- Department of Obstetrics, Huai’an Maternal and Child Health Care Hospital affiliated to Yangzhou University, Huai’an, Jiangsu, China
At the maternal-fetal interface from human early pregnancy, decidual macrophages (dMφs) comprise approximately 20% of the leukocyte population, displaying a distinct immunophenotype characterized by hybrid functional features that transcend conventional M1/M2 polarization paradigms. The dynamic balance between M1-like dMφs and M2-like dMφs in human early pregnancy is closely related to the success of pregnancy. However, the comprehensive subsets profiling of dMφs and the factors influencing polarization haven’t been elucidated until recent years. In this review, we first delineate the dMφs compositional proportion and subsets profiling during early gestation. Second, we clarify the mechanisms underlying dMφs recruitment and tissue residency. Finally, we comprehensively synthesize molecular drivers of dMφs polarization and the functional specialization of polarized dMφs in sustaining successful pregnancy. A comprehensive understanding of the molecular network governing dMφs polarization dynamics and their functional contributions to gestational processes will provide crucial insights for developing targeted therapeutic strategies to address pregnancy-related complications.
1 Introduction
During early human pregnancy, the uterine mucosa undergoes a specialized transformation into the decidua, a receptive tissue that facilitates the implantation of fetal-derived trophoblast cells. This critical biological process initiates a cascade of gestational adaptations, including extensive remodeling of uterine smooth muscle cells and spiral arteries. These coordinated morphological changes ultimately culminate in the establishment of a functional placental organ. Within the placental microenvironment, invasive trophoblasts, decidual stromal cells (DSCs), and specialized immune populations form direct interaction (1). The dynamic crosstalk among these cellular components is essential for maintaining maternal-fetal immune tolerance and ensuring gestational success. Notably, placental macrophages (Mφs), which exhibit distinct phenotypic characteristics compared to their other tissue counterparts, emerge as central regulators in human early pregnancy.
Mφs are generally categorized M1 (classically activated) and M2 (alternatively activated) subtypes (2). M1 Mφs function as pro-inflammatory immune effector characterized by three distinct features: (1) elevated expression of antigen-presenting molecules (MHC-II) and co-stimulatory molecules (CD80, CD86) (3); (2) increased secretion of pro-inflammatory cytokines (interferon-gamma (IFN-γ), reactive oxygen species (ROS), interleukin-12 (IL-12), IL-23, IL-1β); (3) metabolic reprogramming toward glycolysis with concomitant ROS generation (4–6). In contrast, M2 Mφs demonstrate immunosuppressive properties through three complementary mechanisms : (1) immunoregulatory mediator production including IL-10 and transforming growth factor beta (TGF-β); (2) up-regulation of surface marker scavenging receptors and mannose receptor (CD206, CD209, CD163) (7, 8); (3) metabolic shift toward oxidative phosphorylation (OXPHOS) coupled with fatty acid β-oxidation (6). This unique combination enables M2 Mφs to perform tissue-protective functions such as apoptotic cell clearance, extracellular matrix remodeling, and resolution of inflammatory responses.
This functional dichotomy between M1/M2 Mφs is governed by distinct activation pathways. M1 Mφs are typically activated through exposure to pro-inflammatory mediators including tumor necrosis factor-alpha (TNF-α) and IFN-γ, or via engagement of pathogen-associated molecular patterns (PAMPs) such as bacterial lipopolysaccharide (LPS) (9). In contrast, M2 Mφs polarization is orchestrated by anti-inflammatory cytokines, notably IL-4 and IL-13 (8). Pro-inflammatory mediators mediate M1 polarization through engagement of surface receptors, including cytokine receptors and pattern recognition receptors such as toll-like receptor 4 (TLR4). This signaling cascade activates transcription factors like nuclear factor-kappa B (NF-κB) and signal transducer and activator of transcription 1 (STAT1), which drive the expression of genes characteristic of the pro-inflammatory M1 phenotype (10). Conversely, anti-inflammatory cytokines stimulate transcription factors such as STAT6 and peroxisome proliferator-activated receptors (PPARs), facilitating the transcriptional program associated with the immunoregulatory M2 phenotype (11).
Placental Mφs consist of maternal-derived decidual Mφs (dMφs) and fetal-derived Hofbauer cells. Recent years, placental Mφs have become a research hotspot. Hofbauer cells closely resemble alternatively activated M2 Mφs (12), while dMφs display dynamic plasticity and functional heterogeneity that diverge from the classical M1/M2 dichotomy (13). Therefore, in this review, we mainly focus on maternal-derived dMφs. In response to this evolving understanding, the scientific community is increasingly adopting the M1-like and M2-like dMφs. M1-like dMφs and M2-like dMφs denote a broader spectrum of Mφ status that may overlap or transition between these traditional M1 and M2 Mφ categories. The immune status of dMφs is suggested to be dynamic during gestation, with an M1-like status during the peri-implantation period, a mixed M1/M2-like status during early pregnancy followed by an M2-like status during the second trimester, and an M1-like status by the end of pregnancy (3, 14–16). In human early pregnancies, the dynamic balance between M1-like dMφs and M2-like dMφs is closely related to the the success of pregnancy. M1-like dMφs initiate local inflammation and aid embryo implantation and decidualization. M2-like dMφs maintain immune tolerance, phagocytose apoptotic cells and participate in spiral artery remodeling. However, a disruption of the balance of dMφs may result in various adverse pregnancy outcomes including recurrent spontaneous abortion (RSA), pre-eclampsia (PE) and fetal growth restriction (FGR). Only by fully understanding the factors regulating dMφs polarization and roles of dMφs in pregnancy will we be able to develop interventions for the treatment of these various pregnancy complications. Therefore, this systematic review delineates the subsets characteristics of polarized dMφs, mechanisms underlying dMφs recruitment, and molecular drivers of dMφs polarization at the human maternal-fetal interface during early gestation.
2 The dMφs frequency at the maternal-fetal interface
In the human first-trimester pregnancy, the most preponderant maternal immune cells at the maternal-fetal interface are CD56+decidual natural killer (dNK) cells, which account for approximately 60%, and then followed by dMφs at 20% and T cells at 10% (17–19). These findings were typically derived from single-cell suspension techniques. Notably, the inevitable loss of specific cell populations during the isolation procedure may potentially compromise the accuracy of the results. Krop et al. conducted a comparative analysis of immune cell frequencies in the human decidua between tissue sections and single-cell suspensions (20). Their findings revealed significantly higher myeloid cell proportions in tissue sections (35.8%, 52.5%, and 60% during the first, second, and third trimesters, respectively) compared to single-cell suspensions (20%, 26.8%, and 9.4% at corresponding gestational stages) (20). Complementing these findings, a multi-omics study integrating spatial proteomics and transcriptomics demonstrated dynamic shifts in decidual immune composition: while dNK cells predominated at 6 weeks of gestation, dMφs progressively increased from 8 weeks, surpassing dNK cells by 12 weeks (21). Collectively, these results demonstrate that dMφs—the principal antigen-presenting cells (APC) in the human decidua—were substantially underrepresented in conventional analytical approaches, highlighting methodological limitations in assessing their true physiological prevalence.
3 The characteristics of dMφs subsets in human early pregnancy
3.1 Traditional classification of dMφs subsets
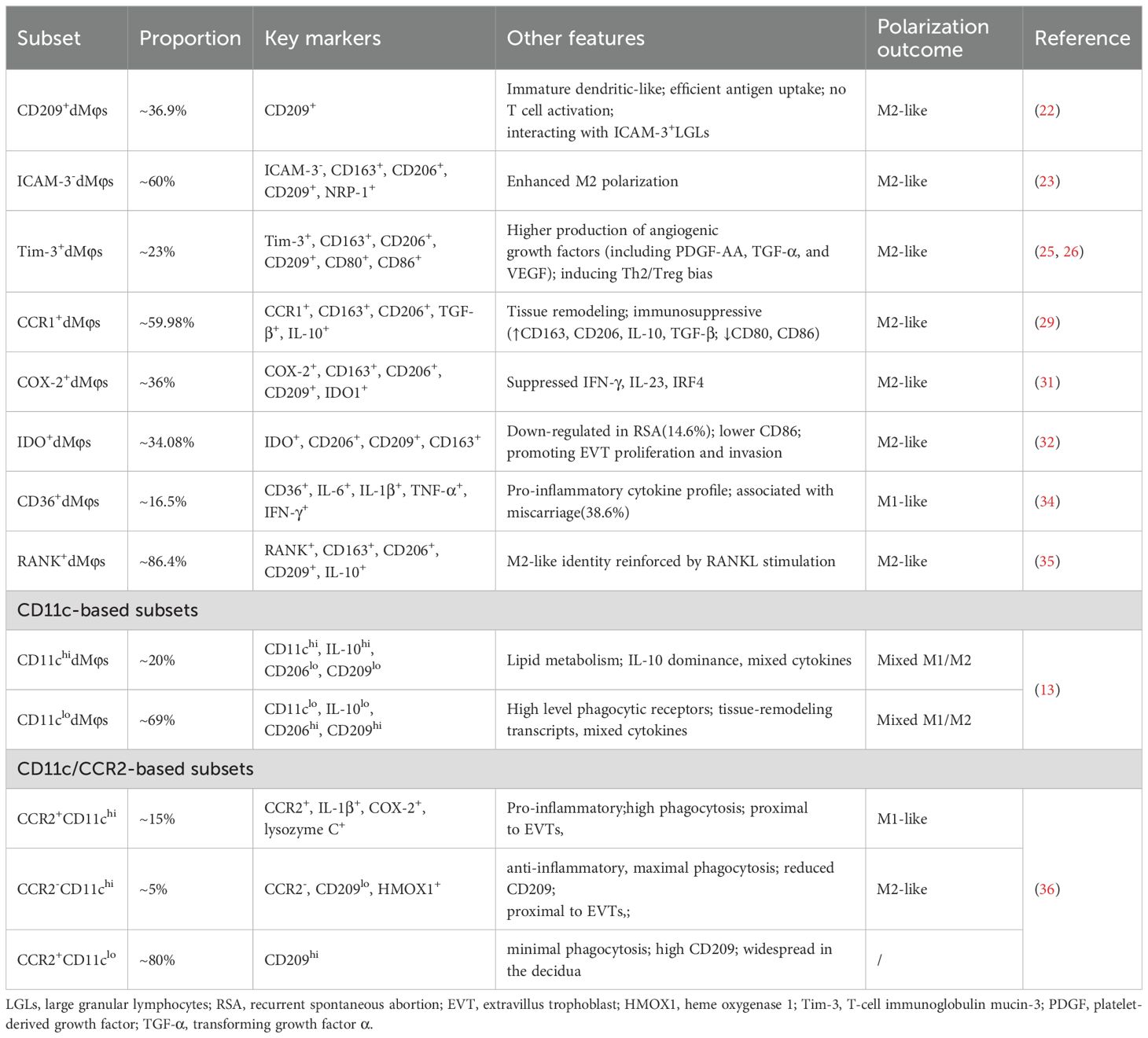
In recent years, several investigators had performed single-cell analysis of human decidual immune cells, either by RNA sequencing or flow cytometric cell sorting. This had led to a more detailed insight into the different M1/M2-like polarized dMφs encountered at the human decidua. The phenotypic and functional heterogeneity of dMφs have been characterized through surface marker profiling including phagocytic receptor CD209(DC-SIGN), intercellular adhesion molecule-3 (ICAM-3), T-cell immunoglobulin mucin-3 (Tim-3), cyclooxygenase-2 (COX-2), chemokine (CC motif) receptor 1 (CCR1), CCR2, indoleamine 2, 3-dioxygenase 1 (IDO1), CD36, receptor activator of nuclear factor kappa B (RANK) and CD11c (Table 1).
