- 1Department of Pain Management, The First Affiliated Hospital of Harbin Medical University, Harbin, China
- 2Department of Neurosurgery, The First Affiliated Hospital of Harbin Medical University, Harbin, China
- 3Department of Neurosurgery, Xi’an Daxing Hospital, Xi’an, China
- 4Department of Anesthesiology, The First Affiliated Hospital of Harbin Medical University, Harbin, China
Introduction: Glioma is one of the most aggressive brain tumors, and its interaction with inflammation has become an emerging research focus. Despite increasing interest in the role of inflammation in glioma progression and therapy, a comprehensive bibliometric analysis of this field is lacking.
Methods: We conducted a systematic bibliometric analysis of glioma and inflammation research using the Web of Science Core Collection (WoSCC). A total of 4,553 publications from 2005 to 2025 were analyzed for research trends, hotspots, key contributors, and emerging directions using CiteSpace and VOSviewer.
Results: Our analysis revealed a significant increase in publications over the past decade. From 2005 to 2025, a total of 4,553 papers related to glioma and inflammation were published. Key contributing countries, institutions, and authors were identified, highlighting the dominance of the U.S. and China in this field. Leading institutions include MD Anderson Cancer Center and Harvard Medical School (USA) and several top Chinese universities. Keyword clustering and co-citation analysis indicated that expression, growth, and survival are major research hotspots. Highly cited papers primarily focused on molecular subtypes, immune modulation, and therapeutic resistance in glioma. ssGSEA analysis revealed that the score based on the 25-gene signature was significantly enriched in GBM and was closely associated with poor prognosis in GBM patients.
Conclusion: Glioma and inflammation research have gained increasing attention, particularly in tumor immunity and microenvironment studies. This study outlines the current research landscape and trends, potentially serving as a reference for exploring future areas of investigation and collaboration.
Introduction
Gliomas represent the most common and aggressive malignant tumors of the central nervous system, with glioblastoma (GBM) being the most lethal subtype. The highly heterogeneous biology of GBM, coupled with its resistance to conventional therapies, contributes to the dismal prognosis associated with this disease. Despite advancements in multimodal treatment approaches, including surgical resection, radiotherapy, and chemotherapy, the median survival of glioblastoma patients remains under two years, with a five-year survival rate that remains exceedingly low (1). With advancements in molecular biology and immunology, researchers have increasingly recognized that gliomas are not merely the result of uncontrolled tumor cell proliferation; rather, the complexity of their microenvironment plays a pivotal role in tumor initiation, progression, and therapeutic resistance. Consequently, a deeper exploration of the key factors influencing glioma progression, particularly inflammation-related mechanisms, is essential for the development of more effective therapeutic strategies.
Inflammation plays a central role in the initiation and progression of gliomas and is regarded as a critical factor shaping the tumor microenvironment and influencing therapeutic responses (2). The glioma microenvironment is enriched with pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α, along with tumor-associated macrophages (TAMs) and microglia. Together, these immune components shape an immunosuppressive milieu that enables tumor cells to evade immune surveillance (3–5). Moreover, inflammatory signaling pathways, including NF-κB, JAK-STAT, and NLRP3, are aberrantly activated within the tumor microenvironment, further driving tumor cell proliferation, invasion, and the development of therapeutic resistance (6–8). In recent years, targeted therapeutic strategies against inflammation-related molecules—such as immune checkpoint inhibitors, anti-inflammatory agents, and drugs targeting tumor-associated macrophages (TAMs)—have emerged as a major focus in glioma research (9–12). Therefore, a systematic review of the current research landscape and emerging trends in glioma-related inflammation can provide deeper insights into their interplay and offer new directions for future investigations.
Bibliometric analysis is a quantitative method based on large-scale literature data, enabling the identification of academic trends, research hotspots, key authors, leading institutions, and emerging frontiers within a specific research field (13). By leveraging bibliometric tools such as CiteSpace and VOSviewer, researchers can analyze glioma and inflammation-related literature from multiple perspectives, including citation networks of high-impact papers, collaboration networks, keyword co-occurrence analysis, and burst term detection. These analyses not only help identify research hotspots but also unveil academic collaboration patterns, interdisciplinary trends, and potential future directions. Furthermore, bibliometric analysis enables the tracking of emerging technologies and research paradigms, providing researchers with a more structured academic resource to support decision-making and strategic research planning.
This study employs a bibliometric approach to systematically analyze the literature on glioma and inflammation from the past two decades, aiming to uncover global research trends, key contributors, influential publications, research hotspots, and emerging advancements in the field. Through high-frequency keyword analysis, we identified core research directions within this domain. Additionally, we examined collaboration networks among research institutions and scholars to assess academic collaboration patterns and the degree of internationalization. The findings of this study not only provide researchers with a comprehensive academic overview but also offer valuable insights for future investigations, facilitating the exploration of inflammation’s role in glioma initiation, progression, and treatment. Furthermore, this study provides a theoretical foundation for optimizing personalized therapeutic strategies.
Materials and methods
Data source and retrieval strategy
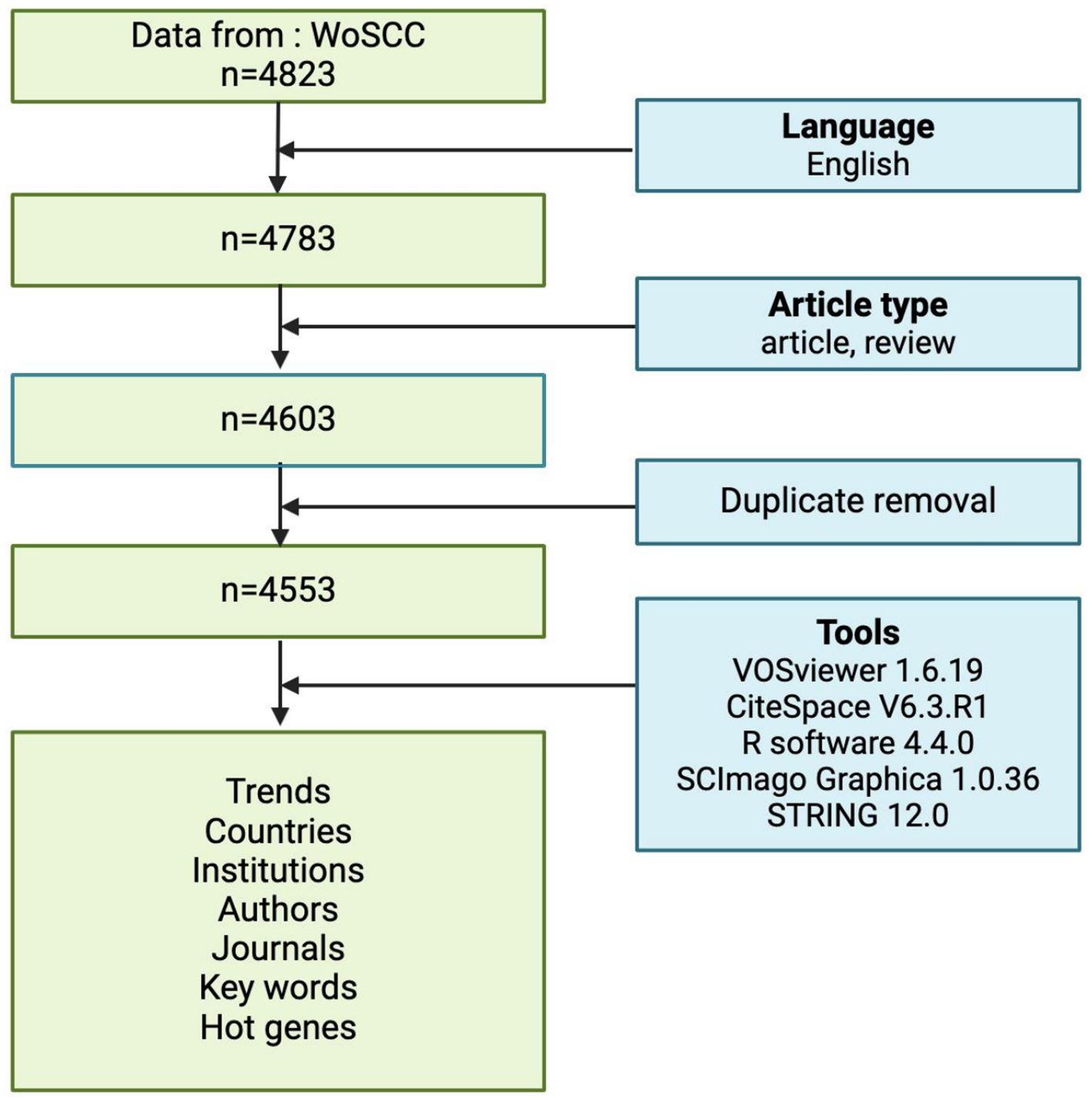
Relevant literature was extracted from the Web of Science Core Collection (WoSCC) database, the most trusted scientific citation database in the world (14), covering the period from 2005 to 2025. The search terms included TS = (“glioma*” OR “glioblastoma*” OR “GBM” OR “glioblastoma multiform*” OR “malignant glioma”) AND TS = (“inflammation*” OR “inflammatory”) AND DOP = (2005-01-01/2025-03-22). The wildcards * represent root word truncation of one or more other characters. For example, glioma* could refers to glioma or gliomas.
The inclusion criteria for the literature sources in this study were: (1) the literature search period spanned from January 1, 2005 to March 22, 2025; (2) the selected publication types of literature were “article’ and ‘review article’, and (3) the selected language was English. The bibliometric process is illustrated in flowchart Figure 1. Finally, 4553 records were retrieved from WoSCC.
Data analysis and visualization
The bibliographic records meeting the above criteria were exported in plain text format, including information such as title, keywords, citation count, publication date, country/region, researchers, affiliated institutions, academic journals, impact factor (IF), and H-index. These records were then used for subsequent visualization and bibliometric analysis. The study employed Biblioshiny with R version 4.4.0 (15), SCImago Graphica (version 1.0.36) (16), CiteSpace (version 6.3.R1) (17) and VOSviewer (version 1.6.19) (18) for data importing, filtering, visualization and trend analysis. Key analyses performed included: (1) Publication trends and citation growth, (2) Co-authorship network analysis (authors, institutions, and countries), (3) Keyword co-occurrence and burst detection, (4) Co-citation analysis of influential references. Additionally, we manually extracted the top 25 hotspot genes and performed Gene Set Enrichment Analysis (GSEA) analysis and protein interaction (PPI) network construction using R language and the STRING (version12.0, https://string-db.org/) online database (19).
