- 1Department of Pulmonary Medicine, Fukushima Medical University School of Medicine, Fukushima, Japan
- 2Department of Otolaryngology-Head and Neck Surgery, Fukushima Medical University School of Medicine, Fukushima, Japan
Immune checkpoint inhibitors (ICIs) enhance antitumor immunity by blocking inhibitory immune signals, but can lead to immune-related adverse events (irAEs). Therefore, effective management of irAEs is crucial during ICI therapy. We report the case of a 50-year-old man who was referred to our department due to cough and abnormal chest shadows. He was diagnosed with hypopharyngeal cancer, and underwent chemoradiotherapy, resulting in complete remission. However, metastatic tumors were detected, and partial lung resection was performed. After one-year, new metastatic tumors and pleural dissemination were identified. Therefore, treatment with pembrolizumab was initiated. After the treatment with pembrolizumab, chest imaging revealed ground-glass opacity (GGO). Laboratory tests showed elevated eosinophils, and fractional exhaled nitric oxide (FeNO). The findings of bronchoscopy revealed eosinophilic infiltration and intraluminal fibrosis, consistent with chronic eosinophilic pneumonia (EP). Based on these findings, he was diagnosed with pembrolizumab-induced chronic EP. Pembrolizumab was temporarily discontinued, and oral corticosteroids (OCS) were initiated. After the treatment of OCS, his symptoms and GGO were dramatically improved. Subsequently, pembrolizumab was resumed, and the hypopharyngeal cancer remains stable without recurrence of EP. This report presents the first pembrolizumab-induced chronic EP during treatment for hypopharyngeal cancer. The chronic EP was effectively managed with systemic corticosteroid therapy. Furthermore, pembrolizumab was resumed with close monitoring of blood eosinophil counts and FeNO levels, without worsening of EP. The results of the current case suggest that ICI-induced chronic EP is manageable, and in cases where ICI therapy exhibits significant efficacy against cancer, its treatment may be continued with careful monitoring of these parameters.
Introduction
Immune checkpoint inhibitors (ICIs) have revolutionized cancer treatment, and are now widely used for various cancers. Anti-programmed cell death 1 (PD-1), anti-programmed cell death ligand 1 (PD-L1), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) are the most frequently used ICIs (1). The mechanism of ICIs involves blocking inhibitory signals that suppress immune responses, thereby enhancing T cell activity to eliminate tumor cells (2). However, their use has revealed a variety of immune-related adverse events (irAEs), most of which are manageable, but some can be unexpected or severe (3). ICIs may disrupt self-tolerance, leading to the emergence of immune-mediated responses against nontumor cells (4). These irAEs can affect any organ system and are classified as part of a broad spectrum of autoimmune disorders induced by monoclonal antibodies targeting immune regulators (5). Among them, ICI-related interstitial lung disease (ILD) is clinically significant, encompassing a broad spectrum of irAEs, ranging from mild to severe, progressive, and potentially life-threatening conditions (1). Therefore, accurate diagnosis and proper management strategies for these events, based on effective information sharing, are crucial, since they may significantly impact the safety, subsequent treatment options, and overall prognosis of cancer patients. Eosinophilic pneumonia (EP) comprises a heterogeneous group of ILD characterized by prominent infiltration of eosinophils into the pulmonary interstitium and alveoli (6). EP is classified into acute EP and chronic EP (6), and can have various causes, including medications or other toxins (7).
Herein, we report, to the best of our knowledge, the first case of chronic EP triggered by pembrolizumab, a PD-1 inhibitor, in a patient with hypopharyngeal cancer, along with a review of the relevant literature.
Case presentation
A 50-year-old man was referred to our department due to cough and abnormal chest shadows. He had a 45 pack-year smoking history, and no history of respiratory or allergic disorders. At the age of 46, he was diagnosed with hypopharyngeal cancer (cT4aN3bM0, cStage IVB), based on the Japanese Clinical Practice Guidelines of Head and Neck Cancer, 4th edition (8). He underwent chemoradiotherapy consisting of three courses of cisplatin (100 mg/m² every 3 weeks) and a total radiation dose of 70 Gy, resulting in complete remission. At the age of 48, metastatic tumors were detected in the posterior segment of the right upper lobe (S2 segment), and partial lung resection was performed under thoracoscopy. At the age of 49, new metastatic tumors in the left upper lobe (S1 + 2 segment) and left pleural dissemination were identified. Therefore, treatment with pembrolizumab at a dose of 200 mg every 3 weeks was initiated as the first-line therapy for recurrent and metastatic hypopharyngeal cancer based on a combined positive score of 5 for PD-L1 in the tumor specimen obtained from the resected lung (9). Pre-treatment chest computed tomography (CT) showed no significant findings other than left metastatic lung nodules and pleural dissemination (Figures 1A, B). One month after starting pembrolizumab therapy, peripheral blood eosinophil levels began to gradually increase without any symptoms (Figure 1). A CT scan taken 2 months after treatment initiation showed a dramatic improvement in the metastatic tumors and left pleural dissemination (Figure 1C), while also revealing a ground-glass opacity (GGO) in the left lower lobe (Figure 1D). As the patient remained asymptomatic, the GGO was observed without any therapeutic intervention. Notably, while the GGO had disappeared on the CT scan obtained 8 months after treatment initiation, a new GGO had appeared in the right lower lobe (Figure 1E), and monitoring was continued without treatment. Of note, after 14 months of pembrolizumab therapy, corresponding to 18 courses, the infiltrative shadow in the right lower lobe had spontaneously disappeared, whereas a new GGO had appeared in the left lower lobe (Figure 1F), which was accompanied by coughing. The patient was subsequently referred to our outpatient clinic.
