- 1National Clinical Research Center for Child Health and Disorders, Ministry of Education Key Laboratory of Child Development and Disorders, Children’s Hospital of Chongqing Medical University, Chongqing, China
- 2Chongqing Key Laboratory of Child Rare Diseases in Infection and Immunity, Children’s Hospital of Chongqing Medical University, Chongqing, China
- 3Department of Rheumatology & Immunology, Children’s Hospital of Chongqing Medical University, Chongqing, China
Mutations in the ELANE gene, which encodes neutrophil elastase, are known to cause cyclic neutropenia (CyN) and severe congenital neutropenia (SCN). Currently, targeting ELANE for insertion-deletion to trigger nonsense-mediated mRNA decay (NMD) may be a simple and universal method to treat SCN caused by ELANE mutations. Here, we present a patient with a heterozygous out-of-frame frameshift variant (-2 frame indel) in exon 4 of the ELANE gene. The patient underwent whole exome sequencing during hospitalization for acute parotitis and bronchitis, and found a variant in ELANE, c.581_588del AGGCCGGC (p.Q194Rfs*93), inherited from the father. The father of the patient, without neutropenia, did not present any clinically significant symptoms and showed no evidence of somatic mosaicism. During the subsequent three-year follow-up period, the patient did not experience any other major infections, and neutrophil counts remained consistently within the normal range. More importantly, bone marrow cytology indicated mild granulocytic hypoplasia without evidence of maturation arrest. This case provides clinical evidence supporting the notion that late exon frameshift -2 frame indel insertions can inhibit mRNA translation efficiency and prevent the production of mutant proteins, thereby promoting neutrophil maturation. It also bolsters the ongoing development of gene therapy for ELANE-related diseases.
Introduction
The ELANE gene codes for neutrophil elastase (NE), a serine protease predominantly localized within neutrophil granules (1). Heterozygous mutations in ELANE cause both cyclic (CyN) and severe congenital neutropenia (SCN) (2). While the pathogenic mechanisms of the ELANE gene are still being investigated, previous hypotheses suggest that mutant polypeptides encoded by ELANE play a significant role. One hypothesis proposes that ELANE mutations induce protein misfolding, triggering stress responses and ultimately leading to apoptosis (3, 4). Another hypothesis suggests that mutated peptides are misrouted but still maintain their destructive proteolytic activity (5). Previous observations suggest that the pathogenicity of ELANE mutations may be due to gain-of-function alterations in mutant NE, rather than a loss of function in normal NE (5, 6).
Here, we present an intriguing case involving a patient with a heterozygous frameshift variant in exon 4 of the ELANE gene. Clinically, the patient does not exhibit symptoms consistent with severe congenital neutropenia or cyclic neutropenia.
Method
Genetic diagnosis
Whole-exome sequencing (WES) was performed on genomic DNA of the patient. Mutations in the ELANE gene were verified by Sanger sequencing for patient and his parents.
Construction of plasmids
7.1-pCMV-3×Flag wild-type human ELANE plasmids were purchased from Unibio (China) (7). As previously described, Point mutation plasmids and frameshift mutation plasmids were generated by overlap PCR method using WT as template. Mutations were confirmed by Sanger sequencing.
Western blotting
HEK 293T (ATCC, CRL-11268) were cultured in Dulbecco’s modified Eagle medium (DMEM;Gibco) supplemented with 10% fetal bovine serum (FBS; Excellbio) at 37°C with 5% CO2. Cells were transiently transfected with either wild-type human ELANE plasmids or mutant ELANE plasmids using PEI (APExBIO) according to the manufacturer’s protocol. The cells were lyzed in radioimmunoprecipitation assay buffer (RIPA) containing phenylmethanesulfonyl fluoride (PMSF) and a protease inhibitor cocktail (PIC). Protein expression was examined by western blotting with an anti-Flag (Sellleck) mouse antibody.
Detection of mutant ELANE-induced ER stress in U2OS cells
U2OS cells (ATCC® HTB-96™) were cultured in DMEM (Gibco) supplemented with 10% fetal bovine serum (FBS; Excellbio) at 37°C with 5% CO2. Cells were transiently transfected with either wild-type human ELANE plasmids or mutant ELANE plasmids using PEI (APExBIO) according to the manufacturer’s protocol. Total RNA was extracted 24 hours post-transfection using TRIzol reagent (Invitrogen). cDNA was synthesized from 1 μg RNA using PrimeScript RT Master Mix (Takara Bio). Quantitative PCR was performed using SYBR Green Supermix (Bio-Rad) with primers specific for ER stress markers (BIP, ATF6) and 18S as an internal control (8).
Statistical analysis
Samples were compared using two-tailed, unpaired Student’s t test with GraphPad Prism 7.00. Error bars were represented by SD. *P < 0.05, **P < 0.01, ***P < 0.001.