Results
Publication trends and citation analysis
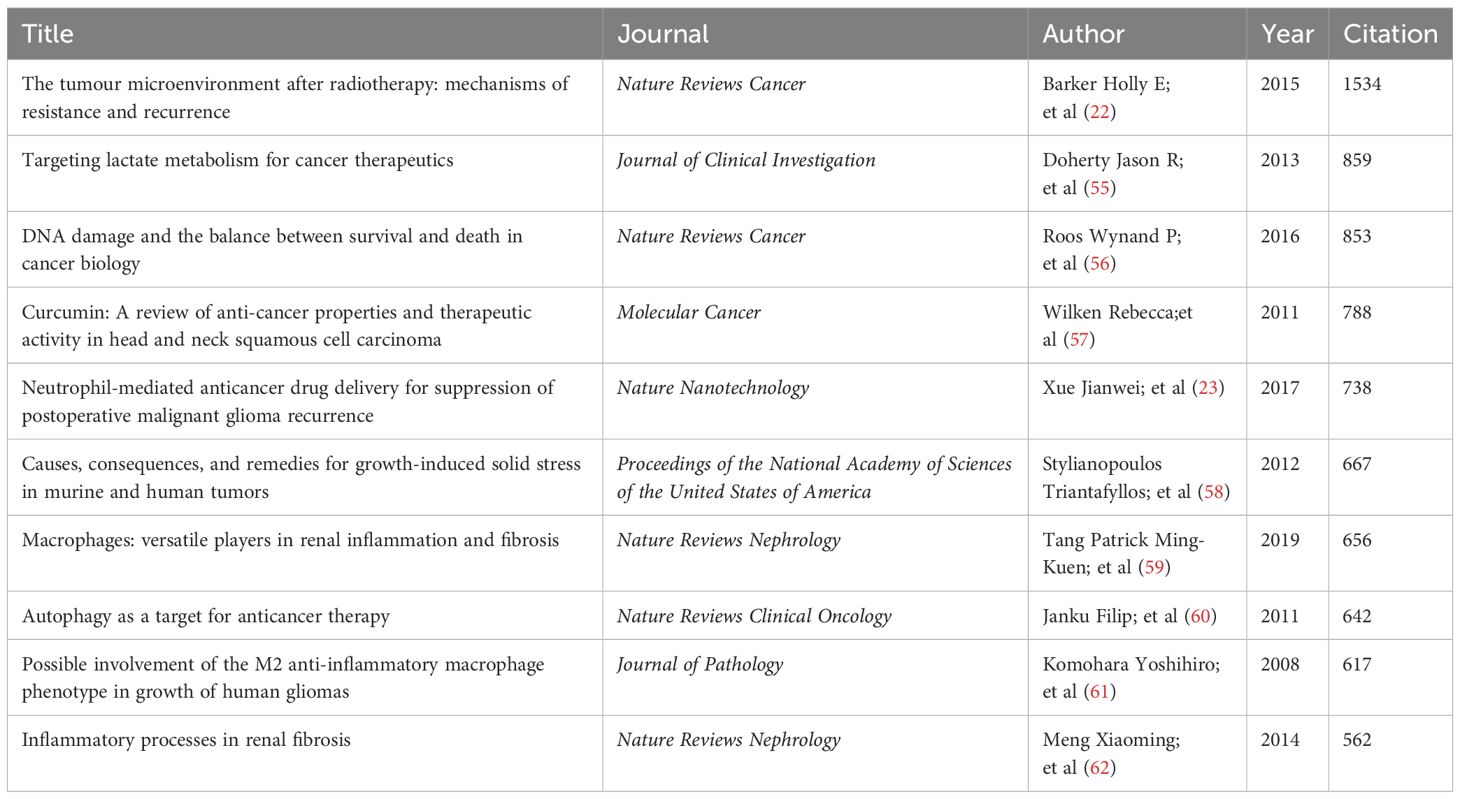
From 2005 to 2025, the Web of Science Core Collection (WoSCC) has indexed a total of 4,553 studies on glioma and inflammation, including 3,600 articles and 933 reviews. Articles account for 79% of the total publications, while reviews make up nearly 21%. Analyzing the historical publication trends in this field, the number of publications has shown a gradual increase over time. Before 2010, fewer than 100 related articles were published annually. Since 2010, the annual publication count reached 100, and from 2017 onward, it surpassed 200 per year. The year 2022 recorded the highest number of publications, reaching 477. Figure 2 presents the top 25 strongest citation bursts, with citation periods primarily concentrated between 2012 and 2025. The earliest among them is a study published by Douglas Hanahan et al. in Cell in 2011, titled “Hallmarks of Cancer: The Next Generation.” (20). The most strongly cited publication appeared in peer-reviewed journal Neuro-Oncology. Authored by David N. Louis et al. in 2021, the study is titled “The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary.” (21). Figure 3 presents the top ten most globally cited documents, covering topics such as the tumor microenvironment, lactate metabolism, DNA damage, neutrophils, macrophages, autophagy, and inflammation. The most highly cited article has been referenced 1,534 times, making it the only one to surpass 1,000 citations. This article, published in Nature Reviews Cancer in 2015, is a review focusing on the tumor microenvironment following radiation therapy (22). Notably, among the ten documents, only one is a research article. This study, authored by Jingwei Xue et al. and published in Nature Nanotechnology in 2017, is titled “Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence.” (23) (Table 1).
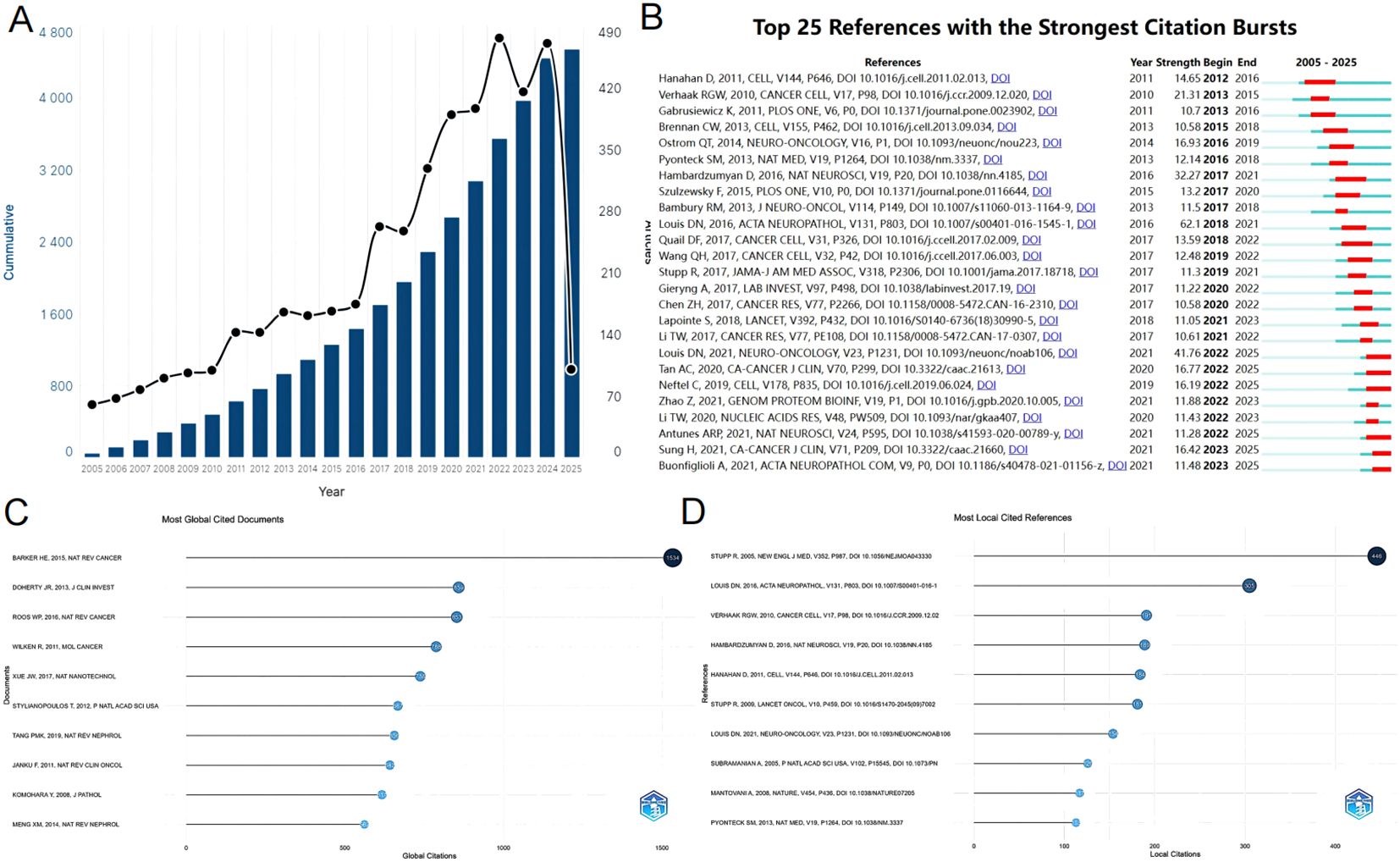
Figure 2. (A) The number of articles published annually and the cumulative number of publications. (B) Top 25 references with the strongest citation bursts. (C) Most global cited documents. (D) Most local cited references.
