- 1Center of Reproductive Medicine, Shengjing Hospital of China Medical University, Shenyang, China
- 2Shenyang Reproductive Health Clinical Medicine Research Center, Shenyang, China
Chronic endometritis (CE) is a persistent inflammatory disorder of the endometrium, associated with infertility, recurrent pregnancy loss, and implantation failure. Diagnosis primarily depends on hysteroscopy and immunohistochemistry, while microbial dysbiosis and antibiotic resistance pose significant challenges to effective management. The pathogenesis of CE involves microbial infections that induce immune dysregulation through TLR/NLR signaling pathways, metabolic reprogramming of immune cells, miRNA-mediated inflammatory responses, and DNA methylation alterations. The activation of pro-inflammatory mediators and the NLRP3 inflammasome further aggravates endometrial dysfunction. Treatment typically includes oral antibiotics and intrauterine therapies, although their efficacy is variable. Probiotics have demonstrated potential in restoring microbial balance. This review outlines the inflammatory mechanisms underlying CE and recent therapeutic advancements, highlighting potential targets for improving treatment outcomes.
1 Introduction
Chronic endometritis (CE) is a persistent inflammatory condition localized to the endometrium, strongly linked to adverse pregnancy outcomes, including infertility, recurrent pregnancy loss, and recurrent implantation failure (1, 2). Diagnosis primarily relies on hysteroscopic examination and immunohistochemical staining. Common hysteroscopic and histological manifestations of CE include stromal edema, focal congestion, increased stromal cell density, and infiltration of abnormal plasma cells in the endometrial stroma (3, 4).
Recent developments have shifted the understanding of CE from a purely infectious etiology to a complex immunological disorder (5). Microbial dysbiosis within the endometrium disrupts microbial balance and triggers dysregulated immune responses involving both innate and adaptive immunity (6, 7). Key inflammatory pathways, such as Toll-like receptors (TLRs), NOD-like receptors (NLRs), and downstream NF-κB signaling, alongside inflammasome activation and metabolic reprogramming of immune cells, are central to the persistence of chronic inflammation and impaired endometrial receptivity (8–10). Therapeutically, empirical antibiotic regimens, such as doxycycline and metronidazole, have shown efficacy in histological resolution and partial improvement in reproductive outcomes (11, 12). Several studies report significant increases in clinical pregnancy and live birth rates following antibiotic treatment in women with CE undergoing in vitro fertilization (IVF) (13). However, other studies indicate that a subset of patients with CE experience persistent inflammation or poor reproductive outcomes despite standard treatment (14, 15), highlighting the heterogeneity of treatment responses and underscoring the need for adjunctive strategies such as intrauterine therapy or immunomodulation.
Despite advancements in characterizing CE pathophysiology and developing management strategies, several knowledge gaps persist. The absence of standardized diagnostic criteria, variability in therapeutic response, and limited understanding of immune-microbiota interactions continue to impede effective clinical translation. This review synthesizes current insights into the immunological mechanisms underlying CE, with a focus on TLR/NLR signaling, immune cell metabolic rewiring, miRNA-mediated inflammation, and epigenetic dysregulation, while evaluating recent therapeutic advances, including antibiotics, intrauterine infusion, and probiotic-based approaches. By summarizing mechanistic and clinical evidence, this review provides a comprehensive framework for guiding future diagnostic and therapeutic innovations in CE.
2 Causes of chronic endometritis
2.1 Microbial infection
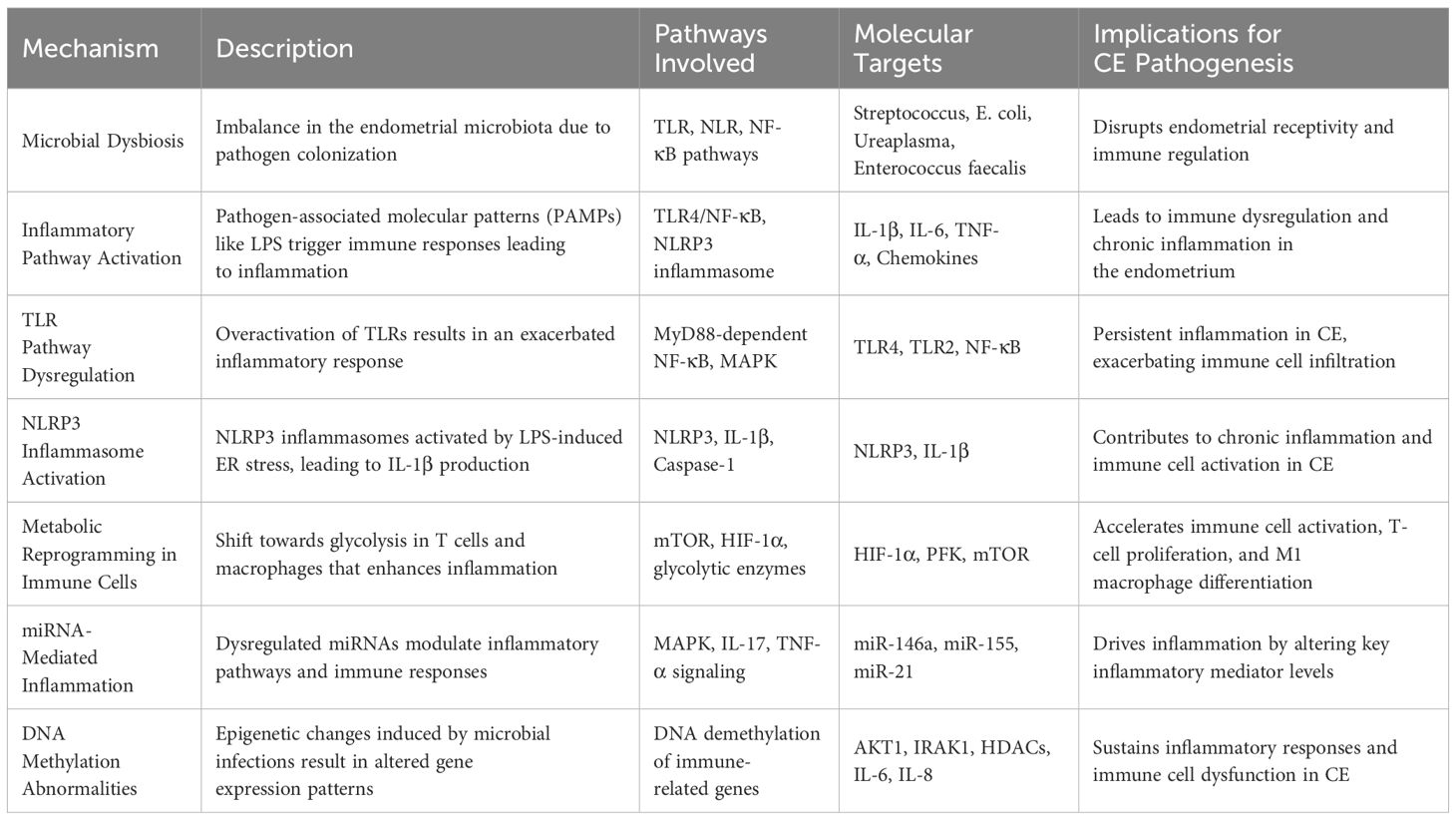
CE is characterized by a localized active infection in the endometrium, disrupting the balance between the uterine microbiome and immune system. Traditional perspectives have emphasized the cervix as a key barrier between the uterus and vagina, with the dominance of lactobacilli in the vaginal microbiota maintaining uterine sterility by suppressing pathogenic microorganisms. Cicinelli et al. (16) used microbial culture techniques to detect a variety of microorganisms in the endometrium of patients with CE, including Streptococcus, Enterococcus faecalis, Escherichia coli, and Ureaplasma urealyticum, thus confirming the presence of microbial communities within the uterine cavity. Common pathogens associated with acute endometritis, such as Chlamydia trachomatis and Neisseria gonorrhoeae, are typically introduced into the uterine cavity through ascension from the vaginal microbiota (17). However, these pathogens are rarely detected in patients with CE, suggesting that the pathogenesis of CE differs from that of acute endometritis. The presence of microorganisms within the uterine cavity is now widely accepted, and given the effectiveness of antibiotic therapy, microbial infection is considered a primary contributor to CE. However, in some cases, endometrial pathogen cultures are negative, and antibiotic treatments fail, implying that multidrug-resistant organisms may play a role in the development of CE (18).
