- Department of Oncology, Affiliated Hospital of North Sichuan Medical College, Nanchong, Sichuan, China
Patients with advanced lung adenocarcinoma lacking driver gene mutations face the clinical dilemma of limited treatment options and poor prognosis in later-line therapy. Although immune checkpoint inhibitors (ICIs) combined with anti-angiogenic agents offer a promising approach, the optimal treatment strategy remains to be explored. In this report, we present a case of advanced lung adenocarcinoma with pancreatic metastasis treated with cadonilimab plus anlotinib and radiotherapy to the pancreatic lesion, resulting in 11 months of progression-free survival (PFS) and only minor side effects. This outcome suggests the potential value of cadonilimab and anlotinib in later-line therapy for advanced lung adenocarcinoma and provides a possible new treatment option for such patients.
Introduction
Lung cancer is the malignant tumor with the highest incidence and mortality worldwide (1). Lung adenocarcinoma has become the most common histological subtype of non-small cell lung cancer (NSCLC) (2). Targeted therapy has significantly improved the prognosis of patients with driver gene-positive tumors (3, 4). However, a considerable proportion of these patients still experience poor outcomes after receiving first-line chemotherapy combined with immune checkpoint inhibitor (ICI) treatment. In recent years, based on the results of the IMpower-150 study, anti-angiogenic therapy combined with immunotherapy has offered a new treatment strategy for advanced lung adenocarcinoma patients without driver gene mutations (5). Cadonilimab, a novel PD-1/CTLA-4 bi-specific antibody, has shown excellent efficiency in solid tumors (6, 7). Furthermore, recent studies have shown that combining antiangiogenic drugs with ICIs can improve the prognosis of patients with mild side effects (8–10). In this study, we report a case of advanced lung adenocarcinoma in a patient who had received standard first-line treatment and could not tolerate second-line docetaxel chemotherapy,. The patient was subsequently treated with cadonilimab plus anlotinib in combination with radiotherapy for pancreatic metastases, resulting in 11 months of PFS with mild side effects. This “triple therapy” approach—immunotherapy, anti-angiogenic therapy, and local radiotherapy—may offer a novel treatment option for advanced pulmonary adenocarcinoma in later-line therapy.
Case presentation
All procedures involving human participants complied with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient.
The patient was admitted to the hospital on December 14, 2023, for postoperative recurrence of lung adenocarcinoma and was receiving later-line therapy after first-line chemotherapy combined with immunotherapy. PET/CT revealed new metabolically active lesions in the pancreatic body (SUVmax = 5.2), (Figure 1A, B). MRI showed a lesion measuring approximately 1.3 cm × 1.1 cm in the pancreatic body by MRI (Figure 1C). The patient refused to undergo pancreatic puncture biopsy. Combined with the PET/CT and MRI results, the pancreatic lesion was considered a metastasis from lung cancer.
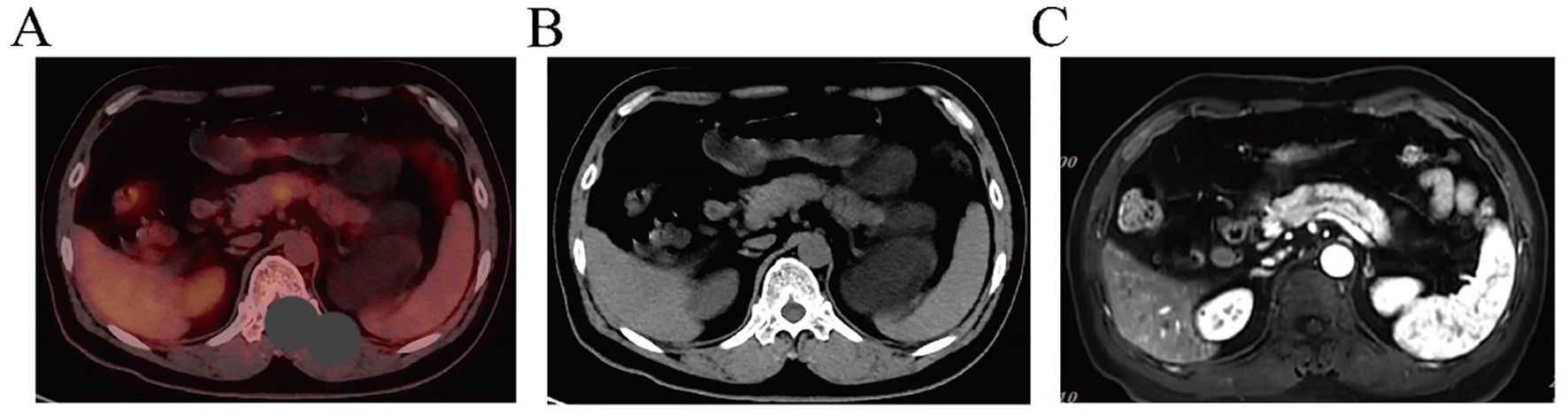
Figure 1. (A, B) PET/CT indicates pancreatic lesion as a metastatic tumor. (C) MRI features suggest that the pancreatic lesion is consistent with metastasis.
