- 1Department of Acupuncture Rehabilitation, Changsha Hospital of Traditional Chinese Medicine (Changsha No.8 Hospital), Changsha, Hunan, China
- 2Department of Neurology, Hunan Provincial Hospital of Integrated Traditional Chinese and Western Medicine (The Affiliated Hospital of Hunan Academy of Traditional Chinese Medicine), Changsha, Hunan, China
- 3Graduate School, Hunan University of Chinese Medicine, Changsha, Hunan, China
Stroke ranks among the most prevalent diseases globally. Ischemic stroke (IS), constituting 87% of all strokes, poses a significant threat to patients’ health. Following the onset of IS, within a few minutes, inflammation is initiated. This inflammation activates immune cells and related signaling pathways, further exacerbating the inflammatory state, eventually leading to irreversible brain injury. Therefore, regulating the inflammatory response can contribute to the treatment of IS. This review delves into the inflammatory mechanism in IS, the role of inflammatory markers, and the research advancements regarding the use of inflammatory markers in treatment. While previous studies often concentrated on a single aspect of the inflammatory mechanism or specific inflammatory markers, this review systematically synthesizes the fragmented information. It offers readers a comprehensive and coherent view, facilitating an in - depth comprehension of the complexity of the inflammatory response in IS. This article not only expounds the value of inflammatory markers in disease diagnosis and prognosis, but also highlights the research progress including traditional Chinese medicine treatment. This has important guiding significance for clinicians to formulate precise treatment plans, and provides a variety of options for clinical practice. The research progress of these treatment strategies presents new opportunities for addressing the challenges in IS treatment, with the potential to improve patients’ prognosis and quality of life. Through a comprehensive overview of inflammation - related studies in IS, this review serves as a valuable reference for research and clinical practice in this field, contributing to the further development of IS treatment research.
1 Introduction
Stroke primarily results from the rupture or occlusion of cerebral blood vessels, which disrupts the normal blood supply to brain tissue, thereby inducing corresponding cerebral injury and neurological deficits. It remains one of the most widespread and debilitating diseases affecting the global population (1). A report indicates that in 2016, stroke prevalence in the United States is 2.5%, affecting approximately 7 million individuals (2). IS mortality in China is 30% and 70% of survivors suffer from sequelae (3). An estimate suggests that the annual cost of stroke during 2014–2015 was $45.5 billion (4). Among various types of stroke, approximately 87% are IS, making ischemia the most common cause (5).
IS is characterized by a series of neuropathological processes, in which a robust and persistent inflammatory response is a key factor in the progression of brain injury. The inflammation following ischemia is associated with the acute impairment of the blood brain barrier (BBB), leading to poorer neurological outcomes. In the initial stage of IS, neuroinflammation aggravates brain damage, however, the inflammatory response may promote neural repair at later stages (6). IS not only trigger local inflammation in the brain but also rapidly induce a strong systemic inflammatory response syndrome(SIRS), characterized by the release of inflammatory mediators and the activation of peripheral immune cells. Emerging evidence suggests that this peripheral inflammation is not merely a consequence of central nervous system (CNS) injury but actively contributes to neuroinflammation (7). Peripheral cytokines, chemokines, and infiltrating leukocytes can exacerbate neuroinflammation by crossing the compromised BBB or signaling through alternative pathways. Recent studies have implicated dysregulation of the gut-brain axis, characterized by altered gut microbiota composition and permeability, as a significant contributor to peripheral inflammation that can modulate CNS inflammation following a stroke.
This article comprehensively details the inflammatory mechanisms in IS, underlines the significance of related inflammatory markers in the diagnosis and treatment of IS, and also emphasizes the integration of multi - domain knowledge, including traditional Chinese medicine approaches. A profound understanding of the neuroinflammatory response in IS will provide a solid foundation for the development of innovative ideas and novel intervention strategies.
2 Pathophysiology of inflammation in IS
Neuroinflammation is recognized as a pivotal component in the pathophysiology of IS. A systematic integration and in depth exploration of the inflammatory response mechanism in IS hold the potential to offer novel concepts and therapeutic targets for the treatment of IS.IS typically occurs when a blood vessel supplying the brain becomes occluded. The abrupt interruption of blood circulation leads to hypoxia in brain tissue, which triggers an inflammatory response. This state disrupts the ionic balance, giving rise to neuronal excitotoxicity, oxidative stress and lipid peroxidation, ultimately culminating in neuronal damage and irreversible neurological impairment (8).
2.1 Inflammatory cell activation
Inflammatory cells are activated after cerebral ischemic injury. Microglia, astrocytes, neutrophils, and monocytes show different characteristics of subtypes, which play pro-inflammatory and neuroprotective functions respectively. Studies have shown that different functional subtypes are related to the stage of the disease, so how to reverse the unfavorable situation is the focus of attention.
2.1.1 Resident cells of the CNS
Microglia, brain-resident macrophages, maintain immune surveillance and synaptic pruning under normal conditions. Following IS, they polarize into M1 (pro-inflammatory) and M2 (anti-inflammatory) phenotypes. M1 microglia secrete proteases, cytokines, and reactive oxygen species (ROS) via p38 MAPK, Notch, and JAK2/STAT3 pathways, exacerbating BBB disruption and neuroinflammation. In contrast, M2 microglia phagocytose debris and release neurotrophic factors to promote repair (9–13). Additionally, in vitro and in vivo studies have demonstrated that gene regulation promotes M2 polarization, which may be related to the inhibition of the TLR4/NFκB/NLRP3 signaling pathway (14–16).
Recent research has introduced a novel M2 microglia-targeting lipid nanoparticle (MLNP) method, which selectively delivers mRNA encoding phenotype-switching interleukin(IL) -10to the ischemic brain. This approach facilitates the transformation of microglia into the M2 phenotype, thereby alleviating neuroinflammation and restoring the BBB compromised by ischemia (17). Additionally, TEPP-46, an agonist of the pyruvate kinase isozyme type M2 (PKM2), has been shown to promote the transition of microglia from the M1 to the M2 phenotype following ischemia/reperfusion injury by inhibiting microglial glycolysis (18). While it is understood that microglia exhibit opposing roles at various stages of cerebral infarction (CI), the phenotypic transition from M1 to M2 is likely critical for patient prognosis. However, the time-dependent nature of this transition complicates the identification of an optimal treatment window. Furthermore, the transformation of microglial phenotypes is intricately linked to the surrounding microenvironment, including interactions with various inflammatory cell types (19). These aspects represent significant challenges that future research must address.
Astrocytes maintain BBB integrity and neurovascular homeostasis. After IS, reactive astrocytes polarize into two subtypes, each associated with distinct functions that correlate with the disease stage. In the acute phase, A1 reactive astrocytes secrete pro-inflammatory cytokines such as vascular endothelial growth factor, matrix metalloproteinase, and lipid carrier protein-2, which exacerbate damage to endothelial cells and disrupt tight junctions. Conversely, A2 reactive astrocytes produce protective factors for endothelial cells, including pentacyclic triterpenoids 3, Sonic hedgehog, and angiopoietin 1. During the recovery phase of IS, both A1 and A2 astrocytes contribute to glial scar formation, albeit through different mechanisms. A1 astrocytes facilitate glial scar formation and inhibit axonal growth via glial fibrillary acidic protein, chondroitin sulfate proteoglycan, and transforming growth factor-β (TGF-β), while A2 astrocytes promote axonal growth through platelet-derived growth factor and play a critical role in vascular remodeling (20).Recent studies have identified that 3-hydroxyquinazoline (3-HKA) can inhibit the activation of A1-type (neurotoxic) astrocytes and promote the polarization of A2-type (neuroprotective) astrocytes, thereby enhancing vascular remodeling post-stroke (21). Furthermore, the latest research has revealed a strong correlation between Lcn2 levels and the polarization of astrocyte phenotypes. By inhibiting Lcn2 to modulate astrocyte phenotype, neuroinflammation can be alleviated, and the repair of the BBB can be promoted (22). Unlike microglia, the “double-edged sword” effect of astrocytes is manifested in both spatial and functional dimensions: different functions may yield both beneficial and detrimental outcomes, complicating regulatory mechanisms. Thus, it is imperative to clearly define the disease stage and employ tailored regulatory strategies for astrocytes. However, whether in experimental or clinical studies, the delineation between the acute and recovery phases remains ambiguous, posing significant challenges for future research.
2.1.2 Recruitment of immune cells at the periphery
Following a stroke, there is a significant increase in the release of peripheral inflammatory markers. This phenomenon occurs not only in the damaged brain tissue but also within the peripheral circulatory system. Urinary tract infections and pneumonia are among the most common complications following a stroke and represent significant sources of peripheral inflammation (23, 24). These peripheral inflammatory responses play a crucial regulatory role in the immune inflammatory response post-stroke, promoting the development of CI and influencing both the severity and progression of the disease.
Neutrophils rapidly infiltrate ischemic brain tissue within 6–12 hours post-stroke, peaking at 2–7 days and persisting for ≥2 weeks. They exacerbate injury by releasing neutrophil extracellular traps (NETs), inflammatory mediators, and matrix metalloproteinases (MMPs), which disrupt the BBB and promote neurotoxicity (25–29).Neutrophils exhibit dual phenotypes: pro-inflammatory N1 cells secrete harmful substances, while protective N2 cells enhance clearance by macrophages and reduce infarct volume. Ischemic neurons induce N1 polarization via conditioned medium, increasing NET formation. TLR4-deficient mice show augmented N2 infiltration and reduced injury, suggesting TLR4 modulates neutrophil phenotype toward protection (30–32).
Lymphocytes play a crucial role in the development of stroke. T cells migrate to ischemic brain regions within 24 hours of stroke, with CD4+ and CD8+ subsets releasing cytotoxic proteins (granzyme/perforin) and cytokines (interferon-γ/IL) that damage neurons and disrupt BBB integrity (33–35). Regulatory T cells (Tregs), however, correlate with reduced infarct volume and improved outcomes via amphiregulin - mediated suppression of neurotoxic astrocyte proliferation. Treg deficiency increases apoptotic neurons and astrocyte neurotoxic gene expression, reversed by Treg adoptive transfer (36–38).
Monocytes migrate to ischemic brain regions early after stroke, with classical monocytes secreting pro-inflammatory cytokines [IL-1/6/tumor necrosis factor(TNF)-α] and MMP-2/9 that disrupt BBB tight junctions and exacerbate injury via CCL2/CCR2 and P2X4R pathways (39–41). Non-classical monocytes secrete IL-10 to counteract inflammation and promote repair (42). Ly6Chi monocytes differentiate into M1 (pro-inflammatory) or M2 (anti-inflammatory) macrophages: M1 produces IL-6/IL-1β/TNF-α, while M2 releases angiogenic cytokines. This M1→M2 phenotypic switch occurs by day 7 post-stroke, suggesting therapeutic potential in accelerating this transition to improve outcomes (43–48).
Neutrophils, lymphocytes, and monocytes are not merely isolated inflammatory cells; rather, they are pivotal in elucidating the pathophysiology of CI and in the development of novel therapeutic strategies. These peripheral recruited immune cells are advantageous due to their accessibility for sample collection, which significantly benefits clinical research. Recent clinical observations indicate that the Neutrophil-to-Lymphocyte Ratio (NLR) and the Lymphocyte-to-Monocyte Ratio (LMR) independently influence the poor prognosis of elderly patients with Acute Ischemic Stroke (AIS). Notably, the area under the ROC curve (AUC) for the combination of NLR and LMR surpasses that of NLR and LMR alone, suggesting that integrating these two indicators enhances predictive accuracy for clinical outcomes in elderly AIS patients (49). Furthermore, the relationship between these inflammatory cells and the brain-gut axis represents an important avenue for contemporary research on CI. Recent studies have shown that gut microbiota significantly influences neutrophil activation in middle cerebral artery occlusion(MCAO) mice, thereby affecting stroke severity. This research implies that targeting the microbiota with antibiotics may mitigate the exacerbation of disease linked to activated neutrophils in stroke patients; however, this hypothesis requires validation through clinical trials (50). Additionally, integrating these peripheral immune cells with factors such as age, gender, underlying diseases, and comorbidities in patients with CI can yield further insights into their interconnections. Moreover, dynamically observing the changes in these inflammatory markers throughout the progression of CI is essential for a comprehensive understanding of the disease.
2.2 Exosomal vesicles
EVs are lipid bilayer nanoparticles secreted by cells, playing a crucial role in mediating neuro-immune communication following ischemic events. EVs released by microglia, astrocytes, and infiltrating immune cells carry proteins, miRNAs, and inflammatory factors, and they bidirectionally regulate injury and repair processes. Vesicles derived from choroid plexus epithelial (CPE) cells contain miR-155-5p, which inhibits the expression of Ras homolog enriched in brain (Rheb) and promotes NLRP3-mediated inflammasome activation, thereby exacerbating cerebral ischemic injury (51). Conversely, numerous studies have demonstrated that in rodent IS models, EVs treatment inhibits the polarization of macrophages and microglia towards pro-inflammatory (M1) phenotypes or promotes anti-inflammatory (M2) phenotypes, thereby improving neurological outcomes (52, 53). Research indicates that M2 microglia deliver miR-124 and TGF-β, which inhibit inflammatory responses and promote neuronal survival (54, 55) Additionally, EVs derived from mesenchymal stem cells (MSCs) and adipose-derived stem cells (ADSCs) alleviate neuroinflammation and inhibit cell apoptosis and pyroptosis through the TLR4/NF-κB pathway and NLRP3/GSDMD signaling pathway (56, 57). However, some studies have reported that in rat ischemia-reperfusion models, MSC-derived EV treatment does not significantly affect the infiltration of immune cells (including CD45+ white blood cells, neutrophils, dendritic cells, macrophages, monocytes, and lymphocytes) in the ipsilateral parenchyma (58, 59). In conclusion, while the extracellular vesicle delivery system may represent a potential therapeutic strategy for IS, controversies persist. The unclear routine usage methods, diverse transplantation doses, and varying observation time points pose significant challenges to its widespread clinical application.
2.3 Activation of inflammasome
Inflammasomes are a complex of diverse proteins that play a crucial role in glia - mediated inflammation within the nervous system. These complexes are involved in the assembly of cytoplasmic pattern - recognition receptors and are essential components of the innate immune system. The function of these inflammasomes is to recruit and activate pro - inflammatory caspase - 1, thereby influencing the maturation and release of cytokines and other inflammatory mediators, including IL-1β and IL-18 (60–62). A critical step in the formation and activation of the NLRP3 inflammasome involves the TLR4/NF - κB signaling pathway (Figure 1). Studies have shown that multicompound can inhibit TLR4/NF - κB signaling pathway, thereby preventing the activation of NLRP3 inflammasome in microglia, and then down-regulating the expression of downstream ACS and caspase-1, reducing the release of pro-inflammatory factors IL-1β, IL-18 and TNF-α, and ultimately alleviating ischemic brain damage (63, 64). In recent years, both in vivo and in vitro experiments have demonstrated that directly targeting NLRP3 can alleviate neuroinflammation associated with stroke. The pathways involved include SIRT3-NLRP3, FPR1/NLRP3, and the NLRP3/caspase-1/IL-1β signaling axis (65–68). In summary, the activation of the inflammasome and the release of pro-inflammatory cytokines are critical factors contributing to further damage during the pathological process of IS, mediated by microglia. Investigating the upstream and downstream signals of inflammasome activation and their interaction mechanisms will aid in the development of new regulators, addressing the limitations of currently available IS therapies and offering new hope to patients.
