- 1Division of General Internal Medicine, Department of Medicine, Tokyo Medical University Ibaraki Medical Center, Ibaraki, Japan
- 2Fujita Health University, Toyoake, Japan
- 3Clinic Grandsoul, Nara, Japan
- 4Meiko CIT Clinic, Tokyo, Japan
- 5Hijikata Clinic, Nagoya, Japan
- 6Biomedica Solution Inc., Osaka, Japan
- 7Research Institute of Pharmaceutical Sciences, University of Musashino, Tokyo, Japan
- 8EpiVax Inc., Providence, RI, United States
- 9Novacellum Inc., Tokyo, Japan
Background: Immune checkpoint inhibitors show limited efficacy in tumors with low tumor mutational burden, partly due to insufficient neoantigen presentation.
Methods: We developed a novel approach for neoantigen identification using circulating tumor cells (CTCs) isolated via leukapheresis and flow cytometry. Peripheral blood mononuclear cells (PBMCs) were collected from 11 stage IV cancer patients and 2 healthy volunteers. CTCs were enriched by depleting CD45+ hematopoietic cells and selecting CD45−Vimentin+ cells, which were confirmed cytologically to contain malignant cells. Hematopoietic lineage analysis showed that over 50% of the CTC fraction consisted of non-hematopoietic cells. DNA extracted from both the CTC and normal hematopoietic fractions underwent exome sequencing. Neoantigens were identified using the Ancer® bioinformatics platform.
Results: In representative patients with gastric and salivary gland cancers, 94,636 and 46,423 CTCs were isolated, respectively. DNA yields were sufficient for exome sequencing without amplification or extensive cell culture. A total of 102 (patient with gastric cancer) and 108 (patient with salivary gland cancer) neoantigens were identified in each subject, including high-ranking T-cell epitopes derived from single nucleotide variants and frameshift mutations. According to the same procedures we could successfully identify a large number of neoantigens from the CTCs of all stage IV cancer patients. This confirms the feasibility of identifying individual patient-specific neoantigens from CTCs without requiring tumor biopsies.
Conclusions: This is the first study to demonstrate successful neoantigen identification using non-amplified CTCs isolated by apheresis and flow cytometry. The approach provides a minimally invasive, scalable alternative for neoantigen discovery and may better capture tumor heterogeneity compared to single-site biopsies. This method holds promise for enabling rapid, personalized immunotherapy strategies, including peptide vaccines, dendritic cell vaccines, and mRNA-based treatments.
Introduction
The advent of immune checkpoint inhibitors, first introduced in the early 2000s for metastatic melanoma patients, marked a significant milestone in cancer treatment and brought immunotherapy into the spotlight (1). Although the complete response (CR) rate for immune checkpoint inhibitors remains low, the overall disease control rate (which includes stable disease, SD) has contributed to prolonged overall survival for many patients (2, 3). However, the disease-control rate is still only 50% (4), highlighting the need for new means of improving therapeutic outcomes. Even though response rates have improved since the early days of immune checkpoint inhibitor development, the estimated response rate for ipilimumab—approved for unresectable or metastatic melanoma – has only improved from 0.14% in 2011 to 12.46% in 2018 (5). The response rate of immune checkpoint inhibitors varies significantly depending on the patient’s tumor mutational burden (TMB), cancer type, and concomitant therapy (6). Immunotherapy drugs have faced a major challenge in treating patients with low TMB whose tumors harbor a limited number of potential neoantigens, the targets of T cells unleashed by checkpoint inhibitors, hindering their efficacy (7). To overcome this limitation, additional strategies are needed to enhance the therapeutic effectiveness of immune checkpoint inhibitors.
Neoantigens are tumor-specific antigens produced by tumor cells through mechanisms such as genomic mutations, dysregulated RNA splicing, abnormal post-translational modifications, and viral open reading frames (8). Neoantigens, unlike tumor-associated antigens (TAAs) which are non-mutated self-antigens, are not subject to central T cell tolerance and have been shown to be highly immunogenic (9). Bypassing central tolerance mechanisms enables the immune system to activate stronger tumor-specific immune responses. As a result, neoantigen-based therapies have the potential to significantly improve treatment outcomes by enabling precise targeting of individual tumors. However, many challenges remain to be overcome for unlocking personalized tumor therapies on a large scale. The collection of tumor biopsies, a critical first step in developing personalized therapies, imposes practical and ethical constraints and restricts treatment to patients with resectable tumors. Circulating tumor cells (CTCs), released into the bloodstream, offer a minimally invasive alternative. CTCs have served as biomarkers for early detection, prognosis, and treatment monitoring of cancers (10). Although methods for CTC enrichment via apheresis and flow cytometry have been reported (11–13), the utility for neoantigen identification remains unexplored. Here, we present a novel workflow combining CTC isolation by FACS and exome sequencing to detect tumor-specific neoantigens directly from blood, offering a new direction for personalized immunotherapy.
Methods
Patient selection
This study was approved by the institutional review board of Medical Cooperation “Isokai” (approval number: 201903), the nonprofit organization “Kodomotachino Kodomotachino Kodomotachinotameni” (approval number: 21-1), and Musashino University (approval number: R1-1). Peripheral blood mononuclear cells (PBMCs) were obtained from eleven stage IV cancer patients (one with lung cancer, two with pancreatic cancer, one with ampullary cancer, two with breast cancer, one with hepatocellular carcinoma, one with biliary tract cancer, one with rhabdomyosarcoma, one with stomach cancer, and one with salivary gland cancer) after written informed consent was obtained. Patients underwent dendritic cell-based immunotherapy pulsed with tumor-associated antigens at Meiko CIT Clinic or Kyushu Koseikai Clinic. PBMCs were also collected from two healthy volunteers as controls after written informed consent was obtained. Leukapheresis was performed on these volunteers at Meiko CIT Clinic.
Leukapheresis
PBMCs were obtained from 5-liter leukapheresis procedures performed on patients and healthy volunteers using the COBE SPECTRA (Cobe Laboratories, Lakewood, CO, USA), as described previously (14, 15). The collected cells were then separated by density gradient centrifugation using Ficoll-Hypaque (Pharmacia Biotech, Uppsala, Sweden). The light-density fraction, located at the 42.5–50% interface, was carefully recovered for further processing. The cells were then re-suspended in cold phosphate-buffered saline (PBS) and incubated on 10 cm plastic dishes (Primaria™™, Becton Dickinson, Mountain View, CA, USA) at 37°C for 30 minutes to allow adherence. After incubation, the non-adherent cells (monocyte-depleted PBMCs; m-PBMCs) were collected and cryopreserved in Cell Banker (ZENOAQ, Fukushima, Japan) for circulating tumor cell (CTC) isolation. The remaining adherent cells were used for dendritic cell (DC) preparation.
Flow cytometric analysis and sorting
After thawing, the cryopreserved cells were washed with PBS containing 2% fetal bovine serum (FBS) and 2 mM ethylenediaminetetraacetic acid (EDTA), then used subsequent staining analysis and cell sorting. Antibodies were added at optimal concentrations, followed by incubation at 4°C for 30 minutes, allowing for staining of the target cell populations. The following antibodies were used in the study: CD45 PE (BioLegend, San Diego, CA, USA), Cell-Surface Vimentin (CSV, clone 84-1) APC (Abnova, Taipei, Taiwan), PE mouse IgG1 κ isotype control and APC mouse IgG2b κ isotype control (BioLegend), Pacific Blue™ anti-human Lineage Cocktail (BioLegend), Human Lineage Cocktail 4 (BD Biosciences Pharmingen), and EpCAM FITC (Biomab, Taipei, Taiwan). To exclude dead cells, propidium iodide (PI; Sigma-Aldrich) staining or 7-Aminoactinomycin D (7AAD) was occasionally used. Cells were analyzed and sorted using an SH800 cell sorter (Sony, Tokyo, Japan).
Papanicolaou staining
About two to three thousand cells that had been obtained as described above were suspended in a small amount of PBS, smeared onto glass slides, and fixed using M-FIX™ Spray (Sigma-Aldrich). After fixation, the cells were subjected to Papanicolaou staining for cytological analysis.
Deoxyribonucleic acid extraction
DNA was extracted from the sorted cell fractions using the QIAamp DNA Mini Kit (Qiagen GmbH, Germany) according to the manufacturer’s instructions. The concentration of DNA in all samples was evaluated by Quantus™ Fluorometer (Promega Corporation, Madison, WI, USA) using QuantiFluor® ONE dsDNA System (Promega Corporation). Samples with a DNA concentration of at least 240 ng were used for exome sequencing analysis.
Exome sequencing
Exome sequencing was outsourced to the Kazusa DNA Research Institute (Chiba, Japan).
Exome sequencing conditions
The Twist exome panel was used, covering an approximate region size of 50 Mbp. Library preparation was performed using ultrasonic fragmentation with the Picoruptor and the KAPA Hyper Prep kit.
