- 1College of Traditional Chinese Medicine, Jiangsu College of Nursing, Huaian, Jiangsu, China
- 2Department of Clinical Pharmacy, Jinling Hospital, Medical school of Nanjing University, Nanjing, China
Background: Current primary tumor treatments include curative resection, chemotherapy, and radiotherapy. However, these conventional methods lack precise drug delivery. Hydrogels, adaptable to the biological characteristics of different tumors, offer potential as drug delivery systems and represent a significant area of research in tumor treatment. In this study, we aimed to conduct a bibliometric analysis to reveal the current progress and future prospects of hydrogels for drug delivery in cancer.
Methods: Publications concerning hydrogels in tumor drug delivery were retrieved from the Web of Science Core Collection (WoSCC) database. Data regarding countries/regions, institutions, journals, authors, and document types were collected. Bibliometric analysis and network visualization were performed using CiteSpace, HisCite, VOSviewer, Alluvial Generator, and R software.
Results: China, the United States, and India were the leading contributing countries. Of the 98 relevant categories, 94 experienced citation bursts between 2000 and 2024. The research team led by Professor Pourmadadi Mehrab demonstrated substantial influence in this field. The International Journal of Biological Macromolecules was the most prolific journal. The top three emergent categories originated in 2020 or later, are "Chemistry, Applied", "Engineering, Environmental", and "Biochemistry & Molecular Biology". "Designing hydrogels for controlled drug delivery" was the most highly cited article. Recent emergent keywords included immunotherapy, immunogenic cell death, carboxymethyl cellulose, and antibacterial. Key concept alluvial flow visualization revealed six terms: peritoneal carcinomatosis, iron oxide nanoparticles, drug delivery, release kinetics, carbon dots, and pathway. Nano-composite hydrogels, immunotherapy, quercetin, pancreatic cancer, and oral cancer exhibited recent activity within the cited article timeline, suggesting these areas as potential future research hotspots.
Conclusion: This bibliometric analysis identifies future research directions within the developing field of hydrogels for drug delivery in cancer. This study provides recommendations and directions for the development of hydrogels as tumor drug delivery systems.
1 Introduction
Cancer remains a significant threat to human health (1). Current tumor treatments include surgery, radiotherapy, chemotherapy, and traditional Chinese medicine, with chemotherapy being a common clinical approach (2, 3). While chemotherapy can improve patient survival, its lack of targeting can damage normal cells, leading to serious side effects (4). To improve chemotherapeutic efficacy, researchers have made significant progress in developing targeted drug delivery systems. These systems can deliver drugs directly to tumor cells through specific targeting mechanisms, thereby reducing side effects on healthy tissues and improving the effectiveness of the treatment (5). Advances in nanotechnology have provided new approaches to drug delivery systems. Nanocarriers can improve drug targeting and release efficiency through a variety of mechanisms (6). For example, pH-sensitive nanosystems can trigger drug release in the acidic tumor microenvironment, thereby improving therapeutic efficiency (7). These intelligent drug delivery systems can more effectively cross biological barriers for targeted intracellular drug delivery, leading to improved therapeutic outcomes. The drug delivery system combines low molecular weight cytotoxic drugs with nanocarriers, and this combination therapy can enhance the anti-tumor potency by promoting more uniform drug distribution within the tumor, thus improving therapeutic efficacy (8, 9). The structure of hydrogels includes polymeric materials with crosslinked 3D networks (10). Based on the raw materials used, hydrogels can be classified as either natural or synthetic (11). Hydrogel technology has experienced increased use in cancer treatment in recent years. Hydrogels, as drug delivery carriers, demonstrate considerable potential in tumor treatment. As semisolid drug delivery systems, they can effectively facilitate the transdermal transport of active drugs (12).
Injectable hydrogels offer minimally invasive in vivo delivery of chemotherapeutic drugs, representing a novel approach to cancer treatment (13). These hydrogels can reduce systemic side effects and improve therapeutic efficacy by increasing drug concentration at tumor sites. In addition, hydrogel nanocomposites can improve drug penetration within tumors, enhancing both penetration and residence time through a combination of active transcytosis and passive diffusion (14).
Bibliometrics employs statistical analysis of published works to evaluate the influence and development trends of scientific research through quantitative indicators. It is widely used across various fields to analyze research status, trends, hotspots, and collaborative patterns. CiteSpace, a tool focused on the visualization of scientific literature, identifies key documents and research frontiers by analyzing document co-citation networks. Researchers can use CiteSpace to construct scientific knowledge maps, facilitating a deeper understanding of the evolution of a given research field (15).
This study employed HisCite, CiteSpace, VOSviewer, and R software to generate a knowledge map. Using a bibliometric approach, we analyzed the knowledge framework, research hotspots, and trends in the development of hydrogels for drug delivery in cancer. This analysis provides further insights into hydrogels as drug delivery systems, especially in cancer treatment, hoping to provide new ideas and directions for targeted tumor delivery.
2 Methods
2.1 Data collection and strategies
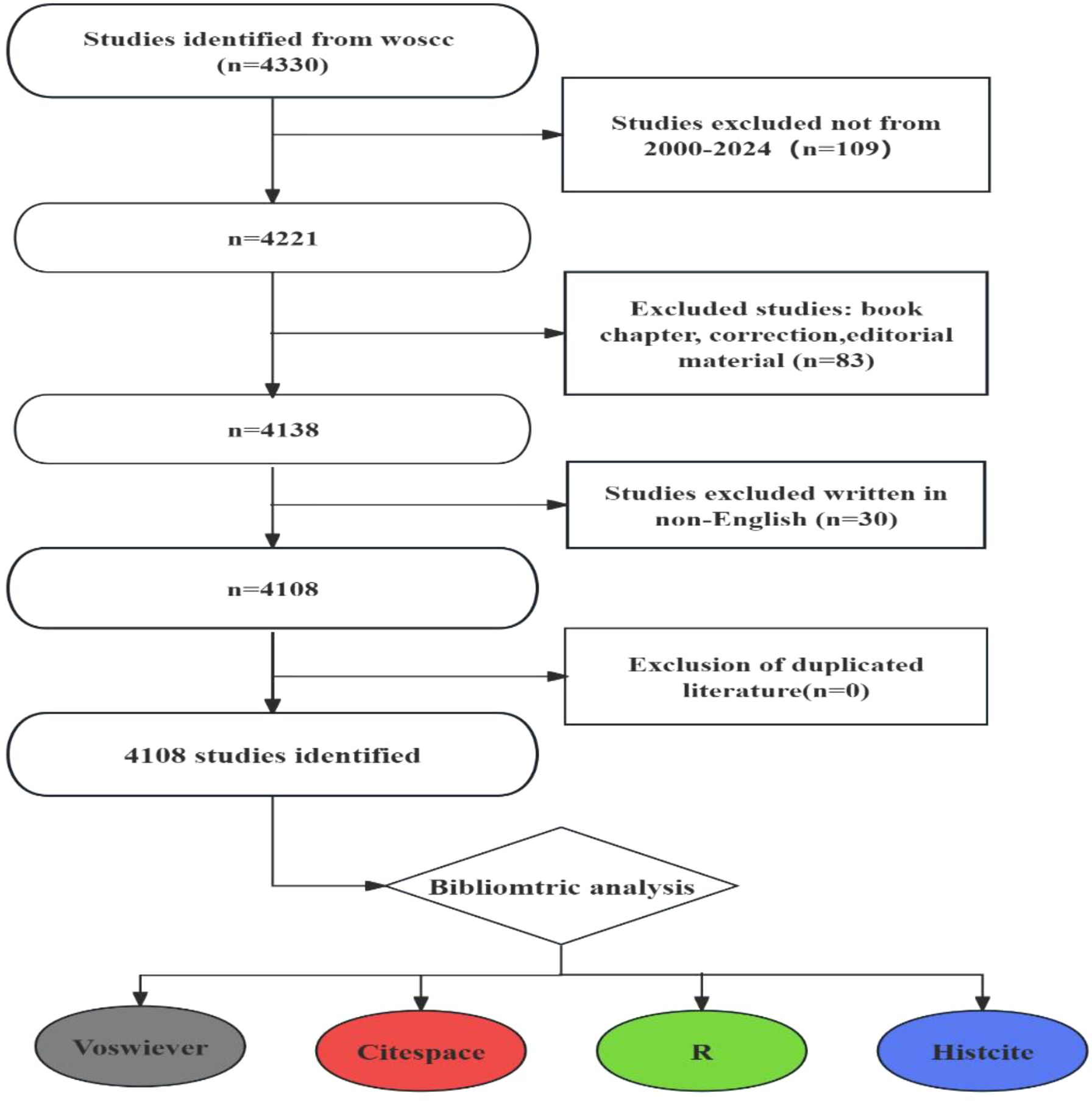
The Web of Science Core Collection (WoSCC), encompassing over 12,000 influential scholarly journals, is a widely recognized and comprehensive, multidisciplinary database offering citation analysis. For this study, we searched the WoSCC database for relevant literature published between 2000 and 2024. The search formula was ((TS=(“Tumor”) OR TS=(“Neoplasm”) OR TS=(“Tumors”) OR TS=(“Neoplasia”) OR TS=(“Neoplasias”) OR TS=(“Cancer”) OR TS=(“Cancers”) OR TS=(“Malignant Neoplasm”) OR TS=(“Malignancy”) OR TS=(“Malignancies”) OR TS=(“Malignant Neoplasms”) OR TS=(“Neoplasm, Malignant”) OR TS=(“Neoplasms, Malignant”) OR TS=(“Benign Neoplasms”) OR TS=(“Benign Neoplasm”) OR TS=(“Neoplasms, Benign”) OR TS=(“Neoplasm, Benign”))AND ((TS=(Hydrogels)) OR TS=(Hydrogel)) OR TS=(“Patterned Hydrogel”) AND (((((TS=(“Drug Delivery Systems”)) OR TS=(“Drug Delivery System”)) OR TS=(“Drug Targeting*”)) OR TS=(“Drug Carrier*”)) OR TS=(“Drug Carriers”)) OR TS=(“drug delivery”). We Retrieved records were downloaded as plain text files in “Full Record and Cited References” format. This data, comprising 4108 publications, was saved for subsequent analysis and designated as DATA (Figure 1). Finally, we collected information, including country, region, institution, journal, author, keywords, and key references. Data statistics were performed using Microsoft Excel 2019.
2.2 Data analysis
CiteSpace uses distinct node and edge colors to differentiate the merged network, with each color representing a different year. This bibliometric analysis software provided three clustering algorithms based on titles, abstracts, and keywords. The resulting cluster maps reflect changes in conceptual clustering over time. Relevant data were imported into CiteSpace (version 6.2.R4). VOSviewer was used to visualize the author collaboration network. HistCite Pro (version 2.1) was used to plot numerical relationships, identify highly cited literature, and readily display the most influential publications. HistCite calculates both local citation scores (LCS) and global citation scores (GCS)) to identify authoritative and influential papers. Relevant data from 4108 research articles on hydrogels in cancer drug delivery were imported into HistCite Pro 2.1. Alluvial flow maps were generated using the Alluvial Flow Generator (http://www.mapequation.org/apps/AlluvialGenerator.html) to illustrate temporal patterns in evolving networks. The donut plot shown in Figure 2 was generated using R (version 4.2.2) with the geom_bar function from the ggplot2 package (version 3.4.4).