Pioneering work by Kammerer et al. revealed unique properties of dMφs in human early pregnancy (22). CD209 is a well-known marker for classic M2 Mφs. Compared to endometrial Mφs, 36.9% of dMφs specifically expressed the phagocytic receptor CD209, exhibiting an immature dendritic cell-like phenotype (Table 1). These CD209+dMφs demonstrated efficient antigen uptake capacity in vitro but failed to stimulate naïve allogeneic T cells.
Further stratification based on ICAM-3, Tim-3, CCR1, COX-2, IDO-1 and CD36 expression revealed functional divergence. ICAM-3, a transmembrane glycoprotein mediating leukocyte adhesion and cellular survival, has not been definitively classified as a marker for either canonical M1 or M2 Mφs subsets. Intriguingly, about 60% of human early pregnancy dMφs lacked ICAM-3 (23) (Table 1). Notably, compared with ICAM-3+dMφs, ICAM-3-dMφs displayed enhanced M2 polarization, with significantly elevated CD163, CD206, CD209, and neuropilin-1 (NRP-1) (23) (Table 1). This inverse correlation between ICAM-3 expression and M2 marker profiles positioned ICAM-3 as a potential identifier of pro-inflammatory M1-like dMφs at the maternal-fetal interface. Tim-3, a checkpoint receptor expressed by a wide variety of immune cells, exerts anti-inflammatory effects through suppression of ROS generation and inflammasome-dependent cytokine secretion (IL-1β, IL-18) in Mφs (24). Strikingly, at the maternal-fetal interface during human early pregnancy, Tim-3+dMφs demonstrated dual functional specialization: (1) pro-angiogenic capacity: enhanced production of growth factors including platelet-derived growth factor-AA (PDGF-AA), TGF-α, and vascular endothelial growth factor (VEGF); (2) immunomodulatory activity: promoting Th2 and Treg bias (25, 26) (Table 1). The functional dynamics of CCR1 in Mφs regulation demonstrated complex tissue-specific and ligand-dependent characteristics (27, 28). Recent study revealed distinct anti-inflammatory properties of CCR1+dMφs in human early pregnancy. Compared to their CCR1- counterparts, CCR1+dMφs displayed elevated expression of scavenger receptors (CD163, CD206), enhanced production of immunoregulatory cytokines (IL-10, TGF-β) (29) (Table 1). The COX-2/PGE2 axis, traditionally associated with M1 polarization (30), exhibited paradoxical regulatory effects in human dMφs. COX-2+dMφs paradoxically exhibited higher levels of CD163, CD206, CD209 and IDO-1, as well as lower levels of interferon regulatory factor 4 (IRF4), IFN-γ and IL-23 than COX-2-dMφs (Table 1), suggesting that COX-2+dMφs presented an M2-like phenotype (31). The immunomodulatory enzyme IDO, primarily expressed by APC including Mφs, mediates tryptophan catabolism via the kynurenine pathway. The percentage of IDO+dMφs from women with normal pregnancy and RSA were 34.08% and 14.6%, respectively (32). IDO+dMφs had higher levels of CD206, CD209 and CD163, and a lower level of CD86 compared with IDO−dMφ (Table 1), suggesting that IDO+dMφs displayed an M2-like phenotype during human early pregnancy (32). CD36, a multifunctional receptor mediating lipoprotein recognition, apoptotic cell clearance, and fatty acid transport, also serves as a pattern recognition receptor. Within the classical M1/M2 polarization framework, CD36 demonstrated preferential association with M2 Mφs through lipid-mediated mechanisms (33). Mechanistically, CD36-dependent triglyceride trafficking facilitated up-regulation of canonical M2 markers (CD206, CD163) (33). Paradoxically, CD36 presented strong link with M1-like dMφs in human early pregnancy (34). Clinical analyses revealed a striking elevation in CD36+dMφs prevalence among RSA patients (38.6% versus 16.5% in normal pregnancies) (Table 1). These CD36+dMφs exhibited amplified pro-inflammatory cytokines (IL-1β, IL-6, TNF-α, IFN-γ) compared to their CD36- counterparts, with comparative analysis showing further up-regulation of pro-inflammatory cytokine in RSA-derived CD36+dMφs relative to normal pregnancy controls (34). This tissue-specific inversion of CD36’s polarization association suggested microenvironment-driven functional plasticity. The molecular mechanisms underlying this phenomenon was systematically clarified in Section 5.
The RANK/RANK ligand (RANKL) axis emerged as another modulator of dMφs plasticity in human early pregnancy. RANK+dMφs, 86.4% at human dMφs from early pregnancy, exhibited up-regulated M2 markers (CD206, CD209, CD163, IL-10) compared with RANK-dMφs (Table 1) (35). RANKL stimulation markedly enhanced the M2 characteristics while suppressing M1 features in RANK+dMφs, demonstrating the RANK/RANKL axis’s pivotal role in controlling dMφs polarization (35).
The CD11c-based classification revealed hybrid phenotype. CD11chidMφs (20%) showed IL-10 dominance, low phagocytic receptors (CD206, CD209) and lipid metabolism gene enrichment, while CD11clodMφs (69%) expressed higher level of CD206, CD209 and tissue-remodeling transcripts as well as low IL-10 (13) (Table 1). Both populations secreted mixed cytokines, reflecting a mixed M1/M2-like dMφs states (13). Further CCR2 stratification of CD11c subsets identified functional gradations (36) (Table 1). CCR2+CD11chidMφs (15%), proximal to EVTs, co-expressed pro-inflammatory mediators (IL-1β, COX-2, lysozyme C) with high phagocytic capacity (36). CCR2-CD11chidMφs (5%) exhibited maximal phagocytosis but reduced CD209, a characteristic feature that is also associated with the CD11chidMφs (36). CCR2-CD11chidMφs, also proximal to EVTs, expressed higher levels of heme metabolism genes indicating its anti-inflammatory role (36). CCR2-CD11clo (80%), widespread in the decidua, showed minimal phagocytic activity and high CD209 correlating well with CD11clodMφs subset (36). Due to the complexity of dMφs, Ning et al. proposed that the function of dMφs in tissue remodeling versus inflammation will not be easily attributable to one or other subset (16).
