- 1Affiliated Hospital of Gansu University of Traditional Chinese Medicine, Lanzhou, China
- 2Gansu University of Traditional Chinese Medicine, Lanzhou, China
Recent advances in bone biology have underscored the essential role of the gut microbiota in maintaining skeletal homeostasis. Gut-derived metabolites, particularly short chain fatty acids and tryptophan derivatives, influence bone metabolism through modulation of immune signaling, inflammation, and endocrine networks. Emerging evidence indicates that these effects are context dependent and dose dependent, rather than uniformly beneficial or detrimental. For instance, butyrate and lipopolysaccharide exhibit biphasic effects on both osteogenesis and osteoclastogenesis, contingent on concentration, immune status, and the local microenvironment. Microbiota-targeted strategies such as probiotics, prebiotics, and fecal microbiota transplantation are under active investigation as innovative interventions for osteoporosis in both preclinical and clinical contexts. However, substantial knowledge gaps persist, including inconsistent therapeutic outcomes, limited mechanistic insight into host–microbiota interactions, and the absence of standardized microbial intervention protocols. In addition, safety concerns related to FMT, particularly in immunocompromised elderly populations, emphasize the need for rigorous donor screening, extended follow-up periods, and personalized risk and benefit assessment models. To advance the field, future studies should incorporate multi-omics platforms and precision medicine tools to identify key microbial targets and enhance therapeutic efficacy. This review consolidates current evidence and proposes a conceptual framework to clarify the context-specific roles of the gut microbiota in bone remodeling. A deeper mechanistic understanding will be crucial for translating microbiota-based strategies into safe and effective treatments for metabolic bone disorders.
1 Introduction
Osteoporosis constitutes a prevalent metabolic bone disorder characterized by diminished bone mass, compromised microarchitecture, and elevated fracture susceptibility (1). With an annual global incidence exceeding 100 million cases and a male-to-female prevalence ratio of approximately 2:3, this condition represents a growing healthcare challenge. Population aging trends compound the osteoporotic burden, compromising patient quality of life while imposing substantial healthcare costs (2, pp. 2005–2010). The insidious nature of early-stage disease, presenting with subtle pain and spinal deformities, often delays diagnosis and increases fracture risk (3).
Human microbiota complexity encompasses diverse microbial ecosystems, with the gastrointestinal tract harboring approximately 70% of total microbial biomass (4, 5). The gut microbiota represents the body’s most extensive microbial ecosystem, containing up to 10^14 microorganisms whose collective genome exceeds human genetic content by 150-fold (6–8). This “microbial organ” maintains critical physiological functions including intestinal barrier integrity, nutrient metabolism, and immunometabolic homeostasis (9, 10). The human gut microbiota comprises predominantly Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria phyla, orchestrating essential processes encompassing nutrient biotransformation, xenobiotic detoxification, immune modulation, and barrier function maintenance (11, 12). Accumulating evidence establishes sophisticated bidirectional communication networks between gut microbiota and skeletal systems, termed the “gut-bone axis” (13). Microbial communities and their metabolic derivatives regulate bone homeostasis through direct and indirect mechanisms, modulating osteoblast-osteoclast dynamics via metabolic, inflammatory, and immune pathways (14). Physiological microbiota stability promotes immune equilibrium, supporting osteocyte function and balanced bone turnover (15, 16). Conversely, microbial dysbiosis disrupts skeletal homeostasis through multiple mechanisms: immune system perturbation altering CD4+ T cell subset ratios and cytokine profiles, particularly receptor activator of nuclear factor kappa-B ligand (RANKL) expression, thereby promoting osteoclastogenesis (15); direct metabolite-mediated regulation of osteoblast and osteoclast differentiation through short chain fatty acids (SCFAs) and bile acid signaling (17); and dysbiosis-induced inflammatory cascades releasing bone-catabolic mediators that disrupt formation-resorption coupling (18). Reciprocally, bone-derived hormones and extracellular vesicles modulate intestinal epithelial function, influencing barrier integrity and microbial homeostasis. Skeletal metabolic perturbations can trigger intestinal stress responses, promoting epithelial damage and microbiota dysbiosis, which perpetuates inflammatory bone loss (19). Elucidating these complex microbiota-bone interactions therefore holds profound therapeutic implications for developing targeted interventions against metabolic bone diseases.
2 Gut microbiota and metabolite associations with osteoporosis
Patients with osteoporosis exhibit pronounced gut microbiota dysbiosis characterized by markedly reduced microbial community diversity (20). Figure 1 illustrates the mechanistic associations between gut microbiota, their metabolites, and osteoporosis pathogenesis. microbiota analyses demonstrate elevated abundances of Bacteroidetes, Bacteroides and Eisenbergiella, alongside increased abundances of Clostridium, Lactobacillus, and Eggerthella species in osteoporotic individuals. Concurrently, relative abundances of Parabacteroides and Flavobacterium increase while Ruminococcus decreases significantly (21). Chen et al. (22) identified substantial reductions in lactobacilli and butyrate-producing bacterial populations. Paradoxically, they observed elevated concentrations of osteogenic metabolites—alkaline phosphatase, runt-related transcription factor 2, and osteoprotegerin—derived from Lactobacillus acidophilus, L. rhamnosus, and butyrate-producing species. These metabolites collectively enhance osteoblast proliferation and differentiation.
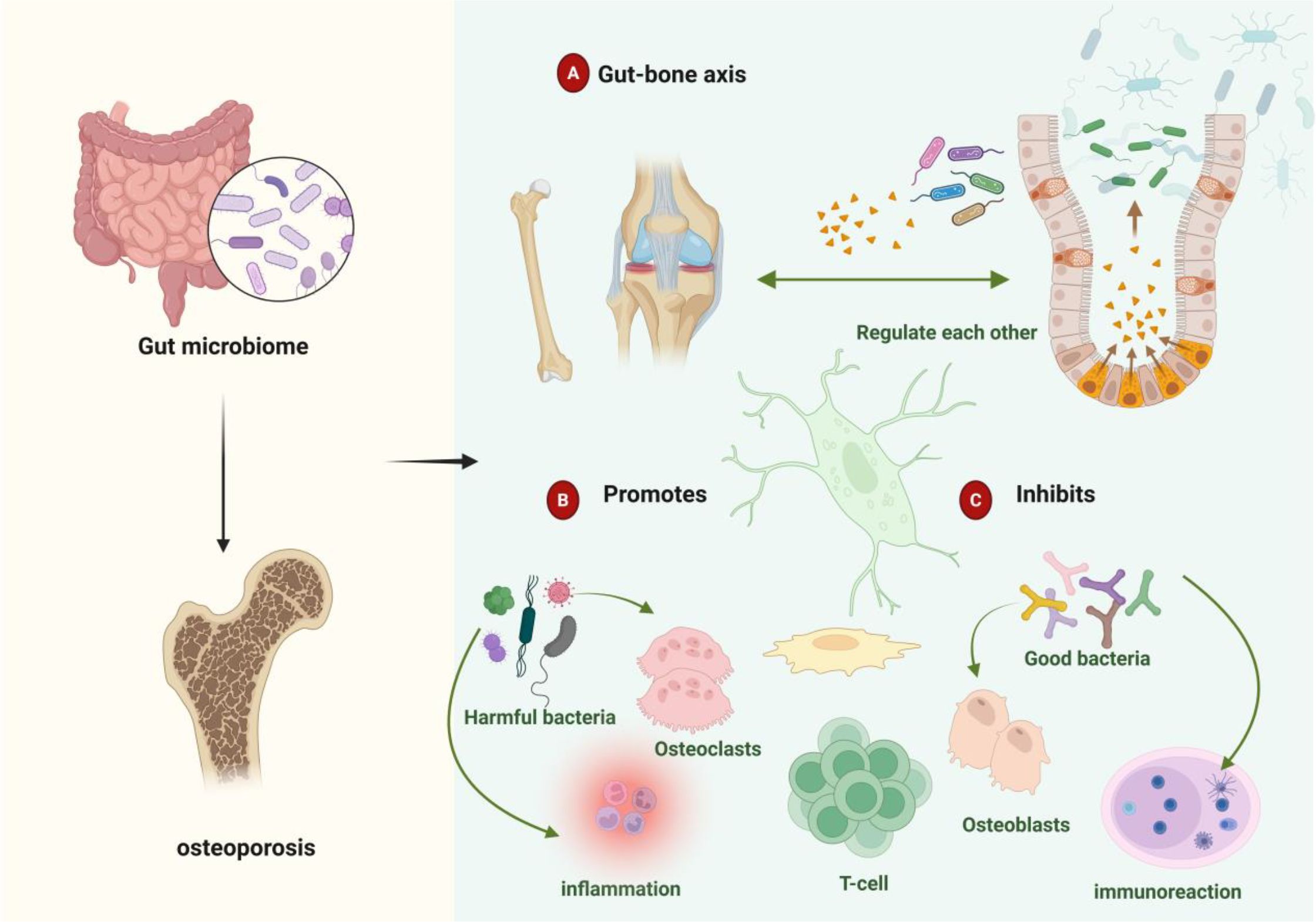
Figure 1. Gut microbiota-metabolite associations with osteoporosis. (A) Bidirectional gut-bone axis communication mediates skeletal homeostasis through direct and indirect microbiota-derived signals. (B) Dysbiotic conditions promote pathogenic bacterial expansion, accelerating osteoclastogenesis and inflammatory bone loss. (C) Beneficial microbes and their metabolites maintain skeletal integrity through immunomodulatory mechanisms that favor bone formation over resorption.
Bacterial taxa demonstrate distinct regulatory effects on bone homeostasis. Beneficial commensals, including Bifidobacterium species, promote regulatory T cells (Treg) differentiation while suppressing excessive bone resorption (23). Conversely, pathogenic bacteria—notably segmented filamentous bacteria and certain Ruminococcus species—exacerbate osteoclastogenesis and bone catabolism through T helper 17 cells (Th17) activation (24, 25). Microbial metabolites serve as critical mediators: butyrate stimulates osteoblast differentiation and bone accrual, whereas lipopolysaccharide (LPS) inhibits osteoblast maturation while promoting osteoclast activation, collectively influencing osteoporotic progression. Investigation of gut virome and mycobiome effects on skeletal health remains in its infancy. These microbial communities modulate both intestinal barrier integrity and bone metabolism through sophisticated immunoregulatory networks (26). Fungal species, particularly Candida and Aspergillus, indirectly influence bone turnover by modulating host immune responses and osteoclast activity (27). However, the precise mechanisms underlying mycobiome-bone interactions require further elucidation. Epidemiological evidence establishes robust associations between gut microbiota dysbiosis and accelerated bone loss, leading to elevated osteoporotic fracture risk (28, 29). Microbial metabolites predominantly derive from incompletely digested dietary substrates and host-secreted mucins (30, 31). These include SCFAs, bile acids, indole derivatives, lipopolysaccharide, vitamins, and polyamines (32, 33). Such bioactive compounds orchestrate complex regulatory networks affecting endocrine signaling, immune cell differentiation, inflammatory cascades, and oxidative stress responses, collectively modulating osteoporotic pathogenesis (34). Host factors—including health status, age, sex, and immunological competence—critically determine microbiota-mediated skeletal effects. Age-related immune senescence and microbiota compositional shifts may fundamentally alter metabolite bioactivity, thereby disrupting bone homeostasis. In elderly populations, dysbiotic conditions can transform typically osteoprotective metabolites into bone-catabolic mediators, accelerating resorptive processes and osteoporotic progression.
