPs preconditioning with MSCs lessened the hepatic apoptosis in septic rats
To investigate hepatic apoptotic events in a rat model of CLP-induced sepsis and the possible anti-apoptotic impact of MSCs and their enhanced forms (E1-MSCs and E2-MSCs), hepatic tissue was analyzed for Bcl-2, Bax, and caspase-3 activity. Rats exposed to the CLP process exhibited significantly higher expression of genes of apoptogenic proteins [caspase-3 (P < 0.0001, F (6, 28) = 75.49) and Bax (P < 0.0001, F (6, 28) = 75.36)] than the control animal, while the level of the anti-apoptotic protein Bcl-2 was considerably lower (P < 0.0001, F = 38.63). In contrast to the untreated CLP levels, treatment with MSCs or their enhanced variants (E1-MSCs and E2-MSCs) significantly (P < 0.05) inhibited the apoptotic cascade. It reversed the changes in apoptotic proteins caused by CLP exposure, suggesting the protective role of MSCs alone or with enhancement against hepatocellular loss. Among the treatments, E2-MSCs demonstrated superior efficacy in restoring apoptotic markers, suggesting enhanced hepatoprotective properties. Based on these findings, E2-MSCs provide the most effective protection against CLP-induced hepatocellular apoptosis (Figure 12).
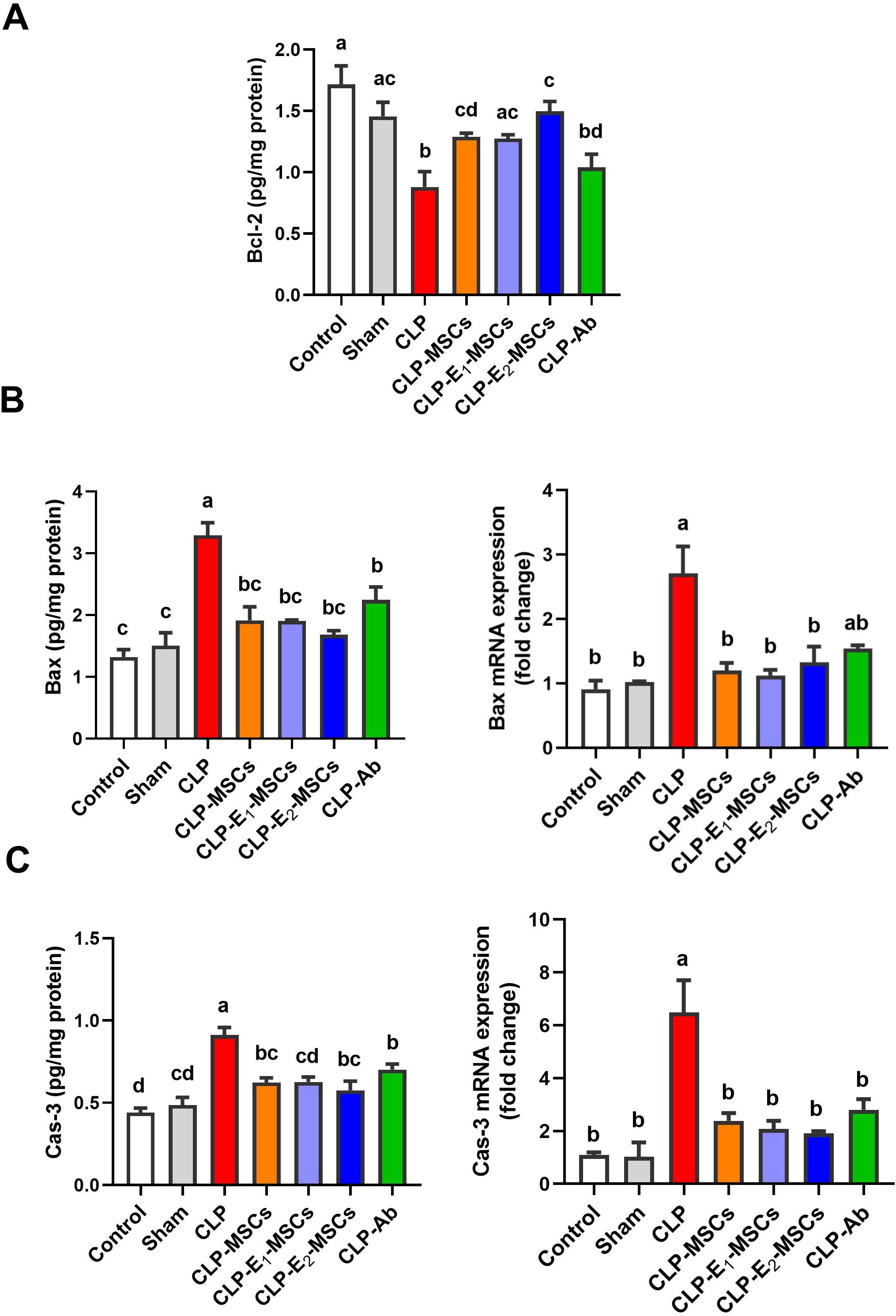
Figure 12. Effects of mesenchymal stem cells (MSCs), mesenchymal stem cells enhanced with Na2SeO3 (E1-MSCs), mesenchymal stem cells enhanced with SeNPs (E2-MSCs), or standard treatment antibiotics (Ab) on the apoptotic markers in damaged liver tissue of septic rats. (A) B-cell lymphoma-2 (Bcl-2), (B) bcl-2-associated X protein (Bax), and (C) caspase-3 (Cas-3). The mean ± SD data displays the biochemical results (n = 5), whereas gene expression findings are expressed as the mean ± SD of triplicate experiments adjusted to GAPDH. Different letters show statistically significant differences between groups by one-way ANOVA, with Tukey’s test at P < 0.05. Groups with the same letter have no significant differences.
4 Discussion
Sepsis is still a serious issue in intensive care today, even with the newest medical advancements and medications. It could be life-threatening, leading to multiple organ failure. The inflammatory response to infection results in symptomatic changes in biology, physiology, and biochemistry, according to Vincent et al. (31). The cecal ligation and puncture model is considered the gold standard for researching sepsis in animals (32). The liver is vital for detoxifying and eliminating endotoxins during sepsis, as it produces inflammatory mediators and is an impacted organ (33). According to Yan et al. (34), reducing liver damage may reduce morbidity and death rates in sepsis patients. MSCs have been explored as a possible treatment for septicemia, considering their potential for regeneration, immunosuppressive features, immunomodulatory abilities, and anti-inflammatory qualities (35, 36). Under culturing conditions, oxidative stress can result in various molecular and cellular changes, including increased apoptosis, cell inactivation, phenotypic changes, diminished stemness and differentiation potential, and accelerated aging (9, 37). Researchers are investigating various approaches to combat oxidative stress and improve MSCs’ capacity to treat inflammatory disorders.
