- Department of Dermatology, Second Affiliated Hospital of Zhejiang University, Hangzhou, China
Background: Cell-free DNA (cfDNA) functions in the early-detection and monitoring of autoimmune diseases including systemic lupus erythematosus and rheumatoid arthritis. However, investigations into cfDNA profiles in dermatomyositis and their potential clinical implications remain scarce.
Objectives: To explore the overall landscape of cfDNA profiles in dermatomyositis and investigate potential roles in discriminating subtypes.
Methods: Following informed consent, 24 treatment-naïve patients diagnosed with dermatomyositis and 16 healthy controls were enrolled. We examined cfDNA concentrations, fragment distribution patterns, 5’-end motif frequencies and genetic variation profiles in all participants and studied potential correlation with laboratory parameters. Moreover, intergroup differences of cfDNA profiles among patients and potential correlation between extracellular DNases levels and cfDNA were investigated.
Results: Compared to healthy controls, dermatomyositis patients exhibited elevated cfDNA concentrations, with significantly longer cfDNA fragments, primarily centered around 180–360 bp; nonetheless, no correlation was witnessed between lab parameters and cfDNA levels. The A-end predominated the 5’-end motif, whereas the C-end was underrepresented, contrasting with the patterns observed in healthy controls. In addition, genetic variations in several genes, including PDE4DIP and BRCA2, were commonly detected in cfDNA from dermatomyositis patients. Notably, end-motif profiles and cfDNA fragment length exhibited variations between anti-transcription intermediary factor 1-gamma positive patients with and without malignancies. However, owing to limited sample size, we failed to draw conclusions regarding extracellular DNase levels.
Conclusions: This study presents the first comprehensive depiction of cfDNA profiles in patients with dermatomyositis. Furthermore, cfDNA features exhibit variability across some sub-phenotypes and may serve as discriminatory indices. Finally, potential involvement of extracellular DNases in cfDNA profiles in dermatomyositis shall be further investigated.
Introduction
Dermatomyositis is a severe condition that can result in significant morbidity and mortality. However, many patients remain undiagnosed based on current diagnostic and classification criteria (1). The delay between initial clinical manifestations and definitive diagnosis is common, with a median delay of 15.5 months (2). Furthermore, although the categories of dermatomyositis are far accurate on the basis of myositis-specific antibodies (MSAs) and other laboratory parameters, heterogeneity remains a significant concern in certain phenotypes, such as concomitant malignant tumors, if any, in dermatomyositis with anti-transcription intermediary factor 1-gamma (TIF1g) antibody (3).
Cell-free DNA (cfDNA) represents a small fraction of the total DNA pool that circulates freely in the bloodstream under both normal and pathological conditions. Altered cfDNA profiles have been observed in numerous autoimmune diseases, including systemic lupus erythematosus and rheumatoid arthritis (4). Recent studies have demonstrated the potential of cfDNA profiles in assisting patient stratification, monitoring therapeutic responses, and predicting disease progression (5).
Prior research has exhibited the potential clinical value of cfDNA levels in monitoring disease severity indices of dermatomyositis including the cutaneous dermatomyositis disease area and severity index (CDASI) and myositis disease activity assessment visual analogue scale (MYOACT) (6, 7). However, these studies have primarily focused on specific phenotypes of dermatomyositis, leaving the overall cfDNA profile of the disease still poorly understood.
The present study aims to explore the overall plasma cfDNA levels, fragmentation profiles and genetic variances landscapes of patients with dermatomyositis. We also seek to investigate correlation between cfDNA and laboratory parameters and compare cfDNA profiles between anti-TIF1g antibody-positive dermatomyositis patients with and without malignancies, as well as other MSA phenotypes, to assess its potential in distinguishing phenotypes. Additionally, we aim to elucidate the role of extracellular DNases, i.e., DNase-1 and DNase1l3, in the cleavage and fragments formation of cfDNA fragments, and their potential correlation with phenotypic variations.
Patients and methods
Patient selection and sample collection
This study enrolled 24 treatment-naïve patients diagnosed with dermatomyositis according to the 2017 EULAR/ACR classification criteria without any active infection or inflammation aside from dermatomyositis and its associated conditions, from the inpatient ward of dermatology, and 16 healthy controls from physical examination center of the Second Affiliated Hospital of Zhejiang University (Hangzhou, China) from 2022 to 2024. Ethical approval was granted by the Ethics Committee of the Second Affiliated Hospital of Zhejiang University (IR2023343). All participants have signed written informed consent. The study adhered to the principles of the Declaration of Helsinki.
Cell-free plasma samples were collected from patients once diagnosed. Patients were subdivided into three subgroups based on MSAs and cancer history: Subgroup 1 (positive anti-TIF1g antibody with tumor history, TWT; 6 patients), Subgroup 2 (positive anti-TIF1g antibody without any malignant tumor up to the most recent follow-up in Nov, 2024, TOT; 5 patients), and Subgroup 3 (other MSAs group, OM; 13 patients). Peripheral blood samples were collected using Streck tubes and were centrifuged within 24h of collection at 350×g for 10min at room temperature and the plasma was further centrifuged again at 3000×g for 15min at 4°C. All plasma samples were collected and stored at -80°C.
cfDNA quantification in plasma and next-generation sequencing
cfDNA extraction and quantification were performed using a standard commercial kit following the manufacturer’s instructions (Plasma cell-free DNA isolation Kit, BunnyMag, Cat. No. TQ01BT0100). The cfDNA concentration was measured by Qubit dsDNA HS Assay Kit (Q32854, Invitrogen). Among them, 10 plasma samples meeting the sequencing requirements (total cfDNA > 20 ng/ml) were additionally processed for library preparation and 650 genes panel exome sequencing to evaluate the molecular characteristics of cell-free DNA. The libraries were constructed using the Hieff NGS Ultima Pro DNA Library Prep Kit for Illumina (Yeasen, Cat. No. 12201ES24). Exome capture was performed using SureSelectXT Human All Exon V6 (Agilent) technology. The sequencing was carried out on the Illumina NovaSeqXplus PE150 platform (150 bp × 2 paired- end format).