2.2 Non-infectious factors
Recent research also highlights that non-infectious factors, including immune dysfunction, endocrine disorders, and environmental influences, may contribute to CE pathogenesis (19–21). Elevated levels of pro-inflammatory cytokines and an increased presence of immune cells, such as T-helper 17 (Th17) cells and M1 macrophages, are commonly observed in CE, indicating that immune system dysfunction may perpetuate the condition (22, 23). Hormonal imbalances, such as those seen in endometriosis and elevated estrogen levels, may influence the susceptibility and severity of CE (24). These endocrine disruptions can affect immune cell function, endometrial receptivity, and inflammatory responses, further contributing to the chronicity of the disease (19). Additionally, exposure to environmental factors, such as smoking (25), may impact immune function and the microbiome (26), potentially exacerbating CE. The interaction between environmental toxins and the immune system may contribute to altered immune responses, increasing the endometrium’s susceptibility to chronic inflammation.
3 Immune cells and cytokines in the pathogenesis of CE
3.1 TLR and NLR in the pathogenesis of chronic endometritis
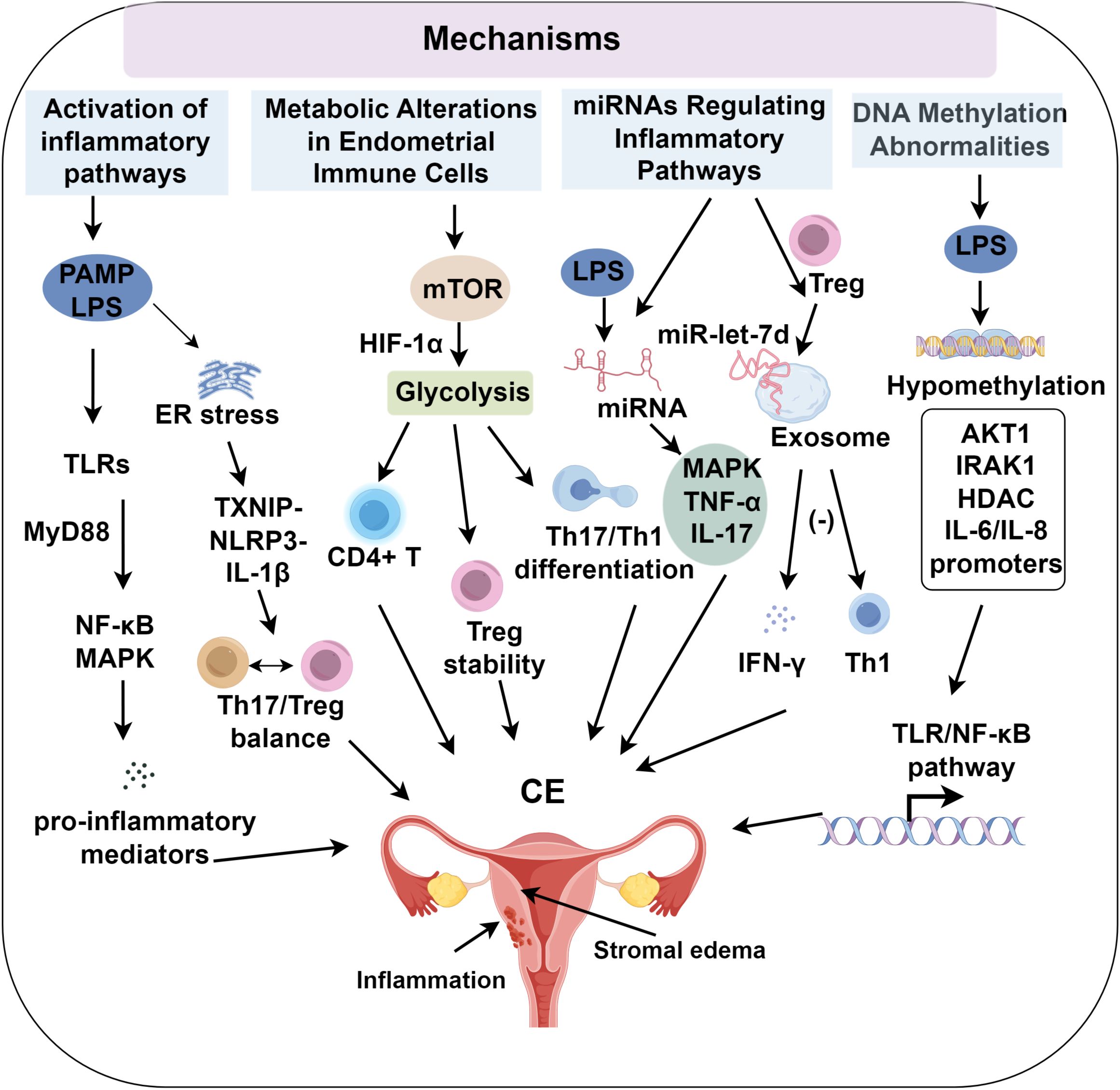
Lipopolysaccharide (LPS) is a key pathogen-associated molecular pattern (PAMP) involved in the pathogenesis of CE. Elevated expression of pro-inflammatory cytokines and chemokines has been observed in both tissues and LPS-stimulated endometrial cells of patients with CE (27). Transcriptomic analyses further reveal the enrichment of inflammation-related gene sets, particularly those involved in TLR and NLR signaling (8). LPS activates pattern recognition receptors (PRRs), triggering the MyD88/NF-κB and TRIF/IRF pathways, resulting in sustained production of IL-6, TNF-α, and CXCL8. This signaling cascade creates a chronic pro-inflammatory microenvironment characterized by cytokine accumulation, immune cell infiltration, and disrupted epithelial-stromal interactions (28–30).
