- Stomatological Hospital, School of Stomatology, Southern Medical University, Guangzhou, China
Osteoarthritis (OA) is a chronic, degenerative joint disease characterized by progressive cartilage degradation and inflammation. Exosomes, small vesicles released by various cell types, play a crucial role in mediating immune responses and inflammation. In OA, exosomes from antigen-presenting cells (APCs) promote synovial inflammation through antigen presentation and cytokine signaling, while those from mesenchymal stem cells (MSCs) modulate inflammation by reprogramming macrophages. Exosomal cargo has shown potential in controlling inflammatory pathways and protecting cartilage from degradation. MSC-derived exosomes have demonstrated therapeutic promise in reducing OA-related inflammation and promoting cartilage regeneration. Despite several reports have outlined the role of exosomes or immune modulation in OA individually, comprehensive reviews integrating their roles in both immune regulation and inflammation repair in OA are still lacking. This knowledge gap hinders the translational application of exosome-based interventions in clinical settings. This review aims to summarize the immunoregulatory roles of exosomes in OA, emphasizing their impact on inflammation and immune responses, and discusses their therapeutic potential in OA treatment. By elucidating the roles of exosomes, the findings of this review could facilitate the development of novel, minimally invasive strategies for improving OA treatment and enhancing inflammation repair.
1 Introduction
Osteoarthritis (OA) is a prevalent, chronic, age-related joint disease characterized by progressive cartilage degeneration, synovial inflammation, and subchondral bone remodeling (1, 2). Traditional therapies for OA are primarily palliative, focusing on symptom relief rather than disease modification. Nonsteroidal anti-inflammatory drugs (NSAIDs) and corticosteroids alleviate pain but are associated with significant adverse effects upon long-term use (3, 4). Intra-articular injections of corticosteroids or hyaluronic acid offer only transient relief, while physical therapy maintains mobility without halting cartilage loss (5, 6). Surgical interventions such as joint arthroplasty are effective in advanced cases but are invasive and costly (7).
In contrast, exosome-based therapies have emerged as a promising regenerative strategy, targeting the immunological and catabolic pathways central to OA pathogenesis (8, 9). MSC-derived exosomes can modulate the inflammatory microenvironment by promoting M2 macrophage polarization and inhibiting NF-κB signaling, thereby promoting cartilage repair (10, 11). These nano-vesicles can also deliver specific miRNAs to restore chondrocyte homeostasis and extracellular matrix synthesis. Although currently in preclinical or early clinical stages (12–14), exosome-based approaches offer minimally invasive, repeatable, and potentially disease-modifying treatment options. Existing literature has predominantly examined either the effects of exosomes or immune regulation in OA separately (15–17), with seldom systematic evaluation of their combined impact on both inflammatory suppression and structural repair. This lack of integrated understanding poses a barrier to the clinical translation of exosome-based therapies. This review aims to highlight recent advances in understanding the immunoregulatory functions of exosomes in OA and their therapeutic implications in inflammation resolution and cartilage regeneration, providing a timely and in-depth evaluation of exosome-based interventions for inflammation resolution and cartilage regeneration in OA.
2 Properties of exosomes
2.1 Structural features
Although exosome molecular composition varies with the cell of origin, microenvironment, developmental stage, epigenetic landscape, and precise biogenetic pathway, exosomes share several conserved features. An exosome comprises an external “shell” and an internal “cargo.” The limiting membrane displays a lipid-raft-like architecture enriched in cholesterol, sphingomyelin, and ceramide, which facilitates vesicular trafficking within the cytosol (18). Canonical surface markers include heat-shock proteins, tetraspanins (CD82, CD81, CD63, and CD9), and major histocompatibility complex (MHC) molecules (19). TSG101, which binds ubiquitinated cargo proteins, serves as a hallmark of endosomal sorting. Initially regarded as metabolic waste, exosome contents are now known to encompass abundant nucleic acids, lipids, and proteins, including long non-coding RNA, microRNA, and mRNA, that play critical roles in intercellular communication and immune regulation.
2.2 Biogenesis and release
Exosome biogenesis and secretion constitute a tightly regulated, multi-step process initiated by the inward budding of the plasma membrane to form early endosomes. These compartments subsequently mature into multivesicular bodies (MVBs) through a secondary invagination process that requires coordinated action of key molecular machinery, including the endosomal sorting complex required for transport (ESCRT), particularly ESCRT-III, which mediates vesicle budding and scission (20). While ESCRT-dependent mechanisms predominate in most cell types, alternative ESCRT-independent pathways involving sphingomyelinase-mediated ceramide generation can also facilitate exosome formation. Following their biogenesis, exosomes are secreted through three primary mechanisms: (i) fusion of MVBs packed with exosomes to the plasma membrane; (ii) direct budding outward from the plasma membrane; (iii) discharge from intracellular plasma-membrane-connected compartments (IPMCs) after relief of export restrictions (21, 22). Sustained mTORC1 activation inhibits exosome secretion, while mTORC1 blockade promotes it, with both processes being linked to autophagy (23).
2.3 Exosomes and immunomodulation
In 1996, immunologists first observed that B lymphocytes transformed by the Epstein-Barr virus could produce exosomes via fusion between MVBs and the plasma membrane (24). Subsequent studies revealed that numerous immune and non-immune cells, including T cells, B cells, dendritic cells (DCs), and macrophages, release exosomes capable of mediating immune activation or suppression (25). Exosomal immunoactivity affects both innate and adaptive immunity by modulating antigen presentation, T cell activation, regulatory T cell polarization, immunosuppression, and anti-inflammatory pathways. Exosomes play a vital role in activating and enhancing immune responses via antigen presentation. Professional antigen-presenting cells, including macrophages, B cells, and dendritic cells, release exosomes containing abundant costimulatory signals and MHC class I/II molecules. These exosome-associated peptide antigens are essential for regulating immune function (26).
3 Synovial inflammation and immune dysregulation in OA
OA is a complex degenerative joint disease involving multiple pathological factors. It progressively destroys articular cartilage, leading to persistent pain and gradual loss of joint function (27). Emerging evidence indicates that low-grade synovial inflammation plays a crucial role in both the initiation and advancement of osteoarthritis (28). The healthy synovium comprises two distinct layers: an outer vascular (sub-intimal) layer and an inner cellular (intimal) layer. Together, these layers secrete synovial fluid, thereby minimizing the coefficient of friction across the articular surface (29). The sub-intimal layer is comparatively thick and consists of adipose tissue interspersed with lymphatic vessels, nerve fibers, dense fibrous tissue, type-I collagen, and microvasculature. The intimal layer is thinner and populated by synovial fibroblasts and synovial macrophages (30). During OA development, the synovium undergoes intimal hyperplasia, stromal fibrosis, and neovascularization, accompanied by marked infiltration of NK cells, plasma cells, B cells, mast cells, T cells, and macrophages (31). Notably, macrophage infiltration is already evident at the earliest stages of disease (32). This inflammatory cascade is initiated by localized chondrocyte injury, increased vascularization, and damage-associated molecular pattern (DAMP) release, which collectively activate synovial macrophages and lymphocytes. The resulting immunocyte activation triggers a feed-forward inflammatory loop characterized by elevated chemokine and cytokine production (33). Concomitantly, dysregulated chondrocytes secrete matrix metalloproteinases, pro-inflammatory cytokines, and prostaglandins, thereby creating a self-perpetuating cycle of cartilage destruction (34).
