- Department of Rheumatology and Immunology, Jiangxi Children’s Hospital, Nanchang Medical College, Nanchang, Jiangxi, China
Objective: This study aimed to investigate the trajectory of complement C3 and C4 levels during tocilizumab (TCZ) treatment in patients with systemic juvenile idiopathic arthritis (sJIA), explore the dynamic relationship between hypocomplementemia and disease activity, and characterize adverse events during long-term TCZ therapy.
Methods: A retrospective analysis was conducted on 19 sJIA patients diagnosed according to the 2019 PRINTO criteria. Clinical data, including C3 and C4 levels, disease activity (sJADAS10-ESR score), and adverse events, were collected at baseline and at intervals from 2 to 96 weeks following TCZ initiation. Statistical analyses were conducted accordingly.
Results: The 19 analyzed patients (6 males, 31.58%; 13 females, 68.42%) had a median (IQR) age of disease onset of 6.75 (4.58-9.42) years and a median (IQR) follow-up duration of 2.08 (1.83-2.67) years. After 2 weeks of TCZ treatment, median (IQR) serum C3 levels declined from 1.45(1.27-1.75) g/L at baseline to 1.11 (0.97-1.18) g/L (a 23.45% reduction, P = 0.009), and C4 levels decreased from 0.29 (0.21-0.38) g/L to 0.13 (0.09-0.17) g/L (a 55.17% reduction, P = 0.005). At week 48, hypocomplementemia was observed in 68.42% of patients for C3 and 26.32% for C4. The mixed linear model revealed significant reductions in C3 (β = -0.058, P < 0.001), C4 (β = -0.061, P = 0.022), and sJADAS10-ESR scores (β = -0.628, P < 0.001) across all time points compared to baseline. Longitudinal Spearman analysis revealed a positive correlation between complement levels and disease activity at specific stages: C3 (r = 0.529, P = 0.029) and C4 (r = 0.577, P = 0.015) were most strongly correlated with sJADAS10-ESR at week 24. Notably, C3 remained significantly correlated at week 48 (r = 0.513, P = 0.025). Acute upper respiratory tract infections were the most common adverse events (occurring in 63.16% of patients), while no serious infections or new autoimmune diseases were reported.
Conclusions: Complement C3 and C4 levels during TCZ treatment follow a trajectory characterized by a rapid early decline followed by a sustained low-level plateau. Long-term hypocomplementemia appears to be well tolerated, with no increased risk of serious infections or autoimmune complications. These findings suggest that hypocomplementemia reflects deep IL-6 signaling inhibition rather than pathological complement consumption.
1 Introduction
Systemic juvenile idiopathic arthritis (sJIA) is a pediatric rheumatic disease characterized by systemic inflammation and immune dysregulation (1). Interleukin-6 (IL-6) plays a pivotal role in its pathogenesis by binding to a receptor complex composed of the IL-6 receptor (IL-6R) and two gp130 molecules (2, 3). This interaction activates the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway, which upregulates pro-inflammatory gene expression and drives disease progression (4). Tocilizumab (TCZ), a humanized monoclonal antibody targeting IL-6R, has become a cornerstone therapy for sJIA. By blocking IL-6 signaling, TCZ effectively reduces systemic inflammation and joint symptoms (5, 6). Consequently, it is now recommended as a first-line biologic agent in international treatment guidelines (7).
The complement system, a key component of innate immunity, contributes to pathogen clearance and regulation of inflammatory responses (8). Complement components C3 and C4, primarily synthesized in the liver, are positively regulated by IL-6 (9). In both rheumatoid arthritis (RA) and juvenile idiopathic arthritis (JIA), C3 and C4 play essential roles in disease pathogenesis. Genetic variations, copy number alterations, and autoantibodies targeting these components have been linked to disease susceptibility, severity, and clinical phenotype (10–13). In RA, complement activation is closely associated with disease progression, with synovial deposition of complement components contributing to exacerbated inflammation and joint damage (14, 15). Recent clinical observations suggest that TCZ treatment induces a persistent decline in serum C3 and C4 levels across various rheumatic diseases (16, 17). However, the temporal dynamics of complement reduction, its relationship with disease activity, and the long-term clinical implications in sJIA remain unclear. To investigate these unclear points, a 96-week longitudinal study was conducted to assess changes in complement C3 and C4 levels during TCZ therapy in sJIA. The investigation focused on (1) the temporal patterns of hypocomplementemia, (2) its correlation with disease activity, and (3) the long-term implications of TCZ-induced complement reduction.
2 Methods
2.1 Patients
This single-center retrospective cohort study included patients newly diagnosed with sJIA who received sequential TCZ treatment at the Department of Rheumatology and Immunology, Jiangxi Children’s Hospital, between January 2021 and May 2024. The study protocol was approved by the Ethics Committee of Jiangxi Children’s Hospital (Approval No: JXSEYY-YXKY-20240174).
2.2 Inclusion and exclusion criteria for study subjects
2.2.1 Inclusion criteria
Patients meeting the following criteria were included: ① Diagnosis of sJIA based on the 2019 criteria established by the Pediatric Rheumatology International Trials Organization (PRINTO) (18). ② Aged ≤ 18 years at the time of diagnosis. ③Received standard TCZ treatment (12 mg/kg for body weight ≤30kg and 8 mg/kg for body weight > 30kg). After 12 weeks, the dosing interval could be extended according to clinical remission status. All patients received at least 12 TCZ infusions, and the treatment duration was ≥48 weeks. ④ Did not receive other biologic agents or drugs known to modulate complement levels during the treatment period.
2.2.2 Exclusion criteria
Patients were excluded if they met any of the following criteria: ①Coexistence of primary conditions known to affect complement levels, including systemic lupus erythematosus, hereditary complement deficiencies, or nephrotic syndrome. ② Baseline hypocomplementemia (C3 < 0.90 g/L or C4 < 0.10 g/L). ③ Development of secondary complement-consuming conditions during treatment, such as sepsis, streptococcal infections, or severe liver failure. ④ Incomplete clinical records, including missing disease activity scores or treatment data, or loss to follow-up.
2.3 Collection and observation indicators
2.3.1 Baseline data
The following baseline characteristics were collected: demographics (age, gender), disease activity (sJADAS10-ESR score), concurrent medication use (glucocorticoid dosage and methotrexate administration), autoantibody profiles, and hepatic and renal function parameters.
