- 1Department of Urology, The Third Affiliated Hospital of Jinzhou Medical University, Jinzhou Medical University, Jinzhou, Liaoning, China
- 2Department of Urology, Affiliated Taikang Xianlin Drum Tower Hospital, Medical School of Nanjing University, Nanjing, Jiangsu, China
- 3Department of Urology, Taikang Xianlin Drum Tower Hospital Clinical College of Wuhan University, Nanjing, Jiangsu, China
- 4Department of Urology, Jinling Hospital, Affiliated Hospital of Nanjing University Medical School, Nanjing, Jiangsu, China
- 5Department of Clinical Medicine, Medical College of Yangzhou University, Yangzhou, Jiangsu, China
- 6Department of Clinical Medicine, Medical College of Soochow University, Suzhou, Jiangsu, China
- 7Shaanxi University of Traditional Chinese Medicine, School of Medicine, Xianyang, Shanxi, China
- 8Department of Nursing, Medical College of Nantong University, Nantong, Jiangsu, China
- 9Department of Clinical Medicine, Medical College of Jiangsu University, Zhenjiang, Jiangsu, China
- 10School of Medicine, Jiangnan University, Wuxi, Jiangsu, China
- 11Department of Clinical Medicine, School of Medicine, Xuzhou Medical University, Xuzhou, Jiangsu, China
- 12Department of Soft Tissue Surgery, The First Affiliated Hospital of Jinzhou Medical University, Jinzhou, Liaoning, China
- 13College of Integrative Medicine, Nanjing University of Chinese Medicine, Nanjing, Jiangsu, China
Background: Oxidative stress plays a central role in the pathogenesis of chronic kidney disease (CKD) and is closely linked to glomerular injury and microvascular endothelial dysfunction. Urinary albumin excretion (UAE) is a sensitive early marker of renal damage and systemic inflammation. Although dietary antioxidants are recognized to modulate oxidative stress, the impact of both their cumulative intake and timing on UAE remains unclear.
Objectives: To investigate the association between the Composite Dietary Antioxidant Index (CDAI)—including both total daily intake and intake at different meals—and the incidence of elevated UAE among adults in the United States. We also aimed to evaluate whether the timing of antioxidant intake, particularly in the evening, modifies this relationship.
Methods: We analysed data from 23,214 adults aged ≥20 years in the U.S. National Health and Nutrition Examination Survey (NHANES) 2009–2018. CDAI was determined using dietary intakes of six antioxidants (vitamins E, A, C, carotenoids, selenium, and zinc) across breakfast, lunch, and dinner. UAE was defined as a urinary albumin-to-creatinine ratio (ACR) >30 mg/g. Weighted multivariable logistic regression, restricted cubic spline analysis, component-independent effect analysis, and analysis of subgroups were used to evaluate the associations and interactions.
Results: Higher CDAI was greatly connected to reduced odds of UAE (fully adjusted OR per SD increase: 0.98; 95% CI: 0.97–0.99; P = 0.041). Antioxidant intake during dinner showed the strongest inverse association with UAE (P < 0.01), while breakfast and lunch intake were not significantly related. The difference between dinner and breakfast CDAI (ΔCDAI) was also inversely associated with UAE. Subgroup analysis revealed effect modification by BMI: the protective association was attenuated in participants with obesity (BMI ≥ 30).
Conclusions: Both the quantity and timing of dietary antioxidant intake are associated with urinary albumin excretion. Evening antioxidant consumption and a higher ΔCDAI may offer enhanced renal protection, potentially via circadian modulation of oxidative stress and inflammation. These findings support a chrononutrition-based approach to kidney health and warrant further interventional studies.
1 Introduction
In addition to being a sign of early kidney illness, elevated urine albumin excretion(UAE) has been demonstrated to be a reliable indicator of the course of cardiovascular risk and chronic kidney disease (CKD) (1–3). The efficiency and ease of the albumin-to-creatinine ratio (ACR) of random urine make it a popular tool for assessing and characterising albuminuria (4, 5). Abnormal elevations in ACR are known to occur at a threshold of 30 mg/g. Albuminuria not only reflects glomerular injury but is also regarded as an early marker of systemic endothelial dysfunction, characterized by increased vascular permeability and impaired barrier integrity (6). According to reports, moderate and/or severe albuminuria (More than 30, less than 300 mg/g) affects 5–19% of the general population; in people with hypertension, the percentage increases to 23%, and in people with diabetes, it can reach 40% (7). Therefore, according to guideline recommendations, patients with diabetes or hypertension should undergo screening once a year. Proteinuria has grown to be a serious public health issue because of its high prevalence and substantial detrimental influence on unfavourable clinical outcomes (8–10).
Consequently, albuminuria should be given high clinical priority. Currently, clinical treatment strategies primarily focus on reducing urinary albumin excretion and delaying the progression of kidney disease (11). Conventional approaches include lifestyle interventions—such as proper dietary control, weight management, and regular physical activity (12–14)—as well as pharmacological treatments, including renin-angiotensin-aldosterone system (RAAS) inhibitors (15), sodium-glucose cotransporter 2 inhibitors (SGLT2i) (16), and mineralocorticoid receptor antagonists (MRAs) (17). In recent years, as understanding of the underlying pathophysiological mechanisms has advanced, emerging therapies such as anti-inflammatory and antioxidant treatments have gained increasing attention, with agents like endothelin receptor antagonists currently undergoing clinical trials. Among these, antioxidant therapy is being actively explored for its potential renoprotective effects (18). Furthermore, precision medicine strategies based on individual risk profiles are considered a promising direction for future interventions to reduce urinary albumin excretion and slow the progression of chronic kidney disease (19).
Oxidative stress plays a central role in the pathogenesis of CKD and is closely linked to both glomerular injury and systemic vascular damage. Beyond its impact on renal function, oxidative stress is a key contributor to cardiovascular diseases, which represent a leading cause of mortality as kidney function deteriorates. Multiple studies have demonstrated that increased oxidative stress accelerates endothelial dysfunction, promotes atherosclerosis, and exacerbates hypertension in individuals with CKD. These interrelated pathways highlight the importance of targeting oxidative stress not only for renal protection but also for cardiovascular risk reduction.
The emergence of novel therapies targeting inflammation and oxidative stress, along with precision medicine approaches based on individual risk profiles, holds great promise in the management of proteinuria. Consequently, there remains an urgent need for reliable and easily applicable indicators to assess the risk of urinary albumin excretion (UAE). To date, numerous studies have demonstrated that the intake of various exogenous dietary antioxidant micronutrients—such as vitamin C, vitamin E, carotenoids, and selenium—is associated with urinary protein excretion, suggesting their potential role as modifiable factors in preventing or managing proteinuria (20–22). However, some conflicting reports indicate that individual dietary antioxidant nutrients may be unrelated to urinary outcomes or may even exert adverse effects. According to a prior study, oral vitamin A supplementation may cause oxidative stress, reduce antioxidant capacity, interfere with redox homeostasis, and make rats more prone to inflammation—all of which run counter to vitamin A’s well-established beneficial antioxidant function (23). The contradictory results imply that isolated chemicals might not be useful in treating oxidative stress-related illnesses (24). Wright et al. developed the Composite Dietary Antioxidant Index (CDAI), a comprehensive metric that accounts for a variety of dietary antioxidants and represents a person’s total antioxidant consumption (25, 26). Based on the combined effect of these nutrients on anti-inflammatory responses, CDAI targets pro-inflammatory indicators, including interleukin-1 beta (IL-1β) and tumour necrosis factor-alpha (TNF-α). These biomarkers are linked to a number of health consequences, such as depression, hypertension, coronary heart disease, cancers, and all-cause mortality (24, 27–30).
Recent research has indicated that the time of meals may affect metabolic and physiological processes, possibly because of the effects of circadian rhythms (31). A main clock in the hypothalamic suprachiasmatic nucleus (SCN) controls the circadian timing system. It communicates with other peripheral clocks, including those in the liver, via neuroendocrine pathways. The light-dark cycle mostly controls the master pacemaker in the SCN, although the liver’s circadian clock is responsive to dietary habits (32). Indeed, dietary patterns may exert direct regulation over the circadian phase of liver function, independently of the SCN and light–dark signalling pathways (33, 34). Therefore, altering eating habits and dietary composition may be a practical way to treat UAE by lowering the body’s levels of oxidative stress. Numerous studies have demonstrated that controlling metabolic status and body weight is significantly influenced by the distribution of vitamins, macronutrients, and minerals throughout the day (35–37). The intricate interplay between the circadian clock and metabolic processes underscores the critical role of meal timing in regulating metabolism (38).
Given these findings, we hypothesize that the intake of exogenous dietary antioxidant micronutrients, as well as the timing of food consumption, may be closely associated with urinary albumin excretion (UAE). This study explored the associations between urinary albumin excretion (UAE) and both daily CDAI levels—measured at breakfast, lunch, dinner, and in total—as well as the intra-day difference in CDAI (ΔCDAI, defined as dinner minus breakfast), using data from participants in the U.S. National Health and Nutrition Examination Survey (NHANES).
2 Resources and procedures
2.1 Sources of information
Making use of a sample of the US civilian population that is not institutionalized, NHANES is a thorough, Stratified, clustered, and multistage probability survey that represents the whole country. The Centres for Disease Control and Prevention’s Institutional Review Board (IRB) has authorised the poll, guaranteeing that ethical guidelines are followed (30). All information and records pertaining to the research are publicly available. Information permission was gained from all subjects through completed consent forms.
Since 1999, NHANES has collected data annually from approximately 5,000 individuals. The survey is conducted in two-year cycles and includes data from questionnaires, laboratory tests, physical examinations, and sociodemographic information. Detailed information about the program is available on its official website.