Sequencing data processing and alignment
Quality control was performed with Fastp (v0.20.0) (8) to trim the sequencing adaptor and eliminate low-quality reads. Subsequently, the cleaned reads were aligned to the human reference genome (GRCh37/HG19) using Burrows Wheeler Aligner (BWA, v0.7.17) as previously described. PCR duplications were identified using Picard-tools (v4.1.1.0) and subsequently removed. Reads with low mapping quality (< 30), multiple alignments, or more than five mismatches were filtered out. Only paired end reads with proper mapping orientations and an insert size below 600 bp were retained for downstream analysis.
Detection and annotation of genetic variances
The SNPs and small fragment insertions/deletions were detected by Mutect2 software from the Genome Analysis Toolkit (GATK, v4.1.1.0), meanwhile, detected VCF files were annotated by Annovar (v201804). The reference database includes COSMIC database, dbSNP Database, 1000 Genomes Project, etc. Annotation included variant location, type, and conservation predictions.
Quantitative analysis of cfDNA
Quantitative levels of cfDNA were measured in haploid genome equivalents per milliliter (hGE/mL), calculated by multiplying the total cfDNA concentration by the mean allele fraction of somatic mutations.
5’ End-motif analysis of cfDNA fragments
End motifs were determined as the terminal nucleotide sequences at each 5’ fragment end of cfDNA molecules. The base content proportions of the end motifs were calculated at each position. Motifs were grouped based on fragment size, and the frequency of each motif was determined for each fragment size.
Quantification of the level of DNase-1 and DNase1l3 in plasma
DNase-1 and DNase1l3 levels in plasma were measured using human DNase-I ELISA kit (Cusabio, CSB-E09068h) and human DNASE1L3 ELISA kit (Cusabio, CSB-EL007052HU) according to the manufacturer’s protocol.
Statistical analysis