2.3.2 Core observation indicators
Dynamic monitoring of complement levels: Serum C3 and C4 levels were measured at baseline and at 2, 4, 8, 24, 48, and 96 weeks after initiation of TCZ treatment. Measurements were performed using immunoturbidimetric assays. Normal reference ranges were defined as follows: C3, 0.90–1.80 g/L; C4, 0.10–0.40 g/L. Hypocomplementemia was defined as C3 < 0.90 g/L and/or C4 < 0.10 g/L.
Disease activity assessment: Disease activity was evaluated using sJADAS10-ESR, which incorporates erythrocyte sedimentation rate (ESR), body temperature, active joint count, and global assessments by both physicians and parents.
Adverse events: Adverse events, including infections, new-onset autoimmune diseases, and malignancies, were recorded during the treatment period. Severe infections were defined as those posing a life-threatening risk or leading to severe complications.
2.4 Statistical analysis
All statistical analyses were conducted using SPSS Statistics version 26.0 (IBM, USA). Continuous variables were expressed as medians with interquartile ranges (IQR), and categorical variables were presented as frequencies and percentages. Data distribution was assessed using the Kolmogorov–Smirnov test in combination with histogram inspection. Results indicated that serum C3 and C4 levels followed a gamma distribution, while sJADAS10-ESR scores conformed to a Poisson distribution. Changes in complement levels (C3 and C4) and sJADAS10-ESR scores before and after TCZ treatment were analyzed using the Wilcoxon signed-rank test. Longitudinal changes in complement levels were assessed using a generalized linear mixed model (gamma regression with log link function), and disease activity was analyzed using a generalized linear mixed model with a Poisson distribution and log link function. Spearman rank correlation analysis was employed to evaluate associations between serum C3/C4 levels and sJADAS10-ESR scores at each post-treatment time point. Adverse events were summarized using cumulative frequencies and the proportion of patients affected. Statistical significance was defined as a two-sided P-value < 0.05.
3 Results
3.1 Clinical characteristics and follow-up
According to the inclusion criteria, 20 patients were initially enrolled in the study, with 1 excluded due to incomplete clinical records. As summarized in Table 1, this retrospective study included 19 patients (6 males, 31.58%; 13 females, 68.42%) with a median (IQR) age of disease onset of 6.75 (4.58-9.42) years. The median (IQR) follow-up duration was 2.08 (1.83-2.67) years. Before TCZ treatment, 94.74% (18/19) of patients were treated with glucocorticoids, and 73.68% (14/19) were treated with methotrexate. The median (IQR) sJADAS10-ESR score was 31.30 (25.20-35.00), indicating high disease activity in all cases. After 48 weeks of TCZ therapy, all patients discontinued glucocorticoid treatment, 36.84% (7/19) discontinued methotrexate treatment, and the sJADAS10-ESR score decreased to 0 (0-5.00).
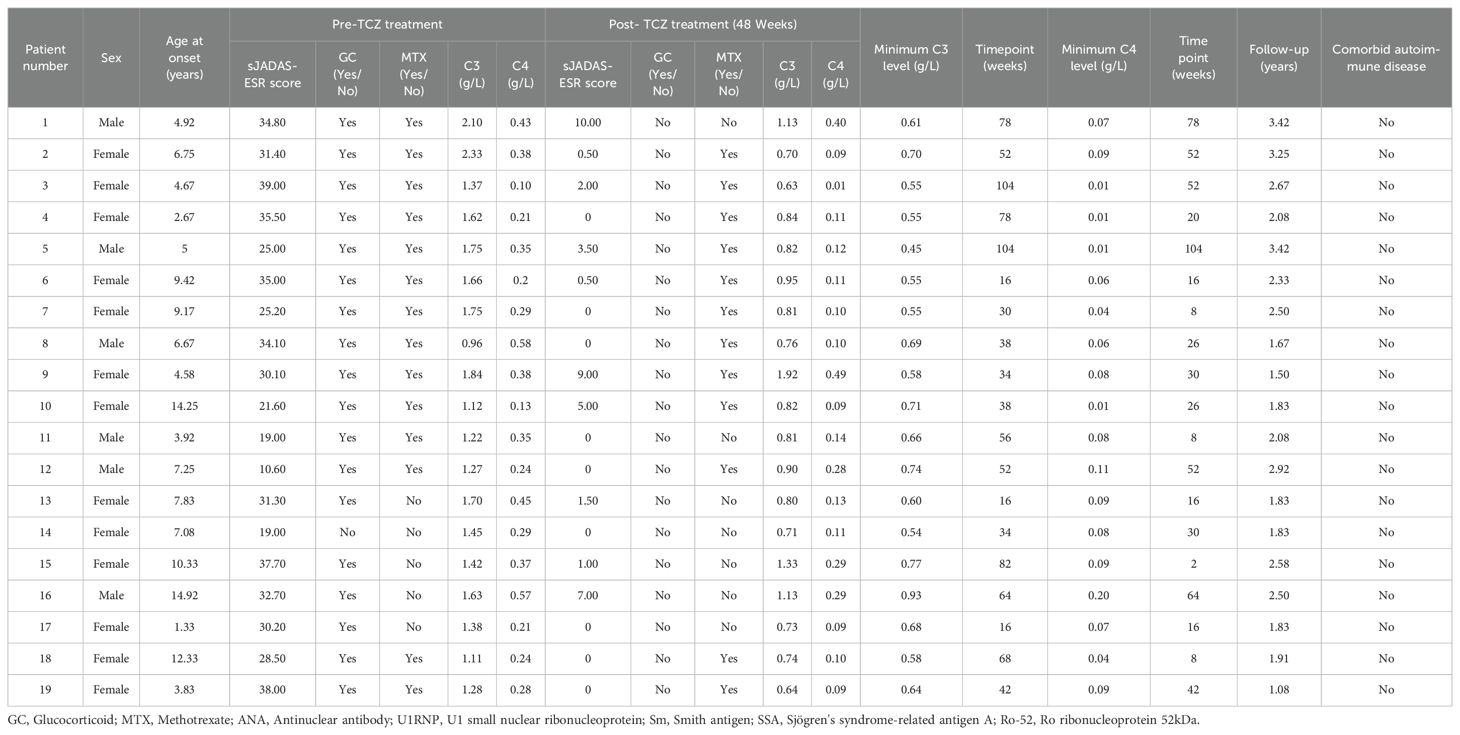
Table 1. Baseline characteristics of systemic juvenile idiopathic arthritis patients treated with tocilizumab (n=19).
