- 1Department of Gynecology, Clinical Oncology School of Fujian Medical University, Fujian Cancer Hospital, Fuzhou, China
- 2Department of Clinical Medical Research Center, Clinical Oncology School of Fujian Medical University, Fujian Cancer Hospital, Fuzhou, China
- 3Department of Pathology, Clinical Oncology School of Fujian Medical University, Fujian Cancer Hospital, Fuzhou, China
- 4Department of Oncology, Fujian Medical University Affiliated Nanping First Hospital, Nanping, China
- 5Department of Obstetrics and Gynecology, The First Hospital Affiliated to Fujian Medical University, Fuzhou, China
- 6Department of Obstetrics and Gynecology, Fujian Provincial Hospital, Fuzhou, China
- 7Department of Obstetrics and Gynecology, Fujian Medical University Union Hospital, Fuzhou, China
- 8Department of Obstetrics and Gynecology, Gutian Hospital, Ningde, China
- 9Department of Gynecology, People’s Hospital Affiliated to Fujian University of Traditional Chinese Medicine, Fuzhou, China
- 10Department of Gynecology, Jiangxi Cancer Hospital, Nanchang, China
- 11Department of Gynecology, Shunde Women and Children’s Hospital (Maternity and Child Healthcare Hospital of Shunde Foshan), Guangdong Medical University, Foshan, China
- 12Department of Obstetrics and Gynecology, Lianyungang Donghai County People’s Hospital, Lianyungang, China
- 13Department of Gynecology, Changsha Maternal and Child Health Hospital, Changsha, China
- 14Department of Gynecology, Pingxiang Maternal and Child Health Hospital, Pingxiang, China
- 15Department of Obstetrics and Gynecology, Huinan County People’s Hospital, Huinan, China
- 16Beijing GenePlus Technology Co. Ltd., Beijing, China
Background: Immunotherapy has become a powerful clinical strategy for treating recurrent or metastatic cervical cancer (R/M CC). Cadonilimab, a novel anti-PD-1/CTLA-4 bispecific antibody, has shown substantial clinical benefits in cancer treatment. However, there is no real-world evidence of cadonilimab with a considerable sample size in R/M CC. Hence, we aim to assess the efficacy and safety of cadonilimab in R/M CC patients and explore its potential mechanism.
Methods: This retrospective real-world study examined a sample of R/M CC patients treated with cadonilimab at 13 large academic medical centers in China from July 6, 2022, to October 1, 2023. The outcomes were objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), overall survival (OS), as well as safety profiles. Additionally, the programmed cell death 1 ligand 1 (PD-L1) was detected by immunohistochemistry to confirm its predictive values. Whole exome sequencing (WES) was also performed to investigate its potential antitumor mechanisms.
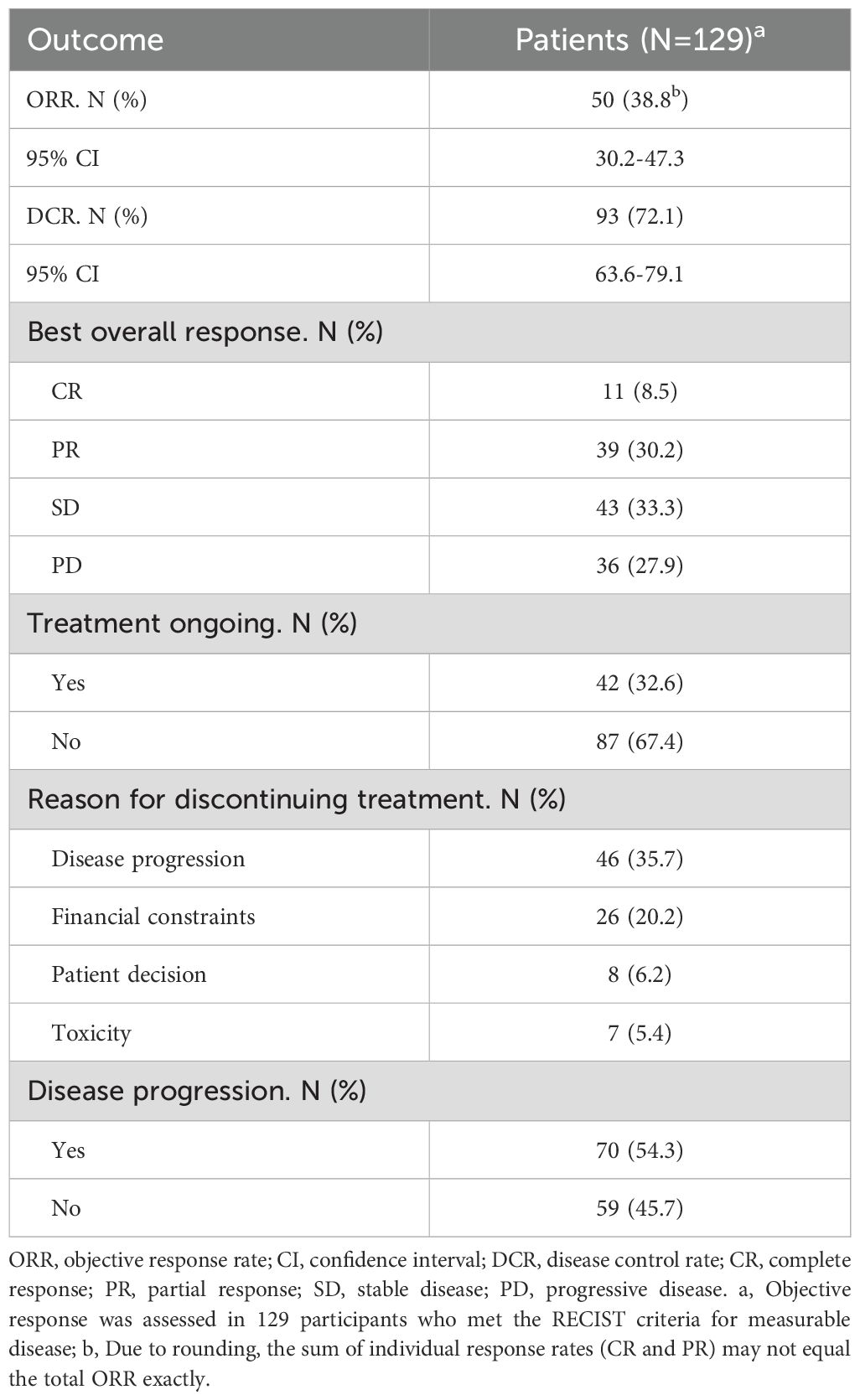
Results: Among the 129 patients with measurable disease, the ORR was 38.8%, consisting of complete and partial responses in 8.5% and 30.2% of patients, respectively. The DCR was 72.1%. The median PFS was 12.4 months, while the median OS has not yet been reached. Subgroup analysis showed a numerical trend toward longer median PFS in patients with PD-L1 CPS ≥ 1 compared with CPS < 1 (14.0 vs. 12.8 months; P = 0.235). Moreover, combined therapy of cadonilimab and radiotherapy was identified as an independent prognostic factor for both OS and PFS. The most common grade 3 or worse adverse event was anemia (28 [20.1%]), decreased white blood cell count (24 [17.2%]), and decreased neutrophil count (20 [14.4%]). The most prevalent genetic variant was PIK3CA, highlighting the importance of the PI3K-AKT pathway in the antitumor mechanism of cadonilimab.
Conclusions: Cadonilimab shows an encouraging tumor response rate, with a manageable safety profile in patients with R/M CC. Notably, cadonilimab is also effective for those with PD-L1 CPS <1, suggesting a broad range of application prospects in R/M CC.
Clinical Trial Registration: https://www.clinicaltrials.gov, identifier NCT06140589.
Introduction
Cervical cancer (CC) is the fourth greatest global burden in terms of both incidence and mortality in women, with the leading cause of cancer death in 37 countries (1). In 2022, Global Cancer Observatory (GLOBOCAN) estimated 661,021 new CC cases and 348,189 CC-related deaths. Notably, China accounts for 22.8% of the worldwide incidence and 16.0% of CC-related mortality, resulting in a tremendous medical burden (2). Although early-stage CC is often amenable to radical surgery or chemoradiotherapy, recurrent or metastatic cervical cancer (R/M CC) patients are incurable and have a dismal prognosis, with a five-year survival rate of only 17% (3). Few effective therapeutic options are left for R/M CC (4). Recent research also emphasizes the urgent need for novel therapeutic strategies in metastatic cancers, as conventional treatments often fail to improve survival outcomes (5).
Recent advances in cancer immunotherapy, which manipulates the immune system to recognize and attack cancer cells (6, 7), have revolutionized the paradigms of CC management (3, 7). Multiple types of immune checkpoint inhibitors (ICIs) targeting programmed death protein-1 (PD-1) and its ligand PD-L1, such as pembrolizumab, camrelizumab, nivolumab, have entered clinical trials successively and exhibited improved efficacy in monotherapy or combination with chemotherapy, radiotherapy, and targeted therapy (8–12). Compared with platinum-based chemotherapy of objective response rate (ORR) ranging from 20% to 30% (13), anti-PD-1 monotherapy could achieve an ORR of 12.2%-33.3%, and reach up to 65.9% when combined with other therapies in late-line treatment of R/M CC (14). Despite this progress, some R/M CC patients still fail to respond to immunotherapy, especially in PD-L1-negative tumors. Hence, considerable strides are urgently needed to improve treatment outcomes.