Differences between groups for continuous variables were assessed using the Mann-Whitney U-test, Wilcoxon test, and Kruskal-Wallis-test. The difference in categorical variables between groups was examined by the Chi-square test or Fisher’s exact test. Spearman co-efficiency analysis was adopted for correlation analysis. Statistical significance was defined as a p-value < 0.05. All statistical analysis was conducted by Prism10 for macOS (version 10.3.1).
Results
Demographic profiles of dermatomyositis patients
A total of 24 adult treatment-naïve Chinese patients diagnosed with dermatomyositis according to the EULAR/ACR classification criteria (2017) were enrolled from 2022 to 2024. Of these patients, 11 were anti-TIF1g antibodies positive, with 6 having a cancer diagnosis and 5 without cancer; while the MSAs of the remaining 13 patients were not TIF1g. Demographic and laboratory data are presented in Table 1, and Figure 1 provides an overview of the study.
Increased cfDNA concentration and possible correlation with lab parameters
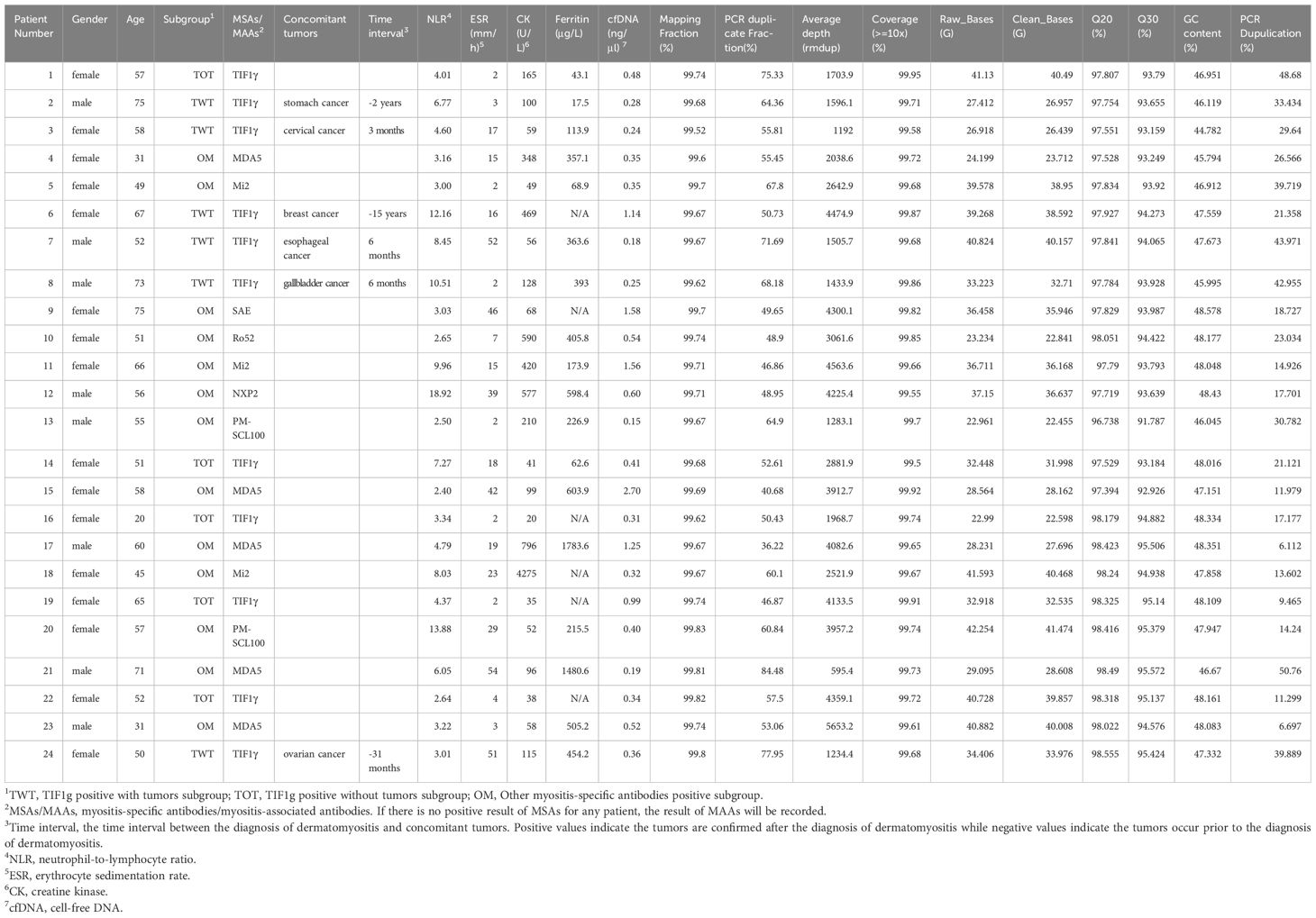
Compared to healthy controls, all enrolled patients displayed an overall higher concentration of cfDNA, with statistical significance, ranging from 0.15 ng/μl to 2.70 ng/μl (Figure 2A). However, no intergroup differences were identified (Figure 2B).
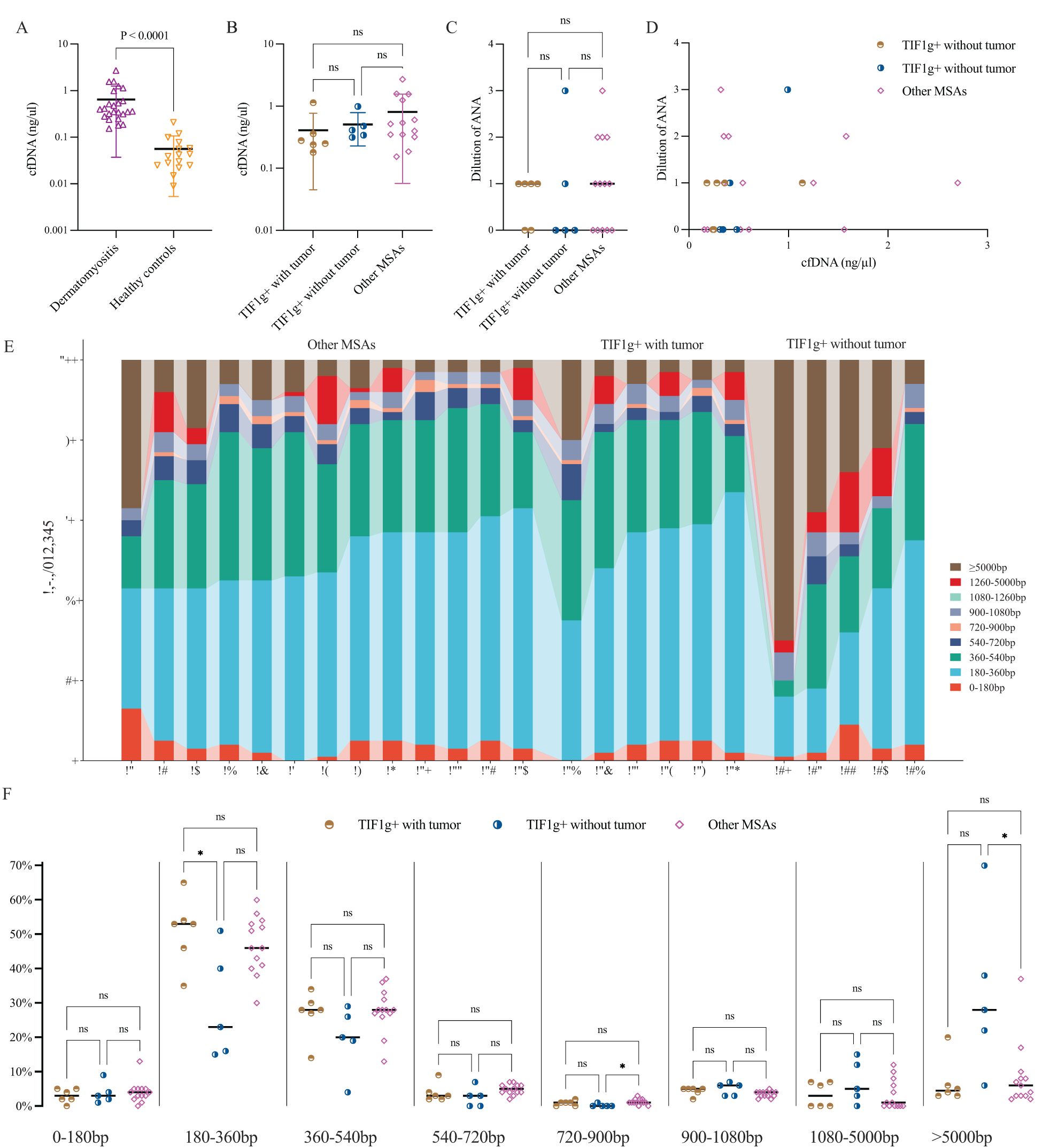
Figure 2. Plasma cfDNA levels, correlation with ANA and cfDNA fragment length distribution profiles in dermatomyositis. Plasma levels of cfDNA were significantly elevated in patients with dermatomyositis compared to healthy controls, but no intergroup variances were found among the subgroups (A, B). ANA dilution showed no notable differences among subgroups and no clear trend was observed between ANA titers and cfDNA levels (C, D). cfDNA fragments assigned to 180~360bp predominate in nearly all patients (E); the intergroup variance was displayed as regards different cfDNA fragment length (F). *P < 0.05. ns, not significant.
