- Department of Pathology, China-Japan Union Hospital, Jilin University, Changchun, Jilin, China
Overexpression of monocarboxylate transporter 1 (MCT1) in tumor cells is often associated with poor prognosis. The established mechanisms through which MCT1 and its mediated lactate transport drive tumor progression are manifold. The classical mechanisms include fostering metabolic symbiosis among tumor cells, dampening the immune function of immune cells, and spurring tumor angiogenesis. Beyond these, new findings of MCT1’s role in tumor progression have emerged. These new findings highlight MCT1’s involvement in mediating the reverse Warburg effect, inhibiting ferroptosis, promoting protective autophagy, and augmenting tumor glycolysis. When acetate serves as a transport substrate for MCT1, additional mechanisms come into play. These encompass MCT1’s participation in the acetylation of histone H3K27 and its role in upregulating c-Myc levels. Several studies have demonstrated that while selective MCT1 inhibitors can effectively impede tumor progression, they also face notable challenges. To address these, combining MCT1 inhibitors with other drugs appears to hold more promise.
Introduction
Aerobic glycolysis is a hallmark of malignant tumor metabolism (1), where tumor cells prefer this process over oxidative phosphorylation for energy production, even in the presence of ample oxygen (2). This metabolic preference leads to the production and extracellular release of lactate, which acidifies the tumor microenvironment (TME) (3). Members of the monocarboxylate transporter (MCT) family, particularly MCT1, play a crucial role in tumor progression by facilitating lactate transport (4). MCT1, encoded by the SLC16A1 gene (5), is one of the 14 MCTs(MCT1-14) family members and is expressed in nearly all human tissues (6). Its expression and function are primarily regulated by p53 and MYC (7). Research by Boidot et al. demonstrated that p53 directly interacted with the MCT1 gene promoter to inhibit its expression (8). In a study involving neuroblastoma and Burkitt’s lymphoma by Gan et al, MYC directly activated transcription of the MCT1 by binding to a specific recognition site of the gene (9). Additionally, MYC repressed the transcription of miR29a and miR29c, which in turn increased the expression of their target protein, MCT1 (9). CD147, a transmembrane glycoprotein (10), is often co-expressed with MCT1 (11). MCT1 can function as a substrate transporter when it binds to CD147 (4). MCT1 facilitates the transmembrane transport of various substrates, including lactate (12), acetate (13), pyruvate (12), butyrate (14), ketone bodies (15), β-hydroxybutyrate (16), and succinate (17). Although MCT1 can transport multiple substrates, its primary physiological role is to mediate lactate entry into cells and, in conjunction with monocarboxylate transporter 4 (MCT4), lactate export out of cells (1). This process is modulated by the cell’s metabolic state and the intra- and extracellular concentrations of lactate and protons (15). Lactate, while not the only substrate for MCT1, is the most extensively studied and prevalent in vivo, particularly in tumors, where concentrations can reach up to 40 mM (4).
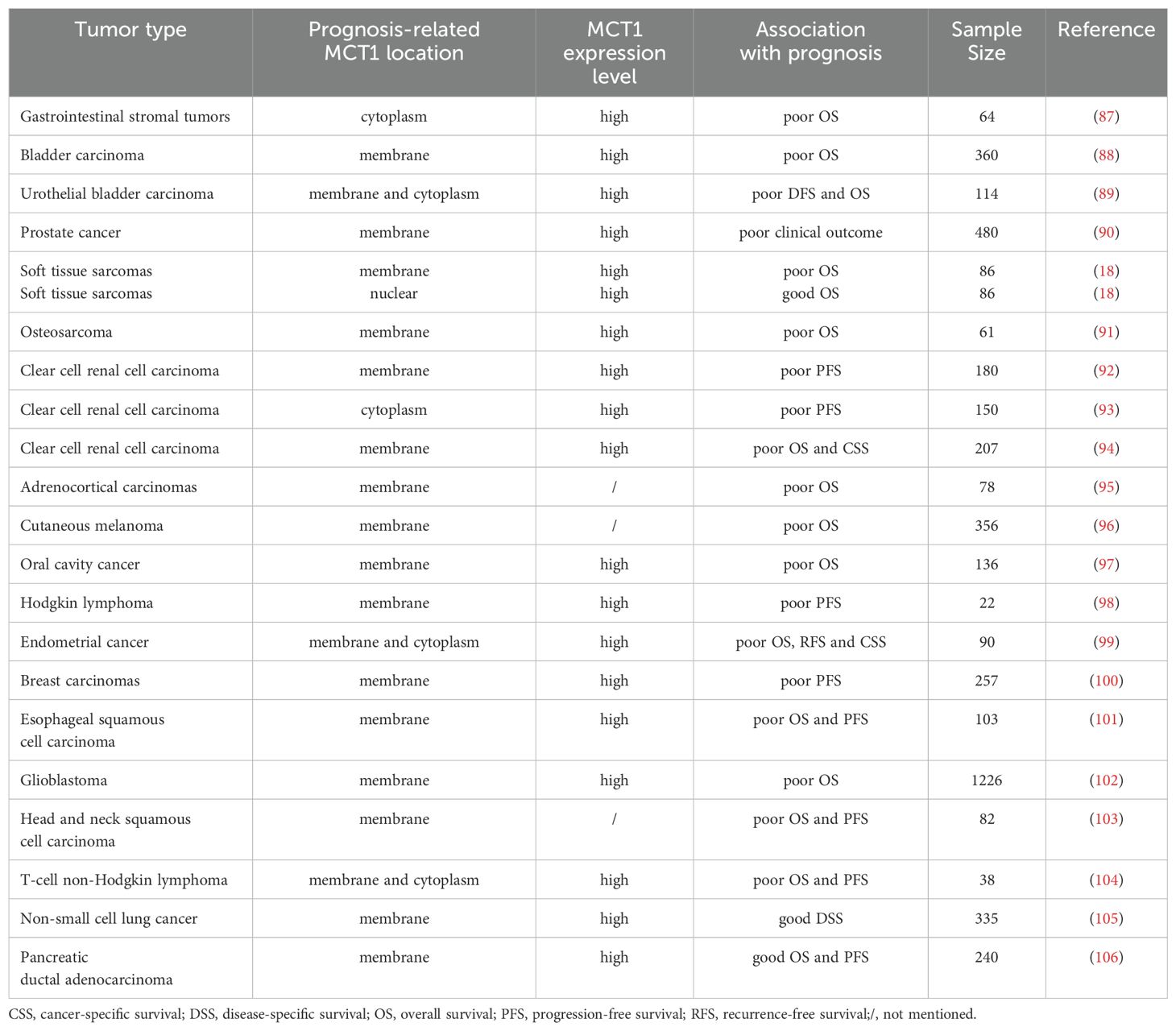
MCT1 is often indicative of a poor prognosis in patients with malignant tumors, as its expression in tumor cells is frequently associated with reduced survival rates. Typically, MCT1 is localized to the cell membrane. However, in some studies, MCT1 has also been detected in the cytoplasm or nucleus. For instance, in a study involving soft tissue sarcomas by Pinheiro et al, different localizations of MCT1 were found to predict varying prognoses: cytomembrane localization of MCT1 was linked to a poor prognosis, while nuclear localization was associated with a better outcome (18). Similarly, in a study involving diffuse large B-cell lymphoma by Afonso et al, MCT1 was found in the nucleus in a few samples (19). Due to the limited number of samples in this research, the relationship between nuclear expression of MCT1 in tumor cells and prognosis remained unclear. Table 1 provides a summary of the relationship between MCT1 expression patterns in tumor cells and the prognosis.
Although the classical mechanisms of MCT1 involvement in tumor progression have been elucidated, tumor-promoting studies of MCT1 through novel mechanisms, such as ferroptosis, remain scattered. In this paper, we will systematically review the multidimensional role of MCT1 in tumor progression and explore potential strategies for its targeted therapy.
Classical mechanisms of MCT1 in tumor progression
SLC16A1, the gene encoding MCT1, appears to function as a proto-oncogene. SLC16A1 induces mutations in mismatch repair (MMR) genes and increases the activity of DNA methyltransferases (DNMTs) in urologic tumors (5). Elevated levels of DNMT are important for tumor progression (20), as are MMR gene mutations (21). In tumor-related studies, MCT1 and its mediated lactate transport can contribute to tumor progression by promoting metabolic symbiosis among tumor cells, inhibiting the immune function of immune cells, and promoting tumor angiogenesis. Figure 1 summarizes the classical mechanisms of MCT1 in tumor progression.
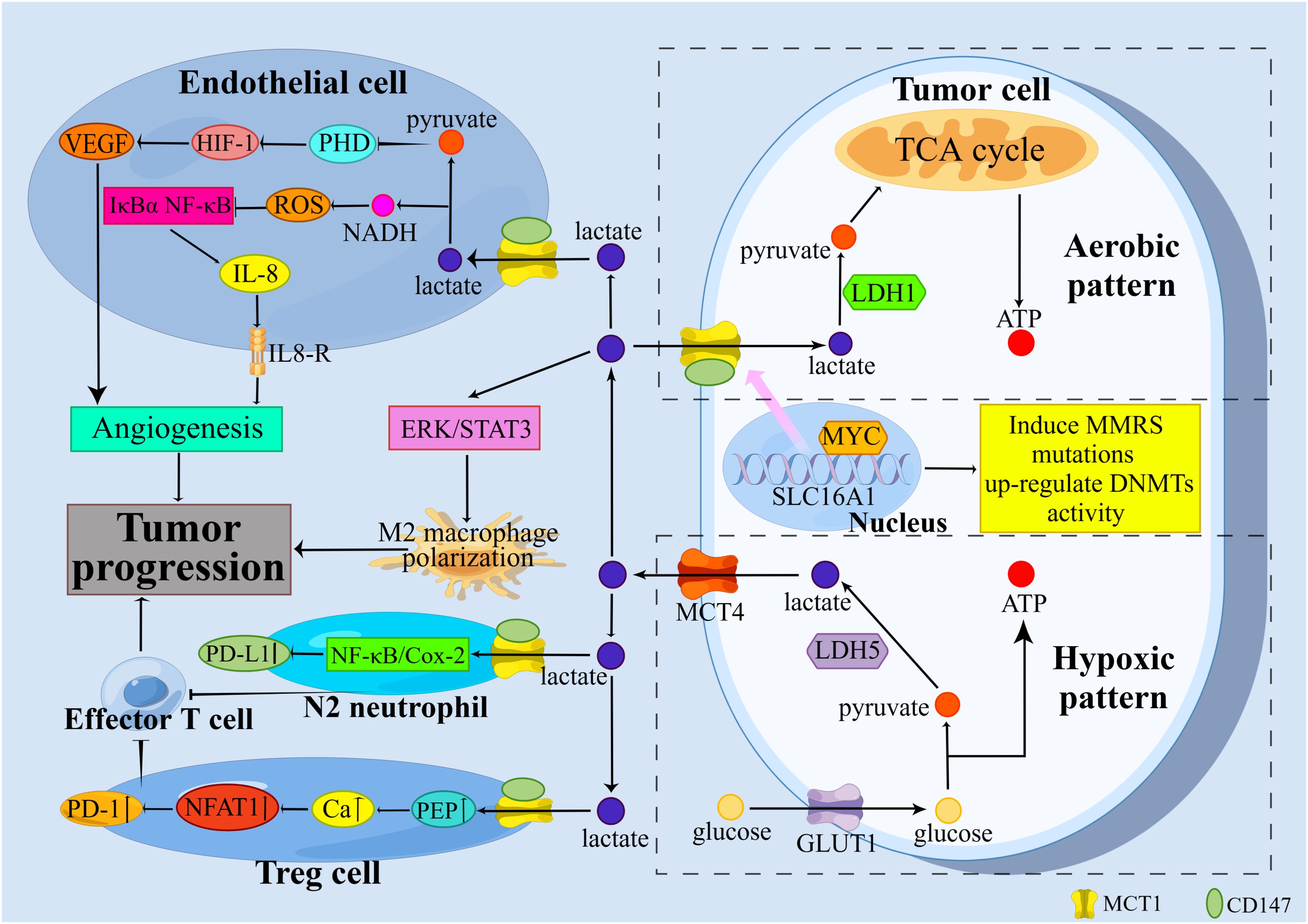
Figure 1. Classical mechanisms by which MCT1 promotes tumor progression. MCT1 promotes tumor progression through the promotion of metabolic symbiosis among tumor cells, the suppression of immune function in immune cells, and the promotion of tumor angiogenesis.
