- Ophthalmologic Center of the Second Hospital, Jilin University, Changchun, China
Noninfectious uveitis (NIU) is a vision-threatening autoimmune disease of the eye, but its pathogenesis is still not fully understood. Recently, accumulating evidence suggests that gut microbiome dysbiosis may affect the development and progression of NIU through potential mechanisms, including translocation, molecular mimicry, and bystander activation. Understanding the mechanisms of gut microbiome-host interactions, especially the gut-eye axis regulation, can offer a theoretical foundation for developing novel therapeutic strategies. We summarized current evidence on the dysregulation of gut microbiome and metabolites in NIU, and explored potential mechanisms involved. Furthermore, possible therapeutic measures are discussed, including probiotics, prebiotics, dietary modifications, antibiotic interventions, as well as fecal microbial transplantation, aiming to exert beneficial effects on NIU progression by reshaping the gut microbial composition.
1 Introduction
Uveitis is a series of intraocular inflammatory diseases involving the iris, ciliary body, choroid and adjacent structures (including the retina and optic nerve). It is one of the common causes of blindness, accounting for 15% of global vision impairment cases and 25% of blindness cases in developing countries (1, 2). Noninfectious uveitis (NIU) is presumed to be autoimmune-mediated uveitis. While a subset of cases is associated with systemic autoimmune diseases, such as Behcet’s disease (BD) and Vogt-Koyanagi-Harada syndrome (VKH), the majority remain idiopathic (3, 4).
The pathogenesis of NIU is thought to involve both genetic and environmental factors. Among genetic predispositions, specific human leukocyte antigen (HLA) genes are strongly implicated, such as HLA-B51 in BD (5), HLA-DR4/DR53 in VKH (6), and HLA-B27 in acute anterior uveitis (AAU) (7). As for environmental factors, increasing evidence has found a critical role for the gut microbiome in modulating immune responses relevant to NIU. However, it can also be modulated by multiple factors, including smoking, diet habits, drugs, age, and psychological stress (Figure 1) (8, 9). The gut microbiome is a complex microecosystem comprising bacteria, viruses, fungi, archaea, and thousands of unique metabolites that collectively shape local and systemic immune responses. Emerging evidence suggests that dysbiosis of the gut microbiome may be associated with disruptions in the balance between pro-inflammatory Th1/Th17 cells and Treg cells (10, 11), which is a key immunological hallmark of NIU. However, whether gut microbiome dysbiosis acts as a trigger and modulator of autoimmune responses in the context of NIU, or is a consequence of inflammation-driven immune imbalance and metabolic alterations during disease progression, remains a subject of debate.
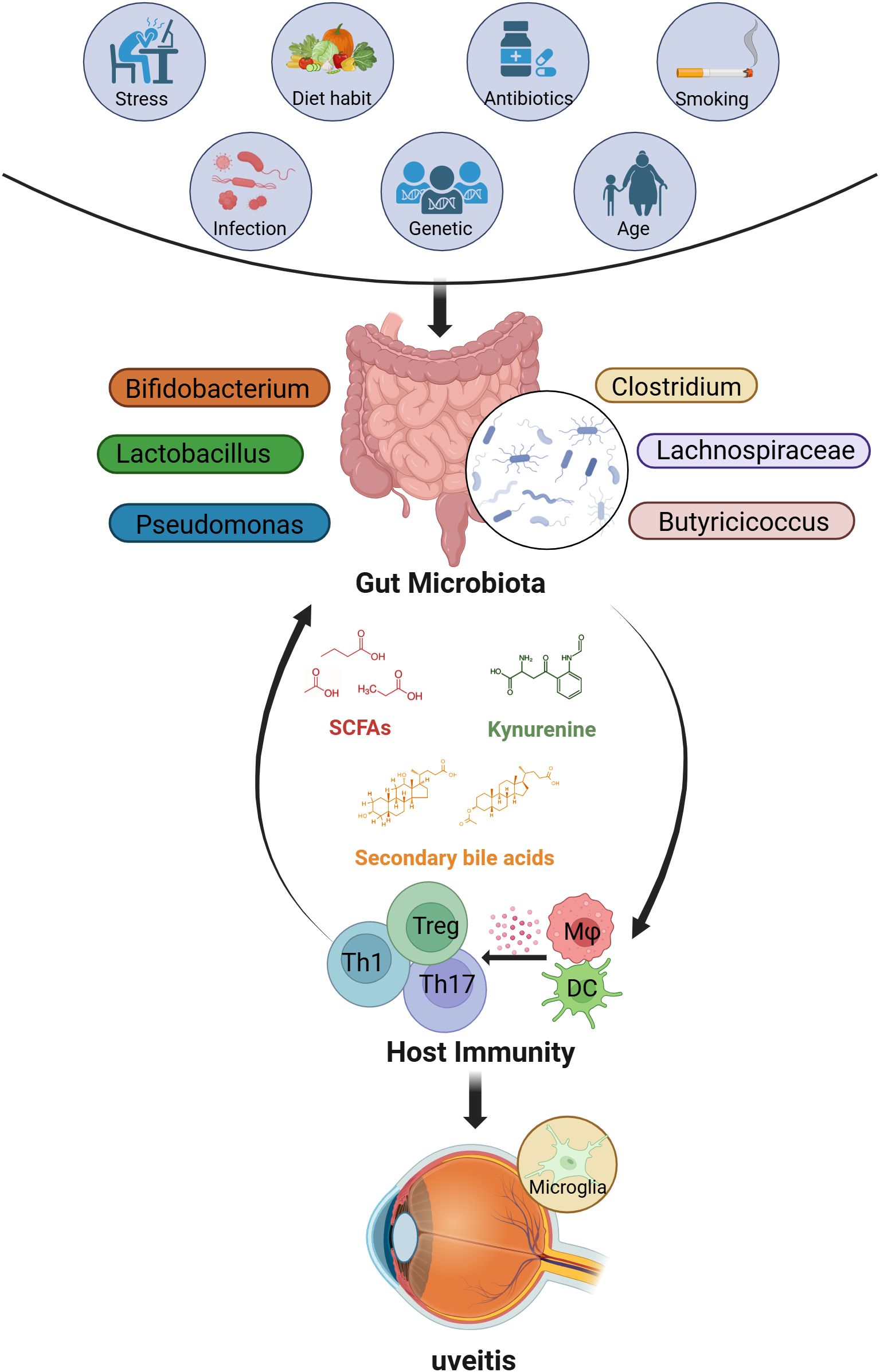
Figure 1. The factors that influence the gut microbiome and possible interactions between the gut and eye in uveitis. Many factors (such as stress, diet, antibiotics, smoking, infections, genetics, and age) may alter the composition of the gut microbiome. In most cases, these factors lead to changes in the abundance of SCFAs-producing bacteria (such as Clostridium, Lachnospiraceae, Butyricicoccus), lactic acid-producing bacteria (such as Bifidobacterium, Lactobacillus), and opportunistic pathogens like Pseudomonas, thereby affecting the levels of related metabolites (including SCFAs, secondary bile acids, and tryptophan metabolites such as kynurenine). Ultimately, an imbalance in the gut microbiome may regulate host immunity and drive the development of uveitis. (Created with BioRender.com) SCFAs, short-chain fatty acids; Th, T helper cells; Treg, regulatory T cells; Mφ, macrophage; DC, Dendritic cells.
Based on current evidence, we hypothesize that dysbiosis of the gut microbiome and its metabolites trigger gut inflammation and increase intestinal permeability (“leaky gut”). This allows the microbiome and its components to translocate into the lamina propria and systemic circulation, promoting aberrant immune activation. Moreover, gut microbiome and metabolites that have entered the lamina propria may further induce cross-reactive T cell responses or amplify the immune activation state through mechanisms such as molecular mimicry and bystander activation, thereby contributing to the initiation and progression of NIU. In this review, we discuss the alterations of gut microbiome and its metabolites in NIU and outline the potential mechanisms linking these changes to the disease. Finally, we propose future directions for microbiota-targeted interventions as emerging therapeutic strategies for NIU.
2 Gut microbiome dysbiosis in NIU
2.1 Dysbiosis of gut bacteriome
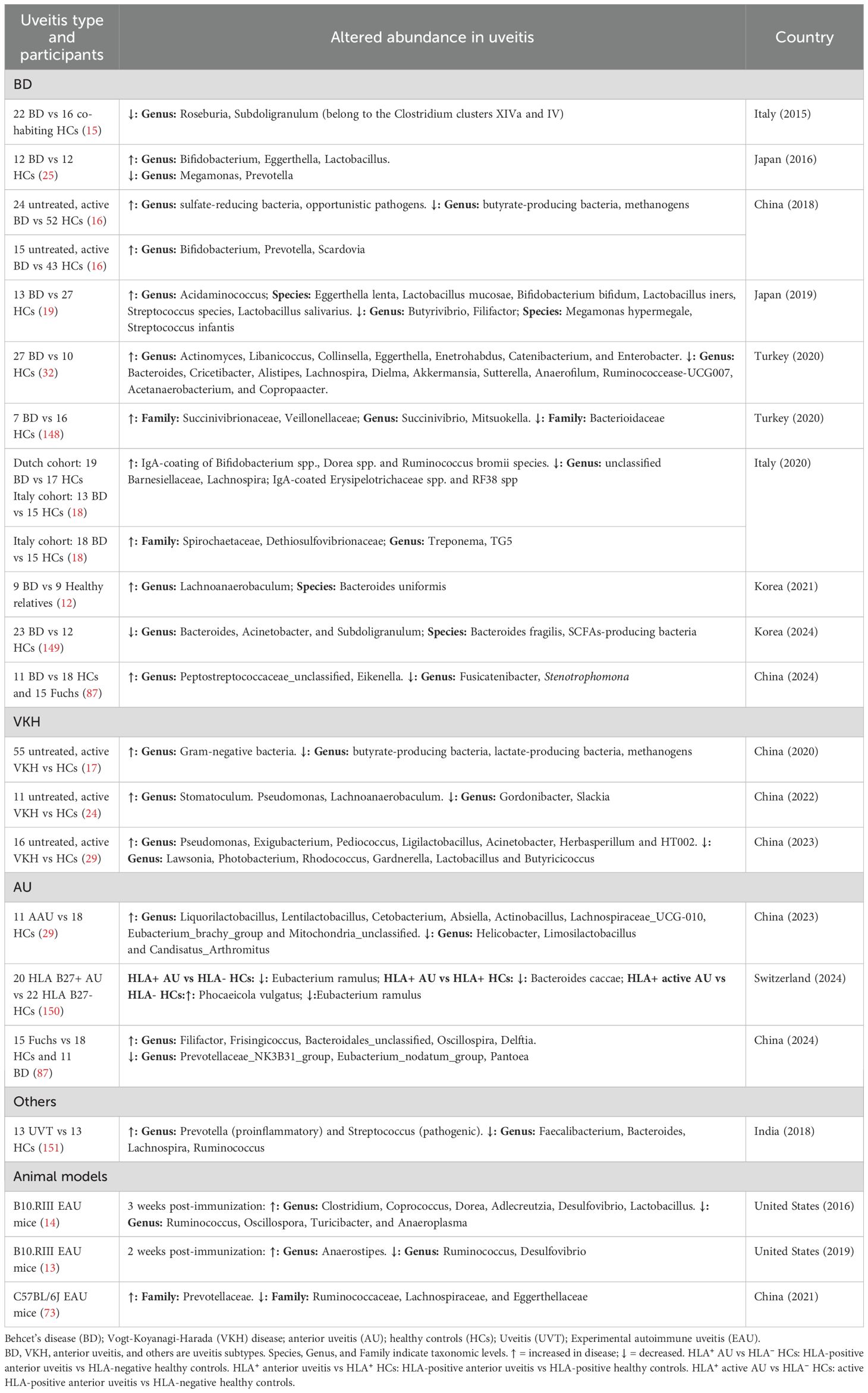
Multiple studies have shown gut microbiome dysbiosis in patients with NIU (Table 1). Although the composition of gut microbiome is influenced by various factors, such as the study population, sample collection, detecting techniques, and geographic region, representative patterns of dysbiosis can still be consistently identified. These patterns primarily include (1) changes in microbial diversity and (2) compositional shifts in specific bacterial taxa.
2.1.1 Changes in microbial diversity
Most studies reported that the overall community structure of patients with uveitis differed from that of healthy controls, with significant changes in both α and β diversity. A longitudinal analysis of BD patients showed that during transition from active to inactive disease stages, α diversity dropped significantly (12). Similar diversity fluctuations have been observed in experimental autoimmune uveitis (EAU) models, indicating dynamic microbial instability during disease progression (13, 14).
2.1.2 Compositional shifts in specific bacterial taxa
A prominent feature of composition dysbiosis in NIU is the reduction of beneficial SCFA-producing bacteria, including Clostridium clusters XIVa and IV (15–17), Lachnospiraceae (18), and Butyricicoccus (19). These bacteria possess anti-inflammatory properties, and their decreased abundance may reduce intestinal SCFA levels, which triggers Th17/Treg immune imbalance and disrupts the intestinal barrier function, affecting immune homeostasis (20, 21).
Another characteristic of composition dysbiosis involves shifts in lactic acid-producing bacteria, which vary across NIU subtypes. Many strains of lactic acid-producing bacteria, such as Bifidobacterium and Lactobacillus, are widely used to maintain gut homeostasis and modulate immune responses (22, 23). In patients with active VKH, the abundance of Bifidobacterium and Lactobacillus is significantly reduced (17, 24), which may impair intestinal barrier function and exacerbate systemic inflammation. However, in BD patients, Bifidobacterium and Lactobacillus are significantly increased (19, 25), and this change may influence the intestinal microenvironment through multiple mechanisms. On one hand, excessive production of lactic acid may lower the intestinal pH, inhibiting the survival of certain commensal bacteria and thereby disrupting gut microbiome balance (26). On the other hand, the abundance of sulfate-reducing bacteria (SRB) is also significantly increased in BD patients (16). SRB competes with butyrate-producing bacteria for substrates, utilizing lactic acid to generate the cytotoxic byproduct hydrogen sulfide, such as H2S, which exacerbates pro-inflammatory responses and impairs intestinal epithelial barrier function (27, 28). Furthermore, different bacterial strains may exhibit distinct metabolic profiles and immune mechanisms. These differences suggest that different types of uveitis may involve distinct patterns and mechanisms of intestinal microbiome imbalance, and provide potential directions for future personalized therapeutic strategies targeting intestinal flora.