3.1.1 Abnormal activation of TLR pathways in chronic endometritis
TLRs play a critical role in pathogen recognition, with TLR4 specifically binding LPS and TLR2 detecting a broader range of microbial PAMPs. Both receptors are mechanistically implicated in CE pathogenesis (31). Endogenous damage-associated molecular patterns (DAMPs), such as HMGB1 and heat shock proteins released from necrotic cells, bind to TLR2, TLR4, or their heterodimeric complexes (32, 33). Moreover, HMGB1-pathogen/DNA complexes interact with advanced glycation end-product (AGE) receptors on antigen-presenting cells, activating TLR7/TLR9 signaling cascades (34). This molecular interaction suggests that microbial infections may trigger the release of modified host-derived molecules that perpetuate inflammatory responses through sustained activation of TLRs and other PRRs, even after pathogen clearance. Such mechanisms may contribute to secondary autoimmune reactions, maintaining chronic inflammation in CE. Pathological overactivation of TLR signaling pathways has been shown to accelerate CE progression (35). Upon PAMP recognition, TLRs initiate downstream signaling through both MyD88-dependent and independent pathways, resulting in NF-κB and MAPK activation (36, 37). These transcriptional regulators subsequently upregulate pro-inflammatory cytokine production, sustaining leukocyte infiltration.
In vitro studies provide evidence for the central role of NF-κB in the pathogenesis of endometrial inflammation (9, 10). Pharmacological studies demonstrate that Epimedium glycosides alleviate LPS-induced endometritis by dual modulation of TLR4/NF-κB inhibition and Nrf2 activation (38). Furthermore, dysregulation of TLR signaling components, such as Akt1 deficiency, enhances MyD88 phosphorylation, potentiating NF-κB and interferon regulatory factor activity and amplifying inflammatory cytokine production (39). This highlights the pivotal role of TLR signaling in CE persistence. Aberrant TLR activation not only initiates inflammation but also perpetuates an imbalanced immune response, reinforcing the chronic nature of CE.
3.1.2 NLR pathway dysregulation in chronic endometritis
NLRs, expressed in both immune and non-immune cells, detect cytoplasmic PAMPs and biomolecules. NLRP1 and NLRP2 recognize bacterial cell wall degradation products, while NLRP3 forms inflammasomes in response to a range of stimuli, activating caspase-1 to promote the release of IL-1β and its precursor. NLRP3-driven inflammation contributes to reproductive pathologies, including endometriosis, polycystic ovary syndrome (PCOS), and RPL. NLRP3 activation has been identified in fibrotic ovarian tissues of PCOS mice and in the endometrial tissues of patients with idiopathic RPL (40), suggesting its involvement in chronic inflammation. In vitro studies of LPS-stimulated bovine endometrial epithelial cells (BEECs), stromal cells, and peripheral blood mononuclear cells (PBMCs) show increased IL-1β secretion, particularly in stromal fibroblasts (41). Inhibition of NLRP3 or caspase-4 through siRNA blocked IL-1β production. A murine CE model confirmed that LPS-induced endoplasmic reticulum (ER) stress activates TXNIP, which in turn triggers NLRP3 and IL-1β expression (42). LPS-exposed goat endometrial stromal cells exhibited upregulated ER stress, autophagy, and inflammatory markers, effects reversible by the ER stress inhibitor 4-phenylbutyrate (43). However, direct evidence of NLRP3 inflammasome activation in human CE endometrial tissue remains absent, with most findings extrapolated from animal models or in vitro studies, limiting their clinical applicability. This lack of clinical evidence constitutes a significant barrier to fully understanding NLRP3’s role in human CE pathogenesis. Addressing this gap through rigorous studies of human endometrial specimens is crucial for validating these pathways and guiding targeted therapeutic approaches.
The regulation of NLRP3 involves multiple mechanisms. ER stress (43), oxidative stress, and inflammation upregulate NLRP3 and pro-IL-1β through TLR pathways, with NLRP3 inflammasome activation occurring once a threshold is reached (44, 45). Co-incubation of HMGB1 with trophoblasts increases NLRP3 expression, indicating that NLR pathway activation drives inflammation (46–48). Elevated extracellular ATP in epithelial cells also activates NLRP3 in uterine macrophages, linking it to sterile inflammation (49, 50). While the precise role of NLRP3 in the initiation of CE remains unclear, it may modulate the Th17/Treg balance, as observed in patients with RPL (51) and CE (52), potentially altering the immune environment of the endometrium. These findings highlight the critical role of NLR pathways, particularly NLRP3 activation, in amplifying innate immune signaling and inflammatory cascades in CE.
3.2 Metabolic alterations in endometrial immune cells
Previous studies suggest significant alterations in immune cell subsets in CE, with notable increases in pro-inflammatory cells such as effector T cells and M1 macrophages (53, 54). Immune cell phenotype stability and function are closely linked to metabolic states, highlighting specific metabolic reprogramming events as central drivers of endometrial immune imbalance. This includes enhanced glycolytic flux in effector T cells and M1 macrophages, coupled with reduced fatty acid oxidation in Tregs and M2 macrophages. These shifts promote a pro-inflammatory environment characterized by Th1/Th17 cell dominance (55), diminished Treg suppressive function, and increased reactive oxygen species production. These changes collectively sustain a chronic inflammatory microenvironment, marked by altered cytokine/chemokine profiles and a disrupted immune cell spatial distribution in CE tissues. Increased glycolysis supports the proliferation and migration of pro-inflammatory effector T cells and M1 macrophages, while inhibiting FOXP3 expression and Treg stability (56), further exacerbating inflammation. PAMPs activate TLRs and T cell receptors, modulating mTOR signaling in macrophages (57). In contrast, TGF-β and IL-4 suppress glycolysis, promoting mitochondrial and fatty acid oxidation to sustain anti-inflammatory Tregs and M2 macrophages (58). In CE, reduced levels of TGF-β/IL-4 may amplify glycolysis, further driving inflammation. Additionally, lipid biosynthesis influences immune responses, as LPS-induced activation of SREBP1 reprograms macrophage lipid metabolism, resolving inflammation through unsaturated fatty acid biosynthesis while suppressing TR4/NF-κB pathway genes (59, 60). However, the role of lipid metabolic changes in CE immune cells and their pathogenic contribution remains unclear. Excessive activation of inflammatory pathways in CE likely modulates immune cell proliferation, differentiation, and function through metabolic shifts, perpetuating immune dysregulation and endometrial inflammation. In summary, immune-metabolic reprogramming sustains CE by promoting pro-inflammatory phenotypes and weakening anti-inflammatory resilience, effectively bridging microbial sensing with persistent immune dysfunction.
3.3 MicroRNA-mediated inflammation development in chronic endometritis
3.3.1 miRNAs regulating inflammatory pathways
miRNAs are small non-coding RNA molecules, typically 20 – 22 nucleotides in length, that primarily regulate gene expression post-transcriptionally by binding to the 3’ untranslated regions (UTRs) of target mRNAs, thereby inhibiting translation (61, 62). Research by Lv et al. (63) demonstrated that LPS stimulation of bovine endometrial stromal cells led to significant differential expression of miRNAs, which were notably enriched in the MAPK, TNF-α, and IL-17 signaling pathways. This suggests that miRNAs contribute to the inflammatory pathogenesis induced by LPS. miRNAs may influence the development of CE by modulating key molecules in these inflammatory pathways, specifically targeting transcripts such as IRAK1, TRAF6, and components of the MAPK and NF-κB pathways. This modulation leads to quantifiable changes in downstream cytokine expression levels and immune cell subset activation, including CD4+ Th1 bias or M1 macrophage polarization (27, 63–65). The regulation of miRNA and mRNA forms a complex network, with much of the current research on miRNAs in CE being conducted at the level of individual cell types (66). However, future studies are necessary to validate the role of miRNAs in CE, particularly through the use of uterine organoids or human endometrial tissues. Collectively, miRNAs represent an epigenetic interface that modulates canonical signaling networks, providing novel targets for diagnostic and therapeutic interventions in CE (Figure 1).