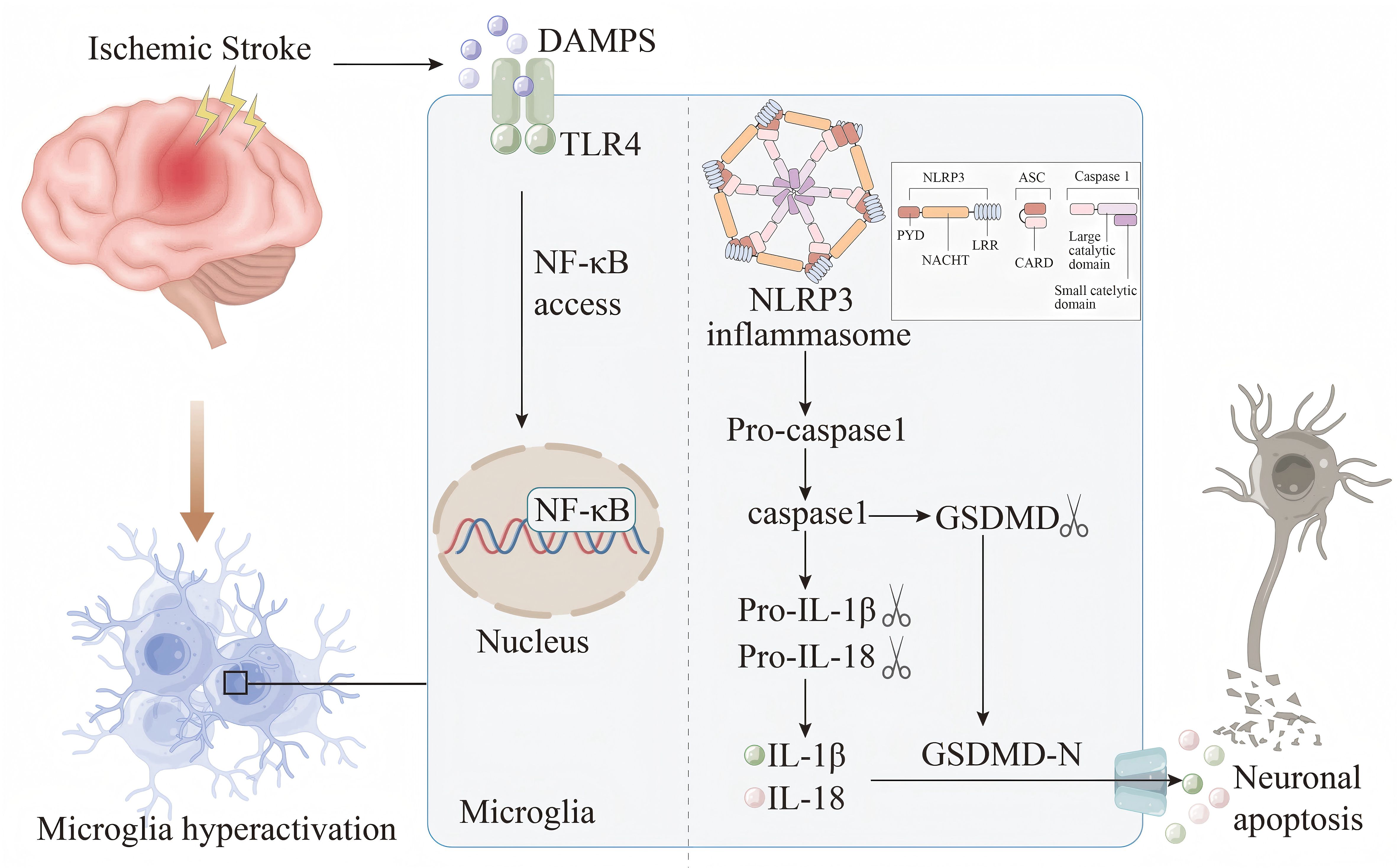
Figure 1. Damp-associated molecular patterns (DAMPs) are released by damaged or necrotic brain cells after IS. DAMPs are subsequently recognized by the TLR4 receptor and trigger the NF-κB pathway.This activation facilitates the assembly and subsequent activation of the NLRP3 inflammasome, setting off a cascade that results in the maturation and secretion of IL-1β and IL-18. Meanwhile, the activated caspase-1 cleaves gasdermin D (GSDMD) to produce GSDMD-N. The active form GSDMD-N is then transported to the plasma membrane, where it interacts with intimal lipids to create pores in the plasma membrane. This process leads to the release of intracellular inflammatory cytokines, which exacerbates the inflammatory response, disrupts the BBB, induces cell apoptosis, and contributes to the progression of IS.
2.4 Brain-gut axis
In recent years, numerous studies have demonstrated that following brain injury, brain-derived damage-associated molecular patterns (DAMPs) enter the systemic circulation through the compromised BBB. Immune cells recognize these DAMPs via pattern recognition receptors, such as Toll-like receptors (TLRs), thereby activating both systemic and local inflammatory responses in the intestine (1). Dysbiosis of the gut microbiota contributes to neuroinflammation and peripheral immune responses post-stroke. Alterations in the microbiota-gut-brain axis (MGBA) subsequent to stroke can ultimately disrupt the gut microbiota and increase intestinal permeability. Such changes may facilitate the translocation of bacteria from the gut to sterile organs, further exacerbating the incidence of stroke-related infections (69, 70). Research indicates that gut microbiota significantly influence neutrophil activation after stroke, directly correlating with stroke severity (50). Additionally, disruption of the gut microbiota impacts the differentiation of intestinal dendritic cells and alters the balance between gut Treg cells and γδ T cells. This imbalance results in a reduction of Treg cells that produce the anti-inflammatory cytokine IL-10 in the gut, alongside an increase in γδ T cells that migrate to the brain injury site and release pro-inflammatory factors such as IFN-γ and IL-17 (71). Furthermore, vagus nerve dysfunction impairs communication between the gut and the brain, exacerbating metabolic imbalances, neuroinflammation, and cognitive impairments following stroke (72). In summary, the brain-gut axis offers a novel perspective for understanding the pathophysiological mechanisms underlying CI, highlighting the significant impact of the gut microbiota on brain outcomes. Although much of the current research in this domain remains at the basic research stage, its therapeutic potential is substantial. Regulating the gut microbiota to enhance outcomes in CI represents a promising treatment strategy.
3 Inflammatory markers
Inflammatory markers are detectable substances generated by cells or present in plasma during an inflammatory reaction. These markers reflect the presence, extent, and nature of inflammation within the body and are of great significance for the diagnosis, monitoring, and prognostic evaluation of inflammatory diseases. A further exploration of the role of inflammatory markers in IS may offer novel insights for the treatment of IS.
3.1 Cytokines
A diverse range of cytokines plays crucial roles in IS, exhibiting both pro-inflammatory and anti-inflammatory properties. Pro-inflammatory cytokines, such as TNF-α, IL-1, and IL-18, contribute to infarct expansion and neuroinflammation, while anti-inflammatory cytokines, including IL-10, IL-2, IL-4, and TGF-β, exert neuroprotective effects (73). Notably, some cytokines, such as IL-6, exhibit context-dependent roles across different phases of IS. The ideal treatment strategy is to precisely inhibit the pro-inflammatory trans-signaling pathways while retaining or even enhancing their beneficial classical signaling pathways. Although the use of soluble gp130 (sgp130) to selectively inhibit trans-signaling pathways is a promising direction (74), achieving this precise regulation in vivo remains challenging. Recently, employing the B/T ratio to determine the time window value for IL-6 signaling in regulating inflammation has attracted significant attention (75). In addition to the challenges associated with intervention during the time window, individual differences also influence the role of IL-6 in neurological functional outcomes. Therefore, future IL-6-targeted therapies are unlikely to adopt a “one-size-fits-all” approach; individualized treatment strategies must be considered (Tables 1, 2).
3.2 Chemokines
Chemokines were initially identified as factors that attract circulating leukocytes to sites of inflammation or injury (95). The role of chemokines in IS is two - fold, presenting both advantages and disadvantages. Chemokines can be categorized into four families, namely CXC, CC, CX3C, and C, based on the number of amino acids separating their first two cysteine residues (96). Nevertheless, research on C - family chemokines is still limited. To date, only one review has suggested that XCL1 is involved in the interaction of neuroimmune cells and can increase the number of neurons, indicating a potential association among XCL1, neurogenesis, and angiogenesis (97). Subsequently, the relationships between the CC, CXC, and CX3C families and IS are summarized as follows (Figure 2).
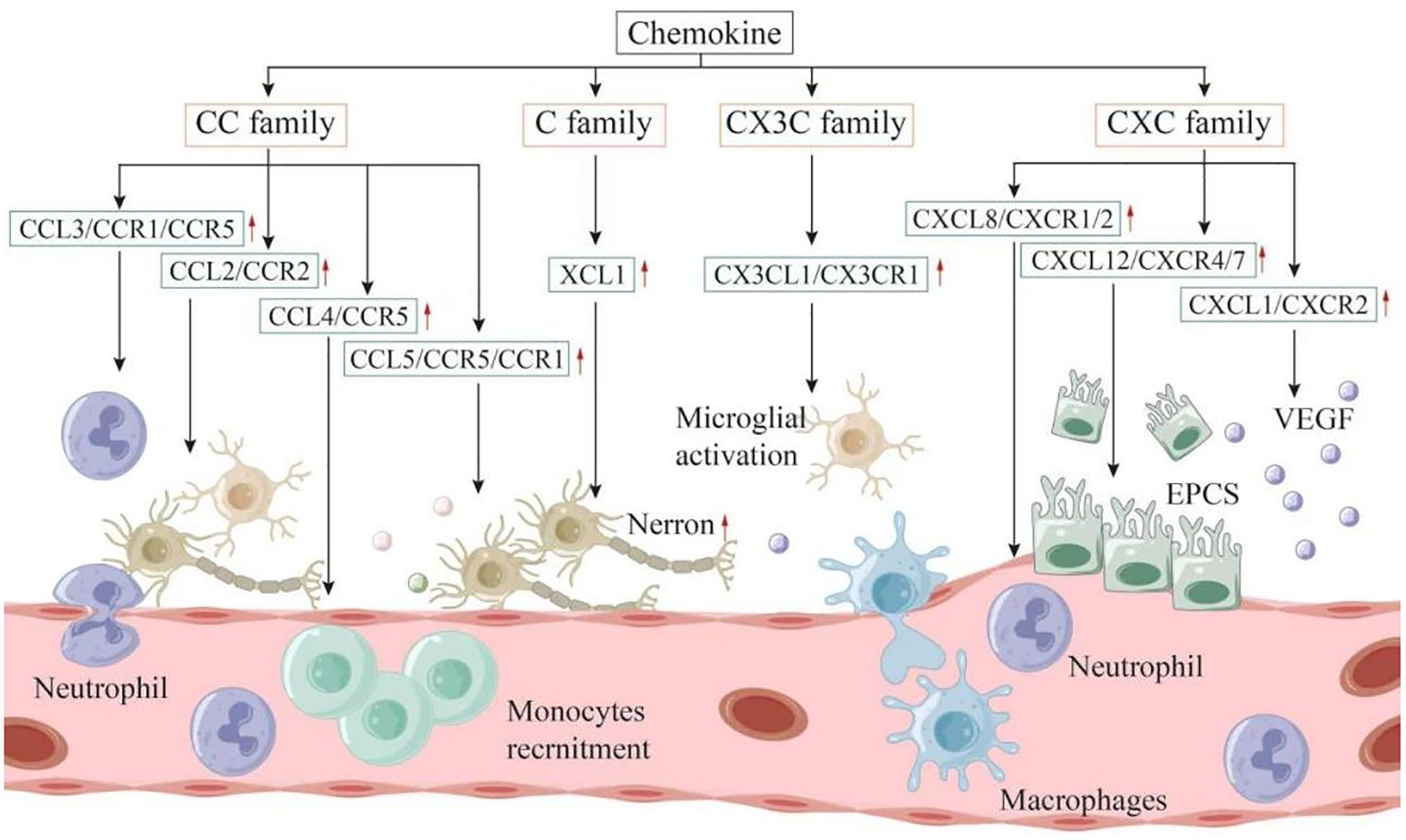
Figure 2. Chemokines can be categorized into four families: CXC, CC, CX3C, and C. These molecules play a dual role in both the onset and progression of IS. Within the inflammatory linked to IS, it is primarily the microglia located in the brain, along with infiltrating immune cells, that produce chemokines. These chemokines contribute to the further recruitment and activation of leukocytes, exacerbating the inflammatory response. On the other hand, following the acute phase, chemokines may also recruit neural progenitor cells, endothelial progenitor cells, and other types of cells that facilitate nerve repair and angiogenesis, ultimately enhancing the neurologic function of the patient.
3.2.1 CC family
CCL2/Monocyte chemotactic protein-1(MCP-1) is upregulated in ischemic penumbra after injury, promoting neuroinflammation by activating microglia and mediating BBB disruption via downregulating tight junction proteins (e.g., occludin, ZO-1). While CCL2/CCR2 antagonists reduce M1 polarization and neuronal damage, CCL2 may also facilitate oligodendrocyte generation and stem cell recruitment, suggesting dual roles in injury and repair (98–101). CCL3 (MIP-1α) drives neutrophil recruitment via CCR1/CCR5, correlating with TNF-α and insulin levels in human studies (102–107). CCL4 (MIP-β) binds CCR5 to recruit monocytes, with elevated serum levels predicting stroke risk (108, 109). CCL5 (RANTES) exhibits paradoxical effects: CCL5 deficiency reduces infarct volume and BBB permeability, yet CCR5 knockout mice show worse injury outcomes, implying protective roles via neuronal survival pathways (110–114). These findings highlight complex, context-dependent functions of CC chemokines in IS.
3.2.2 CXCL family
CXCL1, also known as growth - regulated oncogene (GRO) - α, acts as a chemotactic factor for neutrophils. An animal study investigated that mice with the CXCL1 - infused sponge exhibited a significant reduction in neuroinflammation and infarct size, lower mortality rates, and better neurological function following thromboembolic (TE) compared to those with a sponge infused with 0.9% NaCl (115). An in vitro study showed that microglia inhibited CXCL1 expression in astrocytes through IL-1RA secretion; In addition, animal studies suggest that anti-CXCL1 neutralizing antibody treatment or administration of recombinant IL-1RA protein can reduce the volume of CI and improve the motor coordination ability of mice after ischemia (116).Additionally, anti-atherosclerotic therapies, including candesartan and pioglitazone, were observed to decrease CXCL1 mRNA levels during CI (117). Moreover, activation of the CXCL1/CXCR2 signaling pathway in conjunction with thrombin and thrombin receptor agonist peptides has been shown to protect astrocytes against ceramide - induced apoptosis (118).
CXCL8 (IL-8) promotes neutrophil infiltration and neuroinflammation after CI, exacerbating ischemic injury via NF-κB activation. IL-8 knockout mice exhibit reduced inflammation through PI3K/Akt signaling. Clinically, serum IL-8 correlates with early disability in AIS, suggesting its potential as a severity marker (119–124).
CXCL12/CXCR4 signaling drives recruitment of neural/endothelial progenitors to ischemic sites, enhancing angiogenesis and neurogenesis. Drug-induced CXCL12 upregulation improves recovery in MCAO models by promoting stem cell migration and growth factor expression. CXCL12 also supports neural precursor survival via CXCR7, with plasma levels predicting future stroke risk (125–128).