-Overgrowth of opportunistic pathogens is another characteristic pattern of taxonomic dysbiosis in NIU. Our research group consistently observed a significant enrichment of Pseudomonas in VKH patients across two independent cohorts (24, 29). Further analysis revealed that the abundance of Pseudomonas was significantly higher in VKH patients compared to those with noninfectious scleritis or AAU, suggesting its potential critical role in the onset or progression of VKH. Pseudomonas can produce lipopolysaccharide (LPS) and peptidoglycan (PGN), which act on Toll-like receptor 4 (TLR4) and NOD-like receptors (NLRs), thereby activating host immune responses (30). Additionally, we also found that the abundance of Pseudomonas was significantly negatively correlated with biotin (vitamin B7) levels (29). Long-term biotin deficiency can lead to symptoms such as alopecia and poliosis, which are hallmark clinical manifestations of VKH patients (31).
In addition to baseline dysbiosis, disease stage and treatment status also influence gut microbiota taxonomic composition. By comparing the gut microbiome of VKH patients before and after immunosuppressive treatment (with active intraocular inflammation before treatment and without intraocular inflammation after treatment), Zi et al. found that composition dysbiosis partially recovered after treatment, in parallel with the resolution of intraocular inflammation. The primary bacterial alterations included a decreased abundance of Acidaminococcus sp. BV3L6 (positively correlated with VKH), alongside increased abundance of Proteobacteria bacterium CAG495, Azospirillum sp. CAG260, and Alistipes sp. CAG435 (negatively correlated with VKH) (17). Meanwhile, they also observed that Bacteroides. spp, Prevotella. spp, Paraburkholdria. spp and Listeria. spp were associated with recurrence after treatment (17). Parallel findings emerged in EAU. In B10.RIII mice immunized with interphotoreceptor retinoid-binding protein 161-180 (IRBP161-180), gut microbiota composition was analyzed on day 7 (pre-onset), day 14 (peak onset), and day 21 (chronic phase). The differences in bacterial composition became more pronounced as the disease progressed. Notably, Desulfovibrio was enriched in the non-immunized group during the peak phase, while it was enriched in the immunized group at the chronic phase (13, 14). Subgroup analyses based on clinical phenotypes in BD also revealed distinctive microbial signatures. The skin mucosal group was characterized by Dialister, Intestinomonas, and Marvinbryantia, the vasculitis group by Gemella, and the uveitis group by Lachnospiraceae NK4A136 (32). Another study also found that in patients with active BD, the abundance of Bifidobacterium adolescentis was higher in those with uveitis compared to those without uveitis (12). These findings suggest that while certain dysbiosis patterns are shared among NIU patients, disease activity, treatment, and clinical subtype are important modifiers of gut microbial structure, which may play distinct roles in different inflammatory mechanisms.
2.2 Dysbiosis of gut mycobiome
In healthy individuals, fungi constitute only a minor component of the gastrointestinal microbiome, representing approximately 0.01% to 0.1% of metagenomic reads in fecal samples (33). Compared to the bacterial microbiome, the gut mycobiota displays markedly lower diversity and is predominantly composed of Saccharomyces, Malassezia, and Candida (34). Compared to healthy controls, nine fungal genera were found to be significantly enriched in patients with uveitis. Among them, Aspergillus gracilis, Candida glabrata, Malassezia globosa, M. restricta, and Issatchenkia sp. AUMC 7766 are known opportunistic pathogens (35). Furthermore, gut microbiome interaction network analysis revealed multiple positive or negative correlations between fungal and bacterial taxa in uveitis patients (35), indicating that fungi may contribute to microbial dysbiosis. Similarly, in the EAU mouse model, antibiotic-induced bacterial depletion was accompanied by an overgrowth of gut fungi. This dynamic shift may be associated with reduced bacterial competition, ecological niche vacancy, and changes in host immune responses (36).
2.3 Dysbiosis of gut virome
The human virome is predominantly composed of eukaryotic viruses (viruses that target human cells) and prokaryotic viruses (viruses that infect bacteria and archaea). Eukaryotic viruses account for less than 10% of the virome and primarily include herpesviruses, anelloviruses, and adenoviruses. In most cases, these viruses remain in a latent or dormant state and may contribute to the initiation of immune responses (37). Bacteriophages (also known as bacterial viruses) far exceed the proportion of eukaryotic viruses, accounting for over 90% (37). With advances in high-throughput sequencing technologies (such as metagenomic sequencing) and bioinformatics analyses, numerous studies have revealed the complex relationships between the gut virome—particularly bacteriophages—and human diseases, including metabolic (38, 39) and autoimmune disorders (40, 41). The dominant phages found in the gut include Caudovirales (dsDNA viruses), Microviridae (ssDNA viruses), and cross-assembly phages (crAssphages) (42). These phages may influence human health either by modulating the bacterial microbiome or through direct interactions with the host immune system.
Viral infection may serve as a potential trigger for uveitis. Epstein-Barr virus (EBV) DNA was detected by polymerase chain reaction (PCR) in the vitreous fluid and cerebrospinal fluid of VKH patients (43, 44). In VKH disease, tyrosinase and gp100 are key target antigens involved in the pathogenic immune response. Database screening revealed that the tyrosinase peptide 450–462 shared a homologous amino acid sequence with the cytomegalovirus envelope glycoprotein H peptide (CMV-egH290-302), and further studies confirmed cross-reactive T cell responses between the two peptides (45, 46). Similarly, preliminary evidence suggests that BD may also be associated with viral infections, including EBV, CMV, herpes simplex virus type 1 (HSV-1), varicella-zoster virus (VZV) (47). Early studies have reported the detection of HSV-1 DNA in peripheral blood leukocytes, saliva, and oral ulcer samples of BD patients (48, 49). To date, the most widely accepted theory regarding infectious triggers of BD is that certain microbial antigens share high sequence homology with human proteins, thereby inducing cross-reactive immune responses (50). However, no studies have yet specifically examined the role of the gut virome in uveitis, and its potential role remains to be elucidated.
3 Gut metabolite disturbances in NIU
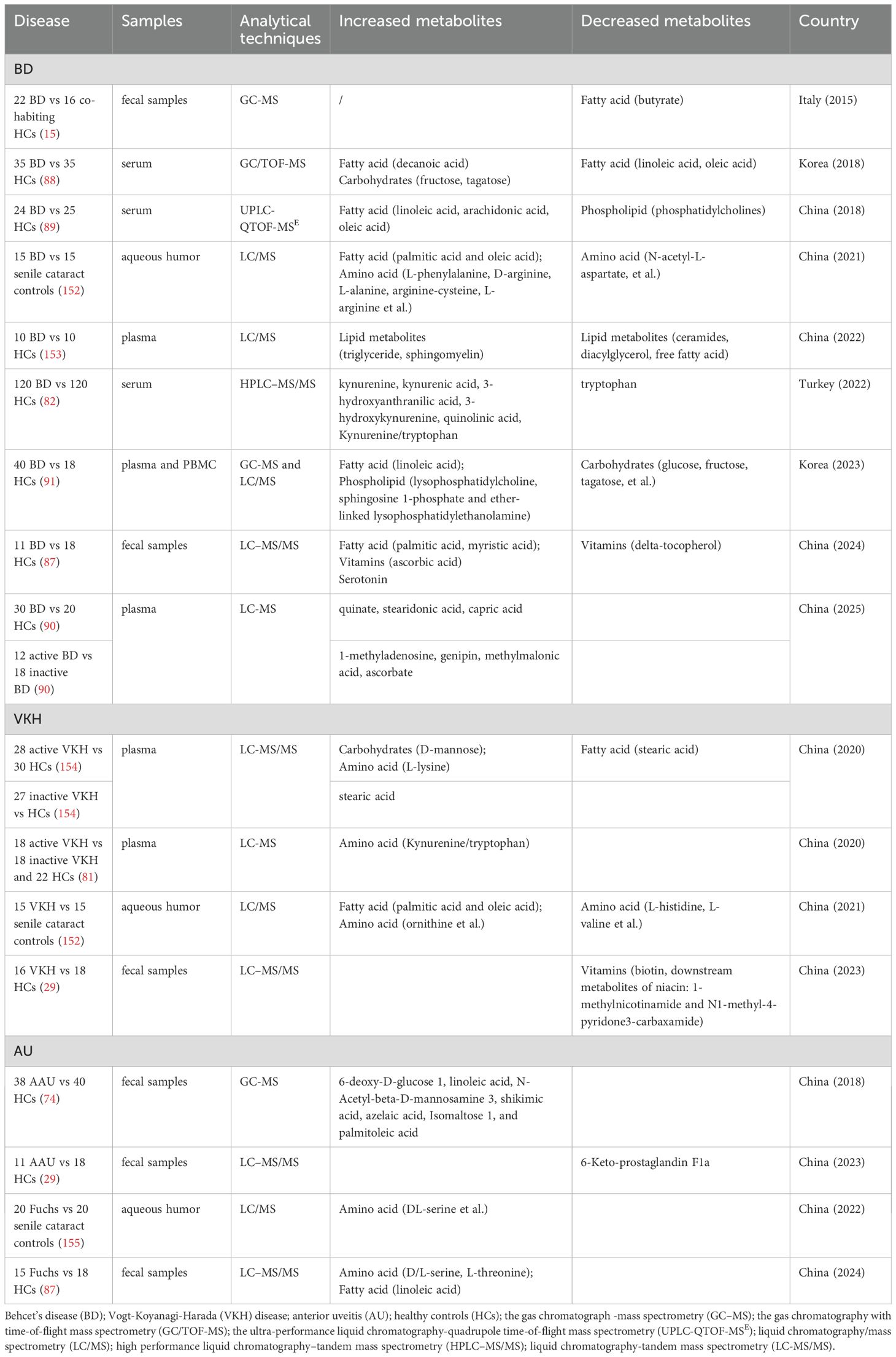
Clinical and experimental evidence indicates that alterations in the gut microbiome composition in patients with uveitis can lead to disturbances in metabolite profiles (Table 2). These metabolic changes were manifested in the metabolism of carbohydrates, fatty acids, amino acids, and bile acids (DAs). Here, we will focus on the changes of several key metabolites and their potential implications (Figure 2).
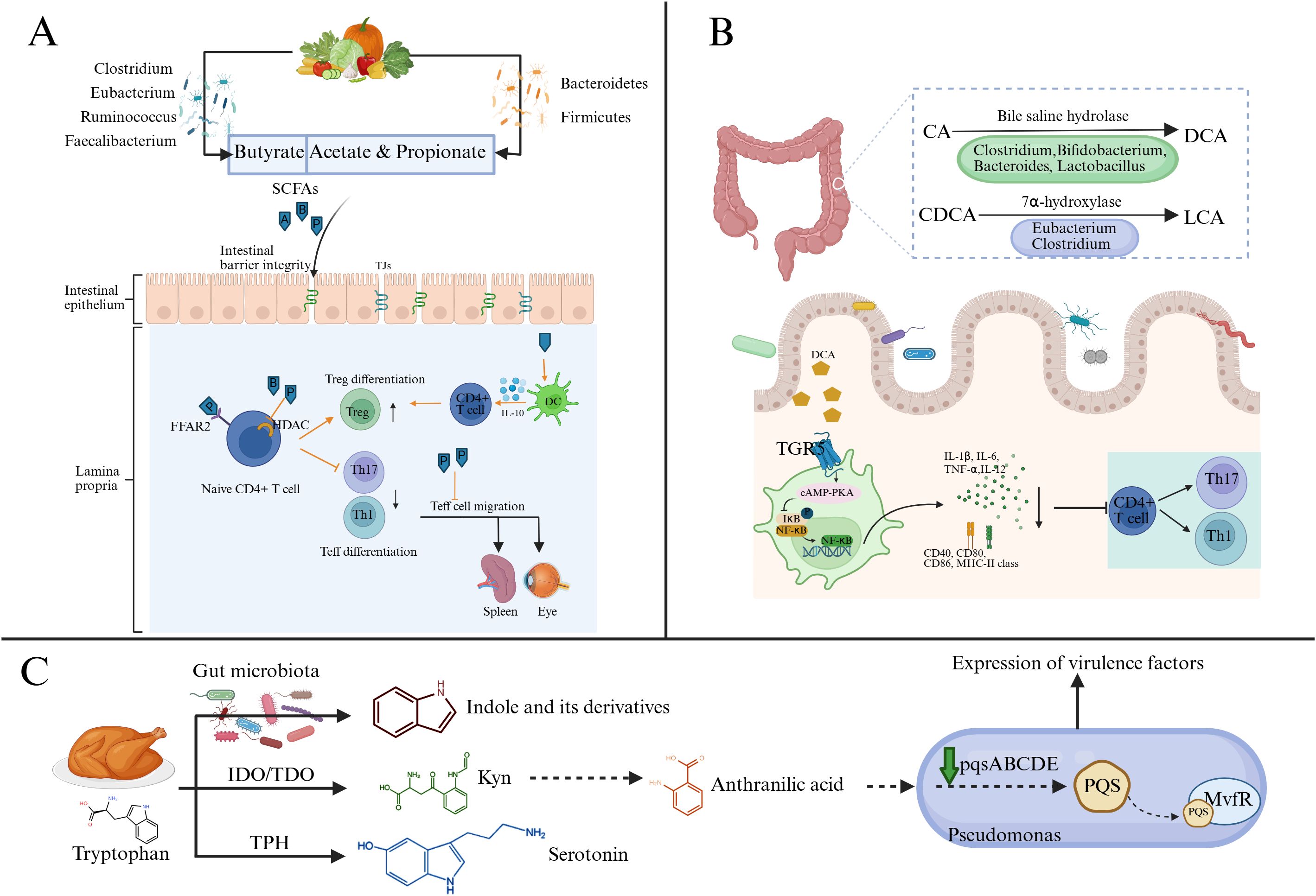
Figure 2. Major microbial metabolic hypothesized pathways to impact uveitis risk and severity. (A) The gut microbiome breaks down host dietary fibers and indigestible carbohydrates to produce SCFAs. SCFAs can maintain the integrity of the intestinal barrier in uveitis and regulate immune cells by binding to FFAR2 or inhibiting HDAC. In addition, propionate can inhibit the migration of Teff cells, especially Th1 cells, from the gut to extra-intestinal lymph nodes. (B) CA and CDCA are metabolized by the gut microbiome to produce DCA and LCA. These two secondary bile acids can bind to TGR5, activate the cAMP-PKA signaling pathway, and inhibit NF-κB-mediated DC activation, thereby reducing the expression of proinflammatory cytokines and costimulatory molecules. (C) The tryptophan metabolism pathway includes the indole pathway, kynurenine pathway, and serotonin pathway. The indole pathway occurs in the intestinal lumen via the gut microbiome. Kynurenine can be further metabolized into anthranilic acid, which is a precursor of the quorum-sensing signal molecule PQS. PQS is synthesized by Pseudomonas through the pqsABCDE gene cluster. Upon binding with MvfR, it regulates the quorum-sensing system of Pseudomonas, thereby influencing the expression of its virulence factors. (Created with BioRender.com) SCFAs, short-chain fatty acids (A, acetate; P, propionate; B, butyrate); Th, T helper cells; Treg, regulatory T cells; DC, Dendritic cells; FFAR2, Free Fatty Acid Receptor 2; HDAC, Histone Deacetylase; CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; LCA, lithocholic acid; TGR5, T G-protein coupled receptor 5; IDO, indoleamine 2,3-dioxygenase; TDO, tryptophan 2,3-dioxygenase; TPH, tryptophan hydroxylase; Kyn, kynurenine; PQS, Pseudomonas Quinolone Signal; MvfR, Multiple Virulence Factor Regulator.
