- 1Department of Burns and Plastic Surgery, Kunming Children′s Hospital, Children′s Hospital Affiliated to Kunming Medical University, Kunming, China
- 2Department of Orthopaedic, The Third Affiliated Hospital of Shenzhen University (Luohu People’s Hospital), Shenzhen, China
- 3Department of Dermatology, The Affiliated Hospital of Yunnan University, Kunming, China
Melanoma frequently develops bone metastases, leading to skeletal-related events and poor survival. The tumor microenvironment (TME) plays a pivotal role in melanoma progression, bone metastasis, and immunotherapy resistance. Key immunosuppressive cells including myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs), regulatory T cells (Tregs), and cancer-associated fibroblasts (CAFs) promote immune evasion and osteolytic bone destruction via RANKL-dependent and -independent mechanisms. Immune checkpoint inhibitors (ICIs), including anti-CTLA-4 and anti-PD-1/PD-L1 therapies, have revolutionized melanoma treatment, yet resistance remains common due to TME immunosuppression. Emerging strategies, such as combination therapies, aim to enhance efficacy by reshaping the TME. This review synthesizes current knowledge on TME-driven immunosuppression, bone metastasis mechanisms, and immunotherapeutic advancements, offering insights into overcoming resistance and improving patient outcomes.
1 Introduction
Melanoma is an aggressive skin cancer characterized by early metastasis and accounts for nearly 90% of deaths from malignant skin tumors despite its relatively low incidence (1, 2). While early-stage cases are surgically curable with favorable outcomes, advanced melanoma exhibits high invasiveness, poor response to radiotherapy and chemotherapy, and a five-year survival rate of only 30% (3, 4). Up to 17% of patients develop metastatic bone disease, leading to skeletal-related events, reduced quality of life, and poorer survival (5). Bone metastasis depends on interactions between tumor cells and the tumor microenvironment (TME), particularly immune components (6, 7).
Recent progress in immunotherapy has offered promising treatment strategies for melanoma (8, 9). Its high immunogenicity enables the immune system to recognize tumor-associated antigens, facilitating immune checkpoint blockade (10). Agents targeting immune checkpoint inhibitors (ICIs), including CTLA-4, PD-1, and PD-L1, have significantly improved survival and are now the standard of care for advanced melanoma (11). However, the immunosuppressive nature of the TME remains a critical barrier, limiting treatment responses and contributing to resistance in many patients (12). Consequently, understanding the mechanisms of immunotherapy and the composition and function of the TME is critical for effectively controlling melanoma progression and improving overall patient survival (13). This review focuses on how the TME shapes melanoma development, bone metastasis, and immunotherapy response, integrating current therapeutic approaches, analyzing their mechanisms and limitations, and providing insight into novel strategies for improving immunotherapy response in melanoma.
2 Immunosuppressive cells in tumor microenvironment of melanoma
2.1 Myeloid-derived suppressor cells
MDSCs represent a heterogeneous population of immature myeloid cells that exert immunosuppressive functions (14). During tumor progression, MDSCs are recruited and activated by multiple proinflammatory cytokines, such as prostaglandin E2 (PGE2), granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), and CCR5, which are released within the TME (15). In melanoma, MDSCs produce immunosuppressive molecules including nitric oxide synthase, reactive oxygen species, and arginase-1, thereby inhibiting T-cell activation and inducing T-cell apoptosis and cell-cycle arrest (16). They also activate the STAT3 pathway, promoting epithelial–mesenchymal transition and expediting tumor immune escape (16). In addition to their immunomodulatory functions, MDSCs exert tumor-promoting effects through mechanisms independent of immune regulation, including stimulation of angiogenesis and establishment of a premetastatic niche (16). Clinically, an increased abundance of MDSCs within the tumor microenvironment has been correlated with poor responsiveness to immune checkpoint blockade in patients with melanoma (17). Accordingly, targeting MDSCs hold potential to improve treatment outcomes. Blattner et al. (18) showed that a CCR5-Ig fusion protein blocked CCR5–CCL5 interactions, inhibiting MDSC recruitment and prolonging survival in patients with melanoma. STAT3 inhibitor napabucasin induced MDSC apoptosis and extended survival in a murine melanoma model (16). These findings underscore the value of targeting MDSCs in melanoma therapy.
2.2 Tumor-associated macrophages
TAMs constitute a major component of the TME and can be broadly classified into M1 and M2 phenotypes based on their function and activation states (19). During melanoma progression, the recruitment of M2 macrophages exceeds that of M1 macrophages. M1-type TAMs secrete classical inflammatory cytokines that induce tumor cell necrosis, promote immune-cell infiltration into the TME, and eliminate tumor cells through phagocytosis and destruction (20), whereas M2-type TAMs exhibit immunosuppressive properties, facilitating tumor progression and distant metastasis through multifaceted mechanisms. First, they enhance tumor cell proliferation and invasion by secreting cytokines such as tumor necrosis factor beta (TNF-β), cyclooxygenase-2 (COX-2), and interleukin-10 (IL-10), along with matrix metalloproteinases (MMPs) that degrade the extracellular matrix and facilitate melanoma cell dissemination (20). Second, they promote angiogenesis by modulating adrenomedullin secretion, hypoxia-inducible factor-1α, and vascular endothelial growth factor (VEGF)-A (21). Third, they contribute to immune evasion by recruiting regulatory T cells (Tregs) and secreting immunosuppressive molecules such as IL-10, indoleamine 2,3-dioxygenase (IDO), and PD-L1 expression, which collectively suppress effector T cell (Teff) activity (21). Besides, they mediate resistance to targeted therapies via TNF-α-induced activation of the nuclear factor-κB pathway and upregulation of Sox family transcription factors in BRAF/MEK inhibitor-resistant melanoma models (22). Collectively, M2-type TAMs drive melanoma progression via the secretion of a broad range of bioactive mediators, making their selective inhibition or reprogramming toward the antitumor M1 phenotype an attractive therapeutic strategy. Han et al. (20) demonstrated that a baicalin-loaded nanocomplex targeting M2-type TAMs effectively inhibited melanoma growth by inducing proinflammatory M1 polarization and reshaping the TME, underscoring the therapeutic potential of TAM phenotype modulation.
2.3 Tregs and CD8+ T cells
Tregs contribute to immunosuppression and weak responsiveness to ICIs. They achieve immunosuppression by secreting inhibitory cytokines such as IL-10, IL-35, and TGF-β, as well as perforin and granzyme, which hamper the activation and proliferation of Teff and neutrophils (12, 23). Tregs also express multiple inhibitory checkpoint receptors, including lymphocyte-activation gene 3 (LAG-3), PD-1, and CTLA-4, thereby promoting immune tolerance (24). Hence, depleting Tregs within the TME has emerged as a promising therapeutic approach. Studies have shown that targeting Tregs in the TME restores Teff function and bolsters antitumor immunity in mouse models of melanoma (23). Notably, the intratumoral Teff/Treg ratio has been proposed as a predictive biomarker for immunotherapy outcomes (20). Cytotoxic CD8+ T cells recognize tumor antigens via MHC-I molecules and eliminate malignant cells through perforin and granzyme B-mediated apoptosis. They also secrete IFN-γ and TNF, which sustain antigen presentation and amplify T-cell responses (25). However, the immunosuppressive milieu of the TME often impairs CD8+ T-cell activity, facilitating tumor immune evasion.