4 Exosomes in the immunoregulation of OA
Both exosomes and OA are intimately linked to immune homeostasis; exploiting this nexus offers new therapeutic avenues (35). Following uptake by target cells, distinct exosome populations elicit discrete functional outcomes. Exosomes may interact directly with the immune system by presenting antigens or, alternatively, modulate cellular behavior via cargo microRNAs (miRNAs) (36). Moreover, exosomes can fuse with endosomes within recipient cells, undergoing self-degradation or being re-secreted extracellularly.
4.1 APC-derived exosomes and OA
4.1.1 Lymphocyte-derived exosomes
Upon T-cell-receptor engagement, murine CD4+CD25+Foxp3+ Tregs release exosomes (37). Treg-derived vesicles carrying miRNAs suppress T helper cell type 1 (Th1) responses via non-cell-autonomous gene silencing (38). Activated CD8+CD25+Foxp3+ T cells likewise secrete exosomes that inhibit CD8+ cytotoxic T-lymphocyte activity, representing an intrinsic negative-feedback mechanism to forestall excessive inflammation. In OA, this pathway manifests as the slow evolution of synovitis: despite strong activation signals from APC-derived exosomes, T-cell hyper-responsiveness is restrained, preventing precipitous inflammatory escalation (39). Compared to DC-derived exosomes, B cell-derived exosomes have been less extensively studied. B-cell exosomes are detectable very early after antigenic challenge—even earlier than DC exosomes—and can activate APCs. These vesicles are enriched in B7-1/B7-2, MHC class I and II molecules, and intercellular adhesion molecule 1 (ICAM-1), facilitating CD4+ T-cell activation and antigen presentation (40). In early OA, B cell-derived exosomes contribute to synovitis. Functional studies show that integrins expressed on these vesicles mediate adhesion to extracellular matrix components and cytokine-primed fibroblasts, suggesting a novel long-distance conduit for adhesive signaling during inflammation. B-cell exosomes also enhance C3 deposition and T-cell reactivity, thereby intensifying synovitis and fueling OA progression (41).
4.1.2 Dendritic cell-derived exosomes
Investigations into exosomal immunomodulation were initiated with DC exosomes, which are now well characterized. Exosomes from mature DCs display MHC class II molecules and co-stimulatory ligands such as B7-2 and ICAM-1, enabling direct T-cell activation (42). In contrast to their mature counterparts, immature DC-derived exosomes exhibit distinct immunomodulatory properties. Rather than directly activating T cells, these vesicles primarily facilitate antigen distribution to other antigen-presenting cells (43, 44) or mediate the transfer of MHC/antigen complexes to DC surface receptors, thereby indirectly promoting CD8+ T cell polarization (45). Mechanistically, immature DC exosomes are characterized by reduced expression of co-stimulatory and adhesion molecules, while displaying up-regulated immunosuppressive factors (TGF-β, NKG2D ligands, Galectin-9) and CD95L, features that collectively induce T cell apoptosis and suppress immune activation (46, 47). Within the osteoarthritic joint, while both DC subsets contribute to T cell-mediated inflammation, immature DC exosomes demonstrate a paradoxical protective capacity by attenuating synovial inflammatory cell infiltration and potentially slowing cartilage degeneration (48).
4.1.3 Monocyte macrophage-derived exosomes
Macrophage-derived exosomes play a pivotal role in propagating inflammation in osteoarthritis through their immunomodulatory effects on innate and adaptive immune cells. Following bacterial infection, these exosomes exert pro-inflammatory effects by activating naïve macrophages, promoting dendritic cell maturation, and stimulating CD4+ and CD8+ T cell responses. This occurs via the presentation of bacterial antigens, such as immunogenic proteins, which trigger DC activation and subsequent cytokine release (49). Although initially characterized in infectious contexts, such antigen presentation constitutes a broader mode of inter-immune-cell communication. In OA, persistent cartilage damage and osteophyte formation sustain a chronic inflammatory microenvironment, where macrophage-derived exosomes contribute significantly. Exosomes isolated from OA synovial fluid polarize macrophages toward an M1 phenotype, driving the production of pro-inflammatory mediators, including chemokines (CCL20, CCL15, CXCL1), cytokines, and matrix metalloproteinases (MMP-12, MMP-7) (50, 51) (Figure 1A).
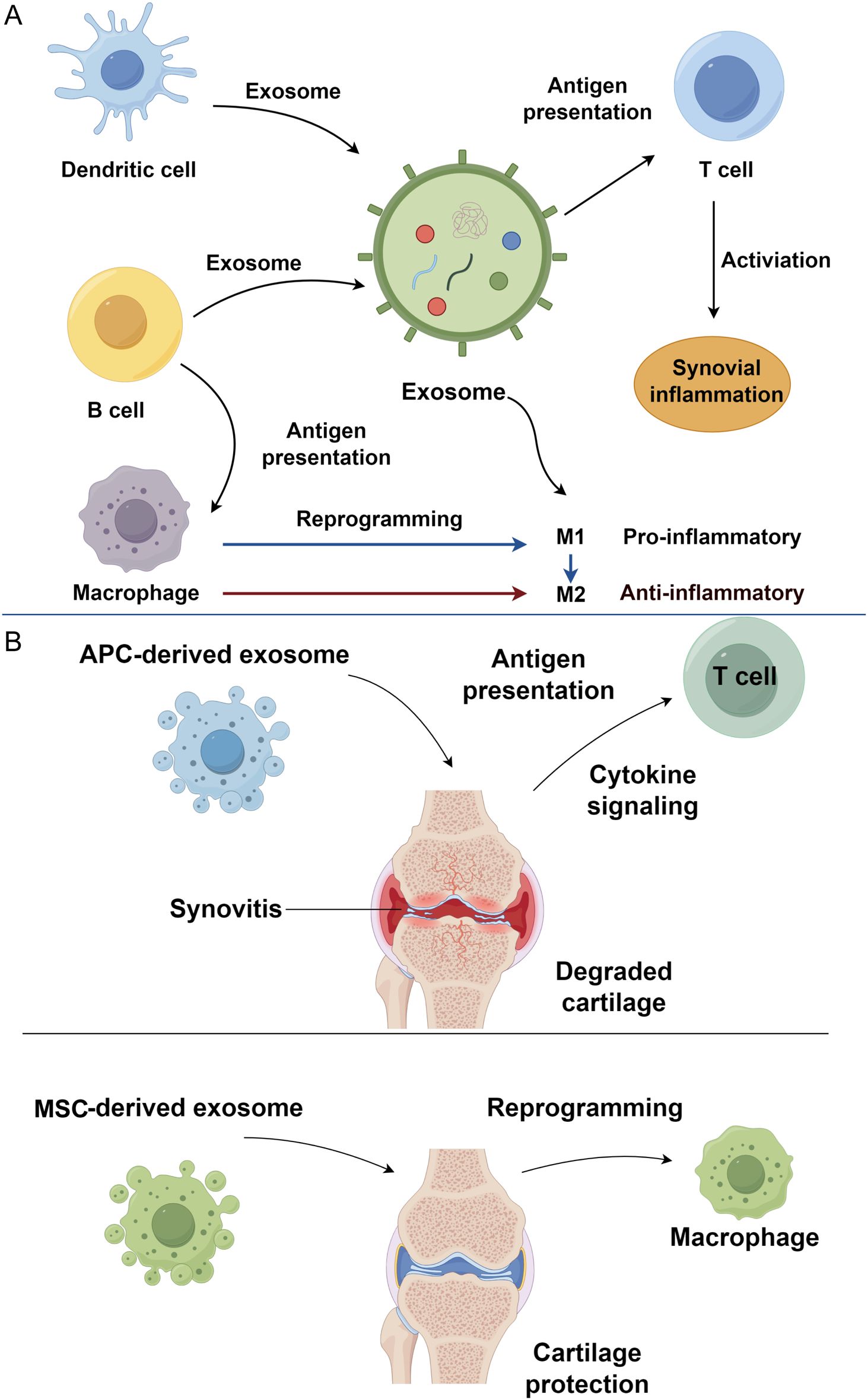
Figure 1. Immune cell-derived exosomes in osteoarthritis progression. (A) Exosomes derived from immune cells, including dendritic cells, B cells, and macrophages, mediate immune communication and synovial inflammation in osteoarthritis. (B) APC-derived exosomes contribute to osteoarthritis progression. In contrast, MSC-derived exosomes exert therapeutic effects by reprogramming macrophages toward an anti-inflammatory phenotype, thereby protecting cartilage and alleviating inflammation. APC, antigen-presenting cell; MSC, mesenchymal stem cell.