3.1 The metabolism of short chain fatty acids
SCFAs, such as acetate (C2), propionate (C3), and butyrate (C4), are produced through the fermentation of dietary fibers and indigestible carbohydrates (such as resistant starch) by the gut microbiome (51). Different types of SCFAs are metabolized by different bacterial taxa. Eubacterium, Ruminococcus, Faecalibacterium, and Clostridium clusters IV and XIVa dominate the production of butyrate, while acetate and propionate are associated with Bacteroidetes and Firmicutes (52–55). The immunomodulatory effects of SCFAs have been comprehensively reviewed (51, 56). In addition to serving as energy sources for the host and gut microbes (57), the possible mechanisms include: 1) maintaining the integrity of the intestinal barrier (58); 2) improving the inflammatory environment by inhibiting the expression of pro-inflammatory cytokines (IL-8, IL-6, IL-1β and TNFα) and promoting the production of anti-inflammatory cytokines (IL-10, TGF-β) (59); 3) inducing tolerance and an anti-inflammatory phenotype in various immune cells (including neutrophils, macrophages, Foxp3+ Treg cells, B cells, and microglia) through G protein-coupled receptors (GPCRs, GPR41, GPR43, and GPR109a) or by inhibiting histone deacetylases (HDAC) (60–64).
Studies have found that, compared to healthy relatives, the levels of butyrate in fecal samples from BD patients were significantly reduced and positively correlated with Roseburia (15). Furthermore, the transplantation of fecal samples from patients with active BD into B10.RIII mice led to a reduction in the concentrations of propionate, butyrate, and valerate (65). Exogenous administration of SCFAs, particularly propionate and butyrate, significantly alleviated ocular inflammation in C57BL/6 EAU mice (66, 67). Nakamura et al. demonstrated using Kaede transgenic mice (expressing photoconvertible fluorescent Kaede protein) that propionate suppressed the migration of effector T cells (Teff) between intestinal and extraintestinal tissues. However, no protective effect of propionate was observed in B10.RIII EAU mice, which may involve multiple mechanisms, including mouse strain-specific gut microbiome composition. A high-pectin diet can increase the concentrations of acetate and propionate in the gut, exerting effects on EAU mice similar to propionate administration. Notably, pectin supplementation can upregulate the expression of the SCFA receptor FFAR2/GPR43 in the ileum during the peak phase of uveitis (68). In addition, the inhibition of Th17 differentiation mediated by butyrate may be achieved through the activation of nuclear factor erythroid 2-related factor 2 (Nrf2)/heme oxygenase 1 (HO-1) pathway and the suppression of IL-6 receptor (IL-6R) expression (67). In BD patients, a butyrate-rich diet was also observed to modulate blood redox status and enhance fibrinolysis. However, it did not significantly alter gut microbiome composition or SCFA production, which may require a longer period of nutritional intervention (69). The above evidence highlights the role of gut microbiome-induced changes in SCFAs in the immune regulation of NIU.
3.2 The metabolism of bile acids
Cholesterol is metabolized into two primary bile acids in the liver: cholic acid (CA) and chenodeoxycholic acid (CDCA) (70). After entering the intestine, CA and CDCA are metabolized to deoxycholic acid (DCA) and lithocholic acid (LCA) by microbial bile saline lyase (BSH, mainly expressed by Clostridium, Bacteroides, Bifidobacterium, and Lactobacillus) and 7α-dehydroxylase (mainly expressed by Eubacterium and Clostridium), respectively (70, 71). BAs play a key role in the host immune response through several GPCRs or nuclear receptors, such as farnesoid X receptor (FXR). Among them, CDCA was identified as the most effective FXR ligand, while DCA and LCA were the preferred agonists of Takeda G protein-coupled receptor 5 (TGR5) (70).
The expression of TGR5 in M1 macrophages of patients with active VKH is significantly lower than that of healthy controls (72). Further experiments showed that TGR5 activation could induce the transformation of M1 (inflammatory) to M2 (anti-inflammatory) macrophages and inhibit the differentiation of Th1 and Th17 cells (72). The same research group also found that the levels of secondary bile acids (DCA, LCA, etc.) were reduced in the feces and serum of C57BL/6 EAU mice, and the proportion of secondary bile acids was positively correlated with the level of Clostridium scindens in the feces (73). C. scindens can convert primary bile acids into secondary bile acids, and its levels were reduced in patients with BD and AAU (16, 74). Studies have shown that the colonization of C. scindens restored the composition of bile acids in the feces and reduced the severity of EAU (73). Additionally, a diet rich in DCA or LCA significantly alleviated ocular inflammation in EAU mice (73, 75). This protective effect is closely related to the activation of the TGR5 receptor mediated by DCA, which inhibits nuclear factor kB (NF-kB)-mediated DC activation via the cyclic AMP (cAMP)-protein kinase A (PKA) signaling pathway, thereby reducing inflammation levels (73). However, the specific mechanisms of bile acid metabolism in uveitis still require further validation.
3.3 The metabolism of tryptophan
Tryptophan (Trp) is an essential amino acid for the human body and can be metabolized by the gut microbiome into various indole-containing compounds. For example, Escherichia coli, Clostridium, and Bacteroides can produce indole through tryptophanase (76). These indole derivatives can act as effective immune regulators by binding to the aryl hydrocarbon receptor (AhR) (77). Additionally, tryptophan can also be metabolized via the kynurenine (Tyn) and serotonin pathways. Pseudomonas can utilize the kynurenine pathway to generate anthranilate, and then synthesize the quorum-sensing signaling molecule 2-heptyl-3-hydroxy-4-quinolone (Pseudomonas quinolone signal, PQS). Studies have shown that PQS regulated the immune response in arthritis by inhibiting the differentiation of CD4+ IFNγ+ cells (78).
The blood Kyn/Trp ratio is commonly used as a marker to evaluate the activity of indoleamine 2, 3-dioxygenase (IDO) in chronic immune activation (79). Previous studies have shown that the Kyn/Trp ratio and Kyn concentration in serum and urine of patients with active uveitis were significantly higher than those of healthy controls, and the Kyn/Trp ratio is even higher in patients with remission uveitis (80). Another study observed a similar trend in VKH patients, except that the Kpn/Trp ratio of VKH patients in the remission phase was similar to that of the control group (81). This may be due to the fact that the former study included multiple uveitis entities, while the latter only focused on VKH patients. In vitro experiments have shown that IDO can affect the antigen-presenting function by enhancing the expression of the cell surface marker CD86, whereas the downregulation of IDO may lead to a reduction in Tregs and an expansion of CD4+ T cells (81). Additionally, dysregulation of the kynurenine metabolism pathway has also been observed in BD patients, characterized by increased tryptophan degradation, along with elevated levels of kynurenine (KYN), kynurenic acid (KYNA), 3-hydroxyanthranilic acid (3HAA), 3-hydroxykynurenine (3HK), and quinolinic acid (QUIN) in serum. These metabolites have been implicated in BD disease activity, clinical manifestations, and inflammatory burden (82). Further research is needed to identify the key tryptophan metabolites involved in the immune response of different uveitis and their potential roles.
Although current studies have predominantly focused on the individual immunoregulatory functions of metabolites, emerging evidence indicates that these metabolites may interact through shared signaling pathways or have overlapping effects on immune homeostasis. For example, SCFAs could influence tryptophan metabolism by promoting the production of AhR ligands, which are known to exert anti-inflammatory effects and enhance mucosal barrier integrity (83). On the other hand, BAs regulate immune responses via FXR and TGR5 and may also modulate the gut bacteria composition involved in tryptophan degradation (84, 85). Furthermore, spore-forming bacteria, known producers of SCFAs, have also been implicated in stimulating serotonin biosynthesis from tryptophan by enhancing tryptophan hydroxylase (TPH)1 expression in colonic enterochromaffin cells (86). These findings suggest that SCFAs, BAs, and tryptophan metabolites may form an interlinked metabolic network that collectively shapes immune responses in the gut and potentially at distal sites such as the eye. However, the precise mechanisms underlying their crosstalk and relevance in NIU remain to be fully elucidated.
Additionally, our studies have highlighted the potential of gut microbiome-derived metabolites as non-invasive biomarkers in NIU. In BD, 19 fecal differential metabolites showed AUC values above 80% (87). Similarly, 31 metabolites distinguished Fuchs syndrome from controls with high accuracy, including serotonin (87). Moreover, two metabolites (–),-gallocatechin and vanillin, effectively differentiated VKH, while norfloxacin showed an AUC of 82% in distinguishing AAU from controls (29). Notably, other studies have also identified metabolite profiles from serum or plasma (88–91) and urine (92) as potential diagnostic biomarkers for BD. These findings suggest that microbiome-related metabolites may serve as accessible biomarkers for NIU diagnosis and stratification, although further validation in larger cohorts is needed.
4 The hypothesized mechanism of gut microbiome dysbiosis in NIU
4.1 Increased intestinal permeability and gut microbial translocation
Alterations in the gut microbiome may affect intestinal permeability and lead to leaky gut, which would allow the translocation of gut microbes and their products into the systemic circulation, thereby triggering innate and/or adaptive immune responses. Our study found that serum Zonulin levels in patients with BD and Fuchs syndrome were significantly higher than those in healthy controls (87). Zonulin is a physiological regulator released by intestinal epithelial cells after exposure to microorganisms or a gluten diet and can degrade intercellular tight junctions in the intestinal barrier (93). Moreover, early studies also reported increased intestinal permeability in BD patients without symptoms or signs of gastrointestinal disease (94). Patients with AU were also found to have chronic intestinal inflammation by ileocolonoscopy, which was associated with the recurrence rate of uveitis, but not with HLA-B27 status, sacroiliitis, or Nonsteroidal Anti-inflammatory drug (NSAID) intake (95). These findings were confirmed in B10.RIII EAU mice. The maximum changes in intestinal morphology (including ileal villus length, crypt depth, submucosal thickness, and muscular layer thickness) occurred prior to the peak of uveitis, which coincided with the peak expression of intestinal Zonula-occludens-1 (ZO-1), increased production of antimicrobial peptides (AMPs), and a reduction in cytokine production. These changes were reversed at the peak of uveitis. However, the increase of intestinal permeability and the difference of intestinal bacteria was paralleled to the course of uveitis, both reaching the peak on day 14 after immunization (13). Interestingly, Wang et al. also revealed intestinal barrier disruption in mice receiving feces from patients with active BD, as shown by decreased expression of three tight junction proteins (Claudin1, Claudin4, and Occludin) in colon tissues and a significant increase in LPS in serum (65). The above evidence suggests that increased intestinal permeability may be an early marker of uveitis and is closely related to the changes in the intestinal microbiome.
In LPS-induced uveitis (EIU) models, high-dose 13C-labeled propionate administered intraperitoneally can be detected in the ocular tissues. Unlabeled propionate was also detected in the eye, which may be produced by gut bacteria and enter the eye via the systemic circulation, or generated by ocular cells metabolizing pyruvate (96). These results suggest the existence of the gut-eye axis. The EIU model is an animal model resembling human AAU. Therefore, future studies are needed to further investigate whether orally 13C-labeled SCFA can translocate through the gut and be transferred to the eye in EAU mice (posterior uveitis model). Notably, the translocation of bacterial components or products, such as LPS and SCFA, has only been observed so far. No study has reported the translocation of intact bacteria in patients or mouse models of uveitis, which may be due to the existence of the intestinal vascular barrier (97) and blood-retinal barrier (BRB) (98), which limit the systemic spread of intact bacteria and reach the target organ, the eye.
The translocation of lymphocytes or other inflammatory cells from the gut to the eye is also considered one of the mechanisms involved in the pathogenesis of uveitis. The migration of gut-derived pathogen-associated molecular patterns (PAMPs) increases antigen exposure in the lamina propria, thereby promoting the differentiation of intestinal immune cell subsets. These immune cells may subsequently migrate to extra-intestinal target organs, and lower the threshold for extra-intestinal inflammation (99, 100). As previously described, cell motility in vivo can be detected using the photoconvertible fluorescent protein Kaede (101). Building on this methodology, Nakamura et al. demonstrated that lymphocyte migration from the gut to extra-intestine lymph nodes and the eye was enhanced in Kaede transgenic C57BL/6J EAU mice (66). Additionally, the research group also found that Th1 and Th17 cells in the mesenteric lymph nodes (MLN) of B10.RIII EAU mice increased on day 7 post-immunization, accompanied by a decrease in intestinal cytokine levels. This intestinal change may contribute to the migration of leukocytes from the gut to extra-intestine lymph nodes (13).