2.4 NK cells and dendritic cells
NK cells can recognize melanoma cells that are resistant to T cell–mediated cytotoxicity and thus play an auxiliary role in anti-cancer immunity. Conversely, melanoma cells can suppress NK-cell function to evade immune surveillance. Studies have shown that melanoma cells secrete IDO and PGE2 to downregulate the expression of activating receptors (NKp30, NKp44, and NKG2D) on NK cells, thereby impairing their tumor-killing activity (26). Furthermore, Lee et al. (27) demonstrated that tumor cells can bind immunosuppressive receptors on the NK-cell surface to inhibit NK-cell activation. Consequently, promoting NK-cell infiltration and activation, via binding to tumor-cell surface ligands, targeting NK-cell–activating receptors, or blocking inhibitory receptors on NK cells, can enhance NK-cell–mediated tumor immunity and immune surveillance. Dendritic cells (DCs), the most potent antigen-presenting cells, orchestrate antitumor immunity through efficient cross-presentation and T cell priming. In melanoma, mature DCs expressing CD80 and CD86 are essential for activating tumor-specific T cells (28). However, melanoma cells within the TME secrete IL-6, IL-10, VEGF, and TGF-β, which disrupt DC recruitment and maturation, thereby impairing T-cell activation and promoting melanoma progression (28). However, tumor-derived cytokines and growth factors, notably IL-10, VEGF, and TGF-β, can skew DC differentiation toward a tolerogenic phenotype characterized by reduced expression of costimulatory molecules, impaired antigen presentation, and increased secretion of immunosuppressive cytokines (29–31). These tolerogenic DCs suppress effector T cell activation, promote the expansion of regulatory T cells, and contribute to immune evasion. Prokopi et al. (32) observed a significant reduction in DCs in human primary melanoma lesions, which was associated with poorer prognosis. A study by Tucci et al. (33) showed that metastatic melanoma patients have lower DC counts than non-metastatic patients; the number of DCs was negatively correlated with Treg count and positively correlated with low melanoma recurrence risk. Hence, enhancing DC activity in the TME is an effective therapeutic approach. Prokopi et al. (32) further developed a DC-boosting therapy that increases both the quantity and activation status of intratumoral DCs and Teff cells, thereby augmenting tumor immunogenicity and sensitizing melanoma to immunotherapy.
2.5 Cancer-associated fibroblasts
CAFs are the most abundant stromal cells in the TME of cutaneous malignant melanoma. They are highly heterogeneous and plastic, and can influence melanoma initiation, progression, metastasis, and drug resistance in various ways. First, CAFs secrete cytokines that favor melanoma invasion, including IL-6, IL-8, transforming TGF-β, β-catenin, fibroblast growth factor-2 (FGF-2), and VEGF (34). Second, CAFs suppress CD8+ T cells and NK cells. Érsek et al. (12) reported that CAFs inhibited CD8+ T cell cytotoxicity by depleting L-arginine. Romano et al. (34) further showed that CAF-derived matrix metalloproteinases and prostaglandin E2 reduced expression of activation receptors on NK cells, resulting in NK-cell inactivation. Third, CAFs promote resistance to immunotherapy and targeted therapy. Zhao et al. (35) discovered that CAFs secreted MMP9, which cleaved PD-L1 on the surface of melanoma cells and contributed to diminished responses to anti–PD-1 therapy. Diazzi et al. (36) found that CAFs produced neuregulin 1, along with large amounts of collagen and fibronectin, rendering melanoma cells unresponsive to MAPK inhibitors. Targeting CAFs may therefore offer a novel therapeutic strategy by improving antitumor immunity and immune surveillance in melanoma. Indeed, inhibiting MMP9/TGF-β expression reversed CAF-induced resistance to anti–PD-1 therapy and increased the ratio of CD8+ T cells to Tregs in vivo (35).
3 The role of immune cells in melanoma bone metastasis
Importantly, beyond their role in immune evasion, immunosuppressive cells such as MDSCs and TAMs also actively shape the metastatic niche in bone (37, 38). These cells secrete pro-osteoclastogenic cytokines (IL-6, TNF-α) and growth factors that promote osteoclast differentiation, thereby facilitating bone resorption (39, 40). Specifically, M2-polarized TAMs drive osteoclastogenesis via converging mechanisms. They secrete RANKL, M-CSF, IL-6 and TNF-α, which directly induce the differentiation and activation of osteoclast precursors. Concurrently, the release of matrix metalloproteinases and VEGF facilitates bone matrix remodeling and generates permissive niches for osteoclast function. Moreover, by shaping a cytokine-rich microenvironment, these TAMs sustain osteoclastic activity and promote persistent bone resorption (41, 42). TAMs, particularly the M2 phenotype, accumulate in the bone microenvironment where they enhance osteoclast activation (43, 44), while MDSCs serve as osteoclast precursors that, under the influence of RANKL and inflammatory cytokines, differentiate into mature osteoclasts and secrete IL-1β and cathepsin K, further amplifying bone resorption (45, 46). This dual role in immune suppression and skeletal remodeling establishes a permissive microenvironment for melanoma bone metastasis. Melanoma cells secrete various factors that induce TAMs recruitment, including VEGF-C, GM-CSF, M-CSF, and MCP-1 (47). Tumor-associated macrophages represent the predominant inflammatory cell population within both primary and metastatic melanoma lesions.
3.1 RANKL-dependent osteoclast formation
Clinical studies have demonstrated a significant correlation between increased TAM infiltration and enhanced melanoma aggressiveness (48). Functionally, these TAMs secrete a variety of pro-tumorigenic mediators such as IL-8, VEGF, and fibroblast growth factor (FGF), which collectively contribute to tumor progression and neovascularization in melanoma (49–51). Metastatic melanoma lesions are primarily osteolytic, driven by osteoclasts rather than tumor cells themselves (52, 53). Osteoclasts originate from hematopoietic mononuclear progenitor cells and belong to the mononuclear phagocyte system (54). These osteoclast precursor cells circulate among monocytes and exhibit characteristic monocyte/macrophage surface markers (52, 55). Their differentiation process is regulated by two essential factors: M-CSF and the RANK-RANKL signaling pathway. Osteoclast precursors expressing RANK interact with RANKL-presenting cells in bone tissue, while this interaction can be negatively regulated by osteoprotegerin secreted by osteoblasts and other cell types (56, 57). TAMs in melanoma metastases express CD14 but lack osteoclast markers (TRAP, VNR) and resorptive capacity until exposed to RANKL and M-CSF, inducing TRAP+ VNR+ multinucleated osteoclast formation. Melanoma-stromal interactions critically influence tumor progression and metastasis. Melanoma-associated fibroblasts may promote osteoclast formation and activation via soluble RANKL, akin to fibroblasts in giant cell tumors of bone (58), unlike normal fibroblasts from skin or bone marrow stroma (59–61). Tumor-associated stromal cells in primary melanoma not only promote cancer progression but also potentially contribute to bone metastasis by inducing osteoclast differentiation and subsequent bone destruction (62). The precise mechanisms underlying osteolytic lesions in melanoma remain unclear but involve RANKL-dependent crosstalk between tumor cells, stromal components, and osteoclast precursors.