4.2 Exosomal cargos in osteoarthritis pathogenesis
To contextualize the roles of exosomal cargos in OA pathogenesis, it is essential to examine how epigenetic alterations mediated by these molecules drive disease progression. The molecular payload enclosed within exosomes is intimately involved in the immunomodulation of osteoarthritis. Throughout disease onset and progression, epigenetic alterations, including miRNA repression, histone modification and DNA methylation (52), perturb multiple transcriptional programs and the synthesis of proteolytic factors that govern the anabolic–catabolic equilibrium, such as ADAMTS5, MMP-13 and RUNX2. Among these epigenetic regulators, miRNAs have attracted particular attention in OA pathobiology (53).
Within the joint, miRNAs exert their effects by targeting key mediators of cartilage homeostasis. Current evidence shows that miR-140 is down-regulated in osteoarthritis cartilage and in chondrocytes stimulated with IL-1β, underscoring its chondroprotective function (54). Mice lacking miR-140 exhibit proteoglycan loss and cartilage fibrillation, whereas transgenic over-expression confers resistance to experimental arthritis (55). This regulatory role is further emphasized by the inverse relationship between miR-140 and catabolic enzymes. In osteoarthritis tissues, low level of miR-140 expression is typically accompanied by increased MMP-13 and ADAMTS5, thereby accelerating disease progression (56, 57). Mechanistically, miR-140 exerts its protective effects primarily by directly targeting the 3′-UTR of ADAMTS5 and MMP-13 mRNA, suppressing their translation and thus attenuating matrix degradation (58). Furthermore, miR-140 negatively regulates RALA and SMAD3, which concurrently modulates chondrocyte hypertrophy and reduces catabolic gene expression (59). Exosomal delivery of miR−140 to chondrocytes enhances COL2A1 expression while simultaneously inhibiting IL−1β-induced activation of the NF-κB pathway, leading to reduced synthesis of pro-inflammatory mediators such as inducible nitric oxide synthase (iNOS) and cyclo−oxygenase−2 (COX-2) (60, 61). Moreover, miR−140-containing exosomes prevent oxidative stress-induced apoptosis by targeting P38 MAPK signaling in articular cartilage (62). Together, these multifaceted mechanisms underscore the central role of exosomal miR−140 in restoring the anabolic-catabolic balance within the joint microenvironment and protecting against OA-associated cartilage erosion. Additional miRNAs, including miR-139 and miR-9, similarly disrupt the anabolic–catabolic balance, precipitating extracellular-matrix breakdown and chondrocyte injury (63).
The dysregulation of exosomal miRNAs in OA is not merely a bystander phenomenon but actively contributes to disease pathology. The miRNA composition of exosomes differs markedly between patients with OA and healthy individuals (64). miRNA profiling of synovial-fluid–derived exosomes reveal the miR-200c overexpression in OA, which suppresses ZEB1 and consequently diminishes type II collagen synthesis (65). Further evidence of exosomal miRNA dysregulation comes from inflammatory stimulation experiments: IL-1β stimulation of normal synovial fibroblasts up-regulates 340 and down-regulates 24 distinct miRNAs relative to unstimulated controls, whereas exosomes released by OA chondrocytes contain 22 up-regulated and 29 down-regulated miRNAs compared with those from normal chondrocytes (66). Collectively, these findings highlight the bidirectional relationship between exosomal cargos and OA progression. Such changes reflect both an altered joint microenvironment and the host’s immunoregulatory response; they, in turn, feed back into disease control—for example, elevated miR-140 dampens local inflammatory cytokine release and mitigates immune activation (67). Exosomes thus occupy a pivotal position in the immunological circuitry of OA.
4.3 Additional links between exosomes and OA
Beyond immunoregulation, exosomes intersect with several other facets of OA pathogenesis, notably synovial angiogenesis. Exosomes released by synovial fibroblasts augment vascular endothelial growth factor (VEGF) secretion, thereby stimulating angiogenesis and driving pathological progression (68, 69). Human umbilical-vein endothelial cells cultured with these exosomes display enhanced migration, tube formation and overall angiogenic capacity. Chondrocyte-derived exosomes are also implicated in osteophyte formation (68). Vesicles 20 – 200 nm in diameter, present within nascent cartilage and bone outgrowths, share exosomal features and carry mediators such as bone morphogenetic proteins (BMPs) that are indispensable for calcification and osteophyte development (70). Exosomes further influence chondrocyte metabolism. Reduced mitochondrial mass in human OA chondrocytes relative to healthy controls, whereas elevated reactive oxygen species (ROS), signifying concurrent mitochondrial dysfunction and ROS accumulation (71–73). Exosome treatment restores mitochondrial integrity, evidenced by increased intracellular ATP, while lowering ROS levels. Intra-articular administration of exosomes can therefore attenuate OA progression by rectifying chondrocyte metabolic defects (74).
5 MSC−derived exosomes and OA
5.1 Anti−inflammatory mechanisms of MSC−derived exosomes
The therapeutic potential of MSC-derived exosomes in OA hinges on their ability to reprogram the inflammatory microenvironment. MSC-derived exosomes contain bioactive components, including trophic factors and apoptosis inhibitors, that modulate the injury microenvironment by shifting the balance from pro-inflammatory to anti-inflammatory responses (75). This shift is particularly relevant in OA, where synovial macrophages play a central role in disease progression (76, 77). In OA pathogenesis, both clinical observations and experimental models demonstrate significant inflammatory cell accumulation within the synovial tissue, particularly highlighting the crucial role of synovial macrophages (78). Notably, macrophage polarization dictates their functional impact: during inflammatory processes, macrophages undergo functional polarization into two distinct subsets: the pro-inflammatory M1 phenotype and the anti-inflammatory M2 phenotype, which play counterregulatory roles in disease progression (79).
