- Department of Orthopedic Surgery, School of Medicine, University of California, San Diego, San Diego, CA, United States
Nutrient availability is a strong determinant of cell function. Immune cells, which must rapidly activate transcriptional, proteomic, and metabolic programs to fulfill their functional roles, depend on nutrient supply to generate the building blocks needed for the production of immune effectors. While glucose, glutamine, and amino acids are well-recognized as critical energy sources and carbon donors during immune activation, the contribution of choline, a vitamin-like metabolite, has been overlooked. Once taken up by cells, choline plays a vital role in several biological processes. It is a precursor for phosphatidylcholine, the primary phospholipid in cellular membranes, and is also essential for synthesizing the neurotransmitter acetylcholine. Additionally, when directed toward mitochondria and betaine synthesis, choline serves as a methyl donor for histone and protein methylation, key processes that regulate gene expression and cellular activity. In this review, we examine the latest research on how immune cells utilize and metabolize choline, as well as its broader implications for immune-related disorders and overall human health. We also discuss recent and ongoing clinical studies investigating the effects of choline supplementation and the potential use of choline-derived metabolites as biomarkers for therapy response.
1 Introduction
Nutrient availability is essential for maintaining cellular homeostasis, supplying the building blocks necessary for cellular processes and functions. While cells can generate some nutrients through metabolic and catabolic pathways, these endogenous sources are insufficient to meet cellular demands, making dietary intake the primary source (1). In recent decades, the field of immunometabolism has significantly advanced our understanding of nutrient utilization by immune cells. Notably, studies have demonstrated the critical roles of glucose, amino acids, fatty acids, and vitamins in immune cell maturation, differentiation, and function (1, 2). However, while choline has been extensively studied in cancer due to its essential role in cell proliferation and tumor growth, its functions in immunometabolism are only beginning to be defined. Recent evidence has identified choline as a key metabolite contributing to immune cell function in various pathological settings (3, 4). Here, we will discuss the major findings on how choline availability and mobilization towards phosphatidylcholine (PC), acetylcholine (ACh), or betaine synthesis affect immune cell function, emphasizing its impact on human health and disease.
1.1 Choline uptake and utilization
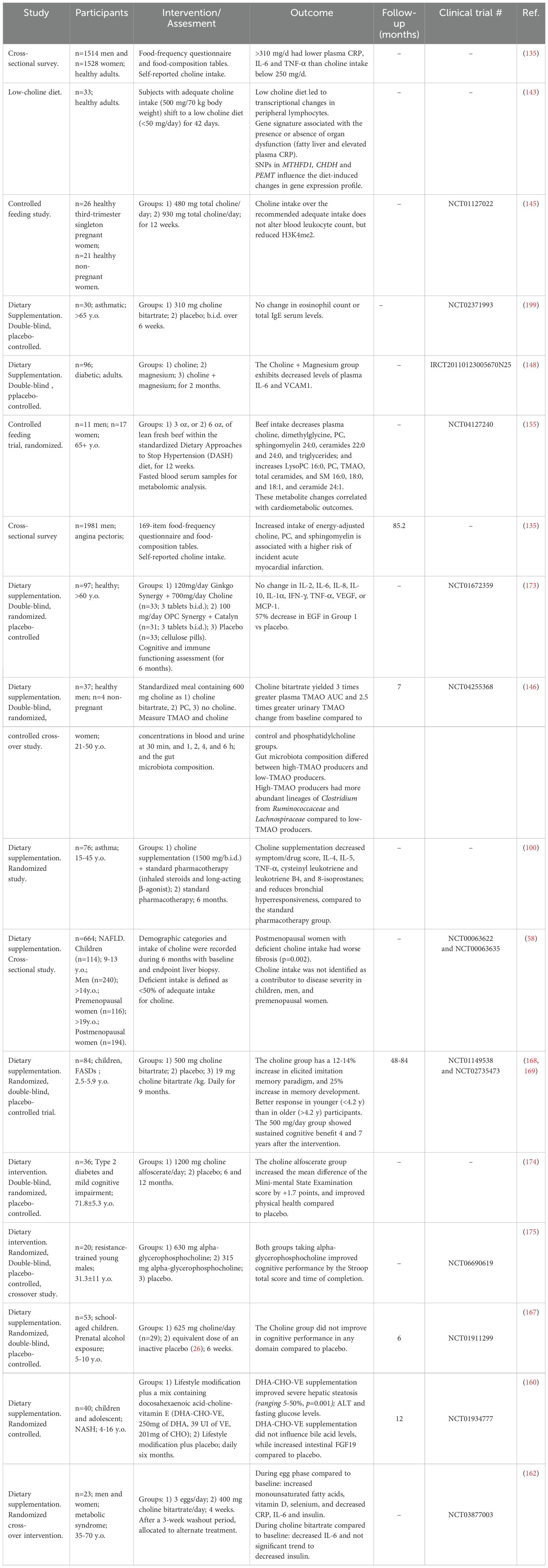
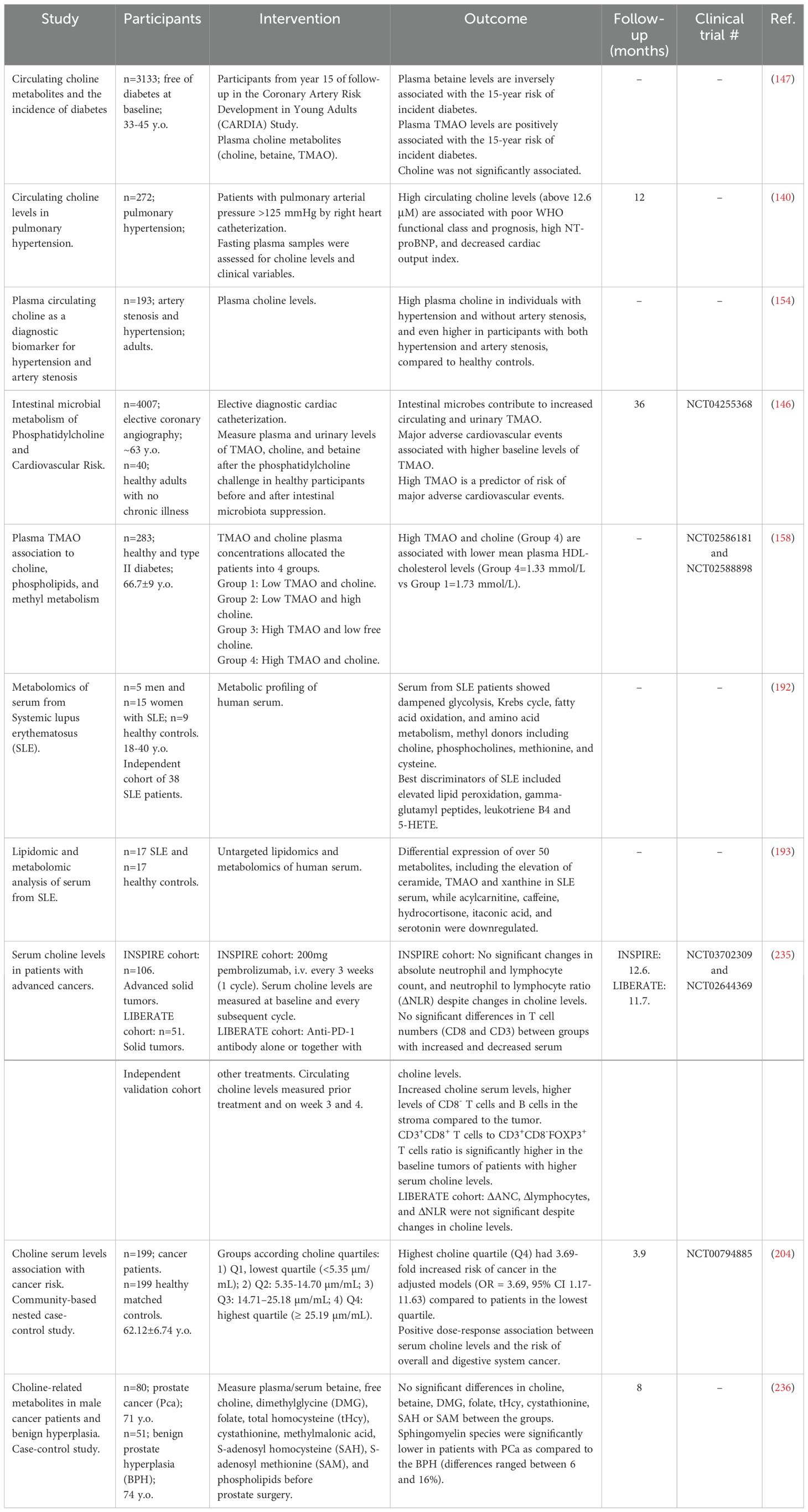
Choline is a vitamin-like essential quaternary ammonium salt present in the diet as free choline and phospholipid-bound forms. Dietary choline is partially absorbed by intestinal cells or metabolized by gut bacteria (5, 6) (Figure 1). While choline does not compete with other nutrients for enterocyte transport, gut microbes can limit its bioavailability (7). In the large intestine, gut microbiota, including Firmicutes and Proteobacteria, convert choline into trimethylamine (TMA) (8), which is subsequently absorbed and oxidized in the liver by flavin monooxygenase 3 (FMO3) into trimethylamine-N-oxidase (TMAO) (9). In Escherichia coli, Pseudomonas aeruginosa, and Acinetobacter baumannii, choline uptake is mainly mediated by the BetT transporter, whose expression and transport activity are upregulated by hyperosmotic stress (10). Bacteria use choline primarily as a precursor to glycine betaine, a potent osmoprotectant (11), and as a carbon and nitrogen source (12). In contrast, in eukaryotic cells, choline transport is more complex and relies on various protein transport systems, including choline transporter-like proteins (CTLs), high-affinity choline-specific transporters (CHTs), and organic cation transporters (OCTs) (13) (Figure 2). The preference for a specific transport system is cell-dependent and aligns with the anticipated metabolic fate of choline. Thus, CTLs facilitate choline transport across the plasma and mitochondrial membranes, aiding in the production of phosphatidylcholine (PC) and betaine, respectively (14). CHTs mainly mediate sodium-dependent choline uptake in nervous tissues and aid the synthesis of the neurotransmitter acetylcholine (ACh) (15), while OCT2 plays a role in ACh recycling in cholinergic neurons in the presynaptic terminals (16). Recent evidence has identified mitochondrial choline import via the orphan solute carrier SLC25A48, leading to the production of betaine (17–19), a choline-derived methyl donor, and the synthesis of purine nucleotides (18–20). Loss or a single nucleotide polymorphism on the SLC25A48 gene inhibits mitochondrial choline import, increases reactive oxygen species, disrupts lipid balance, and impairs cell proliferation (18–20).
Figure 1. Choline intake, distribution, and metabolism. Intake of choline-rich foods provides choline bound to lipids or as a free soluble metabolite for reabsorption by intestinal cells or poured into the circulation. Intestinal bacteria uptake choline to generate the metabolite trimethylamine (TMA) that through the portal vein reaches the liver to be converted into trimethylamine N-oxide (TMAO). Circulating and tissue-resident immune cells are exposed to choline-containing lipids, free-choline, TMA, and TMAO, and respond to changes in physiological levels. Created in BioRender. Sanchez Lopez, E. (2025) https://BioRender.com/cs12pz8.
Figure 2. Choline transporters and utilization. Choline is transported into the cells via high- (CHTS), intermediate- (CTLs), and low-affinity (OCTs) linked to distinct metabolic fates. Left panel: CTLs mediate the uptake of choline for PC production through the Kennedy pathway. Choline is phosphorylated by Choline kinase (ChoK) to form phosphocholine, then converted into CDP-choline by the CTP:phosphocholine cytidylyltransferase (CCT). CDP-choline is converted into PC, the main phospholipid in cellular membranes, by the choline/ethanolamine phosphotransferase (CEPT). CTLs also localize to the mitochondrial membrane where, along with SLC25A48, they mediate choline import into the mitochondria for the generation of betaine, a precursor of methionine and dimethylglycine (DMG), s-adenosylmethionine (SAM), s-adenosylhomocysteine (SAH), and homocysteine (Hcys), all key methylation intermediates for DNA and protein methylation. Middle panel: High-affinity CHTS supports choline uptake for acetylcholine (ACh) synthesis through the choline acetyltransferase (CHAT), a reaction that can be reversed by acetylcholine esterase (AChE), releasing choline and acetate. Acetylcholine acts on both nicotinic and muscarinic receptors to mediate cholinergic signaling. Right panel: Low-affinity organic cation transporters contribute to choline uptake for both ACh and PC synthesis. However, their role in mitochondrial choline utilization and methionine synthesis remains unknown. PPi, inorganic pyrophosphate; DAG, Diacylglycerol; AC, adenylyl cyclase; IP3, inositol 1,4,5-triphosphate. Created in BioRender. Sanchez Lopez, E. (2025). https://BioRender.com/ahp7xe9.
Once intracellular, choline is directed into various metabolic pathways, including 1) its oxidation to form mitochondrial betaine, 2) its acetylation to produce ACh, and 3) its initial phosphorylation followed by mobilization in the Kennedy pathway to generate cholie-containing phospholipids (Figure 2). Choline undergoes irreversible oxidation to betaine through a two-step process mediated by choline dehydrogenase or choline oxidase. Betaine serves as an osmolyte and methyl group donor, playing a role in the re-methylation of homocysteine into methionine, a precursor of S-adenosylmethionine (SAM), essential for numerous methylation reactions, including DNA epigenetics (21–24). In the human body, choline is primarily utilized for synthesizing essential lipids such as sphingomyelin and phosphatidylcholine (PC), which are major components of cellular membranes (25). In particular, PC accounts for approximately 95% of the total cellular choline pool in most cells. It is synthesized from choline via the Kennedy Pathway, involving cytidine diphosphate-choline and a lipid anchor such as diacylglycerol (26). De novo PC synthesis begins with the phosphorylation of choline by the enzyme choline kinase (ChoK) into phosphocholine (27) (Figure 2). Phosphocholine is then converted into cytidine diphosphate-choline by phosphocholine cytidylyltransferase (CTP), which, using either diacylglycerol or alkyl-acylglycerol as a lipid anchor, is ultimately converted into PC (26). Additionally, choline contributes to the production of lipid mediators such as lysophosphatidylcholine (lysoPC), sphingomyelin, and platelet-activating factor (28).
Beyond lipid metabolism, choline plays a critical role in non-metabolic functions. Choline is also the precursor of the neurotransmitter ACh, whose synthesis is catalyzed by choline acetyltransferase (ChAT), transferring an acetyl group from acetyl-coenzyme A to choline, resulting in ACh and coenzyme A production (Figure 2). After being released and bound to its receptors, ACh is rapidly hydrolyzed into acetate and choline by acetylcholinesterase. Then, free choline is transported back by CHTs for further ACh synthesis (29). Cholinergic signaling in immune cells appears to regulate cytokine synthesis, influencing both the initiation and termination of inflammation (e.g., IL-2 in T cells, TNFα in macrophages, and IL-8 in dendritic cells). Modulation of immune cell cholinergic activity, by regulating ChAT activity, ACh breakdown or choline reuptake, regulates physiological responses such as blood pressure control or anti-viral immune reaction (17, 30–34).
1.2 Choline demands throughout the lifespan and choline supplementation
Choline is an essential nutrient throughout life, with particularly high demands during periods of rapid cell proliferation, such as pregnancy. Choline is actively transported across the placenta, leading to fetal plasma choline levels six to seven times higher than maternal blood levels, and amniotic fluid concentrations nearly ten times higher (35). This underscores the critical role of choline in fetal development, particularly in neural tube formation and hippocampal development (36, 37). To prevent deficiency-related complications, health organizations, including the National Institutes of Health (NIH) and the European Food Safety Authority (EFSA), recommend a daily choline intake of 550 mg/day for men and 425 mg/day for women, increasing to 450 mg/day during pregnancy and 550 mg/day during lactation (22). Deficiencies during pregnancy can impair long-term potentiation and memory, while prenatal and early-life choline supplementation has been proposed as an intervention to enhance cognitive outcomes, such as improving cognitive function and mitigating age-related memory decline, enhancing long-term memory, and sustained attention (38–46). Choline supplementation (5 g choline chloride/kg) in rats during pregnancy also protects against gestational inflammation mediated by LPS challenge, reducing the frequency of loss of fetuses, normalizing placenta weights, and attenuating LPS-induced NF-κB activation and TNF-α, IL-1β, IL-6, and IL-17A levels in the placenta (47). Maternal dietary choline can be delivered to the offspring through lactation. Pups from phosphatidylcholine (PC)-fed (egg lecithin) dams have increased concentrations of PC in the plasma and spleen and a lower frequency of antigen-presenting cells (48). However, splenocytes from pups from PC-fed dams produced more IL-2, IL-6, and IFN-γ after stimulation with concanavalin A and LPS (48). Additionally, postnatal choline supplementation (100 mg/kg/day) for 20 consecutive days has been shown to mitigate the long-term effects of prenatal ethanol exposure on hippocampal inflammation and peripheral immune responses in rats (49, 50).
In females, as estrogen levels decline, the choline metabolism and utilization undergo significant changes. A study examining choline intake in 664 subjects enrolled in the cross-sectional study Nonalcoholic Steatohepatitis (NASH) Clinical Research Network (NCT00063622 and NCT00063635) showed that postmenopausal women with self-reported choline intake less than 50% of the adequate intake, had faster progression of NASH as shown by increased liver fibrosis, while no associations were found in children, men, and premenopausal women (51). Dietary choline deprivation led to fatty liver and muscle damage in 77% of men and 80% of postmenopausal women, whereas only 44% of the premenopausal women exhibited signs of organ dysfunction (52). These findings highlight that choline deficiency may become more severe in certain populations, such as postmenopausal women. This is partly due to the loss of ability to maintain the expression of genes with estrogen-response elements involved in choline metabolism. One such gene is phosphatidylethanolamine N-methyltransferase (PEMT) (53), which encodes the enzyme that catalyzes the conversion of phosphatidylethanolamine to PC, through a three-step methylation process using S-adenosylmethionine (SAM) as methyl donor.
In the same line with prenatal choline intake and its effect on cognition and memory, in adult individuals, sustained choline intake between 187.6-399.50 mg/day, is linked to reduced risk of cognitive decline, improved learning ability, verbal fluency, working memory, mental processing speed, and attention span, compared to individuals consuming less than 187.6 mg/kg of total choline (54). Other epidemiological studies have revealed that plasma choline levels are inversely correlated with anxiety (55) and risk of depressive symptoms (56), supporting a potential positive role of choline in mental health as well. While insufficient choline intake is linked to liver dysfunction, neurodegeneration, and muscle damage, excessive intake primarily results in mild cholinergic side effects such as sweating, diarrhea, hypotension, and fishy body odor, with a tolerable upper intake level of 3.5 g/day in adult individuals (57–59). Despite recommendations, a significant portion of the population consumes insufficient choline, and both patients with cirrhosis and those critically ill on parenteral nutrition often experience severe choline deficiencies negatively affect their outcomes (60–62). However, even while consumption levels are optimal, choline depletion may occur locally in specific disease contexts, such as within the tumor microenvironment or at sites of infection by choline-consuming pathogens (63) or systemically, such as in critically ill patients on parenteral nutrition (62). It remains uncertain whether dysregulated choline distribution affects disease progression, and significant gaps exist in understanding how cells adapt to impaired choline availability or metabolism locally.