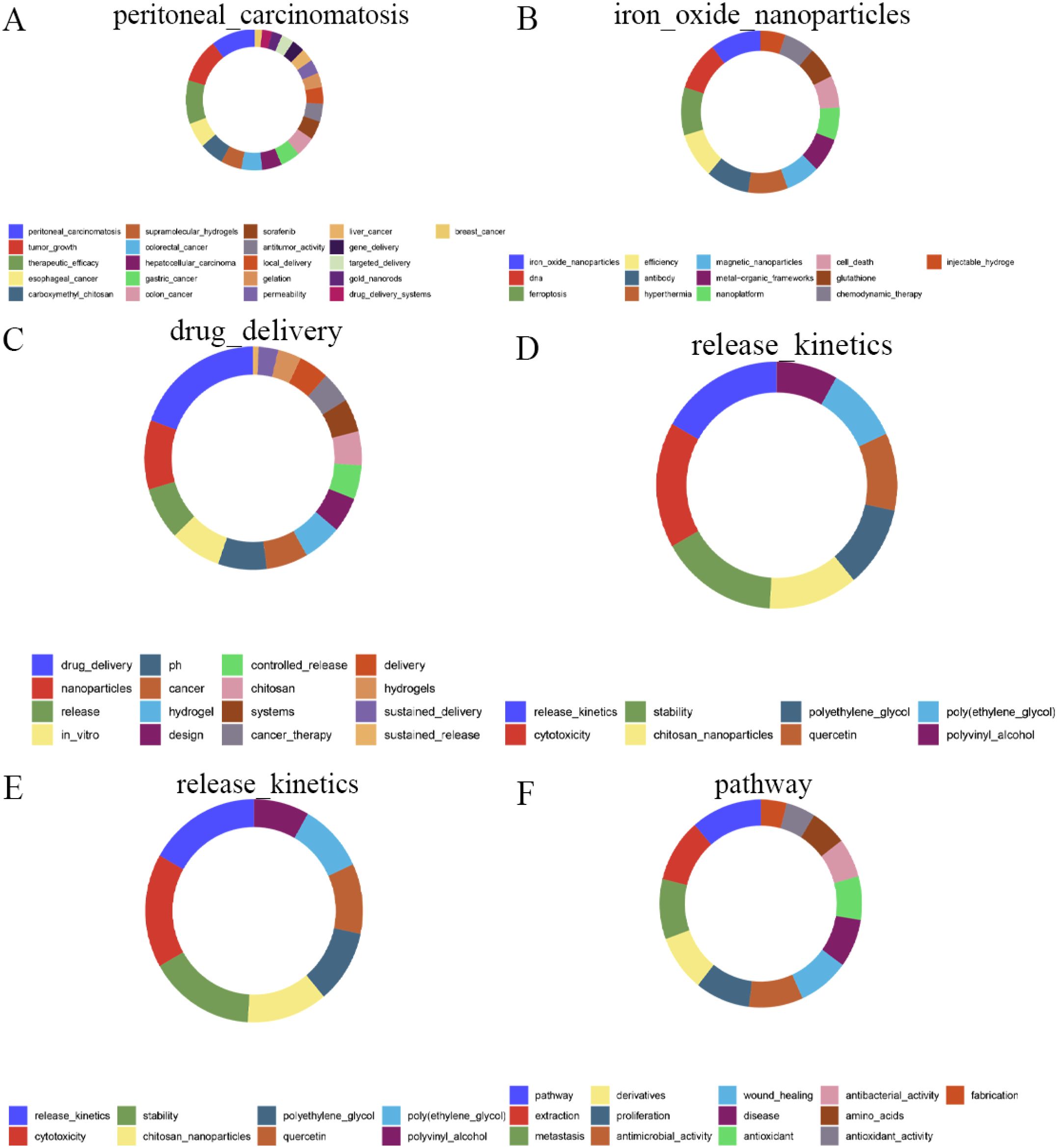
Figure 2. The keywords of top 5 modules in 2024. (A) Module 1. (B) Module 2. (C) Module 3. (D) Module 4. (E) Module 5. (F) Module 6.
3 Results
3.1 The historical overview of hydrogel research in cancer drug delivery
3.1.1 Distribution of publications
The increase or decline in publications over time can reveal research interest in specific fields, providing quantitative insights for subject research and publication. In this study, a total of 4108 publications related to hydrogels in oncology drug delivery were retrieved, including 3110 research articles and 998 review articles, with 15,496 participating authors and 3293 participating institutions, published in 664 journals in 98 scientific categories.
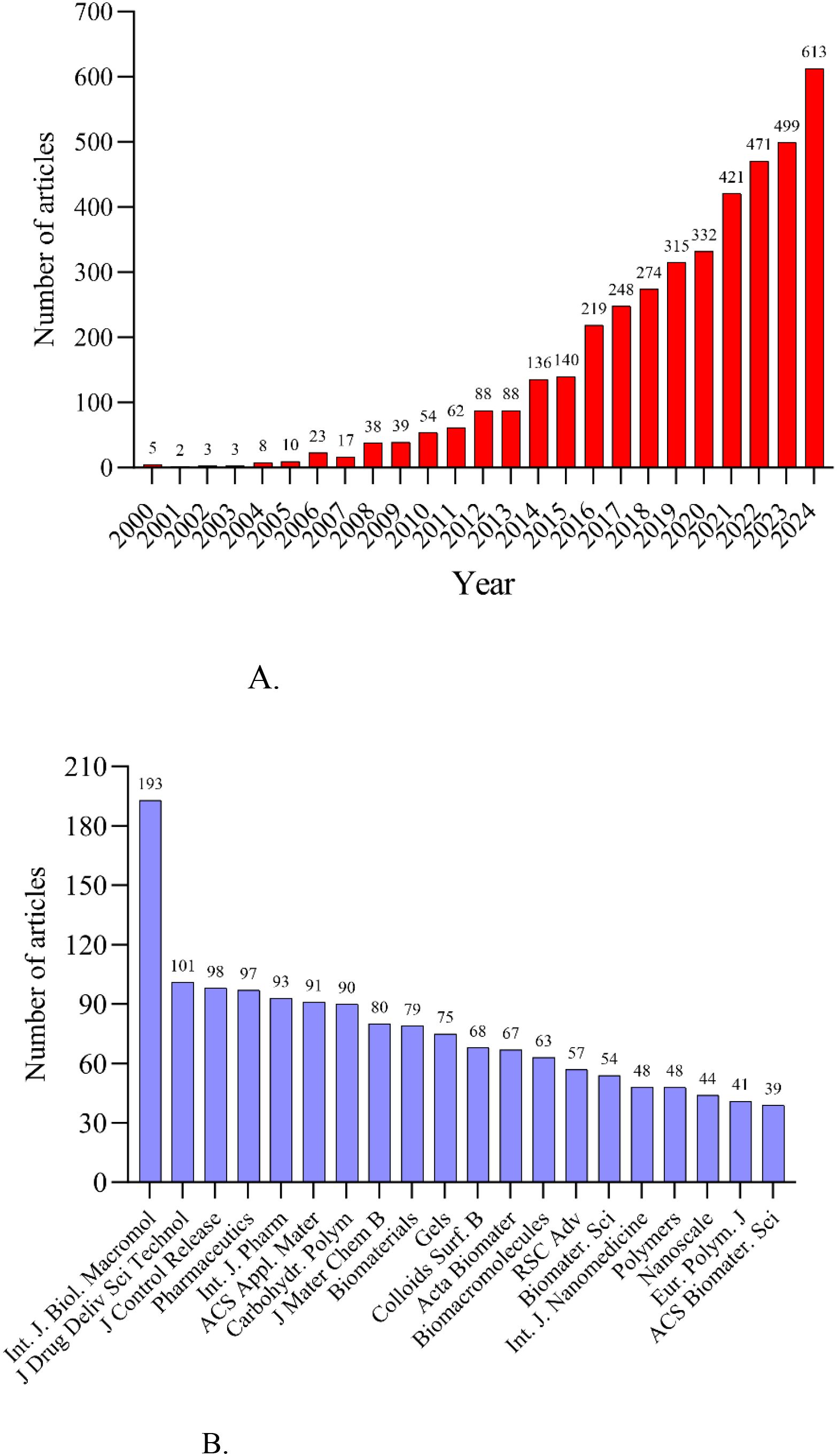
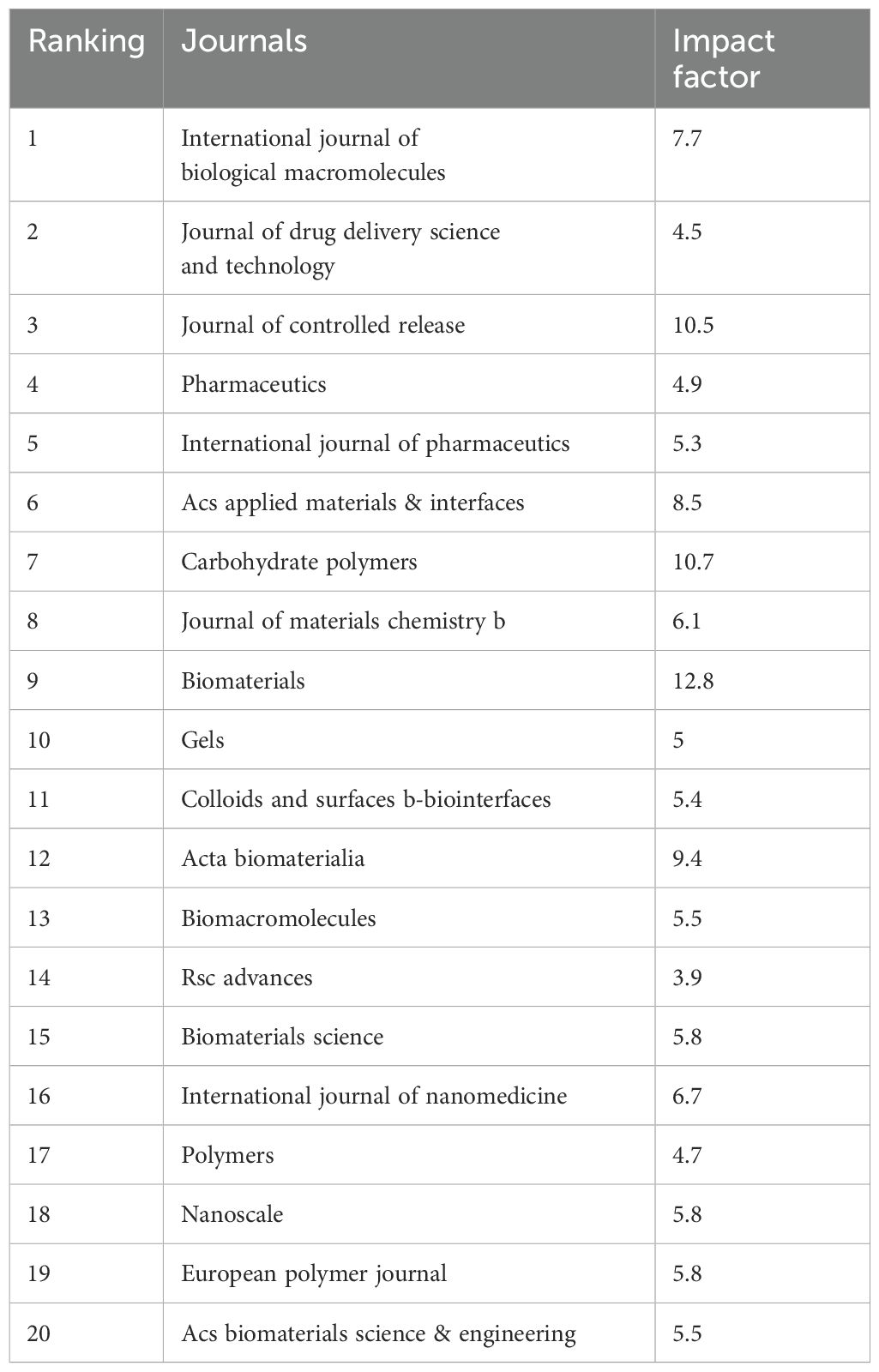
Annual research output is shown in Figure 3A. Five papers related to hydrogels in oncology drug delivery were published in 2000, and two in 2001. Publication numbers remained low from 2000 to 2007, followed by a rapid increase from 2008 to 2015. The growth rate further accelerated after 2016, peaking in 2024. The International Journal of Biological Macromolecules was the most prolific journal (n=193 publications), followed by the Journal of Drug Delivery Science and Technology (n=101) and the Journal of Controlled Release (n=98). Figure 3B and Table 1 list the top 20 most productive journals, providing a useful reference for researchers considering manuscript submissions.
