- 1College of Animal Science and Technology, Jilin Agricultural University-Borui Dairy Science and Technology R&D Center, Key Laboratory of Animal Nutrition and Feed Science of Jilin Province, Key Laboratory of Animal Production Product Quality and Security Ministry of Education, Jilin Agricultural University, Changchun, China
- 2College of Animal Science and Technology, Ningxia University, Yinchuan, China
- 3Postdoctoral Scientific Research Workstation, Feed Engineering Technology Research Center of Jilin Province, Changchun Borui Science & Technology Co., Ltd., Changchun, China
- 4College of Agriculture and Natural Resources, Dilla University, Dilla, Ethiopia
- 5College of Life Science, Jilin Agricultural University, Changchun, China
- 6Tongliao Agricultural and Animal Husbandry Science Research Institute, Tongliao, China
- 7Ningxia Agricultural Reclamation Helan Mountain Dairy Co., Ltd., Yinchuan, China
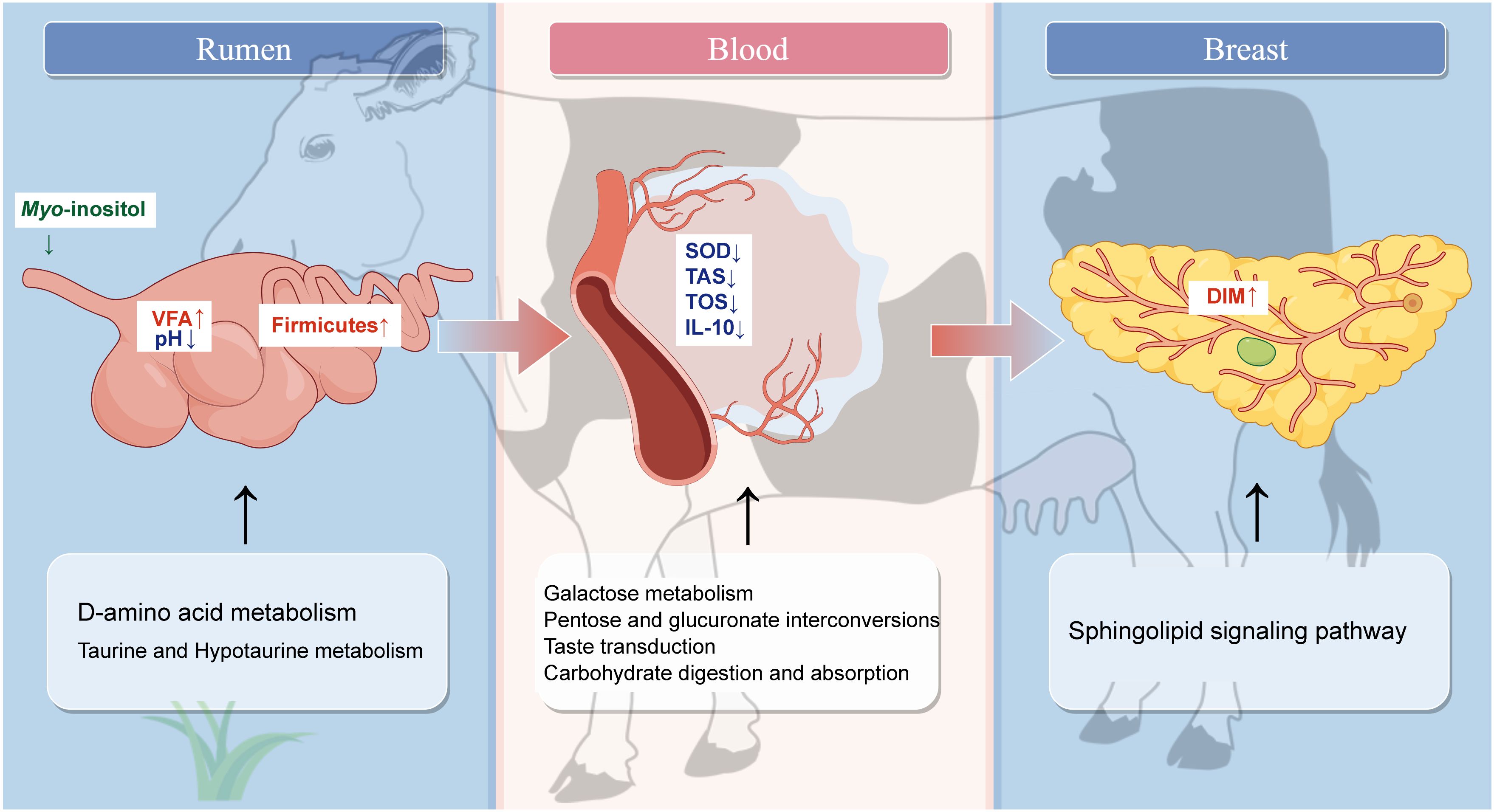
Introduction: Yeast culture (YC) has become a crucial microecological preparation for regulating the ruminal environment and improving ruminant health. Myo-inositol is an effective substance in YC, our study aimed to investigate whether myo-inositol can regulate metabolism and influence the health of dairy cows. Therefore, this study explored the effects of myo-inositol on the health, metabolome, and lactation performance of peripartum dairy cows using both in vitro and in vivo models.
Methods: The ANKOM RFS gas production system was used for the in vitro experiment; Twenty four healthy Holstein cows were assigned to CON group (n = 4; no supplementation of YC or myo-inositol), YC group (n = 10; 2 g/kg YC), and IN65 group (n = 10; 2 g/kg YC + 16 mg/kg myo-inositol) for in vivo experiment.
Results: The concentrations of five volatile fatty acids (acetic acid, butyric acid, isobutyric acid, valeric acid, and isovaleric acid), and total volatile fatty acids were significantly higher in the IN65 group compared to the CON group at 6 h in vitro (P < 0.01). Therefore, 2 g/kg YC plus 16 mg/kg myo-inositol was selected for in vivo experiment. It was found that the average milk production of the IN65 group increased by 4.00% and 4.05% respectively, compared to the control group and YC group on day 21 postpartum. The levels of superoxide dismutase (SOD), total antioxidant status (TAS), and total oxidative status (TOS) were significantly higher on day 21 compared to day 1 postpartum (P < 0.01). However, a trend indicating a decrease in immune function was observed in the IN65 group compared to the CON group. These findings suggested that myo-inositol may improve the health of postpartum dairy cows. Additionally, Mantel analysis showed acetic acid was positively correlated with D-glyceric acid and xanthosine. Similarly, D-glyceric acid, inosine 5-monophosphate, and xanthosine were positively correlated with microbiota at the genus, such as Bulleidia and Moryella.
Discussion: In summary, myo-inositol influences energy metabolism by modulating rumen microbial abundance, improving dairy cow health, and increasing milk production.
1 Introduction
The peripartum period of dairy cows lasts from three weeks before calving to three weeks after calving. This period includes pregnancy, calving, and lactation (1). During this period, the nutritional demands of dairy cows often exceed available nutrients, leading to a negative energy balance (2) and peripartum metabolic disorders (3). This condition is characterized by decreased glucose and insulin levels (4), increased growth hormone concentration (5), excessive mobilization of fat, proteins, glycogen, and minerals; alterations in immune function; and reduced feed intake. During this period dairy cows are immunosuppressive state, making them highly susceptible to diseases such as milk fever, retained placenta, metritis, ketosis, abomasum displacement, lameness, and clinical mastitis (6). Changes in immune cell populations and cytokines, along with reduced antibody levels, contribute to this susceptibility. Suppressive T cells are transported preferentially during calving (7), and postpartum cows rely on T cells for disease resistance (8).
Yeast culture (YC) is a product derived from active yeast through fermentation, concentration, and drying processes. Active yeasts are considered to have probiotic effects, while YC components are believed to have both probiotic and prebiotic effects (9). YC is composed of yeast cell walls (β-glucans and mannan oligosaccharides), soluble cellular substances, vitamins, proteins, peptides, AA, nucleotides, lipids, organic acids, oligosaccharides, esters, and alcohols (10). It can help maintain a healthy rumen environment by regulating pH and reducing the risk of ruminal acidosis (11). Additionally, YC can help balance rumen microbiota (12) and significantly improve the ruminal environment. Over a century and a half ago, German chemist Joseph Scherer first isolated myo-inositol from muscle tissue and named it after the Greek word for muscle (13). Myo-inositol consists of nine isomers, with myo-inositol being the most commonly found isomer (14). Different inositol compounds have significant biochemical importance. The enzymes IRE1α and JNK, which rely on inositol, are involved in metabolic regulation. Targeting the IRE1α-JNK axis can help regulate lipogenesis and fatty acid oxidation, leading to a decrease in lipid accumulation induced by non-esterified fatty acids in bovine hepatocytes (15). As a result, inositol and its components affect fat metabolism in dairy cows. Although there have been many studies on myo-inositol in fish, there is a lack of research on ruminants, specifically peripartum dairy cows (16).
The effects of myo-inositol on the health and productivity of dairy cows during the peripartum period remain unknown. This study aimed to investigate the effects of myo-inositol supplementation on the health, metabolome, and lactation performance of peripartum dairy cows. The hypothesis was that myo-inositol would improve milk production and health of peripartum dairy cows.
2 Materials and methods
2.1 Experimental design and animal husbandry
Animal experiments, both in vitro and in vivo, were conducted in accordance with the guidelines approved by the Jilin Agricultural University Animal Research Ethics Committee (approval number: JLAU-ACUC2022-004).