Clinical studies have shown elevated serum CXCL16 levels are closely associated with atherosclerotic IS (129).Moreover, another study demonstrated that elevated serum CXCL16 levels in individuals with carotid artery stenosis after AIS were significantly associated with microembolic signals, suggesting that high CXCL16 concentrations could serve as a biomarker for predicting stroke occurrence and may be involved in plaque instability (130).
CXCL10 recruits T cells/NK cells and mediates 67% of IL-6 signaling effects on atherosclerotic stroke risk, linking genetic IL-6 downregulation to reduced CXCL10 and lower cardiovascular disease risk (131). CXCL2 upregulated in ET-1 stroke models at 24-48h post-ischemia. Microglial lncRNA U90926 knockdown reduces neutrophil infiltration via CXCL2 downregulation, highlighting its role in ischemic inflammation (132, 133).
In vitro studies showed that anti-CXCL5 antibody promoted the proliferation and decreased the permeability of oxygen-glucose deprivation (OGD) model cells, as indicated by the improvement of transepial electrical resistance (TEER), suggesting that silencing CXCL5 attenuates ischemia/hypoxia-induced damage to human brain microvascular endothelial cells (BMECs).Moreover, the p38 inhibitor SB203580 significantly reduced the activity of CXCL5 in the model cells, suggesting that CXCL5 may influence BMECs function via the p38 pathway (134).
In summary, the CXCL chemokine family, including several key molecules such as CXCL1, CXCL8, CXCL12, and CXCL16, plays a significant role in the onset and progression of CI and is strongly associated with atherosclerosis.
3.2.3 CX3C family
CX3CL1, also known as Fractalkine, is the sole member of the CX3C chemokine family and functions as a transmembrane chemokine. This protein is synthesized by neurons and is crucial for communication between neurons and glial cells. Notably, CX3CL1 is unique within its family in that it interacts exclusively with a single receptor, CX3CR1 (135). During IS, CX3CR1 influences several factors, including the volume of the infarct area, the integrity of the BBB, angiogenesis, and neural recovery. A study showed that CX3CR1 knockout (KO) mice exhibited a larger damage area compared to both wild - type (WT) and CCR2 KO mice 48 hours post - stroke. In the chronic phase, these same CX3CR1 KO mice displayed reduced brain injury/BBB degradation and enhanced angiogenesis, thereby facilitating recovery (136). The loss of CX3CR1 reduces the recruitment of monocytes, the proliferation of microglia, and the inflammatory capacity of these two cell types (137).
3.3 Other inflammatory mediators
3.3.1 hs - CRP
High-sensitivity C-reactive protein (hs-CRP) has been extensively studied in acute infection or acute phase of disease, but lacks specificity (138). Studies have shown that the level of hs-CRP is related to the volume of CI (139, 140) and disease outcome (141), and people with high levels of hs-CRP may be at risk of IS (142).
3.3.2 MMPs
MMPs constitute an important group of proteolytic enzymes capable of degrading all components of the extracellular matrix (143). MMPs can damage the BBB and promote nerve recovery in the acute phase and recovery phase of IS, respectively (144). In the acute stage of IS, MMPs impair the integrity of the BBB by degrading the basement membrane and compromising blood vessel stability. Palm et al. (145) found that there was a correlation between serum MMP-8 concentration and the severity and etiology of stroke in patients with AIS. Twenty - one days post - stroke, an increase in MMP - 2 expression was observed in astrocytes located at the periphery of the peri - infarction region, where the ends of these astrocytes were in contact with blood vessels, suggesting a potential role for MMP - 2 in the repair processes following inflammatory injury (146). MMP-9 expression peaks within 24 hours post-IS in rats, exacerbating BBB disruption and hemorrhagic transformation when combined with tPA (147, 148). Other MMPs (MMP-2, MMP-3, MMP-10, MMP-13) also increase post-stroke, with MMP-3 linked to improved neurological recovery. MMP inhibition reduces infarct size and edema, while MMP-9 knockout mice show smaller infarcts compared to wild-type controls. MMP-2 may facilitate neovascularization, as MMP-2 knockout does not affect infarct size. Serum MMP-9 levels correlate with initial stroke severity and clinical outcomes, suggesting its potential as a prognostic marker (149–155).
3.3.3 S - 100β
S-100β, a calcium-binding protein secreted by astrocytes, promotes neuroinflammation after brain injury by stimulating astrocyte/microglial release of inflammatory factors and nitric oxide. Elevated cerebrospinal fluid/serum S-100β correlates with AIS severity and neurological impairment. Animal studies demonstrate that reducing S-100β levels via pharmacological or genetic interventions decreases infarct volume and improves functional outcomes in MCAO models. These findings highlight S-100β as a potential therapeutic target and prognostic biomarker for IS (156–166).
3.3.4 NSE
Neuron - specific enolase (NSE) is a glycolytic enzyme present in cells. Specifically, it is a dimeric isoenzyme of enolase, predominantly found in neurons and neuroendocrine cells. Under normal conditions, NSE participates in glycolysis within the cytoplasm of nerve cells, contributing to the physiological function of brain tissue (167). However, elevated levels of NSE can cause the degradation and rupture of the basal membrane of the BBB. The results of a clinical study by Bharosay et al. (23) showed that serum NSE concentrations were significantly associated with the degree of disability and neurological decline in AIS patients. In addition, animal studies have found that serum NSE activity IS correlated with cerebral infarct volume in rats, and the treatment of IS by regulating NSE may be related to the regulation of the TLR4/MyD88/NF-κB signaling pathway (24). Collectively, these investigations suggest that targeting NSE expression could be a promising therapeutic approach for the management of IS.
3.3.5 Lipoprotein - associated phospholipase A2
Lipoprotein - associated phospholipase A2 (Lp - PLA2) is an important enzyme involved in the pro - inflammatory actions of oxidized low - density lipoproteins and is significantly associated with the progression of atherosclerosis and the occurrence of strokes (168). Research by Gorelick (169) indicated that individuals with elevated Lp - PLA2 levels had an incidence of first strokes and recurrences that was twice that of healthy controls.
4 Clinical applications
Inflammation is involved in all stages of IS. Accumulating evidence has demonstrated that inflammatory mediators play a crucial role in the diagnosis, prognosis, and disease management of IS. A deeper understanding of the relationship between inflammation and IS is not only beneficial for clinical application but also holds significant clinical implications.
4.1 Inflammatory markers and diagnosis
A research study analyzed five inflammatory markers within the first nine hours after stroke onset. These markers included MMP-9, protein S100β, von Willebrand factor, B - type neurotrophic growth factor, and MCP-1. When three or more of these markers exceeded their respective cutoff values, the result was classified as positive, yielding a sensitivity and specificity of 93% for the diagnosis of IS (158). In another study involving 889 acute stroke patients from eight original investigations, 312 patients developed post - stroke depression (PSD), while 577 did not. This research found that the serum concentrations of IL - 6 and TNF - α in the PSD group were significantly higher than those in the non - PSD group. This suggests that IL - 6 and TNF - α could potentially serve as biomarkers for PSD during the acute phase of stroke, providing a theoretical basis for the early prevention and treatment of PSD (170).
4.2 Inflammatory markers and disease assessment
A research investigation analyzed the variations in procalcitonin (PCT) and hs - CRP levels during the acute phase of IS, exploring the relationship between these biomarkers and long - term functional outcomes and mortality. The results showed that patients with poor functional prognoses and higher mortality at admission had significantly elevated levels of both PCT and hs - CRP. This indicates that PCT may function as an independent prognostic marker for one - year functional outcomes and mortality in patients with AIS (171).
Moreover, additional research focused on the serum levels of pro - inflammatory cytokines, such as IL - 6 and TNF - α, in IS patients, examining their potential as novel markers for predicting the functional outcomes of thrombolytic therapy. The findings revealed a correlation between the serum levels of IL - 6 and TNF - α and the degree of neurological deficits in subacute IS patients, suggesting that these biomarkers could serve as prognostic indicators for thrombolytic therapy in this context (172).
Another investigation included IS patients within 24 hours of onset and identified high - sensitivity hs-CRP, IL - 6, and TNF - α as inflammation markers at admission and 28 days after the event. This analysis concluded that elevated IL - 6 levels were associated with a poorer prognosis. Additionally, the results indicated that increased TNF - α levels in patients with lacunar infarction were related to the severity of neurological impairment at admission, suggesting that TNF - α might reflect underlying arteriolar damage (139). Youn et al. (140) investigated the relationship between hs - CRP levels and the quartile of infarction volume evaluated by diffusion - weighted imaging (DWI). They found a significant positive correlation between hs - CRP levels and DWI infarction volume. This research suggests that increased hs - CRP levels may indicate a larger CI volume and could serve as a serological marker for assessing the severity of AIS. Overall, these findings imply that elevated inflammatory markers in AIS patients may reflect the severity of the disease.
In another study, 500 AIS patients were divided into the early neurological deterioration(END) group (111 cases) and the non-END group (389 cases) according to the presence or absence of END within 7 days of onset, and serum Lp-PLA2 and MMP-9 levels and clinical baseline data were compared between the two groups. Logistic regression analysis was performed to determine the independent predictors of END, and the predictive effects of Lp-PLA2 and MMP-9 levels on END were assessed by subject work characteristics (ROC) curves. Multivariate logistic regression analysis showed that Lp-PLA2 and MMP-9 levels were independent influencing factors of END in AIS patients, and ROC curve analysis showed that the area under the curve of Lp-PLA2, MMP-9 and their combination to predict END were 0.730, 0.763 and 0.831, respectively. The inflammatory markers Lp-PLA2 and MMP-9 were both independent predictors of END in AIS patients, and the combined predictive value of the two was higher (P ≤ 0.05) (173).
4.3 Inflammatory markers and prediction of recurrence
Inflammatory biomarkers have demonstrated significant value in predicting the likelihood of vascular event recurrence after IS. A recent study focused on evaluating the prognostic significance of inflammatory molecular markers associated with the risk of vascular disease recurrence in IS patients not receiving anticoagulants. It was revealed that elevated levels of IL - 6, specifically those exceeding 5 pg/dl, and vascular cell adhesion molecule type 1 (VCAM - 1) exceeding 1350 ng/dl, were associated with 20 - fold and 3 - fold increases, respectively (174). Furthermore, individuals undergoing statin therapy showed a significant reduction in both vascular events and vascular - related mortality, which was associated with decreased levels of various inflammatory molecular markers, including IL - 6, MMP - 9, and cellular fibronectin (172). Additional research has shown that serum concentrations of hs - CRP are closely related to the severity of atherosclerosis and serve as a risk factor for IS recurrence at both 1 - year and 2 - year follow - ups (175).
In addition, a clinical study in China looked at the relationship between serum CXCL12 levels and stroke recurrence in AIS patients and found that elevated circulating CXCL12 levels at admission were strongly associated with IS recurrence (176). In the follow-up population, the median CXCL12 level was significantly higher in patients with relapse than in those without relapse [24.2 ng/mL (IQR 15.4-33.7) vs. 6.5 ng/mL (IQR 3.4-10.2); p < 0.05]. Z=8.258, p < 0.0001], and serum CXCL12 level ≥12.15 ng/mL was associated with an increased risk of stroke recurrence (OR 9.122, 95%CI 6.103-15.104).
A prospective study of 3401 AIS patients was conducted and serum Lp-PLA2 levels were measured. Among the 3278 patients who completed 1 year of follow-up, 188 patients had all-cause mortality, and Kaplan-Meier survival curve showed that the cumulative incidence of all-cause mortality increased in the quartile group of Lp-PLA2 content (log-rank p=0.018). These results indicate that serum Lp-PLA2 level is associated with prognosis within 1 year after AIS (177).
4.4 Inflammatory markers and other vascular diseases
Inflammation is a crucial mechanism in IS and also plays an essential role in atherosclerosis. Atherosclerosis is widely recognized as the primary cause of most cardiovascular diseases, such as coronary artery disease (CAD), heart failure, and stroke. The atherogenic process typically begins when cholesterol - rich low - density lipoprotein (LDL) is deposited in the endothelial lining of blood vessels, leading to endothelial activation. This activation promotes the recruitment of leukocyte adhesion molecules and chemokines, facilitating the recruitment of monocytes and T cells. Monocytes then differentiate into macrophages and upregulate the expression of pattern recognition receptors, including scavenger and toll - like receptors. The scavenger receptor is responsible for the internalization of lipoproteins, resulting in the formation of foam cells. Meanwhile, toll - like receptors transmit activation signals that trigger the release of cytokines, proteases, and vasoactive substances. In the lesions, T cells recognize local antigens, exacerbating inflammation and promoting plaque growth through the secretion of pro - inflammatory cytokines.
An enhanced inflammatory response can lead to localized proteolysis, plaque rupture, and thrombosis, ultimately resulting in ischemia and infarction (178). Research has shown that increased levels of circulating hs-CRP or IL - 6 are closely associated with the occurrence of future vascular events in both primary and secondary prevention, regardless of established risk factors (179). Moreover, additional studies have suggested that inflammatory markers are associated with the classification of IS. The median plasma levels of TNF - α, IL - 6, and IL - 1β in patients with the cardioembolic subtype of the Trial of Org 10172 in acute stroke Treatment (TOAST) were significantly higher than those in patients with lacunar IS. This indicates that inflammation may play a vital role in the development of ischemic death (180).
5 Therapeutic perspectives
Inflammatory markers play a crucial role in the treatment of IS and have been the focus of numerous studies. Existing research has indicated that the inhibition of cytokines, inflammatory cells, chemokines, and inflammasomes can exert beneficial effects on IS. Moreover, recent investigations have revealed that traditional Chinese medicine can be effective in treating IS by suppressing the inflammatory response. Therefore, understanding the role of inflammatory markers in the treatment of IS is of great clinical significance.
5.1 Inhibition of cytokines
Peripheral inflammation plays a significant role in neuroinflammation following a stroke and influences the prognosis of stroke patients. Therefore, inhibiting peripheral cytokines presents a promising therapeutic strategy. Currently, most research focuses on TNF-α inhibitors and IL-1 antagonists. The former includes drugs such as infliximab and etanercept, while the latter is represented by canakinumab. These medications are commonly used to treat inflammatory diseases, including rheumatoid arthritis and ankylosing spondylitis. Studies have demonstrated that the TNF-α inhibitor infliximab alleviates damage to the BBB following stroke in RA mice, reducing the expression of MMP-3 and MMP-9, and improving neurological function (181). Another study showed that intravenous injection of soluble TNF receptor I significantly mitigated ischemic cortical microvascular perfusion damage and cortical infarction in hypertensive rats post-stroke (182). This suggests that TNF-α inhibitors may represent a viable strategy for reducing the burden of stroke. Furthermore, a phase IIa double-blind, randomized, placebo-controlled trial involving 28 participants indicated that the initiation of sustained-release niacin tablets for 24 weeks, starting 3 to 7 days after a stroke, did not yield functional improvements (183). Additionally, administering 150 mg of canakinumab every three months for anti-inflammatory treatment targeting the innate immune pathway of IL-1β significantly reduced the recurrence rate of cardiovascular events (184). Moreover, the CANTOS trial, which also focused on cardiovascular issues, provided insights regarding its anti-inflammatory mechanism that could inform stroke prevention and treatment (185). In conclusion, while suppressing peripheral cytokine levels is crucial, current evidence supporting the use of cytokine inhibitors for treating IS remains insufficient. Further multicenter, large-scale clinical trials are necessary, and a careful evaluation of the associated risks and benefits is warranted.