MSC-derived exosomes directly influence macrophage polarization to attenuate inflammation. Exosomes derived from MSCs, particularly those containing elevated levels of miR-135b, inhibit MAPK6 expression, which facilitates the polarization of synovial macrophages toward the M2 phenotype and subsequently reduces cartilage degeneration (80, 81). Another key mechanism involves the suppression of NF-κB signaling, a master regulator of inflammation. Targeted inhibition of NF−κB is considered a promising strategy for controlling OA−associated inflammation (82). Upon stimulation with inflammatory mediators, NF-κB translocates to the nucleus and up regulates a repertoire of inflammatory genes encoding proteins, including COX-2, MMPs, and iNOS, culminating in chondrocyte death and exacerbation of OA pathology. MSC exosomes counteract this process via specific miRNAs: phosphorylation−dependent degradation of IκB−α is a pivotal step in NF−κB activation; MSC−derived exosomes carrying miR−147b inhibit TNF−α and IL−1β- driven expression of inflammatory mediators and prevent IκB−α degradation (83, 84) (Supplementary Table S1).
Multiple RNAs contained in MSC exosomes modulate inflammatory signaling in OA. For example, miR−222 targets HDAC4 and thereby down−regulates MMP−13 protein (85, 86); miR−199a−5p lowers IL−6 and TNF−α levels, limiting inflammation and cartilage destruction; miR−140−5p targets Toll−like receptor 4 (TLR4), restraining proliferation of synovial fibroblasts and reducing IL−6 and IL−8 secretion, thus fostering cartilage regeneration (87, 88). Additional miRNAs contribute to the resolution of oxidative stress and inflammation. miR−9−5p down−regulates SDC1, diminishing expression of IL−1, IL−6, TNF−α, MMP−13, alkaline phosphatase (ALP), cartilage oligomeric matrix protein (COMP) and C−reactive protein (CRP), while increasing superoxide dismutase (SOD), NO, malondialdehyde (MDA), iNOS and COX−2, collectively alleviating cartilage injury and curbing inflammatory and oxidative stress damage (89, 90). Beyond miRNAs, long non-coding RNAs also play a role: the long non−coding RNA MALAT1 up−regulates miR−19b via the Wnt/β−catenin and NF−κB pathways, protecting chondrocytes from lipopolysaccharide−induced inflammatory injury (91). In addition, miR−181c, miR−146a, and miR−21 contained in MSC exosomes can reverse the pathological inflammatory milieu characteristic of OA (92, 93) (Figure 1B).
5.2 Recent advances in exosome-based OA therapy
The reparative function of MSCs and their exosomes can be modulated by diverse pre−conditioning strategies, encompassing both biomaterial−based and physical interventions. Biomaterials such as hyaluronic acid, sodium−alginate Janus microspheres and related carriers enhance MSC adhesion to cartilage and enable targeted delivery that accelerates cartilage repair (94, 95). Recent advances in exosome-based OA therapies highlight innovative strategies for cartilage repair. Preconditioning MSCs with cytokines and biomaterials, such as hyaluronic acid and Janus microspheres, enhances exosome yield and therapeutic efficacy (64, 96). Engineered exosomes, such as CRISPR/Cas9-loaded CAP-modified hybrids (CAP/FGF18-hyEXO) and fucoidan-primed exosomes (F-MSCs-Exo), promote chondrogenesis and autophagy via miR-146b-5p (97). Hydrogel encapsulation enables sustained, targeted delivery. Conversely, pathogenic FLS-derived exosomes exacerbate OA via HIF1A-driven glycolysis, which is reversible with 2-DG (98). Macrophage-derived miR-26b-5p exosomes and placental exosomes further modulate inflammation and anabolism (50). These approaches underscore exosomes’ potential in precision OA therapy through engineering, priming, and advanced delivery systems.
6 Conclusion
In conclusion, exosomes have emerged as pivotal mediators in the complex immunological and inflammatory processes underlying OA. These nano-sized vesicles, which are released by various cells, including antigen-presenting cells and MSCs, play dual roles in OA pathogenesis by both propagating and mitigating inflammation. APC-derived exosomes, particularly from dendritic cells, are integral in enhancing immune activation, facilitating antigen presentation, and driving synovial inflammation through cytokine signaling. These exosomes contribute to the activation of T cells and macrophages, thereby accelerating disease progression. On the other hand, exosomes derived from MSCs exhibit a counter-regulatory function, promoting anti-inflammatory responses and cartilage protection. By carrying specific microRNAs and other bioactive molecules, MSC-derived exosomes can reprogram macrophages, shifting their polarization towards the anti-inflammatory M2 phenotype, thus attenuating cartilage degradation and fostering tissue repair.
The therapeutic potential of exosomes in OA treatment is significant, particularly in their ability to influence the joint microenvironment. However, despite this promise, several challenges hinder their clinical translation. Standardization of exosome production, including isolation methods, quantification metrics, and quality control, remains unresolved, leading to batch variability and inconsistent therapeutic effects. Optimal dosing regimens, frequency of administration, and delivery routes are yet to be established. Moreover, exosome-based therapies may elicit unforeseen immunogenicity, especially when derived from allogeneic sources, necessitating rigorous safety evaluations. Additionally, the scale-up of exosome manufacturing under GMP-compliant conditions is still technically and economically challenging. Addressing these translational barriers is crucial for transforming exosome-based therapies from experimental platforms into viable clinical interventions. The integration of exosomes with biomaterial scaffolds and physical stimuli will offer a promising avenue for developing effective, multi-faceted treatments for OA. Furthermore, designing “smart” exosomes with targeted delivery capabilities or artificial intelligence (AI)-engineered cargos may enhance therapeutic efficacy and specificity. Ultimately, exosome research in OA stands at a promising yet formative stage. By addressing fundamental scientific questions and overcoming technical and regulatory barriers, future investigations can unlock the full therapeutic potential of exosomes for immune modulation and tissue regeneration in OA.
