- 1School of Sports Medicine and Health, Chengdu Sports University, Chengdu, China
- 2Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
Background: Tumor immune escape, a defining hallmark of malignant tumors, enables cancer cells to thrive within the host by evading detection and attack by the immune system. While immune checkpoint inhibitors, such as PD-1/PD-L1 antibodies, have delivered significant clinical advances, their effectiveness is tempered by modest response rates and a growing challenge of drug resistance. In this study, we aimed to explore the development process and trend of tumor immune escape, analyze the current hot spots, and predict the future research directions.
Methods: A bibliometric analysis was conducted in this study to retrieve and analyze 1839 publications from January 1, 2009 to February 14, 2025 related to tumor immune escape. Literature was obtained from Web of Science Core Collection (WoSCC) and data visualization and trend analysis were performed using VOSviewer, CiteSpace, Bibliometrix software package.
Results: The bibliometric analysis indicates that research on tumor immune escape has primarily focused on China, the United States, and European countries. China ranks first in research output and impact, with notable contributions from institutions like the Sun Yat-sen University System and the University of Texas System. The journal with the most publications is Frontiers in Immunology, while the most cited article globally is Jiang P’s 2018 publication in Nature Medicine, titled “Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response.” Keyword co-occurrence and burst analysis indicate that the field has undergone a thematic evolution. Early research centered around classical immune checkpoint molecules and T cell exhaustion, while more recent trends have shifted toward the tumor microenvironment (TME), multi-target combination immunotherapies, and mechanisms of immune evasion involving metabolic reprogramming and the microbiome. The integration of artificial intelligence (AI) and machine learning (ML) in immunotherapy prediction and biomarker discovery has also gained momentum, highlighting a growing cross-disciplinary approach.
Conclusion: This bibliometric study provides a comprehensive overview of the intellectual landscape, research hotspots, and developmental trajectory of tumor immune escape research over the past 14 years. By mapping influential nation, authors, core journals, reference, and keyword bursts, this work not only summarizes major contributions in the field but also helps researchers better understand its evolution and emerging directions. Based on the observed patterns, we propose three key areas that warrant further exploration: (1) advancing interdisciplinary research at the intersection of the microbiome, metabolism, and immune regulation; (2) integrating artificial intelligence and multi-omics data to enhance predictive modeling and therapeutic precision; and (3) combining multi-modal therapeutic strategies to overcome immune escape more effectively.
1 Introduction
Cancer has become a globally prevalent and serious economic and social problem, with increasing incidence and high mortality rates (1). Although the traditional three main therapies (surgery, radiotherapy, and chemotherapy) remain the cornerstone of clinical treatment, their efficacy is limited by significant toxicities and patient response heterogeneity. Targeted therapies have achieved significant breakthroughs in treating specific malignancies by blocking key oncogenic signaling pathways, such as those involving the epidermal growth factor receptor, HER2, estrogen receptor, vascular endothelial growth factor recepto, and multikinase inhibitors (2). However, issues of acquired drug resistance and inadequate therapeutic efficacy remain unresolved, particularly in cancers with complex pathophysiologic mechanisms.
The advent of cancer immunotherapies has revolutionized treatment approaches, particularly with immune checkpoint inhibitors like anti-PD-1/PD-L1 antibodies marking a landmark advancement. This breakthrough, awarded the 2018 Nobel Prize in Physiology or Medicine, combats tumor immune evasion by enhancing T-cell-mediated antitumor immune responses (3). However, despite these advances, not all patients benefit clinically due to the dynamic complexity and spatial heterogeneity of TME (4). Recent studies have demonstrated that malignant cells employ various strategies to create immune-evasive microenvironments, including metabolic reprogramming, secretion of immunosuppressive factors, and epigenetic modulation of antigen-presentation mechanisms (5, 6).
Tumor immune escape is a phenomenon where tumor cells avoid immune system recognition and attack, enabling them to grow and metastasize. This is a key strategy for tumor survival and progression (7). The interaction between immunity and cancer in regulating tumor growth is considered a cancer hallmark. Anti-tumor immunity involves innate and adaptive immune responses that control cancer development and proliferation. Tumor immune escape poses a major obstacle to effective anticancer therapy (8). Many factors induce tumor immune escape, including low tumor cell immunogenicity, tumor-specific antibody recognition as self-antigens, tumor surface antigen regulation, tumor-induced immune privilege, and tumor-induced immunosuppression. Research mainly focuses on the latter factors. Cancer cells can evade the immune system by activating immune checkpoints, altering the surrounding microenvironment, causing antigen presentation and recognition abnormalities, and undergoing metabolic reprogramming to inhibit T-cell activity. This allows cancer cells to survive and proliferate within the host (9). This mechanism is significantly influenced by programmed death receptor 1/programmed death receptor-ligand 1 (PD-1/PD-L1), which regulates immune tolerance and escape within TME (7, 10–12). When the PD-1 receptor on activated T cells interacts with the PD-L1 receptor on cancer cells, it weakens cytotoxic T lymphocyte effects, helping malignant cells resist immune attacks and promoting immune escape (13).
In recent years, research into tumor immune escape mechanisms and their role in cancer progression has surged exponentially. Reviews have explored key issues in this field from molecular pathways and clinical interventions perspectives (4, 14). However, a systematic overview of the discipline’s development and knowledge structure evolution is still lacking, as is a clear definition of research foci and potential blind spots. Bibliometrics, an effective tool for assessing discipline dynamics, can objectively identify core contributing countries, institutions, and scholars, and reveal landmark high-impact literature. It can also track historical changes in research hotspots, capture emerging frontier directions, and locate under - explored scientific issues (15). Such analyses have been successfully applied to TME (16), checkpoint inhibitor development (17) and other immunotherapy - related fields. Notably, while bibliometric studies on the PD-1/PD-L1 signaling axis or CAR-T cell therapies have been reported (18), there remains a lack of comprehensive and systematic analyses specifically focused on the field of tumor immune escape. Therefore, this study aims to comprehensively analyze the research landscape, evolutionary pathways, and future trends in tumor immune escape using a multidimensional bibliometric approach. This will provide a data - driven decision - making basis for optimizing immunotherapeutic strategies and basic research directions.
2 Materials and methods
2.1 Data collection and sources
Bibliometric analysis offers a systematic framework for identifying developmental trends and research hotspots within a discipline over a defined period. The selection of an appropriate database is critical to ensuring data reliability and analytical rigor. Among available options, the Web of Science (WoS) stands out for its multidisciplinary coverage of high-impact scientific journals and robust citation indexing. Compared to Scopus and MEDLINE/PubMed, WoS provides more comprehensive information that is particularly well-suited for bibliometric analysis (17). In this study, we selected the Web of Science Core Collection as our primary data source, as it is widely recognized for its depth, accuracy, and authority in indexing peer-reviewed literature (19). Its extensive journal coverage ensures that the retrieved publications reflect contemporary research trajectories in immunology and oncology. This choice enables accurate, representative data extraction and supports a thorough exploration of valuable research insights.
2.2 Search strategy and criteria
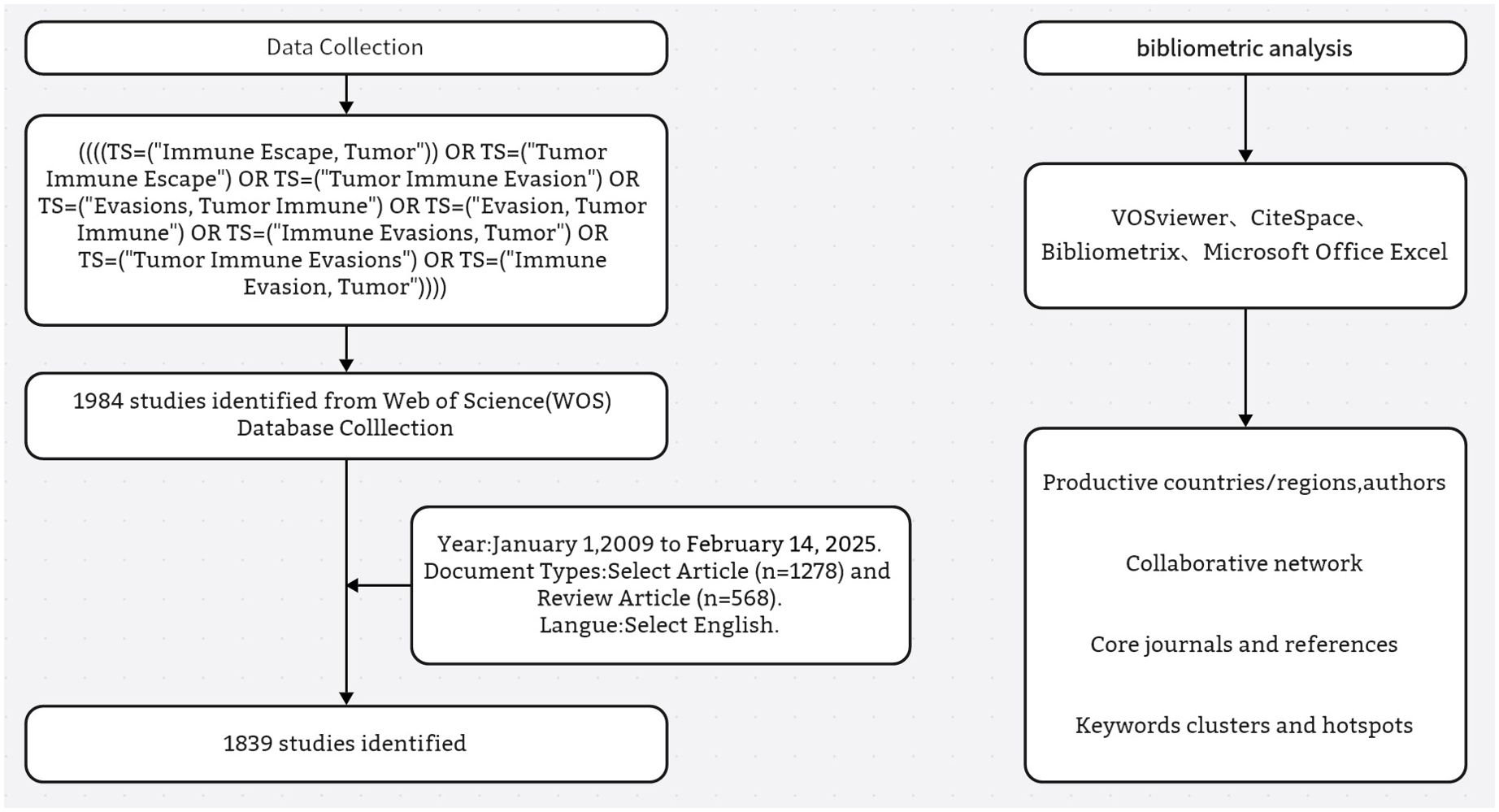
A literature search was conducted on February 14, 2025, to retrieve original articles and reviews on tumor immune escape published between 2009 and February 14, 2025. To avoid temporal bias due to real-time database updates, the search was completed in a single day. The search strategy was as follows: ((((TS=(“Immune Escape, Tumor”)) OR TS=(“Tumor Immune Escape”) OR TS=(“Tumor Immune Evasion”) OR TS=(“Evasions, Tumor Immune”) OR TS=(“Evasion, Tumor Immune”) OR TS=(“Immune Evasions, Tumor”) OR TS=(“Tumor Immune Evasions”) OR TS=(“Immune Evasion, Tumor”)))). Only journal articles and reviews published in English were included in this analysis. Other publication types—such as letters, editorials, conference abstracts, meeting reports—and all non-English publications were excluded to ensure consistency and comparability of the bibliometric dataset. The eligible records were exported in plain-text format with the “Full Record and Cited References” option selected to enable comprehensive metadata extraction. The final dataset contained information on publication counts, citations, titles, authors, affiliations, countries, keywords, and journals. In total, 1,839 records met the inclusion criteria. The detailed screening process is presented in Figure 1.
2.3 Data analysis
For data processing and analysis, we used Microsoft Excel in combination with three specialized tools: Bibliometrix 4.3.3 (an R-based package), VOSviewer 1.6.20, and CiteSpace 6.4.R1.
VOSviewer, developed by van Eck and Waltman, generates bibliometric network visualizations using node-link diagrams. It visualizes collaboration patterns by clustering nodes chromatically, where node size represents publication volume and edge thickness indicates collaboration strength between entities (e.g., countries or institutions).
CiteSpace, created by Chaomei Chen, is a Java-based software for detecting research frontiers. It employs timeline mapping and citation burst detection, with keyword clustering to reveal thematic domains. Clustering reliability is validated when silhouette values exceed 0.5 and modularity Q-values exceed 0.3, indicating strong internal consistency and significant structural separation.
Bibliometrix, an R-integrated package, enables statistical analysis of scholarly outputs including publication frequencies, citation metrics, and national contributions. Its algorithms support cross-comparison among journals and countries, contributing to a quantitative understanding of academic productivity.
3 Result
3.1 Trends in publications and citations
As per the formulated research strategy, 1,839 tumor immune escape - related publications were obtained from the WoSCC database between 2009 and 14 February 2025. Figure 2 presents the annual publication and citation counts for tumor immune escape research from 2009 to 14 February 2025.
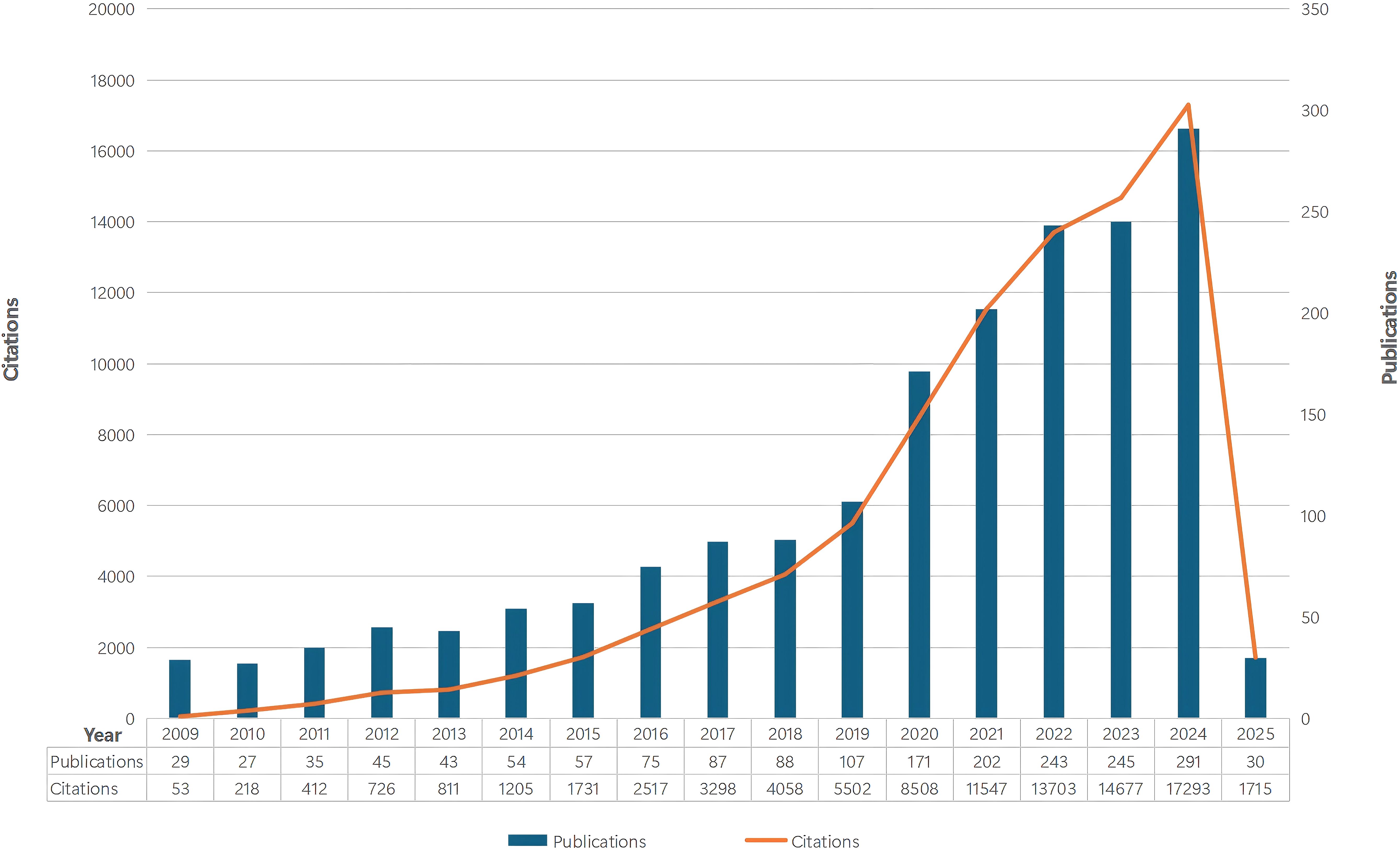
Figure 2. The number of annual papers and citations on tumor immune escape research has been steadily increasing from 2009 to 2025.