2.2 Study population
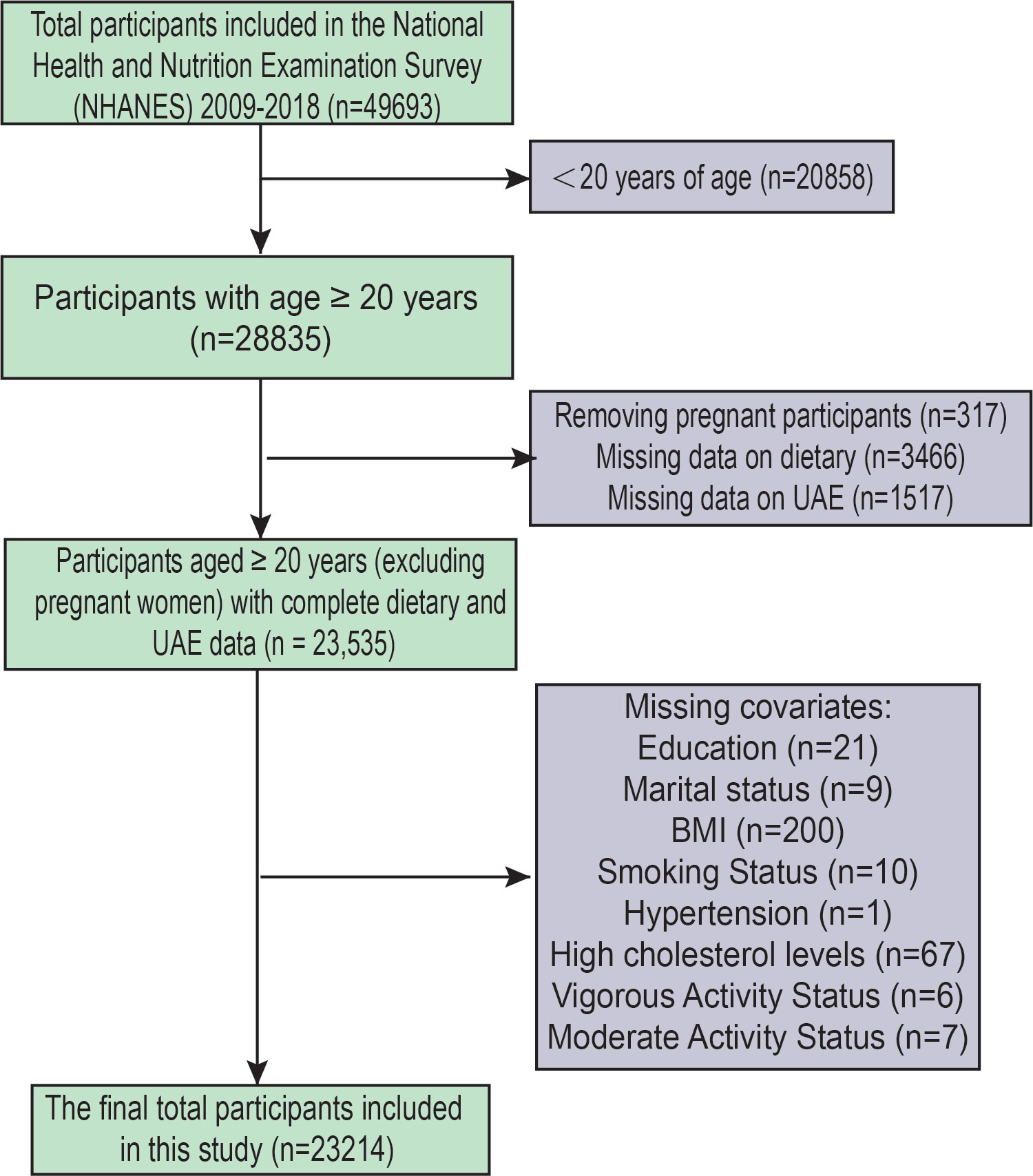
Five-cycle data (2009–2018) from NHANES were analysed, involving 49,693 participants (Figure 1). Participants under 20 years of age were excluded to align with common practice in NHANES-based studies focusing on adult populations, and because key variables related to UAE and dietary assessments were more consistently available for individuals aged 20 years and older. We excluded Participants under the age of twenty (n=20,858). Other factors for exclusion were: (1) Pregnant participants (317); (2) lacking information regarding dietary (n=3466); (3) missing UAE data (n=1517); (4) lacking status of education (n=21); (5) unknown marital status (n=9); (6) lacking data on BMI (n=200); (7) lack of smoking status (n=10); (8) lacking data on hypertension (n=1); (9) No data on high cholesterol levels (67); (10) No data on physical activity (13). After the screening, 23,214 participants were included in this study.
2.3 Measurement of exogenous dietary antioxidant micronutrient intake
The 24-hour Interview on dietary recall is the present component of nutritional assessment in NHANES. Trained Interviewers for diets, proficient in English and Spanish, conducted the in-person interviews. NHANES collects participants’ dietary intake data using two non-consecutive 24-hour dietary recalls. The initial interview is conducted in a Mobile Examination Center (MEC), where each center includes a dietary interview room equipped with standardized measurement protocols. The second dietary recall is conducted by telephone three to ten days after the first interview.
To reduce bias and improve the data’ dependability, we averaged two measurements. CDAI for each participant was calculated using a modified version developed in previous studies (25). The six dietary antioxidants that were part of CDAI were carotenoids, zinc, selenium, and vitamins E, C, and A. Each micronutrient was standardised by calculating its Z-score, which was obtained by subtracting the mean intake from the individual value and dividing by the overall standard deviation. The Z-scores of the six nutrients were then summed up to generate the composite CDAI value. The following is the formula for the calculation:
Based on when the meals and macronutrients were consumed, the variable “Name of eating occasion” separated them into breakfast, lunch, and dinner. The event label that was used by the responder to describe the eating events influenced the decisions made about their classification. This method provides thorough definitions that consider various cultural customs and social norms (36). The exposure variables in this study included the meal timing of CDAI (total, break-fast, lunch, and dinner) and the difference between dinner CDAI and breakfast CDAI (Δ =dinner − breakfast).
2.4 Evaluation of UAE
NHANES participants provided blood and urine samples at standardised Mobile Examination Centers (MECs), and the modified Jaffe kinetic technique and solid-phase fluorescence immunoassay were used to measure urinary albumin and creatinine, respectively. Urine samples for UACR measurement were obtained as random spot samples during MEC visits. The albumin-to-creatinine ratio (ACR) was calculated by dividing the urinary albumin concentration (mg) by the urinary creatinine concentration (g). Increased urinary albumin excretion (albuminuria) was defined as ACR > 30 mg/g (39, 40). Albuminuria was regarded as an outcome variable in our study.
2.5 Evaluation of covariates
Based on prior research, we selected potential confounders that may affect UAE, including gender, age, race, education, BMI (Body Mass Index), marital status, PIR (the ratio of family income to poverty), total cholesterol levels, alcohol use, smoking, diabetes, hypertension, status of exercise, eGFR (Estimated Glomerular Filtration Rate), ALT (Alanine Aminotransferase), AST (Aspartate Aminotransferase), triglyceride, creatinine, and uric acid (41–51).
Urinary albumin, urinary creatinine, ALT, AST, triglycerides, serum creatinine, and uric acid were treated as continuous variables. Gender, race, education, and marital status were considered as categorical variables. PIR was categorized into two groups: less than 2 and greater than or equal to 2. BMI was categorized into three groups: less than 25, 25 to less than 30, and 30 or greater. Patients were identified as smokers if they responded “yes” to the question (SMQ020) on smoking. Alcohol use was determined using a drinking question (ALQ130), identifying people as drinkers if they drank 12 or more drinks annually and as non-drinkers if they drank less than 12.
Individuals were classified as having hypertension if their average systolic blood pressure from three readings was ≥140 mmHg, diastolic blood pressure was ≥90 mmHg, they were taking antihypertensive medication, or they answered “yes” to being diagnosed with hypertension. Diabetes was identified based on a “yes” response to a diabetes diagnosis, the use of glucose-lowering medication or insulin, or meeting diagnostic thresholds for glycosylated haemoglobin (≥6.5%) and fasting blood glucose (≥126 mg/dl). There were two categories for total cholesterol levels: low (<240 mg/dl) and high (≥240 mg/dl). Previously told by a doctor that they had hypercholesterolemia or were on lipid-lowering medication, they were considered to have a hypercholesterolemic state, otherwise, they were considered to not be associated with hypercholesterolemia.
2.6 Analysis of statistics
During data processing, NHANES sample weights were applied to ensure national representation of the study population. Categorical variables were analyzed using weighted percentages (%) with a chi-square test, while using weighted linear regression, continuous variables were compared, and the results were shown as mean (± SD).
First, we conducted a population description based on CDAI quartiles and performed logistic regression analysis under three models for comparison. Smooth curve fitting and RCS analysis were then applied. Subsequently, a threshold effect analysis was carried out to examine the relationship between CDAI and UAE, and a generalised additive model (GAM) was employed to verify the link between dose and response along with multivariable logistic regression. We then performed multivariable logistic regression models and employed them to assess the correlations of morning, midday, evening, and ΔCDAI with UAE across different models. Independent effect analyses were also conducted for each component of CDAI. In addition, subgroup analyses were performed to explore the stratified associations between CDAI and UAE, and interaction tests were conducted to strengthen the robustness of the results.
To account for multiple comparisons, particularly in component-specific and subgroup analyses, we applied the Benjamini-Hochberg false discovery rate (FDR) correction. An FDR-adjusted p-value of <0.05 was considered statistically significant.
Every statistical analysis was carried out with R (version 4.4.0), with statistical significance characterized by a two-sided p-value < 0.05.
3 Result
3.1 Features of the population
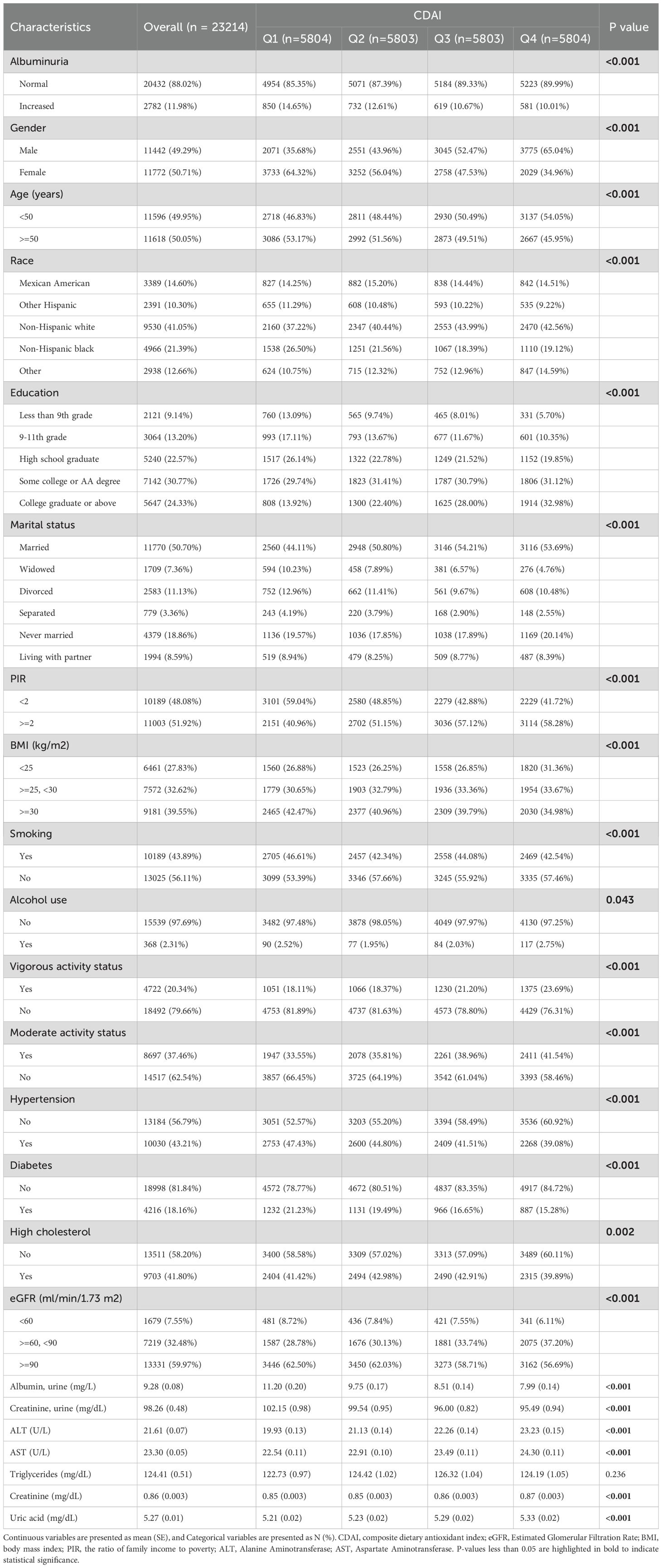
From NHANES 2009–2018, 23214 suitable participants were chosen based on the screening criteria (Figure 1). 2,782 individuals were diagnosed with UAE, while 20,432 individuals did not have UAE. The study population’s baseline characteristics are represented by weighted estimates in Table 1. Compared to participants in the first to third quartiles, those in the highest CDAI quartile were more likely to be younger, male, highly educated, non-smokers, physically active (vigorous or moderate), and without hypertension or diabetes. Additionally, the prevalence of UAE decreased as CDAI levels increased. Notable variations in the baseline attributes were also observed across CDAI quartiles in Regarding PIR, marital status, ethnicity, BMI, alcohol consumption, cholesterol levels, eGFR, urinary protein, urinary creatinine, ALT, AST, triglycerides, serum creatinine, and uric acid.
