- 1School of Exercise and Health, Shanghai University of Sport, Shanghai, China
- 2Human Performance Laboratory, North Carolina Research Campus, Appalachian State University, Kannapolis, NC, United States
- 3School of Athletic Performance, Shanghai University of Sport, Shanghai, China
- 4Shanghai Key Laboratory of Human Performance, Shanghai University of Sport, Shanghai, China
- 5Research Institute for Doping Control, Shanghai University of Sport, Shanghai, China
- 6Department of Rheumatology and Immunology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
The total exercise workload is an important factor influencing immune health. Appropriately prescribed physical activity can mitigate the detrimental effects of chronic inflammation, bolster the body’s defenses against both infectious and non-infectious diseases, and decelerate the immunosenescence process. Physiological and immune system responses to moderate and strenuous exercise workloads vary markedly. This narrative review summarizes current findings on the impacts of moderate-intensity exercise, high-intensity interval training, and strenuous and prolonged exercise on immune health, elucidating their specific effects and underlying mechanisms. Additionally, the role of challenging environmental conditions in shaping immune responses to exercise is also briefly considered. The insights presented here are intended to guide healthy individuals in selecting evidence-based exercise training protocols that are compatible with both health promotion and immune health. Moreover, this review offers prospective research directions, particularly regarding personalized exercise regimens and the interaction between exercise and environmental factors, providing valuable perspectives for scholars within the field of exercise immunology.
1 Introduction
The immune system is a key defensive mechanism that safeguards human health. It identifies and eliminates foreign pathogens, while monitoring and maintaining internal homeostasis. This complex system comprises innate and adaptive immunity, jointly forming the body’s first and second lines of defense against infections and disease (1–3). Modern lifestyle changes, such as prolonged sedentary behavior, irregular sleep patterns, and unhealthy diets have significantly influenced the immune system, resulting in immune dysfunction and heightened disease risk. According to the World Health Organization (WHO), the global incidence of measles reached 10.3 million cases in 2023, representing an almost 60% increase from the previous year. Cancer incidence continues to rise, with approximately 20 million new cases and about 9.7 million deaths worldwide in 2022, and is projected to increase by 77% by 2050 (4). Exercise, as an effective regulatory measure, has been shown to enhance immune cell activity and restore immune system balance, thereby bolstering disease resistance. Consequently, exercise interventions have emerged as an important strategy to confront modern lifestyle challenges, improve immune health, and prevent diseases (5–7).
The immunomodulatory effects of exercise depend on exercise intensity and the total exercise workload (8). Moderate-intensity exercise at recommended workloads (150–300 minutes per week as defined by multiple public health agencies) has proven effective in enhancing immune cell function, strengthening the body’s anti-infection capacity, and improving overall defense. For instance, regular moderate-intensity training can lower the risk of upper respiratory tract infections (URTIs) (9), improve survival rates and reduce inflammatory cytokine levels in mice with acute pneumonia (10), and even mitigate age-related declines in immune function (11). High-intensity interval training (HIIT) at recommended levels has emerged as an effective protocol for improving exercise performance, skeletal muscle capacity (12), cardiovascular health (13), and immune health (14). Strenuous and prolonged exercise may lead to immunosuppression lasting from 3 to 72 hours, during which immune cell counts and functions decline in peripheral blood, increasing susceptibility to infections (8, 15–17). Thus, different exercise workloads exert notably distinct effects on immune health, underscoring the need to select appropriate exercise intensities and workloads to maximize immune health benefits and minimize potential immune impairment.
In this context, the present study aims to review the existing literature to analyze the effects of different types of exercise workloads on immune cell function (Figure 1), cytokine profiles (e.g., IL–6, TNF–α, IL–10), mucosal immunity, and immunoglobulin production. The goal is to improve scientific understanding regarding the mechanisms underlying the interactions between exercise and immunity, and to explore how optimized exercise interventions can effectively enhance immune function, including innate cellular responses, regulatory cytokines, and antibody production.
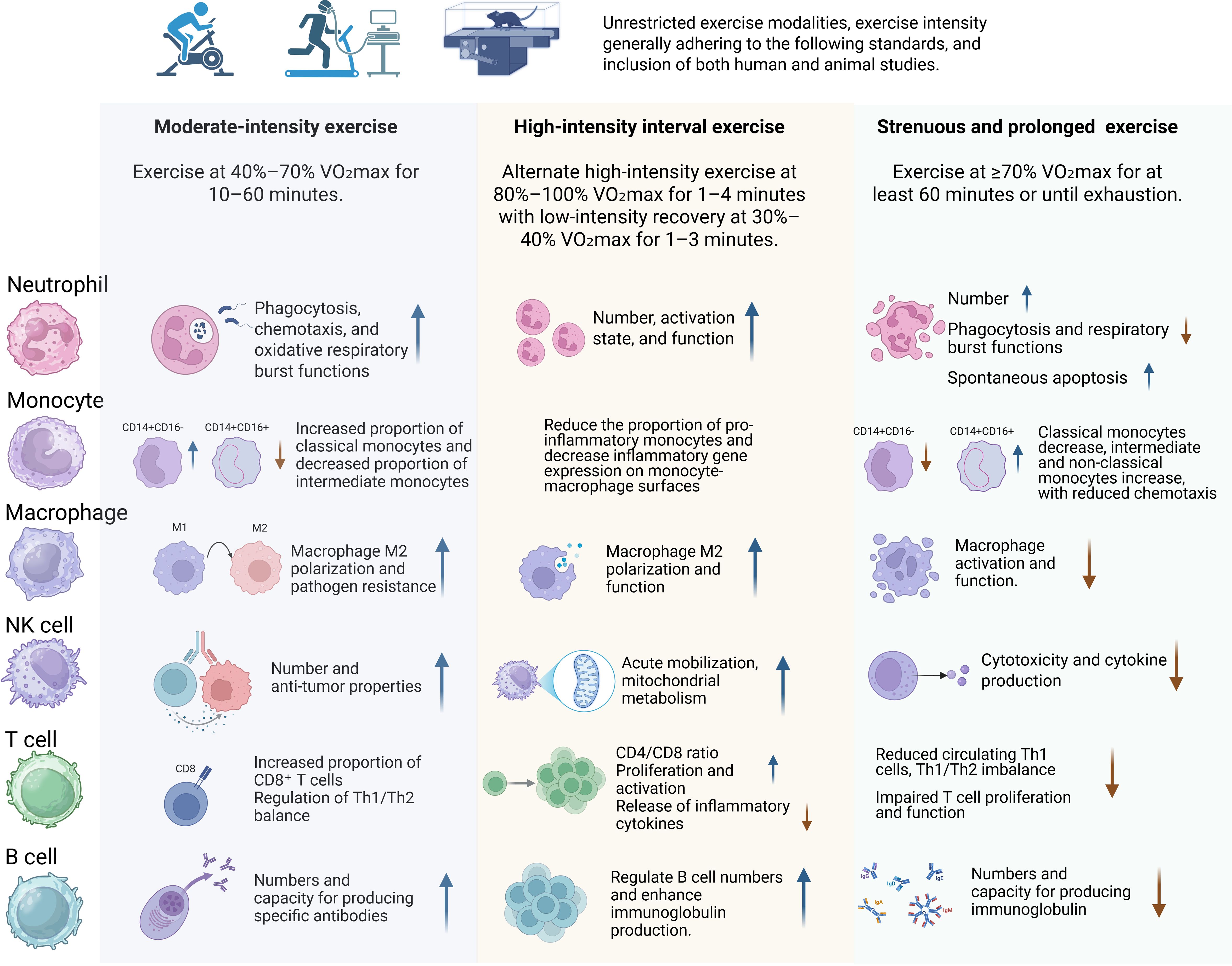
Figure 1. The impact of various exercise workloads on immune cells in both human populations and mice models.
To compile relevant literature for this review, we conducted a structured search of the PubMed, Web of Science, and Scopus databases to identify articles published between January 1990 and March 2025. The following Boolean search string was applied:(‘exercise’ OR ‘physical activity’ OR ‘training’) AND (‘immune function’ OR ‘cytokine’ OR ‘interleukin’ OR ‘TNF’ OR ‘IL-6’ OR ‘IL-10’ OR ‘immunoglobulin’ OR ‘NK cells’ OR ‘T lymphocytes’ OR ‘B lymphocytes’ OR ‘neutrophils’ OR ‘macrophages’ OR ‘inflammation’) AND (‘moderate intensity’ OR ‘HIIT’ OR ‘high-intensity interval training’ OR ‘strenuous’ OR ‘prolonged’ OR ‘endurance’ OR ‘resistance’). Inclusion criteria required studies to be published in English, to report original experimental or observational data (human or animal) on exercise-induced changes in immune parameters, and to clearly define the type and intensity of exercise involved. To ensure completeness, additional relevant studies were retrieved by manually reviewing the cited references of core articles identified in the initial search.
2 Defining exercise workloads and intensity
According to the American College of Sports Medicine (18), supported by international guidelines (19, 20), exercise intensity is commonly categorized as follows: low intensity is defined as <40% VO2max, moderate intensity ranges from 40% to 70% VO2max, and high intensity typically spans from 60% to 84% VO2max.
Low-intensity exercise is generally suitable for beginners and individuals in recovery, as it does not induce significant fatigue. Common activities include leisurely walking and slow cycling. Moderate-intensity exercise is appropriate for most healthy adults and helps improve cardiovascular function and aerobic capacity (21). High-intensity exercise, on the other hand, demands a certain level of physical fitness. During such exercise, individuals experience rapid breathing and typically struggle to maintain a conversation, but high-intensity training can significantly improve VO2max and enhance performance. High-intensity interval training (HIIT), characterized by alternating between brief bursts of near-maximal effort and recovery periods, is a key example of high-intensity exercise that elicits significant physiological responses. Research indicates that, compared to sustained moderate-intensity exercise, HIIT can significantly improve VO2max in a shorter duration (22). However, other studies suggest that intense and prolonged high-intensity exercise may temporarily suppress the immune system, increasing the risk of infection, particularly in competitive environments (17). Therefore, understanding the differences in immune responses to various exercise workloads is crucial for designing training programs that optimize immune health and disease prevention.
3 The impact of different exercise workloads and intensities on the immune system
3.1 Effects of moderate-intensity exercise on the immune system
Acute and chronic moderate-intensity exercise training is generally regarded as an immune system adjuvant that has multiple beneficial effects in stimulating both innate and adaptive immunity and lowering the risk for infectious diseases (17).
3.1.1 Effects on neutrophils
Multiple studies have revealed positive effects of regular moderate-intensity exercise on neutrophil function. Such exercise enhances neutrophil phagocytic activity and oxidative burst capacity, significantly improving their function (23). In healthy women, moderate-intensity exercise improves neutrophil chemotaxis, phagocytosis, and bactericidal capacity more distinctly than high-intensity exercise (24). In overweight adults with cardiovascular risk factors, moderate-intensity training helps maintain neutrophil bactericidal activity (25). Moreover, moderate-intensity exercise increases total glutathione (GSH) levels, elevates antioxidant reserves, enhances mitochondrial membrane potential, and delays neutrophil apoptosis (26).
Research by Mooren et al. showed that the combined effect of fasting and moderate-intensity exercise significantly enhances oxidative burst activity in neutrophils post-exercise (27). Hypoxic conditions can further augment exercise-induced immune effects, increasing neutrophil phagocytosis of E. coli and activating NADPH oxidase, thereby enhancing oxidative burst and pathogen clearance (28). The duration of moderate-intensity exercise is also important; acute exercise (30 minutes of moderate-intensity walking) can increase circulating neutrophils in patients with chronic kidney disease (CKD), boosting their anti-infective potential, whereas long-term regular exercise (30 minutes/day, 5 times a week for 6 months) may not significantly influence neutrophil degranulation (29). Furthermore, acute exercise significantly upregulated the gene expression of several cytokines in skeletal muscle, particularly the monocyte chemoattractant CCL2 (MCP-1) and the neutrophil-attracting CXCL2 (Gro-β), suggesting that skeletal muscle may attract immune cells through the secretion of these signaling molecules to promote inflammation and tissue repair post-exercise (30). Collectively, moderate-intensity exercise exerts multifaceted positive effects on neutrophils, improving their phagocytic and bactericidal functions, antioxidant defenses, and environment-dependent immune responses, ultimately strengthening the body’s immune defenses.
3.1.2 Effects on monocyte-macrophage populations
Moderate-intensity exercise training can affect the distribution of monocyte subsets and macrophage function. After 10 weeks of moderate-intensity exercise in healthy adults, the proportion of classical monocytes (CD14++/CD16-) in peripheral blood increased, while intermediate monocytes (CD14+/CD16+) and non-classical monocytes (CD14-/CD16++) decreased. Additionally, the expression of CD16, TLR2, and TLR4 on monocyte surfaces was reduced, weakening the pro-inflammatory phenotype of monocytes (31), indicating that sustained moderate exercise promotes immune regulation.
Long-term moderate-intensity exercise induces metabolic rewiring in macrophages, enhancing mitochondrial function and shifting macrophage responses toward a more anti-inflammatory state by modulating biosynthetic and bioenergetic processes (32). Studies have also shown that exercise training can regulate the immune microenvironment, promoting the polarization of macrophages toward the M2 phenotype (33). For example, a study by Knudsen et al. demonstrated that endurance training induces systemic increases in IL-13, which acts via the IL-13Rα1–Stat3 axis to reprogram skeletal muscle metabolism. This signaling promotes mitochondrial biogenesis and fatty acid oxidation, and indirectly facilitates a shift toward an anti-inflammatory M2 macrophage phenotype (34).