The steady publication increase from 2009 to 2018 shows great attention and interest in this field. The steeper growth curve from 2018 onwards indicates significant expansion, likely due to the 2018 Nobel Prize in Physiology or Medicine awarded to Professors James P. Allison and Tasuku Honjo for their work on the CTLA - 4 and PD - 1/PD - L1 pathways. The rising citation trend suggests ongoing research impact and the need for more prospective studies to highlight its global relevance.
3.2 National and institutional analyses
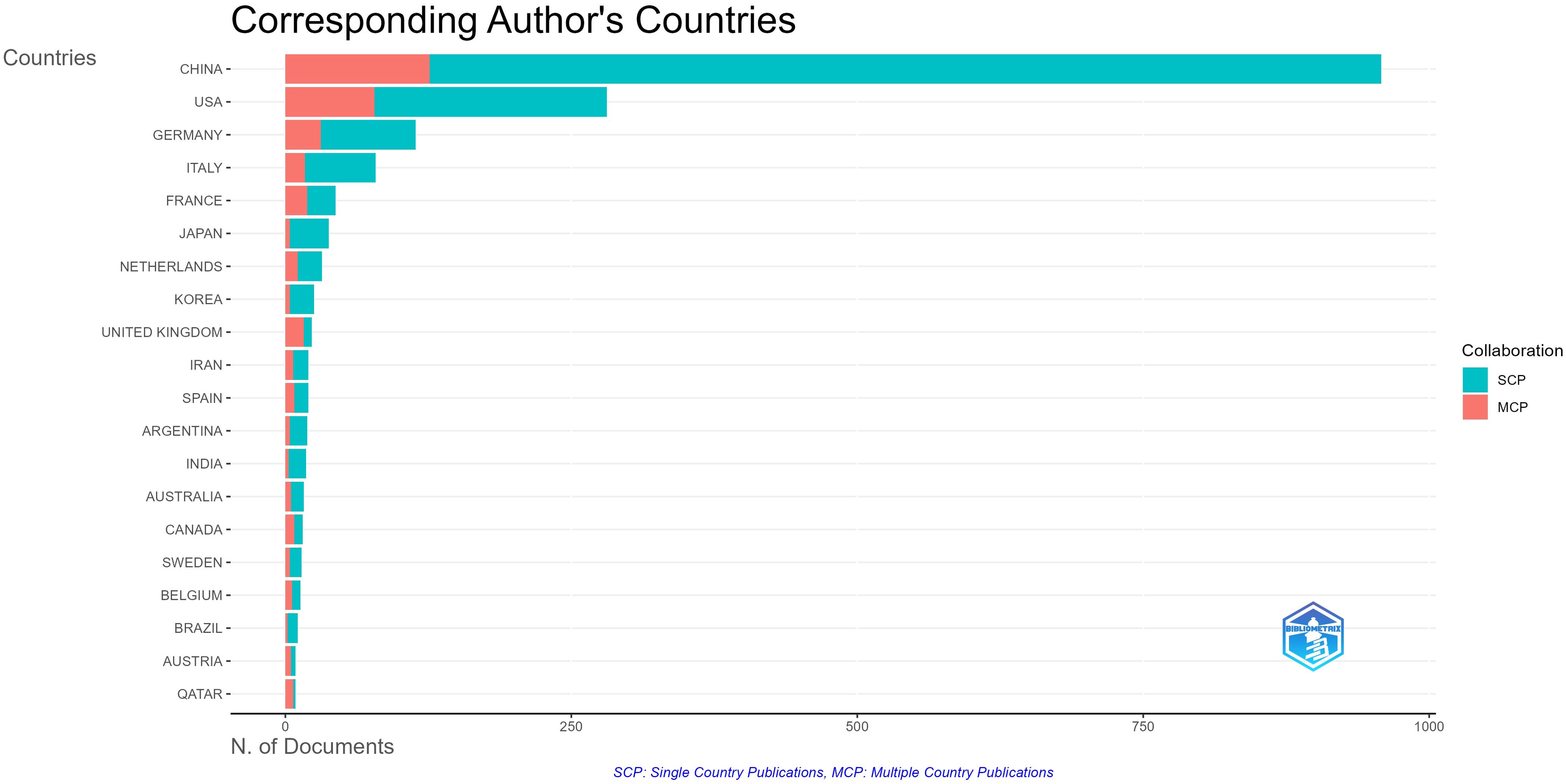
A total of 65 countries and 2,184 institutions participated in tumor immune escape research. Table 1 ranks the top ten countries by number of publications and total citations. China (n = 958) was the most productive country, accounting for 52.1 per cent of the total number of publications, followed by the United States (n = 281, 15.3 per cent), and Germany (n = 114, 6.2 per cent). The US and China have nearly identical total citations, 28,052 and 28,142 respectively, far surpassing other nations and highlighting their influence in this field. The UK has the highest average citations per publication at 131. Multiple country publication (Figure 3) refers to the proportion of publications in this field involving contributions from multiple countries and is used to assess international collaboration levels within a research area in a given country. China has the highest number of publications, but the proportion of multiple country publication with other countries is relatively low at 13.2%. However, France (43.2%) and the UK (69.6%) have a high proportion of multiple country publication, indicating their significant contributions to international cooperation.
A minimum threshold of 7 articles was set to filter out 30 countries meeting the criteria, as shown in Figures 4A, B. This reveals a wide - ranging network of international cooperation, with the US, China, and various European countries serving as key hubs. The United States led international collaboration with the highest total link strength at 303, underscoring its central role in the global tumor immune-escape network. China followed at 204, and Germany at 117, together highlighting these nations’ pivotal contributions to cross-border research and knowledge exchange in the field. Notably, the closest collaboration exists between the US and China. Additionally, publication timelines were analyzed through a VOSviewer - based visual map of organizational collaboration overlays (Figure 4B). It is worth noting that China started publishing later than most other leading countries in this field.
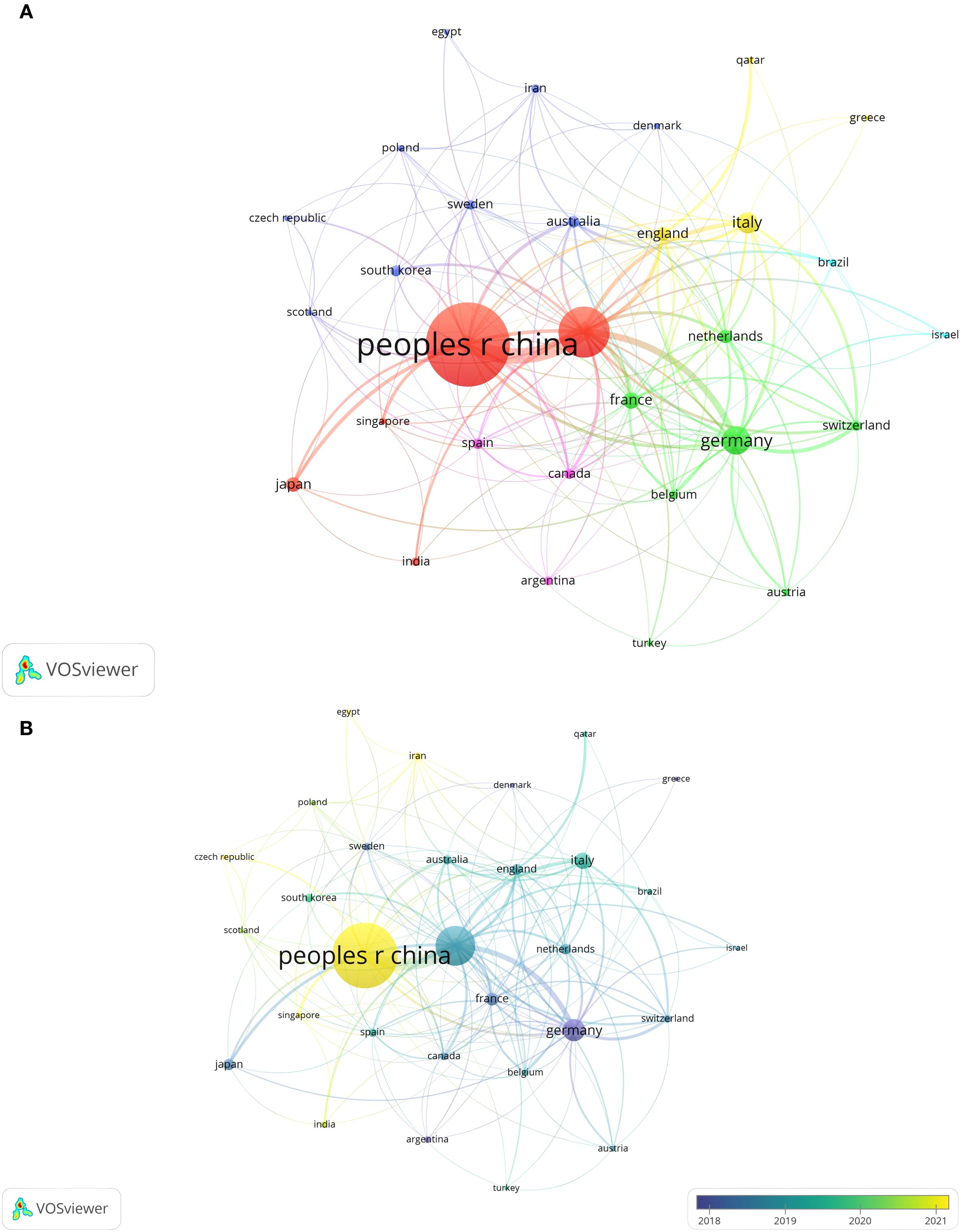
Figure 4. (A) Visual map of national/regional citation networks. Each circle/node’s size shows the number of papers published. The connection strength between circles/nodes is indicated by the line thickness, with colors representing clusters of related objects in the network. Each circle/node stands for a separate country/region. (B) Visualisation map of the country/region citation overlay. Purple nodes are organisations that started research in this area earlier, while yellow nodes are those that began later.
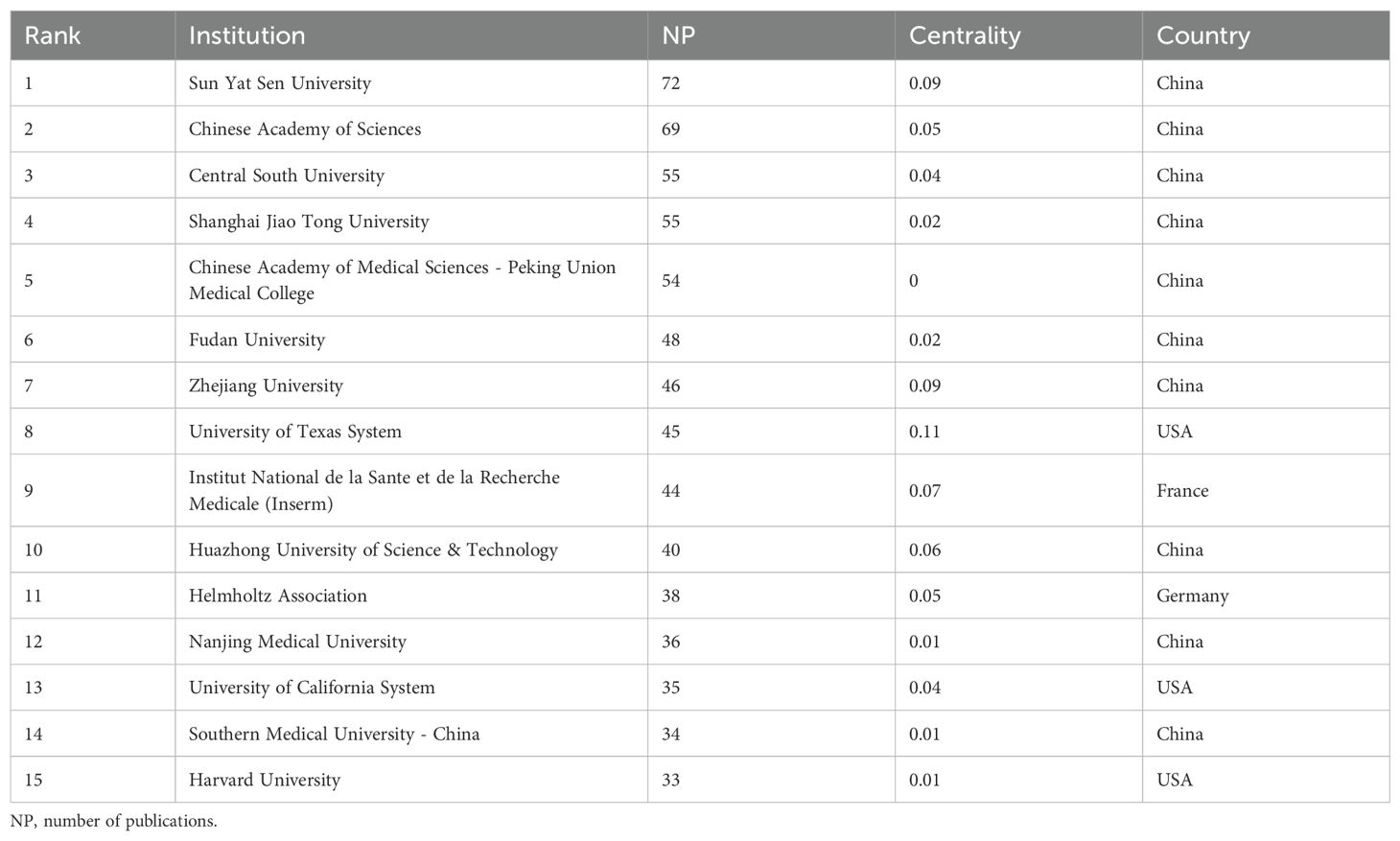
Among the top 15 institutions ranked by publication count (Table 2), Sun Yat-sen University leads with 72 publications, followed by the Chinese Academy of Sciences with 69. This indicates Sun Yat-sen University has the greatest international influence. These institutions are significant not only in publication quantity but also in impact. Notably, the University of Texas System, despite ranking eighth in publication count (45 publications), holds the top spot in betweenness centrality (0.11 centrality), suggesting its research is highly collaborative internationally and highly influential in tumor immune escape.