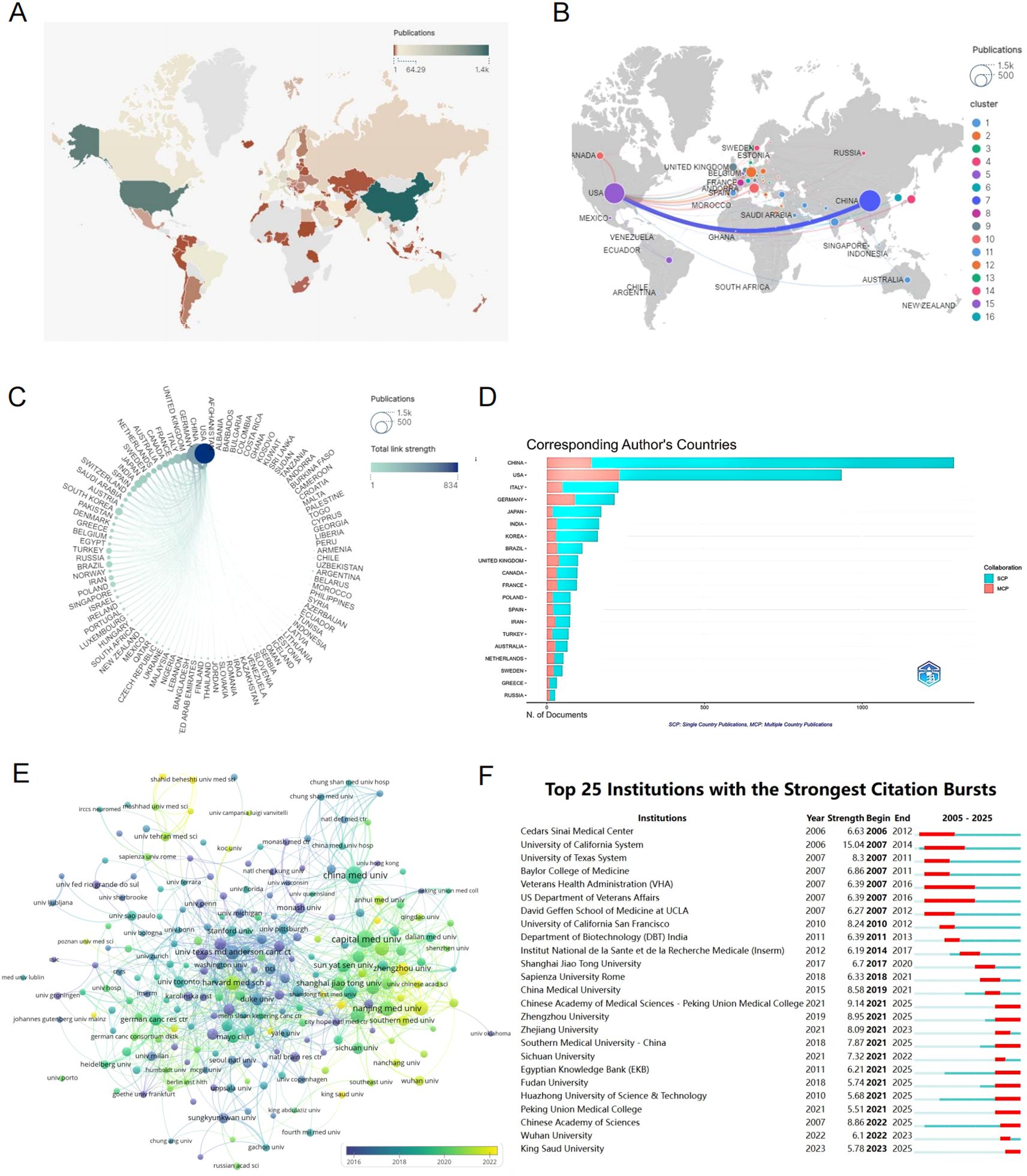
Figure 3. (A) A world map showing the distribution of publication quantities per country represented by different colors. (B) National and Regional Distribution of Publication Volume and Collaboration: Each node represents a country (region), with the node size indicating the number of published articles from that country (region). The lines between nodes represent the extent of collaborative activities between countries (regions). (C) A circular diagram of collaborative relationships between countries (regions). Each node symbolizes a country (region). The size of each node indicates the number of articles published within that country (region). The color of the nodes and the connecting lines represent the total number of interactions between the relevant nodes and other nodes. (D) Corresponding author country statistics chart. (E) Institutional clustering and temporal overlay map of publications. (F) Top 25 institutions with the strongest citation bursts.
Analysis of publication countries and institutions
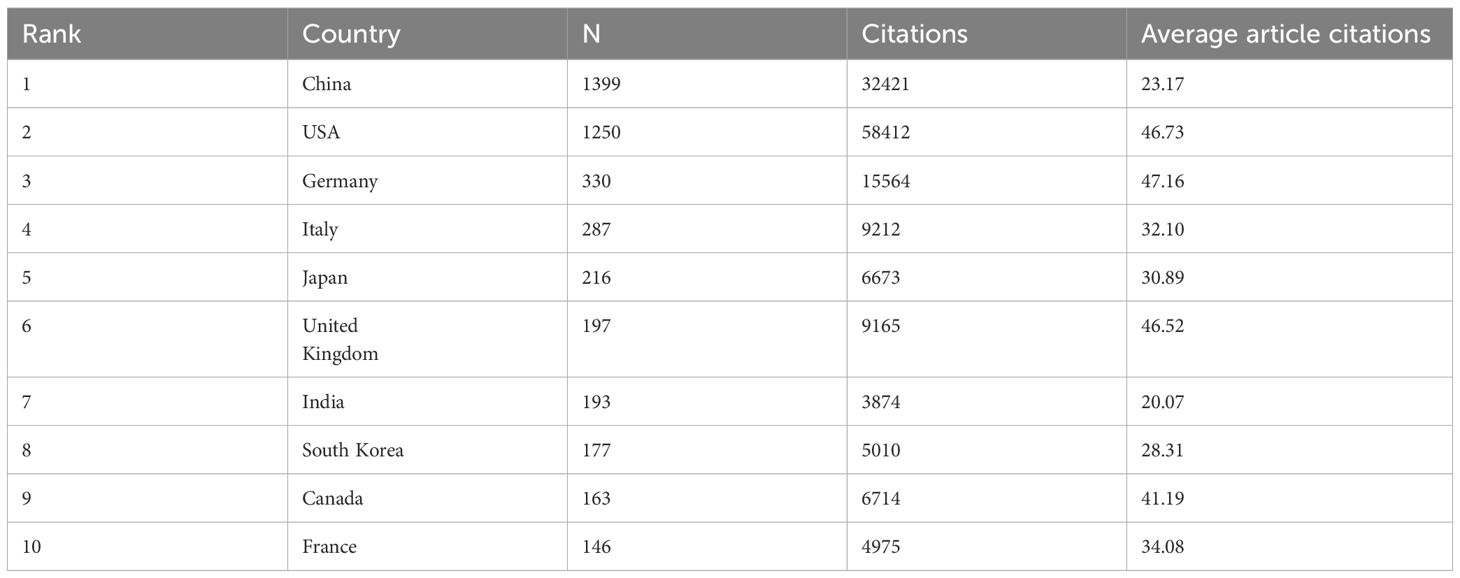
A total of 97 countries or regions and 261 institutions have made significant contributions to the field of glioma and inflammation research. The top ten contributing countries span three continents, including Asia (4 countries), Europe (4 countries), and North America (2 countries). Among them, China ranks first in the number of published articles, with 1,399 publications, followed by the United States with 1,250 publications. However, in terms of total citations, the United States leads with 58,412 citations, followed closely by China with 32,421 citations (Table 2). These findings indicate that China and the United States have made significant contributions to glioma and inflammation research. While the United States currently holds a greater influence in terms of citations, China exhibits a stronger research momentum, showing a trend of catching up and potentially surpassing in the future.
The geographical distribution map (Figures 3A) highlights the central roles of China and the United States in this field, with European countries also holding a considerable share. The chord diagram (Figure 3) further reveals that China and the United States dominate international collaborations, whereas connections among other countries remain relatively weak, suggesting that global cooperation in glioma and inflammation research still needs to be strengthened. By analyzing the corresponding authors’ national affiliations, we find that a large proportion of U.S. publications involve international collaborations. China ranks second in terms of the number of multinational collaborative publications. However, in terms of proportion, German scientists show a stronger tendency to collaborate internationally (Figure 3).
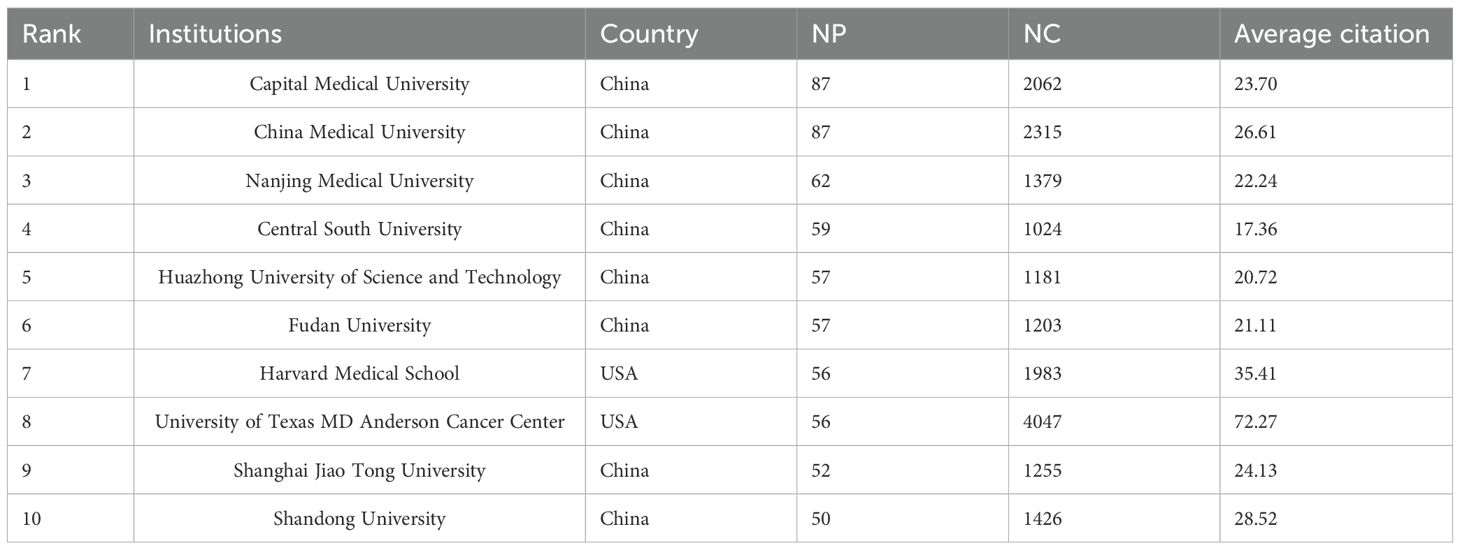
The institutional analysis reveals that the top ten institutions by publication volume are all based in China and the United States, including Capital Medical University (87), China Medical University (87), Nanjing Medical University (62), Central South University (59), Huazhong University of Science and Technology (57), Fudan University (57), Harvard University (56), and MD Anderson Cancer Center, University of Texas (56), among others. From a citation analysis, it is found that MD Anderson Cancer Center has the highest total citations, with 4,047 citations and an average of 72.27 citations per article. China Medical University ranks second with 2,315 total citations. Harvard University has a total of 1,983 citations, ranking fourth, but its average citation of 35.41 is the second highest. These results suggest that the two U.S. institutions have the highest citation and average citation numbers, indicating their core position in the glioma and inflammation research field. Meanwhile, Capital Medical University and China Medical University from China have the highest publication volumes, and their total citations and average citations also rank highly (Table 3).
The institutional clustering diagram shows that institutions publishing on glioma and inflammation are mainly concentrated in Capital Medical University, China Medical University in China, and MD Anderson Cancer Center and Harvard University in the United States. These institutions collaborate on publications, forming a complex publication network (Figure 3). Figure 3 highlights the top 25 strongest citation bursts by institution. Notably, since 2006, Cedars-Sinai Medical Center in the U.S. entered the citation burst phase. The Chinese Academy of Sciences started in this field in 2007, but it wasn’t until 2022 that it entered the burst phase.
