- 1Department of Obstetrics and Gynecology, Shenzhen Longhua District Central Hospital, Shenzhen, China
- 2Department of Pharmacy, Shenzhen Longhua District Central Hospital, Shenzhen, China
- 3Department of Breast and Thyroid Surgery, Shenzhen Longhua District Central Hospital, Shenzhen, China
The immune interactions within the gut–brain axis represent a critical etiological factor in psychiatric disorders. The gut microbiota and their metabolites serve as biological mediators that regulate neuroimmune activation and suppression in the central nervous system (CNS). During intestinal immune activation, pro-inflammatory cytokines (e.g., IL-6, TNF-α) propagate to the CNS via compromised blood–brain barrier (BBB) integrity or vagal afferent fibers, disrupting neurotransmitter metabolism and inducing microglial hyperactivation, thereby exacerbating neuroinflammation. Microglia, the principal immune sentinels of the CNS, adopt a pro-inflammatory phenotype upon peripheral inflammatory signaling characterized by morphological transformations, excessive chemokine/cytokine production (e.g., IL-1β, IL-6), and dysregulated neurotransmitter dynamics. These mechanisms are strongly implicated in neuropsychiatric conditions such as major depressive disorder, anxiety disorders, autism spectrum disorder, and schizophrenia. Emerging microbiota-targeted therapies, including probiotic interventions and fecal microbiota transplantation, demonstrate therapeutic potential by restoring tryptophan homeostasis and modulating systemic inflammation. This review synthesizes current evidence on the regulatory role of the gut microbiota in inflammation-related psychiatric disorders, specifically emphasizing the microbial modulation of neuroimmune crosstalk and neurotransmitter synthesis (e.g., serotonin, dopamine). Mechanistic insights into microbial metabolites, such as short-chain fatty acids and tryptophan derivatives, are critically evaluated for their dual roles in psychiatric disorders. These findings advance a unified framework for managing psychiatric comorbidities through precision modulation of the gut–brain axis.
1 Introduction
The gut–brain axis orchestrates bidirectional communication between the gut microbiota and central nervous system (CNS) through integrated neural, immune, and endocrine pathways (1). Central to this interaction, microbial metabolites such as short-chain fatty acids (SCFAs) and tryptophan derivatives critically regulate neurotransmitter homeostasis (e.g., serotonin [5-HT] and dopamine synthesis) and modulate neuroinflammatory cascades, thereby shaping mood, cognition, and behavior (2). This microbiota–gut–brain axis (MGBA) operates via dynamic cross-talk; specifically, gastrointestinal microbes influence neural plasticity and inflammatory cytokine release, whereas CNS-derived signals reciprocally reshape microbial composition and metabolic activity (3, 4).
Inflammation is a central mediator of gut–brain dysregulation (5). Emerging evidence highlights gut inflammation as a pivotal driver of neuropsychiatric pathology (6, 7). Intestinal-derived inflammatory cytokines (e.g., IL-6, TNF-α) elevate cerebral glutamine levels and activate vagal signaling, concurrently increasing blood ammonia content via hepatic metabolism (8, 9). This cascade compromises blood–brain barrier (BBB) integrity. Subsequent BBB permeability facilitates the entry of cytokines and chemokines into the brain, in which they interact with neural receptors. These processes directly impair neuronal function through reductions in 5-HT, dopamine, and norepinephrine levels, thereby exacerbating inflammation-related psychiatric disorders (10, 11). These cytokines disrupt neurotransmitter metabolism, such as inhibiting tryptophan hydroxylase, thereby diverting tryptophan from 5-HT synthesis toward neurotoxic kynurenine derivatives, and induce microglial hyperactivation, amplifying neuroinflammation (12, 13). Microglia, constituting 5%–12% of brain cells, serve as the CNS’s primary immune sentinels (14). Upon activation by peripheral inflammatory signals, these cells adopt a pro-inflammatory phenotype characterized by morphological changes, chemokine/cytokine overproduction (e.g., IL-1β, IL-6), and dysregulated neurotransmitter dynamics. This microglial dysfunction is mechanistically linked to neuropsychiatric disorders including depression (15), anxiety (16), autism (17), and schizophrenia (18). Critically, gut dysbiosis exacerbates this cycle by impairing BBB integrity and priming both peripheral and central immune systems for sustained cytokine release (2, 19).
Chronic inflammation is a transdiagnostic nexus in gut–brain disorders. Persistent inflammation has emerged as a shared pathogenic thread across gastrointestinal and psychiatric conditions. Notably, 38.9% of patients with inflammatory bowel disease (IBD) exhibit comorbid depression during active flares, with the prevalence of anxiety soaring to 80% (20). Even in remission, patients with IBD retain a 2–3-fold higher risk of depression/anxiety than the general population, with symptom severity correlating directly with intestinal inflammation intensity and disease chronicity (20). Mechanistically, IL-6, the levels of which are elevated systemically in IBD, permeates the BBB or relays signals via vagal pathways to suppress hippocampal neurogenesis and induce depression-like behaviors in preclinical models (21). Clinical validation of this axis is underscored by robust correlations between serum IL-6 levels and Hamilton Depression Rating Scale scores in IBD cohorts (22). These findings position gut-derived inflammation as both a biomarker and therapeutic target for neuropsychiatric comorbidities.
2 Gut microbial signatures in major psychiatric disorders
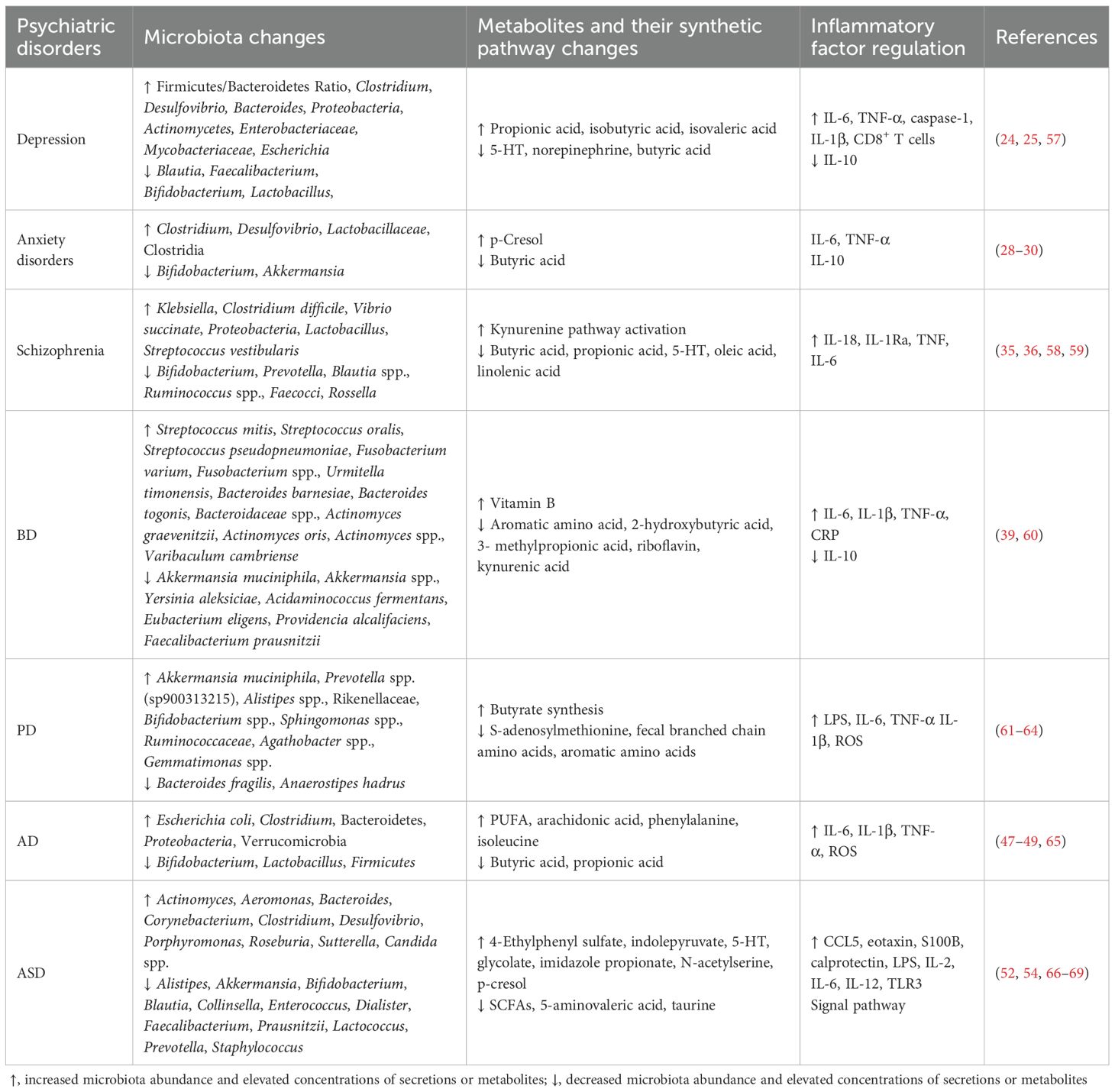
Growing evidence indicates that gut dysbiosis can influence brain function and contribute to neurological disorders via the gut–brain axis. Dysregulation of this axis is increasingly recognized as a pathophysiological basis for cognitive and psychiatric impairments (23). Consequently, targeted modulation of disrupted microbial ecosystems has emerged as a promising therapeutic strategy for such conditions. Table 1 summarizes gut microbial alterations and inflammatory mediator profiles across major psychiatric disorders.
