- 1First Clinical Medical College, Shandong University of Traditional Chinese Medicine, Jinan, Shandong, China
- 2Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, Shandong, China
- 3College of Acupuncture and Tuina, Shandong University of Traditional Chinese Medicine, Jinan, Shandong, China
- 4Rehabilitation Medicine College, Shandong University of Traditional Chinese Medicine, Jinan, Shandong, China
- 5Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, China
Lactate, a key metabolic byproduct of the Warburg effect, has lately been recognized as a regulator of histone lysine lactylation, a unique post-translational modification that plays a crucial role in essential biological processes, including the regulation of gene transcription. Lactylation plays a crucial regulatory role in macrophage biology by influencing inflammatory responses, tumor immune evasion, and fibrotic development. This review methodically investigates the molecular mechanisms of lactate metabolism and lactylation modification, focusing on their roles in macrophage activation and polarization in relation to gastrointestinal disorders, such as gastric cancer, colorectal carcinoma, ulcerative colitis, postoperative ileus, and bacterial and viral gastrointestinal infections. We clarify the molecular switching role of lactylation in regulating macrophage polarization under pathological settings by integrating current developments in epigenetic regulation and metabolic reprogramming. Current evidence demonstrates the dual regulatory role of lactylation in macrophage-mediated immune responses: it fosters anti-inflammatory and reparative phenotypes, yet may paradoxically expedite tumor progression and induce immunosuppressive conditions in certain gastrointestinal microenvironments. This review emphasizes that exploring lactylation as a novel therapeutic target offers new insights into gastrointestinal pathogenesis and lays a molecular groundwork for formulating precision therapeutic strategies against inflammatory diseases and malignant tumors.
1 Introduction
Gastrointestinal diseases include a range of medical problems, such as functional dyspepsia, celiac disease, inflammatory bowel disease (IBD), and gastrointestinal cancers (1). About half of the gastrointestinal symptoms seen in primary care are due to real medical issues, which are divided into two groups: organic disorders (like chronic atrophic gastritis, peptic ulcers, inflammatory bowel disease, and gastrointestinal cancers) and functional disorders (2–4). Data from the American Cancer Society indicates that by 2025, there will be roughly 30,300 new instances of gastric cancer and 154,270 new instances of colorectal cancer in the United States. We will classify these cancers as the third most prevalent kind, accounting for 42.6% of gastrointestinal tumors. The incidence rate among adults aged 50 and above is rising at an annual rate of 2.4% (5). In the United States, over 1% of the population, or 1 in every 100 individuals, is afflicted by inflammatory bowel disease (IBD), with ulcerative colitis (UC) and Crohn’s disease (CD) exhibiting similar incidence rates. The global prevalence of inflammatory bowel disease (IBD) positively corresponds with the level of industrialization, with developing nations witnessing an increasing incidence (6). The pathogenesis of these diseases involves multidimensional mechanisms, including pro-inflammatory cytokine cascades, gut microbiota dysbiosis, immune dysregulation, epithelial barrier repair dysfunction, and tumor microenvironment remodeling (7). Thus, clarifying the molecular interactions that drive these disease processes and creating precision therapies aimed at the microenvironment have become essential focuses in translational medicine research.
Lactate, functioning as a pivotal metabolic node in the glycolytic and mitochondrial oxidative phosphorylation homeostatic network, is dynamically regulated by the equilibrium between the Warburg effect and reverse Warburg effect (8). Groundbreaking studies have redefined lactate not merely as an end-product of hypoxic metabolism, but as a recyclable carbon source among multicellular populations within the tumor microenvironment (TME), while also serving as a metabolic signaling molecule that participates in immunometabolic regulation through epigenetic reprogramming (9, 9). Research has shown that under conditions of hypoxia, increased metabolic activity, and inflammation, anaerobic yeasts produce a large amount of lactate through glycolysis (10). Mechanistic investigations demonstrate that under hypoxic stress, hyperactive energy metabolism, and chronic inflammation, the glycolytic rate-limiting enzyme LDHA (lactate dehydrogenase A) undergoes significant activation, driving exponential lactate production (11). This pathological lactate accumulation bidirectionally modulates NF-κB-mediated inflammatory cascades and PD-L1-associated tumor immune evasion mechanisms via GPR81/AMPK signaling axis activation (12, 13). As a novel lactate-derived post-translational modification (PTM) (14). The molecular basis of lactylation was first elucidated by Zhang et al. (8) in 2019. Their seminal work demonstrated that lactate covalently modifies histone H3K18 sites (H3K18la), establishing an epigenetic regulatory interface that dictates macrophage polarization fate (M1/M2 switching) and immune checkpoint molecule expression profiles. Cutting-edge research reveals that histone lactylation remodels 3D chromatin architecture to specifically activate metabolic stress response networks (e.g., HIF-1α, mTORC1 pathways), while regulating key effectors involved in immune activation (CD8+ T cell infiltration), metabolic reprogramming (glutaminolysis), and tissue regeneration (VEGF signaling) (15).
Macrophages, as key members of the mononuclear phagocyte system, play a crucial role in immune defense, tissue homeostasis maintenance, and anti-tumor immune surveillance (16, 17). With their high plasticity, macrophages perform a variety of functions in both homeostasis and immune responses. Through polarization, macrophages carry out immune functions responsible for microbial defense, while also participating in the disease processes of autoimmune diseases and malignant tumors (18). Notably, in gastrointestinal pathologies, macrophage polarization exhibits dual regulatory characteristics—M1 polarization exacerbates mucosal damage via NF-κB/STAT3 signaling in inflammatory bowel disease (IBD) (19), whereas tumor-associated macrophage (TAM) M2 polarization promotes angiogenesis and immune evasion through VEGF/PD-L1 axis in gastric and colorectal cancer microenvironments (20, 21). The molecular switch mechanism governing macrophage transition from pro-inflammatory to reparative phenotypes remains incompletely elucidated. Emerging mechanistic studies reveal lactate’s multimodal regulation of macrophage activation: (1) as a HIF-1α stabilizer inducing M2 polarization;(2) via GPR81-mediated NLRP3 inflammasome suppression; (3) through histone H3K18 lactylation-mediated chromatin accessibility remodeling (22). These discoveries establish a theoretical foundation for developing novel therapeutic strategies targeting the lactate-macrophage axis, particularly highlighting its translational potential in rebalancing intestinal immune microenvironments and reversing tumor immunosuppression (Figure 1).
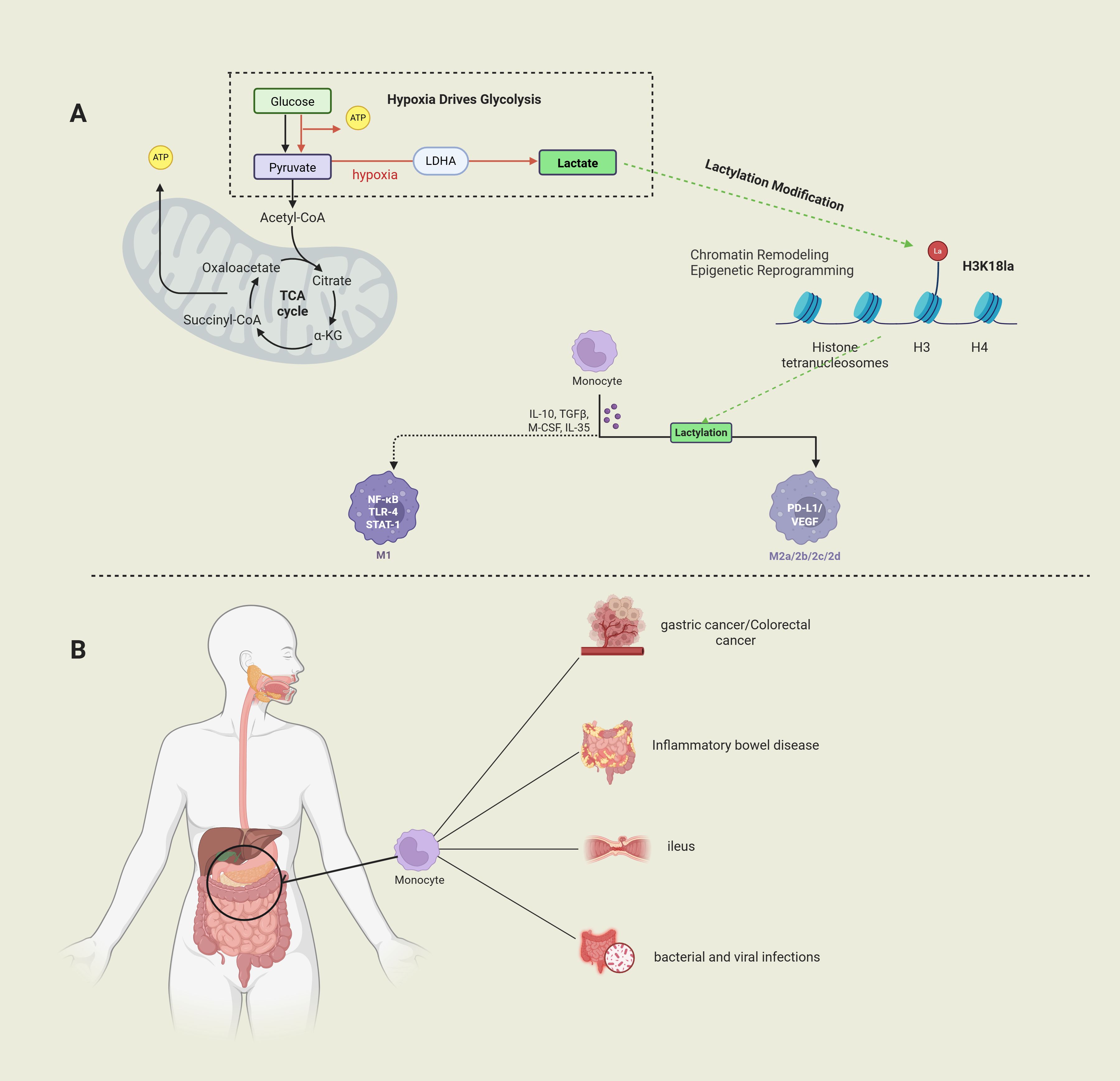
Figure 1. (Image 1 by Biorender) Part (A) Under hypoxic conditions, glucose generates pyruvate through glycolysis and is converted to lactate; lactate regulates histone function through lactation modifications (e.g., H3K18la) and regulates macrophage polarization. Part (B) Macrophages play a key role in diseases such as gastric cancer, colorectal cancer, inflammatory bowel disease, and bacterial/viral infections and may be involved in the pathological process by modulating the immune microenvironment.
2 Lactylation: a metabolic-epigenetic crosstalk hub
Metabolites and intermediates not only play important roles in metabolic processes but also possess non-metabolic functions in cellular signal transduction. Lactate, traditionally viewed as an end-product of glycolysis, has been redefined as a multidimensional signaling molecule that regulates cell fate determination through epigenetic reprogramming (23). Lactylation, a post-translational modification (PTM) driven by intracellular lactate accumulation, is biochemically characterized by the covalent conjugation of a lactyl group to the ϵ-amino group of lysine residues on target proteins (24). In 2019, the Zhang team first reported the lactylation modification of histone H3K18 and elucidated its molecular mechanism in regulating macrophage polarization and inflammatory responses by modulating chromatin’s three-dimensional conformation (8, 25). The discovery of histone lactylation modification reveals lactate-dependent, dynamic, and reversible epigenetic changes that can regulate gene expression and precisely control cellular metabolism (26). Lactylation is currently considered to have two isomers, namely L-lactyl (K(L-la)) and D-lactyl (K(D-la)) configurations (8, 27).
Lactate (C3H6O3), the terminal product of glycolysis, is a hydroxycarboxylic acid generated through NADH-dependent reduction of pyruvate catalyzed by lactate dehydrogenase (LDH). Its stereochemical configurations include L- (levorotatory), D- (dextrorotatory), and racemic DL-forms, with bioactive L-lactate constituting over 95% of the mammalian lactate pool (14, 28, 29). Pyruvate, a pivotal glucose catabolism intermediate, resides at a metabolic branch point: mitochondrial conversion to acetyl-CoA via the pyruvate dehydrogenase complex (PDC) for oxidative phosphorylation, or cytosolic LDH-mediated reduction to lactate (14, 30). Lactate metabolism has dual pathways: Lactate metabolism bifurcates into: ① mitochondrial import via monocarboxylate transporters (MCTs) and reconversion to pyruvate by LDHB for TCA cycle entry; ② hepatic/renal gluconeogenesis (Cori cycle) (31).
The seminal Warburg effect in oncology reveals tumor cells’ preferential glycolysis despite oxygen sufficiency, yielding copious lactate with rapid ATP generation (32). Among them, LDHA is one of the key enzymes for glucose metabolism reprogramming in the TME. It promotes the conversion of pyruvate to lactate, and its activity is positively correlated with the Warburg effect (33), which directly or indirectly activates signal transduction pathways and modulates immune responses to be involved in tumorigenesis and progression (34). The Warburg effect explains the central role of lactate in tumor metabolism. As a novel epigenetic mechanism, lactate triggers histone lysine lactylation modifications, thereby controlling various biological processes such as tumor initiation, progression, immune evasion, and cancer cell metabolic reprogramming (35, 36). During inflammation, tissue repair requires a large amount of energy, with glycolysis being abnormally active to ensure that lactate concentrations inside and outside the cells are higher than those in resting-state cells. Meanwhile, lactate also serves as fuel for mitochondrial metabolism, providing the large amounts of energy required. In summary, lactate accumulation is an inevitable result of inflammatory diseases and tumors (36–38). Conversely, it can influence the occurrence and development of diseases by regulating immune responses and tumor immunity (39). In addition to the Warburg effect in cancer cells, it is also present in proliferating T cells, macrophages, and fibroblasts (40). Lactate homeostasis is maintained by the lactate shuttle system, comprising: ① Monocarboxylate Transporters (MCT) isoforms (MCT1 for uptake, MCT4 for efflux); ② Proton gradient-dependent cotransport (41, 42). Based on the lactate shuttle hypothesis, lactate is described as a carrier linking glycolysis and oxidative metabolism. The association between lactate in the glycolytic pathway and aerobic pathways can occur continuously under fully aerobic conditions. The lactate shuttle can overcome the cellular compartmentalization barrier (43). System dynamics are governed by spatiotemporal expression of LDH isozymes (LDHA favoring lactate production, LDHB promoting oxidation) and MCT subtypes (44).
