- 1Shenzhen Futian Third People’s Hospital, Shenzhen, China
- 2Medical School, Shenzhen University, Shenzhen, China
- 3Shenzhen Longhua District Central Hospital, Shenzhen, China
The gut microbiota is often termed the “second genome” of the human body. It has been shown to be one of the most significant environmental factors (non-genetic) influencing the onset, progression, and prognosis of various neurological and psychiatric disorders through its interactions with the host immune, nervous, and endocrine systems. Changes in the function and composition of the gut microbiota are strongly associated with amyotrophic lateral sclerosis, autism spectrum disorder, depression, Parkinson’s disease, and Alzheimer’s disease. This review summarizes the research regarding the associations and regulatory mechanisms between the gut microbiota and the central nervous system in order to explore the role of the gut microbiota in maintaining neural homeostasis.
1 Introduction
The mammalian gastrointestinal tract harbors trillions of diverse microorganisms, including fungi, viruses, and bacteria, collectively referred to as the gut microbiota (1). The luminal and mucosal gut microbiota and their derived products interact with the host intestinal barrier and immune cells to maintain intestinal homeostasis (2). In addition, the gut microbiome is essential for host immune function (3), influencing both peripheral and central nervous system (CNS) immune homeostasis via microbial components (4) and metabolites like lipopolysaccharides (LPS) (5), tryptophan metabolism derivatives (6), and short-chain fatty acids (SCFAs) (7). This review examines this “gut-neuroimmune axis”, highlighting the mechanisms through which modulation of this axis may impact host neurological disorders.
2 Structures involved in regulating neuroimmune responses in the gut-brain axis
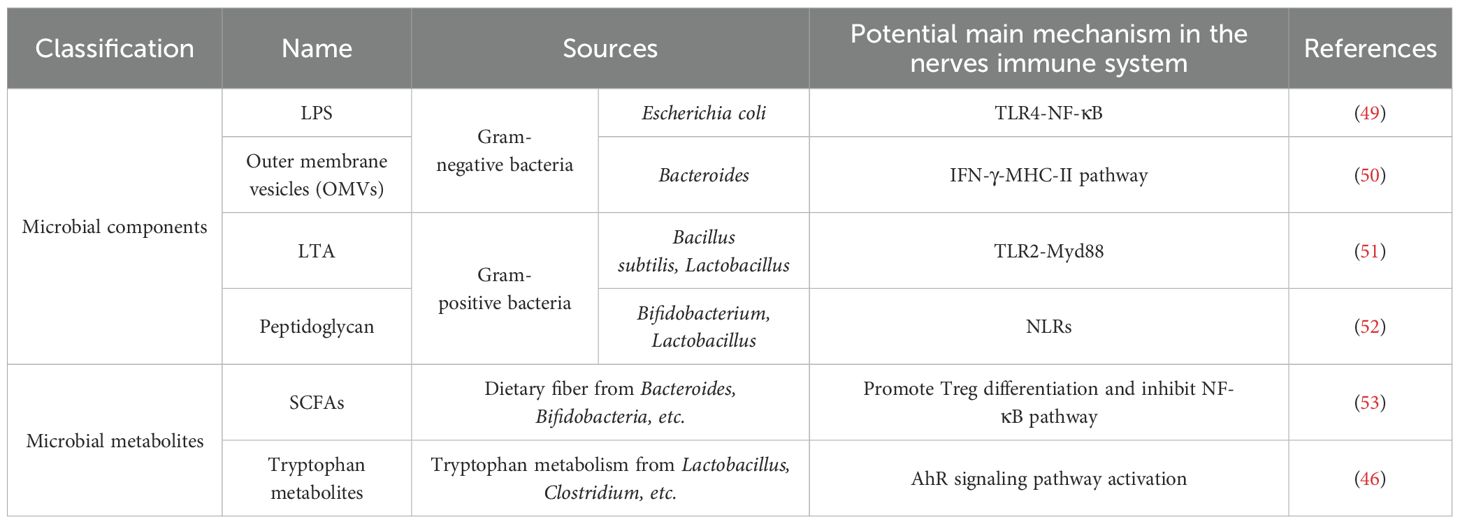
2.1 Microglia
Microglia are the resident myeloid cells of the CNS, comprising ~5-12% of brain cells (8). They are essential for maintaining brain homeostasis via neurogenesis, neurotransmission, synaptic remodeling, neuroinflammation, and injury repair (9). Microglia play a pivotal role in neuroinflammation by producing diverse molecular initiators and mediators (Figure 1A). They participate in neuroinflammatory events, regulate neural patterning, and mediate synaptic pruning (10). Upon activation, microglia release chemokines, cytokines, and antigenic markers, modulate neurotransmitter production and release, and undergo morphological changes (11). They maintain equilibrium by activating phenotypic responses and releasing both pro- and anti-inflammatory cytokines.
Figure 1. This schematic systematically illustrates the physiological structural basis of the microbiota-gut-brain axis (MGBA) mediated by bidirectional multi-channel communication, where interactions among microglia (A), astrocytes (B), the blood-brain barrier (C), gut microbiota (D), spinal cord (E) and the vagus nerve exhibits a dual-directional (bipolar) regulatory mechanism (F).
Under healthy conditions, microglia remain in an immunologically quiescent resting state due to inhibitory signals from cell-surface receptors and soluble ligands derived from surrounding neurons (12). During this state, microglia exhibit a highly branched morphology and they contribute to brain homeostasis by eliminating or remodeling synapses (13), supporting myelin renewal, monitoring neural activity, and actively clearing pathogens or localized tissue damage (14). Upon detecting brain injury, microglia undergo microglial activation and retract their processes, transitioning to an amoeboid morphology (15). It is widely accepted that microglial activation is initiated by the removal of inhibitory neuronal signals and the engagement of pattern recognition receptors (PRRs) by exogenous pathogen-associated molecular patterns (PAMPs) and/or endogenous damage-associated molecular patterns (DAMPs).
Activated microglia can adopt diverse and complex phenotypes, displaying distinct cell-surface and intracellular markers, secreting different cytokines, and performing specialized functions (16). These encompass the pro-inflammatory M1 phenotype and the anti-inflammatory M2 phenotype, which is further divided into M2a, M2b, and M2c subtypes. The early response following injury is characterized by pro-inflammatory activity driven by M1-polarized microglia (17). Eventually, M2-polarized microglia initiate an anti-inflammatory response crucial for tissue repair and a return to homeostasis. In contrast, microglia remain activated in an M1 phenotype by persistent pro-inflammatory stimuli during the chronic neuroinflammation characteristic of numerous neurodegenerative diseases (18). Persistently active microglia produce inflammatory cytokines and reactive oxygen/nitrogen species, leading to neuronal death (19).
2.2 Astrocytes
Astrocytes are tissue-resident stromal cells in the CNS that promote normal brain development and function by providing structural support, metabolite synthesis, neurotransmission regulation, and assisting in immune-related activities (20) (Figure 1B). Under physiological conditions, astrocytes also perform critical roles in pH, ion, and redox buffering, as well as regulating blood flow, neurotransmitter recycling, and energy homeostasis in the CNS. In pathological states, astrocytes undergo morphological and molecular changes, transitioning to a reactive state. During CNS inflammation and neurodegeneration, the cellular state of astrocytes correlates with the activation or suppression of specific genomic modules in response to disease-specific stimuli. For example, single-cell RNA sequencing (scRNA-seq) combined with proteomics has identified that under homeostatic conditions, astrocytes express tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in response to interferon-γ (IFN-γ) produced by natural killer (NK) cells. These astrocytes limit inflammation at CNS borders by inducing T-cell apoptosis.
In the CNS, microglia and infiltrating peripheral immune cells, such as T cells, primarily mediate inflammatory responses. However, macroglia, including astrocytes, serve as critical downstream effectors. Astrocytes can react to immunomodulatory cytokines and influence microglial activity by releasing pro- or anti-inflammatory cytokines (21). The crosstalk between astrocytes and microglia is important in maintaining CNS homeostasis. For example, it has been shown that astrocyte-derived IL-33 can facilitate microglia-mediated synaptic pruning during development, underscoring the critical role of astrocyte-microglia interactions in neural circuit formation (22). Conversely, microglia-derived cytokines influence pathogenic astrocyte functions in CNS inflammation. For example, Bezzi et al. showed that TNF-α from microglia triggered SDF-1-CXCR4 signaling in astrocytes, leading to the secretion of glutamate and causing neuronal death. Additionally, microglial-derived TNF-α, IL-1α, and C1q are known to trigger a neurotoxic astrocyte phenotype, and microglial-derived VEGF-B and TGF-α distinctly modulate pro-inflammatory gene expression in astrocytes during EAE and MS. Microglial VEGF-B amplifies pathogenic astrocyte activity in EAE by facilitating NF-κB activation through the VEGF receptor 1 (FLT-1), while microglial TGF-α mitigates EAE progression through the activation of ErbB1 signaling. Astrocytes also respond to microbiome-modulated systemic and central processes. Dietary tryptophan metabolites cross the blood-brain barrier, influencing aryl hydrocarbon receptor signaling in microglia and astrocytes, thereby regulating VEGF-B and TGF-α production and suggesting a novel mechanism through which the gut-brain axis regulates cells of the CNS. Another metabolite, D-β-hydroxybutyrate suppresses microglial activation, reducing IL-6 and TNF-α production and mitigating neuroinflammation (23). Similarly, indole-3-propionic acid (IPA) modulates TNF-α levels in activated microglia while supporting neuronal function (24).
The role of astrocytes in secreting chemokines to recruit lymphocytes is noteworthy. Research indicates that astrocytes generate chemokines like CXCL10, CXCL12, and CCL2, which play a crucial role in guiding immune cells to sites of inflammation (25). For example, CXCL10+ astrocytes are observed perivascularly in MS patients, suggesting a role in recruiting lymphocytes to the CNS and underscoring the potential for astrocytes to modulate disease progression via chemokine secretion. The influence of astrocytes on T-cell responses occurs through the release of cytokines like interleukin (IL)-6, IL-2, and TGF-β, which play pivotal roles in coordinating immune responses and regulating neuroinflammation. For instance, IL-6 exacerbates CNS autoimmunity, while IL-2 is essential for the expansion of neuroprotective regulatory T cells (Treg).
Neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis contribute to inflammation in the CNS, although their underlying mechanisms are not fully understood. Compelling data suggest that in some neurodegenerative disorders, initial pro-inflammatory stimuli may originate from neurons, triggering secondary inflammatory responses mediated by astrocyte-microglia crosstalk to drive disease progression. Astrocyte-centric therapies require an understanding of how local and remote triggers integrate to induce diverse astrocyte response phenotypes.
2.3 Blood-brain barrier
The blood-brain barrier (BBB) is a crucial regulator of neuroimmune interactions, forming a dynamic interface consisting of brain microvascular endothelial cells, pericytes, neurons, astrocytes, and the extracellular matrix (26). Endothelial cells express tight junction proteins, solute carriers, and receptors to limit paracellular diffusion of water-soluble substances and to facilitate selective transport of nutrients and metabolites from blood to the brain. Under normal conditions, the BBB serves as a crucial physical barrier, restricting interactions between the peripheral immune system and the CNS (26) (Figure 1C). In pathological conditions involving BBB dysfunction, increased permeability may exacerbate neuroinflammatory responses due to immune cell infiltration and pro-inflammatory signaling. Nonetheless, acute neuroinflammation may play a crucial role in tissue repair and recovery (27), and understanding the molecular regulators of BBB-mediated neuroimmune interactions is crucial due to the context-dependent regulation of BBB integrity.
Tight junction proteins, such as claudin-5, occludin, ZO-1, and ZO-2, are essential for the function of the BBB. Germ-free (GF) or antibiotic-treated mice exhibit reduced expression of tight junction proteins and increased BBB permeability. Increased BBB permeability allows immunomodulatory blood components and immune cells to enter the CNS, promoting systemic immune-CNS interactions. Interventions like butyrate supplementation, early-life low-dose penicillin exposure to enhance SCFA-producing bacteria, or gut colonization with Clostridium tyrobutyricum or Bacteroides thetaiotaomicron can restore claudin-5 and occludin expression, thereby reinforcing BBB integrity to support CNS health.