Journal analysis
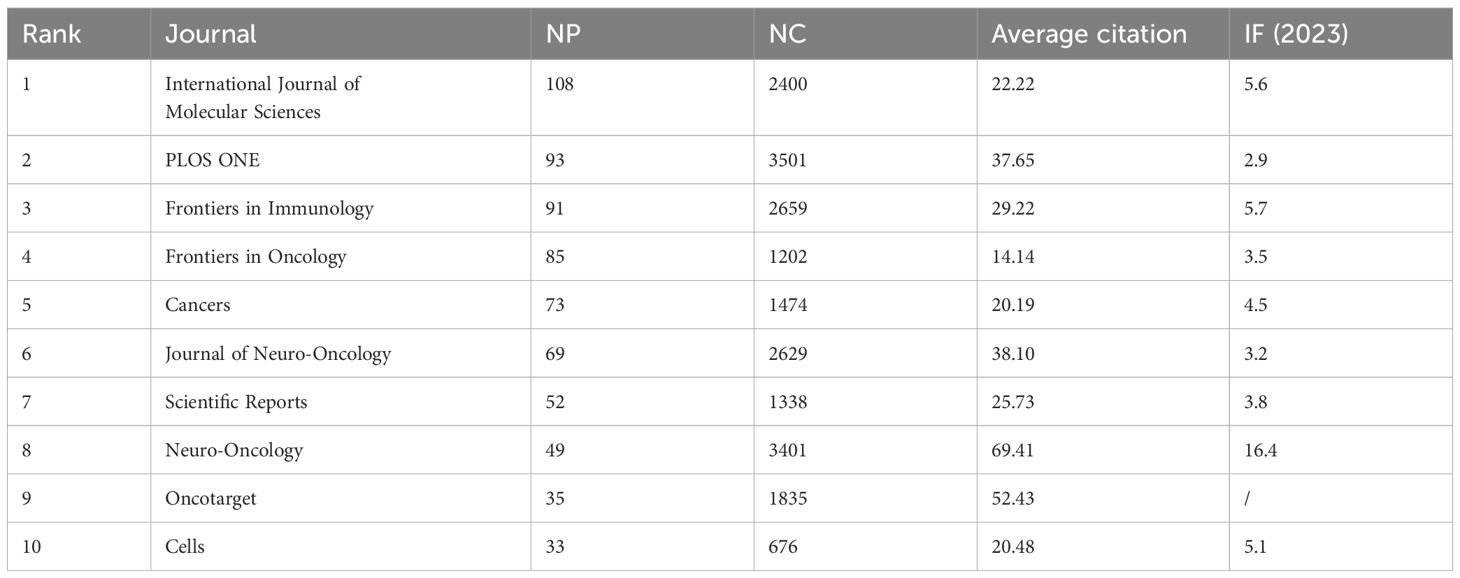
Exploring the distribution of research across different journals provides valuable insights for researchers and supports academic publishing in the field of glioma and inflammation. The journal clustering visualization (Figure 4) reveals the distribution of publications across various journals, with the International Journal of Molecular Sciences, PLOS ONE, and Frontiers in Immunology leading in publication volume. The citation analysis of journals (Figure 4) highlights the core journals in this field, with Cancer Research, Neuro-Oncology, and PLOS ONE standing out as central to the discourse on glioma and inflammation. Figure 4 displays the top ten journals by publication volume, with the International Journal of Molecular Sciences publishing the most related studies, totaling 108 articles, ranking first. Figure 4 shows the top ten journals by H-index, with PLOS ONE ranking first with an H-index of 38. Table 4 presents detailed information on the top 10 journals in terms of total publication volume, total citations, average citations, and impact factor. It shows that PLOS ONE has the highest total citations (3,501), likely due to its large volume of published articles. However, Neuro-Oncology published 49 articles and has a citation total of 3,401, with an average citation of 69.41, ranking first in terms of average citations. Additionally, Neuro-Oncology has the highest impact factor among these journals, further emphasizing its significant influence in the field of glioma and inflammation. These results highlight the importance of Neuro-Oncology in the glioma and inflammation research landscape, both in terms of publication influence and citation impact.
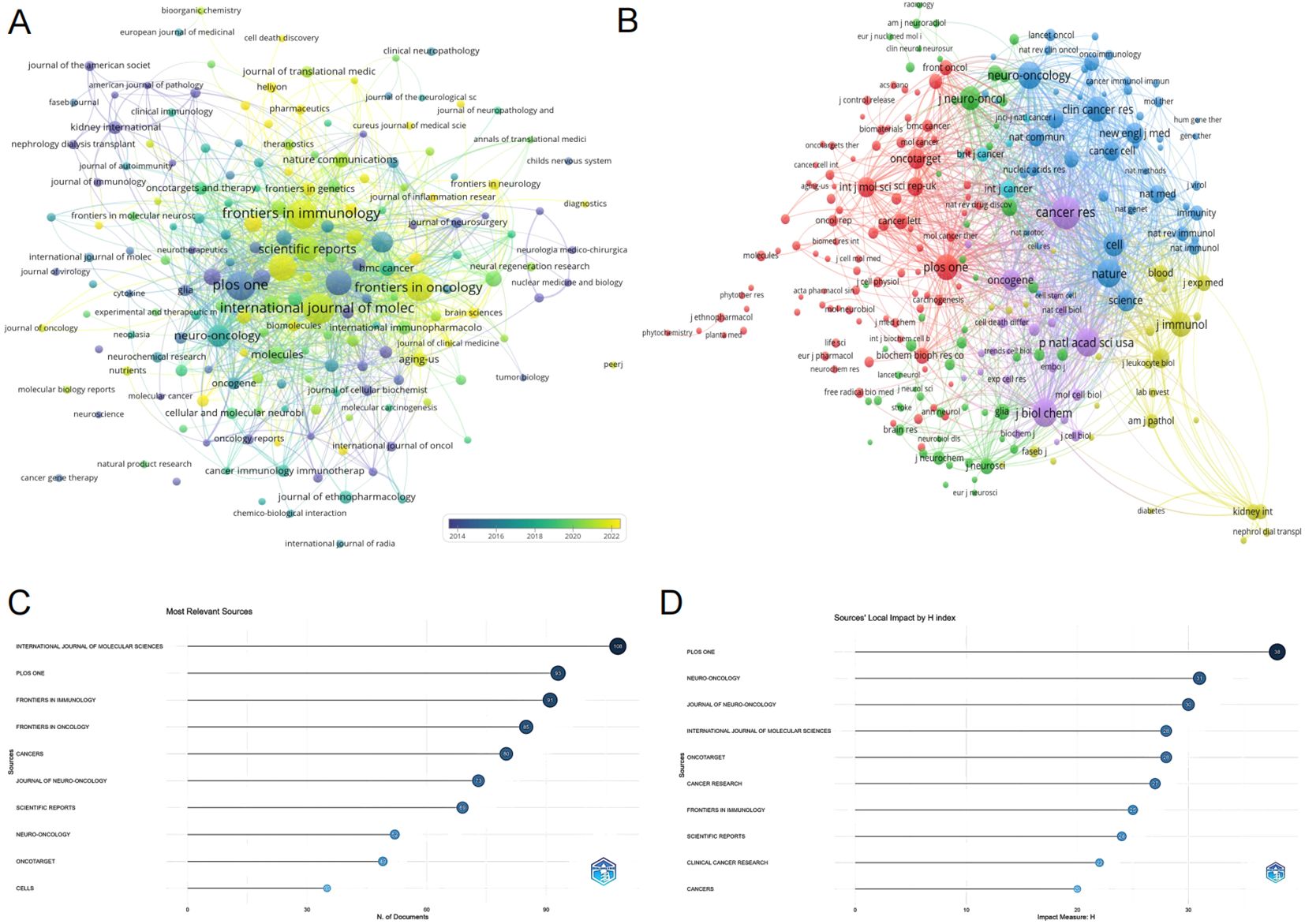
Figure 4. (A) Journal clustering and time overlay map of publications. (B) Journal co-citation analysis graph. (C) Journal publication volume chart. (D) Journal H-index statistics chart.
Author analysis
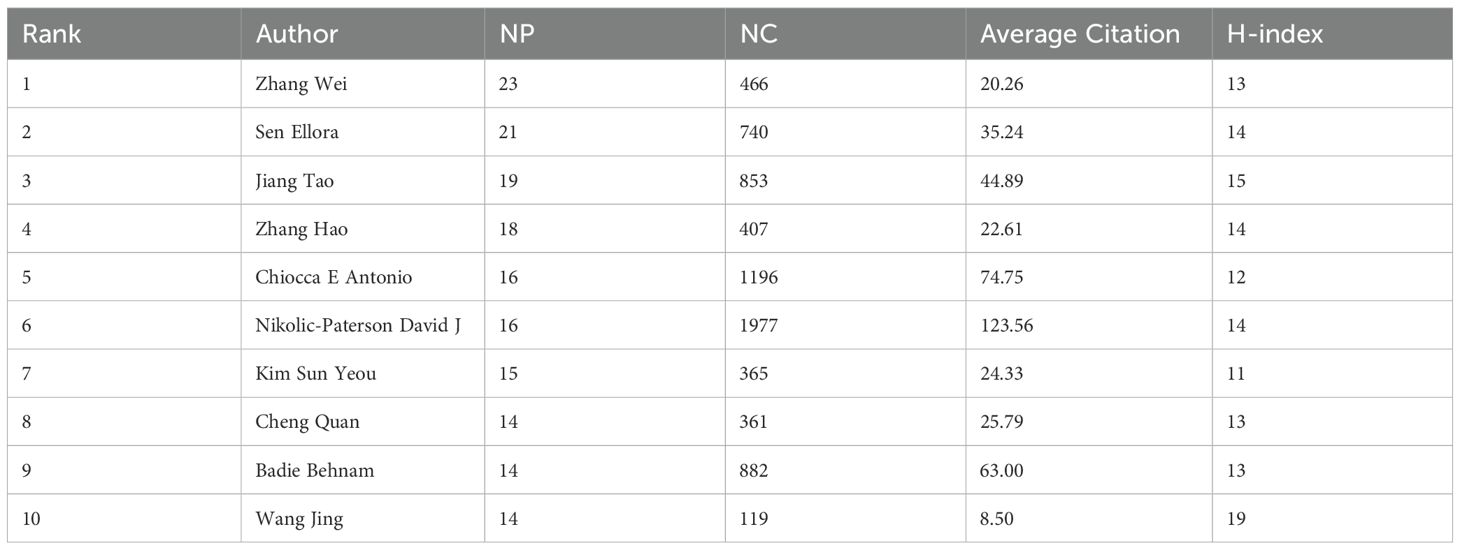
We conducted an analysis of authors publishing in the field of glioma and inflammation, ranking them by total publication volume (Table 5). The top three authors are Zhang Wei (23 publications), Sen Ellora (21 publications), and Jiang Tao (19 publications). Two of these authors are affiliated with Beijing Tiantan Hospital, China, while one is from the National Brain Research Centre, Manesar, India. Figure 5 presents a visualization of author collaborations, where authors are divided into 7 colors based on shared collaboration patterns. Authors with the same color have similar characteristics in their co-authorship networks. Notably, experts in glioma research such as Zhang Wei, Jiang Tao, and Wu Anhua are prominent in the visualization. Figure 5 illustrates the publication trends of different authors over time, showing the number of articles published and their citation impact. This analysis highlights the steady increase in the publication output and citations for key authors in the field. Figure 5 shows the relationship between authors, their highly cited articles, and keywords, indicating the areas of focus and expertise of the leading researchers in glioma and inflammation. This network analysis provides insights into the connections between prominent authors and their contributions to the field.