2.1 Depression
In major depressive disorder (MDD), the most affected bacterial phyla include Firmicutes, Actinobacteria, and Bacteroidetes, leading to an elevated Bacteroidetes/Firmicutes ratio (24). Characteristic changes involve the enrichment of Bacteroides and depletion of Blautia, Faecalibacterium, and Coprococcus. In addition, an increased abundance of Eggerthella and reduced abundance of Sutterella are consistently observed in patients with MDD. A pathological vicious cycle is supported by evidence revealing an elevated abundance of pro-inflammatory genera (e.g., Escherichia) in depression (25). These microbial shifts could drive MDD pathogenesis. Research on post-intervention outcomes of intestinal probiotics demonstrated that supplementation with Bre1025 (Bifidobacterium longum) restores 5-HT levels in the brain while concurrently suppressing serum corticosterone and pro-inflammatory cytokine expression (e.g., IL-1β, IL-6) and elevating the expression of the anti-inflammatory cytokine IL-10 (26). Furthermore, JB-1 (Lactobacillus rhamnosus) supplementation alters gamma-aminobutyric acid (GABA) neurotransmission via the vagus nerve, thereby ameliorating depressive symptoms (27).
2.2 Anxiety disorders
Patients with anxiety disorders exhibit an increased Firmicutes/Bacteroidetes ratio, marked by the overgrowth of Clostridium and Desulfovibrio alongside reductions in the abundance of SCFA-producing genera (e.g., Bifidobacterium, Lactobacillus) (28). Clostridium species can exacerbate anxiety-like behaviors by generating neurotoxic metabolites (e.g., p-cresol), which disrupt dopamine and 5-HT metabolism. Recent studies revealed distinct gut microbial community structures across anxiety states, with the abundance of Akkermansia being inversely correlated with anxiety severity (29, 30). Akkermansia muciniphila synergizes with lactate to restore the tryptophan metabolic balance, promoting 5-HT synthesis and alleviating anxiety (29).
2.3 Schizophrenia
Schizophrenia is associated with distinct gut microbiota alterations characterized by reduced α-diversity, featuring an increased abundance of Proteobacteria and Lactobacillus alongside diminished levels of anti-inflammatory commensals such as Prevotella (31). The condition exhibits a pro-inflammatory microbial profile with an increased abundance of Lachnoclostridium and a decreased abundance of SCFA-producing Blautia spp. and Ruminococcus spp (32)., corresponding with elevated lipopolysaccharide (LPS) (32) and reduced superoxide dismutase-1 levels (33). Notably, the abundance of Lachnoclostridium might predict poor cognitive improvement (34), whereas acute-phase patients display Streptococcus vestibularis enrichment associated with cognitive decline (35). Mechanistically, impaired SCFA synthesis (butyrate/propionate) compromises immunomodulation, and dysregulated tryptophan metabolism (via the kynurenine pathway) reduces 5-HT content, exacerbating the neurotransmitter imbalance (36). Gut dysbiosis further activates the vagus nerve–hypothalamic–pituitary–adrenal axis, inducing hippocampal microglial M1 polarization, whereas SCFA deficiency directly impairs neuronal mitochondrial function, amplifying oxidative stress (37). These findings collectively suggest that gut microbiota dysbiosis plays a multifaceted role in schizophrenia pathogenesis through immune–metabolic–neural pathways.
2.4 Bipolar disorder
Patients with BD exhibit gut dysbiosis, including disrupted Firmicutes/Bacteroidetes ratios, reduced α-diversity, and altered β-diversity. The elevated abundance of Streptococcaceae and Bacteroidaceae contrasts with the depletion of A. muciniphila and F. prausnitzii (38). Functional analyses revealed significant differences in amino acid metabolism and vitamin synthesis pathways (39). Notably, the abundance of Faecalibacterium (an anti-inflammatory, gram-positive commensal) is reduced in BD, IBD, and depression, suggesting gut dysbiosis can broadly disrupt CNS physiology.
2.5 Parkinson’s disease
PD is associated with gut microbial dysbiosis characterized by an increased abundance of Lactobacillus and Bifidobacterium but reduced levels of Faecalibacterium, Coprococcus, and Blautia (40, 41). Probiotic interventions (e.g., L. casei) mitigate β-amyloid deposition and cognitive decline, highlighting microbial metabolites as potential therapeutic targets (42, 43). Altered branched-chain and aromatic amino acid levels in fecal samples are correlated with PD progression (44, 45). Recent clinical studies indicated that patients with PD exhibit a reduced abundance of Blautia and diminished fecal levels of the SCFA butyrate. The abundance of Blautia is correlated with the clinical severity of PD. The RAS-related pathway, a pivotal inflammatory signaling pathway modulated by butyrate, has emerged as a key mechanism inhibiting microglial activation in PD. Alterations in the RAS–NF-κB pathway have been observed in patients with PD. Furthermore, butyrate derived from B. producta inhibited microglial activation by regulating the RAS–NF-κB pathway (46).
2.6 Alzheimer’s disease
The gut microbiota drives AD pathology through the “leaky gut–systemic inflammation–neuroimmune activation” axis, in which intestinal barrier dysfunction triggers systemic inflammation, which ultimately activates neuroimmune responses (47). In patients with AD, this process is characterized by reduced microbial diversity, with decreased levels of probiotics (e.g., Bifidobacterium) and increased levels of opportunistic pathogens (e.g., E. coli, Clostridium) and poly-unsaturated fatty acids (PUFAs), along with diminished levels of anti-inflammatory SCFAs, thereby exacerbating neuroinflammation (48, 49). Therapeutic strategies targeting this axis include probiotic supplementation (e.g., Lactobacillus) and high-fiber diets to boost SCFAs and suppress pro-inflammatory pathways, short-term antibiotic regimens or fecal microbiota transplantation (FMT), which has displayed efficacy in murine models (although long-term safety validation is required), and gut–brain axis-targeted drugs such as GV-971 that remodel the microbial balance while inhibiting neuroinflammation and improving cognitive function (50). These microbiota-modulating approaches represent promising strategies for delaying AD progression.