Results
Case presentation
Here, we present an intriguing case involving a 4-year-and-4-month-old boy born to non-consanguineous parents, exhibiting a heterozygous frameshift variant in exon 4 of the ELANE gene, despite lacking evidence of congenital neutropenia (Figure 1A). At five months of age, he presented to a local hospital with an left neck mass. Physical examination revealed a left neck mass with mildly elevated local skin temperature, without erythema or ulceration. His hematologic profile revealed a consistent reduction in peripheral blood lymphocytes and neutrophils (Absolute neutrophil count range: 0.16-1.16×109/L, absolute lymphocyte count range: 0.73-3.19×109/L) (Figure 1B). Bone marrow cytology suggested mild granulocytic hypoplasia without maturation arrest. Further evaluations, including neck ultrasound and MRI, led to considerations of left acute suppurative parotitis, primary immunodeficiency, and congenital neutropenia. Based on these diagnoses, the local hospital initiated anti-infective treatment and performed incision and drainage of the neck mass. Postoperative purulent puncture culture results were positive for Staphylococcus aureus.
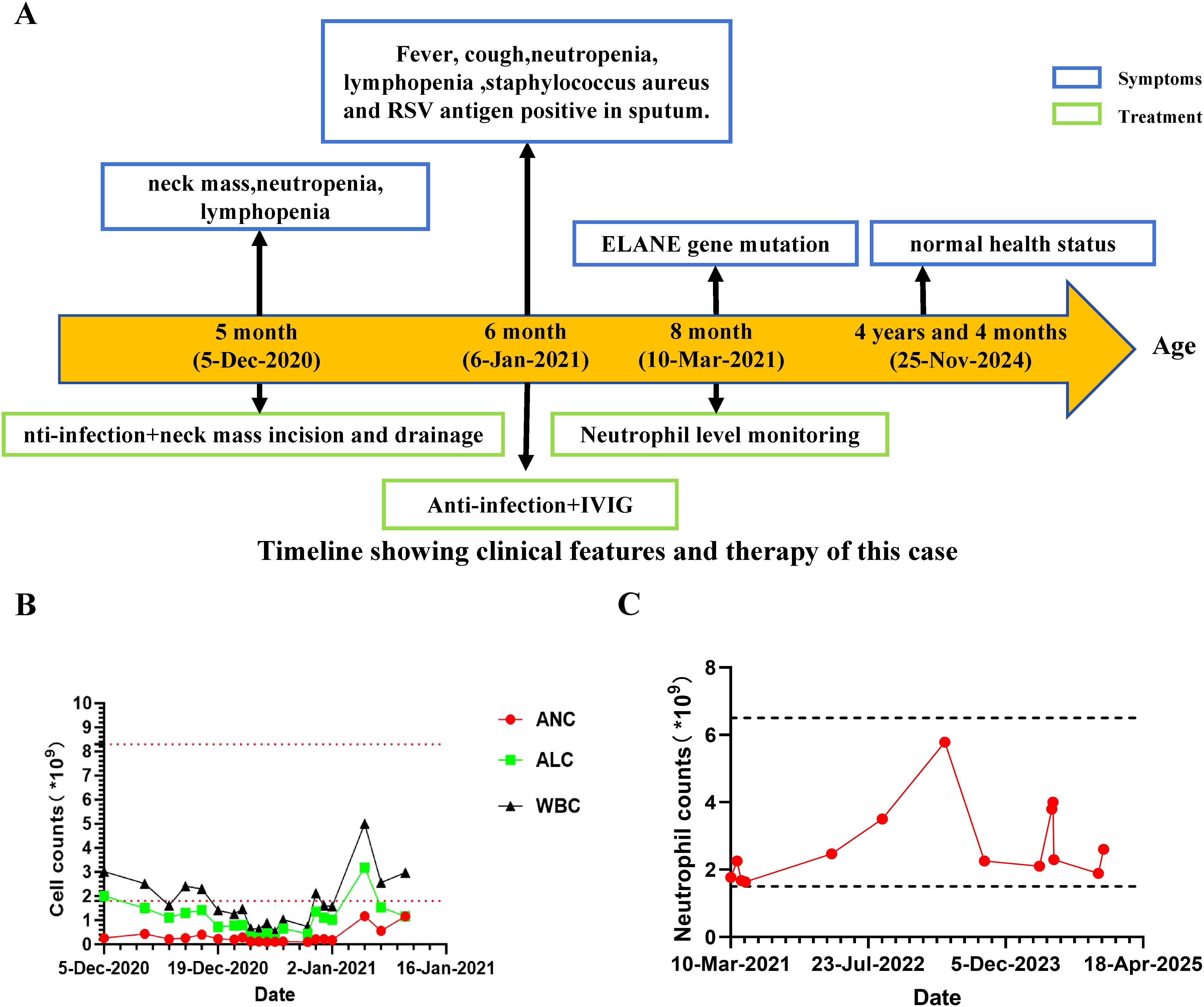
Figure 1. Clinical manifestation of this patient. (A) Clinical course of the patient. (B) Absolute number of peripheral blood white blood cells (WBC), lymphocytes, and neutrophils during follow-up. Horizontal dashed lines indicate upper and lower reference values of ANC. (C) Absolute number of peripheral blood neutrophils during the three-year follow-up.