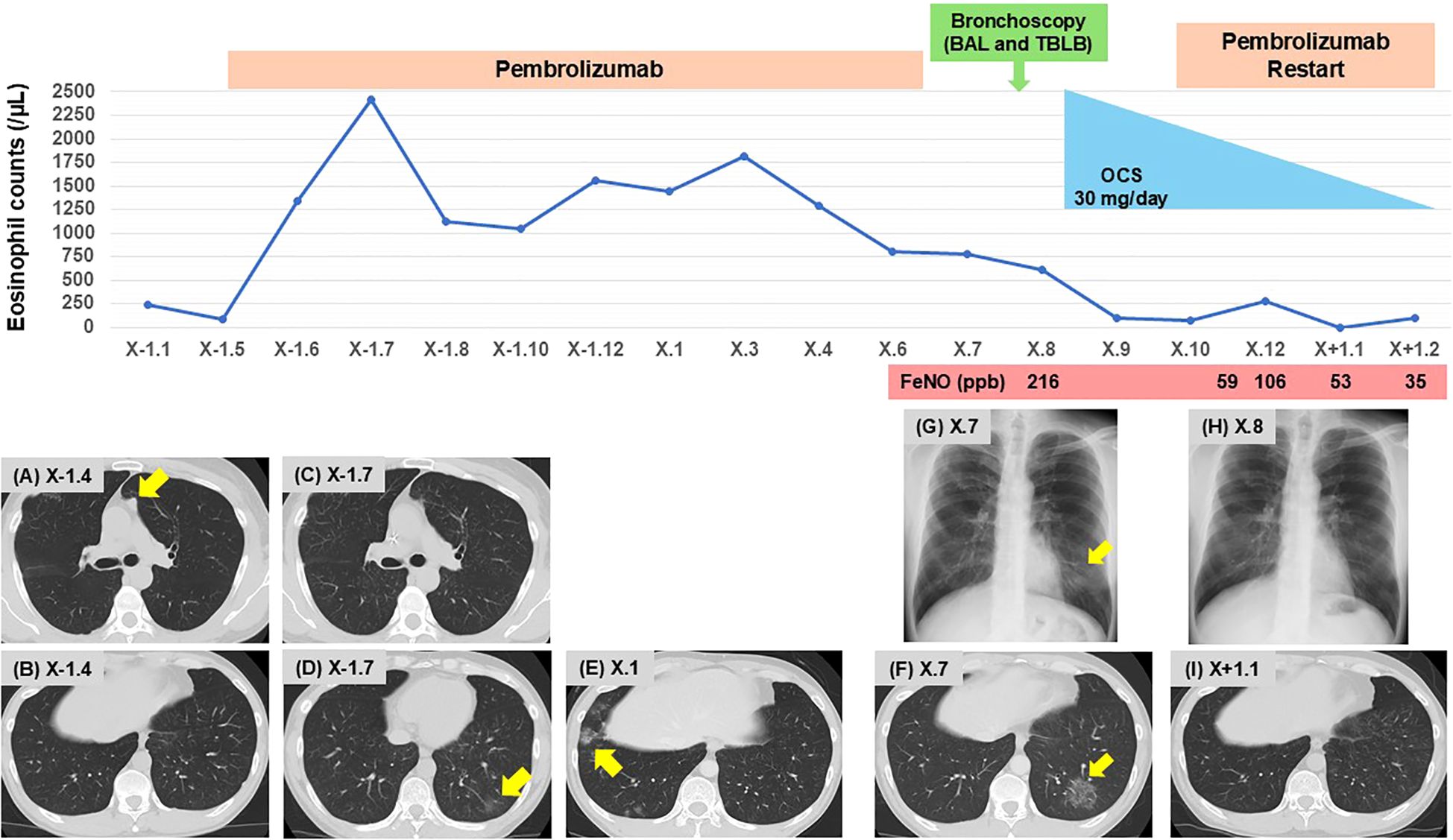
Figure 1. Clinical course of the patient. Changes in eosinophil counts, and imaging findings on chest CT (A–F, I) and radiography (G, H). (A, B) Pre-treatment with pembrolizumab; (A) metastatic lung nodules in the left upper lobe (S1 + 2, arrow), and (B) no signs of pneumonia. (C, D) Two months after initiating pembrolizumab; (C) improvement of metastatic lung nodules, and (D) onset of GGO in the left lower lobe (arrow). (E) Eight months after initiating pembrolizumab; onset of GGO in the right lower lobe (arrow). (F) Fourteen months after initiating pembrolizumab; onset of GGO in the left lower lobe (arrow). (G) Upon presentation to our clinic; GGO in the left lower lung field (arrow). (H, I) Following OCS therapy; improvement of GGO in the left lower lobe. BAL, bronchoalveolar lavage; CT, computed tomography; GGO, ground-glass opacity; FeNO. fractional exhaled nitric oxide; OCS, oral corticosteroids; TBLB, transbronchial lung biopsy.