3.2 RANKL-independent osteoclast formation
Emerging evidence suggests that osteoclastogenesis can be activated through RANKL-independent mechanisms mediated by various cytokines and growth factors, such as TNF-α, IL-6, IL-8, and TGF-β, which promote the differentiation of both bone marrow-derived and circulating osteoclast precursors (63–65). Notably, these cytokine-induced osteoclasts exhibit distinct morphological and functional differences compared to their RANKL-stimulated counterparts. While RANKL stimulation typically produces large multinucleated osteoclasts capable of extensive lacunar resorption, exposure to TNF-α and IL-1 results in the formation of significantly smaller osteoclasts containing fewer than four nuclei (48). These cytokine-derived osteoclasts demonstrate limited resorptive capacity, typically creating only single resorption pits, reflecting their alternative differentiation pathway. In the melanoma TME, these pro-osteoclastogenic cytokines activate intracellular signaling pathways that further amplify bone destruction and immune suppression (6). TNF-α engages TNF receptor 1/2, leading to activation of the NF-κB pathway through the IκB kinase (IKK) complex, which induces nuclear translocation of NF-κB subunits and transcription of osteoclastogenic and inflammatory genes (66, 67). IL-6 signals primarily via the gp130/JAK complex, activating both the JAK/STAT3 and MAPK (ERK1/2) pathways, thereby promoting osteoclast precursor differentiation and survival (68, 69). Similarly, CXCL8 interacts with CXCR1/CXCR2 receptors, triggering downstream PI3K–Akt and MAPK cascades, which synergize with NF-κB to enhance osteoclast maturation and the release of pro-angiogenic factors (70–72). These pathways not only promote osteoclastogenesis but also contribute to melanoma cell survival, invasiveness, and immune evasion by reshaping the bone metastatic niche (73, 74). Thus, cytokine-driven NF-κB and MAPK activation represents a crucial molecular bridge between immunosuppression and bone destruction in metastatic melanoma (75).
4 Melanoma-associated immunotherapy
4.1 Monotherapy with immune checkpoint inhibitors
CTLA-4, an inhibitory checkpoint receptor, suppresses T-cell activation by competing with CD28 for ligands CD80/CD86, depriving costimulatory signals (76). Ipilimumab, the first FDA-approved ICI, blocks CTLA-4-ligand interaction, enhancing tumor-infiltrating T-cells while suppressing Tregs in the TME (77). It significantly improves OS in metastatic melanoma versus chemotherapy, enabling durable disease control (11). PD-1/PD-L1 inhibitors are widely used ICIs. PD-1, expressed on T-cells, binds PD-L1 on tumor cells to inhibit T-cell function, facilitating immune evasion. Melanomas often overexpress PD-L1, correlating with poor prognosis. Anti-PD-1/PD-L1 antibodies (nivolumab, pembrolizumab, atezolizumab) disrupt this axis, enhancing antitumor immunity. Pembrolizumab shows superior response rates, PFS, and OS versus ipilimumab with reduced toxicity (78). Since 2017, anti-PD-1 monotherapy has served as adjuvant therapy for high-risk resected melanoma (50), though predictive biomarkers remain elusive. Despite these advances, the immunosuppressive TME frequently leads to primary or acquired ICI resistance (79). Mechanistically, melanoma cells upregulate alternative checkpoint receptors such as TIM−3, LAG−3, and TIGIT, which maintain T−cell exhaustion even after PD−1/PD−L1 or CTLA−4 blockade (80–82). In addition, activation of WNT/β−catenin signaling excludes dendritic cells and effector T cells from tumor lesions, generating a “cold” microenvironment that fails to respond to ICIs (83). Furthermore, metabolic suppressive pathways, including IDO1–mediated tryptophan depletion and arginase−1–driven arginine catabolism, diminish T−cell proliferation and effector function, further reinforcing immune evasion. Beyond PD-1/PD-L1 and CTLA-4, other checkpoints like TIM-3, TIGIT, and VISTA are co-expressed in melanoma TME, particularly on Tregs, marking them as potential targets (12) (Figure 1).
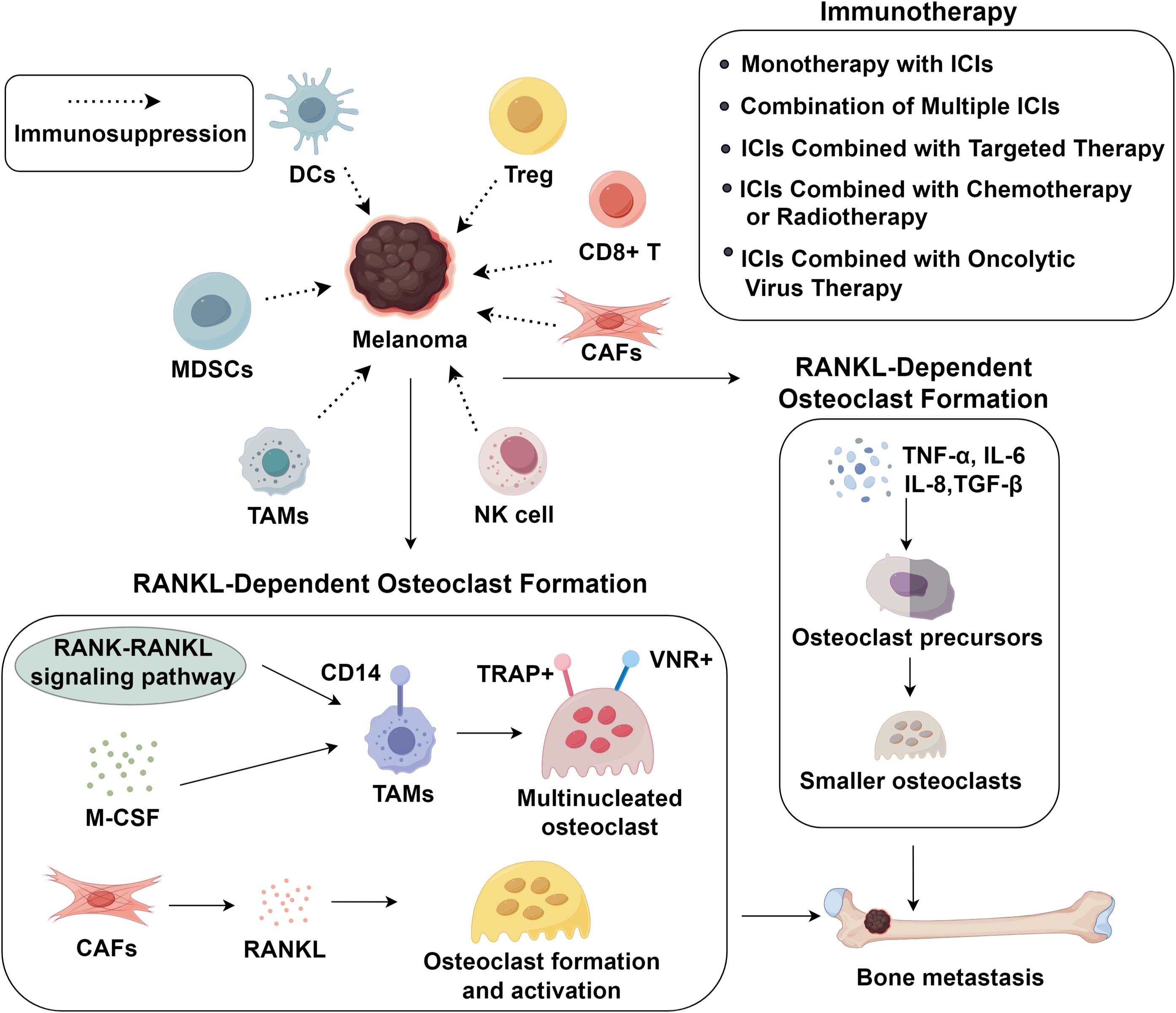
Figure 1. Immunosuppressive tumor microenvironment and advance in immunotherapy in melanoma bone metastasis.
