- 1Department of Gastrointestinal Surgery, Hainan General Hospital (Hainan Affiliated Hospital of Hainan Medical University), Haikou, China
- 2Department of Hepatobiliary and Pancreatic Surgery, Hainan General Hospital (Hainan Affiliated Hospital of Hainan Medical University), Haikou, China
- 3Department of Pathology, Hainan General Hospital (Hainan Affiliated Hospital of Hainan Medical University), Haikou, China
Synchronous multiple gastric cancers (SMGC) represent a rare clinical entity with no established treatment guidelines. We report a 76-year-old female with two synchronous poorly differentiated adenocarcinomas (dMMR/MSI-H phenotype) in the gastric lesser curvature, clinically staged as cT4bN2M0. Following three cycles of neoadjuvant immunochemotherapy, the patient demonstrated remarkable tumor regression (RECIST 1.1 partial response) and subsequently underwent R0 distal gastrectomy. Histopathological examination confirmed a pathological complete response (ypT0N0, TRG 0).To our knowledge, this represents the first documented case of SMGC achieving pCR with neoadjuvant immunochemotherapy. Our findings suggest that PD-1 inhibition combined with chemotherapy may induce profound tumor regression in SMGC, even in cases with high tumor burden, potentially converting unresectable to resectable disease. This case provides compelling evidence for incorporating immunotherapy in SMGC management and warrants further investigation through clinical trials.
Background
Synchronous multiple gastric cancer (SMGC), defined as ≥2 distinct primary gastric malignancies occurring simultaneously (1), accounting for 6%–14% of all gastric cancer cases (2). The pathogenesis involves complex interactions between field cancerization, tumor microenvironment heterogeneity, and genetic predisposition (3–5). Current treatment paradigms extrapolate from solitary gastric cancer protocols, despite evidence suggesting SMGC exhibits more aggressive biology and poorer chemotherapy responses (6, 7).
The advent of immune checkpoint inhibitors (ICIs) has revolutionized management of microsatellite instability-high (MSI-H) gastrointestinal malignancies. While recent trials demonstrate promising efficacy of neoadjuvant immunochemotherapy in gastric cancer (8), SMGC-specific data remains absent due to routine exclusion from clinical studies. This knowledge gap is particularly significant given potential inter-lesional heterogeneity in treatment response.
We present the first documented case of SMGC achieving pathological complete response (pCR) following neoadjuvant PD-1 inhibition combined with chemotherapy, providing critical insights into the management of this challenging clinical scenario.
Case presentation
A 76-year-old female with 12 months of intermittent epigastric pain with well-controlled type 2 diabetes presented and 10 kg unintentional weight loss. No family history of malignancy was reported.
Diagnostic evaluation
Endoscopy
Extensive mucosal ulceration was observed in the lesser curvature to the antrum, with two irregularly elevated ulcerative lesions (Figure 1).