This was considered a progressive disease after multiple lines of therapy in a driver gene-negative patient. Based on recent study results, the patient began treatment with cadonilimab (730mg, d1) plus (allotinib, 10mg D1-14, every 3 weeks) on January 23, 2024. Radiotherapy was also performed to the pancreatic metastasis at a dose of 45 Gy/15 Fx (Figure 2). CT scans performed after the second and fifth cycles of treatment showed stable disease (SD) in the pancreatic lesions. Elevated urinary microalbumin levels (1,810 mg) were observed after two cycles of treatment but returned to normal after 6 weeks of treatment with piperazine ferulate (50 mg, once daily). The patient continued receiving the cadonilimab plus anlotinib regimen.
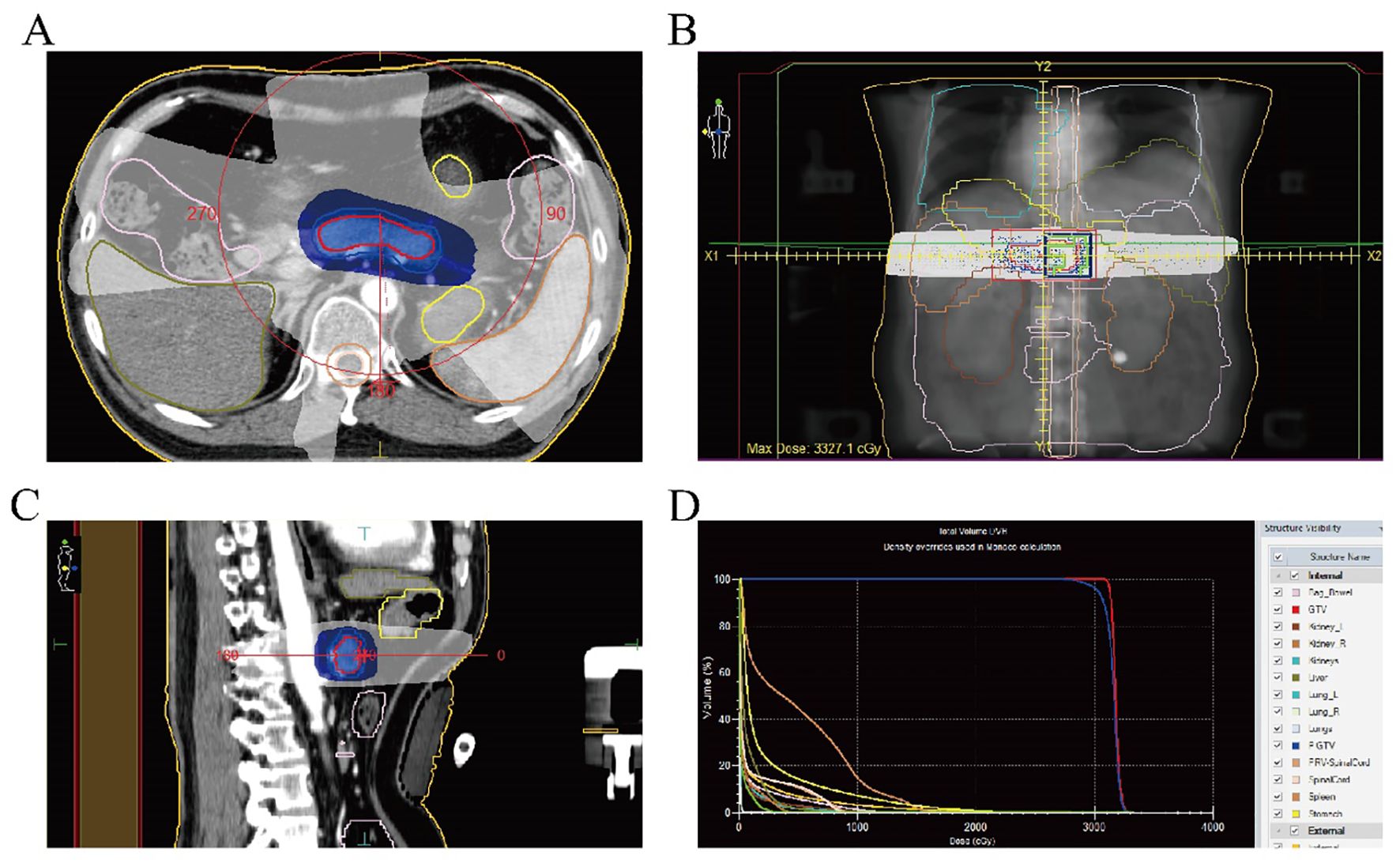
Figure 2. (A) Radiotherapy target area of the pancreatic metastasis (cross section); (B) Radiotherapy target of the pancreatic metastases (coronal view); (C) Radiotherapy target of the pancreatic metastases (sagittal view); (D) Dose-volume histogram of radiotherapy for the pancreatic metastasis.
Follow-up CT scans were performed at the eighth and tenth cycles, both indicating stable disease. After 13 cycles, CT and MRI were performed on November 28, 2024, and the patient’s tumor response remained stable (Figures 3, 4). According