3.2 Emerging multi-dimensional classification of dMφ subsets
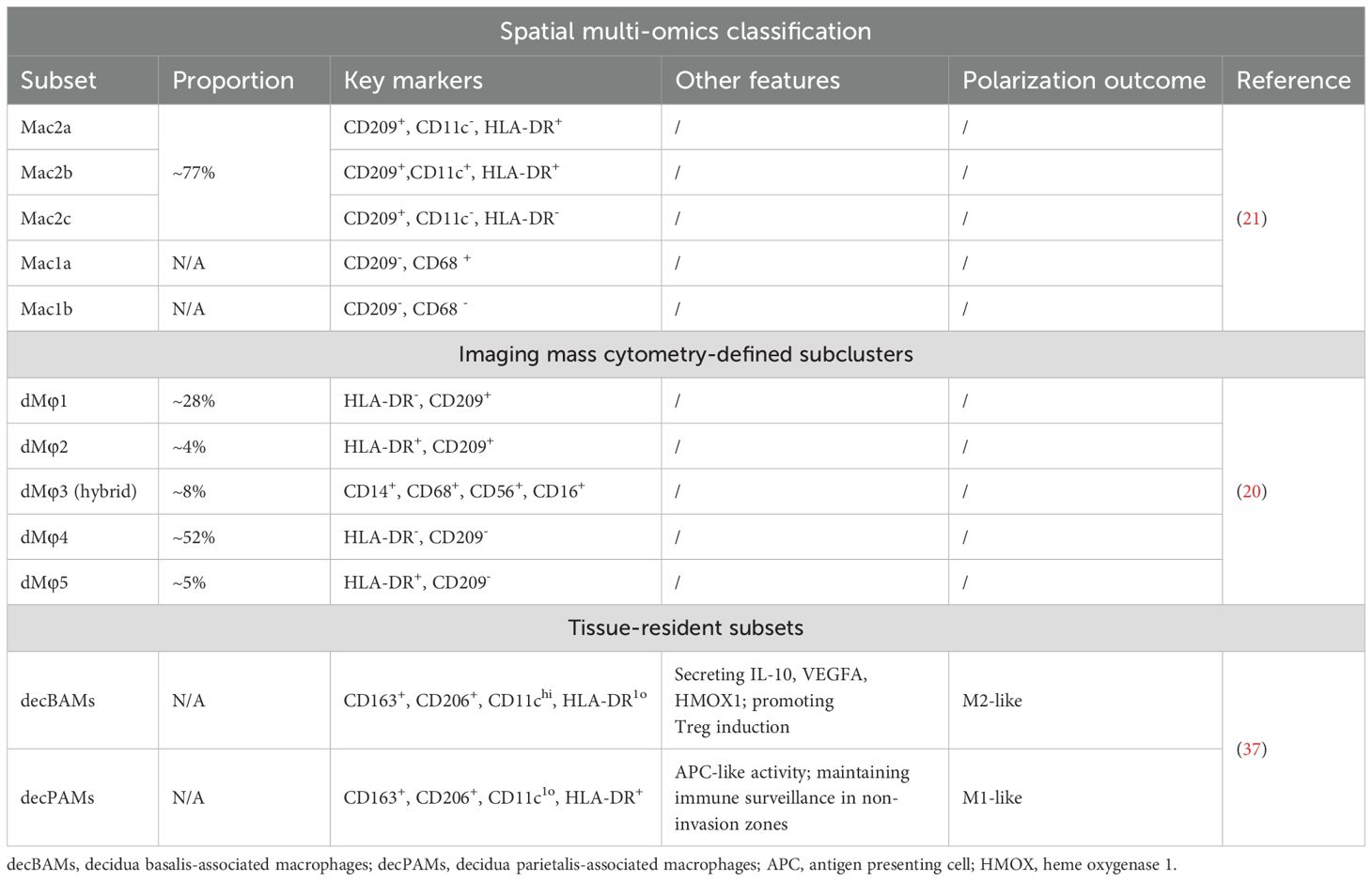
While traditional classification of dMφs relies on one or two surface markers, recent advances in spatial multi-omics and single-cell technologies unveiled a far more complex landscape, emphasizing the necessity of multi-parameter stratification. Spatial proteomic/transcriptomic studies resolved CD209+dMφs (77% prevalence in early pregnancy) into three functionally distinct subsets:Mac2a (CD11c-HLA-DR+), Mac2b (CD11c+HLA-DR+) and Mac2c (CD11c-HLA-DR-) (21) (Table 2). CD209–dMφs were subclustered on the basis of CD68 expression: Mac1a (CD68+) and Mac1b (CD68-) (21). Imaging mass cytometry (IMC) have further resolved the heterogeneity of dMφs, identifying six distinct subclusters during early pregnancy. Among these, four subclusters were definitively stratified by combinatorial expression of HLA-DR and CD209: dMφ1 (HLA-DR-CD209+), dMφ2 (HLA-DR+CD209+), dMφ4 (HLA-DR-CD209-) and dMφ5 (HLA-DR+CD209-) (20) (Table 2). Notably, dMφ1 and dMφ4—both lacking HLA-DR expression—constituted the dominant populations in first-trimester decidua, suggesting their potential roles in early gestational immune modulation. A novel hybrid subset dMφ3 exhibited dual expression of myeloid markers (CD14, CD68) and NK cell markers (CD56), a phenotype previously uncharacterized in decidual tissue (20). This unique co-expression pattern hinting its trans-differentiation potential.
3.3 Spatial classification of dMφs subsets
The dMφs are not uniformly confronted with placental tissues. According to resident tissue, dMφ were categorized into decidua basalis-associated macrophages (decBAMs) and decidua parietalis-associated macrophages (decPAMs) (37) (Table 2). The decBAMs (CD163+CD206+CD11chiHLA-DRlo) secreted pregnancy-sustaining factors (IL-10, VEGFA, HMOX1) and promoted Treg induction, aligning with transcriptional profiles of scRNA-seq-defined CD11chi dMφs. The decPAMs (CD163+CD206+CD11cloHLA-DR+) displayed APC-like activity (phagocytosis, T cell activation), likely maintaining immune surveillance in non-invasion zones.
Above results showed that the dMφs subset were complex and were affected by techniques, markers, and tissue collection strategies. There are also some significant discrepancies regarding the distribution of sepcific dMφs subsets. For example, Kammerer et al. observed that 36.9% of dMφs expressed CD209 (22), whereas Greenbaum et al. reported a significantly higher proporation of CD209+dMφs, which accounted for 77% (21). However, Krop et al. demonstrated a contrasting predominance of CD209-dMφs (52%) (20). In the trophoblast cell microenvironment from the human first-trimester, IMC showed that dMφs localized proximal to EVT were two HLA-DR- subclusters (dMφ1 and dMφ4) (20). However, spatial proteomics and transcriptomics showed that HLA-DR+Mac2a were detected close to EVT (21).
In conclusion, the in-depth understanding of subsets provides an opportunity to open an avenue for the significance of dMφs during pregnancy.
4 Factors influencing dMφs recruitment and residence
DMφs are often detected in close vicinity of invading trophoblasts and in the vessel wall of the actively remodeling vessels (38). However, the factors affecting recruitment and residence of dMφs in these sites have not been fully clarified. Previous reviews described that the constitution of adult tissue Mφs includes long-lived Mφs from yolk sac erythro-myeloid progenitors (EMP) and fetal liver hematopoietic stem cells (HSC) as well as short-lived bone marrow HSCs-derived monocytes (1, 6, 39). So far, the studies of dMφs originated from long-lived Mφs were limited (16). Thus, in this part, we will mainly review the recruitment and residence of dMφs originated from short-lived peripheral monocyte (pMo) cells.
4.1 Chemokines and growth factors
Chemokines, categorized into C, CC, CXC, and CX3C subfamilies based on conserved cysteine motifs, regulate immune cell trafficking through interactions with G-protein-coupled receptors (40). At the maternal-fetal interface, trophoblasts and DSCs secrete multiple chemokines during human early pregnancy (41–46). First-trimester trophoblasts produced CXCL16 (47), which engaged CXCR6 receptors expressed on pMo and dMφs (48). Functional studies confirmed that the CXCL16/CXCR6 axis critically mediated pMo recruitment (48) (Figure 1). Notably, CXCR6 expression declined after pMo differentiate into dMφs (87.92%→47.74%) (48), suggesting this signaling may primarily mediate monocyte recruitment rather than post-differentiation retention. Concurrently, DSCs secreted CCL8 and CCL2, which cooperatively enhanced dMφs chemotaxis through CCR1 and CCR2 receptors, respectively (29, 49, 50) (Figure 1). In addition, it was confirmed that CCL2 level was regulated by VEGFA (Figure 1). For example, VEGFA promoted the secretion of CCL2 from DSCs in hypoxia environment (50). In addition, VEGFA up-regulated the expression of adhesion molecules (ICAM-1, ICAM-5) in DSCs and thus facilitated dMφs anchorage to decidual tissues (50) (Figure 1). M-CSF/CCR2 interaction also induced the dMφs recruitment (51) (Figure 1).
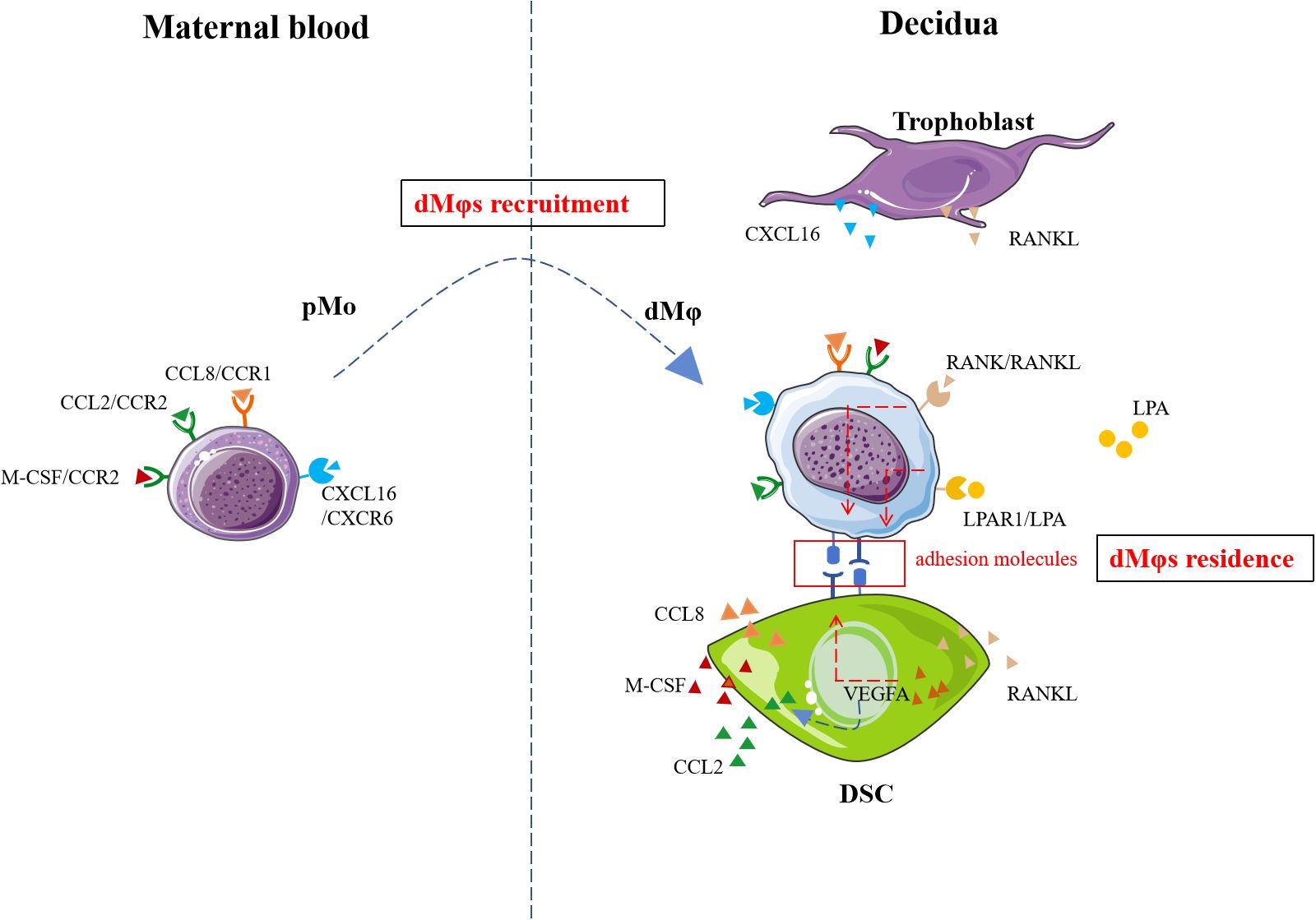
Figure 1. The recruitment and residence mechanisms of dMφs. Recruitment signaling including CXCL16/CXCR6, CCL8/CCR1, CCL2/CCR2, M-CSF/CCR2 and VEGFA promoted the migration of pMo cells to decidua. RANKL/RANK, LPA/LPAR1 and VEGFA facilitate the residence of dMφs. pMo, peripheral blood monocytes; CXCL16, CXC motif chemokine ligand 16; CCL8, CC chemokine ligand 8; CCR1, chemokine-receptor 1; LPA, lysophosphatidic acid; LPAR1, LPA receptors; VEGFA, vascular endothelial growth factor A; RANKL, receptor activator of nuclear factor-kappa B ligand; RANK, receptor activator of nuclear factor-kappa B; M-CSF, macrophage-colony stimulating factor; DSC, decidual stromal cell. The figure was produced by Microsoft Office PowerPoint.