3.2 Complement level changes and correlation with disease activity
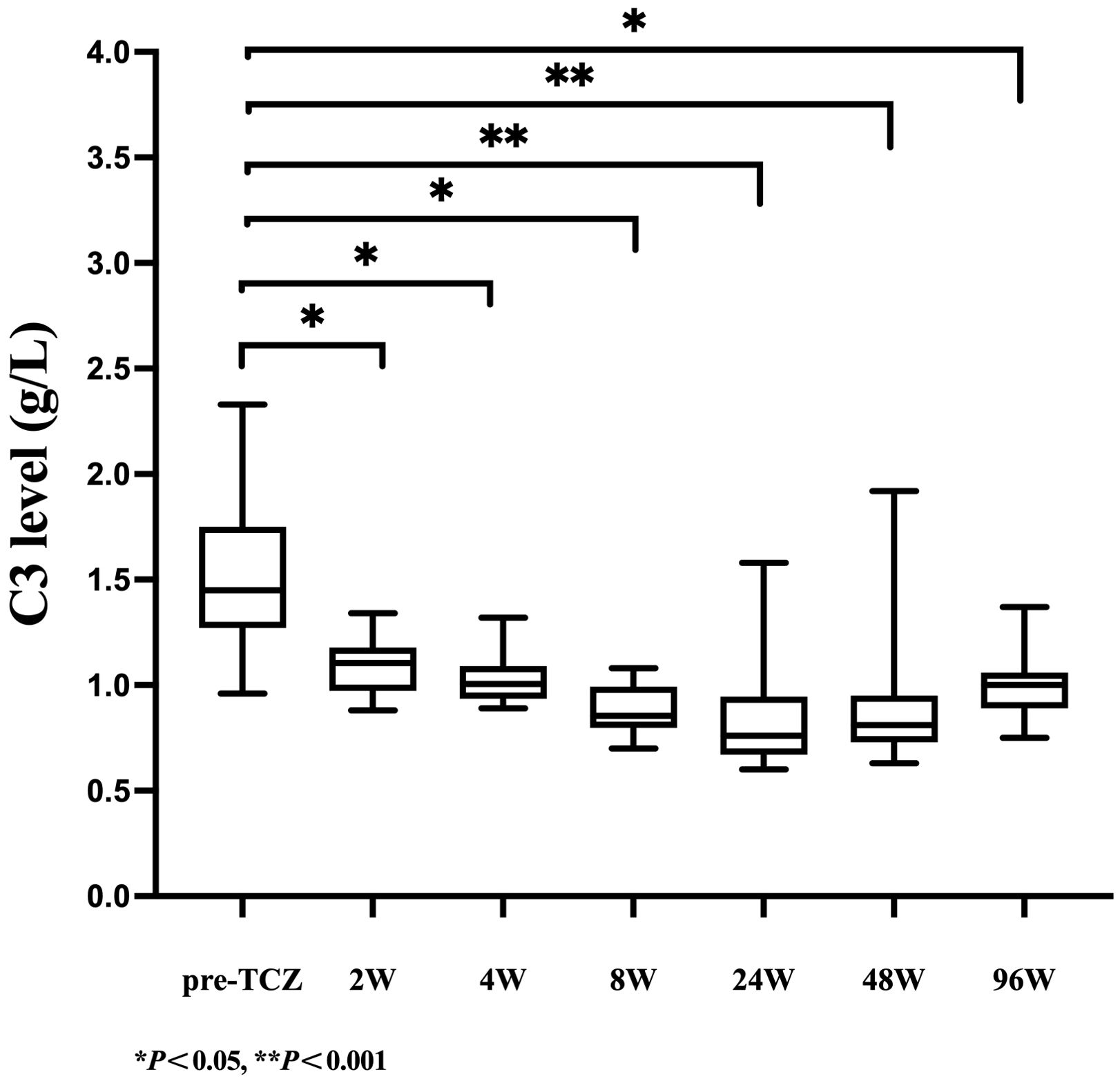
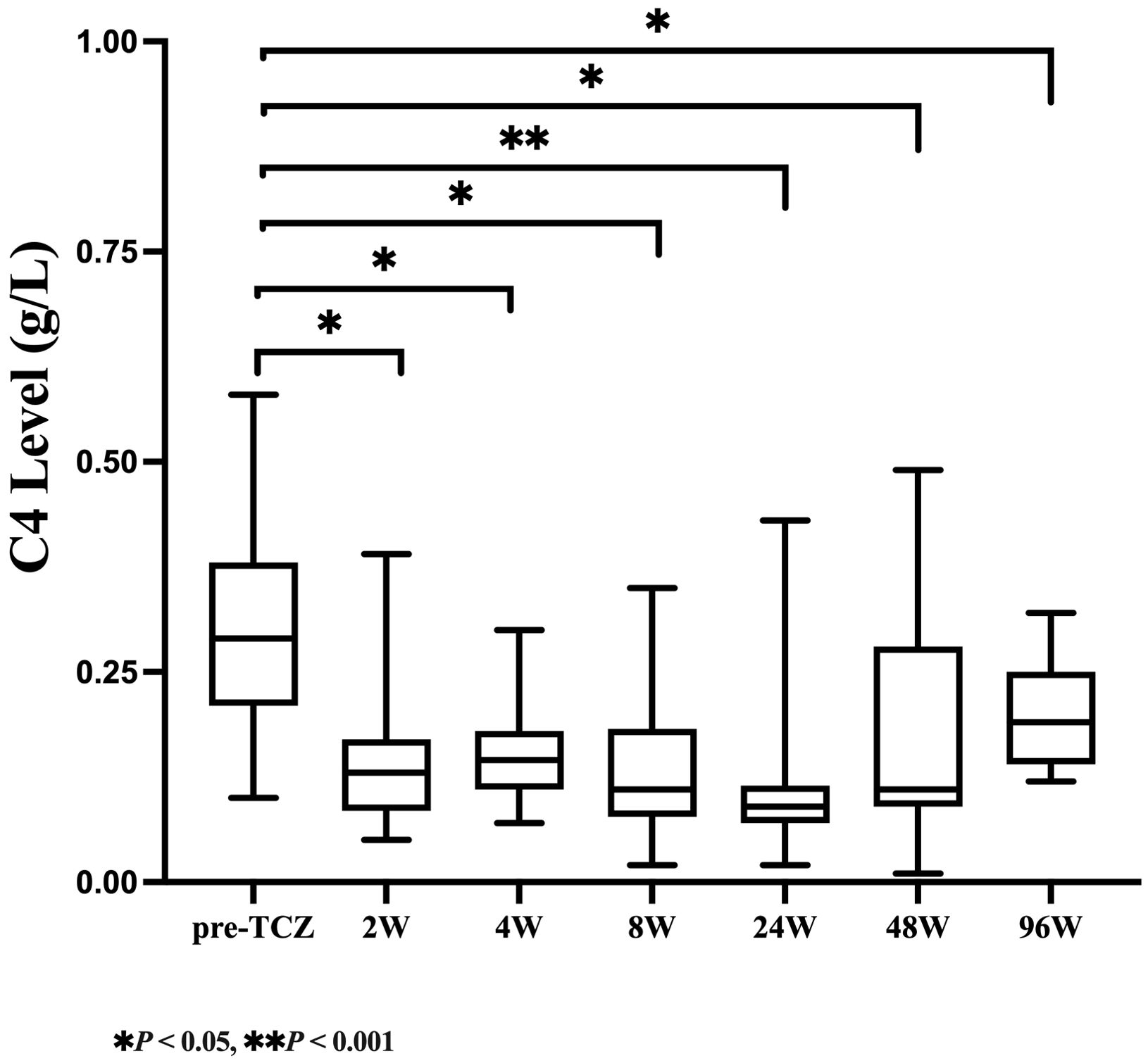
As shown in Table 2, serum C3 levels declined rapidly after 2 weeks of TCZ treatment, with the median (IQR) value decreasing from 1.45 (1.27-1.75) g/L at baseline to 1.11 (0.97-1.18) g/L (a 23.45% reduction, P=0.009). Over the same period, a more pronounced decrease was observed in C4 levels, which dropped from 0.29 (0.21-0.38) g/L to 0.13 (0.09-0.17) g/L (a 55.17% reduction, P = 0.005. At week 48, the median (IQR) C3 level remained at 0.81 (0.73-0.95) g/L, with 68.42% of patients developing C3 hypocomplementemia. The median (IQR) C4 level was 0.11 (0.09-0.28) g/L, and 26.32% of patients exhibited C4 hypocomplementemia. By week 96, both C3 and C4 levels had slightly recovered to 1.00 (0.89-1.06) g/L and 0.19 (0.14-0.25) g/L, respectively, but remained significantly lower than baseline (with reductions of 31.03% and 34.48%, respectively, P < 0.05). As illustrated