3.1.2 Co-citation network analysis of the evolution of hydrogel research in cancer drug delivery
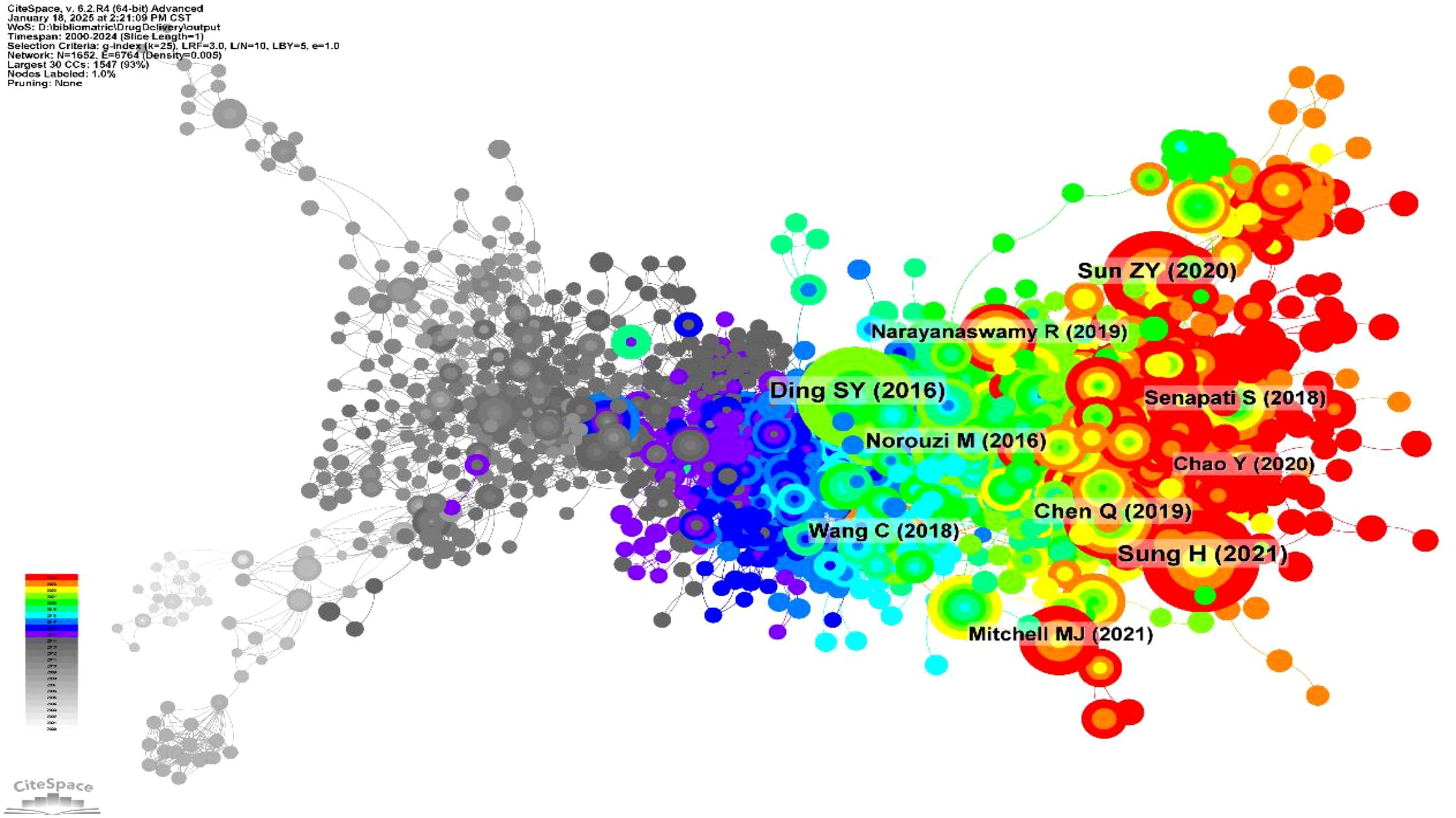
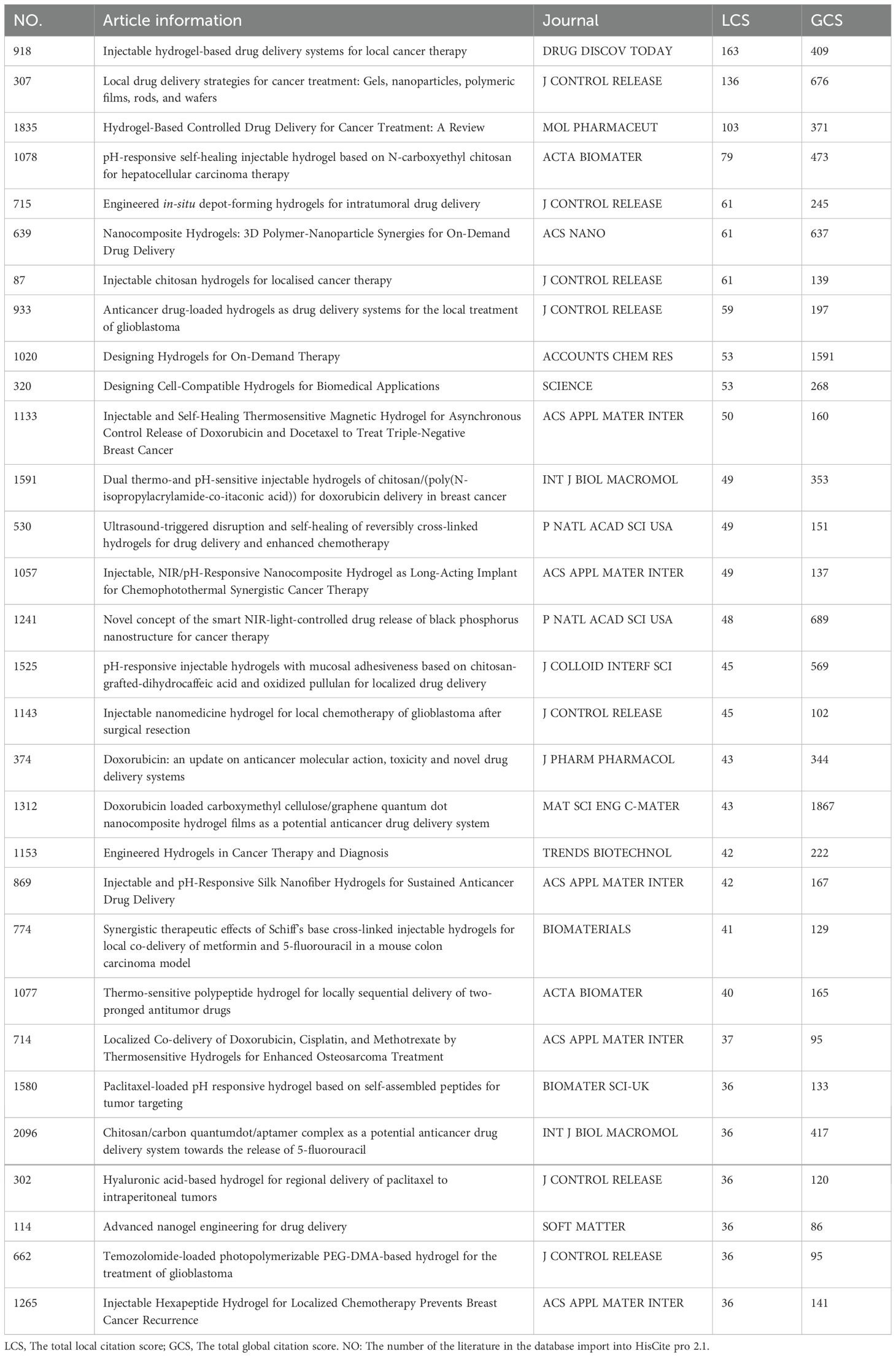
A co-citation network was generated (Figure 4) to illustrate the relationships between publications in the field of hydrogels for oncology drug delivery from 2000 to 2024. The network comprised 1652 nodes and 6764 links, indicating extensive interconnections within this research area. Early literature (2000-2010), represented by densely interconnected gray nodes, forms the foundational “roots”, supporting the field’s subsequent growth. During the mid-period (2011-2017), blue-marked nodes became more dispersed, forming the main research branches. In the later period (2018-2024), nodes develop into tighter clusters, indicating concentration and differentiation within the field. Key publications driving this research, with their respective co-citation frequencies, included Sung H (2021) (16) (n=120), Ding SY (2016) (17) (n=116), Sun ZY (2020) (18) (n=99), Chen Q (2019) (19) (n=79), Norouzi M (2016) (20) (n=68), Wang C (2018) (21) (n=64), Senapati S (2018) (22) (n=61), Narayanaswamy R (2019) (23) (n=57), Mitchell MJ (2021) (24) (n=55), and Chao Y (2020) (25) (n=54). This concentration and differentiation of research clusters were further clarified in subsequent reference timeline plots. Citation histories of these research articles, visualized using HisCite Pro 2.1, are summarized in Table 2. The top three cited papers were “Injectable hydrogel-based drug delivery systems for local cancer therapy” (20), “Local drug delivery strategies for cancer treatment: Gels, nanoparticles, polymeric films, rods, and wafers” (26), and “Hydrogel-based controlled drug delivery for cancer treatment: A Review” (18). Larger nodes in the graph indicate greater citation frequency and, thus, greater importance of the referenced work.
3.1.3 Scientific cooperation
Figure 5 presents the scientific cooperation networks for countries, institutions, and authors, generated using CiteSpace and VOSviewer. The national collaboration network (Figure 5A) comprised 98 nodes and 788 links, with node size reflecting the volume of publications, ranging from China (largest) to the United States, India, Iran, and South Korea. The institutional collaboration network (Figure 5B) consists of 534 nodes and 660 links, with the Chinese Academy of Sciences, Sichuan University, the Indian Institutes of Technology System (IIT System), and Tabriz University of Medical Sciences representing prominent nodes. The author collaboration network is shown in Figure 5C. Pourmadadi Mehrab, Rahdar Abbas, Qian Zhiyong, and Abdouss Majid exhibited the highest publication counts in this field. The dense interconnections among these authors signify substantial collaborative activity (Supplementary Table S1 in the Supplementary Materials). Notably, a clustering effect was observed among the nodes representing Pourmadadi Mehrab, Rahdar Abbas, and Pandey Sadanand, indicating a close collaborative group. Similarly, Qian Zhiyong, Zhang Yu, and their co-authors formed another distinct cluster.
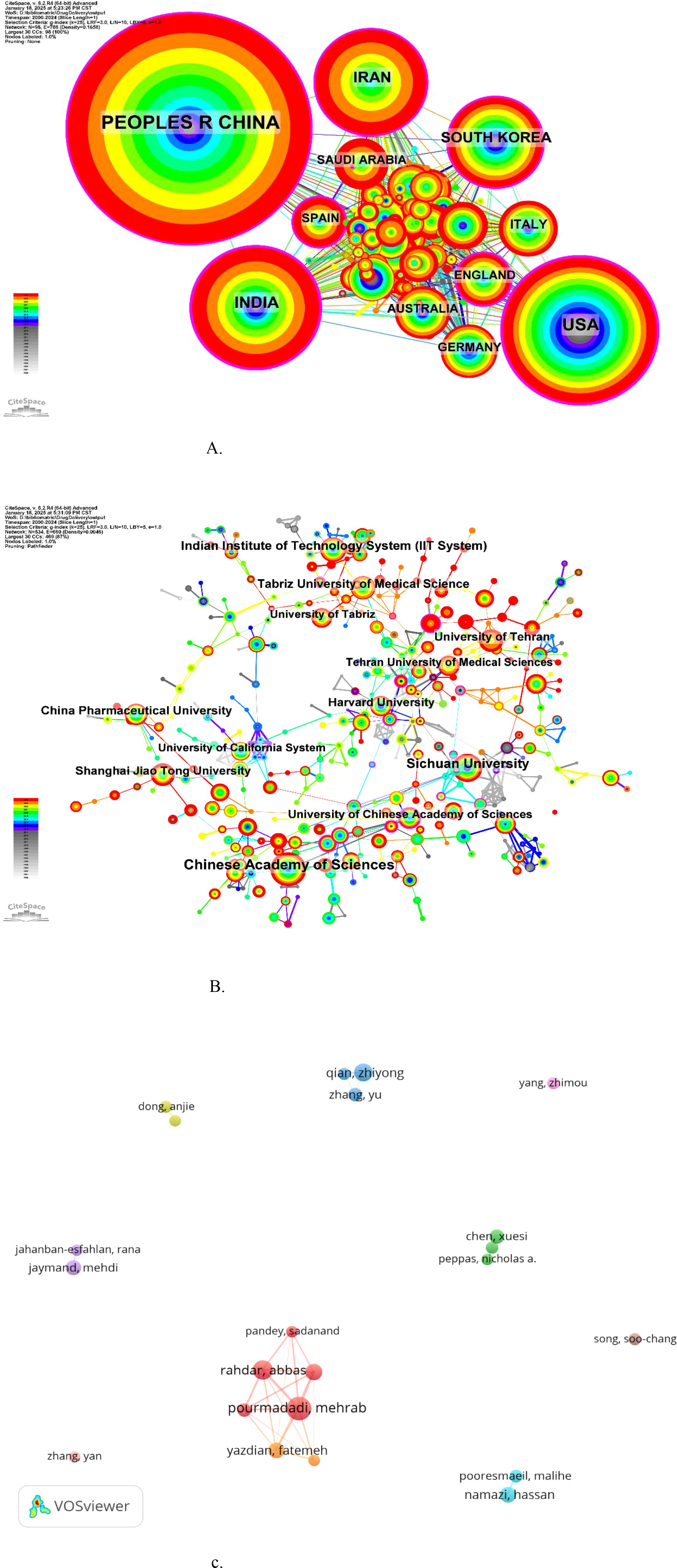
Figure 5. (A) The cooperation network of countries. (B) The cooperation network of institutions. (c) The cooperation network of authors.