2.1.1 In vitro experiment
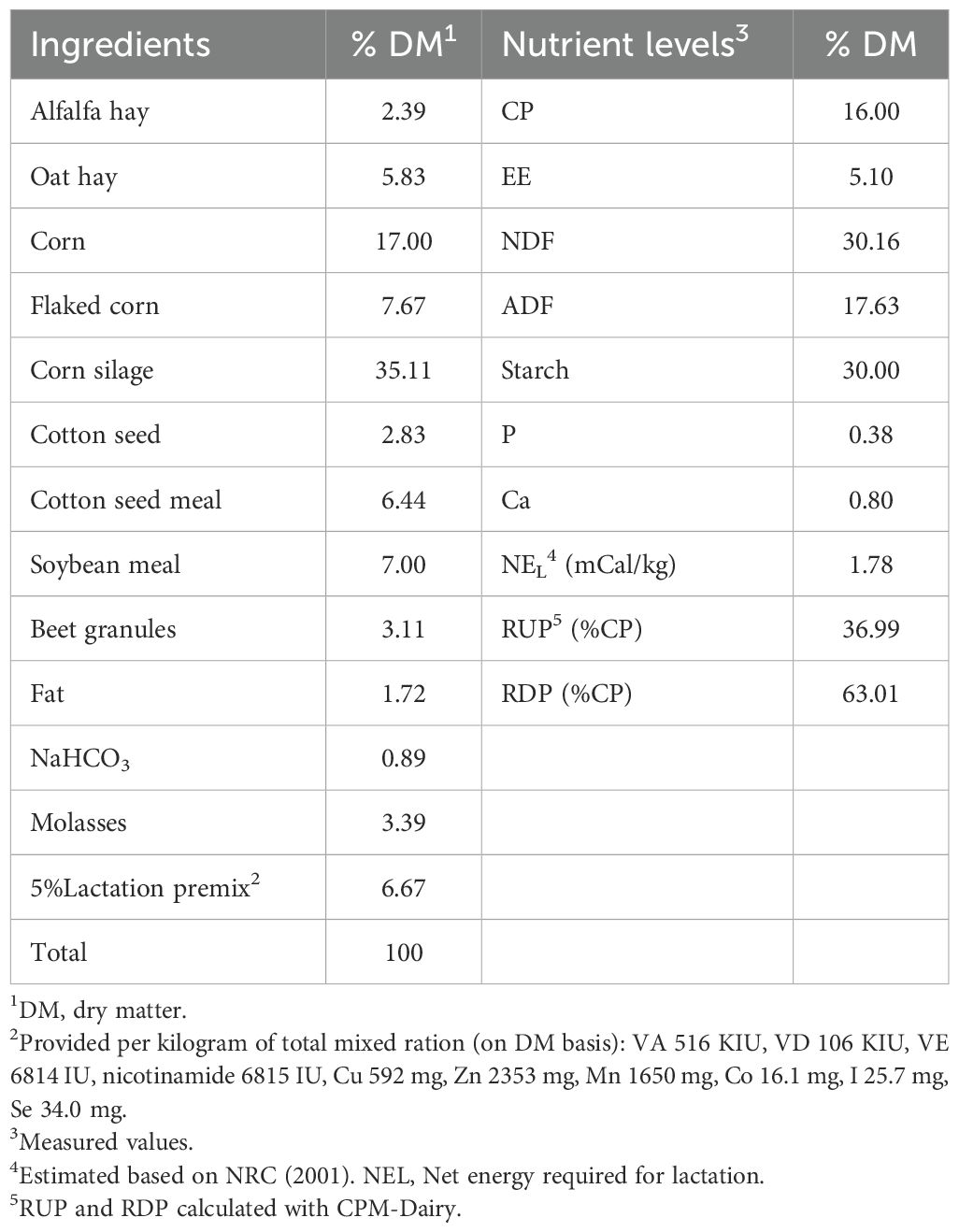
Ruminal fluid was collected from three dairy cows with ruminal fistulas, all of which had free access to clean water and fed a TMR. The in vitro experiment was followed a single-factor design and included six treatment groups: CON, YC, IN65, IN85, IN125, and IN205. The CON group did not supplement with any YC or myo-inositol. The YC group was supplemented with 2 g/kg YC (per kilogram of feed), the IN65 group with 2 g/kg YC (per kilogram of feed) + 16 mg/kg myo-inositol (per kilogram of feed), the IN85 group with 2 g/kg YC (per kilogram of feed) + 36 mg/kg myo-inositol (per kilogram of feed), the IN125 group with 2 g/kg YC (per kilogram of feed)+ 76 mg/kg myo-inositol (per kilogram of feed), and the IN205 group with 2 g/kg YC (per kilogram of feed) + 156 mg/kg myo-inositol (per kilogram of feed). Our previous studies found that the myo-inositol content was 49 mg in 2 g YC (17). Therefore, the myo-inositol levels in the CON, YC, IN65, IN85, IN125, and IN205 groups were 0, 49, 65, 85, 125, and 205 mg/kg, respectively. Details of the basal diets are presented in Table 1.
The myo-inositol (purity > 99%, CAS: 87-89-8) used in the in vitro experiments was purchased from Anhui Yuanzheng Biological (Anhui, China). Before morning feeding, rumen fluid was collected through ruminal cannulas from dairy cows, ensuring sampling from multiple rumen locations. Immediately after collection, rumen fluid was transferred into thermos bottles preheated to 39°C and pre-flushed with CO2 to maintain anaerobic conditions. In the laboratory, rumen fluid was filtered through four-layer cheesecloth and mixed with buffer solution at a ratio of 1:2, and then divided into culture bottles and incubated in a 40 °C water bath shaker (80 rpm), utilized 120 bottles [one substrates × six doses (0, 49, 65, 85, 125, and 205 mg/kg) × four time points (3, 6, 12, or 24 h) × five replicates]. Detailed experimental procedures and buffer solution preparation refer to Dong et al. (18).
2.1.2 In vivo experiment
In this study, 24 healthy Holstein cows in the early stages of pregnancy were carefully selected from a commercial dairy farm in Ning Xia, China, based on similar body condition scores (3.0 – 3.5) and met the necessary quarantine requirements. The feeding management and dietary composition were consistent with those used in the in vitro experiments. Twenty-four cows were randomly assigned to 3 groups as follows: CON (n = 4; no supplementation of YC or myo-inositol), YC (n = 10; 2 g/kg YC), and IN65 (n = 10; 2 g/kg YC + 16 mg/kg myo-inositol). The YC and myo-inositol were supplemented and mixed uniformly in the TMR. They were housed in large groups, and the barn was cleaned three times daily to provide ample drinking water, sodium bicarbonate, and salt. The cowshed is sufficiently spacious to allow the cows to move freely, and the temperature within the cowshed is maintained at 10°C ± 2°C.
2.2 Collection of rumen fluid samples for in vitro experiments
Rumen fermentation fluid samples were collected after 3, 6, 12, and 24 h of cultivation and divided into five parts: 1.5 mL for VFA concentration, 1 mL for ammonia nitrogen (NH3-N) concentration, 2 mL for microbial flora analysis, and 2 mL for metabolomics. The remaining fluid was used for pH determination. Samples for VFA and NH3-N determination were frozen at -40°C, while those for microbial flora and metabolomics were frozen at -80°C. Rumen fluid for VFA determination was centrifuged at 10,000 g for 10 minutes at 4°C. Subsequently, 1 mL of the supernatant and 0.2 mL of a 25% metaphosphate solution were added to a 2 mL centrifuge tube and allowed to precipitate overnight. Afterward, the mixture was centrifuged again at 10,000 g for 10 minutes at 4°C, and the supernatant was collected in a new 2 mL centrifuge tube for further analysis.
2.3 Collection of milk and serum samples for in vivo experiments
2.3.1 Milk
The cows were milked using an automated system, and milk production was recorded manually. Approximately 10 mL of milk was collected each time and stored in a liquid nitrogen tank. Milk samples were collected on the 1st, 7th, 14th, and 21st days after delivery. Before pretreatment, 1 mL of milk sample was collected at three time points (morning, mid-day, and evening) and mixed for metabolomic analysis.
2.3.2 Serum
Blood samples were collected from the tail vein on the day of calving and 21 days postpartum. The samples were processed in vacutainers to measure inflammation, immunity, antioxidant levels, and metabolome. After centrifugation at 3000 ×g for 15 min at 4°C, the samples were stored in liquid nitrogen for further analysis. Processing was completed within 30 min.
2.4 Determination of rumen fermentation parameters in vitro and serum
VFA concentrations were measured using a gas chromatograph (7890 B; Agilent Technologies, Santa Clara, CA, USA) (19). The pH was measured using a pH meter (MP523-04; Shanghai Sanxin Instrument Co. Ltd., Shanghai, China). NH3-N levels were quantified using a spectrophotometer (UV-1201, Shimadzu Corporation, Kyoto, Japan) (20).
Blood indicators including inflammatory ( IL-6, IL-10, IL-1β, TNF-α, and IFN-γ), immune (IgA and IgM), and oxidation/antioxidant (CAT, MDA, GSH-Px, SOD, TAS, and TOS). Indicators were measured using enzyme-linked immunosorbent assay kits (Lengton, Shanghai, China), and the tests were performed in accordance with the manufacturer’s instructions.
2.5 Determination of rumen microbial community in vitro
The bacterial 16S rRNA gene V3–V4 region was amplified by PCR using the forward primer (F): ACTCCTACGGGAGGCAGCA and reverse primer (R): GGACTACHVGGGTWTCTAAT. Sample-specific 7-bp barcodes were included in the primers for multiplex sequencing. The PCR mixture included 5 μL of buffer (5×), 0.25 μL of fast pfu DNA polymerase (5U/μL), 2 μL (2.5 mM) of dNTPs, 1 μL (10 µM) of each forward and reverse primer, 1 μL of DNA template, and 14.75 μL of ddH2O. The thermal cycling protocol consisted of an initial denaturation at 98°C for 5 min, followed by 25 cycles of denaturation at 98°C for 30 s, annealing at 53°C for 30 s, and extension at 72°C for 45 s, with a final extension at 72°C for 5 min. PCR amplicons were purified using Vazyme VAHTSTM DNA Clean Beads (Vazyme, Nanjing, China), quantified with a Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen, Carlsbad, CA, USA), and then pooled in equal amounts for pair-end 250-bp sequencing on the Illumina NovaSeq platform with the NovaSeq 6000 SP Reagent Kit (500 cycles) at Shanghai Personal Biotechnology Co., Ltd (Shanghai, China). Tax4Fun was employed to predict functional characteristics consolidated according to the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway classification system. The data presented in the study are deposited in the NCBl Sequence Read Archive repository, accession number PRJNA1101182.
2.6 Metabolomics analysis methods
The sample (100 μL) was mixed with an extraction solution (MeOH: ACN, 1:1[v/v], 400 μL) containing deuterated internal standards. The mixture was vortexed for 30 s, sonicated for 10 min in a 4°C water bath, and incubated for 1 h at 40°C for 1 h to precipitate proteins. After centrifugation at 12000 rpm (RCF=13800 [× g], R=8.6 cm) for 15 min at 4°C, the supernatant was transferred to a fresh glass vial for further analysis. Quality control samples were prepared by mixing equal aliquots of the supernatants.
LC-MS analyses were performed using an Ultra-High Performance Liquid Chromatography system (Vanquish, Thermo Fisher Scientific) with a Waters ACQUITY UPLC BEH Amide (2.1 mm × 50 mm, 1.7 μm) coupled to Orbitrap Exploris 120 mass spectrometer (Orbitrap MS, Thermo). The mobile phase consisted of 25 mmol/L ammonium acetate and 25 mmol/L ammonia hydroxide in water (pH 9.75) (A) and acetonitrile (B). The auto-sampler temperature was 4°C, and the injection volume was 2 μL. An Orbitrap Exploris 120 mass spectrometer was used to acquire MS/MS spectra in information-dependent acquisition mode under the control of an acquisition software (Xcalibur, Thermo). In this mode, the acquisition software continuously evaluated the full-scan MS spectrum. The Electrospray ionization source conditions were set as follows: sheath gas flow rate at 50 Arb, Aux gas flow rate at 15 Arb, capillary temperature at 320°C, full MS resolution at 60000, MS/MS resolution at 15000, collision energy: SNCE 20/30/40, and spray voltage at 3.8 kV (positive) or 3.4 kV (negative), respectively.
The raw data were converted to the mzXML format using ProteoWizard and subsequently processed with an in-house program developed in R, which is based on XCMS for peak detection, extraction, alignment, and integration.
2.7 Statistical analysis
Data were analyzed using SPSS Statistics software (version 22.0, IBM). The experiment used a one-way analysis of variance for data analysis. Spearman’s correlation coefficient was utilized to analyze the correlation between microbial communities at the genus level and fermentation indicators, and Mantel’s test was used to explore the relationship between differential metabolites, microbial communities, and fermentation indicators.
