- 1Medical Oncology Department of Thoracic Cancer (II), Cancer Hospital of China Medical University Liaoning Cancer Hospital and Institute, Cancer Hospital of Dalian University of Technology, Shenyang, China
- 2Department of Child Healthcare, Shenyang Children’s Hospital, Shenyang, China
- 3Abdominal Radiotherapy Ward II, Cancer Hospital of China Medical University Liaoning Cancer Hospital and Institute, Cancer Hospital of Dalian University of Technology, Shenyang, China
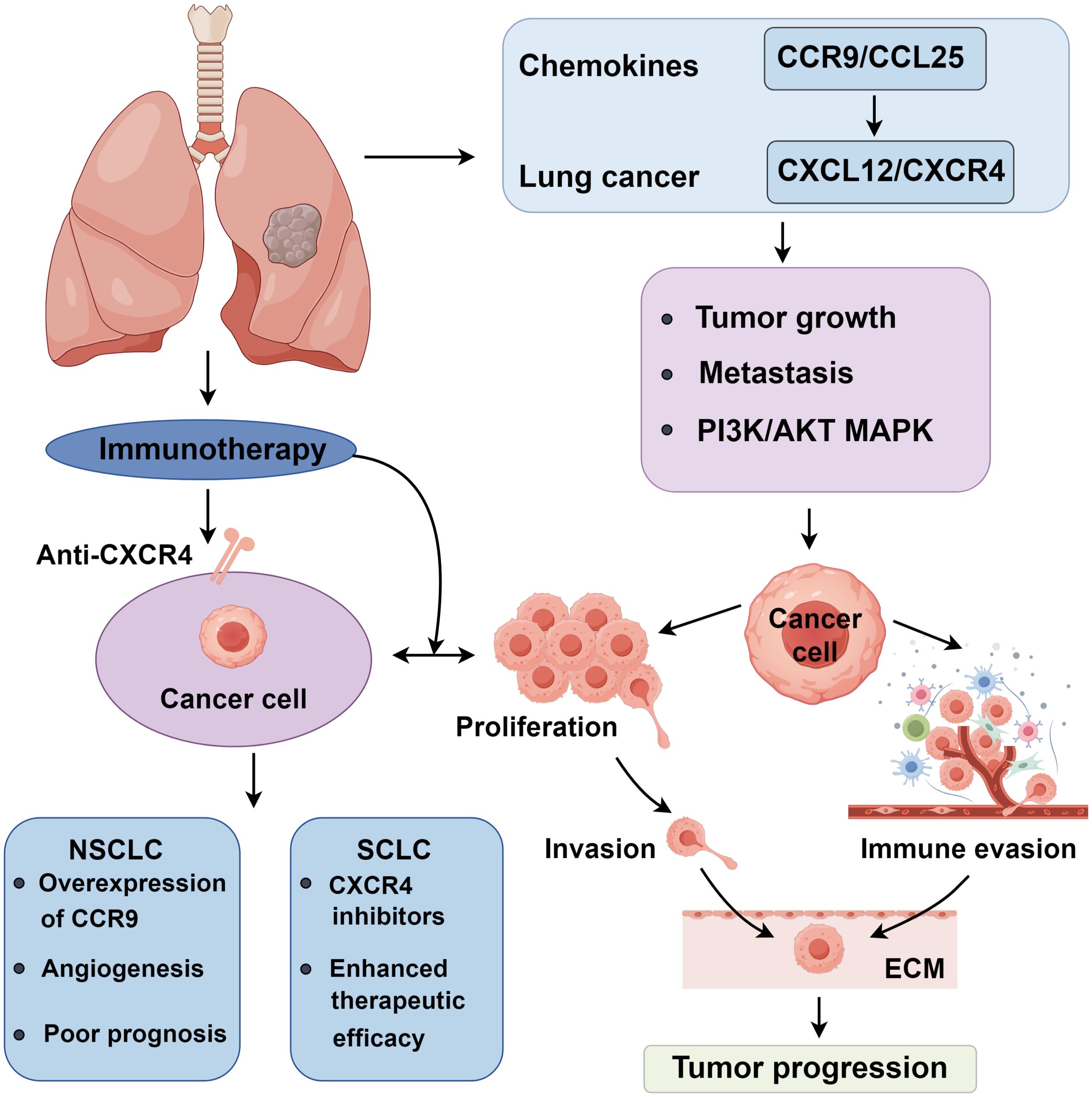
Chemokines are key molecules that regulate immune cell migration and play critical roles in the tumor microenvironment. In lung cancer, chemokine dysregulation is closely linked to tumor progression. They promote immune cell infiltration and interact with tumor cells, enhancing tumor invasiveness and metastatic potential. This review highlights chemokine-mediated mechanisms, focusing on CCR9/CCL25 and CXCL12/CXCR4 axes, which promote tumor growth, metastasis, and immune evasion via PI3K/AKT and MAPK signaling. Elevated expression of these pathways correlates with poor outcomes and aggressive phenotypes. In SCLC, CXCR4 inhibitors show therapeutic promise when combined with chemotherapy or immunotherapy. This review summarizes the prognostic and therapeutic relevance of chemokines in lung cancer progression.
1 Introduction
Lung cancer remains the leading cause of cancer-related mortality worldwide, characterized by its high incidence, insidious onset, and poor prognosis (1). Lung cancer is notable for its rapid proliferation, early metastasis, and resistance to conventional therapies (2). Recent research has revealed that chemokines (3), small secreted proteins that regulate immune cell migration, exert dual roles in the tumor microenvironment (TME), acting as both promoters of tumor progression and modulators of antitumor immune responses (4). On the one hand, tumor-derived chemokines can recruit immunosuppressive cells such as regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs), thereby facilitating immune evasion, angiogenesis, and metastasis (5, 6). On the other hand, chemokines also orchestrate the trafficking of cytotoxic lymphocytes, dendritic cells, and other effector cells that mediate antitumor immunity (7). Among these, CCL25 and its receptor CCR9 (8), as well as CXCL12 and its receptor CXCR4 (9), have drawn significant attention due to their involvement in key oncogenic signaling pathways. These ligand-receptor axes not only mediate tumor cell survival and metastasis but also shape immune responses within the TME, making them promising targets for prognostic assessment and therapeutic intervention in lung cancer (10–13).