4.2 Molecular mimicry and bystander activation
Molecular mimicry may represent another mechanism by which the gut microbiome contributes to the pathogenesis of uveitis. The spontaneous uveitis mouse model (R161H) expresses specific T cell receptors (TCR) against IRBP peptides on B10.RIII mice background. Disease signs appear around weaning age, and all mice develop uveitis by 2 months of age in R161H mice. Unlike the induced model (EAU), in the spontaneous model, retina antigens are sequestered within the eye, while retina-specific T cells in the peripheral circulation must be activated before crossing BRB to initiate intraocular inflammation. Therefore, the question of where pathogenic T cells are activated has attracted attention. Activated Th17 cells have been detected in the intestinal lamina propria of R161H mice before the onset of ocular inflammation. In addition, R161H-Rbp3-/- mice do not develop uveitis due to the lack of target antigen. However, they have a high frequency of Th17 cells in the gut, similar to that of R161H-WT mice, and the transfer of activated T cells into naive wild type (WT) mice can induce uveitis. Moreover, in vitro studies have also found that R161H T cells are activated in an MHC class II-dependent manner by gut contents rich in bacteria (102). These observations all support gut microbiome as a trigger of uveitis. Nakamura et al. treated EAU mice with different antibiotics (metronidazole, vancomycin, neomycin, and ampicillin) to narrow down the gut bacteria species that influenced the severity of uveitis. Studies have shown that oral administration of metronidazole or vancomycin alone significantly reduces the clinical score of uveitis, accompanied by a reduction in the abundance of Coprococcus, Dorea, Clostridium, and Lactobacillus, which may be potential sources of mimic antigens (14). However, the other two antibiotics did not ameliorate uveitis. The team of Caspi identified a series of sequences homologous to IRBP161-180, which derived from the gut bacterium Turicibacter. However, these sequences failed to induce T cell proliferation from R161H mice and did not provoke disease when immunized in mice. The mimic antigens that induce disease may vary in different uveitis entities (103). Therefore, more studies are needed to identify microbiome peptides that have homologous sequences with retinal antigens (including IRBP and retinal S-antigen) and melanocyte antigens (target antigens of VKH disease, including tyrosinase and gp100).
The gut microbiome can also modulate immune responses via adjuvant effects. EAU (induced model) mice rely on active immunization by emulsifying the IRBP antigen in complete Freund’s adjuvant (CFA, such as heat-killed Mycobacterium tuberculosis). CFA strongly stimulates the innate immune response by activating antigen-presenting cells through pathogen recognition receptors, thereby presenting the IRBP antigen in this context and inducing autoreactive T cells (103). Fecal transplantation from patients with active BD did not induce ocular inflammation in naïve mice, but it affected the composition of the gut microbiome, the intestinal barrier, and T cell differentiation in the mesenteric lymph nodes (MLN) and spleen (65). However, EAU induction after fecal transplantation aggravated the development of uveitis (16, 65). They also found that Stenotrophomonas, which was significantly enriched in BD patients, encodes a microbial peptide SteTDR9-17 (YVQPGNTIL) that is homologous to IRBP161-180 (YLHPGNTIL). This peptide can stimulate PBMCs from BD patients or lymphocytes from EAU mice to produce IFN-γ and IL-17. However, similar to the antigen peptides identified by Caspi, SteTDR9–17 cannot induce uveitis directly in mice, but it can synergize with IRBP, amplifying the activated state in a bystander manner, thereby exacerbating EAU (104).
4.3 Blood-retinal barrier
BRB serves to sequester retinal antigens within the eye, thereby preventing their recognition by the peripheral immune system and inhibiting the entry of unactivated T cells into the retina, thus averting retinal inflammation (98, 102). However, in cases of gut microbiome imbalance, retina-specific T cells can become activated, entering the peripheral circulation and potentially inducing inflammation through the BRB (102). Notably, in EAU mice, an increase in BRB permeability was observed, as evidenced by widespread Evans blue staining leakage in retinal vessels (105, 106). Xiang et al. also reported a reduction in occludin levels, a key component contributing to BRB integrity, in the retina of EAU mice (106). Intriguingly, EAU mice treated with propionate or antibiotics exhibited decreased BRB permeability (14, 66, 107). These observations suggest a potential link between gut microbiome dysregulation and altered BRB permeability, implying that antibiotics or SCFAs may offer beneficial effects in preserving BRB integrity.
4.4 Immune responses
Alterations in the gut microbiota can also impact immune responses. Th17 cells are a crucial pathogenic subset in autoimmune uveitis. In R161H mice, broad-spectrum antibiotic treatment reduced intestinal Th17 cell activation and alleviated uveitis, while in vitro studies demonstrated that bacteria-rich intestinal extracts directly activated IRBP-specific T cells, highlighting the role of gut microbial antigens in driving autoreactive T cells (102). Similarly, germ-free (GF) mice exhibited decreased IFN-γ and IL-17 production and increased Tregs in ocular draining lymph nodes compared to conventionally housed EAU mice (108). Nakamura et al. further reported that, prior to the onset of uveitis, expansion of Th1 and Th17 cells in mesenteric lymph nodes (MLN), along with suppressed intestinal cytokines, may promote the migration of pathogenic lymphocytes from the gut to peripheral tissues and the eye (13, 66). Furthermore, colonization of C. scindens associated with secondary bile acid production was found to diminish the proportions of Th1 cells, Th17 cells, and DCs in the spleen of EAU mice, emphasizing the systemic immunomodulatory effects of specific gut microbes (73).
Besides adaptive immunity, gut microbiota dysbiosis also affects innate immune responses in NIU. For instance, fecal microbiota from patients with BD can induce neutrophil activation when transferred to mice, which contributes to the differentiation of pathogenic T cells (65). Microglia, the resident tissue-specific macrophages in the central nervous system, including the retina, are also influenced by gut microbiota. Activated microglia can disrupt the integrity of the BRB and recruit peripheral leukocytes (109, 110). In EAU mice, minocycline treatment inhibits microglial activation and reduces IL-1β production by microglia/macrophages (107). These findings collectively support the crucial role of gut microbiota in orchestrating both innate and adaptive immune responses contributing to the pathogenesis of NIU.
Overall, it is not contradictory that the gut microbiome may be the source of both antigens and adjuvants, making it a trigger and/or amplifying signal for uveitis-specific T cells. Cross-reactive antigens are not provided by a single species but are the result of the collective action of multiple microbes. The gut microbiome that provides mimic antigens remains to be identified. Based on these hypothesized mechanisms, several potential intervention strategies can be considered. Restoring intestinal barrier function may prevent microbial translocation and systemic immune activation, such as by modulating Zonulin signaling. Moreover, targeting gut microbiome dysbiosis through selective antibiotics, probiotics, or dietary interventions may help restore microbial balance and limit bacteria capable of producing mimic antigens or pro-inflammatory metabolites. These strategies may complement existing immunosuppressive therapies and offer novel microbiome-targeted approaches for NIU management.
5 Potential therapeutic strategies for NIU based on gut microbiome
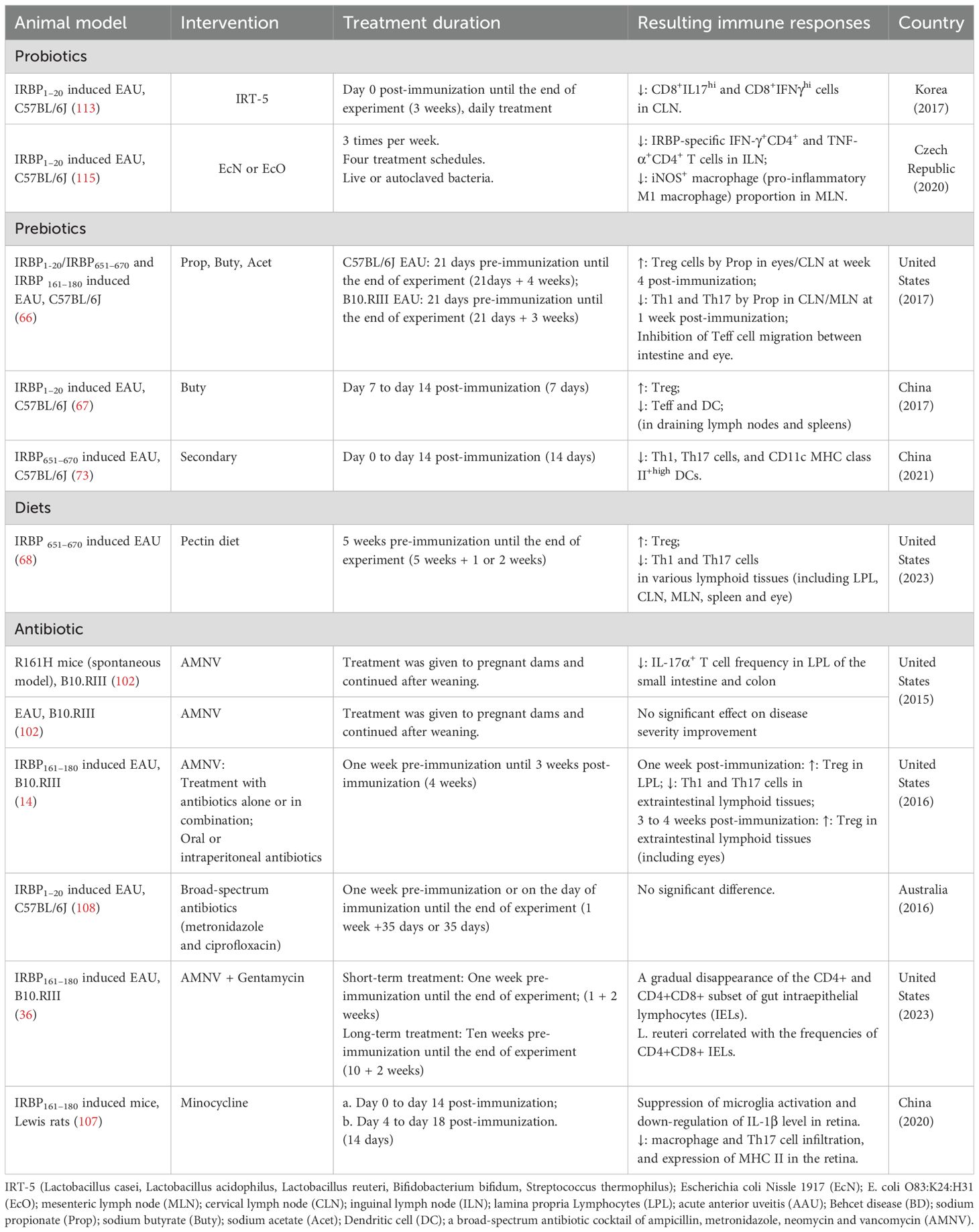
Currently, widely used systemic treatments for uveitis include corticosteroids, immunosuppressants (such as cyclosporine, methotrexate, mycophenolate mofetil), and biologics. However, these drugs have significant side effects and can greatly affect the composition of the gut microbiome. For example, mycophenolate mofetil (MMF) and methotrexate (MTX) treatment of EAU mice induced significant but distinct changes in intestinal bacterial composition (111). MMF may alleviate uveitis by expanding the Treg subsets in the MLN and the eye. In contrast, MTX significantly suppresses the Teff subsets in most tissues, maintaining the disease in a quiescent state, but the risk of relapse may be higher after drug withdrawal (111). Therefore, in this section, we will discuss the impact of probiotics, prebiotics, dietary modifications, antibiotics, and fecal microbiome transplantation (FMT) on immune responses (Table 3), gut microbiome, and disease activity (Figure 3).