3.2 Relationship between CDAI and UAE
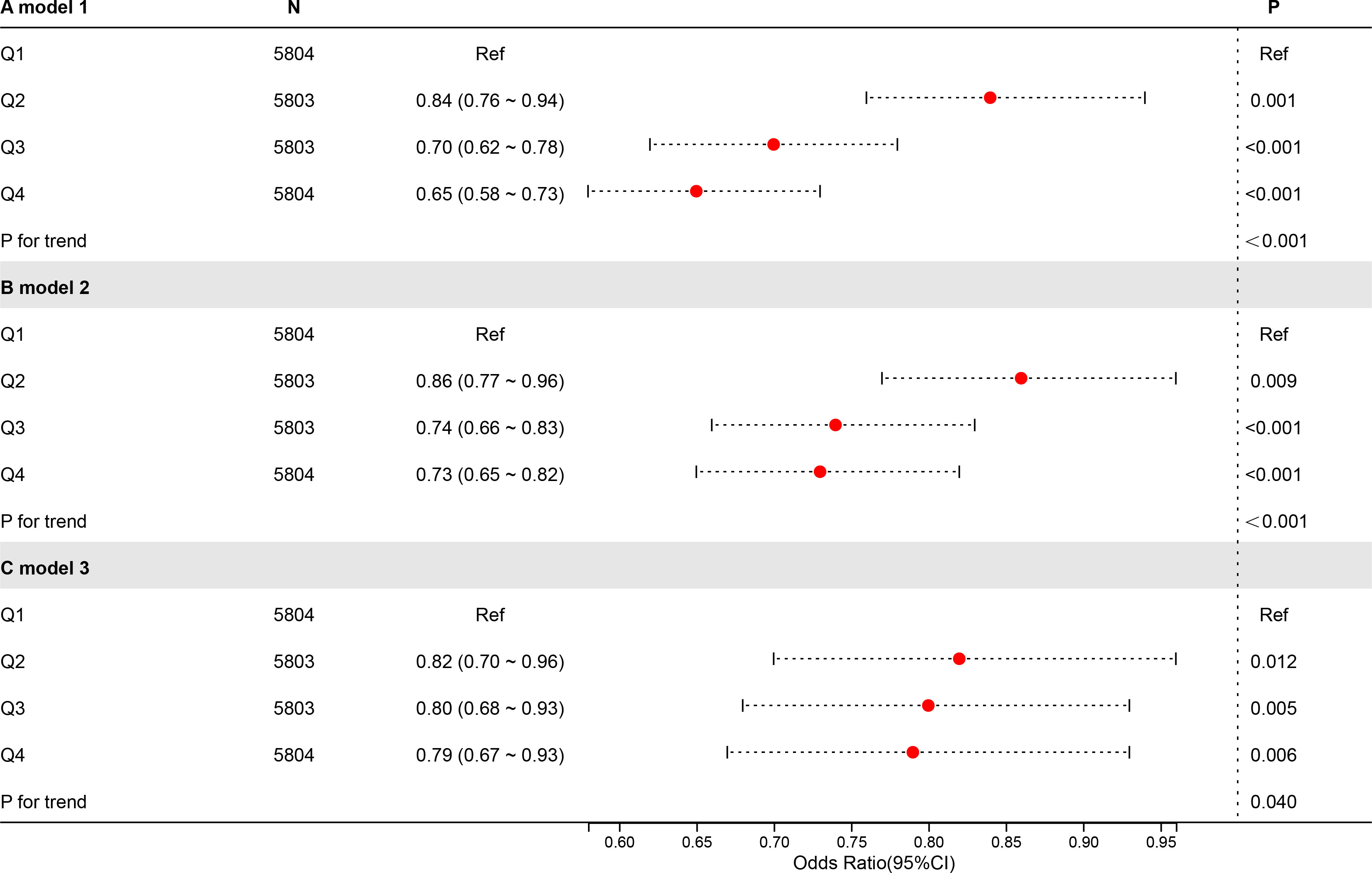
To investigate the connection between CDAI and UAE, an analysis of weighted logistic regression has been carried out using 3 models (Figure 2). There were no additional variables in Model 1. In Model 2, age, gender, education, PIR, and race had been considered. Model 3 was constructed utilising Model 2, with modifications made for marital status, diabetes, alcohol use, smoking, hypertension, vigorous activity, moderate activity, eGFR, and total cholesterol level. Quartiles (Q1-4) were created from CDAI; Q1 served as a reference.
Regardless of whether covariates were adjusted for, higher CDAI levels were related to a lower risk of UAE. In Model 3 (totally modified model), the highest CDAI quartile (Q4) was notably negatively connected to UAE contrasted with the lowest quartile (Q1) (OR = 0.79, P = 0.006). Trend tests across all models were significant in terms of statistics (P < 0.05), indicating that the negative connection between higher UAE risk and CDAI is statistically robust.
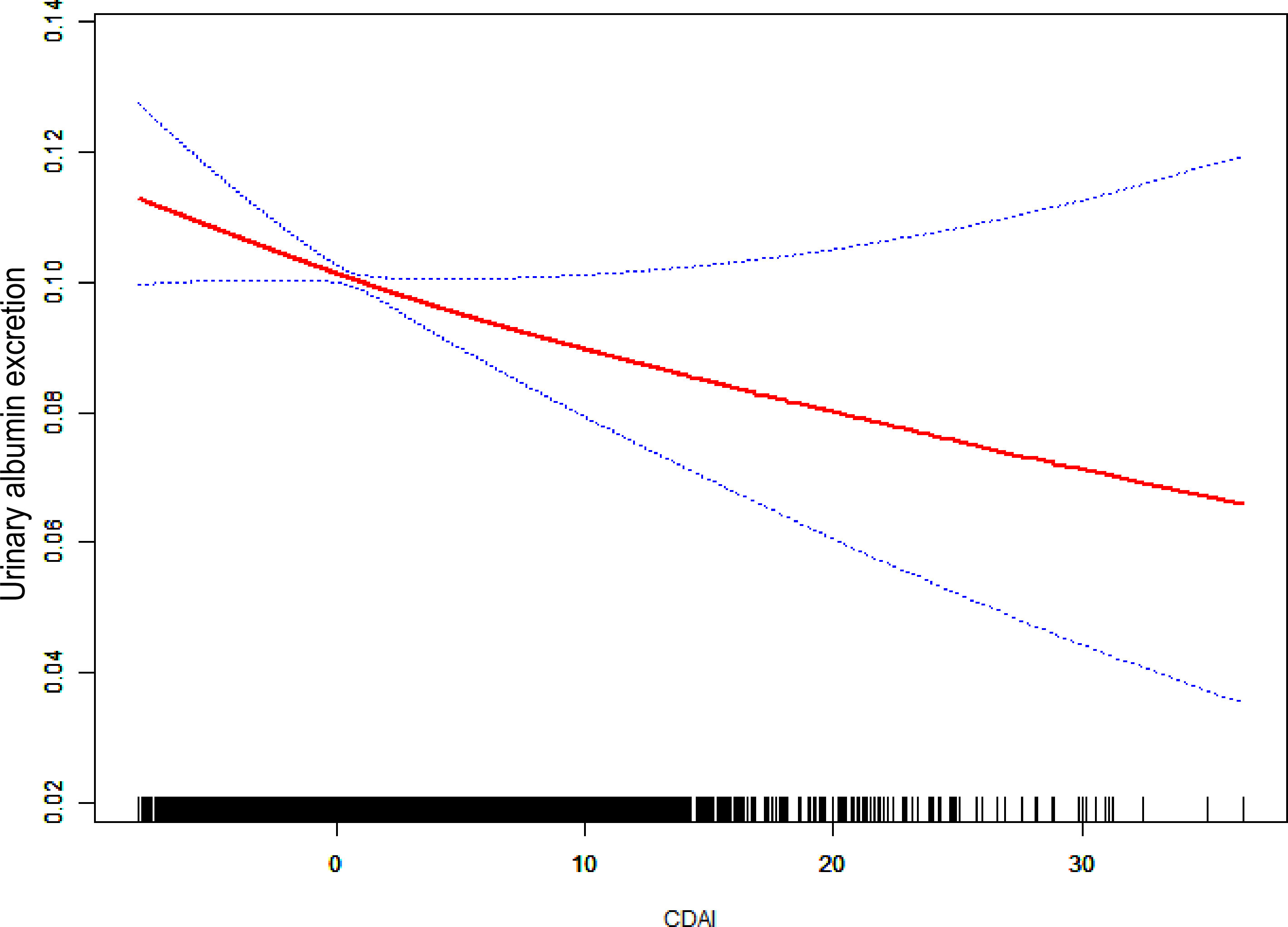
In a completely modified model, the connection between CDAI and UAE was investigated using smoothed curve fitting (Figure 3). A substantial negative relationship was identified between UAE and CDAI, indicating that higher dietary antioxidant intake was associated with decreased urinary albumin excretion. The urinary albumin excretion decreased sharply with an increase in CDAI at lower CDAI levels, whereas this decreasing trend became more moderate at higher CDAI values. Additionally, the 95% confidence interval widened considerably at higher CDAI levels, reflecting greater uncertainty in estimating urinary albumin excretion when CDAI was elevated. Supplementary Figure S1 shows a noteworthy negative correlation has been observed between the CDAI and UAE (P for overall = 0.005), although no significant nonlinearity was detected (P for nonlinear = 0.144). The odds ratio gradually decreased as CDAI increased, indicating a potential protective effect of higher dietary antioxidant intake. Wider confidence intervals at higher CDAI values indicate greater uncertainty.