This review systematically summarizes the biological functions and molecular mechanisms of the CCR9/CCL25 and CXCL12/CXCR4 axes in lung cancer. Specifically, we describe in detail how chemokines influence proliferation, apoptosis, invasion, metastasis, and immune modulation via PI3K/AKT, MAPK, and other signaling pathways. Furthermore, we summarize current research progress on the clinical implications of chemokine expression and the emerging potential of chemokine-targeted therapies, particularly CXCR4 inhibitors, in improving patient outcomes.
2 Chemokines in regulation of NSCLC
CCR9, serves as the specific receptor for CCL25 (14), preferentially marks naive T cells and subsets of mature dendritic cells. Initially recognized for its role in recruiting immune cells, and act as a homing marker for lymphocytes involved in inflammatory responses, immune reactions, and leukocyte regulation, also influencing the chemotactic activity of CD4+ T lymphocytes (15). Studies have indicated that the organ-specific metastasis of tumors is associated with CCR9/CCL25, which is overexpressed in various malignancies, impacting patient prognosis (14, 16).
The PI3K/AKT signaling pathway is integral to the regulation of cellular proliferation throughout the body, primarily through the activation of signals from specific ligand-receptor interactions that inhibit apoptosis (17, 18). Normally, this pathway starts with PI3K, with AKT as a crucial hub, phosphorylating and thereby activating or inhibiting downstream targets such as EGFR, BCL-2, VEGFR, and MMP-9 to ensure normal cell proliferation and growth differentiation (19–21). In malignant tissues, aberrations such as amplification of PI3K, hyperactivation of AKT, or loss of regulatory factors can disrupt the balance of the PI3K/AKT pathway, leading to cancer cell invasion and metastasis (22, 23). In NSCLC, the PI3K subunit p85α is overexpressed and silencing the chemokine receptor CCR9 can reduce CCL25-induced PI3Kp85 and p-AKT production (24, 25). Activation of AKT, observable in transformed bronchial epithelial cells, induces malignant lung tumors, with higher AKT expression correlating with poorer prognoses in NSCLC patients. Studies by Li (24) also demonstrate that high expression of activated AKT in cancer tissues of NSCLC patients suggests a significant role of the PI3K pathway in the pathogenesis and progression of lung cancer (26).
2.1 Regulation of NSCLC proliferation and apoptosis by CCR9 and CCL25
Li (24) demonstrated that the CCR9/CCL25 axis facilitates the transition of cells from G1 to S phase, maintaining cell cycle continuity. Disruption of this axis results in decreased expression of cyclins in NSCLC tumor cells (27). It has been shown through the use of PI3K inhibitors that blocking the CCR9/CCL25 axis ensures the continuity of the cell cycle, upregulates cyclin expression, and activates PI3K and AKT. BCL-2, a downstream protein of AKT, promotes apoptosis by binding with Bad; however, activated AKT can prevent the association of Bad with BCL-2, thereby inhibiting apoptosis. Additionally, the anti-apoptotic factor BCL-2 can prevent the activation of Caspase-3 by releasing cytochrome C, thus blocking apoptosis. Another apoptotic factor, Survivin, under the downstream of BCL-2, blocks the Caspase-3 protease directly, exerting an anti-apoptotic effect. Expression levels of BCL-2 in lung cancer tissue are consistent with those of survivin (28). Sharma and Li (28, 29)found that inhibiting the CCR9/CCL25 axis could downregulate proteins such as PI3K, AKT, ERK1/2, and GSK-3β, while upregulating Caspase-3, thereby driving apoptosis. Zhong (30) research indicates that the expression of CCR9 in NSCLC correlates with BCL-2 and Survivin, suggesting that CCR9’s role in NSCLC may be through the inhibition of apoptosis.
2.2 CCR9 and CCL25 in NSCLC invasion and metastasis
From pathological and physiological perspectives, both angiogenesis (the formation of new blood vessels) and lymphangiogenesis (the formation of new lymphatic vessels) are essential for tumor maturation and lymph node metastasis. Vascular endothelial growth factor (VEGF), primarily located in the cytoplasm of cancer cells, not only induces endothelial cell mitosis to promote angiogenesis and lymphangiogenesis but also enhances plasminogen and collagenase secretion, increasing vascular permeability to support tumor nutrition. Niu (27) found that VEGF expression in peri-tumoral tissues exceeds that in tumor tissues, with its positivity rate closely linked to pathological types and lymph node metastasis. VEGF expression is higher in tissues with large lymph node metastases (≥1 cm) than in those with small metastases (≤1 cm), making it a valuable marker for assessing tumor malignancy, differentiation, and metastatic potential. VEGF also binds to Vascular Endothelial Growth Factor Receptor 3, promoting lymphangiogenesis via the MAPK and PI3K pathways (27, 31).
Matrix metalloproteinases (MMPs), particularly MMP-2 and MMP-9, play a critical role in tumor metastasis by degrading the extracellular matrix. Overexpression of MMP-9 in lung adenocarcinoma is associated with lymph node metastasis and is an independent risk factor for patient survival (32, 33). Li et al. demonstrated that the PI3K/AKT pathway drives MMP-2 expression in malignant tumors (34), while Lee et al. showed that activated AKT increases MMP-9 and MMP-2 expression, enhancing cancer cell invasion (35). Gupta (36) reported that MMP-2 promotes CCR9/CCL25 interactions, facilitating lung cancer cell invasion and selective metastasis. MMP-2 exhibits greater reactivity to CCL25 in lung adenocarcinoma compared to lung SCC, likely due to differential MMP activity and CCR9 phosphorylation (36). In normal tissues, MMPs and tissue inhibitors of matrix metalloproteinases (TIMPs) maintain extracellular matrix balance. However, in malignant tumors, activated MMP-2 and MMP-9 degrade the matrix, promoting cancer cell infiltration. While TIMPs were initially considered anti-cancer proteins, Bourboulia found that TIMP-2 overexpression stimulates lung adenocarcinoma cell proliferation (37–39). Gupta further revealed that lung adenocarcinoma cells produce TIMP-1 and TIMP-2, whereas lung SCC cells only express TIMP-2, suggesting that MMP and TIMP differential expression, mediated by the CCR9/CCL25 axis, influences lung cancer metastasis and invasion (36).