MCT1 mediates metabolic symbiosis
The Warburg effect, characterized by a preference for aerobic glycolysis over oxidative phosphorylation, significantly enhances the aggressiveness of tumor cells exhibiting this metabolic phenotype (3). According to the metabolic symbiosis hypothesis, tumor cells can be divided into two distinct subtypes: oxygenated tumor cells, which are located near blood vessels, and hypoxic tumor cells, which are situated further away from blood vessels (22, 23). To address ATP deficiency and enhance glucose uptake, hypoxic tumor cells upregulate the expression of GLUT1. Hypoxia-inducible factor-1 (HIF-1) facilitates the conversion of glucose to pyruvate and subsequently to lactate in the presence of lactate dehydrogenase-5 (LDH-5). This process promotes the regeneration of nicotinamide adenine dinucleotide (NAD+), which is essential for maintaining a high glycolytic flux. Lactate and hypoxia-stimulated hypoxia-inducible factor-1α (HIF-1α) or WNT/β-catenin signaling prevent pyruvate from entering the TCA cycle and converting to acetyl-CoA by upregulating pyruvate dehydrogenase kinase 1 (PDK1) and then inhibiting pyruvate dehydrogenase complex (PDC). This drives tumor cells to gain energy through aerobic glycolysis. Finally, MCT4 removes lactate produced in hypoxic tumor cells to avoid intracellular acidification. Oxygenated tumor cells transport lactate produced by hypoxic tumor cells through MCT1 and produce ATP by oxidizing lactate (24).
Oxidative lactate metabolism offers several advantages to oxygenated tumor cells compared to glucose-dependent respiration. Specifically, ATP production through lactate oxidation is 7.5 times greater than that produced through aerobic glycolysis. Tumor cells prioritize lactate utilization to conserve energy required for the synthesis and maintenance of glycolytic enzymes, as well as for the phosphorylation of glucose and fructose-6-phosphate (F6P) during glycolysis. Additionally, the oxidation of lactate by LDH-1 generates ample nicotinamide adenine dinucleotide (NADH), which serves as a fuel source for the mitochondrial electron transport chain (ETC). Ultimately, the LDH-1 catalyzed reaction may facilitate lysosomal acidification and the maturation of autophagy vesicles. Autophagy allows oxidized proteins and organelles to be recycled (4).
MCT1 leads to immunosuppression
In addition to its role in metabolic regulation, MCT1 also promotes tumor progression by modulating the immune microenvironment.
Macrophages can differentiate into either M1 or M2 phenotypes (25), M1 macrophages exhibit anti-tumor properties, while M2 macrophages promote tumor growth (26). Tumor cells release lactate via MCT1 or MCT4, which can influence the polarization of M2 macrophages (27). In a study involving breast cancer by Mu et al, it was observed that tumor-derived lactate facilitated the polarization of M2 macrophages through the activation of the extracellular regulated protein kinase (ERK)/signal transducer and activator of transcription 3 (STAT3) signaling pathway (28). In addition, M2 macrophages were found to release immunosuppressive cytokines that can stimulate the development of regulatory T cells (Treg cells) (29).
Treg cells suppress anti-tumor immune responses, while effector T cells play a crucial role in combating tumors (30, 31). The TME facilitates the recruitment and differentiation of Treg cells by upregulating forkhead box proteins P3 (FOXP3) and MCT1. Increased FOXP3 expression enhances the adaptability of Treg cells to the high-lactate TME by suppressing c-Myc and glycolysis, while promoting oxidative phosphorylation (OXPHOS) and increasing NAD+ oxidation (32). Treg cells uptake lactate via MCT1, where it is metabolized intracellularly to phosphoenolpyruvate (PEP). This metabolic process leads to an increase in cytoplasmic calcium ion concentration, facilitating the translocation of nuclear factor of activated T-cells 1 (NFAT1) to the nucleus. This enhances the expression of programmed death-1 (PD-1), while PD-1 expression in effector T cells is inhibited (33). PD-1 blockade invigorates PD-1-expressing Treg cells, leading to treatment failure (33). Activated cytotoxic T lymphocytes (CTLs) predominantly utilize glycolysis for energy production, with the resulting lactate being excreted via MCT1 to sustain their cytokine production and cytotoxic functions (34). Elevated lactate levels within the TME impede T cell function by suppressing lactate efflux (35), thereby creating a conducive environment for tumor cell survival.
Neutrophils, similar to macrophages, exhibit two distinct phenotypes: N1 and N2 (36). The N1 phenotype is characterized by cytotoxicity and anti-inflammatory properties, while the N2 phenotype demonstrates immunosuppressive capabilities (36). It has been shown that the transforming growth factor-β (TGF-β)/Smad3 signaling pathway plays a crucial role in the transformation of neutrophils into the N2 phenotype (37). Tumor-derived lactate enters neutrophils via MCT1 and triggers PD-L1 expression through the nuclear factor-kappa B (NF-κB)/cyclooxygenase-2 (COX-2) pathway. Neutrophils expressing PD-L1 have the potential to suppress T cell cytotoxicity (38), thereby facilitating tumor progression.
MCT1 mediates tumor angiogenesis
In oxygenated tumor cells and endothelial cells, lactate is transported into the cell via MCT1 and subsequently oxidized by LDH-1 to produce pyruvate. Pyruvate is characterized as a pseudo-hypoxic signal that influences HIF prolyl hydroxylases (PHDs), particularly PHD2. When oxygen, α-ketoglutarate, and vitamin C are present, this family of enzymes catalyzes the hydroxylation of HIF-1α on two proline residues, targeting this HIF-1 subunit to proteasome-mediated degradation. This hydroxylation leads to the proteasome-mediated degradation of the HIF-1α subunit. Thus, even in the presence of sufficient oxygen, PHDs can be inhibited by oxidants or α-ketoglutarate competitors. The inhibitory effect of pyruvate on PHDs varies by cell type. In oxygenated tumor cells and endothelial cells, pyruvate stably oxidizes HIF-1α, thereby activating HIF-1 and triggering the transcription of vascular endothelial growth factor A (VEGF-A) in tumor cells and VEGF receptor 2 (VEGFR2) as well as basic fibroblast growth factor (bFGF) in endothelial cells (4). Additionally, endothelial cells possess an autocrine pathway for angiogenesis. Lactate uptake by endothelial cells induces the phosphorylation of the inhibitor of κBα (IκBα), leading to the activation of the NF-κB/interleukin-8 (IL-8) signaling pathway, which promotes angiogenesis in tumor cells (22, 39). VEGF-A, VEGFR2, bFGF, and IL-8 are all pro-angiogenic factors that activate their respective receptors to promote neovascularization (24).
New findings that MCT1 promotes tumor progression
Beyond the classical mechanisms of tumor progression that have been previously described, recent studies have unveiled novel roles of MCT1, thereby further elucidating its multifaceted contributions to tumor biology. Figure 2 summarizes the new findings that MCT1 promotes tumor progression.