in Figures 1, 2, complement levels followed a trajectory characterized by a rapid early decline and a sustained low-level plateau, without evidence of rebound over time or in response to extended TCZ dosing intervals.
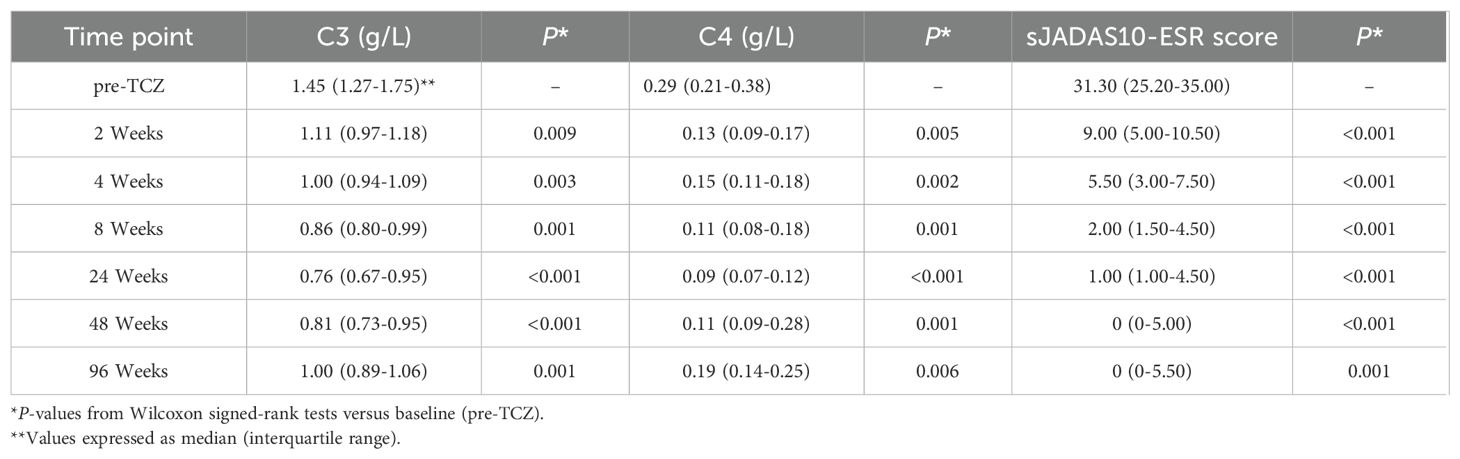
Table 2. Longitudinal changes in serum C3/C4 levels and disease activity during tocilizumab treatment.
The mixed linear model (Table 3) confirmed significant downward trends over time in C3 (β = - 0.058, P < 0.001), C4 (β = -0.061, P = 0.022), and sJADAS10-ESR (β = -0.628, P < 0.001). Longitudinal Spearman correlation analysis revealed stage-specific associations. As shown in Table 4, C3 levels were positively correlated with sJADAS10 - ESR at both week 24 (r = 0.529, P = 0.029) and week 48 (r = 0.513, P = 0.025). As shown in Table 5, a significant correlation between C4 and disease activity was observed only at week 24 (r = 0.577, P = 0.015). However, no significant correlations were observed at baseline or at weeks 2, 4, 8, or 96(all P > 0.05).