3 Mechanistic pathways of gut microbiota-mediated osteoporotic progression
Gut microbiota orchestrate complex physiological networks encompassing metabolic regulation, biosynthetic processes, and immunomodulation (35), fundamentally influencing disease pathogenesis across multiple organ systems. In osteoporotic pathophysiology, gut microbial communities and their bioactive metabolites drive skeletal deterioration through sophisticated regulatory mechanisms targeting bone metabolism, immune cell dynamics, and endocrine signaling networks. Figure 2 illustrates the mechanistic framework whereby gut microbiota promote osteoporotic progression.
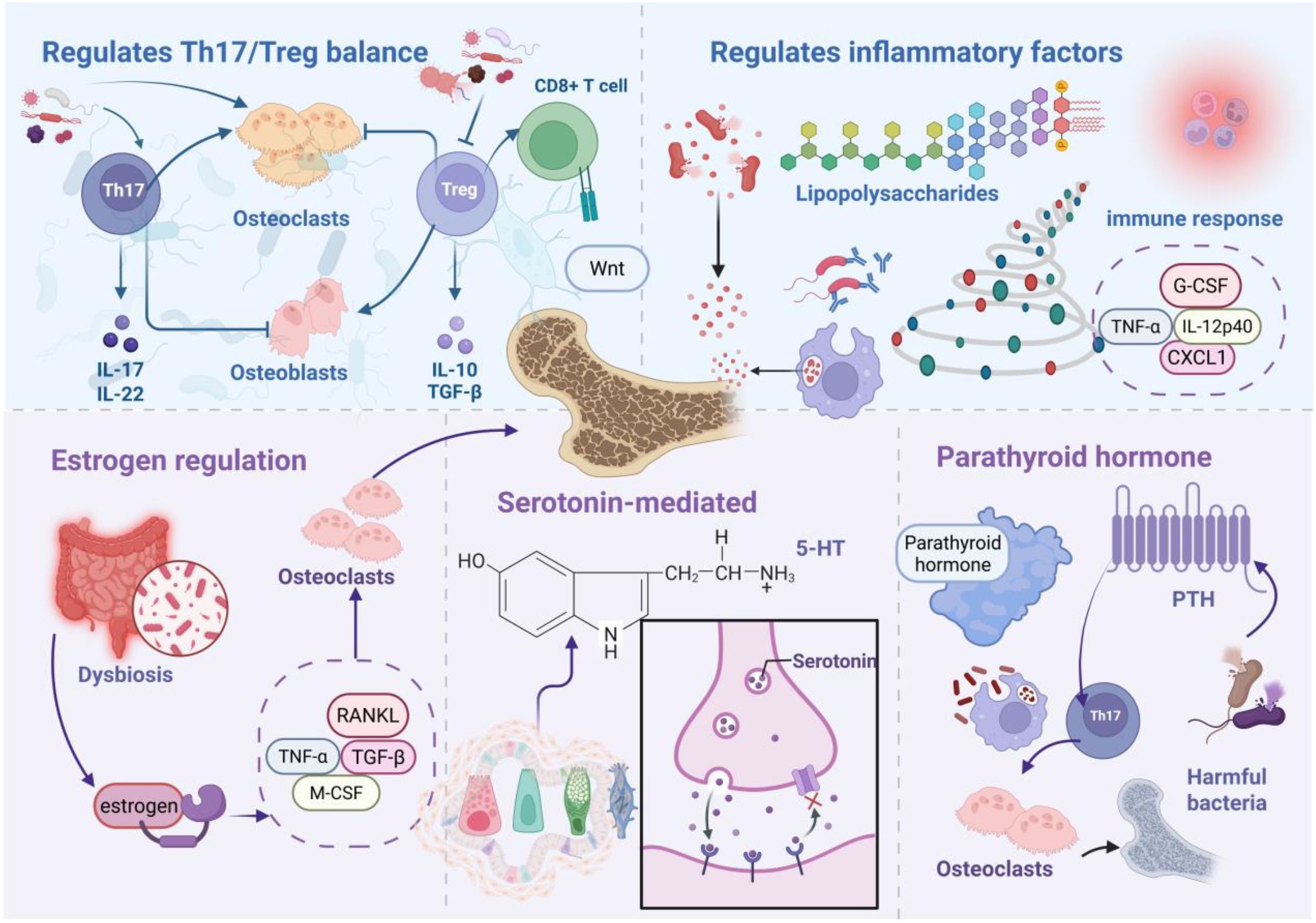
Figure 2. Gut microbiota-driven osteoporotic mechanisms. Microbial dysbiosis promotes pathogenic bacterial expansion, with resulting microorganisms and metabolites orchestrating osteoporotic progression through immune cell modulation, Th17/Treg imbalance, and inflammatory mediator dysregulation. Additionally, microbiota-derived signals drive skeletal catabolism via endocrine pathway perturbations, including estrogen, serotonin, and parathyroid hormone (PTH) dysregulation.
3.1 Immunoregulatory mechanisms
Gut microbial communities modulate bone tissue immune architecture through sophisticated interactions with intestinal dendritic cells and immune effectors, thereby controlling skeletal homeostasis (36, 37). Germ-free mouse studies demonstrate profound reductions in osteoclast populations alongside diminished bone expression of pro-inflammatory mediators Interleukin-6, tumor necrosis factor alpha (TNF-α), and RANKL. microbiota reconstitution restores immune functionality, establishing gut microbiota as fundamental regulators of bone-immune crosstalk (38).
3.1.1 Th17/Treg axis modulation
Th17 cells drive osteoclastogenesis via RANKL-dependent pathways (39). Bone marrow-resident Th17 TNF-α+ populations promote osteoclast differentiation independently of exogenous osteoclastogenic stimuli while stimulating mesenchymal stem cells to secrete chemokines monocyte chemoattractant protein-1, macrophage Inflammatory protein-1 alpha, and RANKL, facilitating inflammatory monocyte recruitment and bone catabolism. Zaiss and colleagues elucidated Treg functions in skeletal homeostasis, particularly through osteoclastogenesis suppression. This seminal work established immune-bone interactions, highlighting Treg-mediated bone protection. Mechanistically, Treg cells inhibit osteoclast differentiation via cytotoxic T-lymphocyte-associated protein 4/CD80/86 and IDO/tryptophan signaling while promoting osteoblast maturation (40, 41). Treg populations suppress osteoclastogenesis through Interleukin-4, Interleukin-10, and transforming growth factor-beta (TGF-β) secretion while enhancing bone formation (42). Furthermore, Treg cells stimulate CD8+ T cell Wnt10b expression, activating osteoblast Wnt signaling to promote bone formation (43). Specific microbial taxa modulate Th17/Treg balance to influence bone turnover. Clostridium clusters IV, XIVa, and XVIII activate intestinal epithelial TGF-β production (44), while Bacteroides fragilis drives Th17 differentiation via Janus Kinase/Signal Transducer and Activator of Transcription 3 activation (45). Firmicutes populations suppress Treg differentiation while promoting Th17 expansion, upregulating stromal RANKL expression and expanding osteoclast precursor pools (46). B cells, as primary RANKL and osteoprotegerin (OPG) producers, respond to microbial regulation (47). Clostridium/Bifidobacterium imbalances suppress mechanistic target of rapamycin) signaling, elevating RANKL/OPG ratios. Bacteroides fragilis downregulates OPG via Wnt/β-catenin interference (48), promoting osteoclast differentiation. Bile acid metabolites demonstrate opposing effects: 3-oxoLCA inhibits Th17 differentiation through RAR-related Orphan Receptor Gammat binding, while isoalloLCA promotes Treg expansion via mitochondrial reactive oxygen species (ROS) induction (49), collectively orchestrating bone immune microenvironments.
3.1.2 Inflammatory mediator regulation
Gram-negative bacterial LPS exerts context-dependent bone effects: promoting phagocyte differentiation from osteoclast precursors in RANKL-depleted conditions while enhancing osteoclastogenesis via Toll-like receptor activation in RANKL-sufficient environments (50). Compromised intestinal barrier integrity facilitates systemic LPS translocation, triggering immune activation and accelerated bone loss (51). RANKL functions as a critical immune-bone interface molecule expressed in activated T cells and mesenchymal lineages. Clostridium-derived secondary bile acids modulate inflammatory responses by suppressing TNF-α-induced immunity and inflammasome activation (52). Colitis models demonstrate elevated chemokine and cytokine levels (granulocyte colony-stimulating factor), TNF-α, Interleukin 12 p40 subunit, monocyte chemoattractant protein-1/C-C motif ligand 2, regulated on activation, normal T cell expressed and secreted/C-C Motif Chemokine Ligand 5, C-X-C motif chemokine ligand 1), stimulating osteoclast precursor proliferation and disrupting bone homeostasis (53).
3.2 Endocrine modulation
Gut microbiota regulate skeletal homeostasis through bioactive compound production, influencing endocrine networks governing bone metabolism (54, 55). Microbial communities orchestrate bone remodeling via estrogen-dependent and independent signaling cascades.
3.2.1 Estrogen signaling
Estrogen receptor alpha mediates estrogen-dependent bone formation (56, 57), with estrogen receptor alpha deficiency reducing femoral length in female mice (58). Estrogen maintains bone mass through immune response regulation and osteoblast-osteoclast balance (59), mechanistically inhibiting RANKL expression in CD3+ T cells and CD20+ B cells while promoting osteoblast OPG production (60). Estrogen-deficient bone loss correlates with T cell-mediated TNF-α upregulation, indirectly enhancing osteoclastogenesis (61, 62). Gut microbiota critically influence estrogen-deficient osteoporosis. Ovariectomized rat models exhibit profound microbiota dysbiosis with elevated Firmicutes/Bacteroidetes ratios. microbiota profiling reveals positive correlations between Ruminococcus, Clostridium, and Coprococcus abundances with bone loss, contrasting with negative Bacteroidetes associations (63). Notably, germ-free and antibiotic-treated mice resist estrogen-deficient bone loss (64). Mechanistically, increased intestinal permeability drives dysbiosis and pathogen translocation, stimulating myeloid stromal cell secretion of TGF-β, RANKL, and M-CSF to promote osteoclast maturation.
