- 1Department of Neurosurgery, Cancer Hospital of China Medical University, Liaoning Cancer Hospital & Institute, Shenyang, Liaoning, China
- 2Department of Integrated Traditional Chinese and Western Medicine Medical Oncology, Cancer Hospital of China Medical University, Liaoning Cancer Hospital & Institute, Shenyang, Liaoning, China
- 3Liaoning University of Traditional Chinese Medicine, Shenyang, Liaoning, China
- 4College of Acupuncture and Massage, Liaoning University of Traditional Chinese Medicine, Shenyang, Liaoning, China
- 5Dalian Medical University, Dalian, Liaoning, China
- 6Phase I Clinical Trial, Cancer Hospital of China Medical University, Liaoning Cancer Hospital & Institute, Shenyang, Liaoning, China
- 7Department of Interventional Medicine, Liaoning Provincial People’s Hospital, Shenyang, Liaoning, China
- 8China Medical University, Shenyang, Liaoning, China
The complexity of the tumor immune microenvironment (TIME), which is composed of mainly tumor cells, immune cells, and cytokines, is a major obstacle limiting the effectiveness of immunotherapy, and the interactions among these factors in the TIME determine the efficacy of antitumor immunity. Over the past few years, nanomaterials, owing to their unique physicochemical properties, multifunctionality, and good targeting ability, have gradually become important tools for modulating the immune microenvironment. By precisely delivering immunomodulatory factors, nanomaterials can effectively activate dendritic cells (DCs), enhance the function of effector T cells, and reverse the immunosuppressive state of tumor-associated macrophages (TAMs). In addition, nanomaterials can alleviate the local hypoxic and acidic tumor microenvironment, which in turn promotes immune cell function and enhances the antitumor immune effect. In light of the aforementioned associations, we summarize the existing studies, systematically describe the latest research progress on the use of nanomaterials in regulating the tumor immune microenvironment, and analyze the potential applications and challenges in tumor immunotherapy, with the goal of providing new therapeutic directions and strategies for tumor immunotherapy.
1 Introduction
Tumor immunotherapy, a major breakthrough in cancer treatment in recent years, works by activating the body’s immune defense system so that it can recognize and remove tumor cells. Immune cells constitute the cytological basis of immunotherapy. Therefore, understanding immune infiltration in the tumor immune microenvironment (TIME) is key to improving the potency of immunotherapy and developing new immunotherapeutic approaches (1). The tumor microenvironment is defined by cytokines and chemokines that facilitate immune suppression and an inflammatory condition. TME influences tumor differentiation, spread, and immune evasion. The TIME is a part of the tumor microenvironment (TME), and the TIME changes dynamically; as the tumor progresses, the immune system shifts from an immunosurveillance state to an immunosuppressive state. Initially, immune cells in the immune microenvironment attempt to attack tumor cells. On the other hand, as the cancer progresses, it alters the microenvironment, thereby suppressing the immune response (2).Nanoparticles possess unique properties (3, 4), and their precise delivery and targeting abilities and modifiability have made them important tools for modulating the TIME. These properties enable nanoparticles to deliver therapeutics directly to specific immune cells at the tumor site or in the tumor microenvironment, thus improving targeting. In addition, nanoparticles can be optimized to adjust the immune response, both by boosting antitumor immune cells and by suppressing immunosuppressive components of the tumor microenvironment (5). Not only do nanomaterials have many advantages, but nanomedicines are also advancing immuno-oncology research through their ability to deliver a variety of payloads and favorable molecular pharmacokinetics (6). The aforementioned benefits demonstrate that nanomaterials can effectively target drug delivery, influence immune cell activity, enhance drug delivery efficiency, and alter the tumor microenvironment. In recent years, the role of tumor-associated immune cells has led to new advances in tumor immunotherapy; thus, remodeling the TIME is a useful strategy for cancer treatment and immunotherapy (7, 8).
2 Characterization of the tumor immune microenvironment
The tumor microenvironment is a highly complex system consisting mainly of tumor cells, infiltrating immune cells, cancer-associated stromal cells, endothelial cells and adipocytes (9). In contrast, multiple immune cell populations in the tumor microenvironment, including inherent and adaptive immune cells, such as myeloid and lymphoid cells, constitute a major part of the TIME (10). In addition, other cell types, such as cancer-associated fibroblasts (CAFs), are also present in the TIME; the interactions between these cells and their functions together determine the role of the TIME in tumor progression (11, 12).
2.1 Cellular composition
The TIME consists of a complex network of multiple immune cell subtypes, cancer cells and stromal cells, which play key roles in tumor growth and disease progression (13). T lymphocytes, B lymphocytes, natural killer cells, dendritic cells, macrophages, myeloid-derived suppressor cells (MDSCs), fibroblasts and endothelial cells interact with tumor cells via sophisticated signaling pathways, forming a dynamic microenvironment (14, 15). The tumor immune microenvironment (TIME) is primarily composed of different immune cell populations found within the tumor microenvironment (TME), such as myeloid cells and lymphocytes, which include both innate and adaptive immune cells (16). IL-10 and analogous anti-inflammatory cytokines and chemokines can suppress the cytotoxic function of T cells and NK cells (17).
2.2 Immune escape
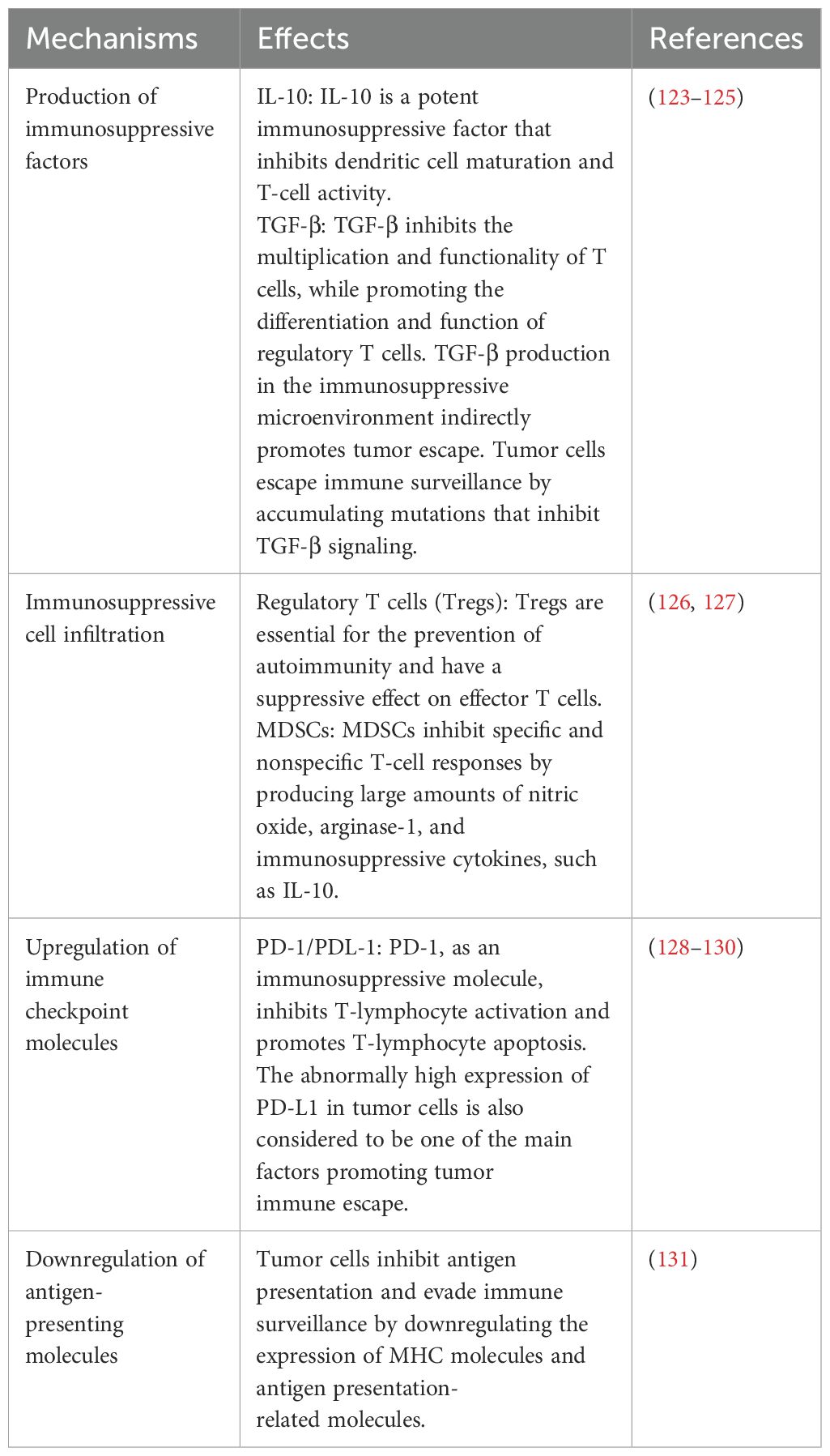
The immune system shifts from a surveillance state to an immunosuppressive state as the tumor progresses (18). Tumor cells evade immune surveillance through a variety of mechanisms, which together result in the formation of an immunosuppressive tumor microenvironment. The main mechanisms are shown in Table 1 (Figure 1).
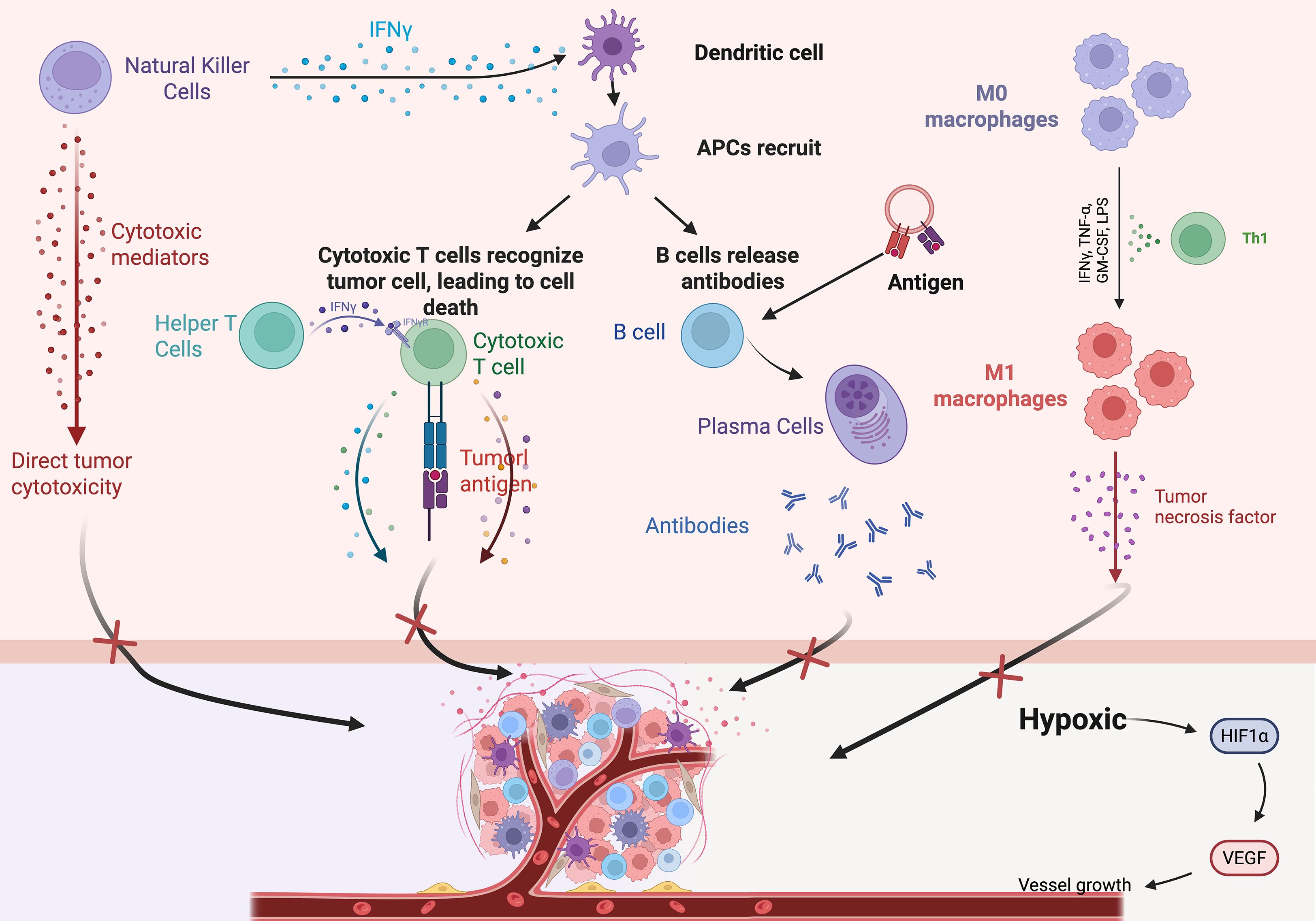
Figure 1. Schematic diagram of the tumor immune microenvironment, TIME major immune cells and their interactions. NK cells, helper T cells, IFNγ, cytotoxic T cells, DCs, etc.
3 Tumor immunotherapy
Tumor immunotherapy is a therapeutic approach that mobilizes the body’s own immune system to attack tumor cells. In the last several years, immunotherapy has achieved remarkable advancements in the treatment of a wide range of cancers, making it one of the most important Strategies for the treatment of cancer.
Immune checkpoints, such as cytotoxic T-lymphocyte antigen-4 (CTLA4) and programmed cell death-1 (PD-1), are cell surface proteins that mainly control the initiation, duration and intensity of the immune response (19). The interaction between CTLA-4, PD-1 and PD-L1 increases the activity of protein phosphatase 2 and Src homology region 2 domain-containing phosphatases, inhibiting T-cell activity (20). In contrast, immune checkpoint blockade (ICB) therapy, targets T-cell regulatory pathways with immune checkpoint inhibitors to enhance antitumor immune responses, and this treatment has been shown to significantly improve patient survival compared with conventional cancer therapy (21). Chimeric antigen receptor T-cell (CAR-T) therapy is another type of cancer immunotherapy. Chimeric antigen receptor (CAR) refers to a synthetic receptor that enables lymphocytes to recognize and destroy cells expressing a specific target antigen. CAR activates T cells and enhances immune responses by binding to target antigens expressed on the cell surface independently of MHC receptors (22). However, CAR-T-cell therapy still has several limitations, including life-threatening CAR-T-cell-related toxicity, inhibition of malignant B cells, antigen escape, and poor durability (23). Therefore, there is an urgent need to identify new therapeutic means. In addition, tumor vaccines have been put into clinical use as a therapy to activate T cells and thus enhance immune responses. Currently, there are three main therapeutic tumor vaccines: cellular vaccines, peptide vaccines, and nucleic acid vaccines. The primary function of tumor vaccines is mainly to increase the infiltration of tumor-infiltrating lymphocytes in the tumor microenvironment or to increase their antitumor activity (24). However, some limitations in the clinical application of tumor vaccines remain. In addition, some cytokines in the tumor microenvironment such as interleukin-2 (IL-2), interferon-α (interferon-α, IFNα) and interferon-γ (interferon-γ, IFNγ) have also been used in immunotherapy approaches (25). Hypoxia and cytokine signaling pathways, which involve proinflammatory mediators such TNF-α and IFN-γ, are the main adaptive signaling pathways that upregulate immune checkpoint molecules (26).Consequently, it aids in preserving immunological tolerance and preventing assaults from the immune system. However, the clinical application of these therapies has also been hampered by their short duration and strong toxicity (27).
Although the above therapies have certain therapeutic effects, they still have certain limitations and shortcomings, so the search for new therapeutic means is one of the urgent clinical problems to be solved (Figure 2).