to the treatment timeline (Figure 5), the patient achieved 11 months of progression-free survival (PFS) with only minor side effects while receiving cadonilimab plus anlotinib and radiotherapy for pancreatic metastasis in later-line therapy.
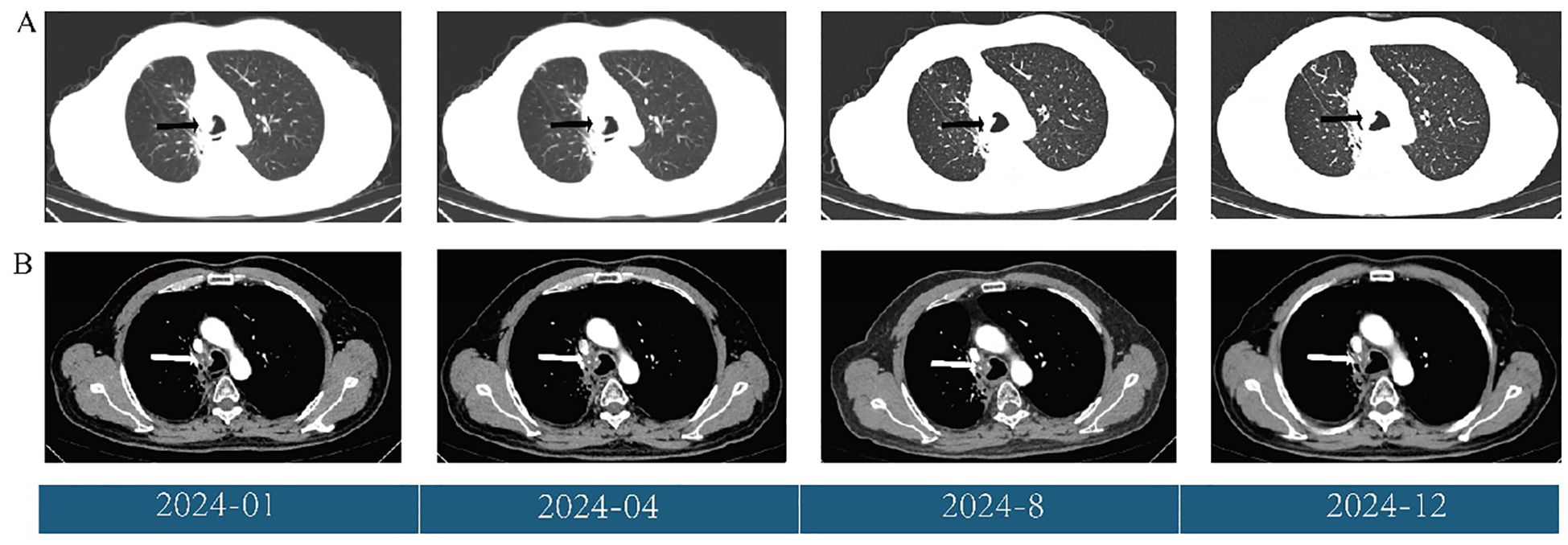
Figure 3. (A) Changes in the primary lung lesions (black arrow) during treatment, shown on CT (lung window). (B) Changes in the primary lung lesions (white arrow) during treatment, shown on CT (mediastinal window).
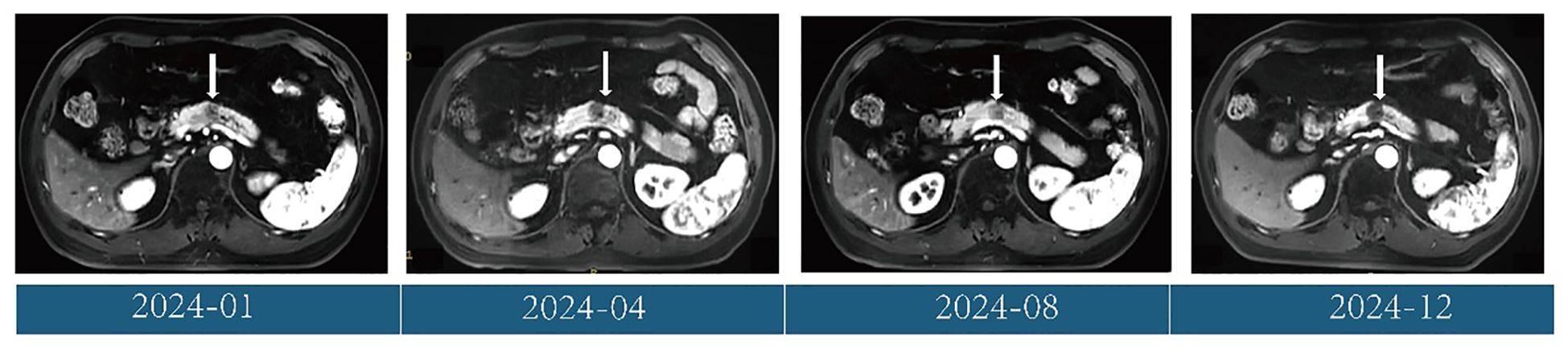
Figure 4. Changes in the pancreatic metastases (white arrow) during treatment, shown on MRI (T1 weighted image).