3 Results
3.1 Analysis of rumen fermentation parameters in vitro
The effects of myo-inositol on in vitro ruminal fermentation are summarized in Table 2. After 3 h of in vitro fermentation, the pH of the YC, IN65, IN85, and IN205 groups were significantly lower than the CON group (P < 0.01). After 6 h of in vitro fermentation, the pH of the IN85 and IN205 groups were significantly lower compared to the CON group (P < 0.05). After 12 h of in vitro fermentation, the pH of the IN125 group was significantly lower than that of the YC group (P < 0.01). Following 24 h of in vitro fermentation, the pH of the YC, IN65, and IN85 groups were significantly higher than that of the CON group (P < 0.01).
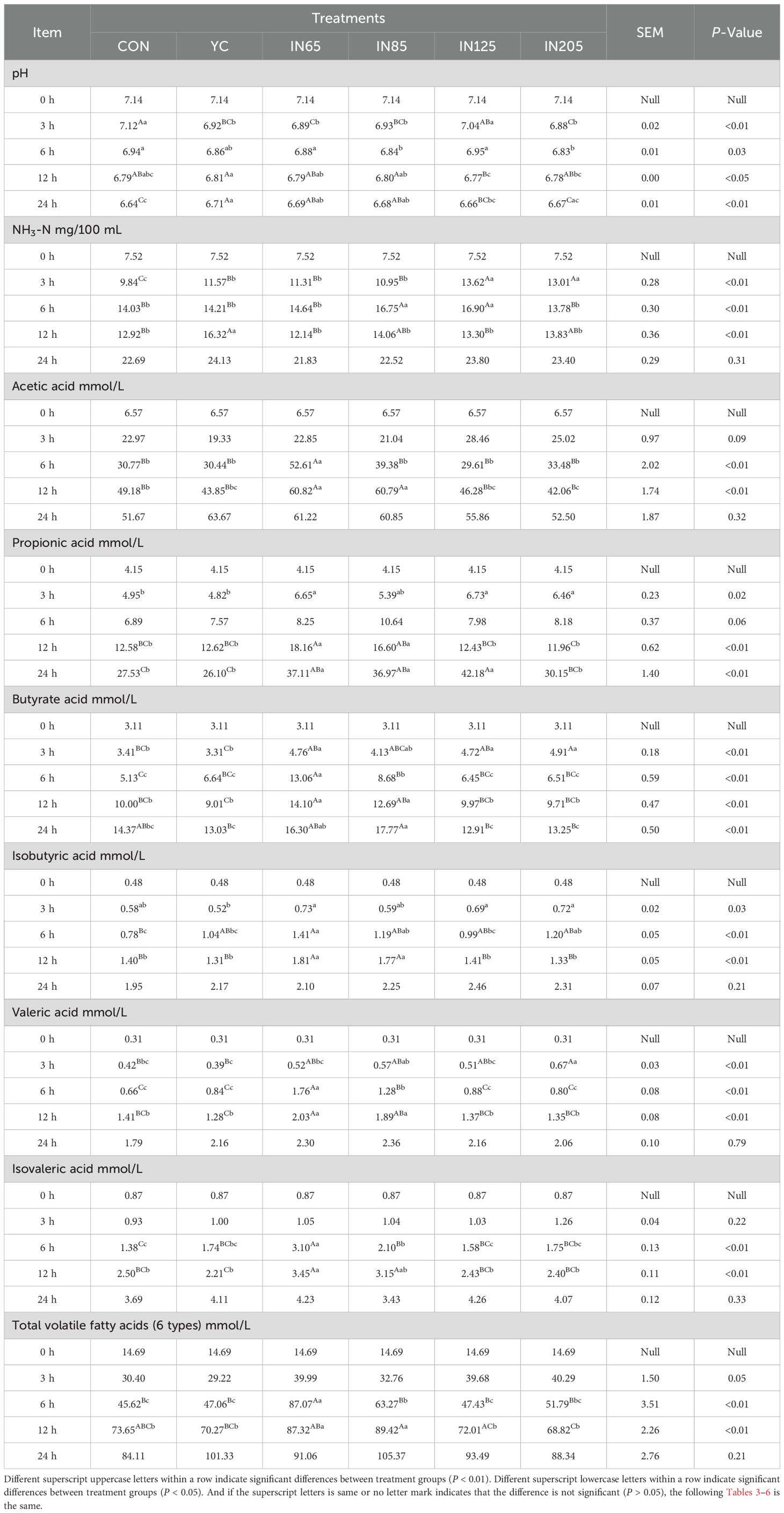
Table 2. Effects of YC and myo-inositol supplementation on pH, NH3-N (mg/100 mL) and VFAs concentration (mmol/L) in vitro.
After 3 h of in vitro fermentation, NH3-N concentrations in YC, IN65, IN85, IN125, and IN205 were significantly higher than those in the CON group (P < 0.01), with NH3-N concentrations in IN125 and IN205 being significantly higher than those in the YC group (P < 0.01). After 6 h of in vitro fermentation, NH3-N concentrations in IN85 and IN125 were significantly higher than those in the CON and YC groups (P < 0.01). After 12 h of in vitro fermentation, the NH3-N concentration in the YC group was significantly higher than that in the CON group (P < 0.01). Notably, after 6 h of in vitro fermentation, the acetic acid/propionic acid ratio (A/P ratio), six VFA, 3type TVFA and 6type TVFA in the IN65 group were significantly higher than those in the CON group (Table 2; Figures 1A, B).
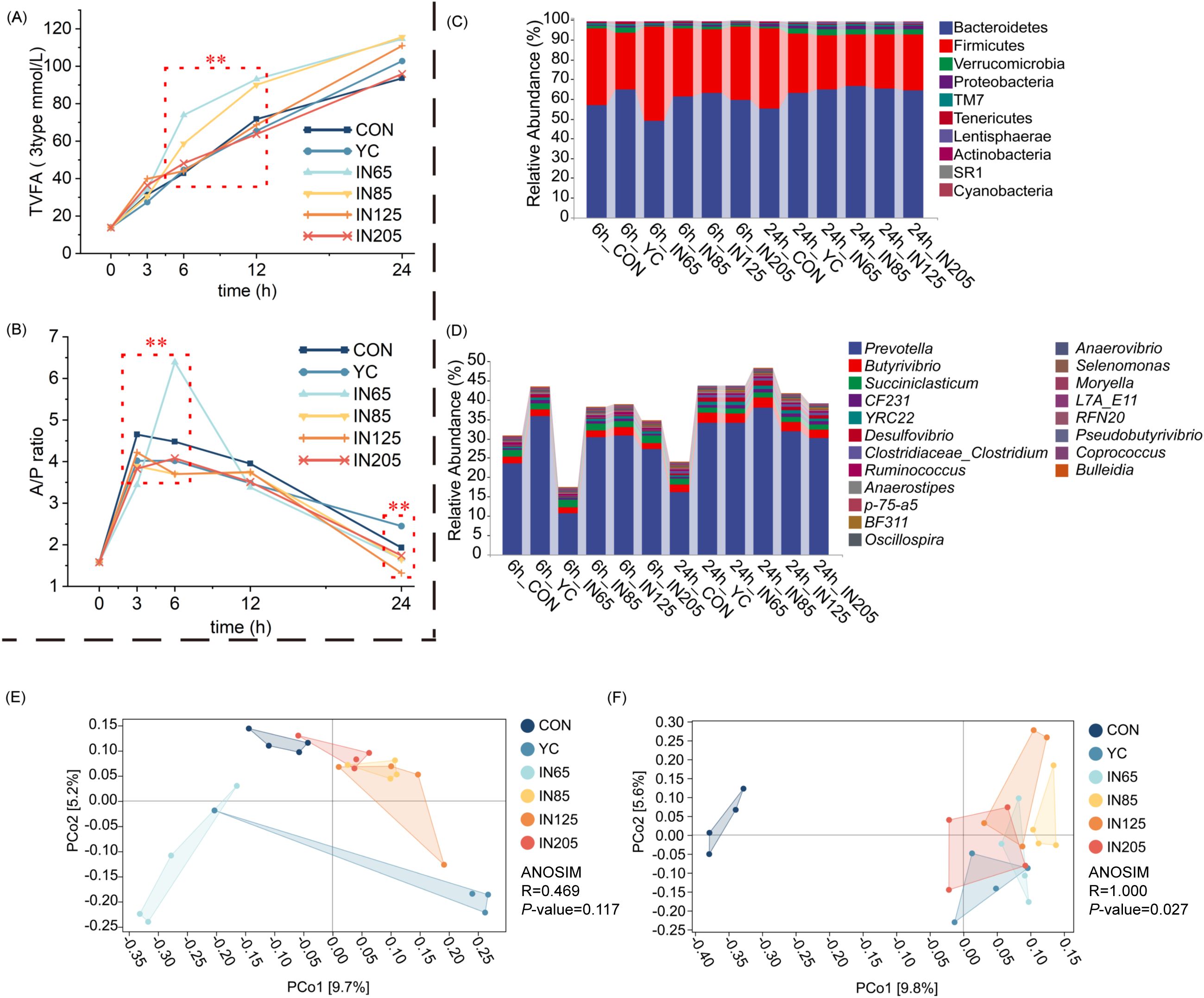
Figure 1. Effects of YC and myo-inositol supplementation on TVFA (3 types) (A), A/P ratio (B), ruminal bacterial community at phylum levels (C) and genus levels (D), the beta diversity at 6 h (E) and 24 h (F) in vitro.
3.2 Analysis of rumen microbial composition in vitro
Alpha diversity analysis was conducted to assess the effect of YC and myo-inositol on rumen microbial abundance. The Chao1, Faith_pd, Shannon, and Simpson indices were used to evaluate the richness, diversity, and evenness of the microbiota. After 6 h of in vitro fermentation, the Shannon index was significantly higher in the IN65 group compared to the YC group (P < 0.05). After 24 h of in vitro fermentation, the Chao1 and Faith_pd indices were significantly lower in the IN85 and IN125 groups compared to the YC group (P < 0.01). The Simpson indices of the YC, IN65, IN85, IN125, and IN205 groups were all significantly higher than the CON group, with the IN85 group showing a significantly lower Simpson index than the YC group (P < 0.01; Table 3).