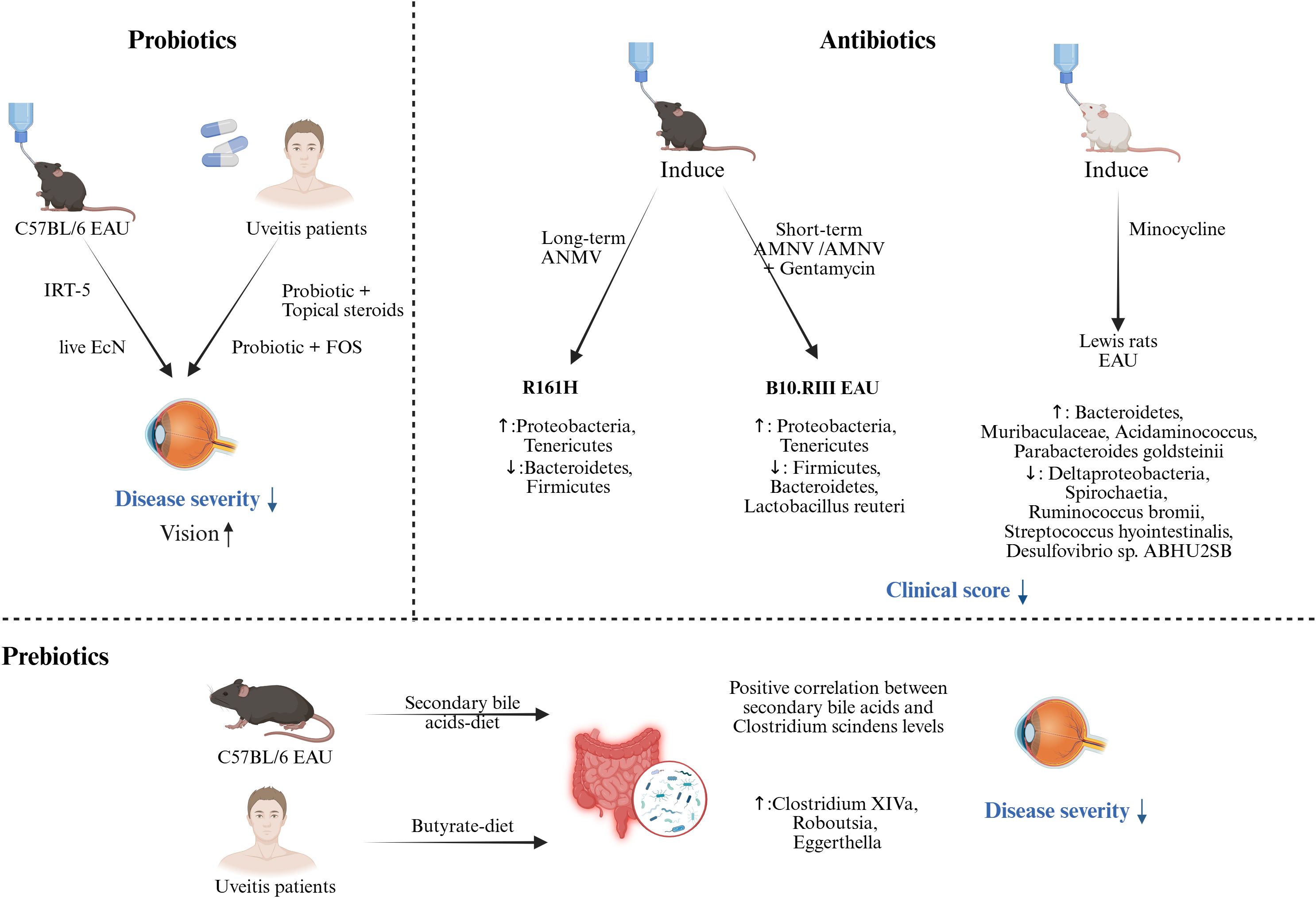
Figure 3. Probiotics, prebiotics, and antibiotics administration as a potentially beneficial strategy against uveitis. Probiotics. Preclinical studies through the EAU model have shown that probiotics used before or during immune induction can significantly reduce clinical scores. Clinical studies have found that combining conventional treatment (topical steroids) or prebiotics with probiotics can also significantly reduce disease severity and improve vision. Prebiotics. A significant positive correlation between secondary bile acid levels and Clostridium scindens was found in EAU mice. Clinical studies have highlighted changes in gut microbiome composition, with relative increases in Clostridium XIVa, Roboutsia, and Eggerthella, following supplementation with a butyrate-rich diet. Antibiotics. Preclinical studies have shown that long-term (oral administration of ANMV from maternal pregnancy to offspring after weaning) administration of ANMV in R161H mice significantly affected the gut microbiome composition (increased Proteobacteria, Tenericutes, and decreased Bacteroidetes, Firmicutes). Short-term administration of ANMV or ANMV+gentamicin (from one week before induction to the end of the experiment) also significantly affected the gut microbiome in B10.RIII induced EAU mice. (Created with BioRender.com) IRT-5 (Lactobacillus casei, Lactobacillus acidophilus, Lactobacillus reuteri, Bifidobacterium bifidum, Streptococcus thermophilus); fructo-oligosaccharide (FOS); Escherichia coli Nissle 1917 (EcN); a broad-spectrum antibiotic cocktail of ampicillin, metronidazole, neomycin and vancomycin (AMNV); Experimental autoimmune uveitis (EAU).
5.1 The intervention of probiotics and prebiotics
Probiotics are living microorganisms that benefit host health when administered in sufficient amounts (112). After reducing the gut microbiome load with antibiotics in advance, IRT-5 probiotics (Lactobacillus casei, Lactobacillus acidophilus, Lactobacillus reuteri, Bifidobacterium bifidum, and Streptococcus thermophilus) supplementation on the day of immunization in EAU mice alleviated the severity of uveitis and significantly inhibited the expansion of CD8+T cells in cervical lymph nodes (CLN) (113). Antigen-specific CD8+ T cells can also induce autoimmunity in uveitis models, although CD4+ T cells are predominant (114). Additionally, administration of live Escherichia coli Nissle 1917 (EcN) only before or during disease induction significantly reduced clinical scores in EAU, indicating that the immunoregulatory effects of EcN preceded the development of EAU and influenced the initial antigen presentation and T cell (IRBP-specific CD4+ T cell) activation in the draining lymph nodes of immune sites (115). Despite the transient colonization of EcN in the gut, administration of EcN from 2 weeks before immunization to the day of immunization similarly prevented uveitis, suggesting that the beneficial effects of EcN are not limited to local effects on the gut. The protective effect of EcN may lie in the improvement of intestinal barrier function and the extension of anti-inflammatory regulation of intestinal mucosal immunity to innate and adaptive immunity, thus having a long-term impact on host health (115). A case report on AAU patients showed that local corticosteroids combined with probiotic supplements can alleviate inflammation and reduce the frequency of recurrences (116). Similarly, in a BD patient with AAU, synbiotics (a combination of probiotics and prebiotics) demonstrated similar therapeutic effects (117).
Prebiotics can selectively stimulate the growth and/or activity of one or a limited number of bacteria in the gut microbiome, thereby improving host health (118). Common prebiotics include galacto-oligosaccharides, fructans, and inulin, which can be metabolized by intestinal bacteria to produce metabolites such as SCFA, secondary bile acids, and folate. These metabolites further regulate the composition of the gut microbiome and interact with the immune cells of the host (119). As previously mentioned, supplementation with SCFAs, secondary bile acids, or diets that produce SCFAs can significantly reduce the severity of inflammation in uveitis.
Taken together, preclinical studies suggest that different probiotic strains may trigger distinct immune mechanisms to alleviate uveitis. However, large cohort randomized controlled trials are needed to validate their preventive and therapeutic efficacy in clinical settings. Although probiotics and prebiotics may transiently modulate immune responses and improve symptoms, their long-term effects remain controversial. Prolonged colonization of probiotics may disrupt gut microbiome structure, reduce beneficial commensals, and replace native microbes essential for immune balance (120, 121). Additionally, if intestinal barrier integrity is compromised, probiotics may translocate into the systemic circulation, leading to invasive infections (120). Notably, in a clinical trial of patients with severe acute pancreatitis, synbiotic prophylaxis containing Bifidobacterium, Lactobacillus, cornstarch, and maltodextrin was associated with a 2.5-fold increase in mortality and bowel ischemia compared to placebo (122). Therefore, while short-term use appears beneficial, the chronic application of these interventions should be cautiously evaluated, with more data needed to assess their safety and efficacy in NIU patients over extended periods.
5.2 The intervention of dietary modifications
Disease activity in patients with uveitis may also be related to the daily diet. It has been observed that a diet rich in vitamins and polyunsaturated fatty acids may alleviate disease progression by regulating immune responses (123). In vitro studies have found that linoleic acid (LA) can inhibit the antigen presentation function of DCs by reducing the expression of co-stimulatory molecules. Meanwhile, LA can also decrease the differentiation of Th cells in both humans and mice, and suppress the secretion of inflammatory cytokines by retinal pigment epithelial cells [110]. Nevertheless, LA has traditionally been regarded as a proinflammatory factor, and its downstream metabolite, arachidonic acid, is capable of converting into pro-inflammatory eicosanoids such as prostaglandin E2 (124). The level of LA was also significantly elevated in AU and BD patients (74, 87, 89, 91, 125). These different results suggest that the increase of LA in uveitis may be a feedback mechanism for the immune response suppression, which requires further investigation. Moreover, a double-blind randomized controlled trial found that combined vitamin C and E supplementation could improve visual acuity in AAU patients (126). Additionally, Li et al. found that caloric restriction increased Treg cells, altered the metabolic state of immune cells, and downregulated glycolysis-related genes by single-cell RNA sequencing. Further flow cytometry analysis confirmed that caloric restriction modulates the PI3K/AKT/c-Myc signaling axis and reduces granulocyte-macrophage colony-stimulating factor (GM-CSF) production in Th17 cells, thereby promoting CD4+ T cell balance (127). Similarly, Duan et al. demonstrated through single-cell RNA sequencing that a ketogenic diet (high fat, low carbohydrate) increased Treg cells, reduced Th17 cells, and restored the Th17/Treg balance, thereby alleviating EAU progression. The diet also partially reversed inflammatory responses in retinal immune cells, especially CD4+ T cells (128).
Overall, dietary interventions, such as fermentable fiber-enriched diets, ketogenic diets, or caloric restriction, have demonstrated promising immunomodulatory effects in preclinical uveitis models. These strategies may help maintain immune balance and prolong remission periods in NIU. However, long-term dietary intake also affects gut microbial structure and function (129). Cross-sectional studies have shown that long-term dietary intake, rather than short-term intake, is strongly associated with enterotype distribution, with high-protein and animal fat diets favoring Bacteroides-dominant enterotypes, while high-carbohydrate diets are linked to Prevotella-dominant enterotypes (129). Nevertheless, strict adherence to specific dietary patterns over prolonged periods may lead to nutritional deficiencies, metabolic disturbances. For example, although low-carbohydrate diets may promote weight loss and metabolic benefits (130), insufficient carbohydrate intake can reduce the abundance of butyrate-producing bacteria and fecal butyrate concentrations, potentially impairing gut and systemic immune homeostasis (131). Therefore, individualized dietary planning, coupled with close nutritional and clinical monitoring, is essential to ensure safety and effectiveness over extended periods.
5.3 The intervention of antibiotics
In several studies of uveitis models, raising mice under germ-free conditions or depleting the gut microbiome with antibiotics significantly reduced the severity of the disease (14, 36, 102, 107, 108). Notably, EAU models suggest that the beneficial effects of antibiotics in uveitis are largely dependent on early intervention, supporting their role as a prophylactic rather than a therapeutic agent. This preventive capacity appears to depend on several factors: 1) the time point of antibiotic administration, 2) the duration of antibiotic treatment, 3) the type of antibiotic used, and 4) the method of antibiotic administration. Heissigerova et al. found that metronidazole and ciprofloxacin treatment started on the day of EAU induction did not significantly affect the clinical score of uveitis, while intervention one week in advance alleviated the disease, suggesting that pre-existing gut microenvironment may affect EAU susceptibility (108). In a separate study, Seidler Stangova et al. demonstrated that metronidazole monotherapy administered two weeks before disease induction significantly attenuated EAU severity, further supporting its role as a prophylactic agent through early intervention (132). In R161H mice, oral administration of broad-spectrum antibiotics (ampicillin, metronidazole, neomycin, and vancomycin, AMNV) starting from the pregnancy of the dam, and continuing the treatment after weaning in the offspring, significantly delayed the development of uveitis. Meanwhile, the frequency of IL-17α+T cells and the cytokines IFN-γ and IL-22 in the lamina propria of the small intestine were decreased after AMNV intervention, but no expansion of Treg cells was observed. Under the same experimental conditions, EAU mice treated with AMNV were not significant improved (102). In contrast, Nakamura et al., also using AMNV treatment, showed significant improvement in uveitis when oral rather than intraperitoneal antibiotics were started one week before immunization. Moreover, this study also found that short-term antibiotic intervention could increase the frequency of Tregs in the intestinal lamina propria at one week post-immunization and increase Tregs in the extra-intestine lymphoid tissue at 3 or 4 weeks post-immunization, while Teff cells were reduced in the extra-intestine lymphoid tissue at the early stage of disease (14). Based on the different findings from two studies, Caspi and colleagues further investigated the impact of antibiotic treatment duration on EAU. The results showed that short-term antibiotic intervention could eliminate the microbiome stimulating the disease, thereby providing a protective effect. However, with prolonged antibiotic intervention, the protective microbiome was also gradually depleted and became resistant, along with secondary depletion of regulatory intestinal intraepithelial lymphocytes (IEL, which inhibit IRBP-specific T cell activation), and the protective effect was reversed. IELs are microbiome-dependent and are significantly reduced or absent in GF mice. After antibiotic intervention, the reduction of CD4+CD8+ IELs lagged behind the depletion of Lactobacillus reuteri (36). Nakamura et al. also found that metronidazole or vancomycin alone could also reduce inflammation and screened different kinds of protective microbes (14). In addition to regulating T cell differentiation, antibiotics can also suppress the activation of retinal microglia, thereby reducing the release of pro-inflammatory cytokines and the recruitment of inflammatory cells, ultimately inhibiting the onset and progression of uveitis (107). Under GF conditions, uveitis could be alleviated, but the disease still progressed after cohousing with SPF mice (102). Moreover, continuous antibiotic intervention led to dynamic and progressive changes in both the gut microbiome and immune cells, accompanied by a gradual increase in disease scores (36). These results suggest that microbial exposure is not the only trigger of disease development.
Generally, preclinical studies suggest that targeted short-term antibiotic interventions may ameliorate uveitis by reshaping the gut microbiome and modulating systemic immune responses. However, prolonged or repeated antibiotic exposure poses significant risks, including depletion of protective commensals, emergence of resistant strains, disruption of immune homeostasis, and loss of microbiome resilience (133, 134). Additionally, such interventions may increase susceptibility to opportunistic infections and exacerbate systemic immune dysregulation in susceptible individuals (134). Therefore, more targeted narrow-spectrum antibiotics are developed and used, and combined probiotics are considered to support microbiome recovery.
5.4 Fecal microbiome transplantation
FMT is the transplantation of fecal microbiome from healthy donors into the gastrointestinal tract of patients to compete for the niche with pathogenic strains, thereby reshaping the intestinal microbiome (135). FMT has become a recommended treatment for recurrent Clostridioides difficile infection (136, 137), as it helps restore gut diversity, improve metabolic function, and modulate the immune system (138). Due to its significant therapeutic potential, FMT has also been gradually applied to intervene in autoimmune diseases (139–141)]. However, FMT protocols involving different doses and routes of administration can cause a variety of clinical responses and lack reproducibility (142). In addition to bacteria, the fecal material used in FMT also contains viruses, fungi, archaea, and various metabolites. While more precise approaches such as fecal virome transplantation (FVT) and washed microbiota transplantation (WMT) have been developed to improve safety by minimizing unwanted components (143, 144), these methods are still experimental, and their long-term impacts remain unclear.
Notably, FMT has been associated with transmission of multidrug-resistant organisms, fungi, and viruses in certain cases (145). Moreover, persistent alterations of the gut microbiome induced by FMT may disrupt immune homeostasis, potentially leading to disease flares in patients with underlying immune disorders, such as IBD (146, 147). To mitigate these risks, next-generation FMT strategies are focusing on the transplantation of more defined microbial components, including WMT, bacterial spore formulations, and FVT, which aim to enrich beneficial taxa while minimizing undesirable elements and reducing the risk of adverse events (142). Additionally, advances in multi-omics technologies and refined donor-recipient matching models are expected to enhance donor screening precision and optimize FMT efficacy and safety in the future (142).