Dual-targeted immunotherapy is a clinically validated strategy for enhancing antitumor activity compared to anti-PD-1 monotherapy. However, the associated side effects can sometimes be intolerable (15). Cadonilimab (AK-104) is a novel bispecific antibody targeting PD-1 and CTLA-4 with favorable safety profile. It features a unique symmetric tetravalent structure that enhances binding activity within the tumor microenvironment, thereby inhibiting immunosuppressive pathways and augmenting T-cell-mediated responses (16). The emerging class of bispecific antibodies has shown promising results in multiple cancers by enhancing T-cell responses and overcoming tumor immune evasion (17). Cadonilimab became the first bispecific antibody approved for patients with R/M CC who were resistant to platinum-based chemotherapy in China in June 2022, marking a significant advancement in cervical cancer therapy. This approval was based on the COMPASSION-03 trial conducted by Gao et al., in which 111 patients who had failed platinum-based chemotherapy were treated with cadonilimab monotherapy. Cadonilimab achieved an overall response rate (ORR) of 32.3% (32/99), with a median progression-free survival (PFS) of 3.71 months. The ORR in the PD-L1+ cohort was 43.8% (28/64), compared with 16.7% (3/18) in the PD-L1- cohort (18). Additionally, grade ≥3 immune-related adverse events (irAEs) were reported in only 4.5% of patients. Furthermore, modulation of the tumor microenvironment through nanotechnology and molecular engineering may synergize with such bispecific antibodies (19).
Despite such encouraging results, no real-world studies have yet focused on cadonilimab in R/M CC. Against this backdrop, our study presents the first real-world evidence from 13 academic medical centers in China, evaluating the effectiveness and safety of cadonilimab in treating R/M CC. Additionally, we investigate biomarkers such as PD-L1, tumor mutational burden (TMB), and integrated genomic profiling to predict response sensitivity to immunotherapy in R/M CC.
Materials and methods
Study design and participants
This multicenter study evaluated the antitumor activity and safety of cadonilimab in R/M CC patients at 13 large academic medical centers in five provinces in China (Fujian, Jiangxi, Guangdong, Hunan, Jiangsu). Patients were included if they had: 1) histologically confirmed R/M CC with pathological types such as squamous cell carcinoma, adenocarcinoma, adenosquamous carcinoma, or neuroendocrine carcinoma; 2) at least one cycle of cadonilimab without restriction on the concurrent use of radiotherapy, chemotherapy, or antiangiogenic therapy. The key exclusion criteria were a history of another malignancy, concurrent malignancies, and incomplete clinical data. All participants were followed up for at least six months after treatment initiation, unless death occurred earlier.
This study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practices Guidelines. The study protocol was approved by the Ethics Committee of Fujian Cancer Hospital (K2023-102-01), with subsequent approvals from all participating centers’ ethics committees. All patients provided written informed consent.
Treatment
Detailed information on treatment strategies, dosage adjustments, evaluation intervals, and treatment cessation were retrieved from physicians and patients. Based on the physician’s decision, cadonilimab was administered intravenously at 10 mg/kg every three weeks or 6 mg/kg every two weeks. Treatment would be suspended or discontinued due to tumor progression, intolerable AEs, such as severe myocarditis and pancreatitis, or the decision of the patient or physician. In selected cases, patients with ECOG 3 received cadonilimab based on physician judgment and shared decision-making. Their poor performance status was mainly due to reversible tumor-related symptoms, and treatment was initiated with careful monitoring.
Outcome measures
Responses were assessed by investigators and radiologists according to RECIST version 1.1 (20). The primary outcome measures were ORR and disease control rate (DCR). ORR was defined as the proportion of patients with measurable disease achieving complete response (CR) or partial response (PR). DCR included patients achieving PR, CR, or stable disease (SD). The secondary endpoints were PFS and OS. PFS was measured from initial cadonilimab administration to progressive disease (PD) or death. OS was defined as the time from treatment initiation to death from any cause. Additionally, AEs were recorded in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0 (21), as were irAEs (22).
Biomarker exploratory
PD-L1 status was assessed in 74 patients using immunohistochemical staining (detailed in the Data Supplementary) and measured by the combined positive score (CPS), defined as the number of PD-L1-positive cells divided by the total number of viable tumor cells, multiplied by 100 (23). CPS ≥1 was considered positive. Whole-exome sequencing was performed on 14 patients, with details provided in the Data Supplementary. Sequencing was conducted on the Geneplus-2000 platform (Geneplus, Beijing, China). TMB was classified as high when ≥9 mutations per megabase (mut/Mb) (24). HRD was considered positive with a score of 34 or higher (25).
Statistical analysis
R version 4.3.2 was used for data analysis. For normal distributions, continuous variables were described as means and ranges. Medians and ranges were used for skewed distributions. Group comparisons were performed using the Chi-square test, Fisher’s exact test, Student’s t-test, or Mann-Whitney U test. The Kaplan-Meier method was used to display the OS and PFS. A P-value <0.05 was considered statistically significant.
Results
Patients and treatment
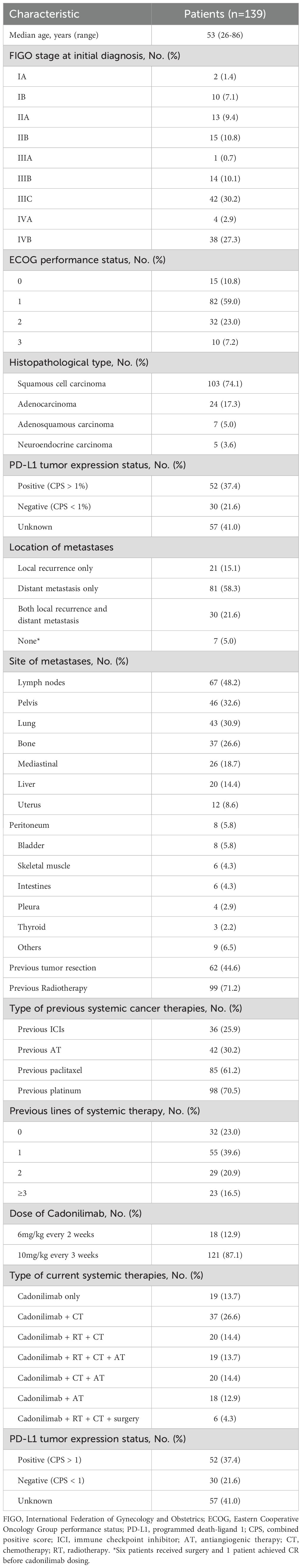
From July 6, 2022, to October 1, 2023, a total of 139 patients with R/M CC were enrolled. Of these, 129 were available for efficacy analysis, while all 139 were included in the safety analysis. The demographic, clinical, and pathological characteristics are summarized in Table 1. The median age was 53 years (range: 26-86), with 41.0% at Stage III and 30.2% at Stage IV. The most common metastasis sites were lymph nodes (67, 48.2%), pelvis (46, 33.1%), and lungs (43, 30.9%). Ninety-eight (70.5%) patients were previously treated with platinum-based chemotherapy, and 85 (61.2%) with paclitaxel chemotherapy. Specifically, 36 (25.9%) were previously treated with anti-PD-1 monotherapy, and 42 (30.2%) had received antiangiogenic therapy. As of April 5, 2024, 49 (35.3%) patients still continued with cadonilimab; while 90 (64.8%) patients discontinued due to disease progression (47, 33.8%), treatment-related toxicity (8, 5.8%); financial constraints (27, 19.4%), and patient/physician’s decision (8, 5.8%).
Among patients receiving chemotherapy, the most common regimens were paclitaxel combined with carboplatin (n = 76) or cisplatin (n = 16). Less frequently used regimens included albumin-bound paclitaxel plus cisplatin (n = 4), cisplatin plus ifosfamide (n = 1), irinotecan plus carboplatin (n = 1), gemcitabine plus nedaplatin (n = 1), and cisplatin monotherapy (n = 3). Additionally, 57 patients received targeted therapies, including bevacizumab (n = 45) and anlotinib (n = 12). Radiotherapy was administered to the primary tumor and regional lymph nodes in newly diagnosed advanced cases, or to metastatic lesions in patients with recurrent disease. In most cases, radiotherapy was delivered concurrently with cadonilimab, typically within seven days before or after the initiation of immunotherapy. Sequential radiotherapy was administered to 7 patients based on clinical judgment.
Regarding baseline performance status, ECOG scores were 0 in 15 patients (10.8%), 1 in 82 (59.0%), 2 in 32 (23.0%), and 3 in 10 (7.2%). Among the 10 patients with ECOG 3, 9 were evaluable for treatment response. Of these, five showed improvement in performance status during therapy, while 4 remained at ECOG 3 throughout the follow-up period. The objective response rate (ORR) in this subgroup was 33.0%, the disease control rate (DCR) was 55.6%, and the median progression-free survival (PFS) was 5.9 months. As expected, these outcomes were generally poorer than those observed in patients with ECOG 0–2.