3.3.2 Exosome-derived miRNAs modulating endometrial inflammation
Exosomes are extracellular vesicles secreted by host cells, including epithelial cells, stromal cells, and immune cells, as well as by microbes (67, 68). These vesicles carry proteins, lipids, mRNAs, and miRNAs, facilitating intercellular communication by transferring these molecules to target cells and modulating their functions (69). For instance, Treg cells release exosomes that transfer exosome-derived miR-let-7d to Th1 cells, inhibiting cell proliferation and γ-interferon secretion, thereby suppressing inflammation (70). Exosome-derived miRNAs in the uterine cavity fluid play a significant role in regulating inflammation in CE (61). Exosome-derived miRNAs in cattle with endometritis undergo dysregulation. For example, the secretion of miR-218 by BEEC exosomes decreases, reducing its inhibitory effect on MIP-1 expression in target cells, thus promoting inflammation. Furthermore, exosomes can exert substantial immune-modulatory effects by transferring PAMPs and other antigenic substances, contributing to the regulation of inflammation (27). Therefore, exosomes serve as important mediators of cell-to-cell communication, playing pivotal roles in immune dysregulation and the inflammatory response in CE. Exosome-derived miRNAs, as potent intercellular messengers, reinforce the inflammatory milieu in CE by linking intracellular regulation with extracellular communication.
3.4 DNA methylation abnormalities
Microbial infections can induce host cell DNA demethylation. LPS alters DNA methylation in BEECs, primarily causing hypomethylation and upregulation of protein-coding genes involved in immune function, inflammation, proliferation, apoptosis, adhesion, and extracellular matrix remodeling (71). These changes, including hypomethylation of AKT1 and IRAK1, activate the TLR/NF-κB pathway, contributing to LPS-induced endometrial inflammation (71). Moreover, LPS demethylates the promoters of IL-6 and IL-8, thereby enhancing their expression. Hypomethylation of HDAC genes leads to the upregulation of HDACs, exacerbating inflammation through modulation of lymphocyte signaling, stabilization of HIF-1α, and acetylation of TLR pathway molecules (72). Persistent infection-induced methylation changes in regulatory regions may drive chronic endometrial inflammation in patients with CE. The role of DNA methylation in the pathogenesis of CE remains unclear, although studies have shown menstrual cycle-dependent methylation dynamics in healthy endometria, with distinct patterns observed in endometriosis and carcinoma (73). Epigenetic reprogramming via DNA methylation acts as a persistent memory of inflammation, and its integration with miRNA and immune pathway data could provide valuable insights into the chronic progression of CE (Table 1).
4 Treatment of chronic endometritis
4.1 Oral antibiotic eradication therapy
Management of CE has proven effective in normalizing endometrial histopathological features and improving reproductive outcomes in affected patients (74, 75). Current therapeutic strategies for CE involve three main approaches: empirical systemic antibiotic administration, intrauterine antimicrobial instillation, and probiotic supplementation to restore microbial balance (76, 77). Among these, oral antibiotic regimens remain the most widely used clinical approach. Cicinelli et al. (78) conducted a thorough evaluation of antibiotic protocols tailored for CE individuals with RIF. Johnston-MacAnanny et al. (79) reported that monotherapy with oral doxycycline resulted in clinical resolution in approximately 70% of RIF individuals with confirmed CE. In cases with doxycycline resistance, combination therapy using ciprofloxacin and metronidazole was effective in eliminating plasma cell infiltration from the endometrial stroma, as confirmed by histopathological examination of endometrial biopsies (80). However, despite appropriate antibiotic treatment, RIF individuals with CE consistently showed lower embryo implantation rates compared to non-CE counterparts. Current clinical guidelines, as outlined in the 2021 Sexually Transmitted Infections Treatment Guidelines, recommend an antibiotic regimen initially developed for pelvic inflammatory disease, including endometritis, which combines doxycycline with metronidazole (81).
4.2 Intrauterine infusion therapy
Intrauterine infusion represents a targeted therapeutic approach that enables direct medication delivery into the uterine cavity, overcoming the limitations of prolonged oral antibiotic regimens (77, 82). This localized delivery system offers several clinical advantages, including enhanced drug concentration at the target site, reduced systemic exposure, and improved cost-effectiveness (83). A clinical study assessing the efficacy of intrauterine antibiotic infusion combined with dexamethasone showed promising reproductive outcomes (84, 85). Comparative analysis revealed superior therapeutic results in patients with CE treated with intrauterine antibiotics compared to those receiving conventional oral combination antibiotic therapy (77). These findings suggest that the combined use of intrauterine antibiotics and corticosteroids constitutes an effective strategy for CE management, leading to improved pregnancy rates (85).
Beyond conventional antibiotic therapies, emerging evidence supports the use of intrauterine platelet-rich plasma (PRP) infusion as an effective treatment for CE (86). PRP, an autologous biological preparation containing concentrated platelets and bioactive molecules such as VEGF, PDGF, and TGF-β, exerts multiple therapeutic effects, including endometrial regeneration, anti-inflammatory action, and promotion of angiogenesis (87, 88). Clinical observations have demonstrated that PRP modulates the uterine immune environment by reducing endometrial populations of CD8+ T cells, CD56+ NK cells, Foxp3+ Treg cells, and T-bet+ Th1 cells in refractory CE cases (86). This immunomodulatory reprogramming correlates with enhanced endometrial receptivity and improved reproductive outcomes, even in antibiotic-resistant cases (86, 89). Notably, successful pregnancies have been reported following PRP treatment after failed antibiotic therapy (90), highlighting its potential as a salvage treatment.
5 Conclusion
CE is a multifactorial condition driven by a complex interplay of microbial infections, immune dysregulation, and epigenetic modifications, all contributing to impaired endometrial receptivity and adverse reproductive outcomes. The pathogenic mechanisms encompass pathogen-induced activation of TLR and NLR signaling pathways, metabolic reprogramming of endometrial immune cells, miRNA-mediated amplification of inflammatory responses, and aberrant DNA methylation patterns that sustain chronic inflammation.
Despite notable therapeutic advances, particularly the use of broad-spectrum antibiotics, persistent CE, treatment resistance, and recurrent reproductive failure continue to pose significant clinical challenges. Alternative approaches such as intrauterine infusion therapies, immunomodulatory strategies, and microbiome-based interventions have shown promising preliminary results. However, no unified consensus exists on treatment protocols, especially regarding the optimal antibiotic regimens, criteria for selecting intrauterine therapies, or the clinical application of emerging interventions like PRP. This lack of standardization contributes to considerable variability in treatment responses, limiting the comparability of outcomes across studies and complicating clinical decision-making. Future efforts must focus on addressing these gaps by establishing standardized diagnostic criteria and conducting multicenter randomized trials to evaluate combinatorial therapies and refine clinical management.