Analysis conditions
The analysis was conducted at the DNA Chip Research Institute. Coverage ranged from approximately 100X to 200X (Seq QC standard), with a target of 10 Gb and surface coverage of 200X. The reference genome used was hg38. Tumor-normal somatic analysis was performed using Strelka, and HLA typing was carried out with Kourami. The custom exome panel covered the combined gene regions of TWIST Biosciences’ TWIST Alliance VCGS Exome and TWIST Exome 2.0. Sequencing was run on Illumina’s NextSeq2000 in 150 base PE mode, generating VCF files.
Neoantigen identification
VCF files generated from the exome sequencing were analyzed with the Ancer® platform (EpiVax Therapeutics) to identify patient-specific neoantigens (16). Briefly, paired normal/mutated amino acid segments were extracted for each mutation yielding non-synonymous changes. Paired sequences were then assessed by EpiMatrix for the presence of putative HLA Class I and HLA Class II T-cell epitopes restricted by the patients’ HLAs. Predicted T-cell epitopes that were newly detected in tumor sequences or that exhibited a significant change in EpiMatrix score, as compared to their normal counterpart, were labeled as neoepitopes. Neoepitopes were further screened with JanusMatrix to flag and remove those sequences with extensive cross-conservation with the human proteome. Source mutated sequences were then trimmed to generate 14- to 25-mer neoantigen sequences containing non-self HLA Class I and/or HLA Class II neoepitopes. Neoantigens were subsequently ranked based on their predicted immunogenicity and assigned an Ancer score.
Results
Flow cytometric analysis of m-PBMCs
Figure 1 shows the results of the flow cytometric analysis of m-PBMCs from a stage IV patient with ampullary cancer, stained with anti-EpCAM antibody (Figure 1A) and anti-Vimentin antibody (Figure 1B). The proportions of CD45−EpCAM+ and CD45−Vimentin+ cells were 0.43% and 0.80%, respectively. The intensity of CD45−EpCAM+ cells staining was considerably lower than CD45−Vimentin+ cells, making it difficult to conclusively determine the presence of CD45−EpCAM+ cells in Figure 1A. The proportion of CD45−EpCAM+ cells and CD45−Vimentin+ cells were found to be very low in samples collected from two healthy volunteers, with values of 0.057% and 0.083%, respectively, observed for the first volunteer (Figures 2A, B), and 0.072% and 0.057%, respectively, for the second volunteer.
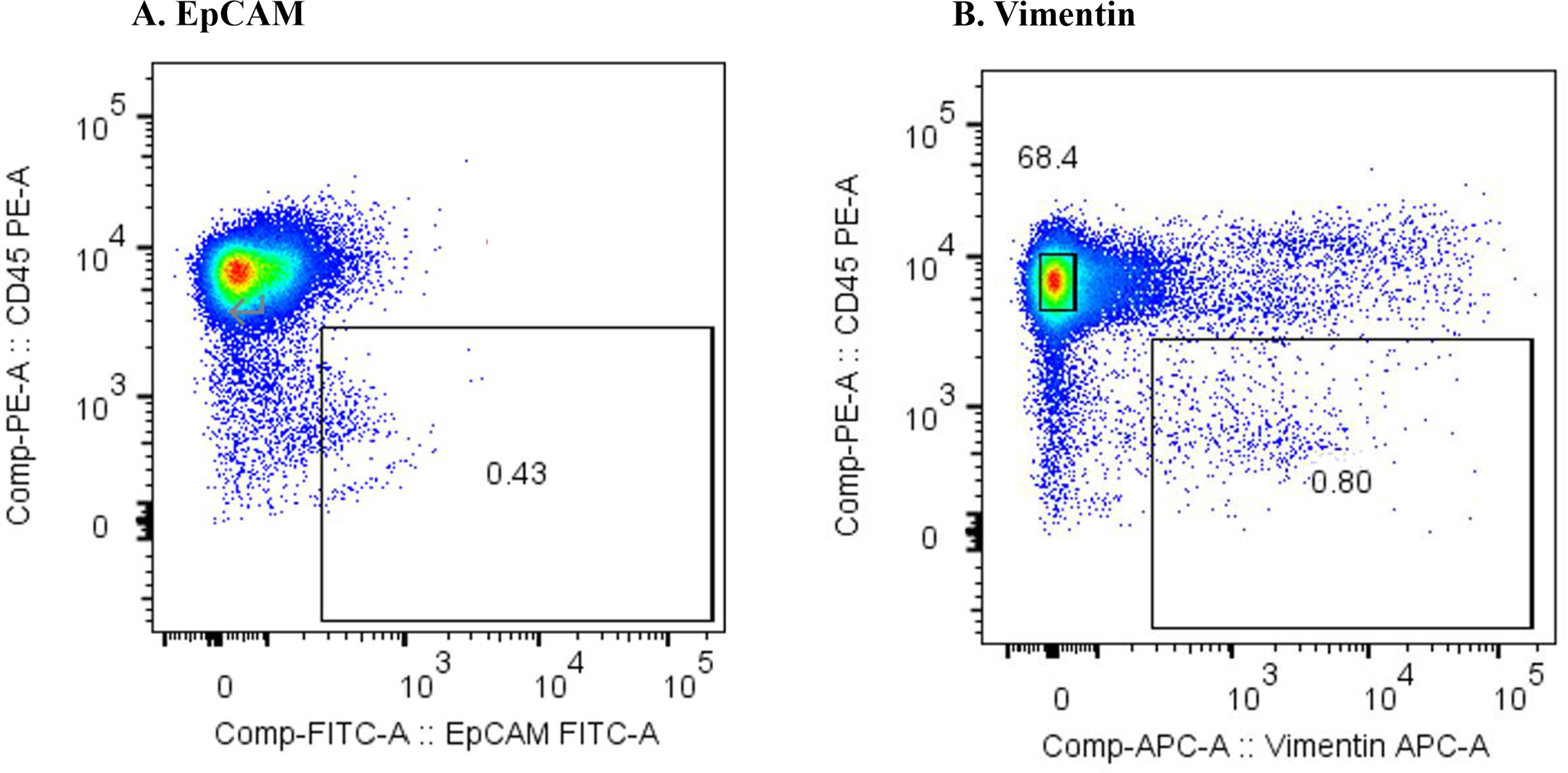
Figure 1. (A) Flow cytometric analysis of monocyte-depleted PBMCs(m-PBMCs) obtained from an ampullary cancer patient. The y-axis indicates the intensity of CD45 PE and the x-axis, that of EpCAM FITC. (B) Flow cytometric analysis of m-PBMC obtained from the same patient. The y-axis indicates the intensity of CD45 PE and the x-axis, that of vimentin APC.
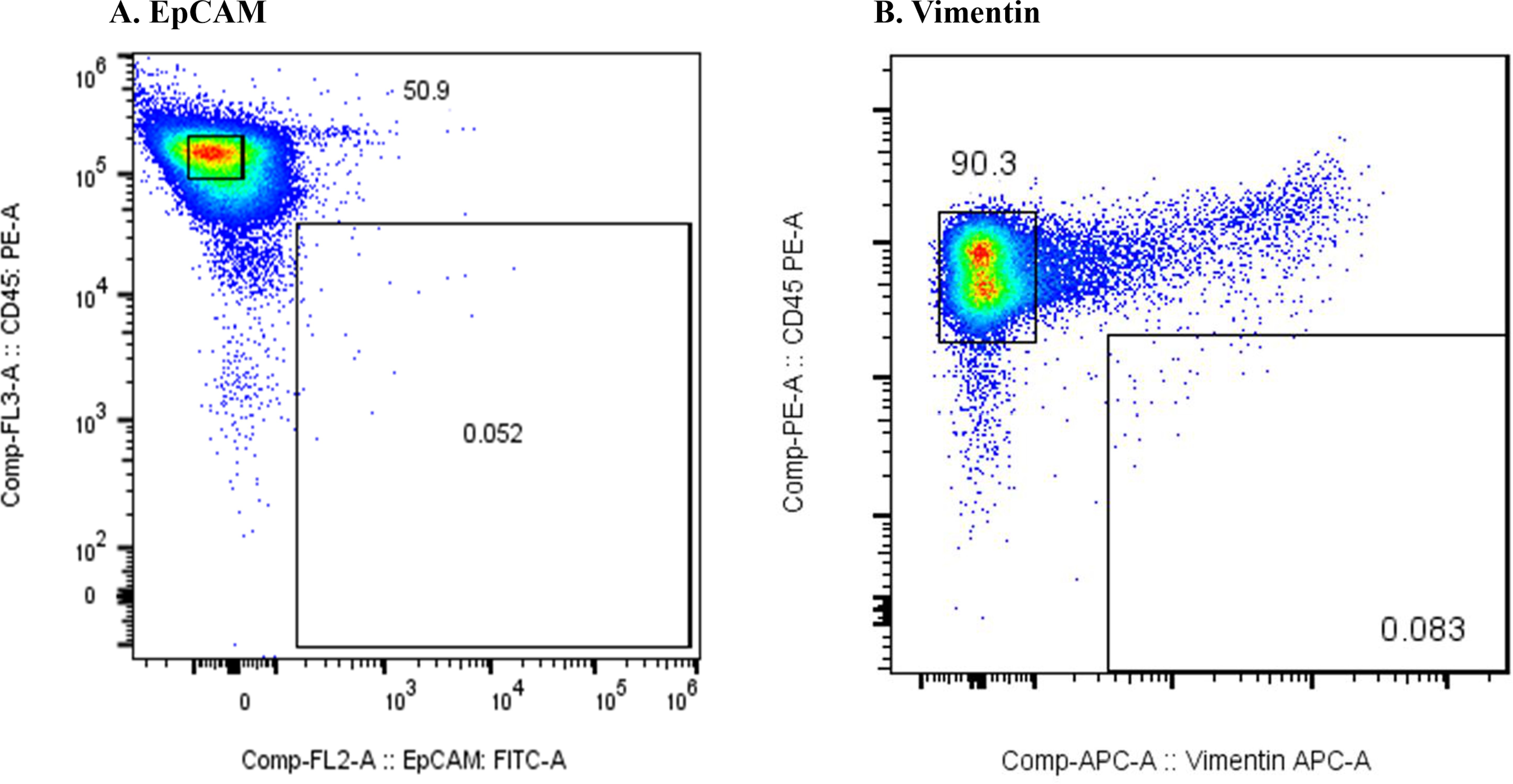
Figure 2. (A) Flow cytometric analysis of monocyte-depleted PBMCs(m-PBMCs) obtained from a healthy volunteer. The y-axis indicates the intensity of CD45 PE and the x-axis, that of EpCAM FITC. (B) Flow cytometric analysis of m-PBMC obtained from the same subject. The y-axis indicates the intensity of CD45 PE and the x-axis, that of vimentin APC.