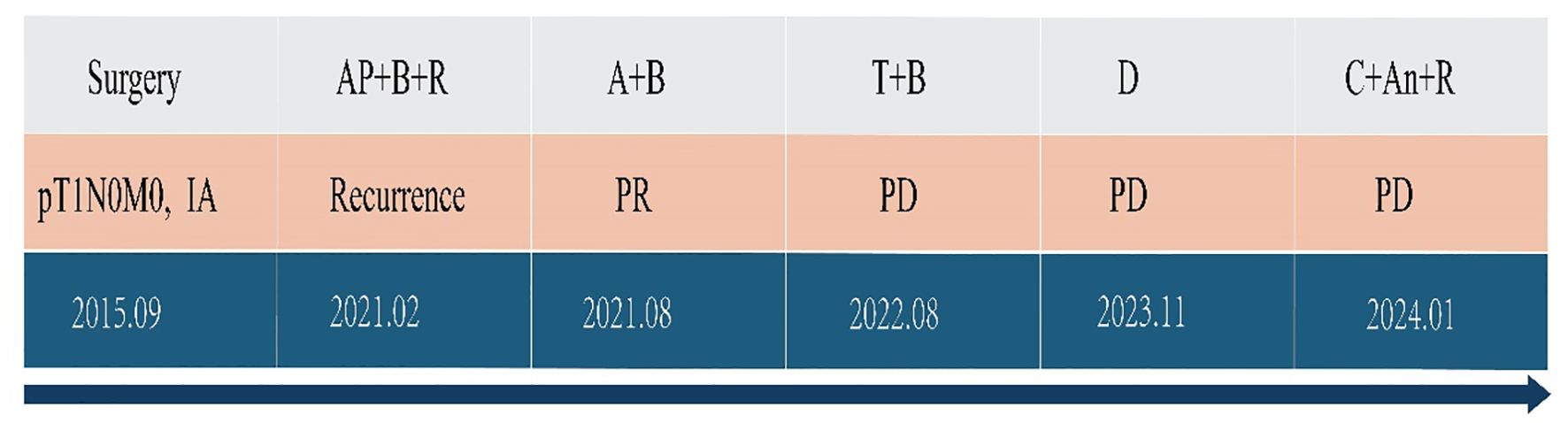
Figure 5. Antitumor treatment timeline. A, pemetrexed; P, cisplatin; B, bevacizumab; T, tislelizumab; C, cadonilimab; An, anlotinib; R, radiotherapy; PR, partial response; PD, progressive disease.
Discussion
Chemotherapy combined with ICIs remains the standard protocol for advanced NSCLC patients who are driver gene-negative and have low PD-L1 expression (tumor proportion score [TPS] < 5%). The KEYNOTE-189, IMpower150, and CameL studies have confirmed the survival advantage of this approach in non-squamous NSCLC (11, 12). However, in-depth analyses indicate that clinical benefits are limited in patients with low PD-L1 expression, and treatment-related toxicity—such as immune-related pneumonia, hepatitis, and chemotherapy-induced myelosuppression—is increased (13). Therefore, identifying more effective and less toxic treatment strategies has become a key focus of clinical research.
Compared with traditional PD-1+CTLA-4 monoclonal antibody combination therapies, cadonilimab can accurately target tumor-infiltrating lymphocytes due to its unique molecular structure. This improves efficacy while significantly reducing peripheral immunotoxicity (14). This advantage has been demonstrated in cervical cancer (7). However, its efficacy in other solid tumors, such as NSCLC, still requires validation through additional phase III clinical studies.
Previous studies have suggested that a “chemo-free” approach—combining anti-angiogenic therapy with immunotherapy —can improve outcomes in patients who are intolerant to chemoimmunotherapy. In this case, cadonilimab plus anlotinib was used for systemic treatment, with radiotherapy directed at pancreatic metastases. To date, the patient has achieved 11 months of PFS, which exceeds the 6-month PFS reported by Wang X et al. (15). The extended PFS in this case may be attributed to the addition of radiotherapy to the treatment plan.
As a rare metastatic site of lung adenocarcinoma, studies have shown that receiving local pancreatic radiotherapy can improve patients’ overall survival (OS). In this case, the patient received radiotherapy for pancreatic metastasis followed by systemic treatment and achieved a progression-free survival (PFS) of 11 months (16). Common side effects of pancreatic radiotherapy include gastrointestinal toxicity (such as ulcers and bleeding) and exocrine pancreatic insufficiency (17). In addition, if the radiation dose to the proximal duodenum or jejunum exceeds 50 Gy, long-term complications such as intestinal wall fibrosis, stenosis, or even obstruction may occur, with an incidence of 2–9%. Severe cases may require surgical intervention (18). In this case, the radiotherapy dose was 45 Gy, which not only achieved good control of pancreatic metastasis, but also showed no obvious gastrointestinal side effects and pancreatic exocrine function injury.