Author contributions
SL: Writing – original draft. CZ: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1611718/full#supplementary-material
References
1. Zhang Y, Jin W, Chen J, Wei S, Cai W, Zhong Y, et al. Gastrodin alleviates rat chondrocyte senescence and mitochondrial dysfunction through Sirt3. Int Immunopharmacol. (2023) 118:110022. doi: 10.1016/j.intimp.2023.110022
2. Cai W, Zhang Y, Jin W, Wei S, Chen J, Zhong C, et al. Procyanidin B2 ameliorates the progression of osteoarthritis: An in vitro and in vivo study. Int Immunopharmacol. (2022) 113:109336. doi: 10.1016/j.intimp.2022.109336
3. Haugen IK, Gløersen M, Mulrooney E, and Mathiessen A. Inflammation as a treatment target in hand osteoarthritis: A review of previous studies and future perspectives. J Rheumatol. (2025). doi: 10.3899/jrheum.2025-0206
4. JiaoYi P, YongQi S, KeChun G, XingYu L, ZeZhong L, Jin Shuai D, et al. Assessing the efficacy and safety of different nonsteroidal anti-inflammatory drugs in the treatment of osteoarthritis: A systematic review and network meta-analysis based on RCT trials. PloS One. (2025) 20:e0320379. doi: 10.1371/journal.pone.0320379
5. Bugday B, Bingol H, Yildirim M, and Alatas B. Enhancing knee osteoarthritis detection with AI, image denoising, and optimized classification methods and the importance of physical therapy methods. PeerJ Comput Sci. (2025) 11:e2766. doi: 10.7717/peerj-cs.2766
6. Cömert Kiliç S, Babayev U, and Kiliç N. Is intra-articular injection of hyaluronic acid, corticosteroid or platelet-rich plasma following medical ozone superior to medical ozone alone in the treatment of temporomandibular joint osteoarthritis? J Oral Maxillofac Surg. (2025). doi: 10.1016/j.joms.2025.05.009
7. Zhang X, Han Y, Bai Q, Li T, and Wang F. Clinical efficacy of unicompartmental knee arthroplasty on limb swelling, pain, and functional rehabilitation in knee osteoarthritis patients. J Orthop Surg Res. (2025) 20:616. doi: 10.1186/s13018-025-05917-7
8. Zhang L, Xiang K, Li J, Hao M, Zhu Z, Wang S, et al. Photoactivatable exosenolytics activate natural killer cells for delaying osteoarthritis. ACS Nano. (2025) 19:23028–45. doi: 10.1021/acsnano.5c03344
9. Wu Y, Feng Y, Hu F, Zheng X, Ding Y, Liu X, et al. Engineered stem cell clusters for extracellular vesicles-mediated gene delivery to rejuvenate chondrocytes and facilitate chondrogenesis in osteoarthritis therapy. Adv Sci (Weinh). (2025) 12:e2500964. doi: 10.1002/advs.202500964
10. Shen X, Qin J, Wei Z, and Liu F. Bone marrow mesenchymal stem cell exosome-derived lncRNA TUC339 influences the progression of osteoarthritis by regulating synovial macrophage polarization and chondrocyte apoptosis. BioMed Pharmacother. (2023) 167:115488. doi: 10.1016/j.biopha.2023.115488
11. Liao Q, Li BJ, Li Y, Xiao Y, Zeng H, Liu JM, et al. Low-intensity pulsed ultrasound promotes osteoarthritic cartilage regeneration by BMSC-derived exosomes via modulating the NF-κB signaling pathway. Int Immunopharmacol. (2021) 97:107824. doi: 10.1016/j.intimp.2021.107824
12. Wang Y, Kong Y, Du J, Qi L, Liu M, Xie S, et al. Injection of human umbilical cord mesenchymal stem cells exosomes for the treatment of knee osteoarthritis: from preclinical to clinical research. J Transl Med. (2025) 23:641. doi: 10.1186/s12967-025-06623-y
13. Shao L, Ding L, Li W, Zhang C, Xia Y, Zeng M, et al. Let-7a-5p derived from parathyroid hormone (1-34)-preconditioned BMSCs exosomes delays the progression of osteoarthritis by promoting chondrocyte proliferation and migration. Stem Cell Res Ther. (2025) 16:299. doi: 10.1186/s13287-025-04416-0
14. Sankaranarayanan J, Kim HK, Kang JY, Kuppa SS, Yang HY, and Seon JK. Comparative efficacy of exosomes derived from different mesenchymal stem cell sources in osteoarthritis models: an in vitro and ex vivo analysis. Int J Mol Sci. (2025) 26:5447. doi: 10.3390/ijms26125447
15. Yin B, Ni J, Witherel CE, Yang M, Burdick JA, Wen C, et al. Harnessing tissue-derived extracellular vesicles for osteoarthritis theranostics. Theranostics. (2022) 12:207–31. doi: 10.7150/thno.62708
16. Zhang H, Cai D, and Bai X. Macrophages regulate the progression of osteoarthritis. Osteoarthritis Cartilage. (2020) 28:555–61. doi: 10.1016/j.joca.2020.01.007
17. Nedunchezhiyan U, Varughese I, Sun AR, Wu X, Crawford R, and Prasadam I. Obesity, inflammation, and immune system in osteoarthritis. Front Immunol. (2022) 13:907750. doi: 10.3389/fimmu.2022.907750
18. Chen YF, Luh F, Ho YS, and Yen Y. Exosomes: a review of biologic function, diagnostic and targeted therapy applications, and clinical trials. J BioMed Sci. (2024) 31:67. doi: 10.1186/s12929-024-01055-0
19. Cunha ERK, Ying W, and Olefsky JM. Exosome-mediated impact on systemic metabolism. Annu Rev Physiol. (2024) 86:225–53. doi: 10.1146/annurev-physiol-042222-024535
20. Chen X, Liu S, Wang H, Liu Y, Xiao Y, Li K, et al. Extracellular vesicles deliver thioredoxin to rescue stem cells from senescence and intervertebral disc degeneration via a feed-forward circuit of the NRF2/AP-1 composite pathway. Acta Pharm Sin B. (2025) 15:1007–22. doi: 10.1016/j.apsb.2024.12.013
21. Bebelman MP, Crudden C, Pegtel DM, and Smit MJ. The convergence of extracellular vesicle and GPCR biology. Trends Pharmacol Sci. (2020) 41:627–40. doi: 10.1016/j.tips.2020.07.001
22. Verweij FJ, Bebelman MP, Jimenez CR, Garcia-Vallejo JJ, Janssen H, Neefjes J, et al. Quantifying exosome secretion from single cells reveals a modulatory role for GPCR signaling. J Cell Biol. (2018) 217:1129–42. doi: 10.1083/jcb.201703206
23. Zubkova E, Kalinin A, Bolotskaya A, Beloglazova I, and Menshikov M. Autophagy-dependent secretion: crosstalk between autophagy and exosome biogenesis. Curr Issues Mol Biol. (2024) 46:2209–35. doi: 10.3390/cimb46030142
24. Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. (1996) 183:1161–72. doi: 10.1084/jem.183.3.1161