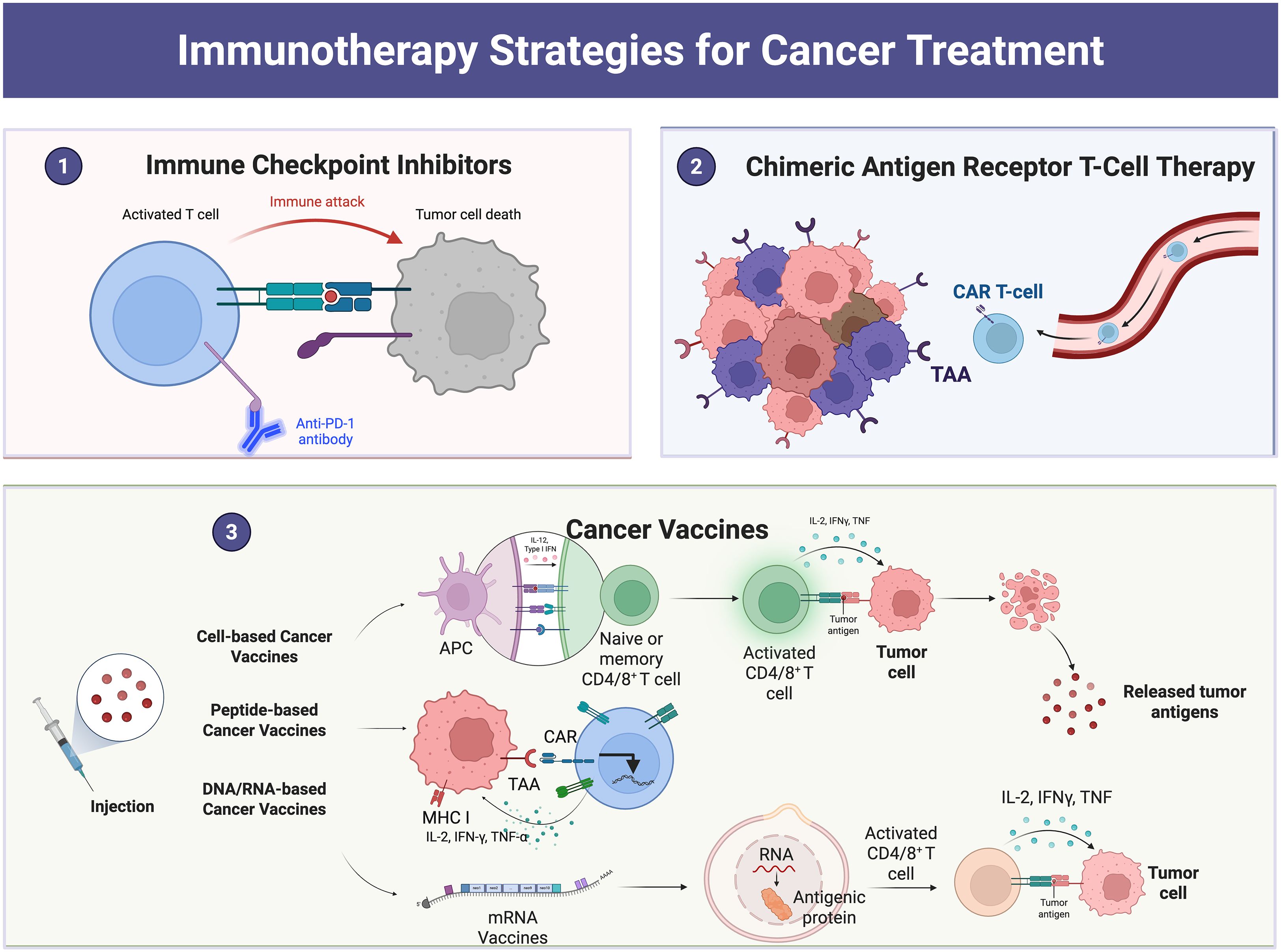
Figure 2. Examples of cancer treatment using immunotherapy: The figure shows immune checkpoint blockade therapy, CAR-T-cell therapy, tumor vaccines, etc.
4 Application of nanomaterials in modulating the tumor immune microenvironment
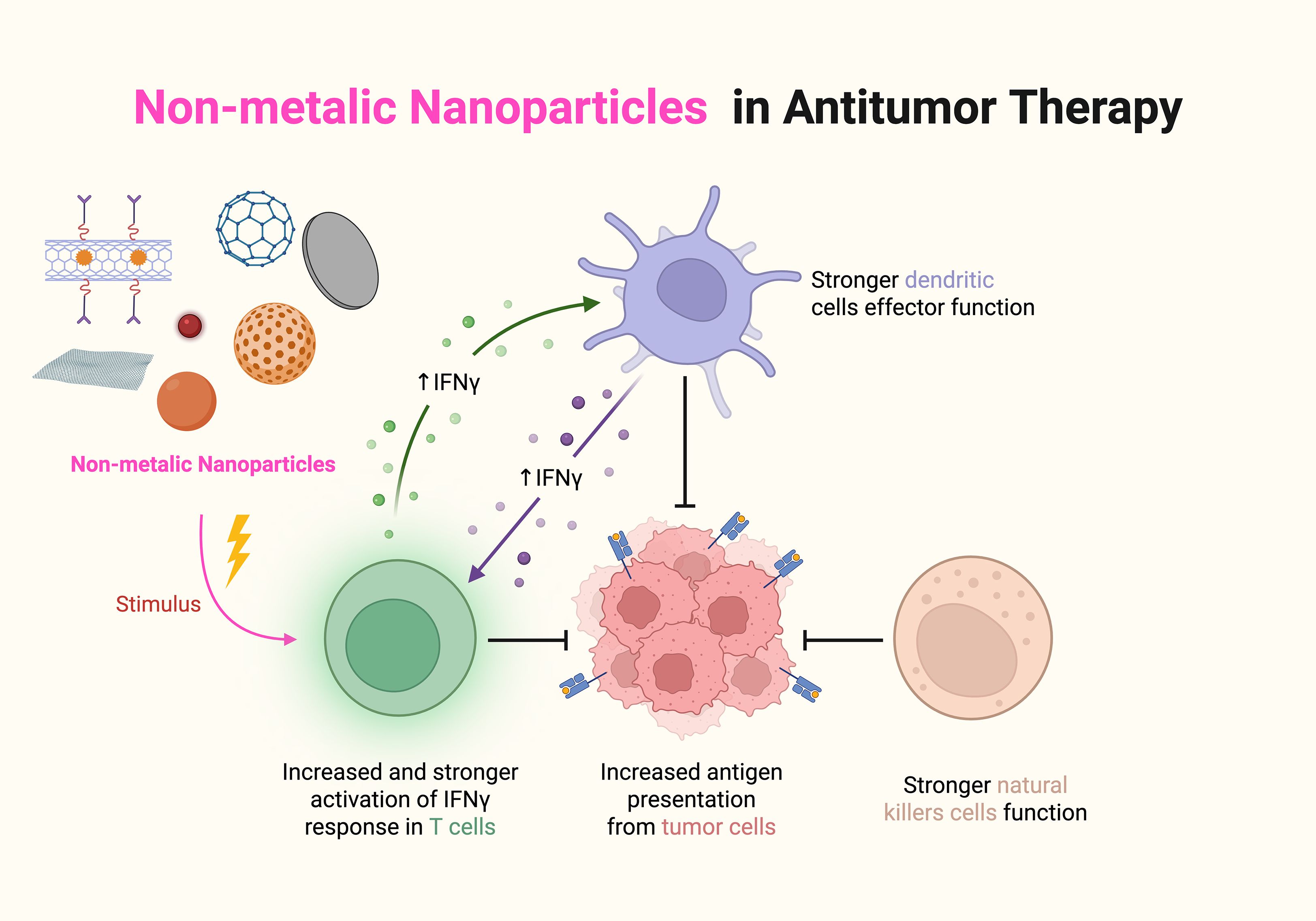
Owing to their small size and excellent physical, chemical and biological properties, nanomaterials have good potential for applications in medicine, energy, environmental protection, and electronics (28). Nanomaterials can act on complex and variable environments in the TIME and can specifically bind to immunosuppressive cells, thus improving immunotherapy efficacy. In addition, drug delivery systems constructed with nanoparticles have become favorable strategies for cancer treatment; for example, the main advantage of the utilization of nanoparticles as vehicles for drugs is that they can encapsulate the drug and transport it directly to tumor cells, which not only reduces drug toxicity but also maximizes the efficacy of the drug. Therefore, nanoparticles can be specifically developed to target components of the tumor microenvironment (TME) and disrupt the immunosuppressive TME, thereby enhancing the efficacy of cancer immunotherapy. Nanomaterials are categorized into organic nanomaterials, inorganic nanomaterials, and composite nanomaterials according to their composition (29). Therefore, in this work, we describe the regulatory effects of the above three different types of nanomaterials on the TIME in detail and analyze the potential application of these nanomaterials in immunotherapy (Figure 3).
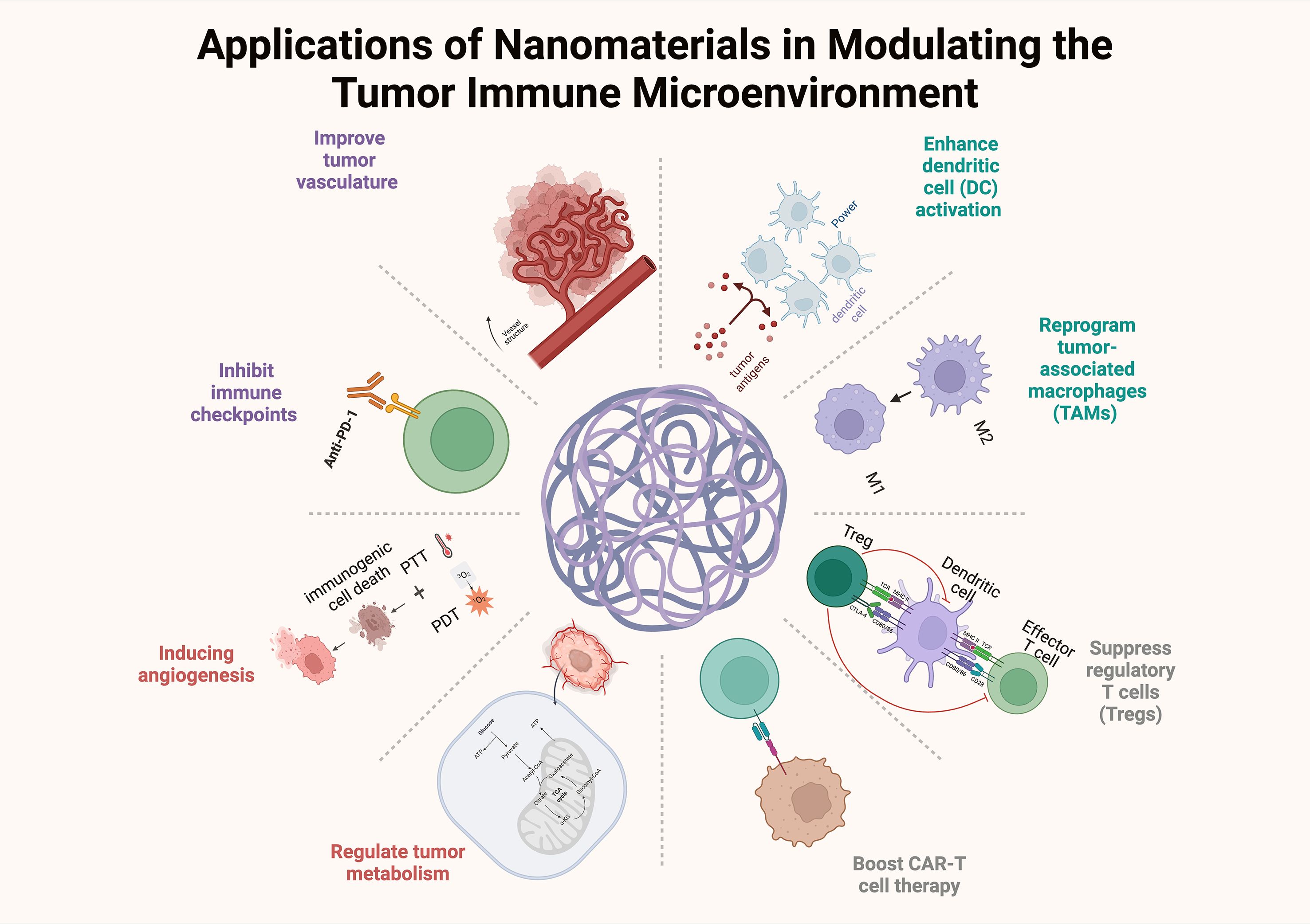
Figure 3. The effects of nanomaterials on the TIME, including the reprogramming of tumor-associated macrophages, inhibition of immune checkpoints, and CAR-T cell therapy.
4.1 Organic nanomaterials
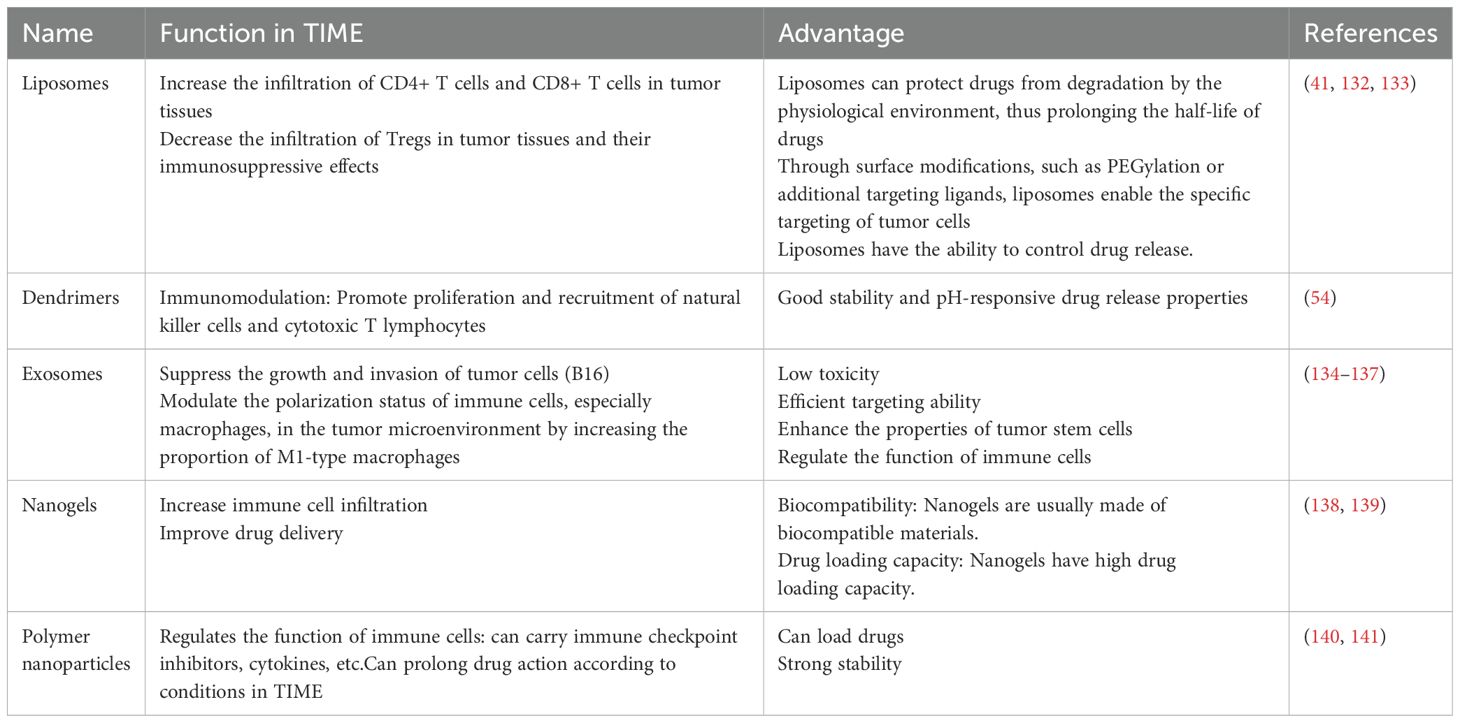
Organic nanomaterials usually consist of naturally occurring organisms or compounds. Compared with inorganic materials, they are less cytotoxic and biodegradable (30). Organic nanomaterials are mainly composed of carbon, hydrogen, oxygen, and nitrogen; the biosafety of organic nanoparticles is a prominent advantage, and their biodegradability mitigates their accumulation in the body (31). Common types include polymer nanoparticles, liposomes, and dendrimers (Figure 4).