A deeper understanding of endometrial microbiota-immune interactions may facilitate the development of personalized therapies, improving pregnancy outcomes in patients with CE. Additionally, standardized diagnostic protocols and well-designed randomized trials assessing combination treatments are critical to optimizing clinical management and improving reproductive success. Exploring the causal relationships between specific microbial species and immune dysfunction through integrative multi-omics approaches could provide valuable mechanistic insights and support the development of targeted therapies.
Author contributions
XY: Writing – original draft. JJ: Writing – original draft, Writing – review & editing. XW: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the National Key Research and Development Program of China (No.2023YFC2705402), and the National Natural Science Foundation of China (No.82401929).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tsonis O, Gkrozou F, Dimitriou E, Barmpalia Z, Tsonis K, Vatopoulou A, et al. Hysteroscopic features suggestive of chronic endometritis: a systematic review. Hum Fertil (Camb). (2023) 26:1530–43. doi: 10.1080/14647273.2023.2265155
2. Kalaitzopoulos DR, Catena U, Schwartz AK, Schoretsanitis G, Leeners B, Drakopoulos P, et al. Chronic endometritis and endometriosis: two sides of the same coin? Reprod Sci. (2025) 32:474–87. doi: 10.1007/s43032-025-01785-y
3. Riemma G, Parry JP, De Franciscis P, Carugno J, Lettieri D, Cobellis L, et al. Hysteroscopic criteria for the diagnosis of chronic endometritis: a systematic review and diagnostic test accuracy meta-analysis. Am J Obstet Gynecol. (2025) 233:12–24. doi: 10.1016/j.ajog.2025.03.005
4. Furui M, Ito A, Fukuda Y, Sekiguchi M, Nakaoka K, Hayashi Y, et al. Endometrial congestion is the only hysteroscopic finding indicative of chronic endometritis. PloS One. (2024) 19:e03:03041. doi: 10.1371/journal.pone.0303041
5. Kitaya K and Yasuo T. Commonalities and disparities between endometriosis and chronic endometritis: therapeutic potential of novel antibiotic treatment strategy against ectopic endometrium. Int J Mol Sci. (2023) 24:2059. doi: 10.3390/ijms24032059
6. Negishi Y, Shima Y, Takeshita T, and Morita R. Harmful and beneficial effects of inflammatory response on reproduction: sterile and pathogen-associated inflammation. Immunol Med. (2021) 44:98–115. doi: 10.1080/25785826.2020.1809951
7. Chen P, Chen P, Guo Y, Fang C, and Li T. Interaction between chronic endometritis caused endometrial microbiota disorder and endometrial immune environment change in recurrent implantation failure. Front Immunol. (2021) 12:748447. doi: 10.3389/fimmu.2021.748447
8. Cluxton D, Petrasca A, Moran B, and Fletcher JM. Differential regulation of human treg and th17 cells by fatty acid synthesis and glycolysis. Front Immunol. (2019) 10:115. doi: 10.3389/fimmu.2019.00115
9. Ma W, Wang L, Pan Y, Wang M, Wang J, Feng M, et al. Beclin1 regulates yak endometrial inflammation and TLR4/NF-κB signaling pathway through autophagy/non-autophagy function. Int Immunopharmacol. (2025) 147:113940. doi: 10.1016/j.intimp.2024.113940
10. Cheng F, Li D, Ma X, Wang Y, Lu L, Hu B, et al. Liriodendrin exerts protective effects against chronic endometritis in rats by modulating gut microbiota composition and the arginine/nitric oxide metabolic pathway. Int Immunopharmacol. (2024) 126:111235. doi: 10.1016/j.intimp.2023.111235
11. Zou Y, Ming L, Ding J, Xiao Z, Li S, Yang J, et al. Low dosage of prednisone acetate combined with doxycycline in the treatment of chronic endometritis in patients with repeated implantation failure. Am J Reprod Immunol. (2023) 89:e13713. doi: 10.1111/aji.13713
12. Gay C, Hamdaoui N, Pauly V, Rojat Habib MC, Djemli A, Carmassi M, et al. Impact of antibiotic treatment for chronic endometritis on unexplained recurrent pregnancy loss. J Gynecol Obstet Hum Reprod. (2021) 50:102034. doi: 10.1016/j.jogoh.2020.102034
13. Vaduva CC, Sandulescu MS, Tenovici M, Siminel MA, and Novac MB. Results of in vitro fertilization after diagnosis and treatment of chronic endometritis. Eur Rev Med Pharmacol Sci. (2023) 27:1069–76. doi: 10.26355/eurrev_202302_31203
14. Duan H, Li X, Hao Y, Shi J, and Cai H. Risk of spontaneous abortion after antibiotic therapy for chronic endometritis before in vitro fertilization and intracytoplasmic sperm injection stimulation. Fertil Steril. (2022) 118:337–46. doi: 10.1016/j.fertnstert.2022.04.026
15. Cheng X, Huang Z, Xiao Z, and Bai Y. Does antibiotic therapy for chronic endometritis improve clinical outcomes of patients with recurrent implantation failure in subsequent IVF cycles? A systematic review and meta-analysis. J Assist Reprod Genet. (2022) 39:1797–813. doi: 10.1007/s10815-022-02558-1
16. Cicinelli E, Matteo M, Tinelli R, Pinto V, Marinaccio M, Indraccolo U, et al. Chronic endometritis due to common bacteria is prevalent in women with recurrent miscarriage as confirmed by improved pregnancy outcome after antibiotic treatment. Reprod Sci. (2014) 21:640–7. doi: 10.1177/1933719113508817
17. Vicetti Miguel RD, Chivukula M, Krishnamurti U, Amortegui AJ, Kant JA, Sweet RL, et al. Limitations of the criteria used to diagnose histologic endometritis in epidemiologic pelvic inflammatory disease research. Pathol Res Pract. (2011) 207:680–5. doi: 10.1016/j.prp.2011.08.007
18. Cicinelli E, Matteo M, Trojano G, Mitola PC, Tinelli R, Vitagliano A, et al. Chronic endometritis in patients with unexplained infertility: Prevalence and effects of antibiotic treatment on spontaneous conception. Am J Reprod Immunol. (2018) 79:10. doi: 10.1111/aji.12782
19. Bouic PJ. Endometriosis and infertility: the hidden link between endometritis, hormonal imbalances and immune dysfunctions preventing implantation! JBRA Assist Reprod. (2023) 27:144–6. doi: 10.5935/1518-0557.20230015