3.2.2 Serotonin-mediated pathways
Serotonin 5-hydroxytryptamine (5-HT) signaling critically regulates skeletal development and maintenance (65). Both osteoblasts and osteoclasts express 5-HT receptors, with elevated serotonin levels correlating with bone loss (66). Synthetic 5-HT inhibitors prevent ovariectomy-induced bone loss (67), with forkhead box O1 serving as a key mediator of intestinal 5-HT effects on osteoblast proliferation (68). Gut microbiota regulate bone metabolism via intestinal chromaffin cell 5-HT synthesis modulation (68). Luminal SCFAs promote tryptophan hydroxylase 1 mRNA expression and chromaffin cell 5-HT synthesis (69). Specifically, Lactobacillus, Streptococcus, and Escherichia coli metabolites induce tryptophan hydroxylase 1 expression in chromaffin cells, elevating peripheral 5-HT in germ-free mice (70). Chromaffin-derived 5-HT activates osteoblast precursor 5-HT1B receptors, promoting osteoclastogenesis while inhibiting osteoblast proliferation and bone formation (71).
3.2.3 Parathyroid hormone signaling
Primary hyperparathyroidism, characterized by excessive PTH secretion (72), drives osteoporosis through osteocyte-derived RANKL and T cell-derived Interleukin-17A-mediated bone catabolism (73). Continuous PTH fails to induce bone loss in antibiotic-treated or germ-free mice. Segmented filamentous bacteria enable PTH-mediated expansion of intestinal TNF+ T cells and Th17 populations with subsequent bone marrow recruitment. Bone marrow TNF+ T cells upregulate C-C motif chemokine ligand 20 expression, facilitating intestinal-to-bone marrow Th17 cell migration (74). Intermittent PTH enhances bone formation through osteoblast Wnt pathway activation, promoting osteoblast formation, survival, and bone lining cell reactivation. Butyrate critically mediates intermittent PTH-induced skeletal anabolism (75), binding dendritic cell G protein-coupled receptor 43 to induce Treg differentiation. Subsequently, Treg cells promote bone marrow CD8+ T cell Wnt10b expression, activating Wnt-dependent bone formation (75).
4 Protective mechanisms of gut microbiota and metabolites against osteoporotic progression
Osteoporotic pathogenesis involves complex regulatory imbalances across multiple molecular networks and signaling cascades. Emerging evidence demonstrates that targeted modulation of bone metabolism regulators, inflammatory mediators, and immune system components effectively attenuates osteoporotic progression. Figure 3 illustrates the mechanistic framework whereby gut microbiota and their metabolites confer skeletal protection.
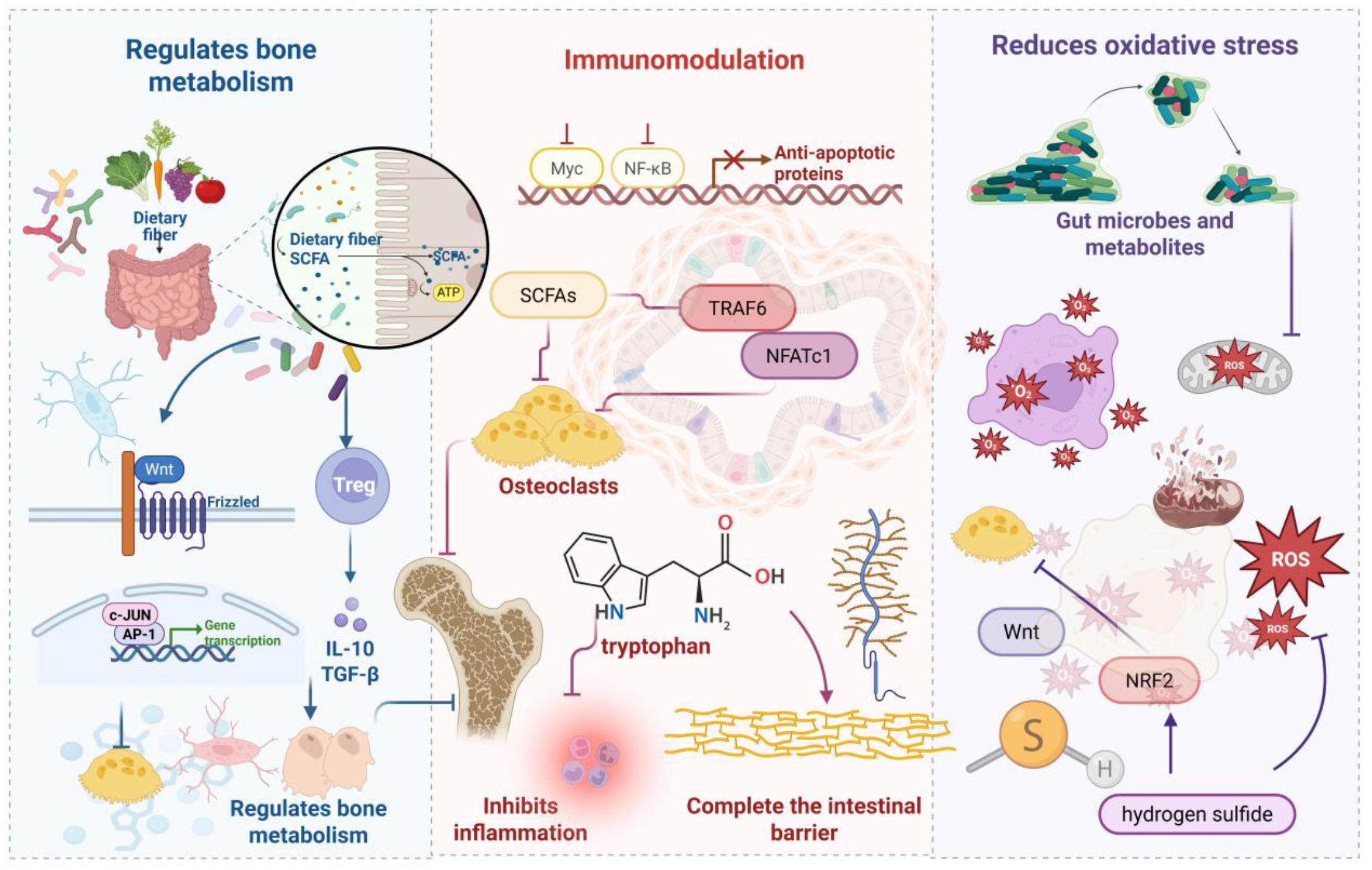
Figure 3. Gut microbiota-mediated osteoprotective mechanisms. SCFAs represent critical microbial metabolites that regulate bone homeostasis via Wnt signaling and regulatory T cell modulation. Additionally, tryptophan derivatives and SCFAs attenuate inflammatory responses while upregulating tight junction proteins to preserve intestinal barrier integrity, collectively inhibiting osteoporotic progression. microbiota-derived compounds reduce oxidative stress levels, providing anti-inflammatory and antioxidant benefits that suppress bone catabolism.
4.1 Bone metabolic regulation
SCFAs constitute pivotal microbial metabolites orchestrating bone homeostasis through multi-pathway mechanisms, principally via osteoclastogenesis inhibition and osteoblast function enhancement. Butyrate promotes stromal cell osteogenic differentiation and mineralization nodule formation (76, 77), primarily through Wnt signaling pathway activation—a central regulator of bone mass acquisition and maintenance (78). Gain-of-function Wnt mutations generate high bone mass phenotypes, whereas loss-of-function variants cause low bone mass and premature osteoporosis (79). SCFAs induce naïve CD4+ T cell differentiation toward regulatory Tregs, with probiotics such as Lactobacillus rhamnosus GG conferring bone protection via the SCFAs-Tregs-bone metabolism axis. L. rhamnosus GG supplementation expands SCFA-producing bacterial populations, elevating intestinal and systemic butyrate concentrations to promote bone formation and increase trabecular volume (43). Enhanced lactobacilli and bifidobacteria levels facilitate mineral absorption, improving bone density (80). Gut microbiota composition modulates mineral bioavailability, particularly calcium, through intestinal pH regulation. Furthermore, gut microbiota participate in vitamin B and K biosynthesis alongside bile acid metabolism (81). Vitamins B and K represent essential bone health modulators (82), while bile acids critically regulate calcium absorption. The gut microbiota degrades macromolecular substrates into bioavailable components, supporting bone health and metabolic homeostasis while inhibiting osteoporotic progression (83).
4.2 Immunomodulation
Indole derivatives, as key tryptophan metabolic products, maintain gut microecological balance through multifaceted mechanisms. Molecularly, these compounds upregulate tight junction protein and mucoprotein expression in intestinal epithelial cells while enhancing anti-inflammatory Interleukin-10 production and suppressing pro-inflammatory Interleukin-8 expression, thereby modulating bone metabolism (84). Indole stimulates antimicrobial peptide and mucoprotein production, promotes intestinal villus cell proliferation, and inhibits pathogenic expansion to preserve mucosal barrier integrity (85). Concurrently, indole exerts anti-inflammatory effects through immune response modulation (86), with appropriate supplementation effectively attenuating inflammatory responses and optimizing microbiota composition (87). SCFAs represent another critical class of anti-inflammatory metabolites derived from indigestible dietary fiber fermentation. These compounds suppress autoimmune inflammation by inhibiting nuclear factor κB activation in B cells (88). Specifically, propionate and butyrate inhibit osteoclastogenesis and bone resorption through TNF receptor-associated factor 6 and nuclear factor of activated T-cells, cytoplasmic 1 downregulation, promoting bone density enhancement (14, 17). Butyrate significantly suppresses osteoclast activity via metabolic reprogramming in osteoclast precursors, enhancing glycolysis while downregulating key osteoclastic genes at the expense of oxidative phosphorylation (77). Additionally, SCFAs regulate osteoclast precursor survival, inducing programmed cell death to improve bone density without compromising osteoblast function (77), thereby critically modulating bone metabolic equilibrium.