2 Choline and choline-containing metabolites contribute to immune cell activation and function
Metabolic reprogramming and lipidomic remodeling are hallmarks of immune cell activation and are closely intertwined with functional responses (64–66). However, how choline utilization and phospholipid homeostasis are regulated during these metabolic shifts remains incompletely understood. Increasing evidence suggests that abnormal choline uptake and metabolism represent a metabolic hallmark associated with immune cell activation and inflammation (67–69). Disruptions in choline metabolism, whether due to impaired uptake or blockade of de novo PC synthesis by pharmacological inhibition of ChoK or CTP:phosphocholine cytidylyltransferase alpha (CCTα), alter the cellular phospholipid pool composition, the integrity of the mitochondrial membrane, and the overall cellular activity, leading to immune dysfunction and dysregulated inflammation across species (67, 69–72). Choline has been shown to regulate cytokine levels following lipopolysaccharide (LPS) treatment (67, 69, 70) and modulate inflammatory markers and homocysteine concentration (21). Dietary supplementation with different forms of choline has varying effects on immune system maturation, with phosphatidylcholine (PC)-rich diets demonstrating stronger immunomodulatory effects than free choline (73). For instance, supplementation of choline as PC is associated with increased T-cell proliferation, higher IL-2, IL-6, and TNF-α production (73), and supports both maternal immune function and the development of the offspring’s immune system (74, 75). However, the effects of other choline-containing lipids, such as sphingomyelin, on immune system development remain largely unexplored. Given the role of choline in immune regulation, in this section of the review, we will discuss its role across various immune cell types.
2.1 Myeloid cells
Myeloid cells are frontline immune defenders that restore tissue homeostasis after infection or tissue damage. Dysfunction of these cells contributes to chronic inflammatory and autoimmune diseases such as gout, rheumatoid arthritis (RA), osteoarthritis (OA), cryopyrin-associated periodic syndromes (CAPS), inflammatory bowel disease, and neurodegeneration. Their functional plasticity, shifting between inflammatory, repair, or anti-inflammatory states, depends on metabolic adaptation and lipid remodeling, including choline uptake and phospholipid reorganization (66, 76). Therefore, disrupting choline metabolism can significantly alter myeloid cell function and disease outcomes.
2.1.1 Macrophages and dendritic cells
Macrophage functions, such as cytokine and chemokine secretion, phagocytosis, and organelle biogenesis, are intricately linked to membrane phospholipid composition, curvature, and charge (77, 78). In fact, distinct stimuli (e.g., Poly[I: C], LPS, IFN-γ, IFNβ, or IL-4) remodel the macrophage lipid composition in a signal-specific manner, altering glycerophospholipids, sphingolipids, cholesterol, and fatty acid composition (70, 79) (Figure 3). Choline metabolism plays a central role in macrophage activation towards both pro-inflammatory and anti-inflammatory functions (67, 69). In LPS-induced macrophage polarization, which initiates the inflammatory program, there is an augmented rate of choline uptake and PC synthesis, facilitated by the upregulation of the choline transporter CTL1 (69, 70). Pharmacologically- or antibody-mediated inhibition of choline uptake favors diacylglycerol (DAG) accumulation and protein kinase C activation, resulting in altered cytokine secretion in response to LPS (70) (Figure 3). Similarly, when phosphatidylcholine (PC) synthesis is compromised by myeloid-specific deletion of CTP:phosphocholine cytidylyltransferase alpha (CCTα), the rate-limiting enzyme in PC synthesis, macrophages fail to secrete the pro-inflammatory cytokines TNF-α and IL-6 in response to LPS, most likely due to secretory defects of the ER and Golgi (78). Through a distinct mechanism, choline uptake and PC synthesis regulate IL-1β production mediated by NLRP3 inflammasome activation (69) (Figure 3). Macrophages exposed to choline deficiency or ChoKα inhibitors (MN58b or RMS932A) exhibit poor PC and sphingomyelin mitochondrial membrane composition, leading to defective mitochondrial ATP synthesis that boosts AMPK activation and mitophagy (Figure 3). This eventually prevents mitochondrial damage and the cytosolic release of oxidized mtDNA, a direct activator of NLRP3, limiting the processing of mature IL-1β69. Similarly, both in vivo feeding with a choline-rich diet and in vitro trimethylamine-N-oxidase (TMAO) stimulation increased TMAO-dependent NLRP3 inflammasome activation and expression of IL-1β, IL6, TNF-α, CXCL9, and CXCL10, supporting that NLRP3 is a key proteolytic activator in the macrophage response to a high choline and TMAO production (80) (Figure 3). Indeed, in a model of graft-versus-host disease (GVHD), T-cell-depleted bone marrow transplants fed with TMAO-rich diet exhibited a significant increase in the frequency of F4/80+CD11b+CD16/32+ inflammatory macrophages relative to the F4/80+CD11b+ whole population, worsening disease severity, and increasing mortality (80).
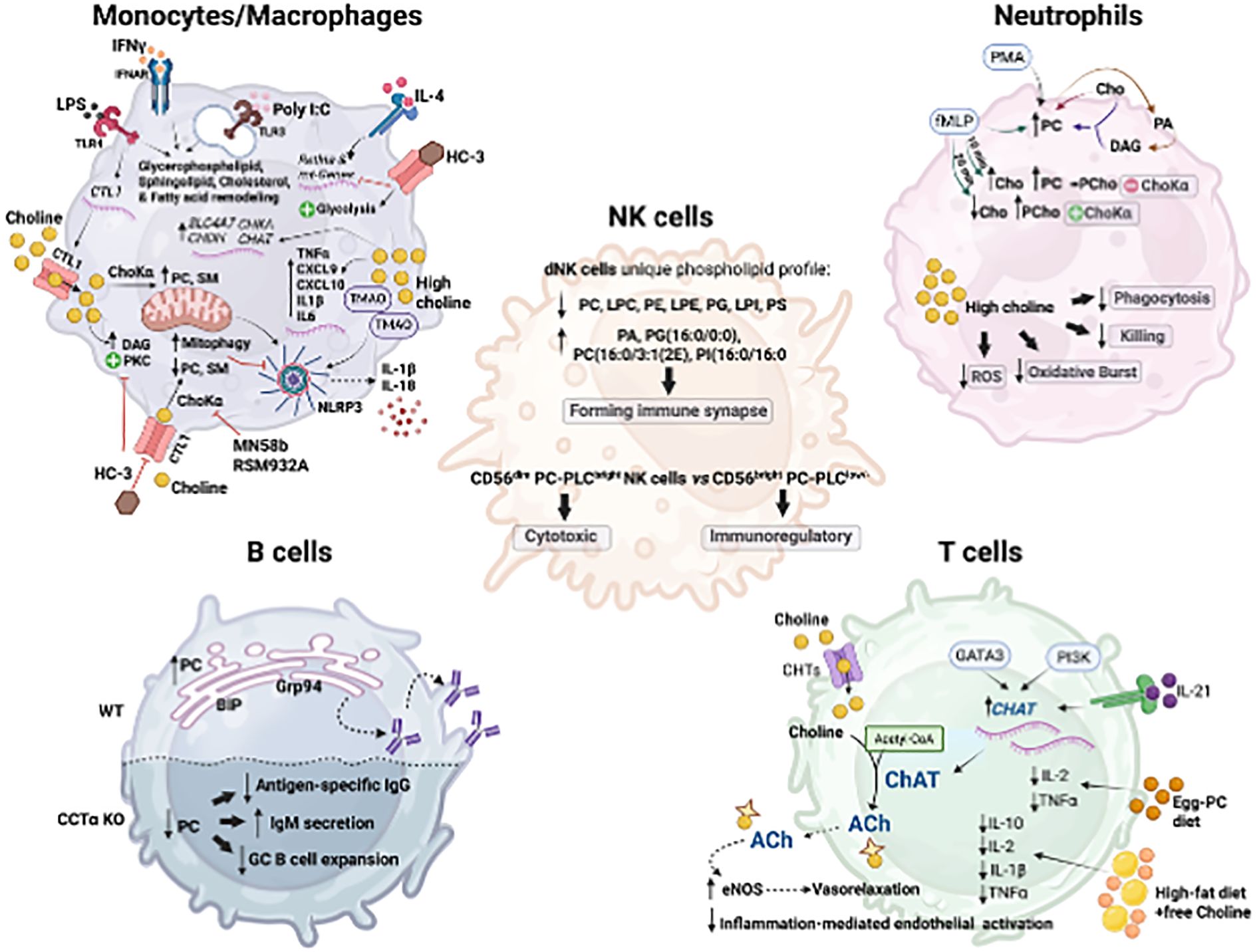
Figure 3. Central mechanisms of choline utilization and metabolism in the different immune cell types. Macrophage/Monocytes: Activation of macrophages (e.g., LPS, IFNy, Poly(I:C), IL-4) increases choline uptake via choline transporter-like protein 1 (CTL1) and PC synthesis. Impaired choline uptake or PCho production disrupts mitochondrial PC and SM, increasing mitophagy, restraining NLRP3 inflammasome activation and IL-1b and IL-18 production; and favors DAG accumulation and PKC activation; and suppresses IL-4-induced mitochondrial genes and Retina expression. Choline supplementation increased SLC4A7, CHDH, CHKA, and CHAT. Neutrophils: PMA and fMLP increase PC from the condensation reaction of choline and DAG formed from PC- derived phosphatidic acid. fMLP also increases PC dependent and independent of Choka activity. High choline diminishes neutrophils' phagocytic and killing capacities, reduces oxidative burst capacity, and decreases ROS production. NK cells: Human decidual NK (dNK) cells display a unique high saturated phospholipid profile compared to blood NK cells (decreased PC, LPC, PE, LPE, PG, LPI, and PS; increased PA, PG (16:0/0:0), PC(16:0/3:1 (2E)) and PI(16:0/16:0)), which difficult forming immune synapses. CD56dim PC- phospholipase C (PLC) bright NK cells are associated with cytotoxic function, whereas CD56brightPC-PLClow- cells exhibit immunoregulatory properties. B cells: Increased synthesis of PC supports rough endoplasmic reticulum (ER) expansion and upregulation of ER chaperones such as BIP and GRP94, immunoglobulin synthesis, and assembly. CCTa-deficient B cells show impaired class switching, reducing antigen-specific IgG1 production while increasing IgM secretion upon antigen challenge. T cells: Viral infection increases ChAT expression in CD4+ and CD8+ T cells in an IL21-dependent manner and via PI3K signaling cascade activation and the Th2-associated master regulator GATA3. T-cell-derived ACh boosts endothelial nitric oxide synthase (eNOS) activity and vasorelaxation and reduces inflammation. Created in BioRender. Sanchez Lopez, E. (2025). https://BioRender.com/6mvmfzs.
Beyond the inflammatory response, choline metabolism is also implicated in IL-4-induced macrophage polarization, critical for immunity against intestinal helminth infection, resolution of inflammation, and tissue repair. IL-4 causes a rapid increase in choline import, phosphocholine production, and PC biosynthesis (67). The inhibition of choline transport and metabolism, using hemicholinium (HC-3), and RSM932A, which target the choline transporter and ChoKα, respectively, dramatically impacted macrophage responses to IL-4. Specifically, inhibition of choline metabolism suppresses mitochondrial gene expression, shifts metabolism toward glycolysis, and inhibits IL-4-induced expression of Retnla (67), a resisting-like molecule involved in type 2 immunity and tissue repair. In a model of intestinal infection with the parasite Heligmosomoides polygyrus, ChoKα inhibition diminished peritoneal macrophage and B-1 lymphocyte frequency, causing compromised immunity against the parasite (67). In the human monocytic cell line U937, IL-4 receptor engagement leads to ChoK-independent increases of phosphocholine, resulting from the degradation of membrane PC into DAG, indicating enhanced PC-specific phospholipase C activity (81). Choline availability may also regulate and support the expression of the machinery necessary for its processing and signaling, as dietary choline supplementation increases the expression of choline receptor SLC4A7, choline dehydrogenase (CHDH), choline kinase alpha (CHKA), choline acetyltransferase (CHAT), and genes related to acetylcholine (ACh)-dependent signaling such as the muscarinic (CHRM1 and CHRM5) and nicotinic (CHRNA7) ACh receptors (82) (Figure 3). Indeed, small peritoneal macrophages maintain peritoneal ACh levels through choline acetyltransferase expression driven by MyD88 pathway activation. This increased macrophage-mediated acetylcholine (ACh) release facilitates the clearance of apoptotic neutrophils and enables the resolution of acute peritonitis (83). In addition, intestinal macrophages respond to ACh released by nerve fibers in the intestinal myenteric plexus, ameliorating inflammation in vivo, in a mechanism that involves the activation of Jak2/STAT3 and the transactivation of STAT3-responsive DNA elements (84). The stimulation of the vagus nerve in rats at 10 Hz, suppresses endotoxin-induced serum TNF-α levels in vivo. Similarly, in vitro exposure of macrophages to 10 μM of ACh prior to endotoxin challenge reduces TNF-α production by increasing adenylyl cyclase 6 activity, leading to cAMP formation, CREB phosphorylation, and the expression of c-Fos, a known inhibitor of TNF transcription (85). This inhibitory effect on monocytes’ TNF and IL-1β production was confirmed in human whole blood and human monocyte/macrophages using a selective inhibitor of α7 nicotinic acetylcholine receptor (α7nAChR) (86). Together, all these findings emphasize the essential role of choline availability in macrophage preparedness for an efficient immune response to diverse triggers and reveal that the impact of impaired choline metabolism and utilization on inflammatory molecular pathways is cell- and stimulus-specific.
It is worth noting that the different choline dietary forms differ in their effect. The offspring from dams fed with mixed choline sources with lower free choline (12.5-25%) but high glycerophosphocholine (25-75%), exhibited a decrease in macrophage and dendritic cell frequencies in the spleen and produced less IL-1β, IL-6 and IFN-γ in response to mitogenic immune challenge with either concanavalin A or LPS, compared to those from dams with higher free choline (100%) intake (87). In the context of obesity-related inflammation, adipose tissue macrophages isolated from obese mice and humans often exhibit increased de novo PC biosynthesis (71). Interestingly, macrophage-specific deletion of CCTα alleviated obesity-induced white adipose tissue (WAT) inflammation and insulin resistance. However, despite reduced CCTα activity, PC levels remain unchanged due to a compensatory reduction in PC degradation, resulting in slower PC turnover that allows for greater remodeling of PC species enriched in polyunsaturated fatty acid (PUFA), which likely protects against endoplasmic reticulum stress and inflammation (71). These establish a causal relationship between obesity-associated increases in the de novo PC synthesis, accelerated PC turnover, and proinflammatory adipose tissue macrophage activation (71).
In dendritic cells, ACh modulates immune function by upregulating HLA-DR and CD86 expression and stimulating TNF-α and IL-8 production (34). However, when combined with LPS stimulation, ACh partially suppresses HLA-DR and TNF-α/IL-12 production, suggesting that its effect depends on dendritic cell maturation status (34). Despite these findings, the influence of choline metabolism on dendritic cell adaptation, particularly in high-choline-demand environments such as tumors, remains unexplored. The gut microbiota also contributes to choline-mediated immune modulation. Enterobacter ludwiggi, an abundant commensal bacterial species in mice, enhances dendritic cell immune tolerance and promotes Treg differentiation via choline metabolism (88). Choline produced by E. ludwiggi protects against DSS-induced colitis by enhancing the choline/α7nAChR-mediated dendritic cell immune tolerance, leading to increased Foxp3+ Treg differentiation (88). CD103+ dendritic cells from Enterobacter ludwiggi-treated mice exhibited higher expression of tolerogenic markers Tgfb1, Tgfb2, Aldh1a2, and Pdl1, and co-culture with naïve CD4+ T cells enhances Treg conversion (88). Moreover, mice receiving dendritic cells exposed to Enterobacter ludwiggi exhibited reduced colitis severity and expanded Treg populations in the mesenteric lymph nodes and spleen, highlighting choline’s role in shaping gut-immune interactions.
Together, these findings highlight the complex role of choline metabolism in shaping antigen-presenting cell function and inflammatory responses across diverse physiological and pathological contexts. A deeper understanding of how choline-derived metabolites influence innate immune signaling may uncover new therapeutic targets and biomarkers for inflammatory disease management and precision immunomodulation.
2.1.2 Neutrophils and eosinophils
Granulocytes play a pivotal role in the initial immune response to pathogen infection or tissue damage, contributing to both inflammation and tissue homeostasis. Among them, neutrophils are the most abundant and are essential for detecting pathogens and initiating immune cascades through processes such as swarming, cytokine production, degranulation, phagocytosis, and the formation of neutrophil extracellular traps (NET) (89, 90). Two decades ago, Tronchère et al. described that neutrophil activation by Phorbol 12-myristate 13-acetate (PMA) and formyl-methionyl-leucyl-phenylalanine (fMLP) results in increased choline incorporation into phosphatidylcholine (PC), dependent solely on diacylglycerol formed from PC-derived phosphatidic acid (91) (Figure 3). This provides evidence for an activated PC cycle in human neutrophils linking phospholipase D and cytidyltransferase activation. However, these findings were later challenged by Pédruzzi et al. (92), who showed that while fMLP and PMA stimulation altered choline and PC levels in neutrophils, phosphorylcholine content remained unchanged for at least 10 minutes, indicating that phospholipase C-mediated PC breakdown was not the primary mechanism. Notably, prolonged fMLP exposure (20 minutes) increased phosphocholine while decreasing choline levels, implicating ChoKα activity rather than phospholipase C-mediated PC degradation (92) (Figure 3). Interestingly, PMA stimulation showed a distinct response, showing a decline in phosphocholine between 10 and 15 minutes, indicating a stimulus-dependent, distinct regulatory mechanism of phosphocholine metabolism (92). Despite early evidence linking choline and PC metabolism to neutrophil activation, the broader impact of choline and its metabolites on neutrophil function remains poorly understood. Studies on bovine neutrophils suggest that increasing choline concentrations linearly diminish their phagocytic and killing capacities (82). In neutrophils from early lactation cows, dietary choline supplementation increased the expression of SLC5A7, CHDH, CHKA, CHAT, CHRM5, and CHRNA7. Choline significantly reduced neutrophil oxidative burst capacity in a dose-dependent manner, and the higher choline concentration (13.2 μM) led to a quadratic decrease in E. coli phagocytosis and a linear reduction in reactive oxygen species (ROS) production per neutrophil (82). Inflammatory gene expression, including TLR4, NFKB1, TNFA, neutrophil elastase (ELANE), H2A, CASP3, and CASP7, was largely unaffected by choline, though a greater reduction in TLR4 expression was observed at higher doses. TNFα levels also tended to decrease following choline supplementation (Figure 3).
Eosinophils contribute to various inflammatory conditions, including asthma, rhinitis, eosinophilic gastroenteritis, and inflammatory bowel disease. While choline exhibits anti-inflammatory activity, its role in eosinophilic inflammation remains unclear. Dietary choline supplementation effectively suppressed airway inflammation in an allergen-induced mouse model of airway hyperreactivity by reducing eosinophil accumulation and eosinophilic peroxidase activity in bronchoalveolar lavage fluid, likely through nicotinic acetylcholine receptor activation via the cholinergic anti-inflammatory pathway (93). Later studies by the same group confirmed these findings, showing that co-administration of choline and α-lipoic acid further reduced eosinophilic infiltration, peroxidase activity, and oxidative stress, suggesting a role for redox status modulation (93). Similarly, asthma patients receiving conventional therapy alongside six months of oral choline supplementation exhibited significant reductions in circulating eosinophils and factors involved in bronchial hyperreactivity, such as IL-5 and cysteinyl leukotrienes (Cys-LT) (94). Choline, as a precursor of PC, a major component of lung surfactants, may help compensate for eosinophilic phospholipases-pulmonary surfactant dysfunction in asthma, thereby reducing airway inflammation and disease severity (95). However, further mechanistic studies are needed to determine the direct effects of choline availability on eosinophil activation and function and to assess the therapeutic potential of dietary choline supplements (95) in managing hyperreactive eosinophilic conditions, such as eosinophilic myositis or gastrointestinal disorders.