Antitumor activity
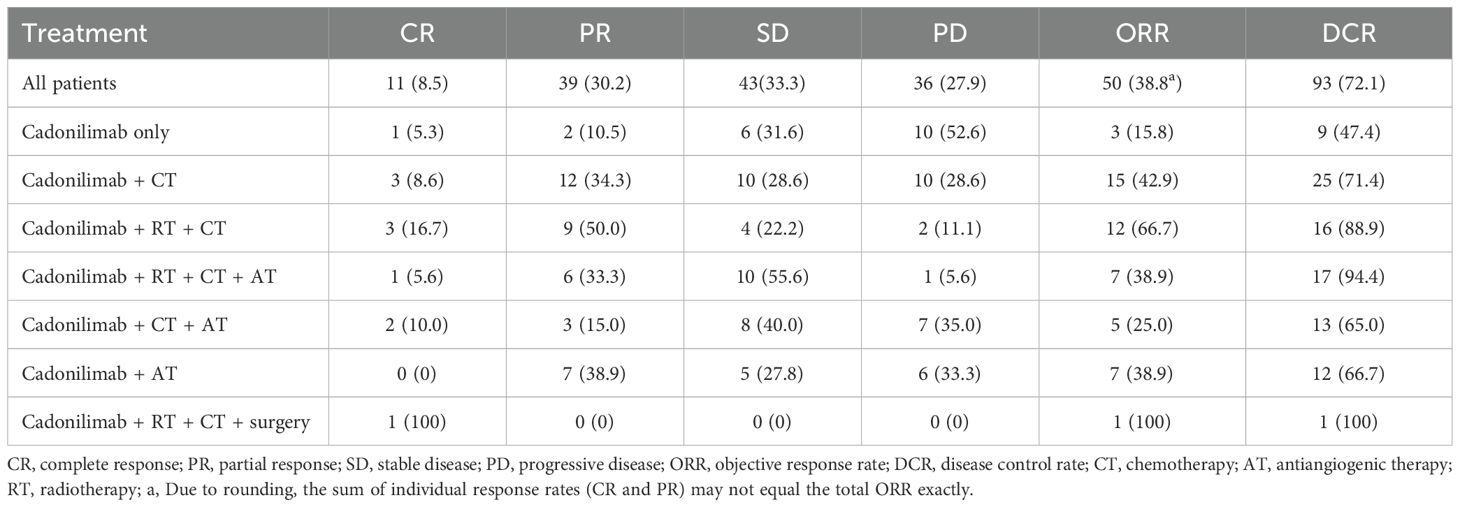
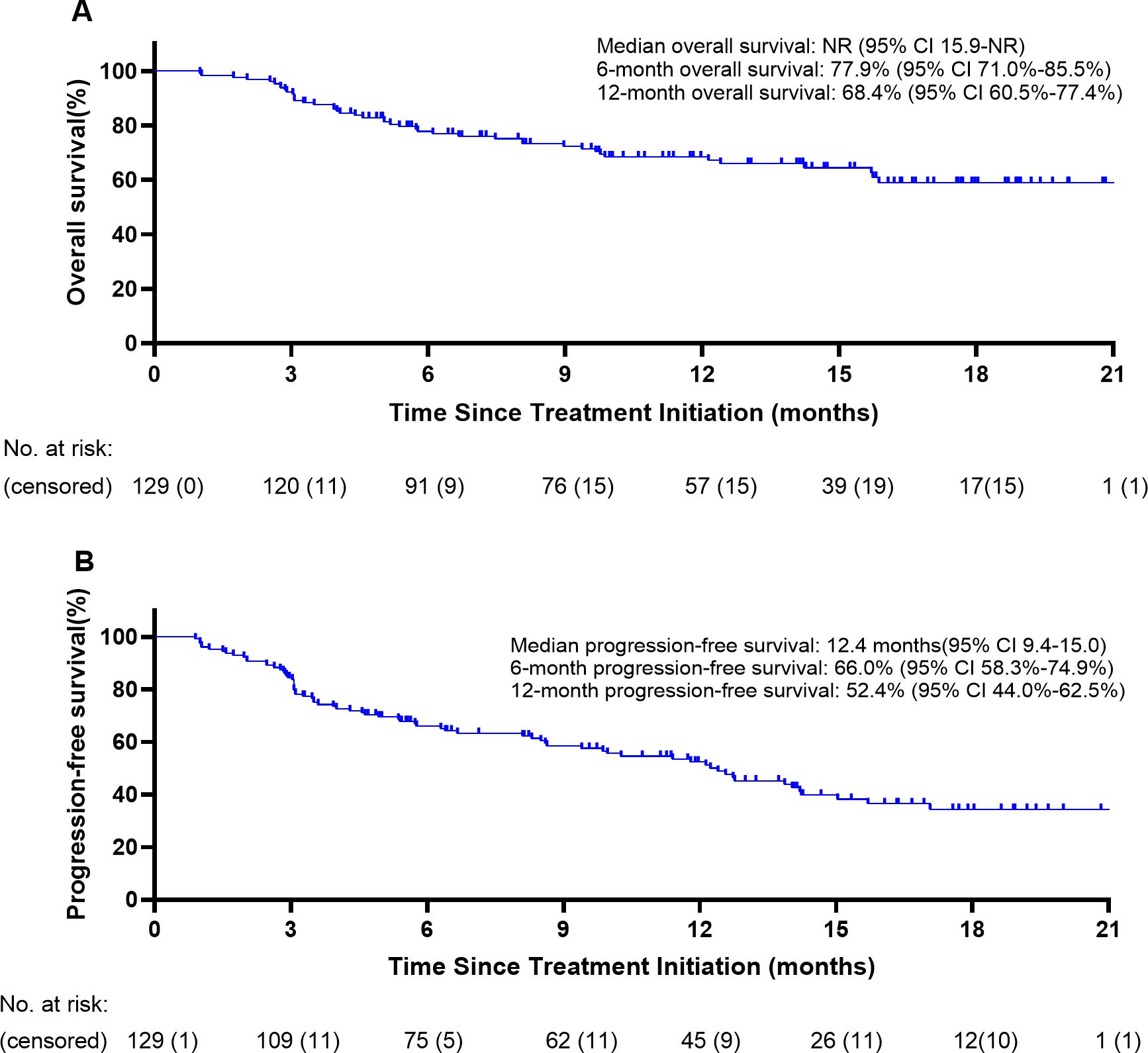
One hundred twenty-nine patients were evaluable for clinical response. Detailed information about therapy modalities and treatment response is presented in Table 2. Up to April 5, 2024, the median follow-up time was 10.2 months (range: 1–21 months). There were 11 patients (8.5%) who achieved a best response of CR, 39 (30.2%) with PR, 43 (33.3%) with SD, and 36 (27.9%) with PD as their best overall response (Figure 1), resulting in an ORR of 38.8% and a DCR of 72.1% (Table 3). At the end of the follow-up period, PD was observed in 72 patients (51.8%), and 45 patients (32.4%) died from the disease. The 6-month and 12-month PFS rates were 66.0% (95% CI, 58.3%-74.9%) and 52.4% (95% CI, 44.0%-62.5%), with a median PFS of 12.4 months (95% CI, 9.4-15.0). The 6-month and 12-month OS rates were 77.9% (95% CI, 71.0%-85.5%) and 68.4% (95% CI, 60.5%-77.4%), with a median OS, which has not been reached (95% CI, 15.9 to not estimable) (Figure 2). Furthermore, multivariate analysis identified that combined therapy of cadonilimab and radiotherapy was an independent prognostic factor for both OS and PFS (HR for OS = 0.37 [95% CI, 0.15–0.94], P = 0.036; HR for PFS = 0.51 [95% CI, 0.27–0.96], P = 0.038). Surprisingly, the status of PD-L1 CPS did not significantly impact patient prognosis, indicating the powerful anti-tumor function of cadonilimab in R/M CC patients, regardless of the PD-L1 CPS status (Figure 3).