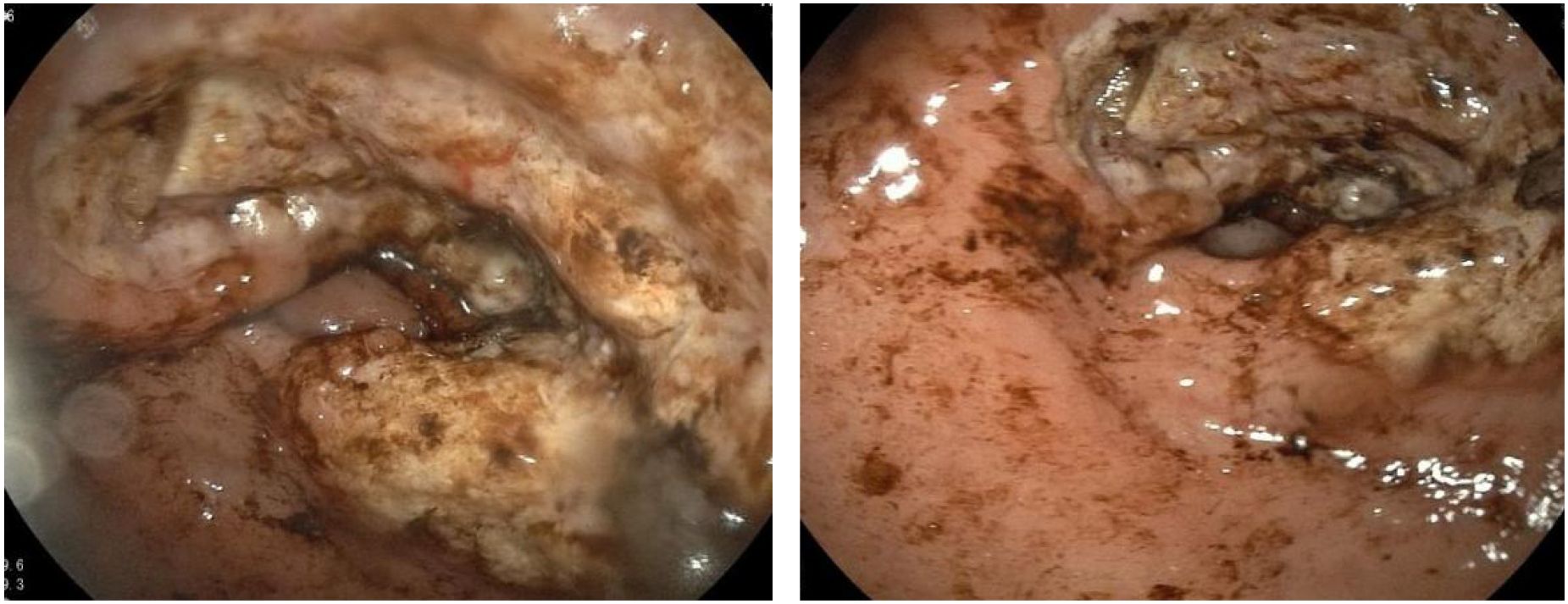
Figure 1. Extensive mucosal ulceration from the lesser curvature of the fundus to the gastric antrum was observed by gastroscopy.
Histopathology
Both lesions demonstrated poorly differentiated adenocarcinoma (Lauren’s diffuse type) with identical immunohistochemical profiles: MSH2(+), MSH6(+), MLH1(-), PMS2(-), HER2 (1+), Claudin18.2(-) (Figure 2).
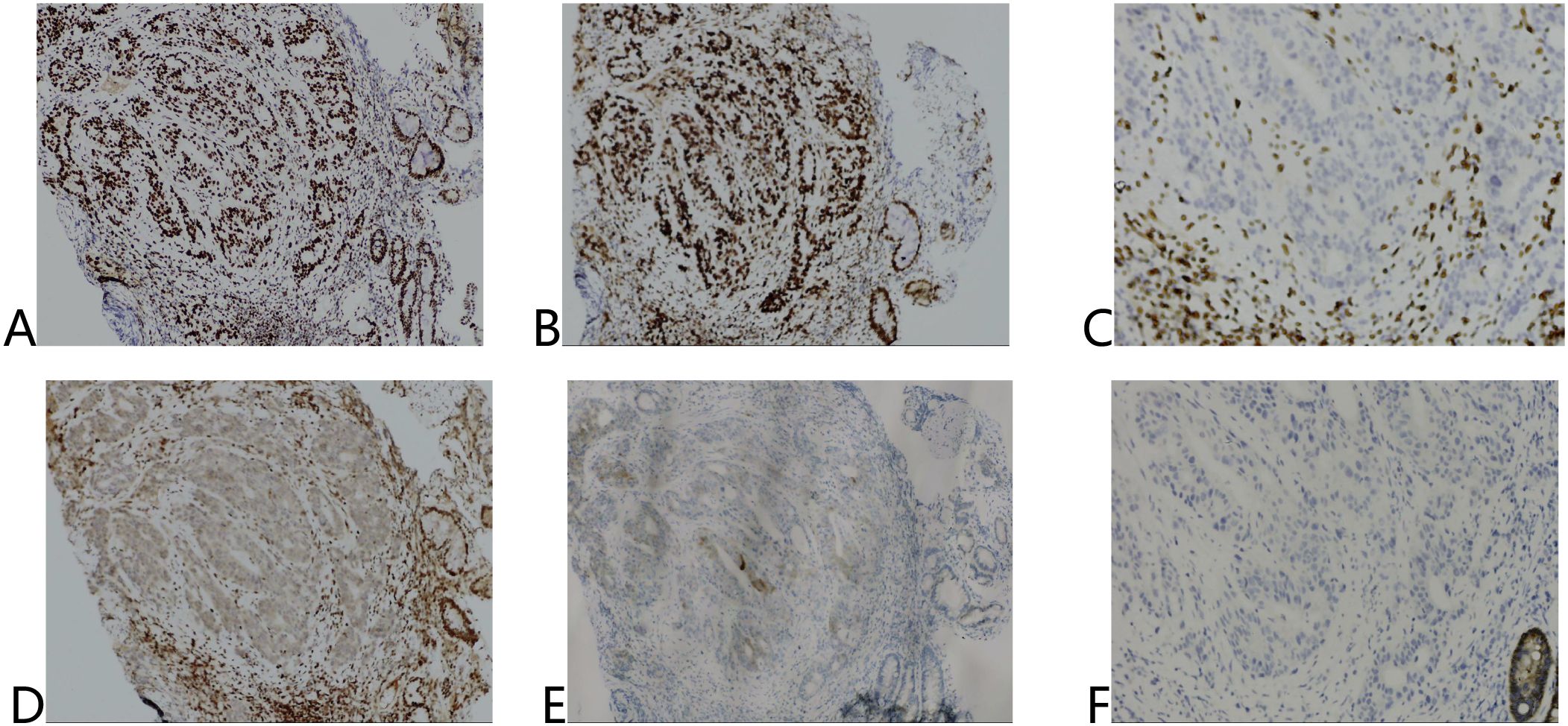
Figure 2. Immunohistochemical results of tumor tissue before treatment. (A) MSH2 (+), (B) MSH6 (+), (C) MLH1 (-), (D) PMS2 (-), (E) HER2 (1+), (F) Claudin18.2 (-).
Radiological staging (CT)
Gastric wall thickening in the lesser curvature with pancreatic invasion, multiple enlarged lymph nodes (maximum: 4.1 cm × 2.7 cm) and no distant metastases. Final clinical stage: cT4bN2M0 (AJCC 8th ed. stage IVA) (Figures 3A-C).