3.3 Dose-response connectivity between CDAI and the UAE
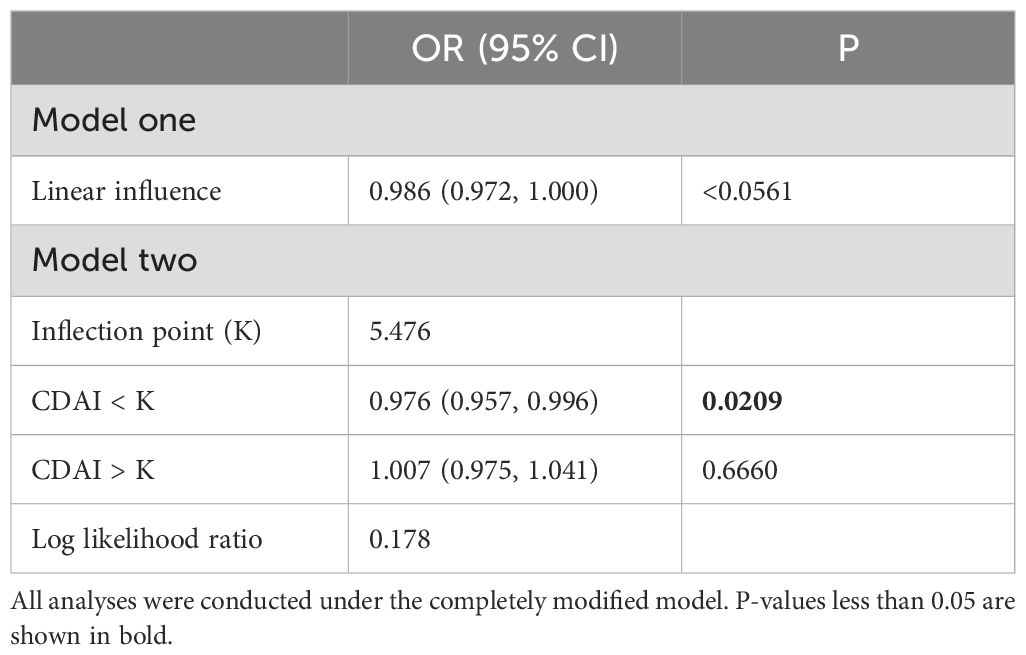
We used an examination of the threshold effect to examine the connection between CDAI and UAE (Table 2). In the linear model (Model 1), CDAI was marginally connected with lower odds of albuminuria (OR = 0.986, 95%, P = 0.0561). The piecewise regression model (Model two) identified an inflection point at CDAI = 5.476. Below this threshold, CDAI was significantly connected with reduced risk (OR = 0.976, P = 0.0209), while no significant association was found above the threshold (OR = 1.007, P = 0.6660). The log-likelihood ratio test showed no significant improvement of the threshold model over the linear model (P = 0.178).
We extracted data from participants with CDAI less than 5.476 and conducted further analysis. As shown in Figure 4, the odds ratio for the UAE decreased linearly with increasing CDAI (P < 0.001; P for nonlinearity = 0.395), indicating a significant inverse linear relationship. Higher dietary antioxidant levels were associated with reduced odds of UAE prevalence.
3.4 Logistic regression analysis of CDAI and UAE
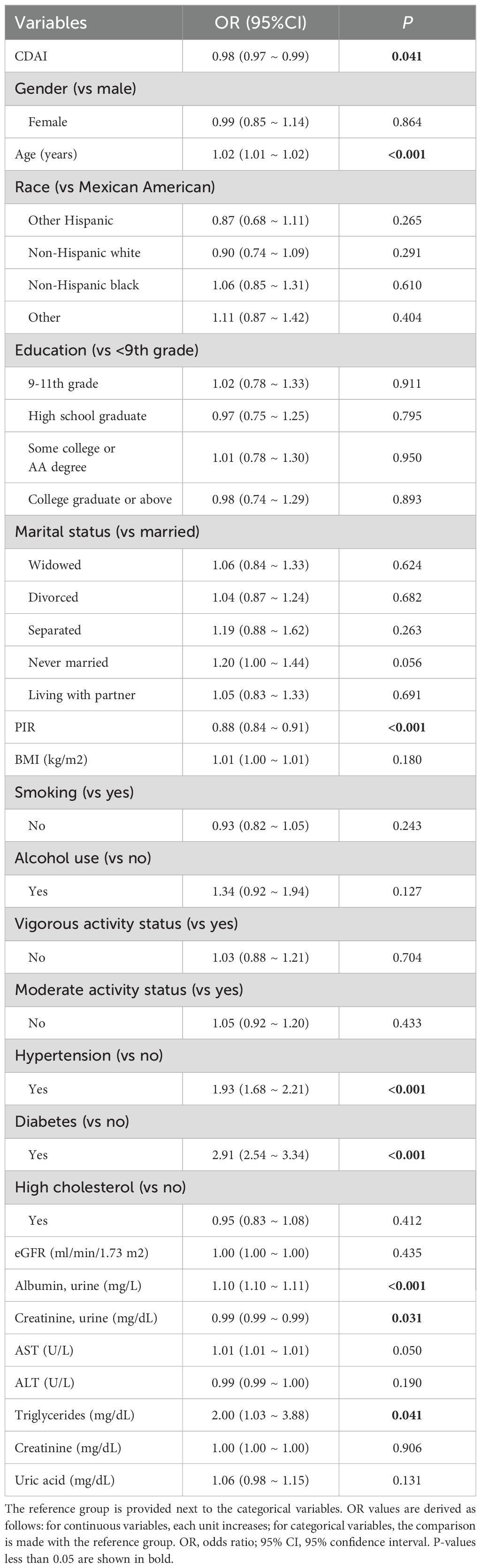
In the multivariable logistic regression analysis (Table 3), CDAI was significantly associated with reduced odds of albuminuria after accounting for every possible confounding factor (OR = 0.98, P = 0.041). After applying the FDR correction, the associations between total CDAI, dinner-specific CDAI, and ΔCDAI with UAE remained statistically significant. The corresponding FDR-adjusted p-values are presented in Supplementary Table S1a. This indicates that higher dietary antioxidant intake may be protective against albuminuria. In addition, factors such as age, hypertension, diabetes, lower income (PIR), and elevated triglycerides were independently associated with increased risk.
The univariate logistic regression analysis revealed a significant inverse relationship between CDAI and albuminuria (OR = 0.96, P < 0.001) (Supplementary Table S1). Other factors such as older age, hypertension, diabetes, higher BMI, and elevated triglycerides were positively associated with albuminuria risk, while higher PIR and non-smoking status were protective.
3.5 Relationship between UAE and the timing of dietary antioxidant micronutrient intake
As shown in Figure 5, no significant relationship was observed between CDAI at breakfast or lunch and albuminuria. However, higher CDAI during dinner was substantially linked to a decreased risk of albuminuria. Moreover, the difference between dinner and breakfast CDAI (ΔCDAI) showed a clear inverse relationship with albuminuria, suggesting that antioxidant intake in the evening, rather than the morning, may have stronger protective effects on renal health.
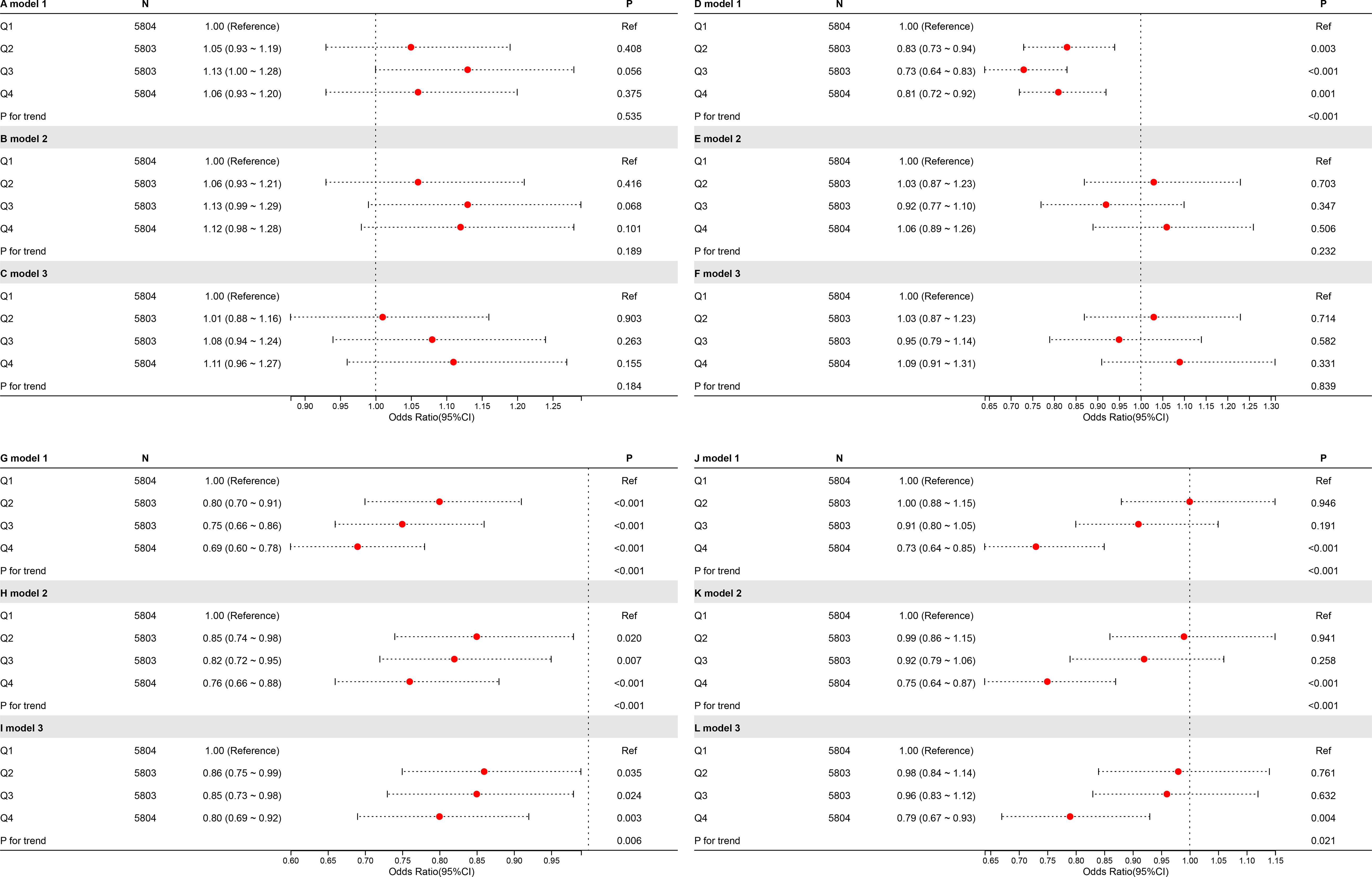
Figure 5. RCS plots of the relationship between meal-specific CDAI and the possibility of albuminuria. The breakfast group is represented by the letters “A,” “B,” and “C”; the lunch group by “D,” “E,” and “F”; the dinner group by “G,” “H,” and “I”; and the Δ group by “J,” “K,” and “L.”.
3.6 Component-independent impacts of the CDAI
The six antioxidant elements comprising the CDAI had their Z-score determined. As shown in the correlation heatmap (Figure 6), CDAI was moderately and positively correlated with its components, particularly zinc (r = 0.68), selenium (r = 0.66), and vitamin A (r = 0.64), indicating a well-constructed composite index. CDAI was weakly but negatively correlated with urinary albumin excretion (r = -0.17), consistent with earlier findings. Among individual antioxidants, vitamin E (r = -0.23), zinc (r = -0.21), and selenium (r = -0.17) showed the strongest inverse correlations with UAE.