2.4 Meningeal immunity
The meninges are the immunologically active barrier tissues that form the outer defense of the CNS. The dura mater, arachnoid mater, and pia mater form the multilayered meningeal structures that cover the CNS surface, crucial for immune surveillance, neuroinflammatory responses, and injury repair (27). Meningeal immune cells mainly consist of macrophage subtypes, along with various innate and adaptive immune cells, including dendritic cells, neutrophils, NK cells, T cells, B cells, and innate lymphoid cells. Located near the brain parenchyma, these immune cells release diverse pro- and anti-inflammatory cytokines that interact with receptors on neurons and glial cells (27). Meningeal immune cells, both resident and transient, can affect parenchymal cell function by reacting to peripheral signals.
The dural sinus system efficiently drains venous blood, while lymphatic vessels bridge the brain with cervical lymph nodes, facilitating immune communication. During localized inflammation, distinct meningeal layers coordinate precise immune cell recruitment and homing, with stromal cells acting as “commanders” to release regulatory factors for fine-tuned immune modulation. For example, γδ T cells are critical in regulating anxiety-like behaviors (28), whereas IgA+ plasma cells form a robust defense against fungal invasion. Specific T cell subsets, such as IFN-γ- (Th1) and IL-4-producing (Th2) cells, are essential for maintaining neuronal circuit stability and cognitive function (29). The meninges also serve as a niche for immature B cell development and selectively eliminate reactive B cells that threaten CNS integrity via apoptosis.
Growing evidence highlights the gut microbiome as a key determinant of meningeal immune function (30). The meningeal immune repertoire is replenished by circulating immune cells “trained” by the gut microbiota. Recent studies demonstrate that GF and antibiotic-treated mice exhibit impaired meningeal immune function due to reduced cell frequencies or suppressed secretory products. The gut microbiota influences meningeal humoral immunity, akin to the microbiome-guided development of IgA+ plasma cells in the gut (31). Reduced meningeal IgA+ B cells heighten CNS susceptibility to bloodborne pathogens. Colonization with specific pathogen-free (SPF) microbiota, Citrobacter rodentium, or segmented filamentous bacteria (SFB) restores IgA+ B cell levels, mitigating pathogen susceptibility (32). Suppressed IFN-γ expression in meningeal NK cells impairs astrocyte-mediated T cell apoptosis (via LAMP1 and TRAIL), weakening autoimmune control of the CNS (33, 34). Similarly, reduced IL-17a production by meningeal γδ17 T cells alleviates anxiety-like behaviors by limiting IL-17a signaling to cortical glutamatergic neurons (28).
In summary, the meninges and CNS border regions are emerging as critical hubs for gut-brain axis neuroimmune communication, though the functional diversity of their immune cell repertoire remains underexplored. Future studies should develop techniques to selectively manipulate and track meningeal immune subtypes to dissect their impact on neural function. Further investigation into the gut microbiome as a regulator of meningeal immunity may yield novel therapies targeting meningeal neuroimmune interactions for neurological disorders.
2.5 Microbiota-gut-brain axis
Similar to the BBB, the microbiome and gut mucosa form a barrier that critically shapes immune responses in the gut and distant organs (Figure 1D). As discussed, the gut microbiome is central to regulating microglial physiology, and targeting gut dysbiosis—common in neurological disorders—may restore microglial function. Reduced microbial diversity correlates with microglial deficits in morphology, maturation, activation, and pathogen responses, deficits which are reversible upon microbiota recolonization. GF mice exhibit increased BBB permeability, while restoring a pathogen-free microbiota normalizes BBB integrity. Microbiome abnormalities are linked to neurobehavioral disorders: early comparative studies revealed that GF mice display immature microglia with attenuated immune-related transcriptional programs (e.g., type I interferon signaling, pathogen recognition, antigen presentation) but enhanced proliferation (Ki67, Ddit4) and survival (Csf1r, Pu.1) signals compared to SPF mice (35). Intriguingly, microglia from GF mice show upregulated expression of MAFB, a key transcription factor driving microglial maturation, suggesting developmental abnormalities may be microbiome-dependent (35). Consistent with this, four-week broad-spectrum antibiotic treatment (cefoxitin, gentamicin, metronidazole, vancomycin) in adult SPF mice replicates GF-like microglial immaturity, underscoring the necessity of continuous microbial signals for microglial homeostasis (35).
Beyond microbiome-BBB interactions in homeostasis, studies have evaluated microbial impacts on BBB integrity in neurological disease models. In experimental autoimmune encephalomyelitis (EAE), SPF microbiota transplantation corrects dysbiosis, reduces disease severity, and improves BBB function, as evidenced by reduced peripheral dye leakage into the brain and increased claudin-5 expression (36). In genetic hypertensive stroke models, cross-fostering with normotensive controls demonstrates that passive microbiota transfer enhances BBB integrity as measured via reduced IgG leakage and reverses stroke susceptibility (37). In murine models of traumatic brain injury (TBI), Clostridium butyricum and butyrate treatment restores BBB integrity by reversing the downregulation of occludin and zonula occludens-1 (ZO-1) observed following TBI (38). Probiotic combinations (e.g., Bifidobacterium animalis lactis, Lactobacillus casei) mitigate inflammation, improve BBB integrity, and enhance memory in aging and post-operative cognitive dysfunction models (39). Although mechanisms remain unclear, enhanced BBB integrity may limit systemic interactions of peripheral solutes, including antibodies, cytokines, and microbial metabolites, that may exacerbate neuroinflammation.
Emerging evidence suggests the microbiome may also regulate neuroprotective immune infiltration via BBB-independent pathways. Antibiotic-induced dysbiosis enhances BBB disruption (ZO-1/2 and occludin loss) while increasing monocyte infiltration in a CCR2-dependent manner (40). Conversely, probiotic mixture VSL#3 suppresses monocyte recruitment in inflammatory behavioral models (41). It should be noted that VSL#3 probiotic formulation after 2016 differs from the De Simone Formulation, which was commercially available under the trademark VSL#3® until 2016 (42). These findings highlight microbiome-BBB crosstalk, but rigorous mechanistic studies are needed to dissect these interactions in health and disease.
2.6 Neural pathway regulation
Unlike cellular and humoral immune responses, neural pathways provide critical signaling mechanisms for gut-brain neuroimmune communication. Immune mediators and key cells, including microglia, endothelial cells, and astrocytes, play pivotal roles in brain development, plasticity, synaptic maintenance, and repair. Neuroinflammation is implicated in various diseases, including Parkinson’s disease, Alzheimer’s disease, and depression. Microbiome alterations, particularly in the gut, influence brain function and behavior, likely through neuroinflammatory mechanisms. The vagus nerve represents a crucial neuroimmune interface that mediates bidirectional communication between the gut and brain. When gut-derived lipopolysaccharide (LPS) from bacteria such as Escherichia fergusonii activates vagal afferent fibers through brainstem nucleus tractus solitarius (NST) neurons, it initiates a dual pathological cascade (43). First, LPS-stimulated vagal signaling upregulates hippocampal tumor necrosis factor-alpha (TNF-α) expression via α7 nicotinic acetylcholine receptor (α7nAChR)-dependent pathways, leading to a disruption in synaptic plasticity (44). Simultaneously, this process suppresses brain-derived neurotrophic factor (BDNF) production, impairing neurogenesis and memory consolidation (45). The pathway is further amplified by microbial tryptophan metabolites signaling through the aryl hydrocarbon receptor (AHR) in both vagal nerve terminals and hippocampal cells (46, 47). Importantly, surgical vagotomy completely abolishes these LPS-induced effects, confirming the vagus nerve’s essential role in this neuroimmune axis (48). This gut-brain communication pathway provides a compelling example of how peripheral immune signals can fundamentally reshape central nervous system functions.
The vagus nerve serves as a critical bidirectional communication pathway between the gut and the central nervous system. This neural conduit transmits various microbially-derived neuroactive compounds including γ-aminobutyric acid (GABA), serotonin (5-HT), dopamine, and acetylcholine (ACh), which activate specific receptors such as 5-HT3 receptors and interact with bacterial components including LPS and SCFAs. The gut microbiota actively modulates vagal nerve activity through multiple mechanisms: Toll-like receptors (TLRs) detect microbial products and activate nodose ganglia neurons, leading to serotonin release that subsequently influences vagal afferent fibers. This neural reflex circuit can exert either anti-inflammatory or pro-inflammatory effects on the gut microbiome. Importantly, intestinal inflammation creates a feedback loop by altering microbial composition, activating endotoxemia pathways, compromising intestinal barrier integrity, and facilitating bacterial product translocation - collectively reinforcing the bidirectional nature of gut-vagus interactions (Figure 1E).
From an efferent perspective, vagal motor neurons project to the intestinal wall where they release ACh to stimulate Brunner’s glands in the duodenal submucosa, promoting mucus secretion that supports commensal microbial colonization (Figure 1F). Vagal efferents also regulate intestinal absorption through mechanisms yet to be fully elucidated. During stress responses, sympathetic activation leads to norepinephrine (NE) release which: (i) modulates epithelial barrier function via α2A-adrenergic receptor (α2A-AR) signaling, and (ii) directly impacts microbial community dynamics. This complex neuroimmune-endocrine network exemplifies the sophisticated integration between the nervous system and gut function (Figure 1E).
3 Material basis of neuroimmune regulation in the MGBA
The nervous and immune systems both demonstrate adaptive plasticity and memory-like responses to external stimuli. While the nervous system reacts rapidly (within seconds) with localized signaling, the immune system exhibits slower but more widespread mobilization. Emerging evidence suggests that gut microbiota can influence astrocyte function through direct and indirect pathways; however, rigorous validation of these findings and deeper mechanistic insights into molecular and cellular interactions remain essential. Critical research priorities include characterizing the biological effects of microbial metabolites, such as indole derivatives that modulate cerebral AHR signaling, and deciphering the molecular mechanisms underlying microbiota-microglia/astrocyte crosstalk to mitigate neurological disorders. Structural components and metabolites derived from gut microorganisms are key mediators of immune regulation along the gut-brain axis. A comprehensive summary of recent advances in this field is provided in Table 1.
3.1 Short-chain fatty acids
SCFAs are the primary metabolites of the gut microbiota and play a central role in gut-brain axis signaling. These metabolites enter the brain via systemic circulation and lymphatic drainage, modulating immune and neurotransmitter activity to influence higher-order brain functions such as mood and cognition. SCFAs traverse the BBB to regulate microglial inflammatory responses, ameliorating neuroinflammation in Alzheimer’s disease (AD), autism, and Parkinson’s disease (PD). They also regulate neurotransmitter synthesis, exerting anxiolytic and antidepressant effects (Table 1). Major SCFA-producing gut bacteria include Akkermansia muciniphila, Bacteroides, Bifidobacterium, Eubacterium, Streptococcus, and Lactobacillus.
SCFAs are microbial fermentation byproducts of dietary fiber and potent modulators of host physiology. Mice treated with antibiotics and GF mice show significantly decreased SCFA levels, which are also associated with impaired microglial development (54). The SCFA butyrate also functions as a histone deacetylase inhibitor (HDACi) and enhances macrophage antimicrobial activity by suppressing HDAC3 (55). Acetate, the predominant short-chain fatty acid in the brain (56), also inhibits HDAC activity and expression while promoting histone hyperacetylation by acting as a substrate for histone acetyltransferases (57). Acetate supplementation counteracts LPS-induced H3K9 hypoacetylation and non-histone protein acetylation, thereby reducing inflammatory signaling in microglia (57). In rat models of neuroinflammation, acute acetate administration increases brain acetyl-CoA levels and decreases glial activation by 40–50% (58). Acetyl-CoA, a key metabolic intermediate in the TCA cycle and oxidative phosphorylation, is associated with macrophage polarization and neuroprotection (59). While further studies are needed to delineate direct vs. indirect SCFA effects on microglia in vivo, these findings suggest SCFAs modulate microglial function via epigenetic intermediates.