4.2 Combination therapies with ICIs
4.2.1 Combination of multiple ICIs
Despite the survival benefits of ICI monotherapy, low response rates often require combination strategies. CTLA-4 and PD-1 inhibit T-cell activation via distinct mechanisms: CTLA-4 modulates early priming (lymph nodes), while PD-1 suppresses effector-phase proliferation (84). Their complementary actions suggest dual blockade may yield synergistic effects. A phase III trial demonstrated improved overall survival (OS) with ipilimumab-nivolumab combination versus monotherapy, but severe (grade 3/4) treatment-emergent adverse events (TEAEs) rose to 55.0% versus 27.3% with ipilimumab alone (85). This toxicity likely reflects systemic immune overactivation, necessitating optimization of dosing and sequencing to mitigate off-target effects. Beyond PD-1/CTLA-4 combinations, Opdualag (relatlimab-nivolumab) for unresectable/metastatic melanoma, targeting LAG-3, a next-generation checkpoint suppressing immunity via: (1) MHC II binding on antigen-presenting cells and (2) interaction with liver sinusoidal endothelial cell lectin on tumors, inhibiting CD4+/CD8+ T-cell function (86, 87). This innovation highlights the potential of novel dual-checkpoint strategies to broaden therapeutic efficacy while underscoring the need for improved safety profiles. Oncolytic virus (OV) therapy offers a distinct modality by selectively lysing tumor cells and initiating systemic immune activation. Upon intratumoral replication, OVs release tumor antigens and virions, triggering adaptive immunity against surrounding malignancies (88). The first OV for unresectable melanoma, has been shown to enhance T-cell infiltration and reverse PD-L1–mediated immunosuppression (88). Hence, combining OVs with ICIs may overcome drug resistance caused by high PD-L1 expression and restore antitumor immune responses (89).
4.2.2 ICIs combined with chemotherapy or radiotherapy
Due to melanoma’s limited chemosensitivity, combining ICIs with chemotherapy is often used in advanced cases resistant to PD-1 blockade (90). Chemotherapeutics like dacarbazine, temozolomide, and platinum agents induce immunogenic cell death (ICD), releasing DAMPs and converting immunologically “cold” tumors into “hot” ones, enhancing ICI efficacy (88, 91). Preclinical studies show that ipilimumab combined with melphalan improves survival, reduces Tregs, and increases CD8+/Treg ratios in melanoma models (92). However, chemotherapy’s nonspecific cytotoxicity risks leukopenia, careful evaluation is warranted when combining ICIs and chemotherapy. Radiotherapy also exhibits immunomodulatory effects, promoting antigen presentation, type I interferon release, and a pro-inflammatory TME (93). Notably, radiotherapy induces the release of tumor-specific antigens, thus boosting T-cell–mediated tumor recognition (93). Clinical data indicate that radiotherapy and ICIs have synergistic effects. Saieg et al. (94) observed both local tumor regression and abscopal responses with ipilimumab-radiotherapy co-treatment. In unresectable or locally advanced melanoma, such combinations improved objective response and disease control without significantly increasing severe toxicity (95). For BRAFV600E-mutant melanoma, MAPK inhibitors achieve rapid responses, yet median progression-free survival remains under 12 months (96). In contrast, ICI offer more durable immunologic memory. Mechanistically, BRAF inhibition enhances tumor antigen presentation, synergizing with PD-1/PD-L1 blockade (97). The IMspire150 phase III trial confirmed that adding atezolizumab to vemurafenib and cobimetinib significantly improved median progression-free survival over dual-target therapy (98).
5 Conclusion
Melanoma’s aggressive progression and bone metastasis are orchestrated by a dynamic interplay between tumor cells and the immunosuppressive TME. Immunosuppressive cells including MDSCs, M2-polarized TAMs, Tregs, and CAFs drive immune evasion, osteoclast activation, and therapy resistance. While ICIs have transformed melanoma management, their efficacy is limited by the TME’s inhibitory landscape. Combination strategies, such as dual checkpoint blockade, ICI-targeted therapy, or oncolytic viruses, show promise in overcoming resistance by modulating immune cell function and enhancing antigen presentation. Future research should focus on identifying predictive biomarkers, optimizing therapeutic sequencing, and developing novel TME-targeted agents to improve durable responses. A deeper understanding of TME-immune crosstalk will be critical for advancing precision immunotherapy and mitigating skeletal complications in metastatic melanoma.
