- 1Department of Radiation Oncology, Jiangxi Cancer Hospital and Institute, Jiangxi Clinical Research Center for Cancer, The Second Affiliated Hospital of Nanchang Medical College, Nanchang, Jiangxi, China
- 2Department of Oncology, Nanchang University, Jiangxi Cancer Hospital and Institute, Nanchang, Jiangxi, China
- 3Department of Pathology, Jiangxi Cancer Hospital and Institute, Jiangxi Clinical Research Center for Cancer, The Second Affiliated Hospital of Nanchang Medical College, Nanchang, Jiangxi, China
We present a case of a 68-year-old male with advanced non-small cell lung cancer (NSCLC), PD-L1 negative and driver gene negative, who exhibited a significant abscopal effect following radiotherapy combined with systemic immunotherapy (sintilizumab) and chemotherapy. The patient achieved complete remission (CR) of intracranial metastases without cranial irradiation, suggesting a systemic immune response triggered by the combination of radiotherapy and immunotherapy. This case highlights the potential of radiotherapy combined with immuno-chemotherapy to induce abscopal effects, even in PD-L1 negative patients, and underscores the importance of further investigation into this therapeutic strategy. This case challenges traditional paradigms in NSCLC management and aligns with emerging theragnostic approaches that integrate localized treatment with systemic immune modulation.
Introduction
The management of brain metastases in NSCLC traditionally relies on cranial irradiation (1), but emerging evidence supports synergistic effects of radiotherapy (RT) and immunotherapy. The abscopal effect, a phenomenon where localized RT induces systemic tumor regression at distant sites, has been increasingly reported in the era of immunotherapy (2). This effect is thought to be mediated by the activation of the immune system, particularly through the release of tumor antigens and subsequent immune response (3, 4). While abscopal effects are rare, they have been observed in various cancers, including NSCLC (5), especially when radiotherapy is combined with immune checkpoint inhibitors. Recent genomic studies highlight that homologous recombination deficiency (HRD) may enhance immunogenicity in driver-negative NSCLC (6), while PD-L1 negativity typically correlates with reduced immunotherapy response. Here, we report a PD-L1 negative NSCLC case achieving rapid intracranial remission through abscopal effects.