5.2 Modulating the brain-gut axis
Numerous studies in recent years have demonstrated that cardiovascular diseases and strokes, which are associated with atherosclerosis, correlate with alterations in the intestinal microbiota and an increase in intestinal bacteria (186). Recent investigations have highlighted the pivotal role of the gut-brain axis in neuroinflammation following a stroke, suggesting that this could lead to novel therapeutic strategies for stroke prevention and treatment. Research indicates that supplementation with Lactobacillus, particularly Lactobacillus rhamnosus GMNL-89 and Lactobacillus acidophilus GMNL-133, can reduce CI volume in the MCAO mouse model and enhance neurological function, thereby exhibiting neuroprotective effects (187). Furthermore, the protective effects of Lactobacillus on IS may be linked to the inhibition of TLR-4/NF-κB signaling, the mitigation of inflammatory responses, and the suppression of neuronal apoptosis (188). Additionally, fecal microbiota transplantation (FMT) has been shown to significantly alter the composition of intestinal microbiota in IS, reduce pathogenic bacteria, increase beneficial bacteria, markedly diminish neurological impairment, eliminate brain edema, and decrease infarct size (189). Moreover, by modulating intestinal microbiota and sphingolipid metabolism, FMT can alleviate multi-organ damage caused by stroke-induced pneumonia (190). Recent studies have also indicated that vagus nerve stimulation (VNS) can rectify gastrointestinal microbiota imbalance and diminish intestinal and neural inflammation by inhibiting mast cell degranulation and reducing trypsin secretion. This regulation not only enhances the integrity of the intestinal and BBB and improves gastrointestinal function post-stroke but also mitigates systemic inflammatory responses (191). Future research should concentrate on elucidating the precise mechanisms underlying gut-brain communication after stroke and translating these insights into safe and effective therapies targeting peripheral drivers of central inflammation. Furthermore, clinical investigations have shown that probiotic supplementation may exert anti-inflammatory, antioxidant, metabolic, and gut microbiota regulatory effects in the context of cardiac remodeling (192). This suggests that the anti-inflammatory mechanisms of probiotics may represent a potential strategy for the prevention and treatment of strokes. Ultimately, the proactive prevention and treatment of infections following a stroke remains a crucial clinical strategy for alleviating adverse peripheral inflammation.
5.3 Regulation of glial cell polarization
Following the occurrence of IS, both microglia and astrocytes become highly activated, releasing various inflammatory factors that exacerbate brain injury. Thus, inhibiting the over - activation of microglia and astrocytes may mitigate this damage and could be a key approach in the treatment of IS.
Studies have shown that subcutaneous injection of cottonseed oil can suppress the TLR4/NF - κB signaling pathway, reduce the activation levels of neurotoxic type A1 astrocytes, and weaken the interactions between microglia and astrocytes (193). Additionally, stephanine, an alkaloid, has been found to improve the outcomes in MCAO rats by inhibiting the TLR4/NF - κB signaling pathway. This, in turn, alleviates neuronal damage and reduces microglial over - activation (194). Moreover, aleglitazar, a dual agonist targeting peroxisome proliferator - activated receptors (PPARα and PPARγ), has been demonstrated to inhibit the migration of microglia and the secretion of cytokines and adhesion molecules in MCAO rats (195).
Recently, a lipid nanoparticle approach targeting M2 microglia (termed MLNP) has been developed. This approach selectively delivers mRNA encoding phenotype - switching IL - 10 to the ischemic brain, creating a beneficial feedback loop that promotes microglial polarization toward a protective M2 phenotype (17). It also enhances the homing of MLNP loaded with mIL - 10 (mIL - 10@MLNPs) to the ischemic region. In a transient MCAO mouse model of IS, intravenous injection of mIL - 10@MLNPs induced the production of IL - 10 and enhanced the M2 polarization of microglia, thereby improving the disruption of the BBB after IS.
The activation of glial cells significantly contributes to the exacerbation of the inflammatory response. By inhibiting the over - activation of these cells and regulating glial polarization, brain damage can be mitigated, and neurons can be protected. Therefore, targeting glial cells offers a novel strategy for treating IS, and how to promote the conversion of glial cells to a favorable phenotype is worth exploring in depth next. Moreover, the related pathways and targets require further investigation.
5.4 Inhibition of chemokines
Chemokines play a vital role in the pathogenesis of IS, and their inhibition is beneficial for controlling the inflammatory response in IS. In a specific study, the CCR5 antagonist maraviroc (MVC, APEXBIO, UK - 427857) was administered to mice undergoing MCAO. In vivo experiments showed that the mice received intraperitoneal (i.p.) doses of MVC at 20 mg/kg for three consecutive days after MCAO. The administration of MVC exerted a neuroprotective effect, as evidenced by the reduction in neurological deficits and infarct volume after MCAO. Moreover, treatment with MVC significantly decreased apoptosis and inflammation levels after cerebral ischemia/reperfusion (CI/R) injury (196).
In another study, using SH - SY5Y cells and a rat MCAO model, the function and mechanism of microRNA (miR) - 532 - 5p in CI/R injury were explored. The results indicated that intraventricular injection of a miR - 532 - 5p agonist led to a reduction in CI size, lower cerebral water content, and reduced neuronal apoptosis. It also inhibited the CXCL1/CXCR2/NF - κB signaling pathway in rats with the MCAO model. These findings suggest that the overexpression of miR - 532 - 5p significantly alleviates CI/R injury both in vitro and in vivo by targeting CXCL1 (197). In summary, targeting chemokines may provide a new therapeutic strategy for IS, which merits further research.
5.5 Inhibition of inflammasome
During AIS, the activation of the NLRP3 inflammasome drives neuroinflammation in neurons. Research has demonstrated that early inhibition of NLRP3 can protect against ischemia/reperfusion (I/R) injury by reducing inflammation and maintaining the integrity of the BBB (198).
Robinin (5,7 - dihydroxy - 4’-methoxyflavone) is considered a promising neuroprotective compound. A study aimed to establish a cerebral ischemia - reperfusion injury model in C57BL/6 mice using a modified thread - plug technique to explore the impact of robinin on the NLRP3 inflammasome after cerebral ischemia - reperfusion injury. In this investigation, one hour after the occlusion of the middle cerebral artery, either 25 mg/kg of robinin (the robinin group) or the same volume of normal saline (0.1 mL/10 g, the MCAO group) was administered via intraperitoneal injection for reperfusion. The infarction volume and neurological function scores were assessed using 2,3,5 - triphenyltetrazolium chloride staining and the Zea - Longa scoring system. The results showed that both the neurological function scores and CI volumes were significantly lower in the robinin group compared to the MCAO group. Western blot analysis revealed that the expression levels of toll - like receptor 4, NF - κB, NLRP3, procaspase - 1, caspase - 1, pro - IL - 1β, and IL - 1β were significantly decreased in the robinin group compared with the MCAO group. These results imply that robinin may protect against cerebral ischemia - reperfusion injury, potentially by modulating the NLRP3 signaling pathway (199).
Furthermore, other research has suggested that NLRP3 inflammasomes play a critical role in mediating inflammation and pyroptosis in cerebral endothelial cells, ultimately leading to the impairment of the BBB during cerebral ischemia (CI). Studies have shown that inhibiting NLRP3 inflammasomes can reduce hypoxia - induced death of endothelial cells in vitro and help maintain the integrity of the BBB in rat models after a stroke (200).
5.6 TCM treatment of ischemic stroke
Recent research has demonstrated the effectiveness of traditional Chinese medicine in treating IS. In animal models, it can reduce both infarct volume and nerve function deficits by inhibiting the inflammatory response, and multiple paths involved. Studies have shown that ginsenoside Rg1, curcumin and notoginsenoside R1 (NG-R1) can inhibit the release of pro-inflammatory cytokines through TLR4/MyD88/NF-κb, TLR4/p38/MAPK and other pathways, reduce CI volume and improve neurological function in MCAO/R models (201–204). Quercetin (Que), baicalein and Psoralatin (PAP-1) inhibit the M1 polarization of microglia and promote the activation of M2 microglia by affecting TLR4/NF-κB, PI3K/Akt/mTOR pathway or inhibiting potassium channel Kv1.3. Finally, it shows neuroprotective properties in hypoxic-ischemic brain damage (205–207).In addition, the therapeutic effects of panax notoginseng saponins in IS have also been demonstrated in clinical trials. The meta-analysis included 46 randomized controlled trials involving 7957 AIS patients. The results showed that panax notoginseng saponins (Xuesaitong, XST) could improve long-term functional outcome by reducing the modified Rankin scale (mRS), improving activities of daily living and neurological deficits. In addition, there were no significant differences between the XST and control groups in the rates of death from any cause or adverse events (208).
6 Summary and outlook
Stroke presents a substantial challenge to global health, imposing a significant burden on both families and society. The inflammatory response is a crucial factor in the occurrence and development of IS. The core of acute-phase anti-inflammatory treatment lies in targeting the key pathways of neuroinflammation: by inhibiting the NLRP3 inflammasome, the maturation and release of IL-1β and IL-18 can be blocked, thereby alleviating early neuroinflammatory damage; at the same time, by antagonizing the CCL2/CCR2 or CXCL1 signaling pathways, the infiltration of neutrophils and monocytes into the brain parenchyma can be effectively inhibited, and the expansion of the inflammatory cascades can be delayed. After entering the repair phase, the reprogramming of the immune microenvironment becomes the focus: anti-inflammatory factors such as IL-4 and IL-10 can induce microglia to polarize to the M2 phenotype, promoting angiogenesis and synaptic remodeling; in addition, the activation of the CXCL12/CXCR4 axis helps recruit neural precursor cells to migrate to the ischemic area, enhancing neurogenesis and tissue repair. The entire strategy presents a logical progression from “inhibiting damage” to “promoting regeneration”, reflecting the dynamics and target specificity of neuro-immune regulation in different stages of stroke. Therefore, the development of novel anti-inflammatory or pro-reparative drugs tailored to the specific temporal phases of IS is crucial.
Furthermore, the inflammatory response within the CNS is influenced to some extent by peripheral inflammatory responses. This underscores the necessity of managing peripheral inflammation as a crucial component of IS treatment. Therapeutic strategies should extend beyond the CNS to mitigate the harmful cycle between peripheral inflammation—particularly driven by SIRS and gut dysbiosis/leakage—and neuroinflammation. Accelerating the transition from a pro-inflammatory to an anti-inflammatory phenotype, both centrally and peripherally, is of immense clinical significance. Additionally, interventions that target the gut-brain axis, such as microbiota modulation and barrier fortification, represent promising avenues for enhancing stroke outcomes. Future research must prioritize elucidating the intricate interactions within this extended neuro-immune network to identify novel, effective, and phase-specific therapeutic targets.
Research on biomarkers in IS continues to face limitations and challenges. (1)Technical Bottlenecks: Numerous potential biomarkers, such as transcriptional RNA-derived fragments (tRFs), have emerged as promising candidates for differentiating stroke subtypes. However, technical and computational challenges may compromise the reliability of tRF expression data obtained from RNA sequencing (209). Therefore, technological innovation and methodological optimization are crucial. Additionally, strict standardization and normalization of detection methods for biomarkers—including sample collection, processing, detection procedures, and data analysis—are necessary to enhance comparability and reproducibility across different studies. (2)Dynamic Changes and Time Window Challenges: The expression levels of biomarkers may fluctuate dynamically following a stroke, and their diagnostic and prognostic values may vary depending on the timing of the study (210). Utilizing appropriate biomarkers and selecting optimal blood sampling time points for diagnosing the early stages of IS are critical for facilitating more effective treatment. The development of precise and high-performance detection systems or kits will create new opportunities for rapid and accurate point-of-care testing. (3)Translational Application and Cost-Effectiveness Considerations: Many newly identified biomarkers, such as various mRNAs or autoantibodies, demonstrate favorable AUC values (e.g., the four-gene diagnostic model with AUC > 0.9) (211). However, their high costs hinder widespread adoption. 4. Heterogeneity and Individual Differences: Patients with CI exhibit significant heterogeneity in terms of etiology, lesion location, and clinical manifestations, complicating the universal applicability of a single biomarker. Addressing this issue requires close integration with clinical practice, necessitating strengthened longitudinal studies and clinical translation. Conducting large-scale, multicenter, prospective clinical cohort studies with long-term dynamic sample collection and follow-up is essential to elucidate the changes in biomarkers throughout the disease course and their association with prognosis. Furthermore, clinical research designs should be more standardized, clearly defining inclusion criteria and outcome assessment methods, while also conducting stratified studies based on disease heterogeneity to provide precise prevention and treatment evidence.
Numerous existing studies have focused on the relationship between stroke and inflammation. Future research should emphasize the following key areas: (1)The integration of inflammatory characteristics with multimodal neuroimaging. This involves combining inflammatory markers found in serum and cerebrospinal fluid with multimodal imaging techniques to analyze changes in inflammation, brain structure, and function from the acute phase to the recovery phase. Maier B.highlights the use of PET-MRI technology to identify specific inflammatory targets and visualize the spatiotemporal evolution of neural inflammation (212). Candelario-Jalil E asserts that the combination of inflammation and neuroimaging is crucial for predicting brain injury progression, assessing the extent of BBB disruption, and evaluating the potential for neural repair, thereby providing a reliable foundation for personalized anti-inflammatory treatments (213). (2)Multi-omics-driven analysis of inflammatory mechanisms. Given that stroke is a complex, multifactorial disease, regulating a single molecular factor is unlikely to sufficiently mitigate or reverse stroke pathology. In this context, integrated omics is anticipated to facilitate the identification of biologically interrelated processes in stroke. Montaner J provides an example of using multi-omics to explore therapeutic drug targets (214). Additionally, combining proteomics and metabolomics can help identify protein interaction networks and metabolic alterations in key inflammatory pathways. Montaner J. notes that transforming these findings into clinically applicable diagnostic tools or effective treatment methods necessitates a lengthy process of validation, optimization, and clinical trials. (3)AI-enabled classification of inflammation and prognosis prediction. This approach integrates clinical data (e.g., NIHSS score), inflammatory markers (e.g., CXCL12, MMP-9), and imaging features (e.g., infarction volume, white matter integrity) using deep learning techniques, such as the Transformer model, to predict the risk of stroke recurrence and functional outcomes, as proposed by Shlobin.NA (215). Furthermore, developing AI-driven treatment response models could optimize the timing and dosage of anti-inflammatory drugs based on dynamic changes in inflammatory marker levels, thereby avoiding the detrimental effects of indiscriminate anti-inflammatory therapy on recovery.
Author contributions
LiX: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. YH: Conceptualization, Supervision, Writing – review & editing. LW: Visualization, Writing – original draft. SZ: Investigation, Writing – review & editing. CQ: Visualization, Writing – original draft. XL: Conceptualization, Supervision, Writing – review & editing. LeX: Resources, Investigation, Writing – review & editing. DW: Funding acquisition, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This review was funded by the following projects: Research Project of Changsha Municipal Health Commission (KJ-B2023078;KJ-B2023078);National Natural Science Foundation of China - Youth Fund Project (82505562); Hunan Provincial Natural Science Foundation - Youth Fund(2025JJ60801).