Figure 5A shows the top 15 institutions with citation outbreaks. Shandong First Medical University & Shandong Academy of Medical Sciences have recently experienced citation bursts, indicating significant potential in tumor immune escape research. In the co - occurrence graph (Figure 5B), node size represents co - occurrence frequency, and links show co - occurrence relationships. Nodes with purple rounded corners have high betweenness centrality (≥0.1), such as the University of Texas System and UTMD Anderson Cancer Center, which play key roles in connecting diverse research communities.
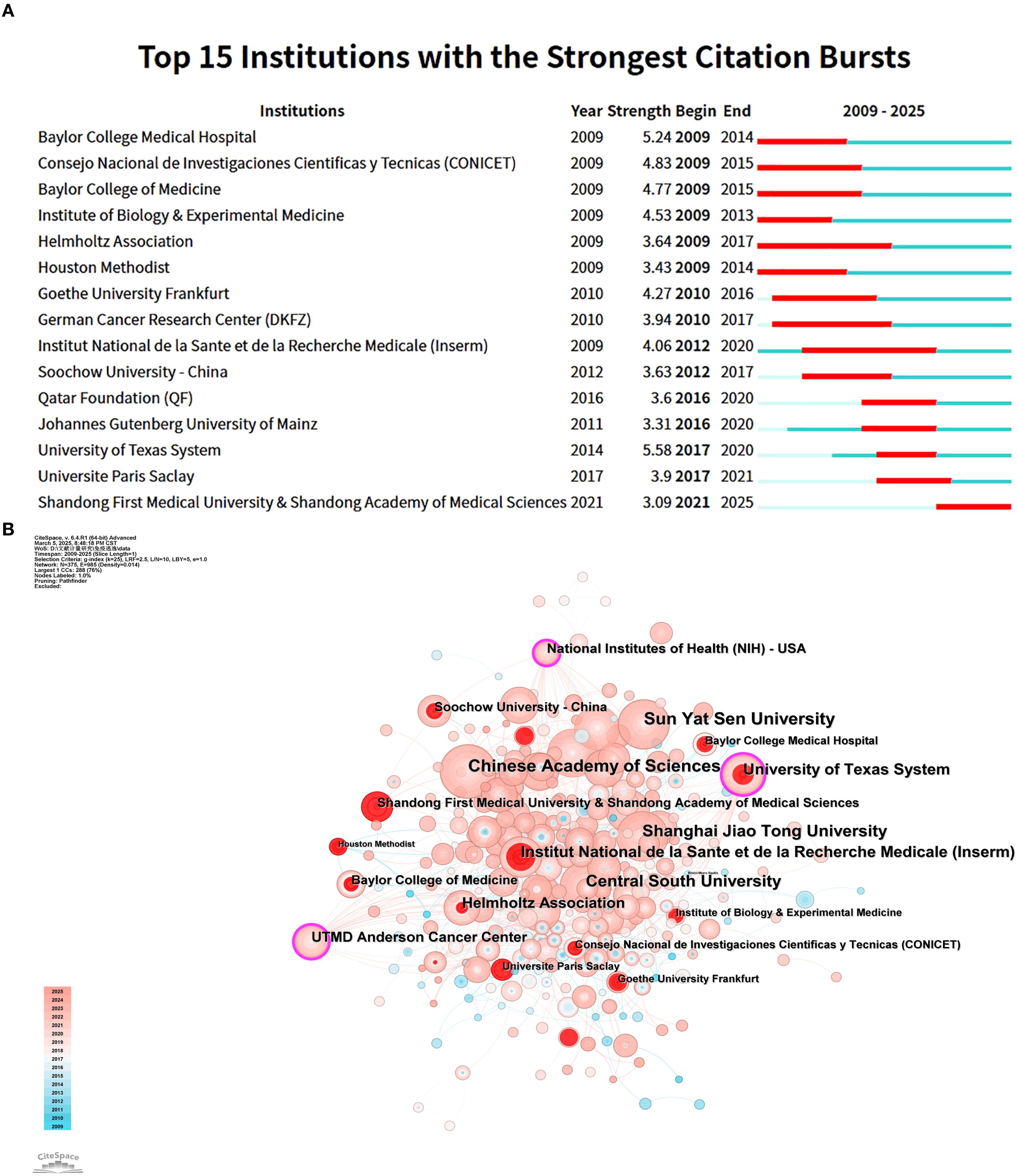
Figure 5. This analysis focuses on research institutes related to tumor immune escape. (A) It visualises co - author and research institution collaborations in this field. (B) It presents a co - occurrence mapping of research institutions. Here, node size shows co - occurrence frequency, and links indicate co - occurrence relationships. Nodes with purple circles have high betweenness centrality (≥0.1).
3.3 Analysis of journals
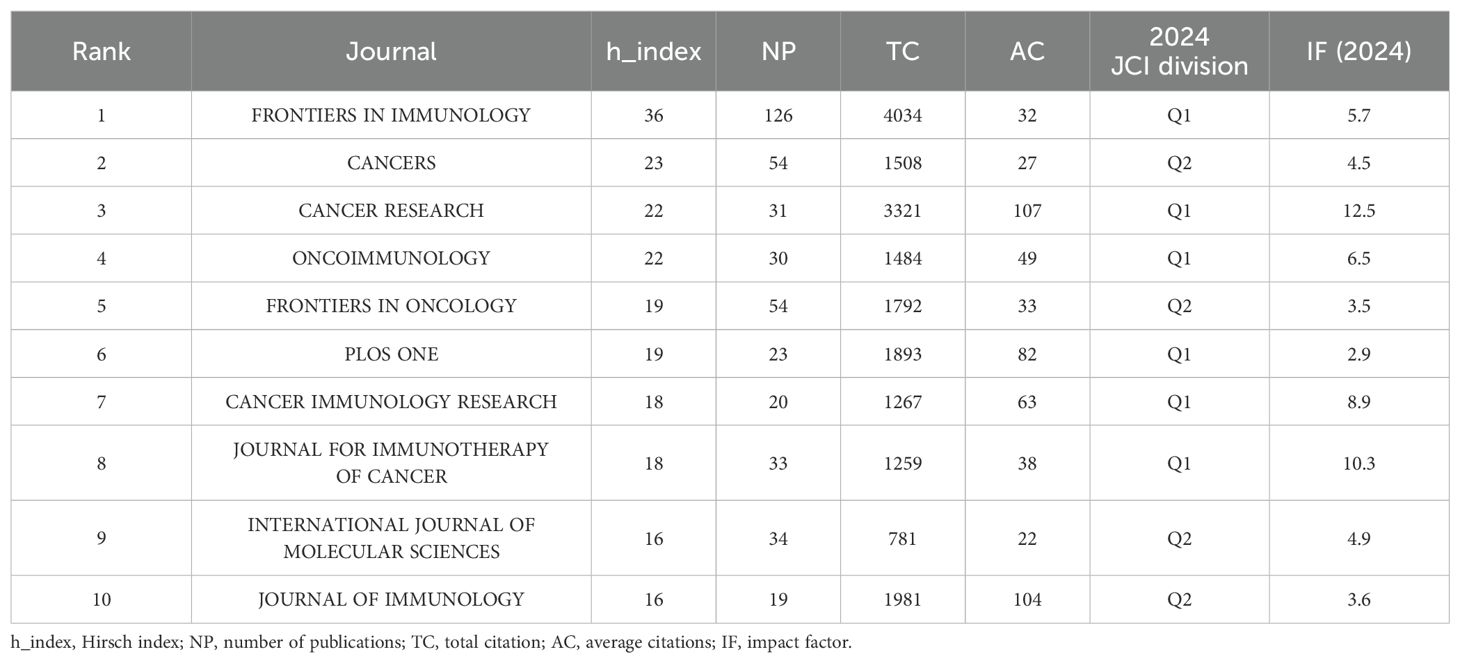
To identify active and influential journals in tumor immune escape, a visual analysis of published journals was done, uncovering 1,839 related publications in 472 academic journals. FRONTIERS IN IMMUNOLOGY had the most publications (126), followed by CANCERS (54) and CANCER RESEARCH (31) (see Table 3). Notably, CANCER RESEARCH has the highest impact factor (12.5) and average citations (107) among the top 10 journals, underscoring its significant impact in tumor immunology.
Figure 6 Application of Bradford’s Law showing core journals for tumor immune escape research.
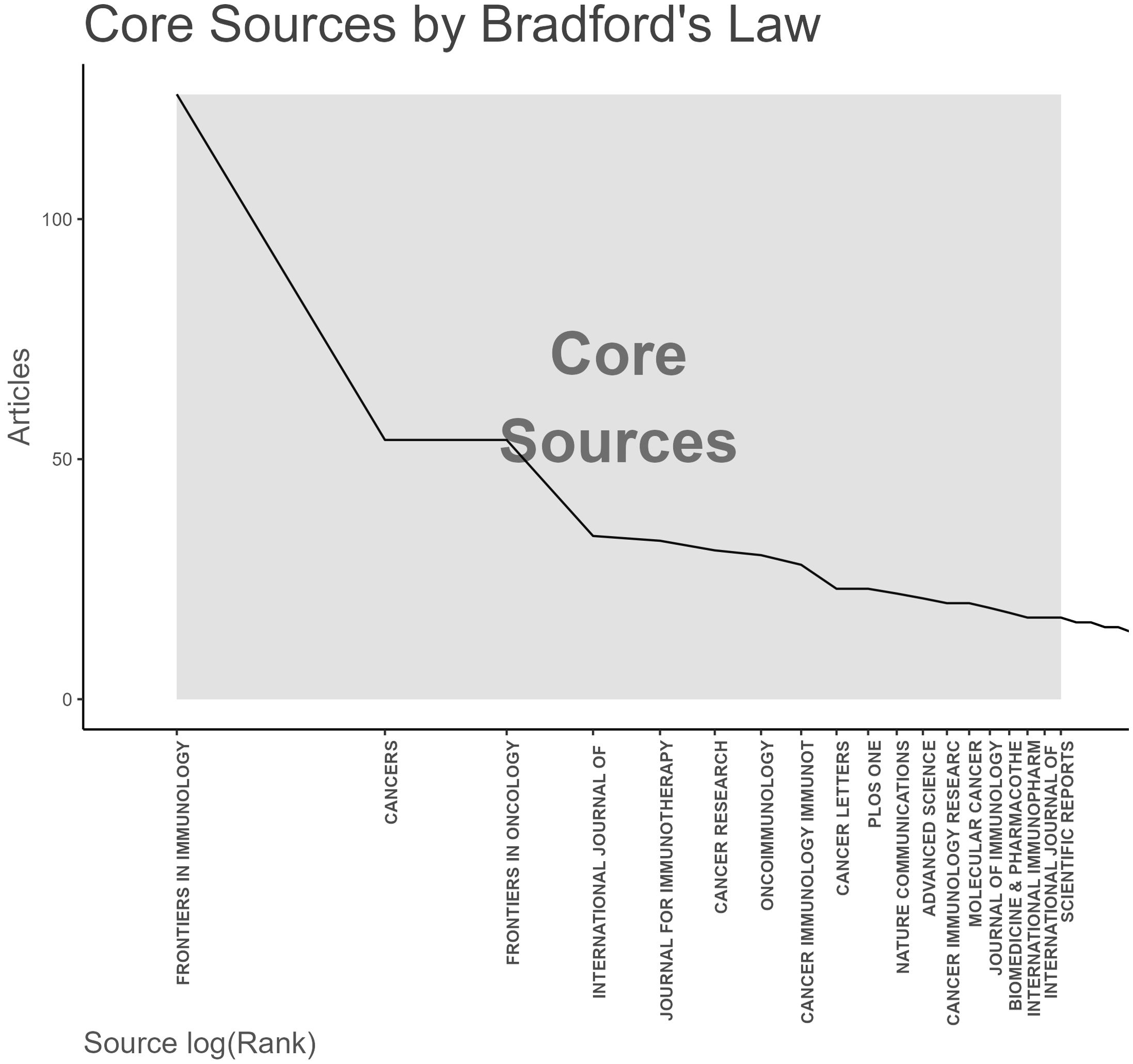
Figure 6. Analysis of academic journals related to tumor immune escape. Bradford’s law in academic journals.
The double figure overlay reveals a single citation pathway in numerous inter - field links between journals (Figure 7). Interestingly, publications on MOLECULAR, BIOLOGY, GENETICS are mainly cited by publications on MOLECULAR, BIOLOGY, IMMUNOLOGY and MEDICINE, MEDICAL, CLINICAL.
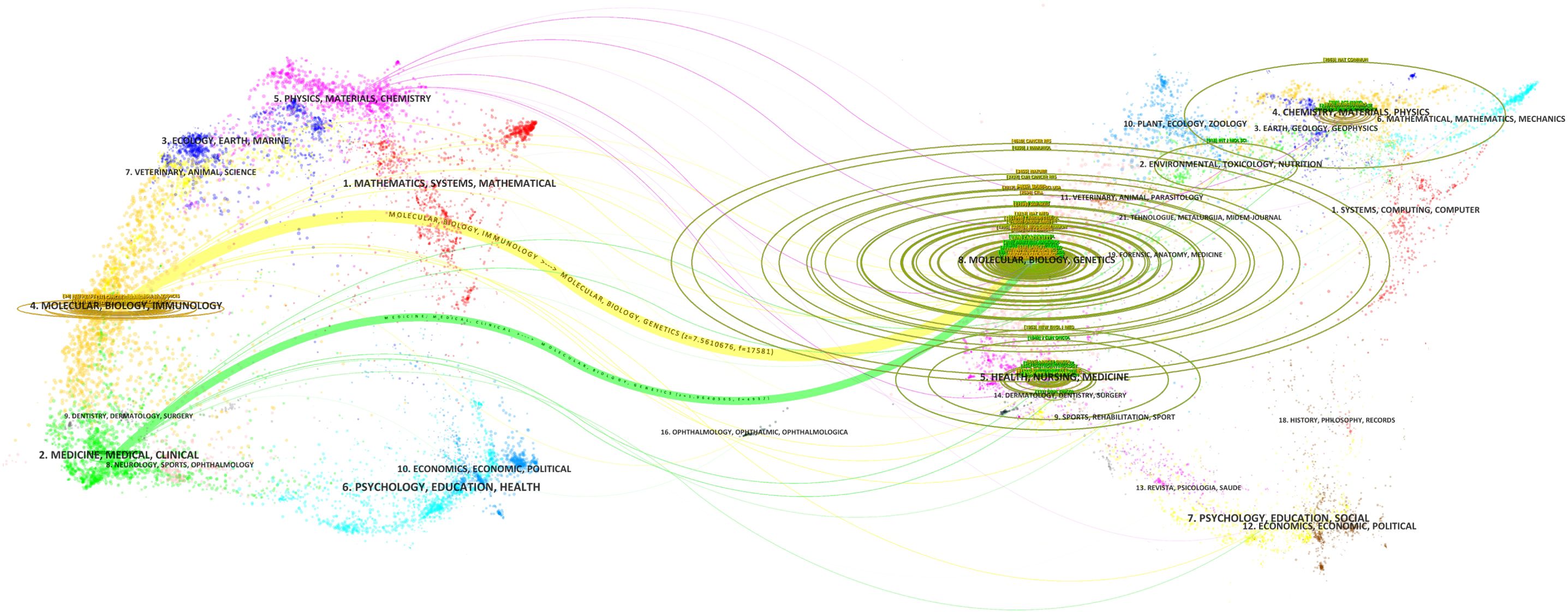
Figure 7. Double image overlay of journals in tumor immune escape research. This overlay visualises citation relationships between journals in this field. The lower graph shows citing journals on the left, cited journals on the right, with coloured lines indicating citation paths.