4.2 RANKL-RANK
Liao et al. showed that RANK+dMφs from human early pregnancy was the dominating subset with higher adhesion molecules expression (CD29, CD31, CD54, CD62L) (52). The interaction of RANKL secreted by DSCs and RANK on dMφs increased the expression of adhesion molecules on dMφs (Figure 1), which in turn allowed dMφs to infiltration into the decidua (52).
4.3 Lysophosphatidic acid metabolism
A previous report by microarray data analysis indicated genes PPARγ was highly expressed by CD11chidMφs from human early pregnancy (13). However, the potential function was unknown. Recently, metabolomics analysis in human dMφs indicated an increased lysophosphatidic acid (LPA) metabolism and high levels of LPA receptors including specific cell-surface G protein coupled receptors LPAR1 and the intracellular receptor PPARγ (53). Yang et al. confirmed that the activation of LPA/LPAR1 or LPA/PPARγ signaling promoted dMφs adhesion to DSCs in a dose-dependent manner by up-regulating adhesion molecules including E-cadherin, E-selectin, L-selectin and integrinαV in vitro (Figure 1). Mechanistically, this process was mediated through activation of the macroautophagy/autophagy, and further up-regulation of multiple adhesion factors (cadherins and selectins) in a claudin 7-dependent manner (53).
5 Factors influencing dMφs polarization and function
Previous findings have consistently shown that the number of M1-like dMφs is higher in women with pregnancy complications such as RSA and PE (54, 55). These observations suggest that a balance between M1/M2-like dMφs is crucial in maintaining decidual homeostasis. Mφs may be shaped by the tissues in which they reside, and they are able to change their functions in response to different microenvironments, forming a broad repertoire of Mφs functions. However, factors involved in M1/M2-like dMφs homeostasis are largely unknown. Recently, advances about regulatory networks underlying dMφs polarization at the human maternal-fetal interface have been achieved, which will provide novel opportunities for manipulating various pregnancy complications.
5.1 Cytokines
Human first-trimester decidual cells secrete many colony-stimulating factors (CSFs), which then acted as potent inducers of Mφs proliferation, differentiation, and activation. The pre-eclamptic decidua contained an excess of both GM-CSF and M-CSF (56, 57). In response to pro-inflammatory stimulation in vitro, human first-trimester decidual cells (leukocyte-free) also enhanced GM-CSF and M-CSF expression (56, 57) (Figure 2). GM-CSF drove human pMo cells toward an M1-like subtype, while M-CSF polarized human pMo cells toward an M2-like subtype (23) (Figure 2). During the pathogenesis of PE, GM-CSF promoted pro-inflammatory M1-like dMφs being the predominant subtype while M-CSF induced immunosuppressive M2-like phenotype serving as a compensatory response to modulate the decidual immune balance (23, 56, 57). IL-34, a second ligand for the M-CSF receptor, was produced by first-trimester DSCs (Figure 2). IL-34, in vitro, was able to polarize pMo cells toward an M2-like phenotype (58).
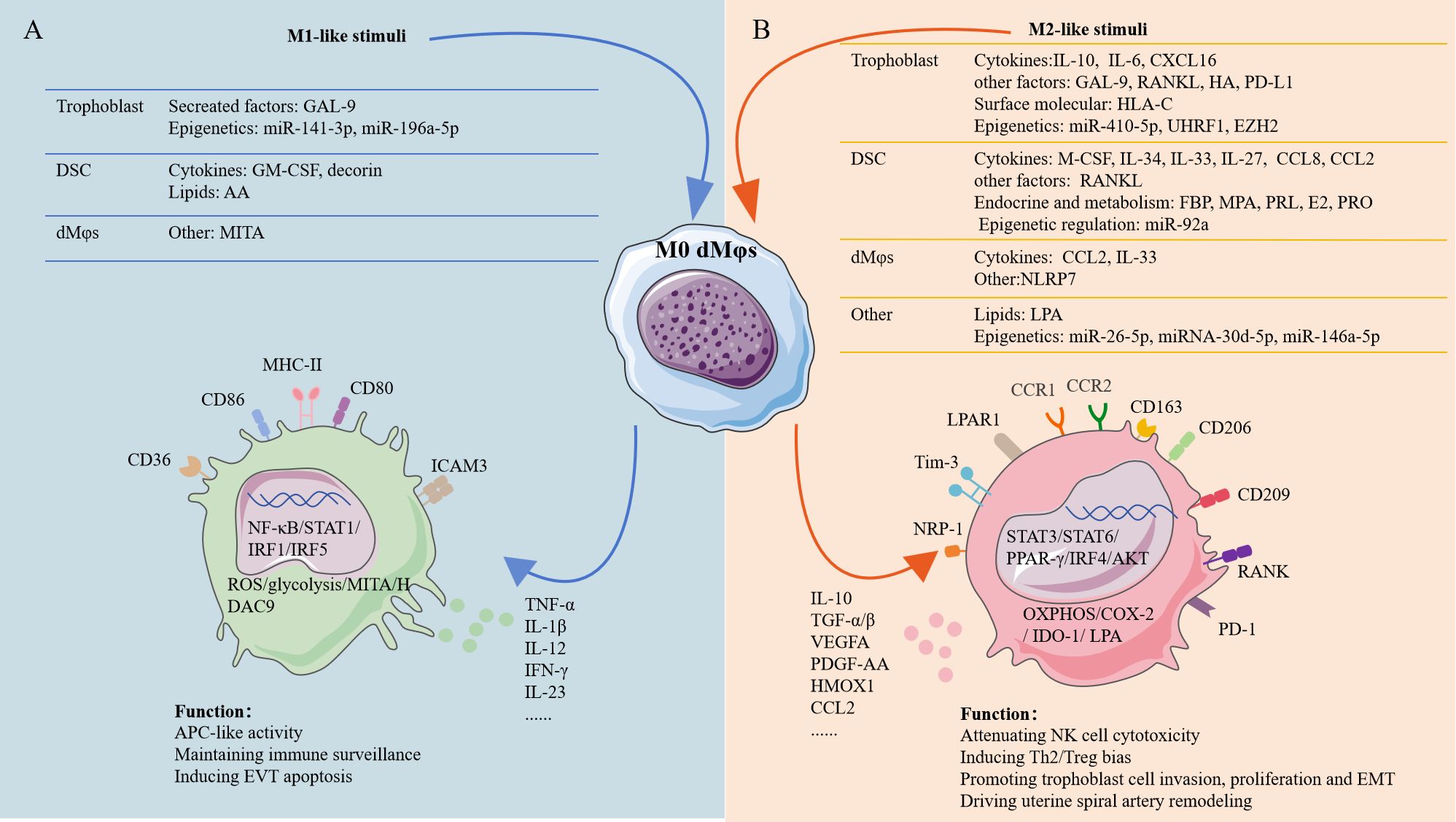
Figure 2. The factors shaping dMφs polarization at the human early maternal-fetal interface. Stimuli of M1-like dMφs and M2-like dMφs were mainly originated from fetal trophoblast, maternal DSCs an dMφs. (A) Transcription factors in M1-like dMφs mainly involve NF-κB, STAT1, IRF1 and IRF5. M1-like dMφs phenotype: high expression of CD80, CD86, MHC-II, CD36 and ICAM-3 in cell surface as well as ROS, MITA and HDAC9 inside the cell; secreting high level of TNF-α, IL-1β, IL-12, IFN-γ and IL-23; activated glycolysis. (B) Transcription factors in M2-like dMφs mainly involve STAT3, STAT6, PPAR-γ and IRF4; M2-lile dMφs phenotype: high expression of CD163, CD209, CD206, RANK, PD-1, CCR1, CCR2, NRP1, Tim-3 and LPAR1 in cell surface as well as COX-2 and IDO-1 inside the cell; secreting high level of IL-10, TGF-β, VEGFA, CCL2 and HMOX1; activated OXPHOS and LPA metabolism. AA, arachidonic acid; PRL; prolactin; HA, hyaluronic acid; FBP, fructose-1,6-bisphosphate; MPA, medroxyprogesterone acetate; E2; estradiol; PRO, progesterone; IRF1, interferon regulatory factor 1; PPAR-γ, peroxisome proliferator-activated receptor gamma; ICAM-3, intercellular adhesion molecule 3; NRP1, neuropilin 1; HMOX1, heme oxygenase 1; COX-2, cyclooxygenase-2; IDO-1, indoleamine 2,3-dioxygenase 1; T-cell immunoglobulin mucin-3 (Tim-3); PDGF, platelet-derived growth factor; EMT, epithelial-to-mesenchymal transition. The figure was produced by Microsoft Office PowerPoint.