Although anlotinib has shown remarkable efficacy in the treatment of solid tumors such as NSCLC, its potential side effects require close attention. In this case, early signs of kidney injury were observed after two treatment cycles. Previous studies have shown that anlotinib can increase the risk of proteinuria in cancer patients (19). Proposed mechanisms include podocyte injury, hemodynamically mediated glomerular damage, and thrombotic microangiopathy (20–22). Therefore, early monitoring and effective management play a crucial role.
Conclusion
We report a case of a patient with driver gene-negative primary lung adenocarcinoma who experienced disease progression following standard first- and second-line therapy, and subsequently achieved 11 months of PFS after treatment with cadonilimab plus anlotinib in combination with radiotherapy for pancreatic metastasis. This case highlights the potential value of a synergistic treatment regimen—immunotherapy + anti-angiogenesis + precision radiotherapy—for advanced driver gene-negative lung adenocarcinoma in later-line therapy, particularly in cases with pancreatic metastasis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Research Ethics Committee of the Affiliated Hospital of North Sichuan Medical College. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JY: Writing – original draft. ZL: Writing – original draft. XP: Writing – original draft. YZ: Supervision, Writing – review & editing. BZ: Supervision, Writing – review & editing. YG: Writing – review & editing. DM: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Nanchong Social Science Federation Project (NO. NC24B260) and the High-Level Talent Research Start-up Project of the Affiliated Hospital of North Sichuan Medical College (No. 2023-2GC016).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Siegel RL, Giaquinto AN, and Jemal A. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
3. Rolfo C, Mack P, Scagliotti GV, Aggarwal C, Arcila ME, Barlesi F, et al. Liquid biopsy for advanced NSCLC: A consensus statement from the international association for the study of lung cancer. J Thorac Oncol. (2021) 16:1647–62. doi: 10.1016/j.jtho.2021.06.017
4. Frankell AM, Dietzen M, Al BM, Lim EL, Karasaki T, Ward S, et al. Author Correction: The evolution of lung cancer and impact of subclonal selection in TRACERx. Nature. (2024) 631:E15. doi: 10.1038/s41586-024-07738-w
5. Nogami N, Barlesi F, Socinski MA, Reck M, Thomas CA, Cappuzzo F, et al. IMpower150 final exploratory analyses for atezolizumab plus bevacizumab and chemotherapy in key NSCLC patient subgroups with EGFR mutations or metastases in the liver or brain. J Thorac Oncol. (2022) 17:309–23. doi: 10.1016/j.jtho.2021.09.014
7. Gao X, Xu N, Li Z, Shen L, Ji K, Zheng Z, et al. Safety and antitumour activity of cadonilimab, an anti-PD-1/CTLA-4 bispecific antibody, for patients with advanced solid tumours (COMPASSION-03): a multicentre, open-label, phase 1b/2 trial. Lancet Oncol. (2023) 24:1134–46. doi: 10.1016/S1470-2045(23)00411-4
8. Liu S, Qin T, Liu Z, Wang J, Jia Y, Feng Y, et al. anlotinib alters tumor immune microenvironment by downregulating PD-L1 expression on vascular endothelial cells. Cell Death Dis. (2020) 11:309. doi: 10.1038/s41419-020-2511-3
9. Yang Y, Li L, Jiang Z, Wang B, and Pan Z. Anlotinib optimizes anti-tumor innate immunity to potentiate the therapeutic effect of PD-1 blockade in lung cancer. Cancer Immunol Immunother. (2020) 69:2523–32. doi: 10.1007/s00262-020-02641-5