Clinical evidence shows dysbiosis in PD patients correlates with weakened SCFA signaling (60). In a 6-hydroxydopamine PD model, propionate supplementation improves motor function and reduces dopaminergic neuron loss. In AD models, butyrate restores synaptic plasticity, accompanied by reduced pro-inflammatory cytokine (TNF-α, IL-6, IL-1β) expression in the hippocampus and cortex (61). However, interventions targeting SCFA pathways in neurodegenerative mouse models yield inconsistent outcomes. These early studies suggest SCFA dysregulation may tilt the balance between neurotoxicity and neuroprotection by altering microglial function.
In summary, current evidence highlights the dominance of acetate, propionate, and butyrate (constituting 90% of total SCFAs) in key physiological processes, including modulation of intestinal pH, promotion of symbiont growth, suppression of appetite, lowering of cholesterol, reduction in fat storage, enhancement of gut barrier integrity, and mitigation of neuroinflammation. Specific microbial-metabolite interactions are summarized in Table 2.
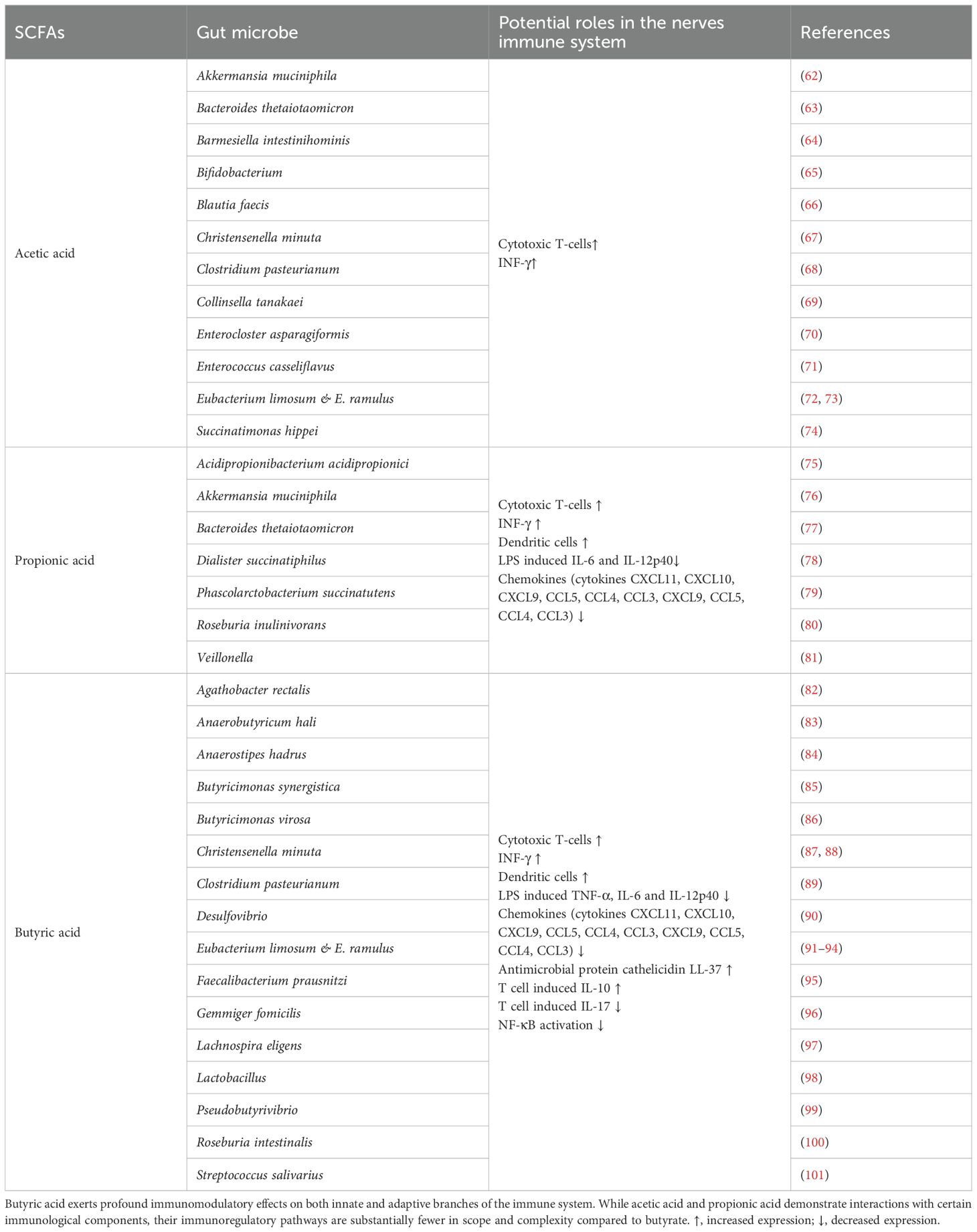
Table 2. The interaction between gut microbiota and short-chain fatty acids has significant implications for maintaining immune homeostasis in the host nervous system.
3.2 Lipopolysaccharides
In humans, Gram-negative bacteria comprise up to 47.5% of the fecal microbiota, with Bacteroidetes being the dominant phylum (1, 102). Notably, LPS derived from Bacteroides spp. (LPS-BS) exhibits significantly lower endotoxic activity compared to Escherichia coli-derived LPS (LPS-E) and may represent the predominant form of LPS in the human gut (103). Although LPS is widely used to induce pro-inflammatory microglial responses and exacerbate CNS disorders, the diversity of effects induced by LPS (priming versus tolerance) must be considered, as these depend on LPS molecular heterogeneity, dose, timing, route of administration, and contextual gene-environment interactions, injury, or disease states (104–106). Intriguingly, systemic LPS preconditioning activates CNS tolerance mechanisms, mitigating subsequent brain injury and neuroinflammation (107). LPS exerts protective effects against cryogenic brain injury via microglial TLR4 activation, suggesting LPS-induced tolerance may originate within the CNS. Repeated low-dose LPS exposure induces a neuroprotective phenotype in the C8-B4 microglial cell line and primary peritoneal macrophages, potentially mediated by TRIF signaling and epigenetic reprogramming (108). However, it remains unclear whether gut-derived LPS or diverse LPS molecules from Gram-negative commensals can elicit CNS immunomodulatory effects.
Toll-like receptors (TLRs), best known for their role in innate immunity, are widely expressed on multiple cell types. In the CNS, TLRs are expressed by neural stem cells, neurons, oligodendrocytes, astrocytes, and microglia, regulating neurodevelopmental, neuroplastic, and neurodegenerative processes (109). Surprisingly, SPF mice with global TLR2/3/4/7/9 deficiencies exhibit normal parenchymal microglial density, morphology, and maturation, indicating that gut microbiota do not regulate microglial development or maintenance via TLR signaling. Nevertheless, GF mice display microglial functional defects, including impaired TLR responsiveness. Compared to SPF mice, GF mice exhibit attenuated microglial innate immune responses (reduced cytokine/chemokine production) following systemic or intracerebral LPS administration.
Notably, oral supplementation studies mimicking gut LPS exposure demonstrate that dietary LPS-E modulates neural functions, including anxiety-like behavior (110) and taste responses (111). Dietary LPS from Pantoea agglomerans ameliorates high-fat diet-induced memory deficits linked to β-amyloid accumulation, potentially via enhanced microglial phagocytosis (112). In GF mice, two-week LPS-E supplementation fully activates microglial antigen presentation and protects against neurotropic mouse hepatitis virus (JHMV) infection (113). These effects are mediated by microglial TLR4 signaling, as mice with microglia-specific TLR4 deletion show blunted LPS priming and exacerbated JHMV pathology. Consistent with the microglia-specific role of TLR4, bone marrow transplantation from TLR4-deficient mice to wild-type recipients does not alter microglial responses to oral LPS or sustained protection against JHMV (113). Collectively, these findings indicate that gut microbiota regulate microglial function via direct LPS-TLR4 interactions.
While gut LPS-induced microglial priming protects against infections, chronic neuroinflammatory diseases may be exacerbated by aberrant microbial antigen exposure. Elevated blood endotoxin levels promote systemic inflammation and blood-brain barrier (BBB) disruption, exposing microglia to peripheral pro-inflammatory mediators and amplifying neuroinflammation (114). Indeed, elevated LPS levels are observed in patients with amyotrophic lateral sclerosis (ALS) (115), Alzheimer’s disease (AD) (116) and severe autism (117). In healthy volunteers, intravenous LPS enhances systemic inflammation, activates microglia, and induces sickness behavior (118). However, whether gut-derived endotoxin directly activates microglia to drive neuroinflammation remains unresolved.
3.3 Aromatic compounds
Aromatic amino acids such as phenylalanine and tyrosine are metabolized by gut microbiota into diverse aromatic compounds. For example, tyrosine is metabolized to 4-hydroxyphenylpyruvic acid, 4-hydroxyphenyllactic acid, 4-methylphenol (p-cresol), and 4-hydroxyphenylethanol (tyrosol), which serve as critical mediators of host-microbiota crosstalk. Neurotransmitters derived from phenylalanine/tyrosine (e.g., dopamine, norepinephrine, melanin) and tryptophan (e.g., serotonin) exhibit pleiotropic roles in the gut-brain axis. A common microbial pathway for phenylalanine/tyrosine metabolism involves AAA aminotransferase-mediated transamination, yielding compounds such as 4-hydroxyphenylpyruvic acid (4H-PAA), 4-hydroxyphenyllactic acid, p-cresol, and tyrosol.
Tryptophan, an essential amino acid obtained solely from dietary sources, is another aromatic compound with significant neuroregulatory roles (Table 1, Figure 2A). Tryptophan metabolites, such as indoleacetic acid and indoleethanol, exhibit neuroprotective, antioxidant, and anti-inflammatory properties, and also regulate neurotransmission (e.g., serotonin). Tryptophan undergoes three major metabolic pathways in the gut: (a) microbial conversion to diverse indole derivatives; (b) serotonin (5-HT) synthesis via tryptophan hydroxylase in enterochromaffin cells; (c) degradation via the kynurenine pathway by indoleamine 2,3-dioxygenase 1 (IDO1) and tryptophan 2,3-dioxygenase (TDO) (119, 120). Notably, tryptophan acts as a key mood modulator and therapeutic target in cancer, autoimmune diseases, and neurological disorders (121, 122).