Epigenetic modifications refer to biological processes that regulate gene expression through DNA sequence-independent mechanisms, characterized by spatiotemporal control of gene activity via chromatin structural remodeling (45). As a pivotal epigenetic regulatory modality, histone acylation exhibits reversibility, spatiotemporal dynamics, and evolutionary conservation, orchestrating embryogenesis, tissue differentiation, and cellular stress responses to maintain organismal homeostasis (46). These modifications are catalyzed by specific acyltransferases that covalently conjugate acyl groups to histone lysine residues, with major types including acetylation, methylation, phosphorylation, and the newly discovered lactylation (47).
The dynamic equilibrium of histone lactylation is co-regulated by “writers” (lactyltransferases) and “erasers” (delactylases), involving the covalent addition and removal of lactyl groups and chromatin structure remodeling. In eukaryotes, the chromatin core structural unit is the nucleosome—a disk-like structure formed by a histone octamer (two each of H2A, H2B, H3, H4) wrapped with 147bp DNA, organized into higher-order chromatin fibers via linker histone H1 (48, 49). Cutting-edge research has unveiled the competitive binding between lactylation and acetylation, shedding light on the metabolic regulatory dimension of the histone code: when lactate exceeds a critical threshold, lactylation at the H3K18 locus (H3K18la) replaces acetylation at the H3K18 locus (H3K18ac), thereby inhibiting the recruitment of bromodomain-containing protein 4 (BRD4) and subsequent oncogene transcription (50). Lactyltransferases catalyze the covalent conjugation of lactyl groups to lysine residues, while delactylases mediate the reversible demodification process (48, 51). The initiation of histone lactylation depends on the activity of lactyltransferases, among which proteins of the p300/CBP family are key executors. As dual-functional enzymes with both acetyltransferase and lactyltransferase activities, p300 can utilize lactyl coenzyme A (lactyl-CoA) as a substrate to transfer lactyl groups to histone lysine residues (52). In HEK293T cells models, p300 overexpression significantly elevates histone lactylation levels, whereas shRNA-mediated p300 knockdown reduces H3K18la to 35% of controls (53). Zhang’s team further demonstrated in bone marrow-derived macrophages (BMDMs) that p300 deficiency abolishes lactate-induced H3K18la modification and suppresses pro-inflammatory cytokine (e.g., IL-1β) expression (8, 35). This catalytic specificity is associated with the bromodomain of p300, which preferentially recognizes lactyl groups bearing hydroxyl groups (54). Zhao (55) et al. identified class I histone deacetylases (HDAC1-3) as efficient erasers for both L/D-lactyllysine. Cellular studies revealed HDAC1 specifically regulates nuclear histone lactylation modifications (such as H4K12la), while HDAC3 is responsible for eliminating non-histone lactylation in the cytoplasm. Moreover, HDAC3 exhibits higher selectivity for lactylation modifications over acetylation modifications, as its catalytic domain can recognize the spatial conformation of lactyl-lysine and preferentially remove lactyl groups (56). Lactylation modification exhibits multi-compartmental distribution and broad target specificity: although initially discovered in histones, it is widely present in the nucleus, Lysosome (57), mitochondria (58), endoplasmic reticulum (59), and cytoskeleton (60, 61) (Table 1), and can modify not only histones but also non-histone proteins. In 2020, Gao et al. (62) discovered through their research that lactylation modification can occur on nuclear histones, cytoplasmic kinases, and mitochondrial enzyme complexes. For example, lactylation at the K147 site of Aldolase A (ALDOA) occurs most frequently and negatively regulates glycolysis formation through feedback inhibition (49). Additionally, lactate inhibits the Warburg effect by activating Pyruvate Kinase M2 (PKM2). Lactylation at the K62 site activates enzyme activity via an allosteric effect, driving the conversion of macrophages from a pro-inflammatory phenotype (M1) to a repair phenotype (M2), thus promoting IL-10-mediated inflammation resolution and wound healing (63).
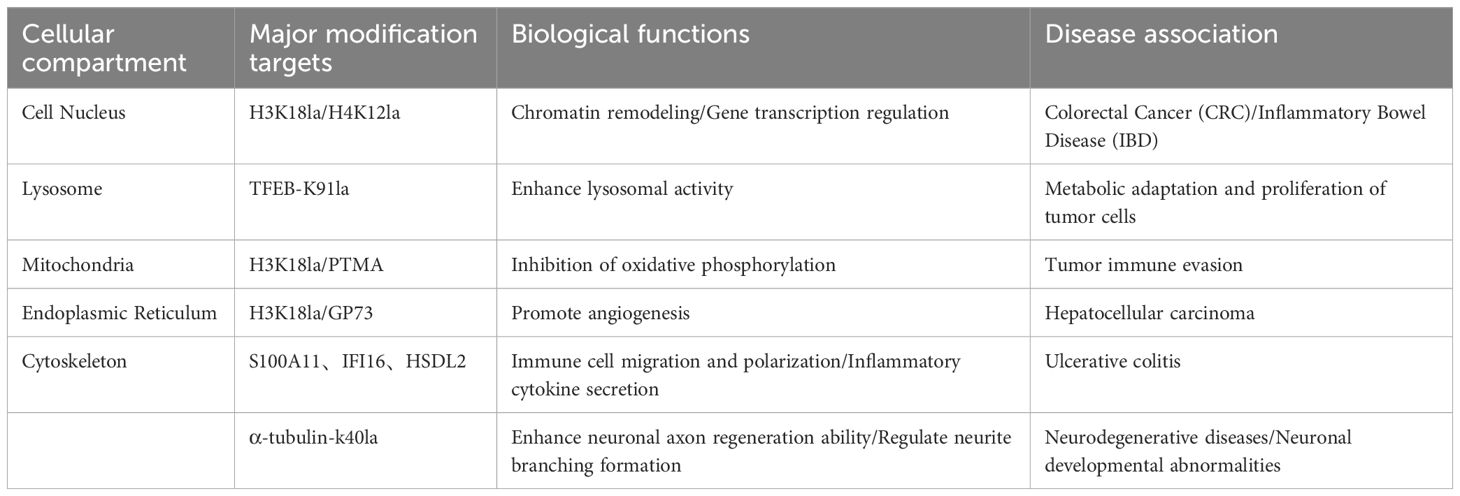
Table 1. Relationship between major modification targets and disease in different cellular compartments.
In the nuclear compartment, the main modification targets are H3K18la/H4K12la, which are involved in chromatin remodeling and gene transcription regulation. Dysfunction of these targets is associated with the development of colorectal cancer and inflammatory bowel disease. In the lysosomal compartment, TFEB-K91la serves as the primary modification target. By enhancing lysosomal activity, it promotes metabolic adaptation and proliferation of tumor cells. In the mitochondrial compartment, modifications of H3K18la and PTMA inhibit oxidative phosphorylation, thereby leading to tumor immune escape and affecting the tumor microenvironment. In the endoplasmic reticulum compartment, H3K18la modification and the GP73 target promote angiogenesis, a process closely linked to the progression of hepatocellular carcinoma. In the cytoskeletal compartment, modification targets such as S100A11, IFI16, HSDL2, and α-tubulin-K40la regulate immune cell migration and polarization, cytokine secretion, and enhancement of neuronal axon regeneration capacity, respectively. Abnormalities in these targets are associated with diseases such as ulcerative colitis and neurodegenerative disorders (57–60).
3 Macrophage heterogeneity and activation regulatory networks
Macrophages, as innate immune cells with phenotypic plasticity and functional heterogeneity, play pivotal roles in maintaining tissue homeostasis and immune regulation. Based on developmental origin and functional states, tissue macrophages can be categorized into: ① tissue-resident macrophages (TRMs) of embryonic origin with self-renewal capacity; ② monocyte-derived macrophages (MDMs) differentiated from infiltrating circulating monocytes (64, 65). Macrophage activation refers to the dynamic phenotypic reprogramming in response to microenvironmental stimuli, such as metabolic remodeling (e.g., lactylation) and epigenetic modifications, whereas macrophage polarization refers to the activation state at a specific point in time that determines their functional phenotype (66), while macrophage polarization refers to the activation state of macrophages at a single point in time (67). In the in vitro characterization of macrophages, they can be distinguished into the classical activated M1 phenotype and the alternatively activated M2 phenotype based on surface receptor expression, secretion profiles, and functional activity (68, 69). Of course, some scholars believe that there are more than two phenotypes for macrophages based on their activation states. Therefore, the classification of macrophage biological behaviors remains an area that requires further research (70).
Classically activated M1 macrophages refer to those activated by IFN-γ secreted by TH1 cells and LPS (which induces TNF-α) stimulation. Alternatively activated M2 macrophages refer to those activated by IL-4 and IL-13 secreted by TH2 cells following in vitro stimulation (67, 71). M1 macrophages are believed to be pro-inflammatory (65).They can secrete high levels of pro-inflammatory cytokines (such as TNF-α, IL-1, IL-6, IL-23, etc.), enhance the microbicidal activity, and play an important role in anti-tumor immunity. M1 macrophages increase their cytotoxic activity by producing substances like superoxide anion, oxygen radicals, and nitrogen radicals, thereby promoting the inflammatory response. However, prolonged M1 activation can lead to tissue damage. In contrast, M2 macrophages secrete a variety of anti-inflammatory factors (e.g., IL-10, mannose receptor C-type 1), inhibit the levels of pro-inflammatory cytokines, promote the resolution of inflammation, and exert immunosuppressive effects. They play a role in preventing excessive inflammation and promoting tissue repair (68, 72–74).
In addition, M2 macrophages activate TGF-β by promoting the Th2 response, further facilitating fibrosis, which is closely associated with tissue remodeling (75). Persistent M2 phenotype can suppress the immune system, potentially increasing the risk of secondary infections or tumor development (76, 77). It is important to note that M2 macrophages can be further classified into subgroups based on the different stimuli they receive and the transcriptional changes that occur. Notably, according to the stimuli received and the transcriptional changes that occur, M2-type macrophages can be further divided into multiple subsets, including M2a, M2b, M2c, and M2d, with each subset playing distinct roles in immune responses and tissue repair processes (78). Currently, the most widely studied is the M2a macrophage subgroup, which has the functions of sensing and clearing pathogens and tissue remodeling (79). Macrophages can be polarized into the M2a phenotype by IL-3/IL-4 cytokines. The binding of pathogen-associated receptors to PRR triggers activation of clearance activity and activates downstream signaling cascades, secreting IL-10 to inhibit inflammatory responses (80, 81). Fibrosis is considered a potential biomarker of M2 macrophages. M2a expresses high levels of Fibronectin, and the produced chitinase-like substances play important roles in tissue reorganization (65, 82). In addition, M2a macrophages can assist tumor cells in growth through the IL-4/STAT6-mediated pathway, and IL-4 released by tumor cells and M2a macrophages further promotes the polarization of M2 macrophages into M2a, thus forming a positive feedback pathway (83). M2b macrophages, characterized by both immunomodulatory and pro-inflammatory properties, are also referred to as regulatory macrophages. These cells are induced by the classical M2b inducers lipopolysaccharide (LPS) plus immune complexes (ICs). Unlike other M2 subsets, M2b macrophages express Fcγ receptors (FcγR), which drive the secretion of high levels of the anti-inflammatory cytokine IL-10 and low levels of IL-12. They predominantly skew Th1 cell responses toward Th2 cell responses through IL-4 secretion. Polarization of macrophages toward the M2b phenotype requires two stimuli and involves multiple signal transduction events mediated by NF-κB, PI3K/Akt, IRF, and MAPK pathways (79, 84). In tumor progression, M2b macrophages gradually occupy the M1 cell population through the CC L1/CCR8 axis, thereby forming an immunosuppressive microenvironment (85).M2c macrophages, also known as acquired inactivated macrophages (86), are macrophages stimulated by IL-10, TGF-β, or glucocorticoids, characterized by secreting pro-inflammatory cytokines IL-10 and TGF-β, as well as chemokines CCL16, CCL18, and CXCL13 (79). In terms of L-arginine metabolism, M2c macrophages share the same metabolic state as M2a and produce Arg1, participating in fibrotic repair and progression, as well as wound healing (84, 87). M2d is an M2 subgroup of macrophages polarized by IL-6 and LIF, also exhibiting typical cytokine production characteristics of M2 subgroups (IL-10 high, IL-12 low) (88, 89). During M2d polarization, IL-6 induces M2d macrophage differentiation by activating JAK-STAT3-mediated cell signal transduction. In this process, macrophages consume M-CSF in an autocrine manner, and IL-6 and LIF play important roles in promoting this M-CSF consumption (88, 89). Additionally, M2d-polarized macrophages stimulated by adenosine, IL-6 and tumor cells secrete proteases (such as MMP-2), cytokines (such as VEGF) and anti-inflammatory factors (such as TGF-β and IL-10) to promote angiogenesis and tumor immunosuppression (90).
Macrophages in the gastrointestinal tract are classified into three subtypes based on their function and location: Subtype I (monocyte-derived mature macrophages), Subtype II (monocyte-derived inflammatory macrophages), and Subtype III (self-maintaining macrophages). These subtypes are involved in maintaining mucosal homeostasis, mediating acute inflammation, and regulating intestinal motility (91). Subtypes I and II of gastrointestinal macrophages are primarily located in the lamina propria. They are replenished by monocytes from the bloodstream and undergo a cascade of differentiation from P1 (newly recruited monocytes) to P4 (fully differentiated resident macrophages) (Figure 2). This differentiation process, which involves sequential stages from P1 to P4, is referred to as the monocyte waterfall (68, 93). Circulating Ly6ChiCCR2+ monocytes (P1) migrate to lamina propria via CX3CR1, sequentially acquire MHCII expression (P2), downregulate Ly6C (P3), and differentiate into mature CX3CR1highCD64+ macrophages (P4) over 7 days (91, 93–95). Tamoutounour et al. reported that macrophage differentiation is attenuated during inflammation, with macrophages in the lamina propria and lymphatic vessels only reaching the P2 stage (96). P2-stage macrophages express inducible nitric oxide synthase (iNOS) and secrete pro-inflammatory cytokines. However, the lack of CX3CR1 expression hinders the generation of fully tolerogenic IL-10-producing macrophages, leading to a predominantly pro-inflammatory phenotype (97). Studies have shown that mice deficient in CX3CR1+ cells are more susceptible to intestinal inflammation and more prone to developing intestinal inflammatory diseases (98). Subtype III is specifically found in the lamina propria and the outer layer of the muscularis in the gut. It is mainly composed of tissue-resident self-renewing macrophages, with additional replenishment from circulating monocyte-derived macrophages. Studies have shown that subtype III macrophages interact with the enteric nervous system (ENS), regulating intestinal motility and secretion. When subtype III cells are depleted, it can lead to a range of issues such as damage to the submucosal vascular network and weakened intestinal motility (78, 79, 99).