It promotes a shift toward M2-related gene expression and reduces M1-related gene expression, driving macrophages toward an M2 phenotype and lowering inflammation. For example, after 8 weeks of moderate-intensity exercise, sedentary women showed an increase in M2 markers (such as Dectin-1 and IL-10) and a decrease in M1 markers (such as MCP-1) on monocytes, thereby influencing macrophage polarization (35). Blanks et al. further demonstrated that habitual exercisers displayed lower CCR2 expression on intermediate monocytes and a greater propensity for M2 macrophage polarization following moderate-intensity cycling (36). These effects were partially sex-dependent, as shown by Blanks et al. (37), who linked post-exercise CCR2 downregulation to ERK1/2 signaling specifically in female participants (37). Moreover, moderate exercise can lower the expression of CXCR2 on CD14+/CD16+ monocytes in the peripheral blood of obese adults, reducing inflammatory monocyte infiltration into tissues (such as adipose tissue) and alleviating obesity-related chronic low-grade inflammation (38).
Although long-term endurance training and the late recovery phase after acute bouts tend to promote M2-like macrophage polarization, the early phase following acute exercise is dominated by the mobilization of pro-inflammatory monocytes and an increase in M1-like macrophage infiltration, particularly in skeletal muscle tissues (39–41). Voskoboynik et al. also indicated in their study that long-term training favors the M2 phenotype to promote recovery and metabolic remodeling, while acute exercise initiates M1 activation to respond to immune stress (42). This biphasic response underscores the need to consider temporal dynamics when evaluating immune adaptations to exercise. In addition, acute aerobic exercise induces robust macrophage infiltration and chemokine (e.g., CXCL12) release in skeletal muscle, especially in individuals with type 2 diabetes. The altered immune profile highlights the role of exerkines and cross-talk between myocytes and immune cells in tissue adaptation (43).
While above human trials animal studies provide insights into the underlying mechanisms, similar effects on macrophage function have been observed in animal studies. In mouse models, moderate-intensity training improves macrophage function and enhances disease resistance. Regular moderate-intensity training can reverse age-related declines in antiviral characteristics of peritoneal and alveolar macrophages (11). Pre-exercise training for 6 days strengthens macrophage resistance to HSV-1 in mice (44), and 8 weeks of moderate exercise increases alveolar and interstitial macrophage counts in mice with acute pneumonia, altering expression of alveolar macrophage markers CD64 and MHC II, thereby creating an anti-inflammatory healing environment (10). Furthermore, in aging contexts, exercise exerts systemic immunoregulatory effects across multiple tissues. A recent study in elderly mice found that aerobic training reduces inflammation-related pathways (e.g., NF-κB, TNF) and restores communication between stem cells and immune populations in muscle, brain, and blood. These changes reestablished regenerative niches and reinforced anti-inflammatory macrophage phenotypes, indicating a rejuvenation of the immune microenvironment (45).
These findings highlight the key role of moderate-intensity exercise in improving macrophage distribution, function, and phenotype, thus enhancing immune defense and reducing chronic inflammation.
3.1.3 Effects on natural killer cells
Moderate-intensity exercise mainly enhances NK cell number, activity, and antitumor properties, thereby improving immune surveillance and tumor defense. Acute moderate-intensity exercise transiently increases NK cell counts, which return to baseline within a few hours (46). NK cells, integral to innate immunity, play a pivotal role in tumor surveillance (47, 48). Chronic moderate-intensity exercise has demonstrated a mobilizing effect on NK cells, hence augmenting their activity and anti-tumor capabilities (49). Gannon et al. found that moderate-intensity cycling significantly increased peripheral NK cell counts and NK cytotoxic activity (NKCA) (50). Six weeks of moderate-intensity exercise also increased the cytotoxic NK cell subset in whole blood, implying enhanced antitumor potential (51).
In addition to their roles in cytotoxic defense against viruses and tumors, NK cells are also critically involved in the clearance of senescent cells, a process that contributes to tissue homeostasis and aging regulation. Recent human studies show that even short durations of moderate-intensity exercise (e.g., 15 minutes at ~55% heart rate reserve) can mobilize cytotoxic NK cells into the bloodstream (52). These effects extend to functionally relevant subsets, such as CD56dim and CD57+ NK cells, which have been observed to increase after moderate exercise in pediatric hematopoietic stem cell transplant recipients (53) and cancer patients undergoing chemotherapy (54). Moreover, muscle-derived cytokines like IL-15 appear to promote NK cell proliferation and cytotoxicity, including against senescent targets (55). Supporting this, a recent meta-analysis demonstrated that senescent NK cell subsets (e.g., CD57+) remain elevated for more than an hour post-exercise, reinforcing the hypothesis that physical activity may contribute to immune-mediated senolysis (56).
These findings further support the concept that moderate-intensity exercise not only enhances immune surveillance of NK cells in the context of tumor defense but may also contribute to the clearance of senescent cells, thus playing a role in aging and tissue homeostasis.
3.1.4 Effects on T lymphocytes
Moderate-intensity exercise can alter T-cell subset distribution and enhance specific T-cell subset functions. Acute moderate exercise significantly increases CD8+ T-cell counts in lymphoma patients, with levels returning to baseline within 30 minutes post-exercise (57). Regular moderate-intensity training prevents declines in CD4+ T-cell counts observed after strenuous exercise (58) and enhances CD4+ T-cell proliferation and cytokine production following vaccination, improving vaccine efficacy (59). CD4+ and CD8+ T cells can differentiate into Th1 or Th2 subsets, with Th1 cells producing pro-inflammatory cytokines (IL-2, IFN-γ) for cell-mediated immunity and Th2 cells producing anti-inflammatory cytokines (IL-4, IL-10) for humoral immunity (60). Moderate-intensity training improves both Th1 and Th2 functions, maintaining Th1/Th2 balance. Studies show that acute moderate exercise increases the IL-2/IL-4 ratio in allergic rhinitis patients, reducing inflammation (61), while 2 months of moderate training in healthy males significantly increased IFN-γ and IL-2 levels in PBMC cultures, enhancing Th1 responses (62). In older adults, regular moderate-intensity exercise increases CD28 expression and Th1 cell count, improving overall immunity (63). Furthermore, Drela et al. observed that moderate exercise enhances IL-2 production, mitigating immunosenescence in older individuals (64).
Animal models confirm that moderate-intensity exercise can adjust Th1/Th2 balance, boost IFN-γ production, reduce IL-4 and IL-10, and favor Th1-mediated responses to intracellular pathogens. For example, 8 weeks of moderate exercise significantly raised the IFN-γ/IL-4 ratio in mice, enhancing Th1 responses (65). Kohut et al. found that 8 weeks of moderate-intensity training in aged mice augmented Th1 functions and cytokine release, improving infection resistance (66). In influenza-infected mice, moderate-intensity training reduced pulmonary IFN-γ gene expression and shifted a Th1 inflammatory to a Th2 anti-inflammatory response, increasing survival rates (67).
Overall, moderate-intensity exercise supports T-cell function and sustains Th1/Th2 equilibrium, promoting a balanced immune response that enhances both cell-mediated immunity (Th1) and humoral immunity (Th2). This shift is essential for maintaining immune function, enhancing immune defense, and promoting healthy aging by preventing excessive inflammatory responses (68, 69).
3.1.5 Effects on B lymphocytes
Moderate-intensity exercise positively influences B-cell function and antibody production, strengthening immune defense. Acute moderate-intensity training increased total immunoglobulin E (IgE) levels in allergic rhinitis patients (61) and enhanced immunoglobulin M (IgM) and immunoglobulin G (IgG) levels following influenza vaccination in older adults (70), improving vaccine responses. In obese postmenopausal women, 15 weeks of moderate-intensity training significantly elevated serum IgG, IgM, and IgA (71), confirming its beneficial effects on immunoglobulin production.
Regular moderate-intensity exercise also increases salivary secretory IgA (SIgA) in older adults (72, 73), bolstering mucosal immunity as a first line of defense. In animal models, prolonged moderate-intensity training reduced B-cell numbers in the duodenal lamina propria but significantly raised IgA levels, improving resistance to gut pathogens (74). In older mice, moderate exercise increased B-cell and IgA+ B-cell counts and factors related to IgA synthesis, thus strengthening intestinal IgA production and immune function (75). Hence, moderate-intensity exercise fosters B-cell proliferation and antibody generation, enhancing mucosal immunity and pathogen defense.
3.1.6 Effects on cytokines
Moderate-intensity exercise exerts a wide range of effects on cytokines, especially in the modulation of critical cytokines like IL-6, TNF-α, and IL-10.The release of IL-6 from contracting skeletal muscle, first identified as a ‘myokine’ nearly two decades ago, represents a hallmark cytokine response to endurance exercise. IL-6 levels can rise up to 100-fold during prolonged activity and play key roles in hepatic glucose output, adipose tissue lipolysis, and anti-inflammatory signaling (76, 77). Moderate-intensity exercise has a dual effect on IL-6, encompassing both short-term immune activation and long-term anti-inflammatory adaptations. Acute moderate-intensity exercise typically triggers a significant increase in IL-6 release due to skeletal muscle contractions, marking a short-term immune response. This increase in IL-6 usually returns to baseline levels within 24 hours (78). Additionally, IL-6 acts not only as a pro-inflammatory mediator during this initial response but also helps modulate anti-inflammatory factors such as IL-10, maintaining immune homeostasis after exercise (79). This mechanism is essential for regulating post-exercise immune function. In contrast, long-term moderate-intensity exercise produces distinct effects. Sustained moderate exercise helps regulate IL-6 levels, promoting immune balance, alleviating chronic low-grade inflammation, and improving metabolic health (80). For example, 6 weeks of regular training reduced the IL-6/IL-10 ratio in patients with CKD (29). One year of moderate-intensity combined with resistance training (MICT+RT) significantly lowered plasma IL-6 in type 2 diabetes patients (81). Animal studies similarly show that moderate exercise lowers IL-6 in septic and diabetic rats, mitigating inflammation and improving survival (82, 83).
Moderate exercise also lowers TNF-α levels, reducing inflammation. Acute exercise decreases TNF-α production by monocytes, mediated by the sympathetic nervous system and β2-adrenergic receptors (84). Prolonged moderate training can maintain long-term suppression of TNF-α, diminishing chronic inflammation. For instance, 6 months of training lowered TNF-α mRNA expression in fluorosis-exposed mice (85). In humans, moderate exercise reduces TNF-α in individuals with high-fat diets or obesity, decreasing low-grade chronic inflammation (86, 87). Moreover, moderate exercise enhances IL-10 secretion, fostering an anti-inflammatory environment. In patients with chronic fatigue syndrome, older adults, and those with systemic lupus erythematosus, moderate-intensity exercise significantly increases IL-10 levels and reduces inflammation (88–90). Animal models corroborate this effect, showing that moderate exercise increases IL-10 concentration and improves immune function while reducing inflammation (74, 86, 91).
Although moderate-intensity exercise has been widely studied for its acute immunological effects, it is important to note that not all individuals can safely or consistently perform at this level. For such populations, low-intensity physical activity may offer an accessible and physiologically beneficial alternative.
While low-intensity physical activity (e.g., walking, stretching, tai chi, Pilates) may not trigger the acute and measurable immune shifts observed with higher-intensity modalities, emerging research suggests it confers significant long-term health benefits. Studies have shown that low-intensity exercise avoids post-exercise immunosuppression (92), promotes anti-inflammatory cytokine expression such as IL-4 (93), enhances dendritic cell and T cell proliferation (94), and elevates mucosal sIgA levels in older adults (95). Furthermore, programs such as low-impact Pilates have been found to alter adaptive immune cell distributions (96), while regular participation may help mitigate age-related immune decline (97). These findings underscore the potential of low-intensity physical activity as a safe and sustainable immunoregulatory strategy, particularly for vulnerable populations.
3.2 Effects of high-intensity interval training on the immune system
The number of HIIT studies investigating immune responses in human participants is limited but expanding. Most studies indicate that immune responses to HIIT at recommended levels are rapid but modest and transient, similar to the beneficial responses to moderate, sustained exercise bouts.
3.2.1 Effects on neutrophils
HIIT has a positive role in increasing neutrophil counts, enhancing their functionality, activating innate immune responses, and bolstering pathogen defense. Acute HIIT significantly increases neutrophil counts (98) and elastase release upon lipopolysaccharide (LPS) stimulation (99), indicating greater neutrophil activation and function. Compared to continuous moderate exercise, HIIT activates neutrophil-mediated innate immune responses earlier (100). Moreover, HIIT improves neutrophil phagocytic and respiratory burst capacities (31), chemotaxis, and mitochondrial function (101). Bartlett et al. found that HIIT improves neutrophil function in rheumatoid arthritis patients, including migration accuracy, enhanced phagocytosis, and increased reactive oxygen species (ROS) production (14). HIIT also reduces excessive neutrophil extracellular trap formation (NETosis) in older men, potentially preventing inflammatory diseases (102).
Animal studies show that HIIT enhances phagocytic capacity in type 1 diabetic rats (103) and reduces lung neutrophil infiltration in acute lung injury models by lowering bronchoalveolar lavage fluid (BALF) MPO levels, thereby alleviating inflammatory damage (104). These findings underscore the positive effects of HIIT on neutrophil function and innate immunity.
3.2.2 Effects on monocyte-macrophage populations
Acute High-Intensity Interval Training (HIIT) triggers a temporary immune activation in monocytes. Studies show that a single session of HIIT significantly decreases TLR2 expression on classical and CD16+ monocytes, indicating its anti-inflammatory properties and the ability to modulate monocyte immune responses (105). Additionally, acute HIIT also indirectly influences monocyte activation by decreasing postprandial triglyceride levels, increasing CD11b and reducing CD36 expression on monocytes (106).