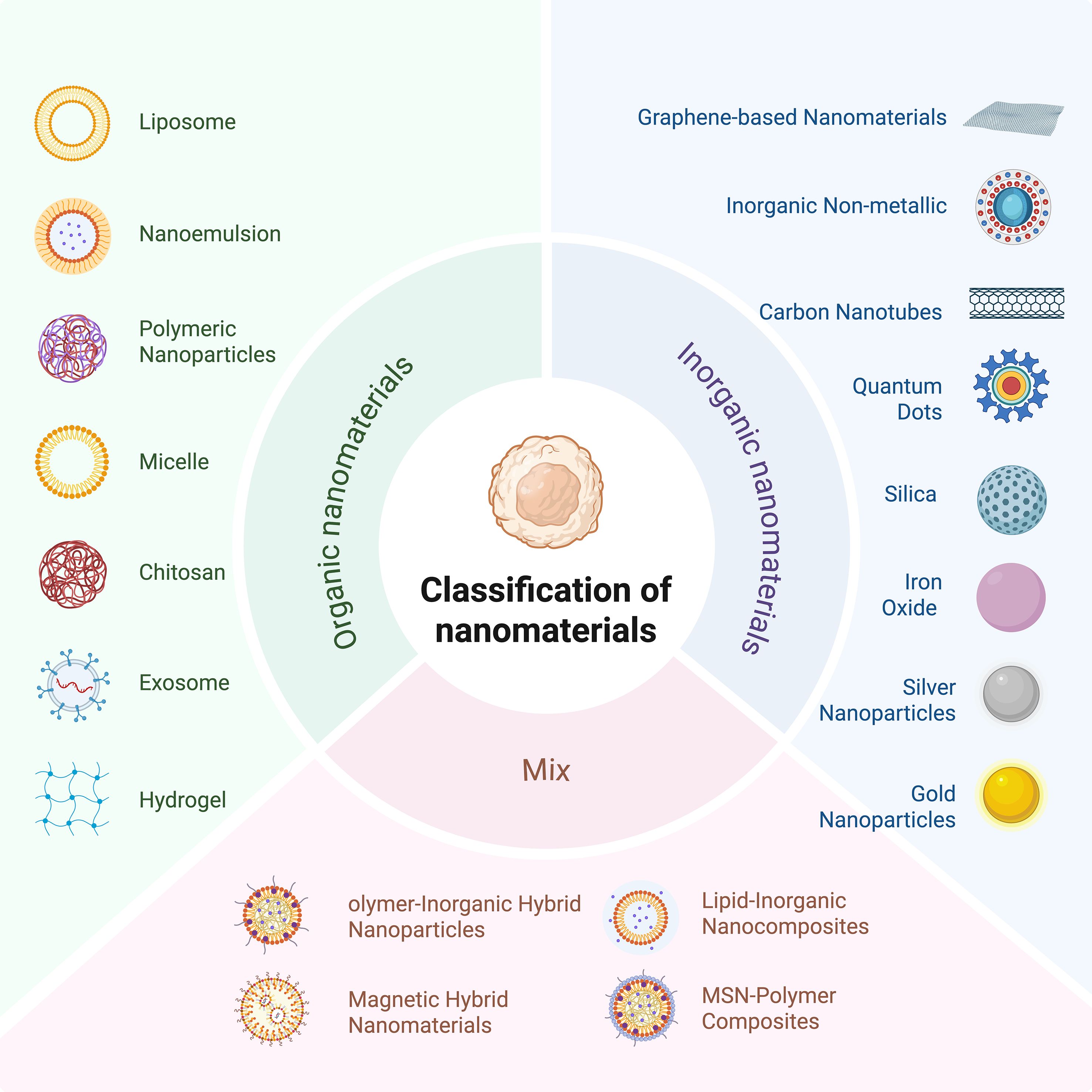
Figure 4. Classification of nanomaterials into organic nanomaterials, inorganic materials, and composite nanomaterials according to their composition.
4.1.1 Liposomes
Liposomes consist of sterols, surfactants and natural or synthetic phospholipids, such as those that can be obtained from egg yolk, soybean and hydrogenated phosphatidylcholine, which are degradable, nontoxic and suitable for industrial production (32). In addition, liposomes are spherical vesicles with bilayer membranes (33). These bilayers are spontaneously formed by phospholipids dispersed in an aqueous phase medium, and the amphiphilic nature of liposomes makes them effective drug carriers (34) and significantly improves the transport of drugs across various lipid-based barriers (35). In addition, liposomes can improve the therapeutic efficacy of drugs by increasing drug solubility and reducing drug toxicity (36).
Liposomes have been used in a variety of therapeutic applications, such as anticancer drugs, antifungal drugs, antibiotics, gene therapy, anesthetic drugs, and anti-inflammatory drugs. Previous studies have revealed the ability of nanodrug delivery systems to modulate the tumor immune microenvironment and enhance the antitumor immune response (37, 38). Gu et al. (39)summarized the many advantages of liposomes as carriers, including their use as vaccines that stimulate immune responses, their ability to selectively deliver drugs to the tumor microenvironment, and their ability to be used in conjunction with other therapies, including chemotherapy, radiotherapy and photothermal therapy. Cheng et al. (40)mixed hydrogenated (soybean) L-α-phosphatidylcholine and 1,2-dioleoyl-3-trimethylammonium propane in a 100:1 weight ratio, dissolved them in chloroform and dried them with a rotary evaporator to form a lipid film. The liposomal nanoparticles were able to deliver cGAMP to macrophages via STING receptors in triple-negative breast cancer. cGAMP reprogrammed M2-like macrophages in the TME to M1-like macrophages and increased CD4+ and CD8+ T-cell infiltration, thereby enhancing the efficacy of breast cancer immunotherapy. In addition, many factors in the TME, such as temperature and pH, can influence the action of the TME; therefore, Fu et al. (41) developed a temperature-sensitive liposome-based delivery system, which was able to remodel the immune microenvironmental of tumor lymph nodes and induce immunogenic cell death in tumor cells after stimulation by photothermal therapy, thus increasing the levels of calmodulin and the secretion of high-mobility protein B1 to promote dendritic cell maturation. Fu et al. (41) also demonstrated strong antitumor immunotherapy against hot and cold tumors when used in combination with PD-L1 therapy. In addition to temperature-sensitive liposomes, pH-sensitive liposomes have been used for similar functions. CAFs are prevalent and engage with cancer cells and the adjacent tumor microenvironment (TME), significantly influencing tumor development, proliferation, and metastasis (42). Moreover, CAFs restructure the extracellular matrix by the secretion of growth factors, chemokines, and other tumor-enhancing substances (43). Consequently, there is a growing emphasis on CAFs and their integration with nanomaterials to modify the tumor microenvironment and improve the effectiveness of immunotherapy. Jia et al. (44) fabricated liposomes encapsulated in CAFs and tumor cell membranes that were able to specifically target CAFs and tumor cells. In addition to drug delivery, liposomal vaccines have shown great potential; a previous study revealed that a liposomal vaccine consisting of the cationic lipid DOTAP and loaded with mRNA was able to induce an immune response against melanoma (45).
4.1.2 Lipid nanoparticles
Lipid nanoparticles (LNPs) are colloidal nanocarriers ranging from 1–100 nm in size. Compared with metal nanoparticles, LNPs are more easily removed and accumulate less in the human body; thus, LNPs are less toxic to organisms (46).
LNPs have been extensively studied for use in small interfering RNA (siRNA) and messenger RNA (mRNA) delivery. SiRNA largely addresses cancer by silencing genes, which is essential for tumor development. Among siRNA delivery techniques, liposomal nanoparticles are the most thoroughly investigated. Most siRNA nanoparticle delivery strategies depend on the increased permeability and retention (EPR) effect to increase tumor accumulation. The EPR effect can enhance the diffusion of siRNA nanoparticles to several organs (47).
Compared with cationic liposomes, these lipids are neutral at physiological pH, are protonated at low pH, and have the advantages of greater biocompatibility and lower cytotoxicity (48). CRISPR/Cas is a gene editing technology, the RNA and protein constituents of the CRISPR/Cas9 system are prone to degradation in the in vivo milieu. Lipid nanoparticles (LNPs) safeguard these components against degradation by nucleases and proteases via their lipid shell, thus extending their stability within the body. Altering the surface of lipid nanoparticles (LNPs) can diminish their identification by the immune system, hence reducing the immunogenicity of the CRISPR/Cas9 system in vivo. It has been shown that by embedding the CRISPR/Cas system into the LNP system, nanocarriers can be target and deliver the CRISPR/Cas system to cancerous tissues; moreover, by incorporating specific antibodies, nanocarriers can reduce off-target effects and enhance their targeting ability (49). Mennati et al. (50) prepared heterotrimeric LNPs loaded with siRNA and lycopene and demonstrated that blockade of the IGF-1R signaling pathway could inhibit cell proliferation and lead to programmed cell death and that LNPs significantly induced apoptosis and blocked the cell cycle in MCF-7 breast cancer cells. Zhang et al. (51) formulated an mRNA vaccine utilizing lipid analogs and designed and screened specific LNPs that activated Toll-like receptor 4 and induced T-cell activation; the results showed that the nanovaccine could deliver mRNAs to the DCs and promote antigen presentation; in addition, LNPs also have good immunogenicity and can induce T-cell activation, thus improving the immunotherapeutic effect on tumors (Figure 5).
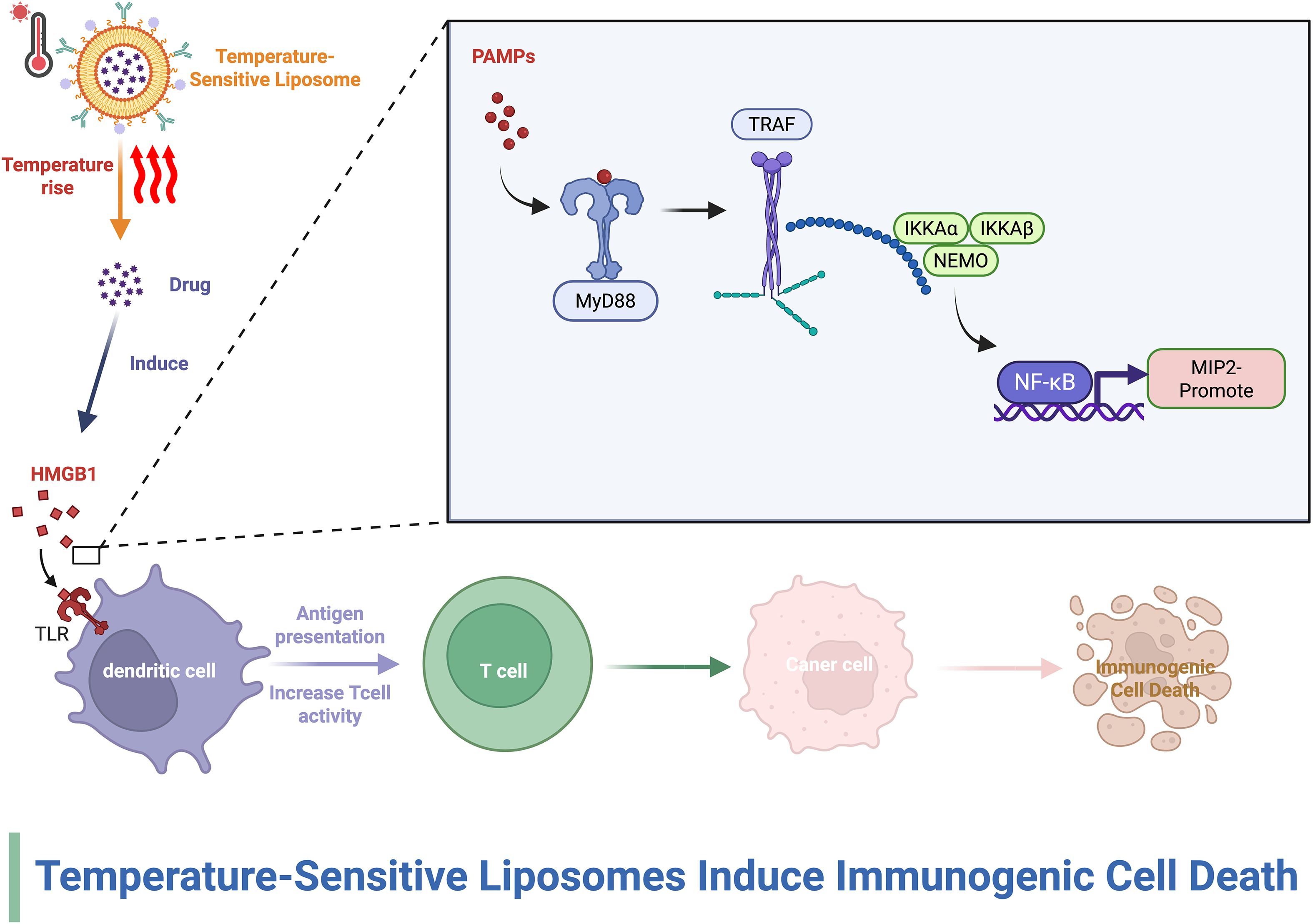
Figure 5. The effect of liposomes on the TIME. This figure demonstrates that thermosensitive liposomes release medicines in response to hyperthermia, thereby causing immunogenic cell death (ICD) in tumor cells.
4.1.3 Dendritic macromolecules
Dendritic macromolecules are nanoscale molecules with radial symmetry as well as well-defined, homogeneous, monodisperse structures. Dendritic polymers are usually between 4 and 20 nm in diameter, which is smaller than most nanoparticles and liposomes; this feature makes them potentially more advantageous in terms of interstitial diffusivity, cellular uptake volume and tissue penetration depth (52). Dendrimers are usually used as nucleic acid carriers, and in the treatment of cancer, nucleic acid therapy has a great advantage (53).
Zhan et al. (54) designed a nanomicelle composed of a phosphorus dendrimer and the chemotherapeutic drug doxorubicin, and the material combined with aPD-L1 treatment inhibited tumor growth, enhanced the apoptosis of B16 cells, induced immunogenic cell death, and promoted the proliferation and activation of natural killer cells, which in turn affected the TIME in a mouse melanoma model. Xiang et al. (55) developed an amphiphilic PAMAM G5 dendrimer that enhances the pH sensitivity of drugs in the TME. While Huang et al. (56) also developed the above dendrimer material and found that it was able to release Fe3+ and Cu2+ in a weakly acidic TME. Moreover, the targeted MR imaging of triple-negative breast cancer was achieved by modulating the TME with enhanced iron death and chemodynamic therapy. The TME contains a high concentration of glutathione (GSH), and glutathione enzymes can participate in the development of chemotherapeutic resistance, thus reducing the efficacy of conventional chemotherapeutic drugs (57). Zhang et al. (58) developed a smart nanomedicine formulation based on redox-responsive dendritic macromolecular nanogels, which not only has good biocompatibility and the ability to reduce the high concentration of GSH in the TME but also has the ability to activate tumor-infiltrating CD 4+ and CD 8+ T cells, in addition to being able to resist PD-L1 immune checkpoint blockade therapy, remodel the TIME and inhibit tumor growth.
4.1.4 Exosomes
Exosomes, also known as extracellular vesicles, are the smallest organelles, with a size of approximately 40–150 nm (59). Exosomes play important roles in human growth and development, immunomodulation, and tissue homeostasis (60), and they serve as versatile drug delivery vehicles that can be used to regulate immunity (61). In addition, although exosomes are similar to liposomes in shape and function, compared with liposomes, exosomes are retained in body fluids for a longer period of time and are not easily eliminated by macrophages or reticuloendothelial cells (62). In recent years, the application of exosomes in tumor immunity has become increasingly widespread; relevant studies have shown that miR-155 and miR-125b2 in exosomes can be used to reprogram tumor-associated macrophages, thereby inhibiting tumor progression (63). Fu et al. reported that exosomes derived from DCs can be loaded with a variety of peptide antigens and that they stimulate both CD4+ and CD8+ T cells, which participate in antitumor responses (64). M1-like macrophage-derived exosomes (M1-Exos) have inflammation-directed tumor-targeting ability, and relevant studies have shown their role in reprogramming the immunosuppressive TME (65). Zhen (66) developed an exosome bound to liposomes that could effectively alleviate hypoxia, increase ROS levels, promote the release of proinflammatory cytokines from M1-Exos and reprogram the immunosuppressive TME. Lv et al. (65) also developed a nanovesicle composed of exosomes and AS1411 aptamer-modified liposomes, which not only generate a large amount of reactive oxygen species to induce the apoptosis of tumor cells via near-infrared laser irradiation but also polarize TAMs and promote the infiltration of T lymphocytes, thus enhancing the antitumor immune response (Figure 6).