2.3 Regulation of NSCLC via the JAK/STAT and NF-κB pathways by chemokines
In addition to the PI3K/AKT and MAPK signaling pathways, chemokines also exert their oncogenic effects in lung cancer through modulation of the JAK/STAT and NF-κB signaling cascades. The JAK/STAT pathway, a critical mediator of cytokine signaling, has been implicated in tumor immune evasion, proliferation, and survival. Studies have shown that CXCL12/CXCR4 activation leads to downstream STAT3 phosphorylation, promoting transcription of genes related to tumor growth and angiogenesis, such as VEGF, MCL-1, and BCL-XL. Overactivation of STAT3 contributes to resistance against apoptosis and enhances immune suppression within the tumor microenvironment. In NSCLC, aberrant CXCL12/CXCR4-JAK/STAT3 signaling has been associated with poor clinical outcomes and therapeutic resistance. Similarly, the NF-κB signaling pathway, well known for its role in inflammation and immunity, is often constitutively activated in lung cancer. Engagement of CCR9/CCL25 or CXCL12/CXCR4 can trigger IκB degradation, leading to nuclear translocation of NF-κB subunits such as p65. This results in transcriptional activation of genes involved in proliferation (cyclin D1), anti-apoptosis (BCL-2, Survivin), and metastasis (MMP-9, VEGF). CXCR4 activation has also been shown to amplify NF-κB-mediated transcription via interaction with downstream intermediates such as TRAF6 and TAK1, promoting a pro-tumorigenic microenvironment (48, 49). Notably, NF-κB activation also upregulates CXCL12 and CCR9 expression, creating a positive feedback loop that sustains tumor-promoting inflammation and chemokine signaling.
3 Chemokines CCR9/CCL25 and CXCL12/CXCR4 in NSCLC
3.1 The prognostic impact of CCR9/CCL25 in NSCLC
The CCR9/CCL25 axis critically contributes to NSCLC progression by activating the PI3K/AKT pathway and serves as a robust prognostic indicator. Immunohistochemical analyses reveal consistent CCR9 overexpression in NSCLC tissues compared to adjacent non-tumorous and normal tissues, with particularly high levels observed in the squamous cell carcinoma line NCI-H157 relative to normal bronchial epithelial cells (40). Functional studies demonstrate that CCR9 silencing attenuates cancer cell proliferation and induces apoptosis, confirming its pro-tumorigenic role (24). Clinically, elevated CCR9 expression correlates with advanced tumor grade, lymph node metastasis (41), and poor survival, independent of histologic subtype (28, 34). Notably, CCR9 is more prevalent in adenocarcinomas than in squamous cell carcinomas (30), potentially explaining the former’s aggressive behavior and worse prognosis. This disparity may arise from histology-specific modulation of downstream effectors: adenocarcinomas exhibit higher serum CCL25 levels (20), while CCR9-driven upregulation of VEGF, MMP-2, and MMP-9 promotes angiogenesis and metastatic dissemination (41). Intriguingly, CCR9 also modulates anti-tumor immunity. Although peripheral CD4+ T cells from NSCLC patients show reduced CCR9 expression compared to healthy controls, post-surgical T cell populations exhibit CCR9 upregulation (42, 43). In vitro, CCL25 enhances CD4+; T cell migration, suggesting a dual role for this axis in both tumor progression and immune surveillance. Thus, assessing CCR9 and CCL25 expression may provide prognostic insights and improve postoperative immune function in NSCLC patients. Therefore, these chemokines serve as promising prognostic biomarkers and potential therapeutic targets, offering valuable insights for personalized treatment strategies in NSCLC and SCLC.
3.2 Biological properties of CXCL12/CXCR4 in relation to the tumor microenvironment
The tumor microenvironment (TME), comprising stromal and immune cells, plays a critical role in tumor formation, invasion, and (44–47). Chemokines act as central mediators within the TME, orchestrating the recruitment, spatial organization, and functional modulation of immune cell populations (48–51). Among these, CXCL12/CXCR4 signaling shapes a complex immunological landscape that supports tumor immune escape and progression (9, 52). T cells, particularly Tregs and exhausted CD8+; T cells, are recruited by the CXCL12 gradient in the TME, leading to an immunosuppressive milieu. Studies have shown that CXCL12-enriched zones can sequester effector T cells away from tumor nests, limiting cytotoxic responses (53). CXCR4 expression on CD8+; T cells are also associated with a dysfunctional phenotype characterized by reduced cytokine production and increased PD-1 expression (54).
The CXCL12/CXCR4 axis plays a multifaceted role in tumor progression through interactions with stromal and immune components. Vascular endothelial growth factor (VEGF) induces CXCL12-associated vascular gene expression in myofibroblasts, thereby recruiting monocyte-derived macrophages to target tissues and fostering a pro-tumorigenic microenvironment that supports tumor proliferation (55). In HER-2-positive breast cancer, HER-2 signaling upregulates CXCR4 expression while simultaneously inhibiting its degradation, a mechanism that contributes to enhanced invasive potential (56). Similarly, in lung cancer, elevated epidermal growth factor receptor (EGFR) expression has been tentatively linked to CXCR4 induction, correlating with poor clinical outcomes; however, further mechanistic validation is required to establish this relationship conclusively (56). The hypoxic tumor microenvironment further modulates CXCR4 activity, as HIF-1α drives CXCL12/CXCR4 upregulation under low oxygen conditions (57). Functionally, CXCR4 not only recruits immunosuppressive cells, such as neutrophils, tumor-associated macrophages, but also engages with transcription factors to activate the NF-κB pathway, collectively reshaping the TME to promote immune evasion and metastatic dissemination (58).
3.3 Association of CXCL12/CXCR4 with tumor growth
The unrestrained proliferation of tumor cells defines the essence of tumor growth, with proliferative signaling pathways often aberrantly activated within tumor cells. Studies highlight the critical role of CXCR4 in chronic lymphocytic leukemia by facilitating tumor migration. In triple-negative breast cancer, CXCR4 is crucial, operating via the LASP1-Ago2 axis. MicroRNA Let-7a can regulate the signaling of the CXCR4/LASP1 axis through competitive binding (59). Dąbrowska examined the expression of CXCL12 and CXCR4 in 100 breast cancer patients, revealing that analyzing the expression levels of CXCL12/CXCR4 can enhance the accuracy of breast cancer diagnosis (60). Research by Li suggests that CXCR4 is overexpressed in tumor tissues compared to normal tissues, with its expression correlating with B cell and CD8+ cell infiltration. Further studies associate high CXCR4 expression with poor prognosis in gastric cancer (58). Ottaviano et al. analyzed the relationship between CXCR4 expression and clinical parameters and prognosis in colon cancer, incorporating 78 patients. They found a 66.7% positivity rate for CXCR4 expression, which was prevalent in right-sided colon cancers and higher-grade tumors. Moreover, high CXCR4 expression could more effectively predict the efficacy of first-line treatments and was associated with worse outcomes in colon cancer patients (61) (Figure 1).