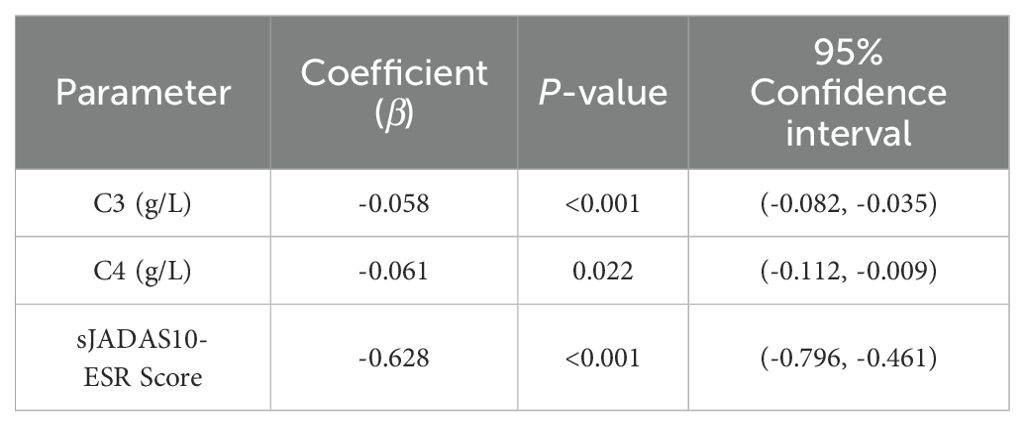
Table 3. Mixed linear models comparing C3- C4, and sJADAS10-ESR levels across treatment time points relative to baseline.
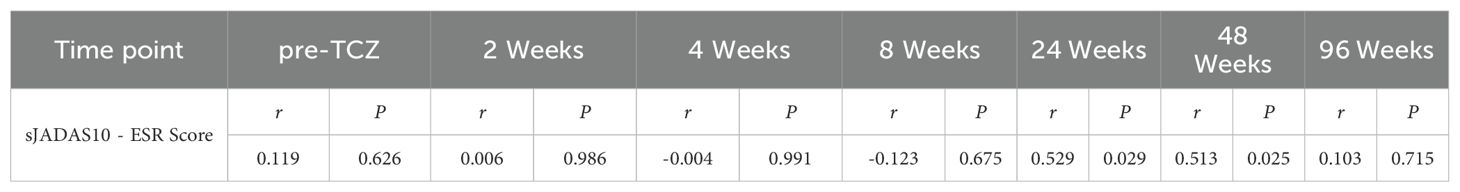
Table 4. Spearman correlation between serum C3 and disease activity (sJADAS10-ESR) during tocilizumab therapy.
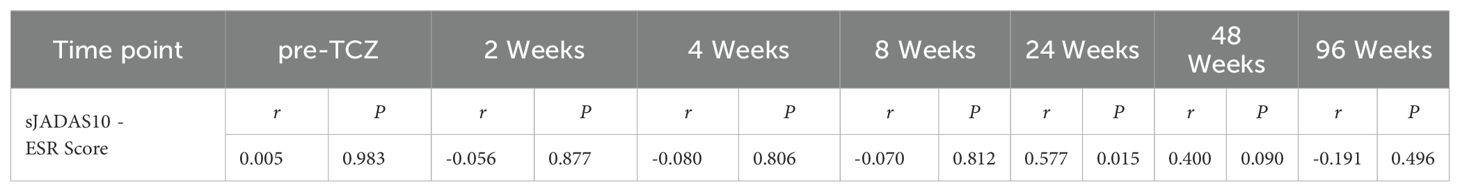
Table 5. Spearman correlation between serum C4 and disease activity (sJADAS10-ESR) during tocilizumab therapy.
3.3 Adverse events
A total of 62 adverse events were documented during a median (IQR) follow-up period of 2.08 (1.83-2.67) years, as detailed in Table 6. Infectious events were primarily composed of acute upper respiratory tract infections, reported in 63.16% of patients (12/19), followed by acute bronchitis in 42.11% (8/19) and pneumonia in 10.53% (2/19). Among non-infectious events, the most frequent were abnormal liver function in 21.05% (4/19) and leukopenia in 15.80% (3/19). No cases of severe infection, new-onset autoimmune disease, or malignancy were observed during the follow-up period. One patient (5.26%) developed a duodenal bulb ulcer with gastrointestinal bleeding, which was attributed to the combined use of glucocorticoids and non-steroidal anti-inflammatory drugs.
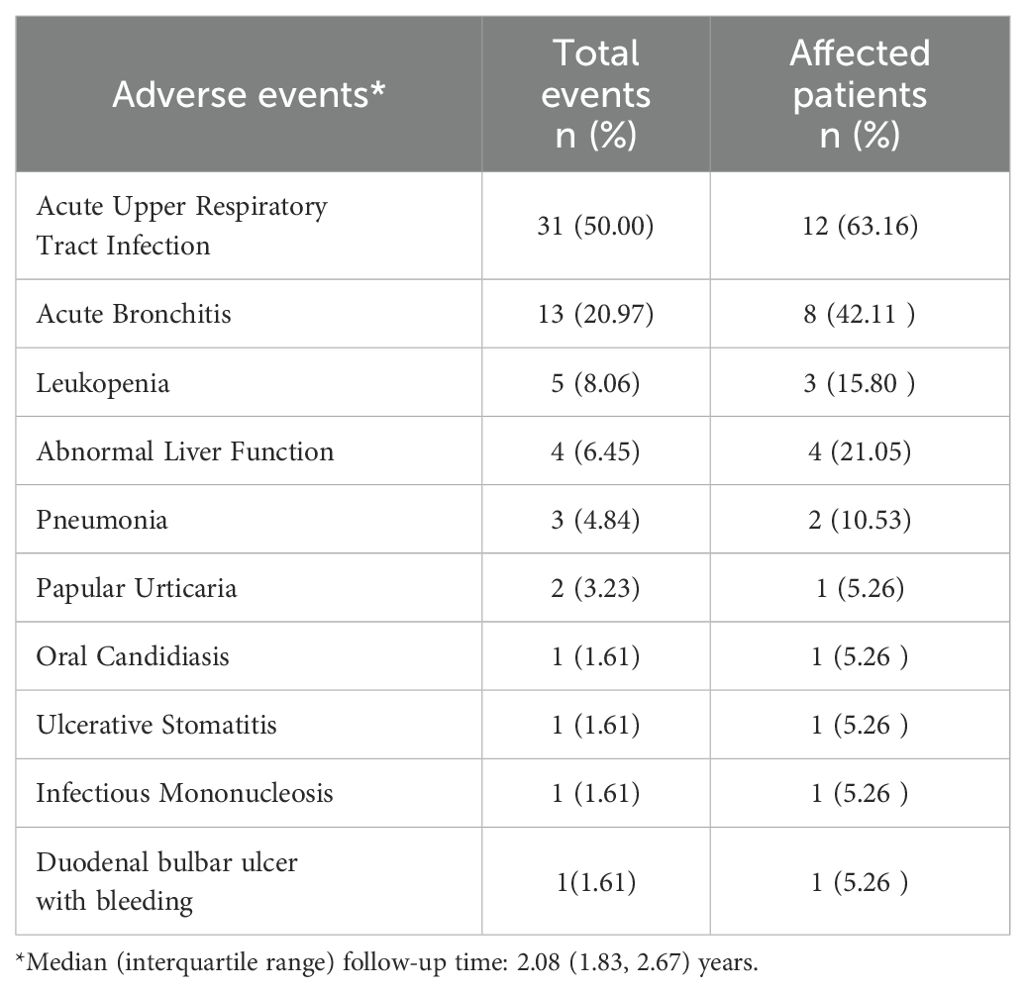
Table 6. Adverse events during tocilizumab treatment in systemic juvenile idiopathic arthritis (n=19).