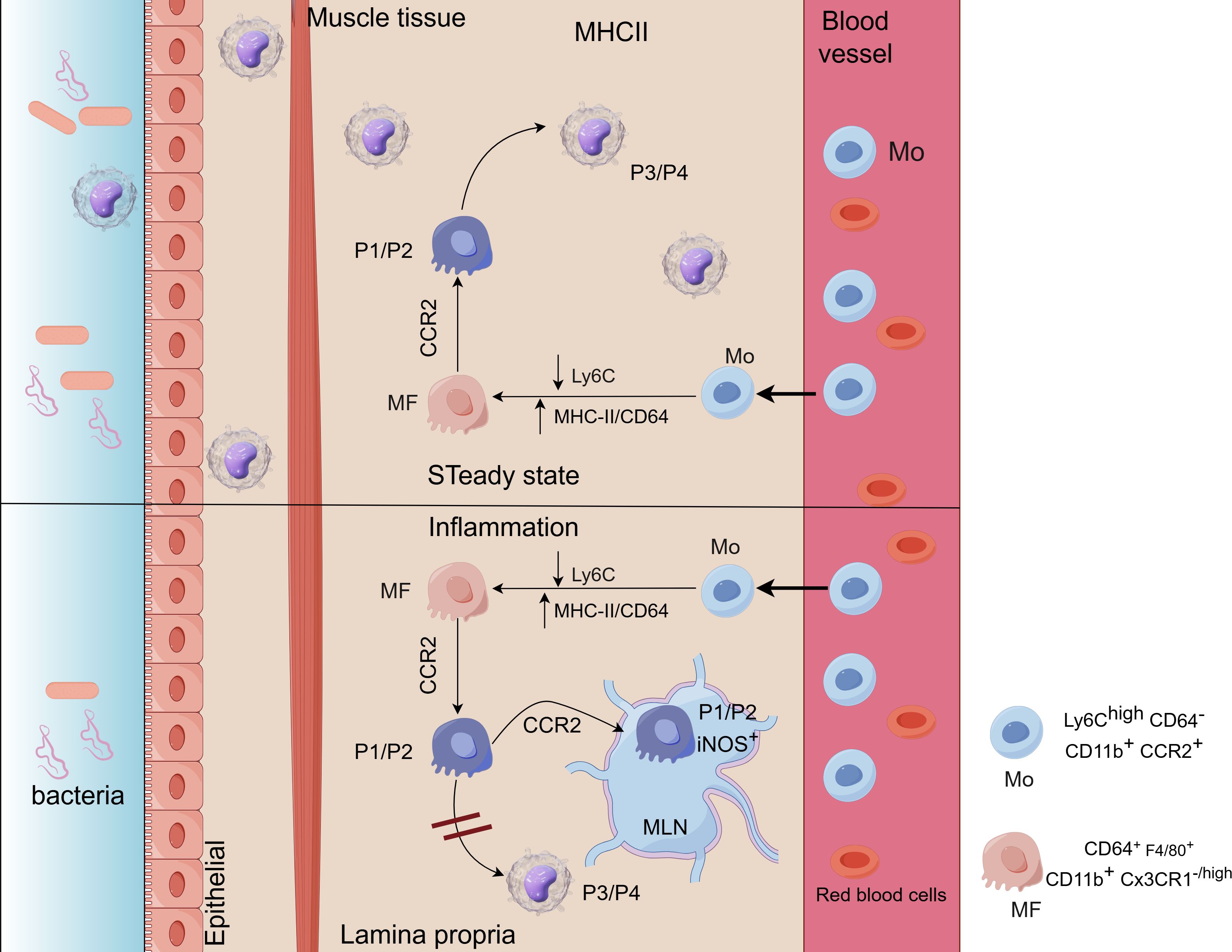
Figure 2. (Image 2 By Figdraw) Ly6Chi blood monocytes (Mo) enter the intestinal lamina propria (LP) where they downregulate Ly6C and acquire the expression of MHC-II and CD64, becoming macrophages (MF). Up: under steady state conditions, Mo enter to the LP in a CCR2-dependent manner. They first go through P1/P2 stages (CD64low/+F4/80negCX3CR1−/int) to finally become P3/P4 tolerogenic IL-10-producing monocytes (MF) (CD64+F4/80+CX3CR1high). Down: during inflammation MF differentiation is blunted, reaching only the P2-MF stage and becoming pro-inflammatory iNOS+ TNFα+ MF (92).
In colorectal cancer (CRC) progression, tumor-associated macrophages (TAMs) undergo phenotypic remodeling into M2-like immunosuppressive subsets, contributing to immune evasion and promoting tumor growth. Single-cell transcriptomics revealed significant upregulation of the immune checkpoint molecule CD155 in TAMs. This upregulation promotes IL-10 and TGF-β secretion through TIGIT-CD155 signaling, while suppressing IL-12p70 production. This creates a pro-tumorigenic microenvironment (100–102). Xu et al. (2021) first demonstrated that Fusobacterium nucleatum activates TLR4/NF-κB/miR-1322 signaling cascade to upregulate CCL20, driving monocyte differentiation into CCR6+ TAMs, which subsequently induce M2 polarization through IL-6/STAT3 pathway, upregulating Arg1 levels and down regulating iNOS levels, ultimately enhancing CRC liver metastasis (102, 103).
In gastric cancer (GC) microenvironments, M2 TAMs suppress CD8+ T cell cytotoxicityand NK cell IFN-γ secretion via PD-L1/IDO dual signaling. Clinical specimen analysis revealed positive correlation between TAM density and VEGF-C expression, promoting lymphangiogenesis (104, 105). Conversely, M1 macrophages induce GC cell ferroptosis through iNOS-dependent ROS production, while anti-CSF-1R antibody treatment significantly reduces TAM infiltration and reverses M2/M1 ratio (105).
According to statistics (106), more than half of patients with Crohn’s disease experience complications related to intestinal fibrosis. During Crohn’s disease (CD)-associated intestinal fibrogenesis, adherent-invasive E. coli (AIEC) activates TLR4/MYD88 signaling in intestinal epithelial cells, causing aberrant let-7b downregulation and subsequent loss of TGF-βR2 inhibition, driving macrophage transition towards profibrotic phenotypes. Animal models confirm that let-7b mimic administration reduces collagen deposition (106–109).
4 Metabolic reprogramming in macrophage polarization
The macrophage polarization process is accompanied by significant reprogramming of glucose metabolism and plays an important role in this process, characterized by dynamic shifts in energy metabolism pathways between M1 and M2 phenotypes. Emerging evidence suggests that metabolic reprogramming not only supplies energy and biosynthetic precursors but also determines cellular functional phenotypes through metabolism-epigenetics crosstalk. M1 macrophages rely predominantly on glycolysis and the pentose phosphate pathway (PPP), characterized by upregulated activities of HK2 and PFKFB3. Oxidative phosphorylation (OXPHOS) is suppressed via HIF-1α-mediated activation of PDK1, thereby disrupting the tricarboxylic acid (TCA) cycle. OXPHOS is suppressed via HIF-1α-mediated PDK1 activation, disrupting TCA cycle (110, 111). M2 macrophages exhibit enhanced OXPHOS and promotes fatty acid β-oxidation (FAO) via CPT1A, generating 2.5-fold more acetyl-CoA to support anti-inflammatory cytokine secretion (110, 112). HIF-1α is markedly upregulated in LPS-activated M1 macrophages, which binds promoters of glycolytic genes (e.g., GLUT1, LDHA) to enhance glycolysis and induces pro-inflammatory cytokines (IL-1β, TNF-α) (110). The pyruvate kinase M2 (PKM2) dimer forms a complex with HIF-1α, translocates to the nucleus to inhibit STAT1 phosphorylation, downregulating M1 markers (iNOS, IL-6) while promoting M2 signature gene (Arg1, IL-10) transcription (113, 114). In M1 macrophages, glycolytic activity is significantly enhanced, and ATP generation proceeds rapidly through the glycolysis pathway to provide sufficient energy for their antimicrobial and pro-inflammatory functions. This metabolic pattern is similar to the Warburg effect in tumor cells, where glycolysis is favored for rapid energy production despite adequate oxygen supply (110). The rapid progression of glycolysis leads to the accumulation of lactate, which not only provides energy for the cells but is also exported extracellularly via MCT4, altering the extracellular pH. This, in turn, inhibits T cell proliferation. At this stage, lactate can shuttle between cells and cellular compartments, serving as a metabolic substrate to provide energy (113). Polarization drives divergent arginine metabolism pathways: M1 macrophages express iNOS, while M2 macrophages express Arg1. Arginine is converted by iNOS and Arg1 into NO (nitric oxide), citrulline, ornithine, and urea, respectively (113).
5 Molecular mechanisms of lactylation in macrophage activation
Unlike histone methylation and acetylation, lactate, as a key metabolic regulatory molecule, regulates macrophage M1 phenotype polarization and the transition from M1 to M2 phenotypes through histone lactonylation modification and synergistic interaction with multiple signaling pathways (Figure 3) (115, 116).The “lactate clock” model proposed by Zhang et al. Zhang et al. proposed the “lactate clock” model, showing that M1-type macrophages accumulate lactate via the Warburg effect in the early phase of inflammation; subsequently, both extracellular lactate taken up by MCTs and enzymatically converted lactate-CoA can be enzymatically reacted to generate lactoyl-CoA, which can inhibit the expression of M1-related genes (e.g., TNF-α) and activate the repair program by mediating histone lactonylation via the p300/p53 complex (8, 113). This shifts macrophages from the P2 phase to the P3/P4 phase, enhances CX3CR1 expression, and reduces the secretion of inflammatory molecules, as well as the polarization of M2 macrophages. Zhang et al. (117) In pathological states such as spinal cord injury (SCI), microglia (brain-resident macrophages) have reduced CX3CR1 gene expression, decreased glycolysis, and reduced lactate production, leading to down-regulation of lactylation-related genes (e.g., Fabp5, Lgals1) expression, which results in an M1-like pro-inflammatory phenotype. While exogenous supplementation of lactate can inhibit the expression of pro-inflammatory genes (e.g., IL-1β, iNOS) in microglia through lactoylation modification, prompting the expression of CX1CR1 gene, and at the same time activate anti-inflammatory repair genes (e.g., Arg-1, CD206), promoting their polarization to M2 type (anti-inflammatory repair phenotype), thus reducing inflammation and improving the recovery of neurological function (117). In addition, related studies have found that CX3CL1 expression levels are gradually upregulated with increasing lactate concentration in gastric cancer cell lines and colon cancer cell lines (118). It is specifically enriched in the promoter region of repair genes during the late inflammatory phase, and promotes IL-10 secretion by inhibiting NF-κB phosphorylation, while activating the STAT6-driven transcription of the M2-type marker arginase-1, thereby attenuating the onset of inflammation (8, 115, 119). IL-4-induced M2-type polarization utilizes LDH1 to convert lactate into pyruvate, generating acetyl-coenzyme A, driving the histone acetylation-dependent gene expression (120). Lactate promotes vascular endothelial growth factor (VEGF) secretion by activating the mTORC1 signaling pathway and inhibiting ATP6V0d2-mediated degradation of HIF-2α lysosomes (121); lactate activates the mTORC2-AKT and ERK signaling pathways in tumor-associated macrophages (TAMs) leading to the up-regulation of PD-L1 expression (122); and lactate induces the expression of HF2α in tumor-associated macrophages (TAMs) through MCT1 endocytosis. MCT1 endocytosis induces K28 lactonylation of HMGB1 and inhibits SIRT1 deacetylase activity, thereby enhancing the transcription of DNA repair genes (123); other studies have found that lactic acid modifies lipopolysaccharides (LPS) in a time-dependent manner to activate macrophage histones and promote gene expression in M2-like macrophages, but interestingly, signature genes of traditional M2-type macrophages (However, it is interesting to note that the signature genes of traditional M2 type macrophages (e.g., Mrc1, Fn1, Retnla, etc.) are not significantly up-regulated, or may even be down-regulated, by LPS stimulation, which induces IL-6 secretion through the MyD88-dependent signaling pathway. IL-6 activates JAK2-STAT3 signaling through an autocrine/paracrine mode, which in turn promotes the expression of Arg1 and regulates arginine metabolism, resulting in macrophage polarization to the M2 phenotype and the process is dominated by glycolysis, and this is a major factor in the development of the M2 phenotype. This process is a specific glycolysis-dominated phenomenon independent of typical M2 polarization (124, 125). In sepsis models, lactate is transported from the extracellular space into macrophages via MCT1, which regulates the acetylation level of HMGB1 by inhibiting β-arrestin2-mediated nuclear translocation of p300/CBP acetyltransferase via the Hippo/YAP signaling axis (8, 24). Lactoylation of methyl-CpG-binding protein 2 (MeCP2 K271) leads to increased chromatin accessibility and transcriptional repression of RUNX1 in cellular models, promotes pro-repair M2 macrophage polarization, and maintains atherosclerotic plate stabilization (126). BCAP articulinic protein induces lactoylation of H4K12 via MyD88-IRAK4 signaling to activate ALOX15-mediated inflammatory reduction (127); furthermore, lactoylation activates DRP1, which induces mitochondrial fission to drive anti-inflammatory conversion (128). A study in traditional Chinese medicine found that Ge Gen Baicalin Lian Tang (GQD) inhibited HDAC3 and reduced lactoylation levels to alleviate DSS-induced colitis (129). Low pH in TME can independently alter macrophage phenotype and function. Lactate can promote lactoylation of lysine 18th position of histone H3 and activate CCL18 expression through macrophage Gpr132-mediated signaling pathway, while inducing polarization of M2 phenotype, which in turn promotes tumor proliferation and metastasis (8, 130). In addition, under hypoxic conditions, tumor necrosis factor superfamily member 9 (TNFSF9) expression is upregulated through a histone lactoylation-dependent mechanism, inducing polarization of M2-type macrophages and leading to malignant progression of tumor cells (131).