4.3 Oxidative stress attenuation
Oxidative stress—characterized by imbalanced ROS generation and clearance—represents a pathological driver of cellular damage. Accumulating evidence establishes oxidative stress as a critical mediator of osteoporotic pathogenesis. Elevated ROS levels directly compromise bone tissue integrity through mitochondrial dysfunction, DNA fragmentation, and protein oxidative modifications, culminating in osteoblast apoptosis and diminished bone formation capacity. Simultaneously, ROS activate nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and mitogen-activated protein kinase signaling cascades, upregulating RANKL expression and enhancing osteoclast differentiation and activity. This process accelerates bone resorption, disrupting formation-resorption coupling and ultimately causing bone loss with trabecular architectural deterioration (89, 90). Within this pathological framework, antioxidant mechanisms function as negative regulatory circuits providing essential bone protection. Gut microbiota and their metabolites attenuate oxidative stress through multiple pathways, indirectly preventing osteoporotic progression. Probiotic strains including lactobacilli and bifidobacteria upregulate key antioxidant enzymes—superoxide dismutase, catalase, and glutathione peroxidase—enhancing host free radical scavenging capacity. These strains additionally produce small-molecule antioxidants such as glutathione and extracellular polysaccharides, further augmenting antioxidant defenses (91). Gut-derived metabolites demonstrate substantial antioxidant regulatory potential. Butyrate activates nuclear factor erythroid 2–related factor 2/heme oxygenase-1 pathways to promote antioxidant factor synthesis, significantly reducing ROS levels while protecting bone cells from oxidative damage. Propionate suppresses endogenous ROS generation via nicotinamide adenine dinucleotide Phosphate oxidase inhibition while upregulating mitochondrial antioxidant enzyme expression, preserving mitochondrial homeostasis and bone cell functionality. Hydrogen sulfide, another key metabolite, promotes bone marrow mesenchymal stem cell proliferation and osteogenic differentiation through Wnt/β-catenin pathway activation, while demonstrating bone-protective effects via RANKL expression suppression and osteoclast formation inhibition (92).
4.4 Contextual and dose-dependent effects of gut metabolites
An increasing body of evidence indicates that certain canonical gut-derived metabolites do not consistently exert osteoanabolic or anti-resorptive effects on bone metabolism. Instead, they exhibit marked dose dependence and sensitivity to host-specific physiological contexts. Their biological effects are regulated in a dynamic manner by multiple factors, including metabolite concentration, the inflammatory microenvironment, endocrine signaling, and immune equilibrium. These context-dependent phenomena are especially pronounced in SCFAs, such as butyrate, and endotoxins like LPS (93). Butyrate, a SCFA possessing significant immunomodulatory and tissue-regenerative properties, demonstrates a characteristic biphasic influence on bone metabolism. At low concentrations (<1 mM), it inhibits histone deacetylase activity, activates osteogenic Wnt signaling pathways, promotes Wnt10b expression, and induces osteogenic differentiation in bone marrow mesenchymal stem cells, thus enhancing osteogenesis (93). Additionally, butyrate contributes indirectly to skeletal homeostasis by expanding Tregs and suppressing Th17-mediated inflammation (94). However, at higher concentrations (>5 mM), butyrate facilitates the release of proinflammatory cytokines such as TNF-α and IL-6 from monocyte–macrophage lineages, leading to RANKL-mediated osteoclastogenesis (93). Under inflammatory conditions, it may further amplify osteoclast precursor activation and bone resorption via macrophage pyroptosis (93). This dose-dependent shift in bioactivity underscores the need for precise dosage control and personalized contextual evaluation when considering butyrate as a therapeutic agent. Similarly, LPS—a major constituent of the Gram-negative bacterial outer membrane—can translocate into the systemic circulation in the event of impaired gut barrier integrity and significantly reshape the bone immune microenvironment. At low doses, in the absence of RANKL co-stimulation, LPS primarily drives precursor cells toward phagocytic differentiation with limited osteoclastogenic capacity, potentially contributing to immune homeostasis. In contrast, elevated LPS concentrations activate the TLR4/MyD88/NF-κB signaling cascade, leading to overproduction of RANKL and proinflammatory cytokines and thereby accelerating osteoclastogenesis and bone resorption. Importantly, these dose–response effects are not universally consistent across physiological states. They are strongly influenced by host immune profiles, hormonal status, and gut microbiota composition. For example, under estrogen-deficient conditions, increased gut permeability and Treg/Th17 imbalance may amplify the bone-resorptive potential of inflammatory metabolites. Concurrently, changes in gut pH can alter the ionization state and membrane transport efficiency of SCFAs, thereby modifying their bioactivity within the bone microenvironment (94). Collectively, the modulatory roles of gut metabolites in bone metabolism can be conceptualized as a tri-axial “dose–effect–context” interaction model. This framework not only elucidates the bidirectional nature of their biological actions but also provides a theoretical foundation for the development of dose-optimized, host-tailored microbiota-targeted therapeutic strategies.
5 Translational applications of gut microbiota in osteoporosis management
Contemporary osteoporosis therapeutics encompass bone resorption inhibitors and formation promoters, including bisphosphonates, calcitonin, and estrogen replacement therapy. However, adverse effects and prohibitive treatment costs limit clinical implementation. Recent advances in gut microbiota-bone health research have established microbiota modulation as a promising therapeutic target for osteoporotic intervention. Figure 4 illustrates gut microbiota-based versus conventional therapeutic approaches.
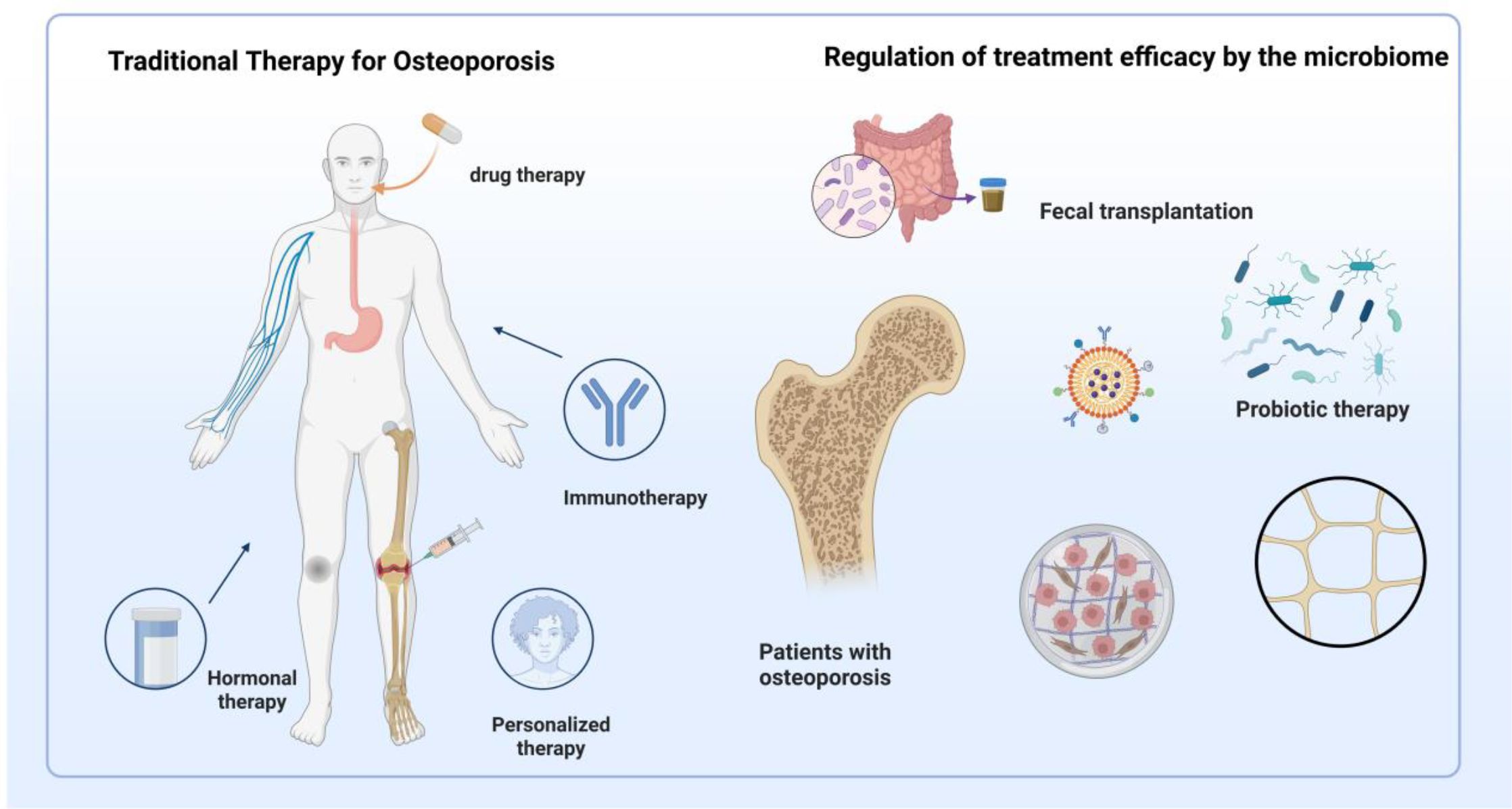
Figure 4. Traditional therapies and microbiota-based interventions for osteoporosis treatment. This figure illustrates two primary approaches to osteoporosis management. On the left, traditional therapies include drug therapy (e.g., bisphosphonates, hormone replacement), immunotherapy, hormonal regulation, and personalized medical strategies targeting bone turnover. On the right, microbiota-targeted interventions are shown to influence therapeutic efficacy through mechanisms such as fecal microbiota transplantation (FMT), probiotic supplementation, and microbial metabolite regulation. These strategies aim to restore gut microecological balance, enhance intestinal barrier integrity, modulate immune responses, and regulate endocrine factors, thereby contributing to improved skeletal health in osteoporotic patients.
5.1 Microbiota-based osteoporosis prevention
Probiotic interventions prevent osteoporotic progression through gut microbiota homeostasis restoration, facilitating vitamin and mineral absorption, immune system enhancement, and bioactive metabolite production, including organic acids and amino acids (74). Clostridium butyricum CBM588 metabolizes dietary fiber to generate protective SCFAs, attenuating bone loss. Faecalibacterium prausnitzii prevents inflammation-mediated bone catabolism via NF-κB inhibition and Interleukin-8 suppression while upregulating protective Interleukin-10 and Interleukin-12 expression (74). Preclinical studies demonstrate that lactobacilli and bifidobacteria effectively prevent ovariectomy-induced bone loss through tartrate-resistant acid phosphatase positive, receptor activator of nuclear factor κB positive, and RANKL+ cell formation inhibition. Mechanistically, propionate and butyrate reduce osteoclast populations and serum C-terminal telopeptide of type I collagen levels, while acetate suppresses osteoclastogenesis via T cell and B cell functional modulation (74).
Microbiota-mediated nutrient absorption regulation represents a fundamental mechanism influencing bone remodeling (95). Gut microbiota orchestrate food digestion, energy recovery, and vitamin biosynthesis and absorption. Comparative germ-free versus conventional mouse studies reveal microbiota-enhanced intestinal monosaccharide absorption and improved dietary energy extraction efficiency (96). Additionally, gut communities synthesize essential vitamins K and B complex, maintaining physiological vitamin requirements. Microbiota further regulate calcium bioavailability through intestinal pH modulation and calcium solubility enhancement (97), promoting skeletal health.