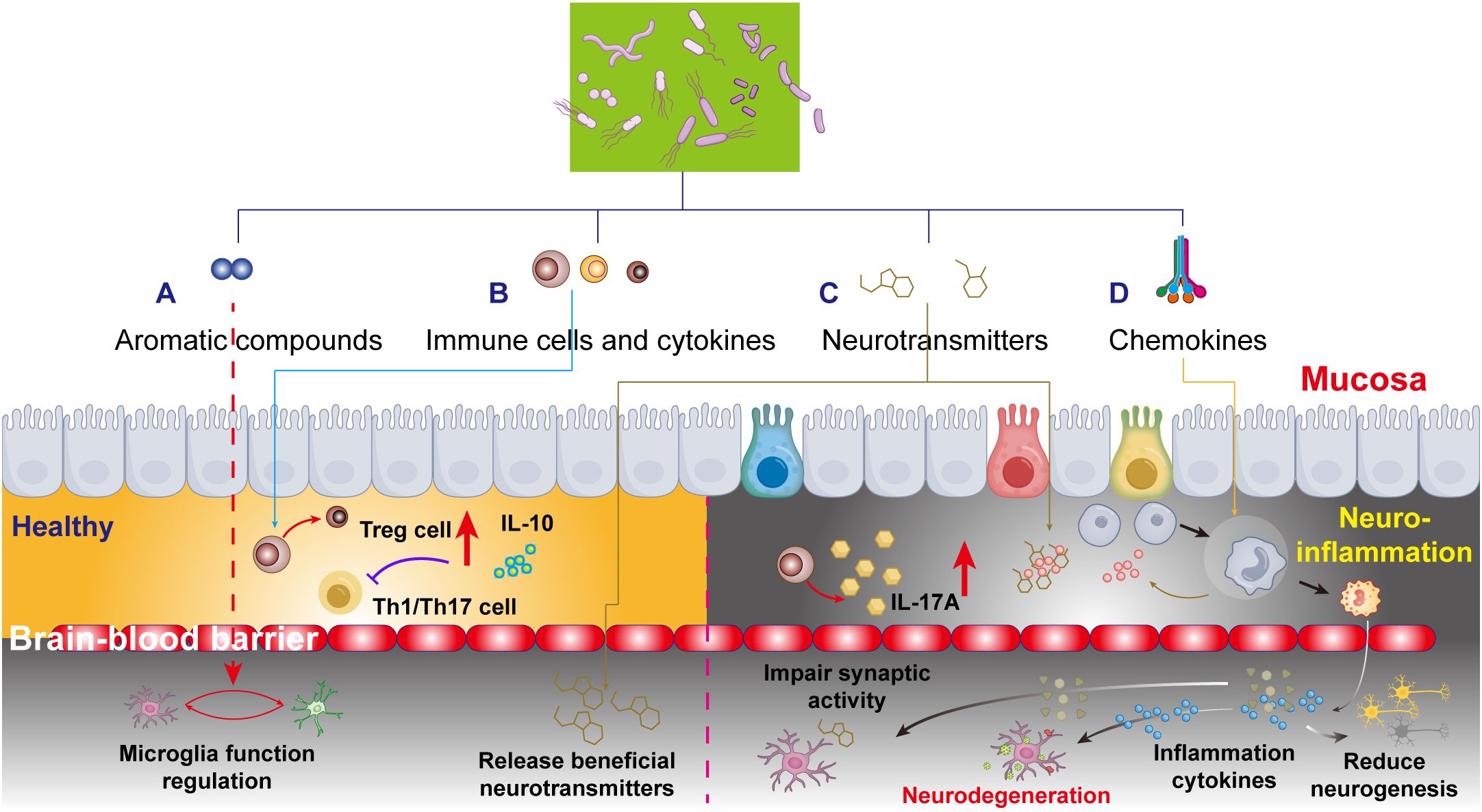
Figure 2. Schematic depiction of the microbiota-gut-brain axis and neuroinflammatory responses, emphasizing four key pathways by which aromatic compounds, cytokines, neurotransmitters, and gut chemokines interact with the blood-brain barrier (BBB) to influence brain function. (A) Aromatic compounds in healthy brain development. Mechanisms by which aromatic compounds promote brain development, emphasizing their regulatory roles in neurogenesis and neural circuit formation. (B) Gut-derived cytokines and neurodegeneration: functional alterations in gut immune cytokines and their impact on neurodegenerative pathologies, focusing on the secretory mechanisms of anti-inflammatory and pro-inflammatory cytokines. (C) Neurotransmitter regulation during brain development: the interplay between gut microbiota metabolites and neuronal function, revealing direct modulation of neural activity via microbial-derived neurotransmitters. (D) Gut chemokines in accelerated brain aging: pathological mechanisms linking gut chemokines to brain aging, including BBB permeability changes and neuroinflammatory cascades.
4 Potential mechanisms of MGBA in neuroinflammation
4.1 Microbiome-dependent T cells in multiple sclerosis
MS is a classical neuroinflammatory disorder characterized by disruption of the BBB, peripheral immune cell activation and infiltration, gliosis, and T cell-dependent demyelination. Its high heterogeneity arises from over 100 genetic susceptibility variants and environmental factors, such as vitamin D deficiency, circadian disruption, viral infections, and gut dysbiosis (123). Compared to healthy controls, the intestinal microbiome of patients with MS exhibits reduced abundances of Prevotella, Faecalibacterium prausnitzii, Bacteroides coprophilus, and Bacteroides fragilis, alongside elevated abundances of Methanobrevibacter and Akkermansia muciniphila (124). Impaired regulatory T cell (Treg) function, marked by reduced IL-10 secretion, diminishes their ability to suppress pro-inflammatory Th1/Th17-mediated neuroinflammation and contributing to the pathogenesis of MS (125). Clinically, MS patients show elevated levels of Th17 cells in cerebrospinal fluid (CSF) and the gut mucosa (126), characterized by increased IL-17a levels in serum and CSF (127) (Figure 2B).
4.2 Gut microbiota-associated neurotransmitters
Neurodegenerative pathophysiology involves dysregulation of neurotransmitter systems, including dopaminergic, cholinergic, serotonergic, glutamatergic, and GABAergic pathways. Critically, the gut microbiota modulates these systems to influence brain function (Figure 2C).
Serotonergic System: Dysregulation of serotonin (5-HT) signaling is implicated in Alzheimer’s disease (AD), affecting amyloid precursor protein (APP) processing and Aβ deposition. Patients with mild cognitive impairment (MCI) exhibit reduced availability of the brain serotonin transporter and higher cortical Aβ burden compared to controls. AD patients also show significantly lower urinary and serum serotonin levels. Notably, selective serotonin reuptake inhibitors (SSRIs) suppress Aβ levels in both human and AD mouse models.
GABAergic System: γ-Aminobutyric acid (GABA) is the primary inhibitory neurotransmitter. It is involved in regulating brain states, cognition (learning, memory, sensory processing), circadian rhythms, and motor function. Dysregulated tonic GABA currents are linked to Parkinson’s disease (PD) and Huntington’s disease (HD). The gut microbiota influences GABA production, and an overgrowth of Escherichia in patients with autism spectrum disorder (ASD) has been strongly correlated with aberrant GABA metabolism. Elevated GABA/glutamate (Glu) ratios serve as metabolic biomarkers for mild ASD.
Dopaminergic System: Microbiota-targeted therapies alleviate dopaminergic damage and motor deficits in PD models. Probiotic supplementation increases serum dopamine levels and improves motor function in PD patients. Berberine stimulates gut microbial production of L-DOPA and dopamine via tyrosine hydroxylase and DOPA decarboxylase activation, enabling dopamine synthesis in the brain.
Cholinergic System: Acetylcholine (ACh) is a key neurotransmitter modulated by the gut microbiota. For example, Lactobacillus plantarum MTCC1325 restores ACh levels in a rat model of AD induced by D-galactose treatment by reducing acetylcholinesterase (AChE) activity. Similarly, prebiotic fructooligosaccharides counteract AChE elevation, rescuing cholinergic dysfunction in AD models.
4.3 AHR signal pathway
Tryptophan is an essential amino acid that is mainly obtained from the diet. The gut microbiota is crucial in regulating intestinal tryptophan metabolism and its key derivative pathways, such as kynurenine, serotonin, indole precursors, and aryl hydrocarbon receptor (AHR) ligands (128). Gut bacteria with tryptophanase convert dietary tryptophan into indole, which the host uses to synthesize AHR agonists like indoxyl sulfate and indole-3-propionic acid (IPA) (128). Notably, indoxyl sulfate and IPA are undetectable in GF mice, confirming that their bioavailability depends on the gut microbiota.
AHR is broadly expressed in both CNS-resident and peripheral immune cells, as extensively reviewed in the literature (129). In murine models of various diseases, including experimental autoimmune encephalomyelitis (129), ischemic stroke (130), intracerebral hemorrhage (131), and LPS-induced neuroinflammation (131), AHR expression is increased in various brain-resident cells, including microglia. However, the precise function of microglial AHR signaling in disease pathology is not yet fully understood. Systemic AHR deficiency exacerbates microglial activation in models of experimental autoimmune uveitis (132) and retinal degeneration (133), consistent with studies showing that microbiota-derived AHR ligands (e.g., urolithin A, indoxyl sulfate) suppress pro-inflammatory markers in microglia (134).
Recent studies, however, suggest dual pro- and anti-inflammatory roles for microglial AHR signaling. AHR silencing or activation via ligands (e.g., formylindolo [3,2-b] carbazole or 3-methylcholanthrene) both inhibit LPS-induced microglial activation (135). This aligns with reports that AHR antagonists (6,2,4′-trimethoxyflavone) protect against ischemic stroke by blocking microglial activation and preserving neurogenesis (130, 136), while indoxyl sulfate promotes neurotoxic environments in glial co-cultures. Conditional AHR knockout in neural stem/progenitor cells attenuates astrogliosis and microgliosis in ischemic stroke models, whereas microglia- or astrocyte-specific AHR deletion worsens EAE (137). These findings indicate that AHR signaling mediates microbiota-neuroimmune crosstalk, with effects dependent on neuroinflammatory context, microbial agonist availability, and contributions from other AHR-expressing CNS/peripheral cells. The complexity of CNS AHR signaling underscores the importance of cell-cell interactions (e.g., microglia-astrocyte crosstalk) during neuroinflammation.
In astrocytes, AHR signaling collaborates with the microbiota and neuroimmune networks to regulate neuroinflammation. Depleting AHR ligand-producing microbes with ampicillin weakens astrocytic AHR signaling, exacerbates EAE, and enhances NF-κB-driven pro-inflammatory gene transcription, leading to microglial activation (138). Similarly, microglia-specific AHR signaling suppresses astrocyte reactivity by modulating ligand ratios (TGF-α) for astrocyte receptors ERBB1 and FLT1 (137). Reduced TGF-α ratios in chronic MS lesions suggest dysregulated tryptophan metabolism and AHR signaling contribute to MS pathology. This aligns with findings that IPA inhibits LPS-induced inflammation in human astrocyte cultures (139), while TGF-α and VEGF-B suppress or amplify pro-inflammatory gene expression, respectively (137).
Clinically, potent synthetic AHR agonists (e.g., laquinimod) demonstrate therapeutic potential by targeting microbial tryptophan metabolism. In preclinical studies, laquinimod alleviates EAE symptoms and neuroinflammation via systemic and CNS immunomodulation (140, 141). Transplanting wild-type bone marrow into AHR-deficient mice partially reinstates laquinimod efficacy (140), independent of LysM+ immune cells (141). Astrocyte-specific AHR knockout significantly, but incompletely, blocks the effect of laquinimod (141). These results suggest optimal anti-inflammatory outcomes in CNS autoimmunity require systemic AHR signaling but highlight CNS-restricted AHR targeting (the primary site of EAE suppression) to minimize off-target effects.
4.4 Role of gut chemokines in neuroinflammation
The gut, as a highly innervated organ replete with immune cells, maintains a delicate equilibrium where integrity of the gut microbiota is essential for preserving intestinal barrier function. While systemic inflammation can compromise the BBB, oral antibiotic-induced dysbiosis further exacerbates BBB permeability through microbial metabolite-mediated mechanisms. Pathological conditions like “leaky gut” syndrome permit microbial byproducts and circulatory factors to stimulate peripheral immune cells and chemokine release, subsequently recruiting neutrophils, monocytes, and other inflammatory cells to affected sites. These chemokines orchestrate immune cell trafficking while dynamically regulating the functions of regulatory T cells (Tregs), type 3 innate lymphoid cells (ILC3s), and macrophages (131). The resultant cytokine milieu (including IL-10 and IL-22) profoundly influences neuronal viability, synaptic plasticity, and neurodegenerative processes (Figure 2D).
Chemokine-directed gut-to-brain immune cell migration operates through specialized molecular cascades that shape neurological disease progression. The CCL20-CCR6 axis exemplifies this mechanism, where intestinal epithelial-derived CCL20 guides ILC3s across compromised BBB regions into the CNS, where the production of neuroprotective IL-22 by the ILC3a mitigates demyelination in MS models (142, 143). In Alzheimer’s pathology, CXCR3+ immune cells facilitate the transport of gut-derived Aβ aggregates across both vascular and neural barriers, accelerating cerebral amyloidosis (144). Similarly, CCL2 overexpression disrupts BBB integrity in Parkinson’s disease, driving monocyte infiltration into the CNS while CCL5-mediated Th17 cell recruitment exacerbates dopaminergic neuron loss (145, 146). These chemokine networks not only regulate T cell differentiation and Th1/Th2 balance but also determine neuroinflammatory severity, as evidenced by the pivotal role of CCL2 in modulating the progression of MS through leukocyte trafficking control (147).