IL-10 was expressed by fetal trophoblasts at the human maternal-fetal interface, increasing from 7.33 pg/mL at 5 weeks to 9.99 pg/mL at≥9 weeks of gestation (23). IL-10 and M-CSF both promoted dMφs polarization with higher CD14, CD163, CD206 and CD209 expression and decreased ICAM-3 expression in vitro (23) (Figure 2). Compared with M-CSF, IL-10 was more potential inducer of M2-like dMφs. M-CSF plus IL-10 induced Mφs that displayed the closest phenotype to dMφs. Unexpectedly, classical Th2 cytokines (IL-4, IL-13), orchestrating the classic M2 polarization, were not able to promote the polarization of M2-like dMφs in vitro (23). Recently, Wang et al. further demonstrated that M2-like dMφs induced by IL-10 were linked with OXPHOS changes in mice (59) (Figure 2B). However, the related mechanism in human remains needs to be explored.
IL-6 is a multifunctional cytokine, which promoted M2 polarization in solid tumors and inflammatory environments (60, 61). IL-6 was recently found as a potential driver for M2-like dMφs in human pregnancy (62). Reduced jupiter microtubule-associated homolog 2 (JPT2) in RSA patients, correlated with down-regulated M1-like dMφs. Mechanistically, JPT2-deficient trophoblasts exhibited impaired IL-6 secretion, triggering M1 polarization and ROS overproduction—reversed by IL-6 supplementation (62) (Figure 2A). IL-27 interacts with a heterodimeric receptor composed of IL-27Rα and gp130 (63), presenting a wide spectrum of different functions ranging from promoting or curbing inflammatory diseases, cancers, and viral infections (64, 65). At the human early pregnancy, IL-27 from DSCs interacted with IL-27R on dMφs induced the COX-2+dMφs presenting an M2-like phenotype (31) (Figure 2B). Consistently, lower IL-27 in DSCs and a lower percentage of M2-like COX-2+dMφs from RSA patients were detected. However, excessive COX-2 in dMφs induced by excessive arachidonic acid (AA) metabolism from RSA patients leaded to severe inflammation by accumulating PGE2 and IL-1β (34).
IL-33, a member of the IL-1 family, is widely expressed under normal physiological conditions. IL-33 activates both the innate and adaptive immune systems through binding to the ST2 receptor. IL-33 and its orphan receptor ST2 were found to be co-expressed by DSCs and dMφs in human first-trimester pregnancy (Figure 2) (66). In RSA patients, decreased IL-33 was observed in DSCs and dMφs. In vitro, inhibited IL-33/ST2 signaling drove classical M1-like dMφs polarization (66).
5.2 Chemokines
In addition to being involved in cell recruitment and residence of dMφs, chemokines also play a pivotal role in dMφs polarization.
Trophoblasts from human early pregnancy secreted substantial quantities of CXCL16, with CXCR6 serving as its exclusive receptor (47, 48). This trophoblast-derived chemokine polarized primary human pMo cells toward an immunoregulatory phenotype, up-regulating M2-associated markers (CD163, CD206, IL-10) while suppressing M1-related molecules (CD80, CD86, IL-12) (Figure 2). Consequently, this phenotype shift reduced IL-15 production, thereby attenuating NK cell cytotoxicity (47).
CCR1+dMφs in human early pregnancy exhibited a significant M2-like phenotype. Furthermore, DSCs from human early pregnancy exhibited elevated expression of CCL8 (29), which functioned as cognate ligand for CCR1. Recently, elevated CCL8 from DSCs was confirmed as a regulator of CCR1+dMφs as indicated that CCL8 recruited peripheral CCR1+pMo cells, educated CCR1+pMo into CCR1+dMφs-like immunosuppressive subsets, and reinforced the CCR1+dMφs- exerted modulation of trophoblasts in vitro (29) (Figure 2). In RSA patients, CCL8 expression in DSCs was decreased and epithelial-to-mesenchymal transition (EMT) of trophoblast was defective.
At the human first-trimester decidua, expression of CCL2 was mainly detected in dMφs and DSCs (3). A previous finding that dMφs could be divided into three subsets based on CCR2 and CD11c showed that CCL2/CCR2 axis was essential for dMφs subpopulations (36). Wei et al. found that the anti-inflammatory status of dMφs was dependent on the CCL2/CCR2 signaling because the CCR2 inhibitor decreased CD163 expression of dMφs, whereas CD80 and CD86 expression were unaffected (3) (Figure 2). Therefore, CCL2 might influence the immune status of dMφs at the maternal-fetal interface in an autocrine and paracrine manner.
5.3 Extracellular matrix
Hyaluronan (HA) is found ubiquitously in the extracellular matrix (ECM) of all mammalian tissues. Beyond its well-established structural contributions to ECM organization and tissue homeostasis, accumulating experimental evidence demonstrates that HA actively participates in immunomodulatory processes (67, 68). CD44 is the principal receptor of HA (69), and HA/CD44 signaling has long been known to play a role in immune regulation. In human early pregnancy, primary trophoblasts could secreted high molecular weight HA (HMW-HA) continuously and about 80% of dMφs express CD44 (70). Wang et al. confirmed that treatment of dMφs from human early pregnancies with HMW-HA significantly up-regulated M2-associated markers while down-regulated M1-associated markers through CD44-mediated activation of the PI3K/AKT and STAT3/STAT6 signaling pathways (70) (Figure 2B).
Decorin is a member of proteoglycan family and involved in regulating collagen fibrillogenesis (71). In human early pregnancy, decorin was expressed by DSCs and significantly up-regulated in DSCs from RSA patients (72). Aberrant decorin level was related to various pregnancy complications (73). A positive correlation between decorin content and the proportion of M1-like dMφs was also observed in the decidua of early normal pregnant women (72). In murine Mφs, decorin treatment induced M1-like Mφs polarization, which was related to enhanced glycolysis, increased mitochondrial membrane potential and intracellular ROS levels (Figure 2A).
5.4 Immune checkpoints
Multiple immune checkpoints dynamically regulated dMφs polarization such as galectin-9 (Gal-9)/Tim-3 and PD-1/PD-L1 signaling (Figure 2). As mentioned above, Tim-3+dMφs demonstrated pro-angiogenic capacity and immunomodulatory activity (25, 26). Higher Tim-3 expression on dMφs was dependent on HLA-C on trophoblast during normal pregnancy (25) (Figure 2). Consistently, Gal-9/Tim-3 signaling alleviated inflammation by inducing M2-like polarization in rodent models of PE (74) (Figure 2). However, Gal-9/CD44 signaling promoted M1-like polarization associated with vascular dysfunction and PE risk in human pregnancy (55) (Figure 2). This functional dichotomy suggested receptor-dependent modulation of Gal-9. The PD-1/PD-L1 signaling further reinforced polarization homeostasis, where down-regulated PD-1 expression on dMφs and attenuated PD-L1 expression in placental villous tissues in RSA correlated with M1-like dMφs dominance (54). Experimental blockade studies confirmed PD-1 signaling inhibition critically promoted M1-like dMφs polarization by enhancing glycolysis and IRF5 activation (54) (Figure 2A).
5.5 Other factors
The RANK/RANKL signaling in osteoclasts regulates bone resorption via activating NF-κB pathway. However, the RANK/RANKL signaling predominantly drove M2-like polarization via AKT/STAT6/IRF4 signaling (35) (Figure 2B). When stimulated with RANKL, RANK+dMφs from human early pregnancy promoted Th2 bias but had no effect on decidual Treg cell differentiation (35).
5.6 Endocrine and metabolism
Mφs metabolic activity is an essential factor regulating their polarization and function (75, 76). Compared with human pMo cells, human dMφs from early pregnancy were significantly rich in LPA metabolism and expressed higher LPA receptor including specific cell-surface G protein coupled receptors LPAR1 and the intracellular receptor PPARγ (53) (Figure 2B). In pregnant mouse model, LPA deficiency promoted M1 polarization (53). Further research about whether LPA was involved in M2-like polarization of human dMφs is needed.
In the classical M1/M2 paradigm, CD36-dependent triglyceride transport is indispensable for M2 Mφs polarization. Contrasting this paradigm, CD36 paradoxically marked pro-inflammatory M1-like dMφs during early pregnancy, a functional shift mechanistically linked to its AA transport activity. In RSA patients, excessive AA accumulation was frequently observed in both DSCs and dMφs (34). Excessive accumulated AA was transferred from DSCs to dMφs via CD36 on dMφs, which excessively activated COX-2/PGE2/IL-1β signaling and promoted M1-like polarization in vitro (34) (Figure 2A). While in normal pregnancy, higher prolactin (PRL) from human DSCs was detected, which down-regulated CD36 expression in human dMφs, inhibiting lipid influx and the inflammatory phenotype of dMφs (34). This bidirectional regulation highlighted how microenvironmental cues reprogrammed CD36 functionality.
In addition to lipid accumulation, fructose-1,6-bisphosphate (FBP) was also accumulated in DSCs during human early pregnancy (31). FBP is considered responsible for sustaining glycolysis and increasing ATP production, eventually accelerating the decidualization. As mentioned above, IL-27 was identified as a new inducer of M2-like dMφs during early pregnancy. Further, IL-27 secreted by human DSCs was mainly promoted by FBP (Figure 2). Previously, it was considered that neither progesterone (PRO) nor estradiol (E2) showed any effects on the differentiation of M2-like dMφs induced from isolated CD14+pMo cells (23). However, PRO was found to promote the enrichment of FBP and IL-27 in DSCs isolated from first-trimester decidua (31) (Figure 2). Above results indicated the indirect role of FBP and PRO on regulating M2-like dMφs polarization. IDO+dMφ displayed a M2-like dominate phenotype during early pregnancy (32). Expression of IDO was increased remarkably after treatment dMφs with medroxyprogesterone acetate (MPA) or E2 (32) (Figure 2), suggesting that the endocrine environment contributed to the high level of IDO in dMφs during early pregnancy.