The potential pathogenicity of abnormally elevated extracellular DNA is of interest, with some studies attributing it to the formation of antinuclear antibodies (ANA) (9). Considering these findings, we explored whether cfDNA concentration correlates with ANA titers and inflammatory markers in all patients.
To streamline subsequent research, ANA titers were classified as 1:40 (dilution 1), 1:80 (dilution 2), and so on, with negative ANA as dilution 0. Generally, 14 patients were reported to be ANA positive (dilution 1 or higher), but no significant differences were found across subgroups, and no correlation between cfDNA levels and ANA titers was observed (Figures 2C, D).
Meanwhile, spearman co-efficiency analysis of erythrocyte sedimentation rate, ferritin and neutrophil-lymphocyte ratio with cfDNA levels showed no significant associations. Furthermore, no correlation was found between cfDNA and creatine kinase, a marker of muscle damage.
Longer cell-free DNA fragments are documented in patients with dermatomyositis
In principle, a cfDNA molecule consists of one or more nucleosome core (146bp), H1-bound/free linker DNA segments and unbound linker DNA (10). In order to investigate the overall characteristics of cfDNA fragments, the profile of cfDNA length was roughly divided into multiples of 180bp. In healthy individuals, most cfDNA was within the nucleosome-core category, with a median length of 167 bp. In contrast, dermatomyositis patients had a predominance of cfDNA fragments between 180 and 360 bp, ranging from 15% to 65% (Figure 2E).
Subsequently, we compared cfDNA lengths among three subgroups using the Kruskal-Wallis-test. Significant differences were observed at 180–360 bp (P = 0.035), 720–900 bp (P = 0.043) and >5,000 bp (P = 0.027). The proportion of 180–360 bp cfDNA fragments varies significantly between the TWT and TOT subgroups, which may serve as an effective indicator of concomitant tumors in dermatomyositis patients with anti-TIF1g antibodies (Figure 2F).
The end-motif landscape of cell-free DNA in dermatomyositis
To provide a comprehensive characterization of cfDNA fragment end profiles, the frequencies of 4-mer end-motif at each 5’ fragment end of cfDNA molecules were calculated. The 4-mer end-motif was defined as the terminal first 4-nt sequence at each 5’ fragment end of cfDNA molecules in alphabetical order, resulting in 256 categories. The top 30 4-mer end-motifs were then summarized as Figure 3A, with AAAA, TTTT and AAGA occupying the top three positions.
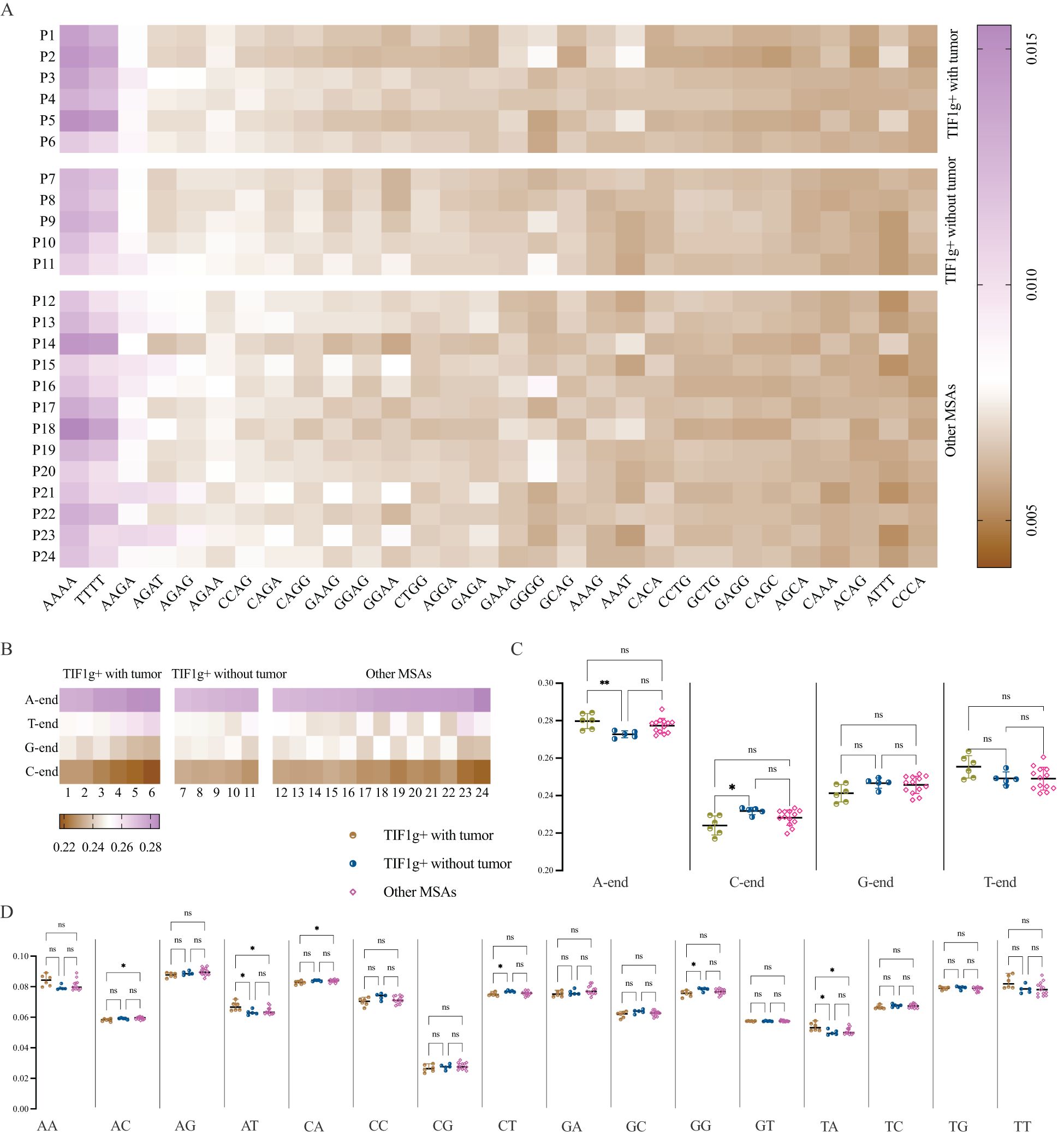
Figure 3. End-motif profiles of cfDNA. Heatmap of the top 30 four-mer end motifs across patient subgroups (A). A-end predominates in all patients, while proportion of C-end is far lower in TWT subgroup compared to TOT subgroup (B, C). The intergroup variances as regards 2-mer end-motifs (D). *P < 0.05, **P < 0.01. ns, not significant.