4.5 Gut microbiota dysbiosis in neurological disorders
The core neuroinflammatory mechanism induced by gut dysbiosis involves increased intestinal permeability allowing endotoxins (e.g., LPS) to enter circulation. This triggers pro-inflammatory cytokine release, subsequently disrupting the blood-brain barrier (BBB) and inducing neuroinflammation. In Alzheimer’s disease, acute enteritis may paradoxically reduce cerebral Aβ deposition (potentially via enhanced Aβ efflux into blood), but chronic dysbiosis interferes with Aβ clearance mechanisms, compromising its potential protective function as an antimicrobial peptide (148–150). Specific microbiota (e.g., Klebsiella pneumoniae) can invade the brain via the gut, exacerbating tau-mediated neurodegeneration in an ApoE genotype-dependent manner (151). Clinically, AD patients exhibit significantly reduced gut microbial diversity, with alterations in specific taxa (e.g., decreased Ruminococcaceae; increased Bacteroidaceae) correlating with cognitive impairment severity.
For Parkinson’s disease, dysbiosis-induced gut barrier damage promotes abnormal aggregation of misfolded α-synuclein (α-syn) in the enteric nervous system. This pathological protein undergoes retrograde transmission via the vagus nerve to the substantia nigra, forming Lewy bodies (152). Germ-free mouse models demonstrate that even with α-syn overexpression, the absence of gut microbiota completely prevents motor deficits and neuronal loss. In contrast, transplanting the microbiota from PD patients into wild-type mice induces α-syn aggregation and dopaminergic neuron death (153).
In MS, the specific eradication of the newly discovered gut bacterium Erysipelotrichaceae OTU002 selectively reduces T cell activity, thereby diminishing the adhesion of myelin oligodendrocyte glycoprotein (MOG) to neuronal myelin and preventing the MS (154). Clinical studies indicate that microbial dysbiosis, characterized by an increase in Alistipes, is significantly correlated with worsened disability in MS, while elevated levels of short-chain fatty acid-producing bacteria such as Eubacterium hallii, Butyricoccaceae, and Blautia improve cognitive function and quality of life (155).
5 Summary and perspectives
This review introduces a novel perspective on the gut-neuroimmune axis by emphasizing the intricate mechanisms through which the gut microbiota influences the CNS. It goes beyond traditional views by delving into the specific roles of microbiota-derived components and metabolites in modulating the maturation and function of the BBB and CNS-resident immune and glial cells. By elucidating these molecular pathways, the study uncovers novel functional microbial species and effector molecules that have not been previously explored in depth. This in-depth exploration of the gut-brain connection not only advances our understanding of the microbiota’s role in neuroimmune regulation but also opens new avenues for innovative therapeutic interventions.
The clinical implications of this review are profound. By highlighting the key role of the gut microbiota in maintaining neuroimmune homeostasis, the study provides a theoretical foundation for the development of microbiota-based therapies for neurological disorders. By identifying specific microbial species and metabolites that regulate neuroimmune interactions, researchers can target these molecules to restore balance in the gut-brain axis, potentially mitigating the symptoms of diseases such as MS, PD, HD, and AD. This innovative approach holds the promise of more effective and personalized treatment options for patients, ultimately leading to improved quality of life and outcomes.
Author contributions
SZ: Writing – original draft, Investigation. DF: Investigation, Writing – original draft, Formal Analysis, Methodology. YL: Funding acquisition, Project administration, Conceptualization, Writing – review & editing. XS: Validation, Conceptualization, Writing – original draft. XZ: Writing – review & editing, Investigation, Formal Analysis, Project administration. XKW: Funding acquisition, Formal Analysis, Writing – review & editing, Writing – original draft, Investigation. XZW: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Guangdong Basic and Applied Basic Research Foundation (No. 2023A1515111116), the Shenzhen Foundation of Science and Technology (No. JCYJ20230807151308018), the Zhanjiang Science and Technology Project (2023B01176), the Futian Healthcare Research Project (No.FTWS088), the Shenzhen Health Economics Society Project (Nos. 202521, 2025211), the Key Clinical Specialties in Futian District, the Shenzhen Longhua District Science and Technology Innovation Fund Projects (Nos. 2022045, 2022051, 2022056, 2022095, 2022123, 2021105, 2021115 and 2020036) and the Research Foundation of Shenzhen Longhua District Central Hospital (No. 202303).
Acknowledgments
We sincerely thank the reviewers for their valuable feedback on this paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rao J, Qiu P, Zhang Y, and Wang X. Gut microbiota trigger host liver immune responses that affect drug-metabolising enzymes. Front Immunol. (2024) 15:1511229. doi: 10.3389/fimmu.2024.1511229
2. Camara-Lemarroy CR, Metz L, Meddings JB, Sharkey KA, and Wee Yong V. The intestinal barrier in multiple sclerosis: implications for pathophysiology and therapeutics. Brain. (2018) 141:1900–16. doi: 10.1093/brain/awy131
3. Aghara H, Patel M, Chadha P, Parwani K, Chaturvedi R, and Mandal P. Unraveling the gut-liver-brain axis: microbiome, inflammation, and emerging therapeutic approaches. Mediators Inflammation. (2025) 2025:6733477. doi: 10.1155/mi/6733477
4. Zhernakova A, Yassour M, Hall LJ, and Collado MC. Unlocking the power of human milk and infant feeding: Understanding how nutrition and early microbiota interaction shapes health programming. Cell Host Microbe. (2025) 33:820–35. doi: 10.1016/j.chom.2025.05.014
5. Tanaka S, Kawakita M, Yasui H, Sudo K, Itoh F, Sasaki M, et al. An immune-adrenergic pathway induces lethal levels of platelet-activating factor in mice. Commun Biol. (2024) 7:782. doi: 10.1038/s42003-024-06498-7
6. Kang JW, Vemuganti V, Kuehn JF, Ulland TK, Rey FE, and Bendlin BB. Gut microbial metabolism in Alzheimer’s disease and related dementias. Neurotherapeutics. (2024) 21:e00470. doi: 10.1016/j.neurot.2024.e00470
7. Pang S, Ren Z, Ding H, and Chan P. Short-chain fatty acids mediate enteric and central nervous system homeostasis in Parkinson’s disease: Innovative therapies and their translation. Neural Regener Res. (2026) 21:938–56. doi: 10.4103/NRR.NRR-D-24-01265
8. Tahmasebi F and Barati S. The role of microglial depletion approaches in pathological condition of CNS. Cell Mol Neurobiol. (2023) 43:2459–71. doi: 10.1007/s10571-023-01326-8
9. Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. (2008) 57:178–201. doi: 10.1016/j.neuron.2008.01.003
10. Nguyen LT, Aprico A, Nwoke E, Walsh AD, Blades F, Avneri R, et al. Mertk-expressing microglia influence oligodendrogenesis and myelin modelling in the CNS. J Neuroinflamm. (2023) 20:253. doi: 10.1186/s12974-023-02921-8
11. Rodrigues-Neves AC, Ambrósio AF, and Gomes CA. Microglia sequelae: brain signature of innate immunity in schizophrenia. Transl Psychiatry. (2022) 12:493. doi: 10.1038/s41398-022-02197-1
12. Subbarayan MS, Joly-Amado A, Bickford PC, and Nash KR. CX3CL1/CX3CR1 signaling targets for the treatment of neurodegenerative diseases. Pharmacol Ther. (2022) 231:107989. doi: 10.1016/j.pharmthera.2021.107989
13. Lv K, Luo Y, Liu T, Xia M, Gong H, Zhang D, et al. Inactivation of microglial LXRβ in early postnatal mice impairs microglia homeostasis and causes long-lasting cognitive dysfunction. Proc Natl Acad Sci U.S.A. (2025) 122:e2410698122. doi: 10.1073/pnas.2410698122
14. Choi I, Heaton GR, Lee Y-K, and Yue Z. Regulation of α-synuclein homeostasis and inflammasome activation by microglial autophagy. Sci Adv. (2022) 8:eabn1298. doi: 10.1126/sciadv.abn1298
15. Brown FN, Iwasawa E, Shula C, Fugate EM, Lindquist DM, Mangano FT, et al. Early postnatal microglial ablation in the Ccdc39 mouse model reveals adverse effects on brain development and in neonatal hydrocephalus. Fluids Barriers CNS. (2023) 20:42. doi: 10.1186/s12987-023-00433-4
16. Zhou X, He J, Song H, Zhao W, Li R, Han W, et al. Regulation of macrophage efferocytosis by the CLCF1/NF-κB pathway improves neurological and cognitive impairment following CO poisoning. Brain Behav Immun. (2025) 127:126–46. doi: 10.1016/j.bbi.2025.03.003
17. Zhu Z, Jin L, Wang Q, Shi H, Cheng K, and Mao Z. Inhalable ce nanozyme-backpacked phage aims at ischemic cerebral injury by M1-microglia hitchhiking. Adv Mater. (2025) 37:e2419903. doi: 10.1002/adma.202419903
18. Ebrahimi R, Shahrokhi Nejad S, Falah Tafti M, Karimi Z, Sadr SR, Ramadhan Hussein D, et al. Microglial activation as a hallmark of neuroinflammation in Alzheimer’s disease. Metab Brain Dis. (2025) 40:207. doi: 10.1007/s11011-025-01631-9
19. Morris G, Fernandes BS, Puri BK, Walker AJ, Carvalho AF, and Berk M. Leaky brain in neurological and psychiatric disorders: Drivers and consequences. Aust N Z J Psychiatry. (2018) 52:924–48. doi: 10.1177/0004867418796955
20. Han RT, Kim RD, Molofsky AV, and Liddelow SA. Astrocyte-immune cell interactions in physiology and pathology. Immunity. (2021) 54:211–24. doi: 10.1016/j.immuni.2021.01.013
21. Vainchtein ID, Chin G, Cho FS, Kelley KW, Miller JG, Chien EC, et al. Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science. (2018) 359:1269–73. doi: 10.1126/science.aal3589
22. Lee H-G, Lee J-H, Flausino LE, and Quintana FJ. Neuroinflammation: An astrocyte perspective. Sci Transl Med. (2023) 15:eadi7828. doi: 10.1126/scitranslmed.adi7828
23. Zhang Y, Liu K, Li Y, Ma Y, Wang Y, Fan Z, et al. D-beta-hydroxybutyrate protects against microglial activation in lipopolysaccharide-treated mice and BV-2 cells. Metab Brain Dis. (2023) 38:1115–26. doi: 10.1007/s11011-022-01146-7
24. Kim C-S, Jung S, Hwang G-S, and Shin D-M. Gut microbiota indole-3-propionic acid mediates neuroprotective effect of probiotic consumption in healthy elderly: A randomized, double-blind, placebo-controlled, multicenter trial and in vitro study. Clin Nutr. (2023) 42:1025–33. doi: 10.1016/j.clnu.2023.04.001