Figure 3 presents the results of the flow cytometric analysis of m-PBMCs from a stage IV pancreatic cancer patient. The CD45−EpCAM+ fraction (Figure 3A) contained very few cells (0.05%), whereas the CD45−Vimentin+ fraction (Figure 3B) had a higher cell count at 0.32%. CD45−Vimentin+ cell staining exhibited higher intensity than CD45−EpCAM+ cells, a pattern that was consistent in m-PBMCs across all stage IV cancer patients analyzed in this study.
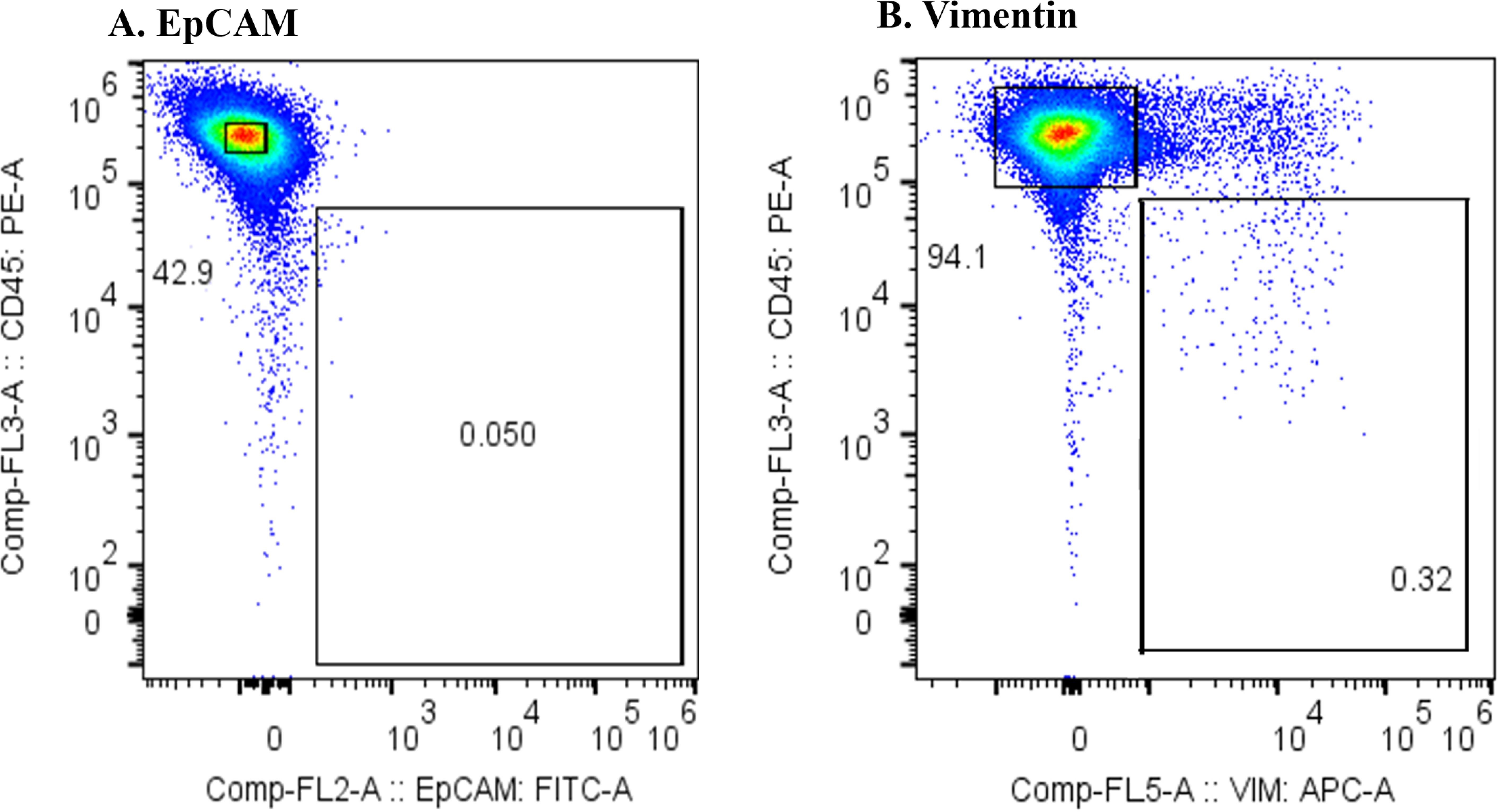
Figure 3. (A) Flow cytometric analysis of monocyte-depleted PBMCs(m-PBMCs) obtained from a pancreatic cancer patient. The y-axis indicates the intensity of CD45 PE and the x-axis, that of EpCAM FITC. (B) Flow cytometric analysis of m-PBMC obtained from the same patient. The y-axis indicates the intensity of CD45 PE and the x-axis, that of vimentin APC.
Epithelial malignant tumor cells typically express the epithelial marker EpCAM on their surface; however, they often undergo epithelial-mesenchymal transition (EMT), leading to the loss of EpCAM and the expression of mesenchymal markers such as vimentin. EMT is known to occur frequently in pancreatic cancer (17), which aligns with the results in Figure 3. It is likely that CTCs from the cancer patients included in this study had undergone substantial EMT, reducing the number of cells detected with the anti-EpCAM antibody. Furthermore, reports suggest that CTCs are more effectively captured by the anti-Vimentin antibody than by the anti-EpCAM antibody (18). Therefore, the subsequent study was conducted using the anti-Vimentin antibody. In the flow cytometric analysis of m-PBMCs obtained from all stage IV cancer patients via apheresis, a significant number of cells were observed in the CD45−Vimentin+ fractions, whereas these fractions contained very few cells in healthy individuals. This suggested that the cells in the CD45−Vimentin+ fractions may represent circulating tumor cells (CTCs). To confirm this hypothesis, the following experiments were conducted.
Cytology and hematopoietic lineage investigation of CD45-Vimentin+ fraction cells
Next, we examined whether cancer cells were present in the CD45-Vimentin+ fraction by cytological examination. After sorting the cells in the CD45+Vimentin- and CD45-Vimentin+ fractions, smeared and fixed cells were stained with Papanicolaou stain, as shown in Figure 4. Figure 4A shows the results from a stage IV breast cancer patient: all cells in the CD45+Vimentin- fraction were normal lymphocytes. In contrast, the CD45-Vimentin+ fraction contained a cluster of atypical cells, with a pathological diagnosis of Class V. Figure 4B shows cells from a stage IV hepatocellular carcinoma patient. Similar to Figure 4A, only normal lymphocytes (small and large lymphocytes) were observed in the CD45+Vimentin- fraction. In the CD45-Vimentin+ fraction, however, a cluster of atypical cells was also seen, with again a pathological diagnosis of Class V. Based on these results, the CD45-Vimentin+ fraction, which we presumed to be the CTC fraction, indeed contains cancer cells.