Chronic HIIT exerts more pronounced effects on monocytes and macrophages, especially in individuals with obesity and metabolic disorders. De Matos et al. demonstrated that 8 weeks of regular HIIT reduced the proportion of non-classical monocytes (CD14-/CD16++) and intermediate monocytes (CD14+/CD16+) in obese adults, restoring the balance of monocyte subsets (107). Moreover, 12 weeks of HIIT in obese men with non-alcoholic fatty liver disease (NAFLD) altered monocyte subset distribution and activation states, decreasing inflammatory gene expression (TLR4, CD11b, and CD14), thereby alleviating inflammation (108). Animal studies further support that long-term HIIT can reduce pro-inflammatory monocyte subsets in the liver and stimulate anti-inflammatory responses, markedly improving the inflammatory condition of non-alcoholic steatohepatitis (NASH). This effect was more pronounced compared to long-term moderate-intensity training (109). These findings suggest that HIIT is effective in ameliorating obesity-related immune dysfunction and inflammation.
Regarding macrophage polarization, long-term HIIT reduces M1 markers (CD86) while increasing M2 markers (CD206), which helps mitigate liver inflammation (110). Animal studies also show that regular HIIT can modulate the activation of monocyte-macrophages in muscle tissue, reducing M1 macrophages and increasing M2 macrophages (111), while also reversing high-fat diet-induced M1 polarization and further promoting M2 polarization, thereby improving metabolic and immune status (112).
In summary, acute HIIT triggers short-term immune activation in monocytes, while chronic HIIT offers lasting anti-inflammatory benefits by balancing monocyte subsets and promoting macrophage polarization towards the M2 phenotype, especially in individuals with obesity and metabolic disorders.
3.2.3 Effects on natural killer cells
Acute HIIT rapidly mobilizes natural killer (NK) cells into the peripheral blood, particularly cytotoxic subpopulations expressing CD57, CD158a, NKG2D, TIM-3, and CXCR3 (cNK), which return to baseline levels after one hour, thereby balancing the distribution of NK cell subsets (54). Furthermore, both animal and human studies confirm that prolonged HIIT training increases circulating NK cell numbers (113).
Additionally, both acute and long-term HIIT have been shown to enhance NK cell activity and function. A single session of HIIT boosts NK cell activity by elevating the activities of antioxidant enzymes and their biological roles (98). Lin et al. demonstrated that 6 weeks of HIIT enhances NK cell mitochondrial membrane potential, reduces mitochondrial oxidative burden (MOB), and increases perforin and granzyme B levels, thereby improving NK cell cytotoxicity against tumor cells (51). In cancer patients, HIIT has been shown to increase NK cell counts and tumor infiltration, augmenting antitumor immunity (114, 115), while higher training doses yield better tumor NK cell infiltration (115). Thus, HIIT supports NK cell mobilization, activity, mitochondrial function, and antitumor cytotoxicity, strengthening immune surveillance.
3.2.4 Effects on T lymphocytes
Acute HIIT has a significant impact on the quantity and function of T cells. Studies show that acute HIIT increases the number of CD4+ and CD8+ T cells in peripheral blood and raises the CD4/CD8 ratio, but this increase typically returns to near pre-exercise levels within 3 hours post-exercise (99). Additionally, acute HIIT rapidly boosts the number of circulating regulatory T cells (Tregs), exerting a short-term immunoregulatory effect. Krüger et al. (116) found that acute HIIT significantly increased Treg numbers in peripheral blood immediately after exercise and up to 3 hours post-exercise, indicating that HIIT can quickly enhance immune regulation through Treg modulation (116). However, acute HIIT may also induce redox imbalance in T cells, leading to reduced T cell proliferation in response to superantigen (SEB) stimulation, although this does not affect early activation or apoptosis (117).
The effects of long-term HIIT on T cells are more persistent, particularly in individuals with low cardiorespiratory fitness (CRF). Dorneles et al. found that long-term HIIT significantly increased the proportion of CD4+CD25highCD127low Tregs and CD39+ memory Tregs in obese men with low CRF, enhancing Treg immunoregulatory function through upregulation of CD39 and CD73 (118). Furthermore, after 3 weeks of HIIT training, the CD4/CD8 T cell ratio in peripheral blood was significantly increased compared to moderate-intensity training, suggesting that HIIT may be more effective than sustained moderate-intensity exercise in regulating immune cell ratios and reducing inflammation (111). Câmara et al.observed that after 5 weeks of HIIT, IL-4 (Th2 cytokine) levels decreased, while the IFN-γ/IL-4 ratio significantly increased, indicating a shift towards Th1 cells, which enhanced CD4+ T cell autophagy and anti-apoptotic capacity, mitigating hypoxia-induced damage (119). Although long-term HIIT improves T cell function, it may exacerbate immune aging in certain populations, such as older women at high risk for breast cancer, leading to a decrease in CD4+ T cell counts and a lower CD4/CD8 ratio, suggesting that HIIT may promote immune aging (120).
In conclusion, acute HIIT exerts a rapid immunoregulatory effect by increasing Tregs and modulating T cell subsets. Long-term HIIT improves T cell subset distribution and enhances immune regulation. However, the effects of HIIT may vary across different populations, particularly in terms of immune aging, and long-term HIIT may need to be tailored to individual conditions.
3.2.5 Effects on B lymphocytes
HIIT acutely alters B-cell counts, leading to a transient decrease immediately post-exercise, followed by a return to baseline within 30 minutes (117). HIIT also increases immunoglobulin production, enhancing mucosal immunity. A single HIIT session significantly increased salivary sIgA levels especially in female athletes (121). Three weeks of HIIT elevated sIgA secretion rates in healthy males, maintaining mucosal immunity (122). Correspondingly, in obese women, 8 weeks of HIIT raised serum IgA, improving mucosal defense (123). These studies collectively suggest that acute HIIT boosts mucosal immunity temporarily by increasing sIgA, while chronic HIIT can lead to more sustained improvements in serum IgA and immune defense.
3.2.6 Effects on cytokines
The effects of high-intensity interval training on cytokines are characterized by a combination of acute responses and prolonged adaptive modifications. Acutely, HIIT elevates IL-6, TNF-α, and IL-10, reflecting an initial immune activation. However, the magnitude of increase in plasma cytokine levels following recommended levels of HIIT is modest, especially when compared to responses following prolonged, strenuous running or cycling. Healthy individuals and patients with colorectal cancer, chronic heart failure, or type 1 diabetes show significant but moderate increases in IL-6 and TNF-α after a single HIIT session, returning to baseline within several hours to a day (124–129). IL-10 also rises acutely, initiating anti-inflammatory processes (130, 131). Long-term HIIT training reduces chronic low-grade inflammation similar to the effects of long-term moderate aerobic exercise training regimens. Twelve weeks of HIIT lower IL-6 and TNF-α in obese adolescents and type 2 diabetes patients (132, 133), while enhancing IL-10 secretion to support anti-inflammatory capacity (134, 135). Animal studies similarly confirm that 8 weeks of HIIT reduce IL-6 and TNF-α in kidney-injured mice, improving inflammatory status (136, 137).
Furthermore, chronic HIIT training diminishes acute immune fluctuations and modulates cytokine expression in various disease models, promoting health. In obesity, type 2 diabetes, and Parkinson’s disease, HIIT effectively lowers TNF-α and alleviates chronic inflammation (138–140). It also consistently elevates IL-10, enhancing anti-inflammatory capacity in obese men, type 2 diabetes patients, and post-percutaneous coronary intervention (PCI) patients (135, 141, 142). Basic research further supports IL-10 enhancement by HIIT, mitigating age-related inflammation and improving immunity (139, 143) (144). Collectively, HIIT training can effectively enhance immune function and modulate inflammation status by regulating levels of IL-6, TNF-α, and IL-10, thereby having significant benefits for health and disease management.
3.3 Effects of prolonged and strenuous exercise on the immune system
Prolonged and intensive exercise induces physiological and metabolic stress and a corresponding and profound effect on both innate and adaptive immunity.
3.3.1 Effects on neutrophils
Strenuous or exhaustive exercise elicits pronounced innate immune responses. After exhaustive exercise, neutrophil numbers significantly increase (neutrophilia), particularly after prolonged (over 2 hours) activity (145). This surge is transient but accompanies declines in phagocytic and oxidative burst functions (146). For instance, during militarized training, the number of neutrophils in healthy men increased significantly and sustained at elevated levels (147). Marathon running increases peripheral band neutrophils, indicating bone marrow release (148). Furthermore, in animal experiments, it was observed that elevated neutrophil infiltration into muscle, kidney, and liver may exacerbate tissue injury (149, 150), and neutrophil depletion can be a factor alleviating liver damage following exhaustive exercise (150).
Acute strenuous exercise also impairs neutrophil phagocytic function and increases ROS levels (151), causing oxidative stress, reducing mitochondrial membrane potential, and accelerating neutrophil apoptosis (26). In elite male athletes, neutrophil phagocytic function significantly declined 2 hours post-exercise and persisted at 6 and 24 hours (152). Furthermore, high-intensity exercise to fatigue affects degranulation responses, which return to normal within 24 hours, whereas persistent functional suppression occurs after prolonged low-intensity exercise to exhaustion (153). Such findings illustrate the complexity of strenuous exercise’s impact on neutrophils, potentially contributing to post-exercise immunosuppression.
3.3.2 Effects on monocyte-macrophage populations
Acute and long-term intense/exhaustive exercise affects the monocyte-macrophage system through different mechanisms, activating pro-inflammatory responses, disrupting immune function, and causing tissue damage, thereby increasing susceptibility to infections. Studies have found that after a single bout of exhaustive exercise in healthy subjects, the number of classical monocytes (CD14++/CD16-) decreases, while the numbers of intermediate (CD14+/CD16+) and non-classical monocytes (CD14-/CD16++) increase. Additionally, LPS stimulation leads to reduced IL-6 and IL-10 production by monocytes, while TNF-α secretion increases, indicating a shift to a pro-inflammatory state (154). Acute intense exercise also increases the expression of TLR2 and TLR4 on monocyte surfaces and decreases HLA-DR expression, weakening the antigen-presenting ability of monocytes (155). Animal studies show that after acute exhaustive exercise, M1 macrophages in the liver are activated (156), and macrophages migrate to muscle, liver, and kidney, promoting the release of pro-inflammatory cytokines and exacerbating tissue damage (157–159). Moreover, acute exhaustive exercise reduces macrophage survival and antiviral capacity (160). These studies suggest that acute intense exercise activates the monocyte-macrophage system, amplifying the inflammatory response and increasing tissue damage. Chronic intense exercise primarily affects monocytes by weakening their chemotaxis and altering immune function. Studies have shown that three weeks of intense exercise training may impair monocyte chemotaxis (161). Furthermore, chronic intense exercise can impair macrophage microbial killing mechanisms, increasing infection susceptibility (162).
3.3.3 Effects on natural killer cells
Strenuous exercise significantly affects NK cell counts and activity. During acute strenuous exercise, NK cell counts rise, possibly due to catecholamine-mediated demargination (163, 164). However, after over one hour of intense activity, NK cell counts drop below baseline levels and may remain low for up to 8 hours, returning to resting levels after 24 hours (152, 165). While NK cell activity may initially surge, it eventually becomes suppressed after exercise, prolonging the immunosuppression window and increasing infection risk (16, 166). Studies have confirmed that after acute intense exercise, NK cell function is suppressed, with a decrease in IFN-γ production upon in vitro stimulation, and this suppressive effect persists for up to 2 hours post-exercise (163). Eda et al. found that exercising at 75% V̇O2max for 60 minutes significantly reduced NK cell activity in healthy men, with the suppression persisting until the following day (167). Kohut’s study also showed that exhaustive exercise reduces NK cell cytotoxicity in mice (168). Overall, the decrease in NK cell activity after intense exercise is a key factor in temporary immune suppression.
However, prolonged high-intensity exercise has less impact on NK cell numbers but also leads to NK cell functional suppression, particularly in cases of overtraining. This functional exhaustion results in a reduced immune response, increasing the risk of infections.
3.3.4 Effects on T lymphocytes
Strenuous exercise induces complex effects on T cells, including temporary changes in T-cell numbers, subset shifts, functional adjustments, and altered cytokine production. After acute high-intensity or prolonged exercise, both CD4+ and CD8+ T cell counts in the blood increase, with a more significant rise in CD8+ T cells. During the recovery phase (30 minutes to 6 hours post-exercise), CD8+ T cell counts rapidly decline, possibly even below baseline levels, leading to lymphocytopenia. Within 24 hours, CD8+ T cell counts generally return to resting levels. Meanwhile, Th1 cytokine production (e.g., IFN-γ) decreases, while Th2 cytokines (e.g., IL-10) increase, shifting the immune response toward Th2, which may impair antiviral and antitumor immunity (60, 68). Studies by Lancaster et al. found that, after acute intense exercise, the number of Th1 cells significantly decreased, and their ability to produce IFN-γ was notably reduced, while Th2 cell numbers and IL-4 production remained unchanged (69, 169). Kakanis et al.confirmed that after strenuous cycling, Th2 cytokines increased in peripheral blood, while Th1 cytokine production increased at 4 and 8 hours post-exercise, showing a transient shift from Th1 to Th2 (170). Mouse studies also indicated that after acute intense exercise to exhaustion, Th1 cytokines (e.g., IFN-γ) decreased, while Th2 cytokines (e.g., IL-4) either remained unchanged or increased, potentially weakening immunity against intracellular pathogens and raising infection risk (168). Acute high-intensity exercise also increases Tregs and FoxP3+ Tregs in the peripheral blood of adolescent swimmers (171), suggesting immune regulation may change post-exercise. However, long-term high-intensity training has been shown to enhance Treg-mediated immune suppression, which may reduce excessive immune responses but also increase infection risk (60).