One such approach is adding antioxidants to the MSC culture medium (38). Selenium has protective properties for MSCs and can scavenge ROS (39, 40). It serves as an enzyme cofactor for GPX and TrxR as well as an antioxidant. Moreover, it is involved in redox regulation, and the selenium nanoform can enhance the proliferation of stem cells (39). In the current research, we investigated the possible function of MSCs alone or enhanced with sodium selenite (Na2SeO3, which is E1-MSCs) or selenium nanoparticles (SeNPs, which are E2-MSCs) in a CLP rat model. The synthesized SeNPs (Figures 2A, B) exhibited a uniform size distribution (PDI = 0.068) and high stability (zeta potential = 19.5 ± 0.11 mV) and were subsequently utilized to enhance MSCs. MSCs’ therapeutic potential was ensured by flow cytometry, which verified their purity by showing that 82.5% of cells expressed CD44 but lacked CD45/CD19 (Figures 3B–D). Our research revealed that MSCs (both alone and enhanced) have promising effects on sepsis. Reducing pro-inflammatory mediators and cytokines, preventing liver damage, reducing oxidative stress, strengthening the antioxidant defense system, significant immunomodulation, and preserving vital tissues are considered potential roles of MSCs—an overall reduction in the morbidity and death caused by sepsis.
Regarding the hepatoprotective effects of MSCs alone or enhanced with Na2SeO3 (E1-MSCs) or SeNPs (E2-MSCs) in treating changes observed in septic rats, our findings indicate significant alterations in liver tissues due to CLP, evident at both histological and biochemical levels. Our histological examination verified the hepatic damage, as CLP led to the emergence of steatosis, hepatocellular necrosis, significant inflammatory cell infiltration, central vein dilatation and congestion, and Kupffer cell hyperplasia (Figure 10). These results align with earlier research (7, 19, 41). Biochemically, the serum levels of ALT, AST, and ALP were significantly elevated, confirming the extensive liver damage caused by CLP. Luo et al. (19) and Jeschke {Jeschke, 2009 #25794} reported that these hepatic enzymes are cytoplasmic in origin, but when a liver injury occurs, they leak into the bloodstream, reflecting a loss of the liver’s functional integrity. Other researchers (42, 43) also reported comparable results. Fortunately, in the current study, treating CLP-induced sepsis in liver tissue with MSCs alone or combined with sodium selenite (E1-MSCs) or selenium nanoparticles (E2-MSCs) has significantly improved the liver’s overall structural and functional alterations, which is consistent with the earlier study by Selim et al. (44). Following treatment, liver tissue revealed nearly standard architecture, reduced hepatocyte necrosis and fatty alterations, and lessened central hepatic vein enlargement and congestion. Hepatic enzyme levels (Figure 4; ALT, AST, and ALP) significantly decreased after treatment, indicating potential hepatoprotective benefits of MSCs on their own or with enhancement against the CLP septic model (19). Similarly, Zhao et al. {Zhao, 2012 #25798} previously showed that bone marrow-derived stem cells can lower serum transaminases in chemically induced acute liver injury. According to our research, MSCs alone or in combination with selenium-enhancing substances may be a viable treatment option for liver damage brought on by sepsis. In line with their established antioxidant and immunomodulatory functions, these histological and biochemical advancements confirm that SeNPs-MSCs simultaneously target inflammation, oxidative stress, and tissue damage. More studies are required to clarify the precise mechanisms underlying these hepatoprotective effects and to improve treatment regimens.
Sepsis-induced hepatic impairment is caused mainly by elevated oxidative stress (45). In line with earlier studies (46, 47). We noticed elevated lipid peroxidation, GSH depletion, and suppression of antioxidant enzyme activity in the septic rat group, confirming a state of severe oxidative stress. These alterations are due to the adverse effects triggered by ROS (reactive oxygen species) and RNS (reactive nitrogen species) on the mitochondria of hepatocytes, which can potentially result in apoptosis, necrosis, and liver failure (48, 49).
Malondialdehyde is a byproduct of lipid peroxidation and a recognized indicator of oxidative stress. It is also a typical indicator of elevated oxidative stress (50, 51). Simultaneously, GSH depletion is crucial for maintaining the stability of lipids and proteins and keeping cellular redox balance (52, 53). Additionally, superoxide dismutase, catalase, and glutathione peroxidase impairment have been shown to support the overloaded antioxidant system in septic livers (33, 54). These outcomes reveal the potential therapeutic benefits of antioxidant therapies for the management of impairment of the liver caused by sepsis.
Our research demonstrated that all treatment groups had a restricted level of lipid peroxidation (Figure 5A), significantly higher GSH content, and antioxidant enzyme activities (Figure 6). This is consistent with MSCs’ well-established antioxidant properties, which, as reported by Stavely and Nurgali (55), include the ability to directly scavenge reactive oxygen species (ROS) and donate functioning mitochondria or indirectly stimulate the antioxidant systems in other cells and modulate the bioenergetics. The MSC treatment in our study—whether used alone or with Na2SeO3 or SeNPs—worked better than regular antibiotics at lowering oxidative damage. The improved effectiveness might come from the combined protective benefits of MSC-derived antioxidants and selenium’s ability to boost MSC function, as shown by Rahimi et al. (56). Further, these effects positively correlate with the MSC-induced recovery in histopathology and liver functioning. Restoring redox balance likely helps explain the overall benefits of MSCs in treating sepsis, as oxidative stress can worsen organ failure and inflammation. Our findings show that MSCs have two important roles: they help reduce ROS and improve the body’s antioxidant systems, which explains how they protect against sepsis.
Moreover, sepsis induces parenchymal cells and vascular dysfunction, which leads to neutrophils infiltrating the site of damage or infection (42). Myeloperoxidase also indicates increased neutrophils and inflammatory cell infiltration (57). The production of MPO and other molecules by activated neutrophils leads to the overproduction of ROS (58). The current study revealed a significant rise in MPO activity, which implies a prominent infiltration of neutrophils and ongoing inflammatory processes in the hepatic tissue of the CLP rats. At the same time, MPO activity significantly decreased due to MSC administration (59, 60). Interestingly, our study introduced a novel approach of combining MSCs with SeNPs to improve the outcome. The combined therapy group (E2-MSCs) has shown a significant decrease in MPO activity, suggesting a substantial reduction in neutrophil infiltration and liver damage. Further evidence to support the result was obtained from histopathological investigation, which showed a decrease in neutrophil infiltration in the hepatic tissues in the combined therapy group. The correlation between MPO activity and histological changes highlights the potential of combined therapy for MSCs and SeNPs to attenuate sepsis-induced liver damage, underscoring the relevance of our findings.