5.2 Microbiota-based osteoporosis therapeutics
Traditional osteoporotic management strategies focus on bone density improvement and fracture risk reduction through anti-resorptive and anabolic agents, yet demonstrate inherent limitations. Emerging gut homeostasis-targeting approaches offer unique therapeutic advantages, providing innovative osteoporotic intervention paradigms.
5.2.1 Microbiota-mediated therapeutic mechanisms
Gut microbial metabolites demonstrate substantial osteoprotective potential. Tryptophan derivatives, including indole-3-acetic acid and indole propionic acid, modulate intestinal barrier integrity via aryl hydrocarbon receptor activation, influencing bone catabolism. SCFAs critically regulate osteoporotic progression (C. 98), with acetate restoring aged bone marrow mesenchymal stem cell osteogenic capacity through cytoplasmic acetyl-coenzyme A restoration and chromatin accessibility enhancement. Akkermansia and Eubacterium species generate propionate, while Clostridiaceae and Eubacteriaceae produce butyrate, promoting bone formation via Treg-dependent mechanisms (98). Plant fiber-enriched dietary supplementation elevates SCFA levels, supporting skeletal homeostasis maintenance.
5.2.2 Microbiota-targeting therapeutic strategies
Probiotic interventions exert therapeutic effects through three principal mechanisms: intestinal epithelial barrier strengthening via tight junction protein regulation and permeability reduction (99); immunomodulation through Treg/Th17 balance restoration, exemplified by Bacillus clausii-mediated bone marrow and splenic Treg expansion alongside Th17 suppression, and Lactobacillus rhamnosus GG/L. reuteri-mediated Treg functional enhancement (100); and sex hormone regulation through sterol microbiota modulation, whereby microbial β-glucuronidase and β-glucosidase encoding influences estrogen metabolism and local/systemic hormone levels (100). Clinical applications demonstrate therapeutic efficacy: Bacteroides vulgatus ATCC 8482 reduces TNF-α via LPS/TLR4/NF-κB pathway downregulation (101), while Lactobacillus plantarum NK3/Bifidobacterium longum NK49 combinations suppress NFκB/TNF-α signaling (102). Clinical trials confirm that probiotic supplementation reduces bone resorption markers and enhances bone density in postmenopausal women (103). Fracture healing applications show promise: Akkermansia muciniphila facilitates healing through intestinal permeability reduction and inflammatory attenuation (104), while bifidobacteria promote barrier function, alleviate fracture-associated inflammation, and accelerate callus cartilage remodeling. Clinical microbiota therapy demonstrates significant potential. Prebiotic fibers, including inulin and fructooligosaccharides, demonstrate bone health benefits through beneficial bacterial proliferation promotion, intestinal barrier enhancement, and immune response modulation, indirectly supporting bone metabolism. These compounds increase bone density and reduce resorption, particularly benefiting elderly and osteoporotic populations (105, 106). Synbiotic formulations combining probiotic-prebiotic advantages represent emerging osteoporotic interventions, demonstrating superior efficacy compared to individual components through synergistic gut health improvement and beneficial bacterial growth promotion. However, clinical translation challenges persist in microbiota osteoporotic therapy. Despite preclinical validation in animal models, clinical application verification remains insufficient. Existing trials predominantly feature small sample sizes with inadequate statistical power, compromising conclusion reliability and generalizability. Future investigations require large-scale, randomized controlled, long-term clinical trials to ensure microbiota intervention efficacy and safety.
5.2.3 Gut microecology reconstruction
FMT represents a novel therapeutic modality that reconstructs gut microecological balance through healthy donor microbiota transfer to patients (107). Successful applications provide innovative osteoporotic microbial treatment paradigms, with FMT influencing bone metabolism through microbiota compositional regulation, intestinal barrier improvement, and immune system modulation (108). Nevertheless, FMT osteoporotic applications face substantial challenges. Primary safety concerns include segmented filamentous bacteria introduction potentially inducing Th17 development via Interleukin-17/Interleukin-23 pathways, exacerbating bone loss (109). Donor microbiota heterogeneity may generate therapeutic variability, necessitating standardized screening criteria and transplantation protocol optimization. FMT-probiotic combination strategies require investigation for enhanced therapeutic outcomes through optimized microbiota composition, though clinical data remain limited. FMT clinical implementation involves safety risks, including infectious disease transmission, adverse immune reactions, and long-term dysbiosis-related complications. While rigorous donor screening, pathogen detection, and standardized preparation protocols mitigate risks, protocol variability across institutions maintains safety concerns. In 2023, the U.S. Food and Drug Administration issued a safety alert highlighting incidents of severe infections, including sepsis, in recipients of FMT due to multidrug-resistant organisms, emphasizing the critical need for stringent screening and quality control of donor microbiota in clinical settings (110). Elderly individuals, a high-risk group for osteoporosis, exhibit age-related declines in immune function, increasing their susceptibility to FMT-associated adverse events (111). Therefore, it is recommended that future clinical trials evaluating the efficacy of FMT extend follow-up durations beyond 12 months to monitor potential long-term risks 112). Further research may focus on constructing a quantitative framework to assess risk–benefit profiles based on host microbiota composition, immune function, and donor–recipient compatibility, thereby enabling personalized safety evaluations in bone metabolic disorders. Notably, variations in donor screening and preparation protocols across institutions pose persistent challenges to the standardization of FMT. Future investigations should validate its safety under more stringent clinical conditions and develop harmonized and standardized procedural guidelines.
5.2.4 Clinical translation
microbiota-based interventions have emerged as a promising strategy for the clinical management of osteoporosis. In a randomized, double-blind, placebo-controlled clinical trial, Nilsson et al. ‘s randomized, double-blind, placebo-controlled trial revealed Lactobacillus reuteri-mediated bone density enhancement in elderly populations (113). Lambert et al. demonstrated that probiotic-red clover extract combinations (rich in isoflavone aglycones) significantly attenuate estrogen deficiency-induced bone loss through beneficial estrogen metabolite promotion. Synergistic effects with calcium, magnesium, and calcitonin supplementation exceed monotherapy efficacy (114), establishing theoretical foundations for probiotic-prebiotic-mineral matrix combinations. Ovariectomy-induced osteoporosis models reveal reduced aryl hydrocarbon receptor ligand levels, prompting indole acetic acid and indole propionic acid supplementation strategies. Integrated 16S rRNA sequencing, targeted HPLC-QQQ-MS metabolomics, and biological assessments elucidate tryptophan metabolite-mediated gut-bone axis improvements, providing novel osteoporotic therapeutic development frameworks (98). Table 1 is summary of clinical and translational studies on microbiota-based Interventions for bone health. Despite these encouraging findings, existing clinical trials are constrained by limited sample sizes, brief follow-up periods, and considerable heterogeneity in probiotic strains and dosing regimens. Consequently, the overall quality of evidence remains low to moderate. There is an urgent need for large-scale, multicenter, long-term randomized controlled trials to rigorously assess the effects of microbiota-targeted therapies on critical clinical outcomes, including bone mineral density and fracture incidence. Furthermore, the incorporation of the grading of recommendations assessment, development and evaluation framework is essential to enhance the scientific rigor and translational credibility of evidence supporting microbiota-based approaches for osteoporosis treatment.
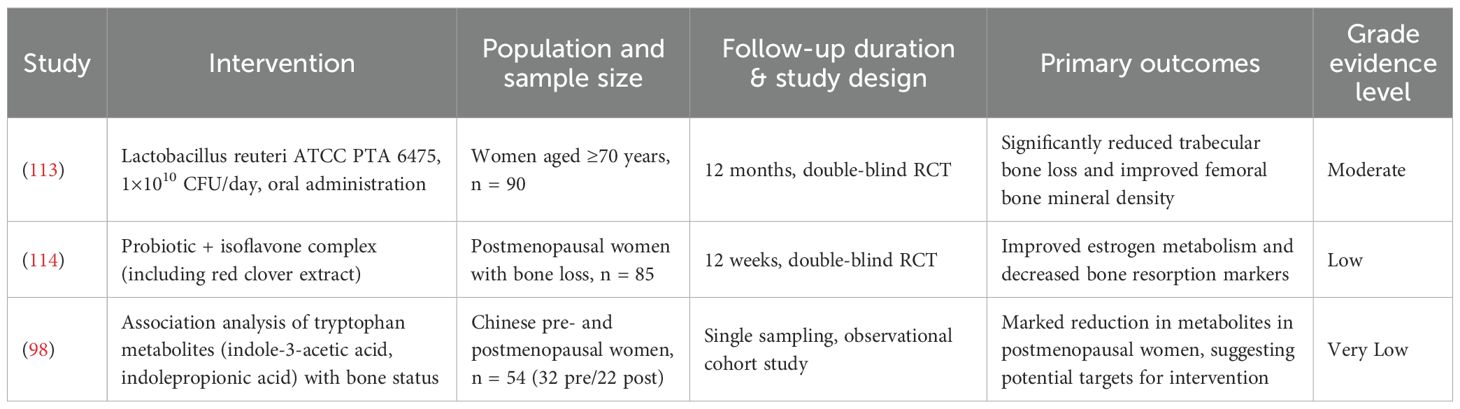
Table 1. Summary of clinical and translational studies on microbiota-based interventions for bone health.
6 Summary and prospects
Gut microbiota and their metabolites significantly influence osteoporotic pathogenesis, establishing causal relationships between microbial dysbiosis and disease development. Pathogenic bacteria and associated metabolites demonstrate elevated levels in osteoporotic patients, promoting disease progression through inflammatory induction, osteoclastogenesis enhancement, and endocrine disruption. Conversely, Lactobacillaceae strains, bifidobacteria, and SCFAs show reduced abundance in osteoporotic individuals yet exhibit protective effects through inflammation suppression, osteoclast inhibition, and bone remodeling optimization.
Gut microbiota and metabolites demonstrate therapeutic potential in osteoporosis management, with probiotics and postbiotics showing promising applications in disease prevention and treatment. Specific microorganisms with anti-osteoporotic properties represent potential pharmacological targets for therapeutic intervention. Despite preliminary mechanistic insights into gut microbiota-osteoporosis interactions, specific regulatory mechanisms require further elucidation. Current research predominantly examines individual bacterial species or metabolites, while gut microbiota constitute complex ecosystems featuring intricate inter-microbial and host-microbe interaction networks. Future investigations must comprehensively characterize bacterial species interactions and host immune-metabolic pathway integration to understand microbiota-mediated osteoporotic regulation. Critical knowledge gaps persist regarding microbiota metabolite cellular recognition and downstream signaling cascade regulation. While existing research reveals preliminary bone metabolic effects, specific cellular mechanisms and signaling pathways—particularly bone resorption-formation balance regulation—require clarification. Future studies should precisely elucidate metabolite-mediated bone metabolism regulatory mechanisms across diverse physiological contexts.