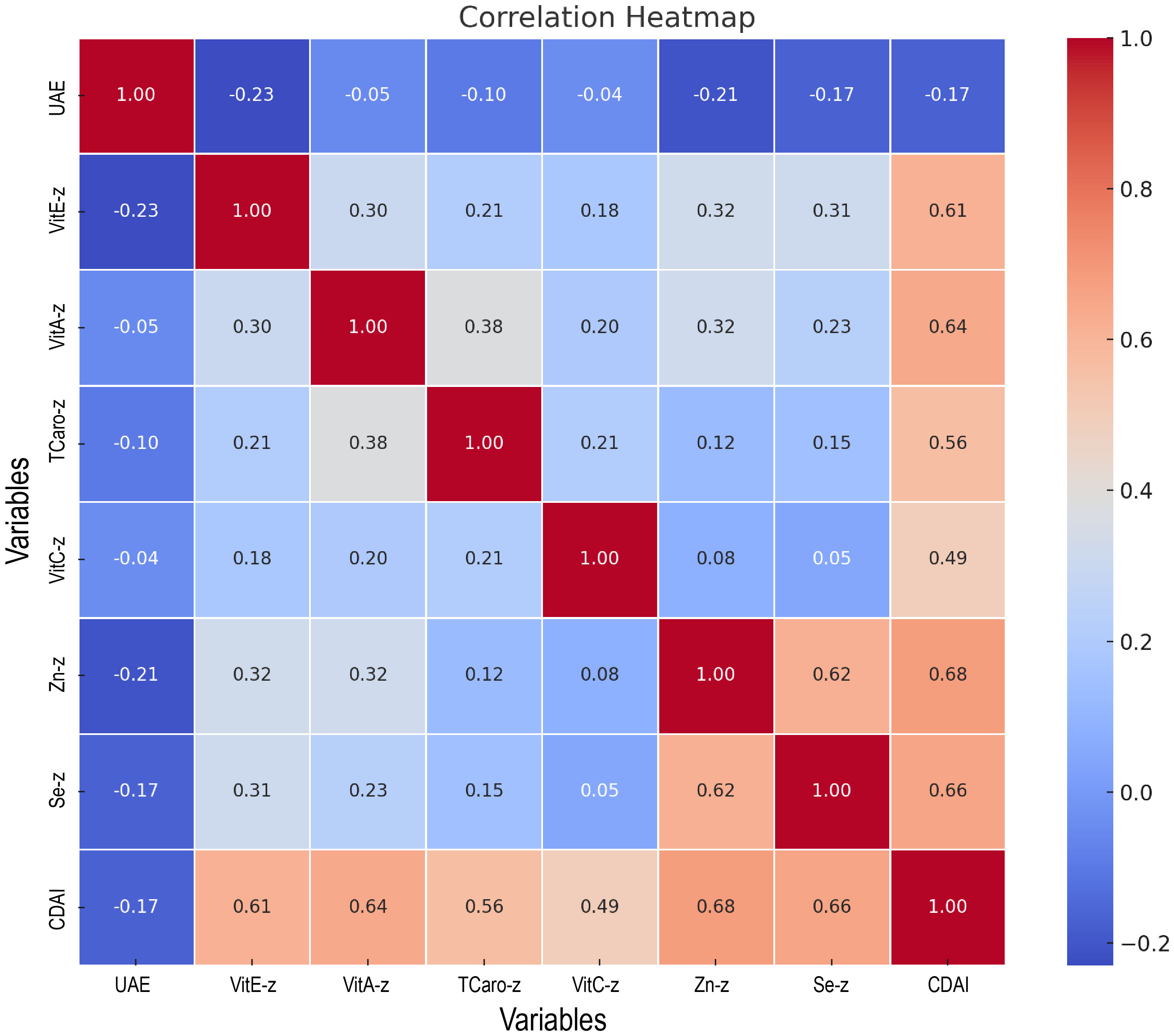
Figure 6. Heatmap of the association between CDAI and its components with urinary albumin excretion.
Regression analyses of dinner-specific CDAI and total CDAI, along with their components, were conducted under the fully modified model (Supplementary Table S2), and the outcomes were visualised (Figure 7). Both total and dinner-specific CDAI were substantially linked with reduced odds of albuminuria (P = 0.001 for both). Among the individual components, vitamin E, zinc, and selenium showed consistent and significant protective associations for both total and dinner intake (all P < 0.01). Interestingly, vitamin A was only significant when derived from dinner intake (P = 0.035), while vitamin C was not significantly associated in either model. These results suggest that not only the quantity but also the timing of antioxidant intake may influence renal outcomes.
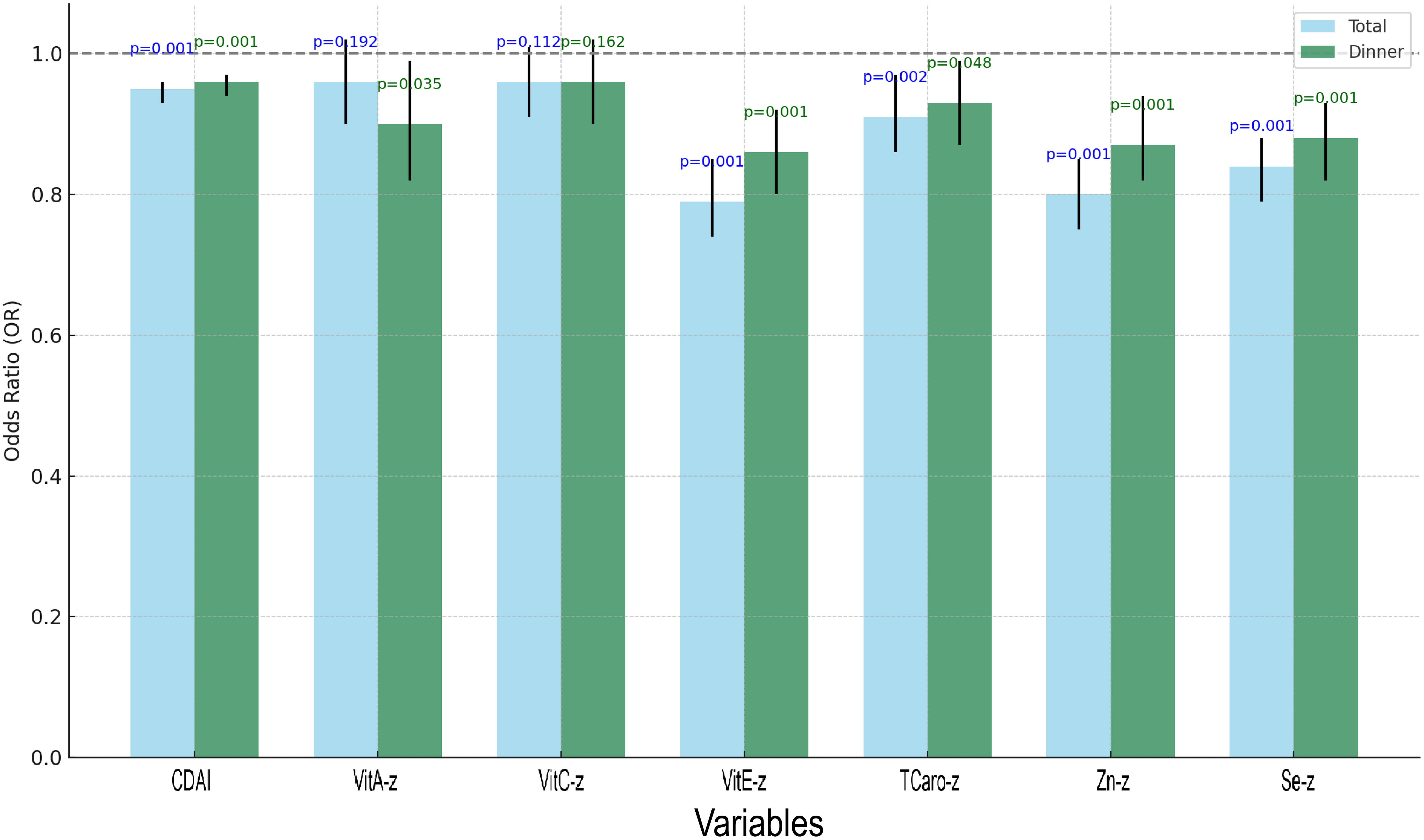
Figure 7. Grouped bar chart of regression analysis for total CDAI, dinner-specific CDAI, and the constituents involved.
3.7 Assessment of the relationship between CDAI and UAE across subgroups
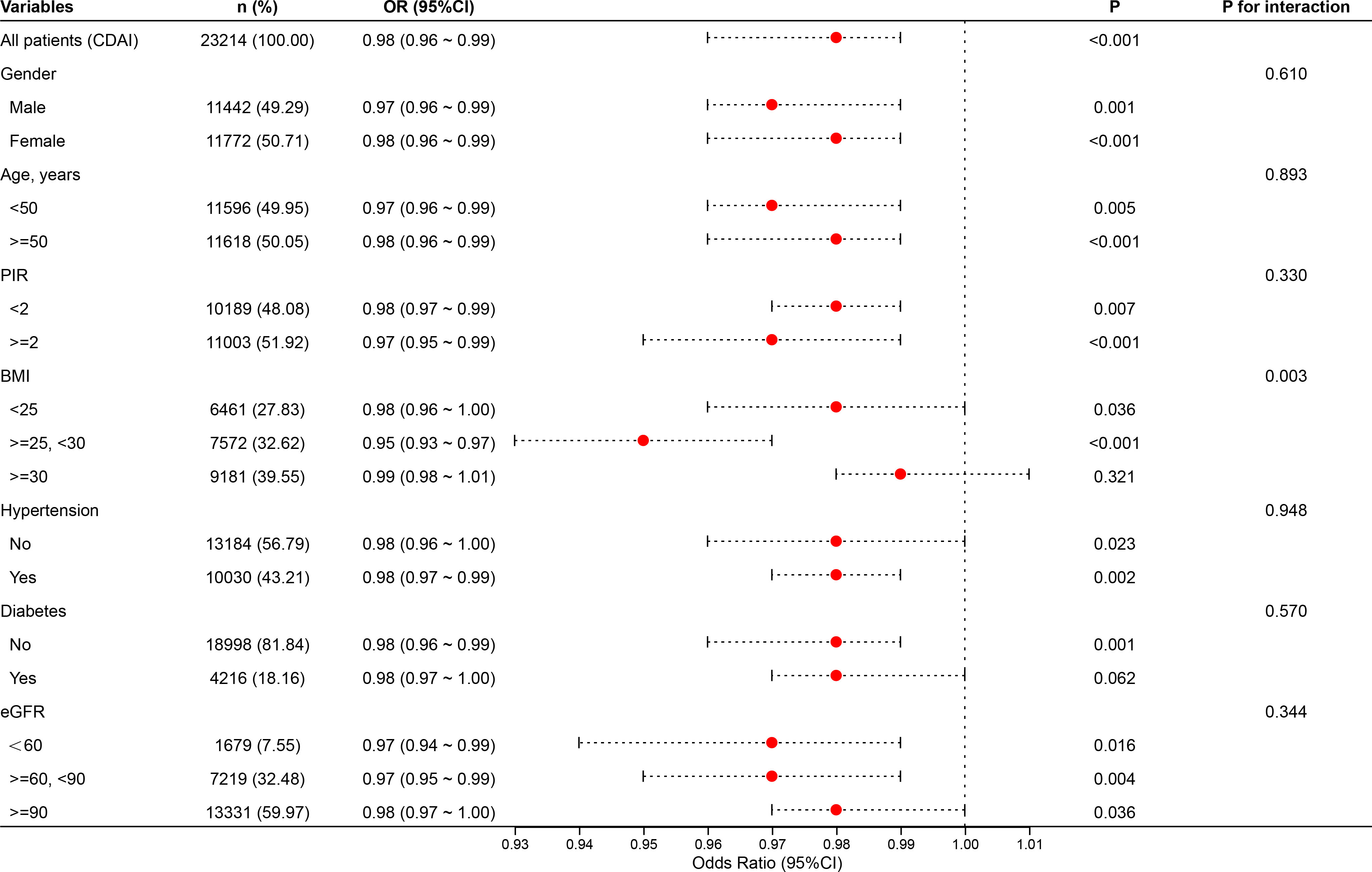
The study conducted stratified analyses based on sex, age, PIR, BMI, diabetes, hypertension, and eGFR (Figure 8). Subgroup analyses revealed that the inverse association between CDAI and albuminuria remained robust across various population strata, including gender, age, poverty-income ratio (PIR), hypertension, diabetes status, and kidney function (eGFR), with all P for interaction values exceeding 0.05. This suggests that the protective effect of dietary antioxidants is generally stable and not modified by these factors. Notably, significant interaction was observed in the BMI subgroup (P for inter = 0.003). The protective relationship between CDAI and albuminuria was evident among individuals with BMI <30, but this association was attenuated and no longer statistically significant among those with BMI ≥30. This finding may imply that obesity could blunt the antioxidant benefit, potentially due to altered oxidative stress pathways, chronic low-grade inflammation, or antioxidant bioavailability in adipose tissue.
