- 1University of California Davis Health, Division of Surgical Oncology, Department of Surgery, Sacramento, CA, United States
- 2Comprehensive Cancer Center, University of California, Davis, Sacramento, CA, United States
Novel cellular therapies have shown practice changing results in a range of hematologic malignancies, though success against solid tumors has been limited. Key factors limiting success of these therapies against solid tumors are homing to the site(s) of disease, engraftment, maintenance of function, and persistence. The inhospitable tumor microenvironment appears to provide barriers at every step of this process. The liver, a unique organ with diverse immunoregulatory functions, is a common site for metastatic disease from solid cancers of the gastrointestinal (GI) tract. Although the complex interplay between hepatocytes, circulatory and tissue resident immune cells, and the enterohepatic circulation has been investigated for some time, many unanswered questions about the immunobiology of the liver remain. More so, novel imaging techniques provide unparalleled insight into these interactions and shed light on these complex processes that can lead to an improved understanding of the tumor microenvironment in the liver and opportunities for improving homing of cellular therapy against liver tumors. In this review, we will provide a focused assessment of this burgeoning field and focus on the emerging tools for studying homing of these therapies and how they may be enhanced to better treat liver metastases.
1 Introduction
The use of autologous chimeric antigen receptor (CAR) T cells has revolutionized the treatment of select hematologic malignancies. The success of CAR T therapy in these B-cell malignancies has sparked investigation into their use in solid tumors. Among gastrointestinal tumors, CAR T cells targeting claudin 18.2 has arisen as one of the most promising therapies against advanced or metastatic disease. Recent long-term results from a Phase I/II clinical trial investigating CAR T cells against claudin 18.2 for advanced GI tumors showed a 39% overall response rate among the 98 patients treated, though there were different cohorts undergoing different treatment sequencing and immunotherapy adjuncts. However, it is notable that the investigators detected significantly worse outcomes in patients with liver metastases (median PFS 3.9 months versus 7.1 months) (1). This observation may highlight unique features of the liver and show how CAR cell therapy may function differently within this organ. Several clinical trials are underway (Table 1) aiming to treat advanced GI malignancies with cellular therapy and with different augmentation strategies.
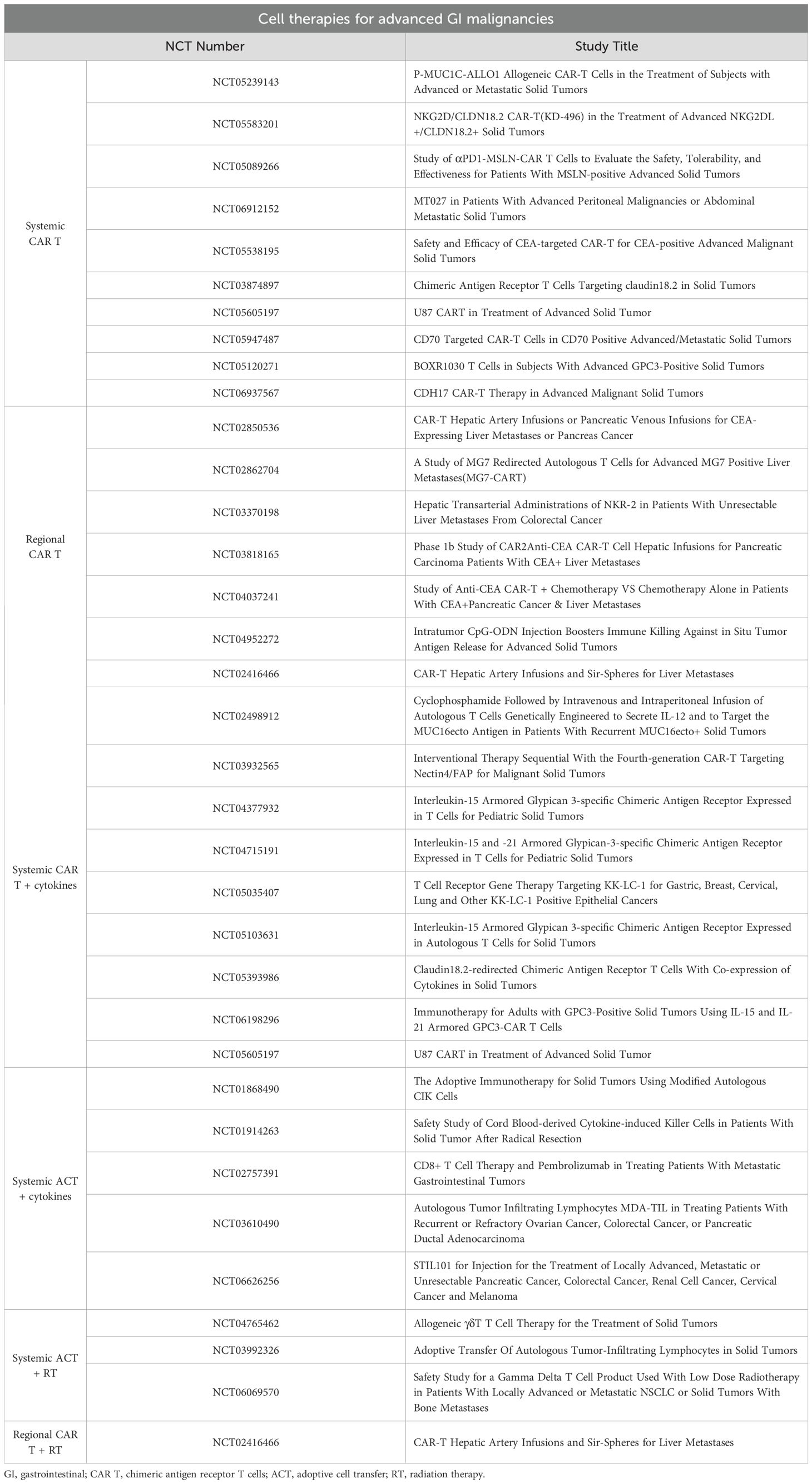
Table 1. Ongoing clinical trials investigating cellular therapies for advanced gastrointestinal malignancies.