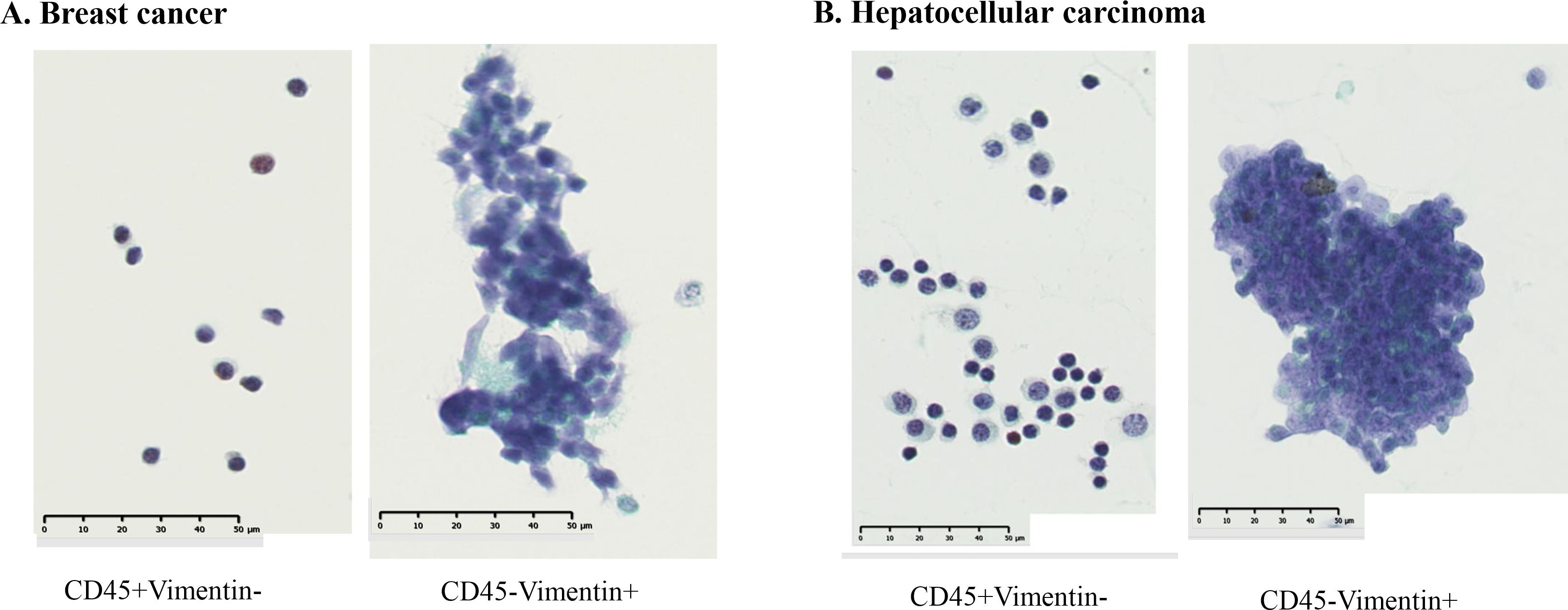
Figure 4. Cytological examination of CD45+Vimentin- and CD45-Vimentin+ fraction cells. Papanicolaou staining was done on smeared and fixed cells. (A) Cells of CD45+Vimentin- and CD45-Vimentin+ fraction obtained from a stage IV breast cancer patient. (B) Cells of CD45+Vimentin- and CD45-Vimentin+ fraction obtained from a stage IV breast hepatocellular carcinoma patient.
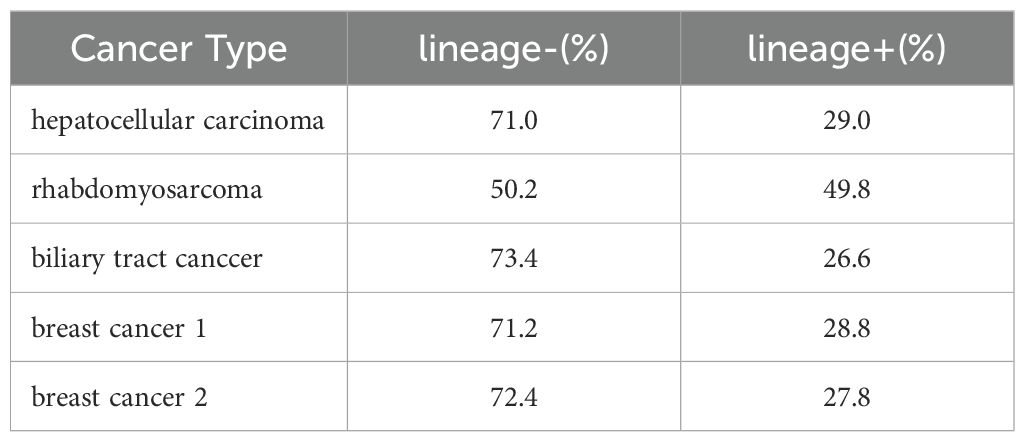
We next examined the proportion of cancer cells within the CD45-Vimentin+ fraction (CTC fraction). In this analysis, we used the Human Lineage Cocktail 4 (containing antibodies against CD2, CD3, CD4, CD7, CD8, CD10, CD11b, CD14, CD19, CD20, CD56, and CD235a), which reacts with most hematopoietic cells, to assess the extent of hematopoietic cell contamination in the CTC fraction. This would enable the estimation of the proportion of cancer cells within this fraction. Figure 5 shows the lineage analysis of hematopoietic cells in the CTC fraction of a hepatocellular carcinoma patient. The CTC fraction of this patient (Figure 5A) was reanalyzed with anti-CD45 antibody and anti-human Lineage Cocktail 4, revealing that 71.0% of the cells were non-hematopoietic (Figure 5B: CD45+Vimentin-, Figure 5C: CD45-Vimentin+), suggesting that these cells are circulating cancer cells. Table 1 presents the results of hematopoietic lineage analysis in the m-PBMCs of four additional patients, showing that 50.2% to 73.4% of the cells in the CTC fraction could be categorized as cancer cells. In summary, these results confirm the presence of cancer cells in the CTC fraction, with a purity of at least 50%.
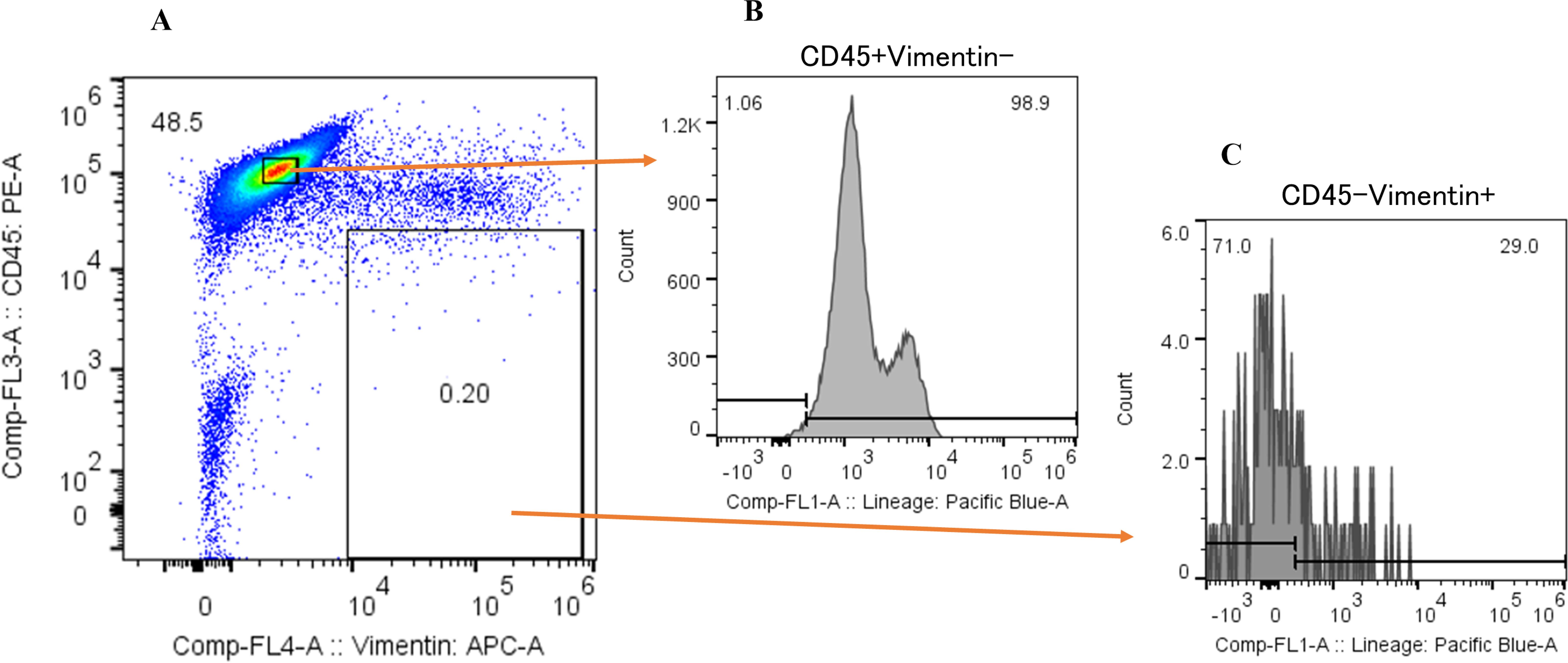
Figure 5. Hematopoietic lineage investigation of CD45-Vimentin+ fraction cells. (A) Flow cytometric analysis of m-PBMC obtained from a hepatocellular carcinoma patient. The y-axis indicates the intensity of CD45 PE and the x-axis, that of vimentin APC. (B) Flow cytometric lineage analysis of CD45+Vimentin- fraction cells in (A). The y-axis indicates the event of cells and the x-axis, the intensity of human lineage cocktail 4 Pacific blue. (C) Flow cytometric lineage analysis of CD45-Vimentin+ fraction cells in (A). The y-axis indicates the event of cells and the x-axis, the intensity of human lineage cocktail 4 Pacific blue.