The Patient was admitted to hospital at 6 months of age with a history of intermittent fever for over 1 month, left neck mass for 1 month, and cough for 4 days. Physical examination revealed a body temperature of 36.5°C, hyperpigmentation of the left cervical skin (approximately 6cm × 5cm), and slightly coarse breath sounds bilaterally. Chest CT plain scan demonstrated mild interstitial changes in both lungs. Combined with additional investigations (Supplementary Figure 1, Supplementary Table 1), the patient was diagnosed with primary immunodeficiency, suppurative parotitis (recovery phase), and acute bronchitis. He has exhibited good growth and development, and during the subsequent three-year follow-up, he has not experienced any major infections apart from occasional upper respiratory tract infections.
Genetic diagnosis
Suspected immune deficiency prompted WES, which identified a novel heterozygous frameshift variant in the ELANE gene, c.581_588del AGGCCGGC (p.Q194Rfs*93), inherited from his father (Figure 2A, Supplementary Table 2). This variant, not previously reported, was predicted to be likely pathogenic (Figures 2B–D, Supplementary Figure 2). Consistent with ACMG guidelines, this variant is classified as a loss-of-function allele (frameshift mutation) that is expected to disrupt gene function. Its absence in population databases (gnomAD frequency = 0) supports its preliminary classification as Likely Pathogenic (9).This variant lead to an out-of-frame heterozygous frameshift variant in exon 4 of the ELANE gene, and the -2 frame indel in the late exon 4 has led to a C-terminal peptide extended by 18 amino acids (aa) (Figure 2B, Supplementary Figure 2). Given that we failed to obtain sufficient patient samples for analysis, we overexpressed ELANE and its mutant variants in vitro. The results revealed significantly reduced protein expression in the ELANE p.Q194Rfs*93 with normal mRNA levels (Figures 3A, B). To validate whether ELANE variant-induced protein misfolding triggers endoplasmic reticulum (ER) stress response, we examined the mRNA expression levels of ER stress markers immunoglobulin heavy chain binding protein (BiP) and activating transcription factor 6 (ATF6). Compared with wild-type, human U2OS cells transiently expressing SCN-associated (P139L) ELANE mutant exhibited higher expression of both BiP and ATF6, whereas transient expression of this patient’s ELANE variant (c.581_588delAGGCCGGC) failed to induce ER stress in U2OS cells (Figure 3C).