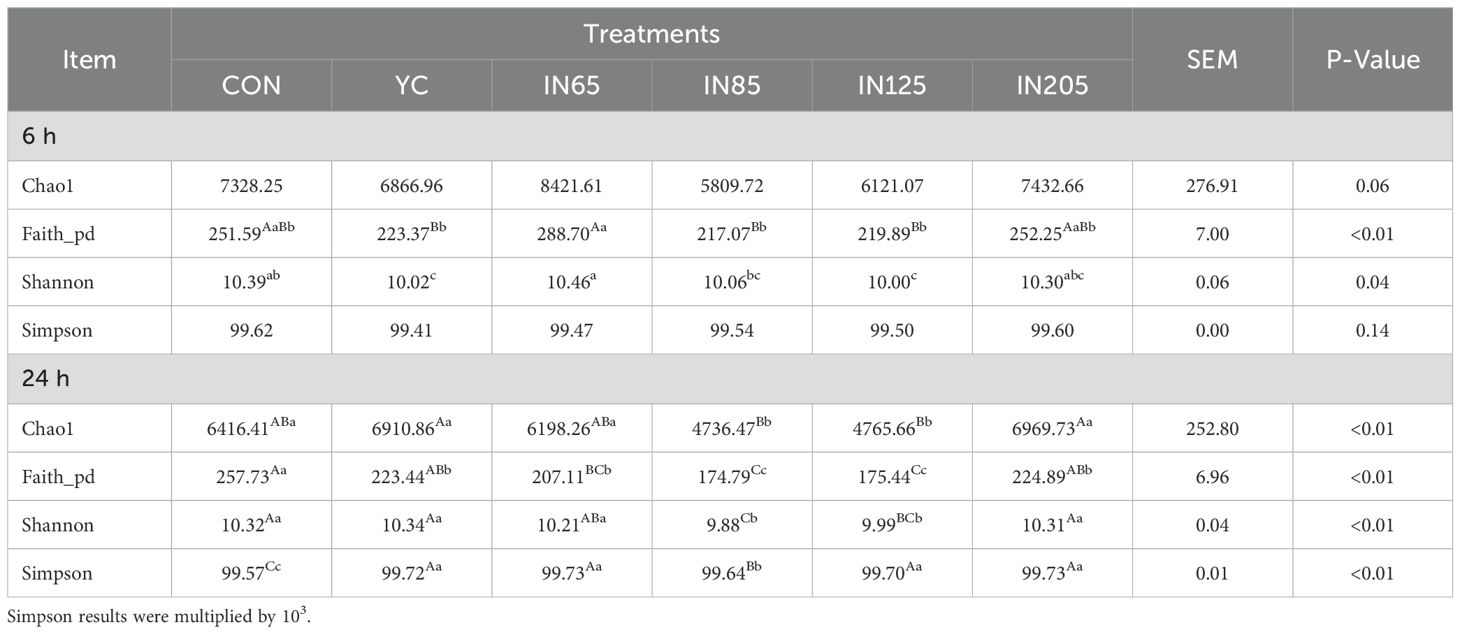
Table 3. Effects of YC and myo-inositol supplementation on alpha diversity of rumen bacterial communities in vitro.
The 16S rRNA gene was sequenced using amplicon sequencing, resulting in 3,473,478 high-quality sequences. On average, each sample had 289,457 sequences. The main microbial phyla consisted of 10 taxa with relative abundances > 0.10%. The most abundant phyla were Bacteroidetes (61.10%), Firmicutes (33.29%), Verrucomicrobia (1.84%), and Proteobacteria (1.11%) (Figure 1C). At the genus level, the main microbiota included 20 genera with relative abundances exceeding 0.10%. The most abundant genera were Prevotella (28.51%), Butyrivibrio (2.10%), and Succinivibrio (1.51%) (figure 1D).
As shown in Table 4, the microbiota at the phylum level were significantly affected by YC and myo-inositol. After 6 h of in vitro fermentation, the Firmicutes abundance in the IN65 group was significantly higher compared to the YC group (P < 0.01), whereas the relative abundances of Bacteroidetes and Lentisphaerae were significantly lower than those in the YC group (P < 0.01). After 24 h in vitro fermentation, the IN65 group showed significantly higher relative abundances of Bacteroidetes, Verrucomicrobia, Proteobacteria, and Lentisphaerae compared to the CON group (P < 0.01), whereas the relative abundances of Firmicutes, and SR1 were significantly lower than that in the CON group (P < 0.01). As shown in Table 5, at 6 h of in vitro, the IN65 group showed a significantly higher relative abundance of Moryella at the genus level compared to the YC group and CON group (P < 0.01); at 24 h of in vitro, the relative abundances of Prevotella, CF231, BF311, YRC22, Selenomonas, and Desulfovibrio in the IN65 group were significantly higher than that in the CON group (P < 0.01).
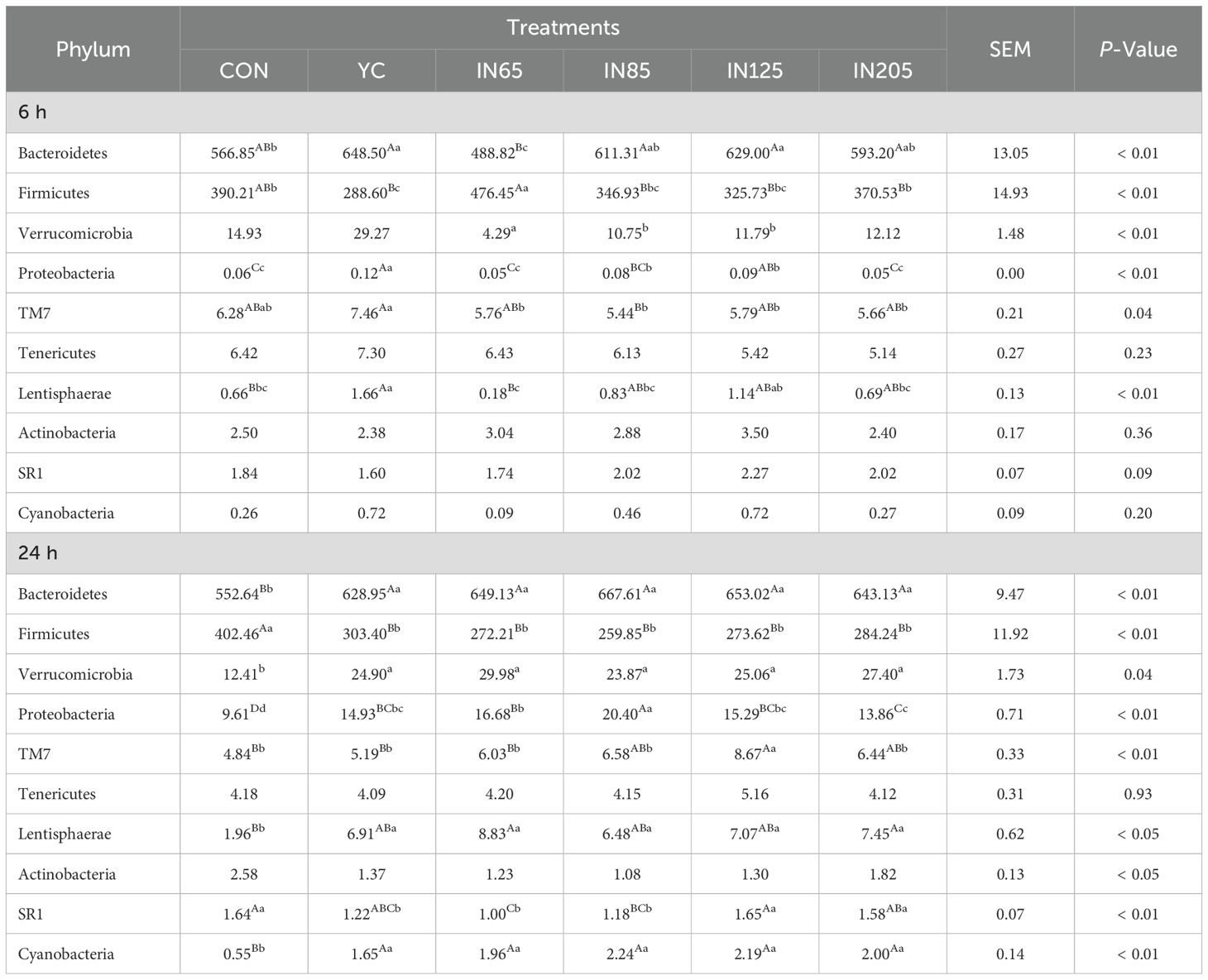
Table 4. Effects of YC and myo-inositol supplementation on rumen bacterial communities at phylum levels in vitro.
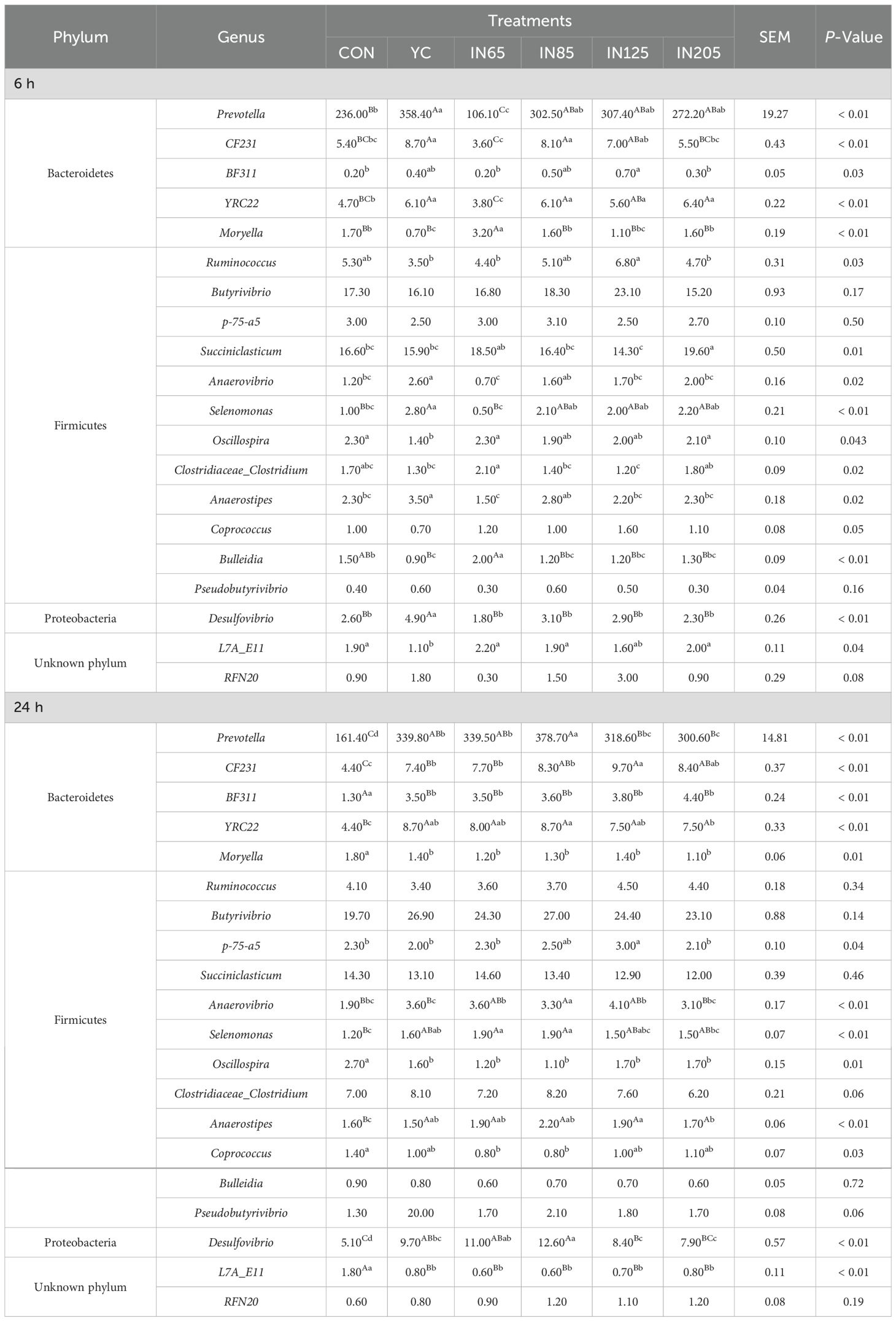
Table 5. Effects of YC and myo-inositol supplementation on ruminal bacterial community at genus levels in vitro.