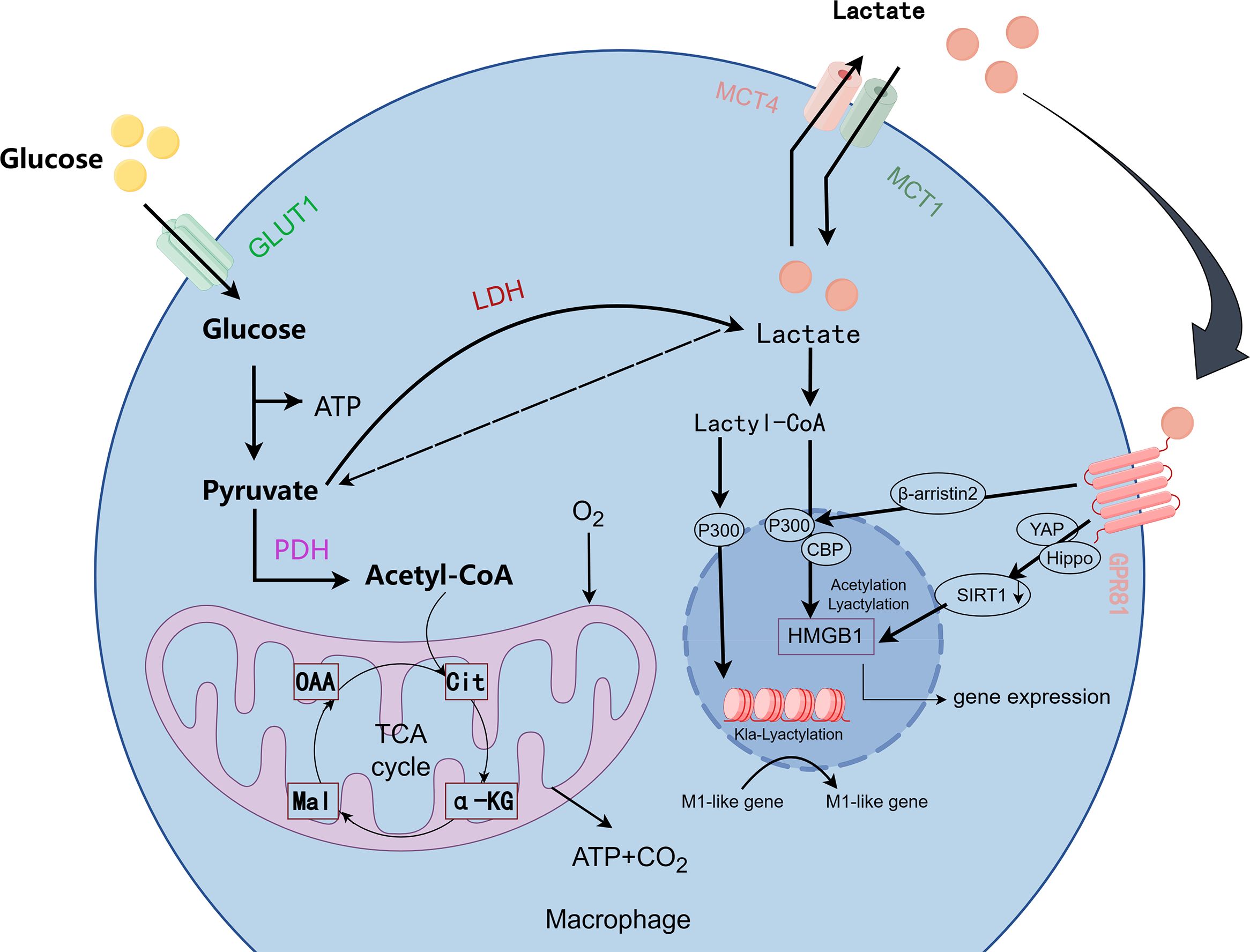
Figure 3. Metabolic pathways and lactylation in macrophages. (Image 3 by Figdraw) In the metabolic process of macrophages, glucose is converted into pyruvate, which then enters the mitochondria to generate ATP, or, under hypoxic conditions, is converted into lactate via lactate dehydrogenase (LDH). Lactate is exported out of the cell through the MCT1 and MCT4 transporters and can be converted into lactyl-CoA. Lactyl-CoA mediates the lactylation of proteins, including HMGB1, which regulates gene expression associated with M1 macrophage activation. Additionally, key signaling molecules such as GPR81, YAP, Hippo, and SIRT1 are involved in regulating these metabolic and immune responses.
In the tumor microenvironment, lactate suppresses anti-tumor immune responses through negative feedback regulation of innate and adaptive tumor-infiltrating immune cells, and both the acidity of the TME and the increase in lactate affect macrophage polarization (132, 133). In glycolytic tumors, lactate concentrations can be elevated up to 40 mmol/g (134). Lactate produced by the Warburg effect in cancer cells becomes a key signal in the TME to induce M2 macrophage polarization (135). In normal tissues, pHi (intracellular pH) and pHe (extracellular pH) are around 7.2-7.4, compared to which tumors are able to maintain pHi around 7.4 and pHe down to around 6.5 (136). At pH 6.8, macrophage acidosis decreases the gene expression of pro-inflammatory markers Nos2, Ccl2 and Il-6 in IFN-γ/LPS-polarized macrophages (M1), while increasing the expression of anti-inflammatory markers Cd206, Arg1 and Reltna, as well as angiogenesis-related genes in IL-4-polarized macrophages (M2) (133). Moreover, the expression of CD206 and Arg1 in tumor-associated macrophages (TAM) was significantly reduced at physiological level of PH=7.4. The phosphorylation of AKT/ERK (mTOR downstream target) in macrophages was found to be enhanced in a lactate concentration-dependent manner; low concentrations of lactate (0–2 mmol/L) did not stimulate AKT-ERK well, while high concentrations of lactate (5–20 mmol/L) significantly activated the AKT-ERK signaling pathway; oxalate, a lactate inhibitor, reduced the concentration of LA in conditioned medium (CM) and counteracted high concentrations of LA. concentration in conditioned medium (CM) and counteracted the stimulatory effect of high LA concentration on this signaling pathway (137).
In pancreatic cancer, tumor cells were able to upregulate the lactate/METTL3/OAS3 (2,5’-oligoadenylate synthase 3) axis promoting M2d polarization (138). Moreover, in the context of lupus erythematosus, when macrophages are polarized to the M2b phenotype, notable alterations in macrophage glucose metabolism transpire, with enhanced glycolysis and the conversion of pyruvate to lactate by lactate dehydrogenase, leading to elevated lactate levels both intracellularly and extracellularly (139). Although the above shows that lactate produces some effects in M2b and M2d subtype polarization, the mechanism of lactate modification on the polarization of each subtype of M2 has yet to be thoroughly investigated. It is important to explore the mechanistic relationship between lactate modification on M2 macrophage subtypes for future target therapy of clinical diseases.
Triplex motif-containing protein 29 (TRIM29), a member of the TRIM family, is abundantly present in macrophages (140), mediating DNA binding, protein-protein interactions, and ubiquitin ligases, and is expressed in cancer, diabetic nephropathy, and immune-related disorders (141). PERK is a key metabolic hub for macrophage immune-suppressant function, and deletion of PERK signaling prevents mitochondrial respiration and lipid oxidation in M2 macrophages, impeding M2 macrophage polarization and promoting macrophage immunosuppression (142). Both play a role in inflammatory diseases of the gastrointestinal tract. One study (143) found that TRIM29 can control intestinal RNA virus-induced intestinal inflammation by targeting the NLRP6 and NLRP9b signaling pathways, while TRIM29 can also regulate PERK (144), which drives glucose metabolism and promotes macrophage immune-suppressing activity through histone lactylation (145), and thus regulates gastrointestinal inflammation. Thus, TRIM29 could alleviate gastrointestinal diseases by modulating inflammasome activation and lactylation-mediated PERK-ER stress immunosuppression. Su et al. found that G6PT-deficient macrophages induced lactate accumulation and reduced the activation of NLRP3 inflammatory vesicles through the lactylation-ALKBH5-m6A-NLRP3 pathway an enhancement of ALKBH5 expression and alleviate IBD (146, 147). In addition, in characteristic dermatitis (AD) studies, UV-treated riboflavin was found to inhibit the activation of NLRP3 inflammatory vesicles in macrophages by inhibiting the H3K9 lactation of NLRP3 and ASC, leading to a reduction in IL-1β secretion and M1 macrophage polarization, as well as a reduction in TSLP secretion by keratin-forming cells to attenuate AD progression (148).
6 Pathological mechanisms of lactylation-regulated macrophage activation in gastrointestinal diseases
Lactylation plays a crucial role in regulating macrophage activation and polarization, particularly in gastrointestinal diseases, where metabolic changes in lactate are closely linked to macrophage function. Gastrointestinal diseases such as ulcerative colitis (UC), Crohn’s disease (CD), and gastrointestinal cancers are strongly associated with immune cell dysfunction and inflammatory responses. Lactylation, by modulating macrophage immune responses, provides a new direction for research into the treatment of these diseases (Table 2).
6.1 Inflammatory bowel disease
Inflammatory bowel disease (IBD), which encompasses ulcerative colitis (UC) and Crohn’s disease (CD), is an incurable chronic inflammatory gastrointestinal disorder characterized by chronic intestinal inflammation, immune dysregulation, and prominent metabolic-epigenetic crosstalk (149, 150). While causing damage to the intestine, it can also significantly impair the patient’s quality of life (151). Single-cell metabolomics reveals glycolytic reprogramming in intestinal macrophages of IBD patients (152). Studies have found that histone lactylation modifications enhance the expression of genes involved in inflammatory responses (153). H3K18 lactylation enhances YTHDF2/Kcnk6 complex stability, activating NLRP3 inflammasome (2.3-fold ASC oligomerization) and promoting IL-1β secretion (154). In ulcerative colitis, lactate in its ionic form downregulates cyclic AMP (cAMP) and protein kinase A (PKA) signaling through the GBR81 receptor, inhibiting the expression of the M1 marker iNOS. At the same time, it activates PPARγ to promote the transcription of M2-type Arg1, thereby alleviating the occurrence of inflammation (155). Lactate accumulation in macrophages lactylates the PKM2 K305 site to stabilize the tetrameric conformation, providing negative feedback inhibition of glycolytic flux and blocking M1 polarization (27, 63). Additionally, studies have found that TAK-242 inhibits the recruitment of MyD88 and TRIF to TLR4 by binding to the TIR domain of MyD88/TRIF, reducing NF-κB phosphorylation and MAPK activation. At the same time, it promotes the expression of anti-inflammatory genes mediated by H3K9la, facilitating tissue repair (153, 156, 157). It has been reported that the lactate content in the gastrointestinal tract of Crohn’s disease (CD) patients is significantly elevated (158). Macrophages exhibit high levels of lactylation, and the expression of SIRT1 is notably reduced, negatively correlating with the degree of oxidative phosphorylation. It is also negatively correlated with the infiltration of pro-inflammatory immune cells, such as Th17 cells, which are regulated by the monocarboxylate transporter encoded by SLC16A1 (24, 113). In a study by Sun et al. (159) on lactate-producing yeast in ulcerative colitis, it was found that lactate from the yeast upregulates NLRP3 transcription via MCT1, while inhibiting H3K9 acetylation and promoting H3K18 lactylation, thus alleviating DSS-induced colitis damage. Additionally, under the guidance of traditional Chinese medicine theory, Gegen Qinlian Decoction (GQD) is an effective prescription for treating ulcerative colitis. Xu et al. experimentally verified that GQD inhibits HDAC3 activity, reducing histone H3/H4 lactylation levels and reversing the imbalance between M1/M2 polarization, providing molecular evidence for the use of traditional Chinese medicine in treating IBD (129).
6.2 Lactylation regulatory mechanisms in postoperative ileus
Postoperative ileus (POI) is one of the common complications following gastrointestinal surgery. The occurrence of POI can affect gastrointestinal function and prolong postoperative recovery time for patients (160–162). According to a report by Grocott, 92% of general surgery patients experience gastrointestinal postoperative complications (163). Surgical trauma activates resident macrophages in the intestinal muscular layer, leading to the release of cytokines and chemokines, and the recruitment of leukocytes (160, 164). Among these, the M1 macrophages, in response to mechanical stimuli, release circulating chemokine ligand 1 (CXCL1) via the TLR4/MyD88 signaling axis. The increase in CXCL1 leads to increased intestinal tension, a reduction in contraction amplitude, and neutrophil infiltration (163). Postoperative inflammation enhances glycolysis, thereby increasing lactate production. Lactylation of H3K18, by enhancing the accessibility of the VCAM1 promoter, activates the AKT/mTOR signaling pathway, promoting the continuous secretion of CXCL1. This leads to the formation of a malignant cycle of inflammation and motility dysfunction (55, 165, 166). STAT3 K685 lactylation strengthens DNA binding, promoting IL-6 autocrine loops and enteric glia activation (125). Therefore, we can treat or prevent postoperative ileus by employing therapeutic strategies that target lactylation regulation:1. Inhibiting MCT1-mediated lactate influx2. Blocking HDAC3-dependent histone lactylation3. Modulating STAT3 lactylation.
6.3 Lactylation regulatory network in gastrointestinal malignancies
Gastrointestinal cancers, including colorectal cancer (CRC) and gastric cancer (GC), remain leading causes of global cancer-related mortality (167). Epigenetic alterations and metabolic reprogramming play pivotal roles in oncogenesis (168). The tumor microenvironment (TME), a heterogeneous ecosystem, harbors diverse tumor-associated macrophage (TAM) populations classically categorized into anti-tumor M1 and pro-tumorigenic M2 subtypes (169, 170). Emerging evidence reveals that pro-tumor TAMs exhibit cancer type-specific molecular signatures (171), with lactate gradients potentially driving their polarization through GPR81-mediated mTORC1 signaling (135). Lactate, a key glycolytic byproduct in cancer metabolism, functions as an epigenetic modulator via histone lysine residue lactylation, thereby influencing tumor progression (8, 22, 172, 173). Notably, crosstalk between lactylation and histone acetylation has been identified; for instance, H3K18 lactylation may competitively inhibit HDAC3 activity, amplifying H3K27 acetylation to synergistically activate oncogenic transcription (8).