Author contributions
YM: Writing – original draft. LZ: Writing – original draft. WL: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Garbe C, Amaral T, Peris K, Hauschild A, Arenberger P, Basset-Seguin N, et al. European consensus-based interdisciplinary guideline for melanoma. Part 1: Diagnostics: Update 2022. Eur J Cancer. (2022) 170:236–55. doi: 10.1016/j.ejca.2022.03.008
2. Wilczak M, Surman M, and PrzybyłO M. Melanoma-derived extracellular vesicles transfer proangiogenic factors. Oncol Res. (2025) 33:245–62. doi: 10.32604/or.2024.055449
3. Huang J, Chan SC, Ko S, Lok V, Zhang L, Lin X, et al. Global incidence, mortality, risk factors and trends of melanoma: A systematic analysis of registries. Am J Clin Dermatol. (2023) 24:965–75. doi: 10.1007/s40257-023-00795-3
4. Zhou JG, Liang R, Wang HT, Jin SH, Hu W, Frey B, et al. Identification and characterization of circular RNAs as novel putative biomarkers to predict anti-PD-1 monotherapy response in metastatic melanoma patients - Knowledge from two independent international studies. Neoplasia. (2023) 37:100877. doi: 10.1016/j.neo.2023.100877
5. Shimizu MR, de Groot TM, Twining PK, Kobes T, Ferrone M, Raskin K, et al. Factors associated with skeletal-related events in patients with bone metastatic melanoma: A retrospective study of 481 patients. J Surg Oncol. (2024) 130:310–21. doi: 10.1002/jso.27731
6. Fornetti J, Welm AL, and Stewart SA. Understanding the bone in cancer metastasis. J Bone Miner Res. (2018) 33:2099–113. doi: 10.1002/jbmr.3618
7. Xie H, Xi X, Lei T, Liu H, and Xia Z. CD8(+) T cell exhaustion in the tumor microenvironment of breast cancer. Front Immunol. (2024) 15:1507283. doi: 10.3389/fimmu.2024.1507283
8. Luo W, Song D, He Y, Song J, and Ding Y. Tumor vaccines for Malignant melanoma: progress, challenges, and future directions. Oncol Res. (2025) 33:1875–93. doi: 10.32604/or.2025.063843
9. Chandra S, Wilson JC, Good D, and Wei MQ. mRNA vaccines: a new era in vaccine development. Oncol Res. (2024) 32:1543–64. doi: 10.32604/or.2024.043987
10. Garbe C, Amaral T, Peris K, Hauschild A, Arenberger P, Basset-Seguin N, et al. European consensus-based interdisciplinary guideline for melanoma. Part 2: Treatment - Update 2022. Eur J Cancer. (2022) 170:256–84. doi: 10.1016/j.ejca.2022.04.018
11. Carlino MS, Larkin J, and Long GV. Immune checkpoint inhibitors in melanoma. Lancet. (2021) 398:1002–14. doi: 10.1016/S0140-6736(21)01206-X
12. Simiczyjew A, Dratkiewicz E, Mazurkiewicz J, Ziętek M, Matkowski R, and Nowak D. The influence of tumor microenvironment on immune escape of melanoma. Int J Mol Sci. (2020) 21:8359. doi: 10.3390/ijms21218359
13. Sikorski H, Żmijewski MA, and Piotrowska A. Tumor microenvironment in melanoma-characteristic and clinical implications. Int J Mol Sci. (2025) 26:6778. doi: 10.3390/ijms26146778
14. Deng Y, Shi M, Yi L, Naveed Khan M, Xia Z, and Li X. Eliminating a barrier: Aiming at VISTA, reversing MDSC-mediated T cell suppression in the tumor microenvironment. Heliyon. (2024) 10:e37060. doi: 10.1016/j.heliyon.2024.e37060
15. Li K, Shi H, Zhang B, Ou X, Ma Q, Chen Y, et al. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct Target Ther. (2021) 6:362. doi: 10.1038/s41392-021-00670-9
16. Bitsch R, Kurzay A, Özbay Kurt F, de la Torre C, Lasser S, Lepper A, et al. STAT3 inhibitor Napabucasin abrogates MDSC immunosuppressive capacity and prolongs survival of melanoma-bearing mice. J Immunother Cancer. (2022) 10:e004384. doi: 10.1136/jitc-2021-004384
17. Ozbay Kurt FG, Lasser S, Arkhypov I, Utikal J, and Umansky V. Enhancing immunotherapy response in melanoma: myeloid-derived suppressor cells as a therapeutic target. J Clin Invest. (2023) 133:e170762. doi: 10.1172/JCI170762
18. Blattner C, Fleming V, Weber R, Himmelhan B, Altevogt P, Gebhardt C, et al. et al: CCR5(+) myeloid-derived suppressor cells are enriched and activated in melanoma lesions. Cancer Res. (2018) 78:157–67. doi: 10.1158/0008-5472.CAN-17-0348
19. Zhang Y, Qin N, Wang X, Liang R, Liu Q, Geng R, et al. Glycogen metabolism-mediated intercellular communication in the tumor microenvironment influences liver cancer prognosis. Oncol Res. (2024) 32:563–76. doi: 10.32604/or.2023.029697
20. Han S, Wang W, Wang S, Yang T, Zhang G, Wang D, et al. Tumor microenvironment remodeling and tumor therapy based on M2-like tumor associated macrophage-targeting nano-complexes. Theranostics. (2021) 11:2892–916. doi: 10.7150/thno.50928
21. Nasrollahzadeh E, Razi S, Keshavarz-Fathi M, Mazzone M, and Rezaei N. Pro-tumorigenic functions of macrophages at the primary, invasive and metastatic tumor site. Cancer Immunol Immunother. (2020) 69:1673–97. doi: 10.1007/s00262-020-02616-6
22. Smith MP, Sanchez-Laorden B, O’Brien K, Brunton H, Ferguson J, Young H, et al. The immune microenvironment confers resistance to MAPK pathway inhibitors through macrophage-derived TNFα. Cancer Discov. (2014) 4:1214–29. doi: 10.1158/2159-8290.CD-13-1007
23. Noyes D, Bag A, Oseni S, Semidey-Hurtado J, Cen L, Sarnaik AA, et al. Tumor-associated Tregs obstruct antitumor immunity by promoting T cell dysfunction and restricting clonal diversity in tumor-infiltrating CD8+ T cells. J Immunother Cancer. (2022) 10:e004605. doi: 10.1136/jitc-2022-004605
24. McRitchie BR and Akkaya B. Exhaust the exhausters: Targeting regulatory T cells in the tumor microenvironment. Front Immunol. (2022) 13:940052. doi: 10.3389/fimmu.2022.940052
25. Durgeau A, Virk Y, Corgnac S, and Mami-Chouaib F. Recent advances in targeting CD8 T-cell immunity for more effective cancer immunotherapy. Front Immunol. (2018) 9:14. doi: 10.3389/fimmu.2018.00014
26. van Vliet AA, Georgoudaki AM, Raimo M, de Gruijl TD, and Spanholtz J. Adoptive NK cell therapy: A promising treatment prospect for metastatic melanoma. Cancers (Basel). (2021) 13:4722. doi: 10.3390/cancers13184722
27. Lee H, Da Silva IP, Palendira U, Scolyer RA, Long GV, and Wilmott JS. Targeting NK cells to enhance melanoma response to immunotherapies. Cancers (Basel). (2021) 13:1363. doi: 10.3390/cancers13061363
28. Marzagalli M, Ebelt ND, and Manuel ER. Unraveling the crosstalk between melanoma and immune cells in the tumor microenvironment. Semin Cancer Biol. (2019) 59:236–50. doi: 10.1016/j.semcancer.2019.08.002
29. Harrell CR, Pavlovic D, Miloradovic D, Stojanovic MD, Djonov V, and Volarevic V. Derived multiple allogeneic protein paracrine signaling (d-MAPPS)” Enhances T cell-driven immune response to murine mammary carcinoma. Anal Cell Pathol (Amst). (2022) 2022:3655595. doi: 10.1155/2022/3655595