10. Su Y, Luo B, Lu Y, Wang D, Yan J, Zheng J, et al. Anlotinib induces a T cell-inflamed tumor microenvironment by facilitating vessel normalization and enhances the efficacy of PD-1 checkpoint blockade in neuroblastoma. Clin Cancer Res. (2022) 28:793–809. doi: 10.1158/1078-0432.CCR-21-2241
11. Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. (2018) 378:2078–92. doi: 10.1056/NEJMoa1801005
12. Socinski MA, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, et al. IMpower150 final overall survival analyses for atezolizumab plus bevacizumab and chemotherapy in first-line metastatic nonsquamous NSCLC. J Thorac Oncol. (2021) 16:1909–24. doi: 10.1016/j.jtho.2021.07.009
13. Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med. (2021) 9:305–14. doi: 10.1016/S2213-2600(20)30365-9
14. Pang X, Huang Z, Zhong T, Zhang P, Wang ZM, Xia M, et al. Cadonilimab, a tetravalent PD-1/CTLA-4 bispecific antibody with trans-binding and enhanced target binding avidity. MAbs. (2023) 15:2180794. doi: 10.1080/19420862.2023.2180794
15. Wang X, Yang K, Yang Y, Wang X, and Yuan K. Immunotherapy rechallenge of advanced lung adenocarcinoma with cadonilimab (PD-1/CTLA-4 Bi-specific antibody): a case report. Anticancer Drugs. (2024) 35:288–91. doi: 10.1097/CAD.0000000000001557
16. Moore C, Hsu CC, Chen WM, Chen BPC, Han C, Story M, et al. Personalized ultrafractionated stereotactic adaptive radiotherapy (PULSAR) in preclinical models enhances single-agent immune checkpoint blockade. Int J Radiat Oncol Biol Phys. (2021) 110:1306–16. doi: 10.1016/j.ijrobp.2021.03.047
17. Shiba S, Miyasaka Y, Okamoto M, Komatsu S, Okazaki S, Shibuya K, et al. Deterioration of pancreatic exocrine function in carbon ion radiotherapy for pancreatic cancer. Clin Transl Radiat Oncol. (2021) 31:80–5. doi: 10.1016/j.ctro.2021.09.007
18. Kavanagh BD, Pan CC, Dawson LA, Das SK, Li XA, Ten Haken RK, et al. Radiation dose-volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys. (2010) 76:S101–7. doi: 10.1016/j.ijrobp.2009.05.071
19. Huang NS, Wei WJ, Xiang J, Chen J, Guan Q, Lu Z, et al. The efficacy and safety of anlotinib in neoadjuvant treatment of locally advanced thyroid cancer: A single-arm phase II clinical trial. Thyroid. (2021) 31:1808–13. doi: 10.1089/thy.2021.0307
20. Wu Q and Finley SD. Mathematical model predicts effective strategies to inhibit VEGF-eNOS signaling. J Clin Med. (2020) 9. doi: 10.3390/jcm9051255
21. Eroglu E, Saravi S, Sorrentino A, Steinhorn B, and Michel T. Discordance between eNOS phosphorylation and activation revealed by multispectral imaging and chemogenetic methods. Proc Natl Acad Sci U S A. (2019) 116:20210–7. doi: 10.1073/pnas.1910942116
Keywords: cadonilimab, anlotinib, lung adenocarcinoma, pancreatic metastasis, radiotherapy
Citation: Yang J, Li Z, Pang X, Zhang Y, Zeng B, Gui Y and Ma D (2025) Case Report: Cadonilimab plus anlotinib with radiotherapy for lung adenocarcinoma with pancreatic metastasis in later-line therapy. Front. Immunol. 16:1610710. doi: 10.3389/fimmu.2025.1610710
Received: 12 April 2025; Accepted: 23 June 2025;
Published: 18 July 2025.
Edited by:
Stefano Cavalieri, Fondazione IRCCS Istituto Nazionale dei Tumori, ItalyReviewed by:
Yunhuan Liu, Tongji University, ChinaShuang Wu, The University of Chicago, United States
Copyright © 2025 Yang, Li, Pang, Zhang, Zeng, Gui and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Gui, bnNjbWNneUAxNjMuY29t; Daiyuan Ma, bXNjbWNwaGR5anFAMTYzLmNvbQ==
†These authors share first authorship
 Jianquan Yang†
Jianquan Yang† Daiyuan Ma
Daiyuan Ma