In CRC, Li et al. demonstrated that Warburg effect-derived lactate promotes H3K18 lactylation, suppresses macrophage RARγ expression, disrupts TRAF6 interactions, elevates IL-6 levels, and activates STAT3 signaling, collectively driving colorectal tumorigenesis (124). Single-cell metabolomics further revealed that M2-TAM subsets preferentially uptake lactate via upregulated monocarboxylate transporter 1 (MCT1), relying on lactate dehydrogenase A (LDHA) to sustain their pro-tumor phenotype (174). Proprotein convertase subtilisin/kexin type 9 (PCSK9) enhances colon cancer progression by modulating epithelial-mesenchymal transition (EMT) and PI3K/AKT signaling while skewing macrophage polarization (175). Additionally, gut microbiota-derived lactate reprograms ATM glycolysis, induces RIG-I K852 lactylation to inhibit RIG-I-MAVS-NF-κB signaling, and synergizes with cathepsin K to establish an immunosuppressive niche favoring CRC metastasis (176, 177). It is worth noting that this microorganism-host metabolic interaction is cancer-type specific. For example, in ductal carcinoma of the breast, stellate cells, rather than microorganism-derived lactate, drive TAM immunosuppressive function through the CCL5-CCR5 signaling axis (178).
While RIG-I lactylation promotes M2-like polarization by rewiring macrophage metabolism and inflammatory pathways (176), paradoxical evidence suggests that localized lactate accumulation at high concentrations may transiently suppress tumor growth via caspase-1-mediated pyroptosis (179). Concurrently, diminished anti-tumor activity of regulatory T cells (Tregs) and CD8+ T cells further compromises immune surveillance. LDHA inhibitors (e.g., FX-11) reverse TAM polarization and synergize with PD-1 blockade, offering novel combinatorial strategies for gastrointestinal malignancies. In GC models, lactate-induced H3K18 lactylation upregulates VCAM1 transcription, activates AKT-mTOR signaling, enhances CXCL1 secretion, and expands GC-derived mesenchymal stem cells and M2 macrophages, collectively accelerating gastric cancer progression (55). Nevertheless, technical limitations persist, particularly the lack of spatial metabolomics integrated with single-cell transcriptomics to resolve TAM metabolic heterogeneity (180).
The role of lactate in different diseases and its potential therapeutic targets. In inflammatory bowel disease (IBD), lactate inhibits the pro-inflammatory activity of M1 macrophages by interacting with GPR81 and promotes the reparative transformation of M2 macrophages, thereby alleviating the inflammatory response (155). In postoperative intestinal obstruction (PIO), increased lactate leads to histone lactylation, which promotes CXCL1 secretion through the AKT-mTOR signaling pathway, increasing the risk of inflammation and obstruction (163). In gastrointestinal tumors, lactate accumulation in the tumor microenvironment promotes the transformation of macrophages to the M2 phenotype, enhancing tumor growth and metastasis. Potential therapeutic targets include GPR81, the lactate/LDH1 pathway, VCAM1, and the AKT-mTOR signaling pathway (55).
6.4 Bacterial and viral infectious gastrointestinal diseases
Unlike non-infectious gastrointestinal diseases, lactation modifications also play an important role in the pathogenesis of bacterial and viral infectious gastrointestinal diseases by controlling macrophage polarization. In recent years, it has been demonstrated that viruses and bacteria are capable of disrupting host metabolic homeostasis during the first phase of infection, specifically the “Warburg” effect (181).There seems to be a relationship between lactonization and bacterial metabolism. One study (182) found that lysine lactonization can regulate the metabolic pathways of Streptococcus mutans. And when we used some catalysts to promote lysine lactylation in E. coli (183), we found that E. coli enhanced the production of lactic acid, which mediates the polarization of M2 macrophages through inhibition of nuclear factor κB gene binding, and undergoes a pro-inflammatory response that leads to liver metastasis of colorectal cancer (176). Additional studies (184) have also found that protein lactylation modulates the intestinal microflora, leading to cancer cell migration and metastasis. Therefore, it has been investigated (159) that by modulating the intestinal microbiota, regulating macrophage polarization status and inhibiting the expression of pro-inflammatory cytokines, inhibiting the over-activation of NLRP3 inflammatory vesicles and the downstream caspase-1 pathway in macrophages, and, at the same time, promoting protein lactonization, attenuates inflammation in ulcerative colitis.There is an association between lactonization and the viral life cycle. A study (185–188) identified a correlation between lactation and the replication and reactivation of herpes simplex virus, severe fever with thrombocytopenia syndrome virus, and Kaposi’s sarcoma-associated herpesvirus, with many of these viruses increasing cellular lactate levels. It has also been found that viruses modulate protein lactylation, leading to disease progression (192). The two appear to be a reciprocal relationship. However, there are no studies that have found any relationship between lactic acidification and viruses associated with gastrointestinal diseases, which could be a direction for future research.
7 Conclusion
Since the groundbreaking discovery of histone lactylation by the Zhang (8) research team in 2019, this field has rapidly transitioned from foundational mechanistic exploration to translational therapeutic development. Emerging evidence has systematically elucidated the dual roles of lactate: functioning not only as a metabolic intermediate to facilitate energy supply but also as an epigenetic regulator that remodels the immune microenvironment through lactylation. As central effector cells of the innate immune system, macrophages exhibit spatiotemporally specific regulation of polarization states (M1/M2 dynamic equilibrium) during gastrointestinal pathogenesis, with lactylation acting as a pivotal mediator via metabolic-epigenetic crosstalk mechanisms. This review comprehensively discusses the impact of lactate-mediated lactylation on macrophage polarization in gastrointestinal diseases. Deciphering the mechanistic interplay between lactylation and macrophage polarization holds significant promise for developing innovative therapeutic strategies.
As a nascent research direction, several critical challenges remain unresolved:The precise regulatory mechanisms underlying lactylation-dependent modulation of macrophage polarization require further elucidation. The regulatory networks governing lactylation, particularly the identification of tissue-specific enzymatic systems (e.g., lactyltransferases and delactylases), remain incompletely characterized. The interplay between lactylation and other post-translational modifications (PTMs), such as acetylation and succinylation, demands systematic investigation to clarify their synergistic or antagonistic effects. Biomarker discovery based on lactylation signatures for disease staging and therapeutic monitoring represents an unmet need. Whether lactylation modifications could provide novel insights into the therapeutic mechanisms of traditional Chinese medicine (TCM) warrants interdisciplinary exploration.
In conclusion, advancing our understanding of lactylation-mediated macrophage polarization in gastrointestinal pathophysiology will accelerate the identification of druggable targets and the development of combinatorial therapeutic regimens, ultimately opening new frontiers for the treatment of gastrointestinal malignancies.
Author contributions
XZC: Methodology, Writing – original draft. YiZ: Writing – review & editing. XQC: Writing – review & editing. GX: Methodology, Writing – review & editing. JL: Investigation, Writing – original draft, Writing – review & editing. CX: Writing – original draft. CZ: Writing – original draft. YoZ: Supervision, Writing – review & editing. XY: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Shandong Provincial Health Commission (Grant No. Z-2023025).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ranjbar R, Ghasemian M, Maniati M, Khatami SH, Jamali N, and Taheri-Anganeh M. Gastrointestinal disorder biomarkers. Clin Chim Acta. (2022) 530:13–26. doi: 10.1016/j.cca.2022.02.013
2. Keely S, Walker MM, Marks E, and Talley NJ. Immune dysregulation in the functional gastrointestinal disorders. Eur J Clin Invest. (2015) 45:1350–9. doi: 10.1111/eci.12548
3. Andréasson K, Ohlsson B, and Mandl T. Elevated levels of faecal calprotectin in primary Sjögren’s syndrome is common and associated with concomitant organic gastrointestinal disease. Arthritis Res Ther. (2016) 18:9. doi: 10.1186/s13075-015-0907-8
4. Häuser W and Andresen V. Funktionelle gastrointestinale Störungen [Functional gastrointestinal disorders. Dtsch Med Wochenschr. (2022) 147:595–604. doi: 10.1055/a-1554-1739
5. Siegel RL, Kratzer TB, Giaquinto AN, Sung H, and Jemal A. Cancer statistics, 2025. CA Cancer J Clin. (2025) 75:10–45. doi: 10.3322/caac.21871
6. Bruner LP, White AM, and Proksell S. Inflammatory bowel disease. Prim Care. (2023) 50:411–27. doi: 10.1016/j.pop.2023.03.009
7. Guan Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J Immunol Res. (2019) 2019:7247238. doi: 10.1155/2019/7247238
8. Zhang D, Tang Z, Huang H, Zhou G, Cui C, Weng Y, et al. Metabolic regulation of gene expression by histone lactylation. Nature. (2019) 574:575–80. doi: 10.1038/s41586-019-1678-1
9. Xin Q, Wang H, Li Q, Liu S, Qu K, Liu C, et al. Lactylation: a passing fad or the future of posttranslational modification. Inflammation. (2022) 45:1419–29. doi: 10.1007/s10753-022-01637-w
10. Fan H, Yang F, Xiao Z, Luo H, Chen H, Chen Z, et al. Lactylation: novel epigenetic regulatory and therapeutic opportunities. Am J Physiol Endocrinol Metab. (2023) 324:E330–8. doi: 10.1152/ajpendo.00159.2022
11. Xie Y, Hu H, Liu M, Zhou T, Cheng X, Huang W, et al. The role and mechanism of histone lactylation in health and diseases. Front Genet. (2022) 13:949252. doi: 10.3389/fgene.2022.949252
12. Wei Y, Guo H, Chen S, and Tang XX. Regulation of macrophage activation by lactylation in lung disease. Front Immunol. (2024) 15:1427739. doi: 10.3389/fimmu.2024.1427739
13. Wang G, Zou X, Chen Q, Nong W, Miao W, Luo H, et al. The relationship and clinical significance of lactylation modification in digestive system tumors. Cancer Cell Int. (2024) 24:246. doi: 10.1186/s12935-024-03429-8
14. Qu J, Li P, and Sun Z. Histone lactylation regulates cancer progression by reshaping the tumor microenvironment. Front Immunol. (2023) 14:1284344. doi: 10.3389/fimmu.2023.1284344
15. Wang N, Wang W, Wang X, Mang G, Chen J, Yan X, et al. Histone lactylation boosts reparative gene activation post-myocardial infarction. Circ Res. (2022) 131:893–908. doi: 10.1161/CIRCRESAHA.122.320488
16. Ngambenjawong C, Gustafson HH, and Pun SH. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv Drug Delivery Rev. (2017) 114:206–21. doi: 10.1016/j.addr.2017.04.010
17. Li M, Yang Y, Xiong L, Jiang P, Wang J, and Li C. Metabolism, metabolites, and macrophages in cancer. J Hematol Oncol. (2023) 16:80. doi: 10.1186/s13045-023-01478-6
18. Viola A, Munari F, Sánchez-Rodríguez R, Scolaro T, and Castegna A. The metabolic signature of macrophage responses. Front Immunol. (2019) 10:1462. doi: 10.3389/fimmu.2019.01462
19. Grainger JR, Konkel JE, Zangerle-Murray T, and Shaw TN. Macrophages in gastrointestinal homeostasis and inflammation. Pflugers Arch. (2017) 469:527–39. doi: 10.1007/s00424-017-1958-2
20. Oya Y, Hayakawa Y, and Koike K. Tumor microenvironment in gastric cancers. Cancer Sci. (2020) 111:2696–707. doi: 10.1111/cas.14521
21. Wang H, Tian T, and Zhang J. Tumor-associated macrophages (TAMs) in colorectal cancer (CRC): from mechanism to therapy and prognosis. Int J Mol Sci. (2021) 22:8470. doi: 10.3390/ijms22168470
22. Chen S, Xu Y, Zhuo W, and Zhang L. The emerging role of lactate in tumor microenvironment and its clinical relevance. Cancer Lett. (2024) 590:216837. doi: 10.1016/j.canlet.2024.216837
23. Liu X, Zhang Y, Li W, and Zhou X. Lactylation, an emerging hallmark of metabolic reprogramming: Current progress and open challenges. Front Cell Dev Biol. (2022) 10:972020. doi: 10.3389/fcell.2022.972020