Case presentation
In August 2020, a 68-year-old male patient presented with a right lower lobe pulmonary nodule. Following guidelines at the time, he underwent surgical resection of the primary tumor. The postoperative pathological diagnosis was stage IA (pT1N0M0) right lower lobe adenocarcinoma. No adjuvant therapy was administered. RNA sequencing testing revealed no detectable alterations in the tested driver genes (EGFR, ALK, ROS1, RET, KRAS, BRAF, MET, HER2, and NTRK were all negative). Due to limitations in economic resources and access to advanced clinical testing, extended molecular profiling—including Tumor Mutational Burden (TMB), Microsatellite Instability-High (MSI-H), or Mismatch Repair Deficiency (dMMR)—could not be performed. However, immunohistochemistry (IHC) confirmed negative PD-L1 expression (Supplementary Figure 1).
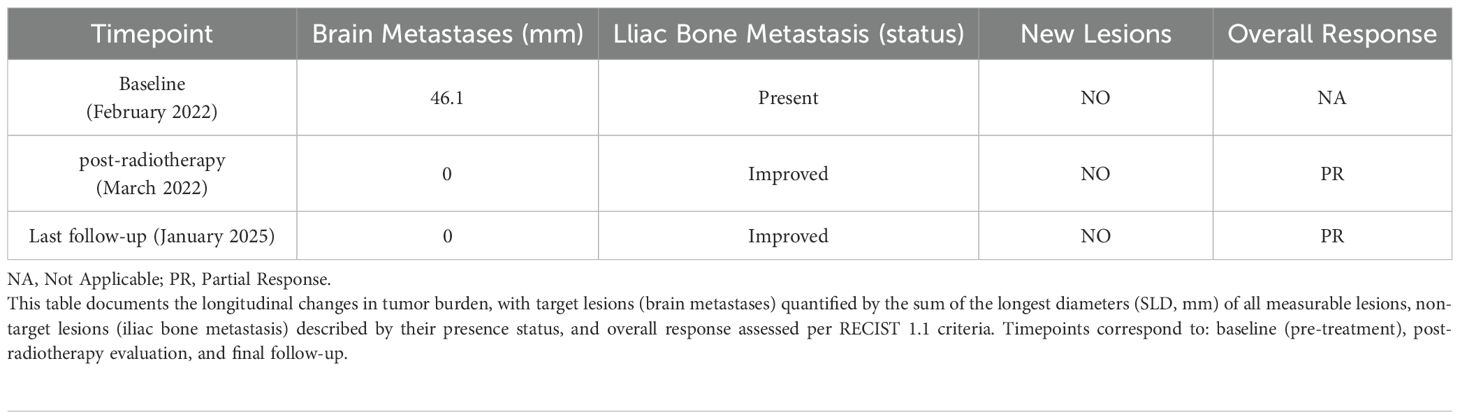
In February 2022, the patient developed left iliac bone metastasis and multiple intracranial metastases (Figure 1), with no new lesions detected at other sites. Given the patient’s left iliac bone pain but absence of central nervous system symptoms, we employed intensity-modulated radiation therapy (IMRT) targeting only the left iliac bone metastatic lesion with a total dose of 36 Gy in 12 fractions to alleviate pain symptoms. (Supplementary Figure 2). During the treatment of RT, the patient received one cycle of pemetrexed disodium(500mg/m2), cisplatin(75mg/m2), and sintilizumab (200mg,an anti-PD-1 antibody). Notably, follow-up MRI revealed complete remission (CR) of multiple brain metastases (Figure 1), despite the absence of cranial irradiation one month later.
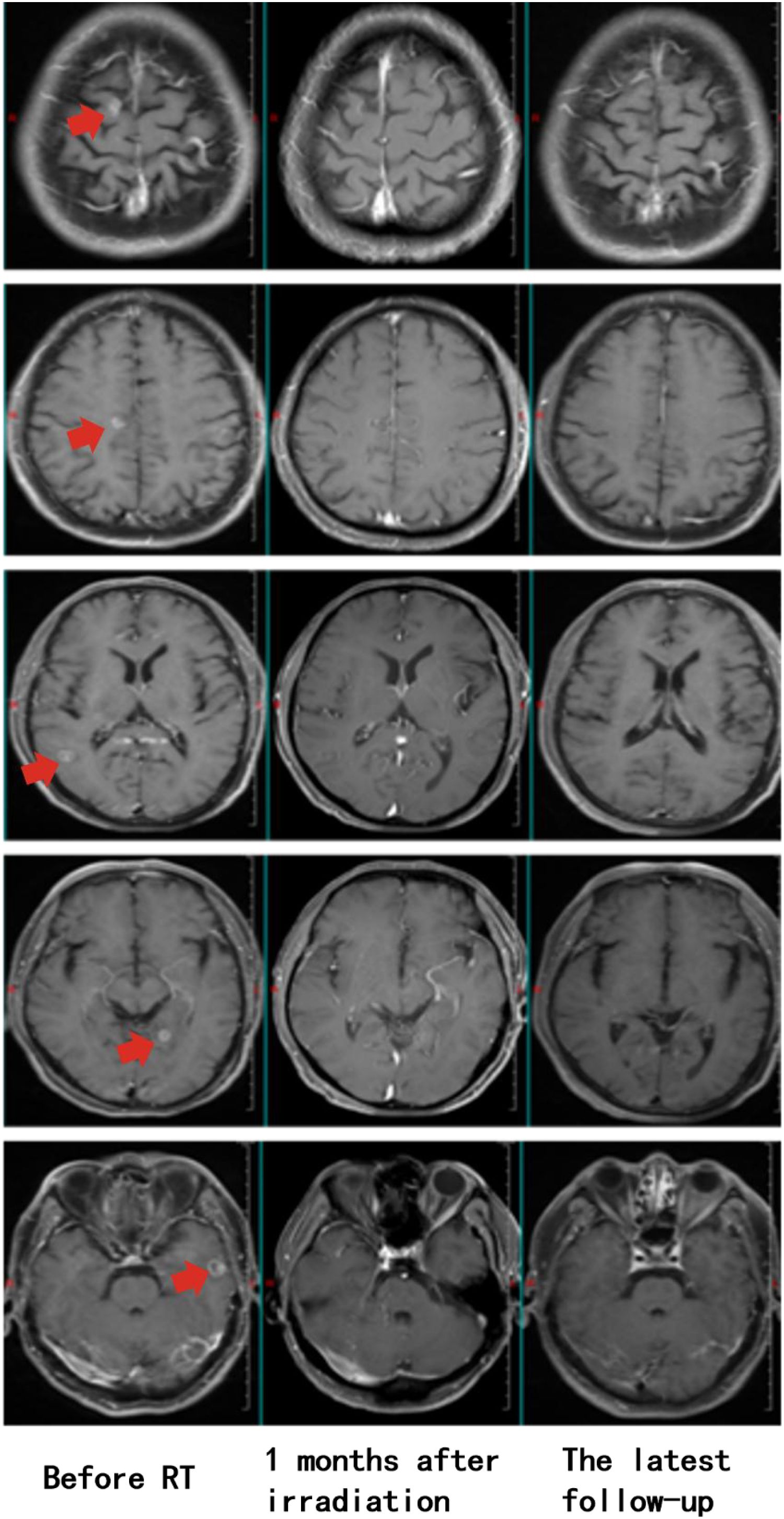
Figure 1. Longitudinal assessment of brain metastasis status following radiotherapy. Pre-treatment imaging (February 7, 2022) confirmed the presence of multiple intracranial metastases prior to radiotherapy for the left iliac bone lesion (Left). Post-therapeutic evaluation at one-month follow-up (March 12, 2022) demonstrated complete radiological response, with no detectable metastatic lesions (Middle). Sustained complete remission was maintained throughout the entire follow-up period, with no evidence of disease recurrence on final imaging studies (January 23, 2025, 35 months post-treatment) (Right).