2.7 Autism spectrum disorder
ASD, a neurodevelopmental disorder marked by social deficits and repetitive behaviors, is linked to gut–brain crosstalk via microbial metabolites (51). Higher concentrations of p-cresol exhibit a significant link to increased symptom severity in ASD, demonstrating a strong correlation with both intensified behavioral symptoms and developmental regression patterns. P-cresol contributes to the pathogenesis of ASD by inducing dopamine accumulation and enhancing dopamine metabolism in the brain. This effect is partly explained by evidence identifying p-cresol as an inhibitor of dopamine β-hydroxylase (DBH)—the enzyme responsible for converting dopamine (DA) into norepinephrine (NE). By blocking dopamine’s transformation into norepinephrine, p-cresol further amplifies dopamine accumulation and promotes heightened dopaminergic metabolic activity within neural systems and reward circuitry (52, 53). Emerging evidence indicates that Candida spp. contribute to immune dysregulation, behavioral abnormalities, and alterations in brain activity, corroborated by their elevated prevalence in the feces of individuals with ASD. This genus might exacerbate hyperserotonemia through enhanced peripheral 5-HT production coupled with impaired brain 5-HT synthesis from tryptophan, thereby aggravating neurobehavioral symptoms (54). Biomarkers include brain-derived neurotrophic factor (BDNF), calprotectin, S100B, and dysregulated cytokines (e.g., CCL5, eotaxin), underscoring the role of gut–microbiota–immune interactions in ASD pathogenesis (55, 56). Specific ASD-related microbial shifts and potential differential mechanisms are summarized in Table 1.
3 Gut-mediated mechanisms in inflammation-related psychiatric disorders
The vagus nerve, serving as the principal bidirectional neural conduit between the gut and brain, mediates the transmission of inflammatory signals and metabolic information through its afferent and efferent fibers (70). This nerve constitutes a critical neuroanatomical bridge in gut–brain axis communication, directly regulating inflammatory-related psychiatric disorders via its synaptic connections to limbic structures (e.g., hippocampus, amygdala) and hypothalamic nuclei (71). In parallel, the microbiota–immune–neural circuit interaction mechanism exhibits greater complexity in modulating inflammation-associated psychiatric conditions (72). This tripartite crosstalk involves microbial metabolite signaling (e.g., SCFAs modulating microglial activation), neuroimmune synchronization (cytokine-mediated TLR4–NF-κB pathway activation), and enteroendocrine regulation (5-HT/dopamine synthesis influenced by gut microbes), as presented in Figure 1.
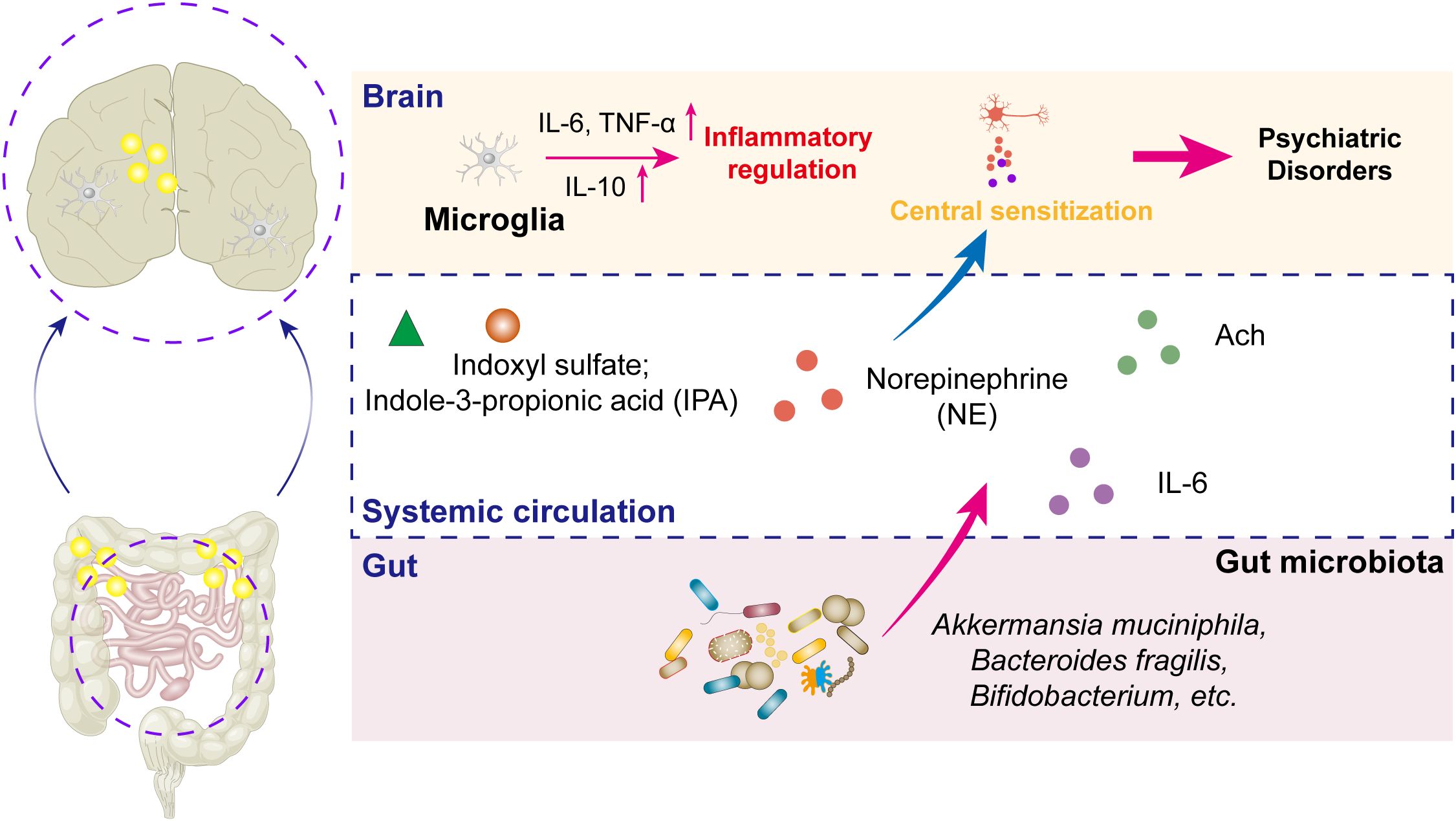
Figure 1. Schematic diagram of the molecular mechanisms underlying inflammation-related psychiatric disorders via the MGBA. Inflammation serves critical bridging roles within the MGBA, mediating the onset and progression of psychiatric disorders. Furthermore, the gut microbiota and their metabolites regulate norepinephrine secretion, which acts synergistically with acetylcholine (Ach) released from parasympathetic neurons to modulate central sensitization capacity within neural circuits, thereby significantly contributing to the neurobiological mechanisms of psychiatric pathologies.
3.1 Bidirectional communication via the gut–brain axis
3.1.1 Neural regulatory pathways
The vagus nerve serves as a direct neural conduit between the gut and brain, facilitating the bidirectional transmission of inflammatory signals and metabolic information (70). The vagus nerve facilitates the real-time gut-to-brain transmission of inflammatory signals and mechanical distension while enabling the brain-to-gut modulation of intestinal motility, secretion, and immune function via vagal efferents. Clinical evidence indicates that vagotomy increases the incidence of psychiatric disorders, whereas vagus nerve stimulation improves mood disorders (73, 74). Complementing this pathway, the enteric nervous system functions as an autonomous “second brain” through self-contained neuronal networks that process local reflexes independently while producing neurotransmitters such as dopamine and 5-HT to concurrently regulate gut functions (e.g., peristalsis) and participate in mood modulation (47).