Sepsis induces a complicated, inflammatory response involving infiltrating immune cells and liver resident cells. According to Yan et al. (34), these cells produce a variety of inflammatory mediators that contribute to organ damage caused by sepsis, such as TNF-α, interleukin-1β, interleukin-8, nitric oxide, and ROS. TNF-α is believed to be an essential mediator in sepsis, primarily generated by Kupffer cells (61). Septic patients and animal models generally show elevated levels (62). TNF-α stimulates specific transmembrane receptors, resulting in immune cell activation and the subsequent release of chemicals causing inflammation. This cycle may result in oxidative stress-induced DNA damage (33). Like TNF-α, IL-1β is produced by activated macrophages and is essential for numerous cellular processes, including differentiation, apoptosis, and proliferation (63). IL-8, another chemokine also generated by macrophages and other cells, acts as a neutrophil chemotactic agent, attracting neutrophils to the site of inflammation. Elevated IL-8 levels have been detected in patients with sepsis (64). Our findings revealed that pro-inflammatory cytokines (TNF-α, IL-1β, IL-8) significantly increased in septic rats, indicating systemic inflammation. Immunohistochemistry revealed TNF-α upregulation in hepatic cells, featuring strong staining in cytoplasm and nuclei, indicating serious tissue injury. Nevertheless, the injection of MSCs, either alone or in combination with selenium (especially as E2-MSC nanoparticles), showed a notable decrease in cytokine levels and limited TNF-α to the cytoplasm (IHC), demonstrating the anti-inflammatory and tissue-regeneration abilities of MSCs and combined therapy (65, 66). Moreover, the study of Abdel-Salam et al. (67) showed that AT-MSCs decrease TNF-α immunoreactivity in the liver tissues compared to the LPS control group. The coordinated inhibition of cytokines (IL-1β/IL-8) and pathogenic TNF-α translocation demonstrates MSCs’ ability to combat inflammation at both systemic and cellular levels. Given the strong relationship between inflammation and oxidative stress in sepsis, the observed decrease in oxidative damage could account for the concurrent decline in pro-inflammatory markers, as demonstrated by our findings (Figures 7A–C). This evidence indicates that MSCs reduce oxidative injury and may play a role in overall tissue protection by interrupting the cycle of oxidative stress and inflammation.
The study also observed a marked reduction in hepatic levels of NO and gene expression of inducible nitric oxide synthase in the CLP groups receiving MSC therapy. Pro-inflammatory cytokines like TNF-α and IL-1β cause sustained large quantities of NO generation via stimulating iNOS, according to Kamanaka et al. (68) and Celep and Gedikli (69). This excess NO may disrupt endothelial, neuronal, and epithelial cells, resulting in liver damage, according to a study by Hua et al. (70) who showed that amnion-derived MSCs (A-MSCs) restricted iNOS activity, NO production, and inflammatory cytokine synthesis while triggering an anti-inflammatory response in liver macrophages. Selenium supplements efficiently inhibit cytokine-induced NF-κB and iNOS upregulation, reducing NO levels (71, 72). Selenium can control NO generation through GPx’s inhibitory effect on iNOS expression (73). Similarly, our results demonstrate that the co-administered therapy attained recovery from hepatic inflammation and maintained redox equilibrium.
In septic rats, we observed significant NF-κB activation, characterized by increased levels measured by ELISA (Figure 7F) and intense nuclear and cytoplasmic staining via immunohistochemistry (Figures 8, 9), confirming its involvement in mediating inflammation. The NF-κB nuclear translocation is crucial for upregulating pro-inflammatory genes (e.g., TNF-α, IL-1β) and ROS-producing enzymes, worsening tissue damage. This supports NF-κB’s known role in regulating cytokine gene expression (74). MSCs have anti-inflammatory effects on acute liver injury by inhibiting the active NF-κB pathway, an essential mediator during serious infections (75). Our results showed that MSC alone or with enhancement treatment decreased NF-κB protein levels and inhibited its nuclear translocation (IHC), localizing it to the cytoplasm. Our findings align with those of previous authors who suggested that MSC-driven suppression of the NF-κB pathway is a crucial therapeutic approach for liver damage resulting from sepsis (76). Additionally, selenite-cultured cells have demonstrated that selenium reduces NF-κB activation and modifies the expression of inflammatory cytokine genes (77). This suppression was associated with reduced oxidative stress (lower lipid peroxidation, restored GSH) and diminished cytokine release, indicating that MSCs interrupt the NF-κB-mediated connection between inflammation and ROS generation. In summary, administration of MSCs, either by themselves or in cooperation with SeNPs (E2-MSCs), assists in keeping pro- and anti-inflammatory cytokines in balance in septic rats (78).
According to our research, MSCs and Na2SeO3, or SeNPs, work together synergistically to modulate the immune system and reduce inflammation in the hepatic tissues of septic rats. Even though the inflammatory response is essential for struggling with infection, an overreaction to it in late sepsis can inhibit the immune system and cause multiple organ failure or even death (79). Regarding potential mechanisms of inflammation, the newest research has shown the significance of cyclooxygenase-2 in producing excessive amounts of prostaglandin E2 during the inflammatory process (80). The pathway that typically controls the release of COX-2 in inflammation involves the mitogen-activated protein kinases (81). The MAPK subgroup JNK2 isoform (MAPK9) mainly engages in inflammation and liver metabolism. Moreover, its upregulation induces neutrophils and macrophages to infiltrate the liver and produce inflammatory cytokines (82). Our findings support previous reports (83); notable decreases were observed in levels of COX-2 and PGE2, as well as a noteworthy downregulation in Mapk9 gene expression in hepatic tissues treated with MSCs in either mono- or combined therapy (E1-MSCs/E2-MSCs). The positive outcomes of MSCs were mainly related to paracrine behaviors, releasing signaling factors for tissue regeneration (84, 85). Prostaglandin E2, one of these paracrine impacts, is an immunosuppressive cytokine that regulates inflammation and immunological responses (60).
Additionally, MSCs inhibit dendritic cells’ capacity to activate T cells, interfere with their differentiation, maturation, and function, and generate cytokines that are more anti-inflammatory and less pro-inflammatory (86, 87). Consequently, MSCs inhibit the MAPK pathway to reduce the production of COX-2 and other inflammatory mediators. Preserving the immune system’s stability and piercing tissues and organs to perform further functions (88). Furthermore, insufficient selenium may impair selenoprotein expression and GPx activity, indirectly affecting COX expression through the MAPK pathway and COX-2 (89). Conversely, selenium supplementation boosts anti-inflammatory mechanisms by reducing these intermediaries, which can mitigate these inflammatory alterations (90, 91). It is crucial to observe that preconditioning SeNPs for MSCs significantly reduced the production of pro-inflammatory cytokines in the hepatic tissue compared to MSCs alone, suggesting that SeNPs have enhanced the anti-inflammatory effects of MSCs.