Furthermore, after prolonged intense training, peripheral blood CD8+ T cell numbers decrease, and the CD4/CD8 ratio is downregulated. CD8+ T cells may also exhibit aging phenotypes (e.g., CD57+ KLRG1+), accompanied by a Th1/Th2 shift towards Th2, weakening immune responses and increasing the risk of infections (60, 68). For instance, a 12-year longitudinal study on Olympic athletes found that long-term high-intensity training could lead to abnormal T cell characteristics, with a Th2-dominant immune response (172).
In addition to these shifts in T-cell subsets, regulatory T cells (Tregs) have emerged as central mediators of skeletal muscle adaptation to exercise. Langston et al. showed that both acute and chronic endurance exercise rapidly increase the accumulation of CD4+Foxp3+ Tregs in skeletal muscle, where they suppress IFN-γ–mediated mitochondrial damage and preserve oxidative metabolism. Functional Treg depletion impaired muscle bioenergetics and reduced endurance performance, confirming their critical role in maintaining tissue integrity during exercise adaptation (173). This dual function of Tregs—modulating inflammation and supporting metabolic homeostasis—has also been highlighted in a systematic review by Proschinger et al. (174). The review found that chronic aerobic or resistance training elevates circulating Tregs and enhances their suppressive capacity (e.g., IL-10, TGF-β secretion), while acute exercise responses vary depending on sampling time, intensity, and phenotypic markers used. Furthermore, Becker et al. identified IL-6Rα signaling as essential for Treg maturation during exercise. Mice lacking IL-6Rα in T cells showed impaired muscle regeneration and reduced Treg activity following training. These findings suggest that muscle–immune communication during exercise requires both cytokine signaling and Treg coordination to ensure proper immune function and tissue repair (175).
These studies underscore the importance of Tregs in both regulating the immune response and supporting metabolic adaptation in response to exercise. Tregs contribute to the preservation of muscle integrity and mitochondrial function, playing an essential role in the body’s long-term adaptation to physical activity.
3.3.5 Effects on B lymphocytes
Both acute and chronic intense exercise affect B-cell function. Acute exercise typically leads to a decrease in immunoglobulin levels, while prolonged intense exercise can induce an immune suppression window, impairing immune defense and increasing infection risk. Specifically, acute prolonged strenuous exercise lowers B-cell numbers (147), compromises antibody production (146, 165), and affects delayed-type hypersensitivity (176). For example, 5–7 days of intensive military training reduced serum IgG, IgA, and IgM in healthy men (147). Chronic intense exercise, such as training in elite athletes, is often associated with significant reductions in IgG, IgA, and IgM, particularly after high-intensity, prolonged endurance training, leading to temporary immune suppression (177). This immune suppression primarily affects B-cell function and reduces sIgA levels, weakening mucosal immunity and increasing infection risk (178). In male cyclists, IgA and IgM levels significantly dropped post-exercise, correlating with higher respiratory infection susceptibility (179). Rugby and elite swimming training likewise lowered sIgA levels and secretion rates, raising URTIs incidence (180, 181). These findings indicate that intense exercise can impair B-cell function and mucosal immunity, increasing infection risk.
3.3.6 Effects on cytokines
The effects of strenuous exercise on cytokines mirror the acute immune response that is triggered by exercise. Strenuous exercise triggers a rapid and large increase in IL-6 and TNF-α levels post-exercise, which can last for around 24 hours, particularly after extreme events like ultramarathons and triathlons where IL-6 and TNF-α levels significantly surpass baseline values (182, 183). Exhaustive exercise also increases cytokine expression in the liver, heart, skeletal muscle, and intestines, exacerbating inflammation and tissue damage (157, 184–187). Nutritional interventions including carbohydrate supplementation can modulate IL-6 changes, helping regulate inflammation and recovery (188). However, chronic strenuous exercise may intensify inflammation, as TNF-α levels rise with repeated bouts of intense activity (65, 189). IL-10 responses vary after strenuous exercise. Some studies show that excessive exercise boosts IL-10 as a counter-regulatory mechanism (190, 191), while others find reduced IL-10 production in certain athletes (192). Notably, the ratio of IL-10 to TNF-α varies across different tissues, exhibiting an anti-inflammatory impact in muscle while adipose tissue tends to be pro-inflammatory (193). These studies suggest that the effects of strenuous exercise on cytokines exhibit temporal and tissue-specific characteristics, with significant implications for the regulation of inflammatory responses.
Although strenuous exercise is linked to transient immunosuppression, the clinical and performance effects in athletic groups are debated (6). De Araújo et al. found that both long-term moderate-training and intensive-training lifestyles in elderly men were associated with stronger antibody responses to influenza vaccination compared to sedentary peers (194). Experimental studies also indicate that strenuous swimming after rabies vaccination does not reduce antibody titers or survival rates (195). More research is needed to better understand the impact of long-term prolonged and intensive training on immune health, but thus far there is little indication that endurance athletes experience higher rates of infectious disease or immune-related disorders. However, a consistent finding is that URTI rates are higher in endurance athletes during periods that include intensified training and competitive events (196).
3.4 Comparative immune signatures across exercise intensities
Immune responses to exercise follow intensity-specific trajectories across multiple effector systems. Moderate-intensity exercise generally promotes immune surveillance and homeostasis, characterized by enhanced neutrophil phagocytosis, M2-skewed macrophage polarization, increased NK cytotoxic activity, improved T cell proliferation, and elevated IL-10 production. These effects are associated with reduced chronic inflammation and improved resistance to infection. In contrast, high-intensity interval training (HIIT) generates acute immune activation, increasing neutrophil, monocyte, and NK cell mobilization, as well as short-term inflammatory markers such as IL-6 and TNF-α. However, long-term HIIT may enhance immunoregulatory capacity depending on baseline fitness.
Strenuous or prolonged exercise, especially when unaccustomed or sustained, tends to cause transient immune dysfunction, including neutrophil degranulation, NK cell suppression, and Th1/Th2 imbalance. This can lead to prolonged inflammatory cytokine elevation, heightening infection susceptibility. Hence, while high-intensity and strenuous exercise have specific adaptive advantages, they must be carefully controlled to avoid immunosuppressive effects.
3.5 Environmental modifiers of exercise-induced immune responses
In addition to internal workload characteristics, recent studies have highlighted the importance of extrinsic environmental conditions—such as air pollution, heat, and urban climate factors—in modulating exercise-induced immune responses. These environmental exposures can independently or synergistically affect inflammation, oxidative stress, and mucosal immunity during and after physical activity.
For instance, combined exposure to fine particulate matter (PM2.5), ozone (O3), and nitrogen dioxide (NO2) during endurance exercise has been shown to significantly enhance both airway and systemic inflammation, elevate circulating neutrophil levels, and impair mucosal immune protection (197, 198). Likewise, exercising in high-heat environments (≥35 °C) can amplify the acute release of IL–6 and TNF–α, while delaying immunoregulatory cytokine responses such as IL–10 (199). Heat stress and oxidative damage can compromise epithelial integrity, increase permeability, and lead to immune dysfunction (200, 201). In urban settings, wildfire smoke can compound inflammatory responses and lengthen the immune suppression window (202, 203).
These findings emphasize the necessity of integrating environmental exposure into the design of exercise programs, especially for populations with pre-existing inflammatory conditions or respiratory sensitivity.
4 Conclusions and future perspectives
This review underscores the multifaceted effects of varying exercise workloads on the immune system. In summary, moderate-intensity exercise consistently provides the most balanced immune benefits, promoting immune surveillance, improving cellular functions, and reducing chronic inflammation. In contrast, HIIT offers beneficial acute immune activation, enhancing NK cell cytotoxicity and modulating inflammatory markers, though its long-term effects on immune regulation are still under investigation. Prolonged or strenuous exercise, while capable of temporarily boosting immune cell mobilization, is often linked to post-exercise immunosuppression and increased infection risk due to cytokine imbalances. These findings suggest that moderate-intensity exercise is optimal for sustaining immune health, especially for those at risk of metabolic disorders or immune decline.
Future research should focus on inter-individual differences in immune responses under various exercise intensities and explore the interplay between exercise type, individual characteristics (e.g., age, health status, genetic endowment), and immune adaptation. Additionally, understanding how exercise interventions can be optimized to enhance immune function in specific disease states (e.g., cancer, diabetes) remains a crucial research avenue. Moreover, future studies are needed into the effects of exercise on immune cell subpopulations, immune markers, and their functions, as well as investigate the potential mechanisms of diverse exercise modalities and personalized exercise regimens on immune health. Prospective clinical trials and mechanistic studies will help clarify the roles of varying exercise intensities in long-term immune adaptation, ultimately providing valuable evidence-based guidelines for sports medicine and exercise nutrition.
5 Limitations
This review has several limitations that should be acknowledged. First, the studies included span a broad range of exercise modalities, populations, and outcome measures, which introduces substantial heterogeneity and limits direct comparability. Second, while moderate- and high-intensity exercise were well-represented, data on low-intensity physical activity remain sparse and often lack robust immunological endpoints. Finally, due to space limitations, certain immunological markers, such as dendritic cell function or immunoglobulin production, were not extensively discussed. These limitations highlight the need for standardized protocols, expanded biomarker profiling, and longitudinal human trials in future research.
Author contributions
XS: Writing – review & editing, Writing – original draft. LH: Writing – original draft. DN: Writing – review & editing, Conceptualization, Supervision. FL: Writing – review & editing, Conceptualization, Investigation. PC: Supervision, Writing – review & editing. HS: Conceptualization, Methodology, Writing – review & editing. YS: Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, and/or publication of this article. This study was supported by Shanghai Key Lab of Human Performance (Shanghai University of Sport) (NO. 11DZ2261100) and research project of Shanghai University of Sport(NO.2025STD003).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gray DW. An overview of the immune system with specific reference to membrane encapsulation and islet transplantation. Ann N Y Acad Sci. (2001) 944:226–39. doi: 10.1111/j.1749-6632.2001.tb03835.x
2. Marshall JS, Warrington R, Watson W, and Kim HL. An introduction to immunology and immunopathology. Allergy Asthma Clin Immunol. (2018) 14:49. doi: 10.1186/s13223-018-0278-1
3. Pradeu T, Thomma B, Girardin SE, and Lemaitre B. The conceptual foundations of innate immunity: taking stock 30 years later. Immunity. (2024) 57:613–31. doi: 10.1016/j.immuni.2024.03.007
4. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
5. Boeira M, Dorneles GP, Junior WF, and Peres A. The influence of physical activity level and cytomegalovirus serostatus on the cytokine levels of young individuals. Immunol Lett. (2023) 256-257:28–33. doi: 10.1016/j.imlet.2023.03.006
6. Campbell JP and Turner JE. Debunking the myth of exercise-induced immune suppression: redefining the impact of exercise on immunological health across the lifespan. Front Immunol. (2018) 9:648. doi: 10.3389/fimmu.2018.00648
7. van der Geest KSM, Wang Q, Eijsvogels TMH, Koenen HJP, Joosten I, Brouwer E, et al. Changes in peripheral immune cell numbers and functions in octogenarian walkers - an acute exercise study. Immun Ageing. (2017) 14:5. doi: 10.1186/s12979-017-0087-2
8. Simpson RJ, Campbell JP, Gleeson M, Krüger K, Nieman DC, Pyne DB, et al. Can exercise affect immune function to increase susceptibility to infection? Exerc Immunol Rev. (2020) 26:8–22.