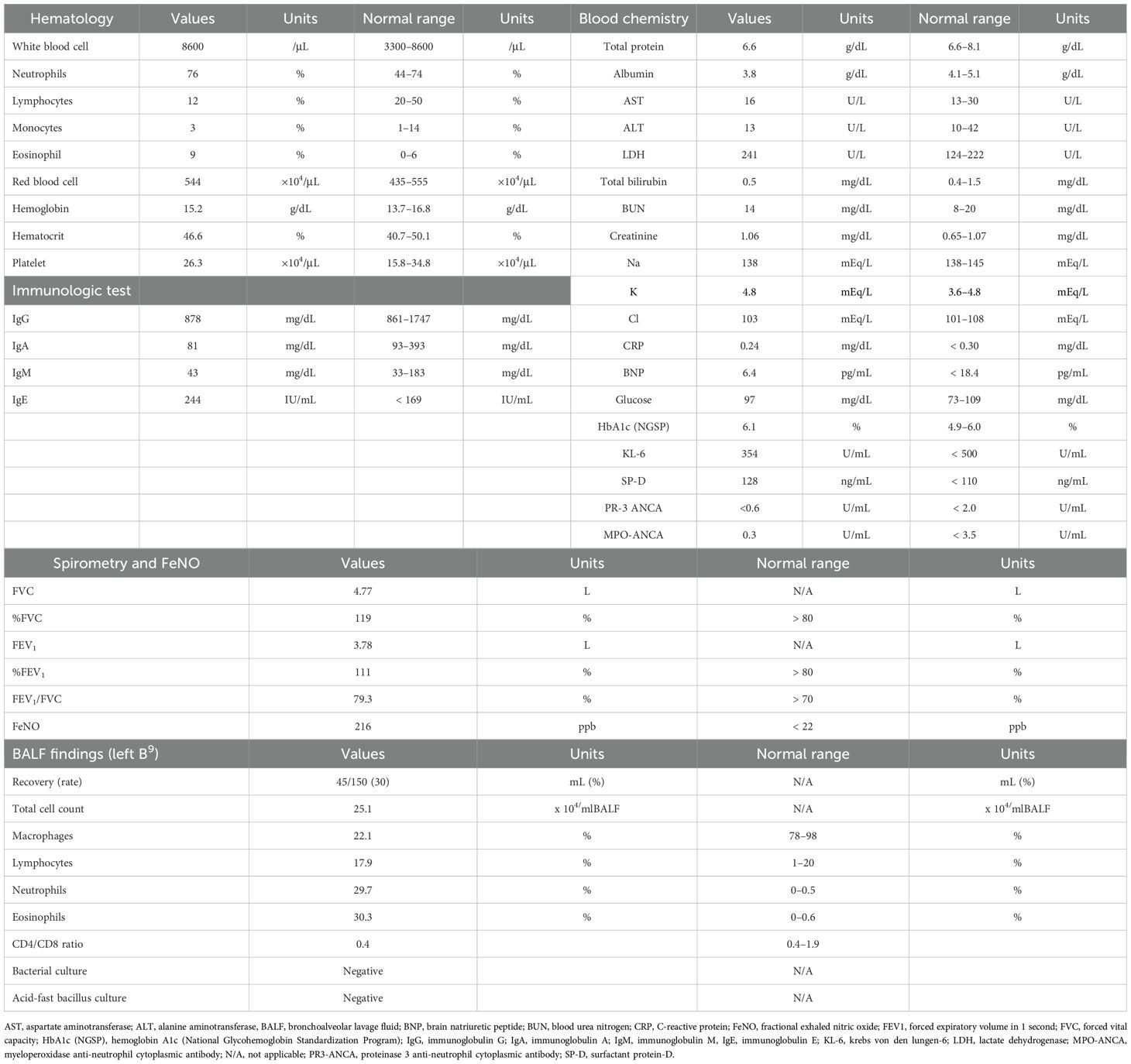
Chest radiograph on initial presentation showed a GGO in the left lower lung field (Figure 1G). Physical examination revealed no crackles or wheezing, and the oxygen saturation was 98% on room air. As shown in Table 1, laboratory examinations revealed increased blood eosinophils of 774/µL (maximum of 2,418/µL during pembrolizumab therapy), immunoglobulin E of 244 IU/mL, and surfactant protein D (SP-D) of 128 ng/mL (normal range < 110 ng/mL). C-reactive protein was 0.24 mg/dL, and sialylated carbohydrate antigen KL-6 was 354 U/mL (normal range < 500 U/mL), which were both within the normal range. There was no elevation in antinuclear antibodies, proteinase 3 anti-neutrophil cytoplasmic antibodies, or myeloperoxidase anti-neutrophil cytoplasmic antibodies. Pulmonary function tests were within the normal range, including small airway parameters such as V50/V25, whereas fractional exhaled nitric oxide (FeNO) level was remarkably elevated at 216 ppb.