To examine broader trends, the frequency of both 1-mer and 2-mer end motifs was also calculated. The results demonstrate that the 5’ A-end predominates, while the C-end is the least prevalent end-motif in all patients with dermatomyositis enrolled in the present study (Figure 3B). Patients in the TWT subgroup showed higher frequencies of A-end (P = 0.0088) and lower frequencies of C-end (P = 0.0148) compared to the TOT subgroup, though no differences were found between the TOT and OM subgroups (Figure 3C).
Regarding 2-mer end-motifs, significant intergroup variances were observed for AC-, AT-, CA-, CT-, GG-, and TA- end-motifs frequencies. The TWT subgroup had higher frequencies of AT- and TA- motifs compared to the TOT and OM subgroups, while showing lower frequencies of CT- and GG- motifs than the TOT subgroup. It is noteworthy that the percentage of AC- and CA- end-motifs was similar between the TWT and TOT subgroups, despite overall intergroup variances (Figure 3D).
Genetic variance profiles with high frequencies in patients with dermatomyositis
The mean sequencing depth exceeded 1000x, ensuring a minimum data size of 22GB. A comprehensive summary of all the sequencing parameters is provided in Table 1 and Figure 4A. Among the 24 patients enrolled, 17 genes were identified as mutated in half the patients or more (Figure 4B). PDE4DIP was identified as the most highly mutated gene (19/24), irrespective of the subgroups, followed by BRCA2 (15/24). Furthermore, genetic variances of BCLAF1, KMT2A and KMT2C were detected in 14 patients. Regarding the absolute variance numbers, KMT2D ranked first with 66 reported variances, followed by BRCA2 and SPEN. The majority of genetic variances were multi-hit combinations in patients, with frame-shift insertion genetic variances ranking highest in terms of mutation types. These findings suggest that these genes and their coding proteins may be involved in the pathogenesis of dermatomyositis.
Figure 4. Results of high throughput cfDNA sequencing. Circos plot represents the chromosome, sequencing coverage, and the variance density from TOT, OM and TWT subgroup, respectively, from the outermost layer to the innermost (A). Genetic variances for all enrolled patients (B), TWT subgroup (C) and TOW subgroup (D). Levels of genetic variances number and variant allele frequency of each subgroup (E, F). Association between variant allele frequency and cfDNA levels (G). ns, not significant.
Subsequently, we investigated differences in genetic mutations between the TWT and TOT subgroups (Figures 4C, D). No genetic variance of either SPEN or KDM5A was detected in the TWT subgroup, and the variance frequencies remained high in the remaining two subgroups. Furthermore, gene variances in ATRX were detected in all patients allocated to the TOT subgroup (5/5), but rarely in the TWT subgroup (1/6). In addition to the above finding, genetic variance frequencies of BTK, FANCM, and PIK3C2B differ among subgroups. This finding provides a foundation for further investigation into the potential roles of these genes.