Jaccard dissimilarity analysis showed significant differences in microbiota composition among six groups with the same fermentation time. The addition of YC and myo-inositol affected the richness, diversity, and uniformity of the rumen microbial communities. At 6 h of in vitro fermentation, the YC group closely resembled was close to the IN65 group, while the other four groups clustered together, suggesting that adding a small amount of myo-inositol and a specific amount of YC had a significant effect on rumen microorganisms. The IN85 group, IN125 group, IN205 group, and YC group were similar to the CON group, indicating a minimal effect of these four groups on rumen microorganisms. At 24 h of in vitro, the treatment groups with myo-inositol addition showed similarities but differed significantly from the CON group, suggesting little variation among the IN65 group, the IN85 group, the IN125 group, and the IN205 group, and a large difference between CON and the treatment groups with myo-inositol addition (Figures 1E, F).
3.3 Effects of myo-inositol on milk yield and serum parameters
At 21 days postpartum, compared with the control group and YC group, a numerically higher milk yield (+4.00% and +4.05%) was observed in the IN65 group, though statistical significance was not achieved due to limited sample size and high variability (Figure 2A). Table 6 shows no significant differences in oxidation, immune, or inflammatory indicators at calving. On the 21 days postpartum, GSH-Px level in the IN65 group was significantly lower compared to the YC and CON groups (P < 0.01), and SOD level was significantly lower than that in the CON group (P < 0.01). These findings suggested that myo-inositol affects oxidative stress status in peripartum dairy cows. Following a period of YC or myo-inositol feeding, TNF-α levels in the IN65 group were significantly lower than those in the YC group (P < 0.01), and IgM levels in the YC group were significantly higher than those in the CON group (P < 0.01). From day 1 to day 21 postpartum, SOD, TAS, TOS and IL-10 levels in the CON group showed a significant increase (P < 0.01). Notably, this trend was significantly mitigated after the addition of myo-inositol (Table 7). The findings indicate that myo-inositol affected oxidative stress in peripartum dairy cows (Figure 2B).
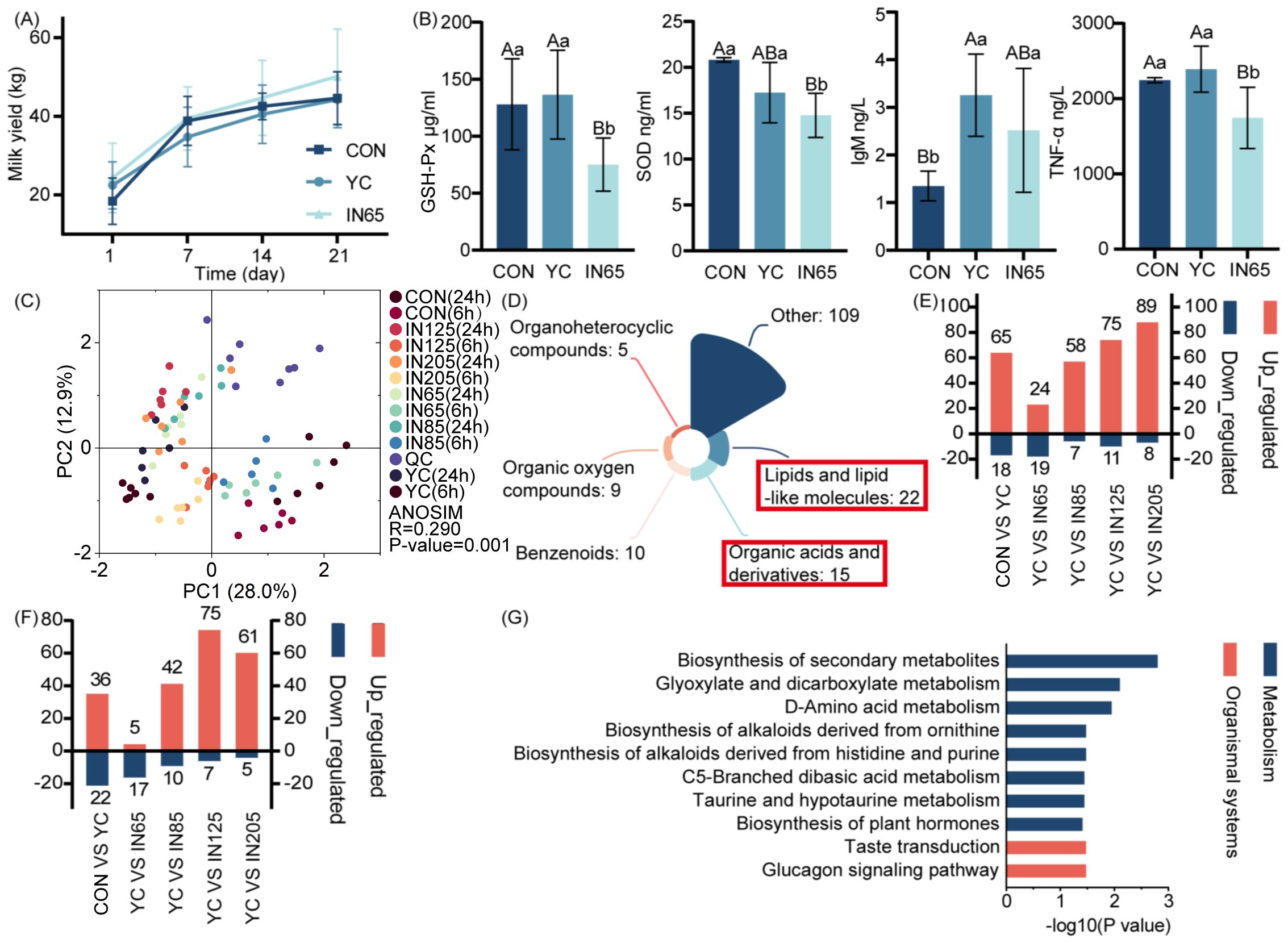
Figure 2. Effects of YC and myo-inositol supplementation on milk yields (A), GSH-Px, SOD, TNF-α levels in serum (B), rumen fluid metabolism PCA (C), super class of differential metabolites of the rumen fluid in vitro (D), up-regulated and down-regulated metabolites of rumen fluid metabolomics at 6 h (E) and 24 h (F), KEGG pathway enriched by differential metabolites in the myo-inositol treatment groups compared with YC group in vitro (G).
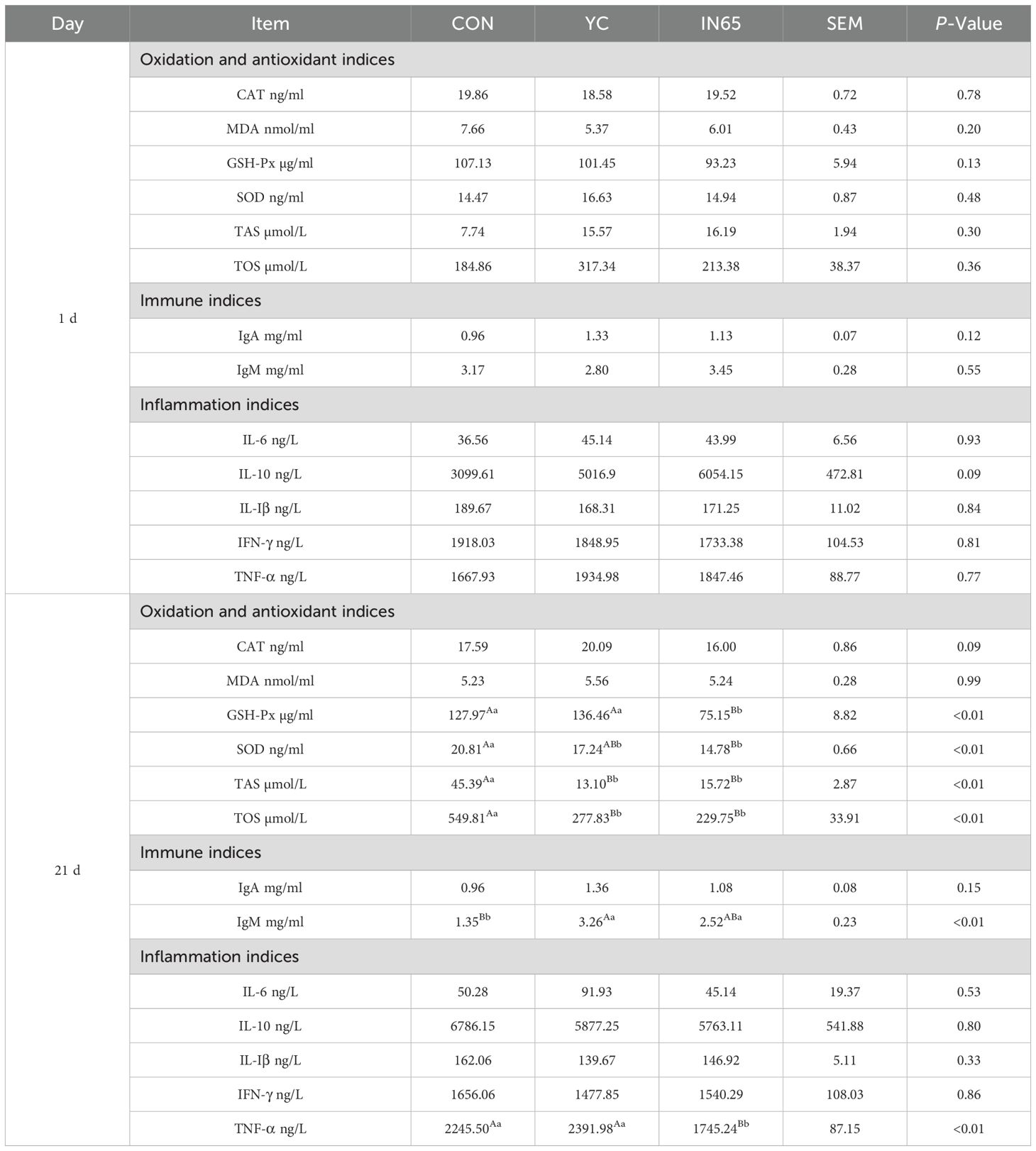
Table 6. Oxidation and antioxidant, immune, and inflammation indices in serum of CON cows, YC cows and IN65 cows.