Bronchoscopy was performed, and bronchoalveolar lavage fluid (BALF) from the left lower lobe revealed an increased total cell count (25.1 x 104/mlBALF) and a high proportion of eosinophils (30.3%), with no malignant cells or microorganisms detected. Transbronchial lung biopsy samples obtained from left B9 and B10 showed eosinophilic infiltration with degranulation, not only within the blood vessels but also around the alveoli (Figure 2A). In addition, Elastica-Masson staining revealed intraluminal fibrosis (Figure 2B). These findings were all indicative of chronic EP rather than acute EP. Based on the clinical, laboratory, radiological, and pathological findings, along with pembrolizumab treatment, the patient was finally diagnosed with pembrolizumab-induced chronic EP. Pembrolizumab was temporarily discontinued, and oral corticosteroids (OCS) at a dose of 30 mg/day (0.5 mg/kg/day) were initiated on an outpatient basis. Soon after starting OCS treatment, the patient’s cough dramatically improved. Subsequently, chest radiography demonstrated a tendency toward improvement in GGO (Figure 1H), and blood eosinophil counts and SP-D levels returned to normal within 14 days. Furthermore, FeNO levels rapidly dropped to 59 ppb after starting OCS, along with the improvement of the GGO (Figure 1I). Since pembrolizumab had been highly effective for his hypopharyngeal cancer, it was resumed while tapering OCS to a dose of 10 mg/day until day 55. No recurrence of EP has been observed to date, and the therapeutic response of hypopharyngeal cancer has been sustained, with no evidence of tumor progression.
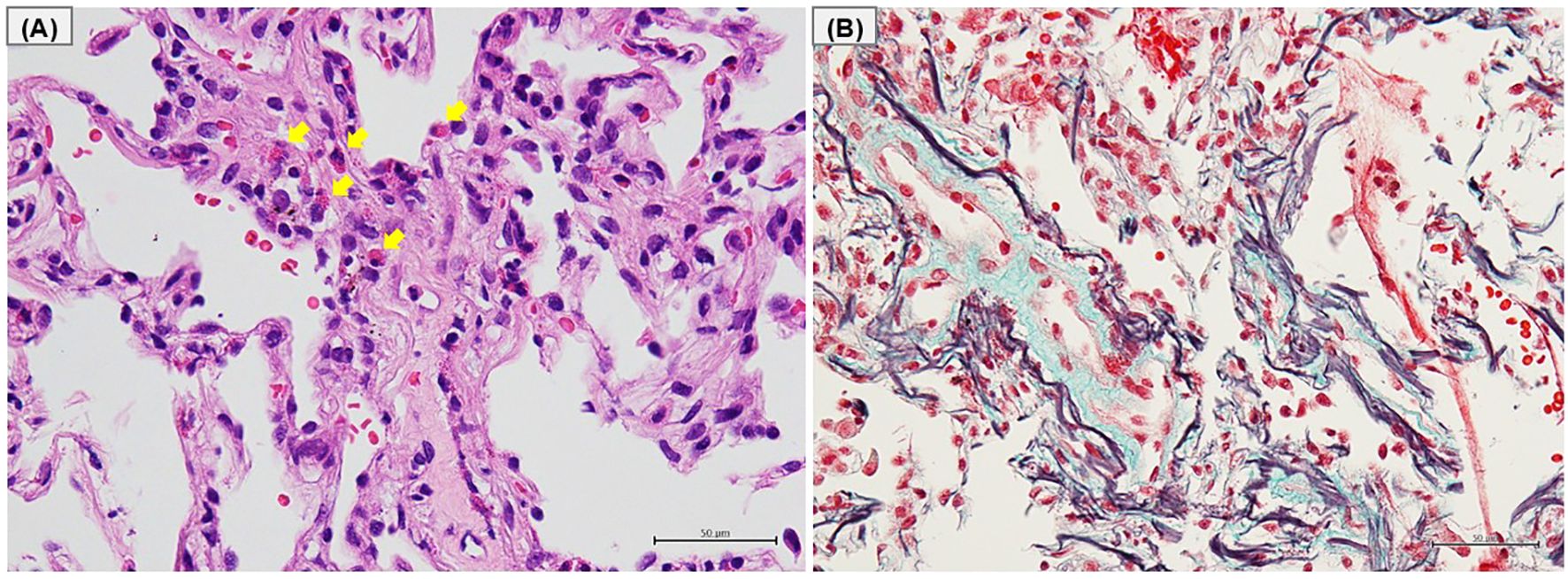
Figure 2. Histological findings of transbronchial lung biopsy. (A) Hematoxylin-eosin staining and (B) Elastica-Masson staining of the left lower lobe tissue obtained by transbronchial lung biopsies. (A) Eosinophilic infiltration with degranulation in the blood vessels and around the alveoli (arrows). (B) Deposition of collagen fibers on the luminal side of the alveoli.
Discussion
EP is characterized by abnormal eosinophil infiltration due to CD4+ T helper type 2 inflammation in the lungs and prompt response to corticosteroid treatment (6). Although both acute EP and chronic EP share several pathophysiological features, such as marked eosinophilic infiltration in the lung parenchyma and a rapid response to corticosteroid therapy (10), they differ not only in the pathological findings but also clinical presentation and disease course (11). To the best of our knowledge, this is the first report of chronic EP caused by pembrolizumab, one of the ICIs available in clinical practice.