Subsequently, the patient underwent two cycles of systemic treatment with pemetrexed disodium (500mg/m2), cisplatin (75mg/m2), and sintilimab (200mg), during which one episode of Grade 3 rash and gastrointestinal reaction occurred. The treatment was then switched to two cycles of carboplatin (AUC=5) combined with pemetrexed disodium (500mg/m2) and sintilimab (200mg). Finally, Patients received maintenance treatment with sintilimab (200mg) for 24 months until the last follow-up with or without pemetrexed disodium (500mg/m2) alternatively, during which no Grade 2 or higher adverse reactions have occurred. Until the last follow-up, the patient’s lung lesions (Supplementary Figure 3), bone metastases (Supplementary Figure 4), and brain metastases (Figure 1) remained stable, with the progression-free survival (PFS) of 35 months and an overall survival (OS) exceeding 40 months (Table 1). Prior to each treatment session, peripheral blood tumor markers—including carcinoembryonic antigen (CEA) and cytokeratin 19 fragment (CYFRA 21-1)-were routinely monitored. During the post-treatment surveillance phase after completing the two-year therapeutic regimen, assessment frequency was reduced to quarterly intervals. Results demonstrated progressive decline of these biomarkers, ultimately stabilizing at low levels (Figure 2). Concurrently, peripheral CD8+ T-cell counts were evaluated at identical time points using flow cytometry (BD FACSCanto II) with CD3+/CD8+ antibodies, with data indicating persistently elevated levels throughout the observation period (Figure 2). Next-generation sequencing of archival tumor tissue identified a TP53 mutation (VAF 4.8%).The sustained remission of both irradiated and non-irradiated lesions, particularly the brain metastases, underscores the potential of this combined approach to achieve durable disease control in advanced NSCLC, even in PD-L1 negative patients.
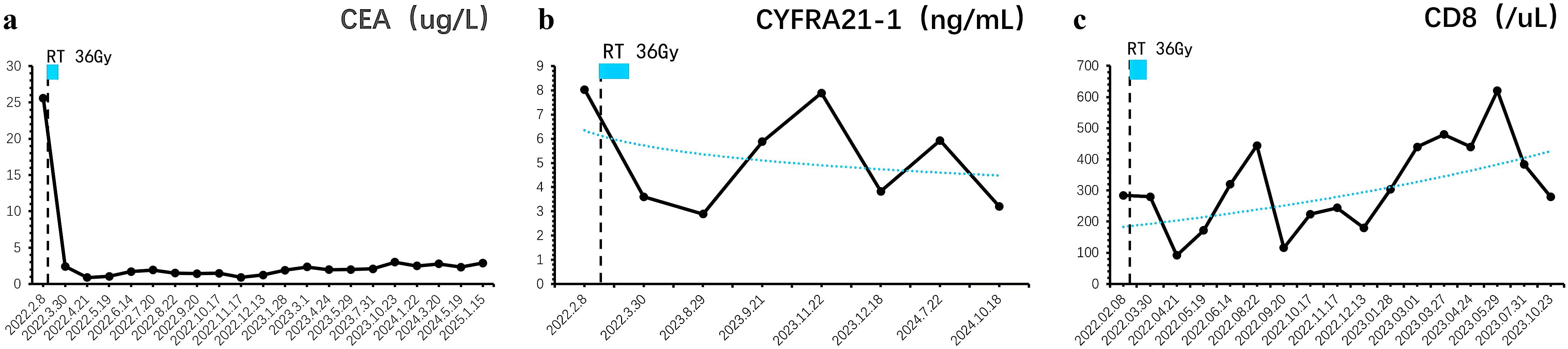
Figure 2. Longitudinal monitoring of tumor markers and immune response dynamics. (a) Carcinoembryonic antigen (CEA) levels exhibited a progressive decline post-treatment, eventually stabilizing at baseline values. (b) Similarly, cytokeratin 19 fragment (CYFRA 21-1) concentrations demonstrated a sustained reduction, reaching undetectable or minimal levels during follow-up. (c) Peripheral immunophenotyping revealed persistently elevated CD8+ T-cell counts, indicative of a robust and durable antitumor immune response.