25. Liu C, Luo Y, Zhou H, Lin M, Zang D, and Chen J. Immune cell-derived exosomal non-coding RNAs in tumor microenvironment: Biological functions and potential clinical applications. Chin J Cancer Res. (2025) 37:250–67. doi: 10.21147/j.issn.1000-9604.2025.02.10
26. Zheng C, Hei H, Zhai Y, Gong W, Zhang R, and Zhang S. CAFs-released exosomal CREB1 promotes cell progression and immune evasion in thyroid cancer via the positive regulation of CCL20. Autoimmunity. (2025) 58:2458324. doi: 10.1080/08916934.2025.2458324
27. Yao Q, Wu X, Tao C, Gong W, Chen M, Qu M, et al. Osteoarthritis: pathogenic signaling pathways and therapeutic targets. Signal Transduct Target Ther. (2023) 8:56. doi: 10.1038/s41392-023-01330-w
28. Terkawi MA, Ebata T, Yokota S, Takahashi D, Endo T, Matsumae G, et al. Low-grade inflammation in the pathogenesis of osteoarthritis: cellular and molecular mechanisms and strategies for future therapeutic intervention. Biomedicines. (2022) 10:1109. doi: 10.3390/biomedicines10051109
29. Roškar S, Mihalič R, Mihelič A, and Trebše R. Synovial fluid viscosity with synovial fluid cell count, valuable diagnostic marker of prosthetic joint infections. Sci Rep. (2025) 15:16223. doi: 10.1038/s41598-025-00760-6
30. Knab K, Chambers D, and Krönke G. Synovial macrophage and fibroblast heterogeneity in joint homeostasis and inflammation. Front Med (Lausanne). (2022) 9:862161. doi: 10.3389/fmed.2022.862161
31. Aitchison AH, Allen NB, O’Neill CN, Droz LG, Patel P, Anastasio AT, et al. Synovial fluid immune cell composition following intraarticular fracture may contribute to posttraumatic osteoarthritis. Int J Mol Sci. (2024) 25:12037. doi: 10.3390/ijms252212037
32. Tang W, Yin JB, Lin RG, Wu CY, Huang JL, Zhu JJ, et al. Rapgef3 modulates macrophage reprogramming and exacerbates synovitis and osteoarthritis under excessive mechanical loading. iScience. (2025) 28:112131. doi: 10.1016/j.isci.2025.112131
33. Shao B, Xu Y, Jia M, Li CX, and Gong ZC. Association of HMGB1 levels in synovial fluid with the severity of temporomandibular joint osteoarthritis. BMC Musculoskelet Disord. (2023) 24:183. doi: 10.1186/s12891-023-06208-0
34. Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis)! Osteoarthritis Cartilage. (2013) 21:16–21. doi: 10.1016/j.joca.2012.11.012
35. Chen X, Tian B, Wang Y, Zheng J, and Kang X. Potential and challenges of utilizing exosomes in osteoarthritis therapy (Review). Int J Mol Med. (2025) 55:43. doi: 10.3892/ijmm.2025.5484
36. Pakdaman Kolour SS, Nematollahi S, Dehbozorgi M, Fattahi F, Movahed F, Esfandiari N, et al. Extracecellulr vesicles (EVs) microRNAs (miRNAs) derived from mesenchymal stem cells (MSCs) in osteoarthritis (OA); detailed role in pathogenesis and possible therapeutics. Heliyon. (2025) 11:e42258. doi: 10.1016/j.heliyon.2025.e42258
37. Basak U, Chakraborty S, Mukherjee S, Pati S, Khan P, Ghosh S, et al. Breast cancer stem cells convert anti-tumor CD4(+) T cells to pro-tumor T regulatory cells: Potential role of exosomal FOXP3. Cell Immunol. (2025) 409-410:104931. doi: 10.1016/j.cellimm.2025.104931
38. Ding M, Zhang C, Wang W, Wang P, Pei Y, Wang N, et al. Silica-exposed macrophages-secreted exosomal miR125a-5p induces Th1/Th2 and Treg/Th17 cell imbalance and promotes fibroblast transdifferentiation. Ecotoxicol Environ Saf. (2023) 267:115647. doi: 10.1016/j.ecoenv.2023.115647
39. Tsuchiya H, Ota M, Sumitomo S, Ishigaki K, Suzuki A, Sakata T, et al. Parsing multiomics landscape of activated synovial fibroblasts highlights drug targets linked to genetic risk of rheumatoid arthritis. Ann Rheum Dis. (2021) 80:440–50. doi: 10.1136/annrheumdis-2020-218189
40. Ling HY, Yang Z, Wang PJ, Sun Y, Ju SG, Li J, et al. Diffuse large B-cell lymphoma-derived exosomes push macrophage polarization toward M2 phenotype via GP130/STAT3 signaling pathway. Chem Biol Interact. (2022) 352:109779. doi: 10.1016/j.cbi.2021.109779
41. Papp K, Végh P, Prechl J, Kerekes K, Kovács J, Csikós G, et al. B lymphocytes and macrophages release cell membrane deposited C3-fragments on exosomes with T cell response-enhancing capacity. Mol Immunol. (2008) 45:2343–51. doi: 10.1016/j.molimm.2007.11.021
42. Zhang M, Yu Q, Tang W, Wu Y, Lv J, Sun L, et al. Epithelial exosomal contactin-1 promotes monocyte-derived dendritic cell-dominant T-cell responses in asthma. J Allergy Clin Immunol. (2021) 148:1545–58. doi: 10.1016/j.jaci.2021.04.025
43. Zhang B, Yin Y, Lai RC, and Lim SK. Immunotherapeutic potential of extracellular vesicles. Front Immunol. (2014) 5:518. doi: 10.3389/fimmu.2014.00518
44. Aloi N, Drago G, Ruggieri S, Cibella F, Colombo P, and Longo V. Extracellular vesicles and immunity: at the crossroads of cell communication. Int J Mol Sci. (2024) 25:1205. doi: 10.3390/ijms25021205
45. Pang XL, Wang ZG, Liu L, Feng YH, Wang JX, Xie HC, et al. Immature dendritic cells derived exosomes promotes immune tolerance by regulating T cell differentiation in renal transplantation. Aging (Albany NY). (2019) 11:8911–24. doi: 10.18632/aging.102346
46. Hwang I. Cell-cell communication via extracellular membrane vesicles and its role in the immune response. Mol Cells. (2013) 36:105–11. doi: 10.1007/s10059-013-0154-2
47. Luo X, Du G, Long Y, Zheng M, Chen B, Li W, et al. Programmed death ligand-1-overexpressing donor exosomes mediate donor-specific immunosuppression by delivering co-inhibitory signals to donor-specific T cells. Adv Healthc Mater. (2023) 12:e2300670. doi: 10.1002/adhm.202300670
48. Kim GB, Shon OJ, Seo MS, Choi Y, Park WT, and Lee GW. Mesenchymal stem cell-derived exosomes and their therapeutic potential for osteoarthritis. Biol (Basel). (2021) 10:285. doi: 10.3390/biology10040285
49. Bizzell E, Sia JK, Quezada M, Enriquez A, Georgieva M, and Rengarajan J. Deletion of BCG Hip1 protease enhances dendritic cell and CD4 T cell responses. J Leukoc Biol. (2018) 103:739–48. doi: 10.1002/JLB.4A0917-363RR