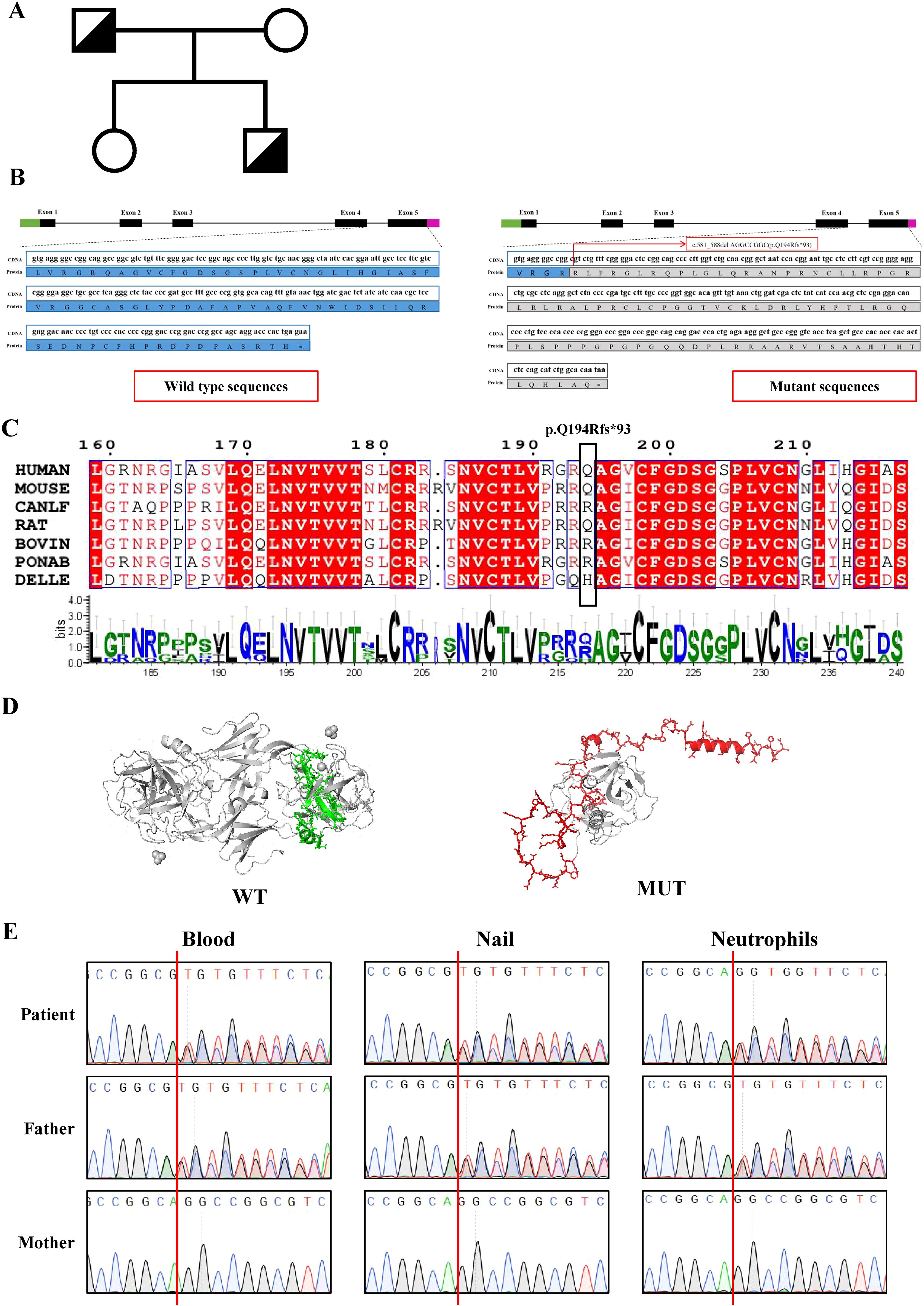
Figure 2. Identification and characterization of ELANE mutations in the patient’s family. (A) Family pedigree. ELANE Q194Rfs*93 mutation in patient and his father is indicated. (B) Schematic representation of the ELANE gene mutation (c.581_588delAGGCCGGC) and its predicted protein impact. (C) Multiple sequence alignment of ELANE across seven mammalian species shows complete conservation of the frameshift mutation region (red arrow indicates PQ194Rfs93 site). Conserved catalytic residues are marked with asterisks. (D) The wild-type ELANE structure (PDB: 7CBK) and AlphaFold3-predicted mutant are shown, with specifically altered residues colored (wild-type: green; mutant: red). (E) Sanger sequencing of the ELANE gene in patient and family members. Genomic DNA from blood, nails and neutrophils was used.
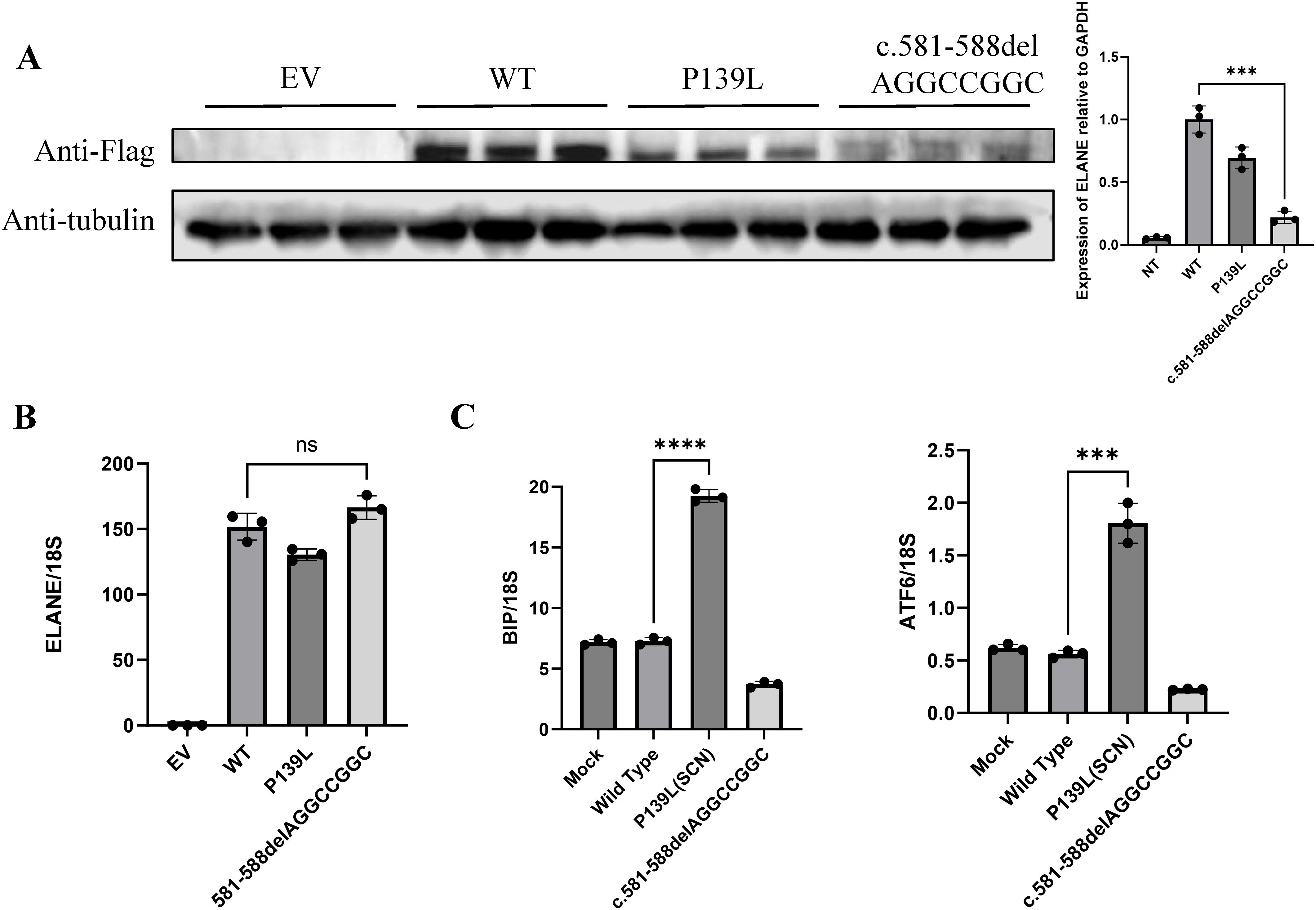
Figure 3. The ELANE c.581_588delAGGCCGGC variant lacks pathogenic evidence. (A, B) Representative images of mutant ELANE protein and mRNA expression across EV, WT, P139L, and c.581-588del AGGCCGGC. from three independent experiments. (C) Endoplasmic reticulum stress induction by mutant ELANE, representative data from two to three independent experiments are shown. "***" means "p<0.001"; "****" means "p<0.0001", and "ns" means "not significant".
Notably, the patient’s parents and sister were healthy, with no history of recurrent infections or neutropenia. Particularly, his father had no childhood periodic neutropenia. Mosaic analysis revealed no mosaic phenomenon in the patient or his father (Figure 2E).
Treatment and outcomes