5.7 Post-transcriptional and epigenetic regulation
Post-transcriptional and epigenetic regulation are key mechanisms controlling gene expression. Post-transcriptional regulation is partly mediated by miRNAs, while epigenetic regulation—primarily involving DNA methylation (and active demethylation via hydroxymethylation intermediates) and histone modifications—can produce heritable phenotypic changes without altering the DNA sequence. Emerging evidence highlights their critical role in maintaining decidual immune homeostasis during early gestation. For instance, trophoblasts modulated dMφs polarization through miRNA-mediated pathways: miR-410-5p enhanced M2-like dMφs polarization by suppressing STAT1 (77) (Figure 2). Notably, miR-410-5p expression was significantly reduced in RSA patients compared to normal pregnancies (77) (Figure 2). Conversely, hypoxia-preconditioned trophoblasts (mimicking PE) and RSA-derived trophoblasts exhibited elevated miR-141-3p (78) and miR-196a-5p (79) levels, respectively. These miRNAs drove M1-like dMφs polarization via NF-κB pathway activation (78, 79) (Figure 2A). Additional miRNAs contributed to dMφs polarization: miR-92a (DSC-derived) (80), miR-26-5p (seminal plasma-derived) (81), and placental miRNAs of unclear origin (miR-146a-5p, miR-30d-5p) (82, 83) promoted M2-like dMφs polarization (Figure 2). Mechanistically, miR-30d-5p targeted histone deacetylase 9 (HDAC9), whose knockdown enhanced M2-like dMφs polarization (83).
Enhancer of zeste homolog 2 (EZH2) is a histone methyltransferase, which mediates the transcriptional silencing of target genes via H3K27me3 (84). Ubiquitin like with PHD and ring finger domains 1 (UHRF1) maintains DNA methylation status (85). Both EZH2 and UHRF1 were expressed by trophoblasts and down-regulated in RSA patients. The conditioned medium from EZH2 or UHRF1 knockdown trophoblasts both promoted M1-like dMφs polarization, indicating an indirect effect (86, 87).
5.8 NLRP7 and pyroptosis
The NOD-like receptor (NLR) family, a critical class of pattern recognition receptors (PRRs), typically mediates inflammasome assembly (NLRP1, NLRP3), pro-inflammatory cytokine release (IL-1β, IL-18), and pyroptosis—hallmarks of M1 Mφs activation (88). However, the role of NLRP7 seems different in dMφs. Unlike canonical NLRs, NLRP7 demonstrated preferential expression in M2-like dMφs compared to M1-like dMφs in the human first-trimester endometrial tissues (89). Functional studies revealed NLRP7 overexpression suppressed M1 markers while enhancing M2 polarization signatures.
Pyroptosis, a marker of M1 Mφs, was also regulated by mitochondrial adaptor protein MITA (90). Liu et al. showed that M1-like dMφs maintained elevated MITA levels to promote pyroptosis, while M2-like dMφs employed TRIM38 mediated K48-linked ubiquitination to degrade MITA, effectively suppressing pyroptosis (91). This polarization-dependent mechanism was clinically validated in RSA cases, where decidual tissues exhibited enhanced pyroptotic markers, higher MITA expression and impaired M2-like dMφs polarization (91).
6 Conclusion
The balance between pro-inflammatory (M1-like) and anti-inflammatory (M2-like) dMφs subsets emerges as a linchpin for maintaining immune homeostasis, with perturbations in this equilibrium linked to adverse pregnancy outcomes. This review systematically characterized the unique characteristics of dMφs subsets—distinct from classical Mφ polarization characteristics—and explored their potential utility in clinical diagnostics for distinguishing M1-like/M2-like dMφs subsets.
The recruitment mechanisms and factors controlling dMφs polarization offer actionable therapeutic targets. For instance, enhancing M2-like polarization via STAT3 and STAT6 activation or modulating placental-derived signals could mitigate excessive inflammation in RSA or PE. Furthermore, interventions targeting dMφs recruitment (CCR2/CCL2 signaling) or tissue-residency programs could restore decidual immune balance.
By bridging mechanistic insights with clinical translation, this synthesis underscores that precision modulation of dMφs dynamics—through small molecules, biologics, or cell-based therapies—holds transformative potential for treating pregnancy complications. Future research should prioritize validating these targets in preclinical models and developing biomarker-driven strategies to tailor interventions, ultimately advancing personalized care for gestational disorders.
Author contributions
HL: Writing – original draft. LZ: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sun F, Wang S, and Du M. Functional regulation of decidual macrophages during pregnancy. J Reprod Immunol. (2021) 143:103264. doi: 10.1016/j.jri.2020.103264
2. Mills CD, Kincaid K, Alt JM, Heilman MJ, and Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. (2000) 164:6166–73. doi: 10.4049/jimmunol.164.12.6166
3. Wei CY, Li MQ, Zhu XY, and Li DJ. Immune status of decidual macrophages is dependent on the CCL2/CCR2/JAK2 pathway during early pregnancy. Am J Reprod Immunol. (2021) 86:e13480. doi: 10.1111/aji.13480
4. Atri C, Guerfali FZ, and Laouini D. Role of human macrophage polarization in inflammation during infectious diseases. Int J Mol Sci. (2018) 19:1801. doi: 10.3390/ijms19061801
5. Palmieri EM, Gonzalez-Cotto M, Baseler WA, Davies LC, Ghesquiere B, Maio N, et al. Nitric oxide orchestrates metabolic rewiring in M1 macrophages by targeting aconitase 2 and pyruvate dehydrogenase. Nat Commun. (2020) 11:698. doi: 10.1038/s41467-020-14433-7
6. Guan F, Wang R, Yi Z, Luo P, Liu W, Xie Y, et al. Tissue macrophages: origin, heterogenity, biological functions, diseases and therapeutic targets. Signal Transduct Target Ther. (2025) 10:93. doi: 10.1038/s41392-025-02124-y
7. Schlundt C, Fischer H, Bucher CH, Rendenbach C, Duda GN, and Schmidt-Bleek K. The multifaceted roles of macrophages in bone regeneration: A story of polarization, activation and time. Acta Biomater. (2021) 133:46–57. doi: 10.1016/j.actbio.2021.04.052
8. Anders HJ and Ryu M. Renal microenvironments and macrophage phenotype determine progression or resolution of renal inflammation and fibrosis. Kidney Int. (2011) 80:915–25. doi: 10.1038/ki.2011.217
9. Toshchakov V, Jones BW, Perera PY, Thomas K, Cody MJ, Zhang S, et al. TLR4, but not TLR2, mediates IFN-beta-induced STAT1alpha/beta-dependent gene expression in macrophages. Nat Immunol. (2002) 3:392–8. doi: 10.1038/ni774
10. Kany S, Vollrath JT, and Relja B. Cytokines in inflammatory disease. Int J Mol Sci. (2019) 20:6008. doi: 10.3390/ijms20236008
11. Wautier JL and Wautier MP. Pro- and anti-inflammatory prostaglandins and cytokines in humans: A mini review. Int J Mol Sci. (2023) 24:9647. doi: 10.3390/ijms24119647
12. Reyes L and Golos TG. Hofbauer cells: their role in healthy and complicated pregnancy. Front Immunol. (2018) 9:2628. doi: 10.3389/fimmu.2018.02628
13. Houser BL, Tilburgs T, Hill J, Nicotra ML, and Strominger JL. Two unique human decidual macrophage populations. J Immunol. (2011) 186:2633–42. doi: 10.4049/jimmunol.1003153
14. Brown MB, von Chamier M, Allam AB, and Reyes L. M1/M2 macrophage polarity in normal and complicated pregnancy. Front Immunol. (2014) 5:606. doi: 10.3389/fimmu.2014.00606
15. Zhang YH, He M, Wang Y, and Liao AH. Modulators of the balance between M1 and M2 macrophages during pregnancy. Front Immunol. (2017) 8:120. doi: 10.3389/fimmu.2017.00120
16. Ning F, Liu H, and Lash GE. The role of decidual macrophages during normal and pathological pregnancy. Am J Reprod Immunol. (2016) 75:298–309. doi: 10.1111/aji.12477
17. van der Zwan A, van Unen V, Beyrend G, Laban S, van der Keur C, Kapsenberg HJM, et al. Visualizing dynamic changes at the maternal-fetal interface throughout human pregnancy by mass cytometry. Front Immunol. (2020) 11:571300. doi: 10.3389/fimmu.2020.571300
18. Bulmer JN, Morrison L, Longfellow M, Ritson A, and Pace D. Granulated lymphocytes in human endometrium: histochemical and immunohistochemical studies. Hum Reprod. (1991) 6:791–8. doi: 10.1093/oxfordjournals.humrep.a137430
19. Gomez-Lopez N, Guilbert LJ, and Olson DM. Invasion of the leukocytes into the fetal-maternal interface during pregnancy. J Leukoc Biol. (2010) 88:625–33. doi: 10.1189/jlb.1209796
20. Krop J, van der Zwan A, Ijsselsteijn ME, Kapsenberg H, Luk SJ, Hendriks SH, et al. Imaging mass cytometry reveals the prominent role of myeloid cells at the maternal-fetal interface. iScience. (2022) 25:104648. doi: 10.1016/j.isci.2022.104648