20. Yan X, Jiao J, and Wang X. The pathogenesis, diagnosis, and treatment of chronic endometritis: a comprehensive review. Front Endocrinol (Lausanne). (2025) 16:1603570. doi: 10.3389/fendo.2025.1603570
21. Wang Y and Li R. Call attention to the reproductive disorders of chronic endometritis. Zhonghua Yi Xue Za Zhi. (2024) 104:3631–5. doi: 10.3760/cma.j.cn112137-20240724-01698
22. You S, Zhu Y, Li H, He F, Liu S, Yang X, et al. Recombinant humanized collagen remodels endometrial immune microenvironment of chronic endometritis through macrophage immunomodulation. Regener Biomater. (2023) 10:rbad033. doi: 10.1093/rb/rbad033
23. Wang Q, Sun Y, Fan R, Wang M, Ren C, Jiang A, et al. Role of inflammatory factors in the etiology and treatment of recurrent implantation failure. Reprod Biol. (2022) 22:100698. doi: 10.1016/j.repbio.2022.100698
24. Gawron I, Derbisz K, Jach R, Trojnarska D, Milian-Ciesielska K, and Pietrus M. Pelvic peritoneal endometriosis is linked to the endometrial inflammatory profile: a prospective cohort study. BMC Womens Health. (2025) 25:94. doi: 10.1186/s12905-025-03632-3
25. Lin M, Xu H, and Qiu J. Inflammation in recurrent miscarriage-a comprehensive perspective from uterine microenvironment and immune cell imbalance to therapeutic strategies. Ginekol Pol. (2024) 95:266–75. doi: 10.5603/gpl.97320
26. Bashiri A, Halper KI, and Orvieto R. Recurrent Implantation Failure-update overview on etiology, diagnosis, treatment and future directions. Reprod Biol Endocrinol. (2018) 16:121. doi: 10.1186/s12958-018-0414-2
27. Wang X, Yao X, Xie T, Chang Z, Guo Y, and Ni H. Exosome-derived uterine miR-218 isolated from cows with endometritis regulates the release of cytokines and chemokines. Microb Biotechnol. (2020) 13:1103–17. doi: 10.1111/1751-7915.13565
28. Geng X, Xia X, Liang Z, Li S, Yue Z, Zhang H, et al. Tropomodulin1 exacerbates inflammatory response in macrophages by negatively regulating LPS-induced TLR4 endocytosis. Cell Mol Life Sci. (2024) 81:402. doi: 10.1007/s00018-024-05424-8
29. Wang YT, Sansone A, Smirnov A, Stallings CL, and Orvedahl A. Myeloid autophagy genes protect mice against fatal TNF- and LPS-induced cytokine storm syndromes. Autophagy. (2023) 19:1114–27. doi: 10.1080/15548627.2022.2116675
30. Wang YF, Zhang WL, Li ZX, Liu Y, Tan J, Yin HZ, et al. METTL14 downregulation drives S100A4(+) monocyte-derived macrophages via MyD88/NF-κB pathway to promote MAFLD progression. Signal Transduct Target Ther. (2024) 9:91. doi: 10.1038/s41392-024-01797-1
31. Huang G, Yao D, Yan X, Zheng M, Yan P, Chen X, et al. Emerging role of toll-like receptors signaling and its regulators in preterm birth: a narrative review. Arch Gynecol Obstet. (2023) 308:319–39. doi: 10.1007/s00404-022-06701-2
32. Ogbodo E, Michelangeli F, and Williams JHH. Exogenous heat shock proteins HSPA1A and HSPB1 regulate TNF-α, IL-1β and IL-10 secretion from monocytic cells. FEBS Open Bio. (2023) 13:1922–40. doi: 10.1002/2211-5463.13695
33. He M, Bianchi ME, Coleman TR, Tracey KJ, and Al-Abed Y. Exploring the biological functional mechanism of the HMGB1/TLR4/MD-2 complex by surface plasmon resonance. Mol Med. (2018) 24:21. doi: 10.1186/s10020-018-0023-8
34. Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. (2007) 8:487–96. doi: 10.1038/ni1457
35. Ju J, Li L, Xie J, Wu Y, Wu X, and Li W. Toll-like receptor-4 pathway is required for the pathogenesis of human chronic endometritis. Exp Ther Med. (2014) 8:1896–900. doi: 10.3892/etm.2014.1990
36. Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. (2003) 301:640–3. doi: 10.1126/science.1087262
37. Wu Y, Wang Q, Li M, Lao J, Tang H, Ming S, et al. SLAMF7 regulates the inflammatory response in macrophages during polymicrobial sepsis. J Clin Invest. (2023) 133:e150224. doi: 10.1172/JCI150224
38. Khan MZ, Chen W, Liu X, Kou X, Khan A, Khan RU, et al. An overview of bioactive compounds’ Role in modulating the nrf2/keap1/NF-κB pathway to alleviate lipopolysaccharide-induced endometritis. Int J Mol Sci. (2024) 25:10319. doi: 10.3390/ijms251910319
39. Shaukat A, Shaukat I, Rajput SA, Shukat R, Hanif S, Huang S, et al. Icariin alleviates escherichia coli lipopolysaccharide-mediated endometritis in mice by inhibiting inflammation and oxidative stress. Int J Mol Sci. (2022) 23:10219. doi: 10.3390/ijms231810219
40. Zhou F, Li C, and Zhang SY. NLRP3 inflammasome: a new therapeutic target for high-risk reproductive disorders? Chin(Engl). (2020) 134:20–7. doi: 10.1097/CM9.0000000000001214
41. Kelly P, Meade KG, and O’Farrelly C. Non-canonical inflammasome-mediated IL-1β Production by primary endometrial epithelial and stromal fibroblast cells is NLRP3 and caspase-4 dependent. Front Immunol. (2019) 10:102. doi: 10.3389/fimmu.2019.00102
42. Hu X, Li D, Wang J, Guo J, Li Y, Cao Y, et al. Melatonin inhibits endoplasmic reticulum stress-associated TXNIP/NLRP3 inflammasome activation in lipopolysaccharide-induced endometritis in mice. Int Immunopharmacol. (2018) 64:101–9. doi: 10.1016/j.intimp.2018.08.028
43. Mohamed AAA, Yang D, Liu S, Lin P, Mohamad OAA, and Jin Y. Endoplasmic reticulum stress is involved in lipopolysaccharide-induced inflammatory response and apoptosis in goat endometrial stromal cells. Mol Reprod Dev. (2019) 86:908–21. doi: 10.1002/mrd.23152
44. Zeng G, Li J, Wang Y, Su J, Lu Z, Zhang F, et al. Polystyrene microplastic-induced oxidative stress triggers intestinal barrier dysfunction via the NF-κB/NLRP3/IL-1β/MCLK pathway. Environ pollut. (2024) 345:123473. doi: 10.1016/j.envpol.2024.123473
45. Yuan C, Liu L, Zhao Y, and Wang K. Puerarin inhibits Staphylococcus aureus-induced endometritis through attenuating inflammation and ferroptosis via regulating the P2X7/NLRP3 signalling pathway. J Cell Mol Med. (2024) 28:e18550. doi: 10.1111/jcmm.18550