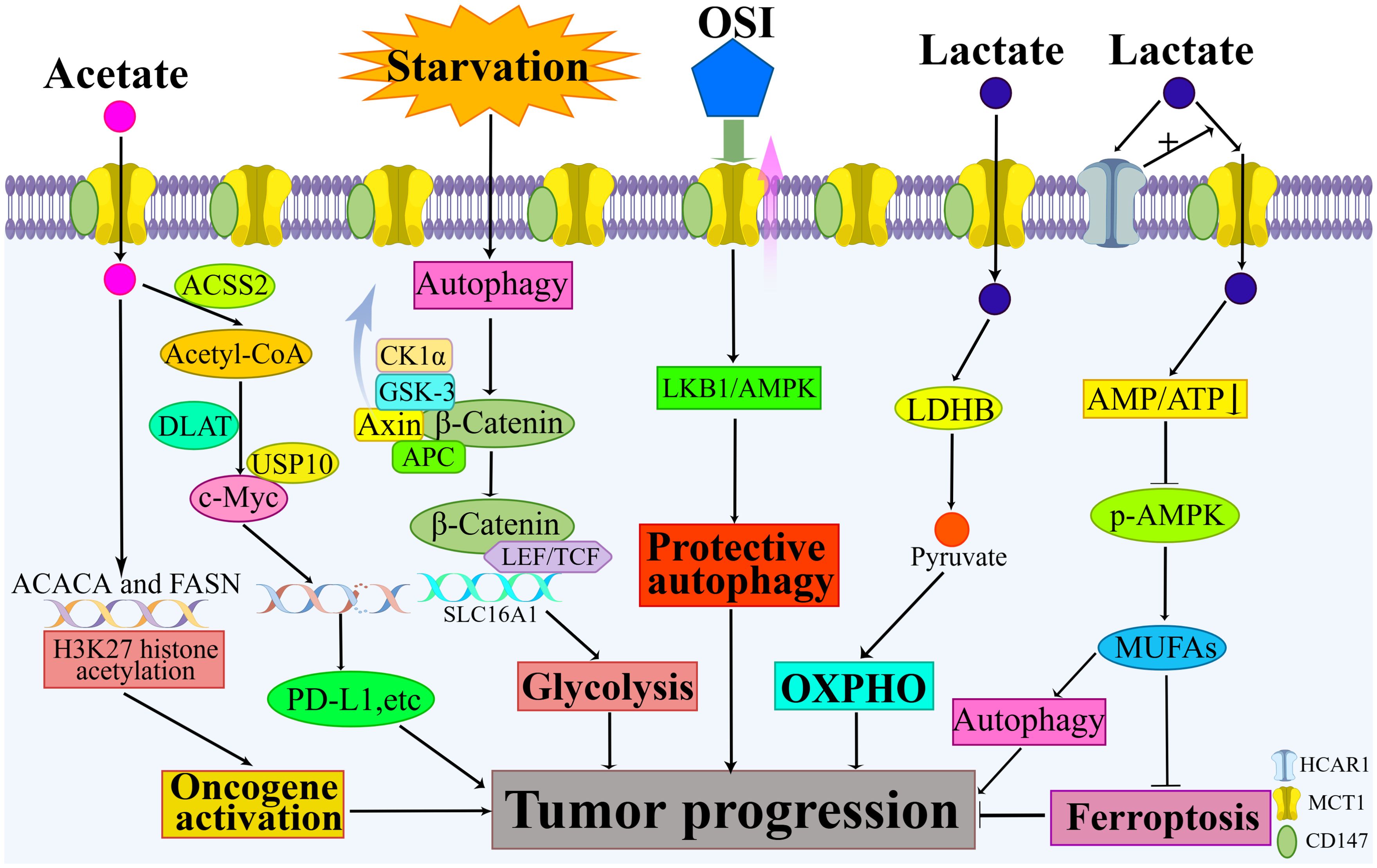
Figure 2. New findings on how MCT1 promotes tumor progression. New findings highlight MCT1’s involvement in mediating the reverse Warburg effect, inhibiting ferroptosis, promoting protective autophagy, and augmenting tumor glycolysis. When acetate serves as a transport substrate for MCT1, additional mechanisms come into play, including MCT1’s participation in the acetylation of histone H3K27 and its role in upregulating c-Myc levels.
MCT1 mediates reverse Warburg effect
The reverse Warburg effect is observed in the interaction between tumor cells and stromal cells. Hydrogen peroxide released by tumor cells induces oxidative stress in stromal cells. This oxidative stress activates hypoxia-inducible factor-1α (HIF-1α) and nuclear factor-kappa B (NF-κB). HIF-1α not only triggers aerobic glycolysis and angiogenesis but also induces autophagy and lysosomal degradation, leading to the loss of caveolin-1 (Cav-1). The loss of Cav-1 amplifies oxidative stress through a positive feed-forward control mechanism and contributes to metabolic changes in stromal cells. As a result, proteins such as MCT1 are highly activated. Lactate produced by stromal cells enters tumor cells via MCT1 for metabolism (40). This metabolic interaction promotes the creation of a nutrient-rich microenvironment that allows tumor cells to meet their metabolic needs (41).
MCT1 promotes tumor progression through associated programmed cell death
Obstruction of ferroptosis favors tumor progression. Ferroptosis is a novel form of programmed cell death (42). The primary feature of ferroptosis is the buildup of lipid peroxides (43). Cellular lipids and lipid metabolism play a pivotal role in regulating ferroptosis (44). Hydroxy-carboxylic acid receptor 1 (HCAR1), a member of the G protein-coupled receptor family (45), acts as a receptor for lactate and modulates its metabolism (46). HCAR1 is expressed on the membrane of various cells, including tumor cells, and its activation through lactate binding results in increased MCT1 expression, thereby facilitating lactate uptake by tumor cells (45). In a study involving hepatocellular carcinoma cells by Zhao et al, these cells exhibited resistance to ferroptosis induced by common ferroptosis inducers. The lactate taken up by MCT1 potentially led to increased ATP production and a decreased AMP: ATP ratio within cellular compartments (47). Disruption of the AMP: ATP balance by lactate can deactivate adenosine monophosphate-activated protein kinase (AMPK), upregulate the expression of sterol regulatory element-binding protein 1 (SREBP1) and its target stearoyl-CoA desaturase 1 (SCD1), ultimately resulting in increased synthesis of anti-ferroptosis monounsaturated fatty acids (MUFAs) and reduced lipid peroxidation (47). Furthermore, MUFAs have been shown to enhance the fluidity and curvature of the lipid bilayer, promote the formation of autophagosomes on the endoplasmic reticulum, and activate autophagy (48). Autophagy can counteract cellular lipotoxicity to some extent and is particularly important for the survival of tumor cells (48).
Evidence suggests that autophagy is indispensable for the progression of malignant tumors in numerous instances (49). Osimertinib (OSI), an epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) (50), has been found to upregulate MCT1 expression in colorectal cancer (CRC) cells (51). This upregulation activated the liver kinase B1 (LKB1)/AMPK signaling pathway, inducing protective autophagy in CRC cells (51). In a study involving hepatocellular carcinoma by Colozza et al, researchers discovered that starvation induced autolysosome production, which activated the Wnt/β-catenin signaling pathway and subsequently upregulated MCT1 expression. This enhanced glycolysis and facilitated tumor metastasis (52). Mechanistically, β-catenin served as a crucial second messenger in the classical Wnt signaling pathway. Under normal physiological conditions, the destruction complex—comprising Axin, adenomatous polyposis coli (APC), glycogen synthase kinase 3 (GSK3), and casein kinase 1α (CK1α)—actively facilitated β-catenin turnover via a proteasome-dependent mechanism. GSK3β-mediated phosphorylation led to the eventual degradation of β-catenin (53). Activation of Wnt signaling led to the binding of the Wnt ligand to its cognate receptors, frizzled (FZD) and lipoprotein receptor-related protein 5 and 6 (LRP5/6). This binding initiated the assembly of a multiprotein complex known as the signalosome and suppressed the activity of the destruction complex (53). The subsequent internalization of the signalosome into early endosomes (EEs), which then matured into multivesicular bodies (MVBs), was essential for the propagation of Wnt signals (53). Consequently, β-catenin was stabilized and translocated to the nucleus, where it cooperated with T-cell factor/lymphoid enhancer-binding factor (TCF/LEF) to activate the transcription and translation of the MCT1 gene (53).
MCT1-mediated acetate transport promotes tumor progression
When used as a substrate for MCT1, acetate can promote tumor progression through various mechanisms. In a study involving clear cell renal cell carcinoma (ccRCC) by Li et al, researchers identified MCT1 as a significant facilitator of ccRCC, with its role in metabolic reprogramming mediated through acetate transport (54). The transportation of acetate into the cell via MCT1, followed by its translocation into the nucleus, resulted in the activation of oncogenes by enhancing the acetylation of histone H3K27 in the promoter region of the FASN and ACACA genes, ultimately promoting tumor progression (54). In a study involving non-small cell lung cancer by Wang et al, the uptake of acetate by tumor cells, facilitated by high expression of MCT1 and catalyzed by ACSS2 to produce acetyl-CoA, increased lipid synthesis in the tumor cells and led to elevated levels of lysine acetylation at position 148 of c-Myc (13). Most importantly, the researchers found that dihydrolipoamide S-acetyltransferase (DLAT) within the pyruvate dehydrogenase complex (PDC) performed a nonclassical metabolic function and mediated the acetylation of c-Myc (13). Acetylated c-Myc increased binding to the deubiquitinating enzyme USP10, promoting c-Myc protein stabilization and subsequent transcriptional activation of PD-L1, LDHA, MCT1, and Cyclin D1 expression (13). Both the mouse in situ tumorigenic lung cancer model and the subcutaneous tumorigenic model demonstrated that the consumption of acetate-containing drinking water inhibits CD8+ T cell infiltration and promotes tumor growth (13).
Advances in MCT1-related inhibitors
Inhibition of MCT1 impedes tumor progression
Given its pivotal role in tumor progression, MCT1 has emerged as a promising therapeutic target. Various research groups have explored strategies to inhibit MCT1, employing both genetic knockdown techniques and pharmacological inhibitors.
Inhibiting MCT1 disrupts the metabolic symbiosis between tumor cells. When MCT1 is inhibited using α-cyano-4-hydroxycinnamate (CHC) or siRNA, lactate transport in oxygenated tumor cells ceases. As a result, these oxygenated tumor cells must increase glucose consumption to compensate for the lack of lactate. This disruption in metabolic symbiosis between oxygenated and hypoxic tumor cells leads to increased glucose uptake by oxygenated tumor cells from nearby blood vessels. Consequently, hypoxic tumor cells experience glucose deprivation, leading to apoptosis. The inhibition of MCT1 in oxygenated tumor cells indirectly causes the death of hypoxic tumor cells (55). This underscores MCT1 as a promising target for tumor therapy, as its inhibition not only eliminates hypoxic tumor cells but also allows for the treatment of more vulnerable oxygenated tumor cells through methods like chemotherapy or radiation (23).
Inhibition of MCT1 can suppress tumor angiogenesis. Since MCT1 is a key transporter for lactate uptake by endothelial cells, a study has employed CHC and siRNA to inhibit MCT1, thereby effectively suppressing lactate-induced tumor angiogenesis. Mechanistically, targeted inhibition of MCT1 in endothelial cells directly impeded angiogenesis by reducing HIF-1 activity (56).
Inhibition of MCT1 holds potential to impede tumor progression by reducing immunosuppression. Lactate, a hallmark metabolite of TME (57), actively promotes immune evasion of tumor cells by inhibiting immune cell toxicity and proliferation (58). Tumor-derived lactate has been identified as an inhibitor of CD8+ T cell toxicity (59). A nanomedicine containing MCT1 inhibitors released the drug at low pH, inhibiting MCT1 to curb lactate release and thereby enhancing the anti-tumor activity of CD8+ T cells (60).
Moreover, inhibiting MCT1 prevents tumor cells from absorbing lactate from stroma cells, thereby diminishing the role of stroma cells in promoting tumor cell proliferation. Additionally, inhibiting MCT1 using siRNA or MCT1 inhibitors promotes ferroptosis, an effect that is primarily mediated through the regulation of intracellular lipid metabolism (24).