21. Greenbaum S, Averbukh I, Soon E, Rizzuto G, Baranski A, Greenwald NF, et al. A spatially resolved timeline of the human maternal-fetal interface. Nature. (2023) 619:595–605. doi: 10.1038/s41586-023-06298-9
22. Kammerer U, Eggert AO, Kapp M, McLellan AD, Geijtenbeek TB, Dietl J, et al. Unique appearance of proliferating antigen-presenting cells expressing DC-SIGN (CD209) in the decidua of early human pregnancy. Am J Pathol. (2003) 162:887–96. doi: 10.1016/S0002-9440(10)63884-9
23. Svensson J, Jenmalm MC, Matussek A, Geffers R, Berg G, and Ernerudh J. Macrophages at the fetal-maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. J Immunol. (2011) 187:3671–82. doi: 10.4049/jimmunol.1100130
24. Du X, Wu Z, Xu Y, Liu Y, Liu W, Wang T, et al. Increased Tim-3 expression alleviates liver injury by regulating macrophage activation in MCD-induced NASH mice. Cell Mol Immunol. (2019) 16:878–86. doi: 10.1038/s41423-018-0032-0
25. Li M, Sun F, Xu Y, Chen L, Chen C, Cui L, et al. Tim-3(+) decidual Mphis induced Th2 and Treg bias in decidual CD4(+)T cells and promoted pregnancy maintenance via CD132. Cell Death Dis. (2022) 13:454. doi: 10.1038/s41419-022-04899-2
26. Cui L, Sun F, Xu Y, Li M, Chen L, Chen C, et al. Tim-3 coordinates macrophage-trophoblast crosstalk via angiogenic growth factors to promote pregnancy maintenance. Int J Mol Sci. (2023) 24:1538. doi: 10.3390/ijms24021538
27. Li M, Sun X, Zhao J, Xia L, Li J, Xu M, et al. CCL5 deficiency promotes liver repair by improving inflammation resolution and liver regeneration through M2 macrophage polarization. Cell Mol Immunol. (2020) 17:753–64. doi: 10.1038/s41423-019-0279-0
28. Nakano H, Kirino Y, Takeno M, Higashitani K, Nagai H, Yoshimi R, et al. GWAS-identified CCR1 and IL10 loci contribute to M1 macrophage-predominant inflammation in Behcet’s disease. Arthritis Res Ther. (2018) 20:124. doi: 10.1186/s13075-018-1613-0
29. Sang Y, Li Y, Xu L, Chen J, Li D, and Du M. Dysfunction of CCR1(+) decidual macrophages is a potential risk factor in the occurrence of unexplained recurrent pregnancy loss. Front Immunol. (2022) 13:1045532. doi: 10.3389/fimmu.2022.1045532
30. Tang X, Li Y, Zhao J, Liang L, Zhang K, Zhang X, et al. Heme oxygenase-1 increases intracellular iron storage and suppresses inflammatory response of macrophages by inhibiting M1 polarization. Metallomics. (2023) 15:mfad062. doi: 10.1093/mtomcs/mfad062
31. Zhou WJ, Yang HL, Mei J, Chang KK, Lu H, Lai ZZ, et al. Fructose-1,6-bisphosphate prevents pregnancy loss by inducing decidual COX-2(+) macrophage differentiation. Sci Adv. (2022) 8:eabj2488. doi: 10.1126/sciadv.abj2488
32. Huang HL, Yang HL, Lai ZZ, Yang SL, Li MQ, and Li DJ. Decidual IDO(+) macrophage promotes the proliferation and restricts the apoptosis of trophoblasts. J Reprod Immunol. (2021) 148:103364. doi: 10.1016/j.jri.2021.103364
33. Chen Y, Zhang X, Huang S, and Febbraio M. Hidden features: CD36/SR-B2, a master regulator of macrophage phenotype/function through metabolism. Front Immunol. (2024) 15:1468957. doi: 10.3389/fimmu.2024.1468957
34. Chen J, Yin T, Hu X, Chang L, Sang Y, Xu L, et al. CD36-mediated arachidonic acid influx from decidual stromal cells increases inflammatory macrophages in miscarriage. Cell Rep. (2024) 43:114881. doi: 10.1016/j.celrep.2024.114881
35. Meng YH, Zhou WJ, Jin LP, Liu LB, Chang KK, Mei J, et al. RANKL-mediated harmonious dialogue between fetus and mother guarantees smooth gestation by inducing decidual M2 macrophage polarization. Cell Death Dis. (2017) 8:e3105. doi: 10.1038/cddis.2017.505
36. Jiang X, Du MR, Li M, and Wang H. Three macrophage subsets are identified in the uterus during early human pregnancy. Cell Mol Immunol. (2018) 15:1027–37. doi: 10.1038/s41423-018-0008-0
37. Vondra S, Hobler AL, Lackner AI, Raffetseder J, Mihalic ZN, Vogel A, et al. The human placenta shapes the phenotype of decidual macrophages. Cell Rep. (2023) 42:111977. doi: 10.1016/j.celrep.2022.111977
38. Krop J, Tian X, van der Hoorn ML, and Eikmans M. The mac is back: the role of macrophages in human healthy and complicated pregnancies. Int J Mol Sci. (2023) 24:5300. doi: 10.3390/ijms24065300
39. Park M, Kim YS, and Song H. Macrophages: a double-edged sword in female reproduction and disorders. Exp Mol Med. (2025) 57:285–97. doi: 10.1038/s12276-025-01392-6
40. Hughes CE and Nibbs RJB. A guide to chemokines and their receptors. FEBS J. (2018) 285:2944–71. doi: 10.1111/febs.14466
41. Drake PM, Gunn MD, Charo IF, Tsou CL, Zhou Y, Huang L, et al. Human placental cytotrophoblasts attract monocytes and CD56(bright) natural killer cells via the actions of monocyte inflammatory protein 1alpha. J Exp Med. (2001) 193:1199–212. doi: 10.1084/jem.193.10.1199
42. Red-Horse K, Drake PM, Gunn MD, and Fisher SJ. Chemokine ligand and receptor expression in the pregnant uterus: reciprocal patterns in complementary cell subsets suggest functional roles. Am J Pathol. (2001) 159:2199–213. doi: 10.1016/S0002-9440(10)63071-4
43. Wu X, Jin LP, Yuan MM, Zhu Y, Wang MY, and Li DJ. Human first-trimester trophoblast cells recruit CD56brightCD16- NK cells into decidua by way of expressing and secreting of CXCL12/stromal cell-derived factor 1. J Immunol. (2005) 175:61–8. doi: 10.4049/jimmunol.175.1.61
44. Hanna J, Wald O, Goldman-Wohl D, Prus D, Markel G, Gazit R, et al. CXCL12 expression by invasive trophoblasts induces the specific migration of CD16- human natural killer cells. Blood. (2003) 102:1569–77. doi: 10.1182/blood-2003-02-0517
45. Roca H, Varsos ZS, Sud S, Craig MJ, Ying C, and Pienta KJ. CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem. (2009) 284:34342–54. doi: 10.1074/jbc.M109.042671
46. O’Connor T, Borsig L, and Heikenwalder M. CCL2-CCR2 signaling in disease pathogenesis. Endocr Metab Immune Disord Drug Targets. (2015) 15:105–18. doi: 10.2174/1871530315666150316120920
47. Wang XQ, Zhou WJ, Hou XX, Fu Q, and Li DJ. Trophoblast-derived CXCL16 induces M2 macrophage polarization that in turn inactivates NK cells at the maternal-fetal interface. Cell Mol Immunol. (2018) 15:1038–46. doi: 10.1038/s41423-018-0019-x
48. Huang Y, Zhu XY, Du MR, and Li DJ. Human trophoblasts recruited T lymphocytes and monocytes into decidua by secretion of chemokine CXCL16 and interaction with CXCR6 in the first-trimester pregnancy. J Immunol. (2008) 180:2367–75. doi: 10.4049/jimmunol.180.4.2367
49. He YY, Du MR, Guo PF, He XJ, Zhou WH, Zhu XY, et al. Regulation of C-C motif chemokine ligand 2 and its receptor in human decidual stromal cells by pregnancy-associated hormones in early gestation. Hum Reprod. (2007) 22:2733–42. doi: 10.1093/humrep/dem208
50. Qin XY, Shen HH, Zhang XY, Zhang X, Xie F, Wang WJ, et al. Hypoxia-mediated chemotaxis and residence of macrophage in decidua by secreting VEGFA and CCL2 during normal pregnancy. Reproduction. (2023) 165:543–55. doi: 10.1530/REP-22-0473
51. Tagliani E, Shi C, Nancy P, Tay CS, Pamer EG, and Erlebacher A. Coordinate regulation of tissue macrophage and dendritic cell population dynamics by CSF-1. J Exp Med. (2011) 208:1901–16. doi: 10.1084/jem.20110866
52. Liao HQ, Han MT, Cheng W, Zhang C, Li H, Li MQ, et al. Decidual-derived RANKL facilitates macrophages accumulation and residence at the maternal-fetal interface in human early pregnancy. Am J Reprod Immunol. (2021) 86:e13406. doi: 10.1111/aji.13406
53. Yang HL, Lai ZZ, Shi JW, Zhou WJ, Mei J, Ye JF, et al. A defective lysophosphatidic acid-autophagy axis increases miscarriage risk by restricting decidual macrophage residence. Autophagy. (2022) 18:2459–80. doi: 10.1080/15548627.2022.2039000
54. Zhang Y, Ma L, Hu X, Ji J, Mor G, and Liao A. The role of the PD-1/PD-L1 axis in macrophage differentiation and function during pregnancy. Hum Reprod. (2019) 34:25–36. doi: 10.1093/humrep/dey347
55. Li Y, Sang Y, Chang Y, Xu C, Lin Y, Zhang Y, et al. A galectin-9-driven CD11c(high) decidual macrophage subset suppresses uterine vascular remodeling in preeclampsia. Circulation. (2024) 149:1670–88. doi: 10.1161/CIRCULATIONAHA.123.064391
56. Li M, Piao L, Chen CP, Wu X, Yeh CC, Masch R, et al. Modulation of decidual macrophage polarization by macrophage colony-stimulating factor derived from first-trimester decidual cells: implication in preeclampsia. Am J Pathol. (2016) 186:1258–66. doi: 10.1016/j.ajpath.2015.12.021