To date, a total of eight cases of EP following ICI administration have been reported, including three cases that were presented in conference papers (12–19) (Table 2). The associated ICIs were nivolumab, ipilimumab, or durvalumab, with nivolumab being the most common. The underlying diseases included lung cancer in three cases, and one case each of malignant pleural mesothelioma, renal cell carcinoma, melanoma, esophagogastric adenocarcinoma, and gastric adenocarcinoma. Although the time from ICI initiation to acute EP onset varied, no cases have been reported during the first ICI administration. The diagnosis of acute EP was based solely on BALF analysis in all cases except one, and histopathological examination was not conducted in any of the cases. In the present report, the hallmark pathological findings of chronic EP based on TBLB are presented for the first time, providing valuable insights into ICI-induced EP. Regarding treatment, one previous case was managed solely by discontinuing ICIs, while the others, including the present case, were treated with systemic corticosteroids in addition to ICI discontinuation, all showing significant improvement. However, except for one previous case (13) and the present case, ICI treatment was not resumed. It remains unclear whether the improvement of EP in the present case was mainly due to corticosteroid therapy, ICI discontinuation, or both. Since the number of reported cases is still limited, and treatment responses may vary depending on disease activity, further accumulation of cases is needed to clarify the most effective management approach for ICI-induced EP. However, in patients with symptomatic EP where early resumption of ICI therapy is clinically desirable because of a remarkable response to cancer therapy, corticosteroid treatment may be a reasonable and effective option to achieve prompt symptom relief.
Drug-induced ILD (DIILD) associated with ICIs has been reported across all classes of ICIs, with an overall incidence of 3–6%, of which 1–2% were grade 3–4 adverse events as defined by the Common Terminology Criteria for Adverse Events (CTCAE) (20). DIILD encompasses a broad and heterogeneous group of diseases (21). Additionally, the characteristic radiological findings of DIILD show various patterns, including GGO, consolidation, and reticulonodular shadows, making it challenging to distinguish drug-induced EP from other types of drug-induced ILD (22, 23). Therefore, accurate diagnosis of elevated eosinophil counts in BALF via bronchoscopy is crucial not only for determining the need for specific treatment on DIILD, but also deciding whether to continue chemotherapy. However, bronchoscopy may not always be feasible due to the patient’s general condition. In such cases, peripheral blood eosinophil counts and FeNO may serve as useful adjuncts in the diagnosis of EP. Peripheral blood eosinophilia during ICI therapy has been observed with relatively high frequency (approximately 20%) (24). However, some can lead to EP, although there have been extremely limited number of reports of EP or asthma as irAEs associated with ICIs. This discrepancy suggests that eosinophil-associated irAE may be under-recognized or under-reported in clinical practice. Therefore, in cases where peripheral blood eosinophilia is observed during ICI therapy, elevated FeNO levels may serve as a useful surrogate biomarker to support the diagnosis of ICI-associated EP. An increase in FeNO levels occurs when eosinophils infiltrate the airways and lung tissues, where they promote the expression of inducible nitric oxide synthase via interleukin (IL)-13 signaling (25). Previous studies have suggested that FeNO measurement may serve as a substitute for bronchoscopy in diagnosing acute and chronic EP (26), and that FeNO levels can predict patient response to corticosteroid therapy (27). In the present case, both FeNO levels and blood eosinophil counts were markedly elevated at the time of diagnosis, and decreased after the initiation of OCS treatment, which is consistent with the results of previous reports. While these markers may serve as indicators of, and are commonly observed in EP, no biomarkers specific to ICI-induced EP have been established to date. Eosinophilic granule proteins (eosinophil cationic protein, eosinophil derived neurotoxin, eosinophil peroxidase, major basic protein, and galectin-10) have been implicated in EP as other candidate biomarkers, but their predictive value remains uncertain (28). Further research is needed to establish reliable and specific biomarkers for the early detection and risk stratification of ICI-induced EP.
Effective management of ICI-induced EP, an irAE, is crucial for improving prognosis and overall survival. In addition, effective intervention is essential to minimize complications and ensure optimal outcomes. EP typically responds well to systemic corticosteroids, as demonstrated in the present case. Prompt initiation of corticosteroid therapy, along with discontinuation of suspected drugs, is particularly important in cases of DIILD with CTCAE grade 2 or higher, where patients present with respiratory symptoms and abnormal chest radiograph or CT findings. Timely treatment in such cases can reduce the risk of further lung damage and improve patient outcomes. On the other hand, the management of ICI-induced EP raises the issue of rechallenging with ICI. Balancing the need to control EP with the potential benefits of continued cancer treatment remains a challenging aspect of clinical decision-making. A previous analysis of 12 cases of rechallenging ICIs after resolving ICI-induced ILD showed that recurrence occurred in 11% (1/9) of patients with Grade 1 ILD, while the recurrence rate increased to 67% (2/3) in those with Grade 2 ILD (29). In the present case, where the patient had Grade 2 ILD, careful consideration was required before rechallenging with ICIs due to the higher risk of EP recurrence. However, an important point in this case was that pembrolizumab demonstrated high efficacy against hypopharyngeal cancer, which led to the decision to restart the treatment. Moreover, using both FeNO and blood eosinophil levels as non-invasive, adjunctive biomarkers to monitor EP progression may have provided valuable information when considering ICI rechallenge (26). As a result, pembrolizumab was successfully resumed and continued without worsening of EP during OCS treatment while carefully monitoring FeNO and blood eosinophil levels in the present case. This is supported by previous reports that have described cases of ICI-induced asthma, where appropriate asthma management allowed continued ICI treatment (30–32).
