- 1Regina Berkovich MD, PhD Inc (MS Center and Research Institute), West Hollywood, CA, United States
- 2Department of Neurology, Minnesota Center for Multiple Sclerosis, Plymouth, MN, United States
- 3Department of Neurology, MS Center of Greater Washington, Vienna, VA, United States
- 4Department of Neurology, Rocky Mountain MS Clinic, Salt Lake City, UT, United States
- 5Department of Neurology, Ruhr-University Bochum, St. Josef-Hospital, Bochum, Germany
- 6Department of Neurology, The Elliot Lewis Center, Wellesley, MA, United States
- 7Atlantic Medical Group Multiple Sclerosis Comprehensive Care Center, Bridgewater, NJ, United States
A Corrigendum on:
Switching to ublituximab from prior anti-CD20 monoclonal antibody therapy: a case report series
by Berkovich R, Calkwood J, Crayton H, Erwin A, Faissner S, Gold R, Katz J and Leekoff M (2025). Front. Immunol. 16:1527102. doi: 10.3389/fimmu.2025.1527102
In the published article, there was an error in affiliation 1. Instead of “Berkovich MS Center and Research Institute, West Hollywood, CA, United States,” it should be “Regina Berkovich MD, PhD Inc (MS Center and Research Institute), West Hollywood, CA, United States.”
Also, there was an error in Figure 1, Figure 3, and Figure 4 as published. The y-axis titles of these figures contained a typo listing the unit of measure as “cells/¼ L”; the correct unit of measure is “cells/µL.” The corrected Figure 1, Figure 3, and Figure 4 and their captions appear below.
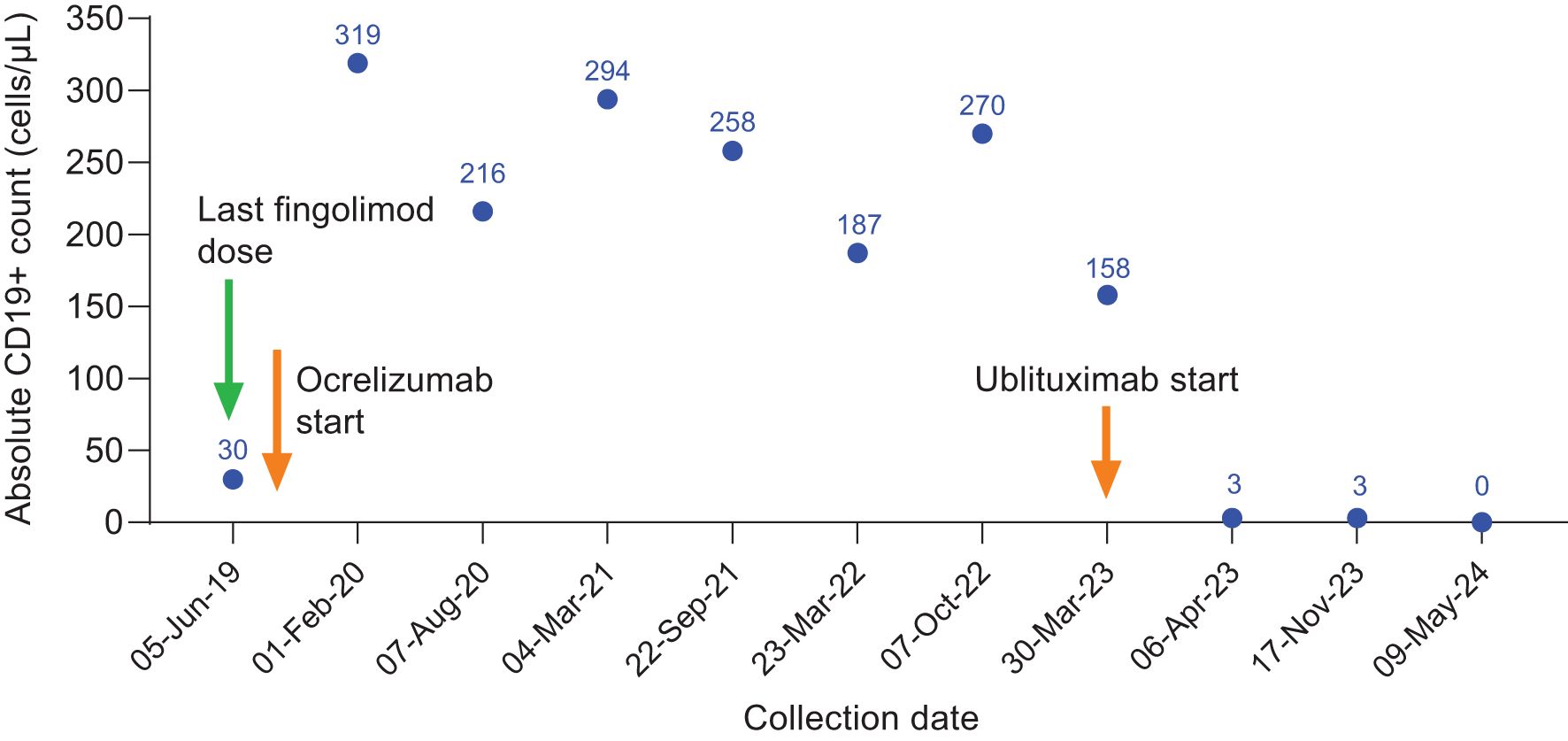
Figure 1. CD19+ counts in Case 1 during ocrelizumab treatment and after switch to ublituximab treatment. Ocrelizumab treatment was initiated in July 2019, with last ocrelizumab dose in October 2022. Ublituximab treatment was started in March 2023, with robust B-cell depletion (3 cells/μL) observed at 1 week after first ublituximab dose.
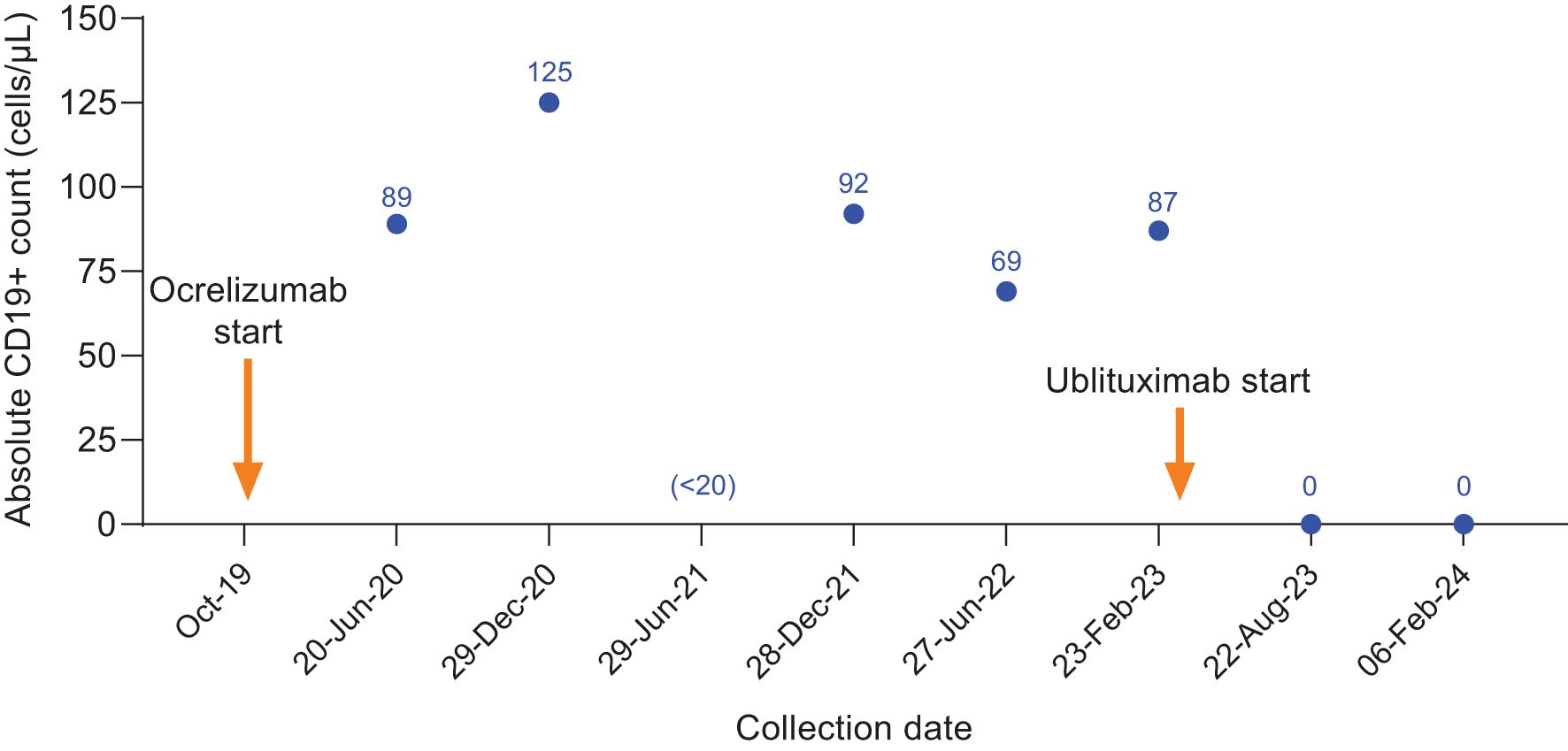