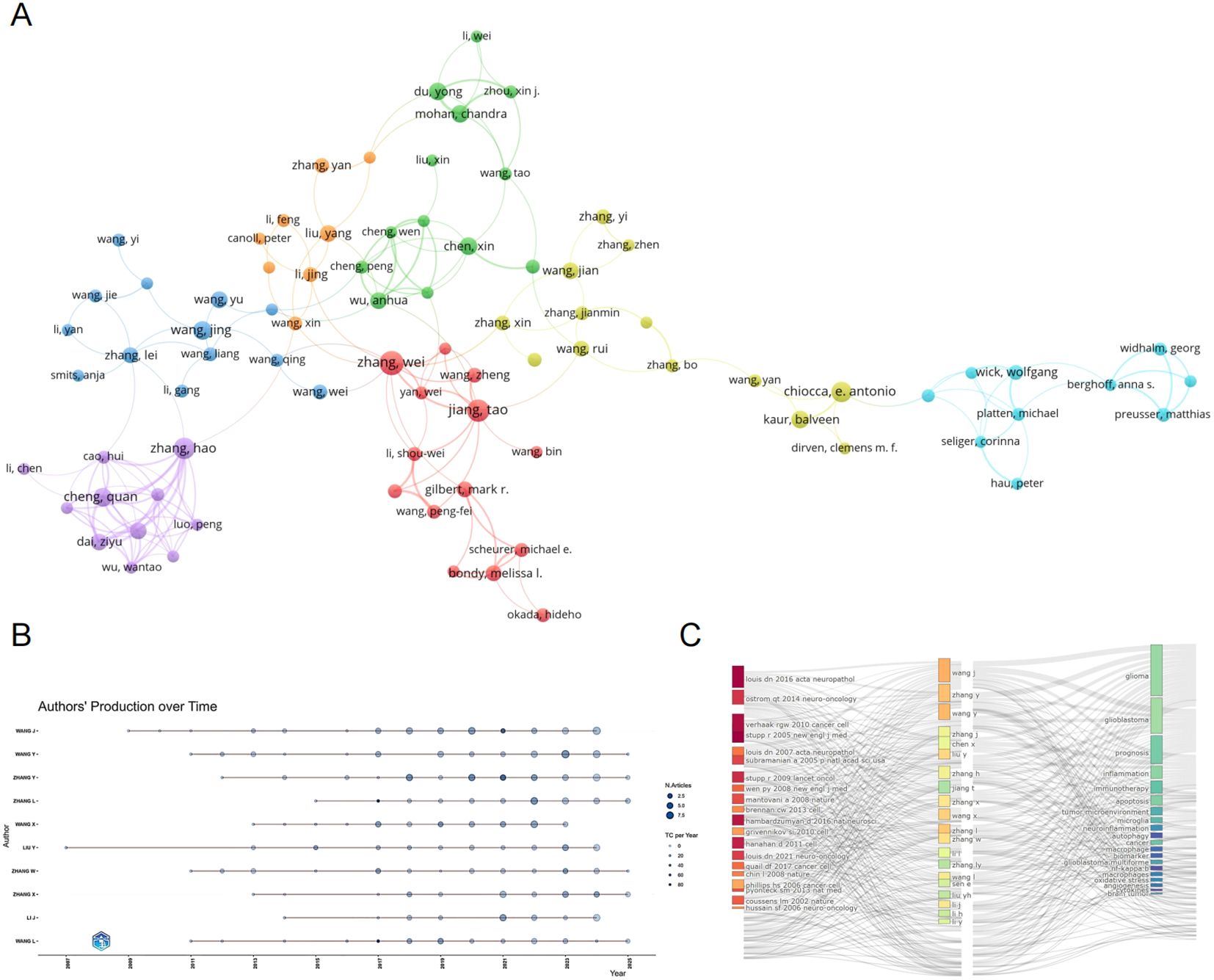
Figure 5. (A) Author collaboration network graph. (B) Author collaboration network graph. (C) Topic evolution map analysis.
Keyword analysis
Keywords are labels for research content, and they can reveal research hotspots and trends. We performed a treemap analysis of keywords, with different colors representing different keywords, and the size of the area indicating the frequency of keyword occurrence. The analysis shows that expression, cancer, and inflammation are the most dominant keywords (Figure 6). Figure 6 presents the visualization of keyword clustering analysis, where the size of the nodes represents the frequency of keyword occurrences, and the distance between nodes indicates the strength of the connection between keywords. Keywords that are close together in distance are clustered, reflecting the primary research focus in the field of glioma and inflammation. The clusters can be roughly divided into three categories: The first group, represented by blue, includes keywords such as glioma, hypoxia, survival, and TMZ. The second group, represented by red, includes keywords like expression, inflammation, growth, and cancer. The third group, represented by green, includes keywords such as apoptosis, angiogenesis, NF-kappaB, and migration. Figure 6 is a timeline chart, showing that research in glioma and inflammation primarily revolves around 14 key themes. We observed that keywords such as cytotoxic effect, inducible nitric oxide synthase, and hedgehog signaling have been the most frequently appearing keywords in recent years.
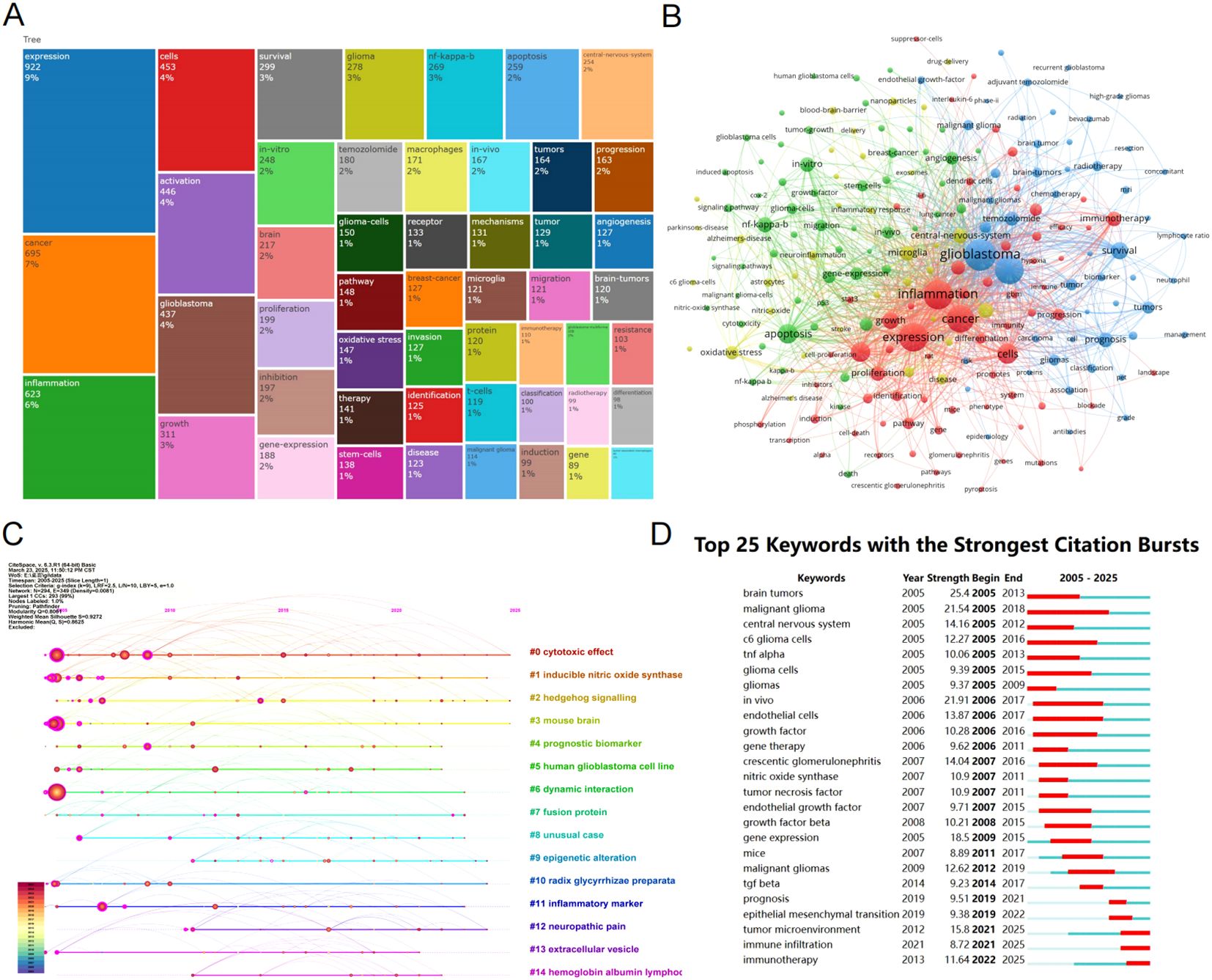
Figure 6. (A) Keyword tree map analysis. (B) Keyword clustering map. (C) Timeline graph of reference co-citation analysis. (D) Top 25 keywords with the strongest citation bursts.
To further understand the emergence and duration of keywords, we used CiteSpace for a keyword burst analysis (Figure 6). We found that malignant glioma appeared the earliest and has had the longest burst duration. In contrast, in recent years, tumor microenvironment, immune infiltration, and immunotherapy have emerged as the most popular research topics.