9. Matthews CE, Ockene IS, Freedson PS, Rosal MC, Merriam PA, and Hebert JR. Moderate to vigorous physical activity and risk of upper-respiratory tract infection. Med Sci Sports Exerc. (2002) 34:1242–8. doi: 10.1097/00005768-200208000-00003
10. Vermeersch V, Léon K, Caillard A, Szczesnowski A, Albacete G, Marec N, et al. Moderate exercise modulates inflammatory responses and improves survival in a murine model of acute pneumonia. Crit Care Med. (2024) 52:e142–e51. doi: 10.1097/ccm.0000000000006166
11. Kohut ML, SenChina DS, Madden KS, Martin AE, Felten DL, and Moynihan JA. Age effects on macrophage function vary by tissue site, nature of stimulant, and exercise behavior. Exp Gerontol. (2004) 39:1347–60. doi: 10.1016/j.exger.2004.07.001
12. Gibala MJ, Little JP, Van Essen M, Wilkin GP, Burgomaster KA, Safdar A, et al. Short-term sprint interval versus traditional endurance training: similar initial adaptations in human skeletal muscle and exercise performance. J Physiol. (2006) 575:901–11. doi: 10.1113/jphysiol.2006.112094
13. Weston KS, Wisløff U, and Coombes JS. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: A systematic review and meta-analysis. Br J Sports Med. (2014) 48:1227–34. doi: 10.1136/bjsports-2013-092576
14. Bartlett DB, Willis LH, Slentz CA, Hoselton A, Kelly L, Huebner JL, et al. Ten weeks of high-intensity interval walk training is associated with reduced disease activity and improved innate immune function in older adults with rheumatoid arthritis: A pilot study. Arthritis Res Ther. (2018) 20:127. doi: 10.1186/s13075-018-1624-x
15. Nieman DC. Exercise, infection, and immunity. Int J Sports Med. (1994) 15 Suppl 3:S131–41. doi: 10.1055/s-2007-1021128
16. Pedersen BK and Ullum H. Nk cell response to physical activity: possible mechanisms of action. Med Sci Sports Exerc. (1994) 26:140–6. doi: 10.1249/00005768-199402000-00003
17. Nieman DC and Wentz LM. The compelling link between physical activity and the body’s defense system. J Sport Health Sci. (2019) 8:201–17. doi: 10.1016/j.jshs.2018.09.009
18. Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. American college of sports medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. (2011) 43:1334–59. doi: 10.1249/MSS.0b013e318213fefb
19. Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, et al. World health organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. (2020) 54:1451–62. doi: 10.1136/bjsports-2020-102955
20. Norton K, Norton L, and Sadgrove D. Position statement on physical activity and exercise intensity terminology. J Sci Med Sport. (2010) 13:496–502. doi: 10.1016/j.jsams.2009.09.008
21. Gormley SE, Swain DP, High R, Spina RJ, Dowling EA, Kotipalli US, et al. Effect of intensity of aerobic training on vo2max. Med Sci Sports Exerc. (2008) 40:1336–43. doi: 10.1249/MSS.0b013e31816c4839
22. Arboleda-Serna VH, Feito Y, Patiño-Villada FA, Vargas-Romero AV, and Arango-Vélez EF. Effects of high-intensity interval training compared to moderate-intensity continuous training on maximal oxygen consumption and blood pressure in healthy men: A randomized controlled trial. Biomedica. (2019) 39:524–36. doi: 10.7705/biomedica.4451
23. Raidal SL, Love DN, Bailey GD, and Rose RJ. Effect of single bouts of moderate and high intensity exercise and training on equine peripheral blood neutrophil function. Res Vet Sci. (2000) 68:141–6. doi: 10.1053/rvsc.1999.0349
24. Giraldo E, Garcia JJ, HinChado MD, and Ortega E. Exercise intensity-dependent changes in the inflammatory response in sedentary women: role of neuroendocrine parameters in the neutrophil phagocytic process and the pro-/anti-inflammatory cytokine balance. Neuroimmunomodulation. (2009) 16:237–44. doi: 10.1159/000212384
25. Hill AM, Worthley C, Murphy KJ, Buckley JD, Ferrante A, and Howe PRC. N-3 fatty acid supplementation and regular moderate exercise: differential effects of a combined intervention on neutrophil function. Br J Nutr. (2007) 98:300–9. doi: 10.1017/s0007114507707286
26. Syu GD, Chen HI, and Jen CJ. Severe exercise and exercise training exert opposite effects on human neutrophil apoptosis via altering the redox status. PloS One. (2011) 6:e24385. doi: 10.1371/journal.pone.0024385
27. Mooren FC, Krueger K, Ringseis R, Eder K, Liebisch G, Conrad K, et al. Combined effects of moderate exercise and short-term fasting on markers of immune function in healthy human subjects. Am J Physiol Regul Integr Comp Physiol. (2020) 318:R1103–R15. doi: 10.1152/ajpregu.00341.2019
28. Wang J-S and Chiu Y-T. Systemic hypoxia enhances exercise-mediated bactericidal and subsequent apoptotic responses in human neutrophils. J Appl Physiol. (2009) 107:1213–22. doi: 10.1152/japplphysiol.00316.2009
29. Viana JL, Kosmadakis GC, Watson EL, Bevington A, Feehally J, Bishop NC, et al. Evidence for anti-inflammatory effects of exercise in ckd. J Am Soc Nephrol. (2014) 25:2121–30. doi: 10.1681/asn.2013070702
30. Pillon NJ, Gabriel BM, Dollet L, Smith JAB, Sardón Puig L, Botella J, et al. Transcriptomic profiling of skeletal muscle adaptations to exercise and inactivity. Nat Commun. (2020) 11:470. doi: 10.1038/s41467-019-13869-w
31. Bartlett DB, Shepherd SO, Wilson OJ, Adlan AM, Wagenmakers AJM, Shaw CS, et al. Neutrophil and monocyte bactericidal responses to 10 weeks of low-volume high-intensity interval or moderate-intensity continuous training in sedentary adults. Oxid Med Cell Longevity. (2017) 2017:8148742. doi: 10.1155/2017/8148742
32. Murugathasan M, Jafari A, Amandeep A, Hassan SA, Chihata M, and Abdul-Sater AA. Moderate exercise induces trained immunity in macrophages. Am J Physiol Cell Physiol. (2023) 325:C429–c42. doi: 10.1152/ajpcell.00130.2023
33. Van der Stede T, Van de Loock A, Turiel G, Hansen C, Tamariz-Ellemann A, Ullrich M, et al. Cellular deconstruction of the human skeletal muscle microenvironment identifies an exercise-induced histaminergic crosstalk. Cell Metab. (2025) 37:842–56.e7. doi: 10.1016/j.cmet.2024.12.011
34. Knudsen NH, Stanya KJ, Hyde AL, Chalom MM, Alexander RK, Liou YH, et al. Interleukin-13 drives metabolic conditioning of muscle to endurance exercise. Science. (2020) 368:eaat3987. doi: 10.1126/science.aat3987
35. Ruffino JS, Davies NA, Morris K, Ludgate M, Zhang L, Webb R, et al. Moderate-intensity exercise alters markers of alternative activation in circulating monocytes in females: A putative role for pparγ. Eur J Appl Physiol. (2016) 116:1671–82. doi: 10.1007/s00421-016-3414-y
36. Blanks AM, Wagamon TT, Lafratta L, Sisk MG, Senter MB, Pedersen LN, et al. Impact of physical activity on monocyte subset ccr2 expression and macrophage polarization following moderate intensity exercise. Brain Behav Immun Health. (2020) 2:100033. doi: 10.1016/j.bbih.2019.100033
37. Blanks AM, Pedersen LN, Bohmke N, Mihalick VL, and Franco RL. Sex differences in monocyte ccr2 expression and macrophage polarization following acute exercise. Life Sci. (2022) 299:120557. doi: 10.1016/j.lfs.2022.120557
38. Barry JC, Simtchouk S, Durrer C, Jung ME, and Little JP. Short-term exercise training alters leukocyte chemokine receptors in obese adults. Med Sci Sports Exercise. (2017) 49:1631–40. doi: 10.1249/mss.0000000000001261
39. Malm C, Nyberg P, Engstrom M, Sjodin B, Lenkei R, Ekblom B, et al. Immunological changes in human skeletal muscle and blood after eccentric exercise and multiple biopsies. J Physiol. (2000) 529 Pt 1:243–62. doi: 10.1111/j.1469-7793.2000.00243.x
40. Malm C, Sjödin TL, Sjöberg B, Lenkei R, Renström P, Lundberg IE, et al. Leukocytes, cytokines, growth factors and hormones in human skeletal muscle and blood after uphill or downhill running. J Physiol. (2004) 556:983–1000. doi: 10.1113/jphysiol.2003.056598
41. Marklund P, Mattsson CM, Wåhlin-Larsson B, Ponsot E, Lindvall B, Lindvall L, et al. Extensive inflammatory cell infiltration in human skeletal muscle in response to an ultraendurance exercise bout in experienced athletes. J Appl Physiol (1985). (2013) 114:66–72. doi: 10.1152/japplphysiol.01538.2011
42. Voskoboynik Y, McCulloch AD, and Sahoo D. Macrophages on the run: exercise balances macrophage polarization for improved health. Mol Metab. (2024) 90:102058. doi: 10.1016/j.molmet.2024.102058
43. Pillon NJ, Smith JAB, Alm PS, Chibalin AV, Alhusen J, Arner E, et al. Distinctive exercise-induced inflammatory response and exerkine induction in skeletal muscle of people with type 2 diabetes. Sci Adv. (2022) 8:eabo3192. doi: 10.1126/sciadv.abo3192
44. Davis JM, Murphy EA, Brown AS, Carmichael MD, Ghaffar A, and Mayer EP. Effects of moderate exercise and oat beta-glucan on innate immune function and susceptibility to respiratory infection. Am J Physiol Regul Integr Comp Physiol. (2004) 286:R366–72. doi: 10.1152/ajpregu.00304.2003
45. Cai Y, Xiong M, Xin Z, Liu C, Ren J, Yang X, et al. Decoding Aging-Dependent Regenerative Decline across Tissues at Single-Cell Resolution. Cell Stem Cell. (2023) 30:1674–91.e8. doi: 10.1016/j.stem.2023.09.014
46. Hanson ED, Sakkal S, Que S, Cho E, Spielmann G, Kadife E, et al. Natural killer cell mobilization and egress following acute exercise in men with prostate cancer. Exp Physiol. (2020) 105:1524–39. doi: 10.1113/ep088627
47. Chester C, Fritsch K, and Kohrt HE. Natural killer cell immunomodulation: targeting activating, inhibitory, and co-stimulatory receptor signaling for cancer immunotherapy. Front Immunol. (2015) 6:601. doi: 10.3389/fimmu.2015.00601
48. Farnault L, Sanchez C, Baier C, Le Treut T, and Costello RT. Hematological Malignancies escape from nk cell innate immune surveillance: mechanisms and therapeutic implications. Clin Dev Immunol. (2012) 2012:1–8. doi: 10.1155/2012/421702
49. Bartlett DB, Brander DM, and Sitlinger A. Impact of exercise on the immune system and outcomes in hematologic Malignancies. Blood Adv. (2020) 4:1801–11. doi: 10.1182/bloodadvances.2019001317
50. Gannon GA, Rhind SG, Suzui M, Zamecnik J, Sabiston BH, Shek PN, et al. Beta-endorphin and natural killer cell cytolytic activity during prolonged exercise. Is there a connection? Am J Physiol. (1998) 275:R1725–34. doi: 10.1152/ajpregu.1998.275.6.R1725
51. Lin M-L, Hsu C-C, Fu T-C, Lin Y-T, Huang Y-C, and Wang J-S. Exercise training improves mitochondrial bioenergetics of natural killer cells. Med Sci Sports Exercise. (2022) 54:751–60. doi: 10.1249/mss.0000000000002842
52. Hunt R, Ali K, Banh R, Bolden M, Laurea K, McBride L, et al. Can just 15 minutes of moderate-intensity exercise mobilize cytotoxic nk cells into circulation? Physiology. (2024) 39:2061. doi: 10.1152/physiol.2024.39.S1.2061
53. Chamorro-Viña C, Valentín J, Fernández L, González-Vicent M, Pérez-Ruiz M, Lucía A, et al. Influence of a moderate-intensity exercise program on early nk cell immune recovery in pediatric patients after reduced-intensity hematopoietic stem cell transplantation. Integr Cancer Ther. (2017) 16:464–72. doi: 10.1177/1534735416679515
54. Parent-Roberge H, Fontvieille A, Poirier L, Tai LH, Pavic M, Fülöp T, et al. Acute natural killer cells response to a continuous moderate intensity and a work-matched high intensity interval exercise session in metastatic cancer patients treated with chemotherapy. Brain Behav Immun Health. (2024) 40:100825. doi: 10.1016/j.bbih.2024.100825
55. Pan H, Meng R, Jia Z, Zhang J, Ma W, Liu Y, et al. Exercise: A non-drug strategy of nk cell activation. Braz J Med Biol Res. (2024) 57:e14144. doi: 10.1590/1414-431X2024e14144
56. Sardeli AV, Mori MA, and Lord JM. Effect of exercise on acute senescent lymphocyte counts: A systematic review and meta-analysis. Gerontology. (2022) 68:961–75. doi: 10.1159/000520528
57. Koivula T, Lempiäinen S, Rinne P, Hollmén M, Sundberg CJ, Rundqvist H, et al. Acute exercise mobilizes Cd8+ Cytotoxic T cells and nk cells in lymphoma patients. Front Physiol. (2023) 13:1078512. doi: 10.3389/fphys.2022.1078512
58. Fu SC, Qin L, Leung CK, Chan BP, and Chan KM. Regular moderate exercise training prevents decrease of Cd4+ T-lymphocytes induced by a single bout of strenuous exercise in mice. Can J Appl Physiol. (2003) 28:370–81. doi: 10.1139/h03-027
59. Rogers CJ, Zaharoff DA, Hance KW, Perkins SN, Hursting SD, Schlom J, et al. Exercise enhances vaccine-induced antigen-specific T cell responses. Vaccine. (2008) 26:5407–15. doi: 10.1016/j.vaccine.2008.07.081
60. Shaw DM, Merien F, Braakhuis A, and Dulson D. T-cells and their cytokine production: the anti-inflammatory and immunosuppressive effects of strenuous exercise. Cytokine. (2018) 104:136–42. doi: 10.1016/j.cyto.2017.10.001
61. Tongtako W, Klaewsongkram J, Jaronsukwimal N, Buranapraditkun S, Mickleborough TD, and Suksom D. The effect of acute exhaustive and moderate intensity exercises on nasal cytokine secretion and clinical symptoms in allergic rhinitis patients. Asian Pac J Allergy Immunol. (2012) 30:185–92.
62. Zamani A, Salehi I, and Alahgholi-Hajibehzad M. Moderate exercise enhances the production of interferon-Γ and interleukin-12 in peripheral blood mononuclear cells. Immune Netw. (2017) 17:186–91. doi: 10.4110/in.2017.17.3.186
63. Shimizu K, Kimura F, Akimoto T, Akama T, Tanabe K, Nishijima T, et al. Effect of moderate exercise training on T-helper cell subpopulations in elderly people. Exerc Immunol Rev. (2008) 14:24–37.