3.1.2 Immune regulatory network
The disruption of gut microbiota homeostasis (dysbiosis) initiates systemic low-grade inflammatory responses via immune system activation, manifesting as an imbalance between elevated levels of pro-inflammatory cytokines (e.g., IL-6, TNF-α) and deficient anti-inflammatory mediators (e.g., IL-10) (75–77). These inflammatory signals cross the BBB or propagate through vagal afferents, subsequently activating microglial cells, exacerbating neuroinflammatory processes, and compromising prefrontal cortex-mediated executive functions including decision-making and emotional regulation (78). Recent mechanistic studies revealed that the gut microbiota directly influence neuroimmune crosstalk by modulating TRPV1-expressing sensory neurons (79). These neurons release calcitonin gene-related peptide, which orchestrates regulatory T cell (Treg) differentiation and functional activity, thereby maintaining the critical Th17/Treg equilibrium (80, 81). Furthermore, gut dysbiosis induces CD8+ T cell activation and the subsequent release of cytotoxic effector molecules (perforin and granzyme B), which in turn stimulate the colonic epithelium to produce the chemokine CXCL9, establishing a pro-inflammatory feedback loop (82).
Pro-inflammatory cytokines disrupt neurotransmitter metabolism (e.g., inhibiting the conversion of tryptophan to 5-HT) and drive microglial activation, exacerbating neuroinflammation. Microglia play pivotal roles in neuroinflammatory cascades by releasing chemokines, cytokines, and reactive oxygen species (ROS) upon activation (83). Their phenotypic shift from surveillance to pro-inflammatory states disrupts neurotransmitter balance and synaptic plasticity.
Gut-derived LPS, a TLR4 ligand, activates NF-κB and MAPK pathways to promote cytokine release (75, 76, 84). In BD, elevated TLR4 expression in peripheral monocytes is correlated with disease severity (85). Targeting the gut microbiota (e.g., probiotics) or TLR4 signaling might therefore ameliorate neuroinflammation in BD.
3.1.3 Metabolite modulation
The gut microbiota profoundly influences emotional regulation through its production of key neurotransmitters such as 5-HT and dopamine that act via the gut–brain axis. Although only 5% of the body’s 5-HT originates in the brainstem, in which it regulates cognition, behavior, and metabolic functions as a neurotransmitter, the remaining 95% is synthesized peripherally in the gastrointestinal tract, in which it primarily functions as a vasoconstrictor and intestinal motility regulator (20, 54). Microbiota-derived SCFAs such as butyrate exert neuroprotective effects by inhibiting histone deacetylases and strengthening BBB integrity against inflammatory mediators. Importantly, gut microbes critically influence tryptophan metabolism. When preferentially shunted toward the kynurenine pathway rather than 5-HT synthesis, this process produces neurotoxic metabolites such as quinolinic acid that contribute to depression and cognitive impairment (86, 87). In mood disorders such as BP, circulating microbial metabolites (including SCFAs and LPS) can cross the BBB through vascular or neural pathways, disrupting monoaminergic neurotransmitter systems (5-HT and dopamine) and microglial activity, ultimately exacerbating mood instability. This multifaceted communication network highlights the gut microbiota’s central role in neuropsychiatric health through both direct neurotransmitter production and the indirect modulation of metabolic and immune pathways.
3.2 Microbiota–immune–neural circuit interactions
3.2.1 Gut microbiota-mediated regulation of immunity
The gut microbiota modulate intestinal immunity through metabolites (SCFAs, tryptophan derivatives) and cell wall components (e.g., LPS) (88). Key mechanisms include Treg expansion (Clostridia-derived butyrate induces dendritic cells to secrete TGF-β, promoting Treg differentiation) and Th17 suppression (Treg-secreted IL-10 inhibits Th17-mediated pro-inflammatory effects [e.g., IL-17 release], maintaining immune homeostasis) (89).
3.2.2 Immune–neuroinflammatory cascades
The peripheral immune status influences central neuroinflammation via three pathways. In the cytokine-to-brain axis, Th17/Treg imbalance elevates the expression of IL-6 and IL-1β, which cross the compromised BBB to activate microglia (90). Upon microglial polarization, pro-inflammatory cytokines drive M1 microglial polarization, releasing ROS and TNF-α to exacerbate neuronal damage (e.g., synaptic pruning defects in autism) (90). Conversely, butyrate promotes M2 polarization via PPAR-γ activation, enhancing anti-inflammatory and reparative functions (88). Following BBB disruption, TNF-α upregulates matrix metalloproteinases, which degrade tight junction proteins (e.g., occludin) and increase BBB permeability. This allows peripheral inflammatory mediators and microbial metabolites to infiltrate the brain parenchyma, inducing prefrontal cortical dysfunction (91).
In the amygdala, inflammatory mediators trigger microglial activation, leading to glutamatergic hyperexcitability and GABAergic synaptic impairment, which underlie anxiety-like behaviors and emotional dysregulation (92). Chronic neuroinflammation reduces synaptic plasticity in the prefrontal cortex and hippocampus, driving cognitive decline and mood disorders (93). Notably, butyrate enhances cognitive performance by modulating dorsal striatal activity (94).
3.2.3 MGBA in psychiatric disorders
The MGBA mechanism in inflammation-related psychiatric disorders manifests as dysregulation across microbial, immune, and neural pathways, with detailed classifications and functional mechanisms presented in Table 1. In ASD and BD, microbiota–immune–neural dysregulation manifests as compositional shifts (reduced Bacteroidetes/Firmicutes ratio [e.g., in ASD] decreases butyrate synthesis and increases inflammatory cytokines) (95, 96), pathogenic metabolites (Clostridia-derived p-cresol inhibits dopamine β-hydroxylase, blocking dopamine-to-norepinephrine conversion and inducing mood instability) (89), and tryptophan metabolism (microbiota-induced indoleamine 2,3-dioxygenase activation diverts tryptophan toward the kynurenine pathway, generating neurotoxic metabolites [e.g., quinolinic acid]) (88).
4 Summary and perspectives
The widespread adoption of modern lifestyles has led to increasing tolerance to conventional pharmacotherapies in many patients. Future research should integrate multiomics datasets (metagenomic, metabolomic, and immunological profiling) to decipher the spatiotemporal dynamics of microbiota–host interactions. Personalized therapeutic strategies, particularly those leveraging microbiota signatures to predict anti-inflammatory treatment responses (e.g., stratified application of probiotics or anti-cytokine therapies), are emerging as critical frontiers. Furthermore, comparative analyses of microbiota-driven inflammatory signatures across psychiatric disorders might reveal transdiagnostic therapeutic targets, offering a unified approach for managing psychiatric comorbidities.
4.1 Microbiota-targeted therapies
Probiotic interventions demonstrate multifaceted therapeutic potential. Bifidobacterium enhances SCFA production, lowers intestinal pH to suppress pathogenic overgrowth, and upregulates BDNF to improve hippocampal neuroplasticity. L. casei mitigates cognitive decline by inhibiting β-amyloid aggregation and slowing disease progression in PD. F. prausnitzii, a keystone anti-inflammatory species, ameliorates CNS dysfunction in both IBD and depression. A. muciniphila synergizes with lactate to restore tryptophan metabolic balance, promoting 5-HT synthesis and alleviating anxiety.