Discussion and conclusion
This case report describes a 68-year-old male with advanced, PD-L1 negative, driver gene-negative NSCLC who achieved complete remission of intracranial metastases after a single cycle of combined radiotherapy, immunotherapy (sintilizumab), and chemotherapy, without cranial irradiation. The treatment, which included IMRT to a left iliac bone metastasis, triggered a systemic immune response, leading to durable disease control and a PFS of 35 months. This notable outcome highlights the potential of combining radiotherapy with immuno-chemotherapy to induce abscopal effects, even in traditionally less responsive PD-L1 negative patients.
The case underscores the importance of multimodal approaches in achieving long-term survival and challenges current paradigms in the management of advanced NSCLC. While the abscopal effect—where localized RT induces systemic tumor regression—has been reported in NSCLC (7–10), the speed of intracranial response in this case is exceptional. The likely mechanism involves RT-induced immunogenic cell death, releasing tumor antigens and damage-associated molecular patterns (DAMPs) that activate dendritic cells and prime tumor-specific T cells (11). The addition of sintilizumab, a PD-1 inhibitor, further amplified this immune response by reversing T cell exhaustion, enabling systemic tumor control, including in the brain (12). Additionally, the patient’s TP53 mutation, which is associated with increased tumor mutational burden and immunogenicity, may have contributed to the robust abscopal effect by enhancing the presentation of neoantigens and promoting a stronger immune response post-radiotherapy (13).Although the rapid intracranial response observed in this case strongly suggests an abscopal effect, we acknowledge that systemic immunotherapy (sintilimab) and chemotherapy may have contributed to the control of brain metastases. Previous studies indicate that PD-1 inhibitors can cross the blood-brain barrier and exert effects on brain metastases (14, 15). Furthermore, pemetrexed, when combined with platinum-based chemotherapy, has also been reported to exhibit limited intracranial activity (16). However, the observation of a complete response within one month following radiotherapy and just one cycle of systemic therapy is more consistent with the characteristics of a radiation-induced abscopal effect—as the typical response time for systemic therapy alone is usually longer. This case highlights the synergistic potential of radiotherapy combined with immunochemotherapy in achieving rapid and durable systemic responses, even in challenging cases such as advanced non-small cell lung cancer with brain metastases.
The second highlight of this case is the remarkable PFS of 35 months, achieved through maintenance therapy with pemetrexed and sintilimab following initial treatment. The sustained high levels of CD8+ T cells in the patient’s peripheral blood, coupled with low tumor burden, likely contributed to this durable response. RT-induced immunogenic cell death and sintilizumab’s blockade of PD-1/PD-L1 signaling may have synergistically maintained CD8+ T cell activation, preventing T cell exhaustion and promoting continuous anti-tumor immunity (10, 17–19). Additionally, it is worth noting that pemetrexed may enhance immune efficacy through a triple mechanism: (1) up-regulating PD-L1 expression in tumor cells (20); (2) Reduce Treg cell infiltration (21, 22); (3) enhance the sensitivity of tumor cells to T cell killing (23). These mechanisms may work synergistically with radiotherapy and immunotherapy. Previous studies have shown that high peripheral CD8+ T cell levels correlate with improved survival in NSCLC patients receiving immunotherapy (24, 25), as these cells play a critical role in tumor cell recognition and elimination. This case underscores the importance of combining radiotherapy, immunotherapy, and chemotherapy to sustain immune activation and achieve long-term disease control, even in advanced NSCLC with high-risk features.