50. Qian Y, Chu G, Zhang L, Wu Z, Wang Q, Guo JJ, et al. M2 macrophage-derived exosomal miR-26b-5p regulates macrophage polarization and chondrocyte hypertrophy by targeting TLR3 and COL10A1 to alleviate osteoarthritis. J Nanobiotechnol. (2024) 22:72. doi: 10.1186/s12951-024-02336-4
51. Domenis R, Zanutel R, Caponnetto F, Toffoletto B, Cifù A, Pistis C, et al. Characterization of the proinflammatory profile of synovial fluid-derived exosomes of patients with osteoarthritis. Mediators Inflammation. (2017) 2017:4814987. doi: 10.1155/2017/4814987
52. Chen D, Shen J, and Hui T. Epigenetic and microRNA regulation during osteoarthritis development. F1000Res. (2015) 4:1092. doi: 10.12688/f1000research
53. Gu J, Rao W, Huo S, Fan T, Qiu M, Zhu H, et al. MicroRNAs and long non-coding RNAs in cartilage homeostasis and osteoarthritis. Front Cell Dev Biol. (2022) 10:1092776. doi: 10.3389/fcell.2022.1092776
54. Luobu Z, Wang L, Jiang D, Liao T, Luobu C, and Qunpei L. CircSCAPER contributes to IL-1β-induced osteoarthritis in vitro via miR-140-3p/EZH2 axis. Bone Joint Res. (2022) 11:61–72. doi: 10.1302/2046-3758.112.BJR-2020-0482.R2
55. Zhang L, Qiu J, Shi J, Liu S, and Zou H. MicroRNA-140-5p represses chondrocyte pyroptosis and relieves cartilage injury in osteoarthritis by inhibiting cathepsin B/Nod-like receptor protein 3. Bioengineered. (2021) 12:9949–64. doi: 10.1080/21655979.2021.1985342
56. Duan L, Liang Y, Xu X, Xiao Y, and Wang D. Recent progress on the role of miR-140 in cartilage matrix remodelling and its implications for osteoarthritis treatment. Arthritis Res Ther. (2020) 22:194. doi: 10.1186/s13075-020-02290-0
57. Si HB, Yang TM, Li L, Tian M, Zhou L, Li DP, et al. miR-140 attenuates the progression of early-stage osteoarthritis by retarding chondrocyte senescence. Mol Ther Nucleic Acids. (2020) 19:15–30. doi: 10.1016/j.omtn.2019.10.032
58. Jiang L, Lin J, Zhao S, Wu J, Jin Y, Yu L, et al. ADAMTS5 in osteoarthritis: biological functions, regulatory network, and potential targeting therapies. Front Mol Biosci. (2021) 8:703110. doi: 10.3389/fmolb.2021.703110
59. Tang S, Zhang C, Oo WM, Fu K, Risberg MA, Bierma-Zeinstra SM, et al. Osteoarthritis. Nat Rev Dis Primers. (2025) 11:10. doi: 10.1038/s41572-025-00594-6
60. He K, Huang X, Shan R, Yang X, Song R, Xie F, et al. Intra-articular injection of lornoxicam and microRNA-140 co-loaded cationic liposomes enhanced the therapeutic treatment of experimental osteoarthritis. AAPS Pharm Sci Tech. (2021) 23:9. doi: 10.1208/s12249-021-02149-w
61. Kamar SS, ShamsEldeen AM, Hosny SA, El-Shafei AA, Rashid LA, Hassanein RT, et al. Comparing effectiveness of hyaluronic acid-chitosan nanoparticles encapsulation versus hyaluronic acid monotherapy in osteoarthritis rat model: microarray screening for miR-140. Microsc Microanal. (2023) 29:686–97. doi: 10.1093/micmic/ozac048
62. Cheleschi S, Tenti S, Bedogni G, and Fioravanti A. Circulating Mir-140 and leptin improve the accuracy of the differential diagnosis between psoriatic arthritis and rheumatoid arthritis: a case-control study. Transl Res. (2022) 239:18–34. doi: 10.1016/j.trsl.2021.08.001
63. Hu W, Zhang W, Li F, Guo F, and Chen A. miR-139 is up-regulated in osteoarthritis and inhibits chondrocyte proliferation and migration possibly via suppressing EIF4G2 and IGF1R. Biochem Biophys Res Commun. (2016) 474:296–302. doi: 10.1016/j.bbrc.2016.03.164
64. Chen M, Liu Y, Cao Y, Zhao C, Liu Q, Li N, et al. Remodeling the proinflammatory microenvironment in osteoarthritis through interleukin-1 beta tailored exosome cargo for inflammatory regulation and cartilage regeneration. ACS Nano. (2025) 19:4924–41. doi: 10.1021/acsnano.4c16785
65. Asghar S, Litherland GJ, Cole JJ, McInnes IB, Meek RMD, Lockhart JC, et al. Small extracellular vesicles derived from synovial fibroblasts contain distinct miRNA profiles and contribute to chondrocyte damage in osteoarthritis. Arthritis Res Ther. (2024) 26:167. doi: 10.1186/s13075-024-03398-3
66. Rogers EL, Reynard LN, and Loughlin J. The role of inflammation-related genes in osteoarthritis. Osteoarthritis Cartilage. (2015) 23:1933–8. doi: 10.1016/j.joca.2015.01.003
67. Tang Y, Sun Y, Zeng J, Yuan B, Zhao Y, Geng X, et al. Exosomal miR-140-5p inhibits osteogenesis by targeting IGF1R and regulating the mTOR pathway in ossification of the posterior longitudinal ligament. J Nanobiotechnol. (2022) 20:452. doi: 10.1186/s12951-022-01655-8
68. Fan WJ, Liu D, Pan LY, Wang WY, Ding YL, Zhang YY, et al. Exosomes in osteoarthritis: Updated insights on pathogenesis, diagnosis, and treatment. Front Cell Dev Biol. (2022) 10:949690. doi: 10.3389/fcell.2022.949690
69. Liu Y, Zeng Y, Si HB, Tang L, Xie HQ, and Shen B. Exosomes derived from human urine-derived stem cells overexpressing miR-140-5p alleviate knee osteoarthritis through downregulation of VEGFA in a rat model. Am J Sports Med. (2022) 50:1088–105. doi: 10.1177/03635465221073991
70. Yerneni SS, Adamik J, Weiss LE, and Campbell PG. Cell trafficking and regulation of osteoblastogenesis by extracellular vesicle associated bone morphogenetic protein 2. J Extracell Vesicles. (2021) 10:e12155. doi: 10.1002/jev2.12155
71. Zhang M, Wu J, Cai K, Liu Y, Lu B, Zhang J, et al. From dysfunction to healing: advances in mitochondrial therapy for Osteoarthritis. J Transl Med. (2024) 22:1013. doi: 10.1186/s12967-024-05799-z
72. Zhang L, Chen X, Cai P, Sun H, Shen S, Guo B, et al. Reprogramming mitochondrial metabolism in synovial macrophages of early osteoarthritis by a camouflaged meta-defensome. Adv Mater. (2022) 34:e2202715. doi: 10.1002/adma.202202715
73. Sanchez-Lopez E, Coras R, Torres A, Lane NE, and Guma M. Synovial inflammation in osteoarthritis progression. Nat Rev Rheumatol. (2022) 18:258–75. doi: 10.1038/s41584-022-00749-9
74. José Alcaraz M. Control of articular degeneration by extracellular vesicles from stem/stromal cells as a potential strategy for the treatment of osteoarthritis. Biochem Pharmacol. (2024) 228:116226. doi: 10.1016/j.bcp.2024.116226