The mechanisms by which ICIs induce eosinophilic inflammatory disorders remain unclear. ICIs exert their effects by targeting proteins such as PD-1, PD-L1, or CTLA-4, which enhance T cell activation to mediate robust anticancer responses (2). Among these, pembrolizumab specifically targets PD-1, a receptor on T cells. While blocking the interaction between PD-1 and its ligands [PD-L1 and programmed cell death ligand 2 (PD-L2)], pembrolizumab facilitates the recognition of tumor antigens by tumor-specific T cells, thereby promoting their effector functions to eliminate tumor cells (33). Other PD-1/PD-L1 inhibitors share a similar mechanism of action, leading to the enhancement of T cell–mediated antitumor immunity. However, PD-1 and its ligands (PD-L1 and PD-L2), have also been implicated in allergic diseases (34). Pulmonary dendritic cells express PD-L1 and PD-L2 upon recognition and activation (35). While both ligands regulate airway and lung inflammation, disruption of the interaction between PD-1 and PD-L2 can occasionally promote Th2 inflammation in the lung (36–38). Th2 cells, in turn, produce Th2 cytokines such as IL-4, IL-5, and IL-13, which are critical for activating and recruiting eosinophils to inflammatory sites, potentially causing EP as an irAE. Although pathophysiological mechanisms underlying EP remain poorly understood, this heightened Th2 activity and cytokine release may contribute to eosinophilic activation and inflammation, ultimately leading to EP as an irAE in some patients. Further investigation is needed to elucidate the precise mechanisms underlying ICI-induced EP, through the accumulation of both acute and chronic cases.
In conclusion, not only acute but also chronic EP should be considered as irAEs following ICI therapy. In addition, in cases where ICIs demonstrate significant antitumor efficacy, chronic EP may be manageable with continued ICI administration under careful monitoring of blood eosinophil counts and FeNO levels. Further accumulation of cases is warranted to validate the effectiveness of this approach.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author/s.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YaS: Conceptualization, Writing – original draft, Investigation, Writing – review & editing. JS: Writing – review & editing, Investigation, Writing – original draft, Conceptualization, Supervision, Visualization. SK: Writing – review & editing, Writing – original draft. MI: Writing – original draft, Writing – review & editing. MR: Writing – review & editing, Writing – original draft. RY: Writing – original draft, Writing – review & editing. TK: Writing – review & editing, Writing – original draft. RTa: Writing – review & editing, Writing – original draft. KK (9th Author): Writing – original draft, Writing – review & editing. KS: Writing – original draft, Writing – review & editing. RH: Writing – review & editing, Writing – original draft. RS: Writing – review & editing, Writing – original draft. HT: Writing – review & editing, Writing – original draft. NW: Writing – review & editing, Writing – original draft. TU: Writing – original draft, Writing – review & editing. RTo: Writing – original draft, Writing – review & editing. YuS: Writing – original draft, Writing – review & editing. TN: Writing – review & editing, Writing – original draft. XW: Writing – original draft, Writing – review & editing. KK (20th Author): Writing – review & editing, Writing – original draft. YT: Writing – review & editing, Writing – original draft. SM: Writing – review & editing, Supervision, Writing – original draft. YoS: Writing – original draft, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank the Scientific English Editing Section of Fukushima Medical University for their fruitful discussion and their linguistic assistance in proofreading this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BALF, bronchoalveolar lavage fluid; CT, computed tomography; CTCAE, Common Terminology Criteria for Adverse Events; CTLA-4; cytotoxic T-lymphocyte-associated protein 4; DIILD, drug-induced interstitial lung disease; EP, eosinophilic pneumonia; FeNO, fractional exhaled nitric oxide; GGO, ground-glass opacity; ICIs, immune checkpoint inhibitors; IL, interleukin; ILD, interstitial lung disease; irAEs, immune-related adverse events; OCS, oral corticosteroids; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1; PD-L2, programmed cell death ligand 2; SP-D, surfactant protein D.