Although the interaction by kidney function (eGFR) was not statistically significant (P = 0.344), the consistent inverse associations across all eGFR categories highlight that the renal benefit of higher CDAI persists even among individuals with mildly to moderately impaired kidney function. These results support the potential universal relevance of antioxidant-rich diets for renal protection, regardless of baseline renal status or cardiometabolic conditions.
4 Discussion
This study, leveraging a huge, nationally typical NHANES sample, provides novel evidence that higher composite dietary antioxidant intake (CDAI) is associated with a lower risk of urinary albumin excretion (UAE), a key marker of endothelial dysfunction and early renal injury. Importantly, the timing of antioxidant intake emerged as a significant modifier of this relationship—dinner-specific CDAI and ΔCDAI (dinner minus breakfast) showed the most robust inverse associations with UAE. These findings introduce two novel aspects not reported in previous NHANES-based analyses: the identification of dinner-specific antioxidant intake as the most protective timing pattern for reducing UAE, and the introduction of ΔCDAI as a practical and novel indicator reflecting intra-day antioxidant distribution and its relevance to renal health.
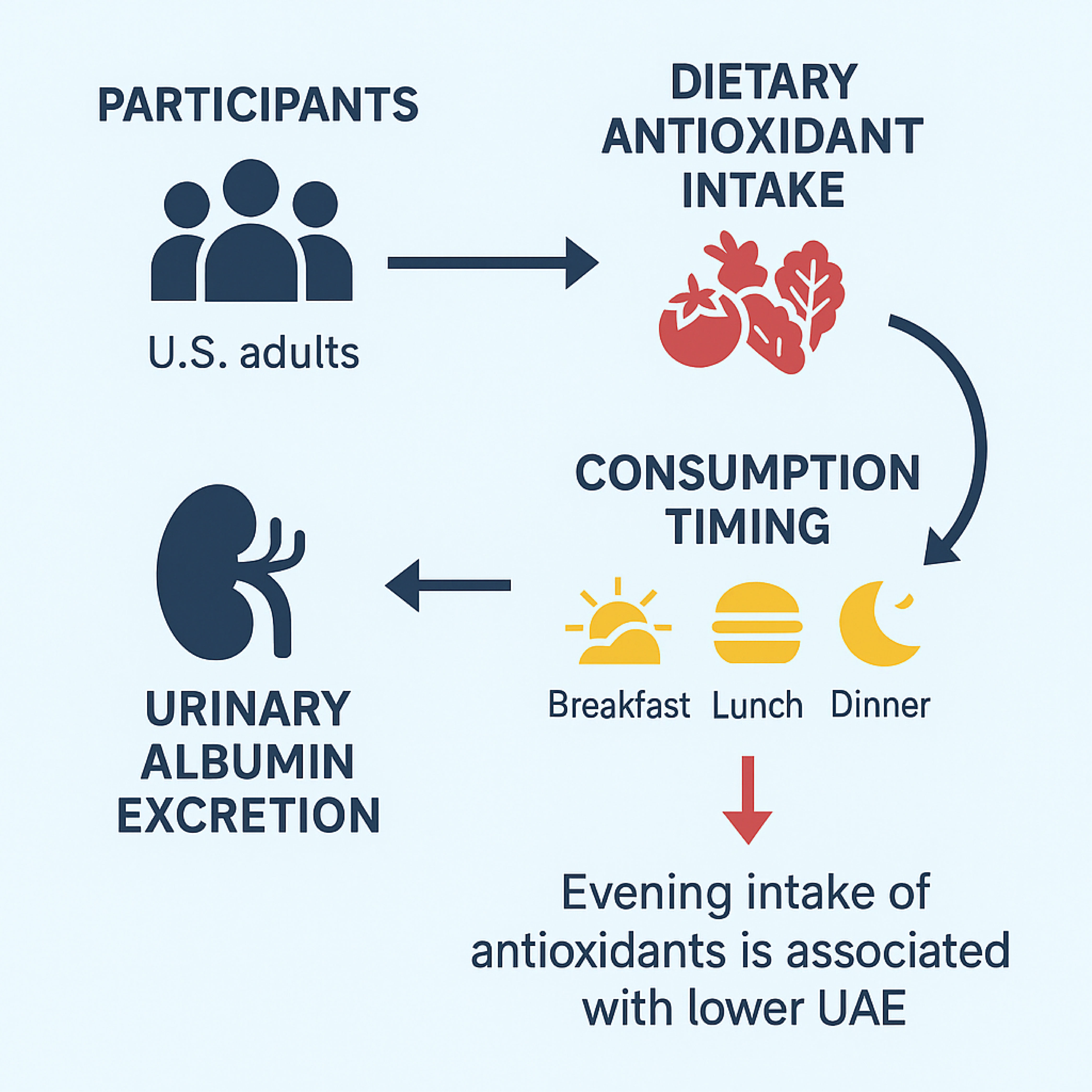
A graphical summary illustrating the study design and key findings is presented in Figure 9.
Prior research investigations have demonstrated that oxidative stress and low-grade inflammation lead to albuminuria via impairment of the glomerular filtration barrier (52). Antioxidant micronutrients such as vitamin E, zinc, and selenium may modulate these pathways by downregulating pro-inflammatory mediators and scavenging reactive oxygen species, including TNF-α and IL-1β—key immune regulators (53–55). While supplementation trials have yielded inconsistent results, likely due to limited synergism, CDAI reflects integrated antioxidant exposure and may thus offer greater explanatory power. Our findings align with emerging interest in diet-induced immune modulation (56). Antioxidant-rich diets may indirectly modulate immune-metabolic cross-talk and endothelial activation, both of which are relevant to subclinical kidney inflammation and albumin leakage (57–59).
Oxidative stress promotes endothelial dysfunction through multiple interconnected mechanisms. Elevated reactive oxygen species (ROS) levels activate inflammatory pathways, disrupt endothelial nitric oxide synthesis, and damage tight junction proteins within the glomerular filtration barrier (60). This cascade leads to increased vascular permeability and subsequent urinary albumin excretion. Targeting oxidative stress may therefore offer dual benefits in mitigating both renal and cardiovascular risks (61).
The stronger association of dinnertime CDAI with UAE reduction suggests that synchronizing antioxidant intake with circadian-regulated immune and oxidative stress responses could enhance renal resilience (62). Feeding-fasting cycles entrain peripheral clocks, particularly in the kidney and liver, which are highly responsive to metabolic cues (63). Evening antioxidant intake may provide timely defense against nocturnal oxidative peaks, enhancing anti-inflammatory responses and protecting the glomerular endothelium (64). ΔCDAI may serve as a practical biomarker for identifying chrononutritional imbalance in antioxidant distribution. Records show that high levels of several important antioxidants in the human body, containing superoxide dismutase (SOD), glutathione peroxidase (GSH-PX), and malondialdehyde (MDA), and melatonin, as well as white blood cell types like neutrophils, monocytes, and lymphocytes, occur at night or shortly before dawn (65). The circadian expression patterns of several antioxidant enzymes, including glutathione reductase (GR), catalase (CAT), and glutathione peroxidase (GPx), have also been demonstrated to be influenced by vitamin A (66).
Subgroup analyses confirmed the protective association across diverse populations; however, the attenuation of antioxidant benefits observed in individuals with obesity (BMI ≥30) may reflect several underlying mechanisms. Obesity is characterized by chronic low-grade inflammation, increased oxidative stress, and altered antioxidant bioavailability. Specifically, excessive adipose tissue may sequester fat-soluble antioxidants such as vitamins A and E, reducing their systemic availability (67, 68). Additionally, obesity-related changes in gut absorption, hepatic metabolism, and circulating lipid profiles can further influence antioxidant distribution and function (69). These factors may collectively diminish the renoprotective effects of dietary antioxidants in obese individuals (70). While these explanations are supported by prior studies, our cross-sectional design and lack of biomarker data preclude direct validation. Future investigations combining dietary assessments with serum antioxidant measurements and inflammatory markers are warranted to clarify these pathways.
This study’s strengths include its large sample size, nationally representative data, and novel focus on both antioxidant quantity and timing. However, several limitations must be considered. First, dietary antioxidant intake was assessed using self-reported 24-hour dietary recalls, which may introduce recall bias and measurement error. Although we applied the average of two non-consecutive 24-hour recalls mitigating variability, this method is inherently limited by participants’ memory and reporting accuracy. Moreover, serum antioxidant biomarkers, which could provide objective measures of antioxidant status, were not consistently available across all NHANES cycles included in our analysis. We have therefore not incorporated biomarker data in this study. Future longitudinal research integrating both detailed dietary assessments and biomarker measurements is warranted to validate and extend our findings, particularly in relation to the timing of antioxidant intake and its physiological effects. Studies integrating dietary patterns with immunophenotyping, circadian transcriptomics, and inflammation biomarkers could clarify the mechanistic underpinnings of these associations and advance precision nutrition approaches in renal-immunometabolic health.
Our findings suggest that in addition to focusing on the amount of antioxidant intake, paying attention to its timing—particularly emphasizing evening consumption—may be important for renal protection. These insights highlight a potential role for personalized chrononutrition strategies in reducing albuminuria risk and improving both renal and cardiovascular outcomes. Although our study is observational, it provides a basis for future interventional research and suggests actionable dietary recommendations that could complement existing clinical practice guidelines.