The patient received active anti-infective treatment and intravenous immunoglobulin (IVIG) (supportive treatment during the acute phase of infection), and showed improvement upon discharge. Throughout the course of the illness, a decline in the absolute number of WBC, lymphocytes, and neutrophils was observed in this patient (Figure 1B). Interestingly, following the treatment of the patient’s parotitis, the number of peripheral blood lymphocytes and neutrophils gradually increased, and long-term follow-up post-discharge revealed sustained normal absolute neutrophil counts (Absolute neutrophil count range: 1.64-5.78×109/L) (Figure 1C). Importantly, he subsequently experienced no significant infection history or need for hematologic disorder treatment.
Discussions
A notable characteristic of ELANE gene mutations is their typically autosomal dominant nature, with the majority of SCN-associated ELANE mutations being missense mutations. Despite the presence of a normal ELANE allele, the aberrant NE protein still leads to neutropenia (10). Since NE appears non-essential, targeting ELANE for indel mutations to trigger NMD may be a straightforward and universal method to treat SCN caused by ELANE mutations (11). Previous studies have shown that introducing indels in ELANE exon 2 can restore neutrophil maturation in ELANE-mutant cells in vitro (12). SCN-associated frameshift mutations are limited to the penultimate exon (exon 4) and the last exon (exon 5) (10). According to the 50-nt rule, these frameshift mutations escape NMD (13). Rao et al. similarly found that efficient gene editing of early exons induced NMD, overcoming the neutrophil maturation arrest in HSPCs of ELANE-mutant SCN patients and producing normal hematopoietic engraftment (11). They observed that only -1 frame insertion or deletion (indel) of late exon (exon 4 and exon 5) can lead to the generation of premature termination codons (PTCs), escaping NMD and affecting normal protein function and causing neutrophil maturation arrest. In contrast, a -2 frame indel does not Express mutant protein, allowing normal neutrophil development. Their study demonstrates that a -2 frameshift indel generates additional amino acids, resulting in a shorter 3’ UTR. This alteration causes ELANE mRNA to shift from polysomes (actively translating) to monosomes (translationally inactive), leading to reduced translation efficiency. Both in vivo and in vitro experiments consistently showed significant enrichment of this variant in mature neutrophils.