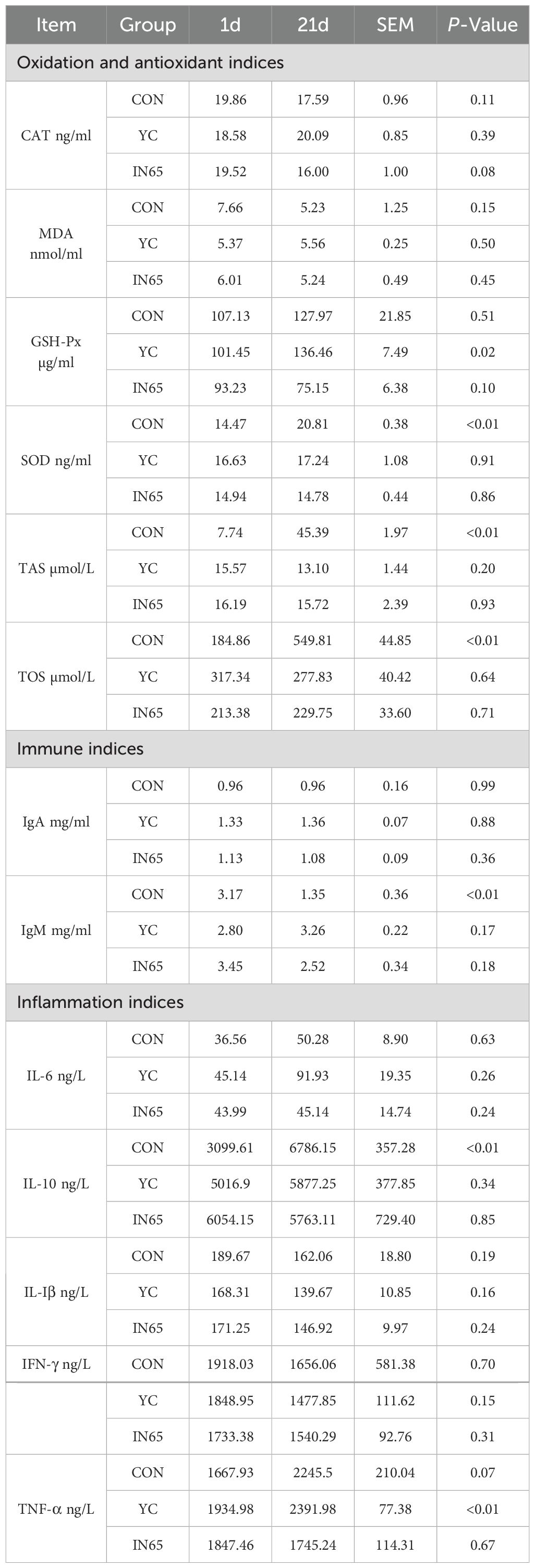
Table 7. Oxidation and antioxidant, immune, and inflammation indices in serum of CON cows, YC cows and IN65 cows from D 1 and D 21 postpartum.
3.4 Analysis of rumen fluid metabolite and pathway in vitro
Principal component analysis (PCA) revealed a clear separation of metabolites at 6 h and 24 h (Figure 2C). A total of 170 differential metabolites were identified between the 6 h and 24 h groups compared to the CON group, with variable importance in projection (VIP) > 1 and P < 0.05. These metabolites were lipids and lipid-like molecules and organic acids and derivatives (Figure 2D). At 6 h of in vitro fermentation, 65 metabolites were upregulated in the YC group compared to the CON group (Figure 2E). At 24 h of in vitro fermentation, 36 differential metabolites were found to be upregulated (Figure 2F).
The study highlights the need for a comprehensive evaluation of the impact of YC and myo-inositol on ruminal fermentation in dairy cows. Further analysis of rumen metabolites and their pathways revealed key metabolic pathways with significant impact. Enrichment analysis identified pathways with P < 0.05 and impact > 0.2 as crucial in understanding the effects of these supplements.
The effects of supplementing myo-inositol on in vitro rumen fermentation in dairy cows were shown by examining the differential metabolites and enriched metabolic pathways between the YC and CON groups. After 6 h of in vitro fermentation, analysis of Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways showed that YC mainly affects the biosynthesis of plant secondary metabolites and unsaturated fatty acids. During 24 h of in vitro fermentation, KEGG pathway enrichment analysis demonstrated that YC primarily affected the biosynthesis of plant secondary metabolites, terpenoids, and steroids.
Analysis of the differential metabolites and enriched differential metabolic pathways between the YC group and the myo-inositol-supplemented (IN65, IN85, IN125, and IN205) groups indicated that the inclusion of myo-inositol in the diet affected rumen fermentation in dairy cows. KEGG pathway enrichment analysis after 6 h of in vitro fermentation revealed that myo-inositol mainly affected AA metabolism, particularly D-amino acid metabolism, and taurine and hypotaurine metabolism (Figure 2G). After 24 h of in vitro fermentation, KEGG pathway enrichment analysis indicated that myo-inositol predominantly affected styrene degradation and fatty acid biosynthesis.
3.5 Differential serum metabolite and pathway analysis
PCA analysis showed a clear separation between metabolites from cows at day 1 and day 21 postpartum (Figure 3A). In the serum samples of the three groups of dairy cows 21 days after calving, 307 differential metabolites with VIP > 1 and P < 0.05 were identified and then classified. The most abundant differential metabolites included lipids and lipid-like molecules, and organic acids and their derivatives (Figure 3B). In comparison to the CON group, the YC group had 62 upregulated and 141 downregulated differential metabolites (Figure 3C). Metabolic pathways with P < 0.05 and impact > 0.2, such as galactose metabolism and other differential metabolic pathways, were identified through enrichment analysis of the pathways where the differential metabolites were located (Figure 3E).
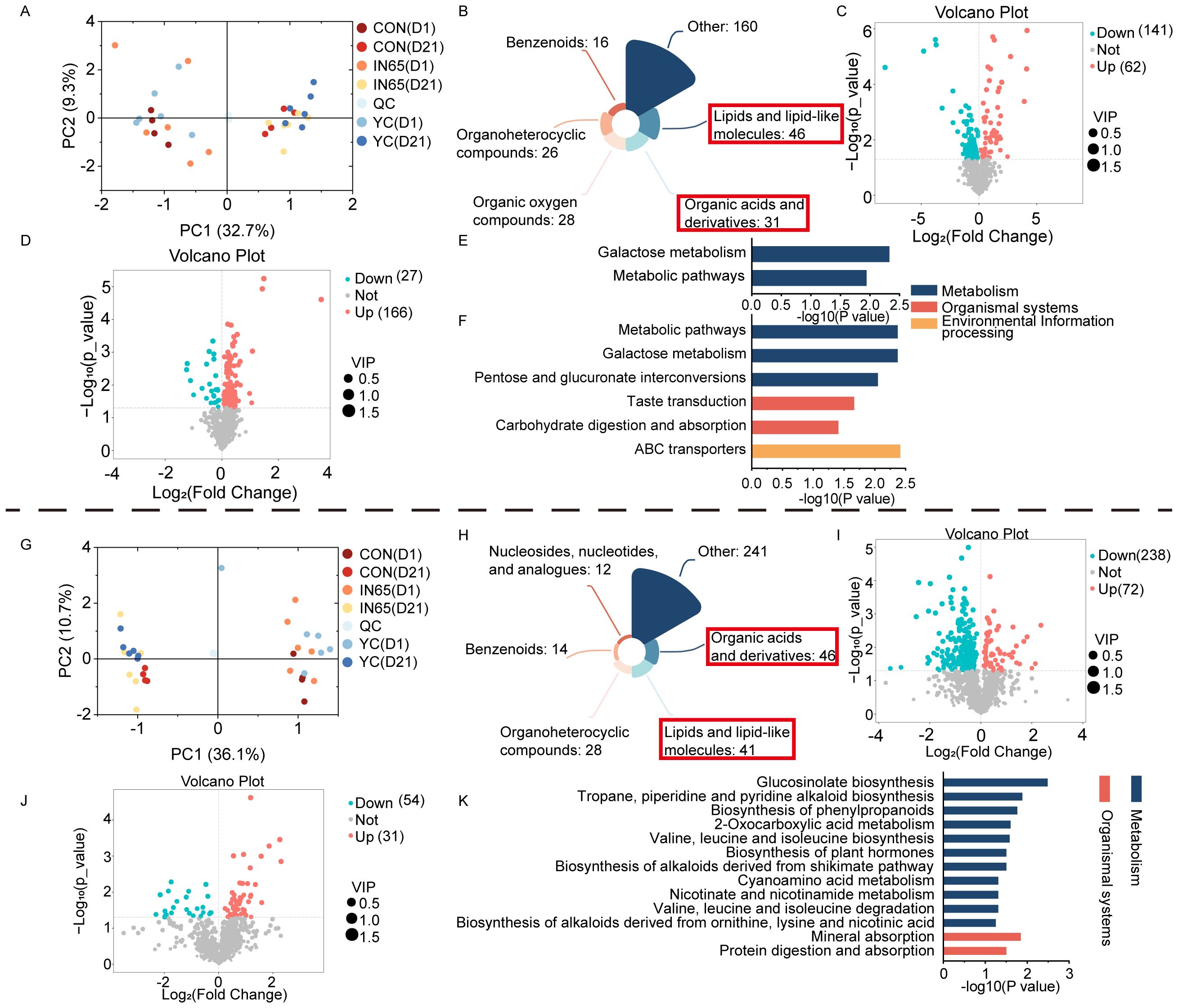
Figure 3. Serum metabolism PCA (A), super class of differential serum metabolites (B), volcano maps of serum metabolites in the YC vs CON (C) and IN65 vs YC (D), significantly different KEGG pathways of serum in the YC vs CON (E) and IN65 vs CON (F), milk metabolism PCA (G), super class of differential milk metabolites (H), volcano maps of milk metabolites in the YC vs CON (I) and IN65 vs YC (J), significantly different KEGG pathways of milk in the IN65 vs CON (K).
In the IN65 group, 166 differential metabolites were upregulated and 27 were downregulated compared to the YC group (Figure 3D). Enrichment analysis of the differential metabolites between IN65 and CON showed significant metabolic pathways such as galactose metabolism, pentose and glucuronate interconversions, taste transduction, and carbohydrate digestion and absorption (Figure 3F).
3.6 Differential milk metabolite and pathway analysis
PCA analysis showed a clear separation between metabolites from cows at day 1 and day 21 postpartum (Figure 3G). In the milk samples collected from the three groups of cows 21 days after calving, a total of 382 differential metabolites with VIP > 1 and P < 0.05 were screened and then classified. The most differentially metabolites included organic acids and derivatives, lipids and lipid-like molecules (Figure 3H). In comparison to the CON group, the YC group had 72 upregulated and 238 downregulated differential metabolites (Figure 3I). In comparison to the YC group, the IN65 group had 54 upregulated and 31 downregulated differential metabolites (Figure 3J). Enrichment analysis of differential metabolites between the CON and YC groups revealed significant metabolic pathways with P < 0.05 and impact > 0.2, such as glucosinolate biosynthesis and tropane, piperidine, and pyridine alkaloid biosynthesis (Figure 3K). Additionally, metabolic pathways with P < 0.05 and impact > 0.2 were selected through enrichment analysis, including the sphingolipid signaling pathway.
Correlation analyses were performed between the differential metabolites in rumen fluid, fermentation indicators, and rumen bacteria (Figure 4). Acetic acid was positively correlated with D-glyceric acid and xanthosine (Figures 4A, C). Similarly, D-glyceric acid, inosine 5-monophosphate, and xanthosine were positively correlated with genus, such as Bulleidia and Moryella (Figures 4D, F). This suggested that myo-inositol affects the production of acetic acid and butyric acid, by regulating the composition of rumen bacteria and their metabolism.