3.4 Author contributions and co-occurrence
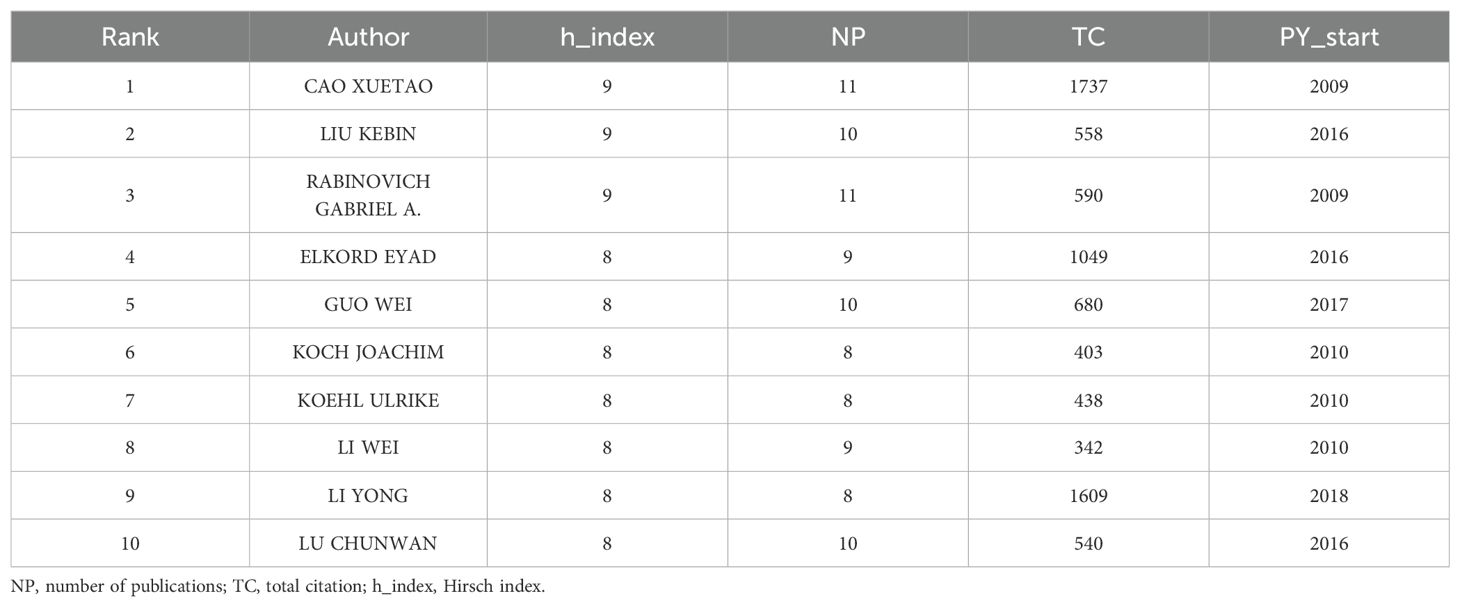
In this study, 12,322 authors were involved in the study. Table 4 lists the top 10 most prominent authors in tumor immune escape research. Xuitao Cao leads the list with 11 articles and 1737 citations, followed by Kebin Liu with 10 articles and 558 citations. Notably, Li Yong has published fewer articles but has the second highest number of citations. His 2018 publication was the first article on tumor immune escape, marking him as a highly promising emerging figure in the field (Table 4).
Figure 8 illustrates the collaborative network of 45 authors who have published five or more articles. These authors are clustered into five distinct collaborative groups. While each group demonstrates strong internal collaboration, there is limited interaction between groups. This pattern indicates a relative lack of intergroup communication and suggests the need to strengthen inter-institutional and international collaboration within the field.
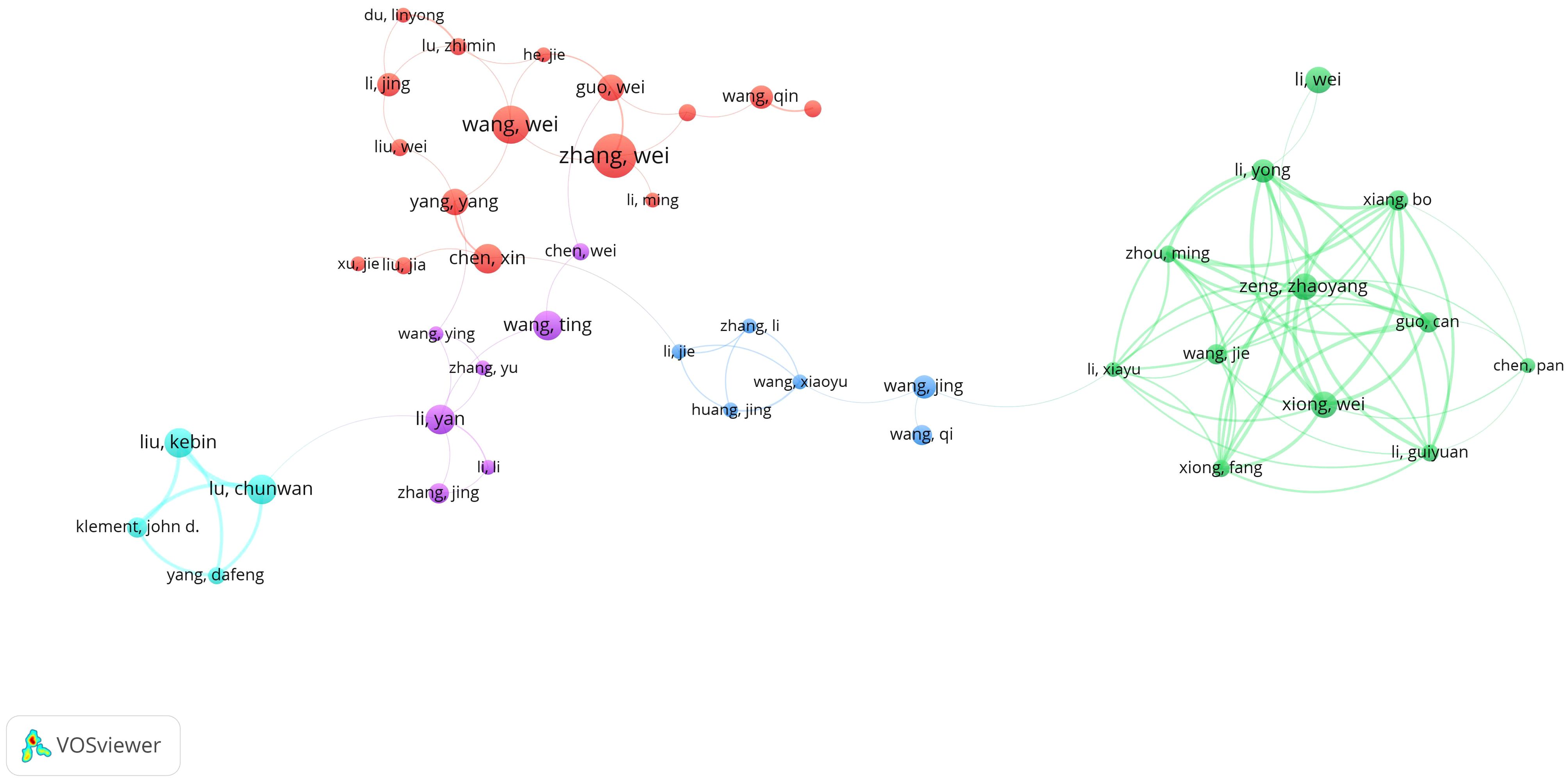
Figure 8. Authors’ tumor immune escape study blood infection co-occurrence graph. 744 different colored nodes reflect authors in different clusters. Node size indicates co-occurrence frequency and links indicate co-occurrence relationships between authors.
3.5 Citation and reference analyses
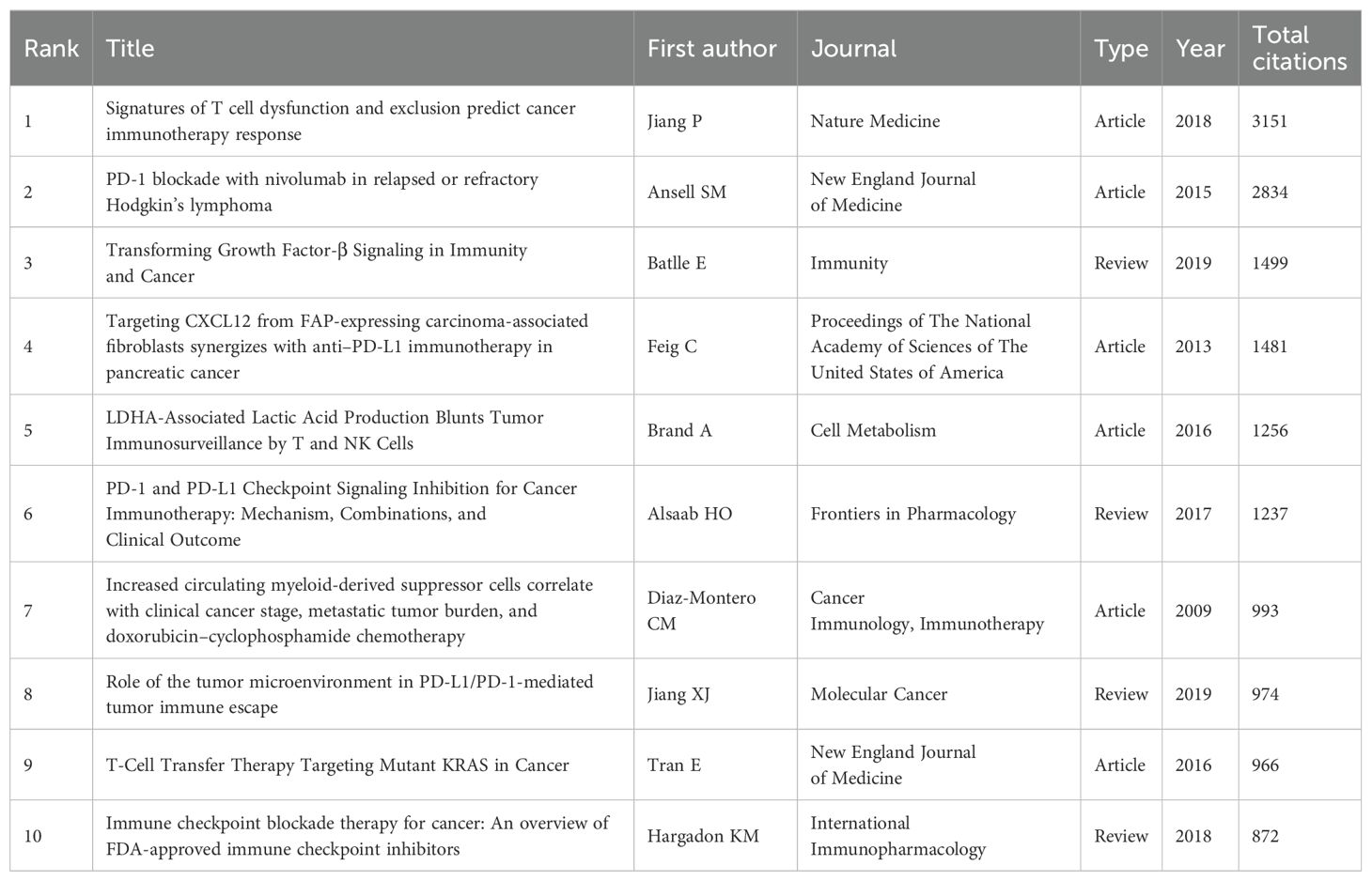
Table 5 resents the top 10 most cited articles on tumor immune escape. The first two articles, each with over 2,800 citations, lead significantly over the remaining entries, underscoring their substantial influence in the field.
The most cited article globally is ‘Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response’ by Jiang P, published in Nature Medicine in 2018 with 3,151 citations. This article introduces TIDE, an alternative biomarker for predicting immune checkpoint blockade (ICB) response, offering novel ideas for immune checkpoint blockade prediction and laying the foundation for immunotherapy prognosis forecasting (20).
The second most cited article globally is Ansell SM’s ‘PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma’, published in the New England Journal of Medicine in 2015. This study presents nivolumab as a new PD-1 blockade antibody and is the first to evaluate its efficacy and safety in relapsed or refractory Hodgkin’s lymphoma, providing a crucial basis for subsequent clinical applications in combating tumor immune escape (21).
Figure 9A highlights 20 core publications that experienced significant citation bursts, underscoring their influence and cutting-edge contributions to the tumor immune escape field during the analyzed timeframe. Early foundational literature, such as the work by Rabinovich et al. (22), experienced a dramatic citation surge between 2009 and 2011. This article became a landmark in tumor immunology by synthesizing previously fragmented immune escape mechanisms into a unified conceptual framework. It identified key immunotherapeutic targets, addressed unmet needs in immunotherapy research, and catalyzed the translation of basic science into clinical practice. Building upon earlier conceptual frameworks, Hanahan D provided a comprehensive synthesis of the hallmarks of cancer, in which immune evasion was recognized as an emerging hallmark and the TME was emphasized as a critical component influencing tumor progression and therapeutic resistance (23). This conceptual integration laid important theoretical groundwork for subsequent research into the mechanisms of tumor immune escape.
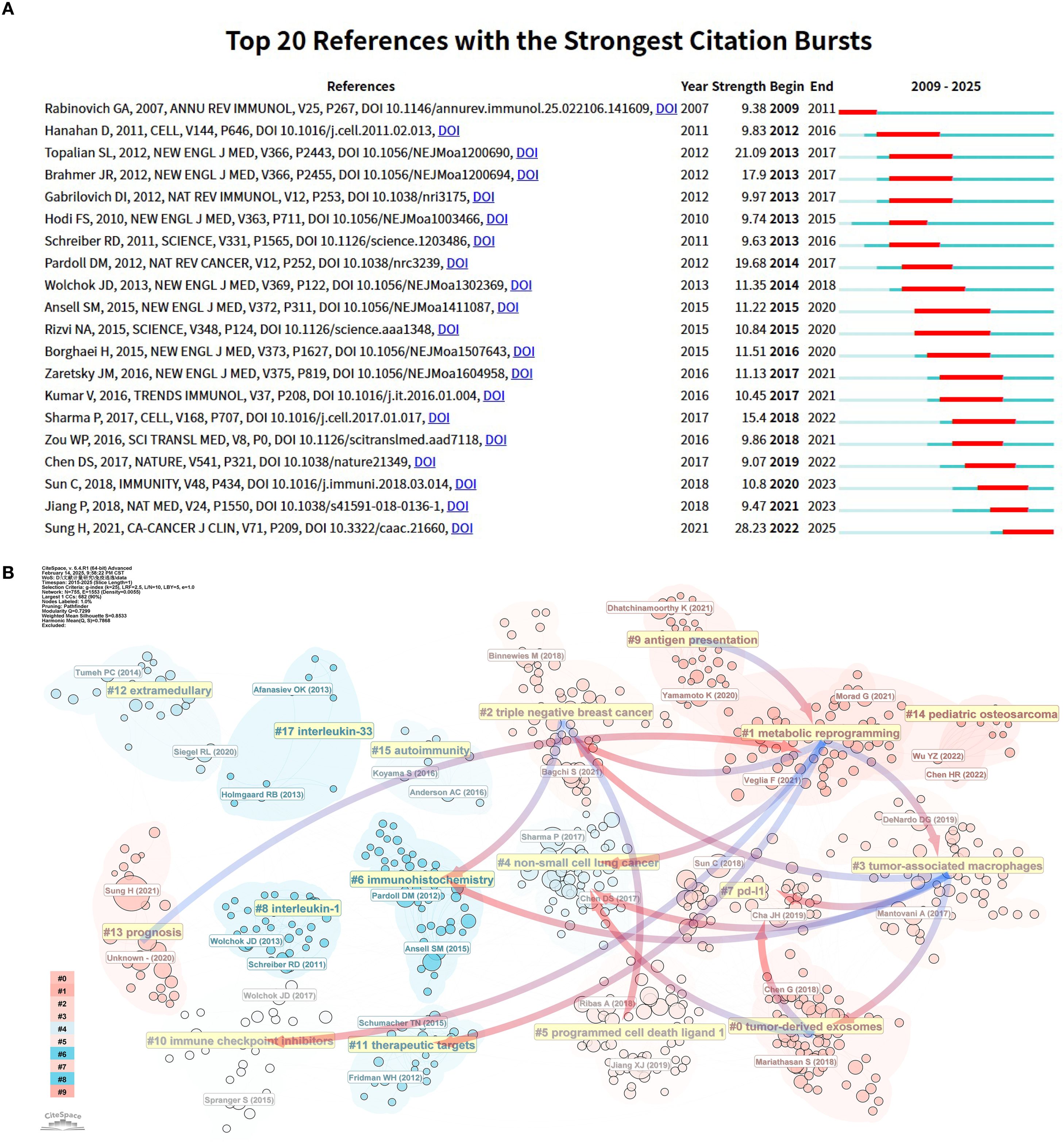
Figure 9. Analysis of references related to tumor immune escape. (A) The top 20 references with a significant increase in citation frequency. (B) Clustering of references according to similarity. Topics include #0 Tumorderived exosomes, #1 Metabolic reprogramming, #2 Triple-negative breast cancer, #3 Tumor-associated macrophages, #4 Non-small cell lung cancer, #5 PD-L1, #6 Immunohistochemistry, #7 PD-L1, #8 Interleukin-1 and so on. Linkage represents connections between different clusters, and the blue groups on the line evolve from the red ones.