2.1.3 Microglia
Microglia constitute specialized immune sentinels of the central nervous system that exhibit similar plasticity to macrophages, transitioning between inflammatory and anti-inflammatory phenotypes. Under stress conditions, activated microglia release inflammatory factors that drive neuroinflammation, whereas in a tissue repair state, they promote neuroprotection (96). The stimulation of the α7nAChR has been shown to exert anti-inflammatory effects by inhibiting cytokine production and release, such as TNF-α, which is neurotoxic, by microglia (97, 98). However, the role of choline uptake and the generation of choline-derived metabolites in microglia-mediated neuroinflammation remains incomplete. Besides acetylcholine (ACh) receptors, microglia also expressed choline transporter CTL1, primarily located in the plasma membrane, facilitating extracellular choline transport, and CTL2 mainly in the mitochondria, suggesting a role in betaine production (99). Microglia activation with either LPS or IL-4 enhances choline uptake and phosphatidylcholine (PC) synthesis. Notably, CTL1 inhibition or choline deprivation suppresses LPS-induced IL-1β and IL-6 but boosts the expression of arginase-1 upon IL-4 stimulation (99). This indicates that, as in macrophages, choline metabolism also modulates microglial inflammatory responses, and manipulating choline metabolism may promote a neuroprotective phenotype. Furthering this concept, research on licochalcone E (Lico E), a β-amyloid aggregation inhibitor, has revealed that CTL1-mediated choline uptake is involved in its neuroprotective effects (100). While stimulation of the microglia cell line SIM-A9 with Aβ1–42 significantly increased TNF-α mRNA expression, this effect was suppressed by choline deficiency and Lico E treatment. Furthermore, Lico E also restored arginase 1 expression, supporting its neuroprotective role. Additionally, IL-4-induced Arg1 expression was further upregulated by choline deprivation and Lico E treatment, reinforcing the hypothesis that CTL1 inhibition fosters a neuroprotective anti-inflammatory phenotype promoting Aβ degradation (100).
The role of choline metabolism in inflammation extends beyond microglia. In acid sphingomyelinase knock-out mice, which mimic a neurovisceral acid sphingomyelinase deficiency (ASMD) characterized by cellular accumulation of sphingomyelin, the effects of a choline-free diet decreased the activation of liver macrophages and microglia, but it did not significantly alter sphingomyelin levels due to compensatory mechanisms involving methionine metabolism (101). While in this model, choline deprivation altered lipid composition in the liver, decreasing sphingomyelin and specific glycerophospholipids with 34:1 fatty acids, leading to reduced inflammation, its impact on brain lipid metabolism was less pronounced (101). This suggests that additional dietary modifications, such as methionine restriction, may be needed to modulate neuroinflammation more effectively. Although growing evidence highlights the impact of choline metabolism on macrophages and microglia, there is a knowledge gap regarding its effects on other tissue-resident immune sentinels, such as Kupffer and Langerhans cells. Further research is necessary to elucidate the broader implications of choline metabolism in immune regulation and inflammatory diseases.
2.2 Lymphocytes
The phospholipid remodeling and cholinergic anti-inflammatory system play a well-established role in immune homeostasis and the regulation of inflammatory and autoimmune diseases. Most immune cells, including CD4+ T cells, B cells, and NK cells, upregulate genes related to the cholinergic system synthesis in response to inflammatory cues, which is pivotal for the maintenance of immunological homeostasis (31–33, 86, 102). Below, we summarize studies that remark the importance of the cholinergic immune signaling and circulating choline-containing lipids on lymphocyte differentiation, activation, and function.
2.2.1 B cells
B cell maturation and differentiation into plasma cells require robust phospholipid synthesis and remodeling to sustain multiple rounds of proliferation, clonal expansion, and antibody production (103). A hallmark of this differentiation process is the increased synthesis of phospholipids, particularly phosphatidylcholine (PC) (104), which supports the expansion of the intracellular membrane network, including the rough endoplasmic reticulum, where immunoglobulins are synthesized and assembled (105, 106) (Figure 3). Plasma cell differentiation is accompanied by endoplasmic reticulum enlargement, increased membrane-bound ribosomes, upregulation of endoplasmic reticulum chaperones such as BIP and GRP94, and transcription factors like XBP-1 (106). The inhibition of choline mobilization through the Kennedy pathway has been examined using B cell-specific CTP: phosphocholine cytidyltransferase (CCTα, Pcyt1a gene) deficient mice. Despite that low penetrance, partial deficiency of CCTα in B cells resulted in decreased peritoneal and splenic B cell numbers, inducing a compromised proliferation, especially in the periphery, decreasing serum IgG concentration, and increasing the incidence of IgM-secreting cells (107). CCTα-deficient B cells stimulated with LPS triggered early activation of the unfolded protein response (UPR)-mediated splicing of Xbp-1, and impaired class switching, reducing antigen-specific IgG1 production while increasing IgM secretion upon antigen challenge (104) (Figure 3). This highlights the requirement of PC synthesis for germinal center B cell expansion and antibody production (76, 104).
Various immune cells, such as T and B lymphocytes, and Natural Killer (NK) cells possess all the necessary components to constitute an independent cholinergic system, including ChAT and acetylcholinesterase, and both muscarinic and nicotinic ACh receptors (31, 34, 83, 108–113). In particular, the production of acetylcholine (ACh) by choline-acetyltransferase (ChAT)-expressing B cells, which seems to be essential for efficient liver regeneration in a mouse model of partial hepatectomy (112). B-cell specific ablation of ChAT increases the mortality of mice subjected to partial hepatectomy compared to their wildtype counterpart, due to dysregulation of α7nAChR expressing Kupffer cells and CD8+ T cells, limiting their regenerative capacity and producing harmful IFN-γ, respectively (112). Similarly, ACh-producing B cells contribute to the regulation of TNF-α production by α7nAChR-expressing interstitial lung macrophages in mice subjected to influenza infection (113). Altogether, these findings support antibody-independent immune regulatory functions of B cells and expand the immunomodulatory mechanisms associated with ACh production.
Aberrant B cell expansion and survival contribute to B cell malignancies, such as multiple myeloma, diffuse large B cell lymphoma, and chronic lymphocytic leukemia, which are frequently associated with TRAF3 gene deletions or inactivating mutations (114–116). Recent studies identified TRAF3 as a regulator of key metabolites, lipids, and enzymes involved in choline metabolism (117). In particular, TRAF3 ablation boosts phosphocholine and PC biosynthesis, promoting B cell survival (117). Pharmacological inhibition of ChoKα by MN58B and RSM932A effectively reduced the survival of TRAF3-deficient B cells both in vivo and in vitro (117). Metabolomic, lipidomic, and transcriptomic studies indicate that TRAF3 exerts broad regulatory effects on B cell metabolism, including interconnecting choline and ethanolamine pathways. Reconstitution of TRAF3 in human multiple myeloma cell lines inhibited ChoKα expression, suppressed the Kennedy pathway, and induced apoptosis, underscoring the role of elevated choline metabolism in sustaining the phenotype of TRAF3-deficient malignant B cells.
2.2.2 T cells
T follicular helper (TFH) cells display distinct lipid metabolic profiles, characterized by the localization of phosphatidylethanolamine predominantly on the outer plasma membrane. Similar to B cells, phosphatidylethanolamine colocalizes with the chemokine receptor CXCR5 (118). De novo phosphatidylethanolamine synthesis via the cytidine diphosphate-ethanolamine pathway is critical for maintaining CXCR5 surface expression by preventing its internalization and degradation. T cell-specific genetic ablation of Pcyt2, which encodes CTP: phosphoethanolamine cytidylyltransferase, but not of Pcyt1a, which mediates the cytidine diphosphate-choline pathway, impairs TFH cell differentiation, leading to diminished humoral immune responses (118). Indeed, splenocyte incubation with lysoPC, a circulating bioavailable form of PC, enhances proliferation and IL-2 secretion (48), suggesting that PC positively modulates T-cell function and may counteract immune dysfunction.
T cell-derived acetylcholine (ACh) has long been studied as a crucial immune regulator. T and B cell lymphocytes express choline acetyltransferase (ChAT), high-affinity choline transporters (ChRM and ChRN), acetylcholine transferase, and can produce and release ACh (102, 119, 120). In vitro studies show that ACh and other ChRM and ChRN agonists enhance T cell cytotoxicity, B and T cell intracellular Ca2+, c-fos expression, nitric oxide, and IL-2 production, cyclic guanosine monophosphate (cGMP), and inositol-1,4,5-triphosphate (IP3) levels, and modulate DNA synthesis and cell proliferation (102, 119, 121–123). As mentioned above, ChAT-expressing T cells affect blood pressure (30) and regulate the release of inflammatory cytokines (33). During lymphocytic choriomeningitis virus (LCMV) infection, ChAT expression is strongly induced in both CD4+ and CD8+ T cells in an IL-21-dependent manner (31) (Figure 3). Using ChAT-GFP reporter mice allowed for tracking a massive expansion of CD4+ and CD8+ T cells at day 8 post-infection, followed by a rapid decline of Chat-GFP+ splenic virus-specific T cells after LCMV clearance (31). In this context, IL-21, a key cytokine in chronic infection, drives ChAT expression in T cells, facilitating their migration into infected tissues. In human T cells, ChAT mRNA expression is induced via the activation of the PI3K signaling cascade (124). In particular, ChAT expression is induced by the Th2-associated master regulator GATA3 and the suppression of RE-1 silencing transcription factor (REST)-mediated methylation of the ChAT promoter (124) (Figure 3). T cell-derived ACh potentiates endothelial nitric oxide synthase (eNOS) activity, facilitating vasorelaxation, improving endothelial barrier integrity, and reducing inflammation-associated endothelial activation (124). Furthermore, in a cohort of patients with severe circulatory failure, improved survival positively correlated with their relative frequency of circulating ChAT+CD4+ T cells (124).
Supplementation with dietary choline from egg-phosphatidylcholine (PC) mitigates T-cell dysfunction associated with diet-induced obesity (125, 126). The egg-PC diet lowered the frequency of CD3+ T cells with no significant differences in helper (CD3+CD4+) and cytotoxic (CD3+CD8+) T cells as well as activated T cells (CD3+CD25+), reducing as well the production of IL-2 and TNF-α 125) (Figure 3). In a follow-up study (125), high-fat diet supplementation with free choline exhibits a reduction in splenocyte T-cell proliferation following stimulation with anti-CD3/anti-CD28, and decreased production of IL-1β, IL-2, IL-10, and TNF-α in splenocytes and mesenteric lymph nodes (126) (Figure 3). Diet supplementation with mixed choline sources, with low free choline but high glycerophosphocholine, increases the frequency of cytotoxic CD8+ T cells expressing CD27, CD71, and CD127, as well as total B cells (CD45RA+) and dendritic cells (OX6+OX62+) (87). Additionally, pups of choline-supplemented dams exhibited lymphocytes that produced lower IL-6 and IFN-γ following concanavalin A stimulation, compared to those from the 100% free choline group (87).
2.2.3 NK cells
Natural killer (NK) cells are innate lymphocytes that contribute to the immune response against malignancies and viral infections. Unlike the T and B cells, NK cells do not undergo receptor gene rearrangement, making them a key component of the first line of immune response.
NK cells exhibit strong metabolic flexibility upon short-term cytokine stimulation, but prolonged activation leads to increased mitochondrial metabolism and glycolysis involving mammalian target of rapamycin complex 1 (mTORC1) (127, 128). Activated NK cells rely on enhanced glycolysis and the citrate-malate shuttle, which facilitates glucose-driven mitochondrial citrate export to the cytosol. There, citrate is converted to malate before re-entering the mitochondria to fuel the electron transport chain (129). Additionally, citrate serves as a precursor for acetyl-CoA, which is essential for lipid synthesis and protein acetylation. While choline uptake and utilization in NK cells remain unexplored, choline-containing lipids play a crucial role in NK cell cytotoxicity and function.
Metabolomic and lipidomic studies have identified distinct alterations in glycerophospholipids among different NK cell subsets (130). Notably, human decidual NK (dNK) cells, which are located at the maternal-fetal interface, display a unique PL profile compared to blood NK cells (130). In dNK cells, total phosphatidylcholine (PC), lysoPC, phosphatidylethanolamine, lysophosphatidylethanolamine, phosphatidylglycerol, lysophosphatidylinositol, and phosphatidylserine are significantly downregulated (Figure 3). In contrast, metabolites such as phosphatidic acid, phosphatidylglycerol (16:0/0:0), PC (16:0/3:1(2E)), and phosphatidylinositol (16:0/16:0) are significantly upregulated, suggesting a high saturation of glycerophospholipids. This metabolic shift may indicate difficulty in forming immune synapses, which are critical for NK cell function (130). Intratumoral NK cells exhibit reduced sphingomyelin content, and inhibition of sphingomyelin synthase 1, the enzyme responsible for converting ceramide and PC into sphingomyelin, disrupts NK cell function (131). Since sphingomyelin is necessary for immune synapse formation, its inhibition impairs NK cell recognition and killing of tumor cells by altering membrane topology and cytotoxicity (131). A reduction in choline availability to the intratumoral NK cells may have effects similar to sphingomyelin biosynthesis inhibition, though this possibility remains unexplored. Moreover, it remains unknown whether increased PC synthesis in NK cells is mediated by choline mobilization to the Kennedy pathway or through increased PC-PLC activity. Both NK-cell-mediated cytotoxicity and lytic granule exocytosis require an increase in PC-PLC (132). Among the NK cells subset, CD56dim PC-phospholipase Cbright cells are associated with cytotoxic function, whereas CD56bright PC-phospholipase Clow/- cells exhibit immunoregulatory properties (133) (Figure 3). Indeed, PC-phospholipase C expression on the NK cell membrane correlates closely with CD16 receptor expression, suggesting a potential relationship between enzyme externalization, NK cell maturation, and CD16-mediated cytolytic process (134). The mechanistic underpinnings of choline-mediated regulation of lymphocytes, including B, T, and NK cells, remain insufficiently defined. There are significant gaps in our understanding of how choline metabolism integrates with lymphocyte function in acute and chronic inflammatory conditions.
3 The influence of choline on health and disease outcomes
Although the human body can synthesize choline in the liver, this endogenous production is insufficient to meet physiological demands, making dietary supplementation necessary. Choline is primarily obtained from animal-derived foods (e.g., meat, dairy, eggs), as well as plant-based sources (e.g., beans, nuts, seeds). Imbalances in choline intake, whether insufficient or excessive, can lead to various health issues, including cardiovascular disease, neurological disorders, and organ dysfunction (3, 22, 23, 135, 136). Moreover, elevated circulating or local choline metabolism has been observed in inflammatory diseases, such as arthritis, cancer, and cardiovascular diseases (68, 137–141). The knowledge gained from in vitro, preclinical, and animal studies has encouraged a variety of clinical trials investigating the role of dietary choline supplementation in disease progression (Table 1), as well as the association between circulating choline-containing metabolites and disease prognosis or clinical symptoms (Table 2). However, there are few studies investigating the impact of choline intake on immune responses in healthy individuals. A cross-sectional survey that enrolled healthy men (n=1514) and women (1528) who reported intakes of choline and betaine calculated from food-frequency questionnaire and food-composition tables found that participants who consumed more than 310 mg/d had lower plasma C-reactive protein (CRP), IL-6 and TNF-α than participants reporting choline intake below 250 mg/d (142). In subjects (n=33) with adequate choline intake (500 mg choline/70 kg body weight), a shift to a low-choline diet (<50 mg/d) for 42 days led to substantial transcriptomic changes in peripheral lymphocytes (152 down- and 107 up-regulated genes), providing a unique signature (including CHEK1, GBE1, and KIF20A), that could be used for segregating the participants according to the absence or presence of signs of organ dysfunction caused by the low choline intake, including fatty liver or elevated plasma CPK (143). Importantly, these diet-induced changes in gene expression profiles were influenced by SNPs within the genes involved in choline metabolism, such as MTHFD1, CHDH, and PEMT (143). This is important, as the prevalence of SNPs in genes that increase susceptibility to choline-related organ dysfunction in the general population is not known. A parallel study revealed that all participants fed with a low choline diet had significant lymphocyte DNA damage compared to the phase in which they were fed with adequate choline amounts. The increase in lymphocyte apoptosis was more pronounced in participants fed with low diet who developed organ dysfunction (144). The impact of choline intake on transcriptional regulation has also been observed in a 12-week controlled feeding study, in 26 healthy third-trimester singleton pregnant women and 21 non-pregnant control women fed with either 480 or 930 mg total choline/day (NCT01127022) (145). In particular, the epigenetic mark histone 3 lysine 4 di-methylation (H3K4me2), which has been associated with increased transcription, was lower among pregnant women consuming higher levels of choline (145). However, further studies are needed to identify the specific genes regulated by H3K4me2 that are sensitive to choline availability.
In addition to facilitating nutrient availability in the circulation, diet is a modifiable factor that shapes the composition of the gut microbiome. Different choline sources can exert distinct actions on healthy individuals. In healthy adults (ages 21 to 50), fasted for 10 hours before consuming a standardized meal containing choline bitartrate (free choline), phosphatidylcholine (PC), or a no-choline diet control (NCT04255368) (146), only free choline intake resulted in a three-fold increase in plasma trimethylamine-N-oxidase (TMAO) and a 2.5-fold increase in urinary TMAO compared to control and PC groups. Notably, high-TMAO producers in urine had distinct gut microbiota beta-diversity compared to the low-TMAO producers, characterized by increased lineages of Clostridium species belonging to the Ruminococcaceae and Lachnospiraceae families within the phylum Firmicutes (146). One important unanswered question is how choline-mediated changes in gut microbiome affect intestinal resident immune cells and their capacity to maintain homeostasis and prevent excessive immune responses to commensal bacteria. The organ dysfunction associated with choline deficiency has limited the scope of human studies. As a result, most human research on disease progression has focused on dietary supplementation with different choline sources. Next, we will highlight studies that report associations between choline and inflammatory or immune responses, related molecules, or disease outcomes.
3.1 Cardiometabolic diseases
Cardiometabolic diseases include conditions such as diabetes, fatty liver disease, and cardiovascular diseases. Epidemiological studies on circulating choline metabolites and the incidence of diabetes in 3,133 individuals aged 33–45 years found that TMAO levels positively and betaine inversely correlated with the 15-year risk of incident diabetes (147). In a randomized, doubled blind placebo placebo-controlled parallel trial in 96 diabetic patients subjected to dietary supplement intervention that included choline, magnesium or both for 2 months (IRCT20110123005670N25), circulating levels of the inflammation and endothelial factors IL-6 and VCAM-1 decreased significantly in the choline and magnesium group compared to the other groups even after adjusting for potential cofounders (148).