3.2 Variation of the most active topics
3.2.1 Subject category burst
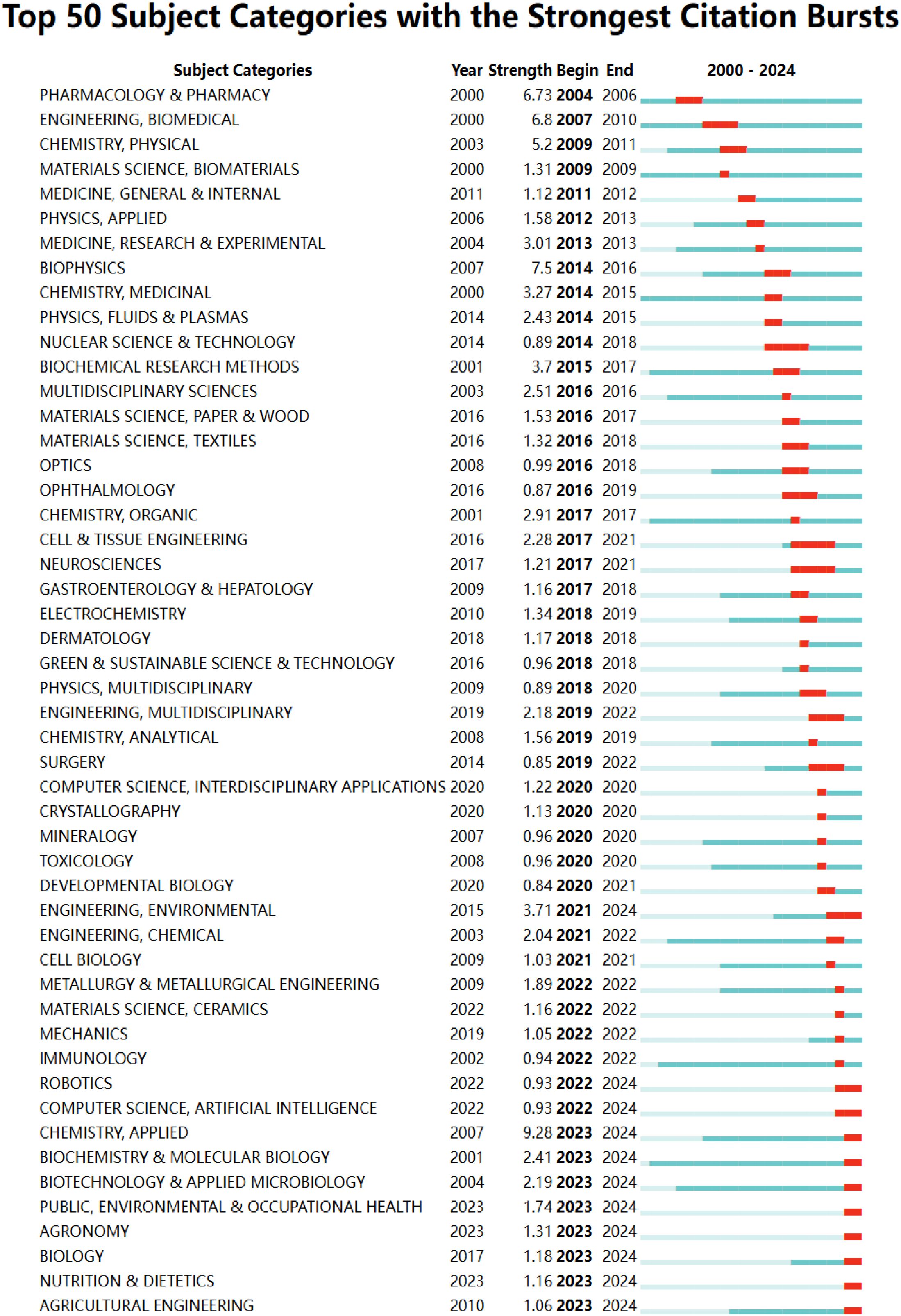
Of the 98 relevant categories, 94 experienced citation bursts between 2000 and 2024. Blue lines indicate the time interval, while red portions highlight the burst timeframe. Figure 6 presents the top 50 categories with the highest burst intensity over time. The “Engineering, Biomedical” category exhibited a burst between 2007 and 2010, with a peak intensity of 6.8. Notably, the subject category bursts have diversified over time, as seen with “Chemistry, Physical” (2009-2011), “Physics, Applied” (2012-2013), “Nuclear Science & Technology” (2014-2018), “Physics, Multidisciplinary” (2018-2020), and “Computer Science, Artificial Intelligence” (2022-2024). This shift in emergent categories reflects the multidisciplinary nature of the field. In addition, 20 emergent categories originated in 2020 or later (Supplementary Table S2 in the Supplementary Materials). The top three, based on recent burst activity, are “Chemistry, Applied” (2023-2024), “Engineering, Environmental” (2021-2024), and “Biochemistry & Molecular Biology” (2023-2024).
3.2.2 Keyword burst
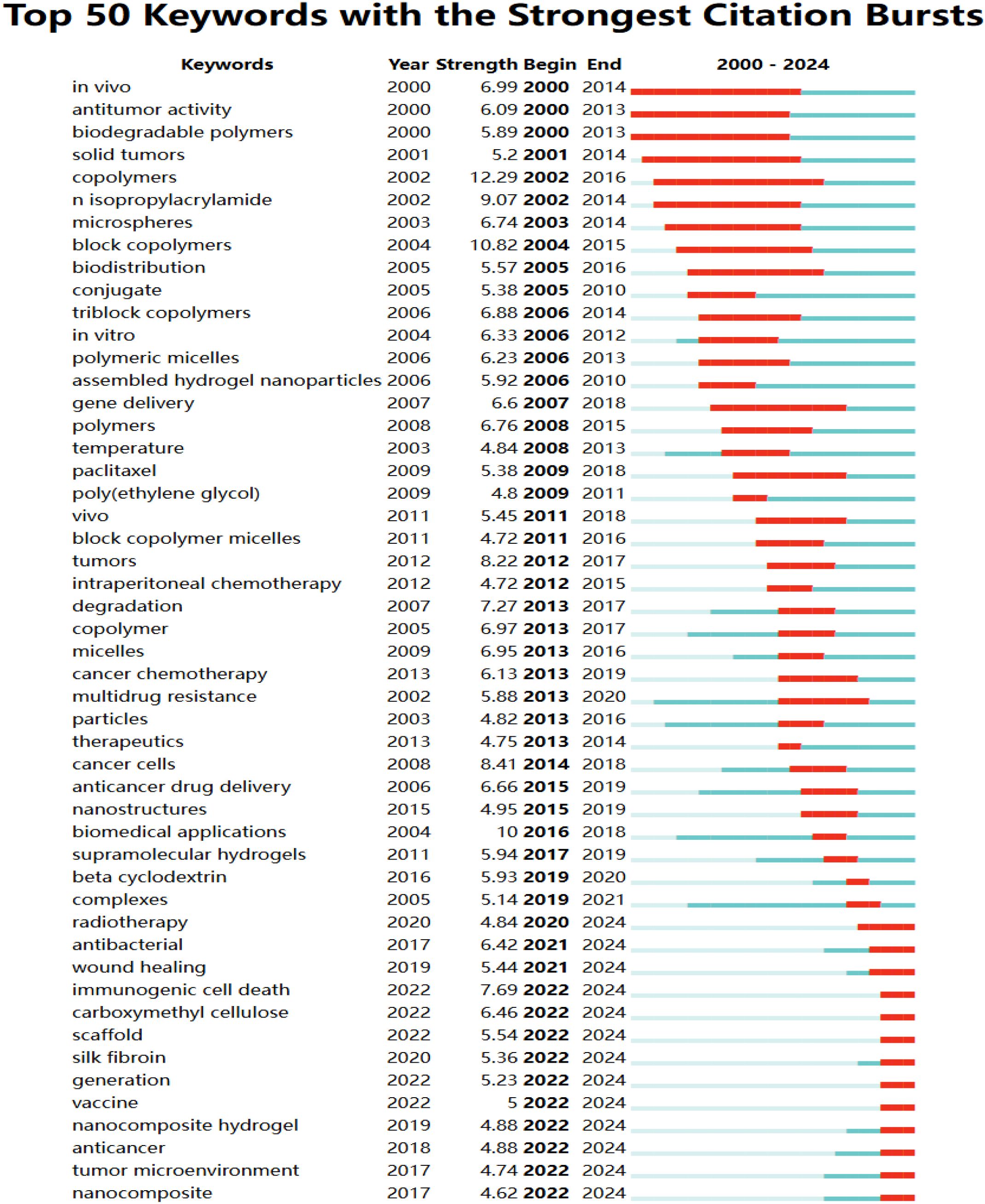
A more detailed analysis of active hydrogel content within oncology drug delivery was conducted by examining keyword burst patterns from 2000 to 2024. Of the 759 keywords that exhibited bursts at various times, the top 50 with the highest burst intensity are presented in Figure 7. “Copolymers” showed the highest burst intensity (12.29) between 2002 and 2016, followed by “block copolymers” (10.82) between 2004 and 2015, and “n-isopropylacrylamide” (9.07) between 2002 and 2014. Notably, 20 keywords remained active in 2024, suggesting potential future research hotspots. Examples include “immunotherapy” (11.07, 2023-2024), “immunogenic cell death” (7.69, 2022-2024), “carboxymethyl cellulose” (6.46, 2022-2024), and “antibacterial” (6.46, 2022-2024). The burst intensity for “antibacterial” specifically between 2021 and 2024 was 6.42 (Supplementary Table S2, Supplementary Materials).
3.2.3 Reference burst analysis
A total of 1,534 references were identified as having burst citations. The 30 most frequently cited references between 2000 and 2024 are shown in Table 3. Li JY (2016) (17) exhibited the highest citation burst rate, spanning from 2019 to 2021. The article concluded that hydrogel drug delivery systems provide beneficial therapeutic effects and have been used in clinical settings. Mura S (2013) (27) experienced a citation burst from 2015 to 2018, noting the recent surge in interest in advanced nanoscale drug delivery systems, particularly within nanomedicine. Norouzi M (2016) (20) had the third highest citation burst rate, attracting considerable attention upon publication and continuing for four years, from 2017 to 2021.
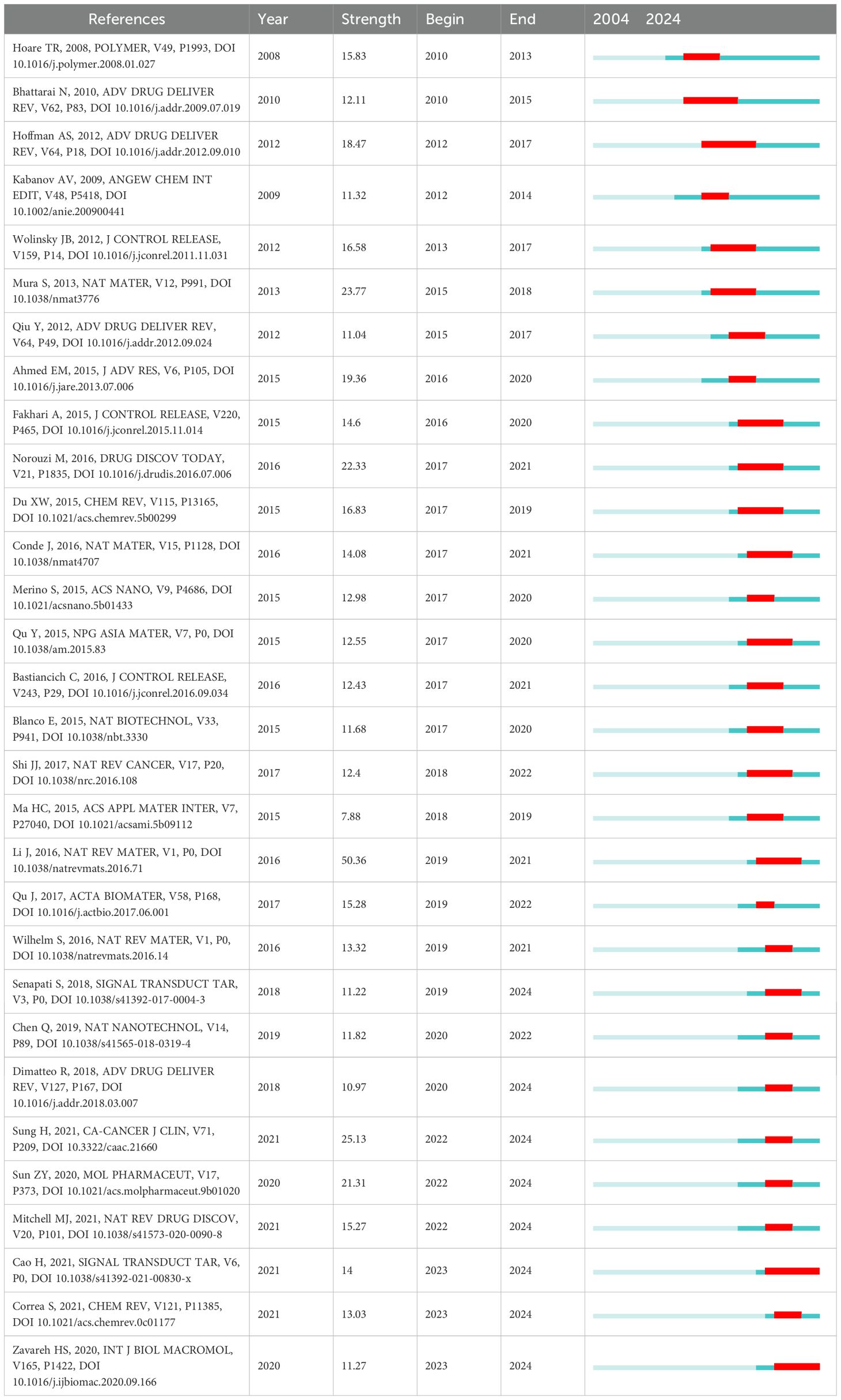
From 2004 onwards, 187 references exhibited citation bursts, with the top 20 strongest bursts detailed in Table 4. Of these, six were review articles and 14 were original research articles. Notably, all these publications experienced citation bursts either in the year of publication or the following year. The review articles provide valuable guidance for research on hydrogels in oncology drug delivery, while the research articles serve as excellent references for applications of hydrogels in this field. This observation highlights the importance of researchers in the field of hydrogels for oncology drug delivery paying close attention to this rapidly evolving body of literature.