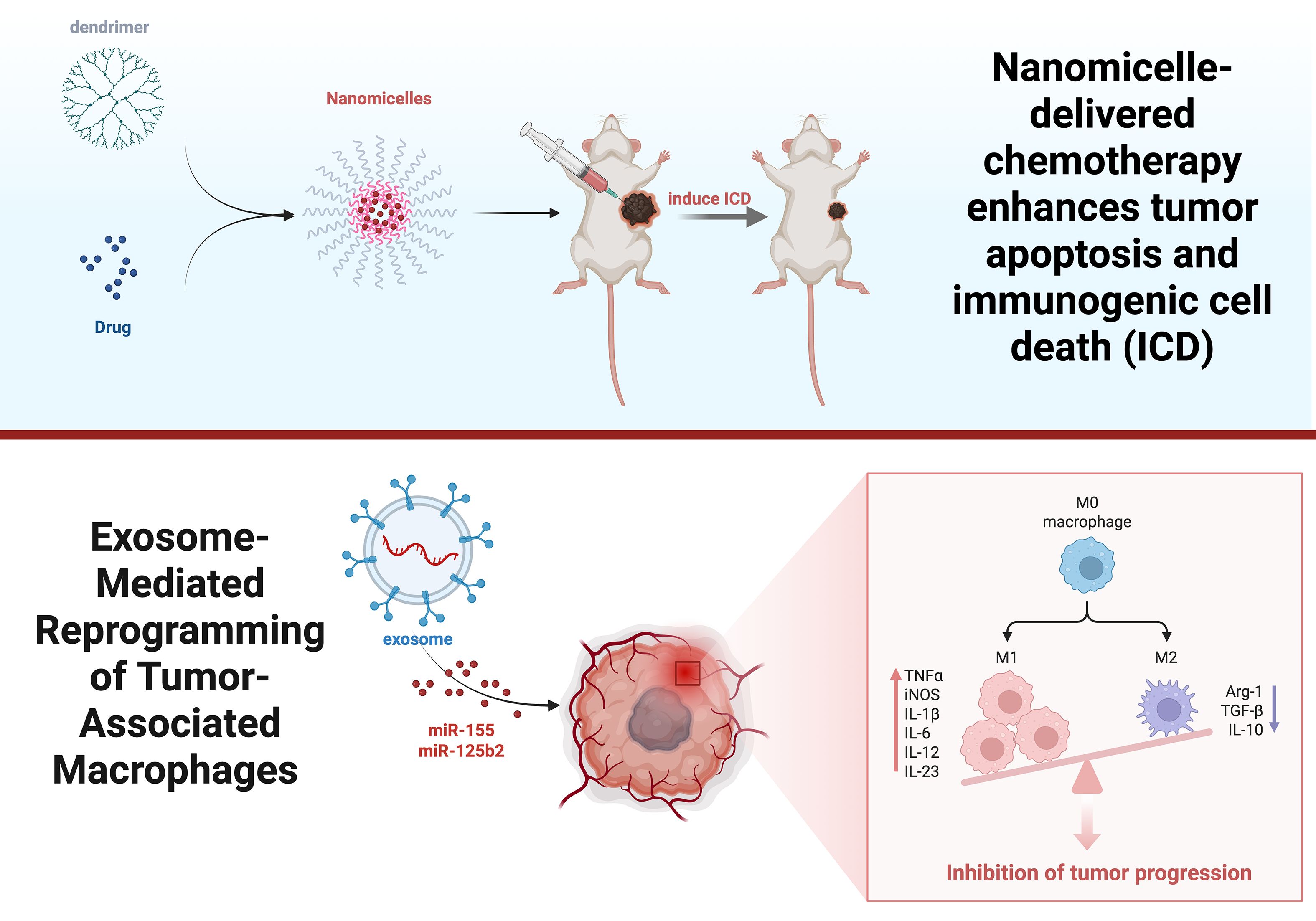
Figure 6. Effects of dendrimer macromolecules and exosomes on the TIME. This picture clarifies how dendrimers and nanomicelles facilitate ICD by drug delivery, subsequently activating anticancer immune responses. Exosomes containing miRNAs reprogram TAMs.
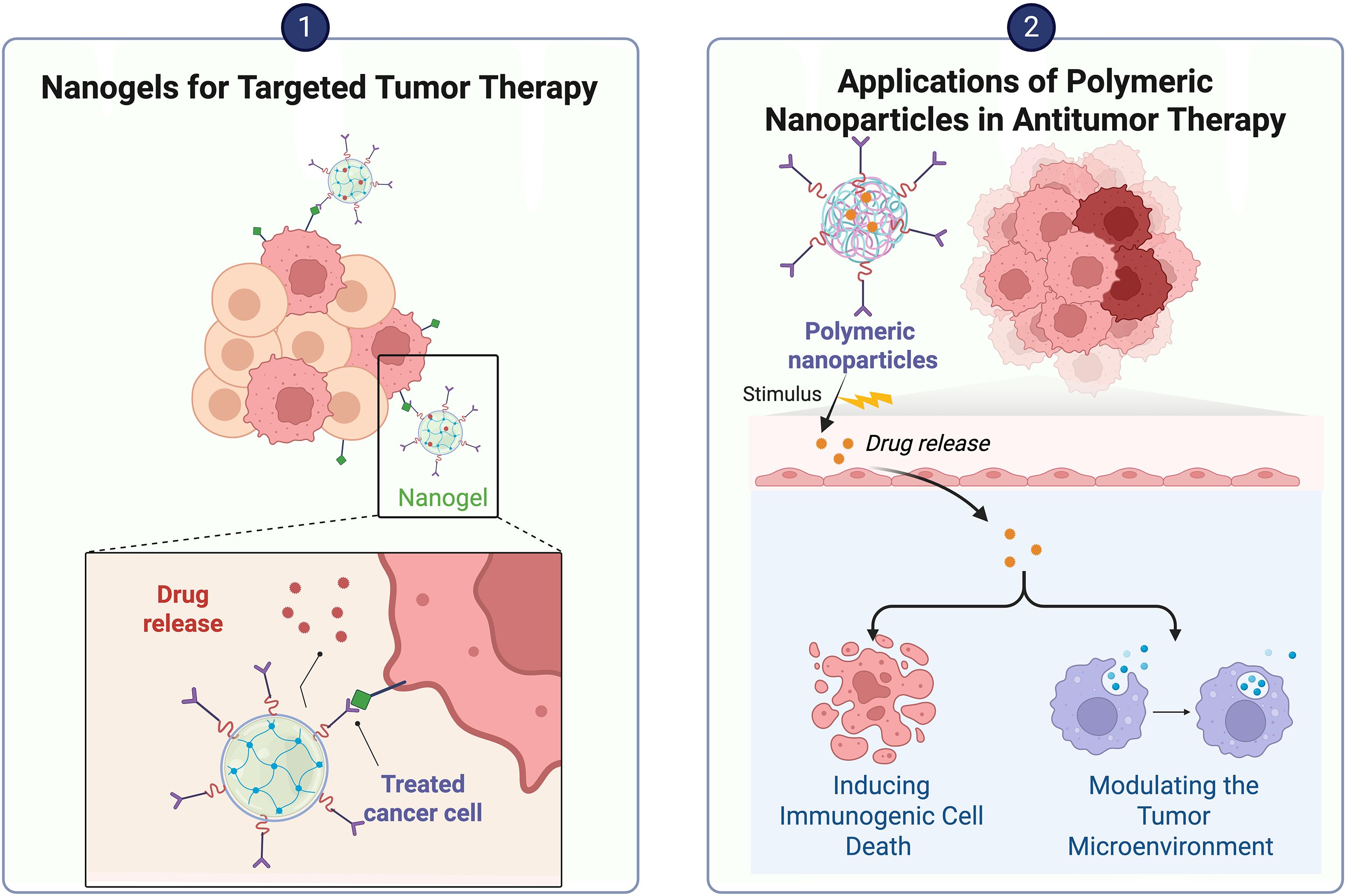
4.1.5 Nanogels
Nanogels can be used as nanocarriers for systemic drug delivery. Nanogels take advantage of the combination of nanotechnology with gel-based materials such as hydrogels (67). Nanogels have unique physical and chemical properties, such as nanoscale size, tunable properties, significant hydration, good biocompatibility, etc. (68). Compared with conventional nanoparticles, they have variable particle sizes and shapes as well as greater sensitivity to external stimuli such as pH, temperature, ionic strength and redox conditions (69).
Bai’s team (70) developed a nanogel that responds to matrix metalloproteinase-2 in the TME and releases two types of liposomes at the tumor site; it not only promotes apoptosis but also triggers immunogenic cell death and promotes the maturation of DCs and T-cell infiltration, thus altering the immune-suppressive state of the TME and enhancing the therapeutic effect. In contrast, Li et al. (71) designed a biocompatible alginate-based hydrogel for codelivery of dextran nanoparticles encapsulated with pessidatinib and platelets modified with an anti-PD-1 antibody; this material was able to inhibit macrophage aggregation, significantly alleviate the immunosuppressive tumor microenvironment, promote infiltration of effector CD8+ T cells, and enhance the immunotherapeutic effect of tumors by depleting TAMs. In addition, Jiang et al. (72) developed an injectable hydrogel for the delivery of macrophage CAR gene-loaded nanocarriers and anti-CD47 antibodies capable of targeting glioma stem cells and triggering an antitumour immune response. Tian et al. (73) prepared a self-degradable nanogel that efficiently facilitated the infiltration and activation of CD8+ T cells and remodeled the immunosuppressive TME. Gao et al. (74) developed Vir-Gel, a membrane-encapsulated nucleic acid nanogel embedded with therapeutic miRNAs, which could induce the calibration of proinvasive M2 macrophages to antitumor M1 macrophages and enhance the immunotherapeutic efficacy in glioma.
4.1.6 Polymeric nanoparticles
Polymeric nanoparticles (PNPs) are entities whose size falls within the range of 1 to 1000 nm, mainly including nanospheres, nanocapsules, and polymeric micelles (75). Polymeric nanoparticles are usually characterized by good biocompatibility, modifiability, etc., and can be either derived from nature or artificially synthesized. Chitosan, as a type of polymer nanoparticle, is derived mainly from the deacetylated form of chitin; it is one of the best drug carriers for the treatment of cancer (76). Micelles are nanocolloidal particles formed by the assembly of amphiphilic polymers. Targeting CAFs is one strategy in tumor immunotherapy, and Cheng et al. (77) demonstrated that polymeric micelles can noncovalently bind CAF-targeted antibody fragments, thus improving the efficacy of immunotherapy. Wang et al. (78) integrated paclitaxel, thioridazine and the small-molecule PD-1/PD-L1 inhibitor HY19991 into a dual-enzymatic pH-sensitive polymer structure that was able to release paclitaxel in the acidic TME. This approach was almost always able to alleviate the immunosuppressive TME in a mouse model of MCF-7 metastatic breast cancer (79). In addition, Wan (80) synthesized a micellar system assembled with gemcitabine-conjugated polymer (PGEM), a CCR2 antagonist PF-6309, for cancer therapy; PGEM was shown to activate the STING signaling pathway in DCs, which enhanced natural killer cell and adaptive antitumor T-cell responses and reduced the aggregation of TAMs. Groettrup et al. (81) created a new type of cancer nanovaccine using PLGA and riboxxim. This vaccine can activate the immune response by triggering endosomal Toll-like receptor 3. Dacoba et al. (82) designed nanocomplexes capable of targeting and delivering the Toll-like receptor 3 agonist poly(I:C), and in vitro experiments revealed that poly(I:C) delivered by this nanocarrier was more efficiently internalized by macrophages, which facilitated macrophage polarization toward the M1 phenotype, thereby enhancing their antitumor effects.
Guo et al. (83) prepared organic complex nanoparticles with photothermal capability, which, while being controllable for photothermal therapy and reducing damage to the surrounding tissues, were also able to significantly increase the infiltration of CD4+ and CD8+ T cells in the TME, downregulate the expression of PD-L1, and increase the proliferation and activity of cytotoxic T lymphocytes, thus improving the antitumor immune response of the organism (See Table 2) (Figure 7).
4.2 Inorganic nanomaterials
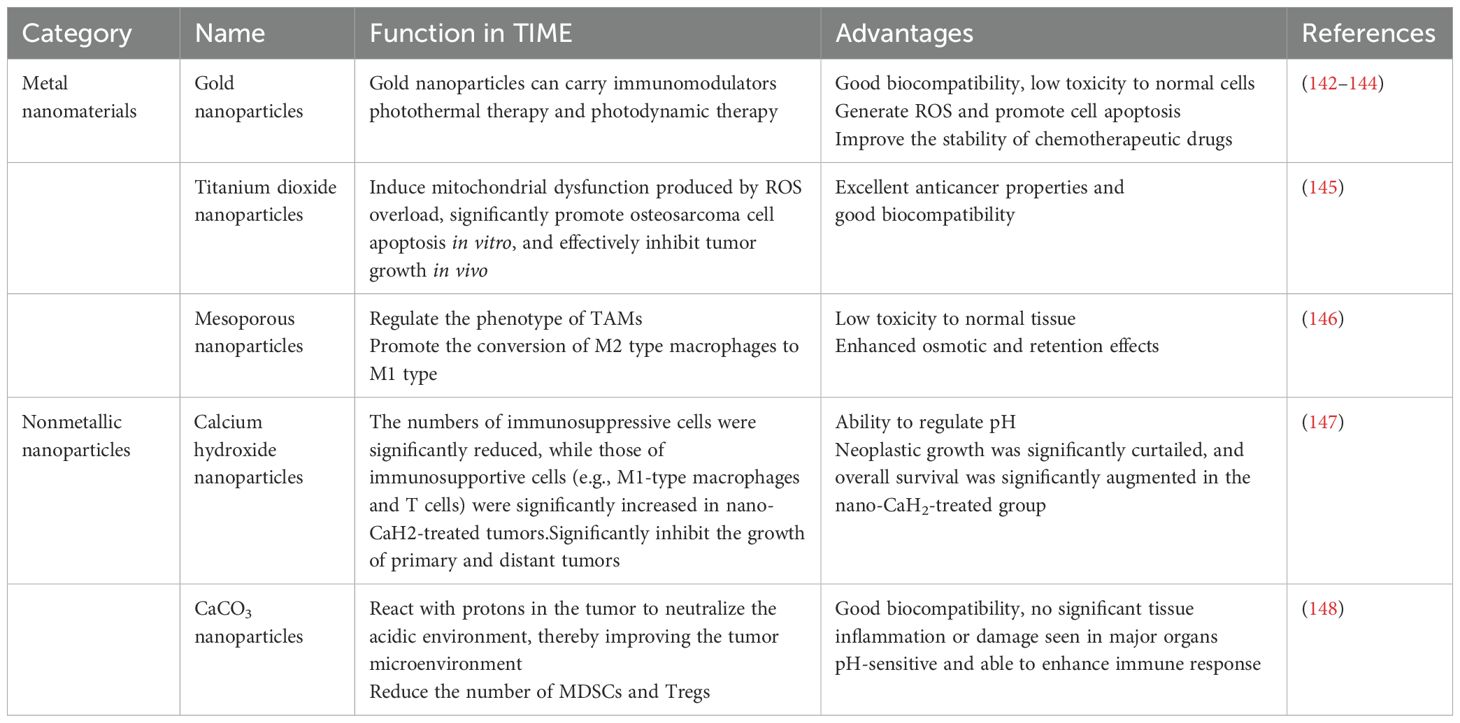
Inorganic nanomaterials not only have controllable shapes and sizes but also well-defined chemical properties and excellent optical, electrical, and magnetic properties, thus showing great advantages in tumor immunotherapy (84, 85). The research and application of inorganic non-metallic biomaterials cover a wide range of fields. In addition to tissue engineering, they also include the diagnosis and treatment of diseases and drugs, such as imaging technology, tumor radiosensitization treatment, immune regulation, sterilization, etc. The inorganic non-metallic materials mainly used in these aspects are at the nanoscale (86). Inorganic nanomaterials are mainly classified into metallic and nonmetallic nanomaterials.
4.2.1 Metallic nanoparticles
Metal ions are vital to tumor immunomodulation, and some scholars have proposed the use of cancer metal immunotherapy (87). Combining metal ions with nanomaterials and preparing corresponding metal nanoparticles can modulate the immune response to tumors. Metallic nanoparticles can be designed to enhance biocompatibility and reduce toxicity to normal tissues by modifying their surface. In addition, metallic nanoparticles can act on tumor cells, immune cells and the extracellular matrix simultaneously to modulate the TME and enhance therapeutic effects.
Among many metal nanoparticles, gold nanoparticles are widely used because of their good biocompatibility and stability (88). Gold nanoparticles can induce an effective immune response at low doses (89). Liang et al. (90) subcutaneously injected gold nanomaterials encapsulated in liposomes into tumor-bearing mice, and 12 hours after the injection, the composite nanoparticles reached maximum accumulation in local lymph nodes and significantly promoted the maturation of dendritic cells, indicating that gold nanomaterials can be used to activate immune cells and enhance immune responses.
In contrast, among inorganic nanomaterials, only iron-based nanoparticles are approved by the FDA for medical use (91). Iron-based nanomaterials can enhance the killing effect on tumor cells through the Fenton reaction (92). Zhang et al. (93) developed nanomaterials consisting of Fe/Cu-based metal–organic frameworks, lactic acid oxidase and hyaluronic acid, which were able to increase the level of intracellular reactive oxygen species (ROS) via the glycolysis, which then promoted the repolarization of TAMs and enhanced the efficacy of immunotherapy.
CAFs inhibit the activity of immune cells by secreting immunomodulatory factors, which in turn promote the accumulation of immunosuppressive cells, such as Tregs and MDSCs, thereby helping tumor cells evade immune surveillance (94). Therefore, Bromma et al. (95) assayed gold nanoparticles modified with polyethylene glycol and arginine-glycine-aspartic acid peptides on the surface inside tumor cells and reported that the nanoparticles were able to increase cellular uptake and significantly reduce the tumor volume. In photodynamic therapy (PDT), photosensitizers are used to generate reactive oxygen species (ROS) under specific light irradiation, which is able to induce tumor cell death and amplify the efficacy of tumor immunotherapy (96). Zhou et al. (97) prepared ferritin nanoparticles, which are able to deliver photosensitizers to CAFs, significantly increase their photosensitizer accumulation at the tumor site, and also generate an immune response toward CAFs, thus enhancing the effect of immunotherapy. Ding et al. (98) formulated a vaccine (ZPM@OVA-CpG) that was able to release Zn2+ in a controlled manner and improve immunotherapy efficacy. Deng et al. (99) demonstrated the ability of a calcium-manganese bionic hybrid nanostimulant to activate both apoptosis and the innate immune response in ferrocytes, which provided a new therapeutic idea for the effective immunotherapy of triple-negative breast cancer. Cen et al. developed a functional immunotherapy for triple-negative breast cancer.