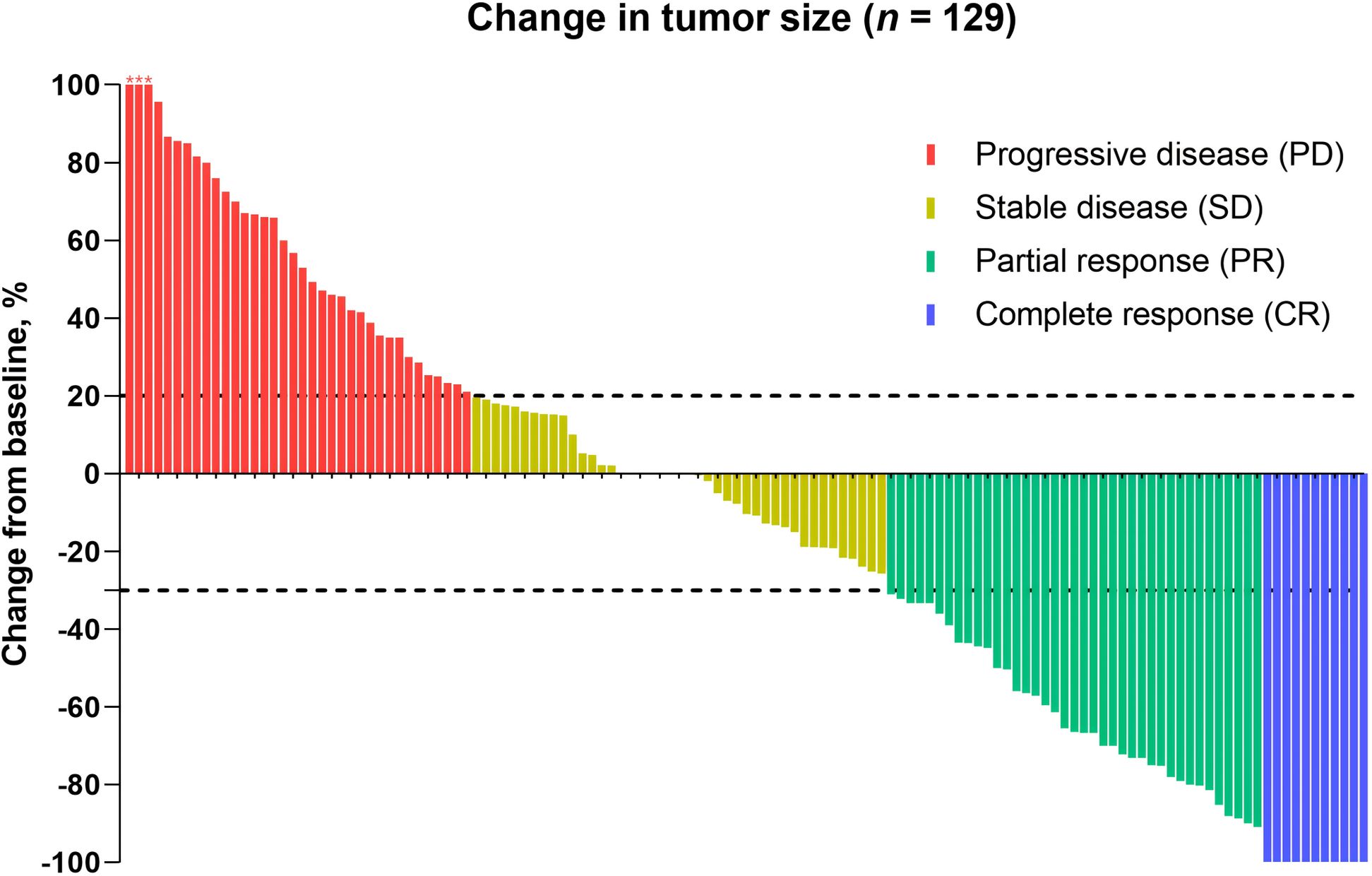
Figure 1. Antitumor activity. Patients who were eligible for the evaluation of treatment efficacy were included (n = 129). The dashed line at +20% change signifies the RECIST version 1.1 cutoff for defining stable disease or progressive disease, whereas the -30% change indicates the cutoff for identifying partial or complete response. *, three patients experienced tumor enlargement exceeding 100%, with increases of 229%, 200%, and 153%, respectively.
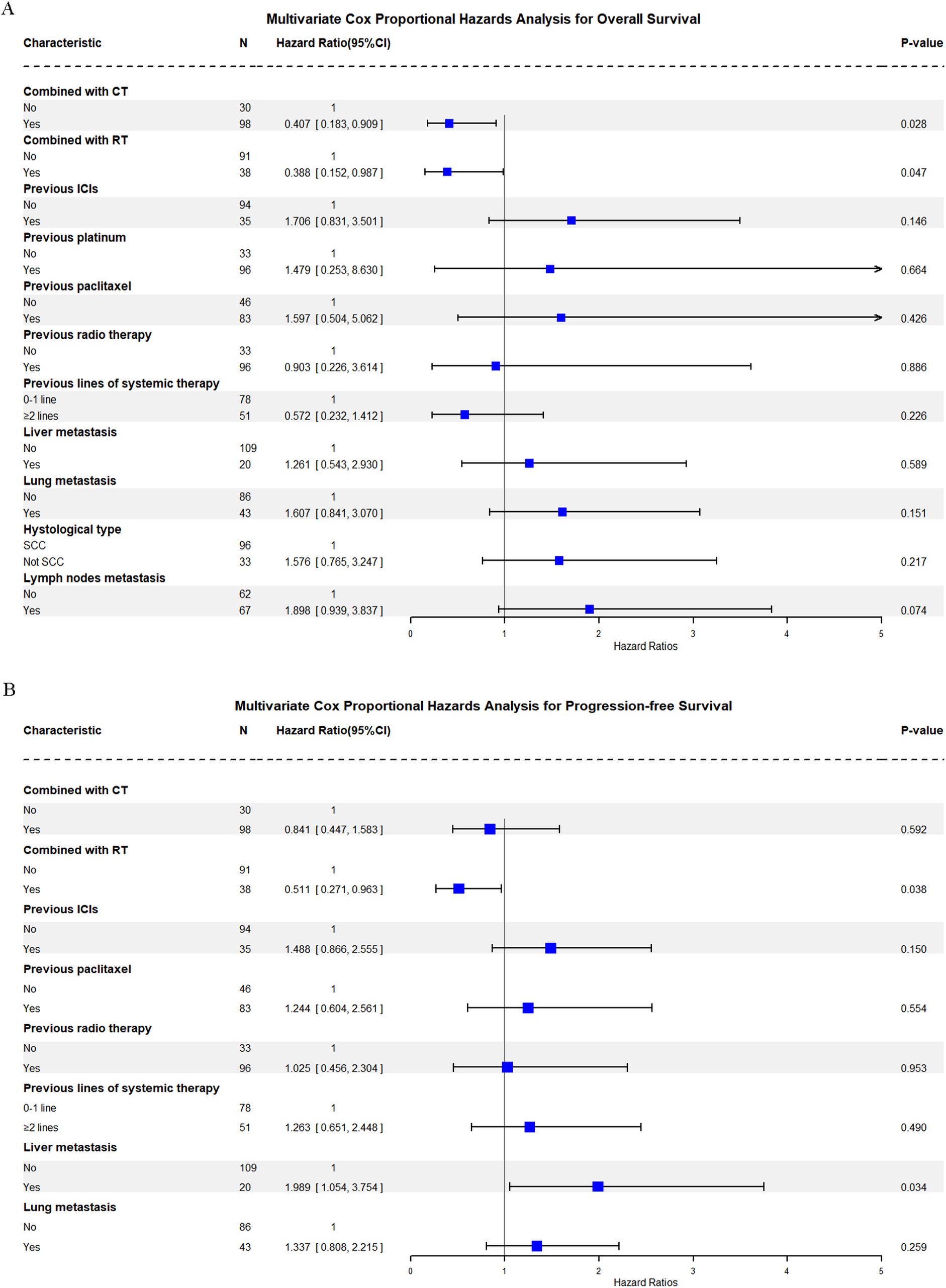
Figure 3. Multivariate Cox proportional hazards analysis for overall and progression-free survival. (A) Overall Survival (OS) Multivariate Cox Analysis; (B) Progression-free Survival (PFS).