The liver contains a diverse repertoire of immune and non-immune cells including liver sinusoid endothelial cells (LSEC), hepatic stellate cells, hepatocytes, Kupffer cells (KCs), monocyte-derived macrophages, myeloid-derived suppressor cells (MDSCs), natural killer (NK) cells, neutrophils, and T lymphocytes. These cell populations have broad and often opposing roles within the context of liver metastases. For example, Kupffer cells and monocyte-derived macrophages can demonstrate anti-tumor effects through direct phagocytosis of cancer cells, the production and pro-inflammatory cytokines such as TNF-a and the recruitment of cytotoxic T lymphocytes and NK cells (2). However, these same cells also demonstrate pro-tumorigenic actions. KCs have been shown to participate in the pre-metastatic niche formation (3), production of various cytokines and tumorigenic growth factors including IL-6, hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), and matrix metalloproteinases (MMP) (2, 4), while monocyte-derived macrophages can be polarized to M2 where they also secrete growth factors such as VEGF (5), and induce regulatory T cells which can inhibit cytotoxic T cells via IL-10 and TGF-b (6). This diverse and highly complex environment is further complicated in the context of cellular therapies such as CAR T, where the interplay between these therapeutic cells and the liver milieu are less understood. Given the limited ability of CAR T cell therapy to successfully treat solid tumors metastatic to the liver, further insights into the unique nature of liver metastases and how CAR cells may, or may not, be homing and functioning within the tumor-bearing liver are needed.
2 Liver metastases from gastrointestinal cancers
Malignancies of the GI tract represent some of the most common cancers in the United States and globally (7, 8). These include malignancies of the colon, esophagus, pancreas, rectum, and stomach with the most common histology being adenocarcinoma. Similar across these disease sites is the prevalent rate of liver metastases from the primary tumor (9). Observational data from the SEER database indicate that about 5% of patients present with synchronous liver metastases at the time of their diagnosis. Malignancies with the highest rate of synchronous liver metastases are cancers of the pancreas (36%), colon and rectum (27%), small bowel (15%), stomach (14%), and esophagus (14%) (10). Among all age groups, the highest incidence of liver metastases at diagnosis was among patients with cancers of the pancreas, colon and rectum. Better treatment of liver metastases is therefore a critical and urgent unmet need.
The current approach to treating liver metastases from GI cancers is mainly based on the primary cancer site. Treatment may include systemic therapy, radiation, or resection, though this depends on the extent of disease. In colorectal cancer, management of isolated liver metastases focuses on resection or other regional therapies such as radiofrequency ablation, arterial directed therapies, and selective internal radiation. Results from a large, multi-centered randomized clinical trial showed no improvement in overall survival (OS) when adding systemic chemotherapy to resection compared to resection alone in the management of colorectal liver metastases (median OS 61.3 months vs. 54.3 months) (11). This is in contrast to pancreas cancer where systemic chemotherapy is standard for patients with metastatic disease, and small case series investigating the role of simultaneous pancreas and liver resection in well-selected patients with low volume liver metastases identified no difference in OS compared to patients receiving palliative bypass without curative surgery and adjuvant chemotherapy (12). Apart from systemic therapy, and unique to the management of liver metastases, is the role of liver-directed, regional therapies. This was recently highlighted in the management of pancreatic cancer with limited liver or lung metastases where patients were randomized to chemotherapy or chemotherapy with targeted radiation to the metastatic sites and investigators detected improved PFS in the group receiving metastasis-directed RT (13). More so, these investigators analyzed patient peripheral blood and identified changes in circulating immune cells and cytokines that were positively associated with better PFS, highlighting the role of the immune system as a putative mediator of the anti-tumor effects following regional RT.
3 Liver homing of systemic cellular therapies
In the initial report of the clinical efficacy of the autologous CD19 CAR T therapy, CTL019 (tisaganleucel), for CD19+ lymphoid malignancies (14), responders had a median peak expansion of the cell therapy product at 8 days and 14/16 responders had consistently detectable levels of CTL019 DNA in their peripheral blood between 6 and 24 months after infusion. While peripheral blood is frequently the source for measuring CAR cell persistence in hematologic malignancies, peripheral blood may not be the most representative source for CAR cell persistence and function in the setting of solid tumors. Detecting the presence of CAR cells within a tumor-bearing solid organ is a challenging clinical issue and is either completed using a biopsy or requires investigation at autopsy. An earlier Phase I safety trial evaluating a second-generation CD19 CAR T cell therapy against B-cell leukemias investigated CAR T cell presence in other tissue sites following patient death. In a patient with CLL, researchers evaluated tumor contained within the lymph nodes, liver and bone marrow and identified the presence of these modified cells 44 hours after CAR T infusion (15). These data suggest that CAR T cells can home to sites of disease when given systemically. However, although CAR T cells can traffic to solid organs and tissues, it is not clear what effect infiltration into solid organs and tumors has on CAR T cell function. While there is an association between persistence of the cell therapy product and improved long term clinical outcomes (16), this does not appear necessary to prevent disease relapse. Additionally, relapse can still occur in the presence of persisting CAR T cells. In a recent review investigating long-term outcomes after CAR T therapy for hematologic malignancies, researchers cite various examples where durable responses have occurred in patients with no long-term evidence of CAR T persistence, and the setting where disease recurs while CAR T cells remain detectable (17). Using techniques to maximize homing of the cell therapy to sites of disease may lead to increased function and persistence by placing the CAR in proximity to the intended antigen. While these data show that systemically administered CAR cells can home to the liver, the larger question is whether this represents passive circulation of the CAR T cells through a highly vascular organ, or rather an active process where the CAR T cells preferentially traffic to the liver based on antigen recognition, chemokine or cytokine gradients, or some other pro-infiltrative signal.