We also examined whether there were differences in the total number of somatic mutations across subgroups. The mean number of each subgroup was found to be approximately 1,000, with no significant differences (P = 0.967, Figure 4E). Variant allele frequency (VAF) is a widely utilized metric for disease monitoring and prognosis in cancer. Despite the absence of intergroup divergence in terms of VAF among each subgroup (Figure 4F), a significant positive correlation was observed between VAF and concentration of cfDNA (Spearman r = 0.5530, P = 0.0062, Figure 4G). Analysis within subgroups revealed a significant positive correlation in the OM subgroup (Spearman r = 0.6272, P = 0.0245).
Discussion
This study provides a comprehensive overview of cfDNA characteristics in dermatomyositis patients, irrespective of MSAs. Furthermore, the study has highlighted patients with positive anti-TIF1g antibody, with or without concomitant tumors, have distinct plasma cfDNA fragmentation profiles.
We observed significantly higher cfDNA levels in dermatomyositis patients compared to healthy individuals, where cfDNA levels are typically undetectable or very low (11). Observed in several autoimmune diseases such as systemic lupus erythematosus, this phenomenon is possibly attributed to tissue damage in disease status (12).
The equilibrium between cfDNA generation and clearance is crucial in both health and disease (13). Impaired clearance mechanisms may be responsible for the abnormally accumulated cfDNA (4). Potential mechanisms of cfDNA clearance in vivo include direct degradation by nucleases; to date, DNA fragmentation factor B (DFFB), DNase-1, and DNase1l3 are the only three nucleases that have been shown to affect cfDNA levels and/or cfDNA fragment characteristics (14). In this study, we aimed to ascertain the involvement of the extracellular nucleases, specifically DNase1l3 and DNase-1, in the cfDNA profiles of dermatomyositis patients. However, due to the variability in the concentration of cfDNA in the collected peripheral blood samples, only 27 ELISA assays were conducted on serial plasma samples from nine patients (one from TWT, four from TOT and four from the OM subgroup) and 16 assays were conducted on samples from eight healthy controls (data not shown). Owing to the very limited sample size and deteriorated power, we failed to conclude any concrete roles of DNase1l3 and DNase-1 in cfDNA patterns.
The frequencies of longer cfDNA fragments in dermatomyositis is higher than normal controls. In health states, the size of cfDNA fragments differs due to variable length of intranucleosomal linker DNA (15), with the main peak at around 166 bp (16). In disease conditions, apoptotic cells produce mononucleosomal fragments, and necrotic cells generate larger fragments exceeding 10 kb (17). Besides, DNase1l3 deficiency leads to an increased amount of longer cfDNA fragments as it targets nucleosomal DNA present in extracellular space (18). In mice models, DNase1l3 deficient individuals have higher frequencies of longer sized cfDNA, indicating the pivotal role of DNase1l3 in digesting cfDNA to nucleosomal size (19). Owing to limited sample size, whether DNase1l3 contributes to longer cfDNA fragments and other underlying mechanisms shall be further investigated.
End-motif analysis, which holds promise for precision medicine (20), revealed non-random production of cfDNA fragments (21). The linker region between core nucleosomes, rather than the DNA wrapping around the nucleosome core, is more likely to be cut (22). In contrast to our current findings, an over- and underrepresentation of C- and A-end motif fragments was documented in healthy states, corresponding with the distribution of nucleosome-occupied and open-chromatin regions (13, 23). This discrepancy may be attributed to the A-end preference seen in longer cfDNA fragments (>200 bp) (14), or the loss of typical CC-end motif preference in DNase1l3-deficient models (14, 19, 24).
Consistent with previous findings from malignant tumor patients (25), over- and underrepresentation of A- and C-end motif fragments are observed between the TWT and TOT subgroups. Nonetheless, contrary to typical short cfDNA fragments (145bp) predominance found in cancer patients (26, 27), no significant intergroup variance of ~180bp cfDNA fragments was observed between TOT and TWT. Hypomethylation in tumor cells is responsible for more alternative cleavage sites and thus leading to shortened cfDNA fragments (28). Further research on cfDNA methylation profiles in dermatomyositis is needed.
Our study also highlighted the potential involvement of specific genes in dermatomyositis. Up to now, scholars only reported somatic variances of JAK2 and TIF1g (TRIM33) that may possibly correlate with dermatomyositis, but most of the studies were case reports or from malignancy-associated dermatomyositis (29, 30).
The limitations of this study include the small sample size and single ethnicity, necessitating further validation through larger, international multi-center studies. Meanwhile, the panel-based cfDNA sequencing may have missed some information compared to whole-exome sequencing. Additionally, due to the study design and insufficient follow-up data on patient prognosis, we could not draw conclusions about the relationship between cfDNA profiles and clinical outcomes such as concomitant symptoms, survival time, treatment response or relapse rates. Therefore, further long-term follow-up and continuous investigation are required to address these issues.
In summary, our study firstly portraits the general landscape of cfDNA in dermatomyositis, which encompass concentration, fragments distribution in length, end motifs and genetic variances panoramic picture. Besides, our findings stressed the potential utility of cfDNA fragmentomes in discriminating sub-phenotypes. However, we still have a long way to go as far more data are needed for a comprehensive model in early diagnosis, therapeutical surveillance or even phenotype discrimination. In addition, the underlying mechanism contributing to such cfDNA profiles including the patterns of extracellular DNase shall be further unraveled.