25. Cui W, Bai H, Guo C, Zhou J, Feng D, Zhang S, et al. Interferon regulatory factor-1-expressing astrocytes are epigenetically controlled and exacerbate TBI-associated pathology in mice. Sci Transl Med. (2025) 17:eadr5300. doi: 10.1126/scitranslmed.adr5300
26. Pérez-López A, Torres-Suárez AI, Martín-Sabroso C, and Aparicio-Blanco J. An overview of in vitro 3D models of the blood-brain barrier as a tool to predict the in vivo permeability of nanomedicines. Adv Drug Delivery Rev. (2023) 196:114816. doi: 10.1016/j.addr.2023.114816
27. Banjara M and Ghosh C. Sterile neuroinflammation and strategies for therapeutic intervention. Int J Inflam. (2017) 2017:8385961. doi: 10.1155/2017/8385961
28. Alves de Lima K, Rustenhoven J, Da Mesquita S, Wall M, Salvador AF, Smirnov I, et al. Meningeal γδ T cells regulate anxiety-like behavior via IL-17a signaling in neurons. Nat Immunol. (2020) 21:1421–9. doi: 10.1038/s41590-020-0776-4
29. Wang S, Li G, Liang X, Wu Z, Chen C, Zhang F, et al. Small extracellular vesicles derived from altered peptide ligand-loaded dendritic cell act as A therapeutic vaccine for spinal cord injury through eliciting CD4+ T cell-mediated neuroprotective immunity. Adv Sci (Weinh). (2024) 11:e2304648. doi: 10.1002/advs.202304648
30. Vashishth S, Ambasta RK, and Kumar P. Deciphering the microbial map and its implications in the therapeutics of neurodegenerative disorder. Ageing Res Rev. (2024) 100:102466. doi: 10.1016/j.arr.2024.102466
31. Posner DA, Lee CY, Portet A, and Clatworthy MR. Humoral immunity at the brain borders in homeostasis. Curr Opin Immunol. (2022) 76:102188. doi: 10.1016/j.coi.2022.102188
32. Jie Z, Yang J-Y, Gu M, Wang H, Xie X, Li Y, et al. NIK signaling axis regulates dendritic cell function in intestinal immunity and homeostasis. Nat Immunol. (2018) 19:1224–35. doi: 10.1038/s41590-018-0206-z
33. Kichev A, Rousset CI, Baburamani AA, Levison SW, Wood TL, Gressens P, et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) signaling and cell death in the immature central nervous system after hypoxia-ischemia and inflammation. J Biol Chem. (2014) 289:9430–9. doi: 10.1074/jbc.M113.512350
34. Sanmarco LM, Wheeler MA, Gutiérrez-Vázquez C, Polonio CM, Linnerbauer M, Pinho-Ribeiro FA, et al. Gut-licensed IFNγ+ NK cells drive LAMP1+TRAIL+ anti-inflammatory astrocytes. Nature. (2021) 590:473–9. doi: 10.1038/s41586-020-03116-4
35. Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. (2015) 18:965–77. doi: 10.1038/nn.4030
36. Li K, Wei S, Hu L, Yin X, Mai Y, Jiang C, et al. Protection of fecal microbiota transplantation in a mouse model of multiple sclerosis. Mediators Inflammation. (2020) 2020:2058272. doi: 10.1155/2020/2058272
37. Castillo-Ruiz A, Mosley M, George AJ, Mussaji LF, Fullerton EF, Ruszkowski EM, et al. The microbiota influences cell death and microglial colonization in the perinatal mouse brain. Brain Behav Immun. (2018) 67:218–29. doi: 10.1016/j.bbi.2017.08.027
38. Li H, Sun J, Du J, Wang F, Fang R, Yu C, et al. Clostridium butyricum exerts a neuroprotective effect in a mouse model of traumatic brain injury via the gut-brain axis. Neurogastroenterol Motil. (2018) 30:e13260. doi: 10.1111/nmo.13260
39. Luck B, Engevik MA, Ganesh BP, Lackey EP, Lin T, Balderas M, et al. Bifidobacteria shape host neural circuits during postnatal development by promoting synapse formation and microglial function. Sci Rep. (2020) 10:7737. doi: 10.1038/s41598-020-64173-3
40. Sun N, Hu H, Wang F, Li L, Zhu W, Shen Y, et al. Antibiotic-induced microbiome depletion in adult mice disrupts blood-brain barrier and facilitates brain infiltration of monocytes after bone-marrow transplantation. Brain Behav Immun. (2021) 92:102–14. doi: 10.1016/j.bbi.2020.11.032
41. D’Mello C, Ronaghan N, Zaheer R, Dicay M, Le T, MacNaughton WK, et al. Probiotics improve inflammation-associated sickness behavior by altering communication between the peripheral immune system and the brain. J Neurosci. (2015) 35:10821–30. doi: 10.1523/JNEUROSCI.0575-15.2015
42. De Simone C. Letter: what gastroenterologists should know about VSL3. Aliment Pharmacol Ther. (2018) 47:698–9. doi: 10.1111/apt.14515
43. Jakob MO, Murugan S, and Klose CSN. Neuro-immune circuits regulate immune responses in tissues and organ homeostasis. Front Immunol. (2020) 11:308. doi: 10.3389/fimmu.2020.00308
44. Rojas-Colón LA, Dash PK, Morales-Vías FA, Lebrón-Dávila M, Ferchmin PA, Redell JB, et al. 4R-cembranoid confers neuroprotection against LPS-induced hippocampal inflammation in mice. J Neuroinflamm. (2021) 18:95. doi: 10.1186/s12974-021-02136-9
45. Jackson A, Forsyth CB, Shaikh M, Voigt RM, Engen PA, Ramirez V, et al. Diet in parkinson’s disease: critical role for the microbiome. Front Neurol. (2019) 10:1245. doi: 10.3389/fneur.2019.01245
46. Ma N, He T, Johnston LJ, and Ma X. Host-microbiome interactions: the aryl hydrocarbon receptor as a critical node in tryptophan metabolites to brain signaling. Gut Microbes. (2020) 11:1203–19. doi: 10.1080/19490976.2020.1758008
47. Qiao C-M, Ma X-Y, Tan L-L, Xia Y-M, Li T, Wu J, et al. Indoleamine 2, 3-dioxygenase 1 inhibition mediates the therapeutic effects in Parkinson’s disease mice by modulating inflammation and neurogenesis in a gut microbiota dependent manner. Exp Neurol. (2025) 385:115142. doi: 10.1016/j.expneurol.2025.115142
48. Tracey KJ. Consolidating roles of neuroimmune reflexes: specificity of afferent, central, and efferent signals in homeostatic immune networks. Genes Dev. (2024) 38:805–7. doi: 10.1101/gad.352287.124
49. Sardar P, Beresford-Jones BS, Xia W, Shabana O, Suyama S, Ramos RJF, et al. Gut microbiota-derived hexa-acylated lipopolysaccharides enhance cancer immunotherapy responses. Nat Microbiol. (2025) 10:795–807. doi: 10.1038/s41564-025-01930-y
50. Yang T, Hu X, Cao F, Yun F, Jia K, Zhang M, et al. Targeting symbionts by apolipoprotein L proteins modulates gut immunity. Nature. (2025) 643:210–8. doi: 10.1038/s41586-025-08990-4
51. Hou Q, Jia J, Lin J, Zhu L, Xie S, Yu Q, et al. Bacillus subtilis programs the differentiation of intestinal secretory lineages to inhibit Salmonella infection. Cell Rep. (2022) 40:111416. doi: 10.1016/j.celrep.2022.111416
52. Kekessie I, Goncharov T, Kőműves LG, Vucic D, and Song A. A solid-phase approach for the synthesis of muramyl dipeptide conjugates for detection of NOD2. Bioorg Chem. (2021) 116:105360. doi: 10.1016/j.bioorg.2021.105360
53. Gao J, He Y, Shi F, Hou F, Wu X, Yi Y, et al. Activation of Sirt6 by icariside II alleviates depressive behaviors in mice with poststroke depression by modulating microbiota-gut-brain axis. J Adv Res. (2025), 1–13. doi: 10.1016/j.jare.2025.03.002
54. Chu C, Murdock MH, Jing D, Won TH, Chung H, Kressel AM, et al. The microbiota regulate neuronal function and fear extinction learning. Nature. (2019) 574:543–8. doi: 10.1038/s41586-019-1644-y
55. Diaz Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U.S.A. (2011) 108:3047–52. doi: 10.1073/pnas.1010529108
56. Dalile B, Van Oudenhove L, Vervliet B, and Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. (2019) 16:461–78. doi: 10.1038/s41575-019-0157-3
57. Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. (2012) 74:691–705. doi: 10.1016/j.neuron.2012.03.026
58. Stephan AH, Barres BA, and Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci. (2012) 35:369–89. doi: 10.1146/annurev-neuro-061010-113810
59. Fonseca MI, Chu S-H, Hernandez MX, Fang MJ, Modarresi L, Selvan P, et al. Cell-specific deletion of C1qa identifies microglia as the dominant source of C1q in mouse brain. J Neuroinflamm. (2017) 14:48. doi: 10.1186/s12974-017-0814-9
60. Rubinstein MR, Burgueño AL, Quiroga S, Wald MR, and Genaro AM. Current understanding of the roles of gut-brain axis in the cognitive deficits caused by perinatal stress exposure. Cells. (2023) 12:1–24. doi: 10.3390/cells12131735
61. Yoshiya K, Lapchak PH, Thai T-H, Kannan L, Rani P, Dalle Lucca JJ, et al. Depletion of gut commensal bacteria attenuates intestinal ischemia/reperfusion injury. Am J Physiol Gastrointest Liver Physiol. (2011) 301:G1020–30. doi: 10.1152/ajpgi.00239.2011
62. Ma J, Liu Z, Gao X, Bao Y, Hong Y, He X, et al. Gut microbiota remodeling improves natural aging-related disorders through Akkermansia muciniphila and its derived acetic acid. Pharmacol Res. (2023) 189:106687. doi: 10.1016/j.phrs.2023.106687
63. Wang J-S, Xue K, Li Z, Ssempebwa J, Wamuyu-Maina G, Musinguzi G, et al. Peanut supplementation affects compositions and functions of gut microbiome in Ugandan children. Food Funct. (2024) 15:4365–74. doi: 10.1039/d3fo04645a
64. Ye H, Ghosh TS, Hueston CM, Vlckova K, Golubeva AV, Hyland NP, et al. Engraftment of aging-related human gut microbiota and the effect of a seven-species consortium in a pre-clinical model. Gut Microbes. (2023) 15:2282796. doi: 10.1080/19490976.2023.2282796
65. Zhang R, Zou S, Cen Q, Hu W, Tan F, Chen H, et al. Effects of Ganoderma lucidum fermentation on the structure of Tartary buckwheat polysaccharide and its impact on gut microbiota composition. Int J Biol Macromol. (2025) 306:140944. doi: 10.1016/j.ijbiomac.2025.140944
66. Nieto JA, Rosés C, Viadel B, Gallego E, Romo-Hualde A, Milagro FI, et al. Sourdough bread enriched with exopolysaccharides and gazpacho by-products modulates in vitro the microbiota dysbiosis. Int J Biol Macromol. (2024) 272:132906. doi: 10.1016/j.ijbiomac.2024.132906
67. Gao X, Zhang Q, and Zhu H. High rejection rate of polysaccharides by microfiltration benefits Christensenella minuta and acetic acid production in an anaerobic membrane bioreactor for sludge fermentation. Bioresour Technol. (2019) 282:197–201. doi: 10.1016/j.biortech.2019.03.015
68. Zhang C, Sharma S, Wang W, and Zeng A-P. A novel downstream process for highly pure 1,3-propanediol from an efficient fed-batch fermentation of raw glycerol by Clostridium pasteurianum. Eng Life Sci. (2021) 21:351–63. doi: 10.1002/elsc.202100012
69. Nagai F, Watanabe Y, and Morotomi M. Slackia piriformis sp. nov. and Collinsella tanakaei sp. nov., new members of the family Coriobacteriaceae, isolated from human faeces. Int J Syst Evol Microbiol. (2010) 60:2639–46. doi: 10.1099/ijs.0.017533-0
70. Abbondio M, Tanca A, De Diego L, Sau R, Bibbò S, Pes GM, et al. Metaproteomic assessment of gut microbial and host functional perturbations in Helicobacter pylori-infected patients subjected to an antimicrobial protocol. Gut Microbes. (2023) 15:2291170. doi: 10.1080/19490976.2023.2291170