3.3 Emerging trends and new developments
3.3.1 The temporal variation of keyword clusters
There exists a close relationship between different keywords. These keywords can generate different clusters, and the identification of these clusters can indicate various subfields of hydrogel in cancer drug delivery research. The past 24 years were divided into four six-year phases, with keyword clustering snapshots for each phase shown in Figure 8. The first clustering (2000-2006), based on 54 papers, yielded 9 clusters, including #0 cancer therapy, #1 polyethylene glycol (PEG), and #2 localization (Figure 8A). The second clustering (2007-2012), analyzing 298 papers, produced 11 clusters, including #0 systems, #1 nanocomposites, and #2 DNA (Figure 8B). The third clustering (2013-2018), encompassing 1105 papers, generated 9 clusters, such as #0 drug delivery, #1 thermosensitive hydrogel, #2cross linking, and 9 other clusters (Figure 8C). The fourth clustering (2019-2024), including 2651 papers, resulted in 8 clusters, including #0 tissue engineering, #1 immunogenic cell death, and #2 nanocomposite hydrogel (Figure 8D). While classical areas like cancer therapy and drug delivery remain active research areas, emerging clusters such as #0 tissue engineering, #1 immunogenic cell death, #2 nanocomposite hydrogel, #3 skin cancer, #5 cancer immunotherapy, #6 graphene quantum dots, and #7 glioblastoma have gained increasing attention compared to the previous 15 years.
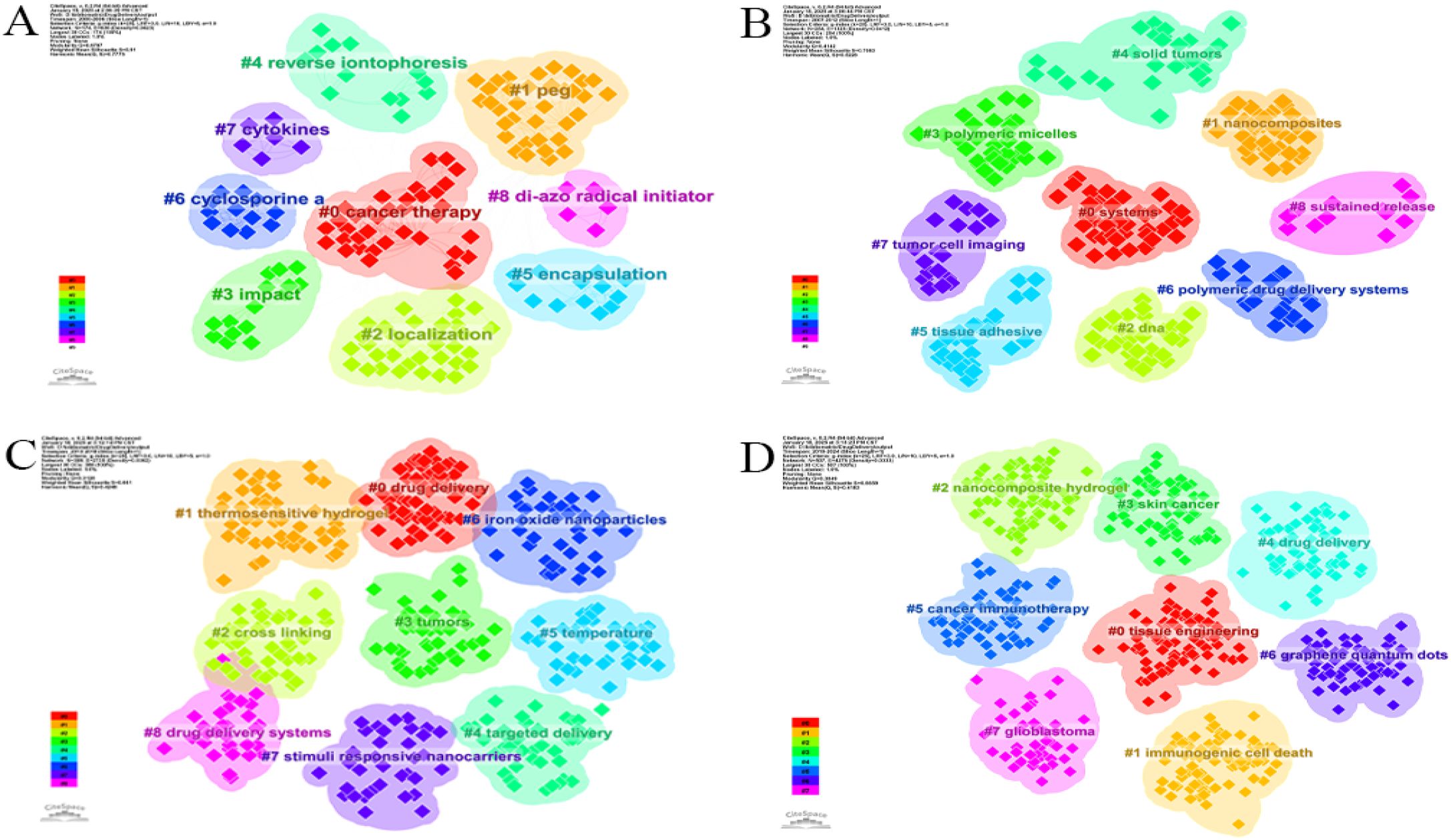
Figure 8. Knowledge map of keyword clustering. (A) 2000-2006, (B) 2007-2012, (C) 2013-2018, (D) 2019-2024.
Analysis of emerging literature clusters revealed a focus on key areas within hydrogel research for oncology drug delivery. Cluster 0#, “tissue engineering,” comprises 77 articles. Cluster 1#, “immunogenic cell death,” and Cluster 5#, “cancer immunotherapy,” contain 76 and 58 articles, respectively, reflecting research into various cell death mechanisms and the role of hydrogels in tumor immunotherapy. Cluster 2#, “nanocomposite hydrogel,” and Cluster 6#, “graphene quantum dots,” with 71 and 56 articles, respectively, explore different nanomaterials for tumor drug delivery. Cluster 3#, “skin cancer,” and Cluster 7#, “glioblastoma,” with 61 and 47 articles, respectively, examine the application of hydrogels in treating specific tumor types. Supplementary Table S3 (Supplementary Materials) provides detailed data for the fourth clustering period (2018-2024), including “representative keywords within the clusters” that identify core research areas within the most recent phase of hydrogel research for oncology drug delivery.”
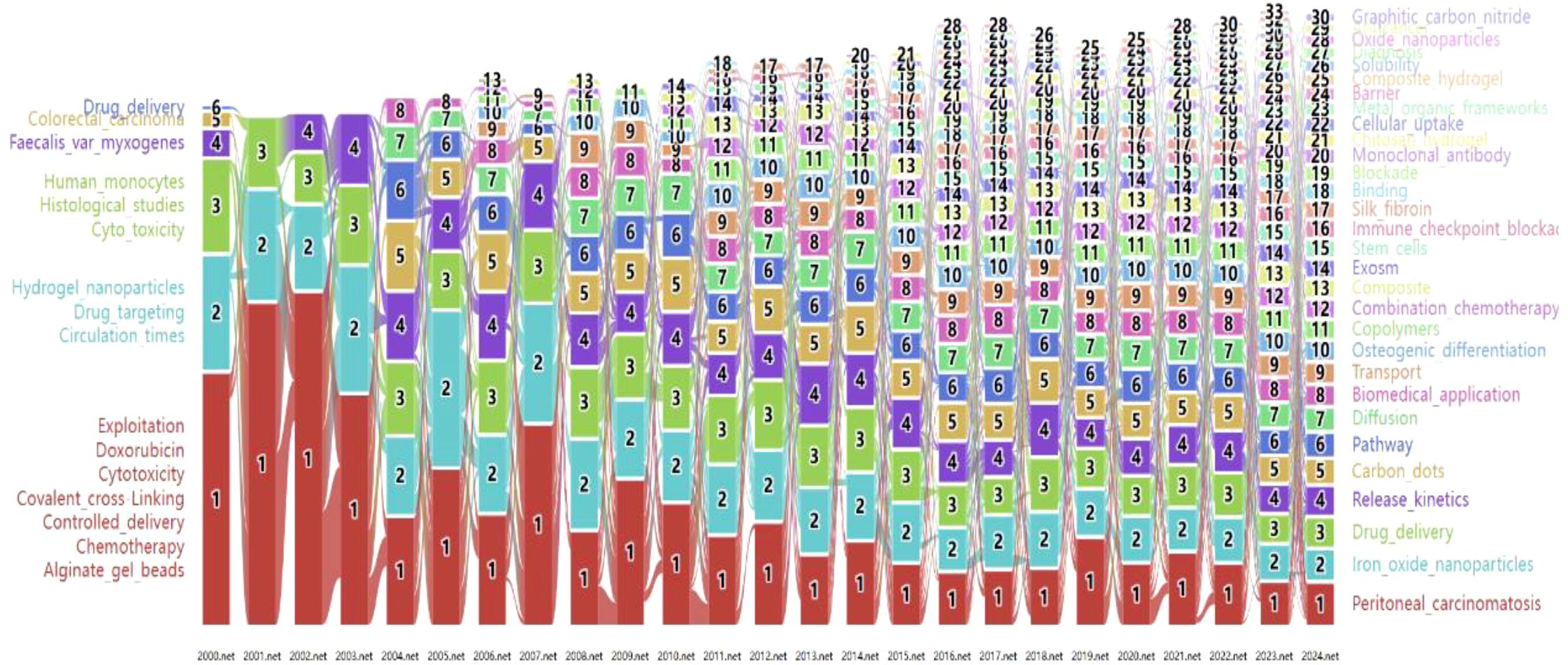
3.3.2 The keyword alluvial flow visualization
As shown in Figure 9, linked keywords form specific research modules, and keywords form new modules over time. Over the past 25 years, some high-traffic keywords have persisted, some have increased in importance and popularity, while others have disappeared. Supplementary Table S4 (Supplementary Materials) lists the top five highest-traffic module keywords for each year. Notably, the keywords within Module 1 in 2024 diverged or converged at this research turning point, forming the largest research tributary (highlighted in red). This suggests that Module 1 represents the most enduring research module. Figure 2 maps all keywords for the top six modules in 2024. Module 1, designated “peritoneal_carcinomatosis”, encompasses 21 keywords, such as tumor growth, therapeutic efficacy, and esophageal cancer (Figure 2A). Module 2, named “iron_oxide_nanoparticles”, contains 13 keywords, such as DNA, ferroptosis, efficiency, and antibody (Figure 2B). Module 3, “drug_delivery”, comprises 16 keywords such as nanoparticles, release, in vitro, and pH (Figure 2C). Module 4, “release_kinetics”, contains 8 keywords, such as cytotoxicity, stability, chitosan nanoparticles, and polyethylene glycol (Figure 2D). Module 5, “carbon_dots”, includes 13 keywords, such as floating hydrogel, classification, and cyclodextrin (Figure 2E). Finally, Module 6, “pathway”, encompasses 13 keywords, including extraction, metastasis, derivatives, and proliferation (Figure 2F). These modules likely represent emerging trends in hydrogels for oncology drug delivery over the next five years and potentially beyond.
3.3.3 The timeline visualization of references
A timeline visualization, based on citation time spans, identifies emerging, classic, and outdated research topics. The timeline plot of hydrogel studies in oncology drug delivery comprised 19 clusters, arranged in descending order of size (Figure 10A). Clusters #1 (tumor cell imaging), #2 (injectable), #4 (multispectral fluorescence), and #5 (self-assembly) represent classic topics. While not necessarily the most recent, these topics were intrinsically linked to other clusters. Clusters #6 (self-organized nanogels), #7 (elp), #9 (cytarabine), #11 (covalent cross-linking), #12 (cytarabine), #13 (in situ forming), #14 (long-term stability), #15 (bioadhesion), #16 (biodegradable), and #17 (vaccination) are considered relatively outdated, exhibiting few connections to other clusters and limited subsequent development. Clusters #0 (nanocomposite hydrogels), #3 (immunotherapy), #8 (quercetin), #10 (pancreatic cancer), and #18 (oral cancer) were emerging topics, demonstrating continued activity on the timeline since their emergence. This suggested their potential to become future research hotspots. Supplementary Table S5 (Supplementary Materials) provides further details on these emerging clusters. In addition, some classical papers (large nodes with red circles) played a very important role in advancing the subfields (Figure 10B).