Acknowledgments
This is a short text to acknowledge the contributions of specific colleagues, institutions, or agencies that aided the efforts of the authors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Iadecola C, Buckwalter MS, and Anrather J. Immune responses to stroke: mechanisms, modulation, and therapeutic potential. J Clin Invest. (2020) 130:2777–88. doi: 10.1172/JCI135530
2. Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics-2020 update: A report from the american heart association. Circulation. (2020) 141:e139–596. doi: 10.1161/CIR.0000000000000757
3. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Association of hypertension with stroke recurrence depends on ischemic stroke subtype. Stroke. (2013) 44:1232–7. doi: 10.1161/STROKEAHA.111.000302
4. Wang Y, Xu J, Zhao X, Wang D, Wang C, Liu L, et al. Ischemic stroke. Am J Med. (2021) 134:1457–64. doi: 10.1016/j.amjmed.2021.07.027
5. Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2013) 44:2064–89. doi: 10.1161/STR.0b013e318296aeca
6. DeLong JH, Ohashi SN, O’Connor KC, and Sansing LH. Inflammatory responses after ischemic stroke. Semin Immunopathol. (2022) 44:625–48. doi: 10.1007/s00281-022-00943-7
7. Simats A and Liesz A. Systemic inflammation after stroke: implications for post-stroke comorbidities. EMBO Mol Med. (2022) 14:e16269. doi: 10.15252/emmm.202216269
8. Petrovic-Djergovic D, Goonewardena SN, and Pinsky DJ. Inflammatory disequilibrium in stroke. Circ Res. (2016) 119:142–58. doi: 10.1161/CIRCRESAHA.116.308022
9. Yang X, Xu S, Qian Y, and Xiao Q. Resveratrol regulates microglia M1/M2 polarization via PGC-1α in conditions of neuroinflammatory injury. Brain Behav Immun. (2017) 64:162–72. doi: 10.1016/j.bbi.2017.03.003
10. Moehle MS and West AB. M1 and M2 immune activation in Parkinson’s Disease: Foe and ally? Neuroscience. (2015) 302:59–73. doi: 10.1016/j.neuroscience.2014.11.018
11. Cherry JD, Olschowka JA, and O’Banion MK. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation. (2014) 11:98. doi: 10.1186/1742-2094-11-98
12. Wolf SA, Boddeke HW, and Kettenmann H. Microglia in physiology and disease. Annu Rev Physiol. (2017) 79:619–43. doi: 10.1146/annurev-physiol-022516-034406
13. Voet S, Mc Guire C, Hagemeyer N, Martens A, Schroeder A, Wieghofer P, et al. A20 critically controls microglia activation and inhibits inflammasome-dependent neuroinflammation. Nat Commun. (2018) 9:2036. doi: 10.1038/s41467-018-04376-5
14. Lu E, Wang Q, Li S, Chen C, Wu W, Xu YXZ, et al. Profilin 1 knockdown prevents ischemic brain damage by promoting M2 microglial polarization associated with the RhoA/ROCK pathway. J Neurosci Res. (2020) 98:1198–212. doi: 10.1002/jnr.24607
15. Kang R, Gamdzyk M, Luo Y, Tang H, Huang L, Lenahan C, et al. Three days delayed recanalization improved neurological function in pMCAO rats by increasing M2 microglia-possible involvement of the IL-4R/STAT6/PPARγ Pathway. Transl Stroke Res. (2023) 14:250–62. doi: 10.1007/s12975-022-01032-5
16. Luo L, Liu M, Fan Y, Zhang J, Liu L, Li Y, et al. Intermittent theta-burst stimulation improves motor function by inhibiting neuronal pyroptosis and regulating microglial polarization via TLR4/NFκB/NLRP3 signaling pathway in cerebral ischemic mice. J Neuroinflammation. (2022) 19:141. doi: 10.1186/s12974-022-02501-2
17. Gao M, Li Y, Ho W, Chen C, Chen Q, Li F, et al. Targeted mRNA nanoparticles ameliorate blood-brain barrier disruption postischemic stroke by modulating microglia polarization. ACS Nano. (2024) 18:3260–75. doi: 10.1021/acsnano.3c09817
18. Xia X, Chen W, Zhou T, Zhou F, Lu C, Yan Z, et al. TEPP-46 inhibits glycolysis to promote M2 polarization of microglia after ischemic stroke. Int Immunopharmacol. (2025) 149:114148. doi: 10.1016/j.intimp.2025.114148
19. Zheng Y, Ren Z, Liu Y, Yan J, Chen C, He Y, et al. T cell interactions with microglia in immune-inflammatory processes of ischemic stroke. Neural Regener Res. (2025) 20:1277–92. doi: 10.4103/NRR.NRR-D-23-01385
20. Cheng J, Zheng Y, Cheng F, Wang C, Han J, Zhang H, et al. Different roles of astrocytes in the blood-brain barrier during the acute and recovery phases of stroke. Neural Regener Res. (2026) 21:1359–72. doi: 10.4103/NRR.NRR-D-24-01417
21. Chen JM, Shi G, Yu LL, Shan W, Sun JY, Guo AC, et al. 3-HKA promotes vascular remodeling after stroke by modulating the activation of A1/A2 reactive astrocytes. Adv Sci (Weinh). (2025) 12:e2412667. doi: 10.1002/advs.202412667
22. Xiao R, Pan J, Yang M, Liu H, Zhang A, Guo X, et al. Regulating astrocyte phenotype by Lcn2 inhibition toward ischemic stroke therapy. Biomaterials. (2025) 317:123102. doi: 10.1016/j.biomaterials.2025.123102
23. Bharosay A, Bharosay VV, Varma M, Saxena K, Sodani A, Saxena R, et al. Correlation of brain biomarker neuron specific enolase (NSE) with degree of disability and neurological worsening in cerebrovascular stroke. Indian J Clin Biochem. (2012) 27:186–90. doi: 10.1007/s12291-011-0172-9
24. Chen HD, Jiang MZ, Zhao YY, Li X, Lan H, Yang WQ, et al. Effects of breviscapine on cerebral ischemia-reperfusion injury and intestinal flora imbalance by regulating the TLR4/MyD88/NF-κB signaling pathway in rats. J Ethnopharmacol. (2023) 300:115691. doi: 10.1016/j.jep.2022.115691
25. Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. (2009) 40:1849–57. doi: 10.1161/STROKEAHA.108.534503
26. Kim SW, Lee H, Lee HK, Kim ID, and Lee JK. Neutrophil extracellular trap induced by HMGB1 exacerbates damages in the ischemic brain. Acta Neuropathol Commun. (2019) 7:94. doi: 10.1186/s40478-019-0747-x
27. Perez-de-Puig I, Miró-Mur F, Ferrer-Ferrer M, Gelpi E, Pedragosa J, Justicia C, et al. Neutrophil recruitment to the brain in mouse and human ischemic stroke. Acta Neuropathol. (2015) 129:239–57. doi: 10.1007/s00401-014-1381-0
28. Kang L, Yu H, Yang X, Zhu Y, Bai X, Wang R, et al. Neutrophil extracellular traps released by neutrophils impair revascularization and vascular remodeling after stroke. Nat Commun. (2020) 11:2488. doi: 10.1038/s41467-020-16191-y
29. Jickling GC, Liu D, Ander BP, Stamova B, Zhan X, and Sharp FR. Targeting neutrophils in ischemic stroke: translational insights from experimental studies. J Cereb Blood Flow Metab. (2015) 35:888–901. doi: 10.1038/jcbfm.2015.45
30. Cuartero MI, Ballesteros I, Moraga A, Nombela F, Vivancos J, Hamilton JA, et al. N2 neutrophils, novel players in brain inflammation after stroke: modulation by the PPARγ agonist rosiglitazone. Stroke. (2013) 44:3498–508. doi: 10.1161/STROKEAHA.113.002470
31. Cai W, Liu S, Hu M, Huang F, Zhu Q, Qiu W, et al. Functional dynamics of neutrophils after ischemic stroke. Transl Stroke Res. (2020) 11:108–21. doi: 10.1007/s12975-019-00694-y
32. García-Culebras A, Durán-Laforet V, Peña-Martínez C, Moraga A, Ballesteros I, Cuartero MI, et al. Role of TLR4 (Toll-like receptor 4) in N1/N2 neutrophil programming after stroke. Stroke. (2019) 50:2922–32. doi: 10.1161/STROKEAHA.119.025085
33. Cai W, Shi L, Zhao J, Xu F, Dufort C, Ye Q, et al. Neuroprotection against ischemic stroke requires a specific class of early responder T cells in mice. J Clin Invest. (2022) 132:e157678. doi: 10.1172/JCI157678
34. Fan L, Zhang CJ, Zhu L, Chen J, Zhang Z, Liu P, et al. FasL-PDPK1 pathway promotes the cytotoxicity of CD8+ T cells during ischemic stroke. Transl Stroke Res. (2020) 11:747–61. doi: 10.1007/s12975-019-00749-0
35. Chu HX, Kim HA, Lee S, Moore JP, Chan CT, Vinh A, et al. Immune cell infiltration in Malignant middle cerebral artery infarction: comparison with transient cerebral ischemia. J Cereb Blood Flow Metab. (2014) 34:450–9. doi: 10.1038/jcbfm.2013.217
36. Santamaría-Cadavid M, Rodríguez-Castro E, Rodríguez-Yáñez M, Arias-Rivas S, López-Dequidt I, Pérez-Mato M, et al. Regulatory T cells participate in the recovery of ischemic stroke patients. BMC Neurol. (2020) 20:68. doi: 10.1186/s12883-020-01648-w
37. Guo S and Luo Y. Brain Foxp3+ regulatory T cells can be expanded by Interleukin-33 in mouse ischemic stroke. Int Immunopharmacol. (2020) 81:106027. doi: 10.1016/j.intimp.2019.106027
38. Ito M, Komai K, Mise-Omata S, Iizuka-Koga M, Noguchi Y, Kondo T, et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature. (2019) 565:246–50. doi: 10.1038/s41586-018-0824-5
39. Zhang H, Yang K, Chen F, Liu Q, Ni J, Cao W, et al. Role of the CCL2-CCR2 axis in cardiovascular disease: Pathogenesis and clinical implications. Front Immunol. (2022) 13:975367. doi: 10.3389/fimmu.2022.975367
40. Geng H, Chen L, Tang J, Chen Y, and Wang L. The role of CCL2/CCR2 axis in cerebral ischemia-reperfusion injury and treatment: from animal experiments to clinical trials. Int J Mol Sci. (2022) 23:3485. doi: 10.3390/ijms23073485
41. Verma R, Cronin CG, Hudobenko J, Venna VR, McCullough LD, and Liang BT. Deletion of the P2X4 receptor is neuroprotective acutely, but induces a depressive phenotype during recovery from ischemic stroke. Brain Behav Immun. (2017) 66:302–12. doi: 10.1016/j.bbi.2017.07.155
42. Piepke M, Clausen BH, Ludewig P, Vienhues JH, Bedke T, Javidi E, et al. Interleukin-10 improves stroke outcome by controlling the detrimental interleukin-17Aresponse. J Neuroinflammation. (2021) 18:265. doi: 10.1186/s12974-021-02316-7
43. Wicks EE, Ran KR, Kim JE, Xu R, Lee RP, and Jackson CM. The translational potential of microglia and monocyte-derived macrophages in ischemic stroke. Front Immunol. (2022) 13:897022. doi: 10.3389/fimmu.2022.897022
44. Zhao M, Tuo H, Wang S, and Zhao L. The roles of monocyte and monocyte-derived macrophages in common brain disorders. BioMed Res Int. (2020) 2020:9396021. doi: 10.1155/2020/9396021
45. Clausen BH, Wirenfeldt M, Høgedal SS, Frich LH, Nielsen HH, Schrøder HD, et al. Characterization of the TNF and IL-1 systems in human brain and blood after ischemic stroke. Acta Neuropathol Commun. (2020) 8:81. doi: 10.1186/s40478-020-00957-y
46. Zarruk JG, Greenhalgh AD, and David S. Microglia and macrophages differ in their inflammatory profile after permanent brain ischemia. Exp Neurol. (2018) 301:120–32. doi: 10.1016/j.expneurol.2017.08.011
47. Yang J, Zhang L, Yu C, Yang XF, and Wang H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. biomark Res. (2014) 2:1. doi: 10.1186/2050-7771-2-1
48. Qiu YM, Zhang CL, Chen AQ, Wang HL, Zhou YF, Li YN, et al. Immune cells in the BBB disruption after acute ischemic stroke: targets for immune therapy? Front Immunol. (2021) 12:678744. doi: 10.3389/fimmu.2021.678744
49. Wang J, Zhao Y, Lv C, and Li F. The prognosis of neutrophil-to-lymphocyte ratio and lymphocyte-to-monocyte ratio in elderly with acute ischemic stroke. Clin Interv Aging. (2024) 19:1715–20. doi: 10.2147/CIA.S491753
50. Tuz AA, Ghosh S, Karsch L, Antler M, Lakovic V, Lohmann S, et al. Gut microbiota deficiency reduces neutrophil activation and is protective after ischemic stroke. J Neuroinflamm. (2025) 22:137. doi: 10.1186/s12974-025-03448-w
51. Yang Z, Shi X, Gao Z, and Chu L. miR-155-5p in extracellular vesicles derived from choroid plexus epithelial cells promotes autophagy and inflammation to aggravate ischemic brain injury in mice. Oxid Med Cell Longev. (2022) 2022:8603427. doi: 10.1155/2022/8603427
52. Venkat P, Cui C, Chopp M, Zacharek A, Wang F, Landschoot-Ward J, et al. MiR-126 mediates brain endothelial cell exosome treatment-induced neurorestorative effects after stroke in type 2 diabetes mellitus mice. Stroke. (2019) 50:2865–74. doi: 10.1161/STROKEAHA.119.025371
53. Zheng Y, He R, Wang P, Shi Y, Zhao L, and Liang J. Exosomes from LPS-stimulated macrophages induce neuroprotection and functional improvement after ischemic stroke by modulating microglial polarization. Biomater Sci. (2019) 7:2037–49. doi: 10.1039/c8bm01449c.Q3.5.7
54. Song Y, Shi R, Liu Y, Cui F, Han L, Wang C, et al. M2 Microglia Extracellular Vesicle miR-124 Regulates Neural Stem Cell Differentiation in Ischemic Stroke via AAK1/NOTCH. Stroke. (2023) 54:2629–39. doi: 10.1161/STROKEAHA.122.041611
55. Zhang L, Wei W, Ai X, Kilic E, Hermann DM, Venkataramani V, et al. Extracellular vesicles from hypoxia-preconditioned microglia promote angiogenesis and repress apoptosis in stroke mice via the TGF-β/Smad2/3 pathway. Cell Death Dis. (2021) 12:1068. doi: 10.1038/s41419-021-04363-7
56. Wang J, Tang H, Tian J, Xie Y, and Wu Y. Extracellular vesicles of ADSCs inhibit ischemic stroke-induced pyroptosis through Gbp3 regulation: A role for the NLRP3/GSDMD signaling pathway. Int Immunopharmacol. (2025) 146:113881. doi: 10.1016/j.intimp.2024.113881
57. Qiu L, Cai Y, Geng Y, Yao X, Wang L, Cao H, et al. Mesenchymal stem cell-derived extracellular vesicles attenuate tPA-induced blood-brain barrier disruption in murine ischemic stroke models. Acta Biomater. (2022) 154:424–42. doi: 10.1016/j.actbio.2022.10.022
58. Doeppner TR, Herz J, Görgens A, Schlechter J, Ludwig AK, Radtke S, et al. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl Med. (2015) 4:1131–43. doi: 10.5966/sctm.2015-0078
59. Hu B, Chen S, Zou M, He Z, Shao S, and Liu B. Effect of extracellular vesicles on neural functional recovery and immunologic suppression after rat cerebral apoplexy. Cell Physiol Biochem. (2016) 40:155–62. doi: 10.1159/000452533
60. Martinon F, Burns K, and Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. (2002) 10:417–26. doi: 10.1016/s1097-2765(02)00599-3
61. Schroder K and Tschopp J. The inflammasomes. Cell. (2010) 140:821–32. doi: 10.1016/j.cell.2010.01.040
62. Fu C, Zhang X, Lu Y, Wang F, Xu Z, Liu S, et al. Geniposide inhibits NLRP3 inflammasome activation via autophagy in BV-2 microglial cells exposed to oxygen-glucose deprivation/reoxygenation. Int Immunopharmacol. (2020) 84:106547. doi: 10.1016/j.intimp.2020.106547