The impact of choline and trimethylamine-N-oxidase (TMAO) on cardiovascular diseases has been investigated in both experimental and human studies. TMAO enhances cholesterol accumulation in macrophages, has been associated with increased cardiovascular disease risk (6, 149), and has a negative impact on disease outcome (150–153). Patients diagnosed with pulmonary hypertension (n=272) with high circulating choline levels (based on the 50th quartile of circulating choline levels, defined as 12.6 µM), exhibit worse key indicators of severe disease progression, including WHO functional class, higher N-terminal pro-B-type natriuretic peptide levels, and reduced cardiac output index, and predicts their prognosis (140). Plasma choline as a diagnostic biomarker for hypertension and artery stenosis was evaluated in 193 individuals, revealing that plasma choline levels were high in patients with hypertension without artery stenosis, and even higher in patients with hypertension with artery stenosis compared to healthy controls (154). The study of the impact of the Dietary Approaches to Stop Hypertension (DASH) diet on plasma choline, choline metabolites, and ceramides in obese older adults revealed a connection between choline metabolites and cardiometabolic outcomes (NCT04127240) (155). The participants in this study consumed either 3 or 6 oz of beef, with rich choline content, within a standardized DASH diet for 12 weeks (155). In response to the DASH diet, with beef intake groups combined, significant changes were observed in plasma biomarkers, including the decreased of plasma choline (by 9.6%); dimethylglycine (10%); phosphatidylcholine (PC) (51%); and triglycerides (18%); and the increase of total lysoPC (by 281%); TMAO (26.5%); total ceramide (22.1%). Around 20 LysoPC species were significantly increased, with lysoPC 16:0 being the most pronounced response. In addition, sphingomyelin 16:0, 18:0, and 18:1 increased by 10.4%, 22.5%, and 24%, respectively, and ceramide 24:1 by 36.8%; whereas sphingomyelin 24:0 significantly decreased by 10%, and ceramides 22:0 and 24:0 declined by 27.6% and 10.9%, respectively (155). These changes in choline and choline metabolites correlated with cardiometabolic outcomes, underscoring the importance of choline in older humans and the role of diet in modulating circulating lysoPC, sphingomyelin, and ceramide species (155). Recently, the study of the effects of L-alpha glycerophosphocholine, a nutritional supplement that has been demonstrated to improve neurological functions, on cardiovascular events in mice has revealed that glycerophosphocholine increases the risk of stroke by shifting the gut microbiota towards abundant Parabacteroides, Ruminococcus, and Bacteroides, while reducing the abundance of Akkermansia, Lactobacillus, and Roseburia (156). These changes reflected an increased relative abundance of choline TMA lyase (cutC). Moreover, glycerophosphocholine supplement also increased the proinflammatory effectors CXC13 and TIMP-1, and activated NF-κB and MAPK signaling pathways in human coronary artery endothelial cells (156). In mice, oral glycerophosphocholine supplementation promotes increased plasma TMAO, phenylalanine, betaine, leucine, and valine (156).
The incidence of acute myocardial infarction was examined in 1981 male patients with stable angina pectoris follow-up during 7.5 years, and subjected to a 169-item food frequency questionnaire to monitor the dietary choline intake, showed that increased intakes of energy-adjusted choline, PC, and sphingomyelin were associated with a higher risk of incident acute myocardial infarction (135). Similarly, a study of 4007 participants (average age of 63) undergoing elective diagnostic cardiac catheterization (NCT04255368) revealed higher baseline TMAO levels in individuals experiencing major adverse cardiovascular events (5.0 μM) compared to those individuals without events (3.5 μM) (157). Participants in the highest TMAO quartile (>6.18 μM) had a 2.54-fold increased hazard ratio compared to controls (157). The prognostic value of elevated plasma TMAO for cardiovascular risk remained significant in various subgroups associated with a reduced overall risk of major cardiovascular events. Notably, a three-year follow-up confirmed elevated plasma TMAO as a significant predictor of major adverse cardiovascular events (157). Another study on cardiometabolic risk factors in a diabetes case-control study (NCT02588898) and a vitamin-supplementation trial (NCT02586181) found high plasma concentrations of metabolites, including TMAO and choline, correlated with lower cholesterol and plasma phospholipid levels, suggesting TMAO may aid in cholesterol solubilization and macrophage cholesterol (158).
Several studies have reported an inverse association between dietary choline supplementation and both the incidence and severity of non-alcoholic fatty liver disease (NAFLD) or steatohepatitis (NASH) (159, 160). Steatosis, or fat accumulation in the liver, which is a direct effect of choline deficiency, can lead to inflammation and cause more severe conditions like fibrosis, cirrhosis and liver cancer (161). Findings from the Framingham Heart Study, a large community-based cohort including offspring and third-generation participants, revealed that choline intake, calculated as the sum of dietary choline-containing compounds including phosphocholine, sphingomyelin, free choline, glycerophosphocholine, and PC, was inversely associated with NAFLD risk (159). A randomized controlled clinical trial (NCT01934777) involving children with non-alcoholic steatohepatitis (NASH) evaluated the effects of combined supplementation with docasahexaenoic acid, choline, and vitamin E (DHA-CHO-VE) (160). Participants underwent a lifestyle counseling along with either a daily supplement containing DHA-CHO-VE (250 mg of DHA, 39 UI of VE, 201 mg of choline) or placebo for six months. The DHA-CHO-VE group showed a marked reduction in severe hepatic steatosis (ranging from 5-50%), alongside improved serum ALT and fasting glucose levels (159). While DHA-CHO-VE supplementation did not affect bile acid concentrations, it did increase intestinal fibroblast growth factor 19 (FGF19), a key regulator of bile acid synthesis and metabolism (160). The role of dietary choline supplementation in adult patients with metabolic syndrome was examined in a randomized crossover intervention in 23 individuals supplemented with either 3 eggs/day or 400 mg choline per day for 3 weeks, followed by a 4-week washout, and then the alternate interventions (NCT03877003) (162). During the egg-derived choline feeding phase, there was a stronger effect on metabolic and inflammatory plasma biomarkers, including an increase in monounsaturated fatty acids, vitamin D, selenium, and a decrease in CRP, IL-6, and insulin (162), while during choline bitartrate feeding, only a decrease in IL-6 was observed (162). This distinct effect may be caused by differences in the lower intestinal absorption of choline bitartrate compared to the choline from eggs (including egg-PC), or by the effect of additional nutrients in the eggs. Collectively, these findings support the idea that different sources of choline have distinct effects, suggesting that a more personalized selection of choline forms, based on individual’s underlying conditions and characteristics, may lead to greater health benefits.
3.2 Cognitive decline and Alzheimer’s disease
Recent human and animal research has emphasized the importance of choline intake in preventing neurodegenerative diseases such as Alzheimer’s disease (163). High choline intake in early life has been shown to improve outcomes in a mouse model of Alzheimer’s disease, regulating hyperexcitability, preserving hilar neurons, and enhancing spatial memory (164). Further evidence comes from studies using the 3xTg-Alzheimer’s disease mouse model, which replicates key features of human Alzheimer’s disease progression. In this model, choline-deficient diet from 3 to 12 months of age disrupted liver and heart normal function, and altered neural networks associated with microtubule stability and postsynaptic membrane regulation in the hippocampus, elevated soluble and insoluble Amyloid-β levels, increased Thioflavin S structures, and tau hyperphosphorylation at various pathological epitopes in the hippocampus and cortex (163). This system-wide dysfunction in mice fed with choline-deficient choline was observed in the circulation, as it modulates plasma proteins associated with inflammation, immune response, and metabolic processes, including insulin metabolism, mitochondrial function, inflammation, and fructose metabolic processing (163). Lifelong choline supplementation in another Alzheimer’s disease mouse model (APP/PS1 transgenic mice), significantly diminished amyloid-β plaque load and decreased activated microglia, thereby mitigating the detrimental effects of brain inflammation associated with Alzheimer’s disease (44). The use of the cholinergic neurotransmission-enhancing agent choline alphoscerate has also shown protection from antibody-mediated neurotoxicity, directly activating the a7nAChR receptor in microglia, leading to phenotype switching towards a less inflammatory state (165). Human studies further support the neuroprotective role of choline. A study with 3,224 participants found that low choline intake was associated with an increased risk of incident dementia and Alzheimer’s disease (136). Similarly, a study with 125,594 participants showed that moderate dietary choline intake, ranging from 332.89 mg/d to 353.93 mg/d, is associated with lower odds of dementia and better cognitive performance (166).
In the context of fetal alcohol spectrum disorders (FASDs), 625 mg of choline was administered to school-aged children (5–10 years old, n=29 choline and n=26 placebo) for six months (NCT01911299), showing no effect on cognitive performance (167). However, in another study in younger children with weight-adjusted dosing, the dietary choline intake increased the memory scores by 12-14% (NCT01149538 and NCT02735473) (168, 169). Indeed, an inverse relation between choline dose (mg/kg) and memory improvement suggested weight-adjusted doses as preferable to fixed doses (168). Follow-up studies for 4 and 7 years after the intervention with 500 mg/day for 9 months showed that sustained cognitive benefit was associated with potential improvements in associated white matter microstructure in the choline group (170, 171), which suggests that choline supplementation has the potential to alter brain architecture. The participant in the group supplemented with choline showed an 12-25% increase of the elicited imitation memory paradigm, 8% higher verbal IQ, 29% higher visual-spatial reasoning, 36% higher crossmodal learning, 27% higher non-verbal working memory 4 years after the treatment at a mean of 8.6 year of age. Moreover, an improvement in lower-order executive function skills (eg, information processing speed) was found in a subset of participants who returned 7 years after completing a choline trial, at a mean age of 11 years (170, 171). While these findings are promising, other studies in pediatric patients (aged 7–12 years) diagnosed with ADHD did not show statistically significant differences between the use of citicoline (cytidine diphosphate-choline supplement) and placebo. If the absence of effect is due to the choline form used, or the age of the individual is not known (172).
In the adult population, a double-blind randomized clinical trial examined choline dietary supplementation on cognitive and immune function in 97 healthy older adults over six months (NCT01672359) (173). The participants were divided into three groups: group 1 (n=33) received 120 mg/day of Ginkgo Synergy plus 700 mg/day of choline; group 2 (n=31) received 100 mg/day OPC Synergy plus Catalyn; group 3 (n=33) received a placebo (173). While no significant changes were observed in cytokine levels (IL-2, IL-6, IL-8, IL-10, IL-1α, IFN-γ, TNF-α, VEGF, and MCP1), the Ginkgo Synergy plus choline group exhibited a significant reduction (57%) in epidermal growth factor (EGF), a protein that is often overexpressed in individuals with mild cognitive impairment or Alzheimer’s disease (173). However, the absence of follow-up studies limited the ability to conclude the effect on the disease progression.
The effect on cognitive function has also been examined in 36 individuals with Type 2 diabetes mellitus and mild cognitive impairment assessed by the Mini-Mental State Examination (MMSE) score. The group provided 1200 mg/day of choline alfoscerate for 12 months, significantly showed better physical health and an increase mean difference in the MMSE score (+1.7 between the two groups), which would support its use as an adjunct therapy for managing early cognitive decline (174). The effect on physical performance has also been observed in a recent randomized, double blind, placebo-controlled crossover approach in 20 resistance-trained young males (31.3 ± 11 years) who consumed either a placebo, 630 mg alpha-glycerophosphocholine, or 315 mg of alpha-glycerophosphocholine (NCT06690619). Both groups taking alpha-glycerophosphocholine increased the cognitive performance (Stroop total score and time of completion) (175).
3.3 Inflammatory and autoimmune diseases
In inflammatory disorders, the increased metabolic demands for immune activation and cellular proliferation necessitate an elevated supply of phospholipids, such as phosphatidylcholine (PC). High plasma and tissue choline concentrations are common in diseases characterized by chronic low-grade inflammation, supporting the role of choline in modulating immune cell activation and tissue damage (176–182).
3.3.1 Autoimmune and degenerative diseases
For instance, in rheumatoid diseases, lipidomic studies in synovial fluid have reported altered phospholipid profiles with enrichment of choline-containing lipids, such as PC (183–186), which correlate with enhanced ChoKα expression and activity. This has positioned ChoKα as a major enzyme involved in the anomalous cellular lipid metabolic profile of inflammatory disorders, such as RA (25). In rheumatoid arthritis (RA), levels of PC increase in response to inflammatory mediators such as TNF-α, PDGF, and IL-1β (68). In RA, Fibroblast-like synoviocytes (FLS) contribute to synovial inflammation by producing inflammatory mediators and recruiting and activating immune cells, and ChoKα is highly expressed in both osteoarthritis and RA synovial tissue and cultured FLS (187). Exposure to inflammatory mediators such as TNF-α and PDGF, increased ChoKα expression and activity in FLS, suggesting activation of this pathway in the RA synovial environment. The inhibition of ChoKα suppressed the pathogenic behavior of RA-FLS, limiting cell migration and resistance to apoptosis, which may contribute to cartilage destruction in RA. In vivo evidence for the role of choline metabolism in RA comes from studies using the K/BxN serum-transfer mouse model of inflammatory arthritis, where treatment with the choline kinase alpha (ChoKα) inhibitor MN58b (3 mg/kg) prevented disease onset (68). Notably, when administered after disease establishment, MN58b also drastically reduced joint swelling, supporting the idea that ChoKα inhibition could serve as an effective adjuvant to current RA therapies by targeting the pathogenic activity of FLS (68).
Other recent studies have investigated the lipidomic profile of arthritis patients in different phases of the disease to understand the correlation between lipid alteration and the severity of local inflammation (188–190). Untargeted lipidomics analysis of synovial fluid and serum from RA patients across various clinical stages, ranging from preclinical to active and sustained phases, showed that despite normal erythrocyte sedimentation rate and CRP at pre-clinical stages, the lipidomic profile of preclinical RA joint fluid closely resembled that of active RA (191). Specifically, alterations in a set of lysoPC, PC, phosphatidylethanolamine, and sphingomyelin subclasses correlated with RA activity (189). Indeed, a strong association was found between lipidome profile in the arthritic joint fluids of RA patients and the severity of synovitis. The sensitivity of lipid profiles in reflecting RA activity and response to disease-modifying anti/rheumatic drugs (DMARDs) (191). Therefore, the lipidome profiles may be considered as a potential biomarker tool to predict the progression of preclinical to established RA disease and facilitate monitoring of disease activity and treatment outcomes (191).
In systemic lupus erythematosus (SLE), which is characterized by chronic activation of self-reactive lymphocytes and myeloid cells, untargeted lipidomics using LC/MS and GC/MS has revealed altered serum concentrations of choline, ACh, phosphocholine, and specific species of PC, lysoPC, and sphingomyelin (192–194). The ACh derived from fibroblastic reticular cells in the lymph nodes is an essential regulator of autoreactive B cell responses. In particular, ACh enhanced B cell differentiation into IgG-producing plasma cells by increasing lipid influx via CD36 and boosting mitochondrial respiration and fatty acid oxidation, leading to an autoreactive phenotype (195). Indeed, the hypomethylation of CD40L in T cells has also been associated with increased disease activity in SLE patients (196, 197). In female patients with SLE, the increase of 168 mg choline per day was associated with a 10% higher methylation of CD40L promoter (198), Besides these findings, it has not been evaluated whether different forms of dietary choline can modify disease progression or the frequency of flares in SLE patients, neither if the selective inhibition of ACh signaling in B cells can prevent autoreactivity and the severity of organ-specific manifestations.
3.3.2 Asthma and pulmonary disease
Studies focused on clinical outcomes in asthma patients who received dietary choline supplementation have been reported in small cohorts with opposing results. In a double-blind, placebo-controlled, crossover trial (NCT02371993) (199) on asthma in elderly individuals (n=30 participants aged >65 years), intake of 310 mg choline bitartrate twice daily for six weeks, did not show significant effects on peripheral blood eosinophil count or total serum IgE levels compared to placebo (199). However, in another study asthma patients (ages 15-45) receiving oral choline supplementation (1500 mg b.i.d.) with inhaled steroids (Budesonide; 400 μg twice daily) and long-acting β-agonist (LABA; formoterol fumarate; 6 μg twice daily) for six months (94), required less additional therapy and had improved bronchial hyperreactivity with a reduction in eosinophil count and total IgE, IL-4, IL-5, TNF-α, and airway inflammatory lipid mediators such as Cys-LT, LTb4 and 8-isoprostanes with no significant changes in IL-10 and IFN-γ, compared to the group with standard pharmacotherpay alone (94). Similarly, other studies in this same line have found a positive impact of high choline supplementation in decreasing symptom scores, the number of asymptomatic days (200). Although these findings suggest that choline can be used at higher doses as a prophylactic intervention or adjuvant to standard therapy in the management of asthma, the exact mechanism by which choline attenuates airway inflammation has not been completely explained.
3.4 Cancer progression and anti-tumor immunity
The role of choline metabolism in cancer progression and growth has been extensively studied and revised elsewhere (201, 202). Enhanced choline uptake and metabolism, and increased serum choline are hallmarks of many cancers and correlate with a higher risk of overall cancer with a poor prognosis (203, 204). Notably, elevated choline kinase alpha (ChoKα) expression and activity are often associated with malignant transformation, invasion, and metastasis in some human cancers, making ChoKα a promising therapeutic target in oncology and choline radiotracers a reasonable tool for monitoring cancer growth and therapy response (205–213). Moreover, ChoKα has been recognized as a prognostic marker in various cancers (208, 214–216). High ChoKα expression correlates with early-stage non-small-cell lung cancer (NSCLC) patients at risk of recurrence, whereas lower expression identifies patients with favorable outcomes, potentially allowing for less aggressive treatment approaches (215). Over recent decades, numerous ChoKα inhibitors have been developed and tested for cancer therapy (217–219). However, the efficacy and predictive value of choline metabolism-related signatures for patients’ prognosis, immune microenvironment, and chemotherapy response remain incompletely understood. Recent evidence using several public datasets from The Cancer Genome Atlas (TCGA), Kyoyo Encyclopedia of Genes and Genomes (KEGG), AmiGO (2) and Reactome Pathways databases has identified two choline metabolism-related genes (choline kinase β, CHKB, and phosphatidylethanolamine N-methyltransferase, PEMT) as key genes involved in the pathogenesis of human colorectal cancer (220, 221). Patients were stratified into high- and low-risk groups based on the optimal cutoff value of the choline metabolism-related risk score to assess the prognostic accuracy of the choline metabolism-related signature (222). The overall survival of patients in the high-risk group was significantly worse than that of patients in the low-risk group. The examination of sc-RNAseq revealed that CHKB expression was mainly in endothelial cells, while PEMT was highly expressed in CD4+ and CD8+ T cells (220). Indeed, there were notable differences in immune microenvironment composition, immune checkpoint gene expression, and chemotherapy response between the two risk groups (220).
Oncogenic MYC drives aberrant choline metabolism by transcriptionally upregulating CTP:phosphocholine cytidylyltransferase-α (PCYT1A) (223), a key enzyme in phosphatidylcholine (PC) de novo biosynthesis. In patients with diffuse large B-cell lymphoma (DLBCL), elevated PCYT1A expression, accompanied by increased MYC levels and decreased serum PC, correlates with a higher international prognostic index risk classification, suggesting that co-expression of MYC and PCYT1A may serve as a biomarker for disease progression (223). The use of histone deacetylase inhibitors (HDACI) modulates lipid metabolism and survival pathways in DLBCL, particularly by gaining dependency on the choline pathway and PI3K signaling activation (224), which results in a decline in the antineoplastic effects of the HDACI (224). In part, the aberrant choline metabolism in cancer is driven by molecular alterations in enzymes such as phospholipases C and D, ethanolamine kinase-α, glycerophosphocholine phosphodiesterases, and choline transporters (211). Tumors exhibit elevated phospholipid levels, characterized by an increase in phosphocholine and total choline-containing metabolites, along with an altered glycerophosphocholine/phosphocholine ratio (208, 210, 225, 226). As the major phospholipid in eukaryotic membranes, PC is essential for cancer cell proliferation, tumor progression, and invasion (209). Increased total choline signal detected by 1H nuclear magnetic resonance spectroscopy (1H-NMR) is currently being considered as a diagnostic marker in multiple cancers (137, 210, 227, 228). Choline plays a critical role in cancer diagnosis and monitoring. The use of (11) C-choline positron emission tomography/computed tomography (PET/TC) has provided insights into choline metabolism in tumors, including lung, liver, ovarian, and prostate cancers (216, 229–234). Both (1)H-NMR and choline PET imaging are being explored to evaluate treatment responses. The increased expression and activity of choline transporters and enzymes, such as CTL1 and ChoKα, respectively, have led to the development of radiolabeled choline analogs as PET imaging tracers (211).