4 PET imaging for tracking cellular therapies
Positron emission tomography (PET) is a nuclear medicine imaging technique that utilizes radioactive tracers to track both typical and atypical metabolic activity. PET/CT is commonly used for staging, assessment of therapeutic responses, and surveillance for different cancer types, including GI malignancies (18). The most commonly used PET radiotracer is 2-deoxy-2-[18F] fluorodeoxyglucose ([18F]FDG) which identified tissues with elevated glycolysis by radiotracer uptake. PET imaging can also be used to track cellular therapies in vivo. Techniques for tracking CAR cell therapy have been driven in large part due to advances in novel PET imaging probes. These technologies have allowed researchers to evaluate the variables of time and dose as a function of CAR homing and persistence, however these studies can be limited by the short half-life of these tracers. In one such study, investigators used CD19-tPSMA CAR T cells tagged with 18-fluorinated-DCFPyL against an acute lymphoblastic leukemia (ALL) mouse model to characterize CAR T durability and persistence (19). While the CAR T cells homed to tumor and had a profound anti-tumor effect, CAR T infiltration into tumor did not correlate with the concentration of CAR T cells within the peripheral blood or bone marrow, suggesting that peripheral blood levels may not represent what’s occurring within the sites of disease. These results highlight the challenges in using peripheral blood as a surrogate in solid tumors.
Other PET-based imaging modalities are also being investigated to track cellular therapies in vivo. Immuno-positron emission tomography (ImmunoPET) is increasingly used in cellular therapies by combining the specificity of monoclonal antibodies with the high sensitivity and resolution of PET through utilizing radiolabeled antibodies and antibody fragments. These immunoPET probes such as the 89Zr-DFO anti-ICOS tracer have been used to track human CAR T cells in vivo (20). Other strategies include Immuno-PET/SPECT imaging which combines immunoPET with single-photon emission computed tomography (SPECT) to track CAR T cell therapies within solid tumors. This method has also been demonstrated to detect CAR T distribution, engraftment, and clearance within the setting of solid tumors (21). While these antibody-based imaging strategies have shown to be quite advantageous, the choice of antibody conjugation strategy and radionuclide half-life can determine the effectiveness of these methods and are important considerations when designing these experiments. The goal of these imaging techniques is to further enhance our understanding of cellular therapy in the setting of solid tumors and give insight into how to better improve these therapies. Various avenues have been investigated in order to improve these cellular therapies including route of administration, as well as enhancement with immunomodulatory agents such as radiation therapy and cytokines.
5 Regional administration to enhance liver homing
In clinical practice certain regional therapies have exploited the unique vasculature of the liver. This is most notable with the use of hepatic artery infusion therapy that utilizes high potency chemotherapeutics instilled directly into the hepatic artery to target liver metastases. Since the chemotherapy undergoes rapid first-pass metabolism in the liver, the agents can deliver targeted doses of chemotherapy to the liver before entering the systemic circulation via hepatic venous outflow as an inert chemotherapy byproduct. Several prior studies have clearly demonstrated that liver metastases are preferentially fed by the hepatic arterial system, as compared to the portal venous system. More so, pre-clinical studies have shown that higher concentrations of active chemotherapy are detected within liver tumors when the chemotherapy is administered via the hepatic artery compared to the portal vein (22). While these observations make a clear case for utilizing the hepatic arterial system for infusion of high potency chemotherapy, portal venous infusion appears to be the preferred route of administration for islet cell transplantation for the treatment and prevention of diabetes (23). While the reason for this observation remains unknown, those prior investigators posited that it may be related to relative ischemia at the end arterioles limiting islet cell survival and persistence. Nonetheless, there appears to be relevant differences between these two vascular systems in how the inflow arrives and is metabolized within the liver parenchyma, and this may be especially true when dealing with a cellular therapy.
5.1 Hepatic artery and portal vein delivery
Hepatic artery infusion therapy has been used for decades with favorable results (24). While this platform is most frequently utilized to deliver high-dose chemotherapy directly to liver tumors, new treatment options have arisen with the advent of novel therapies, including immunotherapy and cellular therapy. More recently, CAR T cells targeting the CEA antigen have been administered via the hepatic artery without or with systemic interleukin (IL)-2 support to target GI cancers metastatic to the liver. In a phase I trial of 8 patients, investigators showed the safety and feasibility of CAR T infusion into the hepatic arterial system for unresectable liver metastases from diverse GI malignancies (25). Investigators also collected a targeted and non-targeted liver biopsy at the time of the third CAR T infusion and evaluated the persistence of the product. The authors noted that nearly 1% of the normal liver mononuclear cells were CAR+ and 6.6% of the intratumoral mononuclear cells were CAR+, suggesting at least some preferential homing to the site of disease. Additionally, in four of the patients CAR T cells were not detectable in peripheral blood, suggesting that either the cell therapy product does not consistently enter systemic circulation, or that there is poor persistence of the cells within the periphery.
While most current CAR T or CAR NK studies are not utilizing regional therapy, prior investigators examined the effect of infusing lymphokine activated killer (LAK) cells into the liver for treating metastases from various primary sources. Using peripheral blood mononuclear cells exposed to IL-2 in vitro, LAK cells were generated and administered. Investigators delivered LAK cells into either the portal vein or hepatic arterial system in patients with liver metastases from melanoma (26). In a subset of patients, these LAK cells were radio-labelled and followed in vivo with imaging. In vivo distribution was monitored 24 and 120 hours after infusion and showed that over 80% of the radioactivity remained in the liver while the remainder of the radioactivity was in the spleen. This observation was noted to be similar between portal venous and hepatic arterial administration at both timepoints. The authors did not report a difference in anti-tumor effect between these two routes. While these results do not show a difference in homing between the route of regional delivery, it does support regional delivery as a technique to maximize cellular engraftment into the liver when targeting liver metastases.