4 Discussion
This study is the first to systematically characterize the dynamic trajectory of complement C3 and C4 levels during TCZ treatment in sJIA patients. The results identified a distinct pattern: a rapid early decline in complement levels followed by a prolonged plateau at low levels. These findings offer important longitudinal insights into the impact of TCZ on the complement system and add valuable details to the literature regarding long-term complement dynamics in sJIA.
The observed trends are consistent with findings from previous studies involving other autoimmune diseases. In 2018, Romano et al. conducted a small-scale investigation in RA patients treated with TCZ, reporting significant reductions in serum C3 and C4 levels, particularly in C4, as early as four weeks after treatment initiation. The proportion of patients with complement levels below the normal range increased with treatment duration (16). Similarly, Bieber et al. retrospectively analyzed 108 patients with various autoimmune conditions, including 8 sJIA patients, and found that 30% experienced C4 reductions and 21% experienced C3 reductions following TCZ therapy (17). In patients with Takayasu arteritis, TCZ treatment was also associated with significant post-treatment declines in C3 and C4 levels, correlating with improvements in inflammatory markers and disease activity (19). Furthermore, Iván Ferraz-Amaro et al. performed serial functional complement assays in 27 RA patients, demonstrating decreased activity in both the classical and alternative pathways after TCZ treatment, while the lectin pathway remained unaffected (20). Collectively, these findings indicate that TCZ-associated reductions in complement levels represent a common biological response across multiple autoimmune diseases. The observed complement-lowering effect appears to be mechanistically distinct from those of other commonly used treatments, such as glucocorticoids or JAK inhibitors. The findings suggest that TCZ-induced hypocomplementemia likely reflects deep suppression of IL-6 signaling, which may exert unique immunomodulatory effects not shared by other agents.
The reduction in complement C3 and C4 levels observed during TCZ treatment is likely closely associated with inhibition of the IL-6 signaling pathway. IL-6 plays a pivotal role in stimulating hepatic synthesis of acute-phase proteins, including C3 and C4 (21, 22). A study on COVID-19 and complement regulation demonstrated enhanced activation of the complement system in infected patients, particularly through the alternative pathway. IL-6 was identified as a key upstream regulator, and treatment targeting IL-6 resulted in decreased levels of complement activity and related components (23). Similarly, data from clinical trials investigating TCZ in patients with systemic lupus erythematosus (SLE) have shown continued reductions in C3 and C4 levels despite clinical improvement. These trials also reported significant declines in complement activation products, such as iC3b and C5b-9, suggesting that the reductions reflect suppressed synthesis rather than pathological consumption (24–26). The complement system interacts with innate immune sensors, including Toll-like receptors (TLRs). In particular, TLR4 activation can upregulate IL-6 family cytokines, further amplifying inflammation (27). In sJIA, systemic inflammation may enhance IL-6 production through TLRs, complement receptors, and other innate immune alarm receptors (28). Inhibiting IL-6 signaling with TCZ may disrupt this pro-inflammatory equilibrium, thereby influencing complement synthesis. To date, no clinical studies have specifically reported hypocomplementemia following the use of other IL-6 receptor antagonists in rheumatic diseases. However, recent findings involving sarilumab, another IL-6 receptor blocker, demonstrated that its administration attenuated chondrocyte senescence induced by the terminal complement complex (TCC) following trauma or exposure to human serum. These data suggest that IL-6 may be involved in TCC-mediated stress-induced premature senescence (29).
In this study, progressive TCZ treatment was accompanied by a gradual decline in sJADAS10-ESR scores, indicating reduced disease activity, alongside continuous reductions in C3 and C4 levels. At weeks 24 and 48, a significant positive correlation was observed between C3 levels and sJADAS10-ESR scores, suggesting a potential link between complement dynamics and disease activity in sJIA. These findings are consistent with previous research. E. Conticini et al. demonstrated that C3 and C4 levels were associated with disease activity in patients with giant cell arteritis. In the TCZ-treated group, reductions in C3 (P =0.001) and C4 (P <0.001) were more pronounced and occurred independently of CRP or ESR levels, indicating that C4 might serve as a potential biomarker for monitoring disease activity and therapeutic response (30). However, conflicting evidence exists. Iván Ferraz-Amaro et al. found no significant correlation between changes in disease activity and functional alterations in the three complement pathways among RA patients treated with TCZ. Additionally, complement activation appears to vary across different JIA subtypes (20). Hackl et al. analyzed 60 JIA patients (including 7 sJIA patients), and found that terminal complement complex (TCC) levels were significantly elevated during the acute phase in certain subtypes (e.g., polyarticular JIA and extended oligoarticular JIA). However, sJIA patients showed no complement activation in TCC or COMPL300 analyses, suggesting a distinct immunological profile (31). The hypocomplementemia observed during TCZ treatment is likely due to IL-6-mediated suppression of complement synthesis rather than complement depletion through immune activation. Consequently, the decline in C3 and C4 levels during treatment may partially reflect clinical improvement. However, current evidence remains insufficient to support their use as standalone biomarkers for continuous monitoring of disease activity in sJIA.