Between 2013 and 2017, three citation burst references were identified, all of which were pivotal clinical trials (24–26). This period marked a significant turning point, as immune checkpoint inhibitors (ICIs) transitioned from preclinical exploration to clinical application. Among these, the landmark study by Hodi FS et al. (26) demonstrated that ipilimumab significantly improved overall survival in patients with advanced melanoma (median OS increased from 6.4 to 10.1 months), leading to its FDA approval in 2011 for metastatic melanoma. Ipilimumab thereby became the first immune checkpoint inhibitor approved globally, inaugurating a new era in cancer immunotherapy. Four additional citation burst articles identified between 2015 and 2020 (21, 27–29) focused on PD-1 inhibitors, likely reflecting the momentum generated by the FDA approvals of nivolumab and pembrolizumab in 2014. These approvals marked a major breakthrough in immunotherapy and spurred a surge of clinical and translational research into immune checkpoint blockade strategies. In 2018, Jiang P and colleagues (20) developed TIDE (Tumor Immune Dysfunction and Exclusion), a computational framework designed to model the two major mechanisms of tumor immune escape and predict responses to ICI therapy. Beyond its direct predictive value, TIDE played a pioneering role in bridging AI and tumor immunology, setting the stage for subsequent applications of machine learning in decoding immune evasion. Notably, this study attracted widespread attention between 2021 and 2023 and remains the most cited article in the field to date, underscoring its foundational significance and groundbreaking impact. The article exhibiting the most intense citation burst was “Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries” by Sung H et al. (30), published in 2021 in CA: A Cancer Journal for Clinicians (impact factor: 503.1). Utilizing GLOBOCAN 2020 data, this study provided a comprehensive overview of the global cancer burden, highlighting substantial regional differences in cancer incidence and mortality, and exploring the underlying epidemiological factors. The publication has since served as both an authoritative data source and an essential reference for global oncology research and clinical practice.
The use of co - citation cluster analysis offers an objective illustration of the knowledge structure within a research area. For a more detailed description of the co - cited reference groups, a network diagram was generated. The degree of association between articles was categorized into 17 groups, which formed the basis for the clustering classification. The co - citation cluster analysis, as shown in the diagram, clearly reveals the knowledge structure of the research area. To fully describe the co - cited literature groups, a complex network diagram was constructed (as shown in Figure 9B). Research topics were classified into 17 categories based on co - citation relationships, forming a clear cluster structure. In this diagram, (1) metabolic reprogramming, being the largest cluster, indicates that metabolic reprogramming - related research holds a central position in the field and carries extensive academic influence.
Evidence of evolution over time among the study clusters is also apparent. For instance, the gradual evolution of (4) non-small cell lung cancer clusters into the emergence of (0) tumor-derived exosome, (3) tumor-associated macrophages and (1) metabolic reprogramming clusters. In the field of non-small cell lung cancer research, the transition from conventional to immunotherapeutic approaches has catalyzed the rapid evolution of tumor immunotherapy. Additionally, triple negative breast cancer is the most aggressive breast cancer type, having limited treatment choices and a poor prognosis (31). The figure shows that the triple negative breast cancer group gradually evolved into metabolic reprogramming and tumor-associated macrophage groups. This means immunotherapy breakthroughs bring hope to triple negative breast cancer clinical treatment.
The (7) PD-L1 cohort has gradually evolved into the (3) tumor-associated macrophages and (0) tumor-derived exosomes cohorts, reflecting the research process of tumor immune escape, which further promotes the exploration of the tumor microenvironment due to the differentiated efficacy of PD-L1-targeted drugs. It reflects the in-depth exploration of the mechanism of tumor development and the continuous optimization of immunotherapy strategies in the field of tumor immunity.
Beyond the main evolutionary trends, several smaller groups indicate the ongoing development of specific research directions. Notably, (1) metabolic reprogramming cohorts have gradually evolved into (9) prognosis cohorts. This shift shows that as tumor metabolomics advances, researchers are paying more attention to predicting patient prognosis. Metabolic reprogramming is a key strategy for tumor cells to adapt to harsh microenvironments and maintain rapid proliferation and survival (32, 33). This metabolic alteration not only supports tumor growth but also closely correlates with tumor malignancy and poor patient prognosis (34), providing a solid theoretical basis for the evolution of metabolic reprogramming research towards prognosis. In clinical practice, accurately predicting patient prognosis is crucial for developing personalized treatment plans and improving survival rates.
3.6 Keywords co-occurrence analysis
Figures 10A, B highlight key themes in tumor immune escape research, including ‘immunotherapy’, ‘pd-l1’, ‘cancer’, ‘expression’, ‘tumor microenvironment’, ‘dendritic cells’, and ‘t-cells’. These keywords are strongly interconnected, reflecting their central role in the research community. The density visualization reveals intense research activity around these themes, with warmer colors indicating areas of high interest.
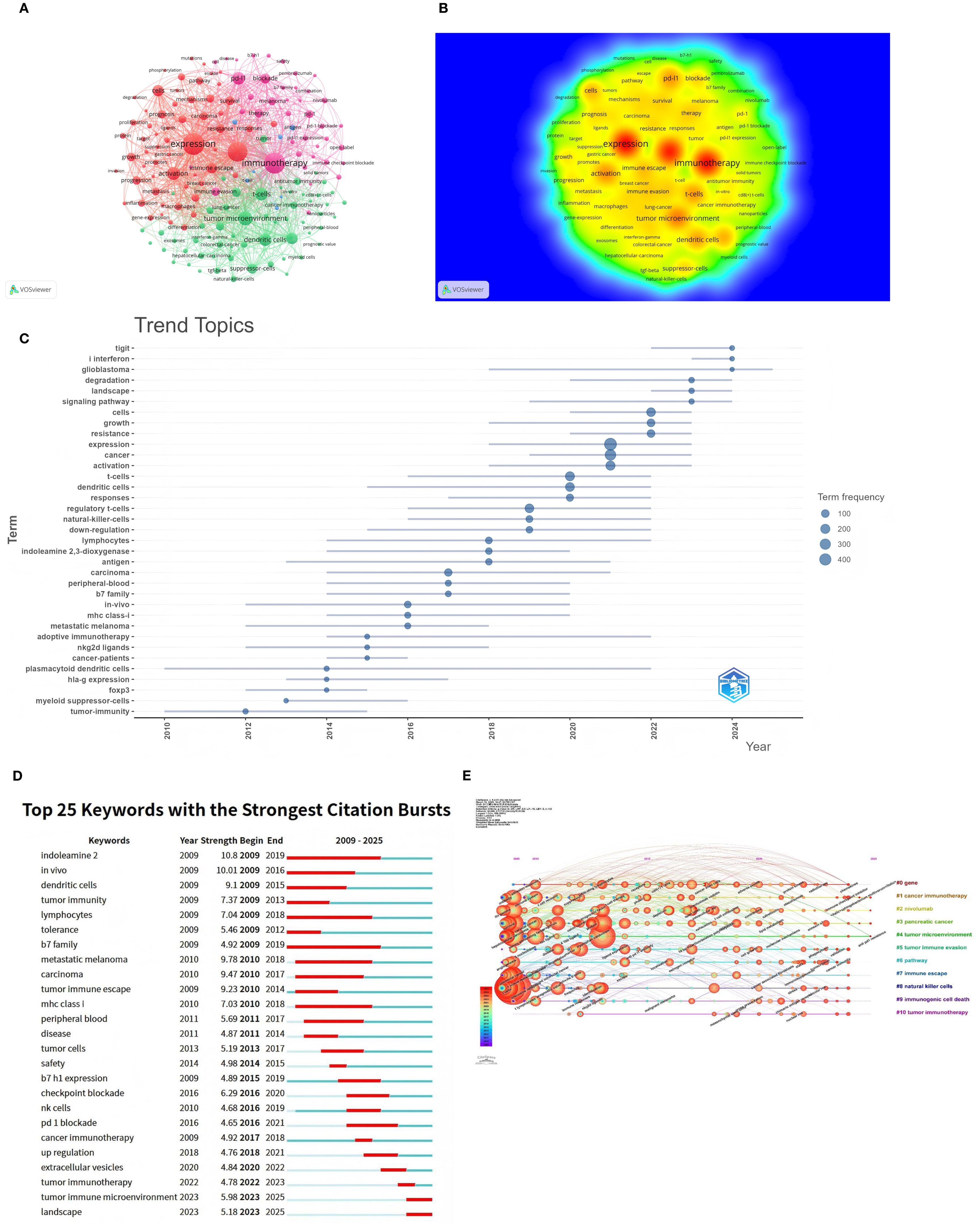
Figure 10. Keyword co-occurrence analysis in tumor immune escape studies. (A) Network visualisation for keyword co-occurrence analysis (n>5). Each node in the network represents a keyword, with the size of the node indicating the number of times the keyword occurs. Lines between nodes indicate co-occurrence between keywords; the larger the node size, the higher the frequency of the keyword. (B) Density visualisation of keyword co-occurrence analysis. This visualisation methodically illustrates the density and intensity of research themes within the designated field. Heat maps are utilised to accentuate areas of varying research intensity, with warmer colours denoting higher activity and stronger connections. (C) Trend themes from 2009 to 2025. The timeline illustrates the temporal progression of pivotal research themes within the field. The relative prominence of these research themes undergoes substantial fluctuations over the course of time, with larger nodes denoting elevated frequency and significance. (D) The 25 keywords with the strongest citation bursts are displayed. The blue line indicates the time axis, with the red segments denoting the start year, end year, and duration of each burst. (E) Timeline of keyword co-occurrence analysis. The timeline visualises the temporal evolution of key research topics in the field. The salience of each keyword undergoes a change over time, with larger and more concentrated nodes representing higher frequency and importance. The keywords are then organised into clusters on the right-hand side of the figure.
Figures 10C, D depict the evolution of research topics in tumor immune evasion. Figure 10C analysis of theme word trends from 2009 to 2025 shows that “TIGIT,” “type I interferon,” and “glioblastoma” are the next few years’ research Frontiers. Keyword burst analysis delineates three pivotal evolutionary stages in tumor immune escape research (Figure 10D). The pronounced bursts of “dendritic cells” and “lymphocytes” during 2009–2015 underscored foundational investigations into antigen presentation machinery and T-cell activation dynamics. Concurrently, sustained bursts of “indoleamine 2,3-dioxygenase” and “B7 family”(2009–2019) signaled the emergence of metabolic checkpoint pathways as critical regulators. The subsequent phase (2016–2021) witnessed transformative clinical advances, where bursts in “checkpoint blockade” and “PD-1 blockade correlated with therapeutic breakthroughs in immune checkpoint inhibitors, while renewed focus on “B7-H1 expression” (2015-2019) reflected its consolidation as a predictive biomarker. Current research has shifted toward tumor microenvironmental orchestration, exemplified by the burst in extracellular vesicles (2020–2022) highlighting exosome-mediated immune remodeling. Dominant ongoing bursts for tumor immune microenvironment and landscape (2023–2025) reveal accelerating adoption of spatial multi-omics and integrative biology frameworks to deconvolute immune evasion ecosystems. Notably, persistent attention to MHC class I (2010–2018) reflects enduring challenges in antigen presentation defects as core resistance mechanisms. Figure 10E categorizes keywords into 11 groups arranged chronologically, showing the most recent and prevalent keywords as ‘Immune Checkpoint Inhibitors’, ‘Sphingobacterium multivorum’, and ‘Anti PD-1 Resistance’.
4 Discussion
4.1 Overall distribution
Research on tumor immune evasion has sustained growth from 2009 to 2024, with no sign of abating in 2024. This reflects growing interest and attention in the field (30). Immunotherapy has become a major strategy for cancer treatment. However, tumor immune escape remains a significant challenge to the efficacy of anticancer therapies. To understand the mechanism of tumor immune escape, many targeted approaches have been explored, and some drugs have been clinically applied and achieved better efficacy (35, 36). Globally, China, the United States, and European countries are major contributors to research on tumor immune evasion. These countries’ research efforts have a clear advantage in addressing the global cancer threat. China leads in research output with 958 (52.1%) research papers published, showing the breadth and depth of its research and its great global influence. This is closely related to China’s comprehensive cancer screening and registration system and the rising cancer mortality rate (37, 38). Meanwhile, the USA and Germany have published 281(15.3%) and 114(6.2%) publications respectively, showing that the USA and Europe also have a large influence in the field of tumor immune escape. China’s cancer screening program, initiated in 1958, has significantly expanded its coverage over the past decade. This provides robust and credible primary data supporting research in the field of tumor immune escape (38). Presently, China faces a substantial cancer burden. According to the latest World Health Organization (WHO) estimates, the country accounts for 24% of global cancer incidence cases, with cancer mortality rates exceeding the global average (1, 39, 40). Consequently, the “Healthy China 2030” initiative designates cancer prevention and control as a strategic priority. Coupled with sustained investment in scientific research, these policies have enabled China to achieve significant progress in cancer prevention and treatment, while making substantial contributions to tumor immune escape research (41).
In the field of research institutions, China’s top institutions excel in output and influence. All seven institutions with the highest number of relevant publications are in China. Sun Yat-sen University leads with 72 publications and a betweenness centrality of 0.09, showing its significant position and global collaborative contribution in tumor immune evasion research. Although the University of Texas System has fewer publications, its betweenness centrality of 0.11 reflects substantial contributions to global cooperation in this field. Notably, Shandong First Medical University & Shandong Academy of Medical Sciences has rapidly risen to become a prominent player in recent years. Despite strong domestic collaborations among Chinese institutions, international cooperation remains relatively limited, which may hinder overall progress in tumor immune evasion research. Therefore, strengthening international collaborations between research institutions is crucial for accelerating global research efforts and addressing the worldwide cancer challenge.