5.2 Technique and safety of regional delivery
The dual blood supply to the liver allows administration of regional therapies via either the hepatic arterial or portal venous systems. For both routes, direct and indirect access are possible. Access to the portal vein is commonly performed via either image-guided percutaneous venous access (often trans-hepatic) or direct needle cannulation at the time of surgery. Access to the hepatic arterial system is commonly performed via selective cannulation following peripheral arterial access, image-guided percutaneous arterial access, or through a hepatic arterial infusion device placed during a surgical procedure. For percutaneous portal vein access, most experience is from trans-jugular intrahepatic portosystemic shunt (TIPS) and islet cell transplantation. In a large series from Japan investigating complications after transhepatic portal vein access, the overall complication rate was 16.5% (bleeding, pleural effusion, bile leak, liver dysfunction), highlighting that bleeding is the most common complication (27). In a more contemporary series of surgical portal vein access for islet cell transplantation following total pancreatectomy, investigators measured a post-operative portal vein thrombosis rate of 6.6% (12/183), with most resolving after anti-coagulation therapy (28). This low thrombosis rate may be related to the large size and high flow volumes of the portal vein and support the safety of direct access during surgery.
Hepatic arterial access is most often completed using selective cannulation or through placement of a hepatic arterial infusion (HAI) device. Selective cannulation is a common technique and is most often utilized for trans-arterial embolization (TAE) for primary liver tumors. In one large study of nearly 5000 hepatic artery catheterizations for TAE, the incidence of arterial dissection of the celiac or its major branches was only 1.3% (61/4791) (29). This approach appears safe, but typically only allows for single treatment sessions. HAI therapy, where a permanent catheter is placed into a major hepatic arterial branch, allowing for continuous infusions and has been used for decades. In a large single-center series evaluating complications in HAI pump placement, the overall complication rate was 22% (120/544) with hepatic arterial thrombosis having occurred in 33 patients (6% total rate) (30), Notably, this thrombosis rate is similar to the portal venous thrombosis rate in the prior study. These results highlight the overall safety of these interventions however, it is important to note that these low complication rates were reported in high-volume centers with significant institutional experience in these procedures.
6 Radiation and cytokines to enhance liver homing
Radiation therapy (RT) has well established benefits in the multimodality management of solid tumors. RT has also been shown to demonstrate a variety of immunomodulatory effects on tumors and is now being investigated for its ability to enhance the engraftment and function of CAR therapies (31, 32). While there are currently few clinical studies investigating the combination of RT with CAR therapies, there is good evidence demonstrating the synergistic effects and enhanced antitumor efficacy with other immunotherapies such as PD-1/PD-L1 blockade (33). RT represents an additional locoregional strategy to improve cellular therapy against solid tumors, specifically cancers of the GI tract.
It has recently been shown that RT has a variety of immunomodulatory effects including direct effects on the tumor microenvironment (34, 35). It has also been shown to improve immune cell homing into the tumor via modulation of endothelial adhesion molecules intracellular adhesion molecule 1 (ICAM-1) and vascular-cell adhesion molecule 1 (VCAM-1), facilitating increased adherence and extravasation of lymphocytes out of circulation and into the tumor (36, 37). RT has also been shown to mediate increased T cell infiltration by inducing T cell attracting chemokines such as CXCL9, CXCL11, CCL5, and CCL8 in tumor cells (38, 39). Furthermore, the combination of RT with CAR T therapy has demonstrated increased CAR T infiltration into tumors with increased efficacy in a number of preclinical studies of GI malignancies. Amit and colleagues demonstrated that proton radiation boosts efficacy of CAR T therapy against pancreatic cancer. Using an orthotopic pancreatic cancer model, investigators treated mice with RT and subsequently injected mesothelin targeted CAR T cells (40). Not only did they observe an increase of CAR T infiltration into tumors but also saw an increase in tumor mesothelin expression following RT, further augmenting CAR-mediated antitumor responses. In a similar pancreatic cancer model, investigators showed that RT in combination with CAR T therapy increased CAR T efficacy and increased antigen-negative tumor susceptibility to CAR therapy (41). Jin and colleagues exploited radiation-induced IL-8 expression from tumors by utilizing CAR T cells expressing IL-8 receptors (CXCR1 or CXCR2). This resulted in enhanced migration and persistence of the CAR T cells in the tumor and was accompanied by tumor regression in pre-clinical models of pancreatic cancer (42). These pre-clinical studies demonstrate that combining RT with CAR T could enhance CAR T trafficking and have synergistic antitumor effects. In the context of GI liver metastases, RT can be directed specifically towards the liver and represents a promising modality to enhance the efficacy of cellular therapy towards solid tumors.