75. Harrell CR, Jovicic N, Djonov V, Arsenijevic N, and Volarevic V. Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells. (2019) 8:1605. doi: 10.3390/cells8121605
76. Zhang J, Rong Y, Luo C, and Cui W. Bone marrow mesenchymal stem cell-derived exosomes prevent osteoarthritis by regulating synovial macrophage polarization. Aging (Albany NY). (2020) 12:25138–52. doi: 10.18632/aging.104110
77. Li P, Lv S, Jiang W, Si L, Liao B, Zhao G, et al. Exosomes derived from umbilical cord mesenchymal stem cells protect cartilage and regulate the polarization of macrophages in osteoarthritis. Ann Transl Med. (2022) 10:976. doi: 10.21037/atm-22-3912
78. Yao Z, Li Y, Mai H, Wang Z, Zhang H, Cai D, et al. Comprehensive multiomics analysis identifies PYCARD as a key pyroptosis-related gene in osteoarthritis synovial macrophages. Front Immunol. (2025) 16:1558139. doi: 10.3389/fimmu.2025.1558139
79. Thomson A and Hilkens CMU. Synovial macrophages in osteoarthritis: the key to understanding pathogenesis? Front Immunol. (2021) 12:678757. doi: 10.3389/fimmu.2021.678757
80. Wang R and Xu B. TGF-β1-modified MSC-derived exosomal miR-135b attenuates cartilage injury via promoting M2 synovial macrophage polarization by targeting MAPK6. Cell Tissue Res. (2021) 384:113–27. doi: 10.1007/s00441-020-03319-1
81. Wu Y, Li J, Zeng Y, Pu W, Mu X, Sun K, et al. Exosomes rewire the cartilage microenvironment in osteoarthritis: from intercellular communication to therapeutic strategies. Int J Oral Sci. (2022) 14:40. doi: 10.1038/s41368-022-00187-z
82. Kang W, Xu Q, Dong H, Wang W, Huang G, and Zhang J. Eriodictyol attenuates osteoarthritis progression through inhibiting inflammation via the PI3K/AKT/NF-κB signaling pathway. Sci Rep. (2024) 14:18853. doi: 10.1038/s41598-024-69028-9
83. Miao C, Zhou W, Wang X, and Fang J. The Research Progress of Exosomes in Osteoarthritis, With Particular Emphasis on the Mediating Roles of miRNAs and lncRNAs. Front Pharmacol. (2021) 12:685623. doi: 10.3389/fphar.2021.685623
84. Kim M, Shin DI, Choi BH, and Min BH. Exosomes from IL-1β-primed mesenchymal stem cells inhibited IL-1β- and TNF-α-mediated inflammatory responses in osteoarthritic SW982 cells. Tissue Eng Regener Med. (2021) 18:525–36. doi: 10.1007/s13770-020-00324-x
85. Song J, Jin EH, Kim D, Kim KY, Chun CH, and Jin EJ. MicroRNA-222 regulates MMP-13 via targeting HDAC-4 during osteoarthritis pathogenesis. BBA Clin. (2015) 3:79–89. doi: 10.1016/j.bbacli.2014.11.009
86. Hu Q and Ecker M. Overview of MMP-13 as a promising target for the treatment of osteoarthritis. Int J Mol Sci. (2021) 22:1742. doi: 10.3390/ijms22041742
87. Li H, Guan SB, Lu Y, and Wang F. MiR-140-5p inhibits synovial fibroblasts proliferation and inflammatory cytokines secretion through targeting TLR4. BioMed Pharmacother. (2017) 96:208–14. doi: 10.1016/j.biopha.2017.09.079
88. Wu MH, Tsai CH, Huang YL, Fong YC, and Tang CH. Visfatin Promotes IL-6 and TNF-α Production in Human Synovial Fibroblasts by Repressing miR-199a-5p through ERK, p38 and JNK Signaling Pathways. Int J Mol Sci. (2018) 19:190. doi: 10.3390/ijms19010190
89. Jin Z, Ren J, and Qi S. Exosomal miR-9-5p secreted by bone marrow-derived mesenchymal stem cells alleviates osteoarthritis by inhibiting syndecan-1. Cell Tissue Res. (2020) 381:99–114. doi: 10.1007/s00441-020-03193-x
90. Huang Y, Hou X, Wang Y, Cao Y, Zhou Y, Chen Y, et al. MicroRNA-9-5p alleviates oxidative stress, inflammation, and apoptosis in cerebral ischemia-reperfusion injury by targeting NOX4 in vitro. Curr Mol Med. (2025). doi: 10.2174/0115665240337045241210064142
91. Li L, Zhang J, Li H, Qin L, Wu H, Li Z, et al. Targeted inhibition of JMJD2C/MALAT1 axis compensates for the deficiency of metformin in reversing ovarian cancer platinum resistance. Life Sci. (2025) 373:123663. doi: 10.1016/j.lfs.2025.123663
92. Li X, Liu L, Yang J, Yu Y, Chai J, Wang L, et al. Exosome derived from human umbilical cord mesenchymal stem cell mediates miR-181c attenuating burn-induced excessive inflammation. EBioMedicine. (2016) 8:72–82. doi: 10.1016/j.ebiom.2016.04.030
93. Morente-López M, Mato-Basalo R, Lucio-Gallego S, Gil C, Carrera M, Fafián-Labora JA, et al. Effect of miR-21 in mesenchymal stem cells-derived extracellular vesicles behavior. Stem Cell Res Ther. (2023) 14:383. doi: 10.1186/s13287-023-03613-z
94. Zhu T, Wan L, Li R, Zhang M, Li X, Liu Y, et al. Janus structure hydrogels: recent advances in synthetic strategies, biomedical microstructure and (bio)applications. Biomater Sci. (2024) 12:3003–26. doi: 10.1039/D3BM02051G
95. Wang Z, Li X, Jiang Y, Wu T, Guo S, and Li T. Preparation of hydrogel microsphere and its application in articular cartilage injury. Mater Today Bio. (2025) 31:101641. doi: 10.1016/j.mtbio.2025.101641
96. Chen M, Lu Y, Liu Y, Liu Q, Deng S, Liu Y, et al. Injectable microgels with hybrid exosomes of chondrocyte-targeted FGF18 gene-editing and self-renewable lubrication for osteoarthritis therapy. Adv Mater. (2024) 36:e2312559. doi: 10.1002/adma.202312559
97. Lou C, Jiang H, Lin Z, Xia T, Wang W, Lin C, et al. MiR-146b-5p enriched bioinspired exosomes derived from fucoidan-directed induction mesenchymal stem cells protect chondrocytes in osteoarthritis by targeting TRAF6. J Nanobiotechnol. (2023) 21:486. doi: 10.1186/s12951-023-02264-9
Keywords: osteoarthritis, immune regulation, inflammation repair, synovial inflammation, exosomes, mesenchymal stem cells
Citation: Lan S and Zhang C (2025) Roles of exosomes in immune regulation of osteoarthritis and their applications in inflammation repair. Front. Immunol. 16:1611718. doi: 10.3389/fimmu.2025.1611718
Received: 14 April 2025; Accepted: 18 August 2025;
Published: 04 September 2025.
Edited by:
Yongqiang Chen, University of Manitoba, CanadaReviewed by:
Sara Shamdani, Hôpital Paul Brousse, FranceCopyright © 2025 Lan and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Zhang, MjY0NTQ5MTc4MUBxcS5jb20=
 Shuquan Lan
Shuquan Lan Chao Zhang
Chao Zhang