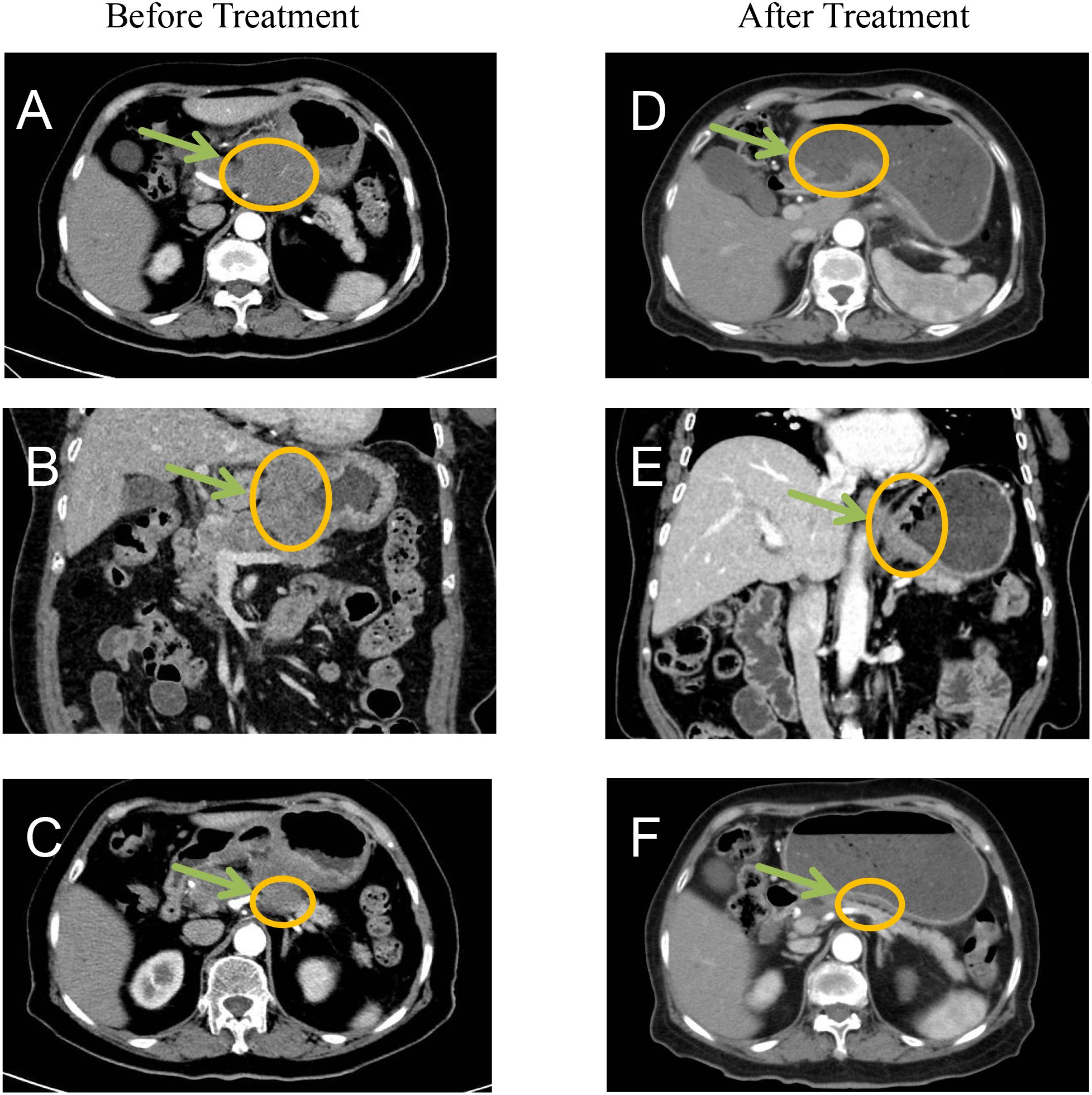
Figure 3. Before treatment CT imaging demonstrated: (A, B) Marked thickening and nodularity along the gastric curvature with contrast enhancement, showing poorly defined margins between the stomach and pancreas. (C) Significant enlargement of lesser curvature lymph nodes. After treatment imaging revealed: (D-F) Substantial reduction in both the primary tumor mass and associated lymphadenopathy, indicating favorable treatment response.
Multidisciplinary decision-making
A multidisciplinary team (MDT) determined that R0 resection was unlikely due to pancreatic involvement and confluent lymph node metastases. The patient received neoadjuvant therapy with SOX (Oxaliplatin + tegafur/gimeracil/octeracil (S-1)) combined with tislelizumab (200 mg on day 1), every 3 weeks, for three cycles, with no significant adverse effects.
Therapeutic response
Post-treatment CT demonstrated significantly reduction in primary lesions and lymph node (Figures 3D-F).