Although microbiota modulation demonstrates osteoporotic improvement potential in animal studies, clinical mechanisms and efficacy require validation. Future research should establish comprehensive animal models integrating transcriptomics, proteomics, and multi-omics technologies for systematic microbiota-metabolite mechanism investigation. This comprehensive approach will reveal condition-specific microbiota functional and metabolic changes affecting osteoporosis, providing precise theoretical foundations for clinical interventions. Multi-level investigations encompassing small molecule screening, animal validation, and clinical trials should evaluate gut microbiota-based therapeutic strategies. Exploring microbiota-drug metabolism interactions will provide individualized treatment insights. Through comprehensive microbiota regulatory strategy exploration, future research may establish novel osteoporotic therapeutic targets and interventions, advancing clinical field applications.
Author contributions
XX: Funding acquisition, Writing – original draft, Writing – review & editing, Conceptualization, Methodology. HL: Writing – original draft, Writing – review & editing. KW: Writing – review & editing. JL: Writing – review & editing. PQ: Writing – review & editing, Methodology, Writing – original draft, Investigation.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was supported by the National Nature Fund Regional Project (82160911, 81860864), Central University Basic Scientific Research Business Expenses Project (31920210041), The special project of science and technology development under the guidance of the central government (YDZX20206200002356).
Acknowledgments
The authors acknowledge the use of Biorender to create schematic representations in Figures 1-3. The agreement numbers associated with this use are XM28K1B2TH, KT28K1C2VR, AW28K1CKYB, VO28K1A2D3.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Eastell R and Szulc P. Use of bone turnover markers in postmenopausal osteoporosis. Lancet Diabetes Endocrinol. (2017) 5:908–23. doi: 10.1016/S2213-8587(17)30184-5
2. Zhang Y-W, Lu P-P, Li Y-J, Dai G-C, Chen M-H, Zhao Y-K, et al. Prevalence, characteristics, and associated risk factors of the elderly with hip fractures: A cross-sectional analysis of NHANES 2005-2010. Clin Interventions Aging. (2021) 16:177–85. doi: 10.2147/CIA.S291071
3. Chevalier C, Kieser S, Çolakoğlu M, Hadadi N, Brun J, Rigo D, et al. Warmth prevents bone loss through the gut microbiota. Cell Metab. (2020) 32:575–90.e7. doi: 10.1016/j.cmet.2020.08.012
4. Almeida A, Mitchell AL, Boland M, Forster SC, Gloor GB, Tarkowska A, et al. A new genomic blueprint of the human gut microbiota. Nature. (2019) 568:499–504. doi: 10.1038/s41586-019-0965-1
5. Sekirov I, Russell SL, Antunes LCM, and Finlay BB. Gut microbiota in health and disease. Physiol Rev. (2010) 90:859–904. doi: 10.1152/physrev.00045.2009
6. Parker A, Romano S, Ansorge R, Aboelnour A, Le Gall G, Savva GM, et al. Fecal microbiota transfer between young and aged mice reverses hallmarks of the aging gut, eye, and brain. Microbiome. (2022) 10:68. doi: 10.1186/s40168-022-01243-w
7. Xiao W, Su J, Gao X, Yang H, Weng R, Ni W, et al. The microbiota-gut-brain axis participates in chronic cerebral hypoperfusion by disrupting the metabolism of short-chain fatty acids. Microbiome. (2022) 10:62. doi: 10.1186/s40168-022-01255-6
8. Zhang T, Cheng J-K, and Hu Y-M. Gut microbiota as a promising therapeutic target for age-related sarcopenia. Ageing Res Rev. (2022) 81:101739. doi: 10.1016/j.arr.2022.101739
9. Allam-Ndoul B, Castonguay-Paradis S, and Veilleux A. Gut microbiota and intestinal trans-epithelial permeability. Int J Mol Sci. (2020) 21:6402. doi: 10.3390/ijms21176402
10. Qi P, Lv J, Bai L-H, Yan X-D, and Zhang L. Effects of hypoxemia by acute high-altitude exposure on human intestinal flora and metabolism. Microorganisms. (2023) 11:2284. doi: 10.3390/microorganisms11092284
11. Díaz-Garrido N, Badia J, and Baldomà L. Microbiota-derived extracellular vesicles in interkingdom communication in the gut. J Extracellular Vesicles. (2021) 10:e12161. doi: 10.1002/jev2.12161
12. Song M, Chan AT, and Sun J. Influence of the gut microbiome, diet, and environment on risk of colorectal cancer. Gastroenterology. (2020) 158:322–40. doi: 10.1053/j.gastro.2019.06.048
13. Lu L, Chen X, Liu Y, and Yu X. Gut microbiota and bone metabolism. FASEB J. (2021) 35:e21740. doi: 10.1096/fj.202100451R
14. Li P, Ji B, Luo H, Sundh D, Lorentzon M, and Nielsen J. One-year supplementation with Lactobacillus reuteri ATCC PTA 6475 counteracts a degradation of gut microbiota in older women with low bone mineral density. NPJ Biofilms Microbiomes. (2022) 8:84. doi: 10.1038/s41522-022-00348-2
15. Indrio F and Salatto A. Gut microbiota-bone axis. Ann Nutr Metab. (2025) 81:47–56. doi: 10.1159/000541999
16. Novince CM, Whittow CR, Aartun JD, Hathaway JD, Poulides N, Chavez MB, et al. Commensal gut microbiota immunomodulatory actions in bone marrow and liver have catabolic effects on skeletal homeostasis in health. Sci Rep. (2017) 7:5747. doi: 10.1038/s41598-017-06126-x
17. Kondo T, Chiba T, and Tousen Y. Short-chain fatty acids, acetate and propionate, directly upregulate osteoblastic differentiation. Int J Food Sci Nutr. (2022) 73:800–8. doi: 10.1080/09637486.2022.2078285
18. Uchida Y, Irie K, Fukuhara D, Kataoka K, Hattori T, Ono M, et al. Commensal microbiota enhance both osteoclast and osteoblast activities. Molecules (Basel Switzerland). (2018) 23:1517. doi: 10.3390/molecules23071517
19. Pacifici R. Bone remodeling and the microbiome. Cold Spring Harbor Perspect Med. (2018) 8:a031203. doi: 10.1101/cshperspect.a031203
20. Xu Z, Xie Z, Sun J, Huang S, Chen Y, Li C, et al. Gut microbiome reveals specific dysbiosis in primary osteoporosis. Front Cell Infection Microbiol. (2020) 10:160. doi: 10.3389/fcimb.2020.00160
21. Wei M, Li C, Dai Y, Zhou H, Cui Y, Zeng Y, et al. High-throughput absolute quantification sequencing revealed osteoporosis-related gut microbiota alterations in Han Chinese elderly. Front Cell Infection Microbiol. (2021) 11:630372. doi: 10.3389/fcimb.2021.630372
22. Chen C, Dong B, Wang Y, Zhang Q, Wang B, Feng S, et al. The role of Bacillus acidophilus in osteoporosis and its roles in proliferation and differentiation. J Clin Lab Anal. (2020) 34:e23471. doi: 10.1002/jcla.23471
23. Sun S, Luo L, Liang W, Yin Q, Guo J, Rush AM, et al. Bifidobacterium alters the gut microbiota and modulates the functional metabolism of T regulatory cells in the context of immune checkpoint blockade. Proc Natl Acad Sci United States America. (2020) 117:27509–15. doi: 10.1073/pnas.1921223117
24. Yu M, Malik Tyagi A, Li J-Y, Adams J, Denning TL, Weitzmann MN, et al. PTH induces bone loss via microbial-dependent expansion of intestinal TNF+ T cells and Th17 cells. Nat Commun. (2020) 11:468. doi: 10.1038/s41467-019-14148-4
25. Chen B, Ye D, Luo L, Liu W, Peng K, Shu X, et al. Adhesive bacteria in the terminal ileum of children correlates with increasing Th17 cell activation. Front Pharmacol. (2020) 11:588560. doi: 10.3389/fphar.2020.588560
26. Li K, Jiang Y, Wang N, Lai L, Xu S, Xia T, et al. Traditional Chinese medicine in osteoporosis intervention and the related regulatory mechanism of gut microbiome. Am J Chin Med. (2023) 51:1957–81. doi: 10.1142/S0192415X23500866
27. Wang L, Zhang K, Zeng Y, Luo Y, Peng J, Zhang J, et al. Gut mycobiome and metabolic diseases: The known, the unknown, and the future. Pharmacol Res. (2023) 193:106807. doi: 10.1016/j.phrs.2023.106807
28. Chen C-Y, Rao S-S, Yue T, Tan Y-J, Yin H, Chen L-J, et al. Glucocorticoid-induced loss of beneficial gut bacterial extracellular vesicles is associated with the pathogenesis of osteonecrosis. Sci Adv. (2022) 8:eabg8335. doi: 10.1126/sciadv.abg8335
29. Li W, Lai K, Chopra N, Zheng Z, Das A, and Diwan AD. Gut-disc axis: A cause of intervertebral disc degeneration and low back pain? Eur Spine J. (2022) 31:917–25. doi: 10.1007/s00586-022-07152-8
30. Elam RE, Bůžková P, Barzilay JI, Wang Z, Nemet I, Budoff MJ, et al. Trimethylamine N-oxide and hip fracture and bone mineral density in older adults: The cardiovascular health study. Bone. (2022) 161:116431. doi: 10.1016/j.bone.2022.116431
31. Liu T, Yu H, Wang S, Li H, Du X, and He X. Chondroitin sulfate alleviates osteoporosis caused by calcium deficiency by regulating lipid metabolism. Nutr Metab. (2023) 20:6. doi: 10.1186/s12986-023-00726-3
32. Coker OO, Liu C, Wu WKK, Wong SH, Jia W, Sung JJY, et al. Altered gut metabolites and microbiota interactions are implicated in colorectal carcinogenesis and can be non-invasive diagnostic biomarkers. Microbiome. (2022) 10:35. doi: 10.1186/s40168-021-01208-5
33. Xu X, Zhan G, Chen R, Wang D, Guan S, and Xu H. Gut microbiota and its role in stress-induced hyperalgesia: Gender-specific responses linked to different changes in serum metabolites. Pharmacol Res. (2022) 177:106129. doi: 10.1016/j.phrs.2022.106129