3.4 Expression and regulatory mechanisms of CXCR4 in SCLC
Small Cell Lung Cancer is highly malignant and rapidly progressive. CXCL12/CXCR4 plays a significant role in the oncogenesis and progression of tumors (62). Stumpf et al. analyzed 58 lung cancer patients, including cases of adenocarcinoma, squamous cell carcinoma, and SCLC, applying immunohistochemistry to assess CXCR4 protein expression. The results showed a 100% positivity rate for SCLC, 63.6% for adenocarcinoma, and 90.5% for squamous cell carcinoma. CXCR4 gene expression was closely linked to the expression of SST2 (63). Studies by Kijima et al. indicated high CXCR4 expression in SCLC cell lines, closely associated with tumor cell adhesion and migration, and correlated with p-AKT levels (64). Long non-coding RNAs (lncRNAs) also play a crucial role in regulating CXCR4 expression (65–67). LncRNA NORAD can downregulate CXCR4 via the RhoA/ROCK signaling pathway, inhibiting cell migration. LncRNA00922 accelerates lung cancer cell proliferation, invasion, and metastasis by regulating miRNA204/CXCR4. High CXCR4 expression in lung cancer correlates not only with cell invasion and metastasis but also with poor prognosis. Katsura et al. included 140 operable non-small cell lung cancer patients and found that high CXCR4 expression correlated with worse patient outcomes using immunohistochemical methods (68). Liang et al. included 2037 patients, showing that CXCR4 expression correlates with lymph node metastasis, distant metastasis, tumor staging, and survival, with higher expression associated with shorter survival (69). In summary, CXCR4 serves as a key driver of SCLC pathogenesis and advancement, positioning it as a potential candidate for targeted therapy in advanced disease.
3.5 Role of CXCR4 inhibitors in small cell lung cancer
The therapeutic landscape of lung cancer has evolved significantly with the identification of chemokine receptors as potential targets, particularly CXCR4 (70, 71). While CXCR4 inhibitors have shown promise in SCLC, emerging studies highlight their potential in NSCLC as well. In NSCLC, overexpression of CXCR4 is associated with poor prognosis and immune evasion (72). Recent studies indicate that CXCR4 expression increases with advancing NSCLC stages (73). Peptide R, a novel CXCR4 inhibitor, has demonstrated dual efficacy, it suppresses the dissemination of metastasis-initiating cells, enhances CD8+ T-cell activity, and reduces regulatory T-cell (Treg) infiltration in NSCLC models (74). These findings suggest its potential as a combinatorial therapeutic target to improve treatment outcomes. Additionally, co-delivery of miR-126-3p mimics and miR-221-3p inhibitors via lipid nanoparticles has been shown to inhibit tumor growth and metastasis by blocking AKT and CXCR4 signaling pathways (75). CXCR4 overexpression promotes tumor spheroid formation and epithelial-mesenchymal transition (EMT), whereas CXCR4-knockout murine models exhibit significantly smaller NSCLC tumor lesions compared to CXCR4-high counterparts (76).
In SCLC, progress in targeted therapies remains limited, but CXCR4 has emerged as a viable candidate. Salgia (77) conducted a Phase II study evaluating the efficacy of a CXCR4 inhibitor combined with etoposide and cisplatin in extensive-stage SCLC. This study included 90 patients and assessed CXCR4 expression in circulating tumor cells. Results indicated that combining a CXCR4 inhibitor with chemotherapy prolonged survival, with higher CXCR4 expression in circulating tumor cells predicting better treatment efficacy and longer disease-free and overall survival times. This suggests that CXCR4 inhibitors have potential as effective therapeutic targets in SCLC. A recent study demonstrated that targeting CXCR4 could enhance the efficacy of PD-1 immunotherapy by modulating the tumor microenvironment, primarily through the reduction of myeloid-derived suppressor cells. The COMBAT trial showed that a CXCR4 antagonist combined with PD-1 inhibitors and chemotherapy could improve disease control and prolong survival in pancreatic cancer, mainly through increased CD8+ cell infiltration and reduced myeloid-derived suppressor cells, suggesting that CXCR4 inhibitors combined with traditional therapies can improve prognosis for tumor patients (78, 79) (Supplementary Table S1).
4 Conclusion
Chemokines, particularly the CCR9/CCL25 and CXCL12/CXCR4 axes, play pivotal roles in lung cancer progression, immune cell recruitment, and tumor microenvironment remodeling. In NSCLC, CCR9/CCL25 drives proliferation, metastasis, and immune evasion through upregulation of VEGF, MMPs, and anti-apoptotic proteins, with its overexpression correlating with advanced disease and poor prognosis. Similarly, CXCL12/CXCR4 orchestrates immunosuppression by recruiting Tregs and MDSCs while promoting angiogenesis and EMT. In SCLC, CXCR4 is nearly ubiquitously expressed and linked to aggressive phenotypes, making it a compelling therapeutic target. Clinical studies highlight the potential of CXCR4 inhibitors to enhance chemotherapy and immunotherapy efficacy, as seen in the COMBAT trial, where CXCR4 blockade improved CD8+ T cell infiltration and reduced MDSCs. These findings underscore chemokines as dual-functional biomarkers for prognosis and promising targets for precision therapy.
Future research should prioritize translational applications, including the development of robust chemokine-based diagnostic panels and combinatorial regimens integrating CXCR4 inhibitors with immune checkpoint blockers or conventional therapies. Challenges such as off-target effects and optimal dosing require further investigation, particularly in SCLC, where treatment options remain limited. Additionally, exploring the crosstalk between chemokine networks and emerging resistance mechanisms could unveil novel therapeutic vulnerabilities. Clarifying the spatiotemporal dynamics of chemokine signaling across lung cancer subtypes may enable the development of subtype-specific therapeutic strategies, ultimately enhancing lung cancer patients’ survival and quality of life.