Hepatocellular apoptosis spurred by sepsis induces organ malfunction and destruction (92). According to Fu et al. (93) and Klingensmith et al. (94), Bcl-2 and Bax are essential mediators of the apoptotic process. Also, it is characterized by cleaved caspase-3 (95). Consequently, apoptosis regulation suggests a therapeutic strategy for preventing liver damage (33). Our findings showed that sepsis markedly increased the liver’s pro-apoptotic pathways, as evidenced by increased protein levels of the proteins Caspase-3 and Bax and RT-PCR production of their related mRNA (Figures 11B, C). On the other hand, a shift toward apoptosis was shown by a substantial decrease in Bcl-2 protein levels (Figure 11A). MSC treatment effectively addressed this imbalance, normalizing the expression of Caspase-3 and Bax at both the protein and mRNA levels, while also restoring Bcl-2 protein levels. This is consistent with earlier research indicating that MSCs inhibit apoptosis by modulating pro- and anti-apoptotic factors (96, 97), and the MSCs can treat various illnesses by boosting tissue cell proliferation and blocking atypical apoptosis (98, 99). Similarly, selenium can alleviate cardiovascular diseases by modulating apoptosis, elevating the expression of the anti-apoptotic Bcl-2, and correcting the elevated levels of the pro-apoptotic Bax and Caspase-3 (100, 101). Furthermore, selenium shields cells against immune suppression, cytotoxicity, and intrinsic apoptosis by scavenging ROS (102, 103). These results indicate that MSCs, especially when combined with selenium, regulate multiple important apoptotic pathways to decrease sepsis-induced apoptosis and contribute to the simultaneous reduction of oxidative damage and inflammation, suggesting an interconnected protective mechanism.
5 Conclusions
According to the study’s findings, sepsis-induced acute liver damage is probably caused by a variety of processes. This study showed that the combination of Na2SeO3 or SeNPs-MSCs is more effective than either treatment alone in improving sepsis-induced acute liver injury, hepatic function, and outcomes in a rodent model. This was primarily due to the reduction of fibrosis and apoptosis, the suppression of ROS generation and oxidative stress, the inhibition of inflammation and inappropriate immune response, the upregulation of antioxidant expressions and potential liver-organ regeneration, and the preservation of hepatic architecture. These findings have crucially clarified the therapeutic potential of Na2SeO3/SeNPs and MSC combination therapy in reducing liver damage brought on by CLP.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by the Institutional Animal Care and Use Committee (IACUC) of Helwan University, Zoology and Entomology Department, and all procedures adhered to ethical guidelines for animal research (authorized no. HU-IACUC/Z/AEA0920-1). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
AAEA: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. ME-K: Data curation, Funding acquisition, Project administration, Resources, Writing – review & editing. AAM: Conceptualization, Data curation, Investigation, Supervision, Validation, Writing – review & editing. SA: Formal analysis, Supervision, Writing – review & editing. FA: Supervision, Validation, Writing – review & editing. MA: Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The authors would like to extend their sincere appreciation to the Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R23), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Acknowledgments
The authors would like to extend their sincere appreciation to the Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R23), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. Also, the authors would like to extend their sincere appreciation to Dr. Manoswini Dash, Centre for Aging, School of Medicine, Tulane University, United States, for editing and reviewing the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1602994/full#supplementary-material
Abbreviations
Ab, Antibiotics; ALP, Alkaline phosphatase; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; Bax, Bcl-2-associated X protein; Bcl2, B-cell lymphoma-2; Cas-3, Caspase-3; CAT, catalase; CLP, cecal ligation and puncture; COX-2, Cyclooxygenase 2; GPx, glutathione peroxidase; GR, glutathione reductase; GSH, glutathione reduced; IL-1β, Interleukin-1β; IL-8, Interleukin-8; iNOS, Inducible nitric oxide synthase; MAPK9, Mitogen-activated protein kinase 9; MDA, Malondialdehyde; MPO, Myeloperoxidase; MSCs, Mesenchymal stem cells; Na2SeO3, Sodium selenite; NF-κB, Nuclear factor kappa B; NO, Nitric oxide; OS, oxidative stress; PGE2, Prostaglandin E2; RNS, Reactive Nitrogen Species; ROS, Reactive Oxygen Species; Se, Selenium; SeNPs, Selenium nanoparticles; SOD, superoxide dismutase; SRLI, Sepsis-Related Liver Injury; TNF-α, Tumor necrosis factor-alpha.
References
1. Mushtaq A and Kazi F. Updates in sepsis management. Lancet Infect Dis. (2022) 22:24. doi: 10.1016/S1473-3099(21)00773-8
2. Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. (2020) 395:200–11. doi: 10.1016/S0140-6736(19)32989-7
3. Silva CMS, Wanderley CWS, Veras FP, Sonego F, Nascimento DC, Goncalves AV, et al. Gasdermin D inhibition prevents multiple organ dysfunction during sepsis by blocking NET formation. Blood. (2021) 138:2702–13. doi: 10.1182/blood.2021011525
4. Margraf A, Lowell CA, and Zarbock A. Neutrophils in acute inflammation: current concepts and translational implications. Blood. (2022) 139:2130–44. doi: 10.1182/blood.2021012295
5. Guo H, Ni M, Xu J, Chen F, Yao Z, Yao Y, et al. Transcriptional enhancement of GBP-5 by BATF aggravates sepsis-associated liver injury via NLRP3 inflammasome activation. FASEB journal: Off Publ Fed Am Societies Exp Biol. (2021) 35:e21672. doi: 10.1096/fj.202100234R
6. Xiang J-x, Liu P, Yang L-f, Su J-b, Zhang X-f, Liu X-m, et al. Hepatic differentiation of bone marrow mesenchymal stem cells induced by additional growth factors inhibits chronic liver fibrosis. Chin J Tissue Eng Res. (2018) 22:5286–91. doi: 10.3969/j.issn.2095-4344.0662