Cytokines represent an additional strategy by which to enhance the homing, engraftment, function, and persistence of CAR T therapy within solid GI tumors. While cytokine administration, namely IL-2, has historically been shown to have anti-tumor activity on their own (43), they are becoming increasingly used within the context of cellular immunotherapy, either via systemic administration or encoded within the cellular product to enhance therapeutic efficacy. In the context of CAR T therapy, 4th generation CARs or TRUCKs (T cell Redirected Universal Cytokine-mediated Killing) are being developed that secrete cytokines to signal in an autocrine fashion for increased efficacy and anti-tumor activity (44). Examples of cytokines currently under investigation include IL-2, IL-7, IL-12, IL-15, IL-18, IL-21, among others (45–50). There are also a number of clinical trials that are investigating cytokine-armored CAR T therapies for solid GI malignancies including IL-12, IL-15/IL-21, IL-7, CCL19 (NCT02498912, NCT06198296, NCT05035407). Allen and colleagues engineered CAR T cells with a synthetic Notch receptor which secrets IL-2 upon tumor recognition. In orthotopic PDAC models using immunocompetent mice, these authors demonstrated that the addition of the IL-2 circuit to the CAR T not only enhanced anti-tumor efficacy but specifically increased the infiltration of CAR T cells into the tumors, demonstrating the importance of cytokines in CAR T infiltration into solid tumors (46). Chemokine gradients have also been investigated to enhance the trafficking of CAR T therapies to solid tumors. Wang and colleagues developed an anti-mesothelin CAR T that co-expresses the chemokine receptor CCR2b for treatment of preclinical models of non-small-cell lung carcinoma which demonstrated superior tumor infiltration compared to the Msln-CAR alone (51). This demonstrates translational potential to liver tumors as CCR2 has been shown to be involved in multiple stages of liver pathology including tumor progression (52). Thus, cytokines represent a promising strategy for enhancing the trafficking and function of CAR T therapy to solid tumors, specifically GI malignancies. While combination of CAR T therapy with strategies such as RT and cytokines do represent great promise for enhancing the efficacy of CAR T therapy against solid tumors, it is important to acknowledge the limitations of these strategies, primarily the safety of these methods. CAR T therapy alone has well documented risks of cytokine release syndrome and neurotoxicity and when combined with RT and/or cytokines which also have individual risks of toxicities, it’s important to be cognizant of these risks and develop strategies that aim to mitigate these toxicities.
7 Conclusion
Chimeric antigen receptor therapies have revolutionized the treatment of hematological malignancies, but there is still much work to be done in optimizing these therapies for solid tumors. Several techniques are being investigated to optimize cell therapy function (Figure 1). Fundamental barriers to their success in solid tumors include homing, engraftment and persistence within the organ of disease. The liver is a common site of metastasis of GI malignancies and provides a unique opportunity to investigate the complex interactions between the host immune system, tumor microenvironment and cellular therapies. Novel liver-directed therapies are needed to improve cellular therapies in the context of GI liver metastases. Locoregional delivery of cellular therapies via hepatic arterial infusion or portal venous infusion represent two strategies for enhancing the homing, engraftment, and persistence of cellular therapy in the liver. While these strategies have long been tested in the application of chemotherapy, they represent a promising avenue for use in the setting of cellular therapy, specifically CAR T therapy for liver-directed treatments. Radiation therapy represents a second promising approach to enhancing the engraftment of CAR T therapy into solid tumors, specifically the liver. Studies investigating the combination of RT with CAR T therapy continue to emerge and have demonstrated promising success in the preclinical setting as a method of enhancing CAR T engraftment into solid GI malignancies. Utilizing cytokines in combination with CAR T therapy represents an additional strategy for enhancing homing and function of CAR T therapy into solid tumors. Lastly, tracking cellular therapy in real time is crucial in furthering our understanding of cellular therapy in the setting of solid tumors so that we may improve our approaches. Positron emission tomography, specifically immunoPET, has emerged as an innovative method to track CAR T therapy and has potential to provide vital information regarding the successes and failures of CAR T therapy. These tools can be utilized together to shed light on the complex interactions between host immunity, cellular therapy and the tumor microenvironment thus leading to improved cellular therapy against solid tumors.