Author contributions
XG: Writing – original draft. XW: Writing – original draft. JJ: Writing – original draft. ZG: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1607225/full#supplementary-material
References
1. Li C, Lei S, Ding L, Xu Y, Wu X, Wang H, et al. Global burden and trends of lung cancer incidence and mortality. Chin Med J (Engl). (2023) 136:1583–90. doi: 10.1097/CM9.0000000000002529
2. Appadurai MI, Chaudhary S, Shah A, Natarajan G, Alsafwani ZW, Khan P, et al. ST6GalNAc-I regulates tumor cell sialylation via NECTIN2/MUC5AC-mediated immunosuppression and angiogenesis in non-small cell lung cancer. J Clin Invest. (2025) 135:e186863. doi: 10.1172/JCI186863
3. Chen P, Wang H, Tang Z, Shi J, Cheng L, Zhao C, et al. Selective depletion of CCR8+Treg cells enhances the antitumor immunity of cytotoxic T cells in lung cancer by dendritic cells. J Thorac Oncol. (2025). doi: 10.1016/j.jtho.2025.02.029
4. Zhao X, Liu B, William WN, Tsanov KM, Ho YJ, Barriga FM, et al. Interferon-ϵ loss is elusive 9p21 link to immune-cold tumors, resistant to immune-checkpoint therapy and endogenous CXCL9/10 induction. J Thorac Oncol. (2024). doi: 10.1016/j.jtho.2024.12.020
5. Jeong H, Koh J, Kim S, Yim J, Song SG, Kim H, et al. Cell-intrinsic PD-L1 signaling drives immunosuppression by myeloid-derived suppressor cells through IL-6/Jak/Stat3 in PD-L1-high lung cancer. J Immunother Cancer. (2025) 13:e010612. doi: 10.1136/jitc-2024-010612
6. Yang L, Li A, Yu W, Wang H, Zhang L, Wang D, et al. Blockade of purine metabolism reverses macrophage immunosuppression and enhances anti-tumor immunity in non-small cell lung cancer. Drug Resist Update. (2025) 78:101175. doi: 10.1016/j.drup.2024.101175
7. Lim RJ, Salehi-Rad R, Tran LM, Oh MS, Dumitras C, Crosson WP, et al. CXCL9/10-engineered dendritic cells promote T cell activation and enhance immune checkpoint blockade for lung cancer. Cell Rep Med. (2024) 5:101479. doi: 10.1016/j.xcrm.2024.101479
8. Xu B, Deng C, Wu X, Ji T, Zhao L, Han Y, et al. CCR9 and CCL25: A review of their roles in tumor promotion. J Cell Physiol. (2020) 235:9121–32. doi: 10.1002/jcp.v235.12
9. Yang Y, Li J, Lei W, Wang H, Ni Y, Liu Y, et al. CXCL12-CXCR4/CXCR7 axis in cancer: from mechanisms to clinical applications. Int J Biol Sci. (2023) 19:3341–59. doi: 10.7150/ijbs.82317
10. Zhang W, Wang H, Sun M, Deng X, Wu X, Ma Y, et al. CXCL5/CXCR2 axis in tumor microenvironment as potential diagnostic biomarker and therapeutic target. Cancer Commun (London England). (2020) 40:69–80. doi: 10.1002/cac2.v40.2-3
11. Lee JM, Lee MH, Garon E, Goldman JW, Salehi-Rad R, Baratelli FE, et al. Phase I trial of intratumoral injection of CCL21 gene-modified dendritic cells in lung cancer elicits tumor-specific immune responses and CD8(+) T-cell infiltration. Clin Cancer Res. (2017) 23:4556–68. doi: 10.1158/1078-0432.CCR-16-2821
12. Salehi-Rad R, Lim RJ, Du Y, Tran LM, Li R, Ong SL, et al. CCL21-DC in situ vaccination in murine NSCLC overcomes resistance to immunotherapy and generates systemic tumor-specific immunity. J Immunother Cancer. (2023) 11:e006896. doi: 10.1136/jitc-2023-006896
13. Oh MS, Dumitras C, Salehi-Rad R, Tran LM, Krysan K, Lim RJ, et al. Characteristics of a CCL21 gene-modified dendritic cell vaccine utilized for a clinical trial in non-small cell lung cancer. Mol Cancer Ther. (2025) 24:286–98. doi: 10.1158/1535-7163.MCT-24-0435
14. Zaballos A, Gutiérrez J, Varona R, Ardavín C, and Márquez G. Cutting edge: identification of the orphan chemokine receptor GPR-9–6 as CCR9, the receptor for the chemokine TECK. J Immunol. (1999) 162:5671–5. doi: 10.4049/jimmunol.162.10.5671
15. Chen H, Cong X, Wu C, Wu X, Wang J, Mao K, et al. Intratumoral delivery of CCL25 enhances immunotherapy against triple-negative breast cancer by recruiting CCR9(+) T cells. Sci Adv. (2020) 6:eaax4690. doi: 10.1126/sciadv.aax4690
16. Tu Z, Xiao R, Xiong J, Tembo KM, Deng X, Xiong M, et al. CCR9 in cancer: oncogenic role and therapeutic targeting. J Hematol Oncol. (2016) 9:10. doi: 10.1186/s13045-016-0236-7
17. Fu NJ, Sheng YW, Fan Z, Wu Z, Li LY, Xi RY, et al. Synthetic lethality of SHP2 and XIAP suppresses proliferation and metastasis in KRAS-mutant nonsmall cell lung cancer. Adv Sci (Weinh). (2025) 12:e2411642. doi: 10.1002/advs.202411642
18. Chen S, Hao Q, Gan Y, Tong J, Xiong C, Liao Q, et al. p53 transcriptionally activates DCP1B to suppress tumor progression and enhance tumor sensitivity to PI3K blockade in non-small cell lung cancer. Cell Death Differ. (2025). doi: 10.1038/s41418-025-01501-y
19. Vokes NI, Le X, and Yap TA. PIKing up and AKTing on resistance mutations in osimertinib-treated EGFR-mutated NSCLC. Clin Cancer Res. (2024) 30:3968–70. doi: 10.1158/1078-0432.CCR-24-1188