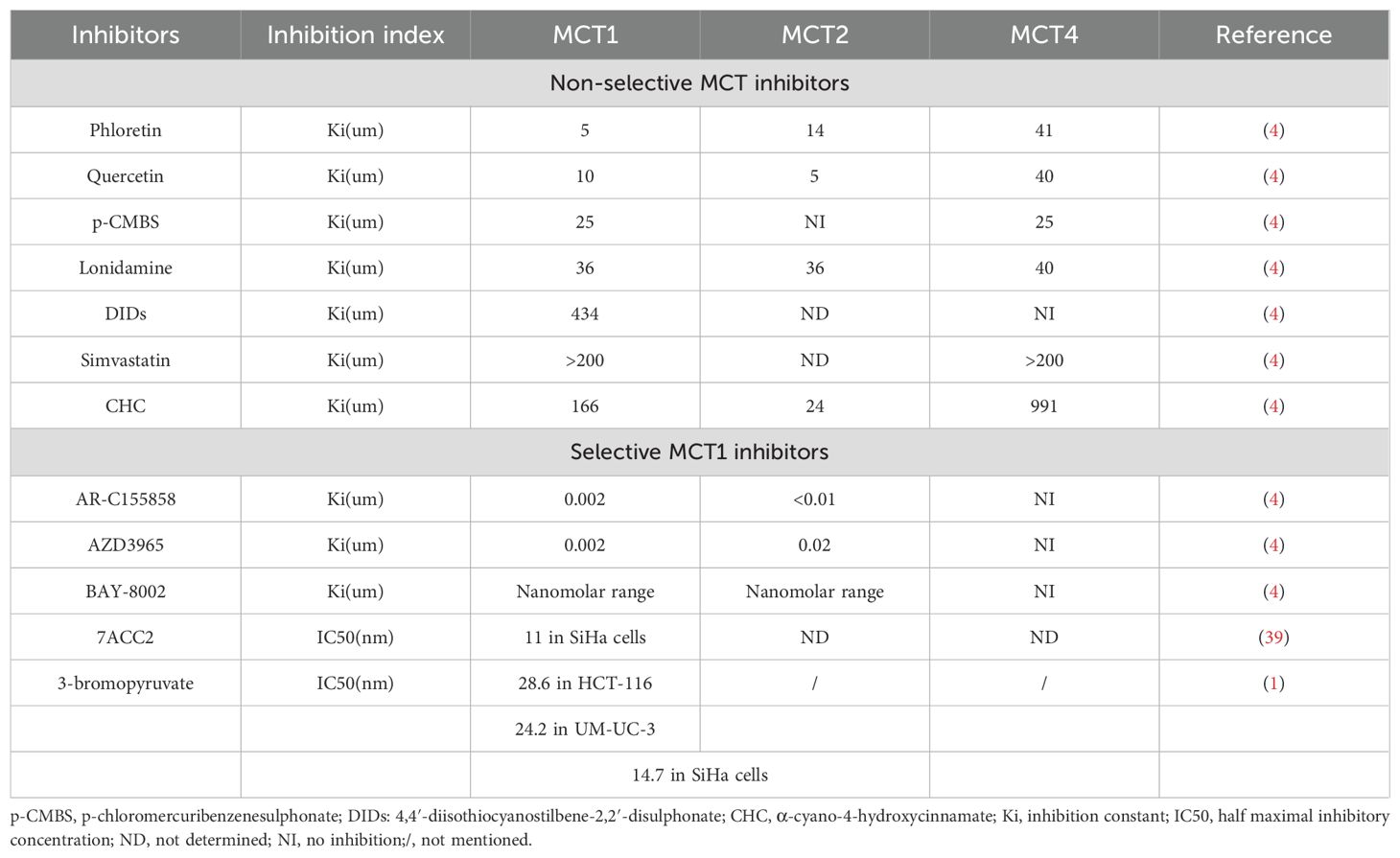
The aforementioned CHC is categorized as a non-selective MCT inhibitor, a group that also encompasses compounds such as phloretin, quercetin, p-CMBS, lonidamine, DIDs, and simvastatin (4). These non-selective MCT inhibitors are characterized by their limited specificity (61). Consequently, the development and utilization of selective MCT1 inhibitors assume heightened significance. AZD3965 stands out as one of the selective MCT1 inhibitors. Other selective MCT1 inhibitors include AR-C155858, BAY-8002, and 7ACC2 (24). Moreover, 3-bromopyruvate (3-BrPA) has been shown to induce an epigenetically driven loss of MCT1 (1). Table 2 summarizes the efficacy of MCTs inhibitors.
AZD3965, BAY-8002, and 7ACC2 induce distinct conformational changes in MCT1, resulting in an outward-open conformation in the presence of BAY-8002 and AZD3965, and an inward-open conformation in the presence of 7ACC2 (61). All three inhibitors directly occupy the substrate binding site, achieving inhibition of MCT1 through direct competition with the substrate binding site and inhibition of conformational changes in the transporter protein (61). 3-BrPA treatment induces silencing of MCT1 by hypermethylating the promoter of the SLC16A1 gene (62). AR-C155858 interacts with transmembrane helices 7–10 in the inward-open conformation of MCT1, resulting in its inhibition (4). Additionally, microRNAs (miRNAs) such as miR-342-3p and miR-124 are found to downregulate MCT1 expression by targeting its messenger RNA (mRNA) (63, 64). MiR-146a indirectly inhibits the expression of MCT1 by inhibiting CD147 (65). The mechanism of action of MCT1 inhibitors is summarized in Figure 3.
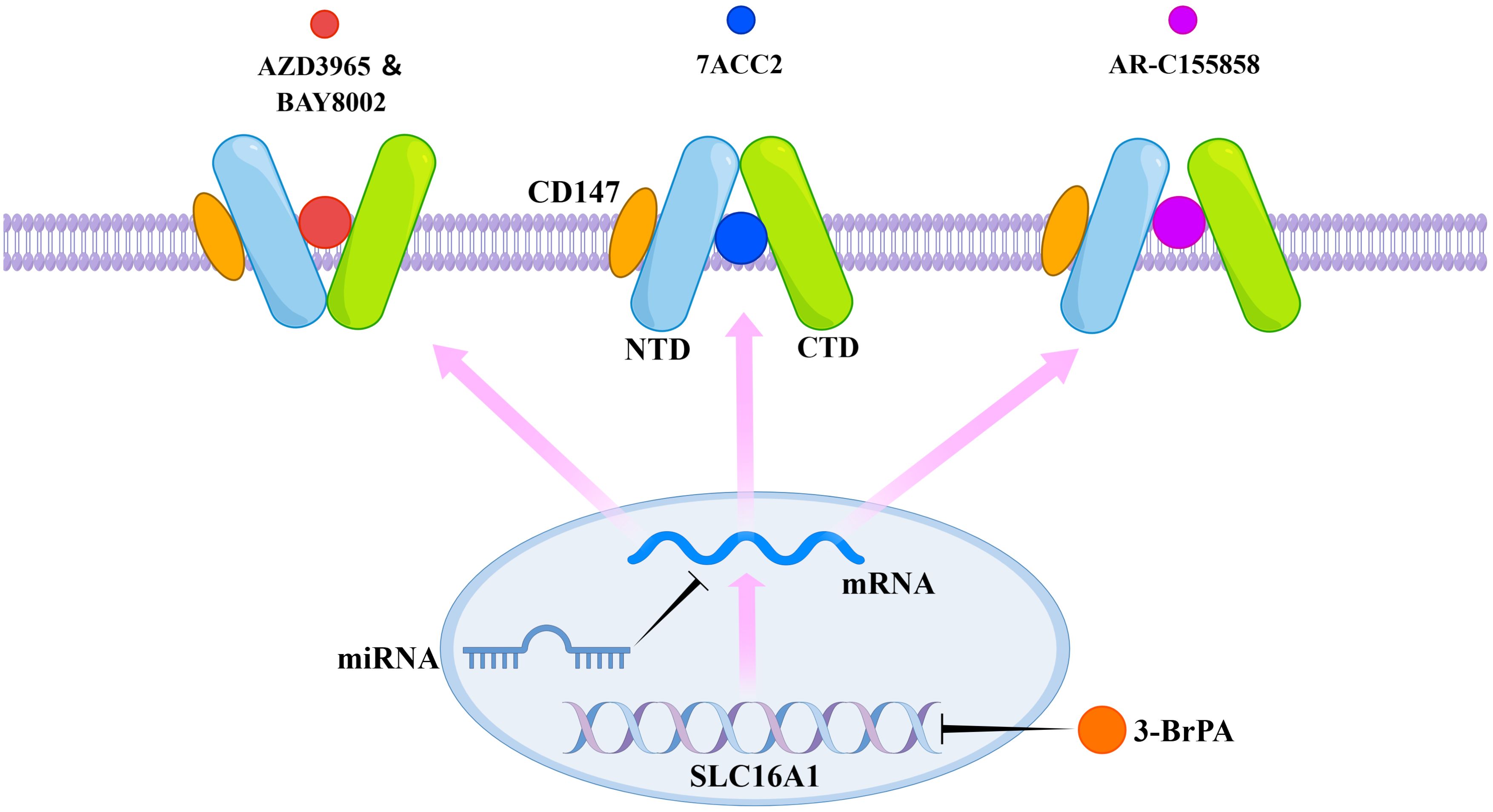
Figure 3. Mechanism of action of selective MCT1 inhibitors. AZD3965, BAY-8002, 7ACC2, and AR-C155858 exert their inhibitory effects by binding to specific sites of MCT1. 3-BrPA acts by inducing methylation of the gene coding for MCT1. MicroRNAs act by inhibiting the associated mRNAs.
Relevant applications of selective MCT1 inhibitors
AZD3965
AZD3965 is a pyrrole pyrimidine derivative (66). In a study involving B-cell lymphoma by Beloueche-Babari et al, treatment of immunodeficient mice with Raji xenografts using AZD3965 resulted in tumor growth inhibition and a reduction in choline levels (67). Mechanistically, intracellular lactate accumulation induced by AZD3965 led to decreased expression of choline kinase α (ChoKα) and its mRNA, consequently inhibiting de novo synthesis of choline phosphate (67). Simultaneously, there was an increase in the infiltration of dendritic cells (DCs) and natural killer cells (NKs) (67). In a systematic review assessing the anti-tumor properties of AZD3965 in a murine model, investigators determined that AZD3965 effectively augmented tumor responsiveness to radiation and chemotherapeutic agents (3).