64. Drela N, Kozdron E, and Szczypiorski P. Moderate exercise may attenuate some aspects of immunosenescence. BMC Geriatr. (2004) 4:8. doi: 10.1186/1471-2318-4-8
65. Gholamnezhad Z, Boskabady MH, Hosseini M, Sankian M, and Khajavi Rad A. Evaluation of immune response after moderate and overtraining exercise in wistar rat. Iran J Basic Med Sci. (2014) 17:1–8.
66. Kohut ML, Boehm GW, and Moynihan JA. Moderate exercise is associated with enhanced antigen-specific cytokine, but not igm antibody production in aged mice. Mech Ageing Dev. (2001) 122:1135–50. doi: 10.1016/s0047-6374(01)00255-x
67. Lowder T, Padgett DA, and Woods JA. Moderate exercise early after influenza virus infection reduces the th1 inflammatory response in lungs of mice. Exerc Immunol Rev. (2006) 12:97–111.
68. Simpson RJ, Kunz H, Agha N, and Graff R. Exercise and the regulation of immune functions. Prog Mol Biol Transl Sci. (2015) 135:355–80. doi: 10.1016/bs.pmbts.2015.08.001
69. Lancaster GI, Khan Q, Drysdale PT, Wallace F, Jeukendrup AE, Drayson MT, et al. Effect of prolonged exercise and carbohydrate ingestion on type 1 and type 2 T lymphocyte distribution and intracellular cytokine production in humans. J Appl Physiol. (2005) 98:565–71. doi: 10.1152/japplphysiol.00754.2004
70. Bachi AL, Suguri VM, Ramos LR, Mariano M, Vaisberg M, and Lopes JD. Increased production of autoantibodies and specific antibodies in response to influenza virus vaccination in physically active older individuals. Results Immunol. (2013) 3:10–6. doi: 10.1016/j.rinim.2013.01.001
71. Nehlsen-Cannarella SL, Nieman DC, Balk-Lamberton AJ, Markoff PA, Chritton DB, Gusewitch G, et al. The effects of moderate exercise training on immune response. Med Sci Sports Exerc. (1991) 23:64–70. doi: 10.1249/00005768-199101000-00011
72. Shimizu K, Kimura F, Akimoto T, Akama T, Otsuki T, Nishijima T, et al. Effects of exercise, age and gender on salivary secretory immunoglobulin a in elderly individuals. Exerc Immunol Rev. (2007) 13:55–66.
73. Akimoto T, Kumai Y, Akama T, Hayashi E, Murakami H, Soma R, et al. Effects of 12 months of exercise training on salivary secretory Iga levels in elderly subjects. Br J Sports Med. (2003) 37:76–9. doi: 10.1136/bjsm.37.1.76
74. Viloria M, Lara-Padilla E, Campos-Rodríguez R, Jarillo-Luna A, Reyna-Garfias H, López-Sánchez P, et al. Effect of moderate exercise on Iga levels and lymphocyte count in mouse intestine. Immunol Invest. (2011) 40:640–56. doi: 10.3109/08820139.2011.575425
75. Hernández-Urbán AJ, Drago-Serrano M-E, Reséndiz-Albor AA, Sierra-Ramírez JA, Guzmán-Mejía F, Oros-Pantoja R, et al. Moderate aerobic exercise induces homeostatic Iga generation in senile mice. Int J Mol Sci. (2024) 25:8200. doi: 10.3390/ijms25158200
76. Pedersen BK and Febbraio M. Muscle-derived interleukin-6–a possible link between skeletal muscle, adipose tissue, liver, and brain. Brain Behav Immun. (2005) 19:371–6. doi: 10.1016/j.bbi.2005.04.008
77. Pedersen BK and Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. (2008) 88:1379–406. doi: 10.1152/physrev.90100.2007
78. Febbraio MA and Pedersen BK. Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. FASEB J. (2002) 16:1335–47. doi: 10.1096/fj.01-0876rev
79. Pedersen BK, Steensberg A, and Schjerling P. Exercise and interleukin-6. Curr Opin Hematol. (2001) 8:137–41. doi: 10.1097/00062752-200105000-00002
80. Pedersen BK, Steensberg A, Fischer C, Keller C, Keller P, Plomgaard P, et al. Searching for the exercise factor: is Il-6 a candidate? J Muscle Res Cell Motil. (2003) 24:113–9. doi: 10.1023/a:1026070911202
81. Magalhães JP, Santos DA, Correia IR, Hetherington-Rauth M, Ribeiro R, Raposo JF, et al. Impact of combined training with different exercise intensities on inflammatory and lipid markers in type 2 diabetes: A secondary analysis from a 1-year randomized controlled trial. Cardiovasc Diabetol. (2020) 19:169. doi: 10.1186/s12933-020-01136-y
82. Osuru HP, Ikeda K, Atluri N, and Thiele RH. Moderate exercise-induced dynamics on key sepsis-associated signaling pathways in the liver. Crit Care. (2023) 27:266. doi: 10.1186/s13054-023-04551-1
83. Belotto MF, Magdalon J, Rodrigues HG, Vinolo MA, Curi R, Pithon-Curi TC, et al. Moderate exercise improves leucocyte function and decreases inflammation in diabetes. Clin Exp Immunol. (2010) 162:237–43. doi: 10.1111/j.1365-2249.2010.04240.x
84. Dimitrov S, Hulteng E, and Hong S. Inflammation and exercise: inhibition of monocytic intracellular tnf production by acute exercise via B2-adrenergic activation. Brain Behav Immun. (2017) 61:60–8. doi: 10.1016/j.bbi.2016.12.017
85. Fu R, Niu R, Zhao F, Wang J, Cao Q, Yu Y, et al. Exercise alleviated intestinal damage and microbial disturbances in mice exposed to fluoride. Chemosphere. (2022) 288:132658. doi: 10.1016/j.chemosphere.2021.132658
86. Günbatar N, Bulduk B, Bezgin S, Oto G, Bayıroğlu F, and Bulduk M. The effect of moderate-intensity physical exercise on some serum inflammation markers and the immune system in rats fed intermittent fasting with a high-fat diet. Medicina. (2023) 59:1687. doi: 10.3390/medicina59091687
87. Gerosa-Neto J, Antunes BMM, Campos EZ, Rodrigues J, Ferrari GD, Neto JCR, et al. Impact of long-term high-intensity interval and moderate-intensity continuous training on subclinical inflammation in overweight/obese adults. J Exercise Rehabil. (2016) 12:575–80. doi: 10.12965/jer.1632770.385
88. Light AR, White AT, Hughen RW, and Light KC. Moderate exercise increases expression for sensory, adrenergic, and immune genes in chronic fatigue syndrome patients but not in normal subjects. J Pain. (2009) 10:1099–112. doi: 10.1016/j.jpain.2009.06.003
89. Santos RVT, Viana VAR, Boscolo RA, Marques VG, Santana MG, Lira FS, et al. Moderate exercise training modulates cytokine profile and sleep in elderly people. Cytokine. (2012) 60:731–5. doi: 10.1016/j.cyto.2012.07.028
90. Perandini LA, Sales-de-Oliveira D, Mello SBV, Camara NO, Benatti FB, Lima FR, et al. Exercise training can attenuate the inflammatory milieu in women with systemic lupus erythematosus. J Appl Physiol. (2014) 117:639–47. doi: 10.1152/japplphysiol.00486.2014
91. de Araújo CC, Silva JD, Samary CS, Guimarães IH, Marques PS, Oliveira GP, et al. Regular and moderate exercise before experimental sepsis reduces the risk of lung and distal organ injury. J Appl Physiol. (2012) 112:1206–14. doi: 10.1152/japplphysiol.01061.2011
92. Suzuki K and Hayashida H. Effect of exercise intensity on cell-mediated immunity. Sports (Basel). (2021) 9:8. doi: 10.3390/sports9010008
93. Choi1 E-J, Lee C-J, Park H-H, and So W-Y. Effect of 12-week low-intensity exercise on interleukin-2, interferon-gamma and interleukin-4 cytokine production in rat spleens. J Men’s Health. (2018) 14:14–9. doi: 10.22374/1875-6859.14.3.1
94. Hou Q. Effect of physical exercise on the proliferation and inactivation capacity of biological immune cells. Mol Cell Biomechanics. (2025) 22:713. doi: 10.62617/mcb713
95. Sakamoto Y, Ueki S, Shimanuki H, Kasai T, Takato J, Ozaki H, et al. Effects of low-intensity physical exercise on acute changes in resting saliva secretory Iga levels in the elderly. Geriatr Gerontol Int. (2005) 5:202–6. doi: 10.1111/j.1447-0594.2005.00297.x
96. Balogh L, Szabó K, Pucsok JM, Jámbor I, Gyetvai Á, Mile M, et al. The effect of aerobic exercise and low-impact pilates workout on the adaptive immune system. J Clin Med. (2022) 11:6814. doi: 10.3390/jcm11226814
97. Xia S. Effects of sports exercises on immunity system in aging. J Wuhan Institute Phys Educ. (2005) 39:63.
98. Fisher G, Schwartz DD, Quindry J, Barberio MD, Foster EB, Jones KW, et al. Lymphocyte enzymatic antioxidant responses to oxidative stress following high-intensity interval exercise. J Appl Physiol. (2011) 110:730–7. doi: 10.1152/japplphysiol.00575.2010
99. Lee T-T, Li T-L, Ko B-J, and Chien L-H. Effect of acute high-intensity interval training on immune function and oxidative stress in canoe/kayak athletes. Biology. (2023) 12:1144. doi: 10.3390/biology12081144
100. Slusher AL, Whitehurst M, Maharaj A, Dodge KM, Fico BG, Mock JT, et al. Plasma pentraxin 3 and glucose kinetics following acute high-intensity interval exercise versus continuous moderate-intensity exercise in healthy men. Appl Physiol Nutr Metab. (2018) 43:1233–8. doi: 10.1139/apnm-2018-0039
101. Bartlett DB, Slentz CA, Willis LH, Hoselton A, Huebner JL, Kraus VB, et al. Rejuvenation of neutrophil functions in association with reduced diabetes risk following ten weeks of low-volume high intensity interval walking in older adults with prediabetes – a pilot study. Front Immunol. (2020) 11:729. doi: 10.3389/fimmu.2020.00729
102. Vidal-Seguel N, Cabrera C, Ferrada L, Artigas-Arias M, Alegría-Molina A, Sanhueza S, et al. High-intensity interval training reduces the induction of neutrophil extracellular traps in older men using live-neutrophil imaging as biosensor. Exp Gerontol. (2023) 181:112280. doi: 10.1016/j.exger.2023.112280
103. Rocha RE, Coelho I, Pequito DC, Yamagushi A, Borghetti G, Yamazaki RK, et al. Interval training attenuates the metabolic disturbances in type 1 diabetes rat model. Arq Bras Endocrinol Metabol. (2013) 57:594–602. doi: 10.1590/s0004-27302013000800003
104. Rajizadeh MA, Hosseini MH, Bahrami M, Bahri F, Rostamabadi F, Bagheri F, et al. High-intensity intermittent training ameliorates methotrexate-induced acute lung injury. BMC Pulm Med. (2024) 24:45. doi: 10.1186/s12890-024-02853-w
105. Durrer C, Francois M, Neudorf H, and Little JP. Acute high-intensity interval exercise reduces human monocyte toll-like receptor 2 expression in type 2 diabetes. Am J Physiol Regul Integr Comp Physiol. (2017) 312:R529–r38. doi: 10.1152/ajpregu.00348.2016
106. Gabriel BM, Pugh J, Pruneta-Deloche V, Moulin P, Ratkevicius A, and Gray SR. The effect of high intensity interval exercise on postprandial triacylglycerol and leukocyte activation–monitored for 48 H post exercise. PloS One. (2013) 8:e82669. doi: 10.1371/journal.pone.0082669
107. de Matos MA, Garcia BCC, Vieira DV, de Oliveira MFA, Costa KB, Aguiar PF, et al. High-intensity interval training reduces monocyte activation in obese adults. Brain Behav Immun. (2019) 80:818–24. doi: 10.1016/j.bbi.2019.05.030
108. Oh S, So R, Shida T, Matsuo T, Kim B, Akiyama K, et al. High-intensity aerobic exercise improves both hepatic fat content and stiffness in sedentary obese men with nonalcoholic fatty liver disease. Sci Rep. (2017) 7:43029. doi: 10.1038/srep43029
109. Fredrickson G, Barrow F, Dietsche K, Parthiban P, Khan S, Robert S, et al. Exercise of high intensity ameliorates hepatic inflammation and the progression of nash. Mol Metab. (2021) 53:101270. doi: 10.1016/j.molmet.2021.101270
110. Sheikh R, Shakerian S, Fatemi Tabatabaei SR, and Habibi A. Moderate and high-intensity interval training protect against diabetes-induced modulation of hepatic Cd86 and Cd206 expression associated with the amelioration of insulin resistance and inflammation in rats. Immunobiology. (2023) 228:152745. doi: 10.1016/j.imbio.2023.152745
111. Luo L, Liu M, Xie H, Fan Y, Zhang J, Liu L, et al. High-intensity interval training improves physical function, prevents muscle loss, and modulates macrophage-mediated inflammation in skeletal muscle of cerebral ischemic mice. Mediators Inflammation. (2021) 2021:1–28. doi: 10.1155/2021/1849428
112. Shanaki M, Khosravi M, Khoshdooni-Farahani A, Dadashi A, Heydari MF, Delfan M, et al. High-intensity interval training reversed high-fat diet-induced M1-macrophage polarization in rat adipose tissue via inhibition of notch signaling. J Inflammation Res. (2020) 13:165–74. doi: 10.2147/jir.S237049
113. Barra NG, Fan IY, Gillen JB, Chew M, Marcinko K, Steinberg GR, et al. High intensity interval training increases natural killer cell number and function in obese breast cancer-challenged mice and obese women. J Cancer Prev. (2017) 22:260–6. doi: 10.15430/jcp.2017.22.4.260
114. Coletta AM, Agha NH, Baker FL, Niemiro GM, Mylabathula PL, Brewster AM, et al. The Impact of High-Intensity Interval Exercise Training on Nk-Cell Function and Circulating Myokines for Breast Cancer Prevention among Women at High Risk for Breast Cancer. Breast Cancer Res Treat. (2021) 187:407–16. doi: 10.1007/s10549-021-06111-z
115. Djurhuus SS, Simonsen C, Toft BG, Thomsen SN, Wielsøe S, Røder MA, et al. Exercise training to increase tumour natural killer-cell infiltration in men with localised prostate cancer: A randomised controlled trial. BJU Int. (2022) 131:116–24. doi: 10.1111/bju.15842
116. KrÜGer K, Alack K, Ringseis R, Mink L, Pfeifer E, Schinle M, et al. Apoptosis of T-cell subsets after acute high-intensity interval exercise. Med Sci Sports Exercise. (2016) 48:2021–9. doi: 10.1249/mss.0000000000000979
117. Zissel G, Tossige-Gomes R, Costa KB, Ottone V, Magalhães F, Amorim FT, et al. Lymphocyte redox imbalance and reduced proliferation after a single session of high intensity interval exercise. PloS One. (2016) 11:e0153647. doi: 10.1371/journal.pone.0153647
118. Dorneles GP, da Silva IM, Peres A, and Romão PRT. Physical fitness modulates the expression of Cd39 and Cd73 on Cd4(+) Cd25(-) and Cd4(+) Cd25(+) T cells following high intensity interval exercise. J Cell Biochem. (2019) 120:10726–36. doi: 10.1002/jcb.28364
119. Câmara Niels OS, Weng T-P, Huang S-C, Chuang Y-F, and Wang J-S. Effects of interval and continuous exercise training on Cd4 lymphocyte apoptotic and autophagic responses to hypoxic stress in sedentary men. PloS One. (2013) 8:e80248. doi: 10.1371/journal.pone.0080248
120. Niemiro GM, Coletta AM, Agha NH, Mylabathula PL, Baker FL, Brewster AM, et al. Salutary Effects of Moderate but Not High Intensity Aerobic Exercise Training on the Frequency of Peripheral T-Cells Associated with Immunosenescence in Older Women at High Risk of Breast Cancer: A Randomized Controlled Trial. Immun Ageing. (2022) 19:30. doi: 10.1186/s12979-022-00266-z
121. Monje C, Rada I, Castro-Sepulveda M, Peñailillo L, Deldicque L, and Zbinden-Foncea H. Effects of a high intensity interval session on mucosal immune function and salivary hormones in male and female endurance athletes. J Sports Sci Med. (2020) 19:436–43.