This case represents a rare and remarkable example of rapid intracranial remission and long-term survival in a PD-L1 negative, driver gene-negative NSCLC patient treated with a combination of RT and immuno-chemotherapy. The rapid speed of intracranial response and sustained disease control highlight the potential of multimodal therapy to induce systemic immune activation and achieve durable outcomes in traditionally challenging cases. The integration of localized RT with systemic immuno-chemotherapy exemplifies a theragnostic approach (26), where molecular imaging (e.g., CXCR4-targeted PET) could non-invasively monitor immune activation during combined modality therapy. Such approaches may optimize RT/immunotherapy sequencing in PD-L1 negative NSCLC. Future studies could utilize patient-derived organoids (PDOs) to model such abscopal responses. As demonstrated in NSCLC PDOs (27), these models can recapitulate tumor-immune interactions and predict combinatorial therapy efficacy, potentially identifying biomarkers for patient selection.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Jiangxi Cancer Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
QW: Writing – original draft, Data curation. WW: Data curation, Writing – original draft. KZ: Writing – original draft, Visualization. CP: Writing – review & editing, Methodology. ZL: Supervision, Writing – review & editing. LW: Writing – review & editing, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1613974/full#supplementary-material
References
1. Nardone V, Romeo C, D’Ippolito E, et al. The role of brain radiotherapy for EGFR- and ALK-positive non-small-cell lung cancer with brain metastases: a review. Radiol Med. (2023) 128:316–29. doi: 10.1007/s11547-023-01602-z
2. Mondini M, Levy A, Meziani L, et al. Radiotherapy-immunotherapy combinations - perspectives and challenges. Mol Oncol. (2020) 14:1529–37. doi: 10.1002/1878-0261.12658
3. Mclaughlin M, Patin EC, Pedersen M, et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat Rev Cancer. (2020) 20:203–17. doi: 10.1038/s41568-020-0246-1
4. Coleman CN, Eke I, Makinde AY, et al. Radiation-induced adaptive response: new potential for cancer treatment. Clin Cancer Res. (2020) 26:5781–90. doi: 10.1158/1078-0432.CCR-20-0572
5. Wang Y, Ma Y, He L, et al. Clinical and molecular significance of homologous recombination deficiency positive non-small cell lung cancer in Chinese population: An integrated genomic and transcriptional analysis. Chin J Cancer Res. (2024) 36:282–97. doi: 10.21147/j.issn.1000-9604.2024.03.05
6. Brix N, Tiefenthaller A, Anders H, et al. Abscopal, immunological effects of radiotherapy: Narrowing the gap between clinical and preclinical experiences. Immunol Rev. (2017) 280:249–79. doi: 10.1111/imr.12573
7. Golden EB, Demaria S, Schiff PB, et al. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res. (2013) 1:365–72. doi: 10.1158/2326-6066.CIR-13-0115
8. Britschgi C, Riesterer O, Burger IA, et al. Report of an abscopal effect induced by stereotactic body radiotherapy and nivolumab in a patient with metastatic non-small cell lung cancer. Radiat Oncol. (2018) 13:102. doi: 10.1186/s13014-018-1049-3
9. Sakaguchi T, Ito K, Fujiwara K, et al. An oldest-old non-small cell lung cancer patient with abscopal effect in a single lesion. Thorac Cancer. (2022) 13:2267–70. doi: 10.1111/1759-7714.14551
10. Morita Y, Saijo A, Nokihara H, et al. Radiation therapy induces an abscopal effect and upregulates programmed death-ligand 1 expression in a patient with non-small cell lung cancer. Thorac Cancer. (2022) 13:1079–82. doi: 10.1111/1759-7714.14330
11. Liu T, Pei P, Shen W, et al. Radiation-induced immunogenic cell death for cancer radioimmunotherapy. Small Methods. (2023) 7:e2201401. doi: 10.1002/smtd.202201401
12. Chow A, Perica K, Klebanoff CA, et al. Clinical implications of T cell exhaustion for cancer immunotherapy. Nat Rev Clin Oncol. (2022) 19:775–90. doi: 10.1038/s41571-022-00689-z