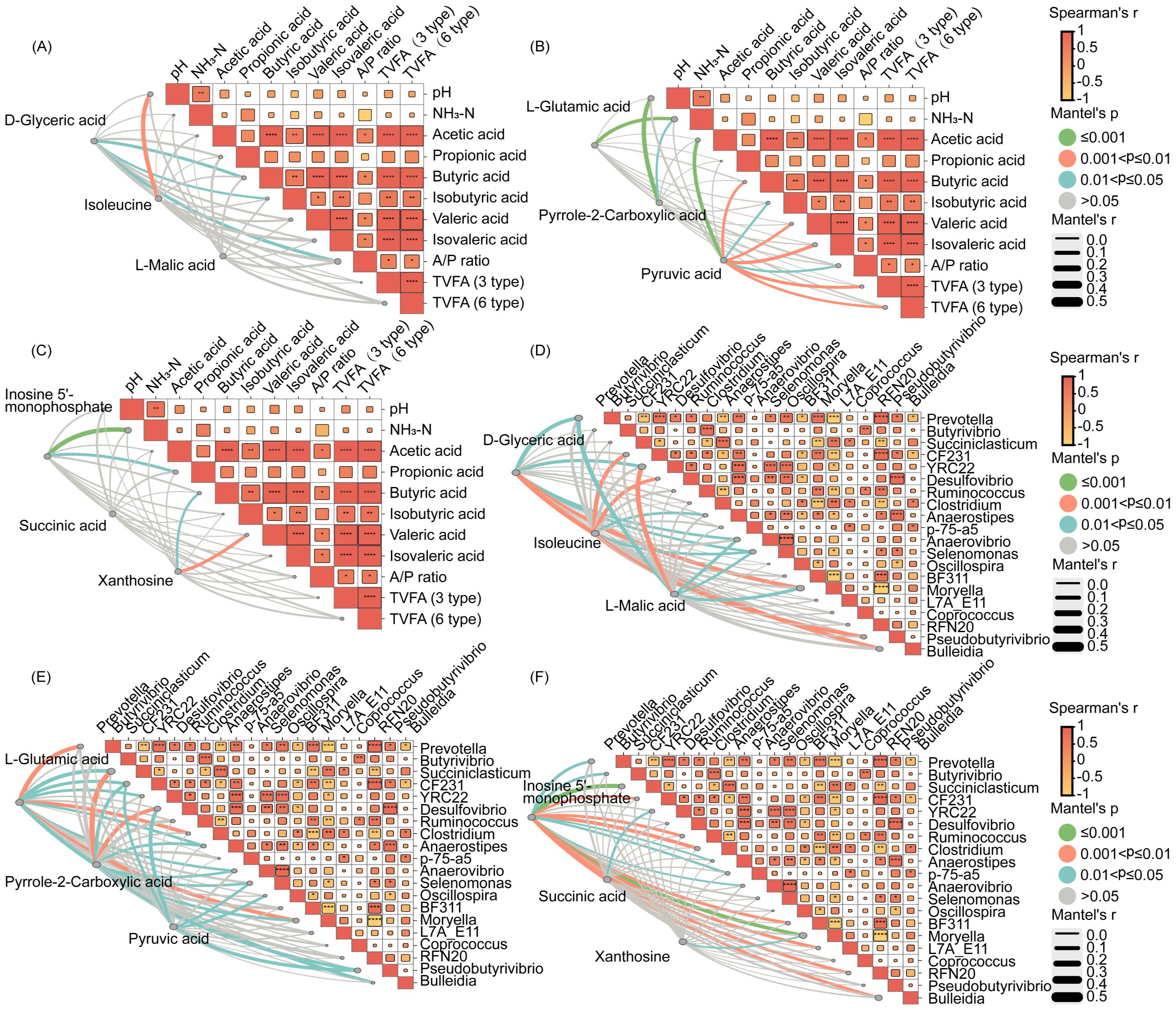
Figure 4. Correlation between fermentation index was analyzed by the Spearman correlation analyses, correlation between fermentation index indicators and nine differential metabolites was analyzed by the Mantel test (A–C). Correlation between microbial genera was analyzed by the Spearman correlation analyses, correlation between nine differential metabolites indicators and microbial genera was analyzed by the Mantel test (D–F).
4 Discussion
YC is widely used to regulate the ruminal environment in dairy cows. However, as a functional substance in YC, myo-inositol is primarily used in aquaculture and is rarely in ruminants. Our research findings indicated that the myo-inositol had distinct impacts on rumen fermentation (in vitro), health, and milk production compared to the CON group.
Research has indicated that supplementing dairy cow diets with nitrogen sources leads to an increase in the proportion of fiber-degrading bacteria in the rumen (21). In this study, the NH3-N levels at 12 h were lower than that at 6 h, possibly because of the increased utilization of NH3-N in the rumen by fiber-degrading bacteria.
In this study, the pH of the YC, IN65, IN85, and IN205 groups were lower than that of the CON group after 3 h of in vitro fermentation. However, after 24 h of fermentation, the pH of these groups were higher than that of the CON. These findings indicate that myo-inositol, helps stabilize rumen pH. Numerous studies have shown that incorporating yeast supplements into the diet can increase the levels of TVFA in the rumen of dairy cows (22). Myo-inositol, an effective substance in YC, may have diverse effects. Research has indicated that Anaerostipes can metabolize dietary myo-inositol to produce propionic and acetic acids (23). In the present study, it was found that IN65 and IN85 had a significant effect on the production of NH3-N, acetic acid, butyric acid, isobutyric acid, valeric acid, and isovaleric acid. These effects were most pronounced at 6 h and 12 h. Based on these findings, we conclude that myo-inositol affects rumen flora, resulting in changes in the concentrations of several VFA. Genus-level investigations showed that Moryella, Succiniclasticum, Clostridiaceae_Clostridium, and Bulleidia had higher relative abundances in the IN65 group compared to the YC group. Among these, Moryella can use carbohydrates and proteins to produce lactic, butyric, and short-chain fatty acids (24). Succiniclasticum, Clostridiaceae_Clostridium, and Bulleidia belong to the Firmicutes. They share common functions and can convert cellulose into VFA, thereby promoting digestion, growth, and development (25).
Changes in the metabolism of different AA have been observed in the rumen fluid metabolic pathways. After the addition of myo-inositol, a variety of metabolic pathways were changed, affecting differential metabolites like glutamate, ornithine, and histidine, which were found to be enriched in the KEGG differential metabolic pathways. Glutamate are essential for nitrogen metabolism and the TCA cycle in the rumen of ruminants (26). Glutamate, a recognized natural antioxidant, is an AA component of glutathione, another well-known natural antioxidant (27, 28). Ornithine is involved in the synthesis of polyamines in the rumen, which play a crucial role in the growth and activity of microorganisms (29). Histidine residues in proteins can act as buffers to help maintain the pH balance in the rumen. Intravenous injection of different amounts of histidine into lactating cows can lead to changes in feed intake and milk production (30). Mantel analysis was conducted on differentially expressed metabolites, fermentation indices, and microbial abundance to assess their correlation. The result showed a strong correlation between differential metabolites, fermentation indices, and microbial abundance. Therefore, these AA are closely associated with ruminal metabolism in dairy cows.
This study identified several important differential metabolic pathways by enriching differential metabolites from the IN65 vs. YC group. One of these pathways is pentose and glucuronate interconversion, which involves the transport and metabolism of key intermediates in the pentose phosphate pathway and the glucuronide pathway. These processes are essential for supporting cellular functions, detoxification, and redox balance. Research has shown that in mice with a complete knockout of the glutamate-cysteine ligase modifying subunit gene, exposure to the liver results in increased glutamate production, activation of the glucuronide pathway, and stimulation of nucleotide biosynthesis. The increased glutamate production suggests enhanced GSH-Px biosynthesis. The induction of nucleotide biosynthesis can provide nutrients for hexosamine biosynthesis and the glucuronide pathway (31). This pathway is essential for the production of NADPH2 and ribose-5-phosphate, which are essential for antioxidant defense and nucleotide synthesis, respectively. The glucuronide pathway involves the conversion of glucose derivatives to glucuronic acid, which plays a key role in the detoxification and conjugation of various substances (32). The study found that the GSH-Px index of the IN65 group was significantly lower compared to the YC and CON groups, possibly due to the activity of pentose and glucuronate interconversions in the blood. Myo-inositol can affect the transport of proteins. ABC transporters participate in the transport of nutrients, antioxidants, and metabolites into blood (33). Research on Holstein cows has shown that the proportion of ABC transporters also increases significantly as cows move from the dry period to the lactation period, peripartumly accounting for the increase in milk production observed in the IN65 group 21 days after delivery in this study (15). To explore the specific metabolic changes in milk, metabolomic analysis was performed on milk samples from the IN65, YC, and CON groups.
Analysis of milk metabolites in the IN65 and YC groups demonstrated that inositol influences the sphingolipid signaling pathway in milk. In this study, the main differential metabolites enriched in the sphingolipid signaling pathway in the IN65 group were C14 dihydroceramide, C16 ceramide, and C18 dihydroceramide. Interestingly, ceramide formation can be induced by different stimuli, such as TNF-α (34, 35). Ceramide can also be produced by sphingomyelin hydrolysis, a process affected by TNF-α or oxidative stress (36). This suggests that the reduction in TNF-α in the IN65 group was achieved by affecting the sphingolipid signaling pathway.
This study confirmed the effects of myo-inositol on rumen fermentation indicators, microbial flora, and metabolism through in vitro research. Additionally, it also determined the most effective dosage of myo-inositol supplementation through in vitro experiments. This optimal dosage was subsequently applied in animal experiments, and a metabolomic analysis of blood and milk was conducted to evaluate its effects on oxidative stress, immune response, and inflammatory indicators.
5 Conclusion
The IN65 was an optimal dose in vitro, which led to an increase in the concentration of VFA and the relative abundance of ruminal Bacteroidetes. Furthermore, myo-inositol was observed to primarily affect amino acid metabolism in the rumen, glucose metabolism in the blood, and sphingolipid metabolism in milk. These findings provide a theoretical basis for the use of myo-inositol as an additive to increase milk production in peripartum dairy cows.
Data availability statement
In this study, all the data generated or analysed are included in this paper. The 16s rRNA data presented in the study are deposited in the NCBl Sequence Read Archive repository, accession number PRJNA1101182.
Ethics statement
Animal experiments, both in vitro and in vivo, were conducted in accordance with the guidelines approved by the Jilin Agricultural University Animal Research Ethics Committee (approval number: JLAU-ACUC2022-004). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
C-zW: Writing – original draft, Formal analysis, Writing – review & editing, Methodology, Software. J-nD: Writing – original draft, Writing – review & editing, Software, Methodology. W-wS: Writing – review & editing. KQ: Writing – review & editing, Methodology. Z-cL: Writing – review & editing, Software. NA: Validation, Writing – review & editing. YZ: Writing – review & editing, Validation. ZS: Writing – review & editing, Conceptualization, Validation. NT: Formal analysis, Writing – review & editing, Software. Z-kZ: Methodology, Supervision, Writing – review & editing. Y-jL: Writing – review & editing, Resources. W-gZ: Writing – review & editing, Resources. WZ: Writing – review & editing, Resources. G-xQ: Writing – review & editing, Resources. Y-gZ: Conceptualization, Methodology, Writing – review & editing. TW: Project administration, Writing – review & editing, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Key Research and Development Program of China (Grant No. 2023YFD1300904) and the Scientific and Technological Developing Scheme of Jilin Province (20210202037NC).
Conflict of interest
Authors W-wS, ZS, W-gZ, WZ, Y-gZ, and TW were employed by the company Changchun Borui Science & Technology Co., Ltd. Author Y-jL was employed by the company Ningxia Agricultural Reclamation Helan Mountain Dairy Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
YC, yeast culture; SOD, superoxide dismutase; TAS, total antioxidant status; TOS, total oxidative status; NH3-N, ammonia nitrogen; CAT, catalase; MDA, malondialdehyde; GSH-Px, glutathione peroxidase; TNF-α, tumor necrosis factor-α; IFN-γ, interferon-γ; VFA, volatile fatty acid; KEGG, Kyoto Encyclopedia of Genes and Genomes.