122. Born DP, Zinner C, and Sperlich B. The mucosal immune function is not compromised during a period of high-intensity interval training. Is it time to reconsider an old assumption? Front Physiol. (2017) 8:485. doi: 10.3389/fphys.2017.00485
123. Nobari H, Gandomani EE, Reisi J, Vahabidelshad R, Suzuki K, Volpe SL, et al. Effects of 8 weeks of high-intensity interval training and spirulina supplementation on immunoglobin levels, cardio-respiratory fitness, and body composition of overweight and obese women. Biology. (2022) 11:196. doi: 10.3390/biology11020196
124. Zwetsloot K, John C, Lawrence M, Battista R, and Shanely RA. High-intensity interval training induces a modest systemic inflammatory response in active, young men. J Inflammation Res. (2014) 7:9–17. doi: 10.2147/jir.S54721
125. Aktitiz S, Atakan MM, Turnagöl HH, and Koşar ŞN. Interleukin-6, undercarboxylated osteocalcin, and brain-derived neurotrophic factor responses to single and repeated sessions of high-intensity interval exercise. Peptides. (2022) 157:170864. doi: 10.1016/j.peptides.2022.170864
126. Devin JL, Hill MM, Mourtzakis M, Quadrilatero J, Jenkins DG, and Skinner TL. Acute high intensity interval exercise reduces colon cancer cell growth. J Physiol. (2019) 597:2177–84. doi: 10.1113/jp277648
127. Taylor AG, Ignaszewski AI, Bredin SSD, Hill JS, Shellington EM, and Warburton DER. High intensity interval training leads to similar inflammatory activation as seen with traditional training in chronic heart failure. Front Cardiovasc Med. (2022) 8:752531. doi: 10.3389/fcvm.2021.752531
128. Wu C, Deng J, and Gao C. Effects of pre-sleep protein supplementation on plasma markers of muscle damage and inflammatory cytokines resulting from sprint interval training in trained swimmers. J Int Soc Sports Nutr. (2023) 20:2244478. doi: 10.1080/15502783.2023.2244478
129. Hall B, Żebrowska A, Sikora M, Siatkowski S, and Robins A. The effect of high-intensity interval exercise on short-term glycaemic control, serum level of key mediator in hypoxia and pro-inflammatory cytokines in patients with type 1 diabetes—an exploratory case study. Nutrients. (2023) 15:3749. doi: 10.3390/nu15173749
130. Lira FS, dos Santos T, Caldeira RS, Inoue DS, Panissa VLG, Cabral-Santos C, et al. Short-term high- and moderate-intensity training modifies inflammatory and metabolic factors in response to acute exercise. Front Physiol. (2017) 8:856. doi: 10.3389/fphys.2017.00856
131. Liu S, Wen D, Feng C, Yu C, Gu Z, Wang L, et al. Alteration of gut microbiota after heat acclimation may reduce organ damage by regulating immune factors during heat stress. Front Microbiol. (2023) 14:1114233. doi: 10.3389/fmicb.2023.1114233
132. Zhao H, He Z, Yun H, Wang R, and Liu C. A meta-analysis of the effects of different exercise modes on inflammatory response in the elderly. Int J Environ Res Public Health. (2022) 19:10451. doi: 10.3390/ijerph191610451
133. Garneau L, Terada T, Mistura M, Mulvihill EE, Reed JL, and Aguer C. Exercise training reduces circulating cytokines in male patients with coronary artery disease and type 2 diabetes: A pilot study. Physiol Rep. (2023) 11:e15634. doi: 10.14814/phy2.15634
134. Dorneles GP, Haddad DO, Fagundes VO, Vargas BK, Kloecker A, Romão PRT, et al. High intensity interval exercise decreases Il-8 and enhances the immunomodulatory cytokine interleukin-10 in lean and overweight–obese individuals. Cytokine. (2016) 77:1–9. doi: 10.1016/j.cyto.2015.10.003
135. Gerosa-Neto J, Monteiro PA, Inoue DS, Antunes BM, Batatinha H, Dorneles GP, et al. High- and moderate-intensity training modify lps-induced ex-vivo interleukin-10 production in obese men in response to an acute exercise bout. Cytokine. (2020) 136:155249. doi: 10.1016/j.cyto.2020.155249
136. Leite AB, Lima HN, Flores C, Oliveira CA, Cunha LEC, Neves JL, et al. High-intensity interval training is more effective than continuous training to reduce inflammation markers in female rats with cisplatin nephrotoxicity. Life Sci. (2021) 266:118880. doi: 10.1016/j.lfs.2020.118880
137. Hu Z, Li X, Yang Y, Zhang Z, and Ding S. High-intensity interval training ameliorates high-fat diet-induced metabolic disorders via the cyclic gmp-amp synthase-stimulator of interferon gene signaling pathway. Int J Mol Sci. (2023) 24:13840. doi: 10.3390/ijms241813840
138. Malczynska-Sims P, Chalimoniuk M, Wronski Z, Marusiak J, and Sulek A. High-intensity interval training modulates inflammatory response in Parkinson’s disease. Aging Clin Exp Res. (2022) 34:2165–76. doi: 10.1007/s40520-022-02153-5
139. Rajizadeh MA, Khoramipour K, Joukar S, Darvishzadeh-Mahani F, Iranpour M, Bejeshk MA, et al. Lung molecular and histological changes in type 2 diabetic rats and its improvement by high-intensity interval training. BMC Pulmon Med. (2024) 24:37. doi: 10.1186/s12890-024-02840-1
140. Hooshmand Moghadam B, Golestani F, Bagheri R, Cheraghloo N, Eskandari M, Wong A, et al. The effects of high-intensity interval training vs. Moderate-intensity continuous training on inflammatory markers, body composition, and physical fitness in overweight/obese survivors of breast cancer: A randomized controlled clinical trial. Cancers. (2021) 13:4386. doi: 10.3390/cancers13174386
141. Joukar S, Rajizadeh MA, Bejeshk MA, Alavi SS, Bagheri F, Rami M, et al. Atp releasing channels and the ameliorative effects of high intensity interval training on diabetic heart: A multifaceted analysis. Sci Rep. (2024) 14:7113. doi: 10.1038/s41598-024-57818-0
142. Munk PS, Breland UM, Aukrust P, Ueland T, Kvaløy JT, and Larsen AI. High intensity interval training reduces systemic inflammation in post-pci patients. Eur J Cardiovasc Prev Rehabil. (2011) 18:850–7. doi: 10.1177/1741826710397600
143. Sun L, Li FH, Li T, Min Z, Yang LD, Gao HE, et al. Effects of high-intensity interval training on adipose tissue lipolysis, inflammation, and metabolomics in aged rats. Pflugers Arch. (2020) 472:245–58. doi: 10.1007/s00424-020-02351-y
144. Ge Z, Wu S, Qi Z, and Ding S. Exercise modulates polarization of tams and expression of related immune checkpoints in mice with lung cancer. J Cancer. (2022) 13:3297–307. doi: 10.7150/jca.76136
145. Peake JM, Neubauer O, Walsh NP, and Simpson RJ. Recovery of the immune system after exercise. J Appl Physiol. (2017) 122:1077–87. doi: 10.1152/japplphysiol.00622.2016
146. Gleeson M. Immune function in sport and exercise. J Appl Physiol (1985). (2007) 103:693–9. doi: 10.1152/japplphysiol.00008.2007
147. Bøyum A, Wiik P, Gustavsson E, Veiby OP, Reseland J, Haugen AH, et al. The effect of strenuous exercise, calorie deficiency and sleep deprivation on white blood cells, plasma immunoglobulins and cytokines. Scand J Immunol. (1996) 43:228–35. doi: 10.1046/j.1365-3083.1996.d01-32.x
148. Suzuki K, Nakaji S, Yamada M, Liu Q, Kurakake S, Okamura N, et al. Impact of a competitive marathon race on systemic cytokine and neutrophil responses. Med Sci Sports Exerc. (2003) 35:348–55. doi: 10.1249/01.Mss.0000048861.57899.04
149. Mizokami T, Shimada M, and Suzuki K. Neutrophil depletion attenuates acute renal injury after exhaustive exercise in mice. Exp Physiol. (2024) 109:588–99. doi: 10.1113/ep091362
150. Mizokami T and Suzuki K. Neutrophil depletion attenuates acute liver stress after exhaustive exercise in mice. Med Sci Sports Exerc. (2023) 55:670–9. doi: 10.1249/mss.0000000000003094
151. Nunes-Silva A, Bernardes PT, Rezende BM, Lopes F, Gomes EC, Marques PE, et al. Treadmill exercise induces neutrophil recruitment into muscle tissue in a reactive oxygen species-dependent manner. An intravital microscopy study. PloS One. (2014) 9:e96464. doi: 10.1371/journal.pone.0096464
152. Kakanis MW, Peake J, Brenu EW, Simmonds M, Gray B, Hooper SL, et al. The open window of susceptibility to infection after acute exercise in healthy young male elite athletes. Exerc Immunol Rev. (2010) 16:119–37. doi: 10.1016/j.jsams.2010.10.642
153. Robson PJ, Blannin AK, Walsh NP, Castell LM, and Gleeson M. Effects of exercise intensity, duration and recovery on in vitro neutrophil function in male athletes. Int J Sports Med. (1999) 20:128–35. doi: 10.1055/s-2007-971106
154. Slusher AL, Zúñiga TM, and Acevedo EO. Maximal exercise alters the inflammatory phenotype and response of mononuclear cells. Med Sci Sports Exerc. (2018) 50:675–83. doi: 10.1249/mss.0000000000001480
155. Booth S, Florida-James GD, McFarlin BK, Spielmann G, O’Connor DP, and Simpson RJ. The impact of acute strenuous exercise on Tlr2, Tlr4 and Hla.Dr expression on human blood monocytes induced by autologous serum. Eur J Appl Physiol. (2010) 110:1259–68. doi: 10.1007/s00421-010-1616-2
156. Zhou X, Yi L, Lang H, Zhang J, Zhang Q, Yu L, et al. Dihydromyricetin-encapsulated liposomes inhibit exhaustive exercise-induced liver inflammation by orchestrating M1/M2 macrophage polarization. Front Pharmacol. (2022) 13:887263. doi: 10.3389/fphar.2022.887263
157. Kawanishi N, Mizokami T, Niihara H, Yada K, and Suzuki K. Macrophage depletion by clodronate liposome attenuates muscle injury and inflammation following exhaustive exercise. Biochem Biophys Rep. (2016) 5:146–51. doi: 10.1016/j.bbrep.2015.11.022
158. Mizokami T and Suzuki K. Involvement of neutrophils and macrophages in exhaustive exercise-induced liver, kidney, heart, and lung injuries. Exerc Immunol Rev. (2024) 30:49–62.