46. Liu C, She Y, Huang J, Liu Y, Li W, Zhang C, et al. HMGB1-NLRP3-P2X7R pathway participates in PM(2.5)-induced hippocampal neuron impairment by regulating microglia activation. Ecotoxicol Environ Saf. (2022) 239:113664. doi: 10.1016/j.ecoenv.2022.113664
47. Lei C, Chen K, Gu Y, Li Y, Wang L, Zhu X, et al. HMGB1/TLR4 axis promotes pyroptosis after ICH by activating the NLRP3 inflammasome. J Neuroimmunol. (2024) 393:578401. doi: 10.1016/j.jneuroim.2024.578401
48. Yin H, Wu M, Lu Y, Wu X, Yu B, Chen R, et al. HMGB1-activatied NLRP3 inflammasome induces thrombocytopenia in heatstroke rat. PeerJ. (2022) 10:e13799. doi: 10.7717/peerj.13799
49. Guo M, Wu H, Zhang J, Zhao L, He J, Yu Z, et al. Baicalin n-butyl ester alleviates inflammatory bowel disease and inhibits pyroptosis through the ROS/ERK/P-ERK/NLRP3 pathway. Vivo vitro. BioMed Pharmacother. (2025) 186:118012. doi: 10.1016/j.biopha.2025.118012
50. Yang C, Lei L, Collins JWM, Briones M, Ma L, Sturdevant GL, et al. Chlamydia evasion of neutrophil host defense results in NLRP3 dependent myeloid-mediated sterile inflammation through the purinergic P2X7 receptor. Nat Commun. (2021) 12:5454. doi: 10.1038/s41467-021-25749-3
51. Lu M, Ma F, Xiao J, Yang L, Li N, and Chen D. NLRP3 inflammasome as the potential target mechanism and therapy in recurrent spontaneous abortions. Mol Med Rep. (2019) 19:1935–41. doi: 10.3892/mmr.2019.9829
52. Li Y, Yu S, Huang C, Lian R, Chen C, Liu S, et al. Evaluation of peripheral and uterine immune status of chronic endometritis in patients with recurrent reproductive failure. Fertil Steril. (2020) 113:187–196.e181. doi: 10.1016/j.fertnstert.2019.09.001
53. Wang B, Yu R, Zhang Z, Peng Y, and Li L. Exosomes secreted from adipose-derived stem cells inhibit M1 macrophage polarization ameliorate chronic endometritis by regulating SIRT2/NLRP3. Mol Cell Biochem. (2025) 480:4781–96. doi: 10.1007/s11010-025-05283-2
54. Huang Q, Yang Y, Yuan L, Zhao Y, and Qin A. Oil-based contrast for hysterosalpingography-regulated Th1/Th2-type cytokines and alleviated inflammation in rats with LPS-induced chronic endometritis. J Obstet Gynaecol Res. (2023) 49:243–52. doi: 10.1111/jog.15451
55. Lin Y, Xue K, Li Q, Liu Z, Zhu Z, Chen J, et al. Cyclin-dependent kinase 7 promotes th17/th1 cell differentiation in psoriasis by modulating glycolytic metabolism. J Invest Dermatol. (2021) 141:2656–2667.e2611. doi: 10.1016/j.jid.2021.04.018
56. Kazmi S, Khan MA, Shamma T, Altuhami A, Assiri AM, and Broering DC. Therapeutic nexus of T cell immunometabolism in improving transplantation immunotherapy. Int Immunopharmacol. (2022) 106:108621. doi: 10.1016/j.intimp.2022.108621
57. Coillard A, Guyonnet L, De Juan A, Cros A, and Segura E. TLR or NOD receptor signaling skews monocyte fate decision via distinct mechanisms driven by mTOR and miR-155. Proc Natl Acad Sci U.S.A. (2021) 118:e2109225118. doi: 10.1073/pnas.2109225118
58. Choi G, Na H, Kuen DS, Kim BS, and Chung Y. Autocrine TGF-β1 maintains the stability of foxp3(+) regulatory T cells via IL-12Rβ2 downregulation. Biomolecules. (2020) 10:819. doi: 10.3390/biom10060819
59. Wang Z, Chao Z, Wang Q, Zou F, Song T, Xu L, et al. EXO1/P53/SREBP1 axis-regulated lipid metabolism promotes prostate cancer progression. J Transl Med. (2024) 22:104. doi: 10.1186/s12967-023-04822-z
60. Fei X, Huang J, Li F, Wang Y, Shao Z, Dong L, et al. The Scap-SREBP1-S1P/S2P lipogenesis signal orchestrates the homeostasis and spatiotemporal activation of NF-κB. Cell Rep. (2023) 42:112586. doi: 10.1016/j.celrep.2023.112586
61. Chen Y, Zheng S, Zhao X, Zhang Y, Yu S, and Wei J. Unveiling the protective effects of BMSCs/anti-miR-124-3p exosomes on LPS-induced endometrial injury. Funct Integr Genomics. (2024) 24:32. doi: 10.1007/s10142-024-01303-4
62. Di Pietro C, Caruso S, Battaglia R, Iraci Sareri M, La Ferlita A, Strino F, et al. MiR-27a-3p and miR-124-3p, upregulated in endometrium and serum from women affected by Chronic Endometritis, are new potential molecular markers of endometrial receptivity. Am J Reprod Immunol. (2018) 80:e12858. doi: 10.1111/aji.12858
63. Lv H, Yan C, Deng L, Peng Z, Yang D, Hu W, et al. Role of microRNAs in protective effects of forsythoside A against lipopolysaccharide-induced inflammation in bovine endometrial stromal cells. Front Vet Sci. (2021) 8:642913. doi: 10.3389/fvets.2021.642913
64. Umar T, Ma X, Yin B, Umer S, Zahoor A, Akhtar M, et al. miR-424-5p overexpression inhibits LPS-stimulated inflammatory response in bovine endometrial epithelial cells by targeting IRAK2. J Reprod Immunol. (2022) 150:103471. doi: 10.1016/j.jri.2021.103471
65. Yan C, Lv H, Peng Z, Yang D, Shen P, Yu J, et al. Analysis of miRNA expression changes in bovine endometrial stromal cells treated with lipopolysaccharide. Theriogenology. (2021) 167:85–93. doi: 10.1016/j.theriogenology.2021.03.012
66. Ferlita A, Battaglia R, Andronico F, Caruso S, Cianci A, Purrello M, et al. Non-coding RNAs in endometrial physiopathology. Int J Mol Sci. (2018) 19:2120. doi: 10.3390/ijms19072120
67. Wang B, Li L, and Yu R. Exosomes from adipose-derived stem cells suppress the progression of chronic endometritis. Cell Transplant. (2023) 32:9636897231173736. doi: 10.1177/09636897231173736
68. Zhao C, Li J, Cai H, Wu D, Tao S, Pi C, et al. An inject able hydrogel scaffold with IL-1β-activated MSC-derived exosomes for the treatment of endometritis. Biomater Sci. (2023) 11:1422–36. doi: 10.1039/D2BM01586B
69. Arya SB, Collie SP, and Parent CA. The ins-and-outs of exosome biogenesis, secretion, and internalization. Trends Cell Biol. (2024) 34:90–108. doi: 10.1016/j.tcb.2023.06.006
70. Okoye IS, Coomes SM, Pelly VS, Czieso S, Papayannopoulos V, Tolmachova T, et al. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity. (2014) 41:503. doi: 10.1016/j.immuni.2014.08.008