Throughout TCZ treatment, adverse events including infections, leukopenia, and abnormal liver function were documented in our patients. However, the incidence of these events was not significantly higher than that reported in previous studies (26, 32, 33). Early case reports described an RA patient developing new-onset glomerulonephritis with positive dsDNA and low complement levels following TCZ treatment (34). However, Romano et al. examined 19 patients treated with TCZ and found no clinical signs of glomerulonephritis or increased incidence of immune complex–related complications, such as recurrent fever (16). Similarly, Illei et al. found no correlation between complement levels and infection risk in TCZ-treated lupus patients (24). Furthermore, Bieber et al. also found that low complement levels during treatment were not associated with an increased incidence of infections, secondary immunodeficiency, or malignancies (17). Although long-term hypocomplementemia was frequently observed in the present study, no significant increase in infection risk was detected. One possible explanation is that TCZ suppresses the classical complement pathway while preserving lectin pathway activity, which may provide partial compensation for innate immune function. Nonetheless, rare adverse events warrant continued vigilance. Given that the median follow-up duration was 2.08 years, extended observation periods of five years or longer are needed to exclude delayed complications such as malignancies or autoimmune sequelae. Given the critical role of the complement system in immune surveillance, patients with persistently reduced complement levels during prolonged TCZ therapy should undergo close monitoring, particularly for infectious complications.
The study demonstrated that TCZ-induced hypocomplementemia serves as a biomarker of profound IL-6 pathway inhibition, rather than a direct indicator of pathological complement consumption or disease activity. Although certain adverse events occurred during TCZ therapy, no significant increase in infection risk was observed, supporting the continued use of TCZ for disease management under hypocomplementemic conditions. However, due to methodological limitations, including a single-center retrospective design and small sample size, serum C3 and C4 levels cannot be regarded as reliable longitudinal markers for monitoring disease activity in sJIA. Future investigations should prioritize multicenter, large-scale prospective studies to clarify the compensatory mechanisms of the complement system. In addition, further exploration of the relationship between dynamic changes in complement fragments (such as C3a and C5b-9) and organ-specific injury may offer deeper insight into complement alterations during TCZ treatment and their clinical relevance in sJIA.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of Jiangxi Children’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this study did not involve the collection or use of identifiable patient data.
Author contributions
JL: Project administration, Writing – review & editing. QZ: Writing – original draft. FX: Formal analysis, Investigation, Writing – review & editing. XL: Project administration, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
Thanks for the cooperation of all departments in the progress of treatment.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1607101/full#supplementary-material
References
1. Pardeo M, Vastert SJ, and De Benedetti F. It is about time: the first validated biomarker for early diagnosis of systemic juvenile idiopathic arthritis. Rheumatol (Oxford). (2022) 61:2724–5. doi: 10.1093/rheumatology/keab948
2. Ambler WG, Nanda K, Onel KB, and Shenoi S. Refractory systemic onset juvenile idiopathic arthritis: current challenges and future perspectives. Ann Med. (2022) 54:1839–50. doi: 10.1080/07853890.2022.2095431
3. Sozeri B, Demir F, Barut K, Atalay E, Pac Kisaarslan A, Ozdel S, et al. Evaluation of clinical outcomes in systemic juvenile idiopathic arthritis patients treated with biological agents in Turkey: the TURSIS study. Clin Exp Rheumatol. (2024) 42:194–201. doi: 10.55563/clinexprheumatol/j611kr
4. De Benedetti F, Meazza C, Oliveri M, Pignatti P, Vivarelli M, Alonzi T, et al. Effect of IL-6 on IGF binding protein-3: a study in IL-6 transgenic mice and in patients with systemic juvenile idiopathic arthritis. Endocrinology. (2001) 142:4818–26. doi: 10.1210/endo.142.11.8511
5. MaChado SH and Xavier RM. Safety of tocilizumab in the treatment of juvenile idiopathic arthritis. Expert Opin Drug Saf. (2017) 16:493–500. doi: 10.1080/14740338.2017.1303479
6. Hinze CH, Foell D, and Kessel C. Treatment of systemic juvenile idiopathic arthritis. Nat Rev Rheumatol. (2023) 19:778–89. doi: 10.1038/s41584-023-01042-z
7. Onel KB, Horton DB, Lovell DJ, Shenoi S, Cuello CA, Angeles-Han ST, et al. 2021 American college of rheumatology guideline for the treatment of juvenile idiopathic arthritis: therapeutic approaches for oligoarthritis, temporomandibular joint arthritis, and systemic juvenile idiopathic arthritis. Arthritis Care Res (Hoboken). (2022) 74:521–37. doi: 10.1002/acr.24853
8. Ding X, Qamar A, and Liu H. The complement system testing in clinical laboratory. Clin Chim Acta. (2023) 541:117238. doi: 10.1016/j.cca.2023.117238
9. Tanaka T, Narazaki M, and Kishimoto T. Interleukin (IL-6) immunotherapy. Cold Spring Harb Perspect Biol. (2018) 10:a028456. doi: 10.1101/cshperspect.a028456
10. Rupert KL, Rennebohm RM, and Yu CY. An unequal crossover between the RCCX modules of the human MHC leading to the presence of a CYP21B gene and a tenascin TNXB/TNXA-RP2 recombinant between C4A and C4B genes in a patient with juvenile rheumatoid arthritis. Exp Clin Immunogenet. (1999) 16:81–97. doi: 10.1159/000019099
11. Holers VM and Banda NK. Complement in the initiation and evolution of rheumatoid arthritis. Front Immunol. (2018) 9:1057. doi: 10.3389/fimmu.2018.01057