A review of 26 frameshift mutations in the ELANE gene associated with congenital neutropenia revealed that nearly all insertion-deletion mutations lead to a single-frame shift. Moreover, among these 26 mutation types, all except one, which manifested as cyclic neutropenia, were categorized as severe congenital neutropenia (Table 1) (10, 14–16). In patient P1, a unique frameshift mutation is observed in the ELANE gene. The genotype of P1 is c.269_277del (p.R91Afs*175), occurring in exon 3, resulting in a -2 frame indel. Although P1’s clinical presentation is SCN, P1’s ELANE mutation is a compound heterozygous mutation, with the other mutation being a missense mutation in an early exon, c.278A>G (p.E93G) (14). Despite the existence of one patient with a -2 frame indel, owing to the compound heterozygous nature of the mutation and the absence of specific hematologic data, it is still presumed that in previous instances of congenital neutropenia caused by ELANE frameshift mutations, the mutations entail single-frame shifts in late exons. Analysis of ELANE frameshift variants in gnomAD (Supplementary Table 3) reveals distinct positional patterns: early exons harbor diverse frameshift types (0, -1, and -2 frame insertion or deletion), while late exons predominantly exhibit -2 frameshifts. These population frequency observations corroborate the mechanistic framework proposed by Rao et al.
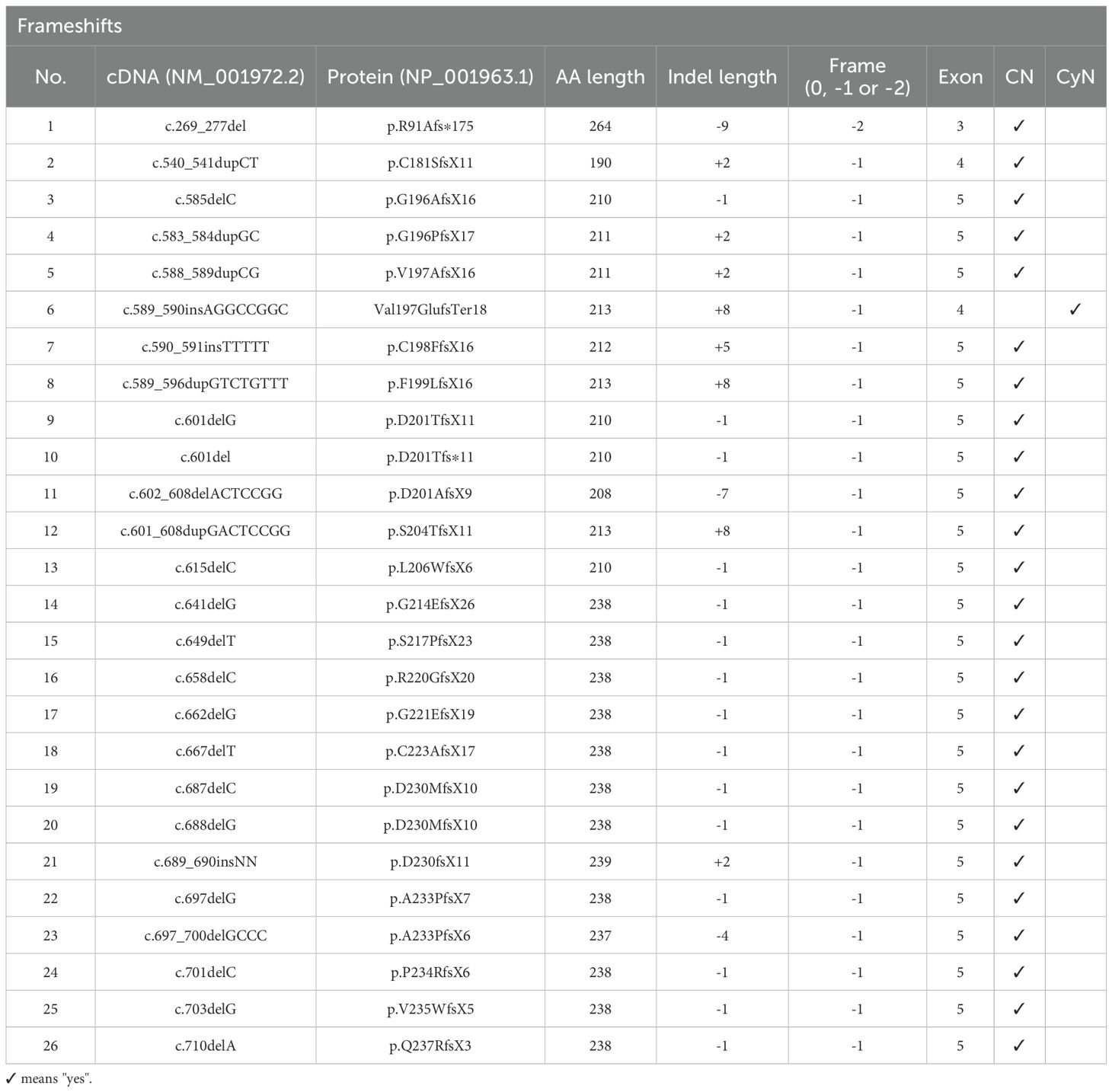
Table 1. Frameshift ELANE mutations identified in congenital neutropenia patients and Cyclic neutropenia patients.