Safety
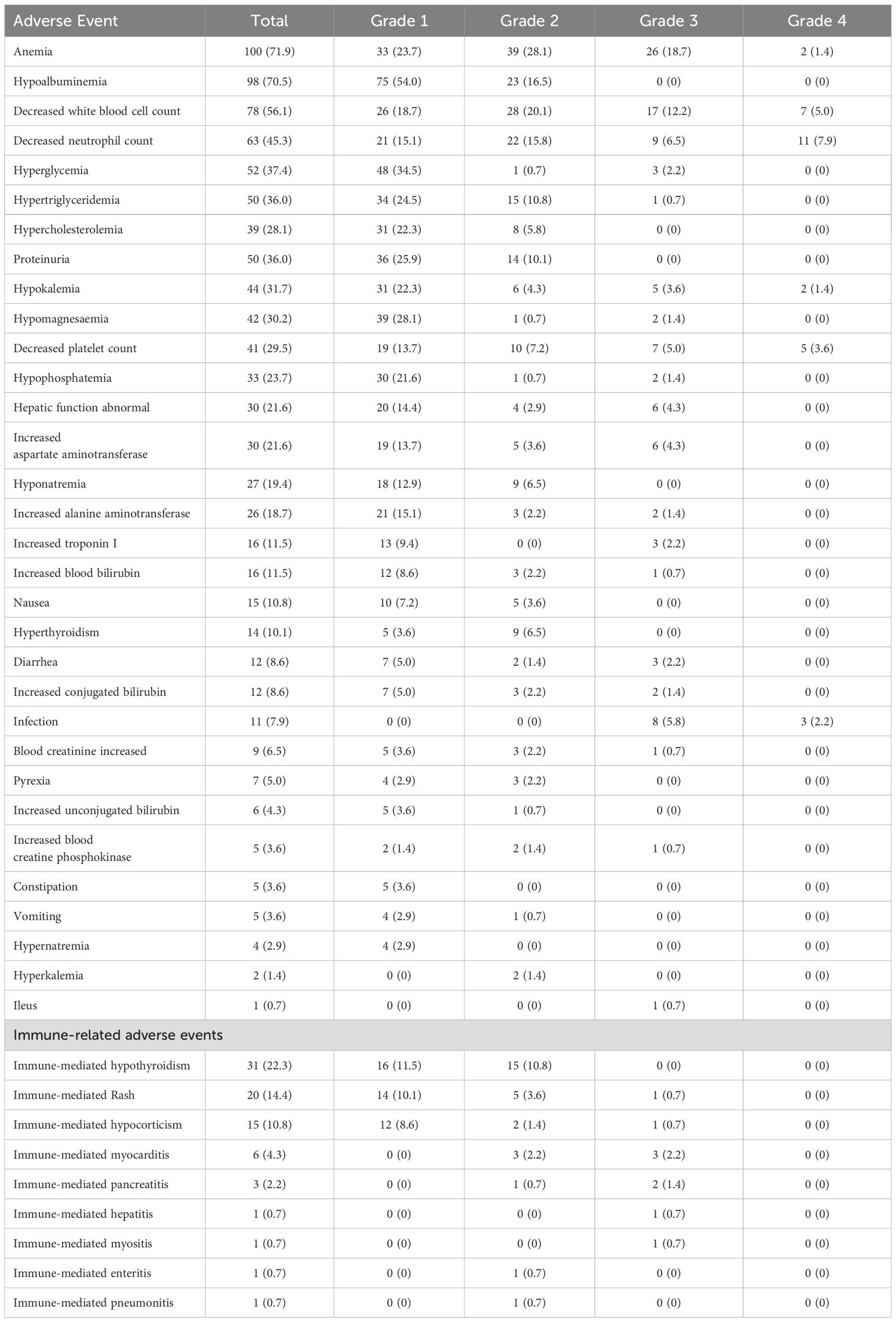
During treatment, 133 (95.7%) patients experienced treatment-related adverse events of any grade, with no unexpected AEs recorded. Fifty-six (40.3%) patients developed grade 3–4 adverse events (Table 4). The most common grade 3 or worse adverse events were anemia (28 [20.1%]), decreased white blood cell count (24 [17.2%]), and decreased neutrophil count (20 [14.4%]). IrAEs occurred in 79 (56.8%) patients, with the most frequent being hypothyroidism (31 [22.3%]) and rash (20 [14.4%]), followed by hypercorticism (15 [10.8%]) and hyperthyroidism (14 [10.1%]). Seven (5.3%) patients developed grade 3 severe irAEs during treatment, including myocarditis (3 [2.2%]), pancreatitis (2 [1.4%]), hypocorticism (1 [0.7%]), hepatitis (1 [0.7%]), rash (1 [0.7%]), and myositis (1 [0.7%]). Notably, most grade 3 or higher irAEs were successfully managed with therapeutic interventions.
Biomarkers and potential mechanism
PD-L1 expression was evaluable in 74 patients, and 46 patients had a PD-L1 CPS ≥1. Individuals with a PD-L1 CPS ≥1 trended to exhibit a higher ORR (26 of 46, 56.5%) compared to those with a CPS <1 (10 of 28, 35.7%). However, this difference was not significant (P = 0.082). Moreover, PFS did not differ significantly between these two groups (P = 0.235). The median PFS was longer in the PD-L1 CPS ≥1 group of 14.0 months (95% CI: 12.1-NR) compared to 12.8 months (95% CI: 6.3-NR) for the PD-L1 CPS <1 group. These results suggest that cadonilimab may also be effective in patients with PD-L1 CPS <1. Further investigations are merited to determine its role in the PD-L1 CPS <1 subgroup.
Whole-exome sequencing was performed on 14 patients using qualified control samples. The most frequently altered genes were PIK3CA, MUC16, and ANK2, each identified in 4 patients (4 of 14, 28.6%) (Figure 4A). All PIK3CA mutations were missense variants located in exon 9, including E542K (c.1624G>A) and E545K (c.1633G>A), and were detected only in patients with complete or partial response. No PIK3CA mutations were observed in patients with stable or progressive disease (Figures 4B, C). KEGG pathway enrichment analysis showed that these mutations were enriched in the PI3K-AKT signaling pathway, often co-occurring with COL6A6 alterations. Moreover, significantly higher median TMB values were detected in patients who achieved CR or PR compared to those with SD or PD (4.8 vs. 1.1, P=0.002; Figure 4D). ORR exhibited no significant differences between participants with HRD-positive (66.7%; n=3) and HRD-negative ones (54.5%; n=11) (P = 0.615; Figure 4E).
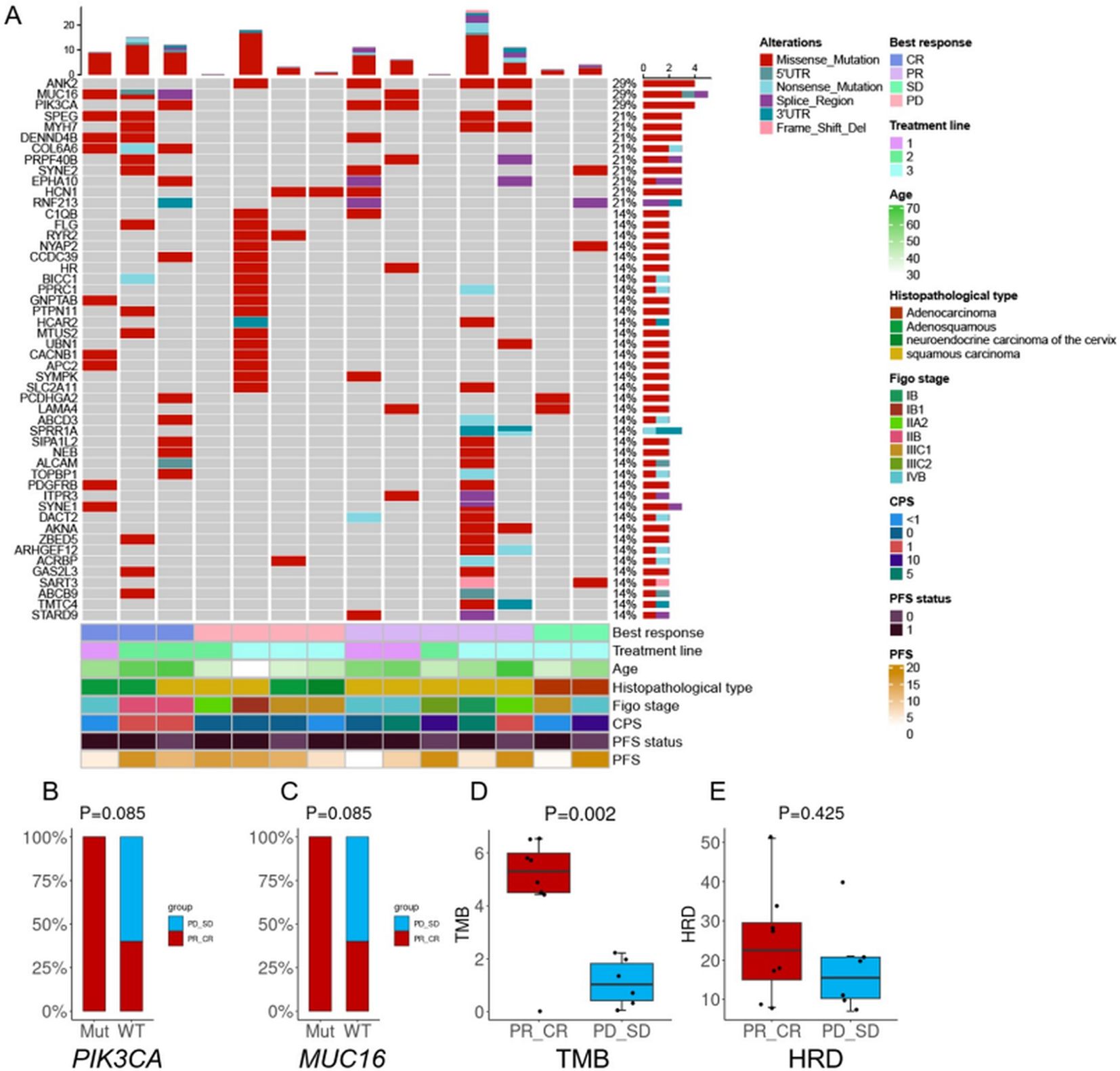
Figure 4. Biomarker analyses. (A) OncoPrint of functional driver mutations in 14 patients with cervical cancer. Rows represent genes; columns represent samples. Glyphs and color coding display genomic alterations (mutations, copy number alterations, changes in gene expression) and best response, treatment line, age, histopathological type, FIGO stage, combined positive score (CPS), and progression-free survival (PFS); (B) Comparison of tumor mutational burden (TMB) by treatment response; (C) Comparison of homologous recombination deficiency (HRD) by treatment response; (D) Treatment response in patients with altered vs. wild-type PIK3CA; (E) Treatment response in patients with altered vs. wild-type MUC16. Abbreviations: CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Discussion
Cadonilimab yielded favorable response rates without compromising safety profiles in R/M CC management. To our knowledge, this multicenter study represents the first real-world assessment of cadonilimab in R/M CC patients. Cadonilimab exhibited an ORR of 38.8% and a DCR of 72.1%. Among those with PD-L1 CPS ≥1, the ORR improved to 56.5%, and the median PFS reached 14.0 months. Notably, patients with PD-L1 CPS <1 also demonstrated significant benefits, with an ORR of 35.7% and a median PFS of 12.8 months, indicating cadonilimab could also provide encouraging anti-tumor activity even in PD-L1 CPS <1 subgroup. Moreover, only 56 (40.3%) patients developed grade 3–4 adverse events in our study, comparable to or lower than the previous study (14), indicating its well-tolerated nature. These results are consistent with prior findings that novel immune agents can offer a favorable safety profile compared to traditional checkpoint inhibitors (26). However, the long-term evaluation of cadonilimab needs an expanded sample and further follow-up.