Analysis of key genes in glioma and inflammation research
To further identify the key research genes in the field of glioma and inflammation, we ranked genes based on total citation counts and manually selected the top 25 most influential genes. To explore the roles of these genes in glioma, we performed Gene Set Enrichment Analysis (GSEA) on the gene set composed of these 25 genes using TCGA-GBM data. The results showed that the ssGSEA score of this pathway was significantly higher in GBM tumor tissues compared to normal brain tissues and was markedly enriched in GBM (Figures 7A). In the TCGA-GBM cohort, survival analysis based on the ssGSEA score revealed that patients in the high-score group had significantly worse overall survival (OS) and progression-free survival (PFS) than those in the low-score group (Figures 7C), indicating that elevated pathway activity is closely associated with poor prognosis. We further evaluated the diagnostic performance of this score in GBM using a receiver operating characteristic (ROC) curve, which yielded an AUC of 0.946 (95% CI: 0.910–0.977), demonstrating strong discriminatory power (Figure 7). To further clarify their functional mechanisms in GBM, we conducted a protein-protein interaction (PPI) analysis using the STRING online database (Figure 7). The results revealed that CSF1R, TGFBI, and IDO1 serve as key nodes in the protein interaction network, suggesting their critical roles in glioma progression and tumor-associated inflammation.
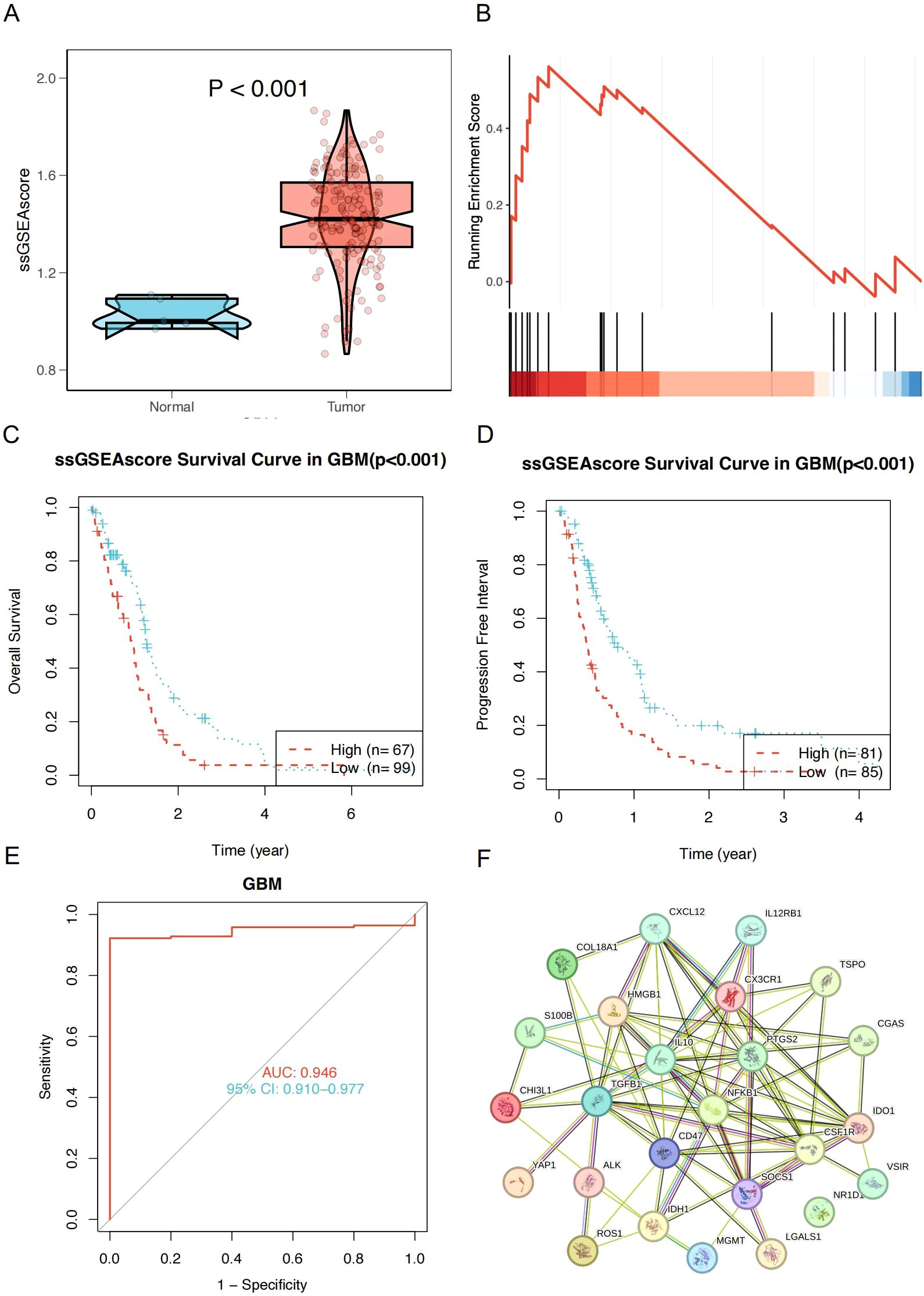
Figure 7. (A) ssGSEA scores were significantly higher in GBM tumor tissues compared to normal tissues (P < 0.001). (B) GSEA Analysis Plot of Top 25 Hot Genes. (C) Kaplan–Meier overall survival curves for GBM patients stratified by high and low ssGSEA scores (P < 0.001). (D) Progression-free survival curves for GBM patients stratified by high and low ssGSEA scores (P < 0.001). (E) Receiver operating characteristic (ROC) curve of the ssGSEA score in diagnosing GBM; AUC = 0.946, 95% CI: 0.910–0.977. (F) Protein-Protein Interaction (PPI) Network Diagram.
Discussion
Bibliometric analysis, as an important research method, has been widely applied across multiple fields. By conducting quantitative analysis of publications, researchers can uncover research trends, hotspots, and academic impact within a specific domain (24). This method not only helps researchers gain a better understanding of the knowledge structure within a specific field but also offers insights into emerging trends and potential areas of interest for future research. However, bibliometric analysis has not yet been conducted in the field of glioma and inflammation. In this study, we performed a systematic literature search using the WoSCC database and, after screening, included 4,553 publications from the past two decades related to glioma and inflammation for bibliometric analysis and visualization. By systematically analyzing the research landscape in this field, we aim to reveal development trends, research hotspots, and emerging directions, enabling researchers to quickly grasp the evolution of this domain and providing valuable references for future studies.
We found that the most locally cited article is “Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma” (446 citations) (1). This clinical study demonstrated that combining radiotherapy with temozolomide (TMZ) after surgery extended the median survival of GBM patients from 12.1 months to 14.6 months. This research laid the foundation for the radiotherapy + TMZ regimen as the standard treatment for GBM, significantly transforming the clinical management of the disease.
While the radiotherapy + TMZ regimen has become the standard of care, its efficacy remains limited, highlighting the need for future research to explore more effective therapeutic strategies, such as combination immunotherapy and personalized precision medicine. In the citation burst analysis, we identified that the earliest highly cited paper with the strongest citation burst was published in 2010: “Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1.” This study played a pivotal role in glioblastoma classification, revealing distinct molecular subtypes based on genetic alterations in PDGFRA, IDH1, EGFR, and NF1. Its strong citation burst in the early stages suggests that molecular subtyping was a major research focus at the time, laying the groundwork for subsequent advancements in targeted therapy and personalized treatment approaches for GBM (25). This groundbreaking study pioneered the molecular subtyping of GBM, significantly advancing the application of precision medicine in GBM research (26–28).
The regional distribution map reveals that the United States and China are the leading countries in terms of publication volume in the field of glioma and inflammation research, with their output significantly higher than that of other countries. However, despite both China and the U.S. leading in publication volume, total citations and average citations per paper show that the U.S. significantly outperforms China. This disparity points to differences in academic influence and research quality between the two countries in the glioma and inflammation field. For China to further enhance its academic influence, it will need to focus more on research innovation, international collaboration, and the publication of high-quality papers, aiming to increase the global academic impact of its research.
The distribution of corresponding authors by country reveals that China has the highest number of domestic collaboration publications (single-country publications), while the number of international collaboration publications (involving multiple countries) is lower than that of the United States. This phenomenon reflects the differences in academic collaboration models between China and the U.S., as well as the collaboration trend and academic influence of different countries in the field of glioma and inflammation research. From another perspective, due to the U.S.’s leading position, scholars worldwide tend to collaborate with U.S. researchers. In the future, China can enhance international cooperation and promote the publication of high-impact papers internationally to further strengthen its global academic influence in the glioma and inflammation research field. Author analysis helps us identify the core scientists, academic networks, collaboration patterns, research hotspots, and future trends in glioma and inflammation research. Through this analysis, we found that collaboration among researchers is primarily concentrated within the same country, institution, and field. This indicates that the research relies heavily on internal collaborations within laboratories or research teams, such as close ties between large medical research centers or university laboratories. The advantage of this collaboration model is that it allows for clear division of labor and easy resource sharing. However, it may also limit the breadth of research perspectives.
Inflammatory processes play a pivotal role in a wide range of diseases and are widely recognized as key drivers—and even initiators—of pathological changes. In tumors of the central nervous system, gliomas represent the most common and aggressive type, and their initiation and progression are closely associated with chronic inflammation (29). A growing body of evidence indicates that inflammatory signaling plays a critical role in the initiation, progression, immune evasion, and therapeutic resistance of glioma (30, 31). With the advancement of high-throughput sequencing technologies, single-cell RNA sequencing (scRNA-seq) has emerged as a powerful tool for investigating the heterogeneity of the tumor microenvironment and intercellular interactions. Through single-cell transcriptomic and spatial transcriptomic analyses of glioma patient samples, murine glioma models, and organoids, researchers have uncovered how glioma cells interact with myeloid-derived cells—including tumor-associated macrophages (TAMs), tumor-associated neutrophils, microglia, and other immune cell types—to establish a highly immunosuppressive microenvironment that further promotes tumor progression and therapeutic resistance (32–35). Emerging evidence highlights the complex interplay between glioma cells and the inflammatory microenvironment. In this context, inflammation is not merely a host response to the tumor but also an active participant in shaping glioma biology. Tumor-associated macrophages (TAMs), along with cytokines such as IL-1β, IL-6, and transforming growth factor-β (TGF-β), contribute to the establishment of a highly immunosuppressive and tumor-promoting niche (36). Activation of inflammatory pathways such as NF-κB and STAT3 not only promotes glioma cell invasion and angiogenesis but also sustains the stem-like properties of glioma stem-like cells (GSCs), ultimately contributing to tumor recurrence and poor prognosis (37, 38).
Despite the detrimental role of inflammation in glioma progression, it also offers potential therapeutic opportunities. Targeting key inflammatory mediators—such as the CCL2/CCR2 axis, CSF1R, or the IL-1β signaling pathway—has demonstrated promising anti-tumor effects in preclinical models (39, 40). Similarly, reprogramming tumor-associated macrophages (TAMs) from a tumor-promoting M2-like phenotype to a tumor-suppressive M1-like phenotype has emerged as a viable strategy to overcome the immunosuppressive tumor microenvironment (41). Moreover, the combination of standard therapy with anti-inflammatory agents, such as ruxolitinib, has already entered phase II clinical trials for the treatment of glioma patients. However, due to the dual and context-dependent role of inflammation in glioma biology, translating these approaches into clinical benefit remains a significant challenge.