Identification of neoantigens using cells from the CTC fraction and normal hematopoietic cells
To achieve accurate neoantigen identification, exome sequencing must be performed by amplification of DNA or culturing of CTCs obtained from the circulating tumor cells. This requires both high purity of the cell population, and a sufficient quantity of CTCs. The CTC fraction sorted in this study met these conditions without nucleic acid amplification or cell culture, allowing us to proceed with exome sequencing and neoantigen identification. Figure 6 shows the flow cytometric analysis of m-PBMCs from a stage IV gastric cancer patient. The m-PBMCs from this patient were divided into four vials, with the analysis of one vial shown in Figure 6. Sorting was performed to separate the CD45+Vimentin- fraction (normal hematopoietic fraction), consisting of normal hematopoietic cells, and the CD45-Vimentin+ fraction (CTC fraction), mainly composed of CTCs. A total of 45,665 cells were obtained from the CTC fraction. Sorting of the normal hematopoietic fraction was stopped once 3 million cells were collected. Another vial of m-PBMCs was similarly sorted, yielding 48,971 cells from the CTC fraction. In total, 94,636 cells were collected from the CTC fraction contained in two vials of m-PBMCs, and 360 ng of DNA was extracted from these isolated CTC. Next, 1,260 ng of DNA was extracted from the 3 million cells in the normal hematopoietic fraction.
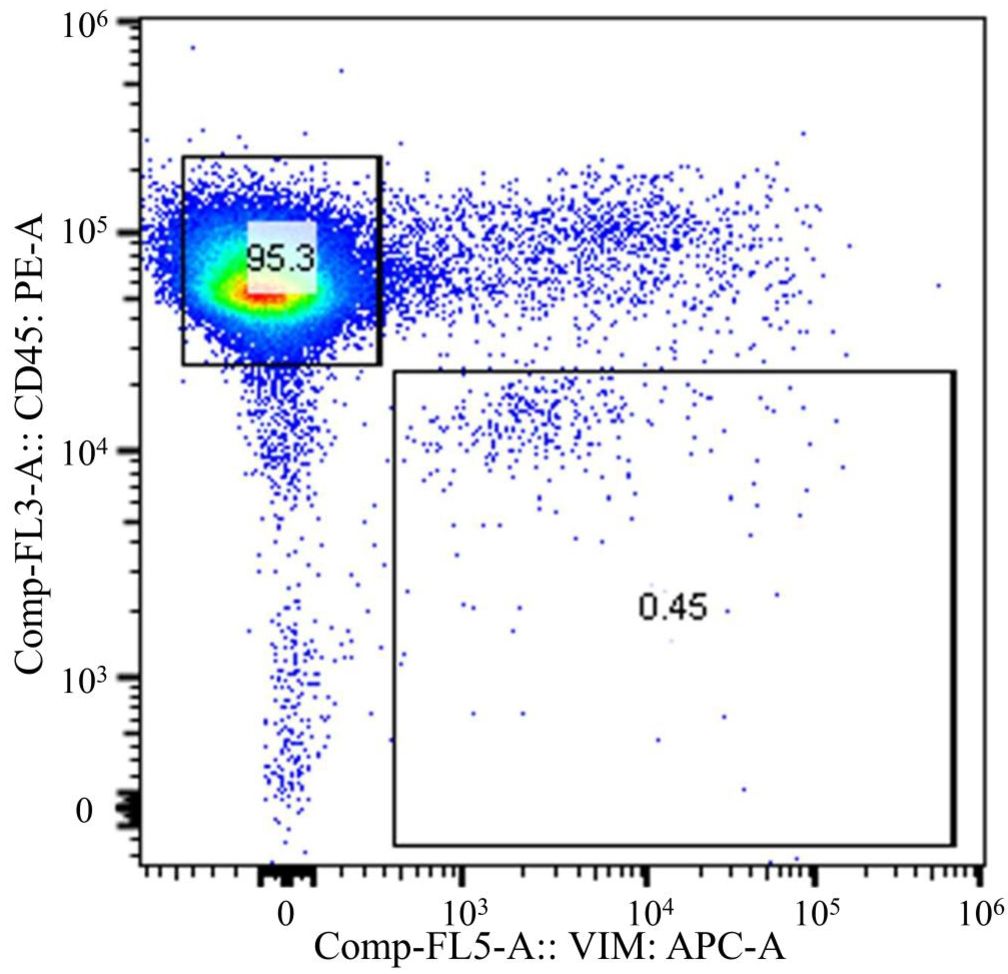
Figure 6. Flow cytometric analysis of monocyte-depleted PBMCs(m-PBMCs) obtained from a stage IV gastric cancer patient. The y-axis indicates the intensity of CD45 PE and the x-axis, that of vimentin APC.
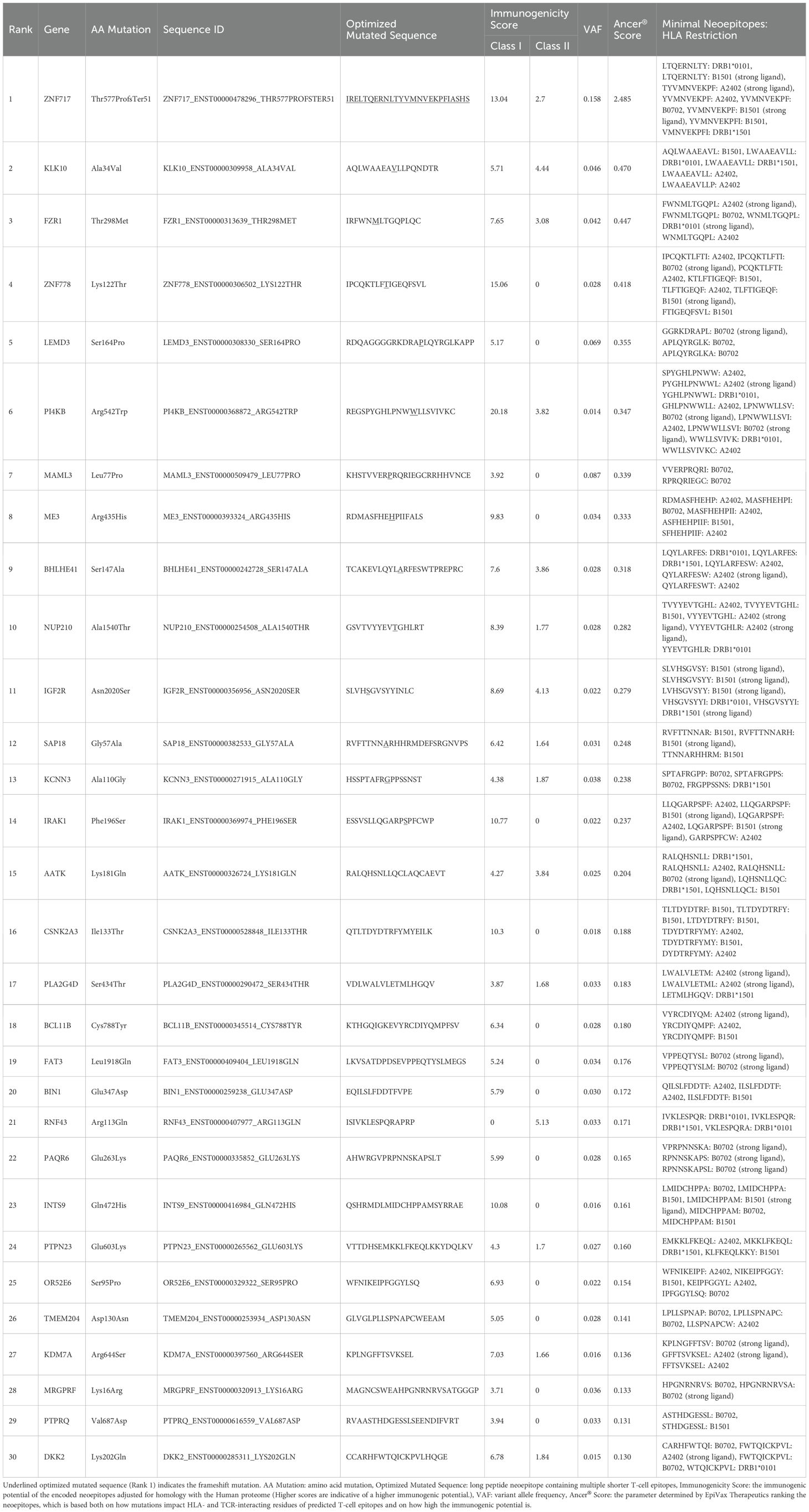