FMT and probiotic formulations hold promise as alternatives to conventional pharmacotherapies, particularly in restoring tryptophan homeostasis and reducing peripheral IL-6 levels. Given their mechanistic versatility and safety profile, microbiota-targeted therapies are poised to gain clinical traction.
4.2 Synergistic anti-inflammatory drug applications
With more than 6.8 million patients globally affected by Crohn’s disease and ulcerative colitis, current therapies (e.g., immunosuppressants, biologics) remain palliative, often causing drug resistance and opportunistic infections with prolonged use (97). Notably, approximately 30% of patients with IBD develop anxiety or depression, underscoring the urgent need for dual-action therapies that concurrently resolve gut inflammation and modulate the gut–brain axis.
Preclinical evidence has highlighted the potential of synergistic strategies. Lamotrigine, a mood stabilizer, attenuates neuroinflammation by suppressing glutamatergic hyperactivity. Duloxetine, when co-administered with lamotrigine, potentiates 5-HT/norepinephrine reuptake inhibition, disrupting the inflammation–depression cycle. Such combinatorial approaches exemplify the paradigm shift toward targeting gut–brain axis dysregulation in psychiatric comorbidities. Future innovations could combine anti-inflammatory biologics (e.g., IL-6/IL-17 inhibitors) with neuromodulatory agents to achieve sustained remission.
Author contributions
LZ: Methodology, Investigation, Writing – original draft, Formal analysis, Data curation. QW: Writing – original draft, Formal analysis, Investigation, Methodology. LJ: Writing – original draft, Methodology, Investigation. JR: Writing – original draft, Methodology. JG: Investigation, Validation, Writing – original draft, Methodology. FZ: Methodology, Investigation, Writing – original draft, Supervision. XW: Project administration, Methodology, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the the Guangdong Basic and Applied Basic Research Foundation (No. 2023A1515111116), the Shenzhen Foundation of Science and Technology (No. JCYJ20230807151308018), the Zhanjiang Science and Technology Project (2023B01176), the Shenzhen Longhua District Science and Technology Innovation Fund Projects (Nos. 2024013, 2022045, 2022051, 2022056, 2022095, 2022123, 2021105, 2021115 and 2020036), the Key Disciplines of Shenzhen Longhua District Central Hospital and the Research Foundation of Shenzhen Longhua District Central Hospital (No. 202303).
Acknowledgments
We sincerely thank the reviewers for their valuable feedback on this paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ohara TE and Hsiao EY. Microbiota-neuroepithelial signalling across the gut-brain axis. Nat Rev Microbiol. (2025) 23:371–84. doi: 10.1038/s41579-024-01136-9
2. Ochoa-Repáraz J, Ramelow CC, and Kasper LH. A gut feeling: the importance of the intestinal microbiota in psychiatric disorders. Front Immunol. (2020) 11:510113. doi: 10.3389/fimmu.2020.510113
3. Keane L, Clarke G, and Cryan JF. A role for microglia in mediating the microbiota-gut-brain axis. Nat Rev Immunol. (2025) 1–15. doi: 10.1038/s41577-025-01188-9
4. Needham BD, Kaddurah-Daouk R, and Mazmanian SK. Gut microbial molecules in behavioural and neurodegenerative conditions. Nat Rev Neurosci. (2020) 21:717–31. doi: 10.1038/s41583-020-00381-0
5. Zundler S, Günther C, Kremer AE, Zaiss MM, Rothhammer V, and Neurath MF. Gut immune cell trafficking: inter-organ communication and immune-mediated inflammation. Nat Rev Gastroenterol Hepatol. (2023) 20:50–64. doi: 10.1038/s41575-022-00663-1
6. Thomann AK, Schmitgen MM, Stephan JC, Ebert MP, Thomann PA, Szabo K, et al. Associations between brain morphology, inflammatory markers, and symptoms of fatigue, depression, or anxiety in active and remitted crohn’s disease. J Crohns Colitis. (2024) 18:1767–79. doi: 10.1093/ecco-jcc/jjae078
7. Varghese SM, Patel S, Nandan A, Jose A, Ghosh S, Sah RK, et al. Unraveling the role of the blood-brain barrier in the pathophysiology of depression: recent advances and future perspectives. Mol Neurobiol. (2024) 61:10398–447. doi: 10.1007/s12035-024-04205-5
8. Jiao W, Lin J, Deng Y, Ji Y, Liang C, Wei S, et al. The immunological perspective of major depressive disorder: unveiling the interactions between central and peripheral immune mechanisms. J Neuroinflamm. (2025) 22:10. doi: 10.1186/s12974-024-03312-3
9. Hang C-H, Shi J-X, Li J-S, Li W-Q, and Wu W. Expressions of intestinal NF-kappaB, TNF-alpha, and IL-6 following traumatic brain injury in rats. J Surg Res. (2005) 123:188–93. doi: 10.1016/j.jss.2004.08.002
10. Bekhbat M, Block AM, Dickinson SY, Tharp GK, Bosinger SE, and Felger JC. Neurotransmitter and metabolic effects of interferon-alpha in association with decreased striatal dopamine in a non-human primate model of cytokine-Induced depression. Brain Behav Immun. (2025) 125:308–18. doi: 10.1016/j.bbi.2025.01.010
11. Klein RS and Hunter CA. Protective and pathological immunity during central nervous system infections. Immunity. (2017) 46:891–909. doi: 10.1016/j.immuni.2017.06.012
12. Veen C, Myint AM, Burgerhout KM, Schwarz MJ, Schütze G, Kushner SA, et al. Tryptophan pathway alterations in the postpartum period and in acute postpartum psychosis and depression. J Affect Disord. (2016) 189:298–305. doi: 10.1016/j.jad.2015.09.064
13. Park H-J, Shim H-S, An K, Starkweather A, Kim KS, and Shim I. IL-4 inhibits IL-1β-induced depressive-like behavior and central neurotransmitter alterations. Mediators Inflammation. (2015) 2015:941413. doi: 10.1155/2015/941413
14. Spangenberg EE and Green KN. Inflammation in Alzheimer’s disease: Lessons learned from microglia-depletion models. Brain Behav Immun. (2017) 61:1–11. doi: 10.1016/j.bbi.2016.07.003
15. Chen Y, Yao X, Wang C, Zhuang H, Xie B, Sun C, et al. Minocycline treatment attenuates neurobehavioural abnormalities and neurostructural aberrations in the medial prefrontal cortex in mice fed a high-fat diet during adolescence. Brain Behav Immun. (2025) 128:83–98. doi: 10.1016/j.bbi.2025.03.035
16. Delorme TC, Arcego DM, Penichet D, O’Toole N, Huebener N, Silveira PP, et al. Large-scale effects of prenatal inflammation and early life circadian disruption in mice: Implications for neurodevelopmental disorders. Brain Behav Immun. (2025) 127:409–22. doi: 10.1016/j.bbi.2025.03.023