30. Sakabe R, Onishi K, Mochizuki J, Toshimitsu T, Shimazu T, Kishino S, et al. Regulation of IL-10 production in dendritic cells is controlled by the co-activation of TLR2 and Mincle by Lactiplantibacillus plantarum OLL2712. Microbiol Spectr. (2025) 13:e0119624. doi: 10.1128/spectrum.01196-24
31. Mo Z, Yu F, Han S, Yang S, Wu L, Li P, et al. New peptide MY1340 revert the inhibition effect of VEGF on dendritic cells differentiation and maturation via blocking VEGF-NRP-1 axis and inhibit tumor growth in vivo. Int Immunopharmacol. (2018) 60:132–40. doi: 10.1016/j.intimp.2018.04.025
32. Prokopi A, Tripp CH, Tummers B, Hornsteiner F, Spoeck S, Crawford JC, et al. Skin dendritic cells in melanoma are key for successful checkpoint blockade therapy. J Immunother Cancer. (2021) 9:e000832. doi: 10.1136/jitc-2020-000832
33. Tucci M, Stucci LS, Mannavola F, Passarelli A, D’Oronzo S, Lospalluti L, et al. Defective levels of both circulating dendritic cells and T-regulatory cells correlate with risk of recurrence in cutaneous melanoma. Clin Transl Oncol. (2019) 21:845–54. doi: 10.1007/s12094-018-1993-2
34. Romano V, Belviso I, Venuta A, Ruocco MR, Masone S, Aliotta F, et al. Influence of tumor microenvironment and fibroblast population plasticity on melanoma growth, therapy resistance and immunoescape. Int J Mol Sci. (2021) 22:5283. doi: 10.3390/ijms22105283
35. Zhao F, Evans K, Xiao C, DeVito N, Theivanthiran B, Holtzhausen A, et al. Stromal fibroblasts mediate anti-PD-1 resistance via MMP-9 and dictate TGFβ Inhibitor sequencing in melanoma. Cancer Immunol Res. (2018) 6:1459–71. doi: 10.1158/2326-6066.CIR-18-0086
36. Diazzi S, Tartare-Deckert S, and Deckert M. Bad neighborhood: fibrotic stroma as a new player in melanoma resistance to targeted therapies. Cancers (Basel). (2020) 12:1364. doi: 10.3390/cancers12061364
37. Li Z, Xia Q, He Y, Li L, and Yin P. MDSCs in bone metastasis: Mechanisms and therapeutic potential. Cancer Lett. (2024) 592:216906. doi: 10.1016/j.canlet.2024.216906
38. Meng C, Lin K, Shi W, Teng H, Wan X, DeBruine A, et al. Histone methyltransferase ASH1L primes metastases and metabolic reprogramming of macrophages in the bone niche. Nat Commun. (2025) 16:4681. doi: 10.1038/s41467-025-59381-2
39. Li Z, Zhao Y, Chen Z, Katz J, Michalek SM, Li Y, et al. Age-related expansion and increased osteoclastogenic potential of myeloid-derived suppressor cells. Mol Immunol. (2021) 137:187–200. doi: 10.1016/j.molimm.2021.07.004
40. Yan L, Liang M, Yang T, Ji J, Jose Kumar GS Sreena, Hou X, et al. The immunoregulatory role of myeloid-derived suppressor cells in the pathogenesis of rheumatoid arthritis. Front Immunol. (2020) 11:568362. doi: 10.3389/fimmu.2020.568362
41. Sousa S and Määttä J. The role of tumour-associated macrophages in bone metastasis. J Bone Oncol. (2016) 5:135–8. doi: 10.1016/j.jbo.2016.03.004
42. Sun Y, Li J, Xie X, Gu F, Sui Z, Zhang K, et al. Macrophage-osteoclast associations: origin, polarization, and subgroups. Front Immunol. (2021) 12:778078. doi: 10.3389/fimmu.2021.778078
43. Batoon L and McCauley LK. Cross talk between macrophages and cancer cells in the bone metastatic environment. Front Endocrinol (Lausanne). (2021) 12:763846. doi: 10.3389/fendo.2021.763846
44. Mendoza-Reinoso V, McCauley LK, and Fournier PGJ. Contribution of macrophages and T cells in skeletal metastasis. Cancers (Basel). (2020) 12:1014. doi: 10.3390/cancers12041014
45. Wu MY, Li CJ, Yiang GT, Cheng YL, Tsai AP, Hou YT, et al. Molecular regulation of bone metastasis pathogenesis. Cell Physiol Biochem. (2018) 46:1423–38. doi: 10.1159/000489184
46. Zhang H, Huang Y, Wang S, Fu R, Guo C, Wang H, et al. Myeloid-derived suppressor cells contribute to bone erosion in collagen-induced arthritis by differentiating to osteoclasts. J Autoimmun. (2015) 65:82–9. doi: 10.1016/j.jaut.2015.08.010
47. Skobe M, Hamberg LM, Hawighorst T, Schirner M, Wolf GL, Alitalo K, et al. Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am J Pathol. (2001) 159:893–903. doi: 10.1016/S0002-9440(10)61765-8
48. Torisu H, Ono M, Kiryu H, Furue M, Ohmoto Y, Nakayama J, et al. Macrophage infiltration correlates with tumor stage and angiogenesis in human Malignant melanoma: possible involvement of TNFalpha and IL-1alpha. Int J Cancer. (2000) 85:182–8. doi: 10.1002/(SICI)1097-0215(20000115)85:2%3C182::AID-IJC6%3E3.0.CO;2-M
49. Nesbit M, Schaider H, Miller TH, and Herlyn M. Low-level monocyte chemoattractant protein-1 stimulation of monocytes leads to tumor formation in nontumorigenic melanoma cells. J Immunol. (2001) 166:6483–90. doi: 10.4049/jimmunol.166.11.6483
50. Shimizu T, Abe R, Nakamura H, Ohkawara A, Suzuki M, and Nishihira J. High expression of macrophage migration inhibitory factor in human melanoma cells and its role in tumor cell growth and angiogenesis. Biochem Biophys Res Commun. (1999) 264:751–8. doi: 10.1006/bbrc.1999.1584
51. Callejo SA, Marshall JC, Cools-Lartigue J, Saraiva VS, and Burnier MN Jr. Macrophage-derived soluble factor enhances melanoma inhibitory activity expression by uveal melanoma cells in vitro. Melanoma Res. (2004) 14:91–5. doi: 10.1097/00008390-200404000-00003
52. Mundy GR. Mechanisms of osteolytic bone destruction. Bone. (1991) 12:S1–6. doi: 10.1016/8756-3282(91)90057-P
53. Clohisy DR, Perkins SL, and Ramnaraine ML. Review of cellular mechanisms of tumor osteolysis. Clin Orthop Relat Res. (2000) 373:104–14. doi: 10.1097/00003086-200004000-00013
54. Lázár-Molnár E, Hegyesi H, Tóth S, and Falus A. Autocrine and paracrine regulation by cytokines and growth factors in melanoma. Cytokine. (2000) 12:547–54. doi: 10.1006/cyto.1999.0614
55. Minkin C. Bone acid phosphatase: tartrate-resistant acid phosphatase as a marker of osteoclast function. Calcif Tissue Int. (1982) 34:285–90. doi: 10.1007/BF02411252
56. Ogg GS, Dunbar PR, Cerundolo V, McMichael AJ, Lemoine NR, and Savage P. Sensitization of tumour cells to lysis by virus-specific CTL using antibody-targeted MHC class I/peptide complexes. Br J Cancer. (2000) 82:1058–62. doi: 10.1054/bjoc.1999.1042
57. Perez M, Migliaccio S, Taranta A, Festuccia C, Orrù L, Brama M, et al. Melanoma cells stimulate osteoclastogenesis, c-Src expression and osteoblast cytokines. Eur J Cancer. (2001) 37:629–40. doi: 10.1016/S0959-8049(00)00436-6
58. Quinn JM, Matsumura Y, Tarin D, McGee JO, and Athanasou NA. Cellular and hormonal mechanisms associated with Malignant bone resorption. Lab Invest. (1994) 71:465–71.