71. Jhamat N, Niazi A, Guo Y, Chanrot M, Ivanova E, Kelsey G, et al. LPS-treatment of bovine endometrial epithelial cells causes differential DNA methylation of genes associated with inflammation and endometrial function. BMC Genomics. (2020) 21:385. doi: 10.1186/s12864-020-06777-7
72. Liu YF, Hu R, Zhang LF, Fan Y, Xiao JF, and Liao XZ. Effects of dexmedetomidine on cognitive dysfunction and neuroinflammation via the HDAC2/HIF-1α/PFKFB3 axis in a murine model of postoperative cognitive dysfunction. J Biochem Mol Toxicol. (2022) 36:e23044. doi: 10.1002/jbt.23044
73. Retis-Resendiz AM, González-García IN, León-Juárez M, Camacho-Arroyo I, Cerbón M, and Vázquez-Martínez ER. The role of epigenetic mechanisms in the regulation of gene expression in the cyclical endometrium. Clin Epigenet. (2021) 13:116. doi: 10.1186/s13148-021-01103-8
74. Singh N and Sethi A. Endometritis-Diagnosis,Treatment and its impact on fertility-A Scoping Review. JBRA Assist Reprod. (2022) 26:538–46. doi: 10.5935/1518-0557.20220015
75. Cao W, Fu X, Zhou J, Qi Q, Ye F, Li L, et al. The effect of the female genital tract and gut microbiome on reproductive dysfunction. Biosci Trends. (2024) 17:458–74. doi: 10.5582/bst.2023.01133
76. Kato H, Yamagishi Y, Hagihara M, Hirai J, Asai N, Shibata Y, et al. Systematic review and meta-analysis for impacts of oral antibiotic treatment on pregnancy outcomes in chronic endometritis patients. J Infect Chemother. (2022) 28:610–5. doi: 10.1016/j.jiac.2022.01.001
77. Luncan M, Huniadi A, Bimbo-Szuhai E, Botea M, Zaha I, Stefan L, et al. The effectiveness of intrauterine antibiotic infusion versus oral antibiotic therapy in the treatment of chronic endometritis in patients(in vitro fertilization) procedures. BMC Womens Health. (2022) 22:529. doi: 10.1186/s12905-022-02128-8
78. Cicinelli E, Matteo M, Tinelli R, Lepera A, Alfonso R, Indraccolo U, et al. Prevalence of chronic endometritis in repeated unexplained implantation failure and the IVF success rate after antibiotic therapy. Hum Reprod. (2015) 30:323–30. doi: 10.1093/humrep/deu292
79. Johnston-MacAnanny EB, Hartnett J, Engmann LL, Nulsen JC, Sanders MM, and Benadiva CA. Chronic endometritis is a frequent finding in women with recurrent implantation failure after in vitro fertilization. Fertil Steril. (2010) 93:437–41. doi: 10.1016/j.fertnstert.2008.12.131
80. Jwa SC, Kuroda K, Shirasawa H, Harada M, Osuga Y, and Yamada M. Variation in diagnostic methods, criteria, and treatment for chronic endometritis: A nationwide survey in Japan. J Obstet Gynaecol Res. (2024) 50:1479–84. doi: 10.1111/jog.16051
81. Workowski KA, Bachmann LH, Chan PA, Johnston CM, Muzny CA, Park I, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. (2021) 70:1–187. doi: 10.15585/mmwr.rr7004a1
82. Abhang A and Burgess DJ. Recent advancements and future applications of intrauterine drug delivery systems. Expert Opin Drug Delivery. (2025) 22:841–56. doi: 10.1080/17425247.2025.2490266
83. Gvozdeva Y and Staynova R. pH-dependent drug delivery systems for ulcerative colitis treatment. Pharmaceutics. (2025) 17:226. doi: 10.3390/pharmaceutics17020226
84. Ma N, Li J, Zhang J, Jin Y, Wang J, Qin W, et al. Combined oral antibiotics and intrauterine perfusion can improve in vitro fertilization and embryo transfer pregnancy outcomes in patients with chronic endometritis and repeated embryo implantation failure. BMC Womens Health. (2023) 23:344. doi: 10.1186/s12905-023-02443-8
85. Zhang Y, Xu H, Liu Y, Zheng S, Zhao W, Wu D, et al. Confirmation of chronic endometritis in repeated implantation failure and success outcome in IVF-ET after intrauterine delivery of the combined administration of antibiotic and dexamethasone. Am J Reprod Immunol. (2019) 82:e13177. doi: 10.1111/aji.13177
86. Chen X, Chen M, Liu M, Qi L, Liu Z, Chen C, et al. Intrauterine infusion of autologous platelet-rich plasma modulates endometrial immune status and improves pregnancy outcomes in patients with persistent chronic endometritis. Front Immunol. (2025) 16:1528522. doi: 10.3389/fimmu.2025.1528522
87. Beitia M, Delgado D, Mercader J, Sánchez P, López de Dicastillo L, and Sánchez M. Action of platelet-rich plasma on. In Vitro Cell Bioactivity: More than Platelets. Int J Mol Sci. (2023) 24:5367. doi: 10.3390/ijms24065367
88. Kim H, Shin JE, Koo HS, Kwon H, Choi DH, and Kim JH. Effect of autologous platelet-rich plasma treatment on refractory thin endometrium during the frozen embryo transfer cycle: A pilot study. Front Endocrinol (Lausanne). (2019) 10:61. doi: 10.3389/fendo.2019.00061
89. Li J, Li X, Ding J, Zhao J, Chen J, Guan F, et al. Analysis of pregnancy outcomes in patients with recurrent implantation failure complicated with chronic endometritis. Front Cell Dev Biol. (2023) 11:1088586. doi: 10.3389/fcell.2023.1088586
90. Sfakianoudis K, Simopoulou M, Nitsos N, Lazaros L, Rapani A, Pantou A, et al. Successful implantation and live birth following autologous platelet-rich plasma treatment for a patient with recurrent implantation failure and chronic endometritis. In Vivo. (2019) 33:515–21. doi: 10.21873/invivo.11504
Keywords: chronic endometritis, endometrial microbiome, TLR/NF-κB pathway, NLRP3 inflammasome, DNA methylation, antibiotic resistance
Citation: Yan X, Jiao J and Wang X (2025) Inflammatory mechanisms and therapeutic advances in chronic endometritis. Front. Immunol. 16:1616217. doi: 10.3389/fimmu.2025.1616217
Received: 22 April 2025; Accepted: 20 August 2025;
Published: 05 September 2025.
Edited by:
Dmitry Aleksandrovich Zinovkin, Gomel State Medical University, BelarusReviewed by:
Ruochun Lian, Shenzhen Zhongshan Urological Hospital, ChinaBinggang Liu, The Central Hospital of Yongzhou, China
Tatsiana Liatkouskaya, Belarussian State Medical University, Belarus
Copyright © 2025 Yan, Jiao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiuxia Wang, d2FuZ3h4c2pAc2luYS5jbg==; Jiao Jiao, MTM4ODkyODQ3OTZAMTYzLmNvbQ==
 Xinyang Yan
Xinyang Yan Jiao Jiao1,2*
Jiao Jiao1,2* Xiuxia Wang
Xiuxia Wang