159. Mizokami T, Shimada M, and Suzuki K. Macrophage depletion attenuates acute renal damage after exhaustive exercise in mice. Int J Sports Med. (2022) 43:964–70. doi: 10.1055/a-1827-3261
160. Brown AS, Davis JM, Murphy EA, Carmichael MD, Carson JA, Ghaffar A, et al. Gender differences in macrophage antiviral function following exercise stress. Med Sci Sports Exerc. (2006) 38:859–63. doi: 10.1249/01.mss.0000218125.21509.cc
161. Czepluch FS, Barrès R, Caidahl K, Olieslagers S, Krook A, Rickenlund A, et al. Strenuous physical exercise adversely affects monocyte chemotaxis. Thromb Haemost. (2011) 105:122–30. doi: 10.1160/th10-06-0363
162. Guimarães TT, Gomes SMR, Albuquerque R, Lima AKC, Braga GF, Souza JB, et al. Chronic aerobic training at different volumes in the modulation of macrophage function and in vivo infection of balb/C mice by leishmania major. Front Microbiol. (2021) 12:734355. doi: 10.3389/fmicb.2021.734355
163. Starkie RL, Rolland J, and Febbraio MA. Effect of adrenergic blockade on lymphocyte cytokine production at rest and during exercise. Am J Physiol Cell Physiol. (2001) 281:C1233–40. doi: 10.1152/ajpcell.2001.281.4.C1233
164. Shephard RJ and Shek PN. Effects of exercise and training on natural killer cell counts and cytolytic activity: A meta-analysis. Sports Med. (1999) 28:177–95. doi: 10.2165/00007256-199928030-00003
165. Pedersen BK and Toft AD. Effects of exercise on lymphocytes and cytokines. Br J Sports Med. (2000) 34:246–51. doi: 10.1136/bjsm.34.4.246
166. Gleeson M and Bishop NC. The T cell and nk cell immune response to exercise. Ann Transplant. (2005) 10:43–8.
167. Eda N, Tabata H, Sone R, Fukuchi M, Kawai R, Harakuma K, et al. Yoga Improves Immunosuppression after A prolonged Intense Exercise. Physiol Int. (2025) 112:85–100. doi: 10.1556/2060.2025.00466
168. Kohut ML, Martin AE, SenChina DS, and Lee W. Glucocorticoids produced during exercise may be necessary for optimal virus-induced Il-2 and cell proliferation whereas both catecholamines and glucocorticoids may be required for adequate immune defense to viral infection. Brain Behav Immun. (2005) 19:423–35. doi: 10.1016/j.bbi.2005.04.006
169. Lancaster GI, Halson SL, Khan Q, Drysdale P, Wallace F, Jeukendrup AE, et al. Effects of acute exhaustive exercise and chronic exercise training on type 1 and type 2 T lymphocytes. Exerc Immunol Rev. (2004) 10:91–106.
170. Kakanis MW, Peake J, Brenu EW, Simmonds M, Gray B, and Marshall-Gradisnik SM. T helper cell cytokine profiles after endurance exercise. J Interferon Cytokine Res. (2014) 34:699–706. doi: 10.1089/jir.2013.0031
171. Wilson LD, Zaldivar FP, Schwindt CD, Wang-Rodriguez J, and Cooper DM. Circulating T-regulatory cells, exercise and the elite adolescent swimmer. Pediatr Exerc Sci. (2009) 21:305–17. doi: 10.1123/pes.21.3.305
172. Bonini M, Gramiccioni C, Fioretti D, Ruckert B, Rinaldi M, Akdis C, et al. Asthma, allergy and the olympics. Curr Opin Allergy Clin Immunol. (2015) 15:184–92. doi: 10.1097/aci.0000000000000149
173. Langston PK, Sun Y, Ryback BA, Mueller AL, Spiegelman BM, Benoist C, et al. Regulatory T cells shield muscle mitochondria from interferon-Γ-mediated damage to promote the beneficial effects of exercise. Sci Immunol. (2023) 8:eadi5377. doi: 10.1126/sciimmunol.adi5377
174. Proschinger S, Winker M, Joisten N, Bloch W, Palmowski J, and Zimmer P. The effect of exercise on regulatory T cells: A systematic review of human and animal studies with future perspectives and methodological recommendations. Exerc Immunol Rev. (2021) 27:142–66.
175. Becker M, Joseph SS, Garcia-Carrizo F, Tom RZ, Opaleva D, Serr I, et al. Regulatory T cells require Il6 receptor alpha signaling to control skeletal muscle function and regeneration. Cell Metab. (2023) 35:1736–51.e7. doi: 10.1016/j.cmet.2023.08.010
176. Bruunsgaard H, Hartkopp A, Mohr T, Konradsen H, Heron I, Mordhorst CH, et al. In vivo cell-mediated immunity and vaccination response following prolonged, intense exercise. Med Sci Sports Exerc. (1997) 29:1176–81. doi: 10.1097/00005768-199709000-00009
177. Nieman DC and Nehlsen-Cannarella SL. The effects of acute and chronic exercise of immunoglobulins. Sports Med. (1991) 11:183–201. doi: 10.2165/00007256-199111030-00003
178. Kurowski M, Seys S, Bonini M, Del Giacco S, Delgado L, Diamant Z, et al. Physical exercise, immune response, and susceptibility to infections-current knowledge and growing research areas. Allergy. (2022) 77:2653–64. doi: 10.1111/all.15328
179. Mackinnon LT, Chick TW, van As A, and Tomasi TB. The effect of exercise on secretory and natural immunity. Adv Exp Med Biol. (1987) 216a:869–76. doi: 10.1007/978-1-4684-5344-7_102
180. Fahlman MM and Engels H-J. Mucosal iga and urti in american college football players: A year longitudinal study. Med Sci Sports Exercise. (2005) 37:374–80. doi: 10.1249/01.Mss.0000155432.67020.88
181. Gleeson M, McDonald WA, Pyne DB, Cripps AW, Francis JL, Fricker PA, et al. Salivary Iga levels and infection risk in elite swimmers. Med Sci Sports Exerc. (1999) 31:67–73. doi: 10.1097/00005768-199901000-00012
182. Borchers J, Merle CL, Schöneborn DD, Lyko LR, Thouet T, Wolfarth B, et al. Salivary diagnostic for monitoring strenuous exercise-a pilot study in a cohort of male ultramarathon runners. Int J Environ Res Public Health. (2022) 19:16110. doi: 10.3390/ijerph192316110
183. Huang W-C, Wei C-C, Huang C-C, Chen W-L, and Huang H-Y. The beneficial effects of lactobacillus plantarum ps128 on high-intensity, exercise-induced oxidative stress, inflammation, and performance in triathletes. Nutrients. (2019) 11:353. doi: 10.3390/nu11020353
184. Ruhee RT, Ma S, and Suzuki K. Protective effects of sulforaphane on exercise-induced organ damage via inducing antioxidant defense responses. Antioxidants. (2020) 9:136. doi: 10.3390/antiox9020136
185. Zhang J, Luo Z, Zhang Z, Zhao M, Tong C, Cong P, et al. Protective effect and mechanism of cannabidiol on myocardial injury in exhaustive exercise training mice. Chem Biol Interact. (2022) 365:110079. doi: 10.1016/j.cbi.2022.110079
186. Aral LA, Pinar L, Göktaş G, Deveden EY, and Erdoğan D. Comparison of hippocampal interleukin-6 immunoreactivity after exhaustive exercise in both exercise-trained and untrained rats. Turk J Med Sci. (2014) 44:560–8. doi: 10.3906/sag-1305-23
187. Hou P, Zhou X, Yu L, Yao Y, Zhang Y, Huang Y, et al. Exhaustive exercise induces gastrointestinal syndrome through reduced Ilc3 and Il-22 in mouse model. Med Sci Sports Exercise. (2020) 52:1710–8. doi: 10.1249/mss.0000000000002298
188. Ma S, Tominaga T, Kanda K, Sugama K, Omae C, Hashimoto S, et al. Effects of an 8-week protein supplementation regimen with hyperimmunized cow milk on exercise-induced organ damage and inflammation in male runners: A randomized, placebo controlled, cross-over study. Biomedicines. (2020) 8:51. doi: 10.3390/biomedicines8030051
189. Dorneles GP, da Silva IM, Santos MA, Elsner VR, Fonseca SG, Peres A, et al. Immunoregulation induced by autologous serum collected after acute exercise in obese men: A randomized cross-over trial. Sci Rep. (2020) 10:21735. doi: 10.1038/s41598-020-78750-z
190. Abbasi A, Fehrenbach E, Hauth M, Walter M, Hudemann J, Wank V, et al. Changes in spontaneous and lps-induced ex vivo cytokine production and mrna expression in male and female athletes following prolonged exhaustive exercise. Exerc Immunol Rev. (2013) 19:8–28.
191. Svendsen IS, Killer SC, and Gleeson M. Influence of hydration status on changes in plasma cortisol, leukocytes, and antigen-stimulated cytokine production by whole blood culture following prolonged exercise. ISRN Nutr. (2014) 2014:1–10. doi: 10.1155/2014/561401
192. Smits HH, Grünberg K, Derijk RH, Sterk PJ, and Hiemstra PS. Cytokine release and its modulation by dexamethasone in whole blood following exercise. Clin Exp Immunol. (1998) 111:463–8. doi: 10.1046/j.1365-2249.1998.00482.x
193. Rosa Neto JC, Lira FS, Oyama LM, Zanchi NE, Yamashita AS, Batista ML Jr., et al. Exhaustive exercise causes an anti-inflammatory effect in skeletal muscle and a pro-inflammatory effect in adipose tissue in rats. Eur J Appl Physiol. (2009) 106:697–704. doi: 10.1007/s00421-009-1070-1
194. de Araújo AL, Silva LCR, Fernandes JR, Matias M, Boas LS, MaChado CM, et al. Elderly men with moderate and intense training lifestyle present sustained higher antibody responses to influenza vaccine. Age. (2015) 37:105. doi: 10.1007/s11357-015-9843-4
195. Xia L, Li M, Zhang Y, Ruan J, Pei J, Shi J, et al. Exhaustive exercise does not affect humoral immunity and protection after rabies vaccination in a mouse model. Virol Sin. (2018) 33:241–8. doi: 10.1007/s12250-018-0026-1
196. Nieman DC. Exercise is medicine for immune function: implication for Covid-19. Curr Sports Med Rep. (2021) 20:395–401. doi: 10.1249/jsr.0000000000000867
197. Zhao T, Markevych I, Standl M, Schikowski T, Berdel D, Koletzko S, et al. Short-term exposure to ambient ozone and inflammatory biomarkers in cross-sectional studies of children and adolescents: results of the giniplus and lisa birth cohorts. Environ pollut. (2019) 255:113264. doi: 10.1016/j.envpol.2019.113264
198. Alexis NE, Huang YC, Rappold AG, Kehrl H, Devlin R, and Peden DB. Patients with asthma demonstrate airway inflammation after exposure to concentrated ambient particulate matter. Am J Respir Crit Care Med. (2014) 190:235–7. doi: 10.1164/rccm.201401-0126LE
199. Starkie RL, Hargreaves M, Rolland J, and Febbraio MA. Heat stress, cytokines, and the immune response to exercise. Brain Behav Immun. (2005) 19:404–12. doi: 10.1016/j.bbi.2005.03.005
200. Wang Q, Guo XL, Noel G, and Ogle C. Heat shock stress ameliorates cytokine mixture-induced permeability by downregulating the nitric oxide and signal transducer and activator of transcription pathways in caco-2 cells. Shock. (2007) 27:179–85. doi: 10.1097/01.shk.0000238070.66900.0e
201. Kuehu DL, Fu Y, Nasu M, Yang H, Khadka VS, and Deng Y. Use of microalgae-derived astaxanthin to improve cytoprotective capacity in the ileum of heat-induced oxidative stressed broilers. Anim (Basel). (2024) 14:1932. doi: 10.3390/ani14131932
202. Frumento D and Ţãlu Ş. Effects of wildfire exposure on the human immune system. Fire. (2024) 7:469. doi: 10.3390/fire7120469
Keywords: exercise intensity, immune health, moderate-intensity exercise, immune cell function, cytokine regulation
Citation: Shi X, Hu L, Nieman DC, Li F, Chen P, Shi H and Shi Y (2025) Exercise workload: a key determinant of immune health – a narrative review. Front. Immunol. 16:1617261. doi: 10.3389/fimmu.2025.1617261
Received: 24 April 2025; Accepted: 01 July 2025;
Published: 24 July 2025.
Edited by:
Bartolo Ferraro, Ludwig Maximilian University of Munich, GermanyReviewed by:
Paul Kent Langston, Harvard Medical School, United StatesCarlos Farinha, Polytechnic Institute of Castelo Branco, Portugal
Marcin Kurowski, Medical University of Lodz, Poland
Copyright © 2025 Shi, Hu, Nieman, Li, Chen, Shi and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yue Shi, c2hpeXVlOTEwQHNpbmEuY29t; Hui Shi, c2hpaHVpX3NqdHVAc2luYS5jb20=
†These authors have contributed equally to this work
 Xinxin Shi
Xinxin Shi Lunuo Hu
Lunuo Hu David C. Nieman
David C. Nieman Fei Li
Fei Li Peijie Chen
Peijie Chen Hui Shi
Hui Shi Yue Shi
Yue Shi