6 Conclusion
Despite the accumulating evidence linking gut microbiome dysbiosis to NIU pathogenesis, the translation of these findings into clinical practice remains challenging. Current insights propose that targeting the gut-eye axis through interventions such as modulating gut microbiome composition and key metabolites, as well as restoring intestinal barrier function, may offer novel adjunctive strategies alongside conventional immunosuppressive therapies. Specifically, strategies including dietary interventions, probiotics, prebiotics, or defined microbial communities warrant further exploration in NIU. However, future research should also focus on the following priorities to facilitate clinical translation. First, the specific pathogenic or protective microbial species, microbial-derived metabolites, and mimic antigens relevant to different NIU subtypes remain to be identified. Second, establishing causality between gut microbiome alterations and NIU onset, activity, or prognosis requires longitudinal clinical studies, mechanistic investigations using humanized animal models. Third, individualized interventions based on microbiome profiles and the immune status of patients should be developed. Addressing these priorities will help bridge the current gap between basic research and clinical translation and may open new avenues for precision medicine strategies in NIU management.
Author contributions
ML: Writing – original draft, Writing – review & editing, Visualization, Investigation. JG: Writing – original draft, Writing – review & editing, Investigation. TL: Writing – review & editing, Investigation, Data curation. XL: Validation, Investigation, Writing – review & editing, Supervision, Funding acquisition, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by National Natural Science Foundation of China (No. 82371043); and the Project of Outstanding Talents in Scientific and Technological Innovation and Entrepreneurship for Middle-aged and Young Scientists of Jilin Provincial Science and Technology Department (No.20240601014RC).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tsirouki T, Dastiridou A, Symeonidis C, Tounakaki O, Brazitikou I, Kalogeropoulos C, et al. A focus on the epidemiology of uveitis. Ocular Immunol Inflammation. (2018) 26:2–16. doi: 10.1080/09273948.2016.1196713
2. E Cunningham ET and Zierhut M. Vision loss in uveitis. Ocular Immunol Inflammation. (2021) 29:1037–9. doi: 10.1080/09273948.2021.2017152
3. Burkholder BM and Jabs DA. Uveitis for the non-ophthalmologist. BMJ (Clinical Res ed). (2021) 372:m4979. doi: 10.1136/bmj.m4979
4. Prete M, Dammacco R, Fatone MC, and Racanelli V. Autoimmune uveitis: clinical, pathogenetic, and therapeutic features. Clin Exp Med. (2016) 16:125–36. doi: 10.1007/s10238-015-0345-6
5. Su G, Zhong Z, Zhou Q, Du L, Ye Z, Li F, et al. Identification of novel risk loci for behçet's disease-related uveitis in a chinese population in a genome-wide association study. Arthritis Rheumatol (Hoboken NJ). (2022) 74:671–81. doi: 10.1002/art.41998
6. Hou S, Du L, Lei B, Pang CP, Zhang M, Zhuang W, et al. Genome-wide association analysis of vogt-koyanagi-harada syndrome identifies two new susceptibility loci at 1p31.2 and 10q21.3. Nat Genet. (2014) 46:1007–11. doi: 10.1038/ng.3061
7. Huang XF, Li Z, De Guzman E, Robinson P, Gensler L, Ward MM, et al. Genomewide association study of acute anterior uveitis identifies new susceptibility loci. Invest Ophthalmol Visual Sci. (2020) 61:3. doi: 10.1167/iovs.61.6.3
8. Laursen MF, Bahl MI, and Licht TR. Settlers of our inner surface - factors shaping the gut microbiota from birth to toddlerhood. FEMS Microbiol Rev. (2021) 45:fuab001. doi: 10.1093/femsre/fuab001
9. Matenchuk BA, Mandhane PJ, and Kozyrskyj AL. Sleep, circadian rhythm, and gut microbiota. Sleep Med Rev. (2020) 53:101340. doi: 10.1016/j.smrv.2020.101340
10. Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal th17 cells by segmented filamentous bacteria. Cell. (2009) 139:485–98. doi: 10.1016/j.cell.2009.09.033
11. Nutsch KM and Hsieh CS. T cell tolerance and immunity to commensal bacteria. Curr Opin Immunol. (2012) 24:385–91. doi: 10.1016/j.coi.2012.04.009
12. Kim JC, Park MJ, Park S, and Lee ES. Alteration of the fecal but not salivary microbiome in patients with behçet's disease according to disease activity shift. Microorganisms. (2021) 9:1449. doi: 10.3390/microorganisms9071449
13. Janowitz C, Nakamura YK, Metea C, Gligor A, Yu W, Karstens L, et al. Disruption of intestinal homeostasis and intestinal microbiota during experimental autoimmune uveitis. Invest Ophthalmol Visual Sci. (2019) 60:420–9. doi: 10.1167/iovs.18-24813
14. Nakamura YK, Metea C, Karstens L, Asquith M, Gruner H, Moscibrocki C, et al. Gut microbial alterations associated with protection from autoimmune uveitis. Invest Ophthalmol Visual Sci. (2016) 57:3747–58. doi: 10.1167/iovs.16-19733
15. Consolandi C, Turroni S, Emmi G, Severgnini M, Fiori J, Peano C, et al. Behçet's syndrome patients exhibit specific microbiome signature. Autoimmun Rev. (2015) 14:269–76. doi: 10.1016/j.autrev.2014.11.009
16. Ye Z, Zhang N, Wu C, Zhang X, Wang Q, Huang X, et al. A metagenomic study of the gut microbiome in behcet's disease. Microbiome. (2018) 6:135. doi: 10.1186/s40168-018-0520-6
17. Ye Z, Wu C, Zhang N, Du L, Cao Q, Huang X, et al. Altered gut microbiome composition in patients with vogt-koyanagi-harada disease. Gut Microbes. (2020) 11:539–55. doi: 10.1080/19490976.2019.1700754
18. van der Houwen TB, van Laar JAM, Kappen JH, van Hagen PM, de Zoete MR, van Muijlwijk GH, et al. Behçet's disease under microbiotic surveillance? A combined analysis of two cohorts of behçet's disease patients. Front Immunol. (2020) 11:1192. doi: 10.3389/fimmu.2020.01192
19. Shimizu J, Kubota T, Takada E, Takai K, Fujiwara N, Arimitsu N, et al. Relative abundance of megamonas hypermegale and butyrivibrio species decreased in the intestine and its possible association with the T cell aberration by metabolite alteration in patients with behcet's disease (210 characters). Clin Rheumatol. (2019) 38:1437–45. doi: 10.1007/s10067-018-04419-8
20. Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. (2013) 504:451–5. doi: 10.1038/nature12726
21. Peng L, Li ZR, Green RS, Holzman IR, and Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of amp-activated protein kinase in caco-2 cell monolayers. J Nutr. (2009) 139:1619–25. doi: 10.3945/jn.109.104638
22. Liévin V, Peiffer I, Hudault S, Rochat F, Brassart D, Neeser JR, et al. Bifidobacterium strains from resident infant human gastrointestinal microflora exert antimicrobial activity. Gut. (2000) 47:646–52. doi: 10.1136/gut.47.5.646
23. Tanabe S. The effect of probiotics and gut microbiota on th17 cells. Int Rev Immunol. (2013) 32:511–25. doi: 10.3109/08830185.2013.839665
24. Li M, Yang L, Cao J, Liu T, and Liu X. Enriched and decreased intestinal microbes in active vkh patients. Invest Ophthalmol Visual Sci. (2022) 63:21. doi: 10.1167/iovs.63.2.21
25. Shimizu J, Kubota T, Takada E, Takai K, Fujiwara N, Arimitsu N, et al. Bifidobacteria abundance-featured gut microbiota compositional change in patients with behcet's disease. PloS One. (2016) 11:e0153746. doi: 10.1371/journal.pone.0153746
26. Flint HJ, Duncan SH, Scott KP, and Louis P. Links between diet, gut microbiota composition and gut metabolism. Proc Nutr Soc. (2015) 74:13–22. doi: 10.1017/s0029665114001463
27. Marquet P, Duncan SH, Chassard C, Bernalier-Donadille A, and Flint HJ. Lactate has the potential to promote hydrogen sulphide formation in the human colon. FEMS Microbiol Lett. (2009) 299:128–34. doi: 10.1111/j.1574-6968.2009.01750.x
28. Szabó C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. (2007) 6:917–35. doi: 10.1038/nrd2425
29. Li M, Liu M, Wang X, Wei H, Jin S, and Liu X. Comparison of intestinal microbes and metabolites in active vkh versus acute anterior uveitis associated with ankylosing spondylitis. Br J Ophthalmol. (2025) 109:353–61. doi: 10.1136/bjo-2023-324125
30. Di Stefano A, Ricciardolo FLM, Caramori G, Adcock IM, Chung KF, Barnes PJ, et al. Bronchial inflammation and bacterial load in stable copd is associated with tlr4 overexpression. Eur Respir J. (2017) 49:1602006. doi: 10.1183/13993003.02006-2016
31. Yang Y, Yang JY, and Chen XJ. Biotinidase deficiency characterized by skin and hair findings. Clinics Dermatol. (2020) 38:477–83. doi: 10.1016/j.clindermatol.2020.03.004
32. Yasar Bilge NS, Pérez Brocal V, Kasifoglu T, Bilge U, Kasifoglu N, Moya A, et al. Intestinal microbiota composition of patients with behçet's disease: differences between eye, mucocutaneous and vascular involvement. Rheuma-Biota Study Clin Exp Rheumatol. (2020) 38 Suppl 127:60–8.
33. Auchtung TA, Fofanova TY, Stewart CJ, Nash AK, Wong MC, Gesell JR, et al. Investigating colonization of the healthy adult gastrointestinal tract by fungi. mSphere. (2018) 3:e00092-18. doi: 10.1128/mSphere.00092-18
34. Nash AK, Auchtung TA, Wong MC, Smith DP, Gesell JR, Ross MC, et al. The gut mycobiome of the human microbiome project healthy cohort. Microbiome. (2017) 5:153. doi: 10.1186/s40168-017-0373-4
35. Jayasudha R, Kalyana Chakravarthy S, Sai Prashanthi G, Sharma S, Tyagi M, and Shivaji S. Implicating dysbiosis of the gut fungal microbiome in uveitis, an inflammatory disease of the eye. Invest Ophthalmol Visual Sci. (2019) 60:1384–93. doi: 10.1167/iovs.18-26426
36. Salvador R, Horai R, Zhang A, Jittayasothorn Y, Tang J, Gupta A, et al. Too much of a good thing: extended duration of gut microbiota depletion reverses protection from experimental autoimmune uveitis. Invest Ophthalmol Visual Sci. (2023) 64:43. doi: 10.1167/iovs.64.14.43
37. Cao Z, Sugimura N, Burgermeister E, Ebert MP, Zuo T, and Lan P. The gut virome: A new microbiome component in health and disease. EBioMedicine. (2022) 81:104113. doi: 10.1016/j.ebiom.2022.104113
38. de Jonge PA, Wortelboer K, Scheithauer TPM, van den Born BH, Zwinderman AH, Nobrega FL, et al. Gut virome profiling identifies a widespread bacteriophage family associated with metabolic syndrome. Nat Commun. (2022) 13:3594. doi: 10.1038/s41467-022-31390-5
39. Vatanen T, Franzosa EA, Schwager R, Tripathi S, Arthur TD, Vehik K, et al. The human gut microbiome in early-onset type 1 diabetes from the teddy study. Nature. (2018) 562:589–94. doi: 10.1038/s41586-018-0620-2
40. Mangalea MR, Paez-Espino D, Kieft K, Chatterjee A, Chriswell ME, Seifert JA, et al. Individuals at risk for rheumatoid arthritis harbor differential intestinal bacteriophage communities with distinct metabolic potential. Cell Host Microbe. (2021) 29:726–39.e5. doi: 10.1016/j.chom.2021.03.020
41. Tomofuji Y, Kishikawa T, Maeda Y, Ogawa K, Nii T, Okuno T, et al. Whole gut virome analysis of 476 Japanese revealed a link between phage and autoimmune disease. Ann rheumatic Dis. (2022) 81:278–88. doi: 10.1136/annrheumdis-2021-221267
42. Shete O and Ghosh TS. Normal gut microbiomes in diverse populations: clinical implications. Annu Rev Med. (2025) 76:95–114. doi: 10.1146/annurev-med-051223-031809
43. Minoda H, Sakai J, Sugiura M, Imai S, Osato T, and Usui M. High inducibility of epstein-barr virus replication in B lymphocytes in vogt-koyanagi-harada disease. Nippon Ganka Gakkai zasshi. (1999) 103:289–96.