Figure 3. CD19+ counts in Case 2 during ocrelizumab treatment and after switch to ublituximab treatment. Ocrelizumab treatment was initiated in October 2019, with last ocrelizumab dose received in June 2022. The individual skipped the December 2022 ocrelizumab infusion with the intent to switch to ublituximab and initiated ublituximab treatment in March 2023, with complete B-cell depletion observed at the subsequent preinfusion blood collections.
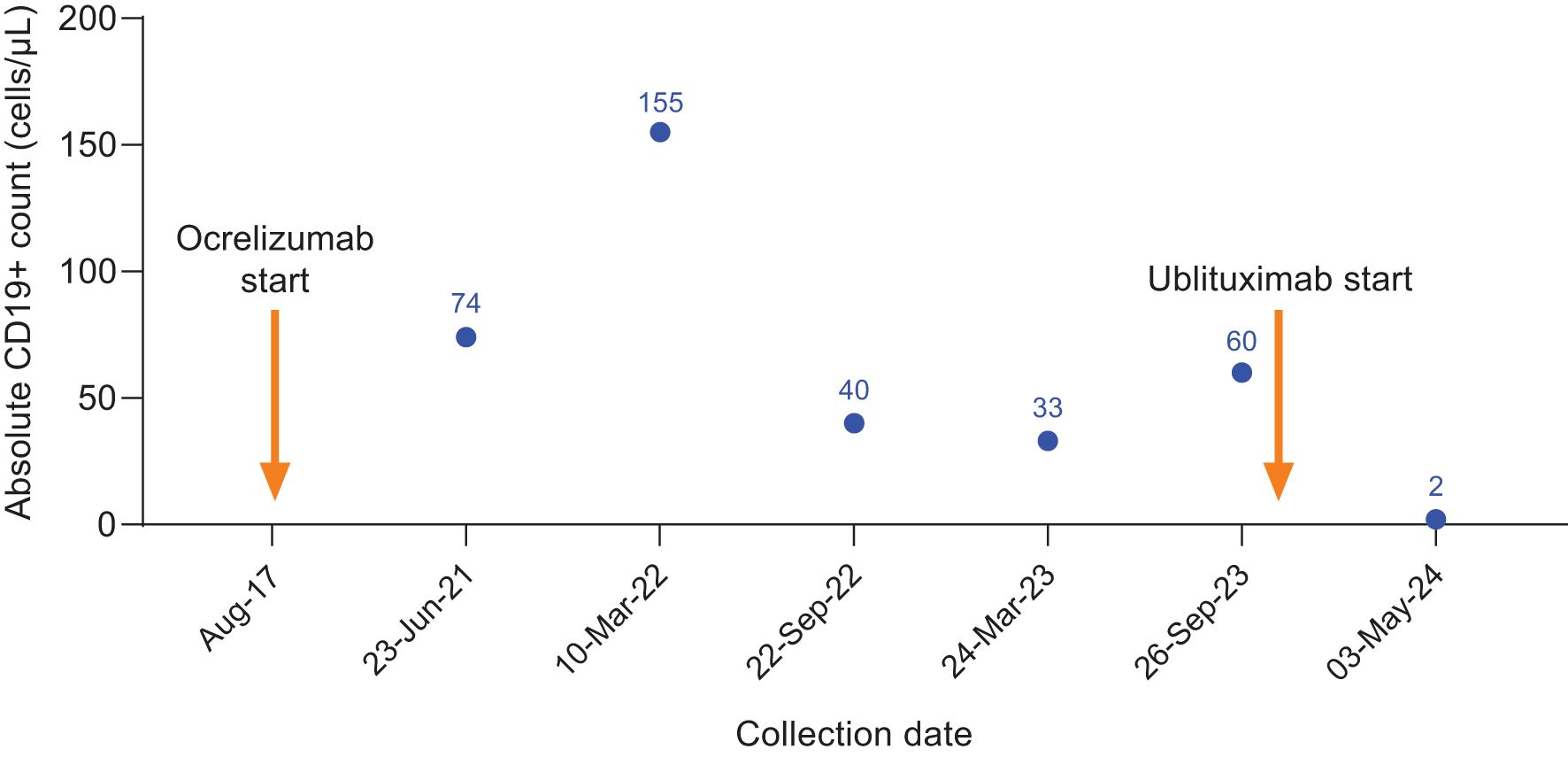
Figure 4. CD19+ counts in Case 4 during ocrelizumab treatment and after switch to ublituximab treatment. Ocrelizumab treatment was initiated in August 2017, with last ocrelizumab dose in March 2023. Ublituximab treatment was started in October 2023, with complete B-cell depletion observed at the subsequent preinfusion blood collection.
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: anti-CD20, B-cell depletion, disability, magnetic resonance imaging, multiple sclerosis, ocrelizumab, rituximab, ublituximab
Citation: Berkovich R, Calkwood J, Crayton H, Erwin A, Faissner S, Gold R, Katz J and Leekoff M (2025) Corrigendum: Switching to ublituximab from prior anti-CD20 monoclonal antibody therapy: a case report series. Front. Immunol. 16:1609054. doi: 10.3389/fimmu.2025.1609054
Received: 09 April 2025; Accepted: 26 May 2025;
Published: 13 June 2025.
Edited and Reviewed by:
Anne Haney Cross, Washington University in St. Louis, United StatesCopyright © 2025 Berkovich, Calkwood, Crayton, Erwin, Faissner, Gold, Katz and Leekoff. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Regina Berkovich, cmVnaW5hYmVya292aWNobWRAZ21haWwuY29t
†These authors have contributed equally to this work
 Regina Berkovich
Regina Berkovich Jonathan Calkwood2†
Jonathan Calkwood2† Heidi Crayton
Heidi Crayton April Erwin
April Erwin Simon Faissner
Simon Faissner Ralf Gold
Ralf Gold Joshua Katz
Joshua Katz Mark Leekoff
Mark Leekoff