4.2.2 Metal oxide nanoparticles
Metal oxide nanomaterials are nanoscale materials composed of metal and oxygen, mainly zinc oxide, iron oxide and other nanomaterials. Sun et al (100). prepared a hyaluronic acid-optimized magnetic nanoparticles (Fe3O4), which can not only efficiently penetrate into the interior of tumors but also adhere to CD44 receptors on the surface of tumor cells via hyaluronic acid to achieve specific targeting of tumor cells. In addition, these nanoparticles can regulate TAM repolarization and stimulate tumor cells and macrophages to secrete more chemokines. In contrast, Yang’s team (101) engineered a novel biomimetic magnetic nanoparticle, Fe3O4-SAS@PLT, which not only elicits tumor-specific immune responses but also effectively repolarizes macrophages from the immunosuppressive M2 phenotype to the antitumor M1 phenotype, in addition to depleting glutathione (GSH).
ROS can modulate the TIME and promote the infiltration and activation of immune cells, thereby enhancing antitumor responses. ROS usually act as intermediate signaling molecules, affecting the differentiation, function, and secretion of TAMs (102). Previous studies have shown that ROS-scavenging nanozymes can inhibit the transformation of macrophages into M2 TAMs by suppressing the activity of the ERK and STAT3 signaling pathways (103). Therefore, Gong et al (104). used bimetallic oxide FeWOX nanosheets in combination with a CTLA-4 checkpoint blocker and reported that ROS-mediated inflammation induced by FeWOX nanosheets in the TME can activate the immune system and trigger a strong immune response, which not only promotes the maturation of DCs, increases the number of T cells, and decreases the number of Tregs but also induces the repolarization of TAMs, thus effectively inhibiting tumor growth. Wu et al. (105) prepared a composite metal oxide nanomaterial of titanium carbide-chitosan-manganese iron oxide (MnFe2O4) that was able to catalyze the oxidation of glutathione to oxidized glutathione, thus depleting excess glutathione in the TME and increasing ROS generation and therapeutic effects (Figure 8).
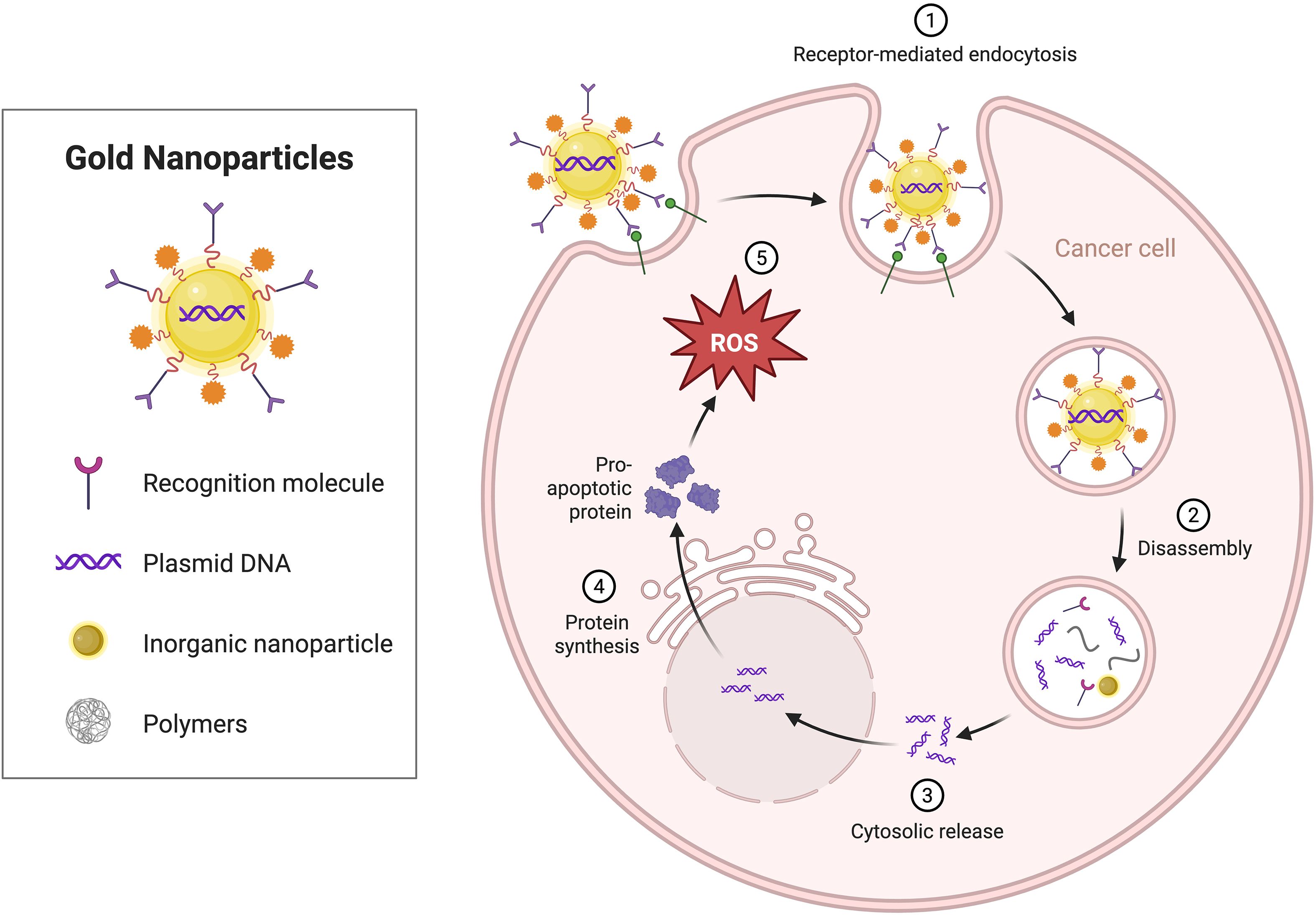
Figure 8. The effect of metallic nanomaterials on the TIME. Gold nanoparticles are modified on their surfaces with recognition molecules, such as plasmid DNA and polymers, enabling their entry into cancer cells via receptor-mediated endocytosis.
4.2.3 Nonmetallic nanomaterials
Non-metallic nanoparticles are particles at the nanoscale composed of non-metallic elements or non-metallic compounds. Graphene oxide nanosheets prepared by Guo et al. (106) were not only able to stimulate the polarization of M1-type macrophages but also were able to increase the levels of proinflammatory cytokines and chemokines, such as IL-12 and TNF-α, in the TME, thereby enhancing the antitumor immune response. A graphene quantum dot capable of forming a heterogeneous structure by combining with copper-based nanomaterials was prepared by Yan et al. and was able to promote the activation of immune cells directly or indirectly through its own properties, in addition to targeting and damaging DNA, thereby releasing tumor-associated antigens and promoting the maturation of DCs and the activation of T cells. In addition, selenium nanoparticles prepared by Xiong et al. (107) were able to promote ROS generation in tumor cells, leading to elevated levels of oxidative stress and thereby inhibiting tumor cell growth and proliferation. Xu et al. (108) synthesized PAP-SeNPs by integrating selenium nanoparticles with polysaccharides derived from Pholiota adiposa. Utilizing the diverse benefits of polysaccharides, including their degradability and biocompatibility, they improved the stability of selenium nanoparticles. These nanoparticles effectively targeted M2 TAMs and induced their polarization into the M1 phenotype, which exhibits anticancer properties. Li et al. (109) created a nanoplatform (Nano-Bi2Se3@MnCaP). This platform’s photodynamic treatment (PDT) can efficiently induce immunogenic cell death, stimulate the immune system, facilitate dendritic cell maturation, and activate T cells, therefore augmenting the antitumor immune response.
Mesoporous silica nanoparticles have excellent drug-carrying capacity and good hydrophilicity and have great potential for development (110). Zhao et al. (111) loaded acidic, environment-responsive CCM-encapsulated mesoporous silica nanoparticles with dacarbazine and combined them with aPD1. These nanoparticles not only induced an antitumor immune response in T cells but also effectively inhibited the growth and metastasis of melanoma. Wang et al. (112) prepared hollow mesoporous silica (HMS) nanospheres, which were able to promote the maturation of DCs. In addition, the HMS cancer vaccine exerted a synergistic effect with anti-PD-L1 antibodies on the tumor, which effectively increased the levels of CD4+ and CD8+ T cells and ultimately inhibited the growth of tumors (see Table 3) (Figure 9).
4.3 Hybrid nanomaterials
Hybrid nanomaterials refer to new materials formed by combining two or more different materials through a specific method. Gyu et al. (113) fabricated a composite nanomaterial of galactose-rich polysaccharides extracted from plant cell walls, which induced the conversion of M2-type tumor-associated macrophages to M1-type phenotypes, thereby enhancing the tumor immune response and mitigating the immunosuppressive TME. Gao et al. (114) used AMD3100-modified poly(lactic-coethanolic acid) nanoparticles as sorafenib carriers and prepared ADOPSor nanoparticles, which were shown to be able to effectively inhibit the infiltration of TAMs in hepatocellular carcinoma. Zhang et al. (115) prepared composite nanomaterials consisting of gold nanorods, manganese dioxide, and silicon dioxide, which enabled the loading of glucose oxidase; subsequently, they applied cancer cell starvation therapy and photothermal therapy to detect tumor cells; the results revealed that the material was not only able to increase the oxygen level in the TME but also to enrich more efficiently at the tumor site, thus improving the therapeutic effect.
Feng et al. (116) prepared a nanomaterial consisting of bacterial outer membrane vesicles combined with CD47 nanoantibodies; it not only specifically binds to CD47 molecules on the surface of tumor cells and Toll-like receptors on the surface of macrophages but also promotes the maturation of DCs as well as the recruitment of a variety of immune cells into tumor tissues, thus remodeling the TME. Chen et al. (117) loaded ovalbumin and copper sulfide into PLGA nanoparticles to form nanocomplexes with a core-shell structure and revealed that the nanomaterials were able to activate CD8+ T cells and induce a tumor immune response.
5 Clinical trials related to nanomaterials regulating the immune microenvironment
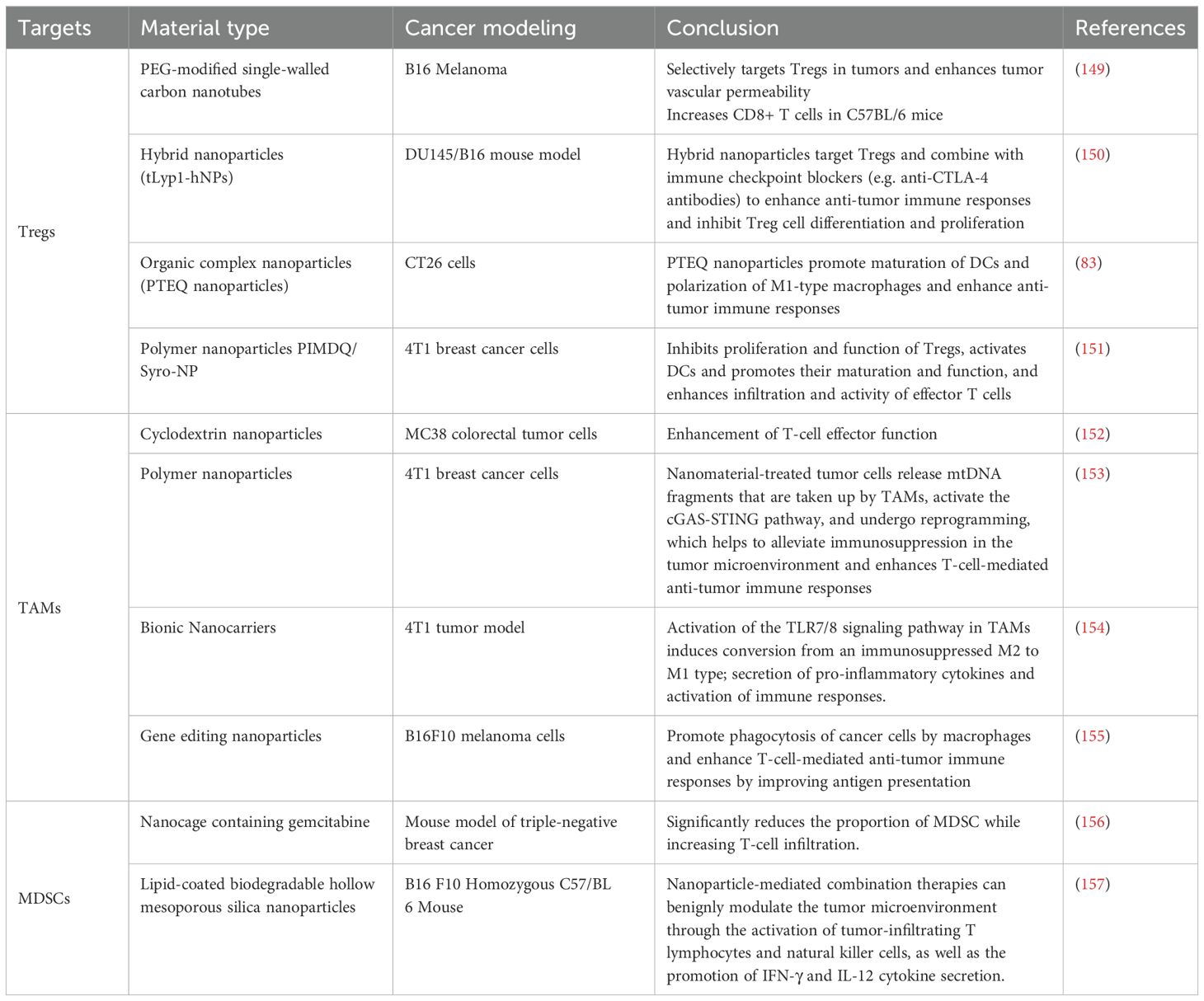
Nanomaterials have demonstrated remarkable potential in influencing the tumor immune microenvironment and have subsequently become a hot topic in research (33). During the past few years, several clinical studies have been carried out to explore the application of nanomaterials in immunotherapy to improve its effects, including amplifying immune cell activity, the promotion of antigen presentation, and the alleviation of immunosuppression. Therefore, we collated the targets of different types of nanomaterials in immunotherapy and their ability to improve the tumor immune microenvironment (TIME) (see Table 4) (Figure 10).
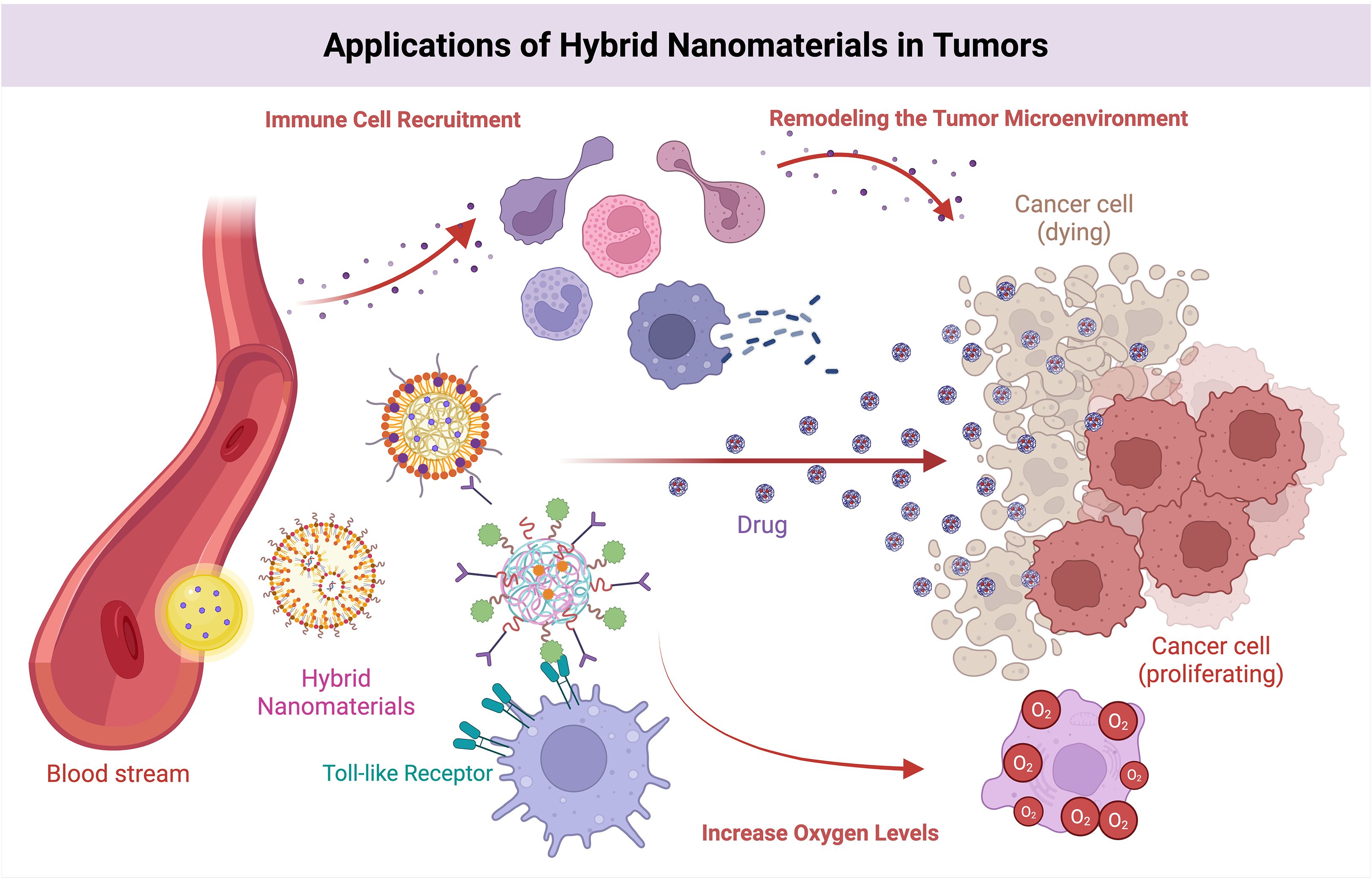
Figure 10. Effects of hybrid nanomaterials on the TIME. Hybrid nanomaterials stimulate the immune system via TLRs, facilitate targeted drug delivery to eradicate cancer cells, attract immune cells to modify the microenvironment.