44. Bassili SS, Peyman GA, Gebhardt BM, Daun M, Ganiban GJ, and Rifai A. Detection of epstein-barr virus DNA by polymerase chain reaction in the vitreous from a patient with vogt-koyanagi-harada syndrome. Retina (Philadelphia Pa). (1996) 16:160–1. doi: 10.1097/00006982-199616020-00013
45. Sugita S, Takase H, Taguchi C, Imai Y, Kamoi K, Kawaguchi T, et al. Ocular infiltrating cd4+ T cells from patients with vogt-koyanagi-harada disease recognize human melanocyte antigens. Invest Ophthalmol Visual Sci. (2006) 47:2547–54. doi: 10.1167/iovs.05-1547
46. Sugita S, Takase H, Kawaguchi T, Taguchi C, and Mochizuki M. Cross-reaction between tyrosinase peptides and cytomegalovirus antigen by T cells from patients with vogt-koyanagi-harada disease. Int Ophthalmol. (2007) 27:87–95. doi: 10.1007/s10792-006-9020-y
47. Sciascia S, Arbrile M, Trunfio M, Calcagno A, Radin M, Roccatello D, et al. The role of bacteria and viruses in behçet syndrome: should we move towards new paradigms? Autoimmun Rev. (2023) 22:103237. doi: 10.1016/j.autrev.2022.103237
48. Studd M, McCance DJ, and Lehner T. Detection of hsv-1 DNA in patients with behçet's syndrome and in patients with recurrent oral ulcers by the polymerase chain reaction. J Med Microbiol. (1991) 34:39–43. doi: 10.1099/00222615-34-1-39
49. Lee S, Bang D, Cho YH, Lee ES, and Sohn S. Polymerase chain reaction reveals herpes simplex virus DNA in saliva of patients with behçet's disease. Arch Dermatol Res. (1996) 288:179–83. doi: 10.1007/bf02505221
50. Greco A, De Virgilio A, Ralli M, Ciofalo A, Mancini P, Attanasio G, et al. Behçet's disease: new insights into pathophysiology, clinical features and treatment options. Autoimmun Rev. (2018) 17:567–75. doi: 10.1016/j.autrev.2017.12.006
51. Sun M, Wu W, Liu Z, and Cong Y. Microbiota metabolite short chain fatty acids, gpcr, and inflammatory bowel diseases. J Gastroenterol. (2017) 52:1–8. doi: 10.1007/s00535-016-1242-9
52. Louis P, Young P, Holtrop G, and Flint HJ. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-coa : acetate coa-transferase gene. Environ Microbiol. (2010) 12:304–14. doi: 10.1111/j.1462-2920.2009.02066.x
53. Ze X, Duncan SH, Louis P, and Flint HJ. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J. (2012) 6:1535–43. doi: 10.1038/ismej.2012.4
54. Louis P, Hold GL, and Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. (2014) 12:661–72. doi: 10.1038/nrmicro3344
55. Reichardt N, Duncan SH, Young P, Belenguer A, McWilliam Leitch C, Scott KP, et al. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. (2014) 8:1323–35. doi: 10.1038/ismej.2014.14
56. Parada Venegas D, de la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, et al. Short chain fatty acids (Scfas)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. (2019) 10:277. doi: 10.3389/fimmu.2019.00277
57. Wong JM, de Souza R, Kendall CW, Emam A, and Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. (2006) 40:235–43. doi: 10.1097/00004836-200603000-00015
58. Zhao Y, Chen F, Wu W, Sun M, Bilotta AJ, Yao S, et al. Gpr43 mediates microbiota metabolite scfa regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mtor and stat3. Mucosal Immunol. (2018) 11:752–62. doi: 10.1038/mi.2017.118
59. Vinolo MA, Rodrigues HG, Nachbar RT, and Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. (2011) 3:858–76. doi: 10.3390/nu3100858
60. Lin MY, de Zoete MR, van Putten JP, and Strijbis K. Redirection of epithelial immune responses by short-chain fatty acids through inhibition of histone deacetylases. Front Immunol. (2015) 6:554. doi: 10.3389/fimmu.2015.00554
61. Vinolo MA, Rodrigues HG, Hatanaka E, Sato FT, Sampaio SC, and Curi R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J Nutr Biochem. (2011) 22:849–55. doi: 10.1016/j.jnutbio.2010.07.009
62. Haghikia A, Jörg S, Duscha A, Berg J, Manzel A, Waschbisch A, et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity. (2015) 43:817–29. doi: 10.1016/j.immuni.2015.09.007
63. Ishikawa T and Nanjo F. Dietary cycloinulooligosaccharides enhance intestinal immunoglobulin a production in mice. Bioscience biotechnology Biochem. (2009) 73:677–82. doi: 10.1271/bbb.80733
64. Soliman ML, Puig KL, Combs CK, and Rosenberger TA. Acetate reduces microglia inflammatory signaling in vitro. J neurochemistry. (2012) 123:555–67. doi: 10.1111/j.1471-4159.2012.07955.x
65. Wang Q, Yi S, Su G, Du Z, Pan S, Huang X, et al. Changes in the gut microbiome contribute to the development of behcet's disease via adjuvant effects. Front Cell Dev Biol. (2021) 9:716760. doi: 10.3389/fcell.2021.716760
66. Nakamura YK, Janowitz C, Metea C, Asquith M, Karstens L, Rosenbaum JT, et al. Short chain fatty acids ameliorate immune-mediated uveitis partially by altering migration of lymphocytes from the intestine. Sci Rep. (2017) 7:11745. doi: 10.1038/s41598-017-12163-3
67. Chen X, Su W, Wan T, Yu J, Zhu W, Tang F, et al. Sodium butyrate regulates th17/treg cell balance to ameliorate uveitis via the nrf2/ho-1 pathway. Biochem Pharmacol. (2017) 142:111–9. doi: 10.1016/j.bcp.2017.06.136
68. Nakamura YK, Metea C, Llorenç V, Karstens L, Balter A, and Lin P. A diet rich in fermentable fiber promotes robust changes in the intestinal microbiota, mitigates intestinal permeability, and attenuates autoimmune uveitis. Sci Rep. (2023) 13:10806. doi: 10.1038/s41598-023-37062-8
69. Emmi G, Bettiol A, Niccolai E, Ramazzotti M, Amedei A, Pagliai G, et al. Butyrate-rich diets improve redox status and fibrin lysis in behçet's syndrome. Circ Res. (2021) 128:278–80. doi: 10.1161/circresaha.120.317789
70. Fiorucci S, Biagioli M, Zampella A, and Distrutti E. Bile acids activated receptors regulate innate immunity. Front Immunol. (2018) 9:1853. doi: 10.3389/fimmu.2018.01853
71. Fiorucci S and Distrutti E. Bile acid-activated receptors, intestinal microbiota, and the treatment of metabolic disorders. Trends Mol Med. (2015) 21:702–14. doi: 10.1016/j.molmed.2015.09.001
72. Yang J, Hu J, Feng L, Yi S, Ye Z, Lin M, et al. Decreased expression of tgr5 in vogt-koyanagi-harada (Vkh) disease. Ocular Immunol Inflammation. (2020) 28:200–8. doi: 10.1080/09273948.2018.1560477
73. Hu J, Wang C, Huang X, Yi S, Pan S, Zhang Y, et al. Gut microbiota-mediated secondary bile acids regulate dendritic cells to attenuate autoimmune uveitis through tgr5 signaling. Cell Rep. (2021) 36:109726. doi: 10.1016/j.celrep.2021.109726
74. Huang X, Ye Z, Cao Q, Su G, Wang Q, Deng J, et al. Gut microbiota composition and fecal metabolic phenotype in patients with acute anterior uveitis. Invest Ophthalmol Visual Sci. (2018) 59:1523–31. doi: 10.1167/iovs.17-22677
75. Hu J, Zhang Y, Yi S, Wang C, Huang X, Pan S, et al. Lithocholic acid inhibits dendritic cell activation by reducing intracellular glutathione via tgr5 signaling. Int J Biol Sci. (2022) 18:4545–59. doi: 10.7150/ijbs.71287
76. Williams BB, Van Benschoten AH, Cimermancic P, Donia MS, Zimmermann M, Taketani M, et al. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe. (2014) 16:495–503. doi: 10.1016/j.chom.2014.09.001
77. Rothhammer V, Borucki DM, Garcia Sanchez MI, Mazzola MA, Hemond CC, Regev K, et al. Dynamic regulation of serum aryl hydrocarbon receptor agonists in ms. Neurology(R) neuroimmunology Neuroinflamm. (2017) 4:e359. doi: 10.1212/nxi.0000000000000359
78. Ogbechi J, Huang YS, Clanchy FIL, Pantazi E, Topping LM, Darlington LG, et al. Modulation of immune cell function, ido expression and kynurenine production by the quorum sensor 2-heptyl-3-hydroxy-4-quinolone (Pqs). Front Immunol. (2022) 13:1001956. doi: 10.3389/fimmu.2022.1001956
79. Schröcksnadel K, Wirleitner B, Winkler C, and Fuchs D. Monitoring tryptophan metabolism in chronic immune activation. Clinica chimica acta; Int J Clin Chem. (2006) 364:82–90. doi: 10.1016/j.cca.2005.06.013
80. Palabiyik SS, Keles S, Girgin G, Arpali-Tanas E, Topdagi E, and Baydar T. Neopterin release and tryptophan degradation in patients with uveitis. Curr eye Res. (2016) 41:1513–7. doi: 10.3109/02713683.2015.1133830
81. Zhang L, Huang Y, Cui X, Tan X, Zhu Y, Zhou W, et al. Increased expression of indoleamine 2,3-dioxygenase (Ido) in vogt-koyanagi-harada (Vkh) disease may lead to a shift of T cell responses toward a treg population. Inflammation. (2020) 43:1780–8. doi: 10.1007/s10753-020-01252-7
82. Eryavuz Onmaz D, Tezcan D, Abusoglu S, Sivrikaya A, Kuzu M, Yerlikaya FH, et al. Elevated serum levels of kynurenine pathway metabolites in patients with behçet disease. Amino Acids. (2022) 54:877–87. doi: 10.1007/s00726-022-03170-4
83. Tillett BJ, Dwiyanto J, Secombe KR, George T, Zhang V, Anderson D, et al. Scfa biotherapy delays diabetes in humanized gnotobiotic mice by remodeling mucosal homeostasis and metabolome. Nat Commun. (2025) 16:2893. doi: 10.1038/s41467-025-58319-y
84. Agus A, Clément K, and Sokol H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut. (2021) 70:1174–82. doi: 10.1136/gutjnl-2020-323071
85. Lavelle A and Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. (2020) 17:223–37. doi: 10.1038/s41575-019-0258-z
86. Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. (2015) 161:264–76. doi: 10.1016/j.cell.2015.02.047
87. Liu M, Li M, Jin S, Wang X, Geng J, and Liu X. Differential intestinal microbes and metabolites between behcet's uveitis and fuchs syndrome. Heliyon. (2024) 10:e39393. doi: 10.1016/j.heliyon.2024.e39393
88. Ahn JK, Kim J, Hwang J, Song J, Kim KH, and Cha HS. Potential metabolomic biomarkers for reliable diagnosis of behcet's disease using gas chromatography/ time-of-flight-mass spectrometry. Joint Bone Spine. (2018) 85:337–43. doi: 10.1016/j.jbspin.2017.05.019
89. Zheng W, Wu X, Goudarzi M, Shi J, Song W, Li C, et al. Metabolomic alterations associated with behçet's disease. Arthritis Res Ther. (2018) 20:214. doi: 10.1186/s13075-018-1712-y
90. Hou CC, Bao HF, She CH, Chen HY, Pan GX, Chen HN, et al. Specific plasma metabolite profile in intestinal behçet's syndrome. Orphanet J rare Dis. (2025) 20:21. doi: 10.1186/s13023-024-03484-4
91. Park SJ, Park MJ, Park S, Lee ES, and Lee DY. Integrative metabolomics of plasma and pbmcs identifies distinctive metabolic signatures in behçet's disease. Arthritis Res Ther. (2023) 25:5. doi: 10.1186/s13075-022-02986-5
92. Ahn JK, Kim J, Hwang J, Song J, Kim KH, and Cha HS. Urinary metabolomic profiling to identify potential biomarkers for the diagnosis of behcet's disease by gas chromatography/time-of-flight-mass spectrometry. Int J Mol Sci. (2017) 18:2309. doi: 10.3390/ijms18112309
93. Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev. (2011) 91:151–75. doi: 10.1152/physrev.00003.2008
94. Fresko I, Hamuryudan V, Demir M, Hizli N, Sayman H, Melikoğlu M, et al. Intestinal permeability in behçet's syndrome. Ann rheumatic Dis. (2001) 60:65–6. doi: 10.1136/ard.60.1.65
95. Bañares AA, Jover JA, Fernández-Gutiérrez B, Benítez del Castillo JM, García J, González F, et al. Bowel inflammation in anterior uveitis and spondyloarthropathy. J Rheumatol. (1995) 22:1112–7.