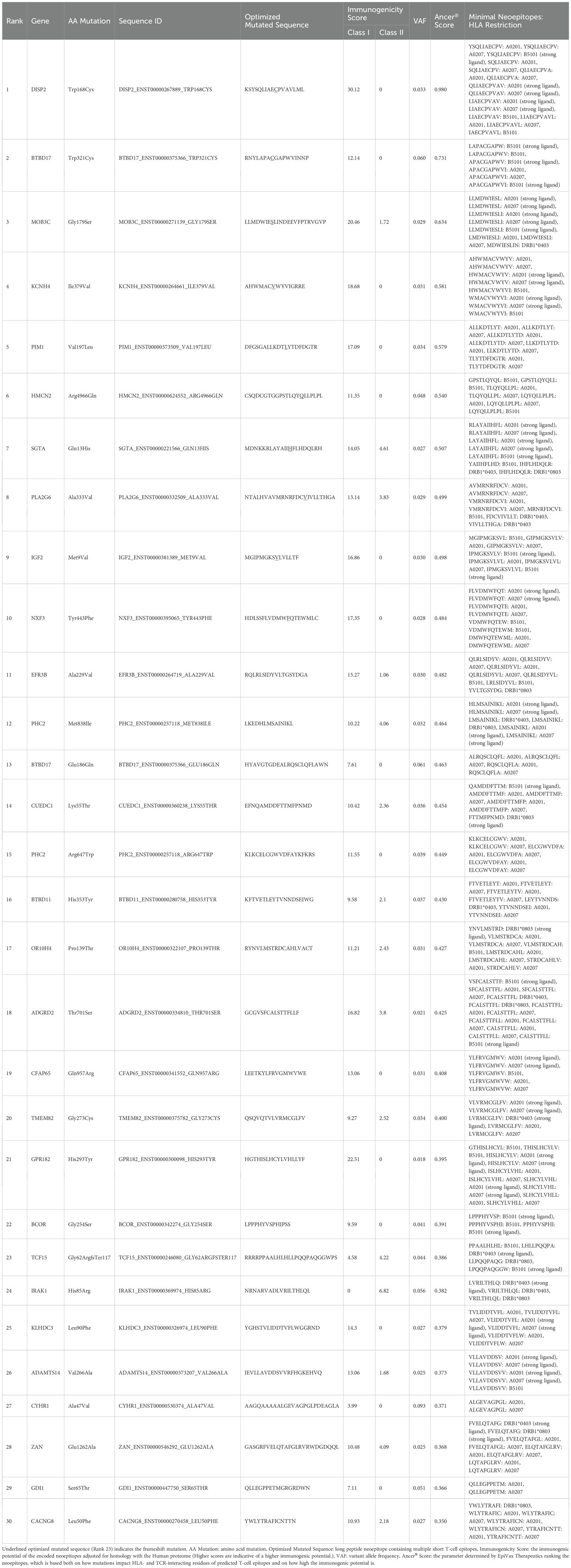
Exome sequencing was performed on DNA obtained from the normal hematopoietic fraction and the CTC fraction, followed by neoantigen identification. Exome sequencing of the CD45+Vimentin- cells (normal hematopoietic fraction) and the CD45-Vimentin+ cells (CTC fraction) were carried out, and 102 neoantigens were identified using the Ancer platform in the patient with gastric cancer. The top ranked 30 neoantigens identified with Ancer are shown in Table 2, where long neoantigen peptides may contain multiple overlapping (short) HLA-binding cores. Most neoantigens were derived from single nucleotide variations (SNVs), although one neoantigen (ranked #1) was identified from a frameshift mutation. Figure 7 shows the flow cytometric profile of a stage IV salivary gland cancer patient, from which CTC were collected. A total of 603 ng of DNA was extracted from the 46,423 cells in the CTC fraction of this patient. Exome sequencing of the CD45+Vimentin- cells (normal hematopoietic fraction) and the CD45-Vimentin+ cells (CTC fraction) were carried out, and 108 neoantigens were identified using the Ancer platform in the patient with salivary gland cancer. The top ranked 30 neoantigens are shown in Table 3 similar to Table 2. A neoantigen derived from frameshift mutation (ranked #21) was also detected. According to the above procedures we could successfully identify a large number of neoantigens from the CTCs of all stage IV cancer patients examined to date.
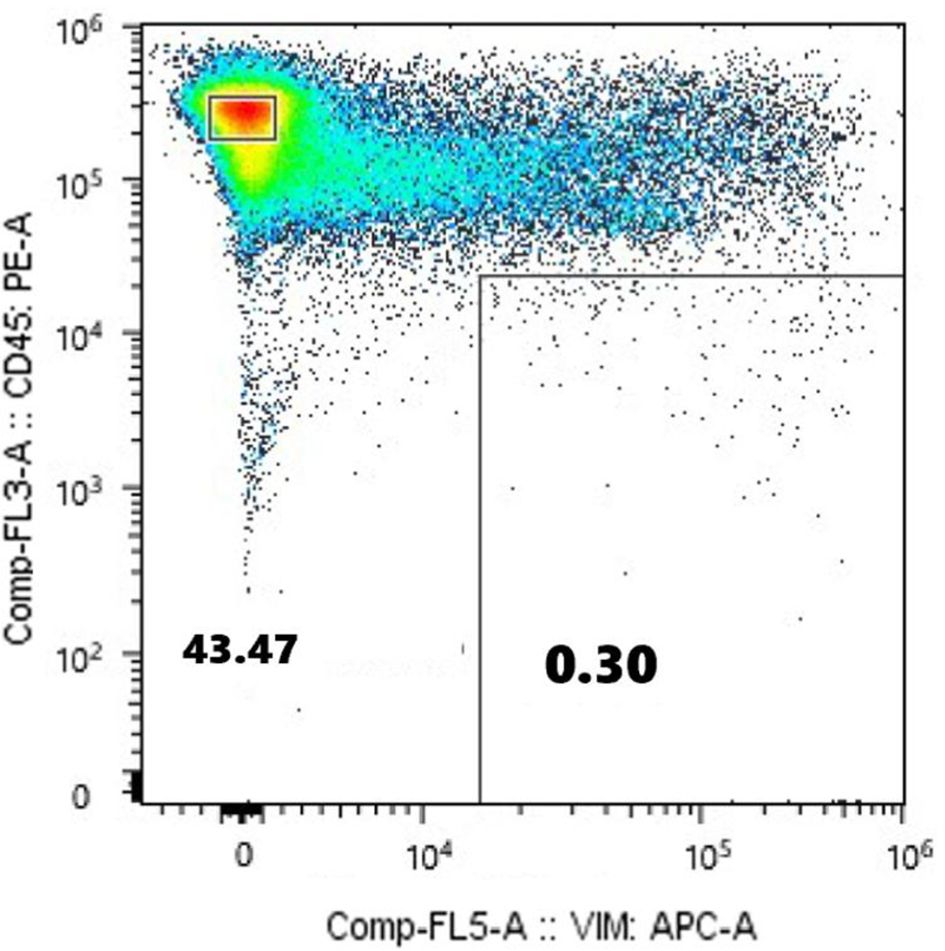
Figure 7. Flow cytometric analysis of monocyte-depleted PBMCs(m-PBMCs) obtained from a stage IV salivary gland cancer patient. The y-axis indicates the intensity of CD45 PE and the x-axis, that of vimentin APC.