In the INSPIRE cohort of 106 patients with advanced solid tumors treated with pembrolizumab (anti-PD-1 antibody) (NCT03702309) (235), higher serum choline levels were associated with augmented CD8- T cells and B cells in the tumor stroma, as well as an elevated CD3+CD8+ cytotoxic T cells to CD3+CD8-FOXP3+ T regulatory cells ratio in the tumors (235). A validation study on 51 patients receiving anti-PD-1 therapy alone or in combination (NCT02644369) (235), found no association between choline levels and changes in absolute neutrophil count (ΔANC), lymphocytes, and neutrophil-to-lymphocyte ratio (ΔNLR) (235). Notably, higher serum choline levels correlated with improved progression-free survival and a trend toward better overall survival (235). A case-control study (NCT00794885), suggested that high serum choline levels were linked to overall cancer risk, particularly among older male smokers (204). A case-control study investigating the differences in circulating concentrations of choline metabolites between elderly men with prostate cancer or benign prostatic hyperplasia (236). While no significant differences were found in choline, betaine, dimethylglycine, folate, total homocysteine, cystathionine, methylmalonic acid, S-adenosylhomocysteine (SAH), S-adenosylmethionine (SAM), the presence of 11 sphingomyelin species was significantly reduced in patients with prostate cancer as compared to benign hyperplasia (236).
In triple-negative breast cancer, a very aggressive disease with a poor prognosis, the commensal microbiota Clostridiales and its metabolite trimethylamine-N-oxide (TMAO) colonize the mammary gland, being more abundant in tumors exhibiting an activated immune microenvironment (237). TMAO was identified as a driver of antitumor immunity, providing a foundation for potential TMAO-based therapeutic strategies. Patients with higher plasma TMAO levels achieved better responses to immunotherapy due to TMAO-induced pyroptosis in tumor cells and enhanced CD8+ T cell-mediated antitumor immunity (237). An unbiased metabolic screening using liquid chromatography-tandem mass spectrometry identified TMAO, as a key gut microbiome-derived metabolite that enhances antitumor immunity in pancreatic ductal adenocarcinoma (PDAC) (238). Administration of TMAO, either intraperitoneally or via dietary choline supplement, in PDAC-bearing mice significantly reduced tumor growth. This antitumor effect was associated with a shift toward an immunostimulatory tumor-associated macrophage (TAM) phenotype and an enhanced effector T cell response within the tumor microenvironment, mediated by activation of the type I Interferon pathway (238). Consistently, delivering intravenously TMAO-primed macrophages yields similar tumor-suppressive effects (238). Indeed, combining TMAO with immune checkpoint blockade therapy, such as anti-PD1 and anti-TIM3 antibodies, further reduced tumor burden and improved survival compared to monotherapies alone. Additionally, higher levels of gut bacteria expressing CutC, the enzyme responsible for generating trimethylamine, correlated with longer survival in PDAC patients and enhanced responses to anti-PD1 immunotherapy in melanoma patients (238). Collectively, these findings highlight TMAO as a promoter of antitumor immunity across multiple cancer types. By enhancing immune cell function, reshaping the tumor microenvironment, and boosting responses to immunotherapy, TMAO holds promise as a potential adjuvant to improve the efficacy of cancer immunotherapy.
3.5 Sepsis
Sepsis is a life-threatening clinical condition arising from an excessive immune response to infection, leading to high morbidity and mortality rates (239). A major challenge in intensive care medicine is managing severe infections with multiple organ dysfunction, commonly referred to as sepsis shock (240). The Systemic Inflammatory Response Syndrome (SIRS) is triggered by endotoxin, which stimulates the release of inflammatory cytokines, such as TNF-α; IL-1α/β, and IL-17 into the circulation. These cytokines, in turn, prompt hepatic cells to release acute-phase proteins such as CRP for immunological regulation (241). Matrix metalloproteinases (MMPs) are up-regulated by pro-inflammatory cytokines, as well as by acute phase proteins like serum amyloid A (SAA), with counter-regulatory inhibition by IL-4 and IL-13, and released into the circulation from damaged vascular endothelium. An increased MMPs/tissue inhibitor MMPs (TIMPs) ratio is more strongly associated with tissue response to LPS-linked injury than with the acute-phase reaction (242, 243). Choline-deficient diet has been linked to increased hepatic injury and mortality in endotoxemic shock (239). In a canine model of sepsis induced by intravenous injection of 0.2 mg/kg LPS from E. coli, choline intravenous administration suppressed the elevation of circulating MMPs and TIMPs, preserved serum IgG and IgM levels, and was associated with reduced acute-phase reaction and multi-organ failure (244).
In a study using the gold-standard experimental model of polymicrobial sepsis, the cecal ligation and puncture (245), metabolic analysis of plasma and urine identified 14 plasma and 11 urine metabolites related to central carbon and choline metabolism (including choline, betaine, methylamine, and creatinine). These metabolites significantly differed between septic mice with and without signs of acute kidney injury (AKI), as defined by Kidney Disease Improving Global Outcomes (KDIGO), as creatinine levels tripled the average of sham mice. These were further validated in 7 pediatric patients with severe sepsis and AKI, and 13 with sepsis with stage 1 or no AKI. Plasma analysis revealed 10 differentially expressed metabolites more abundant in sepsis-associated AKI patients, including succinate, pyruvate, lactate, betaine, dimethylglycine, and choline, resembling the findings in mouse plasma (245). These metabolic changes were supported by alterations in the gene expression of choline metabolism-related enzymes, such as choline dehydrogenase (Chdh), betaine-homocysteine methyltransferase (Bhmt), and DMG dehydrogenase (Dmgdh), and glycerophosphocholine phosphodiesterase 1 (Gpcpd1). Notably, flavin-containing monooxygenase 3 (Fmo3), the enzyme responsible for TMAO production in the liver, was reduced in sepsis-associated AKI. Mice that received choline intraperitoneally before and during sepsis exhibit lower levels of plasma blood urea nitrogen (BUN) and creatinine, as well as urine neutrophil gelatinase-associated lipocalin (NGAL) levels compared to septic mice treated with vehicle. While these findings support that choline may protect the kidneys during sepsis, the survival rate was not affected by choline supplementation (245). A metabolic study using NMR spectroscopy found distinct metabolic profiles between sepsis survivors and non-survivors, those who succumbed at day 0 of hospitalization. Non-survivors exhibited significantly higher levels of creatine, phosphocreatine, choline, betaine, tyrosine, histidine, and phenylalanine compared to survivors (246). However, another study reported that individuals with sepsis had lower concentrations of phosphatidylcholine (PC), phosphatidylserine, lysophosphatidylethanolamine, and lysoPC but higher creatinine and C17-sphinganine, compared to healthy controls (247).
Animal models have demonstrated that choline supplementation improves survival and reduces TNF-α production (248). In endotoxin-infused mice, free serum choline levels dropped by 49% at low endotoxin administration but increased by 98% within 48 hours after high endotoxin exposure (1 mg/kg). Despite these fluctuations, phospholipid-bound choline levels increased regardless of the endotoxin dose, accompanied by an increase in biochemical markers of tissue injury and organ dysfunction (249). However, another study using the CLP mouse model found that administering choline before and after sepsis initiation reduced serum inflammatory mediators such as TNF-α and HMGB1, and improved survival via the α7nAChR-dependent mechanism (250, 251). The anti-inflammatory effect of choline was confirmed ex vivo, where endotoxin-activated human whole blood and macrophages exposed to supraphysiological choline concentrations (between 1–50 mM) exhibited reduced TNF-α production (250). Additionally, elevated plasma choline acetyltransferase (ChAT) concentrations were observed in patients with sepsis, with the highest levels detected in those who succumbed to infection (252). The increase in circulating ChAT coincides with the decline in TNF-α (252), showing its potential anti-inflammatory. However, ChAT activity did not correlate with circulating choline or acetylcholine (ACh) concentration, which suggests additional regulatory mechanisms.
Sepsis is characterized by profound metabolic alterations, with choline metabolism playing a key role in inflammatory regulation and organ function. While experimental and clinical data suggest that choline supplementation may protect against sepsis-associated AKI and modulate inflammation, its effect on overall sepsis survival remains inconclusive. Further research is needed to determine the therapeutic potential of choline in sepsis management, particularly in modulating immune responses and metabolic homeostasis.
4 Conclusion
Choline metabolism plays a critical role in shaping both the innate and adaptive immune responses, with its effect varying by cell type, metabolite state, and immune environment. While choline shows therapeutic potential, such as reducing inflammation and supporting cognitive and liver health, it can also contribute to disease, including increased risk of cardiovascular events or cancer progression. Despite the growing interest in examining the specific impact of choline on the activation and function of immune cells, the precise downstream molecular signaling and pathways regulated by choline availability remain elusive. Collectively, animal and human studies investigating dietary choline supplementation emphasize the idea of divergent effects based on the choline source, the subject’s genetic and physiological characteristics, and the tissue or cell type studied, suggesting that a more context-specific selection of choline forms may lead to greater health benefits.
Future clinical studies should account for baseline choline status, microbiome profiles, genetic polymorphism in choline metabolic pathways, and organ-specific outcomes to define optimal strategies for leveraging the use of modulating choline availability and metabolism in personalized therapeutic interventions.
Author contributions
CM: Writing – original draft, Investigation, Resources, Writing – review & editing, Conceptualization. CF: Writing – original draft, Investigation, Resources, Conceptualization, Writing – review & editing. ES-L: Methodology, Data curation, Resources, Visualization, Project administration, Conceptualization, Validation, Funding acquisition, Investigation, Supervision, Writing – original draft, Software, Writing – review & editing, Formal Analysis.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. ES-L receives support from NIAMS/NIH (K01AR077111).
Acknowledgments
The authors thank the Department of Orthopedic Surgery for the academic and administrative support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Newsholme P. Cellular and metabolic mechanisms of nutrient actions in immune function. Nutr Diabetes. (2021) 11:22. doi: 10.1038/s41387-021-00162-3
2. Munteanu C and Schwartz B. The relationship between nutrition and the immune system. Front Nutr. (2022) 9:1082500. doi: 10.3389/fnut.2022.1082500
3. Jieru P, Zhang S, Cai L, Long W, Wang Y, Zhang L, et al. Dietary choline intake and health outcomes in U.S. adults: exploring the impact on cardiovascular disease, cancer prevalence, and all-cause mortality. J Health Popul Nutr. (2024) 43:59. doi: 10.1186/s41043-024-00528-0
4. Gallo M and Gamiz F. Choline: an essential nutrient for human health. Nutrients. (2023) 15(13):2900. doi: 10.3390/nu15132900
5. Rath S, Rud T, Pieper DH, and Vital M. Potential TMA-producing bacteria are ubiquitously found in mammalia. Front Microbiol. (2019) 10:2966. doi: 10.3389/fmicb.2019.02966
6. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. (2011) 472:57–63. doi: 10.1038/nature09922
7. Romano KA, Vivas EI, Amador-Noguez D, and Rey FE. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio. (2015) 6:e02481. doi: 10.1128/mBio.02481-14
8. Arias N, Arboleya S, Allison J, Kaliszewska A, Higarza SG, Gueimonde M, et al. The relationship between choline bioavailability from diet, intestinal microbiota composition, and its modulation of human diseases. Nutrients. (2020) 12(8):2340. doi: 10.3390/nu12082340
9. Shih DM, Wang Z, Lee R, Meng Y, Che N, Charugundla S, et al. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J Lipid Res. (2015) 56:22–37. doi: 10.1194/jlr.M051680
10. Yang T, Nian Y, Lin H, Li J, Lin X, Li T, et al. Structure and mechanism of the osmoregulated choline transporter BetT. Sci Adv. (2024) 10:eado6229. doi: 10.1126/sciadv.ado6229
11. Wargo MJ. Homeostasis and catabolism of choline and glycine betaine: lessons from Pseudomonas aeruginosa. Appl Environ Microbiol. (2013) 79:2112–20. doi: 10.1128/AEM.03565-12
12. Salvano MA, Lisa TA, and Domenech CE. Choline transport in Pseudomonas aeruginosa. Mol Cell Biochem. (1989) 85:81–9. doi: 10.1007/BF00223517
13. Michel V, Yuan Z, Ramsubir S, and Bakovic M. Choline transport for phospholipid synthesis. Exp Biol Med (Maywood). (2006) 231:490–504. doi: 10.1177/153537020623100503
14. Michel V and Bakovic M. The solute carrier 44A1 is a mitochondrial protein and mediates choline transport. FASEB J. (2009) 23:2749–58. doi: 10.1096/fj.08-121491
15. Hedtke V and Bakovic M. Choline transport for phospholipid synthesis: An emerging role of choline transporter-like protein 1. Exp Biol Med (Maywood). (2019) 244:655–62. doi: 10.1177/1535370219830997
16. Nakata T, Matsui T, Kobayashi K, Kobayashi Y, and Anzai N. Organic cation transporter 2 (SLC22A2), a low-affinity and high-capacity choline transporter, is preferentially enriched on synaptic vesicles in cholinergic neurons. Neuroscience. (2013) 252:212–21. doi: 10.1016/j.neuroscience.2013.08.011
17. Bernard DJ, Pangilinan F, Mendina C, Desporte T, Wincovitch SM, Walsh DJ, et al. SLC25A48 influences plasma levels of choline and localizes to the inner mitochondrial membrane. Mol Genet Metab. (2024) 143:108518. doi: 10.1016/j.ymgme.2024.108518
18. Patil S, Borisov O, Scherer N, Wirth C, Schlosser P, Wuttke M, et al. The membrane transporter SLC25A48 enables transport of choline into human mitochondria. Kidney Int. (2024) 107(2):296–301. doi: 10.1016/j.kint.2024.06.022
19. Verkerke ARP, Shi X, Li M, Higuchi Y, Yamamuro T, Katoh D, et al. SLC25A48 controls mitochondrial choline import and metabolism. Cell Metab. (2024) 36:2156–2166.e2159. doi: 10.1016/j.cmet.2024.07.010
20. Khan A, Unlu G, Lin P, Liu Y, Kilic E, Kenny TC, et al. Metabolic gene function discovery platform GeneMAP identifies SLC25A48 as necessary for mitochondrial choline import. Nat Genet. (2024) 56:1614–23. doi: 10.1038/s41588-024-01827-2
21. Chiuve SE, Giovannucci EL, Hankinson SE, Zeisel SH, Dougherty LW, Willett WC, et al. The association between betaine and choline intakes and the plasma concentrations of homocysteine in women. Am J Clin Nutr. (2007) 86:1073–81. doi: 10.1093/ajcn/86.4.1073
22. Wiedeman AM, Barr SI, Green TJ, Xu Z, Innis SM, and Kitts DD. Dietary choline intake: current state of knowledge across the life cycle. Nutrients. (2018) 10(10):1513. doi: 10.3390/nu10101513
23. Zeisel SH. Dietary choline deficiency causes DNA strand breaks and alters epigenetic marks on DNA and histones. Mutat Res. (2012) 733:34–8. doi: 10.1016/j.mrfmmm.2011.10.008
24. Stead LM, Brosnan JT, Brosnan ME, Vance DE, and Jacobs RL. Is it time to reevaluate methyl balance in humans? Am J Clin Nutr. (2006) 83:5–10. doi: 10.1093/ajcn/83.1.5
25. Miriam Jose A and Rasool M. Choline kinase: An underappreciated rheumatoid arthritis therapeutic target. Life Sci. (2022) 309:121031. doi: 10.1016/j.lfs.2022.121031
26. Gibellini F and Smith TK. The Kennedy pathway–De novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life. (2010) 62:414–28. doi: 10.1002/iub.337
27. Aoyama C, Liao H, and Ishidate K. Structure and function of choline kinase isoforms in mammalian cells. Prog Lipid Res. (2004) 43:266–81. doi: 10.1016/j.plipres.2003.12.001
28. Saito RF, Andrade LNS, Bustos SO, and Chammas R. Phosphatidylcholine-derived lipid mediators: the crosstalk between cancer cells and immune cells. Front Immunol. (2022) 13:768606. doi: 10.3389/fimmu.2022.768606
29. Gu X and Wang X. An overview of recent analysis and detection of acetylcholine. Anal Biochem. (2021) 632:114381. doi: 10.1016/j.ab.2021.114381
30. Olofsson PS, Steinberg BE, Sobbi R, Cox MA, Ahmed MN, Oswald M, et al. Blood pressure regulation by CD4(+) lymphocytes expressing choline acetyltransferase. Nat Biotechnol. (2016) 34:1066–71. doi: 10.1038/nbt.3663
31. Cox MA, Duncan GS, Lin GHY, Steinberg BE, Yu LX, Brenner D, et al. Choline acetyltransferase-expressing T cells are required to control chronic viral infection. Science. (2019) 363:639–44. doi: 10.1126/science.aau9072
32. Jiang W, Li D, Han R, Zhang C, Jin WN, Wood K, et al. Acetylcholine-producing NK cells attenuate CNS inflammation via modulation of infiltrating monocytes/macrophages. Proc Natl Acad Sci U S A. (2017) 114:E6202–11. doi: 10.1073/pnas.1705491114
33. Rosas-Ballina M, Olofsson PS, Ochani M, Valdes-Ferrer SI, Levine YA, Reardon C, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. (2011) 334:98–101. doi: 10.1126/science.1209985
34. Salamone G, Lombardi G, Gori S, Nahmod K, Jancic C, Amaral MM, et al. Cholinergic modulation of dendritic cell function. J Neuroimmunol. (2011) 236:47–56. doi: 10.1016/j.jneuroim.2011.05.007
35. Zeisel SH and Niculescu MD. Perinatal choline influences brain structure and function. Nutr Rev. (2006) 64:197–203. doi: 10.1111/j.1753-4887.2006.tb00202.x
36. Mehedint MG, Craciunescu CN, and Zeisel SH. Maternal dietary choline deficiency alters angiogenesis in fetal mouse hippocampus. Proc Natl Acad Sci U S A. (2010) 107:12834–9. doi: 10.1073/pnas.0914328107
37. Fisher MC, Zeisel SH, Mar MH, and Sadler TW. Perturbations in choline metabolism cause neural tube defects in mouse embryos in vitro. FASEB J. (2002) 16:619–21. doi: 10.1096/fj.01-0564fje