References
1. Cui C, Zhang S, Ren X, Cui W, and Wang Y. Immune-related interstitial lung disease induced by different immune checkpoint inhibitors regimens: A real-world study from 2014 to 2022 based on faers databases. Eur J Pharmacol. (2023) 946:175561. doi: 10.1016/j.ejphar.2023.175561
2. Toor SM, Sasidharan Nair V, Decock J, and Elkord E. Immune checkpoints in the tumor microenvironment. Semin Cancer Biol. (2020) 65:1–12. doi: 10.1016/j.semcancer.2019.06.021
3. Ramos-Casals M and Sisó-Almirall A. Immune-related adverse events of immune checkpoint inhibitors. Ann Intern Med. (2024) 177:Itc17–itc32. doi: 10.7326/aitc202402200
4. Morad G, Helmink BA, Sharma P, and Wargo JA. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell. (2021) 184:5309–37. doi: 10.1016/j.cell.2021.09.020
5. Pérez-De-Lis M, Retamozo S, Flores-Chávez A, Kostov B, Perez-Alvarez R, Brito-Zerón P, et al. Autoimmune diseases induced by biological agents. A review of 12,731 cases (Biogeas registry). Expert Opin Drug Saf. (2017) 16:1255–71. doi: 10.1080/14740338.2017.1372421
6. Suzuki Y and Suda T. Eosinophilic pneumonia: A review of the previous literature, causes, diagnosis, and management. Allergol Int. (2019) 68:413–9. doi: 10.1016/j.alit.2019.05.006
7. Allen J and Wert M. Eosinophilic pneumonias. J Allergy Clin Immunol In Pract. (2018) 6:1455–61. doi: 10.1016/j.jaip.2018.03.011
8. Homma A, Ando M, Hanai N, Harada H, Honma Y, Kanda T, et al. Summary of Japanese clinical practice guidelines for head and neck cancer - 2022 update edited by the Japan society for head and neck cancer. Auris Nasus Larynx. (2024) 51:174–88. doi: 10.1016/j.anl.2023.07.003
9. Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G Jr., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (Keynote-048): A randomised, open-label, phase 3 study. Lancet. (2019) 394:1915–28. doi: 10.1016/s0140-6736(19)32591-7
10. Carbone RG, Puppo F, Mattar E, Roden AC, and Hirani N. Acute and chronic eosinophilic pneumonia: an overview. Front Med (Lausanne). (2024) 11:1355247. doi: 10.3389/fmed.2024.1355247
11. Mochimaru H, Kawamoto M, Fukuda Y, and Kudoh S. Clinicopathological differences between acute and chronic eosinophilic pneumonia. Respirology (Carlton Vic). (2005) 10:76–85. doi: 10.1111/j.1440-1843.2005.00648.x
12. Jodai T, Yoshida C, Sato R, Kakiuchi Y, Sato N, Iyama S, et al. A potential mechanism of the onset of acute eosinophilic pneumonia triggered by an anti-pd-1 immune checkpoint antibody in a lung cancer patient. Immun Inflammation Dis. (2019) 7:3–6. doi: 10.1002/iid3.238
13. Hattori Y, Matsuyama K, Shu E, and Seishima M. Eosinophilic pneumonia and esophagitis in a patient with Malignant melanoma treated with nivolumab. J Dermatol. (2019) 46:e454–e5. doi: 10.1111/1346-8138.15030
14. Hara K, Yamasaki K, Tahara M, Kimuro R, Yamaguchi Y, Suzuki Y, et al. Immune checkpoint inhibitors-induced eosinophilic pneumonia: A case report. Thorac Cancer. (2021) 12:720–4. doi: 10.1111/1759-7714.13848
15. Shinada K, Murakami S, Sugimoto H, and Saito H. Acute eosinophilic pneumonia after changing dosing schedule of nivolumab. Jpn J Clin Oncol. (2021) 51:1766–7. doi: 10.1093/jjco/hyab130
16. Mouri A, Hashimoto K, Naitou E, Miura Y, Shiono A, Yamaguchi O, et al. A case of eosinophilic pneumonia after immune checkpoint inhibitor treatment following concurrent chemoradiotherapy. J Japan Soc Respir Endoscopy. (2022) 44:342–7.
17. Selvan K, Strykowski RK, Lee C, Strek ME, and Jablonski R. Eosinophilic pneumonia secondary to immune checkpoint inhibitor therapy with pembrolizumab. Chest. (2022) 162:A1282–A3. https://www.sciencedirect.com/science/article/pii/S0012369222023832?via%3Dihub (Accessed May 28, 2025).
18. Golden M, Babinski P, and Gamino A. A case of pembrolizumab-induced acute eosinophilic pneumonia. Chest. (2024) 166:A3199–A200. https://journal.chestnet.org/article/S0012-3692(24)02719-3/fulltext (Accessed May 28, 2025).