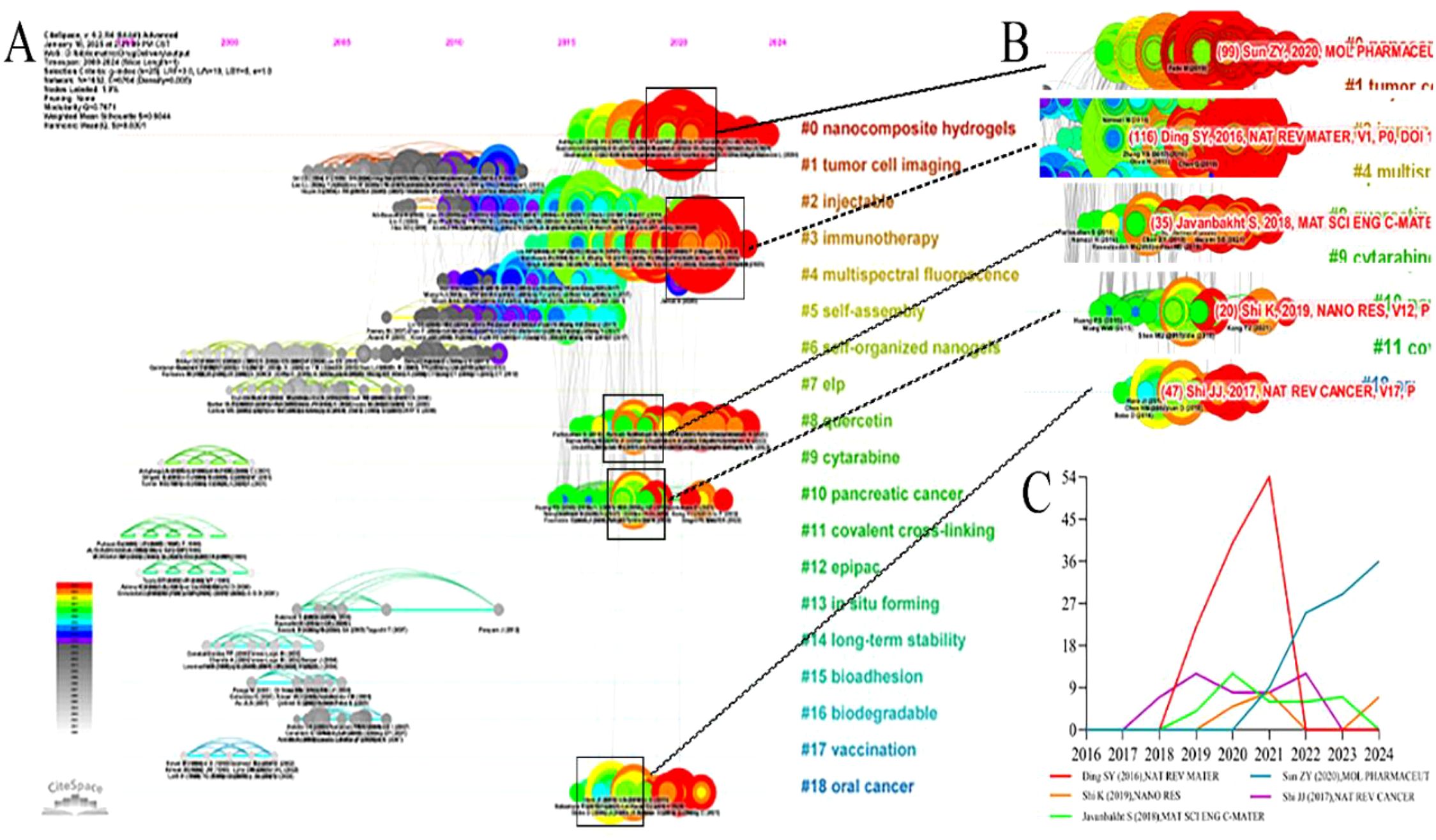
Figure 10. The reference clusters map. (A) the citation timeline visualization, (B) The burst citation in #0, #3, #8, #10, and #18, (C) citation frequency distribution of the burst citation, X-axis: Year, Y-axis: Cited frequency.
Cirillo G’s 2019 publication, “Injectable Hydrogels for Cancer Therapy over the Last Decade”, was attributed to group 0 and exhibited a co-citation frequency of 99 (28). This article highlighted research efforts over the past decade focused on injectable hydrogels for cancer therapy. Many researchers are developing sophisticated injectable drug delivery systems suitable for combination chemotherapy, radiation therapy, and thermal and photothermal ablation, aiming to overcome limitations of current conventional treatments.
The study “Designing hydrogels for controlled drug delivery” (15), published by Li JY, belonged to group 3 and exhibited a co-occurrence frequency of 116. This research demonstrated the ability of hydrogels to control drug release both spatially and temporally, encompassing various drug types such as small molecule drugs, large molecule drugs, and cells. Hydrogels, possessing controlled degradability and the capacity to protect labile drugs from degradation, can be used as a platform on which interactions with encapsulated drugs occur to achieve controlled drug release. They also presented different mechanisms for the design of hydrogel drug delivery systems, especially the physical and chemical properties and the interaction of the hydrogel with the drug at the network, reticular and molecular scales. In addition, they discussed the interplay and integration of these mechanisms for precise spatiotemporal control of drug release.
Javanbakht S authored a publication titled “Doxorubicin loaded carboxymethyl cellulose/graphene quantum dot nanocomposite hydrogel films as a potential anticancer drug delivery system”, which belonged to cluster 8 and exhibited a co-occurrence frequency of 35 (29). In this study, they designed a novel hydrogel nanocomposite film with anticancer properties by incorporating graphene quantum dot (GQD) nanoparticles in carboxymethyl cellulose (CMC) hydrogel with doxorubicin (DOX) as a drug model. The prepared nanocomposite hydrogel films showed improved in vitro solubility, degradation, water vapor permeability and pH-sensitive drug delivery properties. The nanocomposite hydrogel films could be used as anticancer films and drug delivery systems.
The article “Functional hydrogels as wound dressing to enhance wound healing” by Liang YP et al. (30) was attributed to group 3. Hydrogels possess favorable biochemical and mechanical properties, offering significant advantages in wound dressing applications. This paper mainly introduced the antibacterial, anti-inflammatory, and antioxidant properties of hydrogel dressings, along with their material transport and self-healing capabilities. It also summarized the application of hydrogel wound dressing in the treatment of different types of wounds, demonstrating their effectiveness in promoting wound healing.
Shi K et al. (31) reported on the sustained co-delivery of gemcitabine and cisplatin using a biodegradable, thermo-sensitive hydrogel for synergistic combination therapy of pancreatic cancer. They developed a biodegradable, thermosensitive copolymer hydrogel for the combined delivery of gemcitabine (GEM) and cisplatin (DDP) in pancreatic cancer treatment. This hydrogel exists as a free-flowing liquid at room temperature, transitioning to a semi-solid state at physiological temperatures upon injection. In vitro and in vivo drug release studies demonstrated sustained drug release from this system. The hydrogel system exhibited a stronger synergistic therapeutic effect against pancreatic cancer, both in vitro and in vivo, compared to hydrogels loaded with single drugs.
Shi et al.’s 2017 publication, “Cancer nanomedicine: progress, challenges and opportunities” (32), was attributed to group 18. The limitations of traditional cancer therapies have spurred in-depth research into nanotechnology for more effective and safer cancer treatment, giving rise to the field of cancer nanomedicine. This study primarily reviewed the development of cancer nanomedicine, concluding that a deeper understanding of tumor biology and nanobiology is crucial for developing improved cancer nanotherapies. Analysis of the citation distribution of these five articles over recent years (Figure 10C) suggested that these publications were likely to continue to be cited in the future.
4 Discussion
4.1 General information
Cancer is a complex and life-threatening disease with numerous, often imperfect, treatment options. Precisely delivering therapeutic drugs to tumor tissue for release can significantly improve cancer therapy efficacy. Drug delivery systems enhance drug pharmacokinetics and tumor site accumulation, playing a crucial role in cancer therapy (33). In recent years, hydrogels have been used as drug delivery systems in tumor therapy and have gained widespread attention due to their unique physical and chemical properties (34, 35).
This study analyzed hydrogels for drug delivery in cancer using bibliometric methods, employing CiteSpace, HisCite, the Alluvial Generator, and R software. The 4108 retrieved articles related to hydrogels in oncology drug delivery were published in 664 journals across 98 scientific categories. From 2014 to 2024, the number of annual publications related to hydrogels in cancer drug delivery consistently increased, exceeding 100 annually (Figure 1A). 2024 saw the highest number of publications, with 613 articles, clearly indicating growing interest in hydrogels as drug delivery systems in tumor therapy. The top three publishing journals were the International Journal of Biological Macromolecules, the Journal of Drug Delivery Science and Technology, and the Journal of Controlled Release (Figure 3). Researchers should therefore pay close attention to these journals. Co-occurrence frequency analysis identified highly influential countries, institutions, and active authors, providing valuable insights into the field of hydrogels for drug delivery in cancer. Among national collaborations, China, the United States, India, Iran, and South Korea frequently occupied central positions within international cooperation networks (Figure 5A), with China exhibiting the highest co-occurrence frequency. Analysis of institutional collaborations revealed primarily domestic collaborations, with limited direct and in-depth international exchange (Figure 5B). China also hosted the top two institutions, the Chinese Academy of Sciences and Sichuan University, exhibiting high co-occurrence frequency in this field (Supplementary Table S1). In addition, the analysis revealed a limited number of high-yield authors and a low degree of collaboration among them.
4.2 Research topics and trends
Burst detection of subject categories and keywords from 2000 to 2024 was performed to analyze the development trends and evolving characteristics of hydrogels in tumor drug delivery. Polypeptide self-assembled materials, a type of synthetic biomaterial, offer advantages such as simple synthesis, good biocompatibility, low immunogenicity, and low toxicity (36). Hydrogels, composed of hydrophilic polymers, possess a three-dimensional network structure that allows for significant water absorption and drug carrying capacity (37). The burst period for the biomedical discipline occurred between 2007 and 2010, indicating an early focus on biomedical applications (Figure 6). Over time, the research focus has shifted towards chemistry, engineering, environmental science, and biochemistry & molecular biology. Hydrogels can also be combined with metal-organic frameworks (MOFs) to create composite materials, forming stimulus-responsive local drug delivery systems with dynamic structural characteristics. This approach enables localized and sustained drug release in vivo, effectively inhibiting tumor growth (38). In contrast to conventional substrates, biochemically responsive hydrogels modulate the interplay between biological events, hydrogel properties, and cellular behaviors (39). Interdisciplinary research on hydrogels enhances our understanding of their application potential in tumor drug delivery and promotes further advancements in cancer treatment. Keyword burst analysis, based on frequently occurring keywords within specific time periods, revealed research hotspots and trends in hydrogels for tumor drug delivery. Current research keywords identified include immunotherapy, immunogenic cell death, carboxymethyl cellulose, and antibacterial, reflecting current research hotspots in the field.
4.2.1 Immunotherapy
Conventional tumor therapy focuses on the tumor itself, but given the complexity of the tumor microenvironment, immunotherapy has emerged as a promising approach. Immunotherapy leverages the body’s immune system to attack tumors, wherein immune cells target and destroy tumor cells within the tumor microenvironment (40, 41). Tumor immunotherapy is a guideline-recommended treatment modality for gastrointestinal tumors, demonstrating good efficacy and fewer side effects (42). Hydrogels, capable of drug loading, controlled drug release, and delivery of immunomodulatory molecules, immune cells, and environmental regulators, can improve the overall response rate of immunotherapy. This has led to their emergence as multifunctional materials in biomedical applications (43). Yang Y et al. developed a shear-thinning injectable hydrogel that enhances local immunotherapy for gastric cancer by repolarizing tumor-associated macrophages (44). Their results demonstrated favorable tumor growth inhibition after a single injection. Similarly, Seo HS et al. developed a multifunctional ethylene glycol methacrylate-chitosan (MGC) hydrogel that effectively inhibited tumor recurrence and metastasis, enhanced immunotherapy efficacy, and minimized side effects (45). These findings indicate that hydrogel-based drug delivery systems combined with cancer immunotherapy could significantly improve local and systemic treatment outcomes, providing more precise and effective treatment strategies.
4.2.2 Immunogenic cell death
Immunogenic cell death (ICD) is a form of programmed cancer cell death wherein dying cancer cells release immune-stimulating factors, prompting the immune system to recognize and eliminate tumors (46). ICD not only promotes the release of tumor-associated antigens but also activates the immune response of T cells through the maturation of antigen-presenting cells (47). The occurrence of ICD is characterized by changes in surface molecules, the release of inflammatory factors, and antigen activation, all of which contribute to enhancing the anti-tumor response of the immune system. Studies have shown that nanomaterials can enhance tumor immunogenicity by inducing ICD, thereby improving the efficacy of immunotherapy (48). Shen et al. designed a copper (Cu)-induced injectable hydrogel. This ion-induced self-assembled hydrogel containing NO can enhance immunotherapy by amplifying ICD and regulating cancer-associated fibroblasts (49). A pH-responsive polyamidoamine dendritic nanocarrier-incorporated hydrogel can serve as a drug delivery system to bind immunochemotherapy drugs, induce immunogenic cell death-related cytokines, and trigger CD8+ T cell-mediated immune responses to enhance immunogenic cell death and reverse immunosuppression, leading to significant tumor growth inhibition (50). An in-depth study of ICD will improve our understanding of the role of hydrogel drug delivery systems in the tumor immune microenvironment and facilitate the development of more effective immunotherapy strategies.