With the rapid advancement of artificial intelligence (AI), machine learning (ML) and deep learning (DL) techniques have been widely applied in various aspects of glioma management, including diagnosis, grading, prognosis prediction, and molecular subtype classification. In the field of imaging diagnosis, deep learning models—particularly convolutional neural networks (CNNs) based on MRI and PET images—have demonstrated the ability to perform automatic tumor segmentation, boundary delineation, and grade classification, thereby significantly improving diagnostic efficiency and accuracy (42). At the pathological diagnostic level, AI models can automatically analyze digitized histopathological slides to predict tumor grade, quantify cell density, and assess cellular atypia, thereby assisting pathologists in making more objective and consistent evaluations (43).
In this study, we manually selected the 25 most prominent genes from the most popular research, including S100B, IDO, VEGFA, MGMT, TGFBI, CGAS, CSF1R, PTGS2, HMGB1, CHI3L1, ROS1, NR1D1, IDH1, VISTA, SOCS1, NFKB1, LGALS1, CXCL12, IL12, ALK, TSPO, CD47, YAP, IL10, CX3CR1. These genes have gained significant attention in the field of glioma and inflammation. Studies have shown that VEGFA plays a critical role in promoting angiogenesis, stemness maintenance, and immune evasion in glioma. Bevacizumab has already been used in the treatment of certain glioma patients, targeting VEGFA to inhibit blood vessel formation and tumor growth (44–47). IDH1 mutations are commonly found in low-grade gliomas, but are rare in glioblastomas (GBM). These mutations are associated with a relatively better prognosis for patients with low-grade gliomas (38–40). MGMT (O-6-methylguanine-DNA methyltransferase) methylation in patients significantly enhances the efficacy of temozolomide (TMZ) treatment. Moreover, MGMT also plays a role in regulating angiogenesis and might represent a potential predictive biomarker for glioma patients’ response to radiotherapy (48–52). TGFBI (Transforming Growth Factor Beta Induced) plays a crucial role in maintaining glioma stemness and promoting tumor growth. It is involved in regulating the tumor microenvironment, cell signaling pathways, and cellular interactions that support the growth and maintenance of glioma stem cells, which are critical for the tumor’s aggressive nature and resistance to treatment (38, 53). CSF1R (Colony Stimulating Factor 1 Receptor) promotes the recruitment of immune-suppressive macrophages to the tumor site, contributing to the immunosuppressive microenvironment that facilitates glioma progression and resistance to therapies. Targeting and blocking CSF1R has emerged as a promising therapeutic strategy for glioma, as it could inhibit macrophage recruitment, reprogram the immune landscape, and potentially enhance the effectiveness of other treatments, such as immunotherapy (40, 54). However, the presence of the immunosuppressive microenvironment in glioma makes its treatment less effective. In conclusion, the research focus in the field of glioma and inflammation is gradually shifting towards tumor immunity and the tumor microenvironment, which may serve as a potential breakthrough for glioma treatment.
Limitation
Several limitations of this study should be acknowledged. First, the analysis was confined to publications written in English. This decision was made to ensure consistency and to facilitate accurate interpretation of the bibliometric data; however, it may have resulted in the exclusion of relevant research published in other languages, introducing a potential language bias. Second, the data source was limited to the Web of Science Core Collection (WoSCC), which was selected because of its rigorous indexing standards, comprehensive citation information, and widespread use in bibliometric research. Nevertheless, this choice may have led to the omission of pertinent studies indexed in other databases, such as Scopus, PubMed, or regional repositories. While these constraints were necessary to maintain the feasibility and comparability of the analysis, they may limit the completeness and generalizability of the findings. Future studies could address these limitations by incorporating literature from multiple databases and including non-English publications to provide a more comprehensive overview of the research landscape.
Conclusion
Overall, research on glioma and inflammation is receiving increasing attention, largely because tumor immunity and the tumor microenvironment are seen as promising solutions to the challenges in glioma treatment. This article summarizes the key papers, core journals, keywords, research institutions, and authors in the field of glioma and inflammation. In conclusion, inflammation is a double-edged sword in glioma pathogenesis. While it supports tumor growth and immune escape, it also provides potential vulnerabilities that can be exploited therapeutically. Future research should focus on deciphering the spatiotemporal dynamics of inflammatory signals in gliomas and developing precise strategies to modulate the glioma-associated inflammation for therapeutic gain.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
XC: Formal Analysis, Writing – original draft. JWa: Writing – review & editing, Formal Analysis. SL: Data curation, Supervision, Writing – review & editing. QH: Software, Validation, Writing – review & editing. HZ: Methodology, Resources, Writing – review & editing. JWu: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the following funding: (1) Innovation Fund of the First Affiliated Hospital of Harbin Medical University (2021M24). (2) Heilongjiang Provincial Key R&D Program (Guided Project). (3) Heilongjiang Welfare Fund Organization of Disabled Persons (HJ2022-land2023HX037).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. (2005) 352:987–96. doi: 10.1056/NEJMoa043330
2. Bazan NG, Reid MM, Flores VAC, Gallo JE, Lewis W, and Belayev L. Multiprong control of glioblastoma multiforme invasiveness: blockade of pro-inflammatory signaling, anti-angiogenesis, and homeostasis restoration. Cancer Metastasis Rev. (2021) 40:643–7. doi: 10.1007/s10555-021-09987-x
3. Zhang L, Jiang Y, Zhang G, and Wei S. The diversity and dynamics of tumor-associated macrophages in recurrent glioblastoma. Front Immunol. (2023) 14:1238233. doi: 10.3389/fimmu.2023.1238233
4. Khan F, Pang L, Dunterman M, Lesniak MS, Heimberger AB, and Chen P. Macrophages and microglia in glioblastoma: heterogeneity, plasticity, and therapy. J Clin Invest. (2023) 133. doi: 10.1172/JCI163446
5. Ulasov I, Singh V, Laevskaya A, Timashev P, and Kharwar RK. Inflammatory mediators and GBM Malignancy: current scenario and future prospective. Discov Med. (2023) 35:458–75. doi: 10.24976/Discov.Med.202335177.47
6. Li D, Wang X, Chen K, Shan D, Cui G, Yuan W, et al. IFI35 regulates non-canonical NF-κB signaling to maintain glioblastoma stem cells and recruit tumor-associated macrophages. Cell Death Differ. (2024) 31:738–52. doi: 10.1038/s41418-024-01292-8
7. Ius T, Ciani Y, Ruaro ME, Isola M, Sorrentino M, Bulfoni M, et al. An NF-κB signature predicts low-grade glioma prognosis: a precision medicine approach based on patient-derived stem cells. Neuro Oncol. (2018) 20:776–87. doi: 10.1093/neuonc/nox234
8. Doucette TA, Kong LY, Yang Y, Ferguson SD, Yang J, Wei J, et al. Signal transducer and activator of transcription 3 promotes angiogenesis and drives Malignant progression in glioma. Neuro Oncol. (2012) 14:1136–45. doi: 10.1093/neuonc/nos139
9. Lee-Chang C and Lesniak MS. Next-generation antigen-presenting cell immune therapeutics for gliomas. J Clin Invest. (2023) 133. doi: 10.1172/JCI163449
10. Arrieta VA, Dmello C, McGrail DJ, Brat DJ, Lee-Chang C, Heimberger AB, et al. Immune checkpoint blockade in glioblastoma: from tumor heterogeneity to personalized treatment. J Clin Invest. (2023) 133. doi: 10.1172/JCI163447
11. Tomiyama A and Ichimura K. Signal transduction pathways and resistance to targeted therapies in glioma. Semin Cancer Biol. (2019) 58:118–29. doi: 10.1016/j.semcancer.2019.01.004
12. Zhu C, Kros JM, Cheng C, and Mustafa D. The contribution of tumor-associated macrophages in glioma neo-angiogenesis and implications for anti-angiogenic strategies. Neuro Oncol. (2017) 19:1435–46. doi: 10.1093/neuonc/nox081
13. Jiang S, Liu Y, Zheng H, Zhang L, Zhao H, Sang X, et al. Evolutionary patterns and research frontiers in neoadjuvant immunotherapy: a bibliometric analysis. Int J Surg. (2023) 109:2774–83. doi: 10.1097/JS9.0000000000000492
14. Zeng X, Shu B, Zeng Q, Wang X, Li K, Wu J, et al. A bibliometric and visualization analysis of global research status and frontiers on autophagy in cardiomyopathies from 2004 to 2023. Int J Surg. (2024) 110:7687–700. doi: 10.1097/JS9.0000000000001876
15. Derviş H. Bibliometric analysis using bibliometrix an R package. J Scientometric Res. (2019) 8:156–60.
16. Hassan-Montero Y, De-Moya-Anegón F, and Guerrero-Bote VP. SCImago Graphica: a new tool for exploring and visually communicating data. Profesional La Información. (2022) 31. doi: 10.3145/EPI
17. Chen C. CiteSpace: a practical guide for mapping scientific literature. NY, USA: Nova Science Publishers Hauppauge (2016).