Surgical intervention
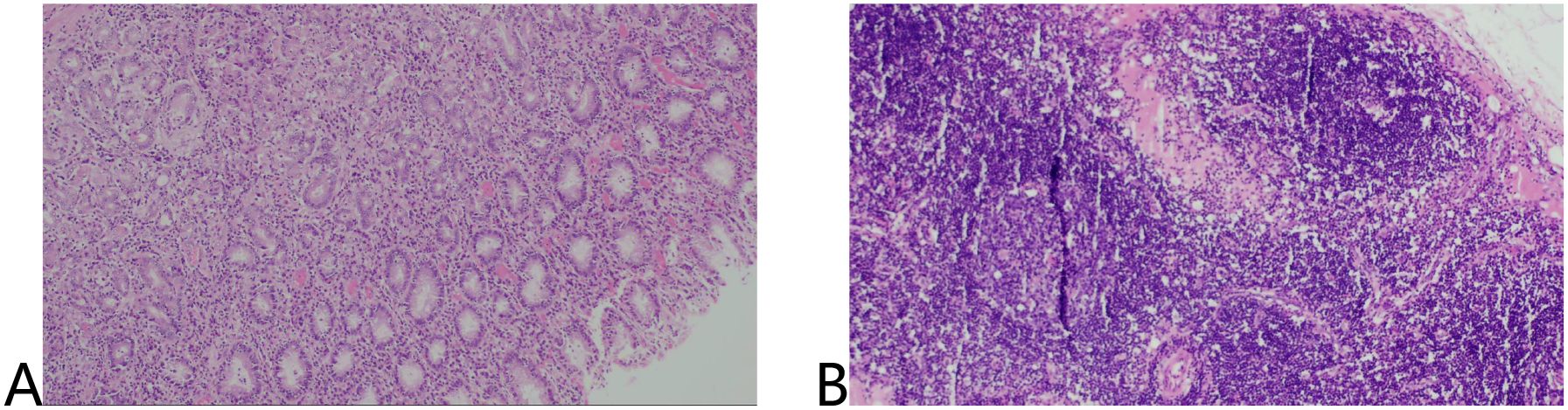
Laparoscopic distal gastrectomy with D2 lymphadenectomy (R0) was performed. Intraoperative findings revealed Two fibrotic ulcer beds (1.7cm×2.4 cm; 3.8cm×2.1 cm) with significant post-treatment scarring (Figure 4).
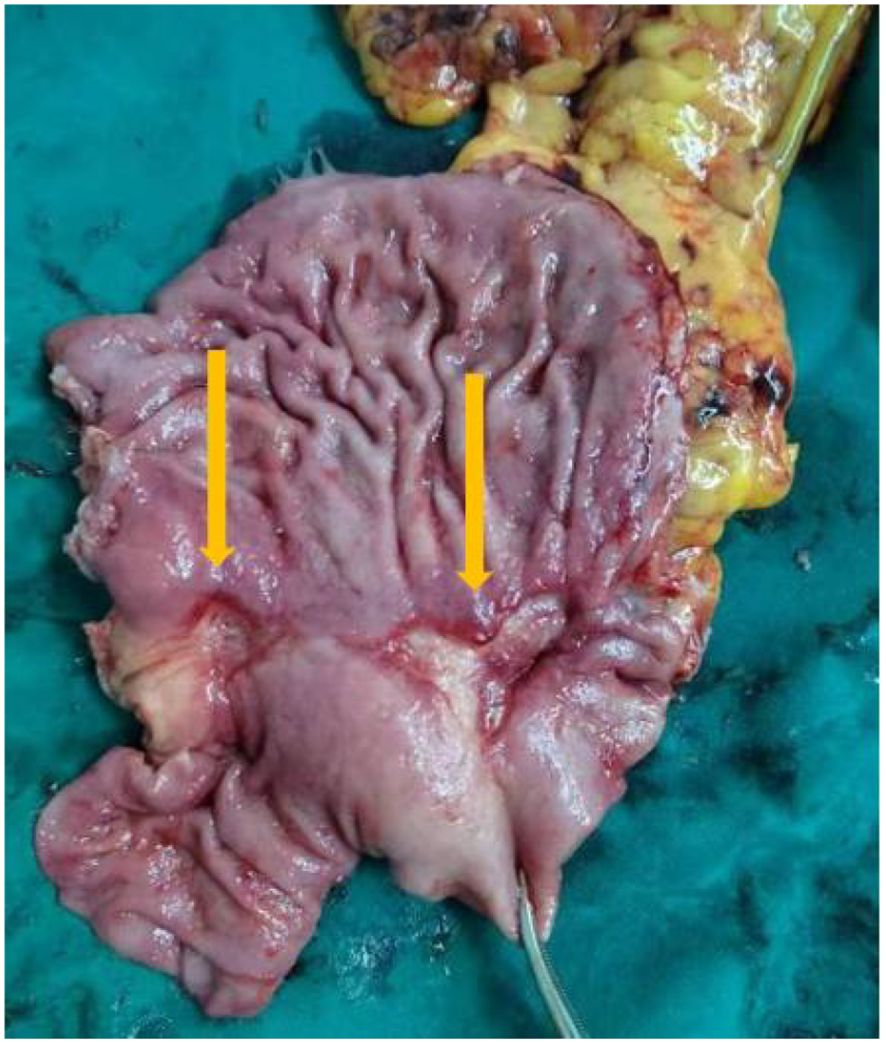
Figure 4. Surgically removed specimens showed two ulcers in the lesser curvature of the stomach and significant receding scars after neoadjuvant therapy.
Pathological evaluation
No residual cancer cells were detected in the ulcers or lymph nodes (ypT0N0). Tumor regression grade (TRG): 0 (Ryan criteria) (Figure 5).
Discussion
Comprehensive genomic profiling has established gastric cancer as a molecularly heterogeneous disease comprising distinct subtypes, each exhibiting unique molecular characteristics and clinical behaviors. Per the Cancer Genome Atlas (TCGA) classification system, gastric cancer can be categorized into four molecular subtypes: microsatellite instability (MSI), chromosomal instability (CIN), Epstein-Barr virus (EBV)-positive, and genomically stable (GS) tumors (9, 10). Of these, the MSI subtype has emerged as a particularly noteworthy entity.
Microsatellites (MS), defined as short, repetitive DNA sequences ubiquitously distributed throughout the human genome, are highly prone to replication errors (11). The DNA mismatch repair (MMR) system serves as the primary mechanism for detecting and correcting such errors. Consequently, genetic or epigenetic alterations in MMR genes may compromise MMR function (dMMR), thereby inducing a high microsatellite instability (MSI-H) phenotype. This molecular signature is associated with genomic instability and an increased tumor mutational burden (12–14).