4.2.3 Cellulose
Cellulose, a renewable polymer, and its derivatives are widely used in various medical applications, including tissue engineering, artificial blood vessels, and drug carriers, with many applications related to the treatment of cancer (51). Carboxymethyl cellulose (CMC), a readily available chemical product, possesses thickening and relative stability properties, making it an ideal hydrogel carrier (52). This hydrogel exhibits biocompatibility, biodegradability, and antibacterial properties. CMC hydrogel can deliver the anticancer drug 5-fluorouracil, and adjusting the electric field can influence the drug’s diffusion coefficient, enabling precise drug release (53). Combining CMC with other materials can further enhance hydrogel properties. Nano-composite hydrogel beads formed by combining CMC with starch and ZnO nanoparticles have been investigated for the controlled release of doxorubicin. This composite material can prolong the drug release and control it more accurately by adjusting the content of ZnO nanoparticles (54). Nanocomposite hydrogel beads composed of CMC, starch, and zinc oxide nanoparticles (ZnO-NPs) have also been studied for the controlled release of doxorubicin. Increasing the ZnO nanoparticle content can prolong drug release time, and this composite has also demonstrated toxicity to human colon cancer cells in vitro (54). CMC-based hydrogels have diverse applications in drug delivery systems and can achieve precise drug release control and enhanced biocompatibility through various combinations and modifications.
4.2.4 Antimicrobial hydrogels
In recent years, with increased understanding of tumor biology, tumor-targeted drugs have seen rapid development in cancer treatment. However, viral reactivation can occur in patients undergoing targeted therapy (55), necessitating viral prophylaxis and anti-inflammatory measures post-treatment. The development and design of bioactive materials with both anti-tumor and anti-bacterial properties has gained attention due to the established link between bacteria and tumors. Antimicrobial hydrogels possess excellent antimicrobial properties, good biocompatibility, water absorption and retention, and high oxygen permeability, making them widely applicable in biomedicine, smart textiles, cosmetics, and medical dressings (56, 57). By modifying the properties of hydrogel, its antibacterial, vascularization, anti-inflammatory and antioxidant properties can be enhanced (58). In one study, a nanocomposite dual network (NDN) hydrogel with tumor-targeting effects was found to improve the therapeutic efficacy of radiation therapy and eliminate potentially pathogenic bacteria to prevent postoperative wound infections (59).
4.2.5 Chitosan
Chitosan, the only naturally occurring alkaline polysaccharide, is derived from chitin through deacetylation and possesses immunomodulatory, anti-cancer, and lipid metabolism-regulating properties (60). Its favorable biocompatibility, biodegradability, and blood compatibility make chitosan hydrogels widely applicable in medicine (61). One study developed a chitosan-based thermosensitive hydrogel containing adriamycin liposomes for localized cancer therapy. This hydrogel rapidly forms a gel at body temperature, significantly enhancing anti-tumor activity while reducing systemic toxicity (62). A modified chitosan thermosensitive hydrogel, administered via intratumoral injection, achieves sustained paclitaxel release and significantly enhances anti-tumor activity (63). Composite hydrogels, formed by combining chitosan with other materials, exhibit excellent drug-release properties and biocompatibility. For example, oxyamino chitosan, as a co-delivery carrier for genes and drugs, effectively facilitates anti-cancer therapy (64). Combining and functionally modifying chitosan hydrogels with other materials to enhance their potential in drug delivery systems leads to more effective oncology drug therapy.
4.2.6 Nanohydrogels
Nanoscale drug delivery systems enhance the retention, accumulation, and penetration of immunotherapeutic drugs at the tumor site, while also enabling controlled drug release. Incorporating micron- and nanoscale materials into hydrogels can improve their mechanical and functional properties, resulting in composite gels. Nanohydrogels can be classified as either conventional or environmentally responsive based on their phase transition mechanisms (65). Environmentally responsive nanohydrogels can respond to various external stimuli, enabling controlled drug release, enhanced penetration and retention of chemotherapeutic drugs, and improved therapeutic efficacy (65). Furthermore, combining nanohydrogels with gene therapy via intelligent drug delivery systems can further enhance tumor therapy effectiveness (66). Composite hydrogels are versatile and tunable, finding wide applications in tumor model reconstruction, tumor diagnosis, and tumor therapy.
4.2.7 Injectable hydrogels
Injectable hydrogel systems have applications in the postoperative management of tumors (67). Implantable hydrogel scaffolds can be designed for drug release or to facilitate immune cell infiltration. For example, an injectable, thermosensitive hydrogel based on black phosphorus nanosheets and adriamycin has been developed for photothermal chemotherapy of bone tumors, which can continuously release adriamycin to improve the anti-tumor efficacy and reduce systemic toxicity (68). Injectable hydrogels, prepared using appropriate physical and chemical crosslinking methods, possess both injectability and self-healing capabilities, achieving a balance between ease of preparation and material functionality. At present, clinically used hydrogel products are primarily administered via oral mucosa, oral, vaginal, transdermal, and ocular routes (69).
4.3 Strength and limitations
Unlike traditional review articles, this study employed bibliometric analysis to comprehensively examine hydrogel drug delivery systems in oncology therapy, considering institutional collaborations, subject categories, keywords, and highly cited literature. Compared to previous reviews, this approach identified and presented the most valuable articles and keywords using visual charts and graphs, providing a clear overview of the trajectory of hydrogel drug delivery systems in oncology therapy. Some high-quality review articles have a high citation rate, and their citation can reflect the influence and importance of related research in this field to some extent. However, this study has limitations that warrant further investigation. First, the WoSCC database was the sole data source. While the WoSCC database is continuously updated and provides authoritative, relevant, and comprehensive information, relying on a single database may introduce bias. Second, the use of multiple analysis software packages, each with its own filtering algorithms, may have resulted in the omission of some information, potentially influencing the results. Furthermore, some high-quality articles were published late, resulting in their low citation counts, and these need to be focused on in future research.
4.4 Conclusion
Overall, hydrogels have gained widespread use as emerging drug carriers for oncology drug delivery. This study characterized the distribution of publications, identified subject categories, keywords, and reference bursts, and highlighted developing trends and frontiers in hydrogels for cancer drug delivery. Over the past two decades, publications on hydrogels for cancer drug delivery have increased significantly, particularly since 2016, with annual publication numbers reaching 200. China and the Chinese Academy of Sciences ranked first in co-occurrence frequency. Emerging research directions include tumor immunotherapy, immunogenic cell death, carboxymethyl cellulose hydrogels, antimicrobial hydrogels, chitosan hydrogels, nanohydrogels, and hydrogels with varied delivery modes. Overall, this study provides researchers with potential guidance for future applications of hydrogels in the medical field.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
XL: Software, Data curation, Writing – original draft, Methodology, Writing – review & editing. QZ: Visualization, Validation, Formal Analysis, Writing – review & editing, Funding acquisition. YY: Project administration, Methodology, Writing – review & editing, Investigation. XW: Project administration, Writing – original draft, Resources. JC: Software, Supervision, Writing – review & editing. RW: Visualization, Writing – original draft, Resources, Validation. EC: Visualization, Writing – original draft, Conceptualization, Validation, Writing – review & editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors are grateful for the support provided by Department of Clinical Pharmacy, Jinling Hospital, Medical school of Nanjing University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1607240/full#supplementary-material
References
1. Kalluru P, Vankayala R, Chiang CS, and Hwang KC. Nano-graphene oxide-mediated In vivo fluorescence imaging and bimodal photodynamic and photothermal destruction of tumors. Biomaterials. (2016) 95:1–10. doi: 10.1016/j.biomaterials.2016.04.006
2. He X, Liao Y, Liu J, and Sun S. Research progress of natural small-molecule compounds related to tumor differentiation. Molecules. (2022) 27:2128. doi: 10.3390/molecules27072128
3. Wei YH, He YZ, Lin XY, Ren FX, Zhu HB, Cheng Y, et al. Regional injection of CAR-T cells for the treatment of refractory and recurrent diffuse large B cell lymphoma: A case report. Front Cell Dev Biol. (2020) 8:333. doi: 10.3389/fcell.2020.00333
4. Rismanbaf A. Improving targeted small molecule drugs to overcome chemotherapy resistance. Cancer Rep (Hoboken). (2023) 7:e1945. doi: 10.1002/cnr2.1945
5. Davodabadi F, Sajjadi SF, Sarhadi M, Mirghasemi S, Nadali Hezaveh M, Khosravi S, et al. Cancer chemotherapy resistance: Mechanisms and recent breakthrough in targeted drug delivery. Eur J Pharmacol. (2023) 958:176013. doi: 10.1016/j.ejphar.2023.176013
6. Kaul S, Gulati N, Verma D, Mukherjee S, and Nagaich U. Role of nanotechnology in cosmeceuticals: A review of recent advances. J Pharmaceutics. (2018) 2018:3420204. doi: 10.1155/2018/3420204
7. Liu J, Huang Y, Kumar A, Tan A, Jin S, Mozhi A, et al. pH-sensitive nano-systems for drug delivery in cancer therapy. Biotechnol Adv. (2013) 32:693–710. doi: 10.1016/j.bioteChadv.2013.11.009
8. Wang Q, Alshaker H, Böhler T, Srivats S, Chao Y, Cooper C, et al. Core shell lipid-polymer hybrid nanoparticles with combined docetaxel and molecular targeted therapy for the treatment of metastatic prostate cancer. Sci Rep. (2017) 7:5901. doi: 10.1038/s41598-017-06142-x
9. Kratz F and Warnecke A. Finding the optimal balance: challenges of improving conventional cancer chemotherapy using suitable combinations with nano-sized drug delivery systems. J Control Release. (2012) 164:221–35. doi: 10.1016/j.jconrel.2012.05.045
10. Dai Z and Huang S. Functional dynamics inside nano- or microscale bio-hybrid systems. Front Chem. (2018) 6:621. doi: 10.3389/fchem.2018.00621
11. Peng H, Liu Y, Xiao F, Zhang L, Li W, Wang B, et al. Research progress of hydrogels as delivery systems and scaffolds in the treatment of secondary spinal cord injury. Front Bioeng Biotechnol. (2023) 11:1111882. doi: 10.3389/fbioe.2023.1111882
12. Teaima MH, Mohamed MAA, Abd El Rehem RT, Tayel SA, El-Nabarawi MA, and Fouad SA. Enhanced transdermal delivery of bisoprolol hemifumarate via combined effect of iontophoresis and chemical enhancers: ex vivo permeation/in vivo pharmacokinetic studies. Pharmaceutics. (2021) 13:682. doi: 10.3390/pharmaceutics13050682
13. Cheng Y, Zhang H, Wei H, and Yu CY. Injectable hydrogels as emerging drug-delivery platforms for tumor therapy. Biomater Sci-UK. (2024) 12:1151–70. doi: 10.1039/d3bm01840g
14. Luo FQ, Xu W, Zhang JY, Liu R, Huang YC, Xiao C, et al. An injectable nanocomposite hydrogel improves tumor penetration and cancer treatment efficacy. Acta Biomater. (2022) 147:235–44. doi: 10.1016/j.actbio.2022.05.042
15. Li C, Ji X, and Luo X. Phytoremediation of heavy metal pollution: A bibliometric and scientometric analysis from 1989 to 2018. Int J Environ Res Public Health. (2019) 16:4755. doi: 10.3390/ijerph16234755
16. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