12. Banda NK, Deane KD, Bemis EA, Strickland C, Seifert J, Jordan K, et al. Analysis of complement gene expression, clinical associations, and biodistribution of complement proteins in the synovium of early rheumatoid arthritis patients reveals unique pathophysiologic features. J Immunol. (2022) 208:2482–96. doi: 10.4049/jimmunol.2101170
13. Coss SL, Zhou D, Chua GT, Aziz RA, Hoffman RP, Wu YL, et al. The complement system and human autoimmune diseases. J Autoimmun. (2023) 137:102979. doi: 10.1016/j.jaut.2022.102979
14. Jia Y, Feng B, Ji X, Tian X, Zhao L, Zhou J, et al. Complement factor H attenuates TNF-alpha-induced inflammation by upregulating EIF3C in rheumatoid arthritis. J Transl Med. (2023) 21:846. doi: 10.1186/s12967-023-04730-2
15. Sturfelt G and Truedsson L. Complement in the immunopathogenesis of rheumatic disease. Nat Rev Rheumatol. (2012) 8:458–68. doi: 10.1038/nrrheum.2012.75
16. Romano C, Del Mastro A, Sellitto A, Solaro E, Esposito S, and Cuomo G. Tocilizumab reduces complement C3 and C4 serum levels in rheumatoid arthritis patients. Clin Rheumatol. (2018) 37:1695–700. doi: 10.1007/s10067-018-3992-7
17. Bieber A, Markovits D, Toledano K, Tavor Y, Mader R, Balbir-Gurman A, et al. Hypocomplementemia during tocilizumab treatment: Long-term follow-up results. Med (Baltimore). (2022) 101:e29528. doi: 10.1097/MD.0000000000029528
18. Martini A, Ravelli A, Avcin T, Beresford MW, Burgos-Vargas R, Cuttica R, et al. Toward new classification criteria for juvenile idiopathic arthritis: first steps, pediatric rheumatology international trials organization international consensus. J Rheumatol. (2019) 46:190–7. doi: 10.3899/jrheum.180168
19. Rongyi C, Xiaojuan D, Jinghua W, Lingying M, Xiaomin D, Lili M, et al. High level of serum complement 3 is a risk factor for vascular stenosis progression in TA patients receiving tocilizumab: a prospective observational study. Arthritis Res Ther. (2023) 25:137. doi: 10.1186/s13075-023-03106-7
20. Ferraz-Amaro I, Santos-Concepcion S, Castro-Hernandez J, Hernandez-Hernandez MV, Tejera Segura B, Luna C, et al. Tocilizumab modulates the activity of the classical and alternative complement pathways in rheumatoid arthritis patients. Front Immunol. (2025) 16:1486588. doi: 10.3389/fimmu.2025.1486588
21. Schmidt-Arras D and Rose-John S. IL-6 pathway in the liver: From physiopathology to therapy. J Hepatol. (2016) 64:1403–15. doi: 10.1016/j.jhep.2016.02.004
22. Mihara M, Hashizume M, Yoshida H, Suzuki M, and Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci (Lond). (2012) 122:143–59. doi: 10.1042/CS20110340
23. Van Damme KFA, Hoste L, Declercq J, De Leeuw E, Maes B, Martens L, et al. A complement atlas identifies interleukin-6-dependent alternative pathway dysregulation as a key druggable feature of COVID-19. Sci Transl Med. (2023) 15:eadi0252. doi: 10.1126/scitranslmed.adi0252
24. Illei GG, Shirota Y, Yarboro CH, Daruwalla J, Tackey E, Takada K, et al. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheumatol. (2010) 62:542–52. doi: 10.1002/art.27221
25. De Matteis A, Sacco E, Celani C, Uva A, Messia V, Nicolai R, et al. Tocilizumab for massive refractory pleural effusion in an adolescent with systemic lupus erythematosus. Pediatr Rheumatol Online J. (2021) 19:144. doi: 10.1186/s12969-021-00635-w
26. Li C, Tang X, Zhou Z, Sun L, Lu M, Zhou W, et al. Efficacy and safety of tocilizumab in Chinese patients with systemic juvenile idiopathic arthritis: a multicentre phase IV trial. Clin Rheumatol. (2024) 43:3457–67. doi: 10.1007/s10067-024-07126-9
27. Zhang M, Gu J, and Zhang C. Hepatitis B virus X protein binding to hepsin promotes C3 production by inducing IL-6 secretion from hepatocytes. Oncotarget. (2016) 7:7780–800. doi: 10.18632/oncotarget.6846
28. Barone P, Pignataro R, Garozzo MT, and Leonardi S. IL-6 blockers in systemic onset juvenile idiopathic arthritis. Immunotherapy. (2016) 8:79–87. doi: 10.2217/imt.15.104
29. Ruths L, Hengge J, Teixeira GQ, Haffner-Luntzer M, Ignatius A, and Riegger J. Terminal complement complex deposition on chondrocytes promotes premature senescence in age- and trauma-related osteoarthritis. Front Immunol. (2024) 15:1470907. doi: 10.3389/fimmu.2024.1470907
30. Conticini E, Hellmich B, Frediani B, Csernok E, and Loffler C. Utility of serum complement factors C3 and C4 as biomarkers during therapeutic management of giant cell arteritis. Scand J Rheumatol. (2023) 52:276–82. doi: 10.1080/03009742.2022.2047311
31. Hackl L, Giner T, Albrecht J, Liebensteiner M, Zlamy M, and Brunner J. Complement activation profiles in patients with juvenile idiopathic arthritis. Biomed Trans Sci. (2021) 1:1–13. doi: 10.33425/2768-4911.1020
32. Gazda A, Naishtetik I, Kolodziejczyk B, Rybak K, Manczak M, Wojtowicz J, et al. Clinical outcomes of tocilizumab therapy in polyarticular and systemic juvenile idiopathic arthritis: a single-center analysis (2018-2022). Rheumatol Int. (2024) 44:2949–59. doi: 10.1007/s00296-024-05711-4
33. Yokota S, Imagawa T, Mori M, Miyamae T, Takei S, Iwata N, et al. Longterm safety and effectiveness of the anti-interleukin 6 receptor monoclonal antibody tocilizumab in patients with systemic juvenile idiopathic arthritis in Japan. J Rheumatol. (2014) 41:759–67. doi: 10.3899/jrheum.130690
Keywords: systemic juvenile idiopathic arthritis, tocilizumab, hypocomplementemia, disease activity, infection
Citation: Ling J, Zhou Q, Xie F and Liu X (2025) Hypocomplementemia dynamics during tocilizumab therapy in systemic juvenile idiopathic arthritis: a retrospective longitudinal study. Front. Immunol. 16:1607101. doi: 10.3389/fimmu.2025.1607101
Received: 07 April 2025; Accepted: 04 August 2025;
Published: 29 August 2025.
Edited by:
Arturo Borzutzky, Pontificia Universidad Católica de Chile, ChileReviewed by:
Ciro Romano, University of Campania Luigi Vanvitelli, ItalyZhuoguang Li, Shenzhen Children’s Hospital, China
Copyright © 2025 Ling, Zhou, Xie and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohui Liu, ODUxNzI0ODQ2QHFxLmNvbQ==; Fang Xie, ODA1MjE5Njc1QHFxLmNvbQ==
 Jiayun Ling
Jiayun Ling Qingfang Zhou
Qingfang Zhou Xiaohui Liu
Xiaohui Liu