Data availability statement
The variation data reported in this paper have been deposited in the Genome Variation Map (GVM) in National Genomics Data Center, Beijing Institute of Genomics, Chinese Academy of Sciences and China National Center for Bioinformation, under accession number GVM000955. (https://bigd.big.ac.cn/gvm/getProjectDetail?Project=GVM000955).
Ethics statement
The studies involving humans were approved by Ethics Committee of the Second Affiliated Hospital of Zhejiang University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
Z-lT: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. P-YC: Resources, Software, Visualization, Writing – original draft, Writing – review & editing. HZ: Investigation, Resources, Software, Writing – review & editing. H-LC: Data curation, Formal analysis, Writing – review & editing. RD: Investigation, Resources, Writing – review & editing. Y-CL: Resources, Software, Writing – review & editing. Y-HS: Data curation, Resources, Writing – review & editing. YZ: Resources, Writing – review & editing. X-YC: Software, Writing – review & editing. M-JZ: Resources, Visualization, Writing – review & editing. Y-QW: Resources, Writing – review & editing. X-YM: Conceptualization, Funding acquisition, Investigation, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was financially supported by grants from the National Natural Science Foundation of China (No. 82230104, 81930089, 81773318, 82404117, 82103709 and 82303999).
Acknowledgments
We sincerely appreciate all the participants who donate their peripheral blood and are willing to report their desensitized information to our research. Besides, we thank the technical support in cfDNA sequencing from Wuhan Kindstar Clinical Diagnostic Co., China. Meanwhile, we thank Bensch Carl Tristan for minor revision in Methods section.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Iaccarino L, Ghirardello A, Bettio S, Zen M, Gatto M, Punzi L, et al. The clinical features, diagnosis and classification of dermatomyositis. J Autoimmun. (2014) 48–49:122–7. doi: 10.1016/j.jaut.2013.11.005
2. Da Silva DM, Patel B, and Werth VP. Dermatomyositis: A diagnostic dilemma. J Am Acad Dermatol. (2018) 79:371–3. doi: 10.1016/j.jaad.2017.12.074
3. Zhao Q, Chen Y, Diao L, Zhang S, Wu D, Xue F, et al. Identification of distinct cytokine/chemokine profiles in dermatomyositis with anti-transcriptional intermediary factor 1-γ antibody. Rheumatol (Oxford). (2022) 61:2176–84. doi: 10.1093/rheumatology/keab625
4. Zhu H, Kong B, Che J, Zhao Y, and Sun L. Bioinspired nanogels as cell-free DNA trapping and scavenging organelles for rheumatoid arthritis treatment. Proc Natl Acad Sci U S A. (2023) 120:e2303385120. doi: 10.1073/pnas.2303385120
5. Duvvuri B and Lood C. Cell-free DNA as a biomarker in autoimmune rheumatic diseases. Front Immunol. (2019) 10:502. doi: 10.3389/fimmu.2019.00502
6. Wang Y, Zhao Y, Gang Q, Hao H, Gao F, Deng J, et al. Circulating Cell-Free DNA Promotes Inflammation in Patients with Dermatomyositis with Anti-NXP2 Antibodies via the cGAS/STING Pathway. Rheumatol (Oxford). (2024) 64(4):2272–81. doi: 10.1093/rheumatology/keae425
7. Ishino T, Myangat TM, Sawamura S, Kajihara I, Shimada S, Kanemaru H, et al. Elevation of cell-free DNA in patients with clinically amyopathic dermatomyositis. J Dermatol. (2023) 50(6):e189–e191. doi: 10.1111/1346-8138.16714
8. Chen S, Zhou Y, Chen Y, and Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. (2018) 34:i884–90. doi: 10.1093/bioinformatics/bty560
9. Stochmal A, Czuwara J, Trojanowska M, and Rudnicka L. Antinuclear antibodies in systemic sclerosis: an update. Clin Rev Allergy Immunol. (2020) 58:40–51. doi: 10.1007/s12016-018-8718-8
10. Cutter AR and Hayes JJ. A brief review of nucleosome structure. FEBS Lett. (2015) 589:2914–22. doi: 10.1016/j.febslet.2015.05.016
11. Truszewska A, Foroncewicz B, and Pączek L. The role and diagnostic value of cell-free DNA in systemic lupus erythematosus. Clin Exp Rheumatol. (2017) 35:330–6.