Bibliometric analysis shows the USA and France excel in international collaboration, especially in cross - national publications. The UK, despite fewer publications, maintains strong research networks with other countries, highlighting its role in advancing global tumor immune escape research. The United States not only demonstrates strong research output in the field of tumor immune escape research, but also maintains a high proportion of multinational collaborative publications. That edge traces back to decades of steady investment by the National Cancer Institute in fostering worldwide oncology partnerships (42). Additionally, the Cancer Genome Atlas (TCGA) (43) have established accessible genomic databases that have catalyzed the formation of multinational research consortia and promoted collaborative discoveries. These collaborations facilitate knowledge sharing and enhance international synergies, enabling effective responses to global cancer challenges. Notably, low - and middle - income countries, largely due to China’s contributions, have significantly contributed to this research, helping overcome resource deficiencies and high cancer risks in these areas (44). Nevertheless, cross - border collaboration between research institutions remains limited, which may impede research progress. Enhanced global research collaboration and resource sharing could facilitate tumor immune escape research in these regions and provide more diverse perspectives and data support in the global fight against cancer.
Among journals, Frontiers in Immunology publishes the most tumor immune escape studies, far exceeding other academic journals. While Cancer Research publishes fewer articles, it has the highest average citations and impact factor (12.5), underscoring its substantial influence in this field. Among authors, Cao XT from China is the most prolific and cited. He and his team summarize the role of tumor - associated macrophages (TAMs) in promoting tumor progression and drug resistance (45), explore antibody variable region engineering applications, and discuss future antibody engineering directions to enhance cancer therapy (46).
4.2 Evolution of research focus and translational impact
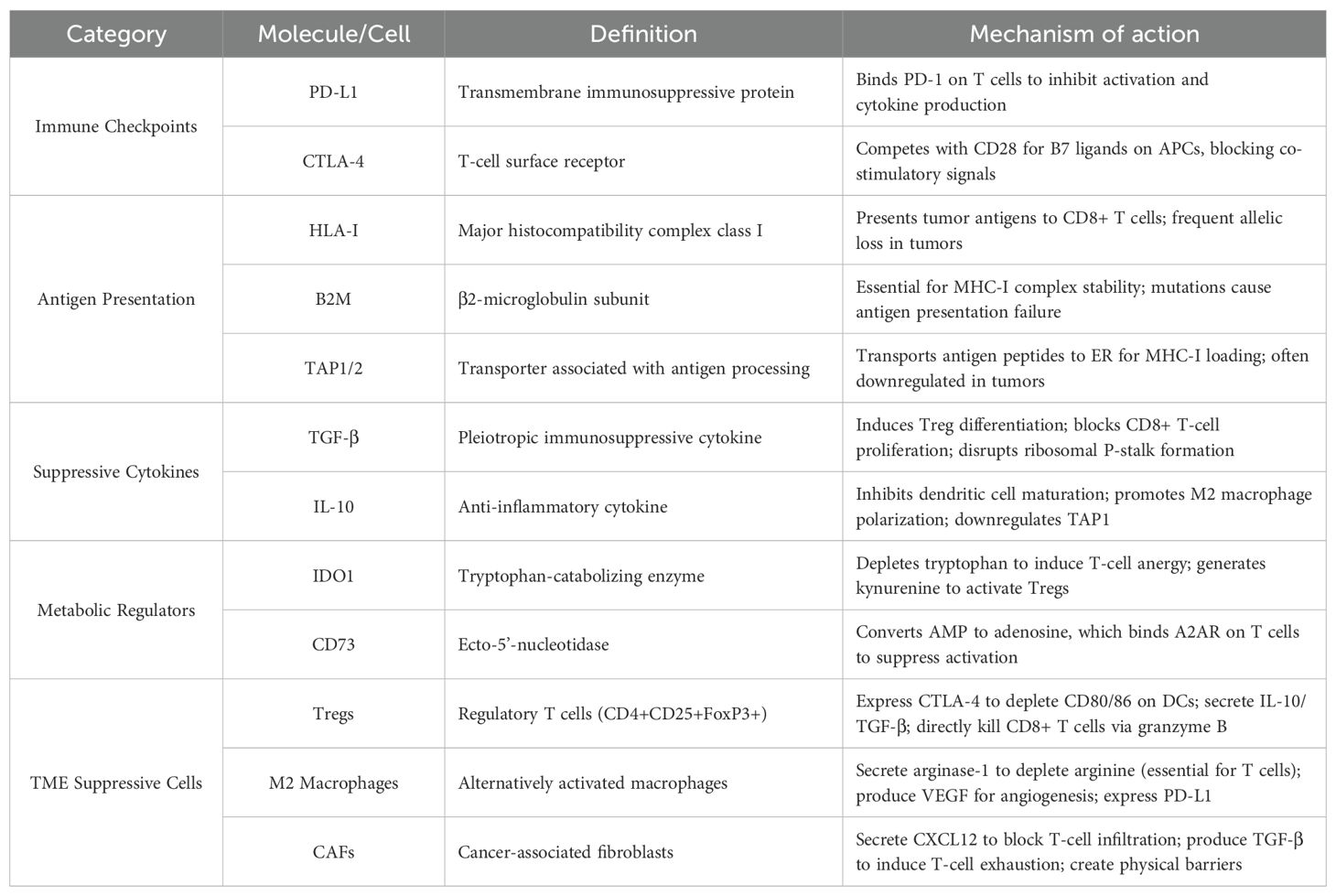
Our bibliometric analysis shows that tumor immune escape research has evolved from focusing on classical checkpoints like PD-1/PD-L1 to exploring more complex mechanisms such as T cell exclusion, antigen presentation loss, and TME dynamics. (Table 6) Recently, emerging hotspots include combination immunotherapies, AI-assisted predictive modeling, and the cross-talk between metabolic reprogramming and the microbiome—an area gaining notable traction.
These trends have clear translational value. Insights into immune evasion are driving the development of multi-target therapeutic strategies, particularly for tumors resistant to standard immunotherapies, such as glioblastoma (GBM) and MSS colorectal cancer. Advances in single-cell profiling, spatial transcriptomics, and AI tools are further enabling precision immune phenotyping and personalized treatment planning.
At the policy level, the increasing relevance of immune escape calls for integrating immune profiling into clinical workflows and national treatment guidelines. Promoting international collaboration and investment in emerging technologies will be key to accelerating progress and improving global cancer outcomes.
4.3 Research hotspots
Bibliometrics is crucial for processing and analyzing large-scale data to offer researchers insights into trends. Analyzing frequent keywords and subject terms can uncover changing trends and key themes, which are vital for understanding the field’s evolution. Based on the above analysis, current major hotspots focus on areas like Immune Checkpoint Inhibitors, tumor immune microenvironment, and landscape. An in-depth analysis of these research hotspots can help better understand the progress of tumor immune escape research and predict future developments in the context of current studies.
4.3.1 Current research hotspots
4.3.1.1 Novel immune checkpoint discovery and multi-target combination strategies
Antibodies targeting immune checkpoints such as the programmed death-1 (PD-1) receptor, its ligand PD-L1, or cytotoxic T lymphocyte-associated-4 (CTLA-4) have transformed the treatment of many tumor types. However, only a small percentage of patients produce a durable response. Consequently, researchers are actively exploring new immune checkpoints to target and combining therapies to achieve enhanced therapeutic efficacy.
TIGIT, a member of the poliovirus receptor (PVR)/nectin family, is expressed on T cells, NK cells, and Tregs (47, 48). It features an extracellular IgV domain, transmembrane region, and cytoplasmic ITIM/ITT motifs. By binding to CD155 (PVR) with high affinity, TIGIT competitively inhibits the co-stimulatory receptor DNAM-1 (CD226), thus suppressing the activation of T and NK cells (49, 50). The signaling pathway involved in this process, mediated by Grb2/SHIP1, results in the disruption of the MAPK and NF-κB pathways. In clinical trials, the anti-TIGIT antibody tiragolumab, when combined with the anti-PD-L1 antibody atezolizumab, demonstrated an improved overall response rate (ORR) of 37.3%, compared to 20.6% with monotherapy, in patients with PD-L1-high non-small cell lung cancer (NSCLC) (51).
LAG-3 (Lymphocyte Activation Gene 3), a member of the immunoglobulin (Ig) superfamily located on chromosome 12, is expressed on CD4+/CD8+ T cells, natural killer (NK) cells, regulatory T cells (Tregs), and plasmacytoid dendritic cells (52, 53). It inhibits T cell activation by binding with high affinity to MHC-II molecules on antigen-presenting or tumor cells, suppressing IL-2 and IFN-γ secretion. The intracellular domain of LAG-3 contains the S484, KIEELE, and EP motifs, which regulate cellular localization and TCR signaling (54, 55). In clinical trials, relatlimab (anti-LAG-3 monoclonal antibody) combined with nivolumab (anti-PD-1) achieved FDA approval for advanced melanoma (Phase III RELATIVITY-047 trial), demonstrating superior progression-free survival (PFS: 10.1 vs. 4.6 months) (56). Future multi-targeted combination therapies have great potential in the treatment of cancer.
4.3.1.2 NETs–TME interactions: a hotspot in tumor immunology
The tumor microenvironment (TME) is now regarded as crucial in cancer development, progression, and treatment (57). This heterogeneous system consists of a chemical TME (marked by acidic pH, hypoxia, and low nutrition), a cellular TME (including tumor cells, stromal cells, pericytes, endothelial cells, immune cells, and the extracellular matrix), and various signaling molecules like cytokines, chemokines, and growth factors within the microenvironment (58, 59). These components interact closely and consistently with tumor cells, thereby enabling the tumor to evade the immune system through different mechanisms (60).
Among the immune cells within the TME, neutrophils play a central role in all stages of cancer progression (61). Neutrophils contribute to tumor metastasis through the formation of extracellular traps (NETs), which protect tumors from effector T cell-mediated elimination (62). However, NETs may also have a dual role in the TME. In certain acute inflammatory conditions, NETs have been shown to inhibit melanoma cell migration and promote tumor lysis, suggesting that they can contribute to tumor elimination (63). Interestingly, depletion of the immune checkpoint receptor CD276 has been found to significantly reduce the expression of CXCL1, which ultimately diminishes neutrophil infiltration into tumors, thereby decreasing NET formation through the CXCL1-CXCR2 axis. This reduction in neutrophil-driven immune suppression can enhance NK cell infiltration, which may play a pivotal role in halting the progression of esophageal squamous cell carcinoma (64). Chronic stress has been shown to disrupt the normal circadian rhythm of neutrophils, leading to increased formation of neutrophil extracellular traps (NETs) via elevated glucocorticoid release. This alteration progressively shapes a TME that favors metastatic cancer progression (65).
Understanding the TME and the role of neutrophil extracellular traps (NETs) is essential for advancing cancer immunology. The TME serves not only as a physical and biochemical scaffold for tumor growth but also as a dynamic immunological hub that orchestrates immune evasion, metastatic potential, and therapeutic resistance. Moving forward, deeper investigation into the mechanistic crosstalk between NETs and the TME is critical. Unraveling these interactions will inform the development of novel immunotherapeutic strategies, including NET-targeted interventions, which may reshape the immunosuppressive landscape of solid tumors and improve clinical outcomes.
4.3.1.3 Type I interferon as a promising strategy to overcome ICB resistance
Despite the transformative success of immune checkpoint blockade therapies, their clinical efficacy remains highly heterogeneous across patient populations (66). A considerable proportion of patients exhibit primary resistance or develop adaptive resistance during treatment, often due to a highly immunosuppressive TME, insufficient tumor immunogenicity, or impaired effector immune responses (67). Type I interferons (IFN-I) have emerged as key modulators of antitumor immunity. IFN-I signaling coordinates a range of immune-regulatory processes, including dendritic cell (DC) maturation, CD8+ T cell activation, macrophage polarization, and the induction of tumor cell senescence and apoptosis (68). Notably, IFN-I can also act directly on natural killer T (NKT) cells, enhancing their infiltration into the TME and further amplifying the immune response (69). A promising approach to overcoming ICB resistance involves combining checkpoint inhibitors with IFN-I–activating strategies, particularly in tumors characterized as immunologically “cold.” (70).
Borui Tang et al. identified daurisoline (DS)—a bioactive alkaloid extracted from the rhizomes of the traditional Chinese medicinal herb Ban Yue Zi—as a potent inducer of IFN-I signaling. Mechanistically, DS stimulates IFN-I production via a TANK–TBK1–dependent pathway in tumor cells. The IFN-I released subsequently promotes NKT cell recruitment, enhancing antitumor immune activity (71). Importantly, their study demonstrated that combination therapy using DS with either anti–PD-1 antibodies or the STING agonist diABZI significantly remodeled the immune landscape of the TME. These findings suggest that DS-based combinations may serve as a viable strategy to overcome resistance in ICB-refractory tumors.
Similarly, Ruixuan Liu et al. engineered a bacterial strain, VNP-C-C, that co-expresses CCL2 and CXCL9, thereby facilitating immune cell mobilization and establishing a pro-inflammatory TME. This strategy induces immunogenic cell death (ICD) and activates the cGAS–STING pathway, resulting in elevated IFN-I production and a strengthened antitumor response (72). Interestingly, following VNP-C-C treatment, a marked upregulation of PD-1 expression on tumor-infiltrating T cells was observed—indicative of robust immune activation but also potential T cell exhaustion and immune escape. These findings highlight VNP-C-C as a potential priming agent for ICB-based combination immunotherapy.
In summary, these preclinical studies underscore the emerging role of type I interferon signaling as a central modulator of resistance to immune checkpoint therapies. While both DS and VNP-C-C have shown promising immunomodulatory effects in experimental models, neither has yet advanced to clinical trials. Nevertheless, the ability of IFN-I–targeted interventions to convert “cold” tumors into “hot” ones positions this axis as a compelling focus for future translational research and therapeutic development.
4.3.2 Future research hotspots
4.3.2.1 Cross-cutting studies of metabolic reprogramming and microbiome
The intersection of the microbiome and metabolic reprogramming has emerged as a prominent research focus in the field of tumor immune evasion, particularly in the context of colorectal cancer. The microbiome plays a pivotal role in shaping the TME by modulating inflammation and immune responses (73, 74). It influences cancer initiation, progression, metastasis, and immune escape mechanisms through both microbial actions and their metabolites (75, 76). Microbial metabolites such as short-chain fatty acids (SCFAs) and bile acids reprogram metabolic processes within the TME, either enhancing or inhibiting immune responses. For example, SCFAs, particularly butyrate, produced by beneficial bacteria like Bacteroides thetaiotaomicron, help maintain immune balance by promoting regulatory T cell (Treg) differentiation, which can suppress inflammation and support immune homeostasis (77). In contrast, dysbiosis—often linked to poor dietary habits—can favor pathogenic bacteria like Fusobacterium nucleatum, which promotes immune evasion by inducing M2 macrophage polarization, suppressing T cell responses, and enhancing inflammation, ultimately accelerating cancer progression (78). Furthermore, the metabolic competition within the TME between cancer cells, immune cells, and microbes adds another layer of complexity to immune evasion. Tumor cells reprogram their metabolism to favor glycolysis, thus depriving immune cells of essential nutrients like glucose and glutamine. This metabolic shift impairs T cell function and promotes immune suppression, facilitating tumor progression (75). The crosstalk between tumor metabolism, microbial metabolites, and immune responses underscores the potential for targeting these metabolic pathways to improve the efficacy of immunotherapies (79). In conclusion, the intersection of metabolic reprogramming and the microbiome offers a promising avenue for cancer research, particularly in tumor immune evasion. By understanding how microbial metabolites influence tumor metabolism and immune responses, this area could lead to new therapies that enhance immunotherapy effectiveness. Further exploration of this cross-cutting research will be key to developing personalized treatments that combine microbiome and metabolic strategies to overcome immune suppression.