Cadonilimab treatment led to significantly longer PFS (12.4 months) than cisplatin-based combination therapy (5.8 months), followed by non-platinum drugs (2–3 months) among R/M CC patients (27). Compared with the investigator’s choice of cisplatin-based chemotherapy, cadonilimab treatment resulted in a 9.7% higher ORR (27). Similarly to our study, the COMPASSION-03 study of the CC cohort also demonstrated cadonilimab with a high ORR of 32.3% in R/M cases (18). Compared with another generic drug (QL1706), cadonilimab also showed higher ORR in CC treatment (28). Hence, the anti-PD-1/CTLA-4 bispecific antibodies, like cadonilimab, have become an important component of the treatment regimen for R/M CC. Moreover, compared to dual PD-1 and CTLA-4 checkpoint blockade combinations in CC treatment, such as the CheckMate 358 study (29) and a Phase II trial of balstilimab and zalifrelimab combination (NCT03495882) (30), cadonilimab showed a more favorable anti-tumor power with higher ORR and lower toxicity, underscoring its enormous potential in CC management.
The significant antitumor activity of cadonilimab is likely due to its tetravalent design. Preclinical studies have suggested its tetravalent structure could enhance the binding avidity of high densities of PD-1 and CTLA-4, leading to increased immune responses to antitumors (16). Mechanistically, blocking the binding of PD-1 and PD-L1 could maintain the active status of tumor-reactive T cells, which would be becoming inactivated after persistent chronic stimulation. On the other hand, CTLA-4 blockade could activate effector T cells and inactivate regulatory T cells, thereby enhancing antitumor immunity. The unique complementary mechanisms of blockade of the PD-1 and CTLA-4 pathways underlie the improved antitumor activities of cadonilimab (31).
An increasing body of evidence also supported the synergistic effect of radiotherapy and immunotherapy in treating malignant tumors, such as non-small cell lung cancer (32) and esophageal or gastroesophageal junction cancer (33), as it can eliminate the primary tumors and induce host immunity to control distant metastases (34). Consistent with previous studies, we found that patients treated with cadonilimab in combination with radiotherapy demonstrated a higher ORR of 52.6% and a DCR of 86.8%, further identifying the combination of radiotherapy as an independent prognostic factor for both OS and PFS in R/M CC. Previous studies have also confirmed that combination strategies, including immunotherapy and radiation, can enhance anti-tumor efficacy via synergistic mechanisms (35). Preclinical studies show that radiotherapy can induce tumor cell death and antigen release, enhancing the immune response by altering the tumor microenvironment and making tumor cells more recognizable to immune cells (36). In addition, ICIs could promote tumor vascular normalization in a T-cell-dependent manner through interferon (IFN)-mediated signaling between T cells and endothelial cells, improving tissue perfusion and reducing intratumoral hypoxia and acidosis, thereby sensitizing tumors to ionizing radiation (37). Hence, the combination of cadonilimab and radiotherapy has shown significant potential in treating R/M CC and might emerge as an important therapeutic strategy in the future.
The safety profile of cadonilimab was consistent with that previously reported for the drug and combined therapy in patients with different tumor types (38–40). In our study, cadonilimab, combined with other therapies, generally reflects a manageable safety profile with mostly grade 1–2 TRAEs, even under the circumstance of prolonged exposure to cadonilimab. It is worth noting that the incidence of grade 3–4 irAEs among participants treated with cadonilimab monotherapy was only 31.6% (6/19), comparable to the 28% reported in the COMPASSION-03 study and lower than MEDI5752 (38%), another bispecific anti-PD-1/CTLA-4 antibody (18, 41). When combined with other treatments, only 46.6% of patients experienced grade ≥3 TRAEs, which is also lower than the reported rate of 73.3% (18), underscoring cadonilimab’s favorable safety profiles. Mechanistically, cadonilimab’s favorable safety profile is likely attributed to its unique Fc-null design. The Fc-null design inhibits binding to Fc receptors, significantly reducing antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and the release of interleukins-6 and -8 (42). Immune-related toxicity profiles might also be influenced by monocyte/macrophage repolarization, a mechanism gaining attention in cancer immunotherapy (43). These properties collectively contribute to the low toxicity observed in clinical settings.
In-depth analysis of the PIK3CA mutation landscape in our cohort revealed that all alterations were restricted to canonical helical domain hotspots—E542K and E545K—which are among the most frequently reported oncogenic mutations in solid tumors, including cervical cancer (44). These mutations are known to promote constitutive activation of the PI3K-AKT pathway by relieving inhibitory interactions with the p85 regulatory subunit, thereby enhancing downstream proliferative and survival signaling (45). Both E542K and E545K have been implicated in increased immune evasion and tumor aggressiveness (46). Furthermore, KEGG pathway enrichment analysis in our cohort demonstrated that PIK3CA mutations often co-occurred with COL6A6 alterations and were significantly enriched in the PI3K–AKT signaling pathway. This pathway not only drives cell cycle progression and metabolic adaptation but also shapes the tumor immune microenvironment by regulating apoptosis resistance, cytokine secretion, and immune cell recruitment (47, 48). Notably, our findings are consistent with a prior report by Xu et al. (49), which demonstrated that patients harboring PIK3CA mutations exhibited a significantly higher objective response rate (ORR) to anti-PD-1 therapy (91.7%) compared to those with wild-type PIK3CA (46.2%; P = 0.012). In our study, all four patients with PIK3CA mutations responded to cadonilimab (CR or PR), whereas no mutations were detected in the non-responder group. Although this trend did not reach statistical significance (P = 0.084), likely due to the limited sample size, the observed pattern suggests a potential predictive role of PIK3CA mutations for immunotherapy responsiveness in selected patients. These findings are exploratory in nature and warrant validation in larger, independent cohorts. Emerging evidence also suggests that traditional compounds, including herbal derivatives, may modulate tumor immunogenicity and complement checkpoint-based approaches (50). Furthermore, future mechanistic studies are needed to explore whether aberrant PI3K–AKT pathway activation influences cadonilimab sensitivity by modulating immune evasion or the tumor microenvironment.
Of note, to our knowledge, this study represents the largest real-world data set to date evaluating cadonilimab as a therapeutic modality in patients with R/M CC. On the one hand, compared to the limited use of ICIs targeting PD-1 in PD-L1-negative populations with a low ORR ranging from 0-16.7% (9, 51–54). Cadonilimab demonstrated significant antitumor activity even in a PD-L1-negative population, with an ORR of 35.7% and an mPFS of 12.8 months, indicating the broad application of cadonilimab in the PD-L1-negative population. On the other hand, 8.5% of the population achieved CR, giving us hope that recovery from disseminated tumors and maintaining a normal life are no longer a luxuries for late-stage CC patients in the era of immunotherapy. Further in-depth research and fundamental experiments are necessary to explore the potential roles of cadonilimab in the low PD-L1 expression subgroup and its antitumor mechanism.
Despite the encouraging results, several limitations should be acknowledged. First, this was a retrospective study conducted in China, which led to selection bias and limited generalizability. Second, the long-term benefits and late toxicities were unavailable due to the relatively short follow-up time. Third, many challenges remain in the clinical use of cadonilimab due to financial constraints. However, with growing real-world evidence, we believe cadonilimab will soon be covered by Chinese medical insurance and benefit more R/M CC patients. Moreover, treatment regimens in our study were heterogeneous, and baseline characteristics varied across subgroups. Due to the limited sample size, we did not perform propensity score matching or multivariable adjustment. Therefore, subgroup analyses—such as those based on PD-L1 CPS—should be considered exploratory and interpreted with caution. Lastly, the limited number of patients who underwent PD-L1 testing and whole-exome sequencing reduced the statistical power to assess the predictive value of biomarkers such as PD-L1 expression, TMB, and PIK3CA mutations. These exploratory findings should be interpreted with caution and validated in larger prospective studies.
Conclusion
In sum, R/M CC is a life-threatening disease with limited treatment options available. In our study, cadonilimab demonstrated promising efficacy with acceptable safety profile, even in the low PD-L1 population. Cadonilimab has the potential to become a new standard of care in treating CC. Further investigations involving larger-scale randomized clinical trials and real-world studies are warranted.