20. Liu Y, Zhang T, Deng J, Huang Q, Yang C, and Cheng Z. The cytotoxicity of (γδ)T cells in non-small cell lung cancer mediated via coordination of the BCL-2 and AKT pathways. Oncogene. (2023) 42:3648–54. doi: 10.1038/s41388-023-02852-x
21. Jouida A, O’Callaghan M, Mc Carthy C, Fabre A, Nadarajan P, and Keane MP. Exosomes from EGFR-mutated adenocarcinoma induce a hybrid EMT and MMP9-dependant tumor invasion. Cancers (Basel). (2022) 14:3776. doi: 10.3390/cancers14153776
22. Liu L, Wuyun T, Sun X, Zhang Y, Cha G, and Zhao L. Therapeutic efficacy of TMTP1-modified EVs in overcoming bone metastasis and immune resistance in PIK3CA mutant NSCLC. Cell Death Dis. (2025) 16:367. doi: 10.1038/s41419-025-07685-y
23. Fan G, Li D, Liu J, Tao N, Meng C, Cui J, et al. HNRNPD is a prognostic biomarker in non-small cell lung cancer and affects tumor growth and metastasis via the PI3K-AKT pathway. Biotechnol Genet Eng Rev. (2024) 40:1571–90. doi: 10.1080/02648725.2023.2196155
24. Li B, Wang Z, Zhong Y, Lan J, Li X, and Lin H. CCR9–CCL25 interaction suppresses apoptosis of lung cancer cells by activating the PI3K/Akt pathway. Med Oncol. (2015) 32:1–9. doi: 10.1007/s12032-015-0531-0
25. Li X, Wu Y, Xie B, Xu M, Xie T, Yue W, et al. SPP1 promotes NSCLC brain metastasis via sequestration of ubiquitin ligase RNF114 to facilitate P85α Ubiquitination. Mol Carcinog. (2025) 64:829–41. doi: 10.1002/mc.23866
26. Xu X, Zhang J, Yao T, Zhao X, Wu Q, Lu C, et al. Differential prognostic impact and potential molecular mechanisms of PCDHGA12 expression in lung adenocarcinoma and squamous cell carcinoma. Int Immunopharmacol. (2024) 139:112727. doi: 10.1016/j.intimp.2024.112727
27. Niu Y, Tang D, Fan L, Gao W, and Lin H. CCL25 promotes the migration and invasion of non-small cell lung cancer cells by regulating VEGF and MMPs in a CCR9-dependent manner. Exp Ther Med. (2020) 19:3571–80. doi: 10.3892/etm.2020.8635
28. Li B, Wang Z, Zhong Y, Lan J, Li X, and Lin H. CCR9-CCL25 interaction suppresses apoptosis of lung cancer cells by activating the PI3K/Akt pathway. Med Oncol (Northwood London England). (2015) 32:66. doi: 10.1007/s12032-015-0531-0
29. Sharma PK, Singh R, Novakovic KR, Eaton JW, Grizzle WE, and Singh S. CCR9 mediates PI3K/AKT-dependent antiapoptotic signals in prostate cancer cells and inhibition of CCR9-CCL25 interaction enhances the cytotoxic effects of etoposide. Int J Cancer (2010) 127:2020–30. doi: 10.1002/ijc.v127:9
30. Zhong Y, Jiang L, Lin H, Li B, Lan J, Liang S, et al. Expression of CC chemokine receptor 9 predicts poor prognosis in patients with lung adenocarcinoma. Diagn Pathol. (2015) 10:101. doi: 10.1186/s13000-015-0341-x
31. Meng-li G, Cai-e W, Yi-meng D, and Jian-gang W. Toxicology, Gecko crude peptides inhibit migration and lymphangiogenesis by down regulating the expression of VEGF-C in human hepatocellular carcinoma cells and human lymphatic endothelial cells. Chinese J Pharmacol and Toxicol. (2017) 31:958–9. doi: 10.3867/j.issn.1000‑3002.2017.10.025
32. Pittayapruek P, Meephansan J, Prapapan O, Komine M, and Ohtsuki M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int J Mol Sci. (2016) 17:868. doi: 10.3390/ijms17060868
33. Cai X, Zhu H, and Li Y. PKCζ, MMP−2 and MMP−9 expression in lung adenocarcinoma and association with a metastatic phenotype. Mol Med Rep. (2017) 16:8301–6. doi: 10.3892/mmr.2017.7634
34. Li H, Zhang B, Liu Y, and Yin C. EBP50 inhibits the migration and invasion of human breast cancer cells via LIMK/cofilin and the PI3K/Akt/mTOR/MMP signaling pathway. Med Oncol. (2014) 31:1–10. doi: 10.1007/s12032-014-0162-x
35. Guan BZ, Yan RL, Huang JW, Li FL, Zhong YX, Chen Y, et al. Activation of G protein coupled estrogen receptor (GPER) promotes the migration of renal cell carcinoma via the PI3K/AKT/MMP-9 signals. Cell Adh Migr. (2018) 12:109–17. doi: 10.4161/19336918.2014.990781
36. Gupta P, Sharma PK, Mir H, Singh R, Singh N, Kloecker GH, et al. CCR9/CCL25 expression in non-small cell lung cancer correlates with aggressive disease and mediates key steps of metastasis. Oncotarget (2014) 5:10170. doi: 10.18632/oncotarget.2526
37. Kaczorowska A, Miękus N, Stefanowicz J, and Adamkiewicz-Drożyńska E. Selected matrix metalloproteinases (MMP-2, MMP-7) and their inhibitor (TIMP-2) in adult and pediatric cancer. Diagn (Basel Switzerland). (2020) 10:547. doi: 10.3390/diagnostics10080547
38. Safranek J, Pesta M, Holubec L, Kulda V, Dreslerova J, Vrzalova J, et al. Expression of MMP-7, MMP-9, TIMP-1 and TIMP-2 mRNA in lung tissue of patients with non-small cell lung cancer (NSCLC) and benign pulmonary disease. Anticancer Res. (2009) 29:2513–7.