63. Ye Y, Jin T, Zhang X, Zeng Z, Ye B, Wang J, et al. Meisoindigo protects against focal cerebral ischemia-reperfusion injury by inhibiting NLRP3 inflammasome activation and regulating microglia/macrophage polarization via TLR4/NF-κB signaling pathway. Front Cell Neurosci. (2019) 13:553. doi: 10.3389/fncel.2019.00553
64. Liu J, Ma W, Zang CH, Wang GD, Zhang SJ, Wu HJ, et al. Salidroside inhibits NLRP3 inflammasome activation and apoptosis in microglia induced by cerebral ischemia/reperfusion injury by inhibiting the TLR4/NF-κB signaling pathway. Ann Transl Med. (2021) 9:1694. doi: 10.21037/atm-21-5752
65. Prakash R, Waseem A, Siddiqui AJ, Naime M, Khan MA, Robertson AA, et al. MCC950 mitigates SIRT3-NLRP3-driven inflammation and rescues post-stroke neurogenesis. BioMed Pharmacother. (2025) 183:117861. doi: 10.1016/j.biopha.2025.117861
66. Gao J, Su G, Liu J, Song J, Chen W, Chai M, et al. A novel compound ligusticum cycloprolactam alleviates neuroinflammation after ischemic stroke via the FPR1/NLRP3 signaling axis. CNS Neurosci Ther. (2024) 30:e70158. doi: 10.1111/cns.70158
67. Ma CS, Ma YP, Han B, Duan WL, Meng SC, Bai M, et al. Apelin-13-loaded macrophage membrane-encapsulated nanoparticles for targeted ischemic stroke therapy via inhibiting NLRP3 inflammasome-mediated pyroptosis. Int J Nanomedicine. (2024) 19:9175–93. doi: 10.2147/IJN.S475915
68. Xu L, Li S, Qi J, Mi Y, Zhang Y, Yang Y, et al. Effusol ameliorates ischemic stroke by targeting NLRP3 protein to regulate NLRP3 inflammasome-mediated pyroptosis. Phytomedicine. (2025) 136:156253. doi: 10.1016/j.phymed.2024.156253
69. Wang M, Liu Y, Zhong L, Wu F, and Wang J. Advancements in the investigation of gut microbiota-based strategies for stroke prevention and treatment. Front Immunol. (2025) 16:1533343. doi: 10.3389/fimmu.2025.1533343
70. Xie X, Wang L, Dong S, Ge S, and Zhu T. Immune regulation of the gut-brain axis and lung-brain axis involved in ischemic stroke. Neural Regener Res. (2024) 19:519–28. doi: 10.4103/1673-5374.380869
71. Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat Med. (2016) 22:516–23. doi: 10.1038/nm.4068
72. Zhang T, Yue Y, Li C, Wu X, and Park S. Vagus nerve suppression in ischemic stroke by carotid artery occlusion: implications for metabolic regulation, cognitive function, and gut microbiome in a gerbil model. Int J Mol Sci. (2024) 25:7831. doi: 10.3390/ijms25147831
73. Monsour M, Croci DM, Agazzi S, and Borlongan CV. Contemplating IL-6, a double-edged sword cytokine: Which side to use for stroke pathology? CNS Neurosci Ther. (2023) 29:493–7. doi: 10.1111/cns.14041
74. Hall C, Nguyen DT, Mendoza K, Tan C, and Chauhan A. Inhibition of IL-6 trans-signaling promotes post-stroke functional recovery in a sex and dose-dependent manner. J Neuroinflammation. (2025) 22:52. doi: 10.1186/s12974-025-03365-y
75. Ziegler L, Wallen H, Aspberg S, de Faire U, and Gigante B. IL6 transsignaling associates with ischemic stroke but not with atrial fibrillation. BMC Neurol. (2021) 21:306. doi: 10.1186/s12883-021-02321-6
76. Laflamme N, Lacroix S, and Rivest S. An essential role of interleukin-1beta in mediating NF-kappaB activity and COX-2 transcription in cells of the blood-brain barrier in response to a systemic and localized inflammation but not during endotoxemia. J Neurosci. (1999) 19:10923–30. doi: 10.1523/JNEUROSCI.19-24-10923.1999
77. Srinivasan D, Yen JH, Joseph DJ, and Friedman W. Cell type-specific interleukin-1beta signaling in the CNS. J Neurosci. (2004) 24:6482–8. doi: 10.1523/JNEUROSCI.5712-03.2004
78. McColl BW, Rothwell NJ, and Allan SM. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. J Neurosci. (2007) 27:4403–12. doi: 10.1523/JNEUROSCI.5376-06.2007
79. Furukawa T, Miyake Y, Ito H, Ogata A, Maeyama H, Nakahara Y, et al. Interleukin-27 deletion has neuroprotective effects in the acute ischemic stage of cerebral infarction. Biochem Biophys Res Commun. (2025) 755:151581. doi: 10.1016/j.bbrc.2025.151581
80. Gerdes N, Sukhova GK, and Libby P. Expression of interleukin (IL)-18 and functional IL-18 receptor on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for atherogenesis. J Exp Med. (2002) 195:245–57. doi: 10.1084/jem.20011022
81. Chen AQ, Fang Z, Chen XL, Yang S, Zhou YF, Mao L, et al. Microglia-derived TNF-α mediates endothelial necroptosis aggravating blood brain-barrier disruption after ischemic stroke. Cell Death Dis. (2019) 10:487. doi: 10.1038/s41419-019-1716-9
82. Wang M, Dufort C, Du Z, Shi R, Xu F, Huang Z, et al. IL-33/ST2 signaling in monocyte-derived macrophages maintains blood-brain barrier integrity and restricts infarctions early after ischemic stroke. J Neuroinflammation. (2024) 21:274. doi: 10.1186/s12974-024-03264-8
83. Sharma S, Yang B, Xi X, et al. IL-10 directly protects cortical neurons by activating PI-3 kinase and STAT-3 pathways. Brain Res. (2011) 1373:189–94. doi: 10.1016/j.brainres.2010.11.096
84. Bodhankar S, Chen Y, Vandenbark AA, et al. Treatment of experimental stroke with IL-10-producing B-cells reduces infarct size and peripheral and CNS inflammation in wild-type B-cell-sufficient mice. Metab Brain Dis. (2014) 29:59–73. doi: 10.1007/s11011-013-9474-3
85. Zhang H, Xia Y, Ye Q, et al. In vivo expansion of regulatory T cells with IL-2/IL-2 antibody complex protects against transient ischemic stroke. J Neurosci. (2018) 38:10168–79. doi: 10.1523/JNEUROSCI.3411-17.2018
86. Zhou YX, Wang X, Tang D, et al. IL-2mAb reduces demyelination after focal cerebral ischemia by suppressing CD8+ T cells. CNS Neurosci Ther. (2019) 25:532–43. doi: 10.1111/cns.13084
87. Liu X, Liu J, Zhao S, et al. Interleukin-4 is essential for microglia/macrophage M2 polarization and long-term recovery after cerebral ischemia. Stroke. (2016) 47:498–504. doi: 10.1161/STROKEAHA.115.012079
88. Tian Y, Zhu P, Liu S, et al. IL-4-polarized BV2 microglia cells promote angiogenesis by secreting exosomes. Adv Clin Exp Med. (2019) 28:421–30. doi: 10.17219/acem/91826
89. Bonde C, Kristensen BW, Blaabjerg M, et al. GDNF and neublastin protect against NMDA-induced excitotoxicity in hippocampal slice cultures. Neuroreport. (2000) 11:4069–73. doi: 10.1097/00001756-200012180-00032
90. Nicole O, Ali C, Docagne F, et al. Neuroprotection mediated by glial cell line-derived neurotrophic factor: involvement of a reduction of NMDA-induced calcium influx by the mitogen-activated protein kinase pathway. J Neurosci. (2001) 21:3024–33. doi: 10.1523/JNEUROSCI.21-09-03024.2001
91. Xin W, Pan Y, Wei W, et al. TGF-β1 decreases microglia-mediated neuroinflammation and lipid droplet accumulation in an in vitro stroke model. Int J Mol Sci. (2023) 24:17329. doi: 10.3390/ijms242417329
92. Gertz K, Kronenberg G, Kälin RE, et al. Essential role of interleukin-6 in post-stroke angiogenesis. Brain. (2012) 135:1964–80. doi: 10.1093/brain/aws075
93. Yao H, Zhang Y, Shu H, et al. Hyperforin promotes post-stroke neuroangiogenesis via astrocytic IL-6-mediated negative immune regulation in the ischemic brain. Front Cell Neurosci. (2019) 13:201. doi: 10.3389/fncel.2019.00201
94. Choi BR, Johnson KR, Maric D, and McGavern DB. Monocyte-derived IL-6 programs microglia to rebuild damaged brain vasculature. Nat Immunol. (2023) 24:1110–23. doi: 10.1038/s41590-023-01521-1
95. Williams JL, Holman DW, and Klein RS. Chemokines in the balance: maintenance of homeostasis and protection at CNS barriers. Front Cell Neurosci. (2014) 8:154. doi: 10.3389/fncel.2014.00154
96. Réaux-Le Goazigo A, Van Steenwinckel J, Rostène W, and Mélik Parsadaniantz S. Current status of chemokines in the adult CNS. Prog Neurobiol. (2013) 104:67–92. doi: 10.1016/j.pneurobio.2013.02.001
97. Lin YT, Chen HD, Ai QD, Yang YT, Zhang Z, Chu SF, et al. Characteristics and pathogenesis of chemokines in the post-stroke stage. Int Immunopharmacol. (2023) 116:109781. doi: 10.1016/j.intimp.2023.109781
98. Xu J, Dong H, Qian Q, Zhang X, Wang Y, Jin W, et al. Astrocyte-derived CCL2 participates in surgery-induced cognitive dysfunction and neuroinflammation via evoking microglia activation. Behav Brain Res. (2017) 332:145–53. doi: 10.1016/j.bbr.2017.05.066
99. Strecker JK, Minnerup J, Schütte-Nütgen K, Gess B, Schäbitz WR, Schilling M, et al. Monocyte chemoattractant protein-1-deficiency results in altered blood-brain barrier breakdown after experimental stroke. Stroke. (2013) 44:2536–44. doi: 10.1161/STROKEAHA.111.000528
100. Ma Y, Zheng K, Zhao C, et al. Microglia LILRB4 upregulation reduces brain damage after acute ischemic stroke by limiting CD8+ T cell recruitment. J Neuroinflammation. (2024) 21:214. doi: 10.1186/s12974-024-03206-4
101. Lee S, Kim OJ, Lee KO, Jung H, Oh SH, and Kim NK. Enhancing the therapeutic potential of CCL2-overexpressing mesenchymal stem cells in acute stroke. Int J Mol Sci. (2020) 21:7795. doi: 10.3390/ijms21207795
102. Ottonello L, Montecucco F, Bertolotto M, Arduino N, Mancini M, Corcione A, et al. CCL3 (MIP-1alpha) induces in vitro migration of GM-CSF-primed human neutrophils via CCR5-dependent activation of ERK 1/2. Cell Signal. (2005) 17:355–63. doi: 10.1016/j.cellsig.2004.08.002
103. Jiang L, Newman M, Saporta S, Chen N, Sanberg C, Sanberg PR, et al. MIP-1alpha and MCP-1 induce migration of human umbilical cord blood cells in models of stroke. Curr Neurovasc Res. (2008) 5:118–24. doi: 10.2174/156720208784310259
104. Cheng SS, Lai JJ, Lukacs NW, and Kunkel SL. Granulocyte-macrophage colony stimulating factor up-regulates CCR1 in human neutrophils. J Immunol. (2001) 166:1178–84. doi: 10.4049/jimmunol.166.2.1178
105. Reichel CA, Rehberg M, Lerchenberger M, Berberich N, Bihari P, Khandoga AG, et al. Ccl2 and Ccl3 mediate neutrophil recruitment via induction of protein synthesis and generation of lipid mediators. Arterioscler Thromb Vasc Biol. (2009) 29:1787–93. doi: 10.1161/ATVBAHA.109.193268
106. Montecucco F, Steffens S, Burger F, Da Costa A, Bianchi G, Bertolotto M, et al. Tumor necrosis factor-alpha (TNFalpha) induces integrin CD11b/CD18 (Mac-1) up-regulation and migration to the CC chemokine CCL3 (MIP-1alpha) on human neutrophils through defined signaling pathways. Cell Signal. (2008) 20:557–68. doi: 10.1016/j.cellsig.2007.11.008
107. Montecucco F, Bianchi G, Bertolotto M, Viviani G, Dallegri F, and Ottonello L. Insulin primes human neutrophils for CCL3-induced migration: crucial role for JNK 1/2. Ann N Y Acad Sci. (2006) 1090:399–407. doi: 10.1196/annals.1378.043
108. Montecucco F, Burger F, Mach F, and Steffens S. CB2 cannabinoid receptor agonist JWH-015 modulates human monocyte migration through defined intracellular signaling pathways. Am J Physiol Heart Circ Physiol. (2008) 294:H1145–55. doi: 10.1152/ajpheart.01328.2007
109. Tatara Y, Ohishi M, Yamamoto K, Shiota A, Hayashi N, Iwamoto Y, et al. Macrophage inflammatory protein-1beta induced cell adhesion with increased intracellular reactive oxygen species. J Mol Cell Cardiol. (2009) 47:104–11. doi: 10.1016/j.yjmcc.2009.03.012
110. Terao S, Yilmaz G, Stokes KY, Russell J, Ishikawa M, Kawase T, et al. Blood cell-derived RANTES mediates cerebral microvascular dysfunction, inflammation, and tissue injury after focal ischemia-reperfusion. Stroke. (2008) 39:2560–70. doi: 10.1161/STROKEAHA.107.513150
111. Ubogu EE, Callahan MK, Tucky BH, and Ransohoff RM. CCR5 expression on monocytes and T cells: modulation by transmigration across the blood-brain barrier in vitro. Cell Immunol. (2006) 243:19–29. doi: 10.1016/j.cellimm.2006.12.001
112. Shahrara S, Park CC, Temkin V, Jarvis JW, Volin MV, and Pope RM. RANTES modulates TLR4-induced cytokine secretion in human peripheral blood monocytes. J Immunol. (2006) 177:5077–87. doi: 10.4049/jimmunol.177.8.5077
113. Sorce S, Bonnefont J, Julien S, Marq-Lin N, Rodriguez I, Dubois-Dauphin M, et al. Increased brain damage after ischemic stroke in mice lacking the chemokine receptor CCR5. Br J Pharmacol. (2010) 160:311–21. doi: 10.1111/j.1476-5381.2010.00697.x
114. Rostène W, Kitabgi P, and Parsadaniantz SM. Chemokines: a new class of neuromodulator? Nat Rev Neurosci. (2007) 8:895–903. doi: 10.1038/nrn2255
115. Stamatovic SM, Phillips CM, Keep RF, and Andjelkovic AV. A novel approach to treatment of thromboembolic stroke in mice: Redirecting neutrophils toward a peripherally implanted CXCL1-soaked sponge. Exp Neurol. (2020) 330:113336. doi: 10.1016/j.expneurol.2020.113336
116. Huang X, Guo M, Zhang Y, Xie J, Huang R, Zuo Z, et al. Microglial IL-1RA ameliorates brain injury after ischemic stroke by inhibiting astrocytic CXCL1-mediated neutrophil recruitment and microvessel occlusion. Glia. (2023) 71:1607–25. doi: 10.1002/glia.24359
117. Schmerbach K, Schefe JH, Krikov M, Müller S, Villringer A, Kintscher U, et al. Comparison between single and combined treatment with candesartan and pioglitazone following transient focal ischemia in rat brain. Brain Res. (2008) 1208:225–33. doi: 10.1016/j.brainres.2008.02.032
118. Wang Y, Luo W, Stricker R, and Reiser G. Protease-activated receptor-1 protects rat astrocytes from apoptotic cell death via JNK-mediated release of the chemokine GRO/CINC-1. J Neurochem. (2006) 98:1046–60. doi: 10.1111/j.1471-4159.2006.03950.x
119. Kostulas N, Kivisäkk P, Huang Y, Matusevicius D, Kostulas V, and Link H. Ischemic stroke is associated with a systemic increase of blood mononuclear cells expressing interleukin-8 mRNA. Stroke. (1998) 29:462–6. doi: 10.1161/01.str.29.2.462
120. Kostulas N, Pelidou SH, Kivisäkk P, Kostulas V, and Link H. Increased IL-1beta, IL-8, and IL-17 mRNA expression in blood mononuclear cells observed in a prospective ischemic stroke study. Stroke. (1999) 30:2174–9. doi: 10.1161/01.str.30.10.2174
121. Domac FM and Misirli H. The role of neutrophils and interleukin-8 in acute ischemic stroke. Neurosci (Riyadh). (2008) 13:136–41.