The advent of immune checkpoint inhibitors (ICIs) targeting programmed death-1 (PD-1) and programmed cell death ligand 1 (PD-L1) has revolutionized cancer treatment paradigms (9). Accumulating evidence has demonstrated a strong association between MSI status and ICI efficacy, and more and more studies have begun to pay attention to the effect of ICIs in neoadjuvant therapy for gastric cancer (15, 16).
Notably, the recently published NEOSUMMIT-01 trial reported a pathological complete response (pCR) rate of 22.2% in locally advanced gastric cancer patients receiving neoadjuvant immunochemotherapy (the PD-1 inhibitor tislelizumab plus SOX regimen), representing a significant improvement over chemotherapy alone (7.4%) (8). However, this study specifically excluded patients with SMGC, leaving the efficacy of immunotherapy in this population unexplored.
To our knowledge, this represents the first documented case of SMGC achieving pCR following neoadjuvant immunochemotherapy. Notably, despite presenting with extensive lymph node metastasis at diagnosis, postoperative pathological examination revealed complete tumor regression, suggesting that immunotherapy may eradicate micrometastases through systemic immune activation. Intraoperative findings demonstrated significant fibrosis along the lesser curvature, potentially attributable to immunotherapy-induced fibroblast activation and collagen deposition. While these changes may obscure surgical planes and increase procedural complexity, they are considered favorable prognostic indicators. Furthermore, current evidence indicates that cancer stage, rather than lesion multiplicity, serves as the primary determinant of SMGC prognosis (17).