Discussion
CTC collection using apheresis
The development and application of apheresis to isolate circulating tumor cells (CTCs) represents a significant advancement in cancer research and therapeutic strategies. Most studies utilizing apheresis for CTC collection have focused on evaluating treatment efficacy (19–21), prognostic predictions (22, 23), and liquid biopsies (24). The diagnostic leukapheresis significantly improved the detection frequency of CTCs, making it a clinically safe and effective method (25). Another study further emphasized the non-invasive nature of apheresis for collecting a substantial number of CTCs (26). While recent investigations have extended into RNA sequencing (27) and transplantation of cultured mammospheres into nude mice (28), our study is the first to successfully identify neoantigens using CTCs without nucleic acid amplification or extensive cell culture. The existence of malignant cells in CTC fractions was confirmed by cytological examination, and the lineage investigation by flow cytometric analysis revealed that at least 50% of cells in CTC fractions were not of hematopoietic origin, but were instead composed of cancer cells in relatively high purity. We could determine neoantigens from cancer patients without obtaining tumor tissue using cells sorted from the CTC fraction and normal blood cells. This success was enabled by the ability of our apheresis approach to yield a sufficient number of highly pure CTCs.
Intratumoral and intertumoral heterogeneity
Cancer exhibits both intratumoral and intertumoral heterogeneity, which complicates the identification of neoantigens that reflect the tumor’s overall profile (29, 30). Studies have shown that only a small fraction of neoantigens is shared across multiple metastatic sites, with primary and metastatic lesions sharing an average of 19.6% of neoantigens in lung cancer cases (31). Another study revealed that only 4.4% of neoantigens were shared across all metastatic and primary lesions in a single patient (32). These findings underscore the necessity of incorporating information from multiple tumor sites to identify neoantigens that represent the entirety of the tumor’s genetic landscape. Previous study demonstrated that CTCs can monitor tumor heterogeneity and provide diagnostic value (11). In this context, CTCs offer a significant advantage by capturing genetic variations not detected in bulk analyses of primary tumors, and the ability of CTCs to provide comprehensive genomic information is particularly noteworthy. Several studies also indicate that CTCs may capture genetic variations not detected in bulk tumor analyses. For example, analyses of melanoma and breast cancer patients have demonstrated that driver mutations present in both primary and metastatic lesions can be detected in CTCs, as well as additional mutations from micrometastatic sites (33, 34). This evidence suggests that neoantigens identified using CTCs have the potential to represent the overall heterogeneity of the patient’s cancer, thereby enhancing the efficacy of personalized immunotherapy.
Advantages of CTC-derived neoantigen identification
The identification of neoantigens using apheresis-derived circulating tumor cells (CTCs) provides several distinct advantages in the field of cancer immunotherapy. First, this approach enables non-invasive tumor sampling, eliminating the need for invasive biopsies of tumor tissues, which can be challenging and risky for patients. Second, it facilitates expedited initiation of personalized treatment strategies, as fresh cancer cells can be collected promptly for analysis and therapeutic planning. Third, the identified neoantigens have broad applicability across various therapeutic modalities, including peptide-based therapies, dendritic cell vaccines, and mRNA-based treatments, thereby expanding their potential utility. Lastly, neoantigen-based therapies have the potential to be combined with immune checkpoint inhibitors, enhancing their therapeutic efficacy. These features collectively establish CTC-derived neoantigen identification as a transformative tool in advancing the precision and effectiveness of cancer immunotherapy.
While promising, our study has several limitations. First, the extent to which CTCs reflect the genetic heterogeneity of all tumor lesions remains to be comprehensively validated. Although our findings align with existing evidence supporting the representational capacity of CTCs, further studies are required to confirm this observation across various cancer types. Second, it should be noted that CTC isolation by apheresis and FACS may introduce impurities and sampling bias, as leukocyte contamination and selection based on surface markers can affect both yield and clonality. Bulk sequencing may further mask intra-sample heterogeneity by averaging signals from mixed cell populations (35). Additionally, CTCs themselves are known to be highly heterogeneous, reflecting only a snapshot of dynamic clonal evolution and possibly underrepresenting minor subclones present in primary or metastatic sites (36). To address these issues, future work should leverage single−cell sequencing or employ multiple marker panels during isolation to more accurately capture CTC diversity and purity across cancer types. Third, the integration of neoantigen identification with other omics approaches, such as proteomics and transcriptomics, may provide additional insights into tumor biology and therapeutic targets. The EpiMatrix and JanusMatrix tools that are used in Ancer® have been described in detail, previously (37). Fourth, optimizing the apheresis protocol to ensure the reproducibility and scalability of CTC collection is essential for widespread clinical application. Although this study did not assess the therapeutic utility of neoantigens identified from CTCs, our future plans include conducting clinical studies or clinical trials with larger cohorts to obtain clinical data and evaluate T cell responses against these neoantigens. We anticipate that such analyses will enable assessment of the immunogenicity of CTC-derived neoantigens. Furthermore, while this study did not compare neoantigens identified from CTCs with those derived from surgically resected tumor tissues, this comparison represents a critical next step. We intend to undertake this investigation in future research.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the institutional review board of Medical Cooperation “Isokai” (approval number: 201903); the nonprofit organization “Kodomotachino Kodomotachino Kodomotachinotameni” (approval number: 21-1); Musashino University (approval number: R1-1). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
DK: Writing – original draft, Writing – review & editing, Visualization. TK: Investigation, Writing – review & editing, Supervision. QL: Supervision, Investigation, Writing – review & editing. SF: Supervision, Writing – review & editing, Investigation. YH: Investigation, Writing – review & editing, Supervision. KT: Investigation, Supervision, Writing – review & editing. TN: Writing – review & editing, Investigation, Supervision. NmY: Supervision, Writing – review & editing, Investigation. GR: Investigation, Writing – review & editing, Supervision. AD: Supervision, Writing – review & editing, Investigation. NhY: Methodology, Conceptualization, Investigation, Writing – review & editing, Resources, Supervision, Funding acquisition, Project administration, Data curation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Novacellum Inc. The corresponding author, Dr. Yamashita, originated and planned this study. Initially, as an Emeritus Professor at the University of Tokyo, he conducted joint research with Musashino University. Based on those findings, he established Novacellum Inc. After its establishment, funds were raised to advance the research, during which Dr. Yamashita was actively involved in the design of the study, the collection, analysis, and interpretation of data, the writing of the report, and the decision to submit the paper for publication. A patent related to this study was also filed and granted (Patent 7426165), with Dr. Yamashita listed as the inventor. Therefore, Dr. Yamashita has been deeply involved in this research, contributing both financial support through Novacelum Inc. and significant intellectual input into all aspects of the study.
Acknowledgments
We thank Mr. Joshua Ho for continuous encouragement of this research.
Conflict of interest
Author KT was employed by the company Biomedica Solution Inc. Authors GR and AD were employed by the company EpiVax Inc. Author NY was employed by the company Novacellum Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This research was conducted with a research fund from Novacellum Inc. Naohide Yamashita is the chairman of Novacellum Inc.
Generative AI statement
The authors used AI to edit English, because some of authors are not native for English. The author(s) declare that Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. (2003) 100:4712–7. doi: 10.1073/pnas.0830997100
2. Hammershoi Madsen AM, Lovendahl Eefsen RH, Nielsen D, and Kumler I. Targeted treatment of metastatic triple-negative breast cancer: A systematic review. Breast J. (2024) 2024:9083055. doi: 10.1155/2024/9083055
3. Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. (2020) 6:e204564. doi: 10.1001/jamaoncol.2020.4564
4. Cao J, Ding X, Ji J, Zhang L, and Luo C. Efficacy and safety of immune checkpoint inhibitors rechallenge in advanced solid tumors: a systematic review and meta-analysis. Front Oncol. (2024) 14:1475502. doi: 10.3389/fonc.2024.1475502
5. Haslam A and Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. (2019) 2:e192535. doi: 10.1001/jamanetworkopen.2019.2535
6. McGrail DJ, Pilie PG, Rashid NU, Voorwerk L, Slagter M, Kok M. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann Oncol. (2021) 32:661–72. doi: 10.1016/j.annonc.2021.02.006
7. Jardim DL, Goodman A, de Melo Gagliato D, and Kurzrock R. The challenges of tumor mutational burden as an immunotherapy biomarker. Cancer Cell. (2021) 39:154–73. doi: 10.1016/j.ccell.2020.10.001
8. Xie N, Shen G, Gao W, Huang Z, Huang C, and Fu L. Neoantigens: promising targets for cancer therapy. Signal Transduct Target Ther. (2023) 8:9. doi: 10.1038/s41392-022-01270-x
9. Sahin U, Oehm P, Derhovanessian E, Jabulowsky RA, Vormehr M, Gold M. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature. (2020) 585:107–12. doi: 10.1038/s41586-020-2537-9
10. Salu P and Reindl KM. Advancements in circulating tumor cell research: bridging biology and clinical applications. Cancers (Basel). (2024) 16(6):1213. doi: 10.3390/cancers16061213
11. Rieckmann LM, Spohn M, Ruff L, Agorku D, Becker L, Borchers A, et al. Diagnostic leukapheresis reveals distinct phenotypes of NSCLC circulating tumor cells. Mol Cancer. (2024) 23:93. doi: 10.1186/s12943-024-01984-2
12. Mihalcioiu C, Li J, Badescu D, Camirand A, Kremer N, Bertos N. Improved platform for breast cancer circulating tumor cell enrichment and characterization with next-generation sequencing technology. Am J Cancer Res. (2023) 13:25–44.