38. Bahnfleth CL, Strupp BJ, Caudill MA, and Canfield RL. Prenatal choline supplementation improves child sustained attention: A 7-year follow-up of a randomized controlled feeding trial. FASEB J. (2022) 36:e22054. doi: 10.1096/fj.202101217R
39. Maurer SV, Kong C, Terrando N, and Williams CL. Dietary choline protects against cognitive decline after surgery in mice. Front Cell Neurosci. (2021) 15:671506. doi: 10.3389/fncel.2021.671506
40. Mellott TJ, Huleatt OM, Shade BN, Pender SM, Liu YB, Slack BE, et al. Perinatal choline supplementation reduces amyloidosis and increases choline acetyltransferase expression in the hippocampus of the APPswePS1dE9 alzheimer’s disease model mice. PLoS One. (2017) 12:e0170450. doi: 10.1371/journal.pone.0170450
41. Mone P, Trimarco V, Kansakar U, Izzo R, Santulli G, and Trimarco B. Combining choline bitartrate and vitamin B12 ameliorates cognitive impairment in hypertensive elders with cognitive frailty. Pharmacol Res. (2024) 201:107103. doi: 10.1016/j.phrs.2024.107103
42. Pyapali GK, Turner DA, Williams CL, Meck WH, and Swartzwelder HS. Prenatal dietary choline supplementation decreases the threshold for induction of long-term potentiation in young adult rats. J Neurophysiol. (1998) 79:1790–6. doi: 10.1152/jn.1998.79.4.1790
43. Shahraki S, Esmaeilpour K, Shabani M, Sepehri G, Rajizadeh MA, Maneshian M, et al. Choline chloride modulates learning, memory, and synaptic plasticity impairments in maternally separated adolescent male rats. Int J Dev Neurosci. (2022) 82:19–38. doi: 10.1002/jdn.10155
44. Velazquez R, Ferreira E, Knowles S, Fux C, Rodin A, Winslow W, et al. Lifelong choline supplementation ameliorates Alzheimer’s disease pathology and associated cognitive deficits by attenuating microglia activation. Aging Cell. (2019) 18:e13037. doi: 10.1111/acel.13037
45. Velazquez R, Ferreira E, Winslow W, Dave N, Piras IS, Naymik M, et al. Maternal choline supplementation ameliorates Alzheimer’s disease pathology by reducing brain homocysteine levels across multiple generations. Mol Psychiatry. (2020) 25:2620–9. doi: 10.1038/s41380-018-0322-z
46. Wang Y, Guan X, Chen X, Cai Y, Ma Y, Ma J, et al. Choline supplementation ameliorates behavioral deficits and alzheimer’s disease-like pathology in transgenic APP/PS1 mice. Mol Nutr Food Res. (2019) 63:e1801407. doi: 10.1002/mnfr.201801407
47. Zhang M, Han X, Bao J, Yang J, Shi SQ, Garfield RE, et al. Choline supplementation during pregnancy protects against gestational lipopolysaccharide-induced inflammatory responses. Reprod Sci. (2018) 25:74–85. doi: 10.1177/1933719117702247
48. Lewis ED, Richard C, Goruk S, Dellschaft NS, Curtis JM, Jacobs RL, et al. The form of choline in the maternal diet affects immune development in suckled rat offspring. J Nutr. (2016) 146:823–30. doi: 10.3945/jn.115.225888
49. Baker JA, Breit KR, Bodnar TS, Weinberg J, and Thomas JD. Choline supplementation modifies the effects of developmental alcohol exposure on immune responses in adult rats. Nutrients. (2022) 14(14):2868. doi: 10.3390/nu14142868
50. Kwan STC, Ricketts DK, Presswood BH, Smith SM, and Mooney SM. Prenatal choline supplementation during mouse pregnancy has differential effects in alcohol-exposed fetal organs. Alcohol Clin Exp Res. (2021) 45:2471–84. doi: 10.1111/acer.14730
51. Guerrerio AL, Colvin RM, Schwartz AK, Molleston JP, Murray KF, Diehl A, et al. Choline intake in a large cohort of patients with nonalcoholic fatty liver disease. Am J Clin Nutr. (2012) 95:892–900. doi: 10.3945/ajcn.111.020156
52. Fischer LM, daCosta KA, Kwock L, Stewart PW, Lu TS, Stabler SP, et al. Sex and menopausal status influence human dietary requirements for the nutrient choline. Am J Clin Nutr. (2007) 85:1275–85. doi: 10.1093/ajcn/85.5.1275
53. Resseguie M, Song J, Niculescu MD, da Costa KA, Randall TA, and Zeisel SH. Phosphatidylethanolamine N-methyltransferase (PEMT) gene expression is induced by estrogen in human and mouse primary hepatocytes. FASEB J. (2007) 21:2622–32. doi: 10.1096/fj.07-8227com
54. Liu L, Qiao S, Zhuang L, Xu S, Chen L, Lai Q, et al. Choline intake correlates with cognitive performance among elder adults in the United States. Behav Neurol. (2021) 2021:2962245. doi: 10.1155/2021/2962245
55. Bjelland I, Tell GS, Vollset SE, Konstantinova S, and Ueland PM. Choline in anxiety and depression: the Hordaland Health Study. Am J Clin Nutr. (2009) 90:1056–60. doi: 10.3945/ajcn.2009.27493
56. Li J, Kang X, Zhang L, Luo J, and Zhang D. Dietary choline is inversely associated with depressive symptoms: A cross-sectional study of the National Health and Nutrition Examination Survey (NHANES) 2011 to 2018. J Affect Disord. (2022) 301:23–9. doi: 10.1016/j.jad.2022.01.013
57. Elango R. Tolerable upper intake level for individual amino acids in humans: A narrative review of recent clinical studies. Adv Nutr. (2023) 14:885–94. doi: 10.1016/j.advnut.2023.04.004
58. Wortmann SB and Mayr JA. Choline-related-inherited metabolic diseases-A mini review. J Inherit Metab Dis. (2019) 42:237–42. doi: 10.1002/jimd.12011
59. Zeisel SH and da Costa KA. Choline: an essential nutrient for public health. Nutr Rev. (2009) 67:615–23. doi: 10.1111/j.1753-4887.2009.00246.x
60. Bernhard W, Bockmann KA, Minarski M, Wiechers C, Busch A, Bach D, et al. Evidence and perspectives for choline supplementation during parenteral nutrition-A narrative review. Nutrients. (2024) 16(12):1873. doi: 10.3390/nu16121873
61. Shronts EP. Essential nature of choline with implications for total parenteral nutrition. J Am Diet Assoc. (1997) 97:639–646, 649; quiz 647-638. doi: 10.1016/S0002-8223(97)00161-2
62. Buchman AL. The addition of choline to parenteral nutrition. Gastroenterology. (2009) 137:S119–128. doi: 10.1053/j.gastro.2009.08.010
63. Romano KA, Martinez-Del Campo A, Kasahara K, Chittim CL, Vivas EI, Amador-Noguez D, et al. Metabolic, epigenetic, and transgenerational effects of gut bacterial choline consumption. Cell Host Microbe. (2017) 22:279–290 e277. doi: 10.1016/j.chom.2017.07.021
64. Galvan-Pena S and O’Neill LA. Metabolic reprograming in macrophage polarization. Front Immunol. (2014) 5:420. doi: 10.3389/fimmu.2014.00420
65. Peyssonnaux C, Datta V, Cramer T, Doedens A, Theodorakis EA, Gallo RL, et al. HIF-1alpha expression regulates the bactericidal capacity of phagocytes. J Clin Invest. (2005) 115:1806–15. doi: 10.1172/JCI23865
66. Sieow JL, Gun SY, and Wong SC. The sweet surrender: how myeloid cell metabolic plasticity shapes the tumor microenvironment. Front Cell Dev Biol. (2018) 6:168. doi: 10.3389/fcell.2018.00168
67. Ghorbani P, Kim SY, Smith TKT, Minarrieta L, Robert-Gostlin V, Kilgour MK, et al. Choline metabolism underpins macrophage IL-4 polarization and RELMalpha up-regulation in helminth infection. PLoS Pathog. (2023) 19:e1011658. doi: 10.1371/journal.ppat.1011658
68. Guma M, Sanchez-Lopez E, Lodi A, Garcia-Carbonell R, Tiziani S, Karin M, et al. Choline kinase inhibition in rheumatoid arthritis. Ann Rheum Dis. (2015) 74:1399–407. doi: 10.1136/annrheumdis-2014-205696
69. Sanchez-Lopez E, Zhong Z, Stubelius A, Sweeney SR, Booshehri LM, Antonucci L, et al. Choline uptake and metabolism modulate macrophage IL-1beta and IL-18 production. Cell Metab. (2019) 29:1350–1362 e1357. doi: 10.1016/j.cmet.2019.03.011
70. Snider SA, Margison KD, Ghorbani P, LeBlond ND, Nunes JRC, et al. Choline transport links macrophage phospholipid metabolism and inflammation. J Biol Chem. (2018) 293:11600–11. doi: 10.1074/jbc.RA118.003180
71. Petkevicius K, Virtue S, Bidault G, Jenkins B, Cubuk C, Morgantini C, et al. Accelerated phosphatidylcholine turnover in macrophages promotes adipose tissue inflammation in obesity. Elife. (2019) 8:e47990. doi: 10.7554/eLife.47990
72. Yuan ZH, Feng L, Jiang WD, Wu P, Liu Y, Jiang J, et al. Dietary choline-enhanced skin immune response of juvenile grass carp might be related to the STAT3 and NF-kB signaling pathway (Ctenopharyngodon idella). Front Nutr. (2021) 8:652767. doi: 10.3389/fnut.2021.652767
73. Azarcoya-Barrera J, Lewis ED, Field CJ, Goruk S, Makarowski A, Pouliot Y, et al. The lipid-soluble forms of choline enhance ex vivo responses from the gut-associated immune system in young female rat offspring. J Nutr. (2022) 152:2604–14. doi: 10.1093/jn/nxac180
74. Caudill MA. Pre- and postnatal health: evidence of increased choline needs. J Am Diet Assoc. (2010) 110:1198–206. doi: 10.1016/j.jada.2010.05.009
75. Dellschaft NS, Richard C, Lewis ED, Goruk S, Jacobs RL, Curtis JM, et al. The dietary form of choline during lactation affects maternal immune function in rats. Eur J Nutr. (2018) 57:2189–99. doi: 10.1007/s00394-017-1493-0
76. Udalova IA, Mantovani A, and Feldmann M. Macrophage heterogeneity in the context of rheumatoid arthritis. Nat Rev Rheumatol. (2016) 12:472–85. doi: 10.1038/nrrheum.2016.91
77. Bohdanowicz M and Grinstein S. Role of phospholipids in endocytosis, phagocytosis, and macropinocytosis. Physiol Rev. (2013) 93:69–106. doi: 10.1152/physrev.00002.2012
78. Tian Y, Pate C, Andreolotti A, Wang L, Tuomanen E, Boyd K, et al. Cytokine secretion requires phosphatidylcholine synthesis. J Cell Biol. (2008) 181:945–57. doi: 10.1083/jcb.200706152
79. Hsieh WY, Zhou QD, York AG, Williams KJ, Scumpia PO, Kronenberger EB, et al. Toll-like receptors induce signal-specific reprogramming of the macrophage lipidome. Cell Metab. (2020) 32:128–143 e125. doi: 10.1016/j.cmet.2020.05.003
80. Wu K, Yuan Y, Yu H, Dai X, Wang S, Sun Z, et al. The gut microbial metabolite trimethylamine N-oxide aggravates GVHD by inducing M1 macrophage polarization in mice. Blood. (2020) 136:501–15. doi: 10.1182/blood.2019003990
81. Ho JL, Zhu B, He S, Du B, and Rothman R. Interleukin 4 receptor signaling in human monocytes and U937 cells involves the activation of a phosphatidylcholine-specific phospholipase C: a comparison with chemotactic peptide, FMLP, phospholipase D, and sphingomyelinase. J Exp Med. (1994) 180:1457–69. doi: 10.1084/jem.180.4.1457
82. Garcia M, Mamedova LK, Barton B, and Bradford BJ. Choline regulates the function of bovine immune cells and alters the mRNA abundance of enzymes and receptors involved in its metabolism in vitro. Front Immunol. (2018) 9:2448. doi: 10.3389/fimmu.2018.02448
83. Luo S, Lin H, Wu C, Zhu L, Hua Q, Weng Y, et al. Cholinergic macrophages promote the resolution of peritoneal inflammation. Proc Natl Acad Sci U S A. (2024) 121:e2402143121. doi: 10.1073/pnas.2402143121
84. de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. (2005) 6:844–51. doi: 10.1038/ni1229
85. Tarnawski L, Reardon C, Caravaca AS, Rosas-Ballina M, Tusche MW, Drake AR, et al. Adenylyl cyclase 6 mediates inhibition of TNF in the inflammatory reflex. Front Immunol. (2018) 9:2648. doi: 10.3389/fimmu.2018.02648
86. Rosas-Ballina M, Goldstein RS, Gallowitsch-Puerta M, Yang L, Valdes-Ferrer SI, Patel NB, et al. The selective alpha7 agonist GTS-21 attenuates cytokine production in human whole blood and human monocytes activated by ligands for TLR2, TLR3, TLR4, TLR9, and RAGE. Mol Med. (2009) 15:195–202. doi: 10.2119/molmed.2009.00039
87. Lewis ED, Richard C, Goruk S, Wadge E, Curtis JM, Jacobs RL, et al. Feeding a mixture of choline forms during lactation improves offspring growth and maternal lymphocyte response to ex vivo immune challenges. Nutrients. (2017) 9(7):713. doi: 10.3390/nu9070713
88. Li Q, Sun X, Yu K, Lv J, Miao C, Yang J, et al. Enterobacter ludwigii protects DSS-induced colitis through choline-mediated immune tolerance. Cell Rep. (2022) 40:111308. doi: 10.1016/j.celrep.2022.111308
89. Lee M, Lee SY, and Bae YS. Emerging roles of neutrophils in immune homeostasis. BMB Rep. (2022) 55:473–80. doi: 10.5483/BMBRep.2022.55.10.115
90. Lu BZ. Identification of H1 and H2 receptors in the lung tissue of the Guinea pig and their changes in experimental allergic asthma. Zhonghua Yi Xue Za Zhi. (1987) 67:313–316, 320.
91. Tronchere H, Planat V, Record M, Terce F, Ribbes G, and Chap H. Phosphatidylcholine turnover in activated human neutrophils. Agonist-induced cytidylyltransferase translocation is subsequent to phospholipase D activation. J Biol Chem. (1995) 270:13138–46. doi: 10.1074/jbc.270.22.13138
92. Pedruzzi E, Hakim J, Giroud JP, and Perianin A. Analysis of choline and phosphorylcholine content in human neutrophils stimulated by f-Met-Leu-Phe and phorbol myristate acetate: contribution of phospholipase D and C. Cell Signal. (1998) 10:481–9. doi: 10.1016/s0898-6568(97)00174-5
93. Mehta AK, Gaur SN, Arora N, and Singh BP. Effect of choline chloride in allergen-induced mouse model of airway inflammation. Eur Respir J. (2007) 30:662–71. doi: 10.1183/09031936.00019307
94. Mehta AK, Singh BP, Arora N, and Gaur SN. Choline attenuates immune inflammation and suppresses oxidative stress in patients with asthma. Immunobiology. (2010) 215:527–34. doi: 10.1016/j.imbio.2009.09.004
95. Kwatia MA, Doyle CB, Cho W, Enhorning G, and Ackerman SJ. Combined activities of secretory phospholipases and eosinophil lysophospholipases induce pulmonary surfactant dysfunction by phospholipid hydrolysis. J Allergy Clin Immunol. (2007) 119:838–47. doi: 10.1016/j.jaci.2006.12.614
96. Zhang L, Zhang J, and You Z. Switching of the microglial activation phenotype is a possible treatment for depression disorder. Front Cell Neurosci. (2018) 12:306. doi: 10.3389/fncel.2018.00306
97. Gamage R, Wagnon I, Rossetti I, Childs R, Niedermayer G, Chesworth R, et al. Cholinergic modulation of glial function during aging and chronic neuroinflammation. Front Cell Neurosci. (2020) 14:577912. doi: 10.3389/fncel.2020.577912
98. Ke P, Shao BZ, Xu ZQ, Chen XW, Wei W, and Liu C. Activating alpha7 nicotinic acetylcholine receptor inhibits NLRP3 inflammasome through regulation of beta-arrestin-1. CNS Neurosci Ther. (2017) 23:875–84. doi: 10.1111/cns.12758
99. Okada T, Muto E, Yamanaka T, Uchino H, and Inazu M. Functional expression of choline transporters in microglia and their regulation of microglial M1/M2 polarization. Int J Mol Sci. (2022) 23(16):8924. doi: 10.3390/ijms23168924
100. Muto E, Okada T, Yamanaka T, Uchino H, Inazu M, and Licochalcone E. a beta-Amyloid Aggregation Inhibitor, Regulates Microglial M1/M2 Polarization via Inhibition of CTL1-Mediated Choline Uptake. Biomolecules. (2023) 13(2):191. doi: 10.3390/biom13020191
101. Gaudioso A, Moreno-Huguet P, Casas J, Schuchman EH, and Ledesma MD. Modulation of dietary choline uptake in a mouse model of acid sphingomyelinase deficiency. Int J Mol Sci. (2023) 24(11):9756. doi: 10.3390/ijms24119756
102. Reardon C, Duncan GS, Brustle A, Brenner D, Tusche MW, Olofsson PS, et al. Lymphocyte-derived ACh regulates local innate but not adaptive immunity. Proc Natl Acad Sci U S A. (2013) 110:1410–5. doi: 10.1073/pnas.1221655110
103. Ji X, Wu L, Marion T, and Luo Y. Lipid metabolism in regulation of B cell development and autoimmunity. Cytokine Growth Factor Rev. (2023) 73:40–51. doi: 10.1016/j.cytogfr.2023.06.008
104. Brewer JW, Solodushko V, Aragon I, and Barrington RA. Phosphatidylcholine as a metabolic cue for determining B cell fate and function. Cell Immunol. (2016) 310:78–88. doi: 10.1016/j.cellimm.2016.08.002
105. Fagone P, Sriburi R, Ward-Chapman C, Frank M, Wang J, Gunter C, et al. Phospholipid biosynthesis program underlying membrane expansion during B-lymphocyte differentiation. J Biol Chem. (2007) 282:7591–605. doi: 10.1074/jbc.M608175200
106. Wiest DL, Burkhardt JK, Hester S, Hortsch M, Meyer DI, and Argon Y. Membrane biogenesis during B cell differentiation: most endoplasmic reticulum proteins are expressed coordinately. J Cell Biol. (1990) 110:1501–11. doi: 10.1083/jcb.110.5.1501
107. Fagone P, Gunter C, Sage CR, Gunn KE, Brewer JW, and Jackowski S. CTP:phosphocholine cytidylyltransferase alpha is required for B-cell proliferation and class switch recombination. J Biol Chem. (2009) 284:6847–54. doi: 10.1074/jbc.M807338200
108. Fujii T, Mashimo M, Moriwaki Y, Misawa H, Ono S, Horiguchi K, et al. Expression and function of the cholinergic system in immune cells. Front Immunol. (2017) 8:1085. doi: 10.3389/fimmu.2017.01085
109. Costa P, Auger CB, Traver DJ, and Costa LG. Identification of m3, m4 and m5 subtypes of muscarinic receptor mRNA in human blood mononuclear cells. J Neuroimmunol. (1995) 60:45–51. doi: 10.1016/0165-5728(95)00051-3
110. Ricci A, Amenta F, Bronzetti E, Mannino F, Mariotta S, and Tayebati SK. Expression of peripheral blood lymphocyte muscarinic cholinergic receptor subtypes in airway hyperresponsiveness. J Neuroimmunol. (2002) 129:178–85. doi: 10.1016/s0165-5728(02)00177-7
111. Zheng C, Snow BE, Elia AJ, Nechanitzky R, Dominguez-Brauer C, Liu S, et al. Tumor-specific cholinergic CD4(+) T lymphocytes guide immunosurveillance of hepatocellular carcinoma. Nat Cancer. (2023) 4:1437–54. doi: 10.1038/s43018-023-00624-w
112. Modares NF, Hendrikse LD, Smith LK, Paul MS, Haight J, Luo P, et al. B cell-derived acetylcholine promotes liver regeneration by regulating Kupffer cell and hepatic CD8(+) T cell function. Immunity. (2025) 58:1201–1216 e1207. doi: 10.1016/j.immuni.2025.04.002
113. Cembellin-Prieto A, Luo Z, Kulaga H, and Baumgarth N. B cells modulate lung antiviral inflammatory responses via the neurotransmitter acetylcholine. Nat Immunol. (2025) 26:775–89. doi: 10.1038/s41590-025-02124-8
114. Nagel I, Bug S, Tonnies H, Ammerpohl O, Richter J, Vater I, et al. Biallelic inactivation of TRAF3 in a subset of B-cell lymphomas with interstitial del(14)(q24.1q32.33). Leukemia. (2009) 23:2153–5. doi: 10.1038/leu.2009.149
115. Walker BA, Boyle EM, Wardell CP, Murison A, Begum DB, Dahir NM, et al. Mutational spectrum, copy number changes, and outcome: results of a sequencing study of patients with newly diagnosed myeloma. J Clin Oncol. (2015) 33:3911–20. doi: 10.1200/JCO.2014.59.1503
116. Zhang B, Calado DP, Wang Z, Frohler S, Kochert K, Qian Y, et al. An oncogenic role for alternative NF-kappaB signaling in DLBCL revealed upon deregulated BCL6 expression. Cell Rep. (2015) 11:715–26. doi: 10.1016/j.celrep.2015.03.059
117. Gokhale S, Lu W, Zhu S, Liu Y, Hart RP, Rabinowitz JD, et al. Elevated choline kinase alpha-mediated choline metabolism supports the prolonged survival of TRAF3-deficient B lymphocytes. J Immunol. (2020) 204:459–71. doi: 10.4049/jimmunol.1900658