4.3.2.2 Intelligent decoding of the tumor immune evasion landscape
As tumor immunology advances toward increasingly personalized and dynamic paradigms, the concept of an “immune evasion landscape” has emerged as a critical framework to describe the intricate, multidimensional interplay between tumors and the host immune system. Recent progress in high-throughput technologies—such as spatial transcriptomics (80), single-cell technologies (81, 82), and multi-omics integration (83)—has enabled unprecedented resolution in mapping this landscape. AI, empowered by access to high-dimensional biological datasets and breakthroughs in computational power and deep learning architectures, offers a transformative approach to decoding these complex interactions (84, 85). Several recent studies exemplify this trend. For instance, Hanqi Li et al. (86) integrated four histological dimensions to define three molecular subtypes of hepatocellular carcinoma (HCC), establishing an MSRS model validated through single-cell RNA sequencing, spatial transcriptomics, and functional assays. This model demonstrated robust prognostic capability and potential for guiding individualized therapy. In another study (87), researchers applied imaging mass cytometry and a graph-based AI model to compare non-small cell lung TMEs in people with and without HIV. Leveraging PageRank and diffusion maps, the model achieved 84.6% accuracy in classifying HIV-associated tumors and identified key immunosuppressive markers, such as PD-L2 on tumor-associated macrophages and CD25 on infiltrating T cells. Additionally, Liu et al. (88) developed a self-supervised learning (SSL) framework based on the Barlow Twins method to analyze over 1,600 H&E-stained colon cancer slides from TCGA-COAD and AVANT cohorts. Their model, trained without manual annotations, extracted latent features to define 47 histomorphological phenotype clusters (HPCs) that reflect immune infiltration, stromal disorganization, and tumor necrosis. The HPCs proved predictive of survival outcomes and treatment response, demonstrating how SSL can be leveraged for label-free, interpretable profiling of the TME. In summary, these advances highlight the growing synergy between AI and tumor immunology. By enabling mechanistic, data-driven characterization of the immune evasion landscape, AI models are not only enhancing prognostic precision but also uncovering biologically meaningful therapeutic targets (89). Looking ahead, the integration of multi-modal data—including spatial, transcriptomic, proteomic, and morphological inputs—into unified AI frameworks will likely revolutionize our ability to anticipate tumor immune dynamics and design next-generation precision immunotherapies tailored to individual patients.
4.3.2.3 The future of glioblastoma: combination immunotherapy
GBM is the most common and aggressive form of primary brain tumor, characterized by a complex network of survival mechanisms that promote therapeutic resistance and immune evasion (90). Within its highly immunosuppressive TME, GBM stem cells—notorious for their intrinsic drug resistance—remain key contributors to treatment failure and disease recurrence (91). The blood–brain barrier further restricts the delivery of therapeutics, while tumor antigenic heterogeneity, limited neoantigen presentation, and T cell exclusion add layers of immune resistance (92). Thus, the most urgent challenge lies in designing integrated therapeutic strategies that concurrently target multiple immune escape mechanisms and reinforce the overall antitumor immune response (93).
The team led by Arrieta VA (94) used low-intensity pulsed ultrasound (LIPU) and intravenous microbubbles to open the blood-brain barrier and increase the concentration of liposomal doxorubicin and PD-1 blocking antibody. Additionally, it was found that when administered with LIPU/MB, doxorubicin’s efficacy surpassed simple drug delivery; it significantly modulated the TME, potentially improving the presentation of tumor antigens to T cells, thereby enhancing the efficacy of T cell-based immunotherapy (including PD-1 blockade).
Luo F et al. (95) found that LRRC15 expression was elevated in GBM patients who did not respond to anti-PD-1 therapy. Therefore, they believe that targeting LRRC15 may provide a new strategy to enhance anti-PD-1 therapy and overcome immune therapy resistance in GBM.
In the preclinical model developed by the Xing YL team (96), it was found that BRAFi+MEKi can synergize with ICI by enhancing T cell activity and antigen presentation, thereby increasing the intrinsic sensitivity of tumors. However, the combination therapy has significant toxicity. Therefore, they propose incorporating galectin-3 inhibitors into treatment regimens for these gliomas as a promising strategy to improve treatment efficacy while controlling toxicity, thereby enhancing patients’ overall quality of life.
In summary, while GBM remains highly resistant to current therapies, progress in blood–brain barrier-penetrating delivery systems, TME modulation, and biomarker-driven combinations has opened new avenues for immunotherapy. Future research should prioritize dissecting the immune evasion mechanisms—particularly those involving GBM stem cells, myeloid cells, and stromal factors—while advancing precision delivery technologies to enhance treatment efficacy. These efforts will be key to developing the next GBM of effective, personalized immunotherapeutic strategies for glioblastoma.
4.4 Limitations
This bibliometric analysis provides a comprehensive overview of tumor immune escape research, though several methodological limitations warrant acknowledgment. First, the keyword strategy, while designed for thematic specificity, may have inadvertently excluded conceptually related topics, leading to potential omissions in the broader immuno-oncology landscape. Second, exclusive reliance on the Web of Science Core Collection—though beneficial for standardization—may underrepresent applied or interdisciplinary studies more extensively indexed in databases such as PubMed or Scopus. Third, citation-based metrics are inherently time-sensitive, often disadvantaging recent publications and reflecting academic rather than translational impact. Fourth, the exclusion of non-English publications to ensure language consistency may introduce geographic bias, potentially overlooking contributions from non-English-speaking countries. Lastly, a certain degree of subjectivity is unavoidable in the interpretation and synthesis of bibliometric findings.
5 Conclusion and future perspectives
This bibliometric study provides a comprehensive overview of the intellectual landscape, research hotspots, and developmental trajectory of tumor immune escape research over the past 14 years. By mapping influential nation, authors, core journals, reference, and keyword bursts, this work not only summarizes major contributions in the field but also helps researchers better understand its evolution and emerging directions. Based on the observed patterns, we propose three key areas that warrant further exploration: (1) advancing interdisciplinary research at the intersection of the microbiome, metabolism, and immune regulation; (2) integrating artificial intelligence and multi-omics data to enhance predictive modeling and therapeutic precision; and (3) combining multi-modal therapeutic strategies to overcome immune escape more effectively.
Looking ahead, future research should emphasize translating mechanistic discoveries into clinically actionable strategies, particularly in identifying biomarkers that predict immune evasion and therapy resistance. Greater investment in large-scale, real-world immunotherapy data, along with the development of open-access, cross-platform analytical tools, will further support reproducibility and innovation. Moreover, fostering stronger international collaboration among researchers, institutions, and countries will be vital to accelerating discovery in this field and promoting the global advancement of cancer immunotherapy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
HZ: Formal Analysis, Funding acquisition, Methodology, Writing – original draft. YH: Data curation, Formal Analysis, Methodology, Software, Writing – original draft. XW: Formal Analysis, Investigation, Methodology, Validation, Writing – original draft. WX: Data curation, Formal Analysis, Investigation, Methodology, Software, Writing – original draft. YX: Resources, Supervision, Validation, Writing – review & editing, Funding acquisition.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank all the authors for their contributions and people other than the authors for their help and support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1604216/full#supplementary-material
Abbreviations
TME, tumor microenvironment; AI, artificial intelligence; ML, machine learning; GBM, Glioblastoma.
References
1. Wang Y, Yan Q, Fan C, Mo Y, Wang Y, Li X, et al. Overview and countermeasures of cancer burden in China. Sci China Life Sci. (2023) 66:2515–26. doi: 10.1007/s11427-022-2240-6
2. Andre F, Ismaila N, Allison KH, Barlow WE, Collyar DE, Damodaran S, et al. Biomarkers for adjuvant endocrine and chemotherapy in early-stage breast cancer: ASCO guideline update. J Clin Oncol. (2022) 40:1816–37. doi: 10.1200/JCO.22.00069
3. Kraehenbuehl L, Weng CH, Eghbali S, Wolchok JD, and Merghoub T. Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat Rev Clin Oncol. (2022) 19:37–50. doi: 10.1038/s41571-021-00552-7
4. Wang H, Zhou F, Qin W, Yang Y, Li X, and Liu R. Metabolic regulation of myeloid-derived suppressor cells in tumor immune microenvironment: targets and therapeutic strategies. Theranostics. (2025) 15:2159–84. doi: 10.7150/thno.105276
5. Przygodzka P, Szulc-Kielbik I, Kielbik M, Pacholczyk M, and Klink M. Neuromedin U in the tumor microenvironment - Possible actions in tumor progression. Biochim Biophys Acta Rev cancer. (2025) 1880:189269. doi: 10.1016/j.bbcan.2025.189269
6. Huang S, Shi J, Shen J, and Fan X. Metabolic reprogramming of neutrophils in the tumor microenvironment: Emerging therapeutic targets. Cancer letters. (2025) 612:217466. doi: 10.1016/j.canlet.2025.217466
7. Jiang X, Wang J, Deng X, Xiong F, Ge J, Xiang B, et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol cancer. (2019) 18:10. doi: 10.1186/s12943-018-0928-4
8. Liu X, To KKW, Zeng Q, and Fu L. Effect of extracellular vesicles derived from tumor cells on immune evasion. Advanced Sci (Weinheim Baden-Wurttemberg Germany). (2025) 12:e2417357. doi: 10.1002/advs.202417357
9. Jiang T, Jin H, Ji X, Zheng X, Xu CX, and Zhang PJ. Drivers of centrosome abnormalities: Senescence progression and tumor immune escape. Semin Cancer Biol. (2025) 110:56–64. doi: 10.1016/j.semcancer.2025.01.008
10. Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. (2018) 560:382–6. doi: 10.1038/s41586-018-0392-8
11. Lee DY, Im E, Yoon D, Lee YS, Kim GS, Kim D, et al. Pivotal role of PD-1/PD-L1 immune checkpoints in immune escape and cancer progression: Their interplay with platelets and FOXP3+Tregs related molecules, clinical implications and combinational potential with phytochemicals. Semin Cancer Biol. (2022) 86:1033–57. doi: 10.1016/j.semcancer.2020.12.001
12. Juneja VR, McGuire KA, Manguso RT, LaFleur MW, Collins N, Haining WN, et al. PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J Exp Med. (2017) 214:895–904. doi: 10.1084/jem.20160801
13. Nihira NT, Kudo R, and Ohta T. Inflammation and tumor immune escape in response to DNA damage. Semin Cancer Biol. (2025) 110:36–45. doi: 10.1016/j.semcancer.2025.02.005
14. Cote AL, Munger CJ, and Ringel AE. Emerging insights into the impact of systemic metabolic changes on tumor-immune interactions. Cell Rep. (2025) 44:115234. doi: 10.1016/j.celrep.2025.115234
15. Ninkov A, Frank JR, and Maggio LA. Bibliometrics: Methods for studying academic publishing. Perspect Med education. (2022) 11:173–6. doi: 10.1007/S40037-021-00695-4
16. Kang Y, Kang Y, Zhang D, and Yao J. Antiangiogenic therapy exerts antitumor effects by altering the tumor microenvironment: bibliometric analysis. Front Immunol. (2024) 15:1460533. doi: 10.3389/fimmu.2024.1460533
17. Zhao Z, Xu K, Hu B, Jiang Y, Xu X, and Liu Y. A bibliometric study on the impact of gut microbiota on the efficacy of immune checkpoint inhibitors in cancer patients: analysis of the top 100 cited articles. Front Immunol. (2024) 15:1519498. doi: 10.3389/fimmu.2024.1519498
18. Liu M, Gao Y, Yuan Y, Shi S, Wu J, Tian J, et al. An evidence mapping and scientometric analysis of the top-100 most cited clinical trials of anti-PD-1/PD-L1 drugs to treat cancers. Biomed pharmacother = Biomed pharmacotherapie. (2021) 143:112238. doi: 10.1016/j.biopha.2021.112238
19. Plana NM, Massie JP, Bekisz JM, Spore S, Diaz-Siso JR, and Flores RL. Variations in databases used to assess academic output and citation impact. New Engl J Med. (2017) 376:2489–91. doi: 10.1056/NEJMc1616626
20. Jiang P, Gu S, Pan D, Fu J, Sahu A, Hu X, et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat Med. (2018) 24:1550–8. doi: 10.1038/s41591-018-0136-1
21. Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. New Engl J Med. (2015) 372:311–9. doi: 10.1056/NEJMoa1411087
22. Rabinovich GA, Gabrilovich D, and Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. (2007) 25:267–96. doi: 10.1146/annurev.immunol.25.022106.141609
23. Hanahan D and Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
24. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. New Engl J Med. (2012) 366:2443–54. doi: 10.1056/NEJMoa1200690
25. Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. New Engl J Med. (2012) 366:2455–65. doi: 10.1056/NEJMoa1200694
26. Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. New Engl J Med. (2010) 363:711–23. doi: 10.1056/NEJMoa1003466
27. Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Sci (New York NY). (2015) 348:124–8. doi: 10.1126/science.aaa1348
28. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. New Engl J Med. (2015) 373:1627–39. doi: 10.1056/NEJMoa1507643
29. Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. New Engl J Med. (2016) 375:819–29. doi: 10.1056/NEJMoa1604958
30. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
31. Nedeljković M, Vuletić A, and Mirjačić Martinović K. Divide and conquer-targeted therapy for triple-negative breast cancer. Int J Mol Sci. (2025) 26(4):1396. doi: 10.3390/ijms26041396