AR-C155858
AR-C155858 is also a pyrrole pyrimidine derivative (66). The use of AR-C155858 in combination with anti-CD19 chimeric antigen receptor (CAR)-T cell therapy has shown promising results in the treatment of B-cell malignancies (68). In a study focusing on B-cell lymphoma by Lopez et al, the inhibition of MCT1 by AR-C155858, in conjunction with CAR-T cells, led to enhanced in vitro cytotoxicity and improved anti-tumor control in a mouse model. Mechanistically, B-cell lymphoma cells primarily relied on MCT1 for lactate export and were susceptible to inhibition, with minimal to no expression of MCT4. Conversely, CAR-T cells exhibited elevated levels of both MCT1 and MCT4 following activation, rendering them functionally resistant to AR-C155858 inhibition (69). Furthermore, in the context of breast cancer by Guan et al, AR-C155858 has been shown to reduce tumor cell proliferation in vitro (70).
7ACC2
Malignant pleural effusion (MPE) is a common complication in advanced malignant tumors (71). FOXP3, a pivotal transcription factor, plays a crucial role in the regulation of immune responses. Within MPE, FOXP3 natural killer T (NKT)-like cells leveraged their elevated expression of MCT1 and LDHB to uptake and metabolize lactate, thereby maintaining their immunosuppressive capabilities within the effusion (72). In vitro studies have shown that 7ACC2 significantly reduced FOXP3 expression in NKT-like cells, which can inform the development of NKT-like cell-based therapies aimed at controlling MPE progression (72). In a study involving pancreatic cancer by Sandforth et al, tumor cells expressing MCT1 were protected against gemcitabine-induced apoptosis in a MCT1-dependent manner (73). The administration of 7ACC2 can counteract this protective effect and induce apoptosis in the tumor cells (73).
BAY-8002
The DNA-dependent protein kinase catalytic subunit (DNA-PKcs) is known for its pleiotropic protein kinase activity and is often linked to poor prognosis in tumor patients (74). Wagner et al. revealed that overlocalization of DNA-PKcs can confer protection against lentiviral transduction, such as human immunodeficiency virus-1 (HIV-1). Meanwhile, BAY-8002, which inhibited lactate flux, enhanced the nuclear localization of DNA-PKcs, thereby reducing the efficacy of lentiviral transduction (75). In the context of prostate cancer by Matheux et al, MCT1 has been identified as a transporter for afatinib. The inhibition of MCT1 by BAY-8002 partially diminished the sensitivity of 22Rv1 cells, which expressed the pregnane X receptor, to afatinib (76).
Challenges and coping strategies for MCT1 inhibitors
Although selective MCT1 inhibitors can effectively impede tumor progression, they also face notable challenges. For instance, in clinical trials, although AZD3965 was well tolerated, it was associated with side effects such as nausea and fatigue (1). In breast cancer research by Guan et al, AR-C155858 has been found to be ineffective in treating 4T1 xenograft breast tumor models, possibly due to alterations in the immune status of these preclinical models (70). Another study revealed that elevated levels of AR-C155858 can only impede cell proliferation without inducing cell death (77). Moreover, selective MCT1 inhibitors like AZD3965 and AR-C155858 have been shown to hinder lactate transport in tumor cells, but they did not significantly impede tumor progression (24).
Given these challenges, the integration of selective MCT1 inhibitors with other pharmaceutical agents may be a promising strategy in the realm of tumor therapy.
Selective MCT1 inhibitors have demonstrated promising therapeutic effects when used in combination with other drugs or treatment modalities. For instance, in a study involving hepatocellular carcinoma by Zhou et al, the combination of AR-C155858 with anti-PD-1 antibodies effectively suppressed tumor growth in xenograft models (78). Similarly, in the Raji Burkitt lymphoma model, combining AZD3965 with doxorubicin or rituximab resulted in decreased tumor growth (79). Furthermore, the combination of AZD3965 with various radiotherapy modalities has shown superior efficacy in treating tumor xenografts (80).
Another significant challenge faced by selective MCT1 inhibitors is their ineffectiveness in the presence of MCT4 overexpression (77). This may be due to reduced competition between MCT1 and MCT4 for CD147 (4). This limitation is particularly concerning because MCT4 is often co-expressed with MCT1 in tumors (81). Although AZD0095, a specific MCT4 inhibitor, has been developed (82), its efficacy has yet to be evaluated in clinical trials. Selective inhibitors targeting either MCT1 or MCT4 alone have proven ineffective against tumors that co-express both transporters, highlighting the need for further research into the development of dual MCT1 and MCT4 inhibitors for potential antitumor therapeutics (83). Syrosingopine, identified as a dual inhibitor of MCT1 and MCT4 (84), has demonstrated anti-proliferative effects in acute myeloid leukemia (85). Additionally, metformin, a drug commonly used to treat diabetes mellitus, has shown promise in the field of antitumor therapy (86). Metformin acts as an inhibitor of mitochondrial NADH dehydrogenase. NAD+ is essential for the glycolysis step that generates ATP, and it can be regenerated from NADH by either mitochondrial NADH dehydrogenase or lactate dehydrogenase. Syrosingopine increases intracellular lactate levels, which inhibits LDH activity. When metformin is combined with Syrosingopine, it blocks NAD+ regeneration, leading to glycolysis inhibition, ATP depletion, and ultimately, tumor cell death (77).
Conclusive remarks
Overexpression of MCT1 in tumor cells is mostly associated with poor prognosis. MCT1 is thus implicated in promoting tumor progression via multiple mechanisms. Extensive research has elucidated that MCT1 and its mediated substrate transport not only sustain tumor progression through the maintenance of metabolic symbiosis but also promote angiogenesis, suppress the immune response of relevant immune cells, and engage in additional mechanisms that are conducive to tumor progression. A finding indicated that MCT1 expression in the cell membrane and MCT1 expression in the nucleus may predict divergent prognoses for patients. The nuclear localization of MCT1 may be associated with the binding of novel chaperone proteins. However, the precise mechanism underlying the nuclear localization of MCT1 remains to be elucidated and warrants further in-depth investigation. Moreover, it is also essential to explore whether other transport substrates of MCT1, beyond lactate and acetate, can promote tumor progression through specific mechanisms.
Given its pivotal role in tumor progression, MCT1 has garnered considerable attention as a potential therapeutic target for tumor treatment. Numerous studies have demonstrated that selective MCT1 inhibitors can inhibit tumor progression to a certain extent. However, these inhibitors also face several challenges. Therefore, further comprehensive studies are imperative to facilitate the development of MCT1-targeted therapeutic strategies.
Author contributions
ZX: Project administration, Writing – original draft. XMW: Supervision, Writing – review & editing. HC: Supervision, Writing – review & editing. JL: Supervision, Writing – review & editing. XZ: Supervision, Writing – review & editing. XJW: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work is supported by the Young Scientists Fund of the National Natural Science Foundation of China (grant No.81700198), the National Science and Technology Major Project of the Ministry of Science and Technology of China (grant No.2017YFC0110105 and 20210101328JC), the Norman Bethune Project of Jilin University (grant No. 2018B17), the Clinical Research Project of Wu Jieping Medical Foundation (grant No.320.6750.2023-3-55), Engineering Sciences and Comprehensive Interdisciplinary Programs, Ministry of Science and Technology of the People’s Republic of China (2024YFF0507403), Health Research Talent Special Program of Jilin Province (2024SCZ01), Natural Science Foundation of Jilin Province (YDZJ202201ZYTS243 and YDZJ202401698ZYTS), and Scientific Research Program of Jilin Provincial Department of Education(JJKH20250190KJ).
Acknowledgments
We thank other members of the Wang laboratory for the critical reading of the manuscript and useful discussions. We would also like to thank Figdraw, as all the images in the article were drawn by Figdraw. We thank Kimi(kimi.moonshot.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Some paragraphs and statements in the article were touched up using AI.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
3-BrPA: 3-Bromopyruvate
AMPK: adenosine monophosphate-activated protein kinase
APC: adenomatous polyposis coli
ATP: adenosine triphosphate
bFGF: basic fibroblast growth factor
CAR: chimeric antigen receptor
Cav-1: Caveolin-1
ccRCC: clear cell renal cell carcinoma
CHC: α-cyano-4-hydroxycinnamate
ChoKα: choline kinase α
CK1α: casein kinase 1 α
COX-2: cyclooxygenase-2
CRC: colorectal cancer
CTL: cytotoxic T lymphocyte
DC: dendritic cell
DLAT: dihydrolipoamide S-acetyltransferase
DNA-PKcs: DNA-dependent protein kinase catalytic subunit
DNMT: DNA methyltransferase
EE: endosome
EGFR-TKI: epidermal growth factor receptor tyrosine kinase inhibitor
ERK: extracellular regulated protein kinase
ETC: electron transport chain
FOXP3: forkhead box proteins P3
FZD: frizzled
G6P: fructose-6-phosphate
GLUT1: glucose transporter 1
GSK3: glycogen synthase kinase 3
HCAR1: hydroxy-carboxylic acid receptor 1
HIF-1: hypoxia-inducible factor 1
HIF-1α: hypoxia-inducible factor 1 α
HIV-1: human immunodeficiency virus-1
IκBα: inhibitor of κBα
IL-8: interleukin-8
LDH: lactate dehydrogenase
LKB1: liver kinase B1
LRP5/6: lipoprotein receptor‐related protein 5 and 6
MCT: monocarboxylate transporter
MCT1: monocarboxylate transporter 1
MCT4: monocarboxylate transporter 4
miRNA: microRNA
MMP: matrix metalloproteinase
MMR: mismatch repair
MPE: malignant pleural effusion
mRNA: messenger RNA
MUFA: monounsaturated fatty acid
MVB: multivesicular body
NAD: nicotinamide adenine dinucleotide
NADH: nicotinamide adenine dinucleotide
NFAT1: nuclear factor of activated T-cells 1
NF-κB: nuclear factor-kappa B
NK: natural killer cell
NKT: natural killer T
OSI: Osimertinib
OXPHOS: oxidative phosphorylation
PD-1: programmed death-1
PDC: pyruvate dehydrogenase complex
PDK1: pyruvate dehydrogenase kinase 1
PEP: phosphoenolpyruvate
PHD: prolylhydoxylase
SCD1: stearoyl-CoA desaturase 1
Smad3: recombinant mothers against decapentaplegic homolog 3
SREBP1: sterol regulatory element binding protein 1
STAT3: signal transducer and activator of transcription 3
TCF/LEF: T‐cell factor/lymphoid enhancer‐binding factor
TGF-β: transforming growth factor-β
TME: tumor microenvironment
Treg cell regulatory: T cell
VEGF-A: vascular endothelial growth factor A
VEGFR2: VEGF receptor 2.