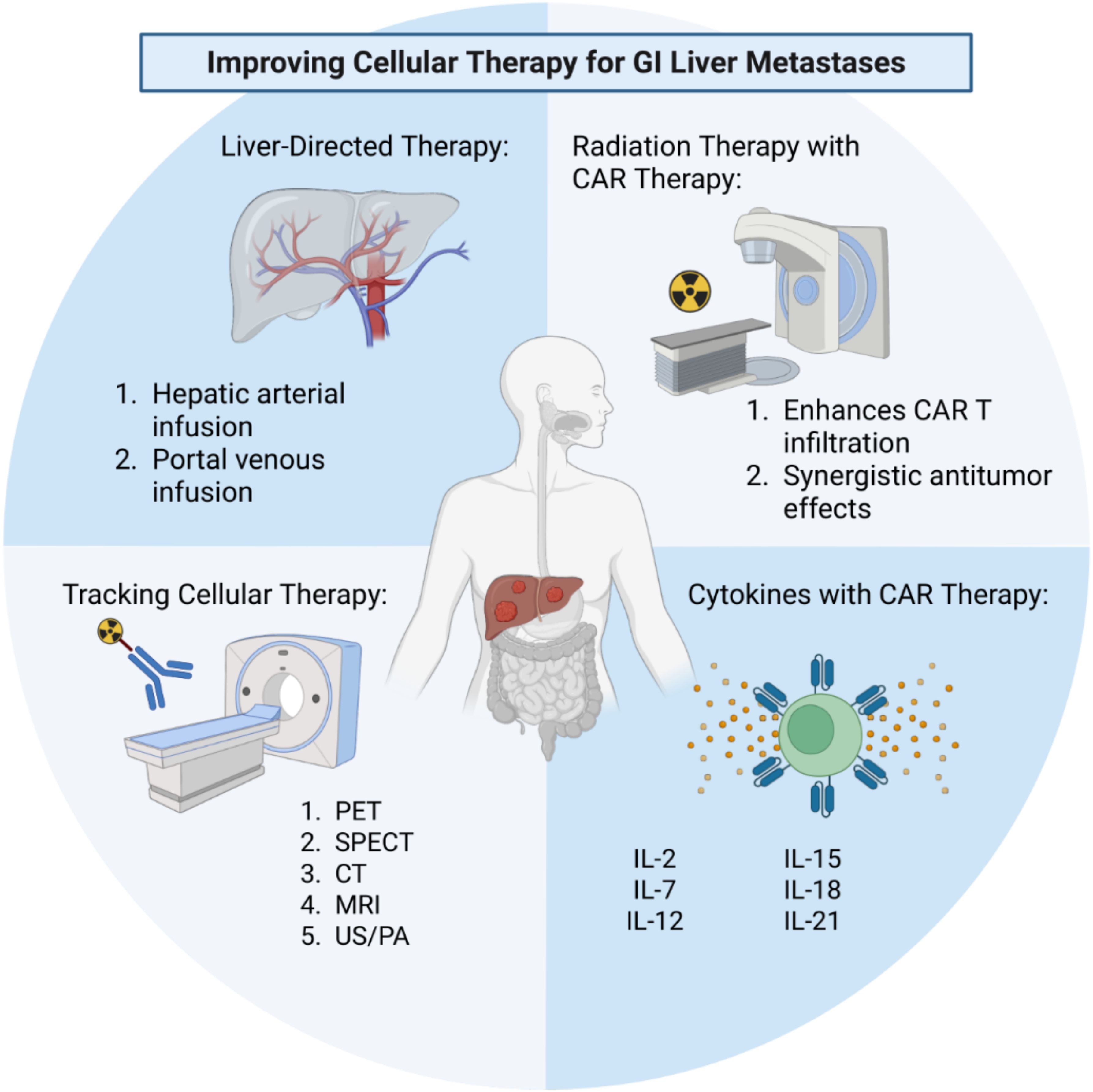
Figure 1. Strategies and techniques to improve cellular therapy for liver metastases of gastrointestinal (GI) origin. These include liver-directed regional therapies, combinations with external beam radiation therapy, augmenting CAR therapy with cytokines, and tracking CAR therapy using modern imaging modalities and novel probes. CAR, chimeric antigen receptor; PET, positron emission tomography; SPECT, single-photon emission computed tomography; CT, computed tomography; MRI, magnetic resonance imaging; US/PA, ultrasound/photoacoustic imaging. Created in BioRender. Purl, M. (2025) https://BioRender.com/4givi1o.
Author contributions
MP: Conceptualization, Data curation, Visualization, Writing – original draft, Writing – review & editing. AS: Writing – original draft, Writing – review & editing. RC: Conceptualization, Writing – original draft, Writing – review & editing. SJ: Conceptualization, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the V Foundation and the following National Institutes of Health/National Cancer Institute Grants: R03CA270854, R03CA252793, and NIH Training Grant 1T32CA251007. SJJ is supported in part by the UC Davis Paul Calabresi Career Development Award for Clinical Oncology as funded by the National Cancer Institute/National Institutes of Health through grant #5K12-CA138464.
Acknowledgments
The authors would like to thank all members of the lab for their thoughtful feedback on this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Qi C, Liu C, Gong J, Liu D, Wang X, Zhang P, et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial final results. Nat Med. (2024) 30:2224–34. doi: 10.1038/s41591-024-03037-z
2. Brodt P. Role of the microenvironment in liver metastasis: from pre- to prometastatic niches. Clin Cancer Res. (2016) 22:5971–82. doi: 10.1158/1078-0432.CCR-16-0460
3. Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. (2015) 17:816–26. doi: 10.1038/ncb3169
4. Dey A, Allen J, and Hankey-Giblin. PA. Ontogeny and polarization of macrophages in inflammation: blood monocytes versus tissue macrophages. Front Immunol. (2014) 5:683. doi: 10.3389/fimmu.2014.00683
5. Mills CD. Anatomy of a discovery: M1 and M2 macrophages. Front Immunol. (2015) 6:212. doi: 10.3389/fimmu.2015.00212
6. Mills CD, Lenz LL, and Harris. RA. A breakthrough: macrophage-directed cancer immunotherapy. Cancer Res. (2016) 76:513–165. doi: 10.1158/0008-5472.CAN-15-1737
7. Siegel RL, Miller KD, Wagle NS, and Jemal. A. Cancer statistics 2023. CA: A Cancer J Clin. (2023) 73:17–485. doi: 10.3322/caac.21763
8. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. (2021) 71:209–495. doi: 10.3322/caac.21660
9. Tsilimigras DI, Brodt P, Clavien P-A, Muschel RJ, D’Angelica MI, Endo I, et al. Liver metastases. Nat Rev Dis Primers. (2021) 7:275. doi: 10.1038/s41572-021-00261-6
10. Horn SR, Stoltzfus KC, Lehrer EJ, Dawson LA, Tchelebi L, Gusani NJ, et al. Epidemiology of liver metastases. Cancer Epidemiol. (2020) 67:101760. doi: 10.1016/j.canep.2020.101760
11. Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. (2013) 14:1208–15. doi: 10.1016/S1470-2045(13)70447-9
12. Gleisner AL, Assumpcao L, Cameron JL, Wolfgang CL, Choti MA, Herman JM, et al. Is resection of periampullary or pancreatic adenocarcinoma with synchronous hepatic metastasis justified? Cancer. (2007) 110:2484–925. doi: 10.1002/cncr.23074
13. Ludmir EB, Sherry AD, Fellman BM, Liu S, Bathala T, Haymaker C, et al. Addition of metastasis-directed therapy to systemic therapy for oligometastatic pancreatic ductal adenocarcinoma (EXTEND): A multicenter, randomized phase II trial. J Clin Oncol. (2024) 42:32. doi: 10.1200/JCO.24.00081
14. Schuster Stephen J, Jakub S, Chong Elise A, Nasta Sunita D, Mato Anthony R, Özlem A, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. (2017) 377:2545–54. doi: 10.1056/NEJMoa1708566
15. Brentjens RJ, Rivière I, Park JH, Davila ML, Wang X, Stefanski J, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. (2011) 118:4817–28. doi: 10.1182/blood-2011-04-348540
16. Melenhorst JJ, Chen GM, Wang M, Porter DL, Chen C, Collins MA, et al. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature. (2022) 602:503–9. doi: 10.1038/s41586-021-04390-6