57. Wu ZM, Yang H, Li M, Yeh CC, Schatz F, Lockwood CJ, et al. Pro-inflammatory cytokine-stimulated first-trimester decidual cells enhance macrophage-induced apoptosis of extravillous trophoblasts. Placenta. (2012) 33:188–94. doi: 10.1016/j.placenta.2011.12.007
58. Lindau R, Mehta RB, Lash GE, Papapavlou G, Boij R, Berg G, et al. Interleukin-34 is present at the fetal-maternal interface and induces immunoregulatory macrophages of a decidual phenotype in vitro. Hum Reprod. (2018) 33:588–99. doi: 10.1093/humrep/dey037
59. Wang H, Wang L, Gong G, Lin X, Luo J, Liu C, et al. Interleukin-10: a novel metabolic inducer of macrophage differentiation and subsequently contributing to improved pregnancy outcomes of mice by orchestrating oxidative phosphorylation metabolismdagger. Biol Reprod. (2024) 111:76–91. doi: 10.1093/biolre/ioae041
60. Mauer J, Chaurasia B, Goldau J, Vogt MC, Ruud J, Nguyen KD, et al. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol. (2014) 15:423–30. doi: 10.1038/ni.2865
61. Wang Q, He Z, Huang M, Liu T, Wang Y, Xu H, et al. Vascular niche IL-6 induces alternative macrophage activation in glioblastoma through HIF-2alpha. Nat Commun. (2018) 9:559. doi: 10.1038/s41467-018-03050-0
62. Chen X, Song QL, Ji R, Wang JY, Cao ML, Guo DY, et al. JPT2 affects trophoblast functions and macrophage polarization and metabolism, and acts as a potential therapeutic target for recurrent spontaneous abortion. Adv Sci (Weinh). (2024) 11:e2306359. doi: 10.1002/advs.202306359
63. Andres-Martin F, James C, and Catalfamo M. IL-27 expression regulation and its effects on adaptive immunity against viruses. Front Immunol. (2024) 15:1395921. doi: 10.3389/fimmu.2024.1395921
64. Shahi A, Afzali S, Salehi S, Aslani S, Mahmoudi M, Jamshidi A, et al. IL-27 and autoimmune rheumatologic diseases: The good, the bad, and the ugly. Int Immunopharmacol. (2020) 84:106538. doi: 10.1016/j.intimp.2020.106538
65. Soler MF, Abaurrea A, Azcoaga P, Araujo AM, and Caffarel MM. New perspectives in cancer immunotherapy: targeting IL-6 cytokine family. J Immunother Cancer. (2023) 11:e007530. doi: 10.1136/jitc-2023-007530
66. Sheng YR, Hu WT, Wei CY, Tang LL, Liu YK, Liu YY, et al. IL-33/ST2 axis affects the polarization and efferocytosis of decidual macrophages in early pregnancy. Am J Reprod Immunol. (2018) 79:e12836. doi: 10.1111/aji.12836
67. Donato A, Belluzzi E, Mattiuzzo E, Venerando R, Cadamuro M, Ruggieri P, et al. Anti-inflammatory and pro-regenerative effects of hyaluronan-chitlac mixture in human dermal fibroblasts: A skin ageing perspective. Polymers (Basel). (2022) 14:1817. doi: 10.3390/polym14091817
68. Chanmee T, Ontong P, and Itano N. Hyaluronan: A modulator of the tumor microenvironment. Cancer Lett. (2016) 375:20–30. doi: 10.1016/j.canlet.2016.02.031
69. Heldin P, Karousou E, Bernert B, Porsch H, Nishitsuka K, and Skandalis SS. Importance of hyaluronan-CD44 interactions in inflammation and tumorigenesis. Connect Tissue Res. (2008) 49:215–8. doi: 10.1080/03008200802143323
70. Wang S, Sun F, Han M, Liu Y, Zou Q, Wang F, et al. Trophoblast-derived hyaluronan promotes the regulatory phenotype of decidual macrophages. Reproduction. (2019) 157:189–98. doi: 10.1530/REP-18-0450
71. Muller M, Laxton C, Briscoe J, Schindler C, Improta T, Darnell JE Jr., et al. Complementation of a mutant cell line: central role of the 91 kDa polypeptide of ISGF3 in the interferon-alpha and -gamma signal transduction pathways. EMBO J. (1993) 12:4221–8. doi: 10.1002/j.1460-2075.1993.tb06106.x
72. Wang L, Wang H, Luo J, Xie T, Mor G, and Liao A. Decorin promotes decidual M1-like macrophage polarization via mitochondrial dysfunction resulting in recurrent pregnancy loss. Theranostics. (2022) 12:7216–36. doi: 10.7150/thno.78467
73. Halari CD, Zheng M, and Lala PK. Roles of two small leucine-rich proteoglycans decorin and biglycan in pregnancy and pregnancy-associated diseases. Int J Mol Sci. (2021) 22:10584. doi: 10.3390/ijms221910584
74. Li ZH, Wang LL, Liu H, Muyayalo KP, Huang XB, Mor G, et al. Galectin-9 alleviates LPS-induced preeclampsia-like impairment in rats via switching decidual macrophage polarization to M2 subtype. Front Immunol. (2018) 9:3142. doi: 10.3389/fimmu.2018.03142
75. Minhas PS, Latif-Hernandez A, McReynolds MR, Durairaj AS, Wang Q, Rubin A, et al. Restoring metabolism of myeloid cells reverses cognitive decline in ageing. Nature. (2021) 590:122–8. doi: 10.1038/s41586-020-03160-0
76. Vitale I, Manic G, Coussens LM, Kroemer G, and Galluzzi L. Macrophages and metabolism in the tumor microenvironment. Cell Metab. (2019) 30:36–50. doi: 10.1016/j.cmet.2019.06.001
77. Yang J, Li L, Wang L, Chen R, Yang X, Wu J, et al. Trophoblast-derived miR-410-5p induces M2 macrophage polarization and mediates immunotolerance at the fetal-maternal interface by targeting the STAT1 signaling pathway. J Transl Med. (2024) 22:19. doi: 10.1186/s12967-023-04831-y
78. Wu D, Zhou B, Hong L, Cen H, Wang L, Ma Y, et al. Trophoblast cell-derived extracellular vesicles regulate the polarization of decidual macrophages by carrying miR-141-3p in the pathogenesis of preeclampsia. Sci Rep. (2024) 14:24529. doi: 10.1038/s41598-024-76563-y
79. Zhang J, Tao Y, Cai R, and Wang Y. miR-196a-5p-rich extracellular vesicles from trophoblasts induce M1 polarization of macrophages in recurrent miscarriage. J Immunol Res. (2022) 2022:6811632. doi: 10.1155/2022/6811632
80. Zhou H, Wang H, Liu X, Liu B, Che Y, and Han R. Downregulation of miR-92a in decidual stromal cells suppresses migration ability of trophoblasts by promoting macrophage polarization. DNA Cell Biol. (2023) 42:507–14. doi: 10.1089/dna.2022.0510
81. Zhang Y, Chen X, Li J, Chen X, Zhao J, Liu Q, et al. Seminal plasma exosome derived miR-26-5p can regulate decidual macrophage polarization via PTEN/PI3K/AKT signaling pathway. Sci Rep. (2025) 15:9192. doi: 10.1038/s41598-025-92880-2
82. Ye HX, Liao GN, Dong YJ, Li L, Wang XM, Shu J, et al. miR-146a-5p enhances embryo survival in unexplained recurrent spontaneous abortion by promoting M2 polarization of decidual macrophages. Int Immunopharmacol. (2022) 110:108930. doi: 10.1016/j.intimp.2022.108930
83. Bai K, Li J, Lin L, Zhang Q, Zhong J, Liu X, et al. Placenta exosomal miRNA-30d-5p facilitates decidual macrophage polarization by targeting HDAC9. J Leukoc Biol. (2023) 113:434–44. doi: 10.1093/jleuko/qiad022
84. Vire E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelot C, et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. (2006) 439:871–4. doi: 10.1038/nature04431
85. Bostick M, Kim JK, Esteve PO, Clark A, Pradhan S, and Jacobsen SE. UHRF1 plays a role in maintaining DNA methylation in mammalian cells. Science. (2007) 317:1760–4. doi: 10.1126/science.1147939
86. Shang Y, Wu S, Li S, Qin X, Chen J, Ding J, et al. Downregulation of EZH2 in trophoblasts induces decidual M1 macrophage polarization: a potential cause of recurrent spontaneous abortion. Reprod Sci. (2022) 29:2820–8. doi: 10.1007/s43032-021-00790-1
87. Liu H, Wang LL, Xu QH, Wang J, Zhang YJ, Luo J, et al. UHRF1 shapes both the trophoblast invasion and decidual macrophage differentiation in early pregnancy. FASEB J. (2022) 36:e22247. doi: 10.1096/fj.202101647RR
88. Lan J, Xu B, Shi X, Pan Q, and Tao Q. WTAP-mediated N(6)-methyladenosine modification of NLRP3 mRNA in kidney injury of diabetic nephropathy. Cell Mol Biol Lett. (2022) 27:51. doi: 10.1186/s11658-022-00350-8
89. Tsai PY, Chen KR, Li YC, and Kuo PL. NLRP7 is involved in the differentiation of the decidual macrophages. Int J Mol Sci. (2019) 20:5994. doi: 10.3390/ijms20235994
90. Liu Z, Wang M, Wang X, Bu Q, Wang Q, Su W, et al. XBP1 deficiency promotes hepatocyte pyroptosis by impairing mitophagy to activate mtDNA-cGAS-STING signaling in macrophages during acute liver injury. Redox Biol. (2022) 52:102305. doi: 10.1016/j.redox.2022.102305
Keywords: decidual macrophages, M1-like dMφs, M2-like dMφs, human early pregnancy, polarization
Citation: Liu H and Zhang L (2025) Decidual macrophage subsets and polarization puzzle during the human early pregnancy. Front. Immunol. 16:1610891. doi: 10.3389/fimmu.2025.1610891
Received: 13 April 2025; Accepted: 26 June 2025;
Published: 14 July 2025.
Edited by:
Nandor Gabor Than, Hungarian Academy of Sciences (MTA), HungaryReviewed by:
Andrea Balogh, Hungarian Academy of Sciences (MTA), HungaryÉva Pállinger, Semmelweis University, Hungary
Copyright © 2025 Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liping Zhang, NjIzODQ2OTkwQHFxLmNvbQ==
 Huiling Liu
Huiling Liu Liping Zhang
Liping Zhang