39. Lizárraga F, Maldonado V, and Meléndez-Zajgla J. Tissue inhibitor of metalloproteinases-2 growth-stimulatory activity is mediated by nuclear factor-kappa B in A549 lung epithelial cells. Int J Biochem Cell Biol. (2004) 36:1655–63. doi: 10.1016/j.biocel.2004.02.004
40. Fan J, Zhang L, and long Wang Q. Chemokine receptor 9 high-expression involved in the migration and invasion of the non-small-cell lung cancer cells. Asian Biomed. (2011) 5:69–76. doi: 10.5372/1905-7415.0501.008
41. Zappa C and Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res. (2016) 5:288. doi: 10.21037/tlcr.2016.06.07
42. Liu F and Wu H. CC chemokine receptors in lung adenocarcinoma: the inflammation-related prognostic biomarkers and immunotherapeutic targets. J Inflammation Res. (2021) 14:267–85. doi: 10.2147/JIR.S278395
43. Korbecki J, Grochans S, Gutowska I, Barczak K, and Baranowska-Bosiacka I. CC chemokines in a tumor: a review of pro-cancer and anti-cancer properties of receptors CCR5, CCR6, CCR7, CCR8, CCR9, and CCR10 ligands. Int J Mol Sci. (2020) 21:7619. doi: 10.3390/ijms21207619
44. Kanaya N, Seddiq W, Chen KS, Kajiwara Y, Moreno Lama L, Borges P, et al. Engineered allogeneic stem cells orchestrate T lymphocyte driven immunotherapy in immunosuppressive leptomeningeal brain metastasis. J Natl Cancer Inst. (2025) 117:1151–65. doi: 10.1093/jnci/djaf006
45. Guo W, Qiao T, Li H, Zhao Y, Qin J, Zhang C, et al. Peripheral CD8(+)PD-1(+) T cells as novel biomarker for neoadjuvant chemoimmunotherapy in humanized mice of non-small cell lung cancer. Cancer Lett. (2024) 597:217073. doi: 10.1016/j.canlet.2024.217073
46. Alsaed B, Lin L, Son J, Li J, Smolander J, Lopez T, et al. Intratumor heterogeneity of EGFR expression mediates targeted therapy resistance and formation of drug tolerant microenvironment. Nat Commun. (2025) 16:28. doi: 10.1038/s41467-024-55378-5
47. Wang J, Jia Y, Liu T, Liu X, Yin S, Chen J, et al. Tumor cell-intrinsic BIN1 deficiency promotes the immunosuppression and impedes ferroptosis of non-small cell lung cancer via G3BP1-mediated degradation of STAT1. J Exp Clin Cancer Res. (2025) 44:141. doi: 10.1186/s13046-025-03404-9
48. Onder L, Papadopoulou C, Lütge A, Cheng HW, Lütge M, Perez-Shibayama C, et al. Fibroblastic reticular cells generate protective intratumoral T cell environments in lung cancer. Cell. (2025) 188:430–446.e20. doi: 10.1016/j.cell.2024.10.042
49. Qu J, Wu B, Chen L, Wen Z, Fang L, Zheng J, et al. CXCR6-positive circulating mucosal-associated invariant T cells can identify patients with non-small cell lung cancer responding to anti-PD-1 immunotherapy. J Exp Clin Cancer Res. (2024) 43:134. doi: 10.1186/s13046-024-03046-3
50. Luo J, Cheng K, Ji X, Gao C, Zhu R, Chen J, et al. Anlotinib enhanced CD8(+) T cell infiltration via induction of CCL5 improves the efficacy of PD-1/PD-L1 blockade therapy in lung cancer. Cancer Lett. (2024) 591:216892. doi: 10.1016/j.canlet.2024.216892
51. Huang L, Wang D, Xu M, Qian D, Cao Y, Wu X, et al. Mixed radiation with different doses induces CCL17 to recruit CD8(+)T cell to exert anti-tumor effects in non-small cell lung cancer. Front Immunol. (2024) 15:1508007. doi: 10.3389/fimmu.2024.1508007
52. Shi Y, Riese DJ 2nd, and Shen J. The role of the CXCL12/CXCR4/CXCR7 chemokine axis in cancer. Front Pharmacol. (2020) 11:574667. doi: 10.3389/fphar.2020.574667
53. Steele MM, Jaiswal A, Delclaux I, Dryg ID, Murugan D, Femel J, et al. T cell egress via lymphatic vessels is tuned by antigen encounter and limits tumor control. Nat Immunol. (2023) 24:664–75. doi: 10.1038/s41590-023-01443-y
54. Cao C, Xu M, Wei Y, Peng T, Lin S, Liu X, et al. CXCR4 orchestrates the TOX-programmed exhausted phenotype of CD8(+) T cells via JAK2/STAT3 pathway. Cell Genomics. (2024) 4:100659. doi: 10.1016/j.xgen.2024.100659
55. Liang Z, Brooks J, Willard M, Liang K, Yoon Y, Kang S, et al. CXCR4/CXCL12 axis promotes VEGF-mediated tumor angiogenesis through Akt signaling pathway. Biochem Biophys Res Commun. (2007) 359:716–22. doi: 10.1016/j.bbrc.2007.05.182
56. Phillips RJ, Mestas J, Gharaee-Kermani M, Burdick MD, Sica A, Belperio JA, et al. Epidermal growth factor and hypoxia-induced expression of CXC chemokine receptor 4 on non-small cell lung cancer cells is regulated by the phosphatidylinositol 3-kinase/PTEN/AKT/mammalian target of rapamycin signaling pathway and activation of hypoxia inducible factor-1α. (2005) 280:22473–81. doi: 10.1074/jbc.M500963200
57. Smit MJ, Schlecht-Louf G, Neves M, van den Bor J, Penela P, Siderius M, et al. The CXCL12/CXCR4/ACKR3 axis in the tumor microenvironment: signaling, crosstalk, and therapeutic targeting. Annu Rev Pharmacol Toxicol. J Biol Chem. (2021) 61:541–63. doi: 10.1146/annurev-pharmtox-010919-023340
58. Qin R, Ren W, Ya G, Wang B, He J, Ren S, et al. Role of chemokines in the crosstalk between tumor and tumor-associated macrophages. Clin Exp Med. (2023) 23:1359–73. doi: 10.1007/s10238-022-00888-z
59. Tilley AM, Howard CM, Sridharan S, Subramaniyan B, Bearss NR, Alkhalili S, et al. The CXCR4-dependent LASP1-Ago2 interaction in triple-negative breast cancer. Cancers (Basel) (2020) 12:2455. doi: 10.3390/cancers12092455
60. Dąbrowska E, Przylipiak A, Zajkowska M, Piskor BM, Sidorkiewicz I, Szmitkowski M, et al. Possible diagnostic application of CXCL12 and CXCR4 as tumor markers in breast cancer patients. Anticancer Res. (2020) 40:3221–9. doi: 10.21873/anticanres.14303
61. Ottaiano A, Scala S, Normanno N, Botti G, Tatangelo F, Di Mauro A, et al. Prognostic and predictive role of CXC chemokine receptor 4 in metastatic colorectal cancer patients. Appl Immunohistochem Mol Morphol. (2020) 28:755–60. doi: 10.1097/PAI.0000000000000828
62. Wu J, Liu X, Wu J, Lou C, Zhang Q, Chen H, et al. CXCL12 derived from CD248-expressing cancer-associated fibroblasts mediates M2-polarized macrophages to promote nonsmall cell lung cancer progression, Biochimica et biophysica acta. Mol Basis Dis. (2022) 1868:166521. doi: 10.1016/j.bbadis.2022.166521
63. Stumpf C, Kaemmerer D, Neubauer E, Sänger J, Schulz S, and Lupp A. Somatostatin and CXCR4 expression patterns in adenocarcinoma and squamous cell carcinoma of the lung relative to small cell lung cancer. J Cancer Res Clin Oncol. (2018) 144:1921–32. doi: 10.1007/s00432-018-2722-5
64. Kijima T, Maulik G, Ma PC, Tibaldi EV, Turner RE, Rollins B, et al. Regulation of cellular proliferation, cytoskeletal function, and signal transduction through CXCR4 and c-Kit in small cell lung cancer cells. Cancer Res. (2002) 62:6304–11.