6 Summary and discussion
The tumor microenvironment is a highly complex ecosystem comprising a diverse array of cell types, including immune cells, as well as various cytokines, chemokines, and signaling molecules. The key cell types include mainly macrophages, tumor-associated fibroblasts, myeloid-derived suppressor cells (MDSCs), dendritic cells, natural killer cells and effector T cells; of these, macrophages usually tend to be protumorigenic with an M2 phenotype, promoting tumor growth and angiogenesis (118), whereas regulatory T cells and MDSCs promote immunosuppression through the inhibition of effector T cells and natural killer (NK) cells, thus creating a supportive tumor environment for tumor development (119). The immunotherapy of tumors often involves these cytokines; therefore, targeting these cytokines can improve therapeutic efficacy.
Methods for targeting the TME to address this problem have been widely investigated; however, improving therapeutic efficacy more efficiently and rapidly is particularly important. Although conventional treatments are effective against tumors, they still have some shortcomings. Nanomaterials have made great progress as a novel means (92), and the rise of nanomaterials not only improves therapeutic efficacy but also improves precision and reduces toxic side effects (120). Owing to the unique physical and chemical properties of nanomaterials, the infiltration and activation of immune cells can be effectively enhanced to improve antitumor immune responses. This paper summarizes the main mechanisms by which different types of nanomaterials modulate the TME. Nanomaterials can not only deliver immune checkpoint inhibitors, such as anti-CD47 antibodies but also enhance the ability of immune cells to kill tumor cells (121). In addition, nanomaterials can be combined with a variety of therapies based on graphene materials, such as graphene oxide-polyethylene glycol-polyethyleneimine-CpG nanocomplexes loaded with CpG ODN, which utilize the near-infrared light absorption of graphene oxide, thus enhancing intracellular delivery and achieving a perfect combination of photothermal and immunotherapy (122).
Despite the good biocompatibility of nanomaterials, further long-term assessments of their toxicity in living organisms and clinical trials are needed. The main limitations of currently employed immunotherapy strategies are the inability of most immunotherapeutic drugs to reactivate T cells and expand them in vitro or in vivo and the various complicating factors that lead to T-cell exhaustion in the context of tumor–TME interactions and the acquisition of immune resistance (33). In addition, the clearance mechanism of nanomaterials in vivo has not yet been clarified, and in the future, we can focus on their retention in vivo and whether they affect organ function. Finally, the drug delivery rate of nanomaterials still needs to be optimized, and owing to the complexity of the TME, the distribution of nanomaterials in tumor tissues is still limited, even though some drugs can be delivered effectively to the intended targets by nanomaterials. Emerging technologies, particularly artificial intelligence, possess significant potential. They are not only efficient and precise but also possess the capability to forecast therapy outcomes. In the future, further developing technologies may be incorporated with nanomaterials to augment medicinal effects.
In summary, nanomaterials have potential in regulating the TIME and offer new therapeutic tools for the immunotherapy of tumors. However, issues such as the biotoxicity and drug delivery rate of nanomaterials still need to be further explored. Therefore, in the future, more efficient and safer nanomaterials could be translated into the clinic, and multidisciplinary intersections could be applied to provide patients with more precise and effective therapeutic options.
Author contributions
HP: Formal analysis, Writing – original draft. YJ: Writing – review & editing, Conceptualization, Writing – original draft. SJ: Writing – original draft, Investigation. JS: Software, Visualization, Writing – original draft. JY: Writing – original draft, Data curation. WW: Writing – review & editing, Project administration. ZD: Writing – review & editing, Supervision. HY: Writing – review & editing, Supervision, Project administration. QL: Writing – review & editing, Software. NL: Visualization, Writing – review & editing. JF: Resources, Writing – review & editing. YS: Resources, Writing – review & editing. ML: Project administration, Conceptualization, Writing – original draft, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by China National Natural Science Foundation (82104838), China Promotion Foundation Spark Program (XH-D001) and Liaoning Provincial Key Research and Development Programme(2024JH2/102500062).
Acknowledgments
The authors would like to express their gratitude to all members of Mingzhu Li ‘s group for them for their support and help. This manuscript was supported by China National Natural Science Foundation (82104838), China Promotion Foundation Spark Program (XH-D001) and Liaoning Provincial Key Research and Development Programme(2024JH2/102500062), which is gratefully acknowledged. The Figures 1-10 were drawn using biorender.
Conflict of interest
The reviewer LY declared a shared parent affiliation with the authors HP, YJ, WW, HY, NL, ML to the handling editor at the time of review.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Seager RJ, Hajal C, Spill F, Kamm RD, and Zaman MH. Dynamic interplay between tumour, stroma and immune system can drive or prevent tumour progression. Converg Sci Phys Oncol. (2017) 3. doi: 10.1088/2057-1739/aa7e86
2. Valencia JC, Erwin-Cohen RA, Clavijo PE, Allen C, Sanford ME, Day CP, et al. Myeloid-derived suppressive cell expansion promotes melanoma growth and autoimmunity by inhibiting CD40/IL27 regulation in macrophages. Cancer Res. (2021) 81:5977–90. doi: 10.1158/0008-5472.CAN-21-1148
3. Lin G and Zhang M. Ligand chemistry in antitumor theranostic nanoparticles. Acc Chem Res. (2023) 56:1578–90. doi: 10.1021/acs.accounts.3c00151
4. Lin G, Revia RA, and Zhang M. Inorganic nanomaterial-mediated gene therapy in combination with other antitumor treatment modalities. Adv Funct Mater. (2021) 31. doi: 10.1002/adfm.202007096
5. Song Y, Bugada L, Li R, Hu H, Zhang L, Li C, et al. Albumin nanoparticle containing a PI3Kγ inhibitor and paclitaxel in combination with α-PD1 induces tumor remission of breast cancer in mice. Sci Transl Med. (2022) 14:eabl3649. doi: 10.1126/scitranslmed.abl3649
6. Bae YH. Drug targeting and tumor heterogeneity. J Control Release. (2009) 133:2–3. doi: 10.1016/j.jconrel.2008.09.074
7. Sceneay J, Griessinger CM, Hoffmann SHL, Wen SW, Wong CSF, Krumeich S, et al. Tracking the fate of adoptively transferred myeloid-derived suppressor cells in the primary breast tumor microenvironment. PloS One. (2018) 13:e0196040. doi: 10.1371/journal.pone.0196040
8. Du X, Yang X, Zhang Y, Gao S, Liu S, Ji J, et al. Transdermal delivery system based on heparin-modified graphene oxide for deep transportation, tumor microenvironment regulation, and immune activation. Nano Today. (2022) 46:101565. doi: 10.1016/j.nantod.2022.101565
9. Giraldo NA, Sanchez-Salas R, Peske JD, Vano Y, Becht E, Petitprez F, et al. The clinical role of the TME in solid cancer. Br J Cancer. (2019) 120:45–53. doi: 10.1038/s41416-018-0327-z
10. Zhang Y, Liu Q, and Liao Q. Long noncoding RNA: a dazzling dancer in tumor immune microenvironment. J Exp Clin Cancer Res. (2020) 39:231. doi: 10.1186/s13046-020-01727-3
11. Fu T, Dai LJ, Wu SY, Xiao Y, Ma D, Jiang YZ, et al. Spatial architecture of the immune microenvironment orchestrates tumor immunity and therapeutic response. J Hematol Oncol. (2021) 14:98. doi: 10.1186/s13045-021-01103-4
12. Tien FM, Lu HH, Lin SY, and Tsai HC. Epigenetic remodeling of the immune landscape in cancer: therapeutic hurdles and opportunities. J BioMed Sci. (2023) 30:3. doi: 10.1186/s12929-022-00893-0
13. Tie Y, Tang F, Wei YQ, and Wei XW. Immunosuppressive cells in cancer: mechanisms and potential therapeutic targets. J Hematol Oncol. (2022) 15:61. doi: 10.1186/s13045-022-01282-8
14. Hanahan D and Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
15. Gubin MM, Esaulova E, Ward JP, Malkova ON, Runci D, Wong P, et al. High-dimensional analysis delineates myeloid and lymphoid compartment remodeling during successful immune-checkpoint cancer therapy. Cell. (2018) 175:1443. doi: 10.1016/j.cell.2018.11.003
16. Lei X, Lei Y, Li JK, Du WX, Li RG, Yang J, et al. Immune cells within the tumor microenvironment: Biological functions and roles in cancer immunotherapy. Cancer Lett. (2020) 470:126–33. doi: 10.1016/j.canlet.2019.11.009
17. Grossman JG, Nywening TM, Belt BA, Panni RZ, Krasnick BA, DeNardo DG, et al. Recruitment of CCR2(+) tumor associated macrophage to sites of liver metastasis confers a poor prognosis in human colorectal cancer. Oncoimmunology. (2018) 7:e1470729. doi: 10.1080/2162402X.2018.1470729
18. Li X, Ramadori P, Pfister D, Seehawer M, Zender L, and Heikenwalder M. The immunological and metabolic landscape in primary and metastatic liver cancer. Nat Rev Cancer. (2021) 21:541–57. doi: 10.1038/s41568-021-00383-9
19. Carlino MS, Larkin J, and Long GV. Immune checkpoint inhibitors in melanoma. Lancet. (2021) 398:1002–14. doi: 10.1016/S0140-6736(21)01206-X
20. Schildberg FA, Klein SR, Freeman GJ, and Sharpe AH. Coinhibitory pathways in the B7-CD28 ligand-receptor family. Immunity. (2016) 44:955–72. doi: 10.1016/j.immuni.2016.05.002
21. Larkin J, Minor D, D’Angelo S, Neyns B, Smylie M, Miller WH Jr., et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator’s choice chemotherapy in CheckMate 037: A randomized, controlled, open-label phase III trial. J Clin Oncol. (2018) 36:383–90. doi: 10.1200/JCO.2016.71.8023
22. Sadelain M, Brentjens R, and Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discov. (2013) 3:388–98. doi: 10.1158/2159-8290.CD-12-0548
23. Sterner RC and Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. (2021) 11:69. doi: 10.1038/s41408-021-00459-7
24. Rui R, Zhou L, and He S. Cancer immunotherapies: advances and bottlenecks. Front Immunol. (2023) 14:1212476. doi: 10.3389/fimmu.2023.1212476
25. Briukhovetska D, Dörr J, Endres S, Libby P, Dinarello CA, and Kobold S. Interleukins in cancer: from biology to therapy. Nat Rev Cancer. (2021) 21:481–99. doi: 10.1038/s41568-021-00363-z
26. Cha JH, Chan LC, Song MS, and Hung MC. New approaches on cancer immunotherapy. Cold Spring Harb Perspect Med. (2020) 10. doi: 10.1101/cshperspect.a036863
27. Lasek W, Zagożdżon R, and Jakobisiak M. Interleukin 12: still a promising candidate for tumor immunotherapy? Cancer Immunol Immunother : CII. (2014) 63:419–35. doi: 10.1007/s00262-014-1523-1
28. Gade A, Ingle A, Whiteley C, and Rai M. Mycogenic metal nanoparticles: progress and applications. Biotechnol Lett. (2010) 32:593–600. doi: 10.1007/s10529-009-0197-9
29. Li J, Lu W, Yang Y, Xiang R, Ling Y, Yu C, et al. Hybrid nanomaterials for cancer immunotherapy. Adv Sci (Weinh). (2023) 10:e2204932. doi: 10.1002/advs.202204932
30. Alves ACS, Bruinsmann FA, Guterres SS, and Pohlmann AR. Organic nanocarriers for bevacizumab delivery: an overview of development, characterization and applications. Molecules. (2021) 26. doi: 10.3390/molecules26144127
31. Cheng H, Liao J, Ma Y, Sarwar MT, and Yang H. Advances in targeted therapy for tumor with nanocarriers: A review. Mater Today Bio. (2025) 31:101583. doi: 10.1016/j.mtbio.2025.101583
32. Large DE, Abdelmessih RG, Fink EA, and Auguste DT. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv Drug Delivery Rev. (2021) 176:113851. doi: 10.1016/j.addr.2021.113851
33. Zhu X and Li S. Nanomaterials in tumor immunotherapy: new strategies and challenges. Mol Cancer. (2023) 22:94. doi: 10.1186/s12943-023-01797-9
34. Guimarães D, Cavaco-Paulo A, and Nogueira E. Design of liposomes as drug delivery system for therapeutic applications. Int J Pharm. (2021) 601:120571. doi: 10.1016/j.ijpharm.2021.120571
35. Filipczak N, Pan J, Yalamarty SSK, and Torchilin VP. Recent advancements in liposome technology. Adv Drug Delivery Rev. (2020) 156:4–22. doi: 10.1016/j.addr.2020.06.022
36. Yuan Z, Gottsacker C, He X, Waterkotte T, and Park YC. Repetitive drug delivery using Light-Activated liposomes for potential antimicrobial therapies. Adv Drug Delivery Rev. (2022) 187:114395. doi: 10.1016/j.addr.2022.114395
37. Gupta J, Safdari HA, and Hoque M. Nanoparticle mediated cancer immunotherapy. Semin Cancer Biol. (2021) 69:307–24. doi: 10.1016/j.semcancer.2020.03.015
38. Irvine DJ and Dane EL. Enhancing cancer immunotherapy with nanomedicine. Nat Rev Immunol. (2020) 20:321–34. doi: 10.1038/s41577-019-0269-6
39. Gu Z, Da Silva CG, van der Maaden K, Ossendorp F, and Cruz LJ. Liposome-based drug delivery systems in cancer immunotherapy. Pharmaceutics. (2020) 12. doi: 10.3390/pharmaceutics12111054
40. Cheng N, Watkins-Schulz R, Junkins RD, David CN, Johnson BM, Montgomery SA, et al. A nanoparticle-incorporated STING activator enhances antitumor immunity in PD-L1-insensitive models of triple-negative breast cancer. JCI Insight. (2018) 3. doi: 10.1172/jci.insight.120638
41. Fu S, Chang L, Liu S, Gao T, Sang X, Zhang Z, et al. Temperature sensitive liposome based cancer nanomedicine enables tumour lymph node immune microenvironment remodelling. Nat Commun. (2023) 14:2248. doi: 10.1038/s41467-023-38014-6
42. Zhang C, Fei Y, Wang H, Hu S, Liu C, Hu R, et al. CAFs orchestrates tumor immune microenvironment-A new target in cancer therapy? Front Pharmacol. (2023) 14:1113378. doi: 10.3389/fphar.2023.1113378
43. Han C, Liu T, and Yin R. Biomarkers for cancer-associated fibroblasts. biomark Res. (2020) 8:64. doi: 10.1186/s40364-020-00245-w