In the present case, the achieved pathological pCR following immunotherapy may be associated with multiple factors including systemic immune activation, elevated tumor mutational burden (TMB), MSI-H status, and dynamic tumor microenvironment remodeling. Moreover, the establishment of immunological memory might facilitate eradication of minimal residual disease and potentially mitigate recurrence risk.
Extensive research has been conducted on laparoscopic gastrectomy following neoadjuvant chemotherapy for gastric cancer. Although neoadjuvant chemotherapy induces significant tissue edema and fibrosis, increasing surgical complexity, advancements in surgical instrumentation (e.g., ultrasonic dissectors) and refined operative techniques have substantially minimized iatrogenic damage to normal tissues (18). The safety and feasibility of this approach have been robustly validated in multiple clinical studies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of Hainan General Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from primarily isolated as part of your previous study for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
Y-HS: Writing – original draft, Writing – review & editing. LC: Writing – original draft. H-RL: Data curation, Writing – review & editing. YM: Data curation, Writing – review & editing. X-WL: Data curation, Writing – review & editing. X-HH: Supervision, Writing – review & editing. K-JZ: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was Supported by Joint Program on Health Science & Technology Innovation of Hainan Province, No. WSJK2024MS236.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1611281/full#supplementary-material
References
1. Kanaya N, van Schaik TA, Aoki H, Sato Y, Taniguchi F, Shigeyasu K, et al. High risk of multiple gastric cancers in Japanese individuals with Lynch syndrome. Ann Gastroenterol Surg. (2024) 8:1008–16. doi: 10.1002/ags3.12809
2. Lee HJ, Lee YJ, Lee JY, Kim ES, Chung WJ, Jang BK, et al. Characteristics of synchronous and metachronous multiple gastric tumors after endoscopic submucosal dissection of early gastric neoplasm. Clin Endosc. (2018) 51:266–73. doi: 10.5946/ce.2017.109
3. Yoon JH, Choi BJ, Nam SW, and Park WS. Gastric cancer exosomes contribute to the field cancerization of gastric epithelial cells surrounding gastric cancer. Gastric Cancer. (2022) 25:490–502. doi: 10.1007/s10120-021-01269-3
4. Balkwill FR, Capasso M, and Hagemann T. The tumor microenvironment at a glance. J Cell Sci. (2012) 125:5591–6. doi: 10.1242/jcs.116392
5. Baghban R, Roshangar L, Jahanban-Esfahlan R, Seidi K, Ebrahimi-Kalan A, Jaymand M, et al. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signal. (2020) 18:59. doi: 10.1186/s12964-020-0530-4
6. Isobe T, Hashimoto K, Kizaki J, Murakami N, Aoyagi K, Koufuji K, et al. Characteristics and prognosis of synchronous multiple early gastric cancer. World J Gastroenterol. (2013) 19:7154–9. doi: 10.3748/wjg.v19.i41.7154
7. Kim JH, Jeong SH, Yeo J, Lee WK, Chung DH, Kim KO, et al. Clinicopathologic similarities of the main and minor lesions of synchronous multiple early gastric cancer. J Korean Med Sci. (2016) 31:873–8. doi: 10.3346/jkms.2016.31.6.873
8. Yuan SQ, Nie RC, Jin Y, Liang CC, Li YF, Jian R, et al. Perioperative toripalimab and chemotherapy in locally advanced gastric or gastro-esophageal junction cancer: a randomized phase 2 trial. Nat Med. (2024) 30:552–9. doi: 10.1038/s41591-023-02721-w