122. Lv H, Li J, and Che YQ. CXCL8 gene silencing promotes neuroglial cells activation while inhibiting neuroinflammation through the PI3K/Akt/NF-κB-signaling pathway in mice with ischemic stroke. J Cell Physiol. (2019) 234:7341–55. doi: 10.1002/jcp.27493
123. Zhang L, Xu D, Zhang T, Hou W, and Yixi L. Correlation between interleukin-6, interleukin-8, and modified early warning score of patients with acute ischemic stroke and their condition and prognosis. Ann Palliat Med. (2021) 10:148–55. doi: 10.21037/apm-20-2200
124. Shaheen HA, Daker LI, Abbass MM, and Abd El Fattah AA. The relationship between the severity of disability and serum IL-8 in acute ischemic stroke patients. Egypt J Neurol Psychiatr Neurosurg. (2018) 54:26. doi: 10.1186/s41983-018-0025-z
125. Li L, Chu L, Ren C, Wang J, Sun S, Li T, et al. Enhanced migration of bone marrow-derived mesenchymal stem cells with tetramethylpyrazine and its synergistic effect on angiogenesis and neurogenesis after cerebral ischemia in rats. Stem Cells Dev. (2019) 28:871–81. doi: 10.1089/scd.2018.0254
126. Shyu WC, Liu DD, Lin SZ, Li WW, Su CY, Chang YC, et al. Implantation of olfactory ensheathing cells promotes neuroplasticity in murine models of stroke. J Clin Invest. (2008) 118:2482–95. doi: 10.1172/JCI34363
127. Bakondi B, Shimada IS, Peterson BM, and Spees JL. SDF-1α secreted by human CD133-derived multipotent stromal cells promotes neural progenitor cell survival through CXCR7. Stem Cells Dev. (2011) 20:1021–9. doi: 10.1089/scd.2010.0198
128. Schutt RC, Burdick MD, Strieter RM, Mehrad B, and Keeley EC. Plasma CXCL12 levels as a predictor of future stroke. Stroke. (2012) 43:3382–6. doi: 10.1161/STROKEAHA.112.660878
129. Ma A, Pan X, Xing Y, Wu M, Wang Y, and Ma C. Elevation of serum CXCL16 level correlates well with atherosclerotic ischemic stroke. Arch Med Sci. (2014) 10:47–52. doi: 10.5114/aoms.2013.39200
130. Ma A, Yang S, Wang Y, Wang X, and Pan X. Increase of serum CXCL16 level correlates well to microembolic signals in acute stroke patients with carotid artery stenosis. Clin Chim Acta. (2016) 460:67–71. doi: 10.1016/j.cca.2016.06.026
131. Prapiadou S, Živkoviç L, Thorand B, George MJ, van der Laan SW, Malik R, et al. Proteogenomic data integration reveals CXCL10 as a potentially downstream causal mediator for IL-6 signaling on atherosclerosis. Circulation. (2024) 149:669–83. doi: 10.1161/CIRCULATIONAHA.123.064974
132. Wolinski P and Glabinski A. Chemokines and neurodegeneration in the early stage of experimental ischemic stroke. Mediators Inflamm. (2013) 2013:727189. doi: 10.1155/2013/727189
133. Chen J, Jin J, Zhang X, Yu H, Zhu X, Yu L, et al. Microglial lnc-U90926 facilitates neutrophil infiltration in ischemic stroke via MDH2/CXCL2 axis. Mol Ther. (2021) 29:2873–85. doi: 10.1016/j.ymthe.2021.04.025
134. Yu M, Ma X, Jiang D, Wang L, Zhan Q, and Zhao J. CXC chemokine ligand 5 (CXCL5) disrupted the permeability of human brain microvascular endothelial cells via regulating p38 signal. Microbiol Immunol. (2021) 65:40–7. doi: 10.1111/1348-0421.12854
135. Subbarayan MS, Joly-Amado A, Bickford PC, and Nash KR. CX3CL1/CX3CR1 signaling targets for the treatment of neurodegenerative diseases. Pharmacol Ther. (2022) 231:107989. doi: 10.1016/j.pharmthera.2021.107989
136. Cisbani G, Le Behot A, Plante MM, Préfontaine P, Lecordier M, and Rivest S. Role of the chemokine receptors CCR2 and CX3CR1 in an experimental model of thrombotic stroke. Brain Behav Immun. (2018) 70:280–92. doi: 10.1016/j.bbi.2018.03.008
137. Tang Z, Gan Y, Liu Q, Yin JX, Liu Q, Shi J, et al. CX3CR1 deficiency suppresses activation and neurotoxicity of microglia/macrophage in experimental ischemic stroke. J Neuroinflammation. (2014) 11:26. doi: 10.1186/1742-2094-11-26
138. Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. (2003) 107:499–511. doi: 10.1161/01.cir.0000052939.59093.45
139. Nakase T, Yamazaki T, Ogura N, Suzuki A, and Nagata K. The impact of inflammation on the pathogenesis and prognosis of ischemic stroke. J Neurol Sci. (2008) 271:104–9. doi: 10.1016/j.jns.2008.03.020
140. Youn CS, Choi SP, Kim SH, Oh SH, Jeong WJ, Kim HJ, et al. Serum highly selective C-reactive protein concentration is associated with the volume of ischemic tissue in acute ischemic stroke. Am J Emerg Med. (2012) 30:124–8. doi: 10.1016/j.ajem.2010.11.006
141. Gussekloo J, Schaap MC, Frölich M, Blauw GJ, and Westendorp RG. C-reactive protein is a strong but nonspecific risk factor of fatal stroke in elderly persons. Arterioscler Thromb Vasc Biol. (2000) 20:1047–51. doi: 10.1161/01.atv.20.4.1047
142. Elkind MS, Luna JM, Moon YP, Liu KM, Spitalnik SL, Paik MC, et al. High-sensitivity C-reactive protein predicts mortality but not stroke: the Northern Manhattan Study. Neurology. (2009) 73:1300–7. doi: 10.1212/WNL.0b013e3181bd10bc
143. Vandooren J, Van Damme J, and Opdenakker G. On the structure and functions of gelatinase B/matrix metalloproteinase-9 in neuroinflammation. Prog Brain Res. (2014) 214:193–206. doi: 10.1016/B978-0-444-63486-3.00009-8
144. Morancho A, Rosell A, García-Bonilla L, and Montaner J. Metalloproteinase and stroke infarct size: role for anti-inflammatory treatment? Ann N Y Acad Sci. (2010) 1207:123–33. doi: 10.1111/j.1749-6632.2010.05734.x
145. Palm F, Pussinen PJ, Safer A, Tervahartiala T, Sorsa T, Urbanek C, et al. Serum matrix metalloproteinase-8, tissue inhibitor of metalloproteinase and myeloperoxidase in ischemic stroke. Atherosclerosis. (2018) 271:9–14. doi: 10.1016/j.atherosclerosis.2018.02.012
146. Chang JJ, Stanfill A, and Pourmotabbed T. The role of matrix metalloproteinase polymorphisms in ischemic stroke. Int J Mol Sci. (2016) 17:1323. doi: 10.3390/ijms17081323
147. Park KP, Rosell A, Foerch C, Xing C, Kim WJ, Lee S, et al. Plasma and brain matrix metalloproteinase-9 after acute focal cerebral ischemia in rats. Stroke. (2009) 40:2836–42. doi: 10.1161/STROKEAHA.109.554824
148. Golab P, Kielbus M, Bielewicz J, and Kurzepa J. The effect of recombinant tissue plasminogen activator on MMP-2 and MMP-9 activities in vitro. Neurol Res. (2015) 37:9–13. doi: 10.1179/1743132814Y.0000000412
149. Lenglet S, Montecucco F, Mach F, Schaller K, Gasche Y, and Copin JC. Analysis of the expression of nine secreted matrix metalloproteinases and their endogenous inhibitors in the brain of mice subjected to ischemic stroke. Thromb Hemost. (2014) 112:363–78. doi: 10.1160/TH14-01-0007
150. Yang Y, Jalal FY, Thompson JF, Walker EJ, Candelario-Jalil E, Li L, et al. Tissue inhibitor of metalloproteinases-3 mediates the death of immature oligodendrocytes via TNF-α/TACE in focal cerebral ischemia in mice. J Neuroinflammation. (2011) 8:108. doi: 10.1186/1742-2094-8-108
151. Cuadrado E, Rosell A, Borrell-Pagès M, García-Bonilla L, Hernández-Guillamon M, Ortega-Aznar A, et al. Matrix metalloproteinase-13 is activated and is found in the nucleus of neural cells after cerebral ischemia. J Cereb Blood Flow Metab. (2009) 29:398–410. doi: 10.1038/jcbfm.2008.130
152. Yang Y and Rosenberg GA. Matrix metalloproteinases as therapeutic targets for stroke. Brain Res. (2015) 1623:30–8. doi: 10.1016/j.brainres.2015.04.024
153. Dang B, Duan X, Wang Z, He W, and Chen G. A therapeutic target of cerebral hemorrhagic stroke: matrix metalloproteinase- 9. Curr Drug Targets. (2017) 18:1358–66. doi: 10.2174/1389450118666170427151657
154. Ma F, Rodriguez S, Buxo X, Morancho A, Riba-Llena I, Carrera A, et al. Plasma matrix metalloproteinases in patients with stroke during intensive rehabilitation therapy. Arch Phys Med Rehabil. (2016) 97:1832–40. doi: 10.1016/j.apmr.2016.06.007
155. Abdelnaseer MM, Elfauomy NM, Esmail EH, Kamal MM, and Elsawy EH. Matrix metalloproteinase-9 and recovery of acute ischemic stroke. J Stroke Cerebrovasc Dis. (2017) 26:733–40. doi: 10.1016/j.jstrokecerebrovasdis.2016.09.043
156. Nishi M, Kawata M, and Azmitia EC. S100beta promotes the extension of microtubule associated protein2 (MAP2)-immunoreactive neurites retracted after colchicine treatment in rat spinal cord culture. Neurosci Lett. (1997) 229:212–4. doi: 10.1016/s0304-3940(97)00443-6
157. Meske V, Hamker U, Albert F, and Ohm TG. The effects of beta/A4-amyloid and its fragments on calcium homeostasis, glial fibrillary acidic protein and S100beta staining, morphology and survival of cultured hippocampal astrocytes. Neuroscience. (1998) 85:1151–60. doi: 10.1016/s0306-4522(98)00008-6
158. Wunderlich MT, Ebert AD, Kratz T, Goertler M, Jost S, and Herrmann M. Early neurobehavioral outcome after stroke is related to release of neurobiochemical markers of brain damage. Stroke. (1999) 30:1190–5. doi: 10.1161/01.str.30.6.1190
159. Li K, Jia J, Wang Z, and Zhang S. Elevated serum levels of NSE and S-100β Correlate with increased risk of acute cerebral infarction in asian populations. Med Sci Monit. (2015) 21:1879–88. doi: 10.12659/msm.893615
160. Yardan T, Erenler AK, Baydin A, et al. Usefulness of S100B protein in neurological disorders. J Pak Med Assoc. (2011) 61:276–81.
161. Büttner T, Weyers S, Postert T, Sprengelmeyer R, and Kuhn W. S-100 protein: serum marker of focal brain damage after ischemic territorial MCA infarction. Stroke. (1997) 28:1961–5. doi: 10.1161/01.str.28.10.1961
162. Petzold A, Michel P, Stock M, and Schluep M. Glial and axonal body fluid biomarkers are related to infarct volume, severity, and outcome. J Stroke Cerebrovasc Dis. (2008) 17:196–203. doi: 10.1016/j.jstrokecerebrovasdis.2008.02.002
163. Jiang D, Wang Y, Zang Y, Liu X, Zhao L, Wang Q, et al. Neuroprotective effects of rhGLP-1 in diabetic rats with cerebral ischemia/reperfusion injury. Drug Dev Res. (2016) 77:124–33. doi: 10.1002/ddr.21297
164. Serarslan Y, Bal R, Altuğ ME, et al. Caffeic acid phenethyl ester decreases the level of S-100B protein after middle cerebral artery [correction for after] occlusion in rabbits. Pak J Pharm Sci. (2009) 22:313–6.