5 Conclusions
This study emphasises the possible contribution of both the quantity and timing of antioxidant micronutrient intake in mitigating urinary albumin excretion among U.S. adults. The observed benefits of evening antioxidant consumption open new avenues for targeted dietary strategies to prevent or manage early kidney damage. These findings may contribute to the development of personalized nutrition interventions aligned with circadian biology to improve renal outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
HL: Methodology, Validation, Project administration, Supervision, Formal analysis, Software, Data curation, Writing – original draft, Visualization, Investigation, Funding acquisition, Resources, Conceptualization, Writing – review & editing. JG: Writing – original draft, Project administration, Writing – review & editing, Supervision. WT: Writing – original draft, Methodology, Validation, Investigation, Supervision, Writing – review & editing. PC: Project administration, Supervision, Writing – original draft, Writing – review & editing. ZL: Methodology, Validation, Formal analysis, Writing – original draft, Writing – review & editing, Resources, Visualization. PS: Methodology, Writing – review & editing, Project administration, Writing – original draft. SL: Methodology, Writing – original draft, Writing – review & editing, Visualization. LS: Methodology, Writing – original draft, Visualization, Writing – review & editing. YC: Writing – review & editing, Formal analysis, Writing – original draft, Visualization, Methodology. YL: Visualization, Writing – original draft, Writing – review & editing, Methodology, Supervision. JW: Methodology, Writing – review & editing, Writing – original draft, Formal analysis, Visualization. JL: Visualization, Validation, Methodology, Writing – original draft, Writing – review & editing. LM: Visualization, Validation, Writing – review & editing, Methodology, Writing – original draft. XJ: Investigation, Writing – review & editing, Methodology, Supervision, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We appreciate the National Centre for Health Statistics (NCHS) and the Centres for Disease Control and Prevention (CDC) for providing public access to NHANES data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1607456/full#supplementary-material
References
1. Johnson DW, Jones GR, Mathew TH, Ludlow MJ, Chadban SJ, Usherwood T, et al. Chronic kidney disease and measurement of albuminuria or proteinuria: a position statement. Med J Aust. (2012) 197:224–5. doi: 10.5694/mja11.11468
2. Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. (2010) 375:2073–81. doi: 10.1016/s0140-6736(10)60674-5
3. Perkovic V, Verdon C, Ninomiya T, Barzi F, Cass A, Patel A, et al. The relationship between proteinuria and coronary risk: a systematic review and meta-analysis. PloS Med. (2008) 5:e207. doi: 10.1371/journal.pmed.0050207
4. Eknoyan G, Hostetter T, Bakris GL, Hebert L, Levey AS, Parving HH, et al. Proteinuria and other markers of chronic kidney disease: a position statement of the national kidney foundation (NKF) and the national institute of diabetes and digestive and kidney diseases (NIDDK). Am J Kidney Dis. (2003) 42:617–22. doi: 10.1016/s0272-6386(03)00826-6
5. Miller WG, Bruns DE, Hortin GL, Sandberg S, Aakre KM, McQueen MJ, et al. Current issues in measurement and reporting of urinary albumin excretion. Clin Chem. (2009) 55:24–38. doi: 10.1373/clinchem.2008.106567
6. Salmon AH and Satchell SC. Endothelial glycocalyx dysfunction in disease: albuminuria and increased microvascular permeability. J Pathol. (2012) 226:562–74. doi: 10.1002/path.3964
7. Thoenes M, Bramlage P, Khan BV, Schieffer B, Kirch W, and Weir MR. Albuminuria: pathophysiology, epidemiology and clinical relevance of an emerging marker for cardiovascular disease. Future Cardiol. (2007) 3:519–24. doi: 10.2217/14796678.3.5.519
8. Comper WD, Hilliard LM, Nikolic-Paterson DJ, and Russo LM. Disease-dependent mechanisms of albuminuria. Am J Physiol Renal Physiol. (2008) 295:F1589–600. doi: 10.1152/ajprenal.00142.2008
9. de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation. (2004) 110:921–7. doi: 10.1161/01.Cir.0000139860.33974.28
10. Kong AP, So WY, Szeto CC, Chan NN, Luk A, Ma RC, et al. Assessment of glomerular filtration rate in addition to albuminuria is important in managing type II diabetes. Kidney Int. (2006) 69:383–7. doi: 10.1038/sj.ki.5000061
11. Tu QM, Jin HM, and Yang XH. Lipid abnormality in diabetic kidney disease and potential treatment advancements. Front Endocrinol (Lausanne). (2025) 16:1503711. doi: 10.3389/fendo.2025.1503711
12. Keyzer CA, van Breda GF, Vervloet MG, de Jong MA, Laverman GD, Hemmelder MH, et al. Effects of vitamin D receptor activation and dietary sodium restriction on residual albuminuria in CKD: the ViRTUE-CKD trial. J Am Soc Nephrol. (2017) 28:1296–305. doi: 10.1681/asn.2016040407
13. Stasi A, Cosola C, Caggiano G, Cimmarusti MT, Palieri R, Acquaviva PM, et al. Obesity-related chronic kidney disease: principal mechanisms and new approaches in nutritional management. Front Nutr. (2022) 9:925619. doi: 10.3389/fnut.2022.925619
14. Robinson ES, Fisher ND, Forman JP, and Curhan GC. Physical activity and albuminuria. Am J Epidemiol. (2010) 171:515–21. doi: 10.1093/aje/kwp442
15. Ruggenenti P, Cravedi P, and Remuzzi G. The RAAS in the pathogenesis and treatment of diabetic nephropathy. Nat Rev Nephrol. (2010) 6:319–30. doi: 10.1038/nrneph.2010.58
16. Neuen BL, Heerspink HJL, Vart P, Claggett BL, Fletcher RA, Arnott C, et al. Estimated lifetime cardiovascular, kidney, and mortality benefits of combination treatment with SGLT2 inhibitors, GLP-1 receptor agonists, and nonsteroidal MRA compared with conventional care in patients with type 2 diabetes and albuminuria. Circulation. (2024) 149:450–62. doi: 10.1161/circulationaha.123.067584
17. Pitt B, Kober L, Ponikowski P, Gheorghiade M, Filippatos G, Krum H, et al. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J. (2013) 34:2453–63. doi: 10.1093/eurheartj/eht187
18. Smeijer JD, Kohan DE, Dhaun N, Noronha IL, Liew A, and Heerspink HJL. Endothelin receptor antagonists in chronic kidney disease. Nat Rev Nephrol. (2025) 21:175–88. doi: 10.1038/s41581-024-00908-z
19. Gerstein HC. Epidemiologic analyses of risk factors, risk indicators, risk markers, and causal factors. The example of albuminuria and the risk of cardiovascular disease in diabetes. Endocrinol Metab Clin North Am. (2002) 31:537–51. doi: 10.1016/s0889-8529(02)00019-1
20. Chen J, Wu J, Kong D, Yang C, Yu H, Pan Q, et al. The effect of antioxidant vitamins on patients with diabetes and albuminuria: A meta-analysis of randomized controlled trials. J Ren Nutr. (2020) 30:101–10. doi: 10.1053/j.jrn.2019.06.011
21. Suzuki K, Honjo H, Ichino N, Osakabe K, Sugimoto K, Yamada H, et al. Association of serum carotenoid levels with urinary albumin excretion in a general Japanese population: the Yakumo study. J Epidemiol. (2013) 23:451–6. doi: 10.2188/jea.je20130058
22. Reddi AS and Bollineni JS. Selenium-deficient diet induces renal oxidative stress and injury via TGF-beta1 in normal and diabetic rats. Kidney Int. (2001) 59:1342–53. doi: 10.1046/j.1523-1755.2001.0590041342.x
23. Petiz LL, Girardi CS, Bortolin RC, Kunzler A, Gasparotto J, Rabelo TK, et al. Vitamin A oral supplementation induces oxidative stress and suppresses IL-10 and HSP70 in skeletal muscle of trained rats. Nutrients. (2017) 9(4):353. doi: 10.3390/nu9040353
24. Jin X, Tong W, Sun L, Lu S, Xu T, Sun P, et al. Composite dietary antioxidant index in relation to urge urinary incontinence in US men. Front Nutr. (2024) 11:1514320. doi: 10.3389/fnut.2024.1514320
25. Wright ME, Mayne ST, Stolzenberg-Solomon RZ, Li Z, Pietinen P, Taylor PR, et al. Development of a comprehensive dietary antioxidant index and application to lung cancer risk in a cohort of male smokers. Am J Epidemiol. (2004) 160:68–76. doi: 10.1093/aje/kwh173
26. Jin X, Sun L, Li H, and Liu Y. Association between the composite dietary antioxidant index and erectile dysfunction in US men: a cross-sectional study. J Health Popul Nutr. (2024) 43:184. doi: 10.1186/s41043-024-00653-w
27. Jin X, Tong W, Sun L, Lu S, Sun P, Li H, et al. Association of composite dietary antioxidant index with high risk of prostate cancer in middle-aged and elderly men: insights from NHANES. Front Immunol. (2025) 16:1530174. doi: 10.3389/fimmu.2025.1530174
28. Qin H, Shen L, and Xu D. Association of composite dietary antioxidant index with mortality in adults with hypertension: evidence from NHANES. Front Nutr. (2024) 11:1371928. doi: 10.3389/fnut.2024.1371928
29. Ma R, Zhou X, Zhang G, Wu H, Lu Y, Liu F, et al. Association between composite dietary antioxidant index and coronary heart disease among US adults: a cross-sectional analysis. BMC Public Health. (2023) 23:2426. doi: 10.1186/s12889-023-17373-1
30. Xu Q, Qian X, Sun F, Liu H, Dou Z, and Zhang J. Independent and joint associations of dietary antioxidant intake with risk of post-stroke depression and all-cause mortality. J Affect Disord. (2023) 322:84–90. doi: 10.1016/j.jad.2022.11.013
31. Asher G and Sassone-Corsi P. Time for food: the intimate interplay between nutrition, metabolism, and the circadian clock. Cell. (2015) 161:84–92. doi: 10.1016/j.cell.2015.03.015
32. Marjot T, Tomlinson JW, Hodson L, and Ray DW. Timing of energy intake and the therapeutic potential of intermittent fasting and time-restricted eating in NAFLD. Gut. (2023) 72:1607–19. doi: 10.1136/gutjnl-2023-329998
33. Stokkan KA, Yamazaki S, Tei H, Sakaki Y, and Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. (2001) 291:490–3. doi: 10.1126/science.291.5503.490
34. Kula-Eversole E, Lee DH, Samba I, Yildirim E, Levine DC, Hong HK, et al. Phosphatase of regenerating liver-1 selectively times circadian behavior in darkness via function in PDF neurons and dephosphorylation of TIMELESS. Curr Biol. (2021) 31:138–49. doi: 10.1016/j.cub.2020.10.013