24. Yang K, Fan M, Wang X, Xu J, Wang Y, Tu F, et al. Lactate promotes macrophage HMGB1 lactylation, acetylation, and exosomal release in polymicrobial sepsis. Cell Death Differ. (2022) 29:133–46. doi: 10.1038/s41418-021-00841-9
25. Hagihara H, Shoji H, Otabi H, Toyoda A, Katoh K, Namihira M, et al. Protein lactylation induced by neural excitation. Cell Rep. (2021) 37:109820. doi: 10.1016/j.celrep.2021.109820
26. Huo M, Zhang J, Huang W, and Wang Y. Interplay among metabolism, epigenetic modifications, and gene expression in cancer. Front Cell Dev Biol. (2021) 9:793428. doi: 10.3389/fcell.2021.793428
27. Gaffney DO, Jennings EQ, Anderson CC, Marentette JO, Shi T, Schou Oxvig AM, et al. Non-enzymatic lysine lactoylation of glycolytic enzymes. Cell Chem Biol. (2020) 27:206–13.e6. doi: 10.1016/j.chembiol.2019.11.005
28. Manosalva C, Quiroga J, Hidalgo AI, Alarcón P, Ansoleaga N, Hidalgo MA, et al. Corrigendum: Role of lactate in inflammatory processes: friend or foe. Front Immunol. (2025) 16:1553925. doi: 10.3389/fimmu.2025.1553925
29. Schütterle DM, Hegner R, Temovska M, Ortiz-Ardila AE, and Angenent LT. Exclusive D-lactate-isomer production during a reactor-microbiome conversion of lactose-rich waste by controlling pH and temperature. Water Res. (2024) 250:121045. doi: 10.1016/j.watres.2023.121045
30. Gargallo-Garriga A, Preece C, Sardans J, Oravec M, Urban O, and Peñuelas J. Root exudate metabolomes change under drought and show limited capacity for recovery. Sci Rep. (2018) 8:12696. doi: 10.1038/s41598-018-30150-0
31. Kes MMG, Van den Bossche J, Griffioen AW, and Huijbers EJM. Oncometabolites lactate and succinate drive pro-angiogenic macrophage response in tumors. Biochim Biophys Acta Rev Cancer. (2020) 1874:188427. doi: 10.1016/j.bbcan.2020.188427
32. Wu H, Huang H, and Zhao Y. Interplay between metabolic reprogramming and post-translational modifications: from glycolysis to lactylation. Front Immunol. (2023) 14:1211221. doi: 10.3389/fimmu.2023.1211221
33. Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, et al. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A. (2010) 107:2037–42. doi: 10.1073/pnas.0914433107
34. Cheng CS, Tan HY, Wang N, Chen L, Meng Z, Chen Z, et al. Functional inhibition of lactate dehydrogenase suppresses pancreatic adenocarcinoma progression. Clin Transl Med. (2021) 11:e467. doi: 10.1002/ctm2.467
35. Li W, Zhou C, Yu L, Hou Z, Liu H, Kong L, et al. Tumor-derived lactate promotes resistance to bevacizumab treatment by facilitating autophagy enhancer protein RUBCNL expression through histone H3 lysine 18 lactylation (H3K18la) in colorectal cancer. Autophagy. (2024) 20:114–30. doi: 10.1080/15548627.2023.2249762
36. Li X, Yang Y, Zhang B, Lin X, Fu X, An Y, et al. Lactate metabolism in human health and disease. Signal Transduct Target Ther. (2022) 7:305. doi: 10.1038/s41392-022-01151-3
37. Linares JF, Cid-Diaz T, Duran A, Osrodek M, Martinez-Ordoñez A, Reina-Campos M, et al. The lactate-NAD+ axis activates cancer-associated fibroblasts by downregulating p62. Cell Rep. (2022) 39:110792. doi: 10.1016/j.celrep.2022.110792
38. Chen L, Huang L, Gu Y, Cang W, Sun P, and Xiang Y. Lactate-lactylation hands between metabolic reprogramming and immunosuppression. Int J Mol Sci. (2022) 23:11943. doi: 10.3390/ijms231911943
39. Moreno-Yruela C, Zhang D, Wei W, Bæk M, Liu W, Gao J, et al. Class I histone deacetylases (HDAC1-3) are histone lysine delactylases. Sci Adv. (2022) 8:eabi6696. doi: 10.1126/sciadv.abi6696
40. Lemons JM, Feng XJ, Bennett BD, Legesse-Miller A, Johnson EL, Raitman I, et al. Quiescent fibroblasts exhibit high metabolic activity. PloS Biol. (2010) 8:e1000514. doi: 10.1371/journal.pbio.1000514
41. Brooks GA, Arevalo JA, Osmond AD, Leija RG, Curl CC, and Tovar AP. Lactate in contemporary biology: a phoenix risen. J Physiol. (2022) 600:1229–51. doi: 10.1113/JP280955
42. Sun S, Li H, Chen J, and Qian Q. Lactic acid: no longer an inert and end-product of glycolysis. Physiol (Bethesda). (2017) 32:453–63. doi: 10.1152/physiol.00016.2017
43. Brooks GA. The science and translation of lactate shuttle theory. Cell Metab. (2018) 27:757–85. doi: 10.1016/j.cmet.2018.03.008
44. Kocianova E, Piatrikova V, and Golias T. Revisiting the Warburg effect with focus on lactate. Cancers (Basel). (2022) 14:6028. doi: 10.3390/cancers14246028
45. Allis CD and Jenuwein T. The molecular hallmarks of epigenetic control. Nat Rev Genet. (2016) 17:487–500. doi: 10.1038/nrg.2016.59
46. Leslie KA, Lekka C, Richardson SJ, Russell MA, and Morgan NG. Regulation of STAT1 signaling in human pancreatic β-cells by the lysine deacetylase HDAC6: A new therapeutic opportunity in type 1 diabetes? Diabetes. (2024) 73:1473–85. doi: 10.2337/db24-0008
47. Cheng Z, Cheng Z, Zhang Y, and Zhang S. Intrinsic disorder-protein modification-LLPS-tumor” regulatory axis: From regulatory mechanisms to precision medicine. Biochim Biophys Acta Rev Cancer. (2025) 1880:189242. doi: 10.1016/j.bbcan.2024.189242
48. Xu H, Wu M, Ma X, Huang W, and Xu Y. Function and mechanism of novel histone posttranslational modifications in health and disease. BioMed Res Int. (2021) 2021:6635225. doi: 10.1155/2021/6635225
49. Sun Z, Song Y, Li J, Li Y, Yu Y, and Wang X. Potential biomarker for diagnosis and therapy of sepsis: Lactylation. Immun Inflammation Dis. (2023) 11:e1042. doi: 10.1002/iid3.1042
50. Yang W, Wang P, Cao P, Wang S, Yang Y, Su H, et al. Hypoxic in vitro culture reduces histone lactylation and impairs pre-implantation embryonic development in mice. Epigenet Chromatin. (2021) 14:57. doi: 10.1186/s13072-021-00431-6
51. Meng Q, Sun H, Zhang Y, Yang X, Hao S, Liu B, et al. Lactylation stabilizes DCBLD1 activating the pentose phosphate pathway to promote cervical cancer progression. J Exp Clin Cancer Res. (2024) 43:36. doi: 10.1186/s13046-024-02943-x
52. Liu R, Ren X, Park YE, Feng H, Sheng X, Song X, et al. Nuclear GTPSCS functions as a lactyl-CoA synthetase to promote histone lactylation and gliomagenesis. Cell Metab. (2025) 37:377–94.e9. doi: 10.1016/j.cmet.2024.11.005
53. Tchurikov NA, Klushevskaya ES, Alembekov IR, Bukreeva AS, Kretova AN, Chechetkin VR, et al. Fragments of rDNA Genes Scattered over the Human Genome Are Targets of Small RNAs. Int J Mol Sci. (2022) 23:3014. doi: 10.3390/ijms23063014
54. Zhang F, Zhou J, Lu P, Zhang X, Yang L, Wu J, et al. Lactylation of histone by BRD4 regulates astrocyte polarization after experimental subarachnoid hemorrhage. J Neuroinflammation. (2024) 21:186. doi: 10.1186/s12974-024-03185-6
55. Zhao Y, Jiang J, Zhou P, Deng K, Liu Z, Yang M, et al. H3K18 lactylation-mediated VCAM1 expression promotes gastric cancer progression and metastasis via AKT-mTOR-CXCL1 axis. Biochem Pharmacol. (2024) 222:116120. doi: 10.1016/j.bcp.2024.116120
56. Li X, Chen M, Chen X, He X, Li X, Wei H, et al. TRAP1 drives smooth muscle cell senescence and promotes atherosclerosis via HDAC3-primed histone H4 lysine 12 lactylation. Eur Heart J. (2024) 45:4219–35. doi: 10.1093/eurheartj/ehae379
57. Huang Y, Luo G, Peng K, Song Y, Wang Y, Zhang H, et al. Lactylation stabilizes TFEB to elevate autophagy and lysosomal activity. J Cell Biol. (2024) 223:e202308099. doi: 10.1083/jcb.202308099
58. Yin X, Xing W, Yi N, Zhou Y, Chen Y, Jiang Z, et al. Comprehensive analysis of lactylation-related gene sets and mitochondrial functions in gastric adenocarcinoma: implications for prognosis and therapeutic strategies. Front Immunol. (2024) 15:1451725. doi: 10.3389/fimmu.2024.1451725
59. Ye J, Gao X, Huang X, Huang S, Zeng D, Luo W, et al. Integrating single-cell and spatial transcriptomics to uncover and elucidate GP73-mediated pro-angiogenic regulatory networks in hepatocellular carcinoma. Res (Wash D C). (2024) 7:387. doi: 10.34133/research.0387
60. Sun S, Xu Z, He L, Shen Y, Yan Y, Lv X, et al. Metabolic regulation of cytoskeleton functions by HDAC6-catalyzed α-tubulin lactylation. Nat Commun. (2024) 15:8377. doi: 10.1038/s41467-024-52729-0
61. Yang Y, Sun X, Liu B, Zhang Y, Xie T, Li J, et al. Identifying Lactylation-related biomarkers and therapeutic drugs in ulcerative colitis: insights from machine learning and molecular docking. BMC Pharmacol Toxicol. (2025) 26:103. doi: 10.1186/s40360-025-00939-7
62. Gao M, Zhang N, and Liang W. Systematic analysis of lysine lactylation in the plant fungal pathogen Botrytis cinerea. Front Microbiol. (2020) 11:594743. doi: 10.3389/fmicb.2020.594743
63. Wang J, Yang P, Yu T, Gao M, Liu D, Zhang J, et al. Lactylation of PKM2 suppresses inflammatory metabolic adaptation in pro-inflammatory macrophages. Int J Biol Sci. (2022) 18:6210–25. doi: 10.7150/ijbs.75434
64. Wynn TA, Chawla A, and Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. (2013) 496:445–55. doi: 10.1038/nature12034
65. Mosser DM and Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. (2008) 8:958–69. doi: 10.1038/nri2448
66. Funes SC, Rios M, Escobar-Vera J, and Kalergis AM. Implications of macrophage polarization in autoimmunity. Immunology. (2018) 154:186–95. doi: 10.1111/imm.12910
67. Kawane K, Fukuyama H, Kondoh G, Takeda J, Ohsawa Y, Uchiyama Y, et al. Requirement of DNase II for definitive erythropoiesis in the mouse fetal liver. Science. (2001) 292:1546–9. doi: 10.1126/science.292.5521.1546
68. Boutilier AJ and Elsawa SF. Macrophage polarization states in the tumor microenvironment. Int J Mol Sci. (2021) 22:6995. doi: 10.3390/ijms22136995
69. Yeung OW, Lo CM, Ling CC, Qi X, Geng W, Li CX, et al. Alternatively activated (M2) macrophages promote tumour growth and invasiveness in hepatocellular carcinoma. J Hepatol. (2015) 62:607–16. doi: 10.1016/j.jhep.2014.10.029
70. Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. (2014) 41:14–20. doi: 10.1016/j.immuni.2014.06.008
71. Gordon S. Alternative activation of macrophages. Nat Rev Immunol. (2003) 3:23–35. doi: 10.1038/nri978
72. Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. (2011) 121:985–97. doi: 10.1172/JCI44490
73. Verreck FA, de Boer T, Langenberg DM, Hoeve MA, Kramer M, Vaisberg E, et al. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci U S A. (2004) 101:4560–5. doi: 10.1073/pnas.0400983101
74. Allavena P, Sica A, Solinas G, Porta C, and Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. (2008) 66:1–9. doi: 10.1016/j.critrevonc.2007.07.004
75. Wynn TA and Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. (2016) 44:450–62. doi: 10.1016/j.immuni.2016.02.015
76. Guo L, Akahori H, Harari E, Smith SL, Polavarapu R, Karmali V, et al. CD163+ macrophages promote angiogenesis and vascular permeability accompanied by inflammation in atherosclerosis. J Clin Invest. (2018) 128:1106–24. doi: 10.1172/JCI93025
77. Meng EX, Verne GN, and Zhou Q. Macrophages and gut barrier function: guardians of gastrointestinal health in post-inflammatory and post-infection responses. Int J Mol Sci. (2024) 25:9422. doi: 10.3390/ijms25179422
78. Ma S, Zhang J, Liu H, Li S, and Wang Q. The role of tissue-resident macrophages in the development and treatment of inflammatory bowel disease. Front Cell Dev Biol. (2022) 10:896591. doi: 10.3389/fcell.2022.896591
79. Zhang Q and Sioud M. Tumor-associated macrophage subsets: shaping polarization and targeting. Int J Mol Sci. (2023) 24:7493. doi: 10.3390/ijms24087493
80. Lugo-Villarino G, Troegeler A, Balboa L, Lastrucci C, Duval C, Mercier I, et al. The C-type lectin receptor DC-SIGN has an anti-inflammatory role in human M(IL-4) macrophages in response to mycobacterium tuberculosis. Front Immunol. (2018) 9:1123. doi: 10.3389/fimmu.2018.01123
81. Junttila IS. Tuning the cytokine responses: an update on interleukin (IL)-4 and IL-13 receptor complexes. Front Immunol. (2018) 9:888. doi: 10.3389/fimmu.2018.00888
82. da Costa Santos MAR, Dos Reis JS, do Nascimento Santos CA, da Costa KM, Barcelos PM, de Oliveira Francisco KQ, et al. Expression of O-glycosylated oncofetal fibronectin in alternatively activated human macrophages. Immunol Res. (2023) 71:92–104. doi: 10.1007/s12026-022-09321-9
83. Fu C, Jiang L, Hao S, Liu Z, Ding S, Zhang W, et al. Activation of the IL-4/STAT6 signaling pathway promotes lung cancer progression by increasing M2 myeloid cells. Front Immunol. (2019) 10:2638. doi: 10.3389/fimmu.2019.02638
84. Zhao L, Tang S, Chen F, Ren X, Han X, and Zhou X. Regulation of macrophage polarization by targeted metabolic reprogramming for the treatment of lupus nephritis. Mol Med. (2024) 30:96. doi: 10.1186/s10020-024-00866-z
85. Sironi M, Martinez FO, D’Ambrosio D, Gattorno M, Polentarutti N, Locati M, et al. Differential regulation of chemokine production by Fcgamma receptor engagement in human monocytes: association of CCL1 with a distinct form of M2 monocyte activation (M2b, Type 2). J Leukoc Biol. (2006) 80:342–9. doi: 10.1189/jlb.1005586