59. Quinn JM and Athanasou NA. Tumour infiltrating macrophages are capable of bone resorption. J Cell Sci. (1992) 101:681–6. doi: 10.1242/jcs.101.3.681
60. Quinn JM, Horwood NJ, Elliott J, Gillespie MT, and Martin TJ. Fibroblastic stromal cells express receptor activator of NF-kappa B ligand and support osteoclast differentiation. J Bone Miner Res. (2000) 15:1459–66. doi: 10.1359/jbmr.2000.15.8.1459
61. Quinn JM, McGee JO, and Athanasou NA. Human tumour-associated macrophages differentiate into osteoclastic bone-resorbing cells. J Pathol. (1998) 184:31–6. doi: 10.1002/(SICI)1096-9896(199801)184:1<31::AID-PATH962>3.0.CO;2-V
62. Sabokbar A, Itonaga I, Sun SG, Kudo O, and Athanasou NA. Arthroplasty membrane-derived fibroblasts directly induce osteoclast formation and osteolysis in aseptic loosening. J Orthop Res. (2005) 23:511–9. doi: 10.1016/j.orthres.2004.10.006
63. Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. (1997) 89:309–19. doi: 10.1016/S0092-8674(00)80209-3
64. Spatz A, Ruiter DJ, Busch C, Theodorovic I, and Oosterhuis JW. The role of the EORTC pathologist in clinical trials: achievements and perspectives. European Organisation for Research and Treatment of Cancer. Eur J Cancer. (2002) 38:S120–124. doi: 10.1016/s0959-8049(01)00445-2
65. Sun SG, Lau YS, Itonaga I, Sabokbar A, and Athanasou NA. Bone stromal cells in pagetic bone and Paget’s sarcoma express RANKL and support human osteoclast formation. J Pathol. (2006) 209:114–20. doi: 10.1002/path.1953
66. Murphy JM, Jeong K, Cioffi DL, Campbell PM, Jo H, Ahn EE, et al. Focal adhesion kinase activity and localization is critical for TNF-α-induced nuclear factor-κB activation. Inflammation. (2021) 44:1130–44. doi: 10.1007/s10753-020-01408-5
67. Cheng HM, Xing M, Zhou YP, Zhang W, Liu Z, Li L, et al. HSP90β promotes osteoclastogenesis by dual-activation of cholesterol synthesis and NF-κB signaling. Cell Death Differ. (2023) 30:673–86. doi: 10.1038/s41418-022-01071-3
68. Heinrich PC, Behrmann I, Müller-Newen G, Schaper F, and Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. (1998) 334:297–314. doi: 10.1042/bj3340297
69. Ilamathi M, Prabu PC, Ayyappa KA, and Sivaramakrishnan V. Artesunate obliterates experimental hepatocellular carcinoma in rats through suppression of IL-6-JAK-STAT signalling. BioMed Pharmacother. (2016) 82:72–9. doi: 10.1016/j.biopha.2016.04.061
70. Meng ZW, Zhang L, Cai XR, Wang X, She FF, and Chen YL. IL-8 is a novel prometastatic chemokine in intrahepatic cholangiocarcinoma that induces CXCR2-PI3K/AKT signaling upon CD97 activation. Sci Rep. (2023) 13:18711. doi: 10.1038/s41598-023-45496-3
71. Zhu Y, Yang S, Zhao N, Liu C, Zhang F, Guo Y, et al. CXCL8 chemokine in ulcerative colitis. BioMed Pharmacother. (2021) 138:111427. doi: 10.1016/j.biopha.2021.111427
72. Li Y, Zhang K, Ai X, Zhang Q, Jiang L, Long J, et al. et al: A Biomimetic Peptide Functions as Specific Extracellular Matrix for Quiescence of Stem Cells against Intervertebral Disc Degeneration. Small. (2023) 19:e2300578. doi: 10.1002/smll.202300578
73. Tamura T, Yamamoto T, Kogure A, Yoshioka Y, Yamamoto Y, Sakamoto S, et al. Extracellular vesicles from prostate cancer-corrupted osteoclasts drive a chain reaction of inflammatory osteolysis and tumour progression at the bone metastatic site. J Extracell Vesicles. (2025) 14:e70091. doi: 10.1002/jev2.70091
74. Dawalibi A, Alosaimi AA, and Mohammad KS. Balancing the scales: the dual role of interleukins in bone metastatic microenvironments. Int J Mol Sci. (2024) 25:8163. doi: 10.3390/ijms25158163
75. Tang R, Xu X, Yang W, Yu W, Hou S, Xuan Y, et al. et al: MED27 promotes melanoma growth by targeting AKT/MAPK and NF-κB/iNOS signaling pathways. Cancer Lett. (2016) 373:77–87. doi: 10.1016/j.canlet.2016.01.005
76. Budden T, Gaudy-Marqueste C, Porter A, Kay E, Gurung S, Earnshaw CH, et al. Ultraviolet light-induced collagen degradation inhibits melanoma invasion. Nat Commun. (2021) 12:2742. doi: 10.1038/s41467-021-22953-z
77. Robert C, Ribas A, Schachter J, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. (2019) 20:1239–51. doi: 10.1016/S1470-2045(19)30388-2
78. Chang CY, Park H, Malone DC, Wang CY, Wilson DL, Yeh YM, et al. Immune checkpoint inhibitors and immune-related adverse events in patients with advanced melanoma: A systematic review and network meta-analysis. JAMA Netw Open. (2020) 3:e201611. doi: 10.1001/jamanetworkopen.2020.1611
79. Xu H, Li S, Liu Y, Sung YY, Zhou Y, and Wu H. A novel pH-sensitive nanoparticles encapsulating anti-PD-1 antibody and MDK-siRNA overcome immune checkpoint blockade resistance in HCC via reshaping immunosuppressive TME. J Exp Clin Cancer Res. (2025) 44:148. doi: 10.1186/s13046-025-03396-6