35. Han T, Gao J, Wang L, Li C, Qi L, Sun C, et al. The association of energy and macronutrient intake at dinner versus breakfast with disease-specific and all-cause mortality among people with diabetes: the U.S. National health and nutrition examination survey, 2003-2014. Diabetes Care. (2020) 43:1442–8. doi: 10.2337/dc19-2289
36. St-Onge MP, Ard J, Baskin ML, Chiuve SE, Johnson HM, Kris-Etherton P, et al. Meal timing and frequency: implications for cardiovascular disease prevention: A scientific statement from the American Heart Association. Circulation. (2017) 135:e96–e121. doi: 10.1161/cir.0000000000000476
37. Xu X, Wei W, Xu J, Huang J, Li L, Han T, et al. The association of minerals intake in three meals with cancer and all-cause mortality: the U.S. National Health and Nutrition Examination Survey, 2003-2014. BMC Cancer. (2021) 21:912. doi: 10.1186/s12885-021-08643-5
38. Kessler K and Pivovarova-Ramich O. Meal timing, aging, and metabolic health. Int J Mol Sci. (2019) 20(8):1911. doi: 10.3390/ijms20081911
39. Cho YT, Chen CW, Chen MP, Hu JL, Su H, Shiea J, et al. Diagnosis of albuminuria by tryptic digestion and matrix-assisted laser desorption ionization/time-of-flight mass spectrometry. Clin Chim Acta. (2013) 420:76–81. doi: 10.1016/j.cca.2012.12.016
40. American Diabetes Association. Standards of medical care in diabetes-2016: summary of revisions. Diabetes Care. (2016) 39 Suppl 1:S4–5. doi: 10.2337/dc16-S003
41. O’Neill MS, Diez-Roux AV, Auchincloss AH, Franklin TG, Jacobs DR Jr., Astor BC, et al. Airborne particulate matter exposure and urinary albumin excretion: the Multi-Ethnic Study of Atherosclerosis. Occup Environ Med. (2008) 65:534–40. doi: 10.1136/oem.2007.035238
42. Qin Z, Chang K, Yang Q, Yu Q, Liao R, and Su B. The association between weight-adjusted-waist index and increased urinary albumin excretion in adults: A population-based study. Front Nutr. (2022) 9:941926. doi: 10.3389/fnut.2022.941926
43. Stehouwer CD, Nauta JJ, Zeldenrust GC, Hackeng WH, Donker AJ, and den Ottolander GJ. Urinary albumin excretion, cardiovascular disease, and endothelial dysfunction in non-insulin-dependent diabetes mellitus. Lancet. (1992) 340:319–23. doi: 10.1016/0140-6736(92)91401-s
44. Holl RW, Grabert M, Heinze E, and Debatin KM. Objective assessment of smoking habits by urinary cotinine measurement in adolescents and young adults with type 1 diabetes. Reliability of reported cigarette consumption and relationship to urinary albumin excretion. Diabetes Care. (1998) 21:787–91. doi: 10.2337/diacare.21.5.787
45. Chai S, Wu S, Xin S, Yuan N, Sun J, Zhang X, et al. Negative association of time in range and urinary albumin excretion rate in patients with type 2 diabetes mellitus: a retrospective study of inpatients. Chin Med J (Engl). (2022) 135:1052–6. doi: 10.1097/cm9.0000000000001914
46. Perez-Hernandez J, Olivares MD, Solaz E, Martinez F, Martínez-Hervas S, Pichler G, et al. Urinary podocyte-associated molecules and albuminuria in hypertension. J Hypertens. (2018) 36:1712–8. doi: 10.1097/hjh.0000000000001747
47. Montelpare WJ, Klentrou P, and Thoden J. Continuous versus intermittent exercise effects on urinary excretion of albumin and total protein. J Sci Med Sport. (2002) 5:219–28. doi: 10.1016/s1440-2440(02)80006-8
48. Anyanwagu U, Donnelly R, and Idris I. Individual and Combined Relationship between Reduced eGFR and/or Increased Urinary Albumin Excretion Rate with Mortality Risk among Insulin-Treated Patients with Type 2 Diabetes in Routine Practice. Kidney Dis (Basel). (2019) 5:91–9. doi: 10.1159/000493731
49. Lu M, Yin J, Xu T, Dai X, Liu T, Zhang Y, et al. Fuling-Zexie formula attenuates hyperuricemia-induced nephropathy and inhibits JAK2/STAT3 signaling and NLRP3 inflammasome activation in mice. J Ethnopharmacol. (2024) 319:117262. doi: 10.1016/j.jep.2023.117262
50. Miao M, Deng X, Wang Z, Jiang D, Lai S, Yu S, et al. Cardiometabolic index is associated with urinary albumin excretion and renal function in aged person over 60: Data from NHANES 2011-2018. Int J Cardiol. (2023) 384:76–81. doi: 10.1016/j.ijcard.2023.04.017
51. Tseng CH. Correlation of uric acid and urinary albumin excretion rate in patients with type 2 diabetes mellitus in Taiwan. Kidney Int. (2005) 68:796–801. doi: 10.1111/j.1523-1755.2005.00459.x
52. Whaley-Connell AT, Chowdhury NA, Hayden MR, Stump CS, Habibi J, Wiedmeyer CE, et al. Oxidative stress and glomerular filtration barrier injury: role of the renin-angiotensin system in the Ren2 transgenic rat. Am J Physiol Renal Physiol. (2006) 291:F1308–14. doi: 10.1152/ajprenal.00167.2006
53. Villalón-García I, Álvarez-Córdoba M, Povea-Cabello S, Talaverón-Rey M, Villanueva-Paz M, Luzón-Hidalgo R, et al. Vitamin E prevents lipid peroxidation and iron accumulation in PLA2G6-Associated Neurodegeneration. Neurobiol Dis. (2022) 165:105649. doi: 10.1016/j.nbd.2022.105649
54. Hübner C and Haase H. Interactions of zinc- and redox-signaling pathways. Redox Biol. (2021) 41:101916. doi: 10.1016/j.redox.2021.101916
55. Hariharan S and Dharmaraj S. Selenium and selenoproteins: it’s role in regulation of inflammation. Inflammopharmacology. (2020) 28:667–95. doi: 10.1007/s10787-020-00690-x
56. Kolodziejczyk AA, Zheng D, and Elinav E. Diet-microbiota interactions and personalized nutrition. Nat Rev Microbiol. (2019) 17:742–53. doi: 10.1038/s41579-019-0256-8
57. Gostner JM, Becker K, Ueberall F, and Fuchs D. The good and bad of antioxidant foods: An immunological perspective. Food Chem Toxicol. (2015) 80:72–9. doi: 10.1016/j.fct.2015.02.012
58. Hwang JH, Heo W, Park JI, Kim KM, Oh HT, Yoo GD, et al. Endothelial TAZ inhibits capillarization of liver sinusoidal endothelium and damage-induced liver fibrosis via nitric oxide production. Theranostics. (2023) 13:4182–96. doi: 10.7150/thno.83714
59. Mehta RB, Tandukar S, Jorgensen D, Randhawa P, Sood P, Puttarajappa C, et al. Early subclinical tubulitis and interstitial inflammation in kidney transplantation have adverse clinical implications. Kidney Int. (2020) 98:436–47. doi: 10.1016/j.kint.2020.03.028
60. Reidy K, Kang HM, Hostetter T, and Susztak K. Molecular mechanisms of diabetic kidney disease. J Clin Invest. (2014) 124:2333–40. doi: 10.1172/jci72271
61. Sarnak MJ, Amann K, Bangalore S, Cavalcante JL, Charytan DM, Craig JC, et al. Chronic kidney disease and coronary artery disease: JACC state-of-the-art review. J Am Coll Cardiol. (2019) 74:1823–38. doi: 10.1016/j.jacc.2019.08.1017
62. Depner CM, Melanson EL, McHill AW, and Wright KP Jr. Mistimed food intake and sleep alters 24-hour time-of-day patterns of the human plasma proteome. Proc Natl Acad Sci U S A. (2018) 115:E5390–e5399. doi: 10.1073/pnas.1714813115
63. Gagliano O, Luni C, Li Y, Angiolillo S, Qin W, Panariello F, et al. Synchronization between peripheral circadian clock and feeding-fasting cycles in microfluidic device sustains oscillatory pattern of transcriptome. Nat Commun. (2021) 12:6185. doi: 10.1038/s41467-021-26294-9
64. Lananna BV and Musiek ES. The wrinkling of time: Aging, inflammation, oxidative stress, and the circadian clock in neurodegeneration. Neurobiol Dis. (2020) 139:104832. doi: 10.1016/j.nbd.2020.104832
65. Sennels HP, Jørgensen HL, and Fahrenkrug J. Diurnal changes of biochemical metabolic markers in healthy young males - the Bispebjerg study of diurnal variations. Scand J Clin Lab Invest. (2015) 75:686–92. doi: 10.3109/00365513.2015.1080385
66. Navigatore-Fonzo LS, Delgado SM, Gimenez MS, and Anzulovich AC. Daily rhythms of catalase and glutathione peroxidase expression and activity are endogenously driven in the hippocampus and are modified by a vitamin A-free diet. Nutr Neurosci. (2014) 17:21–30. doi: 10.1179/1476830513y.0000000062
67. Cox AJ, West NP, and Cripps AW. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. (2015) 3:207–15. doi: 10.1016/s2213-8587(14)70134-2
68. Münzberg H and Morrison CD. Structure, production and signaling of leptin. Metabolism. (2015) 64:13–23. doi: 10.1016/j.metabol.2014.09.010
69. Miyazawa T, Burdeos GC, Itaya M, Nakagawa K, and Miyazawa T. Vitamin E: regulatory redox interactions. IUBMB Life. (2019) 71:430–41. doi: 10.1002/iub.2008
70. Nederveen JP, Mastrolonardo AJ, Xhuti D, Di Carlo A, Manta K, Fuda MR, et al. Novel multi-ingredient supplement facilitates weight loss and improves body composition in overweight and obese individuals: A randomized, double-blind, placebo-controlled clinical trial. Nutrients. (2023) 15(17):3693. doi: 10.3390/nu15173693
Keywords: exogenous dietary antioxidant micronutrient, meal timing, UAE, albuminuria, NHANES
Citation: Li H, Ge J, Tong W, Chen P, Sun P, Lu S, Sun L, Chen Y, Li Y, Wang J, Li J, Mao L, Li Z and Jin X (2025) The association of exogenous dietary antioxidant micronutrient intake and consumption timing with urinary albumin excretion among U.S. adults. Front. Immunol. 16:1607456. doi: 10.3389/fimmu.2025.1607456
Received: 07 April 2025; Accepted: 28 July 2025;
Published: 23 September 2025.
Edited by:
Ennio Polilli, Azienda USL di Pescara, ItalyReviewed by:
Hui Bao, Tongji University, ChinaDorota Formanowicz, Poznan University of Medical Sciences, Poland
Copyright © 2025 Li, Ge, Tong, Chen, Sun, Lu, Sun, Chen, Li, Wang, Li, Mao, Li and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuefeng Jin, amlueHVlZmVuZzcxQGdtYWlsLmNvbQ==; Zizhi Li, bGl6aXpoaUBqem11LmVkdS5jbg==
†Present address: Xuefeng Jin, Organ Transplantation Clinical Medical Center of Xiamen University, Department of General Surgery, Xiang’an Hospital of Xiamen University, School of Medicine, Xiamen University, Xiamen, China
Xiamen Key Laboratory of Regeneration Medicine,School of Medicine, Organ Transplantation Institute of Xiamen University, Xiamen Human Organ Transplantation Quality Control Center, Xiamen University, Xiamen, China
Fujian Provincial Key Laboratory of Organ and Tissue Regeneration, School of Medicine, Organ Transplantation Institute of Xiamen University, Xiamen Human Organ Transplantation Quality Control Center, Xiamen University, Xiamen, China
 Hangxu Li
Hangxu Li Jingping Ge2,3,4
Jingping Ge2,3,4 Wenhui Tong
Wenhui Tong Pengpeng Chen
Pengpeng Chen Pan Sun
Pan Sun Sujue Lu
Sujue Lu Li Sun
Li Sun Yang Chen
Yang Chen Yangyang Li
Yangyang Li Jun Wang
Jun Wang Xuefeng Jin
Xuefeng Jin