86. Patik I, Redhu NS, Eran A, Bao B, Nandy A, Tang Y, et al. The IL-10 receptor inhibits cell extrinsic signals necessary for STAT1-dependent macrophage accumulation during colitis. Mucosal Immunol. (2023) 16:233–49. doi: 10.1016/j.mucimm.2023.02.006
87. Sahu R, Bethunaickan R, Singh S, and Davidson A. Structure and function of renal macrophages and dendritic cells from lupus-prone mice. Arthritis Rheumatol. (2014) 66:1596–607. doi: 10.1002/art.38410
88. Ito I, Bhopale KK, Nishiguchi T, Lee JO, Herndon DN, Suzuki S, et al. The polarization of M2b monocytes in cultures of burn patient peripheral CD14+ Cells treated with a selected human CCL1 antisense oligodeoxynucleotide. Nucleic Acid Ther. (2016) 26:269–76. doi: 10.1089/nat.2016.0617
89. Fu XL, Duan W, Su CY, Mao FY, Lv YP, Teng YS, et al. Interleukin 6 induces M2 macrophage differentiation by STAT3 activation that correlates with gastric cancer progression. Cancer Immunol Immunother. (2017) 66:1597–608. doi: 10.1007/s00262-017-2052-5
90. Anders CB, Lawton TMW, Smith HL, Garret J, Doucette MM, and Ammons MCB. Use of integrated metabolomics, transcriptomics, and signal protein profile to characterize the effector function and associated metabotype of polarized macrophage phenotypes. J Leukoc Biol. (2022) 111:667–93. doi: 10.1002/JLB.6A1120-744R
91. Yip JLK, Balasuriya GK, Spencer SJ, and Hill-Yardin EL. The role of intestinal macrophages in gastrointestinal homeostasis: heterogeneity and implications in disease. Cell Mol Gastroenterol Hepatol. (2021) 12:1701–18. doi: 10.1016/j.jcmgh.2021.08.021
92. De Calisto J, Villablanca EJ, and Mora JR. FcγRI (CD64): an identity card for intestinal macrophages. Eur J Immunol. (2012) 42:3136–40. doi: 10.1002/eji.201243061
93. Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. (2013) 6:498–510. doi: 10.1038/mi.2012.89
94. Schridde A, Bain CC, Mayer JU, Montgomery J, Pollet E, Denecke B, et al. Tissue-specific differentiation of colonic macrophages requires TGFβ receptor-mediated signaling. Mucosal Immunol. (2017) 10:1387–99. doi: 10.1038/mi.2016.142
95. Bain CC and Mowat AM. Macrophages in intestinal homeostasis and inflammation. Immunol Rev. (2014) 260:102–17. doi: 10.1111/imr.12192
96. Tamoutounour S, Henri S, Lelouard H, de Bovis B, de Haar C, van der Woude CJ, et al. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur J Immunol. (2012) 42:3150–66. doi: 10.1002/eji.201242847
97. Kaya B, Doñas C, Wuggenig P, Diaz OE, Morales RA, Melhem H, et al. Lysophosphatidic acid-mediated GPR35 signaling in CX3CR1+ Macrophages regulates intestinal homeostasis. Cell Rep. (2020) 32:107979. doi: 10.1016/j.celrep.2020.107979
98. Jeffrey MP, Saleem L, MacPherson CW, Tompkins TA, Clarke ST, and Green-Johnson JM. A Lacticaseibacillus rhamnosus secretome induces immunoregulatory transcriptional, functional and immunometabolic signatures in human THP-1 monocytes. Sci Rep. (2024) 14:8379. doi: 10.1038/s41598-024-56420-8
99. De Schepper S, Verheijden S, Aguilera-Lizarraga J, Viola MF, Boesmans W, Stakenborg N, et al. Self-maintaining gut macrophages are essential for intestinal homeostasis. Cell. (2018) 175:400–15.e13. doi: 10.1016/j.cell.2018.07.048
100. Zhu X, Liang R, Lan T, Ding D, Huang S, Shao J, et al. Tumor-associated macrophage-specific CD155 contributes to M2-phenotype transition, immunosuppression, and tumor progression in colorectal cancer. J Immunother Cancer. (2022) 10:e004219. doi: 10.1136/jitc-2021-004219
101. Pathria P, Louis TL, and Varner JA. Targeting tumor-associated macrophages in cancer. Trends Immunol. (2019) 40:310–27. doi: 10.1016/j.it.2019.02.003
102. Xu C, Fan L, Lin Y, Shen W, Qi Y, Zhang Y, et al. Fusobacterium nucleatum promotes colorectal cancer metastasis through miR-1322/CCL20 axis and M2 polarization. Gut Microbes. (2021) 13:1980347. doi: 10.1080/19490976.2021.1980347
103. Yang Y, Weng W, Peng J, Hong L, Yang L, Toiyama Y, et al. Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating toll-like receptor 4 signaling to nuclear factor-κB, and up-regulating expression of microRNA-21. Gastroenterology. (2017) 152:851–66.e24. doi: 10.1053/j.gastro.2016.11.018
104. Zhang J, Hu C, Zhang R, Xu J, Zhang Y, Yuan L, et al. The role of macrophages in gastric cancer. Front Immunol. (2023) 14:1282176. doi: 10.3389/fimmu.2023.1282176
105. Costa NL, Valadares MC, Souza PP, Mendonça EF, Oliveira JC, Silva TA, et al. Tumor-associated macrophages and the profile of inflammatory cytokines in oral squamous cell carcinoma. Oral Oncol. (2013) 49:216–23. doi: 10.1016/j.oraloncology.2012.09.012
106. Rieder F, Fiocchi C, and Rogler G. Mechanisms, management, and treatment of fibrosis in patients with inflammatory bowel diseases. Gastroenterology. (2017) 152:340–50.e6. doi: 10.1053/j.gastro.2016.09.047
107. Palmela C, Chevarin C, Xu Z, Torres J, Sevrin G, Hirten R, et al. Adherent-invasive Escherichia coli in inflammatory bowel disease. Gut. (2018) 67:574–87. doi: 10.1136/gutjnl-2017-314903
108. Viladomiu M, Metz ML, Lima SF, Jin WB, Chou L, JRI Live Cell Bank, et al. Adherent-invasive E. coli metabolism of propanediol in Crohn’s disease regulates phagocytes to drive intestinal inflammation. Cell Host Microbe. (2021) 29:607–19. doi: 10.1016/j.chom.2021.01.002
109. Xu Y, Qian W, Huang L, Wen W, Li Y, Guo F, et al. Crohn’s disease-associated AIEC inhibiting intestinal epithelial cell-derived exosomal let-7b expression regulates macrophage polarization to exacerbate intestinal fibrosis. Gut Microbes. (2023) 15:2193115. doi: 10.1080/19490976.2023.2193115
110. Abdel-Wahab AF, Mahmoud W, and Al-Harizy RM. Targeting glucose metabolism to suppress cancer progression: prospective of anti-glycolytic cancer therapy. Pharmacol Res. (2019) 150:104511. doi: 10.1016/j.phrs.2019.104511
111. Palsson-McDermott EM, Curtis AM, Goel G, Lauterbach MAR, Sheedy FJ, Gleeson LE, et al. Pyruvate kinase M2 regulates hif-1α Activity and IL-1β Induction and is a critical determinant of the Warburg effect in LPS-activated macrophages. Cell Metab. (2015) 21:347. doi: 10.1016/j.cmet.2015.01.017
112. Van den Bossche J, Baardman J, and de Winther MP. Metabolic characterization of polarized M1 and M2 bone marrow-derived macrophages using real-time extracellular flux analysis. J Vis Exp. (2015) 105):53424. doi: 10.3791/53424
113. Xu B, Liu Y, Li N, and Geng Q. Lactate and lactylation in macrophage metabolic reprogramming: current progress and outstanding issues. Front Immunol. (2024) 15:1395786. doi: 10.3389/fimmu.2024.1395786
114. Wiese EK, Hitosugi S, Loa ST, Sreedhar A, Andres-Beck LG, Kurmi K, et al. Enzymatic activation of pyruvate kinase increases cytosolic oxaloacetate to inhibit the Warburg effect. Nat Metab. (2021) 3:954–68. doi: 10.1038/s42255-021-00424-5
115. Ivashkiv LB. The hypoxia-lactate axis tempers inflammation. Nat Rev Immunol. (2020) 20:85–6. doi: 10.1038/s41577-019-0259-8
116. Mantovani A, Allavena P, Sica A, and Balkwill F. Cancer-related inflammation. Nature. (2008) 454:436–44. doi: 10.1038/nature07205
117. Zhang B, Li F, Shi Y, Ji C, Kong Q, Sun K, et al. Single-cell RNA sequencing integrated with bulk RNA sequencing analysis reveals the protective effects of lactate-mediated lactylation of microglia-related proteins on spinal cord injury. CNS Neurosci Ther. (2024) 30:e70028. doi: 10.1111/cns.70028
118. Su J, Mao X, Wang L, Chen Z, Wang W, Zhao C, et al. Lactate/GPR81 recruits regulatory T cells by modulating CX3CL1 to promote immune resistance in a highly glycolytic gastric cancer. Oncoimmunology. (2024) 13:2320951. doi: 10.1080/2162402X.2024.2320951
119. Zhang Y, Jiang H, Dong M, Min J, He X, Tan Y, et al. Macrophage MCT4 inhibition activates reparative genes and protects from atherosclerosis by histone H3 lysine 18 lactylation. Cell Rep. (2024) 43:114180. doi: 10.1016/j.celrep.2024.114180
120. Noe JT, Rendon BE, Geller AE, Conroy LR, Morrissey SM, Young LEA, et al. Lactate supports a metabolic-epigenetic link in macrophage polarization. Sci Adv. (2021) 7:eabi8602. doi: 10.1126/sciadv.abi8602
121. Liu N, Luo J, Kuang D, Xu S, Duan Y, Xia Y, et al. Lactate inhibits ATP6V0d2 expression in tumor-associated macrophages to promote HIF-2α-mediated tumor progression. J Clin Invest. (2019) 129:631–46. doi: 10.1172/JCI123027
122. Zhang A, Xu Y, Xu H, Ren J, Meng T, Ni Y, et al. Lactate-induced M2 polarization of tumor-associated macrophages promotes the invasion of pituitary adenoma by secreting CCL17. Theranostics. (2021) 11:3839–52. doi: 10.7150/thno.53749
123. Zhu L, Zheng Q, Liu X, Ding H, Ma M, Bao J, et al. HMGB1 lactylation drives neutrophil extracellular trap formation in lactate-induced acute kidney injury. Front Immunol. (2025) 15:1475543. doi: 10.3389/fimmu.2024.1475543
124. Li XM, Yang Y, Jiang FQ, Hu G, Wan S, Yan WY, et al. Histone lactylation inhibits RARγ expression in macrophages to promote colorectal tumorigenesis through activation of TRAF6-IL-6-STAT3 signaling. Cell Rep. (2024) 43:113688. doi: 10.1016/j.celrep.2024.113688
125. Dichtl S, Lindenthal L, Zeitler L, Behnke K, Schlösser D, Strobl B, et al. Lactate and IL6 define separable paths of inflammatory metabolic adaptation. Sci Adv. (2021) 7:eabg3505. doi: 10.1126/sciadv.abg3505
126. Chen L, Zhang M, Yang X, Wang Y, Huang T, Li X, et al. Methyl-CpG-binding 2 K271 lactylation-mediated M2 macrophage polarization inhibits atherosclerosis. Theranostics. (2024) 14:4256–77. doi: 10.7150/thno.94738
127. Irizarry-Caro RA, McDaniel MM, Overcast GR, Jain VG, Troutman TD, and Pasare C. TLR signaling adapter BCAP regulates inflammatory to reparatory macrophage transition by promoting histone lactylation. Proc Natl Acad Sci U.S.A. (2020) 117:30628–38. doi: 10.1073/pnas.2009778117
128. Susser LI, Nguyen MA, Geoffrion M, Emerton C, Ouimet M, Khacho M, et al. Mitochondrial fragmentation promotes inflammation resolution responses in macrophages via histone lactylation. Mol Cell Biol. (2023) 43:531–46. doi: 10.1080/10985549.2023.2253131
129. Xu ZP, Shan SY, Cai EW, and Wu YY. Gegen Qinlian decoction inhibited M1 macrophage polarization and ulcerative colitis progression through regulating histone lactylation. Tissue Cell. (2024) 89:102468. doi: 10.1016/j.tice.2024.102468
130. Sun J, Feng Q, He Y, Wang M, and Wu Y. Lactate activates CCL18 expression via H3K18 lactylation in macrophages to promote tumorigenesis of ovarian cancer. Acta Biochim Biophys Sin (Shanghai). (2024) 56:1373–86. doi: 10.3724/abbs.2024111
131. Li M, Sun P, Tu B, Deng G, Li D, and He W. Hypoxia conduces the glioma progression by inducing M2 macrophage polarization via elevating TNFSF9 level in a histone-lactylation-dependent manner. Am J Physiol Cell Physiol. (2024) 327:C487–504. doi: 10.1152/ajpcell.00124.2024
132. Wang JX, Choi SYC, Niu X, Kang N, Xue H, Killam J, et al. Tumor microenvironment suppress anticancer immunity. Int J Mol Sci. (2020) 21:8363. doi: 10.3390/ijms21218363
133. El-Kenawi A, Gatenbee C, Robertson-Tessi M, Bravo R, Dhillon J, Balagurunathan Y, et al. Acidity promotes tumour progression by altering macrophage phenotype in prostate cancer. Br J Cancer. (2019) 121:556–66. doi: 10.1038/s41416-019-0542-2
134. Certo M, Tsai CH, Pucino V, Ho PC, and Mauro C. Lactate modulation of immune responses in inflammatory versus tumour microenvironments. Nat Rev Immunol. (2021) 21:151–61. doi: 10.1038/s41577-020-0406-2
135. Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. (2014) 513:559–63. doi: 10.1038/nature13490
136. García-Cañaveras JC, Chen L, and Rabinowitz JD. The tumor metabolic microenvironment: lessons from lactate. Cancer Res. (2019) 79:3155–62. doi: 10.1158/0008-5472.CAN-18-3726
137. Zhou H, Yao J, Zhong Z, Wei H, He Y, Li W, et al. Lactate-induced CCL8 in tumor-associated macrophages accelerates the progression of colorectal cancer through the CCL8/CCR5/mTORC1 axis. Cancers (Basel). (2023) 15:5795. doi: 10.3390/cancers15245795
138. Zhang S, Xu X, Zhang K, Lei C, Xu Y, Zhang P, et al. Targeting OAS3 for reversing M2d infiltration and restoring anti-tumor immunity in pancreatic cancer. Cancer Immunol Immunother. (2024) 74:37. doi: 10.1007/s00262-024-03898-w
139. Zhao H, Wen Z, and Xiong S. Activated lymphocyte-derived DNA drives glucose metabolic adaptation for inducing macrophage inflammatory response in systemic lupus erythematosus. Cells. (2023) 12:2093. doi: 10.3390/cells12162093