96. Chen N, Wu J, Wang J, Piri N, Chen F, Xiao T, et al. Short chain fatty acids inhibit endotoxin-induced uveitis and inflammatory responses of retinal astrocytes. Exp eye Res. (2021) 206:108520. doi: 10.1016/j.exer.2021.108520
97. Spadoni I, Zagato E, Bertocchi A, Paolinelli R, Hot E, Di Sabatino A, et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Sci (New York NY). (2015) 350:830–4. doi: 10.1126/science.aad0135
98. Mölzer C, Heissigerova J, Wilson HM, Kuffova L, and Forrester JV. Immune privilege: the microbiome and uveitis. Front Immunol. (2020) 11:608377. doi: 10.3389/fimmu.2020.608377
99. Christovich A and Luo XM. Gut microbiota, leaky gut, and autoimmune diseases. Front Immunol. (2022) 13:946248. doi: 10.3389/fimmu.2022.946248
100. Honda K and Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. (2016) 535:75–84. doi: 10.1038/nature18848
101. Tomura M, Yoshida N, Tanaka J, Karasawa S, Miwa Y, Miyawaki A, et al. Monitoring cellular movement in vivo with photoconvertible fluorescence protein "Kaede" Transgenic mice. Proc Natl Acad Sci United States America. (2008) 105:10871–6. doi: 10.1073/pnas.0802278105
102. Horai R, Zárate-Bladés CR, Dillenburg-Pilla P, Chen J, Kielczewski JL, Silver PB, et al. Microbiota-dependent activation of an autoreactive T cell receptor provokes autoimmunity in an immunologically privileged site. Immunity. (2015) 43:343–53. doi: 10.1016/j.immuni.2015.07.014
103. Zárate-Bladés CR, Horai R, Mattapallil MJ, Ajami NJ, Wong M, Petrosino JF, et al. Gut microbiota as a source of a surrogate antigen that triggers autoimmunity in an immune privileged site. Gut Microbes. (2017) 8:59–66. doi: 10.1080/19490976.2016.1273996
104. Wang Q, Wu S, Ye X, Tan S, Huang F, Su G, et al. Gut microbial signatures and their functions in behcet's uveitis and vogt-koyanagi-harada disease. J Autoimmun. (2023) 137:103055. doi: 10.1016/j.jaut.2023.103055
105. Du Z, Wang Q, Huang X, Yi S, Mei S, Yuan G, et al. Effect of berberine on spleen transcriptome and gut microbiota composition in experimental autoimmune uveitis. Int Immunopharmacol. (2020) 81:106270. doi: 10.1016/j.intimp.2020.106270
106. Xiang S, Chen J, Deng M, Wang Z, Li X, Lin D, et al. Celastrol ameliorates experimental autoimmune uveitis through stat3 targeting and gut microenvironment reprofiling. Int Immunopharmacol. (2024) 127:111339. doi: 10.1016/j.intimp.2023.111339
107. Zhou J, Yang J, Dai M, Lin D, Zhang R, Liu H, et al. A combination of inhibiting microglia activity and remodeling gut microenvironment suppresses the development and progression of experimental autoimmune uveitis. Biochem Pharmacol. (2020) 180:114108. doi: 10.1016/j.bcp.2020.114108
108. Heissigerova J, Seidler Stangova P, Klimova A, Svozilkova P, Hrncir T, Stepankova R, et al. The microbiota determines susceptibility to experimental autoimmune uveoretinitis. J Immunol Res. (2016) 2016:5065703. doi: 10.1155/2016/5065703
109. Liu Y, Zhao C, Meng J, Li N, Xu Z, Liu X, et al. Galectin-3 regulates microglial activation and promotes inflammation through tlr4/myd88/nf-kb in experimental autoimmune uveitis. Clin Immunol (Orlando Fla). (2022) 236:108939. doi: 10.1016/j.clim.2022.108939
110. Okunuki Y, Mukai R, Nakao T, Tabor SJ, Butovsky O, Dana R, et al. Retinal microglia initiate neuroinflammation in ocular autoimmunity. Proc Natl Acad Sci United States America. (2019) 116:9989–98. doi: 10.1073/pnas.1820387116
111. Llorenç V, Nakamura Y, Metea C, Karstens L, Molins B, and Lin P. Antimetabolite drugs exhibit distinctive immunomodulatory mechanisms and effects on the intestinal microbiota in experimental autoimmune uveitis. Invest Ophthalmol Visual Sci. (2022) 63:30. doi: 10.1167/iovs.63.3.30
112. Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. (2014) 11:506–14. doi: 10.1038/nrgastro.2014.66
113. Kim J, Choi SH, Kim YJ, Jeong HJ, Ryu JS, Lee HJ, et al. Clinical effect of irt-5 probiotics on immune modulation of autoimmunity or alloimmunity in the eye. Nutrients. (2017) 9:1166. doi: 10.3390/nu9111166
114. Lee RW, Nicholson LB, Sen HN, Chan CC, Wei L, Nussenblatt RB, et al. Autoimmune and autoinflammatory mechanisms in uveitis. Semin immunopathology. (2014) 36:581–94. doi: 10.1007/s00281-014-0433-9
115. Dusek O, Fajstova A, Klimova A, Svozilkova P, Hrncir T, Kverka M, et al. Severity of experimental autoimmune uveitis is reduced by pretreatment with live probiotic escherichia coli nissle 1917. Cells. (2020) 10:23. doi: 10.3390/cells10010023
116. Napolitano P, Filippelli M, D'Andrea L, Carosielli M, dell'Omo R, and Costagliola C. Probiotic supplementation improved acute anterior uveitis of 3-year duration: A case report. Am J Case Rep. (2021) 22:e931321. doi: 10.12659/ajcr.931321
117. Askari G and Moravejolahkami AR. Synbiotic supplementation may relieve anterior uveitis, an ocular manifestation in behcet's syndrome. Am J Case Rep. (2019) 20:548–50. doi: 10.12659/ajcr.912023
118. Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients. (2013) 5:1417–35. doi: 10.3390/nu5041417
119. Enam F and Mansell TJ. Prebiotics: tools to manipulate the gut microbiome and metabolome. J Ind Microbiol Biotechnol. (2019) 46:1445–59. doi: 10.1007/s10295-019-02203-4
120. Merenstein D, Pot B, Leyer G, Ouwehand AC, Preidis GA, Elkins CA, et al. Emerging issues in probiotic safety: 2023 perspectives. Gut Microbes. (2023) 15:2185034. doi: 10.1080/19490976.2023.2185034
121. Suez J, Zmora N, Zilberman-Schapira G, Mor U, Dori-Bachash M, Bashiardes S, et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous fmt. Cell. (2018) 174:1406–23.e16. doi: 10.1016/j.cell.2018.08.047
122. Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, et al. Probiotic prophylaxis in patients with predicted severe acute pancreatitis: A randomised, double-blind, placebo-controlled trial. Nederlands tijdschrift voor geneeskunde. (2008) 152:685–96.
123. Islam MA, Khandker SS, Kotyla PJ, and Hassan R. Immunomodulatory effects of diet and nutrients in systemic lupus erythematosus (Sle): A systematic review. Front Immunol. (2020) 11:1477. doi: 10.3389/fimmu.2020.01477
124. Dicks LMT. How important are fatty acids in human health and can they be used in treating diseases? Gut Microbes. (2024) 16:2420765. doi: 10.1080/19490976.2024.2420765
125. Huang X, Yi S, Hu J, Du Z, Wang Q, Ye Z, et al. Linoleic acid inhibits in vitro function of human and murine dendritic cells, cd4(+)T cells and retinal pigment epithelial cells. Graefe's Arch Clin Exp Ophthalmol = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. (2021) 259:987–98. doi: 10.1007/s00417-020-04972-6
126. van Rooij J, Schwartzenberg SG, Mulder PG, and Baarsma SG. Oral vitamins C and E as additional treatment in patients with acute anterior uveitis: A randomised double masked study in 145 patients. Br J Ophthalmol. (1999) 83:1277–82. doi: 10.1136/bjo.83.11.1277
127. Li Z, Duan R, Jiang Q, Liu J, Chen J, Jiang L, et al. Dietary caloric restriction protects experimental autoimmune uveitis by regulating teff/treg balance. iScience. (2024) 27:111279. doi: 10.1016/j.isci.2024.111279
128. Duan R, Wang T, Li Z, Jiang L, Yu X, He D, et al. Ketogenic diet modulates immune cell transcriptional landscape and ameliorates experimental autoimmune uveitis in mice. J Neuroinflamm. (2024) 21:319. doi: 10.1186/s12974-024-03308-z
129. Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Sci (New York NY). (2011) 334:105–8. doi: 10.1126/science.1208344
130. Crowe TC. Safety of low-carbohydrate diets. Obes reviews: an Off J Int Assoc Study Obes. (2005) 6:235–45. doi: 10.1111/j.1467-789X.2005.00196.x
131. Duncan SH, Belenguer A, Holtrop G, Johnstone AM, Flint HJ, and Lobley GE. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl Environ Microbiol. (2007) 73:1073–8. doi: 10.1128/aem.02340-06
132. Seidler Stangova P, Dusek O, Klimova A, Heissigerova J, Kucera T, and Svozilkova P. Metronidazole attenuates the intensity of inflammation in experimental autoimmune uveitis. Folia biologica. (2019) 65:265–74. doi: 10.14712/fb2019065050265
133. Schulfer AF, Battaglia T, Alvarez Y, Bijnens L, Ruiz VE, Ho M, et al. Intergenerational transfer of antibiotic-perturbed microbiota enhances colitis in susceptible mice. Nat Microbiol. (2018) 3:234–42. doi: 10.1038/s41564-017-0075-5
134. Becattini S, Taur Y, and Pamer EG. Antibiotic-induced changes in the intestinal microbiota and disease. Trends Mol Med. (2016) 22:458–78. doi: 10.1016/j.molmed.2016.04.003
135. Weingarden AR and Vaughn BP. Intestinal microbiota, fecal microbiota transplantation, and inflammatory bowel disease. Gut Microbes. (2017) 8:238–52. doi: 10.1080/19490976.2017.1290757
136. Carvalho T. First oral fecal microbiota transplant therapy approved. Nat Med. (2023) 29:1581–2. doi: 10.1038/d41591-023-00046-2
137. Ianiro G, Masucci L, Quaranta G, Simonelli C, Lopetuso LR, Sanguinetti M, et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy plus vancomycin for the treatment of severe refractory clostridium difficile infection-single versus multiple infusions. Alimentary Pharmacol Ther. (2018) 48:152–9. doi: 10.1111/apt.14816
138. Karimi M, Shirsalimi N, Hashempour Z, Salehi Omran H, Sedighi E, Beigi F, et al. Safety and efficacy of fecal microbiota transplantation (Fmt) as a modern adjuvant therapy in various diseases and disorders: A comprehensive literature review. Front Immunol. (2024) 15:1439176. doi: 10.3389/fimmu.2024.1439176
139. Imdad A, Pandit NG, Zaman M, Minkoff NZ, Tanner-Smith EE, Gomez-Duarte OG, et al. Fecal transplantation for treatment of inflammatory bowel disease. Cochrane Database systematic Rev. (2023) 4:Cd012774. doi: 10.1002/14651858.CD012774.pub3
140. Zhang B, Zhou W, Liu Q, Huang C, Hu Z, Zheng M, et al. Effects of fecal microbiota transplant on DNA methylation in patients with systemic lupus erythematosus. J Autoimmun. (2023) 141:103047. doi: 10.1016/j.jaut.2023.103047
141. Kujawa D, Laczmanski L, Budrewicz S, Pokryszko-Dragan A, and Podbielska M. Targeting gut microbiota: new therapeutic opportunities in multiple sclerosis. Gut Microbes. (2023) 15:2274126. doi: 10.1080/19490976.2023.2274126
142. Yu Y, Wang W, and Zhang F. The next generation fecal microbiota transplantation: to transplant bacteria or virome. Advanced Sci (Weinheim Baden-Wurttemberg Germany). (2023) 10:e2301097. doi: 10.1002/advs.202301097
143. Ott SJ, Waetzig GH, Rehman A, Moltzau-Anderson J, Bharti R, Grasis JA, et al. Efficacy of sterile fecal filtrate transfer for treating patients with clostridium difficile infection. Gastroenterology. (2017) 152:799–811.e7. doi: 10.1053/j.gastro.2016.11.010
144. Mao X, Larsen SB, Zachariassen LSF, Brunse A, Adamberg S, Mejia JLC, et al. Transfer of modified gut viromes improves symptoms associated with metabolic syndrome in obese male mice. Nat Commun. (2024) 15:4704. doi: 10.1038/s41467-024-49152-w
145. DeFilipp Z, Bloom PP, Torres Soto M, Mansour MK, Sater MRA, Huntley MH, et al. Drug-resistant E. Coli bacteremia transmitted by fecal microbiota transplant. New Engl J Med. (2019) 381:2043–50. doi: 10.1056/NEJMoa1910437
146. De Leon LM, Watson JB, and Kelly CR. Transient flare of ulcerative colitis after fecal microbiota transplantation for recurrent clostridium difficile infection. Clin Gastroenterol hepatology: Off Clin Pract J Am Gastroenterological Assoc. (2013) 11:1036–8. doi: 10.1016/j.cgh.2013.04.045
147. Fischer M, Kao D, Kelly C, Kuchipudi A, Jafri SM, Blumenkehl M, et al. Fecal microbiota transplantation is safe and efficacious for recurrent or refractory clostridium difficile infection in patients with inflammatory bowel disease. Inflammatory bowel Dis. (2016) 22:2402–9. doi: 10.1097/mib.0000000000000908
148. Tecer D, Gogus F, Kalkanci A, Erdogan M, Hasanreisoglu M, Ergin Ç, et al. Succinivibrionaceae is dominant family in fecal microbiota of behçet's syndrome patients with uveitis. PloS One. (2020) 15:e0241691. doi: 10.1371/journal.pone.0241691
149. Park Y, Ahn JB, Kim DH, Park IS, Son M, Kim JH, et al. Integrated analysis of microbiome and metabolome reveals disease-specific profiles in inflammatory bowel diseases and intestinal behçet's disease. Int J Mol Sci. (2024) 25:6697. doi: 10.3390/ijms25126697
150. Morandi SC, Herzog EL, Munk M, Kreuzer M, Largiadèr CR, Wolf S, et al. The gut microbiome and hla-B27-associated anterior uveitis: A case-control study. J Neuroinflamm. (2024) 21:120. doi: 10.1186/s12974-024-03109-4
151. Kalyana Chakravarthy S, Jayasudha R, Sai Prashanthi G, Ali MH, Sharma S, Tyagi M, et al. Dysbiosis in the gut bacterial microbiome of patients with uveitis, an inflammatory disease of the eye. Indian J Microbiol. (2018) 58:457–69. doi: 10.1007/s12088-018-0746-9
152. Xu J, Su G, Huang X, Chang R, Chen Z, Ye Z, et al. Metabolomic analysis of aqueous humor identifies aberrant amino acid and fatty acid metabolism in vogt-koyanagi-harada and behcet's disease. Front Immunol. (2021) 12:587393. doi: 10.3389/fimmu.2021.587393
153. Tan S, Feng X, Liu Z, Wang Q, Jiang Q, Ye X, et al. The pro-inflammatory effect of triglyceride on human cd4(+) T cells and experimental autoimmune uveitis. Clin Immunol (Orlando Fla). (2022) 240:109056. doi: 10.1016/j.clim.2022.109056
154. Chen L, Chang R, Pan S, Xu J, Cao Q, Su G, et al. Plasma metabolomics study of vogt-koyanagi-harada disease identifies potential diagnostic biomarkers. Exp eye Res. (2020) 196:108070. doi: 10.1016/j.exer.2020.108070
Keywords: noninfectious uveitis, gut microbiome, gut-eye axis, dysregulation, T regulatory cells, T helper 1/17 cell, treatment intervention
Citation: Liu M, Geng J, Liu T and Liu X (2025) Gut microbiome dysregulation in noninfectious uveitis. Front. Immunol. 16:1614304. doi: 10.3389/fimmu.2025.1614304
Received: 18 April 2025; Accepted: 07 July 2025;
Published: 29 July 2025.
Edited by:
Lindsay B Nicholson, University of Bristol, United KingdomReviewed by:
Liping Du, The First Affiliated Hospital of Zhengzhou University, ChinaTimothy Janetos, Northwestern Medicine, United States
Jarmila Heissigerova, Charles University, Czechia
Chaokui Wang, Chongqing Eye Institute, China
Copyright © 2025 Liu, Geng, Liu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoli Liu, bHB3X2x4bEBqbHUuZWR1LmNu
 Mingzhu Liu
Mingzhu Liu Jiawei Geng
Jiawei Geng Tao Liu
Tao Liu Xiaoli Liu
Xiaoli Liu