Moreover, patients with ELANE gene deletions do not exhibit reduced neutrophil counts, providing additional support for the feasibility of inducing NMD through ELANE gene insertions or deletions as a treatment for SCN. Among the 11 documented cases of complete ELANE gene deletions, only one patient has been reported to have neutropenia, and this case lacks supporting evidence, suggesting that ELANE gene deletions do not lead to decreased neutrophil counts in patients (17). Additionally, a six-month-old Caucasian male patient with a de novo nonsense mutation in exon 2 of the ELANE gene, predicted to result in protein truncation or NMD, did not present clinically significant neutropenia (18). This absence of neutropenia may be attributed to early exon editing triggering NMD, thereby averting the maturation arrest of neutrophils, consistent with findings by Nasri et al. and Rao (11, 12).
The patient and his father in this case exhibits an out-of-frame heterozygous frameshift variant in exon 4 of the ELANE gene, and the -2 frame indel in the late exon 4 has led to a C-terminal peptide extended by 18 amino acids (aa). Our results demonstrated that although mRNA levels remain unchanged, the reduction in protein production reflects inefficient translation, and prevents the generation of mutant proteins, thus supporting neutrophil maturation (Figures 3A, B) (11, 13). From another perspective, our failure to observe endoplasmic reticulum stress alterations induced by this mutant variant further substantiates that it does not compromise neutrophil maturation (Figure 3C).
It is evident that our patient’s neutropenic episode is associated with severe acute suppurative mumps, as evidenced by the decrease in both white blood cells and lymphocytes. Importantly, these parameters all returned to normal following treatment, and over the three-year follow-up period, no history of periodic neutropenia or other signs of neutropenia were observed in the patient. While we acknowledge the possibility of other potential immune deficiencies in the patient, or that this variant ELANE c.581_588del; p.Q194Rfs*93 may reduce the efficiency of immune responses to severe infections (possibilities we cannot entirely exclude at present), we can confirm that both the patient and their father carry an out-of-frame shift variant in the ELANE gene (a -2 frame shift corresponding to the end exon), which is not expected to cause congenital neutropenia. These findings support the ongoing development of gene therapy strategies for addressing ELANE-associated diseases.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics statement
The studies involving humans were approved by the ethics committee of Children’s Hospital of Chongqing Medical University (Chongqing, China). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
YL: Data curation, Investigation, Software, Writing – original draft, Writing – review & editing. LY: Data curation, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. YZ: Data curation, Investigation, Writing – original draft. LWZ: Data curation, Investigation, Software, Writing – original draft. LNZ: Project administration, Supervision, Writing – review & editing. XT: Project administration, Resources, Supervision, Writing – review & editing. XZ: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Chongqing medical scientific research project NO.2024GGXM004 (Joint project of Chongqing Health Commission and Science and Technology Bureau).
Acknowledgments
We thank the patient and his parents for participating in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1617868/full#supplementary-material
References
1. Horwitz M, Benson KF, Person RE, Aprikyan AG, and Dale DC. Mutations in ELA2, encoding neutrophil elastase, define a 21-day biological clock in cyclic hematopoiesis. Nat Genet. (1999) 23:433–6. doi: 10.1038/70544
2. Dale DC, Person RE, Bolyard AA, Aprikyan AG, Bos C, Bonilla MA, et al. Mutations in the gene encoding neutrophil elastase in congenital and cyclic neutropenia. Blood. (2000) 96(7):2317–22. doi: 10.1182/blood.V96.7.2317
3. Xia J and Link DC. Severe congenital neutropenia and the unfolded protein response. Curr Opin Hematol. (2008) 15:1–7. doi: 10.1097/MOH.0b013e3282f13cd2