17. Qin Q, Fan L, Zeng X, Zheng D, Wang H, Li M, et al. Mesenchymal stem cell-derived extracellular vesicles alleviate autism by regulating microglial glucose metabolism reprogramming and neuroinflammation through PD-1/PD-L1 interaction. J Nanobiotechnol. (2025) 23:201. doi: 10.1186/s12951-025-03250-z
18. Knight SR, Abbasova L, Zeighami Y, Hansen JY, Martins D, Zelaya F, et al. Transcriptional and neurochemical signatures of cerebral blood flow alterations in individuals with schizophrenia or at clinical high risk for psychosis. Biol Psychiatry. (2025) 98:144–55. doi: 10.1016/j.biopsych.2025.01.028
19. Conesa MPB, Blixt FW, Peesh P, Khan R, Korf J, Lee J, et al. Stabilizing histamine release in gut mast cells mitigates peripheral and central inflammation after stroke. J Neuroinflamm. (2023) 20:230. doi: 10.1186/s12974-023-02887-7
20. Barberio B, Zamani M, Black CJ, Savarino EV, and Ford AC. Prevalence of symptoms of anxiety and depression in patients with inflammatory bowel disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. (2021) 6:359–70. doi: 10.1016/S2468-1253(21)00014-5
21. Basiji K, Sendani AA, Ghavami SB, Farmani M, Kazemifard N, Sadeghi A, et al. The critical role of gut-brain axis microbiome in mental disorders. Metab Brain Dis. (2023) 38:2547–61. doi: 10.1007/s11011-023-01248-w
22. Zhao H, Li W, Xu Q, Chen P, and Wang X. Early Th1-Th2 cytokine imbalance as a predictor for post-stroke depression at 3 months. J Affect Disord. (2025) 389:119650. doi: 10.1016/j.jad.2025.119650
23. Luo K, Zhang M, Tu Q, Li J, Wang Y, Wan S, et al. From gut inflammation to psychiatric comorbidity: mechanisms and therapies for anxiety and depression in inflammatory bowel disease. J Neuroinflamm. (2025) 22:149. doi: 10.1186/s12974-025-03476-6
24. Liu L, Wang H, Chen X, Zhang Y, Zhang H, and Xie P. Gut microbiota and its metabolites in depression: from pathogenesis to treatment. EBioMedicine. (2023) 90:104527. doi: 10.1016/j.ebiom.2023.104527
25. Liu P, Liu Z, Wang J, Wang J, Gao M, Zhang Y, et al. Immunoregulatory role of the gut microbiota in inflammatory depression. Nat Commun. (2024) 15:3003. doi: 10.1038/s41467-024-47273-w
26. Qian X, Li Q, Zhu H, Chen Y, Lin G, Zhang H, et al. Bifidobacteria with indole-3-lactic acid-producing capacity exhibit psychobiotic potential via reducing neuroinflammation. Cell Rep Med. (2024) 5:101798. doi: 10.1016/j.xcrm.2024.101798
27. Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U.S.A. (2011) 108:16050–5. doi: 10.1073/pnas.1102999108
28. Lai Y and Xiong P. Analysis of gut microbiota and depression and anxiety: Mendelian randomization from three datasets. Gen Hosp Psychiatry. (2025) 94:206–18. doi: 10.1016/j.genhosppsych.2025.03.012
29. Pan M, Qian C, Huo S, Wu Y, Zhao X, Ying Y, et al. Gut-derived lactic acid enhances tryptophan to 5-hydroxytryptamine in regulation of anxiety via Akkermansia muciniphila. Gut Microbes. (2025) 17:2447834. doi: 10.1080/19490976.2024.2447834
30. Zhang X, Hou Y, Li Y, Wei W, Cai X, Shao H, et al. Taxonomic and metabolic signatures of gut microbiota for assessing the severity of depression and anxiety in major depressive disorder patients. Neuroscience. (2022) 496:179–89. doi: 10.1016/j.neuroscience.2022.06.024
31. Wu H, Jiawei X, Wen Z, Han Y, Liu Y, Chen S, et al. Microbiome-gut-brain profiles in schizophrenia and their potential link to cognitive performance: findings from a case-control study. Schizophr Bull. (2025) 1–14. doi: 10.1093/schbul/sbaf028
32. Yuan X, Wang Y, Li X, Jiang J, Kang Y, Pang L, et al. Gut microbial biomarkers for the treatment response in first-episode, drug-naïve schizophrenia: a 24-week follow-up study. Transl Psychiatry. (2021) 11:422. doi: 10.1038/s41398-021-01531-3
33. Coughlin JM, Ishizuka K, Kano SI, Edwards JA, Seifuddin FT, Shimano MA, et al. Marked reduction of soluble superoxide dismutase-1 (SOD1) in cerebrospinal fluid of patients with recent-onset schizophrenia. Mol Psychiatry. (2013) 18:10–1. doi: 10.1038/mp.2012.6
34. Manchia M, Paribello P, Pisanu C, Congiu D, Antoniades A, Vogazianos P, et al. A pilot interaction analysis of gut microbiota and peripheral markers of aging in severe psychiatric disorders: A role for lachnoclostridium? Int J Mol Sci. (2023) 24:1–9. doi: 10.3390/ijms242417618
35. Zhu F, Ju Y, Wang W, Wang Q, Guo R, Ma Q, et al. Metagenome-wide association of gut microbiome features for schizophrenia. Nat Commun. (2020) 11:1612. doi: 10.1038/s41467-020-15457-9
36. Zheng F, Yang Y, Lu G, Tan JS, Mageswary U, Zhan Y, et al. Metabolomics insights into gut microbiota and functional constipation. Metabolites. (2025) 15:1–20. doi: 10.3390/metabo15040269
37. Kot M. Aryl hydrocarbon receptor establishes a delicate balance between the level of the trace amine tryptamine and monoamine oxidase activity in the brain and periphery in health and conditions such as neurodegenerative, neurodevelopmental, and psychiatric disorders. Curr Neuropharmacol. (2025) 23:1328–50. doi: 10.2174/011570159X340635241022113450
38. Sublette ME, Cheung S, Lieberman E, Hu S, Mann JJ, Uhlemann A-C, et al. Bipolar disorder and the gut microbiome: A systematic review. Bipolar Disord. (2021) 23:544–64. doi: 10.1111/bdi.13049
39. Li Z, Lai J, Zhang P, Ding J, Jiang J, Liu C, et al. Multi-omics analyses of serum metabolome, gut microbiome and brain function reveal dysregulated microbiota-gut-brain axis in bipolar depression. Mol Psychiatry. (2022) 27:4123–35. doi: 10.1038/s41380-022-01569-9
40. Hasegawa S, Goto S, Tsuji H, Okuno T, Asahara T, Nomoto K, et al. Intestinal dysbiosis and lowered serum lipopolysaccharide-binding protein in parkinson’s disease. PloS One. (2015) 10:e0142164. doi: 10.1371/journal.pone.0142164
41. Unger MM, Spiegel J, Dillmann K-U, Grundmann D, Philippeit H, Bürmann J, et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord. (2016) 32:66–72. doi: 10.1016/j.parkreldis.2016.08.019
42. Kang A, Eor JY, Lee J, Kwak M-J, Lee DJ, Seo E, et al. Lacticaseibacillus casei IDCC 3451 alleviates cognitive and behavioral functions by reshaping the gut microbiome and regulating intestinal barrier integrity in chronic stress animal models. Curr Res Food Sci. (2025) 10:101051. doi: 10.1016/j.crfs.2025.101051
43. Qiao L, Chen Y, Song X, Dou X, and Xu C. Selenium nanoparticles-enriched lactobacillus casei ATCC 393 prevents cognitive dysfunction in mice through modulating microbiota-gut-brain axis. Int J Nanomed. (2022) 17:4807–27. doi: 10.2147/IJN.S374024