18. Van Eck N and Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. scientometrics. (2010) 84:523–38. doi: 10.1007/s11192-009-0146-3
19. Szklarczyk D, Kirsch R, Koutrouli M, Nastou K, Mehryary F, Hachilif R, et al. The STRING database in 2023: protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. (2023) 51:D638–46. doi: 10.1093/nar/gkac1000
20. Hanahan D and Weinberg RA. Hallmarks of cancer: the next generation. cell. (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
21. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro-oncology. (2021) 23:1231–51. doi: 10.1093/neuonc/noab106
22. Barker HE, Paget JT, Khan AA, and Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. (2015) 15:409–25. doi: 10.1038/nrc3958
23. Xue J, Zhao Z, Zhang L, Xue L, Shen S, Wen Y, et al. Neutrophil-mediated anticancer drug delivery for suppression of postoperative Malignant glioma recurrence. Nat Nanotechnol. (2017) 12:692–700. doi: 10.1038/nnano.2017.54
24. Abdelwahab SI, Taha MME, Farasani A, Abdullah SM, Moshi JM, Alshahrani AF, et al. Bibliometric analysis: A few suggestions (Part Two). Curr Probl Cardiol. (2025) 50:102982. doi: 10.1016/j.cpcardiol.2025.102982
25. Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. (2010) 17:98–110. doi: 10.1016/j.ccr.2009.12.020
26. Li S, Chen Y, Xie Y, Zhan H, Zeng Y, Zeng K, et al. FBXO7 Confers Mesenchymal Properties and Chemoresistance in Glioblastoma by Controlling Rbfox2-Mediated Alternative Splicing. Adv Sci (Weinh). (2023) 10:e2303561. doi: 10.1002/advs.202303561
27. Fan Y, Gao Z, Xu J, Wang H, Guo Q, Li B, et al. SPI1-mediated MIR222HG transcription promotes proneural-to-mesenchymal transition of glioma stem cells and immunosuppressive polarization of macrophages. Theranostics. (2023) 13:3310–29. doi: 10.7150/thno.82590
28. Wang L, Jung J, Babikir H, Shamardani K, Jain S, Feng X, et al. A single-cell atlas of glioblastoma evolution under therapy reveals cell-intrinsic and cell-extrinsic therapeutic targets. Nat Cancer. (2022) 3:1534–52. doi: 10.1038/s43018-022-00475-x
29. Alghamri MS, McClellan BL, Hartlage CS, Haase S, Faisal SM, Thalla R, et al. Targeting Neuroinflammation in Brain Cancer: Uncovering Mechanisms, Pharmacological Targets, and Neuropharmaceutical Developments. Front Pharmacol. (2021) 12:680021. doi: 10.3389/fphar.2021.680021
30. Liu H, Sun Y, Zhang Q, Jin W, Gordon RE, Zhang Y, et al. Pro-inflammatory and proliferative microglia drive progression of glioblastoma. Cell Rep. (2021) 36:109718. doi: 10.1016/j.celrep.2021.109718
31. Li S, Guo Y, Hu H, Gao N, Yan X, Zhou Q, et al. TANK shapes an immunosuppressive microenvironment and predicts prognosis and therapeutic response in glioma. Front Immunol. (2023) 14:1138203. doi: 10.3389/fimmu.2023.1138203
32. Klemm F, Maas RR, Bowman RL, Kornete M, Soukup K, Nassiri S, et al. Interrogation of the Microenvironmental Landscape in Brain Tumors Reveals Disease-Specific Alterations of Immune Cells. Cell. (2020) 181:1643–1660.e1617. doi: 10.1016/j.cell.2020.05.007
33. Miller TE, El Farran CA, Couturier CP, Chen Z, D’Antonio JP, Verga J, et al. Programs, origins and immunomodulatory functions of myeloid cells in glioma. Nature. (2025) 640:1072–82. doi: 10.1038/s41586-025-08633-8
34. Kirschenbaum D, Xie K, Ingelfinger F, Katzenelenbogen Y, Abadie K, Look T, et al. Time-resolved single-cell transcriptomics defines immune trajectories in glioblastoma. Cell. (2024) 187:149–165.e123. doi: 10.1016/j.cell.2023.11.032
35. Pombo Antunes AR, Scheyltjens I, Lodi F, Messiaen J, Antoranz A, Duerinck J, et al. Single-cell profiling of myeloid cells in glioblastoma across species and disease stage reveals macrophage competition and specialization. Nat Neurosci. (2021) 24:595–610. doi: 10.1038/s41593-020-00789-y
36. Wang W, Li T, Cheng Y, Li F, Qi S, Mao M, et al. Identification of hypoxic macrophages in glioblastoma with therapeutic potential for vasculature normalization. Cancer Cell. (2024) 42:815–832.e812. doi: 10.1016/j.ccell.2024.03.013
37. Yan Y, Bai S, Han H, Dai J, Niu L, Wang H, et al. Knockdown of trem2 promotes proinflammatory microglia and inhibits glioma progression via the JAK2/STAT3 and NF-κB pathways. Cell Commun Signal. (2024) 22:272. doi: 10.1186/s12964-024-01642-6
38. Peng P, Zhu H, Liu D, Chen Z, Zhang X, Guo Z, et al. TGFBI secreted by tumor-associated macrophages promotes glioblastoma stem cell-driven tumor growth via integrin αvβ5-Src-Stat3 signaling. Theranostics. (2022) 12:4221–36. doi: 10.7150/thno.69605
39. Takacs GP, Kreiger CJ, Luo D, Tian G, Garcia JS, Deleyrolle LP, et al. Glioma-derived CCL2 and CCL7 mediate migration of immune suppressive CCR2(+)/CX3CR1(+) M-MDSCs into the tumor microenvironment in a redundant manner. Front Immunol. (2022) 13:993444. doi: 10.3389/fimmu.2022.993444
40. Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. (2013) 19:1264–72. doi: 10.1038/nm.3337
41. Chryplewicz A, Scotton J, Tichet M, Zomer A, Shchors K, Joyce JA, et al. Cancer cell autophagy, reprogrammed macrophages, and remodeled vasculature in glioblastoma triggers tumor immunity. Cancer Cell. (2022) 40:1111–1127.e1119. doi: 10.1016/j.ccell.2022.08.014
42. van Kempen EJ, Post M, Mannil M, Witkam RL, Ter Laan M, Patel A, et al. Performance of machine learning algorithms for glioma segmentation of brain MRI: a systematic literature review and meta-analysis. Eur Radiol. (2021) 31:9638–53. doi: 10.1007/s00330-021-08035-0
43. Nasrallah MP, Zhao J, Tsai CC, Meredith D, Marostica E, Ligon KL, et al. Machine learning for cryosection pathology predicts the 2021 WHO classification of glioma. Med. (2023) 4:526–540.e524. doi: 10.1016/j.medj.2023.06.002
44. Hiller-Vallina S, Mondejar-Ruescas L, Caamaño-Moreno M, Cómitre-Mariano B, Alcivar-López D, Sepulveda JM, et al. Sexual-biased necroinflammation is revealed as a predictor of bevacizumab benefit in glioblastoma. Neuro Oncol. (2024) 26:1213–27. doi: 10.1093/neuonc/noae033
45. Simon T, Pinioti S, Schellenberger P, Rajeeve V, Wendler F, Cutillas PR, et al. Shedding of bevacizumab in tumour cells-derived extracellular vesicles as a new therapeutic escape mechanism in glioblastoma. Mol Cancer. (2018) 17:132. doi: 10.1186/s12943-018-0878-x
46. Treps L, Perret R, Edmond S, Ricard D, and Gavard J. Glioblastoma stem-like cells secrete the pro-angiogenic VEGF-A factor in extracellular vesicles. J Extracell Vesicles. (2017) 6:1359479. doi: 10.1080/20013078.2017.1359479
47. Hamerlik P, Lathia JD, Rasmussen R, Wu Q, Bartkova J, Lee M, et al. Autocrine VEGF-VEGFR2-Neuropilin-1 signaling promotes glioma stem-like cell viability and tumor growth. J Exp Med. (2012) 209:507–20. doi: 10.1084/jem.20111424
48. Xin L, Tan Y, Zhu Y, Cui X, Wang Q, Zhao J, et al. EPIC-0307-mediated selective disruption of PRADX-EZH2 interaction and enhancement of temozolomide sensitivity to glioblastoma via inhibiting DNA repair and MGMT. Neuro Oncol. (2023) 25:1976–88. doi: 10.1093/neuonc/noad102
49. Mansouri A, Hachem LD, Mansouri S, Nassiri F, Laperriere NJ, Xia D, et al. MGMT promoter methylation status testing to guide therapy for glioblastoma: refining the approach based on emerging evidence and current challenges. Neuro Oncol. (2019) 21:167–78. doi: 10.1093/neuonc/noy132
50. Cen L, Carlson BL, Pokorny JL, Mladek AC, Grogan PT, Schroeder MA, et al. Efficacy of protracted temozolomide dosing is limited in MGMT unmethylated GBM xenograft models. Neuro Oncol. (2013) 15:735–46. doi: 10.1093/neuonc/not010
51. Rivera AL, Pelloski CE, Gilbert MR, Colman H, de la Cruz C, Sulman EP, et al. MGMT promoter methylation is predictive of response to radiotherapy and prognostic in the absence of adjuvant alkylating chemotherapy for glioblastoma. Neuro Oncol. (2010) 12:116–21. doi: 10.1093/neuonc/nop020
52. Chahal M, Xu Y, Lesniak D, Graham K, Famulski K, Christensen JG, et al. MGMT modulates glioblastoma angiogenesis and response to the tyrosine kinase inhibitor sunitinib. Neuro Oncol. (2010) 12:822–33. doi: 10.1093/neuonc/noq017
53. Chen Z, Wang J, Peng P, Liu G, Dong M, Zhang X, et al. Hypoxia-induced TGFBI maintains glioma stem cells by stabilizing EphA2. Theranostics. (2024) 14:5778–92. doi: 10.7150/thno.95141
54. Quail DF, Bowman RL, Akkari L, Quick ML, Schuhmacher AJ, Huse JT, et al. The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science. (2016) 352:aad3018. doi: 10.1126/science.aad3018
55. Doherty JR and Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest. (2013) 123(9):3685–92. doi: 10.1172/JCI69741
56. Roos WP, Thomas AD, and Kaina B. DNA damage and the balance between survival and death in cancer biology. Nat Rev Cancer. (2016) 16(1):20–33. doi: 10.1038/nrc.2015.2
57. Wilken R, Veena MS, Wang MB, and Srivatsan ES. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Cancer. (2011) 10:12. doi: 10.1186/1476-4598-10-12
58. Stylianopoulos T, Martin JD, Chauhan VP, Jain SR, Diop-Frimpong B, Bardeesy N, et al. Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc Natl Acad Sci U S A. (2012) 109(38):15101–8. doi: 10.1073/pnas.1213353109
59. Tang PM, Nikolic-Paterson DJ, and Lan HY. Macrophages: versatile players in renal inflammation and fibrosis. Nat Rev Nephrol. (201) 15(3):144–158. doi: 10.1038/s41581-019-0110-2
60. Janku F, McConkey DJ, Hong DS, and Kurzrock R. Autophagy as a target for anticancer therapy. Nat Rev Clin Oncol. (2011) 8(9):528–39. doi: 10.1038/nrclinonc.2011.71
61. Komohara Y, Ohnishi K, Kuratsu J, and Takeya M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol. (2008) 216(1):15–24. doi: 10.1002/path.2370
Keywords: bibliometrics, glioma, inflammation, VOSviewer, hotspots
Citation: Chen X, Wang J, Liu S, Han Q, Zhang H and Wu J (2025) Research landscape of glioma and inflammation over the past two decades. Front. Immunol. 16:1605346. doi: 10.3389/fimmu.2025.1605346
Received: 03 April 2025; Accepted: 29 July 2025;
Published: 13 August 2025.
Edited by:
Sonam Mittal, Medical College of Wisconsin, United StatesReviewed by:
Zhenyu Gong, Technical University of Munich, GermanyBishnu Joshi, Inc. (A Harvard Innovation Labs Company), United States
Copyright © 2025 Chen, Wang, Liu, Han, Zhang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinrong Wu, d3VqaW5yb25nQGhyYm11LmVkdS5jbg==
 Xiaoli Chen
Xiaoli Chen Jiabin Wang2
Jiabin Wang2