7. Liu P, Qian Y, Liu X, Zhu X, Zhang X, Lv Y, et al. Immunomodulatory role of mesenchymal stem cell therapy in liver fibrosis. Front Immunol. (2022) 13:1096402. doi: 10.3389/fimmu.2022.1096402
8. Alves H, Mentink A, Le B, van Blitterswijk CA, and de Boer J. Effect of antioxidant supplementation on the total yield, oxidative stress levels, and multipotency of bone marrow-derived human mesenchymal stromal cells. Tissue Eng Part A. (2013) 19:928–37. doi: 10.1089/ten.tea.2011.0700
9. Denu RA and Hematti P. Effects of oxidative stress on mesenchymal stem cell biology. Oxid Med Cell Longev. (2016) 2016:2989076. doi: 10.1155/2016/2989076
10. Fatima S, Alfrayh R, Alrashed M, Alsobaie S, Ahmad R, and Mahmood A. Selenium nanoparticles by moderating oxidative stress promote differentiation of mesenchymal stem cells to osteoblasts. Int J Nanomedicine. (2021) 16:331–43. doi: 10.2147/IJN.S285233
11. Khaled AM, Othman MS, Obeidat ST, Aleid GM, Aboelnaga SM, Fehaid A, et al. Green-synthesized silver and selenium nanoparticles using berberine: A comparative assessment of in vitro anticancer potential on human hepatocellular carcinoma cell line (HepG2). Cells. (2024) 13(3):287. doi: 10.3390/cells13030287
12. Elganzoury SS, Abdelfattah MS, Habotta OA, El-Khadragy M, Abdel Moneim AE, and Abdalla MS. Neuro-amelioration of Ficus lyrata (fiddle-leaf fig) extract conjugated with selenium nanoparticles against aluminium toxicity in rat brain: relevance to neurotransmitters, oxidative, inflammatory, and apoptotic events. Environ Sci pollut Res Int. (2023) 30(24):65822–34. doi: 10.1007/s11356-023-26935-0
13. Othman MS, Obeidat ST, Al-Bagawi AH, Fareid MA, Fehaid A, and Moneim AEA. Green-synthetized selenium nanoparticles using berberine as a promising anticancer agent. J Integr Med. (2022) 20:65–72. doi: 10.1016/j.joim.2021.11.002
14. Rodrigues KC, Bortolatto CF, da Motta KP, de Oliveira RL, Paltian JJ, Krüger R, et al. The neurotherapeutic role of a selenium-functionalized quinoline in hypothalamic obese rats. Psychopharmacology. (2021) 238:1937–51. doi: 10.1007/s00213-021-05821-y
15. Zhang H-Y, Zhang A-R, Lu Q-B, Zhang X-A, Zhang Z-J, Guan X-G, et al. Association between fatality rate of COVID-19 and selenium deficiency in China. BMC Infect Dis. (2021) 21:452. doi: 10.1186/s12879-021-06167-8
16. Fujimura N, Sumita S, Aimono M, Masuda Y, Shichinohe Y, Narimatsu E, et al. Effect of free radical scavengers on diaphragmatic contractility in septic peritonitis. Am J Respir Crit Care Med. (2000) 162:2159–65. doi: 10.1164/ajrccm.162.6.9912144
17. Ebert R, Ulmer M, Zeck S, Meissner-Weigl J, Schneider D, Stopper H, et al. Selenium supplementation restores the antioxidative capacity and prevents cell damage in bone marrow stromal cells in vitro. Stem Cells. (2006) 24:1226–35. doi: 10.1634/stemcells.2005-0117
18. Alhadlaq A and Mao JJ. Mesenchymal stem cells: isolation and therapeutics. Stem Cells Dev. (2004) 13:436–48. doi: 10.1089/scd.2004.13.436
19. Luo C, Liu Z, Xu Y, Ning F, Jang W, Zhang X, et al. Protective effects of mesenchymal stem cells on acute liver injury via TLR4/NF-kB signaling pathway in sepsis mice. Int J Clin Exp Med. (2019) 12:1196–205.
20. Patel A, Joseph J, Periasamy H, and Mokale S. Azithromycin in combination with ceftriaxone reduces systemic inflammation and provides survival benefit in a murine model of polymicrobial sepsis. Antimicrobial Agents Chemotherapy. (2018) 62(9): e00752-18. doi: 10.1128/AAC.00752-18
21. Ohkawa H, Ohishi N, and Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. (1979) 95:351–8. doi: 10.1016/0003-2697(79)90738-3
22. Bradley PP, Christensen RD, and Rothstein G. Cellular and extracellular myeloperoxidase in pyogenic inflammation. Blood. (1982) 60:618–22. doi: 10.1182/blood.V60.3.618.618
23. Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. (1959) 82:70–7. doi: 10.1016/0003-9861(59)90090-6
24. Nishikimi M, Appaji N, and Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. (1972) 46:849–54. doi: 10.1016/S0006-291X(72)80218-3
25. Aebi H. Catalase in vitro. Methods Enzymol. (1984) 105:121–6. doi: 10.1016/S0076-6879(84)05016-3
26. Ursini F, Maiorino M, and Gregolin C. The selenoenzyme phospholipid hydroperoxide glutathione peroxidase. Biochim Biophys Acta. (1985) 839:62–70. doi: 10.1016/0304-4165(85)90182-5
27. Carlberg I and Mannervik B. Glutathione reductase. Methods Enzymol. (1985) 113:484–90. doi: 10.1016/S0076-6879(85)13062-4
28. Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, and Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. (1982) 126:131–8. doi: 10.1016/0003-2697(82)90118-X
29. Ahmed A, Al Tamimi DM, Isab AA, Alkhawajah AM, and Shawarby MA. Histological changes in kidney and liver of rats due to gold (III) compound [Au(en)Cl(2)]Cl. PloS One. (2012) 7:e51889. doi: 10.1371/journal.pone.0051889
30. Anu Mary Ealia S and Saravanakumar MP. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf Series: Materials Sci Eng. (2017) 263:32019. doi: 10.1088/1757-899X/263/3/032019
31. Vincent JL, Opal SM, Marshall JC, and Tracey KJ. Sepsis definitions: time for change. Lancet. (2013) 381:774–5. doi: 10.1016/S0140-6736(12)61815-7
32. Doi K, Leelahavanichkul A, Yuen PS, and Star RA. Animal models of sepsis and sepsis-induced kidney injury. J Clin Invest. (2009) 119:2868–78. doi: 10.1172/JCI39421
33. Dkhil MA, Al-Quraishy S, and Abdel Moneim AE. Ziziphus spina-christi leaf extract pretreatment inhibits liver and spleen injury in a mouse model of sepsis via antioxidant and anti-inflammatory effects. Inflammopharmacology. (2018) 26(3):779–91. doi: 10.1007/s10787-017-0439-8
34. Yan J, Li S, and Li S. The role of the liver in sepsis. Int Rev Immunol. (2014) 33:498–510. doi: 10.3109/08830185.2014.889129
35. Ocansey DKW, Pei B, Yan Y, Qian H, Zhang X, Xu W, et al. Improved therapeutics of modified mesenchymal stem cells: an update. J Transl Med. (2020) 18:42. doi: 10.1186/s12967-020-02234-x
36. Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C, et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. (2018) 14:493–507. doi: 10.1038/s41581-018-0023-5
37. Chen F, Liu Y, Wong NK, Xiao J, and So KF. Oxidative stress in stem cell aging. Cell Transplant. (2017) 26:1483–95. doi: 10.1177/0963689717735407