34. Han D, Wang W, Gong J, Ma Y, and Li Y. Microbiota metabolites in bone: Shaping health and Confronting disease. Heliyon. (2024) 10:e28435. doi: 10.1016/j.heliyon.2024.e28435
35. Lynch SV and Pedersen O. The human intestinal microbiome in health and disease. New Engl J Med. (2016) 375:2369–79. doi: 10.1056/NEJMra1600266
36. Erttmann SF, Swacha P, Aung KM, Brindefalk B, Jiang H, Härtlova A, et al. The gut microbiota prime systemic antiviral immunity via the cGAS-STING-IFN-I axis. Immunity. (2022) 55:847–61.e10. doi: 10.1016/j.immuni.2022.04.006
37. Wastyk HC, Fragiadakis GK, Perelman D, Dahan D, Merrill BD, Yu FB, et al. Gut-microbiota-targeted diets modulate human immune status. Cell. (2021) 184:4137–53.e14. doi: 10.1016/j.cell.2021.06.019
38. Schluter J, Peled JU, Taylor BP, Markey KA, Smith M, Taur Y, et al. The gut microbiota is associated with immune cell dynamics in humans. Nature. (2020) 588:303–7. doi: 10.1038/s41586-020-2971-8
39. Raphael I, Nalawade S, Eagar TN, and Forsthuber TG. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine. (2015) 74:5–17. doi: 10.1016/j.cyto.2014.09.011
40. Zaiss MM, Axmann R, Zwerina J, Polzer K, Gückel E, Skapenko A, et al. Treg cells suppress osteoclast formation: A new link between the immune system and bone. Arthritis Rheumatism. (2007) 56:4104–12. doi: 10.1002/art.23138
41. Lei H, Schmidt-Bleek K, Dienelt A, Reinke P, and Volk H-D. Regulatory T cell-mediated anti-inflammatory effects promote successful tissue repair in both indirect and direct manners. Front Pharmacol. (2015) 6:184. doi: 10.3389/fphar.2015.00184
42. Okamoto K, Nakashima T, Shinohara M, Negishi-Koga T, Komatsu N, Terashima A, et al. Osteoimmunology: the conceptual framework unifying the immune and skeletal systems. Physiol Rev. (2017) 97:1295–349. doi: 10.1152/physrev.00036.2016
43. Tyagi AM, Yu M, Darby TM, Vaccaro C, Li J-Y, Owens JA, et al. The microbial metabolite butyrate stimulates bone formation via T regulatory cell-mediated regulation of WNT10B expression. Immunity. (2018) 49:1116–31.e7. doi: 10.1016/j.immuni.2018.10.013
44. Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Sci (New York N.Y.). (2011) 331:337–41. doi: 10.1126/science.1198469
45. Yan J, Herzog JW, Tsang K, Brennan CA, Bower MA, Garrett WS, et al. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci United States America. (2016) 113:E7554–63. doi: 10.1073/pnas.1607235113
46. Guo M, Liu H, Yu Y, Zhu X, Xie H, Wei C, et al. Lactobacillus rhamnosus GG ameliorates osteoporosis in ovariectomized rats by regulating the Th17/Treg balance and gut microbiota structure. Gut Microbes. (2023) 15:2190304. doi: 10.1080/19490976.2023.2190304
47. Wesemann DR. Microbes and B cell development. Adv Immunol. (2015) 125:155–78. doi: 10.1016/bs.ai.2014.09.005
48. Tong X, Gu J, Song R, Wang D, Sun Z, Sui C, et al. Osteoprotegerin inhibit osteoclast differentiation and bone resorption by enhancing autophagy via AMPK/mTOR/p70S6K signaling pathway in vitro. J Cell Biochem. (2019) 120:1630–42. doi: 10.1002/jcb.27468
49. Shim JA, Ryu JH, Jo Y, and Hong C. The role of gut microbiota in T cell immunity and immune mediated disorders. Int J Biol Sci. (2023) 19:1178–91. doi: 10.7150/ijbs.79430
50. Leite FRM, de Aquino SG, Guimarães MR, Cirelli JA, Zamboni DS, Silva JS, et al. Relevance of the myeloid differentiation factor 88 (MyD88) on RANKL, OPG, and nod expressions induced by TLR and IL-1R signaling in bone marrow stromal cells. Inflammation. (2015) 38:1–8. doi: 10.1007/s10753-014-0001-4
51. Li L, Rao S, Cheng Y, Zhuo X, Deng C, Xu N, et al. Microbial osteoporosis: The interplay between the gut microbiota and bones via host metabolism and immunity. MicrobiologyOpen. (2019) 8:e00810. doi: 10.1002/mbo3.810
52. Clark A and Mach N. The crosstalk between the gut microbiota and mitochondria during exercise. Front Physiol. (2017) 8:319. doi: 10.3389/fphys.2017.00319
53. Peek CT, Ford CA, Eichelberger KR, Jacobse J, Torres TP, Maseda D, et al. Intestinal inflammation promotes MDL-1+ Osteoclast precursor expansion to trigger osteoclastogenesis and bone loss. Cell Mol Gastroenterol Hepatol. (2022) 14:731–50. doi: 10.1016/j.jcmgh.2022.07.002
54. Gomes AC, Hoffmann C, and Mota JF. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes. (2018) 9:308–25. doi: 10.1080/19490976.2018.1465157
55. Qi X, Yun C, Pang Y, and Qiao J. The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes. (2021) 13:1–21. doi: 10.1080/19490976.2021.1894070
56. McDougall KE, Perry MJ, Gibson RL, Colley SM, Korach KS, and Tobias JH. Estrogen receptor-alpha dependency of estrogen’s stimulatory action on cancellous bone formation in male mice. Endocrinology. (2003) 144:1994–9. doi: 10.1210/en.2002-0074
57. Samuels A, Perry MJ, Goodship AE, Fraser WD, and Tobias JH. Is high-dose estrogen-induced osteogenesis in the mouse mediated by an estrogen receptor? Bone. (2000) 27:41–6. doi: 10.1016/s8756-3282(00)00289-1
58. Lindberg MK, Alatalo SL, Halleen JM, Mohan S, Gustafsson JA, and Ohlsson C. Estrogen receptor specificity in the regulation of the skeleton in female mice. J Endocrinol. (2001) 171:229–36. doi: 10.1677/joe.0.1710229
59. Carlsten H. Immune responses and bone loss: The estrogen connection. Immunol Rev. (2005) 208:194–206. doi: 10.1111/j.0105-2896.2005.00326.x
60. Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, and Riggs BL. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest. (2003) 111:1221–30. doi: 10.1172/JCI17215
61. Charatcharoenwitthaya N, Khosla S, Atkinson EJ, McCready LK, and Riggs BL. Effect of blockade of TNF-alpha and interleukin-1 action on bone resorption in early postmenopausal women. J Bone Mineral Research: Off J Am Soc Bone Mineral Res. (2007) 22:724–9. doi: 10.1359/jbmr.070207
62. Lee S-K, Kadono Y, Okada F, Jacquin C, Koczon-Jaremko B, Gronowicz G, et al. T lymphocyte-deficient mice lose trabecular bone mass with ovariectomy. J Bone Mineral Res. (2006) 21:1704–12. doi: 10.1359/jbmr.060726
63. Ma S, Qin J, Hao Y, Shi Y, and Fu L. Structural and functional changes of gut microbiota in ovariectomized rats and their correlations with altered bone mass. Aging. (2020) 12:10736–53. doi: 10.18632/aging.103290
64. Li J-Y, Chassaing B, Tyagi AM, Vaccaro C, Luo T, Adams J, et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest. (2016) 126:2049–63. doi: 10.1172/JCI86062
65. Park K-R, Kim E-C, Hong JT, and Yun H-M. Dysregulation of 5-hydroxytryptamine 6 receptor accelerates maturation of bone-resorbing osteoclasts and induces bone loss. Theranostics. (2018) 8:3087–98. doi: 10.7150/thno.24426
66. Kennedy PJ, Cryan JF, Dinan TG, and Clarke G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology. (2017) 112:399–412. doi: 10.1016/j.neuropharm.2016.07.002
67. Yadav VK, Balaji S, Suresh PS, Liu XS, Lu X, Li Z, et al. Pharmacological inhibition of gut-derived serotonin synthesis is a potential bone anabolic treatment for osteoporosis. Nat Med. (2010) 16:308–12. doi: 10.1038/nm.2098
68. Fuhrman BJ, Feigelson HS, Flores R, Gail MH, Xu X, Ravel J, et al. Associations of the fecal microbiome with urinary estrogens and estrogen metabolites in postmenopausal women. J Clin Endocrinol Metab. (2014) 99:4632–40. doi: 10.1210/jc.2014-2222
69. Reigstad CS, Salmonson CE, Rainey JF, Szurszewski JH, Linden DR, Sonnenburg JL, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. (2015) 29:1395–403. doi: 10.1096/fj.14-259598
70. RoshChina VV. New trends and perspectives in the evolution of neurotransmitters in microbial, plant, and animal cells. Adv Exp Med Biol. (2016) 874:25–77. doi: 10.1007/978-3-319-20215-0_2
71. Spohn SN and Mawe GM. Non-conventional features of peripheral serotonin signalling—The gut and beyond. Nat Rev Gastroenterol Hepatol. (2017) 14:412–20. doi: 10.1038/nrgastro.2017.51
72. Muñoz-Torres M and García-Martín A. Primary hyperparathyroidism. Medicina Clinica. (2018) 150:226–32. doi: 10.1016/j.medcli.2017.07.020
73. Li J-Y, Yu M, Tyagi AM, Vaccaro C, Hsu E, Adams J, et al. IL-17 receptor signaling in osteoblasts/osteocytes mediates PTH-induced bone loss and enhances osteocytic RANKL production. J Bone Mineral Res. (2019) 34:349–60. doi: 10.1002/jbmr.3600
74. Lyu Z, Hu Y, Guo Y, and Liu D. Modulation of bone remodeling by the gut microbiota: A new therapy for osteoporosis. Bone Res. (2023) 11:31. doi: 10.1038/s41413-023-00264-x
75. Li J-Y, Yu M, Pal S, Tyagi AM, Dar H, Adams J, et al. Parathyroid hormone-dependent bone formation requires butyrate production by intestinal microbiota. J Clin Invest. (2020) 130:1767–81. doi: 10.1172/JCI133473
76. Chen T-H, Chen W-M, Hsu K-H, Kuo C-D, and Hung S-C. Sodium butyrate activates ERK to regulate differentiation of mesenchymal stem cells. Biochem Biophys Res Commun. (2007) 355:913–8. doi: 10.1016/j.bbrc.2007.02.057
77. Katono T, Kawato T, Tanabe N, Suzuki N, Iida T, Morozumi A, et al. Sodium butyrate stimulates mineralized nodule formation and osteoprotegerin expression by human osteoblasts. Arch Oral Biol. (2008) 53:903–9. doi: 10.1016/j.archoralbio.2008.02.016