65. Dong Q, Chen P, Qiu W, Yang Z, Li Y, Zhou Y, et al. Long non-coding RNA Malat1 modulates CXCR4 expression to regulate the interaction between induced neural stem cells and microglia following closed head injury. Stem Cell Res Ther. (2025) 16:31. doi: 10.1186/s13287-024-04116-1
66. Xie J, Yang Q, Zeng X, Zeng Q, and Xiao H. Dihydromyricetin inhibits injury caused by ischemic stroke through the lncRNA SNHG17/miR-452-3p/CXCR4 axis. PeerJ. (2025) 13:e18876. doi: 10.7717/peerj.18876
67. Ning J, Yang R, Wang H, Ma H, and Cui L. LncRNA MALAT1 silencing represses CXCL12-induced proliferation, invasion, and homing behavior in multiple myeloma by inhibiting CXCR4. Hematol (Amsterdam Netherlands). (2024) 29:2422154. doi: 10.1080/16078454.2024.2422154
68. Katsura M, Shoji F, Okamoto T, Shimamatsu S, Hirai F, Toyokawa G, et al. Correlation between CXCR4/CXCR7/CXCL12 chemokine axis expression and prognosis in lymph-node-positive lung cancer patients. Cancer Sci. (2018) 109:154–65. doi: 10.1111/cas.2018.109.issue-1
69. Liang J-X, Gao W, Liang Y, and Zhou X-M. Chemokine receptor CXCR4 expression and lung cancer prognosis: a meta-analysis. Int J Clin Exp Med. (2015) 8:5163.
70. Perrone C, Bozzano F, Dal Bello MG, Del Zotto G, Antonini F, Munari E, et al. CD34(+)DNAM-1(bright)CXCR4(+) haemopoietic precursors circulate after chemotherapy, seed lung tissue and generate functional innate-like T cells and NK cells. Front Immunol. (2024) 15:1332781. doi: 10.3389/fimmu.2024.1332781
71. Kogue Y, Kobayashi H, Nakamura Y, Takano T, Furuta C, Kawano O, et al. Prognostic value of CXCL12 in non-small cell lung cancer patients undergoing tumor resection. Pharm (Basel). (2023) 16:255. doi: 10.3390/ph16020255
72. Guo W, Huai Q, Zhou B, Guo L, Sun L, Xue X, et al. Comprehensive analysis of the immunological implication and prognostic value of CXCR4 in non-small cell lung cancer. Cancer Immunol Immunother. (2023) 72:1029–45. doi: 10.1007/s00262-022-03298-y
73. Fung AS, Kopciuk K, Dean ML, D’Silva A, Otsuka S, Klimowicz A, et al. CXCR4 expression in lung carcinogenesis: Evaluating gender-specific differences in survival outcomes based on CXCR4 expression in early stage non-small cell lung cancer patients. PloS One. (2021) 16:e0241240. doi: 10.1371/journal.pone.0241240
74. Fortunato O, Belisario DC, Compagno M, Giovinazzo F, Bracci C, Pastorino U, et al. CXCR4 inhibition counteracts immunosuppressive properties of metastatic NSCLC stem cells. Front Immunol. (2020) 11:02168. doi: 10.3389/fimmu.2020.02168
75. Di Paolo D, Pontis F, Moro M, Centonze G, Bertolini G, Milione M, et al. Cotargeting of miR-126-3p and miR-221-3p inhibits PIK3R2 and PTEN, reducing lung cancer growth and metastasis by blocking AKT and CXCR4 signalling. Mol Oncol. (2021) 15:2969–88. doi: 10.1002/1878-0261.13036
76. Jäger B, Klatt D, Plappert L, Golpon H, Lienenklaus S, Barbosa PD, et al. CXCR4/MIF axis amplifies tumor growth and epithelial-mesenchymal interaction in non-small cell lung cancer. Cell Signal. (2020) 73:109672. doi: 10.1016/j.cellsig.2020.109672
77. Salgia R, Weaver RW, McCleod M, Stille JR, Yan SB, Roberson S, et al. Prognostic and predictive value of circulating tumor cells and CXCR4 expression as biomarkers for a CXCR4 peptide antagonist in combination with carboplatin-etoposide in small cell lung cancer: exploratory analysis of a phase II study. Invest New Drugs (2017) 35:334–44. doi: 10.1007/s10637-017-0446-z
78. Li Z, Wang Y, Shen Y, Qian C, Oupicky D, and Sun M. Targeting pulmonary tumor microenvironment with CXCR4-inhibiting nanocomplex to enhance anti–PD-L1 immunotherapy. Sci Adv. (2020) 6:. doi: 10.1126/sciadv.aaz9240
Keywords: chemokines, lung cancer, immunotherapy, CCR9, CXCL12, PI3K pathway
Citation: Gong X, Wang X, Jin J and Gong Z (2025) The novel functions of chemokines in lung cancer progression. Front. Immunol. 16:1607225. doi: 10.3389/fimmu.2025.1607225
Received: 07 April 2025; Accepted: 30 May 2025;
Published: 18 June 2025.
Edited by:
Zhijie Zhao, Shanghai Jiao Tong University, ChinaReviewed by:
Benhua Li, First Affiliated Hospital of Chongqing Medical University, ChinaCopyright © 2025 Gong, Wang, Jin and Gong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiqiang Gong, Z29uZ3poaXFpYW5nQGNhbmNlcmhvc3AtbG4tY211LmNvbQ==
 Xiaorui Gong1
Xiaorui Gong1 Zhiqiang Gong
Zhiqiang Gong