12. Galeazzi M, Morozzi G, Piccini M, Chen J, Bellisai F, Fineschi S, et al. Dosage and characterization of circulating DNA: present usage and possible applications in systemic autoimmune disorders. Autoimmun Rev. (2003) 2:50–5. doi: 10.1016/S1568-9972(02)00101-5
13. Han DSC and Lo YMD. The nexus of cfDNA and nuclease biology. Trends Genet. (2021) 37:758–70. doi: 10.1016/j.tig.2021.04.005
14. Han DSC, Ni M, Chan RWY, Chan VWH, Lui KO, Chiu RWK, et al. The biology of cell-free DNA fragmentation and the roles of DNASE1, DNASE1L3, and DFFB. Am J Hum Genet. (2020) 106:202–14. doi: 10.1016/j.ajhg.2020.01.008
15. Lo YMD, Han DSC, Jiang P, and Chiu RWK. Epigenetics, fragmentomics, and topology of cell-free DNA in liquid biopsies. Science. (2021) 372:eaaw3616. doi: 10.1126/science.aaw3616
16. Cristiano S, Leal A, Phallen J, Fiksel J, Adleff V, Bruhm DC, et al. Genome-wide cell-free DNA fragmentation in patients with cancer. Nature. (2019) 570:385–9. doi: 10.1038/s41586-019-1272-6
17. Hu Z, Chen H, Long Y, Li P, and Gu Y. The main sources of circulating cell-free DNA: Apoptosis, necrosis and active secretion. Crit Rev Oncol Hematol. (2021) 157:103166. doi: 10.1016/j.critrevonc.2020.103166
18. Sisirak V, Sally B, D’Agati V, Martinez-Ortiz W, Özçakar ZB, David J, et al. Digestion of chromatin in apoptotic cell microparticles prevents autoimmunity. Cell. (2016) 166:88–101. doi: 10.1016/j.cell.2016.05.034
19. Serpas L, Chan RWY, Jiang P, Ni M, Sun K, Rashidfarrokhi A, et al. Dnase1l3 deletion causes aberrations in length and end-motif frequencies in plasma DNA. Proc Natl Acad Sci USA. (2019) 116:641–9. doi: 10.1073/pnas.1815031116
20. Qi T, Pan M, Shi H, Wang L, Bai Y, and Ge Q. Cell-free DNA fragmentomics: the novel promising biomarker. Int J Mol Sci. (2023) 24:1503. doi: 10.3390/ijms24021503
21. Snyder MW, Kircher M, Hill AJ, Daza RM, and Shendure J. Cell-free DNA comprises an in vivo nucleosome footprint that informs its tissues-of-origin. Cell. (2016) 164:57–68. doi: 10.1016/j.cell.2015.11.050
22. Zhu D, Wang H, Wu W, Geng S, Zhong G, Li Y, et al. Circulating cell-free DNA fragmentation is a stepwise and conserved process linked to apoptosis. BMC Biol. (2023) 21:253. doi: 10.1186/s12915-023-01752-6
23. Song L, Zhang Z, Grasfeder LL, Boyle AP, Giresi PG, Lee BK, et al. Open chromatin defined by DNaseI and FAIRE identifies regulatory elements that shape cell-type identity. Genome Res. (2011) 21:1757–67. doi: 10.1101/gr.121541.111
24. Chan RWY, Serpas L, Ni M, Volpi S, Hiraki LT, Tam LS, et al. Plasma DNA profile associated with DNASE1L3 gene mutations: clinical observations, relationships to nuclease substrate preference, and in vivo correction. Am J Hum Genet. (2020) 107:882–94. doi: 10.1016/j.ajhg.2020.09.006
25. Liu D, Yehia L, Dhawan A, Ni Y, and Eng C. Cell-free DNA fragmentomics and second Malignant neoplasm risk in patients with PTEN hamartoma tumor syndrome. Cell Rep Med. (2024) 5:101384. doi: 10.1016/j.xcrm.2023.101384
26. Mouliere F, Chandrananda D, Piskorz AM, Moore EK, Morris J, Ahlborn LB, et al. Enhanced detection of circulating tumor DNA by fragment size analysis. Sci Transl Med. (2018) 10:eaat4921. doi: 10.1126/scitranslmed.aat4921
27. Mouliere F and Rosenfeld N. Circulating tumor-derived DNA is shorter than somatic DNA in plasma. Proc Natl Acad Sci U S A. (2015) 112:3178–9. doi: 10.1073/pnas.1501321112
28. Udomruk S, Phanphaisarn A, Kanthawang T, Sangphukieo A, Sutthitthasakul S, Tongjai S, et al. Characterization of cell-free DNA size distribution in osteosarcoma patients. Clin Cancer Res. (2023) 29:2085–94. doi: 10.1158/1078-0432.CCR-22-2912
29. Selva-O’Callaghan A, Ros J, Gil-Vila A, Vila-Pijoan G, Trallero-Araguás E, and Pinal-Fernandez I. Malignancy and myositis, from molecular mimicry to tumor infiltrating lymphocytes. Neuromuscul Disord. (2019) 29:819–25. doi: 10.1016/j.nmd.2019.09.014
Keywords: cell-free DNA, dermatomyositis, fragmentation, 5’ end-motif, genetic variance
Citation: Tang Z-l, Chen P-y, Zhang H, Cao H-l, Dai R, Lou Y-c, Sun Y-h, Zhou Y, Chen X-y, Zhang M-j, Wang Y-q and Man X-y (2025) Cell-free DNA profiles of dermatomyositis and its potential role in discriminating phenotypes. Front. Immunol. 16:1605121. doi: 10.3389/fimmu.2025.1605121
Received: 02 April 2025; Accepted: 28 May 2025;
Published: 17 June 2025.
Edited by:
Zhanjun Guo, Fourth Hospital of Hebei Medical University, ChinaReviewed by:
Shuang Ye, Shanghai Jiao Tong University, ChinaHuifang Guo, Second Hospital of Hebei Medical University, China
Copyright © 2025 Tang, Chen, Zhang, Cao, Dai, Lou, Sun, Zhou, Chen, Zhang, Wang and Man. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-yong Man, bWFueHlAemp1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Zhuang-li Tang
Zhuang-li Tang Peng-yu Chen†
Peng-yu Chen† Hua-li Cao
Hua-li Cao Ru Dai
Ru Dai Yuan Zhou
Yuan Zhou Xue-yan Chen
Xue-yan Chen Xiao-yong Man
Xiao-yong Man