13. Chauhan A, Pal A, Sachdeva M, Boora GS, Parsana M, Bakshi J. A FACS-based novel isolation technique identifies heterogeneous CTCs in oral squamous cell carcinoma. Front Oncol. (2024) 14:1269211. doi: 10.3389/fonc.2024.1269211
14. Nagayama H, Sato K, Morishita M, Uchimaru K, Oyaizu N, Inazawa T, et al. Results of a phase I clinical study using autologous tumour lysate-pulsed monocyte-derived mature dendritic cell vaccinations for stage IV Malignant melanoma patients combined with low dose interleukin-2. Melanoma Res. (2003) 13:521–30. doi: 10.1097/00008390-200310000-00011
15. Thurner B, Roder C, Dieckmann D, Heuer M, Kruse M, Glaser A, et al. Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application. J Immunol Methods. (1999) 223:1–15. doi: 10.1016/S0022-1759(98)00208-7
16. Richard G, Princiotta MF, Bridon D, Martin WD, Steinberg GD, and De Groot AS. Neoantigen-based personalized cancer vaccines: the emergence of precision cancer immunotherapy. Expert Rev Vaccines. (2022) 21:173–84. doi: 10.1080/14760584.2022.2012456
17. Usman S, Waseem NH, Nguyen TKN, Mohsin S, Jamal A, Teh MT, et al. Vimentin is at the heart of epithelial mesenchymal transition (EMT) mediated metastasis. Cancers (Basel). (2021) 13(19):4985. doi: 10.3390/cancers13194985
18. Neal C, Sukumaran S, Gupta V, Jafferji I, Hasegawa D, Melnikova VO, et al. Capture of EpCAM-negative and vimentin-positive circulating cancer cells (CCCs) from blood of metastatic breast cancer patients using ApoStream. J Clin Oncol. (2012) 30:e21029–9. doi: 10.1200/jco.2012.30.15_suppl.e21029
19. Solano C, Badia B, Lluch A, Marugan I, Benet I, Arbona C, et al. Prognostic significance of the immunocytochemical detection of contaminating tumor cells (CTC) in apheresis products of patients with high-risk breast cancer treated with high-dose chemotherapy and stem cell transplantation. Bone Marrow Transpl. (2001) 27:287–93. doi: 10.1038/sj.bmt.1702782
20. Mout L, van Dessel LF, Kraan J, de Jong AC, Neves RPL, Erkens-Schulze S, et al. Generating human prostate cancer organoids from leukapheresis enriched circulating tumour cells. Eur J Cancer. (2021) 150:179–89. doi: 10.1016/j.ejca.2021.03.023
21. Stoecklein NH, Fischer JC, Niederacher D, and Terstappen LW. Challenges for CTC-based liquid biopsies: low CTC frequency and diagnostic leukapheresis as a potential solution. Expert Rev Mol Diagn. (2016) 16:147–64. doi: 10.1586/14737159.2016.1123095
22. Fischer JC, Niederacher D, Topp SA, Honisch E, Schumacher S, Schmitz N. Diagnostic leukapheresis enables reliable detection of circulating tumor cells of nonmetastatic cancer patients. Proc Natl Acad Sci U S A. (2013) 110:16580–5. doi: 10.1073/pnas.1313594110
23. Tamminga M, Andree KC, van den Bos H, Hiltermann TJN, Mentink A, Spierings DCJ. Leukapheresis increases circulating tumour cell yield in non-small cell lung cancer, counts related to tumour response and survival. Br J Cancer. (2022) 126:409–18. doi: 10.1038/s41416-021-01634-0
24. Andree KC, Mentink A, Zeune LL, Terstappen L, Stoecklein NH, Neves RP. Toward a real liquid biopsy in metastatic breast and prostate cancer: Diagnostic LeukApheresis increases CTC yields in a European prospective multicenter study (CTCTrap). Int J Cancer. (2018) 143:2584–91. doi: 10.1002/ijc.31752
25. Fehm TN, Meier-Stiegen F, Driemel C, Jager B, Reinhardt F, Naskou J, et al. Diagnostic leukapheresis for CTC analysis in breast cancer patients: CTC frequency, clinical experiences and recommendations for standardized reporting. Cytometry A. (2018) 93:1213–9. doi: 10.1002/cyto.a.23669
26. Lambros MB, Seed G, Sumanasuriya S, Gil V, Crespo M, Fontes M. Single-cell analyses of prostate cancer liquid biopsies acquired by apheresis. Clin Cancer Res. (2018) 24:5635–44. doi: 10.1158/1078-0432.CCR-18-0862
27. Lightbody ED, Firer DT, Sklavenitis-Pistofidis R, Tsuji J, Agius MP, Dutta AK, et al. Single-cell RNA sequencing of rare circulating tumor cells in precursor myeloma patients reveals molecular underpinnings of tumor cell circulation. Blood. (2022) 140:602–3. doi: 10.1182/blood-2022-162851
28. Alix-Panabieres C, Bartkowiak K, and Pantel K. Functional studies on circulating and disseminated tumor cells in carcinoma patients. Mol Oncol. (2016) 10:443–9. doi: 10.1016/j.molonc.2016.01.004
29. Ramon YCS, Sese M, Capdevila C, Aasen T, De Mattos-Arruda L, Diaz-Cano SJ. Clinical implications of intratumor heterogeneity: challenges and opportunities. J Mol Med (Berl). (2020) 98:161–77. doi: 10.1007/s00109-020-01874-2
30. Wolf Y and Samuels Y. Intratumor heterogeneity and antitumor immunity shape one another bidirectionally. Clin Cancer Res. (2022) 28:2994–3001. doi: 10.1158/1078-0432.CCR-21-1355
31. Jiang T, Cheng R, Pan Y, Zhang H, He Y, Su C, et al. Heterogeneity of neoantigen landscape between primary lesions and their matched metastases in lung cancer. Transl Lung Cancer Res. (2020) 9:246–56. doi: 10.21037/tlcr.2020.03.03
32. Chen R, Li J, Fujimoto J, Hong L, Hu X, Quek K. Immunogenomic intertumor heterogeneity across primary and metastatic sites in a patient with lung adenocarcinoma. J Exp Clin Cancer Res. (2022) 41:172. doi: 10.1186/s13046-022-02361-x
33. Sementsov M, Ott L, Kott J, Sartori A, Lusque A, Degenhardt S. Mutation analysis in individual circulating tumor cells depicts intratumor heterogeneity in melanoma. EMBO Mol Med. (2024) 16:1560–78. doi: 10.1038/s44321-024-00082-6
34. Rothe F, Venet D, Peeters D, Rouas G, Rediti M, Smeets D. Interrogating breast cancer heterogeneity using single and pooled circulating tumor cell analysis. NPJ Breast Cancer. (2022) 8:79. doi: 10.1038/s41523-022-00445-7
35. Jew B, Alvarez M, Rahmani E, Miao Z, Ko A, Garske KM, et al. Accurate estimation of cell composition in bulk expression through robust integration of single-cell information. Nat Commun. (2020) 11:1971. doi: 10.1038/s41467-020-15816-6
36. Lin D, Shen L, Luo M, Zhang K, Li J, Yang Q, et al. Circulating tumor cells: biology and clinical significance. Signal Transduct Target Ther. (2021) 6:404. doi: 10.1038/s41392-021-00817-8
Keywords: apheresis, CTC, neoantigen, CD45, EpCAM, Vimentin
Citation: Kobayashi D, Kosumi T, Lam QLK, Fujita S, Hijikata Y, Takeda K, Narita T, Yamashita N, Richard G, De Groot AS and Yamashita N (2025) A method for identifying neoantigens through isolation of circulating tumor cells using apheresis among patients with advanced-stage cancer. Front. Immunol. 16:1609116. doi: 10.3389/fimmu.2025.1609116
Received: 09 April 2025; Accepted: 19 June 2025;
Published: 11 July 2025.
Edited by:
Peter M. Van Endert, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceReviewed by:
Efthalia Zervoudi, Cardiff University, United KingdomLoulieta Nazerai, University of Copenhagen, Denmark
Copyright © 2025 Kobayashi, Kosumi, Lam, Fujita, Hijikata, Takeda, Narita, Yamashita, Richard, De Groot and Yamashita. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Naohide Yamashita, bi15YW1hc2hpdGFAbm92YWNlbGx1bS5jb20=
 Daiki Kobayashi
Daiki Kobayashi Takuya Kosumi3
Takuya Kosumi3 Yasuki Hijikata
Yasuki Hijikata Naomi Yamashita
Naomi Yamashita Guilhem Richard
Guilhem Richard Anne S. De Groot
Anne S. De Groot Naohide Yamashita
Naohide Yamashita