165. Tateishi N, Mori T, Kagamiishi Y, Satoh S, Katsube N, Morikawa E, et al. Astrocytic activation and delayed infarct expansion after permanent focal ischemia in rats. Part II: suppression of astrocytic activation by a novel agent (R)-(-)-2-propyloctanoic acid (ONO-2506) leads to mitigation of delayed infarct expansion and early improvement of neurologic deficits. J Cereb Blood Flow Metab. (2002) 22:723–34. doi: 10.1097/00004647-200206000-00011
166. Matsui T, Mori T, Tateishi N, Kagamiishi Y, Satoh S, Katsube N, et al. Astrocytic activation and delayed infarct expansion after permanent focal ischemia in rats. Part I: enhanced astrocytic synthesis of s-100beta in the periinfarct area precedes delayed infarct expansion. J Cereb Blood Flow Metab. (2002) 22:711–22. doi: 10.1097/00004647-200206000-00010
167. Wunderlich MT, Lins H, Skalej M, Wallesch CW, and Goertler M. Neuron-specific enolase and tau protein as neurobiochemical markers of neuronal damage are related to early clinical course and long-term outcome in acute ischemic stroke. Clin Neurol Neurosurg. (2006) 108:558–63. doi: 10.1016/j.clineuro.2005.12.006
168. Virani SS and Nambi V. The role of lipoprotein-associated phospholipase A2 as a marker for atherosclerosis. Curr Atheroscler Rep. (2007) 9:97–103. doi: 10.1007/s11883-007-0004-9
169. Gorelick PB. Lipoprotein-associated phospholipase A2 and risk of stroke. Am J Cardiol. (2008) 101:34F–40F. doi: 10.1016/j.amjcard.2008.04.017
170. Chen Y, Pu J, Liu Y, Tian L, Chen X, Gui S, et al. Pro-inflammatory cytokines are associated with the development of post-stroke depression in the acute stage of stroke: A meta-analysis. Top Stroke Rehabil. (2020) 27:620–9. doi: 10.1080/10749357.2020.1755813
171. Wang C, Gao L, Zhang ZG, Li YQ, Yang YL, Chang T, et al. Procalcitonin is a stronger predictor of long-term functional outcome and mortality than high-sensitivity C-reactive protein in patients with ischemic stroke. Mol Neurobiol. (2016) 53:1509–17. doi: 10.1007/s12035-015-9112-7
172. Pawluk H, Kołodziejska R, Grzęsk G, Kozakiewicz M, Woźniak A, Pawluk M, et al. Selected mediators of inflammation in patients with acute ischemic stroke. Int J Mol Sci. (2022) 23:10614. doi: 10.3390/ijms231810614
173. Yu B, Shi G, Yang F, and Xu W. Correlation of LP-PLA2 and MMP-9 with the occurrence of early neurological deterioration in patients with acute ischemic stroke. Med (Baltimore). (2024) 103:e38310. doi: 10.1097/MD.0000000000038310
174. Castillo J, Álvarez-Sabín J, Martínez-Vila E, Montaner J, Sobrino T, and Vivancos J. Inflammation markers and prediction of post-stroke vascular disease recurrence: the MITICO study. J Neurol. (2009) 256:217–24. doi: 10.1007/s00415-009-0058-4
175. Zhang YB, Yin Z, Han X, Wang Q, Zhang Z, and Geng J. Association of circulating highsensitivity C-reactive protein with late recurrence after ischemic stroke. Neuroreport. (2017) 28:598–603. doi: 10.1097/WNR.0000000000000806
176. Gu XL, Liu L, Lu XD, and Liu ZR. Serum CXCL12 levels as a novel predictor of future stroke recurrence in patients with acute ischemic stroke. Mol Neurobiol. (2016) 53:2807–14. doi: 10.1007/s12035-015-9151-0
177. Han L, Zhong C, Bu X, Xu T, Wang A, Peng Y, et al. Prognostic value of lipoprotein-associated phospholipase A2 mass for all-cause mortality and vascular events within one year after acute ischemic stroke. Atherosclerosis. (2017) 266:1–7. doi: 10.1016/j.atherosclerosis.2017.09.013
178. Hansson GK, Robertson AK, and Söderberg-Nauclér C. Inflammation and atherosclerosis. Annu Rev Pathol. (2006) 1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100
179. Ridker PM, Cushman M, Stampfer MJ, Tracy RP, and Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. (1997) 336:973–9. doi: 10.1056/NEJM199704033361401
180. Tuttolomondo A, Di Raimondo D, Pecoraro R, Arnao V, Pinto A, and Licata G. Inflammation in ischemic stroke subtypes. Curr Pharm Des. (2012) 18:4289–310. doi: 10.2174/138161212802481200
181. Bonetti NR, Diaz-Cañestro C, Liberale L, Crucet M, Akhmedov A, Merlini M, et al. Tumor necrosis factor-αInhibition improves stroke outcome in a mouse model of rheumatoid arthritis. Sci Rep. (2019) 9:2173. doi: 10.1038/s41598-019-38670-z
182. Dawson DA, Martin D, and Hallenbeck JM. Inhibition of tumor necrosis factor-alpha reduces focal cerebral ischemic injury in the spontaneously hypertensive rat. Neurosci Lett. (1996) 218:41–4. doi: 10.1016/0304-3940(96)13116-5
183. Russman AN, Silver B, Katramados A, Chopp M, Burmeister C, Schultz L, et al. Abstract WP156: a phase IIa double-blind, placebo controlled study of extended-release niacin for stroke recovery. Stroke. (2024) 49. doi: 10.1161/str.49.suppl_1.WP156
184. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Anti-inflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. (2017) 377:1119–31. doi: 10.1056/NEJMoa1707914
185. Ali M, Girgis S, Hassan A, Rudick S, and Becker RC. Inflammation and coronary artery disease: from pathophysiology to Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Coron Artery Dis. (2018) 29:429–37. doi: 10.1097/MCA.0000000000000625
186. Fusco W, Adolph T, Cammarota G, Gasbarrini A, Ianiro G, and Tilg H. Gut microbiota and atherosclerosis. Gut. (2025) 0:1–11. doi: 10.1136/gutjnl-2025-335610
187. Wang YH, Liao JM, Jan MS, Wang M, Su HH, Tsai WH, et al. Prophylactic use of probiotics as an adjunctive treatment for ischemic stroke via the gut-spleen-brain axis. Brain Behav Immun. (2025) 123:784–98. doi: 10.1016/j.bbi.2024.10.026
188. Wanchao S, Chen M, Zhiguo S, Futang X, and Mengmeng S. Protective effect and mechanism of Lactobacillus on cerebral ischemia reperfusion injury in rats. Braz J Med Biol Res. (2018) 51:e7172. doi: 10.1590/1414-431x20187172
189. Chen R, Xu Y, Wu P, Zhou H, Lasanajak Y, Fang Y, et al. Transplantation of fecal microbiota rich in short chain fatty acids and butyric acid treat cerebral ischemic stroke by regulating gut microbiota. Pharmacol Res. (2019) 148:104403. doi: 10.1016/j.phrs.2019.104403
190. Wang R, Gan C, Gong B, Huang J, Lou Z, Wang D, et al. Tongfu Xingshen capsule alleviates stroke-associated pneumonia-induced multiple organ injuries by modulating the gut microbiota and sphingolipid metabolism. Phytomedicine. (2025) 142:156756. doi: 10.1016/j.phymed.2025.156756
191. Wang Y, Tan Q, Pan M, Yu J, Wu S, Tu W, et al. Minimally invasive vagus nerve stimulation modulates mast cell degranulation via the microbiota-gut-brain axis to ameliorate blood-brain barrier and intestinal barrier damage following ischemic stroke. Int Immunopharmacol. (2024) 132:112030. doi: 10.1016/j.intimp.2024.112030
192. Taslim NA, Yusuf M, Ambari AM, Del Rosario Puling IM, Ibrahim FZ, Hardinsyah H, et al. Anti-inflammatory, antioxidant, metabolic and gut microbiota modulation activities of probiotic in cardiac remodeling condition: evidence from systematic study and meta-analysis of randomized controlled trials. Probiotics Antimicrob Proteins. (2023) 15:1049–61. doi: 10.1007/s12602-023-10105-2
193. Liu M, Xu Z, Wang L, Zhang L, Liu Y, Cao J, et al. Cottonseed oil alleviates ischemic stroke injury by inhibiting the inflammatory activation of microglia and astrocyte. J Neuroinflammation. (2020) 17:270. doi: 10.1186/s12974-020-01946-7
194. Hao T, Yang Y, Li N, Mi Y, Zhang G, Song J, et al. Inflammatory mechanism of cerebral ischemia-reperfusion injury with treatment of stepharine in rats. Phytomedicine. (2020) 79:153353. doi: 10.1016/j.phymed.2020.153353
195. Boujon V, Uhlemann R, Wegner S, Wright MB, Laufs U, Endres M, et al. Dual PPARα/γ agonist aleglitazar confers stroke protection in a model of mild focal brain ischemia in mice. J Mol Med (Berl). (2019) 97:1127–38. doi: 10.1007/s00109-019-01801-0
196. Chen B, Cao P, Guo X, Yin M, Li X, Jiang L, et al. Maraviroc, an inhibitor of chemokine receptor type 5, alleviates neuroinflammatory response after cerebral Ischemia/reperfusion injury via regulating MAPK/NF-κB signaling. Int Immunopharmacol. (2022) 108:108755. doi: 10.1016/j.intimp.2022.108755
197. Shi Y, Yi Z, Zhao P, Xu Y, and Pan P. MicroRNA-532-5p protects against cerebral ischemia-reperfusion injury by directly targeting CXCL1. Aging (Albany NY). (2021) 13:11528–41. doi: 10.18632/aging.202846
198. Franke M, Bieber M, Kraft P, Weber ANR, Stoll G, and Schuhmann MK. The NLRP3 inflammasome drives inflammation in ischemia/reperfusion injury after transient middle cerebral artery occlusion in mice. Brain Behav Immun. (2021) 92:223–33. doi: 10.1016/j.bbi.2020.12.009
199. Bu J, Shi S, Wang HQ, Niu XS, Zhao ZF, Wu WD, et al. Acacetin protects against cerebral ischemia-reperfusion injury via the NLRP3 signaling pathway. Neural Regener Res. (2019) 14:605–12. doi: 10.4103/1673-5374.247465
200. Bellut M, Papp L, Bieber M, Kraft P, Stoll G, and Schuhmann MK. NLPR3 inflammasome inhibition alleviates hypoxic endothelial cell death in vitro and protects blood-brain barrier integrity in murine stroke. Cell Death Dis. (2021) 13:20. doi: 10.1038/s41419-021-04379-z
201. Wang L, Zhao H, Zhai ZZ, and Qu LX. Protective effect and mechanism of ginsenoside Rg1 in cerebral ischemia-reperfusion injury in mice. BioMed Pharmacother. (2018) 99:876–82. doi: 10.1016/j.biopha.2018.01.136
202. Zheng T, Jiang H, Jin R, Zhao Y, Bai Y, Xu H, et al. Ginsenoside Rg1 attenuates protein aggregation and inflammatory response following cerebral ischemia and reperfusion injury. Eur J Pharmacol. (2019) 853:65–73. doi: 10.1016/j.ejphar.2019.02.018
203. Huang L, Chen C, Zhang X, Li X, Chen Z, Yang C, et al. Neuroprotective effect of curcumin against cerebral ischemia-reperfusion via mediating autophagy and inflammation. J Mol Neurosci. (2018) 64:129–39. doi: 10.1007/s12031-017-1006-x
204. Zhang S, Chen Q, Jin M, Ren J, Sun X, Zhang Z, et al. Notoginsenoside R1 alleviates cerebral ischemia/reperfusion injury by inhibiting the TLR4/MyD88/NF-κB signaling pathway through microbiota-gut-brain axis. Phytomedicine. (2024) 128:155530. doi: 10.1016/j.phymed.2024.155530
205. Wu M, Liu F, and Guo Q. Quercetin attenuates hypoxia-ischemia induced brain injury in neonatal rats by inhibiting TLR4/NF-κB signaling pathway. Int Immunopharmacol. (2019) 74:105704. doi: 10.1016/j.intimp.2019.105704
206. Yang S, Wang H, Yang Y, Wang R, Wang Y, Wu C, et al. Baicalein administered in the subacute phase ameliorates ischemia-reperfusion-induced brain injury by reducing neuroinflammation and neuronal damage. BioMed Pharmacother. (2019) 117:109102. doi: 10.1016/j.biopha.2019.109102
207. Chen YJ, Nguyen HM, Maezawa I, Jin LW, Wulff H, et al. Inhibition of the potassium channel Kv1.3 reduces infarction and inflammation in ischemic stroke. Ann Clin Transl Neurol. (2017) 5:147–61. doi: 10.1002/acn3.513
208. Shi X, Feng L, Li Y, Qin M, Li T, Cheng Z, et al. Efficacy and safety of Panax notoginseng saponins (Xuesaitong) for patients with acute ischemic stroke: a systematic review and meta-analysis of randomized controlled trials. Front Pharmacol. (2023) 14:1280559. doi: 10.3389/fphar.2023.1280559
209. Woudenberg T, van der Bent ML, van den Homberg DAL, Nguyen TTM, Wermer MJH, van den Wijngaard IR, et al. Validation of tRNA-derived fragments as diagnostic biomarkers in suspected acute stroke; limitations in analysis and quantification methods. Mol Ther Nucleic Acids. (2025) 36:102553. doi: 10.1016/j.omtn.2025.102553
210. Lakshmipriya T and Gopinath SCB. Clinical markers and diagnostics for diagnosing cerebral infarction. CNS Neurol Disord Drug Targets. (2025) 24, 494–97. doi: 10.2174/0118715273372575250212091813
211. Liu J, Bai C, Yang H, Song L, Xu H, Sun Y, et al. Machine learning-driven identification of blood-based biomarkers and therapeutic agents for personalized ischemic stroke management. J Cardiovasc Transl Res. (2025). doi: 10.1007/s12265-025-10635-w
212. Maïer B, Tsai AS, Einhaus JF, Desilles JP, Ho-Tin-Noé B, Gory B, et al. Neuroimaging is the new “spatial omic”: multi-omic approaches to neuro-inflammation and immuno-thrombosis in acute ischemic stroke. Semin Immunopathol. (2023) 45:125–43. doi: 10.1007/s00281-023-00984-6
213. Candelario-Jalil E, Dijkhuizen RM, and Magnus T. Neuroinflammation, stroke, blood-brain barrier dysfunction, and imaging modalities. Stroke. (2022) 53:1473–86. doi: 10.1161/STROKEAHA.122.036946
214. Montaner J, Ramiro L, Simats A, Tiedt S, Makris K, Jickling GC, et al. Multilevel omics for the discovery of biomarkers and therapeutic targets for stroke. Nat Rev Neurol. (2020) 16:247–64. doi: 10.1038/s41582-020-0350-6
Keywords: stroke, ischemic stroke, inflammation, biomarker, TCM
Citation: Xiao L, Huang Y, Wu L, Zeng S, Qiu C, Li X, Xie L and Wu D (2025) The role of inflammation in Ischemic stroke: from biomarker to treatment. Front. Immunol. 16:1608353. doi: 10.3389/fimmu.2025.1608353
Received: 08 April 2025; Accepted: 03 September 2025;
Published: 26 September 2025.
Edited by:
Luisa F. Duarte, Universidad del Desarrollo, ChileReviewed by:
Ivana Kawikova, National Institute of Mental Health, CzechiaGeetika Mehta, Raj Kumar Goel Institute of Technology (pharmacy), India
Copyright © 2025 Xiao, Huang, Wu, Zeng, Qiu, Li, Xie and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dahua Wu, ODkzMDQ5MzUyQHFxLmNvbQ==
 Liangyi Xiao
Liangyi Xiao Yini Huang1
Yini Huang1 Lingying Wu
Lingying Wu Shanshan Zeng
Shanshan Zeng Le Xie
Le Xie Dahua Wu
Dahua Wu