44. Jia N, Wang Q, Li W, Chen D, and Hu H. Membrane fusion liposomes deliver antifibrotic and chemotherapeutic drugs sequentially to enhance tumor treatment efficacy by reshaping tumor microenvironment. Adv Healthc Mater. (2024) 13:e2400219. doi: 10.1002/adhm.202400219
45. Doonan B, Shaw C, Lee J-H, Manso E, Mendez-Gomez H, Roemeling CV, et al. 772 Novel RNA-nanoparticle vaccine for the treatment of early melanoma recurrence following adjuvant anti-PD-1 antibody therapy. J ImmunoTher Cancer. (2023) 11:A867–A9. doi: 10.1136/jitc-2023-SITC2023.0772
46. Panahi Y, Farshbaf M, Mohammadhosseini M, Mirahadi M, Khalilov R, Saghfi S, et al. Recent advances on liposomal nanoparticles: synthesis, characterization and biomedical applications. Artif Cells Nanomed Biotechnol. (2017) 45:788–99. doi: 10.1080/21691401.2017.1282496
47. Goyal R, Chopra H, Singh I, Dua K, and Gautam RK. Insights on prospects of nano-siRNA based approaches in treatment of Cancer. Front Pharmacol. (2022) 13:985670. doi: 10.3389/fphar.2022.985670
48. Kowalski PS, Rudra A, Miao L, and Anderson DG. Delivering the messenger: advances in technologies for therapeutic mRNA delivery. Mol Ther. (2019) 27:710–28. doi: 10.1016/j.ymthe.2019.02.012
49. Masarwy R, Breier D, Stotsky-Oterin L, Ad-El N, Qassem S, Naidu GS, et al. Targeted CRISPR/Cas9 lipid nanoparticles elicits therapeutic genome editing in head and neck cancer. Adv Sci (Weinh). (2025) 12:e2411032. doi: 10.1002/advs.202411032
50. Mennati A, Rostamizadeh K, Manjili HK, Fathi M, and Danafar H. Co-delivery of siRNA and lycopene encapsulated hybrid lipid nanoparticles for dual silencing of insulin-like growth factor 1 receptor in MCF-7 breast cancer cell line. Int J Biol Macromol. (2022) 200:335–49. doi: 10.1016/j.ijbiomac.2021.12.197
51. Zhang H, You X, Wang X, Cui L, Wang Z, Xu F, et al. Delivery of mRNA vaccine with a lipid-like material potentiates antitumor efficacy through Toll-like receptor 4 signaling. Proc Natl Acad Sci U.S.A. (2021) 118. doi: 10.1073/pnas.2005191118
52. Mittal P, Saharan A, Verma R, Altalbawy FMA, Alfaidi MA, Batiha GE, et al. Dendrimers: A new race of pharmaceutical nanocarriers. BioMed Res Int. (2021) 2021:8844030. doi: 10.1155/2021/8844030
53. Wang K, Hu Q, Zhu W, Zhao M, Ping Y, and Tang G. Structure-invertible nanoparticles for triggered co-delivery of nucleic acids and hydrophobic drugs for combination cancer therapy. Adv Funct Mate. (2015) 25:3380–92. doi: 10.1002/adfm.201403921
54. Zhan M, Qiu J, Fan Y, Chen L, Guo Y, Wang Z, et al. Phosphorous dendron micelles as a nanomedicine platform for cooperative tumor chemoimmunotherapy via synergistic modulation of immune cells. Adv Mater. (2023) 35:e2208277. doi: 10.1002/adma.202208277
55. Xiang Z, Chu C, Xu D, and Chen S. Tuning the protonation sensitivity of weak acidic groups on a zwitterionic dendrimer for selectively targeting GD2-overexpressed tumor cells in an acidic tumor microenvironment. Langmuir. (2024) 40:24106–16. doi: 10.1021/acs.langmuir.4c03503
56. Huang H, Guo H, Liu J, Ni C, Xia L, Cao X, et al. Dendrimer/metal-phenolic nanocomplexes encapsulating CuO(2) for targeted magnetic resonance imaging and enhanced ferroptosis/cuproptosis/chemodynamic therapy by regulating the tumor microenvironment. Acta Biomater. (2024) 183:252–63. doi: 10.1016/j.actbio.2024.05.035
57. Pljesa-Ercegovac M, Savic-Radojevic A, Matic M, Coric V, Djukic T, Radic T, et al. Glutathione transferases: potential targets to overcome chemoresistance in solid tumors. Int J Mol Sci. (2018) 19. doi: 10.3390/ijms19123785
58. Zhang G, Zhan M, Zhang C, Wang Z, Sun H, Tao Y, et al. Redox-responsive dendrimer nanogels enable ultrasound-enhanced chemoimmunotherapy of pancreatic cancer via endoplasmic reticulum stress amplification and macrophage polarization. Adv Sci (Weinh). (2023) 10:e2301759. doi: 10.1002/advs.202301759
59. Buzas EI. The roles of extracellular vesicles in the immune system. Nat Rev Immunol. (2023) 23:236–50. doi: 10.1038/s41577-022-00763-8
60. Han QF, Li WJ, Hu KS, Gao J, Zhai WL, Yang JH, et al. Exosome biogenesis: machinery, regulation, and therapeutic implications in cancer. Mol Cancer. (2022) 21:207. doi: 10.1186/s12943-022-01671-0
61. Campanella C, Caruso Bavisotto C, Logozzi M, Marino Gammazza A, Mizzoni D, Cappello F, et al. On the choice of the extracellular vesicles for therapeutic purposes. Int J Mol Sci. (2019) 20. doi: 10.3390/ijms20020236
62. Soltani F, Parhiz H, Mokhtarzadeh A, and Ramezani M. Synthetic and biological vesicular nano-carriers designed for gene delivery. Curr Pharm Des. (2015) 21:6214–35. doi: 10.2174/1381612821666151027153410
63. Su MJ, Aldawsari H, and Amiji M. Pancreatic cancer cell exosome-mediated macrophage reprogramming and the role of microRNAs 155 and 125b2 transfection using nanoparticle delivery systems. Sci Rep. (2016) 6:30110. doi: 10.1038/srep30110
64. Fu C, Zhou L, Mi QS, and Jiang A. DC-based vaccines for cancer immunotherapy. Vaccines (Basel). (2020) 8. doi: 10.3390/vaccines8040706
65. Lv F, Liu H, Zhao G, Zhao E, Yan H, Che R, et al. Therapeutic exosomal vaccine for enhanced cancer immunotherapy by mediating tumor microenvironment. iScience. (2022) 25:103639. doi: 10.1016/j.isci.2021.103639
66. Zhen X, Li Y, Yuan W, Zhang T, Li M, Huang J, et al. Biointerface-engineered hybrid nanovesicles for targeted reprogramming of tumor microenvironment. Adv Mater. (2024) 36:e2401495. doi: 10.1002/adma.202401495
67. Zhang H, Zhai Y, Wang J, and Zhai G. New progress and prospects: The application of nanogel in drug delivery. Mater Sci Eng C Mater Biol Appl. (2016) 60:560–8. doi: 10.1016/j.msec.2015.11.041
68. Du X, Gao Y, Kang Q, and Xing J. Design and applications of tumor microenvironment-responsive nanogels as drug carriers. Front Bioeng Biotechnol. (2021) 9:771851. doi: 10.3389/fbioe.2021.771851
69. Ahmed S, Alhareth K, and Mignet N. Advancement in nanogel formulations provides controlled drug release. Int J Pharm. (2020) 584:119435. doi: 10.1016/j.ijpharm.2020.119435
70. Bai Z, Yang Y, Cui Z, Liang W, Zhang X, Zhang Z, et al. Double-targeted liposomes coated with matrix metallopeptidase-2-responsive polypeptide nanogel for chemotherapy and enhanced immunotherapy against cervical cancer. Mater Today Bio. (2025) 30:101412. doi: 10.1016/j.mtbio.2024.101412
71. Li Z, Ding Y, Liu J, Wang J, Mo F, Wang Y, et al. Depletion of tumor associated macrophages enhances local and systemic platelet-mediated anti-PD-1 delivery for post-surgery tumor recurrence treatment. Nat Commun. (2022) 13:1845. doi: 10.1038/s41467-022-29388-0
72. Chen C, Jing W, Chen Y, Wang G, Abdalla M, Gao L, et al. Intracavity generation of glioma stem cell-specific CAR macrophages primes locoregional immunity for postoperative glioblastoma therapy. Sci Transl Med. (2022) 14:eabn1128. doi: 10.1126/scitranslmed.abn1128
73. Tian H, Li W, Wang G, Tian Y, Yan J, Zhou S, et al. Self-degradable nanogels reshape immunosuppressive tumor microenvironment via drug repurposing strategy to reactivate cytotoxic CD8(+) T cells. Adv Sci (Weinh). (2023) 10:e2301661. doi: 10.1002/advs.202301661
74. Gao X, Li S, Ding F, Liu X, Wu Y, Li J, et al. A virus-mimicking nucleic acid nanogel reprograms microglia and macrophages for glioblastoma therapy. Adv Mater. (2021) 33:e2006116. doi: 10.1002/adma.202006116
75. Woodman C, Vundu G, George A, and Wilson CM. Applications and strategies in nanodiagnosis and nanotherapy in lung cancer. Semin Cancer Biol. (2021) 69:349–64. doi: 10.1016/j.semcancer.2020.02.009
76. Idrees H, Zaidi SZJ, Sabir A, Khan RU, Zhang X, and Hassan SU. A review of biodegradable natural polymer-based nanoparticles for drug delivery applications. Nanomaterials (Basel). (2020) 10. doi: 10.3390/nano10101970
77. Cheng WJ, Lin SY, Chen M, Chen LC, Ho HO, Chuang KH, et al. Active tumoral/tumor environmental dual-targeting by non-covalently arming with trispecific antibodies or dual-bispecific antibodies on docetaxel-loaded mPEGylated nanocarriers to enhance chemotherapeutic efficacy and minimize systemic toxicity. Int J Nanomed. (2021) 16:4017–30. doi: 10.2147/IJN.S301237
78. Wang C, Wang J, Zhang X, Yu S, Wen D, Hu Q, et al. In situ formed reactive oxygen species-responsive scaffold with gemcitabine and checkpoint inhibitor for combination therapy. Sci Transl Med. (2018) 10. doi: 10.1126/scitranslmed.aan3682
79. Ding M, Fan Y, Lv Y, Liu J, Yu N, Kong D, et al. A prodrug hydrogel with tumor microenvironment and near-infrared light dual-responsive action for synergistic cancer immunotherapy. Acta Biomater. (2022) 149:334–46. doi: 10.1016/j.actbio.2022.06.041
80. Wan Z, Huang H, West RE 3rd, Zhang M, Zhang B, Cai X, et al. Overcoming pancreatic cancer immune resistance by codelivery of CCR2 antagonist using a STING-activating gemcitabine-based nanocarrier. Mater Today (Kidlington). (2023) 62:33–50. doi: 10.1016/j.mattod.2022.11.008
81. Koerner J, Horvath D, Herrmann VL, MacKerracher A, Gander B, Yagita H, et al. PLGA-particle vaccine carrying TLR3/RIG-I ligand Riboxxim synergizes with immune checkpoint blockade for effective anti-cancer immunotherapy. Nat Commun. (2021) 12:2935. doi: 10.1038/s41467-021-23244-3
82. Dacoba TG, Anfray C, Mainini F, Allavena P, Alonso MJ, Torres Andón F, et al. Arginine-based Poly(I:C)-loaded nanocomplexes for the polarization of macrophages toward M1-antitumoral effectors. Front Immunol. (2020) 11:1412. doi: 10.3389/fimmu.2020.01412
83. Guo S, Tang D, Zhang M, Yang H, Zhang T, Hu B, et al. Spatiotemporal-controlled NIR-II immune agonist sensitizes cancer immunotherapy. Adv Mater. (2024) 36:e2400228. doi: 10.1002/adma.202400228
84. Li Y, Younis MH, Wang H, Zhang J, Cai W, and Ni D. Spectral computed tomography with inorganic nanomaterials: State-of-the-art. Adv Drug Delivery Rev. (2022) 189:114524. doi: 10.1016/j.addr.2022.114524
85. Martano S, De Matteis V, Cascione M, and Rinaldi R. Inorganic nanomaterials versus polymer-based nanoparticles for overcoming neurodegeneration. Nanomaterials (Basel). (2022) 12. doi: 10.3390/nano12142337
86. Tang L, Zhang A, Zhang Z, Zhao Q, Li J, Mei Y, et al. Multifunctional inorganic nanomaterials for cancer photoimmunotherapy. Cancer Commun (Lond). (2022) 42:141–63. doi: 10.1002/cac2.12255
87. Wang Y, Gong F, Han Z, Lei H, Zhou Y, Cheng S, et al. Oxygen-deficient molybdenum oxide nanosensitizers for ultrasound-enhanced cancer metalloimmunotherapy. Angew Chem Int Ed Engl. (2023) 62:e202215467. doi: 10.1002/anie.202215467
88. Kesharwani P, Ma R, Sang L, Fatima M, Sheikh A, Abourehab MAS, et al. Gold nanoparticles and gold nanorods in the landscape of cancer therapy. Mol Cancer. (2023) 22:98. doi: 10.1186/s12943-023-01798-8
89. Liu X, Zhang Q, Knoll W, Liedberg B, and Wang Y. Rational design of functional peptide-gold hybrid nanomaterials for molecular interactions. Adv Mater. (2020) 32:e2000866. doi: 10.1002/adma.202000866
90. Liang R, Xie J, Li J, Wang K, Liu L, Gao Y, et al. Liposomes-coated gold nanocages with antigens and adjuvants targeted delivery to dendritic cells for enhancing antitumor immune response. Biomaterials. (2017) 149:41–50. doi: 10.1016/j.biomaterials.2017.09.029
91. Reichel D, Sagong B, Teh J, Zhang Y, Wagner S, Wang H, et al. Near infrared fluorescent nanoplatform for targeted intraoperative resection and chemotherapeutic treatment of glioblastoma. ACS Nano. (2020) 14:8392–408. doi: 10.1021/acsnano.0c02509
92. Guan Y, Zhang W, Mao Y, and Li S. Nanoparticles and bone microenvironment: a comprehensive review for Malignant bone tumor diagnosis and treatment. Mol Cancer. (2024) 23:246. doi: 10.1186/s12943-024-02161-1
93. Li Z, He S, Xie L, Zeng G, Huang J, Wang H, et al. Boosting ferroptosis and immunotherapy for colorectal cancer by lactate-related metabolic reprogramming. Adv Funct Mate. (2024) 34:2411247. doi: 10.1002/adfm.202411247
94. Xie J, Lin X, Deng X, Tang H, Zou Y, Chen W, et al. Cancer-associated fibroblast-derived extracellular vesicles: regulators and therapeutic targets in the tumor microenvironment. Cancer Drug Resist. (2025) 8:2. doi: 10.20517/cdr.2024.152
95. Bromma K, Bannister A, Kowalewski A, Cicon L, and Chithrani DB. Elucidating the fate of nanoparticles among key cell components of the tumor microenvironment for promoting cancer nanotechnology. Cancer Nanotechnol. (2020) 11:8. doi: 10.1186/s12645-020-00064-6
96. Liu J, Movahedi F, Sun B, Sun L, Zhang B, Wang J, et al. Immunostimulatory photochemotherapeutic nanocapsule for enhanced colon cancer treatment. Nanophotonics. (2021) 10:3321–37. doi: 10.1515/nanoph-2021-0202
97. Zhou S, Zhen Z, Paschall AV, Xue L, Yang X, Bebin-Blackwell AG, et al. FAP-targeted photodynamic therapy mediated by ferritin nanoparticles elicits an immune response against cancer cells and cancer associated fibroblasts. Adv Funct Mater. (2021) 31. doi: 10.1002/adfm.202007017
98. Ding L, Liang M, Li Y, Zeng M, Liu M, Ma W, et al. Zinc-organometallic framework vaccine controlled-release Zn2+ regulates tumor extracellular matrix degradation potentiate efficacy of immunotherapy. Adv Sci (Weinh). (2023) 10:e2302967. doi: 10.1002/advs.202302967
99. Deng X, Liu T, Zhu Y, Chen J, Song Z, Shi Z, et al. Ca & Mn dual-ion hybrid nanostimulator boosting anti-tumor immunity via ferroptosis and innate immunity awakening. Bioact Mater. (2024) 33:483–96. doi: 10.1016/j.bioactmat.2023.11.017
100. Sun W, Yang J, Hou M, Xie S, Xiong L, Li B, et al. A nano “Immune-guide” Recruiting lymphocytes and modulating the ratio of macrophages from different origins to enhance cancer immunotherapy. Adv Funct Mate. (2021) 31:2009116. doi: 10.1002/adfm.202009116
101. Jiang Q, Wang K, Zhang X, Ouyang B, Liu H, Pang Z, et al. Platelet membrane-camouflaged magnetic nanoparticles for ferroptosis-enhanced cancer immunotherapy. Small. (2020) 16:e2001704. doi: 10.1002/smll.202001704