71. Bortolini C, Patrone V, Puglisi E, and Morelli L. Detailed analyses of the bacterial populations in processed cocoa beans of different geographic origin, subject to varied fermentation conditions. Int J Food Microbiol. (2016) 236:98–106. doi: 10.1016/j.ijfoodmicro.2016.07.004
72. Kanauchi O, Fukuda M, Matsumoto Y, Ishii S, Ozawa T, Shimizu M, et al. Eubacterium limosum ameliorates experimental colitis and metabolite of microbe attenuates colonic inflammatory action with increase of mucosal integrity. World J Gastroenterol. (2006) 12:1071–7. doi: 10.3748/wjg.v12.i7.1071
73. Kim J-Y, Lee M, Oh S, Kang B, Yasin M, and Chang IS. Acetogen and acetogenesis for biological syngas valorization. Bioresour Technol. (2023) 384:129368. doi: 10.1016/j.biortech.2023.129368
74. Morotomi M, Nagai F, Watanabe Y, and Tanaka R. Succinatimonas hippei gen. nov., sp. nov., isolated from human faeces. Int J Syst Evol Microbiol. (2010) 60:1788–93. doi: 10.1099/ijs.0.015958-0
75. Dishisha T, Jain M, and Hatti-Kaul R. High cell density sequential batch fermentation for enhanced propionic acid production from glucose and glycerol/glucose mixture using Acidipropionibacterium acidipropionici. Microb Cell Fact. (2024) 23:91. doi: 10.1186/s12934-024-02366-5
76. Wang Z, Wang C, Yuan B, Liu L, Zhang H, Zhu M, et al. Akkermansia muciniphila and its metabolite propionic acid maintains neuronal mitochondrial division and autophagy homeostasis during Alzheimer’s disease pathologic process via GPR41 and GPR43. Microbiome. (2025) 13:16. doi: 10.1186/s40168-024-02001-w
77. Liu Z, Wang M, Li J, Guo X, Guo Q, and Zhu B. Differences in utilization and metabolism of Ulva lactuca polysaccharide by human gut Bacteroides species in the in vitro fermentation. Carbohydr Polym. (2025) 351:123126. doi: 10.1016/j.carbpol.2024.123126
78. Loo YT, Howell K, Suleria H, Zhang P, Gu C, and Ng K. Sugarcane polyphenol and fiber to affect production of short-chain fatty acids and microbiota composition using in vitro digestion and pig faecal fermentation model. Food Chem. (2022) 385:132665. doi: 10.1016/j.foodchem.2022.132665
79. Pan L, Wang L, Zeng Z, Zhang Z, Zheng B, and Zhang Y. Chemical structure and prebiotic activity of a Dictyophora indusiata polysaccharide fraction. Food Chem. (2025) 463:1–9. doi: 10.1016/j.foodchem.2024.141086
80. Hao Z, Meng C, Li L, Feng S, Zhu Y, Yang J, et al. Positive mood-related gut microbiota in a long-term closed environment: a multiomics study based on the “Lunar Palace 365” experiment. Microbiome. (2023) 11:88. doi: 10.1186/s40168-023-01506-0
81. Li N, Wang H, Zhao H, Wang M, Cai J, Hao Y, et al. Cooperative interactions between Veillonella ratti and Lactobacillus acidophilus ameliorate DSS-induced ulcerative colitis in mice. Food Funct. (2023) 14:10475–92. doi: 10.1039/d3fo03898j
82. Abdugheni R, Wang W-Z, Wang Y-J, Du M-X, Liu F-L, Zhou N, et al. Metabolite profiling of human-originated Lachnospiraceae at the strain level. Imeta. (2022) 1:e58. doi: 10.1002/imt2.58
83. Lessard-Lord J, Roussel C, Lupien-Meilleur J, Généreux P, Richard V, Guay V, et al. Short term supplementation with cranberry extract modulates gut microbiota in human and displays a bifidogenic effect. NPJ Biofilms Microbiomes. (2024) 10:18. doi: 10.1038/s41522-024-00493-w
84. Song C-H, Kim N, Nam RH, Choi SI, Jang JY, Kim EH, et al. The possible preventative role of lactate- and butyrate-producing bacteria in colorectal carcinogenesis. Gut Liver. (2024) 18:654–66. doi: 10.5009/gnl230385
85. Nuzum ND, Szymlek-Gay EA, Loke S, Dawson SL, Teo W-P, Hendy AM, et al. Differences in the gut microbiome across typical ageing and in Parkinson’s disease. Neuropharmacology. (2023) 235:109566. doi: 10.1016/j.neuropharm.2023.109566
86. Yao H, Wang L, Tang X, Yang Z, Li H, Sun C, et al. Two novel polysaccharides from Solanum nigrum L. exert potential prebiotic effects in an in vitro fermentation model. Int J Biol Macromol. (2020) 159:648–58. doi: 10.1016/j.ijbiomac.2020.05.121
87. Xu Y, Shao M, Fang X, Tang W, Zhou C, Hu X, et al. Antipsychotic-induced gastrointestinal hypomotility and the alteration in gut microbiota in patients with schizophrenia. Brain Behav Immun. (2022) 99:119–29. doi: 10.1016/j.bbi.2021.09.014
88. Huang X, Geng H, Liang C, Xiong X, Du X, Zhuan Q, et al. Leonurine restrains granulosa cell ferroptosis through SLC7A11/GPX4 axis to promote the treatment of polycystic ovary syndrome. Free Radic Biol Med. (2025) 226:330–47. doi: 10.1016/j.freeradbiomed.2024.11.021
89. Gao H, Sun M, Li A, Gu Q, Kang D, Feng Z, et al. Microbiota-derived IPA alleviates intestinal mucosal inflammation through upregulating Th1/Th17 cell apoptosis in inflammatory bowel disease. Gut Microbes. (2025) 17:2467235. doi: 10.1080/19490976.2025.2467235
90. Ballanti M, Antonetti L, Mavilio M, Casagrande V, Moscatelli A, Pietrucci D, et al. Decreased circulating IPA levels identify subjects with metabolic comorbidities: A multi-omics study. Pharmacol Res. (2024) 204:107207. doi: 10.1016/j.phrs.2024.107207
91. Finley JW, Burrell JB, and Reeves PG. Pinto bean consumption changes SCFA profiles in fecal fermentations, bacterial populations of the lower bowel, and lipid profiles in blood of humans. J Nutr. (2007) 137:2391–8. doi: 10.1093/jn/137.11.2391
92. Pogribna M, Freeman JP, Paine D, and Boudreau MD. Effect of Aloe vera whole leaf extract on short chain fatty acids production by Bacteroides fragilis, Bifidobacterium infantis and Eubacterium limosum. Lett Appl Microbiol. (2008) 46:575–80. doi: 10.1111/j.1472-765X.2008.02346.x
93. Litty D and Müller V. Butyrate production in the acetogen Eubacterium limosum is dependent on the carbon and energy source. Microb Biotechnol. (2021) 14:2686–92. doi: 10.1111/1751-7915.13779
94. Flaiz M, Poehlein A, Wilhelm W, Mook A, Daniel R, Dürre P, et al. Refining and illuminating acetogenic Eubacterium strains for reclassification and metabolic engineering. Microb Cell Fact. (2024) 23:24. doi: 10.1186/s12934-024-02301-8
95. Liu X, Lu B, Tang H, Jia X, Zhou Q, Zeng Y, et al. Gut microbiome metabolites, molecular mimicry, and species-level variation drive long-term efficacy and adverse event outcomes in lung cancer survivors. EBioMedicine. (2024) 109:105427. doi: 10.1016/j.ebiom.2024.105427
96. Cao W, Zheng C, Xu X, Jin R, Huang F, Shi M, et al. Clostridium butyricum potentially improves inflammation and immunity through alteration of the microbiota and metabolism of gastric cancer patients after gastrectomy. Front Immunol. (2022) 13:1076245. doi: 10.3389/fimmu.2022.1076245
97. Zhang Q, Hu W-M, Deng Y-L, Wan J-J, Wang Y-J, and Jin P. Dysbiosis of gut microbiota and decreased propionic acid associated with metabolic abnormality in Cushing’s syndrome. Front Endocrinol (Lausanne). (2022) 13:1095438. doi: 10.3389/fendo.2022.1095438
98. Zhang H, Wang X, Zhen L, Chang Q, Cui L, and Xue Z. Composition and metabolite patterns of caproic acid-producing bacteria during pH-mediated pitmud-Huangshui co-fermentation based on multi-database annotation. Food Chem. (2025) 473:143096. doi: 10.1016/j.foodchem.2025.143096
99. Jian C, Silvestre MP, Middleton D, Korpela K, Jalo E, Broderick D, et al. Gut microbiota predicts body fat change following a low-energy diet: a PREVIEW intervention study. Genome Med. (2022) 14:54. doi: 10.1186/s13073-022-01053-7
100. Kang X, Liu C, Ding Y, Ni Y, Ji F, Lau HCH, et al. Roseburia intestinalis generated butyrate boosts anti-PD-1 efficacy in colorectal cancer by activating cytotoxic CD8+ T cells. Gut. (2023) 72:2112–22. doi: 10.1136/gutjnl-2023-330291
101. Ma H, Yu Z, Zhao Y, Li L, Liu Y, and Liu Y. Goat milk fermented with combined lactic acid bacterium alter microbial community structures and levels of the targeted short-chain fatty acids in the large intestine of mice. Food Res Int. (2022) 157:111352. doi: 10.1016/j.foodres.2022.111352
102. Ding M, Ning Y, Song L, Yu X, Yan B, Li P, et al. Clinical features and risk factors of older adults with bloodstream infection. BMC Geriatr. (2025) 25:397. doi: 10.1186/s12877-025-05934-5
103. Lin H-Y, Huang C-C, and Chang K-F. Lipopolysaccharide preconditioning reduces neuroinflammation against hypoxic ischemia and provides long-term outcome of neuroprotection in neonatal rat. Pediatr Res. (2009) 66:254–9. doi: 10.1203/PDR.0b013e3181b0d336
104. Eder K, Vizler C, Kusz E, Karcagi I, Glavinas H, Balogh GE, et al. The role of lipopolysaccharide moieties in macrophage response to Escherichia coli. Biochem Biophys Res Commun. (2009) 389:46–51. doi: 10.1016/j.bbrc.2009.08.082
105. Wang X, Hu X, Ye C, Zhao J, Tan SC, Zhou L, et al. Astragalus Polysaccharide Enhances Voriconazole Metabolism under Inflammatory Conditions through the Gut Microbiota. J Clin Trans Hepatol. (2024) 12:481–95. doi: 10.14218/JCTH.2024.00024
106. Wang X, Ye C, Xun T, Mo L, Tong Y, Ni W, et al. Bacteroides fragilis polysaccharide A ameliorates abnormal voriconazole metabolism accompanied with the inhibition of TLR4/NF-κB pathway. Front In Pharmacol. (2021) 12:663325. doi: 10.3389/fphar.2021.663325
107. Garcia-Bonilla L, Brea D, Benakis C, Lane DA, Murphy M, Moore J, et al. Endogenous protection from ischemic brain injury by preconditioned monocytes. J Neurosci. (2018) 38:6722–36. doi: 10.1523/JNEUROSCI.0324-18.2018
108. Schaafsma W, Zhang X, van Zomeren KC, Jacobs S, Georgieva PB, Wolf SA, et al. Long-lasting pro-inflammatory suppression of microglia by LPS-preconditioning is mediated by RelB-dependent epigenetic silencing. Brain Behav Immun. (2015) 48:205–21. doi: 10.1016/j.bbi.2015.03.013
109. Okun E, Griffioen KJ, and Mattson MP. Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci. (2011) 34:269–81. doi: 10.1016/j.tins.2011.02.005
110. Fields CT, Chassaing B, Castillo-Ruiz A, Osan R, Gewirtz AT, and de Vries GJ. Effects of gut-derived endotoxin on anxiety-like and repetitive behaviors in male and female mice. Biol Sex Differ. (2018) 9:7. doi: 10.1186/s13293-018-0166-x
111. Pittman DW, Dong G, Brantly AM, He L, Nelson TS, Kogan S, et al. Behavioral and neurophysiological taste responses to sweet and salt are diminished in a model of subclinical intestinal inflammation. Sci Rep. (2020) 10:17611. doi: 10.1038/s41598-020-74632-6
112. Kobayashi Y, Inagawa H, Kohchi C, Okazaki K, Zhang R, Kobara H, et al. Effect of lipopolysaccharide derived from pantoea agglomerans on the phagocytic activity of amyloid β by primary murine microglial cells. Anticancer Res. (2017) 37(7):3917–20. doi: 10.21873/anticanres.11774