118. Fu G, Guy CS, Chapman NM, Palacios G, Wei J, Zhou P, et al. Metabolic control of T(FH) cells and humoral immunity by phosphatidylethanolamine. Nature. (2021) 595:724–9. doi: 10.1038/s41586-021-03692-z
119. Kawashima K and Fujii T. The lymphocytic cholinergic system and its contribution to the regulation of immune activity. Life Sci. (2003) 74:675–96. doi: 10.1016/j.lfs.2003.09.037
120. Kawashima K, Fujii T, Watanabe Y, and Misawa H. Acetylcholine synthesis and muscarinic receptor subtype mRNA expression in T-lymphocytes. Life Sci. (1998) 62:1701–5. doi: 10.1016/s0024-3205(98)00131-3
121. Fujii T and Kawashima K. Calcium signaling and c-Fos gene expression via M3 muscarinic acetylcholine receptors in human T- and B-cells. Jpn J Pharmacol. (2000) 84:124–32. doi: 10.1254/jjp.84.124
122. Fujii T and Kawashima K. YM905, a novel M3 antagonist, inhibits Ca2+ signaling and c-fos gene expression mediated via muscarinic receptors in human T cells. Gen Pharmacol. (2000) 35:71–5. doi: 10.1016/s0306-3623(01)00093-3
123. Kimura R, Ushiyama N, Fujii T, and Kawashima K. Nicotine-induced Ca2+ signaling and down-regulation of nicotinic acetylcholine receptor subunit expression in the CEM human leukemic T-cell line. Life Sci. (2003) 72:2155–8. doi: 10.1016/s0024-3205(03)00077-8
124. Tarnawski L, Shavva VS, Kort EJ, Zhuge Z, Nilsson I, Gallina AL, et al. Cholinergic regulation of vascular endothelial function by human ChAT(+) T cells. Proc Natl Acad Sci U S A. (2023) 120:e2212476120. doi: 10.1073/pnas.2212476120
125. Azarcoya-Barrera J, Wollin B, Veida-Silva H, Makarowski A, Goruk S, Field CJ, et al. Egg-phosphatidylcholine attenuates T-cell dysfunction in high-fat diet fed male wistar rats. Front Nutr. (2022) 9:811469. doi: 10.3389/fnut.2022.811469
126. Rusnak T, Azarcoya-Barrera J, Wollin B, Makarowski A, Nelson R, Field CJ, et al. A physiologically relevant dose of 50% Egg-phosphatidylcholine is sufficient in improving gut permeability while attenuating immune cell dysfunction induced by a high-fat diet in male wistar rats. J Nutr. (2023) 153:3131–43. doi: 10.1016/j.tjnut.2023.08.010
127. Donnelly RP, Loftus RM, Keating SE, Liou KT, Biron CA, Gardiner CM, et al. mTORC1-dependent metabolic reprogramming is a prerequisite for NK cell effector function. J Immunol. (2014) 193:4477–84. doi: 10.4049/jimmunol.1401558
128. Sohn H and Cooper MA. Metabolic regulation of NK cell function: implications for immunotherapy. Immunometabolism (Cobham). (2023) 5:e00020. doi: 10.1097/IN9.0000000000000020
129. Assmann N, O'Brien KL, Donnelly RP, Dyck L, Zaiatz-Bittencourt V, Loftus RM, et al. Srebp-controlled glucose metabolism is essential for NK cell functional responses. Nat Immunol. (2017) 18:1197–206. doi: 10.1038/ni.3838
130. Wang P, Liang T, Zhan H, Zhu M, Wu M, Qian L, et al. Unique metabolism and protein expression signature in human decidual NK cells. Front Immunol. (2023) 14:1136652. doi: 10.3389/fimmu.2023.1136652
131. Zheng X, Hou Z, Qian Y, Zhang Y, Cui Q, Wang X, et al. Tumors evade immune cytotoxicity by altering the surface topology of NK cells. Nat Immunol. (2023) 24:802–13. doi: 10.1038/s41590-023-01462-9
132. Ramoni C, Spadaro F, Menegon M, and Podo F. Cellular localization and functional role of phosphatidylcholine-specific phospholipase C in NK cells. J Immunol. (2001) 167:2642–50. doi: 10.4049/jimmunol.167.5.2642
133. Spadaro F, Cecchetti S, Sanchez M, Ausiello CM, Podo F, and Ramoni C. Expression and role of phosphatidylcholine-specific phospholipase C in human NK and T lymphocyte subsets. Eur J Immunol. (2006) 36:3277–87. doi: 10.1002/eji.200635927
134. Cecchetti S, Spadaro F, Lugini L, Podo F, and Ramoni C. Functional role of phosphatidylcholine-specific phospholipase C in regulating CD16 membrane expression in natural killer cells. Eur J Immunol. (2007) 37:2912–22. doi: 10.1002/eji.200737266
135. Van Parys A, Lysne V, Svingen GFT, Ueland PM, Dhar I, Oyen J, et al. Dietary choline is related to increased risk of acute myocardial infarction in patients with stable angina pectoris. Biochimie. (2020) 173:68–75. doi: 10.1016/j.biochi.2019.11.001
136. Yuan J, Liu X, Liu C, Ang AF, Massaro J, Devine SA, et al. Is dietary choline intake related to dementia and Alzheimer’s disease risks? Results from the Framingham Heart Study. Am J Clin Nutr. (2022) 116:1201–7. doi: 10.1093/ajcn/nqac193
137. Al-Saffar NM, Troy H, Ramirez de Molina A, Jackson LE, Madhu B, Griffiths JR, et al. Noninvasive magnetic resonance spectroscopic pharmacodynamic markers of the choline kinase inhibitor MN58b in human carcinoma models. Cancer Res. (2006) 66:427–34. doi: 10.1158/0008-5472.CAN-05-1338
138. Seki M, Kawai Y, Ishii C, Yamanaka T, Odawara M, and Inazu M. Functional analysis of choline transporters in rheumatoid arthritis synovial fibroblasts. Mod Rheumatol. (2017) 27:995–1003. doi: 10.1080/14397595.2017.1280118
139. Hellberg S, Silvola JM, Kiugel M, Liljenback H, Metsala O, Viljanen T, et al. Type 2 diabetes enhances arterial uptake of choline in atherosclerotic mice: an imaging study with positron emission tomography tracer (1)(8)F-fluoromethylcholine. Cardiovasc Diabetol. (2016) 15:26. doi: 10.1186/s12933-016-0340-6
140. Yang Y, Yang B, Liu B, Liang Y, Luo Q, Zhao Z, et al. Circulating choline levels are associated with prognoses in patients with pulmonary hypertension: a cohort study. BMC Pulm Med. (2023) 23:313. doi: 10.1186/s12890-023-02547-9
141. Pan XF, Yang JJ, Shu XO, Moore SC, Palmer ND, Guasch-Ferre M, et al. Associations of circulating choline and its related metabolites with cardiometabolic biomarkers: an international pooled analysis. Am J Clin Nutr. (2021) 114:893–906. doi: 10.1093/ajcn/nqab152
142. Detopoulou P, Panagiotakos DB, Antonopoulou S, Pitsavos C, and Stefanadis C. Dietary choline and betaine intakes in relation to concentrations of inflammatory markers in healthy adults: the ATTICA study. Am J Clin Nutr. (2008) 87:424–30. doi: 10.1093/ajcn/87.2.424
143. Niculescu MD, da Costa KA, Fischer LM, and Zeisel SH. Lymphocyte gene expression in subjects fed a low-choline diet differs between those who develop organ dysfunction and those who do not. Am J Clin Nutr. (2007) 86:230–9. doi: 10.1093/ajcn/86.1.230
144. da Costa KA, Niculescu MD, Craciunescu CN, Fischer LM, and Zeisel SH. Choline deficiency increases lymphocyte apoptosis and DNA damage in humans. Am J Clin Nutr. (2006) 84:88–94. doi: 10.1093/ajcn/84.1.88
145. Jiang X, Bar HY, Yan J, West AA, Perry CA, Malysheva OV, et al. Pregnancy induces transcriptional activation of the peripheral innate immune system and increases oxidative DNA damage among healthy third trimester pregnant women. PloS One. (2012) 7:e46736. doi: 10.1371/journal.pone.0046736
146. Cho CE, Aardema NDJ, Bunnell ML, Larson DP, Aguilar SS, Bergeson JR, et al. Effect of choline forms and gut microbiota composition on trimethylamine-N-oxide response in healthy men. Nutrients. (2020) 12(8):2220. doi: 10.3390/nu12082220
147. Sprinkles JK, Lulla A, Hullings AG, Trujillo-Gonzalez I, Klatt KC, Jacobs DR Jr., et al. Choline metabolites and 15-year risk of incident diabetes in a prospective cohort of adults: coronary artery risk development in young adults (CARDIA) study. Diabetes Care. (2024) 47:1985–94. doi: 10.2337/dc24-1033
148. Rashvand S, Mobasseri M, and Tarighat-Esfanjani A. The effects of choline and magnesium co-supplementation on metabolic parameters, inflammation, and endothelial dysfunction in patients with type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled trial. J Am Coll Nutr. (2019) 38:714–21. doi: 10.1080/07315724.2019.1599745
149. Matsuzawa Y, Nakahashi H, Konishi M, Sato R, Kawashima C, Kikuchi S, et al. Microbiota-derived trimethylamine N-oxide predicts cardiovascular risk after STEMI. Sci Rep. (2019) 9:11647. doi: 10.1038/s41598-019-48246-6
150. Hou CY, Chen YW, Hazeena SH, Tain YL, Hsieh CW, Chen DQ, et al. Cardiovascular risk of dietary trimethylamine oxide precursors and the therapeutic potential of resveratrol and its derivatives. FEBS Open Bio. (2024) 14:358–79. doi: 10.1002/2211-5463.13762
151. Fretts AM, Hazen SL, Jensen P, Budoff M, Sitlani CM, Wang M, et al. Association of trimethylamine N-oxide and metabolites with mortality in older adults. JAMA Netw Open. (2022) 5:e2213242. doi: 10.1001/jamanetworkopen.2022.13242
152. Lee Y, Nemet I, Wang Z, Lai HTM, de Oliveira MC, Lemaitre RN, et al. Longitudinal plasma measures of trimethylamine N-oxide and risk of atherosclerotic cardiovascular disease events in community-based older adults. J Am Heart Assoc. (2021) 10:e020646. doi: 10.1161/JAHA.120.020646
153. Zhou R, Yang M, Yue C, Shi Y, Tan Y, Zhan L, et al. Association between dietary choline intake and cardiovascular diseases: national health and nutrition examination survey 2011-2016. Nutrients. (2023) 15(18):4036. doi: 10.3390/nu15184036
154. Guo F, Qiu X, Zhu Y, Ta Z, Li Z, Ouyang D, et al. Circulating choline is associated with coronary artery stenosis in patients with hypertension: A cross-sectional study of Chinese adults. J Clin Hypertens (Greenwich). (2020) 22:2069–76. doi: 10.1111/jch.14025
155. Tate BN, Van Guilder GP, Aly M, Spence LA, Diaz-Rubio ME, Le HH, et al. Changes in choline metabolites and ceramides in response to a DASH-style diet in older adults. Nutrients. (2023) 15(17):3687. doi: 10.3390/nu15173687
156. Wang Z, Hazen J, Jia X, Org E, Zhao Y, Osborn LJ, et al. The nutritional supplement L-alpha glycerylphosphorylcholine promotes atherosclerosis. Int J Mol Sci. (2021) 22(24):13477. doi: 10.3390/ijms222413477
157. Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. (2013) 368:1575–84. doi: 10.1056/NEJMoa1109400
158. Obeid R, Awwad HM, Rabagny Y, Graeber S, Herrmann W, and Geisel J. Plasma trimethylamine N-oxide concentration is associated with choline, phospholipids, and methyl metabolism. Am J Clin Nutr. (2016) 103:703–11. doi: 10.3945/ajcn.115.121269
159. Yiannakou I, Long MT, Jacques PF, Beiser A, Pickering RT, Moore LL, et al. Eggs, dietary choline, and nonalcoholic fatty liver disease in the framingham heart study. J Nutr. (2025) 155:923–35. doi: 10.1016/j.tjnut.2024.10.026
160. Zohrer E, Alisi A, Jahnel J, Mosca A, Della Corte C, Crudele A, et al. Efficacy of docosahexaenoic acid-choline-vitamin E in pediatric NASH: a randomized controlled clinical trial. Appl Physiol Nutr Metab. (2017) 42:948–54. doi: 10.1139/apnm-2016-0689
161. Mooli RGR and Ramakrishnan SK. Liver steatosis is a driving factor of inflammation. Cell Mol Gastroenterol Hepatol. (2022) 13:1267–70. doi: 10.1016/j.jcmgh.2022.01.007
162. DiBella M, Thomas MS, Alyousef H, Millar C, Blesso C, Malysheva O, et al. Choline intake as supplement or as a component of eggs increases plasma choline and reduces interleukin-6 without modifying plasma cholesterol in participants with metabolic syndrome. Nutrients. (2020) 12(10):3120. doi: 10.3390/nu12103120
163. Dave N, Judd JM, Decker A, Winslow W, Sarette P, Villarreal Espinosa O, et al. Dietary choline intake is necessary to prevent systems-wide organ pathology and reduce Alzheimer’s disease hallmarks. Aging Cell. (2023) 22:e13775. doi: 10.1111/acel.13775
164. Chartampila E, Elayouby KS, Leary P, LaFrancois JJ, Alcantara-Gonzalez D, Jain S, et al. Choline supplementation in early life improves and low levels of choline can impair outcomes in a mouse model of Alzheimer’s disease. Elife. (2024) 21. doi: 10.7554/eLife.89889
165. Cantone AF, Burgaletto C, Di Benedetto G, Pannaccione A, Secondo A, Bellanca CM, et al. Taming Microglia in Alzheimer’s Disease: Exploring Potential Implications of Choline Alphoscerate via alpha7 nAChR Modulation. Cells. (2024) 13(4):309. doi: 10.3390/cells13040309
166. Niu YY, Yan HY, Zhong JF, Diao ZQ, Li J, Li CP, et al. Association of dietary choline intake with incidence of dementia, Alzheimer disease, and mild cognitive impairment: a large population-based prospective cohort study. Am J Clin Nutr. (2025) 121:5–13. doi: 10.1016/j.ajcnut.2024.11.001
167. Nguyen TT, Risbud RD, Mattson SN, Chambers CD, and Thomas JD. Randomized, double-blind, placebo-controlled clinical trial of choline supplementation in school-aged children with fetal alcohol spectrum disorders. Am J Clin Nutr. (2016) 104:1683–92. doi: 10.3945/ajcn.116.142075
168. Wozniak JR, Fuglestad AJ, Eckerle JK, Fink BA, Hoecker HL, Boys CJ, et al. Choline supplementation in children with fetal alcohol spectrum disorders: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. (2015) 102:1113–25. doi: 10.3945/ajcn.114.099168
169. Wozniak JR, Eckerle JK, Gimbel BA, Ernst AM, Anthony ME, Tuominen KA, et al. Choline enhances elicited imitation memory performance in preschool children with prenatal alcohol exposure: a cumulative report of 3 randomized controlled trials. Am J Clin Nutr. (2025) 121:921–31. doi: 10.1016/j.ajcnut.2025.02.009
170. Gimbel BA, Anthony ME, Ernst AM, Roediger DJ, de Water E, Eckerle JK, et al. Long-term follow-up of a randomized controlled trial of choline for neurodevelopment in fetal alcohol spectrum disorder: corpus callosum white matter microstructure and neurocognitive outcomes. J Neurodev Disord. (2022) 14:59. doi: 10.1186/s11689-022-09470-w
171. Wozniak JR, Fink BA, Fuglestad AJ, Eckerle JK, Boys CJ, Sandness KE, et al. Four-year follow-up of a randomized controlled trial of choline for neurodevelopment in fetal alcohol spectrum disorder. J Neurodev Disord. (2020) 12:9. doi: 10.1186/s11689-020-09312-7
172. Hubner IB, Scheibe DB, Marchezan J, and Bucker J. Use of citicoline in attention-deficit/hyperactivity disorder: A pilot study. Clin Neuropharmacol. (2024) 47:146–9. doi: 10.1097/WNF.0000000000000602
173. Lewis JE, Melillo AB, Tiozzo E, Chen L, Leonard S, Howell M, et al. A double-blind, randomized clinical trial of dietary supplementation on cognitive and immune functioning in healthy older adults. BMC Complement Altern Med. (2014) 14:43. doi: 10.1186/1472-6882-14-43
174. Sohn M, Park YH, and Lim S. Effects of choline alfoscerate on cognitive function and quality of life in type 2 diabetes: A double-blind, randomized, placebo-controlled trial. Diabetes Obes Metab. (2025) 27:1350–8. doi: 10.1111/dom.16131
175. Kerksick CM. Acute alpha-glycerylphosphorylcholine supplementation enhances cognitive performance in healthy men. Nutrients. (2024) 16(23):4240. doi: 10.3390/nu16234240
176. Konstantinova SV, Tell GS, Vollset SE, Nygard O, Bleie O, and Ueland PM. Divergent associations of plasma choline and betaine with components of metabolic syndrome in middle age and elderly men and women. J Nutr. (2008) 138:914–20. doi: 10.1093/jn/138.5.914
177. Martin MC. Gonadotropin releasing hormone agonists and the induction or augmentation of ovulation. J Reprod Med. (1989) 34:1034–8.
178. Roe AJ, Zhang S, Bhadelia RA, Johnson EJ, Lichtenstein AH, Rogers GT, et al. Choline and its metabolites are differently associated with cardiometabolic risk factors, history of cardiovascular disease, and MRI-documented cerebrovascular disease in older adults. Am J Clin Nutr. (2017) 105:1283–90. doi: 10.3945/ajcn.116.137158
179. Roivainen A, Parkkola R, Yli-Kerttula T, Lehikoinen P, Viljanen T, Mottonen T, et al. Use of positron emission tomography with methyl-11C-choline and 2-18F-fluoro-2-deoxy-D-glucose in comparison with magnetic resonance imaging for the assessment of inflammatory proliferation of synovium. Arthritis Rheum. (2003) 48:3077–84. doi: 10.1002/art.11282
180. Roivainen A and Yli-Kerttula T. Whole-body distribution of (11)C-choline and uptake in knee synovitis. Eur J Nucl Med Mol Imaging. (2006) 33:1372–3. doi: 10.1007/s00259-006-0184-5
181. Schartum-Hansen H, Pedersen ER, Svingen GF, Ueland PM, Seifert R, Ebbing M, et al. Plasma choline, smoking, and long-term prognosis in patients with stable angina pectoris. Eur J Prev Cardiol. (2015) 22:606–14. doi: 10.1177/2047487314524867
182. Zuo H, Svingen GFT, Tell GS, Ueland PM, Vollset SE, Pedersen ER, et al. Plasma concentrations and dietary intakes of choline and betaine in association with atrial fibrillation risk: results from 3 prospective cohorts with different health profiles. J Am Heart Assoc. (2018) 7(8):e008190. doi: 10.1161/JAHA.117.008190
183. Kang DW, Park MK, Oh HJ, Lee DG, Park SH, Choi KY, et al. Phospholipase D1 has a pivotal role in interleukin-1beta-driven chronic autoimmune arthritis through regulation of NF-kappaB, hypoxia-inducible factor 1alpha, and FoxO3a. Mol Cell Biol. (2013) 33:2760–72. doi: 10.1128/MCB.01519-12
184. Kortekangas P, Aro HT, and Nevalainen TJ. Group II phospholipase A2 in synovial fluid and serum in acute arthritis. Scand J Rheumatol. (1994) 23:68–72. doi: 10.3109/03009749409103030
185. Kosinska MK, Liebisch G, Lochnit G, Wilhelm J, Klein H, Kaesser U, et al. A lipidomic study of phospholipid classes and species in human synovial fluid. Arthritis Rheum. (2013) 65:2323–33. doi: 10.1002/art.38053
186. Panula HE, Lohmander LS, Ronkko S, Agren U, Helminen HJ, and Kiviranta I. Elevated levels of synovial fluid PLA2, stromelysin (MMP-3) and TIMP in early osteoarthrosis after tibial valgus osteotomy in young beagle dogs. Acta Orthop Scand. (1998) 69:152–8. doi: 10.3109/17453679809117617
187. Bartok B and Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. (2010) 233:233–55. doi: 10.1111/j.0105-2896.2009.00859.x
188. Beyer K, Lie SA, Bjorndal B, Berge RK, Svardal A, Brun JG, et al. Lipid, fatty acid, carnitine- and choline derivative profiles in rheumatoid arthritis outpatients with different degrees of periodontal inflammation. Sci Rep. (2021) 11:5332. doi: 10.1038/s41598-021-84122-y
189. Koh RH, Jin Y, Kim J, and Hwang NS. Inflammation-modulating hydrogels for osteoarthritis cartilage tissue engineering. Cells. (2020) 9(2):419. doi: 10.3390/cells9020419
190. Young SP, Kapoor SR, Viant MR, Byrne JJ, Filer A, Buckley CD, et al. The impact of inflammation on metabolomic profiles in patients with arthritis. Arthritis Rheum. (2013) 65:2015–23. doi: 10.1002/art.38021
191. Koh JH, Yoon SJ, Kim M, Cho S, Lim J, Park Y, et al. Lipidome profile predictive of disease evolution and activity in rheumatoid arthritis. Exp Mol Med. (2022) 54:143–55. doi: 10.1038/s12276-022-00725-z
192. Wu T, Xie C, Han J, Ye Y, Weiel J, Li Q, et al. Metabolic disturbances associated with systemic lupus erythematosus. PLoS One. (2012) 7:e37210. doi: 10.1371/journal.pone.0037210
193. Li Y, Liang L, Deng X, and Zhong L. Lipidomic and metabolomic profiling reveals novel candidate biomarkers in active systemic lupus erythematosus. Int J Clin Exp Pathol. (2019) 12:857–66.