Data availability statement
The datasets generated and/or analyzed during the current study are not publicly available due to patient privacy and institutional restrictions. De-identified data may be available from the corresponding author on reasonable request and with appropriate institutional approvals.
Ethics statement
The studies involving humans were approved by Ethics Committee of Fujian Cancer Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Software, Writing – original draft, Writing – review & editing, Resources. HY: Conceptualization, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Y-tL: Formal analysis, Methodology, Project administration, Software, Writing – review & editing. DH: Investigation, Methodology, Project administration, Software, Supervision, Validation, Writing – original draft. LL: Data curation, Formal analysis, Investigation, Project administration, Writing – original draft. RF: Writing – original draft. JZ: Formal analysis, Investigation, Software, Writing – original draft. LZ: Data curation, Investigation, Writing – original draft. YaL: Data curation, Formal analysis, Methodology, Writing – original draft. RLi: Investigation, Methodology, Resources, Writing – original draft. DC: Investigation, Resources, Writing – original draft. XW: Investigation, Resources, Writing – original draft. FS: Investigation, Writing – original draft. SW: Investigation, Writing – original draft. WZ: Investigation, Resources, Writing – original draft. QT: Writing – original draft. YY: Investigation, Resources, Writing – original draft. YC: Investigation, Resources, Writing – original draft. JM: Investigation, Writing – original draft. BZ: Investigation, Writing – original draft. YZ: Investigation, Writing – original draft. FG: Investigation, Writing – original draft. RLa: Resources, Writing – original draft. LY: Investigation, Writing – original draft. YS: Conceptualization, Data curation, Formal analysis, Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Major Scientific Research Program for Young and Middle-aged Health Professionals of Fujian Province, China (Grant number: 2022ZQNZD008). High-level Talents Training Project of Fujian Cancer Hospital (Grant number: 2022YNG04) and Fujian Provincial Health Technology Project (Grant number: 2022QNB001). The funder did not play a role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Acknowledgments
We thank patients and their families and caregivers for participating in the study, as well as participating centers for their support. We also thank Xiaodong Zhu and Yunxing Dai for their helpful suggestions and comments on this research article. The final manuscript was reviewed and revised by a native English speaker for grammatical accuracy and clarity.
Conflict of interest
Author RL was employed by the company Beijing GenePlus Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Generative AI (ChatGPT, OpenAI) was used to assist with improving the grammar, phrasing, and clarity of the manuscript. No scientific content, analysis, or interpretation was generated by AI. The authors take full responsibility for the final content.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1611696/full#supplementary-material
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Han B, Zheng R, Zeng H, Wang S, Sun K, Chen R, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Center. (2024) 4:47–53. doi: 10.1016/j.jncc.2024.01.006
3. Lorusso D, Xiang Y, Hasegawa K, Scambia G, Leiva M, Ramos-Elias P, et al. Pembrolizumab or placebo with chemoradiotherapy followed by pembrolizumab or placebo for newly diagnosed, high-risk, locally advanced cervical cancer (ENGOT-cx11/GOG-3047/KEYNOTE-A18): a randomised, double-blind, phase 3 clinical trial. Lancet. (2024) 403:1341–50. doi: 10.1016/S0140-6736(24)00317-9
4. Abu-Rustum NR, Yashar CM, Arend R, Barber E, Bradley K, Brooks R, et al. NCCN guidelines® Insights: cervical cancer, version 1.2024. J Natl Compr Cancer Network. (2023) 21:1224–33. doi: 10.6004/jnccn.2023.0062
5. Zhang Z, Yang J, Liu R, Wang L, Lin Y, Xu Y, et al. Inhibiting HMGCR represses stemness and metastasis of hepatocellular carcinoma via Hedgehog signaling. Genes Diseases. (2024) 11:101285. doi: 10.1016/j.gendis.2024.101285
6. Kennedy LB and Salama AKS. A review of cancer immunotherapy toxicity. CA: A Cancer J Clin. (2020) 70:86–104. doi: 10.3322/caac.21596
7. Chuah S and Chew V. High-dimensional immune-profiling in cancer: implications for immunotherapy. J ImmunoTher Cancer. (2020) 8(1):e000363. doi: 10.1136/jitc-2019-000363
8. Frenel J-S, Le Tourneau C, O’Neil B, Ott PA, Piha-Paul SA, Gomez-Roca C, et al. Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1–positive cervical cancer: results from the phase ib KEYNOTE-028 trial. J Clin Oncol. (2017) 35:4035–41. doi: 10.1200/JCO.2017.74.5471
9. Chung HC, Ros W, Delord J-P, Perets R, Italiano A, Shapira-Frommer R, et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. (2019) 37:1470–8. doi: 10.1200/JCO.18.01265
10. Monk BJ, Colombo N, Tewari KS, Dubot C, Caceres MV, Hasegawa K, et al. First-line pembrolizumab + Chemotherapy versus placebo + Chemotherapy for persistent, recurrent, or metastatic cervical cancer: final overall survival results of KEYNOTE-826. J Clin Oncol. (2023) 41:5505–11. doi: 10.1200/JCO.23.00914
11. Lan C, Shen J, Wang Y, Li J, Liu Z, He M, et al. Camrelizumab plus apatinib in patients with advanced cervical cancer (CLAP): A multicenter, open-label, single-arm, phase II trial. J Clin Oncol. (2020) 38:4095–106. doi: 10.1200/JCO.20.01920
12. Rodrigues M, Vanoni G, Loap P, Dubot C, Timperi E, Minsat M, et al. Nivolumab plus chemoradiotherapy in locally-advanced cervical cancer: the NICOL phase 1 trial. Nat Commun. (2023) 14(1):3698. doi: 10.1038/s41467-023-39383-8
13. Moore DH. Chemotherapy for advanced, recurrent, and metastatic cervical cancer. J Natl Compr Cancer Network. (2008) 6:53–7. doi: 10.6004/jnccn.2008.0006
14. Li C, Cang W, Gu Y, Chen L, and Xiang Y. The anti-PD-1 era of cervical cancer: achievement, opportunity, and challenge. Front Immunol. (2023) 14. doi: 10.3389/fimmu.2023.1195476
15. Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. (2016) 13:473–86. doi: 10.1038/nrclinonc.2016.58
17. Tang J, He J, Guo H, Yang Z, Zhao J, Zhang Z, et al. PTBP2-mediated alternative splicing of IRF9 controls tumor-associated monocyte/macrophage chemotaxis and repolarization in neuroblastoma progression. Research. (2023) 6:0033. doi: 10.34133/research.0033
18. Gao X, Xu N, Li Z, Shen L, Ji K, Zheng Z, et al. Safety and antitumour activity of cadonilimab, an anti-PD-1/CTLA-4 bispecific antibody, for patients with advanced solid tumours (COMPASSION-03): a multicentre, open-label, phase 1b/2 trial. Lancet Oncol. (2023) 24:1134–46. doi: 10.1016/S1470-2045(23)00411-4
19. Xu G, Li J, Zhang S, Li Z, Zhang C, Deng X, et al. Two-dimensional nano-biomaterials in regulating the tumor microenvironment for immunotherapy. Nano TransMed. (2024) 100045. doi: 10.1016/j.ntm.2024.100045
20. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
21. Dueck AC, Mendoza TR, Mitchell SA, Reeve BB, Castro KM, Rogak LJ, et al. Validity and reliability of the US national cancer institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). JAMA Oncol. (2015) 1(8):1051–9. doi: 10.1001/jamaoncol.2015.2639
22. Yang X, Lin J, Wang D, Zhang L, and Zhao H. Immune-related adverse events (irAEs) predict for clinical efficacy: focusing on organ-specific irAEs and the critical role of steroids. J Thorac Oncol. (2019) 14:e233–e4. doi: 10.1016/j.jtho.2019.05.020
23. Zhu Y, Wen J, Li Q, Chen B, Zhao L, Liu S, et al. Toripalimab combined with definitive chemoradiotherapy in locally advanced oesophageal squamous cell carcinoma (EC-CRT-001): a single-arm, phase 2 trial. Lancet Oncol. (2023) 24:371–82. doi: 10.1016/S1470-2045(23)00060-8