References
1. Singh M, Afonso J, Sharma D, Gupta R, Kumar V, Rani R, et al. Targeting monocarboxylate transporters (MCTs) in cancer: How close are we to the clinics? Semin Cancer Biol. (2023) 90:1–14. doi: 10.1016/j.semcancer.2023.01.007
2. Kang H, Kim B, Park J, Youn H, and Youn B. The Warburg effect on radioresistance: Survival beyond growth. Biochim Biophys Acta Rev Cancer. (2023) 1878:188988. doi: 10.1016/j.bbcan.2023.188988
3. Silva A, Cerqueira MC, Rosa B, Sobral C, Pinto-Ribeiro F, Costa MF, et al. Prognostic value of monocarboxylate transporter 1 overexpression in cancer: A systematic review. Int J Mol Sci. (2023) 24:5141. doi: 10.3390/ijms24065141
4. Payen VL, Mina E, Van Hée VF, Porporato PE, and Sonveaux P. Monocarboxylate transporters in cancer. Mol Metab. (2020) 33:48–66. doi: 10.1016/j.molmet.2019.07.006
5. Zhang L, Song Z-S, Wang Z-S, Guo Y-L, Xu C-G, and Shen H. High expression of SLC16A1 as a biomarker to predict poor prognosis of urological cancers. Front Oncol. (2021) 11:706883. doi: 10.3389/fonc.2021.706883
6. Draoui N and Feron O. Lactate shuttles at a glance: from physiological paradigms to anti-cancer treatments. Dis Model Mech. (2011) 4:727–32. doi: 10.1242/dmm.007724
7. Silva A, Antunes B, Batista A, Pinto-Ribeiro F, Baltazar F, and Afonso J. In vivo anticancer activity of AZD3965: A systematic review. Mol Basel Switz. (2021) 27:181. doi: 10.3390/molecules27010181
8. Boidot R, Végran F, Meulle A, Le Breton A, Dessy C, Sonveaux P, et al. Regulation of monocarboxylate transporter MCT1 expression by p53 mediates inward and outward lactate fluxes in tumors. Cancer Res. (2012) 72:939–48. doi: 10.1158/0008-5472.CAN-11-2474
9. Gan L, Xiu R, Ren P, Yue M, Su H, Guo G, et al. Metabolic targeting of oncogene MYC by selective activation of the proton-coupled monocarboxylate family of transporters. Oncogene. (2016) 35:3037–48. doi: 10.1038/onc.2015.360
10. de la Cruz Concepción B, Bartolo-García LD, Tizapa-Méndez MD, Martínez-Vélez M, Valerio-Diego JJ, Illades-Aguiar B, et al. EMMPRIN is an emerging protein capable of regulating cancer hallmarks. Eur Rev Med Pharmacol Sci. (2022) 26:6700–24. doi: 10.26355/eurrev_202209_29771
11. Xu B, Zhang M, Zhang B, Chi W, Ma X, Zhang W, et al. Embigin facilitates monocarboxylate transporter 1 localization to the plasma membrane and transition to a decoupling state. Cell Rep. (2022) 40:111343. doi: 10.1016/j.celrep.2022.111343
12. Malinowski D, Grzegółkowski P, Piotrowska K, Słojewski M, and Droździk M. Membrane transporters and carriers in human seminal vesicles. J Clin Med. (2022) 11:2213. doi: 10.3390/jcm11082213
13. Wang J, Yang Y, Shao F, Meng Y, Guo D, He J, et al. Acetate reprogrammes tumour metabolism and promotes PD-L1 expression and immune evasion by upregulating c-Myc. Nat Metab. (2024) 6:914–32. doi: 10.1038/s42255-024-01037-4
14. Salvi PS and Cowles RA. Butyrate and the intestinal epithelium: modulation of proliferation and inflammation in homeostasis and disease. Cells. (2021) 10:1775. doi: 10.3390/cells10071775
15. Halestrap AP and Wilson MC. The monocarboxylate transporter family-Role and regulation. IUBMB Life. (2012) 64:109–19. doi: 10.1002/iub.572
16. Adijanto J, Du J, Moffat C, Seifert EL, Hurle JB, and Philp NJ. The retinal pigment epithelium utilizes fatty acids for ketogenesis. J Biol Chem. (2014) 289:20570–82. doi: 10.1074/jbc.M114.565457
17. Bisbach CM, Hass DT, Thomas ED, Cherry TJ, and Hurley JB. Monocarboxylate transporter 1 (MCT1) mediates succinate export in the retina. Invest Ophthalmol Vis Sci. (2022) 63:1. doi: 10.1167/iovs.63.4.1
18. Pinheiro C, Penna V, Morais-Santos F, Abrahão-MaChado LF, Ribeiro G, Curcelli EC, et al. Characterization of monocarboxylate transporters (MCTs) expression in soft tissue sarcomas: distinct prognostic impact of MCT1 sub-cellular localization. J Transl Med. (2014) 12:118. doi: 10.1186/1479-5876-12-118
19. Afonso J, Pinto T, Simões-Sousa S, Schmitt F, Longatto-Filho A, Pinheiro C, et al. Clinical significance of metabolism-related biomarkers in non-Hodgkin lymphoma - MCT1 as potential target in diffuse large B cell lymphoma. Cell Oncol Dordr. (2019) 42:303–18. doi: 10.1007/s13402-019-00426-2
20. Liu P, Yang F, Zhang L, Hu Y, Chen B, Wang J, et al. Emerging role of different DNA methyltransferases in the pathogenesis of cancer. Front Pharmacol. (2022) 13:958146. doi: 10.3389/fphar.2022.958146
21. He Y, Zhang L, Zhou R, Wang Y, and Chen H. The role of DNA mismatch repair in immunotherapy of human cancer. Int J Biol Sci. (2022) 18:2821–32. doi: 10.7150/ijbs.71714
22. Wang X, Liu H, Ni Y, Shen P, and Han X. Lactate shuttle: from substance exchange to regulatory mechanism. Hum Cell. (2022) 35:1–14. doi: 10.1007/s13577-021-00622-z
23. Puri S and Juvale K. Monocarboxylate transporter 1 and 4 inhibitors as potential therapeutics for treating solid tumours: A review with structure-activity relationship insights. Eur J Med Chem. (2020) 199:112393. doi: 10.1016/j.ejmech.2020.112393
24. Duan Q, Zhang S, Wang Y, Lu D, Sun Y, and Wu Y. Proton-coupled monocarboxylate transporters in cancer: From metabolic crosstalk, immunosuppression and anti-apoptosis to clinical applications. Front Cell Dev Biol. (2022) 10:1069555. doi: 10.3389/fcell.2022.1069555
25. Kerneur C, Cano CE, and Olive D. Major pathways involved in macrophage polarization in cancer. Front Immunol. (2022) 13:1026954. doi: 10.3389/fimmu.2022.1026954
26. Gao J, Liang Y, and Wang L. Shaping polarization of tumor-associated macrophages in cancer immunotherapy. Front Immunol. (2022) 13:888713. doi: 10.3389/fimmu.2022.888713
27. Hsieh C-C, Hsieh M-J, Wang Y-H, and Liao Z-X. Macrophage distribution affected by virus-encoded granulocyte macrophage colony stimulating factor combined with lactate oxidase. ACS Omega. (2022) 7:24020–6. doi: 10.1021/acsomega.2c03213
28. Mu X, Shi W, Xu Y, Xu C, Zhao T, Geng B, et al. Tumor-derived lactate induces M2 macrophage polarization via the activation of the ERK/STAT3 signaling pathway in breast cancer. Cell Cycle Georget Tex. (2018) 17:428–38. doi: 10.1080/15384101.2018.1444305
29. Santoni M, Romagnoli E, Saladino T, Foghini L, Guarino S, Capponi M, et al. Triple negative breast cancer: Key role of Tumor-Associated Macrophages in regulating the activity of anti-PD-1/PD-L1 agents. Biochim Biophys Acta Rev Cancer. (2018) 1869:78–84. doi: 10.1016/j.bbcan.2017.10.007
30. Iglesias-Escudero M, Arias-González N, and Martínez-Cáceres E. Regulatory cells and the effect of cancer immunotherapy. Mol Cancer. (2023) 22:26. doi: 10.1186/s12943-023-01714-0
31. Xu D, Wang Y, Chen Y, and Zheng J. Identification of the molecular subtype and prognostic characteristics of pancreatic cancer based on CD8 + T cell-related genes. Cancer Immunol Immunother CII. (2023) 72:647–64. doi: 10.1007/s00262-022-03269-3
32. Chen L, Huang L, Gu Y, Cang W, Sun P, and Xiang Y. Lactate-lactylation hands between metabolic reprogramming and immunosuppression. Int J Mol Sci. (2022) 23:11943. doi: 10.3390/ijms231911943
33. Kumagai S, Koyama S, Itahashi K, Tanegashima T, Lin Y-T, Togashi Y, et al. Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer Cell. (2022) 40:201–218.e9. doi: 10.1016/j.ccell.2022.01.001
34. Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. (2007) 109:3812–9. doi: 10.1182/blood-2006-07-035972
35. Brand A, Singer K, Koehl GE, Kolitzus M, Schoenhammer G, Thiel A, et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. (2016) 24:657–71. doi: 10.1016/j.cmet.2016.08.011
36. Aloe C, Wang H, Vlahos R, Irving L, Steinfort D, and Bozinovski S. Emerging and multifaceted role of neutrophils in lung cancer. Transl Lung Cancer Res. (2021) 10:2806–18. doi: 10.21037/tlcr-20-760
37. Chung JY-F, Tang PC-T, Chan MK-K, Xue VW, Huang X-R, Ng CS-H, et al. Smad3 is essential for polarization of tumor-associated neutrophils in non-small cell lung carcinoma. Nat Commun. (2023) 14:1794. doi: 10.1038/s41467-023-37515-8
38. Deng H, Kan A, Lyu N, He M, Huang X, Qiao S, et al. Tumor-derived lactate inhibit the efficacy of lenvatinib through regulating PD-L1 expression on neutrophil in hepatocellular carcinoma. J Immunother Cancer. (2021) 9:e002305. doi: 10.1136/jitc-2020-002305
39. Sun X, Wang M, Wang M, Yao L, Li X, Dong H, et al. Role of proton-coupled monocarboxylate transporters in cancer: from metabolic crosstalk to therapeutic potential. Front Cell Dev Biol. (2020) 8:651. doi: 10.3389/fcell.2020.00651