19. Clark J and Al-Qadi MO. Immune-checkpoint inhibitor-related eosinophilic pneumonia. Am J Respir Crit Care Med. (2024). doi: 10.1164/ajrccm-conference.2024.209.1
20. Spagnolo P, Bonniaud P, Rossi G, Sverzellati N, and Cottin V. Drug-induced interstitial lung disease. Eur Respir J. (2022) 60(4):210277660. doi: 10.1183/13993003.02776-2021
21. Bridi GDP, Fonseca E, Kairalla RA, Amaral AF, and Baldi BG. Drug-induced lung disease: A narrative review. J Bras Pneumol. (2024) 50:e20240110. doi: 10.36416/1806-3756/e20240110
22. Chuzi S, Tavora F, Cruz M, Costa R, Chae YK, Carneiro BA, et al. Clinical features, diagnostic challenges, and management strategies in checkpoint inhibitor-related pneumonitis. Cancer Manag Res. (2017) 9:207–13. doi: 10.2147/cmar.S136818
23. Bernheim A and McLoud T. A review of clinical and imaging findings in eosinophilic lung diseases. AJR Am J Roentgenol. (2017) 208:1002–10. doi: 10.2214/ajr.16.17315
24. Diamantopoulos PT, Gkoufa A, Anastasopoulou A, Kouzis P, Lyrarakis G, Kyriakakis G, et al. Exploring the dynamics of immune checkpoint inhibitor-induced eosinophilia in advanced/metastatic melanoma: A comprehensive retrospective analysis. Cancer Med. (2025) 14:e70679. doi: 10.1002/cam4.70679
25. Suresh V, Mih JD, and George SC. Measurement of il-13-induced inos-derived gas phase nitric oxide in human bronchial epithelial cells. Am J Respir Cell Mol Biol. (2007) 37:97–104. doi: 10.1165/rcmb.2006-0419OC
26. Lee JE, Rhee CK, Lim JH, Lee SM, Shim YS, Lee CT, et al. Fraction of exhaled nitric oxide in patients with acute eosinophilic pneumonia. Chest. (2012) 141:1267–72. doi: 10.1378/chest.11-1303
27. Furukawa K, Sugiura H, Matsunaga K, Ichikawa T, Koarai A, Hirano T, et al. Increase of nitrosative stress in patients with eosinophilic pneumonia. Respir Res. (2011) 12:81. doi: 10.1186/1465-9921-12-81
28. Tomizawa H, Yamada Y, Arima M, Miyabe Y, Fukuchi M, Hikichi H, et al. Galectin-10 as a potential biomarker for eosinophilic diseases. Biomolecules. (2022) 12(10):1385. doi: 10.3390/biom12101385
29. Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. (2017) 35:709–17. doi: 10.1200/jco.2016.68.2005
30. Maeno K, Fukuda S, Oguri T, and Niimi A. Nivolumab-induced asthma in a patient with non-small-cell lung cancer. Ann Oncol. (2017) 28:2891. doi: 10.1093/annonc/mdx455
31. Deng W, Chen J, and Deng XY. The occurrence of asthma in an extensive-stage small-cell lung cancer patient after combination therapy with atezolizumab and anlotinib: A case report. Front Immunol. (2024) 15:1333850. doi: 10.3389/fimmu.2024.1333850
32. Cordial P, Bentley ID, Horowitz JC, and Ho K. Eosinophilic reactive airways disease after immune checkpoint inhibitor treatment. Respirol Case Rep. (2024) 12:e70022. doi: 10.1002/rcr2.70022
33. Liu J, Chen Z, Li Y, Zhao W, Wu J, and Zhang Z. Pd-1/pd-L1 checkpoint inhibitors in tumor immunotherapy. Front Pharmacol. (2021) 12:731798. doi: 10.3389/fphar.2021.731798
34. Hurrell BP, Helou DG, Howard E, Painter JD, Shafiei-Jahani P, Sharpe AH, et al. Pd-L2 controls peripherally induced regulatory T cells by maintaining metabolic activity and foxp3 stability. Nat Commun. (2022) 13:5118. doi: 10.1038/s41467-022-32899-5
35. Singh AK, Stock P, and Akbari O. Role of pd-L1 and pd-L2 in allergic diseases and asthma. Allergy. (2011) 66:155–62. doi: 10.1111/j.1398-9995.2010.02458.x
36. Zhang Y, Chung Y, Bishop C, Daugherty B, Chute H, Holst P, et al. Regulation of T cell activation and tolerance by pdl2. Proc Natl Acad Sci U.S.A. (2006) 103:11695–700. doi: 10.1073/pnas.0601347103
37. van der Werf N, Redpath SA, Azuma M, Yagita H, and Taylor MD. Th2 cell-intrinsic hypo-responsiveness determines susceptibility to helminth infection. PloS Pathog. (2013) 9:e1003215. doi: 10.1371/journal.ppat.1003215
Keywords: eosinophilic pneumonia, fractional exhaled nitric oxide, immune checkpoint inhibitors, immune-related adverse events, blood eosinophil, corticosteroid, interstitial lung disease
Citation: Suzuki Y, Saito J, Kubota S, Ikeda M, Rikimaru M, Yamada R, Kumanaka T, Tanaka R, Kazama K, Saito K, Harigane R, Sato R, Tomita H, Watanabe N, Umeda T, Togawa R, Sato Y, Nikaido T, Wang X, Kanazawa K, Tanino Y, Murono S and Shibata Y (2025) Successful management of chronic eosinophilic pneumonia triggered by immune checkpoint inhibitor: a case report and literature review. Front. Immunol. 16:1615531. doi: 10.3389/fimmu.2025.1615531
Received: 21 April 2025; Accepted: 06 June 2025;
Published: 22 July 2025.
Edited by:
Zhida Liu, Shanxi Academy of Advanced Research and Innovation, ChinaReviewed by:
Longchao Liu, Chinese Academy of Sciences (CAS), ChinaXuexiang Du, Shandong University, China
Copyright © 2025 Suzuki, Saito, Kubota, Ikeda, Rikimaru, Yamada, Kumanaka, Tanaka, Kazama, Saito, Harigane, Sato, Tomita, Watanabe, Umeda, Togawa, Sato, Nikaido, Wang, Kanazawa, Tanino, Murono and Shibata. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junpei Saito, anVucGVpQGZtdS5hYy5qcA==
 Yasuhito Suzuki
Yasuhito Suzuki Junpei Saito
Junpei Saito Satoshi Kubota2
Satoshi Kubota2 Takahiro Kumanaka
Takahiro Kumanaka Yoshinori Tanino
Yoshinori Tanino Shigeyuki Murono
Shigeyuki Murono