17. Cappell KM and Kochenderfer. JN. Long-term outcomes following CAR T cell therapy: what we know so far. Nat Rev Clin Oncol. (2023) 20:359–715. doi: 10.1038/s41571-023-00754-1
18. Mulgaonkar A, Udayakumar D, Yang Y, Harris S, Öz OK, Geethakumari PR, et al. Current and potential roles of immuno-PET/-SPECT in CAR T-cell therapy. Front Med. (2023) 10:1199146. doi: 10.3389/fmed.2023.1199146
19. Minn Il, Huss DJ, Ahn H-H, Chinn TM, Park A, Jones J, et al. Imaging CAR T cell therapy with PSMA-targeted positron emission tomography. Sci Adv. (2019) 5:eaaw5096. doi: 10.1126/sciadv.aaw5096
20. Simonetta F, Alam IS, Lohmeyer JK, Sahaf B, Good Z, Chen W, et al. Molecular imaging of chimeric antigen receptor T cells by ICOS-immunoPET. Clin Cancer Res. (2021) 27:1058–68. doi: 10.1158/1078-0432.CCR-20-2770
21. Hu Y and Huang J. The chimeric antigen receptor detection toolkit. Front Immunol. (2020) 11:1770. doi: 10.3389/fimmu.2020.01770
22. Archer SG and Gray. BN. Comparison of portal vein chemotherapy with hepatic artery chemotherapy in the treatment of liver micrometastases. Am J Surg. (1990) 159:325–295. doi: 10.1016/S0002-9610(05)81228-0
23. Hirshberg B, Montgomery S, Wysoki MG, Xu H, Tadaki D, Lee J, et al. Pancreatic islet transplantation using the nonhuman primate (Rhesus) model predicts that the portal vein is superior to the celiac artery as the islet infusion site. Diabetes. (2002) 51:2135–40. doi: 10.2337/diabetes.51.7.2135
24. Datta J, Narayan RR, Kemeny NE, and D’Angelica. MI. Role of hepatic artery infusion chemotherapy in treatment of initially unresectable colorectal liver metastases: A review. JAMA Surg. (2019) 154:768–5. doi: 10.1001/jamasurg.2019.1694
25. Katz SC, Burga RA, McCormack E, Wang LJ, Mooring W, Point GR, et al. Phase I hepatic immunotherapy for metastases study of intra-arterial chimeric antigen receptor–modified T-cell therapy for CEA+ Liver metastases. Clin Cancer Res. (2015) 21:3149–59. doi: 10.1158/1078-0432.CCR-14-1421
26. Keilholz U, Scheibenbogen C, Maclachlan D, Brado B, Hunstein W, Brado M, et al. Regional adoptive immunotherapy with interleukin-2 and lymphokine-activated killer (LAK) cells for liver metastases. Eur J Cancer. (1994) 30:103–5. doi: 10.1016/S0959-8049(05)80028-0
27. Ohta M, HashizumeME M, Kawanaka H, Akazawa K, Ueno K, Tomikawa M, et al. Complications of percutaneous transhepatic catheterization of the portal venous system in patients with portal hypertension. J Gastroenterol Hepatol. (1996) 11:630–345. doi: 10.1111/j.1440-1746.1996.tb00305.x
28. Robbins AJ, Skube ME, Bellin MD, Dunn TB, Chapman SA, Berry KL, et al. Portal vein thrombosis after total pancreatectomy and islet autotransplant: prophylaxis and graft impact. Pancreas. (2019) 48(10):1329–33. doi: 10.1097/MPA.0000000000001421
29. Yoon DY, Park JH, Chung JW, Han JK, and Han. MC. Iatrogenic dissection of the celiac artery and its branches during transcatheter arterial embolization for hepatocellular carcinoma: outcome in 40 patients. Cardiovasc Intervent Radiol. (1995) 18:16–195. doi: 10.1007/BF02807349
30. Allen PJ, Nissan A, Picon AI, Kemeny N, Dudrick P, Ben-Porat L, et al. Technical complications and durability of hepatic artery infusion pumps for unresectable colorectal liver metastases: an institutional experience of 544 consecutive cases. J Am Coll Surg. (2005) 201:57–65. doi: 10.1016/j.jamcollsurg.2005.03.019
31. Quach HT, Skovgard MS, Villena-Vargas J, Bellis RY, Chintala NK, Amador-Molina A, et al. Tumor-targeted nonablative radiation promotes solid tumor CAR T-cell therapy efficacy. Cancer Immunol Res. (2023) 11:1314–31. doi: 10.1158/2326-6066.CIR-22-0840
32. Zhong L, Li Y, Achu TM, and Wang. Y. “Combination of CAR−T cell therapy and radiotherapy: opportunities and challenges in solid tumors (Review). Oncol Lett. (2023) 26:2815. doi: 10.3892/ol.2023.13867
33. Gong J, Le TQ, Massarelli E, Hendifar AE, and Tuli. R. Radiation therapy and PD-1/PD-L1 blockade: the clinical development of an evolving anticancer combination. J ImmunoTher Cancer. (2018) 6:46. doi: 10.1186/s40425-018-0361-7
34. Hovhannisyan L, Riether C, Aebersold DM, Medová M, and Zimmer Y. CAR T cell-based immunotherapy and radiation therapy: potential, promises and risks. Mol Cancer. (2023) 22:825. doi: 10.1186/s12943-023-01775-1
35. Monjazeb AM, Schalper KA, Villarroel-Espindola F, Nguyen A, Shiao SL, and Young K. Effects of radiation on the tumor microenvironment. Semin Radiat Oncol. (2020) 30:145–575. doi: 10.1016/j.semradonc.2019.12.004
36. Hallahan D, Kuchibhotla J, and Wyble C. Cell adhesion molecules mediate radiation-induced leukocyte adhesion to the vascular endothelium. Cancer Res. (1996) 56:5150–55.