38. Panahi M, Rahimi B, Rahimi G, Yew Low T, Saraygord-Afshari N, and Alizadeh E. Cytoprotective effects of antioxidant supplementation on mesenchymal stem cell therapy. J Cell Physiol. (2020) 235:6462–95. doi: 10.1002/jcp.29660
39. Ahmed HH, Aglan HA, Mabrouk M, Abd-Rabou AA, and Beherei HH. Enhanced mesenchymal stem cell proliferation through complexation of selenium/titanium nanocomposites. J Mater Sci Mater Med. (2019) 30:24. doi: 10.1007/s10856-019-6224-z
40. Zheng C, Wang J, Liu Y, Yu Q, Liu Y, Deng N, et al. Functional selenium nanoparticles enhanced stem cell osteoblastic differentiation through BMP signaling pathways. Advanced Funct Materials. (2014) 24:6872–83. doi: 10.1002/adfm.201401263
41. Al-Kadi A, Ahmed A-S, El-Tahawy NF, Montaser M, and El-Daly M. Silymarin protects against sepsis-induced acute liver and kidney injury via anti- inflammatory and antioxidant mechanisms in the rat. J advanced Biomed Pharm Sci. (2020) 3:190–7. doi: 10.21608/JABPS.2020.37074.1091
42. Mohamadin AM, Elberry AA, Elkablawy MA, Gawad HS, and Al-Abbasi FA. Montelukast, a leukotriene receptor antagonist abrogates lipopolysaccharide-induced toxicity and oxidative stress in rat liver. Pathophysiology. (2011) 18:235–42. doi: 10.1016/j.pathophys.2011.02.003
43. Sebai H, Sani M, Yacoubi MT, Aouani E, Ghanem-Boughanmi N, and Ben-Attia M. Resveratrol, a red wine polyphenol, attenuates lipopolysaccharide-induced oxidative stress in rat liver. Ecotoxicology Environ Saf. (2010) 73:1078–83. doi: 10.1016/j.ecoenv.2009.12.031
44. Selim SA, El-Baset SAA, Kattaia AAA, Askar EM, and Elkader EA. Bone marrow-derived mesenchymal stem cells ameliorate liver injury in a rat model of sepsis by activating Nrf2 signaling. Histochem Cell Biol. (2019) 151:249–62. doi: 10.1007/s00418-018-1731-4
45. Moradi M, Goodarzi N, Faramarzi A, Cheraghi H, Hashemian AH, and Jalili C. Melatonin protects rats testes against bleomycin, etoposide, and cisplatin-induced toxicity via mitigating nitro-oxidative stress and apoptosis. BioMed Pharmacother. (2021) 138:111481. doi: 10.1016/j.biopha.2021.111481
46. Giustina AD, Danielski LG, NovoChadlo MM, Goldim MPS, Joaquim L, Metzker KLL, et al. Vitamin B6 reduces oxidative stress in lungs and liver in experimental sepsis. Acad Bras Cienc. (2019) 91:e20190434. doi: 10.1590/0001-3765201920190434
47. Larrouyet-Sarto ML, Tamura AS, Alves VS, Santana PT, Ciarlini-Magalhaes R, Rangel TP, et al. P2X7 receptor deletion attenuates oxidative stress and liver damage in sepsis. Purinergic Signal. (2020) 16:561–72. doi: 10.1007/s11302-020-09746-7
48. Zhang W. The mitophagy receptor FUN14 domain-containing 1 (FUNDC1): A promising biomarker and potential therapeutic target of human diseases. Genes Dis. (2021) 8:640–54. doi: 10.1016/j.gendis.2020.08.011
49. Huff LA, Yan S, and Clemens MG. Mechanisms of ataxia telangiectasia mutated (ATM) control in the DNA damage response to oxidative stress, epigenetic regulation, and persistent innate immune suppression following sepsis. Antioxidants (Basel). (2021) 10(7):1146. doi: 10.3390/antiox10071146
50. Petronilho F, Florentino D, Danielski LG, Vieira LC, Martins MM, Vieira A, et al. Alpha-lipoic acid attenuates oxidative damage in organs after sepsis. Inflammation. (2016) 39:357–65. doi: 10.1007/s10753-015-0256-4
51. Zhou YX, Han WW, Song DD, Li ZP, Ding HJ, Zhou T, et al. Effect of miR-10a on sepsis-induced liver injury in rats through TGF-beta1/Smad signaling pathway. Eur Rev Med Pharmacol Sci. (2020) 24:862–9. doi: 10.26355/eurrev_202001_20070
52. Ross D. Glutathione, free radicals and chemotherapeutic agents. Mechanisms of free-radical induced toxicity and glutathione-dependent protection. Pharmacol Ther. (1988) 37:231–49. doi: 10.1016/0163-7258(88)90027-7
53. Coskun AK, Yigiter M, Oral A, Odabasoglu F, Halici Z, Mentes O, et al. The effects of montelukast on antioxidant enzymes and proinflammatory cytokines on the heart, liver, lungs, and kidneys in a rat model of cecal ligation and puncture-induced sepsis. ScientificWorldJournal. (2011) 11:1341–56. doi: 10.1100/tsw.2011.122
54. Zhong W, Qian K, Xiong J, Ma K, Wang A, and Zou Y. Curcumin alleviates lipopolysaccharide induced sepsis and liver failure by suppression of oxidative stress-related inflammation via PI3K/AKT and NF-kappaB related signaling. BioMed Pharmacother. (2016) 83:302–13. doi: 10.1016/j.biopha.2016.06.036
55. Stavely R and Nurgali K. The emerging antioxidant paradigm of mesenchymal stem cell therapy. Stem Cells Transl Med. (2020) 9:985–1006. doi: 10.1002/sctm.19-0446
56. Rahimi B, Panahi M, Saraygord-Afshari N, Taheri N, Bilici M, Jafari D, et al. The secretome of mesenchymal stem cells and oxidative stress: challenges and opportunities in cell-free regenerative medicine. Mol Biol Rep. (2021) 48:5607–19. doi: 10.1007/s11033-021-06360-7
57. Guo RF and Ward PA. Role of oxidants in lung injury during sepsis. Antioxidants Redox Signaling. (2007) 9:1991–2002. doi: 10.1089/ars.2007.1785
58. Kassab RB, Elbaz M, Oyouni AAA, Mufti AH, Theyab A, Al-Brakati A, et al. Anticolitic activity of prodigiosin loaded with selenium nanoparticles on acetic acid–induced colitis in rats. Environ Sci pollut Res. (2022) 29:55790–802. doi: 10.1007/s11356-022-19747-1
59. Gonzalez-Rey E, Anderson P, Gonzalez MA, Rico L, Buscher D, and Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. (2009) 58:929–39. doi: 10.1136/gut.2008.168534
60. Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. (2009) 15:42–9. doi: 10.1038/nm.1905
61. Su GL, Goyert SM, Fan MH, Aminlari A, Gong KQ, Klein RD, et al. Activation of human and mouse Kupffer cells by lipopolysaccharide is mediated by CD14. Am J Physiol Gastrointest Liver Physiol. (2002) 283:G640–645. doi: 10.1152/ajpgi.00253.2001
62. Mera S, Tatulescu D, Cismaru C, Bondor C, Slavcovici A, Zanc V, et al. Multiplex cytokine profiling in patients with sepsis. APMIS. (2011) 119:155–63. doi: 10.1111/j.1600-0463.2010.02705.x
63. Chaudhry H, Zhou J, Zhong Y, Ali MM, McGuire F, Nagarkatti PS, et al. Role of cytokines as a double-edged sword in sepsis. In Vivo. (2013) 27:669–84.