102. Hsieh CY, Lin CC, Huang YW, Chen JH, Tsou YA, Chang LC, et al. Macrophage secretory IL-1β promotes docetaxel resistance in head and neck squamous carcinoma via SOD2/CAT-ICAM1 signaling. JCI Insight. (2022) 7. doi: 10.1172/jci.insight.157285
103. Zhang J, Li H, Wu Q, Chen Y, Deng Y, Yang Z, et al. Tumoral NOX4 recruits M2 tumor-associated macrophages via ROS/PI3K signaling-dependent various cytokine production to promote NSCLC growth. Redox Biol. (2019) 22:101116. doi: 10.1016/j.redox.2019.101116
104. Gong F, Chen M, Yang N, Dong Z, Tian L, Hao Y, et al. Bimetallic oxide FeWO nanosheets as multifunctional cascade bioreactors for tumor microenvironment-modulation and enhanced multimodal cancer therapy. Adv Funct Mate. (2020) 30:2002753. doi: 10.1002/adfm.202002753
105. Wu Y, Xiong W, Wang Z, Wang Y, Sun K-Y, Song X, et al. Self-assembled MXene-based Schottky-junction upon Transition metal oxide for regulated tumor microenvironment and enhanced CDT/PTT/MRI activated by NIR irradiation. Chem Eng J. (2022) 427:131925. doi: 10.1016/j.cej.2021.131925
106. Guo M, Zhao L, Liu J, Wang X, Yao H, Chang X, et al. The underlying function and structural organization of the intracellular protein corona on graphdiyne oxide nanosheet for local immunomodulation. Nano Lett. (2021) 21:6005–13. doi: 10.1021/acs.nanolett.1c01048
107. Xiong Z, He L, Pi F, Yu Y, Xiao Z, and Chen T. Intracellular redox environment determines cancer-normal cell selectivity of selenium nanoclusters. Angew Chem Int Ed Engl. (2025) 64:e202416006. doi: 10.1002/anie.202416006
108. Xu J, Liu Z, Zhang S, Xiang J, Lan H, and Bao Y. Anti-hepatoma immunotherapy of Pholiota adiposa polysaccharide-coated selenium nanoparticles by reversing M2-like tumor-associated macrophage polarization. Int J Biol Macromol. (2024) 277:133667. doi: 10.1016/j.ijbiomac.2024.133667
109. Li Y, Yu H, Ren J, Lu G, Cao Y, Xu Z, et al. Acidic TME-responsive Nano-Bi2 Se3 @MnCaP as a NIR-II-triggered free radical generator for hypoxia-irrelevant phototherapy with high specificity and immunogenicity. Small (Weinheim an der Bergstrasse, Germany). (2022) 18:e2104302. doi: 10.1002/smll.202104302
110. Chen Z, Yue Z, Wang R, Yang K, and Li S. Nanomaterials: A powerful tool for tumor immunotherapy. Front Immunol. (2022) 13:979469. doi: 10.3389/fimmu.2022.979469
111. Zhao P, Qiu L, Zhou S, Li L, Qian Z, and Zhang H. Cancer cell membrane camouflaged mesoporous silica nanoparticles combined with immune checkpoint blockade for regulating tumor microenvironment and enhancing antitumor therapy. Int J Nanomed. (2021) 16:2107–21. doi: 10.2147/IJN.S295565
112. Wang X, Li X, Ito A, Sogo Y, and Ohno T. Synergistic anti-tumor efficacy of a hollow mesoporous silica-based cancer vaccine and an immune checkpoint inhibitor at the local site. Acta Biomater. (2022) 145:235–45. doi: 10.1016/j.actbio.2022.04.001
113. Hyun GH, Jeong DH, Yang YY, Cho IH, Ha YJ, Xing X, et al. Multivalent carbohydrate nanocomposites for tumor microenvironment remodeling to enhance antitumor immunity. ACS Nano. (2023) 17:11567–82. doi: 10.1021/acsnano.3c01645
114. Gao DY, Lin Ts T, Sung YC, Liu YC, Chiang WH, Chang CC, et al. CXCR4-targeted lipid-coated PLGA nanoparticles deliver sorafenib and overcome acquired drug resistance in liver cancer. Biomaterials. (2015) 67:194–203. doi: 10.1016/j.biomaterials.2015.07.035
115. Zhang WX, Zhou ZL, Lv QY, Song X, Chen J, Niu CB, et al. O(2)-generation-enhanced responsive starvation/photothermal synergistic tumor therapy based on the AuNRs@MnO(2)@SiO(2) nanocarrier and thermosensitive biomimetic camouflaging. ACS Appl Bio Mater. (2023) 6:4775–90. doi: 10.1021/acsabm.3c00544
116. Feng Q, Ma X, Cheng K, Liu G, Li Y, Yue Y, et al. Engineered bacterial outer membrane vesicles as controllable two-way adaptors to activate macrophage phagocytosis for improved tumor immunotherapy. Adv Mater. (2022) 34:e2206200. doi: 10.1002/adma.202206200
117. Chen Z, Zhang Q, Zeng L, Zhang J, Liu Z, Zhang M, et al. Light-triggered OVA release based on CuS@poly(lactide-co-glycolide acid) nanoparticles for synergistic photothermal-immunotherapy of tumor. Pharmacol Res. (2020) 158:104902. doi: 10.1016/j.phrs.2020.104902
118. Timperi E, Gueguen P, Molgora M, Magagna I, Kieffer Y, Lopez-Lastra S, et al. Lipid-associated macrophages are induced by cancer-associated fibroblasts and mediate immune suppression in breast cancer. Cancer Res. (2022) 82:3291–306. doi: 10.1158/0008-5472.CAN-22-1427
119. Han SJ, Jain P, Gilad Y, Xia Y, Sung N, Park MJ, et al. Steroid receptor coactivator 3 is a key modulator of regulatory T cell-mediated tumor evasion. Proc Natl Acad Sci U S A. (2023) 120:e2221707120. doi: 10.1073/pnas.2221707120
120. Chen Z, Yue Z, Yang K, Shen C, Cheng Z, Zhou X, et al. Four ounces can move a thousand pounds: the enormous value of nanomaterials in tumor immunotherapy. Adv Healthc Mater. (2023) 12:e2300882. doi: 10.1002/adhm.202300882
121. Jin Y, Huang Y, Ren H, Huang H, Lai C, Wang W, et al. Nano-enhanced immunotherapy: Targeting the immunosuppressive tumor microenvironment. Biomaterials. (2024) 305:122463. doi: 10.1016/j.biomaterials.2023.122463
122. Tao Y, Ju E, Ren J, and Qu X. Immunostimulatory oligonucleotides-loaded cationic graphene oxide with photothermally enhanced immunogenicity for photothermal/immune cancer therapy. Biomaterials. (2014) 35:9963–71. doi: 10.1016/j.biomaterials.2014.08.036
123. Itakura E, Huang RR, Wen DR, Paul E, Wünsch PH, and Cochran AJ. IL-10 expression by primary tumor cells correlates with melanoma progression from radial to vertical growth phase and development of metastatic competence. Mod Pathol. (2011) 24:801–9. doi: 10.1038/modpathol.2011.5
124. Travis MA and Sheppard D. TGF-β activation and function in immunity. Annu Rev Immunol. (2014) 32:51–82. doi: 10.1146/annurev-immunol-032713-120257
125. Korkut A, Zaidi S, Kanchi RS, Rao S, Gough NR, Schultz A, et al. A pan-cancer analysis reveals high-frequency genetic alterations in mediators of signaling by the TGF-β Superfamily. Cell Syst. (2018) 7:422–37.e7. doi: 10.1016/j.cels.2018.08.010
126. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, and Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. (1995) 155:1151–64. doi: 10.4049/jimmunol.155.3.1151
127. Wang Y, Ding Y, Guo N, and Wang S. MDSCs: key criminals of tumor pre-metastatic niche formation. Front Immunol. (2019) 10:172. doi: 10.3389/fimmu.2019.00172
128. Jiang X, Wang J, Deng X, Xiong F, Ge J, Xiang B, et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer. (2019) 18:10. doi: 10.1186/s12943-018-0928-4
129. Tang Y, He Y, Shi L, Yang L, Wang J, Lian Y, et al. Co-expression of AFAP1-AS1 and PD-1 predicts poor prognosis in nasopharyngeal carcinoma. Oncotarget. (2017) 8:39001–11. doi: 10.18632/oncotarget.16545
130. Zhou Y, Miao J, Wu H, Tang H, Kuang J, Zhou X, et al. PD-1 and PD-L1 expression in 132 recurrent nasopharyngeal carcinoma: the correlation with anemia and outcomes. Oncotarget. (2017) 8:51210–23. doi: 10.18632/oncotarget.17214
131. DhatChinamoorthy K, Colbert JD, and Rock KL. Cancer immune evasion through loss of MHC class I antigen presentation. Front Immunol. (2021) 12:636568. doi: 10.3389/fimmu.2021.636568
132. Liu P, Chen G, and Zhang J. A review of liposomes as a drug delivery system: current status of approved products, regulatory environments, and future perspectives. Molecules. (2022) 27. doi: 10.3390/molecules27041372
133. Kozak A, Lavrih E, Mikhaylov G, Turk B, and Vasiljeva O. Navigating the clinical landscape of liposomal therapeutics in cancer treatment. Pharmaceutics. (2025) 17. doi: 10.3390/pharmaceutics17020276
134. Zhang H, Mao Y, Nie Z, Li Q, Wang M, Cai C, et al. Iron oxide nanoparticles engineered macrophage-derived exosomes for targeted pathological angiogenesis therapy. ACS Nano. (2024) 18:7644–55. doi: 10.1021/acsnano.4c00699
135. Yu Z, Fu J, Mantareva V, Blažević I, Wu Y, Wen D, et al. The role of tumor-derived exosomal LncRNA in tumor metastasis. Cancer Gene Ther. (2025) 32:273–85. doi: 10.1038/s41417-024-00852-x
136. Xie Z, Xia J, Jiao M, Zhao P, Wang Z, Lin S, et al. Exosomal lncRNA HOTAIR induces PDL1(+) B cells to impede anti-tumor immunity in colorectal cancer. Biochem Biophys Res Commun. (2023) 644:112–21. doi: 10.1016/j.bbrc.2023.01.005
137. Guo T, Tang XH, Gao XY, Zhou Y, Jin B, Deng ZQ, et al. A liquid biopsy signature of circulating exosome-derived mRNAs, miRNAs and lncRNAs predict therapeutic efficacy to neoadjuvant chemotherapy in patients with advanced gastric cancer. Mol Cancer. (2022) 21:216. doi: 10.1186/s12943-022-01684-9
138. Su YC, Chen YC, Lo YH, Chang CH, and Chen YF. Hydrophobic interactions enhance doxorubicin delivery from hyaluronic acid nanogels. Eur J Pharm Biopharm. (2025) 210:114676. doi: 10.1016/j.ejpb.2025.114676
139. Gomes V, Veloso SRS, Carvalho A, Hilliou L, Coutinho PJG, Moura C, et al. Multifunctional magneto-plasmonic lipogel based on peptide hydrogel for application in combined cancer therapy. J Pept Sci. (2025) 31:e3650. doi: 10.1002/psc.3650
140. Pérez-Herrero E and Fernández-Medarde A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm. (2015) 93:52–79. doi: 10.1016/j.ejpb.2015.03.018
141. Yu Z, Shen X, Yu H, Tu H, Chittasupho C, and Zhao Y. Smart polymeric nanoparticles in cancer immunotherapy. Pharmaceutics. (2023) 15. doi: 10.3390/pharmaceutics15030775
142. Hameed MK, Gul MT, Khan AA, Kanu GA, AbuOdeh RO, Kim S, et al. Enhanced delivery of doxorubicin via transferrin-coated arylated gold nanostars for cancer therapy. Int J Pharm. (2025) 673:125418. doi: 10.1016/j.ijpharm.2025.125418
143. Guinart A, Perry HL, Wilton-Ely J, and Tetley TD. Gold nanomaterials in the management of lung cancer. Emerg Top Life Sci. (2020) 4:627–43. doi: 10.1042/ETLS20200332
144. Velhal K, Sah PM, Naik HS, Raut R, Patil S, Yamgar R, et al. Synergistic nanoformulation: streamlined one-pot synthesis enhances paclitaxel functionalization gold nanoparticles for potent anticancer activity. Cell Biochem Biophys. (2025). doi: 10.1007/s12013-025-01701-w
145. Zhang M, Zhang J, Chen J, Zeng Y, Zhu Z, and Wan Y. Fabrication of curcumin-modified TiO2 nanoarrays via cyclodextrin based polymer functional coatings for osteosarcoma therapy. Adv Healthc Mater. (2019) 8:e1901031. doi: 10.1002/adhm.201901031
146. Zhang H, Zhang X, Ren Y, Cao F, Hou L, and Zhang Z. An in situ microenvironmental nano-regulator to inhibit the proliferation and metastasis of 4T1 tumor. Theranostics. (2019) 9:3580–94. doi: 10.7150/thno.33141
147. Gong F, Xu J, Liu B, Yang N, Cheng L, Huang P, et al. Nanoscale CaH2 materials for synergistic hydrogen-immune cancer therapy. Chem. (2022) 8:268–86. doi: 10.1016/j.chempr.2021.11.020
148. Zhang A, Xiao Z, Liu Q, Li P, Xu F, Liu J, et al. CaCO3 -encapuslated microspheres for enhanced transhepatic arterial embolization treatment of hepatocellular carcinoma. Adv Healthc Mater. (2021) 10:e2100748. doi: 10.1002/adhm.202100748
149. Sacchetti C, Rapini N, Magrini A, Cirelli E, Bellucci S, Mattei M, et al. In vivo targeting of intratumor regulatory T cells using PEG-modified single-walled carbon nanotubes. Bioconjug Chem. (2013) 24:852–8. doi: 10.1021/bc400070q
150. Ou W, Thapa RK, Jiang L, Soe ZC, Gautam M, Chang JH, et al. Regulatory T cell-targeted hybrid nanoparticles combined with immuno-checkpoint blockage for cancer immunotherapy. J Control Release. (2018) 281:84–96. doi: 10.1016/j.jconrel.2018.05.018
151. Liu Y, Li H, Hao YY, Huang LL, Li X, Zou J, et al. Tumor-selective nano-dispatcher enforced cancer immunotherapeutic effects via regulating lactate metabolism and activating toll-like receptors. Small. (2025) 21:e2406870. doi: 10.1002/smll.202406870
152. Rook AH, Gelfand JM, Wysocka M, Troxel AB, Benoit B, Surber C, et al. Topical resiquimod can induce disease regression and enhance T-cell effector functions in cutaneous T-cell lymphoma. Blood. (2015) 126:1452–61. doi: 10.1182/blood-2015-02-630335
153. Tian H, Li W, Wang G, Tian Y, Yan J, Yu X, et al. Metal-Phenolic Nanomaterial with Organelle-Level Precision Primes Antitumor Immunity via mtDNA-dependent cGAS-STING Activation. Angew Chem Int Ed Engl. (2024) 63:e202411498. doi: 10.1002/anie.202411498
154. Huang H, Li N, Wei X, Li Q, Guo J, Yang G, et al. Biomimetic “Gemini nanoimmunoregulators” orchestrated for boosted photoimmunotherapy by spatiotemporally modulating PD-L1 and tumor-associated macrophages. Acta Pharm Sin B. (2024) 14:1345–61. doi: 10.1016/j.apsb.2023.11.005
155. Rao L, Zhao SK, Wen C, Tian R, Lin L, Cai B, et al. Activating macrophage-mediated cancer immunotherapy by genetically edited nanoparticles. Adv Mater. (2020) 32:e2004853. doi: 10.1002/adma.202004853
156. Chen C, Li A, Sun P, Xu J, Du W, Zhang J, et al. Efficiently restoring the tumoricidal immunity against resistant Malignancies via an immune nanomodulator. J Control Release. (2020) 324:574–85. doi: 10.1016/j.jconrel.2020.05.039
Keywords: nanomaterials, tumor microenvironment, tumor immunity, cancer immunotherapy, application
Citation: Piao H, Jiang Y, Jin S, Shi J, Yu J, Wang W, Du Z, Yao H, Liu Q, Li N, Fu J, Shen Y and Li M (2025) Modulation of the immune microenvironment using nanomaterials: a new strategy for tumor immunotherapy. Front. Immunol. 16:1614640. doi: 10.3389/fimmu.2025.1614640
Received: 19 April 2025; Accepted: 16 June 2025;
Published: 02 July 2025.
Edited by:
Teruyoshi Sasayama, Kyushu University, JapanReviewed by:
Li Yu, Shengjing Hospital of China Medical University, ChinaKong Lingkai, Nanjing University, China
Chao Li, Guangxi Medical University, China
Copyright © 2025 Piao, Jiang, Jin, Shi, Yu, Wang, Du, Yao, Liu, Li, Fu, Shen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingzhu Li, bGltaW5nemh1QGNhbmNlcmhvc3AtbG4tY211LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Haozhe Piao
Haozhe Piao Yuxin Jiang
Yuxin Jiang Shengbo Jin
Shengbo Jin Jie Shi
Jie Shi Jun Yu3
Jun Yu3 Huini Yao
Huini Yao Ningxin Li
Ningxin Li Mingzhu Li
Mingzhu Li