40. Fu Y, Liu S, Yin S, Niu W, Xiong W, Tan M, et al. The reverse Warburg effect is likely to be an Achilles’ heel of cancer that can be exploited for cancer therapy. Oncotarget. (2017) 8:57813–25. doi: 10.18632/oncotarget.18175
41. Sakamoto A, Kunou S, Shimada K, Tsunoda M, Aoki T, Iriyama C, et al. Pyruvate secreted from patient-derived cancer-associated fibroblasts supports survival of primary lymphoma cells. Cancer Sci. (2019) 110:269–78. doi: 10.1111/cas.13873
42. Zuo Y-B, Zhang Y-F, Zhang R, Tian J-W, Lv X-B, Li R, et al. Ferroptosis in cancer progression: role of noncoding RNAs. Int J Biol Sci. (2022) 18:1829–43. doi: 10.7150/ijbs.66917
43. Lei G, Zhuang L, and Gan B. Targeting ferroptosis as a vulnerability in cancer. Nat Rev Cancer. (2022) 22:381–96. doi: 10.1038/s41568-022-00459-0
44. Liang D, Minikes AM, and Jiang X. Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol Cell. (2022) 82:2215–27. doi: 10.1016/j.molcel.2022.03.022
45. Shi M, Zhang M-J, Yu Y, Ou R, Wang Y, Li H, et al. Curcumin derivative NL01 induces ferroptosis in ovarian cancer cells via HCAR1/MCT1 signaling. Cell Signal. (2023) 109:110791. doi: 10.1016/j.cellsig.2023.110791
46. Mohammad Nezhady MA, Cagnone G, Joyal J-S, and Chemtob S. Lack of HCAR1, the lactate GPCR, signaling promotes autistic-like behavior. Cell Commun Signal CCS. (2023) 21:196. doi: 10.1186/s12964-023-01188-z
47. Zhao Y, Li M, Yao X, Fei Y, Lin Z, Li Z, et al. HCAR1/MCT1 regulates tumor ferroptosis through the lactate-mediated AMPK-SCD1 activity and its therapeutic implications. Cell Rep. (2020) 33:108487. doi: 10.1016/j.celrep.2020.108487
48. Ascenzi F, De Vitis C, Maugeri-Saccà M, Napoli C, Ciliberto G, and Mancini R. SCD1, autophagy and cancer: implications for therapy. J Exp Clin Cancer Res CR. (2021) 40:265. doi: 10.1186/s13046-021-02067-6
49. Cassidy LD and Narita M. Autophagy at the intersection of aging, senescence, and cancer. Mol Oncol. (2022) 16:3259–75. doi: 10.1002/1878-0261.13269
50. Chmielecki J, Mok T, Wu Y-L, Han J-Y, Ahn M-J, Ramalingam SS, et al. Analysis of acquired resistance mechanisms to osimertinib in patients with EGFR-mutated advanced non-small cell lung cancer from the AURA3 trial. Nat Commun. (2023) 14:1071. doi: 10.1038/s41467-023-35962-x
51. Jin P, Jiang J, Xie N, Zhou L, Huang Z, Zhang L, et al. MCT1 relieves osimertinib-induced CRC suppression by promoting autophagy through the LKB1/AMPK signaling. Cell Death Dis. (2019) 10:615. doi: 10.1038/s41419-019-1844-2
52. Fan Q, Yang L, Zhang X, Ma Y, Li Y, Dong L, et al. Autophagy promotes metastasis and glycolysis by upregulating MCT1 expression and Wnt/β-catenin signaling pathway activation in hepatocellular carcinoma cells. J Exp Clin Cancer Res CR. (2018) 37:9. doi: 10.1186/s13046-018-0673-y
53. Colozza G and Koo B-K. Wnt/β-catenin signaling: Structure, assembly and endocytosis of the signalosome. Dev Growth Differ. (2021) 63:199–218. doi: 10.1111/dgd.12718
54. Li M, Long X, Wan H, Yin M, Yang B, Zhang F, et al. Monocarboxylate transporter 1 promotes proliferation and invasion of renal cancer cells by mediating acetate transport. Cell Biol Int. (2021) 45:1278–87. doi: 10.1002/cbin.11571
55. Sonveaux P, Végran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. (2008) 118:3930–42. doi: 10.1172/JCI36843
56. Sonveaux P, Copetti T, De Saedeleer CJ, Végran F, Verrax J, Kennedy KM, et al. Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS One. (2012) 7:e33418. doi: 10.1371/journal.pone.0033418
57. Zhao J, Tian Z, Zhao S, Feng D, Guo Z, Wen L, et al. Insights into the effect of catalytic intratumoral lactate depletion on metabolic reprogramming and immune activation for antitumoral activity. Adv Sci. (2023) 10:2204808. doi: 10.1002/advs.202204808
58. Hayes C, Donohoe CL, Davern M, and Donlon NE. The oncogenic and clinical implications of lactate induced immunosuppression in the tumour microenvironment. Cancer Lett. (2021) 500:75–86. doi: 10.1016/j.canlet.2020.12.021
59. Elia I, Rowe JH, Johnson S, Joshi S, Notarangelo G, Kurmi K, et al. Tumor cells dictate anti-tumor immune responses by altering pyruvate utilization and succinate signaling in CD8+ T cells. Cell Metab. (2022) 34:1137–1150.e6. doi: 10.1016/j.cmet.2022.06.008
60. Huang T, Feng Q, Wang Z, Li W, Sun Z, Wilhelm J, et al. Tumor-targeted inhibition of monocarboxylate transporter 1 improves T-cell immunotherapy of solid tumors. Adv Healthc Mater. (2021) 10:e2000549. doi: 10.1002/adhm.202000549
61. Wang N, Jiang X, Zhang S, Zhu A, Yuan Y, Xu H, et al. Structural basis of human monocarboxylate transporter 1 inhibition by anti-cancer drug candidates. Cell. (2021) 184:370–383.e13. doi: 10.1016/j.cell.2020.11.043
62. Vander Linden C, Corbet C, Bastien E, Martherus R, Guilbaud C, Petit L, et al. Therapy-induced DNA methylation inactivates MCT1 and renders tumor cells vulnerable to MCT4 inhibition. Cell Rep. (2021) 35:109202. doi: 10.1016/j.celrep.2021.109202
63. Hou L, Zhao Y, Song G-Q, Ma Y-H, Jin X-H, Jin S-L, et al. Interfering cellular lactate homeostasis overcomes Taxol resistance of breast cancer cells through the microRNA-124-mediated lactate transporter (MCT1) inhibition. Cancer Cell Int. (2019) 19:193. doi: 10.1186/s12935-019-0904-0
64. Komoll R-M, Hu Q, Olarewaju O, von Döhlen L, Yuan Q, Xie Y, et al. MicroRNA-342-3p is a potent tumour suppressor in hepatocellular carcinoma. J Hepatol. (2021) 74:122–34. doi: 10.1016/j.jhep.2020.07.039
65. Montes-Mojarro I-A, Steinhilber J, Griessinger CM, Rau A, Gersmann A-K, Kohlhofer U, et al. CD147 a direct target of miR-146a supports energy metabolism and promotes tumor growth in ALK+ ALCL. Leukemia. (2022) 36:2050–63. doi: 10.1038/s41375-022-01617-x
66. Guan X, Rodriguez-Cruz V, and Morris ME. Cellular uptake of MCT1 inhibitors AR-C155858 and AZD3965 and their effects on MCT-mediated transport of L-lactate in murine 4T1 breast tumor cancer cells. AAPS J. (2019) 21:13. doi: 10.1208/s12248-018-0279-5
67. Beloueche-Babari M, Casals Galobart T, Delgado-Goni T, Wantuch S, Parkes HG, Tandy D, et al. Monocarboxylate transporter 1 blockade with AZD3965 inhibits lipid biosynthesis and increases tumour immune cell infiltration. Br J Cancer. (2020) 122:895–903. doi: 10.1038/s41416-019-0717-x
68. Boulch M, Cazaux M, Cuffel A, Ruggiu M, Allain V, Corre B, et al. A major role for CD4+ T cells in driving cytokine release syndrome during CAR T cell therapy. Cell Rep Med. (2023) 4:101161. doi: 10.1016/j.xcrm.2023.101161
69. Lopez E, Karattil R, Nannini F, Weng-Kit Cheung G, Denzler L, Galvez-Cancino F, et al. Inhibition of lactate transport by MCT-1 blockade improves chimeric antigen receptor T-cell therapy against B-cell Malignancies. J Immunother Cancer. (2023) 11:e006287. doi: 10.1136/jitc-2022-006287
70. Guan X, Bryniarski MA, and Morris ME. In vitro and in vivo efficacy of the monocarboxylate transporter 1 inhibitor AR-C155858 in the murine 4T1 breast cancer tumor model. AAPS J. (2018) 21:3. doi: 10.1208/s12248-018-0261-2
71. Trovisco R, Freitas C, Serino M, Ferreira P, Martins B, Coelho D, et al. Predictors of lung entrapment in Malignant pleural effusion. Pulmonology. (2022) S2531-0437(22)00199–4. doi: 10.1016/j.pulmoe.2022.08.001
72. Wang Z-H, Zhang P, Peng W-B, Ye L-L, Xiang X, Wei X-S, et al. Altered phenotypic and metabolic characteristics of FOXP3+CD3+CD56+ natural killer T (NKT)-like cells in human Malignant pleural effusion. Oncoimmunology. (2023) 12:2160558. doi: 10.1080/2162402X.2022.2160558
73. Sandforth L, Ammar N, Dinges LA, Röcken C, Arlt A, Sebens S, et al. Impact of the monocarboxylate transporter-1 (MCT1)-mediated cellular import of lactate on stemness properties of human pancreatic adenocarcinoma cells †. Cancers. (2020) 12:581. doi: 10.3390/cancers12030581
74. Dylgjeri E and Knudsen KE. DNA-PKcs: A targetable protumorigenic protein kinase. Cancer Res. (2022) 82:523–33. doi: 10.1158/0008-5472.CAN-21-1756
75. Wagner W, Sobierajska K, Kania KD, Paradowska E, and Ciszewski WM. Lactate suppresses retroviral transduction in cervical epithelial cells through DNA-PKcs modulation. Int J Mol Sci. (2021) 22:13194. doi: 10.3390/ijms222413194