13. Ar C and Rg B. The p53 protein family and radiation sensitivity: Yes or no? Cancer Metastasis Rev. (2004) 23:237–57. doi: 10.1023/B:CANC.0000031764.81141.e4
14. Yan X, Qu F, and Zhou Y. Progress of immune checkpoint inhibitors therapy for non-small cell lung cancer with brain metastases. Lung Cancer. (2023) 184:107322. doi: 10.1016/j.lungcan.2023.107322
15. Yang G, Xing L, and Sun X. Navigate towards the immunotherapy era: value of immune checkpoint inhibitors in non-small cell lung cancer patients with brain metastases. Front Immunol. (2022) 13:852811. doi: 10.3389/fimmu.2022.852811
16. Barlesi F, Gervais R, Lena H, et al. Pemetrexed and cisplatin as first-line chemotherapy for advanced non-small-cell lung cancer (NSCLC) with asymptomatic inoperable brain metastases: a multicenter phase II trial (GFPC 07-01). Ann Oncol. (2011) 22:2466–70. doi: 10.1093/annonc/mdr003
17. Brooks ED, Schoenhals JE, Tang C, et al. Stereotactic ablative radiation therapy combined with immunotherapy for solid tumors. Cancer J. (2016) 22:257–66. doi: 10.1097/PPO.0000000000000210
18. Galluzzi L, Vitale I, Warren S, et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J Immunother Cancer. (2020) 8. doi: 10.1136/jitc-2019-000337corr1
19. Kong Y, Zhao X, Xu M, et al. PD-1 inhibitor combined with radiotherapy and GM-CSF (PRaG) in patients with metastatic solid tumors: an open-label phase II study. Front Immunol. (2022) 13:952066. doi: 10.3389/fimmu.2022.952066
20. Cavazzoni A, Digiacomo G, Alfieri R, et al. Pemetrexed enhances membrane PD-L1 expression and potentiates T cell-mediated cytotoxicity by anti-PD-L1 antibody therapy in non-small-cell lung cancer. Cancers (Basel). (2020) 12:666. doi: 10.3390/cancers12030666
21. Anraku M TT, Wu L, et al. Synergistic antitumor effects of regulatory T cell blockade combined with pemetrexed in murine Malignant mesothelioma. J Immunol. (2010) 185:956–66. doi: 10.4049/jimmunol.0900437
22. Lu CS, Lin CW, Chang YH, et al. Antimetabolite pemetrexed primes a favorable tumor microenvironment for immune checkpoint blockade therapy. J Immunother Cancer. (2020) 8:e001392. doi: 10.1136/jitc-2020-001392
23. Okimoto T, Kotani H, Iida Y, et al. Pemetrexed sensitizes human lung cancer cells to cytotoxic immune cells. Cancer Sci. (2020) 111:1910–20. doi: 10.1111/cas.14401
24. Thommen DS, Koelzer VH, Herzig P, et al. A transcriptionally and functionally distinct PD-1(+) CD8(+) T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat Med. (2018) 24:994–1004. doi: 10.1038/s41591-018-0057-z
25. Seo AN, Lee HJ, Kim EJ, et al. Tumour-infiltrating CD8+ lymphocytes as an independent predictive factor for pathological complete response to primary systemic therapy in breast cancer. Br J Cancer. (2013) 109:2705–13. doi: 10.1038/bjc.2013.634
26. Wu R, Zhu W, Shao F, et al. Expanding horizons in theragnostics: from oncology to multidisciplinary applications. Radiol Med. (2025) 130:613–28. doi: 10.1007/s11547-025-01971-7
Keywords: NSCLC, brain metastases, abscopal effect, PD-L1 negative, immuno-chemotherapy
Citation: Wen Q, Wang W, Zhang K, Pan C, Liu Z and Wang L (2025) Case Report: Abscopal effect and long-term survival in a PD-L1 negative NSCLC patient treated with radiotherapy and immuno-chemotherapy. Front. Immunol. 16:1613974. doi: 10.3389/fimmu.2025.1613974
Received: 18 April 2025; Accepted: 06 August 2025;
Published: 22 August 2025.
Edited by:
Savvas Lampridis, Imperial College London, United KingdomReviewed by:
Dechao Feng, University College London, United KingdomCarrie Anne Minnaar, Wits University Donald Gordon Medical Centre, South Africa
Copyright © 2025 Wen, Wang, Zhang, Pan, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Wang, d2FuZ2xlaXkwMDFAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Qiang Wen
Qiang Wen Weiqi Wang
Weiqi Wang Ke Zhang
Ke Zhang Chunguo Pan1
Chunguo Pan1 Zhihua Liu
Zhihua Liu