80. Zhang C, Shen H, Yang T, Li T, Liu X, Wang J, et al. A single-cell analysis reveals tumor heterogeneity and immune environment of acral melanoma. Nat Commun. (2022) 13:7250. doi: 10.1038/s41467-022-34877-3
81. Li J, Smalley I, Chen Z, Wu JY, Phadke MS, Teer JK, et al. single-cell characterization of the cellular landscape of acral melanoma identifies novel targets for immunotherapy. Clin Cancer Res. (2022) 28:2131–46. doi: 10.1158/1078-0432.CCR-21-3145
82. Cadiou G, Beauvais T, Marotte L, Lambot S, Deleine C, Vignes C, et al. Differential impact of genetic deletion of TIGIT or PD-1 on melanoma-specific T-lymphocytes. Oncoimmunology. (2024) 13:2376782. doi: 10.1080/2162402X.2024.2376782
83. Zhou Y, Xu J, Luo H, Meng X, Chen M, and Zhu D. Wnt signaling pathway in cancer immunotherapy. Cancer Lett. (2022) 525:84–96. doi: 10.1016/j.canlet.2021.10.034
84. Ascierto PA, Del Vecchio M, Mandalá M, Gogas H, Arance AM, Dalle S, et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. (2020) 21:1465–77. doi: 10.1016/S1470-2045(20)30494-0
85. Bagchi S, Yuan R, and Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. (2021) 16:223–49. doi: 10.1146/annurev-pathol-042020-042741
86. Paik J. Nivolumab plus relatlimab: first approval. Drugs. (2022) 82:925–31. doi: 10.1007/s40265-022-01723-1
87. Thudium K, Selby M, Zorn JA, Rak G, Wang XT, Bunch RT, et al. Preclinical characterization of relatlimab, a human LAG-3-blocking antibody, alone or in combination with nivolumab. Cancer Immunol Res. (2022) 10:1175–89. doi: 10.1158/2326-6066.CIR-22-0057
88. He M, Yang T, Wang Y, Wang M, Chen X, Ding D, et al. Immune checkpoint inhibitor-based strategies for synergistic cancer therapy. Adv Healthc Mater. (2021) 10:e2002104. doi: 10.1002/adhm.202002104
89. Chesney J, Puzanov I, Collichio F, Milhem MM, Hauschild A, Chen L, et al. Patterns of response with talimogene laherparepvec in combination with ipilimumab or ipilimumab alone in metastatic unresectable melanoma. Br J Cancer. (2019) 121:417–20. doi: 10.1038/s41416-019-0530-6
90. Bouchereau S, Chaplain L, Fort M, Beauchet A, Sidibé T, Chapalain M, et al. Impact of prior treatment with immune checkpoint inhibitors on dacarbazine efficacy in metastatic melanoma. Br J Cancer. (2021) 125:948–54. doi: 10.1038/s41416-021-01486-8
91. Pradeep J, Win TT, Aye SN, and Sreeramareddy CT. Efficacy and safety of immune checkpoint inhibitors for advanced Malignant melanoma: A meta-analysis on monotherapy vs combination therapy. J Cancer. (2022) 13:3091–102. doi: 10.7150/jca.72210
92. Ariyan CE, Brady MS, Siegelbaum RH, Hu J, Bello DM, Rand J, et al. Robust antitumor responses result from local chemotherapy and CTLA-4 blockade. Cancer Immunol Res. (2018) 6:189–200. doi: 10.1158/2326-6066.CIR-17-0356
93. Procureur A, Simonaggio A, Bibault JE, Oudard S, and Vano YA. Enhance the immune checkpoint inhibitors efficacy with radiotherapy induced immunogenic cell death: A comprehensive review and latest developments. Cancers (Basel). (2021) 13:678. doi: 10.3390/cancers13040678
94. Saiag P, Molinier R, Roger A, Boru B, Otmezguine Y, Otz J, et al. Efficacy of large use of combined hypofractionated radiotherapy in a cohort of anti-PD-1 monotherapy-treated melanoma patients. Cancers (Basel). (2022) 14:4069. doi: 10.3390/cancers14174069
95. Salama AKS, Palta M, Rushing CN, Selim MA, Linney KN, Czito BG, et al. ipilimumab and radiation in patients with high-risk resected or regionally advanced melanoma. Clin Cancer Res. (2021) 27:1287–95. doi: 10.1158/1078-0432.CCR-20-2452
96. Wang Y, Liu S, Yang Z, Algazi AP, Lomeli SH, Wang Y, et al. Anti-PD-1/L1 lead-in before MAPK inhibitor combination maximizes antitumor immunity and efficacy. Cancer Cell. (2021) 39:1375–1387.e1376. doi: 10.1016/j.ccell.2021.07.023
97. Frederick DT, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res. (2013) 19:1225–31. doi: 10.1158/1078-0432.CCR-12-1630
98. Gutzmer R, Stroyakovskiy D, Gogas H, Robert C, Lewis K, Protsenko S, et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAF(V600) mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. (2020) 395:1835–44. doi: 10.1016/S0140-6736(20)30934-X
Keywords: melanoma, bone metastasis, osteoclasts, tumor microenvironment, immune checkpoint inhibitors, immunotherapy
Citation: Ma Y, Zhang L and Liu W (2025) Immunosuppressive tumor microenvironment and advance in immunotherapy in melanoma bone metastasis. Front. Immunol. 16:1608215. doi: 10.3389/fimmu.2025.1608215
Received: 08 April 2025; Accepted: 06 August 2025;
Published: 27 August 2025.
Edited by:
Jin Bin, Shandong University, ChinaReviewed by:
Benhua Li, Second People’s Hospital of Liangshan Yi Autonomous Prefecture, ChinaCopyright © 2025 Ma, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weimin Liu, ZHJsd20xNUAxNjMuY29t
†These authors have contributed equally to this work
 Yiqun Ma1†
Yiqun Ma1† Lin Zhang
Lin Zhang Weimin Liu
Weimin Liu