78. Kobayashi Y, Uehara S, Udagawa N, and Takahashi N. Regulation of bone metabolism by Wnt signals. J Biochem. (2016) 159:387–92. doi: 10.1093/jb/mvv124
79. Hu J, Lin X, Gao P, Zhang Q, Zhou B, Wang O, et al. Genotypic and phenotypic spectrum and pathogenesis of WNT1 variants in a large cohort of patients with OI/osteoporosis. J Clin Endocrinol Metab. (2023) 108:1776–86. doi: 10.1210/clinem/dgac752
80. Ding Y, Liu W, Zhang X, Xue B, Yang X, Zhao C, et al. Bicarbonate-rich mineral water mitigates hypoxia-induced osteoporosis in mice via gut microbiota and metabolic pathway regulation. Nutrients. (2025) 17:998. doi: 10.3390/nu17060998
81. Clarke G, Stilling RM, Kennedy PJ, Stanton C, Cryan JF, and Dinan TG. Minireview: Gut microbiota: the neglected endocrine organ. Mol Endocrinol (Baltimore Md.). (2014) 28:1221–38. doi: 10.1210/me.2014-1108
82. Villa JKD, Diaz MAN, Pizziolo VR, and Martino HSD. Effect of vitamin K in bone metabolism and vascular calcification: A review of mechanisms of action and evidences. Crit Rev Food Sci Nutr. (2017) 57:3959–70. doi: 10.1080/10408398.2016.1211616
83. Ahire JJ, Kumar V, and Rohilla A. Understanding osteoporosis: human bone density, genetic mechanisms, gut microbiota, and future prospects. Probiotics Antimicrobial Proteins. (2024) 16:875–83. doi: 10.1007/s12602-023-10185-0
84. Szabo A, Kovacs A, Frecska E, and Rajnavolgyi E. Psychedelic N,N-dimethyltryptamine and 5-methoxy-N,N-dimethyltryptamine modulate innate and adaptive inflammatory responses through the sigma-1 receptor of human monocyte-derived dendritic cells. PloS One. (2014) 9:e106533. doi: 10.1371/journal.pone.0106533
85. Niu H, Zhou X, Gong P, Jiao Y, Zhang J, Wu Y, et al. Effect of lactobacillus rhamnosus MN-431 producing indole derivatives on complementary feeding-induced diarrhea rat pups through the enhancement of the intestinal barrier function. Mol Nutr Food Res. (2022) 66:e2100619. doi: 10.1002/mnfr.202100619
86. Tchekalarova J, Stoyanova T, Tzoneva R, Angelova V, and Andreeva-Gateva P. The anticonvulsant effect of a novel indole-related compound in the kainate-induced status epilepticus in mice: the role of the antioxidant and anti-inflammatory mechanism. Neurochemical Res. (2022) 47:327–34. doi: 10.1007/s11064-021-03447-2
87. Xiao H-W, Cui M, Li Y, Dong J-L, Zhang S-Q, Zhu C-C, et al. Gut microbiota-derived indole 3-propionic acid protects against radiation toxicity via retaining acyl-CoA-binding protein. Microbiome. (2020) 8:69. doi: 10.1186/s40168-020-00845-6
88. Mann ER, Lam YK, and Uhlig HH. Short-chain fatty acids: Linking diet, the microbiome and immunity. Nat Rev Immunol. (2024) 24:577–95. doi: 10.1038/s41577-024-01014-8
89. Domazetovic V, Marcucci G, Iantomasi T, Brandi ML, and Vincenzini MT. Oxidative stress in bone remodeling: Role of antioxidants. Clin cases Mineral Bone Metab. (2017) 14:209–16. doi: 10.11138/ccmbm/2017.14.1.209
90. Wang Y, Wu Y, Wang Y, Xu H, Mei X, Yu D, et al. Antioxidant properties of probiotic bacteria. Nutrients. (2017) 9:521. doi: 10.3390/nu9050521
91. Hamer HM, Jonkers DMAE, Bast A, Vanhoutvin SALW, Fischer MAJG, Kodde A, et al. Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin Nutr (Edinburgh Scotland). (2009) 28:88–93. doi: 10.1016/j.clnu.2008.11.002
92. Grassi F, Tyagi AM, Calvert JW, Gambari L, Walker LD, Yu M, et al. Hydrogen sulfide is a novel regulator of bone formation implicated in the bone loss induced by estrogen deficiency. J Bone Mineral Res. (2016) 31:949–63. doi: 10.1002/jbmr.2757
93. Wu Y-L, Zhang C-H, Teng Y, Pan Y, Liu N-C, Liu P-X, et al. Propionate and butyrate attenuate macrophage pyroptosis and osteoclastogenesis induced by CoCrMo alloy particles. Military Med Res. (2022) 9:46. doi: 10.1186/s40779-022-00404-0
94. He J, Chu Y, Li J, Meng Q, Liu Y, Jin J, et al. Intestinal butyrate-metabolizing species contribute to autoantibody production and bone erosion in rheumatoid arthritis. Sci Adv. (2022) 8:eabm1511. doi: 10.1126/sciadv.abm1511
95. Yan J and Charles JF. Gut microbiome and bone: to build, destroy, or both? Curr Osteoporosis Rep. (2017) 15:376–84. doi: 10.1007/s11914-017-0382-z
96. Koh A, De Vadder F, Kovatcheva-Datchary P, and Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. (2016) 165:1332–45. doi: 10.1016/j.cell.2016.05.041
97. Weaver CM. Diet, gut microbiome, and bone health. Curr Osteoporosis Rep. (2015) 13:125–30. doi: 10.1007/s11914-015-0257-0
98. Chen C, Cao Z, Lei H, Zhang C, Wu M, Huang S, et al. Microbial tryptophan metabolites ameliorate ovariectomy-induced bone loss by repairing intestinal AhR-mediated gut-bone signaling pathway. Advanced Sci (Weinheim Baden-Wurttemberg Germany). (2024) 11:e2404545. doi: 10.1002/advs.202404545
99. Park-Min K-H. Mechanisms involved in normal and pathological osteoclastogenesis. Cell Mol Life Sciences: CMLS. (2018) 75:2519–28. doi: 10.1007/s00018-018-2817-9
100. Seely KD, Kotelko CA, Douglas H, Bealer B, and Brooks AE. The human gut microbiota: A key mediator of osteoporosis and osteogenesis. Int J Mol Sci. (2021) 22:9452. doi: 10.3390/ijms22179452
101. Manor O, Dai CL, Kornilov SA, Smith B, Price ND, Lovejoy JC, et al. Health and disease markers correlate with gut microbiome composition across thousands of people. Nat Commun. (2020) 11:5206. doi: 10.1038/s41467-020-18871-1
102. Hayakawa H, Sobue F, Motoyama K, Yoshimura T, and Hemmi H. Identification of enzymes involved in the mevalonate pathway of Flavobacterium johnsoniae. Biochem Biophys Res Commun. (2017) 487:702–8. doi: 10.1016/j.bbrc.2017.04.120
103. Chen KLA, Liu X, Zhao YC, Hieronymi K, Rossi G, Auvil LS, et al. Long-term administration of conjugated estrogen and bazedoxifene decreased murine fecal β-glucuronidase activity without impacting overall microbiome community. Sci Rep. (2018) 8:8166. doi: 10.1038/s41598-018-26506-1
104. Papageorgiou M and Biver E. Interactions of the microbiome with pharmacological and non-pharmacological approaches for the management of ageing-related musculoskeletal diseases. Ther Adv Musculoskeletal Dis. (2021) 13:1759720X211009018. doi: 10.1177/1759720X211009018
105. Yin P, Yi S, Du T, Zhang C, Yu L, Tian F, et al. Dynamic response of different types of gut microbiota to fructooligosaccharides and inulin. Food Funct. (2024) 15:1402–16. doi: 10.1039/d3fo04855a
106. Zhao X, He W, Jakobsen LMA, Panah FM, Barbosa Correia BS, Nielsen DS, et al. Influence of dairy matrix on the prebiotic effects of inulin related to gut metabolic activity and bone health. Food Funct. (2024) 15:11129–40. doi: 10.1039/d4fo01635a
107. Schmidt TSB, Li SS, Maistrenko OM, Akanni W, Coelho LP, Dolai S, et al. Drivers and determinants of strain dynamics following fecal microbiota transplantation. Nat Med. (2022) 28:1902–12. doi: 10.1038/s41591-022-01913-0
108. Li J, Fan R, Zhang Z, Zhao L, Han Y, Zhu Y, et al. Role of gut microbiota in rheumatoid arthritis: Potential cellular mechanisms regulated by prebiotic, probiotic, and pharmacological interventions. Microbiological Res. (2025) 290:127973. doi: 10.1016/j.micres.2024.127973
109. Maseda D and Ricciotti E. NSAID-gut microbiota interactions. Front Pharmacol. (2020) 11:1153. doi: 10.3389/fphar.2020.01153
110. Ng RW, Dharmaratne P, Wong S, Hawkey P, Chan P, and Ip M. Revisiting the donor screening protocol of faecal microbiota transplantation (FMT): A systematic review. Gut. (2024) 73:1029–31. doi: 10.1136/gutjnl-2023-329515
111. Ooijevaar RE, van Nood E, Goorhuis A, Terveer EM, van Prehn J, Verspaget HW, et al. Ten-year follow-up of patients treated with fecal microbiota transplantation for recurrent clostridioides difficile infection from a randomized controlled trial and review of the literature. Microorganisms. (2021) 9:548. doi: 10.3390/microorganisms9030548
113. Nilsson AG, Sundh D, Bäckhed F, and Lorentzon M. Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: A randomized, placebo-controlled, double-blind, clinical trial. J Internal Med. (2018) 284:307–17. doi: 10.1111/joim.12805
114. Lambert MNT, Thybo CB, Lykkeboe S, Rasmussen LM, Frette X, Christensen LP, et al. Combined bioavailable isoflavones and probiotics improve bone status and estrogen metabolism in postmenopausal osteopenic women: A randomized controlled trial. Am J Clin Nutr. (2017) 106:909–20. doi: 10.3945/ajcn.117.153353
Keywords: gut-bone axis, short-chain fatty acids, osteoimmunology, FMT, probiotics, bone mineral density
Citation: Xie X, Liu H, Wan K, Li J and Qi P (2025) The gut microbiota in osteoporosis: dual roles and therapeutic prospects. Front. Immunol. 16:1617459. doi: 10.3389/fimmu.2025.1617459
Received: 24 April 2025; Accepted: 08 August 2025;
Published: 01 September 2025.
Edited by:
Francisco Jose Roig, Universidad San Jorge, SpainReviewed by:
M. Victoria Delpino, CONICET Instituto de Investigaciones Biomédicas en Retrovirus y SIDA (INBIRS), ArgentinaJiale Chen, Chengdu University of Traditional Chinese Medicine, China
Copyright © 2025 Xie, Liu, Wan, Li and Qi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Qi, ODIxODEwMTQ2QHFxLmNvbQ==
†These authors have contributed equally to this work
 Xingwen Xie1†
Xingwen Xie1† Peng Qi
Peng Qi