44. Zhang Y, He X, Qian Y, Xu S, Mo C, Yan Z, et al. Plasma branched-chain and aromatic amino acids correlate with the gut microbiota and severity of Parkinson’s disease. NPJ Parkinsons Dis. (2022) 8:48. doi: 10.1038/s41531-022-00312-z
45. Havelund JF, Heegaard NHH, Færgeman NJK, and Gramsbergen JB. Biomarker research in parkinson’s disease using metabolite profiling. Metabolites. (2017) 7:1–18. doi: 10.3390/metabo7030042
46. Liu J, Lv X, Ye T, Zhao M, Chen Z, Zhang Y, et al. Microbiota-microglia crosstalk between Blautia producta and neuroinflammation of Parkinson’s disease: A bench-to-bedside translational approach. Brain Behav Immun. (2024) 117:270–82. doi: 10.1016/j.bbi.2024.01.010
47. Ray D, Bose P, Mukherjee S, Roy S, and Kaity S. Recent drug delivery systems targeting the gut-brain-microbiome axis for the management of chronic diseases. Int J Pharm. (2025) 680:125776. doi: 10.1016/j.ijpharm.2025.125776
48. Chen C, Liao J, Xia Y, Liu X, Jones R, Haran J, et al. Gut microbiota regulate Alzheimer’s disease pathologies and cognitive disorders via PUFA-associated neuroinflammation. Gut. (2022) 71:2233–52. doi: 10.1136/gutjnl-2021-326269
49. Kesika P, Suganthy N, Sivamaruthi BS, and Chaiyasut C. Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer’s disease. Life Sci. (2021) 264:118627. doi: 10.1016/j.lfs.2020.118627
50. Shen H, Liu K, Kong F, Ren M, Wang X, and Wang S. Strategies for measuring concentrations and forms of amyloid-β peptides. Biosens Bioelectron. (2024) 259:116405. doi: 10.1016/j.bios.2024.116405
51. Yu Y and Zhao F. Microbiota-gut-brain axis in autism spectrum disorder. J Genet Genomics. (2021) 48:755–62. doi: 10.1016/j.jgg.2021.07.001
52. Renaldi R, Wiguna T, Persico AM, and Tanra AJ. p-cresol and p-cresyl sulphate boost oxidative stress: A systematic review of recent evidence. Basic Clin Pharmacol Toxicol. (2025) 137:e70065. doi: 10.1111/bcpt.70065
53. Tran SM-S and Mohajeri MH. The role of gut bacterial metabolites in brain development, aging and disease. Nutrients. (2021) 13:1–40. doi: 10.3390/nu13030732
54. Önal S, SaChadyn-Król M, and Kostecka M. A review of the nutritional approach and the role of dietary components in children with autism spectrum disorders in light of the latest scientific research. Nutrients. (2023) 15:1–22. doi: 10.3390/nu15234852
55. Skogstrand K, Hagen CM, Borbye-Lorenzen N, Christiansen M, Bybjerg-Grauholm J, Bækvad-Hansen M, et al. Reduced neonatal brain-derived neurotrophic factor is associated with autism spectrum disorders. Transl Psychiatry. (2019) 9:252. doi: 10.1038/s41398-019-0587-2
56. Mathew NE, McCaffrey D, Walker AK, Mallitt K-A, Masi A, Morris MJ, et al. The search for gastrointestinal inflammation in autism: a systematic review and meta-analysis of non-invasive gastrointestinal markers. Mol Autism. (2024) 15:4. doi: 10.1186/s13229-023-00575-0
57. Yang B, Wei J, Ju P, and Chen J. Effects of regulating intestinal microbiota on anxiety symptoms: A systematic review. Gen Psychiatr. (2019) 32:e100056. doi: 10.1136/gpsych-2019-100056
58. Zeng C, Yang P, Cao T, Gu Y, Li N, Zhang B, et al. Gut microbiota: An intermediary between metabolic syndrome and cognitive deficits in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. (2021) 106:110097. doi: 10.1016/j.pnpbp.2020.110097
59. Yang M, Cui X, Kong D, Huang X, Zhao G, Li X, et al. The efficacy of Lactobacillus and Bifidobacterium in patients with schizophrenia: a meta-analysis. Eur Arch Psychiatry Clin Neurosci. (2024) 275:1437–51. doi: 10.1007/s00406-024-01935-4
60. Zhou Y, Zheng W, Guo F, Wu S, and Zhong C. The anti-inflammation pharmacodynamics of lithium: Therapy of bipolar disorder. J Psychopharmacol. (2025) 39(6):533–44. doi: 10.1177/02698811251326942
61. Wallen ZD, Demirkan A, Twa G, Cohen G, Dean MN, Standaert DG, et al. Metagenomics of Parkinson’s disease implicates the gut microbiome in multiple disease mechanisms. Nat Commun. (2022) 13:6958. doi: 10.1038/s41467-022-34667-x
62. Zheng S-Y, Li H-X, Xu R-C, Miao W-T, Dai M-Y, Ding S-T, et al. Potential roles of gut microbiota and microbial metabolites in Parkinson’s disease. Ageing Res Rev. (2021) 69:101347. doi: 10.1016/j.arr.2021.101347
63. Bedarf JR, Romano S, Heinzmann SS, Duncan A, Traka MH, Ng D, et al. A prebiotic dietary pilot intervention restores faecal metabolites and may be neuroprotective in Parkinson’s Disease. NPJ Parkinsons Dis. (2025) 11:66. doi: 10.1038/s41531-025-00885-5
64. Hernández-García J, Muro-Reche P, and Orenes-Piñero E. Gut microbiota and microRNAs as biomarkers in Parkinson’s disease: early identification, diagnostic and potential treatments. Mol Cell Biochem. (2025) 480:4573–86. doi: 10.1007/s11010-025-05271-6
65. Doifode T, Giridharan VV, Generoso JS, Bhatti G, Collodel A, Schulz PE, et al. The impact of the microbiota-gut-brain axis on Alzheimer’s disease pathophysiology. Pharmacol Res. (2021) 164:105314. doi: 10.1016/j.phrs.2020.105314
66. Yu R, Hafeez R, Ibrahim M, Alonazi WB, and Li B. The complex interplay between autism spectrum disorder and gut microbiota in children: A comprehensive review. Behav Brain Res. (2024) 473:115177. doi: 10.1016/j.bbr.2024.115177
67. Zarimeidani F, Rahmati R, Mostafavi M, Darvishi M, Khodadadi S, Mohammadi M, et al. Gut microbiota and autism spectrum disorder: A neuroinflammatory mediated mechanism of pathogenesis? Inflammation. (2024) 48:501–19. doi: 10.1007/s10753-024-02061-y
68. Ross FC, Mayer DE, Gupta A, Gill CIR, Del Rio D, Cryan JF, et al. Existing and future strategies to manipulate the gut microbiota with diet as a potential adjuvant treatment for psychiatric disorders. Biol Psychiatry. (2024) 95:348–60. doi: 10.1016/j.biopsych.2023.10.018
69. Mudd AT, Berding K, Wang M, Donovan SM, and Dilger RN. Serum cortisol mediates the relationship between fecal Ruminococcus and brain N-acetylaspartate in the young pig. Gut Microbes. (2017) 8:589–600. doi: 10.1080/19490976.2017.1353849
70. Berthoud H-R, Münzberg H, Morrison CD, and Neuhuber WL. Gut-brain communication: Functional anatomy of vagal afferents. Curr Opin Neurobiol. (2025) 93:103058. doi: 10.1016/j.conb.2025.103058
71. Bitanihirwe BKY, Lizano P, and Woo T-UW. Deconstructing the functional neuroanatomy of the choroid plexus: an ontogenetic perspective for studying neurodevelopmental and neuropsychiatric disorders. Mol Psychiatry. (2022) 27:3573–82. doi: 10.1038/s41380-022-01623-6
72. Wu H, He K, Wang H, Li W, Huo R, Jiang S-H, et al. The gut-brain axis in the context of colorectal cancer. Pharmacol Res. (2025) 217:107816. doi: 10.1016/j.phrs.2025.107816