4. Köllner I, Sodeik B, Schreek S, Heyn H, Von Neuhoff N, Germeshausen M, et al. Mutations in neutrophil elastase causing congenital neutropenia lead to cytoplasmic protein accumulation and induction of the unfolded protein response. Blood. (2006) 108:493–500. doi: 10.1182/blood-2005-11-4689
5. Horwitz MS, Corey SJ, Grimes HL, and Tidwell T. ELANE mutations in cyclic and severe congenital neutropenia. Hematology/Oncology Clinics North America. (2013) 27:19–41. doi: 10.1016/j.hoc.2012.10.004
6. Horwitz MS. Neutrophil elastase: Nonsense lost in translation. Cell Stem Cell. (2021) 28:790–2. doi: 10.1016/j.stem.2021.04.007
7. Sun G, Qiu L, Yu L, An Y, Ding Y, Zhou L, et al. Loss of function mutation in ELF4 causes autoinflammatory and immunodeficiency disease in human. J ClinImmunol. (2022) 42:798–810. doi: 10.1007/s10875-022-01243-3
8. Hirata O, Okada S, Tsumura M, Karakawa S, Matsumura I, Kimura Y, et al. Mosaicism of an ELANE mutation in an asymptomatic mother in a familial case of cyclic neutropenia. J Clin Immunol. (2015) 35:512–6. doi: 10.1007/s10875-015-0165-1
9. Richards CS, Bale S, Bellissimo DB, Das S, Grody WW, Hegde MR, et al. Molecular Subcommittee of the ACMG Laboratory Quality Assurance Committee. ACMG recommendations for standards for interpretation and reporting of sequence variations: revisions 2007. Genet Med: Off J Am Coll Med Genet. (2008) 10:294–300. doi: 10.1097/GIM.0b013e31816b5cae
10. Germeshausen M, Deerberg S, Peter Y, Reimer C, Kratz CP, and Ballmaier M. The spectrum of ELANE mutations and their implications in severe congenital and cyclic neutropenia. Hum Mutat. (2013) 34:905–14. doi: 10.1002/humu.22308
11. Rao S, Yao Y, Soares De Brito J, Yao Q, Shen AH, Watkinson RE, et al. Dissecting ELANE neutropenia pathogenicity by human HSC gene editing. Cell Stem Cell. (2021) 28:833–845.e5. doi: 10.1016/j.stem.2020.12.015
12. Nasri M, Ritter M, Mir P, Dannenmann B, Aghaallaei N, Amend D, et al. CRISPR/Cas9-mediated ELANE knockout enables neutrophilic maturation of primary hematopoietic stem and progenitor cells and induced pluripotent stem cells of severe congenital neutropenia patients. Hematologica. (2020) 105:598–609. doi: 10.3324/haematol.2019.221804
13. Lindeboom RGH, Vermeulen M, Lehner B, and Supek F. The impact of nonsense-mediated mRNA decay on genetic disease, gene editing and cancer immunotherapy. Nat Genet. (2019) 51:1645–51. doi: 10.1038/s41588-019-0517-5
14. Meng X, Zhang H, Dong L, Min Q, Yu M, Li Y, et al. Impact of different genetic mutations on granulocyte development and G-CSF responsiveness in congenital neutropenia. Blood Adv. (2024) 8:1667–82. doi: 10.1182/bloodadvances.2023012171
15. Komvilaisak P, Yudhasompop N, Kanchanakamhaeng K, Hongeng S, Pakakasama S, Anurathapan U, et al. Screening for ELANE, HAX1 and GFI1 gene mutations in children with neutropenia and clinical characterization of two novel mutations in ELANE gene. BMC Pediatr. (2023) 23:592. doi: 10.1186/s12887-023-04428-w
16. Kim H-J, Song M-J, Lee K-O, Kim S-H, and Kim H-J. Paternal somatic mosaicism of a novel frameshift mutation in ELANE causing severe congenital neutropenia: somatic mosaicism of ELANE mutation. Pediatr Blood Cancer. (2015) 62:2229–31. doi: 10.1002/pbc.25654
17. Horwitz MS, Laurino MY, and Keel SB. Normal peripheral blood neutrophil numbers accompanying ELANE whole gene deletion mutation. Blood Adv. (2019) 3:2470–3. doi: 10.1182/bloodadvances.2019000498
Keywords: congenital neutropenia, CYN, SCN, ELANE, frameshift mutation, gene therapy
Citation: Li Y, Yu L, Zhang Y, Zhang L, Zhou L, Tang X and Zhao X (2025) Case Report: Heterozygous out-of-frame frameshift variant in ELANE without evidence of neutropenia. Front. Immunol. 16:1617868. doi: 10.3389/fimmu.2025.1617868
Received: 25 April 2025; Accepted: 04 July 2025;
Published: 25 July 2025.
Edited by:
Eleonora Gambineri, University of Florence, ItalyReviewed by:
Khyber Shinwari, Universidade São Francisco, BrazilXiaochuan Wu, Central South University, China
Copyright © 2025 Li, Yu, Zhang, Zhang, Zhou, Tang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaodong Zhao, emhhb3hkNTMwQGFsaXl1bi5jb20=
†These authors have contributed equally to this work
 Yulin Li
Yulin Li Lang Yu
Lang Yu Yishi Zhang1,2
Yishi Zhang1,2 Lina Zhou
Lina Zhou Xuemei Tang
Xuemei Tang Xiaodong Zhao
Xiaodong Zhao