17. Li J and Mooney DJ. Designing hydrogels for controlled drug delivery. Nat Rev Mater. (2016) 1:16071. doi: 10.1038/natrevmats.2016.71
18. Sun Z, Song C, Wang C, Hu Y, and Wu J. Hydrogel-based controlled drug delivery for cancer treatment: A review. Mol Pharmaceut. (2020) 17:373–91. doi: 10.1021/acs.molpharmaceut.9b01020
19. Chen Q, Wang C, Zhang X, Chen G, Hu Q, Li H, et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat Nanotechnol. (2018) 14:89–97. doi: 10.1038/s41565-018-0319-4
20. Norouzi M, Nazari B, and Miller DW. Injectable hydrogel-based drug delivery systems for local cancer therapy. Drug Discov Today. (2016) 21:1835–49. doi: 10.1016/j.drudis.2016.07.006
21. Wang C, Wang J, Zhang X, Yu S, Wen D, Hu Q, et al. In situ formed reactive oxygen species-responsive scaffold with gemcitabine and checkpoint inhibitor for combination therapy. Sci Transl Med. (2018) 10:eaan3682. doi: 10.1126/scitranslmed.aan3682
22. Senapati S, Mahanta AK, Kumar S, and Maiti P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Tar. (2018) 3:7. doi: 10.1038/s41392-017-0004-3
23. Narayanaswamy R and Torchilin VP. Hydrogels and their applications in targeted drug delivery. Molecules. (2019) 24:603. doi: 10.3390/molecules24030603
24. Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, and Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. (2020) 20:101–24. doi: 10.1038/s41573-020-0090-8
25. Chao Y, Chen Q, and Liu Z. Smart injectable hydrogels for cancer immunotherapy. Adv Funct Mater. (2020) 30:1902785. doi: 10.1002/adfm.201902785
26. Wolinsky JB, Colson YL, and Grinstaff MW. Local drug delivery strategies for cancer treatment: gels, nanoparticles, polymeric films, rods, and wafers. J Control Release. (2011) 159:14–26. doi: 10.1016/j.jconrel.2011.11.031
27. Mura S, Nicolas J, and Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. (2013) 12:991–1003. doi: 10.1038/nmat3776
28. Cirillo G, Spizzirri UG, Curcio M, Nicoletta FP, and Iemma F. Injectable hydrogels for cancer therapy over the last decade. Pharmaceutics. (2019) 11:486. doi: 10.3390/pharmaceutics11090486
29. Javanbakht S and Namazi H. Doxorubicin loaded carboxymethyl cellulose/graphene quantum dot nanocomposite hydrogel films as a potential anticancer drug delivery system. Mat Sci Eng C-Mater. (2018) 87:50–9. doi: 10.1016/j.msec.2018.02.010
30. Liang Y, He J, and Guo B. Functional hydrogels as wound dressing to enhance wound healing. ACS Nano. (2021) 15:12687–722. doi: 10.1021/acsnano.1c04206
31. Shi K, Xue B, Jia Y, Yuan L, Han R, Yang F, et al. Sustained co-delivery of gemcitabine and cis-platinum via biodegradable thermo-sensitive hydrogel for synergistic combination therapy of pancreatic cancer. Nano Res. (2019) 12:1389–99. doi: 10.1007/s12274-019-2342-7
32. Shi J, Kantoff PW, Wooster R, and Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. (2016) 17:20–37. doi: 10.1038/nrc.2016.108
33. Qiu Y, Wu Z, Chen Y, Liao J, Zhang Q, Wang Q, et al. Nano ultrasound contrast agent for synergistic chemo-photothermal therapy and enhanced immunotherapy against liver cancer and metastasis. Adv Sci (Weinh). (2023) 10:e2300878. doi: 10.1002/advs.202300878
34. Ma J, Wang B, Shao H, Zhang S, Chen X, Li F, et al. Hydrogels for localized chemotherapy of liver cancer: a possible strategy for improved and safe liver cancer treatment. Drug Delivery. (2022) 29:1457–76. doi: 10.1080/10717544.2022.2070299
35. Gordon MK, Desantis A, Deshmukh M, Lacey CJ, Hahn RA, Beloni J, et al. Doxycycline hydrogels as a potential therapy for ocular vesicant injury. J Ocul Pharmacol Th. (2010) 26:407–19. doi: 10.1089/jop.2010.0099
36. Wheeldon IR, Gallaway JW, Barton SC, and Banta S. Bioelectrocatalytic hydrogels from electron-conducting metallopolypeptides coassembled with bifunctional enzymatic building blocks. P Natl Acad Sci USA. (2008) 105:15275–80. doi: 10.1073/pnas.0805249105
37. Rehman F, Khan IU, Khalid SH, Asghar S, Irfan M, Khalid I, et al. Optimization, in vitro release and toxicity evaluation of novel pH sensitive itaconic acid-g-poly(acrylamide)/sterculia gum semi-interpenetrating networks. DARU. (2021) 29:171–84. doi: 10.1007/s40199-021-00395-8
38. Zeng Y, Zhang C, Du D, Li Y, Sun L, Han Y, et al. Metal-organic framework-based hydrogel with structurally dynamic properties as a stimuli-responsive localized drug delivery system for cancer therapy. Acta Biomater. (2022) 145:43–51. doi: 10.1016/j.actbio.2022.04.003
39. Li W, Wang J, Ren J, and Qu X. Endogenous signalling control of cell adhesion by using aptamer functionalized biocompatible hydrogel. Chem Sci. (2015) 6:6762–8. doi: 10.1039/c5sc02565f
40. Li K, Wang Q, Bian H, Chen Z, He H, Zhao X, et al. Comprehensive analysis reveals USP45 as a novel putative oncogene in pan-cancer. Front Mol Biosci. (2022) 9:886904. doi: 10.3389/fmolb.2022.886904
41. Ziener ML, Dahlgren S, Thoresen SI, and Lingaas F. Genetics and epidemiology of hypothyroidism and symmetrical onychomadesis in the Gordon setter and the English setter. Canine Genet Epidemiol. (2015) 2:12. doi: 10.1186/s40575-015-0025-6
42. Zheng H, Li M, Wu L, Liu W, Liu Y, Gao J, et al. Progress in the application of hydrogels in immunotherapy of gastrointestinal tumors. Drug Delivery. (2023) 30:2161670. doi: 10.1080/10717544.2022.2161670
43. Cui R, Wu Q, Wang J, Zheng X, Ou R, Xu Y, et al. Hydrogel-by-design: smart delivery system for cancer immunotherapy. Front Bioeng Biotechnol. (2021) 9:723490. doi: 10.3389/fbioe.2021.723490
44. Yang Y, Yang Y, Chen M, Chen J, Wang J, Ma Y, et al. Injectable shear-thinning polylysine hydrogels for localized immunotherapy of gastric cancer through repolarization of tumor-associated macrophages. Biomater Sci-UK. (2021) 9:6597–608. doi: 10.1039/d1bm01053k
45. Seo HS, Han JH, Lim J, Bae GH, Byun MJ, Wang CJ, et al. Enhanced postsurgical cancer treatment using methacrylated glycol chitosan hydrogel for sustained DNA/doxorubicin delivery and immunotherapy. Biomater Res. (2024) 28:8. doi: 10.34133/bmr.0008
46. Cheah YH, Liu CY, Yip BS, Wu CL, Peng KL, and Cheng JW. Strategy to enhance anticancer activity and induced immunogenic cell death of antimicrobial peptides by using non-nature amino acid substitutions. Biomedicines. (2022) 10:1097. doi: 10.3390/biomedicines10051097
47. Sun Y, Feng X, Wan C, Lovell JF, Jin H, and Ding J. Role of nanoparticle-mediated immunogenic cell death in cancer immunotherapy. Asian J Pharm Sci. (2020) 16:129–32. doi: 10.1016/j.ajps.2020.05.004
48. Wang Z, Chen J, Hu J, Zhang H, Xu F, He W, et al. cGAS/STING axis mediates a topoisomerase II inhibitor-induced tumor immunogenicity. J Clin Invest. (2019) 129:4850–62. doi: 10.1172/JCI127471
49. Shen S, Zhang Z, Huang H, Yang J, Tao X, Meng Z, et al. Copper-induced injectable hydrogel with nitric oxide for enhanced immunotherapy by amplifying immunogenic cell death and regulating cancer associated fibroblasts. Biomater Res. (2023) 27:44. doi: 10.1186/s40824-023-00389-4
50. Hanurry EY, Birhan YS, Darge HF, Mekonnen TW, Arunagiri V, Chou HY, et al. PAMAM dendritic nanoparticle-incorporated hydrogel to enhance the immunogenic cell death and immune response of immunochemotherapy. ACS Biomater Sci Eng. (2022) 8:2403–18. doi: 10.1021/acsbiomaterials.2c00171
51. Song SH, Seong KY, Kim JE, Go J, Koh EK, Sung JE, et al. Effects of different cellulose membranes regenerated from Styela clava tunics on wound healing. Int J Mol Med. (2017) 39:1173–87. doi: 10.3892/ijmm.2017.2923
52. Hengge NN, Mallinson SJB, Pason P, Lunin VV, Alahuhta M, Chung D, et al. Characterization of the biomass degrading enzyme guxA from acidothermus cellulolyticus. Int J Mol Sci. (2022) 23:6070. doi: 10.3390/ijms23116070
53. Sangsuriyonk K, Paradee N, and Sirivat A. Electrically controlled release of anticancer drug 5-fluorouracil from carboxymethyl cellulose hydrogels. Int J Biol Macromol. (2020) 165:865–73. doi: 10.1016/j.ijbiomac.2020.09.228
54. Gholamali I and Yadollahi M. Doxorubicin-loaded carboxymethyl cellulose/Starch/ZnO nanocomposite hydrogel beads as an anticancer drug carrier agent. Int J Biol Macromol. (2020) 160:724–35. doi: 10.1016/j.ijbiomac.2020.05.232
55. Law MF, Ho R, Cheung CK, Tam LH, Ma K, So KC, et al. Prevention and management of hepatitis B virus reactivation in patients with hematological Malignancies treated with anticancer therapy. World J Gastroentero. (2016) 22:6484–500. doi: 10.3748/wjg.v22.i28.6484
56. Lei K, Wang K, Sun Y, Zheng Z, and Wang X. Rapid-fabricated and recoverable dual-network hydrogel with inherently anti-bacterial abilities for potential adhesive dressings. Adv Funct Mater. (2020) 31:2008010. doi: 10.1002/adfm.202008010
57. Tavakolian M, Munguia-Lopez JG, Valiei A, Islam MS, Kinsella JM, Tufenkji N, et al. Highly absorbent antibacterial and biofilm-disrupting hydrogels from cellulose for wound dressing applications. ACS Appl Mater Inter. (2020) 12:39991–40001. doi: 10.1021/acsami.0c08784
58. Firlar I, Altunbek M, McCarthy C, Ramalingam M, and Camci-Unal G. Functional hydrogels for treatment of chronic wounds. Gels. (2022) 8:127. doi: 10.3390/gels8020127
59. Wu Y, Yao Y, Zhang J, Gui H, Liu J, and Liu J. Tumor-targeted injectable double-network hydrogel for prevention of breast cancer recurrence and wound infection via synergistic photothermal and brachytherapy. Adv Sci (Weinh). (2022) 9:e2200681. doi: 10.1002/advs.202200681
60. Haniastuti T, Puspasari TA, Hakim ER, and Tandelilin RT. Potential Effect of Giant Freshwater Prawn Shell Nano Chitosan in Inhibiting the Development of Streptococcus mutans and Streptococcus sanguinis Biofilm In Vitro. Int J Dent. (2023) 2023:8890750. doi: 10.1155/2023/8890750
61. Li Q, Chen S, Yan Z, Fang H, Wang Z, and He C. A novel intranasal vaccine with pmpGs + MOMP induces robust protections both in respiratory tract and genital system posting chlamydia psittaci infection. Front Vet Sci. (2022) 9:855447. doi: 10.3389/fvets.2022.855447
62. Wang W, Zhang P, Shan W, Gao J, and Liang W. A novel chitosan-based thermosensitive hydrogel containing doxorubicin liposomes for topical cancer therapy. J Biomat Sci-Polym E. (2013) 24:1649–59. doi: 10.1080/09205063.2013.789357
63. Jiang Y, Meng X, Wu Z, and Qi X. Modified chitosan thermosensitive hydrogel enables sustained and efficient anti-tumor therapy via intratumoral injection. Carbohyd Polym. (2016) 144:245–53. doi: 10.1016/j.carbpol.2016.02.059
64. Kamra M, Moitra P, Ponnalagu D, Karande AA, and Bhattacharya S. New water-soluble oxyamino chitosans as biocompatible vectors for efficacious anticancer therapy via co-delivery of gene and drug. ACS Appl Mater Inter. (2019) 11:37442–60. doi: 10.1021/acsami.9b09485
65. Lu J, Li Y, Hu D, Chen X, Liu Y, Wang L, et al. One-step synthesis of interpenetrating network hydrogels: Environment sensitivities and drug delivery properties. Saudi J Biol Sci. (2015) 23:S22–31. doi: 10.1016/j.sjbs.2015.06.012
66. Yang J, Wang Z, Ma C, Tang H, Hao H, Li M, et al. Advances in hydrogels of drug delivery systems for the local treatment of brain tumors. Gels. (2024) 10:404. doi: 10.3390/gels10060404
67. Zhong Z, Gan L, Feng Z, Wang W, Pan X, Wu C, et al. Hydrogel local drug delivery systems for postsurgical management of tumors: Status Quo and perspectives. Mater Today Bio. (2024) 29:101308. doi: 10.1016/j.mtbio.2024.101308
68. Li S, Qing Y, Lou Y, Li R, Wang H, Wang X, et al. Injectable thermosensitive black phosphorus nanosheet- and doxorubicin-loaded hydrogel for synergistic bone tumor photothermal-chemotherapy and osteogenesis enhancement. Int J Biol MACROMOL. (2023) 239:124209. doi: 10.1016/j.ijbiomac.2023.124209
Keywords: hydrogels, cancer, drug delivery, bibliometrics, CiteSpace
Citation: Liu X, Zhou Q, Yang Y, Wu X, Chen J, Wang R and Chen E (2025) Hydrogels in cancer treatment: mapping the future of precision drug delivery. Front. Immunol. 16:1607240. doi: 10.3389/fimmu.2025.1607240
Received: 07 April 2025; Accepted: 20 June 2025;
Published: 08 July 2025.
Edited by:
Amit K. Tiwari, University of Arkansas for Medical Sciences, United StatesReviewed by:
Christopher Synatschke, Max Planck Institute for Polymer Research, GermanyMingrui Zhu, St. Jude Children’s Research Hospital, United States
Copyright © 2025 Liu, Zhou, Yang, Wu, Chen, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiang Liu, bGl1eF9jcHVAMTYzLmNvbQ==; Erhua chen, Y2hlbmVyaHVhMTk4N0AxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Xiang Liu
Xiang Liu Qiang Zhou
Qiang Zhou Yue Yang1
Yue Yang1