24. Barroso-Sousa R, Jain E, Cohen O, Kim D, Buendia-Buendia J, Winer E, et al. Prevalence and mutational determinants of high tumor mutation burden in breast cancer. Ann Oncol. (2020) 31:387–94. doi: 10.1016/j.annonc.2019.11.010
25. Zhu S, Zhao J, Nie L, Yin W, Zhang Y, Zhao F, et al. Homologous recombination deficiency (HRD) score in aggressive prostatic adenocarcinoma with or without intraductal carcinoma of the prostate (IDC-P). BMC Med. (2022) 20(1):37. doi: 10.1186/s12916-022-02430-0
26. Guo Z, Saw PE, and Jon S. Non-invasive physical stimulation to modulate the tumor microenvironment: unveiling a new frontier in cancer therapy. Bio Integration. (2024) 5:986. doi: 10.15212/bioi-2024-0012
27. Gennigens C, Jerusalem G, Lapaille L, De Cuypere M, Streel S, Kridelka F, et al. Recurrent or primary metastatic cervical cancer: current and future treatments. ESMO Open. (2022) 7(5):100579. doi: 10.1016/j.esmoop.2022.100579
28. Huang Y, Yang Y, Zhao Y, Zhao H, Zhou N, Zhang Y, et al. QL1706 (anti-PD-1 IgG4/CTLA-4 antibody) plus chemotherapy with or without bevacizumab in advanced non-small cell lung cancer: a multi-cohort, phase II study. Signal Transduct Target Ther. (2024) 9(1):23. doi: 10.1038/s41392-023-01731-x
29. Livingstone E, Zimmer L, Hassel JC, Fluck M, Eigentler TK, Loquai C, et al. Adjuvant nivolumab plus ipilimumab or nivolumab alone versus placebo in patients with resected stage IV melanoma with no evidence of disease (IMMUNED): final results of a randomised, double-blind, phase 2 trial. Lancet. (2022) 400:1117–30. doi: 10.1016/S0140-6736(22)01654-3
30. O'Malley DM, Neffa M, Monk BJ, Melkadze T, Huang M, Kryzhanivska A, et al. Dual PD-1 and CTLA-4 Checkpoint Blockade Using Balstilimab and Zalifrelimab Combination as Second-Line Treatment for Advanced Cervical Cancer: An Open-Label Phase II Study. J Clin Oncol. (2022) 40:762–71. doi: 10.1200/JCO.21.02067
31. Wei SC, Levine JH, Cogdill AP, Zhao Y, Anang N-AAS, Andrews MC, et al. Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade. Cell. (2017) 170:1120–33.e17. doi: 10.1016/j.cell.2017.07.024
32. Altorki NK, McGraw TE, Borczuk AC, Saxena A, Port JL, Stiles BM, et al. Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: a single-centre, randomised phase 2 trial. Lancet Oncol. (2021) 22:824–35. doi: 10.1016/S1470-2045(21)00149-2
33. Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. New Engl J Med. (2021) 384:1191–203. doi: 10.1056/NEJMoa2032125
34. Wang M, Rao J, Wang M, Li X, Liu K, Naylor MF, et al. Cancer photo-immunotherapy: from bench to bedside. Theranostics. (2021) 11:2218–31. doi: 10.7150/thno.53056
35. Mokashi A and Bhatia NM. Integrated network ethnopharmacology, molecular docking, and ADMET analysis strategy for exploring the anti-breast cancer activity of ayurvedic botanicals targeting the progesterone receptor. Bio Integration. (2024) 5:970. doi: 10.15212/bioi-2024-0066
36. McLaughlin M, Patin EC, Pedersen M, Wilkins A, Dillon MT, Melcher AA, et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat Rev Cancer. (2020) 20:203–17. doi: 10.1038/s41568-020-0246-1
37. Wang Y, Liu Z-G, Yuan H, Deng W, Li J, Huang Y, et al. The reciprocity between radiotherapy and cancer immunotherapy. Clin Cancer Res. (2019) 25:1709–17. doi: 10.1158/1078-0432.CCR-18-2581
38. Lou H, Cai H, Huang X, Li G, Wang L, Liu F, et al. Cadonilimab combined with chemotherapy with or without bevacizumab as first-line treatment in recurrent or metastatic cervical cancer (COMPASSION-13): A phase 2 study. Clin Cancer Res. (2024) 30:1501–8. doi: 10.1158/1078-0432.CCR-23-3162
39. Chen B, Yao W, Li X, Lin G, Chu Q, Liu H, et al. A phase Ib/II study of cadonilimab (PD-1/CTLA-4 bispecific antibody) plus anlotinib as first-line treatment in patients with advanced non-small cell lung cancer. Br J Cancer. (2023) 130:450–6. doi: 10.1038/s41416-023-02519-0
40. Gao X, Ji K, Jia Y, Shan F, Chen Y, Xu N, et al. Cadonilimab with chemotherapy in HER2-negative gastric or gastroesophageal junction adenocarcinoma: the phase 1b/2 COMPASSION-04 trial. Nat Med. (2024) 30:1943–51. doi: 10.1038/s41591-024-03007-5
41. Bose CK and Basu N. Bispecific immunotherapy MEDI5752 or volrustomig and cervical cancer. J Gynecol Oncol. (2024) 35(4):e82. doi: 10.3802/jgo.2024.35.e82
42. Pang X, Huang Z, Zhong T, Zhang P, Wang ZM, Xia M, et al. Cadonilimab, a tetravalent PD-1/CTLA-4 bispecific antibody with trans-binding and enhanced target binding avidity. mAbs. (2023) 15(1):2180794. doi: 10.1080/19420862.2023.2180794
43. Luo Z, Mei J, Wang X, Jin X, Chen Y, Zhao L, et al. Voluntary exercise sensitizes cancer immunotherapy via the collagen inhibition-orchestrated inflammatory tumor immune microenvironment. Cell Rep. (2024) 43:114697. doi: 10.1016/j.celrep.2024.114697
44. Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. (2017) 23:703–13. doi: 10.1038/nm.4333
45. Burke JE and Williams RL. Synergy in activating class I PI3Ks. Trends Biochem Sci. (2015) 40:88–100. doi: 10.1016/j.tibs.2014.12.003
46. Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, et al. Loss of PTEN promotes resistance to T cell–mediated immunotherapy. Cancer Discovery. (2016) 6:202–16. doi: 10.1158/2159-8290.CD-15-0283
47. Li H, van der Leun AM, Yofe I, Lubling Y, Gelbard-Solodkin D, van Akkooi AC, et al. Dysfunctional CD8 T cells form a proliferative, dynamically regulated compartment within human melanoma. Cell. (2019) 176:775–89.e18. doi: 10.1016/j.cell.2018.11.043
48. Zheng J, Wang S, Xia L, et al. Signal. Transduct Target Ther. (2025) 10(1):35. doi: 10.1038/s41392-024-02075-w
49. Xu Q, Wang J, Sun Y, Lin Y, Liu J, Zhuo Y, et al. Efficacy and safety of sintilimab plus anlotinib for PD-L1–positive recurrent or metastatic cervical cancer: A multicenter, single-arm, prospective phase II trial. J Clin Oncol. (2022) 40:1795–805. doi: 10.1200/JCO.21.02091
50. Qin N, Yang SX, Gao SM, Feng Y, Wang T, Liu J, et al. Celastrol inhibits inflammatory factors expression in glioblastoma. Traditional Med Res. (2024) 9:31. doi: 10.53388/TMR20231207006
51. Doroshow DB, Bhalla S, Beasley MB, Sholl LM, Kerr KM, Gnjatic S, et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol. (2021) 18:345–62. doi: 10.1038/s41571-021-00473-5
52. Naumann RW, Hollebecque A, Meyer T, Devlin M-J, Oaknin A, Kerger J, et al. Safety and efficacy of nivolumab monotherapy in recurrent or metastatic cervical, vaginal, or vulvar carcinoma: results from the phase I/II checkMate 358 trial. J Clin Oncol. (2019) 37:2825–34. doi: 10.1200/JCO.19.00739
53. Tamura K, Hasegawa K, Katsumata N, Matsumoto K, Mukai H, Takahashi S, et al. Efficacy and safety of nivolumab in Japanese patients with uterine cervical cancer, uterine corpus cancer, or soft tissue sarcoma: Multicenter, open-label phase 2 trial. Cancer Science. (2019) 110:2894–904. doi: 10.1111/cas.14148
Keywords: recurrent or metastatic cervical cancer, PD-1, CTLA-4, bi-specific antibody, cadonilimab, real world
Citation: Chen J, Yu H, Lin Y, Hu D, Liu L, Fan R, Zou J, Zang L, Lin Y, Lin R, Chen D, Weng X, Shen F, Wang S, Zeng W, Tian Q, Yi Y, Chen Y, Miao J, Zhang B, Zou Y, Gao F, Lian R, Yang L and Sun Y (2025) Real-world data of cadonilimab in recurrent or metastatic cervical cancer in China: a multicentric study. Front. Immunol. 16:1611696. doi: 10.3389/fimmu.2025.1611696
Received: 14 April 2025; Accepted: 24 June 2025;
Published: 14 July 2025.
Edited by:
Zhiwen Luo, Fudan University, ChinaReviewed by:
Guochun Zhang, Guangdong Provincial People’s Hospital, ChinaHualei Bu, Shandong University, China
Copyright © 2025 Chen, Yu, Lin, Hu, Liu, Fan, Zou, Zang, Lin, Lin, Chen, Weng, Shen, Wang, Zeng, Tian, Yi, Chen, Miao, Zhang, Zou, Gao, Lian, Yang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Sun, c3VueWFuZ2ZqemxAc2luYS5jb20=
†These authors have contributed equally to this work
 Jian Chen
Jian Chen Haijuan Yu
Haijuan Yu Yingtao Lin
Yingtao Lin Dan Hu
Dan Hu Li Liu
Li Liu Renliang Fan4
Renliang Fan4 Lele Zang
Lele Zang Shaoyu Wang
Shaoyu Wang Rong Lian
Rong Lian Yang Sun
Yang Sun