9. Ooki A, Shinozaki E, and Yamaguchi K. Immunotherapy in colorectal cancer: current and future strategies. J Anus Rectum Colon. (2021) 5:11–24. doi: 10.23922/jarc.2020-064
10. Ooki A, Osumi H, Yoshino K, and Yamaguchi. Potent therapeutic strategy in gastric cancer with microsatellite instability-high and/or deficient mismatch repair. Gastric Cancer. (2024) 27:907–31. doi: 10.1007/s10120-024-01523-4
11. Liu B, Shen C, Yin X, Jiang T, Han Y, Yuan R, et al. Perioperative chemotherapy for gastric cancer patients with microsatellite instability or deficient mismatch repair: A systematic review and meta-analysis. Cancer. (2025) 131:e35831. doi: 10.1002/cncr.v131.7
12. Wang CW, Muzakky H, Lee YC, Chung YP, Wang YC, Yu MH, et al. Interpretable multi-stage attention network to predict cancer subtype, microsatellite instability, TP53 mutation and TMB of endometrial and colorectal cancer. Comput Med Imaging Graph. (2025) 121:102499. doi: 10.1016/j.compmedimag.2025.102499
13. Liao Y, Yang R, Wang B, Ruan Y, Cui L, Yang J, et al. Mevalonate kinase inhibits anti-tumor immunity by impairing the tumor cell-intrinsic interferon response in microsatellite instability colorectal cancer. Oncogene. (2025) 44:944–57. doi: 10.1038/s41388-024-03255-2
14. Tsilimigras DI, Kurzrock R, and Pawlik TM. Molecular testing and targeted therapies in hepatobiliary cancers: A review. JAMA Surg. (2025) 160:576–585. doi: 10.1001/jamasurg.2025.0242
15. Le DT, Diaz LJ, Kim TW, Van Cutsem E, Geva R, Jager D, et al. Pembrolizumab for previously treated, microsatellite instability-high/mismatch repair-deficient advanced colorectal cancer: final analysis of KEYNOTE-164. Eur J Cancer. (2023) 186:185–95. doi: 10.1016/j.ejca.2023.02.016
16. Lordick F, Rha SY, Muro K, Yong WP, and Lordick OR. Systemic therapy of gastric cancer-state of the art and future perspectives. Cancers (Basel). (2024) 16:3337. doi: 10.3390/cancers16193337
17. Song DH, Kim N, Jo HH, Kim S, Choi Y, Oh HJ, et al. Analysis of characteristics and risk factors of patients with single gastric cancer and synchronous multiple gastric cancer among 14,603 patients. Gut Liver. (2024) 18:231–44. doi: 10.5009/gnl220491
Keywords: SMGC, neoadjuvant immunochemotherapy, pathological complete response, immune checkpoint inhibitors, microsatellite instability
Citation: Sun Y-h, Ma Y, Chen L, Li H-r, Liang X-W, He X-h and Zou K-j (2025) Case Report: Pathological complete response achieved with neoadjuvant immunochemotherapy in synchronous multiple gastric adenocarcinoma. Front. Immunol. 16:1611281. doi: 10.3389/fimmu.2025.1611281
Received: 14 April 2025; Accepted: 30 June 2025;
Published: 18 July 2025.
Edited by:
Stavros P. Papadakos, Laiko General Hospital of Athens, GreeceReviewed by:
Yuan Tian, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, ChinaTao Li, People’s Liberation Army General Hospital, China
Hesong Wang, Fourth Hospital of Hebei Medical University, China
Copyright © 2025 Sun, Ma, Chen, Li, Liang, He and Zou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xian-Wen Liang, bHh3em5keEAxNjMuY29t; Xiong-hui He, aHhoNzU4MkAxNjMuY29t; Ke-jian Zou, em91a2VqaWFuMTg4QDE2My5jb20=
†These authors share first authorship
 Ya-hui Sun1†
Ya-hui Sun1† Xian-Wen Liang
Xian-Wen Liang