References
1. Horst EA, Kvidera SK, and Baumgard LH. Invited review: the influence of immune activation on transition cow health and performance-a critical evaluation of traditional dogmas. J Dairy Sci. (2021) 104:8380–410. doi: 10.3168/jds.2021-20330
2. Rachah A, Reksen O, Tafintseva V, Stehr FJM, Rukke EO, Prestlokken E, et al. Exploring dry-film ftir spectroscopy to characterize milk composition and subclinical ketosis throughout a cow’s lactation. Foods. (2021) 10:2033. doi: 10.3390/foods10092033
3. Garro CJ, Mian L, and Cobos Roldan M. Subclinical ketosis in dairy cows: prevalence and risk factors in grazing production system. J Anim Physiol Anim Nutr (Berl). (2014) 98:838–44. doi: 10.1111/jpn.12141
4. Sordillo LM and Raphael W. Significance of metabolic stress, lipid mobilization, and inflammation on transition cow disorders. Vet Clin North Am Food Anim Pract. (2013) 29:267–78. doi: 10.1016/j.cvfa.2013.03.002
5. Lucy MC, Verkerk GA, Whyte BE, Macdonald KA, Burton L, Cursons RT, et al. Somatotropic axis components and nutrient partitioning in genetically diverse dairy cows managed under different feed allowances in a pasture system. J Dairy Sci. (2009) 92:526–39. doi: 10.3168/jds.2008-1421
6. Kelton DF, Lissemore KD, and Martin RE. Recommendations for recording and calculating the incidence of selected clinical diseases of dairy cattle. J Dairy Sci. (1998) 81:2502–9. doi: 10.3168/jds.S0022-0302(98)70142-0
7. Sordillo LM, Pighetti GM, and Davis MR. Enhanced production of bovine tumor necrosis factor-alpha during the periparturient period. Vet Immunol Immunopathol. (1995) 49:263–70. doi: 10.1016/0165-2427(95)05465-0
8. Shafer-Weaver KA, Pighetti GM, and Sordillo LM. Diminished mammary gland lymphocyte functions parallel shifts in trafficking patterns during the postpartum period. Proc Soc Exp Biol Med. (1996) 212:271–80. doi: 10.3181/00379727-212-44016
9. Alugongo GM, Xiao J, Wu Z, Li S, Wang Y, and Cao Z. Review: utilization of yeast of saccharomyces cerevisiae origin in artificially raised calves. J Anim Sci Biotechnol. (2017) 8:34. doi: 10.1186/s40104-017-0165-5
10. Jensen G, Patterson K, and Yoon I. Yeast culture has anti-inflammatory effects and specifically activates nk cells. Comp immunol Microbiol Infect Dis. (2008) 31:487–500. doi: 10.1016/j.cimid.2007.08.005
11. Wang D, Wang X, Cheng H, Yin W, An Y, Li M, et al. Effect of yeast culture supplementation on rumen microbiota, regulation pathways, and milk production in dairy cows of different parities. Anim Feed Sci Technol. (2025) 321:116244. doi: 10.1016/j.anifeedsci.2025.116244
12. Halfen J, Carpinelli N, Del Pino F, Chapman J, Sharman E, Anderson J, et al. Effects of yeast culture supplementation on lactation performance and rumen fermentation profile and microbial abundance in mid-lactation holstein dairy cows. J Dairy Sci. (2021) 104:11580–92. doi: 10.3168/jds.2020-19996
13. Derkaczew M, Martyniuk P, Osowski A, and Wojtkiewicz J. Cyclitols: from basic understanding to their association with neurodegeneration. Nutrients. (2023) 15:2029. doi: 10.3390/nu15092029
14. Antonsson B. Phosphatidylinositol synthase from mammalian tissues. Biochim Biophys Acta. (1997) 1348:179–86. doi: 10.1016/s0005-2760(97)00105-7
15. Kessler EC, Gross JJ, Bruckmaier RM, and Albrecht C. Cholesterol metabolism, transport, and hepatic regulation in dairy cows during transition and early lactation. J Dairy Sci. (2014) 97:5481–90. doi: 10.3168/jds.2014-7926
16. Chen J, Jiang W-D, Feng L, Wu P, Liu Y, Jin X-W, et al. Myo-inositol: A potential game-changer in preventing gill cell death and alleviating “Gill rot” in grass carp (Ctenopharyngodon idellus). Fish Shellf Immunol. (2024) 153:109850. doi: 10.1016/j.fsi.2024.109850
17. Chen X, Xiao J, Zhao W, Li Y, Zhao W, Zhang W, et al. Mechanistic Insights into Rumen Function Promotion through Yeast Culture (Saccharomyces Cerevisiae) Metabolites Using in Vitro and in Vivo Models. Front Microbiol. (2024) 15:1407024. doi: 10.3389/fmicb.2024.1407024
18. Dong JN, Li SZ, Chen X, Qin GX, Wang T, Sun Z, et al. Effects of different combinations of sugar and starch concentrations on ruminal fermentation and bacterial-community composition in vitro. Front Nutr. (2021) 8:727714. doi: 10.3389/fnut.2021.727714
19. Callaway E and Martin S. Effects of a saccharomyces cerevisiae culture on ruminal bacteria that utilize lactate and digest cellulose. J Dairy Sci. (1997) 80:2035–44. doi: 10.3168/jds.S0022-0302(97)76148-4
20. Zhu W, Wei Z, Xu N, Yang F, Yoon I, Chung Y, et al. Effects of saccharomyces cerevisiae fermentation products on performance and rumen fermentation and microbiota in dairy cows fed a diet containing low quality forage. J Anim Sci Biotechnol. (2017) 8:36. doi: 10.1186/s40104-017-0167-3
21. Xiao J, Chen T, Alugongo GM, Khan MZ, Li T, Ma J, et al. Effect of the length of oat hay on growth performance, health status, behavior parameters and rumen fermentation of holstein female calves. Metabolites. (2021) 11:890. doi: 10.3390/metabo11120890
22. Makkar HP and McSweeney CS. Methods in Gut Microbial Ecology for Ruminants. Berlin: Springer (2005).
23. Bui TPN, Mannerås-Holm L, Puschmann R, Wu H, Troise AD, Nijsse B, et al. Conversion of dietary inositol into propionate and acetate by commensal anaerostipes associates with host health. Nat Commun. (2021) 12:4798. doi: 10.1038/s41467-021-25081-w
24. Zhao X, Zhang Y, Rahman A, Chen M, Li N, Wu T, et al. Rumen microbiota succession throughout the perinatal period and its association with postpartum production traits in dairy cows: A review. Anim Nutr. (2024) 18:17–26. doi: 10.1016/j.aninu.2024.04.013
25. Jiang F, Gao H, Qin W, Song P, Wang H, Zhang J, et al. Marked seasonal variation in structure and function of gut microbiota in forest and alpine musk deer. Front Microbiol. (2021) 12:699797. doi: 10.3389/fmicb.2021.699797
26. Aragoneses-Cazorla G, Buendia-Nacarino MP, Mena ML, and Luque-Garcia JL. A multi-omics approach to evaluate the toxicity mechanisms associated with silver nanoparticles exposure. Nanomaterials. (2022) 12:1762. doi: 10.3390/nano12101762
27. Hunter G and Eagles BA. Non-protein sulfur compounds in blood. Ii Gluta J Biol Chem. (1927) 72:133–46. doi: 10.1016/S0021-9258(18)84367-8
28. Liu Y-Z, Chen X, Zhao W, Lang M, Zhang X-F, Wang T, et al. Effects of yeast culture supplementation and the ratio of non-structural carbohydrate to fat on rumen fermentation parameters and bacterial-community composition in sheep. Anim Feed Sci Technol. (2019) 249:62–75. doi: 10.1016/j.anifeedsci.2019.02.003
29. Fisher L. Response of lactating cows to the intravenous infusion of amino acids. Can J Anim Sci. (1972) 52:377–84. doi: 10.4141/cjas72-043
30. Shen J, Zheng L, Chen X, Han X, Cao Y, and Yao J. Metagenomic analyses of microbial and carbohydrate-active enzymes in the rumen of dairy goats fed different rumen degradable starch. Front Microbiol. (2020) 11:1003. doi: 10.3389/fmicb.2020.01003
31. Pallotta V, Gevi F, D’Alessandro A, and Zolla L. Storing red blood cells with vitamin C and N-acetylcysteine prevents oxidative stress-related lesions: A metabolomics overview. Blood Transfus. (2014) 12:376–87. doi: 10.2450/2014.0266-13
32. Wang Y-M, Dong W-L, Odah KA, Kong L-C, and Ma H-X. Transcriptome analysis reveals ai-2 relevant genes of multi-drug resistant klebsiella pneumoniae in response to eugenol at sub-mic. Front Microbiol. (2019) 10:1159. doi: 10.3389/fmicb.2019.01159
33. Nigam SK. What do drug transporters really do? Nat Rev Drug Discov. (2015) 14:29–44. doi: 10.1038/nrd4461
34. Kitatani K, Idkowiak-Baldys J, and Hannun YA. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal. (2008) 20:1010–8. doi: 10.1016/j.cellsig.2007.12.006
35. Marchesini N and Hannun YA. Acid and neutral sphingomyelinases: roles and mechanisms of regulation. Biochem Cell Biol. (2004) 82:27–44. doi: 10.1139/o03-091
Keywords: myo-inositol, peripartum dairy cows, metabolomes, milk production, health
Citation: Wang C-z, Dong J-n, Sun W-w, Qu K, Lin Z-c, Aschalew ND, Zhao Y, Sun Z, Ta N, Zhao Z-k, Liu Y-j, Zhang W-g, Zhao W, Qin G-x, Zhen Y-g and Wang T (2025) Myo-inositol affects the health, metabolome, and lactation performance of peripartum dairy cows. Front. Immunol. 16:1605244. doi: 10.3389/fimmu.2025.1605244
Received: 03 April 2025; Accepted: 13 May 2025;
Published: 16 June 2025.
Edited by:
Qian Jiang, Hunan Agricultural University, ChinaReviewed by:
Daming Sun, Nanjing Agricultural University, ChinaYanjiao Li, Jiangxi Agricultural University, China
Zhiyong Hu, Shandong Agricultural University, China
Copyright © 2025 Wang, Dong, Sun, Qu, Lin, Aschalew, Zhao, Sun, Ta, Zhao, Liu, Zhang, Zhao, Qin, Zhen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu-guo Zhen, bmlja3poZW5AMjYzLm5ldA==; Tao Wang, Y2FnZXdhbmdAMTYzLmNvbQ==
†Present address: Chun-zheng Wang, Zhaozhou County Animal Husbandry and Veterinary Service Center, Zhaozhou, China
‡These authors have contributed equally to this work
 Chun-zheng Wang
Chun-zheng Wang Jia-nan Dong
Jia-nan Dong Wu-wen Sun1,3
Wu-wen Sun1,3 Natnael D. Aschalew
Natnael D. Aschalew Zhe Sun
Zhe Sun Zhi-kun Zhao
Zhi-kun Zhao Yu-guo Zhen
Yu-guo Zhen Tao Wang
Tao Wang