76. Matheux A, Gassiot M, Fromont G, Leenhardt F, Boulahtouf A, Fabbrizio E, et al. PXR modulates the prostate cancer cell response to afatinib by regulating the expression of the monocarboxylate transporter SLC16A1. Cancers. (2021) 13:3635. doi: 10.3390/cancers13143635
77. Benjamin D, Robay D, Hindupur SK, Pohlmann J, Colombi M, El-Shemerly MY, et al. Dual inhibition of the lactate transporters MCT1 and MCT4 is synthetic lethal with metformin due to NAD+ Depletion in cancer cells. Cell Rep. (2018) 25:3047–3058.e4. doi: 10.1016/j.celrep.2018.11.043
78. Zhou J, Shao Q, Lu Y, Li Y, Xu Z, Zhou B, et al. Monocarboxylate transporter upregulation in induced regulatory T cells promotes resistance to anti-PD-1 therapy in hepatocellular carcinoma patients. Front Oncol. (2022) 12:960066. doi: 10.3389/fonc.2022.960066
79. Curtis NJ, Mooney L, Hopcroft L, Michopoulos F, Whalley N, Zhong H, et al. Pre-clinical pharmacology of AZD3965, a selective inhibitor of MCT1: DLBCL, NHL and Burkitt’s lymphoma anti-tumor activity. Oncotarget. (2017) 8:69219–36. doi: 10.18632/oncotarget.18215
80. Bola BM, Chadwick AL, Michopoulos F, Blount KG, Telfer BA, Williams KJ, et al. Inhibition of monocarboxylate transporter-1 (MCT1) by AZD3965 enhances radiosensitivity by reducing lactate transport. Mol Cancer Ther. (2014) 13:2805–16. doi: 10.1158/1535-7163.MCT-13-1091
82. Goldberg FW, Kettle JG, Lamont GM, Buttar D, Ting AKT, McGuire TM, et al. Discovery of clinical candidate AZD0095, a selective inhibitor of monocarboxylate transporter 4 (MCT4) for oncology. J Med Chem. (2023) 66:384–97. doi: 10.1021/acs.jmedchem.2c01342
83. Wang Y, Qin L, Chen W, Chen Q, Sun J, and Wang G. Novel strategies to improve tumour therapy by targeting the proteins MCT1, MCT4 and LAT1. Eur J Med Chem. (2021) 226:113806. doi: 10.1016/j.ejmech.2021.113806
84. Pouysségur J, Marchiq I, Parks SK, Durivault J, Ždralević M, and Vucetic M. Warburg effect” controls tumor growth, bacterial, viral infections and immunity - Genetic deconstruction and therapeutic perspectives. Semin Cancer Biol. (2022) 86:334–46. doi: 10.1016/j.semcancer.2022.07.004
85. Saulle E, Spinello I, Quaranta MT, Pasquini L, Pelosi E, Iorio E, et al. Targeting lactate metabolism by inhibiting MCT1 or MCT4 impairs leukemic cell proliferation, induces two different related death-pathways and increases chemotherapeutic sensitivity of acute myeloid leukemia cells. Front Oncol. (2020) 10:621458. doi: 10.3389/fonc.2020.621458
86. Hua Y, Zheng Y, Yao Y, Jia R, Ge S, and Zhuang A. Metformin and cancer hallmarks: shedding new lights on therapeutic repurposing. J Transl Med. (2023) 21:403. doi: 10.1186/s12967-023-04263-8
87. de Oliveira ATT, Pinheiro C, Longatto-Filho A, Brito MJ, Martinho O, Matos D, et al. Co-expression of monocarboxylate transporter 1 (MCT1) and its chaperone (CD147) is associated with low survival in patients with gastrointestinal stromal tumors (GISTs). J Bioenerg Biomembr. (2012) 44:171–8. doi: 10.1007/s10863-012-9408-5
88. Choi J-W, Kim Y, Lee J-H, and Kim Y-S. Prognostic significance of lactate/proton symporters MCT1, MCT4, and their chaperone CD147 expressions in urothelial carcinoma of the bladder. Urology. (2014) 84:245.e9–15. doi: 10.1016/j.urology.2014.03.031
89. Afonso J, Santos LL, Miranda-Gonçalves V, Morais A, Amaro T, Longatto-Filho A, et al. CD147 and MCT1-potential partners in bladder cancer aggressiveness and cisplatin resistance. Mol Carcinog. (2015) 54:1451–66. doi: 10.1002/mc.22222
90. Pértega-Gomes N, Vizcaíno JR, Attig J, Jurmeister S, Lopes C, and Baltazar F. A lactate shuttle system between tumour and stromal cells is associated with poor prognosis in prostate cancer. BMC Cancer. (2014) 14:352. doi: 10.1186/1471-2407-14-352
91. Zhao Z, Wu M-S, Zou C, Tang Q, Lu J, Liu D, et al. Downregulation of MCT1 inhibits tumor growth, metastasis and enhances chemotherapeutic efficacy in osteosarcoma through regulation of the NF-κB pathway. Cancer Lett. (2014) 342:150–8. doi: 10.1016/j.canlet.2013.08.042
92. Kim Y, Choi J-W, Lee J-H, and Kim Y-S. Expression of lactate/H+ symporters MCT1 and MCT4 and their chaperone CD147 predicts tumor progression in clear cell renal cell carcinoma: immunohistochemical and The Cancer Genome Atlas data analyses. Hum Pathol. (2015) 46:104–12. doi: 10.1016/j.humpath.2014.09.013
93. Cao Y-W, Liu Y, Dong Z, Guo L, Kang E-H, Wang Y-H, et al. Monocarboxylate transporters MCT1 and MCT4 are independent prognostic biomarkers for the survival of patients with clear cell renal cell carcinoma and those receiving therapy targeting angiogenesis. Urol Oncol. (2018) 36:311.e15–311.e25. doi: 10.1016/j.urolonc.2018.03.014
94. de Carvalho PA, Bonatelli M, Cordeiro MD, Coelho RF, Reis S, Srougi M, et al. MCT1 expression is independently related to shorter cancer-specific survival in clear cell renal cell carcinoma. Carcinogenesis. (2021) 42:1420–7. doi: 10.1093/carcin/bgab100
95. Pinheiro C, Granja S, Longatto-Filho A, Faria AM, Fragoso MCBV, Lovisolo SM, et al. Metabolic reprogramming: a new relevant pathway in adult adrenocortical tumors. Oncotarget. (2015) 6:44403–21. doi: 10.18632/oncotarget.5623
96. Pinheiro C, Miranda-Gonçalves V, Longatto-Filho A, Vicente ALSA, Berardinelli GN, Scapulatempo-Neto C, et al. The metabolic microenvironment of melanomas: Prognostic value of MCT1 and MCT4. Cell Cycle Georget Tex. (2016) 15:1462–70. doi: 10.1080/15384101.2016.1175258
97. Simões-Sousa S, Granja S, Pinheiro C, Fernandes D, Longatto-Filho A, Laus AC, et al. Prognostic significance of monocarboxylate transporter expression in oral cavity tumors. Cell Cycle Georget Tex. (2016) 15:1865–73. doi: 10.1080/15384101.2016.1188239
98. Mikkilineni L, Whitaker-Menezes D, Domingo-Vidal M, Sprandio J, Avena P, Cotzia P, et al. Hodgkin lymphoma: A complex metabolic ecosystem with glycolytic reprogramming of the tumor microenvironment. Semin Oncol. (2017) 44:218–25. doi: 10.1053/j.seminoncol.2017.10.003
99. Latif A, Chadwick AL, Kitson SJ, Gregson HJ, Sivalingam VN, Bolton J, et al. Monocarboxylate Transporter 1 (MCT1) is an independent prognostic biomarker in endometrial cancer. BMC Clin Pathol. (2017) 17:27. doi: 10.1186/s12907-017-0067-7
100. Johnson JM, Cotzia P, Fratamico R, Mikkilineni L, Chen J, Colombo D, et al. MCT1 in invasive ductal carcinoma: monocarboxylate metabolism and aggressive breast cancer. Front Cell Dev Biol. (2017) 5:27. doi: 10.3389/fcell.2017.00027
101. Chen X, Chen X, Liu F, Yuan Q, Zhang K, Zhou W, et al. Monocarboxylate transporter 1 is an independent prognostic factor in esophageal squamous cell carcinoma. Oncol Rep. (2019) 41:2529–39. doi: 10.3892/or.2019.6992
102. Miranda-Gonçalves V, Gonçalves CS, Granja S, Vieira de Castro J, Reis RM, Costa BM, et al. MCT1 is a new prognostic biomarker and its therapeutic inhibition boosts response to temozolomide in human glioblastoma. Cancers. (2021) 13:3468. doi: 10.3390/cancers13143468
103. Leu M, Kitz J, Pilavakis Y, Hakroush S, Wolff HA, Canis M, et al. Monocarboxylate transporter-1 (MCT1) protein expression in head and neck cancer affects clinical outcome. Sci Rep. (2021) 11:4578. doi: 10.1038/s41598-021-84019-w
104. Zhao H, Chen Y, Liao Y-P, Chen H-M, Yang Q-H, Xiao Y, et al. Immunohistochemical evaluation and prognostic value of monocarboxylate transporter 1 (MCT1) and 4 (MCT4) in T-cell non-Hodgkin lymphoma. Clin Exp Med. (2022). doi: 10.1007/s10238-022-00805-4
105. Eilertsen M, Andersen S, Al-Saad S, Kiselev Y, Donnem T, Stenvold H, et al. Monocarboxylate transporters 1–4 in NSCLC: MCT1 is an independent prognostic marker for survival. PLoS One. (2014) 9:e105038. doi: 10.1371/journal.pone.0105038
Keywords: MCT1, oncogenic mechanism, tumor progression, MCT1 inhibitor, targeted therapy
Citation: Xu Z, Wang X, Cheng H, Li J, Zhang X and Wang X (2025) The role of MCT1 in tumor progression and targeted therapy: a comprehensive review. Front. Immunol. 16:1610466. doi: 10.3389/fimmu.2025.1610466
Received: 12 April 2025; Accepted: 29 May 2025;
Published: 19 June 2025.
Edited by:
Subhadeep Roy, Birla Institute of Technology, Mesra, IndiaReviewed by:
Valentina Vaira, University of Milan, ItalySapna Jain, Translational Health Science and Technology Institute (THSTI), India
Copyright © 2025 Xu, Wang, Cheng, Li, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xueju Wang, eHVlanVAamx1LmVkdS5jbg==
 Zheng Xu
Zheng Xu Xuemei Wang
Xuemei Wang Hongjing Cheng
Hongjing Cheng Jiuling Li
Jiuling Li Xueju Wang
Xueju Wang