37. Krombach J, Hennel R, Brix N, Orth M, Schoetz U, Ernst A, et al. Priming anti-tumor immunity by radiotherapy: dying tumor cell-derived DAMPs trigger endothelial cell activation and recruitment of myeloid cells. Oncoimmunology. (2019) 8:e1523097. doi: 10.1080/2162402X.2018.1523097
38. Kohli K, Pillarisetty VG, and Kim. TS. Key chemokines direct migration of immune cells in solid tumors. Cancer Gene Ther. (2022) 29:10–215. doi: 10.1038/s41417-021-00303-x
39. Dangaj D, Bruand M, Grimm AJ, Ronet C, Barras D, Duttagupta PA, et al. Cooperation between constitutive and inducible chemokines enables T cell engraftment and immune attack in solid tumors. Cancer Cell. (2019) 35:885–900.e10. doi: 10.1016/j.ccell.2019.05.004
40. Amit U, Uslu U, Verginadis II, Kim MM, Motlagh SAO, Diffenderfer ES, et al. Proton radiation boosts the efficacy of mesothelin-targeting chimeric antigen receptor T cell therapy in pancreatic cancer. Proc Natl Acad Sci. (2024) 121:e2403002121. doi: 10.1073/pnas.2403002121
41. DeSelm C, Palomba ML, Yahalom J, Hamieh M, Eyquem J, Rajasekhar VK, et al. Low-dose radiation conditioning enables CAR T cells to mitigate antigen escape. Mol Ther: J Am Soc Gene Ther. (2018) 26:2542–525. doi: 10.1016/j.ymthe.2018.09.008
42. Jin L, Tao H, Karachi A, Long Y, Hou AY, Na M, et al. CXCR1- or CXCR2-modified CAR T cells co-opt IL-8 for maximal antitumor efficacy in solid tumors. Nat Commun. (2019) 10:4016. doi: 10.1038/s41467-019-11869-4
43. Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. (1999) 17:2105–16. doi: 10.1200/JCO.1999.17.7.2105
44. Tang L, Pan S, Wei X, Xu X, and Wei. Q. “Arming CAR-T cells with cytokines and more: innovations in the fourth-generation CAR-T development. Mol Ther. (2023) 31:3146–625. doi: 10.1016/j.ymthe.2023.09.021
45. Zhang Q, Hresko ME, Picton LK, Su L, Hollander MJ, Nunez-Cruz S, et al. A human orthogonal IL-2 and IL-2Rβ System enhances CAR T cell expansion and antitumor activity in a murine model of leukemia. Sci Trans Med. (2021) 13:eabg6986. doi: 10.1126/scitranslmed.abg6986
46. Allen GM, Frankel NW, Reddy NR, Bhargava HK, Yoshida MA, Stark SR, et al. Synthetic cytokine circuits that drive T cells into immune-excluded tumors. Sci (New York NY). (2022) 378:eaba1624. doi: 10.1126/science.aba1624
47. Hurton LV, Singh H, Najjar AM, Switzer KC, Mi T, Maiti S, et al. Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proc Natl Acad Sci U S A. (2016) 113:E7788–97. doi: 10.1073/pnas.1610544113
48. Chmielewski M and Abken H. CAR T cells transform to trucks: chimeric antigen receptor-redirected T cells engineered to deliver inducible IL-12 modulate the tumour stroma to combat cancer. Cancer Immunol Immunother: CII. (2012) 61:1269–775. doi: 10.1007/s00262-012-1202-z
49. He C, Zhou Y, Li Z, Farooq MA, Ajmal I, Zhang H, et al. Co-expression of IL-7 improves NKG2D-based CAR T cell therapy on prostate cancer by enhancing the expansion and inhibiting the apoptosis and exhaustion. Cancers. (2020) 12:1969. doi: 10.3390/cancers12071969
50. Štach M, Ptáčková P, Mucha M, Musil J, Klener P, and Otáhal. P. “Inducible secretion of IL-21 augments anti-tumor activity of piggyBac-manufactured chimeric antigen receptor T cells. Cytotherapy. (2020) 22:744–545. doi: 10.1016/j.jcyt.2020.08.005
51. Wang Y, Wang J, Yang X, Yang J, Lu P, Zhao L, et al. Chemokine receptor CCR2b enhanced anti-tumor function of chimeric antigen receptor T cells targeting mesothelin in a non-small-cell lung carcinoma model. Front Immunol. (2021) 12:628906. doi: 10.3389/fimmu.2021.628906
Keywords: cancer immunotherapy, cellular therapy, immune cell tracking, chimeric antigen receptor, liver metastases, regional therapy
Citation: Purl MC, Shick A, Canter RJ and Judge SJ (2025) Tracking cellular therapies to optimize homing against liver metastases. Front. Immunol. 16:1611861. doi: 10.3389/fimmu.2025.1611861
Received: 14 April 2025; Accepted: 04 June 2025;
Published: 25 June 2025.
Edited by:
Kelsey P. Kubelick, University of Virginia, United StatesReviewed by:
Khan M. Imran, University of North Carolina at Chapel Hill, United StatesAlex Blair, The Ohio State University, United States
Copyright © 2025 Purl, Shick, Canter and Judge. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sean J. Judge, c2pqdWRnZUB1Y2RhdmlzLmVkdQ==
 Megan C. Purl
Megan C. Purl Alexandria Shick
Alexandria Shick Robert J. Canter
Robert J. Canter Sean J. Judge
Sean J. Judge