73. Asker M, Krieger J-P, Maric I, Bedel E, Steen J, Börchers S, et al. Vagal oxytocin receptors are necessary for esophageal motility and function. JCI Insight. (2025) 10:e190108. doi: 10.1172/jci.insight.190108
74. Mörkl S, Narrath M, Schlotmann D, Sallmutter M-T, Putz J, Lang J, et al. Multi-species probiotic supplement enhances vagal nerve function - results of a randomized controlled trial in patients with depression and healthy controls. Gut Microbes. (2025) 17:2492377. doi: 10.1080/19490976.2025.2492377
75. Rao J, Qiu P, Zhang Y, and Wang X. Gut microbiota trigger host liver immune responses that affect drug-metabolising enzymes. Front Immunol. (2024) 15:1511229. doi: 10.3389/fimmu.2024.1511229
76. Wang X, Ye C, Xun T, Mo L, Tong Y, Ni W, et al. Bacteroides fragilis polysaccharide A ameliorates abnormal voriconazole metabolism accompanied with the inhibition of TLR4/NF-κB pathway. Front In Pharmacol. (2021) 12:663325. doi: 10.3389/fphar.2021.663325
77. Deng X, Li Y, Jiang L, Xie X, and Wang X. 1-methylnicotinamide modulates IL-10 secretion and voriconazole metabolism. Front Immunol. (2025) 16:1529660. doi: 10.3389/fimmu.2025.1529660
78. Wang Y-L, Chen L, Zhong X-L, Liu Q-S, Li W-Q, Cheng Y, et al. Antidepressant effects of ershiwei roudoukou pills and its active ingredient Macelignan: Multiple mechanisms involving oxidative stress, neuroinflammation and synaptic plasticity. Transl Psychiatry. (2025) 15:163. doi: 10.1038/s41398-025-03378-4
79. Jaffal SM. Role of TRPV1 in health and disease. J Explor Res Pharmacol. (2023) 8:348–61. doi: 10.14218/jerp.2023.00013
80. Pujo J, De Palma G, Lu J, Galipeau HJ, Surette MG, Collins SM, et al. Gut microbiota modulates visceral sensitivity through calcitonin gene-related peptide (CGRP) production. Gut Microbes. (2023) 15:2188874. doi: 10.1080/19490976.2023.2188874
81. Wheeler MA and Quintana FJ. The neuroimmune connectome in health and disease. Nature. (2025) 638:333–42. doi: 10.1038/s41586-024-08474-x
82. Huang S, Pan L, Pang S, Guo H, Li M, Tian Y, et al. Perforin generated by CD8+ T cells exacerbates IBD-induced depression by promoting CXCL9 production in intestinal epithelial cells. Gastroenterology. (2025) 169:294–307. doi: 10.1053/j.gastro.2025.02.036
83. Park SY, Kim YH, Kim Y, and Lee S-J. Aromatic-turmerone’s anti-inflammatory effects in microglial cells are mediated by protein kinase A and heme oxygenase-1 signaling. Neurochem Int. (2012) 61:767–77. doi: 10.1016/j.neuint.2012.06.020
84. Rieder R, Wisniewski PJ, Alderman BL, and Campbell SC. Microbes and mental health: A review. Brain Behav Immun. (2017) 66:9–17. doi: 10.1016/j.bbi.2017.01.016
85. Liu T, Du D, Zhao R, Xie Q, and Dong Z. Gut microbes influence the development of central nervous system disorders through epigenetic inheritance. Microbiol Res. (2023) 274:127440. doi: 10.1016/j.micres.2023.127440
86. Aziz-Zadeh L, Ringold SM, Jayashankar A, Kilroy E, Butera C, Jacobs JP, et al. Relationships between brain activity, tryptophan-related gut metabolites, and autism symptomatology. Nat Commun. (2025) 16:3465. doi: 10.1038/s41467-025-58459-1
87. Dos Reis RG, Singulani MP, Forlenza OV, Gattaz WF, and Talib LL. Kynurenine pathway metabolite alterations in Down syndrome and Alzheimer’s disease. Alzheimers Dement. (2025) 21:e70197. doi: 10.1002/alz.70197
88. Dodd D, Spitzer MH, Van Treuren W, Merrill BD, Hryckowian AJ, Higginbottom SK, et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature. (2017) 551:648–52. doi: 10.1038/nature24661
89. Yan Y, Ramanan D, Rozenberg M, McGovern K, Rastelli D, Vijaykumar B, et al. Interleukin-6 produced by enteric neurons regulates the number and phenotype of microbe-responsive regulatory T cells in the gut. Immunity. (2021) 54:499–513. doi: 10.1016/j.immuni.2021.02.002
90. Liu M, Blattman SB, Takahashi M, Mandayam N, Jiang W, Oikonomou P, et al. Conserved genetic basis for microbial colonization of the gut. Cell. (2025) 188:2505–20. doi: 10.1016/j.cell.2025.03.010
91. Chen K-H, Lin H-S, Li Y-C, Sung P-H, Chen Y-L, Yin T-C, et al. Synergic effect of early administration of probiotics and adipose-derived mesenchymal stem cells on alleviating inflammation-induced chronic neuropathic pain in rodents. Int J Mol Sci. (2022) 23:1–18. doi: 10.3390/ijms231911974
92. Lama A, Pirozzi C, Severi I, Morgese MG, Senzacqua M, Annunziata C, et al. Palmitoylethanolamide dampens neuroinflammation and anxiety-like behavior in obese mice. Brain Behav Immun. (2022) 102:110–23. doi: 10.1016/j.bbi.2022.02.008
93. Gupta S, Viotti A, Eichwald T, Roger A, Kaufmann E, Othman R, et al. Navigating the blurred path of mixed neuroimmune signaling. J Allergy Clin Immunol. (2024) 153:924–38. doi: 10.1016/j.jaci.2024.02.006
94. Fumagalli A, Castells-Nobau A, Trivedi D, Garre-Olmo J, Puig J, Ramos R, et al. Archaea methanogens are associated with cognitive performance through the shaping of gut microbiota, butyrate and histidine metabolism. Gut Microbes. (2025) 17:2455506. doi: 10.1080/19490976.2025.2455506
95. Zhang M, Ma W, Zhang J, He Y, and Wang J. Analysis of gut microbiota profiles and microbe-disease associations in children with autism spectrum disorders in China. Sci Rep. (2018) 8:13981. doi: 10.1038/s41598-018-32219-2
96. Madany AM, Hughes HK, and Ashwood P. Prenatal maternal antibiotics treatment alters the gut microbiota and immune function of post-weaned prepubescent offspring. Int J Mol Sci. (2022) 23:1–20. doi: 10.3390/ijms232112879
Keywords: gut microbiota, central nervous system, microglia, psychiatric disorders, neuroinflammation
Citation: Zhou L, Wu Q, Jiang L, Rao J, Gao J, Zhao F and Wang X (2025) Role of the microbiota in inflammation-related related psychiatric disorders. Front. Immunol. 16:1613027. doi: 10.3389/fimmu.2025.1613027
Received: 16 April 2025; Accepted: 30 July 2025;
Published: 20 August 2025.
Edited by:
Tommy Regen, Johannes Gutenberg-University Mainz, GermanyReviewed by:
Irena Maria Nalepa, Polish Academy of Sciences, PolandGilberto Pérez-Sánchez, Instituto Nacional de Psiquiatría Ramón de la Fuente Muñiz, Mexico
Copyright © 2025 Zhou, Wu, Jiang, Rao, Gao, Zhao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Zhao, MzE4MzE3NDQ5MUBxcS5jb20=; Xiaokang Wang, a2FuZ3RhZV93b25AaS5zbXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Liying Zhou1†
Liying Zhou1† Jiaoyu Rao
Jiaoyu Rao Xiaokang Wang
Xiaokang Wang