194. Ferreira HB, Melo T, Guerra IMS, Moreira ASP, Laranjeira P, Paiva A, et al. Whole blood and plasma-based lipid profiling reveals distinctive metabolic changes in systemic lupus erythematosus and systemic sclerosis. J Proteome Res. (2023) 22:2995–3008. doi: 10.1021/acs.jproteome.3c00321
195. Zeng Q, Wang S, Li M, Wang S, Guo C, Ruan X, et al. Spleen fibroblastic reticular cell-derived acetylcholine promotes lipid metabolism to drive autoreactive B cell responses. Cell Metab. (2023) 35:837–854 e838. doi: 10.1016/j.cmet.2023.03.010
196. Hurtado C, Acevedo Saenz LY, Vasquez Trespalacios EM, Urrego R, Jenks S, Sanz I, et al. DNA methylation changes on immune cells in Systemic Lupus Erythematosus. Autoimmunity. (2020) 53:114–21. doi: 10.1080/08916934.2020.1722108
197. Vordenbaumen S, Rosenbaum A, Gebhard C, Raithel J, Sokolowski A, Dusing C, et al. Associations of site-specific CD4(+)-T-cell hypomethylation within CD40-ligand promotor and enhancer regions with disease activity of women with systemic lupus erythematosus. Lupus. (2021) 30:45–51. doi: 10.1177/0961203320965690
198. Vordenbaumen S, Sokolowski A, Rosenbaum A, Gebhard C, Raithel J, Dusing C, et al. Methyl donor micronutrients, CD40-ligand methylation and disease activity in systemic lupus erythematosus: A cross-sectional association study. Lupus. (2021) 30:1773–80. doi: 10.1177/09612033211034559
199. Columbo M and Rohr AS. Asthma in the elderly: the effect of choline supplementation. Allergy Asthma Clin Immunol. (2016) 12:15. doi: 10.1186/s13223-016-0121-5
200. Gupta SK and Gaur SN. A placebo controlled trial of two dosages of LPC antagonist–choline in the management of bronchial asthma. Indian J Chest Dis Allied Sci. (1997) 39:149–56.
201. Gokhale S and Xie P. ChoK-full of potential: choline kinase in B cell and T cell Malignancies. Pharmaceutics. (2021) 13(6):911. doi: 10.3390/pharmaceutics13060911
202. Korbecki J, Bosiacki M, Kupnicka P, Barczak K, Zietek P, Chlubek D, et al. Choline kinases: Enzymatic activity, involvement in cancer and other diseases, inhibitors. Int J Cancer. (2025) 156:1314–25. doi: 10.1002/ijc.35286
203. Yao N, Li W, Xu G, Duan N, Yu G, and Qu J. Choline metabolism and its implications in cancer. Front Oncol. (2023) 13:1234887. doi: 10.3389/fonc.2023.1234887
204. Li W, Li C, Liu T, Song Y, Chen P, Liu L, et al. The association of serum choline concentrations with the risk of cancers: a community-based nested case-control study. Sci Rep. (2023) 13:22144. doi: 10.1038/s41598-023-49610-3
205. Garnier L and Albano JP. Preliminary results on the splanchnico-laryngeal reflex in the cat. C R Seances Soc Biol Fil. (1977) 171:1049–53.
206. Arlauckas SP, Popov AV, and Delikatny EJ. Choline kinase alpha-Putting the ChoK-hold on tumor metabolism. Prog Lipid Res. (2016) 63:28–40. doi: 10.1016/j.plipres.2016.03.005
207. Caprifico AE, Vaghi L, Spearman P, Calabrese G, and Papagni A. In vitro detection of cancer cells using a novel fluorescent choline derivative. BMC Med Imaging. (2024) 24:316. doi: 10.1186/s12880-024-01488-x
208. Glunde K, Bhujwalla ZM, and Ronen SM. Choline metabolism in Malignant transformation. Nat Rev Cancer. (2011) 11:835–48. doi: 10.1038/nrc3162
209. Glunde K, Jiang L, Moestue SA, and Gribbestad IS. MRS and MRSI guidance in molecular medicine: targeting and monitoring of choline and glucose metabolism in cancer. NMR BioMed. (2011) 24:673–90. doi: 10.1002/nbm.1751
210. Glunde K, Jie C, and Bhujwalla ZM. Molecular causes of the aberrant choline phospholipid metabolism in breast cancer. Cancer Res. (2004) 64:4270–6. doi: 10.1158/0008-5472.CAN-03-3829
211. Glunde K, Penet MF, Jiang L, Jacobs MA, and Bhujwalla ZM. Choline metabolism-based molecular diagnosis of cancer: an update. Expert Rev Mol Diagn. (2015) 15:735–47. doi: 10.1586/14737159.2015.1039515
212. Jilg CA, Schultze-Seemann W, Drendel V, Vach W, Wieser G, Krauss T, et al. Detection of lymph node metastasis in patients with nodal prostate cancer relapse using (18)F/(11)C-choline positron emission tomography/computerized tomography. J Urol. (2014) 192:103–10. doi: 10.1016/j.juro.2013.12.054
213. Millard T, Chau I, Iyengar S, El-Sharkawi D, Cunningham D, and Sharma B. (18) F-choline radiotracer positron emission tomography as a new means to monitor central nervous system lymphoma. Br J Hematol. (2021) 193:1026. doi: 10.1111/bjh.17374
214. Ramirez de Molina A, Gutierrez R, Ramos MA, Silva JM, Silva J, Bonilla F, et al. Increased choline kinase activity in human breast carcinomas: clinical evidence for a potential novel antitumor strategy. Oncogene. (2002) 21:4317–22. doi: 10.1038/sj.onc.1205556
215. Ramirez de Molina A, Sarmentero-Estrada J, Belda-Iniesta C, Taron M, Ramirez de Molina V, Cejas P, et al. Expression of choline kinase alpha to predict outcome in patients with early-stage non-small-cell lung cancer: a retrospective study. Lancet Oncol. (2007) 8:889–97. doi: 10.1016/S1470-2045(07)70279-6
216. Li M, Peng Z, Liu Q, Sun J, Yao S, and Liu Q. Value of 11C-choline PET/CT for lung cancer diagnosis and the relation between choline metabolism and proliferation of cancer cells. Oncol Rep. (2013) 29:205–11. doi: 10.3892/or.2012.2099
217. Garcia-Molina P, Sola-Leyva A, Luque-Navarro PM, Laso A, Rios-Marco P, Rios A, et al. Anticancer activity of the choline kinase inhibitor PL48 is due to selective disruption of choline metabolism and transport systems in cancer cell lines. Pharmaceutics. (2022) 14(2):426. doi: 10.3390/pharmaceutics14020426
218. Ramirez de Molina A, Gallego-Ortega D, Sarmentero-Estrada J, Lagares D, Gomez Del Pulgar T, Bandres E, et al. Choline kinase as a link connecting phospholipid metabolism and cell cycle regulation: implications in cancer therapy. Int J Biochem Cell Biol. (2008) 40:1753–63. doi: 10.1016/j.biocel.2008.01.013
219. Rubio-Ruiz B, Serran-Aguilera L, Hurtado-Guerrero R, and Conejo-Garcia A. Recent advances in the design of choline kinase alpha inhibitors and the molecular basis of their inhibition. Med Res Rev. (2021) 41:902–27. doi: 10.1002/med.21746
220. Liu C, Liu D, Wang F, Liu Y, Xie J, Xie J, et al. Construction of a novel choline metabolism-related signature to predict prognosis, immune landscape, and chemotherapy response in colon adenocarcinoma. Front Immunol. (2022) 13:1038927. doi: 10.3389/fimmu.2022.1038927
221. Carbon S, Ireland A, Mungall CJ, Shu S, Marshall B, Lewis S, et al. AmiGO: online access to ontology and annotation data. Bioinformatics. (2009) 25:288–9. doi: 10.1093/bioinformatics/btn615
222. Abdelaal MS, Restrepo C, and Sharkey PF. Global perspectives on arthroplasty of hip and knee joints. Orthop Clin North Am. (2020) 51:169–76. doi: 10.1016/j.ocl.2019.11.003
223. Xiong J, Wang L, Fei XC, Jiang XF, Zheng Z, Zhao Y, et al. MYC is a positive regulator of choline metabolism and impedes mitophagy-dependent necroptosis in diffuse large B-cell lymphoma. Blood Cancer J. (2017) 7:e0. doi: 10.1038/bcj.2017.61
224. Pera B, Krumsiek J, Assouline SE, Marullo R, Patel J, Phillip JM, et al. Metabolomic profiling reveals cellular reprogramming of B-cell lymphoma by a lysine deacetylase inhibitor through the choline pathway. EBioMedicine. (2018) 28:80–9. doi: 10.1016/j.ebiom.2018.01.014
225. Iorio E, Mezzanzanica D, Alberti P, Spadaro F, Ramoni C, D'Ascenzo S, et al. Alterations of choline phospholipid metabolism in ovarian tumor progression. Cancer Res. (2005) 65:9369–76. doi: 10.1158/0008-5472.CAN-05-1146
226. Romanska HM, Tiziani S, Howe RC, Gunther UL, Gulzar Z, and Lalani el N. Nuclear magnetic resonance detects phosphoinositide 3-kinase/Akt-independent traits common to pluripotent murine embryonic stem cells and their Malignant counterparts. Neoplasia. (2009) 11:1301–8. doi: 10.1593/neo.09850
227. Iorio E, Podo F, Leach MO, Koutcher J, Blankenberg FG, and Norfray JF. A novel roadmap connecting the (1)H-MRS total choline resonance to all hallmarks of cancer following targeted therapy. Eur Radiol Exp. (2021) 5:5. doi: 10.1186/s41747-020-00192-z
228. Farouqi B, Yahyaoui M, Alaoui FM, Noraz N, Sekkat S, Chkili T, et al. Establishment of T-lymphoid cell lines from Morroccan patients with tropical spastic paraparesis. AIDS Res Hum Retroviruses. (1992) 8:1209–13. doi: 10.1089/aid.1992.8.1209
229. Huang Z, Rui J, Li X, Meng X, and Liu Q. Use of (1)(1)C-Choline positron emission tomography/computed tomography to investigate the mechanism of choline metabolism in lung cancer. Mol Med Rep. (2015) 11:3285–90. doi: 10.3892/mmr.2015.3200
230. Donadon M, Lopci E, Galvanin J, Giudici S, Del Fabbro D, Lanza E, et al. Prognostic value of metabolic imaging data of (11)C-choline PET/CT in patients undergoing hepatectomy for hepatocellular carcinoma. Cancers (Basel). (2021) 13(3):472. doi: 10.3390/cancers13030472
231. Bagnoli M, Granata A, Nicoletti R, Krishnamachary B, Bhujwalla ZM, Canese R, et al. Choline metabolism alteration: A focus on ovarian cancer. Front Oncol. (2016) 6:153. doi: 10.3389/fonc.2016.00153
232. Torizuka T, Kanno T, Futatsubashi M, Okada H, Yoshikawa E, Nakamura F, et al. Imaging of gynecologic tumors: comparison of (11)C-choline PET with (18)F-FDG PET. J Nucl Med. (2003) 44:1051–6.
233. Michaud L, Touijer KA, Mauguen A, Zelefsky MJ, Morris MJ, Lyashschenko SK, et al. (11)C-choline PET/CT in recurrent prostate cancer: retrospective analysis in a large U.S. Patient series. J Nucl Med. (2020) 61:827–33. doi: 10.2967/jnumed.119.233098
234. Welle CL, Cullen EL, Peller PJ, Lowe VJ, Murphy RC, Johnson GB, et al. (1)(1)C-choline PET/CT in recurrent prostate cancer and nonprostatic neoplastic processes. Radiographics. (2016) 36:279–92. doi: 10.1148/rg.2016150135
235. Watson GA, Sanz-Garcia E, Zhang WJ, Liu ZA, Yang SC, Wang B, et al. Increase in serum choline levels predicts for improved progression-free survival (PFS) in patients with advanced cancers receiving pembrolizumab. J Immunother Cancer. (2022) 10(6):e004378. doi: 10.1136/jitc-2021-004378
236. Awwad HM, Ohlmann CH, Stoeckle M, Aziz R, Geisel J, and Obeid R. Choline-phospholipids inter-conversion is altered in elderly patients with prostate cancer. Biochimie. (2016) 126:108–14. doi: 10.1016/j.biochi.2016.01.003
237. Wang H, Rong X, Zhao G, Zhou Y, Xiao Y, Ma D, et al. The microbial metabolite trimethylamine N-oxide promotes antitumor immunity in triple-negative breast cancer. Cell Metab. (2022) 34:581–594 e588. doi: 10.1016/j.cmet.2022.02.010
238. Mirji G, Worth A, Bhat SA, El Sayed M, Kannan T, Goldman AR, et al. The microbiome-derived metabolite TMAO drives immune activation and boosts responses to immune checkpoint blockade in pancreatic cancer. Sci Immunol. (2022) 7:eabn0704. doi: 10.1126/sciimmunol.abn0704
239. Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, and Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. (2001) 29:1303–10. doi: 10.1097/00003246-200107000-00002
240. Laszlo I, Trasy D, Molnar Z, and Fazakas J. Sepsis: from pathophysiology to individualized patient care. J Immunol Res. (2015) 2015:510436. doi: 10.1155/2015/510436
241. Karlsson I, Wernersson S, Ambrosen A, Kindahl H, Sodersten F, et al. Increased concentrations of C-reactive protein but not high-mobility group box 1 in dogs with naturally occurring sepsis. Vet Immunol Immunopathol. (2013) 156:64–72. doi: 10.1016/j.vetimm.2013.09.011
242. Lauhio A, Hastbacka J, Pettila V, Tervahartiala T, Karlsson S, Varpula T, et al. Serum MMP-8, -9 and TIMP-1 in sepsis: high serum levels of MMP-8 and TIMP-1 are associated with fatal outcome in a multicenter, prospective cohort study. Hypothetical impact of tetracyclines. Pharmacol Res. (2011) 64:590–4. doi: 10.1016/j.phrs.2011.06.019
243. Martin G, Asensi V, Montes AH, Collazos J, Alvarez V, Carton JA, et al. Role of plasma matrix-metalloproteases (MMPs) and their polymorphisms (SNPs) in sepsis development and outcome in ICU patients. Sci Rep. (2014) 4:5002. doi: 10.1038/srep05002
244. Kocaturk M, Eralp-Inan O, Tvarijonaviciute A, Cansev M, Ozyigit MO, Ceron JJ, et al. Effects of choline treatment in concentrations of serum matrix metalloproteinases (MMPs), MMP tissue inhibitors (TIMPs) and immunoglobulins in an experimental model of canine sepsis. Vet Immunol Immunopathol. (2016) 180:9–14. doi: 10.1016/j.vetimm.2016.08.011
245. Hasson DC, Watanabe-Chailland M, Romick-Rosendale L, Koterba A, Miner DS, Lahni P, et al. Choline supplementation attenuates experimental sepsis-associated acute kidney injury. Am J Physiol Renal Physiol. (2022) 323:F255–71. doi: 10.1152/ajprenal.00033.2022
246. Naveen Kumar MS, Gupta G, Kumar V, Jagannathan NR, Sinha S, Mewar S, et al. Differentiation between sepsis survivors and sepsis non-survivors through blood serum metabolomics: A proton nuclear magnetic resonance spectroscopy (NMR) study. Magn Reson Imaging. (2022) 89:49–57. doi: 10.1016/j.mri.2022.02.003
247. Peng J, Qiu C, Zhang J, and Xiao X. Serum metabolite profiling reveals metabolic characteristics of sepsis patients using LC/MS-based metabolic profiles: a cross-sectional study. BMC Med Genomics. (2023) 16:224. doi: 10.1186/s12920-023-01666-w
248. Rivera CA, Wheeler MD, Enomoto N, and Thurman RG. A choline-rich diet improves survival in a rat model of endotoxin shock. Am J Physiol. (1998) 275:G862–867. doi: 10.1152/ajpgi.1998.275.4.G862
249. Ilcol YO, Yilmaz Z, and Ulus IH. Endotoxin alters serum-free choline and phospholipid-bound choline concentrations, and choline administration attenuates endotoxin-induced organ injury in dogs. Shock. (2005) 24:288–93. doi: 10.1097/01.shk.0000174018.02688.4b
250. Parrish WR, Rosas-Ballina M, Gallowitsch-Puerta M, Ochani M, Ochani K, Yang LH, et al. Modulation of TNF release by choline requires alpha7 subunit nicotinic acetylcholine receptor-mediated signaling. Mol Med. (2008) 14:567–74. doi: 10.2119/2008-00079.Parrish
251. Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. (2004) 10:1216–21. doi: 10.1038/nm1124
Keywords: choline, acetylcholine (ACh), choline kinase (ChoK), phosphocholine, phosphatidylcholine (PC), inflammation, immune cells, immunity
Citation: Maia C, Fung CW and Sanchez-Lopez E (2025) Choline in immunity: a key regulator of immune cell activation and function. Front. Immunol. 16:1617077. doi: 10.3389/fimmu.2025.1617077
Received: 23 April 2025; Accepted: 14 July 2025;
Published: 01 August 2025.
Edited by:
Henry Kurniawan, University of Luxembourg, LuxembourgReviewed by:
Girdhari Lal, National Center for Cell Science, IndiaLaura Tarnawski, Karolinska Institutet (KI), Sweden
Copyright © 2025 Maia, Fung and Sanchez-Lopez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elsa Sanchez-Lopez, ZXNsMDIzQGhlYWx0aC51Y3NkLmVkdQ==
†These authors have contributed equally to this work
 Catarina Maia
Catarina Maia Chin Wai Fung
Chin Wai Fung Elsa Sanchez-Lopez
Elsa Sanchez-Lopez