64. Livaditi O, Kotanidou A, Psarra A, Dimopoulou I, Sotiropoulou C, Augustatou K, et al. Neutrophil CD64 expression and serum IL-8: sensitive early markers of severity and outcome in sepsis. Cytokine. (2006) 36:283–90. doi: 10.1016/j.cyto.2007.02.007
65. Khosrojerdi A, Soudi S, Hosseini AZ, Eshghi F, Shafiee A, and Hashemi SM. Immunomodulatory and therapeutic effects of mesenchymal stem cells on organ dysfunction in sepsis. Shock. (2021) 55:423–40. doi: 10.1097/SHK.0000000000001644
66. Shu J, He X, Li H, Liu X, Qiu X, Zhou T, et al. The beneficial effect of human amnion mesenchymal cells in inhibition of inflammation and induction of neuronal repair in EAE mice. J Immunol Res. (2018) 2018:5083797. doi: 10.1155/2018/5083797
67. Abdel-Salam OME, Youness ER, Omara EA, and Sleem AA. Effect of adipose tissue-derived mesenchymal stem cell treatment on oxidative stress and inflammatory response following Escherichia coli lipopolysaccharide. Comp Clin Pathol. (2015) 24:343–58. doi: 10.1007/s00580-014-1906-x
68. Kamanaka Y, Kawabata A, Matsuya H, Taga C, Sekiguchi F, and Kawao N. Effect of a potent iNOS inhibitor (ONO-1714) on acetaminophen-induced hepatotoxicity in the rat. Life Sci. (2003) 74:793–802. doi: 10.1016/j.lfs.2003.09.036
69. Celep NA and Gedikli S. Protective effect of silymarin on liver in experimental in the sepsis model of rats. Acta Histochem Cytochem. (2023) 56:9–19. doi: 10.1267/ahc.22-00059
70. Hua D, Ju Z, Gan X, Wang Q, Luo C, Gu J, et al. Human amniotic mesenchymal stromal cells alleviate acute liver injury by inhibiting the pro-inflammatory response of liver resident macrophage through autophagy. Ann Trans Med. (2019) 7:392. doi: 10.21037/atm.2019.08.83
71. Casaril AM, Domingues M, Bampi SR, de Andrade Lourenco D, Padilha NB, Lenardao EJ, et al. The selenium-containing compound 3-((4-chlorophenyl)selanyl)-1-methyl-1H-indole reverses depressive-like behavior induced by acute restraint stress in mice: modulation of oxido-nitrosative stress and inflammatory pathway. Psychopharmacology. (2019) 236:2867–80. doi: 10.1007/s00213-018-5151-x
72. Yun CH, Yang JS, Kang SS, Yang Y, Cho JH, Son CG, et al. NF-kappaB signaling pathway, not IFN-beta/STAT1, is responsible for the selenium suppression of LPS-induced nitric oxide production. Int Immunopharmacol. (2007) 7:1192–8. doi: 10.1016/j.intimp.2007.05.002
73. Chomchan R, Puttarak P, Brantner A, and Siripongvutikorn S. Selenium-rich ricegrass juice improves antioxidant properties and nitric oxide inhibition in macrophage cells. Antioxidants. (2018) 7(4):57. doi: 10.3390/antiox7040057
74. Pereira Beserra F, Sergio Gushiken LF, Vieira AJ, Augusto Bergamo D, Luisa Bergamo P, Oliveira de Souza M, et al. From inflammation to cutaneous repair: topical application of lupeol improves skin wound healing in rats by modulating the cytokine levels, NF-kappaB, Ki-67, growth factor expression, and distribution of collagen fibers. Int J Mol Sci. (2020) 21(14):4952. doi: 10.3390/ijms21144952
75. Jin LY, Li CF, Zhu GF, Wu CT, Wang J, and Yan SF. Effect of siRNA against NF-kappaB on sepsis−induced acute lung injury in a mouse model. Mol Med Rep. (2014) 10:631–7. doi: 10.3892/mmr.2014.2299
76. Liang X, Li T, Zhou Q, Pi S, Li Y, Chen X, et al. Mesenchymal stem cells attenuate sepsis-induced liver injury via inhibiting M1 polarization of Kupffer cells. Mol Cell Biochem. (2019) 452:187–97. doi: 10.1007/s11010-018-3424-7
77. Maehira F, Miyagi I, and Eguchi Y. Selenium regulates transcription factor NF-kappaB activation during the acute phase reaction. Clinica chimica acta; Int J Clin Chem. (2003) 334:163–71. doi: 10.1016/s0009-8981(03)00223-7
78. Abdelnaser M, Alaaeldin R, Attya ME, and Fathy M. Hepatoprotective potential of gabapentin in cecal ligation and puncture-induced sepsis; targeting oxidative stress, apoptosis, and NF-kB/MAPK signaling pathways. Life Sci. (2023) 320:121562. doi: 10.1016/j.lfs.2023.121562
79. Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. (2011) 306:2594–605. doi: 10.1001/jama.2011.1829
80. Ricciotti E and FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. (2011) 31:986–1000. doi: 10.1161/ATVBAHA.110.207449
81. Kyriakis JM and Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev. (2012) 92:689–737. doi: 10.1152/physrev.00028.2011
82. Westenberger G, Sellers J, Fernando S, Junkins S, Han SM, Min K, et al. Function of mitogen-activated protein kinases in hepatic inflammation. J Cell Signal. (2021) 2:172–80.