140. Xing J, Weng L, Yuan B, Wang Z, Jia L, Jin R, et al. Identification of a role for TRIM29 in the control of innate immunity in the respiratory tract [published correction appears in Nat Immunol. 2016 Nov 16;17(12):1479. doi: 10.1038/ni1216-1479a. Nat Immunol. (2016) 17:1373–80. doi: 10.1038/ni.3580
141. Lv K, Li Q, Jiang N, and Chen Q. Role of TRIM29 in disease: What is and is not known. Int Immunopharmacol. (2025) 147:113983. doi: 10.1016/j.intimp.2024.113983
142. Raines LN, Zhao H, Wang Y, Chen HY, Gallart Ayala - H, Hsueh PC, et al. PERK is a critical metabolic hub for immunosuppressive function in macrophages. Nat Immunol. (2022) 23:431–45. doi: 10.1038/s41590-022-01145-x
143. Wang J, Wang L, Lu W, Farhataziz N, Gonzalez A, Xing J, et al. TRIM29 controls enteric RNA virus-induced intestinal inflammation by targeting NLRP6 and NLRP9b signaling pathways. Mucosal Immunol. (2025) 18:135–50. doi: 10.1016/j.mucimm.2024.10.004
144. Wang J, Lu W, Zhang J, Du Y, Fang M, Zhang A, et al. Loss of TRIM29 mitigates viral myocarditis by attenuating PERK-driven ER stress response in male mice. Nat Commun. (2024) 15:3481. doi: 10.1038/s41467-024-44745-x
145. De Leo A, Ugolini A, Yu X, Scirocchi F, Scocozza D, Peixoto B, et al. Glucose-driven histone lactylation promotes the immunosuppressive activity of monocyte-derived macrophages in glioblastoma. Immunity. (2024) 57:1105–23.e8. doi: 10.1016/j.immuni.2024.04.006
146. Su Z, Lan J, Wang Y, Ma N, Yang J, Liang D, et al. Lactylation-driven ALKBH5 diminishes macrophage NLRP3 inflammasome activation in patients with G6PT deficiency. J Allergy Clin Immunol. (2025) 155(6):1783–99.e8. doi: 10.1016/j.jaci.2025.01.028
147. You X, Xie Y, Tan Q, Zhou C, Gu P, Zhang Y, et al. Glycolytic reprogramming governs crystalline silica-induced pyroptosis and inflammation through promoting lactylation modification. Ecotoxicol Environ Saf. (2024) 283:116952. doi: 10.1016/j.ecoenv.2024.116952
148. Ge S, Qiu B, Liu R, Sun L, Yang L, Chen X, et al. Ultraviolet-treated riboflavin alleviates atopic dermatitis by inhibiting NLRP3 inflammasome activation and M1 macrophage polarization via histone lactylation. Biochem Pharmacol. (2025) 236:116879. doi: 10.1016/j.bcp.2025.116879
149. Lee SH, Kwon JE, and Cho ML. Immunological pathogenesis of inflammatory bowel disease. Intest Res. (2018) 16:26–42. doi: 10.5217/ir.2018.16.1.26
150. Olén O, Askling J, Sachs MC, Neovius M, Smedby KE, Ekbom A, et al. Mortality in adult-onset and elderly-onset IBD: a nationwide register-based cohort study 1964-2014. Gut. (2020) 69:453–61. doi: 10.1136/gutjnl-2018-317572
151. Minea H, Singeap AM, Huiban L, Muzica CM, Stanciu C, and Trifan A. Patient and physician factors contributing to delays in inflammatory bowel diseases: Enhancing timely diagnosis. World J Gastroenterol. (2025) 31:100295. doi: 10.3748/wjg.v31.i6.100295
152. Gu J, Zhou J, Chen Q, Xu X, Gao J, Li X, et al. Tumor metabolite lactate promotes tumorigenesis by modulating MOESIN lactylation and enhancing TGF-β signaling in regulatory T cells. Cell Rep. (2022) 39:110986. doi: 10.1016/j.celrep.2022.110986
153. Zhang C and Huang X. Role of TAK-242-induced histone lactylation in modulating repair macrophage transformation in ulcerative colitis. Immunol Invest. (2025) 17:1–19. doi: 10.1080/08820139.2025.2465644
154. Yuan X, Wang Q, Zhao J, Xie H, and Pu Z. The m6A methyltransferase METTL3 modifies Kcnk6 promoting on inflammation associated carcinogenesis is essential for colon homeostasis and defense system through histone lactylation dependent YTHDF2 binding. Int Rev Immunol. (2025) 44:1–16. doi: 10.1080/08830185.2024.2401358
155. Ranganathan P, Shanmugam A, Swafford D, Suryawanshi A, Bhattacharjee P, Hussein MS, et al. GPR81, a cell-surface receptor for lactate, regulates intestinal homeostasis and protects mice from experimental colitis. J Immunol. (2018) 200:1781–9. doi: 10.4049/jimmunol.1700604
156. Yu H, Lin L, Zhang Z, Zhang H, and Hu H. Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct Target Ther. (2020) 5:209. doi: 10.1038/s41392-020-00312-6
157. Huang X, Lin R, Liu H, Dai M, Guo J, Hui W, et al. Resatorvid (TAK-242) ameliorates ulcerative colitis by modulating macrophage polarization and T helper cell balance via TLR4/JAK2/STAT3 signaling pathway. Inflammation. (2024) 47:2108–28. doi: 10.1007/s10753-024-02028-z
158. Zhuang X, Li T, Li M, Huang S, Qiu Y, Feng R, et al. Systematic review and meta-analysis: short-chain fatty acid characterization in patients with inflammatory bowel disease. Inflammation Bowel Dis. (2019) 25:1751–63. doi: 10.1093/ibd/izz188
159. Sun S, Xu X, Liang L, Wang X, Bai X, Zhu L, et al. Lactic Acid-Producing Probiotic Saccharomyces cerevisiae Attenuates Ulcerative Colitis via Suppressing Macrophage Pyroptosis and Modulating Gut Microbiota. Front Immunol. (2021) 12:777665. doi: 10.3389/fimmu.2021.777665
160. Mazzotta E, Villalobos-Hernandez EC, Fiorda-Diaz J, Harzman A, and Christofi FL. Postoperative ileus and postoperative gastrointestinal tract dysfunction: pathogenic mechanisms and novel treatment strategies beyond colorectal enhanced recovery after surgery protocols. Front Pharmacol. (2020) 11:583422. doi: 10.3389/fphar.2020.583422
161. Sui C, Tao L, Bai C, Shao L, Miao J, Chen K, et al. Molecular and cellular mechanisms underlying postoperative paralytic ileus by various immune cell types. Front Pharmacol. (2022) 13:929901. doi: 10.3389/fphar.2022.929901
162. Delfini M, Stakenborg N, Viola MF, and Boeckxstaens G. Macrophages in the gut: Masters in multitasking. Immunity. (2022) 55:1530–48. doi: 10.1016/j.immuni.2022.08.005
163. Docsa T, Bhattarai D, Sipos A, Wade CE, Cox CS Jr, and Uray K. CXCL1 is upregulated during the development of ileus resulting in decreased intestinal contractile activity. Neurogastroenterol Motil. (2020) 32:e13757. doi: 10.1111/nmo.13757
164. Wehner S, Behrendt FF, Lyutenski BN, Lysson M, Bauer AJ, Hirner A, et al. Inhibition of macrophage function prevents intestinal inflammation and postoperative ileus in rodents. Gut. (2007) 56:176–85. doi: 10.1136/gut.2005.089615
165. Zhou J, Xu W, Wu Y, Wang M, Zhang N, Wang L, et al. GPR37 promotes colorectal cancer liver metastases by enhancing the glycolysis and histone lactylation via Hippo pathway. Oncogene. (2023) 42:3319–30. doi: 10.1038/s41388-023-02841-0
166. Yuan PQ and Taché Y. Abdominal surgery induced gastric ileus and activation of M1-like macrophages in the gastric myenteric plexus: prevention by central vagal activation in rats. Am J Physiol Gastrointest Liver Physiol. (2017) 313:G320–9. doi: 10.1152/ajpgi.00121.2017
167. Tong Y, Gao H, Qi Q, Liu X, Li J, Gao J, et al. High fat diet, gut microbiome and gastrointestinal cancer. Theranostics. (2021) 11:5889–910. doi: 10.7150/thno.56157
168. Thakur C and Chen F. Connections between metabolism and epigenetics in cancers. Semin Cancer Biol. (2019) 57:52–8. doi: 10.1016/j.semcancer.2019.06.006
169. Yu H, Liu J, Bu X, Ma Z, Yao Y, Li J, et al. Targeting METTL3 reprograms the tumor microenvironment to improve cancer immunotherapy. Cell Chem Biol. (2024) 31:776–791.e7. doi: 10.1016/j.chembiol.2023.09.001
170. Cassetta L, Fragkogianni S, Sims AH, Swierczak A, Forrester LM, Zhang H, et al. Human tumor-associated macrophage and monocyte transcriptional landscapes reveal cancer-specific reprogramming, biomarkers, and therapeutic targets. Cancer Cell. (2019) 35:588–602.e10. doi: 10.1016/j.ccell.2019.02.009
171. Pittet MJ, Michielin O, and Migliorini D. Author Correction: Clinical relevance of tumour-associated macrophages. Nat Rev Clin Oncol. (2022) 19:424. doi: 10.1038/s41571-022-00632-2
172. Yuneva MO, Fan TW, Allen TD, Higashi RM, Ferraris DV, Tsukamoto T, et al. The metabolic profile of tumors depends on both the responsible genetic lesion and tissue type. Cell Metab. (2012) 15:157–70. doi: 10.1016/j.cmet.2011.12.015
173. Chen B, Deng Y, Hong Y, Fan L, Zhai X, Hu H, et al. Metabolic Recoding of NSUN2-Mediated m5C Modification Promotes the Progression of Colorectal Cancer via the NSUN2/YBX1/m5C-ENO1 Positive Feedback Loop. Adv Sci (Weinh). (2024) 11:e2309840. doi: 10.1002/advs.202309840
174. Goossens P, Rodriguez-Vita J, Etzerodt A, Masse M, Rastoin O, Gouirand V, et al. Membrane cholesterol efflux drives tumor-associated macrophage reprogramming and tumor progression. Cell Metab. (2019) 29:1376–89.e4. doi: 10.1016/j.cmet.2019.02.016
175. Wang L, Li S, Luo H, Lu Q, and Yu S. PCSK9 promotes the progression and metastasis of colon cancer cells through regulation of EMT and PI3K/AKT signaling in tumor cells and phenotypic polarization of macrophages. J Exp Clin Cancer Res. (2022) 41:303. doi: 10.1186/s13046-022-02477-0
176. Gu J, Xu X, Li X, Yue L, Zhu X, Chen Q, et al. Tumor-resident microbiota contributes to colorectal cancer liver metastasis by lactylation and immune modulation. Oncogene. (2024) 43:2389–404. doi: 10.1038/s41388-024-03080-7
177. Li R, Zhou R, Wang H, Li W, Pan M, Yao X, et al. Gut microbiota-stimulated cathepsin K secretion mediates TLR4-dependent M2 macrophage polarization and promotes tumor metastasis in colorectal cancer. Cell Death Differ. (2019) 26:2447–63. doi: 10.1038/s41418-019-0312-y
178. Lin S, Sun L, Lyu X, Ai X, Du D, Su N, et al. Lactate-activated macrophages induced aerobic glycolysis and epithelial-mesenchymal transition in breast cancer by regulation of CCL5-CCR5 axis: a positive metabolic feedback loop. Oncotarget. (2017) 8:110426–43. doi: 10.18632/oncotarget.22786
179. Jia Z, Zhang X, Li Z, Yan H, Tian X, Luo C, et al. Hydrogen sulfide mitigates ox-LDL-induced NLRP3/caspase-1/GSDMD dependent macrophage pyroptosis by S-sulfhydrating caspase-1. Mol Med Rep. (2024) 30:135. doi: 10.3892/mmr.2024.13259
180. Wardlaw CP and Petrini JHJ. ISG15 conjugation to proteins on nascent DNA mitigates DNA replication stress. Nat Commun. (2022) 13:5971. doi: 10.1038/s41467-022-33535-y
181. Pouysségur J, Marchiq I, Parks SK, Durivault J, and Vucetic M. ‘Warburg effect’ controls tumor growth, bacterial, viral infections and immunity - Genetic deconstruction and therapeutic perspectives. Semin Cancer Biol. (2022) 86:334–46. doi: 10.1016/j.semcancer.2022.07.004
182. Li Z, Gong T, Wu Q, Zhang Y, Zheng X, Li Y, et al. Lysine lactylation regulates metabolic pathways and biofilm formation in Streptococcus mutans. Sci Signal. (2023) 16:eadg1849. doi: 10.1126/scisignal.adg1849
183. Dong H, Zhang J, Zhang H, Han Y, Lu C, Chen C, et al. YiaC and CobB regulate lysine lactylation in Escherichia coli. Nat Commun. (2022) 13:6628. doi: 10.1038/s41467-022-34399-y
184. Wang J, Liu Z, Xu Y, Wang Y, Wang F, Zhang Q, et al. Enterobacterial LPS-inducible LINC00152 is regulated by histone lactylation and promotes cancer cells invasion and migration. Front Cell Infect Microbiol. (2022) 12:913815. doi: 10.3389/fcimb.2022.913815
185. Li W, Zhou J, Gu Y, Chen Y, Huang Y, Yang J, et al. Lactylation of RNA m6A demethylase ALKBH5 promotes innate immune response to DNA herpesviruses and mpox virus. Proc Natl Acad Sci U S A. (2024) 121:e2409132121. doi: 10.1073/pnas.24091321216
186. Liu B, Tian X, Li L, Zhang R, Wu J, Jiang N, et al. Severe fever with thrombocytopenia syndrome virus induces lactylation of m6A reader protein YTHDF1 to facilitate viral replication. EMBO Rep. (2024) 25:5599–619. doi: 10.1038/s44319-024-00310-7
187. Yan Q, Zhou J, Gu Y, Huang W, Ruan M, Zhang H, et al. Lactylation of NAT10 promotes N4-acetylcytidine modification on tRNASer-CGA-1–1 to boost oncogenic DNA virus KSHV reactivation. Cell Death Differ. (2024) 31:1362–74. doi: 10.1038/s41418-024-01327-0
Keywords: lactate, histone lactylation, macrophage plasticity, inflammatory bowel disease, postoperative ileus, gastrointestinal oncology
Citation: Che X, Zhang Y, Chen X, Xie G, Li J, Xu C, Zhang C, Zhu Y and Yang X (2025) The lactylation-macrophage interplay: implications for gastrointestinal disease therapeutics. Front. Immunol. 16:1608115. doi: 10.3389/fimmu.2025.1608115
Received: 10 April 2025; Accepted: 05 June 2025;
Published: 09 July 2025.
Edited by:
Junji Xing, Houston Methodist Research Institute, United StatesReviewed by:
Guangchuan Wang, Jinzhou Medical University, ChinaCuncai Guo, Washington University in St. Louis, United States
Copyright © 2025 Che, Zhang, Chen, Xie, Li, Xu, Zhang, Zhu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Zhu, MTUxNjg4ODYzOTZAMTYzLmNvbQ==; Xinyu Yang, MTY5NjIxMjgzN0BxcS5jb20=
 Xinzhen Che
Xinzhen Che Yixin Zhang2
Yixin Zhang2 Xiqi Chen
Xiqi Chen Yong Zhu
Yong Zhu Xinyu Yang
Xinyu Yang