32. Xie D, Li G, Zheng Z, Zhang X, Wang S, Jiang B, et al. The molecular code of kidney cancer: A path of discovery for gene mutation and precision therapy. Mol aspects Med. (2025) 101:101335. doi: 10.1016/j.mam.2024.101335
33. Wan M, Pan S, Shan B, Diao H, Jin H, Wang Z, et al. Lipid metabolic reprograming: the unsung hero in breast cancer progression and tumor microenvironment. Mol cancer. (2025) 24:61. doi: 10.1186/s12943-025-02258-1
34. Vecchiotti D, Clementi L, Cornacchia E, Di Vito Nolfi M, Verzella D, Capece D, et al. Evidence of the link between stroma remodeling and prostate cancer prognosis. Cancers. (2024) 16(18):3215. doi: 10.3390/cancers16183215
35. Li Z, Liu S, Liu D, Yang K, Xiong J, and Fang Z. Multiple mechanisms and applications of tertiary lymphoid structures and immune checkpoint blockade. J Exp Clin Cancer research: CR. (2025) 44:84. doi: 10.1186/s13046-025-03318-6
36. Maksymova L, Pilger YA, Nuhn L, and Van Ginderachter JA. Nanobodies targeting the tumor microenvironment and their formulation as nanomedicines. Mol cancer. (2025) 24:65. doi: 10.1186/s12943-025-02270-5
37. Wei W, Zeng H, Zheng R, Zhang S, An L, Chen R, et al. Cancer registration in China and its role in cancer prevention and control. Lancet Oncol. (2020) 21:e342–e9. doi: 10.1016/S1470-2045(20)30073-5
38. Xia C, Basu P, Kramer BS, Li H, Qu C, Yu XQ, et al. Cancer screening in China: a steep road from evidence to implementation. Lancet Public Health. (2023) 8:e996–e1005. doi: 10.1016/S2468-2667(23)00186-X
39. Qi J, Li M, Wang L, Hu Y, Liu W, Long Z, et al. National and subnational trends in cancer burden in China, 2005-20: an analysis of national mortality surveillance data. Lancet Public Health. (2023) 8:e943–e55. doi: 10.1016/S2468-2667(23)00211-6
40. Diao X, Guo C, Jin Y, Li B, Gao X, Du X, et al. Cancer situation in China: an analysis based on the global epidemiological data released in 2024. Cancer Commun (London England). (2025) 45:178–97. doi: 10.1002/cac2.12627
41. Sun D, Li H, Cao M, He S, Lei L, Peng J, et al. Cancer burden in China: trends, risk factors and prevention. Cancer Biol Med. (2020) 17:879–95. doi: 10.20892/j.issn.2095-3941.2020.0387
42. Parascandola M, Pearlman PC, Eldridge L, and Gopal S. The development of global cancer research at the United States national cancer institute. J Natl Cancer Institute. (2022) 114:1228–37. doi: 10.1093/jnci/djac104
43. Tang Z, Li C, Kang B, Gao G, Li C, and Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. (2017) 45:W98–w102. doi: 10.1093/nar/gkx247
44. Ngoma T, Adewole I, Ainsworth V, Chin D, Chin J, Elzawawy A, et al. Cancer control collaborations between China and African countries. Lancet Oncol. (2024) 25:e164–e72. doi: 10.1016/S1470-2045(23)00634-4
45. Liu J and Cao X. Glucose metabolism of TAMs in tumor chemoresistance and metastasis. Trends Cell Biol. (2023) 33:967–78. doi: 10.1016/j.tcb.2023.03.008
46. Lou H and Cao X. Antibody variable region engineering for improving cancer immunotherapy. Cancer Commun (London England). (2022) 42:804–27. doi: 10.1002/cac2.12330
47. Chauvin JM and Zarour HM. TIGIT in cancer immunotherapy. J immunother Cancer. (2020) 8(2):e000957. doi: 10.1136/jitc-2020-000957
48. Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. (2009) 10:48–57. doi: 10.1038/ni.1674
49. Stanietsky N, Simic H, Arapovic J, Toporik A, Levy O, Novik A, et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci U States A. (2009) 106:17858–63. doi: 10.1073/pnas.0903474106
50. Liu S, Zhang H, Li M, Hu D, Li C, Ge B, et al. Recruitment of Grb2 and SHIP1 by the ITT-like motif of TIGIT suppresses granule polarization and cytotoxicity of NK cells. Cell Death differentiation. (2013) 20:456–64. doi: 10.1038/cdd.2012.141
51. Cho BC, Abreu DR, Hussein M, Cobo M, Patel AJ, Secen N, et al. Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a first-line treatment for PD-L1-selected non-small-cell lung cancer (CITYSCAPE): primary and follow-up analyses of a randomised, double-blind, phase 2 study. Lancet Oncol. (2022) 23:781–92. doi: 10.1016/S1470-2045(22)00226-1
52. Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E, et al. LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med. (1990) 171:1393–405. doi: 10.1084/jem.171.5.1393
53. Chocarro L, Blanco E, Zuazo M, Arasanz H, Bocanegra A, Fernández-Rubio L, et al. Understanding LAG-3 signaling. Int J Mol Sci. (2021) 22(10):5282. doi: 10.3390/ijms22105282
54. Silberstein JL, Du J, Chan KW, Frank JA, Mathews II, YB K, et al. Structural insights reveal interplay between LAG-3 homodimerization, ligand binding, and function. Proc Natl Acad Sci U States A. (2024) 121:e2310866121. doi: 10.1073/pnas.2310866121
55. Ming Q, Celias DP, Wu C, Cole AR, Singh S, Mason C, et al. LAG3 ectodomain structure reveals functional interfaces for ligand and antibody recognition. Nat Immunol. (2022) 23:1031–41. doi: 10.1038/s41590-022-01238-7
56. Tawbi HA, SChadendorf D, Lipson EJ, Ascierto PA, Matamala L, Castillo Gutiérrez E, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. New Engl J Med. (2022) 386:24–34. doi: 10.1056/NEJMoa2109970
57. Kumar A, Nader MA, and Deep G. Emergence of extracellular vesicles as “Liquid biopsy” for neurological disorders: boom or bust. Pharmacol Rev. (2024) 76:199–227. doi: 10.1124/pharmrev.122.000788
58. Meng W, Hao Y, He C, Li L, and Zhu G. Exosome-orchestrated hypoxic tumor microenvironment. Mol cancer. (2019) 18:57. doi: 10.1186/s12943-019-0982-6
59. Shao C, Yang F, Miao S, Liu W, Wang C, Shu Y, et al. Role of hypoxia-induced exosomes in tumor biology. Mol cancer. (2018) 17:120. doi: 10.1186/s12943-018-0869-y
60. Belényesi SK, Patmore S, and O’Driscoll L. Extracellular vesicles and the tumor microenvironment. Biochim Biophys Acta Rev cancer. (2025) 1880:189275. doi: 10.1016/j.bbcan.2025.189275
61. Adrover JM, McDowell SAC, He XY, Quail DF, and Egeblad M. NETworking with cancer: The bidirectional interplay between cancer and neutrophil extracellular traps. Cancer Cell. (2023) 41:505–26. doi: 10.1016/j.ccell.2023.02.001
62. Hirschhorn D, Budhu S, Kraehenbuehl L, Gigoux M, Schröder D, Chow A, et al. T cell immunotherapies engage neutrophils to eliminate tumor antigen escape variants. Cell. (2023) 186:1432–47.e17. doi: 10.1016/j.cell.2023.03.007
63. Schedel F, Mayer-Hain S, Pappelbaum KI, Metze D, Stock M, Goerge T, et al. Evidence and impact of neutrophil extracellular traps in Malignant melanoma. Pigment Cell melanoma Res. (2020) 33:63–73. doi: 10.1111/pcmr.12818
64. Xiong G, Chen Z, Liu Q, Peng F, Zhang C, Cheng M, et al. CD276 regulates the immune escape of esophageal squamous cell carcinoma through CXCL1-CXCR2 induced NETs. J immunother Cancer. (2024) 12. doi: 10.1136/jitc-2023-008662
65. He XY, Gao Y, Ng D, Michalopoulou E, George S, Adrover JM, et al. Chronic stress increases metastasis via neutrophil-mediated changes to the microenvironment. Cancer Cell. (2024) 42:474–86.e12. doi: 10.1016/j.ccell.2024.01.013
66. Di Giacomo AM, Canetta R, Connolly J, Leidner R, Zheng W, Kim Y, et al. Approaches to overcome the current treatment plateau in immunotherapy. Eur J Cancer (Oxford England: 1990). (2025) 226:115605. doi: 10.1016/j.ejca.2025.115605
67. Ju Y, Xu K, Chen X, Wu T, and Yuan Y. Metabolic-immune microenvironment crosstalk mediating ICI resistance in MASH-HCC. Trends Endocrinol metabolism: TEM. (2025) 12(5):e008662. doi: 10.1016/j.tem.2025.06.008
68. Zitvogel L, Galluzzi L, Kepp O, Smyth MJ, and Kroemer G. Type I interferons in anticancer immunity. Nat Rev Immunol. (2015) 15:405–14. doi: 10.1038/nri3845
69. Wu YT, Fang Y, Wei Q, Shi H, Tan H, Deng Y, et al. Tumor-targeted delivery of a STING agonist improvescancer immunotherapy. Proc Natl Acad Sci U States A. (2022) 119:e2214278119. doi: 10.1073/pnas.2214278119
70. Meric-Bernstam F, Larkin J, Tabernero J, and Bonini C. Enhancing anti-tumor efficacy with immunotherapy combinations. Lancet (London England). (2021) 397:1010–22. doi: 10.1016/S0140-6736(20)32598-8
71. Tang B, Wang Y, Li L, Sun C, Dong J, Li R, et al. Daurisoline modulates the TBK1-dependent type I interferon pathway to boost anti-tumor immunity via targeting of LRP1. Res (Washington DC). (2025) 8:0764. doi: 10.34133/research.0764
72. Liu R, Liu Q, Wang Y, Liu T, Zhang Z, Zhao C, et al. Enhanced antitumor immunity of VNP20009-CCL2-CXCL9 via the cGAS/STING axis in osteosarcoma lung metastasis. J immunother Cancer. (2025) 13(7):e012269. doi: 10.1136/jitc-2025-012269
73. Han J, Zhang B, Zhang Y, Yin T, Cui Y, Liu J, et al. Gut microbiome: decision-makers in the microenvironment of colorectal cancer. Front Cell infection Microbiol. (2023) 13:1299977. doi: 10.3389/fcimb.2023.1299977
74. He Y, Huang J, Li Q, Xia W, Zhang C, Liu Z, et al. Gut microbiota and tumor immune escape: A new perspective for improving tumor immunotherapy. Cancers. (2022) 14(21):5317. doi: 10.3390/cancers14215317
75. Ngwa VM, Edwards DN, Philip M, and Chen J. Microenvironmental metabolism regulates antitumor immunity. Cancer Res. (2019) 79:4003–8. doi: 10.1158/0008-5472.CAN-19-0617
76. Liu G, Yang Y, Kang X, Xu H, Ai J, Cao M, et al. A pan-cancer analysis of lipid metabolic alterations in primary and metastatic cancers. Sci Rep. (2023) 13:13810. doi: 10.1038/s41598-023-41107-3
77. Díaz-Basabe A, Lattanzi G, Perillo F, Amoroso C, Baeri A, Farini A, et al. Porphyromonas gingivalis fuels colorectal cancer through CHI3L1-mediated iNKT cell-driven immune evasion. Gut Microbes. (2024) 16:2388801. doi: 10.1080/19490976.2024.2388801
78. Zhang T, Li Y, Zhai E, Zhao R, Qian Y, Huang Z, et al. Intratumoral fusobacterium nucleatum recruits tumor-associated neutrophils to promote gastric cancer progression and immune evasion. Cancer Res. (2025) 85:1819–41. doi: 10.1158/0008-5472.CAN-24-2580
79. Zhou CB, Zhou YL, and Fang JY. Gut microbiota in cancer immune response and immunotherapy. Trends cancer. (2021) 7:647–60. doi: 10.1016/j.trecan.2021.01.010
80. Andrews TS, Nakib D, Perciani CT, Ma XZ, Liu L, Winter E, et al. Single-cell, single-nucleus, and spatial transcriptomics characterization of the immunological landscape in the healthy and PSC human liver. J hepatol. (2024) 80:730–43. doi: 10.1016/j.jhep.2023.12.023
81. Sorin M, Rezanejad M, Karimi E, Fiset B, Desharnais L, Perus LJM, et al. Single-cell spatial landscapes of the lung tumor immune microenvironment. Nature. (2023) 614:548–54. doi: 10.1038/s41586-022-05672-3
82. Liu G, Shi Y, Huang H, Xiao N, Liu C, Zhao H, et al. FPCAM: A weighted dictionary-driven model for single-cell annotation in pulmonary fibrosis. Biology. (2025) 14(5):479. doi: 10.3390/biology14050479
83. Walsh LA and Quail DF. Decoding the tumor microenvironment with spatial technologies. Nat Immunol. (2023) 24:1982–93. doi: 10.1038/s41590-023-01678-9
84. Bhinder B, Gilvary C, Madhukar NS, and Elemento O. Artificial intelligence in cancer research and precision medicine. Cancer discov. (2021) 11:900–15. doi: 10.1158/2159-8290.CD-21-0090
85. Xiao N, Huang X, Wu Y, Li B, Zang W, Shinwari K, et al. Opportunities and challenges with artificial intelligence in allergy and immunology: a bibliometric study. Front Med. (2025) 12:1523902. doi: 10.3389/fmed.2025.1523902
86. Li H, Zhang J, Shi Y, Wang H, Yang R, Wu S, et al. Identification of matrix stiffness-related molecular subtypes in HCC via integrating multi-omics analysis and machine learning algorithms. J Trans Med. (2025) 23:716. doi: 10.1186/s12967-025-06733-7
87. Desai SS, Salahuddin S, Yusuf R, Ranjan K, Gu J, Osmani L, et al. The tumor microenvironment of non-small cell lung cancer impairs immune cell function in people with HIV. J Clin Invest. (2025) 135(14):e177310. doi: 10.1172/JCI177310
88. Liu B, Polack M, Coudray N, Claudio Quiros A, Sakellaropoulos T, Le H, et al. Self-supervised learning reveals clinically relevant histomorphological patterns for therapeutic strategies in colon cancer. Nat Commun. (2025) 16:2328. doi: 10.1038/s41467-025-57541-y
89. Liu G, Zhu Z, Xing Y, Meng H, Shinwari K, Xiao N, et al. WMRCA + : a weighted majority rule-based clustering method for cancer subtype prediction using metabolic gene sets. Hereditas. (2025) 162:121. doi: 10.1186/s41065-025-00487-4
90. Shifman SG, O’Connor JL, Radin DP, Sharma A, Infante L, Ferraresso F, et al. Targeting autophagy and plasminogen activator inhibitor-1 increases survival and remodels the tumor microenvironment in glioblastoma. J Exp Clin Cancer research: CR. (2025) 44:214. doi: 10.1186/s13046-025-03473-w
91. Wu Q, Chen J, Berglund AE, Du D, Macaulay RJ, and Etame AB. The reprogramming impact of SMAC-mimetic on glioblastoma stem cells and the immune tumor microenvironment evolution. J Exp Clin Cancer research: CR. (2025) 44:191. doi: 10.1186/s13046-025-03452-1
92. Li Z, Yang F, Lu S, Wu X, Li S, and Wang M. Neuroimmunology-driven CAR T-cell therapeutics for gliomas: translational challenges and clinical trial paradigm innovation. Cancer letters. (2025) 631:217928. doi: 10.1016/j.canlet.2025.217928
93. Schonfeld E, Choi J, Tran A, Kim LH, and Lim M. The landscape of immune checkpoint inhibitor clinical trials in glioblastoma: A systematic review. Neuro-oncol Adv. (2024) 6:vdae174. doi: 10.1093/noajnl/vdae174
94. Arrieta VA, Gould A, Kim KS, Habashy KJ, Dmello C, Vázquez-Cervantes GI, et al. Ultrasound-mediated delivery of doxorubicin to the brain results in immune modulation and improved responses to PD-1 blockade in gliomas. Nat Commun. (2024) 15:4698. doi: 10.1038/s41467-024-48326-w
95. Luo F, Mei Y, Li Y, Yang J, Xi S, Cao E, et al. CAF-derived LRRC15 orchestrates macrophage polarization and limits PD-1 immunotherapy efficacy in glioblastoma. Neuro-oncology. (2025) noaf157. doi: 10.1093/neuonc/noaf157
Keywords: tumor immune escape, bibliometric analysis, tumors, tumor microenvironments, immune checkpoint inhibitors
Citation: Zhu H, Huang Y, Wang X, Xiang W and Xie Y (2025) Trends and hotspots in research related to tumor immune escape: bibliometric analysis and future perspectives. Front. Immunol. 16:1604216. doi: 10.3389/fimmu.2025.1604216
Received: 01 April 2025; Accepted: 05 August 2025;
Published: 28 August 2025.
Edited by:
Akhileshwar Namani, Sri Shankara Cancer Hospital and Research Centre, IndiaReviewed by:
Giandomenico Roviello, University of Firenze, ItalyGuojun Liu, Ural Federal University, Russia
Xin Wu, Central South University, China
Copyright © 2025 Zhu, Huang, Wang, Xiang and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Xie, eGlleW9uZ25vbEBzaW5hLmNvbQ==
 Houcheng Zhu
Houcheng Zhu Yue Huang
Yue Huang Xiangjin Wang1
Xiangjin Wang1 Wang Xiang
Wang Xiang