83. Pedrazza L, Cubillos-Rojas M, de Mesquita FC, Luft C, Cunha AA, Rosa JL, et al. Mesenchymal stem cells decrease lung inflammation during sepsis, acting through inhibition of the MAPK pathway. Stem Cell Res Ther. (2017) 8:289. doi: 10.1186/s13287-017-0734-8
84. Lee JW, Fang X, Gupta N, Serikov V, and Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci U.S.A. (2009) 106:16357–62. doi: 10.1073/pnas.0907996106
85. Gnecchi M, Zhang Z, Ni A, and Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. (2008) 103:1204–19. doi: 10.1161/CIRCRESAHA.108.176826
86. Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. (2005) 105:4120–6. doi: 10.1182/blood-2004-02-0586
87. Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, and Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. (2006) 177:2080–7. doi: 10.4049/jimmunol.177.4.2080
88. Sun J, Ding X, and Sun T. Mesenchymal stem cells in sepsis: from basic research to clinical application. Intensive Care Res. (2021) 1:2–10. doi: 10.2991/icres.k.210622.001
89. Barchielli G, Capperucci A, and Tanini D. The role of selenium in pathologies: an updated review. Antioxidants (Basel). (2022) 11(2):251. doi: 10.3390/antiox11020251
90. Ala M, Jafari RM, Nematian H, Shadboorestan A, and Dehpour AR. Sodium selenite modulates IDO1/kynurenine, TLR4, NF-kappaB and Bcl2/Bax Pathway and mitigates acetic acid-induced colitis in rat. Cell Physiol Biochem. (2022) 56:24–35. doi: 10.33594/000000504
91. Hogan C and Perkins AV. Selenoproteins in the human placenta: how essential is selenium to a healthy start to life? Nutrients. (2022) 14:(3). doi: 10.3390/nu14030628
92. Gao X, Yan X, Yin Y, Lin X, Zhang Q, Xia Y, et al. Therapeutic targeting of apoptosis inhibitor of macrophage/CD5L in sepsis. Am J Respir Cell Mol Biol. (2019) 60:323–34. doi: 10.1165/rcmb.2018-0272OC
93. Fu H, Wang QS, Luo Q, Tan S, Su H, Tang SL, et al. Simvastatin inhibits apoptosis of endothelial cells induced by sepsis through upregulating the expression of Bcl-2 and downregulating Bax. World J Emerg Med. (2014) 5:291–7. doi: 10.5847/wjem.j.issn.1920-8642.2014.04.009
94. Klingensmith NJ, Fay KT, Lyons JD, Chen CW, Otani S, Liang Z, et al. Chronic alcohol ingestion worsens survival and alters gut epithelial apoptosis and CD8+ T cell function after pseudomonas aeruginosa pneumonia-induced sepsis. Shock. (2019) 51:453–63. doi: 10.1097/SHK.0000000000001163
95. Choudhary GS, Al-Harbi S, and Almasan A. Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis. Methods Mol Biol. (2015) 1219:1–9. doi: 10.1007/978-1-4939-1661-0_1
96. Zhao S, Liu Y, and Pu Z. Bone marrow mesenchymal stem cell-derived exosomes attenuate D-GaIN/LPS-induced hepatocyte apoptosis by activating autophagy in vitro. Drug Des Devel Ther. (2019) 13:2887–97. doi: 10.2147/DDDT.S220190
97. Kholodenko IV, Kholodenko RV, and Yarygin KN. The crosstalk between mesenchymal stromal/stem cells and hepatocytes in homeostasis and under stress. Int J Mol Sci. (2023) 24(20):15212. doi: 10.3390/ijms242015212
98. Cheng T, Ding S, Liu S, Li Y, and Sun L. Human umbilical cord-derived mesenchymal stem cell therapy ameliorates lupus through increasing CD4+ T cell senescence via MiR-199a-5p/Sirt1/p53 axis. Theranostics. (2021) 11:893–905. doi: 10.7150/thno.48080
99. Lee HK, Kim HS, Pyo M, Park EJ, Jang S, Jun HW, et al. Phorbol ester activates human mesenchymal stem cells to inhibit B cells and ameliorate lupus symptoms in MRL.Fas(lpr) mice. Theranostics. (2020) 10:10186–99. doi: 10.7150/thno.46835
100. Sordillo LM, Streicher KL, Mullarky IK, Gandy JC, Trigona W, and Corl CM. Selenium inhibits 15-hydroperoxyoctadecadienoic acid-induced intracellular adhesion molecule expression in aortic endothelial cells. Free Radical Biol Med. (2008) 44:34–43. doi: 10.1016/j.freeradbiomed.2007.09.002
101. Han Q, Wang A, Fu Q, Zhou S, Bao J, and Xing H. Protective role of selenium on ammonia-mediated nephrotoxicity via PI3K/AKT/mTOR pathway: Crosstalk between autophagy and cytokine release. Ecotoxicology Environ Saf. (2022) 242:113918. doi: 10.1016/j.ecoenv.2022.113918
102. Saikiran G, Mitra P, Sharma S, Kumar PK, and Sharma P. Selenium, oxidative stress and inflammatory markers in handicraft workers occupationally exposed to lead. Arch Environ Occup Health. (2022) 77:561–7. doi: 10.1080/19338244.2021.1968780
Keywords: sepsis-related liver injury, mesenchymal stem cells, selenium nanoparticles, inflammation, immunomodulation, NF-κB
Citation: Abd El Aleem AMM, El-Khadragy MF, Abdel Moneim AE, Agwa SH, Abou Zahra F and Abdalla MS (2025) Mesenchymal stem cells enhanced with SeNPs protection against CLP induction associated with liver injury are mediated via antioxidant, anti-inflammatory, and immunomodulatory activities. Front. Immunol. 16:1602994. doi: 10.3389/fimmu.2025.1602994
Received: 30 March 2025; Accepted: 04 August 2025;
Published: 05 September 2025.
Edited by:
Stefania Bruno, University of Turin, ItalyReviewed by:
Rachel Elaine Hewitt, University of Cambridge, United KingdomSerkan Gurgul, Gaziantep University Faculty of Medicine, Türkiye
Naglaa Sarhan, Tanta University, Egypt
Copyright © 2025 Abd El Aleem, El-Khadragy, Abdel Moneim, Agwa, Abou Zahra and Abdalla. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmed E. Abdel Moneim, YWVzdDE5NzdAaG90bWFpbC5jb20=; YWhtZWRfYWJkZWxtb25laW1Ac2NpZW5jZS5oZWx3YW4uZWR1LmVn
 Asmaa M. M. Abd El Aleem
Asmaa M. M. Abd El Aleem Manal F. El-Khadragy
Manal F. El-Khadragy Ahmed E. Abdel Moneim
Ahmed E. Abdel Moneim Sara H. Agwa
Sara H. Agwa Fatma Abou Zahra5
Fatma Abou Zahra5