113. Brown DG, Soto R, Yandamuri S, Stone C, Dickey L, Gomes-Neto JC, et al. The microbiota protects from viral-induced neurologic damage through microglia-intrinsic TLR signaling. Elife. (2019) 8:1–22. doi: 10.7554/eLife.47117
114. Brown GC. The endotoxin hypothesis of neurodegeneration. J Neuroinflamm. (2019) 16:180. doi: 10.1186/s12974-019-1564-7
115. Zhang R, Miller RG, Gascon R, Champion S, Katz J, Lancero M, et al. Circulating endotoxin and systemic immune activation in sporadic amyotrophic lateral sclerosis (sALS). J Neuroimmunol. (2009) 206:121–4. doi: 10.1016/j.jneuroim.2008.09.017
116. Shin SJ, Park YH, Nam Y, Chung H, Kim S, and Moon M. Localization and pathological implications of lipopolysaccharide in the brains of 5XFAD mice. Mol Neurobiol. (2025) 62:1–15. doi: 10.1007/s12035-025-05107-w
117. Mutovina A, Ayriyants K, Mezhlumyan E, Ryabushkina Y, Litvinova E, Bondar N, et al. Unique features of the immune response in BTBR mice. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms232415577
118. Sandiego CM, Gallezot J-D, Pittman B, Nabulsi N, Lim K, Lin S-F, et al. Imaging robust microglial activation after lipopolysaccharide administration in humans with PET. Proc Natl Acad Sci U.S.A. (2015) 112:12468–73. doi: 10.1073/pnas.1511003112
119. Pei T, Li W, Zhou Z, Zhang Q, Yu G, Yin S, et al. The relationship between tryptophan metabolism and gut microbiota: Interaction mechanism and potential effects in infection treatment. Microbiol Res. (2025) 298:128211. doi: 10.1016/j.micres.2025.128211
120. Zhao L-P, Wu J, Quan W, Zhou Y, Hong H, Niu G-Y, et al. DSS-induced acute colitis causes dysregulated tryptophan metabolism in brain: an involvement of gut microbiota. J Nutr Biochem. (2023) 115:109282. doi: 10.1016/j.jnutbio.2023.109282
121. Wu C, Diao M, Yu S, Xi S, Zheng Z, Cao Y, et al. Gut microbial tryptophan metabolism is involved in post-cardiac arrest brain injury via pyroptosis modulation. CNS Neurosci Ther. (2025) 31:e70381. doi: 10.1111/cns.70381
122. Bakker L, Ramakers IHGB, van Greevenbroek MMJ, Backes WH, Jansen JFA, Schram MT, et al. The kynurenine pathway and markers of neurodegeneration and cerebral small vessel disease: The Maastricht Study. J Neurol Sci. (2025) 474:123522. doi: 10.1016/j.jns.2025.123522
123. Di Filippo M, Gaetani L, Centonze D, Hegen H, Kuhle J, Teunissen CE, et al. Fluid biomarkers in multiple sclerosis: from current to future applications. Lancet Reg Health Eur. (2024) 44:101009. doi: 10.1016/j.lanepe.2024.101009
124. Mirza A, Forbes JD, Zhu F, Bernstein CN, Van Domselaar G, Graham M, et al. The multiple sclerosis gut microbiota: A systematic review. Mult Scler Relat Disord. (2020) 37:101427. doi: 10.1016/j.msard.2019.101427
125. Berer K, Gerdes LA, Cekanaviciute E, Jia X, Xiao L, Xia Z, et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci U.S.A. (2017) 114:10719–24. doi: 10.1073/pnas.1711233114
126. Cosorich I, Dalla-Costa G, Sorini C, Ferrarese R, Messina MJ, Dolpady J, et al. High frequency of intestinal TH17 cells correlates with microbiota alterations and disease activity in multiple sclerosis. Sci Adv. (2017) 3:e1700492. doi: 10.1126/sciadv.1700492
127. Balasa R, Barcutean L, Balasa A, Motataianu A, Roman-Filip C, and Manu D. The action of TH17 cells on blood brain barrier in multiple sclerosis and experimental autoimmune encephalomyelitis. Hum Immunol. (2020) 81:237–43. doi: 10.1016/j.humimm.2020.02.009
128. Agus A, Planchais J, and Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. (2018) 23:716–24. doi: 10.1016/j.chom.2018.05.003
129. Gutiérrez-Vázquez C and Quintana FJ. Regulation of the immune response by the aryl hydrocarbon receptor. Immunity. (2018) 48:19–33. doi: 10.1016/j.immuni.2017.12.012
130. Tanaka M, Fujikawa M, Oguro A, Itoh K, Vogel CFA, and Ishihara Y. Involvement of the microglial aryl hydrocarbon receptor in neuroinflammation and vasogenic edema after ischemic stroke. Cells. (2021) 10:1–17. doi: 10.3390/cells10040718
131. Matsumoto K, Kinoshita K, Yoshimizu A, Kurauchi Y, Hisatsune A, Seki T, et al. Laquinimod and 3,3’-diindolylemethane alleviate neuropathological events and neurological deficits in a mouse model of intracerebral hemorrhage. J Neuroimmunol. (2020) 342:577195. doi: 10.1016/j.jneuroim.2020.577195
132. Yang Y, Wang N, Xu L, Liu Y, Huang L, Gu M, et al. Aryl hydrocarbon receptor dependent anti-inflammation and neuroprotective effects of tryptophan metabolites on retinal ischemia/reperfusion injury. Cell Death Dis. (2023) 14:92. doi: 10.1038/s41419-023-05616-3
133. Karmoker JR, Bounds SE, and Cai J. Aryl hydrocarbon receptor (AhR)-mediated immune responses to degeneration of the retinal pigment epithelium. Biochim Biophys Acta Mol Basis Dis. (2024) 1870:167351. doi: 10.1016/j.bbadis.2024.167351
134. Peesh P, Blasco-Conesa MP, El Hamamy A, Khan R, Guzman GU, Honarpisheh P, et al. Benefits of equilibrium between microbiota- and host-derived ligands of the aryl hydrocarbon receptor after stroke in aged male mice. Nat Commun. (2025) 16:1767. doi: 10.1038/s41467-025-57014-2
135. Lee Y-H, Lin C-H, Hsu P-C, Sun Y-Y, Huang Y-J, Zhuo J-H, et al. Aryl hydrocarbon receptor mediates both proinflammatory and anti-inflammatory effects in lipopolysaccharide-activated microglia. Glia. (2015) 63:1138–54. doi: 10.1002/glia.22805
136. Chen W-C, Chang L-H, Huang S-S, Huang Y-J, Chih C-L, Kuo H-C, et al. Aryl hydrocarbon receptor modulates stroke-induced astrogliosis and neurogenesis in the adult mouse brain. J Neuroinflamm. (2019) 16:187. doi: 10.1186/s12974-019-1572-7
137. Rothhammer V, Borucki DM, Tjon EC, Takenaka MC, Chao C-C, Ardura-Fabregat A, et al. Microglial control of astrocytes in response to microbial metabolites. Nature. (2018) 557:724–8. doi: 10.1038/s41586-018-0119-x
138. Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. (2016) 22:586–97. doi: 10.1038/nm.4106
139. Garcez ML, Tan VX, Heng B, and Guillemin GJ. Sodium butyrate and indole-3-propionic acid prevent the increase of cytokines and kynurenine levels in LPS-induced human primary astrocytes. Int J Tryptophan Res. (2020) 13:1178646920978404. doi: 10.1177/1178646920978404
140. Kaye J, Piryatinsky V, Birnberg T, Hingaly T, Raymond E, Kashi R, et al. Laquinimod arrests experimental autoimmune encephalomyelitis by activating the aryl hydrocarbon receptor. Proc Natl Acad Sci U.S.A. (2016) 113:E6145–52. doi: 10.1073/pnas.1607843113
141. Rothhammer V, Kenison JE, Li Z, Tjon E, Takenaka MC, Chao C-C, et al. Aryl hydrocarbon receptor activation in astrocytes by laquinimod ameliorates autoimmune inflammation in the CNS. Neurol Neuroimmunol Neuroinflamm. (2021) 8:1–11. doi: 10.1212/NXI.0000000000000946
142. Barin JG, Talor MV, Diny NL, Ong S, Schaub JA, Gebremariam E, et al. Regulation of autoimmune myocarditis by host responses to the microbiome. Exp Mol Pathol. (2017) 103:141–52. doi: 10.1016/j.yexmp.2017.08.003
143. Yu HB, Yang H, Allaire JM, Ma C, Graef FA, Mortha A, et al. Vasoactive intestinal peptide promotes host defense against enteric pathogens by modulating the recruitment of group 3 innate lymphoid cells. Proc Natl Acad Sci U.S.A. (2021) 118:1–12. doi: 10.1073/pnas.2106634118
144. Martin E and Delarasse C. Complex role of chemokine mediators in animal models of Alzheimer’s Disease. BioMed J. (2018) 41:34–40. doi: 10.1016/j.bj.2018.01.002
145. Grozdanov V, Bliederhaeuser C, Ruf WP, Roth V, Fundel-Clemens K, Zondler L, et al. Inflammatory dysregulation of blood monocytes in Parkinson’s disease patients. Acta Neuropathol. (2014) 128:651–63. doi: 10.1007/s00401-014-1345-4
146. Qu Y, Li J, Qin Q, Wang D, Zhao J, An K, et al. A systematic review and meta-analysis of inflammatory biomarkers in Parkinson’s disease. NPJ Parkinsons Dis. (2023) 9:18. doi: 10.1038/s41531-023-00449-5
147. Vida H, Sahar M, Nikdouz A, and Arezoo H. Chemokines in neurodegenerative diseases. Immunol Cell Biol. (2025) 103:275–92. doi: 10.1111/imcb.12843
148. Zhang B, Wang HE, Bai Y-M, Tsai S-J, Su T-P, Chen T-J, et al. Inflammatory bowel disease is associated with higher dementia risk: a nationwide longitudinal study. Gut. (2021) 70:85–91. doi: 10.1136/gutjnl-2020-320789
149. Navalpur Shanmugam NK, Zamudio F, Vijaya Kumar DK, Barrett KA, VanDoren R, Chen M, et al. Acute experimental colitis in 5xFAD Alzheimer’s disease mice leads to enhanced monocyte infiltration into the brain accompanied by reduced β-amyloid deposition. Alzheimers Dement. (2025) 21:e70292. doi: 10.1002/alz.70292
150. Li Y and Gu BJ. Non-human primate models of alzheimer’s disease. J Explor Res Pharmacol. (2023) 8:199–221. doi: 10.14218/jerp.2023.00006
151. Park G, Kadyan S, Hochuli N, Salazar G, Laitano O, Chakrabarty P, et al. An enteric bacterial infection triggers neuroinflammation and neurobehavioral impairment in 3xTg-AD transgenic mice. J Infect Dis. (2024) 230:S95–S108. doi: 10.1093/infdis/jiae165
152. Munoz-Pinto MF, Candeias E, Melo-Marques I, Esteves AR, Maranha A, Magalhães JD, et al. Gut-first Parkinson’s disease is encoded by gut dysbiome. Mol Neurodegener. (2024) 19:78. doi: 10.1186/s13024-024-00766-0
153. Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of parkinson’s disease. Cell. (2016) 167:1469–80. doi: 10.1016/j.cell.2016.11.018
154. Steimle A, Neumann M, Grant ET, Willieme S, De Sciscio A, Parrish A, et al. Gut microbial factors predict disease severity in a mouse model of multiple sclerosis. Nat Microbiol. (2024) 9:2244–61. doi: 10.1038/s41564-024-01761-3
Keywords: gut microbiota, microglia, microbiota-gut-brain axis, aromatic compounds, neuroinflammation
Citation: Zhao S, Fu D, Lin Y, Sun X, Wang X, Wu X and Zhang X (2025) The role of the microbiome on immune homeostasis of the host nervous system. Front. Immunol. 16:1609960. doi: 10.3389/fimmu.2025.1609960
Received: 11 April 2025; Accepted: 15 July 2025;
Published: 31 July 2025.
Edited by:
Teresa Zelante, University of Perugia, ItalyReviewed by:
Panida Sittipo, Burapha University, ThailandMin Zhang, University of Kentucky, United States
Copyright © 2025 Zhao, Fu, Lin, Sun, Wang, Wu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuejiao Zhang, Mjg3MzUzMzU3QHFxLmNvbQ==; Xuanzhen Wu, dGdfd3h6QDE2My5jb20=
 Shaojuan Zhao
Shaojuan Zhao Danlei Fu2
Danlei Fu2 Xiaokang Wang
Xiaokang Wang